Abstract
Background
Onchocerciasis (“river blindness”) can cause severe morbidity, including vision loss and various skin manifestations, and is targeted for elimination using ivermectin mass drug administration (MDA). We calculated the number of people with Onchocerca volvulus infection and onchocercal skin and eye disease as well as disability-adjusted life years (DALYs) lost from 1990 through to 2030 in areas formerly covered by the African Programme for Onchocerciasis Control.
Methods
Per MDA implementation unit, we collated data on the pre-control distribution of microfilariae (mf) prevalence and the history of control. Next, we predicted trends in infection and morbidity over time using the ONCHOSIM simulation model. DALY estimates were calculated using disability weights from the Global Burden of Disease Study.
Results
In 1990, prior to MDA implementation, the total population at risk was 79.8 million with 26.0 million (32.5%) mf-positive individuals, of whom 17.5 million (21.9%) had some form of onchocercal skin or eye disease (2.5 million DALYs lost). By 2030, the total population was predicted to increase to 236.1 million, while the number of mf-positive cases (about 6.8 million, 2.9%), people with skin or eye morbidity (4.2 million, 1.8%), and DALYs lost (0.7 million) were predicted to decline.
Conclusions
MDA has had a remarkable impact on the onchocerciasis burden in countries previously under the APOC mandate. In the few countries where we predict continued transmission between now and 2030, intensified MDA could be combined with local vector control efforts, or the introduction of new drugs for mopping up residual cases of infection and morbidity.
Author summary
Onchocerciasis, also known as river blindness, is a neglected tropical disease caused by a parasitic worm transmitted through the bite of an infected blackfly. Onchocerciasis is, or used to be, endemic in many West, Central, and East African countries. Mass drug administration (MDA) with ivermectin and vector control have been used to prevent the spread of infection and effectively control onchocerciasis as a public health problem. In Central and East Africa, this was done under the mandate of the African Programme for Onchocerciasis Control (APOC). In 2012, the World Health Organization targeted onchocerciasis for control and elimination. Here, we assess the impact of MDA with ivermectin on the prevalence of onchocercal morbidity in 1990 (pre-control), 2020, and 2030, and calculate the associated burden of disease in terms of disability-adjusted life years (DALYs), a composite measure of life years lost and years lived with disability. By 2030, we expect that 691 thousand DALYs will be lost in countries formerly covered by APOC. This burden is due to onchocercal skin disease in about 3.8 million people and onchocercal eye disease in about 384 thousand people, with most of this burden being concentrated in only a few countries.
Introduction
Human onchocerciasis, also known as river blindness, is a parasitic disease caused by Onchocerca volvulus that is transmitted through the bite of an infected blackfly (genus Simulium). Onchocerciasis has been associated with a high impact on health and socioeconomic status due to blindness and stigmatising scaly, itchy skin manifestations [1]. Before the initiation of mass drug administration (MDA), about 32 million people were estimated to be infected with O. volvulus in countries under the former mandate of the African Programme for Onchocerciasis Control (APOC), which ran from 1995 till 2015 [2]. Another 7.6 million people were estimated to be infected in areas previously under the mandate of the Onchocerciasis Control Programme (OCP, 1974–2002) in West-Africa, prior to the initiation of interventions [3]. In 2019, an estimated 217.2 million people in 30 countries were in need of preventive therapy for onchocerciasis [4], most of whom live in sub-Saharan Africa. Thanks to long-term and large-scale onchocerciasis interventions in Africa, 1.2 million people no longer require MDA [4] and several other regions may be close to elimination [5].
Onchocerciasis is now targeted for elimination [6], and great progress has been achieved in reducing O. volvulus infection to low levels with MDA [5]. However, with the current focus being mostly on infection levels and interruption of transmission, relatively little attention has been paid to the expected impact of MDA on the prevalence of clinical manifestations and overall disease burden. This is relevant because there are likely to be residual infections and disease burden even after elimination targets are met or by the time they should be met. In areas where the targets are met in time and MDA is scaled down, some form of clinical treatment may be needed to address residual cases of infection and disease (e.g. macrofilaricidal drugs or drugs inducing permanent sterilisation of adult female worms). For areas where MDA was implemented relatively recently or less successfully, additional interventions may be needed to speed up progress towards elimination, e.g. intensified MDA efforts, use of moxidectin (longer suppression of skin mf), additional vector control, or new macrofilaricidal or worm-sterilising drug treatments. To understand the potential need for treatments with a new drug to address the residual burden of onchocerciasis, it is necessary to quantify to what extent this burden is affected by MDA with ivermectin. It is important to note that onchocercal morbidity includes a wide range of clinical manifestations, some of which will (partially) persist even after clearance of infection (chronic morbidity, e.g. vision loss and skin depigmentation) and are therefore not, or only marginally, amenable to anti-parasitic treatment.
Here, we estimate the number of infections, number of cases with clinical manifestations, and the disease burden of onchocerciasis in terms of case numbers and disability-adjusted life years (DALYs) lost in 1990, 2020, and 2030 for countries formerly under the APOC mandate. We include a wide spectrum of onchocercal skin (OSD) and eye disease (OED): severe itch, reactive skin disease, skin depigmentation (leopard skin), skin atrophy, hanging groin, subcutaneous onchocercal nodules, and vision loss (visual impairment and blindness). We predict trends over time in the prevalence of infection, OSD, and OED based on data on the pre-control distribution of infection and the history of MDA, and a newly developed version of the mathematical model ONCHOSIM that captures the dynamic association between infection, treatment, and morbidity [7]
Methods
General approach
To estimate the burden of onchocerciasis across countries previously under the APOC mandate, we used published pre-control maps of onchocerciasis prevalence covering all APOC countries. We then coupled the pre-control infection prevalence per APOC project (i.e. implementation unit for MDA) with rural population density data at baseline. We used the mathematical model ONCHOSIM [2,8–10] to predict the prevalence of infection and morbidity over time, taking account of the pre-control distribution of infection levels, bioclimate (forest vs. savanna parasite strain), and history of control (MDA, vector control) of each APOC project. Development of morbidity was modelled using a new version of ONCHOSIM that captures the dynamic association between infection, treatment, and morbidity [7]. DALYs lost due to onchocerciasis were quantified by calculating the number of years lived with disability (YLDs) and years of life lost (YLL) due to blindness-related excess mortality. A detailed description of the methods applied is provided in S1 Text; a high-level overview is provided below.
Pre-control infection level and population size
We used a previously published 1x1 km2 resolution raster map of the pre-control prevalence of onchocercal palpable nodules across 18 former APOC countries (i.e. Angola, Burundi, Cameroon, Central African Republic, Chad, Congo, Democratic Republic of Congo, Equatorial Guinea, Ethiopia, Gabon, Liberia, Malawi, Mozambique, Nigeria, South Sudan, Sudan, Tanzania, Uganda), based on Rapid Epidemiological Mapping of Onchocerciasis (REMO) [11,12]. APOC covered 20 countries in Africa. In Rwanda and Kenya, the prevalence of palpable nodules was virtually zero during REMO surveys [11,12]. As a result, these countries were considered non-endemic for onchocerciasis and we therefore did not include them in our analysis. We converted the pre-control prevalence of palpable nodules in adult males into pre-control microfilariae (mf) prevalence in the population aged ≥5 years, using a published statistical association [13]. We linked each raster cell to an MDA implementation unit, called a “project” from here on, and categorised pixels into six endemicity categories (see S1 Text, Tables A and B). Further, for each project we collated population size estimates from the APOC census database [14,15] and divided the population over the six endemicity levels based on the pixel distribution over the various endemicity levels. See S1 Text, sections 2.1 and 2.2 for more details.
History of control
For each project, we obtained information on the project-specific history of control and expected future treatment scenarios (i.e. MDA start year, achieved coverage, treatment frequency, as well as vector control). S1 Text, section 2.3 provides the relevant details of the data on the history of control per project and assumptions on future MDA scenarios. Briefly, nearly all onchocerciasis hyperendemic and mesoendemic areas had started annual or sometimes bi-annual MDA by 2013 (up to which date we were able to collate information on the history of control [15]). For the few projects that had not yet started MDA by 2013, we consulted the ESPEN Portal [16] to verify the (expected) MDA start year, and adjusted the APOC treatment database where necessary (up to 2017). Hypoendemic areas that are potentially co-endemic for loiasis were assumed not to start MDA before 2025 due to the high risk of serious adverse events related to ivermectin-intake in Loa loa-infected individuals, in line with previous work [14]. We accounted for MDA disruptions due to security issues in countries in civil war (CAR, Liberia, South Sudan) and due to the COVID-19 pandemic. An overview of the history of control used in our analysis can be found in S2 Text.
Mathematical modelling
We predict the prevalence of O. volvulus infection and clinical manifestations per APOC project from 1990 through to 2030, using a newly developed version of the mathematical model ONCHOSIM that captures the dynamic association between infection, treatment, and morbidity [7]. A brief description of ONCHOSIM and its generic morbidity module is provided in S1 Text, sections 3.1 and 3.2. The model was used to predict the mf prevalence in the total population and in individuals aged five years and older, as well as the prevalence of infection with at least one female adult worm in the total population. The model further predicts the prevalence of the following clinical manifestations: severe itch (defined as troublesome itch with insomnia), reactive skin disease (RSD), palpable nodules (which may lead to stigmatisation and shame [17,18]), hanging groin, skin atrophy, depigmentation (two levels of severity: mild and severe), and vision loss (visual impairment and blindness).
Per APOC project (N = 158), we calibrated transmission parameters to reproduce the pre-control mean mf prevalence for each of the six endemicity categories. Next, for each endemicity category in each project, we ran 500 repeated stochastic simulations, accounting for the project-specific MDA history and vector control (S2 Text), assuming that endemicity categories within a project experienced the same history of control. Model predictions were saved by age (0-<2, ≥2-<5, ≥5-<10, ≥10-<20, ≥20-<30, ≥30-<50, ≥50), sex, project, and endemicity category, and were averaged over repeated simulations.
Because ONCHOSIM simulates a single closed community of humans and does not consider mobility of infected humans and flies between multiple communities, it cannot simulate stable hypoendemic infection levels. It is yet unclear which mechanisms contribute the stabilisation of infection across hypoendemic areas, but several hypotheses have been raised, which may not be mutually exclusive: spill-over from adjacent more highly endemic areas, more efficient transmission at low levels through additional density-dependent processes in the blackfly [19], high levels of local individual variation in exposure to blackflies, and/or assortative mixing of small high-risk sub-populations [20]. Rather than explicitly modelling hypoendemic areas making assumptions, we calculated the prevalence of infection and morbidity in hypoendemic areas as a ratio of the model-predicted disease prevalence in mesoendemic areas. We used different ratios for calculating the prevalence of palpable nodules, reversible clinical manifestations (i.e. severe itch and RSD), and irreversible clinical manifestations (i.e. depigmentation, hanging groin, and atrophy) in hypoendemic areas based on a meta-analysis of published data (Fig B in S1 Text) [21,22]. Further information and details are provided in S1 Text, section 3.3.
Burden calculation
The disease burden of onchocerciasis was quantified in terms of DALYs, which are defined as the sum of Years Lived with Disability (YLDs) and Years of Life Lost (YLLs) [23,24]. YLDs were calculated by multiplying the predicted number of prevalent cases with a weight representing the severity level of the condition (disability weight). Disability weights for various subtypes of OSD were defined based on an earlier scheme of disability weights for onchocercal skin morbidity used in the 2010 edition of the Global Burden of Disease (GBD) study [25], as presented in Table C in S1 Text. YLD estimates were corrected for concurrence of multiple types of skin manifestations, using a multiplicative approach [26–29]. This correction was considered important as the mechanics of how some skin manifestations cause a disease burden are similar (e.g. disfigurement) and because, conceptually, the sum of disability weight for concurrent symptoms should not exceed 1.0. A detailed description of how the correction was applied, including a graphical representation, is described in S1 Text, section 4.1. YLLs were calculated for onchocercal blindness only, as blindness is associated with excess mortality [30,31]. S1 Text, section 4.2 provides more details about YLL calculations.
Sensitivity analysis
We assessed the impact of various biological and programmatic assumptions on the estimated number of cases with infection and morbidity by 2030 through univariate sensitivity analyses, including, but not limited to, assumptions about the ratio in morbidity prevalence between hypoendemic and mesoendemic areas, and over-reporting of MDA coverage. Table E in S1 Text provides a full overview of all sensitivity analyses performed.
Results
Number of cases with infection
The total population living in countries previously under the APOC mandate was predicted to increase from 79.8 million in 1990 to 236.1 million people in 2030. Before the initiation of MDA, the prevalence of O. volvulus skin mf-positivity among the population (all ages) was estimated to be 32.5% in 1990 (26.0 million cases), and was predicted to decline to 2.9% by 2030 (6.8 million cases) thanks to MDA, which is a reduction in prevalence of 74%. The prevalence of people infected with at least one adult female worm was predicted to decline from 41.2% in 1990 to 6.6% in 2030, which is a 84% reduction (Table 1). As expected, by 2030, prevalence of mf and worms will be highest in areas that were very hyperendemic before the start of control (Table 2). Further, by 2030 most mf-positive cases will be in the Democratic Republic of Congo (45.4% of all cases), Nigeria (21.6%) and Ethiopia (10.0%). Highest country-level mf prevalence is expected in Gabon (11.9%), where currently no MDA programme is in place due to Loa loa co-endemicity, the Republic of Congo (6.1%), and Mozambique (5.7%) (Table 3). At the project-level, we predict that mf prevalence will be highest in onchocerciasis-hypoendemic areas that are co-endemic for loiasis with an overall mean mf prevalence of 12.7% (4.3 million mf-positive cases among 33.5 million population at risk of infection in P5-projects in 12 (suspected) L. loa-endemic countries) in 2030 (Table W in S3 Text). There are also some hyperendemic foci with high local mf prevalence (>10%) remaining in the Central African Republic, Democratic Republic of Congo, and South Sudan by 2030. See S3 Text for further detailed estimates by year and project, and S4 Text for detailed estimates by APOC-project and country, which also presents country-specific MDA coverages.
Table 1. Population at risk, total number of cases infected and with clinical manifestations, and DALYs lost due to onchocerciasis for 1990, 2020, and 2030.
Absolute numbers (N and DALYs) are presented in thousands.
| 1990 | 2020 | 2030 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| N | % | DALYs | N | % | DALYs | N | % | DALYs | |
| Total population at risk | 79,768 | 100 | - | 180,004 | 100 | - | 236,102 | 100 | - |
| Mf-positive | 25,964 | 32.5 | - | 14,092 | 7.8 | - | 6,813 | 2.9 | - |
| Mf-positive (age 5+) | 25,922 | 32.5 | - | 14,084 | 7.8 | - | 6,812 | 2.9 | - |
| Adult female worm infection | 32,825 | 41.2 | - | 27,073 | 15.2 | - | 15,577 | 6.6 | - |
| Palpable nodules | 7,617 | 9.5 | 81 | 3,810 | 2.1 | 40 | 1,412 | 0.6 | 15 |
| Severe itch | 4,115 | 5.2 | 747 | 1,663 | 0.9 | 302 | 542 | 0.2 | 98 |
| Reactive skin disease | 3,089 | 3.9 | 143 | 845 | 0.5 | 39 | 129 | 0.1 | 6 |
| Total reversible onchocercal skin disease | 7,205 | 9 | 890 | 2,508 | 1.4 | 341 | 670 | 0.3 | 104 |
| Mild depigmentation | 935 | 1.2 | 10 | 1,117 | 0.6 | 12 | 879 | 0.4 | 9 |
| Severe depigmentation | 1,057 | 1.3 | 68 | 1,181 | 0.7 | 76 | 863 | 0.4 | 56 |
| Atrophy | 16 | <0.05 | <0.5 | 3 | <0.05 | <0.5 | <0.5 | <0.05 | <0.5 |
| Hanging groin | 33 | <0.05 | 13 | 27 | <0.05 | 10 | 15 | <0.05 | 6 |
| Total irreversible onchocercal skin disease | 2,041 | 2.6 | 91 | 2,328 | 1.3 | 99 | 1,756 | 0.7 | 71 |
| Total onchocercal skin disease* | 16,862 | 21.1 | 1,062 | 8,647 | 4.8 | 480 | 3,839 | 1.6 | 190 |
| Visual impairment | 421 | 0.5 | 13 | 426 | 0.2 | 13 | 316 | 0.1 | 10 |
| Blindness | 194 | 0.2 | 1,397 | 126 | 0.1 | 903 | 69 | <0.05 | 491 |
| Total onchocercal eye disease | 616 | 0.8 | 1,410 | 552 | 0.3 | 916 | 384 | 0.2 | 501 |
| Total all manifestations | 17,478 | 21.9 | 2,472 | 9,198 | 5.1 | 1,397 | 4,223 | 1.8 | 691 |
* Note: Total onchocercal skin disease is the sum of palpable nodules, reversible and irreversible skin disease.
Table 2. Population at risk and numbers of cases infected and with clinical manifestations by endemicity level for 2030.
Absolute numbers (N and DALYs) are presented in thousands.
| Hypoendemic | Mesoendemic | Hyperendemic | Very hyperendemic | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | % | DALYs | N | % | DALYs | N | % | DALYs | N | % | DALYs | |
| Total population at risk | 145,846 | 100 | - | 67,483 | 100 | - | 18,053 | 100 | - | 4,721 | 100 | - |
| Mf-positive | 4,299 | 2.9 | - | 1,237 | 1.8 | - | 692 | 3.8 | - | 585 | 12.4 | - |
| Mf-positive (age 5+) | 4,299 | 2.9 | - | 1,237 | 1.8 | - | 691 | 3.8 | - | 585 | 12.4 | - |
| Adult female worm infection | 8,735 | 6.0 | - | 3,326 | 4.9 | - | 2,083 | 11.5 | - | 1,432 | 30.3 | - |
| Palpable nodules | 482 | 0.3 | 5 | 419 | 0.6 | 4 | 281 | 1.6 | 3 | 231 | 4.9 | 2 |
| Severe itch | 353 | 0.2 | 64 | 107 | 0.2 | 19 | 56 | 0.3 | 10 | 26 | 0.5 | 5 |
| Reactive skin disease | 89 | 0.1 | 4 | 23 | <0.05 | 1 | 11 | 0.1 | <0.5 | 6 | 0.1 | <0.5 |
| Total reversible onchocercal skin disease | 442 | 0.3 | 68 | 130 | 0.2 | 21 | 67 | 0.4 | 11 | 32 | 0.7 | 5 |
| Mild depigmentation | 366 | 0.3 | 4 | 274 | 0.4 | 3 | 174 | 1 | 2 | 65 | 1.4 | 1 |
| Severe depigmentation | 277 | 0.2 | 18 | 233 | 0.3 | 15 | 238 | 1.3 | 15 | 115 | 2.4 | 7 |
| Atrophy | <0.5 | <0.05 | <0.5 | <0.5 | <0.05 | <0.5 | <0.5 | <0.05 | <0.5 | <0.5 | <0.05 | <0.5 |
| Hanging groin | <0.5 | <0.05 | <0.5 | 1 | <0.05 | <0.5 | 6 | <0.05 | 2 | 8 | 0.2 | 3 |
| Total irreversible onchocercal skin disease | 643 | 0.4 | 22 | 508 | 0.8 | 18 | 418 | 2.3 | 20 | 188 | 4 | 11 |
| Total onchocercal skin disease* | 1,566 | 1.1 | 95 | 1,057 | 1.6 | 43 | 765 | 4.2 | 33 | 451 | 9.5 | 18 |
| Visual impairment | 192 | 0.1 | 6 | 69 | 0.1 | 2 | 38 | 0.2 | 1 | 17 | 0.3 | 1 |
| Blindness | 34 | <0.05 | 227 | 10 | <0.05 | 74 | 14 | 0.1 | 102 | 11 | 0.2 | 87 |
| Total onchocercal eye disease | 226 | 0.2 | 233 | 80 | 0.1 | 77 | 51 | 0.3 | 103 | 27 | 0.6 | 87 |
| Total all manifestations | 1,792 | 1.2 | 329 | 1,137 | 1.7 | 120 | 817 | 4.5 | 137 | 478 | 10.1 | 106 |
* Note: Total onchocercal skin disease is the sum of palpable nodules, reversible and irreversible skin disease.
Table 3. Population at risk and numbers of cases infected and with clinical manifestations by country for 2030.
Absolute numbers (N and DALYs) are presented in thousands.
| Country | Total pop. at risk | Mf infected | Palpable nodules | Reversible skin disease | Irreversible skin disease | Onchocercal eye disease | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | % | N | % | DALYs | N | % | DALYs | N | % | DALYs | N | % | DALYs | ||
| Angola | 3,925 | 101 | 2.6 | 15 | 0.4 | <0.5 | 10 | 0.2 | 2 | 25 | 0.6 | 1 | <0.5 | <0.05 | <0.5 |
| Burundi | 3,944 | 163 | 4.1 | 25 | 0.6 | <0.5 | 17 | 0.4 | 3 | 24 | 0.6 | 1 | <0.5 | <0.05 | <0.5 |
| Cameroon | 15,156 | 426 | 2.8 | 76 | 0.5 | 1 | 46 | 0.3 | 7 | 161 | 1.1 | 7 | 21 | 0.1 | 28 |
| CAR | 3,896 | 62 | 1.6 | 15 | 0.4 | <0.5 | 7 | 0.2 | 1 | 23 | 0.6 | 1 | 20 | 0.5 | 20 |
| Chad | 3,780 | 85 | 2.3 | 18 | 0.5 | <0.5 | 9 | 0.2 | 1 | 15 | 0.4 | <0.5 | 15 | 0.4 | 17 |
| Congo | 2,350 | 144 | 6.1 | 29 | 1.3 | <0.5 | 18 | 0.8 | 3 | 18 | 0.8 | 1 | <0.5 | <0.05 | 1 |
| Democratic Republic of Congo | 68,096 | 3,094 | 4.5 | 795 | 1.2 | 8 | 296 | 0.4 | 46 | 860 | 1.3 | 38 | 35 | 0.1 | 94 |
| Equatorial Guinea | 588 | <0.5 | <0.05 | <0.5 | <0.05 | <0.5 | <0.5 | <0.05 | <0.5 | 3 | 0.5 | <0.5 | <0.5 | <0.05 | <0.5 |
| Ethiopia | 19,911 | 681 | 3.4 | 126 | 0.6 | 1 | 64 | 0.3 | 10 | 163 | 0.8 | 6 | 2 | <0.05 | 4 |
| Gabon | 146 | 17 | 11.9 | 2 | 1.5 | <0.5 | 2 | 1.2 | <0.5 | 1 | 0.9 | <0.5 | <0.5 | <0.05 | <0.5 |
| Liberia | 2,606 | <0.5 | <0.05 | <0.5 | <0.05 | <0.5 | <0.5 | <0.05 | <0.5 | 8 | 0.3 | <0.5 | <0.5 | <0.05 | <0.5 |
| Malawi | 3,469 | <0.5 | <0.05 | <0.5 | <0.05 | <0.5 | <0.5 | <0.05 | <0.5 | 8 | 0.2 | <0.5 | <0.5 | <0.05 | <0.5 |
| Mozambique | 107 | 6 | 5.7 | 1 | 0.6 | <0.5 | 1 | 0.5 | <0.5 | 1 | 0.7 | <0.5 | <0.5 | <0.05 | <0.5 |
| Nigeria | 83,829 | 1,475 | 1.8 | 187 | 0.2 | 2 | 149 | 0.2 | 23 | 298 | 0.4 | 10 | 198 | 0.2 | 207 |
| South Sudan | 11,393 | 323 | 2.8 | 81 | 0.7 | 1 | 28 | 0.2 | 4 | 74 | 0.7 | 3 | 83 | 0.7 | 118 |
| Sudan | 1,083 | 48 | 4.5 | 7 | 0.6 | <0.5 | 5 | 0.5 | 1 | 6 | 0.5 | <0.5 | 7 | 0.6 | 8 |
| Tanzania | 5,444 | 186 | 3.4 | 35 | 0.6 | <0.5 | 19 | 0.3 | 3 | 42 | 0.8 | 2 | 1 | <0.05 | 2 |
| Uganda | 6,379 | <0.5 | <0.05 | <0.5 | <0.05 | <0.5 | <0.5 | <0.05 | <0.5 | 27 | 0.4 | 1 | 1 | <0.05 | 1 |
| Total | 236,102 | 6,813 | 2.9 | 1,412 | 0.6 | 15 | 670 | 0.3 | 104 | 1,756 | 0.7 | 71 | 384 | 0.2 | 501 |
Number of cases with onchocercal morbidity
Prior to MDA implementation (1990), 21.9% of the total population at risk experienced clinical manifestations due to onchocerciasis (17.5 million cases). The most common clinical manifestation was the presence of palpable nodules, followed by acute reversible skin conditions (either severe itch or RSD) (Table 1). The total pre-control prevalence of OED was predicted to be 0.8% (615.5 thousand cases), mostly due to visual impairment in savanna areas (82.3% of all OED cases in 1990; Table G in S3 Text).
By 2030, the total number of cases with onchocercal morbidity was predicted to decline to 4.2 million (1.8% of total population at risk), with palpable nodules and depigmentation contributing most cases (Table 1). Irreversible symptoms of onchocerciasis and, in particular, skin manifestations will be responsible for the majority of the cases: by 2030 there will be 1.8 million cases of irreversible OSD and 384 thousand cases of OED (some cases will have both). In contrast, reversible symptoms only affect 670 thousand people (some of whom will also have irreversible symptoms) by 2030. Cases of OED will be mostly concentrated in savanna areas (87.8% of all OED cases). The highest prevalence of onchocercal morbidity will be in very hyperendemic areas with about 10.1% of the population having clinical manifestations, yet 42.4% of all cases with clinical manifestations due to onchocerciasis were predicted to live in hypoendemic areas where most of the population at risk resides (Table 2). By 2030, the majority of clinical cases will be living in the Democratic Republic of Congo (47.0% of all remaining cases in eastern and central Africa), Nigeria (19.7%), Ethiopia (8.4%), and Cameroon (7.2%) (Table 3). In terms of country-specific morbidity prevalence, Gabon has highest overall morbidity prevalence (3.6%; mainly due to high prevalence of reversible skin disease and assumed absence of MDA), followed by the Democratic Republic of Congo (2.9%), and the Republic of Congo (2.8%), and South Sudan (2.3%). There are also some hyperendemic foci with high overall morbidity prevalence (>7%) remaining in the Democratic Republic of Congo and South Sudan by 2030 (mainly due to palpable nodules). See S3 Text and S4 Text for further detailed estimates by year, project, and country.
DALYs lost due to onchocerciasis
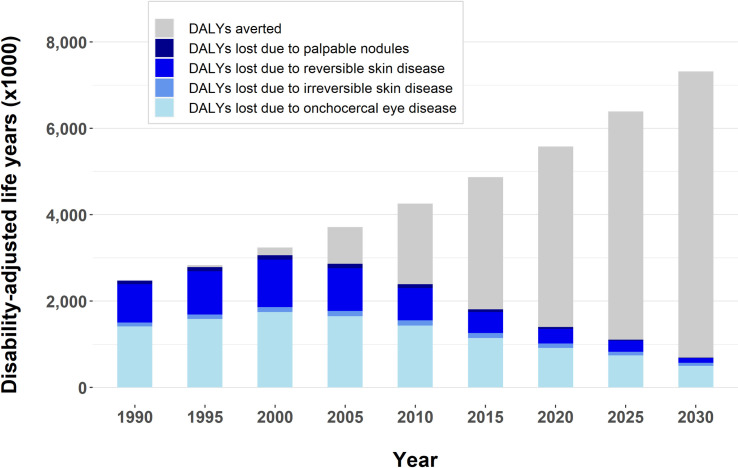
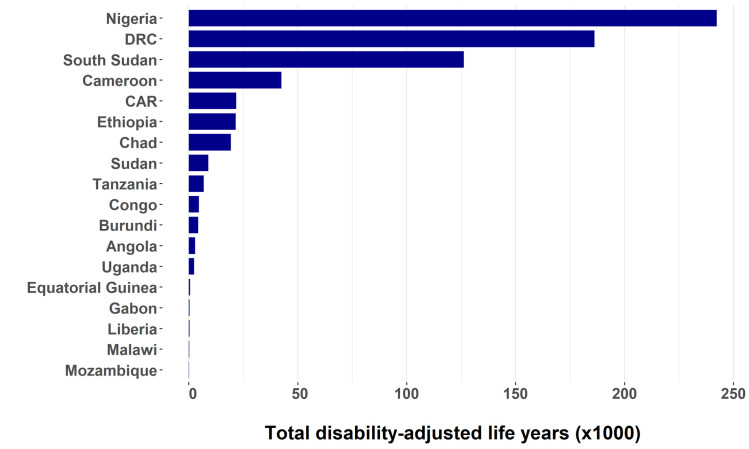
The total number of DALYs lost slightly increased between 1990 and 2000 due to population growth combined with the gradual roll-out of MDA programmes, but from 2000 onwards we predict a steady decline in DALYs lost (Fig 1). We estimate that the total DALYs lost due to onchocerciasis was 2.5 million before MDA started (1990), that this figure declined to 1.4 million in 2020, and will further decline to 691 thousand by 2030. In 1990, most of the onchocercal disease burden was due to blindness (1.4 million DALYs) and severe itch (747 thousand DALYs) (Table 1). By 2030, blindness (491 thousand DALYs) and severe itch (98 thousand DALYs), will account for 71.1% and 14.2% of all DALYs lost, respectively (Table 1). This amounts to a reduction in DALYs lost due to blindness and severe itch of 64.9% and 86.9%, respectively. Nigeria, the Democratic Republic of Congo, and South Sudan will carry the highest disease burden due to onchocerciasis in 2030 (Table 3 and Fig 2), primarily because of the high number of DALYs lost due to blindness in savanna areas and the high numbers of people living in endemic areas in those countries. Overall, thanks to MDA, the annual number of DALYs averted will rise to 6.6 million by 2030 (Fig 1) and around 96.7 million DALYs will have been cumulatively averted between 1990 and 2030.
Fig 1. Total number of disability-adjusted life years (DALYs) lost and averted due to onchocerciasis from 1990 to 2030 in countries formerly covered by the African Programme for Onchocerciasis Control.
DALYs are expressed in thousands (x1000). The total height of the blue part of the bars represents the estimated total number of DALYs lost, with different intensities of blue representing four subcategories of onchocercal morbidity (see legend). The total height of the bars (blue plus grey) represents the DALYs lost in a counterfactual scenario without large-scale MDA. The light grey part of each bar therefore represents the annual number of DALYs averted by MDA.
Fig 2. Total number of disability-adjusted life years (DALYs) lost due to onchocerciasis by country in 2030.
Sensitivity analysis
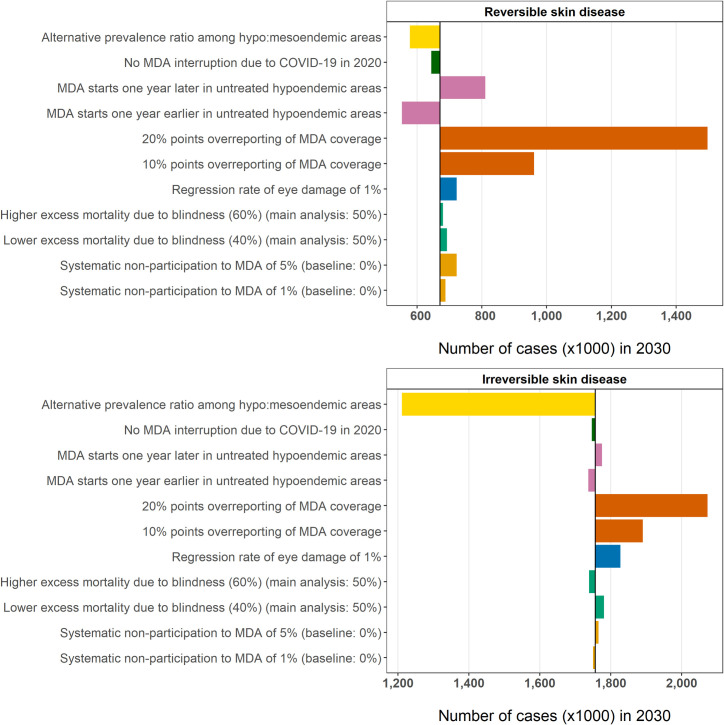
Fig 3 shows the impact of alternative assumptions about the estimated number of cases with reversible and irreversible OSD by 2030. For reversible skin manifestations, the assumptions regarding MDA implementation have the highest impact on case estimates, with up to 826.8 thousand more estimated cases when MDA coverage was systematically reported to be higher (20% points) than the actual distribution. As O. volvulus infection is directly related to the presence of acute, reversible skin conditions, earlier initiation of MDA in untreated areas would reduce case estimates by 117.7 thousand cases. For chronic, irreversible skin manifestations, the effect of systematic over-reporting of MDA coverage would be less pronounced on current case estimates (up to 316.5 thousand more cases if MDA coverage is over-reported by 20% points). Halving the factor with which we calculate morbidity prevalence in hypoendemic as a function of prevalence in mesoendemic areas would considerably influence our case estimates with irreversible subtypes of OSD for 2030 (545.4 thousand fewer cases). As for OSD, the predicted prevalence of skin mf and nodules in 2030 was most sensitive to assumptions about MDA coverage. In contrast, OED estimates for 2030 were only marginally sensitive to MDA coverage but were highly sensitive to assumptions about excess mortality due to blindness and potential reversibility of visual impairment, which led to up to an estimated 7.2% and 93.3% fewer cases of OED respectively by 2030 (S1 Text, section 5). Starting MDA in as yet untreated L. Loa-endemic areas in 2024 instead of 2025, and in untreated non-L. loa-endemic areas in 2022 instead of 2023 would considerably reduce the number of cases with reversible skin manifestations by 2030 (-17.6%), but not the number of cases with irreversible skin morbidity (-1.1%).
Fig 3. Univariate sensitivity analysis for the predicted number of cases with reversible and irreversible onchocercal skin disease by 2030.
Coloured bars represent the difference between the sensitivity analysis and the results of the main analysis (vertical black line). * In the sensitivity analysis, the prevalence of infection and clinical manifestations in hypoendemic areas was assumed to be 1/3 of that in mesoendemic areas (as applied in a previous modelling exercise [2]), instead of ratios based on a more detailed meta-analysis performed for the main analysis (see also Table E in S1 Text).
Discussion
We predict major reductions in the burden of onchocerciasis between 1990 and 2030, both in terms of number of cases as well as in DALYs lost, mainly thanks to the massive impact of MDA with ivermectin. We predict that the annual number of DALYs lost due to onchocerciasis will be more than halved over a 40-year time frame, with around 97 million DALYs cumulatively averted by MDA between 1990 and 2030 in countries formerly covered by APOC. Most of the cases remaining by 2030 will be due to palpable nodules and chronic skin manifestations, and most of the remaining burden will be due to severe itch and blindness. By 2030, almost half of all remaining cases will be located in the Democratic Republic of Congo, where many people live in endemic areas and MDA started relatively late.
The GBD study estimated that the number of DALYs lost due to onchocerciasis was 1.4 million in 1990 and 1.3 million in 2015 [32,33]. The difference with our burden estimates for the same years (2.5 million and 1.8 million DALYs lost in 1990 and 2015, respectively) can be largely explained by the fact that excess mortality due to onchocercal blindness was not considered in the GBD study, while we estimated onchocercal blindness to be responsible for 1.4 million and 1.1 million DALYS lost in 1990 and 2015, respectively. The remaining difference may be explained by a methodological difference: the GBD study relies on statistical models while our study uses a mechanistic mathematical model, which we consider more appropriate for producing forecasts.
We identified areas where we predict high remaining onchocerciasis cases (due to loiasis co-endemicity or very hyperendemic baseline infection levels), as well as areas where onchocerciasis transmission may be close to elimination [5]. Our results are in line with a recent analysis on remaining O. volvulus and L. loa cases by 2025 [14], predicting 19.0% O. volvulus and 5.8% L. loa mf prevalence in these areas. This highlights that particularly hypoendemic areas with loiasis transmission, currently excluded from mass treatment, should rapidly be targeted for onchocerciases elimination mapping and implementation of alternative treatment strategies, such as the Test-and-not-Treat strategy, community-directed vector control (e.g. slash and clear vegetation, ground-based larviciding), or possibly a new macrofilaricidal treatment for onchocerciasis, once available. Intensified interventions could also be considered to bring down infection and morbidity in areas with historically high baseline endemicity levels that are currently still hyperendemic, even after long-term control. We predict that by 2030 the highest remaining prevalence of infection and morbidity is likely to be found in endemic areas of Gabon, Democratic Republic of Congo, and the Republic of Congo. However, the highest burden will be found in countries with the highest number of population at risk or those with the blinding savanna type of onchocerciasis (i.e. Nigeria, DRC, South Sudan, Cameroon). In other countries, the long-standing control interventions in Africa will have reduced infection and morbidity to negligible levels, such as across large areas in Burundi, Equatorial Guinea, and Uganda. For Uganda, these predictions are in line with empirical evidence: 15 out of 17 onchocerciasis-endemic foci in Uganda are currently under post-treatment surveillance or have eliminated onchocerciasis [34,35].
The presented number of cases with infection and morbidity should be considered as indicative only, as the estimates depend on uncertain assumptions regarding baseline endemicity levels, local transmission conditions and programmatic factors. Numbers presented for any hypo-endemic areas left untreated by APOC should be considered with particular caution. The P5 areas include all areas with an estimated nodule prevalence between 5% and 20%, based on a geostatistical analysis of REMO survey data [11]. However, in most areas hypo-endemicity remains to be confirmed in the field through onchocerciasis elimination mapping, although the ESPEN website suggest that elimination mapping may not, or no longer be required in seven former APOC-countries (i.e., Burundi, Chad, Equatorial Guinea, Liberia, Malawi, Tanzania and Uganda). In some of the P5 areas, onchocerciasis infection prevalences may have been reduced thanks to MDA programmes against lymphatic filariasis in loiasis-free areas or the collateral benefits of other drug programmes (e.g., ivermectin against scabies in Ethiopia [36]) may have led to minor overestimations of the aforementioned burden estimates for these areas.
Although we have taken account of most clinical manifestations attributable to O. volvulus infection, there is increasing evidence of an association between high intensity O. volvulus infection and the development of epilepsy during childhood [37–40]. A recent study reported 128 thousand YLDs by 2015 due to onchocerciasis-associated epilepsy in 9 of the 17 countries formerly covered by APOC [41]. This would imply an additional 53.8 thousand DALYs lost due to onchocerciasis (128,000 cases x 0.420 disability weight for uncontrolled epilepsy [25]) by 2015, which is a conservative estimate as it does not capture YLLs caused by excess mortality associated with epilepsy in rural Africa [41]. In addition, MDA with ivermectin will also have a modest impact on off-target diseases like soil-transmitted helminths and scabies [42].
The results presented here are estimates based on the most comprehensive available data from countries formerly covered by APOC. The study was motivated by the extreme paucity of large-scale representative data on actual morbidity levels in (formerly) onchocerciasis-endemic areas. Disease manifestations were modelled using a newly developed disease module within ONCHOSIM that, for the first time, could simultaneously simulate reversible and irreversible clinical manifestations, as well as single- and multi-stage disease, taking into account the impact of excess mortality due to blindness on the trends for prevalence of all these conditions. Model outcomes were externally validated against longitudinal trends in morbidity prevalence, community-level patterns in prevalence of infection and disease manifestations, age-stratified pre-control morbidity prevalence data from savanna communities, and data on the concurrence of clinical manifestations, and it was shown that the model could reasonably well reproduce the morbidity patterns in the field [7]. Also, it is important to note that our analysis builds on estimates of the pre-control distribution of infection levels based on REMO (rapid epidemiological mapping of onchocerciasis) data, which are the most comprehensive and accurate data available, and the basis of the current map of onchocerciasis in the 20 APOC countries. The oldest REMO surveys possibly date from 1993, but many were done more recently. These REMO data provide a good indication of pre-control prevalence in treated areas. The extent of hypoendemic areas is more uncertain; unfortunately, data from recent and ongoing mapping efforts in hypoendemic areas with more sensitive diagnostic tools are not yet published. We further note that we did not capture the impact of potential secular developments, such as climate change, deforestation, economic development, demographic transition, and urbanisation, which may lead or may have led to changes in transmission intensity and lower population densities in endemic areas than assumed here. However, we do not expect this to impact our results with regard to where most of the future onchocercal burden will be. Indeed, the geographical distribution of infection may have changed somewhat over time prior to start of MDA. However, as we aggregated results over a larger geographical area, we expect that this uncertainty does not affect, or only marginally affects, our main findings and conclusions.
We used the mathematical model ONCHOSIM to simulate the impact of ivermectin in treated areas on infection and morbidity according to reported treatment history and future MDA scenarios. ONCHOSIM captures community infection dynamics throughout MDA quite well [2,10,43,44], but relies on adequate input about MDA coverage levels, which may be reported imprecisely or incorrectly [45]. Our sensitivity analysis suggests that a 10%- or 20%-point change (everywhere) in coverage of MDA has a considerable impact on case and burden estimates, highlighting the importance of high-quality MDA coverage data. We based the assumptions of control interventions on information from the APOC treatment database [15] and the ESPEN portal. We might be unaware of acceleration strategies that countries may have implemented in specific implementation units, such as changing MDA frequency from annual to bi-annual [35,46]. Also, apart from (community-based) vector control on the island of Bioko [47–50] and in Uganda [51–53], we did not take account of any other local vector control strategies that may have been implemented in other countries. However, we expect that in general, local interventions other than annual MDA would only have a minimal additional impact on the burden of disease, given the already large effect of annual MDA with ivermectin on prevalence of acute clinical manifestations and incidence of chronic conditions. We have further taken account of major events that may have influenced the continuation of the MDA programme such as civil war in South Sudan, the CAR and Liberia, as well as the COVID-19 pandemic. The WHO recommended in 2020 to suspend all epidemiological surveys and MDA activities for Neglected Tropical Diseases tackled by preventive chemotherapy and transmission control due to the COVID-19 pandemic [54]. A recent modelling paper assessed the impact of MDA disruptions and delays in scaling-up of MDA since 2020, and shows that this affects O. volvulus transmission and infection level in both the short and long term [55].
We used the latest GBD disability weights [23], mapping the clinical manifestations of onchocerciasis to the lay descriptions with corresponding disability weights to estimate DALYs lost due to onchocerciasis. The disability weight for blindness was estimated at 0.187 by the GBD study. Several previous estimates of the blindness disability weight were considerably higher [56]. The difference between earlier and current estimates may be primarily due to the dimensions covered by the disability weight: the GBD disability weights focus only on health loss due to blindness, not capturing other socio-economic consequences of blindness that may occur in Africa (e.g., related to livelihood, employment, accessibility of public resources and infrastructure). Such socio-economic consequences are therefore also not captured in our burden estimates. Quantifying this socio-economic burden was beyond the scope of this work, but it is important to acknowledge that this burden will be high, especially for blindness in the African context [57–59].
Our analysis only covers countries in Central and East Africa formerly covered by APOC, whereas in West Africa the Onchocerciasis Control Programme (OCP) also has been recognised as one of the most successful programmes in the history of development aid [35]. Therefore, a next step is to perform a similar modelling study for countries previously under the OCP mandate to obtain a comprehensive synopsis of health losses due to onchocerciasis in the whole of Africa. A major challenge here will be to collate data on the history of MDA and vector control, which, unlike the case for APOC countries, was not curated centrally but by the individual countries that were part of OCP.
Conclusions
MDA has had a remarkable impact on the onchocerciasis burden in countries previously under the APOC mandate. Yet, by 2030 we still expect over 10 million mf-positive people and almost 20 million individuals harbouring adult worms, with the majority living in only a few countries. If in the future elimination of onchocerciasis is achieved, acute clinical manifestations of onchocerciasis such as reactive skin disease and severe itch will have largely disappeared, although chronic clinical manifestations will linger and only slowly disappear due to demographic turn-over. In the few countries where we predict continued transmission between now and 2030, intensified MDA could be combined with local vector control efforts, or the introduction of new drugs for mopping up residual cases of infection and morbidity.
Supporting information
(PDF)
(PDF)
(PDF)
(PDF)
Acknowledgments
We would like to warmly thank Louise Burrows (DNDi) for editing the final draft of the manuscript. We would like to acknowledge WHO/AFRO for approving the use and publication of the data used in this manuscript. We are grateful to Hans Remme for helping in compiling pixel-level mf prevalence data and stratifying these pixels per APOC project over endemicity levels and coupling to population census data. In addition, we would like to thank Federica Giardina for their statistical advice in the programming of R during the initial modelling and analysis stage.
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
NVSVM, WAS, WvL, BP, and LEC received funding from the United States Agency for International Development (USAID, www.usaid.gov) through the Drugs for Neglected Diseases initiative (DNDi, www.dndi.org) (ref no. AID-OAA-G14-00010). The contents are the responsibility of the authors and do not necessarily reflect the views of USAID or the United States Government. For its overall mission, DNDi receives support from UK aid, UK (www.ukaiddirect.org; MOU 2013-2018 and MOU 2017-2021); Médecins sans Frontières (MSF, www.msf.org; MOU 2014-2018 and MOU 2019-2023); and the Swiss Agency for Development and Cooperation, Switzerland (SDC, www.eda.admin.ch/sdc; contract no. 81017718 and no. 81050394). WAS, SJdV, and LEC gratefully acknowledge funding of the NTD Modelling Consortium by the Bill & Melinda Gates Foundation (www.gatesfoundation.org; grant OPP1184344). LEC further acknowledges funding from the Dutch Research Council (NWO, www.nwo.nl; grant 016.Veni.178.023). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Cox FEG. History of Human Parasitology. Clin Microbiol Rev. 2002;15: 595–595. doi: 10.1128/CMR.15.4.595-612.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Coffeng LE, Stolk WA, Zouré HGM, Veerman JL, Agblewonu KB, Murdoch ME, et al. African Programme for Onchocerciasis Control 1995–2015: model-estimated health impact and cost. PLoS Negl Trop Dis. 2013;7: e2032. doi: 10.1371/journal.pntd.0002032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.O’Hanlon SJ, Slater HC, Cheke RA, Boatin BA, Coffeng LE, Pion SDS, et al. Model-based geostatistical mapping of the prevalence of Onchocerca volvulus in West Africa. PLoS Negl Trop Dis. 2016;10: e0004328. doi: 10.1371/journal.pntd.0004328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.WHO. Elimination of human onchocerciasis: progress report, 2019–2020. Wkly Epidemiol Rec. 2020;45: 545–556. [Google Scholar]
- 5.Tekle A, Zouré H, Noma M, Boussinesq M, Coffeng LE, Stolk WA. Progress towards onchocerciasis elimination in the participating countries of the African Programme for Onchocerciasis Control: epidemiological evaluation results. Infect Dis Poverty. 2016;5. doi: 10.1186/s40249-016-0160-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.World Health Organization. Ending the neglect to attain the Sustainable Development Goals: A road map for neglected tropical diseases 2021–2030. Geneva: World Health Organization. Licence: CC BY-NCSA 3.0 IGO. 2020. [Google Scholar]
- 7.Vinkeles Melchers NVS, Stolk WA, Murdoch ME, Pedrique B, Kloek M, Bakker R, et al. How does onchocerciasis-related skin and eye disease in Africa depend on cumulative exposure to infection and mass treatment? Fairfax KC, editor. PLoS Negl Trop Dis. 2021;15: e0009489. doi: 10.1371/journal.pntd.0009489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Plaisier AP, van Oortmarssen GJ, Remme J, Alley ES, Habbema JD. The risk and dynamics of onchocerciasis recrudescence after cessation of vector control. Bull World Health Organ. 1991;69: 169–78. [PMC free article] [PubMed] [Google Scholar]
- 9.Habbema JD, Plaisier AP, van Oortmarssen GJ, Remme J. Prospective evaluation of onchocerciasis control strategies. Acta Leiden. 1990;59: 387–398. [PubMed] [Google Scholar]
- 10.Stolk WA, Walker M, Coffeng LE, Basáñez M-G, de Vlas SJ. Required duration of mass ivermectin treatment for onchocerciasis elimination in Africa: a comparative modelling analysis. Parasite Vector. 2015;8. doi: 10.1186/s13071-015-1159-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zouré H, Noma M, Tekle AH, Amazigo UV, Diggle PJ, Giorgi E, et al. The geographic distribution of onchocerciasis in the 20 participating countries of the African Programme for Onchocerciasis Control: (2) pre-control endemicity levels and estimated number infected. Parasite Vector. 2014;7. doi: 10.1186/1756-3305-7-326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Noma M, Nwoke BEB, Nutall I, Tambala PA, Enyong P, Namsenmo A, et al. Rapid epidemiological mapping of onchocerciasis (REMO): its application by the African Programme for Onchocerciasis Control (APOC). Ann Trop Med Parasitol. 2002;96 Suppl 1: S29–39. [DOI] [PubMed] [Google Scholar]
- 13.Coffeng LE, Pion SDS, O’Hanlon S, Cousens S, Abiose AO, Fischer PU, et al. Onchocerciasis: the pre-control association between prevalence of palpable nodules and skin microfilariae. PLoS Negl Trop Dis. 2013;7: e2168. doi: 10.1371/journal.pntd.0002168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vinkeles Melchers NVS, Coffeng LE, Boussinesq M, Pedrique B, Pion SDS, Tekle AH, et al. Projected number of people with onchocerciasis-loiasis co-infection in Africa, 1995 to 2025. Clin Infect Dis. 2019;ciz647: 1–9. doi: 10.1093/cid/ciz647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim YE, Remme JHF, Steinmann P, Stolk WA, Roungou J-B, Tediosi F. Control, elimination, and eradication of river blindness: scenarios, timelines, and ivermectin treatment needs in Africa. PLoS Negl Trop Dis. 2015;9: e0003664. doi: 10.1371/journal.pntd.0003664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.ESPEN Portal. Onchocerciasis maps. Expanded Special Project for Elimination of Neglected Tropical Diseases (ESPEN). World Health Organization Regional Office for Africa. Available at: https://espen.afro.who.int/ (Assessed on: 11 May 2021). [Google Scholar]
- 17.Mbanefo EC, Eneanya CI, Nwaorgu OC, Oguoma VM, Otiji MO, Ogolo BA. Onchocerciasis in Anambra State, Southeast Nigeria: clinical and psychological aspects and sustainability of community directed treatment with ivermectin (CDTI). Postgrad Med J. 2010;86: 573–577. doi: 10.1136/pgmj.2010.100248 [DOI] [PubMed] [Google Scholar]
- 18.Amuyunzu-Nyamongo M, Tchounkeu YFL, Oyugi RA, Kabali AT, Okeibunor JC, Manianga C, et al. Drawing and interpreting data: Children’s impressions of onchocerciasis and community-directed treatment with ivermectin (CDTI) in four onchocerciasis endemic countries in Africa. Int J Qual Stud Health Well-being. 2011;6. doi: 10.3402/qhw.v6i2.5918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Basáñez MG, Remme JHF, Alley ES, Bain O, Shelley AJ, Medley GF, et al. Density-dependent processes in the transmission of human onchocerciasis: Relationship between the numbers of microfilariae ingested and successful larval development in the simuliid vector. Parasitology. 1995;110: 409–427. doi: 10.1017/s0031182000064751 [DOI] [PubMed] [Google Scholar]
- 20.de Vos AS, Stolk WA, de Vlas SJ, Coffeng LE. The effect of assortative mixing on stability of low helminth transmission levels and on the impact of mass drug administration: Model explorations for onchocerciasis. PLoS Negl Trop Dis. 2018;12: e0006624. doi: 10.1371/journal.pntd.0006624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Murdoch ME, Murdoch IE, Evans J, Yahaya H, Njepuome N, Cousens S, et al. Pre-control relationship of onchocercal skin disease with onchocercal infection in Guinea Savanna, Northern Nigeria. PLoS Negl Trop Dis. 2017;11: e0005489. doi: 10.1371/journal.pntd.0005489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Murdoch ME, Asuzu MC, Hagan M, Makunde WH, Ngoumou P, Ogbuagu KF, et al. Onchocerciasis: the clinical and epidemiological burden of skin disease in Africa. Ann Trop Med Parasitol. 2002;96: 283–96. doi: 10.1179/000349802125000826 [DOI] [PubMed] [Google Scholar]
- 23.Salomon JA, Haagsma JA, Davis A, Maertens De Noordhout C, Polinder S, Havelaar AH, et al. Disability weights for the Global Burden of Disease 2013 study. Lancet Glob Health. 2015; 3(11): e712–23. doi: 10.1016/S2214-109X(15)00069-8 [DOI] [PubMed] [Google Scholar]
- 24.Bank World. World Development Report 1993: Investing in Health. New York: Oxford University Press. ©World Bank. Available at: https://openknowledge.worldbank.org/handle/10986/5976 (Accessed on: 22 Nov 2019). License: CC BY 3.0 IGO. [Google Scholar]
- 25.Salomon JA, Vos T, Hogan DR, Gagnon M, Naghavi M, Mokdad A, et al. Common values in assessing health outcomes from disease and injury: disability weights measurement study for the Global Burden of Disease Study 2010. Lancet. 2012;380: 2129–43. doi: 10.1016/S0140-6736(12)61680-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mathers CD, Iburg KM, Begg S. Adjusting for dependent comorbidity in the calculation of healthy life expectancy. Popul Health Metr. 2006;4. doi: 10.1186/1478-7954-4-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van Baal PH, Hoeymans N, Hoogenveen RT, de Wit GA, Westert GP. Disability weights for comorbidity and their influence on Health-adjusted Life Expectancy. Popul Health Metr. 2006;4. doi: 10.1186/1478-7954-4-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Flanagan W, McIntosh CN, Le Petit C, Berthelot J-M. Deriving utility scores for co-morbid conditions: a test of the multiplicative model for combining individual condition scores. Popul Health Metr. 2006;4: 13. doi: 10.1186/1478-7954-4-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Haagsma JA, van Beeck EF, Polinder S, Toet H, Panneman M, Bonsel GJ. The effect of comorbidity on health-related quality of life for injury patients in the first year following injury: comparison of three comorbidity adjustment approaches. Popul Health Metr. 2011;9. doi: 10.1186/1478-7954-9-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Prost A, Vaugelade J. Excess mortality among blind persons in the West African savannah zone. Bull World Health Organ. 1981;59: 773–6. [PMC free article] [PubMed] [Google Scholar]
- 31.Pion SDS, Kamgno J, Demanga-Ngangue M, Boussinesq M. Excess mortality associated with blindness in the onchocerciasis focus of the Mbam Valley, Cameroon. Ann Trop Med Parasitol. 2002;96: 181–9. doi: 10.1179/000349802125000718 [DOI] [PubMed] [Google Scholar]
- 32.Hay SI, Abajobir AA, Abate KH, Abbafati C, Abbas KM, Abd-Allah F, et al. Global, regional, and national disability-adjusted life-years (DALYs) for 333 diseases and injuries and healthy life expectancy (HALE) for 195 countries and territories, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. The Lancet. 2017;390: 1260–1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.GHDx. Global Health Data Exchange. GBD results tool. Available at: http://ghdx.healthdata.org/gbd-results-tool (Accessed on: 12 Dec 2019).
- 34.Habomugisha P. Uganda treatments and impact. The Carter Center; River blindness elimination program. Powerpoint presentation 2021. [Google Scholar]
- 35.Katabarwa MN, Lakwo T, Habomugisha P, Unnasch TR, Garms R, Hudson-Davis L, et al. After 70 years of fighting an age-old scourge, onchocerciasis in Uganda, the end is in sight. Int Health. Oxford University Press; 2018. pp. i79–i88. doi: 10.1093/inthealth/ihx044 [DOI] [PubMed] [Google Scholar]
- 36.Enbiale W, Baynie TB, Ayalew A, Gebrehiwot T, Getanew T, Ayal A, et al. “Stopping the itch”: Mass drug administration for scabies outbreak control covered for over nine million people in Ethiopia. J Infect Dev Ctries. 2020; 14(6.1): 28S–35S. doi: 10.3855/jidc.11701 [DOI] [PubMed] [Google Scholar]
- 37.Kaiser C, Pion SDS, Boussinesq M. Case-control studies on the relationship between onchocerciasis and epilepsy: systematic review and meta-analysis. PLoS Negl Trop Dis. 2013;7: e2147. doi: 10.1371/journal.pntd.0002147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pion SDS, Kaiser C, Boutros-Toni F, Cournil A, Taylor MM, Meredith SEO, et al. Epilepsy in onchocerciasis endemic areas: systematic review and meta-analysis of population-based surveys. PLoS Negl Trop Dis. 2009;3: e461. doi: 10.1371/journal.pntd.0000461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mbonye Bo MK, Gumisiriza N, Lawko T, Makumbi I, Idro R, Onen H, et al. Prevalence and annual incidence of nodding syndrome and other forms of epilepsy in onchocerciasis endemic areas in northern Uganda. Abstract 4S64 10th European Conference on Tropical Diseases and International Health 16–20 October 2017, Antwerp, Belgium.
- 40.Levick B, Laudisoit A, Tepage F, Ensoy-Musoro C, Mandro M, Bonareri Osoro C, et al. High prevalence of epilepsy in onchocerciasis endemic regions in the Democratic Republic of the Congo. PLoS Negl Trop Dis. 2017;11: e0005732. doi: 10.1371/journal.pntd.0005732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vinkeles Melchers NVS, Mollenkopf S, Colebunders R, Edlinger M, Coffeng LE, Irani J, et al. Burden of onchocerciasis-associated epilepsy: first estimates and research priorities. Infect Dis Poverty. 2018;7: 101. doi: 10.1186/s40249-018-0481-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Krotneva SP, Coffeng LE, Noma M, Zouré HGM, Bakoné L, Amazigo U V., et al. African Program for Onchocerciasis Control 1995–2010: Impact of annual ivermectin mass treatment on off-target infectious diseases. PLoS Negl Trop Dis. 2015;9: e0004051. doi: 10.1371/journal.pntd.0004051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Plaisier A, van Oortmarssen G, Habbema J, Remme J, Alley E. ONCHOSIM: a model and computer simulation program for the transmission and control of onchocerciasis. Comput Methods Programs Biomed. 1990;31: 43–56. doi: 10.1016/0169-2607(90)90030-d [DOI] [PubMed] [Google Scholar]
- 44.Habbema J, van Oortmarssen G, Plaisier A. The ONCHOSIM model and its use in decision support for river blindness control. Cambridge University Press. 1996; 360–80. [Google Scholar]
- 45.Budge PJ, Sognikin E, Akosa A, Mathieu EM, Deming M. Accuracy of coverage survey recall following an integrated Mass Drug Administration for lymphatic filariasis, schistosomiasis, and soil-transmitted helminthiasis. PLoS Negl Trop Dis. 2016;10: e0004358. doi: 10.1371/journal.pntd.0004358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.National Onchocerciasis Control Program. Ministry of Health. Republic of Uganda. Available at: https://www.health.go.ug/programs/national-onchocerciasis-control-program/ (Accessed on: 11 May 2021).
- 47.Mas J, Ascaso C, Post R, Cheke RA, Meyer R, MacCall P, et al. Oncocercosis and its control in Equatorial Guinea: 1997–2010. Enferm Emerg. 2011;13: S93–S97. [Google Scholar]
- 48.Moya L, Herrador Z, Ta-Tang TH, Rubio JM, Perteguer MJ, Hernandez-González A, et al. Evidence for suppression of onchocerciasis transmission in Bioko Island, Equatorial Guinea. PLoS Negl Trop Dis. 2016;10. doi: 10.1371/journal.pntd.0004829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Herrador Z, Garcia B, Ncogo P, Perteguer MJ, Rubio JM, Rivas E, et al. Interruption of onchocerciasis transmission in Bioko Island: Accelerating the movement from control to elimination in Equatorial Guinea. PLoS Negl Trop Dis. 2018;12: e0006471. doi: 10.1371/journal.pntd.0006471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Traoré S, Wilson MD, Sima A, Barro T, Diallo A, Aké A, et al. The elimination of the onchocerciasis vector from the island of Bioko as a result of larviciding by the WHO African Programme for Onchocerciasis Control. Acta Trop. 2009;111: 211–218. doi: 10.1016/j.actatropica.2009.03.007 [DOI] [PubMed] [Google Scholar]
- 51.Katabarwa MN, Walsh F, Habomugisha P, Lakwo TL, Agunyo S, Oguttu DW, et al. Transmission of onchocerciasis in Wadelai focus of northwestern Uganda has been interrupted and the disease eliminated. J. Parasitol. Res. 2012; 1–7. doi: 10.1155/2012/748540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lakwo TL, Garms R, Rubaale T, Katabarwa M, Walsh F, Habomugisha P, et al. The disappearance of onchocerciasis from the Itwara focus, western Uganda after elimination of the vector Simulium neavei and 19 years of annual ivermectin treatments. Acta Trop. 2013;126: 218–21. doi: 10.1016/j.actatropica.2013.02.016 [DOI] [PubMed] [Google Scholar]
- 53.Jacob BG, Loum D, Lakwo TL, Katholi CR, Habomugisha P, Byamukama E, et al. Community-directed vector control to supplement mass drug distribution for onchocerciasis elimination in the Madi mid-North focus of Northern Uganda. PLoS Negl Trop Dis. 2018;12: e0006702. doi: 10.1371/journal.pntd.0006702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.World Health Organization. COVID-19: WHO Issues Interim Guidance for Implementation of NTD Programmes. Available from: https://www.who.int/neglected_diseases/news/COVID19-WHO-interim-guidance-implementation-NTD-programmes/en/ (Accessed on: 17 May 2021).
- 55.Hamley JID, Blok DJ, Walker M, Milton P, Hopkins AD, Hamill LC, et al. What does the COVID-19 pandemic mean for the next decade of onchocerciasis control and elimination? Trans R Soc Trop Med Hyg. 2021;115: 269–280. doi: 10.1093/trstmh/traa193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Braithwaite T, Taylor H, Bourne R, Keeffe J, Pesudovs K. Does blindness count? Disability weights for vision loss. Clin Exp Ophthalmol. 2017; 45(3): 217–220. doi: 10.1111/ceo.12874 [DOI] [PubMed] [Google Scholar]
- 57.Wagbatsoma VA, Okojie OH. Psychosocial effects of river blindness in a rural community in Nigeria. R Soc Health J. 2004;124: 134–136. [DOI] [PubMed] [Google Scholar]
- 58.Alonso L, Murdoch M, Jofre-Bonet M. Psycho-social and economical evaluation of onchocerciasis: a literature review. Soc Med. 2009;4: 8–31. [Google Scholar]
- 59.Evans TG. Socioeconomic consequences of blinding onchocerciasis in West Africa. Bull World Health Organ. 1995;73: 495–506. [PMC free article] [PubMed] [Google Scholar]