Astrocytes are glial cells of the central nervous system (CNS) that are strategically positioned to control CNS immunity in the context of health and disease. Here, Sanmarco, Polonio, Wheeler, and Quintana review progress in elucidating astrocyte–immune interactions in multiple sclerosis.
Abstract
Astrocytes are abundant glial cells in the central nervous system (CNS) that control multiple aspects of health and disease. Through their interactions with components of the blood–brain barrier (BBB), astrocytes not only regulate BBB function, they also sense molecules produced by peripheral immune cells, including cytokines. Here, we review the interactions between immune cells and astrocytes and their roles in health and neurological diseases, with a special focus on multiple sclerosis (MS). We highlight known pathways that participate in astrocyte crosstalk with microglia, NK cells, T cells, and other cell types; their contribution to the pathogenesis of neurological diseases; and their potential value as therapeutic targets.
Introduction
Complex biological responses involving the coordinated activities of multiple cell types in the central nervous system (CNS) control neurological function and dysfunction. A growing body of evidence points to the importance for CNS physiology of the reciprocal communication between CNS-resident cells and the immune system. Indeed, one area of particular interest is the role of neuroimmune interactions between astrocytes and immune cells in health and disease.
Astrocytes are abundant CNS glial cells that participate in diverse processes ranging from synaptogenesis to neuron metabolic support, the regulation of blood–brain barrier (BBB) function, and behavior (Allen and Lyons, 2018; Ben Haim and Rowitch, 2017; Khakh and Deneen, 2019; Linnerbauer et al., 2020). In addition to the control of these and other functions in the healthy CNS, diverse astrocyte subsets play key roles in multiple neurological diseases, including multiple sclerosis (MS; Kim et al., 2014; Mayo et al., 2014; Rothhammer et al., 2016, 2018; Itoh et al., 2018; Wheeler et al., 2019, 2020; Linnerbauer et al., 2020; Sanmarco et al., 2021), Alzheimer’s disease (AD; Habib et al., 2020; Mathys et al., 2019; Zhou et al., 2020), Huntington’s disease (Diaz-Castro et al., 2019; Jiang et al., 2016; Khakh et al., 2017; Tong et al., 2014; Yu et al., 2018), and acute CNS injury (Anderson et al., 2016; Anderson et al., 2018). Although reactive astrocytes were originally identified over 100 yr ago (Andriezen, 1893), the advent of new technologies to dissect astrocyte molecular and phenotypic heterogeneity in depth has led to a rapid growth in our understanding of astrocyte heterogeneity and its regulation. Consequently, the diverse functions of astrocytes in health and disease are now appreciated to encompass a continuum of cellular states with the potential for plasticity and reprogramming (Anderson et al., 2014; Ben Haim and Rowitch, 2017; Escartin et al., 2021; Khakh and Deneen, 2019; Linnerbauer et al., 2020).
Astrocyte heterogeneity in health and disease
The heterogeneity of astrocytes is best exemplified by their complex morphology. Astrocytes exhibit highly ramified processes; each astrocyte contacts ∼100,000 synapses in mice (Houades et al., 2008) and up to 2,000,000 in humans (Oberheim et al., 2009). Indeed, astrocytes are key components of the tripartite synapse, which encompasses pre- and postsynaptic neuron membranes and surrounding astrocytes (Allen and Eroglu, 2017), buffering neurotransmitter release, controlling neuronal excitability, and modulating synaptic plasticity. Recent reports have linked astrocyte electrical activity via Ca2+ waves to multiple behaviors, including memory, repetitive behavior, and fear (Adamsky et al., 2018; Boisvert et al., 2018; Martin-Fernandez et al., 2017; Nagai et al., 2019; Nagai et al., 2021; Yu et al., 2018), highlighting the physiological roles of these astrocyte–neuron interactions.
Astrocytes are in close contact with the CNS blood supply. Through direct interactions with components of the BBB, including endothelial cells and pericytes, astrocytes not only regulate BBB function but also sense molecules produced by circulating peripheral cells, including cytokines (Sanmarco et al., 2021; Sofroniew, 2015). In fact, roughly 97% of astrocytes are estimated to contact blood vessels (Foo et al., 2011), suggesting that astrocyte responses are continuously modulated by signals originating both within and outside the CNS. In this sense, astrocytes can be considered communication conduits, which in response to peripheral cues sensed via the CNS vasculature adopt specialized cellular states that influence the local microenvironment and consequently CNS physiology, inflammation, and neurodegeneration. Indeed, high-dimensional analyses of gene and protein expression suggest that astrocytes exhibit significant heterogeneity as a function of their location, cell–cell interactions, and peripheral factors provided by the vasculature (Clark et al., 2021; Escartin et al., 2021; Habib et al., 2020; John Lin et al., 2017; Sanmarco et al., 2021; Saunders et al., 2018; Wheeler et al., 2020; Zeisel et al., 2015).
Early descriptions of astrocyte heterogeneity focused on the divergent morphology of white matter (fibrous) and cortical (protoplasmic) astrocytes, which also differ in their expression of canonical astrocyte marker genes such as GFAP (Eng et al., 1971). Recent technologic advances have allowed the analysis of these morphologically distinct populations in greater detail. For example, an early single-cell RNA sequencing brain atlas identified distinct subpial and subcortical astrocyte populations based on the expression of previously underappreciated marker genes (Zeisel et al., 2015). Additional studies identified multiple transcriptionally distinct astrocyte subpopulations across different brain regions (Saunders et al., 2018; Zeisel et al., 2018). Most recently, this molecular heterogeneity was analyzed using multiplex in situ transcriptomic and proteomic approaches, which detected significant astrocyte heterogeneity in cortex layers (Bayraktar et al., 2020), suggesting that astrocyte identity is shaped by interactions with neighboring cells in the local microenvironment.
A pressing question is the role of functional astrocyte heterogeneity in homeostasis and disease (Escartin et al., 2021). Using a combination of transgenic mouse lines, imaging, and electrophysiology, novel astrocyte subsets have been recently identified in the hippocampus and striatum, revealing region-specific transcriptional and functional responses to stimulation (Chai et al., 2017). This work is consistent with elegant reports on the regulation by astrocytes of homotypic, but not heterotypic, medium spiny neuron synapses in the striatum (Martín et al., 2015). Moreover, these data are in agreement with reports of the interaction of specific astrocyte subsets with defined cell types in different brain regions (Molofsky et al., 2014). These recent insights on the spatial heterogeneity of astrocytes highlight the need to define the function and regulatory mechanisms associated with specific astrocyte subsets.
Novel astrocyte subsets have been recently identified in the context of CNS disorders. Pioneering studies reported that astrocytes isolated from a transgenic mouse model of amyotrophic lateral sclerosis induce neuronal death (Di Giorgio et al., 2007). More recently, Deneen and collaborators used transgenic reporter mice in combination with flow cytometry–based antibody screens to define astrocyte subsets based on the expression of surface markers. They identified astrocyte subsets associated with glioblastoma (marked by CD51 and/or CD71 expression) in both preclinical animal models and humans (John Lin et al., 2017). Using a similar approach in combination with in vivo cell-specific CRISPR-driven genetic perturbation studies, we recently identified an astrocyte subset characterized by the expression of TNF-related apoptosis-inducing ligand (TRAIL), which limits T cell–driven CNS inflammation (Sanmarco et al., 2021). Conversely, Barres and coworkers described neurotoxic astrocytes characterized by the expression of the complement protein C3, which were detected in multiple neurological diseases, including AD, Parkinson’s disease, and MS (Liddelow et al., 2017). Similarly, astrocyte subsets characterized by the activation of the unfolded protein response, GM-CSF signaling and/or sphingolipid metabolism promote CNS pathology in MS and prion disease (Chao et al., 2019; Smith et al., 2020; Wheeler et al., 2020). Taken together, these findings identify astrocytes as active players in CNS pathology rather than simply passive or reactive observers (Escartin et al., 2021).
Habib et al. recently identified an astrocyte subset associated with AD and its mouse preclinical model, characterized by the disruption of cholesterol and inflammatory pathways, and the up-regulation of specific genes linked to amyloid plaque pathology, including Serpina3n, Ctsb, Apoe, and Clu (Habib et al., 2020). Similarly, Colonna and coworkers described disease-specific transcriptional responses associated with astrocytes in AD (Zhou et al., 2020), which included the down-regulation of metabolism-associated genes linked to free fatty acid transport and detoxification, concomitant with an up-regulation of genes that promote glial scarring. Based on single-cell RNA sequencing and functional analyses of experimental autoimmune encephalomyelitis (EAE) and human MS samples, we recently identified a novel disease-promoting astrocyte subset controlled by MAFG and MAT2A in response to the T cell–derived cytokine GM-CSF (Wheeler et al., 2020). Collectively, these studies suggest that multiple astrocyte subsets are associated with neurodegeneration and CNS pathology through common and disease-specific mechanisms that include not only the release of neurotoxic molecules but also the activation of neurotoxic programs in microglia/monocytes and/or the decreased production of neurotrophic factors and metabolites involved in neuron support (Chao et al., 2019; Wheeler et al., 2020; Wheeler et al., 2019).
Astrocyte–microglia interactions
The roles of astrocytes and microglia in CNS development and function have been extensively studied (Allen and Lyons, 2018; Alvarez et al., 2011; Chung et al., 2015; Chung et al., 2013; Fields and Stevens-Graham, 2002; Goldmann et al., 2016; Keren-Shaul et al., 2017; Molofsky et al., 2014; Tsai et al., 2012). Although our mechanistic understanding of the complex interactions between astrocytes and microglia is still limited, significant progress has been made lately (Clark et al., 2021; Colombo and Farina, 2016; Goldmann and Prinz, 2013; Liddelow et al., 2017; Rothhammer et al., 2018; Rothhammer et al., 2016; Vainchtein et al., 2018; Vainchtein and Molofsky, 2020).
Cytokines and chemokines are key participants in bidirectional astrocyte–microglia communication. Microglia produce diverse mediators to limit or promote astrocyte pathogenic activities (Bezzi et al., 2001; Clark et al., 2021; Liddelow et al., 2017; Rothhammer et al., 2018). For example, CXCL12, also known as SDF-1α, triggers glutamate production by CXCR4+ astrocytes in a TNF-dependent manner. Indeed, activated microglia respond to CXCL12/SDF-1α by producing TNF, which acts on astrocytes to boost glutamate release and neurotoxicity (Bezzi et al., 2001), important contributors to the pathogenesis of MS and other neurological diseases. However, astrocyte–microglia interactions can also limit CNS pathology. For example, microglial IL-10 triggers TGF-β secretion in astrocytes, which then limits microglial pro-inflammatory responses (Norden et al., 2014).
Additional astrocyte-derived factors also modulate microglial responses (Chao et al., 2019; Mayo et al., 2014; Wheeler et al., 2020). IL-33 is an alarmin originally associated with the regulation of immunity in mucosal tissues (Martin and Martin, 2016). Astrocyte-derived IL-33 contributes to homeostatic synaptic plasticity by acting on neurons (Wang et al., 2021) and microglia (Vainchtein et al., 2018). However, although its levels are increased in serum and cerebrospinal fluid from MS patients (Jafarzadeh et al., 2016), the role of IL-33 in MS is still controversial (Bourgeois et al., 2009; Jiang et al., 2012; Li et al., 2012; Milovanovic et al., 2012; Pomeshchik et al., 2015). These studies highlight the role of cytokines as mediators of astrocyte–microglia bidirectional communication during CNS development and disease.
Dietary components and the gut microbiome have been shown to control CNS inflammation, as well as microglial and astrocyte responses (Berer et al., 2011; Erny et al., 2015; Haghikia et al., 2015; Kadowaki and Quintana, 2020; Ochoa-Repáraz et al., 2009; Rothhammer et al., 2018; Rothhammer et al., 2016; Sanmarco et al., 2021; Yokote et al., 2008). Interestingly, diet–microbiome interactions also modulate microglia–astrocyte communication. For example, we recently established a role for the aryl hydrocarbon receptor (AHR) in the control of microglia and astrocytes by the intestinal microbiome in EAE and, potentially, MS (Rothhammer et al., 2018; Rothhammer and Quintana, 2019; Wheeler et al., 2017). AHR activation by microbial metabolites derived from dietary tryptophan controls the microglial production of Tgfa and Vegfb. AHR activation boosts the production of microglial TGF-α, which acts on astrocytes via its receptor ErbB1 to reduce Ccl2, Il6, and Csf2 expression and consequently limit CNS inflammation and EAE development. Conversely, AHR activation suppresses the microglial production of vascular endothelial growth factor β (VEGFβ), which boosts proinflammatory gene expression in astrocytes via the FLT1 receptor, promoting the recruitment of proinflammatory monocytes, CNS inflammation, and neurodegeneration (Rothhammer et al., 2018). In addition, microbial metabolites can also act directly on astrocytes to control their responses in the context of inflammation (Rothhammer et al., 2016). Thus, diet–microbiome interactions influence not only microglia and astrocyte intrinsic responses but also microglia–astrocyte crosstalk and, consequently, CNS pathology.
Communication between astrocytes and T cells
Meningeal T cells play important roles in CNS physiology (Alves de Lima et al., 2020a; Filiano et al., 2016; Ribeiro et al., 2019), but T cells are rarely detected in the healthy brain parenchyma (Korin et al., 2017). However, T cells are detected in the CNS in MS and other neurological diseases (Gate et al., 2020; Wheeler et al., 2020). In this context, astrocyte–T cell interactions can boost or limit CNS inflammation based on the specific T cell subsets involved (Ito et al., 2019; Kang et al., 2010; Sanmarco et al., 2021; Wheeler et al., 2020; Fig. 1).
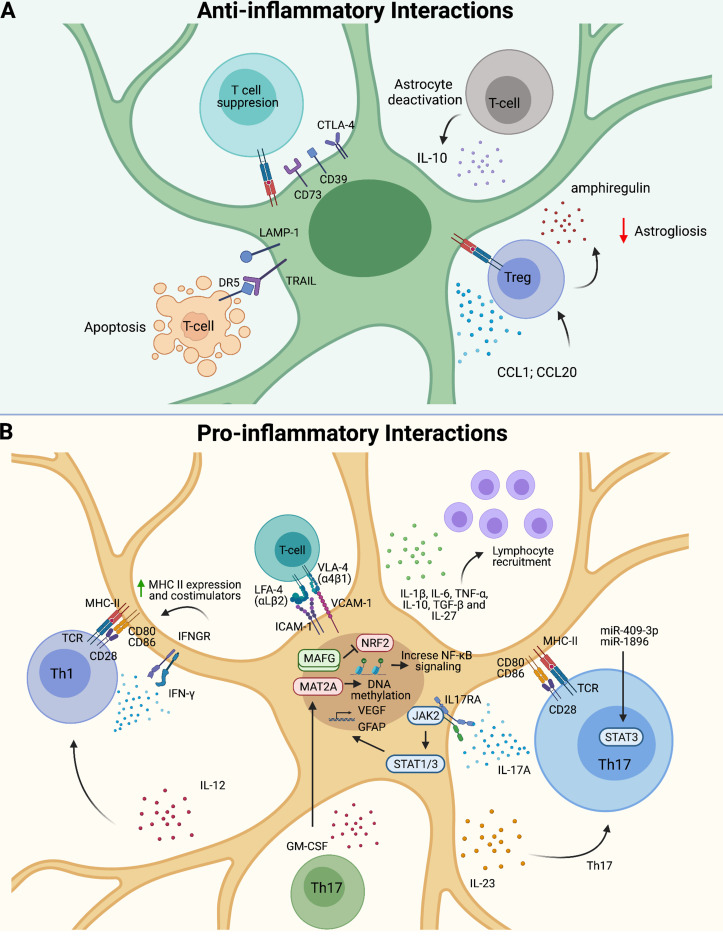
Figure 1.
Astrocyte–T cell bidirectional communication. (A) Regulatory T (Treg) cells limit astrogliosis via the production of amphiregulin and IL-10. Astrocytes express CTLA-4, CD73, and CD39, leading to T cell suppression. LAMP1+TRAIL+ astrocytes induce apoptosis of DR5+ T cells. (B) Astrocyte–T cell interactions via integrins can boost CNS inflammation. Astrocytes release pro-inflammatory cytokines to recruit and polarize pathogenic T cells. Th17 cells induce astrogliosis and VEGF up-regulation, whereas Th1 cells increase MHC class II and costimulation. GM-CSF activates MAFG-driven pathogenic activities in astrocytes.
GM-CSF–producing T helper type 17 cells (Th17 cells) contribute to the pathogenesis of MS and EAE (Codarri et al., 2011; El-Behi et al., 2011; Lee et al., 2012). Pathogenic Th17 cells, characterized by the production of IL-17 and GM-CSF, modify astrocyte responses via contact-independent mechanisms involving secreted neurotrophic factors, pro-inflammatory cytokines, and chemokines (Prajeeth et al., 2017; Wheeler et al., 2020). Indeed, astrocytes express the receptors for IL-17 and GM-CSF. IL-17 produced by T cells activates JAK2–STAT1/3 signaling in astrocytes, inducing astrogliosis and VEGF up-regulation (You et al., 2017). Meanwhile, GM-CSF+ T cells activate MAFG/MAT2A-driven pathogenic activities on astrocytes, resulting in the increased expression of Csf2, Il6, Ccl2, and Il1b, which are known to promote CNS pathology (Wheeler et al., 2020).
Pathogenic Th1 cells express IFNγ and GM-CSF, but IFNγ is also expressed by T cell subsets with anti-inflammatory roles in mice and humans (Groux et al., 1997; Mascanfroni et al., 2015). Indeed, the genetic inactivation of the receptor for IFNγ results in the worsening of EAE (Ferber et al., 1996). Interestingly, the expression of a dominant-negative IFNγ receptor boosts astrocyte activation and worsens CNS inflammation, demyelination, and axonal loss in EAE (Hindinger et al., 2012). A similar worsening of EAE was observed following CRISPR-driven inactivation of both IFNγR1 and IFNγR2 chains of the IFNγ receptor in astrocytes or the inactivation of the transcription factor STAT1 involved in the control of IFNγ-driven transcriptional responses (Sanmarco et al., 2021). These findings suggest an anti-inflammatory role for IFNγ signaling in astrocytes triggered by T cells and other IFNγ-producing cells in the CNS.
Additional molecules can mediate anti-inflammatory interactions between T cells and astrocytes. Regulatory T cells recruited to the CNS in response to CCL1 and CCL20 have been shown to suppress astrocyte neurotoxicity through a mechanism mediated by amphiregulin, limiting CNS pathology associated with stroke (Ito et al., 2019). Similarly, regulatory T cells 1 (Tr1 cells) suppress astrocyte pathogenic activities via the production of IL-10, which reduces pro-inflammatory cytokine and chemokine expression in astrocytes, as well as monocyte recruitment and microglial activation in MS preclinical models (Kenison et al., 2020; Mayo et al., 2016).
Conversely, astrocytes can also modulate T cell responses (Rothhammer and Quintana, 2015; Sanmarco et al., 2021). In the context of neuroinflammation, astrocytes produce pro- and anti-inflammatory cytokines such as IL-1β, IL-6, TNF, IL-10, IL-27, and TGF-β and chemokines involved in T cell recruitment (Dong and Benveniste, 2001; Falsig et al., 2006; Fitzgerald et al., 2007). In addition, the interaction of astrocytes with T cells in an antigen-independent contact-dependent manner induces the up-regulation of the CD39 and CD73 ectoenzymes on T cells, important molecules involved in the regulation of innate and adaptive immune responses (Filipello et al., 2016; Mascanfroni et al., 2015; Mascanfroni et al., 2013; Takenaka et al., 2019; Takenaka et al., 2016). Of note, although IFNγ produced by Th1 cells increases MHC class II expression in astrocytes (Fontana et al., 1984; Gold et al., 1996; Soos et al., 1998), the role of antigen presentation by astrocytes in MS and EAE pathology is still unclear (Stüve et al., 2002).
Astrocytes show diverse effects on T cell polarization. Astrocytes are reported to favor Th1 and Th17 polarization, probably as a result of their expression of IL-12 and IL-23 (Constantinescu et al., 2005). Moreover, the up-regulation of miR-409-3p and miR-1896 microRNA in activated astrocytes induces STAT3 phosphorylation and IL-1β, IL-6, IP-10, MCP-1, and CXCL1 production, which promote Th17 differentiation, enhance Th17 chemotaxis, and worsen EAE (Liu et al., 2019). Conversely, astrocytes also produce IL-27, which can act on Th17 cells and dendritic cells to suppress encephalitogenic T cell responses (Fitzgerald et al., 2007; Mascanfroni et al., 2013; Yang et al., 2012). Of note, although IL-27 drives the differentiation of IL-10–producing anti-inflammatory Tr1 cells, the local hypoxia and increased levels of extracellular ATP that characterize the inflamed CNS microenvironment in EAE and MS interfere with Tr1 cell differentiation (Mascanfroni et al., 2015). However, the expression of CTLA-4, CD39, and CD73 in astrocytes can limit T cell activation (Filipello et al., 2016; Gimsa et al., 2004; Takenaka et al., 2019; Takenaka et al., 2016). Indeed, the deletion in astrocytes of ShcC/Rai augments CD39 and CTLA-4 expression, suppressing T cell responses and demyelination in EAE (Ulivieri et al., 2019; Ulivieri et al., 2016). Finally, as we already mentioned, IFNγ signaling induces TRAIL expression in astrocytes, which limits inflammation by inducing apoptosis of T cells expressing the TRAIL receptor death receptor 5 (DR5)+ (Sanmarco et al., 2021). Taken together, these findings highlight the importance of T cell–astrocyte communication in CNS pathophysiology.
Astrocyte interactions with NK cells
The meninges is a complex tissue made up of three membranes that line and protect the CNS from harmful peripheral agents such as toxins while also participating in the functional modulation of CNS-resident cells (Iliff et al., 2012; Sofroniew, 2015; Rua and McGavern, 2018; Alves de Lima et al., 2020b; Ramaglia et al., 2021). In the steady state, multiple peripheral immune cells reach the meninges, including dendritic cells, neutrophils, monocytes, macrophages, mast cells, CD4+ and CD8+ T cells, B cells, innate lymphoid cells, and natural killer (NK) cells (Coles et al., 2017; Korin et al., 2017; Mrdjen et al., 2018; Alves de Lima et al., 2020b; Rustenhoven et al., 2021). These meningeal immune cells can have a profound impact on astrocyte responses.
Meningeal NK cells have been recently linked to the regulation of astrocyte function (Sanmarco et al., 2021). In the intestine, the gut microbiota license IFN-γ production by NK cells (Ganal et al., 2012; Thiemann et al., 2017). NK cells enter the circulation and reach multiple tissues, including the meninges (Korin et al., 2017; Sanmarco et al., 2021; Fig. 2). Once at the meninges, NK cells producing IFNγ induce the expression of the pro-apoptotic molecule TRAIL on astrocytes located close to the meningeal border, which promote T cell apoptosis to limit pathogenic autoimmune responses in the CNS. Interestingly, TRAIL expression in astrocytes is down-regulated by pro-inflammatory mediators secreted by T cells (e.g., GM-CSF) and microglia (e.g., IL-1, TNF, and C1q; Sanmarco et al., 2021), highlighting the plasticity of astrocyte activation states.
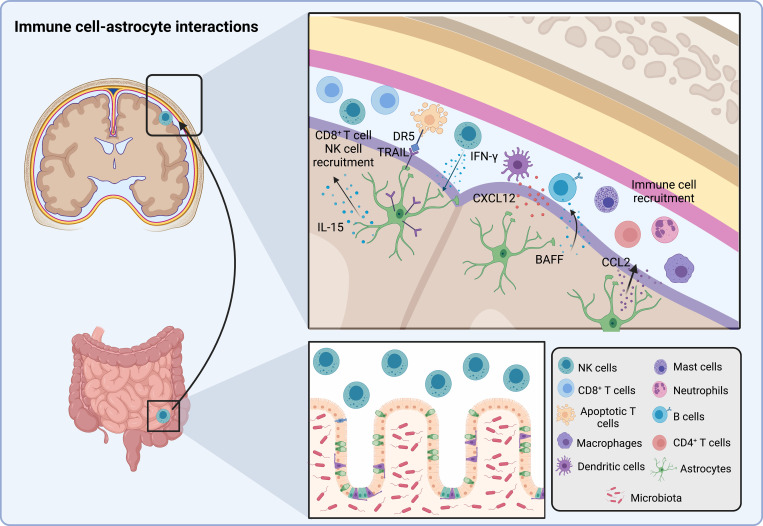
Figure 2.
Astrocyte–immune cell interactions. Microbiota-licensed IFNγ+ NK cells circulate to the meninges. Astrocytes up-regulate TRAIL expression in response to NK cell–produced IFNγ, inducing T cell apoptosis via DR5. Astrocyte-derived cytokines such as CCL2 recruit macrophages, neutrophils, CD4+ T cells, and mast cells to the CNS, whereas CXCL12 attracts dendritic cells and B cells. CD8+ T cells and NK cells are recruited by IL-15. In addition, perivascular astrocytes produce B cell–activating factor of the TNF family (BAFF), which promotes B cell survival and activation.
Conversely, astrocytes can also shape NK cell responses. Under inflammatory conditions, astrocytes produce diverse soluble mediators that, depending on their nature and location, stimulate or suppress NK cell activity (Shi et al., 2011). For example, during ischemia, astrocytes are the main CNS source of the pro-inflammatory cytokine IL-15 (Gómez-Nicola et al., 2008; Li et al., 2017; McInnes and Gracie, 2004), which activates NK cells (Li et al., 2017). Collectively, these findings highlight the bidirectional nature of astrocyte–NK cell crosstalk and its importance for CNS physiology in homeostasis and pathology.
Astrocyte interactions with other peripheral immune cells
Astrocytes secrete a plethora of chemokines that remodel the CNS microenvironment during the course of MS, including CCL2, CXCL1, CCL5, CXCL10, and CXCL12 (Ambrosini and Aloisi, 2004; Ambrosini et al., 2005; Babcock et al., 2003; Cardona et al., 2006; Imitola et al., 2004; Krumbholz et al., 2006; Omari et al., 2006; Ponath et al., 2018; Prins et al., 2014; Sofroniew, 2020). In MS and its preclinical model, EAE, astrocyte-derived CCL2 has been linked to the recruitment of pro-inflammatory monocytes, macrophages, and T cells, as well as microglial activation, axonal loss, and demyelination (Huang et al., 2001; Kim et al., 2014; Moreno et al., 2014; Paul et al., 2014; Ponath et al., 2018; Prins et al., 2014). Astrocyte-derived CXCL12 is linked to the recruitment of dendritic cells to active subcortical lesions in MS patients (Ambrosini et al., 2005). However, CCL2 and CXCL12 are not only associated with immune cell recruitment (Ubogu et al., 2006), but also to the migration of neural progenitor cells during brain development (Tran and Miller, 2003) and CNS inflammation (Belmadani et al., 2006; Imitola et al., 2004). Additional astrocyte-produced chemokines also contribute to CNS repair in MS, as illustrated by the CXCL1-driven recruitment of CXCR2+ oligodendrocytes (Omari et al., 2006). Hence, astrocyte-produced chemokines control CNS pathology and repair.
B cells are now recognized as important participants in CNS pathology (Comi et al., 2021; Zamvil and Hauser, 2021). Astrocyte-produced CXCL12 promotes the recruitment of pathogenic B cells to the CNS (Krumbholz et al., 2006). Moreover, astrocytes produce the B cell–activating factor of the TNF family, which is involved in B cell development, survival, and function (Krumbholz et al., 2005). Indeed, astrocyte conditioned media enhances the survival and activation of B cells isolated from untreated patients with secondary progressive MS (Touil et al., 2018). Conversely, IL-10–producing B cells have been recently reported to migrate from the gut to the CNS to limit inflammation (Pröbstel et al., 2020; Rojas et al., 2019), and IL-10 signaling has been shown to suppress astrocyte pathogenic activities in the context of EAE and, potentially, MS (Kenison et al., 2020; Mayo et al., 2016). Collectively, these findings suggest an important contribution for astrocyte–B cell communication in CNS pathology, but the specific molecular mechanisms involved and the role of these interactions in health and disease are still mostly unknown. The study of the functional interactions among astrocytes, B cells, and other peripheral immune cells will likely identify regulatory mechanisms involved in CNS physiology while guiding novel therapeutic approaches for MS and other neurological diseases.
Future perspectives on the study of astrocyte–cell interactions
Our understanding of astrocyte heterogeneity and regulatory cell interactions has recently benefited from the development of new technologies for their investigation. We recently developed rabies barcode interaction detection followed by sequencing (RABID-seq), a new technique that uses a library of pseudotyped rabies viruses harboring genetically encoded barcodes to trace astrocyte–cell interactions in vivo (Clark et al., 2021; Fig. 3). RABID-seq enables the detection of cell–cell interactions, the mechanisms that mediate them, and the functional effects of these interactions on the cells involved. When applied to the study of MS and its experimental model, EAE, these studies identified Sema4D–PlexinB2 and EphrinB3–EphB3 signaling as novel mediators of microglial–astrocyte communication, defining novel roles for axon guidance molecules in CNS pathology. Moreover, RABID-seq guided the development of novel CNS-penetrant small molecules for the therapeutic modulation of these microglia–astrocyte pathogenic interactions (Clark et al., 2021). The combination of RABID-seq with in situ transcriptomics methods, and the establishment of additional approaches for the study of CNS cell interactions in vivo, will shed light on the regulation of astrocyte heterogeneity and function while opening novel avenues for the treatment of neurological diseases.
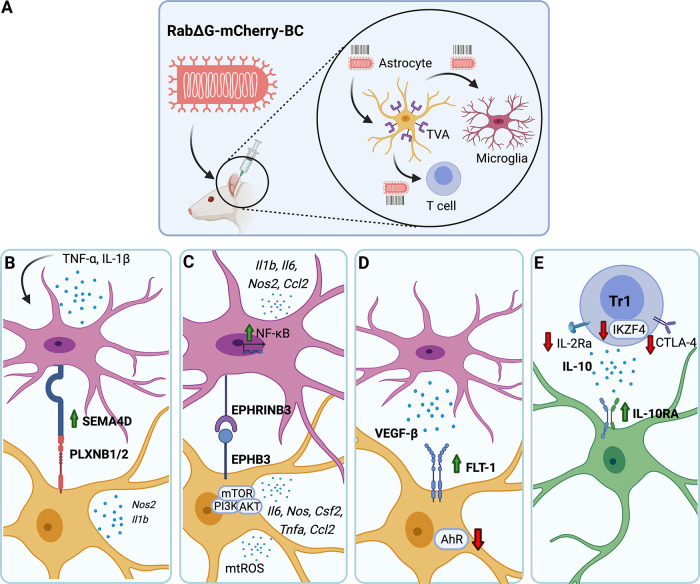
Figure 3.
Barcoded viral tracing of astrocyte–cell–cell interactions.(A) RABID-seq–identified mechanisms of microglia–astrocyte and T cell–astrocyte communication. (B) TNF and IL-1β up-regulate the expression of microglial SEMA4D, which interacts with PLXNB1/2 on astrocytes to induce NOS2 and IL-1β. (C) EPHRINB3–EPHB3 interactions activate PI3K–AKT–mTOR signaling in astrocytes, triggering the production of mtROS and pro-inflammatory mediators, and microglial NF-κB activation. (D) VEGFβ-producing microglia down-regulate AHR expression in FLT1+ astrocytes. (E) Il10ra-expressing astrocytes interact with Tr1 cells expressing Il10, Ctla4, Il2ra, and Ikzf4. BC, barcode; mtROS, mitochondrial reactive oxygen species.
Concluding remarks
Multiple cell interactions involving CNS-resident and recruited peripheral cells shape astrocyte responses in health and disease. In the context of MS and its preclinical model, EAE, molecules produced by microglia and effector T cells promote astrocyte activation states that boost CNS pathology by multiple mechanisms, including the recruitment of pro-inflammatory monocytes, microglial activation, the production of neurotoxic and inflammatory factors and the decreased production of neurotrophic factors and metabolites involved in neuron support. Conversely, NK cell– and regulatory T cell–derived cytokines boost astrocyte anti-inflammatory programs. However, our knowledge in this area is still limited, and important questions remain to be answered: (1) How can we study the repertoire of astrocyte–immune cell interactions in vivo in an unbiased manner? (2) How stable are astrocyte subsets, and what are the mechanisms that control their plasticity in health and disease? (3) Are disease-promoting astrocyte subsets and/or mechanisms of disease pathogenesis shared by multiple neurological diseases, and how can we target them therapeutically? (4) Finally, neurological disorders are also associated with impaired astrocyte homeostatic functions, how can we reestablish them? Understanding the complex cell–cell interactions that control astrocyte phenotype and function may identify novel mechanisms of pathogenesis relevant for MS and other neurological diseases, as well as candidate targets for therapeutic intervention.
Acknowledgments
This work was supported by National Institutes of Health grants NS102807, ES02530, ES029136, AI126880 and AI149699; National Multiple Sclerosis Society grants RG4111A1 and JF2161-A-5; and International Progressive MS Alliance grant PA-1604-08459 (all to F.J. Quintana). M.A. Wheeler was supported by National Institutes of Health grants 1K99NS114111 and F32NS101790, a National Institutes of Health and Dana-Farber Cancer Institute training grant (T32CA207201), a Brigham and Women’s Hospital traveling neuroscience fellowship from the Program in Interdisciplinary Neuroscience, and the Brigham and Women’s Hospital Women’s Brain Initiative. C.M. Polonio was supported by the Fundação de Amparo à Pesquisa do Estado de São Paulo Bolsa, Estágio de Pesquisa no Exterior (fellowship 2019/13731-0).
Author contributions: L.M. Sanmarco, C.M. Polonio, M.A. Wheeler, and F.J. Quintana wrote the manuscript.
References
- Adamsky, A., Kol A., Kreisel T., Doron A., Ozeri-Engelhard N., Melcer T., Refaeli R., Horn H., Regev L., Groysman M., et al. 2018. Astrocytic Activation Generates De Novo Neuronal Potentiation and Memory Enhancement. Cell. 174:59–71.e14. 10.1016/j.cell.2018.05.002 [DOI] [PubMed] [Google Scholar]
- Allen, N.J., and Eroglu C.. 2017. Cell biology of astrocyte-synapse interactions. Neuron. 96:697–708. 10.1016/j.neuron.2017.09.056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen, N.J., and Lyons D.A.. 2018. Glia as architects of central nervous system formation and function. Science. 362:181–185. 10.1126/science.aat0473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez, J.I., Dodelet-Devillers A., Kebir H., Ifergan I., Fabre P.J., Terouz S., Sabbagh M., Wosik K., Bourbonnière L., Bernard M., et al. 2011. The Hedgehog pathway promotes blood-brain barrier integrity and CNS immune quiescence. Science. 334:1727–1731. 10.1126/science.1206936 [DOI] [PubMed] [Google Scholar]
- Alves de Lima, K., Rustenhoven J., Da Mesquita S., Wall M., Salvador A.F., Smirnov I., Martelossi Cebinelli G., Mamuladze T., Baker W., Papadopoulos Z., et al. 2020a. Meningeal γδ T cells regulate anxiety-like behavior via IL-17a signaling in neurons. Nat. Immunol. 21:1421–1429. 10.1038/s41590-020-0776-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alves de Lima, K., Rustenhoven J., and Kipnis J.. 2020b. Meningeal Immunity and Its Function in Maintenance of the Central Nervous System in Health and Disease. 38:597–620. 10.1146/annurev-immunol-020711-075058 [DOI] [PubMed] [Google Scholar]
- Ambrosini, E., and Aloisi F.. 2004. Chemokines and glial cells: a complex network in the central nervous system. Neurochem. Res. 29:1017–1038. 10.1023/B:NERE.0000021246.96864.89 [DOI] [PubMed] [Google Scholar]
- Ambrosini, E., Remoli M.E., Giacomini E., Rosicarelli B., Serafini B., Lande R., Aloisi F., and Coccia E.M.. 2005. Astrocytes produce dendritic cell-attracting chemokines in vitro and in multiple sclerosis lesions. J. Neuropathol. Exp. Neurol. 64:706–715. 10.1097/01.jnen.0000173893.01929.fc [DOI] [PubMed] [Google Scholar]
- Anderson, M.A., Ao Y., and Sofroniew M.V.. 2014. Heterogeneity of reactive astrocytes. Neurosci. Lett. 565:23–29. 10.1016/j.neulet.2013.12.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson, M.A., Burda J.E., Ren Y., Ao Y., O’Shea T.M., Kawaguchi R., Coppola G., Khakh B.S., Deming T.J., and Sofroniew M.V.. 2016. Astrocyte scar formation aids central nervous system axon regeneration. Nature. 532:195–200. 10.1038/nature17623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson, M.A., O’Shea T.M., Burda J.E., Ao Y., Barlatey S.L., Bernstein A.M., Kim J.H., James N.D., Rogers A., Kato B., et al. 2018. Required growth facilitators propel axon regeneration across complete spinal cord injury. Nature. 561:396–400. 10.1038/s41586-018-0467-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andriezen, W.L.1893. The Neuroglia Elements in the Human Brain. BMJ. 2:227–230. 10.1136/bmj.2.1700.227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babcock, A.A., Kuziel W.A., Rivest S., and Owens T.. 2003. Chemokine expression by glial cells directs leukocytes to sites of axonal injury in the CNS. J. Neurosci. 23:7922–7930. 10.1523/JNEUROSCI.23-21-07922.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayraktar, O.A., Bartels T., Holmqvist S., Kleshchevnikov V., Martirosyan A., Polioudakis D., Ben Haim L., Young A.M.H., Batiuk M.Y., Prakash K., et al. 2020. Astrocyte layers in the mammalian cerebral cortex revealed by a single-cell in situ transcriptomic map. Nat. Neurosci. 23:500–509. 10.1038/s41593-020-0602-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belmadani, A., Tran P.B., Ren D., and Miller R.J.. 2006. Chemokines regulate the migration of neural progenitors to sites of neuroinflammation. J. Neurosci. 26:3182–3191. 10.1523/JNEUROSCI.0156-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben Haim, L., and Rowitch D.H.. 2017. Functional diversity of astrocytes in neural circuit regulation. Nat. Rev. Neurosci. 18:31–41. 10.1038/nrn.2016.159 [DOI] [PubMed] [Google Scholar]
- Berer, K., Mues M., Koutrolos M., Rasbi Z.A., Boziki M., Johner C., Wekerle H., and Krishnamoorthy G.. 2011. Commensal microbiota and myelin autoantigen cooperate to trigger autoimmune demyelination. Nature. 479:538–541. 10.1038/nature10554 [DOI] [PubMed] [Google Scholar]
- Bezzi, P., Domercq M., Brambilla L., Galli R., Schols D., De Clercq E., Vescovi A., Bagetta G., Kollias G., Meldolesi J., and Volterra A.. 2001. CXCR4-activated astrocyte glutamate release via TNFalpha: amplification by microglia triggers neurotoxicity. Nat. Neurosci. 4:702–710. 10.1038/89490 [DOI] [PubMed] [Google Scholar]
- Boisvert, M.M., Erikson G.A., Shokhirev M.N., and Allen N.J.. 2018. The Aging Astrocyte Transcriptome from Multiple Regions of the Mouse Brain. Cell Rep. 22:269–285. 10.1016/j.celrep.2017.12.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourgeois, E., Van L.P., Samson M., Diem S., Barra A., Roga S., Gombert J.M., Schneider E., Dy M., Gourdy P., et al. 2009. The pro-Th2 cytokine IL-33 directly interacts with invariant NKT and NK cells to induce IFN-gamma production. Eur. J. Immunol. 39:1046–1055. 10.1002/eji.200838575 [DOI] [PubMed] [Google Scholar]
- Cardona, A.E., Pioro E.P., Sasse M.E., Kostenko V., Cardona S.M., Dijkstra I.M., Huang D., Kidd G., Dombrowski S., Dutta R., et al. 2006. Control of microglial neurotoxicity by the fractalkine receptor. Nat. Neurosci. 9:917–924. 10.1038/nn1715 [DOI] [PubMed] [Google Scholar]
- Chai, H., Diaz-Castro B., Shigetomi E., Monte E., Octeau J.C., Yu X., Cohn W., Rajendran P.S., Vondriska T.M., Whitelegge J.P., et al. 2017. Neural Circuit-Specialized Astrocytes: Transcriptomic, Proteomic, Morphological, and Functional Evidence. Neuron. 95:531–549.e9. 10.1016/j.neuron.2017.06.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao, C.C., Gutiérrez-Vázquez C., Rothhammer V., Mayo L., Wheeler M.A., Tjon E.C., Zandee S.E.J., Blain M., de Lima K.A., Takenaka M.C., et al. 2019. Metabolic Control of Astrocyte Pathogenic Activity via cPLA2-MAVS. Cell. 179:1483–1498.e22. 10.1016/j.cell.2019.11.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung, W.S., Clarke L.E., Wang G.X., Stafford B.K., Sher A., Chakraborty C., Joung J., Foo L.C., Thompson A., Chen C., et al. 2013. Astrocytes mediate synapse elimination through MEGF10 and MERTK pathways. Nature. 504:394–400. 10.1038/nature12776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung, W.S., Allen N.J., and Eroglu C.. 2015. Astrocytes Control Synapse Formation, Function, and Elimination. Cold Spring Harb. Perspect. Biol. 7:a020370. 10.1101/cshperspect.a020370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark, I.C., Gutiérrez-Vázquez C., Wheeler M.A., Li Z., Rothhammer V., Linnerbauer M., Sanmarco L.M., Guo L., Blain M., Zandee S.E.J., et al. 2021. Barcoded viral tracing of single-cell interactions in central nervous system inflammation. Science. 372:eabf1230. 10.1126/science.abf1230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Codarri, L., Gyülvészi G., Tosevski V., Hesske L., Fontana A., Magnenat L., Suter T., and Becher B.. 2011. RORγt drives production of the cytokine GM-CSF in helper T cells, which is essential for the effector phase of autoimmune neuroinflammation. Nat. Immunol. 12:560–567. 10.1038/ni.2027 [DOI] [PubMed] [Google Scholar]
- Coles, J.A., Myburgh E., Brewer J.M., and McMenamin P.G.. 2017. Where are we? The anatomy of the murine cortical meninges revisited for intravital imaging, immunology, and clearance of waste from the brain. Prog. Neurobiol. 156:107–148. 10.1016/j.pneurobio.2017.05.002 [DOI] [PubMed] [Google Scholar]
- Colombo, E., and Farina C.. 2016. Astrocytes: Key Regulators of Neuroinflammation. Trends Immunol. 37:608–620. 10.1016/j.it.2016.06.006 [DOI] [PubMed] [Google Scholar]
- Comi, G., Bar-Or A., Lassmann H., Uccelli A., Hartung H.P., Montalban X., Sørensen P.S., Hohlfeld R., and Hauser S.L.. Expert Panel of the 27th Annual Meeting of the European Charcot Foundation . 2021. Role of B Cells in Multiple Sclerosis and Related Disorders. Ann. Neurol. 89:13–23. 10.1002/ana.25927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantinescu, C.S., Tani M., Ransohoff R.M., Wysocka M., Hilliard B., Fujioka T., Murphy S., Tighe P.J., Das Sarma J., Trinchieri G., and Rostami A.. 2005. Astrocytes as antigen-presenting cells: expression of IL-12/IL-23. J. Neurochem. 95:331–340. 10.1111/j.1471-4159.2005.03368.x [DOI] [PubMed] [Google Scholar]
- Di Giorgio, F.P., Carrasco M.A., Siao M.C., Maniatis T., and Eggan K.. 2007. Non-cell autonomous effect of glia on motor neurons in an embryonic stem cell-based ALS model. Nat. Neurosci. 10:608–614. 10.1038/nn1885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz-Castro, B., Gangwani M.R., Yu X., Coppola G., and Khakh B.S.. 2019. Astrocyte molecular signatures in Huntington’s disease. Sci. Transl. Med. 11:eaaw8546. 10.1126/scitranslmed.aaw8546 [DOI] [PubMed] [Google Scholar]
- Dong, Y., and Benveniste E.N.. 2001. Immune function of astrocytes. Glia. 36:180–190. 10.1002/glia.1107 [DOI] [PubMed] [Google Scholar]
- El-Behi, M., Ciric B., Dai H., Yan Y., Cullimore M., Safavi F., Zhang G.X., Dittel B.N., and Rostami A.. 2011. The encephalitogenicity of T(H)17 cells is dependent on IL-1- and IL-23-induced production of the cytokine GM-CSF. Nat. Immunol. 12:568–575. 10.1038/ni.2031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eng, L.F., Vanderhaeghen J.J., Bignami A., and Gerstl B.. 1971. An acidic protein isolated from fibrous astrocytes. Brain Res. 28:351–354. 10.1016/0006-8993(71)90668-8 [DOI] [PubMed] [Google Scholar]
- Erny, D., Hrabě de Angelis A.L., Jaitin D., Wieghofer P., Staszewski O., David E., Keren-Shaul H., Mahlakoiv T., Jakobshagen K., Buch T., et al. 2015. Host microbiota constantly control maturation and function of microglia in the CNS. Nat. Neurosci. 18:965–977. 10.1038/nn.4030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escartin, C., Galea E., Lakatos A., O’Callaghan J.P., Petzold G.C., Serrano-Pozo A., Steinhäuser C., Volterra A., Carmignoto G., Agarwal A., et al. 2021. Reactive astrocyte nomenclature, definitions, and future directions. Nat. Neurosci. 24:312–325. 10.1038/s41593-020-00783-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falsig, J., Pörzgen P., Lund S., Schrattenholz A., and Leist M.. 2006. The inflammatory transcriptome of reactive murine astrocytes and implications for their innate immune function. J. Neurochem. 96:893–907. 10.1111/j.1471-4159.2005.03622.x [DOI] [PubMed] [Google Scholar]
- Ferber, I.A., Brocke S., Taylor-Edwards C., Ridgway W., Dinisco C., Steinman L., Dalton D., and Fathman C.G.. 1996. Mice with a disrupted IFN-gamma gene are susceptible to the induction of experimental autoimmune encephalomyelitis (EAE). J. Immunol. 156:5–7. [PubMed] [Google Scholar]
- Fields, R.D., and Stevens-Graham B.. 2002. New insights into neuron-glia communication. Science. 298:556–562. 10.1126/science.298.5593.556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filiano, A.J., Xu Y., Tustison N.J., Marsh R.L., Baker W., Smirnov I., Overall C.C., Gadani S.P., Turner S.D., Weng Z., et al. 2016. Unexpected role of interferon-γ in regulating neuronal connectivity and social behaviour. Nature. 535:425–429. 10.1038/nature18626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filipello, F., Pozzi D., Proietti M., Romagnani A., Mazzitelli S., Matteoli M., Verderio C., and Grassi F.. 2016. Ectonucleotidase activity and immunosuppression in astrocyte-CD4 T cell bidirectional signaling. Oncotarget. 7:5143–5156. 10.18632/oncotarget.6914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald, D.C., Ciric B., Touil T., Harle H., Grammatikopolou J., Das Sarma J., Gran B., Zhang G.X., and Rostami A.. 2007. Suppressive effect of IL-27 on encephalitogenic Th17 cells and the effector phase of experimental autoimmune encephalomyelitis. J. Immunol. 179:3268–3275. 10.4049/jimmunol.179.5.3268 [DOI] [PubMed] [Google Scholar]
- Fontana, A., Fierz W., and Wekerle H.. 1984. Astrocytes present myelin basic protein to encephalitogenic T-cell lines. Nature. 307:273–276. 10.1038/307273a0 [DOI] [PubMed] [Google Scholar]
- Foo, L.C., Allen N.J., Bushong E.A., Ventura P.B., Chung W.S., Zhou L., Cahoy J.D., Daneman R., Zong H., Ellisman M.H., and Barres B.A.. 2011. Development of a method for the purification and culture of rodent astrocytes. Neuron. 71:799–811. 10.1016/j.neuron.2011.07.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganal, S.C., Sanos S.L., Kallfass C., Oberle K., Johner C., Kirschning C., Lienenklaus S., Weiss S., Staeheli P., Aichele P., and Diefenbach A.. 2012. Priming of natural killer cells by nonmucosal mononuclear phagocytes requires instructive signals from commensal microbiota. Immunity. 37:171–186. 10.1016/j.immuni.2012.05.020 [DOI] [PubMed] [Google Scholar]
- Gate, D., Saligrama N., Leventhal O., Yang A.C., Unger M.S., Middeldorp J., Chen K., Lehallier B., Channappa D., De Los Santos M.B., et al. 2020. Clonally expanded CD8 T cells patrol the cerebrospinal fluid in Alzheimer’s disease. Nature. 577:399–404. 10.1038/s41586-019-1895-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gimsa, U., ØRen A., Pandiyan P., Teichmann D., Bechmann I., Nitsch R., and Brunner-Weinzierl M.C.. 2004. Astrocytes protect the CNS: antigen-specific T helper cell responses are inhibited by astrocyte-induced upregulation of CTLA-4 (CD152). J. Mol. Med. (Berl.). 82:364–372. 10.1007/s00109-004-0531-6 [DOI] [PubMed] [Google Scholar]
- Gold, R., Schmied M., Tontsch U., Hartung H.P., Wekerle H., Toyka K.V., and Lassmann H.. 1996. Antigen presentation by astrocytes primes rat T lymphocytes for apoptotic cell death. A model for T-cell apoptosis in vivo. Brain. 119:651–659. 10.1093/brain/119.2.651 [DOI] [PubMed] [Google Scholar]
- Goldmann, T., and Prinz M.. 2013. Role of microglia in CNS autoimmunity. Clin. Dev. Immunol. 2013:208093. 10.1155/2013/208093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldmann, T., Blank T., and Prinz M.. 2016. Fine-tuning of type I IFN-signaling in microglia--implications for homeostasis, CNS autoimmunity and interferonopathies. Curr. Opin. Neurobiol. 36:38–42. 10.1016/j.conb.2015.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gómez-Nicola, D., Valle-Argos B., Pita-Thomas D.W., and Nieto-Sampedro M.. 2008. Interleukin 15 expression in the CNS: blockade of its activity prevents glial activation after an inflammatory injury. Glia. 56:494–505. 10.1002/glia.20628 [DOI] [PubMed] [Google Scholar]
- Groux, H., O’Garra A., Bigler M., Rouleau M., Antonenko S., de Vries J.E., and Roncarolo M.G.. 1997. A CD4+ T-cell subset inhibits antigen-specific T-cell responses and prevents colitis. Nature. 389:737–742. 10.1038/39614 [DOI] [PubMed] [Google Scholar]
- Habib, N., McCabe C., Medina S., Varshavsky M., Kitsberg D., Dvir-Szternfeld R., Green G., Dionne D., Nguyen L., Marshall J.L., et al. 2020. Disease-associated astrocytes in Alzheimer’s disease and aging. Nat. Neurosci. 23:701–706. 10.1038/s41593-020-0624-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haghikia, A., Jörg S., Duscha A., Berg J., Manzel A., Waschbisch A., Hammer A., Lee D.H., May C., Wilck N., et al. 2015. Dietary Fatty Acids Directly Impact Central Nervous System Autoimmunity via the Small Intestine. Immunity. 43:817–829. 10.1016/j.immuni.2015.09.007 [DOI] [PubMed] [Google Scholar]
- Hindinger, C., Bergmann C.C., Hinton D.R., Phares T.W., Parra G.I., Hussain S., Savarin C., Atkinson R.D., and Stohlman S.A.. 2012. IFN-γ signaling to astrocytes protects from autoimmune mediated neurological disability. PLoS One. 7:e42088. 10.1371/journal.pone.0042088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houades, V., Koulakoff A., Ezan P., Seif I., and Giaume C.. 2008. Gap junction-mediated astrocytic networks in the mouse barrel cortex. J. Neurosci. 28:5207–5217. 10.1523/JNEUROSCI.5100-07.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, D.R., Wang J., Kivisakk P., Rollins B.J., and Ransohoff R.M.. 2001. Absence of monocyte chemoattractant protein 1 in mice leads to decreased local macrophage recruitment and antigen-specific T helper cell type 1 immune response in experimental autoimmune encephalomyelitis. J. Exp. Med. 193:713–726. 10.1084/jem.193.6.713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iliff, J.J., Wang M., Liao Y., Plogg B.A., Peng W., Gundersen G.A., Benveniste H., Vates G.E., Deane R., Goldman S.A., et al. 2012. A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid β. Sci. Transl. Med. 4:147ra111. 10.1126/scitranslmed.3003748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imitola, J., Raddassi K., Park K.I., Mueller F.J., Nieto M., Teng Y.D., Frenkel D., Li J., Sidman R.L., Walsh C.A., et al. 2004. Directed migration of neural stem cells to sites of CNS injury by the stromal cell-derived factor 1alpha/CXC chemokine receptor 4 pathway. Proc. Natl. Acad. Sci. USA. 101:18117–18122. 10.1073/pnas.0408258102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito, M., Komai K., Mise-Omata S., Iizuka-Koga M., Noguchi Y., Kondo T., Sakai R., Matsuo K., Nakayama T., Yoshie O., et al. 2019. Brain regulatory T cells suppress astrogliosis and potentiate neurological recovery. Nature. 565:246–250. 10.1038/s41586-018-0824-5 [DOI] [PubMed] [Google Scholar]
- Itoh, N., Itoh Y., Tassoni A., Ren E., Kaito M., Ohno A., Ao Y., Farkhondeh V., Johnsonbaugh H., Burda J., et al. 2018. Cell-specific and region-specific transcriptomics in the multiple sclerosis model: Focus on astrocytes. Proc. Natl. Acad. Sci. USA. 115:E302–E309. 10.1073/pnas.1716032115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jafarzadeh, A., Mahdavi R., Jamali M., Hajghani H., Nemati M., and Ebrahimi H.A.. 2016. Increased Concentrations of Interleukin-33 in the Serum and Cerebrospinal Fluid of Patients with Multiple Sclerosis. Oman Med. J. 31:40–45. 10.5001/omj.2016.08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang, H.R., Milovanović M., Allan D., Niedbala W., Besnard A.G., Fukada S.Y., Alves-Filho J.C., Togbe D., Goodyear C.S., Linington C., et al. 2012. IL-33 attenuates EAE by suppressing IL-17 and IFN-γ production and inducing alternatively activated macrophages. Eur. J. Immunol. 42:1804–1814. 10.1002/eji.201141947 [DOI] [PubMed] [Google Scholar]
- Jiang, R., Diaz-Castro B., Looger L.L., and Khakh B.S.. 2016. Dysfunctional Calcium and Glutamate Signaling in Striatal Astrocytes from Huntington’s Disease Model Mice. J. Neurosci. 36:3453–3470. 10.1523/JNEUROSCI.3693-15.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- John Lin, C.-C., Yu K., Hatcher A., Huang T.-W., Lee H.K., Carlson J., Weston M.C., Chen F., Zhang Y., Zhu W., et al. 2017. Identification of diverse astrocyte populations and their malignant analogs. Nat. Neurosci. 20:396–405. 10.1038/nn.4493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadowaki, A., and Quintana F.J.. 2020. The Gut-CNS Axis in Multiple Sclerosis. Trends Neurosci. 43:622–634. 10.1016/j.tins.2020.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang, Z., Altuntas C.Z., Gulen M.F., Liu C., Giltiay N., Qin H., Liu L., Qian W., Ransohoff R.M., Bergmann C., et al. 2010. Astrocyte-restricted ablation of interleukin-17-induced Act1-mediated signaling ameliorates autoimmune encephalomyelitis. Immunity. 32:414–425. 10.1016/j.immuni.2010.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenison, J.E., Jhaveri A., Li Z., Khadse N., Tjon E., Tezza S., Nowakowska D., Plasencia A., Stanton V.P. Jr., Sherr D.H., and Quintana F.J.. 2020. Tolerogenic nanoparticles suppress central nervous system inflammation. Proc. Natl. Acad. Sci. USA. 117:32017–32028. 10.1073/pnas.2016451117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keren-Shaul, H., Spinrad A., Weiner A., Matcovitch-Natan O., Dvir-Szternfeld R., Ulland T.K., David E., Baruch K., Lara-Astaiso D., Toth B., et al. 2017. A Unique Microglia Type Associated with Restricting Development of Alzheimer’s Disease. Cell. 169:1276–1290.e17. 10.1016/j.cell.2017.05.018 [DOI] [PubMed] [Google Scholar]
- Khakh, B.S., and Deneen B.. 2019. The Emerging Nature of Astrocyte Diversity. Annu. Rev. Neurosci. 42:187–207. 10.1146/annurev-neuro-070918-050443 [DOI] [PubMed] [Google Scholar]
- Khakh, B.S., Beaumont V., Cachope R., Munoz-Sanjuan I., Goldman S.A., and Grantyn R.. 2017. Unravelling and Exploiting Astrocyte Dysfunction in Huntington’s Disease. Trends Neurosci. 40:422–437. 10.1016/j.tins.2017.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, R.Y., Hoffman A.S., Itoh N., Ao Y., Spence R., Sofroniew M.V., and Voskuhl R.R.. 2014. Astrocyte CCL2 sustains immune cell infiltration in chronic experimental autoimmune encephalomyelitis. J. Neuroimmunol. 274:53–61. 10.1016/j.jneuroim.2014.06.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korin, B., Ben-Shaanan T.L., Schiller M., Dubovik T., Azulay-Debby H., Boshnak N.T., Koren T., and Rolls A.. 2017. High-dimensional, single-cell characterization of the brain’s immune compartment. Nat. Neurosci. 20:1300–1309. 10.1038/nn.4610 [DOI] [PubMed] [Google Scholar]
- Krumbholz, M., Theil D., Derfuss T., Rosenwald A., Schrader F., Monoranu C.M., Kalled S.L., Hess D.M., Serafini B., Aloisi F., et al. 2005. BAFF is produced by astrocytes and up-regulated in multiple sclerosis lesions and primary central nervous system lymphoma. J. Exp. Med. 201:195–200. 10.1084/jem.20041674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krumbholz, M., Theil D., Cepok S., Hemmer B., Kivisäkk P., Ransohoff R.M., Hofbauer M., Farina C., Derfuss T., Hartle C., et al. 2006. Chemokines in multiple sclerosis: CXCL12 and CXCL13 up-regulation is differentially linked to CNS immune cell recruitment. Brain. 129:200–211. 10.1093/brain/awh680 [DOI] [PubMed] [Google Scholar]
- Lee, Y., Awasthi A., Yosef N., Quintana F.J., Xiao S., Peters A., Wu C., Kleinewietfeld M., Kunder S., Hafler D.A., et al. 2012. Induction and molecular signature of pathogenic TH17 cells. Nat. Immunol. 13:991–999. 10.1038/ni.2416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, M., Li Y., Liu X., Gao X., and Wang Y.. 2012. IL-33 blockade suppresses the development of experimental autoimmune encephalomyelitis in C57BL/6 mice. J. Neuroimmunol. 247:25–31. 10.1016/j.jneuroim.2012.03.016 [DOI] [PubMed] [Google Scholar]
- Li, M., Li Z., Yao Y., Jin W.N., Wood K., Liu Q., Shi F.D., and Hao J.. 2017. Astrocyte-derived interleukin-15 exacerbates ischemic brain injury via propagation of cellular immunity. Proc. Natl. Acad. Sci. USA. 114:E396–E405. 10.1073/pnas.1612930114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liddelow, S.A., Guttenplan K.A., Clarke L.E., Bennett F.C., Bohlen C.J., Schirmer L., Bennett M.L., Münch A.E., Chung W.-S., Peterson T.C., et al. 2017. Neurotoxic reactive astrocytes are induced by activated microglia. Nature. 541:481–487. 10.1038/nature21029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linnerbauer, M., Wheeler M.A., and Quintana F.J.. 2020. Astrocyte Crosstalk in CNS Inflammation. Neuron. 108:608–622. 10.1016/j.neuron.2020.08.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, X., Zhou F., Yang Y., Wang W., Niu L., Zuo D., Li X., Hua H., Zhang B., Kou Y., et al. 2019. MiR-409-3p and MiR-1896 co-operatively participate in IL-17-induced inflammatory cytokine production in astrocytes and pathogenesis of EAE mice via targeting SOCS3/STAT3 signaling. Glia. 67:101–112. 10.1002/glia.23530 [DOI] [PubMed] [Google Scholar]
- Martin, N.T., and Martin M.U.. 2016. Interleukin 33 is a guardian of barriers and a local alarmin. Nat. Immunol. 17:122–131. 10.1038/ni.3370 [DOI] [PubMed] [Google Scholar]
- Martín, R., Bajo-Grañeras R., Moratalla R., Perea G., and Araque A.. 2015. Circuit-specific signaling in astrocyte-neuron networks in basal ganglia pathways. Science. 349:730–734. 10.1126/science.aaa7945 [DOI] [PubMed] [Google Scholar]
- Martin-Fernandez, M., Jamison S., Robin L.M., Zhao Z., Martin E.D., Aguilar J., Benneyworth M.A., Marsicano G., and Araque A.. 2017. Synapse-specific astrocyte gating of amygdala-related behavior. Nat. Neurosci. 20:1540–1548. 10.1038/nn.4649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mascanfroni, I.D., Yeste A., Vieira S.M., Burns E.J., Patel B., Sloma I., Wu Y., Mayo L., Ben-Hamo R., Efroni S., et al. 2013. IL-27 acts on DCs to suppress the T cell response and autoimmunity by inducing expression of the immunoregulatory molecule CD39. Nat. Immunol. 14:1054–1063. 10.1038/ni.2695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mascanfroni, I.D., Takenaka M.C., Yeste A., Patel B., Wu Y., Kenison J.E., Siddiqui S., Basso A.S., Otterbein L.E., Pardoll D.M., et al. 2015. Metabolic control of type 1 regulatory T cell differentiation by AHR and HIF1-α. Nat. Med. 21:638–646. 10.1038/nm.3868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathys, H., Davila-Velderrain J., Peng Z., Gao F., Mohammadi S., Young J.Z., Menon M., He L., Abdurrob F., Jiang X., et al. 2019. Single-cell transcriptomic analysis of Alzheimer’s disease. Nature. 570:332–337. 10.1038/s41586-019-1195-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayo, L., Trauger S.A., Blain M., Nadeau M., Patel B., Alvarez J.I., Mascanfroni I.D., Yeste A., Kivisäkk P., Kallas K., et al. 2014. Regulation of astrocyte activation by glycolipids drives chronic CNS inflammation. Nat. Med. 20:1147–1156. 10.1038/nm.3681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayo, L., Cunha A.P., Madi A., Beynon V., Yang Z., Alvarez J.I., Prat A., Sobel R.A., Kobzik L., Lassmann H., et al. 2016. IL-10-dependent Tr1 cells attenuate astrocyte activation and ameliorate chronic central nervous system inflammation. Brain. 139:1939–1957. 10.1093/brain/aww113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McInnes, I.B., and Gracie J.A.. 2004. Interleukin-15: a new cytokine target for the treatment of inflammatory diseases. Curr. Opin. Pharmacol. 4:392–397. 10.1016/j.coph.2004.04.003 [DOI] [PubMed] [Google Scholar]
- Milovanovic, M., Volarevic V., Ljujic B., Radosavljevic G., Jovanovic I., Arsenijevic N., and Lukic M.L.. 2012. Deletion of IL-33R (ST2) abrogates resistance to EAE in BALB/C mice by enhancing polarization of APC to inflammatory phenotype. PLoS One. 7:e45225. 10.1371/journal.pone.0045225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molofsky, A.V., Kelley K.W., Tsai H.H., Redmond S.A., Chang S.M., Madireddy L., Chan J.R., Baranzini S.E., Ullian E.M., and Rowitch D.H.. 2014. Astrocyte-encoded positional cues maintain sensorimotor circuit integrity. Nature. 509:189–194. 10.1038/nature13161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno, M., Bannerman P., Ma J., Guo F., Miers L., Soulika A.M., and Pleasure D.. 2014. Conditional ablation of astroglial CCL2 suppresses CNS accumulation of M1 macrophages and preserves axons in mice with MOG peptide EAE. J. Neurosci. 34:8175–8185. 10.1523/JNEUROSCI.1137-14.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mrdjen, D., Pavlovic A., Hartmann F.J., Schreiner B., Utz S.G., Leung B.P., Lelios I., Heppner F.L., Kipnis J., Merkler D., et al. 2018. High-Dimensional Single-Cell Mapping of Central Nervous System Immune Cells Reveals Distinct Myeloid Subsets in Health, Aging, and Disease. Immunity. 48:380–395.e6. 10.1016/j.immuni.2018.01.011 [DOI] [PubMed] [Google Scholar]
- Nagai, J., Rajbhandari A.K., Gangwani M.R., Hachisuka A., Coppola G., Masmanidis S.C., Fanselow M.S., and Khakh B.S.. 2019. Hyperactivity with Disrupted Attention by Activation of an Astrocyte Synaptogenic Cue. Cell. 177:1280–1292.e20. 10.1016/j.cell.2019.03.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagai, J., Yu X., Papouin T., Cheong E., Freeman M.R., Monk K.R., Hastings M.H., Haydon P.G., Rowitch D., Shaham S., and Khakh B.S.. 2021. Behaviorally consequential astrocytic regulation of neural circuits. Neuron. 109:576–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norden, D.M., Fenn A.M., Dugan A., and Godbout J.P.. 2014. TGFβ produced by IL-10 redirected astrocytes attenuates microglial activation. Glia. 62:881–895. 10.1002/glia.22647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberheim, N.A., Takano T., Han X., He W., Lin J.H., Wang F., Xu Q., Wyatt J.D., Pilcher W., Ojemann J.G., et al. 2009. Uniquely hominid features of adult human astrocytes. J. Neurosci. 29:3276–3287. 10.1523/JNEUROSCI.4707-08.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochoa-Repáraz, J., Mielcarz D.W., Ditrio L.E., Burroughs A.R., Foureau D.M., Haque-Begum S., and Kasper L.H.. 2009. Role of gut commensal microflora in the development of experimental autoimmune encephalomyelitis. J. Immunol. 183:6041–6050. 10.4049/jimmunol.0900747 [DOI] [PubMed] [Google Scholar]
- Omari, K.M., John G., Lango R., and Raine C.S.. 2006. Role for CXCR2 and CXCL1 on glia in multiple sclerosis. Glia. 53:24–31. 10.1002/glia.20246 [DOI] [PubMed] [Google Scholar]
- Paul, D., Ge S., Lemire Y., Jellison E.R., Serwanski D.R., Ruddle N.H., and Pachter J.S.. 2014. Cell-selective knockout and 3D confocal image analysis reveals separate roles for astrocyte-and endothelial-derived CCL2 in neuroinflammation. J. Neuroinflammation. 11:10. 10.1186/1742-2094-11-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pomeshchik, Y., Kidin I., Korhonen P., Savchenko E., Jaronen M., Lehtonen S., Wojciechowski S., Kanninen K., Koistinaho J., and Malm T.. 2015. Interleukin-33 treatment reduces secondary injury and improves functional recovery after contusion spinal cord injury. Brain Behav. Immun. 44:68–81. 10.1016/j.bbi.2014.08.002 [DOI] [PubMed] [Google Scholar]
- Ponath, G., Lincoln M.R., Levine-Ritterman M., Park C., Dahlawi S., Mubarak M., Sumida T., Airas L., Zhang S., Isitan C., et al. 2018. Enhanced astrocyte responses are driven by a genetic risk allele associated with multiple sclerosis. Nat. Commun. 9:5337. 10.1038/s41467-018-07785-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prajeeth, C.K., Kronisch J., Khorooshi R., Knier B., Toft-Hansen H., Gudi V., Floess S., Huehn J., Owens T., Korn T., and Stangel M.. 2017. Effectors of Th1 and Th17 cells act on astrocytes and augment their neuroinflammatory properties. J. Neuroinflammation. 14:204. 10.1186/s12974-017-0978-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prins, M., Dutta R., Baselmans B., Brevé J.J., Bol J.G., Deckard S.A., van der Valk P., Amor S., Trapp B.D., de Vries H.E., et al. 2014. Discrepancy in CCL2 and CCR2 expression in white versus grey matter hippocampal lesions of Multiple Sclerosis patients. Acta Neuropathol. Commun. 2:98. 10.1186/s40478-014-0098-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pröbstel, A.K., Zhou X., Baumann R., Wischnewski S., Kutza M., Rojas O.L., Sellrie K., Bischof A., Kim K., Ramesh A., et al. 2020. Gut microbiota-specific IgA+ B cells traffic to the CNS in active multiple sclerosis. Sci. Immunol. 5:eabc7191. 10.1126/sciimmunol.abc7191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramaglia, V., Rojas O., Naouar I., and Gommerman J.L.. 2021. The Ins and Outs of Central Nervous System Inflammation-Lessons Learned from Multiple Sclerosis. Annu. Rev. Immunol. 39:199–226. 10.1146/annurev-immunol-093019-124155 [DOI] [PubMed] [Google Scholar]
- Ribeiro, M., Brigas H.C., Temido-Ferreira M., Pousinha P.A., Regen T., Santa C., Coelho J.E., Marques-Morgado I., Valente C.A., Omenetti S., et al. 2019. Meningeal γδ T cell-derived IL-17 controls synaptic plasticity and short-term memory. Sci. Immunol. 4: eaay5199. 10.1126/sciimmunol.aay5199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojas, O.L., Pröbstel A.K., Porfilio E.A., Wang A.A., Charabati M., Sun T., Lee D.S.W., Galicia G., Ramaglia V., Ward L.A., et al. 2019. Recirculating Intestinal IgA-Producing Cells Regulate Neuroinflammation via IL-10. Cell. 177:492–493. 10.1016/j.cell.2019.03.037 [DOI] [PubMed] [Google Scholar]
- Rothhammer, V., and Quintana F.J.. 2015. Control of autoimmune CNS inflammation by astrocytes. Semin. Immunopathol. 37:625–638. 10.1007/s00281-015-0515-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothhammer, V., and Quintana F.J.. 2019. The aryl hydrocarbon receptor: an environmental sensor integrating immune responses in health and disease. Nat. Rev. Immunol. 19:184–197. 10.1038/s41577-019-0125-8 [DOI] [PubMed] [Google Scholar]
- Rothhammer, V., Mascanfroni I.D., Bunse L., Takenaka M.C., Kenison J.E., Mayo L., Chao C.-C., Patel B., Yan R., Blain M., et al. 2016. Type I interferons and microbial metabolites of tryptophan modulate astrocyte activity and central nervous system inflammation via the aryl hydrocarbon receptor. Nat. Med. 22:586–597. 10.1038/nm.4106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothhammer, V., Borucki D.M., Tjon E.C., Takenaka M.C., Chao C.C., Ardura-Fabregat A., de Lima K.A., Gutiérrez-Vázquez C., Hewson P., Staszewski O., et al. 2018. Microglial control of astrocytes in response to microbial metabolites. Nature. 557:724–728. 10.1038/s41586-018-0119-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rua, R., and McGavern D.B.. 2018. Advances in Meningeal Immunity. Trends Mol. Med. 24:542–559. 10.1016/j.molmed.2018.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rustenhoven, J., Drieu A., Mamuladze T., de Lima K.A., Dykstra T., Wall M., Papadopoulos Z., Kanamori M., Salvador A.F., Baker W., et al. 2021. Functional characterization of the dural sinuses as a neuroimmune interface. Cell. 184:1000–1016.e27. 10.1016/j.cell.2020.12.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanmarco, L.M., Wheeler M.A., Gutiérrez-Vázquez C., Polonio C.M., Linnerbauer M., Pinho-Ribeiro F.A., Li Z., Giovannoni F., Batterman K.V., Scalisi G., et al. 2021. Gut-licensed IFNγ+ NK cells drive LAMP1+TRAIL+ anti-inflammatory astrocytes. Nature. 590:473–479. 10.1038/s41586-020-03116-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders, A., Macosko E.Z., Wysoker A., Goldman M., Krienen F.M., de Rivera H., Bien E., Baum M., Bortolin L., Wang S., et al. 2018. Molecular Diversity and Specializations among the Cells of the Adult Mouse Brain. Cell. 174:1015–1030.e16. 10.1016/j.cell.2018.07.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi, F.D., Ljunggren H.G., La Cava A., and Van Kaer L.. 2011. Organ-specific features of natural killer cells. Nat. Rev. Immunol. 11:658–671. 10.1038/nri3065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith, H.L., Freeman O.J., Butcher A.J., Holmqvist S., Humoud I., Schätzl T., Hughes D.T., Verity N.C., Swinden D.P., Hayes J., et al. 2020. Astrocyte Unfolded Protein Response Induces a Specific Reactivity State that Causes Non-Cell-Autonomous Neuronal Degeneration. Neuron. 105:855–866.e5. 10.1016/j.neuron.2019.12.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofroniew, M.V.2015. Astrocyte barriers to neurotoxic inflammation. Nat. Rev. Neurosci. 16:249–263. 10.1038/nrn3898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofroniew, M.V.2020. Astrocyte Reactivity: Subtypes, States, and Functions in CNS Innate Immunity. Trends Immunol. 41:758–770. 10.1016/j.it.2020.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soos, J.M., Morrow J., Ashley T.A., Szente B.E., Bikoff E.K., and Zamvil S.S.. 1998. Astrocytes express elements of the class II endocytic pathway and process central nervous system autoantigen for presentation to encephalitogenic T cells. J. Immunol. 161:5959–5966. 10.1016/S0165-5728(98)91399-6 [DOI] [PubMed] [Google Scholar]
- Stüve, O., Youssef S., Slavin A.J., King C.L., Patarroyo J.C., Hirschberg D.L., Brickey W.J., Soos J.M., Piskurich J.F., Chapman H.A., and Zamvil S.S.. 2002. The role of the MHC class II transactivator in class II expression and antigen presentation by astrocytes and in susceptibility to central nervous system autoimmune disease. J. Immunol. 169:6720–6732. 10.4049/jimmunol.169.12.6720 [DOI] [PubMed] [Google Scholar]
- Takenaka, M.C., Robson S., and Quintana F.J.. 2016. Regulation of the T Cell Response by CD39. Trends Immunol. 37:427–439. 10.1016/j.it.2016.04.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takenaka, M.C., Gabriely G., Rothhammer V., Mascanfroni I.D., Wheeler M.A., Chao C.C., Gutiérrez-Vázquez C., Kenison J., Tjon E.C., Barroso A., et al. 2019. Control of tumor-associated macrophages and T cells in glioblastoma via AHR and CD39. Nat. Neurosci. 22:729–740. 10.1038/s41593-019-0370-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiemann, S., Smit N., Roy U., Lesker T.R., Gálvez E.J.C., Helmecke J., Basic M., Bleich A., Goodman A.L., Kalinke U., et al. 2017. Enhancement of IFNγ Production by Distinct Commensals Ameliorates Salmonella-Induced Disease. Cell Host Microbe. 21:682–694.e5. 10.1016/j.chom.2017.05.005 [DOI] [PubMed] [Google Scholar]
- Tong, X., Ao Y., Faas G.C., Nwaobi S.E., Xu J., Haustein M.D., Anderson M.A., Mody I., Olsen M.L., Sofroniew M.V., and Khakh B.S.. 2014. Astrocyte Kir4.1 ion channel deficits contribute to neuronal dysfunction in Huntington’s disease model mice. Nat. Neurosci. 17:694–703. 10.1038/nn.3691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Touil, H., Kobert A., Lebeurrier N., Rieger A., Saikali P., Lambert C., Fawaz L., Moore C.S., Prat A., Gommerman J., et al. Canadian B Cell Team in MS . 2018. Human central nervous system astrocytes support survival and activation of B cells: implications for MS pathogenesis. J. Neuroinflammation. 15:114. 10.1186/s12974-018-1136-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran, P.B., and Miller R.J.. 2003. Chemokine receptors: signposts to brain development and disease. Nat. Rev. Neurosci. 4:444–455. 10.1038/nrn1116 [DOI] [PubMed] [Google Scholar]
- Tsai, H.H., Li H., Fuentealba L.C., Molofsky A.V., Taveira-Marques R., Zhuang H., Tenney A., Murnen A.T., Fancy S.P., Merkle F., et al. 2012. Regional astrocyte allocation regulates CNS synaptogenesis and repair. Science. 337:358–362. 10.1126/science.1222381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ubogu, E.E., Cossoy M.B., and Ransohoff R.M.. 2006. The expression and function of chemokines involved in CNS inflammation. Trends Pharmacol. Sci. 27:48–55. 10.1016/j.tips.2005.11.002 [DOI] [PubMed] [Google Scholar]
- Ulivieri, C., Savino M.T., Luccarini I., Fanigliulo E., Aldinucci A., Bonechi E., Benagiano M., Ortensi B., Pelicci G., D’Elios M.M., et al. 2016. The Adaptor Protein Rai/ShcC Promotes Astrocyte-Dependent Inflammation during Experimental Autoimmune Encephalomyelitis. J. Immunol. 197:480–490. 10.4049/jimmunol.1502063 [DOI] [PubMed] [Google Scholar]
- Ulivieri, C., De Tommaso D., Finetti F., Ortensi B., Pelicci G., D’Elios M.M., Ballerini C., and Baldari C.T.. 2019. A T Cell Suppressive Circuitry Mediated by CD39 and Regulated by ShcC/Rai Is Induced in Astrocytes by Encephalitogenic T Cells. Front. Immunol. 10:1041. 10.3389/fimmu.2019.01041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vainchtein, I.D., and Molofsky A.V.. 2020. Astrocytes and Microglia: In Sickness and in Health. Trends Neurosci. 43:144–154. 10.1016/j.tins.2020.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vainchtein, I.D., Chin G., Cho F.S., Kelley K.W., Miller J.G., Chien E.C., Liddelow S.A., Nguyen P.T., Nakao-Inoue H., Dorman L.C., et al. 2018. Astrocyte-derived interleukin-33 promotes microglial synapse engulfment and neural circuit development. Science. 359:1269–1273. 10.1126/science.aal3589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, Y., Fu W.Y., Cheung K., Hung K.W., Chen C., Geng H., Yung W.H., Qu J.Y., Fu A.K.Y., and Ip N.Y.. 2021. Astrocyte-secreted IL-33 mediates homeostatic synaptic plasticity in the adult hippocampus. Proc. Natl. Acad. Sci. USA. 118:e2020810118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler, M.A., Rothhammer V., and Quintana F.J.. 2017. Control of immune-mediated pathology via the aryl hydrocarbon receptor. J. Biol. Chem. 292:12383–12389. 10.1074/jbc.R116.767723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler, M.A., Jaronen M., Covacu R., Zandee S.E.J., Scalisi G., Rothhammer V., Tjon E.C., Chao C.C., Kenison J.E., Blain M., et al. 2019. Environmental Control of Astrocyte Pathogenic Activities in CNS Inflammation. Cell. 176:581–596.e18. 10.1016/j.cell.2018.12.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler, M.A., Clark I.C., Tjon E.C., Li Z., Zandee S.E.J., Couturier C.P., Watson B.R., Scalisi G., Alkwai S., Rothhammer V., et al. 2020. MAFG-driven astrocytes promote CNS inflammation. Nature. 578:593–599. 10.1038/s41586-020-1999-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, J.F., Tao H.Q., Liu Y.M., Zhan X.X., Liu Y., Wang X.Y., Wang J.H., Mu L.L., Yang L.L., Gao Z.M., et al. 2012. Characterization of the interaction between astrocytes and encephalitogenic lymphocytes during the development of experimental autoimmune encephalitomyelitis (EAE) in mice. Clin. Exp. Immunol. 170:254–265. 10.1111/j.1365-2249.2012.04661.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokote, H., Miyake S., Croxford J.L., Oki S., Mizusawa H., and Yamamura T.. 2008. NKT cell-dependent amelioration of a mouse model of multiple sclerosis by altering gut flora. Am. J. Pathol. 173:1714–1723. 10.2353/ajpath.2008.080622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- You, T., Bi Y., Li J., Zhang M., Chen X., Zhang K., and Li J.. 2017. IL-17 induces reactive astrocytes and up-regulation of vascular endothelial growth factor (VEGF) through JAK/STAT signaling. Sci. Rep. 7:41779. 10.1038/srep41779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu, X., Taylor A.M.W., Nagai J., Golshani P., Evans C.J., Coppola G., and Khakh B.S.. 2018. Reducing Astrocyte Calcium Signaling In Vivo Alters Striatal Microcircuits and Causes Repetitive Behavior. Neuron. 99:1170–1187.e9. 10.1016/j.neuron.2018.08.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamvil, S.S., and Hauser S.L.. 2021. Antigen Presentation by B Cells in Multiple Sclerosis. N. Engl. J. Med. 384:378–381. 10.1056/NEJMcibr2032177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeisel, A., Muñoz-Manchado A.B., Codeluppi S., Lönnerberg P., La Manno G., Juréus A., Marques S., Munguba H., He L., Betsholtz C., et al. 2015. Brain structure. Cell types in the mouse cortex and hippocampus revealed by single-cell RNA-seq. Science. 347:1138–1142. 10.1126/science.aaa1934 [DOI] [PubMed] [Google Scholar]
- Zeisel, A., Hochgerner H., Lönnerberg P., Johnsson A., Memic F., van der Zwan J., Häring M., Braun E., Borm L.E., La Manno G., et al. 2018. Molecular Architecture of the Mouse Nervous System. Cell. 174:999–1014.e22. 10.1016/j.cell.2018.06.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, Y., Song W.M., Andhey P.S., Swain A., Levy T., Miller K.R., Poliani P.L., Cominelli M., Grover S., Gilfillan S., et al. 2020. Human and mouse single-nucleus transcriptomics reveal TREM2-dependent and TREM2-independent cellular responses in Alzheimer’s disease. Nat. Med. 26:131–142. 10.1038/s41591-019-0695-9 [DOI] [PMC free article] [PubMed] [Google Scholar]