Abstract
Objectives:
Given the differences in prevalence of mild cognitive impairment (MCI) in Black older adults compared to Whites, this study aimed to examine whether overall vascular risk factor (VRF) burden and individual VRF associations with amnestic (aMCI) and non-amnestic (naMCI) MCI risk varied by race.
Methods:
Participants included 2755 older adults without dementia from the ACTIVE trial at baseline. Comprehensive neuropsychological criteria were used to classify cognitively normal, aMCI, and naMCI. VRFs were defined based on subjective report and medication data. Multinomial logistic regression was run predicting MCI subtype.
Results:
Greater VRF burden, high cholesterol, and obesity evinced greater odds of naMCI in Black elders compared to Whites. Across participants, diabetes and hypertension were associated with increased odds of aMCI and naMCI, respectively.
Discussion:
Results may reflect a compound of disadvantage relating to racism/marginalization. Continued efforts toward examining underlying mechanisms and potential disparities contributing to these findings are warranted.
Introduction
Mild cognitive impairment (MCI) is a construct used to capture a prodromal stage of incipient dementia (Albert et al., 2011). MCI has been shown to be associated with biomarkers of neurodegeneration and dysregulation (e.g. Eliassen et al., 2017; Giau, Bagyinszky, & An, 2019; Jack et al., 1999) along with increased risk of Alzheimer’s disease (AD) and other dementias (Smith & Bondi, 2013; Yaffe, Petersen, Lindquist, Kramer, & Miller, 2006). MCI is typically characterized by objective neuropsychological impairment in at least one cognitive domain with relatively intact function in activities of daily living and global cognition (Albert et al., 2011). Older adults with impairment in learning and memory are often classified as amnestic MCI (aMCI), while those with impairment in attention, language, visuospatial, and/or executive functions, in the absence of a memory impairment, are typically classified as non-amnestic MCI (naMCI). Amnestic MCI has been shown to be associated predominantly with AD (Morris, 2006; Petersen, 2004), though there is evidence that aMCI may also be associated with increased risk of other dementias (Ganguli, Dodge, Shen, & DeKosky, 2004). Similarly, while naMCI has been found to be associated with AD, naMCI seems to be more strongly predictive of increased risk of vascular dementia (Mauri et al., 2008; Sudo et al., 2012), dementia with Lewy bodies (Ferman et al., 2013) and other non-AD dementias (Petersen et al., 2001).
There is some evidence for greater risk of MCI and AD/dementia among racial/ethnic minorities, with higher rates among Black elders than non-Hispanic Whites (Mehta & Yeo, 2017). A number of potential underlying factors contributing to these discrepancies have been suggested (Glymour & Manly, 2008), including mistrust of medical researchers (Ighodaro et al., 2017) and less exposure to tests as well as cognitive test biases. Within the Advanced Cognitive Training for Independent and Vital Elderly (ACTIVE) study, Black participants have lower mean-level cognitive scores than White participants; however, there were almost no differences in rate of cognitive change over time by race (Marsiske et al., 2013). These findings are consistent with recent work suggesting that Black participants are more likely to receive dementia diagnoses because of persistent lower cognitive performance rather than accelerated late-life cognitive decline (Weuve et al., 2018). Importantly, within ACTIVE, Aiken-Morgan and colleagues (2010) demonstrated little evidence that specific cognitive measures put either White or Black participants at particular advantage or disadvantage. Given that test bias seems unlikely to account for the level differences in cognitive performance in this sample, more work is needed to understand the potential contributions of health-related and social factors.
One area of interest is the potential contribution of vascular risk factors (VRFs) to cognitive impairment, given that, on average, Black older adults have higher burden of VRFs (Graham, 2015) and more health conditions have been associated with worse cognitive outcomes in Black older adults (Byrd, Thorpe, & Whitfield, 2018). VRFs include a myriad of disorders, including: diabetes, hypertension, high cholesterol, obesity and metabolic syndrome, and a history of and current smoking, all of which have been associated with cognitive decline or greater risk of MCI (Byrd et al., 2018; Cannon et al., 2017; Cervilla, Prince, & Mann, 2000; Feinkohl et al., 2019; Luchsinger et al., 2005; Zou et al., 2014). As such, this study sought to examine the effects of Black/White race and VRFs on MCI subtype status in ACTIVE, and whether the relationships between VRFs and MCI status differed by race.
Methods
Participants
Participants from the ACTIVE study at baseline who had sufficient cognitive test data to determine MCI status were included in analyses (N=2755). Detailed information regarding inclusion criteria for ACTIVE have been described elsewhere (Jobe et al., 2001). Participants were recruited from six sites throughout United States. All procedures were approved by the Institutional Review Boards and informed consent was obtained prior to participation. At baseline, participants were at least 65 years of age, had an Mini Mental Status Exam (Folstein, Folstein, & McHugh, 1975) score >23, had intact basic activities of daily living, and were free of severe sensory impairments and medical conditions likely to impact functioning or significantly increase mortality risk. The current analyses only included participants who identified as either Hispanic (n=3) or non-Hispanic Black (n=718) and Hispanic (n=12) or non-Hispanic White (n=2,022). There were only 15 Hispanic participants, so ethnicity was not explicitly examined. In accordance with other ACTIVE cohort publications (e.g. Marsiske et al., 2013) and given the small number of participants who identified as a race other than Black or White (n=20; 7 Asian; 4 American Indian/Alaskan Native, 9 Biracial), the current analyses excluded these 20 participants due to the small numbers.
Demographic and Vascular Risk Variables
Demographic and background variables.
Baseline demographics included current age, total years of education, sex/gender (women; men) and race (Black; White). Along with cognitive measures, participants were administered Vocabulary (Ekstrom, French, Harman, & Derman, 1976), which involved identifying the synonym of a target word out of a set of five word-choices and may represent a proxy for quality of education.
Individual vascular risk factors.
Objective and subjective health information was collected from all participants. Participants also provided a list of all current prescribed and over-the-counter medications. The American Hospital Formulary Service (AHFS) coding system (AHFS Drug Information, 2015) was used to determine medication classes that were used to inform presence or absence of individual vascular risk factors (VRFs). All individual VRFs were coded as dichotomous predictors (0=absence, 1=presence) if they met at least one of the defining criteria at baseline. Diabetes: self-reported diabetes; use any diabetes-specific medication (e.g. alpha-glucoside inhibitors, biguanide, DPP-4 inhibitors, incretin mimetics, insulin, meglitinides, sulfonylureas, and thiazolidediones). Hypertension: self-reported hypertension; use any antihypertensive medication (e.g. ACE inhibitors, angiotensin II receptor antagonists, aldosterone receptor antagonists, renin inhibitors, beta-adrenergic blockers, vasodilators, central alpha agonists, and calcium channel blockers). High cholesterol: self-reported high cholesterol; use of statins (e.g. HMG-CoA reductase inhibitors). Obesity: body mass index (calculated using baseline height and weight) ≥30. Current smoking: self-reported current smoking status.
Cumulative vascular risk factor burden.
Primary analyses for this study focused on baseline cumulative vascular risk factor burden (VRF). The cumulative VRF variable was the sum of the dichotomous individual VRFs (diabetes, hypertension, high cholesterol, obesity, and current smoking, range=0–5).
MCI Classification
This study utilized a previously published algorithm for classifying MCI subtype and cognitively normal (CN) participants in ACTIVE (see Thomas et al., in press for full details). MCI classification was based on the comprehensive neuropsychological (NP) criteria (Bondi et al., 2014; A. J. Jak et al., 2009; Amy J. Jak et al., 2016), which required performance of <16th percentile on at least two cognitive measures within the same cognitive domain. Seven cognitive test scores were used to determine MCI status and included three memory measures: Hopkins Verbal Learning Test immediate free recall (sum of three learning trials), Rey Auditory Verbal Learning Test (AVLT) immediate free recall (sum of five learning trials) and AVLT delayed recognition (hits - false positives + 35); two reasoning measures: Word Series and Letter Sets total correct; and two speed of processing measures: Digit Symbol Substitution total correct and Useful Field of View (UFOV) Task 2. Each person’s cognitive classification was based on a discrepancy between demographically-adjusted (age, education, sex/gender, Black/White race) expected scores and actual performance.
Participants who met criteria for MCI were then classified into subtypes (Petersen, 2004; Winblad et al., 2004): (1) aMCI if they were impaired in at least the memory domain and (2) naMCI if they were impaired in the reasoning and/or speed domain, but not memory. At baseline, 332 (12.1%) participants met criteria for aMCI, 186 (6.8%) participants met criteria for naMCI. Since dementia was excluded at baseline, the remaining 2,237 (81.2%) participants were considered cognitively normal (CN).
Statistical Analyses
A hierarchical multinomial logistic regression was conducted to determine the main effects of VRF, race, and other demographic factors on MCI odds (CN vs. aMCI; CN vs. naMCI) as well as whether race moderated the relationship between vascular risk and MCI status. Block 1 included age, education, sex/gender, and race. Block 2 added the Vocabulary score. Block 3 added the cumulative VRF score. Block 4 added the interaction between race and VRF.
Decomposition of the race × VRF interaction was conducted via two separate multinomial logistic regression models predicting MCI odds (CN vs aMCI; CN vs naMCI) for Black and White participants, respectively. Covariates included age, education, sex/gender, Vocabulary, and VRF. Exploratory follow-up analyses replicated the above analyses (Blocks 1–4 entered simultaneously) separately for each VRF to determine whether any specific VRFs were associated with MCI status.
Results
Demographic information
Means (standard deviations) and percentages of demographic variables stratified by cognitive status are included in Table 1. Comparisons showed that aMCI participants were older than naMCI and CN; education and Vocabulary were higher in the CN group than the two MCI groups. Black participants were more likely to be classified as aMCI compared to White participants. There were no significant sex/gender differences by group.
Table 1.
Baseline characteristics by MCI subtype
| Total Sample (N = 2,755) | CN (N = 2,237) | aMCI (N = 332) | naMCI (N =186) | F, H, or X2 | ηp2, η2, or V | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | |||
| Age | 73.64 | 5.91 | 73.45a | 5.73 | 75.12bc | 6.64 | 72.80a | 5.71 | 13.685 | 0.010* |
| Education | 13.52 | 2.70 | 13.62ab | 2.68 | 12.98c | 2.75 | 13.12c | 2.72 | 23.480 | 0.009* |
| Women, % | 75.82% | - | 75.19% | - | 76.2% | - | 82.79% | - | 5.449 | 0.044 |
| Black/African American, % | 26.17% | - | 25.20%a | - | 30.42%c | - | 30.65% | - | 6.197 | 0.047 |
| Vocabulary | 12.38 | 3.92 | 12.86ab | 3.70 | 10.14c | 4.31 | 10.42c | 4.11 | 172.590 | 0.063* |
| HVLT Total Recall | 26.07 | 5.01 | 27.37ab | 4.54 | 18.10bc | 4.86 | 24.63ac | 4.81 | 597.446 | 0.303* |
| AVLT Total Recall | 48.60 | 10.36 | 50.87ab | 8.88 | 33.92bc | 8.12 | 48.60ac | 10.36 | 537.894 | 0.281* |
| AVLT Recognition | 40.59 | 8.84 | 42.44ab | 7.58 | 29.23bc | 7.97 | 38.73ac | 8.97 | 518.37 | 0.237* |
| Word Series | 9.47 | 4.88 | 10.34ab | 4.75 | 6.05bc | 3.78 | 5.06ac | 2.66 | 223.528 | 0.140* |
| Letter Sets | 5.74 | 2.80 | 6.20ab | 2.69 | 4.16bc | 2.44 | 3.05ac | 1.99 | 192.617 | 0.123* |
| UFOV Task 2 | 133.64 | 125.10 | 110.13ab | 103.99 | 227.30c | 152.52 | 250.08c | 157.23 | 336.847 | 0.154* |
| Digit Symbol Substitution | 40.38 | 11.14 | 42.30ab | 10.47 | 32.95bc | 9.93 | 30.62ac | 10.30 | 204.608 | 0.130* |
CN=cognitively normal; aMCI=amnestic MCI; naMCI=non-amnestic MCI; SD=standard deviation.; HVLT=Hopkins verbal learning test; AVLT=Rey auditory verbal learning test; UFOV=Useful field of view.
represents significant difference from aMCI
represents significant difference from naMCI
represents significant difference from CN
Partial eta-squared and eta-squared are reported for ANOVA and Kruskal-Wallis tests, respectively, and Cramer’s V is reported for chi-squared tests.
p</= .017
Regarding VRF variables by race (Table 2), Black participants had higher overall VRF burden, along with higher rates of diabetes, hypertension, obesity, and current smoking compared to White participants. There were no differences in rates of high cholesterol.
Table 2.
Prevalence of cumulative and individual vascular risk factors stratified by race
| Black (N = 721) | White (N = 2,034) | F or X2 | ηp2 or V | |||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | |||
| VRF (total) | 1.944 | 1.051 | 1.494 | 1.028 | 101.592 | 0.035 *** |
| Diabetes | 21.70% | - | 10.30% | - | 60.624 | 0.148 *** |
| Hypertension | 79.90% | - | 58.41% | - | 54.990 | 0.141 *** |
| High cholesterol | 44.30% | - | 46.50% | - | 1.060 | 0.020 |
| Obesity | 42.60% | - | 29.90% | - | 37.675 | 0.118 *** |
| Current smoking | 12.90% | - | 5.70% | - | 39.627 | 0.120 *** |
VRF=cumulative vascular risk factors; SD=standard deviation. Partial eta-squared are reported for ANOVA, and Cramer’s V is reported for chi-squared tests.
p< .001
Race and Vascular Risk Associations with MCI
Unique effects of demographic covariates are shown in Table 3. In Block 1, age (OR=1.05, p<.001), education (OR=0.923, p<.001), and race (OR=1.368 p=.019) were significant predictors of aMCI classification but not naMCI (see Table 3). When Vocabulary was added to the model (Block 2), the effect of race on MCI flipped such that Black participants had reduced odds of both aMCI (OR=0.644, p=.021) and naMCI (OR=0.700, p=.018) compared to White participants, while the effects of sex/gender went from non-significant to significant. In general, women had increased odds of naMCI (OR=1.580, p=.027) but not aMCI (OR=1.074, p=.625). Block 3 added cumulative VRF to the overall model. When adjusting for demographic covariates and Vocabulary there was no significant main effect of VRF on aMCI (OR=1.054, p=.433) or naMCI (OR=1.071, p=.405).
Table 3.
Effects of cumulative vascular risk factor score and race on amnestic and non-amnestic MCI odds
| Cognitive Status | Predictor | Block 1 | Block 2 | Block 3 | Block 4 | ||||
|---|---|---|---|---|---|---|---|---|---|
| naMCI | OR | 95% CI | OR | 95% CI | OR | 95% CI | OR | 95% CI | |
| Age | 0.982 | 0.955–1.009 | 0.989 | 0.962–1.016 | 0.991 | 0.964–1.019 | 0.991 | 0.964–1.018 | |
| Education | 0.942 | 0.888–0.999 | 1.073 * | 1.003–1.148 | 1.078 * | 1.008–1.153 | 1.078 * | 1.008–1.154 | |
| Sex/gender (women) | 1.452 | 0.974–2.164 | 1.580 * | 1.055–2.366 | 1.566 * | 1.045–2.347 | 1.565 * | 1.044–2.347 | |
| Race (Black) | 1.178 | 0.844–1.645 | 0.644 ** | 0.442–0.937 | 0.620 * | 0.425–0.906 | 0.287 ** | 0.134–0.614 | |
| Vocabulary | - | - | 0.829 *** | 0.792–0.869 | 0.829 *** | 0.792–0.869 | 0.828 *** | 0.790–0.867 | |
| VRF | - | - | - | - | 1.111 | 0.959–1.287 | 0.986 | 0.825–1.177 | |
| Race × VRF | - | - | - | - | - | - | 1.473 ** | 1.072–2.025 | |
| aMCI | OR | 95% CI | OR | 95% CI | OR | 95% CI | OR | 95% CI | |
| Age | 1.050 *** | 1.03–1.07 | 1.058 *** | 1.037–1.079 | 1.060 *** | 1.039–1.082 | 1.060 *** | 1.039–1.082 | |
| Education | 0.923 *** | 0.882–0.965 | 1.074 *** | 1.019–1.132 | 1.078 ** | 1.023–1.136 | 1.077 ** | 1.022–1.136 | |
| Sex/gender (women) | 0.956 | 0.724–1.261 | 1.074 | 0.807–1.430 | 1.062 | 0.797–1.415 | 1.062 | 0.797–1.415 | |
| Race (Black) | 1.368 * | 1.026–1.333 | 0.700 * | 0.520–0.941 | 0.679 * | 0.504–0.916 | 0.652 | 0.373–1.138 | |
| Vocabulary | - | - | 0.811 *** | 0.782–0.841 | 0.811 *** | 0.782–0.841 | 0.811 *** | 0.782–0.841 | |
| VRF | - | - | - | - | 1.095 | 0.974–1.232 | 1.090 | 0.947–1.254 | |
| Race × VRF | - | - | - | - | - | - | 1.024 | 0.794–1.319 | |
Data represents conditional odds ratios (OR=exp[β]) from stepwise multinomial logistic regression models. naMCI=non-amnestic MCI; aMCI=amnestic MCI; CI=confidence interval (lower bound – upper bound); VRF=cumulative vascular risk factor score. Cognitively normal participants are reference.
p< .001;
p< .01;
p< .05 and are bolded.
Race as a Moderator of VRF burden on MCI
In Block 4, there was a significant VRF × race interaction effect for naMCI (OR=1.473, p=.017), but not aMCI (OR=1.024, p=.856). Follow-up multinomial logistic regression models were then run for Black and White participants separately to examine the VRF effects on MCI status. Greater VRF burden was associated with increased odds of naMCI in Black participants (OR=1.439, p=.008), but not White participants (OR=0.988, p=.896). There were no significant effects of VRF on aMCI for either Black (OR=1.101, p=.385) or White participants (OR=1.101, p=.183).
Individual VRFs Associations with MCI
In exploratory analyses, we examined each of the five VRFs as predictors of cognitive status (see Table 4); having diabetes (OR=2.059, p<.001) and hypertension (OR=1.518, p=.021) were associated with increased odds of aMCI and naMCI, respectively. Regarding the moderating effect of race on individual VRFs, only high cholesterol (OR=2.061, p=.035) and obesity (OR=2.024, p=.048) conferred greater odds of naMCI (but not aMCI) classification for Black participants compared to White. See Figure 1 for all individual VRF effects on MCI odds stratified by race. For naMCI, the pattern of results showed that while high cholesterol and obesity are likely driving the significant total VRF by race interaction, most of the individual VRF odds ratios were qualitatively higher for Black participants than White participants (except diabetes). Conversely, for aMCI, the individual VRF effects are more similar/overlapping by race, which is consistent with the null VRF by race interaction for aMCI.
Table 4.
Effects of Individual vascular risk factors and race on amnestic and non-amnestic MCI odds
| Cognitive Status | Predictor | Diabetes | Hypertension | High Cholesterol | Obesity | Current Smoking | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| naMCI | OR | 95% CI | OR | 95% CI | OR | 95% CI | OR | 95% CI | OR | 95% CI | |
| Age | 0.990 | 0.963–1.018 | 0.987 | 0.960–1.014 | 0.987 | 0.960–1.014 | 0.990 | 0.962–1.018 | 0.987 | 0.959–1.015 | |
| Education | 1.077 * | 1.007–1.152 | 1.077 * | 1.007–1.152 | 1.074 * | 1.003–1.149 | 1.077 * | 1.006–1.153 | 1.070 | 0.999–1.146 | |
| Sex/gender (women) | 1.587 * | 1.059–2.378 | 1.592 * | 1.062–2.388 | 1.609 * | 1.073–2.414 | 1.561 * | 1.034–2.358 | 1.565 * | 1.038–2.359 | |
| Vocabulary | 0.829 *** | 0.791–0.868 | 0.830 *** | 0.792–0.869 | 0.827 *** | 0.790–0.867 | 0.826 *** | 0.788–0.866 | 0.827 *** | 0.789–0.867 | |
| Race (Black) | 0.616 * | 0.406–0.934 | 0.495 | 0.229–1.070 | 0.455 ** | 0.275–0.752 | 0.494 ** | 0.301–0.813 | 0.414–0.918 | ||
| Individual VRF | 1.428 | 0.827–2.465 | 1.581 * | 1.071–2.334 | 0.698 | 0.481–1.014 | 0.789 | 0.517–1.203 | 0.670 | 0.264–1.700 | |
| Race (Black) × Individual vRf | 1.011 | 0.437–2.340 | 1.271 | 0.548–2.948 | 2.061 * | 1.054–4.030 | 2.024 * | 1.007–4.067 | 1.737 | 0.508–5.943 | |
| aMCI | OR | 95% CI | OR | 95% CI | OR | 95% CI | OR | 95% CI | OR | 95% CI | |
| Age | 1.061 *** | 1.040–1.082 | 1.058 *** | 1.037–1.079 | 1.058 *** | 1.037–1.079 | 1.057 *** | 1.035–1.079 | 1.058 *** | 1.037–1.079 | |
| Education | 1.080 ** | 1.024–1.138 | 1.076 ** | 1.021–1.134 | 1.075 ** | 1.020–1.133 | 1.068 * | 1.012–1.127 | 1.081 ** | 1.025–1.140 | |
| Sex/gender (women) | 1.081 | 0.810–1.442 | 1.065 | 0.800–1.419 | 1.067 | 0.800–1.423 | 1.076 | 0.801–1.444 | 1.058 | 0.794- | |
| Vocabulary | 0.809 *** | 0.779–0.839 | 0.810 *** | 0.780–0.840 | 0.811 *** | 0.782–0.841 | 0.813 *** | 0.783–0.844 | 0.806 *** | 0.777–0.837 | |
| Race (Black) | 0.654 * | 0.468–0.914 | 0.701 | 0.422–1.165 | 0.793 | 0.546–1.154 | 0.711 | 0.492–1.026 | 0.669 * | 0.489–0.915 | |
| Individual VRF | 2.059 *** | 1.383–3.065 | 1.086 | 0.810–1.455 | 0.987 | 0.739–1.318 | 1.030 | 0.744–1.425 | 1.023 | 0.528–1.984 | |
| Race (Black) × Individual vRf | 0.916 | 0.484–1.734 | 0.989 | 0.552–1.770 | 0.760 | 0.444–1.303 | 1.048 | 0.604–1.819 | 1.314 | 0.528–3.270 | |
Data represents conditional odds ratios (OR=exp[β]) from multinomial logistic regression models predicting MCI status by race. naMCI=non-amnestic MCI; aMCI=amnestic MCI; CI=confidence interval; Individual VRF=individual vascular risk factor associated with heading. Cognitively normal participants are reference.
p< .001;
p< .01;
p< .05 and are bolded.
Figure 1. Conditional odds ratios for non-amnestic and amnestic MCI stratified by Black/White race.
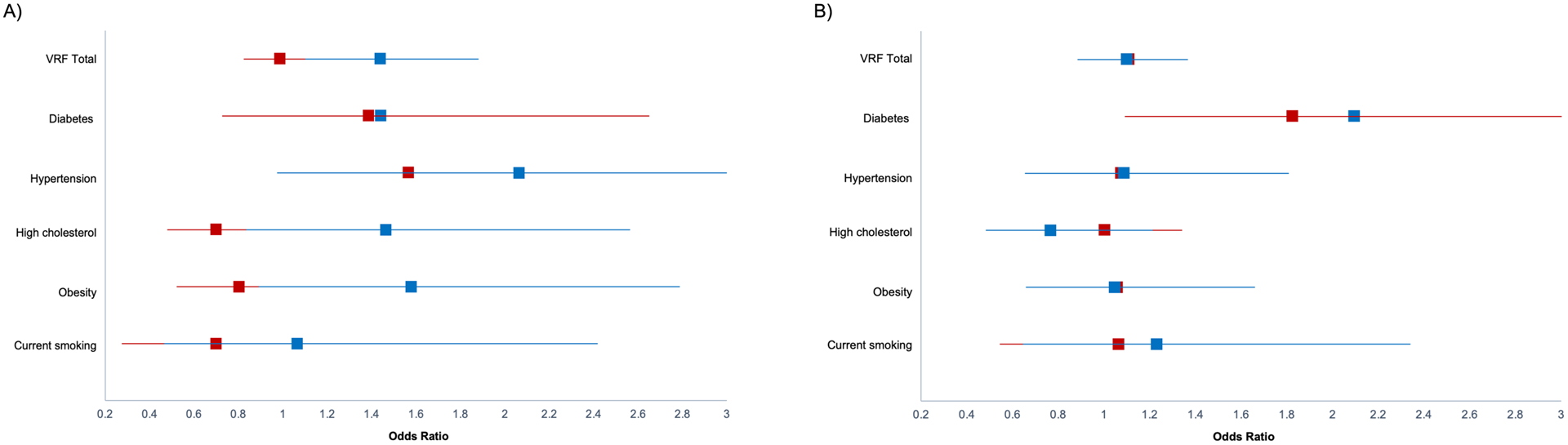
Data represents conditional odds ratios for individual vascular risk factors adjusted for age, years of education, sex/gender, and Vocabulary. Panel A is non-amnestic MCI; Panel B is amnestic MCI. Blue=Black participants; Red=White participants. Lines represent the 95% confidence interval.
Discussion
This study examined the cumulative and individual effects of VRFs on MCI status and explored whether those relationships varied across Black and White community-dwelling older adults from across the United States. The principal findings from this study demonstrated that increasing vascular risk burden was associated with greater naMCI odds for Black participants, but not White participants, after accounting for the effects of age, sex/gender, educational attainment, and Vocabulary (a potential proxy for educational quality). Furthermore, across individual predictors, obesity and high cholesterol independently conferred greater odds of naMCI for Black older adults. While qualitatively most individual VRFs trended toward greater odds for naMCI for Black participants, the pattern of findings for aMCI was less consistent. Taken together, these results suggest that individual predictors and increasing vascular health burden may differentially affect the odds of naMCI diagnosis in Black and White older adults.
These findings are consistent with studies that have found increasing health burden associated with greater declines in cognitive performance, particularly in non-amnestic domains of perceptual/processing speed (Byrd et al., 2018; Carmasin, Mast, Allaire, & Whitfield, 2014) for Black elders. Specifically, Carmasin and colleagues (2014) found vascular burden was associated with both poorer initial and 2.5-year follow-up processing speed performance in a sample of Black older adults. These results may reflect compounding factors including social forces such as healthcare access/treatment quality (Fiscella & Sanders, 2016), neighborhood factors (Clarke, Weuve, Barnes, Evans, & Mendes de Leon, 2015), literacy and quality of education (Manly, Jacobs, Touradji, Small, & Stern, 2002). Other factors include lifelong exposure to racism, discrimination, and stress that may also contribute to higher rates and severity of medical risk factors and cognitive decline (Brewster et al., 2014; Fiscella & Sanders, 2016; Zahodne et al., in press; Zuelsdorff et al., 2020). Notably, adults with cardiovascular disease have been found to experience higher rates of housing insecurity, food insecurity, and financial insecurity compared to adults without cardiovascular disease (Parekh, Desai, Pemmasani, & Cuellar, 2020). As such, these findings may reflect known systemic inequities between Black and White adults on dimensions of social determinants of health impacting vascular health and ultimately cognitive status (Davis, Gebreab, Quarells, & Gibbons, 2014; Havranek et al., 2015).
Our results are also consistent with obesity as a salient risk factor for cognitive impairment in Black older adults (Barnes & Bennett, 2014). In general, prevalence of obesity is higher in Black adults than Whites and the downstream impacts of obesity on cardiometabolic functioning (e.g. diabetes, metabolic syndrome, hypertension) has been found to have greater impact in Black adults (Sturman et al., 2008). While higher prevalence may partially explain this finding, underlying risk factors for obesity, including socioeconomic status (Lakerveld & Mackenbach, 2017), neighborhood segregation (Corral et al., 2012), and greater access to calorically-dense and nutritionally poor foods (Cooksey-Stowers, Schwartz, & Brownell, 2017) may also be contributory. Similarly, high cholesterol has also been found to be associated with increased MCI risk and poorer executive performance (Carvalho et al., 2014; Knopman et al., 2018). While this finding is unlikely due to differential prevalence between Black and White elders as was the case in this study (McIntosh et al., 2013), literature has demonstrated that Black adults may be disproportionately impacted by high cholesterol due to lower rates of statin use and undertreatment (Nanna et al., 2018).
While the focus of the study was on the interaction of VRFs and race on MCI odds, the initial blocks of the analyses showed that while there was a significant main effect of race on aMCI such that despite race being adjusted for in cognitive test z-scores from which MCI status was based, Black participants were at higher odds for aMCI classification, but not naMCI. This relationship, however, flipped once Vocabulary was added to the model such that White participants were then at higher risk for both aMCI and naMCI. Possible explanations for this finding may include cross-over effects (e.g. Elo & Preston, 1997), where at advanced age traditional expectations may flip due to potential underlying factors relating to survivorship and sources of resilience. Beyond the main effects of race and Vocabulary, this study also found that across participants, diabetes and hypertension were associated with increased odds of aMCI and naMCI, respectively, consistent with previous findings (Luchsinger et al., 2007).
A potential critique of this current study relates to the nature of the ACTIVE cohort. Participants were positively selected and included a larger sample of older Black adults than the population prevalence. While this may introduce potential bias, we see including a larger population of Black older adults from multiple sites across the country as a strength given that most aging cohort studies have an overrepresentation of White participants. Despite this larger cohort of Black older adults, our sample was constrained by a paucity of data for other racial/ethnic groups and remains a limitation of the ACTIVE cohort as a whole. Our individual and aggregate VRF burden measures were comprised primarily of self-report and medication data. This type of measurement is unable to capture the potential effects of disease length or severity. Inclusion of disease duration and other objective proxies for vascular health (e.g. A1C, LDL levels) would be helpful in future studies to assist in better understanding these relationships. Additionally, while this study attempted to include previously identified variables which may have contributed to disparities in cognition (e.g. educational attainment, vocabulary as proxy for educational quality), additional exploration of social determinants that likely impact cognition and vascular health will be explored in future studies. This study also only examined MCI prevalence; future studies should examine the associations between VRFs, race, and incident MCI to better capture the longer-term effects in late life.
Although there is a growing body of literature on racial disparities and vascular risk factors in MCI, few studies to date have examined racial disparities, VRFs, and MCI subtype risk in concert. We see the inclusion of interactions to explore these relationships as a relative strength. Further, many studies do not utilize the subtype-specific MCI classification. Use of a comprehensive neuropsychological criteria and inclusion of MCI subtype may assist in better determining primary etiologies of vascular/mixed dementia and/or primarily AD and is of particular importance given neuropathological evidence that even within participants diagnosed with clinical AD, Black participants had a greater mixed pathology (e.g., vascular, Lewy body) than Whites (Barnes et al., 2015; Yaffe et al., 2006). Results from this study highlight the continued need for work on cognitive impairment to be increasingly mindful of representation of diverse elders and including rich data regarding social determinants and health conditions that may drive any observed disparities.
Acknowledgements
The current study is supported by NIA R01 AG056486. The ACTIVE Cognitive Training Trial was supported by grants from the National Institutes of Health to six field sites and the coordinating center, including: Hebrew Senior-Life, Boston (NR04507), the Indiana University School of Medicine (NR04508), the Johns Hopkins University (AG014260), the New England Research Institutes (AG014282), the Pennsylvania State University (AG14263), the University of Alabama at Birmingham (AG14289), and the University of Florida (AG014276). Lindsay Rotblatt is currently supported by the National Institute on Aging Kirschstein Individual Predoctoral Fellowship F31AG063412-01. She previously received support from the National Institute on Aging T32 Predoctoral Training Fellowship AG020499. Dr. Kelsey Thomas is supported by the U.S. Department of Veterans Affairs Clinical Sciences Research and Development Service (Career Development Award-2 1IK2CX001865) and the Alzheimer’s Association (AARF-17-528918). Dr. Adrienne Aiken-Morgan is supported by the National Institute on Aging (Diversity Supplement Award 3RF1AG022018-11S2) and the National Science Foundation (2033926). LJR, AAM, AH, KRT report no disclosures. Dr. Michael Marsiske has received an in-kind contribution of 72 software licenses from the Posit Science Company (licensees of the Useful Field of View testing and training programs described in this manuscript) for another study (funded by the Robert Wood Johnson Foundation). The opinions expressed here are those of the authors and do not necessarily reflect those of the funding agencies, academic, research, governmental institutions, or corporations involved.
Footnotes
Author Note: No conflicts of interest to report.
References
- AHFS Drug Information. (2015). Bethesda, MD: Board of Directors of the American Society of Hospital Pharmacists. [Google Scholar]
- Aiken Morgan AT, Marsiske M, Dzierzewski JM, Jones RN, Whitfield KE, Johnson KE, & Cresci MK (2010). Race-related cognitive test bias in the active study: a mimic model approach. Exp Aging Res, 36(4), 426–452. doi: 10.1080/0361073x.2010.507427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albert MS, DeKosky ST, Dickson D, Dubois B, Feldman HH, Fox NC, … Phelps CH (2011). The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement, 7(3), 270–279. doi: 10.1016/j.jalz.2011.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes LL, & Bennett DA (2014). Alzheimer’s disease in African Americans: risk factors and challenges for the future. Health affairs (Project Hope), 33(4), 580–586. doi: 10.1377/hlthaff.2013.1353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes LL, Leurgans S, Aggarwal NT, Shah RC, Arvanitakis Z, James BD, … Schneider JA (2015). Mixed pathology is more likely in black than white decedents with Alzheimer dementia. Neurology, 85(6), 528–534. doi: 10.1212/WNL.0000000000001834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bondi MW, Edmonds EC, Jak AJ, Clark LR, Delano-Wood L, McDonald CR, … Salmon DP (2014). Neuropsychological criteria for mild cognitive impairment improves diagnostic precision, biomarker associations, and progression rates. J Alzheimers Dis, 42(1), 275–289. doi: 10.3233/jad-140276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewster PW, Melrose RJ, Marquine MJ, Johnson JK, Napoles A, MacKay-Brandt A, … Mungas D (2014). Life experience and demographic influences on cognitive function in older adults. Neuropsychology, 28(6), 846–858. doi: 10.1037/neu0000098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrd D, Thorpe R, & Whitfield K (2018). Greater disease burden, greater risk? Exploring cognitive change and health status among older Blacks. Innovation in Aging, 2(Suppl 1), 645–645. doi: 10.1093/geroni/igy023.2408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon JA, Moffitt P, Perez-Moreno AC, Walters MR, Broomfield NM, McMurray JJV, & Quinn TJ (2017). Cognitive Impairment and Heart Failure: Systematic Review and Meta-Analysis. J Card Fail, 23(6), 464–475. doi: 10.1016/j.cardfail.2017.04.007 [DOI] [PubMed] [Google Scholar]
- Carmasin JS, Mast BT, Allaire JC, & Whitfield KE (2014). Vascular risk factors, depression, and cognitive change among African American older adults. Int J Geriatr Psychiatry, 29(3), 291–298. doi: 10.1002/gps.4007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho JO, Tommet D, Crane PK, Thomas ML, Claxton A, Habeck C, … Romero HR (2014). Deconstructing Racial Differences: The Effects of Quality of Education and Cerebrovascular Risk Factors. The Journals of Gerontology: Series B, 70(4), 545–556. doi: 10.1093/geronb/gbu086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cervilla JA, Prince M, & Mann A (2000). Smoking, drinking, and incident cognitive impairment: a cohort community based study included in the Gospel Oak project. J Neurol Neurosurg Psychiatry, 68(5), 622–626. doi: 10.1136/jnnp.68.5.622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke PJ, Weuve J, Barnes L, Evans DA, & Mendes de Leon CF (2015). Cognitive decline and the neighborhood environment. Annals of epidemiology, 25(11), 849–854. doi: 10.1016/j.annepidem.2015.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooksey-Stowers K, Schwartz MB, & Brownell KD (2017). Food Swamps Predict Obesity Rates Better Than Food Deserts in the United States. International journal of environmental research and public health, 14(11), 1366. doi: 10.3390/ijerph14111366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corral I, Landrine H, Hao Y, Zhao L, Mellerson JL, & Cooper DL (2012). Residential segregation, health behavior and overweight/obesity among a national sample of African American adults. J Health Psychol, 17(3), 371–378. doi: 10.1177/1359105311417191 [DOI] [PubMed] [Google Scholar]
- Davis SK, Gebreab S, Quarells R, & Gibbons GH (2014). Social determinants of cardiovascular health among black and white women residing in Stroke Belt and Buckle regions of the South. Ethnicity & disease, 24(2), 133–143. [PMC free article] [PubMed] [Google Scholar]
- Ekstrom R, French J, Harman H, & Derman D (1976). Kit of factor-referenced cognitive tests (Rev. Ed.). Princeton, NJ: Educational Testing Service. [Google Scholar]
- Eliassen CF, Reinvang I, Selnes P, Grambaite R, Fladby T, & Hessen E (2017). Biomarkers in subtypes of mild cognitive impairment and subjective cognitive decline. Brain and behavior, 7(9), e00776–e00776. doi: 10.1002/brb3.776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elo IT, & Preston SH (1997). Racial and Ethnic Differences in the Health of Older Americans Racial and ethnic differences in American mortality at older ages (pp. 10–43). Washington, D.C. : National Academies Press. [PubMed] [Google Scholar]
- Feinkohl I, Janke J, Hadzidiakos D, Slooter A, Winterer G, Spies C, & Pischon T (2019). Associations of the metabolic syndrome and its components with cognitive impairment in older adults. BMC geriatrics, 19(1), 77–77. doi: 10.1186/s12877-019-1073-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferman TJ, Smith GE, Kantarci K, Boeve BF, Pankratz VS, Dickson DW, … Petersen RC (2013). Nonamnestic mild cognitive impairment progresses to dementia with Lewy bodies. Neurology, 81(23), 2032–2038. doi: 10.1212/01.wnl.0000436942.55281.47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiscella K, & Sanders MR (2016). Racial and Ethnic Disparities in the Quality of Health Care. Annual Review of Public Health, 37(1), 375–394. doi: 10.1146/annurev-publhealth-032315-021439 [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, & McHugh PR (1975). “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res, 12(3), 189–198. [DOI] [PubMed] [Google Scholar]
- Ganguli M, Dodge HH, Shen C, & DeKosky ST (2004). Mild cognitive impairment, amnestic type: an epidemiologic study. Neurology, 63(1), 115–121. doi: 10.1212/01.wnl.0000132523.27540.81 [DOI] [PubMed] [Google Scholar]
- Giau VV, Bagyinszky E, & An SSA (2019). Potential Fluid Biomarkers for the Diagnosis of Mild Cognitive Impairment. International journal of molecular sciences, 20(17), 4149. doi: 10.3390/ijms20174149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glymour MM, & Manly JJ (2008). Lifecourse social conditions and racial and ethnic patterns of cognitive aging. Neuropsychol Rev, 18(3), 223–254. doi: 10.1007/s11065-008-9064-z [DOI] [PubMed] [Google Scholar]
- Graham G (2015). Disparities in cardiovascular disease risk in the United States. Current cardiology reviews, 11(3), 238–245. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havranek EP, Mujahid MS, Barr DA, Blair IV, Cohen MS, Cruz-Flores S, … Yancy CW (2015). Social Determinants of Risk and Outcomes for Cardiovascular Disease: A Scientific Statement From the American Heart Association. Circulation, 132(9), 873–898. doi: 10.1161/cir.0000000000000228 [DOI] [PubMed] [Google Scholar]
- Ighodaro ET, Nelson PT, Kukull WA, Schmitt FA, Abner EL, Caban-Holt A, … Fernander A (2017). Challenges and Considerations Related to Studying Dementia in Blacks/African Americans. J Alzheimers Dis, 60(1), 1–10. doi: 10.3233/jad-170242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CR Jr., Petersen RC, Xu YC, O’Brien PC, Smith GE, Ivnik RJ, … Kokmen E (1999). Prediction of AD with MRI-based hippocampal volume in mild cognitive impairment. Neurology, 52(7), 1397–1403. doi: 10.1212/wnl.52.7.1397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jak AJ, Bondi MW, Delano-Wood L, Wierenga C, Corey-Bloom J, Salmon DP, & Delis DC (2009). Quantification of five neuropsychological approaches to defining mild cognitive impairment. Am J Geriatr Psychiatry, 17(5), 368–375. doi: 10.1097/JGP.0b013e31819431d5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jak AJ, Preis SR, Beiser AS, Seshadri S, Wolf PA, Bondi MW, & Au R (2016). Neuropsychological Criteria for Mild Cognitive Impairment and Dementia Risk in the Framingham Heart Study. J Int Neuropsychol Soc, 22(9), 937–943. doi: 10.1017/S1355617716000199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jobe JB, Smith DM, Ball K, Tennstedt SL, Marsiske M, Willis SL, … Kleinman K (2001). ACTIVE: a cognitive intervention trial to promote independence in older adults. Control Clin Trials, 22(4), 453–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knopman DS, Gottesman RF, Sharrett AR, Tapia AL, DavisThomas S, Windham BG, … Mosley TH Jr. (2018). Midlife vascular risk factors and midlife cognitive status in relation to prevalence of mild cognitive impairment and dementia in later life: The Atherosclerosis Risk in Communities Study. Alzheimers Dement, 14(11), 1406–1415. doi: 10.1016/j.jalz.2018.03.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakerveld J, & Mackenbach J (2017). The Upstream Determinants of Adult Obesity. Obesity facts, 10(3), 216–222. doi: 10.1159/000471489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luchsinger JA, Reitz C, Honig LS, Tang MX, Shea S, & Mayeux R (2005). Aggregation of vascular risk factors and risk of incident Alzheimer disease. Neurology, 65(4), 545–551. doi: 10.1212/01.wnl.0000172914.08967.dc [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luchsinger JA, Reitz C, Patel B, Tang MX, Manly JJ, & Mayeux R (2007). Relation of diabetes to mild cognitive impairment. Arch Neurol, 64(4), 570–575. doi: 10.1001/archneur.64.4.570 [DOI] [PubMed] [Google Scholar]
- Manly JJ, Jacobs DM, Touradji P, Small SA, & Stern Y (2002). Reading level attenuates differences in neuropsychological test performance between African American and White elders. J Int Neuropsychol Soc, 8(3), 341–348. doi: 10.1017/s1355617702813157 [DOI] [PubMed] [Google Scholar]
- Marsiske M, Dzierzewski JM, Thomas KR, Kasten L, Jones RN, Johnson KE, … Rebok GW (2013). Race-related disparities in 5-year cognitive level and change in untrained ACTIVE participants. J Aging Health, 25(8 Suppl), 103s–127s. doi: 10.1177/0898264313497794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauri M, Corbetta S, Pianezzola C, Ambrosoni E, Riboldazzi G, & Bono G (2008). Progression to vascular dementia of patients with mild cognitive impairment: relevance of mild parkinsonian signs. Neuropsychiatric disease and treatment, 4(6), 1267–1271. doi: 10.2147/ndt.s4288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntosh MS, Kumar V, Kalynych C, Lott M, Hsi A, Chang J-L, & Lerman RH (2013). Racial Differences in Blood Lipids Lead to Underestimation of Cardiovascular Risk in Black Women in a Nested observational Study. Global advances in health and medicine, 2(2), 76–79. doi: 10.7453/gahmj.2012.076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta KM, & Yeo GW (2017). Systematic review of dementia prevalence and incidence in United States race/ethnic populations. Alzheimers Dement, 13(1), 72–83. doi: 10.1016/j.jalz.2016.06.2360 [DOI] [PubMed] [Google Scholar]
- Morris JC (2006). Mild Cognitive Impairment Is Early-Stage Alzheimer Disease: Time to Revise Diagnostic Criteria. Arch Neurol, 63(1), 15–16. doi: 10.1001/archneur.63.1.15 [DOI] [PubMed] [Google Scholar]
- Nanna MG, Navar AM, Zakroysky P, Xiang Q, Goldberg AC, Robinson J, … Peterson ED (2018). Association of Patient Perceptions of Cardiovascular Risk and Beliefs on Statin Drugs With Racial Differences in Statin Use: Insights From the Patient and Provider Assessment of Lipid Management Registry. JAMA Cardiology, 3(8), 739–748. doi: 10.1001/jamacardio.2018.1511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parekh T, Desai R, Pemmasani S, & Cuellar A (2020). IMPACT OF SOCIAL DETERMINANTS OF HEALTH ON CARDIOVASCULAR DISEASES. J Am Coll Cardiol, 75(11_Supplement_2), 1989–1989. doi: 10.1016/S0735-1097(20)32616-4 [DOI] [Google Scholar]
- Petersen RC (2004). Mild cognitive impairment as a diagnostic entity. J Intern Med, 256(3), 183–194. doi: 10.1111/j.1365-2796.2004.01388.x [DOI] [PubMed] [Google Scholar]
- Petersen RC, Doody R, Kurz A, Mohs RC, Morris JC, Rabins PV, … Winblad B (2001). Current concepts in mild cognitive impairment. Arch Neurol, 58(12), 1985–1992. doi: 10.1001/archneur.58.12.1985 [DOI] [PubMed] [Google Scholar]
- Smith GE, & Bondi MW (2013). Mild Cognitive Impairment and Dementia: Definitions, Diagnosis, and Treatment. New York, NY: Oxford University Press. [Google Scholar]
- Sturman MT, de Leon CF, Bienias JL, Morris MC, Wilson RS, & Evans DA (2008). Body mass index and cognitive decline in a biracial community population. Neurology, 70(5), 360–367. doi: 10.1212/01.wnl.0000285081.04409.bb [DOI] [PubMed] [Google Scholar]
- Sudo FK, Alves CEO, Alves GS, Ericeira-Valente L, Tiel C, Moreira DM, … Engelhardt E (2012). Dysexecutive syndrome and cerebrovascular disease in non-amnestic mild cognitive impairment: A systematic review of the literature. Dementia & neuropsychologia, 6(3), 145–151. doi: 10.1590/S1980-57642012DN06030006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weuve J, Barnes LL, Mendes de Leon CF, Rajan KB, Beck T, Aggarwal NT, … Evans DA (2018). Cognitive Aging in Black and White Americans: Cognition, Cognitive Decline, and Incidence of Alzheimer Disease Dementia. Epidemiology (Cambridge, Mass.), 29(1), 151–159. doi: 10.1097/EDE.0000000000000747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winblad B, Palmer K, Kivipelto M, Jelic V, Fratiglioni L, Wahlund LO, … Petersen RC (2004). Mild cognitive impairment--beyond controversies, towards a consensus: report of the International Working Group on Mild Cognitive Impairment. J Intern Med, 256(3), 240–246. doi: 10.1111/j.1365-2796.2004.01380.x [DOI] [PubMed] [Google Scholar]
- Yaffe K, Petersen RC, Lindquist K, Kramer J, & Miller B (2006). Subtype of mild cognitive impairment and progression to dementia and death. Dement Geriatr Cogn Disord, 22(4), 312–319. doi: 10.1159/000095427 [DOI] [PubMed] [Google Scholar]
- Zahodne LB, Morris EP, Sharifian N, Zaheed AB, Kraal AZ, & Sol K (in press). Everday Discrimination and Subsequent Cognitive Abilities Across Five Domains Neuropsychology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou Y, Zhu Q, Deng Y, Duan J, Pan L, Tu Q, … Lu Y (2014). Vascular risk factors and mild cognitive impairment in the elderly population in Southwest China. Am J Alzheimers Dis Other Demen, 29(3), 242–247. doi: 10.1177/1533317513517042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuelsdorff M, Okonkwo OC, Norton D, Barnes LL, Graham KL, Clark LR, … Gleason CE (2020). Stressful Life Events and Racial Disparities in Cognition Among Middle-Aged and Older Adults. J Alzheimers Dis, 73(2), 671–682. doi: 10.3233/jad-190439 [DOI] [PMC free article] [PubMed] [Google Scholar]


