Abstract
Background:
Graft choice for pediatric anterior cruciate ligament reconstruction (ACLR) is determined by several factors. There is limited information on the use and outcomes of allograft ACLR in pediatric patients. The purpose of this systematic review and meta-analysis was to quantify reported failure rates of allograft versus autograft ACLR in patients ≤19 years of age with ≥2 years of follow-up. We hypothesized that there would be higher rates of failure for allograft compared with autograft ACLR in this population.
Methods:
PubMed/MEDLINE and Embase databases were systematically searched for literature regarding allograft and autograft ACLR in pediatric/adolescent patients. Articles were included if they described a cohort of patients with average age of ≤19 years, had a minimum of 2 years of follow-up, described graft failure as an outcome, and had a Level of Evidence grade of I to III. Qualitative review and quantitative meta-analysis were performed to compare graft failure rates. A random-effects model was created to compare failure events in patients receiving allograft versus autograft in a pairwise fashion. Data analysis was completed using RevMan 5.3 software (The Cochrane Collaboration).
Results:
The database search identified 1,604 studies; 203 full-text articles were assessed for eligibility. Fourteen studies met the inclusion criteria for qualitative review; 5 studies were included for quantitative meta-analysis. Bone-patellar tendon-bone (BTB) represented 58.2% (n = 1,012) of the autografts, and hamstring grafts represented 41.8% (n = 727). Hybrid allografts (autograft + supplemental allograft) represented 12.8% (n = 18) of all allograft ACLRs (n = 141). The unweighted, pooled failure rate for each graft type was 8.5% for BTB, 16.6% for hamstring, and 25.5% for allograft. Allografts were significantly more likely than autografts to result in graft failure (odds ratio, 3.87; 95% confidence interval, 2.24 to 6.69).
Conclusions:
Allograft ACLR in pediatric and adolescent patients should be used judiciously, as existing studies revealed a significantly higher failure rate for allograft compared with autograft ACLR in this patient population. Additional studies are needed to improve the understanding of variables associated with the high ACLR failure rate among pediatric and adolescent patients.
Level of Evidence:
Therapeutic Level III. See Instructions for Authors for a complete description of levels of evidence.
The incidence of pediatric anterior cruciate ligament (ACL) injury and reconstruction is increasing1-3. Many factors likely contribute to this increase including increased participation in, and intensity of, youth sports4,5. Attitudes regarding early ACL reconstruction (ACLR) in skeletally immature athletes have also shifted as a result of literature showing increased risk of meniscal and chondral injuries associated with delayed reconstruction6-12. Supporting this shift has been literature showing that pediatric ACLR is relatively safe. A recent meta-analysis identified a <5% rate of growth disturbance, with less than one-third of those cases requiring secondary surgery to address this complication13. While reported results of pediatric ACLR are generally favorable14-16, graft rupture has been consistently shown to occur at higher rates in this young, active population17,18. The increased incidence of graft rupture in this group highlights the need to identify and address potentially mitigating factors, including associated injuries (e.g., meniscal tear), postoperative rehabilitation protocols, return to sport clearance/timing, and graft choice19,20.
Graft choice for pediatric ACLR is determined by several factors, including the amount of skeletal growth remaining, patient size, type of sport played, surgical technique, and surgeon preference. While allograft ACLR has the advantages of eliminating donor-site morbidity, control over the desired graft size, and ease of availability, some studies have shown higher failure rates in younger, more active populations when compared with autograft13,21,22.
There is limited information on the use and outcomes of allograft ACLR specifically in pediatric patients, as a majority of the literature has evaluated adult populations. The purpose of this systematic review and meta-analysis was to quantify reported failure rates of allograft versus autograft ACLR in patients ≤19 years of age with ≥2 years of follow-up from the primary ACLR. Specifically, we aimed to answer the following: (1) what are the reported failure rates of allograft and autograft ACLR in pediatric/adolescent patients, and (2) do reported graft failure rates differ significantly between allograft and autograft ACLR in pediatric/adolescent patients? We hypothesized that there would be higher rates of failure for allograft compared with autograft ACLR in this patient population.
Materials and Methods
Literature Search
We utilized methods similar to those of a previous investigation23. The Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) guidelines24 were used to systematically identify literature examining allograft and autograft ACLR in pediatric/adolescent patients. Two university librarians (Brown University, Health Sciences Library) each conducted independent searches of both PubMed (which includes the MEDLINE databases) and Embase databases utilizing search-term combinations described in the Appendix. No time limit ranges were placed on the searches conducted on July 31, 2019, and August 1, 2019.
Eligibility Criteria
All studies found during the initial search were screened by title and abstract. Manuscripts were included in the full-text review if they described autograft and/or allograft ACLR in pediatric patients. If ≥2 studies described the same patient cohort, the most recently published study was included in the final analysis. Manuscripts were excluded if they primarily described revision ACLR, ACLR + meniscal allograft, primary ACL repair, ACLR + osteotomy, synthetic ACL grafts, nonhuman/animal studies, congenital ACL deficiency, multi-ligamentous knee injury, and imaging studies. Case reports, editorials, nonclinical studies (i.e., basic science, cadaveric, biomechanical), non-English-language articles, technique articles, economic-decision analyses, and meeting abstracts were excluded.
Study Selection
After title/abstract screening, full-text articles were reviewed and included in the final analysis if they met the following criteria: described a cohort of pediatric patients (average age of ≤19 years), had a minimum of 2 years of follow-up, adequately described ACL graft failure as an outcome, and had a Level of Evidence grade of I to III25. The average age of ≤19 years was chosen because this age range should be inclusive of those who are high school-aged and younger, an age group that is traditionally thought of as “pediatric/adolescent.” Bibliographies of systematic reviews and meta-analyses were manually reviewed for articles not captured by the initial search.
Data Extraction
After relevant full-text articles were selected, the study authors met and finalized the criteria for data extraction. Eight authors (A.I.C., J.J.B., M.D.E., S.W.M., A.T.P., Z.S.S., C.D.V., H.B.E.) extracted relevant data from included studies and recorded them into a spreadsheet. After initial data extraction, 2 authors (A.I.C., B.B.) confirmed extracted data points from individual full-text articles prior to final analysis. Study variables recorded included authors, journal, publication year, number of study subjects, sex, average age and range, average follow-up duration and range, graft type (allograft or autograft), and number of failed grafts by type. Hybrid grafts (e.g., autograft + allograft augmentation) were classified as allografts for the purposes of meta-analysis (since only 1 category could be chosen for proper analysis). Because of inconsistent reporting, graft diameter was not analyzed. Graft failure was defined as graft rupture/failure explicitly described in each individual study on the basis of clinical or radiographic criteria or a return to the operating room for failed ACLR. All included studies were assimilated in our qualitative analysis21,26-38. All studies included in the qualitative analysis that adequately reported data for our random-effects model were included in our quantitative meta-analysis. These studies presented data on both ACL autograft and allograft reconstruction within the same study cohort21,28,31,35,38. Although Razi et al.26 reported on both autograft and allograft in the same cohort, this study was not included in the meta-analysis because there were 0 events of interest (e.g., graft failure) in either group.
Risk-of-Bias Assessment
The Newcastle-Ottawa Scale (NOS) for assessing quality in nonrandomized studies was used to evaluate the risk of bias within each study39. The NOS is an instrument used to assess the quality of eligible prospective and retrospective cohort studies. The NOS assesses study quality within 3 domains: selection, comparability, and outcome. According to NOS guidelines, stars (0 to 9) were awarded to each study on the basis of methodological quality. Studies with quality scores of 0 to 3 stars, 4 to 6 stars, and 7 to 9 stars were considered to have had high, moderate, and low risk of bias, respectively. Two authors independently assessed study quality (A.I.C., B.B.); any disagreements were resolved by consensus discussion.
Statistical Methods
A random-effects model was created to compare failure events among patients receiving allograft versus autograft in a pairwise fashion. Our model used an inverse variance approach. A random-effects model instead of a fixed-effects model was chosen because there were likely enough underlying differences between study populations (e.g., surgeon characteristics, follow-up duration, postoperative protocols) that it would not be accurate to assume that there was no heterogeneity in the estimates of ACL failure rates in pooled studies40. Data analysis was completed using RevMan 5.3 software (The Cochrane Collaboration).
Results
The database search identified 1,604 studies. After the removal of duplicates and title/abstract screening, 203 full-text articles were assessed for eligibility. Fourteen studies remained after the application of inclusion and exclusion criteria, and 5 studies were included in the quantitative meta-analysis. Figure 1 illustrates the search strategy.
Fig. 1.
Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) flowchart.
Qualitative Analysis
Individual study characteristics are summarized in Tables I and II. The average age of participants in individual studies ranged from 14 years27 to 19 years38, and the average duration of follow-up ranged from 24 months26 to 240 months34. The average age of the pediatric/adolescent cohort could not be determined in 3 studies29,34,36. Shelbourne et al.29 stratified patients by age as <18, 18 to 25, or >25 years but did not describe the average age for the subgroups. Thompson et al.34 stratified patients as ≤18 or >18 years of age and described the average age of the entire study cohort but did not describe the average age of those ≤18 years of age. Webster et al.36 stratified patients as <20 or ≥20 years of age but did not report the average age in the subgroup <20 years of age. This study was not excluded as we postulated that patients <20 years of age in this subgroup still met our inclusion criteria of ≤19 years of age.
TABLE I.
Study Summary Characteristics
| Study | LOE -Study Type* | No. of Participants | Age† (yr) | % Female | Duration of Follow-up† (mo) | Allograft‡ | Autograft‡ | ||||
| No. | No. Failed | % Failed | No. | No. Failed | % Failed | ||||||
| Razi26 (2019) | II - PC | 31 | 14.8 (ND) | 32% | 24 (ND) | 13 | 0 | 0% | 18 (HS) | 0 | 0% |
| Salem31 (2019) | III - CS | 256 | 18.4 (15-25) | 100% | 43 (30-64) | 18 (hybrid autograft/allograft) | 4 | 22% | 175 (BTB), 63 (HS) | 12 (BTB), 7 (HS) | 7% (BTB), 11% (HS) |
| Salmon32 (2018) | III - CC | 39 | 16 (14-18) | 67% | ND (≥240) | 0 | 0 | NA | 39 (HS) | 15 (HS) | 38% (HS) |
| Webster33 (2016) | III - RC | 316 | 17.2 (11-19) | 37% | 60 (36-120) | 0 | 0 | NA | 316 (HS) | 57 (HS) | 18% (HS) |
| Thompson34 (2016) | II - PC | 29 | ND (13-18) | ND | 240 (ND) | 0 | 0 | NA | 14 (BTB), 15 (HS) | 7§ | 24% |
| Engelman35 (2014) | III - CC | 73 | 15.4 (11-18) | 45% | 41.3 (ND) | 38 | 11 | 29% | 35 (HS) | 4 (HS) | 11% |
| Webster36 (2014) | III - CC | 110 | ND (<20) | ND | 57.6 (≥36) | 0 | 0 | NA | 110 (HS) | 15 (HS) | 14% |
| Mascarenhas37 (2012) | III - CC | 46 | 18 (ND) | 57% | 54 (24-120) | 0 | 0 | NA | 23 (BTB), 23 (HS) | 0 | 0% |
| Ellis et al.21 (2012) | III - RC | 79 | 16 (14-18) | 62% | 50.4 (24-135.6) | 20 | 7 | 35% | 59 (BTB) | 2 (BTB) | 3% |
| Pallis38 (2012) | II - PC | 120 (122 knees) | 19 (18-25) | 25% | ND (24-48) | 16 | 7 | 44% | 61 (BTB), 45 (HS) | 7 (BTB), 6 (HS) | 11% (BTB), 13% (HS) |
| Koizumi27 (2013) | III - RC | 15 | 14 (13-16) | 47% | 38# (25-48) | 0 | 0 | NA | 15 (HS) | 2 (HS) | 13% (HS) |
| Barrett28 (2011) | III - RC | 224 | 17.8 (12-25) | ND | ND (≥24) | 24 | 7 | 29% | 152 (BTB), 48 (HS) | 18 (BTB), 12 (HS) | 12% (BTB), 25% (HS) |
| Shelbourne29 (2009) | II - PC | 528** | ND (14-18) | 50% | ND (≥60) | 0 | 0 | NA | 528 (BTB) | 46 (BTB) | 9% (BTB) |
| Sankar30 (2006) | III - RC | 12 | 15.6 (14-17) | 50% | 64 (30-97) | 12 | 0 | 0% | 0 | 0 | NA |
LOE = level of evidence, PC = prospective cohort, CS = case series, CC = case-control, and RC = retrospective cohort.
The values are given as the mean, with the range in parentheses, unless otherwise specified. ND = no data.
NA = not applicable, BTB = bone-patellar tendon-bone, and HS = hamstring.
No data on graft type.
Median.
Patients ≤18 years of age.
TABLE II.
Summary of Concomitant Meniscal Procedures, Associated Ligament Injuries, and Definition of Graft Failure
| Study | Graft Type* (no. [%]) | Definition of Graft Failure | ||
| Meniscal Repair | Meniscectomy | Associated Ligament Injury | ||
| Razi26 (2019) | Allo, 4 (30.8%) Auto, 6 (33.3%) |
Allo, 2 (15.4%) Auto, 5 (27.8%) |
Allo: LCL, 1 (7.7%) MCL, 0 (0%) Auto: LCL, 0 (0%) MCL, 1 (5.6%) |
Not explicitly defined |
| Salem31 (2019) | BTB, 42 (24%) HS, 29 (46%) |
BTB, 32 (18.3%) HS, 15 (23.8%) |
Not reported | “Graft rupture” not explicitly defined |
| Salmon32 (2018) | HS, 20 (10%)† | HS, 20 (10%)† | Not reported‡ | “Graft rupture” not explicitly defined |
| Webster33 (2016) | Not reported | Not reported | Not reported | “Medical records… were initially checked to identify patients who had sustained a second ACL injury…”; “… structured questions regarding any further injuries to the ACL-reconstructed knee or the contralateral knee” |
| Thompson34 (2016) | HS, 10 (11.1%)† BTB, 7 (7.8%)† |
HS, 9 (10%)† BTB, 6 (6.7%)† |
Not reported‡ | “Graft rupture” not explicitly defined |
| Engelman35 (2014) | Allo, 2 (5.3%) Auto, 4 (11.4%) |
Allo, 24 (63.2%) Auto, 12 (34.3%) |
Not reported | Need for revision ACL surgery and/or MRI (magnetic resonance imaging) confirmation of ACL graft failure |
| Webster36 (2014) | Not reported | HS, 207 (36.9%)† | Not reported | Patient self-report. “[They] answered structured questions regarding any further injuries to the ACL-reconstructed knee or the contralateral knee” |
| Mascarenhas37 (2012) | BTB, 3 (13%) HS, 5 (21.7%) |
BTB, 5 (21.7%) HS, 3 (13%) |
None reported (Grade-III collateral ligament injuries excluded) | Not explicitly defined |
| Ellis21 (2012) | Not reported | Not reported | Not reported | Patient self-report. “Subjects were also asked if they had sustained a reinjury to the respective knee or if they had undergone revision ACL surgery” |
| Pallis38 (2012) | Not reported | Not reported | Not reported | ACL injury verified through MRI and/or diagnostic arthroscopy |
| Koizumi27 (2013) | HS, 4 (26.7%) | HS, 2 (13.3%) | Not reported | “Rerupture” not explicitly defined |
| Barrett28 (2011) | Not reported | Not reported | Associated collateral ligament injuries excluded | Reconstruction failure defined as 2+ Lachman, positive pivot shift, and KT-1000 results >5 mm side-to-side difference |
| Shelbourne29 (2009) | Not reported | Not reported | Not reported | “ACL injury was counted if it had been confirmed by physician examination or if the patient reported the ACL injury on a subjective survey or by phone” |
| Sankar30 (2006) | Not reported | Not reported | Allo: MCL, 12 (100%) | Not explicitly defined |
Allo = allograft, Auto = autograft, LCL = lateral collateral ligament, MCL = medial collateral ligament, BTB = bone-patellar tendon-bone autograft, and HS = hamstring.
Percentages of overall cohort (i.e., inclusive of pediatric and adult-aged subgroups).
Exclusion criteria = “associated ligament injury requiring surgical treatment.”
The sex of participants in individual studies ranged from 25% female38 to 100% female31. One study described allograft ACLR only30, 7 studies described autograft ACLR only27,29,32-34,36,37, and 6 studies described both ACL allograft and autograft reconstruction within the same cohort21,26,28,31,35,38.
Regarding autograft ACLR, bone-patellar tendon-bone (BTB) or hamstring grafts were the only autograft types described in the included studies. BTB grafts represented 58.2% (n = 1,012) of the autografts, and hamstring grafts represented 41.8% (n = 727). One study described hybrid ACL allografts and represented 12.8% (n = 18) of all allograft ACLRs (n = 141). The overall pooled autograft failure rate was 12.1%. The overall pooled failure rates for each graft type was 8.5% for BTB and 16.6% for hamstring (with the study by Thompson et al.34 excluded from the calculation because graft failure was not distinguished between BTB or hamstring grafts). This is compared with a pooled allograft failure rate of 25.5% (26.0% if excluding the hybrid grafts; the hybrid grafts had a failure rate of 22.2% [4 of 18]). It should be noted that the graft failure rates described above are unweighted rates and are presented for descriptive purposes only. Statistical comparisons were limited to our quantitative meta-analysis described below.
Quantitative Analysis
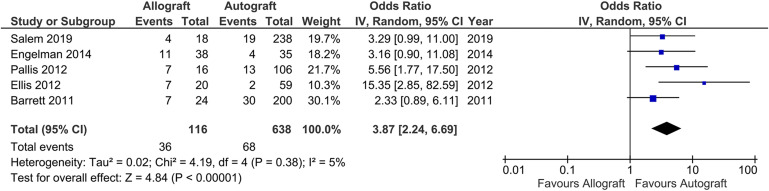
The results of our statistical analysis are summarized in Figure 2. Five studies were included in the quantitative meta-analysis21,28,31,35,38. Allografts were significantly more likely than autografts to result in graft failure (odds ratio [OR], 3.87; 95% confidence interval, 2.24 to 6.69; I2 = 5%; studies = 5).
Fig. 2.
Forest plot examining the 5 studies that were included in the quantitative meta-analysis. The plot shows that allografts were significantly more likely to result in graft failure compared with autografts (odds ratio, 3.87; 95% confidence interval [CI], 2.24 to 6.69). IV = inverse variance, and df = degrees of freedom.
Bias Assessment
Tables III and IV show the NOS bias assessment for the included studies. All 14 studies received 7 to 9 stars (low risk of bias): 6 studies had 9 stars, 6 had 8 stars, and 2 had 7 stars.
TABLE III.
Quality Assessment of the Included Cohort Studies by the Newcastle-Ottawa Scale†
| Study | Selection | Comparability | Outcome | |||||
| Represen-tativeness of Exposed Cohort | Selection of Non-Exposed Cohort | Ascertainment of Exposure | Demonstration That Outcome of Interest Was Not Present at Start of Study | Comparability of Cohort on the Basis of Design or Analysis | Assessment of Outcome | Was Follow-up Long Enough for Outcomes to Occur? | Adequacy of Follow-up of Cohorts | |
| Razi26 (2019) | * | * | * | * | ** | * | * | * |
| Salem31 (2019) | * | * | * | * | * | * | * | * |
| Webster33 (2016) | * | * | * | * | ** | * | * | * |
| Thompson34 (2016) | * | * | * | * | ** | * | * | * |
| Ellis21 (2012) | * | * | * | * | X | * | * | * |
| Pallis38 (2012) | * | * | * | * | * | * | * | * |
| Koizumi27 (2013) | * | * | * | * | * | * | * | * |
| Barrett28 (2011) | * | * | * | * | * | * | * | * |
| Shelbourne29 (2009) | * | * | * | * | ** | * | * | * |
| Sankar30 (2006) | * | * | * | * | X | * | * | * |
Star (*) = item present, and X = item absent. A maximum of 1 star is possible for the Selection and Outcome domains, and a maximum of 2 stars for the Comparability domain.
TABLE IV.
Quality Assessment of the Included Case-Control Studies by the Newcastle-Ottawa Scale†
| Study | Selection | Comparability | Exposure | |||||
| Adequacy of Case Definition | Represen-tativeness of Cases | Selection of Controls | Definition of Controls | Comparability of Cases and Controls on the Basis of the Design or Analysis | Ascertainment of Exposure | Same Method of Ascertainment for Cases and Controls | Non-response Rate | |
| Salmon et al.32 (2018) | * | * | * | * | ** | * | * | X |
| Engelman et al.35 (2014) | * | * | * | * | ** | * | * | X |
| Webster et al.36 (2014) | * | * | * | * | ** | * | * | * |
| Mascarenhas et al.37 (2012) | * | * | * | * | ** | * | * | * |
Star (*) = item present, and X = item absent. A maximum of 1 star is possible for the Selection and Exposure domains, and a maximum of 2 stars for the Comparability domain.
Discussion
The decision between autograft or allograft ACLR is a common topic of discussion between surgeons and patients. The ideal graft would be safe to harvest with minimal morbidity, mimic biomechanical properties of the native ACL, and minimize graft failure rates. Multiple options for grafts are available, each with unique risks and benefits. Allograft tissue has the advantage of less donor-site morbidity and possibly, quicker postoperative recovery. While allograft ACLR has been shown to be relatively safe in adult patients, its use in pediatric patients remains debatable41,42.
With the present systematic review and meta-analysis, we aimed to assess and compare ACL graft failure rates in pediatric/adolescent patients. Among the included studies, we found failure rates of 8.5% for BTB autograft, 16.6% for hamstring autograft, 22.2% for hybrid autograft/allograft, and 25.5% for allograft ACLR. Our meta-analysis found an almost 4-fold increased risk (OR, 3.87) of failure for allograft compared with autograft (BTB/hamstring) ACLR. These findings are consistent with others in the literature. Kaeding et al. examined results of autograft versus allograft ACLR from the MOON (Multicenter Orthopaedic Outcomes Network) cohort, finding the highest risk of graft failure within the age group of 10 to 19 years in those who received allograft reconstruction22. Given these results, the potential benefits of allograft ACLR, compared with autograft reconstruction, in pediatric/adolescent patients are outweighed by the risks of graft failure.
Shanmugaraj et al. also performed a systematic review of allograft use in pediatric patients43. In their review of the literature, the authors found a 7.9% rate of retear following allograft ACLR, nearly double the reported autograft failure rate (4.2%). That study, however, focused only on skeletally immature patients, and as the rates of ACLR have been shown to be highest among older, high school-aged children3,44, this limits the generalizability of their findings to younger patients and not necessarily those most at risk for rerupture as a whole. Shanmugaraj et al. concluded that the reported pooled graft failure rate of 7.9% following allograft ACLR in skeletally immature patients was acceptable43. On the basis of our findings in the current study, however, we caution against concluding that allograft ACLR in those ≤19 years of age has acceptable rates of graft failure compared with autograft. Rather, we conclude that the consistently higher reported rate of allograft failure is unacceptable in this young, active population and would advocate for the limited use of allografts in pediatric/adolescent ACLR.
Systematic reviews examining allograft ACLR in adult populations have found different results than those reported in the current study. Mariscalco et al. performed a systematic review and meta-analysis of autograft versus nonirradiated allograft ACLR in patients with an average age of 24.5 to 32 years41. They found no differences in the graft failure rate, laxity, or patient-reported outcome scores between the 2 groups. Cvetanovich et al. also performed a systematic review and meta-analysis of randomized controlled trials, comparing hamstring autograft and soft-tissue allograft in patients with a mean age of 29.9 years, and found no differences in outcomes42. It is worth noting, however, that the authors of the above studies cautioned against extrapolating their data to younger patient populations.
There was variability in allograft failure rates across the studies evaluated in our investigation, with rates ranging from 0% to 44%. This is likely multifactorial, but the choice of allograft type or allograft sterilization may play a role. Allograft types include all-soft-tissue grafts and soft-tissue-with-bone-block grafts. Our review did not find any studies directly comparing these different allograft types. It has also been shown that irradiated allografts and allografts treated with certain chemical sterilization techniques have poorer outcomes compared with autograft ACLR41,45-47, which may be because of delayed graft incorporation or diminished structural integrity of the graft itself47,48. The studies that reported on allograft use in our analysis inconsistently reported the allograft type, and only 1 study reported on the specific sterilization process21. Because of this, we were unable to elucidate the influence of specific allograft type or sterilization technique on graft failure rates.
Failure rates after ACLR among pediatric/adolescent patients, in general, are higher than those reported for adults, with studies of pediatric ACLR outcomes noting failure rates of 6% to 25%9,17,27,49-54, whereas failure rates of 1% to 8% have generally been found in studies in the adult literature53,55-60. Potential reasons for the higher failure rates among pediatric patients include a return to higher baseline levels of activity, continued musculoskeletal and neuromuscular development, and poorer adherence to activity restrictions during the postoperative rehabilitation period. Given the inherent differences between adult and pediatric/adolescent ACLR outcomes, it is important to distinguish these 2 populations when consolidating and critically evaluating the literature. ACLR in pediatric/adolescent patients should aim to reduce the risk of graft failure in this already vulnerable population, and we believe that this includes avoiding the use of allograft reconstruction, if possible.
Limitations
Limitations of this study include the inherent limitations of systematic reviews and meta-analyses, including the relative paucity of high-quality, nonbiased literature and detailed description of the data reported. We evaluated the included studies with a validated bias-assessment tool39 and found a low risk of bias. Despite the large number of pediatric ACLRs performed each year2, there were limited data meeting our inclusion criteria for final analysis. We included only Level-III or higher evidence in an attempt to improve data quality; however, there were only 4 Level-II studies and no Level-I studies available for inclusion. We pooled allograft data in our analysis because of the variability of allograft types used in individual studies. We therefore cannot comment on variations between allograft types or sterilization techniques, or whether certain allografts could be more desirable in pediatric ACLR. The effect on graft failure rates of other variables, such as surgical technique, graft preparation technique, fixation type, postoperative rehabilitation protocols, duration of follow-up, and return to sport evaluations/timing, could not be analyzed given the variable reporting of these data. Additionally, no weighting or adjustment was performed for autograft type (BTB versus hamstring) in our meta-analysis. These are all important considerations when evaluating the risk of ACLR failure, and authors of primary research studies should strive to consistently report these variables. Additionally, we did not specifically examine other clinical outcomes, such as postoperative radiographic changes or patient-reported outcome measures, and therefore cannot comment on these outcomes. Finally, we were required to rely on the age data presented within each study’s methods section, but we were unable to reliably pool age data given heterogeneous reporting. We could not, therefore, stratify risk of graft failure on the basis of age within this younger population. Additionally, because we included studies with an average patient age of ≤19 years, there may have been some individual patients who were >19 years of age28,31,38. This could have biased the results toward a decreased risk of rerupture in those studies.
Conclusions
Existing studies on ACLR in pediatric/adolescent patients revealed a significantly higher failure rate after allograft reconstruction compared with autograft reconstruction. Allograft ACLR in pediatric/adolescent patients should therefore be used judiciously. Additional studies are needed to improve the understanding of variables associated with the high ACLR failure rate in pediatric/adolescent patients.
Appendix
Supporting material provided by the authors is posted with the online version of this article as a data supplement at jbjs.org (http://links.lww.com/JBJSOA/A239).
Acknowledgments
Note: The authors thank Erin Anthony and Gaelan Adam, Brown University, Health Sciences Library, for their assistance in this project.
Footnotes
Investigation performed at the Department of Orthopaedic Surgery, Warren Alpert Medical School of Brown University, Providence, Rhode Island
Disclosure: The authors indicated that no external funding was received for any aspect of this work. On the Disclosure of Potential Conflicts of Interest forms, which are provided with the online version of the article, one or more of the authors checked “yes” to indicate that the author had a relevant financial relationship in the biomedical arena outside the submitted work (http://links.lww.com/JBJSOA/A238).
References
- 1.Buller LT, Best MJ, Baraga MG, Kaplan LD. Trends in anterior cruciate ligament reconstruction in the United States. Orthop J Sports Med. 2014. Dec 26;3(1):2325967114563664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tepolt FA, Feldman L, Kocher MS. Trends in pediatric ACL reconstruction from the PHIS database. J Pediatr Orthop. 2018. Oct;38(9):e490-4. [DOI] [PubMed] [Google Scholar]
- 3.Dodwell ER, Lamont LE, Green DW, Pan TJ, Marx RG, Lyman S. 20 years of pediatric anterior cruciate ligament reconstruction in New York State. Am J Sports Med. 2014. Mar;42(3):675-80. Epub 2014 Jan 29. [DOI] [PubMed] [Google Scholar]
- 4.Jayanthi NA, LaBella CR, Fischer D, Pasulka J, Dugas LR. Sports-specialized intensive training and the risk of injury in young athletes: a clinical case-control study. Am J Sports Med. 2015. Apr;43(4):794-801. Epub 2015 Feb 2. [DOI] [PubMed] [Google Scholar]
- 5.Frank JS, Gambacorta PL. Anterior cruciate ligament injuries in the skeletally immature athlete: diagnosis and management. J Am Acad Orthop Surg. 2013. Feb;21(2):78-87. [DOI] [PubMed] [Google Scholar]
- 6.Lawrence JTR, Argawal N, Ganley TJ. Degeneration of the knee joint in skeletally immature patients with a diagnosis of an anterior cruciate ligament tear: is there harm in delay of treatment? Am J Sports Med. 2011. Dec;39(12):2582-7. Epub 2011 Sep 14. [DOI] [PubMed] [Google Scholar]
- 7.McCarroll JR, Rettig AC, Shelbourne KD. Anterior cruciate ligament injuries in the young athlete with open physes. Am J Sports Med. 1988. Jan-Feb;16(1):44-7. [DOI] [PubMed] [Google Scholar]
- 8.Dumont GD, Hogue GD, Padalecki JR, Okoro N, Wilson PL. Meniscal and chondral injuries associated with pediatric anterior cruciate ligament tears: relationship of treatment time and patient-specific factors. Am J Sports Med. 2012. Sep;40(9):2128-33. Epub 2012 Jun 22. [DOI] [PubMed] [Google Scholar]
- 9.Anderson AF, Anderson CN. Correlation of meniscal and articular cartilage injuries in children and adolescents with timing of anterior cruciate ligament reconstruction. Am J Sports Med. 2015. Feb;43(2):275-81. Epub 2014 Dec 12. [DOI] [PubMed] [Google Scholar]
- 10.Ekås GR, Moksnes H, Grindem H, Risberg MA, Engebretsen L. Coping with anterior cruciate ligament injury from childhood to maturation: a prospective case series of 44 patients with mean 8 years’ follow-up. Am J Sports Med. 2019. Jan;47(1):22-30. Epub 2018 Nov 26. [DOI] [PubMed] [Google Scholar]
- 11.Crawford EA, Young LJ, Bedi A, Wojtys EM. The effects of delays in diagnosis and surgical reconstruction of ACL tears in skeletally immature individuals on subsequent meniscal and chondral injury. J Pediatr Orthop. 2019. Feb;39(2):55-8. [DOI] [PubMed] [Google Scholar]
- 12.Ramski DE, Kanj WW, Franklin CC, Baldwin KD, Ganley TJ. Anterior cruciate ligament tears in children and adolescents: a meta-analysis of nonoperative versus operative treatment. Am J Sports Med. 2014. Nov;42(11):2769-76. Epub 2013 Dec 4. [DOI] [PubMed] [Google Scholar]
- 13.Wong SE, Feeley BT, Pandya NK. Complications after pediatric ACL reconstruction: a meta-analysis. J Pediatr Orthop. 2019. Sep;39(8):e566-71. [DOI] [PubMed] [Google Scholar]
- 14.Cruz AI, Jr, Fabricant PD, McGraw M, Rozell JC, Ganley TJ, Wells L. All-epiphyseal ACL reconstruction in children: review of safety and early complications. J Pediatr Orthop. 2017. Apr/May;37(3):204-9. [DOI] [PubMed] [Google Scholar]
- 15.Kocher MS, Heyworth BE, Fabricant PD, Tepolt FA, Micheli LJ. Outcomes of physeal-sparing ACL reconstruction with iliotibial band autograft in skeletally immature prepubescent children. J Bone Joint Surg Am. 2018. Jul 5;100(13):1087-94. [DOI] [PubMed] [Google Scholar]
- 16.Cordasco FA, Black SR, Price M, Wixted C, Heller M, Asaro LA, Nguyen J, Green DW. Return to sport and reoperation rates in patients under the age of 20 after primary anterior cruciate ligament reconstruction: risk profile comparing 3 patient groups predicated upon skeletal age. Am J Sports Med. 2019. Mar;47(3):628-39. Epub 2019 Jan 15. [DOI] [PubMed] [Google Scholar]
- 17.Dekker TJ, Godin JA, Dale KM, Garrett WE, Taylor DC, Riboh JC. Return to sport after pediatric anterior cruciate ligament reconstruction and its effect on subsequent anterior cruciate ligament injury. J Bone Joint Surg Am. 2017. Jun 7;99(11):897-904. [DOI] [PubMed] [Google Scholar]
- 18.Dekker TJ, Rush JK, Schmitz MR. What’s new in pediatric and adolescent anterior cruciate ligament injuries? J Pediatr Orthop. 2018. Mar;38(3):185-92. [DOI] [PubMed] [Google Scholar]
- 19.King E, Richter C, Jackson M, Franklyn-Miller A, Falvey E, Myer GD, Strike S, Withers D, Moran R. Factors influencing return to play and second anterior cruciate ligament injury rates in level 1 athletes after primary anterior cruciate ligament reconstruction: 2-year follow-up on 1432 reconstructions at a single center. Am J Sports Med. 2020. Mar;48(4):812-24. Epub 2020 Feb 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Webster KE, Feller JA, Kimp AJ, Whitehead TS. Revision anterior cruciate ligament reconstruction outcomes in younger patients: medial meniscal pathology and high rates of return to sport are associated with third ACL injuries. Am J Sports Med. 2018. Apr;46(5):1137-42. Epub 2018 Jan 30. [DOI] [PubMed] [Google Scholar]
- 21.Ellis HB, Matheny LM, Briggs KK, Pennock AT, Steadman JR. Outcomes and revision rate after bone-patellar tendon-bone allograft versus autograft anterior cruciate ligament reconstruction in patients aged 18 years or younger with closed physes. Arthroscopy. 2012. Dec;28(12):1819-25. Epub 2012 Oct 24. [DOI] [PubMed] [Google Scholar]
- 22.Kaeding CC, Aros B, Pedroza A, Pifel E, Amendola A, Andrish JT, Dunn WR, Marx RG, McCarty EC, Parker RD, Wright RW, Spindler KP. Allograft versus autograft anterior cruciate ligament reconstruction: predictors of failure from a MOON prospective longitudinal cohort. Sports Health. 2011. Jan;3(1):73-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gao B, Dwivedi S, Milewski MD, Cruz AI, Jr. Lack of sleep and sports injuries in adolescents: a systematic review and meta-analysis. J Pediatr Orthop. 2019. May/Jun;39(5):e324-33. [DOI] [PubMed] [Google Scholar]
- 24.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009. Jul 21;339:b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Durieux N, Pasleau F, Howick J; OCEBM Levels of Evidence Working Group. The Oxford 2011 levels of evidence. Oxford Centre for Evidence-Based Medicine. 2012. Accessed 2020 Oct 16. https://www.cebm.ox.ac.uk/resources/levels-of-evidence/ocebm-levels-of-evidence [Google Scholar]
- 26.Razi M, Moradi A, Safarcherati A, Askari A, Arasteh P, Ziabari EZ, Dadgostar H. Allograft or autograft in skeletally immature anterior cruciate ligament reconstruction: a prospective evaluation using both partial and complete transphyseal techniques. J Orthop Surg Res. 2019. Mar 21;14(1):85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Koizumi H, Kimura M, Kamimura T, Hagiwara K, Takagishi K. The outcomes after anterior cruciate ligament reconstruction in adolescents with open physes. Knee Surg Sports Traumatol Arthrosc. 2013. Apr;21(4):950-6. Epub 2012 May 16. [DOI] [PubMed] [Google Scholar]
- 28.Barrett AM, Craft JA, Replogle WH, Hydrick JM, Barrett GR. Anterior cruciate ligament graft failure: a comparison of graft type based on age and Tegner activity level. Am J Sports Med. 2011. Oct;39(10):2194-8. Epub 2011 Jul 22. [DOI] [PubMed] [Google Scholar]
- 29.Shelbourne KD, Gray T, Haro M. Incidence of subsequent injury to either knee within 5 years after anterior cruciate ligament reconstruction with patellar tendon autograft. Am J Sports Med. 2009. Feb;37(2):246-51. Epub 2008 Dec 24. [DOI] [PubMed] [Google Scholar]
- 30.Sankar WN, Wells L, Sennett BJ, Wiesel BB, Ganley TJ. Combined anterior cruciate ligament and medial collateral ligament injuries in adolescents. J Pediatr Orthop. 2006. Nov-Dec;26(6):733-6. [DOI] [PubMed] [Google Scholar]
- 31.Salem HS, Varzhapetyan V, Patel N, Dodson CC, Tjoumakaris FP, Freedman KB. Anterior cruciate ligament reconstruction in young female athletes: patellar versus hamstring tendon autografts. Am J Sports Med. 2019. Jul;47(9):2086-92. Epub 2019 Jun 24. [DOI] [PubMed] [Google Scholar]
- 32.Salmon LJ, Heath E, Akrawi H, Roe JP, Linklater J, Pinczewski LA. 20-year outcomes of anterior cruciate ligament reconstruction with hamstring tendon autograft: the catastrophic effect of age and posterior tibial slope. Am J Sports Med. 2018. Mar;46(3):531-43. Epub 2017 Dec 15. [DOI] [PubMed] [Google Scholar]
- 33.Webster KE, Feller JA. Exploring the high reinjury rate in younger patients undergoing anterior cruciate ligament reconstruction. Am J Sports Med. 2016. Nov;44(11):2827-32. Epub 2016 Jul 7. [DOI] [PubMed] [Google Scholar]
- 34.Thompson SM, Salmon LJ, Waller A, Linklater J, Roe JP, Pinczewski LA. Twenty-year outcome of a longitudinal prospective evaluation of isolated endoscopic anterior cruciate ligament reconstruction with patellar tendon or hamstring autograft. Am J Sports Med. 2016. Dec;44(12):3083-94. Epub 2016 Aug 4. [DOI] [PubMed] [Google Scholar]
- 35.Engelman GH, Carry PM, Hitt KG, Polousky JD, Vidal AF. Comparison of allograft versus autograft anterior cruciate ligament reconstruction graft survival in an active adolescent cohort. Am J Sports Med. 2014. Oct;42(10):2311-8. Epub 2014 Jul 31. [DOI] [PubMed] [Google Scholar]
- 36.Webster KE, Feller JA, Leigh WB, Richmond AK. Younger patients are at increased risk for graft rupture and contralateral injury after anterior cruciate ligament reconstruction. Am J Sports Med. 2014. Mar;42(3):641-7. Epub 2014 Jan 22. [DOI] [PubMed] [Google Scholar]
- 37.Mascarenhas R, Tranovich MJ, Kropf EJ, Fu FH, Harner CD. Bone-patellar tendon-bone autograft versus hamstring autograft anterior cruciate ligament reconstruction in the young athlete: a retrospective matched analysis with 2-10 year follow-up. Knee Surg Sports Traumatol Arthrosc. 2012. Aug;20(8):1520-7. Epub 2011 Nov 3. [DOI] [PubMed] [Google Scholar]
- 38.Pallis M, Svoboda SJ, Cameron KL, Owens BD. Survival comparison of allograft and autograft anterior cruciate ligament reconstruction at the United States Military Academy. Am J Sports Med. 2012. Jun;40(6):1242-6. Epub 2012 Apr 24. [DOI] [PubMed] [Google Scholar]
- 39.Wells GA, Shea B, O’Connell D, Peterson J, Welch V, Losos M, Tugwell P. The Newcastle-Ottawa Scale (NOS) for assessing the quality if nonrandomized studies in meta-analyses. 2012. Accessed 2020 Oct 16. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp [Google Scholar]
- 40.Riley RD, Higgins JPT, Deeks JJ. Interpretation of random effects meta-analyses. BMJ. 2011. Feb 10;342:d549. [DOI] [PubMed] [Google Scholar]
- 41.Mariscalco MW, Magnussen RA, Mehta D, Hewett TE, Flanigan DC, Kaeding CC. Autograft versus nonirradiated allograft tissue for anterior cruciate ligament reconstruction: a systematic review. Am J Sports Med. 2014. Feb;42(2):492-9. Epub 2013 Aug 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cvetanovich GL, Mascarenhas R, Saccomanno MF, Verma NN, Cole BJ, Bush-Joseph CA, Bach BR. Hamstring autograft versus soft-tissue allograft in anterior cruciate ligament reconstruction: a systematic review and meta-analysis of randomized controlled trials. Arthroscopy. 2014. Dec;30(12):1616-24. Epub 2014 Aug 6. [DOI] [PubMed] [Google Scholar]
- 43.Shanmugaraj A, de Sa D, Skelly MM, Duong A, Simunovic N, Musahl V, Peterson DC, Ayeni OR. Primary allograft ACL reconstruction in skeletally immature patients-a systematic review of surgical techniques, outcomes, and complications. J Knee Surg. 2019. Jul;32(7):673-85. Epub 2018 Jul 10. [DOI] [PubMed] [Google Scholar]
- 44.Beck NA, Lawrence JTR, Nordin JD, DeFor TA, Tompkins M. ACL tears in school-aged children and adolescents over 20 years. Pediatrics. 2017. Mar;139(3):e20161877. [DOI] [PubMed] [Google Scholar]
- 45.Zeng C, Gao SG, Li H, Yang T, Luo W, Li YS, Lei GH. Autograft versus allograft in anterior cruciate ligament reconstruction: a meta-analysis of randomized controlled trials and systematic review of overlapping systematic reviews. Arthroscopy. 2016. Jan;32(1):153-63.e18. Epub 2015 Oct 21. [DOI] [PubMed] [Google Scholar]
- 46.Tian S, Wang B, Liu L, Wang Y, Ha C, Li Q, Yang X, Sun K. Irradiated hamstring tendon allograft versus autograft for anatomic double-bundle anterior cruciate ligament reconstruction: midterm clinical outcomes. Am J Sports Med. 2016. Oct;44(10):2579-88. Epub 2016 Jul 27. [DOI] [PubMed] [Google Scholar]
- 47.Lansdown DA, Riff AJ, Meadows M, Yanke AB, Bach BR, Jr. What factors influence the biomechanical properties of allograft tissue for ACL reconstruction? A systematic review. Clin Orthop Relat Res. 2017. Oct;475(10):2412-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Malinin TI, Levitt RL, Bashore C, Temple HT, Mnaymneh W. A study of retrieved allografts used to replace anterior cruciate ligaments. Arthroscopy. 2002. Feb;18(2):163-70. [DOI] [PubMed] [Google Scholar]
- 49.Aichroth PM, Patel DV, Zorrilla P. The natural history and treatment of rupture of the anterior cruciate ligament in children and adolescents. A prospective review. J Bone Joint Surg Br. 2002. Jan;84(1):38-41. [DOI] [PubMed] [Google Scholar]
- 50.Magnussen RA, Meschbach NT, Kaeding CC, Wright RW, Spindler KP. ACL graft and contralateral ACL tear risk within ten years following reconstruction: a systematic review. JBJS Rev. 2015. Jan 20;3(1):01874474-201501000-00002. [DOI] [PubMed] [Google Scholar]
- 51.Nelson IR, Chen J, Love R, Davis BR, Maletis GB, Funahashi TT. A comparison of revision and rerupture rates of ACL reconstruction between autografts and allografts in the skeletally immature. Knee Surg Sports Traumatol Arthrosc. 2016. Mar;24(3):773-9. Epub 2016 Feb 9. [DOI] [PubMed] [Google Scholar]
- 52.Peterson DC, Ayeni OR. Pediatric anterior cruciate ligament reconstruction outcomes. Curr Rev Musculoskelet Med. 2016. Dec;9(4):339-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sanders TL, Pareek A, Hewett TE, Levy BA, Dahm DL, Stuart MJ, Krych AJ. Long-term rate of graft failure after ACL reconstruction: a geographic population cohort analysis. Knee Surg Sports Traumatol Arthrosc. 2017. Jan;25(1):222-8. Epub 2016 Aug 13. [DOI] [PubMed] [Google Scholar]
- 54.Kay J, Memon M, Marx RG, Peterson D, Simunovic N, Ayeni OR. Over 90 % of children and adolescents return to sport after anterior cruciate ligament reconstruction: a systematic review and meta-analysis. Knee Surg Sports Traumatol Arthrosc. 2018. Apr;26(4):1019-36. Epub 2018 Jan 13. [DOI] [PubMed] [Google Scholar]
- 55.Kaeding CC, Pedroza AD, Reinke EK, Huston LJ, Hewett TE, Flanigan DC, Spindler KP; MOON Knee Group. Change in anterior cruciate ligament graft choice and outcomes over time. Arthroscopy. 2017. Nov;33(11):2007-14. Epub 2017 Aug 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wright RW Dunn WR Amendola A Andrish JT Flanigan DC Jones M Kaeding CC Marx RG Matava MJ McCarty EC Parker RD Vidal A Wolcott M Wolf BR Spindler KP; MOON Cohort. Anterior cruciate ligament revision reconstruction: two-year results from the MOON cohort. J Knee Surg. 2007. Oct;20(4):308-11. [DOI] [PubMed] [Google Scholar]
- 57.Spindler KP, Wright RW. Clinical practice. Anterior cruciate ligament tear. N Engl J Med. 2008. Nov 13;359(20):2135-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wright RW, Dunn WR, Amendola A, Andrish JT, Bergfeld J, Kaeding CC, Marx RG, McCarty EC, Parker RD, Wolcott M, Wolf BR, Spindler KP. Risk of tearing the intact anterior cruciate ligament in the contralateral knee and rupturing the anterior cruciate ligament graft during the first 2 years after anterior cruciate ligament reconstruction: a prospective MOON cohort study. Am J Sports Med. 2007. Jul;35(7):1131-4. Epub 2007 Apr 23. [DOI] [PubMed] [Google Scholar]
- 59.Wright RW, Magnussen RA, Dunn WR, Spindler KP. Ipsilateral graft and contralateral ACL rupture at five years or more following ACL reconstruction: a systematic review. J Bone Joint Surg Am. 2011. Jun 15;93(12):1159-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Maletis GB, Chen J, Inacio MCS, Love RM, Funahashi TT. Increased risk of revision after anterior cruciate ligament reconstruction with soft tissue allografts compared with autografts: graft processing and time make a difference. Am J Sports Med. 2017. Jul;45(8):1837-44. Epub 2017 Mar 16. [DOI] [PubMed] [Google Scholar]