Abstract
p62 is a highly conserved, multi-domain, and multi-functional adaptor protein critically involved in several important cellular processes. Via its pronounced domain architecture, p62 binds to numerous interaction partners, thereby influencing key pathways that regulate tissue homeostasis, inflammation, and several common diseases including cancer. Via binding of ubiquitin chains, p62 acts in an anti-inflammatory manner as an adaptor for the auto-, xeno-, and mitophagy-dependent degradation of proteins, pathogens, and mitochondria. Furthermore, p62 is a negative regulator of inflammasome complexes. The transcription factor Nrf2 regulates expression of a bundle of ROS detoxifying genes. p62 activates Nrf2 by interaction with and autophagosomal degradation of the Nrf2 inhibitor Keap1. Moreover, p62 activates mTOR, the central kinase of the mTORC1 sensor complex that controls cell proliferation and differentiation. Through different mechanisms, p62 acts as a positive regulator of the transcription factor NF-κB, a central player in inflammation and cancer development. Therefore, p62 represents not only a cargo receptor for autophagy, but also a central signaling hub, linking several important pro- and anti-inflammatory pathways. This review aims to summarize knowledge about the molecular mechanisms underlying the roles of p62 in health and disease. In particular, different types of tumors are characterized by deregulated levels of p62. The elucidation of how p62 contributes to inflammation and cancer progression at the molecular level might promote the development of novel therapeutic strategies.
Keywords: p62, autophagy, inflammasomes, Nrf2/Keap1, NF-κB, mTORC1, inflammation, cancer
1. Introduction
SQSTM1 (sequestosome 1), the gene coding for the p62 protein, spans about 16 kb and is located on chromosome 5 [1,2]. It is widely expressed and contains eight exons with a short 5′UTR (untranslated region) and an unusually long 3′UTR [3,4] (Figure 1). In humans, due to alternative splicing, SQSTM1 gives rise to two different isoforms. Isoform 1 represents the full-length p62 protein with 440 amino acids, whereas isoform 2 lacks 84 amino acids at the amino terminus in the PB1 domain [5,6,7]. The expression of SQSTM1 is stress-associated and induced by the transcription factors Nrf2, NF-κB, and MiT/TFE [8,9,10]. Apart from their roles in melanocytes, members of the MiT/TFE (microphthalmia/transcription factor E) family of helix-loop-helix transcription factors are activated by different types of internal and external stresses and also in cancer [11] (for Nrf2 see Section 4.2 and for NF-κB Section 4.4).
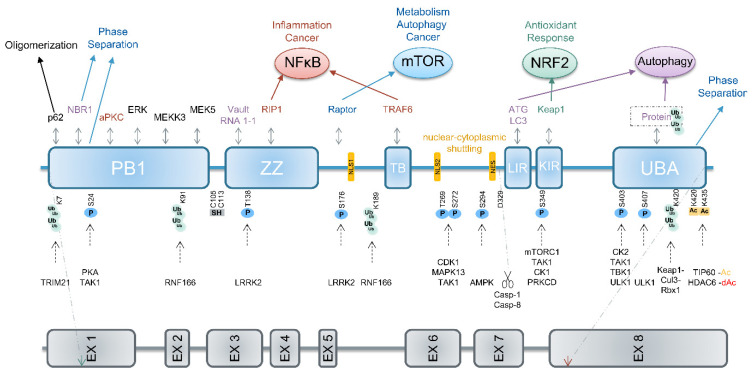
Figure 1.
Structure of SQSTM1 and p62. Domain architecture of p62, posttranslational modifications, binding/modifying proteins and pathways regulated by p62. The PB1 domain mediates oligomerization and activation of p62, UBA-mediated dimerization holds p62 inactive. For phase separation, the PB1 and UBA domains are required. The role of p62 as a cargo receptor in autophagy is mediated by the LIR motif, linking it to LC3 on autophagosomes, and the UBA domain, which binds to ubiquitinated cargos. Upon phosphorylation at Ser349, the KIR motif interacts with Keap1, thereby liberating Nrf2 and inducing Nrf2 target gene expression. When Raptor binds to p62, mTOR is activated inducing metabolic pathways. Binding of RIP1 to the ZZ or TRAF6 to the TB motif supports activation of NF-κB. Cleavage after Asp329 by caspase-8 (casp-8) or caspase-1 (casp-1) generates an amino terminal fragment of p62 activating mTOR. Disulfide bond formation involving Cys105 or Cys113 as well as binding of vault RNA1-1 via the ZZ motif supports autophagy. Please see in the text for details. Abbreviations: Ac: acetylation, dAc: de-acetylation, C: Cys, D: Asp, K: Lys, KIR: Keap1-interacting region, LIR: LC3-interacting region, NES: nuclear export signal/sequence, NLS: nuclear localization signal/sequence, PB1: Phox and Bem1p, S: Ser, T: Thr, TB: TRAF6-binding domain, UBA: ubiquitin-associated, ZZ: Zinc finger, [1,12,13,14].
Deregulated SQSTM1 expression is associated with several human diseases [12,15]. Missense mutations of SQSTM1 cause ALS (amyotrophic lateral sclerosis), PDB (Paget’s disease of bone) or FTLD (frontotemporal lobar degeneration) [16]. ALS is a neurodegenerative disease of motor neurons. The role of p62 in ALS is most likely defined by its autophagy-dependent requirement for proteostasis [17], the dynamic regulation of a functional proteome [18]. Similarly, FTLD is caused by proteotoxic stress-induced neurotoxicity due to the absence of functional SQSTM1 expression [19]. In PDB, a chronic progressive skeletal disorder, p62 mutations induce activation of osteoclasts in a non-physiological manner resulting in increased and deregulated bone turnover [20,21]. Other neurodegenerative diseases, such as Parkinson’s disease and Huntington’s disease, are also characterized by low levels of p62 [22]. In contrast, numerous types of cancer exhibit increased amounts of p62 in the tumor cells, including hepatocellular carcinoma (HCC, see Section 5, ovarian, breast, lung, or pancreatic cancer [1,5,23,24,25,26,27,28]. In these cells, high levels of p62 cause Nrf2 (Section 4.2), mTORC1 (Section 4.3), or/and NF-κB (Section 4.4) activation, which favors the development and proliferation of cancer cells. Interestingly, high levels of p62-positive inclusion bodies, termed Mallory–Denk bodies, are frequently found in patients with chronic liver conditions, such as HCCs, but also in hepatocellular neoplasms, alcoholic and non-alcoholic steatohepatitis, or metabolic disorders [29], and are associated with Nrf2 activation [23,30]. In contrast, p62 expression is reduced in the tumor stroma, for example in cancer-associated fibroblasts of prostate cancer cells [31] or in tumor-associated macrophages [32]. This causes metabolic reprogramming and secretion of IL-6 and TGF-β, both supporting growth and proliferation of tumor cells. Therefore, p62 expression might serve as a diagnostic and prognostic marker in carcinomas [12].
Ablation of expression in mice revealed physiological roles of p62 in osteoclastogenesis and bone remodeling, and in obesity and adipogenesis [33,34]. Upon aging, p62 knockout mice develop obesity caused by hyperphagia due to leptin resistance [35].
This review article aims to summarize what is known about the complex role of p62 in inflammation and inflammation-related diseases, including cancer. The focus is on the underlying molecular mechanisms and pathways. Section 2 describes the domain structure of p62, its post-translational modifications, and interacting proteins. In Section 3, we discuss the role of p62 as a receptor in autophagy and its regulation, and in Section 4, its involvement in signaling pathways, namely inflammasomes (Section 4.1), Nrf2 (Section 4.2), mTOR (Section 4.3), and NF-κB (Section 4.4). In Section 5, we address the mechanisms underlying deregulated expression in cancer and the consequences with HCCs as an example. Our article is based on a literature search using Pubmed (search criteria “p62” and “autophagy”, “inflammasome”, “Nrf2”, “mTOR”, “NF-kappaB” or “cancer/HCC”).
2. p62 Is a Posttranslationally Modified Multi-Domain Protein
p62 consists of 440 amino acids with several domains that influence different pathways upon interaction with distinct binding partners (Figure 1). The roles of p62 in five of these pathways are discussed in more detail below, including autophagy (Section 3), inflammasomes (Section 4.1), Keap1/Nrf2 (Section 4.2), mTORC1 (Section 4.3), and NF-κB (Section 4.4). The activity of p62 is regulated by posttranslational mechanisms, namely ubiquitination, phosphorylation, acetylation, proteolytic processing, and the formation of disulfide bridges (Figure 1).
The amino terminal PB1 domain (Phox1 and Bem1p) of p62 is required for the homotypic oligomerization of inactive p62 dimers, which results in a helical structure required for its role as a cargo receptor in autophagy [36]. The ubiquitin ligase TRIM21 (tripartite motif 21) inhibits oligomerization, and therefore activation of p62 upon ubiquitination via Lys63-linkage at Lys7 of p62. Consequently, ablation of TRIM21 results in Keap1 degradation, Nrf2 activation, and oxidative stress resistance (Section 4.2) [37].
Adjacent to the PB1 domain, p62 harbors a zinc finger (ZZ domain), frequently associated with DNA binding in other proteins. However, evidence for a direct binding of p62 to DNA is missing, although p62 can shuttle between nucleus and cytoplasm via two nuclear localization sequences (NLS1 and NLS2) and one nuclear export signal (NES) [38,39]. In contrast, p62 interacts with the small non-coding vault RNA1-1 via the ZZ domain favoring p62 oligomerization and autophagy [40]. RIP1 (receptor interacting protein 1, also receptor interacting serine/threonine kinase 1 or RIPK1) is an established binding partner of the ZZ domain and this interaction can induce NF-κB activation [41]. Moreover, p62 interacts via its TB domain (TRAF binding domain), located between NLS1 and NLS2, with the E3 ubiquitin ligase TRAF6 (tumor necrosis factor receptor associated factor 6), also leading to NF-κB activation [42].
LC3 (microtubule-associated protein 1A/1B-light chain 3) is a central autophagy protein and interacts with the LIR (LC3-interacting region) of p62, a motif commonly located in autophagy receptors [43,44]. As the carboxy terminal UBA (ubiquitin associated) domain binds and thereby recruits ubiquitinated proteins designated for autophagosomal degradation, p62 functions as a cargo receptor for selective autophagy.
Several kinases, such as mTORC1, TAK1 (TGF-β-activated kinase), CK1 (casein kinase 1), and PKC-delta (protein kinase C delta, PRKCD), phosphorylate p62 at Ser349 [45,46,47,48]. Then, the KIR (KEAP1-interacting region) motif of p62 interacts specifically with the Nrf2 inhibitor Keap1 (Kelch-like ECH-associated protein 1) and induces Nrf2 activation upon its liberation from Keap1 [49]. Interestingly, Mallory–Denk bodies, hallmarks of liver pathogenesis, contain Ser349 phosphorylated p62 as well as Keap1 [50]. Recently, a murine splice variant of p62 was identified lacking the KIR motif [51]. Although this variant is an active autophagy receptor, it is not able to degrade Keap1, and contrary to full-length p62, inhibits Nrf2.
Acetylation of p62 by TIP60 and deacetylation by HDAC6 in the UBA domain at Lys420 and Lys435 regulate binding of p62 to ubiquitin. This increases p62-dependent selective autophagy as well as the assembly of p62 bodies, cytoplasmic aggregates containing ubiquitinated proteins and p62 [52]. Other publications suggest that p62 forms structures with more dynamic liquid-like properties, termed liquid droplets, which allow an exchange of their components with their environment [13,53]. This phase separation is mediated by the UBA and PB1 domains [1,12].
RIP1-dependent cleavage of p62 by caspase-8 [54] results in a stable variant lacking the carboxy terminal KIR and UBA domains that promotes mTORC1 signaling instead of contributing to autophagy. In addition, caspase-1 cleaves p62 after Asp329 [55].
The cysteine residues Cys105 and Cys113 of human p62 are redox-sensitive. Upon oxidative stress, they form intermolecular disulfide bonds crosslinking p62, and this oligomerization supports autophagy and cell survival [56].
3. p62 Is a Cargo Receptor for Autophagy
Autophagy is a central degradative pathway that occurs in all eukaryotic cells and supports survival under stress conditions [57]. At the tissue level, stressors cause inflammation, a complex process that aims to restore tissue homeostasis after its disturbance [58]. Although inflammation is in principle a beneficial and protective response, it can be destructive, when deregulated or/and chronic. Then, inflammation contributes to the pathology of numerous common diseases. Inflammation is also a hallmark in cancer development [59]. Therefore, cellular autophagy is often associated with inflammation but acts in an anti-inflammatory manner [60,61] (see also Section 4.1). Ablation of the essential autophagy-related gene ATG16L1 in mice causes an inflammatory phenotype characterized by high levels of IL-1β and -18 due to increased inflammasome activation (see Section 4.1) [62,63].
The term autophagy is commonly used for the process of macroautophagy, defined as the formation of a cellular structure surrounded by a double membrane that fuses with lysosomes for the digestion of its content [64]. Autophagy is triggered by adverse conditions, such as starvation, infection, oxidative stress, protein aggregation, or inhibition of mTORC1 (see Section 4.3) [65]. Autophagy is also induced by inhibition of the ubiquitin-proteasome system (UPS), as this causes accumulation of ubiquitinated proteins, whereas inhibition of autophagy dampens the UPS [65]. Both degradative pathways are required for cellular proteostasis and p62 connects both. p62 binds ubiquitinated proteins, such as tau, via its UBA domain and causes their proteasomal degradation [66]. However, together with other proteins, such as NBR1, TAX1BP1 and Optineurin, p62 plays a more important role as a cargo receptor in autophagy [61]. In general, Lys48 ubiquitination targets proteins for proteasomal degradation, whereas those with Lys63-linked ubiquitin are sequestered by p62 and delivered to autophagosomes [5]. In addition, p62 is required for mitophagy, the autophagic degradation of damaged mitochondria [67], and for xenophagy, the autophagic destruction of invading pathogens [61]. It has been reported that the intracellular bacteria S. typhimurium, S. flexneri, and M. tuberculosis are targeted by p62 for delivery to autophagosomes [68,69,70].
Under non-stressed conditions, p62 exists in the cell as an inactive homodimer masking its UBA domain from binding to ubiquitin [1,71]. Proteotoxic stress induces phosphorylation of Ser407 by ULK1, causing the liberation of the UBA domain for substrate binding [72]. A subsequent phosphorylation at Ser403 by ULK1, CK2 (casein kinase 2), or TBK1 (TANK-binding kinase 1) enhances the affinity of the UBA domain for ubiquitin [73,74,75]. Upon binding of ubiquitin-conjugated substrates the complex can undergo phase separation by forming a dynamic liquid-like structure [13]. The LIR domain of p62 interacts with LC3 present on autophagosomes (Figure 2). Fusion with lysosomes results in the formation of autolysosomes and the degradation of their content, including p62. Therefore, under conditions where autophagy is impaired, p62 accumulates, and its accumulation levels are proportional to the autophagic impairment, as long as p62 transcription is not strongly downregulated [12,76,77,78].
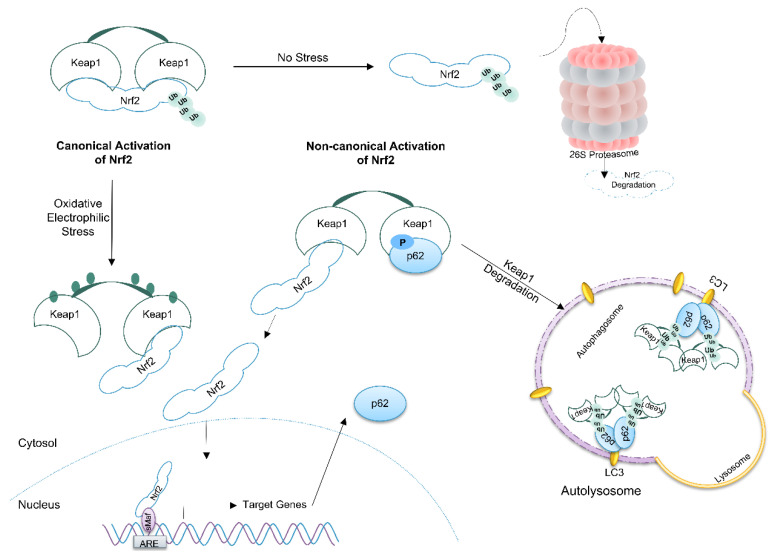
Figure 2.
Non-canonical Nrf2 activation by p62. Under non-stressed conditions, the cytoprotective transcription factor Nrf2 is continuously degraded by the proteasome upon Keap1-mediated ubiquitination. Oxidation of regulatory cysteine residues of Keap1 in situations with oxidative and electrophilic stress inhibits Keap1 and ubiquitination of Nrf2 causing canonical Nrf2 activation and induction of target gene expression. When p62 is phosphorylated at Ser349, it binds to Keap1 causing its autophagic degradation. Afterwards, Nrf2 is free to translocate to the nucleus and to induce target gene expression (non-canonical Nrf2 activation) [79].
4. Roles of p62 in Different Signaling Pathways
4.1. p62 Restricts Inflammasome Signaling
Activation of inflammasomes represents one of the main pathways underlying the induction of an inflammatory response [80]. Moreover, acute and chronic inflammasome activation contributes to the pathogenesis of common diseases and recent research focuses on mechanisms regulating inflammasome activation [81,82]. Increasing evidence demonstrates an important role of p62, autophagy, and the Nrf2/Keap1 pathway (see Section 4.2) in the restriction of the inflammasome pathway [83,84].
Inflammasomes constitute a family of multi-protein complexes that assemble upon sensing of a wide range of stimuli, including DAMPs (damage- or danger-associated molecular patterns) and PAMPs (pathogen-associated molecular patterns) [85]. The sensor protein belongs to the NLR (NOD (nucleotide oligomerization domain)-like receptor) or the ALR (AIM2 (absent in melanoma 2)-like receptor) family. NLRP3 (NLR family pyrin domain containing 3) represents the most prominent inflammasome sensor and its activation is associated with numerous inflammatory diseases, including Alzheimer’s disease, atherosclerosis, and rheumatoid arthritis [86,87]. Once an inflammasome sensor is stimulated by its activator, it recruits the adaptor protein ASC (apoptosis-associated speck-like protein containing a caspase recruitment domain) and induces formation of ASC polymers, termed ASC specks (Figure 3). This causes activation of the protease caspase-1. Subsequently, caspase-1 cleaves and activates the pro-inflammatory cytokines proIL-1β and -18 as well as GSDMD (gasdermin D). Upon oligomerization of its amino terminal fragment, GSDMD forms pores in the outer membrane inducing a lytic type of cell death, termed pyroptosis [88]. As IL-1β and -18 lack a signal peptide for secretion, GSDMD pores play a crucial role in their release and in the induction of an inflammatory response. Whereas the AIM2 inflammasome is activated upon the binding of double stranded pathogen-derived DNA [89], the exact molecular mechanisms underlying NLRP3 activation remain elusive and different models, including pore formation, lysosomal rupture, and mitochondrial dysfunction, are discussed [87].
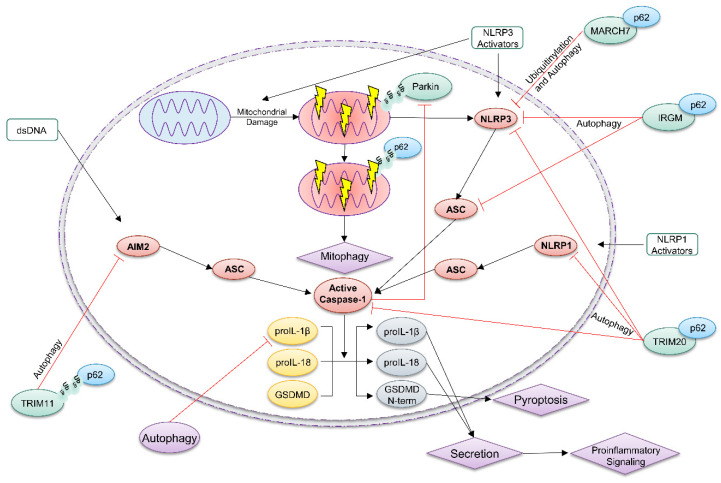
Figure 3.
p62-dependent autophagy antagonizes inflammasomes. The inflammasome sensors NLRP3, NLRP1 and AIM2 sense certain stressors, which induce their activation. This causes oligomerization of the adaptor protein ASC (speck formation) and in turn activation of the protease caspase-1 [81]. Subsequently, caspase-1 activates the proinflammatory cytokine proIL-1β and -18 by proteolytic processing. Release of mature IL-1β and -18 induces an inflammatory response, which is dependent on caspase-1-dependent cleavage and activation of GSDMD. p62 is required for mitophagy of damaged mitochondria that induce NLRP3 activation [90]. Moreover, MARCH7 ubiquitinates NLRP3, inducing its p62-dependent selective autophagic degradation [91]. Together with p62, IRGM regulates degradation of NLRP3 and ASC via autophagy [92]. The E3 ubiquitin ligase TRIM20 ubiquitinates NLRP3, NLRP1 and caspase-1, thereby initiating their p62-dependent autophagic clearance [93]. Ubiquitinated TRIM11 binds with p62 to AIM2 causing its degradation by autophagy [94].
Upon its role as a cargo receptor in autophagy, p62 negatively regulates the inflammasome pathway by different mechanisms [84,95]. Certain NLRP3 inflammasome activators can damage mitochondria [87], which are then ubiquitinated by the E3 ubiquitin ligase Parkin [96,97]. This recruits p62 targeting them for destruction by the autophagic machinery [90]. Therefore, ablation of p62 expression restricts mitophagy and enhances NLRP3 inflammasome activation [90]. On the other hand, caspase-1 (and caspase-8) antagonizes mitophagy by proteolytic cleavage and inactivation of Parkin [98,99]. Furthermore, p62 ablation disturbs proteostasis, which supports inflammasome activation in primary murine macrophages and in mice in vivo [100].
p62 also directly supports the autophagy-dependent degradation of ubiquitinated inflammasome proteins, such as NLRP3, ASC, and AIM2 (Figure 3) [101]. The E3 ubiquitin ligase TRIM20 targets NLRP3, NLRP1, and caspase-1 for autophagic degradation [93]. MARCH7 ubiquitinates NLRP3, marking it for destruction by autophagy [91]. In contrast, TRIM31 induces NLRP3 degradation through the proteasome [102]. Ubiquitinated TRIM11 binds to AIM2 and recruits p62, thus targeting the inflammasome sensor for destruction by autophagosomes [94]. IRGM (immunity-related GTPase M) supports autophagic degradation of NLRP3 and ASC in a p62-dependent manner and protects from gut inflammation in a mouse model of Crohn’s disease [92]. Recently, Zhou et al. demonstrated that Lys63-linked ubiquitination of NLRP3 drives autophagic degradation of the NLRP3 inflammasome in a p62-dependent manner [103].
The cellular level of proIL-1β, the central inducer of inflammation after inflammasome activation, is regulated by autophagy and it is tempting to speculate that proIL-1β is also targeted by p62 [104]. In addition, polyubiquitinated proIL-1α and -1β are also degraded by the proteasome pathway [105]. As mentioned above, IL-1β lacks a signal peptide and is secreted dependent on GSDMD activation but also by mechanisms which are only partially understood [106]. ProIL-1β binds to TRIM16 [107] and some evidence suggests that, upon this interaction, IL-1β is released from the cell by a mechanism involving autophagy, termed secretory autophagy [108,109].
4.2. p62 Activates the Nrf2 Pathway
The transcription factor Nrf2 (nuclear factor E2-related factor 2) is a central regulator of cytoprotection and stress resistance, particularly against ROS and oxidative stress [110,111,112]. Therefore, Nrf2 expression is associated with inflammation and involved in many inflammatory diseases including cancer [113,114]. In general, Nrf2 activation is considered to be anti-inflammatory [115]. p62 activates Nrf2 by a well characterized non-canonical pathway mediated by interaction with and autophagosomal degradation of the Nrf2 inhibitor Keap1 (Kelch-like ECH-associated protein 1) (Figure 2) [79,116]. Furthermore, the Nrf2 pathway is linked to inflammasomes at different levels [117,118].
Nrf2 is a member of the Cap’n’collar family of bZIP (basic leucine zipper) transcription factors and regulates expression of several hundred genes mainly in a positive manner [110,111]. After interaction with sMAFs (small masculoaponeurotic fibrosarcomas), Nrf2 binds to AREs (antioxidant response elements) in the promoter of genes encoding proteins of the glutathione and thioredoxin system, the two most important cellular redox buffers [119]. Furthermore, Nrf2 induces expression of proteins required for detoxification of ROS and xenobiotics. Nrf2 activity is often high in cancer cells, where the transcription factor regulates metabolic reprogramming [23,120]. In addition to these indirect anti-inflammatory effects, Nrf2 is able to repress expression of proinflammatory cytokines, such as proIL1α, -1β, and -6, by a poorly understood mechanism [121]. Transcription of the Nrf2 gene (NFE2L2) is induced by NF-κB, AhR (aryl hydrocarbon receptor), oncogenic pathways, and by Nrf2 itself through a positive feedback loop [112].
The Nrf2 binding partner and inhibitor Keap1 regulates activity of Nrf2 at the posttranslational level. In the cytoplasm, two Keap1 molecules interact with Nrf2 in an asymmetric manner. As Keap1 binds also the E3 ubiquitin ligase complex Cul3/Rbx1 (Cullin 3/RING-box protein 1), this results in constant ubiquitination of Nrf2 and subsequent proteasomal degradation. Consequently, the half-life of Nrf2 is short (about 20 min) and its basic expression level usually low [122]. Keap1 has 27 cysteine residues and some of them are redox sensitive [123]. Oxidation of these cysteines by electrophiles, such as sulforaphane or curcumin, substances found in vegetables, or by glutathione under high ROS conditions, causes a conformational change of Keap1 and an inhibition of Cul3′s E3 ubiquitin ligase activity [111]. After that, newly synthesized Nrf2 bypasses Keap1, translocates to the nucleus, and induces expression of target genes, termed canonical Nrf2 activation (Figure 2).
Binding of p62 via its KIR motif to Keap1 liberates Nrf2 and causes non-canonical Nrf2 activation [79]. The affinity of p62 to Keap1 is strongly increased upon phosphorylation of Ser349 (see Section 3), which occurs while p62 is interacting with cargos [1]. Subsequently, the Keap1-p62-cargo complex is degraded via autophagy (Figure 2). Indeed, Keap1 is degraded mainly by autophagy in a p62-dependent manner [124,125]. Upon p62-induced activation, Nrf2 induces not only the expression of cytoprotective genes, but also the transcription of SQSTM1, resulting in a positive feedback loop. However, the Keap1-Cul3-Rbx1 complex can ubiquitinate p62 at Lys420, which causes the degradation of p62 by autophagy [126].
In general, inhibition of autophagy results in accumulation of p62, which in turn activates Nrf2. This pathway plays an important role in the development of liver cancer (see Section 5.). However, Nrf2 levels are high in different types of cancer induced by several mechanisms, including mutations in the NFE2L2 and KEAP1 genes or Keap1 inhibition by oncometabolites [110,127]. Cancer cells profit from high Nrf2 activity through increased stress and ROS resistance, but also by metabolic reprogramming [23,120]. On the other hand, high levels of Nrf2 can suppress cancer development and neurodegenerative diseases, demonstrating a dual role of Nrf2 in these conditions [5,127].
4.3. p62 Activates mTORC1
Mammalian target of rapamycin (mTOR) is a protein of 289 kDa and a highly conserved member of the PI3K (phosphoinositide 3-kinase)-related kinase family [128]. The serine-threonine kinase plays a complex role as a sensor for the environmental and intracellular status of the cell and regulates central processes, including proliferation, growth, metabolism, and survival [129]. Therefore, the mTOR pathway is an attractive target for immunosuppressive and anti-proliferative therapies for patients after organ transplantation or suffering from cancer, respectively [130,131]. Inhibitors of mTOR, such as rapamycin and rapalogs, are not only tested for their therapeutic potential for cancer patients, but also used in research laboratories as activators of autophagy.
mTORC1 (mTOR complex 1) is composed of the three core proteins mTOR, mLST8 (mammalian lethal with SEC13 protein 8) and Raptor (regulatory-associated protein of mTOR), and the inhibitors PRAS40 (proline-rich AKT1 substrate 1) and DEPTOR (DEP domain-containing mTOR-interacting protein) [130]. In the presence of amino acids, nutrients, energy, and growth factors, mTORC1 induces anabolic processes, such as lipid, nucleotide, and protein synthesis, by phosphorylation of several substrates, thereby supporting cell growth. In addition, mTOR phosphorylates ULK1, a kinase required for initiation of autophagy, leading to the inhibition of catabolic processes [132]. Although p62 acts as a cargo receptor for autophagic degradation, it activates the mTORC1 pathway [133]. p62 interacts with Raptor which is required for assembly, subcellular localization, stability, and substrate recruitment of mTORC1 [130,133]. In the presence of amino acids, MEKK3 (mitogen-activated protein kinase kinase kinase 3) binds to the PB1 domain of p62 and induces phosphorylation of Thr269 and Ser272 [134]. Then, p62 moves to the lysosomal membrane, binds the E3 ubiquitin ligase TRAF6 as well as mTOR, and induces Lys63-linked ubiquitination of mTOR. This p62-TRAF6-dependent ubiquitination causes activation of mTORC1 inducing anabolism and cell growth [134,135].
mTOR is a regulator of immune cell function and contributes to the high proliferation rate of cancer cells [129,131]. Therefore, MTOR itself as well as genes coding for upstream activators of mTORC1, such as AKT1, EGFR, or PTEN, are frequently mutated in cancer [130].
4.4. p62 Activates NF-κB
The NF-κB (nuclear factor-κB) family of transcription factors consist of five members that act as homo- or heterodimers [136,137]. They play a central role in inflammatory processes, numerous (inflammatory) diseases and are involved in all stages of tumor development [138]. p62 activates the NF-κB pathway by different mechanisms and thereby supports inflammation and cancer development [1,139].
In the classical pathway, translocation of the Rel-p50 dimer is regulated by the IKK (IkappaB kinase) complex, which is composed of the regulatory subunit NEMO (NF-kappa-B essential modulator or IKKγ) and the catalytic subunits IKKα and IKKβ. After activation, the IKK complex phosphorylates IκBα that dissociates from Rel-p50 dimers, gets ubiquitinated and degraded by the proteasome. Activation of IKK is induced by activation of several receptors by binding of their ligands, such as TNFα, IL-1 or LPS. The kinase RIP1 induces activation of the IKK complex, and this is supported by interaction with the ZZ domain of p62 (Figure 1) [41]. Activation requires a dimerization of two IKK units, induced by trans-autophosphorylation and Lys63-linked ubiquitination of NEMO. This process is catalyzed by the E3 ubiquitin ligase TRAF6 and supported by p62 via a direct interaction with TRAF6 [140]. A20 is a negative regulator of NF-κB [141]. This ubiquitin-editing protein prevents Lys63 ubiquitination of TRAF6 and promotes Lys48-linked ubiquitination of RIP1, causing proteasomal degradation of the latter [1,142]. p62 promotes degradation of A20 by autophagy.
NF-κB is also a positive transcriptional regulator of p62 expression and supports its own activity via a feed-forward loop. In addition, the transcription factor plays a central role in priming for inflammasome activation by inducing expression of proIL-1β and NLRP3 (Section 4.1) [143]. Nevertheless, via positive regulation of Nrf2 expression (Section 4.2), NF-κB restricts proIL-1β expression. In addition, the NLRP3 inflammasome pathway is also antagonized by NF-κB upon p62-dependent mitophagy [90,144].
5. p62 in Hepatocellular Carcinoma and Other Malignancies
A key role for autophagy, p62, and Nrf2 is well established in the development of different types of cancer, particularly of HCC [145,146]. HCC is the sixth most common cancer and the third leading cause of tumor death.
Different types of stress to the liver and hepatocytes cause liver cirrhosis, a disease characterized by impaired liver function due to the formation of scar tissue as a consequence of chronic tissue repair. This is caused by infection with HBV/HCV (hepatitis B and C virus), chronic alcohol abuse leading to ASH (alcoholic steatohepatitis), NASH (non-alcoholic steatohepatitis) due to obesity, high blood pressure, type 2 diabetes, metabolic syndrome, exposure to chemical carcinogens, or genetic predisposition and diseases [147]. Particularly HBV, HCV, ASH and NASH are known to inhibit autophagy, causing formation of inclusion bodies and accumulation of damaged mitochondria [148]. Low levels of beclin 1 result in autophagy defects in all stages of HCC [149,150]. Beclin 1 is monoallelically deleted and decreased in different cancers, such as prostate, breast and ovarian cancer [151]. Upon decreased rates of autophagy, phosphorylated p62 accumulates with Keap1 in structures of the human liver, indicating Nrf2 activation that contributes to adenoma formation and the development of HCC [152,153]. This is associated with increased NF-κB, mTOR and Wnt/β-catenin signaling. Therefore, p62 represents a hub linking defects in autophagy with critical pathway regulating carcinogenesis, such as inflammation, redox homeostasis and (energy) metabolism [145]. Particularly, metabolic reprogramming supports tumor growth as well as its drug resistance [23,154]. The expression of key players in autophagy provides useful indicators for the prognosis of patients suffering from HCC. High levels of LC3B and ULK1 are associated with a poor prognosis of HCC patients in advanced stages [155,156]. Sorafenib, a tyrosine kinase inhibitor, is a standard drug for liver cancer that enhances autophagy [157,158]. Furthermore, mTOR inhibition by rapamycin/sirolimus has promising anti-tumoral effects in HCC patients [159].
Experiments in mice have confirmed the central role of the autophagy-p62-Nrf2 axis in the development of HCC [1]. Loss of Atg5 (autophagy related 5) or Atg7 in the mouse liver is associated with damaged mitochondria and peroxisomes, lipid droplets and accumulation of protein aggregates with p62 and Keap1, leading to sustained Nrf2 activation that causes benign adenoma [160,161]. Similarly, mice haploinsufficient for beclin 1 are prone to spontaneous tumor development. Furthermore, they are highly susceptible to develop HCC upon infection with HBV [162]. Most likely, ROS derived from degenerated mitochondria and peroxisomes damage DNA causing genomic instability and promoting oncogenesis [163]. The accumulation of p62 in hepatocytes of these mice is sufficient to induce HCC development and allows enhanced proliferation of cancer cells and resistance to anticancer drugs due to Nrf2 activation [23]. Activation of mTORC1 due to TSC (tuberous sclerosis complex) depletion causes HCC development that is reverted by additional p62 deletion [24]. In addition, the simultaneous loss of p62 or Nrf2 in Atg5- or Atg7-deficient mice suppresses tumor development demonstrating the central role of Nrf2 and p62-induced Nrf2 activation in the development of HCC [160,164]. However, it is also known that NF-κB, which is linked both to p62 and to Nrf2, is activated in human HCC and its inhibition as well as activation in mice enhances hepatocarcinogenesis [165,166,167]. Ablation of p62 in hepatocytes is not associated with defects in autophagy, demonstrating that other autophagy receptors can compensate for the lack of p62 [24]. Nonetheless, p62 expression is essential for mitophagy and suppression of the NLRP3 pathway [90]. As discussed above (Section 3), autophagy is an anti-inflammatory stress-induced pathway. Autophagy plays opposing roles at different stages of tumorigenesis. At early stages, autophagy is rather tumor suppressive and cytoprotective, at late stages, tumor cells might need higher levels of autophagy [145,168,169]. This model is supported by the observation that mice lacking expression of Atg5 or Atg7 in hepatocytes develop benign adenomas but not malignant HCC. Moreover, in rats, inhibition of autophagy promotes hepatocarcinogenesis in the dysplastic stage but suppresses tumorigenesis in the tumor-forming stage of HCC [170].
p62 expression is also upregulated in a number of other tumor cells and this list is most likely further increasing (Table 1). Consequently, pharmacological targeting of p62 might represent a successful anti-tumor strategy. First, bioactive molecules were developed that bind to certain domains of p62 and block the pathways and activities regulated by these domains [171].
Table 1.
Human cancers with overexpression of p62 [4].
| Cancer Type | References |
|---|---|
| Thyroid cancer | [172] |
| Lung cancer | [173] |
| Colorectal cancer | [174] |
| Head and neck cancer | [175] |
| Gastric cancer | [176] |
| Liver cancer | [24] |
| Pancreatic cancer | [176] |
| Renal cancer | [177] |
| Urothelial cancer | [178] |
| Prostate cancer | [179] |
| Breast cancer | [180] |
| Ovarian cancer | [181] |
| Endometrial cancer | [182] |
| Melanoma | [183] |
| Glioma | [184] |
6. Summary and Outlook
The p62 protein is a beautiful paradigm for complexity in molecular biology. Via its domain structure, p62 interacts with several proteins regulating key pathways required for the maintenance of homeostasis of cells and tissues, as well as for inflammation and common diseases including cancer (Figure 4). It is likely that further novel binding partners of p62 that are critically involved in other pathways will be revealed in the future. A recent example is the identification of a role of extracellular p62 as an inflammatory mediator in sepsis [185]. p62 is actively secreted from macrophages and monocytes by secretory lysosomes or passively released by pyroptotic cells and binds to the insulin receptor. This causes NF-κB activation and in turn polarization of macrophages contributing to septic death [186]. There is no need to say that p62 requires a specific posttranslational modification (in form of phosphorylation at Ser403) for this role in sepsis [186]. Although research in the past has revealed that p62 is modified and regulated by phosphorylation, ubiquitination, acetylation, proteolytic processing, and the formation of disulfide bridges, we are far away from precisely understanding how the binding of p62 to its numerous interaction partners is regulated. Furthermore, the physical and biological properties of protein aggregates with p62 are incompletely understood. It is clear that p62 plays an important role in inflammation, several neurodegenerative diseases, and cancer. Particularly, the role of p62 in cancer development seems to be complex and double-faced. This is caused by the different pathways linked to p62. p62 is a cargo receptor in auto-, mito-, and xenophagy, as well as an important hub for pro- and anti-inflammatory pathways, such as inflammasomes, NF-κB, Nrf2, and mTORC1. As these pathways have dual or even multiple and in part opposing roles in cancer development depending on the tissue, cell type, and stage of development, it is difficult to estimate whether or not p62 represents a screw, the turning of which might have therapeutic potential. Nevertheless, vaccines for targeting p62 have been developed and tested in humans [187,188]. In addition, the expression, modification, and localization of p62, perhaps in connection with pathways regulated by p62, might be useful as a diagnostic and prognostic marker in cancer and other diseases [145].
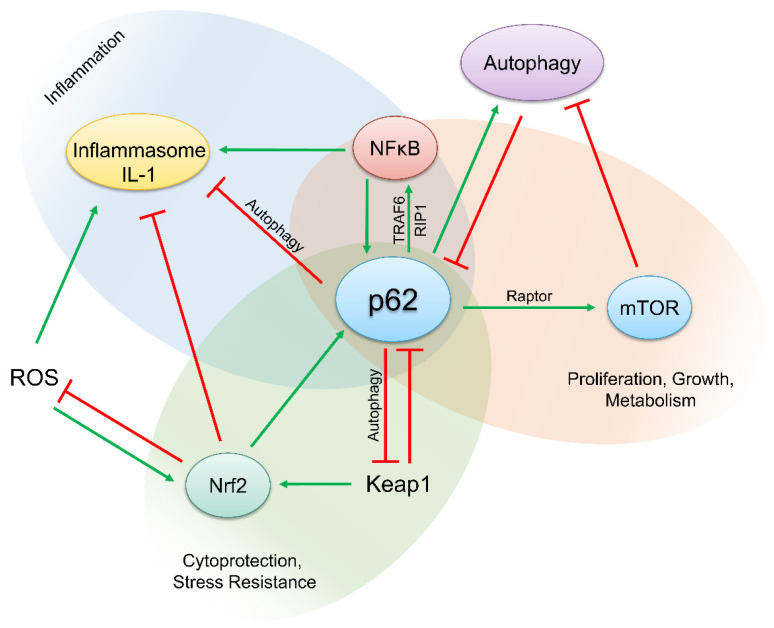
Figure 4.
p62 regulates and links central pathways in inflammation and cancer development. p62 is an important cargo receptor for autophagy and mitophagy and thus inhibits inflammasome activation and inflammation. Moreover, upon Keap1 binding, p62 activates Nrf2 and attenuates inflammation. In contrast, p62 activates NF-κB, a central regulator of inflammation and cancer development, and the kinase mTOR that induces anabolic pathways.
Author Contributions
P.H. and H.-D.B. conceptualized the manuscript; H.-D.B. wrote the text; P.H. revised the text and created the figures with the help of G.F., M.D.F. and T.K. All authors corrected the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
Our research is supported by grants from Swiss Cancer Research (KFS-3940-08-2016 and KFS-5087-08-2020), Wolfermann-Nägeli-Stiftung, Dr. Hans Altschüler-Stiftung, Bangerter-Rhyner-Stiftung (8472), Wilhelm Sander-Stiftung (2019.075.1), Monika Kutzner Stiftung (2019), Monique Dornonville de la Cour-Stiftung (2019) and the Swiss National Science Foundation (310030_197426). H.-D.B. and M.D.F. are members of the SKINTEGRITY.CH collaborative research program. P.H., T.K. and M.D.F. are members of the Life Science Zurich Graduate School.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Sanchez-Martin P., Saito T., Komatsu M. p62/SQSTM1: ’Jack of all trades’ in health and cancer. FEBS J. 2019;286:8–23. doi: 10.1111/febs.14712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Park I., Chung J., Walsh C.T., Yun Y., Strominger J.L., Shin J. Phosphotyrosine-independent binding of a 62-kDa protein to the src homology 2 (SH2) domain of p56lck and its regulation by phosphorylation of Ser-59 in the lck unique N-terminal region. Proc. Natl. Acad. Sci. USA. 1995;92:12338–12342. doi: 10.1073/pnas.92.26.12338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.GeneCards—SQSTM10.05.2021. [(accessed on 1 April 2021)]; Available online: https://www.genecards.org/cgi-bin/carddisp.pl?gene=SQSTM1.
- 4.Protein Atlas—p10.05.2021. [(accessed on 1 April 2021)]; Available online: https://www.proteinatlas.org/ENSG00000161011-SQSTM1.
- 5.Emanuele S., Lauricella M., D’Anneo A., Carlisi D., De Blasio A., Di Liberto D., Giuliano M. p62: Friend or Foe? Evidences for OncoJanus and NeuroJanus Roles. Int. J. Mol. Sci. 2020;21:5029. doi: 10.3390/ijms21145029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang L., Cano M., Handa J.T. p62 provides dual cytoprotection against oxidative stress in the retinal pigment epithelium. Biochim. Biophys. Acta Bioenerg. 2014;1843:1248–1258. doi: 10.1016/j.bbamcr.2014.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.UniProt—p10.05.2021. [(accessed on 1 April 2021)]; Available online: https://www.uniprot.org/uniprot/Q13501.
- 8.Ling J., Kang Y.A., Zhao R., Xia Q., Lee D.F., Chang Z., Li J., Peng B., Fleming J.B., Wang H., et al. KrasG12D-induced IKK2/beta/NF-kappaB activation by IL-1alpha and p62 feedforward loops is required for development of pancreatic ductal adenocarcinoma. Cancer Cell. 2012;21:105–120. doi: 10.1016/j.ccr.2011.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Settembre C., Di Malta C., Polito V.A., Arencibia M.G., Vetrini F., Serkan E., Erdin S.U., Huynh T., Medina D., Colella P., et al. TFEB Links Autophagy to Lysosomal Biogenesis. Science. 2011;332:1429–1433. doi: 10.1126/science.1204592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jain A., Lamark T., Sjøttem E., Larsen K.B., Awuh J.A., Øvervatn A., McMahon M., Hayes J.D., Johansen T. p62/SQSTM1 Is a Target Gene for Transcription Factor NRF2 and Creates a Positive Feedback Loop by Inducing Antioxidant Response Element-driven Gene Transcription. J. Biol. Chem. 2010;285:22576–22591. doi: 10.1074/jbc.M110.118976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.La Spina M., Contreras P.S., Rissone A., Meena N.K., Jeong E., Martina J.A. MiT/TFE Family of Transcription Factors: An Evolutionary Perspective. Front. Cell Dev. Biol. 2021;8:609683. doi: 10.3389/fcell.2020.609683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sánchez-Martín P., Komatsu M. p62/SQSTM1—steering the cell through health and disease. J. Cell Sci. 2018;131:jcs222836. doi: 10.1242/jcs.222836. [DOI] [PubMed] [Google Scholar]
- 13.Berkamp S., Mostafavi S., Sachse C. Structure and function of p62/SQSTM1 in the emerging framework of phase sepa-ration. FEBS J. 2020 doi: 10.1111/febs.15672. [DOI] [PubMed] [Google Scholar]
- 14.Islam A., Sooro M.A., Zhang P. Autophagic Regulation of p62 is Critical for Cancer Therapy. Int. J. Mol. Sci. 2018;19:1405. doi: 10.3390/ijms19051405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Long M., Li X., Li L., Dodson M., Zhang D.D., Zheng H. Multifunctional p62 Effects Underlie Diverse Metabolic Diseases. Trends Endocrinol. Metab. 2017;28:818–830. doi: 10.1016/j.tem.2017.09.001. [DOI] [PubMed] [Google Scholar]
- 16.Rea S., Majcher V., Searle M.S., Layfield R. SQSTM1 mutations—Bridging Paget disease of bone and ALS/FTLD. Exp. Cell Res. 2014;325:27–37. doi: 10.1016/j.yexcr.2014.01.020. [DOI] [PubMed] [Google Scholar]
- 17.Nguyen D.K., Thombre R., Wang J. Autophagy as a common pathway in amyotrophic lateral sclerosis. Neurosci. Lett. 2019;697:34–48. doi: 10.1016/j.neulet.2018.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kumsta C., Chang J.T., Lee R., Tan E.P., Yang S.L.H., Loureiro R., Choy E.H., Lim S.H.Y., Saez I., Springhorn A., et al. The autophagy receptor p62/SQST-1 promotes proteostasis and longevity in C. elegans by inducing autophagy. Nat. Commun. 2019;10:1–12. doi: 10.1038/s41467-019-13540-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Deng Z., Lim J., Wang Q., Purtell K., Wu S., Palomo G.M., Tan H., Manfredi G., Zhao Y., Peng J., et al. ALS-FTLD-linked mutations of SQSTM1/p62 disrupt selective autophagy and NFE2L2/NRF2 anti-oxidative stress pathway. Autophagy. 2020;16:917–931. doi: 10.1080/15548627.2019.1644076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Numan M.S., Amiable N., Brown J.P., Michou L. Paget’s disease of bone: An osteoimmunological disorder? Drug. Des. Devel. Ther. 2015;9:4695–4707. doi: 10.2147/DDDT.S88845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ralston S.H., Layfield R. Pathogenesis of Paget Disease of Bone. Calcif. Tissue Int. 2012;91:97–113. doi: 10.1007/s00223-012-9599-0. [DOI] [PubMed] [Google Scholar]
- 22.Ma S., Attarwala I.Y., Xie X.-Q. SQSTM1/p62: A Potential Target for Neurodegenerative Disease. ACS Chem. Neurosci. 2019;10:2094–2114. doi: 10.1021/acschemneuro.8b00516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Saito T., Ichimura Y., Taguchi K., Suzuki T., Mizushima T., Takagi K., Hirose Y., Nagahashi M., Iso T., Fukutomi T., et al. p62/Sqstm1 promotes malignancy of HCV-positive hepatocellular carcinoma through Nrf2-dependent meta-bolic reprogramming. Nat. Commun. 2016;7:12030. doi: 10.1038/ncomms12030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Umemura A., He F., Taniguchi K., Nakagawa H., Yamachika S., Font-Burgada J., Zhong Z., Subramaniam S., Raghunandan S., Duran A., et al. p62, Upregulated during Preneoplasia, Induces Hepatocellular Carcinogenesis by Maintaining Survival of Stressed HCC-Initiating Cells. Cancer Cell. 2016;29:935–948. doi: 10.1016/j.ccell.2016.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xia M., Yu H., Gu S., Xu Y., Su J., Li H., Kang J., Cui M. p62/SQSTM1 is involved in cisplatin resistance in human ovarian cancer cells via the Keap1-Nrf2-ARE system. Int. J. Oncol. 2014;45:2341–2348. doi: 10.3892/ijo.2014.2669. [DOI] [PubMed] [Google Scholar]
- 26.Ryoo I.-G., Choi B.-H., Ku S.-K., Kwak M.-K. High CD44 expression mediates p62-associated NFE2L2/NRF2 activation in breast cancer stem cell-like cells: Implications for cancer stem cell resistance. Redox Biol. 2018;17:246–258. doi: 10.1016/j.redox.2018.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Todoric J., Antonucci L., Di Caro G., Li N., Wu X., Lytle N.K., Dhar D., Banerjee S., Fagman J.B., Browne C.D., et al. Stress-Activated NRF2-MDM2 Cascade Controls Neoplastic Progression in Pancreas. Cancer Cell. 2017;32:824–839. doi: 10.1016/j.ccell.2017.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huang H., Zhu J., Li Y., Zhang L., Gu J., Xie Q., Jin H., Che X., Li J., Huang C., et al. Upregulation of SQSTM1/p62 contributes to nickel-induced malignant transformation of human bronchial epithelial cells. Autophagy. 2016;12:1687–1703. doi: 10.1080/15548627.2016.1196313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Strnad P., Zatloukal K., Stumptner C., Kulaksiz H., Denk H. Mallory–Denk-bodies: Lessons from keratin-containing hepatic inclusion bodies. Biochim. et Biophys. Acta Mol. Basis Dis. 2008;1782:764–774. doi: 10.1016/j.bbadis.2008.08.008. [DOI] [PubMed] [Google Scholar]
- 30.Shimizu T., Inoue K.-I., Hachiya H., Shibuya N., Aoki T., Kubota K. Accumulation of phosphorylated p62 is associated with NF-E2-related factor 2 activation in hepatocellular carcinoma. J. Hepato Biliary Pancreat. Sci. 2016;23:467–471. doi: 10.1002/jhbp.364. [DOI] [PubMed] [Google Scholar]
- 31.Valencia T., Kim J.Y., Abu-Baker S., Moscat-Pardos J., Ahn C.S., Reina-Campos M., Duran A., Castilla E.A., Metallo C.M., Diaz-Meco M.T., et al. Metabolic Reprogramming of Stromal Fibroblasts through p62-mTORC1 Signaling Promotes Inflammation and Tumorigenesis. Cancer Cell. 2014;26:121–135. doi: 10.1016/j.ccr.2014.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moscat J., Karin M., Diaz-Meco M.T. p62 in Cancer: Signaling Adaptor Beyond Autophagy. Cell. 2016;167:606–609. doi: 10.1016/j.cell.2016.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Durán A., Serrano M., Leitges M., Flores J.M., Picard S., Brown J.P., Moscat J., Diaz-Meco M.T. The Atypical PKC-Interacting Protein p62 Is an Important Mediator of RANK-Activated Osteoclastogenesis. Dev. Cell. 2004;6:303–309. doi: 10.1016/S1534-5807(03)00403-9. [DOI] [PubMed] [Google Scholar]
- 34.Rodriguez A., Durán A., Selloum M., Champy M.-F., Diez-Guerra F.J., Flores J.M., Serrano M., Auwerx J., Diaz-Meco M.T., Moscat J. Mature-onset obesity and insulin resistance in mice deficient in the signaling adapter p. Cell Metab. 2006;3:211–222. doi: 10.1016/j.cmet.2006.01.011. [DOI] [PubMed] [Google Scholar]
- 35.Harada H., Warabi E., Matsuki T., Yanagawa T., Okada K., Uwayama J., Ikeda A., Nakaso K., Kirii K., Noguchi N., et al. Deficiency of p62/Sequestosome 1 Causes Hyperphagia Due to Leptin Resistance in the Brain. J. Neurosci. 2013;33:14767–14777. doi: 10.1523/JNEUROSCI.2954-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lim D., Lee H.S., Ku B., Shin H.-C., Kim S.J. Oligomer Model of PB1 Domain of p62/SQSTM1 Based on Crystal Structure of Homo-Dimer and Calculation of Helical Characteristics. Mol. Cells. 2019;42:729–738. doi: 10.14348/molcells.2019.0096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pan J.-A., Sun Y., Jiang Y.-P., Bott A.J., Jaber N., Dou Z., Yang B., Chen J.-S., Catanzaro J.M., Du C., et al. TRIM21 Ubiquitylates SQSTM1/p62 and Suppresses Protein Sequestration to Regulate Redox Homeostasis. Mol. Cell. 2016;62:149–151. doi: 10.1016/j.molcel.2016.03.015. [DOI] [PubMed] [Google Scholar]
- 38.Lobb I.T., Morin P., Martin K., Thoms H.C., Wills J.C., Lleshi X., Olsen K.C., Duncan R.R., Stark L.A. A Role for the Autophagic Receptor, SQSTM1/p62, in Trafficking NF-kappaB/RelA to Nucleolar Aggresomes. Mol. Cancer Res. 2021;19:274–287. doi: 10.1158/1541-7786.MCR-20-0336. [DOI] [PubMed] [Google Scholar]
- 39.Pankiv S., Lamark T., Bruun J.-A., Øvervatn A., Bjørkøy G., Johansen T. Nucleocytoplasmic Shuttling of p62/SQSTM1 and Its Role in Recruitment of Nuclear Polyubiquitinated Proteins to Promyelocytic Leukemia Bodies. J. Biol. Chem. 2010;285:5941–5953. doi: 10.1074/jbc.M109.039925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Horos R., Buescher M., Kleinendorst R., Alleaume A.-M., Tarafder A., Schwarzl T., Dziuba D., Tischer C., Zielonka E.M., Adak A., et al. The Small Non-coding Vault RNA1-1 Acts as a Riboregulator of Autophagy. Cell. 2019;176:1054–1067. doi: 10.1016/j.cell.2019.01.030. [DOI] [PubMed] [Google Scholar]
- 41.Sanz L., Sanchez P., Lallena M.J., Diaz-Meco M.T., Moscat J. The interaction of p62 with RIP links the atypical PKCs to NF-kappaB activation. EMBO J. 1999;18:3044–3053. doi: 10.1093/emboj/18.11.3044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Moscat J., Diaz-Meco M.T. p62: A versatile multitasker takes on cancer. Trends Biochem. Sci. 2012;37:230–236. doi: 10.1016/j.tibs.2012.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Birgisdottir Å.B., Lamark T., Johansen T. The LIR motif—crucial for selective autophagy. J. Cell Sci. 2013;126:3237–3247. doi: 10.1242/jcs.126128. [DOI] [PubMed] [Google Scholar]
- 44.Khaminets A., Behl C., Dikic I. Ubiquitin-Dependent And Independent Signals In Selective Autophagy. Trends Cell Biol. 2016;26:6–16. doi: 10.1016/j.tcb.2015.08.010. [DOI] [PubMed] [Google Scholar]
- 45.Ichimura Y., Waguri S., Sou Y.-S., Kageyama S., Hasegawa J., Ishimura R., Saito T., Yang Y., Kouno T., Fukutomi T., et al. Phosphorylation of p62 Activates the Keap1-Nrf2 Pathway during Selective Autophagy. Mol. Cell. 2013;51:618–631. doi: 10.1016/j.molcel.2013.08.003. [DOI] [PubMed] [Google Scholar]
- 46.Hashimoto K., Simmons A.N., Kajino-Sakamoto R., Tsuji Y., Ninomiya-Tsuji J. TAK1 Regulates the Nrf2 Antioxidant System Through Modulating p62/SQSTM. Antioxid. Redox Signal. 2016;25:953–964. doi: 10.1089/ars.2016.6663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jiang X., Bao Y., Liu H., Kou X., Zhang Z., Sun F., Qian Z.M., Lin Z., Li X., Liu X., et al. VPS34 stimulation of p62 phosphorylation for cancer progression. Oncogene. 2017;36:6850–6862. doi: 10.1038/onc.2017.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Watanabe Y., Tsujimura A., Taguchi K., Tanaka M. HSF1 stress response pathway regulates autophagy receptor SQSTM1/p62-associated proteostasis. Autophagy. 2017;13:133–148. doi: 10.1080/15548627.2016.1248018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ichimura Y., Komatsu M. Activation of p62/SQSTM1–Keap1–Nuclear Factor Erythroid 2-Related Factor 2 Pathway in Cancer. Front. Oncol. 2018;8:210. doi: 10.3389/fonc.2018.00210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ueno T., Komatsu M. Autophagy in the liver: Functions in health and disease. Nat. Rev. Gastroenterol. Hepatol. 2017;14:170–184. doi: 10.1038/nrgastro.2016.185. [DOI] [PubMed] [Google Scholar]
- 51.Kageyama S., Saito T., Obata M., Koide R.-H., Ichimura Y., Komatsu M. Negative Regulation of the Keap1-Nrf2 Pathway by a p62/Sqstm1 Splicing Variant. Mol. Cell. Biol. 2018;38 doi: 10.1128/MCB.00642-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.You Z., Jiang W.-X., Qin L.-Y., Gong Z., Wan W., Li J., Wang Y., Zhang H., Peng C., Zhou T., et al. Requirement for p62 acetylation in the aggregation of ubiquitylated proteins under nutrient stress. Nat. Commun. 2019;10:1–14. doi: 10.1038/s41467-019-13718-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kageyama S., Gudmundsson S.R., Sou Y.S., Ichimura Y., Tamura N., Kazuno S., Ueno T., Miura Y., Noshiro D., Abe M., et al. p62/SQSTM1-droplet serves as a platform for autophagosome formation and anti-oxidative stress re-sponse. Nat. Commun. 2021;12:16. doi: 10.1038/s41467-020-20185-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sanchez-Garrido J., Sancho-Shimizu V., Shenoy A.R. Regulated proteolysis of p62/SQSTM1 enables differential control of autophagy and nutrient sensing. Sci. Signal. 2018;11:eaat6903. doi: 10.1126/scisignal.aat6903. [DOI] [PubMed] [Google Scholar]
- 55.Jamilloux Y., Lagrange B., Di Micco A., Bourdonnay E., Provost A., Tallant R., Henry T., Martinon F. A proximity-dependent biotinylation (BioID) approach flags the p62/sequestosome-1 protein as a caspase-1 substrate. J. Biol. Chem. 2018;293:12563–12575. doi: 10.1074/jbc.RA117.000435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Carroll B., Otten E.G., Manni D., Stefanatos R., Menzies F.M., Smith G.R., Jurk D., Kenneth N., Wilkinson S., Passos J.F., et al. Oxidation of SQSTM1/p62 mediates the link between redox state and protein homeostasis. Nat. Commun. 2018;9:1–11. doi: 10.1038/s41467-017-02746-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mizushima N., Komatsu M. Autophagy: Renovation of Cells and Tissues. Cell. 2011;147:728–741. doi: 10.1016/j.cell.2011.10.026. [DOI] [PubMed] [Google Scholar]
- 58.Medzhitov R. Origin and physiological roles of inflammation. Nature. 2008;454:428–435. doi: 10.1038/nature07201. [DOI] [PubMed] [Google Scholar]
- 59.Mantovani A., Allavena P., Sica A., Balkwill F.R. Cancer-related inflammation. Nat. Cell Biol. 2008;454:436–444. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- 60.Matsuzawa-Ishimoto Y., Hwang S., Cadwell K. Autophagy and Inflammation. Annu. Rev. Immunol. 2018;36:73–101. doi: 10.1146/annurev-immunol-042617-053253. [DOI] [PubMed] [Google Scholar]
- 61.Sharma V., Verma S., Seranova E., Sarkar S., Kumar D. Selective Autophagy and Xenophagy in Infection and Disease. Front. Cell Dev. Biol. 2018;6:147. doi: 10.3389/fcell.2018.00147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Saitoh T., Fujita N., Jang M.H., Uematsu S., Yang B.G., Satoh T., Omori H., Noda T., Yamamoto N., Komatsu M., et al. Loss of the autophagy protein Atg16L1 enhances endotoxin-induced IL-1beta production. Nature. 2008;45:264–268. doi: 10.1038/nature07383. [DOI] [PubMed] [Google Scholar]
- 63.Gammoh N. The multifaceted functions of ATG16L1 in autophagy and related processes. J. Cell Sci. 2020;133:249227. doi: 10.1242/jcs.249227. [DOI] [PubMed] [Google Scholar]
- 64.Dikic I., Elazar Z. Mechanism and medical implications of mammalian autophagy. Nat. Rev. Mol. Cell Biol. 2018;19:349–364. doi: 10.1038/s41580-018-0003-4. [DOI] [PubMed] [Google Scholar]
- 65.Shin W.H., Park J.H., Chung K.C. The central regulator p62 between ubiquitin proteasome system and autophagy and its role in the mitophagy and Parkinson’s disease. BMB Rep. 2020;53:56–63. doi: 10.5483/BMBRep.2020.53.1.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Babu J.R., Geetha T., Wooten M.W. Sequestosome 1/p62 shuttles polyubiquitinated tau for proteasomal degradation. J. Neurochem. 2005;94:192–203. doi: 10.1111/j.1471-4159.2005.03181.x. [DOI] [PubMed] [Google Scholar]
- 67.Dikic I. Proteasomal and Autophagic Degradation Systems. Annu. Rev. Biochem. 2017;86:193–224. doi: 10.1146/annurev-biochem-061516-044908. [DOI] [PubMed] [Google Scholar]
- 68.Zheng Y.T., Shahnazari S., Brech A., Lamark T., Johansen T., Brumell J.H. The Adaptor Protein p62/SQSTM1 Targets Invading Bacteria to the Autophagy Pathway. J. Immunol. 2009;183:5909–5916. doi: 10.4049/jimmunol.0900441. [DOI] [PubMed] [Google Scholar]
- 69.Mostowy S., Sancho-Shimizu V., Hamon M., Simeone R., Brosch R., Johansen T., Cossart P. p62 and NDP52 Proteins Target Intracytosolic Shigella and Listeria to Different Autophagy Pathways. J. Biol. Chem. 2011;286:26987–26995. doi: 10.1074/jbc.M111.223610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Franco L.H., Nair V.R., Scharn C.R., Xavier R.J., Torrealba J.R., Shiloh M.U., Levine B. The Ubiquitin Ligase Smurf1 Functions in Selective Autophagy of Mycobacterium tuberculosis and An-ti-tuberculous Host Defense. Cell Host Microbe. 2017;22:421–423. doi: 10.1016/j.chom.2017.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Long J., Garner T.P., Pandya M.J., Craven C.J., Chen P., Shaw B., Williamson M.P., Layfield R., Searle M.S. Dimerisation of the UBA domain of p62 inhibits ubiquitin binding and regulates NF-kappaB signalling. J. Mol. Biol. 2010;396:178–194. doi: 10.1016/j.jmb.2009.11.032. [DOI] [PubMed] [Google Scholar]
- 72.Lim J., Lachenmayer M.L., Wu S., Liu W., Kundu M., Wang R., Komatsu M., Oh Y.J., Zhao Y., Yue Z. Proteotoxic Stress Induces Phosphorylation of p62/SQSTM1 by ULK1 to Regulate Selective Autophagic Clearance of Protein Aggregates. PLoS Genet. 2015;11:e1004987. doi: 10.1371/journal.pgen.1004987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Matsumoto G., Shimogori T., Hattori N., Nukina N. TBK1 controls autophagosomal engulfment of polyubiquitinated mitochondria through p62/SQSTM1 phosphorylation. Hum. Mol. Genet. 2015;24:4429–4442. doi: 10.1093/hmg/ddv179. [DOI] [PubMed] [Google Scholar]
- 74.Matsumoto G., Wada K., Okuno M., Kurosawa M., Nukina N. Serine 403 Phosphorylation of p62/SQSTM1 Regulates Selective Autophagic Clearance of Ubiquitinated Proteins. Mol. Cell. 2011;44:279–289. doi: 10.1016/j.molcel.2011.07.039. [DOI] [PubMed] [Google Scholar]
- 75.Pilli M., Arko-Mensah J., Ponpuak M., Roberts E., Master S., Mandell M.A., Dupont N., Ornatowski W., Jiang S., Bradfute S.B., et al. TBK-1 Promotes Autophagy-Mediated Antimicrobial Defense by Controlling Autophagosome Maturation. Immunity. 2012;37:223–234. doi: 10.1016/j.immuni.2012.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bjørkøy G., Lamark T., Pankiv S., Øvervatn A., Brech A., Johansen T. Chapter 12 Monitoring Autophagic Degradation of p62/SQSTM. Methods Enzymol. 2009;452:181–197. doi: 10.1016/s0076-6879(08)03612-4. [DOI] [PubMed] [Google Scholar]
- 77.Jiang P., Mizushima N. LC3- and p62-based biochemical methods for the analysis of autophagy progression in mammalian cells. Methods. 2015;75:13–18. doi: 10.1016/j.ymeth.2014.11.021. [DOI] [PubMed] [Google Scholar]
- 78.Morgan M.J., Thorburn A. Measuring Autophagy in the Context of Cancer. Adv. Exp. Med. Biol. 2016;899:121–143. doi: 10.1007/978-3-319-26666-4_8. [DOI] [PubMed] [Google Scholar]
- 79.Jiang T., Harder B., de la Vega M.R., Wong P.K., Chapman E., Zhang D.D. p62 links autophagy and Nrf2 signaling. Free. Radic. Biol. Med. 2015;88:199–204. doi: 10.1016/j.freeradbiomed.2015.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Strowig T., Henao-Mejia J., Elinav E., Flavell R.A. Inflammasomes in health and disease. Nat. Cell Biol. 2012;481:278–286. doi: 10.1038/nature10759. [DOI] [PubMed] [Google Scholar]
- 81.Broz P., Dixit V.M. Inflammasomes: Mechanism of assembly, regulation and signalling. Nat. Rev. Immunol. 2016;16:407–420. doi: 10.1038/nri.2016.58. [DOI] [PubMed] [Google Scholar]
- 82.Guo H., Callaway J.B., Ting J.P.Y. Inflammasomes: Mechanism of action, role in disease, and therapeutics. Nat. Med. 2015;21:677–687. doi: 10.1038/nm.3893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sun Q., Fan J., Billiar T.R., Scott M.J. Inflammasome and Autophagy Regulation: A Two-way Street. Mol. Med. 2017;23:188–195. doi: 10.2119/molmed.2017.00077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Takahama M., Akira S., Saitoh T. Autophagy limits activation of the inflammasomes. Immunol. Rev. 2018;281:62–73. doi: 10.1111/imr.12613. [DOI] [PubMed] [Google Scholar]
- 85.Vanaja S.K., Rathinam V.A., Fitzgerald K.A. Mechanisms of inflammasome activation: Recent advances and novel insights. Trends Cell Biol. 2015;25:308–315. doi: 10.1016/j.tcb.2014.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.He Y., Hara H., Núñez G. Mechanism and Regulation of NLRP3 Inflammasome Activation. Trends Biochem. Sci. 2016;41:1012–1021. doi: 10.1016/j.tibs.2016.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Mangan M.S.J., Olhava E.J., Roush W.R., Seidel H.M., Glick G.D., Latz E. Targeting the NLRP3 inflammasome in inflammatory diseases. Nat. Rev. Drug Discov. 2018;17:588–606. doi: 10.1038/nrd.2018.97. [DOI] [PubMed] [Google Scholar]
- 88.Broz P., Pelegrín P., Shao F. The gasdermins, a protein family executing cell death and inflammation. Nat. Rev. Immunol. 2020;20:143–157. doi: 10.1038/s41577-019-0228-2. [DOI] [PubMed] [Google Scholar]
- 89.Lugrin J., Martinon F. The AIM2 inflammasome: Sensor of pathogens and cellular perturbations. Immunol. Rev. 2017;281:99–114. doi: 10.1111/imr.12618. [DOI] [PubMed] [Google Scholar]
- 90.Zhong Z., Umemura A., Sanchez-Lopez E., Liang S., Shalapour S., Wong J., He F., Boassa D., Perkins G., Ali S.R., et al. NF-kappaB Restricts Inflammasome Activation via Elimination of Damaged Mitochondria. Cell. 2016;164:896–910. doi: 10.1016/j.cell.2015.12.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Yan Y., Jiang W., Liu L., Wang X., Ding C., Tian Z., Zhou R. Dopamine Controls Systemic Inflammation through Inhibition of NLRP3 Inflammasome. Cell. 2015;160:62–73. doi: 10.1016/j.cell.2014.11.047. [DOI] [PubMed] [Google Scholar]
- 92.Mehto S., Jena K.K., Nath P., Chauhan S., Kolapalli S.P., Das S.K., Sahoo P.K., Jain A., Taylor G.A., Chauhan S. The Crohn’s Disease Risk Factor IRGM Limits NLRP3 Inflammasome Activation by Impeding Its Assembly and by Mediating Its Selective Autophagy. Mol. Cell. 2019;73:429–445. doi: 10.1016/j.molcel.2018.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kimura T., Jain A., Choi S.W., Mandell M.A., Schroder K., Johansen T., Deretic V. TRIM-mediated precision autophagy targets cytoplasmic regulators of innate immunity. J. Cell Biol. 2015;210:973–989. doi: 10.1083/jcb.201503023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Liu T., Tang Q., Liu K., Xie W., Liu X., Wang H., Wang R.-F., Cui J. TRIM11 Suppresses AIM2 Inflammasome by Degrading AIM2 via p62-Dependent Selective Autophagy. Cell Rep. 2016;16:1988–2002. doi: 10.1016/j.celrep.2016.07.019. [DOI] [PubMed] [Google Scholar]
- 95.Biasizzo M., Kopitar-Jerala N. Interplay Between NLRP3 Inflammasome and Autophagy. Front. Immunol. 2020;11:591803. doi: 10.3389/fimmu.2020.591803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lazarou M., Sliter D.A., Kane L.A., Sarraf S.A., Wang C., Burman J.L., Sideris D.P., Fogel A.I., Youle R.J. The ubiquitin kinase PINK1 recruits autophagy receptors to induce mitophagy. Nature. 2015;524:309–314. doi: 10.1038/nature14893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Pickrell A.M., Youle R.J. The Roles of PINK1, Parkin, and Mitochondrial Fidelity in Parkinson’s Disease. Neuron. 2015;85:257–273. doi: 10.1016/j.neuron.2014.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kahns S., Kalai M., Jakobsen L.D., Clark B.F.C., Vandenabeele P., Jensen P.H. Caspase-1 and Caspase-8 Cleave and Inactivate Cellular Parkin. J. Biol. Chem. 2003;278:23376–23380. doi: 10.1074/jbc.M300495200. [DOI] [PubMed] [Google Scholar]
- 99.Yu J., Nagasu H., Murakami T., Hoang H., Broderick L., Hoffman H.M., Horng T. Inflammasome activation leads to Caspase-1-dependent mitochondrial damage and block of mitophagy. Proc. Natl. Acad. Sci. USA. 2014;111:15514–15519. doi: 10.1073/pnas.1414859111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Shin J.N., Fattah E.A., Bhattacharya A., Ko S., Eissa N.T. Inflammasome activation by altered proteostasis. J. Biol. Chem. 2013;288:35886–35895. doi: 10.1074/jbc.M113.514919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Shi C.S., Shenderov K., Huang N.N., Kabat J., Abu-Asab M., Fitzgerald K.A., Sher A., Kehrl J.H. Activation of autophagy by inflammatory signals limits IL-1beta production by targeting ubiquitinated in-flammasomes for destruction. Nat. Immunol. 2012;13:255–263. doi: 10.1038/ni.2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Song H., Liu B., Huai W., Yu Z., Wang W., Zhao J., Han L., Jiang G., Zhang L., Gao C., et al. The E3 ubiquitin ligase TRIM31 attenuates NLRP3 inflammasome activation by promoting proteasomal deg-radation of NLRP. Nat. Commun. 2016;7:13727. doi: 10.1038/ncomms13727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Zhou Z., Zhu X., Yin R., Liu T., Yang S., Zhou L., Pan X., Ma A. K63 ubiquitin chains target NLRP3 inflammasome for autophagic degradation in ox-LDL-stimulated THP-1 macrophages. Aging. 2020;12:1747–1759. doi: 10.18632/aging.102710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Harris J., Hartman M., Roche C., Zeng S.G., O’Shea A., Sharp F.A., Lambe E.M., Creagh E.M., Golenbock D.T., Tschopp J., et al. Autophagy controls IL-1{beta} secretion by targeting pro-IL-1{beta} for degradation. J. Biol. Chem. 2011;286:9587–9597. doi: 10.1074/jbc.M110.202911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ainscough J.S., Gerberick G.F., Zahedi-Nejad M., Lopez-Castejon G., Brough D., Kimber I., Dearman R.J. Dendritic cell IL-1alpha and IL-1beta are polyubiquitinated and degraded by the proteasome. J. Biol. Chem. 2014;289:35582–35592. doi: 10.1074/jbc.M114.595686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kim J., Gee H.Y., Lee M.G. Unconventional protein secretion—New insights into the pathogenesis and therapeutic targets of human diseases. J. Cell Sci. 2018;131:jcs213686. doi: 10.1242/jcs.213686. [DOI] [PubMed] [Google Scholar]
- 107.Munding C., Keller M., Niklaus G., Papin S., Tschopp J., Werner S., Beer H.D. The estrogen-responsive B box protein: A novel enhancer of interleukin-1beta secretion. Cell Death Differ. 2006;13:1938–1949. doi: 10.1038/sj.cdd.4401896. [DOI] [PubMed] [Google Scholar]
- 108.Kimura T., Jia J., Kumar S., Choi S.W., Gu Y., Mudd M., Dupont N., Jiang S., Peters R., Farzam F., et al. Dedicated SNARE s and specialized TRIM cargo receptors mediate secretory autophagy. EMBO J. 2017;36:42–60. doi: 10.15252/embj.201695081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Claude-Taupin A., Bissa B., Jia J., Gu Y., Deretic V. Role of autophagy in IL-1beta export and release from cells. Semin. Cell Dev. Biol. 2018;83:36–41. doi: 10.1016/j.semcdb.2018.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Suzuki T., Yamamoto M. Molecular basis of the Keap1–Nrf2 system. Free. Radic. Biol. Med. 2015;88:93–100. doi: 10.1016/j.freeradbiomed.2015.06.006. [DOI] [PubMed] [Google Scholar]
- 111.Suzuki T., Yamamoto M. Stress-sensing mechanisms and the physiological roles of the Keap1–Nrf2 system during cellular stress. J. Biol. Chem. 2017;292:16817–16824. doi: 10.1074/jbc.R117.800169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Tonelli C., Chio I.I.C., Tuveson D.A. Transcriptional Regulation by Nrf. Antioxid. Redox Signal. 2018;29:1727–1745. doi: 10.1089/ars.2017.7342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Federico A., Morgillo F., Tuccillo C., Ciardiello F., Loguercio C. Chronic inflammation and oxidative stress in human carcinogenesis. Int. J. Cancer. 2007;121:2381–2386. doi: 10.1002/ijc.23192. [DOI] [PubMed] [Google Scholar]
- 114.Hensley K., Robinson K.A., Gabbita S., Salsman S., Floyd R.A. Reactive oxygen species, cell signaling, and cell injury. Free. Radic. Biol. Med. 2000;28:1456–1462. doi: 10.1016/S0891-5849(00)00252-5. [DOI] [PubMed] [Google Scholar]
- 115.Tu W., Wang H., Li S., Liu Q., Sha H. The Anti-Inflammatory and Anti-Oxidant Mechanisms of the Keap1/Nrf2/ARE Signaling Pathway in Chronic Diseases. Aging Dis. 2019;10:637–651. doi: 10.14336/AD.2018.0513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Dodson M., Zhang D.D. Non-Canonical Activation of NRF2: New Insights and Its Relevance to Disease. Curr. Pathobiol. Rep. 2017;5:171–176. doi: 10.1007/s40139-017-0131-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Hennig P., Fenini G., Di Filippo M., Beer H.-D. Electrophiles against (Skin) Diseases: More Than Nrf. Biomolecules. 2020;10:271. doi: 10.3390/biom10020271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Hennig P., Garstkiewicz M., Grossi S., Di Filippo M., French L.E., Beer H.-D. The Crosstalk between Nrf2 and Inflammasomes. Int. J. Mol. Sci. 2018;19:562. doi: 10.3390/ijms19020562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.McEligot A.J., Yang S., Meyskens J.F.L. Redox regulation by intrinsic species and extrinsic nutrients in normal and cancer cells. Annu. Rev. Nutr. 2005;25:261–295. doi: 10.1146/annurev.nutr.25.050304.092633. [DOI] [PubMed] [Google Scholar]
- 120.Mitsuishi Y., Taguchi K., Kawatani Y., Shibata T., Nukiwa T., Aburatani H., Yamamoto M., Motohashi H. Nrf2 Redirects Glucose and Glutamine into Anabolic Pathways in Metabolic Reprogramming. Cancer Cell. 2012;22:66–79. doi: 10.1016/j.ccr.2012.05.016. [DOI] [PubMed] [Google Scholar]
- 121.Kobayashi E.H., Suzuki T., Funayama R., Nagashima T., Hayashi M., Sekine H., Tanaka N., Moriguchi T., Motohashi H., Nakayama K., et al. Nrf2 suppresses macrophage inflammatory response by blocking proinflammatory cytokine transcrip-tion. Nat. Commun. 2016;7:11624. doi: 10.1038/ncomms11624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Ma Q. Role of Nrf2 in Oxidative Stress and Toxicity. Annu. Rev. Pharmacol. Toxicol. 2013;53:401–426. doi: 10.1146/annurev-pharmtox-011112-140320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Kansanen E., Kuosmanen S.M., Leinonen H., Levonen A.-L. The Keap1-Nrf2 pathway: Mechanisms of activation and dysregulation in cancer. Redox Biol. 2013;1:45–49. doi: 10.1016/j.redox.2012.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Taguchi K., Fujikawa N., Komatsu M., Ishii T., Unno M., Akaike T., Motohashi H., Yamamoto M. Keap1 degradation by autophagy for the maintenance of redox homeostasis. Proc. Natl. Acad. Sci. USA. 2012;109:13561–13566. doi: 10.1073/pnas.1121572109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Cloer E.W., Siesser P.F., Cousins E.M., Goldfarb D., Mowrey D.D., Harrison J., Weir S.J., Dokholyan N.V., Major M.B. p62-Dependent Phase Separation of Patient-Derived KEAP1 Mutations and NRF. Mol. Cell. Biol. 2018;38 doi: 10.1128/mcb.00644-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Lee Y., Chou T.F., Pittman S.K., Keith A.L., Razani B., Weihl C.C. Keap1/Cullin3 Modulates p62/SQSTM1 Activity via UBA Domain Ubiquitination. Cell Rep. 2017;20:1994. doi: 10.1016/j.celrep.2017.08.019. [DOI] [PubMed] [Google Scholar]
- 127.Suzuki T., Motohashi H., Yamamoto M. Toward clinical application of the Keap1–Nrf2 pathway. Trends Pharmacol. Sci. 2013;34:340–346. doi: 10.1016/j.tips.2013.04.005. [DOI] [PubMed] [Google Scholar]
- 128.Laplante M., Sabatini D.M. mTOR signaling at a glance. J. Cell Sci. 2009;122:3589–3594. doi: 10.1242/jcs.051011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Saxton R.A., Sabatini D.M. mTOR Signaling in Growth, Metabolism, and Disease. Cell. 2017;169:361–371. doi: 10.1016/j.cell.2017.03.035. [DOI] [PubMed] [Google Scholar]
- 130.Popova N.V., Jücker M. The Role of mTOR Signaling as a Therapeutic Target in Cancer. Int. J. Mol. Sci. 2021;22:1743. doi: 10.3390/ijms22041743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Weichhart T., Hengstschläger M., Linke M. Regulation of innate immune cell function by mTOR. Nat. Rev. Immunol. 2015;15:599–614. doi: 10.1038/nri3901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Kim J., Kundu M., Viollet B., Guan K.L. AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk. Nat. Cell Biol. 2011;13:132–141. doi: 10.1038/ncb2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Duran A., Amanchy R., Linares J.F., Joshi J., Abu-Baker S., Porollo A., Hansen M., Moscat J., Diaz-Meco M.T. p62 Is a Key Regulator of Nutrient Sensing in the mTORC1 Pathway. Mol. Cell. 2011;44:134–146. doi: 10.1016/j.molcel.2011.06.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Linares J.F., Duran A., Reina-Campos M., Aza-Blanc P., Campos A., Moscat J., Diaz-Meco M.T. Amino Acid Activation of mTORC1 by a PB1-Domain-Driven Kinase Complex Cascade. Cell Rep. 2015;12:1339–1352. doi: 10.1016/j.celrep.2015.07.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Linares J.F., Duran A., Yajima T., Pasparakis M., Moscat J., Diaz-Meco M.T. K63 Polyubiquitination and Activation of mTOR by the p62-TRAF6 Complex in Nutrient-Activated Cells. Mol. Cell. 2013;51:283–296. doi: 10.1016/j.molcel.2013.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Vallabhapurapu S., Karin M. Regulation and function of NF-kappaB transcription factors in the immune system. Annu. Rev. Immunol. 2009;27:693–733. doi: 10.1146/annurev.immunol.021908.132641. [DOI] [PubMed] [Google Scholar]
- 137.Zhang Q., Lenardo M.J., Baltimore D. 30 Years of NF-kappaB: A Blossoming of Relevance to Human Pathobiology. Cell. 2009;168:37–57. doi: 10.1016/j.cell.2016.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Grivennikov S.I., Greten F., Karin M. Immunity, Inflammation, and Cancer. Cell. 2010;140:883–899. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Taniguchi K., Karin M. NF-κB, inflammation, immunity and cancer: Coming of age. Nat. Rev. Immunol. 2018;18:309–324. doi: 10.1038/nri.2017.142. [DOI] [PubMed] [Google Scholar]
- 140.Zotti T., Scudiero I., Settembre P., Ferravante A., Mazzone P., D’Andrea L., Reale C., Vito P., Stilo R. TRAF6-mediated ubiquitination of NEMO requires p62/sequestosome-1. Mol. Immunol. 2014;58:27–31. doi: 10.1016/j.molimm.2013.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Shembade N., Harhaj E.W. Regulation of NF-kappaB signaling by the A20 deubiquitinase. Cell. Mol. Immunol. 2012;9:123–130. doi: 10.1038/cmi.2011.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Kanayama M., Inoue M., Danzaki K., Hammer G., He Y.W., Shinohara M.L. Autophagy enhances NFkappaB activity in specific tissue macrophages by sequestering A20 to boost antifungal immunity. Nat. Commun. 2015;6:5779. doi: 10.1038/ncomms6779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Liu T., Zhang L., Joo D., Sun S.C. NF-kappaB signaling in inflammation. Signal Transduct. Target. Ther. 2017;2:1–9. doi: 10.1038/sigtrans.2017.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Greten F.R., Arkan M.C., Bollrath J., Hsu L.C., Goode J., Miething C., Göktuna S.I., Neuenhahn M., Fierer J., Paxian S., et al. NF-kappaB is a negative regulator of IL-1beta secretion as revealed by genetic and pharmacological inhi-bition of IKKbeta. Cell. 2007;130:918–931. doi: 10.1016/j.cell.2007.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Bartolini D., Dallaglio K., Torquato P., Piroddi M., Galli F. Nrf2-p62 autophagy pathway and its response to oxidative stress in hepatocellular carcinoma. Transl. Res. 2018;193:54–71. doi: 10.1016/j.trsl.2017.11.007. [DOI] [PubMed] [Google Scholar]
- 146.Cui J., Gong Z., Shen H.-M. The role of autophagy in liver cancer: Molecular mechanisms and potential therapeutic targets. Biochim. Biophys. Acta Bioenerg. 2013;1836:15–26. doi: 10.1016/j.bbcan.2013.02.003. [DOI] [PubMed] [Google Scholar]
- 147.Ho D.W.-H., Lo C.L.R., Chan L.-K., Ng I.O.-L. Molecular Pathogenesis of Hepatocellular Carcinoma. Liver Cancer. 2016;5:290–302. doi: 10.1159/000449340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Yu S., Wang Y., Jing L., Claret F., Li Q., Tian T., Liang X., Ruan Z., Jiang L., Yao Y., et al. Autophagy in the “inflammation-carcinogenesis” pathway of liver and HCC immunotherapy. Cancer Lett. 2017;411:82–89. doi: 10.1016/j.canlet.2017.09.049. [DOI] [PubMed] [Google Scholar]
- 149.Ding Z.-B., Shi Y.-H., Zhou J., Qiu S.-J., Xu Y., Dai Z., Shi G.-M., Wang X.-Y., Ke A.-W., Wu B., et al. Association of Autophagy Defect with a Malignant Phenotype and Poor Prognosis of Hepatocellular Carcinoma. Cancer Res. 2008;68:9167–9175. doi: 10.1158/0008-5472.CAN-08-1573. [DOI] [PubMed] [Google Scholar]
- 150.Osman N.A.A., El-Rehim D.M.A., Kamal I.M. Defective Beclin-1 and elevated hypoxia-inducible factor (HIF)-1α expression are closely linked to tumorigenesis, differentiation, and progression of hepatocellular carcinoma. Tumor Biol. 2015;36:4293–4299. doi: 10.1007/s13277-015-3068-0. [DOI] [PubMed] [Google Scholar]
- 151.Levine B., Kroemer G. Autophagy in the Pathogenesis of Disease. Cell. 2008;132:27–42. doi: 10.1016/j.cell.2007.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Bao L., Chandra P.K., Moroz K., Zhang X., Thung S.N., Wu T., Dash S. Impaired autophagy response in human hepatocellular carcinoma. Exp. Mol. Pathol. 2014;96:149–154. doi: 10.1016/j.yexmp.2013.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Zatloukal K., Stumptner C., Fuchsbichler A., Heid H., Schnoelzer M., Kenner L., Kleinert R., Prinz M., Aguzzi A., Denk H. p62 Is a Common Component of Cytoplasmic Inclusions in Protein Aggregation Diseases. Am. J. Pathol. 2002;160:255–263. doi: 10.1016/S0002-9440(10)64369-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Hayes J.D., McMahon M. NRF2 and KEAP1 mutations: Permanent activation of an adaptive response in cancer. Trends Biochem. Sci. 2009;34:176–188. doi: 10.1016/j.tibs.2008.12.008. [DOI] [PubMed] [Google Scholar]
- 155.Wu D.-H., Jia C.-C., Chen J., Lin Z.-X., Ruan D.-Y., Li X., Lin Q., Dong M.-, Ma X.-K., Wan X.-B., et al. Autophagic LC3B overexpression correlates with malignant progression and predicts a poor prognosis in hepatocellular carcinoma. Tumor Biol. 2014;35:12225–12233. doi: 10.1007/s13277-014-2531-7. [DOI] [PubMed] [Google Scholar]
- 156.Xu H., Yu H., Zhang X., Shen X., Zhang K., Sheng H., Dai S., Gao H. UNC51-like kinase 1 as a potential prognostic biomarker for hepatocellular carcinoma. Int. J. Clin. Exp. Pathol. 2013;6:711–717. [PMC free article] [PubMed] [Google Scholar]
- 157.Llovet J.M., Ricci S., Mazzaferro V., Hilgard P., Gane E., Blanc J.F., De Oliveira A.C., Santoro A., Raoul J.L., Forner A., et al. Sorafenib in Advanced Hepatocellular Carcinoma. N. Engl. J. Med. 2008;359:378–390. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 158.Shimizu S., Takehara T., Hikita H., Kodama T., Tsunematsu H., Miyagi T., Hosui A., Ishida H., Tatsumi T., Kanto T., et al. Inhibition of autophagy potentiates the antitumor effect of the multikinase inhibitor sorafenib in hepato-cellular carcinoma. Int. J. Cancer. 2012;131:548–557. doi: 10.1002/ijc.26374. [DOI] [PubMed] [Google Scholar]
- 159.Decaens T., Luciani A., Itti E., Hulin A., Roudot-Thoraval F., Laurent A., Zafrani E.S., Mallat A., Duvoux C. Phase II study of sirolimus in treatment-naive patients with advanced hepatocellular carcinoma. Dig. Liver Dis. 2012;44:610–616. doi: 10.1016/j.dld.2012.02.005. [DOI] [PubMed] [Google Scholar]
- 160.Takamura A., Komatsu M., Hara T., Sakamoto A., Kishi C., Waguri S., Eishi Y., Hino O., Tanaka K., Mizushima N. Autophagy-deficient mice develop multiple liver tumors. Genes Dev. 2011;25:795–800. doi: 10.1101/gad.2016211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Inami Y., Waguri S., Sakamoto A., Kouno T., Nakada K., Hino O., Watanabe S., Ando J., Iwadate M., Yamamoto M., et al. Persistent activation of Nrf2 through p62 in hepatocellular carcinoma cells. J. Cell Biol. 2011;193:275–284. doi: 10.1083/jcb.201102031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Qu X., Yu J., Bhagat G., Furuya N., Hibshoosh H., Troxel A., Rosen J., Eskelinen E.-L., Mizushima N., Ohsumi Y., et al. Promotion of tumorigenesis by heterozygous disruption of the beclin 1 autophagy gene. J. Clin. Investig. 2003;112:1809–1820. doi: 10.1172/JCI20039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Yue Z., Jin S., Yang C., Levine A.J., Heintz N. Beclin 1, an autophagy gene essential for early embryonic development, is a haploinsufficient tumor suppressor. Proc. Natl. Acad. Sci. USA. 2003;100:15077–15082. doi: 10.1073/pnas.2436255100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Ni H.-M., Woolbright B.L., Williams J., Copple B., Cui W., Luyendyk J.P., Jaeschke H., Ding W.-X. Nrf2 promotes the development of fibrosis and tumorigenesis in mice with defective hepatic autophagy. J. Hepatol. 2014;61:617–625. doi: 10.1016/j.jhep.2014.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Maeda S., Kamata H., Luo J.-L., Leffert H., Karin M. IKKbeta couples hepatocyte death to cytokine-driven compensatory proliferation that promotes chemical hepatocarcinogenesis. Cell. 2005;121:977–990. doi: 10.1016/j.cell.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 166.Seki E., Brenner D.A. The role of NF-kappaB in hepatocarcinogenesis: Promoter or suppressor? J. Hepatol. 2007;47:307–309. doi: 10.1016/j.jhep.2007.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Taniguchi K., Yamachika S., He F., Karin M. p62/SQSTM1-Dr. Jekyll and Mr. Hyde that prevents oxidative stress but promotes liver cancer. FEBS Lett. 2016;590:2375–2397. doi: 10.1002/1873-3468.12301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Kongara S., Karantza V. The interplay between autophagy and ROS in tumorigenesis. Front. Oncol. 2012;2:171. doi: 10.3389/fonc.2012.00171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Amaravadi R., Kimmelman A.C., White E. Recent insights into the function of autophagy in cancer. Genes Dev. 2016;30:1913–1930. doi: 10.1101/gad.287524.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Sun K., Guo X.-L., Zhao Q.-D., Jing Y.-Y., Kou X.-R., Xie X.-Q., Zhou Y., Cai N., Gao L., Zhao X., et al. Paradoxical role of autophagy in the dysplastic and tumor-forming stages of hepatocarcinoma development in rats. Cell Death Dis. 2013;4:e501. doi: 10.1038/cddis.2013.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Tao M., Liu T., You Q., Jiang Z. p62 as a therapeutic target for tumor. Eur. J. Med. Chem. 2020;193:112231. doi: 10.1016/j.ejmech.2020.112231. [DOI] [PubMed] [Google Scholar]
- 172.Yu F., Ma R., Liu C., Zhang L., Feng K., Wang M., Yin D. SQSTM1/p62 Promotes Cell Growth and Triggers Autophagy in Papillary Thyroid Cancer by Regulating the AKT/AMPK/mTOR Signaling Pathway. Front. Oncol. 2021;11:638701. doi: 10.3389/fonc.2021.638701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Inoue D., Suzuki T., Mitsuishi Y., Miki Y., Suzuki S., Sugawara S., Watanabe M., Sakurada A., Endo C., Uruno A., et al. Accumulation of p62/SQSTM1 is associated with poor prognosis in patients with lung adenocarcinoma. Cancer Sci. 2012;103:760–766. doi: 10.1111/j.1349-7006.2012.02216.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Lei C., Zhao B., Liu L., Zeng X., Yu Z., Wang X. Expression and clinical significance of p62 protein in colon cancer. Medicine. 2020;99:e18791. doi: 10.1097/MD.0000000000018791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Kuo W.L., Sharifi M.N., Lingen M.W., Ahmed O., Liu J., Nagilla M., Macleod K.F., Cohen E.E. p62/SQSTM1 accumulation in squamous cell carcinoma of head and neck predicts sensitivity to phosphati-dylinositol 3-kinase pathway inhibitors. PLoS ONE. 2014;9:e90171. doi: 10.1371/journal.pone.0090171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Mohamed A., Ayman A., Deniece J., Wang T., Kovach C., Siddiqui M.T., Cohen C. P62/Ubiquitin IHC Expression Correlated with Clinicopathologic Parameters and Outcome in Gastroin-testinal Carcinomas. Front. Oncol. 2015;5:70. doi: 10.3389/fonc.2015.00070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Li L., Shen C., Nakamura E., Ando K., Signoretti S., Beroukhim R., Cowley G.S., Lizotte P., Liberzon E., Bair S., et al. SQSTM1 Is a Pathogenic Target of 5q Copy Number Gains in Kidney Cancer. Cancer Cell. 2013;24:738–750. doi: 10.1016/j.ccr.2013.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Li T., Jiang D., Wu K. p62 promotes bladder cancer cell growth by activating KEAP1/NRF2-dependent antioxidative response. Cancer Sci. 2020;111:1156–1164. doi: 10.1111/cas.14321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Kitamura H., Torigoe T., Asanuma H., Hisasue S.-I., Suzuki K., Tsukamoto T., Satoh M., Sato N. Cytosolic overexpression of p62 sequestosome 1 in neoplastic prostate tissue. Histopathology. 2005;48:157–161. doi: 10.1111/j.1365-2559.2005.02313.x. [DOI] [PubMed] [Google Scholar]
- 180.Thompson H.G.R., Harris J.W., Wold B.J., Lin F., Brody J.P. p62 overexpression in breast tumors and regulation by prostate-derived Ets factor in breast cancer cells. Oncogene. 2003;22:2322–2333. doi: 10.1038/sj.onc.1206325. [DOI] [PubMed] [Google Scholar]
- 181.Yan X., Zhong X., Yu S., Zhang L., Liu Y., Zhang Y., Sun L., Su J. p62 aggregates mediated Caspase 8 activation is responsible for progression of ovarian cancer. J. Cell. Mol. Med. 2019;23:4030–4042. doi: 10.1111/jcmm.14288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Iwadate R., Inoue J., Tsuda H., Takano M., Furuya K., Hirasawa A., Aoki D., Inazawa J. High Expression of p62 Protein Is Associated with Poor Prognosis and Aggressive Phenotypes in Endometrial Cancer. Am. J. Pathol. 2015;185:2523–2533. doi: 10.1016/j.ajpath.2015.05.008. [DOI] [PubMed] [Google Scholar]
- 183.Ellis R.A., Horswell S., Ness T., Lumsdon J., Tooze S.A., Kirkham N., Armstrong J.L., Lovat P.E. Prognostic Impact of p62 Expression in Cutaneous Malignant Melanoma. J. Investig. Dermatol. 2014;134:1476–1478. doi: 10.1038/jid.2013.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Deng D., Luo K., Liu H., Nie X., Xue L., Wang R., Xu Y., Cui J., Shao N., Zhi F. p62 acts as an oncogene and is targeted by miR-124-3p in glioma. Cancer Cell Int. 2019;19:280. doi: 10.1186/s12935-019-1004-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Zou B., Liu J., Klionsky D.J., Tang D., Kang R. Extracellular SQSTM1 as an inflammatory mediator. Autophagy. 2020;16:2313–2315. doi: 10.1080/15548627.2020.1843253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Zhou B., Liu J., Zeng L., Zhu S., Wang H., Billiar T.R., Kroemer G., Klionsky D.J., Zeh H.J., Jiang J., et al. Extracellular SQSTM1 mediates bacterial septic death in mice through insulin receptor signalling. Nat. Microbiol. 2020;5:1576–1587. doi: 10.1038/s41564-020-00795-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Venanzi F., Shifrin V., Sherman M.Y., Gabai V., Kiselev O., Komissarov A., Grudinin M., Shartukova M., Romanovskaya-Romanko E.A., Kudryavets Y., et al. Broad-spectrum anti-tumor and anti-metastatic DNA vaccine based on p62-encoding vector. Oncotarget. 2013;4:1829–1835. doi: 10.18632/oncotarget.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Ponomarenko D.M., Klimova I.D., Chapygina Y.A., Dvornichenko V.V., Zhukova N.V., Orlova R.V., Manikhas G.M., Zyryanov A.V., Burkhanova L.A., Badrtdinova I.I., et al. Safety and efficacy of p62 DNA vaccine ELENAGEN in a first-in-human trial in patients with advanced solid tumors. Oncotarget. 2017;8:53730–53739. doi: 10.18632/oncotarget.16574. [DOI] [PMC free article] [PubMed] [Google Scholar]