Abstract
Nearly 20% of genes located on the X chromosome are associated with neurodevelopmental disorders (NDD) due to their expression and role in brain functioning. Given their location, several of these genes are either subject to or can escape X-chromosome inactivation (XCI). The degree to which genes are subject to XCI can influence the NDD phenotype between males and females. We provide a general review of X-linked NDD genes in the context of XCI and detailed discussion of the sex-based differences related to MECP2 and FMR1, two common X-linked causes of NDD that are subject to XCI. Understanding the effects of XCI on phenotypic expression of NDD genes may guide the development of stratification biomarkers in X-linked disorders.
Keywords: X-chromosome inactivation, MECP2, FMR1, Rett syndrome, fragile X syndrome, FXTAS, POI, neurodevelopmental disorders
1. Introduction
Several genes on the X chromosome are specifically expressed in the brain and are essential for neuronal plasticity and cognitive processes [1]. Of these, nearly 20% have been linked to neurodevelopmental disorders (NDD) and the dissimilar phenotype in males and females is due in part to differences in the pattern of gene expression [2]. The hemizygosity of most X-linked genes reveals recessive phenotypes in males, thus accounting for the disproportionately large number of affected males [3], while females with the same pathogenic variant are often unaffected or mildly affected. The sex differences found in X-linked NDDs are influenced by X-chromosome inactivation (XCI), a method of X-chromosome dosage compensation that ensures that X-linked genes are expressed at the same level in females as in males. Very early in female development, random inactivation of either the paternal or the maternal X chromosomes occurs in each cell, and the pattern of inactivation is transmitted to all daughter cells via mitosis. This results in the mosaic expression of X-linked genes in females, which can confer protection against disease. Generally, the ratio of the expression of maternal and paternal alleles is about 50:50 in females; however, deviation from the 50:50 ratio, known as skewed XCI, is also seen. Skewed XCI occurs when the inactivation of one X chromosome is favored over the other, and the ratio is commonly considered skewed if it is ≥65:35.
In this review we provide a general overview of X-linked NDD genes, their phenotype and association with XCI, and a focused discussion of the phenotypes associated with methyl-CpG binding protein 2 (MECP2) and fragile X mental retardation 1 (FMR1), two genes that are subject to XCI.
2. X-Linked NDD Genes
The majority of X-linked NDD genes are subject to XCI, resulting in phenotypic variability between males and females. In Table 1, we show a representative sample of genes that have a well-established association with an NDD phenotype as notated in OMIM [4] and discussed in the review by Migeon [5]. Several factors influence clinical presentation in females; these include whether the gene escapes XCI or is subject to skewing, the variant type, as well as the inheritance pattern. About 15% of the genes on the X chromosome escape inactivation and are expressed from both the active and inactive chromosomes [4]. The degree of the escape from XCI is reported to vary between genes, tissues, and individuals and likely contributes to phenotypic heterogeneity [6,7]. For genes that escape XCI, the NDD phenotype may be lethal in males and is generally more severe when compared with symptomatic females. This pattern is seen in X-linked disorders associated with the following genes: SMC1A, USP9X, LAMP2, IQSEC2, DCX, DDX3X, and OFD1 (Table 1).
Table 1.
Impact of XCI on NDD.
| Gene | X-Linked Disorder | Inheritance | Male | Female | Gene Subject to X-Inactivation | References |
|---|---|---|---|---|---|---|
| ABCD1 | Adrenoleukodystrophy/Adrenomyeloneuropathy | Recessive | Death first decade/progressive stiffness and weakness in the legs, development of cognitive and behavioral disturbance beginning in the 2nd decade | unaffected/late onset adrenomyeloneuropathy | Yes | PMID: 23469258 [9]; PMID: 22280810 [10] |
| AFF2 | Intellectual developmental disorder, X-linked 109 | Recessive | Global developmental delay/ID/behavioral dx | mild or unaffected | N.D. | n/a |
| AIFM1 | Spondyloepimetaphyseal dysplasia, X-linked, with hypomyelinating leukodystrophy | Recessive | hypomyelinating leukodystrophy | unaffected carrier females | Yes | PMID: 32337346 [11] |
| ALG13 | Developmental and epileptic encephalopathy 36 | early-onset epileptic encephalopathy, severe intellectual disability | developmental and epileptic encephalopathy-36; unaffected carrier females | Yes | PMID: 28778787 [12] | |
| AP1S2 | PETTIGREW SYNDROME | Recessive | Intellectual disability | unaffected carrier females | ND | n/a |
| ARHGEF9 | Developmental and epileptic encephalopathy 8 | Recessive | profound ID, epilepsy | intellectual disability, unaffected carriers | Yes | PMID: 33600053 [13] |
| ARSE | Chondrodysplasia punctata 1 | Recessive | Developmental delay/ID | not described | ND | n/a |
| ARX | Early infantile epileptic encephalopathyLISX2, Proud syndrome | Recessive | epilepsy and profound ID; brain abnormalities, abnormal genitalia | unaffected; mild phenotype | Yes | PMID: 21416597 [14] |
| ATP6AP2 | Intellectual Disability, X-linked, syndromic, Hedera type | Recessive | ID, epilepsy parkinsonism, spasticity | unaffected | N.D. | n/a |
| ATP7A | Menkes disease | Recessive | epilepsy, developmental delay | unaffected | N.D. | n/a |
| ATRX | Alpha-thalassemia/ID | Dominant | Severe ID and dysmorphic features | mild ID | Yes | PMID: 16100724 [15] |
| ATRX | ATRX ID syndrome | Recessive | severe ID and dysmorphic features | unaffected | Yes | PMID: 16955409 [16] |
| BRWD3 | Intellectual Disability, X-linked 93 | Recessive | ID | unaffected carrier females | N.D. | n/a |
| CASK | Intellectual Disability and microcephaly with pontine and cerebellar hypoplasia | Dominant | ID, microcephaly, pontine, cerebellar hypoplasia | unaffected carrier females, ASD | Yes | PMID: 28944139 [17] |
| CDKL5 | Early infantile epileptic encephalopathy early death | Dominant | milder phenotype, epilepsy and profound ID | severe ID, early onset epilepsy, microcephaly, less severe | Yes | PMID: 24564546 [18] |
| CLIC2 | Intellectual Disability, X-linked, syndromic 32 | Recessive | no affected males | mild learning disabilities | N.D. | n/a |
| CLCN4 | Raynaud-Claes syndrome | Dominant | Sever ID and epilepsy | milder phenotype | Yes | PMID: 27550844 [19] |
| CNKSR2 | Houge type | ND | epilepsy, microcephaly, developmental delay | mild ID, seizure | Yes | PMID: 31414730 [20] |
| CUL4B | Cullun Ring Cabezas type | Recessive | syndromic ID | learning disability | Yes | PMID: 17273978 [21] |
| CXorf56 | CXorf56-Associated ID | ND | moderate ID | generally unaffected or mild phenotype | Yes | PMID: 31822863 [22] |
| DCX | Lissencephaly | ND | ID, epilepsy, brain malformation | mild epilepsy | Yes | PMID: 12838518 [23] |
| DDX3X | Snijders Blok | Recessive | some males with non-syndromic ID | ID, microcephaly | escapes X inactivation | PMID: 30871455 [24] |
| DLG3 | Intellectual Disability, X-linked 90 | Recessive | moderate - severe ID | not affected and affected | Yes | PMID: 28777483 [25] |
| DMD | Duchenne, Muscular dystrophy | Recessive | mild ID | unaffected | Yes | PMID: 27098336 [26] |
| FAM50A | Intellectual developmental disorder, X-linked, syndromic, Armfield type | ID | unaffected | N.D. | n/a | |
| FDG1 | Aarskog–Scott syndrome | Not reported | Facio-genetial dysmorphisms, ADHD, ID | short stature | N.D. | n/a |
| FGF13 | Developmental and epileptic encephalopathy 90 | Dominant/Recessive | epilepsy, developmental delay | epilepsy, developmental delay | N.D. | n/a |
| FMR1 | Fragile X syndrome | Dominant | ID | mild | Yes | PMID: 8825916 [27] |
| FMR1 | Fragile X Tremor Ataxia | Dominant | late onset tremor, ataxia, cognitive decline | FXTAS in 10% of premutation carriers | Yes | PMID: 26609701 [28] |
| FMR1 | Premature Ovarian Failure | n/a | POI in 25% of premutation carriers | N.D. | PMID: 30098699 [29] | |
| FRMPD4 | Intellectual Disability, X-linked 104 | ID | unaffected | N.D. | n/a | |
| FTSJ1 | Intellectual Disability, X-linked 9/44 | Recessive | ID and mood disorder | unaffected carrier females | N.D. | n/a |
| GRIA3 | Intellectual developmental disorder, X-linked, syndromic, Wu type | Recessive | ID | unaffected carrier females | Yes | PMID: 19449417 [30] |
| GPC3/GPC4 | Simpson-Golabi-Behmel | Recessive | ID, congenital malformation | generally unaffected | Yes | PMID: 30048822 [31] |
| HCFC1 | Methylmalonic acidemia | Recessive | ID | not affected | N.D. | n/a |
| HDAC8 | Cornelia de Lange, 5 | Dominant | syndromic ID | mild | Yes | PMID: 22889856 [32] |
| HPRT | Lesch-Nyhan syndrome | Recessive | ID, spastic cerebral palsy and SIB | not affected | Yes | PMID: 6585829 [33] |
| HUWE1 | Intellectual Disability, X-linked | Not reported | moderate -profound syndromic ID | Chiari malformation, ID, dysmorphism | N.D. | n/a |
| IGBP1 | Corpus callosum, agenesis of, with Intellectual Disability, ocular coloboma and micrognathia | Recessive | ID | unaffected | N.D. | n/a |
| IL1RAPL1 | Intellectual Disability, X-linked 21/34 | Recessive | ID, microcephaly | unaffected carrier females | N.D. | n/a |
| IQSEC2 | Intellectual Disability, X-linked 1/78 | Dominant | non-syndromic ID, epilepsy and non-syndromic ID | some with learning disability, milder ID some with epilepsy | escapes X inactivation | PMID: 32564198 [34] |
| KDM5C/JARIDC/SMCX | Claes-Jensen | Recessive | microcephaly, developmental disability | mild phenotype | Yes | [35] |
| KDM6A (UTX) | Kabuki syndrome 2 | Dominant | syndromic ID | similar to males | escapes X inactivation | PMID: 29022598 [7] |
| KLHL15 | Intellectual Disability, X-linked 103 | Recessive | ID, epilepsy, brain malformation | mild or unaffected | Yes | PMID: 24817631 [36] |
| L1CAM | Hydrocephalus. X-linked aqueductal stenosis | Recessive | ID, spastic paraplegia | mild ID, some are not affected | N.D. | n/a |
| LAMP2 | Danon disease | Dominant | ID and myopathy | late onset | escapes X inactivation | PMID: 30871455 [24] |
| MAOA | Monoamine oxidase A def | Recessive | mild ID, behavioral difficulties | not affected | Yes | PMID: 19684479 [37] |
| MECP2 | Rett syndrome | Dominant | early infantile epileptic encephalopathy/death first 2-4 yrs of life | ID, epilepsy, microcephaly, gait and language disorder | Yes | PMID: 18361425 [38]; PMID: 31427717 [39] |
| MECP2 | MECP2 Dup Syndrome | Recessive | profound Intellectual Disability, infantile hypotonia, autistic features, seizures, progressive spasticity, and recurrent infections | mild neuropsychiatric features, such as anxiety. | Yes | PMID: 29141583 [40] |
| MECP2 | PPMX | Recessive | ID, spasticity, tremor, hyperkinetic behavior | unaffected carrier females | N.D. | n/a |
| MED12 (HOPA) | MED12-Related Disorders | Recessive | moderate ID, marfanoid habitus, ID, ptosis, cryptorchidism | unaffected carrier females or Hardikar syndrome | Yes | PMID: 33244166 [41] |
| NEXMIF (KIA2022) | neurite extension & migration | Dominant | severe ID, epilepsy | unaffected, intractable epilepsy and ID | Yes | PMID: 27358180 [42]; PMID: 29717186 [43] |
| NHS | Nance–Horan Syndrome | Dominant | Congenital cataract, microphthalmia, and mild or moderate | mild vision impairment | N.D. | n/a |
| NKAP | Intellectual developmental disorder, X-linked, syndromic, Hackman-Di Donato type | Recessive | ID | unaffected carrier females | N.D. | n/a |
| NLGN3 | Autism risk | ASD | unaffected carrier females | Yes | PMID: 18361425 [38] | |
| NLGN4X | Intellectual Disability, X-linked | no affected males | ID, epilepsy and language disorder | Yes | PMID: 32564284 [44] | |
| NONO | Intellectual Disability, X-linked, syndromic 34 | ID, congenital cardiac malformation | unaffected carrier females | N.D. | n/a | |
| NSDHL | CK syndrome | Recessive | ID, neonatal seizures | N.D. | n/a | |
| OCRL1 | Lowe syndrome | Recessive | ID, cataracts | not affected | skewed X-inactivation | PMID: 7180850 [45] |
| OFD1 | Simpson-Golabi-Behmel | Recessive | early lethality, severe ID | not affected | escapes X inactivation | PMID: 31243241 [46] |
| OFD1 | Joubert 10 | Recessive | ID, congenital malformation | unaffected carrier females | N.D. | n/a |
| OGT | Intellectual Disability, X-linked | Recessive | syndromic ID | not affected | random X-inactivation | PMID: 25136351 [47] |
| OPHN1 | ID, X-linked Congenital Cerebellar hypoplasia | Recessive | ID, hypotonia, ataxia, seizures, macrocephaly, strabismus, dismorphic features | not reported; mild ID, dysmprohic features, strabismus | Yes | PMID: 24105372 [48] |
| PAK3 | Intellectual Disability, X-linked 30/47 | Recessive | ID | unaffected carrier females | N.D. | n/a |
| PCDH19 | Early infantile epileptic encephalopathy, 9 | Not reported | Mosaic males | ID, autism, infantile seizures | Yes | PMID: 22091964 [49] |
| PDHA1 | PDC deficiency | Dominant | early lethality, brain malformation, infantile/childhood onset Leigh mild ataxia | dysmorphism, brain malformation, epilepsy, spastic cp | Yes | PMID: 31673819 [50] |
| PHF6 | Borjeson–Forssman–Lehmann syndrome | Recessive | ID, epilepsy | mild ID | Yes | PMID: 15994862 [51], PMID: 12415272 [52], PMID: 22190899 [53] |
| PHF8 | Syndromic X-linked intellectual disability Siderius type | Recessive | syndromic ID | not affected | Yes | PMID: 18498374 [54] |
| PLP1 | Pelizaeus-Merzbacher Disease | Recessive | leukodystrophy and spastic diplegia | mild or unaffected | Yes | PMID: 10878666 [55]; PMID: 12297985 [56] |
| PORCN | Focal dermal hypoplasia | Dominant | Mosaic males | syndromic ID | Yes | PMID: 17546030 [57]; PMID: 17546031 [58] |
| PQBP1 | Renpenning syndrome | Recessive | ID, microcephaly | unaffected | Yes | PMID: 15811016 [59]; PMID: 31840929 [60] |
| PRPS1 | Arts syndrome | Recessive | ID, ataxia | milder phenotype | Yes | PMID: 24528855 [61] |
| RLIM (RFN12) | Tonne-Kalscheuer syndrome | Not reported | ID, GDD, Autism, congenital malformation | generally unaffected | Yes | PMID: 29728705 [62] |
| RAB39B | Waisman Syndrome | Recessive | ID, epilepsy, Parkinson disease | later onset Parkinson disease and unaffected | N.D. | n/a |
| RPS6KA3 (RSK2) | Coffin-Lowry syndrome | Dominant | syndromic ID, microcephaly | mild ID | Yes | PMID: 12030896 [63] |
| RPS6KA3 (RSK2) | X-Linked MR19 | Dominant | mod ID | mild nonsyndromic ID | N.D. | n/a |
| SLC35A2 | Congenital disorder of glycosylation, type II | Dominant | males are mosaic | Infantile epileptic encephalopathy | Yes | PMID: 24115232 [64] |
| SLC6A8 | Creatine transporter deficiency | Recessive | ID, epilepsy | mild | Yes | PMID: 20528887 [65] |
| SLC9A6 (NHE6) | Christianson syndrome | X-linked | profound ID, epilepsy | ID, learning differences, ADHD, speech delay | Yes | PMID: 18342287 [66] |
| SLC9A7 | Intellectual developmental disorder, X-linked 108 | Recessive | ID | unaffected carrier females | N.D. | n/a |
| SMC1A | DEE85; Cornelia de Lange, 2 | Dominant | Lethal in males; ID, limb malformations, dysmorphic | ID, midline brain defects, seizures | escape X inactivation | PMID: 30871455 [24] |
| SOX3 | Intellectual Disability, X-linked, with isolated growth hormone deficiency | X-linked | ID, panhypopituitarism | unaffected carrier females | N.D. | n/a |
| STAG2 | Holoprosencephaly 13, X-linked | Dominant, Recessive | early lethality, brain malformation, ID | ID, brain malformation/unaffected | N.D. | n/a |
| SYN1 | Epilepsy, X-linked, with variable learning disabilities and behavior disorders | Dominant, Recessive | ID, epilepsy, ASD | epilepsy and ASD | N.D. | n/a |
| SYP | Intellectual Disability, X-linked 96 | Recessive | ID, epilepsy | unaffected | N.D. | n/a |
| TAF1 | XLID 33 | Recessive | syndromic ID | unaffected | Yes | PMID: 26637982 [67] |
| THOC2 | XLID 12/35 | Recessive | mild - moderate ID | not affected | Yes | PMID: 26166480 [68] |
| TSPAN7 | Intellectual Disability, X-linked 58 | Recessive | ID | unaffected carrier females | N.D. | n/a |
| UBE2A | Intellectual Disability, X-linked syndromic, Nascimento-type | Recessive | ID, epilepsy | unaffected carrier females | Yes | PMID: 16909393 [69] |
| UPF3B | XLID 14 | Recessive | Severe non-syndromic ID, Autism | not affected | Yes | PMID: 19238151 [70] |
| USP9X | Syndromic XLID 99 | Dominant | ID, autism, maybe Lethal in males | mild or unaffected or ID, multiple congenital anomalies | escapes X inactivation | PMID: 29022598 [7] |
| USP27X | Intellectual Disability, X-linked 105 | Recessive | ID | unaffected carrier females | N.D. | n/a |
| WDR45 | NBIA5 | Dominant | lethal, mosaics - affected | static encephalopathy, adult onset neurodegeneration, infantile spasms, developmental delay, ID | Yes | PMID: 23176820 [71] |
| ZDHHC9 | Raymond type XLMR | X-linked | non-syndromic ID | unaffected | N.D. | n/a |
Of the genes that are subject to XCI, skewing towards expression of the allele with the pathogenic variant or the normal allele may occur. When skewing is towards the variant allele, females and males show a similar affected phenotype, as seen in WDR45-related disorders. Conversely, when there is skewing towards expression of the normal allele, females are typically asymptomatic. An exception to this pattern is seen with the ABCD1 gene, where there can be a less severe phenotype in females, despite skewing towards expression of the variant allele [8].
3. MECP2
The MECP2 gene is predominantly expressed in the brain where MeCP2 binds to methylated DNA via methyl-CpG pairs and acts as both a transcriptional repressor and an activator of gene expression [10]. MECP2 is important for prenatal neurogenesis, postnatal development of synaptic connections and function, synaptic plasticity, and adult neural function [72]. Variants involving the MECP2 gene may result in MECP2 duplication syndrome, Rett syndrome (RTT), X-linked intellectual disability or autism spectrum disorder (ASD).
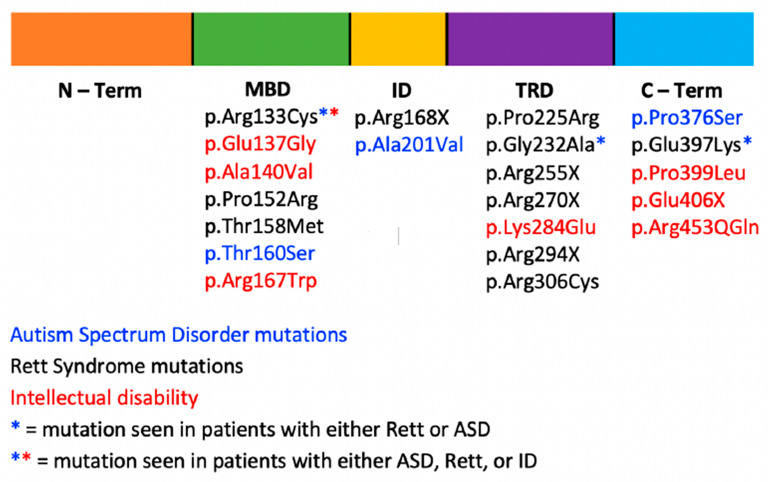
Loss-of-function or missense variants in MECP2 may results in syndromic or non-syndromic intellectual disability, Rett syndrome, or ASD without RTT (Figure 1). RTT is seen almost exclusively in females and is lethal in most males by age 2. Individuals with classic RTT generally present with normal early growth and development followed by developmental stagnation between 6 and 18 months and a period of developmental regression affecting social skills, speech, gait, and purposeful hand use between 1 and 4 years old. During the period of regression, distinct hand movements, seizures, and irregular respirations emerge. In addition to RTT, loss-of-function MECP2 variants can also cause a non-specific X-linked intellectual disability in males and females [73]. Females often have mild intellectual disability, while males may develop mild to severe intellectual disability, including PPM-X syndrome marked by psychosis and bipolar disorder, parkinsonism, increased muscle tone, exaggerated reflexes, and abnormal enlargement of the testes [74].
Figure 1.
Schematic of the MECP2 protein showing phenotypic variability among pathogenic variants and their associated disorders.
The disease severity and variability of the phenotype is influenced both by the location and type of the variant as well as by genetic background and cellular environment [39]. Truncating variants in MECP2 are associated with a more severe phenotype than missense variants, and individuals with truncations show earlier development of hand stereotypies, decreased height z-scores, paucity of speech [75], a higher incidence of awake respiratory dysfunction [76], and overall higher clinical severity [77].
Variant type and location do not adequately explain phenotypic variability, as individuals with the same pathogenic variant have clinical presentations varying from ASD, ID, and RTT (Figure 1), and XCI has been proposed to be an important factor in the onset and severity of RTT [78,79]. Phenotypic variation ranging from classical RTT to normal individuals with protective skewing of the X chromosome have been reported [78]. Zhang et al. described a Chinese family with Rett syndrome and X-linked intellectual disability [79]. They reported eight individuals with MECP2 variants in six families.
A family made up of a mother, daughter, and son had the identical MECP2 variant c.397C > T. The daughter was diagnosed with a preserved speech variant of RTT, the son was diagnosed with X-linked mental retardation (XLMR), while the mother was healthy. XCI studies showed that the mother had skewing towards the normal allele, while the daughter had random XCI. Another mother and daughter pair were found to have the same c.397C > T MECP2 variant. However, although they both had random XCI, the daughter was diagnosed with RTT, and the mother had learning difficulties and autistic behaviors [79]. While the variability in phenotypes between the mothers and their daughters with the same MECP2 variant may be due to the difference in the pattern of XCI, not all clinical presentations can be explained by the pattern of XCI, given that the clinical symptoms of the mother with random XCI were milder than those of her daughter with the same variant and degree of XCI. Consistent with this report, Xiol et al. found no substantial correlation between the XCI patterns in the blood and the clinical presentation of RTT. In their study of 221 RTT patients with nine recurrent MECP2 variants or a large deletion in MECP2, 17 out of 174 patients had a skewed XCI pattern, and there were no consistent increases or decreases in the clinical severity score of RTT patients with a preferential inactivation of the wild-type or mutated alleles [39]. In addition, the XCI pattern in blood and cortex was different for two patients included in their study. A similar finding was reported by Bao et al., who showed no statistically significant relationship between clinical severity and pattern of XCI [80].
MECP2 duplication results in a gain-of-function phenotype that is inherited in a recessive manner, predominantly affects males, and is characterized by severe to profound intellectual disability and limited or absent speech. Individuals with this syndrome have early-onset hypotonia and have progressive spasticity affecting the lower limbs. Additionally, 50% of affected males have epileptic seizures, and many have a predisposition to recurrent infections [81,82,83]. The X chromosome carrying the duplication is often preferentially silenced in most asymptomatic carriers [84]; however, some females have a mild phenotype, despite inactivation of the variant chromosome [85]. Symptomatic females exhibiting random XCI or skewing with preferential expression of the duplicated chromosome may present with varying severity and can exhibit learning disabilities, intellectual disability, autistic features, or psychiatric symptoms [86,87].
4. FMR1
The FMR1 gene encodes the fragile X mental retardation protein (FMRP), an RNA-binding protein that is highly expressed in the brain and reproductive organs. FMRP regulates the translation, transport, and stability of mRNAs and plays important roles in neuronal development and synaptic plasticity [88]. FMR1-related disorders include fragile X syndrome (FXS), fragile X tremor/ataxia syndrome (FXTAS), and premature ovarian insufficiency (POI), and result from expansion of the trinucleotide CGG repeat in the 5′ untranslated region. The repeat is categorized into four groups based on the size of the repeat: normal alleles (5–44 repeats), intermediate alleles (45–54 repeats), premutation alleles (55–200 repeats), and full-mutation alleles (>200 repeats). The FMR1 premutation is associated with FXTAS and POI, while the full mutation is associated with FXS. Normal alleles are typically transmitted from parent to offspring in a stable manner without any increase or decrease in repeat number. Intermediate alleles may expand into the premutation range when transmitted by the mother [89], while premutation alleles are unstable and tend to expand into a full mutation when transmitted from mother to offspring. It is estimated that about 1 in 850 males and 1 in 300 females have the premutation and 1 in 7000 males and 1 in 11,000 females have the FMR1 full mutation [90].
4.1. FMR1 Full Mutation
The phenotype of the full mutation results from hypermethylation of the CGG expanded region, thus causing the loss of FMR1 transcription and the absence of FMRP. Most males with FXS have intellectual disability, macrocephaly, facial dysmorphism, high arched palate, joint hyperlaxity, hypotonia, otitis media, pes planus, connective tissue problems, and pectus excavatum. The behavioral features typically include attention-deficit/hyperactivity disorder (ADHD), anxiety, depression, emotional lability, gaze avoidance, stereotypic movements, echolalia, and sensory processing differences. ASD is present in 50–70% of individuals with FXS [91], and epilepsy is present in 10–20% of individuals and begins between ages 4 and 10 years [92]. The physical and behavioral features seen in males with FXS are present in females with the full mutation but are typically less severe. The female phenotype is more commonly associated with learning disabilities, behavioral problems, anxiety, depression, shyness, and difficulties in establishing social interactions [93], and about half of females with FXS are diagnosed with intellectual disability [91].
The differences in phenotype among females with full FMR1 mutations can be attributed to differences in X-chromosomal inactivation as shown in a case study of three females with the full mutation from the same family [94]. Patient III-1 had complete inactivation of the normal allele and physical traits of FXS and presented with hand-flapping, short attention span, tactile defensiveness, shyness, and poor eye contact. Less than 10% of the normal allele was inactive in patient II-1 who presented with normal intelligence, while 50% of the normal allele was inactive in patient II-2, and she presented with mild physical traits and intellectual disability [94]. Another case study described two sisters with FMR1 full mutations with different fragile X phenotypes. One sister had severe intellectual disability and phenotypic traits like those observed in males with FXS. She had complete inactivation of the normal X chromosome, while her sister with learning disabilities had the normal X chromosome active in 70% of her cells [95]. Martorell et al. described a consanguineous Moroccan family in which the four sisters were compound heterozygote for full and pre-mutation in FMR1. The proband had complete inactivation of normal X chromosome and presented with autistic-like features and had severe intellectual disability, while the sisters had a random XCI pattern and had learning disabilities and emotional problems with mildly affected IQ [96]. These findings support the hypothesis that the different phenotypes in female carriers with full mutations are primarily caused by unequal X-chromosomal inactivation.
4.2. FMR1 Premutation
4.2.1. Premature Ovarian Insufficiency (POI)
Female carriers of the fragile X premutation have an increased risk for development of premature ovarian insufficiency (POI), a condition in which women experience infertility, irregular menstruation, and menopause prior to 40 years old. Although it has been hypothesized that the development of POI in fragile X premutation carriers is due to skewed XCI, this has not been supported by published literature. Using the polymorphic androgen receptor (AR) gene assay, Spath et al. compared the inactivation patterns in female premutation carriers with POI (n = 37) to those of female premutation carriers without POI (n = 64) and women with idiopathic POI (n = 25). They found that the degree of skewed XCI did not differ significantly between female premutation carriers with POI, female premutation carriers without POI, and females with idiopathic POI [97]. Similarly, Rodriguez-Revenga et al., using the same methodologic approach, compared the XCI patterns from 220 control female samples, 40 female premutation carriers with POI, and 220 female premutation carriers without POI. Their results showed no significant difference in the prevalence of skewed XCI among non-POI and POI FMR1 premutation carriers [98]. These findings were further substantiated by a study of monozygotic twins with similar sized FMR1 premutations who had discordant phenotypes for POI and similar X-inactivation ratios [99]. The idea that the development of POI is related to CGG repeat size was proposed by Sullivan et al. In their study of 507 women, they showed that repeat sizes in the medium premutation range (80–99 repeats) were associated with the highest risk for POI, and the risk of developing POI appears to plateau, or perhaps decrease, among women with very high repeats (>or =100 repeats) [100].
4.2.2. Fragile X-Associated Tremor/Ataxia Syndrome (FXTAS)
Fragile X-associated tremor/ataxia syndrome (FXTAS) is a late-onset neurodegenerative disorder that is characterized by intention tremor and gait ataxia, with more variable features of parkinsonism, short-term memory problems, and deficits in executive function. FXTAS symptoms typically affect people over the age of 50 and worsen with age. Male premutation carriers are generally more frequently and severely affected than females. XCI may play a role in facilitating the phenotypic differences between males and females with FXTAS, and it has been hypothesized that the disease severity is inversely related to the activation ratio (AR) for the normal FMR1 allele. Although the data are limited, two case series describe sisters with similar premutation size FMR1 alleles and found that the sisters with the lowest AR of the normal allele had the most severe FXTAS symptoms, while the sisters with the highest AR had no signs of FXTAS [101,102]. These studies suggest that the AR may play a role in the development of FXTAS and its severity in premutation carrier women.
4.2.3. Children with FMR1 Premutation
FMR1 premutation research has primarily focused on FXTAS and POI in adults; however, children with a FMR1 premutation are also at increased risk for several health concerns. Bailey et al. reported on 256 children with a FMR1 premutation and their co-occurring conditions. Premutation males, when compared with the control group, were more likely to have developmental delay, attention problems, aggression, seizures, ASD, and anxiety, while premutation females were more likely to have attention problems, anxiety, depression, and developmental delay [103]. These findings are corroborated by Renda et al. [104] and Farzin et al. [105] but were in contrast to work by Myers et al. [106]. In their study of 28 children, they found no significant difference between children with and without the premutation. Although several medical conditions seem to be related to the FMR1 premutation in children, none of the studies published to date have examined the effect of XCI, thus further study is needed.
5. Conclusions
Symptoms of X-linked disorders are variable among females, with some presenting the full disease phenotype, while others present with a milder phenotype or as asymptomatic carriers. Skewing of XCI provides a mechanism for the diversity of phenotypes observed in X-linked disorders, as shown by our discussion of MECP2- and FMR1-related disorders; however, it does not account for all phenotypic variability as seen in the cases of POI. The lessons learned from these disorders can be extended to other X-linked NDDs, as shown in Table 1, where the phenotypic expression of many X-linked genes is regulated by XCI. Skewed XCI may be required for survival, as it is observed in a majority of heterozygous females [107]; however, the impact of skewed XCI on phenotype is not well understood. The studies included in this review have primarily relied on blood for XCI studies. While blood is the most assessable tissue, the pattern of XCI may not correlate well with XCI in the brain. This, in addition to the small sample size are limitations of the XCI studies and indicates that XCI pattern in blood is not a useful predictor of phenotype.
Despite these challenges, targeted reactivation of genes on the inactive X chromosome could represent a therapeutic approach in heterozygous females affected by X-linked diseases, and several groups are exploring this possibility in rodent models and in-vitro cell lines [108,109]. Sex chromosomal dosage compensation is an important developmental process, and disturbing XCI could have severe consequences for females since overexpression of genes, such as MECP2, results in MECP2 duplication syndrome. While more work remains to be done, these preliminary studies show promise and may lead to meaningful interventions.
Acknowledgments
The authors would like to thank Anna Chassevent for assistance with database queries.
Author Contributions
B.A.B., A.E.B., and C.L.S.-H. contributed to the research and writing of this manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare that there are no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Laumonnier F., Cuthbert P.C., Grant S.G.N. The Role of Neuronal Complexes in Human X-Linked Brain Diseases. Am. J. Hum. Genet. 2007;80:205–220. doi: 10.1086/511441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Firth H.V., Richards S.M., Bevan A.P., Clayton S., Corpas M., Rajan D., Vooren S.V., Moreau Y., Pettett R.M., Carter N.P. DECIPHER: Database of Chromosomal Imbalance and Phenotype in Humans Using Ensembl Resources. Am. J. Hum. Genet. 2009;84:524–533. doi: 10.1016/j.ajhg.2009.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ross M.T., Grafham D.V., Coffey A.J., Scherer S., McLay K., Muzny D., Platzer M., Howell G.R., Burrows C., Bird C.P., et al. The DNA Sequence of the Human X Chromosome. Nature. 2005;434:325–337. doi: 10.1038/nature03440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Online Mendelian Inheritance in Man, OMIM®. [(accessed on 24 April 2021)]; Available online: https://www.omim.org/
- 5.Migeon B.R. X-Linked Diseases: Susceptible Females. Genet. Med. 2020;22:1156–1174. doi: 10.1038/s41436-020-0779-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carrel L., Willard H.F. X-Inactivation Profile Reveals Extensive Variability in X-Linked Gene Expression in Females. Nature. 2005;434:400–404. doi: 10.1038/nature03479. [DOI] [PubMed] [Google Scholar]
- 7.Tukiainen T., Villani A.C., Yen A., Rivas M.A., Marshall J.L., Satija R., Aguirre M., Gauthier L., Fleharty M., MacArthur D.G., et al. Landscape of X chromosome inactivation across human tissues. Nature. 2017;550:244–248. doi: 10.1038/nature24265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Salsano E., Tabano S., Sirchia S.M., Colapietro P., Castellotti B., Gellera C., Rimoldi M., Pensato V., Mariotti C., Pareyson D., et al. Preferential Expression of Mutant ABCD1 Allele Is Common in Adrenoleukodystrophy Female Carriers but Unrelated to Clinical Symptoms. Orphanet J. Rare Dis. 2012;7:10. doi: 10.1186/1750-1172-7-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang Z., Yan A., Lin Y., Xie H., Zhou C., Lan F. Familial Skewed x Chromosome Inactivation in Adrenoleukodystrophy Manifesting Heterozygotes from a Chinese Pedigree. PLoS ONE. 2013;8:e57977. doi: 10.1371/journal.pone.0057977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mellen M., Ayata P., Dewell S., Kriaucionis S., Heintz N. MeCP2 Binds to 5hmc Enriched within Active Genes and Accessible Chromatin in the Nervous System. Cell. 2012;151:1417–1430. doi: 10.1016/j.cell.2012.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pandolfo M., Rai M., Remiche G., Desmyter L., Vandernoot I. Cerebellar Ataxia, Neuropathy, Hearing Loss, and Intellectual Disability due to AIFM1 Mutation. Neurol. Genet. 2020;6:e420. doi: 10.1212/NXG.0000000000000420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hamici S., Bastaki F., Khalifa M. Exome Sequence Identified a c.320A > G ALG13 Variant in a Female with Infantile Epileptic Encephalopathy with Normal Glycosylation and Random X Inactivation: Review of the Literature. Eur. J. Med. Genet. 2017;60:541–547. doi: 10.1016/j.ejmg.2017.07.014. [DOI] [PubMed] [Google Scholar]
- 13.Ghesh L., Besnard T., Nizon M., Trochu E., Landeau-Trottier G., Breheret F., Thauvin-Robinet C., Bruel A.L., Kuentz P., Coubes C., et al. Loss-of-Function Variants in ARHGEF9 are Associated with an X-Linked Intellectual Disability Dominant Disorder. Hum. Mutat. 2021;42:498–505. doi: 10.1002/humu.24188. [DOI] [PubMed] [Google Scholar]
- 14.Conti V., Marini C., Gana S., Sudi J., Dobyns W.B., Guerrini R. Corpus Callosum Agenesis, Severe Mental Retardation, Epilepsy, and Dyskinetic Quadriparesis due to a Novel Mutation in the Homeodomain of ARX. Am. J. Med. Genet. A. 2011;155A:892–897. doi: 10.1002/ajmg.a.33923. [DOI] [PubMed] [Google Scholar]
- 15.Wada T., Sugie H., Fukushima Y., Saitoh S. Non-Skewed X-Inactivation may Cause Mental Retardation in a Female Carrier of X-Linked Alpha-Thalassemia/Mental Retardation Syndrome (ATR-X): X-Inactivation Study of Nine Female Carriers of ATR-X. Am. J. Med. Genet. A. 2005;138:18–20. doi: 10.1002/ajmg.a.30901. [DOI] [PubMed] [Google Scholar]
- 16.Badens C., Martini N., Courrier S., DesPortes V., Touraine R., Levy N., Edery P. ATRX Syndrome in a Girl with a Heterozygous Mutation in the ATRX Zn Finger Domain and a Totally Skewed X-Inactivation Pattern. Am. J. Med. Genet. A. 2006;140:2212–2215. doi: 10.1002/ajmg.a.31400. [DOI] [PubMed] [Google Scholar]
- 17.Seto T., Hamazaki T., Nishigaki S., Kudo S., Shintaku H., Ondo Y., Shimojima K., Yamamoto T. A Novel CASK Mutation Identified in Siblings Exhibiting Developmental Disorders with/without Microcephaly. Intractable Rare Dis Res. 2017;6:177–182. doi: 10.5582/irdr.2017.01031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhao Y., Zhang X., Bao X., Zhang Q., Zhang J., Cao G., Zhang J., Li J., Wei L., Pan H., et al. Clinical Features and Gene Mutational Spectrum of CDKL5-Related Diseases in a Cohort of Chinese Patients. BMC Med. Genet. 2014;15:24. doi: 10.1186/1471-2350-15-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Palmer E.E., Stuhlmann T., Weinert S., Haan E., Van Esch H., Holvoet M., Boyle J., Leffler M., Raynaud M., Moraine C., et al. De Novo and Inherited Mutations in the X-Linked Gene CLCN4 are Associated with Syndromic Intellectual Disability and Behavior and Seizure Disorders in Males and Females. Mol Psychiatry. 2018;23:222–230. doi: 10.1038/mp.2016.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Polla D.L., Saunders H.R., de Vries B.B.A., van Bokhoven H., de Brouwer A.P.M. A De Novo Variant in the X-Linked Gene CNKSR2 is Associated with Seizures and Mild Intellectual Disability in a Female Patient. Mol. Genet Genomic Med. 2019;7:e00861. doi: 10.1002/mgg3.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zou Y., Liu Q., Chen B., Zhang X., Guo C., Zhou H., Li J., Gao G., Guo Y., Yan C., et al. Mutation in CUL4B, which Encodes a Member of Cullin-RING Ubiquitin Ligase Complex, Causes X-Linked Mental Retardation. Am. J. Hum. Genet. 2007;80:561–566. doi: 10.1086/512489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rocha M.E., Silveira T.R.D., Sasaki E., Sás D.M., Lourenço C.M., Kandaswamy K.K., Beetz C., Rolfs A., Bauer P., Reardon W., et al. Novel Clinical and Genetic Insight into CXorf56-Associated Intellectual Disability. Eur. J. Hum. Genet. 2020;28:367–372. doi: 10.1038/s41431-019-0558-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guerrini R., Moro F., Andermann E., Hughes E., D’Agostino D., Carrozzo R., Bernasconi A., Flinter F., Parmeggiani L., Volzone A., et al. Nonsyndromic Mental Retardation and Cryptogenic Epilepsy in Women with Doublecortin Gene Mutations. Ann. Neurol. 2003;54:30–37. doi: 10.1002/ana.10588. [DOI] [PubMed] [Google Scholar]
- 24.Wainer Katsir K., Linial M. Human Genes Escaping X-Inactivation Revealed by Single Cell Expression Data. BMC Genom. 2019;20:201. doi: 10.1186/s12864-019-5507-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gieldon L., Mackenroth L., Betcheva-Krajcir E., Rump A., Beck-Wödl S., Schallner J., Di Donato N., Schröck E., Tzschach A. Skewed X-Inactivation in a Family with DLG3-Associated X-Linked Intellectual Disability. Am. J. Med. Genet. A. 2017;173:2545–2550. doi: 10.1002/ajmg.a.38348. [DOI] [PubMed] [Google Scholar]
- 26.Viggiano E., Ergoli M., Picillo E., Politano L. Determining the Role of Skewed X-Chromosome Inactivation in Developing Muscle Symptoms in Carriers of Duchenne Muscular Dystrophy. Hum. Genet. 2016;135:685–698. doi: 10.1007/s00439-016-1666-6. [DOI] [PubMed] [Google Scholar]
- 27.Kirchgessner C.U., Warren S.T., Willard H.F. X Inactivation of the FMR1 Fragile X Mental Retardation Gene. J. Med. Genet. 1995;32:925–929. doi: 10.1136/jmg.32.12.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Alvarez-Mora M.I., Rodriguez-Revenga L., Feliu A., Badenas C., Madrigal I., Milà M. Skewed X Inactivation in Women Carrying the FMR1 Premutation and Its Relation with Fragile-X-Associated Tremor/Ataxia Syndrome. Neurodegener Dis. 2016;16:290–292. doi: 10.1159/000441566. [DOI] [PubMed] [Google Scholar]
- 29.Miranda-Furtado C.L., Luchiari H.R., Chielli Pedroso D.C., Kogure G.S., Caetano L.C., Santana B.A., Santana V.P., Benetti-Pinto C.L., Reis F.M., Maciel M.A., et al. Skewed X-Chromosome Inactivation and Shorter Telomeres Associate with Idiopathic Premature Ovarian Insufficiency. Fertil. Steril. 2018;110:476–485. doi: 10.1016/j.fertnstert.2018.04.017. [DOI] [PubMed] [Google Scholar]
- 30.Bonnet C., Leheup B., Béri M., Philippe C., Grégoire M.J., Jonveaux P. Aberrant GRIA3 Transcripts with Multi-Exon Duplications in a Family with X-Linked Mental Retardation. Am. J. Med. Genet. A. 2009;149A:1280–1289. doi: 10.1002/ajmg.a.32858. [DOI] [PubMed] [Google Scholar]
- 31.Schirwani S., Novelli A., Digilio M.C., Bourn D., Wilson V., Roberts C., Dallapiccola B., Hobson E. Duplications of GPC3 and GPC4 Genes in Symptomatic Female Carriers of Simpson-Golabi-Behmel Syndrome type 1. Eur. J. Med. Genet. 2019;62:243–247. doi: 10.1016/j.ejmg.2018.07.022. [DOI] [PubMed] [Google Scholar]
- 32.Harakalova M., van den Boogaard M.J., Sinke R., van Lieshout S., van Tuil M.C., Duran K., Renkens I., Terhal P.A., de Kovel C., Nijman I.J., et al. X-Exome Sequencing Identifies a HDAC8 Variant in a Large Pedigree with X-Linked Intellectual Disability, Truncal Obesity, Gynaecomastia, Hypogonadism and Unusual Face. J. Med. Genet. 2012;49:539–543. doi: 10.1136/jmedgenet-2012-100921. [DOI] [PubMed] [Google Scholar]
- 33.Wolf S.F., Jolly D.J., Lunnen K.D., Friedmann T., Migeon B.R. Methylation of the Hypoxanthine Phosphoribosyltransferase Locus on the Human X Chromosome: Implications for X-Chromosome Inactivation. Proc. Natl. Acad. Sci. USA. 1984;81:2806–2810. doi: 10.1073/pnas.81.9.2806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wayhelova M., Ryzí M., Oppelt J., Hladilkova E., Vallova V., Krskova L., Vilemova M., Polackova H., Gaillyova R., Kuglik P. Novel Familial IQSEC2 Pathogenic Sequence Variant Associated with Neurodevelopmental Disorders and Epilepsy. Neurogenetics. 2020;21:269–278. doi: 10.1007/s10048-020-00616-3. [DOI] [PubMed] [Google Scholar]
- 35.Ounap K., Puusepp-Benazzouz H., Peters M., Vaher U., Rein R., Proos A., Field M., Reimand T. A Novel c.2T > C Mutation of the KDM5C/JARID1C Gene in One Large Family with X-Linked Intellectual Disability. Eur. J. Med. Genet. 2012;55:178–184. doi: 10.1016/j.ejmg.2012.01.004. [DOI] [PubMed] [Google Scholar]
- 36.Mignon-Ravix C., Cacciagli P., Choucair N., Popovici C., Missirian C., Milh M., Mégarbané A., Busa T., Julia S., Girard N., et al. Intragenic Rearrangements in X-Linked Intellectual Deficiency: Results of a-CGH in a Series of 54 Patients and Identification of TRPC5 and KLHL15 as Potential XLID Genes. Am. J. Med. Genet. A. 2014;164A:1991–1997. doi: 10.1002/ajmg.a.36602. [DOI] [PubMed] [Google Scholar]
- 37.Stabellini R., Vasques L.R., de Mello J.C., Hernandes L.M., Pereira L.V. MAOA and GYG2 are Submitted to X Chromosome Inactivation in Human Fibroblasts. Epigenetics. 2009;4:388–393. doi: 10.4161/epi.4.6.9492. [DOI] [PubMed] [Google Scholar]
- 38.Gong X., Bacchelli E., Blasi F., Toma C., Betancur C., Chaste P., Delorme R., Durand C.M., Fauchereau F., Botros H.G., et al. Analysis of X Chromosome Inactivation in Autism Spectrum Disorders. Am. J. Med. Genet. B Neuropsychiatr Genet. 2008;147B:830–835. doi: 10.1002/ajmg.b.30688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xiol C., Vidal S., Pascual-Alonso A., Blasco L., Brandi N., Pacheco P., Gerotina E., O’Callaghan M., Pineda M., Armstrong J. X Chromosome Inactivation Does Not Necessarily Determine the Severity of the Phenotype in Rett Syndrome Patients. Sci. Rep. 2019;9:11983. doi: 10.1038/s41598-019-48385-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li X., Xie H., Chen Q., Yu X., Yi Z., Li E., Zhang T., Wang J., Zhong J., Chen X. Clinical and Molecular Genetic Characterization of Familial MECP2 Duplication Syndrome in a Chinese Family. BMC Med. Genet. 2017;18:131. doi: 10.1186/s12881-017-0486-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li D., Strong A., Shen K.M., Cassiman D., Van Dyck M., Linhares N.D., Valadares E.R., Wang T., Pena S.D.J., Jaeken J., et al. De Novo Loss-of-Function Variants in X-Linked MED12 are Associated with Hardikar Syndrome in Females. Genet. Med. 2021;23:637–644. doi: 10.1038/s41436-020-01031-7. [DOI] [PubMed] [Google Scholar]
- 42.De Lange I.M., Helbig K.L., Weckhuysen S., Møller R.S., Velinov M., Dolzhanskaya N., Marsh E., Helbig I., Devinsky O., Tang S., et al. De novo Mutations of KIAA2022 in Females Cause Intellectual Disability and Intractable Epilepsy. J. Med. Genet. 2016;53:850–858. doi: 10.1136/jmedgenet-2016-103909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lambert N., Dauve C., Ranza E., Makrythanasis P., Santoni F., Sloan-Béna F., Gimelli S., Blouin J.L., Guipponi M., Bottani A., et al. Novel NEXMIF Pathogenic Variant in a Boy with Severe Autistic Features, Intellectual Disability, and Epilepsy, and His Mildly Affected Mother. J. Hum. Genet. 2018;63:847–850. doi: 10.1038/s10038-018-0459-2. [DOI] [PubMed] [Google Scholar]
- 44.Vianna E.Q., Piergiorge R.M., Gonçalves A.P., Dos Santos J.M., Calassara V., Rosenberg C., Krepischi A.C.V., Boy da Silva R.T., Dos Santos S.R., Ribeiro M.G., et al. Understanding the Landscape of X-linked Variants Causing Intellectual Disability in Females Through Extreme X Chromosome Inactivation Skewing. Mol. Neurobiol. 2020;57:3671–3684. doi: 10.1007/s12035-020-01981-8. [DOI] [PubMed] [Google Scholar]
- 45.Hittner H.M., Carroll A.J., Prchal J.T. Linkage Studies in Carriers of Lowe Oculo-Cerebro-Renal Syndrome. Am. J. Hum. Genet. 1982;34:966–971. [PMC free article] [PubMed] [Google Scholar]
- 46.Iijima T., Hayami N., Takaichi K., Morisada N., Nozu K., Iijima K., Sawa N., Hoshino J., Ubara Y. An Orofaciodigital Syndrome 1 Patient and Her Mother Carry the Same OFD1 Mutation but Have Different X Chromosome Inactivation Patterns. Intern Med. 2019;58:2989–2992. doi: 10.2169/internalmedicine.2571-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Olivier-Van Stichelen S., Hanover J.A. X-inactivation Normalizes O-GlcNAc Transferase Levels and Generates an O-GlcNAc-Depleted Barr Body. Front Genet. 2014;5:256. doi: 10.3389/fgene.2014.00256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Santos-Rebouças C.B., Belet S., Guedes de Almeida L., Ribeiro M.G., Medina-Acosta E., Bahia P.R., Alves da Silva A.F., Lima dos Santos F., Borges de Lacerda G.C., Pimentel M.M., et al. A Novel in-Frame Deletion Affecting the BAR Domain of OPHN1 in a Family with Intellectual Disability and Hippocampal Alterations. Eur. J. Hum. Genet. 2014;22:644–651. doi: 10.1038/ejhg.2013.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vincent A.K., Noor A., Janson A., Minassian B.A., Ayub M., Vincent J.B., Morel C.F. Identification of Genomic Deletions Spanning the PCDH19 Gene in Two Unrelated Girls with Intellectual Disability and Seizures. Clin. Genet. 2012;82:540–545. doi: 10.1111/j.1399-0004.2011.01812.x. [DOI] [PubMed] [Google Scholar]
- 50.Horga A., Woodward C.E., Mills A., Pareés I., Hargreaves I.P., Brown R.M., Bugiardini E., Brooks T., Manole A., Remzova E., et al. Differential Phenotypic Expression of a Novel PDHA1 Mutation in a Female Monozygotic Twin Pair. Hum. Genet. 2019;138:1313–1322. doi: 10.1007/s00439-019-02075-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Crawford J., Lower K.M., Hennekam R.C., Van Esch H., Mégarbané A., Lynch S.A., Turner G., Gécz J. Mutation Screening in Borjeson-Forssman-Lehmann Syndrome: Identification of a Novel De Novo PHF6 Mutation in a Female Patient. J. Med. Genet. 2006;43:238–243. doi: 10.1136/jmg.2005.033084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lower K.M., Turner G., Kerr B.A., Mathews K.D., Shaw M.A., Gedeon A.K., Schelley S., Hoyme H.E., White S.M., Delatycki M.B., et al. Mutations in PHF6 are Associated with Börjeson-Forssman-Lehmann Syndrome. Nat. Genet. 2002;32:661–665. doi: 10.1038/ng1040. [DOI] [PubMed] [Google Scholar]
- 53.Berland S., Alme K., Brendehaug A., Houge G., Hovland R. PHF6 Deletions May Cause Borjeson-Forssman-Lehmann Syndrome in Females. Mol. Syndromol. 2011;1:294–300. doi: 10.1159/000330111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Qiao Y., Liu X., Harvard C., Hildebrand M.J., Rajcan-Separovic E., Holden J.J., Lewis M.E. Autism-Associated Familial Microdeletion of Xp11.22. Clin. Genet. 2008;74:134–144. doi: 10.1111/j.1399-0004.2008.01028.x. [DOI] [PubMed] [Google Scholar]
- 55.Woodward K., Kirtland K., Dlouhy S., Raskind W., Bird T., Malcolm S., Abeliovich D. X Inactivation Phenotype in Carriers of Pelizaeus-Merzbacher Disease: Skewed in Carriers of a Duplication and Random in Carriers of Point Mutations. Eur. J. Hum. Genet. 2000;8:449–454. doi: 10.1038/sj.ejhg.5200480. [DOI] [PubMed] [Google Scholar]
- 56.Inoue K., Osaka H., Thurston V.C., Clarke J.T., Yoneyama A., Rosenbarker L., Bird T.D., Hodes M.E., Shaffer L.G., Lupski J.R. Genomic Rearrangements Resulting in PLP1 Deletion Occur by Nonhomologous End Joining and Cause Different Dysmyelinating Phenotypes in Males and Females. Am. J. Hum. Genet. 2002;71:838–853. doi: 10.1086/342728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang X., Reid Sutton V., Omar Peraza-Llanes J., Yu Z., Rosetta R., Kou Y.C., Eble T.N., Patel A., Thaller C., Fang P., et al. Mutations in X-linked PORCN, a Putative Regulator of Wnt Signaling, Cause Focal Dermal Hypoplasia. Nat. Genet. 2007;39:836–838. doi: 10.1038/ng2057. [DOI] [PubMed] [Google Scholar]
- 58.Grzeschik K.H., Bornholdt D., Oeffner F., König A., del Carmen Boente M., Enders H., Fritz B., Hertl M., Grasshoff U., Höfling K., et al. Deficiency of PORCN, a Regulator of Wnt Signaling, is Associated with Focal Dermal Hypoplasia. Nat. Genet. 2007;39:833–835. doi: 10.1038/ng2052. [DOI] [PubMed] [Google Scholar]
- 59.Fichera M., Falco M., Lo Giudice M., Castiglia L., Guarnaccia V., Calì F., Spalletta A., Scuderi C., Avola E. Skewed X-Inactivation in a Family with Mental Retardation and PQBP1 Gene Mutation. Clin. Genet. 2005;67:446–447. doi: 10.1111/j.1399-0004.2005.00436.x. [DOI] [PubMed] [Google Scholar]
- 60.Cho R.Y., Peñaherrera M.S., Du Souich C., Huang L., Mwenifumbo J., Nelson T.N., Elliott A.M., Adam S., CAUSES Study. Eydoux P., et al. Renpenning Syndrome in a Female. Am. J. Med. Genet. A. 2020;182:498–503. doi: 10.1002/ajmg.a.61451. [DOI] [PubMed] [Google Scholar]
- 61.Synofzik M., Müller vom Hagen J., Haack T.B., Wilhelm C., Lindig T., Beck-Wödl S., Nabuurs S.B., van Kuilenburg A.B., de Brouwer A.P., Schöls L. X-Linked Charcot-Marie-Tooth Disease, Arts Syndrome, and Prelingual Non-Syndromic Deafness form a Disease Continuum: Evidence from a Family with a Novel PRPS1 Mutation. Orphanet J. Rare Dis. 2014;9:24. doi: 10.1186/1750-1172-9-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Frints S.G.M., Ozanturk A., Rodríguez Criado G., Grasshoff U., de Hoon B., Field M., Manouvrier-Hanu S., E Hickey S., Kammoun M., Gripp K.W., et al. Pathogenic Variants in E3 Ubiquitin Ligase RLIM/RNF12 Lead to a Syndromic X-Linked Intellectual Disability and Behavior Disorder. Mol. Psychiatry. 2019;24:1748–1768. doi: 10.1038/s41380-018-0065-x. [DOI] [PubMed] [Google Scholar]
- 63.Simensen R.J., Abidi F., Collins J.S., Schwartz C.E., Stevenson R.E. Cognitive Function in Coffin-Lowry Syndrome. Clin. Genet. 2002;61:299–304. doi: 10.1034/j.1399-0004.2002.610410.x. [DOI] [PubMed] [Google Scholar]
- 64.Kodera H., Nakamura K., Osaka H., Maegaki Y., Haginoya K., Mizumoto S., Kato M., Okamoto N., Iai M., Kondo Y., et al. De Novo Mutations in SLC35A2 Encoding a UDP-Galactose Transporter Cause Early-Onset Epileptic Encephalopathy. Hum. Mutat. 2013;34:1708–1714. doi: 10.1002/humu.22446. [DOI] [PubMed] [Google Scholar]
- 65.Van de Kamp J.M., Mancini G.M., Pouwels P.J., Betsalel O.T., van Dooren S.J., de Koning I., Steenweg M.E., Jakobs C., van der Knaap M.S., Salomons G.S. Clinical Features and X-Inactivation in Females Heterozygous for Creatine Transporter Defect. Clin. Genet. 2011;79:264–272. doi: 10.1111/j.1399-0004.2010.01460.x. [DOI] [PubMed] [Google Scholar]
- 66.Gilfillan G.D., Selmer K.K., Roxrud I., Smith R., Kyllerman M., Eiklid K., Kroken M., Mattingsdal M., Egeland T., Stenmark H., et al. SLC9A6 Mutations Cause X-Linked Mental Retardation, Microcephaly, Epilepsy, and Ataxia, a Phenotype Mimicking Angelman Syndrome. Am. J. Hum. Genet. 2008;82:1003–1010. doi: 10.1016/j.ajhg.2008.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.O’Rawe J.A., Wu Y., Dörfel M.J., Rope A.F., Au P.Y., Parboosingh .J.S., Moon S., Kousi M., Kosma K., Smith C.S., et al. TAF1 Variants Are Associated with Dysmorphic Features, Intellectual Disability, and Neurological Manifestations. Am. J. Hum. Genet. 2015;97:922–932. doi: 10.1016/j.ajhg.2015.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kumar R., Corbett M.A., van Bon B.W., Woenig J.A., Weir L., Douglas E., Friend K.L., Gardner A., Shaw M., Jolly L.A., et al. THOC2 Mutations Implicate mRNA-Export Pathway in X-Linked Intellectual Disability. Am. J. Hum. Genet. 2015;97:302–310. doi: 10.1016/j.ajhg.2015.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Nascimento R.M., Otto P.A., de Brouwer A.P., Vianna-Morgante A.M. UBE2A, which Encodes a Ubiquitin-Conjugating Enzyme, is Mutated in a Novel X-Linked Mental Retardation Syndrome. Am. J. Hum. Genet. 2006;79:549–555. doi: 10.1086/507047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Laumonnier F., Shoubridge C., Antar C., Nguyen L.S., Van Esch H., Kleefstra T., Briault S., Fryns J.P., Hamel B., Chelly J., et al. Mutations of the UPF3B Gene, which Encodes a Protein Widely Expressed in Neurons, are Associated with Nonspecific Mental Retardation with or without Autism. Mol. Psychiatry. 2010;15:767–776. doi: 10.1038/mp.2009.14. [DOI] [PubMed] [Google Scholar]
- 71.Haack T.B., Hogarth P., Kruer M.C., Gregory A., Wieland T., Schwarzmayr T., Graf E., Sanford L., Meyer E., Kara E., et al. Exome Sequencing Reveals De Novo WDR45 Mutations Causing a Phenotypically Distinct, X-Linked Dominant form of NBIA. Am. J. Hum. Genet. 2012;91:1144–1149. doi: 10.1016/j.ajhg.2012.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Srivastava S., Sahin M., Prock L. Chapter 22—Translational Medicine Strategies in Drug Development for Neurodevelopmental Disorders. In: Nomikos G.G., Feltner D.E., editors. Handbook of Behavioral Neuroscience. Volume 29. Translational Medicine in CNS Drug Development; Elsevier; Amsterdam, The Netherlands: 2019. pp. 309–331. [Google Scholar]
- 73.Orrico A., Lam C.-W., Galli L., Dotti M.T., Hayek G., Tong S.-F., Poon P.M.K., Zappella M., Federico A., Sorrentino V. MECP2 Mutation in Male Patients with Non-Specific X-Linked Mental Retardation. FEBS Lett. 2000;481:285–288. doi: 10.1016/S0014-5793(00)01994-3. [DOI] [PubMed] [Google Scholar]
- 74.Rett Syndrome. [(accessed on 8 June 2020)]; Available online: https://rarediseases.org/rare-diseases/rett-syndrome/
- 75.Weaving L.S., Williamson S.L., Bennetts B., Davis M., Ellaway C.J., Leonard H., Thong M.-K., Delatycki M., Thompson E.M., Laing N., et al. Effects of MECP2 Mutation Type, Location and X-Inactivation in Modulating Rett Syndrome Phenotype. Am. J. Med. Genet. A. 2002;118A:103–114. doi: 10.1002/ajmg.a.10053. [DOI] [PubMed] [Google Scholar]
- 76.Amir R.E., Veyver I.B.V.D., Schultz R., Malicki D.M., Tran C.Q., Dahle E.J., Philippi A., Timar L., Percy A.K., Motil K.J., et al. Influence of Mutation Type and X Chromosome Inactivation on Rett Syndrome Phenotypes. Ann. Neurol. 2000;47:670–679. doi: 10.1002/1531-8249(200005)47:5<670::AID-ANA20>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 77.Cheadle J.P., Gill H., Fleming N., Maynard J., Kerr A., Leonard H., Krawczak M., Cooper D.N., Lynch S., Thomas N., et al. Long-Read Sequence Analysis of the MECP2 Gene in Rett Syndrome Patients: Correlation of Disease Severity with Mutation Type and Location. Hum. Mol. Genet. 2000;9:1119–1129. doi: 10.1093/hmg/9.7.1119. [DOI] [PubMed] [Google Scholar]
- 78.Weaving L., Ellaway C., Gecz J., Christodoulou J. Rett Syndrome: Clinical Review and Genetic Update. J. Med. Genet. 2005;42:1–7. doi: 10.1136/jmg.2004.027730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zhang Q., Zhao Y., Bao X., Luo J., Zhang X., Li J., Wei L., Wu X. Familial Cases and Male Cases with MECP2 Mutations. Am. J. Med. Genet. Part B Neuropsychiatr. Genet. Off. Publ. Int. Soc. Psychiatr. Genet. 2017;174:451–457. doi: 10.1002/ajmg.b.32534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bao X., Jiang S., Song F., Pan H., Li M., Wu X.R. X chromosome inactivation in Rett Syndrome and its correlations with MECP2 mutations and phenotype. J. Child Neurol. 2008;23:22–25. doi: 10.1177/0883073807307077. [DOI] [PubMed] [Google Scholar]
- 81.MECP2 Duplication Syndrome. [(accessed on 23 April 2021)]; Available online: https://rarediseases.org/rare-diseases/mecp2-duplication-syndrome/
- 82.Schwoerer J.S., Laffin J., Haun J., Raca G., Friez M.J., Giampietro P.F. MECP2 Duplication: Possible Cause of Severe Phenotype in Females. Am. J. Med. Genet. A. 2014;164:1029–1034. doi: 10.1002/ajmg.a.36380. [DOI] [PubMed] [Google Scholar]
- 83.Ramocki M.B., Tavyev Y.J., Peters S.U. The MECP2 Duplication Syndrome. Am. J. Med. Genet. A. 2010;152A:1079–1088. doi: 10.1002/ajmg.a.33184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Van Esch H., Bauters M., Ignatius J., Jansen M., Raynaud M., Hollanders K., Lugtenberg D., Bienvenu T., Jensen L.R., Gécz J., et al. Duplication of the MECP2 Region Is a Frequent Cause of Severe Mental Retardation and Progressive Neurological Symptoms in Males. Am. J. Hum. Genet. 2005;77:442–453. doi: 10.1086/444549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ramocki M.B., Peters S.U., Tavyev Y.J., Zhang F., Carvalho C.M., Schaaf C.P., Richman R., Fang P., Glaze D.G., Lupski J.R., et al. Autism and other neuropsychiatric symptoms are prevalent in individuals with MeCP2 duplication syndrome. Ann. Neurol. 2009;66:771–782. doi: 10.1002/ana.21715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Grasshoff U., Bonin M., Goehring I., Ekici A., Dufke A., Cremer K., Wagner N., Rossier E., Jauch A., Walter M., et al. De novo MECP2 duplication in two females with random X-inactivation and moderate mental retardation. Eur. J. Hum. Genet. EJHG. 2011;19:507–512. doi: 10.1038/ejhg.2010.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bijlsma E.K., Collins A., Papa F.T., Tejada M.I., Wheeler P., Peeters E.A., Gijsbers A.C., van de Kamp J.M., Kriek M., Losekoot M., et al. Xq28 duplications including MECP2 in five females: Expanding the phenotype to severe mental retardation. Eur. J. Med. Genet. 2012;55:404–413. doi: 10.1016/j.ejmg.2012.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bagni C., Oostra B.A. Fragile X Syndrome: From Protein Function to Therapy. Am. J. Med. Genet. A. 2013;161:2809–2821. doi: 10.1002/ajmg.a.36241. [DOI] [PubMed] [Google Scholar]
- 89.Nolin S.L., Glicksman A., Ding X., Ersalesi N., Brown W.T., Sherman S.L., Dobkin C. Fragile X Analysis of 1112 Prenatal Samples from 1991 to 2010. Prenat. Diagn. 2011;31:925–931. doi: 10.1002/pd.2815. [DOI] [PubMed] [Google Scholar]
- 90.Hunter J., Rivero-Arias O., Angelov A., Kim E., Fotheringham I., Leal J. Epidemiology of Fragile X Syndrome: A Systematic Review and Meta-Analysis. Am. J. Med. Genet. A. 2014;164:1648–1658. doi: 10.1002/ajmg.a.36511. [DOI] [PubMed] [Google Scholar]
- 91.Hunter J.E., Berry-Kravis E., Hipp H., Todd P.K. FMR1 Disorders. In: Adam M.P., Ardinger H.H., Pagon R.A., Wallace S.E., Bean L.J., Stephens K., Amemiya A., editors. GeneReviews®. University of Washington, Seattle; Seattle, WA, USA: 1993. [Google Scholar]
- 92.Berry-Kravis E., Raspa M., Loggin-Hester L., Bishop E., Holiday D., Bailey D.B. Seizures in Fragile X Syndrome: Characteristics and Comorbid Diagnoses. Am. J. Intellect. Dev. Disabil. 2010;115:461–472. doi: 10.1352/1944-7558-115.6.461. [DOI] [PubMed] [Google Scholar]
- 93.Neri G. Chapter 1—The Clinical Phenotype of the Fragile X Syndrome and Related Disorders. In: Willemsen R., Kooy R.F., editors. Fragile X Syndrome. Academic Press; Cambridge, MA, USA: 2017. pp. 1–16. [Google Scholar]
- 94.Martínez R., Bonilla-Henao V., Jimenez A., Lucas M., Vega C., Ramos I., Sobrino F., Pintado E. Skewed X Inactivation of the Normal Allele in Fully Mutated Female Carriers Determines the Levels of FMRP in Blood and the Fragile X Phenotype. Mol. Diagn. 2005;9:157–162. doi: 10.1007/BF03260084. [DOI] [PubMed] [Google Scholar]
- 95.Heine-Suñer D., Torres-Juan L., Morlà M., Busquets X., Barceló F., Picó G., Bonilla L., Govea N., Bernués M., Rosell J. Fragile-X Syndrome and Skewed X-Chromosome Inactivation within a Family: A Female Member with Complete Inactivation of the Functional X Chromosome. Am. J. Med. Genet. A. 2003;122A:108–114. doi: 10.1002/ajmg.a.20160. [DOI] [PubMed] [Google Scholar]
- 96.Martorell L., Nascimento M.T., Colome R., Genovés J., Naudó M., Nascimento A. Four Sisters Compound Heterozygotes for the Pre- and Full Mutation in Fragile X Syndrome and a Complete Inactivation of X-Functional Chromosome: Implications for Genetic Counseling. J. Hum. Genet. 2011;56:87–90. doi: 10.1038/jhg.2010.140. [DOI] [PubMed] [Google Scholar]
- 97.Spath M.A., Nillesen W.N., Smits A.P.T., Feuth T.B., Braat D.D.M., van Kessel A.G., Yntema H.G. X Chromosome Inactivation Does Not Define the Development of Premature Ovarian Failure in Fragile X Premutation Carriers. Am. J. Med. Genet. A. 2010;152A:387–393. doi: 10.1002/ajmg.a.33243. [DOI] [PubMed] [Google Scholar]
- 98.Rodriguez-Revenga L., Madrigal I., Badenas C., Xunclà M., Jiménez L., Milà M. Premature Ovarian Failure and Fragile X Female Premutation Carriers: No Evidence for a Skewed X-Chromosome Inactivation Pattern. Menopause. 2009;16:944–949. doi: 10.1097/gme.0b013e3181a06a37. [DOI] [PubMed] [Google Scholar]
- 99.Johnston-MacAnanny E.B., Koty P., Pettenati M., Brady M., Yalcinkaya T.M., Schmidt D.W. The First Case Described: Monozygotic Twin Sisters with the Fragile X Premutation but with a Different Phenotype for Premature Ovarian Failure. Fertil. Steril. 2011;95:2431.e13-5. doi: 10.1016/j.fertnstert.2011.01.039. [DOI] [PubMed] [Google Scholar]
- 100.Sullivan A.K., Marcus M., Epstein M.P., Allen E.G., Anido A.E., Paquin J.J., Yadav-Shah M., Sherman S.L. Association of FMR1 Repeat Size with Ovarian Dysfunction. Hum. Reprod. Oxf. Engl. 2005;20:402–412. doi: 10.1093/humrep/deh635. [DOI] [PubMed] [Google Scholar]
- 101.Hall D.A., Robertson-Dick E.E., O’Keefe J.A., Hadd A.G., Zhou L., Berry-Kravis E. X-Inactivation in the Clinical Phenotype of Fragile X Premutation Carrier Sisters. Neurol. Genet. 2016;2:e45. doi: 10.1212/NXG.0000000000000045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Berry-Kravis E., Potanos K., Weinberg D., Zhou L., Goetz C.G. Fragile X-Associated Tremor/Ataxia Syndrome in Sisters Related to X-Inactivation. Ann. Neurol. 2005;57:144–147. doi: 10.1002/ana.20360. [DOI] [PubMed] [Google Scholar]
- 103.Bailey D.B., Raspa M., Olmsted M., Holiday D.B. Co-Occurring Conditions Associated with FMR1 Gene Variations: Findings from a National Parent Survey. Am. J. Med. Genet. A. 2008;146A:2060–2069. doi: 10.1002/ajmg.a.32439. [DOI] [PubMed] [Google Scholar]
- 104.Renda M.M., Voigt R.G., Babovic-Vuksanovic D., Highsmith W.E., Vinson S.S., Sadowski C.M., Hagerman R.J. Neurodevelopmental Disabilities in Children with Intermediate and Premutation Range Fragile X Cytosine-Guanine-Guanine Expansions. J. Child Neurol. 2014;29:326–330. doi: 10.1177/0883073812469723. [DOI] [PubMed] [Google Scholar]
- 105.Farzin F., Perry H., Hessl D., Loesch D., Cohen J., Bacalman S., Gane L., Tassone F., Hagerman P., Hagerman R. Autism Spectrum Disorders and Attention-Deficit/Hyperactivity Disorder in Boys with the Fragile X Premutation. J. Dev. Behav. Pediatr. 2006;27:S137. doi: 10.1097/00004703-200604002-00012. [DOI] [PubMed] [Google Scholar]
- 106.Myers G.F., Mazzocco M.M.M., Maddalena A., Reiss A.L. No Widespread Psychological Effect of the Fragile X Premutation in Childhood: Evidence from a Preliminary Controlled Study. J. Dev. Behav. Pediatr. 2001;22:353–359. doi: 10.1097/00004703-200112000-00001. [DOI] [PubMed] [Google Scholar]
- 107.Morleo M., Franco B. Microphthalmia with Linear Skin Defects Syndrome. In: Adam M.P., Ardinger H.H., Pagon R.A., Wallace S.E., Bean L.J., Stephens K., Amemiya A., editors. GeneReviews®. University of Washington, Seattle; Seattle, WA, USA: 1993. [PubMed] [Google Scholar]
- 108.Guy J., Gan J., Selfridge J., Cobb S., Bird A. Reversal of Neurological Defects in a Mouse Model of Rett Syndrome. Science. 2007;315:1143–1147. doi: 10.1126/science.1138389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Carrette L.L.G., Wang C.-Y., Wei C., Press W., Ma W., Kelleher R.J., Lee J.T. A Mixed Modality Approach towards Xi Reactivation for Rett Syndrome and Other X-Linked Disorders. Proc. Natl. Acad. Sci. USA. 2018;115:E668–E675. doi: 10.1073/pnas.1715124115. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.