Abstract
Haemoparasites of the genus Babesia infect a wide range of domestic and wild animals. Feline babesiosis is considered endemic in South Africa, while data on Babesia spp. infection in felids in Europe is scarce. Using samples from 51 wild felids, 44 Felis silvestris and 7 Lynx lynx, the study aimed to determine the presence and genetic diversity of Babesia spp. in wild felids in Romania by analyzing the 18S rDNA and two mitochondrial markers, cytochrome b (Cytb) and cytochrome c oxidase subunit I (COI) genes. By 18S rDNA analyses, Babesia spp. DNA was detected in 20 European wild felids. All sequences showed 100% similarity to B. canis by BLAST analysis. Conversely, Cytb and COI analyses revealed the presence of two Babesia spp., B. pisicii n. sp., which we herein describe, and B. canis. The pairwise comparison of both mitochondrial genes of B. pisicii n. sp. showed a genetic distance of at least 10.3% from the most closely related species, B. rossi. Phylogenetic analyses of Cytb and COI genes revealed that B. pisicii n. sp. is related to the so-called “large” canid-associated Babesia species forming a separate subclade in a sister position to B. rossi.
Keywords: Babesia pisicii n. sp., European wild felids, piroplasmids, 18S rDNA, mitochondrial genes
1. Introduction
The genus Babesia is composed of apicomplexan tick-transmitted haemoparasites with a remarkable economic, medical, and veterinary impact on domestic and wild animals [1,2,3]. Moreover, Babesia species are gaining increased interest as potential etiological agents of zoonotic diseases [4,5]. Since the first description of the microorganism in erythrocytes of Romanian cattle by Victor Babeş, at the end of the 19th century, more than 100 new species have been described [6,7]. The growing number of available mitochondrial sequences suggests that species diversity of piroplasmids in European wildlife is highly underestimated [8,9]. Robust species differentiation and understanding of their hosts spectrum are necessary for the identification of the parasite in endangered wild species, and for proper diagnostic of the clinical cases in domestic animals.
Feline babesiosis is considered endemic in South Africa, although Babesia spp. have been sporadically reported from various countries from Europe, Asia, or America [10]. In the previous century, several Babesia spp. have been described based only on morphological characteristics or host specificity, including B. felis, B. cati, B. herpailuri, and B. pantherae [11,12,13,14]. However, only B. felis has been molecularly characterized thereafter [15]. Based on molecular data, B. leo, B. lengau, and Babesia species cat Western Cape were also documented in South Africa [15,16,17]. In Asia, B. canis presentii and B. hongkongensis have been reported and molecularly characterized in domestic cats [18,19]. Additionally, B. microti as well as dog-related species such as B. canis, B. gibsoni, and B. vogeli have been identified in felids based on molecular data [20,21,22,23]. The vast majority of deposited sequences are represented by small ribosomal RNA subunit gene (18S rDNA) sequences, while few internal transcribed spacer, 5S rRNA, 28S rRNA, or beta tubulin-like gene sequences are available. However, only one mitochondrial sequence is accessible for comparison (cytochrome b (Cytb) of B. hongkongensis, accession number JQ867357), which may limit phylogenetic analyses of feline specific Babesia spp.
In Europe, data on Babesia spp. infections in felids are sporadic and inconsistent. In European wild felids, Babesia was reported in one individual from Bosnia and Herzegovina and it was molecularly characterized as identical to Babesia sp. previously found in badgers [24]. The presence of intraerythrocytic merozoites compatible with small Babesia/Cytauxzoon spp. was documented in a wild cat in northern Greece; however, the results are not supported by molecular data [25]. In domestic cats, molecular findings of B. canis, B. vogeli, or B. microti were reported in Spain, Portugal, Poland, and Italy [20,22,26,27]. This is in high contrast with numerous reports of Babesia infection in domestic dogs in Europe during the last decades, with prevalence rates ranging from 0.1 to 88.0% [28].
The present study was driven by the lack of relevant data on Babesia in European wild felids and by our findings of B. canis in felids in the course of other studies on feline piroplasms [9]. Therefore, we aimed to understand the diversity of feline Babesia in wild felids in Romania using the 18S rDNA and subsequent molecular characterization by analyzing two mitochondrial markers, Cytb and cytochrome c oxidase subunit I (COI) genes.
2. Materials and Methods
2.1. Samples
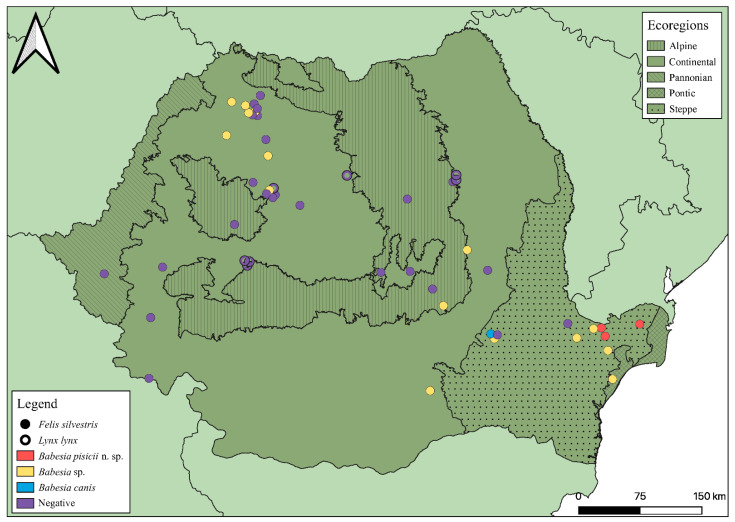
Between February 2011 and February 2020, 51 wild felids carcasses (44 Felis silvestris and 7 Lynx lynx) were examined at the Department of Parasitology and Parasitic Diseases of the University of Agricultural Sciences and Veterinary Medicine of Cluj-Napoca, Romania. The animals were found as road kill or died of natural causes. During necropsy, blood, spleen, liver or heart samples were collected and stored at −20 °C, until further processing. Samples from 42 animals were included in previous studies [9,29], focusing on Cytauxzoon spp. detection and characterization. The wild felid species identification was carried out based on different morphological and pelage characters [30]. If available, data on origin was recorded for each animal (Figure 1). The study area was divided into five ecoregions: continental, steppe, alpine, Pannonian, and Pontic, as previously described [31].
Figure 1.
Geographical distribution of the samples included in the study and their positivity to Babesia sp. (samples that yielded a positive result in the assay targeting 18S rDNA fragment of 376 bp), B. canis, and B. pisicii n. sp. (confirmed by mitochondrial genes analysis).
2.2. DNA Isolation, PCR Amplification, and Phylogenetic Analyses
Genomic DNA was extracted from 200 μL of whole blood or 20 mg of tissue using Isolate II Genomic DNA Kit (Bioline, London, UK), according to manufacturer’s instruction.
A partial fragment of the 18S rDNA of B. canis was amplified using a species-specific nested PCR protocol. Subsequently, all the samples that yielded a positive result were screened by PCR assays targeting a longer fragment of the 18S rDNA, mitochondrial Cytb, and COI genes. Primers, annealing temperatures, and expected length of amplicons are listed in Table 1. Amplification of the first-round reactions were performed in 15 μL reaction mixture, containing 400 nM of each primer, 7.5 μL of 2× PCRBIO Taq Mix Red (PCR Biosystems, London, UK), and 1 μL of template DNA. The second-round reactions were carried out in a total volume of 25 μL, consisting of 400 nM of each primer, 12.5 μL of 2× PCRBIO Taq Mix Red (PCR Biosystems, London, UK), and 1 μL of primary product. PCR products were visualized by electrophoresis on 1.5% agarose gels stained with ECO Safe Nucleic Acid Staining Solution (PacificImage Electronics, New Taipei City, Taiwan).
Table 1.
Primer pairs, annealing temperatures, and expected length of amplicons.
| Genetic Marker | Primers (Forward, Reverse) | Nucleotide Sequence (5′–3′) | Annealing Temperature | Product Size | Reference |
|---|---|---|---|---|---|
| 18S rDNA | Bc_F1 | CGTAGTTGTATTTTTGCGT | 50 °C | ≈430 bp | [33] |
| GR2 | CCAAAGACTTTGATTTCTCTC | ||||
| Bc_F2 | CATTTGGTTGGTTATTTCGTTTT | 53 °C | 376 bp | ||
| Bc_R1 | GTTCCTGAAGGGGTCAAAAA | ||||
| 18S rDNA | BT1F | GGTTGATCCTGCCAGTAGT | 65 °C–55 °C | ≈1730 bp | Modified after [8] |
| BT outer R | GGAAACCTTGTTACGACTTCTC | ||||
| Piro0F2 | GCCAGTAGTCATATGCTTGTCTTA | 65 °C–55 °C | ≈1670 bp | ||
| BT inner R | TTCTCCTTCCTTTAAGTGATAAG | ||||
| Cytb | Bc_cytb_F1 | TGGTCWTGGTATTCWGGAATG | 50 °C | ≈700 bp | [34] |
| Bc_cytb_R1 | AAGMYARTCTYCCTAAACATCC | ||||
| Bc_cytb_F2 | RATKAGYTAYTGGGGAGC | 48 °C | ≈580 bp | ||
| Bc_cytb_R2 | GCTGGWATCATWGGTATAC | ||||
| COI | Bab_For1 | ATWGGATTYTATATGAGTAT | 45 °C | 1250 bp | [34] |
| Bab_Rev1 | ATAATCWGGWATYCTCCTTGG | ||||
| Bab_For2 | TCTCTWCATGGWTTAATTATGATAT | 49 °C | 980 bp | ||
| Bab_Rev2 | TAGCTCCAATTGAHARWACAAAGTG |
PCR products of expected size were excised from gels, purified using Gel/PCR DNA Fragment Extraction Kit (Geneaid Biotech, New Taipei City, Taiwan), and sequenced bi-directionally (Macrogen, Amsterdam, the Netherlands) using the amplification primers. Sequence chromatograms were edited using Geneious 9.1.2 [32] and compared with representative sequences available in the GenBank database by NCBI Basic Local Alignment Search Tool (BLAST) analysis. Alignments of 18S rDNA sequences were generated using ClustalW algorithm.
The phylogenetic trees of Cytb and COI genes were based on sequences acquired in this study and all available sequences of corresponding genes from Babesia sensu stricto species [35] from GenBank longer than 300 nt (sequences containing premature STOP codon in the open reading frame were excluded from the analyses). Four Cytb and three COI gene sequences of Theileria spp. from GenBank were used as an outgroup. The alignments on nucleotide level were guided by amino acid translation (TransAlign, Geneious 9.1.2), restricted to protein coding regions only. The resulting alignments were built from 80 sequences (1440 nt) for COI and 60 sequences (1098 nt) for Cytb. For chosen closest species, alignments were also prepared as described above and their p-distances were computed by Geneious 9.1.2.
All phylogenetic trees were inferred by maximum likelihood method using IQ-TREE v. 1.6.5 [36]. The best-fit evolution models (K3Pu + F + G4 for Cytb gene and GTR + F + I + G4 for COI gene) were chosen based on the Bayesian information criterion (BIC) computed by ModelFinder [37]. Branch supports were assessed by the ultrafast bootstrap (UFBoot) approximation [38] and by SH-like approximate likelihood ratio test (SH-aLRT) [39]. Trees were visualized and edited in FigTree v1.4.4 and Inkscape 0.94.
2.3. Sensitivity of the Assay Targeting 18S rDNA Fragment of 376 bp
The sensitivity of detection of the PCR protocol targeting the 376 bp region of the B. canis 18S rDNA was assessed as described elsewhere [34]. The concentration and purity of the linearized plasmids was evaluated in triplicates by NanoDrop ND-1000 spectrophotometer analyzer (NanoDrop Technologies, Inc., Wilmington, DE, USA). The number of molecules were calculated using the formula:
| (1) |
where dsDNA is the amount of DNA [g/μL], NA is the Avogadro’s number , length is the length of target sequence including vector [bp], and 662 is the average molecular weight of a base pair [g/mol]. Ten-folds dilution series were prepared by combining linearized pGEM®-T Easy plasmid containing insert with DNA isolated from Babesia spp. negative wild cats. The final aliquots, with a concentration of 1 to 105 copies/μL, were used as template in the nested PCR protocol described above. From both PCR rounds, 10 μL of the final product was visualized on 1.5% agarose gels stained by ECO Safe Nucleic Acid Staining Solution, purified and submitted for sequencing on both strands (Macrogen, Amsterdam, The Netherlands).
3. Results
3.1. 18S rDNA Sequence Analyses
Babesia spp. infection was detected by amplification and sequencing of the 376 bp fragment of the 18S rDNA in 20 wild felids (39.2%; 95% CI: 27.0–52.9). All obtained sequences were identical to each other, and the BLAST analysis showed 100% similarity to B. canis isolated from dogs from Lithuania, Iran, or Bosnia and Herzegovina, or red foxes from Poland, etc. (GenBank accession numbers: MN078319-MN078323, MN173223, MN134074, MK107806). All the positive samples originated from European wild cats, while none of seven Eurasian lynxes were found positive. The geographical origin of the samples included in the study and their positivity are shown in Figure 1.
From the 20 positive F. silvestris samples, 14 (27.5%; 95% CI: 17.1–41.0) yielded an amplicon in the assay targeting the 1670 bp fragment of the 18S rDNA gene. However, the presence of B. canis was confirmed only in two samples by direct sequencing (100% identity to B. canis from Romania and Estonia; GenBank accession numbers: KX712122, HQ662634, KT008057). The other amplicons represent members of the genus Cytauxzoon and Hepatozoon, co-amplified by the assay.
3.2. Evaluation of Assay Sensitivity Targeting the 376 bp Fragment of the 18S rDNA
The sensitivity of the nested PCR assay targeting the 376 bp fragment of the 18S rDNA was established to a single molecule in a reaction after the second round of PCR. In the first PCR round, the detection limit was 102 copies of template DNA (Supplementary file: Figure S1). Direct sequencing confirmed the presence of B. canis 18S rDNA in all PCR products that yielded visible bands from both PCR rounds.
3.3. Mitochondrial Genes Analyses
To assess the genetic variability of Babesia spp. present in wild felids, sequence analyses of two mitochondrial genes (Cytb and COI) were performed. Amplification of the Cytb gene fragment was successful in four out of the 20 18S rDNA positive samples (7.8%; CI: 3.1–18.5). Direct sequencing yielded high-quality Babesia spp. consensus sequences of 556–582 nt for all these four samples. The sequence from sample 3569 showed 99.8% identity to B. canis from USA (accession number KC207822) by BLAST analysis. The closest relative of the remaining three sequences (100% identical to each other) was B. rossi with 87.2–89.7% identity (accession number KC207823). All these three sequences originated from wild cats from the steppe ecoregion (Figure 1).
The mitochondrial COI marker was amplified from the same four samples. The sequence from sample 3569 showed 99.7% identity to the aforementioned B. canis isolate from USA (accession number KC207822). The other three sequences (99.9–100% identical to each other) also showed an identity of 84.3–89.7% to the same B. rossi (accession number KC207823).
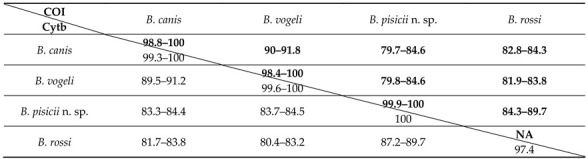
The pairwise comparison of the mitochondrial genes of this new Babesia genotype showed a genetic distance of at least 10.3% in both Cytb and COI genes from the most closely related species, B. rossi. Even higher nucleotide sequence distances were obtained between the new genotype and B. canis or B. vogeli (Table 2, sequence distances on amino acid level are available in the supplementary file: Table S1).
Table 2.
Pairwise nucleotide sequence identities (%) of Cytb (lower left) and COI (upper right) genes for B. canis, B. vogeli, B. pisicii n. sp., and B. rossi.
|
Pairwise nucleotide sequence identities of COI gene are set in bold.
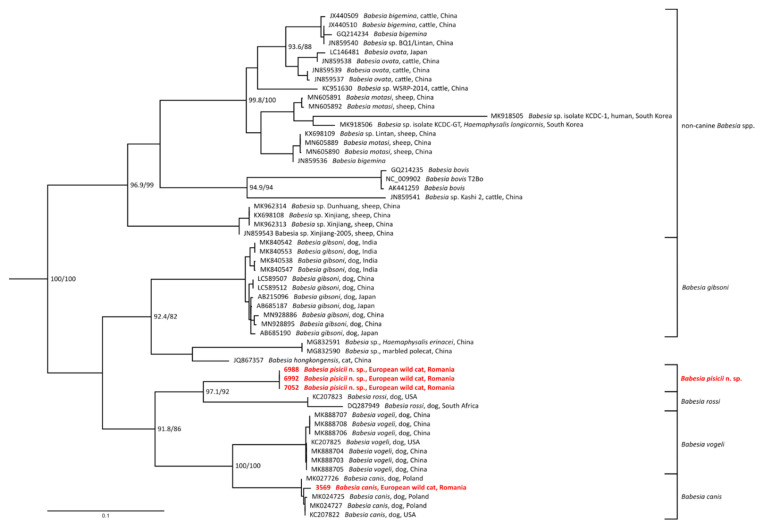
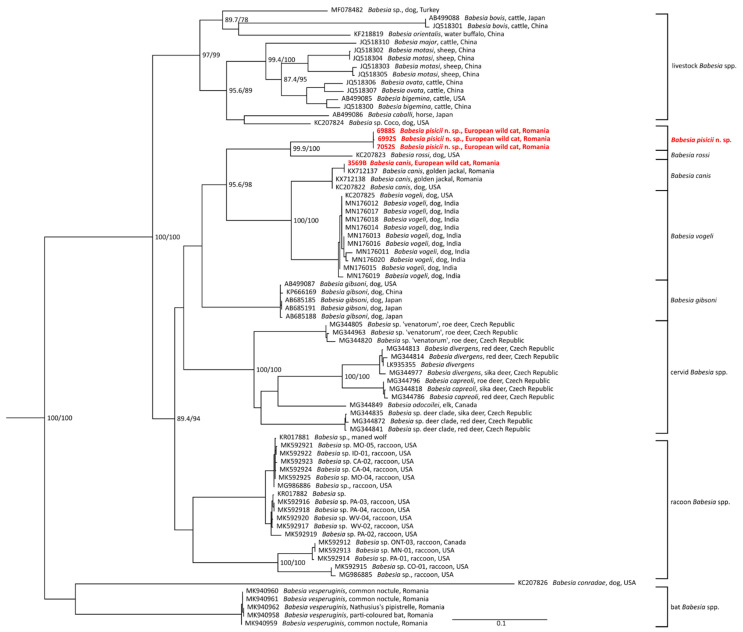
Phylogenetic analyses of both mitochondrial genes were based on GenBank available sequences of Babesia sensu stricto species (clade X sensu Jalovecká et al. [35]) and sequences obtained during the current study. All the new Babesia genotype sequences formed a distinct and highly supported subclade in both Cytb and COI phylogenies, in a sister position to B. rossi (Figure 2 and Figure 3). Furthermore, this subclade is placed more distantly from the sequences representing the B. vogeli and B. canis clade. The B. canis sequences from this study, clustered together with other published Cytb and COI sequences of B. canis.
Figure 2.
Phylogenetic tree assessed by maximum likelihood method based on Cytb sequences of Babesia sensu stricto species (clade X. sensu Jalovecká et al. [35]) and sequences obtained during the current study (highlighted in red); bootstrap values (SH-aLRT/UFB) above the threshold 80/95 are displayed. The scale bar indicates the number of nucleotide substitutions per site.
Figure 3.
Phylogenetic tree assessed by maximum likelihood method based on COI sequences of Babesia sensu stricto species (clade X. sensu Jalovecká et al. [35]) and sequences obtained during the current study (highlighted in red); bootstrap values (SH-aLRT/UFB) above the threshold 80/95 are displayed. The scale bar indicates the number of nucleotide substitutions per site.
All the unique sequences obtained in this study were deposited in GenBank database under the accession numbers MW939359 (18S rDNA), MW938761 (Cytb gene), MW938763 (COI gene) for B. canis and MW939360 (18S rDNA), MW938762 (Cytb gene), MW938764-MW938765 (COI gene) for the new Babesia genotype.
Based on these data, we herein describe this genotype as a new species of Babesia.
3.4. Taxonomic Summary and Species Description
Order Piroplasmida Poche, 1913.
Family Babesiidae du Toit, 1918.
Genus Babesia Starcovici, 1893.
Babesia pisicii n. sp. Panait, Hrazdilová, and Mihalca.
Diagnosis: the organism is a species of piroplasmid protist of the genus Babesia, distinctive from congeners from other carnivores based on DNA sequences and forming a separate clade sister to B. rossi.
Type-host: Felis silvestris Schreber, 1777 (Carnivora: Felidae).
Type-locality: Mila 23, Tulcea (45.22° N, 29.24° E).
Type-material: tissue extract and the total DNA isolated are deposited at the “Grigore Antipa” Natural History Museum, Bucharest, Romania under collection numbers BAB 001 (the tissue) and BAB 002 (the DNA). In correspondence with the ICZN code (Arts 72.5.4, 73.3) [40], the material deposited is considered a hapantotype by its character.
DNA sequences: DNA sequences amplified from the type material are deposited in GenBank under the accession numbers: MW939360 (18S rDNA), MW938762 (Cytb gene), MW938764 (COI gene).
Other localities: Romania: Cataloi, Tulcea (45.10° N, 28.72° E), Somova, Tulcea (45.19° N, 28.67° E).
Prevalence: 3/51 (5.9%)
Etymology: the specific epithet pisicii derives from the Romanian term used for cats. The name is given as a genitive noun, according to the ICZN rules and recommendations.
4. Discussion
Historically, Babesia spp. differentiation was based on the assumed host specificity and phenotypic characteristics, such as the size of intraerythrocytic stages and the number of merozoites observed during microscopic visualization of the blood smears [2]. Currently, the development of molecular techniques and the availability of extensive molecular data have questioned host specificity and allowed species identification [34,41,42].
In Europe, intraerythrocytic parasites of the genus Babesia were molecularly identified in a wide range of mammalian hosts, including bovines [43,44], small ruminants [45,46], different deer species [8], equines [47], swine [48], laboratory rodents [49], hares [50], moles [51], bats [52], and various carnivores such as dogs [34,53], wolves [54], jackals [55], foxes [56], cats [26,27], and mustelids [57].
The frequent use of universal primers detecting the 18S rDNA of a wide range of apicomplexan parasites (Babesia–Theileria–Hepatozoon–Cytauxzoon) [24,58,59,60] may often lead to amplification of other parasites than Babesia spp. in samples co-infected with other blood apicomplexans, such as Cytauxzoon spp. [9,61] and Hepatozoon spp. [24,62]. As a consequence, there is a single report of Babesia sp. in a wild cat [24] in Europe.
Although B. canis is typically considered a canid-associated species, during the last decades, its presence was reported also in non-canid hosts such as bats [63], horses [64], and domestic cats [20,26]. However, all of these reports are based on 18S rDNA detection, which has limitations in distinguishing very closely related Babesia species due to the insufficient sequence variation [2,34,65]. Moreover, the DNA of B. canis was recently demonstrated to be detectable in mice experimentally fed with naturally infected Dermacentor reticulatus ticks [66]. Thus, the detection of B. canis using 18S rDNA assays in felids could be related to non-specific detection of closely related species or to a possible ingestion of B. canis sensu stricto from prays or their ticks.
Piroplasmid species delineation based on mitochondrial sequences has been recently demonstrated and applied for description of closely related species [9]. Even if sequences from 39.2% of the tested samples showed 100% similarity to B. canis after the short fragment of the 18S rDNA analysis, the results obtained in mitochondrial markers assays confirmed the presence of B. canis in only one sample, while B. pisicii n. sp. was identified in three individuals. Therefore, an accurate specific identification of piroplasms should be followed by species confirmation by mitochondrial markers. However, protocols used to amplify mitochondrial genes have 100 to 1000 times lower sensitivities than the specific assay targeting the 376 bp fragment of the B. canis 18S rDNA, as demonstrated by Hrazdilová et al. [34].
In this study, fresh blood was not available for allowing the intraerythrocytic stages examination. However, as previously shown [67], description of new species without morphological identification is becoming a more and more common practice and has been also applied for other piroplasms [9,68], as morphological differences among the intraerythrocytic stages of piroplasms are mostly negligible. Moreover, low parasitemia in naturally infected wild hosts is unlikely to yield microscopically positive blood smears in the future.
The phylogenetic analysis of the mitochondrial genes showed that B. pisicii n. sp. is related to the so called “large” Babesia of dogs, forming a separate subclade in a sister position to B. rossi. The phylogeny based on the 18S rDNA sequences [42] showed that most feline-associated Babesia cluster in separate clades from B. canis, with the exception of B. canis presentii, B. hongkongensis, and Babesia sp. Western Cape. However, the 18S rDNA sequence analysis is able to distinguish between these three Babesia isolates and B. canis, which is not the case for B. pisicii n. sp.
Further studies are needed to clarify the host spectrum of B. pisicii n. sp., mainly its presence and clinical significance in domestic cats. Additionally, the elucidation of its life cycle and tick vector requires future attention. Ixodes ricinus and I. hexagonus were the only species found on wild felids in Romania [69] and both species are present in the area where B. pisicii n. sp. was found. Therefore, the vectorial competence of ticks in transmitting this new Babesia sp. remains to be investigated.
5. Conclusions
The current study indicates that European wild cats from Romania carry at least two Babesia species: B. canis and B. pisicii n. sp. Based on available genetic data, our recommendation is to avoid using the 18S rDNA detection for piroplasmid species differentiation and positive samples identified only based on this gene should be reported as Babesia sp. We encourage the implementation of molecular assays using mitochondrial genes, which, despite their lower sensitivity are able to clearly distinguish even between closely related piroplasm species.
Acknowledgments
This paper was published under the frame of European Social Found, Human Capital Operational Programme 2014–2020, project no. POCU/380/6/13/125171. Georgiana Deak was financially supported in her training as an EVPC resident by the Altius SRL Company in order to promote research and specialization among veterinarians in Romania. We are also grateful to all colleagues involved in sample collection.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/microorganisms9071474/s1, Figure S1: Sensitivity of the nested PCR assay targeting the 18S rDNA fragment of 376 bp. Table S1: Pairwise amino acid sequence identities (%) of Cytb (lower left) and COI (upper right) genes for B. canis, B. vogeli, B. pisicii n. sp. and B. rossi.
Author Contributions
Conceptualization, L.C.P. and A.D.M.; data curation, L.C.P. and K.H.; formal analysis, L.C.P. and K.H.; funding acquisition, K.H. and A.D.M.; investigation, L.C.P. and A.D.M.; methodology, L.C.P., K.H. and A.D.M.; resources, K.H., A.M.I., G.D., G.B.C., C.A., C.M.G. and A.D.M.; supervision, A.D.M.; validation, L.C.P. and K.H.; visualization, L.C.P., K.H. and A.D.M.; writing—original draft, L.C.P., K.H. and A.D.M.; writing—review and editing, L.C.P., K.H., A.M.I., G.D., G.B.C., C.A., C.M.G. and A.D.M. All authors have read and agreed to the published version of the manuscript.
Funding
The research was done under the framework of PCCDI 57/2018 grant, by the Executive Agency for Higher Education, Research, Development and Innovation Funding (UEFISCDI) Grant Agency Romania. In Czech Republic, this study was supported by the Ministry of Education, Youth and Sports of the Czech Republic under the project CEITEC 2020 (LQ1601). Kristýna Hrazdilová was supported by the project No. CZ.02.1.01/0.0/0.0/16_019/0000787 “Fighting Infectious Diseases” provided by the Ministry of Education, Youth and Sports of the Czech Republic.
Data Availability Statement
The datasets supporting the conclusions of this study are included in this published article (and its supplementary information files).
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Alvarado-Rybak M., Solano-Gallego L., Millán J. A review of piroplasmid infections in wild carnivores worldwide: Importance for domestic animal health and wildlife conservation. Parasites Vectors. 2016;9:538. doi: 10.1186/s13071-016-1808-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schnittger L., Rodriguez A.E., Florin-Christensen M., Morrison D.A. Babesia: A world emerging. Infect. Genet. Evol. 2012;12:1788–1809. doi: 10.1016/j.meegid.2012.07.004. [DOI] [PubMed] [Google Scholar]
- 3.Yabsley M.J., Shock B.C. Natural history of zoonotic Babesia: Role of wildlife reservoirs. Int. J. Parasitol. Parasites Wildl. 2013;2:18–31. doi: 10.1016/j.ijppaw.2012.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vannier E., Gewurz B.E., Krause P.J. Human babesiosis. Infect. Dis. Clin. N. Am. 2008;22:469–488. doi: 10.1016/j.idc.2008.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Young K.M., Corrin T., Wilhelm B., Uhland C., Greig J., Mascarenhas M., Waddell L.A. Zoonotic Babesia: A scoping review of the global evidence. PLoS ONE. 2019;14:e0226781. doi: 10.1371/journal.pone.0226781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Babeş V. Sur l’hemoglobinurie bacterienne du boeuf (On the bacterian hemoglobinuria of cattle) (in French) C. R. Hebd. Acad. Sci. 1888;107:692–694. [Google Scholar]
- 7.Mihalca A.D., Cozma V., Şuteu E., Marinculic A., Boireau P. The quest for piroplasms: From Babeş and Smith to molecules. Sci. Parasitol. 2010;11:14–19. [Google Scholar]
- 8.Hrazdilová K., Rybářová M., Široký P., Votypka J., Zintl A., Burgess H., Steinbauer V., Žákovčík V., Modrý D. Diversity of Babesia spp. in cervid ungulates based on the 18S rDNA and cytochrome c oxidase subunit I phylogenies. Infect. Genet. Evol. 2020;77:104060. doi: 10.1016/j.meegid.2019.104060. [DOI] [PubMed] [Google Scholar]
- 9.Panait L.C., Mihalca A.D., Modrý D., Juránková J., Ionică A.M., Deak G., Gherman C.M., Heddergott M., Hodžić A., Veronesi F., et al. Three new species of Cytauxzoon in European wild felids. Vet. Parasitol. 2021;290:109344. doi: 10.1016/j.vetpar.2021.109344. [DOI] [PubMed] [Google Scholar]
- 10.Ayoob A.L., Prittie J., Hackner S.G. Feline babesiosis. J. Vet. Emerg. Crit. Care. 2010;20:90–97. doi: 10.1111/j.1476-4431.2009.00493.x. [DOI] [PubMed] [Google Scholar]
- 11.Davis L. On a piroplasm of the Sudanese wild cat (Felis ocreata) Trans. R. Soc. Trop. Med. Hyg. 1929;22:535–537. doi: 10.1016/S0035-9203(29)90042-0. [DOI] [Google Scholar]
- 12.Mudaliar S.V., Achary G.R., Alwar V.S. On a species of Babesia in an Indian wild cat (Felis catus) Indian Vet. J. 1950;26:392–395. [PubMed] [Google Scholar]
- 13.Dennig H.K. Babesia infections in exotic cats and the significance of these blood parasites for veterinary research. Acta Zool. Pathol. Antverp. 1969;48:361–367. [PubMed] [Google Scholar]
- 14.Dennig H.K., Brocklesby D.W. Babesia pantherae sp. nov., a piroplasm of the leopard (Panthera pardus) Parasitology. 1972;64:525–532. doi: 10.1017/S0031182000045595. [DOI] [PubMed] [Google Scholar]
- 15.Penzhorn B.L., Kjemtrup A.M., Lopez-Rebollar L.M., Conrad P.A. Babesia leo n. sp. from lions in the Kruger National Park, South Africa, and its relation to other small piroplasms. J. Parasitol. 2001;87:681. doi: 10.1645/0022-3395(2001)087[0681:BLNSFL]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 16.Bosman A.-M., Oosthuizen M.C., Peirce M.A., Venter E.H., Penzhorn B.L. Babesia lengau sp. nov., a novel Babesia species in cheetah (Acinonyx jubatus, Schreber, 1775) populations in South Africa. J. Clin. Microbiol. 2010;48:2703–2708. doi: 10.1128/JCM.02266-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bosman A.-M., Penzhorn B.L., Brayton K.A., Schoeman T., Oosthuizen M.C. A novel Babesia sp. associated with clinical signs of babesiosis in domestic cats in South Africa. Parasites Vectors. 2019;12:138. doi: 10.1186/s13071-019-3395-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baneth G., Kenny M.J., Tasker S., Anug Y., Shkap V., Levy A., Shaw S.E. Infection with a proposed new subspecies of Babesia canis, Babesia canis subsp. presentii, in domestic cats. J. Clin. Microbiol. 2004;42:99–105. doi: 10.1128/JCM.42.1.99-105.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wong S.S.Y., Poon R.W.S., Hui J.J.Y., Yuen K.-Y. Detection of Babesia hongkongensis sp. nov. in a free-roaming Felis catus cat in Hong Kong. J. Clin. Microbiol. 2012;50:2799–2803. doi: 10.1128/JCM.01300-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Criado-Fornelio A., Martinez-Marcos A., Buling-Saraña A., Barba-Carretero J. Presence of Mycoplasma haemofelis, Mycoplasma haemominutum and piroplasmids in cats from southern Europe: A molecular study. Vet. Microbiol. 2003;93:307–317. doi: 10.1016/S0378-1135(03)00044-0. [DOI] [PubMed] [Google Scholar]
- 21.André M.R., Denardi N.C.B., de Sousa K.C.M., Gonçalves L.R., Henrique P.C., Ontivero C.R.G.R., Gonzalez I., Nery C.V.C., Chagas C.R.F., Monticelli C., et al. Arthropod-borne pathogens circulating in free-roaming domestic cats in a zoo environment in Brazil. Ticks Tick-Borne Dis. 2014;5:545–551. doi: 10.1016/j.ttbdis.2014.03.011. [DOI] [PubMed] [Google Scholar]
- 22.Spada E., Proverbio D., Galluzzo P., Perego R., De Giorgi G.B., Roggero N., Caracappa S. Frequency of piroplasms Babesia microti and Cytauxzoon felis in stray cats from northern Italy. BioMed Res. Int. 2014;2014:943754. doi: 10.1155/2014/943754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kelly P.J., Köster L., Liza K., Zhang J., Huang K., Branford G.C., Marchi S., Vandenplas M., Wang C. Survey of vector-borne agents in feral cats and first report of Babesia gibsoni in cats on St Kitts, West Indies. BMC Vet. Res. 2017;13:331. doi: 10.1186/s12917-017-1230-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hodžić A., Alić A., Duscher G.G. High diversity of blood-associated parasites and bacteria in European wild cats in Bosnia and Herzegovina: A molecular study. Ticks Tick-Borne Dis. 2018;9:589–593. doi: 10.1016/j.ttbdis.2018.01.017. [DOI] [PubMed] [Google Scholar]
- 25.Diakou A., Dimzas D., Astaras C., Savvas I., Di Cesare A., Morelli S., Neofitos Κ., Migli D., Traversa D. Clinical investigations and treatment outcome in a European wildcat (Felis silvestris silvestris) infected by cardio-pulmonary nematodes. Vet. Parasitol. Reg. Stud. Rep. 2020;19:100357. doi: 10.1016/j.vprsr.2019.100357. [DOI] [PubMed] [Google Scholar]
- 26.Vilhena H., Martinez-Díaz V.L., Cardoso L., Vieira L., Altet L., Francino O., Pastor J., Silvestre-Ferreira A.C. Feline vector-borne pathogens in the north and centre of Portugal. Parasites Vectors. 2013;6:99. doi: 10.1186/1756-3305-6-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maia C., Ramos C., Coimbra M., Bastos F., Martins Â., Pinto P., Nunes M., Vieira M.L., Cardoso L., Campino L. Bacterial and protozoal agents of feline vector-borne diseases in domestic and stray cats from southern Portugal. Parasites Vectors. 2014;7:115. doi: 10.1186/1756-3305-7-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Solano-Gallego L., Sainz Á., Roura X., Peña A.E., Miró G. A review of canine babesiosis: The European perspective. Parasites Vectors. 2016;9:336. doi: 10.1186/s13071-016-1596-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gallusovã¡ M., Jirsovã¡ D., Mihalca A.D., Gherman C.M., D’Amico G., Qablan M.A., Modrý D. Cytauxzoon infections in wild felids from Carpathian-Danubian-Pontic space: Further evidence for a different Cytauxzoon species in European felids. J. Parasitol. 2016;102:377–380. doi: 10.1645/15-881. [DOI] [PubMed] [Google Scholar]
- 30.Kitchener A.C., Yamaguchi N., Ward J.M., Macdonald D.W. A diagnosis for the Scottish wildcat (Felis silvestris): A tool for conservation action for a critically-endangered felid. Anim. Conserv. 2005;8:223–237. doi: 10.1017/S1367943005002301. [DOI] [Google Scholar]
- 31.Petrisor A.-I., Ianos I., Tălângă C. Land cover and use changes focused on the urbanization processes in Romania. Environ. Eng. Manag. J. 2010;9:765–771. doi: 10.30638/eemj.2010.102. [DOI] [Google Scholar]
- 32.Kearse M., Moir R., Wilson A., Stones-Havas S., Cheung M., Sturrock S., Buxton S., Cooper A., Markowitz S., Duran C., et al. Geneious Basic: An integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics. 2012;28:1647–1649. doi: 10.1093/bioinformatics/bts199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sgroi G., Iatta R., Veneziano V., Bezerra-Santos M.A., Lesiczka P., Hrazdilová K., Annoscia G., D’Alessio N., Golovchenko M., Rudenko N., et al. Molecular survey on tick-borne pathogens and Leishmania infantum in red foxes (Vulpes vulpes) from southern Italy. Ticks Tick-Borne Dis. 2021;12:101669. doi: 10.1016/j.ttbdis.2021.101669. [DOI] [PubMed] [Google Scholar]
- 34.Hrazdilová K., Myśliwy I., Hildebrand J., Buńkowska-Gawlik K., Janaczyk B., Perec-Matysiak A., Modrý D. Paralogs vs. genotypes? Variability of Babesia canis assessed by 18S rDNA and two mitochondrial markers. Vet. Parasitol. 2019;266:103–110. doi: 10.1016/j.vetpar.2018.12.017. [DOI] [PubMed] [Google Scholar]
- 35.Jalovecka M., Sojka D., Ascencio M., Schnittger L. Babesia life cycle—When phylogeny meets biology. Trends Parasitol. 2019;35:356–368. doi: 10.1016/j.pt.2019.01.007. [DOI] [PubMed] [Google Scholar]
- 36.Nguyen L.-T., Schmidt H.A., Von Haeseler A., Minh B. Q IQ-TREE: A fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol. Biol. Evol. 2015;32:268–274. doi: 10.1093/molbev/msu300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kalyaanamoorthy S., Minh B.Q., Wong T., Von Haeseler A., Jermiin L.S. ModelFinder: Fast model selection for accurate phylogenetic estimates. Nat. Methods. 2017;14:587–589. doi: 10.1038/nmeth.4285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Minh B.Q., Nguyen M.A.T., Von Haeseler A. Ultrafast approximation for phylogenetic bootstrap. Mol. Biol. Evol. 2013;30:1188–1195. doi: 10.1093/molbev/mst024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Guindon S., Gascuel O. A simple, fast, and accurate algorithm to estimate large phylogenies by maximum likelihood. Syst. Biol. 2003;52:696–704. doi: 10.1080/10635150390235520. [DOI] [PubMed] [Google Scholar]
- 40.ICZN. International Commission on Zoological Nomenclature Amendment of articles 8, 9, 10, 21 and 78 of the International Code of Zoological Nomenclature to expand and refine methods of publication. Bull. Zool. Nomencl. 2012;69:161–169. doi: 10.21805/bzn.v69i3.a8.161. [DOI] [Google Scholar]
- 41.Schreeg M.E., Marr H.S., Tarigo J.L., Cohn L.A., Bird D.M., Scholl E.H., Levy M.G., Wiegmann B.M., Birkenheuer A. Mitochondrial genome sequences and structures aid in the resolution of Piroplasmida phylogeny. PLoS ONE. 2016;11:e0165702. doi: 10.1371/journal.pone.0165702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Penzhorn B.L., Oosthuizen M.C. Babesia species of domestic cats: Molecular characterization has opened Pandora’s box. Front. Vet. Sci. 2020;7:134. doi: 10.3389/fvets.2020.00134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Decaro N., Larocca V., Parisi A., Losurdo M., Lia R.P., Greco M.F., Miccolis A., Ventrella G., Otranto D., Buonavoglia C. Clinical bovine piroplasmosis caused by Babesia occultans in Italy. J. Clin. Microbiol. 2013;51:2432–2434. doi: 10.1128/JCM.00713-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Toma-Naic A., Györk A., Nedișan M.E., Borșan S.D., Berar C., Bojan A., Cozma V. Babesiosis in a 7-week-old calf: Case report. Sci. Parasitol. 2018;19:40–44. [Google Scholar]
- 45.Criado-Fornelio A., Martinez-Marcos A., Buling-Saraña A., Barba-Carretero J. Molecular studies on Babesia, Theileria and Hepatozoon in southern Europe: Part II. Phylogenetic analysis and evolutionary history. Vet. Parasitol. 2003;113:189–201. doi: 10.1016/S0304-4017(03)00078-5. [DOI] [PubMed] [Google Scholar]
- 46.Gray A., Capewell P., Loney C., Katzer F., Shiels B.R., Weir W. Sheep as host species for zoonotic Babesia venatorum, United Kingdom. Emerg. Infect. Dis. 2019;25:2257–2260. doi: 10.3201/eid2512.190459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Manna G., Cersini A., Nardini R., Del Pino L.E.B., Antognetti V., Zini M., Conti R., Lorenzetti R., Veneziano V., Autorino G.L., et al. Genetic diversity of Theileria equi and Babesia caballi infecting horses of Central-Southern Italy and preliminary results of its correlation with clinical and serological status. Ticks Tick-Borne Dis. 2018;9:1212–1220. doi: 10.1016/j.ttbdis.2018.05.005. [DOI] [PubMed] [Google Scholar]
- 48.Zobba R., Parpaglia M.L.P., Spezzigu A., Pittau M., Alberti A. First molecular identification and phylogeny of a Babesia sp. from a symptomatic sow (Sus scrofa Linnaeus 1758) J. Clin. Microbiol. 2011;49:2321–2324. doi: 10.1128/JCM.00312-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Obiegala A., Pfeffer M., Pfister K., Karnath C., Silaghi C. Molecular examinations of Babesia microti in rodents and rodent-attached ticks from urban and sylvatic habitats in Germany. Ticks Tick-Borne Dis. 2015;6:445–449. doi: 10.1016/j.ttbdis.2015.03.005. [DOI] [PubMed] [Google Scholar]
- 50.Rocchigiani G., Ebani V.V., Nardoni S., Bertelloni F., Bascherini A., Leoni A., Mancianti F., Poli A. Molecular survey on the occurrence of arthropod-borne pathogens in wild brown hares (Lepus europaeus) from Central Italy. Infect. Genet. Evol. 2018;59:142–147. doi: 10.1016/j.meegid.2018.02.005. [DOI] [PubMed] [Google Scholar]
- 51.Silaghi C., Woll D., Hamel D., Pfister K., Mahling M., Pfeffer M. Babesia spp. and Anaplasma phagocytophilum in questing ticks, ticks parasitizing rodents and the parasitized rodents—Analyzing the host-pathogen-vector interface in a metropolitan area. Parasites Vectors. 2012;5:191. doi: 10.1186/1756-3305-5-191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Corduneanu A., Hrazdilová K., Sándor A.D., Matei I.A., Ionică A.M., Barti L., Ciocănău M.-A., Măntoiu D.Ș., Coroiu I., Hornok S., et al. Babesia vesperuginis, a neglected piroplasmid: New host and geographical records, and phylogenetic relations. Parasites Vectors. 2017;10:598. doi: 10.1186/s13071-017-2536-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cacciò S.M., Antunovic B., Moretti A., Mangili V., Marinculic A., Baric R.R., Slemenda S.B., Pieniazek N.J. Molecular characterisation of Babesia canis canis and Babesia canis vogeli from naturally infected European dogs. Vet. Parasitol. 2002;106:285–292. doi: 10.1016/S0304-4017(02)00112-7. [DOI] [PubMed] [Google Scholar]
- 54.Erdélyi K., Mezősi L., Vladov S., Földvári G. Fatal acute babesiosis in captive grey wolves (Canis lupus) due to Babesia canis. Ticks Tick-Borne Dis. 2014;5:281–283. doi: 10.1016/j.ttbdis.2013.11.003. [DOI] [PubMed] [Google Scholar]
- 55.Mitková B., Hrazdilová K., D’Amico G., Duscher G.G., Suchentrunk F., Forejtek P., Gherman C.M., Matei I.A., Ionică A.M., Daskalaki A.A., et al. Eurasian golden jackal as host of canine vector-borne protists. Parasites Vectors. 2017;10:183. doi: 10.1186/s13071-017-2110-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hodžić A., Mrowietz N., Cézanne R., Bruckschwaiger P., Punz S., Habler V.E., Tomsik V., Lazar J., Duscher G.G., Glawischnig W., et al. Occurrence and diversity of arthropod-transmitted pathogens in red foxes (Vulpes vulpes) in western Austria, and possible vertical (transplacental) transmission of Hepatozoon canis. Parasitology. 2018;145:335–344. doi: 10.1017/S0031182017001536. [DOI] [PubMed] [Google Scholar]
- 57.Santoro M., Auriemma C., Lucibelli M.G., Borriello G., D’Alessio N., Sgroi G., Veneziano V., Galiero G., Fusco G. Molecular detection of Babesia spp. (Apicomplexa: Piroplasma) in free-ranging canids and mustelids from Southern Italy. Front. Vet. Sci. 2019;6:269. doi: 10.3389/fvets.2019.00269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Carli E., Trotta M., Chinelli R., Drigo M., Sinigoi L., Tosolini P., Furlanello T., Millotti A., Caldin M., Solano-Gallego L. Cytauxzoon sp. infection in the first endemic focus described in domestic cats in Europe. Vet. Parasitol. 2012;183:343–352. doi: 10.1016/j.vetpar.2011.07.025. [DOI] [PubMed] [Google Scholar]
- 59.Veronesi F., Ravagnan S., Cerquetella M., Carli E., Olivieri E., Santoro A., Pesaro S., Berardi S., Rossi G., Ragni B., et al. First detection of Cytauxzoon spp. infection in European wildcats (Felis silvestris silvestris) of Italy. Ticks Tick-Borne Dis. 2016;7:853–858. doi: 10.1016/j.ttbdis.2016.04.003. [DOI] [PubMed] [Google Scholar]
- 60.Díaz-Regañón D., Villaescusa A., Ayllón T., Rodríguez-Franco F., Baneth G., Calleja-Bueno L., García-Sancho M., Agulla B., Sainz Á. Molecular detection of Hepatozoon spp. and Cytauxzoon sp. in domestic and stray cats from Madrid, Spain. Parasites Vectors. 2017;10:112. doi: 10.1186/s13071-017-2056-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Meli M.L., Cattori V., Martínez F., López G., Vargas A., Simón M.A., Zorrilla I., Muñoz A., Palomares F., López-Bao J.V., et al. Feline leukemia virus and other pathogens as important threats to the survival of the critically endangered Iberian lynx (Lynx pardinus) PLoS ONE. 2009;4:e4744. doi: 10.1371/journal.pone.0004744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hodžić A., Alić A., PraŠović S., Otranto D., Baneth G., Duscher G.G. Hepatozoon silvestris sp. nov.: Morphological and molecular characterization of a new species of Hepatozoon (Adeleorina: Hepatozoidae) from the European wild cat (Felis silvestris silvestris) Parasitology. 2017;144:650–661. doi: 10.1017/S0031182016002316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Corduneanu A., Sándor A.D., Mihalca A.D., Hrazdilová K., Modrý D., Hornok S. Molecular evidence of canine pathogens in tissues of European bats; Proceedings of the 17th International Bat Research Conference; Durban, South Africa. 31 July–5 August 2016; pp. 50–51. [Google Scholar]
- 64.Zanet S., Bassano M., Trisciuoglio A., Taricco I., Ferroglio E. Horses infected by Piroplasms different from Babesia caballi and Theileria equi: Species identification and risk factors analysis in Italy. Vet. Parasitol. 2017;236:38–41. doi: 10.1016/j.vetpar.2017.01.003. [DOI] [PubMed] [Google Scholar]
- 65.Uilenberg G., Gray J., Kahl O. Research on Piroplasmorida and other tick-borne agents: Are we going the right way? Ticks Tick-Borne Dis. 2018;9:860–863. doi: 10.1016/j.ttbdis.2018.03.005. [DOI] [PubMed] [Google Scholar]
- 66.Corduneanu A., Ursache T.D., Taulescu M., Sevastre B., Modrý D., Mihalca A.D. Detection of DNA of Babesia canis in tissues of laboratory rodents following oral inoculation with infected ticks. Parasites Vectors. 2020;13:166. doi: 10.1186/s13071-020-04051-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jörger K.M., Schrödl M. How to describe a cryptic species? Practical challenges of molecular taxonomy. Front. Zool. 2013;10:59. doi: 10.1186/1742-9994-10-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Greay T.L., Zahedi A., Krige A.-S., Owens J.M., Rees R.L., Ryan U.M., Oskam C.L., Irwin P.J. Endemic, exotic and novel apicomplexan parasites detected during a national study of ticks from companion animals in Australia. Parasites Vectors. 2018;11:197. doi: 10.1186/s13071-018-2775-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.D’Amico G., Dumitrache M.O., Matei I.A., Ionică A.M., Gherman C.M., Sándor A.D., Modrý D., Mihalca A.D. Ixodid ticks parasitizing wild carnivores in Romania. Exp. Appl. Acarol. 2017;71:139–149. doi: 10.1007/s10493-017-0108-z. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets supporting the conclusions of this study are included in this published article (and its supplementary information files).