Abstract
Objective
To determine whether severe hypoglycemic and hyperglycemic events are associated with longitudinal dementia risk in older adults with type 1 diabetes.
Methods
A longitudinal cohort study followed up 2,821 members of an integrated health care delivery system with type 1 diabetes from 1997 to 2015. Hypoglycemic and hyperglycemic events requiring emergency room or hospitalization were abstracted from medical records beginning January 1, 1996, through cohort entry. Participants were followed up for dementia diagnosis through September 30, 2015. Dementia risk was examined with Cox proportional hazard models adjusted for age (as time scale), sex, race/ethnicity, hemoglobin A1c, depression, stroke, and nephropathy.
Results
Among 2,821 older adults (mean age 56 years) with type 1 diabetes, 398 (14%) had a history of severe hypoglycemia, 335 (12%) had severe hyperglycemia, and 87 (3%) had both. Over a mean 6.9 years of follow-up, 153 individuals (5.4%) developed dementia. In fully adjusted models, individuals with hypoglycemic events had 66% greater risk of dementia than those without a hypoglycemic event (hazard ratio [HR] 1.66, 95% confidence interval [CI] 1.09, 2.53), while those with hyperglycemic events had >2 times the risk (HR 2.11, 95% CI 1.24, 3.59) than those without a hyperglycemic event. There was a 6-fold greater risk of dementia in individuals with both severe hypoglycemia and hyperglycemia vs those with neither (HR 6.20, 95% CI 3.02, 12.70).
Conclusions
For older individuals with type 1 diabetes, severe hypoglycemic and hyperglycemic events are associated with increased future risk of dementia.
Hypoglycemia and hyperglycemia are serious complications among patients with type 1 diabetes (T1D). Severe hypoglycemia is defined as low blood sugar resulting in loss of consciousness or ability to self-manage.1 Severe hyperglycemia results from insulin deficiency combined with metabolic acidosis and the presence of ketones (diabetic ketoacidosis [DKA]) or extremely high blood sugar and dehydration (hyperosmolar hyperglycemic state).1 Among adults with T1D, it is estimated that the annual prevalence of severe (requiring emergency room or hospitalization) hypoglycemia is ≈12%, and the annual prevalence of severe (requiring emergency room or hospitalization) hyperglycemia is ≈5%.2 Both forms of severe glycemic events are diabetic emergencies, and both are largely avoidable. However, when they do occur, they are potentially life-threatening and are associated with a range of immediate, dangerous health outcomes, including coma, increased hospitalization, and death.3
With recent advances in diabetes treatment, people with T1D are living longer than before.4 This increased lifespan places them at risk for aging-related conditions such as dementia. Indeed, prior studies have suggested that those with T1D may be at higher risk for dementia than those without T1D, so identifying modifiable risk factors in this vulnerable patient population is a public health priority.5,6 Glycemic control has emerged as a potentially modifiable factor associated with cognition and dementia, suggesting a future prevention target for brain health.7-9 While studies have established an association between severe glycemic events and increased dementia risk in type 2 diabetes (T2D),10,11 it is unknown whether this is the case in T1D. This is crucial to evaluate considering that patients with T1D are more likely to experience severe hypoglycemic events12,13 and DKA than those with T2D.14 In addition, these severe glycemic events have been shown to have far-reaching cerebrovascular sequelae. Severe hypoglycemia is associated with alterations in the hippocampus and cortex and neuronal loss,15-17 while severe hyperglycemia is associated with reduced N-acetyl aspartate levels (an indicator of neuronal dysfunction), reduced cerebral blood flow, and cerebral edema.18,19 It is important to evaluate whether such events in older individuals with T1D place them at higher future dementia risk. In this study, we leverage data collected over a span of 18 years to examine the associations between severe hyperglycemic and hypoglycemic events and long-term risk of dementia in a large cohort of older adults with T1D.
Methods
Study Population
The source population for this study was identified from the Kaiser Permanente Northern California (KPNC) Diabetes Registry. KPNC is an integrated health care delivery system serving a diverse population of >4 million member representative of the catchment area, with the exception of extremes of the income distribution.20 The KPNC Diabetes Registry identifies members from 4 sources: primary hospital discharge diagnoses of diabetes, ≥2 outpatient visit diagnoses of diabetes, any prescription for a diabetes-related medication, or any record of an elevated hemoglobin A1c (HbA1c) test.
Using the KPNC Diabetes Registry from January 1, 1996, to December 31, 2013, we identified individuals who were ≥50 years of age and had T1D using the following 3 criteria, all of which had to be met: (1) at least 75% of their diagnostic codes indicate T1D, (2) filled insulin prescriptions during the study period, and (3) did not fill prescriptions of any other hypoglycemic agents.21 Patients were considered eligible on the first day on or after January 1, 1996, that he or she was ≥50 years old and met the criteria for T1D described above. Once the patient met eligibility, exposure and covariate status were ascertained from all available data from January 1, 1996, up to 365 days after the date of eligibility. On day 366, patients entered the cohort and follow-up began. Participants were followed up for a maximum of 18 years, with end of follow-up defined as the first of the following: dementia diagnosis, death, a lapse in KPNC membership >90 days, or the end of the study period (September 30, 2015). Individuals were excluded from the cohort if they had evidence of a dementia diagnosis before cohort entry (n = 42). This study was approved by the KPNC Institutional Review Board. The requirement for patient informed consent was waived because analyses were conducted on preexisting data.
Severe Glycemic Events
Severe glycemic events were identified as episodes of hypoglycemia or hyperglycemia that resulted in inpatient or emergency department use. Events were defined as any emergency department visit with a primary diagnosis of hypoglycemia or hyperglycemia or any hospitalization with a principal diagnosis of hypoglycemia or hyperglycemia based on ICD-9-CM codes from the patient's electronic medical records between January 1, 1996, and cohort entry. The following ICD-9 codes were used to identify severe hyperglycemic episodes: secondary diabetes mellitus with hyperosmolarity (249.2), diabetes with ketoacidosis (250.1x), and diabetes with hyperosmolarity (250.2). Severe hypoglycemic events were identified with the following ICD-9 codes based on a modified algorithm: hypoglycemic coma (251.0), other specified hypoglycemia (251.1), and hypoglycemia, unspecified (251.2).22 History of severe hypoglycemic and hyperglycemic events was treated as a binary exposure variable. Patients were also categorized according to combined exposure to both types of glycemic events at baseline as follows: patients without any severe hypoglycemic or hyperglycemic events (no glycemic events), patients with severe hypoglycemic exposure only, patients with severe hyperglycemic exposure only, and patients with both severe hypoglycemic and severe hyperglycemic events (both glycemic events).
Dementia Diagnosis
Consistent with previous studies in this population,8,10 dementia diagnoses were identified in electronic health records from both inpatient and outpatient visits with the following ICD-9 codes: Alzheimer disease (331.0), vascular dementia (290.4x), and other/nonspecific dementia (290.0, 290.1x, 290.2x, 290.3, 294.1, 294.2x, and 294.8).
Death
Deaths were identified through electronic medical records, California death certificates, and Social Security Administration datasets.
Covariates
Age, sex, and self-reported race/ethnicity were captured from KPNC health plan membership databases. A missing indicator was used for participants with missing data on race/ethnicity. For each participant, the HbA1c value closest to, but preceding, cohort entry was extracted from Kaiser Permanente laboratory measures. The following comorbid conditions were captured from electronic medical records between January 1, 1996, and cohort entry (using ICD-9 and Current Procedural Terminology codes) and were categorized as binary indicators (present/absent): depression (296.2, 296.3, 298.0, 300.4, 309.28, 311.x), nephropathy (250.4, 585.x, 583.81, 58.81), stroke (431.x, 433.01, 433.11, 433.21, 433.31, 433.81, 433.91, 434.0, 434.1, 434.9, 435.0, 435.1, 435.3, 435.8, 435.9, 436.x), neuropathy (354.x, 355.x), severe retinopathy (362.02, 362.07, 362.53, 362.83, 67,228, 67,208, 67,210), peripheral artery disease (440.x, 441.x, 442.0, 442.3, 443.81, 443.9), hypertension (401.x, 402.x, 403.x, 404.x, 405.x), and hyperlipidemia (272.x).
Statistical Analysis
We examined the distribution of dementia, demographics, diabetes complications, and HbA1c by severe glycemic event status using p values obtained from Cox proportional hazards models with age as the time scale.23 Age-adjusted incidence rates of dementia were estimated overall and across the following exposure categories: severe hypoglycemic event (yes/no), severe hyperglycemic event (yes/no), and combined glycemic events (neither glycemic event, hypoglycemic event only, hypoglycemic event only, or both glycemic events). Age-adjusted incidence rates of dementia were calculated from the age distribution of the US population from the 2000 US Census across the following age groups: 50 to <55, 55 to <60, 60 to <65, 65 to <70, 70 to <75, 75 to <80, 80 to <85, 85 to 90, and ≥90 years. Person-time during follow-up is allocated across the different age groups on the basis of an individual's age at the start of follow-up; as the person ages during the study, that individual’s person-time is allocated across the different age groups. We also estimated cumulative incidence of dementia in 5-year increments (from 50 to ≥90) conditional on dementia-free survival until age 50 years adjusting for competing risk of death. Cox proportional hazards models with age as the time scale were implemented to estimate the association between severe hypoglycemic and hyperglycemic events and dementia. We fit models with hypoglycemic and hyperglycemic exposures modeled separately and together in the same model to examine the association of a given glycemic exposure while simultaneously adjusting for exposure to the other type of glycemic event. We also used Cox proportional hazards models to examine the risk of dementia among individuals according to their combined exposure to both types of glycemic events (no glycemic events, hypoglycemic event only, hyperglycemic event only, both glycemic events). Models were first adjusted for race/ethnicity and sex (demographics; model 1), then additionally adjusted for HbA1c (model 2), and then the following comorbid conditions that have been shown previously to be associated with dementia and severe glycemic events in populations with diabetes: depression, stroke, and nephropathy (model 3).24-26 To explore the directionality of the association between severe glycemic events and dementia, we conducted a sensitivity analysis in which we restricted the sample to only those participants with ≥2 years of follow-up. This analytic strategy purposefully imposes a greater length of time between exposure and outcome to explore the temporality of the association between glycemic events and the development of dementia. All analyses were performed with SAS 9.4 (SAS Institute Inc, Cary, NC).
Data Availability
Data are available on reasonable request. Deidentified data from participants are available on request/approval from the corresponding author.
Standard Protocol Approvals, Registrations, and Patient Consents
This study was approved by the Institutional Review Board at KPNC.
Results
In this cohort of 2,821 older adults with T1D, over an average of 6.9 years (SD 5.0 years) of follow-up, a total of 153 patients (5.4%) received a diagnosis of dementia (table 1). The mean age at cohort entry was 56.5 years (SD 7.8 years); 48% were female; and the majority of the sample was White (81%). At cohort entry, 14.1% (n = 398) of the sample had experienced a severe hypoglycemic event, 11.9% (n = 335) had experienced a severe hyperglycemic event, and 3.1% (n = 87) had experienced both (table 1). Compared to individuals without prior severe hypoglycemic events, those with prior severe hypoglycemic events were younger and more likely to be Black. They also were more likely to have depression, neuropathy, nephropathy, retinopathy, stroke, peripheral arterial disease, hypertension, and hyperlipidemia. Compared to participants without prior hyperglycemia, those with severe hyperglycemic events were younger, had higher HbA1c levels, and were more likely to be female and Black. They were also more likely to have neuropathy, nephropathy, stroke, peripheral arterial disease, hypertension, and hyperlipidemia (table 1). By the end of follow-up, 153 participants (5.4%) had been diagnosed with dementia, 452 (16.0%) had died, 703 (29.7%) had a lapse in health plan membership, and 1,513 (63.9%) were dementia-free and remained KPNC members.
Table 1.
Baseline Characteristics by Severe Hyperglycemia and Hypoglycemia Exposure Status at Baseline
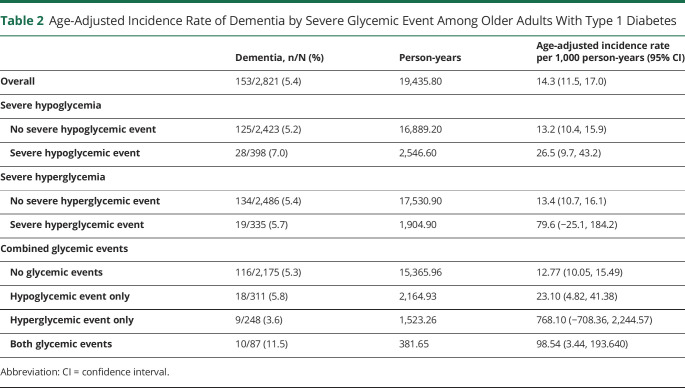
Overall, the age-adjusted incidence rate of dementia was 14.3 per 1,000 person-years (table 2). Across all exposure categories, exposure to severe glycemic events was associated with higher incidence rates of dementia. The age-adjusted incidence rate of dementia was 26.5 per 1,000 person-years among individuals with severe hypoglycemia vs 13.2 among individuals without. The incidence rate of dementia was 79.6 per 1,000 person-years among individuals with severe hyperglycemia vs 13.4 among individuals without. For those with both severe glycemic events, the incidence rate of dementia was 98.5 per 1,000 person-years vs 12.8 among individuals without.
Table 2.
Age-Adjusted Incidence Rate of Dementia by Severe Glycemic Event Among Older Adults With Type 1 Diabetes
The cumulative incidence of dementia at any time after baseline conditional on dementia-free survival to age 50 years was consistently higher among individuals with severe hypoglycemic events compared to those without severe hypoglycemic events (figure), among those with severe hyperglycemic events compared to those without severe hyperglycemic events (figure), and among those with both hypoglycemic and hyperglycemic events compared to those with neither (figure).
Figure. Cumulative Incidence of Dementia Conditional on Dementia-Free Survival Until Age 50 Years Accounting for Competing Risk of Death.

(A) Severe hypoglycemic events among those with a history of a severe hypoglycemic event (red line with red shaded 95% confidence band) vs those without a history of a severe hypoglycemic event (blue line with blue shaded 95% confidence band). (B) Severe hyperglycemic events among those with history of a severe hyperglycemic event (red line with red shaded 95% confidence band) vs those without history of a severe hyperglycemic event (blue line with blue shaded 95% confidence band). (C) Combined glycemic events among those with a history of both glycemic events (brown line with brown shaded 95% confidence band), hypoglycemic event only (red line with red shaded 95% confidence band), hyperglycemic event only (green line with green shaded 95% confidence band), or no history of glycemic events (blue line with blue shaded 95% confidence band).
In Cox proportional hazards models, severe hypoglycemia, severe hyperglycemia, and both glycemic events were associated with increased risk of dementia. In models adjusted for age (as time scale), race/ethnicity, HbA1c, depression, nephropathy, and stroke, individuals with baseline history of severe hypoglycemia had 80% greater risk of developing dementia (hazard ratio [HR] 1.75; 95% confidence interval [CI] 1.15, 2.66; table 3). In models examining the association between severe hyperglycemic events and dementia (adjusted for the same covariates listed above), individuals with baseline history of severe hyperglycemic events had more than double the risk of developing dementia (HR 2.24; 95% CI 1.32, 3.78). Simultaneous adjustment for both glycemic events (e.g., adjusting for hypoglycemic events and hyperglycemic events in the same model) resulted in a slight attenuation of the association, although findings remained significant for both hypoglycemic and hyperglycemic events. In models adjusting for the same set of covariates above (age [as time scale], race/ethnicity, HbA1c, depression, nephropathy, and stroke), compared to those with neither glycemic event at baseline, individuals who experienced both events had a 6-fold greater risk of developing dementia during the course of follow-up (HR 6.20, 95% CI 3.02, 12.70). Among the subset of participants with a minimum of 2 years of follow-up (n = 2,326), the associations between severe hypoglycemia and dementia, severe hyperglycemia and dementia, and both glycemic events and dementia were all slightly attenuated but remained statistically significant (results not shown).
Table 3.
Hazards Ratio of Severe Glycemic Events Predicting Time to Dementia Among Individuals With Type 1 Diabetes
Discussion
In this study of 2,821 older adults with T1D, exposure to severe glycemic events was associated with a significantly increased risk of developing dementia. Individuals with exposure to severe hypoglycemic events were 75% more likely to develop dementia during follow-up, while those with prior exposure to severe hyperglycemic events were more than twice as likely to develop dementia. The risk of developing dementia over the course of follow-up was nearly 6 times higher in individuals with exposure to both types of severe glycemic events (hypoglycemic and hyperglycemic) vs those without exposure to either glycemic event.
In this study, we report an association between severe glycemic events and risk of dementia in T1D. Prior studies have established an association between severe glycemic events and increased dementia risk in T2D.10,11 However, to the best of our knowledge, no prior studies have examined severe hypoglycemic or hyperglycemic events and incident dementia in a T1D population. There are a number of reasons why one could expect to see different patterns of dementia risk in T1D and T2D. Older patients with T1D are very different from older patients with T2D; because dementia diagnosis occurs in later life and T1D typically has a younger age at onset, those with T1D have lived with diabetes for much longer than those with T2D. The influence of decades-long exposure to diabetes on dementia is unknown. In addition to the longer disease duration/younger age at onset, those with T1D have had continuous insulin use since the time of diagnosis; the impact of prolonged insulin use on cognitive function/dementia has not been studied. Additionally, rates of microvascular and macrovascular complications in T1D differ as compared to T2D and the contribution of these complications to dementia risk is unknown.27 Finally, individuals with T1D are more likely to experience severe glycemic events than those with T2D.12-14 In this study, we evaluate the association between severe glycemic events and dementia in T1D.
Pathophysiologic mechanisms by which severe glycemic events may contribute to dementia risk in T1D could result from structural changes in the brain caused by severe events or neuropathologic changes stemming from repeated exposures to dysglycemia. Prior studies, mainly in children, have shown that severe hyperglycemic events produce short-term changes in the brain (neuronal dysfunction, reduced cerebral blood flow, and cerebral edema) and are associated with altered brain growth that is observable up to 4 years after the DKA occurrence.18,28,29 Additional studies, mainly in children and adolescents with T1D, have reported an association between a history of severe hyperglycemia and adverse cognitive effects; possible explanations include increased exposure to oxidative stress, inflammation, or insulin deficiency.30,31 Regarding hypoglycemia in T1D, findings are more heterogeneous. Severe hypoglycemia has been associated with alterations in brain structure, including less gray matter volume; neuronal damage in several regions of the cortex, including the hippocampus; and cortical atrophy.15-17 In children, findings generally support an association between severe hypoglycemia and decreased cognitive performance,32-34 although not in all studies.35,36 In young adults, however, studies examining severe hypoglycemia and cognition have generally found no association.37,38 To the best of our knowledge, only 1 study has examined the association between severe hypoglycemia and cognitive function in older adults with T1D; in that study, severe hypoglycemia during follow-up was associated with greater cognitive decline (median follow-up 4.1 years).39 In T2D, studies have reported an association between severe hypoglycemia and incident dementia and severe hyperglycemia and dementia.10,11 Whether this association is true in T1D was previously unknown.
Because individuals with T1D are only recently living to older ages, optimal glycemic control in this population is not well understood. Individuals with T1D who have survived into older adulthood are a unique population. They have been living with and managing their disease for a long time and are likely to have experienced several severe glycemic events over their lifetime. As these individuals age, managing their health can become more complicated. Studies have shown that even minor changes in cognitive function can translate into significant changes in diabetes self-care; this, in turn, can lead to increased glycemic events and potentially more cognitive decline.40 In addition, impaired awareness of hypoglycemia is a prevalent issue in this population that may contribute to increased occurrences of severe hypoglycemic events.41 The cumulative insult of these events on the aging brain is unknown but underscores the need for improved understanding of optimal glycemic control in this growing population.
There are several strengths of the present study. To the best of our knowledge, this is the first study to investigate the long-term association of severe hypoglycemic and hyperglycemic events with incident dementia among older adults with T1D. The stability of the KPNC health plan membership allowed us to ascertain incident dementia diagnoses over a prolonged period of time and to ensure that glycemic events temporally precede diagnoses of dementia. The size of the health plan allowed us to identify a large cohort of participants with T1D with adequate statistical power to examine the association between these potentially life-threatening events and incident dementia. We were also able to account for a number of potential comorbid conditions to examine the robustness of our findings. Furthermore, the use of KPNC allowed us to identify a cohort of patients with uniform access to medical care; this is especially important because capturing diagnoses relies on health care use. Finally, because exposure, covariate, and outcome data were obtained from patients' medical records, they are not subject to certain biases associated with self-report and recall.
This study also has some limitations. One of the main limitations of our study was the use of clinical diagnoses to identify incident cases of dementia. This may have resulted in underascertainment of the true number of incident cases given results from a recent meta-analysis suggesting high rates of undetected dementia.42 Despite this limitation, identification of dementia cases with ICD-9 codes has been successfully used in prior studies in this population.8,10 In addition, in a 2016 study conducted among members of KPNC using the same set of ICD-9 codes to ascertain dementia,43 age-specific incidence rates in the KPNC sample were comparable to incidence rates reported in recent cohort studies,44 suggesting that the use of clinical diagnoses in our integrated health care system reliably ascertains dementia cases. In a study conducted in a large integrated health care system in Washington State, using the same ICD-9 codes for dementia was reported to have high specificity (95%) and a somewhat lower sensitivity (77%) compared to prospective comprehensive case ascertainment, including cognitive testing, physical examination, informant interviews, and medical records review.45 Another study based in the United Kingdom reported similar sensitivity (78%) and specificity (92%).46 High specificity and lower sensitivity would result in underascertainment of dementia cases, which would bias our estimates toward the null and underestimate the association between glycemic events and dementia. A related limitation is the use of diagnosis codes from the emergency department or inpatient setting that represent the most severe events but do not address the role of less severe but more frequent glycemic episodes on dementia risk. In addition, the observational design limits our ability to make causal inferences. That said, experimental studies on severe glycemic events are infeasible, dangerous, and unethical, and thus, our understanding of the effect of these events on brain health can be informed only by findings from observational studies. A related limitation is the inability to establish temporality between onset of dementia and occurrence of severe glycemic events; despite this limitation, findings were robust when we imposed a lag of at least 2 years between the occurrence of a severe glycemic event and the dementia diagnosis. Another limitation was the lack of information on age at diabetes onset, educational attainment, or area deprivation; this additional information would have been informative in evaluating the role of severe glycemic events on dementia risk. People who had severe glycemic events were also more likely to have a number of comorbid conditions such as depression and stroke; although we adjusted for these comorbid conditions, they could be on the causal pathway between glycemic events and dementia. We plan to explore this in future studies. Finally, because we did not have brain imaging or neuropathology data, we cannot distinguish between different subtypes of dementia and thus cannot makes inferences on whether the pathways between severe glycemic events and dementia are due to neurodegenerative changes or brain vascular injury. This will be a focus of future research.
The increasing incidence of T1D,47,48 coupled with greater life expectancy,4 is resulting in an unprecedented number of older adults living with and managing T1D. As this population continues to grow and age, understanding the impact that a lifetime of chronic disease and associated complications has on brain health will be crucial for informing clinical care for this population and implementing prevention strategies for those with T1D who are still early in the course of their disease. In this study of older patients with T1D, exposure to severe hypoglycemic and hyperglycemic events was independently associated with substantively increased risk of dementia; combined exposure to both hypoglycemic and hyperglycemic events was associated with an even higher risk. This study complements existing literature by extending the association between severe glycemic events and dementia to a previously unstudied population: older adults with T1D who have a much higher prevalence of these events than those with T2D. Understanding the role of glycemic control over the lifetime on brain health in older adulthood will, we hope, raise awareness regarding the importance of optimal self-care throughout the life course. Our findings suggest that exposure to severe glycemic events may have long-term consequences on brain health and should be considered as an additional motivating factor to avoid severe glycemic events throughout one's lifetime.
Glossary
- CI
confidence interval
- DKA
diabetic ketoacidosis
- HbA1c
hemoglobin A1c
- HR
hazard ratio
- ICD-9-CM
International Classification of Diseases, 9th Revision, Clinical Modification
- KPNC
Kaiser Permanente Northern California
- T1D
type 1 diabetes
- T2D
type 2 diabetes
Appendix. Authors

Footnotes
CME Course: NPub.org/cmelist
Study Funding
This work was supported by the NIH (grant R01 AG047500, principal investigator Dr. Whitmer). Dr. Lacy was supported by the University of California, San Francisco Training for Research on Aging and Chronic Disease (T32 AG049663) and contract PPRN-1306-04,709 from the Patient-Centered Outcomes Research Institute.
Disclosure
R.A. Whitmer, P. Gilsanz, C.P. Quesenberry, A.J. Karter, and M.E. Lacy report no disclosures relevant to the manuscript. Go to Neurology.org/N for full disclosures.
References
- 1.Rewers A, Cowie CC, Casagrande SS, et al. Acute metabolic complications in diabetes. In: Diabetes in America, 3rd ed. NIH; 2018. [PubMed] [Google Scholar]
- 2.Weinstock RS, Xing D, Maahs DM, et al. Severe hypoglycemia and diabetic ketoacidosis in adults with type 1 diabetes: results from the T1D Exchange Clinic Registry. J Clin Endocrinol Metab. 2013;98(8):3411-3419. [DOI] [PubMed] [Google Scholar]
- 3.McCoy RG, Van Houten HK, Ziegenfuss JY, Shah ND, Wermers RA, Smith SA. Increased mortality of patients with diabetes reporting severe hypoglycemia. Diabetes Care. 2012;35(9):1897-1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Miller RG, Secrest AM, Sharma RK, Songer TJ, Orchard TJ. Improvements in the life expectancy of type 1 diabetes: the Pittsburgh Epidemiology of Diabetes Complications study cohort. Diabetes. 2012;61(11):2987-2992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Smolina K, Wotton CJ, Goldacre MJ. Risk of dementia in patients hospitalised with type 1 and type 2 diabetes in England, 1998-2011: a retrospective national record linkage cohort study. Diabetologia. 2015;58(5):942-950. [DOI] [PubMed] [Google Scholar]
- 6.Kuo CL, Lu CL, Chang YH, Li CY. Population-based cohort study on dementia risk in patients with type 1 diabetes mellitus. Neuroepidemiology. 2018;50(1-2):57-62. [DOI] [PubMed] [Google Scholar]
- 7.Yaffe K, Blackwell T, Whitmer RA, Krueger K, Barrett Connor E. Glycosylated hemoglobin level and development of mild cognitive impairment or dementia in older women. J Nutr Health Aging. 2006;10(4):293-295. [PubMed] [Google Scholar]
- 8.Lacy ME, Gilsanz P, Karter AJ, Quesenberry CP, Pletcher MJ, Whitmer RA. Long-term glycemic control and dementia risk in type 1 diabetes. Diabetes Care. 2018;41(11):2339-2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Crane PK, Walker R, Larson EB. Glucose levels and risk of dementia. N Engl J Med. 2013;369(6):1863-1864. [DOI] [PubMed] [Google Scholar]
- 10.Whitmer RA, Karter AJ, Yaffe K, Quesenberry CP, Selby JV. Hypoglycemic episodes and risk of dementia in older patients with type 2 diabetes mellitus. JAMA. 2009;301(15):1565-1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen YL, Weng SF, Yang CY, Wang JJ, Tien KJ. Diabetic ketoacidosis further increases risk of Alzheimer's disease in patients with type 2 diabetes. Diabetes Res Clin Pract. 2019;147:55-61. [DOI] [PubMed] [Google Scholar]
- 12.Donnelly LA, Morris AD, Frier BM, et al. Frequency and predictors of hypoglycaemia in Type 1 and insulin-treated type 2 diabetes: a population-based study. Diabet Med. 2005;22(6):749-755. [DOI] [PubMed] [Google Scholar]
- 13.U.K. Hypoglycaemia Study Group. Risk of hypoglycaemia in types 1 and 2 diabetes: effects of treatment modalities and their duration. Diabetologia. 2007;50(6):1140-1147. [DOI] [PubMed] [Google Scholar]
- 14.Zhong VW, Juhaeri J, Mayer-Davis EJ. Trends in hospital admission for diabetic ketoacidosis in adults with type 1 and type 2 diabetes in England, 1998-2013: a retrospective cohort study. Diabetes Care. 2018;41(9):1870-1877. [DOI] [PubMed] [Google Scholar]
- 15.Kalimo H, Olsson Y. Effects of severe hypoglycemia on the human brain neuropathological case reports. Acta Neurol Scand. 2009;62(6):345-356. [DOI] [PubMed] [Google Scholar]
- 16.Perros P, Deary IJ, Sellar RJ, et al. Brain abnormalities demonstrated by magnetic resonance imaging in adult IDDM patients with and without a history of recurrent severe hypoglycemia. Diabetes Care. 1997;20(6):1013-1018. [DOI] [PubMed] [Google Scholar]
- 17.Perros P, Frier B. The long-term sequelae of severe hypoglycemia on the brain in insulin-dependent diabetes mellitus. Horm Metab Res. 1997;29(5):197-202. [DOI] [PubMed] [Google Scholar]
- 18.Cameron FJ, Scratch SE, Nadebaum C, et al. Neurological consequences of diabetic ketoacidosis at initial presentation of type 1 diabetes in a prospective cohort study of children. Diabetes Care. 2014;37(6):1554-1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Glaser N, Ngo C, Anderson S, Yuen N, Trifu A, O'Donnell M. Effects of hyperglycemia and effects of ketosis on cerebral perfusion, cerebral water distribution, and cerebral metabolism. Diabetes. 2012;61(7):1831-1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Krieger N. Overcoming the absence of socioeconomic data in medical records: validation and application of a census-based methodology. Am J Public Health. 1992;82(5):703-710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Klompas M, Eggleston E, McVetta J, Lazarus R, Li L, Platt R. Automated detection and classification of type 1 versus type 2 diabetes using electronic health record data. Diabetes Care. 2013;36(4):914-921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ginde AA, Blanc PG, Lieberman RM, Camargo CA Jr. Validation of ICD-9-CM coding algorithm for improved identification of hypoglycemia visits. BMC Endocr Disord. 2008;8:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Korn EL, Graubard BI, Midthune D. Time-to-event analysis of longitudinal follow-up of a survey: choice of the time-scale. Am J Epidemiol. 1997;145(1):72-80. [DOI] [PubMed] [Google Scholar]
- 24.Gilsanz P, Karter AJ, Beeri MS, Quesenberry CP, Whitmer RA. The bidirectional association between depression and severe hypoglycemic and hyperglycemic events in type 1 diabetes. Diabetes Care. 2018;41(3):446-452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Luitse MJ, Biessels GJ, Rutten GE, Kappelle LJ. Diabetes, hyperglycaemia, and acute ischaemic stroke. Lancet Neurol. 2012;11(3):261-271. [DOI] [PubMed] [Google Scholar]
- 26.Seliger SL, Siscovick DS, Stehman-Breen CO, et al. Moderate renal impairment and risk of dementia among older adults: the Cardiovascular Health Cognition Study. J Am Soc Nephrol. 2004;15(7):1904-1911. [DOI] [PubMed] [Google Scholar]
- 27.Murthy SB, Jawaid A, Qureshi SU, et al. Does diabetes mellitus alter the onset and clinical course of vascular dementia? Behav Neurol. 2010;23(3):145-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Siller AF, Lugar H, Rutlin J, et al. Severity of clinical presentation in youth with type 1 diabetes is associated with differences in brain structure. Pediatr Diabetes. 2017;18(8):686-695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aye T, Mazaika PK, Mauras N, et al. Impact of early diabetic ketoacidosis on the developing brain. Diabetes Care. 2019;42(3):443-449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ghetti S, Lee JK, Sims CE, Demaster DM, Glaser NS. Diabetic ketoacidosis and memory dysfunction in children with type 1 diabetes. J Pediatr. 2010;156(1):109-114. [DOI] [PubMed] [Google Scholar]
- 31.Jessup AB, Grimley MB, Meyer E, et al. Effects of diabetic ketoacidosis on visual and verbal neurocognitive function in young patients presenting with new-onset type 1 diabetes. J Clin Res Pediatr Endocrinol. 2015;7(3):203-210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Naguib JM, Kulinskaya E, Lomax CL, Garralda ME. Neuro-cognitive performance in children with type 1 diabetes: a meta-analysis. J Pediatr Psychol. 2009;34(3):271-282. [DOI] [PubMed] [Google Scholar]
- 33.Hershey T, Craft S, Bhargava N, White NH. Memory and insulin dependent diabetes mellitus (IDDM): effects of childhood onset and severe hypoglycemia. J Int Neuropsychol Soc. 1997;3(6):509-520. [PubMed] [Google Scholar]
- 34.Blasetti A, Chiuri RM, Tocco AM, et al. The effect of recurrent severe hypoglycemia on cognitive performance in children with type 1 diabetes: a meta-analysis. J child Neurol. 2011;26(11):1383-1391. [DOI] [PubMed] [Google Scholar]
- 35.Strudwick SK, Carne C, Gardiner J, Foster JK, Davis EA, Jones TW. Cognitive functioning in children with early onset type 1 diabetes and severe hypoglycemia. J Pediatr. 2005;147(5):680-685. [DOI] [PubMed] [Google Scholar]
- 36.Ferguson SC, Blane A, Perros P, et al. Cognitive ability and brain structure in type 1 diabetes: relation to microangiopathy and preceding severe hypoglycemia. Diabetes. 2003;52(1):149-156. [DOI] [PubMed] [Google Scholar]
- 37.Jacobson AM, Musen G, Ryan CM, et al. Long-term effects of diabetes and its treatment on cognitive function. N Engl J Med. 2007;356:1842-1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Austin EJ, Deary IJ. Effects of repeated hypoglycemia on cognitive function: a psychometrically validated reanalysis of the Diabetes Control and Complications Trial data. Diabetes Care. 1999;22(8):1273-1277. [DOI] [PubMed] [Google Scholar]
- 39.Duinkerken EV, Brands AM, van den Berg E, Henselmans JM, Hoogma RP, Biessels GJ. Cognition in older patients with type 1 diabetes mellitus: a longitudinal study. J Am Geriatr Soc. 2011;59(3):563-565. [DOI] [PubMed] [Google Scholar]
- 40.Sinclair AJ, Girling AJ, Bayer AJ. Cognitive dysfunction in older subjects with diabetes mellitus: impact on diabetes self-management and use of care services. Diabetes Res Clin Pract. 2000;50(3):203-212. [DOI] [PubMed] [Google Scholar]
- 41.Cryer PE. Hypoglycemia begets hypoglycemia in IDDM. Diabetes. 1993;42(12):1691-1693. [DOI] [PubMed] [Google Scholar]
- 42.Lang L, Clifford A, Wei L, et al. Prevalence and determinants of undetected dementia in the community: a systematic literature review and a meta-analysis. BMJ Open. 2017;7(2):e011146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mayeda ER, Glymour MM, Quesenberry CP, Whitmer RA. Inequalities in dementia incidence between six racial and ethnic groups over 14 years. Alzheimers Dement. 2016;12(3):216-224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kukull WA, Higdon R, Bowen JD, et al. Dementia and Alzheimer disease incidence: a prospective cohort study. Arch Neurol. 2002;59(11):1737-1746. [DOI] [PubMed] [Google Scholar]
- 45.Katon WJ, Lin EH, Williams LH, et al. Comorbid depression is associated with an increased risk of dementia diagnosis in patients with diabetes: a prospective cohort study. J Gen Intern Med. 2010;25:423-429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sommerlad A, Perera G, Singh-Manoux A, Lewis G, Stewart R, Livingston G. Accuracy of general hospital dementia diagnoses in England: sensitivity, specificity, and predictors of diagnostic accuracy 2008-2016. Alzheimers Dement. 2018;14(7):933-943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mayer-Davis EJ, Lawrence JM, Dabelea D, et al. Incidence trends of type 1 and type 2 diabetes among youths, 2002-2012. N Engl J Med. 2017;376(15):1419-1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Patterson CC, Dahlquist GG, Gyürüs E, et al. Incidence trends for childhood type 1 diabetes in Europe during 1989-2003 and predicted new cases 2005-20: a multicentre prospective registration study. Lancet. 2009;373:2027-2033. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available on reasonable request. Deidentified data from participants are available on request/approval from the corresponding author.