Abstract
Complex I (NADH dehydrogenase) is the first enzyme in the respiratory chain. It catalyses the electron transfer from NADH to ubiquinone that is associated with proton pumping out of the matrix. In this study, we characterized NADH dehydrogenase activity in seven monoxenous trypanosomatid species: Blechomonas ayalai, Herpetomonas tarakana, Kentomonas sorsogonicus, Leptomonas seymouri, Novymonas esmeraldas, Sergeia podlipaevi and Wallacemonas raviniae. We also investigated the subunit composition of the complex I in dixenous Phytomonas serpens, in which its presence and activity have been previously documented. In addition to P. serpens, the complex I is functionally active in N. esmeraldas and S. podlipaevi. We also identified 24–32 subunits of the complex I in individual species by using mass spectrometry. Among them, for the first time, we recognized several proteins of the mitochondrial DNA origin.
Key words: Monoxenous trypanosomatids, NADH dehydrogenase, Phytomonas
Introduction
NADH:ubiquinone oxidoreductase [EC 7.1.1.2], eukaryotic complex I, is the largest and the most complicated enzyme of the respiratory chain. Its subunits are encoded by both the nuclear and mitochondrial genomes (Chomyn et al., 1985; Walker et al., 1992). It couples the transfer of two electrons from NADH to ubiquinone to the translocation of four protons across the mitochondrial inner membrane. The proposed mechanism includes conformation changes, electrostatic interactions and water molecules that constitute proton-translocation pathways (Grba and Hirst, 2020; Kampjut and Sazanov, 2020). Complex I has an L-shaped structure with a hydrophilic peripheral and a hydrophobic membrane domain. The hydrophilic arm contains two enzymatically distinct regions: the N-module involved in the oxidation of NADH and subsequent electron transport, forming a tip of the arm, and the Q-module, which contains Fe–S clusters, through which electrons are transferred to ubiquinone, forming the interface between two domains. The hydrophobic P-module (composed of the ND1, ND2, ND4 and ND5 multi-protein modules taking part in the proton pumping) is embedded in the inner mitochondrial membrane (Yagi and Matsuno-Yagi, 2003; Brandt, 2006, 2013; Berrisford and Sazanov, 2009). The core of this enzyme consists of 14 essential subunits that are fairly conserved across different domains of life (Gabaldón et al., 2005). Mammalian complex I additionally contains up to 32 accessory subunits that are not directly associated with energy conservation (Carroll et al., 2006; Kmita and Zickermann, 2013). These proteins may be involved in the regulation of enzymatic activity, stability of the complex or auxiliary functions, for example, the fatty acid synthesis (Janssen et al., 2006; Pereira et al., 2013). Two of the most commonly used inhibitors of the mitochondrial complex I are rotenone (stabilizing the semiquinone intermediate within the complex) and capsaicin (antagonizing either formation or release of the quinol product) (Degli Esposti, 1998; Okun et al., 1999).
In addition to complex I, another NADH dehydrogenase, NDH2, has been discovered in the mitochondria of several organisms. It catalyses the transfer of electrons from NADH to ubiquinone without pumping protons out of the matrix (Matus-Ortega et al., 2011). In extreme cases (for example, in Saccharomyces cerevisiae), the complex I is completely missing and its function is taken by the alternative dehydrogenases (Overkamp et al., 2000).
Trypanosomatids (class Kinetoplastea) is a group of obligate parasitic flagellates confined exclusively to insects (monoxenous species) or transmitted by insects or annelids to vertebrates or plants (Lukeš et al., 2018; Maslov et al., 2019). Functionality of the trypanosomatid complex I has long been debated. Bioinformatics analysis identified 29 orthologue genes of the prototypical eukaryotic subunits and further 34 genes encoding unique accessory proteins in genomes of dixenous Trypanosoma brucei, T. cruzi and Leishmania major (Opperdoes and Michels, 2008; Perez et al., 2014; Opperdoes et al., 2016). These genomic data suggest that trypanosomatid complex I is composed of over 60 subunits and its molecular mass is over 2 MDa, which is twice as large as its bovine or yeast counterpart (Abdrakhmanova et al., 2004; Carroll et al., 2006). The mitochondrial DNA of Trypanosoma spp. encodes eight complex I subunits. ND1–ND5 are orthologues to the mitochondrial subunits (which participate in protons pumping and bind ubiquinone and rotenone), while ND7–ND9 are orthologues to the nuclear-encoded subunits NDUFS2 (Fe–S cluster and binding site for ubiquinone), NDUFS8 (two Fe–S clusters) and NDUFS3 in humans. The genes for ND4L and ND6 had been assigned to neither the mitochondrial nor to the nuclear DNA (Opperdoes and Michels, 2008). However, it has been proposed that these proteins are encoded in the mitochondria by CR3 and CR4 genes (Duarte and Tomás, 2014). Recent data demonstrated that trypanosomatids possess all the proteins necessary for NAD+ regeneration by complex I: those involved in electron transfer, ubiquinone binding and reduction and proton pumping. Proteomic analysis confirmed the presence of both canonical and auxiliary subunits encoded in the nuclear genome of T. brucei. It was clearly showed that the complex I subunits are organized into the high molecular weight proteins in trypanosomal mitochondria (Panigrahi et al., 2008; Acestor et al., 2011). However, none of the proteomic studies published to date has been able to detect complex I subunits encoded by the mitochondrial genome of trypanosomatids.
The importance of the complex I in trypanosomatids has been disputed. Indeed, both dyskinetoplastic Trypanosoma evansi and T. equiperdum thrive without it (Schnaufer et al., 2002). The natural T. cruzi mutants with deletions in ND4, ND5 and ND7 genes showed no alterations in mitochondrial bioenergetics compared to the wild type (Carranza et al., 2009). Long-term cultivated isolates of Leishmania tarentolae and Crithidia fasciculata have lost guide RNAs for editing of ND3, ND8 and ND9 genes and no complex I activity had been detected in them (Sloof et al., 1994; Thiemann et al., 1994). Complex I is also not essential in the studied stages of T. brucei. Ablation of NDUFV1 and NDUFS7 in the procyclic and bloodstream forms did not produce any effect on the detected NADH dehydrogenase activity (Verner et al., 2011; Surve et al., 2012), which was also not sensitive to the rotenone (Verner et al., 2014). Of note, the presence of alternative NDH2 has been documented in T. brucei (Fang and Beattie, 2002; Verner et al., 2013) and Phytomonas serpens (Gonzalez-Halphen and Maslov, 2004; Čermáková et al., 2007). Its elimination in both procyclic and bloodstream forms of T. brucei had only a modest effect on the viability of the tested cells (Verner et al., 2013; Surve et al., 2017).
The only trypanosomatid species with essential mitochondrial complex I known to date is P. serpens (Čermáková et al., 2007). However, it lacks respiratory chain complexes III and IV (Nawathean and Maslov, 2000). The size of the complex I in that species is about 2.2 MDa and its NADH dehydrogenase activity, as well as mitochondrial membrane potential are sensitive to rotenone (Moyses and Barrabin, 2004; Verner et al., 2014). It was demonstrated that the complex contains subunits NDUFA6 and NDUFA9 (Čermáková et al., 2007).
Here, we investigated NADH dehydrogenase activity in P. serpens and seven monoxenous trypanosomatids: Blechomonas ayalai (Votýpka et al., 2013), Herpetomonas tarakana (Yurchenko et al., 2016), Kentomonas sorsogonicus (Votýpka et al., 2014), Leptomonas seymouri (Wallace, 1977), Novymonas esmeraldas (Kostygov et al., 2016), Sergeia podlipaevi (Svobodová et al., 2007) and Wallacemonas raviniae (Kostygov et al., 2014). We provide evidence that functional complex I is present in two more trypanosomatids. In these molecular complexes, we detected not only a majority of the nuclear DNA-encoded proteins, but (for the first time) also several subunits derived from the mitochondrial DNA. In all of them we also spotted MURF2, the protein of unknown function (Blum and Simpson, 1990).
Materials and methods
Cultivation of trypanosomatids
Phytomonas serpens (strain 9T) was grown at 27°C in brain heart infusion (BHI) medium (Becton, Dickinson and Co, Sparks, USA) supplemented with 10 μg mL−1 haemin (AppliChem, Darmstadt, Germany) (Lukeš et al., 2006). Herpetomonas tarakana (strain OSR18) was cultivated at 27°C in the complete M199 medium (Sigma-Aldrich, St. Louis, USA) supplemented with 2 μg mL−1 haemin, 10% foetal bovine serum (FBS, Biosera, Kansas City, USA), 100 U mL−1 penicillin, 100 μg mL−1 streptomycin (Sigma-Aldrich), 2 μg mL−1 biopterin (Sigma-Aldrich) and 25 mm HEPES (AppliChem). Blechomonas ayalai (strain B08-376), K. sorsogonicus (strain MF08-01), L. seymouri (strain ATCC30220), N. esmeraldas (strain E262.01), S. podlipaevi (strain CER3) and W. raviniae (strain Mbr-04) were cultured at 23°C in BHI medium supplemented with 10 μg mL−1 haemin, 10% FBS, 100 U mL−1 penicillin, 100 μg mL−1 streptomycin.
Preparation of mitochondrial lysate
The mitochondria-enriched fractions from 5 × 108 cells were isolated by hypotonic lysis as described elsewhere (Horváth et al., 2005). Mitochondria were re-suspended in 0.5 m aminocaproic acid and 2% (w/v) dodecyl maltoside (both AppliChem). Lysis was performed for 1 h on ice and the lysates were centrifuged for 10 min at 20 000 × g at 4°C. The supernatants were recovered, and protein concentration was determined by the Bradford assay (Bradford, 1976).
In silico analyses
The genome of T. brucei [available from the TriTrypDB (Aslett et al., 2010)] was used as a template to search for genes of nucleus-encoded complex I subunits and NDH2 in other trypanosomatid genomes – B. ayalai (Opperdoes et al., 2016), L. seymouri (Kraeva et al., 2015), N. esmeraldas (manuscript in preparation) and W. raviniae (manuscript in preparation) – using BLAST v.2.6.0+ (Camacho et al., 2009).
NADH dehydrogenase activity assay
NADH dehydrogenase activity was measured in 1 mL NDH buffer (50 mm potassium phosphate buffer, pH 7.5, 1 mm EDTA, 0.2 mm KCN), containing 20–30 μg proteins from the mitochondrial lysates and 5 μL of 20 mm NADH (AppliChem). After the addition of 2 μL 10 mm coenzyme Q2 (Sigma-Aldrich), the change in absorbance at 340 nm was followed for 3 min (Čermáková et al., 2019). A unit of activity was defined as the amount of enzyme that catalyses the oxidation of 1 nmol NADH per min, assuming an extinction coefficient of 6.2 L mmol−1 cm−1 (Gonzalez-Halphen and Maslov, 2004). Solutions of the inhibitors were freshly prepared. Capsaicin (Sigma-Aldrich) was dissolved in ethanol, rotenone (Serva, Heidelberg, Germany) and DPI (diphenyl iodonium, Sigma-Aldrich) – in dimethylsulphoxide and methanol, respectively. Rotenone and DPI were added to the assay mixture immediately before the start of the reaction, capsaicin was pre-incubated for 3 min. Native electrophoresis and in-gel activity staining methods were adapted from Zerbetto et al. (1997) and Wittig et al. (2007) and performed as described previously (Verner et al., 2014).
In-gel digestion and mass spectrometry analysis
Procedure was performed as previously described (Shevchenko et al., 2006). Briefly, proteins were separated by native gradient gel, bands of interest were cut into small pieces and incubated in 100 mm ammonium bicarbonate buffer. The samples were reduced in 10 mm DTT (30 min, 56°C) and dehydrated in acetonitrile. Alkylation reaction was performed in the presence of 15 mm iodoacetamide (20 min, room temperature, dark) and samples were dehydrated as described above. For protein digestion, 500 ng of the sequencing grade trypsin (Promega, Madison, USA) and 1 mm CaCl2 were added and the samples were incubated on ice for 30 min (if digestion was incomplete, the reaction was incubated overnight at 37°C). Digested peptides were eluted with acetonitrile and dried in SpeedVac (Thermo Fisher Scientific, Waltham, USA).
For liquid chromatography-mass spectroscopy (LC-MS) analysis, the set of a Nano-trap column (Acclaim PepMap100 C18, 75 μm × 20 mm) and Nano-separation column (Acclaim PepMap C18, 75 μm × 500 mm, both Dionex, Sunnyvale, USA/Thermo Fisher Scientific) attached to the UltiMate 3000 RSLCnano system (Dionex) was used. The peptides were separated for 120 min in a 3–43% gradient of buffer B with two mobile phases used: 0.1% formic acid (v/v) (buffer A) and 80% acetonitrile (v/v) with 0.1% formic acid (buffer B). Spectral data were collected by using the Orbitrap Elite mass spectrometer (Thermo Fisher Scientific) operating in the data-dependent mode using the Top15 strategy for the selection of precursor ions for the HCD fragmentation (Michalski et al., 2012). Obtained datasets were processed by MaxQuant v.1.5.3.30 with built-in Andromeda search engine (Cox et al., 2011). The specific parameters for searching were: carbamidomethylation (C) as permanent modification and oxidation (M) and acetyl (protein N-terminus) as variable modifications. The search was performed against protein datasets of Phytomonas sp. (Hart1), T. brucei (TREU927), T. brucei (Lister 427), L. major (Friedlin) (TriTrypDB, downloaded 10.10.2020) and against a sequence database of U insertion/deletion editing in kinetoplastid mitochondria (Simpson et al., 1998).
Results
We have characterized NADH dehydrogenase activity in P. serpens and seven monoxenous trypanosomatids. We selected species from different clades of Trypanosomatidae (Lukeš et al., 2018). These are the members of the subfamilies Leishmaniinae (Kostygov and Yurchenko, 2017) (L. seymouri and N. esmeraldas), Strigomonadinae (Votýpka et al., 2014) (K. sorsogonicus), Phytomonadinae (Yurchenko et al., 2016) (H. tarakana), Blechomonadinae (Votýpka et al., 2013) (B. ayalai), as well as two genera not formally classified into any subfamily – Sergeia (Svobodová et al., 2007) and Wallacemonas (Kostygov et al., 2014). These species differ not only in host specificity (Dictyoptera, Diptera, Heteroptera or Siphonaptera), but also geographical distribution and particulars of their life cycle. Novymonas esmeraldas and K. sorsogonicus harbour endosymbiotic bacteria, which have been acquired by host species independently in evolution (Kostygov et al., 2017; Silva et al., 2018), while L. seymouri and B. ayalai are heavily infected with dsRNA viruses (Grybchuk et al., 2018a, 2018b).
In silico analyses
We examined the presence of 19 core subunits of both the membrane and peripheral domains of the complex I, whose human orthologues were identified in T. brucei (Duarte and Tomás, 2014), and an alternative pathway enzyme, NDH2, in analysed species of trypanosomatids. The genomic data were available only for four species. The genomes of B. ayalai and L. seymouri are in TriTrypDB and two genomes were sequenced by us: N. esmeraldas (32 Mbp; N50 197 811 bp; 1422 scaffolds) and W. raviniae (27 Mbp; N50 58 925; 1386 scaffolds) (both unpublished data). The correspondent sequences of T. brucei TREU927 from the TriTrypDB (Aslett et al., 2010) were used as queries to search the N. esmeraldas and W. raviniae assemblies with TBLASTN+ v.2.6.0 (Camacho et al., 2009) using a threshold of 10−50. The obtained hits were reciprocally BLASTed against the NCBI database. In all the cases, the genes of interest were located in syntenic genomic positions. All tested genes were detected in the genomes of all analysed trypanosomatids (Table 1 and Supplementary Table 1). Of note, multiple copies for genes encoding subunits NDUFA8, NDUFB10 and NDUFA12 were documented in the genome of W. raviniae.
Table 1.
In silico analysis of the selected complex I genes and alternative dehydrogenase NDH2 encoded by nuclear DNA
| Species | Homo sapiens | Trypanosoma brucei | Blechomonas ayalai | Leptomonas seymouri | Novymonas esmeraldas | Wallacemonas raviniae |
|---|---|---|---|---|---|---|
| Membrane domain | NDUFB1 | Tb927.11.7390 | Baya_011_0530 | Lsey_0055_0260 | + | + |
| NDUFB7 | Tb927.9.11660 | Baya_100_0220 | Lsey_0192_0100 | + | + | |
| NDUFB9 | Tb927.11.15810 | Baya_019_0320 | Lsey_0010_0080 | + | + | |
| NDUFB10 | Tb927.11.9930 | Baya_039_0260 | Lsey_0091_0010 | + | + (2) | |
| NDUFB11 | Tb927.4.440 | Baya_165_0080 | Lsey_0525_0020 | + | + | |
| NDUFAB1 | Tb927.3.860 | Baya_111_0040 | Lsey_0115_0040 | + | + | |
| NDUFS5 | Tb927.3.5340 | Baya_092_0110 | Lsey_0041_0050 | + | + | |
| NDUFA6 | Tb927.10.14860 | Baya_244_0010 | Lsey_0011_0010 | + | + | |
| NDUFA8 | Tb927.10.12930 | Baya_093_0130 | Lsey_0013_0050 | + | + (3) | |
| NDUFA9 | Tb927.10.13620 | Baya_084_0070 | Lsey_0157_0100 | + | + | |
| Peripheral domain | NDUFA13 | Tb927.11.8910 | Baya_029_0060 | Lsey_0071_0190 | + | + |
| NDUFA12 | Tb927.9.12680 | Baya_004_0460 | Lsey_0186_0050 | + | + (2) | |
| NDUFA5 | Tb927.10.4130 | Baya_191_0090 | Lsey_0122_0100 | + | + | |
| NDUFA2 | Tb927.11.16870 | Baya_038_0390 | Lsey_0241_0060 | + | + | |
| NDUFS7 | Tb927.11.1320 | Baya_018_0020 | Lsey_0065_0230 | + | + | |
| NDUFS6 | Tb927.6.4270 | Baya_060_0270 | Lsey_0209_0010 | + | + | |
| NDUFS1 | Tb927.10.12540 | Baya_080_0190 | Lsey_0113_0150 | + | + | |
| NDUFV2 | Tb927.7.6350 | Baya_155_0060 | Lsey_0197_0040 | + | + | |
| NDUFV1 | Tb927.5.450 | Baya_008_1080 | Lsey_0248_0020 | + | + | |
| NDH2 | – | Tb927.10.9440 | Baya_062_0020 | Lsey_0004_0940 | + | + |
All selected genes were detected in all analysed trypanosomatid genomes. The table lists either the names of genes in the TriTrypDB that was used for T. brucei, B. ayalai and L. seymouri or the ‘+’ sign indicating the presence in unannotated databases for N. esmeraldas and W. raviniae. All genes were found in one copy, except for a few genes of W. raviniae, for which a higher copy number is given in parentheses. Names of H. sapiens orthologues are also provided.
NADH dehydrogenase activity
Mitochondrial proteins of the studied strains were separated in 2–12% clear native gradient gel and NADH dehydrogenase activity was detected by in-gel staining (Fig. 1A). In the high molecular weight range, we detected NADH dehydrogenase activity in all species tested. However, the intensity and number of active bands differed significantly. We also noticed significant differences when comparing two different types of native electrophoresis – clear native (Fig. 1A) and blue native (Fig. 1C). NADH dehydrogenase activity in the low molecular weight range was observed for K. sorsogonicus and N. esmeraldas. Its molecular weight around 130 kDa could correspond to the NDH2 dimer. For distinguishing different NADH dehydrogenase activities we performed in-gel staining in the presence of 100 μm DPI (Fig. 1B), which inhibits NDH2 and incomplete complex I (Čermáková et al., 2007). In the case of analysed trypanosomatids, DPI has inhibited most of the signals – strong bands remained visible only in the samples of P. serpens, N. esmeraldas and S. podlipaevi. This suggests that DPI-resistant activity in N. esmeraldas and S. podlipaevi corresponds to the complex I, as was previously shown in P. serpens (Čermáková et al., 2007).
Fig. 1.
In-gel NADH dehydrogenase activity staining. (A, B) Clear native and (C) blue native gradient gel; 100 μg of mitochondrial proteins from Phytomonas serpens (PS), Blechomonas ayalai (BA), Herpetomonas tarakana (HT), Kentomonas sorsogonicus (KS), Leptomonas seymouri (LS), Novymonas esmeraldas (NE), Sergeia podlipaevi (SP) and Wallacemonas raviniae (WR) were applied to each lane. The NADH dehydrogenase activity was detected without (A, C) or with (B) 100 μm DPI. The slices with NADH dehydrogenase activity from blue native gel (C) subjected to MS analysis are marked by numbers 1–4. The positions of molecular weight markers (dimer of BSA and monomer, dimer and trimer of ferritin) are indicated.
NADH dehydrogenase activity was spectrophotometrically measured in four trypanosomatid species (selected based on either the strongest intensity of in-gel staining signal or the presence of activity in low molecular weight range – N. esmeraldas, S. podlipaevi, W. raviniae and K. sorsogonicus) in the absence or presence of specific inhibitors of the eukaryotic complex I (rotenone and capsaicin) and DPI. Our data demonstrated that contribution of the complex I and NDH2 is about equal in P. serpens (Table 2). Although the inhibitory effect of rotenone and capsaicin was comparable in this species, in all other trypanosomatids rotenone did not inhibit NADH dehydrogenase activity. In addition to P. serpens, capsaicin inhibited NADH dehydrogenase activity in N. esmeraldas and S. podlipaevi. A comparable degree of inhibition by capsaicin and DPI in all three species implies the presence of both the functional complex I and the alternative NDH2. Kentomonas sorsogonicus and W. raviniae were not sensitive to capsaicin, while sensitive to DPI, which inhibited over 80% NADH dehydrogenase activity in the W. raviniae and blocked it completely in K. sorsogonicus (Table 2). These results correlate with DPI sensitivity of NADH dehydrogenase determined in the gel (Fig. 1B) and do not indicate the presence of a fully functional complex I in the tested life stage of both W. raviniae and K. sorsogonicus.
Table 2.
Specific NADH dehydrogenase activity with and without inhibitors
| Species | Specific activity (U mg−1) | Inhibitor | Inhibition (%) |
|---|---|---|---|
| Phytomonas serpens | 28 ± 11 | Rotenone | 30 ± 4 |
| Capsaicin | 42 ± 6 | ||
| DPI | 37 ± 4 | ||
| Kentomonas sorsogonicus | 39 ± 8 | Rotenone | 2 ± 3 |
| Capsaicin | 9 ± 8 | ||
| DPI | 100 ± 0 | ||
| N. esmeraldas | 20 ± 9 | Rotenone | 9 ± 7 |
| Capsaicin | 34 ± 12 | ||
| DPI | 35 ± 8 | ||
| Sergeia podlipaevi | 27 ± 10 | Rotenone | 6 ± 3 |
| Capsaicin | 27 ± 9 | ||
| DPI | 20 ± 4 | ||
| W. raviniae | 110 ± 24 | Rotenone | 7 ± 4 |
| Capsaicin | 8 ± 2 | ||
| DPI | 81 ± 13 |
NADH dehydrogenase activity was measured in the mitochondrial lysates of P. serpens, K. sorsogonicus, N. esmeraldas, S. podlipaevi and W. raviniae in the absence or presence of 10 μm rotenone, 300 μm capsaicin and 100 μm DPI. Average values and s.d. of activities and their inhibition (in %) from at least three independent biological replicated (each measured in triplicates) are presented. One unit (U) of NADH dehydrogenase activity catalyses the oxidation of 1 nmol NADH per minute. Specific activity is calculated as U mg−1 of mitochondrial proteins.
Protein composition of the NADH dehydrogenase complex
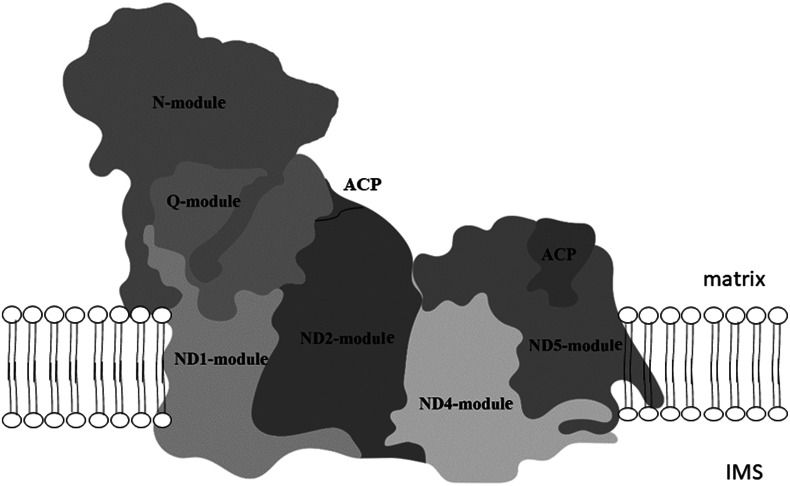
Four native gel's strips in the high molecular weight range of P. serpens, N. esmeraldas and S. podlipaevi and around 130 kDa of N. esmeraldas (Fig. 1C) were subjected to LC-MS analysis (Supplementary Table 2). Most of the returned hits were hypothetical proteins, yet we were able to identify 29 nuclear-encoded subunits of the P. serpens complex I and 22 and 23 subunits of this complex in N. esmeraldas and S. podlipaevi datasets, respectively (Table 3). All the identified subunits were localized to the complex I modules: the N-module forming the peripheral arm, the Q-module binding the ubiquinone, and ND1, ND4 ND5 modules forming the membrane part of the complex I. The only part, from which no subunit has been identified, is the ND2 module. The acyl-carrier protein NDUFAB1 (that is not part of any module) was also detected (Fig. 2, Table 3). In addition to the subunits orthologues to those in other organisms, we also recognized some additional trypanosomatid-specific complex I subunits (Duarte and Tomás, 2014) and a few other proteins that are annotated in the TriTrypDB as NADH dehydrogenase subunits without detailed specification. In addition to the nuclear DNA-encoded complex I subunits, we have also detected several proteins encoded in mitochondrial DNA: ND8, ND7 and ND1. To the best of our knowledge, this is the first experimental detection of mitochondrial DNA-encoded subunits of the complex I at the protein level in trypanosomatids. Moreover, our analysis revealed the presence of the MURF2 (mitochondrial protein with unknown function) in a high molecular weight signals range of NADH dehydrogenase activity. Its detection in all three examined species suggests that this protein may be a part of the trypanosomatids complex I.
Table 3.
Subunits of mitochondrial complex I detected by mass spectrometry analysis
| P. serpens | N. esmeraldas | S. podlipaevi | |
|---|---|---|---|
| N-module | NDUFV1 | NDUFV1 | NDUFV1 |
| NDUFA12 | NDUFA12 | NDUFA12 | |
| NDUFV2 | NDUFV2 | ||
| NDUFA2 | NDUFA2 | NDUFA2 | |
| NDUFS1 | NDUFS1 | NDUFS1 | |
| NDUFA6 | NDUFA6 | NDUFA6 | |
| Q-module | NDUFA5 | NDUFA5 | NDUFA5 |
| NDUFS7 | NDUFS7 | NDUFS7 | |
| NDUFA9 | NDUFA9 | ||
| NDUFS8 (ND8*) | NDUFS8 (ND8*) | NDUFS8 (ND8*) | |
| NDUFS2 (ND7*) | |||
| ND1-module | NDUFA8 | NDUFA8 | NDUFA8 |
| NDUFA13 | NDUFA13 | NDUFA13 | |
| ND1* | ND1* | ||
| ND4-module | NDUFB11 | NDUFB11 | NDUFB11 |
| NDUFB10 | NDUFB10 | NDUFB10 | |
| NDUFB1 | NDUFB1 | ||
| ND5-module | NDUFB7 | NDUFB7 | |
| NDUFB9 | NDUFB9 | ||
| Acyl carrier protein (ACP) | NDUFAB1 | NDUFAB1 | NDUFAB1 |
| Unique trypanosomatids accessory subunits | NDUTB2 | NDUTB2 | |
| NDUTB3 | |||
| NDUTB5 | NDUTB5 | NDUTB5 | |
| NDUTB10 | NDUTB10 | ||
| NDUTB11 | |||
| NDUTB12 | NDUTB12 | NDUTB12 | |
| NDUTB15 | NDUTB15 | NDUTB15 | |
| NDUTB17 | NDUTB17 | ||
| NDUTB25 | |||
| NDUTB26 | NDUTB26 | NDUTB26 | |
| NDUTB31 | NDUTB31 | ||
| Others | Tb927.10.5500 | Tb927.10.5500 | Tb927.10.5500 |
| Tb927.11.7212 | |||
| Tb927.11.15440 | |||
| MURF2* | MURF2* | MURF2* | |
| Total | 32 | 24 | 27 |
Distribution of identified subunits to the modules of complex I is indicated in the left column. Designation of H. sapiens subunits in modules and ACP rows and T. brucei subunits in other rows were used. Subunits encoded by mitochondrial DNA are marked with * (ND1, ND7, ND8 and MURF2).
Fig. 2.
Modular composition of the complex I. The different modules: N-module, Q-module, P- module (composed of ND1, ND2, ND4 and ND5) and acyl carrier protein (ACP) are shown superimposing the structure of bovine complex I. The matrix and intermembrane space (IMS) site of inner mitochondrial membrane are indicated. Adapted from Stroud et al. (2016).
In addition to the complex I proteins, we also identified nine subunits of the ATP synthase, both alternative oxidases (orthologues of Tb927.10.7090 and Tb927.10.9760) and one component of the 2-oxoglutarate dehydrogenase complex (orthologue of Tb927.11.16730) in P. serpens; eight subunits of the ATP synthase, six subunits of the cytochrome c oxidase including mitochondrial DNA-encoded COII and COIII, three subunits of the cytochrome c reductase including apocytochrome b and two subunits of the succinate dehydrogenase in S. podlipaevi; 18 subunits of the ATP synthase including mitochondrial DNA-encoded A6, six subunits of the cytochrome c oxidase, two subunits of the cytochrome c reductase and ten subunits of the succinate dehydrogenase, NDH2 and two components (E2 and E3) of the 2-oxoglutarate dehydrogenase complex in N. esmeraldas (Supplementary Table 2).
Discussion
As was already mentioned above long-term cultivated trypanosomatids L. tarentolae, and C. fasciculata have lost ability to edit some of complex I subunits and do not possess active form of this enzyme (Sloof et al., 1994; Thiemann et al., 1994). It was shown for T. brucei that complex I is not essential for its bloodstream life form (Surve et al., 2012, 2017). Complex I contributes up to 20% of the electron flux of the respiratory chain in the procyclic form of T. brucei but it is also not essential and does not pump protons across the inner mitochondrial membrane (Verner et al., 2011). The main pathways of the electrons entry into the respiratory chain appear to be the complex II (Turrens, 1989; Denicola-Seoane et al., 1992) and/or the alternative enzyme NDH2 (Verner et al., 2013). Although some authors conclude that NDH2 is matrix-oriented (Surve et al., 2017), our previous results strongly suggest that NDH2 is oriented into the intermembrane space and therefore cannot regenerate NAD+ in the matrix (Verner et al., 2013). Within the mitochondria, mitochondrial NADH-dependent fumarate reductase, which converts fumarate to succinate, utilized by complex II, may have this function (Coustou et al., 2005). The only known exception to date was P. serpens, in which the complex I was demonstrated to be not only fully functional, but also the only proton pump in the respiratory chain (Nawathean and Maslov, 2000; Gonzalez-Halphen and Maslov, 2004; Čermáková et al., 2007). Our in silico analysis confirmed the presence of the genes encoding the complex I subunits and the alternative dehydrogenase NDH2 in the genomes of all analysed species (B. ayalai, L. seymouri, N. esmeraldas and W. raviniae). Most genes are present only in one copy, with the exception of W. raviniae, where some subunits are encoded by several genes. However, the mere presence of the genes encoding the complex I subunits is not equal to the functional enzymatic activity. Leishmania tarentolae and C. fasciculata, for example, also possess all the complex I subunit genes in their genomes, and yet their enzymes are not active because the subunits encoded by mitochondrial DNA are not edited (Sloof et al., 1994; Thiemann et al., 1994). Procyclic form of T. brucei has essentially no direct contribution of complex I to the mitochondrial membrane potential (Verner et al., 2011).
Significant differences in NADH dehydrogenase activity within the examined trypanosomatids confirm the statement that complex I is the most controversial enzyme of these parasites (Opperdoes and Michels, 2008; Duarte and Tomás, 2014). Regardless of the strong intensity of some bands, most of them were sensitive to 100 μm DPI, similarly to the case of T. brucei (Verner et al., 2011, 2014). In addition to the expected resistance to DPI of the 2.2 MDa complex of P. serpens (Čermáková et al., 2007), we documented a similar phenomenon only in N. esmeraldas and S. podlipaevi. However, in contrast to P. serpens, these species have also the DPI-resistant activity in the range of about 1.3 MDa (Fig. 1B). It appears that this lower molecular weight complex is even more stable under conditions of native electrophoresis, as its activity was shown to be slightly stronger than that of the upper band under clear native conditions (Fig. 1A) and much stronger in the blue native gel (Fig. 1C). It also differs from the lower P. serpens bands (~600 kDa, DPI-sensitive) in our previous studies, which were suggested to be incomplete forms of the complex I (Čermáková et al., 2007; Verner et al., 2014).
It has been suggested that the 2-oxoglutarate dehydrogenase complex may be responsible for the detected NADH dehydrogenase activity in T. brucei, as up to four proteins of this enzyme were localized to the activity band (Panigrahi et al., 2008; Acestor et al., 2011). Our analysis revealed only one 2-oxoglutarate dehydrogenase subunit in P. serpens, two in N. esmeraldas and none in S. podlipaevi together with the complex I subunits. Therefore, we concluded that 2-oxoglutarate dehydrogenase does not contribute to the NADH dehydrogenase activity in the bands that we have analysed.
In this study, we detected a NADH dehydrogenase signal in the low molecular weight range (around 130 kDa) for the first time in trypanosomatids (K. sorsogonicus and N. esmeraldas). In the yeast Yarrowia lipolytica, the signal in the corresponding range comes from an alternative dehydrogenase (Čermáková et al., 2007). Our results confirm that this is also the case of N. esmeraldas, as we have detected the NDH2 protein in this area by LC-MS analysis. Interestingly, we have also identified NDH2 in the high molecular range along with the complex I subunits in this species. This could suggest that NDH2 functions in association with other proteins. Nevertheless, we revealed it with the complex I only in N. esmeraldas, but not in P. serpens or S. podlipaevi. We explain this discrepancy by either species-specific peculiarities, transient nature of this protein complex, or inconsistencies in databases used for downstream analysis. For example, we used proteome of the exact species N. esmeraldas for Novymonas but had to rely on data from P. serpens isolate Hart1 for the analysis of our model strain, 9T.
Spectrophotometric measurement of enzyme activities is more accurate and quantifiable than in-gel staining. Among the analysed trypanosomatids, sensitivity of the complex I activity to the low and high concentrations of rotenone has been previously documented only for P. serpens (Moyses and Barrabin, 2004; Čermáková et al., 2007) and T. brucei (Beattie and Howton, 1996; Fang et al., 2001), respectively. However, high concentrations of this inhibitor were shown to evoke non-specific effects (Hernandez and Turrens, 1998). It was later demonstrated that lower rotenone concentrations do not affect the NADH dehydrogenase activity of procyclic T. brucei, probably because the complex I is incomplete in this organism (Verner et al., 2011, 2014). In our experiments, rotenone inhibited the NADH dehydrogenase only in P. serpens. This can imply that none of the tested trypanosomatids have the P. serpens-like complex I. However, our experiments with capsaicin (which is another specific inhibitor of the complex I) led a different conclusion. The effect of capsaicin on NADH dehydrogenase activity in P. serpens was comparable to that of rotenone and inversely proportionally correlated with the effect of DPI in four other investigated species. Capsaicin was not effective in K. sorsogonicus and W. raviniae, while DPI inhibited their NADH dehydrogenase activity by 80% or more. The effects of DPI and capsaicin were similar in N. esmeraldas, S. podlipaevi and P. serpens. The resistance to rotenone in N. esmeraldas and S. podlipaevi may be explained by possible amino acid substitutions in NDUFS2, as has been described in other organisms, i.e. a substitution Tyr144Phe leads to 4× lower sensitivity to rotenone in Y. lipolytica (Tocilescu et al., 2010; Angerer et al., 2012). Taken together, our data strongly indicate the presence of a fully functional complex I in N. esmeraldas and S. podlipaevi.
MS analysis of the high molecular weight NADH dehydrogenase activity bands in P. serpens identified 32 subunits of the complex I (29 nuclear and 3 mitochondrial DNA-encoded) (Table 3). The total number of identified subunits is much closer to that of Bos taurus (45 subunits) (Carroll et al., 2006) or Y. lipolytica (42 subunits) (Abdrakhmanova et al., 2004) than to over 60 predicted subunits for trypanosomatids (Duarte and Tomás, 2014). Nevertheless, the complex I of Y. lipolytica migrates at about 880 kDa, which differs from the migration at over 2 MDa for P. serpens (Čermáková et al., 2007) and 1.3 MDa for N. esmeraldas and S. podlipaevi (this study). This can be explained by a higher number of the involved complex I subunits in trypanosomatids, or their significantly higher molecular weight. For example, the NDUFA6 subunit in most eukaryotes is about 15 kDa, whereas its predicted size in trypanosomatids varies from 77 to 83 kDa (Čermáková et al., 2007).
There could be several reasons why we did not detect all the complex I proteins in our analysis: (i) we used protein databases of the related species; (ii) we could not identify unique subunits, similarly to the case of trCOIV subunit of the complex IV (Maslov et al., 2002; Perez et al., 2014) and (iii) some predicted proteins were too short (Duarte and Tomás, 2014) or hydrophobic. A smaller number of subunits identified in N. esmeraldas and S. podlipaevi samples reflects the lower molecular weight form used for the MS analysis. This complex may be depleted of some weaker-bound subunits.
Importantly, we also detected several proteins of complex I encoded by mitochondrial DNA. This is the first experimental evidence for their existence in trypanosomatids. So far, only subunits of the complexes III, IV and V have been detected (Horváth et al., 2000a, 2000b, 2002; Acestor et al., 2011; Škodová-Sveráková et al., 2015a). We identified the ND8 subunit in three analysed species, ND1 in two and ND7 only in S. podlipaevi. We also detected the MURF2 – a mitochondrial protein of unknown function (Blum and Simpson, 1990). Its co-occurrence with other subunits of the complex I in all analysed species strongly suggests that it could be another subunit of this enzyme.
Comparison of bioenergetic metabolism in several trypanosomatid species suggests that these parasites have retained all the essential genes during evolution. Their expression depends on the specific living conditions – the availability of food and host–parasite relationships (Škodová-Sveráková et al., 2015b). Data obtained in this study indicate that the same rules apply to the complex I. Its loss is not only induced by the prolonged cultivation in vitro, but also may be influenced by natural conditions in different trypanosomatid species.
Acknowledgements
We thank Dr Jan Votýpka (Charles University, Prague) for providing cultures of S. podlipaevi and W. raviniae, and Dr Kristína Záhonová (Institute of Parasitology, České Budějovice and Charles University, Prague) for help in analysis of unannotated genomes of N. esmeraldas and W. raviniae and Katarína Behinská (Comenius University, Bratislava) for participating in the measurement of the inhibitory effect of capsaicin.
Ethical standards
Not applicable.
Supplementary material
For supplementary material accompanying this paper visit https://doi.org/10.1017/S0031182020002425.
click here to view supplementary material
Financial support
This study was supported by the Scientific Grant Agency of the Slovak Ministry of Education and the Academy of Sciences (1/0387/17), Slovak Research and Development Agency grants (APVV-0286-12 and APVV-15-0654) to AH, Grant Agency of Czech Republic (20-07186S) and the European Regional Funds (project ‘Centre for Research of Pathogenicity and Virulence of Parasites’ CZ.02.1.01/16_019/0000759) to VY, and by grant ITMS 26240220086 supported by the Research & Development Operational Programme funded by the ERDF. The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
Conflict of interest
The authors declare no conflict of interest.
References
- Abdrakhmanova A, Zickermann V, Bostina M, Radermacher M, Schagger H, Kerscher S and Brandt U (2004) Subunit composition of mitochondrial complex I from the yeast Yarrowia lipolytica. Biochimica et Biophysica Acta 1658, 148–156. [DOI] [PubMed] [Google Scholar]
- Acestor N, Zíková A, Dalley RA, Anupama A, Panigrahi AK and Stuart KD (2011) Trypanosoma brucei mitochondrial respiratome: composition and organization in procyclic form. Molecular and Cellular Proteomics 10, M110006908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angerer H, Nasiri HR, Niedergesass V, Kerscher S, Schwalbe H and Brandt U (2012) Tracing the tail of ubiquinone in mitochondrial complex I. Biochimica et Biophysica Acta 1817, 1776–1784. [DOI] [PubMed] [Google Scholar]
- Aslett M, Aurrecoechea C, Berriman M, Brestelli J, Brunk BP, Carrington M, Depledge DP, Fischer S, Gajria B, Gao X, Gardner MJ, Gingle A, Grant G, Harb OS, Heiges M, Hertz-Fowler C, Houston R, Innamorato F, Iodice J, Kissinger JC, Kraemer E, Li W, Logan FJ, Miller JA, Mitra S, Myler PJ, Nayak V, Pennington C, Phan I, Pinney DF, Ramasamy G, Rogers MB, Roos DS, Ross C, Sivam D, Smith DF, Srinivasamoorthy G, Stoeckert CJ Jr, Subramanian S, Thibodeau R, Tivey A, Treatman C, Velarde G and Wang H (2010) TriTrypDB: a functional genomic resource for the Trypanosomatidae. Nucleic Acids Research 38, D457–D462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beattie DS and Howton MM (1996) The presence of rotenone-sensitive NADH dehydrogenase in the long slender bloodstream and the procyclic forms of Trypanosoma brucei brucei. European Journal of Biochemistry 241, 888–894. [DOI] [PubMed] [Google Scholar]
- Berrisford JM and Sazanov LA (2009) Structural basis for the mechanism of respiratory complex I. Journal of Biological Chemistry 284, 29773–29783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blum B and Simpson L (1990) Guide RNAs in kinetoplastid mitochondria have a nonencoded 3' oligo(U) tail involved in recognition of the preedited region. Cell 62, 391–397. [DOI] [PubMed] [Google Scholar]
- Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry 72, 248–254. [DOI] [PubMed] [Google Scholar]
- Brandt U (2006) Energy converting NADH:quinone oxidoreductase (complex I). Annual Review of Biochemistry 75, 69–92. [DOI] [PubMed] [Google Scholar]
- Brandt U (2013) Inside view of a giant proton pump. Angewandte Chemie 52, 7358–7360. [DOI] [PubMed] [Google Scholar]
- Camacho C, Coulouris G, Avagyan V, Ma N, Papadopoulos J, Bealer K and Madden TL (2009) BLAST+: architecture and applications. BMC Bioinformatics 10, 421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carranza JC, Kowaltowski AJ, Mendonca MA, de Oliveira TC, Gadelha FR and Zingales B (2009) Mitochondrial bioenergetics and redox state are unaltered in Trypanosoma cruzi isolates with compromised mitochondrial complex I subunit genes. Journal of Bioenergetics and Biomembranes 41, 299–308. [DOI] [PubMed] [Google Scholar]
- Carroll J, Fearnley IM, Skehel JM, Shannon RJ, Hirst J and Walker JE (2006) Bovine complex I is a complex of 45 different subunits. Journal of Biological Chemistry 281, 32724–32727. [DOI] [PubMed] [Google Scholar]
- Čermáková P, Verner Z, Man P, Lukeš J and Horváth A (2007) Characterization of the NADH:ubiquinone oxidoreductase (complex I) in the trypanosomatid Phytomonas serpens (Kinetoplastida). FEBS Journal 274, 3150–3158. [DOI] [PubMed] [Google Scholar]
- Čermáková P, Kovalinka T, Ferenczyová K and Horváth A (2019) Coenzyme Q2 is a universal substrate for the measurement of respiratory chain enzyme activities in trypanosomatids. Parasite 26, 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomyn A, Mariottini P, Cleeter MW, Ragan CI, Matsuno-Yagi A, Hatefi Y, Doolittle RF and Attardi G (1985) Six unidentified reading frames of human mitochondrial DNA encode components of the respiratory-chain NADH dehydrogenase. Nature 314, 592–597. [DOI] [PubMed] [Google Scholar]
- Coustou V, Besteiro S, Rivière L, Biran M, Biteau N, Franconi JM, Boshart M, Baltz T and Bringaud F (2005) A mitochondrial NADH-dependent fumarate reductase involved in the production of succinate excreted by procyclic Trypanosoma brucei. Journal of Biological Chemistry 280, 16559–16570. [DOI] [PubMed] [Google Scholar]
- Cox J, Neuhauser N, Michalski A, Scheltema RA, Olsen JV and Mann M (2011) Andromeda: a peptide search engine integrated into the MaxQuant environment. Journal of Proteome Research 10, 1794–1805. [DOI] [PubMed] [Google Scholar]
- Degli Esposti M (1998) Inhibitors of NADH-ubiquinone reductase: an overview. Biochimica et Biophysica Acta 1364, 222–235. [DOI] [PubMed] [Google Scholar]
- Denicola-Seoane A, Rubbo H, Prodanov E and Turrens JF (1992) Succinate-dependent metabolism in Trypanosoma cruzi epimastigotes. Molecular and Biochemical Parasitology 54, 43–50. [DOI] [PubMed] [Google Scholar]
- Duarte M and Tomás AM (2014) The mitochondrial complex I of trypanosomatids – an overview of current knowledge. Journal of Bioenergetics and Biomembranes 46, 299–311. [DOI] [PubMed] [Google Scholar]
- Fang J and Beattie DS (2002) Novel FMN-containing rotenone-insensitive NADH dehydrogenase from Trypanosoma brucei mitochondria: isolation and characterization. Biochemistry 41, 3065–3072. [DOI] [PubMed] [Google Scholar]
- Fang J, Wang Y and Beattie DS (2001) Isolation and characterization of complex I, rotenone-sensitive NADH: ubiquinone oxidoreductase, from the procyclic forms of Trypanosoma brucei. European Journal of Biochemistry 268, 3075–3082. [DOI] [PubMed] [Google Scholar]
- Gabaldón T, Rainey D and Huynen MA (2005) Tracing the evolution of a large protein complex in the eukaryotes, NADH:ubiquinone oxidoreductase (complex I). Journal of Molecular Biology 348, 857–870. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Halphen D and Maslov DA (2004) NADH-ubiquinone oxidoreductase activity in the kinetoplasts of the plant trypanosomatid Phytomonas serpens. Parasitology Research 92, 341–346. [DOI] [PubMed] [Google Scholar]
- Grba DN and Hirst J (2020) Mitochondrial complex I structure reveals ordered water molecules for catalysis and proton translocation. Nature Structural & Molecular Biology 27, 892–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grybchuk D, Akopyants NS, Kostygov AY, Konovalovas A, Lye LF, Dobson DE, Zangger H, Fasel N, Butenko A, Frolov AO, Votýpka J, d'Avila-Levy CM, Kulich P, Moravcová J, Plevka P, Rogozin IB, Serva S, Lukeš J, Beverley SM and Yurchenko V (2018a) Viral discovery and diversity in trypanosomatid protozoa with a focus on relatives of the human parasite Leishmania. Proceedings of the National Academy of Sciences of the United States of America 115, E506–E515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grybchuk D, Kostygov AY, Macedo DH, Votypka J, Lukes J and Yurchenko V (2018b) RNA viruses in Blechomonas (Trypanosomatidae) and evolution of Leishmaniavirus. MBio 9, e01932–e01918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez FR and Turrens JF (1998) Rotenone at high concentrations inhibits NADH-fumarate reductase and the mitochondrial respiratory chain of Trypanosoma brucei and T. cruzi. Molecular and Biochemical Parasitology 93, 135–137. [DOI] [PubMed] [Google Scholar]
- Horváth A, Berry EA and Maslov DA (2000a) Translation of the edited mRNA for cytochrome b in trypanosome mitochondria. Science (New York, N.Y.) 287, 1639–1640. [DOI] [PubMed] [Google Scholar]
- Horváth A, Kingan TG and Maslov DA (2000b) Detection of the mitochondrially encoded cytochrome c oxidase subunit I in the trypanosomatid protozoan Leishmania tarentolae. Evidence for translation of unedited mRNA in the kinetoplast. Journal of Biological Chemistry 275, 17160–17165. [DOI] [PubMed] [Google Scholar]
- Horváth A, Nebohacova M, Lukeš J and Maslov DA (2002) Unusual polypeptide synthesis in the kinetoplast-mitochondria from Leishmania tarentolae. Identification of individual de novo translation products. Journal of Biological Chemistry 277, 7222–7230. [DOI] [PubMed] [Google Scholar]
- Horváth A, Horáková E, Dunajčíková P, Verner Z, Pravdová E, Šlapetová I, Cuninková L and Lukeš J (2005) Downregulation of the nuclear-encoded subunits of the complexes III and IV disrupts their respective complexes but not complex I in procyclic Trypanosoma brucei. Molecular Microbiology 58, 116–130. [DOI] [PubMed] [Google Scholar]
- Janssen RJ, Nijtmans LG, van den Heuvel LP and Smeitink JA (2006) Mitochondrial complex I: structure, function and pathology. Journal of Inherited Metabolic Disease 29, 499–515. [DOI] [PubMed] [Google Scholar]
- Kampjut D and Sazanov LA (2020) The coupling mechanism of mammalian respiratory complex I. Science (New York, N.Y.) 370, eabc4209. [DOI] [PubMed] [Google Scholar]
- Kmita K and Zickermann V (2013) Accessory subunits of mitochondrial complex I. Biochemical Society Transactions 41, 1272–1279. [DOI] [PubMed] [Google Scholar]
- Kostygov AY and Yurchenko V (2017) Revised classification of the subfamily Leishmaniinae (Trypanosomatidae). Folia Parasitologica 64, 020. [DOI] [PubMed] [Google Scholar]
- Kostygov AY, Grybchuk-Ieremenko A, Malysheva MN, Frolov AO and Yurchenko V (2014) Molecular revision of the genus Wallaceina. Protist 165, 594–604. [DOI] [PubMed] [Google Scholar]
- Kostygov A, Dobáková E, Grybchuk-Ieremenko A, Váhala D, Maslov DA, Votýpka J, Lukeš J and Yurchenko V (2016) Novel trypanosomatid – bacterium association: evolution of endosymbiosis in action. MBio 7, e01985–e01915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostygov AY, Butenko A, Nenarokova A, Tashyreva D, Flegontov P, Lukeš J and Yurchenko V (2017) Genome of Ca. Pandoraea novymonadis, an endosymbiotic bacterium of the trypanosomatid Novymonas esmeraldas. Frontiers in Microbiology 8, 1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraeva N, Butenko A, Hlaváčová J, Kostygov A, Myškova J, Grybchuk D, Leštinová T, Votýpka J, Volf P, Opperdoes F, Flegontov P, Lukeš J and Yurchenko V (2015) Leptomonas seymouri: adaptations to the dixenous life cycle analyzed by genome sequencing, transcriptome profiling and co-infection with Leishmania donovani. PLoS Pathogens 11, e1005127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukeš J, Paris Z, Regmi S, Breitling R, Mureev S, Kushnir S, Pyatkov K, Jirků M and Alexandrov K (2006) Translational initiation in Leishmania tarentolae and Phytomonas serpens (Kinetoplastida) is strongly influenced by pre-ATG triplet and its 5' sequence context. Molecular and Biochemical Parasitology 148, 125–132. [DOI] [PubMed] [Google Scholar]
- Lukeš J, Butenko A, Hashimi H, Maslov DA, Votýpka J and Yurchenko V (2018) Trypanosomatids are much more than just trypanosomes: clues from the expanded family tree. Trends in Parasitology 34, 466–480. [DOI] [PubMed] [Google Scholar]
- Maslov DA, Zíková A, Kyselová I and Lukeš J (2002) A putative novel nuclear-encoded subunit of the cytochrome c Oxidase complex in trypanosomatids. Molecular and Biochemical Parasitology 125, 113–125. [DOI] [PubMed] [Google Scholar]
- Maslov DA, Opperdoes FR, Kostygov AY, Hashimi H, Lukeš J and Yurchenko V (2019) Recent advances in trypanosomatid research: genome organization, expression, metabolism, taxonomy and evolution. Parasitology 146, 1–27. [DOI] [PubMed] [Google Scholar]
- Matus-Ortega MG, Salmeron-Santiago KG, Flores-Herrera O, Guerra-Sanchez G, Martinez F, Rendon JL and Pardo JP (2011) The alternative NADH dehydrogenase is present in mitochondria of some animal taxa. Comparative Biochemistry and Physiology. Part D, Genomics & Proteomics 6, 256–263. [DOI] [PubMed] [Google Scholar]
- Michalski A, Damoc E, Lange O, Denisov E, Nolting D, Muller M, Viner R, Schwartz J, Remes P, Belford M, Dunyach JJ, Cox J, Horning S, Mann M and Makarov A (2012) Ultra high resolution linear ion trap Orbitrap mass spectrometer (Orbitrap Elite) facilitates top down LC MS/MS and versatile peptide fragmentation modes. Molecular and Cellular Proteomics 11, O111 013698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moyses DN and Barrabin H (2004) Rotenone-sensitive mitochondrial potential in Phytomonas serpens: electrophoretic Ca2+ accumulation. Biochimica et Biophysica Acta 1656, 96–103. [DOI] [PubMed] [Google Scholar]
- Nawathean P and Maslov DA (2000) The absence of genes for cytochrome c oxidase and reductase subunits in maxicircle kinetoplast DNA of the respiration-deficient plant trypanosomatid Phytomonas serpens. Current Genetics 38, 95–103. [DOI] [PubMed] [Google Scholar]
- Okun JG, Lummen P and Brandt U (1999) Three classes of inhibitors share a common binding domain in mitochondrial complex I (NADH:ubiquinone oxidoreductase). Journal of Biological Chemistry 274, 2625–2630. [DOI] [PubMed] [Google Scholar]
- Opperdoes FR and Michels PA (2008) Complex I of Trypanosomatidae: does it exist? Trends in Parasitology 24, 310–317. [DOI] [PubMed] [Google Scholar]
- Opperdoes FR, Butenko A, Flegontov P, Yurchenko V and Lukeš J (2016) Comparative metabolism of free-living Bodo saltans and parasitic trypanosomatids. Journal of Eukaryotic Microbiology 63, 657–678. [DOI] [PubMed] [Google Scholar]
- Overkamp KM, Bakker BM, Kotter P, van Tuijl A, de Vries S, van Dijken JP and Pronk JT (2000) In vivo Analysis of the mechanisms for oxidation of cytosolic NADH by Saccharomyces cerevisiae mitochondria. Journal of Bacteriology 182, 2823–2830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panigrahi AK, Ziková A, Dalley RA, Acestor N, Ogata Y, Anupama A, Myler PJ and Stuart KD (2008) Mitochondrial complexes in Trypanosoma brucei: a novel complex and a unique oxidoreductase complex. Molecular and Cellular Proteomics 7, 534–545. [DOI] [PubMed] [Google Scholar]
- Pereira B, Videira A and Duarte M (2013) Novel insights into the role of Neurospora crassa NDUFAF2, an evolutionarily conserved mitochondrial complex I assembly factor. Molecular and Cellular Biology 33, 2623–2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez E, Lapaille M, Degand H, Cilibrasi L, Villavicencio-Queijeiro A, Morsomme P, Gonzalez-Halphen D, Field MC, Remacle C, Baurain D and Cardol P (2014) The mitochondrial respiratory chain of the secondary green alga Euglena gracilis shares many additional subunits with parasitic Trypanosomatidae. Mitochondrion 19, 338–349. [DOI] [PubMed] [Google Scholar]
- Schnaufer A, Domingo GJ and Stuart K (2002) Natural and induced dyskinetoplastic trypanosomatids: how to live without mitochondrial DNA. International Journal for Parasitology 32, 1071–1084. [DOI] [PubMed] [Google Scholar]
- Shevchenko A, Tomas H, Havlis J, Olsen JV and Mann M (2006) In-gel digestion for mass spectrometric characterization of proteins and proteomes. Nature Protocols 1, 2856–2860. [DOI] [PubMed] [Google Scholar]
- Silva FM, Kostygov AY, Spodareva VV, Butenko A, Tossou R, Lukes J, Yurchenko V and Alves JMP (2018) The reduced genome of Candidatus Kinetoplastibacterium sorsogonicusi, the endosymbiont of Kentomonas sorsogonicus (Trypanosomatidae): loss of the haem-synthesis pathway. Parasitology 145, 1287–1293. doi: 10.1017/S003118201800046X. [DOI] [PubMed] [Google Scholar]
- Simpson L, Wang SH, Thiemann OH, Alfonzo JD, Maslov DA and Avila HA (1998) U-insertion/deletion edited sequence database. Nucleic Acids Research 26, 170–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Škodová-Sveráková I, Horváth A and Maslov DA (2015a) Identification of the mitochondrially encoded subunit 6 of F1FO ATPase in Trypanosoma brucei. Molecular and Biochemical Parasitology 201, 135–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Škodová-Sveráková I, Verner Z, Skalický T, Votýpka J, Horváth A and Lukeš J (2015b) Lineage-specific activities of a multipotent mitochondrion of trypanosomatid flagellates. Molecular Microbiology 96, 55–67. [DOI] [PubMed] [Google Scholar]
- Sloof P, Arts GJ, van den Burg J, van der Spek H and Benne R (1994) RNA editing in mitochondria of cultured trypanosomatids: translatable mRNAs for NADH-dehydrogenase subunits are missing. Journal of Bioenergetics and Biomembranes 26, 193–203. [DOI] [PubMed] [Google Scholar]
- Stroud DA, Surgenor EE, Formosa LE, Reljic B, Frazier AE, Dibley MG, Osellame LD, Stait T, Beilharz TH, Thorburn DR, Salim A and Ryan MT (2016) Accessory subunits are integral for assembly and function of human mitochondrial complex I. Nature 538, 123–126. [DOI] [PubMed] [Google Scholar]
- Surve S, Heestand M, Panicucci B, Schnaufer A and Parsons M (2012) Enigmatic presence of mitochondrial complex I in Trypanosoma brucei bloodstream forms. Eukaryotic Cell 11, 183–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surve SV, Jensen BC, Heestand M, Mazet M, Smith TK, Bringaud F, Parsons M and Schnaufer A (2017) NADH dehydrogenase of Trypanosoma brucei is important for efficient acetate production in bloodstream forms. Molecular and Biochemical Parasitology 211, 57–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svobodová M, Zídková L, Čepička I, Oborník M, Lukeš J and Votýpka J (2007) Sergeia podlipaevi gen. nov., sp. nov. (Trypanosomatidae, Kinetoplastida), a parasite of biting midges (Ceratopogonidae, Diptera). International Journal of Systematic and Evolutionary Microbiology 57, 423–432. [DOI] [PubMed] [Google Scholar]
- Thiemann OH, Maslov DA and Simpson L (1994) Disruption of RNA editing in Leishmania tarentolae by the loss of minicircle-encoded guide RNA genes. EMBO Journal 13, 5689–5700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tocilescu MA, Fendel U, Zwicker K, Drose S, Kerscher S and Brandt U (2010) The role of a conserved tyrosine in the 49-kDa subunit of complex I for ubiquinone binding and reduction. Biochimica et Biophysica Acta 1797, 625–632. [DOI] [PubMed] [Google Scholar]
- Turrens JF (1989) The role of succinate in the respiratory chain of Trypanosoma brucei procyclic trypomastigotes. Biochemical Journal 259, 363–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verner Z, Čermáková P, Škodová I, Kriegová E, Horváth A and Lukeš J (2011) Complex I (NADH:ubiquinone oxidoreductase) is active in but non-essential for procyclic Trypanosoma brucei. Molecular and Biochemical Parasitology 175, 196–200. [DOI] [PubMed] [Google Scholar]
- Verner Z, Škodová I, Poláková S, Ďurišová-Benkovičová V, Horváth A and Lukeš J (2013) Alternative NADH dehydrogenase (NDH2): intermembrane-space-facing counterpart of mitochondrial complex I in the procyclic Trypanosoma brucei. Parasitology 140, 328–337. [DOI] [PubMed] [Google Scholar]
- Verner Z, Čermáková P, Škodová I, Kováčová B, Lukeš J and Horváth A (2014) Comparative analysis of respiratory chain and oxidative phosphorylation in Leishmania tarentolae, Crithidia fasciculata, Phytomonas serpens and procyclic stage of Trypanosoma brucei. Molecular and Biochemical Parasitology 193, 55–65. [DOI] [PubMed] [Google Scholar]
- Votýpka J, Suková E, Kraeva N, Ishemgulova A, Duží I, Lukeš J and Yurchenko V (2013) Diversity of trypanosomatids (Kinetoplastea: Trypanosomatidae) parasitizing fleas (Insecta: Siphonaptera) and description of a new genus Blechomonas Gen. n. Protist 164, 763–781. [DOI] [PubMed] [Google Scholar]
- Votýpka J, Kostygov AY, Kraeva N, Grybchuk-Ieremenko A, Tesařová M, Grybchuk D, Lukeš J and Yurchenko V (2014) Kentomonas Gen. n., a new genus of endosymbiont-containing trypanosomatids of Strigomonadinae subfam. n. Protist 165, 825–838. [DOI] [PubMed] [Google Scholar]
- Walker JE, Arizmendi JM, Dupuis A, Fearnley IM, Finel M, Medd SM, Pilkington SJ, Runswick MJ and Skehel JM (1992) Sequences of 20 subunits of NADH:ubiquinone oxidoreductase from bovine heart mitochondria. Application of a novel strategy for sequencing proteins using the polymerase chain reaction. Journal of Molecular Biology 226, 1051–1072. [DOI] [PubMed] [Google Scholar]
- Wallace FG (1977) Leptomonas seymouri sp. n. from the cotton stainer Dysdercus suturellus. The Journal of Protozoology 24, 483–484. [DOI] [PubMed] [Google Scholar]
- Wittig I, Karas M and Schagger H (2007) High resolution clear native electrophoresis for in-gel functional assays and fluorescence studies of membrane protein complexes. Molecular and Cellular Proteomics 6, 1215–1225. [DOI] [PubMed] [Google Scholar]
- Yagi T and Matsuno-Yagi A (2003) The proton-translocating NADH-quinone oxidoreductase in the respiratory chain: the secret unlocked. Biochemistry 42, 2266–2274. [DOI] [PubMed] [Google Scholar]
- Yurchenko V, Kostygov A, Havlová J, Grybchuk-Ieremenko A, Ševčíková T, Lukeš J, Ševčík J and Votýpka J (2016) Diversity of trypanosomatids in cockroaches and the description of Herpetomonas tarakana sp. n. Journal of Eukaryotic Microbiology 63, 198–209. [DOI] [PubMed] [Google Scholar]
- Zerbetto E, Vergani L and Dabbeni-Sala F (1997) Quantification of muscle mitochondrial oxidative phosphorylation enzymes via histochemical staining of blue native polyacrylamide gels. Electrophoresis 18, 2059–2064. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
For supplementary material accompanying this paper visit https://doi.org/10.1017/S0031182020002425.
click here to view supplementary material