Abstract
Background:
During sepsis, gram-negative bacteria induce robust inflammation primarily via lipopolysacharride (LPS) signaling through TLR4, a process that involves the glycosylphosphatidylinositol (GPI)-anchored receptor CD14 transferring LPS to the Toll-like receptor 4/myeloid differentiation factor 2 (TLR4/MD-2) complex. Sepsis also triggers the onset of disseminated intravascular coagulation and consumptive coagulopathy.
Objectives:
We investigated the effect of CD14 blockade on sepsis-induced coagulopathy, inflammation, organ dysfunction, and mortality.
Methods:
We used a baboon model of lethal Escherichia (E) coli sepsis to study two experimental groups (n = 5): (a) E coli challenge; (b) E coli challenge plus anti-CD14 (23G4) inhibitory antibody administered as an intravenous bolus 30 minutes before the E coli.
Results:
Following anti-CD14 treatment, two animals reached the 7-day end-point survivor criteria, while three animals had a significantly prolonged survival as compared to the non-treated animals that developed multiple organ failure and died within 30 hours. Anti-CD14 reduced the activation of coagulation through inhibition of tissue factor-dependent pathway, especially in the survivors, and enhanced the fibrinolysis due to strong inhibition of plasminogen activator inhibitor 1. The treatment prevented the robust complement activation induced by E coli, as shown by significantly decreased C3b, C5a, and sC5b-9. Vital signs, organ function biomarkers, bacteria clearance, and leukocyte and fibrinogen consumption were all improved at varying levels. Anti-CD14 reduced neutrophil activation, cell death, LPS levels, and pro-inflammatory cytokines (tumor necrosis factor, interleukin (IL)-6, IL-1β, IL-8, interferon gamma, monocyte chemoattractant protein-1), more significantly in the survivors than non-surviving animals.
Conclusions:
Our results highlight the crosstalk between coagulation/fibrinolysis, inflammation, and complement systems and suggest a protective role of anti-CD14 treatment in E coli sepsis.
Keywords: CD14, coagulation, complement, Escherichia coli, fibrinolysis, immunotherapy, sepsis
1 |. INTRODUCTION
Sepsis and accompanying systemic inflammation have a complex pathophysiology. When progressing to septic shock it becomes a major burden to health-care systems and mortality can reach 30% to 70%.1–3 Except for antibiotics and supportive care, there is no specific treatment for sepsis.
Even if antibiotics successfully kill the bacteria, the pathophysiology of sepsis is driven by the host’s own inflammatory response,4 which can lead to uncontrolled cytokine storm. The joint activation of inflammation and the protease cascades of coagulation, fibrinolysis, and complement will lead to thrombo-inflammation, which when it occurs intravascularly, will progress to disseminated intravascular coagulation (DIC), shock, organ failure, and finally to death.
Bacteria- or pathogen-associated molecular patterns (PAMP) like the lipopolysaccharide (LPS) are procoagulant, triggering the activation of protease cascades and leading to DIC, but are also pro-inflammatory through binding to the CD14 receptor on monocyte/macrophage cell lines and subsequent activation of Toll-like receptors (TLR). TLRs are pattern recognition receptors present in nearly all mammalian cells and especially on cells important for innate immunity signaling such as dendritic cells, leukocytes, and endothelial cells.5
LPS induces inflammatory responses through binding to the soluble LPS-binding protein and transfer to the glycosylphosphatidylinositol (GPI)-anchored CD14 receptor, which associates with TLR4/myeloid differentiation factor 2 (MD-2) complex inducing its dimerization and transmembrane signaling.6,7 TLRs are also activated when damage associated molecular patterns (DAMP) are released from the host necrotic tissue. Endogenous ligands for TLR4 include the prototypic DAMP high mobility group box 1 (HMGB1), heat shock proteins, urate crystals, and defensins.8
Given the pervasiveness of DIC, anticoagulants have been the first choice for sepsis therapy and tens of trials have been conducted but with little success, mainly because of the bleeding risk and interference with bacteria clearance.9
Using the TRL4/MD-2 antagonist eritoran (E5564) in human sepsis to target inflammation did not prolong survival.10 Inhibition of CD14 reduced the inflammatory response and cytokine storm in rabbit,11,12 non-human primate (NHP),13 and porcine14 sepsis models, as well as in CD14 knock-out mice15 and human volunteers administered LPS.16–18 Anti-CD14 antibody increased survival when conjugated with a coagulation factor XIa (FXIa) inhibitor19 or in combination with a complement inhibitor in porcine sepsis.20 A single phase I trial has been performed in human sepsis using anti-CD14,21 but the pattern of inflammatory responses was highly variable among patients and it did not show survival benefit, therefore further investigations are warranted.
Here we hypothesized that antibody-mediated inhibition of CD14 could reduce sepsis-induced coagulopathy and inflammation, as well as improve organ function and survival. For this purpose, we used our well-characterized NHP model of gram-negative coagulopathic sepsis induced by intravenous infusion of LD100 Escherichia coli.22 We found that blocking CD14 had beneficial effects on the pathophysiology and survival in E coli sepsis in baboons.
2 |. MATERIALS AND METHODS
2.1 |. Baboon model of Escherichia coli sepsis
This study was performed in compliance with the Animal Welfare Act, the Guide for the Care and Use of Laboratory Animals,23 and the National Institutes of Health Office of Laboratory Animal Welfare and has received prior approvals from the Institutional Animal Care and Use Committees of both the Oklahoma Medical Research Foundation and the University of Oklahoma Health Sciences Center. Healthy Papio anubis baboons 3 to 4 years of age and 6 to 12 kg body weight, with hemoglobin >10 mg/dL and white blood cell (WBC) counts < 12 × 109/L were randomly distributed between the control and treatment groups: (a) five animals were challenged with E coli (1–2 × 1010 colony forming units [CFU]/kg, LD100 dose, serotype B7–086a:K61; ATCC) through intravenous infusion (IV) over 2 hours; and (b) five animals were treated with anti-CD14 monoclonal antibody (clone 23G4, ATCC, 3 mg/kg) as one IVbolus, 30 minutes before the challenge. The time before and after n hours of E coli infusion is referred to as T − n and T + n hours, respectively. Animals were sedated with pentobarbital administered IV periodically to maintain light anesthesia. The antibiotic gentamicin was given IV (9 mg/kg, 1 hour) after completion of E coli challenge (T + 2), then intramuscular at T + 8 (4.5 mg/kg). Critical care monitoring was done as described.24 Core body temperature, oxygen saturation, mean systemic arterial pressure (MSAP), heart and respiration rates were monitored with a Cardell Max-12 HD Duo monitor (Abaxis Veterinary Diagnostics). Blood samples and physiological parameters were collected while the animal was under anesthesia, and the clinical condition was monitored during the whole duration of the study. After 7 days or when their clinical condition deteriorated, animals were humanely euthanized with euthasol (50 mg/kg, IV).
2.2 |. Bacteria count in the blood
Blood bacteremia expressed as CFU was determined as described.25
2.3 |. Biochemical assays
Blood urea nitrogen (BUN), creatinine, albumin, total protein, and alanine aminotransferase (ALT) were measured using a VetScan VS2 (Abaxis Veterinary Diagnostics) chemistry analyzer.25 Lactate in the blood was measured using Lactate Scout (EKF Diagnostics).
2.4 |. Immunoassays
Plasma cytokines tumor necrosis factor (TNF), interleukin (IL)1β, IL-6, IL-8, interferon (IFN)γ, IL-10, IL-17, IL-1RA, granulocyte-macrophage colony stimulating factor (GM-CSF), and monocyte chemoattractant protein-1 (MCP-1) were measured using the MILLIPLEX MAP Nonhuman Primate Cytokine Magnetic Bead Panel (EMD Millipore) as per the manufacturer’s instructions. Plasma levels of inhibitory complexes of antithrombin (AT) with activated coagulation factors XIa, VIIa, and thrombin (FXIa-AT, FVIIa-AT and thrombin-AT [TAT]) were measured using sandwich enzyme-linked immunosorbent assays (ELISAs) as described.24 Activated protein C (APC) complexes with α1-antitrypsin were determined with a sandwich ELISA using affinity purified antibodies, sheep anti-human protein C (2 μg/mL; Affinity Biologicals) for capture, and biotin-conjugated goat anti-human α1-antitrypsin (1 μg/mL; Affinity Biologicals) for detection. For D-dimer, mouse monoclonal antibody clone DD1 (Novus Biologicals) was used for capture and affinity purified horseradish peroxidase-conjugated polyclonal sheep anti-human fibrinogen (Affinity Biologicals) was used for detection. For plasmin-antiplasmin (PAP) complexes, affinity purified goat anti-plasminogen (2 μg/mL, Affinity Biologicals) was used as capture antibody and horseradish peroxidase-conjugated goat polyclonal anti-antiplasmin antibody was used for detection. Thrombin-activatable fibrinolysis inhibitor (TAFI) was detected with a commercial ELISA kit from Affinity Biologicals. Tissue-type plasminogen activator (t-PA) and plasminogen activator inhibitor 1 (PAI-1) were measured with DuoSet ELISA kits from R&D. C3b and sC5b-9 were measured as described.25 The ELISA kits for HMGB1 and citrullinated histone H3 (Cit H3) were from Thermo Fisher Scientific and Cayman Chemicals, respectively.
2.5 |. Quantitative real-time polymerase chain reaction
Tissue factor (TF) mRNA expression was quantified in leukocytes during the time course of sepsis and in the lung. Leukocytes were isolated by density gradient centrifugation of buffy coats layered on Histopaque 1119/1077 (Sigma-Aldrich), and lysed in Trizol LS (Ambion, Life Technologies). Lung tissue was homogenized in Trizol, and RNA was isolated as per manufacturer’s instruction. DNA digestion and RNA cleanup were performed using RNase free DNase set (Qiagen GmbH) and RNeasy mini kit (Qiagen). cDNA was prepared using Maxima First strand cDNA synthesis kit (Thermo Fisher Scientific). The primers used for qPCR (a) tissue factor- 5′-TGCTTTTACACAGCAGACACAGAGT-3′ (forward), and 5′-AAGACCCGTGCCAAGTACGT-3′ (reverse); (b) Actin- 5′-CACCATTGGCAATGAGCGGTTC-3′ (forward), and 5′-AGGTCTTTGCGGATGTCCACGT-3′ (reverse), were from Prime-Time Integrated DNA Technologies. Quantitative polymerase chain reactions (qPCR) reactions using SYBR Green master mix (Qiagen) were run in CFX96 Real Time System (Bio-Rad Laboratories). TF expression was normalized to housekeeping gene actin.
2.6 |. LPS quantification in plasma
Endotoxin level in plasma was quantified as described.26 Plasma samples diluted in pyrogen-free water were heated at 70°C for 10 minutes to inactivate enzymes that may interfere with the assay. Pyrochrome reagent (Associates of Cape Cod [ACC]) was reconstituted with 3.2 mL Glucashield buffer (ACC). 50 μL diluted plasma samples were mixed with 50 μL Pyrochrome reagent and plate was read at 405 nm in the kinetic mode. Standard endotoxin (ACC) was used as control.
2.7 |. Myeloperoxidase activity assay
Plasma level of myeloperoxidase (MPO) was determined using Fluoro MPO detection kit (Cell Technology).25
2.8 |. Clotting time tests
Activated partial thromboplastin time (aPTT) and prothrombin time (PT) were measured as described.27 Functional fibrinogen was determined using a clotting-based assay.28
2.9 |. Microscopy
Tissues fixed in formalin and embedded in paraffin were used for hematoxylin-eosin and phosphotungstic acid hematoxylin staining to reveal tissue morphology and fibrin deposits.25
Double immunofluorescence staining of PAI-1 on lung cryo-sections was done as previously described.29 We used mouse monoclonal anti PAI-1 (clone 7F5, gift from Paul DeClerck, University of Leuven, Belgium), and rabbit anti von Willebrand factor (DakoCytomation).
2.10 |. Statistical analysis
Statistical analysis was performed using Prism (GraphPad Software 7.0b). Values represent mean ± standard error of the mean. Comparisons between two groups along the time course were performed using a multiple t-tests with Holm-Sidak correction for multiple comparisons. Area under the curve (AUC) was calculated for the treated and non-treated groups for the time interval 0−-24 hours, and for survivor versus non-survivor subgroups in the anti-CD14 treated arm for the 24−-72 hours interval. AUC multiple comparisons were made by one-way analysis of variance followed by Bonferroni test. Results were considered significant at P < .05 (*P < .05, **P < .01, ***P < .001). Comparison of survival data was done using log-rank Mantel–Cox test.
2.11 |. Results presentation
Data presentation follows the first 24 hours for identically challenged untreated animals (control; red symbols and lines), 72 hours for the baboons treated with anti-CD14 antibody who survived at least 3 days but died before 7-day survival end-point (non-survivors, black symbols and lines), and 7 days for the treated animals who reached the end-point of the study (survivors, green symbols and lines). The 24 hours values for the untreated animals comprise the only two animals that survived past 24 hours. Similarly, the 72-hours time-point has non-survivor data for the two out of three baboons treated with anti-CD14 who passed by this time. For the parameters with no significant or trend differences between the survivors and non-survivors, mean values were calculated and presented as pooled anti-CD14 treated group (blue symbols and lines).
3 |. RESULTS
3.1 |. Effect of anti-CD14 antibody on survival after Escherichia coli infusion
Survival was significantly increased in the baboons pretreated with 3 mg/kg anti-CD14 antibody before the IV infusion of a lethal dose of E coli compared to the untreated (Mantel-Cox test P = .0018; Figure 1). The median time to death reached 74 hours in three treated animals compared to 22.5 hours in controls, and two of the treated animals survived and recovered (7 days observation).
FIGURE 1.
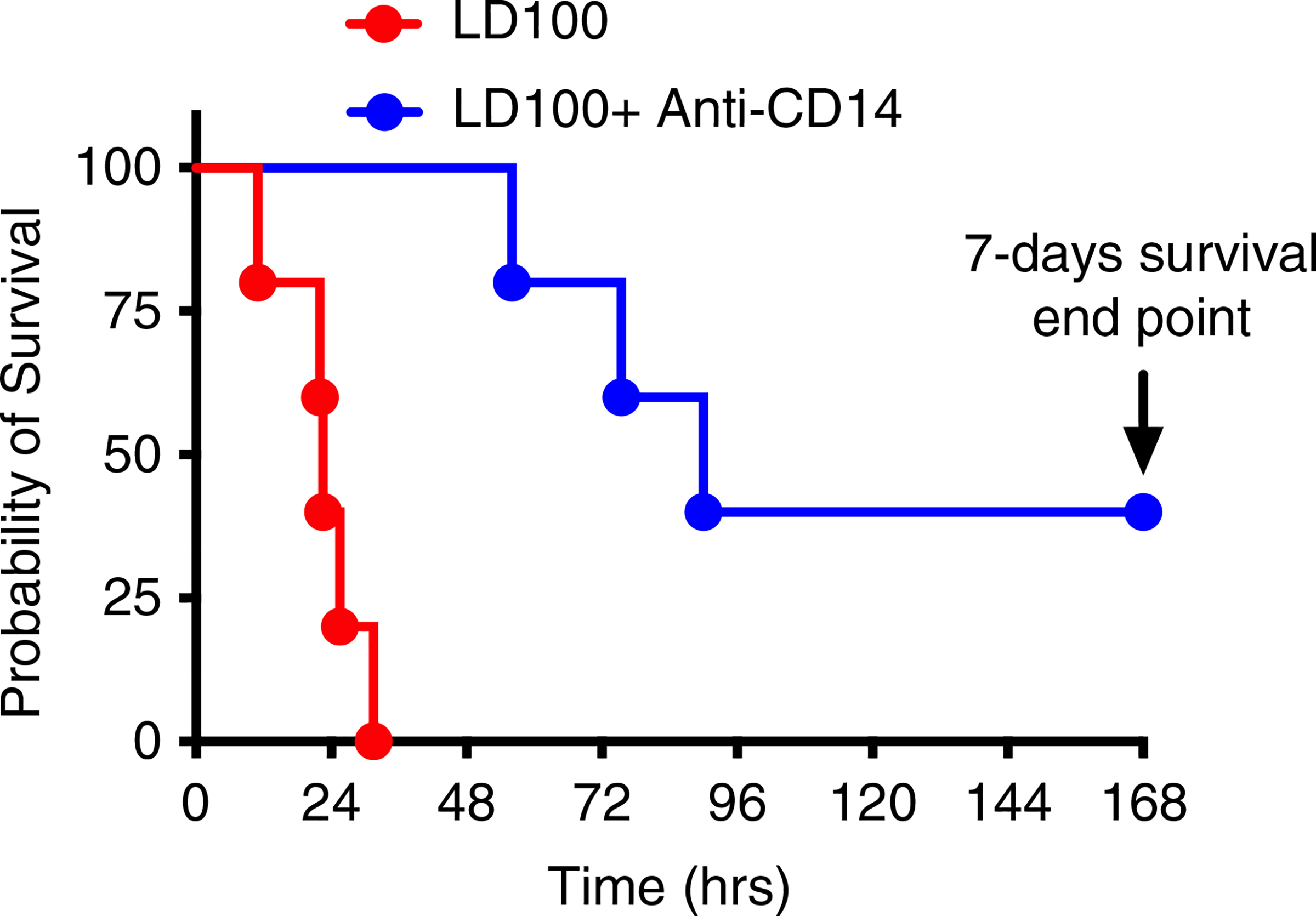
Pretreatment with anti-CD14 antibody (23G4) prolongs survival in lethally Escherichia coli-challenged baboons. Kaplan-Meier survival plots of non-treated control and anti-CD14-treated baboons after LD100 E coli challenge (n = 5 for both groups). Treated animals that survived for 168 hours (7 days) were considered permanent survivors. Survival distribution of the two groups was determined using a log-rank (Mantel-Cox) test and was significantly higher in the anti-CD14 treated group; P = .0018
Animals that died developed multiple organ failure. The organ damage detected at necropsy was consistent with the time of survival. For non-treated controls the lungs, adrenals, spleen, and intestine were the most severely damaged organs. Typically, the lungs show microvascular thrombosis and acute respiratory distress syndrome (ARDS) features (inflammation, diffuse alveolar damage, proteinaceous fluid, blood cells and fibrin accumulation in the alveolae). Anti-CD14 treated animals that succumbed showed prominent kidney damage, including renal microthrombosis and ischemic necrosis.
Next, we addressed possible mechanisms through which anti-CD14 provided partial survival benefit by analyzing effects on markers of coagulation, fibrinolysis, inflammation, and complement activation, and assessment of vital signs and organ function.
3.2 |. Effect of anti-CD14 on the coagulopathic response to Escherichia coli challenge
Infusion of E coli produced rapid increases in aPTT and PT (Figure 2A,B), suggesting induction of a consumptive coagulopathy. There were significant differences in the response to anti-CD14 pretreatment. Mean aPTT values in the treated group were ~50% lower than in the controls (AUC; P < .001), with significant difference between survivors and non-survivors (40% reduction up to 72 hours by AUC; P < .01; Figure 2A). PT values were also reduced by 30% in the treated group (AUC; P < .01; Figure 2B) but with no significant difference between survivors and non-survivors. These findings suggest decreased consumption of clotting factors in animals treated with anti-CD14.
FIGURE 2.
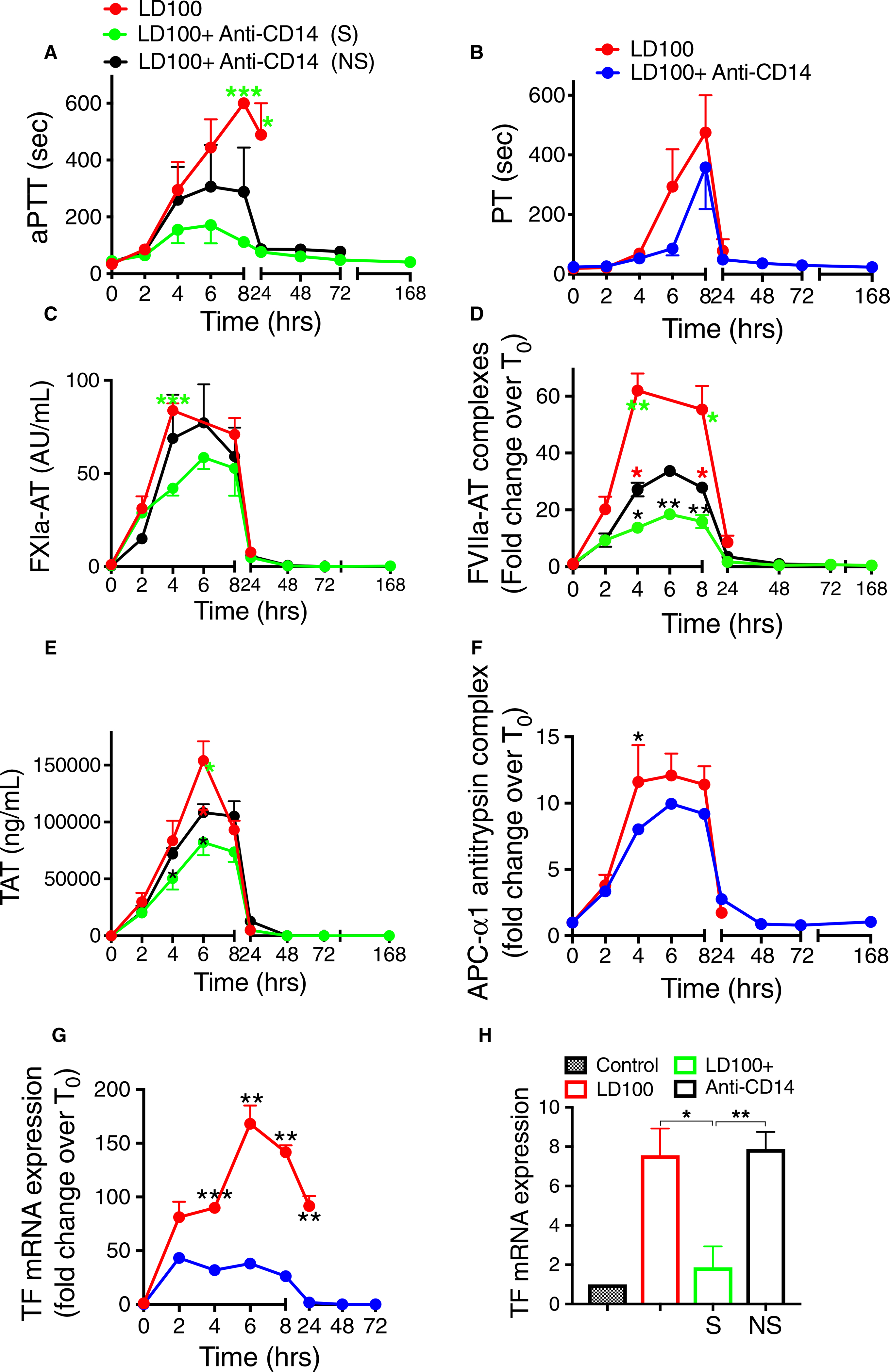
Pretreatment of baboons with anti-CD14 antibody reduced the activation of coagulation following lethal dose of Escherichia coli. Time course dynamics of clotting times activated partial thromboplastin time (aPTT; A) and prothrombin time (PT; B); plasma hemostatic biomarkers activated coagulation factor XIa-antithrombin (FXIa-AT; C), FVIIa-AT (D), TAT (E), and activated protein C (APC)-1-antitrypsin (F) complexes; and TF mRNA in leukocytes (G) and lung (H). AT, antithrombin; TAT, thrombin-antithrombin. Data are presented as mean ± standard error of the mean. Same time points are compared along the time course between LD100 (red) and LD100 + anti-CD14 (pooled values, blue) or survivors (S, green) and non-survivors (NS, black) within the group treated with anti-CD14 using multiple t-tests with Holm-Sidak correction for multiple comparisons. *P < .05, **P < .01, ***P < .001. For panels A, C, D and E, black asterisks mark significant differences between (S) and (NS), green asterisks, between LD100 and (S), and red asterisks, between LD100 and (NS). Panel (H): control bar represents normal (unchallenged) baboon lung. Groups were compared using two-tailed t-tests
Coagulation proteases were activated within 2 to 8 hours after bacterial infusion, as reflected by increased plasma levels of protease-serpin complexes: FXIa-AT, FVIIa-AT, and TAT (Figure 2C–E). Anti-CD14 reduced activation of coagulation, to a lesser extent for the intrinsic pathway (FXIa-AT, AUC reduced by 25%; not significant) but highly significant for the extrinsic pathway (FVIIa-AT, 60% reduction by AUC; P = .0035). Survivors in the anti-CD14 treated group showed significantly lower FXI activation than the controls (AUC; P = .025; Figure 2C), as well as 45% reduction of FVIIa generation versus the non-survivors (AUC; P = .002) and three-fold decrease as compared with the non-treated (AUC; P < .0001; Figure 2D). TAT was 30% lower in the survivors (AUC; P < .05; Figure 2E), indicating inhibition of both the activation (mainly through the extrinsic pathway) and amplification phases of coagulation. Anti-CD14 treatment reduced the levels of complexes of APC with its inhibitor α1-antitrypsin by 15% compared to the control baboons (AUC; P = .04; Figure 2F), which correlates with the decreased thrombin generation (TAT, Figure 2E) and better preservation of endothelial cells’ integrity. Inhibition of the coagulation through the extrinsic pathway (FVIIa) correlates well with the five-fold reduction by the anti-CD14 antibody of E coli-induced TF mRNA expression in circulating blood cells (AUC; P < .0001; Figure 2G). Previously we have shown that TF is strongly induced in monocyte/macrophages of the lung after E coli challenge.29 TF mRNA expression in the lung was induced eight-fold in untreated animals, but significantly reduced in the anti-CD14 treated survivors (Figure 2H).
Consistent with the activation of coagulation, E coli-challenged baboons displayed fibrinogen consumption up to 8 hours regardless of treatment (Figure 3A). After 24 hours, the treated survivors recovered significantly faster than the non-surviving animals (AUC; P < .0001; Figure 3A). The increased clotting times and decreased platelet counts (Figure 4D) suggest development of a consumptive coagulopathy. Plasma markers of fibrinolysis t-PA, PAI-1, plasmin (measured as PAP) and D-dimer, as well as TAFI increased in response to E coli challenge (Figure 3B–F). Double immunostaining showed strong induction of PA-1 in von Willebrand factor-positive endothelial cells of the lung from septic baboons (Figure S1 in supporting information). Overall, CD14 inhibition had a profound effect on fibrinolysis. Although similar between the anti-CD14–treated group and the untreated for the first 8 hours, tPA levels remained high in the treated animals for the next 72 hours (Figure 3B). In parallel, plasma PAI-1 strongly decreased by ~90% (AUC; P = .0001; Figure 3C) leading to a net profibrinolytic response in treated animals. Plasmin generation and fibrin degradation were significantly enhanced in animals treated with anti-CD14, as shown by early and sustained three-fold increase of PAP complexes (AUC; P = .0015; Figure 3D) and ~2-fold more D-dimer (AUC; P = .0038; Figure 3E) in the treated group than in controls. Consistent with the lower thrombin generation observed in the treated survivors, TAFI was also significantly lower in survivors versus non-survivors, treated or not (AUC; P = .006; Figure 3F). Collectively, these data support a pro-fibrinolytic effect of the anti-CD14 antibody treatment.
FIGURE 3.
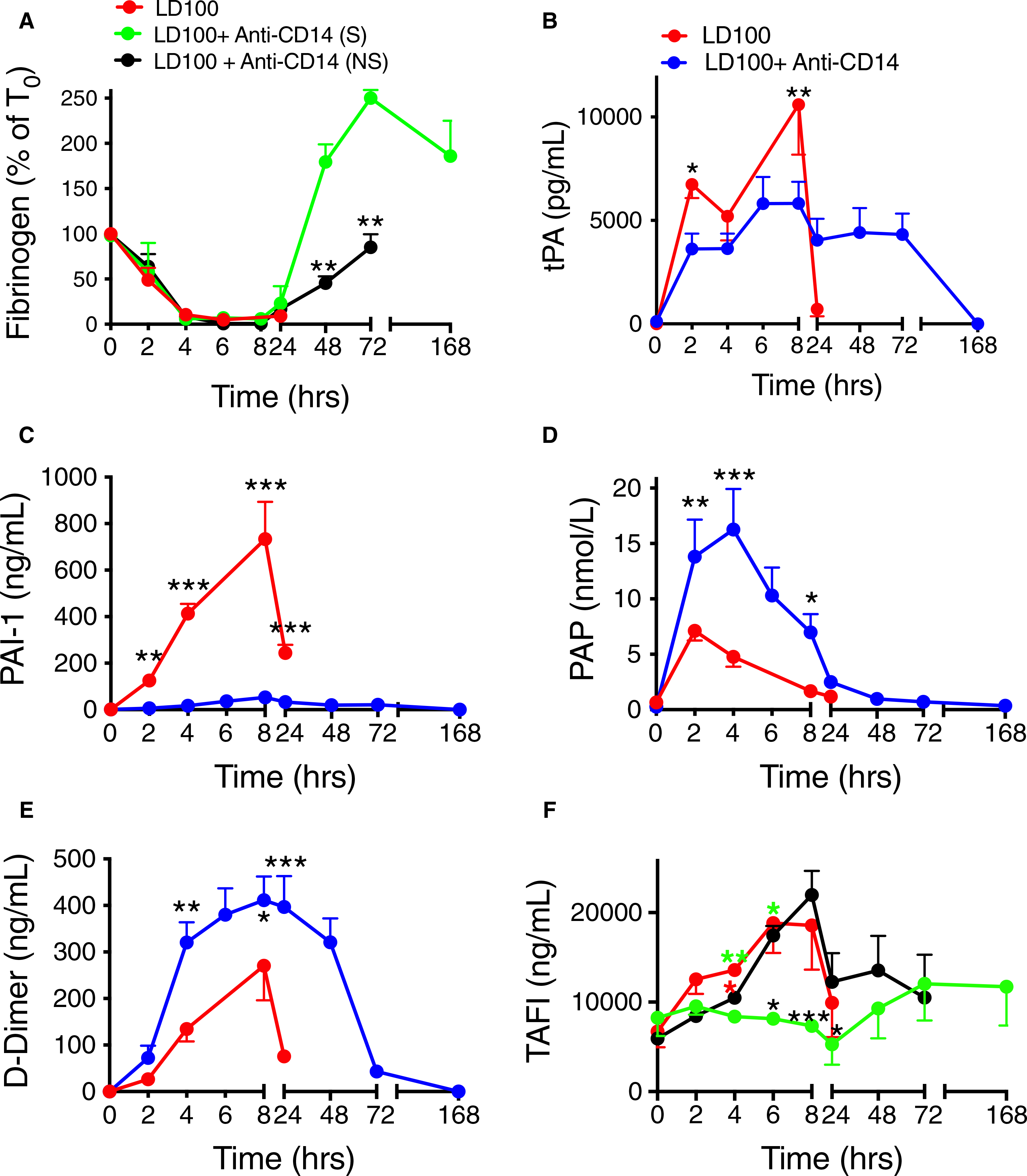
Effect of treatment with anti-CD14 antibody on markers of fibrinolysis in lethal Escherichia coli challenged baboons. A, Time course changes of fibrinogen levels. B--F, Time course changes of plasma biomarkers of fibrinolysis: (B) tissue-type plasminogen activator (tPA), (C) plasminogen activator inhibitor-1 (PAI-1), (D) plasmin-antiplasmin complex (PAP), (E) D-dimer, and (F) thrombin activatable fibrinolysis inhibitor (TAFI), were evaluated following E coli challenge with or without anti-CD14 treatment. Data are presented as mean ± standard error of the mean. Same time points are compared between LD100 (red) and LD100 + anti-CD14 (pooled values, blue) or survivors (S, green) and non-survivors (NS, black) within the group treated with anti-CD14 using multiple t-tests with Holm-Sidak correction for multiple comparisons. *P < .05, **P < .01, ***P < .001. For (A) and (F), black asterisks mark significant differences between (S) and (NS), green asterisks, between LD100 and (S), and red asterisks, between LD100 and (NS)
3.3 |. Effect of anti-CD14 antibody on the blood cells and microenvironment response to Escherichia coli challenge
Fluid loss due to capillary leakage, detected in the control group by decreased total plasma protein and albumin (Figure 4A,B) was ~25% less pronounced in the treated animals during the first 8 hours (AUC; P < .05; Figure 4B) and the total protein completely recovered in the treated survivors (Figure 4A).
FIGURE 4.
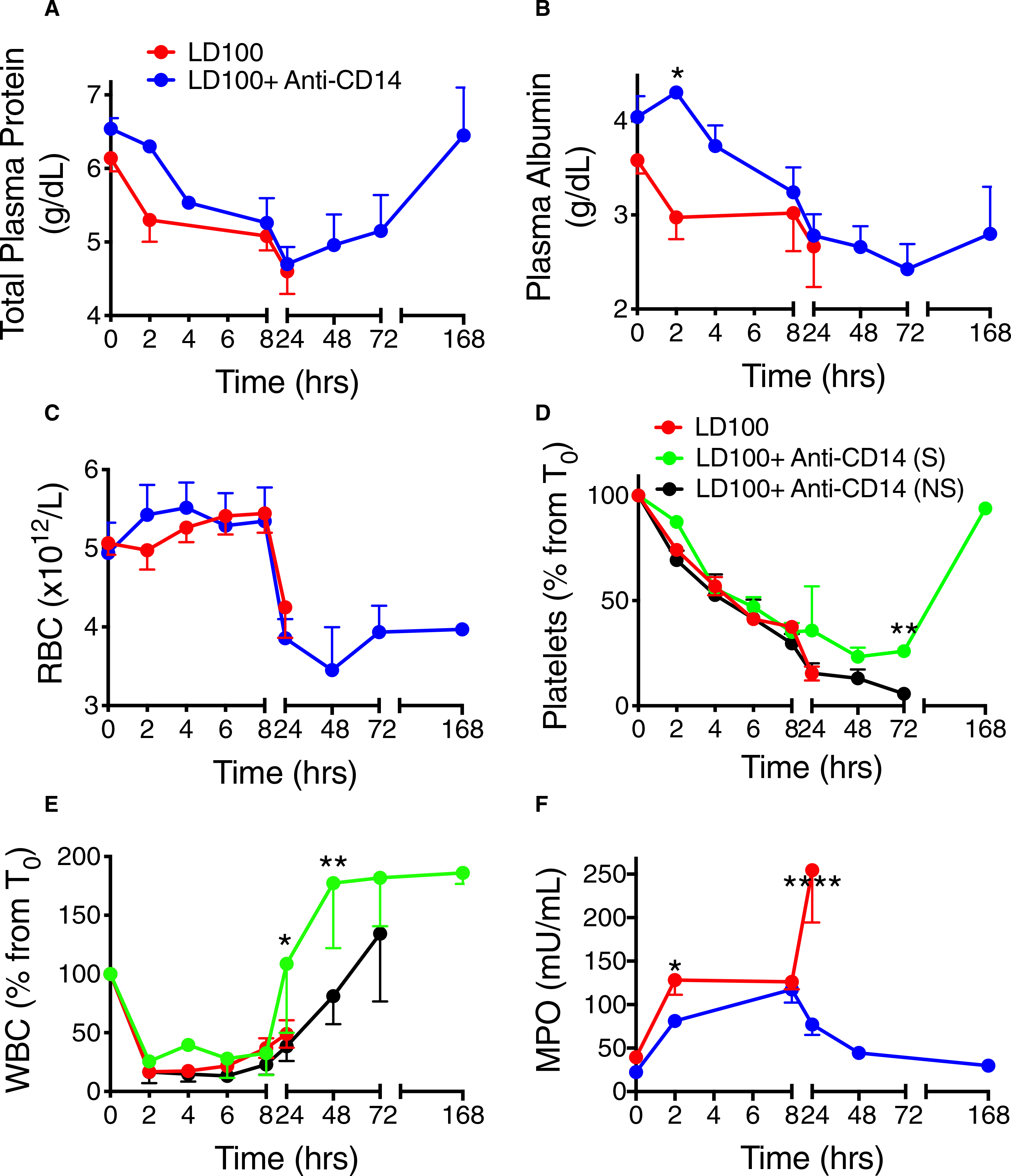
Effect of treatment of baboons with anti-CD14 antibody on capillary leak and blood cells after lethal Escherichia coli challenge. Time course changes of (A) total plasma protein and (B) plasma albumin, and of (C) red blood cells (RBC), (D) platelets, (E) white blood cells (WBC), and (F) myeloperoxidase (MPO) activity in baboons with or without anti-CD14 treatment following E coli challenge. Data are presented as mean ± standard error of the mean. Same time points are compared between LD100 (red) and LD100 + anti-CD14 (pooled values, blue) or survivors (S, green) and non-survivors (NS, black) within the group treated with anti-CD14, using multiple t-tests with Holm-Sidak correction for multiple comparisons. *P < .05, **P < .01, ****P < .0001. For (D) and (E), black asterisks mark significant differences between (S) and (NS), green asterisks, between LD100 and (S), and red asterisks, between LD100 and (NS)
Red blood cell (RBC) counts showed no differences between the groups (Figure 4C). Hematocrit and hemoglobin behaved identical to RBC. Infusion of E coli induced a drop in circulating platelets, which started recovering after 24 hours in the treated survivors but not in the non-survivors (AUC; P < .05; Figure 4D). No bleeding such as petechial or gastrointestinal hemorrhaging was observed. WBC counts fell abruptly and similarly in both groups up to 8 hours and recovered fully in the treated survivors by 24 hours, faster than the non-survivors (45% lower by AUC up to 72 hours; P = .005; Figure 4E). The ability of E coli to induce neutrophil degranulation measured as plasma levels of MPO was ~35% reduced by the anti-CD14 treatment during the first 24 hours (AUC; P < .05; Figure 4F) and any activation of neutrophils disappeared in the survivors.
3.4 |. Effect of anti-CD14 antibody on vital signs and organ failure in response to Escherichia coli
Escherichia coli infusion in baboons induced organ dysfunction and multiple organ failure. The animals displayed hyperdynamic shock characterized by rapid heart rate and drop in blood pressure, likely from low vascular resistance (Figure 5A,B). The animals suffered acute respiratory distress, indicated by increased respiration rate (Figure 5C), a drop in blood oxygen saturation, and increased body temperature (Figure 5D–E). Baboons pretreated with anti-CD14 antibody fared significantly better. Heart rate was ~10% higher and stable in the treated group compared to controls (AUC; P < .0001; Figure 5A). MSAP was partly preserved and more stable through the first 8 hours in the treated group compared to the untreated, which showed a marked fall in the early phase (AUC; P = .0067; Figure 5B). Treated baboons exhibited fewer respiratory problems with more stable respiration rates, better peripheral perfusion and oxygenation as shown by pulse oximetry (AUC; P = .001; Figure 5D), and significantly lower body core temperature after 5 hours (AUC; P = .0008; Figure 5E).
FIGURE 5.
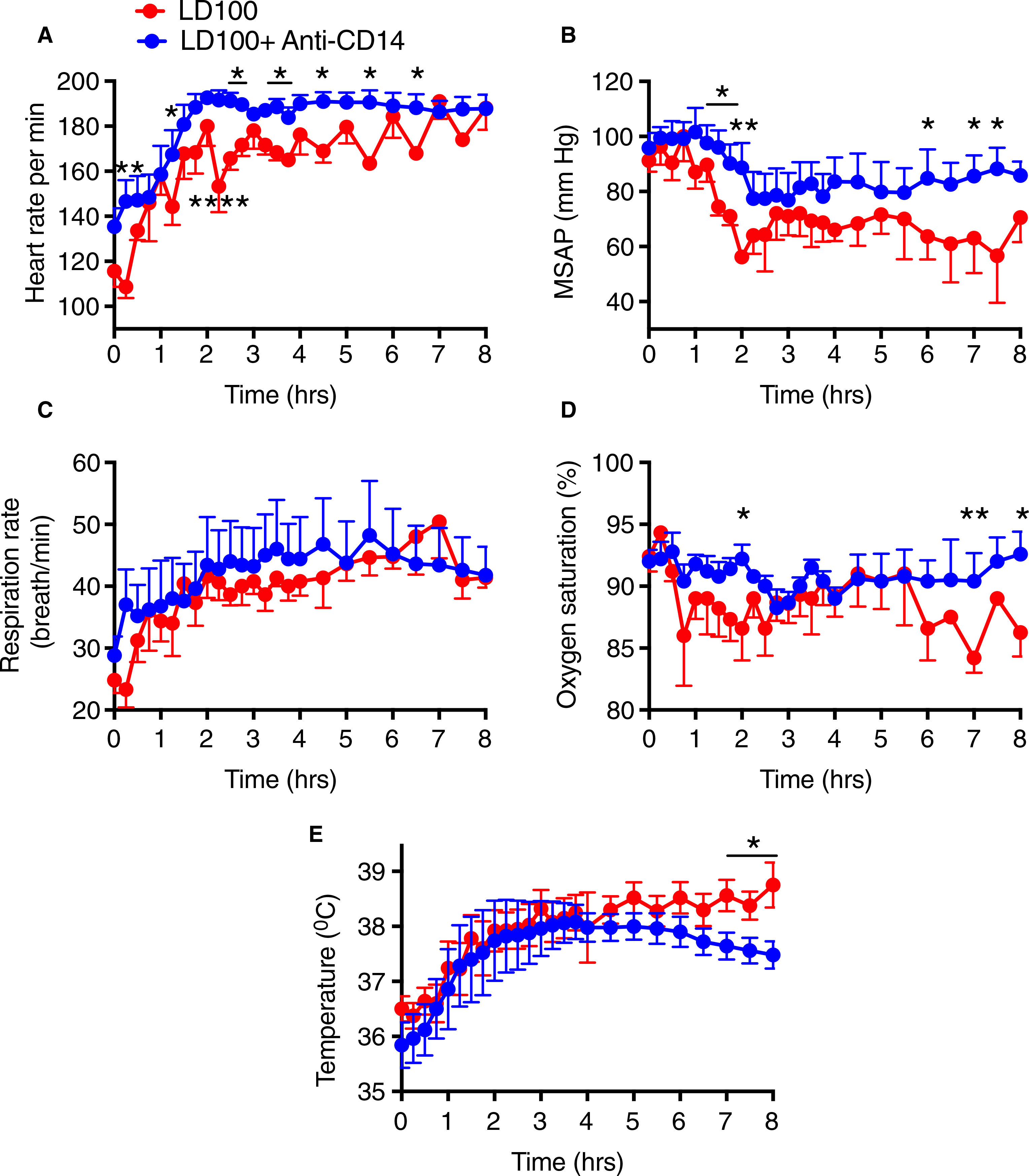
Anti-CD14 antibody treatment improved vital signs following a lethal dose of Escherichia coli. A, Heart rate, (B) mean systemic arterial pressure (MSAP), (C) respiration, (D) oxygen saturation, and (E) core temperature. Data are presented as mean ± standard error of the mean. Same time points are compared between LD100 (red) and LD100 + anti-CD14 using multiple t-tests with Holm-Sidak correction for multiple comparisons. *P < .05, **P < .01, ****P < .0001
Challenge with E coli induced cardiopulmonary dysfunction with hypoperfusion and organ injury as shown by increased lactate (hypoxia marker; Figure 6A), hypoglycemia (Figure 6B), increased creatinine and BUN levels (renal function markers; Figure 6C,D), and high levels of ALT (liver damage marker; Figure 6E). The anti-CD14 treatment prevented organ dysfunction. Lactate was 50% reduced in the treated animals compared to controls, in particular after 8 to 24 hours (AUC; P = .0001; Figure 6A). Anti-CD14 treated animals maintained better glycemic control and the difference between survivors and non-survivors was significant for the 24- to 72-hour interval (AUC; P = .003; Figure 6B). Creatinine was ~2.5-fold lower in the treated group than the untreated during the first 8 hours (AUC; P = .01), and afterward survivors displayed ~2-fold lower values than the non-survivors (AUC; P < .01; Figure 6C). BUN levels were significantly lower in the anti-CD14 treated survivors than in the non-surviving animals (24−-72 hours AUC; P < .0001; Figure 6D). ALT was significantly reduced in the treated survivors as compared to both the untreated animals (AUC; P < .001) and the treated non-survivors (24−-72 hours AUC; P < .0001; Figure 6E).
FIGURE 6.
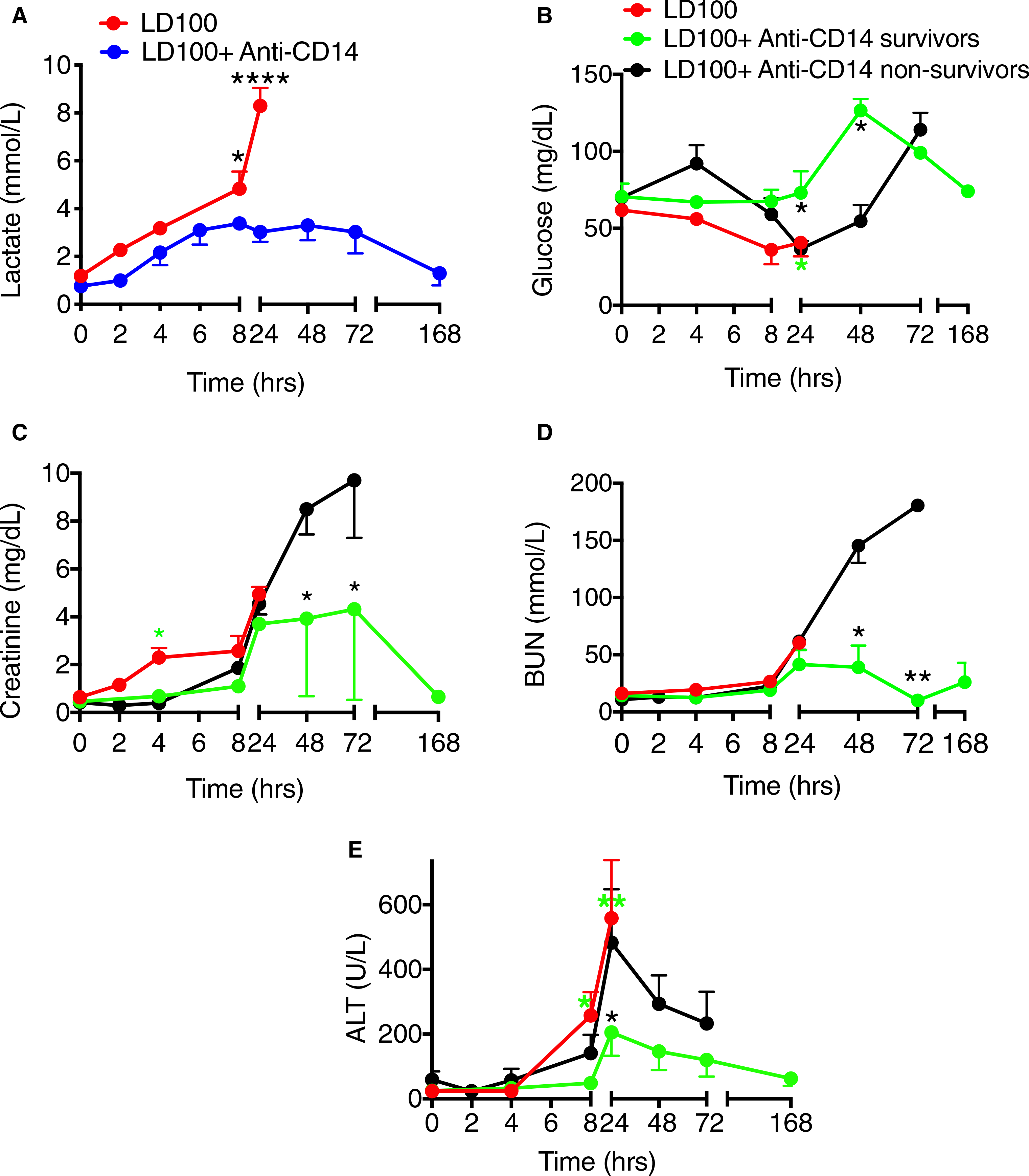
Effect of treatment of baboons with anti-CD14 antibody on organ function parameters in lethal Escherichia coli sepsis. Time course evaluation of plasma biomarkers of: cardiopulmonary hypoperfusion (A) lactate (hypoxia marker); liver and pancreas function (B) glucose; kidney function (C) creatinine and (D) blood urea nitrogen (BUN); liver injury (E) alanine aminotransferase (ALT), during E coli sepsis with or without anti-CD14 treatment. Data are presented as mean ± standard error of the mean. Same time points are compared between LD100 (red) and LD100 + anti-CD14 (pooled values, blue) or survivors (S, green) and non-survivors (NS, black) within the group treated with anti-CD14, using multiple t-tests with Holm-Sidak correction for multiple comparisons. *P < .05, **P < .01, ****P < .0001. For (B), (C), (D), and (E), black asterisks mark significant differences between (S) and (NS); green asterisks, between LD100 and (S);and red asterisks, between LD100 and (NS)
Markers of cell death included Cit H3, which is released from neutrophil extracellular traps, and HMGB1, a non-histone chromatin-binding protein released either passively from necrotic cells, or actively from immune and/or stressed cells. Both increased within 24 hours after E coli challenge (Figure 7A,B). Anti-CD14 reduced their levels suggesting that inhibition of CD14 partially protected against E coli-induced cytolysis. Cit H3 increased identically in the control and treated non-survivors until 24 hours, but not in the survivors (control versus treated survivors AUC; P = .007), who continued to display two times less Cit H3 than the non-survivors (AUC; P < .001; Figure 7A). HMGB1 release was significantly different (AUC; P < .0001) both between control animals and treated survivors, and between survivors and non-survivors in the treated group: survivors showed no increase whereas non-survivors behaved similarly to the non-treated group (Figure 7B).
FIGURE 7.
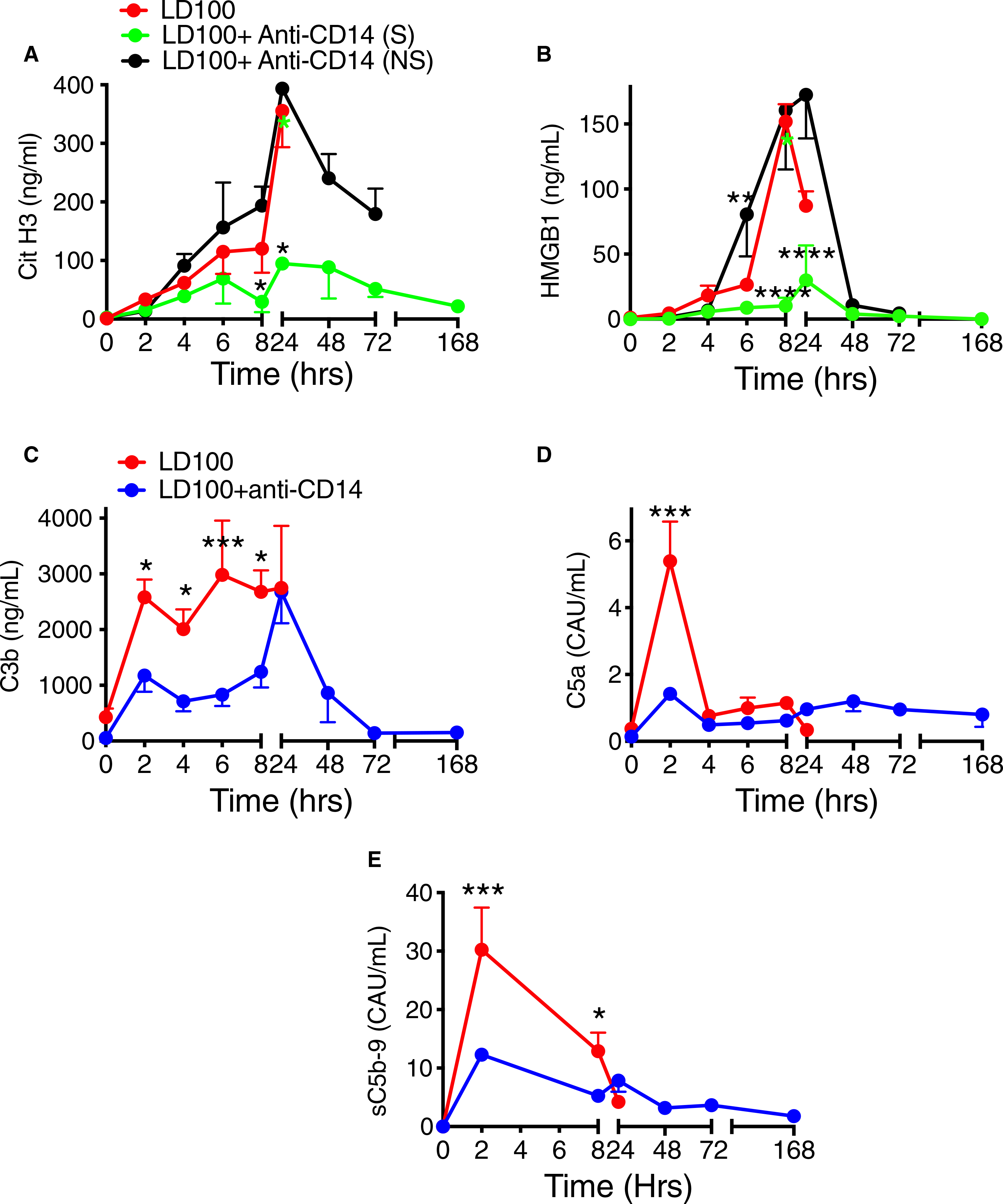
Pretreatment of lethal Escherichia coli-challenged baboons with anti-CD14 antibody reduced tissue damage and complement activation. Time course changes of plasma levels of cell death markers (A) citrullinated (Cit) H3 and (B) high mobility group box1 (HMGB1), and of complement activation markers C3b (C), C5a (D), and soluble terminal complement complex sC5b-9 (E) were evaluated during E coli sepsis. Data are presented as mean ± standard error of the mean. Same time points are compared between LD100 (red) and LD100 + anti-CD14 (pooled values, blue) or survivors (S, green) and non-survivors (NS, black) within the group treated with anti-CD14 using multiple t-tests with Holm-Sidak correction for multiple comparisons. *P < .05, **P < .01, ***P < .001. ****P < .0001. For (A) and (C), black asterisks mark significant differences between (S) and (NS); green asterisks, between LD100 and (S); and red asterisks, between LD100 and (NS)
CD14 inhibition blunted the complement activation induced by E coli in the control group. Plasma level of C3 activation product C3b was reduced by 40% (AUC; P = .007; Figure 7C), C5 activation product C5a by 40% (AUC; P = .03; Figure 7D), and the soluble terminal complex sC5b-9 by 45% (AUC; P = .017; Figure 7E), regardless of the surviving outcome.
Markers of cytokine storm associated with sepsis were observed after E coli challenge, which increased plasma levels of both the pro-inflammatory TNF, IL-6, IL-1β, IL-8, MCP-1, GM-CSF, IL-17a and IFNγ, and anti-inflammatory IL-10 and IL-1RA (Figure 8). In vitro titration of anti-inflammatory effects of the antibody demonstrated dose-dependent inhibition of TNF, IL-6, and in a lower extent of IL-8 (Figure S2 in supporting information). Likewise, anti-CD14 antibody reduced TNF levels ~ 10-fold in survivor baboons (AUC; P = .01) and two times in non-survivors compared to controls. The treated survivors showed four times less TNF than the non-survivors (AUC; P = .023; Figure 8A). A similar pattern of response was observed for IL-6, IL-1β, IL-8, MCP-1, GM-CSF, IL-17a, and IFNγ (Figure 8B–H). Differences in AUC of treated survivors versus controls, respectively, versus the non-survivors, were as follows: IL-6:3-fold (P = .014) and 7-fold lower (P = .006; Figure 8B); IL-1β: ~5-fold for both comparisons (P = .003, respectively P = .017; Figure 8C); IL-8: ~40% reduction (at the limit of significance) and ~ 2-fold lower (P = .009; Figure 8D); MCP-1:30% reduction (ns) and ~ 2-fold lower (P = .01; Figure 8E); GM-CSF: reduced by ~2-fold for both comparisons (P < .05 for both; Figure 8F); IL-17a: ~2-times lower for both comparisons (P < .0001, respectively P < .001; Figure 8G); IFNγ: 4-fold (P = .03) and 2.5-fold lower (P = .025; Figure 8H). For IL-1RA, the lower trending observed for treated survivors was not significant (Figure 8I). The levels of the anti-inflammatory IL-10 were significantly higher (~2-fold) in the anti-CD14 treated animals regardless of survival (AUC for 0−-6 hours; P < .05; Figure 8J).
FIGURE 8.
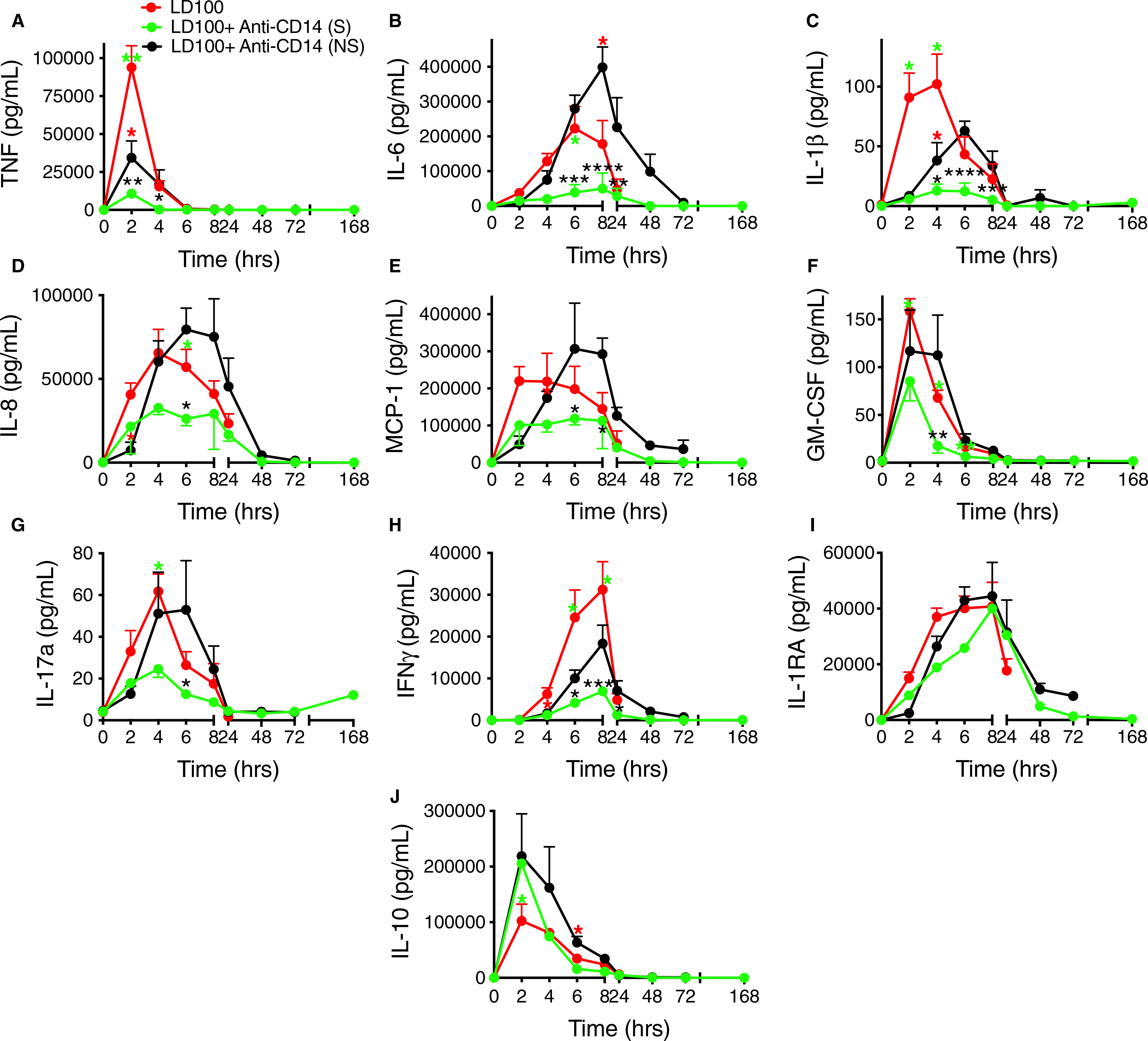
Effect of treatment of baboons with anti-CD14 antibody on circulating pro- and anti-inflammatory cytokines in the LD100 model of Escherichia coli sepsis. Time course evaluation of cytokines in plasma (A) tumor necrosis factor (TNF), (B) interleukin (IL)-6, (C) IL-1, (D) IL-8, (E) monocyte chemoattractant protein-1 (MCP-1), (F) granulocyte-macrophage colony stimulating factor (GM-CSF), (G) IL-17a, (H) interferon (IFN)γ, (I) IL-1RA, and (J) IL-10 during E coli sepsis with or without anti-CD14 treatment. Data are presented as mean ± standard error of the mean. Same time points are compared between LD100 (red) and LD100 + anti-CD14 survivors (S, green) and non-survivors (NS, black) using multiple t-tests with Holm-Sidak correction for multiple comparisons. *P < .05, **P < .01, ***P < .001, ****P < .0001. Black asterisks mark significant differences between (S) and (NS); green asterisks, between LD100 and (S); and red asterisks, between LD100 and (NS)
Histopathological evaluation of the organs postmortem could not be compared temporally due to the differences to the time of death but gave important information, nonetheless. The pathology severity scores of the kidney, spleen, lung, and liver (Figure S3A–D in supporting information) showed that some parameters did improve in the treated group, such as: kidney tubular necrosis, follicular necrosis and medullar congestion in the spleen, capillary leakage in the lung, and liver congestion. A marked and significant protection by the treatment was observed for the adrenals (Figures S3 and S4 in supporting information). Treated animals did not display microthrombosis, cortical necrosis, and cortical hemorrhage, and leukocyte infiltration and cortical congestion were ~50% reduced (29.4 ± 1.4 versus 17.3 ± 1.2 P = .006). Representative images of adrenal histopathology indicating protective effects of the anti-CD14 antibody are shown in Figure S4.
3.5 |. Effect of anti-CD14 treatment on bacteria clearance and LPS levels in response to Escherichia coli
Because CD14 is involved in the phagocytosis of bacteria by various cell types, blocking CD14 may have undesired inhibitory effects on bacteria phagocytosis by immunocompetent cells. In our experimental conditions, E coli counts in the blood fell quickly and similarly in both groups during the first 4 hours, after which the decrease in CFU was almost 10 times more pronounced in the treated group than in the untreated (AUC; P = .014; Figure S5A in supporting information). The abrupt increase in LPS levels by 2 hours was similar in non-treated versus treated non-survivors (Figure S5B). Notably, treated survivors had significantly lower LPS (AUC; P = .008) than the non-survivors, displaying patterns similar to those observed for TNF and IL-6 (Figure S5A,B).
4 |. DISCUSSION
The present data show, to the best of our knowledge, for the first time the effects of inhibiting CD14 in a non-human primate model of sepsis using lethal E coli challenge. The data support the idea that CD14 is a multifunctional protein cross-talking with multiple branches of hemostasis and innate immunity and place it as a key molecule in thrombo-inflammation. Inhibition of CD14 improved cardiopulmonary, renal, liver, and adrenal function; significantly prolonged survival; and reduced mortality. The mechanisms behind these effects involve inhibition of TF-FVIIa-dependent coagulation and strong activation of fibrinolysis, with robust inhibition of the complement system and reduction of capillary leakage. Efficient inhibition of key cytokines like the LPS-induced TNF and downstream IL-6 and IL-1β was observed, which could underlie the partial organ protection as illustrated by lower levels of circulating Cit H3 and HMGB1. Surprisingly, the effect on bacteria clearance was enhanced rather than inhibited.
There are more than 200 putative mediators of sepsis and there have been more than 70 well-designed clinical trials to test the effect of manipulating some of these mediators. The results have been largely disappointing, reflecting the idea that targeting single downstream regulators is not sufficient to resolve such a complex pathology.30,31 Anticoagulation is not currently recommended due to high risk of bleeding and potential impairment of bacteria clearance. Clinical trials targeting inflammation, such as using inhibitors of TNF, IL-6, and IL-1β have all failed. The TLR4/MD-2 inhibitor eritoran (E5564) lacked effect on survival.10 Thus, novel approaches are urgently required.32
CD14 acts as a key orchestrator of the innate immune system and can bind multiple PAMPs and DAMPs including LPS, peptidoglycan, polyI:C, and DNA. Once bound, CD14 chaperones them to the correct TLR out of the 10 different human TLRs identified so far,33 which triggers signaling and downstream inflammatory responses. This places CD14 as a bottleneck integrator molecule for innate immunity recognition and a promising target for attenuating TLR-induced inflammation.
Overall, anti-CD14 treatment had beneficial effects on hemostasis, complement activation, inflammation, and cardiopulmonary physiology and, most important, prolonged survival in our baboon model of lethal E coli challenge.
The effect of CD14 inhibition on coagulation involved decreased activation of the extrinsic pathway, where both TF mRNA, presumably induced by LPS via CD14-TLR4 signaling, and FVIIa-AT complexes in plasma were decreased in treated animals. The survivors displayed significantly blunted activation of coagulation biomarkers, including FXIa (intrinsic pathway) and TAT (thrombin generation) as compared with the non-survivors. Overall, the coagulation was not reduced as measured by fibrinogen consumption, probably due to ongoing activation of the intrinsic system, which was not efficiently counteracted by the treatment except for the two surviving animals. In contrast, fibrinolysis was consistently enhanced, as shown by PAP and D-dimer levels reflecting increase of plasmin generation and fibrin degradation. Typically, E coli sepsis in our model leads to rapid t-PA release and plasmin generation that peaks at 2 hours, while PAI-1 steeply increases after 4 to 8 hours thus dampening fibrinolysis. The hyper-fibrinolytic phenotype of the treated group was likely driven by the strong decrease of PAI-1 and TAFI. Both TF and PAI-1 are regulated by TNF,34 which was also significantly decreased by treatment. Therefore, lower PAI-1 likely reflects decreased synthesis, not increased consumption by APC-mediated inactivation,35 as APC generation was not higher in the treated group. TAFI protects fibrin clots against lysis, so low TAFI in the anti-CD14 treated survivors correlates with enhanced clot lysis. Importantly, despite increased fibrinolysis and/or thrombocytopenia, no bleeding was observed in treated animals. The change of the hemostatic balance toward fibrinolysis may protect against thrombosis and thus partly prevent overt DIC.
One of the most interesting findings was the effect of anti-CD14 antibody on complement activation, whereby formation of C5a anaphylatoxin was completely abolished. C5a is a highly pro-inflammatory mediator that contributes to septic shock. Inhibition of C5a increases survival in mouse studies36 and reduces capillary leakage.37,38 We previously discovered that blocking C5 cleavage had dramatic effects in our lethal E coli baboon model where it increased survival to 100%, most likely through blocking both C5a and sC5b-9 generation.25 The mechanism through which anti-CD14 blocked complement activation is uncertain and difficult to explain through direct effects. More probably, this reflects the organ-protective effects of the treatment by lowering the amounts of DAMPs and acute phase reactants known to activate both complement and coagulation. We reported previously that combined inhibition of CD14 and complement C5 have not only additive, but also synergistic, effects,39 inhibiting inflammation and increasing survival in mouse40 and pig20 sepsis models. While these data highlight the cross-talk between the complement and TLR system,41 the detailed underlying mechanism remains to be elucidated.
The anti-CD14 treatment significantly reduced pro-inflammatory cytokines and chemokines (TNF, IL-1ß, IL-6, IL-8, GM-CSF, MCP-1) and prevented leukocyte degranulation and formation of neutrophil extracellular traps, as shown by low levels of MPO and Cit H3 in the plasma. The almost total inhibition of HMGB1 release in treated survivors suggests a significant correlation between inhibiting CD14 and cytoprotection of vascular cells. The better preservation of blood pressure could be due to the adrenals, whose adrenomedullin production prevents the transition from the hyperdynamic to the hypodynamic phases of sepsis.42 Improvement of microcirculation, as indicated by less capillary leakage in the anti-CD14 treated group may contribute to the better pulmonary function with higher oxygen saturation and could also explain the lower lactate. Anti-CD14 treatment protected against organ injury, as shown by vital signs and organ function biomarkers. Although histopathological evaluation postmortem could not be temporally compared because of different times of death, features indicating better preservation of the organs were observed in the anti-CD14 treated baboons, such as diminished kidney and adrenal necrosis, spleen and liver congestion, leukocyte infiltration, and microthrombosis in the adrenals.
For many of the parameters measured we observed significant differences between the two animals that survived 7 days and recovered fully, and the three lesser responders to treatment that died on average by 76 hours. The reason some animals respond better than others is uncertain. This may reflect the individual genetic heterogeneity of the animals and underscores the necessity of finding biomarkers that could predict surviving/non-surviving outcome.
The interesting observation that CD14 inhibition increased bacterial killing instead of the expected decrease is puzzling. Using a human whole blood in vitro model, we showed that anti-CD14 attenuates both phagocytosis and oxidative burst.43 Anti-CD14 treatment in vivo may functionally protect the phagocytes as indicated by lower levels of MPO and faster recovery of circulating leukocytes. Better surviving phagocytes would be more efficient in bacteria clearance.
Because our model involves intravenous infusion of a large number of bacteria that rapidly release LPS upon lysis by complement,25 we chose to pretreat the animals to preclude CD14 from coming in contact with the released LPS. Different from our model, bacteria release into circulation is slow in many infections (eg, urinary tract infection), therefore we anticipate that targeting CD14 could be protective even when the treatment is given after initiation of bacteremia but may not hold when sepsis is fully developed. Using an in vitro whole blood assay, we observed that anti-CD14 had substantial effect on inflammation even when the anti-CD14 was added after the E coli.44 In our opinion, trials to test the efficacy of blocking CD14 should be done in patients at high sepsis risk, such as seniors who undergo large abdominal surgery and often suffer fecal leakage into the abdomen leading to peritonitis.
In conclusion, despite the limitation of the animal numbers due to animal welfare, the data are robust and consistent and suggest that CD14 inhibition in E coli sepsis has profound anti-inflammatory, anticoagulant, and profibrinolytic effects, thus protecting against the detrimental effects of thrombo-inflammation and could be a therapeutic option in this condition.
Supplementary Material
Essentials.
Gram-negative bacteria activate CD14-dependent signaling leading to inflammation and coagulopathy.
Blocking CD14 in a baboon model of lethal Escherichia coli sepsis decreases thrombo-inflammation.
CD14 inhibition prevents sepsis-induced complement activation.
Anti-CD14 treatment attenuates cardiopulmonary dysfunction and prolongs survival.
ACKNOWLEDGMENTS
The authors thank Dr Fletcher Taylor and Dr Gary Kinasewitz for helpful discussions, and Monalisa Choudhury for technical assistance. This work was supported by grants from The Norwegian Council on Cardiovascular Disease (NCCD-2016 [TEM]) and The Odd Fellow Foundation (OFF-2015 [TEM]); National Institutes of Health, National Institute of General Medical Sciences (GM116184 [FL], GM121601 [FL], GM122775 [FL] and P30GM114731 [FL]), and National Institute of Allergy and Infectious Diseases (U19AI062629 [FL]).
Funding information
The Norwegian Council on Cardiovascular Disease, Grant/Award Number: NCCD-2016; The Odd Fellow Foundation, Grant/Award Number: OFF-2015; National Institutes of Health; National Institute of General Medical Sciences, Grant/Award Number: GM116184, GM121601, GM122775 and P30GM114731; National Institute of Allergy and Infectious Diseases, Grant/Award Number: U19AI062629
Footnotes
CONFLICTS OF INTEREST
The authors declare no competing financial interests.
SUPPORTING INFORMATION
Additional supporting information may be found online in the Supporting Information section.
REFERENCES
- 1.Riedemann NC, Guo RF, Ward PA. The enigma of sepsis. J Clin Invest. 2003;112:460–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Riedemann NC, Guo RF, Ward PA. Novel strategies for the treatment of sepsis. Nat Med. 2003;9:517–524. [DOI] [PubMed] [Google Scholar]
- 3.Oberholzer A, Oberholzer C, Moldawer LL. Sepsis syndromes: understanding the role of innate and acquired immunity. Shock. 2001;16:83–96. [DOI] [PubMed] [Google Scholar]
- 4.van der Poll T, van de Veerdonk FL, Scicluna BP, Netea MG. The immunopathology of sepsis and potential therapeutic targets. Nat Rev Immunol. 2017;17(7):407–442. 10.1038/nri.2017.36 [DOI] [PubMed] [Google Scholar]
- 5.Vallejo JG. Role of toll-like receptors in cardiovascular diseases. Clin Sci (Lond). 2011;121:1–10. CS20100539 [pii]; 10.1042/CS20100539 [DOI] [PubMed] [Google Scholar]
- 6.Heumann D, Roger T. Initial responses to endotoxins and Gram-negative bacteria. Clin Chim Acta. 2002;323:59–72. [DOI] [PubMed] [Google Scholar]
- 7.Dobrovolskaia MA, Vogel SN. Toll receptors, CD14, and macrophage activation and deactivation by LPS. Microbes Infect. 2002;4:903–914. [DOI] [PubMed] [Google Scholar]
- 8.Yu L, Wang L, Chen S. Endogenous toll-like receptor ligands and their biological significance. J Cell Mol Med. 2010;14:2592–2603. 10.1111/j.1582-4934.2010.01127.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van der Poll T, Opal SM. Should all septic patients be given systemic anticoagulation? No. Intensive Care Med. 2017;43:455–457. 10.1007/s00134-016-4607-x [DOI] [PubMed] [Google Scholar]
- 10.Opal SM, Laterre PF, Francois B, et al. Effect of eritoran, an antagonist of MD2-TLR4, on mortality in patients with severe sepsis: the ACCESS randomized trial. JAMA. 2013;309:1154–1162. 1669798 [pii]; 10.1001/jama.2013.2194 [DOI] [PubMed] [Google Scholar]
- 11.Schimke J, Mathison J, Morgiewicz J, Ulevitch RJ. Anti-CD14 mAb treatment provides therapeutic benefit after in vivo exposure to endotoxin. Proc Natl Acad Sci USA. 1998;95:13875–13880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nakamura M, Takeuchi T, Shirakawa K, Furusako S. Anti-human CD14 monoclonal antibody improves survival following sepsis induced by endotoxin, but not following polymicrobial infection. Eur J Pharmacol. 2017;806:18–24. 10.1016/j.ejphar.2017.03.027 [DOI] [PubMed] [Google Scholar]
- 13.Leturcq DJ, Moriarty AM, Talbott G, Winn RK, Martin TR, Ulevitch RJ. Antibodies against CD14 protect primates from endotoxin-induced shock. J Clin Invest. 1996;98:1533–1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thorgersen EB, Hellerud BC, Nielsen EW, et al. CD14 inhibition efficiently attenuates early inflammatory and hemostatic responses in Escherichia coli sepsis in pigs. FASEB J. 2009;24:712–722 fj.09–140798 [pii]; 10.1096/fj.09-140798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ebong SJ, Goyert SM, Nemzek JA, Kim J, Bolgos GL, Remick DG. Critical role of CD14 for production of proinflammatory cytokines and cytokine inhibitors during sepsis with failure to alter morbidity or mortality. Infect Immun. 2001;69:2099–2106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Olszyna DP, Verbon A, Pribble JP, Turner T, Axtelle T. van Deventer SJ, van der PT. Effect of IC14, an anti-CD14 antibody, on plasma and cell-associated chemokines during human endotoxemia. Eur Cytokine Netw. 2003;14:158–162. [PubMed] [Google Scholar]
- 17.Verbon A, Meijers JC, Spek CA, et al. Effects of IC14, an anti-CD14 antibody, on coagulation and fibrinolysis during low-grade endotoxemia in humans. J Infect Dis. 2003;187:55–61. [DOI] [PubMed] [Google Scholar]
- 18.Daubeuf B, Mathison J, Spiller S, et al. TLR4/MD-2 monoclonal antibody therapy affords protection in experimental models of septic shock. J Immunol. 2007;179:6107–6114. [DOI] [PubMed] [Google Scholar]
- 19.Nakamura M, Takeuchi T, Kawahara T, et al. Simultaneous targeting of CD14 and factor XIa by a fusion protein consisting of an anti-CD14 antibody and the modified second domain of bikunin improves survival in rabbit sepsis models. Eur J Pharmacol. 2017;802:60–68. 10.1016/j.ejphar.2017.02.045 [DOI] [PubMed] [Google Scholar]
- 20.Skjeflo EW, Sagatun C, Dybwik K, et al. Combined inhibition of complement and CD14 improved outcome in porcine polymicrobial sepsis. Crit Care. 2015;19:415. 10.1186/s13054-015-1129-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reinhart K, Gluck T, Ligtenberg J, et al. CD14 receptor occupancy in severe sepsis: results of a phase I clinical trial with a recombinant chimeric CD14 monoclonal antibody (IC14). Crit Care Med. 2004;32:1100–1108. [DOI] [PubMed] [Google Scholar]
- 22.Taylor FB Jr, Kinasewitz GT, Lupu F. Pathophysiology, staging and therapy of severe sepsis in baboon models. J Cell Mol Med. 2012;16:672–682. 10.1111/j.1582-4934.2011.01454.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Council NR. Guide for the Care and Use of Laboratory Animals, (8th edn). Washington, DC: The National Academies Press; 2011. [PubMed] [Google Scholar]
- 24.Silasi R, Keshari RS, Lupu C, et al. Inhibition of contact-mediated activation of factor XI protects baboons against S aureus-induced organ damage and death. Blood Adv. 2019;3:658–669. 10.1182/bloodadvances.2018029983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Keshari RS, Silasi R, Popescu NI, et al. Inhibition of complement C5 protects against organ failure and reduces mortality in a baboon model of Escherichia coli sepsis. Proc Natl Acad Sci USA. 2017;114:E6390–E6399. 10.1073/pnas.1706818114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thorgersen EB, Pischke SE, Barratt-Due A, et al. Systemic CD14 inhibition attenuates organ inflammation in porcine Escherichia coli sepsis. Infect Immun. 2013;81:3173–3181. 10.1128/IAI.00390-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Silasi-Mansat R, Zhu H, Popescu NI, et al. Complement inhibition decreases the procoagulant response and confers organ protection in a baboon model of Escherichia coli sepsis. Blood. 2010;116:1002–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Taylor FB Jr, Stearns-Kurosawa DJ, Kurosawa S, et al. The endothelial cell protein C receptor aids in host defense against Escherichia coli sepsis. Blood. 2000;95:1680–1686. [PubMed] [Google Scholar]
- 29.Tang H, Ivanciu L, Popescu N, et al. Sepsis-induced coagulation in the baboon lung is associated with decreased tissue factor pathway inhibitor. Am J Pathol. 2007;171:1066–1077. 10.2353/ajpath.2007.070104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hotchkiss RS, Karl IE. The pathophysiology and treatment of sepsis. N Engl J Med. 2003;348:138–150. [DOI] [PubMed] [Google Scholar]
- 31.Marshall JC. Such stuff as dreams are made on: mediator-directed therapy in sepsis. Nat Rev Drug Discov. 2003;2:391–405. [DOI] [PubMed] [Google Scholar]
- 32.Editorial. Focus on sepsis. Nat Med. 2012;18:997. nm0712–997 [pii]; 10.1038/nm0712-997 [DOI] [PubMed] [Google Scholar]
- 33.Schaefer L Complexity of danger: the diverse nature of damage-associated molecular patterns. J Biol Chem. 2014;289:35237–35245. 10.1074/jbc.R114.619304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Peiretti F, Alessi MC, Henry M, Anfosso F, Juhan-Vague I, Nalbone G. Intracellular calcium mobilization suppresses the TNF-alpha-stimulated synthesis of PAI-1 in human endothelial cells. Indications that calcium acts at a translational level. Arterioscler Thromb Vasc Biol. 1997;17:1550–1560. [DOI] [PubMed] [Google Scholar]
- 35.Urano T, Castellino FJ, Ihara H, et al. Activated protein C attenuates coagulation-associated over-expression of fibrinolytic activity by suppressing the thrombin-dependent inactivation of PAI-1. J Thromb Haemost. 2003;1:2615–2620. 10.1046/j.1538-7836.2003.00443.x [DOI] [PubMed] [Google Scholar]
- 36.Ward PA. The dark side of C5a in sepsis. Nat Rev Immunol. 2004;4:133–142. [DOI] [PubMed] [Google Scholar]
- 37.Liu ZM, Zhu SM, Qin XJ, et al. Silencing of C5a receptor gene with siRNA for protection from Gram-negative bacterial lipopolysaccharide-induced vascular permeability. Mol Immunol. 2010;47: 1325–1333. 10.1016/j.molimm.2009.11.001. [DOI] [PubMed] [Google Scholar]
- 38.Opal SM, van der Poll T. Endothelial barrier dysfunction in septic shock. J Intern Med. 2014;277:277–293. 10.1111/joim.12331 [DOI] [PubMed] [Google Scholar]
- 39.Lau C, Nygard S, Fure H, et al. CD14 and complement crosstalk and largely mediate the transcriptional response to Escherichia coli in human whole blood as revealed by DNA microarray. PLoS One. 2015;10:e0117261. 10.1371/journal.pone.0117261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Huber-Lang M, Barratt-Due A, Pischke SE, et al. Double blockade of CD14 and complement C5 abolishes the cytokine storm and improves morbidity and survival in polymicrobial sepsis in mice. J Immunol. 2014;192:5324–5331. 10.4049/jimmunol.1400341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hajishengallis G, Lambris JD. Crosstalk pathways between Toll-like receptors and the complement system. Trends Immunol. 2010;31:154–163. S1471–4906(10)00003–7 [pii]; 10.1016/j.it.2010.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yang S, Zhou M, Chaudry IH, Wang P. Novel approach to prevent the transition from the hyperdynamic phase to the hypodynamic phase of sepsis: role of adrenomedullin and adrenomedullin binding protein-1. Ann Surg. 2002;236:625–633. 10.1097/01.SLA.0000033040.18139.A2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Skjeflo EW, Christiansen D, Landsem A, et al. Phagocytosis of live and dead Escherichia coli and Staphylococcus aureus in human whole blood is markedly reduced by combined inhibition of C5aR1 and CD14. Mol Immunol. 2019;112:131–139. 10.1016/j.molimm.2019.03.014 [DOI] [PubMed] [Google Scholar]
- 44.Egge KH, Thorgersen EB, Lindstad JK, et al. Post challenge inhibition of C3 and CD14 attenuates Escherichia coli-induced inflammation in human whole blood. Innate Immun. 2014;20:68–77. 10.1177/1753425913482993 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


