Abstract
Simple Summary
Toxoplasmosis is a significant public health issue worldwide, caused by the intracellular protozoan Toxoplasma gondii. It has a heteroxenous life cycle in which felines act as definitive reservoirs and a wide range of warm-blooded animals, including humans, act as intermediate hosts. Due to the complex life cycle, monitoring, prevention and control of this parasite are very difficult. A thorough analysis of the epidemiology of T. gondii in humans, animals and food as well as the risk factors associated with the infection are needed to plan adequate control strategies in a given geographical area. Based on this, an integrated approach for monitoring toxoplasmosis was developed and conducted in an endemic area of southern Italy. The main tasks of this approach were based on the following strategies: parasitological and risk factor analysis for T. gondii in livestock farms, serological and molecular monitoring in meat-producing livestock at slaughterhouses, hospital discharge records (HDRs) analysis and outreach activities. The findings of this study confirmed the spread of T. gondii infection in southern Italy with high prevalence values in ruminants and the need of valid control strategies based on comprehensive and transdisciplinary actions according to the One Health approach.
Abstract
Toxoplasmosis is a widespread worldwide zoonotic infection caused by the intracellular protozoan Toxoplasma gondii. This protozoan infection is considered one of the most important food-borne parasitic zoonoses globally. Beyond its impact on public health, toxoplasmosis has also important veterinary implications, because it causes miscarriage or congenital malformations in livestock with negative economic impacts. An integrated monitoring programme aimed to deepen the epidemiological data on toxoplasmosis and to identify the risk factors that may favour T. gondii infections in animals and humans was conducted in an endemic area of southern Italy. The monitoring activities were based on the following tasks: (i) parasitological analysis and risk factors for T. gondii in livestock (sheep, goat, cattle and water buffalo) farms; (ii) serological and molecular monitoring at slaughterhouse in meat-producing livestock; (iii) analysis of hospital discharge records (HDRs); (iv) outreach activities (information, dissemination and health education) to farmers, vet practitioners and school-age children. The present study confirmed a very high seroprevalence of T. gondii infection in livestock farms (e.g., up to 93.1% in sheep farms) in southern Italy and highlighted the potentially significant public health risk in this area.
Keywords: Toxoplasma gondii, livestock, monitoring, risk factors, humans, Italy, One Health
1. Introduction
Toxoplasmosis is a zoonotic infection with a worldwide distribution caused by the intracellular protozoan Toxoplasma gondii. The definitive hosts of T. gondii are felids, and these play an essential role in the contamination of the environment with oocysts, whereas a wide range of warm-blooded animals, including humans, act as intermediate hosts [1]. Toxoplasmosis is considered one of the most important food-borne parasitic zoonoses globally [2]. Based on the disease burden, the WHO Foodborne Disease Burden Epidemiology Reference Group (FERG) listed the prioritised food-borne parasites, of these T. gondii resulted third with 1.7 million Disability Adjusted Life Years—DALYs [3]. Most infections appear to be asymptomatic in immunocompetent persons; however, the parasite can cause serious disease in humans, especially neonates [4], and immunocompromised people, who are at a risk of developing cerebral toxoplasmosis [5]. Among the several ways of transmission, the consumption of food/water contaminated with oocysts dispersed by cats and other felines, raw/undercooked meat containing tissue cysts or un-pasteurized milk containing tachyzoites, as well as the transplacental route are the most common [6,7].
Beyond its impact on public health, T. gondii infection also has important veterinary implications especially in small ruminants where the protozoa mainly affect the reproductive organs resulting in abortions, fetal mummifications, stillbirths and the birth of weak offspring. Hence, toxoplasmosis may have severe negative socio-economic effects on veterinary and public health [8].
Due to the complex life cycle, monitoring, prevention and control of this parasite are very difficult and require a comprehensive and transdisciplinary approach [9]. A thorough analysis of the epidemiology of T. gondii in humans, animals and food as well as the assessment of risk factors associated with the infection are needed in order to plan adequate control strategies in a given geographical area [10]. For this purpose, an accurate diagnosis, using highly sensitive and specific methods, is crucial. So far, diagnostic tools available to detect T. gondii infection in livestock have included direct (e.g., histopathology, immunohistochemistry, polymerase chain reaction—PCR and bioassays) and indirect methods (serological tests based on the detection of antibodies against the parasite) [11]. Currently, prevalence studies in livestock are mainly based on serological analysis, of these the enzyme-linked immunosorbent assay (ELISA) test is the most cost-effective and convenient diagnostic tool for large-scale surveys although the immunofluorescence antibody test (IFAT) is considered the gold standard. Recently, meat juice has been also suggested as serological matrix for improving meat inspection because to date, the PCR methods still show a low sensitivity [12].
Several studies have been conducted to establish the seroprevalence of T. gondii in livestock across the Italian regions. In central-northern Italy, seroprevalence ranged from 27.5% to 60.6% at individual animal level [13,14] and up to 97.0% [13] at farm level in small ruminants whereas a prevalence value of 68.4% was reported in cattle farms [15]. The same epidemiological scenario was encountered in southern and insular regions, where high prevalence values, up to 87.0%, were reported in small ruminant farms [16]. In the Campania region of southern Italy, prevalence values in livestock have been reported as follows: 77.8% in sheep [17] and 13.7% in water buffalo farms [18], respectively. Furthermore, high prevalence values (39.6%) were recently found in wild boars [19].
So as to reduce the regional spread of toxoplasmosis, since 2019, the Campania government supported and financed “ToxoCamp”, a monitoring programme that, through a multi-institutional approach, aimed at deepening the epidemiological data on toxoplasmosis and to identify the risk factors which may favour T. gondii infections in animals and humans.
This paper describes the main activities and findings of the ToxoCamp programme, highlighting the strategies of actions used in this endemic region of southern Italy. The final goal was to evaluate the real impact of this zoonosis to develop adequate control strategies according to the One Health concept.
2. Materials and Methods
2.1. Study Design
The activities of ToxoCamp were performed from January 2019 to December 2020 in the Campania region. The main tasks carried out in this programme were: (i) parasitological analysis and risk factors for T. gondii in ruminant livestock (sheep, goat, cattle and water buffalo) farms; (ii) serological and molecular monitoring at slaughterhouses in meat-producing livestock; (iii) analysis of hospital discharge records (HDRs); (iv) outreach activities (information, dissemination and health education) to farmers, vet practitioners and school-aged children.
2.2. Task 1. Parasitological Analysis and Risk Factors for T. gondii in Ruminant Livestock Farms
2.2.1. Selection of Livestock Farms
A total of 104 livestock farms (29 sheep, 26 goat, 25 cattle and 24 water buffalo farms) were selected using simple random sampling to ensure a representative sample of farms from the Campania region. Sample size was calculated considering the following parameters: population size, expected farm-prevalence (70%), absolute error (8%) and confidence level (95%) [20]. The calculations determined that a minimum of 96 farms should be sampled for this study.
2.2.2. Serological Analysis (Livestock)
In each farm, blood samples were collected from 15 adult (older than 18 months) and 5 young (4–18 months) animals (when possible). Blood samples were transferred to the laboratory on ice. After centrifugation (1690× g for 10 min), the sera were stored at −20 °C until analysis. Sera samples were tested for T. gondii antibodies by a commercial ELISA kit (ID Screen® Toxoplasmosis Indirect Multi-Species, IDVET, Montpellier, France) according to the manufacturer’s instruction. Positive and negative sera provided with the kit were used as controls. For each sample, the resulting values were calculated by applying the formula supplied in the kit: S/P% = OD sample − OD-negative control/OD-positive control − OD-negative control) × 100. Samples with S/P% ≥ 50% were considered positive.
2.2.3. Serological and Copromicroscopic Analysis (Cats)
In each farm whose livestock resulted serologically positive to T. gondii (see the results section), the cats present were subjected to parasitological examination. Trap cages for cats (https://www.cage-system.com/trappole/ accessed date: 15 June 2021) were used to allow the confinement of cats for 48 h respecting the standards on animal welfare and safety of staff (Figure 1).
Figure 1.
Confinement of a cat by using a trap cage.
After 48 h, blood and faecal samples were collected, and cats were released. The blood samples were processed and analysed using the commercial ELISA kit (ID Screen® Toxoplasmosis Indirect Multi-Species, IDVET, Montpellier, France) as described above. Copromicroscopic exams were performed using the FLOTAC dual technique [21], with sodium chloride (specific gravity – s.g. = 1.20) and zinc sulphate (s.g. = 1.20) as flotation solutions with a detection limit of 2 oocysts per gram of faeces.
2.2.4. Questionnaire Data Collection
A questionnaire was prepared to include questions on different management variables (type of production, number of animals, presence of other domestic animals at farms, presence of resident and/or stray cats, frequency of grazing, transhumance and presence of any control rodent measures) related to farm and pasture typology. The questionnaire was administered by the farm veterinarian at the time of blood sample collection to all participating farmers.
2.2.5. Statistical Analysis
The differences in seroprevalence among the different animal species were analysed by the Chi-square test. Multivariate logistic regression models were used to identify the risk factors for T. gondii seropositivity in livestock farms. Each model was applied at farm level, using all the data recorded (e.g., management variables related to farm and pasture typology; presence of cats and control rodent measures) as independent variables and the T. gondii serological status (positive/negative), as dependent variable. The odds ratio (OR) was used to estimate the strength of the association between each factor included in the study and the positive status to T. gondii. The independent variables considered in the final model were those showing probabilities <0.05. All the statistical data were analyzed using a dedicated software (SPSS, Version 22.0, IBM Corporation, Armonk, NY, USA).
2.3. Task 2. Serological and Molecular Monitoring in Meat-Producing Livestock at slaughterouses
Overall, 193 adult animals (50 sheep, 50 goats, 45 cattle and 48 water buffaloes), slaughtered in five sentinel abattoirs located in the Campania region, were randomly sampled. In addition, a total of 218 pigs, during at-home animal slaughter, were investigated.
For each animal slaughtered, a blood sample was collected from the jugular vein into tubes without anticoagulants, and approximately 50 g of myocardium and diaphragm tissue samples, respectively, were collected and placed into plastic bags suitable for biological samples. Blood and tissue samples were transported to the laboratory within a few hours; blood was centrifuged (1690× g for 10 min), and serum was transferred into Eppendorf tubes and stored at −20 °C until further serological analysis. Each tissue sample, either myocardium or diaphragm, was divided into two aliquots. The first aliquot (25 g) was frozen in a plastic bag overnight, subsequently thawed to extract meat juice, and stored at −20 °C until serological analysis. The second aliquot (25 g) was stored at −20 °C until molecular analysis.
2.3.1. Serological Analysis
Serum and meat juice samples were analyzed for T. gondii antibodies with a commercial ELISA kit (ID Screen® Toxoplasmosis Indirect Multi-Species, IDVET, Montpellier, France), according to the manufacturer’s instruction (see above), using two different dilution ratios, i.e., 1:10 and 1:2, for serum and meat juice samples, respectively.
2.3.2. K-Agreement
The Cohen’s ĸ value, for each animal species, was calculated to evaluate the agreement among different biological matrices (serum, meat juices from myocardium and diaphragm, respectively) used in the ELISA test. The κ values obtained were interpreted as follows: slight agreement (ĸ < 0.2); fair agreement (ĸ = 0.2–0.4); moderate agreement (ĸ = 0.4–0.6); good agreement (ĸ = 0.6–0.8); or very good agreement (ĸ > 0.8) [20]. All statistical analyses were performed using the SPSS software (version 22.0, IBM Corporation, Armonk, NY, USA) and the significance level was set at a p-value of ≤0.05.
2.3.3. Molecular Analysis
Twenty-five grams of each sample were homogenized by means of a stomacher (Interscience, France) and then 25 mg of each lysate sample were subjected to DNA extraction with the commercial kit QIAamp DNA Mini (Qiagen, Hilden, Germany).
The real-time PCR was used to detect the 529 bp RE gene target of T. gondii in a final reaction volume of 20 µL. Briefly, 2 μL of template DNA was added to a reaction mixture containing: 10 μL of TaqMan universal master mix, 500 nM of each primer (forward primer AF1, 5′-CACAGAAGGGACAGAAGT-3′ and reverse primer AF2, 5′-TCGCCTTCATCTACAGTC-3′), 250 nM of TaqMan probe (FAM-5CTCTCCTCCAAGACGGCTGG-BHQ-3′), 2 μL of Exo IPC Mix, 0.4 μL of Exo IPC DNA and H2O to a final volume of 20 μL. The thermal profile was as follows: 50 °C for 2 min, 95 °C for 10 min, 40 cycles: 95 °C for 15 s and 60 °C for 1 min PCR amplifications were performed on CFX96 DeepWell (Bio-Rad, Hercules, CA, USA) [22].
2.4. Task 3. Hospital Discharge Records (HDRs) Analysis
The hospital discharge records (HDRs) data from 2009 to 2013, in anonymous form and free of personal information, were provided by the Italian Department of Health. The HDRs contained an anonymous individual code for tracking patient’s hospital admissions, discharges, and readmissions. Each anonymous individual code identified one patient. Specifically, the database included: patient’s admission and discharge dates, gender, age, domicile code, type of hospitalization (ordinary hospitalization or day hospital).
2.5. Task 4. Outreach Activities
Dissemination meetings as well as webinars for farmers, veterinary practitioners and school-age children were organized throughout the duration of the programme. The objectives of these activities were focused mainly on providing key information regarding: (i) the parasite life cycle; (ii) actions to avoid infection in the animal and human population. In addition, educational materials (brochures and posters) were designed to support the activities of dissemination (Supplementary Materials).
3. Results
3.1. Task 1. Parasitological Analysis and Risk Factors for T. gondii in Ruminant Livestock Farms
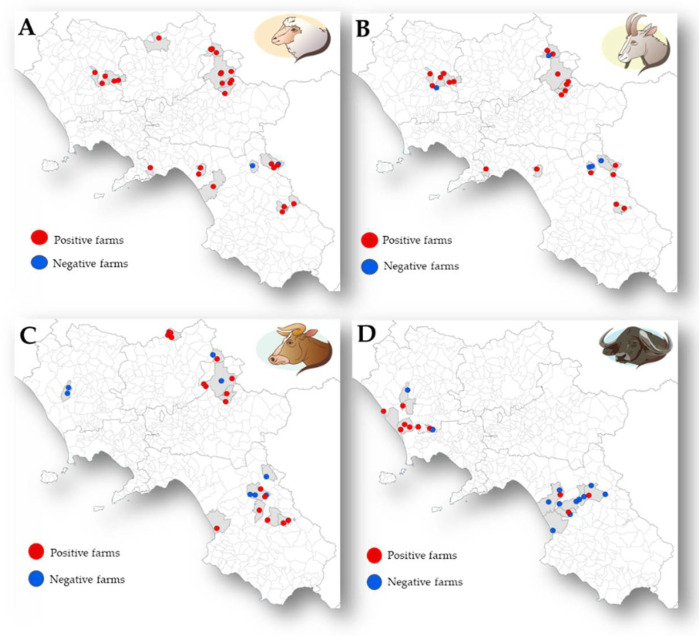
Serum samples were collected from 1127 animals in 104 farms. A total of 426 animals (37.8%; 95% Confidence Interval [CI] = 35.0–40.7) resulted positive for T. gondii with the highest mean S/P% values in sheep and goats (142% and 163%, respectively), while the mean S/P% values in cattle and water buffaloes were 93% and 59%, respectively. Seroprevalence of T. gondii varied significantly (p < 0.05) among ruminant species showing higher values in small (sheep and goats) than in large (cattle and water buffaloes) ruminants. The distribution of T. gondii in the livestock farms analysed is showed in Figure 2. The prevalence obtained from the serological analysis, both at farm and animal level, are showed in Table 1. Furthermore, Table 2 reports the seroprevalence according to the age of animals (youngs and adults) for each ruminant species analysed.
Figure 2.
Distribution of T. gondii in the livestock farms analysed according to animal species: (A) sheep; (B) goats; (C) cattle and (D) water buffaloes.
Table 1.
Overall seroprevalence of T. gondii at farm and animal level according to animal species (sheep, goats, cattle and water buffaloes).
| Animal Species | No. Farms Analysed |
No. Farms Positive |
Prevalence (%) (95%CI) | No. Animals Analysed | No. Animals Positive | Prevalence (%) (95%CI) |
|---|---|---|---|---|---|---|
| Sheep | 29 | 27 | 93.1 (75.8–98.8) | 390 | 221 | 56.7 (51.6–61.2) |
| Goats | 26 | 21 | 80.8 (60.0–92.7) | 241 | 114 | 47.3 (40.9–53.8) |
| Cattle | 25 | 17 | 68.0 (46.4–84.3) | 296 | 48 | 16.2 (12.3–21.0) |
| Water buffaloes | 24 | 11 | 45.8 (26.2–66.8) | 200 | 43 | 21.5 (16.1–28.0) |
| TOTAL | 104 | 76 | 73.1 (63.3–81.1) | 1.127 | 426 | 37.8 (35.0–40.7) |
Table 2.
Overall seroprevalence of T. gondii according to the age (youngs and adults) for each animal species (sheep, goats, cattle and water buffaloes).
| Animal Species | Age | No. Animals Analysed | No. Animals Positive | Prevalence (%) (95%CI) |
|---|---|---|---|---|
| Sheep | Youngs Adults |
78 312 |
32 190 |
41.0 (30.2–52.7) 60.9 (55.2–66.3) |
| Goats | Youngs Adults |
39 202 |
17 97 |
43.6 (28.2–60.2) 48.0 (41.0–55.1) |
| Cattle | Youngs Adults |
62 234 |
19 42 |
30.6 (19.9–43.8) 17.9 (13.4–23.6) |
| Water buffaloes | Youngs Adults |
9 191 |
0 43 |
0.0 22.5 (16.9–29.2) |
In the 76 farms whose ruminants resulted serologically positive to T. gondii, a total of 304 cats were investigated. Of these, 298 cats (98.0%; 95%CI = 95.5–99.2) resulted positive for T. gondii antibodies whereas all of them were negative for T. gondii oocysts (0.0%).
Statistical Analysis
Depending on the animal species, the multivariate logistic regression analysis identified a strong association between the seropositivity to T. gondii and the variables “presence of cats” and “abortion” (p < 0.05). In contrast, the variables “presence of young animals” and “rodent control measures” were associated with a significant low T. gondii seroprevalence (Table 3).
Table 3.
Results of the multivariate logistic regression analysis. Variables associated with seropositivity to T. gondii, according to the animal species.
| Animal Species | Variable | Standard Error | Wald’s Chi-Square | Odds Ratio | p-Value |
|---|---|---|---|---|---|
| Sheep | Young sheep (<12 months) |
0.592 | 5.852 | 0.239 | 0.016 |
| Presence of cats | 0.341 | 11.381 | 3.164 | 0.001 | |
| Goats | Presence of cats | 0.308 | 15.074 | 3.306 | 0.000 |
| Abortion | 0.284 | 6.502 | 2.064 | 0.011 | |
| Cattle | Control rodent measures | 0.608 | 13.602 | 0.106 | 0.000 |
| Water buffaloes | Abortion | 0.476 | 4.796 | 2.837 | 0.029 |
| Control rodent measures | 0.792 | 35.350 | 0.009 | 0.000 |
3.2. Task 2. Serological and Molecular Monitoring of Meat-Producing Livestock at Slaughterouses
Blood, myocardium and diaphragm tissue samples were collected from a total of 411 animals. The prevalence values obtained from serological analysis for each animal species were: 96.0%, 98.0%, 17.8%, 8.3% and 5.5% in sheep, goats, cattle, water buffaloes and pigs, respectively.
Table 4 shows the results obtained from serological and molecular analysis, for each matrix and according to animal species.
Table 4.
Results (positivity to T. gondii) of serological and molecular analysis, for each matrix and according to animal species.
| Animal Species |
No. Animals Analysed | ELISA Test | Real-Time PCR | |||
|---|---|---|---|---|---|---|
| Serum (No. Animals Positive) |
Myocardium (No. Animals Positive) |
Diaphragm (No. Animals Positive) |
Myocardium (No. Animals Positive) |
Diaphragm (No. Animals Positive) |
||
| Sheep | 50 | 48 | 47 | 45 | 1 | 0 |
| Goats | 50 | 49 | 48 | 46 | 0 | 0 |
| Cattle | 45 | 6 | 8 | 7 | 0 | 0 |
| Water buffaloes |
48 | 4 | 3 | 1 | 0 | 0 |
| Pigs | 218 | 12 | 12 | 10 | 2 | 0 |
K-Agreement
The agreements, resulted from the T. gondii antibody detection using different serological matrices (i.e., serum, meat juice from myocardium, meat juice from diaphragm), are shown in Table 5.
Table 5.
Cohen’ ĸ values obtained from the comparison of the T. gondii antibody detection using different serological matrices (i.e., serum, meat juice from myocardium, meat juice from diaphragm) from different livestock species.
| Animal Species | Serum vs. Myocardium (Cohen’s ĸ) | Serum vs. Diaphragm (Cohen’s ĸ) |
|---|---|---|
| Sheep | 0.790 | 0.545 |
| Goats | 0.658 | 0.380 |
| Cattle | 0.831 | 0.910 |
| Water Buffaloes | 0.846 | 0.379 |
| Pigs | 1 | 0.904 |
p < 0.05 is statistically significant.
The best results, with an agreement from good (0.6 < ĸ < 0.8) to very good (ĸ ≥ 0.8), were obtained between serum and meat juice from myocardium for most of the animal species analysed (sheep, goats, water buffaloes and pigs). Only in cattle, a better agreement value was found between serum and diaphragm (ĸ = 0.910) compared to serum and myocardium (ĸ = 0.831).
3.3. Task 3. Hospital Discharge Records (HDRs) Analysis
The HDRs analysis showed an incidence of human toxoplasmosis hospitalizations in the Campania region of approximately 0.72/100,000 inhabitants. Most patients with toxoplasmosis aged less than 1 year, followed by adults aged between 25 and 44 years. No difference was found regarding their gender.
3.4. Task 4. Outreach Activities
A total of 21,000 dissemination materials including brochures and posters about the toxoplasmosis with cartoon pictures tailored for both children and adults were produced and distributed to farmers, veterinarians and school-aged children.
A total of 20 meetings were organized with primary and middle school students (No. = 5), farmers (No. = 10) and veterinarians (No. = 5) to explain the different aspects of toxoplasmosis, as the disease, the life cycle and the best preventive measures.
4. Discussion
This study shed light on the current epidemiological situation of T. gondii in the Campania region of southern Italy, confirming the occurrence of a very high seroprevalence of this infection in livestock farms in southern Italy and highlighting the significant public health risk in this area.
Toxoplasmosis is widespread and its seroprevalence in livestock vary widely, reaching high values in sheep (98.9%), goats (95.2%), cattle (93.5%) and pigs (96.6%) reviewed in [1,23,24,25]. The seroprevalence rates, at individual and farm level, in livestock reported here are in line with the results from different studies conducted in other Italian regions [13,15,16,26]. According to these studies, a higher seroprevalence was recorded in small ruminants (sheep and goats) than in cattle and water buffaloes due probably to differences in susceptibility to T. gondii infection and to differences in farm management.
Since in ruminants the principal route of infection with T. gondii appears to be the ingestion of oocysts contaminating feed, water or the environment, control strategies for stray cat populations must be implemented [27]. To date, the seroprevalence values of T. gondii in domestic cats worldwide range between 10.0% and 84.7% [28]. In Italy, the seroprevalence observed in privately owned and stray cats was 42.3% [29] and 30.5% [30], respectively. Comparing with these studies, the seroprevalence observed in our survey was higher (98.0%). The different methodologies used, different sample sizes and sample populations surveyed may have contributed to these differences; therefore, it is difficult to compare the reported prevalence. Despite the high seroprevalence, no faecal samples of cats resulted positive to T. gondii oocysts, confirming that the copromicroscopic method is not a robust assay for the identification of potentially shedding cats in cross-sectional surveys [29]. Indeed, cats have been shown to excrete T. gondii oocysts for a limited and relatively short period when a primary infection takes place. Then, cats usually develop antibodies to T. gondii 1–2 weeks after they have shed oocysts [31]. This may be the reason why oocysts were not detected in the faecal samples of all the seropositive cats examined in the present study.
In order to reduce the risk of human infection with T. gondii, the knowledge of potential risk factors associated with the infection of farm animals with the parasite is of fundamental importance to implement the Hazard Analysis and Critical Control Points (HACCP), allowing the farmers to develop efficient and sustainable control measures against T. gondii infection in their farms [2].
In this study, the multivariate logistic regression model identified the presence of outdoor domestic cats as a risk factor for infection with T. gondii in farm animals, especially in sheep and goat farms, as previous reported by other authors [11,13,32]. Interestingly, a strong association between seroprevalence and access of cats to water and not to the feed store was found by Cenci-Goga et al. [13], thus supporting the importance of waterborne transmission of T. gondii in sheep farms. In contrast to Cenci-Goga et al. [13], in our study only the presence of stray cats was assessed as significant variable, not their access to water or feed store. Therefore, further analysis should be performed to evaluate the realistic chance of cats to contaminate farmland, feed or water provided to livestock [30].
Young animals in sheep farms were associated with a low seropositivity to T. gondii. Indeed, age was widely identified as a risk factor associated with the spread of T. gondii infection in numerous studies and it was observed that the seroprevalence increased with the age of the animal most likely due to the time of exposure to the infective stages of the parasite [32], thus suggesting that most infections would occur postnatally [33]. On the other hand, a strong association was found between the variable abortion and the seropositivity to T. gondii in goat and in water buffalo farms. The abortive role of Toxoplasma in small ruminants is widely recognized [23,24] whereas it is not common in cattle [34] and water buffaloes [35]. Recently, Ciuca et al. [18] showed that the co-infection by Neospora caninum and T. gondii is significantly associated with abortion in a water buffalo farm located in the same area.
Furthermore, a significant association between the presence of control rodent measures and a low seropositivity to T. gondii was found in cattle and water buffalo farms. The association between seropositivity and lacking adequate biosecurity and pest management practices was widely associated to seropositivity to T. gondii infection in livestock farms [36,37]. No association to T. gondii infection was found with the other farm management factors analysed in this study. Thus, according to the risk factor analysis, particular attention should be given to the hygienic measures and procedures in farms by, for example, keeping indoor animals, denying access to cats at sites of food storage, controlling rodents and other animal pests, and providing clean drinking water to animals. In addition, potential on-farm interventions to control T. gondii should include the vaccination of sheep [38].
To gain more insight into the role of meat as a source of human infection with T. gondii, in this study, seroprevalence in the main meat-producing livestock species was investigated. According to the results obtained in other studies [14,39,40,41], high seroprevalence values were reported during the slaughter’s activities, highlighting the fundamental role that infected meat plays in the T. gondii epidemiology. Of these, the seroprevalence obtained in pigs raised for familiar consumption are of particular importance since the pork products are, usually, consumed raw, processed only by smoking and/or salting and thus, may be potential sources of toxoplasmosis for humans [42]. Furthermore, given the important role of the sylvatic cycle in the spread of the toxoplasmosis, more attention should be paid also to the control of the sylvatic animals such as wild boars, according to the European legislation that included T. gondii in the list of zoonotic agents to be subjected to epidemiological monitoring in wildlife [43].
The results of the K-agreement analysis showed that meat juice from myocardium consistently provided the best agreement with serum results in line with previous findings [44,45], hence providing further evidence that meat juice samples can be used in seroprevalence studies where serum or plasma samples cannot be collected [12].
Compared to the total number of seropositive samples, only a few samples were found to be positive using the real-time PCR. This lower sensitivity has been also reported in other studies [12,40,41]. The low detection may be due to the limited amount of sample that can be tested or to the irregular distribution of tissue cysts in muscles and organs. To improve the sensitivity of molecular detection, a magnetic capture-based DNA extraction has been developed and used for testing large amounts of tissue (up to 100 g), increasing the probability of including a portion of tissue containing parasite DNA [46]. In addition, novel technologies, such as the droplet digital polymerase chain reaction (ddPCR) could be used to provide higher sensitivity compared to the real-time PCR [47].
The results obtained from the HDRs analysis evidenced as toxoplasmosis continues to be a public health problem in this area with an incidence of 0.72/100,000 inhabitants. It is important to note that the HDRs did not include cases managed in an outpatient setting or asymptomatic cases, so the data represent only the tip of the iceberg of the real burden of toxoplasmosis. To obtain a more detailed information about delivery and neonatal care in the Campania region, it would be appropriate to collect data from all deliveries included in the Italian Birth Register-CeDAP and integrate them with HDRs.
During the two years of ToxoCamp, outreach activities (information, dissemination and health education) to farmers, vet practitioners and school-age children have been performed to increase the Toxoplasma-related knowledge, control and prevention as well as the main risk factors. Indeed, it is well known that the effectiveness of a control program is strongly associated with a good education program addressed to the community and aimed to reduce the risk of toxoplasmosis.
5. Conclusions
Reducing T. gondii infection in animals is critical to prevent foodborne transmission of T. gondii to humans according to the One Health perspective. To increase the feasibility of preventing infection in food animals, screening to identify farms with infected animals should be routinely performed.
Finally, due to the impact of toxoplasmosis on public and veterinary health, a greater institutional awareness of the pathways of infection and comprehensive and transdisciplinary actions to control transmission are needed.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/ani11071949/s1, Figure S1: Brochure designed to support the outreach activities. Figure S2: Poster designed to support the outreach activities.
Author Contributions
Conceptualization, L.M., R.P., P.S., G.C. and L.R.; Data curation, P.P., A.B., A.G., A.M. and M.B.; Formal analysis, P.P., A.B., F.C., L.B. and A.M.; Funding acquisition, L.M., R.P. and P.S.; Investigation, P.P., A.B., F.C., L.B., A.G., A.M. and M.B.; Methodology, P.P., A.B., A.G., M.B., G.C. and L.R.; Project administration, L.M., R.P. and P.S.; Resources, F.C., L.B., G.C. and L.R.; Supervision, L.M., R.P., P.S., G.C. and L.R.; Validation, G.C. and L.R.; Writing—original draft, P.P., A.B., F.C. and L.B.; Writing—review & editing, L.M., R.P., P.S., G.C. and L.R. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the Regional Project “Control and reduction of toxoplasmosis in animals and humans- ToxoCamp”, Campania region, Italy.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and the protocol was approved by the Ethics Committee of the University of Naples Federico II (Protocol number: PG/2021/0048459).
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article or supplementary materials.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Dubey J.P., Cerqueira-Cézar C.K., Murata F.H., Kwok O.C., Hill D., Yang Y.R., Su C. All about Toxoplasma gondii infections in pigs: 2009–2020. Vet. Parasitol. 2020;288:109185. doi: 10.1016/j.vetpar.2020.109185. [DOI] [PubMed] [Google Scholar]
- 2.Opsteegh M., Schares G., Blaga R., Van der Giessen J., on behalf of the consortium . Experimental Studies on Toxoplasma Gondii in the Main Livestock Species (GP/EFSA/BIOHAZ/2013/01) EFSA Supporting Publication, EFSA; Parma, Italy: 2016. Final Report. [Google Scholar]
- 3.FERG . WHO Estimates of the Global Burden of Foodborne Diseases: Foodborne Disease Burden Epidemiology Reference Group 2007–2015. World Health Organization; Geneva, Switzerland: 2015. [Google Scholar]
- 4.Peyron F., Wallon M., Kieffer F., Graweg G. Toxoplasmosis. In: Remington J.S., Klein J.O., Wilson C.B., Nizet V., Maldonado Y.A., editors. Infectious Diseases of the Fetus and Newborn Infant. 8th ed. Elsevier Saunders; Philadelphia, PA, USA: 2016. pp. 949–1042. [Google Scholar]
- 5.Schlüter D., Barragan A. Advances and Challenges in Understanding Cerebral Toxoplasmosis. Front. Immunol. 2019;10:242. doi: 10.3389/fimmu.2019.00242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tenter A.M., Heckeroth A.R., Weiss L.M. Toxoplasma gondii: From animals to humans. Int. J. Parasitol. 2000;30:1217–1258. doi: 10.1016/S0020-7519(00)00124-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hill D., Dubey J. Toxoplasma gondii: Transmission, diagnosis and prevention. Clin. Microbiol. Infect. 2002;8:634–640. doi: 10.1046/j.1469-0691.2002.00485.x. [DOI] [PubMed] [Google Scholar]
- 8.Hill D.E., Chirukandoth S., Dubey J.P. Biology and epidemiology of Toxoplasma gondii in man and animals. Anim. Health Res. Rev. 2005;6:41–61. doi: 10.1079/AHR2005100. [DOI] [PubMed] [Google Scholar]
- 9.Djurković-Djaković O., Dupouy-Camet J., Van Der Giessen J., Dubey J.P. Toxoplasmosis: Overview from a One Health perspective. Food Waterborne Parasitol. 2019;15:e00054. doi: 10.1016/j.fawpar.2019.e00054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aguirre A.A., Longcore T., Barbieri M., Dabritz H., Hill D., Klein P.N., Lepczyk C., Lilly E.L., McLeod R., Milcarsky J., et al. The One Health Approach to Toxoplasmosis: Epidemiology, Control, and Prevention Strategies. EcoHealth. 2019;16:378–390. doi: 10.1007/s10393-019-01405-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guo M., Dubey J.P., Hill D., Buchanan R.L., Gamble H.R., Jones J.L., Pradhan A.K. Prevalence and risk factors for Toxo-plasma gondii infection in meat animals and meat products destined for human consumption. J. Food Prot. 2015;78:457–476. doi: 10.4315/0362-028X.JFP-14-328. [DOI] [PubMed] [Google Scholar]
- 12.Hamilton C.M., Kelly P.J., Bartley P.M., Burrells A., Porco A., Metzler D., Crouch K., Ketzis J.K., Innes E.A., Katzer F. Toxoplasma gondii in livestock in St. Kitts and Nevis, West Indies. Parasites Vectors. 2015;8:166. doi: 10.1186/s13071-015-0776-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cenci-Goga B.T., Ciampelli A., Sechi P., Veronesi F., Moretta I., Cambiotti V., Thompson P.N. Seroprevalence and risk factors for Toxoplasma gondii in sheep in Grosseto district, Tuscany, Italy. BMC Vet. Res. 2013;9:25. doi: 10.1186/1746-6148-9-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gazzonis A.L., Zanzani S.A., Villa L., Manfredi M.T. Toxoplasma gondii infection in meat-producing small ruminants: Meat juice serology and genotyping. Parasitol. Int. 2020;76:102060. doi: 10.1016/j.parint.2020.102060. [DOI] [PubMed] [Google Scholar]
- 15.Gazzonis A.L., Marino A.M.F., Garippa G., Rossi L., Mignone W., Dini V., Giunta R.P., Luini M., Villa L., Zanzani S.A., et al. Toxoplasma gondii seroprevalence in beef cattle raised in Italy: A multicenter study. Parasitol. Res. 2020;119:3893–3898. doi: 10.1007/s00436-020-06878-y. [DOI] [PubMed] [Google Scholar]
- 16.Vesco G., Buffolano W., La Chiusa S., Mancuso G., Caracappa S., Chianca A., Villari S., Currò V., Liga F., Petersen E. Toxoplasma gondii infections in sheep in Sicily, southern Italy. Vet. Parasitol. 2007;146:3–8. doi: 10.1016/j.vetpar.2007.02.019. [DOI] [PubMed] [Google Scholar]
- 17.Fusco G., Rinaldi L., Guarino A., Proroga Y.T.R., Pesce A., Giuseppina D.M., Cringoli G. Toxoplasma gondii in sheep from the Campania region (Italy) Vet. Parasitol. 2007;149:271–274. doi: 10.1016/j.vetpar.2007.07.020. [DOI] [PubMed] [Google Scholar]
- 18.Ciuca L., Borriello G., Bosco A., D’Andrea L., Cringoli G., Ciaramella P., Maurelli M.P., Di Loria A., Rinaldi L., Guccione J. Seroprevalence and Clinical Outcomes of Neospora caninum, Toxoplasma gondii and Besnoitia besnoiti Infections in Water Buffaloes (Bubalus bubalis) Animals. 2020;10:532. doi: 10.3390/ani10030532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sgroi G., Viscardi M., Santoro M., Borriello G., D’Alessio N., Boccia F., Pacifico L., Fioretti A., Veneziano V., Fusco G. Genotyping of Toxoplasma gondii in wild boar (Sus scrofa) in southern Italy: Epidemiological survey and associated risk for consumers. Zoonoses Public Health. 2020;67:805–813. doi: 10.1111/zph.12762. [DOI] [PubMed] [Google Scholar]
- 20.Thrusfield M. Veterinary Epidemiology. Blackwell Publishing; Oxford, UK: 2007. [Google Scholar]
- 21.Cringoli G., Rinaldi L., Maurelli M.P., Utzinger J. FLOTAC: New multivalent techniques for qualitative and quantitative copromicroscopic diagnosis of parasites in animals and humans. Nat. Protoc. 2010;5:503–515. doi: 10.1038/nprot.2009.235. [DOI] [PubMed] [Google Scholar]
- 22.Marino A.M.F., Giunta R.P., Salvaggio A., Castello A., Alfonzetti T., Barbagallo A., Aparo A., Scalzo F., Reale S., Buffolano W., et al. Toxoplasma gondii in edible fishes captured in the Mediterranean basin. Zoonoses Public Health. 2019;66:826–834. doi: 10.1111/zph.12630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dubey J., Murata F., Cerqueira-Cézar C., Kwok O., Su C. Economic and public health importance of Toxoplasma gondii infections in sheep: 2009–2020. Vet. Parasitol. 2020;286:109195. doi: 10.1016/j.vetpar.2020.109195. [DOI] [PubMed] [Google Scholar]
- 24.Dubey J.P., Murata F., Cerqueira-Cézar C.K., Kwok O. Public health and economic importance of Toxoplasma gondii infec-tions in goats: The last decade. Res. Vet. Sci. 2020;132:292–307. doi: 10.1016/j.rvsc.2020.06.014. [DOI] [PubMed] [Google Scholar]
- 25.Dubey J.P., Murata F.H.A., Cerqueira-Cézar C.K., Kwok O.C.H., Yang Y.R. Public Health Significance of Toxoplasma gondii Infections in Cattle: 2009–2020. J. Parasitol. 2020;106:772–788. doi: 10.1645/20-82. [DOI] [PubMed] [Google Scholar]
- 26.Gazzonis A., Veronesi F., Di Cerbo A.R., Zanzani S., Molineri G., Moretta I., Moretti A., Fioretti D.P., Invernizzi A., Manfredi M.T. Toxoplasma gondii in small ruminants in Northern Italy—prevalence and risk factors. Ann. Agric. Environ. Med. 2015;22:62–68. doi: 10.5604/12321966.1141370. [DOI] [PubMed] [Google Scholar]
- 27.Opsteegh M., Kortbeek T.M., Havelaar A.H., Van Der Giessen J.W.B. Intervention Strategies to Reduce Human Toxoplasma gondii Disease Burden. Clin. Infect. Dis. 2014;60:101–107. doi: 10.1093/cid/ciu721. [DOI] [PubMed] [Google Scholar]
- 28.Dubey J., Cezar C.C., Murata F.H.A., Kwok O., Yang Y., Su C. All about toxoplasmosis in cats: The last decade. Vet. Parasitol. 2020;283:109145. doi: 10.1016/j.vetpar.2020.109145. [DOI] [PubMed] [Google Scholar]
- 29.Veronesi F., Santoro A., Milardi G.L., Diaferia M., Morganti G., Ranucci D., Gabrielli S. Detection of Toxoplasma gondii in faeces of privately owned cats using two PCR assays targeting the B1 gene and the 529-bp repetitive element. Parasitol. Res. 2017;116:1063–1069. doi: 10.1007/s00436-017-5388-z. [DOI] [PubMed] [Google Scholar]
- 30.Spada E., Proverbio D., della Pepa A., Perego R., Baggiani L., DeGiorgi G.B., Domenichini G., Ferro E., Cremonesi F. Seroprevalence of feline immunodeficiency virus, feline leukaemia virus and Toxoplasma gondii in stray cat colonies in northern Italy and correlation with clinical and laboratory data. J. Feline Med. Surg. 2012;14:369–377. doi: 10.1177/1098612X12437352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dubey J. Toxoplasmosis—A waterborne zoonosis. Vet. Parasitol. 2004;126:57–72. doi: 10.1016/j.vetpar.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 32.Stelzer S., Basso W., Benavides Silván J., Ortega-Mora L.M., Maksimov P., Gethmann J., Conraths F.J., Schares G. Tox-oplasma gondii infection and toxoplasmosis in farm animals: Risk factors and economic impact. Food Waterborne Parasitol. 2019;15:e00037. doi: 10.1016/j.fawpar.2019.e00037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Basso W., Sollberger E., Schares G., Küker S., Ardüser F., Moore-Jones G., Zanolari P. Toxoplasma gondii and Neospora caninum infections in South American camelids in Switzerland and assessment of serological tests for diagnosis. Parasites Vectors. 2020;13:1–18. doi: 10.1186/s13071-020-04128-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Canada N., Meireles C.S., Rocha A., Correia Da Costa J.M., Erickson M.W., Dubey J.P. Isolation of viable Toxoplasma gondii from naturally infected aborted bovine fetuses. J. Parasitol. 2002;88:1247–1248. doi: 10.1645/0022-3395(2002)088[1247:IOVTGF]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 35.De Barros L.D., Garcia J.L., Bresciani K.D.S., Cardim S.T., Storte V.S., Headley S.A. A Review of Toxoplasmosis and Neosporosis in Water Buffalo (Bubalus bubalis) Front. Vet. Sci. 2020;7:455. doi: 10.3389/fvets.2020.00455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dubey J.P., Jones J.L. Toxoplasma gondii infection in humans and animals in the United States. Int. J. Parasitol. 2008;38:1257–1278. doi: 10.1016/j.ijpara.2008.03.007. [DOI] [PubMed] [Google Scholar]
- 37.Hill D.E., Dubey J.P. Toxoplasma gondii as a Parasite in Food: Analysis and Control. Microbiol. Spectr. 2016;4 doi: 10.1128/microbiolspec.PFS-0011-2015. [DOI] [PubMed] [Google Scholar]
- 38.Ducournau C., Moiré N., Carpentier R., Cantin P., Herkt C., Lantier I., Betbeder D., Dimier-Poisson I. Effective Nano-particle-Based Nasal Vaccine Against Latent and Congenital Toxoplasmosis in Sheep. Front. Immunol. 2020;11:2183. doi: 10.3389/fimmu.2020.02183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Papini R., Di Ciccio P., Marangi M., Ghidini S., Zanardi E., Vergara A., Giangaspero A., Nardoni S., Rocchigiani G., Mancianti F., et al. Occurrence of Toxoplasma gondii in Carcasses of Pigs Reared in Intensive Systems in Northern Italy. J. Food Prot. 2017;80:515–522. doi: 10.4315/0362-028X.JFP-16-314. [DOI] [PubMed] [Google Scholar]
- 40.Vismarra A., Barilli E., Miceli M., Mangia C., Genchi M., Brindani F., Kramer L., Bacci C. Toxoplasma gondii in the Cornigliese sheep breed in Italy: Meat juice serology, in vitro isolation and genotyping. Vet. Parasitol. 2017;243:125–129. doi: 10.1016/j.vetpar.2017.06.013. [DOI] [PubMed] [Google Scholar]
- 41.Gazzonis A.L., Marangi M., Villa L., Ragona M.E., Olivieri E., Zanzani S.A., Giangaspero A., Manfredi M.T. Toxoplasma gondii infection and biosecurity levels in fattening pigs and sows: Serological and molecular epidemiology in the intensive pig industry (Lombardy, Northern Italy) Parasitol. Res. 2018;117:539–546. doi: 10.1007/s00436-017-5736-z. [DOI] [PubMed] [Google Scholar]
- 42.Condoleo R., Rinaldi L., Sette S., Mezher Z. Risk Assessment of Human Toxoplasmosis Associated with the Consumption of Pork Meat in Italy. Risk Anal. 2018;38:1202–1222. doi: 10.1111/risa.12934. [DOI] [PubMed] [Google Scholar]
- 43.Ranucci D., Veronesi F., Moretti A., Branciari R., Miraglia D., Manfredi M.T., Piergili F.D. Seroprevalence of Toxoplasma gondii in wild boars (Sus scrofa) from Central Italy. Parasite. 2013;20:48. doi: 10.1051/parasite/2013048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Forbes L.B., Parker S.E., Gajadhar A.A. Performance of commercial ELISA and agglutination test kits for the detection of anti-Toxoplasma gondii antibodies in serum and muscle fluid of swine infected with 100, 300, 500 or 1000 oocysts. Vet. Parasitol. 2012;190:362–367. doi: 10.1016/j.vetpar.2012.06.040. [DOI] [PubMed] [Google Scholar]
- 45.Wallander C., Frössling J., Vågsholm I., Burrells A., Lundén A. “Meat juice” is not a homogeneous serological matrix. Foodborne Pathog. Dis. 2015;12:280–288. doi: 10.1089/fpd.2014.1863. [DOI] [PubMed] [Google Scholar]
- 46.Juránková J., Hůrková-Hofmannová L., Volf J., Baláž V., Piálek J. Efficacy of magnetic capture in comparison with con-ventional DNA isolation in a survey of Toxoplasma gondii in wild house mice. Eur. J. Protistol. 2014;50:11–15. doi: 10.1016/j.ejop.2013.08.002. [DOI] [PubMed] [Google Scholar]
- 47.Hindson C.M., Chevillet J.R., Briggs H.A., Gallichotte E.N., Ruf I.K., Hindson B.J., Vessella R.L., Tewari M. Absolute quantification by droplet digital PCR versus analog real-time PCR. Nat. Methods. 2013;10:1003–1005. doi: 10.1038/nmeth.2633. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are contained within the article or supplementary materials.