To the Editor:
Untreated obstructive sleep apnea (OSA) has been linked to mild cognitive impairment and dementia, raising its significance as a potentially modifiable risk factor for neurodegenerative disorders, but exact mechanisms as well as reversibility with treatment remain undetermined (1–3). During slow-wave sleep (SWS), declarative memory traces are thought to undergo active replay, resulting in their stabilization and strengthening (4). Given that SWS is reduced in patients with OSA, this deficiency could impair the ability to stabilize and strengthen these memories.
Continuous positive airway pressure (CPAP) treatment for OSA restores normal ventilation during sleep, reestablishes sleep architecture, increases SWS-rich deep sleep, and is considered the treatment of choice for OSA (5). A number of studies examining cognitive function before and after CPAP treatment have indicated selective cognitive improvement; however, many larger trials have found inconsistent results.
Here, we first summarize a case–control study, in which we applied a declarative memory test (Verbal Paired-Associates [VPA] task), which selectively relies on SWS, to test the hypothesis that patients with obstructive sleep apnea, although exhibiting normal practice-related learning, lack the memory consolidation benefit normally mediated by SWS-rich sleep. In a subsequent study, we conducted a randomized controlled trial (NCT01800786) to test the hypothesis that the declarative memory impairment improves after 3 months of CPAP therapy. Some of the results of these studies have been previously reported in the form of abstracts (6, 7).
A total of 36 patients with newly diagnosed obstructive sleep apnea (apnea–hypopnea index [AHI] >5/h) and 36 matched healthy control subjects (AHI <5/h) with no subjective sleep complaints were recruited from the community and local sleep clinics. The overnight testing included a training session of the VPA task in the evening followed by a full-night polysomnogram and a VPA recall test in the morning.
The two groups performed similarly in the evening (mean difference, −0.75; 95% confidence interval [95% CI], −1.63 to 0.13; P = 0.15). The following morning, patients with OSA correctly completed on average 74.1% of the word pairs, a 7.1 ± 8.0% (95% CI, 4.25 to 9.64) increase from the evening before, compared with 82.4% in the healthy control group, a 13.9 ± 7.7% (95% CI, 11.27 to 16.51) increase and almost twice that seen in patients with OSA (mean difference, 6.94; 95% CI, 3.35 to 10.64; P = 0.0004). In a combined regression model including oxygen nadir, AHI, and arousal index, only time in N3 sleep predicted overnight performance improvement significantly (t = 3.34, P = 0.001).
Subjects with OSA were then randomly assigned to two groups: a CPAP group and a no-CPAP group. The no-CPAP group was asked to modify their lifestyle with diet and exercise. The CPAP group began treatment with an autotitrating CPAP device as well as implementing lifestyle changes. The purpose of the no-CPAP group was to control for time and common lifestyle changes that often occur with the awareness of a new OSA diagnosis and its health consequences. Of the initial 36 patients with OSA, 4 dropped out and 1 moved out of the area. This attrition left 31 patients, of whom 15 had been assigned to the CPAP group and 16 to the no-CPAP group.
After 3 months, both groups returned for overnight VPA testing with a polysomnogram, including evening training and morning recall using a new set of word pairs. The CPAP group wore their CPAP device during that night.
Compliance data for the CPAP group (using smart cards) revealed a mean nightly adherence to CPAP of 5.68 ± 1.02 hours over the preceding 3 months.
Demographic, questionnaire, and polysomnography data are displayed in Table 1. The CPAP group had more than twice as much N3 sleep compared with the no-CPAP group (15.8% vs. 7.2%; P = 0.036) on this second visit. A one-way repeated measures ANOVA revealed a significant N3 sleep increase from 3 months earlier for the CPAP group (P = 0.01) but not for the no-CPAP group (P = 0.75). As expected, measures of sleep-disordered breathing—AHI, oxygen nadir, and arousal index—were improved in the CPAP group, confirming its therapeutic benefits.
Table 1.
Demographics, Questionnaire Data, and Polysomnography Results (Randomized Controlled Trial)
| No-CPAP (N = 16) | CPAP (N = 15) | P Value | |
|---|---|---|---|
| Age, yr | 51.7 ± 3.7 | 47.5 ± 4.2 | 0.458 |
| Sex, F, n (%) | 3 (20.0) | 3 (21.4) | 0.928 |
| BMI, kg/m2 | 34.8 ± 1.9 | 33.8 ± 1.7 | 0.705 |
| BDI | 3.0 ± 1.0 | 3.4 ± 1.1 | 0.774 |
| WASI full scale | 201 ± 8.1 | 179.6 ± 10.2 | 0.109 |
| WASI percentile | 52.4 ± 7.9 | 43.4 ± 5.3 | 0.361 |
| Epworth sleepiness scale | 7.1 ± 1.1 | 7.8 ± 1.1 | 0.646 |
| Stanford sleepiness scale p.m. | 2.2 ± 0.4 | 2.6 ± 0.3 | 0.384 |
| Stanford sleepiness scale a.m. | 2.5 ± 0.3 | 3.3 ± 0.4 | 0.130 |
| TST, min | 382.9 ± 20.7 | 339.1 ± 22.5 | 0.164 |
| Sleep efficiency, % | 79.1 ± 3.8 | 74.2 ± 4.0 | 0.389 |
| WASO, min | 105.3 ± 20.4 | 154.3 ± 31.3 | 0.201 |
| N1, % | 10.6 ± 2.4 | 5.2 ± 1.3 | 0.060 |
| N2, % | 62.9 ± 3.3 | 61.4 ± 2.8 | 0.740 |
| N3, % | 7.2 ± 2.9 | 15.8 ± 2.8 | 0.036 |
| REM, % | 19.3 ± 1.8 | 17.6 ± 2.3 | 0.571 |
| Arousal index, events/h | 35.3 ± 7.7 | 18.2 ± 3.0 | 0.016 |
| AHI, events/h | 34.7 ± 7.5 | 3.6 ± 0.7 | 0.001 |
| Mean oxygen saturation, % | 92.2 ± 1.0 | 94.1 ± 0.5 | 0.127 |
| Oxygen nadir, % | 78.5 ± 2.3 | 88.1 ± 1.1 | 0.001 |
| PLMS index, events/h | 0.2 ± 0.1 | 0.7 ± 0.6 | 0.431 |
Definition of abbreviations: AHI = apnea–hypopnea index; BDI = Beck Depression Inventory; BMI = body mass index; CPAP = continuous positive airway pressure; PLMS = periodic limb movements of sleep; TST = total sleep time; WASI = Wechsler Abbreviated Scale of Intelligence; WASO = wake after sleep onset.
Values are presented as mean ± SEM. Bold type indicates statistical significance.
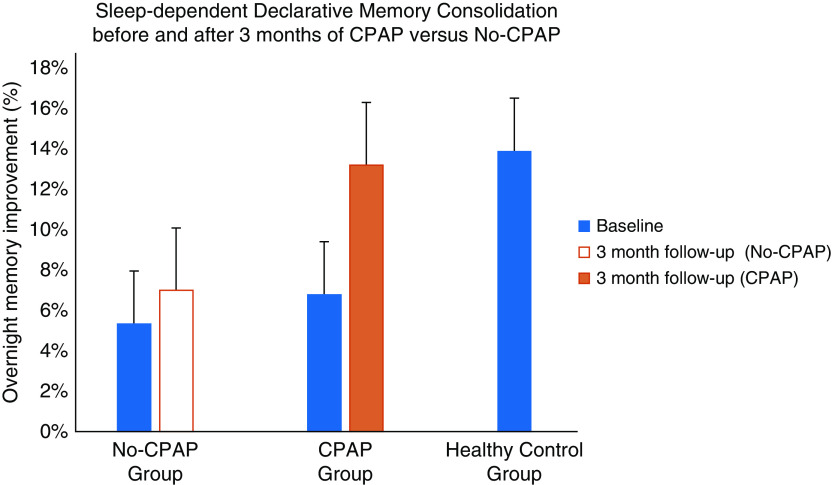
No group difference was observed for evening VPA learning (CPAP: 68.4%; no-CPAP: 70.5%; P = 0.56). The CPAP group improved by 13.2% (95% CI, 9.36 to 17.07) in the morning, similar to the healthy control subjects (13.9%, P = 0.78). The no-CPAP group improved by 7.0% (95% CI, 3.26 to 10.74) in the morning, which was significantly lower than the CPAP group (mean difference, 6.21; 95% CI, 1.08 to 11.34; P = 0.02; Figure 1).
Figure 1.
Overnight improvement on the declarative Verbal Paired-Associates (VPA) task for patients with obstructive sleep apnea (OSA), who were randomized to treatment with continuous positive airway pressure (CPAP) and lifestyle changes for 3 months (CPAP group, n = 15), patients with OSA who implemented lifestyle changes for 3 months (no-CPAP, n = 16), and healthy control subjects (n = 36). Baseline performance for all groups (blue) and performance after 3 months are displayed for the CPAP (orange) and no-CPAP (white) group. Mean overnight VPA improvement between initial baseline and after 3 months of treatment intervention demonstrated a significant improvement for the CPAP group (P = 0.01) but not for the no-CPAP group (P = 0.14), with a significant difference in mean improvement over time between groups (P = 0.02). After 3 months of treatment, the CPAP group achieved performance levels similar to those of healthy control subjects (13.2% vs. 13.9%; P = 0.78).
For the primary outcome analysis, a one-way repeated measures ANOVA on mean overnight VPA improvement between initial and 3-month tests demonstrated a significant increase in overnight improvement for the CPAP group, F (1, 14) = 9.21, P = 0.01 but not for the no-CPAP group, F (1, 15) = 2.45, P = 0.14, with a significant difference in mean improvement across time between groups (P = 0.02). Importantly, increases in N3 sleep from the baseline night predicted the increase in overnight VPA memory improvement between the two test sessions (r = 0.34, P = 0.04).
Our results show that 3 months of nightly therapy with CPAP leads to a resolution of a declarative memory deficit in patients with OSA in proportion to the increase of N3 sleep, with performance levels rising to those of healthy control subjects.
Two important factors have likely contributed to our results. First, the two-time-point assessment of memory retention across a night of sleep has proved to be more sensitive to identifying memory deficits compared with a single daytime memory assessment. This difference in experimental design might explain why several previous studies using single-session neurocognitive testing failed to demonstrate a consistent effect of OSA and CPAP treatment on memory functions (8–10).
Second, adherence to CPAP was higher in comparison with other randomized trials such as the Apnea Positive Pressure Long-term Efficacy Study (APPLES) (4.2 h/night in the active group, 3.4 h in the sham group) and the Sleep Apnea Cardiovascular Endpoints (SAVE) study (average use 3.3 h/night), which may further explain the lack of improvement in these studies (9, 11).
Given that our sample size was limited, we acknowledge that further work will be required to determine the impact of various covariates. We are aware of very few positive randomized clinical trials for CPAP therapy using important outcomes. To our knowledge, this is the first study to show that nightly CPAP therapy can offer an important therapeutic pathway by restoring a pivotal memory system in the brain through augmentation of SWS-rich sleep.
Footnotes
Supported by NIH grant K23HL103850 and American Sleep Medicine Foundation grant 54-JF-10 (I.E.D.). R.S. is funded by NIH grant MH048832. A.M. is funded by the NHLBI. ResMed provided a philanthropic donation to UC San Diego. A.M. reports consulting income from Merck and Livanova.
Author Contributions: Conception and study design: I.E.D., R.S., and A.M. Data acquisition, analysis, and interpretation: I.E.D., M.G., M.I., D.K., R.S., and A.M. Manuscript preparation: I.E.D., M.G., M.I., D.K., R.S., and A.M.
Originally Published in Press as DOI: 10.1164/rccm.202011-4253LE on December 21, 2020
Author disclosures are available with the text of this letter at www.atsjournals.org.
References
- 1.Yaffe K, Laffan AM, Harrison SL, Redline S, Spira AP, Ensrud KE, et al. Sleep-disordered breathing, hypoxia, and risk of mild cognitive impairment and dementia in older women. JAMA. 2011;306:613–619. doi: 10.1001/jama.2011.1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blackwell T, Yaffe K, Laffan A, Redline S, Ancoli-Israel S, Ensrud KE, et al. Osteoporotic Fractures in Men Study Group. Associations between sleep-disordered breathing, nocturnal hypoxemia, and subsequent cognitive decline in older community-dwelling men: the Osteoporotic Fractures in Men Sleep Study. J Am Geriatr Soc. 2015;63:453–461. doi: 10.1111/jgs.13321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Osorio RS, Gumb T, Pirraglia E, Varga AW, Lu SE, Lim J, et al. Alzheimer’s Disease Neuroimaging Initiative. Sleep-disordered breathing advances cognitive decline in the elderly. Neurology. 2015;84:1964–1971. doi: 10.1212/WNL.0000000000001566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wilson MA, McNaughton BL. Reactivation of hippocampal ensemble memories during sleep. Science. 1994;265:676–679. doi: 10.1126/science.8036517. [DOI] [PubMed] [Google Scholar]
- 5.McArdle N, Douglas NJ. Effect of continuous positive airway pressure on sleep architecture in the sleep apnea-hypopnea syndrome: a randomized controlled trial. Am J Respir Crit Care Med. 2001;164:1459–1463. doi: 10.1164/ajrccm.164.8.2008146. [DOI] [PubMed] [Google Scholar]
- 6.Guo M, Malhotra MIA, Stickgold R, Djonlagic I. The effect of obstructive sleep apnea on declarative memory consolidation [abstract] Sleep Med. 2013;14:e33–e34. [Google Scholar]
- 7.Djonlagic IGM, Kishore D, Igue M, Malhotra A, Stickgold R.The effect of CPAP therapy on sleep dependent memory consolidation [abstract] J Sleep Res 201625:301–302 [Google Scholar]
- 8.Quan SF, Budhiraja R, Kushida CA. Associations between sleep quality, sleep architecture and sleep disordered breathing and memory after continuous positive airway pressure in patients with obstructive sleep apnea in the Apnea Positive Pressure Long-term Efficacy Study (APPLES) Sleep Sci. 2018;11:231–238. doi: 10.5935/1984-0063.20180037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kushida CA, Nichols DA, Holmes TH, Quan SF, Walsh JK, Gottlieb DJ, et al. Effects of continuous positive airway pressure on neurocognitive function in obstructive sleep apnea patients: the Apnea Positive Pressure Long-term Efficacy Study (APPLES) Sleep (Basel) 2012;35:1593–1602. doi: 10.5665/sleep.2226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jackson ML, McEvoy RD, Banks S, Barnes M. Neurobehavioral impairment and CPAP treatment response in mild-moderate obstructive sleep apneas. J Clin Sleep Med. 2018;14:47–56. doi: 10.5664/jcsm.6878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McEvoy RD, Antic NA, Heeley E, Luo Y, Ou Q, Zhang X, et al. SAVE Investigators and Coordinators. CPAP for prevention of cardiovascular events in obstructive sleep apnea. N Engl J Med. 2016;375:919–931. doi: 10.1056/NEJMoa1606599. [DOI] [PubMed] [Google Scholar]