Abstract
Polycystic ovary syndrome (PCOS) is the most common endocrinopathy in women of reproductive age associated with long-term metabolic and cardiovascular consequences. A plethora of symptoms and their severity differentiate on an individual level, giving the syndrome numerous phenotypes. Due to menstrual cycle abnormalities, women suffer from irregular menstrual bleeding, difficulty in conception, and infertility. Furthermore, the risk of pregnancy complications such as gestational diabetes mellitus, hypertensive disorders of pregnancy, and preterm birth are higher in women with PCOS than in the general population. Often, women with PCOS have comorbidities such as dyslipidemia, obesity, glucose intolerance or diabetes type 2, non-alcoholic fatty liver disease, and metabolic syndrome, which all influence the treatment plan. Historic insulin-sensitizing agents, although good for some of the metabolic derangements, do not offer long-term cardiovascular benefits; therefore, new treatment options are of paramount importance. Sodium-glucose co-transporter-2 (SGLT-2) inhibitors, a new class of antidiabetic agents with beneficial cardiovascular, bodyweight, and antihyperglycemic effects, although not approved for the treatment of PCOS, might be an attractive therapeutic addition in the PCOS armamentarium. Namely, recent studies with SGLT-2 inhibitors showed promising improvements in anthropometric parameters and body composition in patients with PCOS. It is important to further explore the SGLT-2 inhibitors potential as an early therapeutic option because of the PCOS-related risk of metabolic, reproductive, and psychological consequences.
Keywords: Polycystic ovary syndrome, Sodium-glucose co-transporter-2 inhibitors, Metabolic risk, Cardiovascular risk, Metabolic syndrome, Insulin resistance, Obesity, Type 2 diabetes mellitus, Dyslipidemia
Core Tip: Sodium-glucose co-transporter-2 (SGLT-2) inhibitors are a new class of antidiabetic agents with beneficial cardiovascular, bodyweight, and antihyperglycemic effects. Although not approved for the treatment of polycystic ovary syndrome (PCOS), they might be an attractive therapeutic addition for the related metabolic, reproductive, and psychological consequences. Recent studies with SGLT-2 inhibitors in PCOS patients showed promising improvements in anthropometric parameters and body composition. Thus, it is important to explore the SGLT-2 inhibitors potential as an early therapeutic option in PCOS due to its high cardiometabolic risk.
INTRODUCTION
Polycystic ovary syndrome (PCOS) is a complex condition defined by metabolic, fertility, and psychological consequences, with a prevalence of up to 20% among females of reproductive age[1]. It is diagnosed according to Rotterdam criteria (2 of the following): Oligo- or anovulation, clinical and/or biochemical hyperandrogenemia, or polycystic ovarian morphology on ultrasound[2]. Underlying pathologic processes create a broad spectrum of clinical and laboratory abnormalities. The underlying mechanisms are still inconclusive, however certain genetic traits[3-5], altered gonadotropin secretion[6,7], faulty ovarian follicle maturation[8], and insulin resistance (IR)[9] are considered the most important etiological factors. IR leads to hyperinsulinemia which precipitates hyperandrogenaemia by stimulating ovarian androgen secretion and inhibition of hepatic sex hormone-binding globulin (SHBG) production[10].
A plethora of symptoms and their severity differentiate on an individual level, giving the syndrome numerous phenotypes. Due to menstrual cycle abnormalities, women suffer from irregular menstrual bleeding, difficulty in conception, and infertility. Furthermore, the risk of pregnancy complications such as gestational diabetes mellitus, hypertensive disorders of pregnancy, and preterm birth are higher in women with PCOS than in the general population[11,12].
Although cardiometabolic risk factors are not part of the PCOS diagnostic criteria, they impact the treatment and prognosis[13]. Metabolic issues related to PCOS increase the risk for long-term consequences such as dyslipidemia, obesity, glucose intolerance[14], diabetes type 2[15-17], low-grade chronic inflammation[18], non-alcoholic fatty liver disease[19,20] and metabolic syndrome[21,22]. Consequently, all women with PCOS should be assessed for cardiovascular risk factors and global cardiovascular disease risk[2].
Treatment goals for PCOS include diminishing clinical hyperandrogenism, managing menstrual dysfunction, preventing endometrial hyperplasia and carcinoma, accomplishing ovulation in pursuit of pregnancy, and regulating metabolic issues in the long term. Lifestyle changes and weight loss are the cornerstones of treatment[23]. Oral contraceptives (OCs) are the first line of PCOS pharmacotherapy due to their effect on hyperandrogenism, menstrual irregularity, and endometrial carcinoma prevention[2,24]. In cases of prevalent hyperandrogenism despite OCs, antiandrogens can be added. When pursuing pregnancy, ovulation induction should be considered with clomiphene citrate, letrozole, and, rarely, gonadotropins[25]. If weight loss and ovulation induction are not successful, the next step is in vitro fertilization.
In the case of metabolic derangements, insulin-sensitizing agents, primarily metformin and thiazolidinediones, are widely used as an alternative or add-on to OCs[26,27]. Studies of newer glucose-lowering agents, such as glucagon-like peptide-1 receptor analogs (GLP-1RA) used for the treatment of obese women with PCOS, revealed a reduction of body weight, increase in menstrual frequency, and improvement of hyperandrogenemia and metabolic derangements even more effectively than metformin[28,29]. The down-side of the mentioned therapy might be a subcutaneous way of application.
PCOS AND CARDIOMETABOLIC RISK
One of the most important pathophysiological processes involved in PCOS development includes IR. The prevalence of IR in PCOS is high: it affects 75% of lean and 95% overweight women[30]. IR represents a link towards increased cardiometabolic risk leading to conditions such as hypertension, glucose intolerance or diabetes, dyslipidemia, and obesity[9,31].
Up to 70% of PCOS women demonstrate IR, glucose intolerance, and overt diabetes[32]. An American study on Women's Health Across the Nation showed a higher prevalence of impaired glucose tolerance (IGT) in PCOS (25%) compared to controls (9.2%)[33]. Moreover, a recent meta-analysis in women with PCOS demonstrated an increased prevalence of type 2 diabetes (T2DM) (odds ratio = 2.87, 95%CI: 1.44-5.72)[34]. Interestingly, 15%-36% of all T2DM diagnosed in women, irrespective of age, is found in association with PCOS. Women with PCOS often exhibit insulin secretory impairment, which accelerates the progression from IGT to T2DM 5 to 10-fold compared to the non-PCOS population, leading to prevalence rates of T2DM 5 to 7-fold higher than those reported in population-based studies of women aged 20-44 years[15,35].
In addition, dyslipidemia occurs in up to 70% of women with PCOS, most commonly characterized by high triglyceride, increased small dense LDL-C levels, and low HDL-C levels[36]. Obesity is also highly prevalent in PCOS; up to 60% of women with PCOS have body mass index (BMI) in the overweight or obesity range, which predisposes them to IR, gonadotropin secretion disturbances, hyperandrogenemia, and low SHBG secretion[37-39]. Consequently, metabolic syndrome is commonly found in women with PCOS (prevalence of 33%-47% in the United States, and 8%-25% in other countries)[36].
According to the recent guidelines, all women with PCOS should be screened for cardiovascular risk factors. Androgen Excess and Polycystic Ovary Syndrome (AE-PCOS) Society recommends categorizing PCOS related cardiovascular disease (CVD) risk as patients at risk (PCOS patients with obesity, cigarette smoking, hypertension, dyslipidemia, subclinical vascular disease, IGT, family history of premature CVD) or high risk (PCOS patients with metabolic syndrome, diabetes mellitus or overt vascular/renal disease)[36].
ROLE OF SGLT-2 INHIBITORS IN PCOS
Multiple metabolic disorders are well recognized among PCOS patients, so assessing the glycemic status is essential. If IGT is detected, lifestyle interventions together with insulin-sensitizing agents such as metformin and thiazolidinedione can be added to improve insulin sensitivity[31]. There are no dedicated metformin studies to confirm its effects on superior BMI reduction compared to placebo or decrease in central adiposity, a good marker for metabolic syndrome[26,27]. Incretins, primarily GLP-1RAs, have the potential to overcome metabolic derangements of PCOS and adding cardiovascular benefits. However, their use is limited by the need for subcutaneous application, while dipeptidyl peptidase-4 inhibitors, oral incretin therapy, lack the evidence of cardiovascular protection in recently published dedicated CVOTs[40]. Therefore, there is still a considerable demand for safe and effective therapeutic agents, offering solutions against PCOS's metabolic dysregulation.
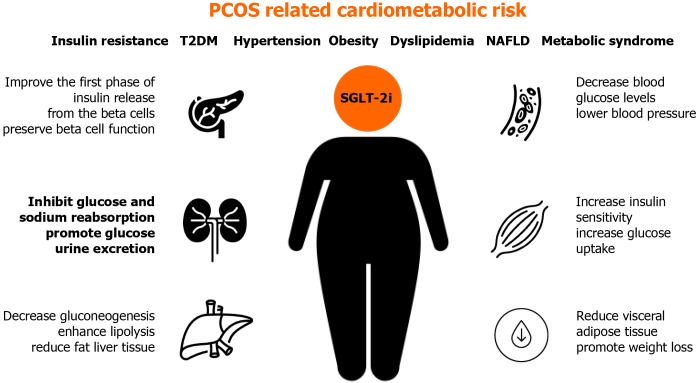
Although SGLT-2 inhibitors are not approved for PCOS treatment, this class of antidiabetic drugs could be useful for PCOS patients due to beneficial glycemic and cardiovascular effects, which are often an issue in women affected by PCOS[41] (Figure 1).
Figure 1.
Potential benefits of sodium-glucose co-transporter-2 inhibitors in treatment of different metabolic and cardiovascular features of polycystic ovary syndrome. NAFLD: Non-alcoholic fatty liver disease; PCOS: Polycystic ovary syndrome; SGLT-2i: Sodium-glucose co-transporter-2 inhibitors; T2DM: Type 2 diabetes.
SGLT receptors are not found on ovaries, but their inhibition can indirectly improve metabolic status disrupted in certain PCOS patients. The role of SGLT-2 inhibitors in the treatment of PCOS is not yet well studied. Their mode of action can contribute to several pathophysiologic disorders in PCOS, including previously mentioned IR, hypertension, obesity, and dyslipidemia. By binding to SGLT-2 receptors in the proximal convoluted tubule of the kidney, gliflozins inhibit glucose and sodium reabsorption, causing a decrease in blood glucose levels, glucosuria, and natriuresis, which contributes to lowering blood pressure. Gliflozins promote glucose urine excretion by 60-80 g per day (approximately 240-320 kcal/d), promoting weight loss by approximately 1.7 kg. The action of SGLT-2 inhibitors does not depend on insulin secretion, beta-cell function, or IR[42,43].
SGLT-2 inhibitors achieve a further reduction in blood glucose levels by increasing insulin sensitivity, increasing glucose uptake in the muscle, decreasing gluconeogenesis in the liver, and improving the first phase of insulin release from the pancreatic beta-cells.
All mentioned processes improve metabolic profiles in diabetic patients, including lipid levels and serum uric acid levels, which could also be beneficial for PCOS patients[44]. Research also suggests the role of SGLT-2 inhibitors in preserving beta-cell function by indirectly reducing insulin secretion and promoting glucagon secretion. The latter plays a role in enhancing lipolysis and reducing the liver and visceral adipose tissue[45].
Besides the expected effect of SGLT-2 inhibitors on glycemic control, they are also shown to be cardioprotective[46], which is an important benefit regarding an increased risk of cardiovascular disease in PCOS.
So far, only one randomized controlled trial compared the effects of empagliflozin (25 mg) vs metformin (1500 mg) on anthropometric and body composition, hormonal and metabolic parameters in 39 women with PCOS. Group treated with empagliflozin showed beneficial effects on weight, BMI, waist circumference and hip circumference, and total body fat in overweight and obese women with PCOS compared to metformin, but no differences were seen in hormonal and metabolic parameters, including IR and androgen levels[47]. The study comparing the effects of another SGLT-2 inhibitor, canagliflozin vs metformin in PCOS, is still underway (Clinical Trial Gov Identifier: NCT04700839).
CONCLUSION
Until more research confirms the positive metabolic effect of SGLT-2 inhibitors in PCOS patients, the mainstream treatment option will be lifestyle intervention, metformin, and oral contraceptive pills[24]. However, this treatment strategy does not successfully address long-term cardiometabolic consequences of PCOS[48], so SGLT-2 inhibitors, due to their mode of action, emerge as a potential new treatment option for PCOS.
ACKNOWLEDGEMENTS
The authors thank Antonije and Hrvojka Dolić for graphic design support.
Footnotes
Conflict-of-interest statement: The authors declare no conflicts of interest.
Manuscript source: Invited manuscript
Peer-review started: March 13, 2021
First decision: March 30, 2021
Article in press: June 22, 2021
Specialty type: Medicine, general and internal
Country/Territory of origin: Croatia
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Morozov S S-Editor: Liu M L-Editor: Filipodia P-Editor: Wang LL
Contributor Information
Jelena Marinkovic-Radosevic, Department of Endocrinology, Diabetes and Metabolism, Sisters of Charity Clinical Hospital Centre, Zagreb 10000, Croatia.
Maja Cigrovski Berkovic, Department of Endocrinology, Diabetes, Metabolism and Clinical Pharmacology, Clinical Hospital Dubrava, Zagreb 10000, Croatia; Department of Kinesiological Anthropology and Methodology, Faculty of Kinesiology, University of Zagreb, Zagreb 10000, Croatia. maja.cigrovskiberkovic@gmail.com.
Egon Kruezi, Department of Gynecology and Obstetrics, Sisters of Charity Clinical Hospital Centre, Zagreb 10000, Croatia.
Ines Bilic-Curcic, Department of Pharmacology, Faculty of Medicine, University of J. J. Strossmayer Osijek, Osijek 31000, Croatia; Clinical Hospital Center Osijek, Osijek 31000, Croatia.
Anna Mrzljak, School of Medicine, University of Zagreb, Zagreb 10000, Croatia; Department of Gastroenterology and Hepatology, University Hospital Centre Zagreb, Zagreb 10000, Croatia.
References
- 1.Bozdag G, Mumusoglu S, Zengin D, Karabulut E, Yildiz BO. The prevalence and phenotypic features of polycystic ovary syndrome: a systematic review and meta-analysis. Hum Reprod . 2016;31:2841–2855. doi: 10.1093/humrep/dew218. [DOI] [PubMed] [Google Scholar]
- 2.Teede HJ, Misso ML, Costello MF, Dokras A, Laven J, Moran L, Piltonen T, Norman RJ International PCOS Network. Recommendations from the international evidence-based guideline for the assessment and management of polycystic ovary syndrome. Fertil Steril . 2018;110:364–379. doi: 10.1016/j.fertnstert.2018.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vink JM, Sadrzadeh S, Lambalk CB, Boomsma DI. Heritability of polycystic ovary syndrome in a Dutch twin-family study. J Clin Endocrinol Metab . 2006;91:2100–2104. doi: 10.1210/jc.2005-1494. [DOI] [PubMed] [Google Scholar]
- 4.Kahsar-Miller MD, Nixon C, Boots LR, Go RC, Azziz R. Prevalence of polycystic ovary syndrome (PCOS) in first-degree relatives of patients with PCOS. Fertil Steril . 2001;75:53–58. doi: 10.1016/s0015-0282(00)01662-9. [DOI] [PubMed] [Google Scholar]
- 5.Legro RS, Driscoll D, Strauss JF 3rd, Fox J, Dunaif A. Evidence for a genetic basis for hyperandrogenemia in polycystic ovary syndrome. Proc Natl Acad Sci USA. 1998;95:14956–14960. doi: 10.1073/pnas.95.25.14956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Balen AH. Hypersecretion of luteinizing hormone and the polycystic ovary syndrome. Hum Reprod . 1993;8 Suppl 2:123–128. doi: 10.1093/humrep/8.suppl_2.123. [DOI] [PubMed] [Google Scholar]
- 7.Jakimiuk AJ, Weitsman SR, Navab A, Magoffin DA. Luteinizing hormone receptor, steroidogenesis acute regulatory protein, and steroidogenic enzyme messenger ribonucleic acids are overexpressed in thecal and granulosa cells from polycystic ovaries. J Clin Endocrinol Metab . 2001;86:1318–1323. doi: 10.1210/jcem.86.3.7318. [DOI] [PubMed] [Google Scholar]
- 8.Fauser BC, Van Heusden AM. Manipulation of human ovarian function: physiological concepts and clinical consequences. Endocr Rev . 1997;18:71–106. doi: 10.1210/edrv.18.1.0290. [DOI] [PubMed] [Google Scholar]
- 9.Osibogun O, Ogunmoroti O, Michos ED. Polycystic ovary syndrome and cardiometabolic risk: Opportunities for cardiovascular disease prevention. Trends Cardiovasc Med . 2020;30:399–404. doi: 10.1016/j.tcm.2019.08.010. [DOI] [PubMed] [Google Scholar]
- 10.Sanchez-Garrido MA, Tena-Sempere M. Metabolic dysfunction in polycystic ovary syndrome: Pathogenic role of androgen excess and potential therapeutic strategies. Mol Metab . 2020;35:100937. doi: 10.1016/j.molmet.2020.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Qin JZ, Pang LH, Li MJ, Fan XJ, Huang RD, Chen HY. Obstetric complications in women with polycystic ovary syndrome: a systematic review and meta-analysis. Reprod Biol Endocrinol . 2013;11:56. doi: 10.1186/1477-7827-11-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sha T, Wang X, Cheng W, Yan Y. A meta-analysis of pregnancy-related outcomes and complications in women with polycystic ovary syndrome undergoing IVF. Reprod Biomed Online . 2019;39:281–293. doi: 10.1016/j.rbmo.2019.03.203. [DOI] [PubMed] [Google Scholar]
- 13.Oliver-Williams C, Vassard D, Pinborg A, Schmidt L. Risk of cardiovascular disease for women with polycystic ovary syndrome: results from a national Danish registry cohort study. Eur J Prev Cardiol . 2020:2047487320939674. doi: 10.1177/2047487320939674. [DOI] [PubMed] [Google Scholar]
- 14.Legro RS, Gnatuk CL, Kunselman AR, Dunaif A. Changes in glucose tolerance over time in women with polycystic ovary syndrome: a controlled study. J Clin Endocrinol Metab . 2005;90:3236–3242. doi: 10.1210/jc.2004-1843. [DOI] [PubMed] [Google Scholar]
- 15.Ehrmann DA, Barnes RB, Rosenfield RL, Cavaghan MK, Imperial J. Prevalence of impaired glucose tolerance and diabetes in women with polycystic ovary syndrome. Diabetes Care . 1999;22:141–146. doi: 10.2337/diacare.22.1.141. [DOI] [PubMed] [Google Scholar]
- 16.Ehrmann DA, Kasza K, Azziz R, Legro RS, Ghazzi MN PCOS/Troglitazone Study Group. Effects of race and family history of type 2 diabetes on metabolic status of women with polycystic ovary syndrome. J Clin Endocrinol Metab . 2005;90:66–71. doi: 10.1210/jc.2004-0229. [DOI] [PubMed] [Google Scholar]
- 17.Lo JC, Feigenbaum SL, Yang J, Pressman AR, Selby JV, Go AS. Epidemiology and adverse cardiovascular risk profile of diagnosed polycystic ovary syndrome. J Clin Endocrinol Metab . 2006;91:1357–1363. doi: 10.1210/jc.2005-2430. [DOI] [PubMed] [Google Scholar]
- 18.Spritzer PM, Lecke SB, Satler F, Morsch DM. Adipose tissue dysfunction, adipokines, and low-grade chronic inflammation in polycystic ovary syndrome. Reproduction . 2015;149:R219–R227. doi: 10.1530/REP-14-0435. [DOI] [PubMed] [Google Scholar]
- 19.Setji TL, Holland ND, Sanders LL, Pereira KC, Diehl AM, Brown AJ. Nonalcoholic steatohepatitis and nonalcoholic Fatty liver disease in young women with polycystic ovary syndrome. J Clin Endocrinol Metab . 2006;91:1741–1747. doi: 10.1210/jc.2005-2774. [DOI] [PubMed] [Google Scholar]
- 20.Rocha ALL, Faria LC, Guimarăes TCM, Moreira GV, Cândido AL, Couto CA, Reis FM. Non-alcoholic fatty liver disease in women with polycystic ovary syndrome: systematic review and meta-analysis. J Endocrinol Invest . 2017;40:1279–1288. doi: 10.1007/s40618-017-0708-9. [DOI] [PubMed] [Google Scholar]
- 21.Ford ES, Giles WH, Mokdad AH. Increasing prevalence of the metabolic syndrome among u.s. Adults. Diabetes Care . 2004;27:2444–2449. doi: 10.2337/diacare.27.10.2444. [DOI] [PubMed] [Google Scholar]
- 22.Apridonidze T, Essah PA, Iuorno MJ, Nestler JE. Prevalence and characteristics of the metabolic syndrome in women with polycystic ovary syndrome. J Clin Endocrinol Metab . 2005;90:1929–1935. doi: 10.1210/jc.2004-1045. [DOI] [PubMed] [Google Scholar]
- 23.Crosignani PG, Colombo M, Vegetti W, Somigliana E, Gessati A, Ragni G. Overweight and obese anovulatory patients with polycystic ovaries: parallel improvements in anthropometric indices, ovarian physiology and fertility rate induced by diet. Hum Reprod . 2003;18:1928–1932. doi: 10.1093/humrep/deg367. [DOI] [PubMed] [Google Scholar]
- 24.Legro RS, Arslanian SA, Ehrmann DA, Hoeger KM, Murad MH, Pasquali R, Welt CK Endocrine Society. Diagnosis and treatment of polycystic ovary syndrome: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab . 2013;98:4565–4592. doi: 10.1210/jc.2013-2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Legro RS, Brzyski RG, Diamond MP, Coutifaris C, Schlaff WD, Casson P, Christman GM, Huang H, Yan Q, Alvero R, Haisenleder DJ, Barnhart KT, Bates GW, Usadi R, Lucidi S, Baker V, Trussell JC, Krawetz SA, Snyder P, Ohl D, Santoro N, Eisenberg E, Zhang H NICHD Reproductive Medicine Network. Letrozole vs clomiphene for infertility in the polycystic ovary syndrome. N Engl J Med . 2014;371:119–129. doi: 10.1056/NEJMoa1313517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Morley LC, Tang T, Yasmin E, Norman RJ, Balen AH. Insulin-sensitising drugs (metformin, rosiglitazone, pioglitazone, D-chiro-inositol) for women with polycystic ovary syndrome, oligo amenorrhoea and subfertility. Cochrane Database Syst Rev . 2017;11:CD003053. doi: 10.1002/14651858.CD003053.pub6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moghetti P, Castello R, Negri C, Tosi F, Perrone F, Caputo M, Zanolin E, Muggeo M. Metformin effects on clinical features, endocrine and metabolic profiles, and insulin sensitivity in polycystic ovary syndrome: a randomized, double-blind, placebo-controlled 6-month trial, followed by open, long-term clinical evaluation. J Clin Endocrinol Metab . 2000;85:139–146. doi: 10.1210/jcem.85.1.6293. [DOI] [PubMed] [Google Scholar]
- 28.Lamos EM, Malek R, Davis SN. GLP-1 receptor agonists in the treatment of polycystic ovary syndrome. Expert Rev Clin Pharmacol . 2017;10:401–408. doi: 10.1080/17512433.2017.1292125. [DOI] [PubMed] [Google Scholar]
- 29.Han Y, Li Y, He B. GLP-1 receptor agonists vs metformin in PCOS: a systematic review and meta-analysis. Reprod Biomed Online . 2019;39:332–342. doi: 10.1016/j.rbmo.2019.04.017. [DOI] [PubMed] [Google Scholar]
- 30.Stepto NK, Cassar S, Joham AE, Hutchison SK, Harrison CL, Goldstein RF, Teede HJ. Women with polycystic ovary syndrome have intrinsic insulin resistance on euglycaemic-hyperinsulaemic clamp. Hum Reprod . 2013;28:777–784. doi: 10.1093/humrep/des463. [DOI] [PubMed] [Google Scholar]
- 31.Goodarzi MO, Dumesic DA, Chazenbalk G, Azziz R. Polycystic ovary syndrome: etiology, pathogenesis and diagnosis. Nat Rev Endocrinol . 2011;7:219–231. doi: 10.1038/nrendo.2010.217. [DOI] [PubMed] [Google Scholar]
- 32.Geller DH, Pacaud D, Gordon CM, Misra M of the Drug and Therapeutics Committee of the Pediatric Endocrine Society. State of the Art Review: Emerging Therapies: The Use of Insulin Sensitizers in the Treatment of Adolescents with Polycystic Ovary Syndrome (PCOS) Int J Pediatr Endocrinol . 2011;2011:9. doi: 10.1186/1687-9856-2011-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Polotsky AJ, Allshouse A, Crawford SL, Harlow SD, Khalil N, Santoro N, Legro RS. Relative contributions of oligomenorrhea and hyperandrogenemia to the risk of metabolic syndrome in midlife women. J Clin Endocrinol Metab . 2012;97:E868–E877. doi: 10.1210/jc.2011-3357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kakoly NS, Khomami MB, Joham AE, Cooray SD, Misso ML, Norman RJ, Harrison CL, Ranasinha S, Teede HJ, Moran LJ. Ethnicity, obesity and the prevalence of impaired glucose tolerance and type 2 diabetes in PCOS: a systematic review and meta-regression. Hum Reprod Update . 2018;24:455–467. doi: 10.1093/humupd/dmy007. [DOI] [PubMed] [Google Scholar]
- 35.Legro RS, Kunselman AR, Dodson WC, Dunaif A. Prevalence and predictors of risk for type 2 diabetes mellitus and impaired glucose tolerance in polycystic ovary syndrome: a prospective, controlled study in 254 affected women. J Clin Endocrinol Metab . 1999;84:165–169. doi: 10.1210/jcem.84.1.5393. [DOI] [PubMed] [Google Scholar]
- 36.Wild RA, Carmina E, Diamanti-Kandarakis E, Dokras A, Escobar-Morreale HF, Futterweit W, Lobo R, Norman RJ, Talbott E, Dumesic DA. Assessment of cardiovascular risk and prevention of cardiovascular disease in women with the polycystic ovary syndrome: a consensus statement by the Androgen Excess and Polycystic Ovary Syndrome (AE-PCOS) Society. J Clin Endocrinol Metab . 2010;95:2038–2049. doi: 10.1210/jc.2009-2724. [DOI] [PubMed] [Google Scholar]
- 37.Azziz R, Woods KS, Reyna R, Key TJ, Knochenhauer ES, Yildiz BO. The prevalence and features of the polycystic ovary syndrome in an unselected population. J Clin Endocrinol Metab . 2004;89:2745–2749. doi: 10.1210/jc.2003-032046. [DOI] [PubMed] [Google Scholar]
- 38.Legro RS. Obesity and PCOS: implications for diagnosis and treatment. Semin Reprod Med . 2012;30:496–506. doi: 10.1055/s-0032-1328878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bajuk Studen K, Pfeifer M. Cardiometabolic risk in polycystic ovary syndrome. Endocr Connect . 2018;7:R238–R251. doi: 10.1530/EC-18-0129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jensterle Sever M, Kocjan T, Pfeifer M, Kravos NA, Janez A. Short-term combined treatment with liraglutide and metformin leads to significant weight loss in obese women with polycystic ovary syndrome and previous poor response to metformin. Eur J Endocrinol . 2014;170:451–459. doi: 10.1530/EJE-13-0797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Moran LJ, Norman RJ, Teede HJ. Metabolic risk in PCOS: phenotype and adiposity impact. Trends Endocrinol Metab . 2015;26:136–143. doi: 10.1016/j.tem.2014.12.003. [DOI] [PubMed] [Google Scholar]
- 42.Satoh H. Pleiotropic effects of SGLT2 inhibitors beyond the effect on glycemic control. Diabetol Int . 2018;9:212–214. doi: 10.1007/s13340-018-0367-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Abdalla MA, Deshmukh H, Atkin S, Sathyapalan T. A review of therapeutic options for managing the metabolic aspects of polycystic ovary syndrome. Ther Adv Endocrinol Metab . 2020;11:2042018820938305. doi: 10.1177/2042018820938305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Verma S, McMurray JJV. SGLT2 inhibitors and mechanisms of cardiovascular benefit: a state-of-the-art review. Diabetologia . 2018;61:2108–2117. doi: 10.1007/s00125-018-4670-7. [DOI] [PubMed] [Google Scholar]
- 45.Hallow KM, Greasley PJ, Helmlinger G, Chu L, Heerspink HJ, Boulton DW. Evaluation of renal and cardiovascular protection mechanisms of SGLT2 inhibitors: model-based analysis of clinical data. Am J Physiol Renal Physiol . 2018;315:F1295–F1306. doi: 10.1152/ajprenal.00202.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Heerspink HJ, Perkins BA, Fitchett DH, Husain M, Cherney DZ. Sodium Glucose Cotransporter 2 Inhibitors in the Treatment of Diabetes Mellitus: Cardiovascular and Kidney Effects, Potential Mechanisms, and Clinical Applications. Circulation . 2016;134:752–772. doi: 10.1161/CIRCULATIONAHA.116.021887. [DOI] [PubMed] [Google Scholar]
- 47.Javed Z, Papageorgiou M, Deshmukh H, Rigby AS, Qamar U, Abbas J, Khan AY, Kilpatrick ES, Atkin SL, Sathyapalan T. Effects of empagliflozin on metabolic parameters in polycystic ovary syndrome: A randomized controlled study. Clin Endocrinol (Oxf) . 2019;90:805–813. doi: 10.1111/cen.13968. [DOI] [PubMed] [Google Scholar]
- 48.Al Khalifah RA, Florez ID, Zoratti MJ, Dennis B, Thabane L, Bassilious E. Efficacy of Treatments for Polycystic Ovarian Syndrome Management in Adolescents. J Endocr Soc . 2021;5:bvaa155. doi: 10.1210/jendso/bvaa155. [DOI] [PMC free article] [PubMed] [Google Scholar]