Supplemental Digital Content is available in the text.
Keywords: acute stroke, fibrin, leukocytes, medical center, thrombectomy
Abstract
Background and Purpose:
Mechanical properties of thromboemboli play an important role in the efficacy of endovascular thrombectomy (EVT) for acute ischemic stroke. However, very limited data on mechanical properties of human stroke thrombi are available. We aimed to mechanically characterize thrombi retrieved with EVT, and to assess the relationship between thrombus composition and thrombus stiffness.
Methods:
Forty-one thrombi from 19 patients with acute stroke who underwent EVT between July and October 2019 were mechanically analyzed, directly after EVT. We performed unconfined compression experiments and determined tangent modulus at 75% strain (Et75) as a measure for thrombus stiffness. Thrombi were histologically analyzed for fibrin/platelets, erythrocytes, leukocytes, and platelets, and we assessed the relationship between histological components and Et75 with univariable and multivariable linear mixed regression.
Results:
Median Et75 was 560 (interquartile range, 393–1161) kPa. In the multivariable analysis, fibrin/platelets were associated with increased Et75 (aβ, 9 [95% CI, 5 to 13]) kPa, erythrocytes were associated with decreased Et75% (aβ, −9 [95% CI, −5 to −13]) kPa. We found no association between leukocytes and Et75. High platelet values were strongly associated with increased Et75 (aβ, 56 [95% CI, 38–73]).
Conclusions:
Fibrin/platelet content of thrombi retrieved with EVT for acute ischemic stroke is strongly associated with increased thrombus stiffness. For thrombi with high platelet values, there was a very strong relationship with thrombus stiffness. Our data provide a basis for future research on the development of next-generation EVT devices tailored to thrombus composition.
See related article, p 2518
Mechanical endovascular thrombectomy (EVT) for acute ischemic stroke (AIS) is among the most effective treatments in clinical medicine. Nevertheless, in ≈20% to 40% of patients who undergo EVT, substantial revascularization is not achieved.1,2 In vitro data with thrombus analogs suggest that the mechanical properties of thrombi play a vital role in the efficacy of EVT.3,4 However, very limited data on mechanical properties of human AIS thrombi are currently available.
Earlier studies have assessed the mechanical properties of thrombus analogs; artificial blood clots, produced from animal or human blood, which resemble acute ischemic stroke thrombi. Thrombus analogs with higher fibrin and platelet content were found to have increased material stiffness4–8 and friction.3 Experimental studies with analogs have shown that the successfulness of EVT depends on the mechanical properties of the thrombus analog: stiffer specimen often led to failed or incomplete recanalization.3,4 Instead of being captured by the EVT device, stiffer analogs often rolled between vessel wall and device, without being retrieved. In line with these findings, clinical studies have shown that fibrin-rich thrombi more often need multiple retrieval attempts, leading to longer EVT times and lower revascularization scores.9,10 Only one previous study has characterized the mechanical properties of human AIS thrombi.11 This study concerned 9 specimen from 4 patients with AIS who underwent EVT, and only qualitative histological analysis was performed.
Substantial data on the mechanical characteristics of stroke thrombi from EVT patients, and the relationship to quantitative thrombus composition, are still lacking. We aimed to mechanically characterize thrombi retrieved during EVT in patients with acute ischemic stroke, and to quantitatively assess the association of thrombus composition with thrombus stiffness. We hypothesized that, in line with results from thrombus analog studies, fibrin and platelet rich thrombi would be associated with higher thrombus stiffness.
Methods
Patient Population
Thrombi were collected from consecutive patients with AIS who underwent EVT from July to October 2019, in the Erasmus University Medical Center in Rotterdam, the Netherlands. Thrombi from patients who met the following criteria were included: 18 years or older, EVT performed for ischemic stroke because of a proximal intracranial occlusion of the anterior or posterior circulation, and at least one thrombus fragment was collected during the procedure. The study was approved by the central medical ethics committee of the Erasmus Medical Center Rotterdam, the Netherlands, and written informed consent from patients was obtained (MEC 2017-366, MEC 2017-367 and MEC 2017-368). Data, syntax files, and output of statistical analyses in STATA are available from the corresponding author on reasonable request.
Thrombus Collection and Mechanical Characterization
We used unconfined compression experiments for the mechanical characterization of the thrombi. Unconfined compression is a widely used technique for the assessment of mechanical properties of soft biological tissue, in which the tissue is mechanically compressed between 2 rigid plates, and the force response is measured. It is commonly used to evaluate mechanical properties of thrombus analogs and thrombi from patients with AIS.4,11
After EVT, thrombi were preserved in DMEM (high glucose, L-glutamine, HEPES, no phenol red; Thermo Fischer, Waltham, MA) at 4 °C to 10 °C. If EVT took place between 9 am and 9 pm, mechanical characterization was performed immediately after EVT. If EVT took place between 9 pm and 9 am, the experiment was performed the following working day, provided that the experiment could be initiated within 12 hours after EVT. Time from EVT to experiment was registered, to be taken into account in the statistical analyses. In preparation for the experiment, thrombi were carefully removed from the stent-retriever using forceps. They were trimmed to a height of 1 millimeter, such that the resulting sample had a flat top and bottom. Thrombi with a diameter of smaller than 1 millimeter were not eligible for the mechanical experiment. If a thrombus was large enough, or if > 1 thrombus was obtained during EVT, multiple samples per patient were prepared for mechanical testing.
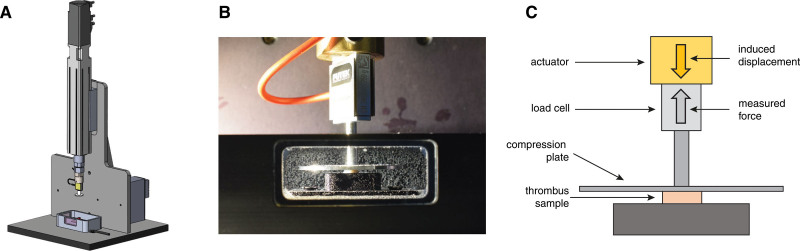
The unconfined compression experiments were performed with a custom-built test setup (Figure 1 and Methods in the Data Supplement), with the test samples submerged in DMEM at body temperature (37 °C) during the experiment. Samples were subjected to 80% compression of their initial height, at a strain rate of 10% per second. The applied deformation (strain) of the sample, and the corresponding force exerted by the tissue against the compression plate, were measured. The initial cross-sectional surface areas of the thrombus samples were measured from photographs of the trimmed samples before the experiment, with ImageJ (1.52a, National Institutes of Health). Stress was obtained by dividing the measured force by the initial cross-sectional surface area of the sample. The mechanical behavior of the tissue was represented in a nominal stress-strain curve. The thrombus tissue was mechanically modeled as a first-order Mooney-Rivlin material. To obtain the material constants of the Mooney-Rivlin material, the model was fitted to the experimental stress-strain data in Abaqus (2016, Dassault Systems, Johnston, Ri). The tangent modulus, defined as the slope of the tangent at any point of interest on the stress-strain curve, was used to describe thrombus stiffness. Since thrombi in clinical practice are subjected to large strains, tangent modulus at 75% strain (Et75) was used.
Figure 1.
Human stroke thrombi were mechanically characterized. Mechanical force tester (A); close-up photo of the tissue bath and the compression head department (B). Thrombus samples were subjected to 80% compression of their initial height, at a strain rate of 10% per second. The applied deformation (strain) of the sample, and the corresponding force exerted by the sample against the compression plate, were measured (C).
Histological Characterization
Mechanically analyzed thrombus samples were incubated in 4% paraformaldehyde at room temperature for 24 to 48 hours, after which they were embedded in paraffin and sectioned into 5 µm sections. Per sample, one section was stained with Hematoxylin and Eosin (H&E, HT110216, Sigma-Aldrich, St. Louis, MO) for the assessment of fibrin/platelets (on H&E, fibrin cannot be easily distinguished from platelets), erythrocytes and leukocytes. A second section per sample was stained with immunohistochemistry for platelets (CD61, CMC16121040, Cell Marque, Rocklin, CA). Microscopical images were acquired with a single slide scanner at 40× magnification (2.0 HT Nanozoomer, Hamamatsu, Japan), after which the quantitative fraction of histological components (relative to the total area of the section) was determined with the use of Orbit Image Analysis (version 3.15, Idorsia Pharmaceuticals Ltd, Allschwil).
Statistical Analysis
The relationship between histological components (fibrin/platelets, erythrocytes, leukocytes and platelets) of the thrombi, with thrombus stiffness (Et75) in kilopascal (kPa) was assessed with linear mixed regression models and reported as coefficients (β) with 95% CIs. We used mixed regression models, to account for the fact that >1 thrombus sample could be derived from the same patient. We performed univariable and multivariable analyses. In the multivariable analyses, we adjusted for age, sex, administration of intravenous thrombolysis and time from end of EVT to start of the mechanical experiment, based on clinical knowledge. Nonlinearity of the relationship of histological components with Et75 was assessed by visual inspection of the crude data. In case of nonlinearity, we used a linear mixed-effects model with 2 linear terms for thrombus composition, and to assess whether the model fit for this binary term was superior to the linear term, we performed a likelihood ratio test. STATA/SE 15.1 (StataCorp, College Station, TX) was used for all statistical analyses.
Results
Of the 48 patients treated from July 1, 2019 to October 3, 2019, 19 could be included in this study. A flow diagram of patient and thrombus selection is shown in Figure I in the Data Supplement.
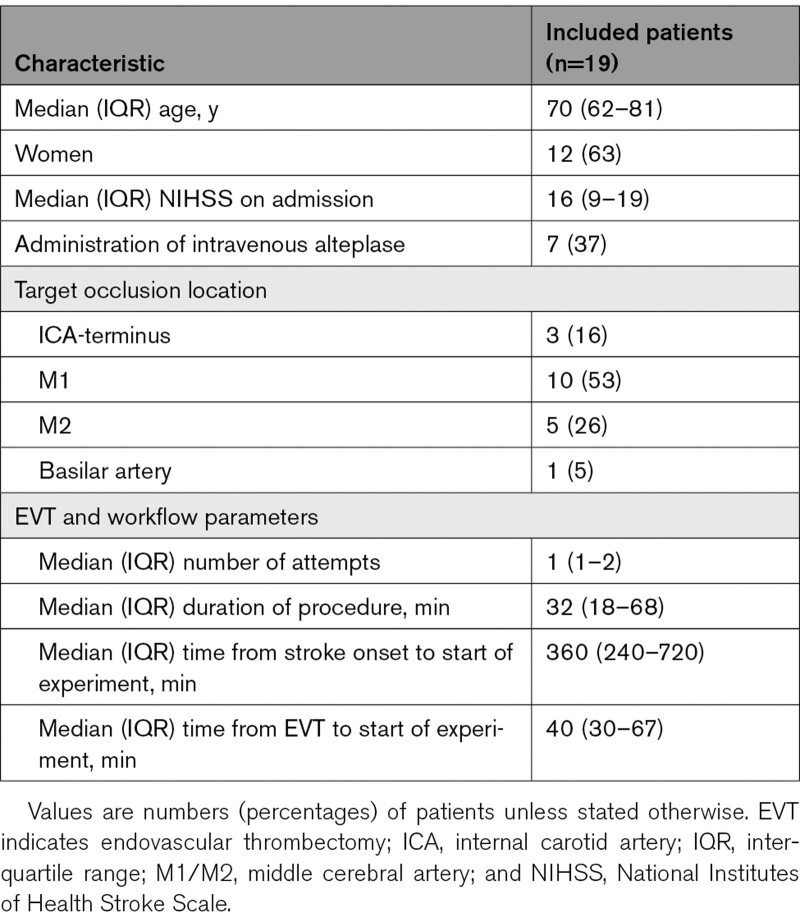
Of the 19 included patients, median age was 70 (interquartile range [IQR], 62–81) years, and 12 (63%) were women. Median National Institutes of Health Stroke Scale on admission was 16 (IQR, 9–19) and 7 (37%) patients received administration of intravenous thrombolysis. In most patients (10, 53%), the occlusion was located in the M1-segment of the middle cerebral artery, followed by the M2-segment of the middle cerebral artery (n=5, 26%) and the internal carotid artery terminus (n=3, 16%). One patient had an occlusion of the basilar artery. The median time from end of EVT to start of the mechanical experiment was 40 (IQR, 30–67) minutes. Clinical and interventional characteristics of all patients are shown in Table 1. Of the 19 included patients, 41 thrombus samples could be trimmed and mechanically analyzed (Table I in the Data Supplement). The median number of analyzed thrombus samples per patient was 2.
Table 1.
Baseline Characteristics of the 19 Included Patients
Thrombus Composition
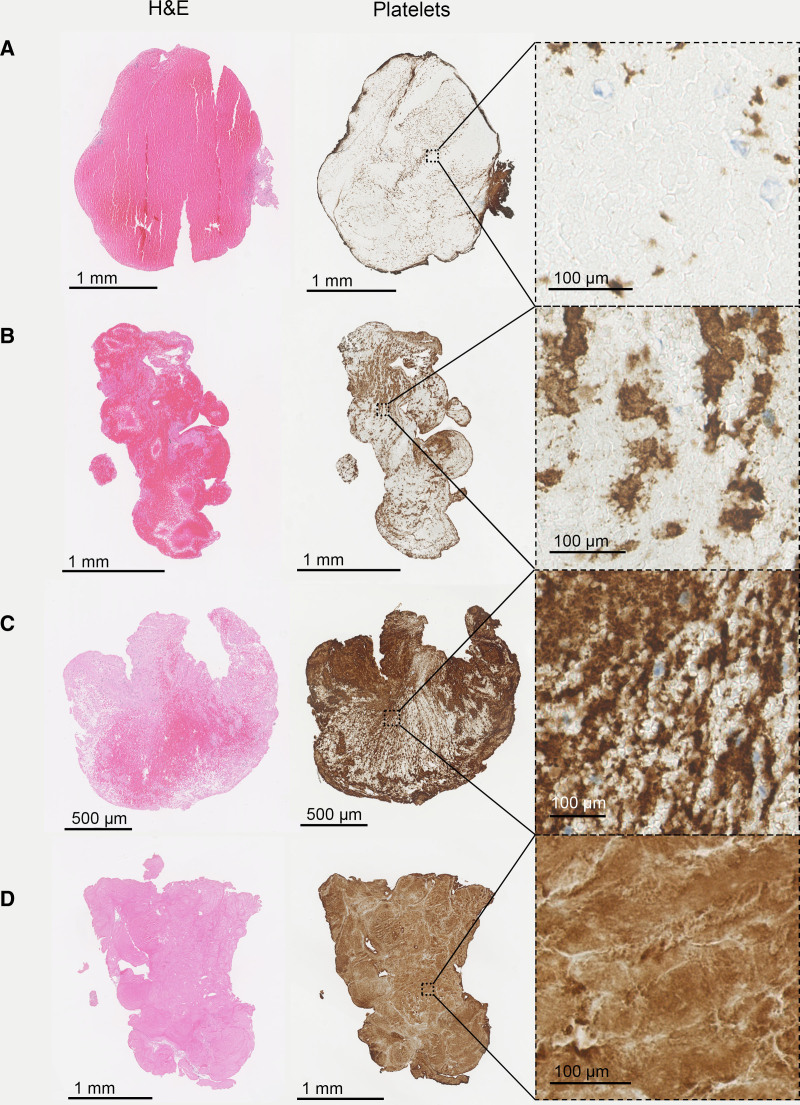
Based on visual inspection of the histological images, thrombi were heterogeneous in shape and organization (Figure 2). There was a large variation in quantified histological composition, as shown in Figure II in the Data Supplement. On H&E, median fibrin/platelet content was 59% (IQR, 38–91), median erythrocyte content was 40% (IQR, 9–60), and median leukocyte content was 1% (IQR, 0–2). On CD61 immunostaining, median platelet content was 68% (IQR, 49–79). Thrombi from later passes were more fibrin/platelet rich and erythrocyte poor (Figure III in the Data Supplement), but these differences were not statistically significant. Thrombus stiffness did not differ according to pass number.
Figure 2.
Histological images of 4 mechanically analyzed thrombi are shown, according to increasing fibrin/platelet content. From top to bottom: 11% (A), 36% (B), 56% (C), and 99% (D) fibrin/platelets. H&E indicates hematoxylin-eosin stain and platelets, immunostaining for platelets by CD61 (platelets are shown in brown).
Relationship Between Thrombus Composition and Thrombus Stiffness
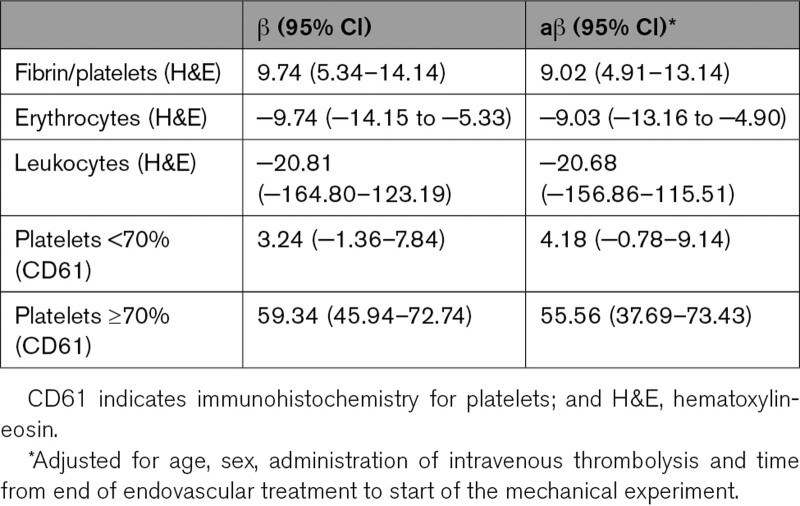
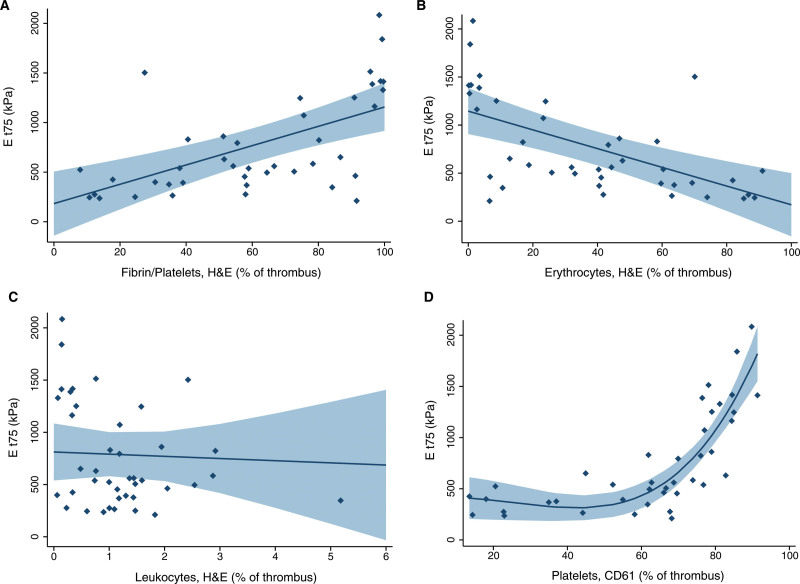
All thrombus samples exhibited nonlinear mechanical behavior. Median thrombus stiffness, defined as Et75, was 560 (IQR, 393–1161) kPa. The pass number to which a thrombus belonged did not influence Et75. Fibrin/platelet content was associated with increased Et75 in the univariable (β, 9.7 [95% CI, 5.3–14.1]) and in the multivariable (β, 9.0 [95% CI, 4.9–13.1]) analysis. In other words, for every 1% increase in fibrin/platelet content, thrombus stiffness increased with 9 kPa. Erythrocyte content was associated with decreased Et75 in the univariable (β, −9.7 [95% CI, −14.2 to −5.3]) and the multivariable (β, −9.0, [95% CI, −13.2 to −4.9]) analysis (Table 2, Figure 3). We found no relationship between leukocytes and Et75 (β, −20.8 [95% CI, −164.8 to 123.2]).
Table 2.
Univariable and Multivariable Linear Mixed Regression for the Relationship Between Histological Parameters and Thrombus Stiffness (Tangent Modulus, Et75) in kPa
Figure 3.
Relationship of thrombus composition with thrombus stiffness (Et75) is shown. Margins plots including 95% confidence intervals for fibrin/platelets (A), erythrocytes (B), leukocytes (C) on H&E with Et75 and fitted curve including 95% confidence interval for platelets on CD61 (D) with Et75.
We observed a nonlinear relationship of platelets with Et75, with an inflection point at ≈70% platelets (Figure 3). Therefore, we assessed the relationship of platelets with Et75 using a linear mixed-effects model with 2 linear terms for platelets: <70% platelets and ≥70% platelets. To assess whether the model fit for this binary term for platelets was superior to the linear term for platelets, we performed a likelihood ratio test, which demonstrated that the binary term was a better fit for our data. For thrombi with <70% platelets, we found no relationship with Et75 (β, 3.2 [95% CI, −1.4 to 7.8]). For thrombi with >70% platelets, however, we found a strong positive relationship with Et75 in the univariable (β, 59.3 [95% CI, 45.9–72.7]) and multivariable (β, 55.6 [95% CI 37.7–73.4]) analysis (Table 2).
Discussion
In this study, we mechanically characterized human AIS thrombi and assessed the association of thrombus composition with thrombus stiffness. We found a median thrombus stiffness (Et75) of 560 (IQR, 393–1161) kPa and have shown that thrombus composition is strongly associated with thrombus stiffness.
We found that fibrin/platelet content was associated with increased thrombus stiffness, and erythrocyte content with decreased thrombus stiffness. For every 1% increase in fibrin/platelets on H&E, and every ≈1% decrease in erythrocytes on H&E, Et75 increased with 9 kPa. One could say that in terms of material stiffness, erythrocyte rich thrombi in our study were comparable to subcutaneous fat, whereas fibrin/platelet rich thrombi had stiffness values comparable to scar tissue.12,13 These findings are in line with studies on thrombus analogs, which have shown that increasing fibrinogen concentrations led to higher thrombus stiffness values.5–7 In addition, we found an interesting relationship between platelet content and thrombus stiffness. For platelet values under ≈70%, as assessed on CD61-immunostaining, we found no relationship with thrombus stiffness. However, for thrombi with platelet values over ≈70%, we found a strong positive relationship with stiffness. Studies with thrombus analogs have shown similar findings: Carr et al5 found that stiffness of thrombus analogs increased with platelet concentration. Previous studies have shown that platelets are the key drivers for thrombus contraction, a mechanism resulting into compact and stiffer thrombi.14 Our results suggest there might be a certain platelet threshold, after which thrombus contraction becomes a strong contributor to thrombus stiffness. The threshold value of 70% platelets, which we used for our regression analyses, however, should not be interpreted as an absolute number, but rather regarded as an estimate.
Interestingly, thrombus stiffness in our study was considerably higher than reported in previous literature. Chueh et al11 mechanically characterized 9 thrombi from 4 patients with AIS, resulting in an average secant modulus at 0% to 75% strain (the slope from zero to the 75% strain on the stress-strain curve) of 40±10 kPa (122±104 kPa in our study). Thrombus analog studies, too, report lower stiffness values than the ones in our study. Malone et al15 reported a tangent modulus at 40% strain of 14 kPa15 (59±63 kPa in our study). Chueh et al11 tested analogs of various compositions, with secant modulus at 0% to 75% strain ranging from 10 to 65 kPa (average 122±104 kPa in our study). There are several possible explanations for finding higher thrombus stiffness values than previously reported. First, all human thrombi that were characterized by Chueh et al were described as red, presumably erythrocyte rich, based on visual inspection of the histological images. This might have resulted in relatively soft thrombi, compared with thrombi in our study, which had a wide histological variability. Also, differences in treatment modality (aspiration thrombectomy in Chueh et al versus stent-retriever thrombectomy in our study) could have influenced results. Some studies have shown that stent-retrievers respond better to fibrin/platelet-rich thrombi than aspiration devices,16,17 which could have resulted in an overrepresentation of fibrin-rich, stiffer thrombi in our study. Another explanation for finding higher thrombus stiffness in real thrombi than in analogs might be differences in microstructure. In contrast to human thrombi, thrombus analogs are homogeneous. Since strong internal thrombus organization has been shown to contribute to thrombus stiffness,6 human thrombi might be stiffer than analogs with a similar composition.
From One Size Fits All to Thrombus-Specific Endovascular Treatment
To develop EVT techniques and devices that specifically target stiff, thrombectomy-resistant thrombi, a basic understanding of the interaction between thrombus properties, vessel wall, and the EVT device is needed. However, representative data on the mechanical properties of human stroke thrombi has been lacking. We have characterized human stroke thrombi with a wide, representative range of histological compositions. Interestingly, thrombus stiffness in our study was considerably higher than reported in previous studies. Therefore, human stroke thrombi might be more thrombectomy-resistant than analogs, used to develop next-generation EVT devices.3,18 Larger studies with human thrombi are needed to confirm this hypothesis. We found a strong positive association between fibrin concentration, platelet concentration, and thrombus stiffness. This implies that it is harder for a stent retriever to penetrate fibrin/platelet rich thrombi8,18 and underlines the importance of taking these histological components into account in the development of thrombus analogs. Stent retrievers with larger radial forces, for example, by changing the material they are made of or with more oval-shaped stent struts, might improve the penetration of fibrin/platelet-rich thrombi. One could also speculate that fibrin/platelet rich thrombi are suitable for completely new retriever designs, making use of the firmness of the thrombus: a retriever design that anchors in the proximal part of the thrombus and subsequently pulls the thrombus, might be one of the potential avenues to explore.
Limitations
This study has several limitations. First, the unconfined compression experiment is designed to be performed on tissue that is firm and large enough to be characterized. Therefore, thrombus material that could not be trimmed to a 1 mm thick sample in preparation for the experiment, was not tested, probably introducing a selection bias. Another limitation of the unconfined compression experiment is the unidirectionality. Thrombus stiffness might vary depending on the direction in which it is loaded19; therefore, anisotropic mechanical properties could not be quantified. Also, the current study reported one parameter to describe thrombus stiffness. The nonlinear mechanical behavior of thrombi could be better described in the future if multiple stiffness parameters would be used.20
Thrombi from patients with unsuccessful recanalization were not included in the study. During the inclusion period, there was however only one patient with unsuccessful recanalization (Figure I in the Data Supplement). In addition, a considerable number of patients were excluded because they were treated during off hours, or because the thrombus was preserved in formalin, instead of DMEM, by the interventionalist. We assume this selection was random and therefore did not influence our results.
There are some limitations to the histological analysis in this study. First, the compression experiment could have altered thrombus composition, as thrombus components might be lost due to the material compression. More advances mechanical models that account for both the solid and fluid character of thrombi would be useful to improve the interpretation of the results of the unconfined compression experiments. We analyzed platelets separately using a CD61 immunostaining, in addition to the H&E staining. Counterintuitively, some platelet values on CD61 exceeded the fibrin/platelet values on H&E. This is possible, as platelets can be underestimated on an H&E stain, since their small diameter can cause them to be obscured by cells. Also, suboptimal distinction between pink and red on H&E could cause underestimation of fibrin/platelets on H&E.
Conclusions
Mechanical characterization of thrombi from patients with acute ischemic stroke who underwent EVT, resulted in a median thrombus stiffness of 560 (IQR, 393–1161) kPa. For every 1% increase in fibrin/platelets on H&E, thrombus stiffness increased with 9 kPa. For high platelet values, we found a very strong relationship with thrombus stiffness. Our data provide a basis for future research on the development of next-generation EVT devices tailored to thrombus composition.
Sources of Funding
This study was funded and carried out by the Erasmus MC, University Medical Center Rotterdam. The study has received funding from the European Union’s Horizon 2020 research and innovation programme under grant agreement No. 777072 (INSIST [IN-Silico trials for treatment of acute Ischemic Stroke]), which played no role in study design and patient enrollment, nor in data collection, analysis, or writing of the article.
Disclosures
Erasmus Medical Center received compensation from Stryker, Siemens Healthineers and GE Healthcare for activities of Dr van der Lugt. Drs Dippel and van der Lugt report grants from Dutch Heart Foundation, grants from Brain Foundation Netherlands, grants from Health Holland Top Sector Life Sciences & Health, grants from The Netherlands Organisation for Health Research and Development and unrestricted grants from Stryker European Operations BV, from Penumbra Inc, from Medtronic, from Thrombolytic Science, LLC, and from Cerenovus outside the submitted work, all paid to institution.
Supplemental Materials
Expanded Methods
Online Table I
Online Figures I–III
Supplementary Material
Nonstandard Abbreviations and Acronyms
- AIS
- acute ischemic stroke
- EVT
- endovascular thrombectomy
- IQR
- interquartile range
N. Boodt and P.R.W. Snouckaert van Schauburg contributed equally.
This manuscript was sent to Kazunori Toyoda, Guest Editor, for review by expert referees, editorial decision, and final disposition.
The Data Supplement is available with this article at https://www.ahajournals.org/doi/suppl/10.1161/STROKEAHA.120.033527.
For Sources of Funding and Disclosures, see page 2516.
Presented in part at the European Stroke Organisation-World Stroke Organization 2020 Virtual Conference, November 7–9, 2020.
Contributor Information
Philip R.W. Snouckaert van Schauburg, Email: p.snouckaert@gmail.com.
Hajo M. Hund, Email: info@hajohund.nl.
Behrooz Fereidoonnezhad, Email: behrooz.fereidoonnezhad@nuigalway.ie.
J. Patrick McGarry, Email: patrick.mcgarry@nuigalway.ie.
Ali C. Akyildiz, Email: a.akyildiz@erasmusmc.nl.
Adriaan C.G.M. van Es, Email: A.C.G.M.van_Es@LUMC.nl.
Simon F. De Meyer, Email: simon.demeyer@kuleuven.be.
Diederik W.J. Dippel, Email: d.dippel@erasmusmc.nl.
Hester F. Lingsma, Email: h.lingsma@erasmusmc.nl.
Heleen M.M. van Beusekom, Email: h.vanbeusekom@erasmusmc.nl.
Aad van der Lugt, Email: a.vanderlugt@erasmusmc.nl.
Frank J.H. Gijsen, Email: f.gijsen@erasmusmc.nl.
References
- 1.Yoo AJ, Andersson T. Thrombectomy in acute ischemic stroke: challenges to procedural success. J Stroke. 2017; 19:121–130. doi: 10.5853/jos.2017.00752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jansen IGH, Mulder MJHL, Goldhoorn RB; MR CLEAN Registry investigators. Endovascular treatment for acute ischaemic stroke in routine clinical practice: prospective, observational cohort study (MR CLEAN Registry). BMJ. 2018; 360:k949. doi: 10.1136/bmj.k949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gunning GM, McArdle K, Mirza M, Duffy S, Gilvarry M, Brouwer PA. Clot friction variation with fibrin content; implications for resistance to thrombectomy. J Neurointerv Surg. 2018; 10:34–38. doi: 10.1136/neurintsurg-2016-012721 [DOI] [PubMed] [Google Scholar]
- 4.Duffy S, Farrell M, McArdle K, Thornton J, Vale D, Rainsford E, Morris L, Liebeskind DS, MacCarthy E, Gilvarry M. Novel methodology to replicate clot analogs with diverse composition in acute ischemic stroke. J Neurointerv Surg. 2017; 9:486–491. doi: 10.1136/neurintsurg-2016-012308 [DOI] [PubMed] [Google Scholar]
- 5.Carr ME, Jr, Carr SL. Fibrin structure and concentration alter clot elastic modulus but do not alter platelet mediated force development. Blood Coagul Fibrinolysis. 1995; 6:79–86. doi: 10.1097/00001721-199502000-00013 [DOI] [PubMed] [Google Scholar]
- 6.Ryan EA, Mockros LF, Weisel JW, Lorand L. Structural origins of fibrin clot rheology. Biophys J. 1999; 77:2813–2826. doi: 10.1016/S0006-3495(99)77113-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ashton JH, Vande Geest JP, Simon BR, Haskett DG. Compressive mechanical properties of the intraluminal thrombus in abdominal aortic aneurysms and fibrin-based thrombus mimics. J Biomech. 2009; 42:197–201. doi: 10.1016/j.jbiomech.2008.10.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weafer FM, Duffy S, Machado I, Gunning G, Mordasini P, Roche E, McHugh PE, Gilvarry M. Characterization of strut indentation during mechanical thrombectomy in acute ischemic stroke clot analogs. J Neurointerv Surg. 2019; 11:891–897. doi: 10.1136/neurintsurg-2018-014601 [DOI] [PubMed] [Google Scholar]
- 9.Maekawa K, Shibata M, Nakajima H, Mizutani A, Kitano Y, Seguchi M, Yamasaki M, Kobayashi K, Sano T, Mori G, et al. Erythrocyte-rich thrombus is associated with reduced number of maneuvers and procedure time in patients with acute ischemic stroke undergoing mechanical thrombectomy. Cerebrovasc Dis Extra. 2018; 8:39–49. doi: 10.1159/000486042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brinjikji W, Duffy S, Burrows A, Hacke W, Liebeskind D, Majoie CBLM, Dippel DWJ, Siddiqui AH, Khatri P, Baxter B, et al. Correlation of imaging and histopathology of thrombi in acute ischemic stroke with etiology and outcome: a systematic review. J Neurointerv Surg. 2017; 9:529–534. doi: 10.1136/neurintsurg-2016-012391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chueh JY, Wakhloo AK, Hendricks GH, Silva CF, Weaver JP, Gounis MJ. Mechanical characterization of thromboemboli in acute ischemic stroke and laboratory embolus analogs. AJNR Am J Neuroradiol. 2011; 32:1237–1244. doi: 10.3174/ajnr.A2485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Geerligs M, Peters GW, Ackermans PA, Oomens CW, Baaijens FP. Linear viscoelastic behavior of subcutaneous adipose tissue. Biorheology. 2008; 45:677–688 [PubMed] [Google Scholar]
- 13.Clark JA, Cheng JC, Leung KS, Leung PC. Mechanical characterisation of human postburn hypertrophic skin during pressure therapy. J Biomech. 1987; 20:397–406. doi: 10.1016/0021-9290(87)90047-9 [DOI] [PubMed] [Google Scholar]
- 14.Cines DB, Lebedeva T, Nagaswami C, Hayes V, Massefski W, Litvinov RI, Rauova L, Lowery TJ, Weisel JW. Clot contraction: compression of erythrocytes into tightly packed polyhedra and redistribution of platelets and fibrin. Blood. 2014; 123:1596–1603. doi: 10.1182/blood-2013-08-523860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Malone F, McCarthy E, Delassus P, Fahy P, Kennedy J, Fagan AJ, Morris L. The mechanical characterisation of bovine embolus analogues under various loading conditions. Cardiovasc Eng Technol. 2018; 9:489–502. doi: 10.1007/s13239-018-0352-3 [DOI] [PubMed] [Google Scholar]
- 16.Madjidyar J, Pineda Vidal L, Larsen N, Jansen O. Influence of thrombus composition on thrombectomy: ADAPT vs. Balloon guide catheter and stent retriever in a flow model. Rofo. 2020; 192:257–263. doi: 10.1055/a-0998-4246 [DOI] [PubMed] [Google Scholar]
- 17.Mokin M, Waqas M, Fifi J, De Leacy R, Fiorella D, Levy EI, Snyder K, Hanel R, Woodward K, Chaudry I, et al. Clot perviousness is associated with first pass success of aspiration thrombectomy in the compass trial. J Neurointerv Surg. 2020; 0:1–7 [DOI] [PubMed] [Google Scholar]
- 18.van der Marel K, Chueh JY, Brooks OW, King RM, Marosfoi MG, Langan ET, Carniato SL, Gounis MJ, Nogueira RG, Puri AS. Quantitative assessment of device-clot interaction for stent retriever thrombectomy. J Neurointerv Surg. 2016; 8:1278–1282. doi: 10.1136/neurintsurg-2015-012209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Collet JP, Shuman H, Ledger RE, Lee S, Weisel JW. The elasticity of an individual fibrin fiber in a clot. Proc Natl Acad Sci USA. 2005; 102:9133–9137. doi: 10.1073/pnas.0504120102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fereidoonnezhad B, O’Connor C, McGarry JP. A new anisotropic soft tissue model for elimination of unphysical auxetic behaviour. J Biomech. 2020; 111:110006. doi: 10.1016/j.jbiomech.2020.110006 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.