Abstract
Study Objectives:
The efficacy of portable-monitor (PM) sleep testing in children is not well understood. While most studies have evaluated PM in a lab setting, the utility of PM in the home environment is relatively unknown. We sought to determine whether home PM accurately diagnoses obstructive sleep apnea in adolescents and to assess patient satisfaction with home PM sleep testing.
Methods:
We evaluated adolescents (age 12–18 years) with suspected obstructive sleep apnea using a PM device. In addition to in-laboratory polysomnography (PSG), all participants had PM testing performed twice, once in their home and once concurrent to in-laboratory PSG. PM was compared to PSG using 2 primary outcomes: the apnea-hypopnea index and oxygen desaturation index. All participants were approached for interview to evaluate their experience with PM sleep testing.
Results:
Twenty adolescents participated. Bland-Altman analysis comparing the apnea-hypopnea index and oxygen desaturation index determined by home or in-laboratory PM to in-laboratory PSG revealed mostly agreement; however, some deviations were observed when either parameter was markedly increased. While PM testing tended to underestimate the apnea-hypopnea index, the diagnostic agreement between home PM and PSG was 80% (by the White-Westbrook method). Most preferred PM to PSG and found PM easy to very easy to set up.
Conclusions:
In a small cohort of adolescents, our study supports the application of home PM in the diagnosis of suspected obstructive sleep apnea. Until studies implementing PM using larger cohorts become readily available, the findings from this preliminary study could contribute to adolescents receiving sleep apnea therapy more promptly.
Clinical Trial Registration:
Registry: ClinicalTrials.gov; Identifier: NCT03748771. At the time of issue publication, this registration is not publicly available because the trial includes a device that is not approved or cleared for use in pediatric populations. Once the device is FDA cleared, the registration will become public.
Citation:
Bhattacharjee R, Benjafield A, Blase A, et al. The accuracy of a portable sleep monitor to diagnose obstructive sleep apnea in adolescent patients. J Clin Sleep Med. 2021;17(7):1379–1387.
Keywords: obstructive sleep apnea, home sleep apnea testing, polysomnography, pediatrics, adolescents, diagnosis
BRIEF SUMMARY
Current Knowledge/Study Rationale: Portable-monitor (PM) sleep testing to diagnose obstructive sleep apnea in children in the home environment is understudied. Here we present 20 adolescents who underwent PM testing performed at home and in the lab, and the results demonstrated agreement of the apnea-hypopnea index and the oxygen desaturation index when PM was compared to polysomnogram using Bland-Altman analysis.
Study Impact: Using the White-Westbrook method, diagnostic agreement between home PM and polysomnogram was 80%. Most adolescents found PM easy to very easy to set up, and preferred PM to polysomnogram. Taken together, this study supports broader application of PM in adolescent patients with suspected obstructive sleep apnea and could contribute to adolescents receiving sleep apnea therapy more promptly.
INTRODUCTION
Obstructive sleep apnea (OSA) in children is a highly prevalent disorder with major health consequences.1,2 Conservative estimates of prevalence are that approximately 2–3% of all children have OSA.3–5 Moreover, given the childhood obesity pandemic and the link to pediatric OSA,6,7 the observed prevalence of OSA is likely to continue increasing.6,8–11
Currently, the gold standard for OSA diagnostic testing consists of overnight attended in-laboratory polysomnography (PSG). With the lack of access to pediatric PSG labs, including the availability of sleep physicians and sleep technologists with pediatric training, the diagnosis of OSA in children remains particularly challenging. In addition, PSG in children is costly and often technically difficult and inconvenient for families.12 Not surprisingly, treatment of OSA in children is most often performed without confirmatory diagnostic PSG.13
Similar issues have impacted PSG diagnostic testing in adults with suspected OSA. Subsequently, this situation has led the American Academy of Sleep Medicine to approve the use of a portable monitor (PM) to conduct home sleep apnea testing (HSAT) as an alternative to PSG.14 HSAT is more appealing compared to PSG since HSAT is less costly, more convenient for patients, and less cumbersome. Thus, HSAT has gained major traction for adult OSA diagnostic testing. The American Academy of Sleep Medicine suggests that HSAT should include measurement of airflow, respiratory effort, and blood oxygenation consistent with what is defined as level 3 sleep testing.14
Several recent studies have evaluated the utility of level 3 PM in children.12,15–21 Although the presentation and treatment of OSA in children differs, OSA among adolescent children, particularly obese children, very much resembles OSA in adults.22 Given this finding, HSAT may be an attractive alternative to in-lab PSG for adolescent children.
The aims of this study were: (1) to evaluate the accuracy of HSAT compared to in-lab PSG to diagnose OSA in adolescent children, and (2) to identify patient satisfaction with HSAT in the comfort of their own home compared to their experience during in-lab PSG.
METHODS
This prospective study was approved by the University of California San Diego Institutional Research Board (#171173). Participants approached for recruitment were patients from Rady Children’s Hospital, a tertiary care pediatric hospital, between January 2019 and January 2020. Children aged 12–18 years undergoing a clinically indicated PSG to rule out OSA were approached for recruitment. A sample size of 20 adolescent patients was targeted. Children with genetic syndromes, craniofacial abnormalities, metabolic storage diseases, and congenital cardiac disease and/or pulmonary hypertension were excluded. All patients who provided written consent were included in the study. Basic demographic characteristics were collected at time of recruitment.
Study design
At time of recruitment, children were randomly selected to undergo HSAT no longer than 1 week prior to their in-lab PSG or to undergo HSAT no later 1 one week following their in-lab PSG.
For HSAT, all participants used the ResMed ApneaLink Air device (ResMed, San Diego, CA). The ApneaLink Air is a PM device that assesses sleep-disordered breathing using 5 channels of recorded information: respiratory effort, pulse and pulse oxygen saturation, nasal flow, body position, and snoring.
In addition to HSAT, all patients underwent PM testing concurrent with their in-lab PSG, termed ApneaLink Lab Testing (ALT). In this configuration, application of PM-belt pulse oximeter was performed in isolation of PSG equipment. However, the nasal cannula flow signal from the patient was split to both the PSG recording device and the PM recording device. Therefore, patients had PM performed twice, once at home (HSAT) and once during their in-laboratory PSG (ALT).
Pediatric sleep studies were conducted at Rady Children’s Hospital Sleep Laboratory and were performed by sleep technicians with considerable experience in performing pediatric sleep studies. In-laboratory PSG utilized Nihon Kohden PSG equipment and software (Tokyo, Japan). Children were studied for up to 10 hours in a quiet, darkened room maintained at an ambient temperature of 24°C in the presence of one of their parents. No drugs were used to induce sleep. The following parameters were measured: chest and abdominal wall movement by inductance plethysmography, heart rate by electrocardiography, and air flow was monitored with a side- stream end-tidal capnograph (Nihon Kohden) that also provided breath-by-breath assessment of end-tidal carbon dioxide levels, a nasal pressure cannula, and an oronasal thermistor. Arterial pulse oxygen saturation was assessed by oximetry (Nihon Kohden), with simultaneous recording of the pulse waveform. The bilateral electro-oculogram, 6 channels of electroencephalogram (2 frontal, 2 occipital, and 2 central leads), chin and anterior tibial electromyograms, and analog output from a body position sensor were also monitored.
All PSG scoring (by technician) and interpretation (by physician) was performed blinded to the results from both HSAT and ALT and used American Academy of Sleep Medicine scoring criteria.23 PSG parameters examined included the total sleep apnea-hypopnea index (AHI), oxygen desaturation index (ODI), percent of total sleep time with oxygen saturation < 90%, percent of total sleep time with end tidal CO2 above 50 mm Hg, and oxygen saturation nadir during sleep. OSA was determined based on the AHI, where mild, moderate, and severe OSA were defined as an AHI of 1.5 to < 5, 5 to < 1,0 and ≥ 10 events/h, respectively.
All HSAT and ALT scoring was performed by a single registered sleep technologist (G.D.) and interpreted by a single pediatric sleep physician (R.B.) using AirView Web-based software. Scoring of HSAT and ALT was completely blinded from each other and blinded from the results of the in-lab PSG.
Following completion of the study, all participants were approached via telephone to survey their experiences with HSAT and compare both home and in-lab testing environments.
Statistical analysis
Descriptive statistics were used to summarize the children’s demographic and polysomnographic characteristics. Comparisons between in-laboratory PSG, HSAT, and ALT outcomes were conducted using one-way ANOVA. Agreement between PSG, HSAT, and ALT was done by measuring the intraclass correlation coefficient (ICC)–two-way mixed model as well as by Bland-Altman analysis of the 2 primary outcomes of interest, the AHI and ODI. For nonparametric data, we used log transformation to linearize the outcomes and we used the White-Westbrook method24 modified for pediatric analyses. The White-Westbrook method is a well-established technique in sleep research to allow comparisons between diagnostic tests that account for the nonlinearity in the AHI scale. Values of AHI > 40 events/h are classified as severe for White-Westbrook in adults, so we used a threshold of 10 events/h to be consistent with the pediatric literature. Diagnostic agreement was defined as an AHI ≥ 10 events/h on both systems (PM and PSG) or, if AHI < 10 events/h on PSG, the AHI was within 5 events/h on both systems. An overestimate of AHI was defined as an AHI of 5 events/h greater on PM than on PSG (both < 10 events/h). An underestimate of AHI was defined as an AHI of 5 events/h less on PM than on PSG (both < 10 events/h). A P-value < .05 was considered statistically significant.
RESULTS
Twenty children were recruited for this study. Eight of 20 children were arbitrarily assigned to have their HSAT performed before their PSG date.
Of the entire cohort (n = 20), the mean ± standard deviation age was 14.5 ± 1.7 years, and 7 of 20 (35%) participants were female. Most patients (15/20 or 75%) were obese with a body-mass index exceeding the 95th percentile for age and sex using US Centers for Disease Control normative data (https://www.cdc.gov/growthcharts/). The mean ± standard deviation body-mass index was 32.0 ± 10.7 kg/m2 (Table 1).
Table 1.
Demographic summary of study population.
| Factor | Values |
|---|---|
| Age (y) | 14.5 ± 1.72 (12.4–17.7) |
| Sex: female | 7 (35%) |
| Sex: male | 13 (65%) |
| BMI (kg/m2) | 32.0 ± 10.7 (14.5–55.8) |
| Nonobese | 5 (25%) |
| Obese | 15 (75%) |
| Race/ethnicity | |
| White non-Hispanic | 7 (35%) |
| Hispanic | 11 (55%) |
| Pacific Islander | 1 (5%) |
| Other | 1 (5%) |
Results expressed as mean ± standard deviation with (range) or number with (percentage). BMI = body mass index.
Evaluation of PSG revealed that average total sleep time was 5 hours and 7 minutes with an average recording time of 6 hours and 28 minutes (Table 2). The mean ± standard deviation sleep efficiency of all 20 participants was 79.2 ± 13.6%. Total recording time from the 20 participants undergoing HSAT revealed a significantly greater recording time at home at 9 hours, 23 minutes compared to PSG (6 hours, 28 minutes, P < .001). Of the 20 patients undergoing PSG, only 19 had available ALT data as 1 ALT recording was not started by the study personnel inadvertently, resulting in an absence of data. Although not statistically significant, the total average recording time of ALT was also slightly higher than PSG (Table 2), but this finding is likely related to the fact that ALT was started by study personnel earlier than the lights were turned off which signifies the start time of PSG.
Table 2.
Summary of study qualities.
| hh:mm | Percent Recording Time | |
|---|---|---|
| Polysomnogram (n = 20) | ||
| Recording time | 6:28 ± 0:31 (5:07–7:29) | |
| Total sleep time | 5:07 ± 0:57 (3:17–6:24) | |
| Sleep efficiency (%) | 79.2 ± 13.6 (49.5–97.6) | |
| Home ApneaLink (n = 20) | ||
| Recording time | 9:23 ± 1:35 (6:02–12:00)* | |
| Flow monitoring time | 6:09 ± 3:01 (0:47–11:46) | 65.2 ± 28.2** |
| SpO2 duration | 8:08 ± 2:19 (2:53–11:49) | 86.7 ± 19.7 |
| Lab ApneaLink (n = 19) | ||
| Recording time | 6:48 ± 1:10 (3:38–12:00) | |
| Flow monitoring time | 5:53 ± 1:18 (3:13–7:48) | 86.9 ± 11.4 |
| SpO2 duration | 5:54 ± 1:37 (2:14–8:10) | 86.3 ± 17.6 |
Results expressed as mean ± standard deviation (range). *P < .001 comparing Home ApneaLink (HSAT) to PSG recording time and to Lab ApneaLink (ALT) recording time. **P = .004 comparing Home ApneaLink to Lab ApneaLink flow monitoring time. hh:mm = hours:minutes, SpO2 = oxygen saturation.
Evaluation of signal quality strength of flow and oximetry from both HSAT and ALT revealed that PM flow monitoring time was significantly reduced at home (HSAT) compared to lab (ALT) testing (65.2% vs 86.9%, P = .004). Total flow monitoring time in ALT was reduced compared to total PSG recording time, reflecting the loss of flow signal even during the in-lab study. Although flow signals were monitored by the sleep technologist during the in-lab PSG and ALT, the net loss of flow signal reflects the cumulative episodes of the nasal cannula being displaced or mouth breathing, which occurs frequently during pediatric PSG.
In contrast, oximetry signal was stable in 86.7 ± 19.7% of HSAT and 86.3 ± 17.6% of ALT, and this was not significantly different (Table 2). Successful HSAT in which the blinded investigator believed there was sufficient signal quality to facilitate an interpretation was observed in 15 of 20 participants (75%), whereas successful ALT was seen in 17 of 19 participants (89%).
The mean ± standard deviation AHI from HSAT was 6.2 ±10.7 events/h; from ALT was 11.6 ± 21.8 events/h and from PSG was 18.6 ± 35.8 events/h (Table 3). The median AHI from HSAT was 2.2 events/h, from ALT was 2.4 events/h, and from PSG was 2.9 events/h. Although the PSG AHI was higher than HSAT or ALT, this difference was not statistically significant. The ODI from HSAT was 10.9 ± 11.4 events/h, from ALT was 16.9 ± 24.0 events/h and from PSG was 13.5 ± 26.7 events/h; there were no observed significant differences across all groups.
Table 3.
Summary of sleep-disordered breathing indices.
| AHI | ODI | Normal (AHI < 1.5 Events/h) | Mild (1.5 < AHI < 5 Events/h) | Moderate (5 < AHI < 10 Events/h) | Severe (AHI > 10 Events/h) | |
|---|---|---|---|---|---|---|
| Polysomnogram (n = 20) | 18.6 ± 35.8(0.7–118.3) [3.0; 11.1] | 13.5 ± 26.7(0.0–85.0) [2.3; 7.4] | 3 (15%) | 12 (60%) | 0 (0%) | 5 (25%) |
| Home ApneaLink (n = 20) | 6.2 ± 10.7(0.2–48.5) [2.6; 6.3] | 10.9 ± 11.4(1.0–49.1) [7.4; 1.0] | – | 7 (35%) | 4 (20%) | 3 (15%) |
| Lab ApneaLink (n = 19) | 11.6 ± 21.8(0.0–73.7) [2.4; 5.6] | 16.9 ± 24.0 (0.4–77.4) [8.5; 10.1] | 7 (35%) | 7 (35%) | 1 (5%) | 4 (20%) |
Results expressed as mean ± standard deviation (range) [median; interquartile range] or number (percentage). AHI = apnea-hypopnea index, ODI = oxygen desaturation index.
From PSG, 17 of 20 children were identified as having OSA with 5 children identified with having either moderate or severe OSA (AHI > 5 events/h) (Table 3). In contrast, HSAT identified 14 of 20 children with OSA and 7 children having moderate or severe OSA. ALT identified 12 of 19 children with OSA and 5 children having moderate or severe OSA.
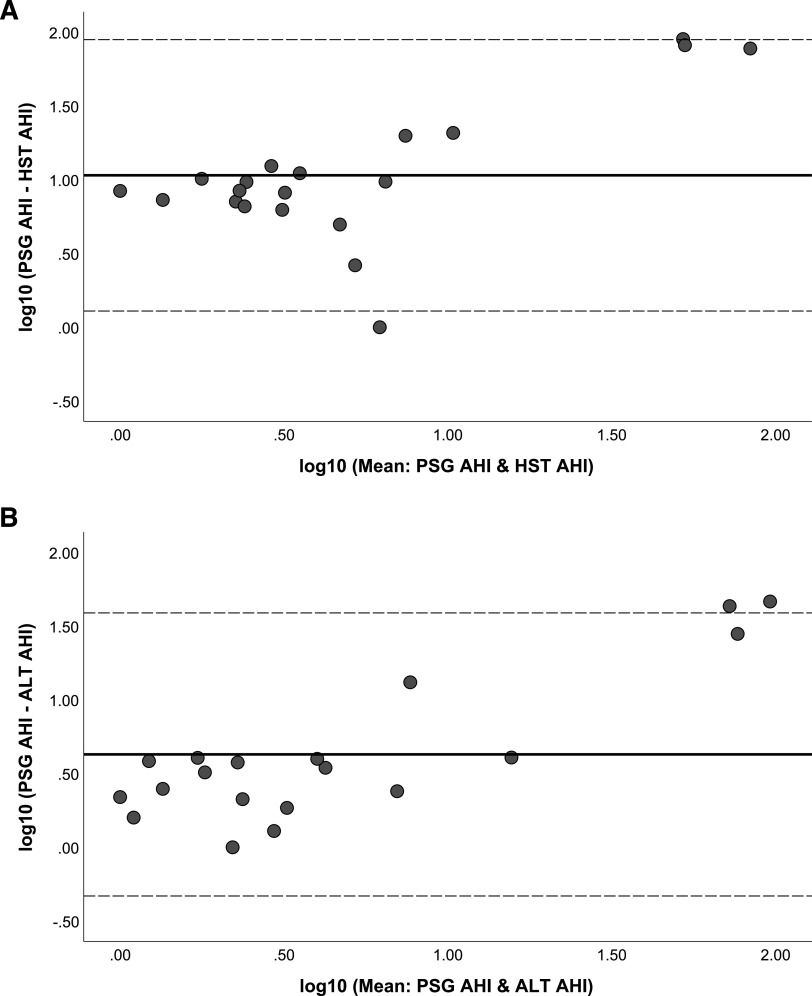
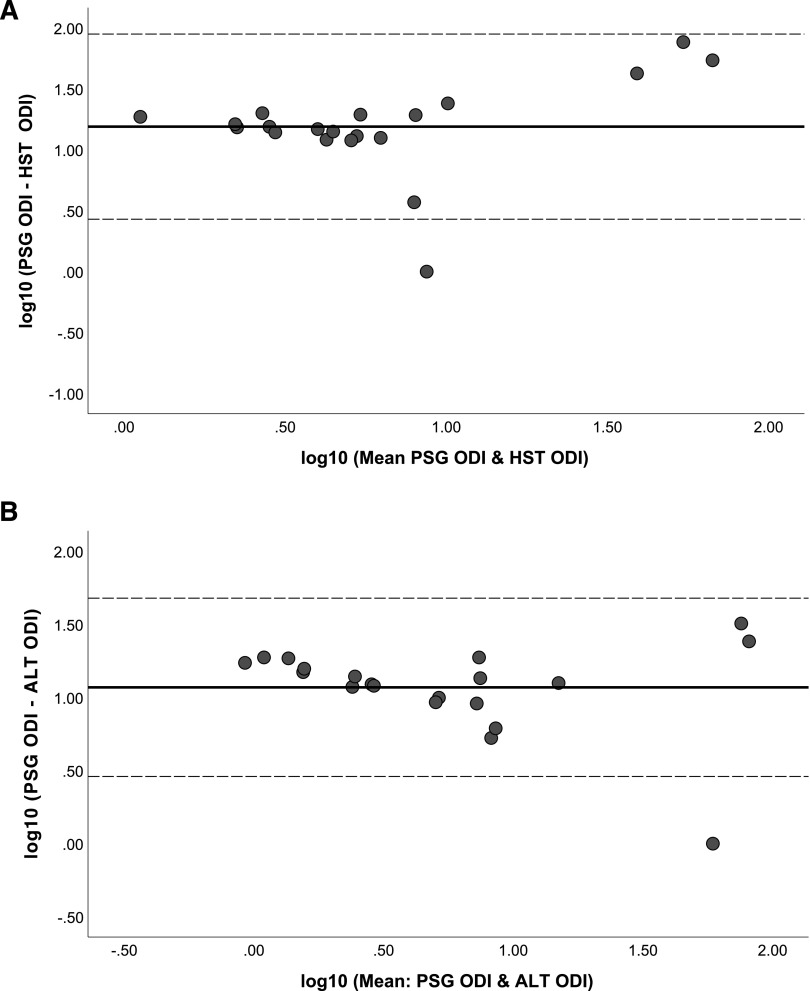
Performing Bland-Altman analysis comparing the primary outcomes, namely PSG-identified parameters of AHI and ODI to HSAT and ALT (Figure 1 and Figure 2), revealed mostly agreement; however, there were deviations in both the AHI and the ODI when either parameter was increased. In general, both the HSAT and the ALT underestimated the AHI compared to the PSG (ie, net positive difference), and this observation was more consistent with the ALT (Figure 1). Comparing the ODI difference when measured from the PSG to either the HSAT or the ALT (Figure 2) did not reveal any marked differences. In general, there was not a consistent underestimation of the ODI (ie, net positive difference) by the HSAT and the ALT as was observed with the AHI (Figure 1).
Figure 1. Bland-Altman analysis evaluating accuracy of AHI measure.
(A) Bland-Altman analysis comparing PSG AHI to HSAT AHI. (B) Bland-Altman analysis comparing PSG AHI to ALT AHI. AHI = apnea-hypopnea index, ALT = ApneaLink Lab Test, HSAT (HST) = home sleep apnea test, PSG = polysomnogram.
Figure 2. Bland-Altman analysis evaluating accuracy of ODI measure.
(A) Bland-Altman analysis comparing PSG ODI to HSAT ODI. (B) Bland-Altman analysis comparing PSG ODI to ALT ODI. ALT = ApneaLink Lab Test, HSAT (HST) = home sleep apnea test, ODI = oxygen desaturation index, PSG = polysomnogram.
Comparing Bland-Altman analysis of pre-PSG participants (n = 8) vs post-PSG HSAT (n = 12) to the in-lab PSG, no differences between both groups were observed (Figure S1 (369KB, pdf) and Figure S2 (369KB, pdf) in the supplemental material). Again, in both cases, when either the observed AHI or ODI increased, there was a marked difference between PSG and HSAT measures (Figure S1 (369KB, pdf) and Figure S2 (369KB, pdf) ).
In all participants, there was a strong correlation between both the AHI (Figure 3A) [ICC: 0.933 (0.805–0.975)] and ODI (Figure 3B) [ICC: 0.979 (0.943–0.992)] when PSG was compared to ALT; however, a weak correlation was observed when HSAT AHI [ICC: 0.592 (0.041–0.833)] and HSAT ODI [ICC: 0.779 (0.442–0.913)] were compared to PSG.
Figure 3. Correlation analysis evaluating accuracy of AHI and ODI measures.
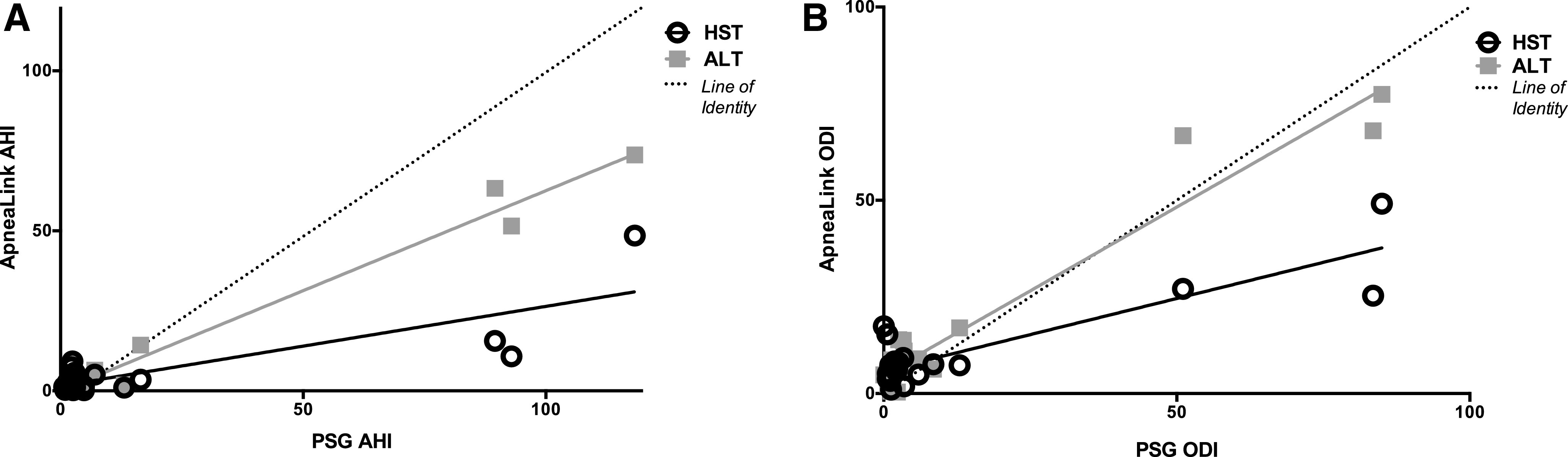
(A) Correlation analysis comparing AHI measured by HSAT and ALT to PSG. HSAT AHI ρ = 0.386, P = .093. ALT AHI ρ = 0.790, P < .001. (B) Correlation analysis comparing ODI measured by HSAT and ALT to PSG. HSAT ODI ρ = 0.334, P = .146. ALT AHI ρ = 0.770, P < .001. AHI = apnea-hypopnea index, ALT = ApneaLink Lab Test, HSAT (HST) = home sleep apnea test, ODI = oxygen desaturation index, PSG = polysomnogram.
Assessing agreement using the modified White-Westbrook method (Table 4) revealed that 80% of HSAT studies attained diagnostic agreement with PSG, with 2 studies underestimating the AHI. There was slighter greater diagnostic agreement with ALT AHI compared to the PSG AHI (Table 4).
Table 4.
Diagnostic agreement between HSAT and ALT with PSG.
| HSAT (n = 20) | ALT (n = 19) | |
|---|---|---|
| Diagnostic agreement (%) | 16 (80%) | 18 (95%) |
| Overestimate of AHI (%) | 2 (10%) | 0 (0%) |
| Underestimate of AHI (%) | 2 (10%) | 1 (5%) |
Results expressed as number (percentage). AHI = apnea-hypopnea index, ALT = ApneaLink Lab Test, HSAT = home sleep apnea test, PSG = polysomnogram.
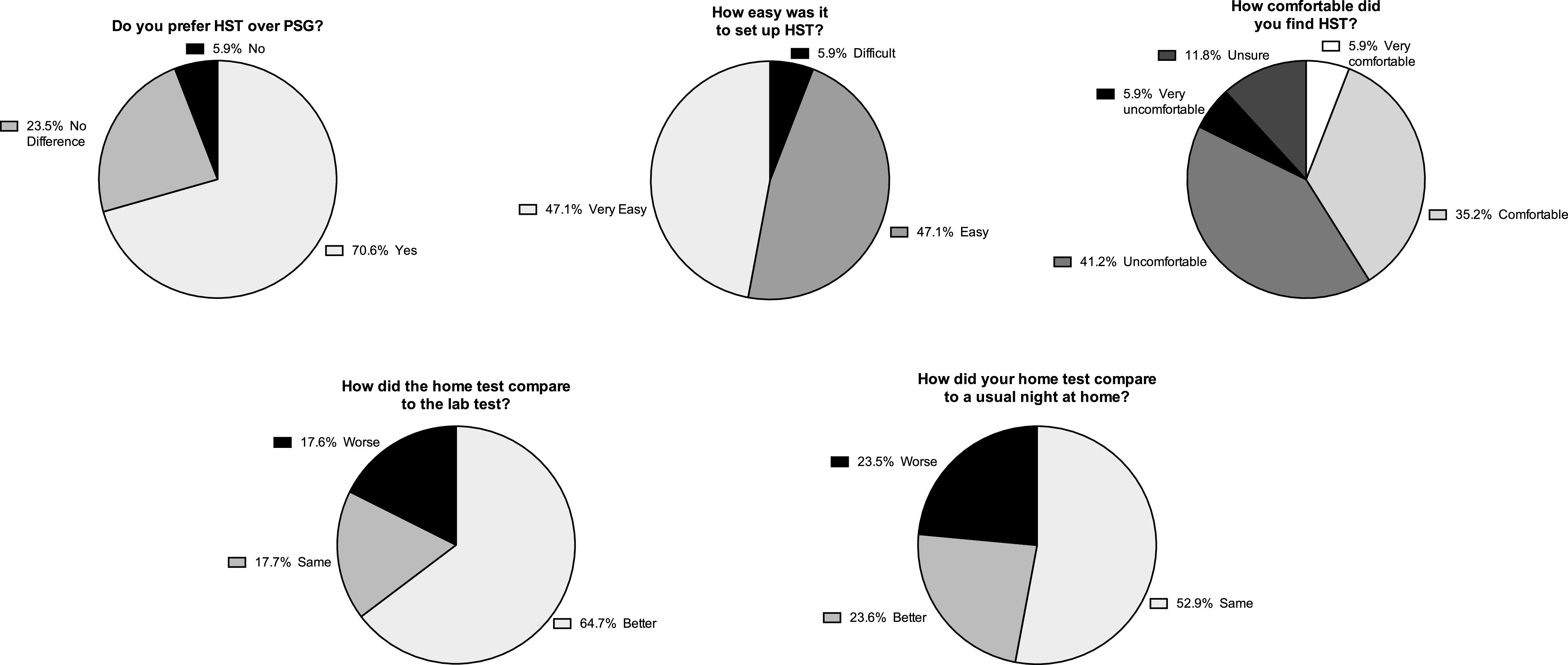
Using telephone surveys, we then evaluated patient satisfaction with and patient preference for HSAT compared to their experience in the sleep lab when undergoing PSG (Figure 4). Only 17 of 20 participants (85%) were reachable for these surveys. As observed in all specific questions addressed, most patients preferred and were satisfied with HSAT compared to in-lab PSG, and the majority of patients felt it was easy to use (Figure 4).
Figure 4. Patient opinions of and preferences for HST compared to PSG. HST = home sleep apnea test, PSG = polysomnogram.
DISCUSSION
Our study adds important findings to the literature for a number of reasons. First, we have observed acceptable values for diagnostic home sleep apnea testing in an adolescent population. These data provide reassurance that in this age group, diagnosis of pediatric sleep apnea does not always require polysomnography. Second, using various analyses, the correspondence between home testing and in-laboratory polysomnography was acceptable. Indeed, gold standard polysomnography was perhaps limited by modest recording time and sleep time; perhaps the home environment, which allows for a greater opportunity to sleep, may align sleep testing with the patient’s own sleep-wake schedule and may yield more “real-life” results. Third, unlike previous studies evaluating the efficacy of PM devices to diagnose pediatric OSA,12,16–18 our study was unique because we evaluated PM in the home environment, with the patients/families themselves setting up the device, in addition to evaluating PM in the lab environment. Recent studies, using a different home sleep apnea testing device, showed favorable accuracy, including PM in a cohort of children with Down syndrome19; however, similar challenges with nasal airflow signal strength were encountered.20 Additionally, we found that there was no difference in study accuracy in patients who were naïve to PSG (pre-PSG group) compared to those who had already undergone PSG (post-PSG group). Finally, we evaluated patient opinions which suggested that sleep testing with the home equipment was generally preferred to in-laboratory testing and was considered less cumbersome by most participants.
The need for home testing of children with sleep disorders is clear based on the finding that the majority of pediatric patients is currently receiving empiric therapy without any diagnostic testing.13 This situation is problematic given the value of establishing a rigorous diagnosis to motivate therapy and to follow objective treatment responses, eg, following adenotonsillectomy or weight loss. Given the large burden of sleep apnea in both adults25 and children, simplified methods to establish the diagnosis seem imperative. Further data will be required to determine the optimal technique to predict disease complications and to optimize OSA management.
Regarding comparison of different diagnostic tests, a number of considerations are worthwhile. The AHI thresholds vary in the literature, particularly in children, and thus we did not focus on a particular AHI cutoff for sensitivity and specificity. Instead, we examined the correspondence between various diagnostic methods such that the practitioner could then use the data in a clinical context depending on what AHI threshold was of interest. We used a number of methods to compare the diagnostic techniques including Bland-Altman analysis. Although some AHI values were somewhat discordant in the high range, one could argue that a diagnosis of severe OSA is established with little clinical impact of a discrepancy, eg, AHI = 60 vs AHI = 80 events/h. To compare techniques, we also used log transformation, which serves to linearize the AHIs and showed good correspondence between techniques. Moreover, we used the well-established White-Westbrook method24 modified for pediatrics, which also showed reasonable diagnostic classification using home testing. Comparing the raw values of AHI and ODI, we did observe significant correlations between ALT and PSG but not with HSAT and PSG. Although this finding did not necessarily impact the diagnostic accuracy using the aforementioned techniques, it may suggest that the accuracy of PM was improved when the study was attended by a sleep technologist. It is also noteworthy that the sleep technologist was not able to monitor PM signals during the recording in our study design.
Notwithstanding, we are reasonably confident that home testing gives a practical estimate of the PSG result, recognizing the latter is also far from perfect. In general, home testing leads to a slight underestimate of AHI compared to PSG, likely reflecting a longer total recording time from HSAT as opposed to measured true total sleep time from PSG. In addition, as we did observe reduced flow monitoring time in HSAT, it is plausible that respiratory events were missed, resulting in an underscoring of the AHI. Comparing our findings to studies assessing the accuracy of HSAT to diagnose OSA in adults suggests that the diagnostic yield is greater with adult patients, when there is more reliable signal strength. Further, unlike pediatric sleep testing, there is guidance for adult HSAT to minimize study failures.26,27
Despite our study’s strength, we acknowledge a number of limitations. First, we conducted a single-center clinic-based study with a modest sample size. Thus, one could argue that the predictive values may differ if testing were done in a different context, eg, community screening of asymptomatic individuals. However, we studied a diverse group of adolescents and found fairly consistent results, suggesting that our findings should generalize to other sleep clinic settings. Nonetheless, we are supportive of further research in young children, non-OSA cohorts, and potentially syndromic children to extend our findings. Second, we did not examine hard outcomes such as PAP adherence, and thus we cannot say with confidence whether home testing results will have the same impact clinically as PSG results. However, the adult literature has shown similar if not improved outcomes with home testing,28 suggesting no good reason to believe that HSAT results will be minimized or ignored. Third, we recognize the limitations of the AHI given varying criteria used in both pediatric and adult literature. In our study design, only pediatric scoring criteria were used, thus all PM was rescored using pediatric scoring rules. There is some validity using adult scoring rules,29 which could possibly improve the agreement between PSG and PM had we used adult scoring rules. In addition, we selected the AHI as opposed to the obstructive AHI outcome measure, a measure that excludes central events (central apneas and central hypopneas) from the AHI. A limitation of the ApneaLink Web-based software is that it only provides the AHI index. Thus, we opted to compare the AHI from ApneaLink to the AHI determined by PSG. Nonetheless, we also focused our comparisons on oxygen desaturation from the various techniques, to allow a fair comparison. We recognize the potential importance of measuring arousals, carbon dioxide levels, and other factors that we did not focus on for our study. We view this limitation as a problem with the AHI in general, rather than a specific weakness of our methodology. However, we are certainly supportive of further efforts to define disease metrics, including patient-reported outcomes and their predictive value. Finally, only 75% of HSAT studies were deemed successful compared to 89% of ALT studies. This finding is likely related to the influence of having a specialized sleep technologist observe and troubleshoot when patients are applying the device at night. Flow monitoring time was significantly greater in ALT studies compared to HSAT. We speculate this result was likely related to having a technologist continuously monitor flow signals from the PSG montage and reposition the nasal cannula should the device come out. Certainly, the presence of a sleep technologist contributed to successful PM studies when conducted in the lab compared to at home. Of note, the ALT device was started slightly earlier (∼20 minutes) than the “lights out” of PSG, thereby extending the total recording time. As a consequence, this may have led to an underestimation of the indices of interest. Despite these limitations, we used all studies, regardless of whether they were deemed a success or a failure. Although 25% of HSAT studies were identified as study failures, in clinical practice, patients would have been re-educated and offered a repeat test at home, possibly further enhancing the diagnostic yield of PM.
Despite these limitations we believe our results are an important addition to the literature and hope that they stimulate further research and perhaps help lead to adolescents receiving therapy for sleep apnea promptly.
DISCLOSURE STATEMENT
All authors have seen and approved the final manuscript. This study was funded by ResMed Corp. Representatives from the study sponsor were involved in the study design, collection, analysis, and interpretation of data; writing of the report; and in the decision to submit the paper for publication. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication. The authors report no conflictsof interest.
SUPPLEMENTARY MATERIAL
ABBREVIATIONS
- AHI
apnea-hypopnea index
- ALT
ApneaLink Lab Test
- HSAT (or HST)
home sleep apnea test
- ICC
intraclass correlation coefficient
- ODI
oxygen desaturation index
- OSA
obstructive sleep apnea
- PM
portable monitor/monitoring
- PSG
polysomnography (polysomnogram)
REFERENCES
- 1.Owens JA. Neurocognitive and behavioral impact of sleep disordered breathing in children. Pediatr Pulmonol. 2009;44(5):417–422. 10.1002/ppul.20981 [DOI] [PubMed] [Google Scholar]
- 2.Bhattacharjee R, Kheirandish-Gozal L, Pillar G, Gozal D. Cardiovascular complications of obstructive sleep apnea syndrome: evidence from children. Prog Cardiovasc Dis. 2009;51(5):416–433. 10.1016/j.pcad.2008.03.002 [DOI] [PubMed] [Google Scholar]
- 3.Young T, Peppard PE, Gottlieb DJ. Epidemiology of obstructive sleep apnea: a population health perspective. Am J Respir Crit Care Med. 2002;165(9):1217–1239. 10.1164/rccm.2109080 [DOI] [PubMed] [Google Scholar]
- 4.Heinzer R, Vat S, Marques-Vidal P, et al. Prevalence of sleep-disordered breathing in the general population: the HypnoLaus study. Lancet Respir Med. 2015;3(4):310–318. 10.1016/S2213-2600(15)00043-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Peppard PE, Young T, Barnet JH, Palta M, Hagen EW, Hla KM. Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol. 2013;177(9):1006–1014. 10.1093/aje/kws342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tauman R, Gozal D. Obesity and obstructive sleep apnea in children. Paediatr Respir Rev. 2006;7(4):247–259. 10.1016/j.prrv.2006.08.003 [DOI] [PubMed] [Google Scholar]
- 7.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of obesity and trends in body mass index among US children and adolescents, 1999-2010. JAMA. 2012;307(5):483–490. 10.1001/jama.2012.40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Redline S, Tishler PV, Schluchter M, Aylor J, Clark K, Graham G. Risk factors for sleep-disordered breathing in children. Associations with obesity, race, and respiratory problems. Am J Respir Crit Care Med. 1999;159(5 Pt 1):1527–1532. 10.1164/ajrccm.159.5.9809079 [DOI] [PubMed] [Google Scholar]
- 9.Verhulst SL, Van Gaal L, De Backer W, Desager K. The prevalence, anatomical correlates and treatment of sleep-disordered breathing in obese children and adolescents. Sleep Med Rev. 2008;12(5):339–346. 10.1016/j.smrv.2007.11.002 [DOI] [PubMed] [Google Scholar]
- 10.Silvestri JM, Weese-Mayer DE, Bass MT, Kenny AS, Hauptman SA, Pearsall SM. Polysomnography in obese children with a history of sleep-associated breathing disorders. Pediatr Pulmonol. 1993;16(2):124–129. 10.1002/ppul.1950160208 [DOI] [PubMed] [Google Scholar]
- 11.Lumeng JC, Chervin RD. Epidemiology of pediatric obstructive sleep apnea. Proc Am Thorac Soc. 2008;5(2):242–252. 10.1513/pats.200708-135MG [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Massicotte C, Al-Saleh S, Witmans M, Narang I. The utility of a portable sleep monitor to diagnose sleep-disordered breathing in a pediatric population. Can Respir J. 2014;21(1):31–35. 10.1155/2014/271061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mitchell RB, Pereira KD, Friedman NR. Sleep-disordered breathing in children: survey of current practice. Laryngoscope. 2006;116(6):956–958. 10.1097/01.MLG.0000216413.22408.FD [DOI] [PubMed] [Google Scholar]
- 14.Collop NA, Anderson WM, Boehlecke B, et al. Clinical guidelines for the use of unattended portable monitors in the diagnosis of obstructive sleep apnea in adult patients. J Clin Sleep Med. 2007;3(7):737–747. 10.5664/jcsm.27032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marcus CL, Traylor J, Biggs SN, et al. Feasibility of comprehensive, unattended ambulatory polysomnography in school-aged children. J Clin Sleep Med. 2014;10(8):913–918. 10.5664/jcsm.3970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lesser DJ, Haddad GG, Bush RA, Pian MS. The utility of a portable recording device for screening of obstructive sleep apnea in obese adolescents. J Clin Sleep Med. 2012;8(3):271–277. 10.5664/jcsm.1912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Choi JH, Lee B, Lee JY, Kim HJ. Validating the Watch-PAT for diagnosing obstructive sleep apnea in adolescents. J Clin Sleep Med. 2018;14(10):1741–1747. 10.5664/jcsm.7386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Masoud AI, Patwari PP, Adavadkar PA, Arantes H, Park C, Carley DW. Validation of the MediByte portable monitor for the diagnosis of sleep apnea in pediatric patients. J Clin Sleep Med. 2019;15(5):733–742. 10.5664/jcsm.7764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ikizoglu NB, Kiyan E, Polat B, Ay P, Karadag B, Ersu R. Are home sleep studies useful in diagnosing obstructive sleep apnea in children with down syndrome? Pediatr Pulmonol. 2019;54(10):1541–1546. 10.1002/ppul.24440 [DOI] [PubMed] [Google Scholar]
- 20.Gudnadottir G, Hafsten L, Redfors S, Ellegård E, Hellgren J. Respiratory polygraphy in children with sleep-disordered breathing. J Sleep Res. 2019;28(6):e12856. 10.1111/jsr.12856 [DOI] [PubMed] [Google Scholar]
- 21.Stehling F, Keull J, Olivier M, Große-Onnebrink J, Mellies U, Stuck BA. Validation of the screening tool ApneaLink® in comparison to polysomnography for the diagnosis of sleep-disordered breathing in children and adolescents. Sleep Med. 2017;37:13–18. 10.1016/j.sleep.2017.05.018 [DOI] [PubMed] [Google Scholar]
- 22.Dayyat E, Kheirandish-Gozal L, Gozal D. Childhood obstructive sleep apnea: one or two distinct disease entities? Sleep Med Clin. 2007;2(3):433–444. 10.1016/j.jsmc.2007.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Berry RB, Quan SF, Abreu AR, et al; for the American Academy of Sleep Medicine. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications. Version 2.6. Darien, IL: American Academy of Sleep Medicine; 2020. [Google Scholar]
- 24.White DP, Gibb TJ, Wall JM, Westbrook PR. Assessment of accuracy and analysis time of a novel device to monitor sleep and breathing in the home. Sleep. 1995;18(2):115–126. 10.1093/sleep/18.2.115 [DOI] [PubMed] [Google Scholar]
- 25.Benjafield AV, Ayas NT, Eastwood PR, et al. Estimation of the global prevalence and burden of obstructive sleep apnoea: a literature-based analysis. Lancet Respir Med. 2019;7(8):687–698. 10.1016/S2213-2600(19)30198-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rosen IM, Kirsch DB, Carden KA, et al. ; American Academy of Sleep Medicine Board of Directors . Clinical use of a home sleep apnea test: an updated American Academy of Sleep Medicine position statement. J Clin Sleep Med. 2018;14(12):2075–2077. 10.5664/jcsm.7540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lux L, Boehlecke B, Lohr KN. Effectiveness of Portable Monitoring Devices for Diagnosing Obstructive Sleep Apnea: Update of a Systematic Review [Internet]. Rockville, MD: Agency for Healthcare Research and Quality (US); 2004. https://www.cms.gov/Medicare/Coverage/DeterminationProcess/Downloads/id24TA.pdf. Accessed March 28, 2021. [PubMed]
- 28.Mulgrew AT, Fox N, Ayas NT, Ryan CF. Diagnosis and initial management of obstructive sleep apnea without polysomnography: a randomized validation study. Ann Intern Med. 2007;146(3):157–166. 10.7326/0003-4819-146-3-200702060-00004 [DOI] [PubMed] [Google Scholar]
- 29.Tapia IE, Karamessinis L, Bandla P, et al. Polysomnographic values in children undergoing puberty: pediatric vs. adult respiratory rules in adolescents. Sleep. 2008;31(12):1737–1744. 10.1093/sleep/31.12.1737 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.