Abstract
Lipoprotein(a) (Lp(a)) is one of the most important risk factors for the development of calcific aortic valve stenosis (CAVS). However, the mechanisms through which Lp(a) causes CAVS are currently unknown. Our objectives were to characterize the Lp(a) proteome and to identify proteins that may be differentially associated with Lp(a) in patients with versus without CAVS. Our second objective was to identify genes that may be differentially regulated by exposure to high versus low Lp(a) levels in explanted aortic valves from patients with CAVS. We isolated Lp(a) from the blood of 21 patients with CAVS and 22 volunteers and performed untargeted label-free analysis of the Lp(a) proteome. We also investigated the transcriptomic signature of calcified aortic valves from patients who underwent aortic valve replacement with high versus low Lp(a) levels (n = 118). Proteins involved in the protein activation cascade, platelet degranulation, leukocyte migration, and response to wounding may be associated with Lp(a) depending on CAVS status. The transcriptomic analysis identified genes involved in cardiac aging, chondrocyte development, and inflammation as potentially influenced by Lp(a). Our multi-omic analyses identified biological pathways through which Lp(a) may cause CAVS, as well as key molecular events that could be triggered by Lp(a) in CAVS development.
Keywords: lipoprotein(a), calcific aortic valve stenosis, aortic valve, proteomics, transcriptomics
1. Introduction
Calcific aortic valve stenosis (CAVS) is the most prevalent valvulopathy worldwide, affecting approximately 2% of the population over 65 years old [1,2]. Its prevalence is increasing with population aging [3]. CAVS results from an active inflammatory process, including lipoprotein infiltration and oxidation, immune cell activation and infiltration into the valve leaflets cells, and osteoblastic transition of valvular interstitial cells (VICs) leading to fibrocalcific remodelling [4,5,6]. CAVS shares many of the risk factors for atherosclerotic cardiovascular diseases (ACVD), such as age, male sex, hypertension, higher low-density lipoprotein (LDL) cholesterol, type 2 diabetes, tobacco use, and elevated lipoprotein(a) (Lp(a)) levels [7,8,9,10,11,12]. However, there is currently no specific pharmacological treatment for the prevention or treatment of CAVS. The only treatment for symptomatic end-stage process disease patients is surgical or transcatheter valve replacement [6].
Many studies sought to identify potential new biomarkers or therapeutic targets for CAVS using different “omic” approaches, such as plasma [13,14,15], heart valve tissue proteomics [16,17,18], transcriptomic [19,20], or genome-wide analysis [11,21,22]. In genome-wide analysis, LPA was identified as one of the main risk factors for the development of aortic valve calcification and CAVS. Lp(a) is an LDL-like particle whereby the highly polymorphic apolipoprotein(a) (encoded by LPA on chromosome 6) is linked to apolipoprotein (apo) B via a disulfide bridge [23]. Apo(a) is composed of different kringle domains (KIV-1 to KIV-10 and KV), all present once, except KIV-2, which can be repeated several times [24]. The copy number of KIV-2 is inversely correlated with Lp(a) concentration and with the risk of ACVD [25]. Lp(a) not only possesses the atherogenic properties of LDL, but it was found to be the preferential carrier of proinflammatory oxidized phospholipids [26] (OxPL). OxPLs have an important role in the initiation and progression of ACVD or CAVS [27], and have been found colocalized with Lp(a) in aortic lesions [28,29,30]. Moreover, it was found that high levels of OxPL and Lp(a) may be independently associated with CAVS progression [31].
In addition to apoB and apo(a), Lp(a) might transport other proteins in the bloodstream. A study identified autotaxin (ATX), a lysophospholipase D which converts lysophosphatidylcholine into lysophosphatidic acid, to be transported by Lp(a) [32]. High Lp(a), combined with high ATX levels, were associated with a faster progression of CAVS [33] in patients with coronary artery disease. Analyses of Lp(a) and LDL proteomes have also identified a potential role of Lp(a) in wound healing and complement activation [34]. In a comparative analysis of the Lp(a) and LDL proteomes isolated from the same individuals, we also recently reported profound differences between these two fractions, with Lp(a) carrying many more proteins that LDLs [35]. It is, however, unknown if the proteome of Lp(a) differs across disease states such as CAVS.
Ex vivo, incubation of human valvular endothelial or interstitial cells with Lp(a) induces the expression of osteogenic genes, such as IL6, BMP2, and RUNX2 [28,29]. This effect may be dependent on OxPLs. To our knowledge, there have been no studies using unbiased transcriptomic approaches to identify the mechanisms through which Lp(a) may cause CAVS.
Multi-omic approaches are increasingly used in basic cardiovascular sciences to optimize risk prediction or patient stratification and to identify new pathobiological mechanisms leading to ACVD or CAVS. Since the majority of people with high Lp(a) levels will not develop CAVS, our first objective was to identify the potential differences in the Lp(a) proteome between individuals with versus without CAVS. Our second objective was to obtain new insight on the mechanisms or pathways through which Lp(a) might cause aortic valve calcification in an unbiased manner. We therefore used a transcriptomic approach on explanted calcified aortic valves, and compared gene expression levels in patients with high versus low Lp(a) levels.
2. Results
2.1. Proteomic Analysis of Lp(a) Proteome from Patients with Versus without CAVS
The label-free analysis of the Lp(a) proteome identified 172 proteins associated with Lp(a) (regardless of CAVS status). These are listed in order of intensity in Table S1. Next, we compared the Lp(a) proteome of patients with CAVS to that of controls with an anatomically normal aortic valve. Participants’ characteristics are presented in Table 1.
Table 1.
Patient characteristics for Lp(a)-isolated particles.
| Clinical Characteristics | Control Lp(a) (n = 22) |
CAVS Lp(a) (n = 21) |
p-Value |
|---|---|---|---|
| Age | 61.2 ± 15.1 | 71.1 ± 4.4 | <0.0001 |
| Men, % (n) | 68.2 (15) | 57.1 (12) | 0.454 |
| Smoking, %, (n) | 42.8 (9) | 66.7 (14) | 0.121 |
| BMI, kg/m2 | 27.6 ± 5.3 | 30.3 ± 5.8 | 0.056 |
| Diabetes, %, (n) | 40.9 (9) | 42.9(9) | 0.897 |
| Blood pressure, mmHg | |||
| Systolic | 129 ± 30.9 | 133.6 ± 18.7 | 0.601 |
| Diastolic | 77.8 ± 19.5 | 72.4 ± 9.8 | 0.148 |
| Cardiovascular disease, %, (n) | 54.5 (12) | 57.1 (12) | 0.864 |
| Statin use, %, (n) | 59.1 (13) | 85.7 (18) | 0.052 |
| Lipoprotein(a), nmol/L | 202.1 ± 84 | 198.3 ± 81.8 | 0.759 |
A total of 13 proteins were differently associated with the Lp(a) of participants with versus without CAVS, with a p-value < 0.05. All the fold changes were found above 1.7. Among these 13 proteins, 9 of them were found to be preferentially associated with the Lp(a) of patients with CAVS: LCAT, NCAM1, VASN, PON3, SerpinG1, LAMP2, MCAM, MADCAM1, and CHL1. Conversely, the four other proteins were found to be preferentially associated with the Lp(a) of controls: beta-2-glycoprotein 1 (β2GPI), IGHM, IGKV2D-30, and JCHAIN;IGJ (Table 2).
Table 2.
List of proteins found to be differentially associated between Lp(a) of subjects with aortic stenosis compared to healthy participants.
| Gene | Protein | CAVS/Control Ratio | Limma p-Value |
|---|---|---|---|
| LCAT | lecithin–cholesterol acyltransferase | 3.4007 | 0.0029 |
| NCAM1 | neural cell adhesion molecule 1 | 2.9069 | 0.0070 |
| VASN | Vasorin | 3.4835 | 0.0072 |
| β2GPI | beta-2-glycoprotein 1 | 0.4060 | 0.0087 |
| PON3 | paraoxonase 3 | 2.3675 | 0.0108 |
| SERPING1 | serpin family G member 1 | 2.0398 | 0.0109 |
| LAMP2 | lysosomal-associated membrane protein 2 | 2.1678 | 0.0270 |
| IGHM | immunoglobulin heavy constant mu | 0.2424 | 0.0285 |
| IGKV2D-30 | immunoglobulin kappa variable 2D-30 | 0.3026 | 0.0343 |
| MCAM | melanoma cell adhesion molecule | 2.9838 | 0.0356 |
| MADCAM1 | mucosal vascular addressin cell adhesion molecule 1 | 2.4532 | 0.0392 |
| CHL1 | cell adhesion molecule L1-like | 1.7736 | 0.0427 |
| JCHAIN;IGJ | joining chain of multimeric IgA and IgM | 0.5021 | 0.0479 |
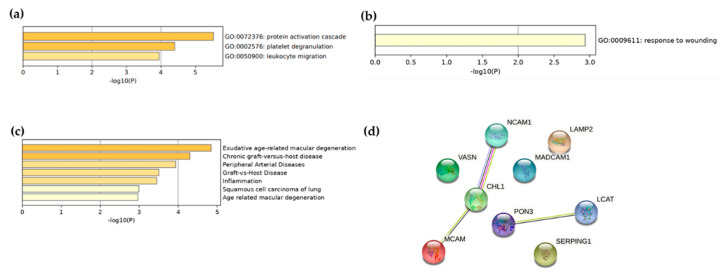
Enrichment pathway analysis performed with Metascape [36] revealed that the 13 proteins identified were mainly involved in protein activation cascade, platelet degranulation, and leukocytes migration pathways (Figure 1a). When performing pathway analyses with the nine proteins associated with the CAVS Lp(a), we found one enriched pathway: response to wounding (Figure 1b). Moreover, a DisGeNET [37] analysis of these nine proteins found them to be associated with several diseases, such as peripheral arterial disease and inflammation (Figure 1c). Finally, using STRING-db, we observed that MCAM (co-expression) and NCAM1 (co-expression, protein homology, and interaction demonstrated experimentally between putative homologs) are known to interact with CHL1, and PON3 with LCAT (co-expression of putative homologs in other organisms) (Figure 1d).
Figure 1.
Enrichment analysis of proteins found to be associated with Lp(a) regardless of (a) CAVS status and (b) in CAVS only. (c) DisGeNET enrichment analysis of proteins found to be associated with CAVS Lp(a) and (d) interaction analysis between the proteins identified as preferentially associated with the CAVS Lp(a) by STRING-db.
2.2. Transcriptomic Analysis of Explanted Calcified Valves from Patients with CAVS
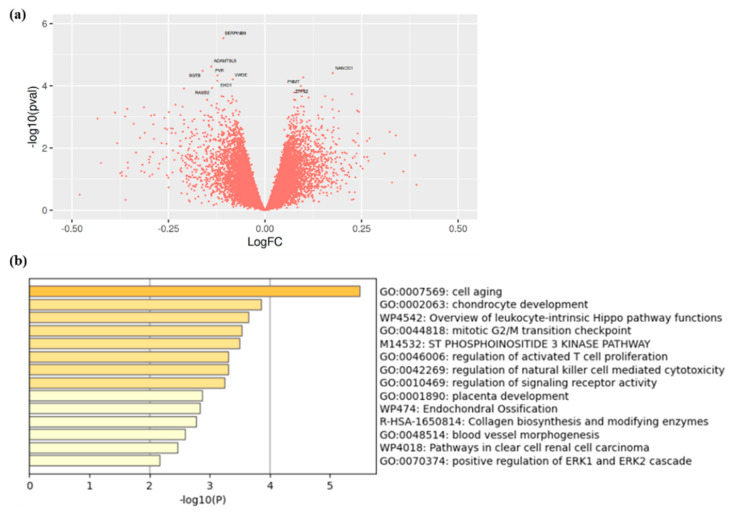
We compared the gene expression signature of calcified aortic valves from CAVS patients with high vs. low Lp(a) levels. Participants were matched for age, sex, tobacco, and statin use. Participant characteristics are shown in Table 3. Lp(a) levels were 29.2 ± 28.6 nmol/L and 217.6 ± 93.1 nmol/L in the low and high Lp(a) group, respectively. Transcriptomic analysis revealed that one gene, SERPINB9, reached an arbitrary threshold of statistical significance (p < 1.0 × 10−5) and may be downregulated in aortic valve tissue from participants with high Lp(a) (Figure 2a and Table S2). However, after FDR correction, no gene passed the statistical significance threshold (p-value for SERPINB9 was 2.94 × 10−6, and 0.070 after FDR correction). We then performed an enrichment pathway analysis on the 100 genes most influenced by Lp(a) levels. Interestingly, among all the pathways identified with Metascape, the most enriched pathways involved cell aging, chondrocyte development, and inflammation (Figure 2b).
Table 3.
Patient characteristics for the explanted valves cohort.
| Clinical Characteristics | Low Lp(a) (n = 59) |
High Lp(a) (n = 59) |
p-Value |
|---|---|---|---|
| Age, years | 73.0 ± 6.6 | 72.8 ± 6.9 | 0.85 |
| Men, % (n) | 61.0 (36) | 61.0 (36) | 1 |
| Smoking, %, (n) | 5.1 (3) | 5.1 (3) | 1 |
| BMI, kg/m2 | 29.9 ± 5.1 | 30.4 ± 6.4 | 0.61 |
| Diabetes, %, (n) | 45.8 (27) | 37.3 (22) | 0.35 |
| Blood pressure, mmHg | |||
| Systolic | 134.2 ± 19.8 | 134.5 ± 20.4 | 0.71 |
| Diastolic | 73.1 ± 9.8 | 73.1 ± 9.6 | 0.51 |
| Cardiovascular disease, % (n) | 57.6 (34) | 74.6 (44) | 0.051 |
| NYHA severity, %, (n) | |||
| 1 | 13.6 (8) | 18.6 (11) | |
| 2 | 47.5 (28) | 50.8 (30) | |
| 3 | 37.3 (22) | 27.1 (16) | |
| 4 | 1.7 (1) | 3.4 (2) | |
| Statin use, %, (n) | 83.1 (49) | 84.7 (49) | 1 |
| Lipoprotein(a), nmol/L | 29.2 ± 28.6 | 217.6 ± 93.1 | <0.0001 |
Figure 2.
(a) Volcano plot showing the genes identified in calcified valve of patients with high versus low Lp(a) and (b) enrichment analysis of genes found to be the most influenced by Lp(a) concentrations in explanted calcified aortic valves of patients with high versus low Lp(a).
3. Discussion
The role of Lp(a) in the development of CAVS is well established. However, in light of the fact that only a minority of people with high Lp(a) levels will develop CAVS, we investigated the potential difference of the Lp(a) proteome in patients with versus without CAVS. Our untargeted proteomic approach identified 172 proteins linked to Lp(a) in the bloodstream and identified nine proteins (LCAT, NCAM1, VASN, PON3, SerpinG1, LAMP2, MCAM, MADCAM1, and CHL1) that may be preferentially associated with the Lp(a) of patients with CAVS. Four proteins (β2GPI, IGHM, IGKV2D-30, and JCHAIN;IGJ) may be found in lower concentrations in the Lp(a) of patients with CAVS. These proteins are involved in several key atherogenic pathways, including protein activation cascade, platelet degranulation, leukocyte migration, and response to wound healing. We also investigated the transcriptome of explanted calcified valves of patients with CAVS with either high or low Lp(a) levels to identify genes and pathways that could be differentially regulated according to Lp(a) levels, and found that exposure to higher Lp(a) levels may be associated with the downregulation of the SERPINB9 gene as well as the dysregulation of genes involved in cell aging, chondrocyte development, and inflammation.
Most of the proteins that were found to be more present on the Lp(a) isolated from patients with CAVS compared to controls appeared to be involved in cell adhesion, immune cell recruitment, and migration, which may be important mechanisms in the progression of CAVS. For instance, vasorin (VASN) was found to modulate arterial response to injury in vivo [38]. It also appears to be involved in cellular migration and proliferation/differentiation [39]. Neural cell adhesion molecule-1 (NCAM1) is involved in cell–cell and matrix adhesion in cardiomyocytes adhesion [40], and is upregulated under metabolic stress [41]. Melanoma cell-adhesion molecule (MCAM) is an adhesion molecule from the immunoglobulin superfamily involved in monocyte transmigration [42,43] and angiogenesis. Its short isoform also produces proinflammatory cytokines [44]. It was reported that MCAM could associate with the monocytes and promote interaction with activated endothelial cells causing atherosclerosis progression [43]. MCAM upregulation also mediates oxidized lipids uptake in macrophages, contributing to macrophage retention in atheroma [45]. Another protein that was found to be higher in the Lp(a) isolated from patients with CAVS compared to controls is mucosal vascular addressin cell adhesion molecule 1 (MADCAM1). This protein is expressed in cardiomyocytes and endothelial cells in vitro and could induce interaction of endothelial cells with leukocytes [46]. Cell adhesion molecule close homolog of L1 (CHL1) has an important role in the development and regeneration of the nervous system [47,48], and is linked to pathologies, such as mental retardation [49] or schizophrenia [50]. Lysosomal-associated membrane protein 2 (LAMP2) is mainly found on the surface of lysosomes, where its role is to maintain lysosomal membrane integrity, lysosome biogenesis, and lysosome fusion with autophagosome in macro autophagy [51]. Recently, it was suggested that LAMP2 was involved in surface expression of RANKL of osteoblast, thus participating in osteoclastogenesis, a process found in aortic valve calcification [52]. LAMP2 deficiency is associated with Danon disease, which features cardiomyopathy, myopathy, and mental retardation [53]. SerpinG1, a protease inhibitor, might help reduce monocyte activation and intimal hyperplasia [54]. It can inactivate several fibrinolytic and coagulation system proteases, making this protein a protector against atherosclerosis, counteracting the role of Lp(a) in the development and progress of atherosclerosis, and so, potentially, CAVS. Interestingly, SerpinG1 was found in control and diseased aortic valves, with decreased levels in the diseased ones [55]. Vasorin and SerpinG1, which were found to be more abundant in the Lp(a) of CAVS patients compared to controls in this study, were already identified in studies investigating the blood proteome of patients with CAVS [13,14]. Initiation of aortic stenosis involves endothelial dysfunction, which is amplified in the context of exposure to elevated levels of apoB-containing lipoproteins. Two of the proteins we identified, LCAT and PON3, are involved in lipoprotein metabolism and modification. The role of LCAT in cardiovascular diseases and atherosclerosis is still controversial, as LCAT deficiency is common in several chronic disorders [56], whereas some studies suggest that an increase in LCAT activity is associated with an increased formation of small and dense LDL [57,58], which are more atherogenic and prone to oxidation. PON3, on the other hand, may prevent oxidative modification of LDL in atherosclerosis by degrading lipoperoxides in lipoproteins [59,60].
We identified four proteins that may be more abundant in the Lp(a) of participants without CAVS, three of them being associated with the immunoglobulin family (IgM, JCHAIN, IGKV-2D-30) and β2GPI. β2GPI activates lipoprotein lipase and triglyceride-rich lipoprotein (TRL) catabolism [61]. High TRL levels have recently been linked with CAVS [62]. β2GPI was found to associate with ox-LDL [63] and, more interestingly, to Lp(a) particles [64]. As this protein also has a role in clearing the non-self particles [65], it was proposed that Lp(a) could be taken up via β2GPI recognition mechanism by macrophages [66]. Mutations in APOH (the gene encoding β2GPI) are also associated with an impaired association of β2GPI with phospholipids [67] and with LDL particle size [68]. Although this would require experimental confirmation, one can hypothesize that the Lp(a) of control subjects might be cleared by a β2GPI-mediated mechanism to a greater extent than the CAVS Lp(a) and/or have a potentially lower OxPL content. Interestingly, a novel variant at the APOH locus was recently found to be associated with increased Lp(a) levels [69]. Finally, IGHM is involved in primary defense mechanisms and has a wide variety of properties, allowing it to participate in different pathophysiologies, such as infection, inflammation, or atherosclerosis [70]. Clinical studies have also shown an inverse relationship between cardiovascular events and specific IGHM [71,72]. IGHM also facilitates apoptotic cell clearance, which could promote inflammation resolution. Interestingly, antigenic targets present in atherosclerotic plaques as ox-LDL are bound to natural IGHM [73,74].
Our transcriptomic approach identified SERPINB9 as being potentially expressed at lower levels in the valves of patients with high Lp(a) compared to those with lower Lp(a) levels. SerpinB9 is an intracellular inhibitor of granzyme B activity, and was discovered in T-cells to protect them from apoptosis [75]. Granzyme B is a serine protease family member which induces cell death by different mechanisms, and is also associated with extracellular matrix degradation [75]. Granzyme B expression was found to be 7-fold higher in calcified aortic valves, compared to aortic valves explanted from patients without CAVS [76]. It was demonstrated that this protein could be reduced in human atherosclerotic lesions [77]. Altogether, this suggests that people with less Lp(a) could be more protected from granzyme B apoptosis by a higher level of SerpinB9 expression, thus indicating a potentially different mechanism in the presence of lifelong exposure to high Lp(a) levels. A pathway analysis of the top genes most influenced by Lp(a) levels revealed that the main pathway identified appeared to involve dysregulation of genes encoding cell aging, chondrocyte development, and inflammatory pathways. These findings support the notion that plasma Lp(a) levels may be a key determinant of healthy aging, as supported by studies showing a strong association between Lp(a) levels and parental lifespan, health span, and all-cause mortality [78,79,80,81]. Pathways linking Lp(a) with cell aging may involve cellular senescence and cell cycle inhibition via the PI3K/AKT/mTOR pathway. Chondrocytes produce and maintain extracellular matrix collagens and proteoglycans, and may eventually differentiate into osteoblasts, which is a key molecular event in the development of aortic valve calcification. These results, therefore, support the notion that Lp(a) levels are robustly associated with macro- and microcalcification [11,82]. Finally, our transcriptomic analysis revealed several inflammatory pathways that may be influenced by lifelong exposure to high Lp(a) levels, including T lymphocyte proliferation and natural killer-cell-mediated lipotoxicity. These results further extend the results of the proteomic study by suggesting that several inflammatory pathways may be influenced by Lp(a) in the context of CAVS.
To our knowledge, our study may be the first study to compare the Lp(a) proteome across disease states and the first comparing the Lp(a) proteome in patients with versus without CAVS. We also could not find another study comparing the aortic valve transcriptomic response to lifelong exposure to high Lp(a) levels in such a large dataset. Limiting our findings however is the fact that, although our analysis could identify several new proteins that could be transported by Lp(a), this analysis was semiquantitative. Additional studies should be performed to confirm these results and to measure the proteins we identified in a quantitative manner. Furthermore, the lack of replication studies either in our proteomic and in our transcriptomic study is another limitation of this work. However, we were the first to perform label-free analysis of isolated Lp(a) fractions of 43 participants, which is the biggest Lp(a) sample cohort to date.
4. Materials and Methods
4.1. Study Participants
We recruited 21 participants with high Lp(a) levels (>125 nmol/L) and mild to severe CAVS at the echocardiography laboratory of the Quebec Heart and Lung Institute. Exclusion criteria included mitral valve stenosis, mitral or aortic insufficiency (moderate or more), heart failure (ejection fraction < 40%), CAVS from rheumatic etiology or cancer requiring radiotherapy in the thoracic area before CAVS diagnosis. Pregnant or lactating women were also excluded from the study. We also recruited 22 controls without CAVS and with high Lp(a) levels (>125 nmol/L) through advertisements. The study protocol was approved by the Ethics Committee of the Quebec Heart and Lung Institute, and all patients signed a written informed consent. Each participant completed detailed questionnaires, as previously described [82]. Plasma Lp(a) levels were measured using a turbidimetric assay, Tina-quant lipoprotein(a) Gen.2 system (Cobas integra 400/800, Roche Diagnostics, Mannheim, Germany).
4.2. Lipoprotein Isolation
Lp(a) subfractions were isolated from these 43 participants, as previously described [35,83,84]. Briefly, fresh serum was ultracentrifugated with a gradient of iodixanol solution (Optiprep). Three subfractions were identified after ultracentrifugation and the fraction enriched with Lp(a), as assessed with Sebia Hydragel LIPO + Lp(a) (Sebia, Lisses, France), was submitted to a combination of size exclusion and affinity chromatography. Lp(a) fraction purity was then assessed with Sebia Hydragel LIPO + Lp(a) to ensure no meaningful amounts of LDL or HDL were found with Lp(a).
4.3. Assessment of Lp(a) Proteome by Nanolc-MS/MS
The proteome of Lp(a) was evaluated as previously described [35]. Briefly, proteins from Lp(a) samples were precipitated overnight with acetone at −20 °C, then resuspended, reduced, alkylated, and, finally, digested with trypsin. An equivalent of 2 μg of each sample was then injected into a Dionex UltiMate 3000 nanoRSLC chromatography system (Thermo Fisher Scientific, Waltham, MA, USA), coupled with a nanoelectrospray source to an Orbitrap Fusion Tribrid mass spectrometer (Thermo Fisher Scientific). The acquired spectra were searched against the Uniprot complete proteome homo sapiens database (21 April 2019, 74,435 entries) using the Andromeda search engine in MaxQuant software v. 1.6.7.0 (Max Planck Institute of Biochemistry, Martinsried, Germany). Intensity values were first normalised by applying a normalization factor calculated from the median of all protein intensities of each sample. Missing values were then imputed with a noise value corresponding to the first percentile of all protein intensities. A protein was considered to be quantifiable only if it possessed at least 75% replicate values (16 of 21 samples in CAVS or 17 of 22 samples in controls) in one group with at least 2 peptides per protein. Statistical analyses were conducted on a mean CAVS Lp(a) intensity/mean control Lp(a) intensity ratio with Limma t-test). A multiple-test-corrected p-value adjusting FDR (set at 1% in MaxQuant) below 0.05 was considered significant. Data analysis was conducted with RStudio 1.1.383.
4.4. Transcriptomic Analysis of Explanted Calcified Valves from Patients with CAVS
Calcified explanted valves were recovered from the Quebec Heart and Lung Institute biobank from 240 subjects who underwent aortic valve replacement. We included all study participants with an Lp(a) level > 125 nmol/L and matched them on a 1:1 ratio with participants with the lowest Lp(a) levels according to age, sex, tobacco use, and statin use. The study protocol was approved by the Ethics Committee of the Quebec Heart and Lung Institute, and all patients signed a written informed consent. We obtained two groups of 59 participants. RNA was extracted from explanted valves leaflets using RNeasy Plus Universal Mini Kit (Qiagen). Gene expression was evaluated using the Illumina HumanHT-12 v4 Expression BeadChip. Standard microarray processing and quality control analysis were performed as previously described [22,85]. Briefly, the raw data were quantile normalized after log2-transformation with the lumi package in R. Probe sequences were mapped to RefSeq B38, GENCODE v24 B38, mRNA B38, and the human genome (GRCh B38) using Bowtie, and probes not mapping to any coding region were removed, leaving a total of 45,699 probes. Robust fitting of linear models function in the R statistical package MASS was used to adjust gene expression data for age and sex. Residual values deviating by more than three standard deviations from the median were then filtered out. The gene expression profile of patients with high versus low Lp(a) is reported using the t-statistic and the Benjamini–Hochberg method to correct for FDR (p-value < 0.05).
5. Conclusions
Our study revealed new potential mechanisms and Lp(a)-associated proteins that may be involved in the development and progression of CAVS. Some proteins seemed to have a protective effect and could counteract the deleterious effects of Lp(a), whereas other proteins, such as cell-adhesion molecules, may be more deleterious. These proteins could be used as potential new biomarkers or therapeutic targets of CAVS. From our large-scale analysis of the aortic valve transcriptome, we conclude that several mechanisms related to aging, ossification and inflammation may be involved in the development of CAVS in the context of lifelong exposure to high Lp(a) levels.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/metabo11070459/s1, Table S1: Characterisation of the proteins found on Lp(a) particles regardless of CAVS status according to their relative abundance, Table S2: Top 10 genes influenced by Lp(a) levels, Table S3: Pathways description and log10(p) from the MCODE algorithm analysis of the top genes identified in the transcriptomic stud. Figure S1: Molecular Complex Detection (MCODE) algorithm network performed for the top genes influenced by Lp(a) levels. MCODE algorithm detects densely connected regions in large protein-protein interaction networks that may represent molecular complexes. MCODE details are represented in Table S3.
Author Contributions
Conceptualization, P.P., P.M., Y.B. and B.J.A.; formal analysis, R.B., J.B., P.M. and B.J.A.; funding acquisition, P.P. and B.J.A.; investigation, R.B., N.P., A.G., P.L.M., C.G., S.B. and N.G.; methodology, R.B., C.G., S.B., C.A.S., N.G., M.B.B., M.L.K. and A.D.; project administration, P.P., P.M. and B.J.A.; resources, A.-A.D., N.P., J.G. and P.L.M.; software, J.B., S.T. and Y.B.; supervision, B.J.A.; visualization, R.B., J.B. and B.J.A.; writing—original draft, R.B., P.L.M. and B.J.A.; writing—review and editing, R.B., J.B., A.-A.D., N.P., J.G., A.G., P.L.M., C.G., S.B., C.A.S., N.G., M.B.B., M.L.K., A.D., S.T., P.M., Y.B. and B.J.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Canadian Institutes of Health Research, grant number FRN155226 and FRN149068. Arsenault and Thériault hold a junior scholar award from the Fonds de recherche du Québec: Santé (FRQS). Després is supported by a master’s training award from the FRQS. Mathieu holds a FRQS Research Chair on the Pathobiology of Calcific Aortic Valve Disease. P.P. holds the Canada Research Chair in Valvular Heart Disease and his research program is supported by a Foundation Scheme Grant from the Canadian Institutes of Health Research (CIHR). Koschinsky is supported by a grant from the Heart and Stroke Foundation of Canada (G-17-0018740) for this work. Bossé holds a Canada Research Chair in Genomics of Heart and Lung Diseases.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Ethics Committee of the Quebec Heart and Lung Institute (protocol code 2016-2542 and 21225, date of approval 2 October 2015).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The microarray gene expression dataset on human aortic valves is available in the Gene Expression Omnibus with accession number GSE102249. The other data available upon reasonable request.
Conflicts of Interest
The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Nkomo V.T., Gardin J.M., Skelton T.N., Gottdiener J.S., Scott C.G., Enriquez-Sarano M. Burden of valvular heart diseases: A population-based study. Lancet. 2006;368:1005–1011. doi: 10.1016/S0140-6736(06)69208-8. [DOI] [PubMed] [Google Scholar]
- 2.Vahanian A., Alfieri O., Andreotti F., Antunes M.J., Baron-Esquivias G., Baumgartner H., Borger M.A., Carrel T.P., De Bonis M., Evangelista A., et al. Guidelines on the management of valvular heart disease (version 2012): The Joint Task Force on the Management of Valvular Heart Disease of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS) Eur. J. Cardiothorac. Surg. 2012;42:S1–S44. doi: 10.1093/ejcts/ezs455. [DOI] [PubMed] [Google Scholar]
- 3.Eveborn G.W., Schirmer H., Heggelund G., Lunde P., Rasmussen K. The evolving epidemiology of valvular aortic stenosis. the Tromso study. Heart. 2013;99:396–400. doi: 10.1136/heartjnl-2012-302265. [DOI] [PubMed] [Google Scholar]
- 4.Helske S., Kupari M., Lindstedt K.A., Kovanen P.T. Aortic valve stenosis: An active atheroinflammatory process. Curr. Opin. Lipidol. 2007;18:483–491. doi: 10.1097/MOL.0b013e3282a66099. [DOI] [PubMed] [Google Scholar]
- 5.Otto C.M., Kuusisto J., Reichenbach D.D., Gown A.M., O’Brien K.D. Characterization of the early lesion of ‘degenerative’ valvular aortic stenosis. Histological and immunohistochemical studies. Circulation. 1994;90:844–853. doi: 10.1161/01.CIR.90.2.844. [DOI] [PubMed] [Google Scholar]
- 6.Lindman B.R., Clavel M.A., Mathieu P., Iung B., Lancellotti P., Otto C.M., Pibarot P. Calcific aortic stenosis. Nat. Rev. Dis. Primers. 2016;2:16006. doi: 10.1038/nrdp.2016.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stewart B.F., Siscovick D., Lind B.K., Gardin J.M., Gottdiener J.S., Smith V.E., Kitzman D.W., Otto C.M. Clinical factors associated with calcific aortic valve disease. Cardiovascular Health Study. J. Am. Coll. Cardiol. 1997;29:630–634. doi: 10.1016/S0735-1097(96)00563-3. [DOI] [PubMed] [Google Scholar]
- 8.Katz R., Wong N.D., Kronmal R., Takasu J., Shavelle D.M., Probstfield J.L., Bertoni A.G., Budoff M.J., O’Brien K.D. Features of the metabolic syndrome and diabetes mellitus as predictors of aortic valve calcification in the Multi-Ethnic Study of Atherosclerosis. Circulation. 2006;113:2113–2119. doi: 10.1161/CIRCULATIONAHA.105.598086. [DOI] [PubMed] [Google Scholar]
- 9.Aronow W.S., Schwartz K.S., Koenigsberg M. Correlation of serum lipids, calcium, and phosphorus, diabetes mellitus and history of systemic hypertension with presence or absence of calcified or thickened aortic cusps or root in elderly patients. Am. J. Cardiol. 1987;59:998–999. doi: 10.1016/0002-9149(87)91144-1. [DOI] [PubMed] [Google Scholar]
- 10.Mohler E.R., Sheridan M.J., Nichols R., Harvey W.P., Waller B.F. Development and progression of aortic valve stenosis: Atherosclerosis risk factors—A causal relationship? A clinical morphologic study. Clin. Cardiol. 1991;14:995–999. doi: 10.1002/clc.4960141210. [DOI] [PubMed] [Google Scholar]
- 11.Thanassoulis G., Campbell C.Y., Owens D.S., Smith J.G., Smith A.V., Peloso G.M., Kerr K.F., Pechlivanis S., Budoff M.J., Harris T.B., et al. Genetic associations with valvular calcification and aortic stenosis. N. Engl. J. Med. 2013;368:503–512. doi: 10.1056/NEJMoa1109034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Arsenault B.J., Boekholdt S.M., Dube M.P., Rheaume E., Wareham N.J., Khaw K.T., Sandhu M.S., Tardif J.C. Lipoprotein(a) levels, genotype, and incident aortic valve stenosis: A prospective Mendelian randomization study and replication in a case-control cohort. Circ. Cardiovasc. Genet. 2014;7:304–310. doi: 10.1161/CIRCGENETICS.113.000400. [DOI] [PubMed] [Google Scholar]
- 13.Olkowicz M., Debski J., Jablonska P., Dadlez M., Smolenski R.T. Application of a new procedure for liquid chromatography/mass spectrometry profiling of plasma amino acid-related metabolites and untargeted shotgun proteomics to identify mechanisms and biomarkers of calcific aortic stenosis. J. Chromatogr. A. 2017;1517:66–78. doi: 10.1016/j.chroma.2017.08.024. [DOI] [PubMed] [Google Scholar]
- 14.Gil-Dones F., Darde V.M., Alonso-Orgaz S., Lopez-Almodovar L.F., Mourino-Alvarez L., Padial L.R., Vivanco F., Barderas M.G. Inside human aortic stenosis: A proteomic analysis of plasma. J. Proteom. 2012;75:1639–1653. doi: 10.1016/j.jprot.2011.11.036. [DOI] [PubMed] [Google Scholar]
- 15.Ljungberg J., Janiec M., Bergdahl I.A., Holmgren A., Hultdin J., Johansson B., Naslund U., Siegbahn A., Fall T., Soderberg S. Proteomic Biomarkers for Incident Aortic Stenosis Requiring Valvular Replacement. Circulation. 2018;138:590–599. doi: 10.1161/CIRCULATIONAHA.117.030414. [DOI] [PubMed] [Google Scholar]
- 16.Schlotter F., Halu A., Goto S., Blaser M.C., Body S.C., Lee L.H., Higashi H., DeLaughter D.M., Hutcheson J.D., Vyas P., et al. Spatiotemporal Multi-Omics Mapping Generates a Molecular Atlas of the Aortic Valve and Reveals Networks Driving Disease. Circulation. 2018;138:377–393. doi: 10.1161/CIRCULATIONAHA.117.032291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Martin-Rojas T., Gil-Dones F., Lopez-Almodovar L.F., Padial L.R., Vivanco F., Barderas M.G. Proteomic profile of human aortic stenosis: Insights into the degenerative process. J. Proteome Res. 2012;11:1537–1550. doi: 10.1021/pr2005692. [DOI] [PubMed] [Google Scholar]
- 18.Martin-Rojas T., Mourino-Alvarez L., Alonso-Orgaz S., Rosello-Lleti E., Calvo E., Lopez-Almodovar L.F., Rivera M., Padial L.R., Lopez J.A., de la Cuesta F., et al. iTRAQ proteomic analysis of extracellular matrix remodeling in aortic valve disease. Sci. Rep. 2015;5:17290. doi: 10.1038/srep17290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Heuschkel M.A., Skenteris N.T., Hutcheson J.D., van der Valk D.D., Bremer J., Goody P., Hjortnaes J., Jansen F., Bouten C.V.C., van den Bogaerdt A., et al. Integrative Multi-Omics Analysis in Calcific Aortic Valve Disease Reveals a Link to the Formation of Amyloid-Like Deposits. Cells. 2020;9:2164. doi: 10.3390/cells9102164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bosse Y., Miqdad A., Fournier D., Pepin A., Pibarot P., Mathieu P. Refining molecular pathways leading to calcific aortic valve stenosis by studying gene expression profile of normal and calcified stenotic human aortic valves. Circ. Cardiovasc. Genet. 2009;2:489–498. doi: 10.1161/CIRCGENETICS.108.820795. [DOI] [PubMed] [Google Scholar]
- 21.Helgadottir A., Thorleifsson G., Gretarsdottir S., Stefansson O.A., Tragante V., Thorolfsdottir R.B., Jonsdottir I., Bjornsson T., Steinthorsdottir V., Verweij N., et al. Genome-wide analysis yields new loci associating with aortic valve stenosis. Nat. Commun. 2018;9:987. doi: 10.1038/s41467-018-03252-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Theriault S., Gaudreault N., Lamontagne M., Rosa M., Boulanger M.C., Messika-Zeitoun D., Clavel M.A., Capoulade R., Dagenais F., Pibarot P., et al. A transcriptome-wide association study identifies PALMD as a susceptibility gene for calcific aortic valve stenosis. Nat. Commun. 2018;9:988. doi: 10.1038/s41467-018-03260-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Boffa M.B., Koschinsky M.L. Lipoprotein (a): Truly a direct prothrombotic factor in cardiovascular disease? J. Lipid Res. 2016;57:745–757. doi: 10.1194/jlr.R060582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van der Hoek Y.Y., Wittekoek M.E., Beisiegel U., Kastelein J.J., Koschinsky M.L. The apolipoprotein(a) kringle IV repeats which differ from the major repeat kringle are present in variably-sized isoforms. Hum. Mol. Genet. 1993;2:361–366. doi: 10.1093/hmg/2.4.361. [DOI] [PubMed] [Google Scholar]
- 25.Erqou S., Thompson A., Di Angelantonio E., Saleheen D., Kaptoge S., Marcovina S., Danesh J. Apolipoprotein(a) isoforms and the risk of vascular disease: Systematic review of 40 studies involving 58,000 participants. J. Am. Coll. Cardiol. 2010;55:2160–2167. doi: 10.1016/j.jacc.2009.10.080. [DOI] [PubMed] [Google Scholar]
- 26.Tsimikas S., Witztum J.L. The role of oxidized phospholipids in mediating lipoprotein(a) atherogenicity. Curr. Opin. Lipidol. 2008;19:369–377. doi: 10.1097/MOL.0b013e328308b622. [DOI] [PubMed] [Google Scholar]
- 27.Mohty D., Pibarot P., Despres J.P., Cote C., Arsenault B., Cartier A., Cosnay P., Couture C., Mathieu P. Association between plasma LDL particle size, valvular accumulation of oxidized LDL, and inflammation in patients with aortic stenosis. Arterioscler. Thromb. Vasc. Biol. 2008;28:187–193. doi: 10.1161/ATVBAHA.107.154989. [DOI] [PubMed] [Google Scholar]
- 28.Zheng K.H., Tsimikas S., Pawade T., Kroon J., Jenkins W.S.A., Doris M.K., White A.C., Timmers N., Hjortnaes J., Rogers M.A., et al. Lipoprotein(a) and Oxidized Phospholipids Promote Valve Calcification in Patients With Aortic Stenosis. J. Am. Coll. Cardiol. 2019;73:2150–2162. doi: 10.1016/j.jacc.2019.01.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yu B., Hafiane A., Thanassoulis G., Ott L., Filwood N., Cerruti M., Gourgas O., Shum-Tim D., Al Kindi H., de Varennes B., et al. Lipoprotein(a) Induces Human Aortic Valve Interstitial Cell Calcification. JACC Basic Transl. Sci. 2017;2:358–371. doi: 10.1016/j.jacbts.2017.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Torzewski M., Ravandi A., Yeang C., Edel A., Bhindi R., Kath S., Twardowski L., Schmid J., Yang X., Franke U.F.W., et al. Lipoprotein(a) Associated Molecules are Prominent Components in Plasma and Valve Leaflets in Calcific Aortic Valve Stenosis. JACC Basic Transl. Sci. 2017;2:229–240. doi: 10.1016/j.jacbts.2017.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Capoulade R., Chan K.L., Yeang C., Mathieu P., Bosse Y., Dumesnil J.G., Tam J.W., Teo K.K., Mahmut A., Yang X., et al. Oxidized Phospholipids, Lipoprotein(a), and Progression of Calcific Aortic Valve Stenosis. J. Am. Coll. Cardiol. 2015;66:1236–1246. doi: 10.1016/j.jacc.2015.07.020. [DOI] [PubMed] [Google Scholar]
- 32.Bouchareb R., Mahmut A., Nsaibia M.J., Boulanger M.C., Dahou A., Lepine J.L., Laflamme M.H., Hadji F., Couture C., Trahan S., et al. Autotaxin Derived From Lipoprotein(a) and Valve Interstitial Cells Promotes Inflammation and Mineralization of the Aortic Valve. Circulation. 2015;132:677–690. doi: 10.1161/CIRCULATIONAHA.115.016757. [DOI] [PubMed] [Google Scholar]
- 33.Nsaibia M.J., Mahmut A., Boulanger M.C., Arsenault B.J., Bouchareb R., Simard S., Witztum J.L., Clavel M.A., Pibarot P., Bosse Y., et al. Autotaxin interacts with lipoprotein(a) and oxidized phospholipids in predicting the risk of calcific aortic valve stenosis in patients with coronary artery disease. J. Intern. Med. 2016;280:509–517. doi: 10.1111/joim.12519. [DOI] [PubMed] [Google Scholar]
- 34.Von Zychlinski A., Kleffmann T., Williams M.J., McCormick S.P. Proteomics of Lipoprotein(a) identifies a protein complement associated with response to wounding. J. Proteom. 2011;74:2881–2891. doi: 10.1016/j.jprot.2011.07.008. [DOI] [PubMed] [Google Scholar]
- 35.Bourgeois R., Girard A., Perrot N., Guertin J., Mitchell P.L., Couture C., Gotti C., Bourassa S., Poggio P., Mass E., et al. A Comparative Analysis of the Lipoprotein(a) and Low-Density Lipoprotein Proteomic Profiles Combining Mass Spectrometry and Mendelian Randomization. CJC Open. 2020 doi: 10.1016/j.cjco.2020.11.019. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhou Y., Zhou B., Pache L., Chang M., Khodabakhshi A.H., Tanaseichuk O., Benner C., Chanda S.K. Metascape provides a biologist-oriented resource for the analysis of systems-level datasets. Nat. Commun. 2019;10:1523. doi: 10.1038/s41467-019-09234-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pinero J., Bravo A., Queralt-Rosinach N., Gutierrez-Sacristan A., Deu-Pons J., Centeno E., Garcia-Garcia J., Sanz F., Furlong L.I. DisGeNET: A comprehensive platform integrating information on human disease-associated genes and variants. Nucleic Acids Res. 2017;45:D833–D839. doi: 10.1093/nar/gkw943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ikeda Y., Imai Y., Kumagai H., Nosaka T., Morikawa Y., Hisaoka T., Manabe I., Maemura K., Nakaoka T., Imamura T., et al. Vasorin, a transforming growth factor beta-binding protein expressed in vascular smooth muscle cells, modulates the arterial response to injury in vivo. Proc. Natl. Acad. Sci. USA. 2004;101:10732–10737. doi: 10.1073/pnas.0404117101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bonnet A.L., Chaussain C., Broutin I., Rochefort G.Y., Schrewe H., Gaucher C. From Vascular Smooth Muscle Cells to Folliculogenesis: What About Vasorin? Front Med. 2018;5:335. doi: 10.3389/fmed.2018.00335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ackermann M.A., Petrosino J.M., Manring H.R., Wright P., Shettigar V., Kilic A., Janssen P.M.L., Ziolo M.T., Accornero F. TGF-beta1 affects cell-cell adhesion in the heart in an NCAM1-dependent mechanism. J. Mol. Cell Cardiol. 2017;112:49–57. doi: 10.1016/j.yjmcc.2017.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nagao K., Ono K., Iwanaga Y., Tamaki Y., Kojima Y., Horie T., Nishi H., Kinoshita M., Kuwabara Y., Hasegawa K., et al. Neural cell adhesion molecule is a cardioprotective factor up-regulated by metabolic stress. J. Mol. Cell Cardiol. 2010;48:1157–1168. doi: 10.1016/j.yjmcc.2009.10.014. [DOI] [PubMed] [Google Scholar]
- 42.Bardin N., Anfosso F., Masse J.M., Cramer E., Sabatier F., Le Bivic A., Sampol J., Dignat-George F. Identification of CD146 as a component of the endothelial junction involved in the control of cell-cell cohesion. Blood. 2001;98:3677–3684. doi: 10.1182/blood.V98.13.3677. [DOI] [PubMed] [Google Scholar]
- 43.Bardin N., Blot-Chabaud M., Despoix N., Kebir A., Harhouri K., Arsanto J.P., Espinosa L., Perrin P., Robert S., Vely F., et al. CD146 and its soluble form regulate monocyte transendothelial migration. Arterioscler. Thromb. Vasc. Biol. 2009;29:746–753. doi: 10.1161/ATVBAHA.108.183251. [DOI] [PubMed] [Google Scholar]
- 44.Kebir A., Harhouri K., Guillet B., Liu J.W., Foucault-Bertaud A., Lamy E., Kaspi E., Elganfoud N., Vely F., Sabatier F., et al. CD146 short isoform increases the proangiogenic potential of endothelial progenitor cells in vitro and in vivo. Circ. Res. 2010;107:66–75. doi: 10.1161/CIRCRESAHA.109.213827. [DOI] [PubMed] [Google Scholar]
- 45.Luo Y., Duan H., Qian Y., Feng L., Wu Z., Wang F., Feng J., Yang D., Qin Z., Yan X. Macrophagic CD146 promotes foAm. cell formation and retention during atherosclerosis. Cell Res. 2017;27:352–372. doi: 10.1038/cr.2017.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Connor E.M., Eppihimer M.J., Morise Z., Granger D.N., Grisham M.B. Expression of mucosal addressin cell adhesion molecule-1 (MAdCAM-1) in acute and chronic inflammation. J. Leukoc. Biol. 1999;65:349–355. doi: 10.1002/jlb.65.3.349. [DOI] [PubMed] [Google Scholar]
- 47.Hillenbrand R., Molthagen M., Montag D., Schachner M. The close homologue of the neural adhesion molecule L1 (CHL1): Patterns of expression and promotion of neurite outgrowth by heterophilic interactions. Eur. J. Neurosci. 1999;11:813–826. doi: 10.1046/j.1460-9568.1999.00496.x. [DOI] [PubMed] [Google Scholar]
- 48.Holm J., Hillenbrand R., Steuber V., Bartsch U., Moos M., Lubbert H., Montag D., Schachner M. Structural featuRes. of a close homologue of L1 (CHL1) in the mouse: A new member of the L1 family of neural recognition molecules. Eur. J. Neurosci. 1996;8:1613–1629. doi: 10.1111/j.1460-9568.1996.tb01306.x. [DOI] [PubMed] [Google Scholar]
- 49.Frints S.G., Marynen P., Hartmann D., Fryns J.P., Steyaert J., Schachner M., Rolf B., Craessaerts K., Snellinx A., Hollanders K., et al. CALL interrupted in a patient with non-specific mental retardation: Gene dosage-dependent alteration of murine brain development and behavior. Hum. Mol. Genet. 2003;12:1463–1474. doi: 10.1093/hmg/ddg165. [DOI] [PubMed] [Google Scholar]
- 50.Sakurai K., Migita O., Toru M., Arinami T. An association between a missense polymorphism in the close homologue of L1 (CHL1, CALL) gene and schizophrenia. Mol. Psychiatry. 2002;7:412–415. doi: 10.1038/sj.mp.4000973. [DOI] [PubMed] [Google Scholar]
- 51.Eskelinen E.L., Illert A.L., Tanaka Y., Schwarzmann G., Blanz J., Von Figura K., Saftig P. Role of LAMP-2 in lysosome biogenesis and autophagy. Mol. Biol. Cell. 2002;13:3355–3368. doi: 10.1091/mbc.e02-02-0114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jansen I.D.C., Tigchelaar-Gutter W., Hogervorst J.M.A., de Vries T.J., Saftig P., Everts V. LAMP-2 Is Involved in Surface Expression of RANKL of Osteoblasts In Vitro. Int. J. Mol. Sci. 2020;21:6110. doi: 10.3390/ijms21176110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mavrogeni S., Markousis-Mavrogenis G., Markussis V., Kolovou G. The Emerging Role of Cardiovascular Magnetic Resonance Imaging in the Evaluation of Metabolic Cardiomyopathies. Horm. Metab. Res. 2015;47:623–632. doi: 10.1055/s-0035-1555913. [DOI] [PubMed] [Google Scholar]
- 54.Shagdarsuren E., Bidzhekov K., Djalali-Talab Y., Liehn E.A., Hristov M., Matthijsen R.A., Buurman W.A., Zernecke A., Weber C. C1-esterase inhibitor protects against neointima formation after arterial injury in atherosclerosis-prone mice. Circulation. 2008;117:70–78. doi: 10.1161/CIRCULATIONAHA.107.715649. [DOI] [PubMed] [Google Scholar]
- 55.ter Weeme M., Vonk A.B., Kupreishvili K., van Ham M., Zeerleder S., Wouters D., Stooker W., Eijsman L., Van Hinsbergh V.W., Krijnen P.A., et al. Activated complement is more extensively present in diseased aortic valves than naturally occurring complement inhibitors: A sign of ongoing inflammation. Eur. J. Clin. Investig. 2010;40:4–10. doi: 10.1111/j.1365-2362.2009.02216.x. [DOI] [PubMed] [Google Scholar]
- 56.Saeedi R., Li M., Frohlich J. A review on lecithin:cholesterol acyltransferase deficiency. Clin. Biochem. 2015;48:472–475. doi: 10.1016/j.clinbiochem.2014.08.014. [DOI] [PubMed] [Google Scholar]
- 57.Holleboom A.G., Kuivenhoven J.A., Vergeer M., Hovingh G.K., van Miert J.N., Wareham N.J., Kastelein J.J., Khaw K.T., Boekholdt S.M. Plasma levels of lecithin:cholesterol acyltransferase and risk of future coronary artery disease in apparently healthy men and women: A prospective case-control analysis nested in the EPIC-Norfolk population study. J. Lipid Res. 2010;51:416–421. doi: 10.1194/P900038-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tani S., Takahashi A., Nagao K., Hirayama A. Association of lecithin-cholesterol acyltransferase activity measured as a serum cholesterol esterification rate and low-density lipoprotein heterogeneity with cardiovascular risk: A cross-sectional study. Heart Vessels. 2016;31:831–840. doi: 10.1007/s00380-015-0678-9. [DOI] [PubMed] [Google Scholar]
- 59.Priyanka K., Singh S., Gill K. Paraoxonase 3: Structure and Its Role in Pathophysiology of Coronary Artery Disease. Biomolecules. 2019;9:817. doi: 10.3390/biom9120817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chistiakov D.A., Melnichenko A.A., Orekhov A.N., Bobryshev Y.V. Paraoxonase and atherosclerosis-related cardiovascular diseases. Biochimie. 2017;132:19–27. doi: 10.1016/j.biochi.2016.10.010. [DOI] [PubMed] [Google Scholar]
- 61.Nakaya Y., Schaefer E.J., Brewer H.B., Jr. Activation of human post heparin lipoprotein lipase by apolipoprotein H (beta 2-glycoprotein I) Biochem. Biophys. Res. Commun. 1980;95:1168–1172. doi: 10.1016/0006-291X(80)91595-8. [DOI] [PubMed] [Google Scholar]
- 62.Kaltoft M., Langsted A., Nordestgaard B.G. Triglycerides and remnant cholesterol associated with risk of aortic valve stenosis: Mendelian randomization in the Copenhagen General Population Study. Eur. Heart J. 2020;41:2288–2299. doi: 10.1093/eurheartj/ehaa172. [DOI] [PubMed] [Google Scholar]
- 63.Hasunuma Y., Matsuura E., Makita Z., Katahira T., Nishi S., Koike T. Involvement of beta 2-glycoprotein I and anticardiolipin antibodies in oxidatively modified low-density lipoprotein uptake by macrophages. Clin. Exp. Immunol. 1997;107:569–573. doi: 10.1046/j.1365-2249.1997.00300.x. [DOI] [PubMed] [Google Scholar]
- 64.Kochl S., Fresser F., Lobentanz E., Baier G., Utermann G. Novel interaction of apolipoprotein(a) with beta-2 glycoprotein I mediated by the kringle IV domain. Blood. 1997;90:1482–1489. doi: 10.1182/blood.V90.4.1482. [DOI] [PubMed] [Google Scholar]
- 65.Chonn A., Semple S.C., Cullis P.R. Beta 2 glycoprotein I is a major protein associated with very rapidly cleared liposomes in vivo, suggesting a significant role in the immune clearance of “non-self” particles. J. Biol. Chem. 1995;270:25845–25849. doi: 10.1074/jbc.270.43.25845. [DOI] [PubMed] [Google Scholar]
- 66.Zioncheck T.F., Powell L.M., Rice G.C., Eaton D.L., Lawn R.M. Interaction of recombinant apolipoprotein(a) and lipoprotein(a) with macrophages. J. Clin. Investig. 1991;87:767–771. doi: 10.1172/JCI115079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sanghera D.K., Wagenknecht D.R., McIntyre J.A., Kamboh M.I. Identification of structural mutations in the fifth domain of apolipoprotein H (beta 2-glycoprotein I) which affect phospholipid binding. Hum. Mol. Genet. 1997;6:311–316. doi: 10.1093/hmg/6.2.311. [DOI] [PubMed] [Google Scholar]
- 68.Bosse Y., Feitosa M.F., Despres J.P., Lamarche B., Rice T., Rao D.C., Bouchard C., Perusse L., Vohl M.C. Detection of a major gene effect for LDL peak particle diameter and association with apolipoprotein H gene haplotype. Atherosclerosis. 2005;182:231–239. doi: 10.1016/j.atherosclerosis.2005.02.008. [DOI] [PubMed] [Google Scholar]
- 69.Hoekstra M., Chen H.Y., Rong J., Dufresne L., Yao J., Guo X., Tsai M.Y., Tsimikas S., Post W.S., Vasan R.S., et al. Genome-Wide Association Study Highlights APOH as a Novel Locus for Lipoprotein(a) Levels-Brief Report. Arterioscler. Thromb. Vasc. Biol. 2021;41:458–464. doi: 10.1161/ATVBAHA.120.314965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ehrenstein M.R., Notley C.A. The importance of natural IgM: Scavenger, protector and regulator. Nat. Rev. Immunol. 2010;10:778–786. doi: 10.1038/nri2849. [DOI] [PubMed] [Google Scholar]
- 71.Sjoberg B.G., Su J., Dahlbom I., Gronlund H., Wikstrom M., Hedblad B., Berglund G., de Faire U., Frostegard J. Low levels of IgM antibodies against phosphorylcholine-A potential risk marker for ischemic stroke in men. Atherosclerosis. 2009;203:528–532. doi: 10.1016/j.atherosclerosis.2008.07.009. [DOI] [PubMed] [Google Scholar]
- 72.Tsimikas S., Brilakis E.S., Lennon R.J., Miller E.R., Witztum J.L., McConnell J.P., Kornman K.S., Berger P.B. Relationship of IgG and IgM autoantibodies to oxidized low density lipoprotein with coronary artery disease and cardiovascular events. J. Lipid Res. 2007;48:425–433. doi: 10.1194/jlr.M600361-JLR200. [DOI] [PubMed] [Google Scholar]
- 73.Shaw P.X., Goodyear C.S., Chang M.K., Witztum J.L., Silverman G.J. The autoreactivity of anti-phosphorylcholine antibodies for atherosclerosis-associated neo-antigens and apoptotic cells. J. Immunol. 2003;170:6151–6157. doi: 10.4049/jimmunol.170.12.6151. [DOI] [PubMed] [Google Scholar]
- 74.Tuominen A., Miller Y.I., Hansen L.F., Kesaniemi Y.A., Witztum J.L., Horkko S. A natural antibody to oxidized cardiolipin binds to oxidized low-density lipoprotein, apoptotic cells, and atherosclerotic lesions. Arterioscler. Thromb. Vasc. Biol. 2006;26:2096–2102. doi: 10.1161/01.ATV.0000233333.07991.4a. [DOI] [PubMed] [Google Scholar]
- 75.Saito Y., Kondo H., Hojo Y. Granzyme B as a novel factor involved in cardiovascular diseases. J. Cardiol. 2011;57:141–147. doi: 10.1016/j.jjcc.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 76.Ohukainen P., Napakangas J., Ohtonen P., Ruskoaho H., Taskinen P., Peltonen T., Rysa J. Expression and Localization of Granzymes and Perforin in Human Calcific Aortic Valve Disease. J. Heart Valve Dis. 2015;24:612–620. [PubMed] [Google Scholar]
- 77.Hendel A., Cooper D., Abraham T., Zhao H., Allard M.F., Granville D.J. Proteinase inhibitor 9 is reduced in human atherosclerotic lesion development. Cardiovasc. Pathol. 2012;21:28–38. doi: 10.1016/j.carpath.2010.12.002. [DOI] [PubMed] [Google Scholar]
- 78.Langsted A., Kamstrup P.R., Nordestgaard B.G. High lipoprotein(a) and high risk of mortality. Eur. Heart J. 2019;40:2760–2770. doi: 10.1093/eurheartj/ehy902. [DOI] [PubMed] [Google Scholar]
- 79.Zenin A., Tsepilov Y., Sharapov S., Getmantsev E., Menshikov L.I., Fedichev P.O., Aulchenko Y. Identification of 12 genetic loci associated with human healthspan. Commun. Biol. 2019;2:41. doi: 10.1038/s42003-019-0290-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Arsenault B.J., Pelletier W., Kaiser Y., Perrot N., Couture C., Khaw K.T., Wareham N.J., Bosse Y., Pibarot P., Stroes E.S.G., et al. Association of Long-term Exposure to Elevated Lipoprotein(a) Levels With Parental Life Span, Chronic Disease-Free Survival, and Mortality Risk: A Mendelian Randomization Analysis. JAMA Netw. Open. 2020;3:e200129. doi: 10.1001/jamanetworkopen.2020.0129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Timmers P.R., Mounier N., Lall K., Fischer K., Ning Z., Feng X., Bretherick A.D., Clark D.W., eQTLGen Consortium. Shen X., et al. Genomics of 1 million parent lifespans implicates novel pathways and common diseases and distinguishes survival chances. Elife. 2019;8:e39856. doi: 10.7554/eLife.39856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Despres A.A., Perrot N., Poulin A., Tastet L., Shen M., Chen H.Y., Bourgeois R., Trottier M., Tessier M., Guimond J., et al. Lipoprotein(a), Oxidized Phospholipids, and Aortic Valve Microcalcification Assessed by 18F-Sodium Fluoride Positron Emission Tomography and Computed Tomography. CJC Open. 2019;1:131–140. doi: 10.1016/j.cjco.2019.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Romagnuolo R., Marcovina S.M., Boffa M.B., Koschinsky M.L. Inhibition of plasminogen activation by apo(a): Role of carboxyl-terminal lysines and identification of inhibitory domains in apo(a) J. Lipid Res. 2014;55:625–634. doi: 10.1194/jlr.M036566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bourgeois R., Devillers R., Perrot N., Despres A.A., Boulanger M.C., Mitchell P.L., Guertin J., Couture P., Boffa M.B., Scipione C.A., et al. Interaction of Autotaxin With Lipoprotein(a) in Patients With Calcific Aortic Valve Stenosis. JACC Basic Transl. Sci. 2020;5:888–897. doi: 10.1016/j.jacbts.2020.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Theriault S., Dina C., Messika-Zeitoun D., Le Scouarnec S., Capoulade R., Gaudreault N., Rigade S., Li Z., Simonet F., Lamontagne M., et al. Genetic Association Analyses Highlight IL6, ALPL, and NAV1 As 3 New Susceptibility Genes Underlying Calcific Aortic Valve Stenosis. Circ. Genom. Precis Med. 2019;12:e002617. doi: 10.1161/CIRCGEN.119.002617. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The microarray gene expression dataset on human aortic valves is available in the Gene Expression Omnibus with accession number GSE102249. The other data available upon reasonable request.