Abstract
Cardiorenal syndrome is a term that defines the complex bidirectional nature of the interaction between cardiac and renal disease. It is well established that patients with kidney disease have higher incidence of cardiovascular comorbidities and that renal dysfunction is a significant threat to the prognosis of patients with cardiac disease. Fibrosis is a common characteristic of organ injury progression that has been proposed not only as a marker but also as an important driver of the pathophysiology of cardiorenal syndromes. Due to the relevance of fibrosis, its study might give insight into the mechanisms and targets that could potentially be modulated to prevent fibrosis development. The aim of this review was to summarize some of the pathophysiological pathways involved in the fibrotic damage seen in cardiorenal syndromes, such as inflammation, oxidative stress and endoplasmic reticulum stress, which are known to be triggers and mediators of fibrosis.
Keywords: cardiorenal syndrome, endoplasmic reticulum stress, fibrosis, heart failure, inflammation, kidney disease, oxidative stress
1. Introduction
The existence of a relationship between the heart and the kidney was first described in the XIX century by Robert Bright, who reported structural changes in the heart in patients with advanced kidney disease [1]. Since then, new discoveries have given insight into the interaction between heart and kidney diseases in terms of shared risk factors (such as hypertension, obesity, diabetes and atherosclerosis) and the pathophysiological pathways involved in each [2,3,4]. Clinically, the shared pathology of the heart and kidneys has a strong impact on the clinical outcome and is associated with increased morbidity and mortality rates [5,6].
The classic definition of cardiorenal syndrome (CRS) was proposed in 2010 by the Acute Dialysis Quality Initiative as a term that gathers the “disorders of the heart and kidneys whereby acute or chronic dysfunction in one organ may induce acute or chronic dysfunction of the other” [7]. In addition, within the term there is further classification into different subtypes according to the primary organ dysfunction and to whether it is an acute or chronic situation [7]. However, the appearance of risk factors that can affect both the heart and the kidney complicate the clinical picture, and with it the causal relationship of one to the other.
2. CRS Classification
2.1. CRS Type 1 or Acute Cardiorenal Syndrome
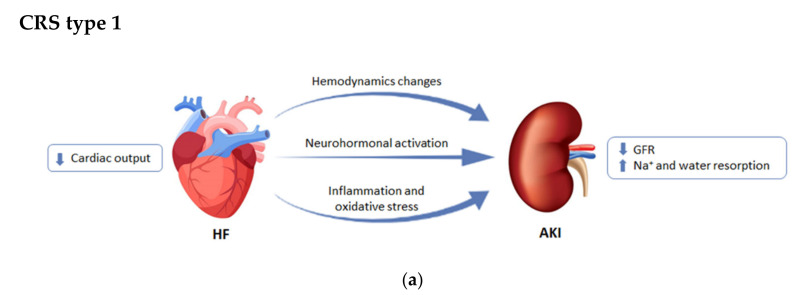
CRS type 1 (CRS-1) is characterized by the worsening of cardiac function leading to acute kidney injury (AKI) and/or dysfunction of both organs [7]. Around 25–30% of patients with acute decompensated heart failure (ADHF) present AKI, often after ischemic or non-ischemic heart disease [8,9,10]. These patients have higher morbi-mortality and lengthier hospitalization [7]. CRS-1 has a complex pathophysiology, with hemodynamic and non-hemodynamic alterations for which the treatments show no improvements [10,11], thus demonstrating the need to discover and understand the mechanisms involved.
Faced with a drop in blood pressure levels due to the development of heart failure (HF), the kidney responds to the decrease in cardiac output by retaining sodium and water. Nevertheless, it has been demonstrated that an elevation of the central venous pressure can result in impairment of renal function and congestion of the kidneys [10,12]. In this context, neurohormonal activation through the Renin–Angiotensin–Aldosterone System (RAAS) also has an important role, as it is both an initially compensatory mechanism for the decrease in volume consequence of the ventricular injury, and a long-term initiator of cardiovascular and renal dysfunction [13,14]. Other non-hemodynamic mechanisms, such as inflammation and oxidative stress, have been established as common pathways for cellular dysfunction in heart and kidney failure [9,10,11,15].
2.2. CRS Type 2 or Chronic Cardiorenal Syndrome
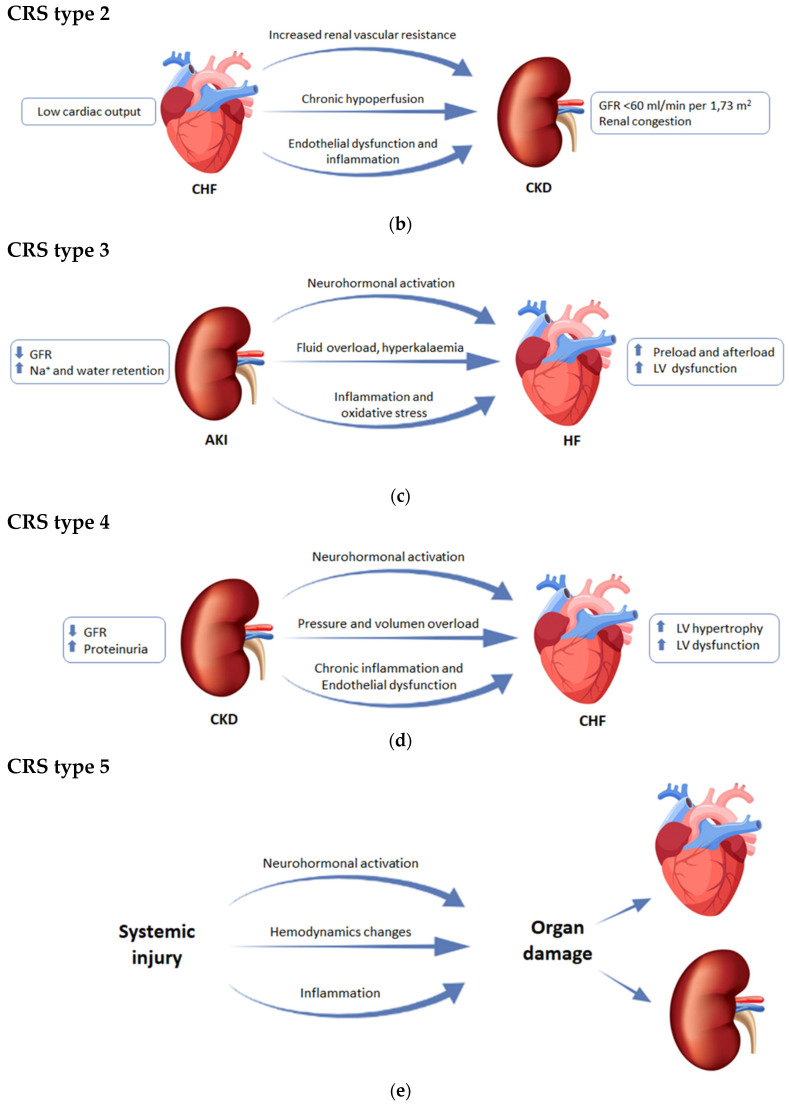
CRS type 2 is defined as chronic cardiac dysfunction that leads to progressive appearance of renal impairment that promotes the development of chronic kidney disease (CKD) [6,16,17]. CKD was defined in 2012 by Kidney Disease: Improving Global Outcomes (KDIGO) as an abnormality in kidney function or structure that is present for more than 3 months and has health implications. It is classified based on cause, a glomerular filtration rate (GFR) of <60 mL/min per 1.73 m2 and the degree of albuminuria [18]. A meta-analysis by Damman et al. showed that almost a third (32%) of the total of 1 million HF patients studied presented CKD, and 23% had worsening renal function [19], confirming that renal dysfunction is an important contributor to the comorbidities in HF.
The pathological process implicated in CKD secondary to HF is a consequence of the renal response to preserve the GFR. The combination of renal congestion, hypoperfusion and the increased right atrium pressure promotes renal dysfunction in HF patients [6,11]. It has been suggested that the correct diagnosis of this CRS should be based on HF aetiology, HF with preserved ejection fraction (HFpEF) or with reduced ejection fraction (HFrEF), and on biochemical parameters of renal dysfunction, such as creatinine levels [20]. However, as the interactions between the heart and kidney are bidirectional, is not always easy to assess the inciting event from the secondary damage, thus making it difficult to differentiate CRS type 2 patients from CRS type 4 ones [11,20].
2.3. CRS Type 3 or Acute Reno-Cardiac Syndrome
CRS type 3 occurs when there is an acute worsening of kidney function secondary to AKI, ischemia, or glomerulonephritis that leads to acute heart injury and/or dysfunction [6,7,11]. AKI may produce cardiac events as a consequence of the fluid overload, hyperkalaemia, or metabolic acidosis, but the exact cause of the damage is difficult to establish, as there are shared comorbidities and variability in the risk factors for AKI [6,11,21,22].
There are multiple definitions of AKI according to urine output and serum creatinine levels (SCr), all of which have limitations in their clinical application [21,23]. It is due to the differing definitions of AKI that make it difficult to identify this type of CRS. Despite the lacking criteria, the incidence of AKI is increasing in hospitalized patients, and is associated with an 86% increased risk of cardiovascular mortality and a 38% increased risk of major cardiovascular events [24].
2.4. CRS Type 4 or Chronic Reno-Cardiac Syndrome
CRS type 4 is characterized by cardiovascular damage in patients with CKD at any stage [7,11]. It is well established that renal dysfunction is an independent risk factor for cardiovascular disease, with the risk for myocardial infection and sudden death being higher in CKD patients [25,26]. Numerous studies have found there is an independent association between the severity of CKD, evaluated by the degree of decline in kidney function, and the subsequent cardiac events [5,27,28], which could suggest that CKD likely accelerates the risk and development of cardiovascular disease [7].
CKD has been demonstrated to be associated with inflammation and other cardiovascular factors, such as hypertension, activation of RAAS, or volume overload, that usually go in parallel with a decline in GFR [26,29]. Pressure and volume overload in CKD patients lead to left ventricular hypertrophy (LVH), which is a common feature that is accompanied by fibrosis and other histological changes. These structural changes consequently cause diastolic dysfunction and increased oxygen demand, which could also explain these patients’ predispositions to arrhythmias and sudden death [6,29,30].
2.5. CRS Type 5 or Secondary Cardiorenal Syndrome
CRS type 5 (CRS-5) represents simultaneous injury and/or dysfunction of the heart and kidneys as a result of a systemic condition, such as sepsis, drug toxicity, lupus, cirrhosis or amyloidosis [7,23,31]. Although many pathways have been proposed, it is challenging to identify the mechanisms that are involved in CRS-5 due to the multitude of contributing factors and the sequence of organ involvement [7,31].
CRS-5 has been divided into four stages according to severity: hyperacute (0–72 h after diagnosis), acute (3–7 days), subacute (7–30 days) and chronic (beyond 30 days) [6,23]. Usually, the existing studies of CRS-5 are those of hyperacute or acute stages, as these evaluate the effects of sepsis. Sepsis, defined as a life-threatening organ dysfunction caused by a deregulated host response to infection [32], is one of the most common causes of death among hospitalized patients [33], among whom the prevalence of CRS-5 is high [7,34].
In the early stages of sepsis, microcirculatory changes are developed despite normal systemic haemodynamics [35]. Those alterations, along with inflammation, are important in the cardiac and renal dysfunction given in this type of CRS [11]. For instance, the increase in pro-inflammatory cytokines during sepsis and the decrease in renal blood flow lead to tubular necrosis, reduction in GFR and severe kidney failure [6,23,26]. Sepsis is also related to autonomic nervous system dysfunction and RAAS activation [23,31]. This complex environment makes differentiating between the cardiorenal crosstalk effects and sepsis effects very difficult.
The different CRSs are summarized in Figure 1.
Figure 1.
Differences among the subtypes of cardiorenal syndrome (CRS). (a) CRS type 1 or acute cardiorenal syndrome; (b) CRS type 2 or chronic cardiorenal syndrome; (c) CRS type 3 or acute reno-cardiac syndrome; (d) CRS type 4 or chronic reno-cardiac syndrome; (e) CRS type 5 or secondary cardiorenal syndrome. GFR: glomerular filtration rate; LV: left ventricular. Modified from [7].
3. Pathophysiology of CRS
Due to the essential role of both the heart and kidney in the maintenance of cardiovascular homeostasis, initial organ damage during a disease state, such as CRS, can induce structural remodelling and functional alterations in the other.
3.1. Cardiac Alterations Associated with CKD
As CKD is considered an important complication associated with higher cardiovascular risk and mortality. This increased risk is partially due to common risk factors such as hypertension, obesity or diabetes [36], but not entirely, as the association between CKD and cardiovascular mortality persists after risk factor adjustment [37,38]. Albuminuria- and creatinine-based estimated GFR (eGFR) are currently considered to be useful measurements for cardiovascular risk prediction, as they improve discrimination for cardiovascular mortality among CKD patients beyond traditional risk factors [38,39].
In patients with CKD there is high prevalence of structural and functional heart alterations from the early stages to end-stage renal disease (ESRD), which includes left ventricular (LV) remodelling, valvular sclerosis, reduction of the ejection fraction (EF) and diastolic dysfunction [40,41,42].
Echocardiographic studies have observed that LV remodelling is prevalent among patients with CKD and has been recognized as an important predictor of poor prognosis [43,44]. There are many factors that influence LV geometry in CKD patients. Pressure overload causes the thickening of the LV walls, which translates into concentric hypertrophy, whereas hypervolemia and anaemia contributes to the development of eccentric hypertrophy [45]. Two studies have reported the existence of associations between LV hypertrophy and renal dysfunction, characterized by low eGFR, which are independent of other risk factors, suggesting that impaired kidney function contributes to LV hypertrophy. In addition, they also describe that LV geometry tends to shift to concentric LV hypertrophy in advanced kidney dysfunction rather than eccentric hypertrophy [44,46]. A recent clinical study showed that the stages are associated with LV remodelling even in milder CKD, as 22% of 90 patients with stages 1 to 3 presented concentric hypertrophy, 19% eccentric hypertrophy and 20% concentric remodelling [47].
Most of the studies that have investigated the association between CKD and cardiac alterations have focused on the assessment of LV mass or hypertrophy, whilst fewer have explored LV function (neither systolic nor diastolic) [48]. In terms of LV systolic function, LVEF has been used in the majority of studies, although subclinical systolic dysfunction can happen in patients with CKD despite normal LVEF [49,50,51]. Diastolic dysfunction usually coexists with systolic dysfunction during LV remodelling and is common in CKD patients [44,52,53,54].
Numerous studies have assessed LV function in patients with CKD by trying to find an association between eGFR or albuminuria and systolic or diastolic function alterations. According to the literature, systolic dysfunction seems to be strongly correlated with albuminuria over low eGFR [55,56,57]. However, there is high variability. Similarly, there seems to be higher association of diastolic dysfunction with albuminuria than eGFR [56,57,58]. Therefore, some studies have found clear association between low eGFR, LV diastolic dysfunction and LVH [44,59].
Despite the advances made in cardiac damage, the increasing incidence and prevalence of HF makes it an important health problem. For that reason, various potential biomarkers that could contribute to diagnosis have been proposed. The gold standard in chronic HF diagnosis and prognosis is the natriuretic peptides, such as atrial natriuretic peptide (ANP) and brain natriuretic peptide (BNP), which are produced within the heart as a response to myocardial stretch as a consequence of volume or pressure overload [60,61]. HF guidelines currently recommends monitoring of BNP and its precursor, N-terminal-proBNP (NT-proBNP), for CHF progression evaluation. It must be acknowledged, however, that age, body mass, renal failure and pulmonary diseases influence its plasmatic concentrations [62]. Other molecules associated with myocyte necrosis or injury have been evaluated as HF biomarkers, such as cardiac troponins (cTn), which are regulatory proteins involved in contraction. The troponin complex is formed by cardiac troponin C (cTnC), I (cTnI) and T (cTnT), which dissociates after Ca2+ binds to cTnC. cTnI and cTnT are considered a reference marker of myocardial injury as its blood concentrations rise after myocyte damage [63,64].
3.2. Renal Alteration Associated to HF
As explained before, CRS-1 and CRS-2 are characterized by progressive kidney damage due to HF. Over 50% of HF patients have been reported to have renal insufficiency [19,65]. Indeed, even a modest reduction in renal function is associated with a higher mortality rate in cardiovascular disease patients [19,66]. The most currently used diagnostic measurements for renal damage are GFR, serum creatinine and urinary output.
The systolic blood pressure and effective arterial volume are reduced once HF develops, which translates into a decrease in renal blood flow as well as GFR [67]. In order to preserve adequate blood flow, the kidneys autoregulate through different mechanisms, including sympathetic nervous system (SNS) and RAAS activation, which would act as vasoconstrictors of the afferent and the efferent arteriole [13,68]. In the long term, this activation or the neurohormonal axis could result in podocyte injury [69,70], loss of mesangial integrity [71,72], tubular and glomerular damage [73,74,75] and kidney dysfunction [76], which are often associated with CKD and ESRD.
It is common to use the term kidney failure in a clinical setting to refer to a situation where there is a persistent decrease in eGFR in the short term [18]. Another important concept is worsening renal function, which is considered to appear in those patients in which the serum creatinine increases by 25% compared to the basal levels or the eGFR decreases by more than 20% in a period of around 25 weeks [77,78]. AKI is characterized by a rapid loss of kidney function that can happen in HF patients when diuresis decreases <0.5 mL/kg/h in 6–12 h or the basal serum creatinine levels increases ≥0.3 mg/dL in 48 h [77,78].
In addition to traditional markers of decreased glomerular filtration, such as creatinine and albuminuria [79,80], other markers, such as cystatin C [81,82] and blood urea nitrogen (BUN) [83,84], also have been proposed as possible biomarkers of tubular damage.
One of these is Neutrophil Gelatinase Associated Lipocalin (NGAL), a small glycoprotein expressed in renal and other cell types to which different functions have been attributed [85]. Its involvement in renal pathologies and its role as a biomarker comes from its rapid release in response to a tubular lesion and its presence in plasma, serum and urine, making it easy to quantify [85,86]. Another proposed molecule is kidney injury molecule-1 (KIM-1), a transmembrane glycoprotein expressed in low levels in healthy kidneys. Shortly after tubular damage, KIM-1 cleavage allows its secretion by the injured cells to the tubule lumen, resulting in detection in the urine, to where it is excreted [87]. Moreover, its role as a biomarker has proved to be associated with inflammation and fibrosis in the injured kidney, which would help monitor the degree of tubular damage [88,89,90]. Interleukin-18 (IL-18) is a proinflammatory cytokine that is expressed in activated macrophages, renal epithelial cells and others [91]. Urinary IL-18 is considered a marker of both short- and long-term injury in AKI, as it increases within 6 h of the insult or at least a day before serum creatinine increase [91,92].
3.3. Fibrosis
Another common structural alteration observed in both heart and kidney remodelling in CRS is fibrosis, which is also considered a key contributor to the progression of cardiac and renal failure [93,94,95]. Fibrosis is an important process that can be contemplated as aberrant wound healing as a consequence of the misbalance between extracellular matrix (ECM) production and degradation [96]. The fibrotic response to injury can be classified into reparative, when the scar is necessary to stabilize the tissue defect, or reactive, when the mechanical stress and the hormonal mediators facilitate the expansion of connective tissue in a remote non-injured zone, compromising the correct function of the organ [97]. The main fibrosis effectors are the fibroblasts and myofibroblasts, both of which are responsible for the synthesis and accumulation of interstitial ECM proteins. While fibroblasts are mesenchymal cells ubiquitous in tissues and organs, myofibroblasts are differentiated cells that are rarely found in non-pathological environments [98,99,100]. The fibrotic scar composition is similar amongst different tissues, predominantly formed by collagens type I and III, fibronectin, proteoglycans and laminin [101,102,103].
As a response to the damaged heart in cardiac ischemia, myocardial remodelling occurs through the secretion of ECM components by the myofibroblasts. Histopathologically speaking, there are three types of cardiac fibrosis: replacement fibrosis, interstitial fibrosis and perivascular fibrosis. Replacement fibrosis provides structural support, as it consists of the removal of necrotic tissue and generation of a fibrotic scar within the infarcted zone that compensates cardiomyocyte loss [104,105]. On the other hand, the widespread deposition of ECM proteins in the endo and perimysium of remote areas of the infarct is what is known as interstitial fibrosis [106]. The term perivascular fibrosis is used to describe the increase in connective tissue around the cardiac microvasculature [107], both of which are types of fibrotic lesions that could not be a consequence of cardiomyocyte death.
The remodelling that follows after MI happens in different phases that partially overlap: First, there is cell death and an inflammatory response (inflammatory phase); secondly, the resolution of inflammation and fibroblast proliferation (proliferative or reparative phase); and lastly, the scar formation and maturation (maturation or remodelling phase) [108]. During the proliferative phase, which usually coexists with the inflammatory and reparative phases, there is an increase in the number of fibroblasts, which will adopt the proliferatory, secretory and migratory myofibroblast phenotype [109]. Following the proliferative phase of cardiac repair, when the scar has been synthesized, there begins a long process known as maturation, in which an organized fibrotic state is formed due to ECM crosslinking [110] and scar reinforcement by other components of the ECM, such as decorin [111,112] and perlecan [113,114]. In addition, during the maturation phase, the activated fibroblasts go through apoptosis and senescence [115]. The presence of a mature fibrotic scar ultimately leads to an increased ventricular stiffness that compromises cardiac output [116,117]. In addition to the impaired cardiac contractility, fibrosis also interferes with the normal electrical signals within the heart, which predisposes to arrhythmias and fibrillation [118,119]. Overall, fibrosis has thus been proposed as a risk factor in HF as it predisposes to ventricular systolic and diastolic dysfunction [120,121,122], cardiomyocyte hypertrophy [122,123,124] and sudden cardiac death [125,126], thereby increasing mortality [127,128].
At the renal level, CKD is characterized by functional loss and deposition of connective tissue that ends up creating a common fibrotic phenotype independently of the initial damage. This happens since tubulointerstitial diseases lead to glomerular injury, and glomerular lesions produce tubulointerstitial damage. Fibrosis is a common manifestation of functional alterations that spreads in response to sustained inflammation and epithelial damage [129,130,131]. Among the events that induce fibrosis, both diabetes and hypertension are considered to be the leading causes of CKD [132,133], as they elevate the glomerular pressure that gradually leads to glomerular damage, endothelial dysfunction [134,135] and other structural changes, such as alterations of the glomerular basement membrane [136,137,138], decrease in podocyte number and mesangial distension [136,139,140]. As a result of such damage, the renal tissue would start a response that resembles wound healing in other tissues. The scar created in the early stage is potentially reversible but with the progression of the damage, the cross-linking of the ECM proteins makes it stiff and resistant to proteolysis [141].
During chronic injury to the kidney in CKD, the excessive accumulation of connective tissue and expansion of interstitial fibroblasts during the reparative stage of the fibrotic scar can happen in all compartments of the kidney, including the glomeruli, usually termed glomerulosclerosis, and the tubules, which is referred to as tubulointerstitial fibrosis [142,143,144]. Such deposition of the fibrotic matrix alters organ structure and function, which could further damage kidney function, as it impairs blood flow in this region of the parenchyma [96,145]. The fibrotic wound is not the only structural change involved since it is usually associated with tubular atrophy, tubular dilation and inflammatory cell infiltration [146,147,148]. Indeed, as the loss of renal cells and its replacement by ECM are common sequelae of renal damage, expansion of cortical fibrosis is considered one of the best histologic predictors of kidney dysfunction loss in CKD along with tubular atrophy (IFTA parameter) [148,149,150]. It is also one of the most common features assessed in biopsies in predicting a progression to ESRD [151,152].
Even though chronic damage to the kidney will naturally converge into histological and functional alterations that are common and lead to glomerulosclerosis and fibrosis, it is important to understand that the fibrotic progression is different depending on where it begins [153]. In glomerular damage, the progression starts with an injury within the Bowman’s Capsule that initially leads to glomerular hyperfiltration for a long period of time until it progresses to decrease the total GFR [154,155]. This reduction in the blood flow results in tubular hypoxia and epithelial cell death normally referred to as tubule atrophy [156,157]. In these circumstances, the inflammation initiated by the damaged tubular cells propitiates the formation of a fibrotic scar to fill the void created by epithelial cell death [158,159]. To form that scar, resident fibroblasts differentiate into the myofibroblast phenotype, which can synthesize different extracellular matrix proteins. Among the ECM components produced by myofibroblasts in order to form the fibrotic scar, the main ones in the kidney are collagen type I, III and IV, as well as fibronectin [160,161,162]. During tubule atrophy, the tubular basement membrane remains, thereby separating the cell death from the interstitium but disappears after the cell-free tubule collapses, at which point we could talk of complete loss of the nephron [163,164,165].
Epithelial damage is heterogeneous in tubular injury, which can be caused by many factors, such as hemodynamic, inflammatory, toxin-related or metabolic alterations. Some cells will instantly go through necrosis or apoptosis, whereas others will survive with different levels of injury, these being the ones that could proliferate and replace the lost cells of the tubular epithelium [166,167,168]. In the cases in which the tubules do not recover, inflammation signalling activates and with it the fibroblasts differentiate into myofibroblasts that will lead to tubulointerstitial fibrosis and tubular atrophy [169,170,171]. Tubulointerstitial fibrosis is the deposition of ECM proteins in the space between the tubular basement membrane and the peritubular capillaries [160], which impairs blood flow and induces ischemic injury in the nephrons of the fibrotic wound [148,172,173].
Inflammation and oxidative stress serve as the initial response to injury although its long-term progression could damage organ structure and function [174,175]. Inflammation is a common process in fibroproliferative diseases that leads to the release of pro-inflammatory mediators that have an important role in tissue damage and could either stimulate or inhibit fibrosis [176,177]. An appropriate level of cytokines and growth factors that mediates the cellular responses is key in normal wound healing. Among the many growth factors involved, transforming growth factor ß (TGF-ß) is considered to be a prototypic profibrotic cytokine that has a central role in organ fibrosis as it binds to its receptors causing the phosphorylation of SMADs, which modulate the expression of the target genes [100,178]. TGF-ß can also activate SMAD-independent pathways in what is called non-canonical signalling [179]. Among the many TGF-ß-mediated responses are cell proliferation and differentiation, ECM production and immune modulation [180,181,182]. Another important mediator is the connective tissue growth factor (CTGF), a downstream factor of TGF ß that has been reported in fibrosis in different organs such as the heart and kidney [93,183,184]. CTGF promotes the TGF-ß-induced excessive ECM production and fibroblast proliferation [185,186], and its expression appears to correlate with the degree of fibrosis [187].
As previously said, a dynamic balance between production and breakdown of ECM regulates the degree of fibrosis. The degradation of the ECM components is performed by the matrix metalloproteinases (MMPs), whose activity is controlled by the tissue inhibitors of MMPs (TIMPs) in order to maintain the homeostasis. MMPs can be classified according to substrate specificity into collagenases, such as MMP-1, MMP-8 and MMP-13 [188,189]; gelatinases, such as MMP-2 and MMP-9 [190,191]; membrane MMPs, such as MMP-14 [192]; and stromelysins, such as MMP-3, MMP-10 and MMP-11 [193]. Interestingly, MMPs can have both inhibitory and stimulatory effects on fibrosis as some of them promote it [194]. For example, the most frequently studied MMPs in HF and kidney damage are MMP-2 and MMP-9, out of which MMP-9 is believed to have a profibrotic effect [195,196,197] whereas MMP-2 has antifibrotic effects [198,199].
In recent years, it has been proved that different metabolic alterations stimulate structural and/or functional alterations, such as fibrosis development. Changes in metabolic regulation, such as that occurring in a situation such as lipotoxicity, defined as the accumulation of lipids in non-adipose tissues, is known to promote the development of fibrosis. This fibrosis is due to an upregulation in ECM protein synthesis, promoted by fibroblasts [200,201]. In this sense, we have observed in a recent study that MI is associated with cardiac lipotoxicity in rats, independently of the presence of obesity. This lipotoxicity was accompanied by alterations in the mitochondrial lipid profile and associated with myocardial fibrosis, suggesting that MI promotes an increase in lipid accumulation in the heart through mechanisms that are currently unknown. Similarly, we observed at the renal level the direct profibrotic role of palmitic acid at renal fibroblasts, as it induced an increase in ECM synthesis mediated by activation of ER stress, suggesting its importance in lipotoxicity-induced fibrosis [202]. These observations are in agreement with another study in which the authors proved that accumulation of lipid droplets accelerates tubulointerstitial fibrosis development in an animal model of kidney disease [200].
4. Mechanisms Involved in Fibrosis Progression
As a wide variety of diseases converge in fibrosis understanding, the pathogenesis involved is important in order to determine potential therapeutic targets. Despite the efforts to acquire insight into the process, the mechanisms involved are not fully established, and the current therapies are either ineffective or only slightly successful [203,204]. The current clinical strategies for CRS are guided towards the treatment of the general processes, such as diuretics, to treat volume overload, or angiotensin converting enzyme (ACE) inhibitors, Angiotensin II receptor blockers, mineralocorticoid receptor antagonist or β-adrenergic blockers to inhibit RAAS activation [17,205]. Due to the complex pathophysiology of CRS, new therapeutic approaches centred in fibrosis have been proposed. For instance, in a recent study, it has been proved that cardiac shock wave therapy significantly reduces cardiac fibrosis in a rat model of MI through the activation of the PI3K/Akt signalling pathway [206]. Despite this, these new experimental approaches are still required in order to have a comprehensive understanding of the pathophysiological mechanisms underlying fibrosis.
4.1. Inflammation
Inflammation can be defined as a defensive immune response that is triggered by damage to a tissue. The acute inflammatory response can be initiated as a consequence of an infection in which the pattern recognition receptors in the innate immune cells interact with the pathogen-associated molecular patterns (PAMPs), or due to the damage-associated molecular patterns (DAMPs) that are released during physical injury [207]. An acute inflammatory response is characterized by vasodilation, vascular leak and leukocyte emigration and, shortly after its induction, secretion of cytokines and chemokines will happen in order to recruit the immune cells to the damaged or infected region. Among the cells recruited, neutrophils are the first to migrate as a means to engulf the pathogens and secrete pro-inflammatory mediators and vasoactive substances [208,209].
In a normal inflammatory response, the activity is temporally restricted, as it resolves once the threat has been dealt with. However, the presence of a prolonged low-grade activity leads to chronic inflammation, which is characterized by the activation of different immune components that lead to major alterations in tissues, increasing the risk of diseases [210]. The clinical consequences of chronic inflammation include type 2 diabetes [211,212], hypertension [213], cardiovascular disease [214,215], chronic kidney disease [216]) and metabolic syndrome [217] among others.
Since both CHF and CKD are associated with a chronic inflammation response, characterized by an increase in the circulating inflammatory mediators, this process has become of interest in the understanding of CRS. A persistent inflammatory trigger is needed in order to activate the wound-healing process. However, if not eliminated quickly, the inflammatory cells could increase the response, leading to the abnormal wound healing and scarring characteristic of fibrosis. Within the wound-healing mechanism that is activated after injury, the first response is coagulation, in which activated platelets release platelet-derived growth factor (PDGF), acting as a chemoattractant for inflammatory cells, and transforming growth factor ß1 (TGF-ß1), which is one of the main drivers of fibrosis as it stimulates ECM synthesis by the fibroblasts of the tissue that was damaged [218,219,220].
Inflammation is known to have an important role in the development and progression of chronic diseases. For example, CKD progression into ESRD is characterized by chronic inflammation in the renal parenchyma, concluding in ECM deposition and loss of renal function [221,222,223]. Independent of the original cause, experimental models and human biopsies have shown that during renal inflammation, cells such as neutrophils and macrophages infiltrate both the glomeruli and tubulointerstitial space in order to remove the cell and matrix components that were damaged during the insult [223,224,225]. In general, M1 macrophages generate the initial response in the diseased organ by generation of pro-inflammatory cytokines, such as tumour necrosis factor α (TNFα) and interleukin-1 (IL-1), whereas M2 macrophages propitiate tissue repair by secretion of immunosuppressive cytokines during the repair phase [223,226]. It is that transition from the M1 to M2 phenotype that promotes fibrosis, as the production of cytokines, chemokines and growth factors alter the ECM balance between production and degradation [214,227,228].
Cytokines are cell-derived polypeptides that mediate the inflammatory response and can have positive or negative effects. It is well known that not all cytokines are involved at all stages of inflammation, but some of them do mediate both acute and chronic responses. This is the case of TNF-α, IL-1 (α and β) and IL-6 [229], which are some of the most studied ones and have been suggested to have an important role in inflammatory modulation during CRS due to its extremely potent proinflammatory effects [94,230,231,232].
It is well established that RAAS activation and the sympathetic nervous system (SNS) promotes the inflammatory response both in the heart and kidneys [233]. Angiotensin II (Ang II), one of the main effectors of RAAS activation, induces endothelial dysfunction, upregulation of adhesion molecules and fibrosis [234,235,236]. These Ang II effects are accompanied by recruitment of infiltrating cells and an increase in proinflammatory cytokines via the angiotensin type 1 (AT1) receptor in cardiorenal disease [230,237]. It has been proved that Ang II produces the accumulation of macrophage in the kidney [238,239], and it was shown in a murine unilateral ureteral obstruction (UUO) model that the macrophages’ AT1 receptor activation impedes polarization towards the M1 phenotype and limits the damage and fibrosis [240]. This shows that an increase in M1 macrophage differentiation makes organs more susceptible to damage whereas the M2 phenotype decreases injury [223,241,242]. Nevertheless, neurohormonal activation is not the only proposed source of inflammation in CRS. Both animal and human studies have shown that congestion may lead to endothelial activation and peripheral release of proinflammatory mediators, as venous congestion itself causes an inflammatory response activation in cells [233,243,244].
Inflammation leads to functional and structural damage in the cardiorenal axis, as the different cytokines, especially TNF-α, which plays a central role in organ dysfunction, are involved in inflammation, cell proliferation [245] and apoptosis [246]. During inflammation, TNF-α has been described to be involved in vasodilation, inflammatory cell adhesion, coagulation and reactive oxygen species (ROS) production, among others [247].
Numerous cytokines have been studied due to their profibrotic or antifibrotic effects [248]. Th2-derived cytokines, such as IL-4, IL-5, IL-6, IL-13 and IL-21, are important in the regulation of organ fibrosis [249,250], out of which the most studied one is IL-13, an interleukin whose profibrotic effect can be enhanced by IL-5 and IL-21, and which can increase its production and its receptor expression [251,252,253]. IL-21 can also promote tissue fibrosis through the induction of differentiation into Th17 cells [254,255], which produce a well-known profibrotic interleukin, IL-17, and which is involved in the development of fibrosis in various organs [256,257,258], although a recent study has suggested IL-17 plays an antifibrotic role in tubulointerstitial fibrosis [259]. On the other hand, Th1 cytokines, such as IL-7 [250,260], IL-10 [261,262], IL-12 [263,264] and IL-22 [265,266], along with IFN-γ [267,268], have been shown to have a suppressive effect on fibrosis. For instance, the inflammatory response in IL-10 KO mice resulted in scar formation rather than wound repair, suggesting IL-10 has an important antifibrotic role [269,270].
Chronic, unresolved inflammation damages renal structure and function, thereby leading to CKD, a state characterized by progressive renal fibrosis. In previous studies, it was reported that circulating levels of fibrinogen, TNF-α and a decrease in serum albumin were associated with loss of kidney function, linking the progression of CKD to the inflammatory response [221,271]. Systemic inflammation and function decline can alter the structure of the kidney, creating an environment in which epithelial damage increases and the factors released by infiltrating macrophages lead to fibrotic expansion [272,273]. Indeed, macrophage depletion has proved to reduce renal fibrosis in an animal model of myocardial infarction [274]. In renal fibrosis, the first process involved is the injury itself, followed by the unresolved inflammation. In the tubulointerstitium, pro-inflammatory cytokines, such as IL-6, TNF-α and IL-1β, promote further inflammatory cell infiltration, propitiating activation of profibrotic cells to differentiate into myofibroblasts and local secretion of fibrotic mediators [275,276,277]. This situation will lead to overproduction and deposition of ECM proteins, disruption of tissue integrity and progressive decline in function. Finally, glomerulosclerosis and tubular atrophy will happen in the latest stages [278,279].
In cardiac injury, as what happens in renal damage, the necrotic cell death within the heart activates tissue cells that will synthetize proinflammatory cytokines to recruit inflammatory cells. In the first phase, the macrophages and neutrophils act to remove the debris and release growth factors and cytokines that propitiate formation of connective tissue. Afterwards, fibroblast activation and cell proliferation will happen in the maturation phase to repair the myocardium by fibrotic wound formation [280,281]. After the phagocytic clearance of the apoptotic cells, macrophages will polarize towards the “reparative” M2 phenotype, releasing anti-inflammatory and profibrotic cytokines such as IL-10 and TGFβ, while proinflammatory cytokines, such as IL-1β or TNF-α, decrease in order to stimulate cardiac fibroblast activation to collagen-secreting myofibroblast [281,282,283]. Having said that, chronic inflammation entails a change in the inflammatory behaviour towards persistent and exacerbated fibrinogenesis, which is a structural feature in chronic injuries. It is due to that characteristic chronic inflammation for which TNF-α has been proposed as an independent predictor of cardiac and non-cardiac mortality in CHF patients [284]. Nonetheless, there is no consensus on the role of cytokines and chemokines, as some studies suggest its aggravating injury effects and others show that they endanger cardioprotective responses. For example, TNF-α ablation has proved to reduce the infarct size in mice with I/R injury [285], but in other studies TNF receptor deficiency increased the ischemic injury during I/R [286].
4.2. Oxidative Stress
Oxidative stress is a general concept that describes the imbalance between the production of ROS and the antioxidant defences. ROS includes both free radicals, which are species with an unpaired electron, such as superoxide anion (O2•−) and hydroxyl radical (∙OH), or non-free radical oxygenated molecules, such as hydrogen peroxide (H2O2) [287,288]. Other reactive species derived from nitrogen or sulphur do exist, but they are less abundant [289,290].
Even in basal conditions, aerobic metabolism involves ROS production, thus making O2•− and H2O2 physiological intracellular metabolites. In low quantities, ROS act as signalling molecules involved in different pathways, such as cell proliferation, apoptosis and gene expression [291,292]. However, the fact that an important increase in oxidants could target almost all substrates implies the impairment and alteration of all biomolecules, resulting in cell damage and death [293,294]. ROS can damage proteins [295] and nucleic acids [296,297], but among all the molecules to undergo oxidation, polyunsaturated fatty acids are the most susceptible, leading to an increase in the markers of lipid peroxidation, such as malondialdehyde or 4-hydroxynonenal [298,299,300].
The endogenous sources of prooxidant species include organelles where there is high oxygen use, such as the mitochondria, peroxisomes, due to the fatty acid β-oxidation [301,302], and the endoplasmic reticulum (ER) [303], although the mitochondria seem to be the major source of ROS production, as around 95% of the breathed oxygen is reduced in the mitochondrial electron chain. Specifically, there are two major sites in the electron transport chain, the NADH dehydrogenase (complex I) and the ubiquinone cytochrome c reductase (complex III), which transfer electrons to coenzyme Q or ubiquinone, creating reduced forms that will ultimately transfer electrons to the molecular oxygen, generating superoxide radicals [304,305]. Through the action of mitochondrial superoxide dismutase (SOD), the superoxide anion is converted to hydrogen peroxide, which can be detoxified by the catalase and glutathione peroxidase [305,306].
In the outer mitochondrial membrane, the monoamine oxidases are another source of ROS that is not related to respiration [307,308]. In this case, the bivalent reduction of oxygen produces H2O2. In order to regulate the levels of ROS, the sources colocalize with the antioxidant response, among which there are enzymes, such as superoxide dismutases, catalase and glutathione peroxidase, as well as non-enzymatic antioxidants, such as vitamin A, bilirubin or reduced coenzyme Q [288,309,310].
Both inflammation and oxidative stress are related to chronic diseases, such as diabetes, hypertension, cardiovascular diseases or CKD [175,311,312,313]. It is known that under chronic damage the inflammatory and hypoxic environment propitiates fibrosis by fibroblast activation and proliferation into myofibroblasts. In this circumstance, ROS formation also occurs, and is considered to have an important role in both inflammation and organ fibrosis [314,315,316]. The bidirectional link between ROS and TGF-β1 is well established, as ROS production and enhanced ROS formation leads to higher activation and expression of TGF-β1 [317,318,319]. One of the possible explanations for this link resides in the action of an important ROS source, such as the different NADPH oxidases (NOX). In normal conditions, the NOX-derived ROS act as modulators of cell growth, proliferation, differentiation and apoptosis, but once it is uncontrolled, oxidative stress damages the DNA, proteins and lipids, inducing organ damage and fibrosis [320,321,322]. Multiple studies have shown the effectiveness of NOX-1 and NOX-4 inhibition in inflammation and fibrosis amelioration in liver and kidney injury [323,324,325], while different studies in the heart have shown that both NOX-2 and NOX-4 mediate the oxidative stress and cardiac injury following I/R [320,326,327]. Indeed, NOX-4 is considered a well-recognized mediator of the transition from fibroblast to myofibroblast, and its inhibition in in vitro studies with renal cells proved to prevent ROS production and myofibroblast differentiation, which would translate into a decrease in fibrosis during damage [328,329].
Multiple factors seem to participate in order to produce the characteristic multiorgan dysfunction of CRS, among which the increase in proinflammatory cytokines, the dysregulation of apoptosis and the increase in oxidative stress have been proposed as key elements of this complex pathophysiology [232,330,331]. Different animal models have shown that an increase in oxidative stress plays a pivotal role in cardiac and renal damage, independently of the CRS type depicted, through activation of the inflammatory response [15,331,332,333]. This can also be seen in patients with CRS, who presented an increase in ROS and RNS, which was accompanied by higher inflammatory cytokines, such as IL-6 [15].
4.3. Endoplasmic Reticulum Stress
The ER is an essential organelle for calcium homeostasis, lipid biosynthesis and protein synthesis and post-translational modifications. To ensure correct protein folding, the ER lumen balance between unfolded and misfolded proteins, and the capability to handle it, must be maintained. Such homeostasis could be altered by both physiological and pathological entities, such as inflammatory cytokines, protein demand or mutant protein expression, which translates into what is called ER stress [334,335].
In response to ER stress, the unfolded protein response (UPR) is initiated by at least one of three different pathways: the ER transmembrane proteins Activating Transcription Factor 6 (ATF6), Inositol-Requiring 1 (IRE1) or PKR-like ER kinase (PERK). In unstressed conditions, the chaperone Immunoglobin Binding Protein (BiP) binds to the luminal domain of ATF6, IRE1 and PERK, keeping them inactive [334,336]. In ER stress conditions, BiP dissociates from the three regulators, activating UPR [337]. Although initially UPR is considered a beneficial adaptive response, if it fails to restore homeostasis, then the UPR pathways guide the damaged cells to apoptosis and the consequent tissue injury [338,339].
Different pathologies, such as diabetes mellitus [340], obesity [341,342], cardiovascular disease [343,344] and CKD [345,346], have been associated with ER stress. In CRS, the activation of ER stress in the heart and kidney could be induced by different factors, such as hemodynamic changes, hormones from the RAAS, inflammation or oxidative stress [346,347,348]. These pathophysiological mediators could directly induce ER stress in the myocardium or renal parenchyma, resulting in apoptotic cell death due to prolonged UPR activation [349,350,351] and the consequent fibrotic wound formation, all of which would eventually lead to structural and functional changes [348,352,353,354]. Our group has recently evaluated the effect of myocardial infarction (MI) at renal level in rats. At 4 weeks post-MI, animals presented renal alterations characterized by tubulointerstitial fibrosis, oxidative stress and upregulation of inflammatory cytokines, such as IL-6 and TNF-α. All these alterations were accompanied by ER stress activation, which correlates with the renal fibrosis, suggesting ER stress relevance in the structural renal damage in CRS type 1 [202].
As ER stress inhibition has proved to ameliorate the fibrotic progression, it has been suggested that its blockade could be a new therapeutic approach for fibrosis [355,356,357]. One of the possible ways in which ER stress could lead to fibrosis is through fibroblast differentiation and collagen formation by TGF-β upregulation, as PERK and IRE1 activation have been seen to increase TGF-β expression [358,359,360]. ER stress activation of fibroblasts during injury at the wounded site triggers their differentiation into myoblasts, so as to restore the area by ECM protein synthesis and secretion [361,362]. Different in vitro studies have described ER-mediated differentiation into different cell types, such as renal tubular cells [363], cardiac cells [364], adipocytes [365,366], plasma cells [367,368] and others [369,370,371]. Additionally, our group’s in vitro studies in kidney fibroblasts, stimulated with the well-known profibrotic factor Ang II in presence of the pharmacological inhibitor of ER stress, 4-phenylbutiric acid (4-PBA), proved to be effective in preventing the increase in collagen I, inflammatory markers and superoxide anion production. All of this suggests the important role of ER stress in fibrosis, inflammation and oxidative stress in renal damage [202].
The explained mechanisms involvement in CRS is depicted in Figure 2.
Figure 2.
Mechanisms involved in the progression of cardiac and renal fibrosis in CRS.
5. Conclusions
Due to the pathogenesis of cardiorenal syndromes, numerous efforts have given insight into the different pathways and mediators involved. This review has summarized evidence that the development of a fibrotic wound has proved to play a central role in both cardiac and renal damage progression, in which inflammation, oxidative stress and ER stress could be relevant players. This makes it crucial to understand the pathogenic basis of fibrosis in order to determine therapeutic targets.
Acknowledgments
We thank Anthony DeMarco for his help in editing.
Author Contributions
Conceptualization: E.M.-M. and V.C.; investigation: B.D.-V., E.M.-M. and V.C.; orgibnal draft preparation: B.D.-V.; review and editing: B.D.-V., E.M.-M. and V.C.; funding acquisition: E.M.-M. and V.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Instituto de Salud Carlos III-Fondo Europeo de Desarrollo Regional (FEDER) [grant numbers PI18/00257; CIBERCV]. B.D.-V. was supported by a grant P-FIS (FI19/00277) and E.M-M was supported by a contract from CAM (Atracción de talento).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Bright R. Cases and Observations Illustrative of Renal Disease, Accompanied with the Secretion of Albuminous Urine. Med. Chir. Rev. 1836;25:23–35. [PMC free article] [PubMed] [Google Scholar]
- 2.Zannad F., Rossignol P. Cardiorenal Syndrome Revisited. Circulation. 2018;138:929–944. doi: 10.1161/CIRCULATIONAHA.117.028814. [DOI] [PubMed] [Google Scholar]
- 3.Cabandugama P.K., Gardner M.J., Sowers J.R. The Renin Angiotensin Aldosterone System in Obesity and Hypertension. Med. Clin. North Am. 2017;101:129–137. doi: 10.1016/j.mcna.2016.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Banerjee S., Panas R. Diabetes and Cardiorenal Syndrome: Understanding the “Triple Threat”. Hell. J. Cardiol. 2017;58:342–347. doi: 10.1016/j.hjc.2017.01.003. [DOI] [PubMed] [Google Scholar]
- 5.McCullough P.A., Jurkovitz C.T., Pergola P.E., McGill J.B., Brown W.W., Collins A.J., Chen S.-C., Li S., Singh A., Norris K.C., et al. Independent Components of Chronic Kidney Disease as a Cardiovascular Risk State. Arch. Intern. Med. 2007;167:1122–1129. doi: 10.1001/archinte.167.11.1122. [DOI] [PubMed] [Google Scholar]
- 6.Raina R., Nair N., Chakraborty R., Nemer L., Dasgupta R., Varian K. An Update on the Pathophysiology and Treatment of Cardiorenal Syndrome. Cardiol. Res. 2020;11:76–88. doi: 10.14740/cr955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ronco C., McCullough P., Anker S.D., Anand I., Aspromonte N., Bagshaw S.M., Bellomo R., Berl T., Bobek I., Cruz D.N., et al. Cardio-Renal Syndromes: Report from the Consensus Conference of the Acute Dialysis Quality Initiative. Eur. Heart J. 2009;31:703–711. doi: 10.1093/eurheartj/ehp507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Heywood J.T., Fonarow G., Costanzo M.R., Mathur V.S., Wigneswaran J.R., Wynne J. High Prevalence of Renal Dysfunction and Its Impact on Outcome in 118,465 Patients Hospitalized with Acute Decompensated Heart Failure: A Report from the ADHERE Database. J. Card. Fail. 2007;13:422–430. doi: 10.1016/j.cardfail.2007.03.011. [DOI] [PubMed] [Google Scholar]
- 9.Ronco C., Cicoira M., McCullough P.A. Cardiorenal Syndrome Type 1. J. Am. Coll. Cardiol. 2012;60:1031–1042. doi: 10.1016/j.jacc.2012.01.077. [DOI] [PubMed] [Google Scholar]
- 10.Prins K.W., Thenappan T., Markowitz J.S., Pritzker M.R. Cardiorenal Syndrome Type 1: Renal Dysfunction in Acute Decom-Pensated Heart Failure. J. Clin. Outcomes Manag. 2015;22:443–454. [PMC free article] [PubMed] [Google Scholar]
- 11.Ronco C., Bellasi A., Di Lullo L. Cardiorenal Syndrome: An Overview. Adv. Chronic Kidney Dis. 2018;25:382–390. doi: 10.1053/j.ackd.2018.08.004. [DOI] [PubMed] [Google Scholar]
- 12.Mullens W., Abrahams Z., Francis G.S., Sokos G., Taylor D.O., Starling R.C., Young J.B., Tang W.W. Importance of Venous Congestion for Worsening of Renal Function in Advanced Decompensated Heart Failure. J. Am. Coll. Cardiol. 2009;53:589–596. doi: 10.1016/j.jacc.2008.05.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ames M.K., Atkins C.E., Pitt B. The renin-angiotensin-aldosterone System and Its Suppression. J. Veter. Intern. Med. 2019;33:363–382. doi: 10.1111/jvim.15454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brewster U.C., Perazella A.M. The Renin-Angiotensin-Aldosterone System and the Kidney: Effects on Kidney Disease. Am. J. Med. 2004;116:263–272. doi: 10.1016/j.amjmed.2003.09.034. [DOI] [PubMed] [Google Scholar]
- 15.Virzì G.M., Clementi A., De Cal M., Brocca A., Day S., Pastori S., Bolin C., Vescovo G., Ronco C. Oxidative Stress: Dual Pathway Induction in Cardiorenal Syndrome Type 1 Pathogenesis. Oxidative Med. Cell. Longev. 2015;2015:1–9. doi: 10.1155/2015/391790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harrison J.C., Smart S.D.G., Besley E.M.H., Kelly J.R., Read M.I., Yao Y., Sammut I. A Clinically Relevant Functional Model of Type-2 Cardio-Renal Syndrome with Paraventricular Changes Consequent to Chronic Ischaemic Heart Failure. Sci. Rep. 2020;10:1–12. doi: 10.1038/s41598-020-58071-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rangaswami J., Bhalla V., Blair J.E., Chang T.I., Costa S., Lentine K.L., Lerma E., Mezue K., Molitch M., Mullens W., et al. Cardiorenal Syndrome: Classification, Pathophysiology, Diagnosis, and Treatment Strategies: A Scientific Statement from the American Heart Association. Circulation. 2019;139:e840–e878. doi: 10.1161/CIR.0000000000000664. [DOI] [PubMed] [Google Scholar]
- 18.Stevens P.E., Levin A. Kidney Disease: Improving Global Outcomes Chronic Kidney Disease Guideline Development Work Group Members. Evaluation and Management of Chronic Kidney Disease: Synopsis of the Kidney Disease: Improving Global Outcomes 2012 Clinical Practice Guideline. Ann. Intern. Med. 2013;158:825–830. doi: 10.7326/0003-4819-158-11-201306040-00007. [DOI] [PubMed] [Google Scholar]
- 19.Damman K., Valente M.A., Voors A.A., O’Connor C.M., Van Veldhuisen D.J., Hillege H.L. Renal impairment, worsening renal function, and outcome in patients with heart failure: An updated meta-analysis. Eur. Heart J. 2014;35:455–469. doi: 10.1093/eurheartj/eht386. [DOI] [PubMed] [Google Scholar]
- 20.De Vecchis R., Baldi C. Cardiorenal Syndrome Type 2: From Diagnosis to optimal Management. Ther. Clin. Risk Manag. 2014;10:949–961. doi: 10.2147/TCRM.S63255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Di Lullo L., Reeves P.B., Bellasi A., Ronco C. Cardiorenal Syndrome in Acute Kidney Injury. Semin. Nephrol. 2019;39:31–40. doi: 10.1016/j.semnephrol.2018.10.003. [DOI] [PubMed] [Google Scholar]
- 22.Bagshaw S.M., Hoste E., Braam B., Briguori C., Kellum J.A., McCullough P.A., Ronco C. Cardiorenal Syndrome Type 3: Pathophysiologic and Epidemiologic Considerations. Contrib. Nephrol. 2013;182:137–157. doi: 10.1159/000349971. [DOI] [PubMed] [Google Scholar]
- 23.Kumar U., Wettersten N., Garimella P.S. Cardiorenal Syndrome. Cardiol. Clin. 2019;37:251–265. doi: 10.1016/j.ccl.2019.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Uduman J. Epidemiology of Cardiorenal Syndrome. Adv. Chronic Kidney Dis. 2018;25:391–399. doi: 10.1053/j.ackd.2018.08.009. [DOI] [PubMed] [Google Scholar]
- 25.Mentzer R.M., Oz M.C., Sladen R.N., Graeve A.H., Hebeler R.F., Luber J.M., Smedira N.G. Effects of Perioperative Nesiritide in Patients with Left Ventricular Dysfunction Undergoing Cardiac Surgery: The NAPA Trial. J. Am. Coll. Cardiol. 2007;49:716–726. doi: 10.1016/j.jacc.2006.10.048. [DOI] [PubMed] [Google Scholar]
- 26.Di Lullo L., Bellasi A., Barbera V., Russo D., Russo L., Di Iorio B., Cozzolino M., Ronco C. Pathophysiology of the Cardio-Renal Syndromes Types 1–5: An Uptodate. Indian Heart J. 2017;69:255–265. doi: 10.1016/j.ihj.2017.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hillege H.L., Nitsch D., Pfeffer M.A., Swedberg K., McMurray J.J., Yusuf S., Granger C.B., Michelson E.L., Ostergren J., Cornel J., et al. Renal Function as a Predictor of Outcome in a Broad Spectrum of Patients with Heart Failure. Circulation. 2006;113:671–678. doi: 10.1161/CIRCULATIONAHA.105.580506. [DOI] [PubMed] [Google Scholar]
- 28.Go A.S., Chertow G.M., Fan D., McCulloch C.E., Hsu C.-Y. Chronic Kidney Disease and the Risks of Death, Cardiovascular Events, and Hospitalization. N. Eng. J. Med. 2004;351:1296–1305. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- 29.Suresh H., Arun B.S., Moger V., Swamy M. Cardiorenal Syndrome Type 4: A Study of Cardiovascular Diseases in Chronic Kidney Disease. Indian Heart J. 2017;69:11–16. doi: 10.1016/j.ihj.2016.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Clementi A., Virzì G.M., Goh C.Y., Cruz D.N., Granata A., Vescovo G., Ronco C. Cardiorenal Syndrome Type 4: A Review. Cardiorenal Med. 2013;3:63–70. doi: 10.1159/000350397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mehta R.L., Rabb H., Shaw A.D., Singbartl K., Ronco C., McCullough P.A., Kellum J.A. Cardiorenal Syndrome Type 5: Clinical Presentation, Pathophysiology and Management Strategies from the Eleventh Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Contrib. Nephrol. 2013;182:174–194. doi: 10.1159/000349970. [DOI] [PubMed] [Google Scholar]
- 32.Singer M., Deutschman C.S., Seymour C.W., Shankar-Hari M., Annane D., Bauer M., Bellomo R., Bernard G.R., Chiche J.-D., Coopersmith C.M., et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3) JAMA. 2016;315:801–810. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vincent J.-L., Rello J., Marshall J.K., Silva E., Anzueto A., Martin C.D., Moreno R., Lipman J., Gomersall C., Sakr Y., et al. International Study of the Prevalence and Outcomes of Infection in Intensive Care Units. JAMA. 2009;302:2323–2329. doi: 10.1001/jama.2009.1754. [DOI] [PubMed] [Google Scholar]
- 34.Mehta R.L., Program to Improve Care in Acute Renal Disease (PICARD) Study Group. Bouchard J., Soroko S.B., Ikizler T., Paganini E.P., Chertow G.M., Himmelfarb J. Sepsis as a Cause and Consequence of Acute Kidney Injury: Program to Improve Care in Acute Renal Disease. Intensiv. Care Med. 2010;37:241–248. doi: 10.1007/s00134-010-2089-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ronco C., McCullough P.A., Anker S.D., Anand I., Aspromonte N., Bagshaw S.M., Bellomo R., Berl T., Bobek I., Cruz D.N., et al. Cardiorenal Syndromes: An Executive Summary from the Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Contrib. Nephrol. 2010;165:54–67. doi: 10.1159/000313745. [DOI] [PubMed] [Google Scholar]
- 36.Evans M., Grams M.E., Sang Y., Astor B.C., Blankestijn P.J., Brunskill N.J., Collins J.F., Kalra P.A., Kovesdy C.P., Levin A., et al. Risk Factors for Prognosis in Patients with Severely Decreased GFR. Kidney Int. Rep. 2018;3:625–637. doi: 10.1016/j.ekir.2018.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Matsushita K., Van Der Velde M., Astor B.C., Woodward M., Levey A.S., De Jong P.E., Coresh J., Gansevoort R.T. Association of Estimated Glomerular Filtration Rate and Albuminuria with All-Cause and Cardiovascular Mortality in General Population Cohorts: A Collaborative Meta-Analysis. Lancet. 2010;375:2073–2081. doi: 10.1016/s0140-6736(10)60674-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Matsushita K., Coresh J., Sang Y., Chalmers J., Fox C., Guallar E., Jafar T., Jassal S.K., Landman G.W.D., Muntner P., et al. Estimated Glomerular Filtration Rate and Albuminuria for Prediction of Cardiovascular Outcomes: A Collaborative Meta-Analysis of Individual Participant Data. Lancet Diabetes Endocrinol. 2015;3:514–525. doi: 10.1016/S2213-8587(15)00040-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.James M.T., Grams M.E., Woodward M., Elley C.R., Green J.A., Wheeler D.C., de Jong P., Gansevoort R.T., Levey A.S., Warnock D.G., et al. A Meta-Analysis of the Association of Estimated GFR, Albuminuria, Diabetes Mellitus, and Hypertension with Acute Kidney Injury. Am. J. Kidney Dis. 2015;66:602–612. doi: 10.1053/j.ajkd.2015.02.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Matsuo H., Dohi K., Machida H., Takeuchi H., Aoki T., Nishimura H., Yasutomi M., Senga M., Ichikawa T., Kakuta K., et al. Echocardiographic Assessment of Cardiac Structural and Functional Abnormalities in Patients with End-Stage Renal Disease Receiving Chronic Hemodialysis. Circ. J. 2018;82:586–595. doi: 10.1253/circj.CJ-17-0393. [DOI] [PubMed] [Google Scholar]
- 41.Otsuka T., Suzuki M., Yoshikawa H., Sugi K. Left Ventricular Diastolic Dysfunction in the Early Stage of Chronic Kidney Disease. J. Cardiol. 2009;54:199–204. doi: 10.1016/j.jjcc.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 42.Dobre M., Roy J., Tao K., Anderson A.H., Bansal N., Chen J., Deo R., Drawz P., Feldman H.I., Hamm L.L., et al. Serum Bicarbonate and Structural and Functional Cardiac Abnormalities in Chronic Kidney Disease - A Report from the Chronic Renal Insufficiency Cohort Study. Am. J. Nephrol. 2016;43:411–420. doi: 10.1159/000446860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shlipak M.G., Fried L.F., Cushman M., Manolio T.A., Peterson D., Stehman-Breen C., Bleyer A., Newman A.B., Siscovick D., Psaty B. Cardiovascular Mortality Risk in Chronic Kidney Disease. JAMA. 2005;293:1737–1745. doi: 10.1001/jama.293.14.1737. [DOI] [PubMed] [Google Scholar]
- 44.Park M., Hsu C.-Y., Li Y., Mishra R.K., Keane M., Rosas S.E., Dries D., Xie D., Chen J., He J., et al. Associations Between Kidney Function and Subclinical Cardiac Abnormalities in CKD. J. Am. Soc. Nephrol. 2012;23:1725–1734. doi: 10.1681/ASN.2012020145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Taddei S., Nami R., Bruno R.M., Quatrini I., Nuti R. Hypertension, Left Ventricular Hypertrophy and Chronic Kidney Disease. Heart Fail. Rev. 2010;16:615–620. doi: 10.1007/s10741-010-9197-z. [DOI] [PubMed] [Google Scholar]
- 46.Matsumoto M., Io H., Furukawa M., Okumura K., Masuda A., Seto T., Takagi M., Sato M., Nagahama L., Omote K., et al. Risk Factors Associated with Increased Left Ventricular Mass Index in Chronic Kidney Disease Patients Evaluated Using Echocardiography. J. Nephrol. 2012;25:794–801. doi: 10.5301/jn.5000066. [DOI] [PubMed] [Google Scholar]
- 47.Pluta A., Stróżecki P., Krintus M., Odrowaz-Sypniewska G., Manitius J. Left Ventricular Remodeling and Arterial Remodeling in Patients with Chronic Kidney Disease Stage 1–3. Ren. Fail. 2015;37:1–6. doi: 10.3109/0886022X.2015.1061669. [DOI] [PubMed] [Google Scholar]
- 48.Matsushita K., Ballew S., Coresh J. Influence of Chronic Kidney Disease on Cardiac Structure and Function. Curr. Hypertens. Rep. 2015;17:1–9. doi: 10.1007/s11906-015-0581-x. [DOI] [PubMed] [Google Scholar]
- 49.Toida T., Toida R., Yamashita R., Komiya N., Uezono S., Komatsu H., Ishikawa T., Kitamura K., Sato Y., Fujimoto S. Grading of Left Ventricular Diastolic Dysfunction with Preserved Systolic Function by the 2016 American Society of Echocardiography/European Association of Cardiovascular Imaging Recommendations Contributes to Predicting Cardiovascular Events in Hemodialysis Patients. Cardiorenal Med. 2019;9:190–200. doi: 10.1159/000496064. [DOI] [PubMed] [Google Scholar]
- 50.Escoli R., Carvalho M.J., Cabrita A., Rodrigues A. Diastolic Dysfunction, an Underestimated New Challenge in Dialysis. Ther. Apher. Dial. 2019;23:108–117. doi: 10.1111/1744-9987.12756. [DOI] [PubMed] [Google Scholar]
- 51.Cai Q.-Z., Lu X.-Z., Lu Y., Wang A.Y.-M. Longitudinal Changes of Cardiac Structure and Function in CKD (CASCADE Study) J. Am. Soc. Nephrol. 2014;25:1599–1608. doi: 10.1681/ASN.2013080899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Franczyk B., Gluba A., Olszewski R., Banach M., Rysz J. Heart Function Disturbances in Chronic Kidney Disease – Echocardiographic Indices. Arch. Med Sci. 2014;10:1109–1116. doi: 10.5114/aoms.2014.47822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vogel M.W., Slusser J.P., Hodge D.O., Chen H.H. The Natural History of Preclinical Diastolic Dysfunction. Circ. Heart Fail. 2012;5:144–151. doi: 10.1161/CIRCHEARTFAILURE.110.959668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shah S., Kitzman D.W., Borlaug B., Van Heerebeek L., Zile M., Kass D.A., Paulus W.J. Phenotype-Specific Treatment of Heart Failure with Preserved Ejection Fraction. Circulation. 2016;134:73–90. doi: 10.1161/CIRCULATIONAHA.116.021884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dekkers I.A., De Mutsert R., Rabelink T.J., Jukema J.W., De Roos A., Rosendaal F.R., Lamb H.J., De Vries A.P. Associations Between Normal Range Albuminuria, Renal Function and Cardiovascular Function in a Population-Based Imaging Study. Atherosclerosis. 2018;272:94–100. doi: 10.1016/j.atherosclerosis.2018.03.029. [DOI] [PubMed] [Google Scholar]
- 56.Shah A.M., Lam C.S., Cheng S., Verma A., Desai A.S., Rocha R.A., Hilkert R., Izzo J., Oparil S., Pitt B., et al. The Relationship Between Renal Impairment and Left Ventricular Structure, Function, and ventricular–arterial Interaction in Hypertension. J. Hypertens. 2011;29:1829–1836. doi: 10.1097/HJH.0b013e32834a4d38. [DOI] [PubMed] [Google Scholar]
- 57.Matsushita K., Kwak L., Sang Y., Ballew S.H., Skali H., Shah A.M., Coresh J., Solomon S. Kidney Disease Measures and Left Ventricular Structure and Function: The Atherosclerosis Risk in Communities Study. J. Am. Heart Assoc. 2017;6:e006259. doi: 10.1161/JAHA.117.006259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhou J., Cui X., Jin X., Zhou J., Zhang H., Tang B., Fu M., Herlitz H., Cui J., Zhu H., et al. Association of Renal Biochemical Parameters with Left Ventricular Diastolic Dysfunction in a Community-Based Elderly Population in China: A Cross-Sectional Study. PLoS ONE. 2014;9:e88638. doi: 10.1371/journal.pone.0088638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kang E., Ryu H., Kim J., Lee J., Lee K., Chae D., Sung S.A., Kim S.W., Ahn C., Oh K. Association Between High-Sensitivity Cardiac Troponin T and Echocardiographic Parameters in Chronic Kidney Disease: Results from the KNOW-CKD Cohort Study. J. Am. Heart Assoc. 2019;8:e013357. doi: 10.1161/JAHA.119.013357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.McCullough P.A., Jefferies J.L. Novel Markers and Therapies for Patients with Acute Heart Failure and Renal Dysfunction. Am. J. Med. 2015;128:312-e1–312.e22. doi: 10.1016/j.amjmed.2014.10.035. [DOI] [PubMed] [Google Scholar]
- 61.Savic-Radojevic A., Pljesa-Ercegovac M., Matic M., Simic D., Radovanovic S., Simic T. Novel Biomarkers of Heart Failure. Adv. Clin. Chem. 2017;79:93–152. doi: 10.1016/bs.acc.2016.09.002. [DOI] [PubMed] [Google Scholar]
- 62.Ponikowski P., Voors A.A., Anker S.D., Bueno H., Cleland J.G.F., Coats A.J.S., Falk V., González-Juanatey J.R., Harjola V.-P., Jankowska E.A., et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. J. Heart Fail. 2016;18:891–975. doi: 10.1002/ejhf.592. [DOI] [PubMed] [Google Scholar]
- 63.Arslan M., Dedic A., Boersma E., A Dubois E. Serial High-Sensitivity Cardiac Troponin T Measurements to Rule Out Acute Myocardial Infarction and a Single High Baseline Measurement for Swift Rule-In: A Systematic Review and Meta-Analysis. Eur. Heart J. Acute Cardiovasc. Care. 2020;9:14–22. doi: 10.1177/2048872618819421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Westermann D., Neumann J.T., Sörensen N.A., Blankenberg D.W.J.T.N.N.A.S.S. High-Sensitivity Assays for Troponin in Patients with Cardiac Disease. Nat. Rev. Cardiol. 2017;14:472–483. doi: 10.1038/nrcardio.2017.48. [DOI] [PubMed] [Google Scholar]
- 65.Hill N.R., Fatoba S.T., Oke J.L., Hirst J., O’Callaghan C.A., Lasserson D., Hobbs R. Global Prevalence of Chronic Kidney Disease—A Systematic Review and Meta-Analysis. PLoS ONE. 2016;11:e0158765. doi: 10.1371/journal.pone.0158765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Vogt L., Bangalore S., Fayyad R., Melamed S., Hovingh G.K., DeMicco D.A., Waters D.D. Atorvastatin Has a Dose-Dependent Beneficial Effect on Kidney Function and Associated Cardiovascular Outcomes: Post Hoc Analysis of 6 Double-Blind Randomized Controlled Trials. J. Am. Heart Assoc. 2019;8:e010827. doi: 10.1161/JAHA.118.010827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dalal R., Bruss Z.S., Sehdev J.S. Physiology, Renal Blood Flow and Filtration. StatPearls; Treasure Island, FL, USA: 2021. [PubMed] [Google Scholar]
- 68.Borovac J.A., D’Amario D., Bozic J., Glavas D. Sympathetic Nervous System Activation and Heart Failure: Current State of Evidence and the Pathophysiology in the Light of Novel Biomarkers. World J. Cardiol. 2020;12:373–408. doi: 10.4330/wjc.v12.i8.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Dlugos C.P., Picciotto C., Lepa C., Krakow M., Stöber A., Eddy M.-L., Weide T., Jeibmann A., Krahn M., Van Marck V., et al. Nephrin Signaling Results in Integrin β1 Activation. J. Am. Soc. Nephrol. 2019;30:1006–1019. doi: 10.1681/ASN.2018040362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lichtnekert J., Kaverina N.V., Eng D.G., Gross K.W., Kutz J.N., Pippin J.W., Shankland S.J. Renin-Angiotensin-Aldosterone System Inhibition Increases Podocyte Derivation from Cells of Renin Lineage. J. Am. Soc. Nephrol. 2016;27:3611–3627. doi: 10.1681/ASN.2015080877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Stoll D., Yokota R., Aragão D.S., Casarini D.E. Both Aldosterone and Spironolactone Can Modulate the Intracellular ACE/ANG II/AT1 and ACE2/ANG (1-7)/MAS Receptor Axes in Human Mesangial Cells. Physiol. Rep. 2019;7:e14105. doi: 10.14814/phy2.14105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gómez G.I., Fernández P., Velarde V., Sáez J.C. Angiotensin II-Induced Mesangial Cell Damage Is Preceded by Cell Membrane Permeabilization Due to Upregulation of Non-Selective Channels. Int. J. Mol. Sci. 2018;19:957. doi: 10.3390/ijms19040957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Xu Z., Li W., Han J., Zou C., Huang W., Yu W., Shan X., Lum H., Li X., Liang G. Angiotensin II Induces Kidney Inflammatory Injury and Fibrosis through Binding to Myeloid Differentiation Protein-2 (MD2) Sci. Rep. 2017;7:srep44911. doi: 10.1038/srep44911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Brankovic M., Akkerhuis K.M., Van Boven N., Manintveld O., Germans T., Brugts J., Caliskan K., Umans V., Constantinescu A., Kardys I. Real-Life Use of Neurohormonal Antagonists and Loop Diuretics in Chronic Heart Failure: Analysis of Serial Biomarker Measurements and Clinical Outcome. Clin. Pharmacol. Ther. 2017;104:346–355. doi: 10.1002/cpt.931. [DOI] [PubMed] [Google Scholar]
- 75.Aggarwal D., Singh G. Effects of Single and Dual RAAS Blockade Therapy on Progressive Kidney Disease Transition to CKD in Rats. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2019;393:615–627. doi: 10.1007/s00210-019-01759-3. [DOI] [PubMed] [Google Scholar]
- 76.Dörr O., Liebetrau C., Möllmann H., Gaede L., Troidl C., Wiebe J., Renker M., Bauer T., Hamm C., Nef H. Long-Term Verification of Functional and Structural Renal Damage After Renal Sympathetic Denervation. Catheter. Cardiovasc. Interv. 2016;87:1298–1303. doi: 10.1002/ccd.26355. [DOI] [PubMed] [Google Scholar]
- 77.Damman K., Tang W.W., Testani J.M., McMurray J.J. Terminology and Definition of Changes Renal Function in Heart Failure. Eur. Heart J. 2014;35:3413–3416. doi: 10.1093/eurheartj/ehu320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Damman K., Testani J.M. The Kidney in Heart Failure: An Update. Eur. Heart J. 2015;36:1437–1444. doi: 10.1093/eurheartj/ehv010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Norris K.C., Smoyer K.E., Rolland C., Van Der Vaart J., Grubb E.B. Albuminuria, Serum Creatinine, and Estimated Glomerular Filtration Rate as Predictors of Cardio-Renal Outcomes in Patients with Type 2 Diabetes Mellitus and Kidney Disease: A Systematic Literature Review. BMC Nephrol. 2018;19:1–13. doi: 10.1186/s12882-018-0821-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Jackson C.E., Solomon S.D., Gerstein H., Zetterstrand S., Olofsson B., Michelson E.L., Granger C.B., Swedberg K., A Pfeffer M., Yusuf S., et al. Albuminuria in Chronic Heart Failure: Prevalence and Prognostic Importance. Lancet. 2009;374:543–550. doi: 10.1016/S0140-6736(09)61378-7. [DOI] [PubMed] [Google Scholar]
- 81.Yang Y., Kim K., Hwang I., Yim T., Do W., Kim M., Lee S., Jung H.-Y., Choi J.-Y., Park S.-H., et al. Cystatin C–Based Equation for Predicting the Glomerular Filtration Rate in Kidney Transplant Recipients. Transplant. Proc. 2017;49:1018–1022. doi: 10.1016/j.transproceed.2017.03.050. [DOI] [PubMed] [Google Scholar]
- 82.Wang D., Feng J.-F., Wang A.-Q., Yang Y.-W., Liu Y.-S. Role of Cystatin C and Glomerular Filtration Rate in Diagnosis of Kidney Impairment in Hepatic Cirrhosis Patients. Medicine. 2017;96:e6949. doi: 10.1097/MD.0000000000006949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Richter B., Sulzgruber P., Koller L., Steininger M., El-Hamid F., Rothgerber D.J., Forster S., Goliasch G., Silbert B.I., Meyer E.L., et al. Blood Urea Nitrogen Has Additive Value Beyond Estimated Glomerular Filtration Rate for Prediction of Long-Term Mortality in Patients with Acute Myocardial Infarction. Eur. J. Intern. Med. 2019;59:84–90. doi: 10.1016/j.ejim.2018.07.019. [DOI] [PubMed] [Google Scholar]
- 84.Seki M., Nakayama M., Sakoh T., Yoshitomi R., Fukui A., Katafuchi E., Tsuda S., Nakano T., Tsuruya K., Kitazono T. Blood Urea Nitrogen Is Independently Associated with Renal Outcomes in Japanese Patients with Stage 3–5 Chronic Kidney Disease: A Prospective Observational Study. BMC Nephrol. 2019;20:115. doi: 10.1186/s12882-019-1306-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Buonafine M., Martinez-Martinez E., Jaisser F. More Than a Simple Biomarker: The Role of NGAL in Cardiovascular and Renal Diseases. Clin. Sci. 2018;132:909–923. doi: 10.1042/CS20171592. [DOI] [PubMed] [Google Scholar]
- 86.Merdler I., Rozenfeld K.-L., Zahler D., Shtark M., Goldiner I., Loewenstein I.S., Fortis L., Hochstadt A., Keren G., Banai S., et al. Neutrophil Gelatinase-Associated Lipocalin for the Early Prediction of Acute Kidney Injury in ST-Segment Elevation Myocardial Infarction Patients Treated with Primary Percutaneous Coronary Intervention. Cardiorenal Med. 2020;10:154–161. doi: 10.1159/000506378. [DOI] [PubMed] [Google Scholar]
- 87.Moresco R.N., Bochi G.V., Stein C.S., de Carvalho J.A.M., Cembranel B.M., Bollick Y.S. Urinary Kidney Injury Molecule-1 in Renal Disease. Clin. Chim. Acta. 2018;487:15–21. doi: 10.1016/j.cca.2018.09.011. [DOI] [PubMed] [Google Scholar]
- 88.Maydan O., McDade P.G., Liu Y., Wu X.-R., Matsell D., Eddy A.A. Uromodulin Deficiency Alters Tubular Injury and Interstitial Inflammation But Not Fibrosis in Experimental Obstructive Nephropathy. Physiol. Rep. 2018;6:e13654. doi: 10.14814/phy2.13654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Nogare A.L., Veronese F.V., Carpio V.N., Montenegro R.M., Pedroso J.A., Pegas K.L., Gonçalves L.F., Manfro R.C. Kidney Injury Molecule-1 Expression in Human Kidney Transplants with Interstitial Fibrosis and Tubular Atrophy. BMC Nephrol. 2015;16:19. doi: 10.1186/s12882-015-0011-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Humphreys B.D., Xu F., Sabbisetti V., Grgic I., Naini S.M., Wang N., Chen D., Xiao S., Patel D., Henderson J.M., et al. Chronic Epithelial Kidney Injury Molecule-1 Expression Causes Murine Kidney Fibrosis. J. Clin. Investig. 2013;123:4023–4035. doi: 10.1172/JCI45361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Edelstein C.L. Biomarkers of Acute Kidney Injury. Adv. Chronic Kidney Dis. 2008;15:222–234. doi: 10.1053/j.ackd.2008.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Parikh C.R., Abraham E., Ancukiewicz M., Edelstein C.L. Urine IL-18 Is an Early Diagnostic Marker for Acute Kidney Injury and Predicts Mortality in the Intensive Care Unit. J. Am. Soc. Nephrol. 2005;16:3046–3052. doi: 10.1681/ASN.2005030236. [DOI] [PubMed] [Google Scholar]
- 93.Szabó Z., Magga J., Alakoski T., Ulvila J., Piuhola J., Vainio L., Kivirikko K.I., Vuolteenaho O., Ruskoaho H., Lipson K., et al. Connective Tissue Growth Factor Inhibition Attenuates Left Ventricular Remodeling and Dysfunction in Pressure Overload–Induced Heart Failure. Hypertension. 2014;63:1235–1240. doi: 10.1161/HYPERTENSIONAHA.114.03279. [DOI] [PubMed] [Google Scholar]
- 94.Zhao Y., Wang C., Hong X., Miao J., Liao Y., Hou F.F., Zhou L., Liu Y. Wnt/β-Catenin Signaling Mediates Both Heart and Kidney Injury in Type 2 Cardiorenal Syndrome. Kidney Int. 2019;95:815–829. doi: 10.1016/j.kint.2018.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lekawanvijit S., Kompa A.R., Zhang Y., Wang B.H., Kelly D.J., Krum H. Myocardial Infarction Impairs Renal Function, Induces Renal Interstitial Fibrosis, and Increases Renal KIM-1 Expression: Implications for Cardiorenal Syndrome. Am. J. Physiol. Circ. Physiol. 2012;302:H1884–H1893. doi: 10.1152/ajpheart.00967.2011. [DOI] [PubMed] [Google Scholar]
- 96.Rockey D.C., Bell P.D., Hill J.A. Fibrosis—A Common Pathway to Organ Injury and Failure. N. Engl. J. Med. 2015;372:1138–1149. doi: 10.1056/NEJMra1300575. [DOI] [PubMed] [Google Scholar]
- 97.Weber K.T., Brilla C.G. Factors Associated with Reactive and Reparative Fibrosis of the Myocardium. Cell. Mol. Alter. Fail. Human Heart. 1992;87(Suppl. S1):291–301. doi: 10.1007/978-3-642-72474-9_25. [DOI] [PubMed] [Google Scholar]
- 98.Baum J., Duffy H.S. Fibroblasts and Myofibroblasts: What Are We Talking About? J. Cardiovasc. Pharmacol. 2011;57:376–379. doi: 10.1097/FJC.0b013e3182116e39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Mack M., Yanagita M. Origin of Myofibroblasts and Cellular Events Triggering Fibrosis. Kidney Int. 2015;87:297–307. doi: 10.1038/ki.2014.287. [DOI] [PubMed] [Google Scholar]
- 100.Györfi A.-H., Matei A.-E., Distler J.H. Targeting TGF-β Signaling for the Treatment of Fibrosis. Matrix Biol. 2018;68–69:8–27. doi: 10.1016/j.matbio.2017.12.016. [DOI] [PubMed] [Google Scholar]
- 101.Valiente-Alandi I., Potter S.J., Salvador A.M., Schafer A.E., Schips T., Carrillo-Salinas F.J., Gibson A.M., Nieman M.L., Perkins C., Sargent M.A., et al. Inhibiting Fibronectin Attenuates Fibrosis and Improves Cardiac Function in a Model of Heart Failure. Circulation. 2018;138:1236–1252. doi: 10.1161/CIRCULATIONAHA.118.034609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zheng Z., Ma T., Lian X., Gao J., Wang W., Weng W., Lu X., Sun W., Cheng Y., Fu Y., et al. Clopidogrel Reduces Fibronectin Accumulation and Improves Diabetes-Induced Renal Fibrosis. Int. J. Biol. Sci. 2019;15:239–252. doi: 10.7150/ijbs.29063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Karsdal M.A., Nielsen S.H., Leeming D.J., Langholm L.L., Nielsen M.J., Manon-Jensen T., Siebuhr A., Gudmann N.S., Ronnow S., Sand J.M., et al. The good and the bad collagens of fibrosis—Their role in signaling and organ function. Adv. Drug Deliv. Rev. 2017;121:43–56. doi: 10.1016/j.addr.2017.07.014. [DOI] [PubMed] [Google Scholar]
- 104.Frangogiannis N.G. Pathophysiology of Myocardial Infarction. Compr. Physiol. 2015;5:1841–1875. doi: 10.1002/cphy.c150006. [DOI] [PubMed] [Google Scholar]
- 105.Frangogiannis N.G. Cardiac Fibrosis: Cell Biological Mechanisms, Molecular Pathways and Therapeutic Opportunities. Mol. Asp. Med. 2019;65:70–99. doi: 10.1016/j.mam.2018.07.001. [DOI] [PubMed] [Google Scholar]
- 106.Frangogiannis N.G. The Extracellular Matrix in Ischemic and Nonischemic Heart Failure. Circ. Res. 2019;125:117–146. doi: 10.1161/CIRCRESAHA.119.311148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ytrehus K., Hulot J.-S., Perrino C., Schiattarella G., Madonna R. Perivascular Fibrosis and the Microvasculature of the Heart. Still Hidden Secrets of Pathophysiology? Vasc. Pharmacol. 2018;107:78–83. doi: 10.1016/j.vph.2018.04.007. [DOI] [PubMed] [Google Scholar]
- 108.Talman V., Ruskoaho H. Cardiac fibrosis in myocardial infarction—from repair and remodeling to regeneration. Cell Tissue Res. 2016;365:563–581. doi: 10.1007/s00441-016-2431-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Shinde A.V., Frangogiannis N.G. Fibroblasts in Myocardial Infarction: A Role in Inflammation and Repair. J. Mol. Cell. Cardiol. 2014;70:74–82. doi: 10.1016/j.yjmcc.2013.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Schilter H., Findlay A.D., Perryman L., Yow T.T., Moses J., Zahoor A., Turner C.I., Deodhar M., Foot J.S., Zhou W., et al. The Lysyl Oxidase Like 2/3 Enzymatic Inhibitor, PXS-5153A, Reduces Crosslinks and Ameliorates Fibrosis. J. Cell. Mol. Med. 2018;23:1759–1770. doi: 10.1111/jcmm.14074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Doi M., Kusachi S., Murakami T., Ninomiya Y., Murakami M., Nakahama M., Takeda K., Komatsubara I., Naito I., Tsuji T. Time-Dependent Changes of Decorin in the Infarct Zone After Experimentally Induced Myocardial Infarction in Rats: Comparison with Biglycan. Pathol. Res. Pract. 2000;196:23–33. doi: 10.1016/S0344-0338(00)80018-7. [DOI] [PubMed] [Google Scholar]
- 112.Li L., Okada H., Takemura G., Kosai K.-I., Kanamori H., Esaki M., Takahashi T., Goto K., Tsujimoto A., Maruyama R., et al. Postinfarction Gene Therapy with Adenoviral Vector Expressing Decorin Mitigates Cardiac Remodeling and Dysfunction. Am. J. Physiol. Circ. Physiol. 2009;297:H1504–H1513. doi: 10.1152/ajpheart.00194.2009. [DOI] [PubMed] [Google Scholar]
- 113.Nakahama M., Murakami T., Kusachi S., Naito I., Takeda K., Ohnishi H., Komatsubara I., Oka T., Ninomiya Y., Tsuji T. Expression of Perlecan Proteoglycan in the Infarct Zone of Mouse Myocardial Infarction. J. Mol. Cell. Cardiol. 2000;32:1087–1100. doi: 10.1006/jmcc.2000.1146. [DOI] [PubMed] [Google Scholar]
- 114.Sasse P., Malan D., Fleischmann M., Roell W., Gustafsson E., Bostani T., Fan Y., Kolbe T., Breitbach M., Addicks K., et al. Perlecan Is Critical for Heart Stability. Cardiovasc. Res. 2008;80:435–444. doi: 10.1093/cvr/cvn225. [DOI] [PubMed] [Google Scholar]
- 115.Fu X., Khalil H., Kanisicak O., Boyer J.G., Vagnozzi R.J., Maliken B.D., Sargent M.A., Prasad V., Valiente-Alandi I., Blaxall B.C., et al. Specialized Fibroblast Differentiated States Underlie Scar Formation in the Infarcted Mouse Heart. J. Clin. Investig. 2018;128:2127–2143. doi: 10.1172/JCI98215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Woodiwiss A.J., Tsotetsi O.J., Sprott S., Lancaster E.J., Mela T., Chung E.S., Meyer T.E., Norton G. Reduction in Myocardial Collagen Cross-Linking Parallels Left Ventricular Dilatation in Rat Models of Systolic Chamber Dysfunction. Circulation. 2001;103:155–160. doi: 10.1161/01.CIR.103.1.155. [DOI] [PubMed] [Google Scholar]
- 117.Santamaria J.G., Villalba M., Busnadiego O., López-Olañeta M.M., Sandoval P., Snabel J., López-Cabrera M., Erler J., Hanemaaijer R., Lara-Pezzi E., et al. Matrix Cross-Linking Lysyl Oxidases Are Induced in Response to Myocardial Infarction and Promote Cardiac Dysfunction. Cardiovasc. Res. 2015;109:67–78. doi: 10.1093/cvr/cvv214. [DOI] [PubMed] [Google Scholar]
- 118.Siebermair J., Suksaranjit P., McGann C.J., Peterson K.A., Kheirkhahan M., Baher A.A., Damal K., Wakili R., Marrouche N.F., Wilson B.D. Atrial Fibrosis in non–atrial Fibrillation Individuals and Prediction of Atrial Fibrillation by Use of Late Gadolinium Enhancement Magnetic Resonance Imaging. J. Cardiovasc. Electrophysiol. 2019;30:550–556. doi: 10.1111/jce.13846. [DOI] [PubMed] [Google Scholar]
- 119.Tan T.C., Koutsogeorgis I.D., Grapsa J., Papadopoulos C., Katsivas A., Nihoyannopoulos P. Left Atrium and the Imaging of Atrial Fibrosis: Catch It If You Can! Eur. J. Clin. Investig. 2014;44:872–881. doi: 10.1111/eci.12305. [DOI] [PubMed] [Google Scholar]
- 120.Yang J., Savvatis K., Kang J.S., Fan P., Zhong H., Schwartz K., Barry V., Mikels-Vigdal A., Karpinski S., Kornyeyev D., et al. Targeting LOXL2 for Cardiac Interstitial Fibrosis and Heart Failure Treatment. Nat. Commun. 2016;7:13710. doi: 10.1038/ncomms13710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.De Gaspari M., Toscano G., Bagozzi L., Metra M., Lombardi C., Rizzo S., Angelini A., Marra M.P., Gerosa G., Basso C. Endomyocardial Fibrosis and Myocardial Infarction Leading to Diastolic and Systolic Dysfunction Requiring Transplantation. Cardiovasc. Pathol. Off. J. Soc. Cardiovasc. Pathol. 2019;38:21–24. doi: 10.1016/j.carpath.2018.10.010. [DOI] [PubMed] [Google Scholar]
- 122.Nucifora G., Muser D., Gianfagna P., Morocutti G., Proclemer A. Systolic and Diastolic Myocardial Mechanics in Hypertrophic Cardiomyopathy and Their Link to the Extent of Hypertrophy, Replacement Fibrosis and Interstitial Fibrosis. Int. J. Cardiovasc. Imaging. 2015;31:1603–1610. doi: 10.1007/s10554-015-0720-0. [DOI] [PubMed] [Google Scholar]
- 123.Shinde A.V., Su Y., Palanski B.A., Fujikura K., Garcia M.J., Frangogiannis N.G. Pharmacologic Inhibition of the Enzymatic Effects of Tissue Transglutaminase Reduces Cardiac Fibrosis and Attenuates Cardiomyocyte Hypertrophy Following Pressure Overload. J. Mol. Cell. Cardiol. 2018;117:36–48. doi: 10.1016/j.yjmcc.2018.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.A Beltrami C., Finato N., Rocco M., A Feruglio G., Puricelli C., Cigola E., Quaini F., Sonnenblick E.H., Olivetti G., Anversa P. Structural Basis of End-Stage Failure in Ischemic Cardiomyopathy in Humans. Circulation. 1994;89:151–163. doi: 10.1161/01.CIR.89.1.151. [DOI] [PubMed] [Google Scholar]
- 125.Shah N.N., Ayyadurai P., Saad M., E Kosmas C., Dogar M.U., Patel U., Vittorio T.J. Galectin-3 and Soluble ST2 As Complementary Tools to Cardiac MRI for Sudden Cardiac Death Risk Stratification in Heart Failure: A Review. JRSM Cardiovasc. Dis. 2020;9 doi: 10.1177/2048004020957840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Kim E.K., Chattranukulchai P., Klem I. Cardiac Magnetic Resonance Scar Imaging for Sudden Cardiac Death Risk Stratification in Patients with Non-Ischemic Cardiomyopathy. Korean J. Radiol. 2015;16:683–695. doi: 10.3348/kjr.2015.16.4.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Kato S., Saito N., Kirigaya H., Gyotoku D., Iinuma N., Kusakawa Y., Iguchi K., Nakachi T., Fukui K., Futaki M., et al. Prognostic Significance of Quantitative Assessment of Focal Myocardial Fibrosis in Patients with Heart Failure with Preserved Ejection Fraction. Int. J. Cardiol. 2015;191:314–319. doi: 10.1016/j.ijcard.2015.05.048. [DOI] [PubMed] [Google Scholar]
- 128.King J.B., Azadani P.N., Suksaranjit P., Bress A.P., Witt D.M., Han F.T., Chelu M., Silver M.A., Biskupiak J., Wilson B.D., et al. Left Atrial Fibrosis and Risk of Cerebrovascular and Cardiovascular Events in Patients with Atrial Fibrillation. J. Am. Coll. Cardiol. 2017;70:1311–1321. doi: 10.1016/j.jacc.2017.07.758. [DOI] [PubMed] [Google Scholar]
- 129.Peddakkulappagari C.S., Saifi M.A., Khurana A., Anchi P., Singh M., Godugu C. Withaferin A Ameliorates Renal Injury Due to Its Potent Effect on Inflammatory Signaling. BioFactors. 2019;45:750–762. doi: 10.1002/biof.1534. [DOI] [PubMed] [Google Scholar]
- 130.Tammaro A., Florquin S., Brok M., Claessen N., Butter L.M., Teske G.J.D., de Boer O., Vogl T., Leemans J.C., Dessing M.C. S100A8/A9 Promotes Parenchymal Damage and Renal Fibrosis in Obstructive Nephropathy. Clin. Exp. Immunol. 2018;193:361–375. doi: 10.1111/cei.13154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Grande M.T., Sanchez-Laorden B., López-Blau C., De Frutos C.A., Boutet A., Arévalo M., Rowe R.G., Weiss S.J., López-Novoa J.M., Nieto M.A. Snail1-Induced Partial Epithelial-to-Mesenchymal Transition Drives Renal Fibrosis in Mice and Can Be Targeted to Reverse Established Disease. Nat. Med. 2015;21:989–997. doi: 10.1038/nm.3901. [DOI] [PubMed] [Google Scholar]
- 132.Xie K., Bao L., Jiang X., Ye Z., Bing J., Dong Y., Gao D., Ji X., Jiang T., Li J., et al. The Association of Metabolic Syndrome Components and Chronic Kidney Disease in Patients with Hypertension. Lipids Health Dis. 2019;18:1–6. doi: 10.1186/s12944-019-1121-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Alicic R.Z., Rooney M.T., Tuttle K. Diabetic Kidney Disease. Clin. J. Am. Soc. Nephrol. 2017;12:2032–2045. doi: 10.2215/CJN.11491116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Mordi I., Mordi N., Delles C., Tzemos N. Endothelial Dysfunction in Human Essential Hypertension. J. Hypertens. 2016;34:1464–1472. doi: 10.1097/HJH.0000000000000965. [DOI] [PubMed] [Google Scholar]
- 135.Shi Y., Vanhoutte P.M. Macro- and Microvascular Endothelial Dysfunction in Diabetes. J. Diabetes. 2017;9:434–449. doi: 10.1111/1753-0407.12521. [DOI] [PubMed] [Google Scholar]
- 136.Moriya T., Yamagishi T., Matsubara M., Ouchi M. Serial Renal Biopsies in Normo- and Microalbuminuric Patients with Type 2 Diabetes Demonstrate That Loss of Renal Function Is Associated with a Reduction in Glomerular Filtration Surface Secondary to Mesangial Expansion. J. Diabetes Complicat. 2019;33:368–373. doi: 10.1016/j.jdiacomp.2019.02.002. [DOI] [PubMed] [Google Scholar]
- 137.Heintz B., Stöcker G., Mrowka C., Rentz U., Melzer H., Stickeler E., Sieberth H.-G., Greiling H., Haubeck H.-D. Decreased Glomerular Basement Membrane Heparan Sulfate Proteoglycan in Essential Hypertension. Hypertension. 1995;25:399–407. doi: 10.1161/01.HYP.25.3.399. [DOI] [PubMed] [Google Scholar]
- 138.Salem R.M., Todd J.N., Sandholm N., Cole J.B., Chen W.-M., Andrews D., Pezzolesi M.G., McKeigue P.M., Hiraki L.T., Qiu C., et al. Genome-Wide Association Study of Diabetic Kidney Disease Highlights Biology Involved in Glomerular Basement Membrane Collagen. J. Am. Soc. Nephrol. 2019;30:2000–2016. doi: 10.1681/ASN.2019030218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Tung C.-W., Hsu Y.-C., Shih Y.-H., Chang P.-J., Lin C.-L. Glomerular Mesangial Cell and Podocyte Injuries in Diabetic Nephropathy. Nephrology. 2018;23:32–37. doi: 10.1111/nep.13451. [DOI] [PubMed] [Google Scholar]
- 140.Puelles V.G., Cullen-McEwen L., Taylor G.E., Li J., Hughson M.D., Kerr P.G., Hoy W.E., Bertram J. Human Podocyte Depletion in Association with Older Age and Hypertension. Am. J. Physiol. Physiol. 2016;310:F656–F668. doi: 10.1152/ajprenal.00497.2015. [DOI] [PubMed] [Google Scholar]
- 141.Liu Y. Cellular and molecular mechanisms of renal fibrosis. Nat. Rev. Nephrol. 2011;7:684–696. doi: 10.1038/nrneph.2011.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Conlin C.C., Huang Y., Gordon B.A.J., Zhang J.L. Quantitative Characterization of Glomerular Fibrosis with Magnetic Resonance Imaging: A Feasibility Study in a Rat Glomerulonephritis Model. Am. J. Physiol. Physiol. 2018;314:F747–F752. doi: 10.1152/ajprenal.00529.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Zhou C., Lou K., Tatum K., Funk J., Wu J., Bartkowiak T., Kagan D., Lou Y. Differentiating Glomerular Inflammation from Fibrosis in a Bone Marrow Chimera for Rat Anti-Glomerular Basement Membrane Glomerulonephritis. Am. J. Nephrol. 2015;42:42–53. doi: 10.1159/000438929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Genovese F., A Manresa A., Leeming D.J., Karsdal M.A., Boor P. The Extracellular Matrix in the Kidney: A Source of Novel Non-Invasive Biomarkers of Kidney Fibrosis? Fibrogenesis Tissue Repair. 2014;7:4. doi: 10.1186/1755-1536-7-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Bohle A., Mackensen-Haen S., Gise H.V. Significance of Tubulointerstitial Changes in the Renal Cortex for the Excretory Function and Concentration Ability of the Kidney: A Morphometric Contribution. Am. J. Nephrol. 1987;7:421–433. doi: 10.1159/000167514. [DOI] [PubMed] [Google Scholar]
- 146.Tervaert T.W.C., Mooyaart A., Amann K., Cohen A.H., Cook H.T., Drachenberg C.B., Ferrario F., Fogo A.B., Haas M., De Heer E., et al. Pathologic Classification of Diabetic Nephropathy. J. Am. Soc. Nephrol. 2010;21:556–563. doi: 10.1681/ASN.2010010010. [DOI] [PubMed] [Google Scholar]
- 147.Boor P., Perkuhn M., Ms M.W., Zok S., Martin W., Ms J.G., Schoth F., Ostendorf T., Kuhl C., Floege J. Diffusion-Weighted MRI Does Not Reflect Kidney Fibrosis in a Rat Model of Fibrosis. J. Magn. Reson. Imaging. 2015;42:990–998. doi: 10.1002/jmri.24853. [DOI] [PubMed] [Google Scholar]
- 148.Zhao J., Wang Z., Liu M., Zhu J., Zhang X., Zhang T., Li S., Li Y. Assessment of Renal Fibrosis in Chronic Kidney Disease Using Diffusion-Weighted MRI. Clin. Radiol. 2014;69:1117–1122. doi: 10.1016/j.crad.2014.06.011. [DOI] [PubMed] [Google Scholar]
- 149.Eadon M.T., Schwantes-An T.-H., Phillips C.L., Roberts A.R., Greene C.V., Hallab A., Hart K.J., Lipp S.N., Perez-Ledezma C., Omar K.O., et al. Kidney Histopathology and Prediction of Kidney Failure: A Retrospective Cohort Study. Am. J. Kidney Dis. 2020;76:350–360. doi: 10.1053/j.ajkd.2019.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Belghasem M.E., A’Amar O., Roth D., Walker J., Arinze N., Richards S.M., Francis J.M., Salant D.J., Chitalia V.C., Bigio I.J. Towards Minimally-Invasive, Quantitative Assessment of Chronic Kidney Disease Using Optical Spectroscopy. Sci. Rep. 2019;9:7168. doi: 10.1038/s41598-019-43684-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Nath K.A. Tubulointerstitial Changes As a Major Determinant in the Progression of Renal Damage. Am. J. Kidney Dis. 1992;20:1–17. doi: 10.1016/S0272-6386(12)80312-X. [DOI] [PubMed] [Google Scholar]
- 152.Howie A.J., Ferreira M.A.S., Adu D. Prognostic Value of Simple Measurement of Chronic Damage in Renal Biopsy Specimens. Nephrol. Dial. Transplant. 2001;16:1163–1169. doi: 10.1093/ndt/16.6.1163. [DOI] [PubMed] [Google Scholar]
- 153.Kaissling B., LeHir M., Kriz W. Renal Epithelial Injury and Fibrosis. Biochim. Biophys. Acta Mol. Basis Dis. 2013;1832:931–939. doi: 10.1016/j.bbadis.2013.02.010. [DOI] [PubMed] [Google Scholar]
- 154.Rosenberg A., Kopp J. Focal Segmental Glomerulosclerosis. Clin. J. Am. Soc. Nephrol. 2017;12:502–517. doi: 10.2215/CJN.05960616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Palatini P. Glomerular Hyperfiltration: A Marker of Early Renal Damage in Pre-Diabetes and Pre-Hypertension. Nephrol. Dial. Transplant. 2012;27:1708–1714. doi: 10.1093/ndt/gfs037. [DOI] [PubMed] [Google Scholar]
- 156.Kriz W., Lehir M. Pathways to Nephron Loss Starting from Glomerular diseases—Insights from Animal Models. Kidney Int. 2005;67:404–419. doi: 10.1111/j.1523-1755.2005.67097.x. [DOI] [PubMed] [Google Scholar]
- 157.Dong J., Li Y., Yue S., Liu X., Wang L., Xiong M., Wang G., Nie S., Xu X. The Profiles of Biopsy-Proven Renal Tubulointerstitial Lesions in Patients with Glomerular Disease. Ann. Transl. Med. 2020;8:1066. doi: 10.21037/atm-20-1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Tang P.M.K., Nikolic-Paterson D.J., Lan H.-Y. Macrophages: Versatile Players in Renal Inflammation and Fibrosis. Nat. Rev. Nephrol. 2019;15:144–158. doi: 10.1038/s41581-019-0110-2. [DOI] [PubMed] [Google Scholar]
- 159.Zhao J.-H. Mesangial Cells and Renal Fibrosis. Adv. Exp. Med. Biol. 2019;1165:165–194. doi: 10.1007/978-981-13-8871-2_9. [DOI] [PubMed] [Google Scholar]
- 160.Bülow R.D., Boor P. Extracellular Matrix in Kidney Fibrosis: More Than Just a Scaffold. J. Histochem. Cytochem. 2019;67:643–661. doi: 10.1369/0022155419849388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Müller G.A., Rodemann H.P. Characterization of Human Renal Fibroblasts in Health and Disease: I. Immunophenotyping of Cultured Tubular Epithelial Cells and Fibroblasts Derived from Kidneys with Histologically Proven Interstitial Fibrosis. Am. J. Kidney Dis. 1991;17:680–683. doi: 10.1016/S0272-6386(12)80351-9. [DOI] [PubMed] [Google Scholar]
- 162.Rodemann H.P., Müller G.A. Characterization of Human Renal Fibroblasts in Health and Disease: II. In Vitro Growth, Differentiation, and Collagen Synthesis of Fibroblasts from Kidneys with Interstitial Fibrosis. Am. J. Kidney Dis. 1991;17:684–686. doi: 10.1016/S0272-6386(12)80352-0. [DOI] [PubMed] [Google Scholar]
- 163.Koesters R., Kaissling B., LeHir M., Picard N., Theilig F., Gebhardt R., Glick A.B., Hähnel B., Hosser H., Gröne H.-J., et al. Tubular Overexpression of Transforming Growth Factor-β1 Induces Autophagy and Fibrosis But Not Mesenchymal Transition of Renal Epithelial Cells. Am. J. Pathol. 2010;177:632–643. doi: 10.2353/ajpath.2010.091012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Kriz W., Hähnel B., Hosser H., Ostendorf T., Gaertner S., Kränzlin B., Gretz N., Shimizu F., Floege J. Pathways to Recovery and Loss of Nephrons in Anti-Thy-1 Nephritis. J. Am. Soc. Nephrol. 2003;14:1904–1926. doi: 10.1097/01.ASN.0000070073.79690.57. [DOI] [PubMed] [Google Scholar]
- 165.Cheng S., Pollock A.S., Mahimkar R., Olson J.L., Lovett D.H. Matrix Metalloproteinase 2 and Basement Membrane Integrity: A Unifying Mechanism for Progressive Renal Injury. FASEB J. 2006;20:1898–1900. doi: 10.1096/fj.06-5898fje. [DOI] [PubMed] [Google Scholar]
- 166.Nadasdy T., Laszik Z., E Blick K., Johnson L.D., Silva F.G. Proliferative Activity of Intrinsic Cell Populations in the Normal Human Kidney. J. Am. Soc. Nephrol. 1994;4:2032–2039. doi: 10.1681/ASN.V4122032. [DOI] [PubMed] [Google Scholar]
- 167.Humphreys B.D., Valerius M.T., Kobayashi A., Mugford J.W., Soeung S., Duffield J.S., McMahon A.P., Bonventre J.V. Intrinsic Epithelial Cells Repair the Kidney After Injury. Cell Stem Cell. 2008;2:284–291. doi: 10.1016/j.stem.2008.01.014. [DOI] [PubMed] [Google Scholar]
- 168.Yang L., Besschetnova T.Y., Brooks C.R., Shah J.V., Bonventre J.V. Epithelial Cell Cycle Arrest in G2/M Mediates Kidney Fibrosis After Injury. Nat. Med. 2010;16:535–543. doi: 10.1038/nm.2144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Grgic I., Campanholle G., Bijol V., Wang C., Sabbisetti V.S., Ichimura T., Humphreys B.D., Bonventre J.V. Targeted Proximal Tubule Injury Triggers Interstitial Fibrosis and Glomerulosclerosis. Kidney Int. 2012;82:172–183. doi: 10.1038/ki.2012.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Xu L., Sharkey D., Cantley L.G. Tubular GM-CSF Promotes Late MCP-1/CCR2-Mediated Fibrosis and Inflammation After Ischemia/Reperfusion Injury. J. Am. Soc. Nephrol. 2019;30:1825–1840. doi: 10.1681/ASN.2019010068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Arfian N., Wahyudi D.A.P., Zulfatina I.B., Citta A.N., Anggorowati N., Multazam A., Romi M.M., Sari D.C.R. Chlorogenic Acid Attenuates Kidney Ischemic/Reperfusion Injury via Reducing Inflammation, Tubular Injury, and Myofibroblast Formation. BioMed Res. Int. 2019;2019:1–10. doi: 10.1155/2019/5423703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Jing W., Vaziri N.D., Nunes A.C.F., Suematsu Y., Farzaneh T., Khazaeli M., Moradi H. LCZ696 (Sacubitril/Valsartan) Ameliorates Oxidative Stress, Inflammation, Fibrosis and Improves Renal Function Beyond Angiotensin Receptor Blockade in CKD. Am. J. Transl. Res. 2017;9:5473–5484. [PMC free article] [PubMed] [Google Scholar]
- 173.Fortrie G., De Geus H.R.H., Betjes M.G.H. The Aftermath of Acute Kidney Injury: A Narrative Review of Long-Term Mortality and Renal Function. Crit. Care. 2019;23:1–11. doi: 10.1186/s13054-019-2314-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Stroo I., Stokman G., Teske G.J.D., Raven A., Butter L.M., Florquin S., Leemans J.C. Chemokine Expression in Renal ischemia/Reperfusion Injury Is Most Profound During the Reparative Phase. Int. Immunol. 2010;22:433–442. doi: 10.1093/intimm/dxq025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Ali B.H., Al-Salam S., Al Suleimani Y., Al Kalbani J., Al Bahlani S., Ashique M., Manoj P., Al Dhahli B., Al Abri N., Naser H.T., et al. Curcumin Ameliorates Kidney Function and Oxidative Stress in Experimental Chronic Kidney Disease. Basic Clin. Pharmacol. Toxicol. 2018;122:65–73. doi: 10.1111/bcpt.12817. [DOI] [PubMed] [Google Scholar]
- 176.Fabre T., Kared H., Friedman S.L., Shoukry N.H. IL-17A Enhances the Expression of Profibrotic Genes through Upregulation of the TGF-β Receptor on Hepatic Stellate Cells in a JNK-Dependent Manner. J. Immunol. 2014;193:3925–3933. doi: 10.4049/jimmunol.1400861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Mack M. Inflammation and Fibrosis. Matrix Biol. 2018;68-69:106–121. doi: 10.1016/j.matbio.2017.11.010. [DOI] [PubMed] [Google Scholar]
- 178.Massagué J. TGFβ Signalling in Context. Nat. Rev. Mol. Cell Biol. 2012;13:616–630. doi: 10.1038/nrm3434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Finnson K., Almadani Y., Philip A. Non-Canonical (non-SMAD2/3) TGF-β Signaling in Fibrosis: Mechanisms and Targets. Semin. Cell Dev. Biol. 2020;101:115–122. doi: 10.1016/j.semcdb.2019.11.013. [DOI] [PubMed] [Google Scholar]
- 180.Sun K.-H., Chang Y., Reed N.I., Sheppard D. α-Smooth Muscle Actin Is an Inconsistent Marker of Fibroblasts Responsible for Force-Dependent TGFβ Activation or Collagen Production across Multiple Models of Organ Fibrosis. Am. J. Physiol. Cell. Mol. Physiol. 2016;310:L824–L836. doi: 10.1152/ajplung.00350.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Walker E.J., Heydet D., Veldre T., Ghildyal R. Transcriptomic Changes During TGF-β-Mediated Differentiation of Airway Fibroblasts to Myofibroblasts. Sci. Rep. 2019;9:1–14. doi: 10.1038/s41598-019-56955-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Chen W., Dijke P.T. Immunoregulation by Members of the TGFβ Superfamily. Nat. Rev. Immunol. 2016;16:723–740. doi: 10.1038/nri.2016.112. [DOI] [PubMed] [Google Scholar]
- 183.Gravning J., Ørn S., Kaasbøll O.J., Martinov V.N., Manhenke C., Dickstein K., Edvardsen T., Attramadal H., Ahmed M.S. Myocardial Connective Tissue Growth Factor (CCN2/CTGF) Attenuates Left Ventricular Remodeling After Myocardial Infarction. PLoS ONE. 2012;7:e52120. doi: 10.1371/journal.pone.0052120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Mao L., Liu L., Zhang T., Wu X., Zhang T., Xu Y. MKL1 Mediates TGF-β-induced CTGF Transcription to Promote Renal Fibrosis. J. Cell. Physiol. 2020;235:4790–4803. doi: 10.1002/jcp.29356. [DOI] [PubMed] [Google Scholar]
- 185.Mori T., Kawara S., Shinozaki M., Hayashi N., Kakinuma T., Igarashi A., Takigawa M., Nakanishi T., Takehara K. Role and in-Teraction of Connective Tissue Growth Factor with Transforming Growth Factor-Beta in Persistent Fibrosis: A Mouse Fibrosis Model. J. Cell Physiol. 1999;181:153–159. doi: 10.1002/(SICI)1097-4652(199910)181:1<153::AID-JCP16>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 186.(96) Frazier K., Williams S., Kothapalli D., Klapper H., Grotendorst G.R. Stimulation of Fibroblast Cell Growth, Matrix Production, and Granulation Tissue Formation by Connective Tissue Growth Factor. J. Investig. Dermatol. 1996;107:404–411. doi: 10.1111/1523-1747.ep12363389. [DOI] [PubMed] [Google Scholar]
- 187.Igarashi A., Nashiro K., Kikuchi K., Sato S., Ihn H., Fujimoto M., Grotendorst G.R., Takehara K. Connective Tissue Growth Factor Gene Expression in Tissue Sections from Localized Scleroderma, Keloid, and Other Fibrotic Skin Disorders. J. Investig. Dermatol. 1996;106:729–733. doi: 10.1111/1523-1747.ep12345771. [DOI] [PubMed] [Google Scholar]
- 188.Inanc S., Keleş D., Oktay G. An Improved Collagen Zymography Approach for Evaluating the Collagenases MMP-1, MMP-8, and MMP-13. Biotechnology. 2017;63:174–180. doi: 10.2144/000114597. [DOI] [PubMed] [Google Scholar]
- 189.Falconer A.M.D., Chan C.M., Gray J., Nagashima I., Holland R.A., Shimizu H., Pickford A.R., Rowan A.D., Wilkinson D.J. Collagenolytic Matrix Metalloproteinases Antagonize Proteinase-Activated Receptor-2 Activation, Providing Insights into Extracellular Matrix Turnover. J. Biol. Chem. 2019;294:10266–10277. doi: 10.1074/jbc.RA119.006974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Toth M., Sohail A., Fridman R. Metastasis Research Protocols. Humana Press; Totowa, NJ, USA: 2012. Assessment of Gelatinases (MMP-2 and MMP-9) by Gelatin Zymography; pp. 163–174. [Google Scholar]
- 191.Vafashoar F., Mousavizadeh K., Poormoghim H., Tavasoli A., Shabestari T.M., JavadMoosavi S.A., Mojtabavi N. Gelatinases Increase in Bleomycin-Induced Systemic Sclerosis Mouse Model. Iran. J. Allergy Asthma Immunol. 2019;18:182–189. doi: 10.18502/ijaai.v18i2.921. [DOI] [PubMed] [Google Scholar]
- 192.Butler G.S., Connor A.R., Sounni N.E., Eckhard U., Morrison C.J., Noel A., Overall C.M. Degradomic and Yeast 2-Hybrid Inactive Catalytic Domain Substrate Trapping Identifies New Membrane-Type 1 Matrix Metalloproteinase (MMP14) Substrates: CCN3 (Nov) and CCN5 (WISP2) Matrix Biol. 2017;59:23–38. doi: 10.1016/j.matbio.2016.07.006. [DOI] [PubMed] [Google Scholar]
- 193.Mirastschijski U., Dinesh N., Baskaran S., Wedekind D., Gavrilovic J., Murray M.Y., Bevan D., Kelm S. Novel Specific Human and Mouse stromelysin-1 (MMP-3) and stromelysin-2 (MMP-10) Antibodies for Biochemical and Immunohistochemical Analyses. Wound Repair Regen. 2019;27:309–323. doi: 10.1111/wrr.12704. [DOI] [PubMed] [Google Scholar]
- 194.Giannandrea M., Parks W.C. Diverse Functions of Matrix Metalloproteinases During Fibrosis. Dis. Model. Mech. 2014;7:193–203. doi: 10.1242/dmm.012062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Wang H., Gao M., Li J., Sun J., Wu R., Han D., Tan J., Wang J., Wang B., Zhang L., et al. MMP-9-positive Neutrophils Are Essential for Establishing Profibrotic Microenvironment in the Obstructed Kidney of UUO Mice. Acta Physiol. 2019;227:e13317. doi: 10.1111/apha.13317. [DOI] [PubMed] [Google Scholar]
- 196.Zhao Y., Qiao X., Tan T.K., Zhao H., Zhang Y., Liu L., Zhang J., Wang L., Cao Q., Wang Y., et al. Matrix Metalloproteinase 9-Dependent Notch Signaling Contributes to Kidney Fibrosis through Peritubular Endothelial-Mesenchymal Transition. Nephrol. Dial. Transplant. 2016;32:781–791. doi: 10.1093/ndt/gfw308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Chiao Y.A., Ramirez T.A., Zamilpa R., Okoronkwo S.M., Dai Q., Zhang J., Jin Y.-F., Lindsey M.L. Matrix Metalloproteinase-9 Deletion Attenuates Myocardial Fibrosis and Diastolic Dysfunction in Ageing Mice. Cardiovasc. Res. 2012;96:444–455. doi: 10.1093/cvr/cvs275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Altieri P., Brunelli C., Garibaldi S., Nicolino A., Ubaldi S., Spallarossa P., Olivotti L., Rossettin P., Barsotti A., Ghigliotti G. Metalloproteinases 2 and 9 Are Increased in Plasma of Patients with Heart Failure. Eur. J. Clin. Investig. 2003;33:648–656. doi: 10.1046/j.1365-2362.2003.01187.x. [DOI] [PubMed] [Google Scholar]
- 199.Takamiya Y., Fukami K., Yamagishi S.-I., Kaida Y., Nakayama Y., Obara N., Iwatani R., Ando R., Koike K., Matsui T., et al. Experimental Diabetic Nephropathy Is Accelerated in Matrix Metalloproteinase-2 Knockout Mice. Nephrol. Dial. Transplant. 2012;28:55–62. doi: 10.1093/ndt/gfs387. [DOI] [PubMed] [Google Scholar]
- 200.Jao T.-M., Nangaku M., Wu C.-H., Sugahara M., Saito H., Maekawa H., Ishimoto Y., Aoe M., Inoue T., Tanaka T., et al. ATF6α Downregulation of PPARα Promotes Lipotoxicity-Induced Tubulointerstitial Fibrosis. Kidney Int. 2019;95:577–589. doi: 10.1016/j.kint.2018.09.023. [DOI] [PubMed] [Google Scholar]
- 201.Jiménez-González S., Marín-Royo G., Jurado-López R., Bartolomé M.V., Miranda A.R., Luaces M., Islas F., Nieto M.L., Martínez-Martínez E., Cachofeiro V. The Crosstalk Between Cardiac Lipotoxicity and Mitochondrial Oxidative Stress in the Cardiac Alterations in Diet-Induced Obesity in Rats. Cells. 2020;9:451. doi: 10.3390/cells9020451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Delgado-Valero B., de la Fuente-Chávez L., Romero-Miranda A., Bartolomé M.V., Ramchandani B., Islas F., Luaces M., Cachofeiro V., Martínez-Martínez E. Role of Endoplasmic Reticulum Stress in Renal Damage After Myocardial Infarction. Clin. Sci. 2021;135:143–159. doi: 10.1042/CS20201137. [DOI] [PubMed] [Google Scholar]
- 203.Nogueira A., Pires M.J., Oliveira P.A. Pathophysiological Mechanisms of Renal Fibrosis: A Review of Animal Models and Therapeutic Strategies. Vivo. 2017;31:1–22. doi: 10.21873/invivo.11019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Sun H.-J. Current Opinion for Hypertension in Renal Fibrosis. Adv. Exp. Med. Biol. 2019;1165:37–47. doi: 10.1007/978-981-13-8871-2_3. [DOI] [PubMed] [Google Scholar]
- 205.Rubinstein J., Sanford D. Treatment of Cardiorenal Syndrome. Cardiol. Clin. 2019;37:267–273. doi: 10.1016/j.ccl.2019.04.002. [DOI] [PubMed] [Google Scholar]
- 206.Wang L., Tian X., Cao Y., Ma X., Shang L., Li H., Zhang X., Deng F., Li S., Guo T., et al. Cardiac Shock Wave Therapy Improves Ventricular Function by Relieving Fibrosis Through PI3K/Akt Signaling Pathway: Evidence from a Rat Model of Post-Infarction Heart Failure. Front. Cardiovasc. Med. 2021;8:693875. doi: 10.3389/fcvm.2021.693875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Zindel J., Kubes P. DAMPs, PAMPs, and LAMPs in Immunity and Sterile Inflammation. Annu. Rev. Pathol. Mech. Dis. 2020;15:493–518. doi: 10.1146/annurev-pathmechdis-012419-032847. [DOI] [PubMed] [Google Scholar]
- 208.Abdulkhaleq L.A., Assi M.A., Abdullah R., Zamri-Saad M., Taufiq-Yap Y.H., Hezmee M.N.M. The Crucial Roles of Inflammatory Mediators in Inflammation: A Review. Veter. World. 2018;11:627–635. doi: 10.14202/vetworld.2018.627-635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Clark R., Kupper T. Old Meets New: The Interaction Between Innate and Adaptive Immunity. J. Investig. Dermatol. 2005;125:629–637. doi: 10.1111/j.0022-202X.2005.23856.x. [DOI] [PubMed] [Google Scholar]
- 210.Furman D., Campisi J., Verdin E., Carrera-Bastos P., Targ S., Franceschi C., Ferrucci L., Gilroy D.W., Fasano A., Miller G.W., et al. Chronic Inflammation in the Etiology of Disease across the Life Span. Nat. Med. 2019;25:1822–1832. doi: 10.1038/s41591-019-0675-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Odegaard A.O., Goff D.R., Jr., Sanchez O.A., Goff D.C., Reiner A.P., Gross M.D. Oxidative Stress, Inflammation, Endothelial Dysfunction and Incidence of Type 2 Diabetes. Cardiovasc. Diabetol. 2016;15:1–12. doi: 10.1186/s12933-016-0369-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Kanazawa I., Tanaka S., Sugimoto T. The Association Between Osteocalcin and Chronic Inflammation in Patients with Type 2 Diabetes Mellitus. Calcif. Tissue Int. 2018;103:599–605. doi: 10.1007/s00223-018-0460-y. [DOI] [PubMed] [Google Scholar]
- 213.Xiao L., Harrison D.G. Inflammation in Hypertension. Can. J. Cardiol. 2020;36:635–647. doi: 10.1016/j.cjca.2020.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Rios F., Zou Z.-G., Harvey A.P., Harvey K.Y., Nosalski R., Anyfanti P., Camargo L.L., Lacchini S., Ryazanov A.G., Ryazanova L., et al. Chanzyme TRPM7 Protects Against Cardiovascular Inflammation and Fibrosis. Cardiovasc. Res. 2020;116:721–735. doi: 10.1093/cvr/cvz164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Ridker P.M., Everett B.M., Thuren T., MacFadyen J.G., Chang W.H., Ballantyne C., Fonseca F., Nicolau J., Koenig W., Anker S.D., et al. Antiinflammatory Therapy with Canakinumab for Atherosclerotic Disease. N. Engl. J. Med. 2017;377:1119–1131. doi: 10.1056/NEJMoa1707914. [DOI] [PubMed] [Google Scholar]
- 216.Mihai S., Codrici E., Popescu I.D., Enciu A.-M., Albulescu L., Necula L.G., Mambet C., Anton G., Tanase C. Inflammation-Related Mechanisms in Chronic Kidney Disease Prediction, Progression, and Outcome. J. Immunol. Res. 2018;2018:1–16. doi: 10.1155/2018/2180373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Torres S., Fabersani E., Marquez A., Gauffin-Cano P. Adipose Tissue Inflammation and Metabolic Syndrome. The Proactive Role of Probiotics. Eur. J. Nutr. 2018;58:27–43. doi: 10.1007/s00394-018-1790-2. [DOI] [PubMed] [Google Scholar]
- 218.Pierce G.F., A Mustoe T., Lingelbach J., Masakowski V.R., Griffin G.L., Senior R.M., Deuel T.F. Platelet-Derived Growth Factor and Transforming Growth Factor-Beta Enhance Tissue Repair Activities by Unique Mechanisms. J. Cell Biol. 1989;109:429–440. doi: 10.1083/jcb.109.1.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Rossaint J., Margraf A., Zarbock A. Role of Platelets in Leukocyte Recruitment and Resolution of Inflammation. Front. Immunol. 2018;9:2712. doi: 10.3389/fimmu.2018.02712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Lichtman M.K., Otero-Vinas M., Falanga V. Transforming Growth Factor Beta (TGF-β) Isoforms in Wound Healing and Fibrosis. Wound Repair Regen. 2015;24:215–222. doi: 10.1111/wrr.12398. [DOI] [PubMed] [Google Scholar]
- 221.Amdur R.L., Feldman H.I., Gupta J., Yang W., Kanetsky P., Shlipak M., Rahman M., Lash J.P., Townsend R.R., Ojo A., et al. Inflammation and Progression of CKD: The CRIC Study. Clin. J. Am. Soc. Nephrol. 2016;11:1546–1556. doi: 10.2215/CJN.13121215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Greenberg J.H., Abraham A.G., Xu Y., Schelling J.R., Feldman H.I., Sabbisetti V.S., Gonzalez M.C., Coca S., Schrauben S.J., Waikar S.S., et al. Plasma Biomarkers of Tubular Injury and Inflammation Are Associated with CKD Progression in Children. J. Am. Soc. Nephrol. 2020;31:1067–1077. doi: 10.1681/ASN.2019070723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Wen Y., Lu X., Ren J., Privratsky J.R., Yang B., Rudemiller N.P., Zhang J., Griffiths R., Jain M.K., Nedospasov S.A., et al. KLF4 in Macrophages Attenuates TNFα-Mediated Kidney Injury and Fibrosis. J. Am. Soc. Nephrol. 2019;30:1925–1938. doi: 10.1681/ASN.2019020111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Brandt S., Ballhause T.M., Bernhardt A., Becker A., Salaru D., Le-Deffge H.M., Fehr A., Fu Y., Philipsen L., Djudjaj S., et al. Fibrosis and Immune Cell Infiltration Are Separate Events Regulated by Cell-Specific Receptor Notch3 Expression. J. Am. Soc. Nephrol. 2020;31:2589–2608. doi: 10.1681/ASN.2019121289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Wang Y.-Y., Jiang H., Pan J., Huang X.-R., Wang Y.-C., Huang H.-F., To K.-F., Nikolic-Paterson D.J., Lan H.-Y., Chen J.-H. Macrophage-to-Myofibroblast Transition Contributes to Interstitial Fibrosis in Chronic Renal Allograft Injury. J. Am. Soc. Nephrol. 2017;28:2053–2067. doi: 10.1681/ASN.2016050573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Kormann R., Kavvadas P., Placier S., Vandermeersch S., Dorison A., Dussaule J.-C., Chadjichristos C.E., Prakoura N., Chatziantoniou C. Periostin Promotes Cell Proliferation and Macrophage Polarization to Drive Repair After AKI. J. Am. Soc. Nephrol. 2020;31:85–100. doi: 10.1681/ASN.2019020113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Simões F.C., Cahill T.J., Kenyon A., Gavriouchkina D., Vieira J.M., Sun X., Pezzolla D., Ravaud C., Masmanian E., Weinberger M., et al. Macrophages Directly Contribute Collagen to Scar Formation During Zebrafish Heart Regeneration and Mouse Heart Repair. Nat. Commun. 2020;11:1–17. doi: 10.1038/s41467-019-14263-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Shen B., Liu X., Fan Y., Qiu J. Macrophages Regulate Renal Fibrosis Through Modulating TGFβ Superfamily Signaling. Inflammation. 2014;37:2076–2084. doi: 10.1007/s10753-014-9941-y. [DOI] [PubMed] [Google Scholar]
- 229.Wright T.M. Cytokines in Acute and Chronic Inflammation. Front. Biosci. 1997;2:d12–d26. doi: 10.2741/A171. [DOI] [PubMed] [Google Scholar]
- 230.Panico K., Abrahão M.V., Sonoda M.T., Muzi-Filho H., Vieyra A., Carneiro-Ramos M.S. Cardiac Inflammation After Ischemia-Reperfusion of the Kidney: Role of the Sympathetic Nervous System and the Renin-Angiotensin System. Cell. Physiol. Biochem. 2019;53:587–605. doi: 10.33594/000000159. [DOI] [PubMed] [Google Scholar]
- 231.Virzì G.M., Breglia A., Castellani C., Ankawi G., Bolin C., De Cal M., Cianci V., Angelini A., Vescovo G., Ronco C. Lipopolysaccharide in Systemic Circulation Induces Activation of Inflammatory Response and Oxidative Stress in Cardiorenal Syndrome Type 1. J. Nephrol. 2019;32:803–810. doi: 10.1007/s40620-019-00613-2. [DOI] [PubMed] [Google Scholar]
- 232.Virzì G.M., Breglia A., Brocca A., De Cal M., Bolin C., Vescovo G., Ronco C. Levels of Proinflammatory Cytokines, Oxidative Stress, and Tissue Damage Markers in Patients with Acute Heart Failure with and without Cardiorenal Syndrome Type 1. Cardiorenal Med. 2018;8:321–331. doi: 10.1159/000492602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Colombo P.C., Ganda A., Lin J., Onat D., Harxhi A., Iyasere J.E., Uriel N., Cotter G. Inflammatory Activation: Cardiac, Renal, and Cardio-Renal Interactions in Patients with the Cardiorenal Syndrome. Heart Fail. Rev. 2012;17:177–190. doi: 10.1007/s10741-011-9261-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Li R., Mi X., Yang S., Yang Y., Zhang S., Hui R., Chen Y., Zhang W. Long-Term Stimulation of Angiotensin II Induced Endothelial Senescence and Dysfunction. Exp. Gerontol. 2019;119:212–220. doi: 10.1016/j.exger.2019.02.012. [DOI] [PubMed] [Google Scholar]
- 235.Du Y., Han J., Zhang H., Xu J., Jiang L., Ge W. Kaempferol Prevents Against Ang II-Induced Cardiac Remodeling Through Attenuating Ang II-Induced Inflammation and Oxidative Stress. J. Cardiovasc. Pharmacol. 2019;74:326–335. doi: 10.1097/FJC.0000000000000713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Lang P.-P., Bai J., Zhang Y.-L., Yang X.-L., Xia Y.-L., Lin Q.-Y., Li H.-H. Blockade of Intercellular Adhesion Molecule-1 Prevents Angiotensin II-Induced Hypertension and Vascular Dysfunction. Lab. Investig. 2019;100:378–386. doi: 10.1038/s41374-019-0320-z. [DOI] [PubMed] [Google Scholar]
- 237.Kalra D., Sivasubramanian N., Mann D.L. Angiotensin II Induces Tumor Necrosis Factor Biosynthesis in the Adult Mammalian Heart Through a Protein Kinase C–Dependent Pathway. Circulation. 2002;105:2198–2205. doi: 10.1161/01.CIR.0000015603.84788.47. [DOI] [PubMed] [Google Scholar]
- 238.Ozawa Y., Kobori H., Suzaki Y., Navar L.G. Sustained Renal Interstitial Macrophage Infiltration Following Chronic Angiotensin II Infusions. Am. J. Physiol. Physiol. 2007;292:F330–F339. doi: 10.1152/ajprenal.00059.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Frenay A.-R.S., Yazdani S., Boersema M., Van Der Graaf A.M., Waanders F., Born J.V.D., Navis G.J., Van Goor H. Incomplete Restoration of Angiotensin II - Induced Renal Extracellular Matrix Deposition and Inflammation Despite Complete Functional Recovery in Rats. PLoS ONE. 2015;10:e0129732. doi: 10.1371/journal.pone.0129732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Zhang J.-D., Patel M.B., Griffiths R., Dolber P.C., Ruiz P., Sparks M.A., Stegbauer J., Jin H., Gomez J.A., Buckley A.F., et al. Type 1 Angiotensin Receptors on Macrophages Ameliorate IL-1 receptor–mediated Kidney Fibrosis. J. Clin. Investig. 2014;124:2198–2203. doi: 10.1172/JCI61368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Wang D., Xiong M., Chen C., Du L., Liu Z., Shi Y., Zhang M., Gong J., Song X., Xiang R., et al. Legumain, an Asparaginyl Endopeptidase, Mediates the Effect of M2 Macrophages on Attenuating Renal Interstitial Fibrosis in Obstructive Nephropathy. Kidney Int. 2018;94:91–101. doi: 10.1016/j.kint.2017.12.025. [DOI] [PubMed] [Google Scholar]
- 242.Witherel C.E., Abebayehu D., Barker T.H., Spiller K.L. Macrophage and Fibroblast Interactions in Biomaterial-Mediated Fibrosis. Adv. Health Mater. 2019;8:e1801451. doi: 10.1002/adhm.201801451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Colombo P.C., Onat D., Harxhi A., Demmer R.T., Hayashi Y., Jelic S., LeJemtel T.H., Bucciarelli L., Kebschull M., Papapanou P.N., et al. Peripheral Venous Congestion Causes Inflammation, Neurohormonal, and Endothelial Cell Activation. Eur. Heart J. 2013;35:448–454. doi: 10.1093/eurheartj/eht456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Colombo P.C., Rastogi S., Onat D., Zacà V., Gupta R.C., Jorde U.P., Sabbah H.N. Activation of Endothelial Cells in Conduit Veins of Dogs with Heart Failure and Veins of Normal Dogs After Vascular Stretch by Acute Volume Loading. J. Card. Fail. 2009;15:457–463. doi: 10.1016/j.cardfail.2008.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Berguetti T.S., Quintaes L.S.P., Pereira T.H., Robaina M.C., Cruz A.L.S., Maia R.C., De Souza P.S., Hancio T. TNF-α Modulates P-Glycoprotein Expression and Contributes to Cellular Proliferation via Extracellular Vesicles. Cells. 2019;8:500. doi: 10.3390/cells8050500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Chen T., Zhang X., Zhu G., Liu H., Chen J., Wang Y., He X. Quercetin Inhibits TNF-α Induced HUVECs Apoptosis and Inflammation via Downregulating NF-KB and AP-1 Signaling Pathway in Vitro. Medicine. 2020;99:e22241. doi: 10.1097/MD.0000000000022241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Zelová H., Hošek J. TNF-α Signalling and Inflammation: Interactions Between Old Acquaintances. Inflamm. Res. 2013;62:641–651. doi: 10.1007/s00011-013-0633-0. [DOI] [PubMed] [Google Scholar]
- 248.Sziksz E., Pap D., Lippai R., Béres N.J., Fekete A., Szabó A.J., Vannay Á. Fibrosis Related Inflammatory Mediators: Role of the IL-10 Cytokine Family. Mediat. Inflamm. 2015;2015:1–15. doi: 10.1155/2015/764641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Wynn T.A. Cellular and Molecular Mechanisms of Fibrosis. J. Pathol. 2007;214:199–210. doi: 10.1002/path.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Shao D., Suresh R., Vakil V., Gomer R., Pilling D. Pivotal Advance: Th-1 Cytokines Inhibit, and Th-2 Cytokines Promote Fibrocyte Differentiation. J. Leukoc. Biol. 2008;83:1323–1333. doi: 10.1189/jlb.1107782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Le Floc’H A., Allinne J., Nagashima K., Scott G., Birchard D., Asrat S., Bai Y., Lim W.K., Martin J., Huang T., et al. Dual Blockade of IL-4 and IL-13 with Dupilumab, an IL-4Rα Antibody, Is Required to Broadly Inhibit Type 2 Inflammation. Allergy. 2019;75:1188–1204. doi: 10.1111/all.14151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Reiman R.M., Thompson R.W., Feng C., Hari D., Knight R., Cheever A.W., Rosenberg H.F., Wynn T.A. Interleukin-5 (IL-5) Augments the Progression of Liver Fibrosis by Regulating IL-13 Activity. Infect. Immun. 2006;74:1471–1479. doi: 10.1128/IAI.74.3.1471-1479.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Pesce J., Kaviratne M., Ramalingam T.R., Thompson R.W., Urban J., Cheever A.W., Young D.A., Collins M., Grusby M.J., Wynn T.A. The IL-21 Receptor Augments Th2 Effector Function and Alternative Macrophage Activation. J. Clin. Investig. 2006;116:2044–2055. doi: 10.1172/JCI27727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Korn T., Bettelli E., Gao W., Awasthi A., Jäger A., Strom T.B., Oukka M., Kuchroo V.K. IL-21 Initiates an Alternative Pathway to Induce Proinflammatory TH17 Cells. Nat. Cell Biol. 2007;448:484–487. doi: 10.1038/nature05970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Nurieva R., Yang X.O., Martinez G., Zhang Y., Panopoulos A., Ma L., Schluns K., Tian Q., Watowich S.S., Jetten A., et al. Essential Autocrine Regulation by IL-21 in the Generation of Inflammatory T Cells. Nat. Cell Biol. 2007;448:480–483. doi: 10.1038/nature05969. [DOI] [PubMed] [Google Scholar]
- 256.Lei L., Zhao C., Qin F., He Z.Y., Wang X., Zhong X.N. Th17 Cells and IL-17 Promote the Skin and Lung Inflammation and Fibrosis Process in a Bleomycin-Induced Murine Model of Systemic Sclerosis. Clin. Exp. Rheumatol. 2016;34(Suppl. S100):14–22. [PubMed] [Google Scholar]
- 257.Wu L., Ong S., Talor M.V., Barin J.G., Baldeviano G.C., Kass D.A., Bedja D., Zhang H., Sheikh A., Margolick J.B., et al. Cardiac Fibroblasts Mediate IL-17A–driven Inflammatory Dilated Cardiomyopathy. J. Exp. Med. 2014;211:1449–1464. doi: 10.1084/jem.20132126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Sommerfeld S.D., Cherry C., Schwab R.M., Chung L., Maestas D.R., Jr., Laffont P., Stein J.E., Tam A., Ganguly S., Housseau F., et al. Interleukin-36γ–producing Macrophages Drive IL-17–mediated Fibrosis. Sci. Immunol. 2019;4:eaax4783. doi: 10.1126/sciimmunol.aax4783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Ramani K., Tan R.J., Zhou D., Coleman B.M., Jawale C.V., Liu Y., Biswas P.S. IL-17 Receptor Signaling Negatively Regulates the Development of Tubulointerstitial Fibrosis in the Kidney. Mediat. Inflamm. 2018;2018:1–14. doi: 10.1155/2018/5103672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Huang M., Sharma S., Zhu L.X., Keane M.P., Luo J., Zhang L., Burdick M.D., Lin Y.Q., Dohadwala M., Gardner B., et al. IL-7 Inhibits Fibroblast TGF-β Production and Signaling in Pulmonary Fibrosis. J. Clin. Investig. 2002;109:931–937. doi: 10.1172/JCI0214685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Demols A., Van Laethem J.-L., Quertinmont E., Degraef C., Delhaye M., Geerts A., Devière J. Endogenous Interleukin-10 Modulates Fibrosis and Regeneration in Experimental Chronic Pancreatitis. Am. J. Physiol. Liver Physiol. 2002;282:G1105–G1112. doi: 10.1152/ajpgi.00431.2001. [DOI] [PubMed] [Google Scholar]
- 262.Shamskhou E.A., Kratochvil M.J., Orcholski M.E., Nagy N., Kaber G., Steen E., Balaji S., Yuan K., Keswani S., Danielson B., et al. Hydrogel-Based Delivery of Il-10 Improves Treatment of Bleomycin-Induced Lung Fibrosis in Mice. Biomaterials. 2019;203:52–62. doi: 10.1016/j.biomaterials.2019.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Guan Q., Weiss C.R., Wang S., Qing G., Yang X., Warrington R.J., Bernstein C.N., Peng Z. Reversing Ongoing Chronic Intestinal Inflammation and Fibrosis by Sustained Block of IL-12 and IL-23 Using a Vaccine in Mice. Inflamm. Bowel Dis. 2018;24:1941–1952. doi: 10.1093/ibd/izy142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Keane M.P., Belperio J.A., Burdick M.D., Strieter R.M. IL-12 Attenuates Bleomycin-Induced Pulmonary Fibrosis. Am. J. Physiol. Cell. Mol. Physiol. 2001;281:L92–L97. doi: 10.1152/ajplung.2001.281.1.L92. [DOI] [PubMed] [Google Scholar]
- 265.Weidenbusch M., Song S., Iwakura T., Shi C., Rodler S., Kobold S., Mulay S.R., Honarpisheh M.M., Anders H.-J. IL-22 Sustains Epithelial Integrity in Progressive Kidney Remodeling and Fibrosis. Physiol. Rep. 2018;6:e13817. doi: 10.14814/phy2.13817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Wang S., Li Y., Fan J., Zhang X., Luan J., Bian Q., Ding T., Wang Y., Wang Z., Song P., et al. Interleukin-22 Ameliorated Renal Injury and Fibrosis in Diabetic Nephropathy through Inhibition of NLRP3 Inflammasome Activation. Cell Death Dis. 2017;8:e2937. doi: 10.1038/cddis.2017.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Lee J.-W., Oh J.E., Rhee K.-J., Yoo B.-S., Eom Y.W., Park S.W., Lee J.H., Son J.-W., Youn Y.J., Ahn M.-S., et al. Co-Treatment with Interferon-γ and 1-Methyl Tryptophan Ameliorates Cardiac Fibrosis through Cardiac Myofibroblasts Apoptosis. Mol. Cell. Biochem. 2019;458:197–205. doi: 10.1007/s11010-019-03542-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Poosti F., Bansal R., Yazdani S., Prakash J., Post E., Klok P., Born J.V.D., de Borst M.H., van Goor H., Poelstra K., et al. Selective Delivery of IFN-γ to Renal Interstitial Myofibroblasts: A Novel Strategy for the Treatment of Renal Fibrosis. FASEB J. 2015;29:1029–1042. doi: 10.1096/fj.14-258459. [DOI] [PubMed] [Google Scholar]
- 269.Liechty K.W., Kim H.B., Adzick N., Crombleholme T.M. Fetal Wound Repair Results in Scar Formation in Interleukin-10–deficient Mice in a Syngeneic Murine Model of Scarless Fetal Wound Repair. J. Pediatr. Surg. 2000;35:866–873. doi: 10.1053/jpsu.2000.6868. [DOI] [PubMed] [Google Scholar]
- 270.Lin W.-R., Lim S.-N., Yen T.-H., Alison M.R. The Influence of Bone Marrow-Secreted IL-10 in a Mouse Model of Cerulein-Induced Pancreatic Fibrosis. BioMed Res. Int. 2016;2016:1–11. doi: 10.1155/2016/4601532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Gupta J., Mitra N., Kanetsky P.A., Devaney J., Wing M.R., Reilly M., Shah V.O., Balakrishnan V.S., Guzman N.J., Girndt M., et al. Association Between Albuminuria, Kidney Function, and Inflammatory Biomarker Profile in CKD in CRIC. Clin. J. Am. Soc. Nephrol. 2012;7:1938–1946. doi: 10.2215/CJN.03500412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Li R., Guo Y., Zhang Y., Zhang X., Zhu L., Yan T. Salidroside Ameliorates Renal Interstitial Fibrosis by Inhibiting the TLR4/NF-κB and MAPK Signaling Pathways. Int. J. Mol. Sci. 2019;20:1103. doi: 10.3390/ijms20051103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Edeling M., Ragi G., Huang S., Pavenstädt H., Susztak K. Developmental Signalling Pathways in Renal Fibrosis: The Roles of Notch, Wnt and Hedgehog. Nat. Rev. Nephrol. 2016;12:426–439. doi: 10.1038/nrneph.2016.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Cho E., Kim M., Ko Y.S., Lee H.Y., Song M., Kim H.-K., Cho W.-Y., Jo S.-K. Role of Inflammation in the Pathogenesis of Cardiorenal Syndrome in a Rat Myocardial Infarction Model. Nephrol. Dial. Transplant. 2013;28:2766–2778. doi: 10.1093/ndt/gft376. [DOI] [PubMed] [Google Scholar]
- 275.Yhee J.-Y., Yu C.-H., Kim J.-H., Sur J.-H. Effects of T Lymphocytes, Interleukin-1, and Interleukin-6 on Renal Fibrosis in Canine End-Stage Renal Disease. J. Veter. Diagn. Investig. 2008;20:585–592. doi: 10.1177/104063870802000508. [DOI] [PubMed] [Google Scholar]
- 276.Van Linthout S., Miteva K., Tschöpe C. Crosstalk Between Fibroblasts and Inflammatory Cells. Cardiovasc. Res. 2014;102:258–269. doi: 10.1093/cvr/cvu062. [DOI] [PubMed] [Google Scholar]
- 277.Black L.M., Lever J.M., Agarwal A. Renal Inflammation and Fibrosis: A Double-Edged Sword. J. Histochem. Cytochem. 2019;67:663–681. doi: 10.1369/0022155419852932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Meng X.-M. Inflammatory Mediators and Renal Fibrosis. Adv. Exp. Med. Biol. 2019;1165:381–406. doi: 10.1007/978-981-13-8871-2_18. [DOI] [PubMed] [Google Scholar]
- 279.Iwano M., Neilson E.G. Mechanisms of Tubulointerstitial Fibrosis. Curr. Opin. Nephrol. Hypertens. 2004;13:279–284. doi: 10.1097/00041552-200405000-00003. [DOI] [PubMed] [Google Scholar]
- 280.Prabhu S.D., Frangogiannis N.G. The Biological Basis for Cardiac Repair After Myocardial Infarction. Circ. Res. 2016;119:91–112. doi: 10.1161/CIRCRESAHA.116.303577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Sun K., Li Y.-Y., Jin J. A Double-Edged Sword of Immuno-Microenvironment in Cardiac Homeostasis and Injury Repair. Signal Transduct. Target. Ther. 2021;6:1–16. doi: 10.1038/s41392-020-00455-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Fadok V.A., Bratton D.L., Konowal A., Freed P.W., Westcott J.Y., Henson P.M. Macrophages That Have Ingested Apoptotic Cells In Vitro Inhibit Proinflammatory Cytokine Production through autocrine/Paracrine Mechanisms Involving TGF-Beta, PGE2, and PAF. J. Clin. Investig. 1998;101:890–898. doi: 10.1172/JCI1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Song E., Ouyang N., Hörbelt M., Antus B., Wang M., Exton M.S. Influence of Alternatively and Classically Activated Macrophages on Fibrogenic Activities of Human Fibroblasts. Cell. Immunol. 2000;204:19–28. doi: 10.1006/cimm.2000.1687. [DOI] [PubMed] [Google Scholar]
- 284.Valgimigli M., Ceconi C., Malagutti P., Merli E., Soukhomovskaia O., Francolini G., Cicchitelli G., Olivares A., Parrinello G., Percoco G., et al. Tumor Necrosis Factor-α Receptor 1 Is a Major Predictor of Mortality and New-Onset Heart Failure in Patients with Acute Myocardial Infarction. Circulation. 2005;111:863–870. doi: 10.1161/01.CIR.0000155614.35441.69. [DOI] [PubMed] [Google Scholar]
- 285.Maekawa N., Wada H., Kanda T., Niwa T., Yamada Y., Saito K., Fujiwara H., Sekikawa K., Seishima M. Improved Myocardial ischemia/Reperfusion Injury in Mice Lacking Tumor Necrosis Factor-α. J. Am. Coll. Cardiol. 2002;39:1229–1235. doi: 10.1016/S0735-1097(02)01738-2. [DOI] [PubMed] [Google Scholar]
- 286.Kurrelmeyer K.M., Michael L.H., Baumgarten G., Taffet G.E., Peschon J.J., Sivasubramanian N., Entman M.L., Mann D.L. Endogenous Tumor Necrosis Factor Protects the Adult Cardiac Myocyte Against Ischemic-Induced Apoptosis in a Murine Model of Acute Myocardial Infarction. Proc. Natl. Acad. Sci. USA. 2000;97:5456–5461. doi: 10.1073/pnas.070036297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Huang M., Li J.-Y. Physiological Regulation of Reactive Oxygen Species in Organisms Based on Their Physicochemical Properties. Acta Physiol. 2020;228:e13351. doi: 10.1111/apha.13351. [DOI] [PubMed] [Google Scholar]
- 288.Pisoschi A.M., Pop A. The Role of Antioxidants in the Chemistry of Oxidative Stress: A Review. Eur. J. Med. Chem. 2015;97:55–74. doi: 10.1016/j.ejmech.2015.04.040. [DOI] [PubMed] [Google Scholar]
- 289.Lau N., Pluth M.D. Reactive Sulfur Species (RSS): Persulfides, Polysulfides, Potential, and Problems. Curr. Opin. Chem. Biol. 2019;49:1–8. doi: 10.1016/j.cbpa.2018.08.012. [DOI] [PubMed] [Google Scholar]
- 290.Deng Z., Hu J., Liu S. Reactive Oxygen, Nitrogen, and Sulfur Species (RONSS)-Responsive Polymersomes for Triggered Drug Release. Macromol. Rapid Commun. 2017;38:10–1002. doi: 10.1002/marc.201600685. [DOI] [PubMed] [Google Scholar]
- 291.Luo Z., Xu X., Sho T., Zhang J., Xu W., Yao J., Xu J. ROS-Induced Autophagy Regulates Porcine Trophectoderm Cell Apoptosis, Proliferation, and Differentiation. Am. J. Physiol. Physiol. 2019;316:C198–C209. doi: 10.1152/ajpcell.00256.2018. [DOI] [PubMed] [Google Scholar]
- 292.Pei J., Wang F., Pei S., Bai R., Cong X., Nie Y., Chen X. Hydrogen Sulfide Promotes Cardiomyocyte Proliferation and Heart Regeneration via ROS Scavenging. Oxidative Med. Cell. Longev. 2020;2020:1–11. doi: 10.1155/2020/1412696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Zhang G., He J., Ye X., Zhu J., Hu X., Shen M., Ma Y., Mao Z., Song H., Chen F. β-Thujaplicin Induces Autophagic Cell Death, Apoptosis, and Cell Cycle Arrest through ROS-Mediated Akt and p38/ERK MAPK Signaling in Human Hepatocellular Carcinoma. Cell Death Dis. 2019;10:1–14. doi: 10.1038/s41419-019-1492-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Kang R., Li R., Dai P., Li Z., Li Y., Li C. Deoxynivalenol Induced Apoptosis and Inflammation of IPEC-J2 Cells by Promoting ROS Production. Environ. Pollut. 2019;251:689–698. doi: 10.1016/j.envpol.2019.05.026. [DOI] [PubMed] [Google Scholar]
- 295.Hawkins C.L., Davies M.J. Detection, Identification, and Quantification of Oxidative Protein Modifications. J. Biol. Chem. 2019;294:19683–19708. doi: 10.1074/jbc.REV119.006217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Kowalska M., Piekut T., Prendecki M., Sodel A., Kozubski W., Dorszewska J. Mitochondrial and Nuclear DNA Oxidative Damage in Physiological and Pathological Aging. DNA Cell Biol. 2020;39:1410–1420. doi: 10.1089/dna.2019.5347. [DOI] [PubMed] [Google Scholar]
- 297.Salehi F., Behboudi H., Kavoosi G., Ardestani S.K. Oxidative DNA Damage Induced by ROS-Modulating Agents with the Ability to Target DNA: A Comparison of the Biological Characteristics of Citrus Pectin and Apple Pectin. Sci. Rep. 2018;8:1–16. doi: 10.1038/s41598-018-32308-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Ayala A., Muñoz M.F., Argüelles S. Lipid Peroxidation: Production, Metabolism, and Signaling Mechanisms of Malondialdehyde and 4-Hydroxy-2-Nonenal. Oxid. Med. Cell. Longev. 2014;2014:1–31. doi: 10.1155/2014/360438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Tsikas D. Assessment of Lipid Peroxidation by Measuring Malondialdehyde (MDA) and Relatives in Biological Samples: Analytical and Biological Challenges. Anal. Biochem. 2017;524:13–30. doi: 10.1016/j.ab.2016.10.021. [DOI] [PubMed] [Google Scholar]
- 300.Gallo G., Sprovierio P., Martino G. 4-Hydroxynonenal and Oxidative Stress in Several Organelles and Its Damaging Effects on Cell Functions. J. Physiol. Pharmacol. Off. J. Pol. Physiol. Soc. 2020;71:10–26402. doi: 10.26402/jpp.2020.1.07. [DOI] [PubMed] [Google Scholar]
- 301.Schrader M., Fahimi H. Peroxisomes and Oxidative Stress. Biochim. Biophys. Acta Bioenerg. 2006;1763:1755–1766. doi: 10.1016/j.bbamcr.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 302.Antonenkov V.D., Grunau S., Ohlmeier S., Hiltunen K. Peroxisomes Are Oxidative Organelles. Antioxid. Redox Signal. 2010;13:525–537. doi: 10.1089/ars.2009.2996. [DOI] [PubMed] [Google Scholar]
- 303.Zeeshan H.M.A., Lee G.H., Kim H.-R., Chae H.-J. Endoplasmic Reticulum Stress and Associated ROS. Int. J. Mol. Sci. 2016;17:327. doi: 10.3390/ijms17030327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Zhao R.-Z., Jiang S., Zhang L., Yu Z.-B. Mitochondrial electron transport chain, ROS generation and uncoupling (Review) Int. J. Mol. Med. 2019;44:3–15. doi: 10.3892/ijmm.2019.4188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Phaniendra A., Jestadi D.B., Periyasamy L. Free Radicals: Properties, Sources, Targets, and Their Implication in Various Diseases. Ind. J. Clin. Biochem. 2015;30:11–26. doi: 10.1007/s12291-014-0446-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Oyewole A.O., Birch-Machin M.A. Mitochondria-targeted Antioxidants. FASEB J. 2015;29:4766–4771. doi: 10.1096/fj.15-275404. [DOI] [PubMed] [Google Scholar]
- 307.Iacovino L.G., Manzella N., Resta J., Vanoni M.A., Rotilio L., Pisani L., Edmondson D.E., Parini A., Mattevi A., Mialet-Perez J., et al. Rational Redesign of Monoamine Oxidase A into a Dehydrogenase to Probe ROS in Cardiac Aging. ACS Chem. Biol. 2020;15:1795–1800. doi: 10.1021/acschembio.0c00366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Kaludercic N., Mialet-Perez J., Paolocci N., Parini A., Di Lisa F. Monoamine Oxidases As Sources of Oxidants in the Heart. J. Mol. Cell. Cardiol. 2014;73:34–42. doi: 10.1016/j.yjmcc.2013.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Dey S., Sidor A., O’Rourke B. Compartment-Specific Control of Reactive Oxygen Species Scavenging by Antioxidant Pathway Enzymes. J. Biol. Chem. 2016;291:11185–11197. doi: 10.1074/jbc.M116.726968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.He L., He T., Farrar S., Ji L., Liu T., Ma X. Antioxidants Maintain Cellular Redox Homeostasis by Elimination of Reactive Oxygen Species. Cell. Physiol. Biochem. 2017;44:532–553. doi: 10.1159/000485089. [DOI] [PubMed] [Google Scholar]
- 311.Dinh Q.N., Drummond G., Sobey C.G., Chrissobolis S. Roles of Inflammation, Oxidative Stress, and Vascular Dysfunction in Hypertension. BioMed Res. Int. 2014;2014:1–11. doi: 10.1155/2014/406960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Chiba T., Peasley K.D., Cargill K.R., Maringer K.V., Bharathi S.S., Mukherjee E., Zhang Y., Holtz A., Basisty N., Yagobian S.D., et al. Sirtuin 5 Regulates Proximal Tubule Fatty Acid Oxidation to Protect Against AKI. J. Am. Soc. Nephrol. 2019;30:2384–2398. doi: 10.1681/ASN.2019020163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Papinska A.M., Rodgers K.E. Long-Term Administration of Angiotensin (1–7) to db/Db Mice Reduces Oxidative Stress Damage in the Kidneys and Prevents Renal Dysfunction. Oxidative Med. Cell. Longev. 2018;2018:1–10. doi: 10.1155/2018/1841046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Kumar S., Wang G., Zheng N., Cheng W., Ouyang K., Lin H., Liao Y., Liu J. HIMF (Hypoxia-Induced Mitogenic Factor)-IL (Interleukin)-6 Signaling Mediates Cardiomyocyte-Fibroblast Crosstalk to Promote Cardiac Hypertrophy and Fibrosis. Hypertension. 2019;73:1058–1070. doi: 10.1161/HYPERTENSIONAHA.118.12267. [DOI] [PubMed] [Google Scholar]
- 315.He T., Guan X., Wang S., Xiao T., Yang K., Xu X., Wang J., Zhao J. Resveratrol Prevents High Glucose-Induced epithelial–mesenchymal Transition in Renal Tubular Epithelial Cells by Inhibiting NADPH oxidase/ROS/ERK Pathway. Mol. Cell. Endocrinol. 2015;402:13–20. doi: 10.1016/j.mce.2014.12.010. [DOI] [PubMed] [Google Scholar]
- 316.Carthy J.M. TGFβ Signaling and the Control of Myofibroblast Differentiation: Implications for Chronic Inflammatory Disorders. J. Cell. Physiol. 2017;233:98–106. doi: 10.1002/jcp.25879. [DOI] [PubMed] [Google Scholar]
- 317.Park S.-A., Kim M.-J., Park S.-Y., Kim J.-S., Lee S.-J., Woo H.A., Kim D.-K., Nam J.-S., Sheen Y.Y. EW-7197 Inhibits Hepatic, Renal, and Pulmonary Fibrosis by Blocking TGF-β/Smad and ROS Signaling. Cell. Mol. Life Sci. 2014;72:2023–2039. doi: 10.1007/s00018-014-1798-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Liu Y., Yuan X., Li W., Cao Q., Shu Y. Aspirin-Triggered Resolvin D1 Inhibits TGF-β1-Induced EMT through the Inhibition of the MTOR Pathway by Reducing the Expression of PKM2 and Is Closely Linked to Oxidative Stress. Int. J. Mol. Med. 2016;38:1235–1242. doi: 10.3892/ijmm.2016.2721. [DOI] [PubMed] [Google Scholar]
- 319.De Bleser P.J., Xu G., Rombouts K., Rogiers V., Geerts A. Glutathione Levels Discriminate Between Oxidative Stress and Transforming Growth Factor-β Signaling in Activated Rat Hepatic Stellate Cells. J. Biol. Chem. 1999;274:33881–33887. doi: 10.1074/jbc.274.48.33881. [DOI] [PubMed] [Google Scholar]
- 320.Matsushima S., Kuroda J., Ago T., Zhai P., Ikeda Y., Oka S., Fong G.-H., Tian R., Sadoshima J. Broad Suppression of NADPH Oxidase Activity Exacerbates Ischemia/Reperfusion Injury Through Inadvertent Downregulation of Hypoxia-Inducible Factor-1α and Upregulation of Peroxisome Proliferator–activated Receptor-α. Circ. Res. 2013;112:1135–1149. doi: 10.1161/CIRCRESAHA.111.300171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Verzola D., Ratto E., Villaggio B., Parodi E.L., Pontremoli R., Garibotto G., Viazzi F. Uric Acid Promotes Apoptosis in Human Proximal Tubule Cells by Oxidative Stress and the Activation of NADPH Oxidase NOX 4. PLoS ONE. 2014;9:e115210. doi: 10.1371/journal.pone.0115210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Buvelot H., Jaquet V., Krause K.-H. Mammalian NADPH Oxidases. Methods Mol. Biol. 2019;1982:17–36. doi: 10.1007/978-1-4939-9424-3_2. [DOI] [PubMed] [Google Scholar]
- 323.Lan T., Kisseleva T., Brenner D.A. Deficiency of NOX1 or NOX4 Prevents Liver Inflammation and Fibrosis in Mice through Inhibition of Hepatic Stellate Cell Activation. PLoS ONE. 2015;10:e0129743. doi: 10.1371/journal.pone.0129743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Muñoz M., López-Oliva M.E., Rodríguez C., Martínez M.P., Sáenz-Medina J., Sánchez A., Climent B., Benedito S., García-Sacristán A., Rivera L., et al. Differential Contribution of Nox1, Nox2 and Nox4 to Kidney Vascular Oxidative Stress and Endothelial Dysfunction in Obesity. Redox Biol. 2020;28:101330. doi: 10.1016/j.redox.2019.101330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Rajaram R.D., Dissard R., Faivre A., Ino F., Delitsikou V., Jaquet V., Cagarelli T., Lindenmeyer M., Jansen-Duerr P., Cohen C., et al. Tubular NOX4 Expression Decreases in Chronic Kidney Disease But Does Not Modify Fibrosis Evolution. Redox Biol. 2019;26:101234. doi: 10.1016/j.redox.2019.101234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Braunersreuther V., Montecucco F., Ashri M., Pelli G., Galan K., Frias M., Burger F., Quinderé A.L.G., Montessuit C., Krause K.-H., et al. Role of NADPH Oxidase Isoforms NOX1, NOX2 and NOX4 in Myocardial ischemia/Reperfusion Injury. J. Mol. Cell. Cardiol. 2013;64:99–107. doi: 10.1016/j.yjmcc.2013.09.007. [DOI] [PubMed] [Google Scholar]
- 327.Cai X., Yang C., Shao L., Zhu H., Wang Y., Huang X., Wang S., Hong L. Targeting NOX 4 by Petunidin Improves anoxia/Reoxygenation-Induced Myocardium Injury. Eur. J. Pharmacol. 2020;888:173414. doi: 10.1016/j.ejphar.2020.173414. [DOI] [PubMed] [Google Scholar]
- 328.Bondi C.D., Manickam N., Lee D.Y., Block K., Gorin Y., Abboud H.E., Barnes J.L. NAD(P)H Oxidase Mediates TGF-β1–Induced Activation of Kidney Myofibroblasts. J. Am. Soc. Nephrol. 2009;21:93–102. doi: 10.1681/ASN.2009020146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.He T., Xiong J., Nie L., Yu Y., Guan X., Xu X., Xiao T., Yang K., Liu L., Zhang D., et al. Resveratrol Inhibits Renal Interstitial Fibrosis in Diabetic Nephropathy by Regulating AMPK/NOX4/ROS Pathway. J. Mol. Med. 2016;94:1359–1371. doi: 10.1007/s00109-016-1451-y. [DOI] [PubMed] [Google Scholar]
- 330.Breglia A., Virzì G.M., Pastori S., Brocca A., De Cal M., Bolin C., Vescovo G., Ronco C. Determinants of Monocyte Apoptosis in Cardiorenal Syndrome Type 1. Cardiorenal Med. 2018;8:208–216. doi: 10.1159/000488949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Caio-Silva W., Dias D.D.S., JunHo C.V.C., Panico K., Neres-Santos R.S., Pelegrino M.T., Pieretti J.C., Seabra A.B., De Angelis K., Carneiro-Ramos M.S. Characterization of the Oxidative Stress in Renal Ischemia/Reperfusion-Induced Cardiorenal Syndrome Type 3. BioMed Res. Int. 2020;2020:1–11. doi: 10.1155/2020/1605358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Fox B.M., Gil H.-W., Kirkbride-Romeo L., Bagchi R., Wennersten S., Haefner K.R., Skrypnyk N.I., Brown C.N., Soranno D.E., Gist K.M., et al. Metabolomics Assessment Reveals Oxidative Stress and Altered Energy Production in the Heart After Ischemic Acute Kidney Injury in Mice. Kidney Int. 2019;95:590–610. doi: 10.1016/j.kint.2018.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Guo H., Xu D., Kuroki M., Lu Z., Xu X., Geurts A., Osborn J.W., Chen Y. Kidney Failure, Arterial Hypertension and Left Ventricular Hypertrophy in Rats with Loss of Function Mutation of SOD3. Free. Radic. Biol. Med. 2020;152:787–796. doi: 10.1016/j.freeradbiomed.2020.01.023. [DOI] [PubMed] [Google Scholar]
- 334.Oslowski C.M., Urano F. Measuring ER Stress and the Unfolded Protein Response Using Mammalian Tissue Culture System. Methods Enzymol. 2011;490:71–92. doi: 10.1016/b978-0-12-385114-7.00004-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Díaz-Villanueva J.F., Díaz-Molina R., García-González V. Protein Folding and Mechanisms of Proteostasis. Int. J. Mol. Sci. 2015;16:17193–17230. doi: 10.3390/ijms160817193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Read A., Schröder M. The Unfolded Protein Response: An Overview. Biology. 2021;10:384. doi: 10.3390/biology10050384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Sanyal A., Zbornik E.A., Watson B.G., Christoffer C., Ma J., Kihara D., Mattoo S. Kinetic and Structural Parameters Governing Fic-Mediated adenylylation/AMPylation of the Hsp70 Chaperone, BiP/GRP78. Cell Stress Chaperon. 2021:1–18. doi: 10.1007/s12192-021-01208-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Song S., Tan J., Miao Y., Li M., Zhang Q. Crosstalk of Autophagy and Apoptosis: Involvement of the Dual Role of Autophagy under ER Stress. J. Cell. Physiol. 2017;232:2977–2984. doi: 10.1002/jcp.25785. [DOI] [PubMed] [Google Scholar]
- 339.Lam M., A Marsters S., Ashkenazi A., Walter P. Misfolded Proteins Bind and Activate Death Receptor 5 to Trigger Apoptosis During Unresolved Endoplasmic Reticulum Stress. eLife. 2020;9:e52291. doi: 10.7554/eLife.52291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Shrestha N., De Franco E., Arvan P., Cnop M. Pathological β-Cell Endoplasmic Reticulum Stress in Type 2 Diabetes: Current Evidence. Front. Endocrinol. 2021;12:650158. doi: 10.3389/fendo.2021.650158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Parks S.Z., Gao T., Awuapura N.J., Ayathamattam J., Chabosseau P.L., Kalvakolanu D.V., Valdivia H.H., Rutter G.A., Leclerc I. The Ca 2+ -binding Protein Sorcin Stimulates Transcriptional Activity of the Unfolded Protein Response Mediator ATF6. FEBS Lett. 2021;10 doi: 10.1002/1873-3468.14101. [DOI] [PubMed] [Google Scholar]
- 342.Baba B., Caliskan M., Boyuk G., Hacisevki A. Chemical Chaperone PBA Attenuates ER Stress and Upregulates SOCS3 Expression as a Regulator of Leptin Signaling. Biochemistry. 2021;86:480–488. doi: 10.1134/s0006297921040088. [DOI] [PubMed] [Google Scholar]
- 343.Yang Y., Zhou Q., Gao A., Chen L., Li L. Endoplasmic Reticulum Stress and Focused Drug Discovery in Cardiovascular Disease. Clin. Chim. Acta. 2020;504:125–137. doi: 10.1016/j.cca.2020.01.031. [DOI] [PubMed] [Google Scholar]
- 344.Ajoolabady A., Wang S., Kroemer G., Klionsky D.J., Uversky V.N., Sowers J.R., Aslkhodapasandhokmabad H., Bi Y., Ge J., Ren J. ER Stress in Cardiometabolic Diseases: From Molecular Mechanisms to Therapeutics. Endocr. Rev. 2021 doi: 10.1210/endrev/bnab006. [DOI] [PubMed] [Google Scholar]
- 345.Hsu Y.-H., Zheng C.-M., Chou C.-L., Chen Y.-J., Lee Y.-H., Lin Y.-F., Chiu H.-W. Therapeutic Effect of Endothelin-Converting Enzyme Inhibitor on Chronic Kidney Disease through the Inhibition of Endoplasmic Reticulum Stress and the NLRP3 Inflammasome. Biomedicine. 2021;9:398. doi: 10.3390/biomedicines9040398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.Lins B.B., Casare F.A.M., Fontenele F.F., Gonçalves G.L., Oliveira-Souza M. Long-Term Angiotensin II Infusion Induces Oxidative and Endoplasmic Reticulum Stress and Modulates Na+ Transporters Through the Nephron. Front. Physiol. 2021;12:642752. doi: 10.3389/fphys.2021.642752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347.Dong Z., Wu P., Li Y., Shen Y., Xin P., Li S., Wang Z., Dai X., Zhu W., Wei M. Myocardial Infarction Worsens Glomerular Injury and Microalbuminuria in Rats with Pre-Existing Renal Impairment Accompanied by the Activation of ER Stress and Inflammation. Mol. Biol. Rep. 2014;41:7911–7921. doi: 10.1007/s11033-014-3685-5. [DOI] [PubMed] [Google Scholar]
- 348.Dickhout J.G., Carlisle R.E., Austin R.C. Interrelationship Between Cardiac Hypertrophy, Heart Failure, and Chronic Kidney Disease. Circ. Res. 2011;108:629–642. doi: 10.1161/CIRCRESAHA.110.226803. [DOI] [PubMed] [Google Scholar]
- 349.Olivares-Silva F., Espitia-Corredor J., Letelier A., Vivar R., Parra-Flores P., Olmedo I., Montenegro J., Pardo-Jiménez V., Díaz-Araya G. TGF-β1 Decreases CHOP Expression and Prevents Cardiac Fibroblast Apoptosis Induced by Endoplasmic Reticulum Stress. Toxicol. Vitr. 2020;70:105041. doi: 10.1016/j.tiv.2020.105041. [DOI] [PubMed] [Google Scholar]
- 350.Yamamoto T., Endo J., Kataoka M., Matsuhashi T., Katsumata Y., Shirakawa K., Isobe S., Moriyama H., Goto S., Shimanaka Y., et al. Palmitate Induces Cardiomyocyte Death via Inositol Requiring Enzyme-1 (IRE1)-Mediated Signaling Independent of X-Box Binding Protein 1 (XBP1) Biochem. Biophys. Res. Commun. 2020;526:122–127. doi: 10.1016/j.bbrc.2020.03.027. [DOI] [PubMed] [Google Scholar]
- 351.Shu S., Zhu J., Liu Z., Tang C., Cai J., Dong Z. Endoplasmic Reticulum Stress Is Activated in Post-Ischemic Kidneys to Promote Chronic Kidney Disease. EBioMedicine. 2018;37:269–280. doi: 10.1016/j.ebiom.2018.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352.Huang D., Yan M.-L., Chen K.-K., Sun R., Dong Z.-F., Wu P.-L., Li S., Zhu G.-S., Ma S.-X., Pan Y.-S., et al. Cardiac-Specific Overexpression of Silent Information Regulator 1 Protects Against Heart and Kidney Deterioration in Cardiorenal Syndrome via Inhibition of Endoplasmic Reticulum Stress. Cell. Physiol. Biochem. 2018;46:9–22. doi: 10.1159/000488404. [DOI] [PubMed] [Google Scholar]
- 353.Liu Y., Wang Y., Ding W., Wang Y. Mito-TEMPO Alleviates Renal Fibrosis by Reducing Inflammation, Mitochondrial Dysfunction, and Endoplasmic Reticulum Stress. Oxidative Med. Cell. Longev. 2018;2018:1–13. doi: 10.1155/2018/5828120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 354.(66) Luo T., Kim J.K., Chen B., Abdel-Latif A., Kitakaze M., Yan L. Attenuation of ER Stress Prevents Post-Infarction-Induced Cardiac Rupture and Remodeling by Modulating Both Cardiac Apoptosis and Fibrosis. Chem. Interact. 2015;225:90–98. doi: 10.1016/j.cbi.2014.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355.Fan Y., Xiao W., Lee K., Salem F., Wen J., He L., Zhang J., Fei Y., Cheng D., Bao H., et al. Inhibition of Reticulon-1A–Mediated Endoplasmic Reticulum Stress in Early AKI Attenuates Renal Fibrosis Development. J. Am. Soc. Nephrol. 2017;28:2007–2021. doi: 10.1681/ASN.2016091001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356.Han J., Pang X., Shi X., Zhang Y., Peng Z., Xing Y. Ginkgo Biloba Extract EGB761 Ameliorates the Extracellular Matrix Accumulation and Mesenchymal Transformation of Renal Tubules in Diabetic Kidney Disease by Inhibiting Endoplasmic Reticulum Stress. BioMed Res. Int. 2021;2021:1–11. doi: 10.1155/2021/6657206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357.Qu J., Li M., Li D., Xin Y., Li J., Lei S., Wu W., Liu X. Stimulation of Sigma-1 Receptor Protects Against Cardiac Fibrosis by Alleviating IRE1 Pathway and Autophagy Impairment. Oxidative Med. Cell. Longev. 2021;2021:1–25. doi: 10.1155/2021/8836818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358.Chen Y.-T., Jhao P.-Y., Hung C.-T., Wu Y.-F., Lin S.-J., Chiang W.-C., Lin S.-L., Yang K.-C. Endoplasmic Reticulum Protein TXNDC5 Promotes Renal Fibrosis by Enforcing TGF-β Signaling in Kidney Fibroblasts. J. Clin. Investig. 2021;131 doi: 10.1172/JCI143645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359.Liu S.-H., Yang C.-C., Chan D.-C., Wu C.-T., Chen L.-P., Huang J.-W., Hung K.-Y., Chiang C.-K. Chemical Chaperon 4-Phenylbutyrate Protects Against the Endoplasmic Reticulum Stress-Mediated Renal Fibrosis in Vivo and in Vitro. Oncotarget. 2016;7:22116–22127. doi: 10.18632/oncotarget.7904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360.Park M.-J., Oh K.-S., Nho J.-H., Kim G.-Y., Kim D.-I. Asymmetric Dimethylarginine (ADMA) Treatment Induces Apoptosis in Cultured Rat Mesangial Cells via Endoplasmic Reticulum Stress Activation. Cell Biol. Int. 2016;40:662–670. doi: 10.1002/cbin.10602. [DOI] [PubMed] [Google Scholar]
- 361.Matsuzaki S., Hiratsuka T., Taniguchi M., Shingaki K., Kubo T., Kiya K., Fujiwara T., Kanazawa S., Kanematsu R., Maeda T., et al. Physiological ER Stress Mediates the Differentiation of Fibroblasts. PLoS ONE. 2015;10:e0123578. doi: 10.1371/journal.pone.0123578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362.Shih Y.-C., Chen C.-L., Zhang Y., Mellor R.L., Kanter E.M., Fang Y., Wang H.-C., Hung C.-T., Nong J.-Y., Chen H.-J., et al. Endoplasmic Reticulum Protein TXNDC5 Augments Myocardial Fibrosis by Facilitating Extracellular Matrix Protein Folding and Redox-Sensitive Cardiac Fibroblast Activation. Circ. Res. 2018;122:1052–1068. doi: 10.1161/CIRCRESAHA.117.312130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 363.Pallet N., Bouvier N., Bendjallabah A., Rabant M., Flinois J.P., Hertig A., Legendre C., Beaune P., Thervet E., Anglicheau D. Cyclosporine-Induced Endoplasmic Reticulum Stress Triggers Tubular Phenotypic Changes and Death. Arab. Archaeol. Epigr. 2008;8:2283–2296. doi: 10.1111/j.1600-6143.2008.02396.x. [DOI] [PubMed] [Google Scholar]
- 364.Li Y., Weng X., Wang P., He Z., Cheng S., Wang D., Li X., Cheng G., Li T. 4-Phenylbutyrate Exerts Stage-Specific Effects on Cardiac Differentiation via HDAC Inhibition. PLoS ONE. 2021;16:e0250267. doi: 10.1371/journal.pone.0250267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 365.Basseri S., Lhoták Š., Sharma A.M., Austin R.C. The Chemical Chaperone 4-Phenylbutyrate Inhibits Adipogenesis by Modulating the Unfolded Protein Response. J. Lipid Res. 2009;50:2486–2501. doi: 10.1194/jlr.M900216-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 366.Akita S., Suzuki K., Yoshimoto H., Ohtsuru A., Hirano A., Yamashita S. Cellular Mechanism Underlying Highly-Active or Antiretroviral Therapy-Induced Lipodystrophy: Atazanavir, a Protease Inhibitor, Compromises Adipogenic Conversion of Adipose-Derived Stem/Progenitor Cells through Accelerating ER Stress-Mediated Cell Death in Differentiating Adipocytes. Int. J. Mol. Sci. 2021;22:2114. doi: 10.3390/ijms22042114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 367.Shaffer A., Shelef M., Iwakoshi N.N., Lee A.-H., Qian S.-B., Zhao H., Yu X., Yang L., Tan B.K., Rosenwald A., et al. XBP1, Downstream of Blimp-1, Expands the Secretory Apparatus and Other Organelles, and Increases Protein Synthesis in Plasma Cell Differentiation. Immunity. 2004;21:81–93. doi: 10.1016/j.immuni.2004.06.010. [DOI] [PubMed] [Google Scholar]
- 368.Gaudette B.T., Jones D.D., Bortnick A., Argon Y., Allman D. MTORC1 Coordinates an Immediate Unfolded Protein Response-Related Transcriptome in Activated B Cells Preceding Antibody Secretion. Nat. Commun. 2020;11:1–16. doi: 10.1038/s41467-019-14032-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 369.Meijer B.J., Smit W.L., Koelink P.J., Westendorp B.F., de Boer R.J., van der Meer J.H.M., Vermeulen J.L.M., Paton J.C., Paton A.W., Qin J., et al. Endoplasmic Reticulum Stress Regulates the Intestinal Stem Cell State through CtBP2. Sci. Rep. 2021;11:1–15. doi: 10.1038/s41598-021-89326-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 370.Tanaka K.-I., Yamaguchi T., Kaji H., Kanazawa I., Sugimoto T. Advanced Glycation End Products Suppress Osteoblastic Differentiation of Stromal Cells by Activating Endoplasmic Reticulum Stress. Biochem. Biophys. Res. Commun. 2013;438:463–467. doi: 10.1016/j.bbrc.2013.07.126. [DOI] [PubMed] [Google Scholar]
- 371.Moon J.Y., Kim H.S. α-Syntrophin Alleviates ER Stress to Maintain Protein Homeostasis During Myoblast Differentiation. FEBS Lett. 2021;595:1656–1670. doi: 10.1002/1873-3468.14088. [DOI] [PubMed] [Google Scholar]