Abstract
The pathogenesis and natural history of intracranial aneurysm (IA) remains poorly understood. To this end, animal models with induced cerebral vessel lesions mimicking human aneurysms have provided the ability to greatly expand our understanding. In this review, we comprehensively searched the published literature to identify studies that endogenously induced IA formation in animals. Studies that constructed aneurysms (i.e. by surgically creating a sac) were excluded. From the eligible studies, we reported information including the animal species, method for aneurysm induction, aneurysm definitions, evaluation methods, aneurysm characteristics, formation rate, rupture rate, and time course. Between 1960 and 2019, 174 articles reported endogenous animal models of IA. The majority used flow modification, hypertension, and vessel wall weakening (i.e. elastase treatment) to induce IAs, primarily in rats and mice. Most studies utilized subjective or qualitative descriptions to define experimental aneurysms and histology to study them. In general, experimental IAs resembled the pathobiology of the human disease in terms of internal elastic lamina loss, medial layer degradation, and inflammatory cell infiltration. After the early 2000s, many endogenous animal models of IA began to incorporate state-of-the art technology, such as gene expression profiling and 9.4T magnetic resonance imaging (MRI) in vivo imaging, to quantitatively analyze the biological mechanisms of IA. Future studies aimed at longitudinally assessing IA pathobiology in models that incorporate aneurysm growth will likely have the largest impact on our understanding of the disease. We believe this will be aided by high-resolution, small animal, survival imaging, in situ live-cell imaging, and next-generation omics technology.
Keywords: Animal model, cerebral aneurysm, intracranial aneurysm, natural history, vascular disease, review
Introduction
Intracranial aneurysm (IA) is a multifaceted disease that is characterized by pathologic outpouchings of the cerebral vascular wall. An estimated 3–5% of the general population harbors an IA, [1] but the exact prevalence is unknown because most are asymptomatic. The rupture of an IA is the most common cause of nontraumatic subarachnoid hemorrhage, a devastating event that carries high rates of mortality, morbidity, and disability, as well as high health care costs.[2] Although IAs are a major public healthcare concern, their pathogenesis and natural history remain poorly understood. Aside from studies involving imaging, the only investigations of human IA pathobiology have been performed using precious clinical samples that are rarely obtained from autopsy or clipped IAs. Furthermore, their examination has only provided biological information about the aneurysm’s endpoint, not its genesis or development over time, which limits delineating mechanisms of natural history. To this end, animal models with induced cerebral vessel aneurysm mimicking the human disease have shown the ability to expand our understanding of IA, affording opportunities to unravel disease mechanisms, identify biomarkers, and develop potential pharmacotherapies.
Generally, animal models of IA can be categorized into two classes. The first class induces aneurysmal lesions spontaneously in the cerebral vasculature in response to manipulations that incite risk factors relevant to the disease. These are what we are calling endogenous IA models. The second class surgically creates an aneurysm pouch, typically in extracranial arteries. These models include the creation of vein pouches and elastase-treated, distally-ligated extracranial artery stumps.[3–5] Such surgical models have been integral in evaluating endovascular IA interventions (e.g. aneurysm coiling and stenting). As they have been thoroughly reviewed elsewhere,[6–8] they will not be discussed here. Instead, this review will focus on endogenous IA models, which have provided the most knowledge of aneurysm pathobiology to date.
Nearly six decades of research have been devoted to the development and implementation of endogenous IA models in various animal species. In the last 20 years, extensive use of these models has led to a deeper understanding of the biological and molecular basis of IA formation and development.
Materials and Methods
For this review, we comprehensively searched the published literature. In PubMed, we used Medical Subject Headings (MeSH) terms to identify studies that used animal models with endogenously induced IAs between 01/01/1960 and 12/31/2019 (Figure 1). We included the MeSH terms “intracranial aneurysm”, “cerebral aneurysm”, “animal model”, “animal models, experimental”, and “animal model, laboratory”, and excluded MeSH terms “surgical”, “structure, surgically created”, and “medical device”. Next, we excluded review articles, as well as re-analyses of existing datasets, letters and commentaries, published datasets, and other articles that did not meet our search terms. In all, we identified 174 original publications between 1960 and 2019 (Table 1), from which we extracted the animal species, the method for aneurysm induction, the definition of aneurysmal lesion(s), IA evaluation methods, aneurysm characteristics, the IA formation and rupture rates, and the developmental time course.
Figure 1: A flowchart detailing the literature survey that yielded the publications analyzed in this study.
In PubMed, we searched for published animal model studies of intracranial aneurysm, excluding those that used surgically created aneurysms (primarily to study medical devices). Furthermore, we manually excluded review articles, as well as other publications (i.e. commentaries on other articles) that did not fit our search criteria.
Table 1:
174 studies involving endogenous animal models of IA.*
| First Author | Year | Journal Abbreviation | Animal | Manipulation | Number of experimental animals | Aneurysm Rate | Rupture Rate | Max. Time of Evaluation (Days) | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Flow Modification | Hypertension | Estrogen Manipulation | Connective Tissue Weakness | Genetics | ||||||||
| White[9] | 1961 | J Neurosurg | Canine | X | 10 | 30% | NR | 21 | ||||
| Hassler[10] | 1963 | J Neurosurg | Rabbit | X | NR | NR | NR | 150 | ||||
| Uchida[87] | 1974 | Kumamoto Med J | Rabbit | X | X | NR | NR | NR | 63 | |||
| Uchida[13] | 1974 | Kumamoto Med J | Rabbit | X | X | NR | NR | NR | 63 | |||
| Lee[11] | 1978 | J Pathol | Rat | X | 14 | 71% | NR | 98 | ||||
| Kido[80] | 1978 | Neuroradiology | Rabbit | X | 4 | 100% | 0% | NR | ||||
| Hashimoto[14] | 1978 | Surg Neurol | Rat | X | X | X | 55 | 11% | 4% | 60 | ||
| Hashimoto[88] | 1979 | Surg Neurol | Rat | X | X | X | 30 | 37% | 3% | 147 | ||
| Hashimoto[162] | 1979 | Surg Neurol | Rat | X | X | X | NR | NR | NR | 147 | ||
| Nagata[119] | 1979 | Surg Neurol | Rat | X | X | X | NR | NR | 0% | 90 | ||
| Hashimoto[163] | 1979 | Neurol Med Chir | Rat | X | X | X | 45 | NR | NR | NR | ||
| Hashimoto[164] | 1979 | Nihon Geka Hokan | Rat | X | X | X | NR | NR | NR | NR | ||
| Nagata[120] | 1980 | Surg Neurol | Rat | X | X | X | NR | 50% | 32% | 112 | ||
| Hashimoto[165] | 1980 | Surg Neurol | Rat | X | X | X | 72 | 40% | NR | 147 | ||
| Suzuki[30] | 1980 | J Neruosurg | Rat | X | X | X | X | 43 | 14% | 0% | 70 | |
| Zhou[94] | 1985 | Chin Med J | Rat | X | X | X | X | 32 | 40% | NR | 126 | |
| Alvarez[37] | 1986 | J Neruosurg | Rat | X | X | 19 | 42% | NR | 112 | |||
| Kojima[118] | 1986 | Stroke | Rat | X | X | 20 | 15% | NR | 84 | |||
| Hazama[166] | 1986 | Am J Pathol | Rat | X | X | 22 | 36% | NR | 90 | |||
| Hashimoto[167] | 1987 | J Neruosurg | Primate | X | X | X | 7 | 29% | 0% | 365 | ||
| Roda[168] | 1988 | Acta Neurochir Suppl | Rat | X | 8 | 38% | NR | 240 | ||||
| Kim[81] | 1988 | Stroke | Rat | X | X | 13 | 15% | NR | 90 | |||
| Kim[169] | 1989 | Surg Neurol | Primate | X | X | X | 7 | 86% | NR | 365 | ||
| Yamazoe[133] | 1990 | Stroke | Rat | X | X | X | 19 | 58% | NR | 112 | ||
| Kang[170] | 1990 | J Neruosurg | Rat | X | X | X | 32 | 13% | NR | 42 | ||
| Nakatani[171] | 1991 | J Neruosurg | Rat | X | X | X | NR | NR | 90 | |||
| Kim[172] | 1992 | Surg Neurol | Primate | X | X | 3 | 67% | NR | 180 | |||
| Nakatani[173] | 1993 | Acta Neurochir | Rat | X | X | X | 12 | 50% | NR | 90 | ||
| Kim[174] | 1993 | Acta Neurochir | Rat | X | X | NR | 0% | NR | 90 | |||
| Futami[175] | 1995 | Stroke | Rat | X | X | 25 | 24% | NR | 90 | |||
| Futami[176] | 1995 | Stroke | Rat | X | X | 15 | 58% | NR | 90 | |||
| Kondo[177] | 1997 | Stroke | Rat | X | X | 35 | 54% | 0% | 365 | |||
| Coutard[49] | 1997 | Stroke | Rat | X | X | 43 | 26% | NR | 405 | |||
| Kondo[178] | 1998 | Stroke | Rat | X | X | 35 | 31% | NR | 180 | |||
| Futami[179] | 1998 | Surg Neurol | Rat | X | X | 23 | 30% | NR | 90 | |||
| Coutard[180] | 1999 | Int J Exp Pathol | Mouse | X | X | 31 | 19% | 3% | 360 | |||
| Fukuda[55] | 2000 | Circulation | Rat | X | X | 8 | 100% | NR | 90 | |||
| Coutard[50] | 2000 | Stroke | Rat | X | X | 142 | 30% | 16% | NR | |||
| Morimoto[181] | 2002 | Stroke | Mouse | X | X | 18 | 33% | NR | 120 | |||
| Zhang[109] | 2003 | Chin Med J | Rat | X | 20 | 10% | NR | 365 | ||||
| Sadamasa[51] | 2003 | Stroke | Mouse | X | X | X | 24 | 38% | NR | 120 | ||
| Jamous[31] | 2005 | J Neurosurg | Rat | X | X | 12 | 60% | NR | 60 | |||
| Jamous[32] | 2005 | J Neurosurg | Rat | X | X | X | 15 | 53% | 0% | 90 | ||
| Jamous[33] | 2005 | J Neurosurg | Rat | X | X | X | 11 | 18% | 0% | 90 | ||
| Kaufmann[73] | 2006 | Neuroradiology | Rat | X | 37 | 19% | NR | 365 | ||||
| Moriwaki[182] | 2006 | Stroke | Mouse | X | X | X | 8 | 50% | NR | 150 | ||
| Aoki[18] | 2007 | Stroke | Rat | X | X | X | 21 | 90% | NR | 90 | ||
| Sadamasa[183] | 2007 | J Neurosurg | Rat | X | X | X | 28 | 100% | NR | 90 | ||
| Aoki[19] | 2007 | Stroke | Rat, Mouse | X | X | X | 16 | 19% | NR | 150 | ||
| Abruzzo[184] | 2007 | Curr Neurovasc Res | Mouse | X | 13 | 31% | NR | 690 | ||||
| Jamous[185] | 2007 | J Neurosurg | Rat | X | X | 40 | 10% | 0% | 60 | |||
| Aoki[22] | 2007 | Circulation | Rat, Mouse | X | X | X | 10 | 30% | NR | 90 | ||
| Aoki[78] | 2008 | Curr Neurovasc Res | Rat | X | X | X | 20 | NR | NR | 30 | ||
| Aoki[79] | 2008 | Stroke | Rat | X | X | 10 | NR | NR | 90 | |||
| Sibon[186] | 2008 | J Neurosurg | Rat | X | X | NR | NR | NR | 180 | |||
| Sadamasa[147] | 2008 | J Vasc Res | Rat | X | X | X | 18 | NR | NR | 90 | ||
| Aoki[23] | 2008 | Int J Mol Med | Rat, Mouse | X | X | X | 25 | 16% | NR | 150 | ||
| Gao[187] | 2008 | Stroke | Rabbit | X | 6 | NR | NR | 84 | ||||
| Aoki[15] | 2008 | Stroke | Rat | X | X | X | 19 | 90% | NR | 90 | ||
| Aoki[16] | 2008 | Int J Mol Med | Rat | X | X | X | 27 | NR | NR | 30 | ||
| Aoki[115] | 2009 | Stroke | Rat, Mouse | X | X | X | 15 | 20% | NR | 90 | ||
| Aoki[188] | 2009 | Neurosurgery | Rat | X | X | 20 | NR | NR | 90 | |||
| Tamura[189] | 2009 | J Hypertens | Rat | X | X | X | 18 | 25% | NR | 90 | ||
| Aoki[17] | 2009 | Arterioscler Thromb Vasc Biol | Rat | X | X | X | NR | NR | NR | 90 | ||
| Aoki[26] | 2009 | Lab Invest | Rat, Mouse | X | X | X | NR | NR | NR | 90 | ||
| Tada[34] | 2009 | Hypertension | Rat | X | X | X | 32 | 59% | NR | 91 | ||
| Aoki[25] | 2009 | Int J Mol Med | Rat | X | X | X | NR | NR | NR | 30 | ||
| Nuki[56] | 2009 | Hypertension | Mouse | X | X | 44 | 77% | NR | 14 | |||
| Eldawoody[35] | 2009 | Brain Res | Rat | X | X | X | 32 | 94% | NR | 90 | ||
| Eldawoody[190] | 2010 | Neurosci Lett | Rat | X | X | X | 16 | 100% | NR | 60 | ||
| Dai[191] | 2010 | AJNR Am J Neuroradiol | Rabbit | X | 30 | 0% | NR | 231 | ||||
| Kimura[192] | 2010 | Brain Res | Rat | X | X | X | 14 | 50% | NR | 90 | ||
| Yagi[193] | 2010 | Neurosurgery | Rat | X | X | X | 20 | 30% | NR | 90 | ||
| Tada[194] | 2010 | J Hypertens | Rat | X | X | X | 14 | NR | NR | 91 | ||
| Metaxa[70] | 2010 | Stroke | Rabbit | X | NR | NR | NR | 5 | ||||
| Aoki[24] | 2010 | J Neurosurg | Rat, Mouse | X | X | X | NR | NR | NR | 90 | ||
| Ishibashi[195] | 2010 | Curr Neruovasc Res | Rat | X | X | X | NR | NR | NR | 30 | ||
| Aoki[20] | 2010 | Gene Ther | Rat | X | X | X | NR | NR | NR | 90 | ||
| Meng[126] | 2011 | J Neurosurg | Rabbit | X | 17 | NR | NR | 189 | ||||
| Kanematsu[196] | 2011 | Stroke | Mouse | X | X | X | 10 | 60% | NR | 21 | ||
| Kolega[127] | 2011 | J Vasc Res | Rabbit | X | NR | NR | NR | 5 | ||||
| Aoki[28] | 2011 | Br J Pharmacol | Rat, Mouse | X | X | X | 36 | 61% | NR | 150 | ||
| Aoki[27] | 2011 | Lab Invest | Rat, Mouse | X | X | X | 37 | 56% | NR | 150 | ||
| Gopal[110] | 2011 | J Thromb Thrombolysis | Mouse | X | X | 12 | 33% | NR | 45 | |||
| Tada[197] | 2011 | Stroke | Rat | X | X | X | 19 | 47% | 0% | 168 | ||
| Xu[198] | 2011 | Neuroscience | Rat | X | X | NR | NR | NR | 90 | |||
| Xu[199] | 2011 | Neruosci Lett | Rat | X | 15 | 20% | NR | 90 | ||||
| Aoki[21] | 2012 | Neurosurgery | Rat | X | X | X | 29 | NR | NR | 60 | ||
| Dolan[200] | 2012 | Am J Physiol Cell Physiol | Rabbit | X | NR | NR | NR | 5 | ||||
| Cai[74] | 2012 | J Clin Neurosci | Rat | X | 15 | 20% | NR | 90 | ||||
| Ishibashi[201] | 2012 | Neurosurgery | Rat | X | X | X | 20 | NR | NR | 30 | ||
| Makino[57] | 2012 | Stroke | Mouse | X | X | 8 | 63% | 50% | 28 | |||
| Mandelbaum[116] | 2013 | PloS One | Rabbit | X | 45 | 100% | 0% | 180 | ||||
| Dolan[134] | 2013 | Am J Physiol Cell Physiol | Rabbit | X | NR | 100% | 0% | 150 | ||||
| Ali[202] | 2013 | J Cereb Blood Flow Metab | Rat | X | X | 24 | 100% | 0% | 14 | |||
| Wada[203] | 2013 | Transl Stroke Res | Mouse | X | X | 34 | 75% | 1% | 28 | |||
| Lee[204] | 2013 | J Korean Neurosurg Soc | Rat | X | X | NR | NR | NR | 90 | |||
| Li[205] | 2014 | West Indian Med J | Rabbit | X | NR | 100% | NR | 90 | ||||
| Hosaka[58] | 2014 | J Neurointerv Surg | Mouse | X | X | X | 40 | 98% | 43% | 90 | ||
| Tutino[117] | 2014 | J Cereb Blood Flow Metab | Rabbit | X | 6 | 100% | NR | 180 | ||||
| Tada[91] | 2014 | Stroke | Mouse | X | X | 16 | 75% | 50% | 28 | |||
| Aoki[144] | 2014 | Acta Neuropathol Comm | Rat, Mouse | X | X | X | 37 | NR | NR | 90 | ||
| Tada[90] | 2014 | Hypertension | Mouse | X | X | 19 | 74% | 58% | 21 | |||
| Starke[53] | 2014 | J Neuroinflammation | Mouse | X | X | 22 | 82% | 68% | 28 | |||
| Liaw[206] | 2014 | PLoS One | Rabbit | X | 7 | 100% | NR | 5 | ||||
| Tada[95] | 2014 | Neurosurgery | Mouse | X | X | NR | 36% | NR | 28 | |||
| Li[207] | 2014 | Brain res | Rat | X | X | NR | NR | NR | 60 | |||
| Fukuda[208] | 2014 | J Pharmacol Sci | Rat, Mouse | X | X | X | 24 | NR | NR | 1 | ||
| Yokoi[68] | 2014 | J Neurosurg | Rat | X | X | 36 | 100% | NR | 90 | |||
| Nowicki[209] | 2014 | Hypertension | Mouse | X | X | X | 15 | 67% | NR | 14 | ||
| Li[67] | 2015 | Neurochem Res | Rat | X | X | 20 | NR | NR | 60 | |||
| Zhao[61] | 2015 | J Clin Neurosci | Rat | X | X | X | 12 | 42% | 16% | 49 | ||
| Pena-Silva[155] | 2015 | Hypertension | Mouse | X | X | 14 | 86% | 64% | 21 | |||
| Lee[54] | 2015 | Circulation | Mouse | X | X | 8 | 60% | NR | 28 | |||
| Makino[156] | 2015 | J Cereb Blood Flow Metab | Mouse | X | X | 10 | 60% | 50% | 14 | |||
| Chu[210] | 2015 | Stroke | Mouse | X | X | 17 | 90% | 83% | 19 | |||
| Tutino[71] | 2015 | Anat Rec | Rabbit | X | X | X | 6 | 100% | NR | 180 | ||
| Shimada[111] | 2015 | J Cereb Blood Flow Metab | Mouse | X | X | 20 | 85% | 76% | 14 | |||
| Zhang[211] | 2015 | J Neuropathol Exp Neurol | Mouse | X | X | X | 30 | NR | NR | NR | ||
| Hasan[112] | 2015 | Hypertension | Mouse | X | X | X | 27 | 81% | 59% | 21 | ||
| Shimada[157] | 2015 | Stroke | Mouse | X | X | 39 | 85% | 79% | 21 | |||
| Pena-Silva[212] | 2015 | Neurosurgery | Mouse | X | X | 11 | 73% | 0% | 21 | |||
| Wu[213] | 2015 | Cell Mol Biol | Rabbit | X | 48 | 27% | NR | 28 | ||||
| Wu[214] | 2015 | Cell Mol Biol | Rabbit | X | NR | NR | NR | NR | ||||
| Wu[215] | 2016 | Turk Neurosurg | Rat | X | X | X | 30 | 90% | NR | 180 | ||
| Jung[216] | 2016 | J Neuropathol Exp Neurol | Rat | X | X | 36 | 78% | 14% | 150 | |||
| Tutino[72] | 2016 | Curr Neruovasc Res | Rat, Rabbit | X | 18 | 50% | NR | 7 | ||||
| Chalouhi[217] | 2016 | Hypertension | Mouse | X | X | X | 14 | 100% | 100% | 19 | ||
| Zhu[124] | 2016 | J Neurointerv Surg | Mouse | X | 38 | 21% | 13% | 28 | ||||
| Sawyer[218] | 2016 | J Neuroinflammation | Mouse | X | X | NR | NR | 100% | 14 | |||
| Aoki[219] | 2016 | Acta Neuropathol Commun | Rat | X | X | X | NR | NR | NR | NR | ||
| Lee[220] | 2016 | J Clin Neurosci | Mouse | X | X | 15 | 100% | 100% | NR | |||
| Liu[221] | 2017 | Stem Cells | Mouse | X | X | NR | 74% | 81% | 21 | |||
| Miyamoto[222] | 2017 | J Cereb Blood Flow Metab | Rat | X | X | X | 21 | 40% | 81% | 90 | ||
| Yamamoto[223] | 2017 | Br J Pharmacol | Rat, Primate | X | X | X | X | 30 | 57% | NR | 364 | |
| Miyata[224] | 2017 | PLoS One | Rat | X | X | 15 | NR | NR | 60 | |||
| Ikedo[125] | 2017 | J Am Heart Assoc | Rat | X | X | X | 7 | NR | NR | 28 | ||
| Aoki[225] | 2017 | J Stroke Cerebrovasc Dis | Rat | X | X | X | NR | NR | NR | 5 | ||
| Liu[226] | 2017 | Shock | Mouse | X | NR | 70% | 75% | 20 | ||||
| Aoki[227] | 2017 | Sci Signal | Rat, Mouse | X | X | X | 24 | NR | NR | 224 | ||
| Maekawa[228] | 2017 | J Neuroinflammation | Rat | X | X | X | NR | 100% | 17% | 84 | ||
| Wu[229] | 2017 | Am J Trans Res | Rabbit | X | 35 | NR | NR | NR | ||||
| Kuwabara[230] | 2017 | Neurosurgery | Mouse | X | X | 11 | 91% | 90% | 21 | |||
| Labeyrie[231] | 2017 | Stroke | Mouse | X | X | 12 | 100% | 67% | 28 | |||
| Starke[151] | 2018 | Anrterioscler Thromb Vasc Biol | Mouse | X | X | 25 | 84% | 71% | 28 | |||
| Suzuki[232] | 2018 | Cerebrovasc Dis | Mouse | X | X | 54 | 67% | 81% | 21 | |||
| Hoh[96] | 2018 | J Am Heart Assoc | Mouse | X | X | X | X | 19 | 65% | NR | NR | |
| Quan[59] | 2018 | Frontier Pharmacol | Rat | X | X | X | 12 | 58% | 29% | 35 | ||
| Kamio[233] | 2018 | Stroke | Mouse | X | X | 22 | 68% | 47% | 21 | |||
| Ikedo[234] | 2018 | Biochem Biophys Res Commun | Rat | X | X | X | NR | NR | NR | NR | ||
| Liu[235] | 2018 | J Photochem Photobiol B | Rat | X | NR | NR | NR | NR | ||||
| Li[236] | 2018 | CNS Neurosci Ther | Rat | X | X | NR | NR | NR | 30 | |||
| Zhang[237] | 2018 | Biochem Biophys Res Commun | Rat | X | X | X | NR | NR | NR | NR | ||
| Hoh[238] | 2018 | J Neurointerv Surg | Mouse | X | NR | NR | NR | 21 | ||||
| Nowicki[239] | 2018 | J Neurointerv Surg | Mouse | X | X | X | NR | 100% | 48% | NR | ||
| Zhao[240] | 2018 | Mol Med Rep | Mouse | X | X | X | 25 | 72% | 44% | 14 | ||
| Shikata[241] | 2019 | Hypertension | Mouse | X | X | 19 | 80% | NR | NR | |||
| Pascale[242] | 2019 | J Cereb Blood Flow Metab | Mouse | X | X | 60 | 70% | 90% | 14 | |||
| Tutino[47] | 2019 | Cur neurovasc res | Mouse | X | 3 | 0% | NR | 80 | ||||
| Rajabzadeh-Oghaz[48] | 2019 | Proc SPIE Int Soc Opt Eng | Mouse | X | 3 | 0% | NR | 80 | ||||
| Miyata[69] | 2019 | Neurol Med Chir | Rat | X | NR | NR | NR | NR | ||||
| Wei[243] | 2019 | Med Sci Monit | Rat | X | X | NR | NR | NR | NR | |||
| Suzuki[63] | 2019 | Stroke | Mouse | X | X | NR | NR | 80% | 21 | |||
| Xiao[64] | 2019 | World Neurosurg | Rat, Rabbit | X | X | NR | NR | NR | NR | |||
| Ikedo[244] | 2019 | World Neurosurg | Rat | X | X | X | 11 | NR | NR | 90 | ||
| Ma[113] | 2019 | Mol red rep | Rat | X | X | NR | NR | NR | 28 | |||
| Yamaguchi[145] | 2019 | J Neurosurg | Rat | X | X | 42 | NR | 14% | 84 | |||
| Lai[245] | 2019 | Biosci Rep | Rat | X | X | 40 | NR | NR | 90 | |||
| Wei[246] | 2019 | Pharmazie | Rat | X | X | 9 | NR | NR | NR | |||
| Shimizu[146] | 2019 | J Neuropathol Exp Neurol | Rat | X | X | X | X | NR | NR | 13% | 28 | |
| Miyata[114] | 2019 | J Nuerosurg | Rat | X | X | X | 13 | 100% | 30% | 70 | ||
| Yang[247] | 2019 | Pharmacology | Rat | X | X | X | 32 | NR | NR | 120 | ||
| Feng[248] | 2019 | Clin Sci | Rat | X | X | NR | NR | 0% | 28 | |||
| Fan[249] | 2019 | Neuropharmacology | Rat | X | X | NR | NR | 0% | 30 | |||
Studies are presented chronologically. In cases were 0% was reported as the IA formation rate, the studies were still included because the authors reported aneurysm-like remodeling that was consistent with other, previous reports. Oftentimes, the rate of 0% was reported the observed lesion did not match strict criteria for IA that the author defined. Abbreviations: IA=intracranial aneurysm, NR=not reported.
In this review, we present a brief history of endogenous IA models and summarize the methods that have been employed for induction, investigation, and characterization of experimental IAs. We will also discuss the strengths and weaknesses of the various approaches and the relevance of these models to human disease.
Results
A History of Endogenous IA Model Development
Endogenous animal models of IA have been studied over the last 60 years. While clinical IA studies during that time largely focused on epidemiologic analyses and interventions, experimental IA investigations have tried to understand the disease pathophysiology, unravel mechanisms of IA formation, identify molecular targets for potential pharmacological therapies, and understand IA rupture. Figure 2 illustrates the chronology of the introduction of aneurysm models in terms of three aspects that will be discussed in this review: the animal species, the factors used to induce aneurysms, and the technology used for manipulation or assessment.
Figure 2: A timeline of important milestones in IA model development.
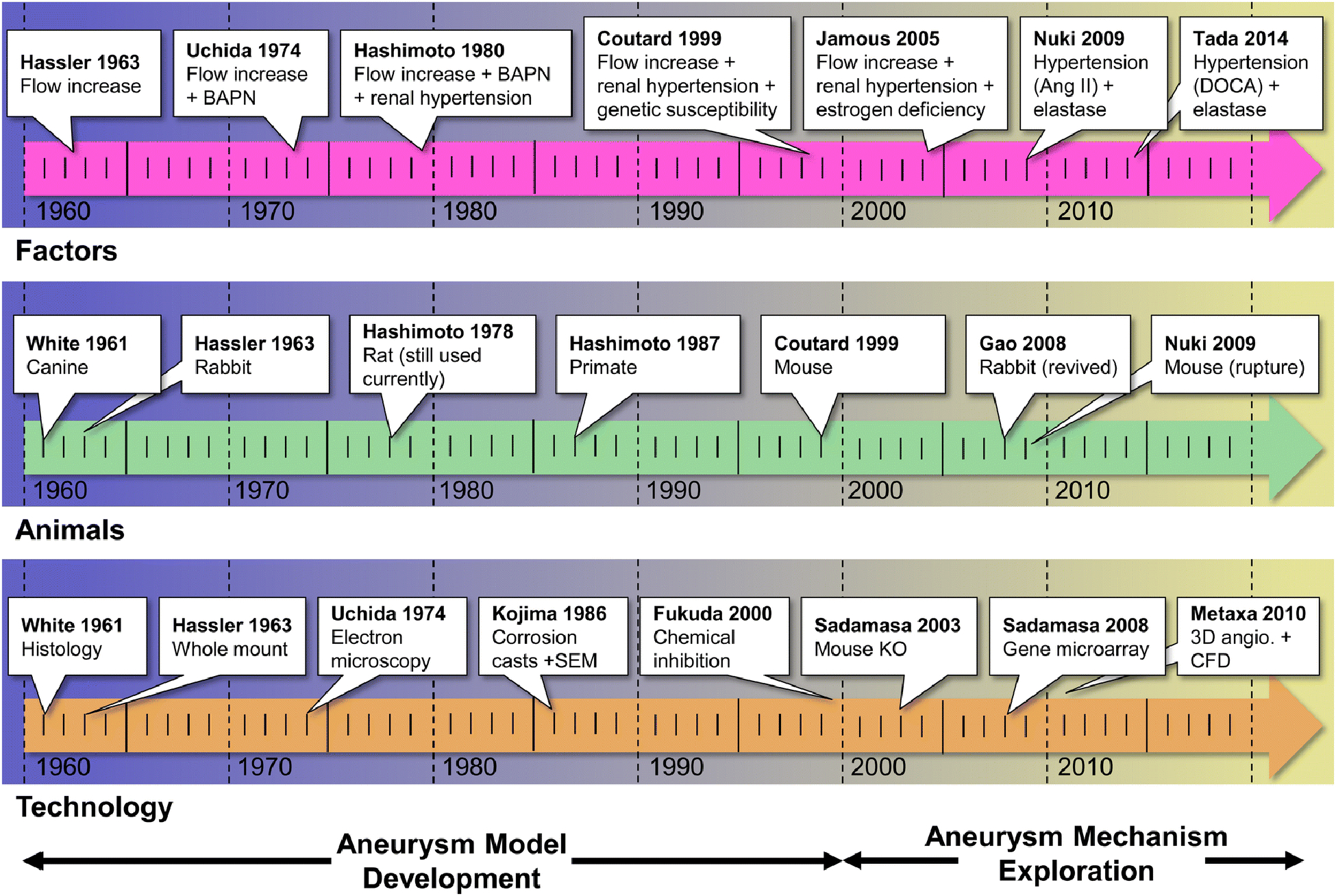
For the most part, endogenous animal models of IA were developed from 1961 through the early 2000s. At the turn of the century there was an exponential growth in research focused on the pathogenesis of the disease. The top row shows noteworthy studies that utilized new methods to incite IA risk factors. The middle row highlights notable first uses of different animal species (many of which are still in use today, i.e. rats). The bottom row highlights the first use of different analytical technologies in the study of animal models of IA. Abbreviations: Ang II=Angiotensin II, angio.=angiography, BAPN= β-Aminopropionitrile, CFD=computational fluid dynamics, DOCA=deoxycorticosterone acetate, IA=intracranial aneurysm, KO=knock-out, SEM=scanning electron microscopy.
The earliest report of an endogenous IA animal model was published by White et al. in 1961.[9] In this study, various noxious chemical solutions, i.e.,. hyaluronidase (150 or 300 USP units/mL), sodium morrhuate (10% or 5 %), plasmocid (1 or 5 mg/mL), and nitrogen mustard (0.5 or 2 mg), were injected into the wall of the left internal carotid artery (ICA) of mongrel canines to induce IA. Hypertonic saline produced the best results, mimicking many features of human IAs, including loss of internal elastic lamina (IEL) and degradation of the medial layer. In 1963, motivated by observations that IAs form in high blood flow regions in the cerebral circulation, Hassler et al.[10] used unilateral common carotid artery (CCA) ligation in rabbits of unspecified strain to induce flow-mediated cerebrovascular damage. The resulting vascular changes were described as “consistent with aneurysms”, including bulging, media thinning, and loss of IEL at the basilar terminus (BT) and other bifurcations. Then in 1978, seeking a small animal model of IA, Lee et al.[11] created micro-aneurysms in rats via hypertension (a known IA risk factor[12]) induced by unilateral nephrectomy, deoxycorticosterone acetate (DOCA) treatment, and high salt diet. They found lesions throughout the vasculature of the cortex and basal ganglia 14 weeks after inducing hypertension. From these early, simpler models, multi-modal models (i.e. models inducing multiple risk factors) began to emerge in the 1970s in an effort to increase the incidence of IA formation. [13; 14] Notably, in 1974, Uchida et al.[13] built upon Hassler’s model [10] by combining β-aminopropionitrile (BAPN) with either unilateral or bilateral CCA ligation in rabbits. With this approach they were able to produce larger and more rapidly developing lesions at the BT. [13]
One of the most important milestones in IA model development was the rat model created by Hashimoto et al.[14] from Kyoto University in Japan. In 1978, this group combined flow increase via unilateral CCA ligation, hypertension induced by renal ligation and DOCA (2.5 mg/100 mg body weight) and salt diet (1% in drinking water), and vessel wall weakening by oral administration of BAPN (0.12%) to incite aneurysm formation in the anterior Circle of Willis (CoW) of male Sprague-Dawley rats. This led to consistent lesion formation at the anterior cerebral artery-olfactory artery (ACA-OA) bifurcation. Histopathological studies confirmed that IAs induced by this model share common features with human IAs, including bulge formation, IEL loss, destructive changes in the medial layer, and intimal hyperplasia.[14] This model has since been employed in a large number of follow-up studies, making it one of the most extensively studied IA animal model.[15–28] It has largely served as the means for the most comprehensive pathobiological research on experimental IAs, which has been led by Tomohiro Aoki from the same group. [15–28] The combination of manipulations in this rat model also laid the foundation for later efforts that added modifications in in different species (e.g. mice), with the goal of increasing aneurysm (and rupture) incidence or elucidating the role of specific proteins and signaling pathways.
Since the incidence of IAs in humans is higher among females [29], Suzuki et al.[30] began investigating the role of estrogen in IA models via feminization of male Sprague-Dawley rats in 1980. Further study on the role of estrogen was continued by Jamous et al. [31–33] from Tokushima University in Japan in 2005. In a series of studies, they built upon the rat model introduced by Hashimoto, but used female rats instead of males, removed BAPN from their diet, and added bilateral oophorectomy (removal of the ovaries). Using these manipulations, they showed that estrogen deficiency increased lesion formation 3-fold (from 20% to 60%) in rats when added to hypertension and CCA ligation.[31–34] Eldewoody et al. [35] from Tohoku University in Japan further fine-tuned this model by optimizing the method of hypertension induction. They found that despite the simplified surgical procedure, posterior renal artery ligation was more effective at inducing IA formation, i.e. led to greater degree and frequency of IA lesions than did posterior and inferior renal artery ligation or the use of spontaneously hypertensive, Dahl salt-sensitive rats.
Given a long-recognized correlation between blood flow and IA pathophysiology in humans, – IAs preferentially form at locations of locations of high fluid shear stress[36] – several groups began studying the effect of hemodynamic insult on aneurysm formation in flow-only experimental models. [37–39] In 1986 Alvarez et al.[37] subjected rats to either left CCA ligation, or left CCA ligation coupled with anastomosis of the proximal left CCA to the right CCA, to increase flow in the right CCA. They found that the latter manipulation resulted in much higher flow rates in the right CCA compared to unilateral ligation alone and higher rates of aneurysm formation at downstream locations - the anterior communicating artery and P1 segment of the left posterior cerebral artery - that presumably experience higher flow.[37]
The critical role for hemodynamics in IA formation was further explored by Meng et al.[38] at the University at Buffalo in New York. In 2006, this group first observed flow-driven aneurysm-like remodeling in surgically-created carotid bifurcations in a canine model (not included in Table 1 as it was not an endogenous model).[40–42] Using computational fluid dynamics (CFD) simulations of flow at the artificial bifurcation and co-mapping flow topology with histology (hemodynamics-histology co-mapping), they demonstrated that the vascular response to flow (namely IEL loss and groove formation) was associated with increased flow acceleration.[43] Subsequently, the same group built upon Hassler’s rabbit model and induced endogenous aneurysmal remodeling at the BT in New Zealand White rabbits after bilateral CCA ligation to increase flow in the BA. The induced remodeling was characterized by IEL loss, medial thinning, and vessel bulging.[39] Using their hemodynamics-histology co-mapping, they showed that the aneurysmal remodeling occurred predominantly in regions where high wall shear stress (WSS) and high positive WSS gradient (WSSG) exceeded a quantitatively-defined threshold level.[44; 45] Using this rabbit model, they observed a critical role for smooth muscle cells (SMCs) in IA genesis, finding that SMCs exhibited proinflammatory behavior in response to hemodynamic insult and produced matrix metalloproteinases (MMPs) in the earliest stages of IA development.[46] In 2019, Tutino et al.[47; 48], demonstrated the feasibility of determining detailed cerebrovascular hemodynamics in mice using CFD and high resolution 9.4 Tesla MRI, which could permit fine-grained analysis of hemodynamic factors in small animals where a much wider range of molecular tools, such as protein antibodies and genetically engineered animals, are available.
Beginning in the late 1990s, genetic factors that increase IA incidence began to be studied in animal models. Genetic predisposition in the Hashimoto model was first investigated by Coutard et al.,[49] who explored the effects of genetic variability as a result of differing strains of rat. Induction of IA formation via unilateral CCA ligation and renal artery ligation in 4 different strains demonstrated that Long Evans rats have over a 5-fold higher IA incidence rate compared to LOU rats and over a 3-fold higher IA incidence compared to Brown Norway rats.[49] Hybrid Brown Norway and Long Evans rats were later shown to have higher incidence rate (~50%) of IA remodeling compared to the original Brown Norway strain (0%), demonstrating that IA incidence could be modulated by genetics.[50] With the rapid evolution of microarray technology in the early 2000s, many began to investigate the RNA expression profiles of endogenously-created IAs in animals,[28; 51] albeit they did not study the role of inherited genetics.[50] These efforts include microarrays implemented on aneurysmal tissue from the Hashimoto rat model[23] and microRNA arrays in flow- and hypertension-driven rat models. [52] More recently, transgenic knock-out (KO) mice have been implemented in several IA models to elucidate the biological mechanisms of key genes/proteins identified in previous animal model studies.[26; 53; 54]
At the turn of the century, IA models had been utilized to extensively explore the roles of suspected molecules in IA formation, delineate mechanistic pathways, and develop targets and drugs for reducing IA formation.[23; 25; 55] After establishment of the rat model in Kyoto, several investigators applied similar manipulations in mice to create aneurysms. In 2009, Nuki et al.[56] reported a new model of IA induction in female C57BL/6J mice, which was distinguished from other endogenous IA models by its aggressive application of elastase (35 mU) and sustained infusion of angiotensin II (500 or 1000 ng/kg/min). They showed that their new model increased incidence of aneurysm formation by 77%, depending on dose of elastase and an angiotensin II injection. One variation of this model [56], introduced by Makino et al.[57], was subsequently used to explore aneurysm rupture and resulting subarachnoid hemorrhage. This model used a combination of stereotactic elastase injection, hypertension by unilateral nephrectomy, implantation of a DOCA-salt pellet, and high salt diet. These manipulations lead to higher rates of IA formation (greater than 60%) and higher rates of aneurysm rupture (greater than 50%) at approximately 1 week after induction. Aneurysms created by this method resembled human IAs in terms of IEL disruption, endothelial cell (EC) loss, inflammatory cell infiltration, and SMC hyperplasia. To increase the rate of IA formation and rupture, Hosaka et al. [58] subsequently modified this model to include ligation of the left CCA to increase flow in the CoW, as well as exacerbating hypertension by angiotensin II infusion and 8% NaCl diet (rather than DOCA) in addition to unilateral renal artery ligation, and weakening extracellular matrix (ECM) by adding BAPN to the diet. Since their creation, these models have been also implemented in rats,[59–61] and have been used extensively to explore IA rupture and to test pharmacological treatments that decrease the incidence of IA formation and rupture.
IA Induction Methods
Endogenous animal models of IA have implemented manipulations that mimic risk factors for human IAs,[62] such as elevated blood flow, hypertension, and estrogen deficiency in several animal species. Figure 3 illustrates these methods and the frequency at which they appear in the literature. We note that manipulations are most often used in combination; only 32 of the 174 publications (18%) surveyed employed a single manipulation. Figure 4 shows the model-generating manipulations with the numbers of publications that use them, either individually (non-overlapping regions) or combined (overlapping regions).
Figure 3: Different methods used for IA model creation.
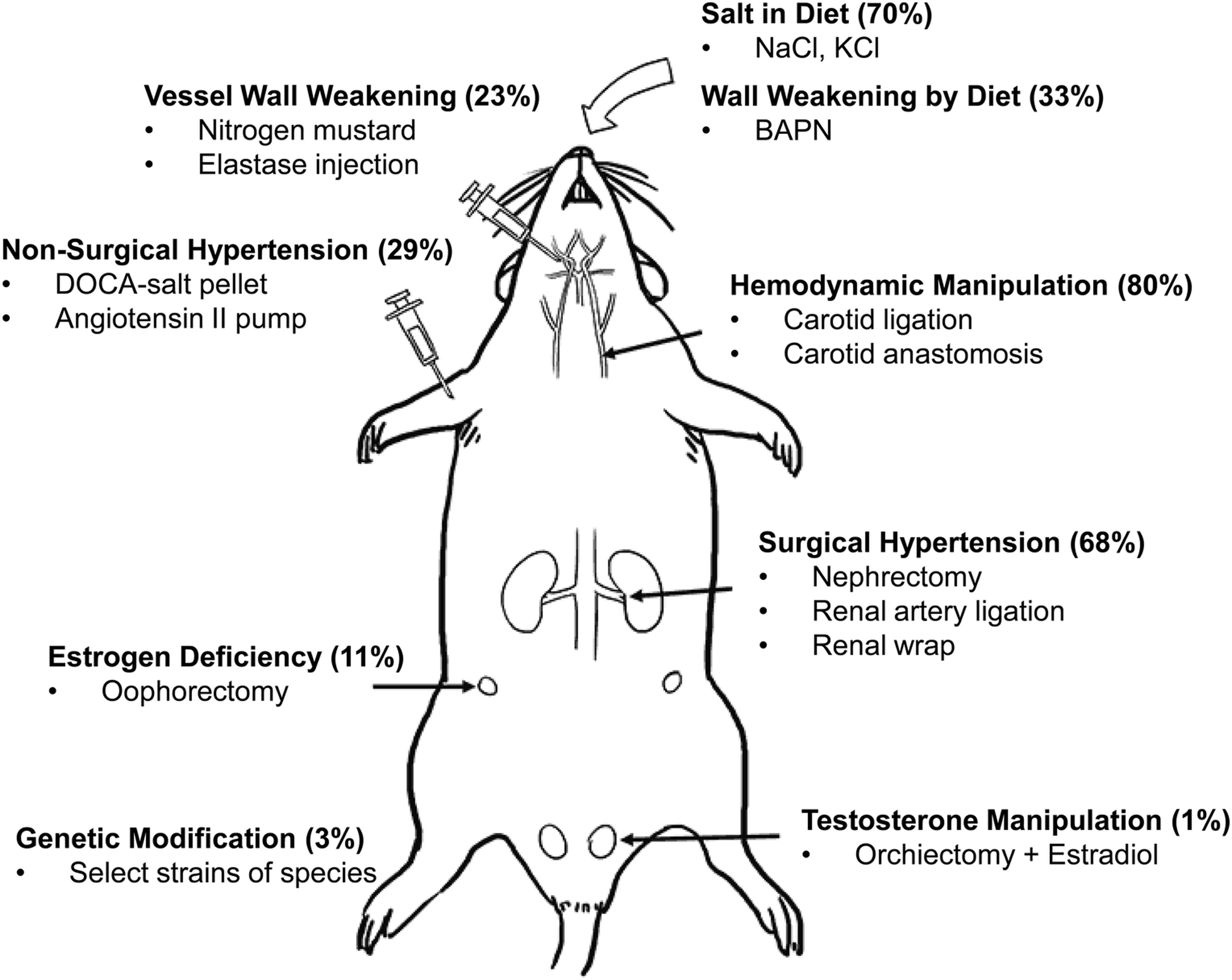
A diagram of a rat with annotations of different surgical and chemical manipulations performed to induce IA-associated risk factors. Abbreviations: BAPN= β-Aminopropionitrile, DOCA=deoxycorticosterone acetate.
Figure 4: The different factors induced in endogenous animal models.
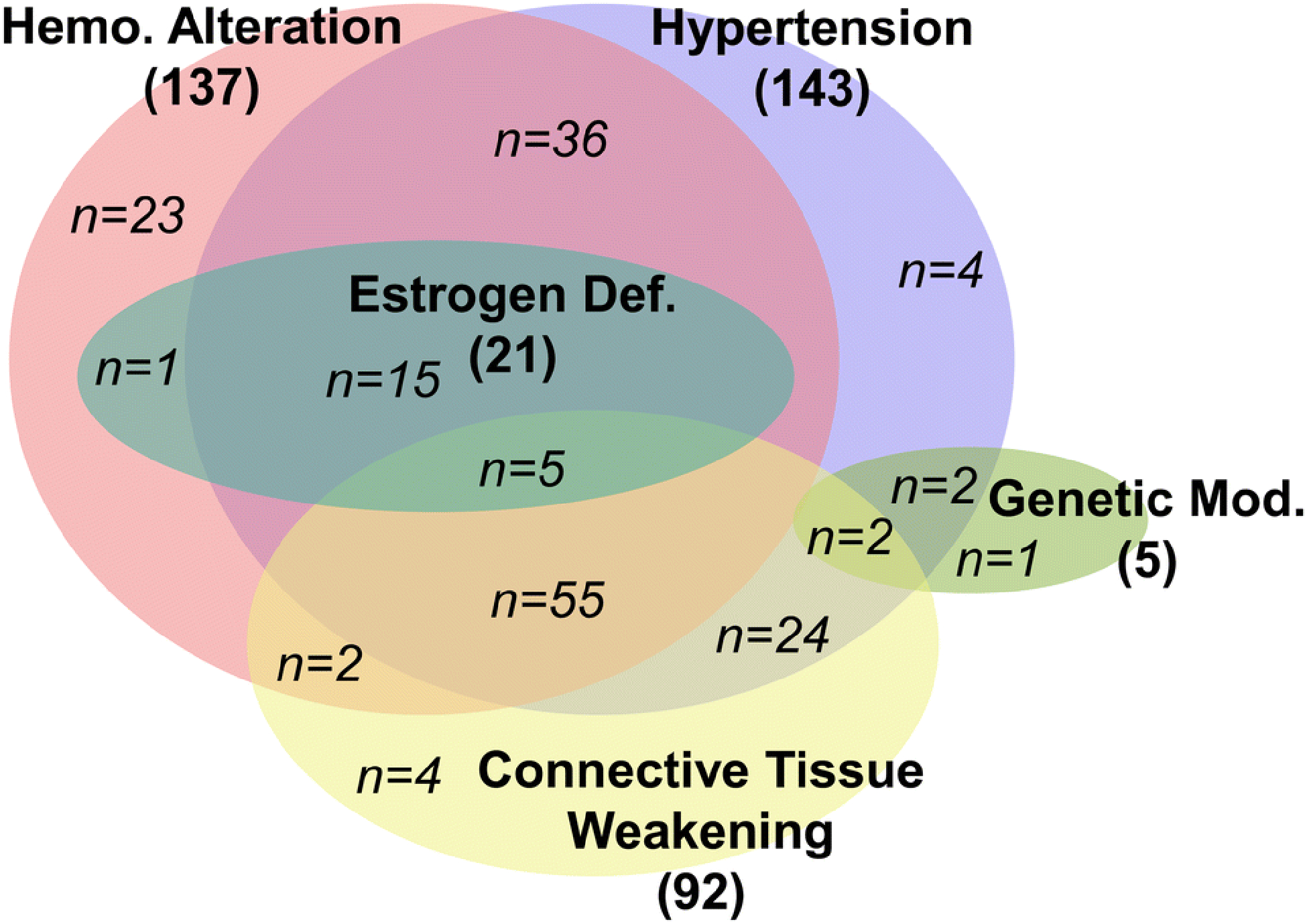
Each main risk factor (in bold) is represented by one oval (hemodynamic alteration - pink, hypertension - violet, estrogen deficiency - teal, connective tissue weakening - yellow, and genetic modification - green) The overlapping of ovals represents studies in which the respective methods were used in conjunction with each other. The number of studies using each method of IA induction is also indicated (italicization indicates the number for each combination of manipulations). The vast majority of the studies have implemented multi-modal models, with flow increase, hypertension, and connective tissue weakening as the predominant factors used.
The above-mentioned manipulations have been implemented in 5 types of animals: canines (in <1% of studies), primates (2%), rabbits (12%), mice (33%), and rats (62%), with rats and mice being used in the vast majority of studies. A total of 7.5% of studies used multiple animal species. In recent years, mice have been increasingly used, likely due to their rapid life cycle, inexpensive housing, and well-established methods for their genetic manipulation that make them ideal candidates for an animal model.[47; 63] New Zealand White rabbits are still used, albeit to a lesser extent, because their cerebral vessels are large enough for imaging by catheter-based digital subtraction angiography, which can enable minimally-invasive vessel morphology measurement and CFD analysis.[64]
Flow Increase –
Anecdotal observations have strongly implicated hemodynamic insult in IA pathogenesis.[65] Saccular IAs are commonly located at bifurcations and along outer curves of cerebral vessels,[43; 45] locations that are exposed to flow impingement and increased wall shear stress (WSS).[45] Clinical reports have demonstrated de novo IA formation in the human Circle of Willis in contralateral regions where compensatory flow increase occurs following carotid occlusion or ligation.[66] In animal models, cerebral blood flow increase is generally achieved by vessel ligation. Of the 174 publications we surveyed, 137 (79%) used flow increase for aneurysm creation, as indicated by the pink circle in Figure 4. Out of these, 23 (13%) used flow increase as the sole manipulation. The most frequently performed manipulation to elevate hemodynamic stress has been unilateral common carotid artery (CCA) ligation, which increases blood flow through the contralateral CCA and the basilar artery.[67; 68] [69] This has typically been performed in rodents, where aneurysms are commonly observed at the ACA-OA bifurcation.
Bilateral CCA ligation has also been performed in order to increase flow more drastically though the BA and create more severe hemodynamic insult at the BT. [10; 39; 70]This has been used extensively in rabbits to elucidate the relationship between hemodynamic insult and IA initiation, even without other risk factors. Tutino et al.[71] demonstrated that combining additional risk factors (hypertension and estrogen deficiency) to bilateral CCA ligation increased the observed aneurysmal remodeling at the BT, as well as other locations in the Circle of Willis. While bilateral CCA ligation has been used with great success in rabbits, this manipulation has failed to produce detectable aneurysm initiation at the rat and mouse BT.[47; 72] This may be because the configuration of the rat and mouse Circles of Willis dictates lesser hemodynamic forces at locations around the basilar artery bifurcation where IAs form in the rabbit. Recently, other flow-only models have been developed in rats.[73; 74] For instance, Cai et al.[74] developed a model that formed aneurysms in the anterior communicating artery following unilateral CCA ligation, and contralateral external carotid artery and pterygopalatine artery ligation. Yet, while this model produced large aneurysmal bulges in the anterior communicating arteries, IA incidence was low (<30%).
Hypertension –
Hypertension is a major risk factor for IAs in humans, as it is present in 43% of those with IA, compared to 20% of the general population.[71] This may be because increased blood pressure causes greater tensile stress in the vessel wall, leading to arterial remodeling that causes stiffening. Increased stiffness makes the arterial wall less mechanically responsive to changes in blood flow, and thus increases hemodynamic stresses,[75] and also disrupts the beneficial production of nitric oxide by endothelial cells in response to flow.[76] Additionally, angiotensin II, a vasoconstrictive hormone involved in the renal initiation of hypertension (it increases Na+ reabsorption), can also cause increased inflammation in the vasculature,[77] which could make vessels more susceptible to pathological remodeling.
As shown by the blue circle in Figure 4, 143 of the 174 surveyed articles (82%) used hypertension in IA model creation. Hypertension is most commonly produced in animal models by surgical means, such as branch ligation of the renal arteries,[78; 79] nephrectomy,36, 53 and silk wrapping of the kidneys.[80] All of these manipulations increase salt retention in the bloodstream and elevate systemic blood pressure. Many models combine these surgeries with addition of salt (in the form of NaCl or KCl, typically at a dose of 1 to 8%) in the animals’ diets.[34; 81] Furthermore, hormonal manipulation has also been used to induce hypertension. Here, animals have been given angiotensin II infusions or corticosteroids (such as DOCA at 2.5 mg/100 mg body weight [11]), either alone or in addition to surgery.[56] While studies have shown that hypertension can lead to formation of micro-aneurysms throughout the cerebral vasculature,[11; 80] it has generally not been sufficient to form lesions resembling human IAs on its own. Indeed, all models used in the last 10 years employ hypertension in conjunction with other IA risk factors to induce IAs.
Connective Tissue Weakening –
Several studies on human IA tissues collected from autopsy or after resection following aneurysm clipping have found that IA walls tend to have degraded IEL. This is even more pronounced in ruptured aneurysms.[82] Studies have also reported that that aneurysm tissues contain weakened and disorganized collagen matrices, likely because of collagen turnover due to remodeling of the IA tissue.[83] This has typically been attributed to inflammatory cells (macrophages, neutrophils, T/B lymphocytes) infiltrating the IA wall and secreting MMPs and reactive oxygen species (ROS), which are associated with smooth muscle cell turnover and destruction of ECM proteins, like elastin and collagen.[84–86]
As indicated by the yellow circle in Figure 4, 92 of the 174 surveyed publications (53%) incorporated connective tissue weakening in IA model creation. To mimic degradation, chemical manipulation of the connective tissues has been previously used. This was first described by White et al.[9] in 1961, who injected noxious materials (i.e. hyaluronidase, sodium morrhuate, plasmocid, nitrogen mustard) into the wall of the ICA, which resulted in formation of berry aneurysms in animals. Since 1974, BAPN has been more commonly used to increase the severity of IA lesions, as it inhibits lysyl oxidase, thus preventing collagen cross-linking and promoting vessel weakening. [13; 87] Early publications by Hashimoto et al.,[14; 88] showed that the addition of BAPN (0.12%) to rats’ high-salt diet increased the rate of IA incidence. Work by the Kyoto group led by Aoki demonstrated that the addition of BAPN increased IA incidence and IA severity. These findings suggest that this manipulation may therefore simulate aneurysm formation in humans with connective tissue disorders (such as Ehlers-Danlos Syndrome[89]). Overall, the combination of connective tissue weakening, hypertension, and flow increase resulted in over a 3-fold increase in IA incidence compared to flow increase plus hypertension or flow increase alone.
More recently, elastase, an enzyme that specifically degrades elastin, has been used to degrade the vessel wall and encourage IA formation. Typically, this has been done by stereotactic injection (typically 25–35 mU) into the basal cistern. To develop a model of IAs that rupture, Nuki et al.[56] combined elastase injection into the basal cistern and osmotic angiotensin II infusion (to simulate hypertension) to create large bulging IAs in the anterior circulation of mice with high frequency (>70%). This was later followed by modifications, such as those by Makino et al. [57] who used elastase treatment and hypertension via unilateral nephrectomy, DOCA-salt pellet implantation, and 1% NaCl in diet. Such modifications enabled 50–60% of created IAs to rupture within a period of approximately 2 weeks. Tada et al. [90; 91] used this model in a series of studies on the effects of certain pharmacotherapies (e.g. doxycycline) on IA formation and rupture. This approach has since been used extensively to study pharmacological treatments aimed at reducing rupture rates. However, due to its quick, aggressive weakening of the IA wall, there are concerns that the manipulation might mask the true biological mechanisms behind rupture.
Estrogen Deficiency –
The formation of IAs is more prevalent in females,[92] particularly in post-menopausal populations.[71] This may be because under normal conditions, estrogen enhances endothelial release of nitric oxide (NO), a vasoprotective molecule that helps regulate vascular tone, dilation, and cell growth, as well as protect vessels from inflammation and injury.[93] Estrogen itself has also been shown to be vasoprotective, as it stimulates the proliferation of endothelial cells, reduces oxidative stress, enhances SMC function, and inhibits MMP-2 and −9. Thus, it has been hypothesized that the reduction in estrogen levels during menopause may increase risk for IA formation.
A total of 21 of the 174 surveyed publications (12%) described IA models that incorporated estrogen deficiency and hormonal modulation to augment IA development, as indicated by the green oval in Figure 4. However, considering the higher IA incidence in women,[1] the majority of investigations opt to use only female animals for experimental IA models although they may not manipulate hormone levels. Early on, feminization of male rats via removal of the testes and administration of estradiol (1mg/kg/7 days) was briefly used to test the role of sex on IA formation,[94] but did not increase the rate of IA formation. Then, in 2005, Jamous et al. [32] introduced a model to form IAs in rats via unilateral CCA ligation, posterior renal artery ligation, and bilateral oophorectomy. They showed that incidence of IA formation in this model is three times higher than in rats that were only subjected to unilateral CCA ligation and posterior renal artery ligation (60% vs 20%). In 2014, Tada et al.[95] used oophorectomized mice to show that stimulation of estrogen receptor-β (but not α) protected against IA genesis. Since these studies, estrogen deficiency has been utilized extensively in rat models and added to a flow-only model in rabbits[71], as well as elastase mouse models from Hoh et al. in 2018.[96]
Genetic Predisposition –
Several genetic factors are associated with IAs. In patients with heritable disorders that affect connective tissue, such as Ehlers-Danlos syndrome, Loeys– Dietz syndrome, and Marfan syndrome, the integrity of the vasculature is severely compromised, and IA development occurs at a far greater frequency than in the general population. [97] Even in the absence of any identified heritable syndrome, a family history of IA is associated with higher IA prevalence (10%) and rupture rates (4%), suggesting that other heritable genetic factors may contribute to IA susceptibility.[98] Furthermore, clinical data indicates higher rates of aneurysmal subarachnoid hemorrhage in certain populations, notably the Japanese and the Finnish.[99] Recent genome-wide association studies (GWAS) have identified several important single nucleotide polymorphisms (SNPs) that occur at greater rates in people that have IAs.[100–108].
In animal models, only 5 of the 174 surveyed studies (3%) used genetic predisposition in IA model creation, as indicated by the green oval in Figure 4. To investigate genetic influences on the tendency to develop IAs, researchers have used selective breeding to obtain animals that preferentially develop more severe IA lesions. Established strains, such as Spontaneously Hypertensive Rats (SHR) have been used along with other manipulations to create IAs.[109] [35] Coutard et al. designed an IA-prone rat strain by crossing Long Evans and Norwegian Brown rat strains.[50] After 3 generations of crosses, IA formation rate following surgical manipulation increased from 24% to 50%. However, genetic manipulation alone was not sufficient to induce spontaneous aneurysms; at least one other manipulation (such as hypertension) has always been used in conjunction with genetics. More recent studies have begun using genetic knock-out mice to study the mechanisms of IA formation.[54; 110] However, these knock-outs have been implemented in existing animal models, and thus far have only been used to study the roles of specific molecules in targeted mechanistic pathways, rather than addressing genetic predisposition to aneurysm.
Methods for Evaluating Experimental IAs
Characterization of experimental IAs has been based not only on aneurysm morphology, but also on biological changes in the vascular wall during IA development. A variety of techniques have been used to process specimens in order to assess the morphology and structures of lesions created in animal models. These techniques include tissue (whole-mount) examination, histology, corrosion casting, and scanning electron microscopy (SEM). Their advantages and disadvantages as well as the frequency in the literature are shown in Figure 5 (for details on which study used each method, see Supplemental Table 1).
Figure 5: Aneurysm tissue examination methods in experimental models.
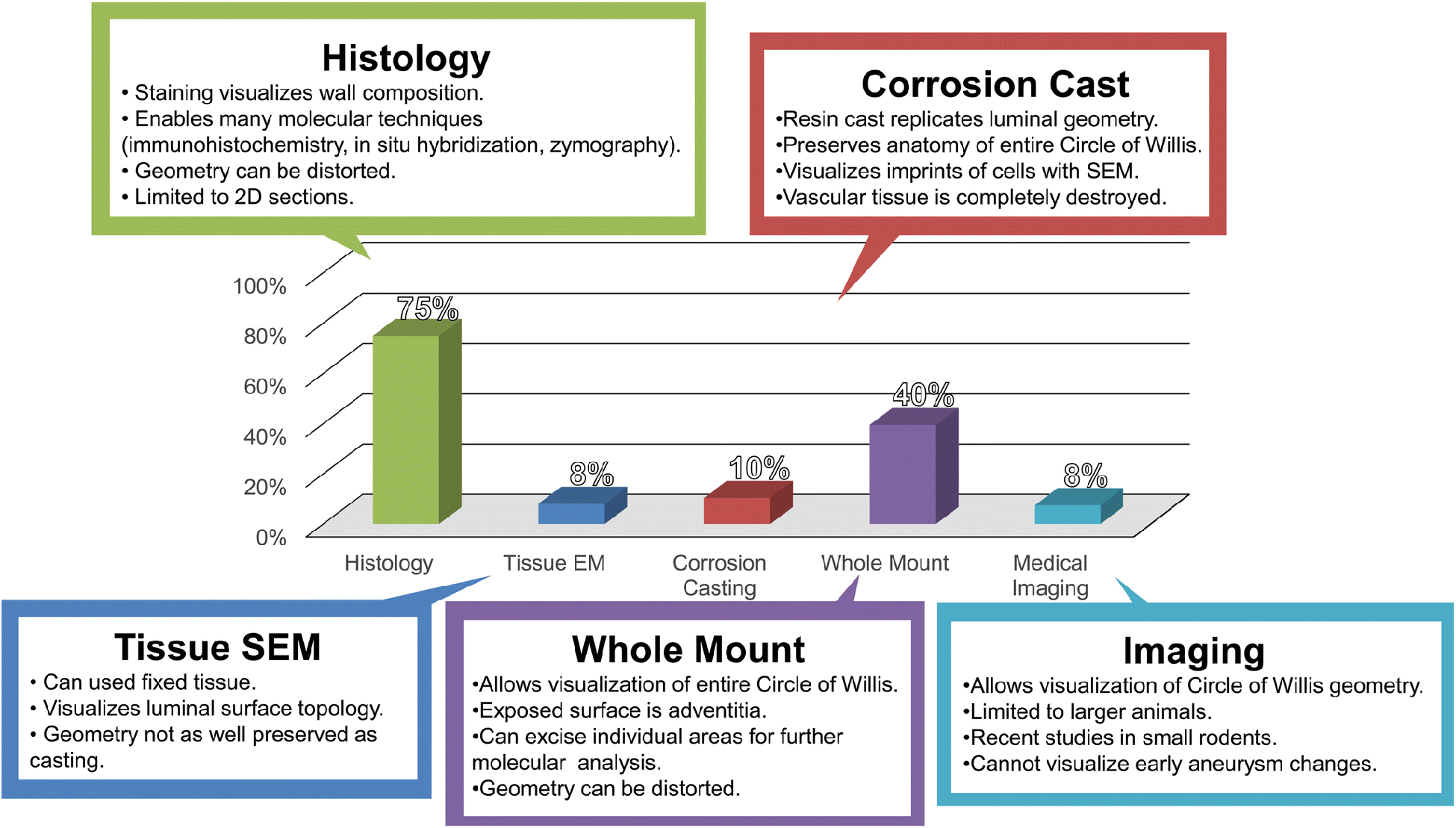
A graph indicating percentage each method was used in the literature. Model development studies tended to use corrosion casting and whole mount analysis, while mechanistic studies have strongly favored histology for analysis. Abbreviations: SEM=scanning electron microscopy.
Cerebral Vessel Tissue Examination –
The most basic form of examining aneurysms in experimental models is the inspection of the whole CoW with a dissecting microscope. In all, 68 of the 174 studies we reviewed (40%) used whole-mount specimen examination. Before knowing the location(s) of lesions produced by a given IA model, many investigators opted to inspect the entire cerebral vasculature for morphological changes. For example, this approach enabled Hashimoto et al.[14; 88] to identify common sites of IA formation, namely the ACA-OA bifurcation, in early studies. This examination method has been key in determining where to cut histological sections for further study. While whole tissue examination limits detailed imaging of the IA wall remodeling, as it only reveals the adventitia to a microscope, the collected tissues of IA lesions are often subjected to secondary molecular analyses. Molecular analyses require additional, destructive processing of aneurysmal material, such as physical or chemical breakdown to extract and stabilize biomolecules (primarily proteins or nucleic acids). Extracted materials have been analyzed by gene expression techniques (e.g. PCR[67; 68; 111; 112]) or protein expression methods (e.g. ELISA[113]), which have begun to lead to a greater understanding of the mechanisms behind the pathological remodeling of IA.
Histological Sections –
The majority of studies, 129 of 174 (74%), have used histological sections of the aneurysmal tissue, with or without whole-mount pre-examination. Tissue can be prepared in many ways for histological sectioning, including chemical fixation and embedding in paraffin or being frozen in optimal cutting temperature compound. It is possible to preserve the vessel geometry more accurately by pressure fixing the vessels with formalin while still in vivo using a perfusion pump or pressure drip. Overall, histological staining can provide cellular information and reveal microscopic lesions that are too small to see with the dissecting microscope. Histological examination allows for visualization of all the vessel layers, and adjacent sections can be stained for different purposes (based on stain used). Traditional histological analyses using H&E stain have been most frequently used to evaluate aneurysm lesions. Besides revealing overall tissue morphology and cell organization, H&E staining shows the presence of cell nuclei (to determine where cells are present in the wall). Further, Van Giesen staining has been used to visualize elastin, and in aneurysmal lesions, plays a pivotal role in determining if there is loss of IEL, a hallmark of IA (which will be discussed further below). Trichrome staining additionally has allowed for better demarcation of connective tissues in the vascular wall.
In addition to identifying vessel remodeling present in the arterial wall (which may or may not be obvious when viewed from the vessel’s exterior surface), another advantage of histological analysis is that it enables molecular investigation of the tissue by immunohistochemistry or immunofluorescence. Immunostaining for individual molecules can be done on individual slides to determine the presence and spatial distribution of protein expression.[114] This technique has been widely employed across almost all endogenous animal models and has greatly contributed to the current knowledge of the molecular mechanism of aneurysmal remodeling. For example, in multiple studies, Aoki et al. have used immunostaining to characterize the inflammatory environment of the IA wall, demonstrating increased MCP-1, MMP-9, and NF-kB protein levels.[18; 22; 115] Additional analyses, such as in situ hybridization to reveal expression of specific mRNAs,[116] have also been employed in histological sections but to a lesser degree.
Corrosion Casting –
Another method of tissue preparation, which has some of the advantages of both whole-mount specimen and histological sections, is the creation of vascular corrosion casts.[72] This has been performed in 18 of the 174 studies we reviewed (10%). By injecting a polymer that hardens inside the arteries, then chemically removing the artery tissue, a cast is created that accurately preserves the geometry of the vascular lumen. Such casts can capture the geometry of the entire CoW, which makes it easy to study morphological changes across all cerebral vessels. The casts can be quickly analyzed via dissecting microscope with oblique illumination or by SEM for accurate morphological assessment at high resolutions to reveal large- and small-scale changes. Furthermore, visualization of the endothelial cell imprints is possible under SEM, which can permit assessment of changes in the endothelial layer.[117] For example, several models employing this technique have found that, early in IA formation, ECs at bifurcations where the aneurysms form change morphology from smooth spindle shapes (aligned with flow) to amorphous, cobblestone shapes.[118] Unfortunately, the tissue is typically destroyed by the casting process, thus limiting further biological analyses of the vessels from these animals.[117]
Scanning Electron Microscopy –
A few studies (14 of the 174 surveyed, 8%) have performed SEM on the vascular tissue itself (not on corrosion casts, as discussed above). Tissue SEM allows for visualization of the intima of the vessel and can allow for direct visualization of endothelial cells and gaps in endothelial cells. SEM has primarily been employed in early model development studies to observe en face vascular damage during IA formation at the highest magnification. For SEM, fixed tissue is prepared by taking the desired vessel section and opening it up to expose the intima. The sample is then coated with a thin layer of conductive material (i.e. carbon or gold) to allow visualization of the tissue via the microscope. Tissue used for SEM cannot undergo the same molecular studies as preparations for histological sections, but some studies have used SEM to visualize inflammatory cells on the inner walls of IAs.[87] However, the vast majority of studies employing SEM have used it to analyze corrosion casting of the cerebral arteries after IA induction as described in previous section.
Cerebrovascular Imaging –
Cerebrovascular imaging, typically by traditional medical imaging modalities, such as digital subtraction angiography or MRI, has been used in a limited number of reported studies, primarily because aneurysmal lesions in animal models are substantially smaller than human IAs. It is not surprising that medical imaging has only been performed in 13 of the 174 reviewed studies (7%). Typically, imaging has been used to confirm presence of IAs, since it cannot reliably be used to analyze aneurysm morphology in most models where both the animals and the lesions are small. Hashimoto was the first to report in vivo imaging of IAs in rats with 2D angiography,[119; 120] but the procedure involved immediate euthanasia of the animal. This was similar to Cai et al. who reported cyclotron imaging of rat aneurysms, but this was also accompanied by termination of the animal. However, recent efforts with MRI have demonstrated survival IA model imaging. In 2015 Makino et al. reported successful serial imaging of the mouse cerebral vasculature via 3T MRI in a mouse IA model. More recently, Tutino et al. [47] and Rajabzadeh-Oghaz et al.[48] used non-invasive, survival 9.4T MRI to track aneurysmal remodeling in mice, demonstrating feasibility of longitudinal IA stu dies using high-resolution MRI in small animals. They also demonstrated the feasibility of generating 3D geometry of cerebrovascular of mice at different time points during the remodeling which allows one to perform CFD and study detailed vascular hemodynamics. These recent efforts show promise of the ability to longitudinally image aneurysmal lesions that can be visualized at a resolution as low as 50 um3.
Characteristics of Experimental IAs
It is important to compare experimentally-induced IAs to the human aneurysms that the animal models aim to recapitulate. Histopathological evaluation of human IAs have shown that, aside from their bulging appearance, the hallmarks of IA tissue are degradation of the IEL (the main load-bearing element of the vessel wall) and degeneration of the ECM, both of which severely weaken the IA wall.[82] Loss of mural cells, mainly SMCs, is also common, as IAs tend to have thinned or absent medial layers,[82; 121; 122] which can lead to anoikis.[123] Inflammatory cells, including macrophages and T lymphocytes, can contribute to aneurysm degeneration via production of MMPs and the release of ROS. These cells are present in aneurysms at a much higher rate (~50%) than in healthy vasculature[121] and have been shown to be more numerous in ruptured IAs.[82] Common characteristics of experimental aneurysms are detailed in Figure 6 (for details on what study reported each characteristic, see Supplemental Table 1). Unlike human IAs, aneurysmal lesions induced in animal models tend to be small with shallow/wide necks. This may be because animal IA models are created within a relatively short time window (a few days to a few months) via a limited number of manipulations, and thus may only recapitulate certain aspects of the human disease. Nonetheless, key features of human IAs are typically investigated in animal models as noted below.
Figure 6: Features observed in endogenously-created animal aneurysms.
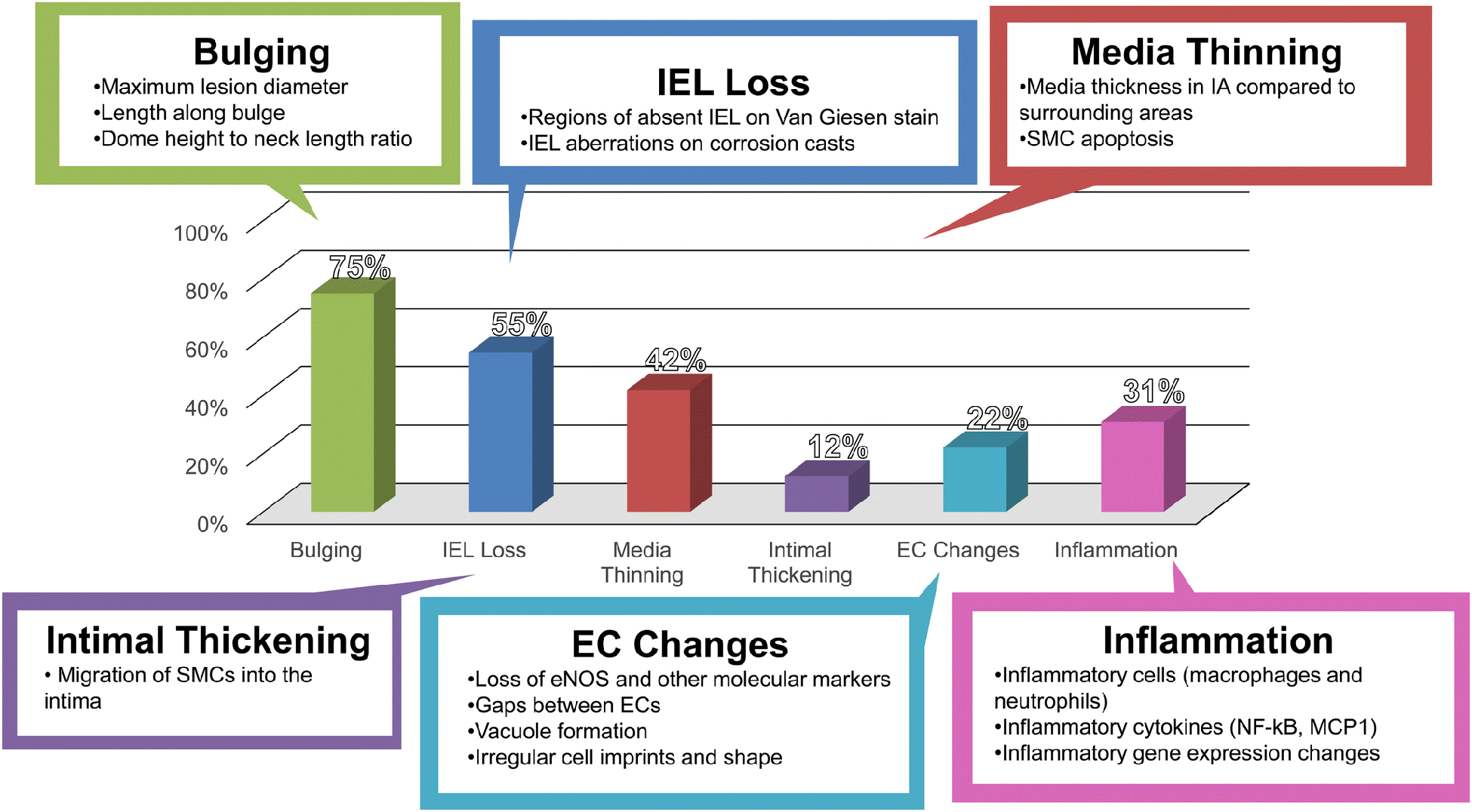
A graph indicating the percentage that each feature was observed in the literature. The three most common IA features are bulging, IEL loss, and medial thinning. Abbreviations: EC=endothelial cell, IEL=internal elastic lamina, SMC=smooth muscle cell.
Bulging –
Bulging is the ultimate defining characteristic of aneurysms, and as such, is commonly used to denote the IA presentation. Of the 174 studies surveyed here, 129 (74%) noted bulging in experimental IA models. Unlike human IAs, however, bulges observed in animal models tend to be microscopic, often only visible after histological processing and imaging. These bulges are measured either by maximal lesion diameter, length along the bulge, or dome height to neck ratio.[124; 125] Aneurysmal bulges in animal models tend to have a larger neck and less rounded shape than human IAs. Using animals sacrificed at different end-points, Meng et al.[126] inferred progressive development of a BT aneurysm due to flow increase in the bilateral CCA ligation rabbit model. The longest time-point was six months after flow increase manipulation, at which time the aneurysm was a wide-necked, thin-walled bulge that was pointed, but not rounded, and exhibited complete loss of media and IEL. Many models that have used combinations of flow increase, hypertension, and estrogen deficiency have created aneurysmal lesions such as these. Recent models of IA formation and rupture that induce aneurysms by multiple risk factors and injection of elastase have shown prominent bulging, berry IAs that have diameters larger than the parent artery, as one would see in humans.
IEL Destruction –
Vessel wall remodeling during IA formation has most notably been shown by the loss of the IEL (as well as medial thinning), and can be seen in the absence of discernable bulging.[126] Typically, the IEL (or its absence) is detected by Van Giesen staining on histological sections, and its loss is the most commonly used marker of IA development other than bulging. Of the 174 publications we studied, 94 (54%) indicated IEL loss associated with IA formation. Many studies have even used IEL loss to define the formation of the aneurysmal lesion. Indeed, IEL loss appears to be one of the first morphological changes during aneurysm development in animal models.[70; 127] This highlights one important strength of the developed models: the ability to reveal early stages of aneurysm genesis and development that are not readily observable in humans (where most studied IAs are terminal). Indeed, even five days after hemodynamic driven IA induction, Tutino et al.[117] found IEL fenestrations, or raised circular lesions on the corrosion cast surface representing indentations in the vessel due to IEL loss or focal weakening of the IEL or matrix degradation, in locations that develop aneurysms.
Smooth Muscle Cell Loss –
In human aneurysms, SMCs undergo phenotypic modulation from a contractile to a proinflammatory phenotype.[128] This is often accompanied by increased SMC turnover and apoptosis that increases as the vessel wall degenerates.[129] Studies of human IA tissues have shown that ruptured IAs have widespread SMC apoptosis with few remaining SMCs, while unruptured IAs still had some thinned SMC layers.[130] Indeed, a loss of media is noted in many IA models either by comparing the thickness of the media in the lesion to the thickness in the adjacent wall, or by investigating surrogate markers, such as apoptosis of SMCs. In all, 72 of the 174 studies we investigated also observed SMC loss associated with experimental IAs. The loss of SMCs is interpreted as evidence of a weakened wall that is susceptible to rupture. In many models, SMC loss and media thinning are commonly observed together with IEL loss on histology.[127] Several studies have even created scores to grade aneurysmal development, which combine the degree of SMC loss and media thinning, along with bulging, e.g. the Aneurysm Development Score.[126]
Endothelial Changes –
The endothelium is responsible for sensing shear forces of flow and communicating with the blood, and as such is critical in the initiation of IAs.[131] EC changes were noted in 38 of the 174 studies investigated as part of this review. Typically, investigators have looked at loss of endothelial nitric oxide synthase production, gaps between endothelial cells, or endothelium irregularities, typical markers of endothelial dysfunction.[132] Early tissue SEM studies demonstrated degeneration of ECs at locations of IA formation, [133] while later corrosion casting experiments by Jamous et al.[31] found that early EC morphology changes were related to changes in hemodynamic shear stress and blood flow patterns. Tutino et al.[117] also showed EC irregularities, deviations from healthy, spindle-shaped, flow-aligned ECs in locations that form IAs in the Meng rabbit model. A rough and irregular endothelial surface has conventionally been interpreted as a sign of EC dysfunction. Dolan et al. further demonstrated altered EC gene expression in these locations.[134]
Intimal Thickening –
Intimal thickening (or intimal hyperplasia) is the process by which the intimal layer becomes enriched with vascular SMCs and proteoglycan-rich ECM components and is typically associated with atherosclerosis.[135] In humans, many IAs are seen with atherosclerotic changes occurring in the sac,[136; 137] and individuals with atherosclerosis are at greatly increased risk of IA formation.[138] Migration of smooth muscle cells or inflammatory cells into the intima of the vessel, which causes thickening of the intima (as observed in humans) has been reported in a number of animal studies. Intimal thickening is commonly observed in OA-ACA aneurysms formed in response to unilateral CCA ligation, renal artery ligation, and salt diet in rats (i.e. the Hashimoto rat model). This feature was observed in experimental aneurysms in 21 of the 171 studies we surveyed here, which mostly included the OA-ACA IAs of the Hashimoto model.
Inflammatory Changes –
Inflammation plays a key role in IA pathophysiology. The natural history of the disease is characterized by an escalating inflammatory response, which accompanies the progressive degradation of the aneurysm wall.[139–141] This process is thought to begin with risk-factor (including elevated blood flow) induced pro-inflammatory responses in ECs and SMCs that lead to local production of MMPs and the initial formation of an aneurysmal sac. Once formed, the IA sac creates a hemodynamic environment characterized by slower recirculating flow that is conducive to leukocyte infiltration into the wall. In the IA tissue, inflammatory cells, such as macrophages and neutrophils, extensively produce MMPs and ROS (via expression of myeloperoxidase) which further degenerate the aneurysm, advancing its growth and rupture.[129] Our knowledge of the presence of inflammation in human IAs has largely been informed by early histological studies.[82; 121; 122] However, recent gene expression studies of IA tissues have demonstrated increased inflammatory processes and production of inflammatory cytokines/chemoattractants in the walls of humans IAs.[142; 143]
Inflammatory cells, primarily macrophages, have been observed infiltrating aneurysmal lesions in animal models. Beginning in the late 2000s and owing to the wide acceptance of inflammation as a main driver in IA natural history, many investigators have begun to directly study inflammation in experimental IA models. In addition to purely inflammatory cell staining, immunostaining for inflammatory cytokines is common in many studies.[22; 115] Inflammation has been uncovered to be among the first responses to IA risk factors, such as increased hemodynamics. Meng et al. demonstrated that during IA formation pro-inflammatory SMCs produce MMPs.[116] Furthermore, the inflammatory pathways involving NF-kB, MCP-1, TNF, and MMP were thoroughly investigated over several studies by Aoki et al[18; 22; 115; 144]. More recent gene profiling studies and qPCR-based investigations have also been used to study inflammatory gene expression in experimental IA tissues.[145] In all, 53 out of 174 studies (30%) specifically denoted inflammatory changes associated with experimental IAs.
Discussion
The pathogenesis and natural history of human IAs is a complex process that remains widely unknown. In order to study this disease and uncover pathobiological mechanisms, endogenous animal models of IA have been implemented over the past 60 years and have enabled investigations of the pathophysiological, cellular, and molecular biology of IA. Most models have implemented multiple manipulations mimicking aspects of the human disease, largely to maximize the rates of aneurysm development. The majority of these were implemented in rats and mice, used histology to study lesion pathobiology, and defined IA remodeling by IEL loss, bulge formation, and medial thinning. While there was a wide range of aneurysm induction techniques, the most common models included hemodynamic stress, hypertension, and connective tissue weakening (typically by elastase injection).
Endogenous IA models were largely developed between the years of 1961 and the early 2000s, starting with relatively large animals (e.g. canines, rabbits and primates) and quickly moving to rodents (i.e. rats and mice) due to their versatility and low cost. Since the turn of the century, several models have been extensively used to study the mechanisms of IA, leading to discoveries about the physical and molecular mechanisms behind IA formation and rupture. The most common models have been the Hashimoto model (rat – unilateral CCA ligation, unilateral nephrectomy, DOCA, salt diet, and BAPN)[14] – or modifications thereof (such as that published by Nagata et al.[120] and Aoki et al.,[19] rat: unilateral CCA ligation, bilateral renal artery ligation, salt diet, and BAPN), to study the molecular biology of aneurysmal tissue, the Jamous model [32] (rat – unilateral CCA ligation, renal artery ligation, oophorectomy) to study the influence of added risk factors, namely estrogen deficiency, the Meng model [70] (Rabbit – bilateral CCA ligation) to study the role of hemodynamics in IA genesis, and the Nuki[56]/Makino[57] model (mouse –elastase injection with either Angiotensin II pump or unilateral nephrectomy and DOCA salt diet, respectively), which has been most recently used to study IA rupture. These models of IA have been very successful at emulating many histological and geometric properties of human IAs. In particular, multimodal models where many etiological factors are employed have shown particularly impressive bulge formation in addition to IEL damage, wall thinning, and apoptosis.[49; 50; 146] On the other hand, other studies with genetic and chemical manipulations have teased out the beginnings of the mechanisms that may lie behind IA formation in humans.[109]
Over the last 20 years, use of these models, in conjunction with new technology, such as high-resolution imaging, microarray technology, and genetic knock-outs has led to the identification of several key molecular pathways involved in IA formation and rupture.[16; 26; 53; 54; 147] One of the most important successes of the endogenous IA models is that they allow study of early pathological events that precipitate IA formation, which is impossible in humans. Kolega et al.[127] used the rabbit bilateral CCA ligation model to investigate mechanisms of IA formation as early as two days after aneurysm induction. They observed the earliest changes in aneurysm genesis reported to date, namely loss of the IEL and degradation of the medial layer, and correlated these changes with changes in smooth muscle differentiation and local expression of matrix proteases.
Despite these successes, there remain many opportunities for improvement that would help further elucidate mechanisms of aneurysm formation and progression. While they share some features with human IAs, aneurysms in animals may have significant pathophysiological differences, since several factors in the development of human IAs have not been incorporated into endogenous animal models. A number of risk factors that influence IA development in humans have only just begun to be explored in animal models. For example, a predominant risk factor for IAs in humans is cigarette smoking,[148; 149] possibly through toxic effects on the vasculature, including increased oxidative stress in the vasculature, damage to the endothelium, and stimulation of the production of pro-inflammatory cytokines.[150] Starke et al.[151] recently explored the role of NOX1-mediated SMC phenotype switching in IA formation in mice that were exposed to cigarette smoke and found that smoke exposure brings about oxidative stress-induced SMC phenotypic modulation during IA formation.
Other risk factors have yet to be studied. Older age, for example, is strongly correlated with IA development,[152] as it is associated with stiffening of the vasculature and decreasing EC responses to vasodilatory factors,[153] which make blood vessels less adaptive to changes in blood flow.[154] While comparisons have been made between different age animals,[10] no group to date has explored animals at an age with documented vascular changes consistent with human old age. The addition of more risk factors into animal models of IA will likely give rise to studies to determine mechanisms behind different induced factors. Animal models can contribute to understanding how factors interact in inciting IA, by varying the manipulations in order to disentangle the contributions of individual factors to the observed traits or pathways. Indeed, several groups have already reported such studies, broadly finding that adding extra risk factors leads to increased rates of aneurysmal development. Throughout their work, Hashimoto et al. added manipulations to their rat IA model, demonstrating that tissue weakening (via BAPN), hypertension, and flow increase increased the formation rate of aneurysmal lesions by more than 300% compared to rats subjected to hypertension and flow increase alone. [14; 88] Jamous et al. [32] also showed that adding estrogen deficiency via bilateral oophorectomy to the Hashimoto model increased IA lesion formation from 20% to 60%. More recently, Tutino et al.[71] investigated IA formation in the rabbit CoW after flow manipulation alone and in combination with risk factors (hypertension and estrogen deficiency), and reported increased IA formation and a greater degree of IEL damage in the model with combined risk factors. Nevertheless, work is still needed to determine how and to what extent individual risk factors contribute differently to IA formation and development.
In addition to combining and exploring the contribution of aneurysm risk factors to IA formation, animal models could elucidate what factors influence progression of the disease, i.e. growth and rupture. One of the most important advancements in IA animal models to emerge in recent years has been the creation of IA rupture models, which hold the promise of revealing how and why an IA progresses towards rupturing.[90; 155–157] With a reported rupture rate of ~50%, their success has been facilitated by the addition of direct injection of elastase to hypertension and hemodynamic manipulation. Compared to other endogenous models, connective tissue weakening brought on by the injection of elastase is the major differentiator that leads to the creation of larger IAs that proceed to rupture. Thus, the observed biological responses in models that include elastase may reflect that of human IAs in patients with connective tissue disorders. For example, Ehlers-Danlos syndrome, a hereditary disease that is associated with higher rates of IA formation and rupture, results in part from SNPs in exonic regions of collagen.[158; 159] Such rupture models have been used to study the effects of certain pharmaceutical drugs on IA formation and rupture.[90; 91]. Still, as the fast delivery of a massive bolus of elastase likely does not occur in humans during IA formation (rather proteases and elastases are released over time by inflammatory cells[160]), these models may better simulate end-stage rupture-prone IAs, rather than the true growth and rupture phenomenon. Furthermore, the massive destruction of elastin that is induced throughout the cerebral vessels causes side-wall IAs that may, in some cases, reflect dissecting aneurysms. The disruption of the IEL may cause blood leakage into the medial layer and ballooning of the artery that may rupture through the adventitia, and could thus produce lesions/ruptures similar to those reported in animal studies of elastase-induced IAs. Future endogenous IA models that incorporate the effect of collagen degradation using more physiological methods are needed.
Conclusions
We believe that future studies aimed at longitudinally assessing IA pathobiology in models that incorporate aneurysm growth will likely have the largest impact on our understanding of the disease. Investigations using the latest developments in vascular imaging may permit the tracking of the entire course of the disease on an individualized basis, as well as the tracking of disease reversal in response to hemodynamic or pharmacological interventions. Putatively, once an aneurysm is established in a growth/rupture model, the success of a treatment can be monitored via imaging, to see if the IA stops growing or even decreases in size. This would enable longitudinal and non-destructive analysis of IA development in an individual before and after IA initiation, which would be uniquely possible in animals. The major limitation of current methods that use tissue examination for assessment is that they are only useable as endpoint assessments. In contrast, intravascular imaging of the IA wall (i.e. via optical coherence tomography[161]) or live cell imaging would allow for longitudinal studies of the IA that can continue even after bulge visualization. Recently Miyata et al.[69] reported real-time in vivo imaging of IAs in transgenic rats with GFP-stained ECs and was able to visualize aneurysm wall movement. However, the procedure was unable to be repeated serially. Future efforts to merge imaging and pathobiology analyses like this could enable longitudinal assessment of biological pathways in IA and would be highly valuable in investigating IA natural history.
Supplementary Material
Acknowledgements
The authors would like to thank Liza Gutierrez, MD, LVT at the Canon Stroke and Vascular Research Center for stimulating discussions during the drafting of this work.
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
Conflicts of Interest/Competing Interests
VMT – Principal investigator: National Science Foundation Award No. 1746694, NIH NINDS award R43 NS115314–0, Clinical and Translational Science Institute grant. Co-founder: Neurovascular Diagnostics, Inc.
HR-O – None.
SSV – None.
KEP – None.
MW – None.
MM – None.
NL – None.
AHS – Financial Interest/Investor/Stock Options/Ownership: Amnis Therapeutics, Apama Medical, BlinkTBI, Inc, Buffalo Technology Partners, Inc., Cardinal Health, Cerebrotech Medical Systems, Inc, Claret Medical, Cognition Medical, Endostream Medical, Ltd, Imperative Care, International Medical Distribution Partners, Rebound Therapeutics Corp., Silk Road Medical, StimMed, Synchron, Three Rivers Medical, Inc., Viseon Spine, Inc.
Consultant/Advisory Board: Amnis Therapeutics, Boston Scientific, Canon Medical Systems USA, Inc., Cerebrotech Medical Systems, Inc., Cerenovus, Claret Medical, Corindus, Inc., Endostream Medical, Ltd, Guidepoint Global Consulting, Imperative Care, Integra, Medtronic, Micro-Vention, Northwest University—DSMB Chair for HEAT Trial, Penumbra, Rapid Medical, Rebound Therapeutics Corp., Silk Road Medical, StimMed, Stryker, Three Rivers Medical, Inc., VasSol, W.L. Gore & Associates. National PI/Steering Committees: Cerenovus LARGE Trial and ARISE II Trial, Medtronic SWIFT PRIME and SWIFT DIRECT Trials, MicroVention FRED Trial & CONFIDENCE Study, MUSC POSITIVE Trial, Penumbra 3D Separator Trial, COMPASS Trial, INVEST Trial. Principal investigator: Cummings Foundation grant.
HM – Principal investigator NIH grant R01NS064592 and NIH grant R01NS091075. Co-founder: Neurovascular Diagnostics, Inc.
JK – None.
References
- [1].Vlak MH, Algra A, Brandenburg R, Rinkel GJ. Prevalence of unruptured intracranial aneurysms, with emphasis on sex, age, comorbidity, country, and time period: a systematic review and meta-analysis. Lancet Neurol 2011; 10(7): 626–36. [DOI] [PubMed] [Google Scholar]
- [2].Hackenberg KA, Hänggi D, Etminan N. Unruptured intracranial aneurysms: contemporary data and management. Stroke 2018; 49(9): 2268–75. [DOI] [PubMed] [Google Scholar]
- [3].Strother CM, Graves VB, Rappe A. Aneurysm hemodynamics: an experimental study. American Journal of Neuroradiology 1992; 13(4): 1089–95. [PMC free article] [PubMed] [Google Scholar]
- [4].Wakhloo AK, Schellhammer F, de Vries J, Haberstroh J, Schumacher M. Self-expanding and balloon-expandable stents in the treatment of carotid aneurysms: an experimental study in a canine model. American Journal of Neuroradiology 1994; 15(3): 493–502. [PMC free article] [PubMed] [Google Scholar]
- [5].Szikora I, Guterman LR, Wells KM, Hopkins LN. Combined use of stents and coils to treat experimental wide-necked carotid aneurysms: preliminary results. American Journal of Neuroradiology 1994; 15(6): 1091–102. [PMC free article] [PubMed] [Google Scholar]
- [6].Brinjikji W, Ding YH, Kallmes DF, Kadirvel R. From bench to bedside: utility of the rabbit elastase aneurysm model in preclinical studies of intracranial aneurysm treatment. J Neurointerv Surg 2016; 8(5): 521–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Fahed R, Raymond J, Ducroux C, et al. Testing flow diversion in animal models: a systematic review. Neuroradiology 2016; 58(4): 375–82. [DOI] [PubMed] [Google Scholar]
- [8].Strange F, Gruter BE, Fandino J, Marbacher S. Preclinical Intracranial Aneurysm Models: A Systematic Review. Brain Sci 2020; 10(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].White JC, Sayre GP, Whisnant JP. Experimental destruction of the media for the production of intracranial arterial aneurysms. Journal of neurosurgery 1961; 18: 741–5. [DOI] [PubMed] [Google Scholar]
- [10].Hassler O EXPERIMENTAL CAROTID LIGATION FOLLOWED BY ANEURYSMAL FORMATION AND OTHER MORPHOLOGICAL CHANGES IN THE CIRCLE OF WILLIS. Journal of neurosurgery 1963; 20: 1–7. [DOI] [PubMed] [Google Scholar]
- [11].Lee J, Berry CL. Cerebral micro-aneurysm formation in the hypertensive rat. The Journal of pathology 1978; 124(1): 7–11. [DOI] [PubMed] [Google Scholar]
- [12].Vlak MH, Rinkel GJ, Greebe P, Algra A. Independent risk factors for intracranial aneurysms and their joint effect: a case-control study. Stroke 2013; 44(4): 984–7. [DOI] [PubMed] [Google Scholar]
- [13].Uchida M Electron microscopical studies on the arteries of brain basis. II. Saccular aneurysm in rabbit and human cases. The Kumamoto medical journal 1974; 27(3): 150–69. [PubMed] [Google Scholar]
- [14].Hashimoto N, Handa H, Hazama F. Experimentally induced cerebral aneurysms in rats. Surg Neurol 1978; 10(1): 3–8. [PubMed] [Google Scholar]
- [15].Aoki T, Kataoka H, Ishibashi R, Nozaki K, Hashimoto N. Cathepsin B, K, and S are expressed in cerebral aneurysms and promote the progression of cerebral aneurysms. Stroke 2008; 39(9): 2603–10. [DOI] [PubMed] [Google Scholar]
- [16].Aoki T, Kataoka H, Ishibashi R, Nozaki K, Hashimoto N. Gene expression profile of the intima and media of experimentally induced cerebral aneurysms in rats by laser-microdissection and microarray techniques. Int J Mol Med 2008; 22(5): 595–603. [PubMed] [Google Scholar]
- [17].Aoki T, Kataoka H, Ishibashi R, Nozaki K, Morishita R, Hashimoto N. Reduced collagen biosynthesis is the hallmark of cerebral aneurysm: contribution of interleukin-1beta and nuclear factor-kappaB. Arteriosclerosis, thrombosis, and vascular biology 2009; 29(7): 1080–6. [DOI] [PubMed] [Google Scholar]
- [18].Aoki T, Kataoka H, Morimoto M, Nozaki K, Hashimoto N. Macrophage-derived matrix metalloproteinase-2 and −9 promote the progression of cerebral aneurysms in rats. Stroke 2007; 38(1): 162–9. [DOI] [PubMed] [Google Scholar]
- [19].Aoki T, Kataoka H, Moriwaki T, Nozaki K, Hashimoto N. Role of TIMP-1 and TIMP-2 in the progression of cerebral aneurysms. Stroke 2007; 38(8): 2337–45. [DOI] [PubMed] [Google Scholar]
- [20].Aoki T, Kataoka H, Nishimura M, Ishibashi R, Morishita R, Miyamoto S. Ets-1 promotes the progression of cerebral aneurysm by inducing the expression of MCP-1 in vascular smooth muscle cells. Gene therapy 2010; 17(9): 1117–23. [DOI] [PubMed] [Google Scholar]
- [21].Aoki T, Kataoka H, Nishimura M, Ishibashi R, Morishita R, Miyamoto S. Regression of intracranial aneurysms by simultaneous inhibition of nuclear factor-kappaB and Ets with chimeric decoy oligodeoxynucleotide treatment. Neurosurgery 2012; 70(6): 1534–43; discussion 43. [DOI] [PubMed] [Google Scholar]
- [22].Aoki T, Kataoka H, Shimamura M, et al. NF-kappaB is a key mediator of cerebral aneurysm formation. Circulation 2007; 116(24): 2830–40. [DOI] [PubMed] [Google Scholar]
- [23].Aoki T, Moriwaki T, Takagi Y, et al. The efficacy of apolipoprotein E deficiency in cerebral aneurysm formation. International journal of molecular medicine 2008; 21(4): 453–9. [PubMed] [Google Scholar]
- [24].Aoki T, Nishimura M, Ishibashi R, Kataoka H, Takagi Y, Hashimoto N. Toll-like receptor 4 expression during cerebral aneurysm formation. Laboratory investigation. Journal of neurosurgery 2010; 113(4): 851–8. [DOI] [PubMed] [Google Scholar]
- [25].Aoki T, Nishimura M, Kataoka H, et al. Role of angiotensin II type 1 receptor in cerebral aneurysm formation in rats. International journal of molecular medicine 2009; 24(3): 353–9. [DOI] [PubMed] [Google Scholar]
- [26].Aoki T, Nishimura M, Kataoka H, Ishibashi R, Nozaki K, Hashimoto N. Reactive oxygen species modulate growth of cerebral aneurysms: a study using the free radical scavenger edaravone and p47phox(−/−) mice. Laboratory investigation; a journal of technical methods and pathology 2009; 89(7): 730–41. [DOI] [PubMed] [Google Scholar]
- [27].Aoki T, Nishimura M, Kataoka H, Ishibashi R, Nozaki K, Miyamoto S. Complementary inhibition of cerebral aneurysm formation by eNOS and nNOS. Laboratory investigation; a journal of technical methods and pathology 2011; 91(4): 619–26. [DOI] [PubMed] [Google Scholar]
- [28].Aoki T, Nishimura M, Matsuoka T, et al. PGE(2)-EP(2) signalling in endothelium is activated by haemodynamic stress and induces cerebral aneurysm through an amplifying loop via NF-kappaB. British journal of pharmacology 2011; 163(6): 1237–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Chason JL, Hindman WM. Berry aneurysms of the circle of Willis; results of a planned autopsy study. Neurology 1958; 8(1): 41–4. [DOI] [PubMed] [Google Scholar]
- [30].Suzuki S, Robertson JT, White RP, Stadlan EM, Popoff N. Experimental intracranial aneurysms in rats. A gross and microscopic study. Journal of neurosurgery 1980; 52(4): 494–500. [DOI] [PubMed] [Google Scholar]
- [31].Jamous MA, Nagahiro S, Kitazato KT, Satoh K, Satomi J. Vascular corrosion casts mirroring early morphological changes that lead to the formation of saccular cerebral aneurysm: an experimental study in rats. J Neurosurg 2005; 102(3): 532–5. [DOI] [PubMed] [Google Scholar]
- [32].Jamous MA, Nagahiro S, Kitazato KT, Satomi J, Satoh K. Role of estrogen deficiency in the formation and progression of cerebral aneurysms. Part I: experimental study of the effect of oophorectomy in rats. Journal of neurosurgery 2005; 103(6): 1046–51. [DOI] [PubMed] [Google Scholar]
- [33].Jamous MA, Nagahiro S, Kitazato KT, Tamura T, Kuwayama K, Satoh K. Role of estrogen deficiency in the formation and progression of cerebral aneurysms. Part II: experimental study of the effects of hormone replacement therapy in rats. Journal of neurosurgery 2005; 103(6): 1052–7. [DOI] [PubMed] [Google Scholar]
- [34].Tada Y, Kitazato KT, Tamura T, et al. Role of mineralocorticoid receptor on experimental cerebral aneurysms in rats. Hypertension (Dallas, Tex : 1979) 2009; 54(3): 552–7. [DOI] [PubMed] [Google Scholar]
- [35].Eldawoody H, Shimizu H, Kimura N, et al. Simplified experimental cerebral aneurysm model in rats: comprehensive evaluation of induced aneurysms and arterial changes in the circle of Willis. Brain research 2009; 1300: 159–68. [DOI] [PubMed] [Google Scholar]
- [36].Alfano JM, Kolega J, Natarajan SK, et al. Intracranial aneurysms occur more frequently at bifurcation sites that typically experience higher hemodynamic stresses. Neurosurgery 2013; 73(3): 497–505. [DOI] [PubMed] [Google Scholar]
- [37].Alvarez F, Roda JM. Experimental model for induction of cerebral aneurysms in rats. Journal of neurosurgery 1986; 65(3): 398–400. [DOI] [PubMed] [Google Scholar]
- [38].Meng H, Swartz DD, Wang ZJ, et al. Development of aneurysm-like remodeling on vessels subjected to impinging flow. The FASEB Journal 2006; 20(5): LB116–LB16. [Google Scholar]
- [39].Meng H, Natarajan SK, Gao L, et al. Aneurysmal Changes at the Basilar Terminus in the Rabbit Elastase Aneurysm Model. American Journal of Neuroradiology 2010; 31(3): E35–E36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Meng H, Swartz DD, Wang Z, et al. A model system for mapping vascular responses to complex hemodynamics at arterial bifurcations in vivo. Neurosurgery 2006; 59(5): 1094–100; discussion 100–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Meng H, Wang Z, Hoi Y, et al. Complex hemodynamics at the apex of an arterial bifurcation induces vascular remodeling resembling cerebral aneurysm initiation. Stroke 2007; 38(6): 1924–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Wang Z, Kolega J, Hoi Y, et al. Molecular alterations associated with aneurysmal remodeling are localized in the high hemodynamic stress region of a created carotid bifurcation. Neurosurgery 2009; 65(1): 169–77; discussion 77–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Meng H, Wang Z, Hoi Y, et al. Complex Hemodynamics at the Apex of an Arterial Bifurcation Induces Vascular Remodeling Resembling Cerebral Aneurysm Initiation. Stroke 2007; 38(6): 1924–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Metaxa E, Tremmel M, Natarajan SK, et al. , Eds. Hemodynamic Factors Contributing to Intracranial Aneurysm Initiation in a Rabbit Model. Proceedings of the Stroke; 2010. LIPPINCOTT WILLIAMS & WILKINS; 530 WALNUT ST, PHILADELPHIA, PA 19106–3621: USA Year. [Google Scholar]
- [45].Metaxa E, Tremmel M, Xiang J, et al. , Eds. High Wall Shear Stress and Positive Wall Shear Stress Gradient Trigger the Initiation of Intracranial Aneurysms. Proceedings of the ASME 2009 Summer Bioengineering Conference; 2009. Year. [Google Scholar]
- [46].Mandelbaum M, Kolega J, Dolan JM, Siddiqui AH, Meng H. A critical role for proinflammatory behavior of smooth muscle cells in hemodynamic initiation of intracranial aneurysm. PloS one 2013; 8(9): e74357–e57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Tutino VM, Rajabzadeh-Oghaz H, Chandra AR, et al. 9.4T Magnetic Resonance Imaging of the Mouse Circle of Willis Enables Serial Characterization of Flow-Induced Vascular Remodeling by Computational Fluid Dynamics. Curr Neurovasc Res 2018; 15(4): 312–25. [DOI] [PubMed] [Google Scholar]
- [48].Rajabzadeh-Oghaz H, Chandra AR, Gutierrez L, et al. High-resolution MRI of the mouse cerebral vasculature to study hemodynamic-induced vascular remodeling. SPIE 2019. [Google Scholar]
- [49].Coutard M, Osborne-Pellegrin M. Genetic susceptibility to experimental cerebral aneurysm formation in the rat. Stroke 1997; 28(5): 1035–41; discussion 42. [DOI] [PubMed] [Google Scholar]
- [50].Coutard M, Huang W, Osborne-Pellegrin M. Heritability of intracerebral hemorrhagic lesions and cerebral aneurysms in the rat. Stroke 2000; 31(11): 2678–84. [DOI] [PubMed] [Google Scholar]
- [51].Sadamasa N, Nozaki K, Hashimoto N. Disruption of gene for inducible nitric oxide synthase reduces progression of cerebral aneurysms. Stroke 2003; 34(12): 2980–4. [DOI] [PubMed] [Google Scholar]
- [52].Lee H-J, Yi J-S, Lee H-J, Lee I-W, Park K-C, Yang J-H. Dysregulated Expression Profiles of MicroRNAs of Experimentally Induced Cerebral Aneurysms in Rats. Journal of Korean Neurosurgical Society 2013; 53(2): 72–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Starke RM, Chalouhi N, Jabbour PM, et al. Critical role of TNF-alpha in cerebral aneurysm formation and progression to rupture. Journal of neuroinflammation 2014; 11: 77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Lee S, Kim IK, Ahn JS, et al. Deficiency of endothelium-specific transcription factor Sox17 induces intracranial aneurysm. Circulation 2015; 131(11): 995–1005. [DOI] [PubMed] [Google Scholar]
- [55].Fukuda S, Hashimoto N, Naritomi H, et al. Prevention of rat cerebral aneurysm formation by inhibition of nitric oxide synthase. Circulation 2000; 101(21): 2532–8. [DOI] [PubMed] [Google Scholar]
- [56].Nuki Y, Tsou TL, Kurihara C, Kanematsu M, Kanematsu Y, Hashimoto T. Elastase-induced intracranial aneurysms in hypertensive mice. Hypertension (Dallas, Tex : 1979) 2009; 54(6): 1337–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Makino H, Tada Y, Wada K, et al. Pharmacological stabilization of intracranial aneurysms in mice: a feasibility study. Stroke 2012; 43(9): 2450–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Hosaka K, Downes DP, Nowicki KW, Hoh BL. Modified murine intracranial aneurysm model: aneurysm formation and rupture by elastase and hypertension. Journal of neurointerventional surgery 2014; 6(6): 474–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Quan K, Li S, Wang D, et al. Berberine Attenuates Macrophages Infiltration in Intracranial Aneurysms Potentially Through FAK/Grp78/UPR Axis. Front Pharmacol 2018; 9: 565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Yang Q, Yu D, Zhang Y. β-Sitosterol Attenuates the Intracranial Aneurysm Growth by Suppressing TNF-α-Mediated Mechanism. Pharmacology 2019; 104(5–6): 303–11. [DOI] [PubMed] [Google Scholar]
- [61].Zhao J, Lin X, He C, Yang GY, Ling F. Study of cerebral aneurysms in a modified rat model: from real-time imaging to histological analysis. J Clin Neurosci 2015; 22(2): 373–7. [DOI] [PubMed] [Google Scholar]
- [62].Etminan N, Rinkel GJ. Unruptured intracranial aneurysms: development, rupture and preventive management. Nature Reviews Neurology 2016; 12(12): 699. [DOI] [PubMed] [Google Scholar]
- [63].Suzuki T, Takizawa T, Kamio Y, et al. Noninvasive Vagus Nerve Stimulation Prevents Ruptures and Improves Outcomes in a Model of Intracranial Aneurysm in Mice. Stroke 2019; 50(5): 1216–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Xiao ZP, Zhao JL, Rong WL, Jiang JW, Li MH. Role of Vascular Endothelial-Cadherin and p120-Catenin in the Formation of Experimental Intracranial Aneurysm in Animals. World neurosurgery 2019; 128: e177–e84. [DOI] [PubMed] [Google Scholar]
- [65].Meng H, Tutino VM, Xiang J, Siddiqui A. High WSS or Low WSS? Complex Interactions of Hemodynamics with Intracranial Aneurysm Initiation, Growth, and Rupture: Toward a Unifying Hypothesis. American Journal of Neuroradiology 2014; 35(7): 1254–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Kono K, Masuo O, Nakao N, Meng H. De novo cerebral aneurysm formation associated with proximal stenosis. Neurosurgery 2013; 73(6): E1080–90. [DOI] [PubMed] [Google Scholar]
- [67].Li S, Wang D, Tian Y, et al. Aspirin Inhibits Degenerative Changes of Aneurysmal Wall in a Rat Model. Neurochemical research 2015; 40(7): 1537–45. [DOI] [PubMed] [Google Scholar]
- [68].Yokoi T, Isono T, Saitoh M, Yoshimura Y, Nozaki K. Suppression of cerebral aneurysm formation in rats by a tumor necrosis factor-alpha inhibitor. Journal of neurosurgery 2014; 120(5): 1193–200. [DOI] [PubMed] [Google Scholar]
- [69].Miyata H, Shimizu K, Koseki H, et al. Real-time Imaging of an Experimental Intracranial Aneurysm in Rats. Neurologia medico-chirurgica 2019; 59(1): 19–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Metaxa E, Tremmel M, Natarajan SK, et al. Characterization of critical hemodynamics contributing to aneurysmal remodeling at the basilar terminus in a rabbit model. Stroke 2010; 41(8): 1774–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Tutino VM, Mandelbaum M, Takahashi A, et al. Hypertension and Estrogen Deficiency Augment Aneurysmal Remodeling in the Rabbit Circle of Willis in Response to Carotid Ligation. Anat Rec (Hoboken) 2015; 298(11): 1903–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Tutino VM, Liaw N, Spernyak JA, et al. Assessment of Vascular Geometry for Bilateral Carotid Artery Ligation to Induce Early Basilar Terminus Aneurysmal Remodeling in Rats. Curr Neurovasc Res 2016; 13(1): 82–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Kaufmann TJ, Marx WF, Kallmes DF. A failure of matrix metalloproteinase inhibition in the prevention of rat intracranial aneurysm formation. Neuroradiology 2006; 48(3): 190–5. [DOI] [PubMed] [Google Scholar]
- [74].Cai J, He C, Yuan F, Chen L, Ling F. A novel haemodynamic cerebral aneurysm model of rats with normal blood pressure. Journal of clinical neuroscience : official journal of the Neurosurgical Society of Australasia 2012; 19(1): 135–8. [DOI] [PubMed] [Google Scholar]
- [75].Torii R, Oshima M, Kobayashi T, Takagi K, Tezduyar TE. Fluid–structure Interaction Modeling of Aneurysmal Conditions with High and Normal Blood Pressures. Computational Mechanics 2006; 38(4): 482–90. [Google Scholar]
- [76].Kohn JC, Zhou DW, Bordeleau F, et al. Cooperative effects of matrix stiffness and fluid shear stress on endothelial cell behavior. Biophys J 2015; 108(3): 471–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Ruiz-Ortega M, Ruperez M, Esteban V, et al. Angiotensin II: a key factor in the inflammatory and fibrotic response in kidney diseases. Nephrol Dial Transplant 2006; 21(1): 16–20. [DOI] [PubMed] [Google Scholar]
- [78].Aoki T, Kataoka H, Ishibashi R, Nozaki K, Hashimoto N. Nifedipine inhibits the progression of an experimentally induced cerebral aneurysm in rats with associated down-regulation of NF-kappa B transcriptional activity. Current neurovascular research 2008; 5(1): 37–45. [DOI] [PubMed] [Google Scholar]
- [79].Aoki T, Kataoka H, Ishibashi R, Nozaki K, Hashimoto N. Simvastatin suppresses the progression of experimentally induced cerebral aneurysms in rats. Stroke 2008; 39(4): 1276–85. [DOI] [PubMed] [Google Scholar]
- [80].Kido DK, Gomez DG, Santos-Buch CA, Caston TV, Potts DG. Microradiographic study of cerebral and ocular aneurysms in hypertensive rabbits. Neuroradiology 1978; 15(1): 21–6. [DOI] [PubMed] [Google Scholar]
- [81].Kim C, Kikuchi H, Hashimoto N, Kojima M, Kang Y, Hazama F. Involvement of internal elastic lamina in development of induced cerebral aneurysms in rats. Stroke 1988; 19(4): 507–11. [DOI] [PubMed] [Google Scholar]
- [82].Frösen J, Piippo A, Paetau A, et al. Remodeling of saccular cerebral artery aneurysm wall is associated with rupture: histological analysis of 24 unruptured and 42 ruptured cases. Stroke 2004; 35(10): 2287–93. [DOI] [PubMed] [Google Scholar]
- [83].Hackenberg KAM, Rajabzadeh-Oghaz H, Dreier R, et al. Collagen Turnover in Relation to Risk Factors and Hemodynamics in Human Intracranial Aneurysms. Stroke 2020; 51(5): 1624–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Kataoka K, Taneda M, Asai T, Kinoshita A, Ito M, Kuroda R. Structural fragility and inflammatory response of ruptured cerebral aneurysms. A comparative study between ruptured and unruptured cerebral aneurysms. Stroke 1999; 30(7): 1396–401. [DOI] [PubMed] [Google Scholar]
- [85].Kockx MM, De Meyer GR, Bortier H, et al. Luminal foam cell accumulation is associated with smooth muscle cell death in the intimal thickening of human saphenous vein grafts. Circulation 1996; 94(6): 1255–62. [DOI] [PubMed] [Google Scholar]
- [86].Kockx MM, De Meyer GR, Muhring J, Jacob W, Bult H, Herman AG. Apoptosis and related proteins in different stages of human atherosclerotic plaques. Circulation 1998; 97(23): 2307–15. [DOI] [PubMed] [Google Scholar]
- [87].Uchida M Electron microscopical studies on the arteries of brain basis. I. Arteriosclerotic changes in human cases and rabbit. The Kumamoto medical journal 1974; 27(3): 130–49. [PubMed] [Google Scholar]
- [88].Hashimoto N, Handa H, Hazama F. Experimentally induced cerebral aneurysms in rats: part II. Surgical neurology 1979; 11(3): 243–6. [PubMed] [Google Scholar]
- [89].Kim ST, Brinjikji W, Kallmes DF. Prevalence of Intracranial Aneurysms in Patients with Connective Tissue Diseases: A Retrospective Study. AJNR Am J Neuroradiol 2016; 37(8): 1422–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Tada Y, Wada K, Shimada K, et al. Estrogen protects against intracranial aneurysm rupture in ovariectomized mice. Hypertension (Dallas, Tex : 1979) 2014; 63(6): 1339–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Tada Y, Wada K, Shimada K, et al. Roles of hypertension in the rupture of intracranial aneurysms. Stroke 2014; 45(2): 579–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Wiebers DO, Whisnant JP, Huston J 3rd, et al. Unruptured intracranial aneurysms: natural history, clinical outcome, and risks of surgical and endovascular treatment. Lancet 2003; 362(9378): 103–10. [DOI] [PubMed] [Google Scholar]
- [93].Gewaltig MT, Kojda G. Vasoprotection by nitric oxide: mechanisms and therapeutic potential. Cardiovasc Res 2002; 55(2): 250–60. [DOI] [PubMed] [Google Scholar]
- [94].Zhou D, Bao YD, Du ZW, Takeda F, Kanno T. Experimental cerebral aneurysms in rats. Experimental method and effect of estradiol. Chinese medical journal 1985; 98(6): 421–6. [PubMed] [Google Scholar]
- [95].Tada Y, Makino H, Furukawa H, et al. Roles of estrogen in the formation of intracranial aneurysms in ovariectomized female mice. Neurosurgery 2014; 75(6): 690–5; discussion 95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Hoh BL, Rojas K, Lin L, et al. Estrogen Deficiency Promotes Cerebral Aneurysm Rupture by Upregulation of Th17 Cells and Interleukin-17A Which Downregulates E-Cadherin. Journal of the American Heart Association 2018; 7(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Schievink WI. Genetics of intracranial aneurysms. Neurosurgery 1997; 40(4): 651–62; discussion 62–3. [DOI] [PubMed] [Google Scholar]
- [98].Ronkainen A, Hernesniemi J, Puranen M, et al. Familial intracranial aneurysms. Lancet 1997; 349(9049): 380–84. [DOI] [PubMed] [Google Scholar]
- [99].Greving JP, Wermer MJ, Brown RD Jr., et al. Development of the PHASES score for prediction of risk of rupture of intracranial aneurysms: a pooled analysis of six prospective cohort studies. Lancet Neurol 2014; 13(1): 59–66. [DOI] [PubMed] [Google Scholar]
- [100].Abrantes P, Santos MM, Sousa I, et al. Genetic Variants Underlying Risk of Intracranial Aneurysms: Insights from a GWAS in Portugal. PLoS One 2015; 10(7): e0133422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Bilguvar K, Yasuno K, Niemela M, et al. Susceptibility loci for intracranial aneurysm in European and Japanese populations. Nature genetics 2008; 40(12): 1472–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Deka R, Koller DL, Lai D, et al. The relationship between smoking and replicated sequence variants on chromosomes 8 and 9 with familial intracranial aneurysm. Stroke 2010; 41(6): 1132–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Foroud T, Koller DL, Lai D, et al. Genome-wide association study of intracranial aneurysms confirms role of Anril and SOX17 in disease risk. Stroke 2012; 43(11): 2846–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Foroud T, Lai D, Koller D, et al. Genome-wide association study of intracranial aneurysm identifies a new association on chromosome 7. Stroke 2014; 45(11): 3194–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Kurki MI, Gaal EI, Kettunen J, et al. High risk population isolate reveals low frequency variants predisposing to intracranial aneurysms. PLoS Genet 2014; 10(1): e1004134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Low SK, Takahashi A, Cha PC, et al. Genome-wide association study for intracranial aneurysm in the Japanese population identifies three candidate susceptible loci and a functional genetic variant at EDNRA. Hum Mol Genet 2012; 21(9): 2102–10. [DOI] [PubMed] [Google Scholar]
- [107].Yasuno K, Bakircioglu M, Low SK, et al. Common variant near the endothelin receptor type A (EDNRA) gene is associated with intracranial aneurysm risk. Proc Natl Acad Sci U S A 2011; 108(49): 19707–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Yasuno K, Bilguvar K, Bijlenga P, et al. Genome-wide association study of intracranial aneurysm identifies three new risk loci. Nat Genet 2010; 42(5): 420–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Zhang D, Zhao J, Sun Y, et al. Pathological observation of brain arteries and spontaneous aneurysms in hypertensive rats. Chinese medical journal 2003; 116(3): 424–7. [PubMed] [Google Scholar]
- [110].Gopal K, Nagarajan P, Raj TA, Jahan P, Ganapathy HS, Mahesh Kumar MJ. Effect of dietary beta carotene on cerebral aneurysm and subarachnoid haemorrhage in the brain apo E−/− mice. Journal of thrombosis and thrombolysis 2011; 32(3): 343–55. [DOI] [PubMed] [Google Scholar]
- [111].Shimada K, Furukawa H, Wada K, et al. Angiotensin-(1–7) protects against the development of aneurysmal subarachnoid hemorrhage in mice. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism 2015; 35(7): 1163–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Hasan DM, Starke RM, Gu H, et al. Smooth Muscle Peroxisome Proliferator-Activated Receptor gamma Plays a Critical Role in Formation and Rupture of Cerebral Aneurysms in Mice In Vivo. Hypertension (Dallas, Tex : 1979) 2015; 66(1): 211–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Ma J, Hou D, Wei Z, et al. Tanshinone IIA attenuates cerebral aneurysm formation by inhibiting the NFkappaBmediated inflammatory response. Molecular medicine reports 2019; 20(2): 1621–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Miyata H, Imai H, Koseki H, et al. Vasa vasorum formation is associated with rupture of intracranial aneurysms. Journal of neurosurgery 2019: 1–11. [DOI] [PubMed] [Google Scholar]
- [115].Aoki T, Kataoka H, Ishibashi R, Nozaki K, Egashira K, Hashimoto N. Impact of monocyte chemoattractant protein-1 deficiency on cerebral aneurysm formation. Stroke 2009; 40(3): 942–51. [DOI] [PubMed] [Google Scholar]
- [116].Mandelbaum M, Kolega J, Dolan JM, Siddiqui AH, Meng H. A critical role for proinflammatory behavior of smooth muscle cells in hemodynamic initiation of intracranial aneurysm. PLoS One 2013; 8(9): e74357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Tutino VM, Mandelbaum M, Choi H, et al. Aneurysmal remodeling in the circle of Willis after carotid occlusion in an experimental model. J Cereb Blood Flow Metab 2014; 34(3): 415–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Kojima M, Handa H, Hashimoto N, Kim C, Hazama F. Early changes of experimentally induced cerebral aneurysms in rats: scanning electron microscopic study. Stroke 1986; 17(5): 835–41. [DOI] [PubMed] [Google Scholar]
- [119].Nagata I, Handa H, Hashimoto N. Experimentally induced cerebral aneurysms in rats: part IV--cerebral angiography. Surgical neurology 1979; 12(5): 419–24. [PubMed] [Google Scholar]
- [120].Nagata I, Handa H, Hashimoto N, Hazama F. Experimentally induced cerebral aneurysms in rats: Part VI. Hypertension. Surg Neurol 1980; 14(6): 477–9. [PubMed] [Google Scholar]
- [121].Chyatte D, Bruno G, Desai S, Todor DR. Inflammation and intracranial aneurysms. Neurosurgery 1999; 45(5): 1137–46; discussion 46–7. [DOI] [PubMed] [Google Scholar]
- [122].Kataoka K, Taneda M, Asai T, Yamada Y. Difference in nature of ruptured and unruptured cerebral aneurysms. Lancet 2000; 355(9199): 203. [DOI] [PubMed] [Google Scholar]
- [123].Michel JB. Anoikis in the cardiovascular system: known and unknown extracellular mediators. Arterioscler Thromb Vasc Biol 2003; 23(12): 2146–54. [DOI] [PubMed] [Google Scholar]
- [124].Zhu YQ, Dai DY, Xing HX, Ding YH, Kadirvel R, Kallmes DF. Concomitant aneurysm detection in an intracranial dolichoectasia mouse model using a MicroFil polymer perfusion technique. Journal of neurointerventional surgery 2017; 9(8): 783–86. [DOI] [PubMed] [Google Scholar]
- [125].Ikedo T, Minami M, Kataoka H, et al. Dipeptidyl Peptidase-4 Inhibitor Anagliptin Prevents Intracranial Aneurysm Growth by Suppressing Macrophage Infiltration and Activation. Journal of the American Heart Association 2017; 6(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Meng H, Metaxa E, Gao L, et al. Progressive aneurysm development following hemodynamic insult. J Neurosurg 2011; 114(4): 1095–103. [DOI] [PubMed] [Google Scholar]
- [127].Kolega J, Gao L, Mandelbaum M, et al. Cellular and molecular responses of the basilar terminus to hemodynamics during intracranial aneurysm initiation in a rabbit model. J Vasc Res 2011; 48(5): 429–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Chalouhi N, Hoh BL, Hasan D. Review of cerebral aneurysm formation, growth, and rupture. Stroke 2013; 44(12): 3613–22. [DOI] [PubMed] [Google Scholar]
- [129].Frösen J, Tulamo R, Paetau A, et al. Saccular intracranial aneurysm: pathology and mechanisms. Acta Neuropathol 2012; 123(6): 773–86. [DOI] [PubMed] [Google Scholar]
- [130].Sakaki T, Kohmura E, Kishiguchi T, Yuguchi T, Yamashita T, Hayakawa T. Loss and apoptosis of smooth muscle cells in intracranial aneurysms. Studies with in situ DNA end labeling and antibody against single-stranded DNA. Acta Neurochir (Wien) 1997; 139(5): 469–74; discussion 74–5. [DOI] [PubMed] [Google Scholar]
- [131].Dolan JM, Kolega J, Meng H. High wall shear stress and spatial gradients in vascular pathology: a review. Ann Biomed Eng 2013; 41(7): 1411–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Sheinberg DL, McCarthy DJ, Elwardany O, et al. Endothelial dysfunction in cerebral aneurysms. Neurosurg Focus 2019; 47(1): E3. [DOI] [PubMed] [Google Scholar]
- [133].Yamazoe N, Hashimoto N, Kikuchi H, Hazama F. Elastic skeleton of intracranial cerebral aneurysms in rats. Stroke 1990; 21(12): 1722–6. [DOI] [PubMed] [Google Scholar]
- [134].Dolan JM, Meng H, Sim FJ, Kolega J. Differential gene expression by endothelial cells under positive and negative streamwise gradients of high wall shear stress. American journal of physiology Cell physiology 2013; 305(8): C854–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Sakakura K, Nakano M, Otsuka F, Ladich E, Kolodgie FD, Virmani R. Pathophysiology of atherosclerosis plaque progression. Heart Lung Circ 2013; 22(6): 399–411. [DOI] [PubMed] [Google Scholar]
- [136].Adamson J, Humphries SE, Ostergaard JR, Voldby B, Richards P, Powell JT. Are cerebral aneurysms atherosclerotic? Stroke 1994; 25(5): 963–6. [DOI] [PubMed] [Google Scholar]
- [137].Fennell VS, Kalani MY, Atwal G, Martirosyan NL, Spetzler RF. Biology of Saccular Cerebral Aneurysms: A Review of Current Understanding and Future Directions. Front Surg 2016; 3: 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Hokari M, Isobe M, Imai T, Chiba Y, Iwamoto N, Isu T. The impact of atherosclerotic factors on cerebral aneurysm is location dependent: aneurysms in stroke patients and healthy controls. J Stroke Cerebrovasc Dis 2014; 23(9): 2301–7. [DOI] [PubMed] [Google Scholar]
- [139].Meng H, Tutino VM, Xiang J, Siddiqui A. High WSS or low WSS? Complex interactions of hemodynamics with intracranial aneurysm initiation, growth, and rupture: toward a unifying hypothesis. AJNR Am J Neuroradiol 2014; 35(7): 1254–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Tutino VM, Poppenberg KE, Jiang K, et al. Circulating neutrophil transcriptome may reveal intracranial aneurysm signature. PLoS One 2018; 13(1): e0191407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Tutino VM, Poppenberg KE, Li L, et al. Biomarkers from circulating neutrophil transcriptomes have potential to detect unruptured intracranial aneurysms. J Transl Med 2018; 16(1): 373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Pera J, Korostynski M, Krzyszkowski T, et al. Gene expression profiles in human ruptured and unruptured intracranial aneurysms: what is the role of inflammation? Stroke 2010; 41(2): 224–31. [DOI] [PubMed] [Google Scholar]
- [143].Tromp G, Weinsheimer S, Ronkainen A, Kuivaniemi H. Molecular basis and genetic predisposition to intracranial aneurysm. Ann Med 2014; 46(8): 597–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144].Aoki T, Fukuda M, Nishimura M, Nozaki K, Narumiya S. Critical role of TNF-alpha-TNFR1 signaling in intracranial aneurysm formation. Acta Neuropathol Commun 2014; 2: 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145].Yamaguchi T, Miyamoto T, Kitazato KT, et al. Time-dependent and site-dependent morphological changes in rupture-prone arteries: ovariectomized rat intracranial aneurysm model. J Neurosurg 2019: 1–9. [DOI] [PubMed] [Google Scholar]
- [146].Shimizu K, Miyata H, Abekura Y, et al. High-Fat Diet Intake Promotes the Enlargement and Degenerative Changes in the Media of Intracranial Aneurysms in Rats. Journal of neuropathology and experimental neurology 2019; 78(9): 798–807. [DOI] [PubMed] [Google Scholar]
- [147].Sadamasa N, Nozaki K, Kita-Matsuo H, et al. Gene expression during the development of experimentally induced cerebral aneurysms. J Vasc Res 2008; 45(4): 343–9. [DOI] [PubMed] [Google Scholar]
- [148].Can A, Castro VM, Ozdemir YH, et al. Association of intracranial aneurysm rupture with smoking duration, intensity, and cessation. Neurology 2017; 89(13): 1408–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [149].Okamoto K, Horisawa R, Ohno Y. The relationships of gender, cigarette smoking, and hypertension with the risk of aneurysmal subarachnoid hemorrhage: a case-control study in Nagoya, Japan. Ann Epidemiol 2005; 15(10): 744–8. [DOI] [PubMed] [Google Scholar]
- [150].Csiszar A, Podlutsky A, Wolin MS, Losonczy G, Pacher P, Ungvari Z. Oxidative stress and accelerated vascular aging: implications for cigarette smoking. Front Biosci (Landmark Ed) 2009; 14: 3128–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [151].Starke RM, Thompson JW, Ali MS, et al. Cigarette Smoke Initiates Oxidative Stress-Induced Cellular Phenotypic Modulation Leading to Cerebral Aneurysm Pathogenesis. Arterioscler Thromb Vasc Biol 2018; 38(3): 610–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [152].Rinkel GJE, Djibuti M, Algra A, Gijn Jv. Prevalence and Risk of Rupture of Intracranial Aneurysms. Stroke 1998; 29(1): 251–56. [DOI] [PubMed] [Google Scholar]
- [153].Seals DR, Jablonski KL, Donato AJ. Aging and vascular endothelial function in humans. Clin Sci (Lond) 2011; 120(9): 357–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [154].Franklin SS, Gustin Wt, Wong ND, et al. Hemodynamic patterns of age-related changes in blood pressure. The Framingham Heart Study. Circulation 1997; 96(1): 308–15. [DOI] [PubMed] [Google Scholar]
- [155].Pena-Silva RA, Chalouhi N, Wegman-Points L, et al. Novel role for endogenous hepatocyte growth factor in the pathogenesis of intracranial aneurysms. Hypertension (Dallas, Tex : 1979) 2015; 65(3): 587–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [156].Makino H, Hokamura K, Natsume T, et al. Successful serial imaging of the mouse cerebral arteries using conventional 3-T magnetic resonance imaging. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism 2015; 35(9): 1523–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [157].Shimada K, Furukawa H, Wada K, et al. Protective Role of Peroxisome Proliferator-Activated Receptor-gamma in the Development of Intracranial Aneurysm Rupture. Stroke 2015; 46(6): 1664–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [158].Byers PH, Duvic M, Atkinson M, et al. Ehlers-Danlos syndrome type VIIA and VIIB result from splice-junction mutations or genomic deletions that involve exon 6 in the COL1A1 and COL1A2 genes of type I collagen. Am J Med Genet 1997; 72(1): 94–105. [DOI] [PubMed] [Google Scholar]
- [159].Caranci F, Briganti F, Cirillo L, Leonardi M, Muto M. Epidemiology and genetics of intracranial aneurysms. Eur J Radiol 2013; 82(10): 1598–605. [DOI] [PubMed] [Google Scholar]
- [160].Strong MJ, Amenta PS, Dumont AS, Medel R. The role of leukocytes in the formation and rupture of intracranial aneurysms. Neuroimmunology and Neuroinflammation 2015; 2: 107–14. [Google Scholar]
- [161].Gounis MJ, Ughi GJ, Marosfoi M, et al. Intravascular Optical Coherence Tomography for Neurointerventional Surgery. Stroke 2018: Strokeaha118022315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [162].Hashimoto N, Handa H, Hazama F. Experimentally induced cerebral aneurysms in rats: Part III. Pathology. Surgical neurology 1979; 11(4): 299–304. [PubMed] [Google Scholar]
- [163].Hashimoto N, Handa H, Nagata I, Hazama F. [Experimental cerebral aneurysms--with reference to the experimental condition and to the etiology (author’s transl)]. Neurologia medico-chirurgica 1979; 19(10): 999–1003. [DOI] [PubMed] [Google Scholar]
- [164].Hashimoto N [Experimental inducement of saccular cerebral aneurysms in rats (author’s transl)]. Nihon geka hokan Archiv fur japanische Chirurgie 1979; 48(6): 667–78. [PubMed] [Google Scholar]
- [165].Hashimoto N, Handa H, Nagata I, Hazama F. Experimentally induced cerebral aneurysms in rats: Part V. Relation of hemodynamics in the circle of Willis to formation of aneurysms. Surgical neurology 1980; 13(1): 41–5. [PubMed] [Google Scholar]
- [166].Hazama F, Kataoka H, Yamada E, et al. Early changes of experimentally induced cerebral aneurysms in rats. Light-microscopic study. The American journal of pathology 1986; 124(3): 399–404. [PMC free article] [PubMed] [Google Scholar]
- [167].Hashimoto N, Kim C, Kikuchi H, Kojima M, Kang Y, Hazama F. Experimental induction of cerebral aneurysms in monkeys. Journal of neurosurgery 1987; 67(6): 903–5. [DOI] [PubMed] [Google Scholar]
- [168].Roda JM, Alvarez F, Garcia-Villalon AL, Ruiz MR, Gutierrez M, Garcia Blazquez M. An increment in unilateral carotid blood flow produces cerebral aneurysms in rats. Acta neurochirurgica Supplementum 1988; 43: 189–92. [DOI] [PubMed] [Google Scholar]
- [169].Kim C, Kikuchi H, Hashimoto N, Hazama F. Histopathological study of induced cerebral aneurysms in primates. Surgical neurology 1989; 32(1): 45–50. [DOI] [PubMed] [Google Scholar]
- [170].Kang Y, Hashimoto N, Kikuchi H, Yamazoe N, Hazama F. Effects of blood coagulation factor XIII on the development of experimental cerebral aneurysms in rats. Journal of neurosurgery 1990; 73(2): 242–7. [DOI] [PubMed] [Google Scholar]
- [171].Nakatani H, Hashimoto N, Kang Y, et al. Cerebral blood flow patterns at major vessel bifurcations and aneurysms in rats. Journal of neurosurgery 1991; 74(2): 258–62. [DOI] [PubMed] [Google Scholar]
- [172].Kim C, Cervos-Navarro J, Kikuchi H, Hashimoto N, Hazama F. Alterations in cerebral vessels in experimental animals and their possible relationship to the development of aneurysms. Surgical neurology 1992; 38(5): 331–7. [DOI] [PubMed] [Google Scholar]
- [173].Nakatani H, Hashimoto N, Kikuchi H, Yamaguchi S, Niimi H. In vivo flow visualization of induced saccular cerebral aneurysms in rats. Acta neurochirurgica 1993; 122(3–4): 244–9. [DOI] [PubMed] [Google Scholar]
- [174].Kim C, Cervos-Navarro J, Kikuchi H, Hashimoto N, Hazama F. Degenerative changes in the internal elastic lamina relating to the development of saccular cerebral aneurysms in rats. Acta neurochirurgica 1993; 121(1–2): 76–81. [DOI] [PubMed] [Google Scholar]
- [175].Futami K, Yamashita J, Tachibana O, Higashi S, Ikeda K, Yamashima T. Immunohistochemical alterations of fibronectin during the formation and proliferative repair of experimental cerebral aneurysms in rats. Stroke 1995; 26(9): 1659–64. [DOI] [PubMed] [Google Scholar]
- [176].Futami K, Yamashita J, Tachibana O, et al. Basic fibroblast growth factor may repair experimental cerebral aneurysms in rats. Stroke 1995; 26(9): 1649–54. [DOI] [PubMed] [Google Scholar]
- [177].Kondo S, Hashimoto N, Kikuchi H, Hazama F, Nagata I, Kataoka H. Cerebral aneurysms arising at nonbranching sites. An experimental Study. Stroke 1997; 28(2): 398–403; discussion 03–4. [DOI] [PubMed] [Google Scholar]
- [178].Kondo S, Hashimoto N, Kikuchi H, Hazama F, Nagata I, Kataoka H. Apoptosis of medial smooth muscle cells in the development of saccular cerebral aneurysms in rats. Stroke 1998; 29(1): 181–8; discussion 89. [DOI] [PubMed] [Google Scholar]
- [179].Futami K, Yamashita J, Higashi S. Do cerebral aneurysms originate at the site of medial defects? Microscopic examinations of experimental aneurysms at the fenestration of the anterior cerebral artery in rats. Surgical neurology 1998; 50(2): 141–6. [DOI] [PubMed] [Google Scholar]
- [180].Coutard M Experimental cerebral aneurysms in the female heterozygous Blotchy mouse. International journal of experimental pathology 1999; 80(6): 357–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [181].Morimoto M, Miyamoto S, Mizoguchi A, Kume N, Kita T, Hashimoto N. Mouse model of cerebral aneurysm: experimental induction by renal hypertension and local hemodynamic changes. Stroke 2002; 33(7): 1911–5. [DOI] [PubMed] [Google Scholar]
- [182].Moriwaki T, Takagi Y, Sadamasa N, Aoki T, Nozaki K, Hashimoto N. Impaired progression of cerebral aneurysms in interleukin-1beta-deficient mice. Stroke 2006; 37(3): 900–5. [DOI] [PubMed] [Google Scholar]
- [183].Sadamasa N, Nozaki K, Takagi Y, et al. Cerebral aneurysm progression suppressed by blockage of endothelin B receptor. Journal of neurosurgery 2007; 106(2): 330–6. [DOI] [PubMed] [Google Scholar]
- [184].Abruzzo T, Kendler A, Apkarian R, Workman M, Khoury JC, Cloft HJ. Cerebral aneurysm formation in nitric oxide synthase-3 knockout mice. Current neurovascular research 2007; 4(3): 161–9. [DOI] [PubMed] [Google Scholar]
- [185].Jamous MA, Nagahiro S, Kitazato KT, et al. Endothelial injury and inflammatory response induced by hemodynamic changes preceding intracranial aneurysm formation: experimental study in rats. Journal of neurosurgery 2007; 107(2): 405–11. [DOI] [PubMed] [Google Scholar]
- [186].Sibon I, Mercier N, Darret D, Lacolley P, Lamaziere JM. Association between semicarbazide-sensitive amine oxidase, a regulator of the glucose transporter, and elastic lamellae thinning during experimental cerebral aneurysm development: laboratory investigation. Journal of neurosurgery 2008; 108(3): 558–66. [DOI] [PubMed] [Google Scholar]
- [187].Gao L, Hoi Y, Swartz DD, Kolega J, Siddiqui A, Meng H. Nascent aneurysm formation at the basilar terminus induced by hemodynamics. Stroke 2008; 39(7): 2085–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [188].Aoki T, Kataoka H, Ishibashi R, et al. Pitavastatin suppresses formation and progression of cerebral aneurysms through inhibition of the nuclear factor kappaB pathway. Neurosurgery 2009; 64(2): 357–65; discussion 65–6. [DOI] [PubMed] [Google Scholar]
- [189].Tamura T, Jamous MA, Kitazato KT, et al. Endothelial damage due to impaired nitric oxide bioavailability triggers cerebral aneurysm formation in female rats. Journal of hypertension 2009; 27(6): 1284–92. [DOI] [PubMed] [Google Scholar]
- [190].Eldawoody H, Shimizu H, Kimura N, et al. Fasudil, a Rho-kinase inhibitor, attenuates induction and progression of cerebral aneurysms: experimental study in rats using vascular corrosion casts. Neuroscience letters 2010; 470(1): 76–80. [DOI] [PubMed] [Google Scholar]
- [191].Dai D, Ding YH, Kadirvel R, Lewis DA, Kallmes DF. Experience with microaneurysm formation at the basilar terminus in the rabbit elastase aneurysm model. AJNR American journal of neuroradiology 2010; 31(2): 300–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [192].Kimura N, Shimizu H, Eldawoody H, et al. Effect of olmesartan and pravastatin on experimental cerebral aneurysms in rats. Brain research 2010; 1322: 144–52. [DOI] [PubMed] [Google Scholar]
- [193].Yagi K, Tada Y, Kitazato KT, Tamura T, Satomi J, Nagahiro S. Ibudilast inhibits cerebral aneurysms by down-regulating inflammation-related molecules in the vascular wall of rats. Neurosurgery 2010; 66(3): 551–9; discussion 59. [DOI] [PubMed] [Google Scholar]
- [194].Tada Y, Yagi K, Kitazato KT, et al. Reduction of endothelial tight junction proteins is related to cerebral aneurysm formation in rats. Journal of hypertension 2010; 28(9): 1883–91. [DOI] [PubMed] [Google Scholar]
- [195].Ishibashi R, Aoki T, Nishimura M, Hashimoto N, Miyamoto S. Contribution of mast cells to cerebral aneurysm formation. Current neurovascular research 2010; 7(2): 113–24. [DOI] [PubMed] [Google Scholar]
- [196].Kanematsu Y, Kanematsu M, Kurihara C, et al. Critical roles of macrophages in the formation of intracranial aneurysm. Stroke 2011; 42(1): 173–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [197].Tada Y, Kitazato KT, Yagi K, et al. Statins promote the growth of experimentally induced cerebral aneurysms in estrogen-deficient rats. Stroke 2011; 42(8): 2286–93. [DOI] [PubMed] [Google Scholar]
- [198].Xu Y, Tian Y, Wei HJ, et al. Erythropoietin increases circulating endothelial progenitor cells and reduces the formation and progression of cerebral aneurysm in rats. Neuroscience 2011; 181: 292–9. [DOI] [PubMed] [Google Scholar]
- [199].Xu Y, Tian Y, Wei HJ, Dong JF, Zhang JN. Methionine diet-induced hyperhomocysteinemia accelerates cerebral aneurysm formation in rats. Neuroscience letters 2011; 494(2): 139–44. [DOI] [PubMed] [Google Scholar]
- [200].Dolan JM, Sim FJ, Meng H, Kolega J. Endothelial cells express a unique transcriptional profile under very high wall shear stress known to induce expansive arterial remodeling. American journal of physiology Cell physiology 2012; 302(8): C1109–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [201].Ishibashi R, Aoki T, Nishimura M, Miyamoto S. Imidapril inhibits cerebral aneurysm formation in an angiotensin-converting enzyme-independent and matrix metalloproteinase-9-dependent manner. Neurosurgery 2012; 70(3): 722–30. [DOI] [PubMed] [Google Scholar]
- [202].Ali MS, Starke RM, Jabbour PM, et al. TNF-alpha induces phenotypic modulation in cerebral vascular smooth muscle cells: implications for cerebral aneurysm pathology. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism 2013; 33(10): 1564–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [203].Wada K, Makino H, Shimada K, Shikata F, Kuwabara A, Hashimoto T. Translational research using a mouse model of intracranial aneurysm. Translational stroke research 2014; 5(2): 248–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [204].Lee HJ, Yi JS, Lee HJ, Lee IW, Park KC, Yang JH. Dysregulated Expression Profiles of MicroRNAs of Experimentally Induced Cerebral Aneurysms in Rats. Journal of Korean Neurosurgical Society 2013; 53(2): 72–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [205].Li MH, Li PG, Huang QL, Ling J. Endothelial injury preceding intracranial aneurysm formation in rabbits. The West Indian medical journal 2014; 63(2): 167–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [206].Liaw N, Fox JM, Siddiqui AH, Meng H, Kolega J. Endothelial nitric oxide synthase and superoxide mediate hemodynamic initiation of intracranial aneurysms. PloS one 2014; 9(7): e101721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [207].Li S, Tian Y, Huang X, et al. Intravenous transfusion of endothelial colony-forming cells attenuates vascular degeneration after cerebral aneurysm induction. Brain research 2014; 1593: 65–75. [DOI] [PubMed] [Google Scholar]
- [208].Fukuda M, Aoki T, Manabe T, et al. Exacerbation of intracranial aneurysm and aortic dissection in hypertensive rat treated with the prostaglandin F-receptor antagonist AS604872. Journal of pharmacological sciences 2014; 126(3): 230–42. [DOI] [PubMed] [Google Scholar]
- [209].Nowicki KW, Hosaka K, He Y, McFetridge PS, Scott EW, Hoh BL. Novel high-throughput in vitro model for identifying hemodynamic-induced inflammatory mediators of cerebral aneurysm formation. Hypertension (Dallas, Tex : 1979) 2014; 64(6): 1306–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [210].Chu Y, Wilson K, Gu H, et al. Myeloperoxidase is increased in human cerebral aneurysms and increases formation and rupture of cerebral aneurysms in mice. Stroke 2015; 46(6): 1651–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [211].Zhang M, Ren Y, Wang Y, et al. Regulation of smooth muscle contractility by competing endogenous mRNAs in intracranial aneurysms. Journal of neuropathology and experimental neurology 2015; 74(5): 411–24. [DOI] [PubMed] [Google Scholar]
- [212].Pena Silva RA, Mitchell IJ, Kung DK, et al. Paradoxical Increase in Mortality and Rupture of Intracranial Aneurysms in Microsomal Prostaglandin E2 Synthase Type 1-Deficient Mice: Attenuation by Aspirin. Neurosurgery 2015; 77(4): 613–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [213].Wu X, Zhang J, Huang Q, Yang P, Chen J, Liu J. Role of Kruppel-like Factor 2 in Intracranial Aneurysm of the Rabbit Model. Cellular and molecular biology (Noisy-le-Grand, France) 2015; 61(7): 33–9. [PubMed] [Google Scholar]
- [214].Wu X, Zhang J, Huang Q, Yang P, Chen J, Liu J. MicroRNA-92a Regulates Expression of Kruppel-like Factor2 in Rabbit Model of Intracranial Aneurysm. Cellular and molecular biology (Noisy-le-Grand, France) 2015; 61(8): 44–8. [PubMed] [Google Scholar]
- [215].Wu C, Liu Y, He M, Zhu L, You C. Single Operation with Simplified Incisions to Build an Experimental Cerebral Aneurysm Model by Induced Hemodynamic Stress and Estrogen Deficiency in Rats. Turkish neurosurgery 2016; 26(1): 62–8. [DOI] [PubMed] [Google Scholar]
- [216].Jung KH, Chu K, Lee ST, et al. Experimental Induction of Cerebral Aneurysms by Developmental Low Copper Diet. Journal of neuropathology and experimental neurology 2016; 75(5): 455–63. [DOI] [PubMed] [Google Scholar]
- [217].Chalouhi N, Starke RM, Correa T, et al. Differential Sex Response to Aspirin in Decreasing Aneurysm Rupture in Humans and Mice. Hypertension (Dallas, Tex : 1979) 2016; 68(2): 411–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [218].Sawyer DM, Pace LA, Pascale CL, et al. Lymphocytes influence intracranial aneurysm formation and rupture: role of extracellular matrix remodeling and phenotypic modulation of vascular smooth muscle cells. Journal of neuroinflammation 2016; 13(1): 185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [219].Aoki T, Yamamoto K, Fukuda M, Shimogonya Y, Fukuda S, Narumiya S. Sustained expression of MCP-1 by low wall shear stress loading concomitant with turbulent flow on endothelial cells of intracranial aneurysm. Acta neuropathologica communications 2016; 4(1): 48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [220].Lee JA, Marshman LA, Moran CS, et al. A small animal model for early cerebral aneurysm pathology. Journal of clinical neuroscience : official journal of the Neurosurgical Society of Australasia 2016; 34: 259–63. [DOI] [PubMed] [Google Scholar]
- [221].Liu J, Kuwabara A, Kamio Y, et al. Human Mesenchymal Stem Cell-Derived Microvesicles Prevent the Rupture of Intracranial Aneurysm in Part by Suppression of Mast Cell Activation via a PGE2-Dependent Mechanism. Stem cells (Dayton, Ohio) 2016; 34(12): 2943–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [222].Miyamoto T, Kung DK, Kitazato KT, et al. Site-specific elevation of interleukin-1beta and matrix metalloproteinase-9 in the Willis circle by hemodynamic changes is associated with rupture in a novel rat cerebral aneurysm model. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism 2017; 37(8): 2795–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [223].Yamamoto R, Aoki T, Koseki H, et al. A sphingosine-1-phosphate receptor type 1 agonist, ASP4058, suppresses intracranial aneurysm through promoting endothelial integrity and blocking macrophage transmigration. British journal of pharmacology 2017; 174(13): 2085–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [224].Miyata H, Koseki H, Takizawa K, et al. T cell function is dispensable for intracranial aneurysm formation and progression. PloS one 2017; 12(4): e0175421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [225].Aoki T, Saito M, Koseki H, et al. Macrophage Imaging of Cerebral Aneurysms with Ferumoxytol: an Exploratory Study in an Animal Model and in Patients. Journal of stroke and cerebrovascular diseases : the official journal of National Stroke Association 2017; 26(10): 2055–64. [DOI] [PubMed] [Google Scholar]
- [226].Liu M, Zhao J, Zhou Q, Peng Y, Zhou Y, Jiang Y. Primary Cilia Deficiency Induces Intracranial Aneurysm. Shock (Augusta, Ga) 2018; 49(5): 604–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [227].Aoki T, Frosen J, Fukuda M, et al. Prostaglandin E2-EP2-NF-kappaB signaling in macrophages as a potential therapeutic target for intracranial aneurysms. Science signaling 2017; 10(465). [DOI] [PubMed] [Google Scholar]
- [228].Maekawa H, Tada Y, Yagi K, et al. Bazedoxifene, a selective estrogen receptor modulator, reduces cerebral aneurysm rupture in Ovariectomized rats. Journal of neuroinflammation 2017; 14(1): 197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [229].Wu X, Zhang JZ, Yang PF, Huang QH, Liu JM. Regulation of Kruppel-like factor 2 (KLF2) in the pathogenesis of intracranial aneurysm induced by hemodynamics. American journal of translational research 2017; 9(12): 5452–60. [PMC free article] [PubMed] [Google Scholar]
- [230].Kuwabara A, Liu J, Kamio Y, et al. Protective Effect of Mesenchymal Stem Cells Against the Development of Intracranial Aneurysm Rupture in Mice. Neurosurgery 2017; 81(6): 1021–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [231].Labeyrie PE, Goulay R, Martinez de Lizarrondo S, et al. Vascular Tissue-Type Plasminogen Activator Promotes Intracranial Aneurysm Formation. Stroke 2017; 48(9): 2574–82. [DOI] [PubMed] [Google Scholar]
- [232].Suzuki T, Kamio Y, Makino H, et al. Prevention Effect of Antiplatelets on Aneurysm Rupture in a Mouse Intracranial Aneurysm Model. Cerebrovascular diseases (Basel, Switzerland) 2018; 45(3–4): 180–86. [DOI] [PubMed] [Google Scholar]
- [233].Kamio Y, Miyamoto T, Kimura T, et al. Roles of Nicotine in the Development of Intracranial Aneurysm Rupture. Stroke 2018; 49(10): 2445–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [234].Ikedo T, Minami M, Kataoka H, et al. Imaging mass spectroscopy delineates the thinned and thickened walls of intracranial aneurysms. Biochemical and biophysical research communications 2018; 495(1): 332–38. [DOI] [PubMed] [Google Scholar]
- [235].Liu Y, Zhang Y, Zheng Z, Liu X. Investigating the efficacy of a new intravenous (IV) nanoemulsified sevoflurane/arginine formulation for maintenance of general anesthesia for embolization of cerebral aneurysm. Journal of photochemistry and photobiology B, Biology 2018; 187: 61–65. [DOI] [PubMed] [Google Scholar]
- [236].Li XG, Wang YB. SRPK1 gene silencing promotes vascular smooth muscle cell proliferation and vascular remodeling via inhibition of the PI3K/Akt signaling pathway in a rat model of intracranial aneurysms. CNS neuroscience & therapeutics 2019; 25(2): 233–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [237].Zhang JZ, Chen D, Lv LQ, et al. miR-448–3p controls intracranial aneurysm by regulating KLF5 expression. Biochemical and biophysical research communications 2018; 505(4): 1211–15. [DOI] [PubMed] [Google Scholar]
- [238].Hoh BL, Fazal HZ, Hourani S, Li M, Lin L, Hosaka K. Temporal cascade of inflammatory cytokines and cell-type populations in monocyte chemotactic protein-1 (MCP-1)-mediated aneurysm healing. Journal of neurointerventional surgery 2018; 10(3): 301–05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [239].Nowicki KW, Hosaka K, Walch FJ, Scott EW, Hoh BL. M1 macrophages are required for murine cerebral aneurysm formation. Journal of neurointerventional surgery 2018; 10(1): 93–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [240].Zhao W, Zhang H, Su JY. MicroRNA29a contributes to intracranial aneurysm by regulating the mitochondrial apoptotic pathway. Molecular medicine reports 2018; 18(3): 2945–54. [DOI] [PubMed] [Google Scholar]
- [241].Shikata F, Shimada K, Sato H, et al. Potential Influences of Gut Microbiota on the Formation of Intracranial Aneurysm. Hypertension (Dallas, Tex : 1979) 2019; 73(2): 491–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [242].Pascale CL, Martinez AN, Carr C, et al. Treatment with dimethyl fumarate reduces the formation and rupture of intracranial aneurysms: Role of Nrf2 activation. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism 2019: 271678x19858888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [243].Wei H, Yang M, Yu K, et al. Atorvastatin Protects Against Cerebral Aneurysmal Degenerative Pathology by Promoting Endothelial Progenitor Cells (EPC) Mobilization and Attenuating Vascular Deterioration in a Rat Model. Medical science monitor : international medical journal of experimental and clinical research 2019; 25: 928–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [244].Ikedo T, Kataoka H, Minami M, et al. Sequential Inward Bending of Arterial Bifurcations is Associated with Intracranial Aneurysm Formation. World neurosurgery 2019; 129: e361–e66. [DOI] [PubMed] [Google Scholar]
- [245].Lai XL, Deng ZF, Zhu XG, Chen ZH. Apc gene suppresses intracranial aneurysm formation and rupture through inhibiting the NF-kappaB signaling pathway mediated inflammatory response. Bioscience reports 2019; 39(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [246].Wei L, Yang C, Li KQ, Zhong CL, Sun ZY. 3-Aminobenzamide protects against cerebral artery injury and inflammation in rats with intracranial aneurysms. Die Pharmazie 2019; 74(3): 142–46. [DOI] [PubMed] [Google Scholar]
- [247].Yang Q, Yu D, Zhang Y. beta-Sitosterol Attenuates the Intracranial Aneurysm Growth by Suppressing TNF-alpha-Mediated Mechanism. Pharmacology 2019; 104(5–6): 303–11. [DOI] [PubMed] [Google Scholar]
- [248].Feng Z, Zhang X, Li L, et al. Tumor-associated macrophage-derived exosomal microRNA-155–5p stimulates intracranial aneurysm formation and macrophage infiltration. Clinical science (London, England : 1979) 2019; 133(22): 2265–82. [DOI] [PubMed] [Google Scholar]
- [249].Fan W, Liu Y, Li C, et al. microRNA-331–3p maintains the contractile type of vascular smooth muscle cells by regulating TNF-alpha and CD14 in intracranial aneurysm. Neuropharmacology 2020; 164: 107858. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


