Abstract
The literature on pharmacogenomics as a tool to support antidepressant precision is burgeoning. Recently, a more active role has been argued for pharmacists in pharmacogenomic testing, with both pharmacists and family physicians perceiving pharmacist‐led testing as a valuable method by which to scale this innovation for depression treatment. In this prospective, single‐blind randomized controlled design, we evaluated the impact of pharmacogenomics guided versus standard antidepressant treatment of depression and anxiety, implemented in three large community pharmacies. Participants were 213 outpatients diagnosed with major depressive disorder and/or generalized anxiety disorder, randomized to receive pharmacogenomics guided (n = 105) or standard antidepressant treatment (n = 108); participants were blinded to the study. Patient reported outcomes of depression, anxiety, disability, and treatment satisfaction were assessed at months 0, 1, 3, and 6. Hypotheses were investigated using mixed effect models on the full data. All clinical outcomes improved significantly. The primary outcome (depression) and two secondary outcomes (generalized anxiety and disability) exhibited significant time by group interactions indicating that they improved for participants who received pharmacogenomics guided treatment more so than they did for participants who received standard treatment. Treatment satisfaction improved similarly for both groups. Results contribute to a growing body of work evaluating the impact of pharmacogenomics testing to inform antidepressant medication treatment for depression and anxiety, and provides important initial evidence for the role of pharmacists in care delivery. Pharmacogenomic testing may be a valuable tool to allow pharmacists to more effectively collaborate in facilitating clinical treatment decisions. ClinicalTrials.gov registration: (NCT03591224).
Study Highlights.
WHAT IS THE CURRENT KNOWLEDGE ON THE TOPIC?
Findings are mixed on whether pharmacogenomic testing as a tool to guide antidepressant treatment improves depression response, remission, and symptom severity.
WHAT QUESTION DID THIS STUDY ADDRESS?
We evaluated the impact of pharmacogenomics guided versus standard antidepressant treatment of depression and anxiety as they occur in the real‐world, implemented by pharmacists in three large community pharmacies.
WHAT DOES THIS STUDY ADD TO OUR KNOWLEDGE?
Results from this study provide initial evidence in support of a pharmacist‐led pharmacogenomic testing program in the treatment of mental health difficulties, as well as evidence for the role of pharmacists in care delivery.
HOW MIGHT THIS CHANGE CLINICAL PHARMACOLOGY OR TRANSLATIONAL SCIENCE?
In supporting the external validity of pharmacogenomic testing, this study has implications for how primary care clinicians may manage antidepressant medication use in their patients. In conjunction with measurement‐based care, pharmacogenomic testing may help pharmacists more confidently and effectively manage patients in a protocolized and evidence‐based manner.
INTRODUCTION
Antidepressant medication represents a first‐line treatment for many depressive and anxiety disorders, including major depressive disorder (MDD) 1 and generalized anxiety disorder (GAD). 2 A high proportion of patients seeking treatment for depression are treated with antidepressant medication, frequently within primary care settings. 3 , 4 Yet, antidepressant treatment is recognized to produce modest remission rates in depression. 5 Indeed, antidepressants are linked to suboptimal rates of response 6 , 7 and a substantial percentage of patients do not achieve remission despite several trials of antidepressant medication. 5 , 8 , 9 Emergent adverse events from antidepressant use present a further challenge, reducing treatment tolerability and adherence. 10 , 11
The literature on pharmacogenomics as a tool to support antidepressant precision is burgeoning. Patient response to antidepressant medication is proposed to be a polygenic trait, with common genetic variants accounting for a sizable proportion (over 40%) of the variability in response. 12 Indeed, the genes encoding for cytochrome P450 (CYP450), a family of hepatic enzymes involved in the metabolism of antidepressant medications, are highly polymorphic and have therefore been a major focus of psychiatric pharmacogenomic research. 13 Previous reviews suggest that functional variants in the CYP2D6 and CYP2C19 genes in particular produce phenotypes characterized by varying levels of activity of hepatic enzymes, which have been linked to parameters of antidepressant medication metabolism. 13 , 14
Only a minority of available pharmacogenomic tests have been subject to empirical investigation in the form of randomized controlled trials (RCTs), nonrandomized case‐control studies, and naturalistic, observational studies. 15 Limitations of many existing investigations have been previously outlined, 15 , 16 including small sample sizes, improper blinding procedures, nonrandomization, and retrospective designs. Findings from these investigations are equivocal with respect to whether pharmacogenomic testing improves depression response, remission, and symptom severity. 15 , 16 , 17 Several, recent RCTs have failed to find a positive effect of pharmacogenetic treatment on their primary outcomes of symptom improvement 18 , 19 or patient‐reported sustained response. 20 On the other hand, recent meta‐analyses have concluded that acute treatment for depression guided by pharmacogenetic testing improved response and remission relative to treatment as usual (TAU), 17 with testing associated with 1.71 greater likelihood of symptom remission following acute treatment. 21
Evidently, further investigation is needed to more accurately assess treatment response and tolerability in response to pharmacogenomic guided treatment. 17 Studies that aim to approximate treatment of depression as it occurs in the real‐world are crucial to build support for the generalizability of positive results. For example, there is support that primary care practitioners are consulted more widely than psychiatrists by patients for mental health issues. 22 , 23 Relatedly, primary care physicians are also noted to prescribe more antidepressant medications relative to psychiatrists. 24 Tanner and colleagues reported that in their naturalistic investigation, improvement in depression severity following testing was seen in patients treated in both primary care and psychiatric settings. 22
Recently, a more active role has been argued for pharmacists in pharmacogenomic testing. 25 Pharmacists may be ideally positioned to interpret pharmacogenomic testing profiles and provide recommendations, owing to their specialized training in pharmacology. 25 , 26 , 27 Indeed, previous research suggests that involvement of pharmacists in depression management, including through patient education and counselling, can improve adherence to antidepressant medications. 27 Moreover, recent work leveraging prescription dispensing data in community pharmacies in the Netherlands estimated that approximately one out of four new prescriptions of common primary care medications will have an actionable gene‐drug interaction, 28 highlighting the potential impact of pharmacogenomic testing in this setting.
In our earlier observational investigation within community pharmacies, pharmacogenomic testing by pharmacists was associated with the successful identification of clinically significant drug therapy problems. 26 In an open label RCT, pharmacist‐led pharmacogenomic testing in a home health sample with a range of health problems was associated with a reduced number of re‐hospitalizations and emergency department visits relative to TAU. 29 Relevant to the present study, a retrospective evaluation of pharmacist facilitated pharmacogenomic testing in depressed inpatients was linked to shorter hospital stays compared to an untested cohort. 30 Taken together, such results provide important support for the feasibility and potential impact of pharmacist‐led pharmacogenomic testing for medication optimization.
Our study aimed to extend the research on pharmacist‐led pharmacogenomic testing in the treatment of mental health difficulties with an RCT design. Specifically, we evaluated the impact of pharmacogenomics guided versus standard antidepressant treatment of depression and anxiety, as implemented by pharmacists in three large community pharmacies. The impact of testing on the identification of drug therapy problems, and on the short‐term and long‐term patient‐reported outcomes (PROs) of depression, anxiety, disability, and treatment satisfaction were assessed, in keeping with recent calls to incorporate PROs in psychiatry outcome research. 17 We hypothesized that participants randomized to the experimental group (pharmacogenomics guided treatment) would report greater improvements in PROs compared to those randomized to the control group (TAU) over a 6‐month period.
METHODS
Participants
This protocol was reviewed and approved by an accredited, independent ethics board and was conducted in accordance with the Declaration of Helsinki of 1975. Participants consisted of 213 outpatients (159 women, 53 men, and 1 did not report), ranging in age from 18 to 77 years (M = 42.69, SD = 14.90). Participants were from two urban community pharmacies in Toronto, Ontario, and a third location in Oakville, Ontario. All participants met the following inclusion criteria: (1) age 18 years or older; (2) prescribed one or more antidepressants; (3) diagnosed with MDD and / or GAD; (4) newly initiated on antidepressant therapy or a recent change in therapy; (5) experiencing adverse drug reactions, suboptimal response, or dissatisfaction with antidepressant therapy; and (6) demonstrated dissatisfaction based on the Treatment Satisfaction with Medicines questionnaire (SATMED‐Q). Participants were excluded from the study if they: (1) had a poor command of English or were unable to provide fully informed consent; (2) had received a liver transplant (as a buccal swab will not detect liver DNA); (3) were nonadherent to prescribed drug therapy due to other failure or refusal to take medication as prescribed (nonmedical influencing factors); and (4) were diagnosed with schizophrenia, bipolar disorder, or dementia.
Measures
All outcome measures were administered at baseline, and at months 1, 3, and 6. Depression was assessed using the Patient Health Questionnaire (PHQ‐9), 31 a nine‐item self‐report questionnaire with a total score of 5, 10, and 15 indicating mild, moderate, and severe symptoms, respectively.
GAD symptom severity was assessed using the 7‐item self‐report, GAD‐7 32 ; total score cutoffs of 5, 10, and 15 similarly indicate mild, moderate, and moderately severe anxiety, respectively. The Sheehan Disability Scale (SDS), 33 a three‐item self‐report questionnaire was used to assess functional disability and impairment. Treatment satisfaction was ascertained using the SATMED‐Q, 34 a 17‐item self‐report questionnaire assessing side effects, drug efficacy, convenience of use, impact on activities of daily living, medical care, and general satisfaction. Internal consistencies of all measures were acceptable according to established cutoffs: PHQ‐9 α = 0.85–0.91; GAD‐7 α = 0.91; SDS α = 0.84–0.95; and SATMED‐Q α = 0.82–0.88.
Procedures
Pharmacists reviewed the pharmacy patient database to identify patients who had been prescribed antidepressants and who may have been dissatisfied with their therapy, identified based on the frequency of or recent changes in their medication, dosage, or documented evidence of noncompliance. Walk‐in patients using antidepressants who met study criteria were also provided the opportunity to participate. Following initial identification of potential participants, patients were prescreened using the SATMED‐Q to establish eligibility. Patients who scored greater than 2 on select items (1–3) and/or less than 2 on others (4–9) were considered to be dissatisfied with their current drug therapy and would benefit from pharmacogenetics screening. All patients who met the remaining eligibility criteria were invited to participate upon consent and received the Pillcheck test, available through GeneYouIn, Inc. in Canada. The Pillcheck test was developed for integration into a clinical pharmacy setting and provides a comprehensive report based on variation in 19 genes, with implications for over 200 nonpsychotropic and psychotropic medications, including antidepressant medications (see Table S1). 35 Recommendations generated by the Pillcheck test are aligned with existing clinical guidelines (i.e., the Clinical Pharmacogenetics Implementation Consortium [CPIC]) 36 and regulatory bodies (i.e., labeling guidelines of the US Food and Drug Administration [FDA]). 26
The Pillcheck report prioritizes the CPIC guidelines for medications with both FDA and CPIC guidelines because the latter has more refined recommendations for specific diplotypes. There are a limited number of drugs with Pharmacogenomics Knowledgebase (PharmGKB) evidence (levels 1 and 2), which are deemed clinically significant by independent expert pharmacists, that are also included in the Pillcheck report. The recommendations are constructed by expert clinicians based on the review of publications annotated by the PharmGKB database. The functional annotation of an enzyme’s activity (i.e., classification of specific diplotypes to poor, intermediate, or normal metabolizer status) is aligned according to the CPIC guidelines for other drugs metabolized by the same enzyme. The Pillcheck reports are updated annually to reflect revisions to the CPIC guidelines and new FDA drug labels. The Pillcheck version 2.1.0 report that was used throughout the study period, reported according to the CPIC guidelines and the FDA drug labels published before January 2018. For example, CYP2C19*1/17 was classified as a rapid metabolizer and CYP2D6 *1/*4 was classified as a normal metabolizer. The Pillcheck test has been linked to the successful identification of drug therapy problems in community pharmacy 26 and nursing home 37 settings.
Prior to the study, pharmacists completed an online pharmacogenetics overview course provided through GeneYouIn, and over 8 h of hands‐on training, including an introduction to CPIC guidelines and report interpretation. Recommendations were based on the integration of patient drug profiles and full medical history with the Pillcheck report.
A prospective, single‐blind randomized controlled design was used. Participants were randomly assigned to either the pharmacogenomics guided group (n = 105) or control group (n = 108) based on a random sequence generated by an online randomizer tool, which only the Principal Investigator had access to. Participant profiles were created on the Pillcheck portal and the pharmacists facilitated the Pillcheck registration and supervised the buccal swab DNA collection process. For participants in the pharmacogenomics guided group, pharmacists received the Pillcheck report 2 weeks following swab collection; this report guided recommendations for optimizing antidepressant drug therapy either through dose adjustment or a change in drug therapy. For participants in the control group, recommendations were made as per standard of care clinical guidelines. Changes in therapy were discussed with participants in both study groups; however, participants were blinded to their group assignment. The pharmacist communicated recommended changes with the prescriber in both study groups via fax or telephone; prescribers were able to accept, decline, or disregard these recommendations. Prescribers were asked to maintain the integrity of the study by withholding knowledge of the pharmacogenomics results and study group assessment until the end of the study. Pillcheck results were not communicated to participants until the end of the study.
Statistical analyses
Mixed effect models were conducted on the full data. The PHQ‐9 total score served as the primary outcome variable, and the GAD‐7, SDS, and SATMED‐Q total scores served as secondary outcomes, in separate models. Time was entered as within‐subject categorical factor with four levels, and study group as between‐subject factor with two levels, as well as their interaction. Mixed effect models have been applied in recent investigations of pharmacogenomics guided treatment. 19 This analysis approach handles missing values using maximum likelihood estimation, which is able to use all available information in all recruited participants, and is associated with the least bias in a comparison of methods for dealing with missing data. 38 We followed the intention‐to‐treat principle and analyzed all randomized participants. In line with recommendations, 39 we conducted models with and without the inclusion of important covariates, including age and sex, pharmacy site, prescriber (family physician, specialist, family physician and specialist, and nurse practitioner), and method of payment (third party, provincial drug coverage, or cash). We also conducted sensitivity analyses, restricted to participants in the pharmacogenomics guided group whose prescribers accepted pharmacist recommendations at any time during the treatment period.
RESULTS
Participant descriptives
Participants were recruited between April 2018 and May 2019, with the follow‐up period ending December 2019. Participants exhibited the following current diagnoses: MDD (n = 169), GAD (n = 165), and other disorders (e.g., anxiety or eating disorders; n = 10); the majority of participants (n = 133) met criteria for more than one current psychiatric diagnosis, with most exhibiting both MDD and GAD (n = 124). There were no differences across study groups in sex (χ2 = 1.13, p = 0.57) or age (t = 0.77, p = 0.44).
Descriptive statistics for participant demographic and clinical features at baseline are presented in Table 1. There were no differences in the primary outcome (PHQ‐9) at baseline across study groups; there were similarly no differences in two secondary outcomes (GAD‐7 and SDS). The SATMED‐Q was greater in the pharmacogenomics guided group at baseline, with a small effect size (d = 0.28). Skew and kurtosis of all outcomes were below established cutoffs.
Table 1.
Participant demographic and clinical features at baseline
|
Total (N = 213) |
Control (n = 108) |
PGx guided (n = 103) |
t (p) | |
|---|---|---|---|---|
| M (SD) | M (SD) | M (SD) | ||
| Age | 42.69 (14.90) | 43.46 (15.26) | 41.90 (14.55) | 0.77 (0.44) |
| Female (%) a | 159 (74.65) | 82 (75.93) | 77 (73.33) | 1.13 (0.57) |
| GAD‐7 | 11.67 (5.54) | 11.57 (5.70) | 11.76 (5.93) | 0.24 (0.81) |
| PHQ‐9 | 13.86 (6.17) | 14.18 (6.31) | 13.52 (6.04) | 0.78 (0.43) |
| SATMED‐Q | 35.16 (10.24) | 33.77 (10.34) | 36.59 (9.99) | 2.02 (0.04) |
| SDS | 17.37 (7.39) | 17.04 (7.64) | 17.72 (7.14) | 0.67 (0.50) |
| Payment: | ||||
| Third party | 113 | 54 | 59 | |
| Provincial drug coverage | 69 | 38 | 31 | |
| Participants | 30 | 16 | 14 | |
| Prescriber: | ||||
| Family physician | 153 | 74 | 79 | |
| Family physician and specialist | 14 | 10 | 4 | |
| Specialist | 41 | 20 | 21 | |
| Nurse practitioner | 5 | 4 | 1 | |
| Prescriptions b : | ||||
| SSRIs | 166 | 83 | 83 | |
| SNRIs | 72 | 31 | 41 | |
| TCAs | 7 | 5 | 2 | |
| Antipsychotics | 32 | 20 | 12 | |
| Other antidepressants | 86 | 46 | 40 |
Abbreviations: GAD‐7, Generalized Anxiety Disorder 7; M, mean; PGx, pharmacogenomics; PHQ‐9, Patient Health Questionnaire 9; SATMED‐Q, Treatment Satisfaction with Medicines Questionnaire; SD, standard deviation; SDS, Sheehan Disability Scale; SNRIs, serotonin and norepinephrine reuptake inhibitors; SSRIs, selective serotonin reuptake inhibitors; TCAs, tricyclic antidepressants.
Signifies that study group differences in sex evaluated via c2.
Signifies total number of prescriptions throughout the study, including at baseline.
Attrition in the pharmacogenomics guided arm and control arm was 16 in each group. Reasons for loss included discontinuation of medication (n = 11), participant withdrawal (n = 12), and lost to follow‐up (n = 9; see Figure S1). Of the 213 participants who completed measures at baseline, 197 completed measures at month 1, 187 at month 3, and 182 at month 6. Family physicians prescribed medication for the majority of participants (n = 153), although family physicians and specialists (n = 14), specialists (n = 41), and nurse practitioners (n = 5) also prescribed medications as well. Medication costs were paid by third parties (i.e., insurance; n = 113), provincial drug programs (n = 69), or participants (n = 30). Medications prescribed throughout the study in the intent‐to‐treat sample can be found in Table S2.
Effect of treatment group on study outcomes
Intent‐to‐treat analyses
All study outcomes exhibited significant effects of time, and the GAD‐7 exhibited a significant effect of treatment; these main effects were moderated by a time by group interaction for three of four outcomes (see Table 2). More specifically, the primary outcome (PHQ‐9) and two secondary outcomes (GAD‐7 and SDS) exhibited significant time by group interactions indicating that they improved for participants who received pharmacogenomics guided treatment more so than they did for participants who received TAU. The SATMED‐Q did not exhibit such a differential improvement across study groups over time, indicating that treatment satisfaction improved similarly for both groups from baseline to month 6 (see Figure 1). Models including covariates demonstrated the same pattern of results (see Table S3). Only age demonstrated significant effects across all outcomes, such that younger age was associated with better outcomes.
Table 2.
Primary and secondary outcomes: mixed effects models
| ITT analyses | Sensitivity analyses | |||||
|---|---|---|---|---|---|---|
| Time | Group | Time * group | Time | Group | Time * group | |
| F (p) | F (p) | F (p) | F (p) | F (p) | F (p) | |
| GAD‐7 | 30.94 (<0.001) | 4.26 (0.04) | 4.17 (0.01) | 30.75 (<0.001) | 7.23 (0.01) | 4.93 (0.002) |
| PHQ‐9 | 23.65 (<0.001) | 2.06 (0.15) | 2.74 (0.04) | 23.16 (<0.001) | 8.50 (0.004) | 2.92 (0.03) |
| SATMED‐Q | 33.14 (<0.001) | 1.07 (0.30) | 0.11 (0.95) | 31.95(<0.001) | 6.55 (0.01) | 0.23 (0.88) |
| SDS | 33.01 (<0.001) | 1.02 (0.31) | 6.25 (<0.001) | 33.13 (<0.001) | 3.41 (0.07) | 7.33(<0.001) |
Abbreviations: F, F‐statistic; GAD‐7, Generalized Anxiety Disorder 7; ITT, intention‐to‐treat; PHQ‐9, Patient Health Questionnaire 9; SATMED‐Q, Treatment Satisfaction with Medicines Questionnaire; SDS, Sheehan Disability Scale.
Figure 1.
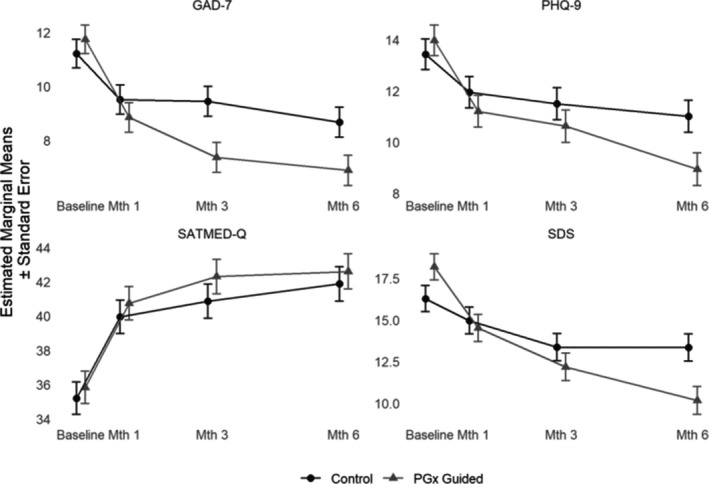
Clinical outcomes over time and study group. Note: PHQ‐9 = Patient Health Questionnaire 9; GAD‐7 = Generalized Anxiety Disorder 7; SDS = Sheehan Disability Scale; SATMED‐Q = Treatment Satisfaction with Medicines Questionnaire; Mth = Month; PGx Guided = Pharmacogenomics guided treatment. Panels illustrate the marginal means and standard errors of control and PGx Guided group at baseline, months 1, 3, and 6 across all clinical outcomes
Sensitivity analyses
Sensitivity analyses were conducted, and restricted to those participants in the pharmacogenomics guided group whose prescribers accepted pharmacist recommendations (n = 79; see Table 2). Again, all study outcomes exhibited significant effects of time, and now the PHQ‐9 and GAD‐7 further exhibited significant effects of treatment. Again, the PHQ‐9, GAD‐7, and SDS demonstrated significant time × group interactions, demonstrating greater improvements for participants receiving pharmacogenomics guided treatment than TAU for these outcomes. Again, treatment satisfaction did not demonstrate such an interaction, exhibiting similar improvements across both study groups. Notably, prescriber acceptance of pharmacist recommendations differed across the study groups (χ2 = 11.64, p = 0.001), with 75.2% acceptance in the pharmacogenomics guided group and 52.8% in the control group. Sensitivity analyses were therefore repeated, and restricted to participants whose prescribers accepted pharmacist recommendations in both groups (79 and 57 participants in the pharmacogenomics guided and control groups, respectively). All significant time × group interactions were replicated (see Table S4).
Accepted recommendations included dose adjustments (e.g., pharmacist recommended increased dosage due to ultrarapid metabolism, resulting in suboptimal response; n = 72 in pharmacogenomics guided group vs. n = 50 in control group), medication switches (e.g., pharmacist recommended switching to a different medication to manage adverse reactions as a result of poor metabolism of original medication; n = 26 in pharmacogenomics guided group vs. n = 16 in control group), addition of a new medication (e.g., pharmacist recommended an adjunctive therapy; n = 10 in pharmacogenomics guided group vs. n = 2 in control group), and medication adherence (e.g., pharmacist recommended that patient continue with medication regimen; n = 5 in pharmacogenomics guided group vs. n = 6 in control group). Recommendations were made for the majority of participants (n = 204); although the number of recommendations per participant ranged from one to five, most received one (n = 124) or two (n = 60). Pharmacist recommendations did not differ across study groups: there was no difference in the number of participants who received pharmacist recommendations (χ2 = 0.37, p = 0.54) or the number of pharmacist recommendations made (χ2 = 1.57, p = 0.81) across study groups. Pharmacist recommendations were also more commonly accepted when medication costs were covered by third parties or provincial drug coverage (χ2=9.54, p = 0.02; see Figure 2). Prescriber acceptance of pharmacist recommendations did not differ across the pharmacy sites (χ2 = 0.29, p = 0.59) or by prescriber (χ2 = 1.53, p = 0.68).
Figure 2.
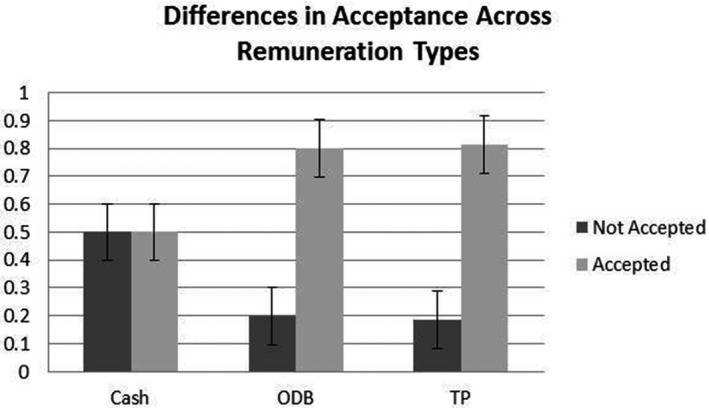
Prescriber acceptance of pharmacist recommendations by payment type. Note: ODB = Ontario Drug Benefit program; TP = third party (i.e., insurance company). Figure illustrates the proportion of participants whose clinicians accepted pharmacist recommendations across remuneration type, or how medication costs were covered
DISCUSSION
Consistent with hypotheses, participants with MDD or GAD randomized to receive pharmacist‐led pharmacogenomics guided treatment reported greater improvements in depression severity over a 6‐month period compared to those randomized to receive TAU. Participants receiving pharmacogenomics guided treatment also reported greater improvements in generalized anxiety and in disability over this period compared to those receiving TAU. Treatment satisfaction did not differ across groups, however. Significant results were not only statistically robust but also clinically meaningful. The overall change in PHQ‐9 total scores over 6 months was 5.03 and 2.42 in the pharmacogenomics guided and control groups, respectively, representing improvements from baseline depression severity of 36% versus 18%. Similarly, improvements in anxiety and disability were two times greater in the pharmacogenomics guided group versus the control group: GAD‐7 improved by 41% versus 23%, and SDS by 44% versus 18% across these groups.
The current investigation responded to recent calls for the use of PROs in pharmacogenomics research, and has strong external validity due to use of these scales in measurement‐based care in clinical settings. Yet, as prescribers were not blinded in our study, the possibility that participants were inadvertently unblinded to group assignment, or that there were potential differences in treatment provided across arms, 21 cannot be overlooked. Thus, biases (e.g., expectancy bias and performance bias) may have influenced results. Blinding of participants, outcome assessors, as well as prescribers 19 would buoy these results in future replications. Notably, the current investigation did not find expected differences in treatment satisfaction across groups. Our capacity to detect effects may have been impacted by a restricted range of scores, as the overall change demonstrated in this study is below the “minimum important difference” demonstrated for this measure. 40 Future research might evaluate other domains and measures of patient satisfaction and experience, to more fully elucidate how this multifaceted outcome might differ across those who do and do not receive pharmacogenomics guided care.
The current investigation thus provides important support for the role of pharmacists in the implementation of pharmacogenomic testing, and associated treatment recommendations. Differential prescriber acceptance of pharmacist recommendations across study groups suggests that pharmacogenomics test results may have increased confidence in these recommendations. Pharmacist‐led pharmacogenomic testing leverages the specialized training of these allied health professionals, and may provide a promising method by which to scale this decision support tool. Pharmacogenomic testing by pharmacists has identified clinically significant drug therapy problems in previous research. 26 The current investigation extends this foundation to the outpatient treatment of mental health difficulties, using a robust prospective, single‐blind randomized controlled design. Notably, differential prescriber acceptance of pharmacist recommendations across payment methods suggest that prescribers may be more inclined to consider these recommendations when the financial implications for their patients are minimized, and underscores the importance of tracking these important practical considerations in this research.
The present study was designed to improve overall participant therapeutic benefits, enabling pharmacists to flag potential gene‐drug interactions and help pharmacists assess risk of drug‐drug interactions in participants with reduced drug metabolism. Results contribute to a growing body of work evaluating the impact of pharmacogenomics testing to inform antidepressant medication treatment of depression and anxiety, and provides important initial evidence for the role of pharmacists in care delivery. Pharmacogenomic testing may be a valuable tool to allow pharmacists to more effectively collaborate in facilitating clinical treatment decisions. As pharmacists’ role and scope expand to include changing dosages to initiating prescription therapies (depending on geographical region), evidence‐based decision making becomes even more critical. Unfortunately, due to the complexity in managing psychiatric conditions and uncertainties in treatment guidelines, pharmacists are often reluctant to intervene on any identified drug therapy problems. Pharmacogenomic testing in conjunction with measurement‐based care, including validated patient‐report scales may help pharmacists to more confidently and effectively manage patients in a protocolized and evidence‐based manner. With the pharmacist’s skillset in both pharmacology and therapeutics, interpreting pharmacogenomic test results and developing an action plan is uniquely best‐suited for pharmacists. Pharmacists may be particularly well‐situated to identify those who may benefit from pharmacogenomic testing due to their high number of patient interactions each day, access to patients’ drug history, and detect reasons of noncompliance, such as adverse drug reactions.
The current investigation boasts numerous strengths. First, this study was characterized by high external validity or generalizability: experimental controls were minimized to permit an investigation of the closest approximation of a real‐world implementation of pharmacist‐led pharmacogenomics guided treatment possible. Eligibility criteria permitted the recruitment of a range of outpatients with depression and anxiety, heterogeneous in their prescriber, payment method, and other important features. Prescribers were free to follow pharmacist recommendations or not, in line with current practice, and widely used brief measures of clinical outcomes were used. Second, participant attrition was fairly limited at only 15%, maintaining the power to detect effects over a 6‐month follow‐up period and demonstrating the feasibility of this approach. Third, results were robust to the inclusion of covariates, and were replicated across both intent‐to‐treat and sensitivity analyses. Finally, by exploring sustained improvements in antidepressant treatment outcomes specific to guided therapy over a 6‐month period, our study addresses a gap in the existing pharmacogenomic literature of studies investigating longer‐term depression outcomes. 19
Limitations
Nevertheless, several limitations are important to note. First, this study did not include blinded outcomes of the assessors or prescribers, which does introduce a risk of bias. Although we are not able to rule out issues associated with blinding, there were no differences in pharmacist recommendations across the study groups. However, this study lacked the power to compare the kinds of recommendations made (e.g., medication switches, dose adjustments, and medication augmentations), precluding the ability to evaluate whether these differed systematically in a way that might have contributed to unblinding. A fulsome demographic and clinical characterization of participants, using clinician‐rated semistructured instruments, for example, was not possible, precluding the evaluation of potential covariates or moderators of effects. In line with gender and sex differences in the prevalence of depression and anxiety, 41 , 42 a greater number of women participated in this research; although sex was incorporated in covariate analyses, we lacked the power to examine sex as a predictor in its own right, including as a potential moderator of effects. Relatedly, participants’ history of medication exposure—a potentially important moderator of the effect of pharmacogenomic testing in depression—was not formally collected in this study. Although this study is characterized by high external validity in many respects, the repeated administration of outcome measures and the 6‐month follow‐up period is not currently a standard of care uniformly implemented across pharmacy settings, and these features may have had therapeutic benefits in their own right. Further, this study was conducted at urban pharmacies, which may not generalize to pharmacies in other communities and settings. Finally, the Pillcheck did not detect all DNA variations that may have altered gene activity. Only specified genetic variations in White patients and major ethnic minority groups were tested.
CONCLUSION
To conclude, the current investigation provides initial evidence in support of a pharmacist‐led pharmacogenomic testing program in the treatment of mental health difficulties. Results contribute to a growing body of work evaluating the impact of pharmacogenomics testing to inform antidepressant medication treatment of depression and anxiety, and provide important initial evidence for the role of pharmacists in care delivery. Results suggest that pharmacogenomic testing may be a promising avenue to increase the ability of pharmacists to be actively involved in patient care and provide evidence‐based and personalized insights regarding patient‐predicted treatment response, thereby emphasizing the importance of pharmacist‐patient communication and interaction. Continued investigation into the benefits of pharmacogenomics guided pharmacotherapy therapy, including healthcare economics analysis and important moderators of effects, is warranted.
CONFLICT OF INTERESTS
Thulasi Thiruchselvam and Esha Jain received a stipend from Green Shield Canada to support their contribution to manuscript preparation. All other authors declared no competing interests for this work.
AUTHOR CONTRIBUTIONS
L.C.Q., T.T., and E.J. wrote the manuscript. J.P., L.C.Q., W.L., P.G., L.M., B. vdB., and N.P. designed the research. J.P., W.L., and B.vdB. performed the research. L.C.Q. and W.L. analyzed the data.
DISCLAIMER
Study tests were purchased from and analyzed by the developer of Pillcheck, GeneYouIn Inc. GeneYouIn Inc. had no other involvement in the study.
Supporting information
Fig S1
Table S1
Table S2
Table S3
Table S4
ACKNOWLEDGMENTS
The authors would like to recognize Fabio De Rango and his pharmacy team at Shoppers Drug Mart, Oakville, Ontario for contributing to the recruitment of patients for the study.
Funding information
Green Shield Canada, a nonprofit organization, provided operating funds toward study execution.
REFERENCES
- 1. Kennedy SH, Lam RW, McIntyre RS, et al. Canadian Network for Mood and Anxiety Treatments (CANMAT) 2016 clinical guidelines for the management of adults with major depressive disorder: Section 3. Pharmacological treatments. Can J Psychiatry. 2016;61:540‐560. 10.1177/0706743716659417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Katzman MA, Bleau P, Blier P, et al. Canadian clinical practice guidelines for the management of anxiety, posttraumatic stress and obsessive‐compulsive disorders. BMC Psychiatry. 2014;14:S1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hockenberry JM, Joski P, Yarbrough C, Druss BG. Trends in treatment and spending for patients receiving outpatient treatment of depression in the United States, 1998–2015. JAMA Psychiatry. 2019;76:810‐817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wong ST, Manca D, Barber D, et al. The diagnosis of depression and its treatment in Canadian primary care practices: an epidemiological study. CMAJ Open. 2014;2:E337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Trivedi MH, Daly EJ. Treatment strategies to improve and sustain remission in major depressive disorder. Dialogues Clin Neurosci. 2008;10:378‐384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Trivedi MH, Rush AJ, Wisniewski SR, et al. Evaluation of outcomes with citalopram for depression using measurement‐based care in STAR*D: implications for clinical practice. Am J Psychiatry. 2006;163:28‐40. [DOI] [PubMed] [Google Scholar]
- 7. Undurraga J, Baldessarini RJ. Randomized, placebo‐controlled trials of antidepressants for acute major depression: thirty‐year meta‐analytic review. Neuropsychopharmacology. 2011;37:851‐864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rush AJ, Trivedi MH, Wisniewski SR, et al. Acute and longer‐term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am J Psychiatry. 2006;163:1905‐1917. [DOI] [PubMed] [Google Scholar]
- 9. Thase ME, Nierenberg AA, Vrijland P, et al. Remission with mirtazapine and selective serotonin reuptake inhibitors: a meta‐analysis of individual patient data from 15 controlled trials of acute phase treatment of major depression. Int Clin Psychopharmacol. 2010;25:189‐198. [DOI] [PubMed] [Google Scholar]
- 10. Masand PS. Tolerability and adherence issues in antidepressant therapy. Clin Ther. 2003;25:2289‐2304. [DOI] [PubMed] [Google Scholar]
- 11. Schatzberg AF. Safety and tolerability of antidepressants: weighing the impact on treatment decisions. J Clin Psychiatry. 2007;68:26‐34. [PubMed] [Google Scholar]
- 12. Tansey KE, Guipponi M, Hu X, et al. Contribution of common genetic variants to antidepressant response. Biol Psychiatry. 2013;73:679‐682. [DOI] [PubMed] [Google Scholar]
- 13. Porcelli S, Fabbri C, Spina E, Serretti A, Ronchi DD. Genetic polymorphisms of cytochrome P450 enzymes and antidepressant metabolism. Expert Opin Drug Metab Toxicol. 2011;7:1101‐1115. [DOI] [PubMed] [Google Scholar]
- 14. Fabbri C, Serretti A. Genetics of treatment outcomes in major depressive disorder: present and future. Clin Psychopharmacol Neurosci. 2020;18:1‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fabbri C, Zohar J, Serretti A. Pharmacogenetic tests to guide drug treatment in depression: comparison of the available testing kits and clinical trials. Prog Neuropsychopharmacol Biol Psychiatry. 2018;86:36‐44. [DOI] [PubMed] [Google Scholar]
- 16. Rosenblat JD, Lee Y, Mcintyre RS. Does pharmacogenomic testing improve clinical outcomes for major depressive disorder? J Clin Psychiatry. 2017;78:720‐729. [DOI] [PubMed] [Google Scholar]
- 17. Rosenblat JD, Lee Y, Mcintyre RS. The effect of pharmacogenomic testing on response and remission rates in the acute treatment of major depressive disorder: a meta‐analysis. J Affect Disord. 2018;241:484‐491. [DOI] [PubMed] [Google Scholar]
- 18. Greden JF, Parikh SV, Rothschild AJ, et al. Impact of pharmacogenomics on clinical outcomes in major depressive disorder in the GUIDED trial: a large, patient‐ and rater‐blinded, randomized, controlled study. J Psychiatr Res. 2019;111:59‐67. [DOI] [PubMed] [Google Scholar]
- 19. Perlis RH, Dowd D, Fava M, Lencz T, Krause DS. Randomized, controlled, participant‐ and rater‐blind trial of pharmacogenomic test‐guided treatment versus treatment as usual for major depressive disorder. Depress Anxiety. 2020;37(9):834‐841. [DOI] [PubMed] [Google Scholar]
- 20. Pérez V, Salavert A, Espadaler J, et al. Efficacy of prospective pharmacogenetic testing in the treatment of major depressive disorder: results of a randomized, double‐blind clinical trial. BMC Psychiatry. 2017;17:1‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bousman CA, Arandjelovic K, Mancuso SG, Eyre HA, Dunlop BW. Pharmacogenetic tests and depressive symptom remission: a meta‐analysis of randomized controlled trials. Pharmacogenomics. 2019;20:37‐47. [DOI] [PubMed] [Google Scholar]
- 22. Tanner J‐A, Davies PE, Voudouris NC, et al. Combinatorial pharmacogenomics and improved patient outcomes in depression: treatment by primary care physicians or psychiatrists. J Psychiatr Res. 2018;104:157‐162. [DOI] [PubMed] [Google Scholar]
- 23. Vasiliadis H‐M, Lesage A, Adair C, Wang PS, Kessler RC. Do Canada and the United States differ in prevalence of depression and utilization of services? Psychiatr Serv. 2007;58:63‐71. [DOI] [PubMed] [Google Scholar]
- 24. Mark TL, Levit KR, Buck JA. Datapoints: psychotropic drug prescriptions by medical specialty. Psychiatr Serv. 2009;60:1167. [DOI] [PubMed] [Google Scholar]
- 25. Weinstein S, Carroll JC, Jukic S, Mcgivney MS, Klatt P. Perspectives of a pharmacist‐run pharmacogenomic service for depression in interdisciplinary family medicine practices. J Am Coll Clin Pharm. 2019;3:417‐424. [Google Scholar]
- 26. Papastergiou J, Tolios P, Li W, Li J. The Innovative Canadian Pharmacogenomic Screening Initiative in Community Pharmacy (ICANPIC) study. J Am Pharm Assoc. 2017;57:624‐629. [DOI] [PubMed] [Google Scholar]
- 27. Readdean KC, Heuer AJ, Parrott JS. Effect of pharmacist intervention on improving antidepressant medication adherence and depression symptomology: a systematic review and meta‐analysis. Res Social Adm Pharm. 2018;14:321‐331. [DOI] [PubMed] [Google Scholar]
- 28. Bank PCD, Swen JJ, Guchelaar HJ. Estimated nationwide impact of implementing a preemptive pharmacogenetic panel approach to guide drug prescribing in primary care in the Netherlands. BMC Med. 2019;17:110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Elliott LS, Henderson JC, Neradilek MB, et al. Clinical impact of pharmacogenetic profiling with a clinical decision support tool in polypharmacy home health patients: a prospective pilot randomized controlled trial. PLoS One. 2017;12:e0170905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bättig VAD, Roll SC, Hahn M. Pharmacogenetic testing in depressed patients and interdisciplinary exchange between a pharmacist and psychiatrists results in reduced hospitalization times. Pharmacopsychiatry. 2020;53:185‐192. [DOI] [PubMed] [Google Scholar]
- 31. Kroenke K, Spitzer RL, Williams JBW. The PHQ‐9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16:606‐613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Spitzer RL, Kroenke K, Williams JBW, Löwe B. A brief measure for assessing generalized anxiety disorder. Arch Intern Med. 2006;166:1092‐1097. [DOI] [PubMed] [Google Scholar]
- 33. Leon AC, Olfson M, Portera L, Farber L, Sheehan DV. Assessing psychiatric impairment in primary care with the Sheehan Disability Scale. Int J Psychiatry Med. 1997;27:93‐105. [DOI] [PubMed] [Google Scholar]
- 34. Ruiz MA, Pardo A, Rejas J, Soto J, Villasante F, Aranguren JL. Development and validation of the “Treatment Satisfaction with Medicines Questionnaire” (SATMED‐Q). Value in Health. 2008;11:913‐926. [DOI] [PubMed] [Google Scholar]
- 35. GeneYouIn Inc., Product Information . Pillcheck (2020). https://www.pillcheck.ca/product‐information/#1572900543001‐eedaa85d‐c1fe.
- 36. Clinical Pharmacogenetics Implementation Consortium . Guidelines. https://cpicpgx.org/guidelines/. Accessed June 24, 2020.
- 37. Dorfman R, London Z, Metias M, Kabakchiev B, Mukerjee G, Moser A. Individualized medication management in Ontario long‐term care clinical impact on management of depression, pain, and dementia. J Am Med Dir Assoc. 2020;21:823‐829. [DOI] [PubMed] [Google Scholar]
- 38. Hallgren KA, Witkiewitz K. Missing data in alcohol clinical trials: a comparison of methods. Alcohol Clin Exp Res. 2013;37:2152‐2160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Simmons JP, Nelson LD, Simonsohn U. False‐positive psychology: undisclosed flexibility in data collection and analysis allows presenting anything as significant. Psychol Sci. 2011;22:1359‐1366. [DOI] [PubMed] [Google Scholar]
- 40. Rejas J, Ruiz MA, Pardo A, Soto J. Minimally important difference of the Treatment Satisfaction with Medicines Questionnaire (SATMED‐Q). BMC Med Res Methodol. 2011;11:142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Salk RH, Hyde JS, Abramson LY. Gender differences in depression in representative national samples: meta‐analyses of diagnoses and symptoms. Psychol Bull. 2017;143:783‐822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Vesga‐López O, Schneier FR, Wang S, et al. Gender differences in generalized anxiety disorder: results from the national epidemiologic survey on alcohol and related conditions (NESARC). J Clin Psychiatry. 2008;69:1606‐1616. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1
Table S1
Table S2
Table S3
Table S4


