Abstract
Habitual smoking of tobacco and marijuana can lead to weight changes and poor diet quality. These effects may be caused by taste changes related to smoking and marijuana use. This study examined the associations among taste perceptions of a bitterant (quinine) and salt, tobacco and marijuana use, and weight status. We conducted a cross-sectional analysis of adults who responded to the National Health and Nutrition Examination Survey in 2013–2014. Participants (n = 2808; female = 51.7%) were adults ≥40 years with an average body mass index (BMI) of 29.6 kg/m2. Participants completed whole mouth and tongue tip assessments of bitter (quinine) and salty (NaCl) tastes, and questionnaires on demographics, cigarette, tobacco, and drug use. Measured height and weight were used to calculate BMI. Compared with never smokers, current smokers reported increased bitter ratings. Smoking status was not associated with salty taste intensity ratings after adjustment for demographic variables. Current marijuana users reported lower tongue tip quine ratings than never users. Among current smokers, current marijuana users had lower whole mouth quinine ratings than never users. Taste perception for salt and quinine for current and former smokers as well as marijuana smokers varied in whole mouth and tongue tip assessment. Changes in taste perception among cigarette smokers and marijuana consumers may be clinically relevant to address to improve diet and weight status.
Keywords: bitter, body mass index, marijuana, obesity, salt, smoking, taste
Introduction
Although tobacco use has decreased in recent years, ~34.2 million (13.7%) adults in the US currently smoke cigarettes, including 16.3% of middle-aged adults (Jamal et al., 2018; Centers for Disease Control and Prevention, 2019a). The prevalence of marijuana use in the United States is increasing (Jamal et al., 2018; Lloyd and Striley, 2018; Weinberger et al., 2019). Furthermore, 57.6% of smokers also use marijuana (Agrawal et al., 2012). Both smoking tobacco and marijuana use are associated with adverse health effects, including cardiovascular disease and weight changes (Audrain-McGovern and Benowitz, 2011; Volkow et al., 2014; Clark et al., 2018; Pradhan et al., 2018).
On average, cigarette smoking is associated with lower body mass index (BMI) compared with never smokers (Dare et al., 2015; Piirtola et al., 2018). The effect of smoking cigarettes on weight status is primarily attributable to nicotine, an appetite suppressant (Bush et al., 2016; Carreras-Torres et al., 2018). The removal of nicotine during cessation of cigarette smoking may lead to changes in eating behavior, including emotional eating and increased caloric intake (Komiyama et al., 2013; Bush et al., 2016; Chao et al., 2019), and is associated with a mean body weight gain of 4.7 kg after 12 months of abstinence (Aubin et al., 2012).
On the other hand, research on the relationship between marijuana use and weight status has yielded mixed findings (Carreras-Torres et al., 2018; Courtemanche et al., 2018; Ngueta and Ndjaboue, 2020). Previous cross-sectional studies in adults indicate that Δ 9-tetrahydrocannabinol (THC) in marijuana increases appetite and caloric intake (Rodondi et al., 2006; Le Foll et al., 2013; Racine et al., 2015; Clark et al., 2018), and may contribute to weight gain in some people. Yet, factors that explain the relationship between weight and smoking tobacco or marijuana remain poorly understood. Smoking tobacco and habitual marijuana use may impact taste perception by altering taste bud structures on the tongue. Increased keratin modules and complicated capillary development in fungiform papillae, as well as, distortions in filiform papillae were found in heavy smokers (Pavlos et al., 2009; Konstantinidis et al., 2010; Pavlidis et al., 2014). These effects may be associated with impaired taste transduction pathways.
The sense of taste has inherited plasticity (Hill, 2004), and its function can be affected by biological and environmental factors such as smoking (Mojet et al., 2003; Narukawa et al., 2017). Taste, particularly bitter taste, is important for the identification of toxic compounds as well as certain desirable foods (e.g., chocolate, coffee, and beer; Meyerhof et al., 2005; Reed and Knaapila, 2010; Kourouniotis et al., 2016; Di Pizio et al., 2019; Higgins et al., 2020) and may become impaired following use of substances, such as tobacco and marijuana (Dovey et al., 2016). Smoking tobacco affects taste bud shape, abundance, and vascularization, which may diminish the ability to taste (Pavlos et al., 2009; Chéruel et al., 2017). For example, chronic smokers have lower density and vascularization differences of fungiform papillae, located on the tongue’s tip (Pavlos et al., 2009; Fischer et al., 2013; Duffy et al., 2019). Several studies have reported that taste alterations occur in individuals who smoke tobacco or marijuana (Dovey et al., 2016; Da Re et al., 2018; Kale et al., 2019). However, the effects of smoking on taste perception are inconsistent. Several studies have reported that decreased taste sensitivity is associated with tobacco smoking (Krut et al., 1961; Fischer et al., 1963; Dovey et al., 2016; Rawal et al., 2016); some studies found that taste sensitivity increases with tobacco smoking (Fischer et al., 2013; Baker et al., 2018); while other studies have reported that taste is not affected by tobacco smoking (Liu et al., 2016). Clarification of these findings is necessary to determine the importance of assessing and addressing taste alterations that may occur with cigarette smoking and marijuana use.
The purpose of this study was to examine the relationships among taste perception, cigarette smoking, marijuana use, and BMI status in adults who participated in the National Health and Nutrition Examination Survey (NHANES) from 2013 to 2014. Particularly, we examined whole mouth and tongue tip tests for bitter and salty tastes. We also explored whether there was an interaction effect between BMI and cigarette smoking, or BMI and marijuana use on taste intensity ratings.
Materials and methods
Study design
This study is an analysis of the NHANES, a multi-wave cross-sectional study designed to assess the health and nutritional status of the US population (Centers for Disease Control and Prevention, 2017). In this study, we used cross-sectional data collected from 2013 to 2014. Data collection for NHANES was approved by the NCHS Research Ethics Review Board. Analysis of de-identified data is considered exempt from federal regulations for the protection of human research participants.
Participants
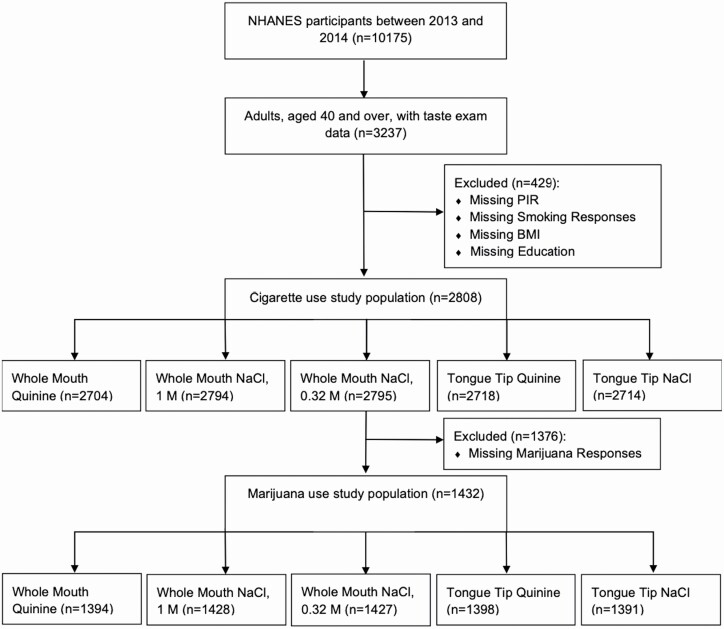
Participants were recruited from 30 survey locations across the United States from 2013 to 2014. Individuals with taste examination data, ranging were included in this sample. The taste examination was performed in adults aged 40 years and older. Our inclusion criteria also required that participants had a recorded BMI and responses to cigarette, tobacco use, and demographic questionnaires (Centers for Disease Control and Prevention, 2017). Participants were excluded from the taste examination if they were pregnant at the time of their examination, breastfeeding, allergic to quinine, or unable to correctly rate the brightness of a standard series of three lights in an LED luminescence panel (Centers for Disease Control and Prevention, 2016). Participants that were or had cancer in the past year of participation in NHANES, as reported in their medical conditions questionnaire, were excluded from the analysis (Centers for Disease Control and Prevention, 2015a). These exclusions left 2808 participants for the cigarette smoking analyses. For analyses assessing the effect of marijuana smoking on taste, 1376 participants were excluded for missing marijuana data, leaving 1432 participants for those analyses.
Measures
Demographics
Gender, age, education level, race, and socioeconomic status were self-reported. An income-to-poverty ratio (PIR) was calculated from household income to the family’s respective poverty threshold while accounting for inflation and family size. This ratio has been widely used as an indicator of socioeconomic status (United States Census Bureau, 2019). A PIR of 1.0 indicates the federal poverty level while income values below 1 represent those below the poverty line.
Cigarette and tobacco use
The Cigarette Use Questionnaire was used to collect participants’ history of use, past 30-day use, and other details related to cigarette smoking. Adults 18 years and older were interviewed by trained administrators via the computer-assisted personal interview (CAPI) system (Centers for Disease Control and Prevention, 2015b). The Recent Tobacco Use Questionnaire focuses on the past 5 days of cigarettes, e-cigarette, pipe, cigar, nicotine substitute, and other forms of tobacco use. Trained interviewers administered the questionnaire in a Mobile Examination Center, via the CAPI system. For the present study, emphasis was placed on data concerning what type of tobacco was used (Centers for Disease Control and Prevention, 2015a).
Marijuana use
The Drug Use Questionnaire assessed the extent and vehicle of marijuana/hashish and other drug usage. This questionnaire was self-administered via the Audio Computer-Assisted Self Interview system. Participants were asked about the use of drugs, including eating foods with marijuana, or smoking or using marijuana or hashish (a form of marijuana that is typically smoked in a pipe) that were not prescribed by a doctor. Participants were asked about the frequency and duration of former and current use (Centers for Disease Control and Prevention, 2016).
Taste assessment
A general labelled magnitude scale (gLMS) measured participants’ perceived intensity of a stimulus. A gLMS is an empirically derived, category-ratio response scale presented as a vertical line graph that measured participants’ perceived intensity of taste stimuli from 0 to 100 units (Bartoshuk et al., 2006). The labels “barely,” “weak,” “moderate,” “strong,” “very strong,” and “strongest of any kind” were spaced at 1, 5, 16, 34, 53, and 100 units, respectively. Following a pre-exam screening process to identify nasal symptoms and other conditions, participants were instructed to practice using the measure by rating their perception of three light intensities. Afterwards, they rated the different light intensities. Failure to correctly rank them in relative order excluded the participant from the taste measures (Centers for Disease Control and Prevention, 2016).
In the NHANES taste protocols, participants had tongue tip taste testing and whole mouth testing (Coldwell et al., 2013; Hoffman et al., 2016). First, Tongue Tip Taste Testing was conducted to assess regional gustatory function. Small volumes of tastant solutions were applied in a standardized manner, via a cotton swab, to the frontmost portion of the participant’s tongue for taste rating. Participants were instructed to leave the tongue out of the mouth, while focusing on the taste sensation for 30 s. A 1 mM quinine (bitter) solution was presented first, followed by a rinse with water, and 30-s break. The process was repeated with a 1 M NaCl (salty) solution. Participants were then asked to identify the taste as sweet, salty, bitter, sour, umami, or no taste (Centers for Disease Control and Prevention, 2016). After regional testing, Whole Mouth Taste Testing was conducted in which participants were given three 10 mL tastant solutions to taste in randomized order (0.32 M NaCl, 1 M NaCl, and 1 mM quinine). Participants were instructed to hold the volume in his/her mouth for 3 s, and immediately spit it out, without swallowing. After rinsing with water and waiting 30 s, participants were prompted to rate each sensation on the gLMS and identify whether the taste was salty, bitter, sour, or no taste. This process was repeated for each of the three solutions (Centers for Disease Control and Prevention, 2016).
Body mass index
A health technologist measured participants’ weight (kg) using a built-in digital floor scale with weight calibration. Height (m) was collected through a stadiometer, with a height adjustment ruler to accommodate different hair styles. These measures were used to calculate BMI in kilogram per meter squared (Centers for Disease Control and Prevention, 2013a).
Statistical analysis
Tobacco smoking classification
All participants were categorized as never, former, or current cigarette smokers. Those who had not smoked 100 or more cigarettes in their lifetimes and who had not smoked tobacco in the 5 days preceding their examinations were considered never smokers. Those who answered “yes” to having smoked 100 or more cigarettes in their lifetimes, who had indicated “not at all” to current cigarette use, and who had not smoked tobacco in the past 5 days were classified as former smokers. Current smokers consisted of those who had smoked at least 100 cigarettes in their lifetimes and who fulfilled either of the two criteria: they currently smoke “every day” or “some days” or they had smoked tobacco in the past 5 days.
Marijuana classification
Participants were classified into one of four classifications based on marijuana use: never, former regular, current, or occasional users. If the participant had indicated that they had never used marijuana or hashish, they were considered never users. Former, regular users were classified as participants who endorsed using marijuana or hashish at least once a month for a year but had not used marijuana in the last thirty days. If participants had used marijuana at least once a month for a year and had used marijuana in the past thirty days, they were considered current users. If the participant reported smoking marijuana before but not regularly (i.e., less than once a month for a year), they were classified as occasional users.
Data were cleaned according to NHANES recommendations (Centers for Disease Control and Prevention, 2013b, 2019b). We employed 2-year sampling weights from the Mobile Examination Center (Centers for Disease Control and Prevention, 2020). We calculated frequency and descriptive statistics for the demographic variables. The number of years of cigarette use for current smokers was calculated as the difference between their current age and the age they started smoking cigarettes regularly. For former smokers, number of years of cigarette use was calculated by subtracting their reported age of last smoking cigarettes regularly and the age they first started smoking cigarettes regularly. The duration of marijuana use was defined as the difference between the participant’s current age, the last time he/she used marijuana regularly in years, and the age he/she started regularly smoking marijuana.
Correlations were evaluated using the Rao-Scott F Adjusted Chi-Square statistic and Wald F-test. Sample-weighted multiple linear regression models were utilized to analyze the associations among weight, smoking status, and taste intensity rating. Model 1 demonstrated the effect of smoking status on each taste intensity rating. Model 2 added BMI. Model 3 incorporated demographic variable adjustments for age, gender, race and ethnicity, federal poverty to income ratio, and education levels. Model 4 included the interaction effect between BMI and smoking status. Similar models were created for marijuana use. However, model 4 also adjusted for cigarette smoking status given the potential overlap in these two behaviors. An exploratory analysis was also conducted that only included current cigarette smokers to examine the effect of co-using marijuana. Data analyses were conducted with SAS version 9.4. Statistical significance was considered at a two-sided P-value of <0.05.
Results
Participant characteristics
Figure 1 shows the participants included in each analysis. In this sample, 51.45% of participants were never smokers, 27.62% were former smokers, and 20.92% were current smokers. Former smokers had smoked for a mean ± SE of 21.22 ± 0.81 years before quitting and quit 22.12 ± 0.81 years before the survey. Current smokers had smoked for an average of 34.17 ± 0.59 years. Over the past 30 days, current smokers reported smoking 13.41 ± 0.67 cigarettes and an average of 26.85 ± 0.37 days.
Figure 1.
CONSORT flow chart.
Tobacco smoking
Demographic characteristics for the smoking subpopulation are in Table 1. Former smokers were significantly older (62.05 ± 0.38; P < 0.001) than never smokers (57.12 ± 0.48) and current smokers (53.41 ± 0.31). A greater percent of never smokers were female (56.14%) and former smokers were more likely to be male (54.98%). Never smokers had significantly higher family PIR (3.47 ± 0.12) than current smokers (2.33 ± 0.20 P < 0.001). Never smokers were more likely to have college or more education than current smokers (P < 0.001). Approximately 13.56% of current smokers were Non-Hispanic Black compared with 6.30% of former smokers and 10.00% of never smokers.
Table 1.
Demographic characteristics by smoking status
| Smoking status | |||||
|---|---|---|---|---|---|
| Total | Never | Former | Current | P-value | |
| N in millions (% of sample) | 119.82 (100) | 61.65 (51.45) | 33.10 (27.62) | 25.07 (20.92) | |
| Unweighted N | 2808 | 1421 | 780 | 607 | |
| Age, years, mean ± SE | 57.71 ± 0.28 | 57.12 ± 0.48 | 62.05 ± 0.38* | 53.41 ± 0.31* ¥ | <0.001 |
| Gender, N (%) | <0.001 | ||||
| Female | 61.97 (51.72) | 34.61 (56.14) | 14.90 (45.02) | 12.45 (49.67) | |
| Male | 57.85 (48.28) | 27.04 (43.86) | 18.20 (54.98) | 12.62 (50.33) | |
| Race, N (%) | <0.001 | ||||
| Non-Hispanic White | 89.25 (74.48) | 44.53 (72.23) | 26.21 (79.19) | 18.51 (73.81) | |
| Non-Hispanic Black | 11.65 (9.72) | 6.16 (10.00) | 2.08 (6.30) | 3.40 (13.56) | |
| Asian | 4.66 (3.89) | 3.35 (5.43) | 0.89 (2.69) | 0.42 (1.68) | |
| Mexican American | 7.09 (5.92) | 4.10 (6.65) | 1.94 (5.86) | 1.05 (4.19)^ | |
| Other Hispanic | 4.54 (3.79) | 2.38 (3.87) | 1.34 (4.04) | 0.82 (3.29) | |
| Other Race | 2.63 (2.19) | 1.13 (1.83) | 0.63 (1.91)^ | 0.87 (3.46)^ | |
| Education, N (%) | <0.001 | ||||
| Less than 9th grade | 4.32 (3.60) | 2.15 (3.49) | 1.21 (3.66) | 0.96 (3.81) | |
| 9 to 11th grade | 11.47 (9.57) | 3.91 (6.35) | 3.08 (9.30) | 4.48 (17.85) | |
| High School Graduate/GED or equivalent | 26.42 (22.05) | 11.09 (17.99) | 7.74 (23.40) | 7.59 (30.25) | |
| Some College or AA degree | 37.81 (31.55) | 18.04 (29.26) | 10.89 (32.91) | 8.87 (35.39) | |
| College Graduate or above | 39.81 (33.23) | 26.46 (42.91) | 10.17 (30.74) | 3.18 (12.69) | |
| Family PIR, mean ± SE | 3.18 ± 0.12 | 3.47 ± 0.12 | 3.30 ± 0.09 | 2.33 ± 0.20* ¥ | <0.001 |
| BMI, kg/m2, mean ± SE | 29.63 ± 0.20 | 29.55 ± 0.30 | 30.39 ± 0.30* | 28.83 ± 0.49¥ | <0.001 |
*P < 0.05 difference compared with never smoker; ¥P < 0.05 difference compared with former smoker; ^SE is >30% of estimate.
Cigarette smoking and taste perception
Quinine
Among former smokers, the length of time since smoking cessation was negatively associated with whole mouth quinine taste intensity ratings (β = −0.15, P = 0.001). No association was found with tongue tip quinine taste intensity ratings (β = −0.01, P = 0.77). For former smokers, the duration of cigarette use was not significantly associated with whole mouth quinine taste intensity ratings (β = 0.10, P = 0.14) or tongue tip quinine taste intensity ratings (β = −0.05, P = 0.35). Among current smokers, the number of cigarettes smoked per day over the past 30 days was negatively associated with tongue tip quinine taste intensity ratings (β = −0.15, P = 0.005), but not significantly associated with whole mouth quinine taste intensity ratings (β = −0.10, P = 0.59). Among current smokers, the duration of cigarette use was not significantly associated with whole mouth quinine taste intensity ratings (β = −0.11, P = 0.16) or tongue tip quinine taste intensity ratings (β = −0.12, P = 0.051).
In Model 1, which assessed the effect of smoking status on whole mouth quinine, current smokers had significantly higher bitterness ratings compared to never smokers (P = 0.003; Table 2). The positive association between current smokers and whole mouth quinine remained significant in Model 2. In Model 2, an increase of 1 BMI unit was associated with a 0.22 ± 0.06 increase in bitterness rating (P = 0.002; Table 2) but was no longer significant when demographic factors were adjusted in Model 3. There was no significant interaction between BMI and smoking status in Model 4. Smoking status was not associated with tongue tip quinine (Table 3) before or after adjusting for demographic factors in Models 1–4.
Table 2.
Cigarette smoking, BMI, and demographic characteristics as correlates of whole mouth taste test ratings
| Quinine, 1 mM (N = 2704) Coef (SE) |
NaCl, 1 M (N = 2794) Coef (SE) |
NaCl, 0.32 M (N = 2795) Coef (SE) |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Model 1 | Model 2 | Model 3 | Model 4 | Model 1 | Model 2 | Model 3 | Model 4 | Model 1 | Model 2 | Model 3 | Model 4 | |
| Intercept | 49.87 (0.82) | 43.25 (1.73) | 53.17 (2.40) | 53.13 (2.91) | 50.56 (0.95) | 46.68 (2.36) | 48.68 (4.07) | 47.89 (4.72) | 31.68 (0.60) | 29.99 (2.00) | 36.83 (3.39) | 35.29 (3.08) |
| Smoking status | ||||||||||||
| Never (ref) | — | — | — | — | — | — | — | — | — | — | — | — |
| Former | 1.53 (0.80) | 1.36 (0.80) | 2.87 (0.87)* | 4.63 (5.05) | −0.28 (1.32) | −0.39 (1.32) | 0.35 (1.28) | 9.80 (6.47) | −0.52 (0.88) | −0.57 (0.87) | −0.29 (0.78) | 6.79 (4.02) |
| Current | 6.68 (1.87)* | 6.85(1.78)* | 5.02 (2.00)* | 3.19 (4.53) | 3.11 (1.32)* | 3.21 (1.26)* | 1.80 (1.25) | −5.51 (4.66) | 2.42 (1.21) | 2.46 (1.20) | 0.68 (1.27) | −0.81 (4.56) |
| BMI | 0.22 (0.06)* | 0.10 (0.06) | 0.10 (0.09) | — | 0.13 (0.08) | 0.04 (0.08) | 0.08 (0.10) | — | 0.06 (0.06) | 0.01 (0.06) | 0.07 (0.06) | |
| Age | −0.11 (0.04)* | −0.12 (0.04)* | −0.00 (0.03) | −0.001 (0.04) | −0.04 (0.03) | −0.05 (0.03) | ||||||
| Gender | ||||||||||||
| Male (ref) | — | — | — | — | — | — | ||||||
| Female | 9.16 (1.30)** | 9.17 (1.32)** | 7.78 (0.56)** | 7.81 (0.57)** | 2.71 (1.05)* | 2.72 (1.05)* | ||||||
| Education | ||||||||||||
| Less than 9th Grade | −−3.00 (2.36) | −2.97 (2.36) | 0.49 (2.23) | 0.53 (2.19) | −1.59 (2.03) | −1.58 (2.02) | ||||||
| 9-11th Grade | −0.67 (2.50) | −0.69 (2.52) | 0.99 (2.02) | 0.90 (2.01) | 2.32 (2.05) | 2.28 (2.06) | ||||||
| High School Graduate/GED or equivalent (ref) | — | — | — | — | — | — | ||||||
| Some college or AA degree | −0.24 (1.65) | −0.24 (1.64) | −0.58 (1.75) | −0.54 (1.75) | −2.28 (1.42) | −2.25 (1.42) | ||||||
| College Graduate or above | −1.19 (1.65) | −1.20 (1.62) | −1.72 (1.05) | −1.76 (1.07) | −1.78 (1.40) | −1.76 (1.38) | ||||||
| Race/ethnicity | ||||||||||||
| Non-Hispanic White (ref) | — | — | — | — | — | — | ||||||
| Mexican American | 3.34 (1.95) | 3.32 (1.95) | −0.44 (1.67) | −0.54 (1.65) | −0.13 (1.21) | −0.19 (1.20) | ||||||
| Non-Hispanic Asian | −0.66 (1.43) | −0.67 (1.48) | −0.29 (2.15) | −0.31 (2.17) | 0.75 (1.30) | 0.81 (1.30) | ||||||
| Non-Hispanic Black | 5.30 (1.11)** | 5.27 (1.10)** | 4.62 (1.34)* | 4.46 (1.30)* | 1.80 (1.00) | 1.66 (1.01) | ||||||
| Other Hispanic | 2.52 (1.42) | 2.51 (1.42) | −4.87 (1.19)** | −4.92 (1.18)** | −1.58 (1.36) | −1.59 (1.39) | ||||||
| Other race, including multi-racial | −2.00 (2.22) | −1.97 (2.21) | 0.75 (3.17) | 0.89 (3.08) | −0.52 (2.48) | −0.41 (2.44) | ||||||
| Family poverty to income ratio | −1.39 (0.52)* | −1.41 (0.52)* | −0.89 (0.21)** | −0.95 (0.21)** | −0.93 (0.35)* | −0.95 (0.35)* | ||||||
| BMI*smoking status | ||||||||||||
| BMI*never (ref) | — | — | — | |||||||||
| BMI*former | −0.06 (0.16) | −0.31 (0.21) | −0.24 (0.14) | |||||||||
| BMI*current | 0.06 (0.16) | 0.25 (0.17) | 0.05 (0.16) | |||||||||
| R 2 | <0.01 | 0.02 | 0.09 | 0.09 | <0.01 | <0.01 | 0.05 | 0.06 | <0.01 | <0.01 | 0.03 | 0.03 |
| Adjusted R2 | <0.01 | 0.02 | 0.09 | 0.09 | <0.01 | <0.01 | 0.05 | 0.05 | <0.01 | <0.01 | 0.02 | 0.03 |
*P < 0.05; **P < 0.001.
Table 3.
Cigarette smoking, BMI, and demographic characteristics as correlates of tongue tip taste test ratings
| Quinine, 1 mM (N = 2718) Coef (SE) |
NaCl, 1 M (N = 2714) Coef (SE) |
|||||||
|---|---|---|---|---|---|---|---|---|
| Model 1 | Model 2 | Model 3 | Model 4 | Model 1 | Model 2 | Model 3 | Model 4 | |
| Intercept | 14.14 | 12.83 | 10.42 | 11.54 | 25.75 (0.53) | 24.04 (1.90) | 22.28 | 20.41 |
| (0.52) | (1.61) | (3.09) | (2.73) | (3.12) | (3.39) | |||
| Smoking status | ||||||||
| Never (ref) | — | — | — | — | — | — | — | — |
| Former | −0.70 | −0.73 | −0.05 | −1.98 | −0.52 | −0.57 (0.89) | −0.35 | 6.51 |
| (0.73) | (0.71) | (0.60) | (4.16) | (0.90) | (0.84) | (3.31) | ||
| Current | −0.60 | −0.57 | −0.27 | −2.92 | 0.36 | 0.40 | −0.42 | 0.05 |
| (0.83) | (0.80) | (0.91) | (2.45) | (1.22) | (1.20) | (1.29) | (4.85) | |
| BMI | 0.04 | 0.02 | −0.01 | 0.06 | 0.02 | 0.09 | ||
| (0.05) | (0.05) | (0.06) | (0.05) | (0.06) | (0.09) | |||
| Age | 0.01 | 0.01 | 0.06 | 0.06 | ||||
| (0.02) | (0.02) | (0.03) | (0.03) | |||||
| Gender | ||||||||
| Male (ref) | — | — | — | — | ||||
| Female | 4.90 | 4.91 (0.69)** | 4.47 (0.83)** | 4.48 | ||||
| (0.68)** | (0.83)** | |||||||
| Education | ||||||||
| Less than 9th grade | −0.98 | −0.96 | −1.24 (1.48) | −1.23 | ||||
| (1.69) | (1.71) | (1.44) | ||||||
| 9–11th grade | −1.19 | −1.20 | 2.03 | 1.98 | ||||
| (0.98) | (0.97) | (1.21) | (1.23) | |||||
| High School Graduate/GED or Equivalent (ref) | — | — | — | — | ||||
| Some college or AA degree | −0.32 | −0.33 | −2.24 (1.09) | −2.22 | ||||
| (1.05) | (1.05) | (1.07) | ||||||
| College graduate or above | −0.41 (1.36) |
−0.46 (1.36) |
−1.05 (1.02) | −1.00 (0.98) |
||||
| Race/ethnicity | ||||||||
| Non-Hispanic White (ref) | — | — | — | — | ||||
| Mexican American | 1.84 | 1.85 | 0.98 | 0.94 | ||||
| (1.12) | (1.13) | (1.06) | (1.04) | |||||
| Non-Hispanic Asian | 3.31 | 3.23 (0.81)** | 1.11 | 1.19 | ||||
| (0.80)** | (1.04) | (1.08) | ||||||
| Non-Hispanic Black | 2.63 | 2.67 (0.57)** | 3.64 (0.72)** | 3.51 | ||||
| (0.55)** | (0.70)** | |||||||
| Other Hispanic | 3.37 (1.12)** | 3.36 | -1.66 (1.03) | -1.65 | ||||
| (1.12)* | (0.99) | |||||||
| Other race, including multi-racial | 0.25 | 0.23 | 0.31 | 0.39 | ||||
| (1.72) | (1.71) | (1.71) | (1.78) | |||||
| Family poverty to income ratio | −0.13 (0.17) | −0.14 | −0.74 (0.20)* | −0.75 | ||||
| (0.17) | (0.21)* | |||||||
| BMI*smoking status | ||||||||
| BMI*never (ref) | — | — | ||||||
| BMI*former | 0.06 | −0.23 | ||||||
| (0.15) | (0.12) | |||||||
| BMI*current | 0.09 | −0.01 | ||||||
| (0.09) | (0.17) | |||||||
| R 2 | <0.01 | <0.01 | 0.04 | 0.04 | <0.01 | <0.01 | 0.05 | 0.05 |
| Adjusted R2 | <0.01 | <0.01 | 0.04 | 0.04 | <0.01 | <0.01 | 0.04 | 0.05 |
*P < 0.05; **P < 0.001.
NaCl
Among former smokers, the duration since quitting smoking was not significantly associated with whole mouth 1 M NaCl taste intensity ratings (β = 0.03, P = 0.56), 0.32 M NaCl taste intensity ratings (β = −0.11, P = 0.07), or tongue tip NaCl taste intensity ratings (β = −0.02, P = 0.70). For former smokers, the number of years of cigarette use was negatively associated with whole mouth 0.32 M NaCl taste intensity ratings (β = 0.12, P = 0.07), but not with whole mouth 1 M NaCl taste intensity ratings (β = 0.03, P = 0.66) or tongue tip NaCl taste intensity ratings (β = 0.14; P = 0.10). Among current smokers, the number of years of cigarette use was not significantly associated with whole mouth 1 M NaCl taste intensity ratings (β = −0.05, P = 0.58), whole mouth 0.32 M NaCl taste intensity ratings (β = −0.04, P = 0.61), or tongue tip NaCl taste intensity ratings (β = 0.01, P = 0.88). Among current smokers, the number of cigarettes smoked per day in the past 30 days was not significantly associated with whole mouth 0.32 M NaCl taste intensity ratings (β = 0.03, P = 0.71), whole mouth 1 M NaCl taste intensity ratings (β = −0.11, P = 0.60), or tongue tip NaCl taste intensity ratings (β = −0.15, P = 0.09).
In Models 1 and 2, whole mouth 1 M NaCl ratings were higher for current smokers compared to never smokers (P = 0.03 and P = 0.02, respectively; Table 2) but did not remain significant once demographic factors were added in Model 3. In Models 1–4, BMI was not significantly associated with whole mouth salt ratings, and there was no significant interaction effect between BMI and smoking status. In all models, no associations between variables of interest and taste ratings were found for the whole mouth 0.32 M NaCl examination (Table 2) or tongue tip examination (Table 3).
Marijuana use
In the marijuana subsample, 38.80% were never users, 19.59% were former regular users, 7.79% were current regular marijuana consumers, and 33.82% were occasional marijuana consumers. Former regular users had last used marijuana 17.22 ± 1.14 years ago and last smoked marijuana regularly 20.79 ± 1.05 years ago. Their average duration of marijuana use was 13.37 ± 1.18 years. Among current marijuana consumers, the age they first tried marijuana was 16.40 ± 0.41 years, and participants reported first using marijuana regularly at 20.02 ± 0.71 years. Their average duration of marijuana use was 26.93 ± 1.33 years. Participants reported using marijuana an average of 14.56 ± 0.70 of the last 30 days. Occasional users last used marijuana an average of 21.67 ± 1.09 years ago. Demographic characteristics for the marijuana use subpopulation are in Table 4. Never marijuana consumers were mostly never cigarette smokers (80.37%). For former regular marijuana consumers, 43.63% were current cigarette smokers, 30% were former cigarette smokers. Among current marijuana consumers, the majority (63.34%) were current cigarette smokers (Table 4). Most occasional marijuana consumers had never smoked cigarettes before (51.37%).
Table 4.
Demographic characteristic by marijuana use
| Marijuana use | |||||
|---|---|---|---|---|---|
| Never | Former | Current | Occasional | P-value | |
| N in millions (% of sample) | 26.34 (38.80) | 13.30 (19.59) | 5.29 (7.79) | 22.96 (33.82) | |
| Unweighted N | 663 | 251 | 108 | 410 | |
| Age, years, mean ± SE | 49.06 ± 0.28 | 50.72 ± 0.54* β | 49.87 ± 0.57* ¥β | 49.48 ± 0.39 | <0.001 |
| Gender, N (%) | <0.001 | ||||
| Female | 14.69 (55.78) | 5.04 (37.90) | 2.20 (41.59) | 12.33 (53.71) | |
| Male | 11.64 (44.22) | 8.26 (62.10) | 3.09 (58.41) | 10.63 (46.29) | |
| Race, N (%) | <0.001 | ||||
| Non-Hispanic White | 14.95 (56.78) | 10.21 (76.77) | 4.14 (78.16) | 18.32 (79.78) | |
| Non-Hispanic Black | 3.14 (11.91) | 1.40 (10.55) | 0.57 (10.79) | 2.15 (9.36) | |
| Asian | 2.32 (8.82) | 0.09 (0.67) | 0.03(0.59) | 0.42 (1.83) | |
| Mexican American | 3.32 (12.61) | 0.77 (5.79) | 0.21 (3.95) | 0.82 (3.57) | |
| Other Hispanic | 2.00 (7.59) | 0.43 (3.21) | 0.16 (3.03) | 0.58 (2.52) | |
| Other Race | 0.61 (2.30) | 0.40 (3.01) | 0.18 (3.49) | 0.68 (2.95) | |
| Education, N (%) | <0.001 | ||||
| Less than 9th grade | 1.45 (5.51) | 0.19 (1.45) | 0.26 (4.89) | 0.20 (0.86) | |
| 9–11th grade | 2.18 (8.29) | 1.34 (10.08) | 0.91 (17.26) | 1.72 (7.48) | |
| High School Graduate/GED or equivalent | 5.68 (21.56) | 3.55 (26.69) | 1.46 (27.63) | 4.31 (18.76) | |
| Some college or AA degree | 7.50 (28.49) | 4.58 (34.43) | 2.01 (38.00) | 6.55 (28.52) | |
| College graduate or above | 9.52 (36.16) | 3.64 (27.35) | 0.65 (12.22) | 10.19 (44.38) | |
| Family PIR, mean ± SE | 3.23 ± 0.11 | 3.25 ± 0.17 | 2.38 ± 0.29* ¥β | 3.53 ± 0.18* | <0.001 |
| BMI, kg/m2, mean ± SE | 30.70 ± 0.58 | 30.12 ± 0.45 | 28.10 ± 0.63* ¥ | 29.44 ± 0.52 | <0.001 |
| Cigarette smoking status, N (%) | <0.001 | ||||
| Never | 21.17 (80.37) | 3.51 (26.37) | 1.13 (21.44) | 11.79 (51.37) | |
| Former | 2.49 (9.47) | 3.99 (30.00) | 0.81 (15.22) | 5.53 (24.10) | |
| Current | 2.67 (10.16) | 5.80 (43.63) | 3.35 (63.34) | 5.63 (24.53) | |
*P < 0.05 difference compared with never smoker; ¥P < 0.05 difference compared with former smoker; ^SE is >30% of estimate; βP < 0.05 difference compared with occasional smoker.
Marijuana use and taste perception
Quinine
Among former marijuana consumers, the duration since they last used marijuana regularly was not significantly associated with whole mouth quinine taste intensity ratings (β = −0.07, P = 0.50) or tongue tip quinine taste intensity ratings (β = −0.09, P = 0.27). Among current marijuana consumers, the duration of marijuana use was not significantly associated with whole mouth quinine taste intensity ratings (β = −0.14, P = 0.37) or tongue tip quinine taste intensity ratings (β = −0.14, P = 0.11). The number of days in which current marijuana consumers used marijuana out of the past 30 days was not significantly associated with whole mouth quinine taste intensity ratings (β = −0.07, P = 0.69) or tongue tip quinine taste intensity ratings (β = −0.13, P = 0.16).
Marijuana use was not significantly associated with whole mouth quinine ratings (Table 5). However, former regular, current, and occasional marijuana consumers had significantly lower tongue tip quinine ratings compared to never users in Models 1 and 2 (P < 0.02, P < 0.01, P < 0.02, respectively; Table 6). After adjusting for demographic characteristics in Model 3, current users continued to have statistically lower tongue tip quinine ratings than never users (P = 0.02). There was no statistically significant interaction between BMI and marijuana use for either whole mouth or tongue tip ratings, as shown in Model 4.
Table 5.
Marijuana use, BMI, cigarette smoking, and demographic characteristics as correlates of whole mouth taste test ratings
|
|
Quinine 1 mM (N = 1394) Coef (SE) |
NaCl, 1 M (N = 1428) Coef (SE) |
NaCl, 0.32 M (N = 1427) Coef (SE) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Model 1 | Model 2 | Model 3 | Model 4 | Model 1 | Model 2 | Model 3 | Model 4 | Model 1 | Model 2 | Model 3 | Model 4 | |
| Intercept | 53.49 (0.75) | 44.91 (2.50) | 52.69 (6.09) | 52.16 (6.63) | 51.83 (0.94) | 44.90 (2.00) | 49.40 (5.60) | 47.66 (6.54) | 33.25 (0.65) | 31.63 (2.44) | 40.96 (5.40) | 43.01 (5.95) |
| Marijuana use | ||||||||||||
| Never (ref) | — | — | — | — | — | — | — | — | — | — | — | — |
| Occasional | −2.58 (1.33) | −2.22 (1.21) | −2.48 (1.38) | −5.01 (5.53) | −3.16 (1.23)* | −2.88 (1.19)* | −2.66 (0.84)* | −4.33 (6.94) | −2.09 (1.12) | −2.02 (1.14) | −1.52 (1.36) | −8.43 (5.95) |
| Former | −0.88 (1.49) | −0.71 (1.40) | −1.48 (1.66) | 7.48 (9.74) | −1.89 (1.38) | −1.76 (1.31) | −1.02 (1.76) | 12.49 (8.45) | −1.71 (1.51) | −1.67 (1.49) | −1.17 (1.82) | 3.61 (6.24) |
| Current | 0.05 (2.29) | 0.76 (2.20) | −2.44 (2.36) | −3.00 (9.73) | 3.53 (2.42) | 4.12 (2.33) | 2.70 (2.89) | 5.75 (6.52) | 2.62 (2.74) | 2.76 (2.76) | 1.72 (2.68) | −4.58 (6.54) |
| BMI |
0.28 (0.08)* | 0.14 (0.08) | 0.16 (0.15) | 0.23 (0.07)* | 0.12 (0.08) | 0.19 (0.14) | 0.05 (0.08) | −0.01 (0.08) | −0.08 (0.11) | |||
| Age |
−0.11 (0.11) | −0.12 (0.11) | −0.07 (0.09) | −0.07 (0.10) | −0.10 (0.09) | −0.10 (0.10) | ||||||
| Gender |
||||||||||||
| Male (ref) | — | — | — | — | — | — | ||||||
| Female | 8.35 (1.71)** | 8.45 (1.74)** | 7.86 (1.14)** | 7.99 (1.14)** | 3.26 (1.39)* | 3.38 (1.45)* | ||||||
| Education |
||||||||||||
|
Less than 9th grade |
−2.32 (3.87) | −2.38 (3.91) | −0.64 (3.65) | −0.64 (3.61) | −2.02 (4.36) | −2.16 (4.30) | ||||||
| 9–11th grade | −2.30 (2.88) | −2.51 (2.87) | 0.89 (2.46) | 0.65 (2.42) | −0.34 (2.61) | −0.64 (2.63) | ||||||
| High School Graduate/ GED or equivalent (ref) | — | — | — | — | — | — | ||||||
| Some college or AA degree | 2.01 (1.79) | 1.98 (1.77) | 1.28 (2.19) | 1.26 (2.21) | −2.92 (2.07) | −2.99 (2.06) | ||||||
| College Graduate or above | −0.64 (2.48) | −0.59 (2.46) | −0.95 (1.85) | −0.86 (1.84) | −3.53 (1.97) | −3.53 (1.96) | ||||||
| Race/ethnicity | ||||||||||||
| Non-Hispanic White (ref) | — | — | — | — | — | — | ||||||
| Mexican American | 3.29 (2.69) | 3.21 (2.63) | 0.04 (1.74) | −0.02 (1.68) | −0.65 (1.49) | −0.81 (1.41) | ||||||
| Non-Hispanic Asian | −0.46 (2.05) | −0.34 (2.06) | 0.70 (2.52) | 1.03 (2.67) | 1.01 (1.64) | 0.67 (1.53) | ||||||
| Non-Hispanic Black | 5.14 (1.46)* | 5.18 (1.40)* | 4.57 (1.58)* | 4.64 (1.55)* | 3.11 (1.37)* | 3.12 (1.37)* | ||||||
| Other Hispanic | 2.72 (2.10) | 2.68 (1.98) | −4.13 (1.49)* | −4.13 (1.46)* | −1.22 (1.54) | −1.46 (1.57) | ||||||
| Other race, including multi-racial | −1.03 (3.26) | −0.93 (3.44) | 0.92 (4.25) | 1.06 (4.46) | 0.75 (3.91) | 0.80 (4.06) | ||||||
| Family PIR | −1.44 (0.58)* | −1.47 (0.56)* | −0.85 (0.32)* | −0.89 (0.33)* | −0.70 (0.62) | −0.75 (0.63) | ||||||
| Smoking status | ||||||||||||
| Never (ref) | — | — | — | — | — | — | ||||||
| Former | 2.81 (1.27)* | 2.86 (1.21)* | −1.21 (1.69) | −1.12 (1.68) | −0.82 (1.43) | −0.84 (1.44) | ||||||
| Current | 5.89 (2.96) | 5.91 (2.91) | 2.16 (2.24) | 2.20 (2.13) | 0.63 (2.08) | 0.60 (2.06) | ||||||
| BMI*marijuana use |
||||||||||||
| BMI*never (ref) | — | — | — | |||||||||
| BMI*occasional | 0.09 (0.18) | 0.06 (0.23) | 0.23 (0.21) | |||||||||
| BMI*former | −0.30 (0.31) | −0.45 (0.25) | −0.16 (0.19) | |||||||||
| BMI*current | 0.02 (0.33) | −0.10 (0.16) | 0.22 (0.21) | |||||||||
| R 2 | <0.01 | 0.01 | 0.09 | 0.09 | <0.01 | 0.01 | 0.06 | 0.07 | <0.01 | <0.01 | 0.03 | 0.03 |
| Adjusted R2 | <0.01 | 0.01 | 0.09 | 0.09 | <0.01 | 0.01 | 0.06 | 0.06 | <0.01 | <0.01 | 0.03 | 0.03 |
*P < 0.05; **P < 0.001.
Table 6.
Marijuana use, BMI, smoking, and demographic characteristics as correlates of tongue tip taste test ratings for quinine and NaCl
| Quinine 1 mM (N = 1398) Coef (SE) |
NaCl, 0.32 mM (N = 1391) Coef (SE) |
|||||||
|---|---|---|---|---|---|---|---|---|
| Model 1 | Model 2 | Model 3 | Model 4 | Model 1 | Model 2 | Model 3 | Model 4 | |
| Intercept | 15.42 (0.75) |
14.54 (1.95) |
14.16 (4.95) | 13.07 (6.77) | 26.77 (0.58) |
23.67 (1.81) |
27.39 (4.00) | 27.05 (3.49) |
| Marijuana use | ||||||||
| Never (ref) | — | — | — | — | — | — | — | — |
| Occasional | −2.07 | −2.04 | −1.50 | −2.52 | −2.75 | −2.64 | −1.87 | −4.53 |
| (0.80)* | (0.76)* | (0.76) | (4.84) | (1.34) | (1.36) | (1.12) | (5.80) | |
| Former | −2.87 | −2.85 | −1.69 | 5.03 | −2.53 | −2.45 | −0.89 | 6.29 |
| (1.11)* | (1.09)* | (1.23) | (5.36) | (1.01)* | (1.01)* | (1.60) | (7.05) | |
| Current | −4.38 | −4.31 | −3.36 | 1.18 | −1.13 | −0.88 | −0.35 | 2.46 |
| (1.47)* | (1.44)* | (1.26)* | (4.76) | (1.91) | (1.92) | (1.84) | (8.87) | |
| BMI | 0.03 | 0.01 | 0.05 | 0.10 | 0.05 | 0.06 | ||
| (0.07) | (0.06) | (0.13) | (0.06) | (0.06) | (0.13) | |||
| Age | −0.07 | −0.07 | −0.07 | −0.08 | ||||
| (0.07) | (0.06) | (0.06) | (0.07) | |||||
| Gender | ||||||||
| Male (ref) | — | — | — | — | ||||
| Female | 5.16 | 5.25 | 5.18 | 5.25 | ||||
| (0.90)** | (0.92)** | (1.17)** | (1.20)** | |||||
| Education | ||||||||
| Less than 9th grade | −1.75 | −1.69 | −2.84 | −2.82 | ||||
| (2.47) | (2.40) | (2.67) | (2.61) | |||||
| 9–11th grade | −1.27 | −1.40 | 0.58 | 0.38 | ||||
| (1.41) | (1.45) | (1.94) | (1.82) | |||||
| High School Graduate/ GED or equivalent (ref) | -- | -- | -- | -- | ||||
| Some College or AA degree | 0.17 | 0.18 | −2.37 | −2.39 | ||||
| (1.60) | (1.62) | (1.93) | (1.92) | |||||
| College Graduate or above | −0.43 | −0.35 | −2.78 | −2.69 | ||||
| (1.76) | (1.73) | (1.76) | (1.71) | |||||
| Race/ethnicity | ||||||||
| Non-Hispanic White (ref) | — | — | — | — | ||||
| Mexican American | 2.08 | 2.05 | 1.35 | 1.29 | ||||
| (1.39) | (1.39) | (1.56) | (1.54) | |||||
| Non-Hispanic Asian | 3.15 | 3.34 | 1.99 | 2.07 | ||||
| (0.95)* | (1.16)* | (1.17) | (1.28) | |||||
| Non-Hispanic Black | 2.27 | 2.31 | 4.75 | 4.81 | ||||
| (0.78)* | (0.78)* | (1.35)* | (1.35)* | |||||
| Other Hispanic | 4.19 | 4.20 | −1.87 | −1.93 | ||||
| (1.74)* | (1.68)* | (1.17) | (1.20) | |||||
| Other race, including multi-racial | 1.85 | 1.89 | 3.34 | 3.40 | ||||
| (2.08) | (1.95) | (2.17) | (2.07) | |||||
| Family PIR | 0.21 | 0.19 | −0.04 | −0.07 | ||||
| (0.22) | (0.22) | (0.32) | (0.32) | |||||
| Smoking status | ||||||||
| Never (ref) | — | — | — | — | ||||
| Former | −0.004 | 0.04 | −2.13 | −2.07 | ||||
| (1.14) | (1.15) | (1.46) | (1.43) | |||||
| Current | 1.16 | 1.18 | −0.31 | −0.28 | ||||
| (1.44) | (1.41) | (1.74) | (1.69) | |||||
| BMI*marijuana use | ||||||||
| BMI*never (ref) | — | — | ||||||
| BMI*occasional | 0.04 | 0.09 | ||||||
| (0.16) | (0.21) | |||||||
| BMI*former | −0.22 | −0.24 | ||||||
| (0.19) | (0.23) | |||||||
| BMI*current | −0.16 | −0.10 | ||||||
| (0.16) | (0.28) | |||||||
| R 2 | 0.01 | 0.01 | 0.06 | 0.06 | <0.01 | <0.01 | 0.06 | 0.06 |
| Adjusted R2 | 0.01 | 0.01 | 0.05 | 0.06 | <0.01 | <0.01 | 0.06 | 0.06 |
*P < 0.05; **P < 0.001.
NaCl
For former marijuana consumers, the duration since they last used marijuana regularly was not significantly associated with whole mouth 1 M NaCl taste intensity ratings (β = −0.05, P = 0.62), whole mouth 0.32 M NaCl taste intensity ratings (β = −0.20, P = 0.06) or tongue tip NaCl taste intensity ratings (β = −0.001, P = 0.99). Among current marijuana consumers, the duration of smoking was significantly associated with tongue tip and whole mouth 0.32 M NaCl taste intensity ratings (β = −0.59, P = 0.03), but not for whole mouth 1 M NaCl taste intensity ratings (β = 0.02, P = 0.91), or tongue tip NaCl taste intensity ratings (β = 0.09, P = 0.59). For current marijuana consumers, the number of days they used marijuana in the past 30 days was not significantly associated with whole mouth 1 M NaCl ratings (β = −0.31, P = 0.13), whole mouth 0.32 M NaCl ratings (β = −0.35, P = 0.06), or tongue tip NaCl taste intensity ratings (β = −0.02, P = 0.91).
Occasional marijuana users had significantly lower NaCl 1 M scores compared with never users (Table 5). There was no association for the lower NaCl scores. Former marijuana users, relative to never users, had significantly lower NaCl scores in Models 1 and 2, but this relationship was no longer significantly after adding demographic variables. There was no statistically significant interaction between BMI and marijuana use.
Current smoking and co-use of marijuana
Among current cigarette smokers, current marijuana users had significantly lower quinine whole mouth taste intensity ratings compared with never marijuana smokers (β = −11.64, P = 0.001). Within the current cigarette smoker group, there was no significant relationships between marijuana use and tongue tip quinine ratings, or for whole mouth or tongue tip NaCl ratings (P > 0.05).
Discussion
The present study examined the relationships among taste, smoking status, marijuana use, and BMI in adults from the NHANES study. Using the gLMS to rate perceived intensities of bitter and salt tastes, we found increased bitter taste intensities in current smokers relative to never smokers in the whole mouth taste test but no significant associations on the tongue tip taste test. Among current smokers, those who were never marijuana users had higher bitter taste intensity ratings compared with current marijuana users. There were no differences in salty taste perception by smoking status. In contrast, our results showed that current marijuana consumers had significantly lower bitter intensity ratings on the tongue tip test. Occasional marijuana consumers also had lower perceptions of salty tastes for NaCl. These results suggest that smoking cigarettes and using marijuana may differentially alter taste perceptions of bitterness and saltiness.
Analyses from the present study showed that individuals who were current cigarette smokers exhibited higher perceived bitter taste intensity than never smokers. Other studies have found the opposite: cigarette smokers displayed lower bitter taste intensity and sensitivity (Krut et al., 1961; Fischer et al., 1963; Tepper et al., 2014; Khukhunaishvili et al., 2016; Risso et al., 2016). These discrepant results could be due to study designs, sample characteristics, and differences in the bitter taste stimuli and procedures used. It is possible that the lower sensitivities and intensities to bitter taste may contribute to an individual’s decision to start and continue using bitter tobacco products. Another explanation is that altered perceptions could result as a consequence of damage related to smoking. Our findings showed that within the current cigarette smoking group, never marijuana users had higher perceived bitter taste intensity. Thus, co-use of marijuana among cigarette smokers appears to influence taste sensitivity and may be important to assess clinically. Our results are supported by Baker et al. (2018), whose study explored smoking status, TAS2R38 genotype, and 6-n-propylthiouracil (PROP) bitterness ratings. While their results demonstrating increased bitter intensity among smokers were contrary to their original hypothesis, Baker et al. (2018) suggested that damaged epithelium or differential expression of bitter receptors in current smokers may increase bitter perception. Similarly, Fischer et al. (2013) report that current smokers showed higher bitter and sour intensities than nonsmokers. As one of the first studies to see this effect, Fisher et al. suggests that this may be due to smoking’s morphological changes on the fungiform papillae, based on results from Konstantinidis et al. (Konstantinidis et al., 2010; Fischer et al., 2013). This study found distortions in smoker fungiform papillae; however, they concluded that these changes did not directly affect subjects’ taste perception (Kourouniotis et al., 2016). Therefore, increased bitter taste perception may be influenced by expression of bitter receptor genes or morphology of fungiform papillae.
Additionally, our results revealed that current cigarette smokers perceived salt taste more intensely than never smokers in the whole mouth 1 M NaCl test, but not after adjusting for demographic variables. Smoking status was not associated with perceptions of the 0.32 M NaCl solution in unadjusted or adjusted models. Studies regarding perceived salt taste intensity and smoking are limited and inconsistent. For example, an older study examining glucose consumption and salt perceived intensity reported no baseline differences between smokers and nonsmokers (Redington, 1984), while a recent study suggests that there are notable differences in smokers (Duffy et al., 2019). Therefore, we suggest that the observed differences in current smokers and salt taste intensity were initiated by oral irritation to the salty solutions, rather than dysfunction of taste. These results are consistent with a similar analysis who noted that higher perceived intensities of concentrated salt solutions were elicited by an irritation response, as opposed to altered dysfunction (Duffy et al., 2019). Previous research reports that salt may cause irritation at higher concentrations compared to lower concentrations (Green and Gelhard, 1989; Gilmore and Green, 1993), which may influence an individual’s capacity to detect specific tastes (taste sensitivity). A previous study, by McCutcheon and Tennissen and reported in Gilmore et al., demonstrated that subjects who received irritating concentrations of 3.0 M NaCl were proficient at discriminating between different tastants (NaCl and citric acid; Gilmore and Green, 1993). Although research exploring the correlation between perceived taste intensity and taste sensitivity has yet to be explored, previous studies reported changes in taste sensitivity among smokers (Baker et al., 1983; Uota et al., 2016). For example, Baker et al. (1983) reported higher salt detection threshold capabilities in smokers compared with nonsmokers, indicating that smokers were more sensitive to salt taste. Ultimately, high concentrations of salt may illicit an irritation response, which is supported by Dias et al.’s study on salt taste receptors, such as TRVP1 (Dias et al., 2013). This may influence individuals’ ability to detect salt tastes.
Relative to never marijuana users, current marijuana consumers reported lower quinine ratings. Although research on taste perception and marijuana is scarce, findings from previous studies lack consistency, many studies have been conducted with small sample sizes and in controlled laboratory settings that may limit external generalizability (Foltin et al., 1986, 1988; Mattes et al., 1994; Dovey et al., 2016). We hypothesize that differences in the method of delivery of marijuana, sample characteristics, and genetic variation of taste genes may underlie the heterogeneity of findings. For example, prior research has reported null findings in taste perception (bitter, umami, salt, and sweet) upon oral THC administration; however, moderate differences in taste perception were reported upon smoking marijuana in small, laboratory-based studies (Foltin et al., 1986, 1988). Also, a study by Dovey et al. (2016) suggested that alterations in taste perception upon smoking a recreational substance, like marijuana, may lead to the inability to perceive specific tastes. Impairments in taste perception can also be influenced by age (Mojet et al., 2003; Krishnaa and Jayaraj, 2017; Barragán et al., 2018). Research on taste and aging has suspected that taste perception depreciates at approximately ages 50 or 60 (Mojet et al., 2003; Krishnaa and Jayaraj, 2017). This depreciation could potentially be induced by the loss of tongue papillae, decreased saliva production, or alterations in taste bud cell membranes (Boyce and Shone, 2006; Krishnaa and Jayaraj, 2017). Additionally, studies have shown that genetic variations in taste genes influence sensitivity and perception of a stimulus (Pilic and Mavrommatis, 2018; Turner et al., 2018). For example, Pilic and Mavrommatis suggested that individuals lacking a genetic predisposition to salt sensitivity may have decreased perception of salt, which thereby increases salt intake (Pilic and Mavrommatis, 2018). In addition, variants of the TAS2R (bitter taste receptor) genes have been associated with quinine taste perception (Reed et al., 2010; Hayes et al., 2015). Thus, the method of delivery, age, or genetic modifications may have influenced the perceived ratings of participants included in the present study. This relationship will be an important facet to consider as legalization of medical and recreational marijuana use continues across the Unites States (Capriotti, 2016).
Our exploratory, cross-sectional approach has limits findings pertaining to causality. Taste intensity ratings may vary across groups due to differences in participant interpretation of rating scales. As with all self-reported data, responses to smoking and marijuana use questions are prone to recall bias and underreporting of socially taboo or illegal behaviors. This study did not focus on the current increasing trends in other methods of drug use such as electronic cigarettes, vaping products, and edible marijuana. As these products and methods become more popular throughout culture, more research will be necessary to examine how they affect taste perception. Studies are necessary to examine whether smoking per se, regardless of the psychoactive substance, negatively influences taste. Furthermore, not all types of taste were measured, only saltiness and bitterness. Other variables of interest and potential covariates could not be included in the regression model due to sample size restrictions and collinearity between predictors. While the beta coefficients were statistically significant, the r-squared values were low, suggesting that much of the variance in the data could not be predicted by the models. Additional research is needed to identify correlates of taste differences.
Conclusion
In conclusion, we identified significant associations with taste perception, smoking status, and marijuana use. Specifically, we reported positive associations among bitter taste perception and current cigarette use. Marijuana use was associated with negatively associated with bitter and salty taste perception. These findings did not differ by weight status. Both sets of findings suggest that changes in taste perception may be influenced by demographics as well as genetic variations and are important factors to assess clinically.
Acknowledgment
The authors would like to thank Dr Joan Austin for her helpful insights and edits.
Author contributions
A.M.C.: Conceptualization; methodology; software; analysis; investigation; data curation; writing; supervision; project administration. Y.Z.: Methodology; software; analysis; investigation; data curation; writing. A.T.F. and B.E.B.: Conceptualization; investigation; writing. P.V.J.: Conceptualization; methodology; investigation; writing; supervision; project administration.
Conflict of interest
A.M.C. reports grants and personal fees from WW International, Inc. (formerly Weight Watchers), outside the submitted work. PVJ, ATF, BEB, and YZ have no conflict of interest to disclose.
Funding
This work was supported by the National Institute of Nursing Research of the National Institutes of Health (grant number K23NR017209 to A.M.C.). Dr P.V.J. is supported by the National Institute on Alcohol Abuse and Alcoholism and the National Institute of Nursing Research, the Office of Workforce Diversity, and the National Institutes of Health Distinguished Scholars Award, and by the Rockefeller University Heilbrunn Nurse Scholar Award. ATF and BEB received Intramural Research Training Awards by the National Institute of Nursing Research, National Institutes of Health, Department of Health and Human Services. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
References
- Agrawal A, Budney AJ, Lynskey MT. 2012. The co-occurring use and misuse of cannabis and tobacco: a review. Addiction. 107(7):1221–1233. doi: 10.1111/j.1360-0443.2012.03837.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aubin HJ, Farley A, Lycett D, Lahmek P, Aveyard P. 2012. Weight gain in smokers after quitting cigarettes: meta-analysis. Br Med J. 345:e4439. doi: 10.1136/bmj.e4439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Audrain-McGovern J, Benowitz NL. 2011. Cigarette smoking, nicotine, and body weight. Clin Pharmacol Ther. 90(1):164–168. doi: 10.1038/clpt.2011.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker KA, Didcock EA, Kemm JR, Patrick JM. 1983. Effect of age, sex and illness on salt taste detection thresholds. Age Ageing. 12(2):159–165. doi: 10.1093/ageing/12.2.159. [DOI] [PubMed] [Google Scholar]
- Baker AN, Miranda AM, Garneau NL, Hayes JE. 2018. Self-reported smoking status, TAS2R38 variants, and propylthiouracil phenotype: an exploratory crowdsourced cohort study. Chem Senses. 43(8):617–625. doi: 10.1093/chemse/bjy053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barragán R, Coltell O, Portolés O, Asensio EM, Sorlí JV, Ortega-Azorín C, González JI, Sáiz C, Fernández-Carrión R, Ordovas JM, et al. 2018. Bitter, sweet, salty, sour and umami taste perception decreases with age: sex-specific analysis, modulation by genetic variants and taste-preference associations in 18 to 80 year-old subjects. Nutrients. 10(10):1539. doi: 10.3390/nu10101539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartoshuk LM, Duffy VB, Hayes JE, Moskowitz HR, Snyder DJ. 2006. Psychophysics of sweet and fat perception in obesity: problems, solutions and new perspectives. Philos Trans R Soc Lond B Biol Sci. 361(1471):1137–1148. doi: 10.1098/rstb.2006.1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyce JM, Shone GR. 2006. Effects of ageing on smell and taste. Postgrad Med J. 82(966):239–241. doi: 10.1136/pgmj.2005.039453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush T, Lovejoy JC, Deprey M, Carpenter KM. 2016. The effect of tobacco cessation on weight gain, obesity, and diabetes risk. Obesity (Silver Spring). 24(9):1834–1841. doi: 10.1002/oby.21582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capriotti T. 2016. Medical marijuana. Home Healthc Now, 34(1):10–15. doi: 10.1097/NHH.0000000000000325. [DOI] [PubMed] [Google Scholar]
- Carreras-Torres R, Johansson M, Haycock PC, Relton CL, Davey Smith G, Brennan P, Martin RM. 2018. Role of obesity in smoking behaviour: Mendelian randomisation study in UK Biobank. Br Med J. 361:k1767. doi: 10.1136/bmj.k1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. 2013a. 2013–2014 Anthropometry Procedures Manual. https://wwwn.cdc.gov/nchs/data/nhanes/2013–2014/manuals/2013_Anthropometry.pdf
- Centers for Disease Control and Prevention. 2013b. Clean & Recode Data. Centers for Disease Control and Prevention. www.cdc.gov/nchs/tutorials/nhanes/preparing/cleanrecode/intro.htm.
- Centers for Disease Control and Prevention. 2015a. 2013–2014 Data Documentation, Codebook, and Frequencies (Medical Conditions). https://wwwn.cdc.gov/Nchs/Nhanes/2013–2014/MCQ_H.htm
- Centers for Disease Control and Prevention. 2015b. 2013–2014 Data Documentation, Codebook, and Frequencies (Smoking – Cigarette Use). https://wwwn.cdc.gov/Nchs/Nhanes/2013–2014/SMQ_H.htm
- Centers for Disease Control and Prevention . 2016. 2013–2014 Data Documentation, Codebook, and Frequencies (Taste & Smell). https://wwwn.cdc.gov/Nchs/Nhanes/2013–2014/CSX_H.htm
- Centers for Disease Control and Prevention. 2017. National Health and Nutrition Examination Survey. https://www.cdc.gov/nchs/nhanes/about_nhanes.htm
- Centers for Disease Control and Prevention. 2019a. Current Cigarette Smoking Among Adults in the United States. U.S. Department of Health and Human Services. https://www.cdc.gov/tobacco/data_statistics/fact_sheets/adult_data/cig_smoking/index.htm#nation
- Centers for Disease Control and Prevention. 2019b. National Health Nutrition and Examination Survey Tutorials. U.S. Department of Health and Human Services. https://wwwn.cdc.gov/nchs/nhanes/tutorials/default.aspx [Google Scholar]
- Centers for Disease Control and Prevention. 2020. Module 3: weighting. U.S. Department of Health and Human Services. https://wwwn.cdc.gov/nchs/nhanes/tutorials/module3.aspx
- Chao AM, Wadden TA, Ashare RL, Loughead J, Schmidt HD. 2019. Tobacco smoking, eating behaviors, and body weight: a review. Curr Addict Rep. 6:191–199. doi: 10.1007/s40429-019-00253-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chéruel F, Jarlier M, Sancho-Garnier H. 2017. Effect of cigarette smoke on gustatory sensitivity, evaluation of the deficit and of the recovery timecourse after smoking cessation. Tob Induc Dis. 15:15. doi: 10.1186/s12971-017-0120-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark TM, Jones JM, Hall AG, Tabner SA, Kmiec RL. 2018. Theoretical explanation for reduced body mass index and obesity rates in cannabis users. Cannabis Cannabinoid Res. 3(1):259–271. doi: 10.1089/can.2018.0045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coldwell SE, Mennella JA, Duffy VB, Pelchat ML, Griffith JW, Smutzer G, Cowart BJ, Breslin PA, Bartoshuk LM, Hastings L, et al. 2013. Gustation assessment using the NIH Toolbox. Neurology, 80(11 Suppl 3):S20–24. doi: 10.1212/WNL.0b013e3182872e38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtemanche C, Tchernis R, Ukert B. 2018. The effect of smoking on obesity: evidence from a randomized trial. J Health Econ. 57:31–44. doi: 10.1016/j.jhealeco.2017.10.006. [DOI] [PubMed] [Google Scholar]
- Da R AF, [TS: Missing font : STIXGeneral]Gurgel LG, Buffon G, Moura WER, Marques Vidor DCG, Maahs MAP. 2018. Tobacco influence on taste and smell: systematic review of the literature. Int Arch Otorhinolaryngol. 22(1):81–87. doi: 10.1055/s-0036-1597921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dare S, Mackay DF, Pell JP. 2015. Relationship between smoking and obesity: a cross-sectional study of 499,504 middle-aged adults in the UK general population. PLoS One. 10(4):e0123579. doi: 10.1371/journal.pone.0123579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dias AG, Rousseau D, Duizer L, Cockburn M, Chiu W, Nielsen D, El-Sohemy A. 2013. Genetic variation in putative salt taste receptors and salt taste perception in humans. Chem Senses. 38(2):137–145. doi: 10.1093/chemse/bjs090. [DOI] [PubMed] [Google Scholar]
- Di Pizio A, Ben Shoshan-Galeczki Y, Hayes JE, Niv MY. 2019. Bitter and sweet tasting molecules: It’s complicated. Neurosci Lett. 700:56–63. doi: 10.1016/j.neulet.2018.04.027. [DOI] [PubMed] [Google Scholar]
- Dovey TM, Boyland EJ, Trayner P, Miller J, Rarmoul-Bouhadjar A, Cole J, Halford JCG. 2016. Alterations in taste perception due to recreational drug use are due to smoking a substance rather than ingesting it. Appetite. 107:1–8. doi: 10.1016/j.appet.2016.07.016. [DOI] [PubMed] [Google Scholar]
- Duffy VB, Glennon SG, Larsen BA, Rawal S, Oncken C, Litt MD. 2019. Heightened olfactory dysfunction and oral irritation among chronic smokers and heightened propylthiouracil (PROP) bitterness among menthol smokers. Physiol Behav. 201:111–122. doi: 10.1016/j.physbeh.2018.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer ME, Cruickshanks KJ, Schubert CR, Pinto A, Klein R, Pankratz N, Pankow JS, Huang GH. 2013. Factors related to fungiform papillae density: the beaver dam offspring study. Chem Senses. 38(8):669–677. doi: 10.1093/chemse/bjt033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer R, Griffin F, Kaplan AR. 1963. Taste thresholds, cigarette smoking, and food dislikes. Med Exp Int J Exp Med. 9:151–167. doi: 10.1159/000135346. [DOI] [PubMed] [Google Scholar]
- Foltin RW, Brady JV, Fischman MW. 1986. Behavioral analysis of marijuana effects on food intake in humans. Pharmacol Biochem Behav. 25(3):577–582. doi: 10.1016/0091-3057(86)90144-9. [DOI] [PubMed] [Google Scholar]
- Foltin RW, Fischman MW, Byrne MF. 1988. Effects of smoked marijuana on food intake and body weight of humans living in a residential laboratory. Appetite. 11(1):1–14 doi: 10.1016/s0195-6663(88)80017-5. [DOI] [PubMed] [Google Scholar]
- Gilmore MM, Green BG. 1993. Sensory irritation and taste produced by NaCl and citric acid: effects of capsaicin desensitization. Chemical Senses. 18(3):257–272. doi: 10.1093/chemse/18.3.257. [DOI] [Google Scholar]
- Green BG, Gelhard B. 1989. Salt as an oral irritant. Chem Senses. 14(2):259–271. doi: 10.1093/chemse/14.2.259. [DOI] [Google Scholar]
- Hayes JE, Feeney EL, Nolden AA, McGeary JE. 2015. Quinine bitterness and grapefruit liking associate with allelic variants in TAS2R31. Chem Senses. 40(6):437–443. doi: 10.1093/chemse/bjv027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins MJ, Bakke AJ, Hayes JE. 2020. Personality traits and bitterness perception influence the liking and intake of pale ale style beers. Food Qual Preference. 86(7):103994. doi: 10.1016/j.foodqual.2020.103994. [DOI] [Google Scholar]
- Hill DL. 2004. Neural plasticity in the gustatory system. Nutr Rev. 62(11 Pt 2):S208–17; discussion S224. doi: 10.1111/j.1753-4887.2004.tb00101.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman HJ, Rawal S, Li CM, Duffy VB. 2016. New chemosensory component in the U.S. National Health and Nutrition Examination Survey (NHANES): first-year results for measured olfactory dysfunction. Rev Endocr Metab Disord. 17(2):221–240. doi: 10.1007/s11154-016-9364-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamal A, Phillips E, Gentzke AS, Homa DM, Babb SD, King BA, Neff LJ. 2018. Current cigarette smoking among adults – United States, 2016. MMWR Morb Mortal Wkly Rep. 67(2):53–59. doi: 10.15585/mmwr.mm6702a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kale YS, Vibhute N, Belgaumi U, Kadashetti V, Bommanavar S, Kamate W. 2019. Effect of using tobacco on taste perception. J Family Med Prim Care. 8(8):2699–2702. doi: 10.4103/jfmpc.jfmpc_457_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khukhunaishvili R, Tskvitinidze S, Koridze M, Nagervadze M, Chelidze N. 2016. Smoking included groups according to the phenotype of the PTC gene. Georgian Med News. (258):59–63. [PubMed] [Google Scholar]
- Komiyama M, Wada H, Ura S, Yamakage H, Satoh-Asahara N, Shimatsu A, Koyama H, Kono K, Takahashi Y, Hasegawa K. 2013. Analysis of factors that determine weight gain during smoking cessation therapy. PLoS One. 8(8):e72010. doi: 10.1371/journal.pone.0072010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konstantinidis I, Chatziavramidis A, Printza A, Metaxas S, Constantinidis J. 2010. Effects of smoking on taste: assessment with contact endoscopy and taste strips. Laryngoscope. 120(10):1958–1963. doi: 10.1002/lary.21098. [DOI] [PubMed] [Google Scholar]
- Kourouniotis S, Keast RSJ, Riddell LJ, Lacy K, Thorpe MG, Cicerale S. 2016. The importance of taste on dietary choice, behaviour and intake in a group of young adults. Appetite. 103:1–7. doi: 10.1016/j.appet.2016.03.015. [DOI] [PubMed] [Google Scholar]
- Krishnaa PK, Jayaraj G. 2017. Effect of age on taste perception: a survey. Int J Orofacial Biol. 1(1):35–38. doi: 10.4103/ijofb.ijofb_10_17. [DOI] [Google Scholar]
- Krut LH, Perrin MJ, Bronte-stewart B. 1961. Taste perception in smokers and non-smokers. Br Med J. 1(5223):384–387. doi: 10.1136/bmj.1.5223.384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Foll B, Trigo JM, Sharkey KA, Le Strat Y. 2013. Cannabis and Δ9-tetrahydrocannabinol (THC) for weight loss?. Med Hypotheses. 80(5):564–567. doi: 10.1016/j.mehy.2013.01.019. [DOI] [PubMed] [Google Scholar]
- Liu G, Zong G, Doty RL, Sun Q. 2016. Prevalence and risk factors of taste and smell impairment in a nationwide representative sample of the US population: a cross-sectional study. BMJ Open. 6(11):e013246. doi: 10.1136/bmjopen-2016-013246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd SL, Striley CW. 2018. Marijuana use among adults 50 years or older in the 21st century. Gerontol Geriatr Med. 4:2333721418781668. doi: 10.1177/2333721418781668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattes RD, Shaw LM, Engelman K. 1994. Effects of cannabinoids (marijuana) on taste intensity and hedonic ratings and salivary flow of adults. Chem Senses. 19(2):125–140. doi: 10.1093/chemse/19.2.125. [DOI] [PubMed] [Google Scholar]
- Meyerhof W, Behrens M, Brockhoff A, Bufe B, Kuhn C. 2005. Human bitter taste perception. Chem Senses. 30Suppl 1:i14-i15. doi: 10.1093/chemse/bjh089. [DOI] [PubMed] [Google Scholar]
- Mojet J, Heidema J, Christ-Hazelhof E. 2003. Taste perception with age: generic or specific losses in supra-threshold intensities of five taste qualities? Chem Senses. 28(5):397–413. doi: 10.1093/chemse/28.5.397. [DOI] [PubMed] [Google Scholar]
- Narukawa M, Kurokawa A, Kohta R, Misaka T. 2017. Participation of the peripheral taste system in aging-dependent changes in taste sensitivity. Neuroscience. 358:249–260. doi: 10.1016/j.neuroscience.2017.06.054. [DOI] [PubMed] [Google Scholar]
- Ngueta G, Ndjaboue R. 2020. Lifetime marijuana use in relation to insulin resistance in lean, overweight, and obese US adults. J Diabetes. 12(1):38–47. doi: 10.1111/1753-0407.12958. [DOI] [PubMed] [Google Scholar]
- Pavlidis P, Gouveris C, Kekes G, Maurer J. 2014. Changes in electrogustometry thresholds, tongue tip vascularization, density and form of the fungiform papillae in smokers. Eur Arch Otorhinolaryngol. 271(8):2325–2331. doi: 10.1007/s00405-014-3003-9. [DOI] [PubMed] [Google Scholar]
- Pavlos P, Vasilios N, Antonia A, Dimitrios K, Georgios K, Georgios A. 2009. Evaluation of young smokers and non-smokers with electrogustometry and contact endoscopy. BMC Ear Nose Throat Disord. 9:9. doi: 10.1186/1472-6815-9-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piirtola M, Jelenkovic A, Latvala A, Sund R, Honda C, Inui F, Watanabe M, Tomizawa R, Iwatani Y, Ordoñana JR, et al. 2018. Association of current and former smoking with body mass index: a study of smoking discordant twin pairs from 21 twin cohorts. PLoS One. 13(7):e0200140. doi: 10.1371/journal.pone.0200140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilic L, Mavrommatis Y. 2018. Genetic predisposition to salt-sensitive normotension and its effects on salt taste perception and intake. Br J Nutr. 120(7):721–731. doi: 10.1017/S0007114518002027. [DOI] [PubMed] [Google Scholar]
- Pradhan RR, Pradhan SR, Mandal S, Pradhan DR. 2018. A systematic review of marijuana use and outcomes in patients with myocardial infarction. Cureus. 10(9):e3333. doi: 10.7759/cureus.3333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Racine C, Vincent M, Rogers A, Donat M, Ojike NI, Necola O, Yousef E, Masters-Israilov A, Jean-Louis G, McFarlane SI. 2015. Metabolic effects of marijuana use among blacks. J Dis Glob Health. 4(1):9–16. [PMC free article] [PubMed] [Google Scholar]
- Rawal S, Hoffman HJ, Bainbridge KE, Huedo-Medina TB, Duffy VB. 2016. Prevalence and risk factors of self-reported smell and taste alterations: results from the 2011–2012 US National Health and Nutrition Examination Survey (NHANES). Chem Senses. 41(1):69–76. doi: 10.1093/chemse/bjv057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redington K. 1984. Taste differences between cigarette smokers and nonsmokers. Pharmacol Biochem Behav. 21(2):203–208. doi: 10.1016/0091-3057(84)90215-6. [DOI] [PubMed] [Google Scholar]
- Reed DR, Knaapila A. 2010. Genetics of taste and smell: poisons and pleasures. Prog Mol Biol Transl Sci. 94:213–240. doi: 10.1016/B978-0-12-375003-7.00008-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed DR, Zhu G, Breslin PA, Duke FF, Henders AK, Campbell MJ, Montgomery GW, Medland SE, Martin NG, Wright MJ. 2010. The perception of quinine taste intensity is associated with common genetic variants in a bitter receptor cluster on chromosome 12. Hum Mol Genet. 19(21):4278–4285. doi: 10.1093/hmg/ddq324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risso DS, Kozlitina J, Sainz E, Gutierrez J, Wooding S, Getachew B, Luiselli D, Berg CJ, Drayna D. 2016. Genetic variation in the TAS2R38 bitter taste receptor and smoking behaviors. PLoS One. 11(10):e0164157. doi: 10.1371/journal.pone.0164157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodondi N, Pletcher MJ, Liu K, Hulley SB, Sidney S; Coronary Artery Risk Development in Young Adults (CARDIA) Study. 2006. Marijuana use, diet, body mass index, and cardiovascular risk factors (from the CARDIA study). Am J Cardiol. 98(4):478–484. doi: 10.1016/j.amjcard.2006.03.024. [DOI] [PubMed] [Google Scholar]
- Tepper BJ, Banni S, Melis M, Crnjar R, Tomassini Barbarossa I. 2014. Genetic sensitivity to the bitter taste of 6-n-propylthiouracil (PROP) and its association with physiological mechanisms controlling body mass index (BMI). Nutrients. 6(9):3363–3381. doi: 10.3390/nu6093363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner A, Veysey M, Keely S, Scarlett C, Lucock M, Beckett EL. 2018. Interactions between bitter taste, diet and dysbiosis: consequences for appetite and obesity. Nutrients. 10(10):1336. doi: 10.3390/nu10101336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- United States Census Bureau. 2019. How the Census Bureau Measures Poverty. https://www.census.gov/topics/income-poverty/poverty/guidance/poverty-measures.html
- Uota M, Ogawa T, Ikebe K, Arai Y, Kamide K, Gondo Y, Masui Y, Ishizaki T, Inomata C, Takeshita H, et al. 2016. Factors related to taste sensitivity in elderly: cross-sectional findings from SONIC study. J Oral Rehabil. 43(12):943–952. doi: 10.1111/joor.12442. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Baler RD, Compton WM, Weiss SR. 2014. Adverse health effects of marijuana use. N Engl J Med. 370(23):2219–2227. doi: 10.1056/NEJMra1402309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberger AH, Delnevo CD, Wyka K, Gbedemah M, Lee J, Copeland J, Goodwin RD. 2020. Cannabis use Is associated with increased risk of cigarette smoking initiation, persistence, and relapse among adults in the United States. Nicotine Tob Res. 22(8):1404–1408. doi: 10.1093/ntr/ntz085. [DOI] [PMC free article] [PubMed] [Google Scholar]