Abstract
The presence of nuts in diets has notably increased due to their composition, and the presence of antioxidants and their unsaturated fatty acid profile has led to a considerable increase in their consumption. The volatile profile of nuts is important from different points of view. It affects consumer’s selection, influences raw material selection for the production of composite foods, dictates variety selection in breeding programs, and, from a quality perspective, its changes can indicate food degradation or alteration. A review of the published bibliography concerning the determination of volatiles in nuts has been carried out. The information retrieved has been divided into four main sections. First, a discussion on the main volatiles present in nuts is performed; next, a revision of the methods used to determine the volatiles is presented; and, finally, two sections describing how harvesting conditions, healthy state and the thermal treatment of nuts modifies their volatile profile are added. Analysis of the published bibliography denoted the complexity of volatile determination and the different variables that can modify the compounds present in the volatile fraction of nuts.
Keywords: nut, volatile, aroma, flavor, key odorants, analytical methods, solid-phase microextraction
1. Introduction
Food odor and aroma have a great influence on consumers’ preference. These attributes are related to different volatile chemicals. Volatiles are a set of compounds with a relatively low molecular weight and high vapor pressure [1]. They include different classes of chemicals such as alcohols, hydrocarbons, esters, terpenes and aldehydes. The characterization of this complex and heterogeneous mix is important in food quality control and consequently, for the food industry [2].
Molecules with low molecular weight are perceived in the nose and mouth sensors faster than others with higher molecular weight and, for this reason, they condition the final flavor of the product. These predominant compounds are known as odorants, and they are very important as some of them are associated with pleasant odors, and others with off-odors. Study of the chemical composition of the volatile profile of foods allows us to understand the optimal conditions for food production and contributes to obtaining valuable information about their composition. Odor molecules arrive into the nose by air, and they are perceived by the olfactory mucosa via orthonasal smell. Meanwhile, aromas are perceived in the nose by the olfactory receptors, in the olfactory epithelium via the retronasal pathway, when the food is put into the mouth and the cells are broken by chewing, thus releasing volatile compounds. Odor and aroma perception is the result of the activation of odorant receptors generating complex signals that are sent to the central nervous system [3]. The amount and type of volatiles released from the food highly depends on the nature of the sample matrix and the state of the food [4]. More specifically, the entire volatile profile reflects the phenotypic or metabolic state of foods. In plants, volatile compounds have diverse and important functions such as to attract pollinators to flower organs, to protect damage plants from the attack of herbivores and to exchange some low molecular weight terpenes during light changes, droughts, or other stress situations for the plant [4].
Nuts are indehiscent dry fruits with one seed and a thick, hard pericarp. In the botanical sense, they are produced by some families of the order Fagales [5]. These families are the Juglandaceae (walnut and pecan nut), the Fagaceae (chestnut), and the Betulaceae (hazelnut). In the culinary sense, the term nut is applied to a wide variety of dried seeds and fruits and to any large, oily kernel found within a shell. These nuts belong to the Fabaceae (peanut), the Rosaceae (almond) and the Anacardiaceae (pistachio) families. From the food composition point of view, nuts are characterized by having a low water content, i.e., usually less than 50% weight, although some exceptions exist [6]. Due to the group’s diversity, nut volatile profiles are expected to be diverse.
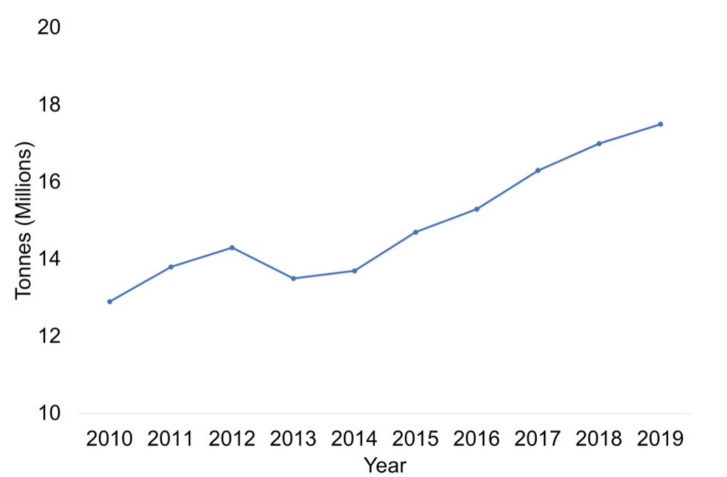
Based on data recorded by the Food and Agriculture Organization of the United Nations (FAOSTAT), worldwide nut production has increased significantly in recent years, from 12.9 Mtons in 2010 up to 17.5 Mtons in 2019 (Figure 1) [7]. In 2019, California was the top worldwide producer of nuts, with an estimated annual production of 27.0% of the total. The United States was the second largest producer at around 17.4%, followed by Turkey (7.5%), Iran (4.9%), Côte d’Ivoire (4.6%), India (4.3%) and Spain (3.3%).
Figure 1.
Worldwide nut production from 2010 to 2019.
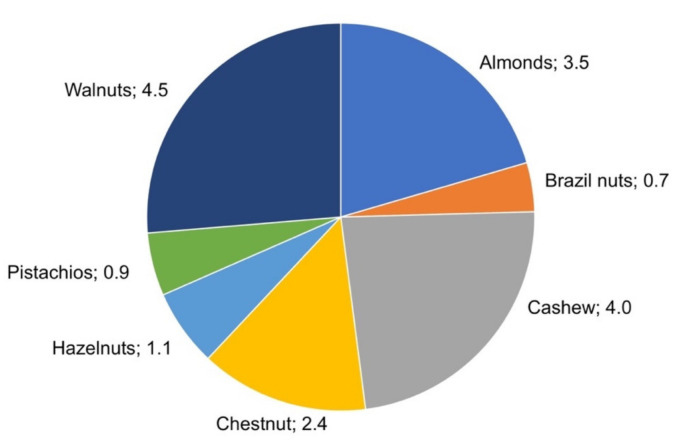
The majority of worldwide nut production in 2019 was registered as walnuts, followed by almonds, cashew, chestnut, hazelnuts, and finally, pistachio and Brazil nuts (Figure 2) [7]. Nuts are seeds showing a high sensory appeal and numerous health benefits due to their composition. In this sense, they present a high content in terms of protein (13–26% total weight) and lipids (43–76% total weight), carbohydrates (9–30% total weight) and fiber (7–12% total weight) with less than 6.4% water [6]. Nuts can be eaten raw, roasted, or salted as snacks. Thus, they are widely added to some important food formulations such as ice creams, chocolates, confectioneries, cookies, cereal bars and cakes. Due to their high level of dry matter and the possibility of undergoing technological processing, the volatile profile of nuts can undergo significant modifications compared to the profile of raw products.
Figure 2.
Worldwide nut production (Mtons) registered by FAOSTAT in 2019.
Today, nuts are important foods whose presence in a healthy diet is recommended. They have relatively high levels of antioxidants and a fatty acid profile that is beneficial in reducing the risk of cardiovascular and degenerative diseases. However, differences have been reported due to their organoleptic properties. It is precisely the amount of certain monounsaturated and polyunsaturated acids that mainly condition their acceptability. This fact influences their flavor, as unsaturated fatty acids are prone to oxidation. Even though oxidative rancidity should be avoided due to off-flavor formation, a mild oxidation reaction is sometimes desirable for the development of characteristic nut volatile odors. Frequently, equilibrium among them is important to define the characteristic volatile profile of a nut [8]. Additionally, it is important to know if there are differences in key odorants among different types of nuts, but also how the processing methods affect the volatile profiles. Finally, a discussion on the most extended analytical methods employed for volatile determination and a brief recompilation of sample preparation and quantification variants will be conducted.
2. Main Volatile Organic Compounds (VOCs) Present in Nuts
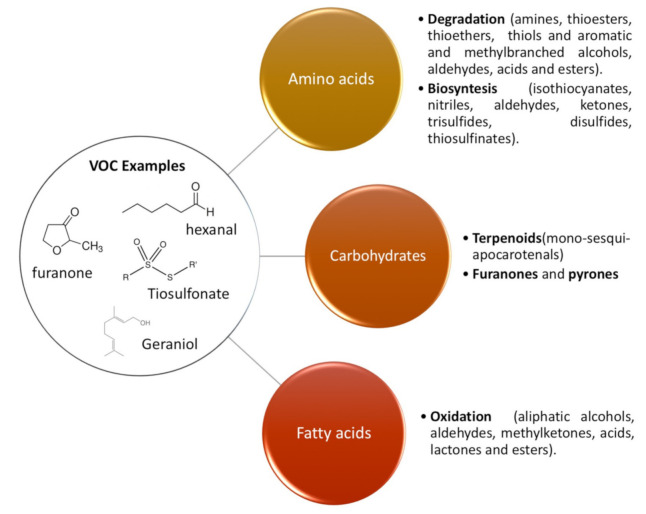
The final flavor of nuts is conditioned by the volatile compounds generated during fruit growth and maturation. Further changes in the volatile profile can occur during the storage after the harvesting of fruits and in the processing and cooking of nuts, thus affecting their sensorial quality [9]. The precursors of the VOCs are mainly fatty acids, carbohydrates and amino acids present in the plants and fruits (Figure 3). Among the VOCs in nuts, it is possible to find saturated and unsaturated molecules with straight, branched or cyclic structures including different functional groups such as alcohols, aldehydes, ketones, esters and ethers and also nitrogen and sulfur [10].
Figure 3.
Scheme showing different pathways from the main precursors of nut volatiles [3,10].
The most representative volatile compounds derived from fatty acid oxidation are aldehydes and alcohols [11]. The concentration level of some of these compounds can be employed to indicate the degree of oxidative deterioration of a food sample. In this sense, in walnut oils, the main ones associated with rancid flavor were 2-octenal, hexanal, 2-heptenal, 1-octen-3-ol, hexanoic acid and nonanal, whereas in almond oils, the total oxidation of lipids was mainly related to 1-pentanol, hexanal, and hexanoic acid [12]. In relation to peanuts, it was found that the compounds that were directly linked to an oxidized flavor were octanal, nonanal, hexanal and 2-pentylpyridine [13]. Hexanal is the most characteristic volatile product formed as a consequence of nut oxidation due to the high concentration of linoleic acid in the fat of the nuts. Another important aldehyde is nonanal, which appears as a by-product of oleic acid degradation [14]. Moreover, the most relevant volatile compounds present in the aroma notes of hazelnuts are in the group of aldehydes such as octanal (soapy), hexanal (grassy or green) and 2-methybutanal (malty). As alcohols, linalool is representative of flowery odor and ketones such as 2-hepten-4-one and 5-methyl-(Z)-hepten-4-one are responsible for nutty and fruity odors and 2,3- butanedione of buttery. Other important volatiles in hazelnuts are pyrazines such as 3,6-dimethyl-2-ethyl pyrazine (roasted and earthy) among other compounds [8].
Some odor notes in nuts are associated with unripe fruits, which is mainly related to hexane derived molecules such as hexanal, cis-3-hexanal, and hexanol, among others, their precursors being linolenic and linoleic fatty acids [15]. The same fatty acids are also precursors of other volatile compounds formed after decarboxylation of the alfa-keto acids to generate aldehydes such as 3-methylbutanal and 2-methylbutanal and other respective alcohols. Additionally, free aromatic amino acids such as phenylalanine can suffer decarboxylation processes and some precursors of floral aromas are formed such as 2-phenylethanol and phenylacetaldehyde [16].
Meanwhile, terpenoids are also present in the volatile profile of nuts, providing the characteristic aroma of fresh nuts. These compounds are molecules with an isopentane skeleton such as valencene, limonene, and linalool. In this sense, linalool is also responsible for the flavor of a lot of fruits such as tomatoes and oranges and some herbs such as lavender and basil [3]. Finally, other components such as carotenoids are also precursors of some fruity and floral smells formed during the maturity stage. Some examples of these compounds are ketones such as 6-methyl-5-hepten-2-one and β-ionone.
3. Analysis of VOCs Present in Nuts
The large number of volatile compounds, usually up to 200, that can be identified in nuts, make their identification and quantification difficult [12]. In this case, a separation technique such chromatography is usually employed. The volatile nature of the compounds determined makes gas chromatography (CG) coupled to mass spectrometry (MS) the preferred chromatographic type utilized for this analysis [17]. By using this technique, volatile compounds are commonly introduced in the chromatograph by using the split or splitless mode but if a higher sample capacity is needed due to a low volatile concentration in the sample, a programmed temperature vaporizer (PTV) is employed as an injection port [18]. In order to identify the main VOCs, the most extended stationary phases are nonpolar phases such as polydimethylsiloxane, columns with 5% phenyl groups or moderately polar phases such as polyethylene glycols. The length of the employed columns can vary between 25 and 30 m, but if complex mixtures of volatile compounds need to be separated, the length of the column must be increased up to 200 m [18]. Finally, the detector selected is usually a mass spectrometer that allows spectral information of the peaks to be obtained for identification of the target analytes. In routine analysis, the flame ionization detector (FID) can also be employed [18].
Prior to the identification and quantification of volatiles in nuts, the extraction of the target compounds must be optimized. A reference method for the extraction of VOCs in food matrices is liquid extraction (LE) with organic solvents. This methodology implies the employment of great amounts of organic solvents and, in many cases, low recoveries are obtained [19]. To solve this problem, liquid–liquid microextraction (LLME), dispersive liquid–liquid extraction (DLLE), stir bar sorptive extraction (SBSE) and solid-phase microextraction (SPME) are also employed. All of these analytical methodologies are commercially available, and extensively used.
SPME is nowadays the most commonly used extraction methodology for the analysis of volatile compounds in food samples, either in direct or in headspace (HS) mode, since it is a solvent-free extraction process that employs lower amounts of sample [17]. The analytical methodology consists of a fiber holder, in which a fiber coated with polymeric material is employed to retain the volatiles. After this, the analytes can be directly desorbed on the injection port of a chromatograph [20]. SPME methodology involves equilibration and extraction steps. Firstly, the sample inside a sealed vial is exposed to a selected temperature for a specific period of time in order to promote analyte volatilization into the HS in the equilibration step. Secondly, the coated fiber is immersed in the HS of the sample, maintaining a constant temperature, for a selected period of time to extract the target compounds. Experimental conditions (time and temperature values) of both steps, equilibration and extraction, commonly employed in VOCs analysis in nut samples are depicted in Table 1.
Table 1.
Experimental SPME conditions (time and temperature values) of both steps, equilibration and extraction, commonly employed in VOCs analysis in nut samples.
| Equilibration Conditions | Extraction Conditions | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Type of Nut | Sample Amount (g) | Fiber | Agitation | Time (min) | Temperature (°C) | Time (min) | Temperature (°C) | Quantification | Column | Ref. |
| Almond | 5.0 * | 1-cm 50/30 µm DVB/CAR/PDMS | No | 40 | 24 | 30 | 24 | (1) | DB-Wax (30 m × 0.25 mm × 0.25 μm) |
[38] |
| Almond | 5.0 * | 1-cm 50/30 µm DVB/CAR/PDMS | Yes | 45 | 40 | 45 | 40 | (3) | DB-Wax (30 m × 0.25 mm × 0.25 μm) |
[21] |
| Almond | 0.250 * | 1-cm 50/30 µm DVB/CAR/PDMS | No | 15 | 25 | 30 | 25 | (2) | DB-Wax (30 m × 0.25 mm × 0.25 μm) |
[37] |
| Almond | 5.0 * | 1-cm 50/30 µm DVB/CAR/PDMS | Yes | 45 | 40 | 45 | 40 | (3) | DB-Wax (30 m × 0.25 mm × 0.25 μm) |
[12] |
| Almond | 3.0 * | 1-cm 50/30 µm DVB/CAR/PDMS | No | 10 | 40 | 30 | 40 | (1) | TRB-5MS (30 m × 0.25 mm × 0.25 μm) |
[22] |
| Beechnut, hazelnut, pistachio and walnut | 10.0 ** | 1-cm 50/30 µm DVB/CAR/PDMS | Yes | 60 | 25 | 60 | 25 | No | RTx-5 (60 m × 0.25 mm × 0.25 μm) | [30] |
| Hazelnut | 0.1 * | 1-cm 75 µm CAR/PDMS |
No | 10 | 60 | 10 | 60 | (3) | DB-Wax (30 m × 0.25 mm × 0.5 μm) |
[15] |
| Hazelnut | 1.5 * | 2-cm 50/30 µm DVB/CAR/PDMS | No | 20 | 50 | 20 | 50 | (3) º | MEGA-WAX™ (30 m × 0.20 mm × 0.20 μm) | [43] |
| Peanut | 5.0 * | PDMS/DVB | No | 30 | 60 | 15 | 60 | (3) | DB-5 | [35] |
| Peanut | 5.0 * | 50/30 µm DVB/CAR/PDMS |
Yes | 1440 | 25 | 20 | 21 | (1) | SUPELCOWAX™ 10 (30 m, 0.25 mm, 0.25 mm) | [25] |
| Peanut | 3.0 ** | 1-cm 65 µm PDMS/DVB |
Yes | 10 | 50 | 40 | 50 | (1) | DB-Wax (30 m × 0.25 mm × 0.25 μm) |
[44] |
| Peanut | 5.0 ** | 2-cm 50/30 µm DVB/CAR/PDMS | No | 30 | 80 | 10 | 80 | (1) | RTX-5MS (30 m × 0.25 mm × 0.25 μm) |
[39] |
| Peanut | 5.0 ** | 1-cm 50/30 µm DVB/CAR/PDMS | Yes | 30 | 50 | 30 | 50 | No | DB-17MS (60 m × 0.25 mm × 0.25 μm) |
[32] |
| Peanut | 0.2 * | 2-cm DVB/CAR/PDMS |
Yes | 8 h | 20 | 50 | 60 | (1) | dB5-MS semi-polar (60 m × 0.32 mm × 1 μm) | [26] |
| Peanut | 5.0 ** | 1-cm 65 µm DVB/CAR/PDMS |
No | 20 | 80 | 60 | 80 | No | HP-5 (30 m × 0.25 mm × 0.25 μm) |
[33] |
| Pistachio | 15.0 * | 50/30 µm DVB/CAR/PDMS |
Yes | 15 | 50 | 120 | 50 | No | HP-5 (30 m × 0.32 mm × 0.25 μm) |
[19] |
| Pistachio | 8.0 * | 50/30 µm DVB/CAR/PDMS |
No | - | - | 60 | 83 | No | HP-5MS (30 m × 0.25 mm × 0.25 μm) | [34] |
| Pistachio | 10.0 * | 50/30 µm DVB/CAR/PDMS |
15 | 50 | 120 | 50 | (1) | Equity-5 (30 m × 0.25 mm × 0.25 μm) | [28] | |
| Pistachio | 24.5 * | PDMS-DVB | No | 30 | 30 | 20 | 30 | No | Agilent DB-1 (60 m × 0.320 mm × 0.25 μm) |
[31] |
| Pistachio | 1.5 ** | 2-cm 50/30 µm DVB/CAR/PDMS | Yes | - | - | 30 | 40 | (1) | DB-Wax (30 m × 0.25 mm × 0.25 μm) |
[2] |
| Walnut | 0.5 * | 50/30 µm DVB/CAR/PDMS |
Yes | 15 | 50 | 30 | 60 | (1) | RTX-5MS (30 m × 0.25 mm × 0.25 μm) |
[27] |
| Walnut | 3.0 ** (mL) | 1-cm 50/30 µm DVB/CAR/PDMS | No | 10 | 50 | 30 | 50 | No | HP-INNOWAX (30 m × 0.25 mm × 0.25 μm) |
[45] |
| Walnut | 1.0 ** | 65 µm PDMS/DVB |
No | - | - | 30 | 50 | No | CP-Wax52CB (30 m × 0.25 mm × 0.25 μm) |
[20] |
| Pecan | 2.0 * | 50/30 µm DVB/CAR/PDMS |
Yes | 30 | 25 | 30 | 65 | (1) | HP-5 (30 m × 0.25 mm × 0.25 μm) |
[36] |
Sample pre-treatment: * Only grinding ** Grinding and oil extraction. Quantification: 1: Semi-quantification with IS, 2: (1) corrected by sample amount, 3: quantification. º: IS was employed. DVB: Divinylbenzene, CAR: Carboxen, PDMS: Polydimethylsiloxane.
SPME results depend on the quantity of sample, and time and temperature extraction values. The influence of both factors—time and temperature—should be analyzed simultaneously since a temperature increase induces a drop in process time in many cases. Consequently, it is reported that higher temperatures reduce the needed equilibration time [21]. However, shorter equilibration times produced higher relative standard deviations (RSD) [19]. Pastorelli et al. tested higher temperatures (50, 60, 70 and 80 °C) and short equilibration times (5–60 min) [15]. Optimal values (60 °C, 10 min) proved a remarkable time reduction at higher temperatures. In addition, ultrasonic extraction has been used in place of the extraction by using temperature for analyzing the volatile compounds present in almonds [22].
Meanwhile, the sample is often agitated using a stirring bar to decrease the time necessary for equilibration [23]. However, as is shown in Table 1, a steady extraction process is commonly used as well. In fact, Pillonel et al. demonstrated that stirring has a minimal effect in the extraction yield of highly volatile compounds [24]. In addition, the solid nature of nut samples makes their agitation difficult, and the addition of water is necessary for homogenization purposes [22]. Moreover, some researchers increased the medium ionic strength in order to modify the nature of the matrix and the extraction efficiency, since this addition could affect the partition coefficient of the analytes [25,26]. Nevertheless, the higher the ionic strength, the lower the solubility of neutral molecules in water and the less likely these molecules are to pass from the solid matrix to the water. As a result, the extraction efficiency of these molecules decreases [19]. Consequently, most authors decided to avoid the addition of salt [19,27,28,29]. It is interesting to highlight that no water addition was required when hexanal was the only target compound [15]. Sample amount is also a key factor in the extraction process of volatile compounds in nuts [15]. As can be seen in Table 1, different sample amounts have been tested, most of them being higher than 0.1 g.
Finally, analyte extraction depends on fiber type. The commercially available fiber materials are carboxen (CAR), polydimethylsiloxane (PDMS) and divinylbenzene (DVB) [21]. In order to choose the most suitable extraction phase composition, the highest affinity of the target analytes is the key factor in the extraction efficiency. The vast majority of authors opt to employ three-phase CAR/PDMS/DVB fiber because of its high extraction capacity, which allows the removal of a high number of odorants [19]. Nevertheless, depending on the target compound, other coatings could also be better for the extraction process. As an example, Pastorelli et al. showed that CAR/PDMS fiber had higher sensitivity for hexanal extraction than PDMS, PDMS/DVB, CAR/DVB, and DVB/PDMS/CAR fibers [15].
Once the results are obtained, some authors directly employ the area of the compound [19,30,31] (or the percentage of areas [32,33,34]) if the analysis has the aim of comparing different varieties or the processing conditions of nuts. However, in some studies, a complete quantification was performed. Different types of quantification approaches are shown in Table 1. Most researchers employ semi-quantification with internal standard (IS), which is described in Equation (1), Equation (2) being an extension of the previous one. In both cases, the procedure is simple due to the fact that an external standard (ext. std.) curve is not required. Actual quantification is depicted in Equation (3). External standards are commonly prepared in deionizer water [35] or in organic solvents (e.g., n-hexane [25]).
| (1) |
| (2) |
| (3) |
Franklin et al. prepared external standards in a devolatized nut matrix, adding a mix solution of deuterated analytes [12,21]. Deuterated compounds were employed as internal standards. Octanal-d16, 2-methylpyrazine-d6, and n-hexyl-d13 alcohol were used as internal standards to determine aldehydes, pyrazines, and alcohols, respectively [36,37,38]. Octanal, 2-methylpyrazine and 1-hexanol were also employed for this purpose [22]. However, other studies used only a compound as the internal standard such as 4-methyl-2-pentanone [2,39] or 2-pentanol in pistachios [28].
Additionally, it is important to keep in mind that there are great differences in the perception threshold concentrations in humans for the different volatile compounds found in nuts. Based on this, to identify the most relevant compounds present in the sensorial profile of nuts, sometimes the odor activity value (OAV) is referenced. This value is calculated as the ratio between the concentration of a volatile compound present in a sample and the perception threshold concentration of the same compound. Volatiles with OAV values higher than 1 are detectable by consumers and, for this reason, are considered key odorants. Chetschik et al. investigated the volatile profile of peanuts and 26 compounds were quantified, but only 11 of them presented OAVs higher than 1 [40]. The compounds with a higher impact on the overall flavor of raw peanuts were 3-isopropyl-2-methoxypyrazine, acetic acid, 3-methylthiopropanal, 2,3-pentanedione and hexanal [40]. Meanwhile, 5-methyl-4-heptanone and 2-methoxy-3,5-dimethylpyrazine were the volatiles with higher OAVs present in raw hazelnuts [41]. On the other hand, 2,3-pentanedione, methional, 2-acetyl-1-pyrroline and 2-Acetyl-3,4,5,6(or 1,4,5,6)-tetrahydropyridine were detected in raw almonds. However, OAVs for raw almonds were significantly lower than the ones obtained for the major representative volatiles in peanuts and hazelnuts [42].
Nevertheless, when thermal treatment is applied to nuts, the OAV profile changes, and a higher number of compounds with values greater than 1 appears. The peanut’s key odorants, responsible for the typical roasted notes, are methanethiol followed by 2,3-pentanedione. Furthermore, some products of the initial steps of the Maillard reaction appeared with high OAV values such as 3-methylthiopropanal, 2-acetyl-1-pyrroline, 3-methylbutanal, and 2-methylbutanal [40]. In the case of pan-roasted hazelnuts, the volatile compounds which presented the highest OAV values resulted to be 3-methylbutanal, followed by distance by 4-hydroxy-2-5-dimethyl-3(2H)-furanone, (E,E)-2,4-decadienal, nonenal, hexenal and octanal [41]. Additionally, in the case of dry- or oil-roasted almonds, the principal biomarkers were 2,3-pentanedione, methional, followed by 2-acetyl-1-pyrroline and 4-hydroxy-2,5-dimethyl-3(2H)-furanone [42], even though other compounds in lower quantities could also be used as biomarkers.
4. Effect of Harvesting Conditions and Healthy State of Nuts on Volatile Profile
The main volatile compounds reported in raw nuts are reviewed in Table 2. As expected, several differences are detected that could be related to nut composition. Additionally, different factors could change the characteristic volatile profile of raw nuts. In this section, the effect of harvesting conditions is reviewed as well as the healthy state of nuts.
Table 2.
Main VOCs of raw nuts.
| Nut | Main VOCs | Ref. |
|---|---|---|
| Almond | 1-Hexanol, 3-methyl-1-butanol, nonanal, 2-methyl-1-propanol, 1-propanol | [37] |
| Benzaldehyde, hexanal, 1,2-propanediol, 1-chloro-2-propanol, 3-methyl-1-butanol, pentanal, 2-heptanone, 1-hexanol. | [38] | |
| Hexanal, 3-methyl-1-butanol, benzaldehyde, heptanal, nonanal, 1-octanol, 2-octanone | [22] | |
| Chestnut | ϒ-terpinene, phenylaldehyde, hexanal, furfural, α-terpinene | [57] |
| Hazelnut | α-tujone, β-tujone, 2-pentanone, acetic acid, 3-methyl-2- butanol, n-decane | [43] |
| Peanut | Hexanoic acid, 2-ethyl-1-hexanol, 1-hexanol, pentanal, hexanal, palmitic acid, 2-ethyl-5-methylpyrazine, heptanal | [44] |
| 2-Propanone, α-pinene, benzene, α-terpinolene, hexanal, d-limonene | [29] | |
| Toluene, α-limonene, γ-terpinene, p-cymene, nonanal, β, pinene, hexanoic acid | [39] | |
| 2,5-Dimethylpyrazine, nonanal, hexanal, 2-ethyl-5-methylpyrazine, octanal, 2,5-dimethyl-3-ethylpyrazine | [33] | |
| Hexanal, benzaldehyde, benzenacetaldehyde, 2,5-dimethylpyrazine, 2-heptenal, 2-ethyl-5-methylpyrazine, trimethylpyrazine, 3-ethyl-2,5-dimethylpyrazine | [26] | |
| Pistachio | 9-Octadecenoic acid, α-pinene, 1-methyl-1H-pyrrole, α-terpinolene, limonene, dimethyl-2H-pyran-2-one, 2-octenal, 2-hexenal | [34] |
| α-Pinene, α-terpinolene, 1H-pyrrole, ethyl-alcohol, limonene, hexane | [28] | |
| α-Pinene, β-pinene, 2-ethyl-1-hexanol, α-terpineol, camphene, hexanoic acid | [2] | |
| Walnut | Hexanal, hexanoic acid, 1-pentanol, 2-octenal, pentanal, 2-pentylfuran, propanoic acid | [45] |
| 2-Octenal, hexanoic acid, hexanal, 2-decenal, 1-octen-3-ol, nonanal | [20] |
The current limitation of water resources is threatening nut productivity, so new sustainable agronomic practices, such as regulated deficit irrigation (RDI), have been implemented. These irrigation strategies do not affect fruit quality [46,47]; however, water stress during the growing of nuts can modify its volatile profile [48]. For example, almonds produced under an RDI strategy presented higher total volatile content. In a study with pistachios, it was found lower water stress conditions produced nuts with higher terpene content such as α-pinene [49,50]. This compound is the most important volatile compound in pistachio samples, and it was observed that weather can influence the biosynthesis of VOCs [51] and also the harvesting time [23,25]. In another study, it was also evidenced that the flavor of pistachios was minimized by irrigation [49].
Moreover, the volatile organic profile of nuts could be modified by the state of health of the nut. The navel orangeworm (NOW) is among the major concerns in the almond industry, due to it being able to cause fungal infection. VOCs emission of almonds and their relationship with NOW have been investigated [52]. Although differences in the volatile profile have been found, the identification of particular compounds and their relationship to NOW have not been addressed. Mature almonds from the Monterey variety were evaluated for their volatile composition after mechanical damage and compared with the volatile composition of undamaged almonds. 3-pentanol and two isomers of a spiroketal chalcogran were found in the damaged almonds. Moreover, the concentration of some compounds such as a spiroketal conophthorin, numerous four-carbon esters and ketones as well as alcohol derivatives, in addition to two eight-carbon chain compounds, increased in the damaged almonds [52,53].
Changes in volatile organic compounds have been evaluated as an indicator of aflatoxin contamination [31,54,55]. Beck et al. studied the volatile emissions of whole and blanched almonds naturally contaminated with aflatoxins. Volatiles indicative of fatty acid decomposition were predominant in the samples that underwent some form of blanching. Moreover, they found an increase in the concentration of some aldehydes (e.g., hexanal, heptanal, octanal) and hexanoic acid [56]. A similar study was carried out with pistachios. A comparison of volatile compounds in healthy and naturally or artificially aflatoxin-contaminated pistachios was carried out by Georgiadou et al. They found some differences in specific compounds such as C-8 alcohols and aldehydes, sesquiterpenes, and monoterpenes, among others. These compounds allowed differentiation among contaminated and healthy pistachios by applying principal component analysis [28].
5. Volatile Profile of Nuts after Thermal Treatments
Processed nuts are consumed as a snack or added to confectionary and bakery products. For this purpose, heat treatments such as roasting and frying are necessary during nut processing to improve their sensory quality, digestibility, and microbiological safety. These heat treatments may significantly affect their properties and quality attributes, obtaining appreciable and desired changes in their texture, color, flavor and taste [58]. Interestingly, when nuts are thermally treated, new VOCs are formed from different reactions that are produced, others disappear, and others increase. Knowledge of the key odorants in thermal treatments of nuts can help to select the best preservation and thermal processing conditions. In this section, the most popular thermal processing methods of nuts and their influence on volatile profile are reviewed (Table 3).
Table 3.
Most popular thermal processing methods of nuts and main VOCs reported in the literature.
| Thermal Processing | Nut | Processing Conditions | Main VOCs | Ref. |
|---|---|---|---|---|
| Hot-air roasting | Hazelnut | 34, 18, 13 min at 130, 140 and 150 °C | 2,3-pentanedione, 2-acetyl-1-pyrroline, dimethyl sulphide, 2-furfurylthiol, 3-methylbutanal, 2-nonenal, 2-decenal, hydroxy-2,5-dimethyl-3(2H)-furanone | [59] |
| 20, 25 and 30 min at 160 °C |
2-methylpropanal, 2-methylbutanal, 3-methylbutanal, 2,5-methylpyrazine | [60] | ||
| 40 min at 140 °C | 2-methylbutanal, 3-methylbutanal, 2,5-methylpyrazine, furfuryl alcohol, 2-methylpropanal, ehtyl acetate, 2,3-pentanedione, 2-methylpyrazine, 2,5-methylpyrazine, furfural, 1-hydroxy-2-propanone | [43] | ||
| Almond | 5 min at 177 °C | Benzyl alcohol, benzaldehyde, 1-octen-3-ol, toluene, dimethylpyrazine, 1-butanol, hexanal | [61] | |
| 5–10 min at 170–190 °C |
Hexanal, 2-methyl-butanal, 2-methyl-pyrazine, 2,5-dimethyl-pyrazine, furfural | [62] | ||
| 10 min at 190 °C | 2,5-dimethyl-pyrazine, trimethylpyrazine | [48] | ||
| 33 min at 138 °C | Hexanal, benzeneacetaldehyde, 2,5-dimethyl-pyrazine, nonanal | [38] | ||
| 28 and 38 min at 138 °C | 2-methylbutanal, 3-methylbutanal, hexanal, benzaldehyde, furfural, 2-phenyl acetaldehyde | [38] | ||
| 28, 33 and 38 min at 138 °C |
2-methylbutanal, 3-methylbutanal, hexanal, benzaldehyde, furfural, 2-phenyl acetaldehyde | [63] | ||
| Chestnut | 25 min at 200 °C | Hexanal, butylacetate, ethylbenzene, 2-hydroxy-2-cyclopenten-1-one | [63] | |
| Pistachio with and without salt | 90 min at 120 °C | α-pinene, limonene, 3-carene | [64] | |
| Cashew | 3 and 9 min at 143 °C |
Methylbutanal, hexanal, acetaldehyde, heptane, ethanol, pentane, acetone | [65] | |
| Microwave roasting | Almond | 120 V for 2 min | Benzyl alcohol, methional, benzaldehyde, dimethylpyrazine, nonanal, undecane, 1-octen-3-ol, 1,4-butyrolactone | [61] |
| Pistachio | 480 or 640 W for 2, 3 and 4 min | α-pinene, limonene, nonanal | [66] | |
| Hot air-assisted radio frequency | Almond | 15 min at 120–130 °C | 2,5-dimethyl-pyrazine, toluene, hexanal and heptane | [67] |
| Deep-frying | Almond | 5 min at 135 °C | Benzyl alcohol, methional, benzaldehyde, 1-butanol, 1-octen-3-ol | [61] |
| 10–15 min at 160–200 °C | Hexanal, 2-methyl-butanal, 3-methyl-butanal, 2,5-dimethyl-pyrazine, 1-pentanol | [62] | ||
| Chestnut | 15 min at 240 °C | Hexanal, octanal, nonanal, furfural, 3-heptanone, 4-hydroxy-2-butanone | [68] |
5.1. Roasting Thermal Processing
The most popular thermal processing method of nuts is hot air roasting. During this process, samples are heated to temperatures of 130–200 °C from 5 to 60 min [69]. As a result, different reactions develop, which affects the nut’s volatile profile. These main reactions are the Maillard reaction, caramelization, fatty acid autoxidation, lipid degradation and the degradation of sulfur-containing amino acids.
The roasting process is associated with non-enzymatic darkening reactions such as, for example, the Maillard and caramelization reactions. The Maillard reaction initiates when amino acids and certain reducing sugars react and produce diverse kinds of compounds depending on the reactants, but also on the pH and the temperature reached during the roasting process. In the Maillard reaction, some heterocyclic volatile compounds such as furans, ketones, pyrazines, pyrroles, aldehydes and pyridines are formed and some of them show an increase compared to raw homologue nuts [62]. As shown in Table 3, some specific compounds related to this reaction in nuts are benzyl alcohol, 2,3-pentanedione, furfural, phenylacetaldehyde, toluene, 2,5-dimethyl-4-hydroxy-3(2H)-furanone, 2-furfurylthiol and some nitrogen-containing compounds including pyrazines such as 2,5-methylpyrazine, 2-methylpyrazine, dimethylpyrazine and trimethylpyrazine [36,59,61]. Some reported compounds are formed by the Strecker degradation reaction of amino acids in the Maillard reaction such as 2-methylbutanal, 3-methylbutanal and 2-acetyl-1-pyrroline [42]. Benzaldehyde is produced from phenylalanine under heat [61]. Benzeneacetaldehyde is a compound formed from phenylalanine by the action of polyphenol oxidase, and has been found in freshly roasted almonds, with honey-like scent, harsh, and hawthorn odor descriptors [22].
One important reaction is the degradation of sugars in the caramelization process [42]. In this process, heterocyclic oxygen furans are formed such as furfural. Another important reaction is fatty acid autoxidation. Hexanal and 2-nonenal, among others, are products of the degradation of linoleic acid; meanwhile, for example, nonanal and 2-decenal come from oleic acid [61]. Moreover, lipid degradation is a very important process for the volatile profiles of nuts, in which some aldehydes, alcohols and ketones are formed, being responsible for the desire toasted flavor [42]. Alcohols were also generated through lipid degradation such as 1-octen-3-ol, which comes from the thermal decomposition of methyl linoleate hydroperoxide, and contributes to a herbaceous aroma, whereas 1-butanol is formed from the decomposition of linolenic acid and is responsible for an unripe apple aroma [61]. Regarding ethanol, it was at high concentration among the volatile compounds in roasted cashew nut, which contributes to the formation of esters that usually have fruity odors [65]. Acetone found in roasted cashew is a result of the reaction of D-xylose with valine, while heptane does not contribute to aroma [65].
Sulfur-containing compounds also increase during the roasting process because of the degradation of sulfur-containing amino acids, such as cysteine and methionine. Sulfur compounds such as dimethyl sulfide give a taste of fresh onions to the oils. In relation with terpene compounds, in a study with pistachios, it was found that VOCs analysis shows that Argentinean pistachio nuts are rich in monoterpenes, mainly limonene (citrus, mint), α-pinene (pine, turpine), and 3-carene (lemon, resin) [64]. According to the mentioned work, it is possible to define the limonene as a marker for unroasted pistachio, while α-pinene and 3-carene seem to be associated with roasted pistachio with and without salt. In this sense, the perception of roasting is associated with increased amounts of α-pinene and 3-carene in roasted pistachio, while the raw sample is associated with higher amounts of limonene. Finally, it seems that any difference was reported due to the addition of salt.
To sum up, changes in the volatile profile due to the roasting treatment significantly affect the quality attributes of nuts, obtaining appreciable and desired changes in flavor. For example, the presence of 4-hydroxy-2,5-dimethyl-3(2H)-furanone in roasted hazelnuts was detected at high concentrations. This compound is formed after the dehydration of reducing monosaccharides. The changes in concentration of this volatile compound explain the differences in sensorial perception (popcorn-like, coffee-like and caramel) after eating roasted hazelnuts; meanwhile, in raw hazelnuts, the perception is associated with a fruity and nutty aroma [59].
5.2. Novel Roasting Treatments
Although hot air roasting is the most popular heat treatment of nuts, this operation is time and energetically expensive. As a consequence, new processing methods have been applied to reduce these drawbacks.
In this context, microwave energy offers the advantages of speed of operation, energy savings, precise process control, and faster start up and shut-down times, compared with conventional heat processes. Microwaves have been used in the roasting of almonds [61], and pistachios [66]. Regarding almonds, microwave heating enhanced the production of volatiles compared to frying and air-hot heating, and the flavor was the most preferred by panelists. Although the main VOCs were similar to those obtained by hot air roasting, two additional compounds were reported. On the one hand, the Strecker aldehyde methional contributes vegetable and creamy aromas. On the other hand, the undecane and 1,4-butyrolactone were obtained from the lipid oxidation of the nut—the lactone contributes to milky and creamy aromas. With respect to pistachios, Hojjati et al. [66] found that limonene, nonanal, and α-pinene were the main components in microwave-roasted pistachios. In this sense, the treatment variables, i.e., time and microwave power, affected the volatile concentrations of the nut. In particular, the total concentration of volatiles increased with the time and power of microwave roasting.
Radio frequency is a novel thermal processing technology with a frequency range of 3 kHz–300 MHz. Heat is generated inside the food products by molecular friction, owing to ionic conduction and dipole rotation during heating. Compared with microwave heating, radio frequency has a deeper penetration depth and a relatively uniform electromagnetic field distribution, which allows the bulk and thick materials to be heated more efficiently [67]. Recently, a roasting processing method of almonds has been reported by using the radio frequency technique for 15 min at 120–130 °C. As a result, similar major volatile compounds were obtained. Moreover, radio frequency roasting produced almonds with desired roasted flavor compounds at a lower roasting temperature.
5.3. Deep-Frying Processing
During the deep-frying process, nuts are immersed in different frying oils at 160–200 °C in the presence of air [69]. Frying involves the immersion of food in hot oil and during this process, some of the moisture is replaced by the frying oil, leading to the formation of pores that allow oil penetration into the created voids [70]. As a consequence, it is expected that differences in the volatile profile and sensory characteristics of nuts could appear. Aldehydes, pyrazines and alcohols were the predominant VOCs present in fried almonds [61,62]. Concerning Maillard products, benzyl alcohol, methional, 2-methylbutanal, 3-methylbutanal and 2,5-dimethylpyrazine were reported, whereas 1-butanol, 1-octen-3-ol, hexanal and 1-pentanol were produced due to lipid oxidation. In this sense, the concentration of 1-pentanol increased as a linoleic acid oxidation product [62]. When the results obtained by the frying process were compared with those obtained by hot air roasting, common obtained VOCs were observed, but fried almonds showed 18 additional volatile compounds (2-ethyl-5-methylpyrazine, 3-ethyl-2,5-dimethylpyrazine, diacetyl, (Z)-1,5-octadien-3-one, acetic acid, (E,E,Z)-2,4,6-nonatrienal, cyclotene and 11 unknown compounds). Meanwhile, nine other compounds were identified in the hot air-roasted almonds and not in the fried ones ((E,Z)-2,6-nonadienal, (E)-2-decenal, (E)-4,5-epoxy-(E)-2-decenal, methyl propyl disulfide and five unknown compounds). In general, fried almonds presented a higher number and a higher concentration of furanones and nitrogen-containing compounds. On the other hand, the hot air-roasted almonds were richer in aldehydes and sulfur compounds. The authors justified this behavior as the differences in energy transference and time and temperature. In this sense, in the fried almonds, the energy transfer is mainly produced by conduction, while in hot air-roasted samples, air convection predominates. As oil heat transfer is more efficient, more Maillard reaction and sugar degradation products are formed [42].
Regarding fried chestnuts, hexanal, octanal, nonanal, furfural, 3-heptanone, and 4-hydroxy-2-butanone were identified as the main VOCs after 15 min of processing at 240 °C [68]. In particular, 3-heptanone and 4-hydroxy-2-butanone were underlined as characteristics of the frying thermal processing of chestnuts.
6. Conclusions
Although the number of compounds present in the volatile profile of nuts is quite high, some conclusions can be drawn from the review of the published bibliography. The volatile profile of nuts depends both on the chemical composition of the nut in question and on the state of maturity of the product. In this respect, besides terpenoids and substances derived from certain amino acids, volatiles originating as a result of the evolution (i.e., oxidation) of the fatty acids present in the fat fraction of the nut are compounds usually present in the volatile profile of nuts. It is important to mention that the volatile profile may be modified depending on the agronomic conditions of nut production and the presence of microorganisms.
For the determination of volatiles, SPME combined with GC–MS is the most frequently used analytical technique. Optimization of the extraction conditions is one of the most critical aspects of the analysis, since the diversity of compounds present hampers simultaneous optimal conditions for all compounds being found. For the same reason, quantification of the analytes is complicated by using external standards and internal standardization.
It is important to mention than some nuts are consumed after having undergone heat treatment. This treatment can have a significant influence on the volatile profile of the product as a result of the reactions that occur during processing. These reactions are the Maillard reaction, caramelization, oxidation of fatty acids and the degradation of sulfur-containing amino acids. The time and duration of the treatment, as well as the use of a lipid medium for heat transmission, cause the appearance of new compounds and the disappearance of compounds initially present in the nut.
Finally, it should be noted that the determination of the volatile profile of nuts is a complex task but offers many possibilities to control different aspects of food production and evolution.
Acknowledgments
The authors would like to acknowledge the Generalitat Valenciana (FEDEGENT/2018/021) for their financial support.
Author Contributions
Conceptualization, A.V.G., R.S.R., A.J.P., S.E.M.P., S.P.M. and A.B.S.; methodology, A.V.G., R.S.R., A.J.P., S.P.M. and A.B.S.; formal analysis, A.V.G., S.E.M.P. and A.B.S.; data curation, A.V.G., R.S.R. and A.B.S.; writing—original draft preparation, A.V.G., R.S.R., A.J.P., S.E.M.P., S.P.M. and A.B.S.; writing—review and editing, A.V.G. and S.E.M.P.; supervision, A.V.G., R.S.R., A.J.P., S.E.M.P., S.P.M. and A.B.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Rowan D.D. Volatile Metabolites. Metabolites. 2011;1:41–63. doi: 10.3390/metabo1010041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ojeda-Amador R.M., Fregapane G., Salvador M.D. Influence of Cultivar and Technological Conditions on the Volatile Profile of Virgin Pistachio Oils. Food Chem. 2020;311 doi: 10.1016/j.foodchem.2019.125957. [DOI] [PubMed] [Google Scholar]
- 3.Sud Ali N., Cano-Lamadrid M., Noguera-Artiaga L., Lipan L., Carbonell-Barrachina Á.A., Sendra E. Flavors and Aromas. In: Yahia E.M., Carrillo-López A., editors. Postharvest Physiology and Biochemistry of Fruits and Vegetables. Elsevier; London, UK: 2019. pp. 385–404. [Google Scholar]
- 4.Mansurova M., Ebert B.E., Blank L.M., Ibáñez A.J. A Breath of Information: The Volatilome. Curr. Genet. 2018;64:959–964. doi: 10.1007/s00294-017-0800-x. [DOI] [PubMed] [Google Scholar]
- 5.Phenol-Explorer Database Phenol-Explorer Database. [(accessed on 15 January 2021)]; Available online: http://phenol-explorer.eu/
- 6.USDA Database. [(accessed on 20 February 2021)]; Available online: Https://Fdc.Nal.Usda.Gov/
- 7.FAOSTAT Food and Agriculture Organization of the United States FAOSTAT Database. [(accessed on 20 February 2021)]; Available online: http://www.fao.org/faostat/en/#data.
- 8.Cialiè Rosso M., Liberto E., Spigolon N., Fontana M., Somenzi M., Bicchi C., Cordero C. Evolution of Potent Odorants within the Volatile Metabolome of High-Quality Hazelnuts (Corylus avellana, L.): Evaluation by Comprehensive Two-Dimensional Gas Chromatography Coupled with Mass Spectrometry. Anal. Bioanal. Chem. 2018;410:3491–3506. doi: 10.1007/s00216-017-0832-6. [DOI] [PubMed] [Google Scholar]
- 9.Beltrán A., Ramos M., Grané N., Martín M.L., Garrigós M.C. Monitoring the Oxidation of Almond Oils by HS-SPME–GC–MS and ATR-FTIR: Application of Volatile Compounds Determination to Cultivar Authenticity. Food Chem. 2011;126:603–609. doi: 10.1016/j.foodchem.2010.11.058. [DOI] [Google Scholar]
- 10.Schwab W., Davidovich-Rikanati R., Lewinsohn E. Biosynthesis of Plant-Derived Flavor Compounds. Plant. J. 2008;54:712–732. doi: 10.1111/j.1365-313X.2008.03446.x. [DOI] [PubMed] [Google Scholar]
- 11.Alasalvar C., Shahidi F. Tree Nuts: Composition, Phytochemicals, and Health Effects. CRC Press; Boca Raton, FL, USA: 2008. [Google Scholar]
- 12.Franklin L.M., King E.S., Chapman D., Byrnes N., Huang G., Mitchell A.E. Flavor and Acceptance of Roasted California Almonds during Accelerated Storage. J. Agric. Food Chem. 2018;66:1222–1232. doi: 10.1021/acs.jafc.7b05295. [DOI] [PubMed] [Google Scholar]
- 13.Wang S., Adhikari K., Hung Y.-C. Acceptability and Preference Drivers of Freshly Roasted Peanuts. J. Food Sci. 2017;82:174–184. doi: 10.1111/1750-3841.13561. [DOI] [PubMed] [Google Scholar]
- 14.Ribeiro S.R., Ribeiro Q.M., Klein B., Duarte dos Santos I., Forgiarini S., Hamann J.J., Cichoski A.J., Fronza D., Brackmann A., Both V., et al. Effect of Low Oxygen on Quality Attributes of ‘Barton’ Pecan Nuts after Long-Term Storage at Different Temperatures. Sci. Hortic. 2020;263:109098. doi: 10.1016/j.scienta.2019.109098. [DOI] [Google Scholar]
- 15.Pastorelli S., Valzacchi S., Rodriguez A., Simoneau C. Solid-Phase Microextraction Method for the Determination of Hexanal in Hazelnuts as an Indicator of the Interaction of Active Packaging Materials with Food Aroma Compounds. Food Addit. Contam. 2006;23:1236–1241. doi: 10.1080/02652030600778744. [DOI] [PubMed] [Google Scholar]
- 16.Tieman D., Taylor M., Schauer N., Fernie A.R., Hanson A.D., Klee H.J. Tomato Aromatic Amino Acid Decarboxylases Participate in Synthesis of the Flavor Volatiles 2-Phenylethanol and 2-Phenylacetaldehyde. Proc. Natl. Acad. Sci. USA. 2006;103 doi: 10.1073/pnas.0602469103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Beltrán A., Maestre S.E., Grané N., Valdés A., Prats M.S. Variability of Chemical Profile in Almonds (Prunus dulcis) of Different Cultivars and Origins. Foods. 2021;10:153. doi: 10.3390/foods10010153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lubes G., Goodarzi M. Analysis of Volatile Compounds by Advanced Analytical Techniques and Multivariate Chemometrics. Chem. Rev. 2017;117:6399–6422. doi: 10.1021/acs.chemrev.6b00698. [DOI] [PubMed] [Google Scholar]
- 19.Aceña L., Vera L., Guasch J., Busto O., Mestres M. Comparative Study of Two Extraction Techniques to Obtain Representative Aroma Extracts for Being Analysed by Gas Chromatography-Olfactometry: Application to Roasted Pistachio Aroma. J. Chromatogr. A. 2010;1217:7781–7787. doi: 10.1016/j.chroma.2010.10.030. [DOI] [PubMed] [Google Scholar]
- 20.Mu H., Gao H., Chen H., Fang X., Zhou Y., Wu W., Han Q. Study on the Volatile Oxidation Compounds and Quantitative Prediction of Oxidation Parameters in Walnut (Carya cathayensis Sarg.) Oil. Eur. J. Lipid Sci. Technol. 2019;121:1–9. doi: 10.1002/ejlt.201800521. [DOI] [Google Scholar]
- 21.Franklin L.M., Chapman D.M., King E.S., Mau M., Huang G., Mitchell A.E. Chemical and Sensory Characterization of Oxidative Changes In Roasted Almonds Undergoing Accelerated Shelf Life. J. Agric. Food Chem. 2017;65:2549–2563. doi: 10.1021/acs.jafc.6b05357. [DOI] [PubMed] [Google Scholar]
- 22.Oliveira I., Malheiro R., Meyer A.S., Pereira J.A., Gonçalves B. Application of Chemometric Tools for the Comparison of Volatile Profile from Raw and Roasted Regional and Foreign Almond Cultivars (Prunus dulcis) J. Food Sci. Technol. 2019;56:3764–3776. doi: 10.1007/s13197-019-03847-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vas G., Vékey K. Solid-Phase Microextraction: A Powerful Sample Preparation Tool Prior to Mass Spectrometric Analysis. J. Mass Spectrom. 2004;39:233–254. doi: 10.1002/jms.606. [DOI] [PubMed] [Google Scholar]
- 24.Pillonel L., Bosset J.O., Tabacchi R. Rapid Preconcentration and Enrichment Techniques for the Analysis of Food Volatile. A Review. LWT Food Sci. Technol. 2002;35:1–14. doi: 10.1006/fstl.2001.0804. [DOI] [Google Scholar]
- 25.Abegaz E.G., Kerr W.L., Koehler P.E. The Role of Moisture in Flavor Changes of Model Peanut Confections during Storage. LWT Food Sci. Technol. 2004;37:215–225. doi: 10.1016/j.lwt.2003.07.007. [DOI] [Google Scholar]
- 26.Lykomitros D., Fogliano V., Capuano E. Drivers of Preference and Perception of Freshness in Roasted Peanuts (Arachis spp.) for European Consumers. J. Food Sci. 2018;83:1103–1115. doi: 10.1111/1750-3841.14095. [DOI] [PubMed] [Google Scholar]
- 27.Lee J., Vázquez-Araújo L., Adhikari K., Warmund M., Elmore J. Volatile Compounds in Light, Medium, and Dark Black Walnut and Their Influence on the Sensory Aromatic Profile. J. Food Sci. 2011;76:C199–C204. doi: 10.1111/j.1750-3841.2010.02014.x. [DOI] [PubMed] [Google Scholar]
- 28.Georgiadou M., Gardeli C., Komaitis M., Tsitsigiannis D.I., Paplomatas E.J., Sotirakoglou K., Yanniotis S. Volatile Profiles of Healthy and Aflatoxin Contaminated Pistachios. Food Res. Int. 2015;74:89–96. doi: 10.1016/j.foodres.2015.03.021. [DOI] [PubMed] [Google Scholar]
- 29.Mexis S.F., Kontominas M.G. Effect of Gamma Irradiation on the Physico-Chemical and Sensory Properties of Raw Shelled Peanuts (Arachis hypogaea, L.) and Pistachio Nuts (Pistacia vera, L.) J. Sci. Food Agric. 2009;89:867–875. doi: 10.1002/jsfa.3526. [DOI] [Google Scholar]
- 30.Stuebiger G., Buchbauer G., Krist S., Bail S., Unterweger H. Characterization of Volatile Compounds and Triacylglycerol Profiles of Nut Oils Using SPME-GC-MS and MALDI-TOF-MS. Eur. J. Lipid Sci. Technol. 2009;111:170–182. doi: 10.1002/ejlt.200800007. [DOI] [Google Scholar]
- 31.Beck J.J., Willett D.S., Mahoney N.E., Gee W.S. Silo-Stored Pistachios at Varying Humidity Levels Produce Distinct Volatile Biomarkers. J. Agric. Food Chem. 2017;65:551–556. doi: 10.1021/acs.jafc.6b04384. [DOI] [PubMed] [Google Scholar]
- 32.Xu L., Yu X., Li M., Chen J., Wang X. Monitoring Oxidative Stability and Changes in Key Volatile Compounds in Edible Oils during Ambient Storage through HS-SPME/GC–MS. Int. J. Food Prop. 2018;20:S2926–S2938. doi: 10.1080/10942912.2017.1382510. [DOI] [Google Scholar]
- 33.Dun Q., Yao L., Deng Z., Li H., Li J., Fan Y., Zhang B. Effects of Hot and Cold-Pressed Processes on Volatile Compounds of Peanut Oil and Corresponding Analysis of Characteristic Flavor Components. LWT Food Sci. Technol. 2019;112:107648. doi: 10.1016/j.lwt.2018.11.084. [DOI] [Google Scholar]
- 34.Kendirci P., Onoǧur T.A. Investigation of Volatile Compounds and Characterization of Flavor Profiles of Fresh Pistachio Nuts (Pistacia vera, L.) Int. J. Food Prop. 2011;14:319–330. doi: 10.1080/10942910903177830. [DOI] [Google Scholar]
- 35.Baker G.L., Cornell J.A., Gorbet D.W., O’Keefe S.F., Sims C.A., Talcott S.T. Determination of Pyrazine and Flavor Variations in Peanut Genotypes during Roasting. J. Food Sci. 2003;68:394–400. doi: 10.1111/j.1365-2621.2003.tb14171.x. [DOI] [Google Scholar]
- 36.Gong Y., Kerrihard A.L., Pegg R.B. Characterization of the Volatile Compounds in Raw and Roasted Georgia Pecans by HS-SPME-GC-MS. J. Food Sci. 2018;83:2753–2760. doi: 10.1111/1750-3841.14365. [DOI] [PubMed] [Google Scholar]
- 37.Rogel-Castillo C., Luo K., Huang G., Mitchell A.E. Effect of Drying Moisture Exposed Almonds on the Development of the Quality Defect Concealed Damage. J. Agric. Food Chem. 2017;65:8948–8956. doi: 10.1021/acs.jafc.7b03680. [DOI] [PubMed] [Google Scholar]
- 38.Lee J., Xiao L., Zhang G., Ebeler S.E., Mitchell A.E. Influence of Storage on Volatile Profiles in Roasted Almonds (Prunus dulcis) J. Agric. Food Chem. 2014;62:11236–11245. doi: 10.1021/jf503817g. [DOI] [PubMed] [Google Scholar]
- 39.Costa De Camargo A., Aparecida Bismara Regitano-d’Arce M., Matias De Alencar S., Guidolin Canniatti-Brazaca S., Ferreira de Souza Vieira T.M., Shahidi F. Chemical Changes and Oxidative Stability of Peanuts as Affected by the Dry-Blanching. J. Am. Oil Chem. Soc. 2016;93:1101–1109. doi: 10.1007/s11746-016-2838-1. [DOI] [Google Scholar]
- 40.Chetschik I., Granvogl M., Schieberle P. Quantitation of Key Peanut Aroma Compounds in Raw Peanuts and Pan-Roasted Peanut Meal. Aroma Reconstitution and Comparison with Commercial Peanut Products. J. Agric. Food Chem. 2010;58:11018–11026. doi: 10.1021/jf1026636. [DOI] [PubMed] [Google Scholar]
- 41.Burdack-Freitag A., Schieberle P. Characterization of the Key Odorants in Raw Italian Hazelnuts (Corylus avellana, L. Var. Tonda Romana) and Roasted Hazelnut Paste by Means of Molecular Sensory Science. J. Agric. Food Chem. 2012;60:5057–5064. doi: 10.1021/jf300908d. [DOI] [PubMed] [Google Scholar]
- 42.Erten E.S., Cadwallader K.R. Identification of Predominant Aroma Components of Raw, Dry Roasted and Oil Roasted Almonds. Food Chem. 2017;217:244–253. doi: 10.1016/j.foodchem.2016.08.091. [DOI] [PubMed] [Google Scholar]
- 43.Nicolotti L., Cordero C., Bicchi C., Rubiolo P., Sgorbini B., Liberto E. Volatile Profiling of High Quality Hazelnuts (Corylus avellana, L.): Chemical Indices of Roasting. Food Chem. 2013;138:1723–1733. doi: 10.1016/j.foodchem.2012.11.086. [DOI] [PubMed] [Google Scholar]
- 44.Liu X.J., Jin Q.Z., Liu Y.F., Huang J.H., Wang X.G., Mao W.Y., Wang S.S. Changes in Volatile Compounds of Peanut Oil during the Roasting Process for Production of Aromatic Roasted Peanut Oil. J. Food Sci. 2011;76:404–412. doi: 10.1111/j.1750-3841.2011.02073.x. [DOI] [PubMed] [Google Scholar]
- 45.Zhou Y., Fan W., Chu F., Wang C., Pei D. Identification of Volatile Oxidation Compounds as Potential Markers of Walnut Oil Quality. J. Food Sci. 2018;83:2745–2752. doi: 10.1111/1750-3841.14342. [DOI] [PubMed] [Google Scholar]
- 46.Lipan L., Martín-Palomo M.J., Sánchez-Rodríguez L., Cano-Lamadrid M., Sendra E., Hernández F., Burló F., Vázquez-Araújo L., Andreu L., Carbonell-Barrachina Á.A. Almond Fruit Quality Can Be Improved by Means of Deficit Irrigation Strategies. Agric. Water Manag. 2019;217 doi: 10.1016/j.agwat.2019.02.041. [DOI] [Google Scholar]
- 47.Lipan L., García-Tejero I.F., Gutiérrez-Gordillo S., Demirbaş N., Sendra E., Hernández F., Durán-Zuazo V.H., Carbonell-Barrachina A.A. Enhancing Nut Quality Parameters and Sensory Profiles in Three Almond Cultivars by Different Irrigation Regimes. J. Agric. Food Chem. 2020;68 doi: 10.1021/acs.jafc.9b06854. [DOI] [PubMed] [Google Scholar]
- 48.Lipan L., Cano-Lamadrid M., Vázquez-Araújo L., Łyczko J., Moriana A., Hernández F., García-García E., Carbonell-Barrachina Á.A. Optimization of Roasting Conditions in HydroSOStainable Almonds Using Volatile and Descriptive Sensory Profiles and Consumer Acceptance. J. Food Sci. 2020;85:3969–3980. doi: 10.1111/1750-3841.15481. [DOI] [PubMed] [Google Scholar]
- 49.Şahan A., Bozkurt H. Effects of Harvesting Time and Irrigation on Aroma Active Compounds and Quality Parameters of Pistachio. Sci. Hortic. 2020;261:108905. doi: 10.1016/j.scienta.2019.108905. [DOI] [Google Scholar]
- 50.Carbonell-Barrachina Á.A., Memmi H., Noguera-Artiaga L., del Carmen Gijón-López M., Ciapa R., Pérez-López D. Quality Attributes of Pistachio Nuts as Affected by Rootstock and Deficit Irrigation. J. Sci. Food Agric. 2015;95 doi: 10.1002/jsfa.7027. [DOI] [PubMed] [Google Scholar]
- 51.Polari J.J., Zhang L., Ferguson L., Maness N.O., Wang S.C. Impact of Microclimate on Fatty Acids and Volatile Terpenes in “Kerman” and “Golden Hills” Pistachio (Pistacia vera) Kernels. J. Food Sci. 2019;84:1937–1942. doi: 10.1111/1750-3841.14654. [DOI] [PubMed] [Google Scholar]
- 52.Beck J.J., Higbee B.S., Merrill G.B., Roitman J.N. Comparison of Volatile Emissions from Undamaged and Mechanically Damaged Almonds. J. Sci. Food Agric. 2008;88 doi: 10.1002/jsfa.3224. [DOI] [Google Scholar]
- 53.Beck J.J., Merrill G.B., Higbee B.S., Light D.M., Gee W.S. In Situ Seasonal Study of the Volatile Production of Almonds (Prunus dulcis) Var. ‘Nonpareil’ and Relationship to Navel Orangeworm. J. Agric. Food Chem. 2009;57 doi: 10.1021/jf9003187. [DOI] [PubMed] [Google Scholar]
- 54.Scott-Thomas A., Chambers S.T. Volatile Organic Compounds: Upcoming Role in Diagnosis of Invasive Mould Infections. Curr. Fungal Infect. Rep. 2017;11 doi: 10.1007/s12281-017-0284-7. [DOI] [Google Scholar]
- 55.Beck J.J., Willett D.S., Gee W.S., Mahoney N.E., Higbee B.S. Differentiation of Volatile Profiles from Stockpiled Almonds at Varying Relative Humidity Levels Using Benchtop and Portable GC-MS. J. Agric. Food Chem. 2016;64 doi: 10.1021/acs.jafc.6b04220. [DOI] [PubMed] [Google Scholar]
- 56.Beck J.J., Mahoney N.E., Cook D., Gee W.S. Volatile Analysis of Ground Almonds Contaminated with Naturally Occurring Fungi. J. Agric. Food Chem. 2011;59 doi: 10.1021/jf200739a. [DOI] [PubMed] [Google Scholar]
- 57.Krist S., Unterweger H., Bandion F., Buchbauer G. Volatile Compound Analysis of SPME Headspace and Extract Samples from Roasted Italian Chestnuts (Castanea sativa Mill.) Using GC-MS. Eur. Food Res. Technol. 2004;219:470–473. doi: 10.1007/s00217-004-0983-5. [DOI] [Google Scholar]
- 58.Valdés A., Beltrán A., Karabagias I.K., Badeka A., Kontominas M.G., Garrigós M.C. Effect of Frying and Roasting Processes on the Oxidative Stability of Sunflower Seeds (Helianthus annuus) under Normal and Accelerated Storage Conditions. Foods. 2021;10:944. doi: 10.3390/foods10050944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yang J., Pan Z., Takeoka G., MacKey B., Bingol G., Brandl M.T., Garcin K., McHugh T.H., Wang H. Shelf-Life of Infrared Dry-Roasted Almonds. Food Chem. 2013;138:671–678. doi: 10.1016/j.foodchem.2012.09.142. [DOI] [PubMed] [Google Scholar]
- 60.Marzocchi S., Pasini F., Verardo V., Ciemniewska-Żytkiewicz H., Caboni M.F., Romani S. Effects of Different Roasting Conditions on Physical-Chemical Properties of Polish Hazelnuts (Corylus avellana, L. Var. Kataloński) LWT Food Sci. Technol. 2017;77:440–448. doi: 10.1016/j.lwt.2016.11.068. [DOI] [Google Scholar]
- 61.Agila A., Barringer S. Effect of Roasting Conditions on Color and Volatile Profile Including HMF Level in Sweet Almonds (Prunus dulcis) J. Food Sci. 2012;77:1–8. doi: 10.1111/j.1750-3841.2012.02629.x. [DOI] [PubMed] [Google Scholar]
- 62.Valdés A., Beltrán A., Karabagias I., Badeka A., Kontominas M.G., Garrigós M.C. Monitoring the Oxidative Stability and Volatiles in Blanched, Roasted and Fried Almonds under Normal and Accelerated Storage Conditions by DSC, Thermogravimetric Analysis and ATR-FTIR. Eur. J. Lipid Sci. Technol. 2015;117:1199–1213. doi: 10.1002/ejlt.201400384. [DOI] [Google Scholar]
- 63.Xiao L., Lee J., Zhang G., Ebeler S.E., Wickramasinghe N., Seiber J., Mitchell A.E. HS-SPME GC/MS Characterization of Volatiles in Raw and Dry-Roasted Almonds (Prunus dulcis) Food Chem. 2014;151:31–39. doi: 10.1016/j.foodchem.2013.11.052. [DOI] [PubMed] [Google Scholar]
- 64.Penci M.C., Martinez M.L., Fabani M.P., Feresin G.E., Tapia A., Ighani M., Ribotta P.D., Wunderlin D.A. Matching Changes in Sensory Evaluation with Physical and Chemical Parameters: A Case Study: Argentinean Pistachio Nuts (Pistachia vera, L. Cv Kerman) Food Bioprocess. Technol. 2013;6:3305–3316. doi: 10.1007/s11947-012-0993-4. [DOI] [Google Scholar]
- 65.Agila A., Barringer S.A. Volatile Profile of Cashews (Anacardium occidentale, L.) from Different Geographical Origins during Roasting. J. Food Sci. 2011;76 doi: 10.1111/j.1750-3841.2011.02180.x. [DOI] [PubMed] [Google Scholar]
- 66.Hojjati M., Noguera-Artiaga L., Wojdyło A., Carbonell-Barrachina Á.A. Effects of Microwave Roasting on Physicochemical Properties of Pistachios (Pistaciavera, L.) Food Sci. Biotechnol. 2015;24:1995–2001. doi: 10.1007/s10068-015-0263-0. [DOI] [Google Scholar]
- 67.Xu Y., Liao M., Wang D., Jiao S. Physicochemical Quality and Volatile Flavor Compounds of Hot Air-Assisted Radio Frequency Roasted Almonds. J. Food Process. Preserv. 2020;44 doi: 10.1111/jfpp.14376. [DOI] [Google Scholar]
- 68.Li Q., Shi X., Zhao Q., Cui Y., Ouyang J., Xu F. Effect of Cooking Methods on Nutritional Quality and Volatile Compounds of Chinese Chestnut (Castanea mollissima Blume) Food Chem. 2016;201:80–86. doi: 10.1016/j.foodchem.2016.01.068. [DOI] [PubMed] [Google Scholar]
- 69.Liu Z., Wang W., Huang G., Zhang W., Ni L. In Vitro and in Vivo Evaluation of the Prebiotic Effect of Raw and Roasted Almonds (Prunus amygdalus) J. Sci. Food Agric. 2016;96:1836–1843. doi: 10.1002/jsfa.7604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dana D., Saguy I.S. Frying of Nutritious Foods: Obstacles and Feasibility. Food Sci. Technol. Res. 2001;7:265–279. doi: 10.3136/fstr.7.265. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.