Abstract
The coronavirus disease 2019 (COVID-19) is an emerged infectious disease characterized by a severe pneumonia leading to death in some cases. Currently, no licensed vaccines, drugs, or biologics have been confirmed to be absolutely effective in prophylaxis or treatment of this novel infection. Therefore, the treatment of this highly contagious disease remains a global concern and emergency. The viral interference is a competition phenomenon by which a primary virus infecting a cell prohibits the infection of the same cell by another (secondary) virus. The phenomenon has recently been indicated to be exploited for antiviral strategies. This strategy, particularly when there is no efficient drug against a viral infection, is of high importance. Some researchers have studied the application of the phenomenon among different viruses. In this paper, I discussed the possibility of the application of interference phenomenon in prophylaxis of the disease.
Keywords: COVID-19, SARS-CoV-2, Interference phenomenon, Prophylaxis, Viral infection
Introduction
The coronavirus disease 2019 (COVID-19)is an emerged infectious disease caused by a novel coronavirus called severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). The infection has begun and developed as a type of pneumonia in Wuhan, China, in December 20191 and rapidly spread by human-to-human transmission across China and almost all other countries and currently became a major global health dilemma.2 On March 11, the World Health Organization (WHO) publicly characterized COVID-19 as a pandemic disease.3
Up to now (30 August 2020), 24 854 140 confirmed cases have been infected with SARS-CoV-2 throughout the world and 838,924 have died from the disease.4
Despite the use of some old drugs as probable options for the treatment of COVID-19 in some involved countries,5,6 as well as some researches and efforts to develop an associated efficient vaccine,7-9 currently no licensed vaccines, drugs, or biologics have been verified to be absolutely effective in prevention or for treatment of COVID-19. Therefore, the prophylaxis and treatment of this highly contagious disease remains a global concern and emergency.
Vaccine development for COVID-19
To date, it is claimed that 78 active vaccine projects have been confirmed from various vaccine developers against COVID-19 throughout the world, of which 73 are currently at pre-clinical stages and five in clinical phase.8 The major target for development of the vaccine is one of the main viral structural proteins, the spike (S) protein which is capable to elicit virus neutralizing antibodies blocking virus uptake via the human angiotensin-converting enzyme 2 (ACE2) receptor.8,9 Some researchers used immunoinformatics analysis of SARS-CoV-2 vaccine candidate antigens to design a prophylactic mRNA or peptide-based vaccine against the disease.10 However, none of these developing vaccines are currently licensed to be efficient in preventing the disease. In fact, the procedure of vaccine development is usually time-consuming with associated potential risks, therefore, achieving to an approved and licensed vaccine available for large-scale distribution requires a long period of time.7
Viral interference
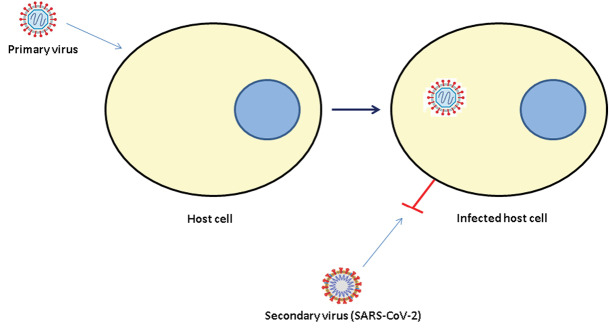
Among prophylaxis options, the “viral interference” has recently been indicated to be exploited for antiviral strategies.11,12 The viral interference is a competition phenomenon by which a primary virus infecting a cell prohibits the infection of the same cell by another (secondary) virus. In fact, the infected cell displays reduced susceptibility to re-infection.13 A good description for this phenomenon can be achieved from a Persian proverb, purporting that “one climate cannot contain two kings” which is equal to the English metaphor: “birds in their little nests agree”.
The viral interference is referred to as homologous (when both the viruses belong to the same family),14 heterotypic (when both the viruses belong to the same species but different serotypes),15 or heterologous (when the viruses are of different families). These can be categorized as co-infections (when there is an interaction between the host cell and the two viruses, at the same time) or super-infections (when one virus interacts with the host cell prior to the second one).16
In general, the phenomenon results in elimination of one virus (exclusion) and survival of the other one (persistence);17 however, in some occasions, the infection with two distinct viruses does not lead to viral interference, and both viruses can coexist in the same host cell (mixed infections); this phenomenon is called viral accommodation.18
Mechanisms involved in viral interference
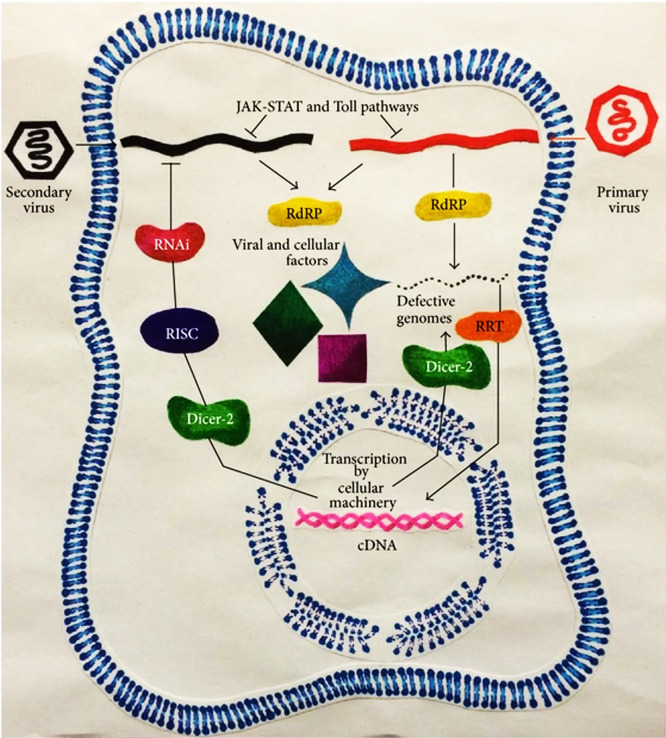
Viral interference is based on competition for cellular factors required for replication and/or translation. However, different mechanisms may be involved in the process. The primary virus might capture the host factors that are critical for the amplification of the secondary virus19,20; this could result in viral interference, particularly during a super-infection. As shown in Fig. 1, the elements of innate immune system, including the Janus kinase-signal transducer and activator of transcription (JAK-STAT) and Toll pathways are implicated in the phenomenon. Viral RNA-dependent RNA polymerase (RdRP) and cellular Retrotransposon Retrotranscriptase (RRT) through the RNase III-like enzyme Dicer-2 are implicated in the formation of viral defective genomes capable of competing for viral and cellular factors that are essential for replication and/or translation of the parental virus. The viral cDNAs made by the RRT could prevent the replication of secondary virus through a pathway involving Dicer-2, RNA-induced silencing complex (RISC), and the RNAi.16
Fig. 1.
A schematic representation of the mechanisms involved in viral interference. Figure adopted from Salas-Benito and Nova-Ocampo.16 RISC: RNA-induced silencing complex.
It has been indicated that the nucleic acid of the interfering virus may be essential for initiating the cellular response that leads to interference. Another probable mechanism by which viral interference occurs is that certain interfering viruses stimulate the cell to produce protein-like substances of non-viral origin called interferons which may prevent super-infection with homologous or heterologous viruses. The interferons can be secreted by an infected cell and transmitted to other cells, thereby rendering them resistant to subsequent infection.21 Consequently, the interference phenomenon occurs generally as a result of occupation or down-regulation of cellular receptors and pathways by the primary infecting virus that inhibits super-infection with a secondary virus.13
The interference phenomenon as an anti-viral tool
Perhaps the first report of viral interference was happened in 1804, when Jenner reported that herpetic infections may inhibit the development of Vaccinia virus lesions.22 Since then, the viral interference has been studied extensively as a phenomenon in a wide variety of animal and plant viruses,23 as well as bacteriophages24 and recently introduced to be exploited as a tool for the treatment of some viral infections.11,12 This strategy, particularly when there is no efficient drug against a viral infection, is of high importance. Moreover, some viruses are often highly pathogenic and routinely counteract and escape from therapeutic intervention through mutations caused by an error-prone genome replication. This type of replication delivers heterogeneous viral strains capable of rapidly adapting to new selection pressures, leading to anti-viral drug resistance.12
Some researchers have studied the application of the phenomenon among different viruses. For example, Ge et al reported that influenza virus had a negative influence on the growth of Newcastle disease virus when they were inoculated simultaneously or consecutively to the chicken embryo.23 It has also been suggested that the continued presence of an influenza virus prevented or modulated the subsequent infection with a different influenza virus.25 Laurie et al stated that infection with one lineage of influenza B virus may benefit in protecting against subsequent infection with the same lineage of this respiratory virus.26 Moreover, this fact that the epidemic peaks of different respiratory viruses occur at different times within populations is due, probably, to the interference phenomenon.11
Kumar et al investigated the viral interference between foot-and-mouth disease virus (FMDV) and peste des petits ruminants virus (PPRV) in goats using BHK-21 cell line. They indicated that the transfection of PPRV RNA as a primary virus, despite Newcastle disease virus (NDV) and rotavirus RNA, resulted in the reduced replication of subsequently transfected FMDV in BHK-21 cell line, suggesting that the interference phenomenon was induced by PPRV RNA against FMDV.17
Another example of viral interference is demonstrated between a common non-pathogenic virus named GB virus C (GBV-C), and HIV. It has been shown that GBV-C replication might cause interference in one or more of the HIV-1 replication steps.27 Moreover, co-infection of HIV-1 and GBV-C has been associated with extended survival among HIV-1 infected patients in clinical studies;28 this may potentially identify an attractive target for the development of novel anti-HIV therapies.29
Applying the interference phenomenon in prophylaxis of COVID-19
According to the aforementioned findings, a proposed solution to overcome the indicated challenges regarding prophylaxis against new coronavirus disease (COVID-19) is to utilize the concept of the interference phenomenon. Perhaps using an appropriate competing non-pathogenic virus or one with low pathogenicity as a primary infecting virus against SARS-CoV-2 may induce and promote the interference phenomenon which may result or help in the prophylaxis of COVID-19 or decrease the morbidity and mortality of the disease.
The application of this phenomenon looks like the application of a live (attenuated) vaccine. However, like other procedures in vaccines development, there are some considerations and limitations ahead. The cost-effectiveness and time-consuming of this proposed approach could be assessed through more experimental researches. First, the type of the primary virus should be determined.
Some instances of primary virus may include the viruses that have common or similar mechanisms of colonization or mode of action in upper respiratory tract compared to SARS-CoV-2. Some examples may include the viruses causing common cold from Coronaviridae family (other than SARS-CoV-2) or Rhinoviruses that have low pathogenicity, as well as other non-pathogenic respiratory viruses, or engineered attenuated ones capable of colonizing the upper respiratory tract. The probable interference between each of these viruses with SARS-CoV-2 should be carefully studied in vitro and intensively investigated using appropriate animal models.
The delivery system of the primary virus is another issue to be determined. The proposed route would be trough respiratory tract inoculation (perhaps as a nasal spray). Moreover, the safety of this interventional method should be considered. Finally, investigation of the prophylactic aspects of this hopeful phenomenon appears to be worthwhile.
In conclusion, although the hypothesis and the mentioned associated findings are encouraging, more related in vitro and in vivo experimental studies, as well as animal model surveys and clinical trials are required to develop and apply this phenomenon as a potential antiviral strategy against SARS-CoV-2 in human. Furthermore, the safety and ethical implications of this type of prophylaxis should be considered and accurately investigated.
Funding sources
There is none to be declared.
Ethical statement
Not applicable.
Competing interests
There is none to be declared.
Report Highlights
What is the current knowledge?
√ The viral interference is a competition phenomenon occurred between some viruses.
√ The phenomenon has recently been indicated to be exploited for antiviral strategies.
√ The coronavirus disease 2019 (COVID-19) is novel emerging infectious disease.
√ Currently no licensed drugs are absolutely effective for the treatment of this disease.
What is new here?
√ This phenomenon would be applied as an alternative option in the prophylaxis of the novel coronavirus infection.
References
- 1.Lu H, Stratton CW, Tang YW. Outbreak of Pneumonia of Unknown Etiology in Wuhan China: the Mystery and the Miracle. J Med Virol. 2020;92:401–2. doi: 10.1002/jmv.25678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lai C-C, Shih T-P, Ko W-C, Tang H-J, Hsueh P-R. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and corona virus disease-2019 (COVID-19): the epidemic and the challenges. Int J Antimicrob Agents. 2020;105924 doi: 10.1016/j.ijantimicag.2020.105924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. WHO Director-General's opening remarks at the media briefing on COVID-19 - 11 March 2020. Available from: https://www.who.int/dg/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---11-march-2020.
- 4. World Health Organization. Coronavirus disease 2019 (COVID-19): Weekly Epidemiological Update, 30 August 2020. Available from: https://www.who.int/publications/m/item/weekly-epidemiological-update---31-august-2020.
- 5. Gao J, Tian Z, Yang X. Breakthrough: Chloroquine phosphate has shown apparent efficacy in treatment of COVID-19 associated pneumonia in clinical studies. Biosci Trends 2020. 10.5582/bst.2020.01047 [DOI] [PubMed]
- 6.Lu H. Drug treatment options for the 2019-new coronavirus (2019-nCoV) Biosci Trends. 2020;14:69–71. doi: 10.5582/bst.2020.01020. [DOI] [PubMed] [Google Scholar]
- 7.Graham BS. Rapid COVID-19 vaccine development. Science. 2020;368:945–6. doi: 10.1126/science.abb8923. [DOI] [PubMed] [Google Scholar]
- 8.Le TT, Andreadakis Z, Kumar A, Roman RG, Tollefsen S, Saville M. et al. The COVID-19 vaccine development landscape. Nat Rev Drug Discov. 2020;19:305–6. doi: 10.1038/d41573-020-00073-5. [DOI] [PubMed] [Google Scholar]
- 9.Wu SC. Progress and Concept for COVID‐19 Vaccine Development. Biotechnol J. 2020;15:e2000147. doi: 10.1002/biot.202000147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pourseif MM, Parvizpour S, Jafari B, Dehghani J, Naghili B, Omidi Y. A domain-based vaccine construct against SARS-CoV-2, the causative agent of COVID-19 pandemic: development of self-amplifying mRNA and peptide vaccines. BioImpacts. 2021;11:65–84. doi: 10.34172/bi.2021.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kovesdi I, Bakacs T. Therapeutic exploitation of viral interference. Infect Disord Drug Targets. 2020;20:423–32. doi: 10.2174/1871526519666190405140858. [DOI] [PubMed] [Google Scholar]
- 12.Tanner EJ, Kirkegaard KA, Weinberger LS. Exploiting genetic interference for antiviral therapy. PLoS Genet. 2016;12:e1005986. doi: 10.1371/journal.pgen.1005986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Remion A, Delord M, Saragosti S, Mammano F. Co-infection, super-infection and viral interference in HIV. Retrovirology. 2013;10:P72. doi: 10.1186/1742-4690-10-S1-P72. [DOI] [Google Scholar]
- 14.Bolling BG, Olea-Popelka FJ, Eisen L, Moore CG, Blair CD. Transmission dynamics of an insect-specific flavivirus in a naturally infected Culex pipiens laboratory colony and effects of co-infection on vector competence for West Nile virus. Virology. 2012;427:90–7. doi: 10.1016/j.virol.2012.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dittmar D, Castro A, Haines H. Demonstration of interference between dengue virus types in cultured mosquito cells using monoclonal antibody probes. J Gen Virol. 1982;59:273–82. doi: 10.1099/0022-1317-59-2-273. [DOI] [PubMed] [Google Scholar]
- 16.Salas-Benito JS, Nova-Ocampo D. Viral interference and persistence in mosquito-borne flaviviruses. J Immunol Res. 2015;2015:873404. doi: 10.1155/2015/873404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kumar N, Barua S, Riyesh T, Chaubey KK, Rawat KD, Khandelwal N. et al. Complexities in isolation and purification of multiple viruses from mixed viral infections: viral interference, persistence and exclusion. PloS one. 2016;11:e156110. doi: 10.1371/journal.pone.0156110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sivaram A, Barde PV, Gokhale MD, Singh DK, Mourya DT. Evidence of co-infection of chikungunya and densonucleosis viruses in C6/36 cell lines and laboratory infected Aedes aegypti (L) mosquitoes. Parasit Vectors. 2010;3:95. doi: 10.1186/1756-3305-3-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Karpf AR, Lenches E, Strauss EG, Strauss JH, Brown DT. Superinfection exclusion of alphaviruses in three mosquito cell lines persistently infected with Sindbis virus. J Virol. 1997;71:7119–23. doi: 10.1128/JVI.71.9.7119-7123.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Singh IR, Suomalainen M, Varadarajan S, Garoff H, Helenius A. Multiple mechanisms for the inhibition of entry and uncoating of superinfecting Semliki Forest virus. Virology. 1997;231:59–71. doi: 10.1006/viro.1997.8492. [DOI] [PubMed] [Google Scholar]
- 21.Wagner RR. Viral interference Some considerations of basic mechanisms and their potential relationship to host resistance. Bacteriol Rev. 1960;24:151. doi: 10.1128/br.24.1.151-166.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jenner E. Letter to the editors of the Medical and Physical Journal. Med Phys J. 1804;12:97. [PMC free article] [PubMed] [Google Scholar]
- 23.Ge S, Zheng D, Zhao Y, Liu H, Liu W, Sun Q. et al. Evaluating viral interference between Influenza virus and Newcastle disease virus using real-time reverse transcription–polymerase chain reaction in chicken eggs. Virol J. 2012;9:128. doi: 10.1186/1743-422X-9-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Delbrück M, Luria SE. Interference between bacterial viruses I Interference between two bacterial viruses acting upon the same host, and the mechanism of virus growth. Arch Biochem. 1942;1:111–41. [Google Scholar]
- 25.Laurie KL, Guarnaccia TA, Carolan LA, Yan AW, Aban M, Petrie S. et al. Interval between infections and viral hierarchy are determinants of viral interference following influenza virus infection in a ferret model. J Infect Dis. 2015;212:1701–10. doi: 10.1093/infdis/jiv260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Laurie KL, Horman W, Carolan LA, Chan KF, Layton D, Bean A. et al. Evidence for viral interference and cross-reactive protective immunity between influenza B virus lineages. J Infect Dis. 2018;217:548–59. doi: 10.1093/infdis/jix509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bagasra O, Bagasra AU, Sheraz M, Pace DG. Potential utility of GB virus type C as a preventive vaccine for HIV-1. Expert Rev Vaccines. 2012;11:335–47. doi: 10.1586/erv.11.191. [DOI] [PubMed] [Google Scholar]
- 28.Bhattarai N, Stapleton JT. GB virus C: the good boy virus? Trends Microbiol. 2012;20:124–30. doi: 10.1016/j.tim.2012.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Timmons CL, Shao Q, Wang C, Liu L, Liu H, Dong X. et al. GB virus type C E2 protein inhibits human immunodeficiency virus type 1 assembly through interference with HIV-1 gag plasma membrane targeting. J Infect Dis. 2013;207:1171–80. doi: 10.1093/infdis/jit001. [DOI] [PMC free article] [PubMed] [Google Scholar]