Abstract
Objective
Next to olfactory function, the nose can also perceive chemestetic sensations mediated by the trigeminal nerve. While olfactory dysfunction as a symptom of COVID-19 is well described, there has been little research on the limitation of other nasal sensory inputs due to SARS-CoV-2 infection. The aim of this study was to determine possible limitations of nasal chemesthesis after COVID-19 infection by a psychophysiological diagnostic tool.
Methods
In 65 patients with a PCR-confirmed, former COVID-19 disease, olfaction was tested by means of a sniffin' sticks test, tasting by taste sprays and chemesthesis with a menthol dilution series. The subjective self-assessment of the patients was recorded via a questionnaire.
Results
We found a restriction of nasal chemesthesis and the extent correlated with the loss of smell, as well as with the values of the taste score, but not with subjective self-assessment.
Conclusion
Not only the ability to smell and taste, but also nasal chemesthesis is affected by COVID-19.
Keywords: SARS-CoV-2, Smell disorders, Trigeminal nerve, Chemesthesis, Sniffin’ Sticks
1. Introduction
More than a year after the outbreak of the new SARS Coronavirus-2 (SARS-CoV-2), olfactory dysfunction as a symptom of COVID-19 is well described as one of the most common symptoms [1] and is even used as a predictive screening factor for diagnosis [2]. However, beyond these symptoms, many patients also complain of a restriction or affection of nasal chemesthesis, such as burning or the sensation of a blocked nose. These sensations are to be distinguished from olfactory disorders [3] and are mediated in the nose via the trigeminal nerve [4]. So far, only patient records and questionnaire-based surveys exist on COVID-19-associated impairment of chemesthesis. The aim of this study was therefore to confirm this sensory impairment in the COVID-19-related olfactory disorder with a psychophysical test.
2. Methods
This study was conducted at a university hospital in Germany. The principles of the Declaration of Helsinki and its subsequent amendments were adhered to and monitored by the local ethics committee. The participants of the study were recruited from the local outpatient clinic for COVID-19 sufferers. Only patients who had received a virus detection by polymerase chain reaction (PCR) during their disease were included. Additional inclusion criteria were an age of at least 18 years and sufficient knowledge of the German language. All participants had an overall mild course without hospitalisation. The onset of the disease was at least two months ago in all subjects (mean: 91.78 days, ± 6.91).
Exclusion criteria were pre-existing nasal conditions such as previous trauma, chronic sinusitis or acute allergic symptoms and as were previously known olfactory and gustatory disorders or chemosensitivity limitations.
Demographic and general data of the test persons as well as data on the course of the disease were collected via a standardised questionnaire. The questionnaire was also used to find out whether the patients had suffered a loss of smell and taste in the course of their disease. The patients were asked to rate their subjective ability to smell and taste on an analogue scale of 1–10 (1 = bad, 10 = good). In addition, the subjective patency of the nose was asked about via a 5-point scale, as nasal patency is a barometer of trigeminal sensitivity [5].
Psychophysical measurements were performed in a quiet, well-ventilated room. Orthonasal olfaction was tested using the Sniffin' Sticks Test (Burghart Messtechnik GmbH, Wedel, Germany). This widely used olfactory test offers the possibility of a differentiated view of olfactory ability, as it determines the olfactory threshold (OT) as well as the ability to differentiate odours (OD) and identify odours (OI) [6]. Odours are presented via felt-tip pens. Olfactory threshold is determined via the subject's ability to detect n-butanol, using a staircase technique based on a three-alternative forced-choice (score range = 1–16) starting with a 4% solution. In the OD test, the patient has to recognise the pen that smells different from the other two from 16 offered triplets. To quantify the OI, a total of 16 common odours are presented, which have to be matched to the appropriate picture cards and words in a multiple-choice procedure. The total score TDI is then summed up from the collected values for OD, OT and OI.
If the subject achieves a score of more than 30.5 points, he/she is normosmic, while with a score between 16.6 and 30.5, he/she is hyposmic. A TDI score of less than 16.5 corresponds to functional anosmia [6]. For the simplicity of the term, we will refer to functional anosmia as anosmia in the following.
As a measure of chemesthesis, the perception threshold for menthol is described in the literature [7], which was determined within this study with the help of a psychophysical method [8]. For this purpose, 16 menthol solutions of decreasing concentration were first prepared by geometric dilution. The initial concentration was 50% g/g (No.1) the following 25% g/g (No.2), and so on.
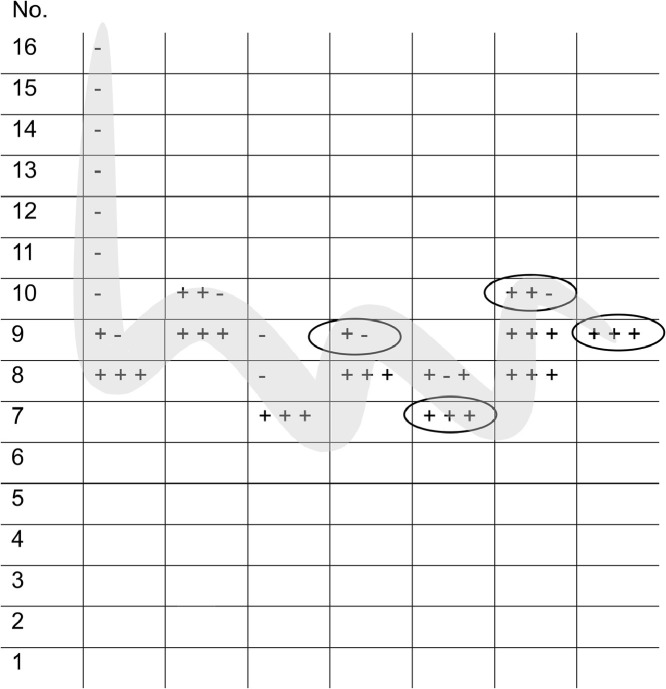
Presentation during the examination was performed using an ascending single-step method, starting with the weakest concentration (0,00,213,623% g/g, No.16). The mucous membranes of the eyes were protected from possible trigeminal stimulation by glasses. After the presentation of the menthol solution under the patients’ nose, they were asked whether they had felt a "burning, stinging, cooling or tickling" sensation, impressions like those triggered by menthol [9]. If this was answered in the negative, the next higher concentration level was presented until the patient felt a sensation three times in succession at one concentration. The staircase was then reversed and the next lower concentration was applied. As soon as the patient did not notice a stimulus, the next turning point was reached. This procedure was followed until a total of seven turning points were set. From the last four turning points, the mean value was calculated as the threshold value (Fig. 1 ). If the test person was not able to feel the highest concentration seven times in a row, a threshold value of 1 was assigned as a substitute value. Higher numbers mean lower threshold values and thus a more sensitive sensation. The interval between two stimulations was 30 s.
Fig. 1.
Schematic procedure for determining the chemesthesis threshold with example values entered. Starting with the weakest concentration of menthol (“1″), the next higher concentration was offered until the subject felt trigeminal irritation three times in a row. This was the first turning point until the subject did not register correct perceptions three times in a row and thus the next turning point towards stronger concentrations was reached. This procedure was repeated until seven turning points were recorded. The threshold was calculated from the mean value of the last four turning points. 1–16 = concentrations of the methanol solution, 1 = strongest, 16 = weakest concentration. + = subject felt a trigeminal sensation (cooling, burning). - = subject did not indicate a trigeminal sensation. The ellipses mark the last four turning points. The grey line follows the direction of the testing.
The taste function was screened with taste sprays of the basic qualities sweet (1 g sucrose per 10 g purified water), sour (0.5 g citric acid per 10 g purified water), salty (0.75 g sodium chloride per 10 g purified water) and bitter (0.005 g quinine hydrochloride per 10 g purified water) [10]. These suprathreshold solutions were sprayed on the subjects' tongues. A score from 0 to 4 was given according to the correctly recognised tastes. Statistical Analysis was performed using SPSS v. 24 (SPSS Inc., Chicago, USA). Data is presented as average values (± standard error of the mean).
3. Results
The study included 65 patients, 24 men (36.9%) and 41 women (63.1%). The mean age was 44.88 years (±11.67 years). On average, 191.78 days (6.91) days had elapsed since disease onset/positive PCR. The mean TDI score of all study participants was 32.90 points. 53 patients (81.5%) were normosmic, 10 patients (15.4%) were hyposmic. Only two patients (3.1%) were anosmic. In the taste test, the mean score was 3.75 (± 0.69). The mean chemesthesis threshold was 9.42 (± 0.42). According to the Kolmogorov-Smirnov test (p = 0.200) and the Shapiro-Wilk test (p = 0.497), the chemesthesis score values were distributed normally.
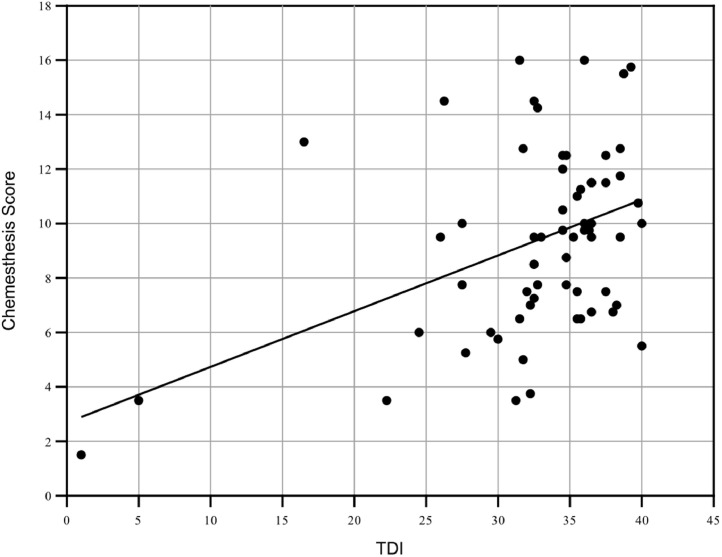
There was a positive correlation between the chemesthesis score and the TDI in the Pearson analysis (r = 0.418, p < 0.01), as well as with all TDI subscores (Table 1 , Fig. 2 ). There was also a good correlation between the self-assessment of olfactory ability and the TDI score (r = 0.574, p < 0.01) and the subjective tasting ability (r = 0.248, p > 0.05). A positive correlation was likewise found between the chemesthesis score and the taste score (r = 0.303, p > 0.05), whereas the subjective self-assessments regarding the ability to smell and taste did not correlate with chemesthesis. No correlation could be determined with regard to the self-assessment of nasal passability either (Table 2 ).
Table 1.
There was a positive correlation between the nasal chemesthesis score and the TDI and all its subscores. The Taste score also correlated with nasal chemesthesis. A two-way correlation according to Pearson was performed, * p < 0.05, ** p < 0.01.
| TDI | OT | OD | OI | Taste Score | |
|---|---|---|---|---|---|
| Chemesthesis score | 0,418⁎⁎ | 0,275* | 0,414⁎⁎ | 0,414⁎⁎ | 0,303* |
Fig. 2.
Except for two subjects with anosmia, the other participants had TDI scores in the hypo- or normosmic range. The graph illustrates the correlation between the TDI and the chemesthesis score.
Table 2.
The subjects' self-assessment correlated well with the TDI, but not with the score for nasal chemesthesis according to Pearson's calculation. The r-value is given, * p < 0.05, ** p < 0.01, two-sided).
| Self-assessment olfaction | Self-assessment taste | Self-assessment nasal passability | |
|---|---|---|---|
| TDI | 0,574⁎⁎ | 0,248* | −0,248 |
| Chemesthesis score | 0,224 | 0,166 | −0,058 |
4. Discussion
Olfactory dysfunction is one of the most common symptoms of COVID-19 and has been described numerously in the literature [11,12]. COVID-19-associated olfactory loss is characterised by a rapid onset and improvement after a short time in about half of patients. However, in about 50% of patients, a reduction in olfaction can be measured even after three months [13]. Olfactory function improves in most patients during the course of the disease, but in some patients limitations of the olfactory function can be measured even 6 months after the disease [14]. Impairments in chemesthesis have been described in SARS-CoV-2 infected patients [3], but data is very limited compared to olfactory impairment. Impairments of other sensory systems such as taste and chemesthesis frequently occur in connection with postinfectious olfactory disorders [15], [16], [17]. Taste impairment has been described in COVID patients and confirmed by psychophysiological tests [13,18]. Chemesthesis has only been physiologically tested by Ferreli et al. so far, limited to ability to recognise menthol in the context of the Sniffin' Stick [19]. To the best of our knowledge, our study is the first to prove the limitation of chemesthesis with a detailed physiological threshold test.
Our study results show that COVID-19-related olfactory dysfunction correlates with a reduction in nasal chemesthesis, and also that study participants with reduced taste showed impaired chemesthesis.
Since both smell and chemesthesis are senses of the nose, these impressions can easily be mixed up by patients and study participants. The difference was explained in detail to the participants of the study and they were instructed to focus on the cooling or burning sensation. The trigeminal stimulus we chose, menthol, is well described in the literature for testing chemesthesis [9,20,21].
The pathomechanism for the reduction of chemesthesis by SARS-CoV-2 remains unclear to date. An obvious mechanism would be the involvement of the trigeminal nerve by the virus. However, in the case of COVID-19-associated olfactory loss, this pathomechanism is controversial. So far, there are only data on preprint servers that suggest a direct attack of the olfactory cells [22], while other authors favour an attack of the sustaining cells due to the lack of expression of ACE-2 on the olfactory nerves [23]. With regard to the trigeminal nerve, however, an invasion of the cells by the viruses via the ACE-2 receptor is assumed in the literature [24]. For SARS-CoV-1 an infection of nerve cells has been shown [25]. In mice, infestation of the trigeminal nerve by coronaviruses has been known for a long time [26] and since the key protein of the virus ACE-2 is expressed in large quantities in the nose [23], infestation of the nerve is suspected. Histological samples from the nose and immunohistochemical staining of the trigeminal nerve branches in SARS-CoV-2 infected persons may provide more detailed information. Of note, there is increasing evidence that patients with an affection of the nasal senses could have a more severe course of the disease [27] or more neurological symptoms [28].
Although we were able to show a clear correlation between olfactory impairment and chemesthesis with this study, it is still not possible to make an absolute statement about the trigeminal system. On the one hand due to the subjective nature of the chosen methodology [8], why the results should be confirmed by objective measurement methods using an olfactometer [29]. On the other hand, due to the lack of a control group, no absolute classification of chemesthesis could be made, but only the correlation of olfactory loss and chemesthesis could be worked out. The control group would be particularly important because, unlike the TDI value, there are no validated standard values for the chemesthesis score. However, recruitment is currently difficult due to the increased risk of infection - especially in hospitals. The above-mentioned future olfactometer studies should, however, also collect these comparative data. In order to investigate the development of the restrictions over time, further measurements should be taken over the long term.
Declaration of Competing interest
The authors received no external funding for this work and declare no conflicts of interest or competing interests.
References
- 1.Meng X., Deng Y., Dai Z., Meng Z. COVID-19 and anosmia: a review based on up-to-date knowledge. Am J Otolaryngol. 2020;41 doi: 10.1016/j.amjoto.2020.102581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Haehner A., Draf J., Dräger S., de With K., Hummel T. Predictive value of sudden olfactory loss in the diagnosis of COVID-19. ORL J Otorhinolaryngol Relat Spec. 2020;82:175–180. doi: 10.1159/000509143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Parma V., Ohla K., Veldhuizen M.G., Niv M.Y., Kelly C.E., Bakke A.J. More than smell—COVID-19 is associated with severe impairment of smell, taste, and chemesthesis. Chem Senses. 2020;45:609–622. doi: 10.1093/chemse/bjaa041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Frasnelli J., Albrecht J., Bryant B., Lundström J.N. Perception of specific trigeminal chemosensory agonists. Neuroscience. 2011;189:377–383. doi: 10.1016/j.neuroscience.2011.04.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Oleszkiewicz A., Schultheiss T., Schriever V.A., Linke J., Cuevas M., Hähner A. Effects of “trigeminal training” on trigeminal sensitivity and self-rated nasal patency. Eur Arch Otorhinolaryngol. 2018;275:1783–1788. doi: 10.1007/s00405-018-4993-5. Off J Eur Fed Oto-Rhino-Laryngol Soc EUFOS Affil Ger Soc Oto-Rhino-Laryngol - Head Neck Surghttps://doi.org/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Oleszkiewicz A., Schriever V.A., Croy I., Hähner A., Hummel T. Updated Sniffin’ Sticks normative data based on an extended sample of 9139 subjects. Eur Arch Otorhinolaryngol. 2019;276:719–728. doi: 10.1007/s00405-018-5248-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Doty R.L., Brugger W.E., Jurs P.C., Orndorff M.A., Snyder P.J., Lowry L.D. Intranasal trigeminal stimulation from odorous volatiles: psychometric responses from anosmic and normal humans. Physiol Behav. 1978;20:175–185. doi: 10.1016/0031-9384(78)90070-7. [DOI] [PubMed] [Google Scholar]
- 8.Frasnelli J., Hummel T. Intranasal trigeminal thresholds in healthy subjects. Environ Toxicol Pharmacol. 2005;19:575–580. doi: 10.1016/j.etap.2004.12.022. [DOI] [PubMed] [Google Scholar]
- 9.Laska M. Perception of trigeminal chemosensory qualities in the elderly. Chem Senses. 2001;26:681–689. doi: 10.1093/chemse/26.6.681. [DOI] [PubMed] [Google Scholar]
- 10.Ahne G., Erras A., Hummel T., Kobal G. Assessment of gustatory function by means of tasting tablets. Laryngoscope. 2000;110:1396–1401. doi: 10.1097/00005537-200008000-00033. [DOI] [PubMed] [Google Scholar]
- 11.Kaye R., Chang C.W.D., Kazahaya K., Brereton J., Denneny J.C. COVID-19 anosmia reporting tool: initial findings. Otolaryngol Head Neck Surg. 2020 doi: 10.1177/0194599820922992. Off J Am Acad Otolaryngol-Head Neck Surg. [DOI] [PubMed] [Google Scholar]
- 12.Lechien J.R., Chiesa-Estomba C.M., Place S., Van Laethem Y., Cabaraux P., Mat Q. Clinical and epidemiological characteristics of 1,420 European patients with mild-to-moderate coronavirus disease 2019. J Intern Med. 2020 doi: 10.1111/joim.13089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Otte M.S., Eckel H.N.C., Poluschkin L., Klussmann J.P., Luers J.C. Olfactory dysfunction in patients after recovering from COVID-19. Acta Otolaryngol (Stockh) 2020;0:1–4. doi: 10.1080/00016489.2020.1811999. [DOI] [PubMed] [Google Scholar]
- 14.Otte M., Bork M., Zimmermann P., Peter Klußmann J., Luers J.-.C. Persisting olfactory dysfunction improves in patients six months after COVID-19 disease. Acta Otolaryngol (Stockh) 2020 doi: 10.1080/00016489.2021.1905178. n.d. [DOI] [PubMed] [Google Scholar]
- 15.Frasnelli J., Schuster B., Hummel T. Interactions between olfaction and the trigeminal system: what can be learned from olfactory loss. Cereb Cortex. 2007;17:2268–2275. doi: 10.1093/cercor/bhl135. [DOI] [PubMed] [Google Scholar]
- 16.Landis B.N., Scheibe M., Weber C., Berger R., Brämerson A., Bende M. Chemosensory interaction: acquired olfactory impairment is associated with decreased taste function. J Neurol. 2010;257:1303–1308. doi: 10.1007/s00415-010-5513-8. [DOI] [PubMed] [Google Scholar]
- 17.Migneault-Bouchard C., Hsieh J.W., Hugentobler M., Frasnelli J., Landis B.N. Chemosensory decrease in different forms of olfactory dysfunction. J Neurol. 2020;267:138–143. doi: 10.1007/s00415-019-09564-x. [DOI] [PubMed] [Google Scholar]
- 18.Vaira L.A., Deiana G., Fois A.G., Pirina P., Madeddu G., De Vito A. Objective evaluation of anosmia and ageusia in COVID-19 patients: single-center experience on 72 cases. Head Neck. 2020 doi: 10.1002/hed.26204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ferreli F., Bari M.D., Gaino F., Albanese A., Politi L.S., Spriano G. Trigeminal features in COVID-19 patients with smell impairment. Int Forum Allergy Rhinol. 2021 doi: 10.1002/alr.22796. n.d.;n/a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eccles R., Jones A.S. The effect of menthol in nasal resistance to air flow. J Laryngol Otol. 1983;97:705–709. doi: 10.1017/S002221510009486X. [DOI] [PubMed] [Google Scholar]
- 21.Schriever V.A., Hummel T. Subjective changes in nasal patency after chewing a menthol-containing gum in patients with olfactory loss. Acta Otolaryngol (Stockh) 2015;135:254–257. doi: 10.3109/00016489.2014.980913. [DOI] [PubMed] [Google Scholar]
- 22.Melo G.D.D., Lazarini F., Levallois S., Hautefort C., Michel V., Larrous F. COVID-19-associated olfactory dysfunction reveals SARS-CoV-2 neuroinvasion and persistence in the olfactory system. BioRxiv. 2020 doi: 10.1101/2020.11.18.388819. 2020.11.18.388819. [DOI] [Google Scholar]
- 23.Fodoulian L., Tuberosa J., Rossier D., Boillat M., Kan C., Pauli V. SARS-CoV-2 receptors and entry genes are expressed in the human olfactory neuroepithelium and brain. IScience. 2020;23 doi: 10.1016/j.isci.2020.101839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Salamanna F., Maglio M., Landini M.P., Fini M. Body localization of ACE-2: on the trail of the keyhole of SARS-CoV-2. Front Med. 2020;7 doi: 10.3389/fmed.2020.594495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xu J., Zhong S., Liu J., Li L., Li Y., Wu X. Detection of severe acute respiratory syndrome coronavirus in the brain: potential role of the chemokine mig in pathogenesis. Clin Infect Dis Off Publ Infect Dis Soc Am. 2005;41:1089–1096. doi: 10.1086/444461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Perlman S., Jacobsen G., Afifi A. Spread of a neurotropic murine coronavirus into the CNS via the trigeminal and olfactory nerves. Virology. 1989;170:556–560. doi: 10.1016/0042-6822(89)90446-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tabari A., Golpayegani G., Tabari A., Saedi B., Mahdkhah A., Amali A. Olfactory dysfunction is associated with more severe clinical course in COVID-19. Indian J Otolaryngol Head Neck Surg. 2021 doi: 10.1007/s12070-021-02507-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cocco A., Amami P., Desai A., Voza A., Ferreli F., Albanese A. Neurological features in SARS-CoV-2-infected patients with smell and taste disorder. J Neurol. 2020 doi: 10.1007/s00415-020-10135-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hummel T., Frasnelli J. Chapter 8 - The intranasal trigeminal system. Doty R.L., editor. Chapter 8 - The intranasal trigeminal systemHandb Clin Neurol. 2019;164 doi: 10.1016/B978-0-444-63855-7.00008-3. vol.Elsevier119-34. [DOI] [PubMed] [Google Scholar]