Abstract
Objective:
Respiratory abnormalities are a hallmark of anxiety symptomatology and may serve as clinically useful modifiers for alleviating anxiety symptoms. However, gold-standard anxiety treatments (e.g., cognitive-behavioral interventions) often do not directly address respiratory components despite their theoretical utility and clinical accessibility. This review examined the clinical effectiveness of respiratory interventions, interventions that directly target respiration abnormalities and processes, in treating trait anxiety symptoms.
Methods:
The final analysis included 40 randomized controlled trials including at least one measure of trait anxiety, a respiratory-focused intervention group, and a non-respiratory control-group (active or inactive treatment). Overall effects of respiratory focused interventions were examined, as well as the effect of hypothesized moderators.
Results:
Respiratory component interventions yielded significantly greater improvements (moderate to large effect) in anxiety symptoms than controls, with the stronger effects observed in comparison to inactive, rather than active, control conditions. Significant heterogeneity in findings suggests that variability in intervention design, population, and control comparison may obfuscate interpretation of findings.
Conclusions:
Evidence supports the clinical utility of respiratory interventions as either an independent anxiety treatment, or as an adjunct to other interventions. Clinical and research implications of findings along with recommendations for ongoing investigations in this domain are discussed.
Keywords: Anxiety, Intervention, Treatment, Respiration, Breathing, Meta-analysis
Anxiety and related disorders are estimated to affect 11.6% (95% CI 7.6–17.7%) of people worldwide (Baxter et al., 2013) and 21.3% of the U.S. adult population ages 18–64 annually (Kessler et al., 2012). Given anxiety disorders’ global health burden and associated impairment (Baxter et al., 2014), extensive efforts have been made to better characterize their etiology and course and to refine extant evidence-based interventions utilized to treat them (e.g., Baxter et al., 2014; Lau & Rapee, 2011; Olatunji et al., 2010). Yet, even among those who receive what is regarded as ‘gold standard’ anxiety treatment (i.e., cognitive-behavioral therapy; CBT), effect sizes are small to medium (Carpenter et al., 2018). In addition, there is a notable treatment gap where, according to one report, just 21.7% of individuals diagnosed with an anxiety disorder have sought treatment from a health care provider in the prior 12 months (Wang et al., 2005). There is thus a need to decrease barriers to treatment (Wang et al., 2005) and to develop easily disseminable and effective treatment packagess that target symptoms central to anxiety pathology and maintenance.
Cognitive-behavioral interventions that have received the most robust empirical support among non-pharmacological interventions for anxiety disorders largely focus on addressing maladaptive beliefs and behaviors that serve to maintain anxiety and associated impairment (Carpenter et al., 2018; Cuijpers et al., 2016; Hofmann et al., 2012; Hofmann & Smits, 2008; Montero-Marin et al., 2018; Olatunji et al., 2010; Olatunji & Wolitzky-Taylor, 2009; Tolin, 2010). However, for many individuals, cognitive-behavioral interventions do not result in clinically significant changes, with smaller effect sizes obtained for posttraumatic stress disorder (PTSD)1, social anxiety disorder (SAD), and panic disorder (PD; Carpenter et al., 2018). One possible explanation for this treatment gap is that CBT may fail to adequately address hyperarousal often observed in these disorders. Exposure, particularly interoceptive exposure, which addresses fear of bodily sensations associated with anxious arousal (Boettcher et al., 2016), tends to serve as the primary empirically supported means by which somatic symptoms are targeted in CBT interventions. Yet, use of exposure is low even amongst therapists who report practicing cognitive-behavioral interventions (Becker et al., 2004; Hipol & Deacon, 2013; Whiteside et al., 2016; Wolitzky-Taylor et al., 2015). This is likely due to a lack of therapist training and/or confidence, or of perceived discomfort on the part of clients who may report that exposures are aversive (Becker et al., 2004; Gunter & Whittal, 2010; van Minnen et al., 2010). Moreover, interoceptive exposures are designed to expose the individual to sympathetic arousal, thus the effective target is psychological distress rather than arousal itself, suggesting that therapists and clients may benefit from alternative intervention approaches (Boettcher et al., 2016).
Respiration is one viable target that may serve as a more direct means of targeting anxiety symptoms, and in particular, those related to anxious arousal (Paulus, 2013). State and clinical anxiety have been found to be closely related to an elevated perception of respiratory dysregulation (Leander et al., 2014), altered respiratory features (Grassi et al., 2013; Stein et al., 1995), and elevated responsivity to respiratory challenges (Papp et al., 1997). Hyperventilation, for example, defined as an arterial blood pH above 7.4 (i.e., respiratory alkalosis) and blood CO2 (i.e., PaCO2) levels less than 30 mmHg (i.e., hypocapnia; Balke & Lillehei, 1956; Cohen, 1988; Gibson, 1978; Lickel et al., 2008), is a core symptom of panic disorder and a diagnostic specifier in other anxiety disorders (American Psychiatric Association [APA], 2013). Importantly, meta-analytic work examining respiratory dysfunction in panic disorder has found that dysfunctional breathing, a term we will use to describe the various respiratory patterns that produce hyperventilation (Boulding et al., 2016; Courtney, 2017; Courtney, Greenwood, & Cohen, 2011; Courtney, van Dixhoorn, et al., 2011), appears to be chronic in this population, even in the absence of a panic attack (Grassi et al., 2013). Also, sensations of dyspnea (i.e., breathlessness or the inability to get an adequate breath) are frequently observed in anxious individuals, such that anxiety appears to precede the onset of, and perhaps contribute to, dyspnea (Neuman et al., 2006). Thus, it seems probable that chronic dysfunctional breathing and related sensations could increase vulnerability to anxiety symptoms when stress or other respiratory stimulants cause blood levels of carbon dioxide (PaCO2) to reach symptom levels.
Although the relation between dysfunctional breathing and anxiety appears to have particular specificity to panic, trait anxiety is also related to respiratory dysregulation (Ben-Amnon et al., 1995; Leander et al., 2014), and through complex processes affects affective and cognitive dysregulation. The characteristic overuse of accessory muscles of the thorax and shoulders during dysfunctional breathing, versus abdominal breathing (Courtney, van Dixhoorn, et al., 2011), may contribute to increases in sympathetic nervous system activity (Delaney et al., 2010; Fatouleh & Macefield, 2013; Hellström et al., 2005; Radovanovic et al., 2015) and increased respiratory drive. Ultimately, this cascade can result in a positive feedback loop where increases in muscle tension lead to increased sympathetic arousal producing afferent feedback to the central nervous system that can compound anxiety and further increase ventilation (Ritz et al., 2013).
The effects of this forward feedback loop may exacerbate dysfunctional breathing and in turn lead to subsequent blood vessel constriction in the brain and body (Bindslev et al., 1986; Malatino et al., 1992), thus decreasing availability of oxygen (Bindslev et al., 1986; Malatino et al., 1992). Resultant increased affinity of hemoglobin to oxygen, i.e., the ‘Bohr effect’ (Bohr et al., 1904) compounds oxygen deprivation to body tissues and the brain, which may impact cognitive impairment (Van Diest et al., 2000) and contribute to dissociation (Michel et al., 1966; Simeon et al., 2003), in addition to its effects on physiological symptoms of anxiety (Van den Bergh et al., 2013). In some cases, these uncomfortable and sometimes frightening symptoms may further contribute to feelings of anxiety and ultimately panic. Therefore, targeting dysfunctional breathing across anxiety pathology may be beneficial.
Additional support for targeting dysfunctional breathing in anxious individuals comes from research on anxiety sensitivity, defined as the fear of anxiety relevant sensations and their potential negative social, mental, and physical outcomes (Reiss et al., 1986). Anxiety sensitivity, the purported target of interoceptive exposures, is an established risk for the onset and maintenance of anxiety and its disorders, in particular those characterized by autonomic arousal, such as panic disorder and posttraumatic stress disorder (Olatunji & Wolitzky-Taylor, 2009). The physical concerns subscale of the anxiety sensitivity index, as compared to mental and social concerns, is correlated with greater bodily vigilance (Zvolensky et al., 2007; Zvolensky et al., 2002). This could magnify the salience of uncomfortable respiratory-related symptoms and further potentiate an anxiety spiral. In support of this, anxiety sensitivity has been found to predict self-reported ratings of dyspnea and dyspnea-related avoidance (Simon et al., 2006). Moreover, the use of interoceptive exposure in the context of cognitive-behavioral therapy has been linked to significant reductions in anxiety sensitivity (Boswell et al., 2013; Collimore & Asmundson, 2014; Wald & Taylor, 2008), suggesting that changes in anxiety sensitivity may mediate treatment effects. It is therefore plausible that among anxious individuals, subjective reports of respiratory distress in the absence of pulmonary pathology may contribute to misinterpretation and avoidance of physiological symptoms. This avoidance may also generalize to other various behaviors that can generate these sensations through increases in respiratory drive, including exercise, exposure to stress, altitude, or elevations in ambient carbon dioxide that might occur in crowded settings. Whereas interoceptive exposures may promote inhibitory learning by altering maladaptive beliefs about anxiety, interventions that target respiratory processes directly may offer an alternative approach toward diminishing anxiety interference.
Together, given the close connection between dysfunctional breathing and anxiety symptoms, therapies that directly target respiration might be expected to have a clinically significant therapeutic effect, both via direct effects on the central and parasympathetic nervous systems, and indirectly via secondary effects on cognitive vulnerabilities. For example: (1) diaphragmatic breathing can reduce the use of thoracic muscles; (2) controlled or paced breathing can regulate dysfunctional breathing via timed inhalation and expiration; (3) heart rate variability biofeedback, whereby individuals are taught to breathe at the resonance frequency of the baroreflex system (approximately 6 breaths/minute), can improve alveolar gas exchange efficiency (Bernardi et al., 1998; Giardino et al., 2004), thereby preventing hyperventilation (Bernardi et al., 2007; Bernardi et al., 1998; Spicuzza et al., 2005), and increase parasympathetic activation (Lehrer et al., 2003); and (4) PaCO2 biofeedback (as approximated by end-tidal CO2 [ETCO2]; Meuret & Ritz, 2010; Meuret et al., 2008) can decrease hypocapnia and improve availability of oxygen to the tissues through the Bohr effect (See Table 1 for descriptions of respiratory intervention types).
Table 1.
Search Terms
| Respiratory Search Terms | breath* retrain*, respirat* retrain*, HeartMath, freeze framer, breathing and meditat*, respir* meditation, ETCO2 biofeedback, zen, tai chi, gigong, chi-gong, qigong, mindful*, emwave, jan van dixhoorn, pranya*, pranaya*, vinyasa*, buteyko, respiratory exercises, stress eraser, cardiosignal*, resp-e-rate, Whole body breathing, capnometry assisted respiratory training, diaphragm* breath*, paced breathing, paced respiration, breathing exercises, slow breathing, slow respiration, abdominal breathing, abdominal respiration, relaxed breathing, relaxed respiration, resonance frequency biofeedback, resonant frequency biofeedback, HRV*, heart rate variability, RSA*, respiratory sinus arrhythmia |
| Anxiety Search Terms | phobi*, panic*, fear*, psychological stress, PTSD, Post-traumatic stress, posttraumatic stress, anxi*, ataque*, taijin kyofusho, khyal, shenkui, grisi sicknis, effort syndrome, neurocirculatory ischemia, ataca de nerviosa, emotional stress, Hypocapn*, Alkalosis, alkylosis |
| Treatment Search Terms | interven*, treat*, feedback, biofeedback, retrain*, train*, therapy, therapeutic, therapies, management* |
indicate truncated versions of each term
Despite the strong theoretical utility of respiratory interventions for the treatment of anxiety, availability of these interventions, and their common use in clinical intervention, to date no one has systematically reviewed literature on their independent clinical effectiveness. Notably, some CBT interventions have included relaxation and breathing retraining as additional techniques to target anxious arousal and comparison of cognitive-behavioral interventions with versus without these components has found no significant difference in clinical outcomes (Siev & Chambless, 2007), or additive value (Hofmann et al., 2012; Olatunji et al., 2010). Yet, breathing retraining is just one means by which to target dysfunctional breathing and these findings do not negate the potential clinical value of respiratory interventions for anxiety. Moreover, we do not anticipate that respiratory interventions should function as standalone treatments for anxiety given the importance of cognitive (e.g., worry) and behavioral (e.g., avoidance) symptoms that may play a more central role in impairment (Stapinski et al., 2010), and the experience of psychophysiological symptoms that are less directly related to dysfunctional breathing (McKinnon et al., 2015; Pollard et al., 2017; Saigo et al., 2014). While respiratory interventions may not adequately address these various symptom domains, a clearer understanding of their utility as a treatment component or adjunct is warranted.
The current meta-analytic review examined the effect size of interventions that target various manifestations of dysfunctional breathing for the treatment of anxiety. In addition to using DSM diagnostic categories (APA, 1994, 2013), we approached anxiety symptoms dimensionally, a perspective that has gained wide acceptance in recent research (Kliem et al., 2016; Kotov et al., 2017; Kotov et al., 2015; Uliaszek et al., 2015), and is supported by extant findings linking dysfunctional breathing to various anxiety symptoms independent of DSM disease categories (Crockett et al., 2016; Hagman et al., 2008; Paulus, 2013; Simon et al., 2006). This dimensional approach allowed us to examine the effects of respiratory interventions on specific symptoms that have a more psychophysiological basis as compared to disorder categories.
Methods
Literature Search
A combination of respiratory, anxiety, and treatment search terms were employed (see Table 3) to capture all interventions that may have used a respiratory component and measured trait anxiety as a primary or secondary outcome of interest. Specifically, search terms within each of the three categories were linked with the Boolean operator ‘or,’ and categories of search terms were linked using the Boolean operator ‘and,’ resulting in capture of manuscripts that included at least one ‘Respiratory,’ ‘Anxiety,’ and ‘Treatment’ search term. We used PsycInfo, Medline, and PubMed search engines to identify potentially relevant studies (date range: through November, 2019). We additionally selectively searched for studies by authors who have published substantially in the area of respiration and psychopathology and cross-checked reference sections of identified articles, with an emphasis on review and theoretical articles. The initial literature search was independently conducted by MV and MJY, yielding identical results. Screening of manuscripts for inclusion in the analyses was completed by MV, MJY, and HB, following training and supervision by TML and PL.
Table 3.
Characteristics of Randomized Controlled Trials of Respiratory Interventions for the Treatment of Anxiety (N=40)
| Author(s), year | Sample Characteristics | Intervention | Control Comparison(s) | Sessions | Treatment Duration (weeks) | Followup (weeks) | Anxiety Measures | Anxiety as Primary Study Outcome? | |
|---|---|---|---|---|---|---|---|---|---|
| Number | Duration (min) | ||||||||
| Berger, 2000 | N=21; PD; Outpatient | Diaphragmatic Breathing (single) | CBTa | 6 | 30–60 | 6 | 4 | PDSS; PA frequency | Yes |
| Bonn et al., 1984 | N=12; Agoraphobia; Outpatient | Diaphragmatic Breathing with Exposure (combined) | Exposurea | 2 | 60 | 2 | 4 & 24 | PA frequency; Global Phobia Score; Agoraphobia Score | Yes |
| Bratton, 2008 | N=20;Pregnant Women; Outpatient | Diaphragmatic Breathing (single) | Mindfulness meditationa | 1 | 90 | 1 | N/A | STAI | Yes |
| Chandl a et al., 2013 | N=96; Medical Students; Non-Clinical | DiaphragmaticBreathing1 (single) | Yoga Asanaa | 42 | 60 | 6 | N/A | HAM-A | Yes |
| Chang et al., 2020 | N=35; Clinical; Acute Ischemic Stroke Patients | HRV Biofeedback (single) | Usual Carei | 4 | 20 | 1 | 4 & 12 | HADS | Yes |
| Chen et al., 2017 | N=30; Clinical; Outpatient | Diaphragmatic Breathing (single) | Breathing Relaxation Practice with Biofeedback Monitoring a | 12 | 30 | 8 | N/A | BAI | Yes |
| Craske et al., 1997 | N=38; PD; Outpatient | Diaphragmatic Breathing w/ cognitive restructuring & in-vivo exposure (combined) | Interoceptive exposure w/ cognitive restructurin g, & invivo exposurea | 12 | 90-120 | 12 | N/A | ADIS-R HAM-A; Situational Fear Question naire | Yes |
| Colgan et al., 2016 | N=102; PTSD; Outpatient | HRV Biofeedback 2 (single) | Body Scan Meditation a; Mindful Breathinga; Sitting Quietlyi | 6 | 20 | 6 | N/A | PCL_C | Yes |
| Dhruv a et al., 2012 | N=16; Chemotherapy patients; Outpatient | Diaphragmatic Breathing1 (single) | Waitlisti | 7 | 60 | 7 | N/A | HADS | Yes |
| Enrigh t et al., 2004 | N=19; Cystic Fibrosis; Outpatient | High Effort Inspiratory Muscle Training (single) | Low Effort Inspiratory Muscle Traininga; No Treatmenti | 24 | 45 | 8 | N/A | HADS | No |
| Fasko et al., 1992 | N=10; Undergrad uate; Non- Clinical | Controlled Breathing3 (single) | Saying ‘ 1’ upon exhalationa | 1 | 30 | 1 | N/A | STAI | Yes |
| Gatche l & Procto r, 1976 | N=36; Undergrad uate; Non- clinical | HR Biofeedback (single) | Light trackingp | 2 | 20 | 2 | N/A | Personal Report of Confide nce as a Speaker Self Reported Anxiety | Yes |
| Gruzel ier et al., 2014 | N=32; Undergrad uate; Non- Clinical | HRV Biofeedback (single) | Alphatheta neurofeedbacka; Choreology Instructionp; No Treatmenti | 10 | 20 | 10 | N/A | DASS | Yes |
| Hayam a & Inoue, 2012 | N=23; Gynecological cancer; Outpatients | Diaphragmatic Breathing (single) | Chemother apy and Nursing Carei | 4 | 10 | 2 | N/A | POMS | Yes |
| Hakke d et al., 2017 | N=27; Competitive Swimmers; Non-Clinical | Yogic Breathing (single) | Waitlisti | 20 | 30 | 4 | N/A | SAS-2 | Yes |
| Henriq ues et al., 2011 | N=30; Undergrad uate; Non- Clinical | HRV Biofeedback (single) | Waitlisti | 4 | 15 | 20 | N/A | MASQ | Yes |
| Hibber t & Chan, 1989 | N=42; PA; Outpatient | Paced Breathing (single) | Obesrving physical anxiety symptomsp | 5 | 30–60 | 6 | N/A | HAM-A | Yes |
| Lee et al., 2015 | N=10; Undergrad uate; Non- Clinical | HRV Biofeedback (single) | No Treatmenti | 4 | 45 | 8 | N/A | STAI | Yes |
| Lin, 2018 | N=82; Online recruited healthy adults; Non-Clinical | HRV Biofeedback (single) | Relaxation Training Groupa; No Treatment i | 4 | 60 | 4 | N/A | BAI | Yes |
| Lin et al., 2019 | N=48; MDD; Outpatient | HRV Biofeedback (single) | Waitlisti | 6 | 60 | 6 | N/A | BAI | Yes |
| Lickel, 2010 | N=57 Undergrad uate; Non- Clinical | Diaphragmatic Breathing with Psychoeducation (combined) | Psychoeducationa | 1 | Not specified | 1 | N/A | BAI | Yes |
| Meuret et al., 2008 | N=37 PD; Community sample | ETCO2 Biofeedback (single) | Waitlisti | 5 | 60 | 4 | N/A | PDSS | Yes |
| Meuret et al., 2010 | N=41 PD; Outpatient | ETCO2 Biofeedback (single) | Cognitive Skill Traininga | 5 | 60 | 4 | N/A | PDSS | Yes |
| Paul & Garg, 2012 | N=30 Basketball Players; Non-Clinical | HRV Biofeedback (single) | viewing motivation al filmp; No Treatmenti | 10 | 20 | 1.5 | 9.5 | STAI | Yes |
| Penzli n et al., 2015 | N=48 AUD; Inpatient | HRV Biofeedback +CBT, MI, and Occupationa lTherapy (single) | CBT, MI, and Occupation al Therapya | 6 | 20 | 2 | 5 & 8 | Symptom Checklist-90-Revised | No |
| Pham et al., 2016 | N=63 Online recruited adults; Non-Clinical | Diaphragmatic Breathing (single) | Waitlisti | Variable | Atleast 1 | 4 | N/A | GAD-7; PDSS | No |
| Smith et al., 2019 | N=169; Technology Service Employees; Non-Clinical | Mindful Breathing (single) | Waitlisti | Variable | Atleast 1 | 4 | N/A | MASQ; CDC Healthy Days-Days Anxious | Yes |
| Stefanaki et al., 2015 | N=46 PCOS; Outpatient | Diaphragmatic Breathing (single) | No Treatmenti | 9 | 30 | 8 | N/A | DASS-21 | Yes |
| Tan et al., 2011 | N=20; PTSD; Outpatient | HRV Biofeedback (single) | Treatment as Usuala | 8 | 30 | 8 | N/A | PCL-S; CAPS | Yes |
| Thoma s et al., 2009 | N=183; Asthma; Outpatient | Diaphragmatic Breathing (single) | Psychoeducationa | 3 | 60 | 7 | 4 & 24 | HADS | No |
| Thurbe r, 2007 | N=14; Undergrad uate; Non- Clinical | HRV Biofeedback (single) | No Treatment i | 4 | 40 | 4 | N/A | STAI | Yes |
| Valenz a et al., 2014 | N=46; COPD; Inpatient | Controlled Breathing (single) | Standard MedicalCarea | 20 | 30 | 2 | N/A | HADS | Yes |
| van der Zwan et al., 2015 | N=75; Community Sample; Non- Clinical | HRV Biofeedback (single) | Mindfulness Meditation a Physical Activitya | 5 | 20 | 1 | 6 | DASS | Yes |
| van der Zwan et al., 2019 | N=50; Pregnant and NonPregnant women; Outpatient | HRV Biofeedback (single) | Waitlisti | 5 | 60–90 | 5 | 5 & 11 | DASS | Yes |
| Van Dixho orn et al., 1990 | N=137; Cardiac Patients; Outpatient | EMG Biofeedback for Inspiratory control+Physical Activity (single) | Physical Activitya | 6 | 60 | 6 | N/A | STAI | Yes |
| Wahbeh et al., 2016 | N=102; PTSD; Combat Veterans | Slow Breathing2 (single) Slow Breathing2 & Mindfulness Meditation (combined) | Mindfulness Meditation a; Sitting Quietlyi | 6 | 20 | 6 | N/A | PCL-C | Yes |
| Wang et al., 2017 | N=55; COPD; Outpatient | Inspiratory Muscle Training + Cycle Ergometer Training (combined) | Cycle Ergometer Traininga | 24 | 44 | 8 | N/A | HADS | Yes |
| White, 2008 | N=36; PTSD; Inpatient | HRV Biofeedback (single) | Progressiv e Muscle Relaxationa | 5 | 20 | 5 | N/A | DAPS | Yes |
| Yekta et al., 2017 | N=60; Clinical; Mastecto my Surgery Patients | Rhythmic Breathing (single) | Benson’s Relaxation Techniquea; No Treatmenti | 2 | 40 | 1 | N/A | CSAQ | Yes |
| Zucker et al., 2009 | N=38; PTSD; Inpatient | HRV Biofeedback (single) | Progressiv e Muscle Relaxationa | 28 | 20 | 4 | N/A | DAPS; PCL-C | Yes |
Note. N/A=Not Available; AUD=Alcohol Use Disorder; COPD=Chronic Obstructive Pulmonary disease; MDD= Major Depressive Disorder; MI=Motivational Interviewing; PA=Panic Attack; PD=Panic Disorder; PCOS=Polycystic Ovary Syndrome; PTSD=Posttraumatic Stress Disorder; CBT=Cognitive Behavioral Therapy; HRV=Heart Rate Variability; BAI (Beck Anxiety Index; (Beck, Epstein, Brown, & Steer, 1988)); CAPS (Clinician-Administered PTSD Scale: (Weathers & Litz, 1994)); CDC Healthy Days-Days Anxious (Center for Disease Control’s Healthy Days Core and Symptoms Modules (CDC HRQOL-14; Centers for Disease Control and Prevention, 2000); CSAQ (Cognitive-Somatic Trait Anxiety Questionnaire: (Schwartz, Davidson, & Goleman, 1978); DAPS (Detailed Assessment of Posttraumatic Stress; (Briere, 2001)); DASS (Depression Anxiety Stress Scale: (Lovibond & Lovibond, 1995); DASS-21 (Depression Anxiety Stress Scale: (Henry & Crawford, 2005)); GAD-7 (Generalized Anxiety Disorder Scale;(Spitzer, Kroenke, Williams, & Löwe, 2006)); HADS= (Hospital Anxiety and Depression Scale, all studies utilized anxiety subscale; (Zigmond & Snaith, 1983)); HAM-A (Hamilton Anxiety Scale: (Hamilton, 1960)); MASQ (Mood and Anxiety Symptom Questionnaire, all studies utilized arousal and distress scale; (Keogh & Reidy, 2000)); PCL-C or S (PTSD Checklist-Civilian or Specific: (Tan et al., 2011)); PDSS (Panic Disorder Severity Scale; (Shear et al., 2001)); POMS (Profile of Mood State; (McNair, Lorr, & Droppleman, 1971)); STAI (State-Trait Anxiety Inventory: (Spielberg, Gorsuch, & Lushene, 1983)); SAS-2 (Sport Anxiety Scale-2: (R. E. Smith, Smoll, Cumming, & Grossbard, 2006)
Active control comparison
Inactive control comparison
Placebo control comparison
Pranayama Yoga
RESPeRATE
Comeditation
Inclusion/Exclusion Criteria for this Study
Primary inclusion criteria required that manuscripts: (1) included a treatment with at least one component targeting respiration; (2) employed a control comparison group that allowed for isolation of the respiratory intervention component; and (3) included an anxiety index as an outcome. In addition, while there were no explicit inclusion restrictions regarding the study design employed, included studies must have indexed anxiety symptoms after the administration of a respiratory intervention. As such, studies that reported pre- and post-intervention trait anxiety symptoms, pre- to post-intervention change in trait anxiety symptoms, or post- intervention trait anxiety symptoms were eligible for inclusion. All manuscripts were required to be written in English due to author language limitations. Lastly, no inclusion restriction was placed based on the year of publication of a manuscript. To minimize the ‘file drawer effect’, whereby studies without significant findings often remain unpublished, we also screened and included unpublished dissertations, when relevant.
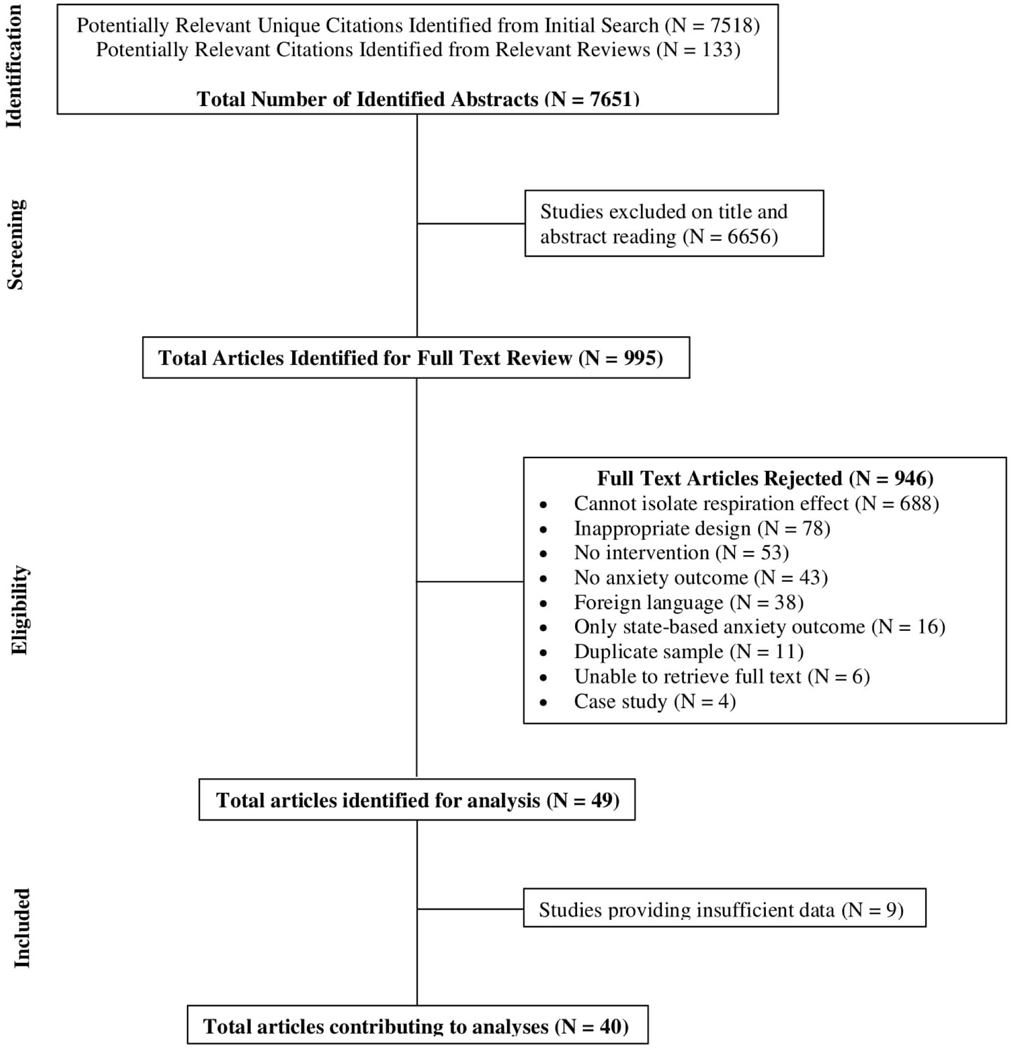
We screened 7,518 unique abstracts to determine initial eligibility. Following initial abstract review, 995 manuscripts were selected for full-text examination. Articles whose abstracts did not provide adequate information to determine if they met the primary inclusion criteria were also retained for full-text review (see Figure 1 for the consort chart of screened articles). Next, full-text articles were retrieved and independently read and coded by MJY, MV, and HB. Where initial reviewers disagreed or were unclear about inclusion of particular studies, the decision was made by TML or PL. We then determined whether studies provided sufficient information to compute effect size estimates (see Data Synthesis). Where studies did not provide this information, authors were directly contacted.
Figure 1.
Consort Chart of Screened Articles
Next, the remaining articles (n=49) were coded for inclusion of appropriate anxiety measurement, composition of experimental and control group and interventions, duration of treatment and assessment, and presentation of data in a manner interpretable for meta-analysis. In total, n=40 studies were identified as meeting our inclusion criteria and provided sufficient data for analyses.
In order to obtain the largest possible pool of studies we included some studies with methodological flaws, such as failing to blind of statistical analysts, lack of behavioral placebo conditions, or absence of double blinding approximations. To partially compensate for this, we included all anxiety measures included in each study, whether or not they were identified as primary outcome measures.
Assessment of Study Quality
Risk of Bias (RoB) of assignment to intervention (i.e., the ‘intention-to-treat, effect) was assessed based on the Cochrane Handbook (Higgins et al., 2019), and supported using available tools (e.g., signaling questions and algorithm; Risk of bias, 2020). RoB as ‘high,’ ‘low,’ or having ‘some concerns’ was assessed across five domains: (1) Domain 1: Bias arising from the randomization process; (2) Domain 2: Bias due to deviations from intended intervention; (3) Domain 3: Bias due to missing outcome data; (4) Domain 4: Bias in measurement of the outcome; and (5) Domain 5: Bias in selection of the reported result. First, raters (HRB, DLH, MJY) were trained (TML) on how to classify studies across the five domains indicated. Next, they completed independent ratings of 12.5% (k=5) studies. Discrepancies were then discussed with TML, adjustments were made to the tool, and ratings were again completed independently, to ensure rater consensus. The remaining studies were randomly assigned for independent review by the three raters with stratification for dissertation studies (i.e., gray literature); a total of 20% (k=8) of all the studies were duplicate reviewed by two raters. Minor discrepancies in RoB for duplicate reviews were noted, consensus was reached, and ratings were adjusted independently according to that consensus. If relevant, conclusions reached during consensus discussions were re-applied to all studies to ensure consistency. When rating domains, sub-criteria are rated as ‘Yes,’ ‘Probably Yes,’ ‘No,’ ‘Probably No,’ and ‘No Information.’ Given the Cochrane criteria were developed with pharmaceutical trials in mind, we found several criteria within domains to be overly conservative for the purposes of the current review. Therefore, several adjustments to the signaling questions and tool algorithm were made following initial discrepancy discussions. First, we opted to remove Domain 2 from our assessment, which is informed by blinding of participant (Criterion 2.1) and interventionist (Criterion 2.2); an impossibility based on the behavioral basis of interventions reviewed such that all studies would have been rated as high RoB in this domain, thus resulting in a high overall RoB assessment. Also, the majority of retained studies were not pre-registered (k=39), which typically results in a ‘some concerns’ RoB rating for Domain 5. To account for this, in the case of no pre-registration of an analytic plan, we instead opted to code RoB in Domain 5 based upon match between the proposed analytic plan and reported results within the manuscript.
Data Synthesis
Data were analyzed using Comprehensive Meta-Analysis, Version 3, using Hedges’ g as a correction to Cohen’s d (Hedges & Olkin, 1985). Hedges’ g values of 0.0 to 0.3 are interpreted as no or minimal difference between the respiratory condition and control conditions, while 0.3 to 0.5, 0.5 to 0.7, and > 0.7 as small to medium, medium to large, and large to very large effect sizes, respectively (Cohen, 1988). Because the studies we reviewed are independent, we used a random effects model (Borenstein et al., 2017; Hedges & Vevea, 1998) and report pooled mean effect size estimates, allowing us to make population-based inferences (Berkeljon & Baldwin, 2009). We additionally examined the influence of outlying studies that may either reflect bias or the presence of unique population or procedural variables, by examining a funnel plot, which allows for greater between-study variability among studies by accounting for sample size. In studies with smaller n’s where the influence of sampling error is expected to be greater (Sterne & Harbord, 2004), we inspected them for unique procedural or population characteristics.
Several indices were used to assess for heterogeneity. We report Cochran’s Q-statistic (i.e., the weighted sum of squared differences between individual study effects and the pooled weighted effect across studies) based on the n for all studies, where Q is distributed as χ2 with k (i.e., number of studies), minus 1 degree of freedom. Here, significant values suggest we reject the null hypothesis of homogeneity, indicating dispersion in treatment effects among various populations. However the Q-statistic is highly sensitive to number of studies and to the cumulative number of participants, so a significant Q with a large sample size, as in the current review, can yield statistical significance for inconsequential heterogeneity (Higgins et al., 2003). We additionally interpret the I2 statistic, which estimates the proportion of the variance reflecting true effect size differences (i.e., the percentage of the variance that would remain if it were possible to remove all within-study sampling error; Borenstein et al., 2017). Finally, we use tau-(τ) and tau-squared to estimate dispersion of true effect sizes between studies (Borenstein et al., 2017). Tau-squared is the variance of effect size parameters across the population of studies (i.e., variance in true effect sizes). Tau represents the standard deviation of true effects across all studies. Tau can also be used to calculate a 95% prediction interval, wherein 95% of new studies would expect to yield g. Because the actual population effect size and variance are unknown a priori, we assumed a non-normal distribution and used an adjusted calculation for the corresponding prediction interval (Borenstein et al., 2017).
Where multiple anxiety outcomes were reported (n=9), an equal weight was assigned to each measure, calculated by dividing the Hedges’ g for each measure by the number of measures used (Rosenthal & Rubin, 1986). This ensured that each sample contributed just one overall anxiety effect size in each study, weighted by number of participants. Where there was more than one ‘control’ comparison (i.e., active and inactive controls; n=7), we averaged effects across control conditions, and later, used both separate meta-analyses and moderator analysis to characterize differences in effect size estimates as a function of treatment comparison. Where available, we used mean and standard deviation values to calculate Hedges’ g effect size estimates, weighted by sample size. When these values were not available (n=8), we calculated g from change scores and SDs (n=1), or from F-, t- or p-values to estimate effect sizes (n=7). Where studies reported both post-test and follow-up data, we averaged the two in our initial analysis. This was done, primarily, because the term “follow-up” has different meanings among studies, where follow-up time in one study sometimes was shorter than post-test time in other studies. Separate analyses were also conducted on post-test or follow-up data separately.
We separately analyzed the effects of respiratory interventions on dimensional anxiety symptom categories (e.g., panic, general anxiety, and post-traumatic stress) population (i.e., non-psychiatric/medical; psychiatric), and diagnostic category of the sample treated. For dimensional anxiety symptoms, we looked separately at inactive control comparisons to evaluate the independent effectiveness of respiratory interventions. Given respiratory biofeedback interventions provide highly specific training using objective physiological assessment of breathing relevant parameters, we used mixed-effects analyses (i.e., random effects) within subgroups to estimate and compare effect sizes of interventions including a biofeedback component and non-biofeedback interventions. Mixed-effects analyses were also used to compare active versus inactive control effects. Random effects meta regression analyses, using weighted least squares to account for variability in sample size, were used to investigate several moderator effects on overall effect size estimates (Borenstein et al., 2017; Stanley & Doucouliagos, 2012; Stanley & Jarrell, 1989). These included treatment duration and number of treatment sessions. Finally, year of publication was included in moderator analyses to estimate bias that may occur in the experimental and peer review processes over time. In all studies, we coded Hedges’ g in the negative direction indicating greater decreases in scores on anxiety scales in the respiratory treatment than in the control groups, or greater increases in scores on tests reflecting freedom from anxiety. For our primary analysis, however, we calculated the combined effect size for all comparisons.
Results
Systematic Review
The final sample of k=40 studies had an aggregated n of 3,047 participants, with treatment group sizes ranging from five to 92. The most common anxiety outcomes were the State Trait Anxiety Index (n=6), Hospital Anxiety and Depression Scale (n=6) Beck Anxiety Inventory (n=4), Panic Disorder Severity Scale (n=4), and the Depression Anxiety and Stress Scale (n=4). There was great variability in the populations examined, with k=10 studies examining a psychiatric population, k=7 focused on various physical conditions, including chronic obstructive pulmonary disease (Valenza et al., 2014), polycystic ovary syndrome (Stefanaki et al., 2015), pregnancy (Bratton, 2008), and chemotherapy patients (Dhruva et al., 2012), and k=12 on various non-psychiatric populations (Table 3), including participants with specific fears (e.g., fear of public speaking; Gatchel & Proctor, 1976), and nonclinical samples with elevated scores on various anxiety inventories, (e.g., Pham et al., 2016) that did not necessarily meet for a clinical diagnosis or were not diagnostically assessed.
The majority of active interventions examined the effects of diaphragmatic breathing (k=12) and heart rate variability (HRV) biofeedback (k=16) on anxiety outcomes. The remaining (k=12) interventions examined breathing modification techniques that did not specify diaphragmatic techniques or employed techniques to specifically affect other physiological processes. These included ETCO2 biofeedback, EMG biofeedback of the frontalis muscle, inspiratory muscle training, paced breathing at frequencies differing from those in HRV biofeedback, deep breathing, slow breathing, slow breathing, and co-meditation2 (Table 3).
Risk of Bias Findings
Our RoB assessment using our adjusted algorithm indicated k=35 studies classified as ‘high’ with the remaining k=5 classified as ‘some concerns’ (Table 4). Here, it is notable that k=33 studies were coded as ‘high’ risk in Domain 4, Outcome Measurement. In this domain k=16 included an inactive control comparison condition and k=4 including active comparator conditions that were not adequately matched to the active condition. For k=20 studies, this was the only domain coded as ‘high’ risk. Another k=6 studies were coded as ‘some concerns’ in Domain 4 due to lack of explicit details regarding how the conditions were presented to participants (e.g., neither inferior to the other). Together this indicates that a major limitation for extant studies of respiratory interventions is the failure to use an appropriate control comparison condition. We also noted that just k=1 study (Valenza et al., 2014) included a pre-registered analytic plan, suggesting that without editing the algorithm used for Domain 5, Selection of Reported Result, the majority would have been classified as ‘some concerns’ bias risk. Our assessment underscores the importance of considering control comparison type in between-group effects and mixed-effects analyses.
Table 4.
Risk of Bias Assessments by Domain and Overall
Note. Risk of Bias as assessed using the Cochrane Risk of Bias Tool; Domain 2 was removed as the impossibility of blinding in behavioral interventions would have resulted in every study being ranked as High risk of bias in this domain; H, high risk of bias; S, some concerns regarding risk of bias; L, low risk of bias.
Trial did not have a pre-registered analytic plan, thus the assessor’s judgment for this domain was changed from the suggested algorithm assessment of “some concerns” to an assessment based on what the algorithm would display if signaling questions were answered based upon match to the analytic plan as specified within the manuscript.
Meta-analysis
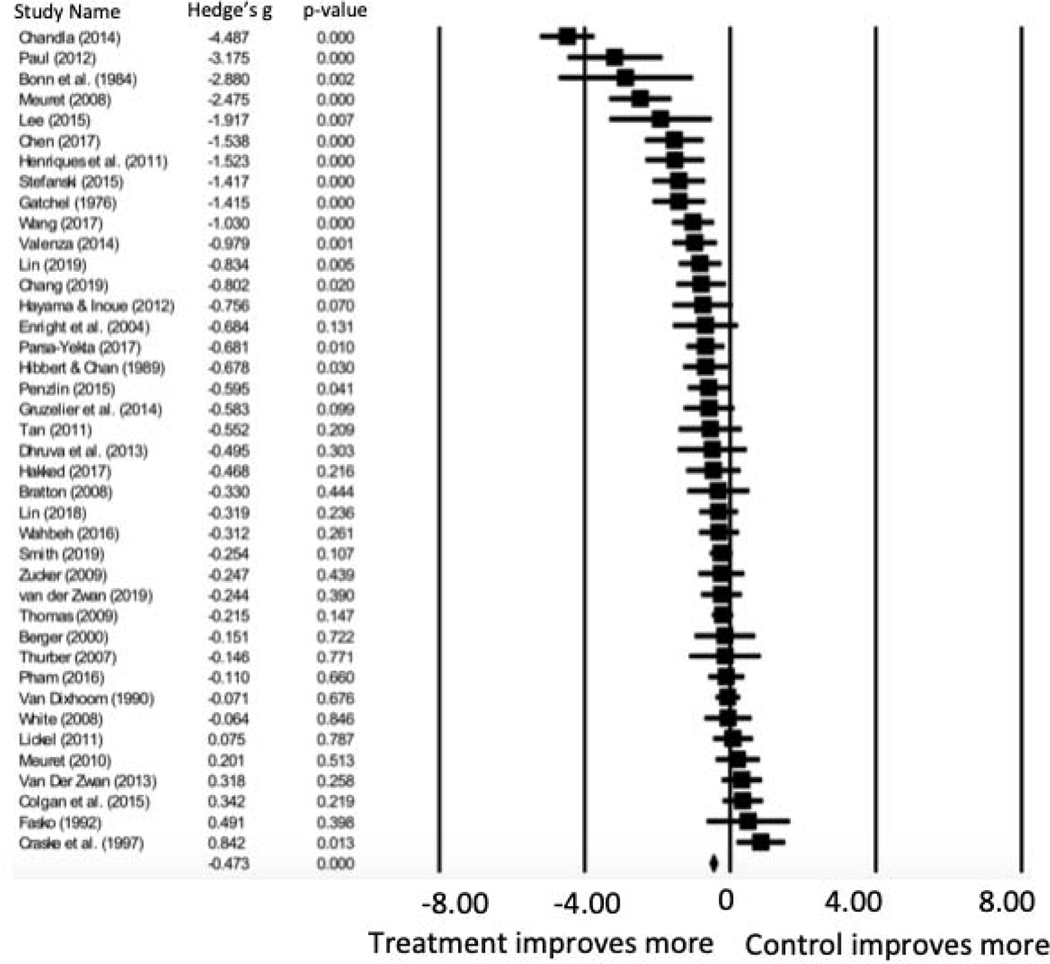
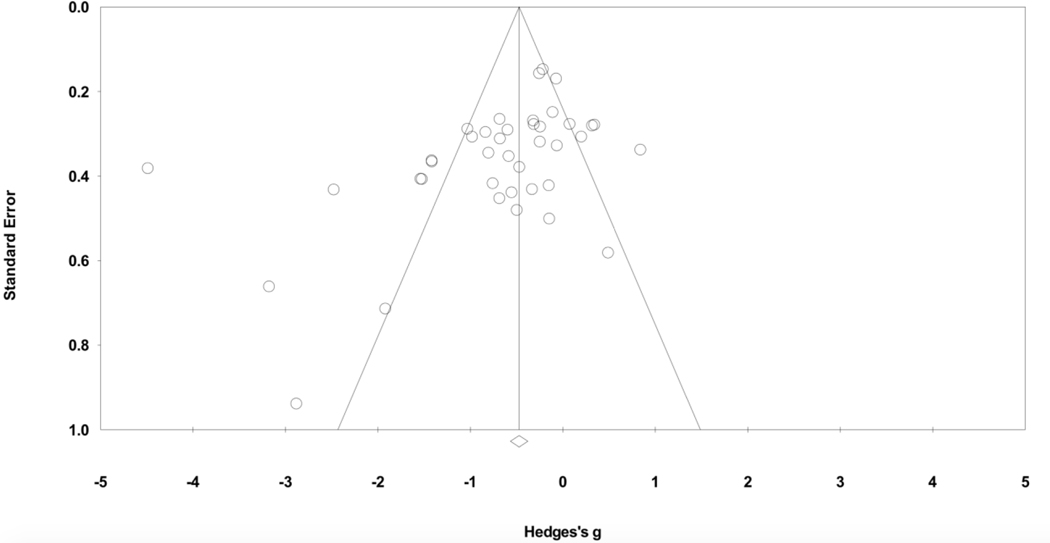
Overall, averaging post-treatment and follow-up ratings of anxiety across both active and inactive control conditions, therapies that included a respiratory component yielded significantly greater decreases in anxiety symptoms compared to controls, indicating a medium to large symptom improvement effect (Hedges’ g= −.678, 95% CI = −0.932, −0.424, p<.001) (see Table 5 and Figure 2). Significant heterogeneity suggests a large proportion of variance is estimated to reflect true variance rather than sample error (I2). Inspection of the funnel plot (Figure 3) identified Chandla et al. (2013) as an outlier. This trial exhibited unique characteristics wherein medical students were randomized to practice either slow paced pranayamic breathing or yoga asanas, led by expert instructors, each morning for about 40 minutes over the course of 6 weeks, totaling 42 sessions. Conversely, the treatment dose for all other studies included ranged from 1 to 28 sessions (Table 3). Due to its outlier status and unique study characteristics, Chandla et al. (2013) was removed from subsequent analyses.
Table 5.
Within-Group Respiratory Intervention Effect Sizes
| Author(s) (Year) | Hedge’s g | Standard Error | Variance | Lower Limit | Upper Limit | Z-Value | P Value |
|---|---|---|---|---|---|---|---|
| Berger (2000) | −0.151 | 0.423 | 0.179 | −0.979 | 0.678 | −0.356 | 0.722 |
| Bonn et al. (1984) | −2.880 | 0.939 | 0.881 | −4.720 | −1.040 | −3.067 | 0.002 |
| Bratton (2008) | −0.330 | 0.431 | 0.186 | −1.176 | 0.515 | −0.766 | 0.444 |
| Chandla et al. (2013) | −4.487 | 0.382 | 0.146 | −5.236 | −3.739 | −11.749 | <.001 |
| Chang et al. (2020) | −0.802 | 0.345 | 0.119 | −1.478 | −0.125 | −2.322 | 0.020 |
| Chen et al. (2017) | −1.538 | 0.407 | 0.166 | −2.335 | −0.740 | −3.778 | <.001 |
| Colgan et al. (2016) | 0.342 | 0.279 | 0.078 | −0.204 | 0.889 | 1.228 | 0.219 |
| Craske et al., 1997 | 0.842 | 0.338 | 0.114 | 0.179 | 1.504 | 2.490 | 0.013 |
| Dhruva et al. (2012) | −0.495 | 0.481 | 0.231 | −1.438 | 0.447 | −1.030 | 0.303 |
| Enright et al. (2004) | −0.684 | 0.453 | 0.205 | −1.572 | 0.204 | −1.510 | 0.131 |
| Fasko et al. (1992) | 0.491 | 0.582 | 0.338 | −0.649 | 1.631 | 0.845 | 0.398 |
| Gatchel et al. (1976) | −1.415 | 0.366 | 0.134 | −2.132 | −0.697 | −3.865 | <.001 |
| Gruzelier et al. (2014) | −0.583 | 0.353 | 0.125 | −1.276 | 0.109 | −1.651 | 0.099 |
| Hakked et al. (2017) | −0.468 | 0.379 | 0.144 | −1.211 | 0.274 | −1.236 | 0.216 |
| Hayama et al. (2012) | −0.756 | 0.417 | 0.174 | −1.574 | 0.062 | −1.811 | 0.070 |
| Henriques et al. (2011) | −1.523 | 0.407 | 0.166 | −2.322 | −0.725 | −3.738 | <.001 |
| Hibbert et al. (1989) | −0.678 | 0.312 | 0.097 | −1.289 | −0.067 | −2.174 | 0.030 |
| Lee et al. (2015) | −1.917 | 0.714 | 0.510 | −3.316 | −0.517 | −2.684 | 0.007 |
| Lickel (2010) | 0.075 | 0.277 | 0.077 | −0.468 | 0.618 | 0.270 | 0.787 |
| Lin (2018) | −0.319 | 0.269 | 0.072 | −0.846 | 0.208 | −1.185 | 0.236 |
| Lin et al. (2019) | −0.834 | 0.296 | 0.088 | −1.415 | −0.253 | −2.814 | 0.005 |
| Meuret et al. (2008) | −2.475 | 0.432 | 0.187 | −3.322 | −1.627 | −5.724 | <.001 |
| Meuret et al. (2010) | 0.201 | 0.307 | 0.094 | −0.401 | 0.803 | 0.654 | 0.513 |
| Parsa-Yekta et al. (2017) | −0.681 | 0.265 | 0.070 | −1.201 | −0.161 | −2.566 | 0.010 |
| Paul et al. (2012) | −3.175 | 0.662 | 0.438 | −4.472 | −1.878 | −4.798 | <.001 |
| Penzlin et al. (2015) | −0.595 | 0.291 | 0.085 | −1.165 | −0.025 | −2.046 | 0.041 |
| Pham et al. (2016) | −0.110 | 0.249 | 0.062 | −0.598 | 0.379 | −0.440 | 0.660 |
| Smith et al. (2019) | −0.254 | 0.157 | 0.025 | −0.562 | 0.054 | −1.614 | 0.107 |
| Stefanaki et al. (2015) | −1.417 | 0.363 | 0.132 | −2.129 | −0.705 | −3.900 | <.001 |
| Tan et al. (2011) | −0.552 | 0.439 | 0.193 | −1.412 | 0.308 | −1.257 | 0.209 |
| Thomas et al. (2009) | −0.215 | 0.148 | 0.022 | −0.504 | 0.075 | −1.452 | 0.147 |
| Thurber (2007) | −0.146 | 0.501 | 0.251 | −1.128 | 0.837 | −0.290 | 0.771 |
| Valenza et al. (2014) | −0.979 | 0.307 | 0.094 | −1.582 | −0.377 | −3.187 | 0.001 |
| van der Zwan et al. (2015) | 0.318 | 0.281 | 0.079 | −0.233 | 0.869 | 1.132 | 0.258 |
| van der Zwan et al. (2019) | −0.244 | 0.284 | 0.080 | −0.800 | 0.312 | −0.860 | 0.390 |
| Van Dixhoorn et al. (1990) | −0.071 | 0.170 | 0.029 | −0.404 | 0.262 | −0.418 | 0.676 |
| Wahbeh et al. (2016) | −0.312 | 0.278 | 0.077 | −0.856 | 0.232 | −1.124 | 0.261 |
| Wang et al. (2017) | −1.030 | 0.289 | 0.083 | −1.595 | −0.464 | −3.568 | <.001 |
| White (2008) | −0.064 | 0.328 | 0.108 | −0.707 | 0.579 | −0.194 | 0.846 |
| Zucker et al. (2009) | −0.247 | 0.319 | 0.102 | −0.873 | 0.379 | −0.774 | 0.439 |
| Average Effect Size | −0.678 | 0.129 | 0.017 | −0.932 | −0.424 | −5.234 | <.001 |
Figure 2.
Forest Plot of Breathing Interventions Pre- to Post-Treatment Change in Anxiety (n=40)
Note. Negative values of Hedges’ g indicate better treatment outcome.
Figure 3.
Funnel Plot of Standard
Error by Hedges’ g for Measures of Anxiety (n=40)
Upon removal of Chandla et al. (2013), we still found a medium effect size in favor of respiratory interventions compared with control interventions (Hedges’ g= −0.550, 95% CI = −0.747, −0.352, p<.001). Heterogeneity also remained significant, with the majority of variance (reflected in I2) reflecting true variance. Borenstein’s (2017) correction of τ resulted in a prediction interval of −1.613 to 0.513 suggesting that another study with a comparable population and method would have a 95% chance of falling between a very large effect size favoring respiratory therapies and a medium effect size favoring a control condition (see Table 6). To characterize whether therapeutic effects were maintained over time, we also examined effect size estimates at follow-up time points, where data were available (k=8) and found a medium to large effect (Hedges’ g= −0.710, 95% CI = −1.251, −0.168, p<.001)). Examination of post-test data (k=37) only, yielded a significant medium effect (Hedges’ g= −0.535, 95% CI = −0.740, −0.331, p<.001).
Table 6.
Main Study Outcomes
| k | Hedge s’ g | SE | Variance | 95%CI | 95%CI | Test of Null | p-value | Q-value | I2 | Prediction Intervals | |
|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|||||||||||
| LL | UL | Z | |||||||||
| All studies | 40 | −0.678 | 0.129 | 0.017 | −0.932 | −0.424 | −5.234 | <.001 | 257.206 | 84.837 | −2.176 to 0.820 |
| Removing Chandla et al. (2003) | 39 | −0.550 | 0.101 | 0.010 | −0.747 | −0.352 | −5.458 | <.001 | 144.9231 | 73.779 | −1.613 to 0.513 |
| Follow-Up Data Only | 8 | −0.710 | 0.276 | 0.076 | −1.251 | −0.168 | −2.570 | .010 | 39.245 | 82.163 | −2.472 to 1.052 |
| Post-Test Data Only | 37 | −0.535 | 0.104 | 0.011 | −0.740 | −0.331 | −5.128 | <.001 | 139.971 | 74.280 | −1.613 to 0.543 |
|
| |||||||||||
| Anxiety Symptom Category (dimensional) | |||||||||||
|
| |||||||||||
| Panic Symptoms | 6 | −0.784 | 0.555 | 0.308 | −1.872 | 0.303 | −1.414 | .157 | 61.285 | 91.841 | −4.586 to 3.018 |
| Inactive control comparis ons only | 2 | −1.220 | 1.229 | 1.511 | −3.629 | 1.189 | −0.993 | .321 | 24.292 | 95.883 | n/a |
| General Anxiety Symptoms | 28 | −0.521 | 0.099 | 0.010 | −0.715 | −0.328 | −5.279 | <.001 | 76.681 | 64.789 | −1.3603 to 0.407 |
| Inactive control comparis ons only | 20 | −0.668 | 0.121 | 0.015 | −0.905 | −0.432 | −5.544 | <.001 | 54.884 | 65.382 | −1.566 to 0.318 |
| Posttraumatic Stress Symptoms | 5 | −0.114 | 0.148 | 0.022 | −0.405 | 0.177 | −0.767 | .443 | 4.378 | 8.644 | −0.675 to 0.447 |
|
| |||||||||||
| Sample Characteristics | |||||||||||
|
| |||||||||||
| Nonpsychiatric and Medical | 26 | −0.572 | 0.108 | 0.012 | −0.784 | −0.359 | −5.279 | <.001 | 78.058 | 67.973 | −1.483 to 0.339 |
| Psychiatric sample | 13 | −0.513 | 0.230 | 0.053 | −0.963 | −0.062 | −2.229 | 0.026 | 66.432 | 81.936 | −2.208 to 1.182 |
| Panic disorder, only | 4 | −0.375 | 0.664 | 0.441 | −1.677 | 0.927 | −0.565 | 0.572 | 38.823 | 92.273 | −6.555 to 5.805 |
Note. k = Number of Studies, SE=Standard Error, CI=Confidence Interval, LL=Lower Limit, UL=Upper Limit
Dimensional Anxiety Symptoms
Between-group effect size estimates on dimensional measures of panic symptoms (k=6) were large, but did not reach statistical significance, with significant heterogeneity (Hedges’ g= −0.784, 95% CI = −1.872, 0.303, p>.05). Between-group effect size estimates on general anxiety symptoms (k=28) were significant and medium (Hedges’ g= −0.521, 95% CI = −0.715, −0.328, p<.001), with a prediction interval of −1.360 to 0.407, suggesting that another study with comparable population and method would have a 95% chance of falling between a very large effect size in favor of respiratory therapies and a small effect size in favor of control interventions (Borenstein et al., 2017). The same approach was used for general anxiety symptoms among studies with inactive control conditions (k=20), only, and yielded a medium to large effect size estimate (Hedges’ g= −0.668, 95% CI = −0.905, −0.432, p<.001). and a prediction interval of −1.566 to 0.318 suggesting that another study with comparable population and method would have a 95% chance of falling between a very large effect size favoring respiratory therapies and a small effect size favoring a an inactive control condition (Borenstein et al., 2017). Effect size estimates for posttraumatic stress symptoms were small and non-significant (Hedges’ g=−0.114, 95% CI = −0.405, 0.177, p>.05). All analyses evidenced significant heterogeneity, with a large portion of variance reflecting true variability in effect sizes (Table 6).
Analysis by Diagnostic Category
Between-group effect size estimates on anxiety outcomes in non-psychiatric and medical populations (k=26) were medium to large and significant (Hedges’ g= −0.572, 95% CI = −0.784, −0.359, p<.001). with a prediction interval of −1.483 to 0.339 suggesting that another study with a comparable population and method would have a 95% chance of falling between a very large effect size favoring respiratory therapies and a small effect size favoring a control condition. We also examined effect size estimates for anxiety outcomes in studies with psychiatric participants (k=13). Here, we found between-group effect size estimates that were medium and significant (Hedges’ g= −0.513, 95% CI = −0.963, 0.062, p<.05) with a prediction interval of −2.208 to 1.182 suggesting that another study with comparable population and method would have a 95% chance of falling between a large effect size favoring respiratory therapies and a large effect size favoring a control condition. A more granular examination of intervention effects in specific diagnostic groups yielded a small to medium non-significant, pooled effect size estimate for panic disorder (k=4; Hedges’ g= −0.375, 95% CI = −1.677, 0.927, p>.05). We did not examine effects in individuals diagnosed with PTSD (k=4) given redundancy with aforementioned effect size estimates for posttraumatic stress symptoms. All analyses evidenced significant heterogeneity, with a large portion of variance reflecting true variability in effect sizes (Table 6).
Mixed-effects and Moderator Analyses: Treatment modality, control comparison, intensity, duration, publication year
Results from mixed-effects and moderator analyses can be found in Tables 7 and 8, respectively. We identified 13 distinct types of respiratory interventions, but the small number of studies for each intervention precluded examination of mean effects within treatment type or examination of effects on overall Hedges’ g via meta-regression. Instead, mixed-effects models were used to estimate and compare random effects within interventions that employed a biofeedback component (k = 19) versus those that did not (k = 20). We first examined this in trials with inactive control comparisons (k=25), which yielded a large effect size for biofeedback studies (B=−0.974, 95% CI = −1.428, −0.520, p<.001) and a medium effect size for other studies (B=−0.476, 95% CI = −0.711, −0.240, p<.05; see Table 7). In this inactive control comparisons analysis, differences between biofeedback and non-biofeedback interventions were marginally significant, suggesting the overall effect of respiratory interventions on anxiety was related to intervention modality (Q(1)=3.648, p=.056). This same analysis, including all available studies (i.e., inactive and active control conditions; k=39), again yielded a medium to large effect size for biofeedback interventions (B=−0.631, 95% CI = −0.961, −0.301, p<.001) and a medium effect size for other studies (B=−0.489, 95% CI = −0.731, −0.247, p<.001). Here, differences between biofeedback and non-biofeedback studies were not significant (Q(1)=0.462, p=.497). Notably, in both the inactive control analysis and analysis with all available studies, significant unexplained variance in true effects (I2) within each subgroup was observed, suggesting most of the within-subgroup variance reflecting true differences in study effects (see Table 7).
Table 7.
Mixed-Effects Subgroup Analyses of Dichotomous Moderators
| Description | k | B | SE | 95%CI | 95%CI | Z | p-value | Between Study Heterogeneity | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
||||||||||
| LL | UL | Q | df | p-value | I2 | ||||||
| Intervention Type | |||||||||||
|
| |||||||||||
| Biofeedback | 19 | −0.631 | 0.168 | −0.961 | −0.301 | −3.749 | <.001 | 87.823 | 18 | <.001 | 79.504 |
| Non-Biofeedback | 20 | −0.489 | 0.123 | −0.731 | −0.247 | −3.966 | <.001 | 57.088 | 19 | <.001 | 66.718 |
| Combined between- group effects | 0.462 | 1 | 0.497 | ||||||||
| Biofeedback (Inactive control comparisons only) | 13 | −0.974 | 0.232 | −1.428 | −0.520 | −4.205 | <.001 | 59.068 | 12 | <.001 | 79.684 |
| Non-Biofeedback (Inactive control comparisons only) | 12 | −0.476 | 0.120 | −0.711 | −0.240 | −3.969 | <.001 | 23.823 | 11 | 0.013 | 53.826 |
| Combined between- group effects | 3.648 | 1 | 0.056 | ||||||||
|
| |||||||||||
| Control Type3 | |||||||||||
|
| |||||||||||
| Active | 25 | −0.279 | .132 | −0.537 | −0.021 | −2.118 | .034 | 55.595 | 19 | <.001 | 66.047 |
|
|
|||||||||||
| Inactive | 20 | −0.709 | .127 | −0.958 | −0.460 | −5.586 | <.001 | 85.433 | 24 | <.001 | 71.908 |
| Combined between-group effects | 5.526 | 1 | 0.019 | ||||||||
Note. k = Number of Studies, SE=Standard Error, CI=Confidence Interval, LL=Lower Limit, UL=Upper Limit
Number of studies listed in dichotomous analyses of control type is greater than analyzed sample of k=39, due to the following studies containing both active and inactive conditions: Colgan et al., 2016; Enright et al., 2004; Gruzelier et al., 2014; Lin, 2018; Wahbeh et al., 2016; Yekta et al., 2017
Table 8.
Results of Moderation Analyses
| Description | No Studies | B | SE | 95%CI | 95%CI | Z | p-value | R2 analogue |
|---|---|---|---|---|---|---|---|---|
|
|
||||||||
| LL | UL | |||||||
| Treatment Duration (# weeks between pre- and post-treatment anxiety ratings) | ||||||||
|
| ||||||||
| All available studies | 34 | 0.003 | 0.042 | −0.078 | 0.085 | 0.08 | 0.937 | 0.00 |
| Inactive comparisons only | 24 | −0.015 | 0.056 | −0.125 | 0.095 | −0.27 | 0.789 | 0.00 |
|
| ||||||||
| Treatment Intensity (# of sessions) | ||||||||
|
| ||||||||
| All available studies | 37 | −0.020 | 0.015 | −0.050 | 0.009 | −1.34 | 0.181 | 0.05 |
| Inactive comparisons only | 23 | −0.020 | 0.024 | −0.067 | 0.027 | −0.83 | 0.407 | 0.01 |
|
| ||||||||
| Publication Year | ||||||||
|
| ||||||||
| All available studies | 39 | −0.000 | 0.010 | −0.021 | 0.020 | −0.02 | 0.983 | 0.00 |
| Inactive comparisons only | 25 | 0.016 | 0.013 | −0.009 | 0.041 | 1.28 | 0.201 | 0.02 |
Note. k = Number of Studies, SE=Standard Error, CI=Confidence Interval, LL=Lower Limit, UL=Upper Limit
Mixed-effects models were also used to examine whether estimated effects differed by inactive (k=25) or active (k=20) treatment comparison type. Inactive controls lacked a specific intervention component or were given a sham intervention component not specifically designed to target anxiety. These included treatment as usual (TAU) as well as placebo, attention, waitlist and no treatment control condition (Table 2). Analyses indicated a medium to large and significant effect for inactive control comparisons (B=0.709, 95% CI = −0.958, −0.460, p<.001) and a small but significant effect for active treatment control comparisons (B=0.279, 95% CI = −0.537, −0.021 p<.001). Differences between inactive and active control comparison conditions were significant (Q(1)=5.526, p<.05), indicating that effect sizes are related to control comparison type. Again, a significant unexplained variance in true effects (I2) within inactive and within active control comparisons was observed suggesting most of the within-subgroup variance reflects true differences in study effects (see Table 3).
Table 2.
Overview of Respiratory Interventions
| Types of Respiratory Intervention | Description |
|---|---|
| Diaphragmatic Breathing | Use of the diaphragm muscle to move air. |
| Controlled or Paced Breathing | Breathing at a given respiratory frequency (i.e., pace). |
| Breathing Retraining | Induction of hyperventilation, which is then contrasted with diaphragmatic breathing, to promote the practice of diaphragmatic breathing. |
| Heart Rate Variability Biofeedback | Breathing at objectively measured individual’s resonance frequency aiming to increase their heart rate variability. Feedback of physiological parameters is provided in real time to promote accuracy. |
| PaCO2 Biofeedback | Breathing that targets a specific PaCO2 level, approximated via end-tidal (i.e., expired) CO2, to optimize gas exchange. Feedback of relevant physiological parameters is provided in real time to promote accuracy. |
In addition, we examined the effect of treatment duration as reflected by the number of weeks between pre- and post-treatment (range = 1 to 20; see Table 3). We found no significant relation between treatment duration and outcomes in the full sample (k=34; B=0.003, 95% CI = −0.078, 0.085, p>.05) or inactive control comparisons only (k = 24; B=−0.015, 95% CI = −0.125, 0.095, p>.05). Similarly, a meta regression analysis of treatment intensity, as reflected by number of treatment sessions (range: 1–28; k=37), did not account for significant variance in effect size estimates in the full sample (B=−0.020, 95% CI = −0.050, 0.009, p>.05) or inactive control comparisons, only (k = 23; B=0.020, 95% CI = −0.067, 0.027, p>.05; Table 8).
Finally, in an attempt to account for biases in a changing peer-review process over the past several decades as well as intervention quality, year of publication was entered into model as a covariate. This did not account for a significant portion of variance in the entire sample including all studies sample (B=−0.00, 95% CI = −0.21, 0.020, p>.05), or just those comparing to an inactive control condition sample (B=−0.016, 95% CI = −0.009, 0.041, p>.05).
Discussion
Respiratory abnormalities are a hallmark of anxiety symptomatology (Grassi et al., 2013; Leander et al., 2014; Papp et al., 1997; Stein et al., 1995) and may provide a direct means by which to target symptoms such as interoceptive sensitivity (Paulus, 2013). Yet, gold standard interventions do not emphasize intervention components that directly target respiration. The aim of this meta-analysis was to estimate the efficacy of respiratory interventions on trait anxiety symptoms. A total of 40 randomized controlled trials met our inclusion criteria. Overall, a medium to large and significant effect size was observed, indicating that, on average, respiratory interventions are better at reducing anxiety symptoms when compared with control conditions. However, a majority of included studies were classified as ‘high’ risk of bias, many due to the inclusion of an inactive control comparison, which contributed to a bias of outcome measurement. When examining respiratory interventions’ effects as compared to active controls, only, effects were small. Significant heterogeneity in our findings suggests that variability in the intervention ingredients and delivery, population, and control comparison may have obscured the clarity of our findings. Yet, given some promising observations, we make several recommendations for ongoing investigations in this domain.
Respiratory interventions yielded the most promising effects for dimensional anxiety symptoms with medium, and medium to large, effects found in comparison to any control condition and inactive control conditions alone, respectively. Very large, but non-significant, effects were found for panic symptoms in a likely under-powered analysis with just six studies reporting panic outcomes. Observed effect sizes for generalized anxiety and panic symptoms are not surprising given that somatic symptoms, in particular cardiopulmonary and musculoskeletal symptoms, are prominent in generalized anxiety and panic (Bekhuis et al., 2015), and subtypes of individuals with panic are in fact characterized by respiratory dysfunction (Boulding et al., 2016; Courtney, 2017; Courtney, Greenwood, et al., 2011; Courtney, van Dixhoorn, et al., 2011). It is possible that respiratory interventions reduce arousal via correcting breathing abnormalities, thereby preventing the onset of panic distress. Interestingly, dismantling studies of CBT for panic (Pompoli et al., 2018; Schmidt et al., 2000) as well as experimental studies targeting panic relevant processes (Lickel et al., 2013) have found that the inclusion of respiratory interventions does not improve clinical outcomes, despite being associated with treatment acceptability. Yet, others have found that anxiety interventions with respiratory components (i.e., relaxation training) are superior to CBT (in older adults; Thorp et al., 2009), and our meta-analytic review indicates that respiratory interventions indeed hold clinical value for the treatment of anxiety, including panic symptoms. We did not identify any trials on individuals diagnosed with generalized anxiety disorder. Given evidence that these interventions reduce general anxiety symptoms, such a line of inquiry is warranted. Conversely, respiratory interventions yielded small and non-significant effects on posttraumatic stress symptoms. Although posttraumatic stress symptoms include physiological components (e.g., hyperarousal), these symptoms are conditional on the type of trauma. Additionally, heterogeneity of posttraumatic symptoms, relative to panic and generalized anxiety (Asmundson et al., 2000; King et al., 1998; Pai et al., 2017), may further dilute, or make it more difficult to detect, the effects of a respiratory based intervention among those with trauma symptoms.
The effects of respiratory interventions were medium to large in non-psychiatric and medical populations, and medium in the populations presenting with a psychiatric condition, suggesting broad intervention utility. Of the trials including psychiatric populations, just two (Meuret et al., 2008; Lin et al. 2019) used an inactive control comparison. Our findings are relatively consistent with previous meta-analyses of CBT for dimensional anxiety that have found low to medium effects in comparison to other forms of psychotherapy (e.g., Hedges’ g= 0.43; Tolin, 2010). Just three of eleven interventions conducted on psychiatric samples included the respiratory intervention as a treatment adjunct (Bonn et al., 1984; Craske et al., 1997; Penzlin et al., 2015). This indicates that respiratory interventions may offer a promising anxiety intervention approach in psychiatric and non-psychiatric samples who do not have access to, are unwilling to engage in, or have not experienced symptom alleviation from other gold-standard interventions. Moreover, respiratory interventions are highly disseminable and can easily be incorporated into primary care settings, and/or administered remotely. For example, many respiratory and respiratory biofeedback interventions are readily accessible via smartphone apps (Pham et al., 2016).
We did not find support for the perspective that treatment effects vary based on modality (e.g., biofeedback), duration, or intensity. This is surprising given biofeedback interventions provide immediate data regarding changes in physiology that are occurring in the context of a respiratory manipulation, which may enhance fidelity, participant engagement, and accuracy of directly targeting mechanisms purported to promote and maintain anxiety interference. Moreover, some respiratory interventions are purported to require extensive practice and mastery in order to obtain an effect (Lehrer & Vaschillo, 2004; Lehrer et al., 2000). Our current findings challenge this notion and elicit further questions regarding mechanisms of treatment and how to enhance intervention effects. However, given the heterogeneity in study methods, our analysis of treatment modality was limited, and should be re-examined in the future.
One possible, but not yet tested, explanation of observed effects is that respiratory interventions improve cognitive processes implicated in the onset and maintenance of anxiety, such as intolerance of uncertainty (i.e., unwillingness to tolerate the possibility of something bad happening in the future) which are posited to be a central feature of various types of anxiety (Gentes & Ruscio, 2011; Holaway et al., 2006). Anxious individuals who are unable to withstand the anxiety that accompanies the possibility of something bad happening in the future may experience respiratory interventions as a means by which to control their physiology. This may generalize to a greater sense of anxiety control and self-efficacy in managing symptoms (Gallagher et al., 2014), while also providing them with a skill to use when feeling anxious. Respiratory interventions may also act upon similar mechanisms to interoceptive exposure, such as anxiety sensitivity. For example, heart rate variability biofeedback requires individuals to dramatically slow their breathing pace, which, while increasing relaxation when done correctly, may initially result in worry that one is not getting enough oxygen, and therefore promote anxiety. Similarly, many respiratory interventions promote increased awareness of respiration and other related physiological changes, which may increase sensitivity to sensations, and initially increase anxiety. Finally, interventions such as breath awareness training may increase tolerance and acceptance of anxiety relevant discomfort. This is consistent with hypothesized mechanisms underlying Acceptance and Commitment Therapy (Hayes et al., 2006) that ask patients to practice attending to present experiences (such as respiration) as a means of more broadly strengthening present-focused awareness of negative thoughts, feelings, and sensations. This in turn allows patients to become more aware of the role that anxiety plays in their behaviors and provides an opportunity for patients to volitionally choose to engage in desired activities despite the presence of anxiety. While assessing the potential mediating role of cognitive processes in explaining the benefits of respiratory interventions is beyond the scope of the current investigation, future trials should index these processes as a means of explicating mechanisms of change in these interventions.
Our meta-analytic review is limited by considerable variability amongst studies with regard to sample characteristics, intervention type, duration and intensity of treatment, and use of clinical or non-clinical (e.g., STAI) outcome measures, as reflected in immense effect size dispersion. Despite this, included interventions have a common target (i.e., respiration), and their heterogeneity may be used to propel several important lines of inquiry. First, although some previous research on clinical samples (e.g., Pompoli et al., 2018; Schmidt et al., 2000) has found that respiratory interventions are inferior to other treatment packages, they present a viable alternative that is acceptable and highly disseminable, and perhaps optimal for certain populations (e.g., older adults; Thorp et al., 2009). Additional research should also determine what type of anxiety symptoms are most effectively addressed by respiratory interventions. Such a line of inquiry may also help researchers and clinicians understand when, and for whom, respiratory adjuncts may be most useful. Future research also is needed to examine possible mechanisms explaining favorable outcomes for respiratory interventions, including arousal reduction, extinction, and modifying appraisal or expectancies. Finally, anxiety is commonly comorbid with a variety of health conditions, and often complicates treatment. Future work would benefit from examining the efficacy of respiratory interventions in other health risk behaviors, such as illicit substance use, alcohol (e.g., ClinicalTrials.gov ID: NCT02579317), and smoking (e.g., ClinicalTrials.gov ID: NCT03972137), where comorbid anxiety is highly prevalent.
Collectively, the current review provides support for respiratory interventions serving as effective treatments for anxiety disorders. In particular, evidence suggests respiratory interventions are efficacious in treating dimensional symptoms of anxiety, notably generalized anxiety and panic, while also being acceptable anxiety interventions for a range of clinical and non-clinical populations. Yet, findings from this review also reflect the predominant use of non-active comparators when evaluating respiratory intervention efficacy, and the need for future trials to incorporate more adequately matched active controls. Moreover, there is a need for increased standardization across methodological designs in addition to more transparent reporting of trial outcome data that is consistent with Cochrane Database assessment tools. In efforts to reduce risk of bias future trials would benefit from pre-registering data analytic strategies, as well as incorporating active comparators matched in treatment intensity and dose (i.e., frequency and duration of in-person sessions, assignment of at-home practice, etc.). Greater standardization across methodological designs may also assist in identifying common and distinct therapeutic elements across respiratory modalities the current review was unable to detect. This may also facilitate in identifying how respiratory elements may differentially relate to and operate on underlying processes of change implicated in anxiety pathology. Given the promise respiratory interventions hold in aiding current anxiety disorder treatments, ongoing research initiatives that address these methodological barriers are highly encouraged.
Highlights.
Respiratory abnormalities are a hallmark of anxiety symptomatology
The effectiveness of respiratory interventions for anxiety is not well established
Respiratory interventions yield significant improvements in anxiety symptoms
Respiratory interventions hold clinical value for the treatment of anxiety
Acknowledgments
Funding: This work was supported by the National Institutes of Health [grant numbers R34 DA043751, R03 DA041556]
Author Bios:
Teresa M. Leyro, Ph.D.: Dr. Teresa Leyro is an Assistant Professor in the Doctoral Program in Clinical Psychology in the Department of Psychology at Rutgers University-New Brunswick.
Mark Versella, M.S.: Mark Versella is a student in the Doctoral Program in Clinical Psychology in the Department of Psychology at Rutgers University-New Brunswick.
Min-Jeong Yang, Ph.D.: Min-Jeong Yang is a post-doctoral fellow in the Department of Health Outcomes and Behavior at Moffitt Cancer Center and former student in the Doctoral Program in Clinical Psychology in the Department of Psychology at Rutgers University-New Brunswick.
Hannah R. Brinkman, M.S.: Hannah Brinkman is a student in the Doctoral Program in Clinical Psychology in the Department of Psychology at Rutgers University-New Brunswick.
Danielle L. Hoyt, M.A.: Danielle Hoyt is a student in the Doctoral Program in Clinical Psychology in the Department of Psychology at Rutgers University-New Brunswick.
Paul Lehrer, Ph.D.: Dr. Paul Lehrer is a Professor, emeritus, in the Department of Psychiatry at Rutgers Robert Wood Johnson Medical School
Appendix A
- Abbott RA, Whear R, Rodgers LR, Bethel A, Coon JT, Kuyken W, Stein K, & Dickens C. (2014). Effectiveness of mindfulness-based stress reduction and mindfulness based cognitive therapy in vascular disease: A systematic review and meta-analysis of randomised controlled trials. Journal of Psychosomatic Research, 76(5), 341–351. 10.1016/j.jpsychores.2014.02.012 [DOI] [PubMed] [Google Scholar]
- Abbott-Anderson K, & Kwekkeboom KL (2012). A systematic review of sexual concerns reported by gynecological cancer survivors. Gynecologic Oncology, 124(3), 477–489. 10.1016/j.ygyno.2011.11.030 [DOI] [PubMed] [Google Scholar]
- Ball EF, Nur Shafina Muhammad Sharizan E, Franklin G, & Rogozinska E. (2017). Does mindfulness meditation improve chronic pain? A systematic review. Current Opinion in Obstetrics & Gynecology 29(6), 359–366. 10.1097/GCO.0000000000000417 [DOI] [PubMed] [Google Scholar]
- Bandelow B, Reitt M, Rover C, Michaelis S, Gorlich Y, & Wedekind D. (2015). Efficacy of treatments for anxiety disorders: a meta-analysis. International Clinical Psychopharmacology, 30(4), 183–192. 10.1097/YIC.0000000000000078 [DOI] [PubMed] [Google Scholar]
- Banks K, Newman E, & Saleem J. (2015). An Overview of the Research on Mindfulness-Based Interventions for Treating Symptoms of Posttraumatic Stress Disorder: A Systematic Review. Journal of Clinical Psychology, 71(10), 935–963. 10.1002/jclp.22200 [DOI] [PubMed] [Google Scholar]
- Bartley CA, Hay M, & Bloch MH (2013). Meta-analysis: aerobic exercise for the treatment of anxiety disorders. Progress in Neuro-Psychopharmacology & Biological Psychiatry, 45, 34–39. 10.1016/j.pnpbp.2013.04.016 [DOI] [PubMed] [Google Scholar]
- Bazzan AJ, Zabrecky G, Monti DA, & Newberg AB (2014). Current evidence regarding the management of mood and anxiety disorders using complementary and alternative medicine. Expert Review of Neurotherapeutics, 14(4), 411–423. 10.1586/14737175.2014.892420 [DOI] [PubMed] [Google Scholar]
- Bennett PN, Ngo T, Kalife C, & Schiller B. (2018). Improving wellbeing in patients undergoing dialysis: Can meditation help? Seminars in Dialysis 31(1), 59–64. 10.1111/sdi.12656 [DOI] [PubMed] [Google Scholar]
- Bluett EJ, Homan KJ, Morrison KL, Levin ME, & Twohig MP (2014). Acceptance and commitment therapy for anxiety and OCD spectrum disorders: An empirical review. Journal of Anxiety Disorders, 28(6), 612–624. 10.1016/j.janxdis.2014.06.008 [DOI] [PubMed] [Google Scholar]
- Blumenthal JA, Wang JT, Babyak M, Watkins L, Kraus W, Miller P, Hinderliter A, & Sherwood A. (2010). Enhancing standard cardiac rehabilitation with stress management training: background, methods, and design for the enhanced study. Journal of Cardiopulmonary Rehabilitation & Prevention, 30(2), 77–84. 10.1097/HCR.0b013e3181d0c1d3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohlmeijer E, Prenger R, Taal E, & Cuijpers P. (2010). The effects of mindfulness-based stress reduction therapy on mental health of adults with a chronic medical disease: a meta-analysis. Journal of Psychosomatic Research, 68(6), 539–544. 10.1016/j.jpsychores.2009.10.005 [DOI] [PubMed] [Google Scholar]
- Bojic S, & Becerra R. (2017). Mindfulness-Based Treatment for Bipolar Disorder: A Systematic Review of the Literature. Europes Journal of Psychology 13(3), 573–598. 10.5964/ejop.v13i3.1138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavicchioli M, Movalli M, & Maffei C. (2018). The Clinical Efficacy of Mindfulness-Based Treatments for Alcohol and Drugs Use Disorders: A Meta-Analytic Review of Randomized and Nonrandomized Controlled Trials. European Addiction Research 24(3):137–162, 2018, 24(3), 137–162. 10.1159/000490762 [DOI] [PubMed] [Google Scholar]
- Chambers CT, Taddio A, Uman LS, & McMurtry CM (2009). Psychological interventions for reducing pain and distress during routine childhood immunizations: a systematic review. Clinical Therapeutics, 31(2), 77–103. 10.1016/j.clinthera.2009.07.023 [DOI] [PubMed] [Google Scholar]
- Chiesa A. (2010). Vipassana meditation: systematic review of current evidence. Journal of Alternative & Complementary Medicine, 16(1), 37–46. doi: 10.1089/acm.2009.0362 [DOI] [PubMed] [Google Scholar]
- Chiesa A, Brambilla P, & Serretti A. (2011). Neuro-imaging of mindfulness meditations: implications for clinical practice. Epidemiology and Psychiatric Sciences, 20(2), 205–210. 10.1017/s204579601100028x [DOI] [PubMed] [Google Scholar]
- Dawson AF, Brown WW, Anderson J, Datta B, Donald JN, Hong K, Galante J. (2019). Mindfulness-Based Interventions for University Students: A Systematic Review and Meta-Analysis of Randomised Controlled Trials. Applied Psychology Health and Well-being, 12(2), 384–410. 10.1111/aphw.12188 [DOI] [PubMed] [Google Scholar]
- De Witte NAJ, Buyck I, & Van Daele T. (2019). Combining Biofeedback with Stress Management Interventions: A Systematic Review of Physiological and Psychological Effects. Applied Psychophysiology and Biofeedback, 44(2), 71–82. 10.1007/s10484-018-09427-7 [DOI] [PubMed] [Google Scholar]
- Dhillon A, Sparkes E, & Duarte RV (2017). Mindfulness-Based Interventions During Pregnancy: a Systematic Review and Meta-analysis. Mindfulness 8(6), 1421–1437. 10.1007/s12671-017-0726-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunning DL, Griffiths K, Kuyken W, Crane C, Foulkes L, Parker J, & Dalgleish T. (2019). Research Review: The effects of mindfulness-based interventions on cognition and mental health in children and adolescents - a meta-analysis of randomized controlled trials. Journal of Child Psychology & Psychiatry & Allied Disciplines 60(3), 244–258. 10.1111/jcpp.12980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escuriex BF, & Labbe EE (2011). Health care providers’ mindfulness and treatment outcomes: A critical review of the research literature. Mindfulness, 2(4), 242–253. 10.1007/s12671-011-0068-z [DOI] [Google Scholar]
- Esper LH, & Gherardi-Donato E. C. d. S. (2019). Mindfulness-based interventions for women victims of interpersonal violence: A systematic review. Archives of Psychiatric Nursing, 33(1), 120–130. 10.1016/j.apnu.2018.09.003 [DOI] [PubMed] [Google Scholar]
- Fish J, Brimson J, & Lynch S. (2016). Mindfulness Interventions Delivered by Technology Without Facilitator Involvement: What Research Exists and What Are the Clinical Outcomes? Mindfulness 7(5):1011–1023, 2016, 7(5), 1011–1023. 10.1007/s1267-1016-0548-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fjorback L, Arendt M, Ornbol E, Fink P, & Walach H. (2011). Mindfulness-Based Stress Reduction and Mindfulness-Based Cognitive Therapy-A systematic review of randomized controlled trials. Acta Psychiatrica Scandinavica, 124(2), 102–119. 10.1111/j.1600-0447.2011.01704.x [DOI] [PubMed] [Google Scholar]
- Franca RD (2015). A meta-analysis of mindfulness based interventions (MBIs) show that MBIs are effective in reducing acute symptoms of depression but not anxiety. Australian Occupational Therapy Journal, 62(2), 147–148. 10.1111/1440-1630.12198 [DOI] [PubMed] [Google Scholar]
- Ghawadra SF, Abdullah KL, Choo WY, & Phang CK (2019). Mindfulness-based stress reduction for psychological distress among nurses: A systematic review. Journal of Clinical Nursing 28(21–22), 3747–3758. 10.1111/jocn.14987 [DOI] [PubMed] [Google Scholar]
- Ghielen I, Rutten S, Boeschoten RE, Houniet-de Gier M, van Wegen EE, van den Heuvel OA, & Cuijpers P. (2019). The effects of cognitive behavioral and mindfulness-based therapies on psychological distress in patients with multiple sclerosis, Parkinson’s disease and Huntington’s disease: Two meta-analyses. Journal of Psychosomatic Research, 122, 43–51. 10.1016/j.jpsychores.2019.05.001 [DOI] [PubMed] [Google Scholar]
- Gilmartin H, Goyal A, Hamati MC, Mann J, Saint S, & Chopra V. (2017). Brief Mindfulness Practices for Healthcare Providers - A Systematic Literature Review. American Journal of Medicine 130(10), 1219.e1211–1219.e1217. 10.1016/j.amjmed.2017.05.041 [DOI] [PubMed] [Google Scholar]
- Goessl VC, Curtiss JE, & Hofmann SG (2017). The effect of heart rate variability biofeedback training on stress and anxiety: a meta-analysis. Psychological Medicine 47(15), 2578–2586. 10.1017/S0033291717001003 [DOI] [PubMed] [Google Scholar]
- Goldman BL, Dormitor PJ, & Murray EJ (1979). Effects of Zen meditation on anxiety reduction and perceptual functioning. Journal of Consulting and Clinical Psychology, 47(3), 551–556. 10.1037/0022-006X.47.3.551 [DOI] [PubMed] [Google Scholar]
- Goyal M, Singh S, Sibinga EM, Gould NF, Rowland-Seymour A, Sharma R, Berger Z, Sleicher D, Maron DD, Shihab HM, Ranasinghe PD, Linn S, Saha S, Bass EB, & Haythornthwaite JA (2014). Meditation programs for psychological stress and well-being: a systematic review and meta-analysis. JAMA Internal Medicine, 174(3), 357–368. 10.1001/jamainternmed.2013.13018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman P, Niemann L, Schmidt S, & Walach H. (2004). Mindfulness-based stress reduction and health benefits: A meta-analysis. Journal of Psychosomatic Research, 57(1), 35–43. 10.1016/S0022-3999(03)00573-7 [DOI] [PubMed] [Google Scholar]
- Guillaumie L, Boiral O, & Champagne J. (2017). A mixed-methods systematic review of the effects of mindfulness on nurses. Journal of Advanced Nursing, 73(5), 1017–1034. 10.1111/jan.13176 [DOI] [PubMed] [Google Scholar]
- Harrison SL, Lee A, Janaudis-Ferreira T, Goldstein RS, & Brooks D. (2016). Mindfulness in people with a respiratory diagnosis: A systematic review. Patient Education & Counseling, 99(3), 348–355. 10.1016/j.pec.2015.10.013 [DOI] [PubMed] [Google Scholar]
- Havermans RC (2011). Does mindfulness meditation treatment really work? Really? Psychologie & Gezondheid, 39(1), 42–46. 10.1007/s12483-011-0008-6 [DOI] [Google Scholar]
- Hazlett-Stevens H, & Oren Y. (2017). Effectiveness of Mindfulness-Based Stress Reduction Bibliotherapy: A Preliminary Randomized Controlled Trial. Journal of Clinical Psychology 73(6):626–637, 2017 Jun, 73(6), 626–637. 10.1002/jclp.22370 [DOI] [PubMed] [Google Scholar]
- Hofmann SG, Sawyer AT, Witt AA, & Oh D. (2010). The effect of mindfulness-based therapy on anxiety and depression: A meta-analytic review. Journal of Consulting and Clinical Psychology, 78(2), 169–183. 10.1037/a0018555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hood MM, & Jedel S. (2017). Mindfulness-Based Interventions in Inflammatory Bowel Disease. Gastroenterology Clinics of North America 46(4):859–874, 2017 12, 46(4), 859–874. 10.1016/j.gtc.2017.08.008 [DOI] [PubMed] [Google Scholar]
- Hopper SI, Murray SL, Ferrara LR, & Singleton JK (2019). Effectiveness of diaphragmatic breathing for reducing physiological and psychological stress in adults: a quantitative systematic review. JBI Database of Systematic Reviews and Implementation Reports, 17(9), 1855–1876. 10.11124/jbisrir-2017-003848 [DOI] [PubMed] [Google Scholar]
- Hopwood TL, & Schutte NS (2017). A meta-analytic investigation of the impact of mindfulness-based interventions on post traumatic stress. Clinical Psychology Review, 57, 12–20. 10.1016/j.cpr.2017.08.002 [DOI] [PubMed] [Google Scholar]
- Ivanovski B, & Malhi GS (2007). The psychological and neurophysiological concomitants of mindfulness forms of meditation. Acta Neuropsychiatrica, 19(2), 76–91. 10.1111/j.1601-5215.2007.00175.x [DOI] [PubMed] [Google Scholar]
- Janssen M, Heerkens Y, Kuijer W, van der Heijden B, & Engels J. (2018). Effects of Mindfulness-Based Stress Reduction on employees’ mental health: A systematic review. PLoS ONE 13(1), e0191332. 10.1371/journal.pone.0191332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones P, Blunda M, Biegel G, Carlson LE, Biel M, & Wiener L. (2013). Can mindfulness-based interventions help adolescents with cancer? Psycho-Oncology, 22(9), 2148–2151. 10.1002/pon.3251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahl KG, Winter L, & Schweiger U. (2012). The third wave of cognitive behavioural therapies: What is new and what is effective? Current Opinion in Psychiatry, 25(6), 522–528. 10.1097/YCO.0b013e328358e531 [DOI] [PubMed] [Google Scholar]
- Kallapiran K, Koo S, Kirubakaran R, & Hancock K. (2015). Review: Effectiveness of mindfulness in improving mental health symptoms of children and adolescents: a meta-analysis. Child & Adolescent Mental Health, 20(4), 182–194. 10.1111/camh.12113 [DOI] [PubMed] [Google Scholar]
- Kearney DJ, & Brown-Chang J. (2008). Complementary and alternative medicine for IBS in adults: mind-body interventions. Nature Clinical Practice Gastroenterology & Hepatology, 5(11), 624–636. 10.1038/ncpgasthep1257 [DOI] [PubMed] [Google Scholar]
- Kelly BD (2008). Meditation, mindfulness, and mental health. Irish Journal of Psychological Medicine, 25(1), 3–4. 10.1017/S0790966700010752 [DOI] [PubMed] [Google Scholar]
- Keng SL, Smoski MJ, & Robins CJ (2011). Effects of mindfulness on psychological health: a review of empirical studies. Clinical Psychology Review, 31(6), 1041–1056. 10.1016/j.cpr.2011.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khoury B, Lecomte T, Fortin G, Masse M, Therien P, Bouchard V, Chapleau MA, Paquin K, & Hofmann SG (2013). Mindfulness-based therapy: a comprehensive meta-analysis. Clinical Psychology Review, 33(6), 763–771. 10.1016/j.cpr.2013.05.005 [DOI] [PubMed] [Google Scholar]
- Khoury B, Lecomte T, Gaudiano BA, & Paquin K. (2013). Mindfulness interventions for psychosis: A meta-analysis. Schizophrenia Research, 150(1), 176–184. 10.1016/j.schres.2013.07.055 [DOI] [PubMed] [Google Scholar]
- Khoury B, Sharma M, Rush SE, & Fournier C. (2015). Mindfulness-based stress reduction for healthy individuals: A meta-analysis. Journal of Psychosomatic Research, 78(6), 519–528. 10.1016/j.jpsychores.2015.03.009 [DOI] [PubMed] [Google Scholar]
- Kim SH, Schneider SM, Kravitz L, Mermier C, & Burge MR (2013). Mind-body practices for posttraumatic stress disorder. Journal of Investigative Medicine, 61(5), 827–834. 10.2310/JIM.0b013e3182906862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishita N, Takei Y, & Stewart I. (2017). A meta-analysis of third wave mindfulness-based cognitive behavioral therapies for older people. International Journal of Geriatric Psychiatry 32(12):1352–1361, 2017 12, 32(12), 1352–1361. 10.1002/gps.4621 [DOI] [PubMed] [Google Scholar]
- Kostova Z, Levin L, Lorberg B, & Ziedonis D. (2019). Mindfulness-based interventions for adolescents with mental health conditions: A systematic review of the research literature. Journal of Child and Family Studies, 28(10), 2633–2649. 10.1007/s10826-019-01477-7 [DOI] [Google Scholar]
- Lakhan SE, & Schofield KL (2013). Mindfulness-based therapies in the treatment of somatization disorders: a systematic review and meta-analysis. PLoS ONE, 8(8), e71834. 10.1371/journal.pone.0071834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalande L, Bambling M, King R, & Lowe R. (2012). Breathwork: An additional treatment option for depression and anxiety? Journal of Contemporary Psychotherapy: On the Cutting Edge of Modern Developments in Psychotherapy, 42(2), 113–119. 10.1007/s10879-011-9180-6 [DOI] [Google Scholar]
- Lamothe M, Rondeau E, Malboeuf-Hurtubise C, Duval M, & Sultan S. (2016). Outcomes of MBSR or MBSR-based interventions in health care providers: A systematic review with a focus on empathy and emotional competencies. Complementary Therapies in Medicine, 24, 19–28. 10.1016/j.ctim.2015.11.001 [DOI] [PubMed] [Google Scholar]
- Langer AI, Ulloa VG, Cangas AJ, Rojas G, & Krause M. (2015). Mindfulness-based interventions in secondary education: A qualitative systematic review. Estudios de Psicologia, 36(3), 533–570. 10.1080/02109395.2015.1078553 [DOI] [Google Scholar]
- Langhorst J, Wulfert H, Lauche R, Klose P, Cramer H, Dobos GJ, & Korzenik J. (2015). Systematic review of complementary and alternative medicine treatments in inflammatory bowel diseases. Journal of Crohn’s & Colitis, 9(1), 86–106. 10.1093/ecco-jcc/jju007 [DOI] [PubMed] [Google Scholar]
- Lever Taylor B, Cavanagh K, & Strauss C. (2016). The Effectiveness of Mindfulness-Based Interventions in the Perinatal Period: A Systematic Review and Meta-Analysis. PLoS ONE, 11(5), e0155720. 10.1371/journal.pone.0155720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G, Yuan H, & Zhang W. (2016). The effects of mindfulness-based stress reduction for family caregivers: Systematic review. Archives of Psychiatric Nursing, 30(2), 292–299. 10.1016/j.apnu.2015.08.014 [DOI] [PubMed] [Google Scholar]
- Li SYH, & Bressington D. (2019). The effects of mindfulness-based stress reduction on depression, anxiety, and stress in older adults: A systematic review and meta-analysis. International Journal of Mental Health Nursing 28(3), 635–656. 10.1111/inm.12568 [DOI] [PubMed] [Google Scholar]
- Liu W, Pan YL, Gao CX, Shang Z, Ning LJ, & Liu X. (2013). Breathing exercises improve post-operative pulmonary function and quality of life in patients with lung cancer: A meta-analysis. Experimental and Therapeutic Medicine, 5(4), 1194–1200. 10.3892/etm.2013.926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomas T, Medina JC, Ivtzan I, Rupprecht S, & Eiroa-Orosa FJ (2019). A systematic review and meta-analysis of the impact of mindfulness-based interventions on the well-being of healthcare professionals. Mindfulness, 10(7), 1193–1216. 10.1007/s12671-018-1062-5 [DOI] [PubMed] [Google Scholar]
- Lomas T, Medina JC, Ivtzan I, Rupprecht S, & Eiroa-Orosa FJ (2019). Mindfulness-based interventions in the workplace: An inclusive systematic review and meta-analysis of their impact upon wellbeing. The Journal of Positive Psychology, 14(5), 625–640. 10.1080/17439760.2018.1519588 [DOI] [Google Scholar]
- Lovas DA, & Schuman-Olivier Z. (2018). Mindfulness-based cognitive therapy for bipolar disorder: A systematic review. Journal of Affective Disorders, 240, 247–261. 10.1016/j.jad.2018.06.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynn SJ, Malakataris A, Condon L, Maxwell R, & Cleere C. (2012). Post-traumatic stress disorder: Cognitive hypnotherapy, mindfulness, and acceptance-based treatment approaches. American Journal of Clinical Hypnosis, 54(4), 311–330. 10.1080/00029157.2011.645913 [DOI] [PubMed] [Google Scholar]
- Marc I, Toureche N, Ernst E, Hodnett ED, Blanchet C, Dodin S, & Njoya MM (2011). Mind-body interventions during pregnancy for preventing or treating women’s anxiety. Cochrane Database of Systematic Reviews, 7, CD007559. 10.1002/14651858.CD007559.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchand WR (2012). Mindfulness-based stress reduction, mindfulness-based cognitive therapy, and Zen meditation for depression, anxiety, pain, and psychological distress. Journal of Psychiatric Practice, 18(4), 233–252. 10.1097/01.pra.0000416014.53215.86 [DOI] [PubMed] [Google Scholar]
- Marchand WR (2013). Mindfulness meditation practices as adjunctive treatments for psychiatric disorders. Psychiatric Clinics of North America, 36(1), 141–152. 10.1016/j.psc.2013.01.002 [DOI] [PubMed] [Google Scholar]
- Matvienko-Sikar K, Lee L, Murphy G, & Murphy L. (2016). The effects of mindfulness interventions on prenatal well-being: A systematic review. Psychology & Health 31(12), 1415–1434. 10.1080/08870446.2016.1220557 [DOI] [PubMed] [Google Scholar]
- McConville J, McAleer R, & Hahne A. (2017). Mindfulness Training for Health Profession Students-The Effect of Mindfulness Training on Psychological Well-Being, Learning and Clinical Performance of Health Professional Students: A Systematic Review of Randomized and Non-randomized Controlled Trials. Explore: The Journal of Science & Healing 13(1), 26–45. 10.1016/j.explore.2016.10.002 [DOI] [PubMed] [Google Scholar]
- McDonnell L, & Bowden ML (1989). Breathing management: a simple stress and pain reduction strategy for use on a pediatric service. Issues in Comprehensive Pediatric Nursing, 12(5), 339–344. 10.3109/01460868909038042 [DOI] [PubMed] [Google Scholar]
- Metcalf CA, & Dimidjian S. (2014). Extensions and mechanisms of mindfulness-based cognitive therapy: A review of the evidence. Australian Psychologist, 49(5), 271–279. 10.1111/ap.12074 [DOI] [Google Scholar]
- Meuret AE, Wilhelm FH, Ritz T, & Roth WT (2003). Breathing Training for Treating Panic Disorder: Useful Intervention or Impediment? Behavior Modification, 27(5), 731–754. 10.1177/0145445503256324 [DOI] [PubMed] [Google Scholar]
- Miro M, Perestelo-Perez L, Perez J, Rivero A, Gonzalez M, de la Fuente J, & Serrano P. (2011). Effectiveness of mindfulness based treatments for anxiety and depressive disorders: A systematic review. Revista de Psicopatologia y Psicologia Clinica, 16(1), 1–14. 10.5944/rppc.vol.16.num.1.2011.10347 [DOI] [Google Scholar]
- Moravec CS (2008). Biofeedback therapy in cardiovascular disease: rationale and research overview. Cleve Clin J Med, 75, S35–38. 10.3949/ccjm.75.suppl_2.s35 [DOI] [PubMed] [Google Scholar]
- Newby JM, McKinnon A, Kuyken W, Gilbody S, & Dalgleish T. (2015). Systematic review and meta-analysis of transdiagnostic psychological treatments for anxiety and depressive disorders in adulthood. Clinical Psychology Review, 40, 91–110. 10.1016/j.cpr.2015.06.002 [DOI] [PubMed] [Google Scholar]
- Niles BL, Mori DL, Polizzi C, Pless Kaiser A, Weinstein ES, Gershkovich M, & Wang C. (2018). A systematic review of randomized trials of mind-body interventions for PTSD. Journal of Clinical Psychology 74(9), 1485–1508. 10.1002/jclp.22634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norton AR, Abbott MJ, Norberg MM, & Hunt C. (2015). A systematic review of mindfulness and acceptance-based treatments for social anxiety disorder. Journal of Clinical Psychology, 71(4), 283–301. 10.1002/jclp.22144 [DOI] [PubMed] [Google Scholar]
- Peltier B. (2009). Psychological treatment of fearful and phobic special needs patients. Special Care in Dentistry, 29(1), 51–57. 10.1111/j.1754-4505.2008.00062.x [DOI] [PubMed] [Google Scholar]
- Petcharat M, & Liehr P. (2017). Mindfulness training for parents of children with special needs: Guidance for nurses in mental health practice. Journal of Child & Adolescent Psychiatric Nursing 30(1), 35–46. 10.1111/jcap.12169 [DOI] [PubMed] [Google Scholar]
- Piet J, Wurtzen H, & Zachariae R. (2012). The effect of mindfulness-based therapy on symptoms of anxiety and depression in adult cancer patients and survivors: A systematic review and meta-analysis. Journal of Consulting and Clinical Psychology, 80(6), 1007–1020. 10.1037/a0028329 [DOI] [PubMed] [Google Scholar]
- Prinsloo GE, Rauch HG, & Derman WE (2014). A brief review and clinical application of heart rate variability biofeedback in sports, exercise, and rehabilitation medicine. Physician & Sportsmedicine, 42(2), 88–99. 10.3810/psm.2014.05.2061 [DOI] [PubMed] [Google Scholar]
- Provencher MD, Hawke LD, & Thienot E. (2011). Psychotherapies for comorbid anxiety in bipolar spectrum disorders. Journal of Affective Disorders, 133(3), 371–380. 10.1016/j.jad.2010.10.040 [DOI] [PubMed] [Google Scholar]
- Regehr C, Glancy D, & Pitts A. (2013). Interventions to reduce stress in university students: a review and meta-analysis. Journal of Affective Disorders, 148(1), 1–11. 10.1016/j.jad.2012.11.026 [DOI] [PubMed] [Google Scholar]
- Regehr C, Glancy D, Pitts A, & LeBlanc VR (2014). Interventions to reduce the consequences of stress in physicians: a review and meta-analysis. Journal of Nervous & Mental Disease, 202(5), 353–359. 10.1097/NMD.0000000000000130 [DOI] [PubMed] [Google Scholar]
- Ren Z, Zhang Y, & Jiang G. (2018). Effectiveness of mindfulness meditation in intervention for anxiety: A meta-analysis. Acta Psychologica Sinica, 50(3), 283–305. 10.3724/SP.J.1041.2018.00283 [DOI] [Google Scholar]
- Reneau M. (2019). Heart Rate Variability Biofeedback to Treat Fibromyalgia: An Integrative Literature Review. Pain Management Nursing. 10.1016/j.pmn.2019.08.001 [DOI] [PubMed] [Google Scholar]
- Rogers CE, Larkey LK, & Keller C. (2009). A review of clinical trials of tai chi and qigong in older adults. Western Journal of Nursing Research, 31(2), 245–279. 10.1177/0193945908327529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues MF, Nardi AE, & Levitan M. (2017). Mindfulness in mood and anxiety disorders: a review of the literature. Trends in Psychiatry & Psychotherapy 39(3), 207–215. 10.1590/2237-6089-2016-0051 [DOI] [PubMed] [Google Scholar]
- Sánchez-Meca J, Rosa-Alcázar AI, Marín-Martínez F, & Gómez-Conesa A. (2010). Psychological treatment of panic disorder with or without agoraphobia: a meta-analysis. Clinical Psychology Review, 30(1), 37–50. 10.1016/j.cpr.2009.08.011 [DOI] [PubMed] [Google Scholar]
- Scott-Sheldon LA, Balletto BL, Donahue ML, Feulner MM, Cruess DG, Salmoirago-Blotcher E, Wing RR, Carey MP (2019). Mindfulness-based interventions for adults living with HIV/AIDS: A systematic review and meta-analysis. AIDS and Behavior, 23(1), 60–75. 10.1007/s10461-018-2236-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sevilla-Llewellyn-Jones J, Santesteban-Echarri O, Pryor I, McGorry P, & Alvarez-Jimenez M. (2018). Web-Based Mindfulness Interventions for Mental Health Treatment: Systematic Review and Meta-Analysis. JMIR Mental Health 5(3), e10278. 10.2196/10278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma M, & Rush SE (2014). Mindfulness-based stress reduction as a stress management intervention for healthy individuals: a systematic review. Journal of Evidence-Based Complementary & Alternative Medicine, 19(4), 271–286. 10.1177/2156587214543143 [DOI] [PubMed] [Google Scholar]
- Shiralkar MT, Harris TB, Eddins-Folensbee FF, & Coverdale JH (2013). A systematic review of stress-management programs for medical students. Academic Psychiatry, 37(3), 158–164. 10.1176/appi.ap.12010003 [DOI] [PubMed] [Google Scholar]
- Singh SK, & Gorey KM (2018). Relative effectiveness of mindfulness and cognitive behavioral interventions for anxiety disorders: Meta-analytic review. Social Work in Mental Health, 16(2), 238–251. 10.1080/15332985.2017.1373266 [DOI] [Google Scholar]
- Stanley S. (2012). Mindfulness: Towards a critical relational perspective. Social and Personality Psychology Compass, 6(9), 631–641. 10.1111/j.1751-9004.2012.00454.x [DOI] [Google Scholar]
- Stratford HJ, Cooper MJ, Di Simplicio M, Blackwell SE, & Holmes EA (2015). Psychological therapy for anxiety in bipolar spectrum disorders: a systematic review. Clinical Psychology Review, 35, 19–34. 10.1016/j.cpr.2014.11.002 [DOI] [PubMed] [Google Scholar]
- Taylor BL, Cavanagh K, & Strauss C. (2016). The effectiveness of mindfulness-based interventions in the perinatal period: A systematic review and meta-analysis. PLoS ONE, 11(5). 10.1371/journal.pone.0155720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thabrew H, Ruppeldt P, & Sollers JJ 3rd. (2018). Systematic Review of Biofeedback Interventions for Addressing Anxiety and Depression in Children and Adolescents with Long-Term Physical Conditions. Applied Psychophysiology & Biofeedback, 43(3), 179–192. 10.1007/s10484-018-9399-z [DOI] [PubMed] [Google Scholar]
- Treanor M. (2011). The potential impact of mindfulness on exposure and extinction learning in anxiety disorders. Clinical Psychology Review, 31(4), 617–625. 10.1016/j.cpr.2011.02.003 [DOI] [PubMed] [Google Scholar]
- van der Riet P, Levett-Jones T, & Aquino-Russell C. (2018). The effectiveness of mindfulness meditation for nurses and nursing students: An integrated literature review. Nurse Education Today, 65, 201–211. 10.1016/j.nedt.2018.03.018 [DOI] [PubMed] [Google Scholar]
- Wu Y, Johnson BT, Acabchuk RL, Chen S, Lewis HK, Livingston J, Park CL, & Pescatello LS (2019). Yoga as Antihypertensive Lifestyle Therapy: A Systematic Review and Meta-analysis. Mayo Clinic Proceedings, 94(3), 432–446. 10.1016/j.mayocp.2018.09.023 [DOI] [PubMed] [Google Scholar]
- Zhang JY, Cui YX, Zhou YQ, & Li YL (2019). Effects of mindfulness-based stress reduction on prenatal stress, anxiety and depression. Psychology Health & Medicine 24(1):51–58, 2019 01, 24(1), 51–58. 10.1080/13548506.2018.1468028 [DOI] [PubMed] [Google Scholar]
- Zhang J, Xu R, Wang B, & Wang J. (2016). Effects of mindfulness-based therapy for patients with breast cancer: A systematic review and meta-analysis. Complementary Therapies in Medicine, 26, 1–10. 10.1016/j.ctim.2016.02.012 [DOI] [PubMed] [Google Scholar]
- Zimmermann FF, Burrell B, & Jordan J. (2018). The acceptability and potential benefits of mindfulness-based interventions in improving psychological well-being for adults with advanced cancer: A systematic review. Complementary Therapies in Clinical Practice, 30, 68–78. 10.1016/j.ctcp.2017.12.014 [DOI] [PubMed] [Google Scholar]
Footnotes
Conflict of Interest
All authors declare that they have no conflicts of interest.
Declarations of interest: none
Extant meta-analytic reviews of anxiety disorder interventions include those targeting PTSD, as it was classified as an anxiety disorder until publication of the DSM-5 (2013).
In co-meditation, participants were instructed to breathe at the pace of a designated partner.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- American Psychiatric Association. (1994). Diagnostic and statistical manual of mental disorders (4th ed. ed.). Washington, D. C.: Author. [Google Scholar]
- American Psychiatric Association. (2013). Diagnostic and statistical manual of mental disorders (5th ed. ed.). Washington, D. C.: Author. [Google Scholar]
- Asmundson GJ, Frombach I, McQuaid J, Pedrelli P, Lenox R, & Stein MB (2000). Dimensionality of posttraumatic stress symptoms: A confirmatory factor analysis of DSM-IV symptom clusters and other symptom models. Behaviour Research and Therapy, 38(2), 203–214. 10.1016/s0005-7967(99)00061-3 [DOI] [PubMed] [Google Scholar]
- Balke B, & Lillehei JP (1956). Effect of hyperventilation on performance. Journal of Applied Physiology, 9(3), 371–374. 10.1152/jappl.1956.9.3.371 [DOI] [PubMed] [Google Scholar]
- Baxter AJ, Scott KM, Vos T, & Whiteford HA (2013). Global prevalence of anxiety disorders: A systematic review and meta-regression. Psychological Medicine, 43(5), 897–910. 10.1017/S003329171200147X [DOI] [PubMed] [Google Scholar]
- Baxter AJ, Vos T, Scott KM, Ferrari AJ, & Whiteford HA (2014). The global burden of anxiety disorders in 2010. Psychological Medicine, 44(11), 2363–2374. 10.1017/S0033291713003243 [DOI] [PubMed] [Google Scholar]
- Beck AT, Epstein N, Brown G, & Steer RA (1988). An inventory for measuring clinical anxiety: Psychometric properties. Journal of Consulting and Clinical Psychology, 56(6), 893–897. 10.1037//0022-006x.56.6.893 [DOI] [PubMed] [Google Scholar]
- Becker CB, Zayfert C, & Anderson E. (2004). A survey of psychologists’ attitudes towards and utilization of exposure therapy for PTSD. Behaviour Research and Therapy, 42(3), 277–292. 10.1016/S0005-7967(03)00138-4 [DOI] [PubMed] [Google Scholar]
- Bekhuis E, Boschloo L, Rosmalen JG, & Schoevers RA (2015). Differential associations of specific depressive and anxiety disorders with somatic symptoms. Journal of Psychosomatic Research, 78(2), 116–122. 10.1016/j.jpsychores.2014.11.007 [DOI] [PubMed] [Google Scholar]
- Ben-Amnon Y, Fux M, Maoz B, & Benjamin J. (1995). Respiratory and other symptoms in panic disorder versus other anxiety disorders. Israel Journal of Psychiatry and Related Sciences, 32(1), 38–43. https://www.ncbi.nlm.nih.gov/pubmed/7622345 [PubMed] [Google Scholar]
- Berger BC (2000). The treatment of panic disorder: A comparative study between breathing retraining and cognitive behavioral therapy. [Doctor of Philosophy Dissertation]. Alliant University, California School of Professional Psychology, San Diego, [Google Scholar]
- Berkeljon A, & Baldwin SA (2009). An introduction to meta-analysis for psychotherapy outcome research. Psychotherapy Research, 19(4–5), 511–518. 10.1080/10503300802621172 [DOI] [PubMed] [Google Scholar]
- Bernardi L, Passino C, Spadacini G, Bonfichi M, Arcaini L, Malcovati L, Bandinelli G, Schneider A, Keyl C, Feil P, Greene RE, & Bernasconi C. (2007). Reduced hypoxic ventilatory response with preserved blood oxygenation in yoga trainees and Himalayan Buddhist monks at altitude: Evidence of a different adaptive strategy? European Journal of Applied Physiology, 99(5), 511–518. 10.1007/s00421-006-0373-8 [DOI] [PubMed] [Google Scholar]
- Bernardi L, Spadacini G, Bellwon J, Hajric R, Roskamm H, & Frey AW (1998). Effect of breathing rate on oxygen saturation and exercise performance in chronic heart failure. The Lancet, 351(9112), 1308–1311. 10.1016/S0140-6736(97)10341-5 [DOI] [PubMed] [Google Scholar]
- Bindslev L, Carlsson J, Santesson J, & Gottlieb I. (1986). Hypoxic pulmonary vasoconstriction in man: Effects of hyperventilation. Survey of Anesthesiology, 30(1), 1. 10.1111/j.1399-6576.1985.tb02251.x [DOI] [PubMed] [Google Scholar]
- Boettcher H, Brake CA, & Barlow DH (2016). Origins and outlook of interoceptive exposure. Journal of Behavior Therapy and Experimental Psychiatry, 53, 41–51. 10.1016/j.jbtep.2015.10.009 [DOI] [PubMed] [Google Scholar]
- Bohr C, Hasselbalch K, & Krogh A. (1904). Über einen in biologischer Beziehung wichtigen Einfluss, den die Kohlensäurespannung des Blutes auf dessen Sauerstoffbindung übt. Acta Physiologica, 16(2), 402–412. 10.1111/j.1748-1716.1904.tb01382.x [DOI] [Google Scholar]
- Bonn JA, Readhead CPA, & Timmons BH (1984). Enhanced adaptive behavioural response in agoraphobic patients pretreated with breathing retraining. The Lancet, 324(8404), 665–669. 10.1016/s0140-6736(84)91226-1 [DOI] [PubMed] [Google Scholar]
- Borenstein M, Higgins JPT, Hedges LV, & Rothstein HR (2017). Basics of meta-analysis: I2 is not an absolute measure of heterogeneity. Research Synthesis Methods, 8(1), 5–18. 10.1002/jrsm.1230 [DOI] [PubMed] [Google Scholar]
- Boswell JF, Farchione TJ, Sauer-Zavala S, Murray HW, Fortune MR, & Barlow DH (2013). Anxiety sensitivity and interoceptive exposure: a transdiagnostic construct and change strategy. Behavior Therapy, 44(3), 417–431. 10.1016/j.beth.2013.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulding R, Stacey R, Niven R, & Fowler SJ (2016). Dysfunctional breathing: A review of the literature and proposal for classification. European Respiratory Review, 25(141), 287–294. 10.1183/16000617.0088-2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bratton BL (2008). Mindfulness in childbirth: An investigation of the effects of mindfulness training on maternal satisfaction with childbirth and obstetric outcomes. [Doctor of Philsophy Dissertation]. Western Michigan University, [Google Scholar]
- Briere J. (2001). DAPS, Detailed Assessment of Posttraumatic Stress: Professional Manual: Psychological Assessment Resources. [Google Scholar]
- Carpenter JK, Andrews LA, Witcraft SM, Powers MB, Smits JAJ, & Hofmann SG (2018). Cognitive behavioral therapy for anxiety and related disorders: A meta-analysis of randomized placebo-controlled trials. Depression and Anxiety, 1–13. 10.1002/da.22728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandla SS, Sood S, Dogra R, Das S, Shukla SK, & Gupta S. (2013). Effect of short-term practice of pranayamic breathing exercises on cognition, anxiety, general well being and heart rate variability. Journal of the Indian Medical Association, 111(10), 662–665. Retrieved from https://www.ima-india.org/ima/left-side-bar.php?pid=347 [PubMed] [Google Scholar]
- Chang WL, Lee JT, Li CR, Davis AHT, Yang CC, & Chen YJ (2020). Effects of Heart Rate Variability Biofeedback in Patients With Acute Ischemic Stroke: A Randomized Controlled Trial. Biological Research for Nursing, 22(1), 34–44. 10.1177/1099800419881210 [DOI] [PubMed] [Google Scholar]
- Chen YF, Huang XY, Chien CH, & Cheng JF (2017). The Effectiveness of Diaphragmatic Breathing Relaxation Training for Reducing Anxiety. Perspectives in Psychiatric Care, 53(4), 329–336. 10.1111/ppc.12184 [DOI] [PubMed] [Google Scholar]
- Cohen J. (1988). Statistical power analysis for the behavioral sciences. In (2nd ed.). Hillsdale, NJ: Erlbaum. [Google Scholar]
- Colgan DD, Christopher M, Michael P, & Wahbeh H. (2016). The body scan and mindful breathing among veterans with PTSD: Type of intervention moderates the relationship between changes in mindfulness and post-treatment depression. Mindfulness, 7(2), 372–383. 10.1007/s12671-015-0453-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collimore KC, & Asmundson GJG (2014). Fearful responding to interoceptive exposure in social anxiety disorder. Journal of Anxiety Disorders, 28(2), 195–202. 10.1016/j.janxdis.2013.10.003 [DOI] [PubMed] [Google Scholar]
- Courtney R. (2017). Breathing training for dysfunctional breathing in asthma: taking a multidimensional approach. ERJ Open Research, 3(4), 00065–02017. 10.1183/23120541.00065-2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtney R, Greenwood KM, & Cohen M. (2011). Relationships between measures of dysfunctional breathing in a population with concerns about their breathing. Journal of Bodywork and Movement Therapies, 15(1), 24–34. 10.1016/j.jbmt.2010.06.004 [DOI] [PubMed] [Google Scholar]
- Courtney R, van Dixhoorn J, Greenwood KM, & Anthonissen ELM (2011). Medically unexplained dyspnea: partly moderated by dysfunctional (thoracic dominant) breathing pattern. Journal of Asthma, 48(3), 259–265. 10.3109/02770903.2011.554942 [DOI] [PubMed] [Google Scholar]
- Craske MG, Rowe M, Lewin M, & Noriega-Dimitri R. (1997). Interoceptive exposure versus breathing retraining within cognitive-behavioural therapy for panic disorder with agoraphobia. British Journal of Clinical Psychology, 36(1), 85–99. 10.1111/j.2044-8260.1997.tb01233.x [DOI] [PubMed] [Google Scholar]
- Crockett JE, Cashwell CS, Tangen JL, Hall KH, & Young JS (2016). Breathing characteristics and symptoms of psychological distress: An exploratory study. Counseling and Values, 61(1), 10–27. 10.1002/cvj.12023 [DOI] [Google Scholar]
- Cuijpers P, Gentili C, Banos RM, Garcia-Campayo J, Botella C, & Cristea IA (2016). Relative effects of cognitive and behavioral therapies on generalized anxiety disorder, social anxiety disorder and panic disorder: A meta-analysis. Journal of Anxiety Disorders, 43, 79–89. 10.1016/j.janxdis.2016.09.003 [DOI] [PubMed] [Google Scholar]
- Delaney EP, Greaney JL, Edwards DG, Rose WC, Fadel PJ, & Farquhar WB (2010). Exaggerated sympathetic and pressor responses to handgrip exercise in older hypertensive humans: Role of the muscle metaboreflex. American Journal of Physiology-Heart and Circulatory Physiology, 299(5), H1318–H1327. 10.1152/ajpheart.00556.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhruva A, Miaskowski C, Abrams D, Acree M, Cooper B, Goodman S, & Hecht FM (2012). Yoga breathing for cancer chemotherapy–associated symptoms and quality of life: Results of a pilot randomized controlled trial. The Journal of Alternative and Complementary Medicine, 18(5), 473–479. 10.1089/acm.2011.0555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enright S, Chatham K, Ionescu AA, Unnithan VB, & Shale DJ (2004). Inspiratory muscle training improves lung function and exercise capacity in adults with cystic fibrosis. CHEST Journal, 126(2), 405–411. 10.1378/chest.126.2.405 [DOI] [PubMed] [Google Scholar]
- Fasko D Jr, Hall G, Osborne MR, & Kornfeld RWBH (1992). Comeditation: An exploratory study of pulse and respiration rates and anxiety. Perceptual and Motor Skills, 74(3), 895–904. 10.2466/pms.1992.74.3.895 [DOI] [PubMed] [Google Scholar]
- Fatouleh R, & Macefield VG (2013). Cardiorespiratory coupling of sympathetic outflow in humans: a comparison of respiratory and cardiac modulation of sympathetic nerve activity to skin and muscle. Experimental Physiology, 98(9), 1327–1336. 10.1113/expphysiol.2013.072421 [DOI] [PubMed] [Google Scholar]
- Gallagher MW, Bentley KH, & Barlow DH (2014). Perceived control and vulnerability to anxiety disorders: A meta-analytic review. Cognitive Therapy and Research, 38(6), 571–584. 10.1007/s10608-014-9624-x [DOI] [Google Scholar]
- Gatchel RJ, & Proctor JD (1976). Effectiveness of voluntary heart rate control in reducing speech anxiety. Journal of Consulting and Clinical Psychology, 44(3), 381–389. 10.1037//0022-006x.44.3.381 [DOI] [PubMed] [Google Scholar]
- Gentes EL, & Ruscio AM (2011). A meta-analysis of the relation of intolerance of uncertainty to symptoms of generalized anxiety disorder, major depressive disorder, and obsessive–compulsive disorder. Clinical Psychology Review, 31(6), 923–933. 10.1016/j.cpr.2011.05.001 [DOI] [PubMed] [Google Scholar]
- Giardino ND, Chan L, & Borson S. (2004). Combined heart rate variability and pulse oximetry biofeedback for chronic obstructive pulmonary disease: Preliminary findings. Applied Psychophysiology and Biofeedback, 29(2), 121–133. 10.1023/B:APBI.0000026638.64386.89 [DOI] [PubMed] [Google Scholar]
- Gibson T. (1978). The Effects of Hypocapnia on Psychomotor and Intellectual Performance. Aviation, Space, and Environmental Medicine, 49(8), 943–946. Retrieved from http://www.asma.org/journal [PubMed] [Google Scholar]
- Grassi M, Caldirola D, Vanni G, Guerriero G, Piccinni M, Valchera A, & Perna G. (2013). Baseline respiratory parameters in panic disorder: A meta-analysis. Journal of Affective Disorders, 146(2), 158–173. 10.1016/j.jad.2012.08.034 [DOI] [PubMed] [Google Scholar]
- Gruzelier JH, Thompson T, Redding E, Brandt R, & Steffert T. (2014). Application of alpha/theta neurofeedback and heart rate variability training to young contemporary dancers: State anxiety and creativity. International Journal of Psychophysiology, 93(1), 105–111. 10.1016/j.ijpsycho.2013.05.004 [DOI] [PubMed] [Google Scholar]
- Gunter RW, & Whittal ML (2010). Dissemination of cognitive-behavioral treatments for anxiety disorders: Overcoming barriers and improving patient access. Clinical Psychology Review, 30(2), 194–202. 10.1016/j.cpr.2009.11.001 [DOI] [PubMed] [Google Scholar]
- Hagman C, Janson C, & Emtner M. (2008). A comparison between patients with dysfunctional breathing and patients with asthma. The Clinical Respiratory Journal, 2(2), 86–91. 10.1111/j.1752-699X.2007.00036.x [DOI] [PubMed] [Google Scholar]
- Hakked CS, Balakrishnan R, & Krishnamurthy MN (2017). Yogic breathing practices improve lung functions of competitive young swimmers. Journal of Ayurveda and Integrative Medicine, 8(2), 99–104. 10.1016/j.jaim.2016.12.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton M. (1960). A rating scale for depression. Journal Neurology, Neurosurgery, and Psychiatry, 23(1), 56–62. 10.1136/jnnp.23.1.56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayama Y, & Inoue T. (2012). The effects of deep breathing on ‘tension–anxiety’and fatigue in cancer patients undergoing adjuvant chemotherapy. Complementary Therapies in Clinical Practice, 18(2), 94–98. 10.1016/j.ctcp.2011.10.001 [DOI] [PubMed] [Google Scholar]
- Hayes SC, Luoma JB, Bond FW, Masuda A, & Lillis J. (2006). Acceptance and commitment therapy: Model, processes and outcomes. Behaviour Research and Therapy, 44(1), 1–25. 10.1016/j.brat.2005.06.006 [DOI] [PubMed] [Google Scholar]
- Hedges L, & Olkin I. (1985). Statistical Methods for Meta-Analysis. New York: Academic Press. [Google Scholar]
- Hedges LV, & Vevea JL (1998). Fixed-and random-effects models in meta-analysis. Psychological Methods, 3(4), 486. 10.1037/1082-989X.3.4.486 [DOI] [Google Scholar]
- Hellström F, Roatta S, Thunberg J, Passatore M, & Djupsjöbacka M. (2005). Responses of muscle spindles in feline dorsal neck muscles to electrical stimulation of the cervical sympathetic nerve. Experimental Brain Research, 165(3), 328–342. 10.1007/s00221-005-2309-7 [DOI] [PubMed] [Google Scholar]
- Henriques G, Keffer S, Abrahamson C, & Horst SJ (2011). Exploring the effectiveness of a computer-based heart rate variability biofeedback program in reducing anxiety in college students. Applied Psychophysiology and Biofeedback, 36(2), 101–112. 10.1007/s10484-011-9151-4 [DOI] [PubMed] [Google Scholar]
- Henry JD, & Crawford JR (2005). The short-form version of the Depression Anxiety Stress Scales (DASS-21): Construct validity and normative data in a large non-clinical sample. British Journal of Clinical Psychology, 44(2), 227–239. 10.1348/014466505X29657 [DOI] [PubMed] [Google Scholar]
- Hibbert GA, & Chan M. (1989). Respiratory control: Its contribution to the treatment of panic attacks. A controlled study. The British Journal of Psychiatry, 154(2), 232–236. 10.1192/bjp.154.2.232 [DOI] [PubMed] [Google Scholar]
- Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, & Welch VA (Eds.). (2019). Cochrane handbook for systematic reviews of interventions. John Wiley & Sons. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins JPT, Thompson SG, Deeks JJ, & Altman DG (2003). Measuring inconsistency in meta-analyses. BMJ: British Medical Journal, 327(7414), 557–560. 10.1136/bmj.327.7414.557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hipol LJ, & Deacon BJ (2013). Dissemination of evidence-based practices for anxiety disorders in Wyoming: A survey of practicing psychotherapists. Behavior Modification, 37(2), 170–188. 10.1177/0145445512458794 [DOI] [PubMed] [Google Scholar]
- Hofmann SG, Asnaani A, Vonk IJ, Sawyer AT, & Fang A. (2012). The efficacy of cognitive behavioral therapy: a review of meta-analyses. Cognitive Therapy and Research, 36(5), 427–440. 10.1007/s10608-012-9476-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann SG, & Smits JA (2008). Cognitive-behavioral therapy for adult anxiety disorders: a meta-analysis of randomized placebo-controlled trials. Journal of Clinical Psychiatry, 69(4), 621–632. 10.4088/jcp.v69n0415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holaway RM, Heimberg RG, & Coles ME (2006). A comparison of intolerance of uncertainty in analogue obsessive-compulsive disorder and generalized anxiety disorder. Journal of Anxiety Disorders, 20(2), 158–174. 10.1016/j.janxdis.2005.01.002 [DOI] [PubMed] [Google Scholar]
- Keogh E, & Reidy J. (2000). Exploring the factor structure of the Mood and Anxiety Symptom Questionnaire (MASQ). Journal of Personality Assessessment, 74(1), 106–125. 10.1207/S15327752JPA740108 [DOI] [PubMed] [Google Scholar]
- Kessler RC, Petukhova M, Sampson NA, Zaslavsky AM, & Wittchen HU (2012). Twelve-month and lifetime prevalence and lifetime morbid risk of anxiety and mood disorders in the United States. International Journal of Methods in Psychiatric Research, 21(3), 169–184. 10.1002/mpr.1359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- King DW, Leskin GA, King LA, & Weathers FW (1998). Confirmatory factor analysis of the Clinician-Administered PTSD Scale: evidence for the dimensionality of posttraumatic stress disorder. Psychological Assessment, 10(2), 90. 10.1037/1040-3590.10.2.90 [DOI] [Google Scholar]
- Kliem S, Kröger C, Foran HM, Mößle T, Glaesmer H, Zenger M, & Brähler E. (2016). Dimensional latent structure of PTSD-symptoms reporting: Is it adding by subtracting? Psychological Assessment, 28(12), 1663–1673. 10.1037/pas0000287 [DOI] [PubMed] [Google Scholar]
- Kotov R, Krueger RF, Watson D, Achenbach TM, Althoff RR, Bagby RM, Brown TA, Carpenter WT, Caspi A, Clark LA, Eaton NR, Forbes MK, Forbush KT, Goldberg D, Hasin D, Hyman SE, Ivanova MY, Lynman DR, Markon K, . . . Zimmerman M. (2017). The Hierarchical Taxonomy of Psychopathology (HiTOP): A dimensional alternative to traditional nosologies. Journal of Abnormal Psychology, 126(4), 454–477. 10.1037/abn0000258 [DOI] [PubMed] [Google Scholar]
- Kotov R, Perlman G, Gámez W, & Watson D. (2015). The structure and short-term stability of the emotional disorders: A dimensional approach. Psychological Medicine, 45(8), 1687–1698. 10.1017/S0033291714002815 [DOI] [PubMed] [Google Scholar]
- Lau EX, & Rapee RM (2011). Prevention of anxiety disorders. Current Psychiatry Reports, 13(4), 258–266. 10.1007/s11920-011-0199-x [DOI] [PubMed] [Google Scholar]
- Leander M, Lampa E, Rask-Andersen A, Franklin K, Gislason T, Oudin A, Svanes C, Torén K, & Janson C. (2014). Impact of anxiety and depression on respiratory symptoms. Respiratory Medicine, 108(11), 1594–1600. 10.1016/j.rmed.2014.09.007 [DOI] [PubMed] [Google Scholar]
- Lee J, Kim JK, & Wachholtz A. (2015). The benefit of heart rate variability biofeedback and relaxation training in reducing trait anxiety. Han’guk Simni Hakhoe chi. Kon’gang= The Korean Journal of Health Psychology, 20(2), 391–408. 10.17315/kjhp.2015.20.2.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehrer P, & Vaschillo E. (2004). Heart rate variability biofeedback: a new tool for improving autonomic homeostasis and treating emotional and psychosomatic diseases (Special Address in 2002). Japanese Journal of Biofeedback Research, 30, 7–16. 10.20595/jjbf.30.0_7 [DOI] [Google Scholar]
- Lehrer PM, Vaschillo E, & Vaschillo B. (2000). Resonant frequency biofeedback training to increase cardiac variability: Rationale and manual for training. Applied Psychophysiology and Biofeedback, 25(3), 177–191. 10.1023/a:1009554825745 [DOI] [PubMed] [Google Scholar]
- Lehrer PM, Vaschillo EG, Vaschillo B, Lu S-E, Eckberg DL, Edelberg R, Shih WJ, Lin Y, Kuusela TA, Tahvanainen K, & Hamer RM (2003). Heart rate variability biofeedback increases baroreflex gain and peak expiratory flow. Psychosomatic Medicine, 65(5), 796–805. 10.1097/01.psy.0000089200.81962.19 [DOI] [PubMed] [Google Scholar]
- Lickel J, Nelson E, Lickel AH, & Deacon B. (2008). Interoceptive exposure exercises for evoking depersonalization and derealization: A pilot study. Journal of Cognitive Psychotherapy, 22(4), 321–330. 10.1891/0889-8391.22.4.321 [DOI] [Google Scholar]
- Lickel JJ (2010). The use of breathing retraining among individuals who fear anxiety-related body sensations: Safety behavior or coping aid? [Doctor of Philosophy Dissertation]. University of Wyoming, [Google Scholar]
- Lickel JJ, Carruthers BR, Dixon LJ, & Deacon BJ (2013). Breathing retraining for individuals who fear respiratory sensations: Examination of safety behavior and coping aid hypotheses. Journal of Cognitive Psychotherapy, 27(2), 111–125. 10.1891/0889-8391.27.2.111 [DOI] [PubMed] [Google Scholar]
- Lin IM (2018). Effects of a cardiorespiratory synchronization training mobile application on heart rate variability and electroencephalography in healthy adults. International Journal of Psychophysiology, 134, 168–177. 10.1016/j.ijpsycho.2018.09.005 [DOI] [PubMed] [Google Scholar]
- Lin IM, Fan SY, Yen CF, Yeh YC, Tang TC, Huang MF, Liu TL, Wang PW, Lin HC, Tsai HY, & Tsai YC (2019). Heart Rate Variability Biofeedback Increased Autonomic Activation and Improved Symptoms of Depression and Insomnia among Patients with Major Depression Disorder. Clinical Psychopharmacology and Neuroscience, 17(2), 222–232. 10.9758/cpn.2019.17.2.222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovibond PF, & Lovibond SH (1995). The structure of negative emotional states: Comparison of the Depression Anxiety Stress Scales (DASS) with the Beck Depression and Anxiety Inventories. Behaviour Research and Therapy, 33(3), 335–343. 10.1016/0005-7967(94)00075-u [DOI] [PubMed] [Google Scholar]
- Malatino LS, Bellofiore S, Costa MP, Manto GL, Finocchiaro F, & Di Maria GU (1992). Cerebral blood flow velocity after hyperventilation-induced vasoconstriction in hypertensive patients. Stroke, 23(12), 1728–1732. 10.1161/01.str.23.12.1728 [DOI] [PubMed] [Google Scholar]
- McKinnon AC, Van Oudenhove L, Tack J, & Jones M. (2015). The association of personality, appraisal, catastrophising and vigilance with gastrointestinal symptom-specific anxiety. Journal of Health Psychology, 20(4), 456–465. 10.1177/1359105313503027 [DOI] [PubMed] [Google Scholar]
- McNair D, Lorr M, & Droppleman L. (1971). Profile of mood state manual. San Diego (CA): Educational and Industrial Testing Service. [Google Scholar]
- Meuret AE, & Ritz T. (2010). Hyperventilation in panic disorder and asthma: Empirical evidence and clinical strategies. International Journal of Psychophysiology, 78(1), 68–79. 10.1016/j.ijpsycho.2010.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meuret AE, Rosenfield D, Seidel A, Bhaskara L, & Hofmann SG (2010). Respiratory and cognitive mediators of treatment change in panic disorder: Evidence for intervention specificity. Journal of Consulting and Clinical Psychology, 78(5), 691–704. 10.1037/a0019552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meuret AE, Wilhelm FH, Ritz T, & Roth WT (2008). Feedback of end-tidal pCO 2 as a therapeutic approach for panic disorder. Journal of Psychiatric Research, 42(7), 560–568. 10.1016/j.jpsychires.2007.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel CC, Lloyd BB, & Cunningham DJC (1966). The in vivo carbon dioxide dissociation curve of true plasma. Respiration Physiology, 1(2), 121–137. 10.1016/0034-5687(66)90011-9 [DOI] [PubMed] [Google Scholar]
- Montero-Marin J, Garcia-Campayo J, Lopez-Montoyo A, Zabaleta-Del-Olmo E, & Cuijpers P. (2018). Is cognitive-behavioural therapy more effective than relaxation therapy in the treatment of anxiety disorders? A meta-analysis. Psychological Medicine, 48(9), 1427–1436. 10.1017/S0033291717003099 [DOI] [PubMed] [Google Scholar]
- Neuman Å, Gunnbjörnsdottir M, Tunsäter A, Nyström L, Franklin KA, Norrman E, & Janson C. (2006). Dyspnea in relation to symptoms of anxiety and depression: a prospective population study. Respiratory Medicine, 100(10), 1843–1849. 10.1016/j.rmed.2006.01.016 [DOI] [PubMed] [Google Scholar]
- Olatunji BO, Cisler JM, & Deacon BJ (2010). Efficacy of cognitive behavioral therapy for anxiety disorders: A review of meta-analytic findings. Psychiatric Clinics, 33(3), 557–577. 10.1016/j.psc.2010.04.002 [DOI] [PubMed] [Google Scholar]
- Olatunji BO, & Wolitzky-Taylor KB (2009). Anxiety sensitivity and the anxiety disorders: A meta-analytic review and synthesis. Psychological Bulletin, 135(6), 974–999. 10.1037/a0017428 [DOI] [PubMed] [Google Scholar]
- Pai A, Suris A, & North C. (2017). Posttraumatic stress disorder in the DSM-5: controversy, change, and conceptual considerations. Behavioral Sciences, 7(1), 7. 10.3390/bs7010007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papp LA, Martinez JM, Klein DF, Coplan JD, Norman RG, Cole R, de Jesus MJ, Ross D, Goetz R, & Gorman JM (1997). Respiratory psychophysiology of panic disorder: Three respiratory challenges in 98 subjects. American Journal of Psychiatry, 154(11), 1557–1565. 10.1176/ajp.154.11.1557 [DOI] [PubMed] [Google Scholar]
- Paul M, & Garg K. (2012). The effect of heart rate variability biofeedback on performance psychology of basketball players. Applied Psychophysiology and Biofeedback, 37(2), 131–144. 10.1007/s10484-012-9185-2 [DOI] [PubMed] [Google Scholar]
- Paulus MP (2013). The breathing conundrum—Interoceptive sensitivity and anxiety. Depression and Anxiety, 30(4), 315–320. 10.1002/da.22076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penzlin AI, Siepmann T, Illigens BM-W, Weidner K, & Siepmann M. (2015). Heart rate variability biofeedback in patients with alcohol dependence: A randomized controlled study. Neuropsychiatric Disease and Treatment, 11, 2619–2627. 10.2147/NDT.S84798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pham Q, Khatib Y, Stansfeld S, Fox S, & Green T. (2016). Feasibility and efficacy of an mHealth game for managing anxiety: “Flowy” randomized controlled pilot trial and design evaluation. Games for Health Journal, 5(1), 50–67. 10.1089/g4h.2015.0033 [DOI] [PubMed] [Google Scholar]
- Pollard KL, Campbell C, Squires M, Palsson O, & van Tilburg MAL (2018). Seasonal association of pediatric functional abdominal pain disorders and anxiety. Journal of Pediatric Gastroenterology and Nutrition, 67(1), 18–22. 10.1097/MPG.0000000000001886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pompoli A, Furukawa TA, Efthimiou O, Imai H, Tajika A, & Salanti G. (2018). Dismantling cognitive-behaviour therapy for panic disorder: a systematic review and component network meta-analysis. Psychological Medicine, 48(12), 1945–1953. 10.1017/S0033291717003919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radovanovic D, Peikert K, Lindström M, & Domellöf FP (2015). Sympathetic innervation of human muscle spindles. Journal of Anatomy, 226(6), 542–548. 10.1111/joa.12309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiss S, Peterson RA, Gursky DM, & McNally RJ (1986). Anxiety sensitivity, anxiety frequency and the prediction of fearfulness. Behaviour Research and Therapy, 24(1), 1–8. 10.1016/0005-7967(86)90143-9 [DOI] [PubMed] [Google Scholar]
- Risk of Bias. (2020). Risk of bias tools. https://www.riskofbias.info/welcome.
- Ritz T, Meuret AE, Bhaskara L, & Petersen S. (2013). Respiratory muscle tension as symptom generator in individuals with high anxiety sensitivity. Psychosomatic Medicine, 75(2), 187–195. 10.1097/PSY.0b013e31827d1072 [DOI] [PubMed] [Google Scholar]
- Rosenthal R, & Rubin DB (1986). Meta-analytic procedures for combining studies with multiple effect sizes. Psychological Bulletin, 99(3), 400–406. 10.1123/jsep.28.4.479 [DOI] [Google Scholar]
- Saigo T, Tayama J, Hamaguchi T, Nakaya N, Tomiie T, Bernick PJ, Kanazawa M, Labus JS, Naliboff BD, Shirabe S, & Fukudo S. (2014). Gastrointestinal specific anxiety in irritable bowel syndrome: Validation of the Japanese version of the visceral sensitivity index for university students. BioPsychoSocial Medicine, 8(1), 10. 10.1186/1751-0759-8-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt NB, Woolaway-Bickel K, Trakowski J, Santiago H, Storey J, Koselka M, & Cook J. (2000). Dismantling cognitive–behavioral treatment for panic disorder: Questioning the utility of breathing retraining. Journal of Consulting and Clinical Psychology, 68(3), 417–424. 10.1037//0022-006x.68.3.417 [DOI] [PubMed] [Google Scholar]
- Schwartz GE, Davidson RJ, & Goleman DJ (1978). Patterning of cognitive and somatic processes in the self-regulation of anxiety: effects of meditation versus exercise. Psychosomatic Medicine, 40(4), 321–328. 10.1097/00006842-197806000-00004 [DOI] [PubMed] [Google Scholar]
- Shear MK, Rucci P, Williams J, Frank E, Grochocinski V,J, Bilt JV, Houck P, & Wang T (2001). Reliability and validity of the Panic Disorder Severity Scale: Replication and extension. Journal of Psychiatric Research, 35(5), 293–296. 10.1016/s0022-3956(01)00028-0 [DOI] [PubMed] [Google Scholar]
- Siev J, & Chambless DL (2007). Specificity of treatment effects: Cognitive therapy and relaxation for generalized anxiety and panic disorders. Journal of Consulting and Clinical Psychology, 75(4), 513. 10.1037/0022-006X.75.4.513 [DOI] [PubMed] [Google Scholar]
- Simeon D, Guralnik O, Knutelska M, Yehuda R, & Schmeidler J. (2003). Basal norepinephrine in depersonalization disorder. Psychiatry Research, 121(1), 93–97. 10.1016/s0165-1781(03)00205-1 [DOI] [PubMed] [Google Scholar]
- Simon NM, Weiss AM, Kradin R, Evans KC, Reese HE, Otto MW, Oppenheimer JE, Smoller JW, Zalta A, Worthington III JJ, & Pollack MH (2006). The relationship of anxiety disorders, anxiety sensitivity and pulmonary dysfunction with dyspnea-related distress and avoidance. The Journal of Nervous and Mental Disease, 194(12), 951–957. 10.1097/01.nmd.0000249062.25829.53 [DOI] [PubMed] [Google Scholar]
- Smith EN, Santoro E, Moraveji N, Susi M, & Crum AJ (2019). Integrating wearables in stress management interventions: Promising evidence from a randomized trial. International Journal of Stress Management. 27(2), 172–182. 10.1037/str0000137 [DOI] [Google Scholar]
- Smith RE, Smoll FL, Cumming SP, & Grossbard JR (2006). Measurement of multidimensional sport performance anxiety in children and adults: The Sport Anxiety Scale-2. Journal of Sport and Exercise Psychology, 28(4), 479–501. 10.1123/jsep.28.4.479 [DOI] [Google Scholar]
- Spicuzza L, Porta C, Bramanti A, Maffeis M, Casucci G, Casiraghi N, & Bernardi L. (2005). Interaction between central-peripheral chemoreflexes and cerebro-cardiovascular control. Clinical Autonomic Research, 15(6), 373–381. 10.1007/s10286-005-0284-5 [DOI] [PubMed] [Google Scholar]
- Spielberg CD, Gorsuch RL, & Lushene RE (1983). Manual for the State-Trait Anxiety Inventory. Palo Alto, CA: Consulting Psychologist Press. Retrieved from https://www.apa.org/pi/about/publications/caregivers/practice-settings/assessment/tools/trait-state [Google Scholar]
- Spitzer RL, Kroenke K, Williams JB, & Löwe B. (2006). A brief measure for assessing generalized anxiety disorder: the GAD-7. Archives of Internal Medicine, 166(10), 1092–1097. 10.1001/archinte.166.10.1092 [DOI] [PubMed] [Google Scholar]
- Stanley TD, & Doucouliagos H. (2012). Meta-regression analysis in economics and business (Vol. 5): Routledge. 10.4324/9780203111710 [DOI] [Google Scholar]
- Stanley TD, & Jarrell SB (1989). Meta-Regression analysis: A quantitative method of literature surveys. Journal of Economic Surveys, 3(2), 161–170. 10.1111/j.1467-6419.1989.tb00064.x [DOI] [Google Scholar]
- Stapinski LA, Abbott MJ, & Rapee RM (2010). Evaluating the cognitive avoidance model of generalised anxiety disorder: Impact of worry on threat appraisal, perceived control and anxious arousal. Behaviour Research and Therapy, 48(10), 1032–1040. 10.1016/j.brat.2010.07.005 [DOI] [PubMed] [Google Scholar]
- Stefanaki C, Bacopoulou F, Livadas S, Kandaraki A, Karachalios A, Chrousos GP, & Diamanti-Kandarakis E. (2015). Impact of a mindfulness stress management program on stress, anxiety, depression and quality of life in women with polycystic ovary syndrome: A randomized controlled trial. Stress, 18(1), 57–66. 10.3109/10253890.2014.974030 [DOI] [PubMed] [Google Scholar]
- Stein MB, Millar TW, Larsen DK, & Kryger MH (1995). Irregular breathing during sleep in patients with panic disorder. The American Journal of Psychiatry, 152(8), 1168–1173. 10.1176/ajp.152.8.1168 [DOI] [PubMed] [Google Scholar]
- Sterne JAC, & Harbord RM (2004). Funnel plots in meta-analysis. Stata Journal, 4, 127–141. 10.1177/1536867X0400400204 [DOI] [Google Scholar]
- Tan G, Dao TK, Farmer L, Sutherland RJ, & Gevirtz R. (2011). Heart rate variability (HRV) and posttraumatic stress disorder (PTSD): A pilot study. Applied Psychophysiology and Biofeedback, 36(1), 27–35. 10.1007/s10484-010-9141-y [DOI] [PubMed] [Google Scholar]
- Thomas M, McKinley RK, Mellor S, Watkin G, Holloway E, Scullion J, Shaw DE, Wardlaw A, Price D, & Pavord I. (2009). Breathing exercises for asthma: A randomised controlled trial. Thorax, 64(1), 55–61. 10.1136/thx.2008.100867 [DOI] [PubMed] [Google Scholar]
- Thurber MR (2007). Effects of heart-rate variability biofeedback training and emotional regulation on music performance anxiety in university students. [Dissertation]. University of North Texas. [Google Scholar]
- Thorp SR, Ayers CR, Nuevo R, Stoddard JA, Sorrell JT, & Wetherell JL (2009). Meta-analysis comparing different behavioral treatments for late-life anxiety. The American Journal of Geriatric Psychiatry, 17(2), 105–115. 10.1097/JGP.0b013e31818b3f7e [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolin DF (2010). Is cognitive–behavioral therapy more effective than other therapies?: A meta-analytic review. Clinical Psychology Review, 30(6), 710–720. 10.1016/j.cpr.2010.05.003 [DOI] [PubMed] [Google Scholar]
- Uliaszek AA, Alden A, & Zinbarg RE (2015). Dimensionality in cognitive-behavioral assessment. In Assessment in cognitive therapy (pp. 149–171). New York, NY: Guilford Press. Retrieved from http://ebookcentral.proquest.com/lib/rutgers-ebooks/detail.action?docID=1760728 [Google Scholar]
- Valenza MC, Valenza-Peña G, Torres-Sánchez I, González-Jiménez E, Conde-Valero A, & Valenza-Demet G. (2014). Effectiveness of controlled breathing techniques on anxiety and depression in hospitalized patients with COPD: A randomized clinical trial. Respiratory Care, 59(2), 209–215. 10.4187/respcare.02565 [DOI] [PubMed] [Google Scholar]
- Van den Bergh O, Zaman J, Bresseleers J, Verhamme P, & Van Diest I. (2013). Anxiety, pCO2 and cerebral blood flow. International Journal of Psychophysiology, 89(1), 72–77. 10.1016/j.ijpsycho.2013.05.011 [DOI] [PubMed] [Google Scholar]
- van der Zwan JE, de Vente W, Huizink AC, Bögels SM, & de Bruin EI (2015). Physical activity, mindfulness meditation, or heart rate variability biofeedback for stress reduction: A randomized controlled trial. Applied Psychophysiology and Biofeedback, 40(4), 257–268. 10.1007/s10484-015-9293-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Zwan JE, Huizink AC, Lehrer PM, Koot HM, & de Vente W. (2019). The Effect of Heart Rate Variability Biofeedback Training on Mental Health of Pregnant and Non-Pregnant Women: A Randomized Controlled Trial. International Journal of Environmental Research and Public Health, 16(6). 10.3390/ijerph16061051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Diest I, Stegen K, Van de Woestijne KP, Schippers N, & Van den Bergh O. (2000). Hyperventilation and attention: Effects of hypocapnia on performance in a Stroop task. Biological Psychology, 53(2–3), 233–252. 10.1016/s0301-0511(00)00045-4 [DOI] [PubMed] [Google Scholar]
- Van Dixhoorn J, Duivenvoorden HJ, Pool J, & Verhage F. (1990). Psychic effects of physical training and relaxation therapy after myocardial infarction. Journal of Psychosomatic Research, 34(3), 327–337. 10.1016/0022-3999(90)90089-m [DOI] [PubMed] [Google Scholar]
- van Minnen A, Hendriks L, & Olff M. (2010). When do trauma experts choose exposure therapy for PTSD patients? A controlled study of therapist and patient factors. Behaviour Research and Therapy, 48(4), 312–320. 10.1016/j.brat.2009.12.003 [DOI] [PubMed] [Google Scholar]
- Wahbeh H, Goodrich E, Goy E, & Oken BS (2016). Mechanistic Pathways of Mindfulness Meditation in Combat Veterans With Posttraumatic Stress Disorder. Journal of Clinical Psychology, 72(4), 365–383. 10.1002/jclp.22255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wald J, & Taylor S. (2008). Responses to interoceptive exposure in people with posttraumatic stress disorder (PTSD): A preliminary analysis of induced anxiety reactions and trauma memories and their relationship to anxiety sensitivity and PTSD symptom severity. Cognitive Behaviour Therapy, 37(2), 90–100. 10.1080/16506070801969054 [DOI] [PubMed] [Google Scholar]
- Wang K, Zeng GQ, Li R, Luo YW, Wang M, Hu YH, Xu W, Zhou L, Chen R, & Chen X. (2017). Cycle ergometer and inspiratory muscle training offer modest benefit compared with cycle ergometer alone: a comprehensive assessment in stable COPD patients. International Journal of Chronic Obstructive Pulmonary Disease, 12, 2655–2668. 10.2147/COPD.S140093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang PS, Lane M, Olfson M, Pincus HA, Wells KB, & Kessler RC. (2005). Twelve-month use of mental health services in the United States: Results from the National Comorbidity Survey Replication. Archives of General Psychiatry, 62(6), 629–640. 10.1001/archpsyc.62.6.629 [DOI] [PubMed] [Google Scholar]
- Weathers F, & Litz B. (1994). Psychometric properties of the Clinician-Administered PTSD Scale, CAPS-1. PTSD Research Quarterly, 5(2), 2–6. https://www.ptsd.va.gov/publications/ptsd_rq.asp [Google Scholar]
- White B. (2008). The effects of heart rate variability biofeedback as an adjunct to therapy on trauma symptoms: ProQuest. [Google Scholar]
- Whiteside SP, Deacon BJ, Benito K, & Stewart E. (2016). Factors associated with practitioners’ use of exposure therapy for childhood anxiety disorders. Journal of Anxiety Disorders, 40, 29–36. 10.1016/j.janxdis.2016.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolitzky-Taylor K, Zimmermann M, Arch JJ, De Guzman E, & Lagomasino I. (2015). Has evidence-based psychosocial treatment for anxiety disorders permeated usual care in community mental health settings? Behaviour Research and Therapy, 72, 9–17. 10.1016/j.brat.2015.06.010 [DOI] [PubMed] [Google Scholar]
- Yekta ZP, Sadeghian F, Larijani TT, & Mehran A. (2017). The comparison of two types of relaxation techniques on postoperative state anxiety in candidates for the mastectomy surgery: a randomized controlled clinical trial. International Journal of Community Based Nursing and Midwifery, 5(1), 61. Retrieved from https://ijcbnm.sums.ac.ir/ [PMC free article] [PubMed] [Google Scholar]
- Zigmond AS, & Snaith RP (1983). The hospital anxiety and depression scale. Acta Psychiatrica Scandinavica, 67(6), 361–370. 10.1111/j.1600-0447.1983.tb09716.x [DOI] [PubMed] [Google Scholar]
- Zucker TL, Samuelson KW, Muench F, Greenberg MA, & Gevirtz RN (2009). The effects of respiratory sinus arrhythmia biofeedback on heart rate variability and posttraumatic stress disorder symptoms: A pilot study. Applied Psychophysiology and Biofeedback, 34(2), 135–143. 10.1007/s10484-009-9085-2 [DOI] [PubMed] [Google Scholar]
- Zvolensky MJ, Bernstein A, Cardenas SJ, Colotla VA, Marshall EC, & Feldner MT (2007). Anxiety sensitivity and early relapse to smoking: A test among Mexican daily, low-level smokers. Nicotine & Tobacco Research, 9(4), 483–491. 10.1080/14622200701239621 [DOI] [PubMed] [Google Scholar]
- Zvolensky MJ, Forsyth JP, Fuse T, Feldner MT, & Leen-Feldner EW (2002). Panic attacks and smoking: An initial investigation of panic-relevant cognitive processes. Cognitive Behaviour Therapy, 31, 170–182. 10.1080/165060702321138564 [DOI] [Google Scholar]