Abstract
Objective
To evaluate the effectiveness of a nurse-led hospital-to-home transitional care intervention versus usual care on mental functioning (primary outcome), physical functioning, depressive symptoms, anxiety, perceived social support, patient experience, and health service use costs in older adults with multimorbidity (≥ 2 comorbidities) and depressive symptoms.
Design and setting
Pragmatic multi-site randomized controlled trial conducted in three communities in Ontario, Canada. Participants were allocated into two groups of intervention and usual care (control).
Participants
127 older adults (≥ 65 years) discharged from hospital to the community with multimorbidity and depressive symptoms.
Intervention
This evidence-based, patient-centred intervention consisted of individually tailored care delivery by a Registered Nurse comprising in-home visits, telephone follow-up and system navigation support over 6-months.
Outcome measures
The primary outcome was the change in mental functioning, from baseline to 6-months. Secondary outcomes were the change in physical functioning, depressive symptoms, anxiety, perceived social support, patient experience, and health service use cost, from baseline to 6-months. Intention-to-treat analysis was performed using ANCOVA modeling.
Results
Of 127 enrolled participants (63-intervention, 64-control), 85% had six or more chronic conditions. 28 participants were lost to follow-up, leaving 99 (47 -intervention, 52-control) participants for the complete case analysis. No significant group differences were seen for the baseline to six-month change in mental functioning or other secondary outcomes. Older adults in the intervention group reported receiving more information about health and social services (p = 0.03) compared with the usual care group.
Conclusions
Although no significant group differences were seen for the primary or secondary outcomes, the intervention resulted in improvements in one aspect of patient experience (information about health and social services). The study sample fell below the target sample (enrolled 127, targeted 216), which can account for the non-significant findings. Further research on the impact of the intervention and factors that contribute to the results is recommended.
Trial registration
clinicaltrials.gov Identifier: NCT03157999.
Introduction
As the population of older adults (≥ 65 years) increases, so too does the number of individuals living with multimorbidity, defined as the co-existence of 2+ (or 3+) chronic conditions in the same person [1]. Worldwide, more than half of older adults have multimorbidity [2], with a mean of five chronic conditions per person [3]. High prevalence rates of multimorbidity have been reported in older adults in Canada (43%) [4], the United States (63%) [5], and the United Kingdom (67%) [6], with a significant increase over the last three decades [7, 8]. The increasing prevalence of multimorbidity is driven by a growing aging population as well as a rise in global life expectancies [9–11]. The global population of older adults is expected to reach approximately 16% in 2050 compared to 9% in 2018 [12]. Of the chronic conditions that co-exist, depression is the single most common condition in older adults [13]. Older adults with multimorbidity are two to three times more likely to have depression compared to those without multimorbidity [14]. Depression is a common and serious problem in its own right that is estimated to affect up to 40% of community-living older adults [15]. Although depression can be successfully treated with antidepressant medications or psychosocial interventions, few older adults receive adequate treatment. Untreated or under-treated depression in older adults is associated with a range of negative outcomes, including reduced quality of life, impaired functional status [16], increased use of healthcare services [17], premature admission to long-term care [18], increased mortality [18, 19], and increased burden on family caregivers [1]. As the number of comorbid chronic conditions increases so too does the likelihood of depression and other mental health conditions [6, 18, 20].
Older adults (≥ 65 years) with multimorbidity and depressive symptoms are arguably the most vulnerable of patient groups. Compared with the general population of older adults, older adults with multimorbidity and depressive symptoms have significantly higher rates of hospital, emergency room, and physician use and costs, and experience frequent transitions between hospital and home [21, 22]. Multiple studies suggest that hospital-to-home care transitions for this population are fragmented and poorly coordinated, resulting in increased hospital readmission rates, adverse medical events, decreased patient satisfaction and safety, and increased caregiver burden [23–31]. Studies in Canada, the USA, and elsewhere have attributed these adverse outcomes to factors such as lack of patient knowledge about available community-based services resulting in suboptimal or delayed utilization of these services [31, 32], conflicting plans of care and instructions from different providers [31, 33–36], medication errors [29–31, 37, 38], lack of timely follow-up with specialists and family physicians after hospital discharge [30, 31, 39], limited engagement of older adults and caregivers in care decisions [29, 40] and preparation for self-care [30, 37, 38, 41–43], lack of support for family caregivers, poor communication and collaboration among providers within and across settings [29, 30, 44], lack of timely and adequate home-based support after hospital discharge [29, 30], untreated or under-treated depressive symptoms [29, 45–47], inadequate community mental health supports [29], and having other unaddressed social and psychological needs during previous hospitalization [30]. Factors related to the social determinants of health, such as lower socioeconomic status, living in rural or remote communities, inadequate housing conditions [30], lack of social support [30], or identifying as ethnocultural minorities, can further exacerbate the challenges associated with hospital-to-home transitions for older adults with multimorbidity and depressive symptoms [48]. Two recent studies conducted in Ontario reported that as many as 44% of patients in Ontario do not attend recommended post-discharge appointments for follow-up care after hospital discharge because of issues such as low health literacy, financial concerns, and a lack of social supports [39, 49].
Transitional care interventions have been recommended to enhance coordination and continuity of health care in community-living older adults with complex needs transitioning from hospital to home, and have been linked to decreasing hospital readmissions and other positive outcomes [31, 44, 50–56]. However, these trials have been largely based on transitional care interventions for single conditions, which often excluded patients with multimorbidity or depressive symptoms [57–61]. As a result, the effectiveness of these interventions for older adults with multimorbidity and depressive symptoms is undetermined [62–64]. Key components of effective hospital-to-home transitional care interventions have been identified, including a comprehensive assessment of patients’ current and evolving health care and support needs; patient, family, and caregiver involvement in transition planning; patient, family, and caregiver education support and training; care coordination and system navigation; medication review and support; coordinated team-based care; holistic, person-centred care; and developing individualized care plans [31, 61, 65]. However, what elements should be included in transitional care interventions for older adults with stroke and multimorbidity and depressive symptoms remains inconclusive.
This team designed and tested a new hospital-to-home transitional care intervention to address this gap in knowledge. The Community Assets Supporting Transitions (CAST) is a 6-month, tailored, nurse-led, patient-and caregiver-centred intervention designed to support families and caregivers and foster collaboration between primary care and other interdisciplinary service providers, both within and outside of the health sector, in delivering home and community services. The intervention was adapted from a previous nurse-led mental health promotion intervention for older home care clients with multimorbidity and depressive symptoms [50]. While this intervention did not specifically focus on transitional care, 60% of the older adult study participants reported one or more hospital admissions in the last six months, thus serving as a strong foundation for the hospital-to-home transitional care intervention tested in this trial. The intervention also included most of the key elements recommended in best practice guidelines for transitional care [31, 40, 65–68]. The results of our feasibility study of the effectiveness of this intervention showed that the intervention was feasible to implement, and resulted in a statistically significant reduction in depressive symptoms, significant improvements in mental and physical functioning, and a statistically significant reduction in the use of hospitalization, ambulance service utilization and emergency room visits [50].
Objectives
Primary Objective: To compare the effect of a 6-month transitional care intervention versus usual care on the primary outcome–mental functioning–in older adults with depressive symptoms and multimorbidity transitioning from hospital-to-home.
Secondary Objective: To compare the effect of a 6-month transitional care intervention versus usual care on secondary outcomes–physical functioning, depressive symptoms, anxiety, perceived social support, patient experience, and healthcare costs–in older adults with depressive symptoms and multimorbidity transitioning from hospital-to-home.
Methods
A multi-site, pragmatic, randomized controlled trial was conducted in Ontario, Canada (ClinicalTrials.gov: NCT03157999). The study was designed to be highly pragmatic, using the criteria described in the Pragmatic Explanatory Continuum Indicator Summary-2 tool [69, 70]. Pragmatic features included the recruitment of participants representative of the population presenting in the hospital setting, the flexible delivery of the intervention by Registered Nurses (RNs) from the setting, the use of patient-relevant outcomes (e.g., quality of life, patient experience), and the use of intention-to-treat analysis. Details of the study design and outcome measures are reported in the published study protocol [71]. The methods, results, and flow of participants through the study are presented here according to the CONSORT statement for pragmatic randomized controlled trials [70]. The key design features are summarized below.
Participants and recruitment
Participants were recruited from three large academic hospitals within three geographical areas in Ontario, Canada. These three communities were selected for their diversity with respect to geography (e.g., rural, urban), socio-economic, and language (i.e., English, French) characteristics. Study recruitment was conducted during 2017–2018 and spanned 5–11 months, depending on the site. Inpatient older adults were screened prior to hospital discharge (n = 825) by a trained recruiter at each hospital for potential inclusion and were eligible to participate if they met the following criteria: 1) aged 65 years or older; 2) planned for discharged from hospital to the community (not long-term care); 3) self-reported at least two chronic conditions; 4) screened positive for depressive symptoms as assessed by a two-item version of the Patient Health Questionnaire (PHQ-2) [72]; 5) not planning to leave the community during the 6-month study period; 6) passed a cognitive screening test (achieved at least 5 correct responses on the Short Portable Mental Status Questionnaire (SPMSQ) [73]; and 7) were competent in English or had an interpreter available who was competent in the English language (this also included French-language in the site with a large Francophone population).
The purpose of the PHQ-2 is not to establish definitively the presence of a depressive disorder, but rather to screen for depressive symptoms as a “first step” approach [72]. Thus, eligible and consenting participants were further evaluated with the Center for Epidemiologic Studies Depression Scale 10-item tool (CESD-10) [74] to determine whether they met the criteria for a depressive disorder (CES-D ≥ 10). A trained Research Assistant (RA) contacted potential participants following discharge from hospital to arrange an in-home interview. The RA obtained written informed consent prior to conducting the baseline in-home interview.
Randomization
Within each study region, participants were assigned to either the intervention or the usual care group following the collection of baseline data, using permuted block randomization administered by a centralized web-based software program (RedCap) that ensured concealment of the allocation from the research team. Participants were allocated to the two groups using a 1:1 ratio and in accordance with the sequence determined by RedCap.
Intervention
Details regarding the CAST intervention are described in the published study protocol [71]. The intervention was developed using the Medical Research Council Framework for developing complex interventions, which highlights the importance of theoretical and empirical evidence [75]. The intervention was based on Bandura’s Social Cognitive Theory [76], where the aim is to build self-efficacy to improve self-management of health conditions and associated risk factors [76], and on research evidence [31, 40, 66–68, 77–82], More importantly, the intervention incorporated input from a range of stakeholders, including patients, providers, and decision-makers from local and provincial health authorities. The stakeholders worked as a team to identify gaps in the delivery of hospital-to-home transitional care which, in turn, informed the core components of the intervention and how they were delivered and tailored in the study settings. The involvement of multiple provider agencies was critical to designing the intervention to ensure that all viewpoints were considered.
The description of the intervention follows the Template for Intervention Description and Replication guidelines [83]. The intervention consisted of usual care plus a 6-month tailored patient- centred intervention delivered by 4 Registered Nurses (RNs) who functioned as Care Transition Coordinators (CTCs) within each of the study regions. The CTCs were not responsible for usual care. All the CTCs had a baccalaureate degree, one CTC had a master’s degree, and all had 2–20 years experience working as an RN in both acute care and community settings.
To support intervention fidelity, the CTCs were provided training by the principal investigators and the research coordinator prior to initiation of the intervention to convey key intervention activities, research study procedures, and underlying theories. A standardized training manual was developed that includes key content pertaining to all aspects of the intervention. Training was adapted to the individual needs of the CTCs and included education and role-playing to enhance skills in problem-solving therapy within the context of multimorbidity. Monthly outreach meetings were conducted to enable the principal investigators and the research coordinator to meet with the CTCs to monitor intervention implementation and strategize to address any challenges [84].
The intervention consisted of up to 6 in-home visits (minimum 2), telephone calls, and system navigation support. The CAST intervention was designed to improve both the quality and experience of hospital-to-home transitions. It was based on a patient-centred model and encapsulates strategies included in effective care transitions interventions [31, 40, 66–68], and recommended in best practice guidelines for system navigation [77], management of depressive symptoms [31, 78, 79], and prevention and management of multimorbidity [80–82].
Each participant was offered monthly in-home visits by the CTCs that were an average of one hour in duration supplemented by telephone calls for 6 months as part of the CAST intervention. The CTCs main activities during the home visits and telephone calls included: 1) conducting a comprehensive assessment of the health and social care needs of older adult participants using standardized tools; 2) identifying and managing depressive symptoms and multimorbidity in accordance with best practice [31, 78–81]; 3) conducting medication review and reconciliation and supporting antidepressant medication management using best practice [85]; 4) providing problem-solving therapy with participants and caregivers using Nezu et al.’s manual [86]; 5) implementing social and behavioural activation strategies tailored to individual needs; 6) providing education to participants and caregivers; and 7) communicating alerts to primary care providers regarding the presence of depressive symptoms, dementia, delirium, suicidal ideation, or medication issues in participants.
During and between home visits, the CTC provided system navigation support that consisted of: 1) identifying and addressing any risk factors for adverse events, e.g., hospital readmissions; 2) arranging community services such as home care and follow-up health-care appointments; 3) facilitating communication between the patient, their family caregiver, and their health care team; 4) supporting linkages and referrals to relevant health and social service providers; 5) developing an individualized patient-centred plan of care [77]; and 6) identifying health care professionals involved in the participant’s circle of care and initiating a plan for regular communication and follow-up with them. Consistent with a pragmatic trial design, the intervention was tailored to patient needs and preferences and the local context. For example, patients could decline any number of home visits, and all participants continued to have access to the programs and services normally offered in their community.
Patient and public involvement
A key component of this patient-oriented research project was the meaningful engagement in all stages of the research process of diverse patients (including family caregivers) who reflected the population of interest. Patient and caregiver research partners with experience in hospital-to-home transitions or depressive symptoms and multimorbidity were actively involved as members on: 1) a Research Steering Committee to provide input on the design of the trial and management oversight, and to inform cross-site implementation of the research; 2) three local Community Advisory Boards to support local implementation of the research at each study site; and 3) the research team as Co-Investigators. Through these structures, patient and caregiver research partners assisted with the identification of the research priorities and questions, selection of patient-relevant outcomes, review of study materials (e.g., consent forms, interview guides), interpretation of study findings, and knowledge dissemination [87]. Patient engagement was grounded in principles of inclusiveness, support, mutual respect, and co-build [88].
Outcomes and measures
Details regarding outcome measures are described in the study protocol [71]. Outcomes were assessed at baseline and at the 6-month and 12-month follow-up through interviewer-administered questionnaires during a structured in-home interview. The primary study outcome (mental functioning) was measured using the Mental Component Score (MCS) score from the Veterans Rand 12-item health survey (VR-12), a reliable and valid patient-reported outcome measure of quality of life [89, 90]. This outcome was consistent with the overall goal of our intervention [90], to enhance the mental functioning of older adults with depressive symptoms and multimorbidity. Secondary outcome measures included physical functioning measured using the Physical Component Score (PCS) score from the VR-12 [90]; depressive symptoms measured using the Center for Epidemiologic Studies Depression Scale 10-item tool (CESD-10) [74, 91], anxiety measured using the Generalized Anxiety Disorder 7-item scale (GAD-7) [92, 93], perceived social support measured using the Personal Resource Questionnaire (PRQ-2000) Part 2 [94–96], and patient experience measured using one question from the Client Centred Care Questionnaire (CCCQ) [97], and the complete Intermediate Care for Older People Home-Based-Integrated Care Patient-Reported Experience Measure (IC-PREM) [98]. These instruments have demonstrated reliability and validity and have been used in our previous trials involving community-living older adults with multimorbidity.
Healthcare use was measured using the Health and Social Services Utilization Inventory (HSSUI) [99], which is a reliable and valid self-report questionnaire that measures the use of health and social services [100, 101]. The HSSUI captures use of primary care, emergency department and specialists, hospital days, other health and social professionals, prescribed medications, and lab services. The cost analysis applied unit costs to the service volumes reported in the HSSUI [102] and assumed a societal perspective to inform the broad allocation of resources in the public interest [103].
Guidelines are available for judging clinical significance for the VR-12, but not the other outcome measures. VR-12 developers suggest a minimally important difference (MID) of 3 for interpreting group mean summary score differences (PCS, MCS) [104]. A recent systematic review of RCTs reporting non-significant results emphasized the importance of interpreting confidence intervals in relation to the MID to distinguish “negative” findings from “inconclusive” ones [105]. We applied this recommendation to our study for the PCS and MCS of the VR-12, which have MIDs.
Blinding
To reduce bias, study participants were blinded to their group allocation (usual care, intervention) and the research assistants who collected the assessment data and statistician who analyzed the data were also blinded. Upon completion of the study at 12-months, participants received a mailed debriefing letter describing the two groups and their group allocation. Usual care providers were also unaware of the participant’s group allocation.
Sample size
The target sample size of 216 (72 from each of the three sites) was calculated for the primary outcome—MCS score of the VR-12 [106]. The calculation assumed 80% power, 2-tailed alpha of 5%, 20% attrition, and a mean (standard deviation) MCS score difference of 6.5 (15.0) as observed in the feasibility study [50]. Using these assumptions, the sample size was 108 (each) for the intervention and control groups.
Statistical analysis
The reporting of this trial follows the CONSORT guideline for pragmatic RCTs [70]. Descriptive statistics were used to summarize outcome values at baseline, 6 months, and 12 months. Means and standard deviations were used for continuous outcomes, and frequency and percentages were used for categorical outcomes. Analysis of covariance (ANCOVA) was used to assess group differences in the change in primary and secondary outcomes from baseline to 6-months (T2) to determine if the intervention was effective over the 6-month intervention period. The ANCOVA model used the 6-month outcome value as the dependent variable, the group indicator as the independent variable, and the baseline outcome value as the covariate. Model results were expressed as mean group differences with accompanying 95% confidence limits. Quantile regression was used to examine the group differences in the change in outcomes from baseline to 6-months across quantiles. This method allows us to relax the common regression slope assumption to explore group differences across the distribution of the dependent variable rather than only at the mean [107, 108]. Assessment of group differences in continuous outcomes from 6-months (T2) to 12-months (T3) were assessed using ANCOVA if statistically significant group differences were achieved in the outcomes from baseline to 6-months, with the purpose being to assess the sustainability of the intervention effects.
Z tests of proportions and McNemar tests will be used to assess changes within each group in the number of participants with acute care episodes and the number of acute care episodes from baseline to T2, and from T2 to T3 (if statistically significant group differences were achieved in these outcomes from baseline to 6-months). Acute care episodes included emergency department visits and hospital admissions. Due to the highly skewed nature of cost data, a non-parametric test was used to compare the change in health and social service costs from baseline to T2 for the two groups. Outliers are particularly common for certain healthcare costs such as hospital admissions, thus we conducted a sensitivity analysis to assess the impact of outliers on the cost comparison.
Subgroup analyses will be conducted for the VR-12 outcomes to determine if the intervention was effective for subgroups of participants from baseline to T2 if the overall trial results achieved statistical significance. The following baseline variables were selected a priori for testing subgroup effects: age, sex, number of chronic conditions, depressive symptoms (CESD-10), study site, and dose of the intervention (number of home visits).
Intention-to-treat analysis was employed. Group differences were examined using both a complete case analysis (n = 99) (participants with a complete record at baseline and six-months), and multiple imputation to address missing data for the primary and secondary outcomes. Joint multiple imputation methods were employed as recommended for small samples with an arbitrary missings pattern [109], and five imputations were conducted and pooled to obtain overall parameter estimates and associated confidence intervals. All data analyses assumed a two-sided alpha of 5% and were performed using SAS Version 9.4.
Ethics approval and consent to participate
The study was conducted in accordance with the Tri-Council Policy Statement, Ethical Conduct for Research Involving Humans [110]. Institutional ethics approval was obtained from: the McMaster University Hamilton Integrated Research Ethics Board (REB) (# 2586); the Office of Research Ethics at the University of Waterloo (#40867); the Laurentian University REB (#6009840), and the REBs from the study sites (Health Sciences North REB # 17–007; Joseph Brant Hospital REB #000-039-17), and renewed yearly as required. Operational approval to conduct the study was obtained from each hospital site. Written informed consent was obtained from all participants by the RA before study enrolment.
Results
Study site characteristics
Table 1 provides information related to the characteristics of the regional health authorities within each of the study sides. Sites 1 and 2 served suburban/rural geographies. Site 3 served an urban geography. All the sites had a higher proportion of older adults than the provincial average. Site 1 had the highest turnover of intervention nurses compared to the other two sites.
Table 1. Study site characteristics.
| Characteristic | Site 1: Sudbury, Ontario | Site 2: Burlington, Ontario | Site 3: Hamilton, Ontario | Ontario |
|---|---|---|---|---|
| Geographic Density1 | Suburban/Rural | Suburban/Rural | Urban | |
| Participants Enrolled | 18 | 36 | 73 | |
| Intervention Nurse Turnover | 2 RNs replaced 4 months into intervention | 1 RN added 8 months into the intervention | 1 RN added 12 months into the intervention | |
| Population | ~165,000 | ~205,000 | ~580,000 | |
| Language | 25% French first language; 39% bilingual (English and French) | 81% English as first language | 23.1% non-official language as first language; 73.5% English as first language | 2.4% French first-language; 26.7% non-official language as first language; 66.9% English as first language |
| Ethnocultural Diversity | 3.8% visible minority. 12.5% FNIM | 9.7% visible minority. 0.7% FNIM | 19.0% visible minority. 2.1% FNIM | 22.3% visible minority. 6.2% FNIM |
| Proportion of older adults | 20.4% | 19.3% | 24.5% | 16.4% |
| Proportion of older adults with low income | 2.3% | 4.3% | 7.4% | 5.1% |
RN: Registered Nurse, FNIM: First Nations, Inuit, or Metis
1 In Canada, the Organisation for Economic Co-operation and Development defines a predominantly rural region as having more than 50% of the population living in rural communities where a rural community" has a population density less than 150 people per square kilometre. Predominantly urban regions have less than 15 percent of their population living in a rural community.
Eligibility rate
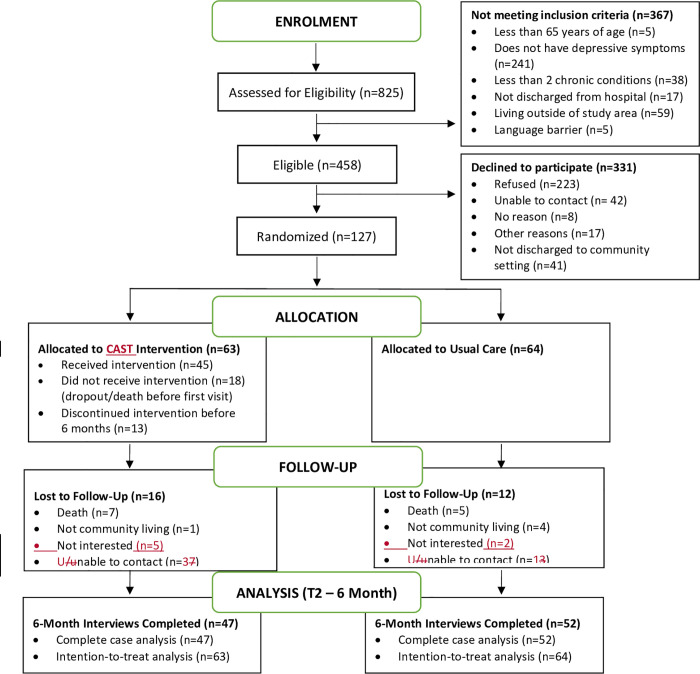
Recruitment ranged from 5 to 11 months depending on the site; significant recruitment challenges were experienced (See discussion). The CONSORT diagram summarizes recruitment, participation, and analysis (Fig 1 and S1 Table). A total of 825 older adults were potentially eligible because they were older adults with a planned discharge to the community and had multimorbidity. Of these older adults, 56% (458/825) were screened for the study and met the remaining eligibility criteria. The most common reason for ineligibility was that potential participants did not screen positive for depressive symptoms (241/367, 66%).
Fig 1. Study flow diagram.
Enrolment rate
In total, 28% (127/458) of eligible older adults consented and entered the study. Among the eligible participants, almost half refused participation in the study (223/458, 49%); another 18% were either unable to be contacted (42/458, 9%) or had moved out of the study region or to long-term care following hospital discharge (41/458, 9%). Lack of interest in having services in their homes, and participating in a research study, coupled with lack of perceived need for services, were the most common reasons for refusal.
Attrition rate
Of the 127 enrolled participants, 99 (78%) successfully completed the six-month follow-up. A total of 28 participants were lost to follow-up at six months, yielding an attrition rate of 22%. Of the 127 enrolled participants, 78 (61%) completed the one-year follow-up interviews. A total of 49 participants were lost to follow-up at one year, yielding an attrition rate of 39%. Reasons for loss to follow-up at six months and one year are shown in Fig 1. Attrition in this study was related to several factors. First, 17 (35%) of participants died over the course of the study, 17 (35%) were unable to be contacted, 7 (15%) were hospitalized, and the remainder of those for whom a reason is known were too unwell to participate.
Comparison between dropouts and completers
The baseline characteristics of participants who completed the six-month follow-up (n = 99) were compared to those who dropped out of the study prior to the six-month follow-up (n = 28). Compared to completers, more dropouts lived in a retirement home or supportive living environment (39.3% vs. 12%), reported a history of depression (50% vs. 29.3%), and had lower scores on the VR: MCS-12 (40.7 vs. 43.7). There was no difference between dropouts and participants who completed the six-month follow-up on any other baseline characteristics.
Baseline characteristics of participants
Baseline characteristics of the participants who completed the six-month follow-up (n = 99) are reported in Table 2. For both groups, approximately 63% of participants were female, 41% were living with a spouse or other family member, 65% were 75 years or older (average of 77 years), 41% were married, and 59% were widowed/divorced. About two-thirds (60%) of the participants had annual incomes of less than CAD$40,000, almost one-third (30%) had less than a high school education, and 38% lived alone. Participants reported VR: PCS-12 and MCS scores at baseline that were significantly lower than published norms for the Canadian population, indicating poor physical and mental functioning [111]. The majority (84.9%) had six or more chronic conditions, with a mean of 8 chronic conditions, and were taking a mean of 8 prescription medications daily. Most participants (79%) self-reported hypertension, and 72% reported arthritis. Depressive symptoms (≥ 10 on CES-D-10) were found in 72% of participants in both groups. For both groups, about 30% of participants self-reported a history of depression, and 31% reported taking an antidepressant. Anxiety symptoms (≥ 5 on GAD-7) were found in 44% of participants.
Table 2. Baseline characteristics of older adults with multimorbidity and depressive symptoms (n = 99) a.
| Characteristic | Total | Intervention Group (n = 47) | Usual Care Group (n = 52) |
|---|---|---|---|
| Sex, n (%) | |||
| Male | 37 (37.4) | 18 (38.3) | 19 (36.5) |
| Female | 62 (62.6) | 29 (61.7) | 33 (63.5) |
| Age in years, n (%) | |||
| 65–69 | 13 (13.3) | 7 (14.9) | 6 (11.8) |
| 70–74 | 21 (21.4) | 10 (21.3) | 11 (21.6) |
| ≥ 75 | 64 (65.3) | 30 (63.8) | 34 (66.7) |
| Type of Accommodation, n (%) | |||
| House or Apartment | 87 (87.9) | 43 (91.5) | 44 (84.6) |
| Retirement Home | 12 (12.1) | 4 (8.5) | 8 (15.4) |
| Marital Status, n (%) | |||
| Married, living together | 41 (41.4) | 21 (44.7) | 20 (38.5) |
| Separated, Divorced, Widowed | 58 (58.6) | 26 (55.3) | 32 (61.5) |
| Education, n (%) | |||
| < High School | 29 (29.6) | 11 (23.9) | 18 (34.6) |
| High School | 32 (32.7) | 14 (30.4) | 18 (34.6) |
| Post-Secondary | 37 (37.8) | 21 (45.7) | 16 (30.8) |
| Annual Income in CAD, n (%) | |||
| < $40,000 | 47 (59.5) | 17 (51.5) | 30 (65.2) |
| ≥ $40,000 | 32 (40.5) | 16 (48.5) | 16 (34.8) |
| Living Arrangement, n (%) | |||
| Live Alone | 37 (37.8) | 17 (36.2) | 20 (39.2) |
| Live with Others | 61 (60.7) | 30 (63.8) | 31 (60.8) |
| Number of Chronic Conditions, n (%) | |||
| 0–5 | 15 (15.2) | 5 (10.6) | 10 (19.2) |
| 6 to 10 | 69 (69.7) | 34 (72.3) | 35 (67.3) |
| ≥ 11 | 15 (15.2) | 8 (17.0) | 7 (13.5) |
| Type of Chronic Conditions, n (%) | |||
| Hypertension | 78 (78.8) | 34 (72.3) | 44 (84.6) |
| Arthritis | 71 (72.0) | 36 (76.6) | 35 (67.3) |
| Anxiety Symptoms, n (%) | |||
| ≥ 5 (GAD-7) | 44 (44.4) | 19 (40.4) | 25 (48.1) |
| < 5 (GAD-7) | 55 (55.6) | 28 (59.6) | 27 (51.9) |
| Depressive Symptoms, n (%) | |||
| ≥ 10 (CESD-10) | 70 (72.1) | 32 (68.1) | 38 (76.0) |
| < 10 (CESD-10) | 27 (27.8) | 15 (31.9) | 12 (24.0) |
| History of Depression, n (%) | |||
| Yes | 29 (29.3) | 17 (36.2) | 12 (23.1) |
| No | 70 (70.7) | 30 (63.8) | 40 (76.9) |
| Antidepressant Medication Use, n (%) | |||
| Yes | 31 (31.3) | 20 (25.5) | 11 (21.2) |
| No | 68 (68.7) | 27 (74.5) | 41 (78.9) |
| Number of Prescription Medications, n (%) | |||
| 0–3 medications | 7 (7.2) | 4 (8.7) | 3 (5.9) |
| 4–7 medications | 22 (22.7) | 9 (19.6) | 13 (25.5) |
| ≥ 8 medications | 68 (70.1) | 33 (71.8) | 35 (68.6) |
| Social supportb, mean (SD) | 83.54 (12.9) | 82.29 (15.2) | 83.84 (10.7) |
| Depressive Symptomsc, mean (SD) | 11.0 (6.60) | 10.6 (6.75) | 11.97 (6.71) |
| Anxiety Symptomsd, mean (SD) | 5.69 (5.71) | 5.28 (5.14) | 6.14 (6.35) |
| Physical Functioninge, mean (SD) | 23.91 (10.97) | 22.23 (10.24) | 26.16 (11.61) |
| Mental Functioning, mean (SD) | 43.67 (12.76) | 43.52 (11.91) | 43.98 (14.23) |
| Number of hospital admissions, last 6 months, mean (SD) | 1.55 (0.94); Range: 0–4 | 1.64 (1.03); Range: 0–4 | 1.46 (0.86); Range: 0–3 |
a Significance tests were independent t-tests except for number of hospital admissions in 6 months (used non-parametric test–Mann Whitney U–reported z-score & associated p-value).
b Measured by Personal Resource Questionnaire (PRQ 2000), scale range 15–105.
c Measured by Centre for Epidemiologic Studies Depression 10-item Scale (CES-D-10), scale range 0–30.
d Measured by Generalized Anxiety Disorder 7-item Scale (GAD-7), scale range 0–21.
e Measured by Physical Component Score (PCS) of the Veterans Rand 12-item Scale (VR-12), scale range 0–100.
f Measured by the Mental Component Score (MCS) of the Veterans Rand 12-item Scale (VR-12), scale range 0–100.
A higher proportion of participants in the intervention group reported a history of depression (36.2% vs. 23.1%) and took antidepressant medications (25.5% vs. 21.2%) compared with the usual care group. Participants in the intervention group also had lower scores on the VR: PCS-12 (22.2 vs. 26.2) compared with the usual care group.
Intervention dose
Of 63 intervention participants enrolled at baseline, 45 (71%) received at least one home visit by the CTC. Reasons for not receiving the intervention (all or in part) are shown in Fig 1. Most of the participants who refused the home visits cited that they were not interested in the study (78%). Thirteen additional participants discontinued the intervention early. Over 6 months, the participants received an average of three home visits by the CTC (offered 6). The average duration of the home visits was one hour. For the 45 participants who received at least one home visit, care coordination and system navigation support were cited as the most frequently delivered activity by the CTC nurse (average 4.2 times per participant). This was followed by providing practical and emotional support (average 2.9 times), clinical assessment and screening (average 2.8 times), self-management support (average 2.2 times), behavioural change support (average 2.4 times), health education (average 2 times), health promotion activities (average 1.4 times), and caregiver support (average 1 time).
Effects of the intervention
Health outcomes
The results of the complete case (n = 99) ANCOVA from baseline to 6-months are provided in Table 3. For the primary outcome (VR: MCS-12), the group difference in mean 6-month scores adjusted for baseline values was not statistically significant (mean difference: 1.09; 95% CI: -3.24–5.41). For the secondary outcomes, there were no significant group differences between the intervention and control groups on the: VR: PCS-12 (mean difference: -1.45; 95% CI: -4.96, 2.07), PRQ-2000 (mean difference: 2.95; 95% CI: -1.93–7.83), GAD-7 (mean difference: 1.34; 95% CI: -0.25–2.92) or CES-D-10 (mean difference: 0.80; 95% CI: -1.43–3.03). Multiple imputation results were consistent with the complete case findings. Subgroup analyses were not done since statistical significance was not achieved (see Statistical Analysis above).
Table 3. Group differences in outcomes from baseline to six-months (n = 99).
| Outcome | Intervention n = 47 | Control n = 52 | ANCOVA | ||
|---|---|---|---|---|---|
| Baseline Mean (SD) | T2 Mean (SD) | Baseline Mean (SD) | T2 Mean (SD) | Mean Diff (95% CI) [t, p-value] | |
| VR: MCS-12 | 43.52 (11.91) | 48.63 (11.62) | 43.98 (14.23) | 47.74 (11.59) | 1.09 (-3.24, 5.41) [0.50, 0.61] |
| PRQ 2000 | 82.29 (15.23) | 83.55 (14.68) | 83.84 (10.71) | 81.59 (13.42) | 2.95 (-1.93, 7.83) [1.2, 0.23] |
| GAD-7 | 5.28 (5.14) | 4.85 (5.28) | 6.14 (6.35) | 3.82 (3.39) | 1.34 (-0.25, 2.92) [1.68, 0.10] |
| CESD-10 | 10.6 (6.75) | 9.82 (7.09) | 11.97 (6.71) | 9.65 (5.25) | 0.80 (-1.43, 3.03) [0.71, 0.48] |
| VR: PCS-12 | 22.23 (10.24) | 24.23 (10.78) | 26.16 (11.61) | 28.07 (10.20) | -1.45 (-4.96, 2.07) [-0.82, 0.42] |
Fig 2 provides a graphic interpretation of the MCS and PCS findings. For the MCS, the findings are inconclusive with either usual care or the intervention being potentially superior, since the CI crosses 0 with the upper CI (favours the intervention) and the lower CI (favours the control) exceeding the MID of 3. For the PCS, the findings are inconclusive regarding the superiority of usual care but rules out the superiority of the intervention, since the CI crosses 0 with the lower CI (favours usual care) exceeding the MID of 3 and the upper CI (favours the intervention) does not reach the MID. The results of the quantile regression analyses showed that the intervention consistently outperformed usual care across most of the response range values (baseline to 6-month change) for the PRQ-2000 (perceived social support) (p = 0.03) (Fig 3), although statistical significance was not achieved. ANCOVA from 6-months to one year was not done since statistically significant group differences were not achieved in the outcomes from baseline to 6-months (see Statistical Analysis above).
Fig 2. Interpreting 95% confidence intervals for PCS and MCS (“inconclusive findings”).
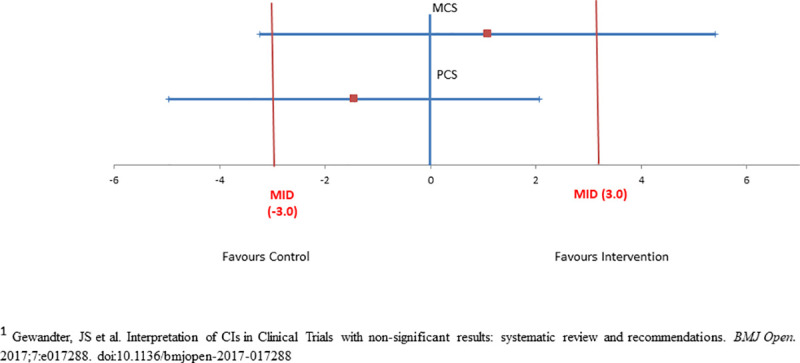
Note: MID: minimally important difference.
Fig 3. Quantile regression results: Baseline to six-month change in PRQ.
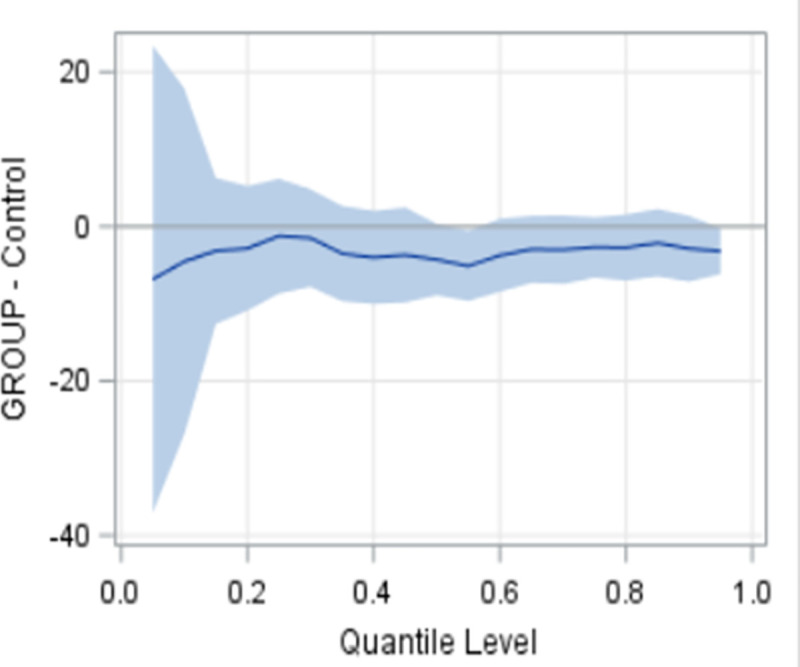
Patient experience
Chi-square analysis was used to compare the two groups (intervention, control) at baseline and 6 months on participant’s care experience, namely one item from the Client Centered Care Questionnaire (CCCQ) [97], and the Intermediate Care for Older People Home-Based-Integrated Care Patient-Reported Experience Measures (IC-PREMs) [98] for the complete cases (n = 99). At 6-months, older adults in the intervention group reported receiving more information about health and social services (p = 0.03) compared with the usual care group. There was no significant group difference between the intervention and control groups on any of the other items on the CCQ or the IC-PREMs Questionnaire (S2 Table).
Health service use costs
The results of the complete case (n = 99) Wilcoxin Rank Sum test from baseline to 6-months are provided in Table 4 for the two groups (intervention, control). The median intervention cost was CAD$449.60 (interquartile range $CAD$0–859.20) per study participant. Despite inclusion of the intervention costs, there was no statistically significant difference between groups in the change in total costs (including or excluding hospital costs) from baseline to 6-months (p = 0.07). For example, cost changes for some services favored the intervention group (family physician and emergency department visits), and others favoured the control group (prescription medications, ambulance, 911 calls, and hospitalization). However, none of these differences were statistically significant. Three extreme outliers were identified (2 in the intervention group and 1 in the control group) that fell well outside the range of health service use. These extreme outliers were excluded from the analysis. The analysis from 6-months to one year was not done since statistically significant group differences were not achieved in the cost of health service use from baseline to 6-months (see Statistical Analysis above).
Table 4. Group difference in health and service costsa from baseline to six-months (n = 99).
| Service | Intervention (n = 47) | Usual Care Group (n = 52) | Group Differences | |||
|---|---|---|---|---|---|---|
| Baseline Median (QI, Q3) | 6-Month Median (Q1, Q3) | Baseline Median (QI, Q3) | 6-Month Median (Q1, Q3) | z | p value | |
| Family Physician Visits | 348.48 (231.60, 799.92) | 350.64 (198.30, 1178.64) | 231.60 (134.31, 511.60) | 303.94 (143.86, 520.31) | 0.13 | 0.90 |
| Specialist Visits | 195.74 (50.35, 380.16) | 241.23 (50.35, 437.47) | 140.34 (0.00, 351.54) | 164.10 (0.00, 379.43) | 0.25 | 0.81 |
| Home Care | 0.00 (0.00. 122.41) | 0.00 (0.00, 315.85) | 0.00 (0.00, 128.61) | 0.00 (0.00, 160.00) | 0.40 | 0.69 |
| Social & Community | 0.00 (0.00, 0.00) | 0.00 (0.00, 0.00) | 0.00 (0.00, 3.00) | 0.00 (0.00, 0.00) | 0.53 | 0.59 |
| Transportation | 0.00 (0.00, 0.00) | 0.00 (0.00, 24.00) | 0.00 (0.00, 0.00) | 0.00 (0.00, 6.00) | -0.54 | 0.59 |
| Prescription Medications | 711.09 (447.01, 11,977.46) | 803.22 (226.86, 16,006.77) | 783.69 (388.72, 3,797.08) | 507.49 (225.57, 10,266.87) | 1.81 | 0.07 |
| Intervention c | 0.00 (0.00, 0.00) | 429.60 (0.00, 859.20) | 0.00 (0.00, 0.00) | 0.00 (0.00, 0.00) | 7.14 | <0.0001 |
| Ambulance & 911 | 264.80 (0.00, 480.00) | 0.00 (0.00, 264.80) | 264.80 (264.80, 480.00) | 0.00 (0.00, 264.80) | 0.88 | 0.28 |
| Emergency Department Visits | 239.31 (239.31, 478.62) | 0.00 (0.00, 478.62) | 239.31 (239.31, 478.62) | 0.00 (0.00, 239.31) | -0.01 | 0.99 |
| Hospitalization | 12,552.00 (6,276.00, 29,811.00) | 0.00 (0.00, 10,983.00) | 15,690.00 (7,845.00, 31,380.00) | 0.00 (0.00, 9,414.00) | 1.29 | 0.20 |
| Total Costs (including Hospital Costs) | 23,369.85 (12,182.52, 44,173.80) | 13,790.06 (2,699.03, 42,379.06) | 24,026.48 (10,420.91, 46,058.39) | 7.354.53 (1,806.41, 28,031.76) | 1.82 | 0.07 |
| Total Costs (excluding Hospital Costs) | 2,172.23 (1,201.49, 15,531.52) | 5.070.81 (2,369.95, 18.367.59) | 1,885.00 (1,303.38, 9,278.66) | 3,074.69 (889,18, 14,427.26) | 1.83 | 0.07 |
a Currency CAD
b Wilcoxon Rank Sum Test used to determine significance of group differences. p-values are 2-sided.
c Includes costs for the intervention group (training, in-home visits). Difference between intervention and control group favours control group.
Discussion
The purpose of this pragmatic RCT was to test the effects of a six-month hospital-to-home, nurse-led, transitional care intervention for older adults with multimorbidity and depressive symptoms on health outcomes (mental and physical functioning, depressive symptoms, anxiety, perceived social support), patient experience, and service use costs. This intervention was based on a patient-centred model, and encapsulated strategies included in effective care transitions interventions [31, 66–68], and recommended in best practice guidelines for management of depressive symptoms [31, 78, 79] and multimorbidity [80–82]. The intervention is well-aligned with health-care reform in Ontario and Canada, which is focused on exploring new health-care models that integrate and coordinate care around the patient and across providers in a way that makes sense for each community and improves patient outcomes [31]. Although the overall benefits of the intervention were inconclusive for mental and physical functioning, the intervention resulted in improvements in one aspect of patient experience, and the potential for significant improvements in perceived social support.
This trial had several strengths. It was designed to be highly pragmatic, using the criteria described in the Pragmatic Explanatory Continuum Indicator Summary-2 tool [69, 70]. As a result, it reflects the effectiveness of the intervention in real-world implementation [112]. Pragmatic features included the recruitment of participants representative of the population presenting in the hospital setting, the flexible delivery of the intervention by RNs from the setting, the use of patient-relevant outcomes, (e.g., quality of life, patient experience), the flexible delivery of the intervention by providers, and the use of intention-to-treat analysis [112]. The baseline rate of depressive symptoms of 72% in the present sample is higher than the 55% rates [113] reported for representative samples of hospitalized older adults at discharge. The use of broad eligibility criteria in a range of diverse study sites with respect to geography (e.g., rural, and urban), socio-economic, and language (e.g., English and French) characteristics enhances external validity. Our study is unique in that it measured the costs of use of a full range of health and social services, from a societal perspective. Previous trials assessing the impact of transitional care interventions provide little information on costs and most have focused on the cost of institutional care (e.g., hospital, ER, long-term care).
This trial had several limitations that may have contributed to the modest study results. First, only 45 (71%) of intervention group participants received at least one home visit by the nurse. Of that number, 32 (71%) completed the six-month intervention and 13 (29%) withdrew prior to six-months. Generally, this engagement rate is in keeping with other studies of older adults with multimorbidity, such as the 3D trial which reported that reach varied across sites from 38% and 94% (median 66%) [10]. The low intervention engagement rate in our study may have diluted the effectiveness of the intervention. Implementation difficulties and deficiencies are not frequently identified in effectiveness evaluations of complex healthcare delivery interventions [10, 114]. We are currently conducting a process evaluation on the implementation of the intervention to understand the influence of contextual factors on study outcomes and inform decisions about wider implementation of the intervention or the need for further research.
Second, only 28% of eligible participants agreed to participate in the trial (enrolled 127, targeted 216) despite extending the recruitment period of the trial. Generally, this recruitment rate is in keeping with other studies of older adults with multimorbidity, such as our previous nurse-led mental health promotion trial that reported a recruitment rate of 29% [50], the 3D trial that reported a recruitment rate of 33% [10], and the Guided Care cluster trial, where 38% agreed to participate [115]. The challenges recruiting older adults with depression are also well documented [116, 117]. An examination of 114 trials involving older adults with depression conducted between 1994 and 2002 showed that less than one-third (31%) reached their original recruitment targets [118]. Our study sample was well below the target sample size of 216, which may account for the study’s non-significant findings and may have led to recruitment bias. The finding that there was no statistically significant difference between groups in per-person health service use costs may not be due to similar cost of the two interventions, but due to a lack of power (small sample size). We invested significant resources and implemented numerous strategies to encourage recruitment that are outlined in detail in the study protocol [71]. However, the results highlight the continued need to identify, test and implement innovative methods to enhance recruitment of this population.
Third, despite randomization, there was some chance imbalance in the groups at baseline. A higher proportion of participants in the intervention group reported a history of depression compared with the usual care group (Table 2). This may have reduced the comparative benefit of the intervention.
Fourth, we should acknowledge the retention challenges encountered in this study. We had attrition rates of 22% at six-months and 39% at one-year. There was a slightly higher loss to follow-up in the intervention group at both six months (25% vs. 19%) and one-year (45% vs. 33%) compared to the control group (see Fig 1). Our retention rate is typical of trials in this population, such as our previous nurse-led mental health promotion trial that reported a retention rate of 61% at one year [50], and the Guided cluster trial where 55% completed the final interview [115]. However, attrition may have resulted in self-selection bias, because the drop-outs differed from those individuals who completed the study in that they were more likely to live in a retirement home or supportive living environment, reported lower levels of mental health functioning, and were more likely to report a history of depression. Based on this, it would appear as if the dropouts were a somewhat lower-functioning group than those who were retained in the study. Future research is warranted to identify effective strategies to recruit and retain vulnerable older adult populations [119]. Finally, the large number of secondary outcomes raises the possibility of false-positive findings due to multiple testing. However, in pragmatic trials, it is important to include a range of outcomes that are relevant to patients, providers, and decision-makers [112]. A recent consensus study identified a core set of outcomes specifically for multimorbidity research, and this trial includes all of these outcomes (health-related quality of life, mental health outcomes, and mortality) [120].
The finding that the overall benefits of the intervention were inconclusive for mental and physical functioning is consistent with several large multimorbidity trials [10, 61, 115]. This is due in part to the heterogeneity in the type of interventions and the characteristics of the study participants included in these trials [10]. The non-significant findings in our study may also be due, in part, to the limitations and challenges of implementing pragmatic trials. The literature suggests that while the features of pragmatic trials support the applicability of the results to real-world practice, they may also reduce effect sizes. In this study, these features include recruiting and retaining heterogeneous populations, lack of a placebo, and suboptimal delivery of the intervention [121].
Nevertheless, it is possible that our transitional care intervention, which is similar to multimorbidity interventions in terms of the components, such as the 3D trial [10], and the Guided Care cluster trial [115], improves older adults’ perceptions of the quality of their care but not the quality of their lives. Older adults in the intervention group reported receiving more information about health and social services (p = 0.03) compared with the usual care group. Information provision regarding available health and social services is a key aspect of patient’s care experience in the IC-PREM [98]. In a recent systematic review of the reliability and validity of eighty-eight patient-reported experience measures, Bull et al. [122] reported several other tools that included information provision as a component of measuring patient experience. However, the fact that older adults received more information does not indicate whether the information was appropriate, and whether lack of information was an issue. Nevertheless, improving patient experience is one of the triple aims of health care [123], and improving patient experience and incorporating patient’s perspectives into the design and delivery of health services has been shown to improve patient health, healthcare service delivery and quality of care [124]. This is particularly important for older adults with multimorbidity, who are managed by different providers, often through many unconnected care episodes [125]. Nevertheless, providing care that is demonstrably more patient-centred is arguably sufficient justification for implementation, especially since the intervention was found to be cost neutral relative to usual care. Previous trials assessing the impact of transitional care interventions have focused on acute care readmission rates as the primary measure of effect, with limited attention to patient-relevant outcomes, such as patient experience.
Exploratory analysis suggests that there was a consistent trend of greater improvement in the level of perceived social support in the intervention group compared to the usual care group over a broader range of values (beyond the mean). This finding is noteworthy given that lower levels of perceived social support are linked to increased hospitalization rates [126]. However, further research is needed to replicate and confirm this exploratory finding.
Conclusion
This pragmatic trial of a nurse-led transitional care intervention for older adults with
multimorbidity and depressive symptoms transitioning from hospital to home demonstrated inconclusive results for mental and physical functioning (MCS and PCS of VR-12), improvements in one aspect of patient experience, and the potential for significant improvements in perceived social support. Further research on the impact of the intervention and the factors that contribute to the results seen is recommended given the high prevalence of depression among older adults with multimorbidity transitioning from hospital to home, and the low rate of recognition and treatment of depressive symptoms in this high-risk population. Future research is also needed to identify strategies to improve recruitment and retention rates to ensure adequate sample size; improve the reach of the intervention; and to understand the influence of these contextual factors on study outcomes to inform decisions about wider implementation of the intervention and the need for further research.
Supporting information
(DOCX)
(DOCX)
(DOCX)
Acknowledgments
We thank the older adults who participated in this study, as well as the Nurse Care Transition Coordinators (CTCs) at the participating communities who provided the intervention. We also thank Hamilton Health Sciences, Joseph Brant Hospital, and Health Sciences North who recruited study participants, one hospital site who supplied and supervised the CTCs who provided the intervention, and the Centre for Rural and Northern Health Research (CRaNHR) who supplied and supervised the RAs. A final thanks to the RAs and the research team in the Aging, Community and Health Research Unit, School of Nursing, McMaster University, Hamilton, Ontario, Canada for supporting this study.
Data Availability
The study data cannot be publicly shared due to ethical restrictions involving potentially identifying information in accordance with the Hamilton Integrated Research Ethics Board (HiREB) for Hamilton Health Sciences and McMaster University’s Faculty of Health Sciences. The participant consent form does not address open public access to the data. Data are available upon request from McMaster University, Faculty of Health Sciences, School of Nursing for researchers who meet the criteria for access to confidential data pending approval from the Hamilton Health Sciences Integrated Research Board. For inquiries, please contact: Dr. Michael McGillion, Associate Professor and Assistant Dean, Research, School of Nursing, Faculty of Health Sciences, McMaster University, Email: mmcgill@mcmaster.ca; Phone: 905-525-9140 x 20275.
Funding Statement
Maureen Markle-Reid and Carrie McAiney received funding from the Ontario SPOR Support Unit IMPACT award (grant no: 60502). Ruta Valaitis, Rebecca Ganann, Maureen Markle-Reid, and Carrie McAiney received funding from the Labarge Foundation, McMaster University. This research was also undertaken, in part, thanks to the funding from Dr. Markle-Reid's Tier 2 CIHR Canada Research Chair.
References
- 1.Birk JL, Kronish IM, Moise N, Falzon L, Yoon S, Davidson KW. Depression and multimorbidity: Considering temporal characteristics of the associations between depression and multiple chronic diseases. Health Psychol. 2019;38(9):802–11. Epub 2019/04/22. doi: 10.1037/hea0000737 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marengoni A, Angleman S, Melis R, Mangialasche F, Karp A, Garmen A, et al. Aging with multimorbidity: a systematic review of the literature. Ageing research reviews. 2011;10(4):430–9. Epub 2011/03/16. doi: 10.1016/j.arr.2011.03.003 . [DOI] [PubMed] [Google Scholar]
- 3.van den Bussche H, Koller D, Kolonko T, Hansen H, Wegscheider K, Glaeske G, et al. Which chronic diseases and disease combinations are specific to multimorbidity in the elderly? Results of a claims data based cross-sectional study in Germany. BMC public health. 2011;11:101. Epub 2011/02/16. doi: 10.1186/1471-2458-11-101 ; PubMed Central PMCID: PMC3050745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Feely A, Lix LM, Reimer K. Estimating multimorbidity prevalence with the Canadian Chronic Disease Surveillance System. Health Promot Chronic Dis Prev Can. 2017;37(7):215–22. Epub 2017/07/14. doi: 10.24095/hpcdp.37.7.02 ; PubMed Central PMCID: PMC5650032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ward BW, Schiller JS. Prevalence of multiple chronic conditions among US adults: estimates from the National Health Interview Survey, 2010. Prev Chronic Dis. 2013;10:E65. Epub 2013/04/27. doi: 10.5888/pcd10.120203 ; PubMed Central PMCID: PMC3652717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barnett K, Mercer SW, Norbury M, Watt G, Wyke S, Guthrie B. Epidemiology of multimorbidity and implications for health care, research, and medical education: a cross-sectional study. Lancet (London, England). 2012;380(9836):37–43. Epub 2012/05/15. doi: 10.1016/s0140-6736(12)60240-2 . [DOI] [PubMed] [Google Scholar]
- 7.Koné Pefoyo AJ, Bronskill SE, Gruneir A, Calzavara A, Thavorn K, Petrosyan Y, et al. The increasing burden and complexity of multimorbidity. BMC public health. 2015;15(1):415. doi: 10.1186/s12889-015-1733-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.King DE, Xiang J, Pilkerton CS. Multimorbidity Trends in United States Adults, 1988–2014. The Journal of the American Board of Family Medicine. 2018;31(4):503–13. doi: 10.3122/jabfm.2018.04.180008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chatterji S, Byles J, Cutler D, Seeman T, Verdes E. Health, functioning, and disability in older adults—present status and future implications. Lancet (London, England). 2015;385(9967):563–75. Epub 2014/12/04. doi: 10.1016/S0140-6736(14)61462-8 ; PubMed Central PMCID: PMC4882096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Salisbury C, Man MS, Bower P, Guthrie B, Chaplin K, Gaunt DM, et al. Management of multimorbidity using a patient-centred care model: a pragmatic cluster-randomised trial of the 3D approach. Lancet (London, England). 2018;392(10141):41–50. Epub 2018/07/03. doi: 10.1016/S0140-6736(18)31308-4 ; PubMed Central PMCID: PMC6041724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kastner M, Cardoso R, Lai Y, Treister V, Hamid JS, Hayden L, et al. Effectiveness of interventions for managing multiple high-burden chronic diseases in older adults: a systematic review and meta-analysis. CMAJ. 2018;190(34):E1004–E12. Epub 2018/08/29. doi: 10.1503/cmaj.171391 ; PubMed Central PMCID: PMC6110649 the Canadian Institutes of Health Research and the Government of Ontario during the conduct of the study; he also reports receiving personal fees from Diabetes Canada, outside the submitted work. Noah Ivers held a volunteer position as Chair of the Ontario Vascular Coalition, which envisioned a multicondition approach to management of chronic diseases and risk factors in primary care. Sharon Marr reports a patent issued for the trademark for the Geriatric Certificate program (February 2013). Geoff Wong reports that his salary is partly supported by The Evidence Synthesis Working Group of the National Institute for Health Research School for Primary Care Research (Project Number 390). He is Deputy Chair of the UK National Institute of Health Research Health Technology Assessment Out-of-Hospital Panel. No other competing interests were declared. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.2018 World Population Data Sheet With Focus on Changing Age Structures â Population Reference Bureau 2018. Available from: https://www.prb.org/2018-world-population-data-sheet-with-focus-on-changing-age-structures/.
- 13.Sinnige J, Braspenning J, Schellevis F, Stirbu-Wagner I, Westert G, Korevaar J. The prevalence of disease clusters in older adults with multiple chronic diseases—a systematic literature review. PloS one. 2013;8(11):e79641. Epub 2013/11/19. doi: 10.1371/journal.pone.0079641 ; PubMed Central PMCID: PMC3823581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Read JR, Sharpe L, Modini M, Dear BF. Multimorbidity and depression: A systematic review and meta-analysis. Journal of affective disorders. 2017;221:36–46. Epub 2017/06/20. doi: 10.1016/j.jad.2017.06.009 . [DOI] [PubMed] [Google Scholar]
- 15.Padayachey U, Ramlall S, Chipps J. Depression in older adults: prevalence and risk factors in a primary health care sample. South African Family Practice. 2017;59(2):61–6. doi: 10.1080/20786190.2016.1272250 [DOI] [Google Scholar]
- 16.Unützer J, Patrick DL, Diehr P, Simon G, Grembowski D, Katon W. Quality adjusted life years in older adults with depressive symptoms and chronic medical disorders. International psychogeriatrics. 2000;12(1):15–33. Epub 2000/05/08. doi: 10.1017/s1041610200006177 . [DOI] [PubMed] [Google Scholar]
- 17.Bock JO, Luppa M, Brettschneider C, Riedel-Heller S, Bickel H, Fuchs A, et al. Impact of depression on health care utilization and costs among multimorbid patients—from the MultiCare Cohort Study. PloS one. 2014;9(3):e91973. Epub 2014/03/19. doi: 10.1371/journal.pone.0091973 ; PubMed Central PMCID: PMC3956806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Strauss R, Kurdyak P, Glazier RH. Mood Disorders in Late Life: A Population-based Analysis of Prevalence, Risk Factors, and Consequences in Community-dwelling Older Adults in Ontario. Canadian journal of psychiatry Revue canadienne de psychiatrie. 2020:706743720927812-. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Blazer DG. Depression in Late Life: Review and Commentary. The Journals of Gerontology: Series A. 2003;58(3):M249–M65. doi: 10.1093/gerona/58.3.m249 [DOI] [PubMed] [Google Scholar]
- 20.Voinov B, Richie WD, Bailey RK. Depression and chronic diseases: it is time for a synergistic mental health and primary care approach. The primary care companion for CNS disorders. 2013;15(2). Epub 2013/08/10. doi: 10.4088/PCC.12r01468 ; PubMed Central PMCID: PMC3733529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Albrecht JS, Gruber-Baldini AL, Hirshon JM, Brown CH, Goldberg R, Rosenberg JH, et al. Depressive symptoms and hospital readmission in older adults. Journal of the American Geriatrics Society. 2014;62(3):495–9. Epub 2014/02/12. doi: 10.1111/jgs.12686 ; PubMed Central PMCID: PMC3959240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Buja A, Claus M, Perin L, Rivera M, Corti MC, Avossa F, et al. Multimorbidity patterns in high-need, high-cost elderly patients. PloS one. 2018;13(12):e0208875–e. doi: 10.1371/journal.pone.0208875 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Neiterman E, Wodchis WP, Bourgeault IL. Experiences of older adults in transition from hospital to community. Can J Aging. 2015;34(1):90–9. doi: 10.1017/S0714980814000518 . [DOI] [PubMed] [Google Scholar]
- 24.Mesteig M HJ, Sletvold O, Rosstad T, Saltvedt I. Unwanted incidents during transition of geriatric patients from hospital to home: a prospective observational study. BMC Health Serv Res. 2010;10(1):1–9. doi: 10.1186/1472-6963-10-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Storm M SI, Laugaland J, Dyrstad DN, Aase K. Quality in transitional care of the elderly: Key challenges and relevant improvement measures. Int J Integr Care. 2014;8(14):e013. doi: 10.5334/ijic.1194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Forster AJ, Clark HD, Menard A, Dupuis N, Chernish R, Chandok N, et al. Adverse events among medical patients after discharge from hospital. Canadian Medical Association Journal. 2004;170(3):345–9. [PMC free article] [PubMed] [Google Scholar]
- 27.Manderson B, McMurray J, Piraino E, Stolee P. Navigation roles support chronically ill older adults through healthcare transitions: a systematic review of the literature. Health Soc Care Community. 2012;20(2):113–27. Epub 2011/10/15. doi: 10.1111/j.1365-2524.2011.01032.x . [DOI] [PubMed] [Google Scholar]
- 28.Gruneir A, Fung K, Fischer HD, Bronskill SE, Panjwani D, Bell CM, et al. Care setting and 30-day hospital readmissions among older adults: a population-based cohort study. Cmaj. 2018;190(38):E1124–e33. Epub 2018/09/27. doi: 10.1503/cmaj.180290 ; PubMed Central PMCID: PMC6157496 Innovations Branch of the Ontario Ministry of Health and Long-Term Care. No other competing interests were declared. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kiran T, Wells D, Okrainec K, Kennedy C, Devotta K, Mabaya G, et al. Patient and caregiver priorities in the transition from hospital to home: results from province-wide group concept mapping. BMJ Qual Saf. 2020;29(5):390–400. Epub 2020/01/08. doi: 10.1136/bmjqs-2019-009993 . [DOI] [PubMed] [Google Scholar]
- 30.Jeffs L, Dhalla I, Cardoso R, Bell CM. The perspectives of patients, family members and healthcare professionals on readmissions: preventable or inevitable? J Interprof Care. 2014;28(6):507–12. Epub 2014/06/11. doi: 10.3109/13561820.2014.923988 . [DOI] [PubMed] [Google Scholar]
- 31.Ontario HQ. Transitions Between Hospital and Home—Health Quality Ontario (HQO) 2020. Available from: https://www.hqontario.ca/Evidence-to-Improve-Care/Quality-Standards/View-all-Quality-Standards/Transitions-Between-Hospital-and-Home. [Google Scholar]
- 32.Almborg AH UK, Thulin A, Berg S. Patients’ perceptions of their participation in discharge planning after acute stroke. J Clin Nurs. 2009;18(2):199–209. doi: 10.1111/j.1365-2702.2008.02321.x [DOI] [PubMed] [Google Scholar]
- 33.Sheikh F GE, Bellatoni M, Christmas C, Lafreniere JP, Arbaje AI. A call to bridge across silos during care transitions. The Joint Commission Journal on Quality and Patient Safety. 2018;44(5):270–8. doi: 10.1016/j.jcjq.2017.10.006 [DOI] [PubMed] [Google Scholar]
- 34.Lowthian J. How do we optimise care transition of frail older people? Age Ageing. 2017;46(1):2–4. doi: 10.1093/ageing/afw171 [DOI] [PubMed] [Google Scholar]
- 35.Hudson R CL, Whichello R. Transitions in a wicked environment. J Nurs Manag. 2014;22(2):201–10. doi: 10.1111/j.1365-2834.2012.1478.x [DOI] [PubMed] [Google Scholar]
- 36.Bauer M FL, Haesler E, Manfrin M. Hospital discharge planning for frail older people and their family. Are we delivering best practice? A review of the evidence. J Clin Nurs. 2009;18(18):2539–46. doi: 10.1111/j.1365-2702.2008.02685.x [DOI] [PubMed] [Google Scholar]
- 37.Jerant AF vF-FM, Moore M. Patients’ perceived barriers to active self-management of chronic conditions. Patient Educ Couns. 2005;57(3):300–7. doi: 10.1016/j.pec.2004.08.004 [DOI] [PubMed] [Google Scholar]
- 38.Schulman-Green D, Jaser SS, Park C, W R. A metasynthesis of factors affecting self-management of chronic illness. J Adv Nurs. 2016;72(7):1469–89. doi: 10.1111/jan.12902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lapointe-Shaw L, Mamdani M, Luo J, Austin PC, Ivers NM, Redelmeier DA, et al. Effectiveness of a financial incentive to physicians for timely follow-up after hospital discharge: a population-based time series analysis. Cmaj. 2017;189(39):E1224–e9. Epub 2017/10/04. doi: 10.1503/cmaj.170092 ; PubMed Central PMCID: PMC5628034 advisory board member to AstraZeneca, Bristol-Myers Squibb, Eli Lilly and Company, GlaxoSmithKline, Hoffman–La Roche, Novartis, Novo Nordisk and Pfizer. Noah Ivers and Muhammad Mamdani have received research grants from the Ontario Ministry of Health and Long-Term Care, unrelated to the current study. Chaim Bell has received consultant fees from the Policy and Innovation Branch of the Ontario Ministry of Health and Long-Term Care. No other competing interests were declared. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bucknall TK, Hutchinson AM, Botti M, McTier L, Rawson H, Hitch D, et al. Engaging patients and families in communication across transitions of care: An integrative review. Patient Education and Counseling. 2020;103(6):1104–17. doi: 10.1016/j.pec.2020.01.017 [DOI] [PubMed] [Google Scholar]
- 41.Naylor MD, Hirschman KB, apos, Connor M, Barg R, Pauly MV. Engaging older adults in their transitional care: what more needs to be done? Journal of Comparative Effectiveness Research. 2013;2:457+. doi: 10.2217/cer.13.58 [DOI] [PubMed] [Google Scholar]
- 42.Ganann R, McAiney C, Johnson W. Engaging older adults as partners in transitional care research. Cmaj. 2018;190(Suppl):S40–s1. Epub 2018/11/09. doi: 10.1503/cmaj.180396 ; PubMed Central PMCID: PMC6472453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Greysen SR, Harrison JD, Kripalani S, Vasilevskis E, Robinson E, Metlay J, et al. Understanding patient-centred readmission factors: a multi-site, mixed-methods study. BMJ Qual Saf. 2017;26(1):33–41. Epub 2016/01/16. doi: 10.1136/bmjqs-2015-004570 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Naylor MD, Hirschman KB, Toles MP, Jarrín OF, Shaid E, Pauly MV. Adaptations of the evidence-based Transitional Care Model in the U.S. Social science & medicine (1982). 2018;213:28–36. Epub 2018/07/29. doi: 10.1016/j.socscimed.2018.07.023 . [DOI] [PubMed] [Google Scholar]
- 45.Brown EL MG, Raue PJ, Moses S, Bruce ML. Recognition of depression among elderly recipients of home care services. Psychiatr Serv. 2003;54(2):208–13. doi: 10.1176/appi.ps.54.2.208 [DOI] [PubMed] [Google Scholar]
- 46.Ell K UJ, Aranda M, Sanchez K, Lee PJ. Routine PHQ-9 depression screening in home health care: depression, prevalence, clinical and treatment characteristics and screening implementation. Home Health Care Serv Q. 2005;24(4):1–19. doi: 10.1300/J027v24n04_01 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Briggs R TK, Kenny RA, Kennelly SP. What is the prevalence of untreated depression and death ideation in older people? Data from the Irish Longitudinal Study on Aging. Int Psychogeriatrics. 2018;30(9):1393–401. doi: 10.1017/S104161021700299X [DOI] [PubMed] [Google Scholar]
- 48.McGilton KS, Vellani S, Yueung L, Chishtie J, Commisso E, Ploeg J, et al. Identifying and understanding the health and social care needs of older adults with multiple chronic conditions and their caregivers: A scoping review. BMC Geriatrics. 2018;231. doi: 10.1186/s12877-018-0925-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lam K, Abrams HB, Matelski J, Okrainec K. Factors associated with attendance at primary care appointments after discharge from hospital: a retrospective cohort study. CMAJ open. 2018;6(4):E587–e93. Epub 2018/12/05. doi: 10.9778/cmajo.20180069 ; PubMed Central PMCID: PMC6277252 Karen Okrainec sits on advisory committees for Health Quality Ontario on care transitions and is principal investigator for a study evaluating the affect of a patient-oriented discharge summary on patient experience and health care use after discharge. No other competing interests were declared. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Markle-Reid M, McAiney C, Forbes D, Thabane L, Gibson M, Browne G, et al. An interprofessional nurse-led mental health promotion intervention for older home care clients with depressive symptoms. BMC Geriatr. 2014;14:62. Epub 2014/06/03. doi: 10.1186/1471-2318-14-62 ; PubMed Central PMCID: PMC4019952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Blewett LA JK, McCarthy T, Lackner T, Brandt B. Improving geriatric transitional care through inter-professional care teams. Journal of Evaluation in Clinical Practice. 2010;16(1):57–63. doi: 10.1111/j.1365-2753.2008.01114.x [DOI] [PubMed] [Google Scholar]
- 52.Coburn KD MS, Lazansky R, Keller M, Davis N. Effect of a community-based nursing intervention on mortality in chronically ill older adults: a randomized controlled trial. PLoS Med. 2012;9(7):e1001265. doi: 10.1371/journal.pmed.1001265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Finlayson K, Chang AM, Courtney MD, Edwards HE, Parker AW, Hamilton K, et al. Transitional care interventions reduce unplanned hospital readmissions in high-risk older adults. BMC Health Serv Res. 2018;18(1):956. doi: 10.1186/s12913-018-3771-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Redmond P, Grimes TC, McDonnell R, Boland F, Hughes C, Fahey T. Impact of medication reconciliation for improving transitions of care (Review). Cochrane Database Syst Rev. 2018;(8). doi: 10.1002/14651858.CD013087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Coleman EA, Parry C, Chalmers S, Min S-J. The Care Transitions Intervention: Results of a Randomized Controlled Trial. Arch Intern Med. 2006;166(17):1822–8. doi: 10.1001/archinte.166.17.1822 [DOI] [PubMed] [Google Scholar]
- 56.Naylor MD. Transitional care for older adults: a cost-effective model. LDI Issue Briefs. 2004;9(6). [PubMed] [Google Scholar]
- 57.Bryant-Lukosius D, Carter N, Reid K, Donald F, Martin-Misener R, Kilpatrick K, et al. The clinical effectiveness and cost-effectiveness of clinical nurse specialist-led hospital to home transitional care: a systematic review. J Eval Clin Pract. 2015;21(5):763–81. Epub 2015/07/03. doi: 10.1111/jep.12401 . [DOI] [PubMed] [Google Scholar]
- 58.Le Berre M, Maimon G, Sourial N, Guériton M, Vedel I. Impact of Transitional Care Services for Chronically Ill Older Patients: A Systematic Evidence Review. Journal of the American Geriatrics Society. 2017;65(7):1597–608. doi: 10.1111/jgs.14828 [DOI] [PubMed] [Google Scholar]
- 59.Toles M, Colón-Emeric C, Naylor MD, Barroso J, Anderson RA. Transitional care in skilled nursing facilities: a multiple case study. BMC Health Serv Res. 2016;16:186. Epub 2016/05/18. doi: 10.1186/s12913-016-1427-1 ; PubMed Central PMCID: PMC4869313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Weeks LE, Macdonald M, Martin-Misener R, Helwig M, Bishop A, Iduye DF, et al. The impact of transitional care programs on health services utilization in community-dwelling older adults: a systematic review. JBI Database System Rev Implement Rep. 2018;16(2):345–84. Epub 2018/02/09. doi: 10.11124/JBISRIR-2017-003486 . [DOI] [PubMed] [Google Scholar]
- 61.Smith SM, Soubhi H, Fortin M, Hudon C, O’Dowd T. Interventions for improving outcomes in patients with multimorbidity in primary care and community settings. Cochrane Database Syst Rev. 2012;(4):Cd006560. Epub 2012/04/20. doi: 10.1002/14651858.CD006560.pub2 . [DOI] [PubMed] [Google Scholar]
- 62.Royal College of Psychiatrists. Suffering in silence: Age inequality in older people’s mental health care. 2018. [Google Scholar]
- 63.Cohen A, Houck PR, Szanto K, Dew MA, Gilman SE, Reynolds CF. Social inequalities in response to antidepressant treatment in older adults. JAMA Psychiatry. 2006;63(1):50–6. doi: 10.1001/archpsyc.63.1.50 [DOI] [PubMed] [Google Scholar]
- 64.Sadana R, Blas E, Budhwani S, Koller T, Paraje G. Healthy ageing: Raising awareness of inequalities, determinants, and what could be done to improve health equity. The Gerontologist. 2016;56(2):S178–93. doi: 10.1093/geronto/gnw034 [DOI] [PubMed] [Google Scholar]
- 65.Naylor MD, Shaid EC, Carpenter D, Gass B, Levine C, Li J, et al. Components of Comprehensive and Effective Transitional Care. Journal of the American Geriatrics Society. 2017;65(6):1119–25. Epub 2017/04/04. doi: 10.1111/jgs.14782 ; PubMed Central PMCID: PMC5497308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kansagara D, Chiovaro JC, Kagen D, Jencks S, Rhyne K, O’Neil M, et al. Transitions of Care from Hospital to Home: An Overview of Systematic Reviews and Recommendations for Improving Transitional Care in the Veterans Health Administration. 2015. [PubMed] [Google Scholar]
- 67.Vedel I, Khanassov V. Transitional Care for Patients With Congestive Heart Failure: A Systematic Review and Meta-Analysis. Ann Fam Med. 2015;13(6):562–71. doi: 10.1370/afm.1844 ; PubMed Central PMCID: PMC4639382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Verhaegh KJ, MacNeil-Vroomen JL, Eslami S, Geerlings SE, de Rooij SE, Buurman BM. Transitional care interventions prevent hospital readmissions for adults with chronic illnesses. Health Aff (Millwood). 2014;33(9):1531–9. doi: 10.1377/hlthaff.2014.0160 . [DOI] [PubMed] [Google Scholar]
- 69.Sox HC, Lewis RJ. Pragmatic trials: Practical answers to “real world” questions. JAMA Guide to Statistics and Methods. 2016;316(11):1205–6. doi: 10.1001/jama.2016.11409 [DOI] [PubMed] [Google Scholar]
- 70.Zwarenstein M, Treweek S, Gagnier JJ, Altman DG, Tunis S, Haynes B, et al. Improving the reporting of pragmatic trials: An extension of the CONSORT statement. BMJ. 2008;337. doi: 10.1136/bmj.a2390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Markle-Reid M, McAiney C., Ganann R., Fisher K., Gafni A., Gauthier A. et al. Study Protocol for a Hospital-to-Home Transitional Care Intervention for Older Adults with Multiple Chronic Conditions and Depressive Symptoms: A Pragmatic Effectiveness-Implementation Trial. BMC Geriatrics. (In Press). doi: 10.1186/s12877-020-01638-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kroenke K, Spitzer RL, Williams JBW. The Patient Health Questionnaire-2: Validity of a two-item depression screener. Medical Care. 2003;41(11):1284–92. doi: 10.1097/01.MLR.0000093487.78664.3C [DOI] [PubMed] [Google Scholar]
- 73.Pfieffer E. A short portable mental status questionnaire for the assessment of organic brain deficit in elderly patients. Journal of the American Geriatrics Society. 1975;23(10):433–41. doi: 10.1111/j.1532-5415.1975.tb00927.x [DOI] [PubMed] [Google Scholar]
- 74.National Institute of Mental Health. Center for Epidemiologic Studies Depression Scale (CES-D) Accessed 27 March 2020. Available from: http://www.chcr.brown.edu/pcoc/cesdscale.pdf.
- 75.Craig P, Dieppe P, Macintyre S, Michie S, Nazareth I, Petticrew M. Developing and evaluating complex interventions: the new Medical Research Council guidance. International journal of nursing studies. 2013;50(5):587–92. Epub 2012/11/20. doi: 10.1016/j.ijnurstu.2012.09.010 . [DOI] [PubMed] [Google Scholar]
- 76.Bandura A. Self-efficacy: toward a unifying theory of behavioral change. Psychol Rev. 1977;84(2):191–215. Epub 1977/03/01. doi: 10.1037//0033-295x.84.2.191 . [DOI] [PubMed] [Google Scholar]
- 77.Carter N, Valaitis RK, Lam A, Feather J, Nicholl J, Cleghorn L. Navigation delivery models and roles of navigators in primary care: a scoping literature review. BMC Health Services Research. 2018;18(1):96. doi: 10.1186/s12913-018-2889-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Canadian Coalition for Senior’s Mental Health. National guidelines for senior’s mental health: The assessment and treatment of depresssion. Toronto, ON: 2006. [Google Scholar]
- 79.National Institute for Clinical Excellence. Depression: Management of depression in primary and secondary care. London: 2004. [Google Scholar]
- 80.Kernick D, Chew-Graham CA, O’Flynn N. Clinical assessment and management of multimorbidity: NICE guideline. The British journal of general practice: the journal of the Royal College of General Practitioners. 2017;67(658):235–6. Epub 2017/04/30. doi: 10.3399/bjgp17X690857 ; PubMed Central PMCID: PMC5409424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Guiding principles for the care of older adults with multimorbidity: an approach for clinicians: American Geriatrics Society Expert Panel on the Care of Older Adults with Multimorbidity. Journal of the American Geriatrics Society. 2012;60(10):E1–e25. Epub 2012/09/22. doi: 10.1111/j.1532-5415.2012.04188.x ; PubMed Central PMCID: PMC4450364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Muth C, Blom JW, Smith SM, Johnell K, Gonzalez-Gonzalez AI, Nguyen TS, et al. Evidence supporting the best clinical management of patients with multimorbidity and polypharmacy: a systematic guideline review and expert consensus. Journal of internal medicine. 2019;285(3):272–88. Epub 2018/10/26. doi: 10.1111/joim.12842 . [DOI] [PubMed] [Google Scholar]
- 83.Hoffmann TC, Glasziou PP, Boutron I, Milne R, Perera R, Moher D, et al. Better reporting of interventions: template for intervention description and replication (TIDieR) checklist and guide. BMJ: British Medical Journal. 2014;348:g1687. doi: 10.1136/bmj.g1687 [DOI] [PubMed] [Google Scholar]
- 84.Powell BJ, Waltz TJ, Chinman MJ, Damschroder LJ, Smith JL, Matthieu MM, et al. A refined compilation of implementation strategies: results from the Expert Recommendations for Implementing Change (ERIC) project. Implementation science: IS. 2015;10:21. Epub 2015/04/19. doi: 10.1186/s13012-015-0209-1 ; PubMed Central PMCID: PMC4328074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Institute CPS. Medication Reconciliation in Home Care 2019. [Google Scholar]
- 86.Nezu AM, Nezu CM, D’Zurilla T. Solving life’s problems: a 5-step guide to enhanced well-being. New York, NY: Springer; 2007. [Google Scholar]
- 87.Kramer RM, Hanna BA, Su S, Wei J. Collective identity, collective trust, and social capital: linking group identification and group cooperation. In: Turner ME, editor. Groups at Work: Theory and Research. 1 ed. New York: Psychology Press; 2014. p. 24. [Google Scholar]
- 88.Strategy for Patient-Oriented Research—CIHR 2018 [updated 2018-06-18]. Available from: https://cihr-irsc.gc.ca/e/41204.html.
- 89.National Committee for Quality Assurance. The Veterans Rand 12-item health survey Accessed 27 March 2020. Available from: https://www.aaos.org/uploadedFiles/PreProduction/Quality/Measures/Veterans%20RAND%2012%20(VR-12).pdf.
- 90.Kazis LE, Rogers WH, Rothendler J, Qian S, Selim A, Edelen MO, et al. Outcome Performance Measure Development for Persons with Multiple Chronic Conditions: RAND Corporation; 2017. [Google Scholar]
- 91.Irwin M, Artin KH, Oxman MN. Screening for depression in the older adult: criterion validity of the 10-item Center for Epidemiological Studies Depression Scale (CES-D). Arch Intern Med. 1999;159(15):1701–4. Epub 1999/08/17. doi: 10.1001/archinte.159.15.1701 . [DOI] [PubMed] [Google Scholar]
- 92.Spitzer RL, Kroenke K, Williams JBW, Lowe B. A brief measure for assessing generalized anxiety disorder: The GAD-7. Archives of Internal Medicine. 2006;166(10):1092–7. doi: 10.1001/archinte.166.10.1092 [DOI] [PubMed] [Google Scholar]
- 93.Löwe B, Decker O, Müller S, Brähler E, Schellberg D, Herzog W, et al. Validation and standardization of the Generalized Anxiety Disorder Screener (GAD-7) in the general population. Med Care. 2008;46(3):266–74. Epub 2008/04/05. doi: 10.1097/MLR.0b013e318160d093 . [DOI] [PubMed] [Google Scholar]
- 94.Weinert C, Brandt P. Measuring social support with the Personal Resource Questionnaire. Western Journal of Nursing Research. 1987;9(4):589–602. doi: 10.1177/019394598700900411 [DOI] [PubMed] [Google Scholar]
- 95.Tawalbeh LI, Ahmad MM. Personal resource questionnaire: a systematic review. J Nurs Res. 2013;21(3):170–7. Epub 2013/08/21. doi: 10.1097/01.jnr.0000432049.31921.ab . [DOI] [PubMed] [Google Scholar]
- 96.Weinert C. Measuring Social Support: PRQ. In: Dilorio C, editor. Measurement of Nursing Outcomes: Self Care and Coping. 2 ed: Springer Publishing Company, 2003; 2003. [Google Scholar]
- 97.de Witte L, Schoot T, Proot I. Development of the Client-Centred Care Questionnaire. Journal of Advanced Nursing. 2006;56(1):62–8. doi: 10.1111/j.1365-2648.2006.03980.x [DOI] [PubMed] [Google Scholar]
- 98.Teale EA, Young JB. A patient reported experience measure (PREM) for use by older people in community services. Age and Ageing. 2015;44(4):667–72. doi: 10.1093/ageing/afv014 [DOI] [PubMed] [Google Scholar]
- 99.Brown G, Gafni A, Roberts J. Approach to the measurement of resource use and costs. Working paper. 2006. [Google Scholar]
- 100.Browne G, Roberts J, Gafni A, Byrne C, Weir R, Majumdar B, et al. Economic evaluations of community-based care: lessons from twelve studies in Ontario. J Eval Clin Pract. 1999;5(4):367–85. Epub 1999/12/01. doi: 10.1046/j.1365-2753.1999.00191.x . [DOI] [PubMed] [Google Scholar]
- 101.Browne G, Roberts J, Byrne C, Gafni A, Weir R, Majumdar B. The costs and effects of addressing the needs of vulnerable populations: results of 10 years of research. Can J Nurs Res. 2001;33(1):65–76. Epub 2002/04/04. . [PubMed] [Google Scholar]
- 102.Markle-Reid M, Gafni A, Ploeg J, Fisher K, Ark P. Health and social service utilization costing manual. McMaster University: Aging, Community and Health Research Unit (ACHRU). 2015;Contract 001. [Google Scholar]
- 103.Drummond M, Weatherly H, Ferguson B. Economic evaluation of health interventions. BMJ. 2008;337:a1204. doi: 10.1136/bmj.a1204 [DOI] [PubMed] [Google Scholar]
- 104.Maruish ME eUsMftS-vHS, 3rd Ed. Lincoln, RI: QualityMetric Inc, 2012.
- 105.Gewandter JS, McDermott MP, Kitt RA, Chaudari J, Koch JG, Evans SR, et al. Interpretation of CIs in clinical trials with non-significant results: systematic review and recommendations. BMJ Open. 2017;7(7):e017288. Epub 2017/07/20. doi: 10.1136/bmjopen-2017-017288 ; PubMed Central PMCID: PMC5726092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Mental Component Summary. In: Preedy VR, Watson R.R., editor. Handbook of Disease Burdens and Quality of Life Measures. New York, NY: Springer; 2010. [Google Scholar]
- 107.Lê Cook B, Manning WG. Thinking beyond the mean: a practical guide for using quantile regression methods for health services research. Shanghai Arch Psychiatry. 2013;25(1):55–9. doi: 10.3969/j.issn.1002-0829.2013.01.011 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Flom P. Quantile regression with PROC QUANTREG2011 November 5, 2020. Available from: https://www.lexjansen.com/nesug/nesug11/sa/sa04.pdf. [Google Scholar]
- 109.McNeish D. Missing data methods for arbitrary missingness with small samples. Journal of Applied Statistics. 2017;44(1):24–39. doi: 10.1080/02664763.2016.1158246 [DOI] [Google Scholar]
- 110.Canadian Institutes of Health Research, Natural Sciences and Engineering Council of Canada, Social Sciences and Humanities Research Council of Canada. Tri-council policy statement: ethical conduct for research involving humans. Ottawa, Ontario: Government of Canada, 2018. [Google Scholar]
- 111.Hopman WM, Towheed T, Anastassiades T, Tenenhouse A, Poliquin S, Berger C, et al. Canadian normative data for the SF-36 health survey. Canadian Medical Association Journal. 2000;163(3):265–71. [PMC free article] [PubMed] [Google Scholar]
- 112.Loudon K, Treweek S, Sullivan F, Donnan P, Thorpe KE, Zwarenstein M. The PRECIS-2 tool: designing trials that are fit for purpose. Bmj. 2015;350:h2147. Epub 2015/05/10. doi: 10.1136/bmj.h2147 . [DOI] [PubMed] [Google Scholar]
- 113.Haines TP, Williams CM, Hill AM, McPhail SM, Hill KD, Brauer SG, et al. Depressive symptoms and adverse outcomes from hospitalization in older adults: secondary outcomes of a trial of falls prevention education. Archives of gerontology and geriatrics. 2015;60(1):96–102. Epub 2014/12/03. doi: 10.1016/j.archger.2014.09.009 . [DOI] [PubMed] [Google Scholar]
- 114.Jansen YJFM, de Bont A, Foets M, Bruijnzeels M, Bal R. Tailoring intervention procedures to routine primary health care practice; an ethnographic process evaluation. BMC Health Services Research. 2007;7(1):125. doi: 10.1186/1472-6963-7-125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Boult C, Leff B, Boyd CM, Wolff JL, Marsteller JA, Frick KD, et al. A matched-pair cluster-randomized trial of guided care for high-risk older patients. Journal of general internal medicine. 2013;28(5):612–21. Epub 2013/01/12. doi: 10.1007/s11606-012-2287-y ; PubMed Central PMCID: PMC3631081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Brown JSL, Murphy C, Kelly J, Goldsmith K. How can we successfully recruit depressed people? Lessons learned in recruiting depressed participants to a multi-site trial of a brief depression intervention (the ‘CLASSIC’ trial). Trials. 2019;20(1):131. doi: 10.1186/s13063-018-3033-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Bower P, Brueton V, Gamble C, Treweek S, Smith CT, Young B, et al. Interventions to improve recruitment and retention in clinical trials: a survey and workshop to assess current practice and future priorities. Trials. 2014;15:399. Epub 2014/10/18. doi: 10.1186/1745-6215-15-399 ; PubMed Central PMCID: PMC4210542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.McDonald AM, Knight RC, Campbell MK, Entwistle VA, Grant AM, Cook JA, et al. What influences recruitment to randomised controlled trials? A review of trials funded by two UK funding agencies. Trials. 2006;7:9. Epub 2006/04/11. doi: 10.1186/1745-6215-7-9 ; PubMed Central PMCID: PMC1475627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Mody L, Miller DK, McGloin JM, Freeman M, Marcantonio ER, Magaziner J, et al. Recruitment and retention of older adults in aging research. Journal of the American Geriatrics Society. 2008;56(12):2340–8. doi: 10.1111/j.1532-5415.2008.02015.x . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Smith SM, Wallace E, Salisbury C, Sasseville M, Bayliss E, Fortin M. A Core Outcome Set for Multimorbidity Research (COSmm). The Annals of Family Medicine. 2018;16(2):132–8. doi: 10.1370/afm.2178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Ford I, Norrie J. Pragmatic Trials. The New England journal of medicine. 2016;375(5):454–63. Epub 2016/08/16. doi: 10.1056/NEJMra1510059 . [DOI] [PubMed] [Google Scholar]
- 122.Bull C, Byrnes J, Hettiarachchi R, Downes M. A systematic review of the validity and reliability of patient-reported experience measures. Health Serv Res. 2019;54(5):1023–35. Epub 2019/06/21. doi: 10.1111/1475-6773.13187 ; PubMed Central PMCID: PMC6736915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Berwick DM, Nolan TW, Whittington J. The triple aim: care, health, and cost. Health Aff (Millwood). 2008;27(3):759–69. Epub 2008/05/14. doi: 10.1377/hlthaff.27.3.759 . [DOI] [PubMed] [Google Scholar]
- 124.Bombard Y, Baker GR, Orlando E, Fancott C, Bhatia P, Casalino S, et al. Engaging patients to improve quality of care: a systematic review. Implementation science: IS. 2018;13(1):98. Epub 2018/07/27. doi: 10.1186/s13012-018-0784-z ; PubMed Central PMCID: PMC6060529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Kuluski K, Peckham A, Gill A, Gagnon D, Wong-Cornall C, McKillop A, et al. What is Important to Older People with Multimorbidity and Their Caregivers? Identifying Attributes of Person Centered Care from the User Perspective. International journal of integrated care. 2019;19(3):4–. doi: 10.5334/ijic.4655 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Chan B, Goldman LE, Sarkar U, Guzman D, Critchfield J, Saha S, et al. High perceived social support and hospital readmissions in an older multi-ethnic, limited English proficiency, safety-net population. BMC Health Services Research. 2019;19(1):334. doi: 10.1186/s12913-019-4162-6 [DOI] [PMC free article] [PubMed] [Google Scholar]