Abstract
Phylum Cnidaria is an ancient venomous group defined by the presence of cnidae, specialised organelles that serve as venom delivery systems. The distribution of cnidae across the body plan is linked to regionalisation of venom production, with tissue-specific venom composition observed in multiple actiniarian species. In this study, we assess whether morphological variants of tentacles are associated with distinct toxin expression profiles and investigate the functional significance of specialised tentacular structures. Using five sea anemone species, we analysed differential expression of toxin-like transcripts and found that expression levels differ significantly across tentacular structures when substantial morphological variation is present. Therefore, the differential expression of toxin genes is associated with morphological variation of tentacular structures in a tissue-specific manner. Furthermore, the unique toxin profile of spherical tentacular structures in families Aliciidae and Thalassianthidae indicate that vesicles and nematospheres may function to protect branched structures that host a large number of photosynthetic symbionts. Thus, hosting zooxanthellae may account for the tentacle-specific toxin expression profiles observed in the current study. Overall, specialised tentacular structures serve unique ecological roles and, in order to fulfil their functions, they possess distinct venom cocktails.
Keywords: Actiniaria, venom, toxin expression, transcriptomics, ecology
1. Introduction
Venom composition tends to be dynamic, varying across geographic locations and ontogenic stages, and between individuals in venomous taxa [1,2,3,4,5,6,7]. Phylum Cnidaria is considered the most ancient venomous lineage and is differentiated from most other venomous lineages by the fact the venom production does not occur within a central venom gland [8,9,10]. Instead, cnidae, the highly specialised organelles responsible for venom production and delivery, are found within ectodermal and endodermal tissue across the entire body plan [11,12,13], creating a unique link between toxin functional ecology and tissue functional morphology [9]. Indeed, differential expression of toxins across life stages and discrete anatomical regions has been reported across several taxa in the order Actiniaria (sea anemones) [13,14,15]. Different toxin profiles have been found in acrorhagi, tentacles, mesenterial filaments and body column [14,15], although these tissue-specific toxin expression profiles vary across species. For example, sea anemone sodium channel inhibitory toxins were observed to be upregulated in the column of Anemonia sulcata and Heteractis crispa, but in the mesenterial filaments of Megalactis griffithsi and the acrorhagi of Actinia tenebrosa [14,15]. However, multiple tentacle subtypes with distinct morphology and ecological roles may be present simultaneously within a species, suggesting that toxin expression profiles may be fine-tuned at an even greater level of detail.
While tentacles are used primarily for prey capture and defence against predators (feeding tentacles), contact with competitors can induce the development of a secondary tentacle type—catch tentacles—in actiniarians [16,17,18,19]. Analogous competitor-induced tentacles (sweeper tentacles) can be observed in scleractinian corals and octocorals [19,20,21,22]. Both catch and sweeper tentacles are specialised for agonistic encounters with competitors, causing necrosis in other polyps, and are morphologically distinct from feeding tentacles [20,22,23]. A recent investigation of gene expression in sweeper and feeding tentacles in the stony coral Galaxea fasicularis revealed that toxin genes are differentially expressed across the two tentacle subsets [24] indicating that venom composition may vary across tentacle types when they fulfil distinct ecological functions. We surmised that toxin expression might similarly differ in a tentacle-specific manner in sea anemones, given that they also possess functionally distinct tentacle subsets.
Actiniarian tentacle morphology is highly variable, and several species possess additional specialised tentacular structures [19,25,26,27]. While tentacles in many species are characterised by simple external morphology, they can vary in structure (e.g., branched) or possess nematocyst-dense structures [28]. The relative size of tentacles can also vary, as observed with the long inner and short outer tentacles of genera Dofleinia and Macrodactyla (Figure 1a,b). Additionally, Dofleinia armata has a discrete battery of cells (papillae) covering their tentacles which are laden with nematocysts [29]. Tentacles co-locate with mesenterial spaces, which are termed endocoels or exocoels depending on whether they arise inside mesenterial pairs or between mesenterial pairs, respectively [30]. In many actiniarian taxa, a single tentacle is associated with each endocoel/exocoel, although in Cryptodendrum adhaesivum and Heterodactyla hemprichii multiple tentacles are associated with each endocoel (Figure 1c–f), with a clear morphological distinction between endocoelic and exocoelic tentacles [26,31,32]. Exocoelic tentacles of C. adhaesivum and H. hemprichii are orally-aborally flattened, branched structures present at the margin of the oral disc [30,31]. In contrast, dendritic endocoelic tentacles of these species are arranged in rows that radiate out from the mouth and are considerably shorter than exocoelic tentacles [31,32].
Figure 1.
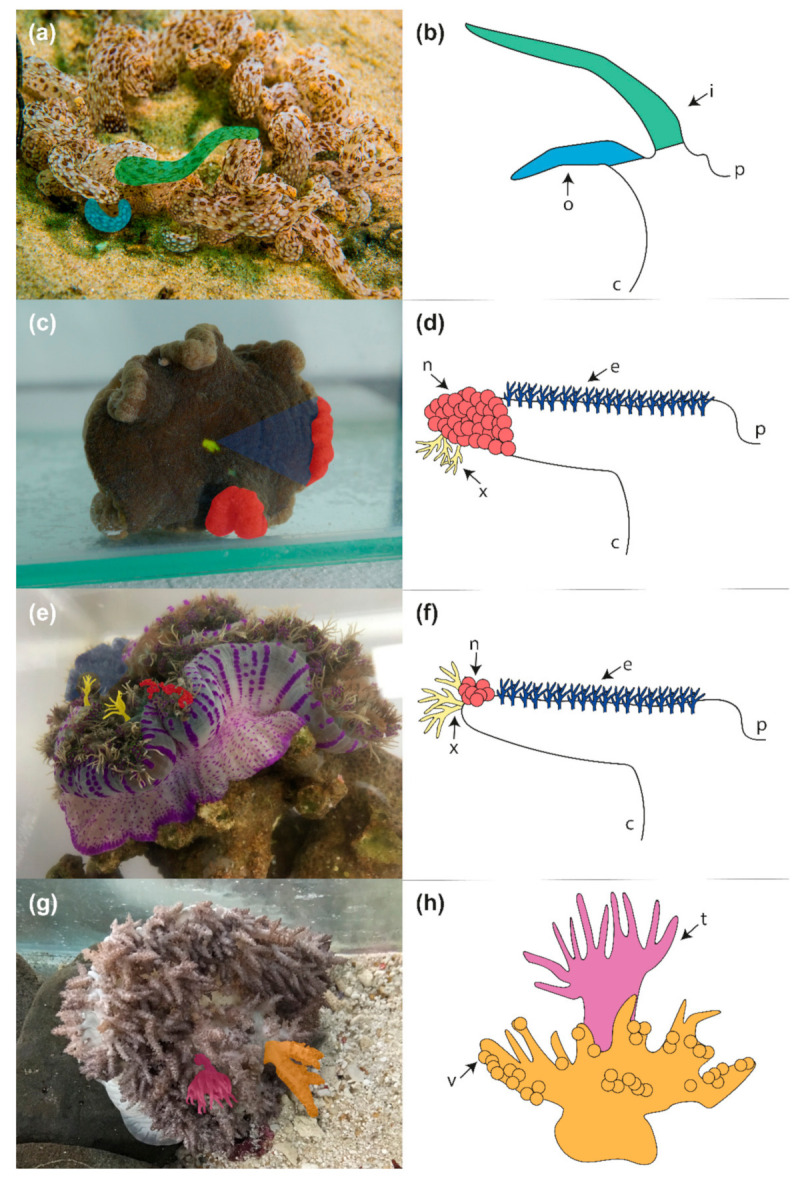
Tentacular structures isolated from sea anemone species: (a,b) inner tentacles (green) and outer tentacles (blue) from D. cf. armata; (c,d) endocoelic tentacles (dark blue) and nematospheres (red) from C. adhaesivum; (e,f) endocoelic tentacles (dark blue), exocoelic tentacles (yellow) and nematospheres (red) from H. hemprichii; (g,h) tentacles (pink) and pseudotentacles with attached vesicles (orange) from P. semoni. Abbreviations: c = body column; p = actinopharynx; I = inner tentacles; o = outer tentacles; n = nematospheres; x = exocoelic tentacles; e = endocoelic tentacles; v = vesicles on pseudotentacles; t = true tentacles. Photo credit: Gary Cranitch (a) and Lauren Ashwood (c,e,g).
In addition to endocoelic and exocoelic tentacles, species from the family Thalassianthidae possess a third tentacle type: nematospheres (Figure 1c–f). Characterized by a spherical morphology, these modified tentacles are taxonomically restricted, although similar globular structures can be observed in other species [26,32,33]. While associated with an endocoel, nematospheres are co-located with exocoelic tentacles at the oral disc margin but likely serve different ecological functions [26].
In H. hemprichii, nematospheres form distinctive grape-like clusters, while in C. adhaesivum the nematospheres form a continuous band adjacent to endocoelic tentacles, although the boundary between the two tentacle types is clearly defined [26,34]. The function of nematospheres has not yet been determined but they are presumed to serve a defensive role [26]. Vesicles in Phyllodiscus semoni are comparably nematocyst-dense spherical structures attached to the pseudotentacles (Figure 1g,h) [26]. Pseudotentacles are restricted to the family Aliciidae and differ from true tentacles in that they are outgrowths of the lower column wall [26,35]. Tentacles and pseudotentacles alternate, extending and retracting in a cyclic manner, with pseudotentacles extending and obscuring the true tentacles during the day [35,36]. The alternating retraction of pseudotentacles and tentacles implies that these structures have specific functions, which may require different venom compositions, resulting in differential expression of toxins across these morphological features.
Given that differences in the functional profiles of structures correlate to the differential expression of toxins in a tissue-specific manner [14,15,24], distinct tentacle-specific toxin expression patterns may be present across tentacle subtypes that serve different ecological functions. Furthermore, there is likely to be a functional basis for morphological variants of tentacles observed in Aliciidae and Thalassianthidae. In this study, we assessed whether morphological variants of tentacles are associated with distinct toxin expression profiles and investigated the functional significance of specialised tentacular structures. Our results provide insight into the underlying morphological basis for the different toxin profiles associated with functionally distinct tissues.
2. Results
2.1. Assembly Statistics
We sequenced and assembled the transcriptomes of five sea anemone species collected from Australian waters: C. adhaesivum, D. cf. armata, H. hemprichii, M. doreensis and P. semoni. More than 142 million paired-end reads were generated for M. doreensis, C. adhaesivum, D. cf. armata and P. semoni, while 240 million single-end reads were produced for H. hemprichii. The library for endocoelic tentacles of C. adhaesivum appeared to have degraded during transport and was not able to be sequenced. Transcriptomes for all taxa had Benchmarking Universal Single-Copy Orthologs (BUSCO) completeness scores between 88.9 and 97.2%. The assembly N50 scores ranged from 609 to 2823 base pairs (bp). Although the N50 for C. adhaesivum (609; Table 1) was significantly lower than the other species, the N50 calculated from only the most highly expressed transcripts (1346; Table 1) was comparable to that of M. doreensis (1493; Table 1).
Table 1.
Transcriptome sequencing and assembly parameters for actiniarian species.
| C. adhaesivum | D. cf. armata | H. hemprichii | M. doreensis | P. semoni | |
|---|---|---|---|---|---|
| Raw Reads | 200.5 million | 160.2 million | 240.3 million | 142.2 million | 305.0 million |
| Transcripts | 628,468 | 164,583 | 101,150 | 108,541 | 208,537 |
| Genes | 451,132 | 97,982 | 74,496 | 64,558 | 107,984 |
| DE 1 Transcripts | 3672 | 156 | 3118 | 271 | 1411 |
| N50 | 609 | 1514 | 1370 | 1493 | 2823 |
| E90 N50 | 1346 | 2068 | 1991 | 1549 | 3241 |
| E90 transcripts | 105,056 | 35,114 | 19,888 | 18,285 | 19,460 |
| BUSCO 2 | 93.9% | 97.2% | 92.0% | 88.9% | 95.0% |
1 DE; differentially expressed defined as greater than 4-fold expression change (false discovery rate [FDR] ≤ 0.05). 2 BUSCO; Benchmarking Universal Single-Copy Orthologs.
2.2. Functional Differences of Structures
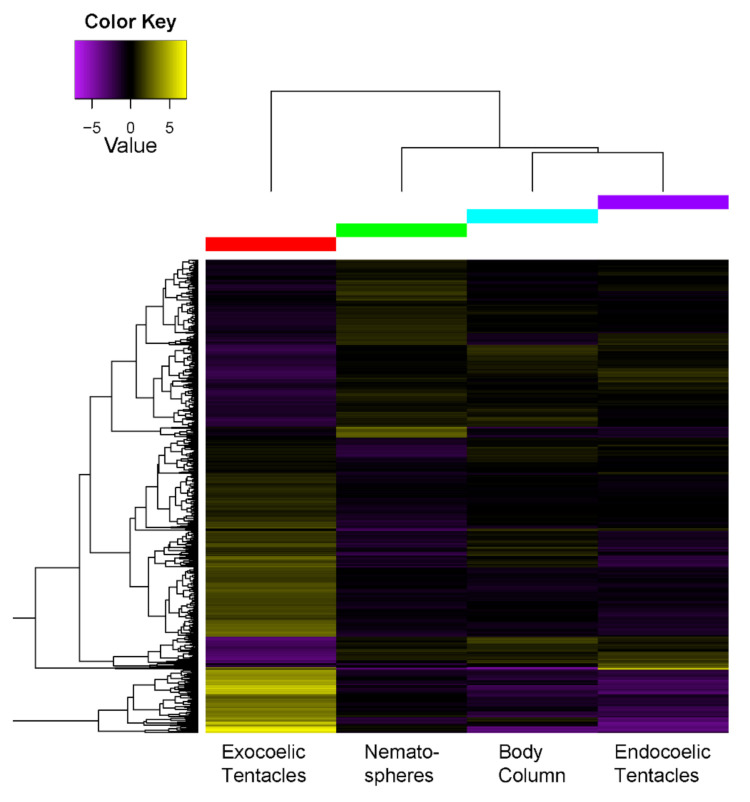
Differential gene expression and Gene Ontology (GO) enrichment analysis supported functionally discrete roles for the structures analysed. Patterns of transcript expression differed significantly across the structures examined in all taxa. When comparing gene expression between inner and outer tentacles of D. cf. armata and M. doreensis, 156 and 271 transcripts were found to be differentially expressed, respectively. Higher numbers of differentially expressed transcripts were observed among structures in C. adhaesivum, H. hemprichii and P. semoni (Table 1). In H. hemprichii, transcript expression in the body column showed the most divergent expression pattern, with 978, 1050 and 1996 genes differentially expressed between the body column and endocoelic tentacles, exocoelic tentacles and nematospheres, respectively (Figure 2). Comparing expression profiles among tentacle types, we found that exocoelic tentacles and nematospheres, which are both located at the marginal edge of the oral disc, had the highest degree of similarity with 327 genes differentially expressed. Conversely, 824 genes were differentially expressed between the endocoelic tentacles and nematospheres. Comparisons between two structures in the remaining species revealed that fewer transcripts were differentially expressed between tentacular structures in P. semoni (1411) than between the body column and tentacles in C. adhaesivum (3672). However, in both species, comparable numbers of transcripts were upregulated in each of the structures examined (Figure S1).
Figure 2.
Heatmap of differentially expressed genes (centered reads per kilobase of transcript per million mapped [RPKM] values) for body column and tentacular structures in H. hemprichii.
GO enrichment analysis of differentially expressed genes also supported greater differences among tentacular structures of H. hemprichii and P. semoni than between inner and outer tentacles of D. cf. armata and M. doreensis. Extracellular region (GO:0005576) was found to be enriched in outer tentacles of D. cf. armata and M. doreensis. While no GO terms were enriched for inner tentacles of D. cf. armata, photosynthesis (GO:0015979) and related terms were enriched in inner tentacles of M. doreensis. Similarly, chlorophyll binding (GO:0016168) was enriched in nematospheres of C. adhaesivum and vesicles of P. semoni. The enrichment of photosynthesis-related terms likely reflects the presence of algal symbionts in C. adhaesivum, M. doreensis and P. semoni. Heterodactyla hemprichii also hosts zooxanthellae species, although the specimen included in this study had undergone bleaching prior to tissue dissection and therefore no photosynthetic-related GO terms were found to be enriched in this individual. Venom-related GO terms, including nematocyst (GO:0042151) and toxin activity (GO:0090729), were significantly enriched in multiple structures across C. adhaesivum, H. hemprichii and P. semoni.
2.3. Venom Repertoire and Toxin Expression
2.3.1. Comparison of Species Venom Arsenal
Transcripts with homology to known toxins (toxin-like) were identified and extracted from the transcriptome assembly of each taxon. The number of toxin-like transcripts compiled for each species ranged from 60 in H. hemprichii to 119 in C. adhaesivum and P. semoni (Table 2), with approximately 0.05% of transcripts categorised as toxin-like.
Table 2.
Venom arsenal of five sea anemone species, C. adhaesivum, D. cf. armata, H. hemprichii, M. doreensis and P. semoni, according to biological function and toxin family. The values listed correspond to the number of toxin-like transcripts identified for each toxin family.
| Category | ToxProt Family | C. adhaesivum | D. cf. armata | H. hemprichii | M. doreensis | P. semoni |
|---|---|---|---|---|---|---|
| Auxiliary | Peptidase M12A | 8 | 3 | 1 | 4 | 35 |
| Membrane-active | Actinoporin family | 15 | 0 | 9 | 9 | 4 |
| Jellyfish toxin family | 0 | 1 | 0 | 0 | 0 | |
| MACPF toxin family | 7 | 3 | 3 | 11 | 29 | |
| Mixed function enzymes | Phospholipase A2 family | 21 | 12 | 10 | 11 | 15 |
| Neurotoxin | Sea anemone type 3 (BDS) potassium channel toxin family | 24 | 11 | 13 | 7 | 2 |
| Sea anemone type 1 potassium channel toxin family | 6 | 2 | 3 | 6 | 2 | |
| Sea anemone type 5 potassium channel toxin family | 1 | 0 | 0 | 0 | 1 | |
| Sea anemone sodium channel inhibitory toxin family | 2 | 1 | 3 | 1 | 5 | |
| Cnidaria small cysteine-rich protein (SCRiP) family | 1 | 1 | 0 | 0 | 2 | |
| Sea anemone short toxin (type III) family | 1 | 0 | 1 | 0 | 0 | |
| Sea anemone structural class 9a family | 0 | 2 | 0 | 3 | 0 | |
| Protease Inhibitor | Venom Kunitz-type family | 14 | 12 | 9 | 6 | 11 |
| Unknown | EGF domain peptide family | 2 | 0 | 1 | 2 | 1 |
| Sea anemone 8 toxin family | 14 | 5 | 6 | 8 | 9 | |
| Acrorhagin | 2 | 3 | 1 | 1 | 0 | |
| Unknown | 0 | 5 | 0 | 3 | 0 | |
| 118 | 61 | 60 | 72 | 116 |
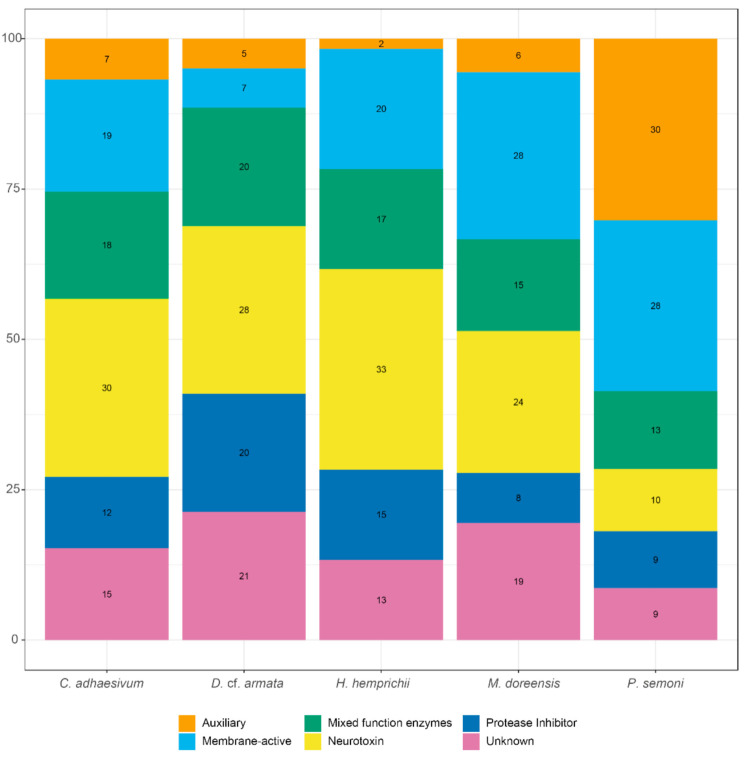
In order to summarise the distinct venom profile of each actiniarian species, toxin-like transcripts were categorised into toxin families and then assigned one of six biological functions: auxiliary, membrane-active, mixed function enzymes, neurotoxin, protease inhibitor and unknown (Table 2 and Figure 3). Toxin families with neurotoxic functions were associated with the largest number of toxin-like transcripts in C. adhaesivum, D. cf. armata and H. hemprichii. In particular, sea anemone type 3 (BDS) potassium channel toxin-like transcripts were numerous in all three species. Conversely, membrane-active toxins were the most abundant category of toxins identified in the transcriptome of M. doreensis. The Membrane Attack Complex/Perforin (MACPF) toxin family is expanded in this species, with MACPF toxin-like transcripts also highly abundant in the transcriptome of P. semoni. Auxiliary toxins comprised a much higher proportion of the venom arsenal in P. semoni compared to the other four species; this can be explained by the large number of toxin-like transcripts with homology to peptidase M12A.
Figure 3.
Composition of toxin arsenal for C. adhaesivum, D. cf. armata, H. hemprichii, M. doreensis and P. semoni. The functional classes of toxins are presented as percentage of identified toxin-like transcripts.
2.3.2. Tissue-Specific Expression of Toxin-Like Transcripts
At least one toxin-like transcript was differentially expressed across tentacle types for all species. Mirroring the pattern observed for all transcripts, the number of toxin-like transcripts differentially expressed across tentacle types was greatly reduced in D. cf. armata and M. doreensis compared to all other species. Only a single toxin-like transcript was found to be significantly differentially expressed (4-fold, false discovery rate [FDR] < 0.05) between inner and outer tentacles. The toxin-like transcript that was upregulated in the inner tentacles of D. cf. armata has high sequence similarity to nematocyst expressed protein 6 (K7Z9Q9), a member of the peptidase M12A toxin family (Figure S2a). Conversely, in M. doreensis, the toxin-like transcript had a significant BLASTp hit to δ-actitoxin-Amc1a (P69929), a boundless beta-hairpin type sea anemone neurotoxin (Figure S2b) [37]. Therefore, only minimal differential expression of toxins is observed in actiniarian species examined here whose tentacles are characterised by a difference in size rather than morphology.
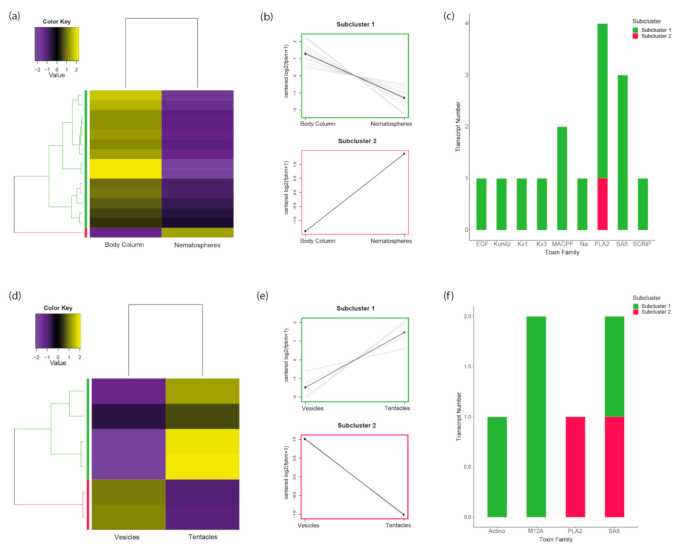
In C. adhaesivum, 15 toxin-like transcripts were differentially expressed between the body column and nematospheres (Figure 4a–c). Cutting the dendrogram at 50% of its height led to partitioning of the toxin-like transcripts into two subclusters: 14 transcripts were upregulated in the body column (subcluster 1) and one transcript was upregulated in nematospheres (subcluster 2). Eight toxin families were upregulated only in the body column, including multiple MACPF-like toxins. 57% of these toxin-like transcripts were neurotoxins or toxins with unknown function. Several genes from the phospholipase A2 family were upregulated in both the body column and nematospheres of this individual. Thus, a more diverse profile of toxins is upregulated in the body column and the toxin significantly upregulated in the nematospheres is enzymatic in nature.
Figure 4.
Toxin expression profiles of tentacular structures in C. adhaesivum and P. semoni: (a) Heatmap of differentially expressed toxin genes (centered fragments per kilobase of transcript per million mapped [FPKM] values) for C. adhaesivum; (b) Plot of the expression profile for each subcluster of differentially expressed toxin-like transcripts for C. adhaesivum; (c) Bar plot of toxin family distribution across subclusters for C. adhaesivum; (d) Heatmap of differentially expressed toxin genes (centered FPKM values) for P. semoni; (e) Plot of the expression profile for each subcluster of differentially expressed toxin-like transcripts for P. semoni; (f) Bar plot of toxin family distribution across subclusters for P. semoni. Abbreviations: Actino = Actinoporin; EGF = endothelial growth factor domain peptide; Kunitz = Venom Kunitz-type; Kv1 = Sea anemone type 1 potassium channel toxin; Kv3 = Sea anemone type 3 (BDS) potassium channel toxin; M12A= Peptidase M12A; MACPF= Membrane-attack complex/perforin; Na = Sea anemone sodium channel inhibitory toxin; PLA2 = Phospholipase A2; SA8 = Sea anemone 8 toxin; SCRiP= Cnidaria small cysteine-rich protein.
In the single P. semoni specimen examined, six toxin-like transcripts were differentially expressed between the true tentacles and vesicles (Figure 4d–f). When cut at 50% of its height, the hierarchically clustered gene tree splits into two subclusters: four transcripts were upregulated in tentacles (subcluster 1) and two transcripts were upregulated in vesicles (subcluster 2). Within the tentacles, toxin families associated with auxiliary, membrane-active and unknown functions were found to be upregulated. Toxin-like transcripts consistently upregulated only in vesicles have homology to phospholipase A2. Only the sea anemone 8 toxin family is upregulated in both the vesicles and tentacles. Overall, these findings reinforce that toxin expression is more likely to diverge when substantial morphological variation is present among tentacular structures.
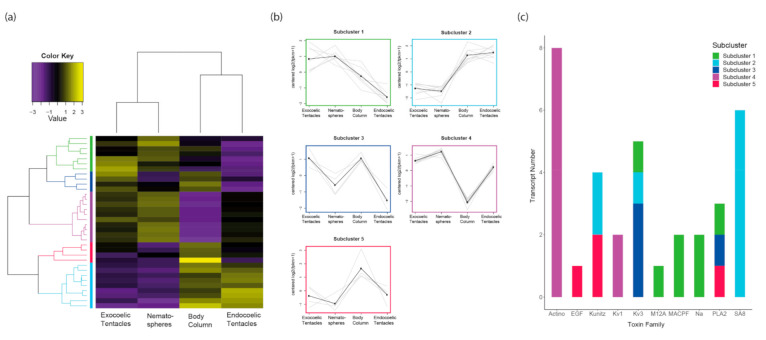
In H. hemprichii, 34 toxin-like transcripts were differentially expressed across the four morphological features: body column, endocoelic tentacles, exocoelic tentacles and nematospheres (Figure 5). A high degree of similarity is observed between exocoelic tentacles and nematospheres, even when examining only transcripts with significant homology to known toxins. Unlike the heatmap for all transcripts, the body column clusters with the endocoelic tentacles when considering only expression of toxin-like transcripts. This suggests that while the body column and endocoelic tentacles are distinct anatomical regions, the venom from both structures may serve similar ecological functions.
Figure 5.
Toxin expression profiles of tentacle structures in H. hemprichii: (a) Heatmap of differentially expressed toxin genes (centered RPKM values) for H. hemprichii; (b) Plot of the expression profile for each subcluster of differentially expressed toxin-like transcripts for H. hemprichii; (c) Bar plot of toxin family distribution across subclusters for H. hemprichii. Abbreviations: Actino = Actinoporin; EGF = endothelial growth factor domain peptide; Kunitz = Venom Kunitz-type; Kv1 = Sea anemone type 1 potassium channel toxin; Kv3 = Sea anemone type 3 (BDS) potassium channel toxin; M12A= Peptidase M12A; MACPF= Membrane-attack complex/perforin; Na = Sea anemone sodium channel inhibitory toxin; PLA2 = Phospholipase A2; SA8 = Sea anemone 8 toxin; SCRiP= Cnidaria small cysteine-rich protein.
Furthermore, a relatively small number of toxin-like transcripts was differentially expressed between the body column and endocoelic tentacles of this individual (10). More toxin-like transcripts were differentially expressed between endocoelic tentacles and nematospheres (15) than between endocoelic tentacles and exocoelic tentacles (13). Overall, the highest number of differentially expressed toxin-like transcripts was observed when comparing the body column and nematospheres (24), and the lowest when comparing exocoelic tentacles and nematospheres (1).
Cutting the dendrogram at 50% of its height led to partitioning of the toxin-like transcripts into five subclusters with the following expression patterns: upregulated in exocoelic tentacles and nematospheres (subcluster 1, seven transcripts); upregulated in the body column and endocoelic tentacles (subcluster 2, nine transcripts); upregulated in exocoelic tentacles and body (subcluster 3, 4 transcripts); upregulated in all tentacle types (subcluster 4, 10 transcripts); and upregulated in the body column (subcluster 5, four transcripts).
When examining the subclusters in conjunction with the biological function of toxins, a pattern emerged in that a single functional category was generally not present in more than two subclusters. The exceptions to this were neurotoxins and mixed function enzymes, which were present in four and three subclusters, respectively. Interestingly, auxiliary function toxin-like transcripts were only found in cluster 5, while the two membrane-active toxin families were consistently upregulated across exocoelic tentacles and nematospheres. Further interrogating the differential expression data, we established that 5, 3, 2, 2, and 3 toxin protein families were found in subclusters 1, 2, 3, 4 and 5, respectively. Additionally, many toxin families were found to be isolated within a single subcluster. Sea anemone type 3 (BDS) potassium channel and phospholipase A2 toxins were found in three subclusters, the most of any toxin family. Thus, most toxin families are associated with distinct expression patterns across the envenomating structures examined in H. hemprichii.
3. Discussion
3.1. Tissue-Specific Toxin Expression Profiles of Tentacles Show Greater Divergence When Morphological Variation Is Present
Differential expression of toxins between discrete anatomical regions has been established in the actiniarian families Actiniidae, Actinodendridae and Stichodactylidae [14,15]. Likewise, we report the differential expression of toxins between the body column and tentacles in a species from Thalassianthidae. Further building on this, we also report that toxin expression can vary significantly across morphologically disparate tentacle types. Comparing expression profiles of morphologically similar inner and outer tentacles, only a single toxin-like transcript was found upregulated for each species from Actiniidae. The inner and outer tentacles of D. cf. armata and M. doreensis are simple unbranched tentacles, differentiated only by size, indicating that morphologically similar tentacles have comparable toxin profiles. As different cnidae type are associated with different venom profiles in cnidarians [13,38], the minimal differences in toxin expression across inner and outer tentacles may suggest that the cnidome does not vary significantly across these tentacles within D. cf. armata and M. doreensis. However, more pronounced differences in gene expression are observed when substantial morphological variation exists within tentacles.
Members of Thalassianthidae possess three morphologically distinct types of tentacles: endocoelic tentacles, exocoelic tentacles and nematospheres [26]. Endocoelic and exocoelic tentacles are branched outgrowths located in rows radiating out from the mouth and along the margin of the oral disc, respectively [26,31,32]. Nematospheres are a specialised form of endocoelic tentacles, characterised by a spherical morphology and high nematocyst density, observed exclusively in Thalassianthidae [26]. Comparison of toxin expression across morphologically distinct tentacle types in one H. hemprichii individual indicates that endocoelic tentacles, exocoelic tentacles and nematospheres are each associated with a unique toxin profile. Differences in toxin expression between endocoelic and the other tentacle types is more pronounced than between exocoelic tentacles and nematospheres. Tissue-specific toxin expression with similar profiles have been observed between functionally similar structures, such as acrorhagi and tentacles in A. tenebrosa [14,15]. Given the high degree of similarity in their toxin expression, it is likely that venom produced by exocoelic tentacles and nematospheres fulfils similar functional demands in H. hemprichii.
3.2. Ecological Significance of Tentacular Structures, and Its Relationship with Toxin Expression Profiles
The location of nematospheres and exocoelic tentacles at the oral disc margin may provide insight into some of the functional demands of these two tentacle types. Actiniarians are soft-bodied creatures that rely on venom for multiple ecological functions, including defence against predators [9,25]. Nevertheless, behavioural responses, such as the retraction of tentacles, can also offer protection from predation [28]. In many actiniarian species, prolonged contact with the nudibranch species Berghia stephania resulted in fully retracted tentacles being concealed by the sphincter [28,39]. However, concealment of tentacles is not possible in Anemonia sulcata due to anatomical differences [39] and is unlikely to occur in a species with an expanded oral disc, such as C. adhaesivum and H. hemprichii. However, it has been observed that the oral disc of C. adhaesivum expands and contracts in response to light conditions. When exposed to low light conditions, the oral disc folds in on itself, with the region bearing nematospheres curling towards the mouth and concealing the majority of endocoelic tentacles [40]. Therefore, it stands to reason that venom from nematospheres and exocoelic tentacles must contain toxins which would deter predators, possibly through pain induction [41].
Similarly, vesicles and pseudotentacles in species from Aliciidae are predicted to have a major role in predator deterrence. During the day, tentacles are retracted in P. semoni and offer no protection against predators [35,36]. Conversely, vesicles and pseudotentacles extend in response to light exposure, obscuring the retracted tentacles [36], and therefore these are the structures that predators are most likely to come into contact with. While technically not a tentacle structure, as they arise from the body column [26], these “tentacle-like” structures appear to be primarily responsible for defence against predators, a function typically assigned to true tentacles. However, P. semoni participates in predatory feeding behaviour at night when the tentacles are extended [35,36], so venom from the tentacle-like structures is unlikely to play a major role in prey capture. The significant differences in the toxin expression profiles of P. semoni tentacle and tentacle-like structures can be explained by the discrete key roles of these structures in predatory and defensive behaviour, respectively. Thus, nematospheres and vesicles share a common functional role in defence, but these structures are taxonomically restricted within two different actiniarian superfamilies (Actinioidea and Metridioidea, respectively) [26,42] and likely differ in their venom content and anatomy.
Phylogenetic analysis conducted by Crowther [26] demonstrated that the spherical defensive structures (nematospheres and vesicles) present in Thalassianthidae and Aliciidae are superficially similar but not homologous structures. The nematocyst content of the nematospheres and vesicles has been found to differ [26] and we report that very few toxin families are upregulated across both morphological features. Even within the same structure across different species, toxin expression profiles differ substantially in actiniarians [15]. Within nematospheres of C. adhaesivum we find an enzymatic toxin family upregulated. Conversely, in H. hemprichii, membrane-active toxins and neurotoxins are the predominant functional classes of toxins significantly upregulated in the nematospheres compared to other structures. Variation in the cnidome of tentacular structures between species may account for these differences, with different cnidae types associated with different venom profiles in cnidarians [13,38]. Basitrichs are common across the tentacles of C. adhaesivum and H. hemprichii but vary in size, with the largest basitrichs observed in the nematospheres [26,43,44]. While microbasic p-mastigophores occur in endocoelic tentacles of both species, they are abundant in C. adhaesivum but rare in H. hemprichii [26,44,45]. Additionally, greater diversity of cnidae is present in the exocoelic tentacles of C. adhaesivum relative to H. hemprichii [26,44]. These differences in the type, size, and abundance of cnidae probably all contribute to differences in the toxin profile of a tentacular structure across species, but the correlation between the cnidome of tentacular structures and their toxin profiles needs to be directly examined in future research. Therefore, while both nematospheres and vesicles function as defensive structures, the toxin cocktails they utilise differ between the two structures and even within a single structure across species.
3.3. Unique Toxin Arsenals Are Required by Structures That Defend Endosymbiont-Hosting Structures
Regionalisation of venom production has been observed in other venomous taxa, but in all instances there is an underlying functional basis for producing multiple venoms with distinct compositions. For example, the assassin bug Pristhesancus plagipennis and the cone snail Conus geographus produce distinct venoms specialised for predation and defence [46,47]. Differences in toxin expression across sweeper and feeding tentacles in corals can likewise be explained by the discrete roles of these two structures in intraspecific combat and prey capture, respectively [24]. While ecological interactions have minimal impact on the toxin gene complement in cnidarian species, they are known to function as keys drivers of toxin gene expression [14,25,48,49]. Consequently, understanding which ecological factors have driven C. adhaesivum, H. hemprichii and P. semoni to possess morphologically diverse tentacular structures will provide insights into the functional basis of the tentacle-specific toxin expression profiles observed in the current study.
Crowther demonstrated that convergent morphological adaptations can be observed in distantly related sea anemone families due to similar ecological demands [26]. Actiniarians are predominantly predatory, although many also rely upon zooxanthellae, intracellular algae symbionts, for nutrients [28]. Branched outgrowths, such as endocoelic tentacles and pseudotentacles, increase surface area, and thereby enable sea anemones to house more zooxanthellae and increase their photosynthetic capacity by increasing access to light [36]. Unfortunately, the H. hemprichii specimen used to generate the transcriptomic dataset had bleached, but the enrichment of photosynthetic-related gene GO terms in the nematospheres of C. adhaevisum supports the presence of zooxanthellae in this structure. The endoderm of tentacles in C. adhaevisum and H. hemprichii has previously been noted to contain a high proportion of zooxanthellae compared to other regions [26]. Zooxanthellae are similarly reported to be dense in the pseudotentacles of Aliciidae species [26] and photosynthetic-related GO terms were found to be enriched in the vesicles of P. semoni. Therefore, morphological diversity of tentacles supports hosting of endosymbionts in the families Thalassianthidae and Aliciidae.
Defensive spheres have convergently evolved at least three times in Actiniaria [26], resulting in nematospheres in Thalassianthidae, vesicles in Aliciidae and Boloceroididae, and acrospheres in Actinodendridae and Haloclavidae [26,50,51]. Species from the Aliciidae and Actinodendridae families can pose a significant health risk to humans, with envenomation by P. semoni and Actinodendron plumosum resulting in severe skin ulceration [52,53,54,55,56]. The painful sting of species from Actinodendridae is why they are collectively known as hell’s fire anemones [57,58], while an instance of death following envenomation by P. semoni has been reported [59]. The potent venom associated with nematospheres and vesicles, which is likely utilised to deter vertebrate predators, in combination with the exposed position of these structures when the oral disc contracts and tentacles retract, ensures these Aliciidae and Thalassianthidae are well armed against the threat of predation. However, the morphology and unique toxin profiles of these structures also serves to protect tissue where endosymbionts are housed in C. adhaesivum, H. hemprichii and P. semoni, and thereby protects a dependable energy source. Thus, hosting zooxanthellae appears to underpin both the morphological variation in tentacular structures and the distinct toxin profiles associated with each tentacular subtype in the subtidal species examined.
Acrospheres are swellings of the tentacles, characterised by a unique cnidome, which are present in orders Actiniaria and Corallimorpharia [26,50,51,60,61]. Within actiniarians, acrospheres represent a third spherical tentacle structure specialised for defence and can be observed in Actinodendron species and the haloclavid genera Anemonactis and Haloclava [26,51]. Additionally, the genus Telmatactis is defined by club-like swellings at the distal portion of tentacles [62] and, while not classified as acrospheres, these club-tips may serve a defensive function. Given the patterns of toxin expression of nematospheres and vesicles observed in the current study, acrospheres and indeed other morphologic variants of tentacles may also be associated with unique toxin profiles driven by underlying functional demands of the structures and therefore warrant further investigation. Obtaining multiple specimens of C. adhaesivum, H. hemprichii and P. semoni proved challenging and thus we were unable to verify findings across individuals from the same species. Future research should employ biological replicates to determine whether the tissue-specific toxin expression profiles associated with tentacular structures is subject to intraspecific variability. Furthermore, investigating the phylogenetic relationship of toxin families differentially expressed across tentacular structures may also provide greater insights into venom diversity within order Actiniaria.
4. Materials and Methods
4.1. Sample Preparation and Sequencing
4.1.1. Heterodactyla hemprichii, Cryptodendrum adhaesivum and Phyllodiscus semoni
Heterodactyla hemprichii, Cryptodendrum adhaesivum and Phyllodiscus semoni were collected by Cairns Marine Pty Ltd. from the Great Barrier Reef, QLD, Australia and housed in holding tanks at the Queensland University of Technology under standard aquarium conditions [63]. Following a 72-h starvation period, anatomically distinct structures were dissected from a single individual for each species, flash frozen and stored at 80 °C for transcriptome sequencing. For H. hemprichii, tissue from the body column, endocoelic tentacles, exocoelic tentacles and nematospheres was isolated (Figure 1e). Tissue from the body column, endocoelic tentacles and nematospheres was also isolated from C. adhaesivum (Figure 1c). For P. semoni, tissue was isolated from the tentacles, pseudotentacles and vesicles (Figure 1g). However, due to the small size of the individual, the pseudotentacles and vesicles were dissected together and not separated for RNA extraction.
Total RNA was extracted from homogenised tissue using the TRIzol/chloroform protocol [64] followed by purification with an ISOLATE II RNA mini kit (Bioline, Sydney, Australia). RNA integrity and quantity were determined using a Bioanalyser 2100 (Agilent, Santa Clara, CA, USA) and NanoDrop 2000 Spectrophotometer (Thermo Fisher Scientific, Carlsbad, CA, USA). Sequencing libraries for H. hemprichii were prepared using a TruSeq RNA Library Prep Kit v2 (Illumina, San Diego, CA, USA) and sequenced using 75 bp single-end chemistry on an Illumina NextSeq 500. Sequencing libraries for C. adhaesivum and P. semoni were prepared using TruSeq Stranded mRNA Library Prep (Illumina, San Diego, CA, USA) and sequenced using 150 bp paired-end chemistry on an Illumina HiSeq X10.
4.1.2. Dofleinia cf. armata
Dofleinia cf. armata (identified by sea anemone taxonomist M.L.M.) was collected from Southport, QLD, Australia under Queensland Museum permit 160782. After a 72-h starvation period, inner and outer tentacles (Figure 1a) from two individual specimens of Dofleinia cf. armata were collected and preserved in RNAlater (Thermo Fisher Scientific, Carlsbad, CA, USA). Preserved tissue was lysed using a TissueLyser II (Qiagen, Hilden, Germany) and total RNA extracted using a QIAGEN RNeasy Mini Kit. Extracted RNA with an integrity number (RIN) above 8.5 was used for library preparation. Individual cDNA libraries were prepared using PolyA-enriched RNA-seq stranded sample prep (Illumina, San Diego, CA, USA). Libraries were sequenced using 100 bp, paired-end sequencing on an Illumina HiSeq 2500. Preserved animals (QM G335858 & QM G335860) are lodged in the Queensland Museum. Full details of D. cf. armata extraction and sequencing methods have been published previously [65].
4.1.3. Macrodactyla doreensis
Two specimens of Macrodactyla doreensis were collected from North Stradbroke Island, QLD, Australia under the permit QS2014/MAN259 and kept in aquaria at The University of Queensland. Tissue from inner and outer tentacles from two individual specimens was collected with tweezers and flash frozen before total RNA was extracted using TRIzol (Thermo Fisher Scientific, Carlsbad, CA, USA) and enriched for mRNA using a DynaBeads Direct mRNA kit (Thermo Fisher Scientific, Carlsbad, CA, USA) as described [66]. cDNA libraries for each sample were then prepared using a TruSeq library kit (Illumina, San Diego, CA, USA) and sequenced on an Illumina NextSeq 500, using 150 bp paired-end chemistry.
4.2. Transcriptome Assembly
Raw reads were quality checked using FastQC (http://www.bioinformatics.babraham.ac.uk/projects/fastqc/, accessed on 23 March 2018) and tissue-specific reads were pooled prior to transcriptome assembly for each species. Sequence reads were filtered (Q > 30, N < 1%), with non-biological sequences and low-quality reads removed using Trimmomatic [67]. The Trinity short read de novo assembler v2.5.1 was used to assemble the remaining high-quality reads into contigs using default parameters for the specific sequencing libraries, with strand-specific RNA-seq read orientation provided for stranded assemblies [68].
Post-assembly, redundant contigs (≥98% similarity) were removed using CD-Hit EST v4.6.4 [69]. BUSCO v3.0.1 was used to assess the completeness of each assembly [70]. Additionally, Trinity scripts were used to compute contig N50 and Ex90N50 statistics for all assemblies.
M. doreensis and species from the families Thalassianthidae (C. adhaesivum and H. hemprichii) and Aliciidae (P. semoni) are known to contain symbiotic dinoflagellate algae [26,35,71]. Therefore, the PsyTrans Python script (https://github.com/sylvainforet/psytrans, accessed on 5 April 2017) was employed to remove potential symbiont contamination from the assembled transcriptomes of M. doreensis and H. hemprichii. Host reference sequences were derived from the Nematostella vectensis genome proteins models [72]. The combined genome protein models of Symbiodinium microadriaticum, S. minutum and S. kawagutii [73,74,75] served as symbiont reference sequences. Default script settings were used to analyse the sea anemone transcriptomes, except for the following script modifications; maxBestEvalue = 1e-30, numberOfSeq = minimum blast hit count, Line 55: Training Proportion = 1.0, Line 566: if nSeqs < options.args.numberOfSeq:, Line 572: for i in xrange(trainingMaxIdx, options.args.numberOfSeq):.
4.3. Tissue-Specific Expression Profiles
4.3.1. Functional Annotation of Transcripts
Functional annotation was conducted using the Trinotate protocol. Open reading frames (ORFs) encoding more than 65 amino acid residues were extracted from assemblies using TransDecoder 3.0.1 [76]. To determine homology to known proteins, translated ORFs were used as BLASTp queries against the UniProt database (accessed on 2 June 2020). Additionally, contigs were used as BLASTx queries against the UniProt database. Significant BLAST hits were defined by a threshold e-value of 1 × E−5. Protein family (Pfam) domains were identified using HMMER 3.1b2 software [77]. Using Trinotate [76], GO terms associated with both BLAST and Pfam domain hits were generated.
4.3.2. Differential Expression Analysis and Gene Ontology Enrichment Analysis
Bowtie2 was used to align raw reads to assembled contigs [78], followed by transcript quantification with RSEM 1.2.30, using a strand-specific flag for C. adhaesivum and P. semoni [79]. Differential gene expression was calculated using RSEM outputs and the edgeR Bioconductor package v3.3.1, with the dispersion value set to 0.1 when no biological replicates were present [80]. Using the Trinity suite, differentially expressed transcripts (4-fold, FDR ≤ 0.05) were extracted and clustered according to expression patterns [76]. These transcripts were then partitioned into subclusters by cutting the hierarchically clustered tree at 50% height of the tree [76].
GO enrichment analysis was performed by running GOSeq on significantly differentially expressed transcripts [81]. Significantly enriched GO terms for each structure (FDR < 0.05) were then summarised using REVIGO with SimRel semantic similarities (0.5 allowed similarity) [82].
4.4. Analysis of Toxin-Like Transcripts
Transcripts with homology to known toxins were identified from BLASTp searches against the UniProt database by filtering for transcripts annotated with the ‘toxin activity’ GO term (GO:0090729), excluding bacterial toxins. To limit results to lineage-specific toxin-like transcripts, the translated ORFs generated by TransDecoder were then used as BLASTp queries against database of cnidarian toxins from ToxProt (accessed on 21 April 2021) using BLAST+ 2.3.0. Output from edgeR was then filtered to include only toxin-like transcripts and using Trinity PtR, heatmaps were generated and used to visualise differentially expressed toxin-like transcripts. Protein family designations for toxin-like transcripts were assigned using data available in the UniProt database and toxin families were further categorised by biological function as in previous work [83,84].
Acknowledgments
The authors would like to thank the QUT Marine group for their help and advice caring for the animals. The authors would like to acknowledge QUT Molecular Genetics Research facility (MGRF) for use of their facilities. Computational resources and services used in this work were provided by the High Performance Computing and Research Support Group, Queensland University of Technology, Brisbane, Australia, and the High Performance Computing Facilities (MILTON) at Walter and Eliza Hall Institute of Medical Research (WEHI), Melbourne, Australia.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/toxins13070452/s1, Figure S1: Heatmap of differentially expressed genes (centered FPKM values) for C. adhaesivum and P. semoni, Figure S2: Patterns of differentially expressed toxins for D. cf. armata and M. doreensis, Table S1: Sequence read archive and BioSample accession numbers.
Author Contributions
Conceptualization, L.M.A. and P.J.P.; methodology, L.M.A.; validation, L.M.A.; formal analysis, L.M.A.; investigation, L.M.A., M.L.M. and B.M.; resources, L.M.A., M.L.M. and B.M.; data curation, L.M.A.; writing—original draft preparation, L.M.A., M.L.M. and P.J.P.; writing—review and editing, L.M.A., M.L.M., B.M., D.A.H., G.F.K., E.A.B.U., R.S.N. and P.J.P.; visualization, L.M.A.; supervision, D.A.H., G.F.K., E.A.B.U., R.S.N. and P.J.P.; project administration, L.M.A.; funding acquisition, G.F.K., E.A.B.U., R.S.N. and P.J.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded in part by ARC Linkage Grants LP150100621 and LP140100832. L.M.A. received an Australian Government Research Training Program Scholarship. M.L.M. received an Australian Government Research Training Program Scholarship, Monash Medicinal Chemistry Faculty Scholarship and Monash University-Museum Victoria Scholarship top-up. R.S.N. received fellowship support from the Australian National Health and Medical Research Council (NHMRC). G.F.K. is supported by Principal Research fellowship APP1136889 from the NHMRC. E.A.B.U. is supported by a Norwegian Research Council FRIPRO-YRT Fellowship no. 287462.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Raw reads for C. adhaesivum, H. hemprichii, M. doreensis and P. semoni are available at the NCBI SRA under BioProject PRJNA715506. Accession numbers for C. adhaesivum, H. hemprichii, M. doreensis and P. semoni can be viewed in Supplementary Materials. Raw reads for D. cf. armata are registered in EMBL-ENA under project accession number PRJEB42903 and ENA sample runs as follows: DA4ITa—ERXS6629214, DA4OTc—ERS6629215, DA5IT—ERS6629216 and DA5OT—ERS6629217.
Conflicts of Interest
The authors declare no conflict of interest.
Key Contribution
We find that morphologically specialised tentacular structures possess structure-specific toxin profiles that may underpin their ecological roles and venom diversity within order Actiniaria.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Augusto-de-Oliveira C., Stuginski D.R., Kitano E.S., Andrade-Silva D., Liberato T., Fukushima I., Serrano S.M., Zelanis A. Dynamic rearrangement in snake venom gland proteome: Insights into Bothrops jararaca intraspecific venom variation. J. Proteome Res. 2016;15:3752–3762. doi: 10.1021/acs.jproteome.6b00561. [DOI] [PubMed] [Google Scholar]
- 2.Dowell N.L., Giorgianni M.W., Kassner V.A., Selegue J.E., Sanchez E.E., Carroll S.B. The deep origin and recent loss of venom toxin genes in rattlesnakes. Curr. Biol. 2016;26:2434–2445. doi: 10.1016/j.cub.2016.07.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goncalves-Machado L., Pla D., Sanz L., Jorge R.J.B., Leitao-De-Araujo M., Alves M.L.M., Alvares D.J., De Miranda J., Nowatzki J., de Morais-Zani K., et al. Combined venomics, venom gland transcriptomics, bioactivities, and antivenomics of two Bothrops jararaca populations from geographic isolated regions within the Brazilian Atlantic rainforest. J. Proteom. 2016;135:73–89. doi: 10.1016/j.jprot.2015.04.029. [DOI] [PubMed] [Google Scholar]
- 4.Huang H.W., Liu B.S., Chien K.Y., Chiang L.C., Huang S.Y., Sung W.C., Wu W.G. Cobra venom proteome and glycome determined from individual snakes of Naja atra reveal medically important dynamic range and systematic geographic variation. J. Proteom. 2015;128:92–104. doi: 10.1016/j.jprot.2015.07.015. [DOI] [PubMed] [Google Scholar]
- 5.Lyons K., Dugon M.M., Healy K. Diet breadth mediates the prey specificity of venom potency in snakes. Toxins. 2020;12:74. doi: 10.3390/toxins12020074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Massey D.J., Calvete J.J., Sanchez E.E., Sanz L., Richards K., Curtis R., Boesen K. Venom variability and envenoming severity outcomes of the Crotalus scutulatus scutulatus (Mojave rattlesnake) from Southern Arizona. J. Proteom. 2012;75:2576–2587. doi: 10.1016/j.jprot.2012.02.035. [DOI] [PubMed] [Google Scholar]
- 7.Mitchell M.L., Tonkin-Hill G.Q., Morales R.A., Purcell A.W., Papenfuss A.T., Norton R.S. Tentacle transcriptomes of the speckled anemone (Actiniaria: Actiniidae: Oulactis sp.): Venom-related components and their domain structure. Mar. Biotechnol. 2020:1–13. doi: 10.1007/s10126-020-09945-8. [DOI] [PubMed] [Google Scholar]
- 8.Jouiaei M., Yanagihara A.A., Madio B., Nevalainen T.J., Alewood P.F., Fry B.G. Ancient venom systems: A review on cnidaria toxins. Toxins. 2015;7:2251–2271. doi: 10.3390/toxins7062251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schendel V., Rash D.L., Jenner A.R., Undheim A.B.E. The diversity of venom: The importance of behavior and venom system morphology in understanding its ecology and evolution. Toxins. 2019;11:666. doi: 10.3390/toxins11110666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Daly M. Functional and genetic diversity of toxins in sea anemones. In: Malhotra A., editor. Evolution of Venomous Animals and Their Toxins. Springer; Dordrecht, The Netherlands: 2017. pp. 87–104. [DOI] [Google Scholar]
- 11.Fautin D. Structural diversity, systematics, and evolution of cnidae. Toxicon. 2009;54:1054–1064. doi: 10.1016/j.toxicon.2009.02.024. [DOI] [PubMed] [Google Scholar]
- 12.Watson G.M., Hessinger D.A. Cnidocyte mechanoreceptors are tuned to the movements of swimming prey by chemoreceptors. Science. 1989;243:1589–1591. doi: 10.1126/science.2564698. [DOI] [PubMed] [Google Scholar]
- 13.Columbus-Shenkar Y.Y., Sachkova M.Y., Macrander J., Fridrich A., Modepalli V., Reitzel A.M., Sunagar K., Moran Y. Dynamics of venom composition across a complex life cycle. eLife. 2018;7:e35014. doi: 10.7554/eLife.35014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Surm J.M., Smith H.L., Madio B., Undheim E.A., King G.F., Hamilton B.R., Van der Burg C.A., Pavasovic A., Prentis P.J. A process of convergent amplification and tissue-specific expression dominates the evolution of toxin and toxin-like genes in sea anemones. Mol. Ecol. 2019;28:2272–2289. doi: 10.1111/mec.15084. [DOI] [PubMed] [Google Scholar]
- 15.Macrander J., Broe M., Daly M. Tissue-specific venom composition and differential gene expression in sea anemones. Genome Biol. Evol. 2016;8:2358–2375. doi: 10.1093/gbe/evw155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Purcell J.E. Aggresive function and induced development of catch tentacles in the sea anemone Metridium Senile (Coelenterata, Actiniaria) Biol. Bull. 1977;153:355–368. doi: 10.2307/1540441. [DOI] [Google Scholar]
- 17.Fukui Y. Catch tentacles in the sea anemone Haliplanella luciae. Mar. Biol. 1986;91:245–251. doi: 10.1007/BF00569440. [DOI] [Google Scholar]
- 18.Watson G.M., Mariscal R.N. Comparative ultrastructure of catch tentacles and feeding tentacles in the sea anemone Haliplanella. Tissue Cell. 1983;15:939–953. doi: 10.1016/0040-8166(83)90059-9. [DOI] [PubMed] [Google Scholar]
- 19.Williams R.B. Acrorhagi, catch tentacles and sweeper tentacles: A synopsis of aggression of actiniarian and scleractinian cnidaria. Hydrobiologia. 1991;216:539–545. doi: 10.1007/BF00026511. [DOI] [Google Scholar]
- 20.Chornesky E.A. Induced development of sweeper tentacles on the reef coral Agaricia agaricites: A response to direct competition. Biol. Bull. 1983;165:569–581. doi: 10.2307/1541466. [DOI] [PubMed] [Google Scholar]
- 21.Lapid E.D., Chadwick N.E. Long-term effects of competition on coral growth and sweeper tentacle development. Mar. Ecol. Prog. Ser. 2006;313:115–123. doi: 10.3354/meps313115. [DOI] [Google Scholar]
- 22.Sebens K.P., Miles J.S. Sweeper tentacles in a Gorgonian octocoral: Morphological modifications for interference competition. Biol. Bull. 1988;175:378–387. doi: 10.2307/1541729. [DOI] [Google Scholar]
- 23.Wellington G.M. Reversal of digestive interactions between Pacific reef corals: Mediation by sweeper tentacles. Oecologia. 1980;47:340–343. doi: 10.1007/BF00398527. [DOI] [PubMed] [Google Scholar]
- 24.Yosef O., Popovits Y., Malik A., Ofek-Lalzer M., Mass T., Sher D. A tentacle for every occasion: Comparing the hunting tentacles and sweeper tentacles, used for territorial competition, in the coral Galaxea fascicularis. BMC Genom. 2020;21:548. doi: 10.1186/s12864-020-06952-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ashwood L.M., Norton R.S., Undheim E.A., Hurwood D.A., Prentis P.J. Characterising functional venom profiles of anthozoans and medusozoans within their ecological context. Mar. Drugs. 2020;18:202. doi: 10.3390/md18040202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Crowther A. Ph.D. Dissertation. University of Kansas; Lawrence, KS, USA: 2013. Character Evolution in Light OF Phylogenetic Analysis and Taxonomic Revision of the Zooxanthellate Sea Anemone Families Thalassianthidae and Aliciidae. [Google Scholar]
- 27.Daly M. The anatomy, terminology, and homology of acrorhagi and pseudoacrorhagi in sea anemones. Zool. Verh. 2003:89–102. [Google Scholar]
- 28.Shick J.M. A Functional Biology of Sea Anemones. Chapman & Hall; London, UK: 1991. [Google Scholar]
- 29.Song J.-I., Cha H.-R. Taxonomy of Actiniidae (Anthozoa, Actiniaria, Thenaria, Endomvaria) from Korea. Anim. Syst. Evol. Divers. 2002;18:253–270. [Google Scholar]
- 30.Carlgren O.H. A Survey of the Ptychodactiaria, Corallimorpharia and Actiniaria. Kungl. Sven. Vetensk. Handl. 1949;1:1–122. [Google Scholar]
- 31.Attaran-Fairman G., Javid P., Shakouri A. Morphology and phylogeny of the sea anemone Stichodactyla haddoni (Cnidaria: Anthozoa: Actiniaria) from Chabahar Bay, Iran. Turk. Zool. Derg. 2015;39:998–1003. doi: 10.3906/zoo-1310-4. [DOI] [Google Scholar]
- 32.Ardelean A. Reinterpretation of some tentacular structures in actinodendronid and thalassianthid sea anemones (Cnidaria: Actiniaria) Zool. Verh. 2003:31–40. [Google Scholar]
- 33.Ardelean A., Fautin D. Variability in nematocysts from a single individual of the sea anemone Actinodendron arboreum (Cnidaria: Anthozoa: Actiniaria) Hydrobiologia. 2004;530:189–197. doi: 10.1007/s10750-004-2662-8. [DOI] [Google Scholar]
- 34.Fautin D.G., Allen G.R. Field Guide to Anemonefishes and Their Host sea Anemones. Western Australian Museum; Perth, Australia: 1992. [Google Scholar]
- 35.Hoeksema B.W., Crowther A.L. Masquerade, mimicry and crypsis of the polymorphic sea anemone Phyllodiscus semoni and its aggregations in South Sulawesi. Contrib. Zool. 2011;80:251–268. doi: 10.1163/18759866-08004003. [DOI] [Google Scholar]
- 36.Gladfelter W.B. Sea anemone with zooxanthellae: Simultaneous contraction and expansion in response to changing light intensity. Science. 1975;189:570. doi: 10.1126/science.189.4202.570. [DOI] [PubMed] [Google Scholar]
- 37.Osmakov D.I., Kozlov S.A., Andreev Y.A., Koshelev S.G., Sanamyan N.P., Sanamyan K.E., Dyachenko I.A., Bondarenko D.A., Murashev A.N., Mineev K.S., et al. Sea anemone peptide with uncommon β-hairpin structure inhibits acid-sensing ion channel 3 (ASIC3) and reveals analgesic activity. J. Biol. Chem. 2013;288:23116–23127. doi: 10.1074/jbc.M113.485516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McClounan S., Seymour J. Venom and cnidome ontogeny of the cubomedusae Chironex fleckeri. Toxicon. 2012;60:1335–1341. doi: 10.1016/j.toxicon.2012.08.020. [DOI] [PubMed] [Google Scholar]
- 39.Edmunds M., Potts G.W., Swinfen R.C., Waters V.L. Defensive behaviour of sea anemones in response to predation by the opisthobranch mollusc Aeolidia papillosa (L.) J. Mar. Biol. Assoc. UK. 1976;56:65–83. doi: 10.1017/S0025315400020440. [DOI] [Google Scholar]
- 40.Ho G. Cryptodendrum Adhaesivum. [(accessed on 7 March 2021)]; Available online: https://www.gbri.org.au//SpeciesList//Cryptodendrumadhaesivum%7CGuenHo.
- 41.Casewell N.R., Wüster W., Vonk F.J., Harrison R.A., Fry B.G. Complex cocktails: The evolutionary novelty of venoms. Trends Ecol. Evol. 2013;28:219–229. doi: 10.1016/j.tree.2012.10.020. [DOI] [PubMed] [Google Scholar]
- 42.Rodríguez E., Barbeitos M.S., Brugler M.R., Crowley L.M., Grajales A., Gusmão L., Häussermann V., Reft A., Daly M. Hidden among sea anemones: The first comprehensive phylogenetic reconstruction of the order Actiniaria (Cnidaria, Anthozoa, Hexacorallia) reveals a novel group of hexacorals. PLoS ONE. 2014;9:e96998. doi: 10.1371/journal.pone.0096998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dunn D.F. The clownfish sea anemones: Stichodactylidae (Coelenterata: Actiniaria) and other sea anemones symbiotic with Pomacentrid fishes. Trans. Am. Philos. Soc. 1981;71:3–115. doi: 10.2307/1006382. [DOI] [Google Scholar]
- 44.Carlgren O.H. Scientific Reports of the Great Barrier Reef Expedition 1928–1929. Volume 5. BM(NH); London, UK: 1950. Actiniaria and Corallimorpharia; pp. 427–457. [Google Scholar]
- 45.Carlgren O.H. A contribution to the knowledge of the structure and distribution of the cnidae in the Anthozoa. K. Fysiogr. Sällskapets Handl. 1940;51:1–62. [Google Scholar]
- 46.Dutertre S., Jin A., Vetter I., Hamilton B., Sunagar K., Lavergne V., Dutertre V., Fry B.G., Antunes A., Venter D.J. Evolution of separate predation-and defence-evoked venoms in carnivorous cone snails. Nat. Commun. 2014;5:3521. doi: 10.1038/ncomms4521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Walker A.A., Mayhew M.L., Jin J., Herzig V., Undheim E.A.B., Sombke A., Fry B.G., Meritt D.J., King G.F. The assassin bug Pristhesancus plagipennis produces two distinct venoms in separate gland lumens. Nat. Commun. 2018;9:1–10. doi: 10.1038/s41467-018-03091-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sachkova M.Y., Macrander J., Surm J.M., Aharoni R., Menard-Harvey S.S., Klock A., Leach W.B., Reitzel A.M., Moran Y. Some like it hot: Population-specific adaptations in venom production to abiotic stressors in a widely distributed cnidarian. BMC Biol. 2020;18:121. doi: 10.1186/s12915-020-00855-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.O’Hara E.P., Caldwell G.S., Bythell J. Equistatin and equinatoxin gene expression is influenced by environmental temperature in the sea anemone Actinia equina. Toxicon. 2018;153:12–16. doi: 10.1016/j.toxicon.2018.08.004. [DOI] [PubMed] [Google Scholar]
- 50.Izumi T., Yanagi K., Fujita T. Comprehensive revision of Anemonactis (Cnidaria: Anthozoa: Actiniaria: Haloclavidae) in Japan: Reestablishment of Anemonactis minuta (Wassilieff, 1908) comb. nov. and description of Anemonactis tohrui sp. nov. Mar. Biodivers. 2020;50:73. doi: 10.1007/s12526-020-01085-5. [DOI] [Google Scholar]
- 51.Rodríguez E., López-González P.J. Stephanthus antarcticus, a new genus and species of sea anemone (Actiniaria, Haloclavidae) from the South Shetland Islands, Antarctica. Helgol. Mar. Res. 2003;57:54–62. doi: 10.1007/s10152-002-0132-0. [DOI] [Google Scholar]
- 52.Mizuno M., Nishikawa K., Yuzawa Y., Kanie T., Mori H., Araki Y., Hotta N., Matsuo S. Acute renal failure after a sea anemone sting. Am. J. Kidney Dis. 2000;36:E10. doi: 10.1053/ajkd.2000.9006. [DOI] [PubMed] [Google Scholar]
- 53.Nakamoto M., Uezato H. Stings of box-jellyfish and sea anemones. Clin. Derm. 1998;52:29–33. [Google Scholar]
- 54.Brush D.E. Marine envenomations. In: Flomenbaum N.E., Goldfrank L.R., Hoffman R.S., Howland M.A., Lewin N.A., Nelson L.S., editors. Goldfrank’s Toxicologic Emergencies. 8th ed. McGraw-Hill Medical; New York, NY, USA: 2006. pp. 1629–1642. [Google Scholar]
- 55.Haddad V., Jr., Lupi O., Lonza J.P., Tyring S.K. Tropical dermatology: Marine and aquatic dermatology. J. Am. Acad. Derm. 2009;61:733–750. doi: 10.1016/j.jaad.2009.01.046. [DOI] [PubMed] [Google Scholar]
- 56.Ulrich H., Landthaler M., Vogt T. Aquatic dermatoses. J. Dtsch. Dermatol. Ges. 2008;6:133–146. doi: 10.1111/j.1610-0387.2007.06590.x. [DOI] [PubMed] [Google Scholar]
- 57.Randall J., Fautin D. Fishes other than anemonefishes that associate with sea anemones. Coral Reefs. 2002;21:188–190. doi: 10.1007/s00338-002-0234-9. [DOI] [Google Scholar]
- 58.Prentis P.J., Pavasovic A., Norton R.S. Sea anemones: Quiet achievers in the field of peptide toxins. Toxins. 2018;10:36. doi: 10.3390/toxins10010036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Erhardt H., Knop D. Corals: Indo-Pacific Field Guide. IKAN Unterwasserarchiv; Frankfurt, Germany: 2005. [Google Scholar]
- 60.Acuña F.H., Garese A. The cnidae of the acrospheres of the corallimorpharian Corynactis carnea (Studer, 1878) (Cnidaria, Corallimorpharia, Corallimorphidae): Composition, abundance and biometry. Belg. J. Zool. 2009;139:50–57. [Google Scholar]
- 61.Riemann-Zürneck K., Iken K. Corallimorphus profundus in shallow Antarctic habitats: Bionomics, histology, and systematics (Cnidaria: Hexacorallia) Zool. Verh. 2003;345:367–386. [Google Scholar]
- 62.den Hartog J.C. The genus Telmatactis Gravier, 1916 (Actiniaria: Acontiaria: Isophelliidae) in Greece and the eastern Mediterranean. Zool. Meded. 1995;69:153–176. [Google Scholar]
- 63.van der Burg C.A., Prentis P.J., Surm J.M., Pavasovic A. Insights into the innate immunome of actiniarians using a comparative genomic approach. BMC Genom. 2016;17:850. doi: 10.1186/s12864-016-3204-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Prentis P.J., Pavasovic A. The Anadara trapezia transcriptome: A resource for molluscan physiological genomics. Mar. Genom. 2014;18:113–115. doi: 10.1016/j.margen.2014.08.004. [DOI] [PubMed] [Google Scholar]
- 65.Mitchell M.L., Shafee T., Papenfuss A.T., Norton R.S. Evolution of cnidarian trans-defensins: Sequence, structure and exploration of chemical space. Proteins. 2019;87:551–560. doi: 10.1002/prot.25679. [DOI] [PubMed] [Google Scholar]
- 66.Madio B., Undheim E.A., King G.F. Revisiting venom of the sea anemone Stichodactyla haddoni: Omics techniques reveal the complete toxin arsenal of a well-studied sea anemone genus. J. Proteom. 2017;166:83–92. doi: 10.1016/j.jprot.2017.07.007. [DOI] [PubMed] [Google Scholar]
- 67.Bolger A.M., Lohse M., Usadel B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics. 2014;30:2114–2120. doi: 10.1093/bioinformatics/btu170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Garber M., Grabherr M.G., Guttman M., Trapnell C. Computational methods for transcriptome annotation and quantification using RNA-seq. Nat. Methods. 2011;8:469–477. doi: 10.1038/nmeth.1613. [DOI] [PubMed] [Google Scholar]
- 69.Huang Y., Niu B., Gao Y., Fu L., Li W. CD-HIT Suite: A web server for clustering and comparing biological sequences. Bioinformatics. 2010;26:680–682. doi: 10.1093/bioinformatics/btq003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Simão F.A., Waterhouse R.M., Ioannidis P., Kriventseva E.V., Zdobnov E.M. BUSCO: Assessing genome assembly and annotation completeness with single-copy orthologs. Bioinformatics. 2015;31:3210–3212. doi: 10.1093/bioinformatics/btv351. [DOI] [PubMed] [Google Scholar]
- 71.Wolstenholme J.K., Wallace C.C. Australian Anemones Final Report Accompanied by Attribution Database of Australian Anemones (on CD rom): Prepared for the Department of Environment and Heritage, Heritage Division. Museum of Tropical Queensland; Townsville, Australia: 2004. [Google Scholar]
- 72.Putnam N.H., Srivastava M., Hellsten U., Dirks B., Chapman J., Salamov A., Terry A., Shapiro H., Lindquist E., Kapitonov V.V., et al. Sea anemone genome reveals ancestral eumetazoan gene repertoire and genomic organization. Science. 2007;317:86. doi: 10.1126/science.1139158. [DOI] [PubMed] [Google Scholar]
- 73.Aranda M., Li Y., Liew Y.J., Baumgarten S., Simakov O., Wilson M.C., Piel J., Ashoor H., Bougouffa S., Bajic V.B., et al. Genomes of coral dinoflagellate symbionts highlight evolutionary adaptations conducive to a symbiotic lifestyle. Sci. Rep. 2016;6:39734. doi: 10.1038/srep39734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lin S., Cheng S., Song B., Zhong X., Lin X., Li W., Li L., Zhang Y., Zhang H., Ji Z., et al. The Symbiodinium kawagutii genome illuminates dinoflagellate gene expression and coral symbiosis. Science. 2015;350:691–694. doi: 10.1126/science.aad0408. [DOI] [PubMed] [Google Scholar]
- 75.Shoguchi E., Shinzato C., Kawashima T., Gyoja F., Mungpakdee S., Koyanagi R., Takeuchi T., Hisata K., Tanaka M., Fujiwara M., et al. Draft assembly of the Symbiodinium minutum nuclear genome reveals dinoflagellate gene structure. Curr. Biol. 2013;23:1399–1408. doi: 10.1016/j.cub.2013.05.062. [DOI] [PubMed] [Google Scholar]
- 76.Haas B.J., Papanicolaou A., Yassour M., Grabherr M., Blood P.D., Bowden J., Couger M.B., Eccles D., Li B., Lieber M. De novo transcript sequence reconstruction from RNA-seq using the Trinity platform for reference generation and analysis. Nat. Protoc. 2013;8:1494. doi: 10.1038/nprot.2013.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Finn R.D., Clements J., Eddy S.R. HMMER web server: Interactive sequence similarity searching. Nucleic Acids Res. 2011;39:W29–W37. doi: 10.1093/nar/gkr367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Langmead B., Salzberg S.L. Fast gapped-read alignment with Bowtie 2. Nat. Methods. 2012;9:357–359. doi: 10.1038/nmeth.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Li B., Dewey C.N. RSEM: Accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinform. 2011;12:323. doi: 10.1186/1471-2105-12-323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Robinson M.D., McCarthy D.J., Smyth G.K. edgeR: A Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics. 2010;26:139–140. doi: 10.1093/bioinformatics/btp616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Young M., Wakefield M., Smyth G., Oshlack A. Gene ontology analysis for RNA-seq: Accounting for selection bias. Genome Biol. 2010;11:R14. doi: 10.1186/gb-2010-11-2-r14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Supek F., Bošnjak M., Škunca N., Šmuc T. REVIGO summarizes and visualizes long lists of gene ontology terms. PLoS ONE. 2011;6:e21800. doi: 10.1371/journal.pone.0021800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Liao Q., Gong G., Poon T.C., Ang I.L., Lei K.M., Siu S.W.I., Wong C.T.T., Rádis-Baptista G., Lee S.M.-Y. Combined transcriptomic and proteomic analysis reveals a diversity of venom-related and toxin-like peptides expressed in the mat anemone Zoanthus natalensis (Cnidaria, Hexacorallia) Arch. Toxicol. 2019;93:1745–1767. doi: 10.1007/s00204-019-02456-z. [DOI] [PubMed] [Google Scholar]
- 84.Klompen A.M., Macrander J., Reitzel A.M., Stampar S.N. Transcriptomic analysis of four cerianthid (Cnidaria, Ceriantharia) venoms. Mar. Drugs. 2020;18:413. doi: 10.3390/md18080413. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Raw reads for C. adhaesivum, H. hemprichii, M. doreensis and P. semoni are available at the NCBI SRA under BioProject PRJNA715506. Accession numbers for C. adhaesivum, H. hemprichii, M. doreensis and P. semoni can be viewed in Supplementary Materials. Raw reads for D. cf. armata are registered in EMBL-ENA under project accession number PRJEB42903 and ENA sample runs as follows: DA4ITa—ERXS6629214, DA4OTc—ERS6629215, DA5IT—ERS6629216 and DA5OT—ERS6629217.