Abstract
The increase in human life expectancy has become a challenge to reduce the deleterious consequences of aging. Nowadays, an increasing number of the population suffer from age-associated neurodegenerative diseases including Parkinson’s disease (PD) and Alzheimer’s disease (AD). These disorders present different signs of neurodegeneration such as mitochondrial dysfunction, inflammation, and oxidative stress. Accumulative evidence suggests that the transcriptional factor nuclear factor (erythroid-derived 2)-like 2 (Nrf2) plays a vital defensive role orchestrating the antioxidant response in the brain. Nrf2 activation promotes the expression of several antioxidant enzymes that exert cytoprotective effects against oxidative damage and mitochondrial impairment. In this context, several studies have proposed a role of Nrf2 in the pathogenesis of PD and AD. Thus, we consider it important to summarize the ongoing literature related to the effects of the Nrf2 pathway in the context of these diseases. Therefore, in this review, we discuss the mechanisms involved in Nrf2 activity and its connection with mitochondria, energy supply, and antioxidant response in the brain. Furthermore, we will lead our discussion to identify the participation of the Nrf2 pathway in mitochondrial impairment and neurodegeneration present in PD and AD. Finally, we will discuss the therapeutic effects that the Nrf2 pathway activation could have on the cognitive impairment, neurodegeneration, and mitochondrial failure present in PD and AD.
Keywords: Alzheimer’s disease, Parkinson’s disease, mitochondria, neurodegeneration, Nrf2 neuroprotection
1. Introduction
In recent years, the interest to study the contribution of the Nrf2 pathway on PD and AD has become a topic of great interest [1,2]. Several studies have suggested that Nrf2 activation could be proposed as a novel strategy to reduce oxidative damage and mitochondrial dysfunction present in PD and AD [3,4]. Oxidative and mitochondrial damage occurs in the early stages of these disorders [5], suggesting that these defects could play a role in the progression of these neurodegenerative diseases (NDs) [6,7].
Mitochondria are essential organelles involved in energy supply and represent a significant source of reactive oxygen species (ROS) production in the cells [8]. The central nervous system (CNS), particularly the brain, presents high energy requirements, making it more vulnerable to mitochondrial dysfunction and oxidative stress [9]. Decreased ATP production is a common hallmark in PD and AD and can also be caused by defects in mitochondrial function such as respiratory imbalance and mitochondrial uncoupling [10].
Nrf2 activation increases the expression of antioxidant enzymes such as NAD(P)H dehydrogenase quinone 1 (NQO1), heme oxygenase 1 (HO-1), and others [9]. Consequently, these actions improve mitochondrial function and ATP synthesis and prevent oxidative damage [11]. Additionally, current studies suggests an important role of Nrf2 regulating mitochondrial biogenesis, dynamics, and mitophagy [11]. Interestingly, Nrf2 activity declines during aging [12], causing a progressive loss of glutathione (GSH) synthesis in aged-rats of 24–28 months of age [13]. The loss of GSH regulation and the existence of a pro-oxidant state in aging cells indicates that the cellular antioxidant defenses could be progressively affected by senescence [14]. In this context, it has been suggested that the Nrf2/ARE pathway is affected by aging and NDs [15]. For example, a meta-analysis of AD and PD gene expression of different tissues revealed that from 54 affected genes, 31 were downregulated genes containing ARE (antioxidant response elements) [16]. Studies from Branca and colleagues also performed in APP/PS1 mice, a transgenic mice strain that produces an excess of β-amyloid plaques and cognitive impairment, showed a decrease in Nrf2 activity and significant reduction in HO-1 levels concomitantly with AD pathology and cognitive impairment [17]. Additionally, studies related to PD pathology showed that Nrf2 expression was significantly impaired in nigral dopaminergic neurons of PD patients [18]. More importantly, this reduction of dopaminergic neurons and inflammatory-mediated microglia activation were enhanced in Nrf2 (−/−) knock out mice [19].
Finally, a growing body of evidence suggests that the activation of Nrf2 pathway can be considered as a valid therapeutic target against PD and AD [20]. Indeed, several drugs or natural compounds with Nrf2 activity have been used for proof-of-concept studies indicating that activation of this pathway could provide a positive outcome against PD [21] and AD [4].
In this review, we present an overview of the Nrf2 pathway and its contribution to oxidative damage and mitochondria failure present in AD and PD. We also discuss evidence that suggests Nrf2 activation as a promising target to ameliorate the neurodegenerative changes present in these disorders.
2. Nrf2 Pathway
2.1. Regulation of Nrf2 Pathway by Protein Stability
Nrf2 is a transcriptional factor encoded by the gene NFE2L2 related to the Cap’n’collar family of transcription factors that regulates the basal and stress-inducible expression of over 250 genes that contain ARE sequence (core sequence: (A/G)TGA(G/C)TCAGCA) [22], in their promoters [23,24,25]. These genes constitute a defensive response against oxidative agents including genes that encode for HO-1, NQO1, glutathione S-transferase (GST), glutamate-cysteine ligase (GCL), glutathione peroxidase (GPx), thioredoxin reductase (TXNRD)1, thioredoxin (TXN)1, and reduced glutathione (GSH) [26,27,28,29]. The activation of the Nrf2-ARE pathway results in increasing cellular energy and redox potential, which reduce oxidative damage [30].
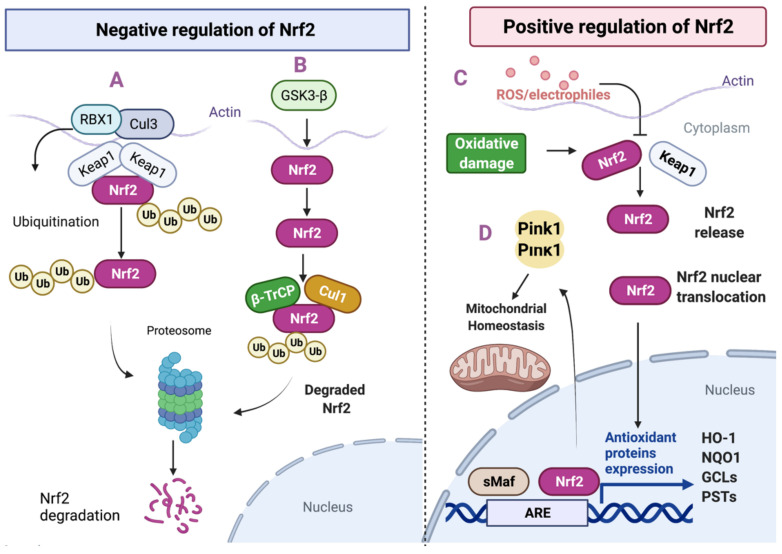
The regulation of Nrf2 occurs mainly by two specific mechanisms: the Kelch-like ECH-associated protein 1 (Keap1), and the participation of b-TrCP with the glycogen synthase kinase-3β (GSK)-3β [31] (Figure 1). In normal cells, Keap1 and the E3 ubiquitin ligase substrate adaptor regulate the Nrf2 protein levels in a redox-dependent manner [32,33]. The protein Keap1 forms a homodimer responsible for sequestering Nrf2 in the cytosol and rendering it inactive [34]. Keap1 also binds Cullin 3 (Cul3), which forms a core with the E3 ubiquitin ligase complex through an association with Ring-box1 protein (Rbx1, also called Roc1) [32,33,35,36]. In homeostatic conditions the Keap1–Cul3-Rbx1 complex can ubiquitinate Nrf2 and target it for proteasome degradation [37,38]. In response to electrophiles and oxidants, that identify and chemically modify specific cysteine residues of Keap1, Nrf2 ubiquitination is inhibited [39] (Figure 1).
Figure 1.
Regulation of the Nrf2 pathway in neuronal cells. (A) Negative regulation of Nrf2. Under basal conditions, Nrf2 expression is maintained at low levels through the proteasome activity. Nrf2 is sequestered by Keap1 in the cytosol leading to ubiquitination through the formation of the Keap1–Cul3-Rbx1 complex, which induces the Nrf2 proteasomal degradation. (B) Negative regulation of Nrf2 by GSK-3β: Nrf2 can also be conducted to degradation by GSK-3β. GSK-3 phosphorylates Nrf2 to create a recognition motif for the E3 ligase adapter β-TrCP. GSK-3/β-TrCP leads to Keap1-independent ubiquitin-proteasome degradation of Nrf2. (C) Positive regulation of Nrf2: in the presence of high ROS levels, Nrf2 is released from Keap1 binding and is translocated to the nucleus and binds to ARE gene sequences, which allows for the activation of antioxidant genes such as HO-1 and NQO1. (D) Mitochondrial homeostasis: PINK1 expression is positively regulated by Nrf2 and promotes mitochondrial homeostasis through several mechanisms such as the removal of damaged mitochondria.
Nrf2 is also subjected to degradation mediated by GSK-3β and TrCP-dependent Cul1-based ubiquitin ligase [40]. GSK-3β phosphorylates Nrf2 at Ser334–338 protein residues, which creates a degradation domain that is recognized by β-TrCP and tagged for proteasomal degradation by the Cullin 1 (Cul1) and Rbx1 complex [40,41]. These actions promote Nrf2 nuclear exclusion and degradation by a Keap1-independent manner [42]. (Figure 1). Under oxidative stress conditions such AD or PD, Nrf2 modulates its activity through Fyn protein phosphorylation induced by GSK-3β [43,44] Phosphorylated Fyn protein translocates and accumulates in the nucleus and phosphorylates Nrf2, leading to its nuclear export, ubiquitination, and degradation [45,46] (Figure 2).
Figure 2.
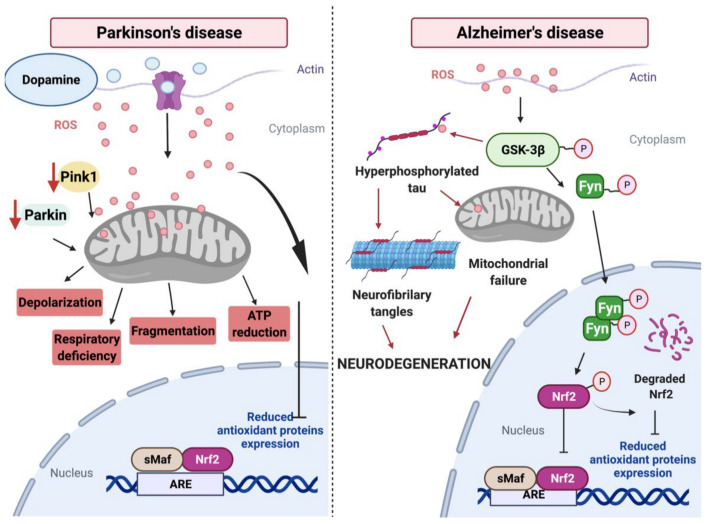
Contribution of the Nrf2 pathway to the pathogenesis of Parkinson’s disease and Alzheimer’s Disease. In PD, an increase in the dopamine release could affect mitochondrial function, producing an increase in ROS levels affecting Nrf2 activity and the response against the oxidative damage. Additionally, the decrease in Parkin and PINK expression levels shown in PD could affect mitochondrial function, inducing depolarization, fragmentation, respiratory deficiency, and ATP reduction. These changes will affect synaptic function, contributing to neurodegeneration and cognitive impairment present in PD. In AD, the GSK-3β protein, which is a kinase that promotes the anomalous phosphorylation of tau protein, promotes Nrf2 degradation by proteasome activity through the Fyn’s phosphorylation. Additionally, during AD, activated GSK-3β induces tau hyperphosphorylation, which could affect mitochondrial function. Later on, the accumulation of pathological forms of tau could lead to the formation of neurofibrillary tangles (NFTs), which is considered a hallmark in AD.
In a typical redox environment, minimal Nrf2 activity maintains the basal expression of some ARE-driven genes [47]. In these conditions, the thiol groups of the redox-sensitive Cys residues located in Keap1 are not modified by the action of electrophiles and pro-oxidant environment present in the cells [48]. Thus, under these conditions, the degradation of Nrf2 occurs mainly via Keap1–Cul3/Rbx1 complexes [43,45,49].
2.2. Transcriptional and Post-Transcriptional Regulation of Nrf2
In addition to the regulation of the Nrf2 protein stability, the genetic products from the Nrf2 pathway can be controlled by post-transcriptional modifications and the availability of binding partners [44]. In this case, the transcriptional factors involved include aryl hydrocarbon receptor (AhR), nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB), and Nrf2 itself [50]. AhR induces Nrf2 activity in response to polycyclic aromatic hydrocarbon exposure [51]. AhR and Nrf2 signaling modulate the expression of genes that affect the metabolism of xenobiotics [51]. Nrf2 gene transcription can be modulated by AhR activation and this signaling is also present in the opposite direction, suggesting that the AhR gene is directly affected by Nrf2 [52]. The response element phase II genes and xenobiotic response element (XRE) may recognize these transcription factors, which are present in the regulatory domains of the same target genes such NQO1 [53]. Furthermore, the Nrf2 gene promoter contains a binding site for NF-κB subunits p50 and p65, which are involved in the transactivation of the Nrf2 gene [54]. NF-κB can trigger Nrf2 signaling and participate in the decrease of NF-κB, indicating an interesting crosstalk between these two pathways [55]. In addition, p65, a canonical NF-κB subunit, can exert a negative regulation on ARE-linked gene expression [56]. p65 participates in increasing the abundance of Keap1 levels, thus decreasing Nrf2 activity [56]. Additionally, the treatment with LPS in a Nrf2 (−/−) mice model with amyotrophic lateral sclerosis (ALS) showed elevated NF-κB activity and an increase in cytokine production that contributed to astrogliosis, neuronal death, and demyelination of neuronal axons [57]. Nrf2 and NF-κB are tightly regulated by redox factors and the lack of Nrf2 is related to an increased oxidative stress, conductive to a high cytokine production and leading to the NF-κB activation in an oxidative environment [58].
Under stress conditions or under the effect of natural compounds, Nrf2 stabilizes and translocates to the nucleus, where it binds (as a heterodimer member of the small Maf family of transcription factors) to the ARE/EpRE sequences in the promoter of its target genes [59] (Figure 2). The Nrf2 cytoprotective response has been described in various mammalian tissues, cultured cells, and other model organisms such as Drosophila melanogaster and Caenorhabditis Elegans [60,61]. Interestingly, these organisms have shown similar antioxidant systems to mammals, indicating that the Nrf2 pathway represents an evolutionarily conserved defense mechanism [62].
Growing evidence has shown that Nrf2 is involved in the regulation of the unfolded protein response (UPR), which is triggered by the accumulation of misfolded proteins in the endoplasmic reticulum [63,64]. Alternatively, studies showed that during endoplasmic reticulum stress, Nrf2 is also regulated by ubiquitination [65]. Nrf2 is ubiquitinated and degraded in a process mediated by the E3 ubiquitin ligase, Hrd1, p62/sequestosome-1 (p62/SQSTM1), and p21Cip1/WAF1, which interfere with the formation of the Keap1–Nrf2 ubiquitination complex regulating the abundance of Nrf2 [66,67]. Thus, the use of electrophilic compounds that target the Nrf2/Keap1 axis could increase the transcriptional activity of Nrf2 and strengthen cellular antioxidant defenses.
Importantly, Nrf2 contributes to removing misfolded or damaged proteins by regulating proteasome degradation [68,69]. In this regard, Nrf2 upregulates the expression of several proteasome subunits, protecting the cell from the accumulation of toxic proteins [70]. Twenty proteasome and ubiquitination-related genes are regulated by Nrf2 through the Keap1-Nrf2-ARE signaling pathway in agreement with a wide microarray analysis from liver RNA that was set up with Nrf2 natural activators, sulforaphane, or inducer 3H1,2-dithiole-3-thione (D3T) [71,72]. These studies showed an enhanced activity of the 26S proteosome promoted by Nrf2 activators [69].
2.3. Nrf2 and the Antioxidant Response in the Brain
Under oxidative stress, brain cells adjust their metabolism and gene expression to maintain redox homeostasis by activating Nrf2 and other stress response pathways [30,73,74]. For example, Kovac and colleagues found that the Keap1–Nrf2 pathway modulates ROS production via NADPH oxidase in primary neurons and brain explant slice cultures [75]. ROS production in Nrf2 (−/−) cells was significantly increased compared to WT cells, indicating that Nrf2 participates in regulating the redox and the intermediary metabolism [75,76]. Furthermore, Nrf2 can induce the expression of the principal enzymes implicated in NADPH generation: isocitrate dehydrogenase 1 (IDH-1), glucose-6-phosphate dehydrogenase (G6PD), malic enzyme 1 (ME-1), and 6-phosphogluconate dehydrogenase (PGD) [76,77,78]. In the brain, Nrf2 also coordinates the activity of thioredoxins (TRXs) and peroxiredoxins (PRXs) necessary for the removal of ROS in the mitochondria [79]. Nrf2 increases the inducible expression of GSH biosynthesis enzymes and its regeneration enzyme GSH reductase, which plays a crucial role in maintaining the mitochondrial GSH pool [80,81]. GSH peroxidases (GPx1 and GPx4) detoxify superoxide-derived hydrogen peroxide to water using GSH and NADPH [82]. Additionally, Nrf2 activation is associated with the induction of mitochondrial antioxidant enzymes like thioredoxin reductase-2 (Txnrd2), peroxiredoxin 3 (Prdx3), 5 (Prdx5), GPx1, and mitochondrial superoxide dismutase 2 (SOD2) [83] However, in this process, the regulatory mechanisms induced by Nrf2 are not entirely elucidated [83,84,85,86].
In this context, Lee and colleagues have shown that increased Nrf2 activity by specific activators enhanced resistance to mitochondrial toxins such as the complex I inhibitor, rotenone, or the complex II inhibitor, 3-nitropropionic acid (3-NP) in primary cortical astrocytes from Nrf2 (−/−) mice [87]. Complementary, these studies showed that astrocytes from Nrf2 (−/−) treated with 3-NP showed a hypersensibility to this drug inducing neurodegeneration and astrocyte activation [88,89]. Likewise, the genetic ablation of Nrf2 reduces the constitutive and inducible expression of cytoprotective genes, thereby enhancing the sensitivity of neurons and astrocytes to the oxidative damage [77].
2.4. Nrf2 and Mitochondrial Function in the Brain
Mitochondria function is essential for cellular bioenergetics, biosynthesis, and other related process [88]. During respiration and oxidative phosphorylation, mitochondria use oxygen to generate ATP by the electron transport chain (ETC) [89,90]. In this process, there is an inevitable generation of ROS [91], which also represents a key signaling molecules [92]. ROS excess is harmful by modifying macromolecules such as proteins, lipids, and DNA, thereby disrupting cellular homeostasis and inducing mitochondrial impairment [89]. Mitochondrial sensitivity to oxidative stress is strongly implicated in the pathophysiology of many diseases and disorders including those affecting the CNS [92,93,94,95]. Vulnerable mitochondrial sites for oxidative stress include metabolic enzymes, electron transport chain, oxidative phosphorylation, DNA, RNA, membrane lipids, Ca2+ handling proteins, and mitochondrial permeability induced by the opening of the mitochondrial permeability transition pore (mPTP) [96]. Opening of mPTP causes mitochondrial depolarization, uncoupling, and mitochondrial metabolite release including pyridine nucleotides and glutathione [97].
An interesting connection between Nrf2 and mitochondria was reported by Lo and colleagues [98]. Their studies showed that Keap1 associates with phosphoglycerate mutase 5 (PGAM5), a protein phosphatase related to mitochondria homeostasis, mitophagy, and cell death [98,99]. Additionally, recent reports indicate that the expression of PGMA5 results in decreased mitochondrial movement, which is particularly essential for the transport of these organelles along the axon [100]. Furthermore, several studies have suggested a link between the Nrf2 pathway and mitochondrial function, which we will discuss in the next sections.
2.4.1. Mitochondrial Bioenergetics
Growing evidence indicates that Nrf2 influences primary metabolism and bioenergetics [101,102]. For instance, the genetic suppression of Nrf2 reduced the expression levels of the malic enzyme, glucose-6-phosphate dehydrogenase (G6PD), transaldolase (TAL), and transketolase (TKT) [28,76]. These enzymes participate in the biosynthesis of primary metabolites including NADPH, glutathione, ribose-5-phosphate (fatty acids), and erythrose-4-phosphate (aromatic amino acids) [76]. Additionally, other studies have shown that genetic ablation of Nrf2 leads to mitochondrial failure, defects in fatty acid oxidation, respiration, and ATP production [103,104]. Moreover, Holmström et al. studied the role of Nrf2 and its repressor Keap-1 on mitochondrial bioenergetics using isolated mitochondria derived from primary neuronal culture of Nrf2 (−/−) mice [105]. They showed defective respiration, mitochondrial uncoupling, and decreased ATP levels in Nrf2 (−/−) neuronal cultures. Interestingly, when the Nrf2/ARE pathway is genetically activated, it restores mitochondrial membrane potential and the respiration rate levels, confirming that the Nrf2-Keap1 pathway modulates cellular energy metabolism through mitochondrial substrate availability.
2.4.2. Mitochondrial Biogenesis
One of the first links between Nrf2 and mitochondrial biogenesis was provided by Piantadosi et al. [106]. They showed that Nrf2 activation resulted in transcriptional upregulation of Nrf1/alpha-PAL and the transactivation of mitochondrial biogenesis genes that encode for NADH dehydrogenase subunit 1 (ND1) and cytochrome c oxidase (COX) subunit I [106]. In addition, other studies have suggested that Nrf2 also influences mitochondrial biogenesis by activating the mitophagy process under stress conditions [75]. This process maintains the organelle integrity by selectively removing damaged mitochondria [107]. One of the critical components for this process is the autophagy adaptor protein sequestosome-1 (SQSTM1/p62) [108]. p62 acts as an adaptor that binds protein aggregates that are conducted to ubiquitination and delivers them to autophagosomes [109]. Interestingly, p62 contains an interacting region (KIR) domain of Keap-1 (349-DPSTGE-354) [110,111], resembling the Keap1-interacting ETGE motif in the Neh2 domain of Nrf2, allowing p62 to sequester Keap1, preventing the ubiquitylation of Nrf2, and favoring its activation [111,112]. Furthermore, Nrf2 promotes mitochondrial biogenesis through the activation of the family of peroxisome proliferator-activated receptor coactivators (PGC) including PGC-1α and PGC-1β, which are involved in the process of mitochondrial biogenesis [113]. Complementary studies showed that Nrf2 could interact with PGC1α, inducing the expression of mitochondrial SOD2, preserving mitochondrial mass, and reducing oxidative damage [1,104].
2.5. Nrf2 and Neuroinflammation
Another important connection between Nrf2 and NDs is related to the neuroinflammation in pathologies like AD and PD [114,115]. Neuroinflammation is a process characterized by changes in the morphology of glial cells including both astrocytes and microglia as well as the inflammatory cytokine release (IL-1β, IL-6, and TNF-α) [116,117]. The prolonged and chronic inflammatory responses in the CNS lead to the augmented release of inflammatory mediators and oxidative stress, thereby maintaining neuroinflammation cascades and accelerating neuronal dysfunction [118].
In this context, ARE-regulated Nrf2-dependent genes are activated in stressed astrocytes, suggesting an interesting link between the impairment of these cells and the role of oxidative stress in neurodegeneration [119]. Additionally, Nrf2 activation maintains redox homeostasis in microglia [114]. For example, the release of CX3CL activates the Nrf2 signaling in microglial cells [116,120]. CX3CR is the receptor for the chemokine fractalkine (CX3CL1), which is a critical pathway for microglia-neuron crosstalk [121]. Interestingly, other studies have investigated the crosstalk between CX3CR1/Nrf2 in the context of tauopathies and neuroinflammation [116]. Here, they observed a significant decrease in the mRNA levels of Nrf2 and its related genes in primary microglia cultures from Cx3cr1 (−/−) mice [116]. Indeed, Nrf2 has been considered as a potential candidate for pharmacological targeting to ameliorate neurodegenerative changes induced by neuroinflammation [122].
3. Neurodegeneration in PD
PD ranks second among the diseases with the highest prevalence occurrence worldwide [123]. While PD has been widely investigated, the primary causes remain unsolved, even though several risk factors have been identified including age, environmental toxin exposure, and genetic mutations [124,125]. One of the most important pathological hallmarks present in PD is the selective loss of dopaminergic neurons in the substantia nigra (SN) pars compacta and the loss of dopamine nerve terminals projecting to the striatum [126]. Another hallmark in PD is the formation of protein inclusions within the cytosol known as Lewy bodies, mainly constituted by aggregates of the protein α-synuclein (α-Syn) [127,128]. Additionally, clinical/pathological studies showed that the progressive neuronal death from the ventrolateral region of the SN represents a clear sign of the PD presence in the brain [129].
Several studies have indicated that the genetic and molecular causes involved in PD are intrinsically related to the mitochondrial impairment, oxidative stress, and endo-lysosomal system dysfunction [130,131]. Additionally, the accumulation of aberrant or misfolded proteins and the impairment of the ubiquitin-proteasome system contributes to the pathogenesis of sporadic and familial PD [130,131]. In addition, some studies have shown a trilateral correlation between mitochondrial failure, α-Syn aggregation, and the impairment of proteasome systems in the neurodegenerative process of PD [132,133].
Transcriptional dysfunction of several genes has been associated with familial PD [134,135]. These defects include changes in the expression of α-Syn, Parkin, leucine-rich repeat kinase 2 (LRRK2), PTEN-induced putative kinase 1 (PINK1), and DJ1 [136]. From this group, the major autosomal dominant PD-related gene is LRRK2, which is considered to be the most common genetic cause of familial and sporadic PD [137]. This genetic deficiency has been demonstrated to cause α-Syn accumulation and autophagy stress and the disturbances in mitochondrial dynamics (fusion/fission), mitochondrial membrane potential, and mtDNA [138]. The loss of Parkin function could harm mitochondrial biogenesis and conduct cell death [128,139]. PINK1 accumulates on dysfunctional mitochondria, and its kinase activity is required for Parkin translocation to mitochondria and the induction of the mitophagy [140,141]. Additionally, the loss of function mutations of the DJ-1 gene has been associated with the recessive early-onset familial PD and late-onset sporadic PD [142]. Changes in DJ-1 expression resulted in defective complex I function, fragmented mitochondria, uncoupling, Ca2+ disturbances, and oxidative damage [143]. Altogether, these studies showed that mutations in these genes affect mitochondrial function, contributing to PD’s neurodegeneration [144].
3.1. PD and ROS
Accumulative damage by an uncontrolled ROS production in the brain contributes significantly to the pathogenesis of PD [145,146]. In physiological conditions, ROS are produced from several sources including complexes I and III of the ETC located in the mitochondria inner membrane and can also be produced by NADPH oxidase induced by Ca+2 influx [8,147]. In a pathological condition such as PD, oxidative damage and mitochondrial impairment contribute to the cascade of events leading to the degeneration of dopaminergic neurons, considered one of the hallmarks of this disease [148] (Figure 2). More importantly, the experimental data suggest that ROS overproduction is a significant contributor to dopaminergic neuronal loss in PD [149] (Figure 2).
In homeostatic conditions, GSH plays a fundamental role in reducing high levels of ROS and minimizing oxidative damage in the brain [150]. In PD, the extended loss of nigral GSH is the most distinctive change that happens in the earliest stage of this progressive disease [151]. Analysis of postmortem brain from PD patients showed a decrease in the amount of GSH in the Sustantia nigra (SN) compared to the controls [152]. Loss of GSH in the SN results in diminished levels of mitochondrial complex I activity [153]. In the same context, accumulation of iron ions (Fe3+) and ferrous iron (Fe2+) can easily react with radical superoxide (O2−) and hydrogen peroxide (H2O2), producing a highly reactive hydroxyl free radical that, together with dopamine oxidation, can trigger neurotoxicity observed in PD [149].
3.2. PD and Mitochondrial Impairment
Oxidative stress is considered one of the critical factors in the etiopathology of PD [145,148]. A significant amount of ROS levels are produced by the inhibition of complex I of the mitochondrial respiratory chain, and this alteration is present in PD patients [154]. During PD, increased ROS production could affect mitochondrial function, inducing calcium uptake by oxidizing thiol groups in protein channels, which later contributes to neuronal death [155]. Dopamine oxidation could affect mitochondrial function by producing an increase in ROS levels [156,157] (Figure 2). Primary or toxin-induced respiratory chain complex dysfunction in PD is tightly linked with ROS generation [145]. During PD, dopaminergic neurons showed a ROS accumulation contributing to mitochondrial impairment, and the inflammatory response shown in PD [158]. How important is mitochondrial impairment for the pathogenesis and neurodegeneration in PD will be a matter of discussion in the next sections.
3.2.1. Mitochondrial Bioenergetics Defects
The first association between PD and mitochondrial dysfunction was suggested in the late 1970s by using the neurotoxic compound MPTP (1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine), a by-product accidentally generated during the synthesis of a meperidine analogue [159]. Treatment with MPTP caused parkinsonian-like symptoms in intravenous drug users [159,160]. When MPTP crosses the blood–brain barrier, this compound is bio-transformed into its toxic form 1-methyl-4-phenylpyridinium (MPP+) by glial monoamine oxidase (MAO) [161]. MPP+ specifically interferes with the activity of mitochondrial respiratory chain complex I (NADH: Ubiquinone oxidoreductase) in dopaminergic neurons (DA), causing selective neurodegeneration in the substantia nigra of both human and mouse models [159,162]. As a consequence of the mitochondrial respiratory complex I defects, the ATP production decreases while the generation of ROS and nitrogen species is increased [163,164]. These actions ultimately lead to neuronal cell death by activation of pro-apoptotic Bcl-2 family members, p53, JNK, and caspases as well as inflammation [165,166].
Furthermore, mitochondrial respiratory complex I activity was significantly reduced in the SN of PD patients [167,168,169]. Additionally, the higher number of mitochondrial DNA (mtDNA) encoded subunits required for complex I assembly indicates that this complex is more likely to be affected by pathogenic mtDNA mutations [167,170]. However, the specific role of complex I deficiency in PD neuronal loss remains elusive [171,172]. In addition, a wide number of clinical trials showed a relation between PD and specific mtDNA-specific mutations. Interestingly, the mitochondrial transcription factor A protein (Tfam), whose expression sequence is part of the promoters within the D-loop region of mtDNA, have been implicated in PD [173]. Studies in Tfam (−/−) knock-out mice showed a decrease in mtDNA expression, respiratory rate deficiency, neuronal death, and the impairment of motor functions in midbrain dopaminergic neurons [174].
3.2.2. Mitophagy
Mitophagy, which is known as the selective degradation of mitochondria through autophagy, is an elemental mechanism for mitochondrial homeostasis [175,176]. This process is particularly essential in post-mitotic and slow-dividing cells (like neurons) as it promotes the renewal of mitochondria, preventing the accumulation of dysfunctional organelles [177]. Alteration of mitophagy leads to the progressive accumulation of defective mitochondria, leading to neurodegeneration and synaptic dysfunction [178,179] (Figure 2). Clinically, the majority of PD cases are late-onset and sporadically caused by a combination of genetic and environmental factors; up to 10% have origin in monogenic forms of the disease [137]. Interestingly, the identification of familial autosomal recessive forms of early-onset PD caused by mutations in PINK150 and PARKIN51 genes has been critical in implicating dysfunctional mitophagy in the PD pathogenesis [178,180,181].
Under physiological conditions, PINK1 localizes with the mitochondria and is quickly translocated toward the inner membrane (IM) to be cleaved and deactivated by IM protease presenilins-associated rhomboid-like protein (PARL) [182]. PINK1 inhibits the fusion of damaged mitochondria, which may serve the purpose of preventing the contamination of healthy mitochondria by those that are damaged [182,183]. In addition to the adverse effects against mitochondrial function and dynamics, PINK1 and Parkin participate in the mitochondrial biogenesis process through an indirect interaction with the cofactor PGC-1α [139,184]. PGC-1α (encoded by PPARGC1A gene) was discovered as the co-regulator of PPARγ, a transcriptional factor that is considered a master regulator of mitochondrial biogenesis [184,185]. The loss of PINK1/Parkin activity reduces clearance and the consequent build-up of the Parkin interacting substrate (PARIS/ZNF746), a transcriptional repressor of PGC-1α [186]. Interestingly, Murata and colleagues found that Nrf2 is also a transcription factor for the PINK1 gene via activation of the ARE sequence in the PINK1 promoter (Figure 1). This Nrf2-PINK1 signaling participates in cell survival and in the maintenance of mitochondrial homeostasis through several mechanisms such as the removal of damaged mitochondria and reduction of ROS [187].
Current studies have indicated a possible explanation of how PINK1 may regulate parkin-mediated mitophagy and how PD-associated PINK1 and parkin mutations result in defective mitophagy [188]. For example, experiments using SH-SY5Y human neuroblastoma cells that were stable knockdown for PINK1 showed an enhancement of the mitophagy process by increasing oxidative stress [189]. In the same context, the overexpression of PINK1 stabilized mitochondrial networking and function in SH-SY5Y neuroblastoma cells [189]. Complementary evidence also demonstrates that Parkin enhanced this protective mitophagy response, indicating that PINK1 and Parkin may cooperate to maintain mitochondrial homeostasis [189,190]. In addition, the suppression or knock-out of Parkin in Drosophila, zebrafish, mice, or human patient cells leads to severe mitochondrial dysfunction including decreased ATP production, mitochondrial depolarization, and altered mitochondrial morphology [191,192]. In particular, one study showed evidence that Parkin interacts with mitochondrial Stomatin-like protein 2 (SLP-2), which is a protein required for the assembly of mitochondrial respiratory chain complexes [193]. These studies suggest that mutations in both Parkin or PINK1 may alter mitochondrial turnover, resulting in the accumulation of abnormal mitochondria that contribute to PD [194,195].
3.3. Nrf2 Activation Prevents Neurodegeneration in PD
Accumulative studies suggest that the use of natural or synthetic compounds that activate the Nrf2 pathway reduce the negative consequences of the oxidative damage and mitochondrial dysfunction present in NDs [196]. Dimethyl fumarate (DMF) is a methyl ester of fumaric acid, and its neuroprotective actions are conducted by modulating the Nrf2 pathway [197]. DMF acts as an Nrf2 activator with the capacity to stimulate a cellular defense to protect neurons from ROS damage [198]. Similarly, other groups have studied the effect of Kolaviron, a mixture of bioflavonoids with neuroprotective effects on the microglia activation [199]. BV2 microglia and HT22 hippocampal neuron co-culture treated with this compound showed a reduction in PGE2/COX-2, and NO/iNOS, and an increase in HO-1 levels promoting an antioxidant environment in these cells [199].
Strong evidence suggests that the activation of Nrf2 could be a therapeutic target against PD and other neurodegenerative diseases such as AD and HD [200,201,202,203]. At least two relevant facts support the importance of the Nrf2 pathway on PD: first, the DJ-1/PARK7 gene, whose deficiency is associated with autonomic recessive parkinsonism, sequesters the Nrf2 inhibitor Keap1 and leads to an increase in Nrf2 activity [204]. DJ-1-deficient patients showed a reduced expression of Nrf2-dependent genes such as NQO1 and GST, increasing the oxidative damage [205,206,207]. The second fact is the association between PD and the polymorphisms of several genes regulated by Nrf2 [16,208,209]. This association was suggested by studies of the microchip analysis of tissues samples of PD patients [16]. Brain PD samples showed a decrease in expression of 31 genes that contained the ARE-sequences in the promoter with an increase in the expression of Nrf2 [16]. Interestingly, other studies have suggested that the Nrf2 pathway is highly activated in PD [210,211]. Moreover, neuronal cells such as astrocytes, endothelial cells, and dopaminergic neurons showed an increase in NQO1 levels in PD post-mortem brain samples [203]
GSK-3β has been implicated in the regulation of several physiological responses by phosphorylating a wide range of nuclear and cytoplasmic proteins [212,213]. Additionally, GSK-3β participates in several processes related to mitochondrial function such as biogenesis, bioenergetics, motility, and permeability [214]. In the PD context, an association between PD and two single nucleotide polymorphisms located in the GSK-3β promoter region, (nt-171 to +29), rs334558 (−50 C/T), and intronic single nucleotide polymorphism, rs6438552, has been reported [215]. These interactions affect GSK-3β function by increasing its expression and activity [215]. In addition, Rojo and colleagues identified GSK-3β as a fundamental element in the downregulation of the antioxidant defense elicited by Nrf2 after oxidant injury [42]. In this case, the GSK-3β inhibition resulted in increased Nrf2 nuclear levels and the expression of antioxidant genes that prevented the oxidation induced by H2O2, 6-hydroxydopamine (6-OHDA), and MPP+ [42,45].
Complementary studies showed that mice neuronal cultures co-transfected with Nrf2 and α-synuclein showed an increase in proteasomal activity, conducting a reduction in the neuronal load of α-synuclein [216]. Interestingly, pioneer studies from Jakel et al. showed that the transplantation of astrocytes overexpressing Nrf2 into the striatum of a Nrf2 (−/−) PD mice model induced by 6-OHDA showed neuroprotection and reduced oxidative damage [217]. Importantly, Nrf2 activation reduced oxidative damage and neuroinflammation of wild type mice treated with MPTP [218]. Additionally, in Nrf2 (−/−), knock out mice submitted to MPTP showed a decrease in Nrf2 activity, which accentuates the PD pathological phenotype [219].
Despite the fact that Nrf2 has not been evaluated for clinical trials in PD, epidemiological evidence indicates that the intake of compounds that activate this pathway such as vitamins C and E are associated with a significant decline in PD risk [220]. Additionally, the treatment during four weeks with N-acetyl cysteine, which activates the Nrf2 pathway, showed better scores on the Unified Parkinson Disease Rating Scale and increased peripheral markers of antioxidant activity [221].
Further studies have shown that the Nrf2 activation by sulforaphane (SFN) induced the expression of antioxidant enzymes, reduced ROS levels, increased mitochondrial biogenesis, and prevented dopaminergic neuronal loss in MPTP-treated mice [222]. SFN is a natural organic isothiocyanate compound isolated from the cruciferous family of vegetables, which includes cauliflower, brussels sprouts, broccoli, white cabbage, and red cabbage [223,224]. Increasing evidence has shown that this compound could act on different targets and modulate antioxidant response in neuronal cells [225,226]. The lipophilic nature and molecular size of SFN make it suitable for passive absorption by neuronal cells, where SFN is attached with GSH by glutathione S-transferase (GST), which leads to the maintenance of a concentration gradient by a continuous uptake of SFN [227].
In this context, studies have reported that neurons treated with SFN altered Keap1 function mainly in the cysteines (Cys-77, Cys-226, Cys-249, Cys-257, Cys-489, Cys-513, Cys-518, and Cys-583) of the Kelch domain of human Keap1 [228]. Furthermore, SFN suppressed the GSK-3β activity and increased the nuclear translocation of Nrf2 [229]. More importantly, SFN pre-treatment decreased membrane damage and DNA fragmentation, and ROS generation caused 6-OHDA treatment on rat nigrostriatal cultures [230]. These positive effects were mediated by Nrf2 activation by increasing enzyme levels of NQO1 [230,231]. Likewise, SFN protected against 6-OHDA treatment in cultures of rat nigrostriatal neurons [232] and human neuroblastoma SH-SY5Y cells [233].
4. Neurodegeneration in AD
AD is the most common type of dementia in the elderly population and is characterized by memory loss and selective neuronal death [234]. AD brain develops two distinguished pathological characteristics: the senile plaques formed by extracellular deposits of the β-amyloid peptide Aβ (1–40), Aβ (1–42) and the neurofibrillary tangles (NFTs) made of intraneuronal aggregations of hyperphosphorylated tau protein [235]. Accumulative evidence has shown extensive mitochondria abnormalities in AD patient’s brains [236]. Additionally, mitochondrial impairment has been established as an early hallmark in the genesis and progression of this disease [236,237]. In addition, the GSK-3β dysregulation is related to the pathogenesis of AD [234]. Elevated GSK-3β activity has been observed in the brains of AD patients [238]. Several studies have proposed a molecular relation between GSK-3β, Aβ, and tau in AD pathogenesis [239]. It has been demonstrated that Aβ activates GSK-3β, which in addition, hyperphosphorylates tau protein (Figure 2) [240]. Mice that overexpress GSK-3β in the forebrain (Tet/GSK-3β mice) exhibit hyperphosphorylation of tau, followed by its accumulation in hippocampal neurons [241].
4.1. ROS in AD
It is well accepted that oxidative stress plays a significant role in the pathogenesis of AD [7,242,243]. In this context, Aβ (1–42) mediates part of its harmful effect against neurons by inducing mitochondrial stress and ROS increase in AD patients [244]. In addition, increase of ROS levels promotes Aβ deposition and the loss of synapses in hippocampal neurons [245]. Studies using live imaging showed an active ROS production followed by neuronal death in the proximity of amyloid plaques in the APP/PS1 double transgenic mice that exhibited elevated production of β-amyloid [246]. Several findings have demonstrated that lipid peroxidation is highly increased in AD [242]. Lipid peroxidation is a process in which ROS attack lipids through a mechanism of free radical chain reaction, generating lipid peroxidation products harmful for neuronal function such as 4-hydroxy-2-nonenal (HNE), which is one of the major products of lipid peroxidation that also contributes to Aβ depositions in AD [242,247]. Protein oxidation by ROS also plays a significant role in the modification of key enzyme activities critical to neuron and glial functions such as glutamine synthetase and creatine kinase, which are significantly reduced in AD brains [248]. In addition, increased levels of 3-nitrotyrosine, a marker for peroxynitrite-mediated protein nitration, have also been found in brain regions of AD patients compared to control samples [249]. Thus, these irreversible protein modifications that include protein nitration and HNE could compromise enzymatic activity and alter energy metabolism in AD.
4.2. AD and Mitochondrial Impairment
Interestingly, a strong connection has been shown between mitochondrial dysfunction and oxidative stress in the progression of AD [250]. These defects represent a critical aspect to explain how oxidative damage and synaptic dysfunction contribute to the early stages of AD [251,252,253]. Oxidative damage is considered an early effect in AD, as recent studies have shown that its onset is frequently preceded by an intermediate phase known as mild cognitive impairment (MCI) when there is no accumulation of senile plaques and NFTs [254]. In addition, increased levels of Aβ could accelerate ROS production by directly binding to mitochondrial membranes, thus affecting mitochondrial dynamics and function, which leads to abnormal energy metabolism and synaptic loss [255].
4.2.1. Mitochondrial Dynamics Defects
Mitochondrial dysfunction is an essential and early feature of AD, and almost all aspects of mitochondrial biology have been reported to be affected during this disease [256]. Mitochondria suffer from continuous fission and fusion (mitochondrial dynamics) events that regulate their morphology and distribution [257]. These processes are coordinated by mitochondrial fission proteins such as dynamin-like protein 1 (DLP1), Fis1 [258], and mitochondrial fusion proteins like mitofusin 1 (Mfn1), mitofusin 2 (Mfn2), and Optic atrophy 1 (OPA1) [259]. Most DLP1 expression is present in the cytoplasm. Nevertheless, during fission, DLP1 moves toward the mitochondrial surface and arises as a dot formation [259]. Fis1, Mfn1, and Mfn2 are mitochondrial transmembrane proteins placed on the outer mitochondrial membrane [257], while OPA1 is located on the inner mitochondrial membrane [260]. In AD, alterations of mitochondrial dynamics have been demonstrated, showing significant changes in the expression of almost all mitochondrial fission and fusion proteins in the brain of AD patients [256]. Mitochondrial DLP1, the critical fraction for mitochondrial fission, increases in the brain with AD [261]. Consistently, the phosphorylation of DLP1 in Ser616 and S-nitrosylation of DLP1 enables DLP1 translocation to mitochondria activating the GTPase activity of DLP1, which induces mitochondrial fission [262]. In addition, structural damage to mitochondria such as the presence of broken ridges and almost complete loss of internal structure represents a frequent sign in AD brains [263]. Furthermore, Wang and colleagues also demonstrated that Aβ production causes an imbalance of mitochondrial fission/fusion, resulting in mitochondrial fragmentation and abnormal distribution [264]. Furthermore, Aβ treatment causes oxidative stress and induces mitochondrial fragmentation through decreased expression of mitofusin-2 (Mfn2) by activating cyclin-dependent cyclin 5 (Cdk5)-mediated phosphorylation of peroxidase 2 (Prx2) [265]. In addition, Aβ mediates the phosphorylation of dynamin-related protein 1 (Drp1) through AKT stimulation, causing an increase in mitochondrial fission and neuronal death [264]. Importantly, significant changes in the size and number of mitochondria have also been found in susceptible AD neurons [266].
4.2.2. Mitochondrial Bioenergetics Defects
It has been established that damaged mitochondria are less efficient in ATP production, but more capable in the generation of ROS, a fact that represents a key source of the oxidative imbalance observed in AD [266]. In this context, exciting studies suggest that mitochondrial damage resulting from increased ROS production could be an essential contributor to the early stages of AD before reaching the onset of clinical symptoms and the appearance of the tau and Aβ pathology [267].
Complementary studies have also shown that Aβ induces ROS generation and impaired calcium homeostasis, leading to mitochondrial dysfunction [236,255]. For example, the overexpression of the mutant beta-amyloid precursor (APP) protein in the hippocampal cell line HT22 results in defective mitochondrial dynamics, changing the structure and function of these organelles [268]. APP can accumulate in the mitochondrial import channels of AD brains and cause mitochondrial dysfunction [269]. Additionally, Aβ can directly affect mitochondrial function, inhibiting some key enzyme activities [270]. For example, Lustbader et al. showed that alcohol dehydrogenase (ABAD) interacts with Aβ and mediates Aβ-induced apoptosis and ROS production in neurons [271]. Other studies have also shown an increase in the levels of voltage-dependent anion-selective channel 1 (VDAC1), Aβ, and hyperphosphorylated tau in AD brain samples [268].
Furthermore, the disruption in glucose metabolism is associated with early mitochondrial defects observed in several studies made in animal models and AD patients [255,272,273]. These studies showed a significant reduction in glucose metabolism consistent with a first feature observed during AD [274,275]. In addition, the examination of the mitochondrial bioenergetics profile in fibroblasts from late-onset AD (LOAD) and age-healthy control match patients demonstrated that the cells from LOAD changed their metabolic activity from the mitochondrial oxidative phosphorylation system (OXPHOS) to glycolysis generating mitochondrial depolarization in LOAD cells [276]. Finally, brain mitochondria fractioned from triple transgenic AD model mice (3xTg-AD) showed mitochondrial depolarization, a decrease in ATP production, and respiratory rate failure [277].
4.2.3. Mitochondrial Transport Defects
Mitochondrial transport is a process that contributes to the proper organelle distribution through the neurons [278]. Recent studies have shown defects in axonal mitochondrial transport, which precedes to the accumulation of toxic protein in AD, altering axonal integrity and synaptic performance [251,279,280]. In fact, the relation between mitochondrial transport and synaptic activity has been investigated when a loss of function of syntabulin, an adaptor protein that mediates presynaptic mitochondrial motility, affects synaptic plasticity [281,282]. While the exact molecular mechanism in the mitochondrial transport inhibition seen in AD is still a matter of research, a disturbance in mitochondrial motility is highly connected with increased levels of both Aβ and hyperphosphorylated tau and oxidative stress [280,283]. For example, Reddy’s group showed alterations in anterograde mitochondrial movement with an increased fission, and mitochondrial depolarization in primary neurons obtained from the Tg2576 mice expressing mutant human APP protein [284].
4.2.4. AD, Tau Pathology, and Mitochondrial Dysfunction
Tau is a neuronal protein that exhibits different post-translational modifications including phosphorylation, glycosylation, acetylation, nitration, methylation, prolyl isomerization, ubiquitylation, sumoylation, and glycation [285,286]. In AD brains, tau is pathologically modified, which causes its separation from microtubule structures, leading to intraneuronal aggregation and the formation of NFTs [287]. Defects in tau could accelerate neurotoxicity or become neurons more susceptible to different stressors including calcium dysregulation, oxidative stress, inflammation, and mitochondrial failure [288]. In this context, transgenic expression of truncated human tau, which is a relevant tau modification in AD [289], affects mitochondrial distribution and decreases neuronal viability under conditions of exogenous oxidative stress [290]. Furthermore, it has been established that mutated tau expression at P301L affects mitochondrial dynamics, respiratory activity, decreases ATP levels, and induces mitochondrial depolarization in SY5Y cells exposed to hydrogen peroxide [291]. In this context, further studies have shown that an important proportion of 20–22 kDa N-terminal tau fragments (NH2hTau) are found preferentially in the AD hippocampus and frontal cortex mitochondria [292,293]. This tau fragment is connected with neurofibrillary degeneration, synaptic damage, and mitochondrial impairment in the AD brain [292]. Interestingly, it has been suggested that this N-terminal tau truncation contributes to disease progression and represents a key step in the toxic cascade that leads to neuronal death [294].
In this context, recent studies have shown that a reduced tau expression could enhance neuronal and mitochondrial function [295]. For example, Lopes et al. showed that genetic reduction of tau prevented impaired working memory, loss of dendritic spine, and synaptic failure induced in a mouse exposed to chronic stress [295]. More importantly, Jara et al. showed that tau knock-out mice presented less oxidative damage, better recognition memory and attention span, and better mitochondrial bioenergetics compared to wild-type mice that expressed physiological tau levels [296]. In addition, reduced tau expression prevented oxidative damage in hippocampal cells and activated the pathways necessary to protect mitochondrial health such as the Nrf-2 and PGC-α pathways [1,296]. Therefore, these studies suggest that stress-induced neuronal damage and cognitive decline depend on an interaction between tau and mitochondria, which could later affect memory and cognition.
In addition, other pathological tau modifications like caspase cleavage play a pivotal role in the pathogenesis of AD [297,298,299]. Tau could be a substrate for caspase-3 and is cleaved at Asp-421, which is located at the protein’s carboxyl-terminus [300]. This cleavage event results in a highly fibrogenic protein form that rapidly assembles into tau filaments in vitro than that of wild-type tau [297]. Different studies made in cell culture models provided evidence that Asp-421 cleaved tau is toxic to neurons [294,300,301].
More importantly, we have carried out several studies investigating the effects of caspase 3-cleaved tau against neuronal viability induced by mitochondrial failure [301,302,303,304]. We showed that immortalized cortical neurons expressing caspase-3-cleaved tau presented severely fragmented mitochondria, high ROS levels, and mitochondrial depolarization when cells were exposed to calcium overload stress [301]. In addition, other studies using a pseudophosphorylated form of tau (PHF-1), a component of NFTs and caspase 3-cleaved tau, showed that these pathological forms contribute differently to mitochondrial impairment in neurons [304]. Neurons expressing the tau pseudophosphorylated form (PHF-1) affected mitochondrial function (potential and ROS production) in mature neurons; in contrast, hippocampal neurons expressing truncated tau affected mitochondrial function regardless of the age of neuronal culture [304]. These results support the hypothesis that truncated tau at Asp-421 is an early contributor to mitochondrial impairment in the AD brain, and these effects could be relevant for the disease progression [297,301].
Interestingly, new findings suggest that pathological forms of tau and specially truncated tau by caspase-3 may also affect mitochondrial transport in hippocampal neurons [303,304,305]. Truncated tau expression reduced the number of moving mitochondrial elements compared with hippocampal neurons expressing full-length tau [304]. In addition, we studied the molecular mechanism involved in the mitochondrial transport failure induced by truncated tau [305]. Interestingly, truncated tau expression affects the expression of TRAK2 protein, which is a critical mitochondrial-associated transport accessory protein [306]. Truncated tau augmented the accumulation of mitochondria in the soma, reducing the mitochondrial population in neuronal processes including the axon [305]. Interestingly, truncated tau reduced TRAK2 expression and increased its interaction with mitochondria compared to cells expressing full-length tau [305]. These novel findings indicate that caspase-cleaved tau may affect mitochondrial transport through increasing TRAK2-mitochondrial binding and also affecting ATP production [305]. Together, these findings reveal the importance of tau participation in the mitochondrial transport in neurons, an event that may contribute to the synaptic failure observed in AD.
4.3. Nrf2 Activation Reduces Tau Pathology in AD
Several studies under the cell-based and in silico high-throughput screens have identified Nrf2-activating natural compounds that control the expression of genes linked with autophagy response and nerve growth factor signaling in the CNS [20]. These wide arrays of functions developed by Nrf2 might also ameliorate disease progression [307]. In this context, evidence obtained by Jo et al. showed that activating the Nrf2 pathway reduced the abnormal accumulation of hyperphosphorylated tau by inducing the expression of autophagy adapter protein NDP52 in neurons [308]. The NDP52 protein presents three ARE in its promoter region and its expression is strongly induced by Nrf2 activation, facilitating the elimination of hyperphosphorylated tau [308]. The polyubiquitination binding protein, p62, can also modulate the Nrf2 activity through Keap1, which targets its degradation by autophagy [108]. Other studies have shown that Nrf2-mediated induction of p62 plays a critical role in the TLR4-driven aggresome formation and the autophagy degradation to maintain host protection [306]. These studies are related to early evidence that showed significantly decreased levels of p62 in the frontal cortex of AD patients [309]. On the other hand, mRNA and p62 protein levels as well as Nrf2 target genes were shown to be augmented in the cortex of AD brains [310,311]. Furthermore, Aβ injection into rat hippocampus induced an increase in the expression levels of LC3-II, beclin1 (both autophagy protein markers), and Keap1 while p62 and Nrf2 levels were decreased in the hippocampus and cortex of these animals [312].
Cuadrado et al. investigated the effect of the expression of pathological forms of tau like P301L on Nrf2/ARE pathway activation in the Mefs cell line (Keap1 −/−) and Nrf2 (−/−) knock out mice [73]. P301L expression was made with the use of recombinant Adenovirus-Associated-Virus vectors (AVV hT-AUp301L) [73]. Furthermore, the Nrf2 (−/−) mice model expressing AVV-hT-AUp301L showed an increase in the levels of tau hyperphosphorylated, and this effect was prevented in Nrf2 (−/−) mice treated with DMF [73]. Treatment with DMF induces the Nrf2 transcriptional activation through KEAP1-dependent and-independent mechanisms both in vitro and in vivo model [313]. These studies suggest that the impairment of the Nrf2 pathway could be involved in the modulation of tau pathology and the formation of neurofibrillary tangles.
4.4. Nrf2 Activation Prevents Neurodegeneration and Mitochondrial Failure in AD
Accumulative evidence has suggested an essential role of the Nrf2 pathway as a useful therapeutic strategy to ameliorate neurodegenerative changes present in AD [314]. Ramsey and colleagues demonstrated a significant expression of Nrf2 in the nucleus of primary hippocampal neurons while the examination of AD brain samples showed a major Nrf2 cytoplasmic presence compared with the control tissue samples [315]. This reduced presence of Nrf2 in the nuclei of hippocampal neurons during AD suggests that Nrf2 is not performing the activities observed in control individuals of the same age [315]. This deficit in nuclear Nrf2 presence is not the result of a generalized loss of the total cytosolic protein, but may reflect an altered nuclear trafficking, as seen with other transcription factors [316,317].
Furthermore, studies made in Nrf2 (−/−) mice’s hippocampus exhibited higher levels of oxidative damage and pro-inflammatory factors compared with wild type mice with endogenous Nrf2 expression [318]. In this context, memory and learning performance was severely affected in Nrf2 (−/−) mice as early as six months and before the appearance of amyloid plaques and fibrillary aggregates formed by tau [319,320].
Current evidence shows that increased antioxidant activity reduces the risk of neurodegenerative diseases [74,321]. Nrf2 appears to be a promising target because several natural Nrf2 activators have shown positive effects in vitro and in vivo models of study for age-related neurodegenerative diseases [20].
The use of natural compounds with mitochondrial boost actions has also been studied to treat neurodegenerative diseases like AD [322,323]. Various vegetable components like SFN from broccoli or spice ingredients like S-allyl-l-cysteine (SAC) from garlic are known to activate the Nrf2 pathway [324]. Complementary studies have shown that the pharmacological activation of Nrf2 with SFN or through the use of lentiviruses for the activation of Nrf2 increases the expression of antioxidant genes regulated by ARE, improving long-term memory defects induced by Aβ peptide both in vitro and in vivo [314,325]. Further studies have examined the effect of elevated expression of the proteasome (i.e., 26S) in cytoprotection by SFN in murine neuroblastoma cells [72]. These studies propose that the proteasome system’s upregulation leads to the cytoprotective effects induced by SFN, in opposition to oxidative stress [72]. Another study from Park et al. reported that the SFN prevents neuronal cells from Aβ42 treatment and also reduces proteasome activity [326]. More importantly, complementary studies showed that SFN improved neurobehavioral deficits and decreased the load of Aβ in the AD transgenic mice model (APP/PS1) where treatment with SFN increased p75 neurotrophin receptor levels by reducing histone deacetylase 1 and 3 expressions [327]. Furthermore, SFN treatment of neuroblastoma cells upregulated autophagy genes, which were suppressed after Nrf2 expression was knocked out, suggesting a relevant role of Nrf2 in neuroprotection against the toxicity of Aβ [69].
Besides SFN, other natural products have shown positive effects in reducing the oxidative damage and mitochondrial injury during AD [328,329]. For example, a compound known as curcumin exerts neuroprotection in AD [328]. Curcumin is a natural phytochemical compound isolated from the rhizome of the plant named turmeric, which has been shown to have multiple positive effects for neuronal function including anti-inflammatory, antioxidant, and anti-protein-aggregate properties [330]. Curcumin exerts neuroprotection for its capacity to scavenge free radicals such as ROS and reactive nitrogen species (RNS) [331]. In addition, curcumin exhibits protection to mitochondria in the prevention of lipid peroxidation and protein carbonylation [332]. Furthermore, curcumin-derived product (CNB-001) protects mitochondrial dysfunction by its capacity to maintain mitochondrial membrane potential (ΔΨm) and mitochondrial respiratory complex activity under physiological levels [333,334].
DMF is another product that has been shown to have positive outcomes against neurodegeneration in PD and AD [21,335]. DMF has been approved by federal drug administration (FDA) mainly for the treatment of multiple sclerosis (MS) and is also considered as a promising agent for the treatment of AD [336,337]. DMF has protective effects against oxidative stress by increasing the production of the NQO1 enzyme, which exerts a neuroprotective function in hippocampal pyramidal neurons in AD patients [338]. In addition, Kume and colleagues used primary striatal cells that showed that DMF treatment reduced oxidative stress in striatal cells exposed to sodium nitroprusside [339]. Interestingly, these positive effects were produced because DMF treatment induced an increased activity of HO-1 and other ARE-related to neuroprotective genes [339]. In addition, DMF induces mitochondrial biogenesis mainly through the activation of Nrf2, as shown by one in vivo human study [340]. Furthermore, treatment with DMF alleviated tauopathy, decreased GSK-3β activity, and promoted neuronal viability by increasing brain-derived neurotrophic factor (BDNF) expression and reduced inflammatory processes in the hippocampus of Nrf2 −/− mice that stereotaxically expressed the human TAUP301L gene, which is a tau modification responsible for frontotemporal dementia [73].
Simultaneously, it is possible to exert negative regulation of the Nrf2 pathway by several kinases constitutively activated or over-expressed in pathological conditions such as chronic inflammation and AD [341,342]. As above-mentioned, GSK-3β can induce the degradation of Nrf2 by the proteasome in a Keap1-independent manner [341]. These are exciting findings because GSK-3β expression is upregulated in the hippocampus of AD patients [343], post-synaptosomal fractions from AD brains [342], and in AD circulating peripheral lymphocytes [343].
Current reports have elucidated the possible links between GSK-3β and Nrf2 in AD pathology, suggesting new neuroprotective therapies [344]. For example, Rojo and colleagues showed that the inhibition of GSK-3β with lithium administration increased the transcriptional activity of Nrf2 in N2A cells [42]. In addition, cortical extracts obtained from the AD mouse model with GSK-3β suppression showed an increase in nuclear Nrf2 localization, an increase in glutathione-S transferase (GST) levels, reduced oxidative damage, and a decrease in tau hyperphosphorylation levels [345]. Additionally, pyrrolidine dithiocarbonate (PDTC), a small molecule with antioxidant properties that inhibits GSK-3β, also showed Nrf2-ARE stimulation and a significant improvement of the cognitive decline present in APP/PS1 mice [346]. Furthermore, the PI3K/Akt pathway, which downregulates GSK-3β and promotes Nrf2 activity [347], was reduced in the brain of patients with AD [348]. Furthermore, the antioxidant puerarin, described to present antihypertensive, antiarrhythmic, antioxidant, anti-apoptotic, and neuroprotective properties [349,350], caused augmented PI3K/Akt stimulation, reduced GSK-3β activity, increased Nrf2 pathway activation, and HO-1 gene expression in APP/PS1 mice hippocampus, with further cognitive improvement [350].
Notably, a new AD therapy that simultaneously inhibits GSK-3β and increases Nrf2 activity has been proposed [344]. Interestingly, therapeutic Nrf2 activation with 5A-T compounds was independent of GSK-3β inhibition in SH-SY5Y human neuroblastoma cells treated with the mitochondrial toxins oligomycin/rotenone [344]. Nowadays, the number of natural and synthetic Nrf2 activators that can be used for a better understanding of the positive effects that Nrf2 may have on AD pathology are still increasing.
5. Conclusions
In this review, we discussed several studies that highlighted the protective role of the Nrf2 pathway against PD and AD. Moreover, upregulation of Nrf2-driven antioxidant enzymes decreased neurodegeneration and mitochondrial dysfunction both in vitro and in vivo models of PD and AD (Figure 2). In this context, we considered that the Nrf2-ARE pathway could be a valid therapeutic target to ameliorate the progression and pathological signs present in these disorders.
Evidence from animal models indicate that a pharmacologic therapy to slow the progression of neurodegenerative diseases might be achieved through the activation of Nrf2. Nrf2-activating compounds are already FDA approved for the treatment in other diseases such as multiple sclerosis, and clinical testing is underway in Friedrich’s ataxia [350]. In addition, Nrf2 activators can also prevent mitochondrial impairment, which is related to synaptic dysfunction in PD and AD. Therefore, activation of the Nrf2 pathway could have positive effects by decreasing oxidative damage and also reducing the cognitive impairment present in neurodegenerative disorders like PD and AD.
Acknowledgments
CONICYT: FONDECYT, Chile. Figures were prepared using the Biorender software.
Author Contributions
Conceived and written by F.V.T. and R.A.Q.; Manuscript editing and figure drawings by F.V.T. and R.A.Q.; Funding by R.A.Q. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by Fondo de Ciencia y Tecnología (FONDECYT), Chile (Grant No. 1200178) (R.A.Q.).
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Gureev A.P., Shaforostova E.A., Popov V.N. Regulation of Mitochondrial Biogenesis as a Way for Active Longevity: Interaction Between the Nrf2 and PGC-1α Signaling Pathways. Front. Genet. 2019;10:435. doi: 10.3389/fgene.2019.00435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Osama A., Zhang J., Yao J., Yao X., Fang J. Nrf2: A dark horse in Alzheimer’s disease treatment. Ageing Res. Rev. 2020;64:101206. doi: 10.1016/j.arr.2020.101206. [DOI] [PubMed] [Google Scholar]
- 3.Joshi G., Johnson J.A. The Nrf2-ARE Pathway: A Valuable Therapeutic Target for the Treatment of Neurodegenerative Diseases. Recent Patents CNS Drug Discov. 2012;7:218–229. doi: 10.2174/157488912803252023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Johnson D.A., Johnson J.A. Nrf2—A therapeutic target for the treatment of neurodegenerative diseases. Free Radic. Biol. Med. 2015;88:253–267. doi: 10.1016/j.freeradbiomed.2015.07.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aruoma O.I. Free radicals, oxidative stress, and antioxidants in human health and disease. J. Am. Oil Chem. Soc. 1998;75:199–212. doi: 10.1007/s11746-998-0032-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim G.H., Kim J.E., Rhie S.J., Yoon S. The Role of Oxidative Stress in Neurodegenerative Diseases. Exp. Neurobiol. 2015;24:325–340. doi: 10.5607/en.2015.24.4.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nunomura A., Perry G., Aliev G., Hirai K., Akeda A., Balraj E.K., Jones P.K., Ghanbari H., Wataya T., Shimohama S., et al. Oxidative Damage Is the Earliest Event in Alzheimer Disease. J. Neuropathol. Exp. Neurol. 2001;60:759–767. doi: 10.1093/jnen/60.8.759. [DOI] [PubMed] [Google Scholar]
- 8.Nickel A., Kohlhaas M., Maack C. Mitochondrial reactive oxygen species production and elimination. J. Mol. Cell. Cardiol. 2014;73:26–33. doi: 10.1016/j.yjmcc.2014.03.011. [DOI] [PubMed] [Google Scholar]
- 9.Moreira P.I., Carvalho C., Zhu X., Smith M.A., Perry G. Mitochondrial dysfunction is a trigger of Alzheimer’s disease pathophysiology. Biochim. Biophys. Acta BBA Mol. Basis Dis. 2010;1802:2–10. doi: 10.1016/j.bbadis.2009.10.006. [DOI] [PubMed] [Google Scholar]
- 10.Burchell V.S., Gandhi S., Deas E., Wood N.W., Abramov A.Y., Plun-Favreau H. Targeting mitochondrial dysfunction in neurodegenerative disease: Part I. Expert Opin. Ther. Targets. 2010;14:369–385. doi: 10.1517/14728221003652489. [DOI] [PubMed] [Google Scholar]
- 11.Dinkova-Kostova A.T., Abramov A.Y. The emerging role of Nrf2 in mitochondrial function. Free. Radic. Biol. Med. 2015;88:179–188. doi: 10.1016/j.freeradbiomed.2015.04.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kirkwood T.B.L. A systematic look at an old problem. Nature. 2008;451:644–647. doi: 10.1038/451644a. [DOI] [PubMed] [Google Scholar]
- 13.Suh J.H., Shenvi S.V., Dixon B.M., Liu H., Jaiswal A.K., Liu R.-M., Hagen T.M. Decline in transcriptional activity of Nrf2 causes age-related loss of glutathione synthesis, which is reversible with lipoic acid. Proc. Natl. Acad. Sci. USA. 2004;101:3381–3386. doi: 10.1073/pnas.0400282101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rebrin I., Kamzalov S., Sohal R.S. Effects of age and caloric restriction on glutathione redox state in mice. Free. Radic. Biol. Med. 2003;35:626–635. doi: 10.1016/S0891-5849(03)00388-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yamazaki H., Tanji K., Wakabayashi K., Matsuura S., Itoh K. Role of the Keap1/Nrf2 pathway in neurodegenerative diseases. Pathol. Int. 2015;65:210–219. doi: 10.1111/pin.12261. [DOI] [PubMed] [Google Scholar]
- 16.Wang Q., Li W.-X., Dai S.-X., Guo Y.-C., Han F.-F., Zheng J.-J., Li G.-H., Huang J.-F. Meta-Analysis of Parkinson’s Disease and Alzheimer’s Disease Revealed Commonly Impaired Pathways and Dysregulation of NRF2-Dependent Genes. J. Alzheimer’s Dis. 2017;56:1525–1539. doi: 10.3233/JAD-161032. [DOI] [PubMed] [Google Scholar]
- 17.Branca C., Ferreira E., Nguyen T.-V., Doyle K., Caccamo A., Oddo S. Genetic reduction of Nrf2 exacerbates cognitive deficits in a mouse model of Alzheimer’s disease. Hum. Mol. Genet. 2017;26:4823–4835. doi: 10.1093/hmg/ddx361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schipperab H.M., Liberman A., Stopa E.G. Neural Heme Oxygenase-1 Expression in Idiopathic Parkinson’s Disease. Exp. Neurol. 1998;150:60–68. doi: 10.1006/exnr.1997.6752. [DOI] [PubMed] [Google Scholar]
- 19.Rojo A.I., Innamorato N.G., Martín-Moreno A.M., De Ceballos M.L., Yamamoto M., Cuadrado A. Nrf2 regulates microglial dynamics and neuroinflammation in experimental Parkinson’s disease. Glia. 2010;58:588–598. doi: 10.1002/glia.20947. [DOI] [PubMed] [Google Scholar]
- 20.Brandes M.S., Gray N.E. NRF2 as a Therapeutic Target in Neurodegenerative Diseases. ASN Neuro. 2020;12:1759091419899782. doi: 10.1177/1759091419899782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lastres-Becker I., García-Yagüe Á.J., Scannevin R.H., Casarejos M.J., Kügler S., Rábano A., Cuadrado A. Repurposing the NRF2 Activator Dimethyl Fumarate as Therapy Against Synucleinopathy in Parkinson’s Disease. Antioxid. Redox Signal. 2016;25:61–77. doi: 10.1089/ars.2015.6549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kobayashi E.H., Suzuki T., Funayama R., Nagashima T., Hayashi M., Sekine H., Tanaka N., Moriguchi T., Motohashi H., Nakayama K., et al. Nrf2 suppresses macrophage inflammatory response by blocking proinflammatory cytokine transcription. Nat. Commun. 2016;7:11624. doi: 10.1038/ncomms11624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hirotsu Y., Katsuoka F., Funayama R., Nagashima T., Nishida Y., Nakayama K., Douglas Engel J., Yamamoto M. Nrf2–MafG heterodimers contribute globally to antioxidant and metabolic networks. Nucleic Acids Res. 2012;40:10228–10239. doi: 10.1093/nar/gks827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Itoh K., Chiba T., Takahashi S., Ishii T., Igarashi K., Katoh Y., Oyake T., Hayashi N., Satoh K., Hatayama I., et al. An Nrf2/Small Maf Heterodimer Mediates the Induction of Phase II Detoxifying Enzyme Genes through Antioxidant Response Elements. Biochem. Biophys. Res. Commun. 1997;236:313–322. doi: 10.1006/bbrc.1997.6943. [DOI] [PubMed] [Google Scholar]
- 25.Moi P., Chan K., Asunis I., Cao A., Kan Y.W. Isolation of NF-E2-related factor 2 (Nrf2), a NF-E2-like basic leucine zipper transcriptional activator that binds to the tandem NF-E2/AP1 repeat of the beta-globin locus control region. Proc. Natl. Acad. Sci. USA. 1994;91:9926–9930. doi: 10.1073/pnas.91.21.9926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hayes J.D., Chanas S.A., Henderson C.J., McMahon M., Sun C., Moffat G.J., Wolf C.R., Yamamoto M. The Nrf2 transcription factor contributes both to the basal expression of glutathione S-transferases in mouse liver and to their induction by the chemopreventive synthetic antioxidants, butylated hydroxyanisole and ethoxyquin. Biochem. Soc. Trans. 2000;28:33–41. doi: 10.1042/bst0280033. [DOI] [PubMed] [Google Scholar]
- 27.Chan J.Y., Kwong M. Impaired expression of glutathione synthetic enzyme genes in mice with targeted deletion of the Nrf2 basic-leucine zipper protein. Biochim. Biophys. Acta BBA Gene Struct. Express. 2000;1517:19–26. doi: 10.1016/S0167-4781(00)00238-4. [DOI] [PubMed] [Google Scholar]
- 28.Mitsuishi Y., Taguchi K., Kawatani Y., Shibata T., Nukiwa T., Aburatani H., Yamamoto M., Motohashi H. Nrf2 Redirects Glucose and Glutamine into Anabolic Pathways in Metabolic Reprogramming. Cancer Cell. 2012;22:66–79. doi: 10.1016/j.ccr.2012.05.016. [DOI] [PubMed] [Google Scholar]
- 29.Moinova H.R., Mulcahy R.T. Up-Regulation of the Human γ-Glutamylcysteine Synthetase Regulatory Subunit Gene Involves Binding of Nrf-2 to an Electrophile Responsive Element. Biochem. Biophys. Res. Commun. 1999;261:661–668. doi: 10.1006/bbrc.1999.1109. [DOI] [PubMed] [Google Scholar]
- 30.Hayes J.D., Dinkova-Kostova A.T. The Nrf2 regulatory network provides an interface between redox and intermediary metabolism. Trends Biochem. Sci. 2014;39:199–218. doi: 10.1016/j.tibs.2014.02.002. [DOI] [PubMed] [Google Scholar]
- 31.Itoh K., Tong K.I., Yamamoto M. Molecular mechanism activating nrf2–keap1 pathway in regulation of adaptive response to electrophiles. Free Radic. Biol. Med. 2004;36:1208–1213. doi: 10.1016/j.freeradbiomed.2004.02.075. [DOI] [PubMed] [Google Scholar]
- 32.Cullinan S.B., Gordan J.D., Jin J., Harper J.W., Diehl J.A. The Keap1-BTB Protein Is an Adaptor That Bridges Nrf2 to a Cul3-Based E3 Ligase: Oxidative Stress Sensing by a Cul3-Keap1 Ligase. Mol. Cell. Biol. 2004;24:8477–8486. doi: 10.1128/MCB.24.19.8477-8486.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kobayashi A., Kang M.-I., Okawa H., Ohtsuji M., Zenke Y., Chiba T., Igarashi K., Yamamoto M. Oxidative Stress Sensor Keap1 Functions as an Adaptor for Cul3-Based E3 Ligase To Regulate Proteasomal Degradation of Nrf2. Mol. Cell. Biol. 2004;24:7130–7139. doi: 10.1128/MCB.24.16.7130-7139.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yamamoto M., Kensler T.W., Motohashi H. The KEAP1-NRF2 System: A Thiol-Based Sensor-Effector Apparatus for Maintaining Redox Homeostasis. Physiol. Rev. 2018;98:1169–1203. doi: 10.1152/physrev.00023.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Furukawa M., Xiong Y. BTB Protein Keap1 Targets Antioxidant Transcription Factor Nrf2 for Ubiquitination by the Cullin 3-Roc1 Ligase. Mol. Cell. Biol. 2005;25:162–171. doi: 10.1128/MCB.25.1.162-171.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang D.D., Lo S.-C., Cross J.V., Templeton D.J., Hannink M. Keap1 Is a Redox-Regulated Substrate Adaptor Protein for a Cul3-Dependent Ubiquitin Ligase Complex. Mol. Cell. Biol. 2004;24:10941–10953. doi: 10.1128/MCB.24.24.10941-10953.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dinkova-Kostova A.T., Holtzclaw W.D., Cole R.N., Itoh K., Wakabayashi N., Katoh Y., Yamamoto M., Talalay P. Direct evidence that sulfhydryl groups of Keap1 are the sensors regulating induction of phase 2 enzymes that protect against carcinogens and oxidants. Proc. Natl. Acad. Sci. USA. 2002;99:11908–11913. doi: 10.1073/pnas.172398899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kobayashi M., Yamamoto M. Nrf2–Keap1 regulation of cellular defense mechanisms against electrophiles and reactive oxygen species. Adv. Enzyme Regul. 2006;46:113–140. doi: 10.1016/j.advenzreg.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 39.McMahon M., Lamont D.J., Beattie K.A., Hayes J.D. Keap1 perceives stress via three sensors for the endogenous signaling molecules nitric oxide, zinc, and alkenals. Proc. Natl. Acad. Sci. USA. 2010;107:18838–18843. doi: 10.1073/pnas.1007387107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Salazar M., Rojo A.I., Velasco D., de Sagarra R.M., Cuadrado A. Glycogen Synthase Kinase-3β Inhibits the Xenobiotic and Antioxidant Cell Response by Direct Phosphorylation and Nuclear Exclusion of the Transcription Factor Nrf2. J. Biol. Chem. 2006;281:14841–14851. doi: 10.1074/jbc.M513737200. [DOI] [PubMed] [Google Scholar]
- 41.Rada P., Rojo A.I., Chowdhry S., McMahon M., Hayes J.D., Cuadrado A. SCF/β-TrCP Promotes Glycogen Synthase Kinase 3-Dependent Degradation of the Nrf2 Transcription Factor in a Keap1-Independent Manner. Mol. Cell. Biol. 2011;31:1121–1133. doi: 10.1128/MCB.01204-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rojo A.I., De Sagarra M.R., Cuadrado A. GSK-3β down-regulates the transcription factor Nrf2 after oxidant damage: Relevance to exposure of neuronal cells to oxidative stress. J. Neurochem. 2008;105:192–202. doi: 10.1111/j.1471-4159.2007.05124.x. [DOI] [PubMed] [Google Scholar]
- 43.Huang H.-C., Nguyen T., Pickett C.B. Regulation of the antioxidant response element by protein kinase C-mediated phosphorylation of NF-E2-related factor 2. Proc. Natl. Acad. Sci. USA. 2000;97:12475–12480. doi: 10.1073/pnas.220418997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tonelli C., Chio I.I.C., Tuveson D.A. Transcriptional Regulation by Nrf2. Antioxid. Redox Signal. 2018;29:1727–1745. doi: 10.1089/ars.2017.7342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jain A.K., Jaiswal A.K. GSK-3β Acts Upstream of Fyn Kinase in Regulation of Nuclear Export and Degradation of NF-E2 Related Factor 2. J. Biol. Chem. 2007;282:16502–16510. doi: 10.1074/jbc.M611336200. [DOI] [PubMed] [Google Scholar]
- 46.Kanninen K., White A.R., Koistinaho J., Malm T. Targeting Glycogen Synthase Kinase-3β for Therapeutic Benefit against Oxidative Stress in Alzheimer’s Disease: Involvement of the Nrf2-ARE Pathway. Int. J. Alzheimer’s Dis. 2011;2011:985085. doi: 10.4061/2011/985085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nguyen T., Sherratt P.J., Nioi P., Yang C.S., Pickett C.B. Nrf2 Controls Constitutive and Inducible Expression of ARE-driven Genes through a Dynamic Pathway Involving Nucleocytoplasmic Shuttling by Keap1. J. Biol. Chem. 2005;280:32485–32492. doi: 10.1074/jbc.M503074200. [DOI] [PubMed] [Google Scholar]
- 48.Wakabayashi N., Dinkova-Kostova A.T., Holtzclaw W.D., Kang M.-I., Kobayashi A., Yamamoto M., Kensler T.W., Talalay P. Protection against electrophile and oxidant stress by induction of the phase 2 response: Fate of cysteines of the Keap1 sensor modified by inducers. Proc. Natl. Acad. Sci. USA. 2004;101:2040–2045. doi: 10.1073/pnas.0307301101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Apopa P.L., He X., Ma Q. Phosphorylation of Nrf2 in the transcription activation domain by casein kinase 2 (CK2) is critical for the nuclear translocation and transcription activation function of Nrf2 in IMR-32 neuroblastoma cells. J. Biochem. Mol. Toxicol. 2008;22:63–76. doi: 10.1002/jbt.20212. [DOI] [PubMed] [Google Scholar]
- 50.Li R., Jia Z., Zhu H. Regulation of Nrf2 Signaling. React. Oxyg. Species. 2019;8:312–322. doi: 10.20455/ros.2019.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Miao W., Hu L., Scrivens P.J., Batist G. Transcriptional Regulation of NF-E2 p45-related Factor (NRF2) Expression by the Aryl Hydrocarbon Receptor-Xenobiotic Response Element Signaling Pathway: Direct Cross-Talk between Phase I and II Drug-Metabolizing Enzymes. J. Biol. Chem. 2005;280:20340–20348. doi: 10.1074/jbc.M412081200. [DOI] [PubMed] [Google Scholar]
- 52.Shin S., Wakabayashi N., Misra V., Biswal S., Lee G.H., Agoston E.S., Yamamoto M., Kensler T.W. NRF2 Modulates Aryl Hydrocarbon Receptor Signaling: Influence on Adipogenesis. Mol. Cell. Biol. 2007;27:7188–7197. doi: 10.1128/MCB.00915-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nioi P., Hayes J.D. Contribution of NAD(P)H:quinone oxidoreductase 1 to protection against carcinogenesis, and regulation of its gene by the Nrf2 basic-region leucine zipper and the arylhydrocarbon receptor basic helix-loop-helix transcription factors. Mutat. Res. Fundam. Mol. Mech. Mutagen. 2004;555:149–171. doi: 10.1016/j.mrfmmm.2004.05.023. [DOI] [PubMed] [Google Scholar]
- 54.Rushworth S.A., Zaitseva L., Murray M.Y., Shah N.M., Bowles K.M., MacEwan D.J. The high Nrf2 expression in human acute myeloid leukemia is driven by NF-κB and underlies its chemo-resistance. Blood. 2012;120:5188–5198. doi: 10.1182/blood-2012-04-422121. [DOI] [PubMed] [Google Scholar]
- 55.Sun C.-C., Li S.-J., Yang C.-L., Xue R.-L., Xi Y.-Y., Wang L., Zhao Q.-L., Li D.-J. Sulforaphane Attenuates Muscle Inflammation in Dystrophin-deficient mdx Mice via NF-E2-related Factor 2 (Nrf2)-mediated Inhibition of NF-κB Signaling Pathway. J. Biol. Chem. 2015;290:17784–17795. doi: 10.1074/jbc.M115.655019. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 56.Yu M., Li H., Liu Q., Liu F., Tang L., Li C., Yuan Y., Zhan Y., Xu W., Li W., et al. Nuclear factor p65 interacts with Keap1 to repress the Nrf2-ARE pathway. Cell. Signal. 2011;23:883–892. doi: 10.1016/j.cellsig.2011.01.014. [DOI] [PubMed] [Google Scholar]
- 57.Neymotin A., Calingasan N.Y., Wille E., Naseri N., Petri S., Damiano M., Liby K.T., Risingsong R., Sporn M., Beal M.F., et al. Neuroprotective effect of Nrf2/ARE activators, CDDO ethylamide and CDDO trifluoroethylamide, in a mouse model of amyotrophic lateral sclerosis. Free Radic. Biol. Med. 2011;51:88–96. doi: 10.1016/j.freeradbiomed.2011.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Thimmulappa R.K., Lee H., Rangasamy T., Reddy S.P., Yamamoto M., Kensler T.W., Biswal S. Nrf2 is a critical regulator of the innate immune response and survival during experimental sepsis. J. Clin. Investig. 2006;116:984–995. doi: 10.1172/JCI25790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Motohashi H., Yamamoto M. Nrf2–Keap1 defines a physiologically important stress response mechanism. Trends Mol. Med. 2004;10:549–557. doi: 10.1016/j.molmed.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 60.Pitoniak A., Bohmann D. Mechanisms and functions of Nrf2 signaling in Drosophila. Free Radic. Biol. Med. 2015;88:302–313. doi: 10.1016/j.freeradbiomed.2015.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hu Q., D’Amora D.R., MacNeil L.T., Walhout A.J.M., Kubiseski T.J. The Oxidative Stress Response in Caenorhabditis elegans Requires the GATA Transcription Factor ELT-3 and SKN-1/Nrf2. Genetics. 2017;206:1909–1922. doi: 10.1534/genetics.116.198788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fuse Y., Kobayashi M. Conservation of the Keap1-Nrf2 System: An Evolutionary Journey through Stressful Space and Time. Molecules. 2017;22:436. doi: 10.3390/molecules22030436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Meakin P.J., Chowdhry S., Sharma R.S., Ashford F.B., Walsh S.V., McCrimmon R.J., Dinkova-Kostova A.T., Dillon J.F., Hayes J.D., Ashford M.L.J. Susceptibility of Nrf2-Null Mice to Steatohepatitis and Cirrhosis upon Consumption of a High-Fat Diet Is Associated with Oxidative Stress, Perturbation of the Unfolded Protein Response, and Disturbance in the Expression of Metabolic Enzymes but Not with Insulin Resistance. Mol. Cell. Biol. 2014;34:3305–3320. doi: 10.1128/mcb.00677-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wang M., Kaufman R.J. The impact of the endoplasmic reticulum protein-folding environment on cancer development. Nat. Rev. Cancer. 2014;14:581–597. doi: 10.1038/nrc3800. [DOI] [PubMed] [Google Scholar]
- 65.Lee S., Hur E.-G., Ryoo I.-G., Jung K.-A., Kwak J., Kwak M.-K. Involvement of the Nrf2-proteasome pathway in the endoplasmic reticulum stress response in pancreatic β-cells. Toxicol. Appl. Pharmacol. 2012;264:431–438. doi: 10.1016/j.taap.2012.08.021. [DOI] [PubMed] [Google Scholar]
- 66.Chen W., Sun Z., Wang X.-J., Jiang T., Huang Z., Fang D., Zhang D.D. Direct Interaction between Nrf2 and p21Cip1/WAF1 Upregulates the Nrf2-Mediated Antioxidant Response. Mol. Cell. 2009;34:663–673. doi: 10.1016/j.molcel.2009.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gureev A.P., Sadovnikova I.S., Starkova N.N., Starkov A.A., Popov V.N. p62-Nrf2-p62 Mitophagy Regulatory Loop as a Target for Preventive Therapy of Neurodegenerative Diseases. Brain Sci. 2020;10:847. doi: 10.3390/brainsci10110847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kapeta S., Chondrogianni N., Gonos E.S. Nuclear Erythroid Factor 2-mediated Proteasome Activation Delays Senescence in Human Fibroblasts. J. Biol. Chem. 2010;285:8171–8184. doi: 10.1074/jbc.M109.031575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kwak M.-K., Wakabayashi N., Greenlaw J.L., Yamamoto M., Kensler T.W. Antioxidants Enhance Mammalian Proteasome Expression through the Keap1-Nrf2 Signaling Pathway. Mol. Cell. Biol. 2003;23:8786–8794. doi: 10.1128/MCB.23.23.8786-8794.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pajares M., Cuadrado A., Rojo A.I. Modulation of proteostasis by transcription factor NRF2 and impact in neurodegenerative diseases. Redox Biol. 2017;11:543–553. doi: 10.1016/j.redox.2017.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jirousek L., Stárka L. DCber das Vorkommen von Trithionen (1,2-dithiacyclopent-4-en-3-thione) in Brassicapflanzen. Naturwissenschaften. 1958;45:386–387. doi: 10.1007/BF00678518. [DOI] [Google Scholar]
- 72.Kwak M.-K., Cho J.-M., Huang B., Shin S., Kensler T.W. Role of increased expression of the proteasome in the protective effects of sulforaphane against hydrogen peroxide-mediated cytotoxicity in murine neuroblastoma cells. Free Radic. Biol. Med. 2007;43:809–817. doi: 10.1016/j.freeradbiomed.2007.05.029. [DOI] [PubMed] [Google Scholar]
- 73.Cuadrado A., Kügler S., Lastres-Becker I. Pharmacological targeting of GSK-3 and NRF2 provides neuroprotection in a preclinical model of tauopathy. Redox Biol. 2018;14:522–534. doi: 10.1016/j.redox.2017.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yang Y., Jiang S., Yan J., Li Y., Xin Z., Lin Y., Qu Y. An overview of the molecular mechanisms and novel roles of Nrf2 in neurodegenerative disorders. Cytokine Growth Factor Rev. 2015;26:47–57. doi: 10.1016/j.cytogfr.2014.09.002. [DOI] [PubMed] [Google Scholar]
- 75.Kovac S., Angelova P.R., Holmström K.M., Zhang Y., Dinkova-Kostova A.T., Abramov A.Y. Nrf2 regulates ROS production by mitochondria and NADPH oxidase. Biochim. Biophys. Acta BBA Gen. Subj. 2015;1850:794–801. doi: 10.1016/j.bbagen.2014.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wu K.C., Cui J.Y., Klaassen C.D. Beneficial Role of Nrf2 in Regulating NADPH Generation and Consumption. Toxicol. Sci. 2011;123:590–600. doi: 10.1093/toxsci/kfr183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lee J.-M., Calkins M.J., Chan K., Kan Y.W., Johnson J.A. Identification of the NF-E2-related Factor-2-dependent Genes Conferring Protection against Oxidative Stress in Primary Cortical Astrocytes Using Oligonucleotide Microarray Analysis. J. Biol. Chem. 2003;278:12029–12038. doi: 10.1074/jbc.M211558200. [DOI] [PubMed] [Google Scholar]
- 78.Thimmulappa R.K., Mai K.H., Srisuma S., Kensler T.W., Yamamoto M., Biswal S. Identification of Nrf2-regulated genes induced by the chemopreventive agent sulforaphane by oligonucleotide microarray. Cancer Res. 2002;62:5196–5203. [PubMed] [Google Scholar]
- 79.Jimenez-Blasco D., Santofimia-Castaño P., Gonzalez A., Almeida A., Bolaños J.P. Astrocyte NMDA receptors’ activity sustains neuronal survival through a Cdk5–Nrf2 pathway. Cell Death Differ. 2015;22:1877–1889. doi: 10.1038/cdd.2015.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wild A.C., Mulcahy R.T. Regulation ofγ-glutamylcysteine synthetase subunit gene expression: Insights into transcriptional control of antioxidant defenses. Free Radic. Res. 2000;32:281–301. doi: 10.1080/10715760000300291. [DOI] [PubMed] [Google Scholar]
- 81.Harvey C.J., Thimmulappa R.K., Singh A., Blake D.J., Ling G., Wakabayashi N., Fujii J., Myers A., Biswal S. Nrf2-regulated glutathione recycling independent of biosynthesis is critical for cell survival during oxidative stress. Free Radic. Biol. Med. 2009;46:443–453. doi: 10.1016/j.freeradbiomed.2008.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ribas V., García-Ruiz C., Fernández-Checa J.C. Glutathione and mitochondria. Front. Pharmacol. 2014;5:151. doi: 10.3389/fphar.2014.00151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ryoo I.-G., Kwak M.-K. Regulatory crosstalk between the oxidative stress-related transcription factor Nfe2l2/Nrf2 and mitochondria. Toxicol. Appl. Pharmacol. 2018;359:24–33. doi: 10.1016/j.taap.2018.09.014. [DOI] [PubMed] [Google Scholar]
- 84.Greco T., Shafer J., Fiskum G. Sulforaphane inhibits mitochondrial permeability transition and oxidative stress. Free Radic. Biol. Med. 2011;51:2164–2171. doi: 10.1016/j.freeradbiomed.2011.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Miyamoto N., Izumi H., Miyamoto R., Kondo H., Tawara A., Sasaguri Y., Kohno K. Quercetin Induces the Expression of Peroxiredoxins 3 and 5 via the Nrf2/NRF1 Transcription Pathway. Investig. Opthalmol. Vis. Sci. 2011;52:1055–1063. doi: 10.1167/iovs.10-5777. [DOI] [PubMed] [Google Scholar]
- 86.Hu B., Wu Y., Liu J., Shen X., Tong F., Xu G., Shen R. GSK-3beta Inhibitor Induces Expression of Nrf2/TrxR2 Signaling Pathway to Protect against Renal Ischemia/Reperfusion Injury in Diabetic Rats. Kidney Blood Press. Res. 2016;41:937–946. doi: 10.1159/000452598. [DOI] [PubMed] [Google Scholar]
- 87.Lee J.-M., Shih A.Y., Murphy T.H., Johnson J.A. NF-E2-related Factor-2 Mediates Neuroprotection against Mitochondrial Complex I Inhibitors and Increased Concentrations of Intracellular Calcium in Primary Cortical Neurons. J. Biol. Chem. 2003;278:37948–37956. doi: 10.1074/jbc.M305204200. [DOI] [PubMed] [Google Scholar]
- 88.Spinelli J.B., Haigis M.C. The multifaceted contributions of mitochondria to cellular metabolism. Nat. Cell Biol. 2018;20:745–754. doi: 10.1038/s41556-018-0124-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Szewczyk A., Wojtczak L. Mitochondria as a pharmacological target. Pharmacol. Rev. 2002;54:101–127. doi: 10.1124/pr.54.1.101. [DOI] [PubMed] [Google Scholar]
- 90.Nunnari J., Suomalainen A. Mitochondria: In Sickness and in Health. Cell. 2012;148:1145–1159. doi: 10.1016/j.cell.2012.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Handy D.E., Loscalzo J. Redox Regulation of Mitochondrial Function. Antioxid. Redox Signal. 2012;16:1323–1367. doi: 10.1089/ars.2011.4123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Finkel T. Reactive oxygen species and signal transduction. IUBMB Life. 2001;52:3–6. doi: 10.1080/15216540252774694. [DOI] [PubMed] [Google Scholar]
- 93.Fiskum G., Murphy A.N., Beal M.F. Mitochondria in Neurodegeneration: Acute Ischemia and Chronic Neurodegenerative Diseases. J. Cereb. Blood Flow Metab. 1999;19:351–369. doi: 10.1097/00004647-199904000-00001. [DOI] [PubMed] [Google Scholar]
- 94.Niizuma K., Endo H., Chan P.H. Oxidative stress and mitochondrial dysfunction as determinants of ischemic neuronal death and survival. J. Neurochem. 2009;109:133–138. doi: 10.1111/j.1471-4159.2009.05897.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Navarro A., Boveris A. Brain mitochondrial dysfunction and oxidative damage in Parkinson’s disease. J. Bioenerg. Biomembr. 2009;41:517–521. doi: 10.1007/s10863-009-9250-6. [DOI] [PubMed] [Google Scholar]
- 96.Lemasters J.J., Theruvath T.P., Zhong Z., Nieminen A.-L. Mitochondrial calcium and the permeability transition in cell death. Biochim. Biophys. Acta BBA Bioenerg. 2009;1787:1395–1401. doi: 10.1016/j.bbabio.2009.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Halestrap A.P., McStay G.P., Clarke S.J. The permeability transition pore complex: Another view. Biochimie. 2002;84:153–166. doi: 10.1016/S0300-9084(02)01375-5. [DOI] [PubMed] [Google Scholar]
- 98.Lo S.-C., Hannink M. PGAM5 tethers a ternary complex containing Keap1 and Nrf2 to mitochondria. Exp. Cell Res. 2008;314:1789–1803. doi: 10.1016/j.yexcr.2008.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ruiz K., Thaker T.M., Agnew C., Miller-Vedam L., Trenker R., Herrera C., Ingaramo M., Toso D., Frost A., Jura N. Functional role of PGAM5 multimeric assemblies and their polymerization into filaments. Nat. Commun. 2019;10:531. doi: 10.1038/s41467-019-08393-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.O’Mealey G.B., Plafker K.S., Berry W.L., Janknecht R., Chan J.Y., Plafker S.M. A PGAM5-KEAP1-Nrf2 complex is required for stress-induced mitochondrial retrograde trafficking. J. Cell Sci. 2017;130:3467–3480. doi: 10.1242/jcs.203216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Dinkova-Kostova A.T., Baird L., Holmström K.M., Meyer C.J., Abramov A.Y. The spatiotemporal regulation of the Keap1–Nrf2 pathway and its importance in cellular bioenergetics. Biochem. Soc. Trans. 2015;43:602–610. doi: 10.1042/BST20150003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Esteras N., Dinkova-Kostova A.T., Abramov A.Y. Nrf2 activation in the treatment of neurodegenerative diseases: A focus on its role in mitochondrial bioenergetics and function. Biol. Chem. 2016;397:383–400. doi: 10.1515/hsz-2015-0295. [DOI] [PubMed] [Google Scholar]
- 103.Holmström K.M., Kostov R.V., Dinkova-Kostova A.T. The Multifaceted Role of Nrf2 in Mitochondrial Function. Curr. Opin. Toxicol. 2016;1:80–91. doi: 10.1016/j.cotox.2016.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ludtmann M.H.R., Angelova P.R., Zhang Y., Abramov A.Y., Dinkova-Kostova A.T. Nrf2 affects the efficiency of mitochondrial fatty acid oxidation. Biochem. J. 2014;457:415–424. doi: 10.1042/BJ20130863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Holmström K.M., Baird L., Zhang Y., Hargreaves I., Chalasani A., Land J.M., Stanyer L., Yamamoto M., Dinkova-Kostova A.T., Abramov A.Y. Nrf2 Impacts Cellular Bioenergetics by Controlling Substrate Availability for Mitochondrial Respiration. Biol. Open. 2013;2:761–770. doi: 10.1242/bio.20134853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Piantadosi C.A., Carraway M.S., Babiker A., Suliman H.B. Heme Oxygenase-1 Regulates Cardiac Mitochondrial Biogenesis via Nrf2-Mediated Transcriptional Control of Nuclear Respiratory Factor-1. Circ. Res. 2008;103:1232–1240. doi: 10.1161/01.RES.0000338597.71702.ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Sun N., Youle R.J., Finkel T. The Mitochondrial Basis of Aging. Mol. Cell. 2016;61:654–666. doi: 10.1016/j.molcel.2016.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Komatsu M., Kurokawa H., Waguri S., Taguchi K., Kobayashi A., Ichimura Y., Sou Y.-S., Ueno I., Sakamoto A., Tong K.I., et al. The selective autophagy substrate p62 activates the stress responsive transcription factor Nrf2 through inactivation of Keap1. Nat. Cell Biol. 2010;12:213–223. doi: 10.1038/ncb2021. [DOI] [PubMed] [Google Scholar]
- 109.Lippai M., Lőw P. The Role of the Selective Adaptor p62 and Ubiquitin-Like Proteins in Autophagy. BioMed Res. Int. 2014;2014:832704. doi: 10.1155/2014/832704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Jain A., Lamark T., Sjøttem E., Larsen K.B., Awuh J.A., Øvervatn A., McMahon M., Hayes J.D., Johansen T. p62/SQSTM1 Is a Target Gene for Transcription Factor NRF2 and Creates a Positive Feedback Loop by Inducing Antioxidant Response Element-driven Gene Transcription. J. Biol. Chem. 2010;285:22576–22591. doi: 10.1074/jbc.M110.118976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ichimura Y., Waguri S., Sou Y.-S., Kageyama S., Hasegawa J., Ishimura R., Saito T., Yang Y., Kouno T., Fukutomi T., et al. Phosphorylation of p62 Activates the Keap1-Nrf2 Pathway during Selective Autophagy. Mol. Cell. 2013;51:618–631. doi: 10.1016/j.molcel.2013.08.003. [DOI] [PubMed] [Google Scholar]
- 112.Cherry A.D., Suliman H.B., Bartz R.R., Piantadosi C.A. Peroxisome Proliferator-activated Receptor γ Co-activator 1-α as a Critical Co-activator of the Murine Hepatic Oxidative Stress Response and Mitochondrial Biogenesis in Staphylococcus aureus Sepsis. J. Biol. Chem. 2014;289:41–52. doi: 10.1074/jbc.M113.512483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Min K.-J., Yang M.-S., Kim S.-U., Jou I., Joe E.-H. Astrocytes Induce Hemeoxygenase-1 Expression in Microglia: A Feasible Mechanism for Preventing Excessive Brain Inflammation. J. Neurosci. 2006;26:1880–1887. doi: 10.1523/JNEUROSCI.3696-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Sivandzade F., Prasad S., Bhalerao A., Cucullo L. NRF2 and NF-κB interplay in cerebrovascular and neurodegenerative disorders: Molecular mechanisms and possible therapeutic approaches. Redox Biol. 2019;21:101059. doi: 10.1016/j.redox.2018.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Castro-Sánchez S., García-Yagüe Á.J., Kügler S., Lastres-Becker I. CX3CR1-deficient microglia shows impaired signalling of the transcription factor NRF2: Implications in tauopathies. Redox Biol. 2019;22:101118. doi: 10.1016/j.redox.2019.101118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ransohoff R.M. How neuroinflammation contributes to neurodegeneration. Science. 2016;353:777–783. doi: 10.1126/science.aag2590. [DOI] [PubMed] [Google Scholar]
- 117.Sochocka M., Diniz B.S., Leszek J. Inflammatory Response in the CNS: Friend or Foe? Mol. Neurobiol. 2017;54:8071–8089. doi: 10.1007/s12035-016-0297-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Scuderi S.A., Ardizzone A., Paterniti I., Esposito E., Campolo M. Antioxidant and Anti-Inflammatory Effect of Nrf2 Inducer Dimethyl Fumarate in Neurodegenerative Diseases. Antioxidants. 2020;9:630. doi: 10.3390/antiox9070630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Inui M., Ishida Y., Kimura A., Kuninaka Y., Mukaida N., Kondo T. Protective Roles of CX3CR1-Mediated Signals in Toxin A-Induced Enteritis through the Induction of Heme Oxygenase-1 Expression. J. Immunol. 2011;186:423–431. doi: 10.4049/jimmunol.1000043. [DOI] [PubMed] [Google Scholar]
- 120.Mecca C., Giambanco I., Donato R., Arcuri C. Microglia and Aging: The Role of the TREM2–DAP12 and CX3CL1-CX3CR1 Axes. Int. J. Mol. Sci. 2018;19:318. doi: 10.3390/ijms19010318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Singh S., Nagalakshmi D., Sharma K.K., Ravichandiran V. Natural antioxidants for neuroinflammatory disorders and possible involvement of Nrf2 pathway: A review. Heliyon. 2021;7:e06216. doi: 10.1016/j.heliyon.2021.e06216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Dorsey E.R., Elbaz A., Nichols E., Abd-Allah F., Abdelalim A., Adsuar J.C., Ansha M.G., Brayne C., Choi J.-Y.J., Collado-Mateo D., et al. Global, regional, and national burden of Parkinson’s disease, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2018;17:939–953. doi: 10.1016/S1474-4422(18)30295-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Ascherio A., Schwarzschild M.A. The epidemiology of Parkinson’s disease: Risk factors and prevention. Lancet Neurol. 2016;15:1257–1272. doi: 10.1016/S1474-4422(16)30230-7. [DOI] [PubMed] [Google Scholar]
- 124.Emamzadeh F.N., Surguchov A. Parkinson’s Disease: Biomarkers, Treatment, and Risk Factors. Front. Neurosci. 2018;12:612. doi: 10.3389/fnins.2018.00612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Spillantini M.G., Schmidt M.L., Lee V.M.-Y., Trojanowski J.Q., Jakes R., Goedert M. α-Synuclein in Lewy bodies. Nature. 1997;388:839–840. doi: 10.1038/42166. [DOI] [PubMed] [Google Scholar]
- 126.Spillantini M.G., Crowther R.A., Jakes R., Hasegawa M., Goedert M. α-Synuclein in filamentous inclusions of Lewy bodies from Parkinson’s disease and dementia with Lewy bodies. Proc. Natl. Acad. Sci. USA. 1998;95:6469–6473. doi: 10.1073/pnas.95.11.6469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Goedert M., Jakes R., Spillantini M.G. The Synucleinopathies: Twenty Years On. J. Parkinsons Dis. 2017;7:S51–S69. doi: 10.3233/JPD-179005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Braak H., Del Tredici K., Rüb U., de Vos R.A.I., Steur E.N.H.J., Braak E. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol. Aging. 2003;24:197–211. doi: 10.1016/S0197-4580(02)00065-9. [DOI] [PubMed] [Google Scholar]
- 129.Iborra S.F., Cuadros T., Parent A., Romero-Gimenez J., Vila M., Perier C. Defective mitochondrial protein import contributes to complex I-induced mitochondrial dysfunction and neurodegeneration in Parkinson’s disease. Cell Death Dis. 2018;9:1122. doi: 10.1038/s41419-018-1154-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Helley M.P., Pinnell J., Sportelli C., Tieu K. Mitochondria: A Common Target for Genetic Mutations and Environmental Toxicants in Parkinson’s Disease. Front. Genet. 2017;8:177. doi: 10.3389/fgene.2017.00177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Devoto V.M.P., Falzone T.L. Mitochondrial dynamics in Parkinson’s disease: A role for α-synuclein? Dis. Models Mech. 2017;10:1075–1087. doi: 10.1242/dmm.026294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Grassi D., Howard S., Zhou M., Diaz-Perez N., Urban N.T., Guerrero-Given D., Kamasawa N., Volpicelli-Daley L.A., LoGrasso P., Lasmézas C.I. Identification of a highly neurotoxic α-synuclein species inducing mitochondrial damage and mitophagy in Parkinson’s disease. Proc. Natl. Acad. Sci. USA. 2018;115:E2634–E2643. doi: 10.1073/pnas.1713849115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Inamdar N., Arulmozhi D., Tandon A., Bodhankar S. Parkinsons Disease: Genetics and Beyond. Curr. Neuropharmacol. 2007;5:99–113. doi: 10.2174/157015907780866893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.International Parkinson Disease Genomics Consortium Imputation of sequence variants for identification of genetic risks for Parkinson’s disease: A meta-analysis of genome-wide association studies. Lancet. 2011;377:641–649. doi: 10.1016/S0140-6736(10)62345-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Lesage S., Brice A. Parkinson’s disease: From monogenic forms to genetic susceptibility factors. Hum. Mol. Genet. 2009;18:R48–R59. doi: 10.1093/hmg/ddp012. [DOI] [PubMed] [Google Scholar]
- 136.Klein C., Westenberger A. Genetics of Parkinson’s Disease. Cold Spring Harb. Perspect. Med. 2012;2:a008888. doi: 10.1101/cshperspect.a008888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Alegre-Abarrategui J., Christian H., Lufino M.M.P., Mutihac R., Venda L.L., Ansorge O., Wade-Martins R. LRRK2 regulates autophagic activity and localizes to specific membrane microdomains in a novel human genomic reporter cellular model. Hum. Mol. Genet. 2009;18:4022–4034. doi: 10.1093/hmg/ddp346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Lee Y., Stevens D.A., Kang S.-U., Jiang H., Lee Y.-I., Ko H.S., Scarffe L.A., Umanah G.E., Kang H., Ham S., et al. PINK1 Primes Parkin-Mediated Ubiquitination of PARIS in Dopaminergic Neuronal Survival. Cell Rep. 2017;18:918–932. doi: 10.1016/j.celrep.2016.12.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Geisler S., Holmström K.M., Skujat D., Fiesel F.C., Rothfuss O.C., Kahle P.J., Springer W. PINK1/Parkin-mediated mitophagy is dependent on VDAC1 and p62/SQSTM1. Nat. Cell Biol. 2010;12:119–131. doi: 10.1038/ncb2012. [DOI] [PubMed] [Google Scholar]
- 140.Narendra D.P., Jin S.M., Tanaka A., Suen D.-F., Gautier C.A., Shen J., Cookson M.R., Youle R.J. PINK1 Is Selectively Stabilized on Impaired Mitochondria to Activate Parkin. PLoS Biol. 2010;8:e1000298. doi: 10.1371/journal.pbio.1000298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Mita Y., Kataoka Y., Saito Y., Kashi T., Hayashi K., Iwasaki A., Imanishi T., Miyasaka T., Noguchi N. Distribution of oxidized DJ-1 in Parkinson’s disease-related sites in the brain and in the peripheral tissues: Effects of aging and a neurotoxin. Sci. Rep. 2018;8:12056. doi: 10.1038/s41598-018-30561-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Strobbe D., Robinson A.A., Harvey K., Rossi L., Ferraina C., De Biase V., Rodolfo C., Harvey R.J., Campanella M. Distinct Mechanisms of Pathogenic DJ-1 Mutations in Mitochondrial Quality Control. Front. Mol. Neurosci. 2018;11:68. doi: 10.3389/fnmol.2018.00068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Zuo L., Motherwell M.S. The impact of reactive oxygen species and genetic mitochondrial mutations in Parkinson’s disease. Gene. 2013;532:18–23. doi: 10.1016/j.gene.2013.07.085. [DOI] [PubMed] [Google Scholar]
- 144.Jenner P. Oxidative stress in Parkinson’s disease. Ann. Neurol. 2003;53:S26–S38. doi: 10.1002/ana.10483. [DOI] [PubMed] [Google Scholar]
- 145.Kolodkin A.N., Sharma R.P., Papa M., Kumar V., Peters B., Skupin A., Alberghina L., Balling R., Westerhoff H.V., Colangelo A.M., et al. ROS networks: Designs, aging, Parkinson’s disease and precision therapies. npj Syst. Biol. Appl. 2020;6:34. doi: 10.1038/s41540-020-00150-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Brennan A.M., Suh S.W., Won S.J., Narasimhan P., Kauppinen T.M., Lee H., Edling Y., Chan P.H., Swanson R.A. NADPH oxidase is the primary source of superoxide induced by NMDA receptor activation. Nat. Neurosci. 2009;12:857–863. doi: 10.1038/nn.2334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Schapira A.H., Jenner P. Etiology and pathogenesis of Parkinson’s disease. Mov. Disord. 2011;26:1049–1055. doi: 10.1002/mds.23732. [DOI] [PubMed] [Google Scholar]
- 148.Jenner P., Olanow C.W. The pathogenesis of cell death in Parkinson’s disease. Neurology. 2006;66:S24–S36. doi: 10.1212/WNL.66.10_suppl_4.S24. [DOI] [PubMed] [Google Scholar]
- 149.Kwon D.H., Cha H.-J., Lee H., Hong S.-H., Park C., Park S.-H., Kim G.-Y., Kim S., Kim H.-S., Hwang H.-J., et al. Protective Effect of Glutathione against Oxidative Stress-induced Cytotoxicity in RAW 264.7 Macrophages through Activating the Nuclear Factor Erythroid 2-Related Factor-2/Heme Oxygenase-1 Pathway. Antioxidants. 2019;8:82. doi: 10.3390/antiox8040082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Smeyne M., Smeyne R.J. Glutathione metabolism and Parkinson’s disease. Free Radic. Biol. Med. 2013;62:13–25. doi: 10.1016/j.freeradbiomed.2013.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Pearce R.K.B., Owen A., Daniel S., Jenner P., Marsden C.D. Alterations in the distribution of glutathione in the substantia nigra in Parkinson’s disease. J. Neural Transm. 1997;104:661–677. doi: 10.1007/BF01291884. [DOI] [PubMed] [Google Scholar]
- 152.Hsu M., Srinivas B., Kumar J., Subramanian R., Andersen J. Glutathione depletion resulting in selective mitochondrial complex I inhibition in dopaminergic cells is via an NO-mediated pathway not involving peroxynitrite: Implications for Parkinson’s disease: Glutathione Affects Mitochondrial Complex I via NO Not ONOO. J. Neurochem. 2005;92:1091–1103. doi: 10.1111/j.1471-4159.2004.02929.x. [DOI] [PubMed] [Google Scholar]
- 153.Haas R.H., Nasirian F., Nakano K., Ward D., Pay M., Hill R., Shults C.W. Low platelet mitochondrial complex I and complex II/III activity in early untreated Parkinson’s disease. Ann. Neurol. 1995;37:714–722. doi: 10.1002/ana.410370604. [DOI] [PubMed] [Google Scholar]
- 154.Görlach A., Bertram K., Hudecova S., Krizanova O. Calcium and ROS: A mutual interplay. Redox Biol. 2015;6:260–271. doi: 10.1016/j.redox.2015.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Burbulla L.F., Song P., Savas J.N., Kiskinis E., Zhuang X., Krüger R., Surmeier D.J., Krainc D., Mazzulli J.R., Zampese E., et al. Dopamine oxidation mediates mitochondrial and lysosomal dysfunction in Parkinson’s disease. Science. 2017;357:1255–1261. doi: 10.1126/science.aam9080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Berman S.B., Hastings T.G. Dopamine Oxidation Alters Mitochondrial Respiration and Induces Permeability Transition in Brain Mitochondria: Implications for Parkinson’s Disease. J. Neurochem. 2001;73:1127–1137. doi: 10.1046/j.1471-4159.1999.0731127.x. [DOI] [PubMed] [Google Scholar]
- 157.Bosco D.A., Fowler D.M., Zhang Q., Nieva J., Powers E.T., Wentworth P., Lerner R.A., Kelly J.W. Elevated levels of oxidized cholesterol metabolites in Lewy body disease brains accelerate α-synuclein fibrilization. Nat. Chem. Biol. 2006;2:249–253. doi: 10.1038/nchembio782. [DOI] [PubMed] [Google Scholar]
- 158.Davis G.C., Williams A.C., Markey S.P., Ebert M.H., Caine E.D., Reichert C.M., Kopin I.J. Chronic parkinsonism secondary to intravenous injection of meperidine analogues. Psychiatry Res. 1979;1:249–254. doi: 10.1016/0165-1781(79)90006-4. [DOI] [PubMed] [Google Scholar]
- 159.Langston J., Ballard P., Tetrud J., Irwin I. Chronic Parkinsonism in humans due to a product of meperidine-analog synthesis. Science. 1983;219:979–980. doi: 10.1126/science.6823561. [DOI] [PubMed] [Google Scholar]
- 160.Nicklas W.J., Vyas I., Heikkila R.E. Inhibition of NADH-linked oxidation in brain mitochondria by 1-methyl-4-phenyl-pyridine, a metabolite of the neurotoxin, 1-methyl-4-phenyl-1,2,5,6-tetrahydropyridine. Life Sci. 1985;36:2503–2508. doi: 10.1016/0024-3205(85)90146-8. [DOI] [PubMed] [Google Scholar]
- 161.Ramsay R.R., Salach J.I., Dadgar J., Singer T.P. Inhibition of mitochondrial NADH dehydrogenase by pyridine derivatives and its possible relation to experimental and idiopathic parkinsonism. Biochem. Biophys. Res. Commun. 1986;135:269–275. doi: 10.1016/0006-291X(86)90972-1. [DOI] [PubMed] [Google Scholar]
- 162.Chan P., DeLanney L.E., Irwin I., Langston J.W., Di Monte D. Rapid ATP Loss Caused by 1-Methyl-4-Phenyl-1,2,3,6-Tetrahydropyridine in Mouse Brain. J. Neurochem. 1991;57:348–351. doi: 10.1111/j.1471-4159.1991.tb02134.x. [DOI] [PubMed] [Google Scholar]
- 163.Drechsel D.A., Patel M. Role of reactive oxygen species in the neurotoxicity of environmental agents implicated in Parkinson’s disease. Free Radic. Biol. Med. 2008;44:1873–1886. doi: 10.1016/j.freeradbiomed.2008.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Dauer W., Przedborski S. Parkinson’s Disease: Mechanisms and Models. Neuron. 2003;39:889–909. doi: 10.1016/S0896-6273(03)00568-3. [DOI] [PubMed] [Google Scholar]
- 165.Hartmann A., Michel P.P., Troadec J.-D., Mouatt-Prigent A., Faucheux B.A., Ruberg M., Agid Y., Hirsch E.C. Is Bax a mitochondrial mediator in apoptotic death of dopaminergic neurons in Parkinson’s disease? J. Neurochem. 2001;76:1785–1793. doi: 10.1046/j.1471-4159.2001.00160.x. [DOI] [PubMed] [Google Scholar]
- 166.Schapira A.H.V., Cooper J.M., Dexter D., Jenner P., Clark J.B., Marsden C.D. Mitochondrial complex I deficiency in Parkinson’s disease. Lancet. 1989;333:1269. doi: 10.1016/S0140-6736(89)92366-0. [DOI] [PubMed] [Google Scholar]
- 167.Janetzky B., Hauck S., Youdim M.B.H., Riederer P., Jellinger K., Pantucek F., Zöchling R., Boissl K.W., Reichmann H. Unaltered aconitase activity, but decreased complex I activity in substantia nigra pars compacta of patients with Parkinson’s disease. Neurosci. Lett. 1994;169:126–128. doi: 10.1016/0304-3940(94)90372-7. [DOI] [PubMed] [Google Scholar]
- 168.Smigrodzki R., Parks J., Parker W.D. High frequency of mitochondrial complex I mutations in Parkinson’s disease and aging. Neurobiol. Aging. 2004;25:1273–1281. doi: 10.1016/j.neurobiolaging.2004.02.020. [DOI] [PubMed] [Google Scholar]
- 169.Parker D.W., Parks J.K., Swerdlow R.H. Complex I Deficiency in Parkinson’s Disease Frontal Cortex. Neurobiol. Aging. 2008;25:1273–1281. doi: 10.1016/j.brainres.2007.10.061. [DOI] [Google Scholar]
- 170.Bury A.G., Pyle A., Elson J.L., Greaves L., Morris C.M., Hudson G., Pienaar I.S. Mitochondrial DNA changes in pedunculopontine cholinergic neurons in Parkinson disease. Ann. Neurol. 2017;82:1016–1021. doi: 10.1002/ana.25099. [DOI] [PubMed] [Google Scholar]
- 171.Flønes I.H., Fernandez-Vizarra E., Lykouri M., Brakedal B., Skeie G.O., Miletic H., Lilleng P.K., Alves G., Tysnes O.-B., Haugarvoll K., et al. Neuronal complex I deficiency occurs throughout the Parkinson’s disease brain, but is not associated with neurodegeneration or mitochondrial DNA damage. Acta Neuropathol. 2018;135:409–425. doi: 10.1007/s00401-017-1794-7. [DOI] [PubMed] [Google Scholar]
- 172.Virbasius J.V., Scarpulla R.C. Activation of the human mitochondrial transcription factor A gene by nuclear respiratory factors: A potential regulatory link between nuclear and mitochondrial gene expression in organelle biogenesis. Proc. Natl. Acad. Sci. USA. 1994;91:1309–1313. doi: 10.1073/pnas.91.4.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Ekstrand M.I., Terzioglu M., Hoffer B., Cullheim S., Mohammed A.H., Olson L., Larsson N.-G., Galter D., Zhu S., Hofstetter C., et al. Progressive parkinsonism in mice with respiratory-chain-deficient dopamine neurons. Proc. Natl. Acad. Sci. USA. 2007;104:1325–1330. doi: 10.1073/pnas.0605208103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Ding W.-X., Yin X.-M. Mitophagy: Mechanisms, pathophysiological roles, and analysis. Biol. Chem. 2012;393:547–564. doi: 10.1515/hsz-2012-0119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Doherty J., Baehrecke E.H. Life, death and autophagy. Nat. Cell Biol. 2018;20:1110–1117. doi: 10.1038/s41556-018-0201-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Barazzuol L., Giamogante F., Brini M., Calì T. PINK1/Parkin Mediated Mitophagy, Ca2+ Signalling, and ER–Mitochondria Contacts in Parkinson’s Disease. Int. J. Mol. Sci. 2020;21:1772. doi: 10.3390/ijms21051772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Kitada T., Asakawa S., Hattori N., Matsumine H., Yamamura Y., Minoshima S., Yokochi M., Mizuno Y., Shimizu N. Mutations in the parkin gene cause autosomal recessive juvenile parkinsonism. Nature. 1998;392:605–608. doi: 10.1038/33416. [DOI] [PubMed] [Google Scholar]
- 178.Khymenets O., Ortuño J., Fitó M., Covas M.I., Farré M., De La Torre R. Evaluation of RNA isolation procedures from human blood and its application for gene expression studies (Sod-1, Sod-2) Anal. Biochem. 2005;347:156–158. doi: 10.1016/j.ab.2005.07.007. [DOI] [PubMed] [Google Scholar]
- 179.Nakamura K., Nemani V.M., Azarbal F., Skibinski G., Levy J.M., Egami K., Munishkina L., Zhang J., Gardner B., Wakabayashi J., et al. Direct Membrane Association Drives Mitochondrial Fission by the Parkinson Disease-associated Protein α-Synuclein. J. Biol. Chem. 2011;286:20710–20726. doi: 10.1074/jbc.M110.213538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Valente E.M., Abou-Sleiman P.M., Caputo V., Muqit M.M.K., Harvey K., Gispert S., Ali Z., Del Turco D., Bentivoglio A.R., Healy D.G., et al. Hereditary Early-Onset Parkinson’s Disease Caused by Mutations in PINK1. Science. 2004;304:1158–1160. doi: 10.1126/science.1096284. [DOI] [PubMed] [Google Scholar]
- 181.Pickrell A.M., Youle R.J. The Roles of PINK1, Parkin, and Mitochondrial Fidelity in Parkinson’s Disease. Neuron. 2015;85:257–273. doi: 10.1016/j.neuron.2014.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Shirihai O.S., Song M., Dorn G.W., II How Mitochondrial Dynamism Orchestrates Mitophagy. Circ. Res. 2015;116:1835–1849. doi: 10.1161/CIRCRESAHA.116.306374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Stevens D.A., Lee Y., Kang H.C., Lee B.D., Lee Y.-I., Bower A., Jiang H., Kang S.-U., Andrabi S.A., Dawson V.L., et al. Parkin loss leads to PARIS-dependent declines in mitochondrial mass and respiration. Proc. Natl. Acad. Sci. USA. 2015;112:11696–11701. doi: 10.1073/pnas.1500624112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Puigserver P., Wu Z., Park C.W., Graves R., Wright M., Spiegelman B.M. A Cold-Inducible Coactivator of Nuclear Receptors Linked to Adaptive Thermogenesis. Cell. 1998;92:829–839. doi: 10.1016/S0092-8674(00)81410-5. [DOI] [PubMed] [Google Scholar]
- 185.Castillo-Quan J.I. Parkin’ control: Regulation of PGC-1α through PARIS in Parkinson’s disease. Dis. Models Mech. 2011;4:427–429. doi: 10.1242/dmm.008227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Murata H., Takamatsu H., Liu S., Kataoka K., Huh N.-H., Sakaguchi M. NRF2 Regulates PINK1 Expression under Oxidative Stress Conditions. PLoS ONE. 2015;10:e0142438. doi: 10.1371/journal.pone.0142438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Wang Y., Liu N., Lu B. Mechanisms and roles of mitophagy in neurodegenerative diseases. CNS Neurosci. Ther. 2019;25:859–875. doi: 10.1111/cns.13140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Dagda R.K., Gusdon A.M., Pien I., Strack S., Green S., Li C., Van Houten B., Cherra S.J., Chu C.T. Mitochondrially localized PKA reverses mitochondrial pathology and dysfunction in a cellular model of Parkinson’s disease. Cell Death Differ. 2011;18:1914–1923. doi: 10.1038/cdd.2011.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Deas E., Wood N.W., Plun-Favreau H. Mitophagy and Parkinson’s disease: The PINK1–parkin link. Biochim. Biophys. Acta BBA Mol. Cell Res. 2011;1813:623–633. doi: 10.1016/j.bbamcr.2010.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Palacino J.J., Sagi D., Goldberg M.S., Krauss S., Motz C., Wacker M., Klose J., Shen J. Mitochondrial Dysfunction and Oxidative Damage in parkin-deficient Mice. J. Biol. Chem. 2004;279:18614–18622. doi: 10.1074/jbc.M401135200. [DOI] [PubMed] [Google Scholar]
- 191.Flinn L., Mortiboys H., Volkmann K., Köster R.W., Ingham P.W., Bandmann O. Complex I deficiency and dopaminergic neuronal cell loss in parkin-deficient zebrafish (Danio rerio) Brain. 2009;132:1613–1623. doi: 10.1093/brain/awp108. [DOI] [PubMed] [Google Scholar]
- 192.Zanon A., Kalvakuri S., Rakovic A., Foco L., Guida M., Schwienbacher C., Serafin A., Rudolph F., Trilck M., Grünewald A., et al. Corrigendum: SLP-2 interacts with Parkin in mitochondria and prevents mitochondrial dysfunction in Parkin-deficient human iPSC-derived neurons and Drosophila. Hum. Mol. Genet. 2019;28:1225. doi: 10.1093/hmg/ddy408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Cherra S.J., III, Dagda R.K., Chu C.T. Review: Autophagy and neurodegeneration: Survival at a cost? Neuropathol. Appl. Neurobiol. 2010;36:125–132. doi: 10.1111/j.1365-2990.2010.01062.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Chu C.T., Plowey E.D., Dagda R.K., Hickey R.W., Cherra S.J., Clark R.S.B. Autophagy in neurite injury and neurodegeneration: In vitro and in vivo models. Methods Enzymol. 2009;453:217–249. doi: 10.1016/S0076-6879(08)04011-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Lim J.L., Wilhelmus M.M.M., De Vries H.E., Drukarch B., Hoozemans J.J.M., Van Horssen J. Antioxidative defense mechanisms controlled by Nrf2: State-of-the-art and clinical perspectives in neurodegenerative diseases. Arch. Toxicol. 2014;88:1773–1786. doi: 10.1007/s00204-014-1338-z. [DOI] [PubMed] [Google Scholar]
- 196.Ahuja M., Kaidery N.A., Yang L., Calingasan N., Smirnova N., Gaisin A., Gaisina I.N., Gazaryan I., Hushpulian D.M., Kaddour-Djebbar I., et al. Distinct Nrf2 Signaling Mechanisms of Fumaric Acid Esters and Their Role in Neuroprotection against 1-Methyl-4-Phenyl-1,2,3,6-Tetrahydropyridine-Induced Experimental Parkinson’s-Like Disease. J. Neurosci. 2016;36:6332–6351. doi: 10.1523/JNEUROSCI.0426-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Campolo M., Casili G., Lanza M., Filippone A., Paterniti I., Cuzzocrea S., Esposito E. Multiple mechanisms of dimethyl fumarate in amyloid β-induced neurotoxicity in human neuronal cells. J. Cell. Mol. Med. 2018;22:1081–1094. doi: 10.1111/jcmm.13358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Onasanwo S.A., Velagapudi R., El-Bakoush A., Olajide O.A. Inhibition of neuroinflammation in BV2 microglia by the biflavonoid kolaviron is dependent on the Nrf2/ARE antioxidant protective mechanism. Mol. Cell. Biochem. 2016;414:23–36. doi: 10.1007/s11010-016-2655-8. [DOI] [PubMed] [Google Scholar]
- 199.Bowling A.C., Beal M.F. Bioenergetic and oxidative stress in neurodegenerative diseases. Life Sci. 1995;56:1151–1171. doi: 10.1016/0024-3205(95)00055-B. [DOI] [PubMed] [Google Scholar]
- 200.Browne S.E., Beal M.F. Oxidative Damage in Huntington’s Disease Pathogenesis. Antioxidants Redox Signal. 2006;8:2061–2073. doi: 10.1089/ars.2006.8.2061. [DOI] [PubMed] [Google Scholar]
- 201.De Vries H.E., Witte M., Hondius D., Rozemuller A.J.M., Drukarch B., Hoozemans J., van Horssen J. Nrf2-induced antioxidant protection: A promising target to counteract ROS-mediated damage in neurodegenerative disease? Free Radic. Biol. Med. 2008;45:1375–1383. doi: 10.1016/j.freeradbiomed.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 202.Van Muiswinkel F.L., Kuiperij H.B. The Nrf2-ARE Signalling Pathway: Promising Drug Target to Combat Oxidative Stress in Neurodegenerative Disorders. Curr. Drug Target CNS Neurol. Disord. 2005;4:267–281. doi: 10.2174/1568007054038238. [DOI] [PubMed] [Google Scholar]
- 203.Clements C.M., McNally R.S., Conti B.J., Mak T.W., Ting J.P.-Y. DJ-1, a cancer- and Parkinson’s disease-associated protein, stabilizes the antioxidant transcriptional master regulator Nrf2. Proc. Natl. Acad. Sci. USA. 2006;103:15091–15096. doi: 10.1073/pnas.0607260103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Li W., Jain M.R., Chen C., Yue X., Hebbar V., Zhou R., Kong A.-N.T. Nrf2 Possesses a Redox-insensitive Nuclear Export Signal Overlapping with the Leucine Zipper Motif. J. Biol. Chem. 2005;280:28430–28438. doi: 10.1074/jbc.M410601200. [DOI] [PubMed] [Google Scholar]
- 205.Malhotra D., Thimmulappa R., Navas-Acien A., Sandford A., Elliott M., Singh A., Chen L., Zhuang X., Hogg J., Paré P., et al. Decline in NRF2-regulated Antioxidants in Chronic Obstructive Pulmonary Disease Lungs Due to Loss of Its Positive Regulator, DJ-1. Am. J. Respir. Crit. Care Med. 2008;178:592–604. doi: 10.1164/rccm.200803-380OC. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 206.Zhou W., Freed C.R. DJ-1 Up-regulates Glutathione Synthesis during Oxidative Stress and Inhibits A53T α-Synuclein Toxicity. J. Biol. Chem. 2005;280:43150–43158. doi: 10.1074/jbc.M507124200. [DOI] [PubMed] [Google Scholar]
- 207.Dick F.D., De Palma G., Ahmadi A., Osborne A., Scott N.W., Prescott G.J., Bennett J., Semple S., Dick S., Mozzoni P., et al. Gene-environment interactions in parkinsonism and Parkinson’s disease: The Geoparkinson study. Occup. Environ. Med. 2007;64:673–680. doi: 10.1136/oem.2006.032078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Singh M., Khan A.J., Shah P.P., Shukla R., Khanna V.K., Parmar D. Polymorphism in environment responsive genes and association with Parkinson disease. Mol. Cell. Biochem. 2008;312:131–138. doi: 10.1007/s11010-008-9728-2. [DOI] [PubMed] [Google Scholar]
- 209.Petrillo S., Schirinzi T., Di Lazzaro G., D’Amico J., Colona V.L., Bertini E., Pierantozzi M., Mari L., Mercuri N.B., Piemonte F., et al. Systemic Activation of Nrf2 Pathway in Parkinson’s Disease. Mov. Disord. 2020;35:180–184. doi: 10.1002/mds.27878. [DOI] [PubMed] [Google Scholar]
- 210.Tufekci K.U., Bayin E.C., Genc S., Genc K. The Nrf2/ARE Pathway: A Promising Target to Counteract Mitochondrial Dysfunction in Parkinson’s Disease. Parkinson’s Dis. 2011;2011:314082. doi: 10.4061/2011/314082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Ali A., Hoeflich K.P., Woodgett J.R. Glycogen Synthase Kinase-3: Properties, Functions, and Regulation. Chem. Rev. 2001;101:2527–2540. doi: 10.1021/cr000110o. [DOI] [PubMed] [Google Scholar]
- 212.Beurel E., Grieco S.F., Jope R.S. Glycogen synthase kinase-3 (GSK3): Regulation, actions, and diseases. Pharmacol. Ther. 2015;148:114–131. doi: 10.1016/j.pharmthera.2014.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Yang K., Chen Z., Gao J., Shi W., Li L., Jiang S., Hu H., Liu Z., Xu D., Wu L. The Key Roles of GSK-3β in Regulating Mitochondrial Activity. Cell. Physiol. Biochem. 2017;44:1445–1459. doi: 10.1159/000485580. [DOI] [PubMed] [Google Scholar]
- 214.Kwok J.B.J., Hallupp M., Loy C.T., Chan D.K.Y., Woo J., Mellick G.D., Buchanan D.D., Silburn P.A., Halliday G.M., Schofield P.R. GSK3B polymorphisms alter transcription and splicing in Parkinson’s disease. Ann. Neurol. 2005;58:829–839. doi: 10.1002/ana.20691. [DOI] [PubMed] [Google Scholar]
- 215.Skibinski G., Hwang V., Ando D.M., Daub A., Lee A.K., Ravisankar A., Modan S., Finucane M.M., Shaby B.A., Finkbeiner S. Nrf2 mitigates LRRK2- and α-synuclein–induced neurodegeneration by modulating proteostasis. Proc. Natl. Acad. Sci. USA. 2017;114:1165–1170. doi: 10.1073/pnas.1522872114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Jakel R.J., Townsend J.A., Kraft A.D., Johnson J.A. Nrf2-mediated protection against 6-hydroxydopamine. Brain Res. 2007;1144:192–201. doi: 10.1016/j.brainres.2007.01.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Williamson T.P., Johnson D.A., Johnson J.A. Activation of the Nrf2-ARE pathway by siRNA knockdown of Keap1 reduces oxidative stress and provides partial protection from MPTP-mediated neurotoxicity. NeuroToxicology. 2012;33:272–279. doi: 10.1016/j.neuro.2012.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Chen P.-C., Vargas M.R., Pani A.K., Smeyne R.J., Johnson D.A., Kan Y.W., Johnson J.A. Nrf2-mediated neuroprotection in the MPTP mouse model of Parkinson’s disease: Critical role for the astrocyte. Proc. Natl. Acad. Sci. USA. 2009;106:2933–2938. doi: 10.1073/pnas.0813361106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Seidl S.E., Potashkin J.A. The Promise of Neuroprotective Agents in Parkinson’s Disease. Front. Neurol. 2011;2:68. doi: 10.3389/fneur.2011.00068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Coles L.D., Tuite P.J., Öz G., Mishra U.R., Kartha R.V., Sullivan K.M., Cloyd J.C., Terpstra M. Repeated-Dose Oral N-Acetylcysteine in Parkinson’s Disease: Pharmacokinetics and Effect on Brain Glutathione and Oxidative Stress. J. Clin. Pharmacol. 2018;58:158–167. doi: 10.1002/jcph.1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Jazwa A., Rojo A.I., Innamorato N.G., Hesse M., Fernández-Ruiz J., Cuadrado A. Pharmacological Targeting of the Transcription Factor Nrf2 at the Basal Ganglia Provides Disease Modifying Therapy for Experimental Parkinsonism. Antioxid. Redox Signal. 2011;14:2347–2360. doi: 10.1089/ars.2010.3731. [DOI] [PubMed] [Google Scholar]
- 222.Guerrero-Beltrán C.E., Mukhopadhyay P., Horváth B., Rajesh M., Tapia E., García-Torres I., Pedraza-Chaverri J., Pacher P. Sulforaphane, a natural constituent of broccoli, prevents cell death and inflammation in nephropathy. J. Nutr. Biochem. 2012;23:494–500. doi: 10.1016/j.jnutbio.2011.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Uddin M.S., Al Mamun A., Jakaria M., Thangapandiyan S., Ahmad J., Rahman M.A., Mathew B., Abdel-Daim M.M., Aleya L. Emerging promise of sulforaphane-mediated Nrf2 signaling cascade against neurological disorders. Sci. Total Environ. 2020;707:135624. doi: 10.1016/j.scitotenv.2019.135624. [DOI] [PubMed] [Google Scholar]
- 224.Angeloni C., Malaguti M., Rizzo B., Barbalace M.C., Fabbri D., Hrelia S. Neuroprotective Effect of Sulforaphane against Methylglyoxal Cytotoxicity. Chem. Res. Toxicol. 2015;28:1234–1245. doi: 10.1021/acs.chemrestox.5b00067. [DOI] [PubMed] [Google Scholar]
- 225.Zhang R., Zhang J., Fang L., Li X., Zhao Y., Shi W., An L. Neuroprotective Effects of Sulforaphane on Cholinergic Neurons in Mice with Alzheimer’s Disease-Like Lesions. Int. J. Mol. Sci. 2014;15:14396–14410. doi: 10.3390/ijms150814396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Sun Y., Yang T., Leak R.K., Chen J., Zhang F. Preventive and Protective Roles of Dietary Nrf2 Activators Against Central Nervous System Diseases. CNS Neurol. Disord. Drug Targets. 2017;16:326–338. doi: 10.2174/1871527316666170102120211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Hong F., Freeman A.M.L., Liebler D.C. Identification of Sensor Cysteines in Human Keap1 Modified by the Cancer Chemopreventive Agent Sulforaphane. Chem. Res. Toxicol. 2005;18:1917–1926. doi: 10.1021/tx0502138. [DOI] [PubMed] [Google Scholar]
- 228.Shang G., Tang X., Gao P., Guo F., Liu H., Zhao Z., Chen Q., Jiang T., Zhang N., Li H. Sulforaphane attenuation of experimental diabetic nephropathy involves GSK-3 beta/Fyn/Nrf2 signaling pathway. J. Nutr. Biochem. 2015;26:596–606. doi: 10.1016/j.jnutbio.2014.12.008. [DOI] [PubMed] [Google Scholar]
- 229.Han J.M., Lee Y.J., Lee S.Y., Kim E.M., Moon Y., Kim H.W., Hwang O. Protective Effect of Sulforaphane against Dopaminergic Cell Death. J. Pharmacol. Exp. Ther. 2007;321:249–256. doi: 10.1124/jpet.106.110866. [DOI] [PubMed] [Google Scholar]
- 230.Zhang M., An C., Gao Y., Leak R.K., Chen J., Zhang F. Emerging roles of Nrf2 and phase II antioxidant enzymes in neuroprotection. Prog. Neurobiol. 2013;100:30–47. doi: 10.1016/j.pneurobio.2012.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Siebert A., Desai V., Chandrasekaran K., Fiskum G., Jafri M.S. Nrf2 activators provide neuroprotection against 6-hydroxydopamine toxicity in rat organotypic nigrostriatal cocultures. J. Neurosci. Res. 2009;87:1659–1669. doi: 10.1002/jnr.21975. [DOI] [PubMed] [Google Scholar]
- 232.Tarozzi A., Angeloni C., Malaguti M., Morroni F., Hrelia S., Hrelia P. Sulforaphane as a Potential Protective Phytochemical against Neurodegenerative Diseases. Oxid. Med. Cell. Longev. 2013;2013:415078. doi: 10.1155/2013/415078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Serrano-Pozo A., Frosch M.P., Masliah E., Hyman B.T. Neuropathological Alterations in Alzheimer Disease. Cold Spring Harb. Perspect. Med. 2011;1:a006189. doi: 10.1101/cshperspect.a006189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Smith M.A. Alzheimer Disease. Int. Rev. Neurobiol. 1998;42:1–54. doi: 10.1016/s0074-7742(08)60607-8. [DOI] [PubMed] [Google Scholar]
- 235.Swerdlow R.H. Mitochondria and Mitochondrial Cascades in Alzheimer’s Disease. J. Alzheimer’s Dis. 2018;62:1403–1416. doi: 10.3233/JAD-170585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Wang X., Wang W., Li L., Perry G., Lee H.-G., Zhu X. Oxidative stress and mitochondrial dysfunction in Alzheimer’s disease. Biochim. Biophys. Acta BBA Mol. Basis Dis. 2014;1842:1240–1247. doi: 10.1016/j.bbadis.2013.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Leroy K., Yilmaz Z., Brion J.-P. Increased level of active GSK-3β in Alzheimer’s disease and accumulation in argyrophilic grains and in neurones at different stages of neurofibrillary degeneration. Neuropathol. Appl. Neurobiol. 2007;33:43–55. doi: 10.1111/j.1365-2990.2006.00795.x. [DOI] [PubMed] [Google Scholar]
- 238.Hernández F., de Barreda E.G., Fuster-Matanzo A., Lucas J.J., Avila J. GSK3: A possible link between beta amyloid peptide and tau protein. Exp. Neurol. 2010;223:322–325. doi: 10.1016/j.expneurol.2009.09.011. [DOI] [PubMed] [Google Scholar]
- 239.Takashima A. GSK-3 is essential in the pathogenesis of Alzheimer’s disease. J. Alzheimer’s Dis. 2006;9:309–317. doi: 10.3233/JAD-2006-9S335. [DOI] [PubMed] [Google Scholar]
- 240.Lucas J.J., Hernández F., Gómez-Ramos P., Morán M.A., Hen R., Avila J. Decreased nuclear beta-catenin, tau hyperphosphorylation and neurodegeneration in GSK-3beta conditional transgenic mice. EMBO J. 2001;20:27–39. doi: 10.1093/emboj/20.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Markesbery W.R. Oxidative Stress Hypothesis in Alzheimer’s Disease. Free Radic. Biol. Med. 1997;23:134–147. doi: 10.1016/S0891-5849(96)00629-6. [DOI] [PubMed] [Google Scholar]
- 242.Christen Y. Oxidative stress and Alzheimer disease. Am. J. Clin. Nutr. 2000;71:621S–629S. doi: 10.1093/ajcn/71.2.621s. [DOI] [PubMed] [Google Scholar]
- 243.Butterfield D.A., Boyd-Kimball D. Oxidative Stress, Amyloid-β Peptide, and Altered Key Molecular Pathways in the Pathogenesis and Progression of Alzheimer’s Disease. J. Alzheimer’s Dis. 2018;62:1345–1367. doi: 10.3233/JAD-170543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Chen Z., Zhong C. Oxidative stress in Alzheimer’s disease. Neurosci. Bull. 2014;30:271–281. doi: 10.1007/s12264-013-1423-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Xie H., Hou S., Jiang J., Sekutowicz M., Kelly J., Bacskai B.J. Rapid cell death is preceded by amyloid plaque-mediated oxidative stress. Proc. Natl. Acad. Sci. USA. 2013;110:7904–7909. doi: 10.1073/pnas.1217938110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Siegel S.J., Bieschke J., Powers E.T., Kelly J.W. The Oxidative Stress Metabolite 4-Hydroxynonenal Promotes Alzheimer Protofibril Formation. Biochemistry. 2007;46:1503–1510. doi: 10.1021/bi061853s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Butterfield D.A., Hensley K., Cole P., Subramaniam R., Aksenov M., Aksenova M., Bummer P.M., Haley B.E., Carney J.M. Oxidatively Induced Structural Alteration of Glutamine Synthetase Assessed by Analysis of Spin Label Incorporation Kinetics: Relevance to Alzheimer’s Disease. J. Neurochem. 2002;68:2451–2457. doi: 10.1046/j.1471-4159.1997.68062451.x. [DOI] [PubMed] [Google Scholar]
- 248.Aksenov M.Y., Aksenova M.V., Butterfield D.A., Geddes J.W., Markesbery W.R. Protein oxidation in the brain in Alzheimer’s disease. Neuroscience. 2001;103:373–383. doi: 10.1016/S0306-4522(00)00580-7. [DOI] [PubMed] [Google Scholar]
- 249.Tönnies E., Trushina E. Oxidative Stress, Synaptic Dysfunction, and Alzheimer’s Disease. J. Alzheimer’s Dis. 2017;57:1105–1121. doi: 10.3233/JAD-161088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Trushina E., Nemutlu E., Dzeja P.P., Poduslo J.F., Zhang S., Christensen T., Camp J., Mesa J., Siddiqui A., Tamura Y., et al. Defects in Mitochondrial Dynamics and Metabolomic Signatures of Evolving Energetic Stress in Mouse Models of Familial Alzheimer’s Disease. PLoS ONE. 2012;7:e32737. doi: 10.1371/journal.pone.0032737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Valla J., Yaari R., Wolf A.B., Kusne Y., Beach T.G., Roher A.E., Corneveaux J.J., Huentelman M.J., Caselli R.J., Reiman E.M. Reduced Posterior Cingulate Mitochondrial Activity in Expired Young Adult Carriers of the APOE ε4 Allele, the Major Late-Onset Alzheimer’s Susceptibility Gene. J. Alzheimer’s Dis. 2010;22:307–313. doi: 10.3233/JAD-2010-100129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Trushina E., Dutta T., Persson X.-M.T., Mielke M.M., Petersen R.C. Identification of Altered Metabolic Pathways in Plasma and CSF in Mild Cognitive Impairment and Alzheimer’s Disease Using Metabolomics. PLoS ONE. 2013;8:e63644. doi: 10.1371/journal.pone.0063644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Price J.L., McKeel D.W., Jr., Buckles V.D., Roe C.M., Xiong C., Grundman M., Hansen L.A., Petersen R.C., Parisi J.E., Dickson D.W., et al. Neuropathology of nondemented aging: Presumptive evidence for preclinical Alzheimer disease. Neurobiol. Aging. 2009;30:1026–1036. doi: 10.1016/j.neurobiolaging.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Beal M.F. Mitochondria take center stage in aging and neurodegeneration. Ann. Neurol. 2005;58:495–505. doi: 10.1002/ana.20624. [DOI] [PubMed] [Google Scholar]
- 255.Wang X., Su B., Zheng L., Perry G., Smith M.A., Zhu X. The role of abnormal mitochondrial dynamics in the pathogenesis of Alzheimer’s disease. J. Neurochem. 2009;109:153–159. doi: 10.1111/j.1471-4159.2009.05867.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Detmer S.A., Chan D.C. Functions and dysfunctions of mitochondrial dynamics. Nat. Rev. Mol. Cell Biol. 2007;8:870–879. doi: 10.1038/nrm2275. [DOI] [PubMed] [Google Scholar]
- 257.Chan D.C. Mitochondria: Dynamic Organelles in Disease, Aging, and Development. Cell. 2006;125:1241–1252. doi: 10.1016/j.cell.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 258.Knott A.B., Perkins G., Schwarzenbacher R., Bossy-Wetzel E. Mitochondrial fragmentation in neurodegeneration. Nat. Rev. Neurosci. 2008;9:505–518. doi: 10.1038/nrn2417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Chen H., Chomyn A., Chan D.C. Disruption of Fusion Results in Mitochondrial Heterogeneity and Dysfunction. J. Biol. Chem. 2005;280:26185–26192. doi: 10.1074/jbc.M503062200. [DOI] [PubMed] [Google Scholar]
- 260.Wang S., Song J., Tan M., Albers K.M., Jia J. Mitochondrial fission proteins in peripheral blood lymphocytes are potential biomarkers for Alzheimer’s disease. Eur. J. Neurol. 2012;19:1015–1022. doi: 10.1111/j.1468-1331.2012.03670.x. [DOI] [PubMed] [Google Scholar]
- 261.Cho D.-H., Nakamura T., Fang J., Cieplak P., Godzik A., Gu Z., Lipton S.A. S-Nitrosylation of Drp1 Mediates β-Amyloid-Related Mitochondrial Fission and Neuronal Injury. Science. 2009;324:102–105. doi: 10.1126/science.1171091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Zhu X., Perry G., Smith M.A., Wang X. Abnormal Mitochondrial Dynamics in the Pathogenesis of Alzheimer’s Disease. J. Alzheimer’s Dis. 2013;33:S253–S262. doi: 10.3233/JAD-2012-129005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Wang X., Su B., Siedlak S.L., Moreira P.I., Fujioka H., Wang Y., Casadesus G., Zhu X. Amyloid-β overproduction causes abnormal mitochondrial dynamics via differential modulation of mitochondrial fission/fusion proteins. Proc. Natl. Acad. Sci. USA. 2008;105:19318–19323. doi: 10.1073/pnas.0804871105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Park J., Choi H., Min J.-S., Kim B., Lee S.-R., Yun J.W., Choi M.-S., Chang K.-T., Lee D.-S. Loss of mitofusin 2 links beta-amyloid-mediated mitochondrial fragmentation and Cdk5-induced oxidative stress in neuron cells. J. Neurochem. 2015;132:687–702. doi: 10.1111/jnc.12984. [DOI] [PubMed] [Google Scholar]
- 265.Castellani R., Hirai K., Aliev G., Drew K.L., Nunomura A., Takeda A., Cash A.D., Obrenovich M.E., Perry G., Smith M.A. Role of mitochondrial dysfunction in Alzheimer’s disease. J. Neurosci. Res. 2002;70:357–360. doi: 10.1002/jnr.10389. [DOI] [PubMed] [Google Scholar]
- 266.Uttara B., Singh A.V., Zamboni P., Mahajan R.T. Oxidative Stress and Neurodegenerative Diseases: A Review of Upstream and Downstream Antioxidant Therapeutic Options. Curr. Neuropharmacol. 2009;7:65–74. doi: 10.2174/157015909787602823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Manczak M., Reddy P.H. Abnormal interaction of VDAC1 with amyloid beta and phosphorylated tau causes mitochondrial dysfunction in Alzheimer’s disease. Hum. Mol. Genet. 2012;21:5131–5146. doi: 10.1093/hmg/dds360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Devi L., Prabhu B.M., Galati D.F., Avadhani N.G., Anandatheerthavarada H.K. Accumulation of Amyloid Precursor Protein in the Mitochondrial Import Channels of Human Alzheimer’s Disease Brain Is Associated with Mitochondrial Dysfunction. J. Neurosci. 2006;26:9057–9068. doi: 10.1523/JNEUROSCI.1469-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Casley C.S., Canevari L., Land J.M., Clark J.B., Sharpe M.A. β-Amyloid inhibits integrated mitochondrial respiration and key enzyme activities. J. Neurochem. 2002;80:91–100. doi: 10.1046/j.0022-3042.2001.00681.x. [DOI] [PubMed] [Google Scholar]
- 270.Lustbader J.W., Cirilli M., Lin C., Xu H.W., Takuma K., Wang N., Caspersen C., Chen X., Pollak S., Chaney M., et al. ABAD Directly Links Aβ to Mitochondrial Toxicity in Alzheimer’s Disease. Science. 2004;304:448–452. doi: 10.1126/science.1091230. [DOI] [PubMed] [Google Scholar]
- 271.Du H., Guo L., Yan S., Sosunov A.A., McKhann G.M., Yan S.S. Early deficits in synaptic mitochondria in an Alzheimer’s disease mouse model. Proc. Natl. Acad. Sci. USA. 2010;107:18670–18675. doi: 10.1073/pnas.1006586107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Cardoso S.M., Santana I., Swerdlow R.H., Oliveira C.R. Mitochondria dysfunction of Alzheimer’s disease cybrids enhances Aβ toxicity. J. Neurochem. 2004;89:1417–1426. doi: 10.1111/j.1471-4159.2004.02438.x. [DOI] [PubMed] [Google Scholar]
- 273.Gordon B.A., Blazey T.M., Su Y., Hari-Raj A., Dincer A., Flores S., Christensen J., McDade E., Wang G., Xiong C., et al. Spatial patterns of neuroimaging biomarker change in individuals from families with autosomal dominant Alzheimer’s disease: A longitudinal study. Lancet Neurol. 2018;17:241–250. doi: 10.1016/S1474-4422(18)30028-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Kapogiannis D., Mattson M.P. Disrupted energy metabolism and neuronal circuit dysfunction in cognitive impairment and Alzheimer’s disease. Lancet Neurol. 2011;10:187–198. doi: 10.1016/S1474-4422(10)70277-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Sonntag K.-C., Ryu W.-I., Amirault K.M., Healy R.A., Siegel A.J., McPhie D.L., Forester B., Cohen B.M. Late-onset Alzheimer’s disease is associated with inherent changes in bioenergetics profiles. Sci. Rep. 2017;7:14038. doi: 10.1038/s41598-017-14420-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Carvalho C., Cardoso S., Correia S.C., Santos R.X., Santos M.S., Baldeiras I., Oliveira C.R., Moreira P.I. Metabolic Alterations Induced by Sucrose Intake and Alzheimer’s Disease Promote Similar Brain Mitochondrial Abnormalities. Diabetes. 2012;61:1234–1242. doi: 10.2337/db11-1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Sheng Z.-H., Cai Q. Mitochondrial transport in neurons: Impact on synaptic homeostasis and neurodegeneration. Nat. Rev. Neurosci. 2012;13:77–93. doi: 10.1038/nrn3156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Stokin G.B., Lillo C., Falzone T.L., Brusch R.G., Rockenstein E., Mount S.L., Raman R., Davies P., Masliah E., Williams D.S., et al. Axonopathy and Transport Deficits Early in the Pathogenesis of Alzheimer’s Disease. Science. 2005;307:1282–1288. doi: 10.1126/science.1105681. [DOI] [PubMed] [Google Scholar]
- 279.Calkins M.J., Manczak M., Mao P., Shirendeb U., Reddy P.H. Impaired mitochondrial biogenesis, defective axonal transport of mitochondria, abnormal mitochondrial dynamics and synaptic degeneration in a mouse model of Alzheimer’s disease. Hum. Mol. Genet. 2011;20:4515–4529. doi: 10.1093/hmg/ddr381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Ma H., Cai Q., Lu W., Sheng Z.-H., Mochida S. KIF5B Motor Adaptor Syntabulin Maintains Synaptic Transmission in Sympathetic Neurons. J. Neurosci. 2009;29:13019–13029. doi: 10.1523/JNEUROSCI.2517-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Cai Q., Davis M.L., Sheng Z.-H. Regulation of axonal mitochondrial transport and its impact on synaptic transmission. Neurosci. Res. 2011;70:9–15. doi: 10.1016/j.neures.2011.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Tammineni P., Ye X., Feng T., Aikal D., Cai Q. Impaired retrograde transport of axonal autophagosomes contributes to autophagic stress in Alzheimer’s disease neurons. eLife. 2017;6:e21776. doi: 10.7554/eLife.21776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Reddy P.H., McWeeney S., Park B.S., Manczak M., Gutala R.V., Partovi D., Jung Y., Yau V., Searles R., Mori M., et al. Gene expression profiles of transcripts in amyloid precursor protein transgenic mice: Up-regulation of mitochondrial metabolism and apoptotic genes is an early cellular change in Alzheimer’s disease. Hum. Mol. Genet. 2004;13:1225–1240. doi: 10.1093/hmg/ddh140. [DOI] [PubMed] [Google Scholar]
- 284.Martin L., Latypova X., Terro F. Post-translational modifications of tau protein: Implications for Alzheimer’s disease. Neurochem. Int. 2011;58:458–471. doi: 10.1016/j.neuint.2010.12.023. [DOI] [PubMed] [Google Scholar]
- 285.Wang Y., Mandelkow E. Tau in physiology and pathology. Nat. Rev. Neurosci. 2016;17:22–35. doi: 10.1038/nrn.2015.1. [DOI] [PubMed] [Google Scholar]
- 286.Alonso A.D.C., Grundke-Iqbal I., Barra H.S., Iqbal K. Abnormal phosphorylation of tau and the mechanism of Alzheimer neurofibrillary degeneration: Sequestration of microtubule-associated proteins 1 and 2 and the disassembly of microtubules by the abnormal tau. Proc. Natl. Acad. Sci. USA. 1997;94:298–303. doi: 10.1073/pnas.94.1.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Gendron T.F., Petrucelli L. The role of tau in neurodegeneration. Mol. Neurodegener. 2009;4:13. doi: 10.1186/1750-1326-4-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Tapia-Rojas C., Cabezas-Opazo F., Deaton C.A., Vergara E.H., Johnson G.V.W., Quintanilla R.A. It’s all about tau. Prog. Neurobiol. 2019;175:54–76. doi: 10.1016/j.pneurobio.2018.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Cente M., Filipcik P., Pevalova M., Novak M. Expression of a truncated tau protein induces oxidative stress in a rodent model of tauopathy. Eur. J. Neurosci. 2006;24:1085–1090. doi: 10.1111/j.1460-9568.2006.04986.x. [DOI] [PubMed] [Google Scholar]
- 290.Schulz K.L., Eckert A., Rhein V., Mai S., Haase W., Reichert A.S., Jendrach M., Müller W.E., Leuner K. A New Link to Mitochondrial Impairment in Tauopathies. Mol. Neurobiol. 2012;46:205–216. doi: 10.1007/s12035-012-8308-3. [DOI] [PubMed] [Google Scholar]
- 291.Amadoro G., Corsetti V., Atlante A., Florenzano F., Capsoni S., Bussani R., Mercanti D., Calissano P. Interaction between NH2-tau fragment and Aβ in Alzheimer’s disease mitochondria contributes to the synaptic deterioration. Neurobiol. Aging. 2012;33:833.e1–833.e25. doi: 10.1016/j.neurobiolaging.2011.08.001. [DOI] [PubMed] [Google Scholar]
- 292.Amadoro G., Corsetti V., Calissano P., Florenzano F., Atlante A., Ciotti M.T., Mongiardi M.P., Bussani R., Nicolin V., Nori S.L., et al. AD-linked, toxic NH2 human tau affects the quality control of mitochondria in neurons. Neurobiol. Dis. 2014;62:489–507. doi: 10.1016/j.nbd.2013.10.018. [DOI] [PubMed] [Google Scholar]
- 293.Fasulo L., Ugolini G., Cattaneo A. Apoptotic effect of caspase-3 cleaved tau in hippocampal neurons and its potentiation by tau FTDP-mutation N279K. J. Alzheimer’s Dis. 2005;7:3–13. doi: 10.3233/JAD-2005-7102. [DOI] [PubMed] [Google Scholar]
- 294.Lopes S., Teplytska L., Vaz-Silva J., Dioli C., Trindade R., Morais M., Webhofer C., Maccarrone G., Almeida O.F.X., Turck C.W., et al. Tau Deletion Prevents Stress-Induced Dendritic Atrophy in Prefrontal Cortex: Role of Synaptic Mitochondria. Cereb. Cortex. 2017;27:2580–2591. doi: 10.1093/cercor/bhw057. [DOI] [PubMed] [Google Scholar]
- 295.Jara C., Aránguiz A., Cerpa W., Tapia-Rojas C., Quintanilla R.A. Genetic ablation of tau improves mitochondrial function and cognitive abilities in the hippocampus. Redox Biol. 2018;18:279–294. doi: 10.1016/j.redox.2018.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Gamblin T.C., Chen F., Zambrano A., Abraha A., Lagalwar S., Guillozet A.L., Lu M., Fu Y., Garcia-Sierra F., Lapointe N., et al. Caspase cleavage of tau: Linking amyloid and neurofibrillary tangles in Alzheimer’s disease. Proc. Natl. Acad. Sci. USA. 2003;100:10032–10037. doi: 10.1073/pnas.1630428100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Rissman R.A., Poon W.W., Blurton-Jones M., Oddo S., Torp R., Vitek M.P., LaFerla F.M., Rohn T.T., Cotman C.W. Caspase-cleavage of tau is an early event in Alzheimer disease tangle pathology. J. Clin. Investig. 2004;114:121–130. doi: 10.1172/JCI200420640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.García-Sierra F., Mondragón-Rodríguez S., Basurto-Islas G. Truncation of Tau Protein and its Pathological Significance in Alzheimer’s Disease. J. Alzheimer’s Dis. 2008;14:401–409. doi: 10.3233/JAD-2008-14407. [DOI] [PubMed] [Google Scholar]
- 299.Chung C.-W., Song Y.-H., Kim I.-K., Yoon W.-J., Ryu B.-R., Jo D.-G., Woo H.-N., Kwon Y.-K., Kim H.-H., Gwag B.-J., et al. Proapoptotic Effects of Tau Cleavage Product Generated by Caspase-3. Neurobiol. Dis. 2001;8:162–172. doi: 10.1006/nbdi.2000.0335. [DOI] [PubMed] [Google Scholar]
- 300.Quintanilla R.A., Matthews-Roberson T.A., Dolan P.J., Johnson G.V.W. Caspase-cleaved Tau Expression Induces Mitochondrial Dysfunction in Immortalized Cortical Neurons: Implications for the Pathogenesis of Alzheimer Disease. J. Biol. Chem. 2009;284:18754–18766. doi: 10.1074/jbc.M808908200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Pérez M.J., Vergara-Pulgar K., Jara C., Cabezas-Opazo F., Quintanilla R.A. Caspase-Cleaved Tau Impairs Mitochondrial Dynamics in Alzheimer’s Disease. Mol. Neurobiol. 2018;55:1004–1018. doi: 10.1007/s12035-017-0385-x. [DOI] [PubMed] [Google Scholar]
- 302.Quintanilla R.A., Dolan P.J., Jin Y.N., Johnson G.V.W. Truncated tau and Aβ cooperatively impair mitochondria in primary neurons. Neurobiol. Aging. 2012;33:619.e25–619.e35. doi: 10.1016/j.neurobiolaging.2011.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Quintanilla R.A., von Bernhardi R., Godoy J.A., Inestrosa N.C., Johnson G.V.W. Phosphorylated tau potentiates Aβ-induced mitochondrial damage in mature neurons. Neurobiol. Dis. 2014;71:260–269. doi: 10.1016/j.nbd.2014.08.016. [DOI] [PubMed] [Google Scholar]
- 304.Quintanilla R.A., Tapia-Monsalves C., Vergara E.H., Pérez M.J., Aranguiz A. Truncated Tau Induces Mitochondrial Transport Failure Through the Impairment of TRAK2 Protein and Bioenergetics Decline in Neuronal Cells. Front. Cell. Neurosci. 2020;14:175. doi: 10.3389/fncel.2020.00175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Brickley K., Stephenson F.A. Trafficking Kinesin Protein (TRAK)-mediated Transport of Mitochondria in Axons of Hippocampal Neurons. J. Biol. Chem. 2011;286:18079–18092. doi: 10.1074/jbc.M111.236018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Bahn G., Jo D.-G. Therapeutic Approaches to Alzheimer’s Disease Through Modulation of NRF2. NeuroMol. Med. 2019;21:1–11. doi: 10.1007/s12017-018-08523-5. [DOI] [PubMed] [Google Scholar]
- 307.Jo C., Gundemir S., Pritchard S., Jin Y.N., Rahman I., Johnson G.V.W. Nrf2 reduces levels of phosphorylated tau protein by inducing autophagy adaptor protein NDP52. Nat. Commun. 2014;5:3496. doi: 10.1038/ncomms4496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Fujita K.-I., Maeda D., Xiao Q., Srinivasula S.M. Nrf2-mediated induction of p62 controls Toll-like receptor-4-driven aggresome-like induced structure formation and autophagic degradation. Proc. Natl. Acad. Sci. USA. 2011;108:1427–1432. doi: 10.1073/pnas.1014156108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Tanji K., Maruyama A., Odagiri S., Mori F., Itoh K., Kakita A., Takahashi H., Wakabayashi K. Keap1 Is Localized in Neuronal and Glial Cytoplasmic Inclusions in Various Neurodegenerative Diseases. J. Neuropathol. Exp. Neurol. 2013;72:18–28. doi: 10.1097/NEN.0b013e31827b5713. [DOI] [PubMed] [Google Scholar]
- 310.Tanji K., Miki Y., Kakita A., Takahashi H., Wakabayashi K., Ozaki T., Maruyama A., Yoshida H., Mimura J., Matsumiya T., et al. Phosphorylation of serine 349 of p62 in Alzheimer’s disease brain. Acta Neuropathol. Commun. 2014;2:50. doi: 10.1186/2051-5960-2-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Zheng X., Wang W., Liu R., Huang H., Zhang R., Sun L. Effect of p62 on tau hyperphosphorylation in a rat model of Alzheimer’s disease. Neural Regen. Res. 2012;7:1304–1311. doi: 10.3969/j.issn.1673-5374.2012.17.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Rojo A.I., Pajares M., García-Yagüe A.J., Buendia I., Van Leuven F., Yamamoto M., López M.G., Cuadrado A. Deficiency in the transcription factor NRF2 worsens inflammatory parameters in a mouse model with combined tauopathy and amyloidopathy. Redox Biol. 2018;18:173–180. doi: 10.1016/j.redox.2018.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Eftekharzadeh B., Maghsoudi N., Khodagholi F. Stabilization of transcription factor Nrf2 by tBHQ prevents oxidative stress-induced amyloid β formation in NT2N neurons. Biochimie. 2010;92:245–253. doi: 10.1016/j.biochi.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 314.Ramsey C.P., Glass C.A., Montgomery M.B., Lindl K.A., Ritson G.P., Chia L.A., Hamilton R.L., Chu C.T., Jordan-Sciutto K.L. Expression of Nrf2 in Neurodegenerative Diseases. J. Neuropathol. Exp. Neurol. 2007;66:75–85. doi: 10.1097/nen.0b013e31802d6da9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Chalovich E.M., Zhu J.-H., Caltagarone J., Bowser R., Chu C.T. Functional Repression of cAMP Response Element in 6-Hydroxydopamine-treated Neuronal Cells. J. Biol. Chem. 2006;281:17870–17881. doi: 10.1074/jbc.M602632200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Kerr F., Sofola-Adesakin O., Ivanov D.K., Gatliff J., Gomez Perez-Nievas B., Bertrand H.C., Martinez P., Callard R., Snoeren I., Cochemé H.M., et al. Direct Keap1-Nrf2 disruption as a potential therapeutic target for Alzheimer’s disease. PLoS Genet. 2017;13:e1006593. doi: 10.1371/journal.pgen.1006593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Gabbita S.P., Lovell M.A., Markesbery W.R. Increased Nuclear DNA Oxidation in the Brain in Alzheimer’s Disease. J. Neurochem. 1998;71:2034–2040. doi: 10.1046/j.1471-4159.1998.71052034.x. [DOI] [PubMed] [Google Scholar]
- 318.Hooper C., Markevich V., Plattner F., Killick R., Schofield E., Engel T., Hernández F., Anderton B., Rosenblum K., Bliss T., et al. Glycogen synthase kinase-3 inhibition is integral to long-term potentiation. Eur. J. Neurosci. 2007;25:81–86. doi: 10.1111/j.1460-9568.2006.05245.x. [DOI] [PubMed] [Google Scholar]
- 319.Praticò D., Clark C.M., Liun F., Rokach J., Lee V.Y.-M., Trojanowski J.Q. Increase of Brain Oxidative Stress in Mild Cognitive Impairment: A Possible Predictor of Alzheimer Disease. Arch. Neurol. 2002;59:972–976. doi: 10.1001/archneur.59.6.972. [DOI] [PubMed] [Google Scholar]
- 320.Engelhart M.J., Geerlings M.I., Ruitenberg A., Van Swieten J.C., Hofman A., Witteman J.C.M., Breteler M.M.B. Dietary Intake of Antioxidants and Risk of Alzheimer Disease. JAMA. 2002;287:3223–3229. doi: 10.1001/jama.287.24.3223. [DOI] [PubMed] [Google Scholar]
- 321.Abdel-Kader R., Hauptmann S., Keil U., Scherping I., Leuner K., Eckert A., Müller W.E. Stabilization of mitochondrial function by Ginkgo biloba extract (EGb 761) Pharmacol. Res. 2007;56:493–502. doi: 10.1016/j.phrs.2007.09.011. [DOI] [PubMed] [Google Scholar]
- 322.Upadhyay S., Dixit M. Role of Polyphenols and Other Phytochemicals on Molecular Signaling. Oxid. Med. Cell. Longev. 2015;2015:504253. doi: 10.1155/2015/504253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Kosuge Y. Neuroprotective mechanisms of S-allyl-L-cysteine in neurological disease (Review) Exp. Ther. Med. 2020;19:1565–1569. doi: 10.3892/etm.2019.8391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Kanninen K., Heikkinen R., Malm T., Rolova T., Kuhmonen S., Leinonen H., Ylä-Herttuala S., Tanila H., Levonen A.-L., Koistinaho M., et al. Intrahippocampal injection of a lentiviral vector expressing Nrf2 improves spatial learning in a mouse model of Alzheimer’s disease. Proc. Natl. Acad. Sci. USA. 2009;106:16505–16510. doi: 10.1073/pnas.0908397106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Park H.-M., Kim J.-A., Kwak M.-K. Protection against amyloid beta cytotoxicity by sulforaphane: Role of the proteasome. Arch. Pharm. Res. 2009;32:109–115. doi: 10.1007/s12272-009-1124-2. [DOI] [PubMed] [Google Scholar]
- 326.Zhang J., Zhang R., Zhan Z., Li X., Zhou F., Xing A., Jiang C., Chen Y., An L. Beneficial Effects of Sulforaphane Treatment in Alzheimer’s Disease May Be Mediated through Reduced HDAC1/3 and Increased P75NTR Expression. Front. Aging Neurosci. 2017;9:121. doi: 10.3389/fnagi.2017.00121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Zheng K., Dai X., Xiao N., Wu X., Wei Z., Fang W., Zhu Y., Zhang J., Chen X. Curcumin Ameliorates Memory Decline via Inhibiting BACE1 Expression and β-Amyloid Pathology in 5×FAD Transgenic Mice. Mol. Neurobiol. 2017;54:1967–1977. doi: 10.1007/s12035-016-9802-9. [DOI] [PubMed] [Google Scholar]
- 328.Gibellini L., Bianchini E., De Biasi S., Nasi M., Cossarizza A., Pinti M. Natural Compounds Modulating Mitochondrial Functions. Evid. Based Complement. Altern. Med. 2015;2015:527209. doi: 10.1155/2015/527209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Cole G.M., Yang F., Lim G.P., Cummings J.L., Masterman D.L., Frautschy S.A. A Rationale for Curcuminoids for the Prevention or Treatment of Alzheimers Disease. Curr. Med. Chem. Immunol. Endocr. Metab. Agents. 2003;3:15–25. doi: 10.2174/1568013033358761. [DOI] [Google Scholar]
- 330.Alisi I.O., Uzairu A., Abechi S.E., Idris S.O. Evaluation of the antioxidant properties of curcumin derivatives by genetic function algorithm. J. Adv. Res. 2018;12:47–54. doi: 10.1016/j.jare.2018.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Hasan W., Kori R.K., Thakre K., Yadav R.S., Jat D. Synthesis, characterization and efficacy of mitochondrial targeted delivery of TPP-curcumin in rotenone-induced toxicity. DARU J. Pharm. Sci. 2019;27:557–570. doi: 10.1007/s40199-019-00283-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Jayaraj R.L., Tamilselvam K., Manivasagam T., Elangovan N. Neuroprotective Effect of CNB-001, a Novel Pyrazole Derivative of Curcumin on Biochemical and Apoptotic Markers Against Rotenone-Induced SK-N-SH Cellular Model of Parkinson’s Disease. J. Mol. Neurosci. 2013;51:863–870. doi: 10.1007/s12031-013-0075-8. [DOI] [PubMed] [Google Scholar]
- 333.Bagheri H., Ghasemi F., Barreto G.E., Rafiee R., Sathyapalan T., Sahebkar A. Effects of curcumin on mitochondria in neurodegenerative diseases. BioFactors. 2020;46:5–20. doi: 10.1002/biof.1566. [DOI] [PubMed] [Google Scholar]
- 334.Albrecht P., Bouchachia I., Goebels N., Henke N., Hofstetter H.H., Issberner A., Kovacs Z., Lewerenz J., Lisak D., Maher P., et al. Effects of dimethyl fumarate on neuroprotection and immunomodulation. J. Neuroinflamm. 2012;9:163. doi: 10.1186/1742-2094-9-163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Bomprezzi R. Dimethyl fumarate in the treatment of relapsing–remitting multiple sclerosis: An overview. Ther. Adv. Neurol. Disord. 2015;8:20–30. doi: 10.1177/1756285614564152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Paraiso H.C., Kuo P.-C., Curfman E.T., Moon H.J., Sweazey R.D., Yen J.-H., Chang F.-L., Yu I.-C. Dimethyl fumarate attenuates reactive microglia and long-term memory deficits following systemic immune challenge. J. Neuroinflamm. 2018;15:100. doi: 10.1186/s12974-018-1125-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Wang Y., Santa–Cruz K., DeCarli C., Johnson J.A. NAD(P)H:quinone oxidoreductase activity is increased in hippocampal pyramidal neurons of patients with alzheimer’s disease. Neurobiol. Aging. 2000;21:525–531. doi: 10.1016/S0197-4580(00)00114-7. [DOI] [PubMed] [Google Scholar]
- 338.Kume T., Suenaga A., Izumi Y., Akaike A. Protective Effect of Dimethyl Fumarate on an Oxidative Stress Model Induced by Sodium Nitroprusside in Mice. Biol. Pharm. Bull. 2016;39:1055–1059. doi: 10.1248/bpb.b16-00134. [DOI] [PubMed] [Google Scholar]
- 339.Hayashi G., Jasoliya M., Sahdeo S., Saccà F., Pane C., Filla A., Marsili A., Puorro G., Lanzillo R., Brescia Morra V., et al. Dimethyl fumarate mediates Nrf2-dependent mitochondrial biogenesis in mice and humans. Hum. Mol. Genet. 2017;26:2864–2873. doi: 10.1093/hmg/ddx167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Chowdhry S., Zhang Y., McMahon M., Sutherland C., Cuadrado A., Hayes J.D. Nrf2 is controlled by two distinct β-TrCP recognition motifs in its Neh6 domain, one of which can be modulated by GSK-3 activity. Oncogene. 2013;32:3765–3781. doi: 10.1038/onc.2012.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Pei J.-J., Tanaka T., Tung Y.-C., Braak E., Iqbal K., Grundke-Iqbal I. Distribution, Levels, and Activity of Glycogen Synthase Kinase-3 in the Alzheimer Disease Brain. J. Neuropathol. Exp. Neurol. 1997;56:70–78. doi: 10.1097/00005072-199701000-00007. [DOI] [PubMed] [Google Scholar]
- 342.Hye A., Kerr F., Archer N., Foy C., Poppe M., Brown R., Hamilton G., Powell J., Anderton B., Lovestone S. Glycogen synthase kinase-3 is increased in white cells early in Alzheimer’s disease. Neurosci. Lett. 2004;373:1–4. doi: 10.1016/j.neulet.2004.10.031. [DOI] [PubMed] [Google Scholar]
- 343.Gameiro I., Michalska P., Tenti G., Cores Á., Buendia I., Rojo A.I., Georgakopoulos N.D., Hernández-Guijo J.M., Ramos M.T., Wells G., et al. Discovery of the first dual GSK3β inhibitor/Nrf2 inducer. A new multitarget therapeutic strategy for Alzheimer’s disease. Sci. Rep. 2017;7:45701. doi: 10.1038/srep45701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Farr S.A., Ripley J.L., Sultana R., Zhang Z., Niehoff M.L., Platt T.L., Murphy M.P., Morley J.E., Kumar V., Butterfield D.A. Antisense oligonucleotide against GSK-3β in brain of SAMP8 mice improves learning and memory and decreases oxidative stress: Involvement of transcription factor Nrf2 and implications for Alzheimer disease. Free Radic. Biol. Med. 2014;67:387–395. doi: 10.1016/j.freeradbiomed.2013.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.Malm T.M., Iivonen H., Goldsteins G., Keksa-Goldsteine V., Ahtoniemi T., Kanninen K., Salminen A., Auriola S., Van Groen T., Tanila H., et al. Pyrrolidine Dithiocarbamate Activates Akt and Improves Spatial Learning in APP/PS1 Mice without Affecting β-Amyloid Burden. J. Neurosci. 2007;27:3712–3721. doi: 10.1523/JNEUROSCI.0059-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.Kang K.W., Lee S.J., Park J.W., Kim S.G. Phosphatidylinositol 3-Kinase Regulates Nuclear Translocation of NF-E2-Related Factor 2 through Actin Rearrangement in Response to Oxidative Stress. Mol. Pharmacol. 2002;62:1001–1010. doi: 10.1124/mol.62.5.1001. [DOI] [PubMed] [Google Scholar]
- 347.Mercado-Gómez O., Hernández-Fonseca K., Villavicencio-Queijeiro A., Massieu L., Chimal-Monroy J., Arias C. Inhibition of Wnt and PI3K Signaling Modulates GSK-3β Activity and Induces Morphological Changes in Cortical Neurons: Role of Tau Phosphorylation. Neurochem. Res. 2008;33:1599–1609. doi: 10.1007/s11064-008-9714-9. [DOI] [PubMed] [Google Scholar]
- 348.Zhu G., Wang X., Wu S., Li X., Li Q. Neuroprotective Effects of Puerarin on 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine induced Parkinson’s Disease Model in Mice. Phytotherapy Res. 2014;28:179–186. doi: 10.1002/ptr.4975. [DOI] [PubMed] [Google Scholar]
- 349.Zhou Y., Xie N., Li L., Zou Y., Zhang X., Dong M. Puerarin alleviates cognitive impairment and oxidative stress in APP/PS1 transgenic mice. Int. J. Neuropsychopharmacol. 2014;17:635–644. doi: 10.1017/S146114571300148X. [DOI] [PubMed] [Google Scholar]
- 350.Abeti R., Baccaro A., Esteras N., Giunti P. Novel Nrf2-Inducer Prevents Mitochondrial Defects and Oxidative Stress in Friedreich’s Ataxia Models. Front. Cell. Neurosci. 2018;12:188. doi: 10.3389/fncel.2018.00188. [DOI] [PMC free article] [PubMed] [Google Scholar]