Abstract
Structural bone allograft transplantation remains one of the common strategies for repair and reconstruction of large bone defects. Due to the loss of periosteum that covers the outer surface of the cortical bone, the healing and incorporation of allograft is extremely slow and limited. To enhance the biological performance of allograft, herein, we report a novel and simple approach for engineering of a periosteum mimetic coating on the surface of structural bone allografts via polymer-mediated electrospray deposition. This approach enables the coating on allograft with precisely controlled composition and thickness. In addition, the periosteum mimetic coating can be tailored to achieve desired drug release profiles by making use of an appropriate biodegradable polymer or polymer blend. The efficacy study in a murine segmental femoral bone defect model demonstrates that the allograft coating composed of poly(lactic-co-glycolic acid) (PLGA) and bone morphogenetic protein-2 (BMP-2) mimicking peptide significantly improves allograft healing as evidenced by decreased fibrotic tissue formation, increased periosteal bone formation, and enhanced osseointegration. Taken together, this study provides a platform technology for engineering periosteum mimetic coating which can greatly promote bone allograft healing. This technology could eventually result in an off-the-shelf and multifunctional structural bone allograft for highly effective repair and reconstruction of large segmental bone defects. The technology can also be used to ameliorate the performance of other medical implants by modifying their surfaces.
Keywords: Bone allograft, Polymer-mediated electrospray deposition, Surface coating, BMP-2 peptide, Periosteum mimetic
Graphical Abstract

1. INTRODUCTION
Bone grafts and substitutes are widely used in orthopedic surgery for repair and reconstruction of bone defects. More than 500,000 bone grafting procedures are performed in the United States annually and 2.2 million procedures worldwide with an estimated market value of 2.4 billion dollars in 2016. 1–2 Although autograft is the gold standard for bone graft transplantation, limited source, donor site morbidity, and potential surgical complications have restricted its usage. Allograft bone remains as a common choice for reconstructive surgery due to its immediate availability, desirable mechanical properties and absence of donor site morbidity. However, allograft transplantation is reported to have a 60%, 10-year post-implantation failure rate due to fibrotic nonunions, infections, and secondary fractures. 3–4 The high failure rate of allograft is largely attributed to the lack of periosteum and the absence of osteogenic and angiogenic activities of the devitalized bone. 5–8 To improve allograft healing and incorporation, strategies have been developed to enhance the osteogenic and angiogenic properties of the structural allografts, including coating bone allografts with bioactive molecules, viral vectors, and osteogenic cells to mimic the biological function of periosteum. 6, 9–13 While some success has been achieved, new strategies are needed to overcome challenges such as i) fibrosis, ii) uneven callus formation, iii) safety of viral vectors, iv) the costly production of transplantable cells, and v) translational hurdles.
The inert nature of the cortical bone allografts has been shown to limit the recruitment and attachment of osteoblastic or osteoclastic cells on their surfaces. 4, 14–15 From this standpoint of view, modification of allograft surface to enhance osteoblast attachment, colonization and differentiation is critical for direct induction of new bone formation on the allograft surface and for osseointegration between allografts and host bone tissues. Using the classic polymer coating technique of dipping and rapid drying, a number of growth factors and bioactive molecules have been delivered to allografts for enhanced allograft healing and incorporation. 9, 16–18 In addition, functional modification of allograft surfaces through binding hydroxyapatite to osteoinductive peptides has shown beneficial healing effects. 19–21 While these methods have demonstrated potential for clinical translation of modified allografts, control of the thickness and uniformity of the surface coating, optimization of the drug loading and release profiles have been problematic. Novel methods to enhance the bio-distribution, reproducibility, and versatility of the surface coating are needed.
Electrospraying has been demonstrated as an efficient method to produce uniformly dispersed droplets/particles ranging from nanometers to micrometers on various surfaces. 22 This technique relies on high electric voltage to disintegrate or atomize a bulk liquid jet into fine liquid droplets of identical charge for surface coating. The basic experimental setup of electrospray is similar to that used in electrospinning. 22 It consists of a high voltage power supply, a syringe pump, and a plastic or glass syringe capped by a metallic needle with a defined diameter, and a grounded collector or substrate for collecting the particles. Electrospray deposition has been used to fabricate various biodegradable films/coatings for sustained drug delivery. 22–23 Compared with other existing coating techniques such as dip-coating, physical adsorption, and growth factor conjugation, 16–18 polymer-mediated electrospray deposition provides several unique features: i) high efficiency; ii) uniform coatings; iii) ease of incorporation of multiple therapeutic agents via layer-by-layer deposition; iv) elimination of cracks associated with other coating methods; v) better control of coating thickness as compared to dip coating; and vi) ease of transition to mass production.
While electrospray has been used in the fabrication of biomaterials in the form of nano/micro particles and thin films, this technology has never been used to engineer periosteum mimetic coating to bone allografts. The objective of this study was to establish electrospinning as a platform technology to modify the surface of structural bone allografts to restore the native function of periosteum for effective repair and reconstruction of segmental bone defects. To this end, an osteogenic BMP-2 mimetic peptide and a biodegradable PLGA polymer were used to create a periosteum mimetic coating on the surface of bone allografts via electrospray deposition (Figure 1). The healing efficacy of coated allografts were examined in a murine segmental bone defect model by performing Micro-CT, histologic, immunofluorescent and biomechanical analyses.
Figure 1.
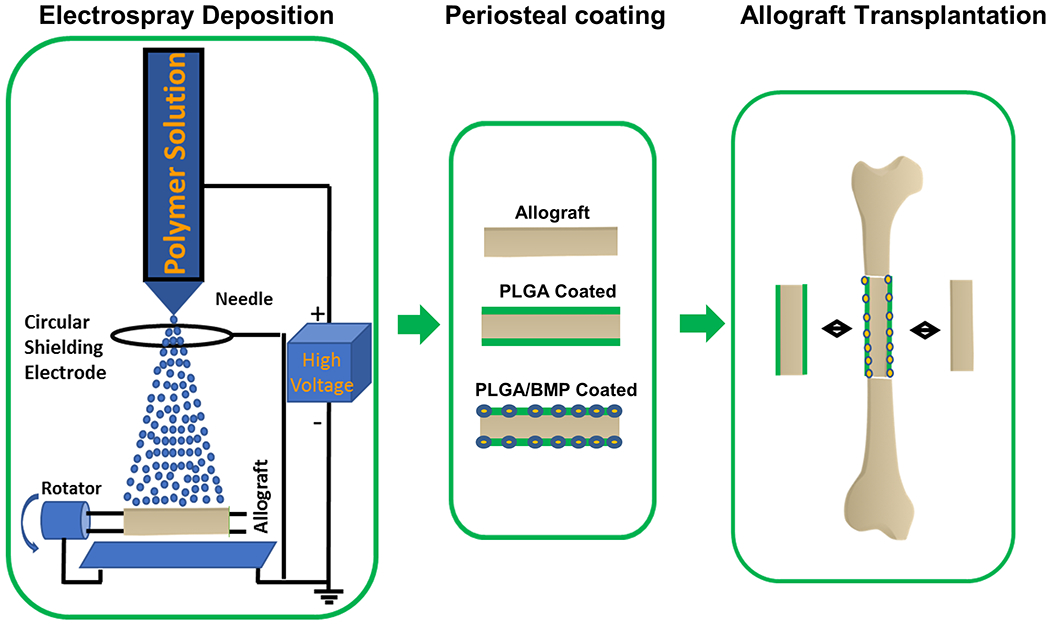
Schematic illustrating fabrication of a periosteum mimetic coating of structural bone allografts via electrospray deposition for repair and reconstruction of large segmental defects.
2. MATERIALS AND METHODS
2.1. Materials.
The following chemicals were used: Poly (lactic-co-glycolic acid) (PLGA, 50:50, Mw 30,000–60,000) (LACTEL absorbable polymers, Birmingham, AL), dichloromethane (DCM, Fisher Scientific, Hampton, NH), poly(ε-caprolactone) (PCL) (Mw=80 kDa) (Sigma Aldrich, St. Louis, MO), gelatin (Sigma Aldrich, St. Louis, MO), and hexafluoro-2-propanol (HFIP) (Oakwood Chemical, Estill, SC).
2.2. Preparation of bone allograft coatings via polymer-mediated electrospray deposition.
Figure 1A shows a schematic illustrating the setup of electrospray deposition. A voltage of 6.0-8.0 kV was applied to the nozzle and a voltage of 4.0 kV was applied to the metal ring to stabilize electrospraying towards the bone allograft on top of the rotating motor (EURO-ST D, Kika Labortechnik, Staufen, Germany). This was necessary as the high viscosity of the polymeric solution often caused partial clogging of the capillary, thus resulting in deviance in spraying direction. The distance between the spinneret and the rotating bone allograft was 3 cm, and the distance between the spinneret and grounded aluminum foil collector was 15 cm. The syringe pump (Stoelting Co., Chicago, IL) was set at 1 mL/h for the fabrication of all samples. The stable cone-jet was formed during electrospraying. Following electrospray deposition, the coated bone samples were left overnight in the hood and then freeze-dried to ensure complete removal of the solvent.
To stimulate osteogenic differentiation and bone formation, we incorporated a short-chain BMP-2 mimetic peptide (KIPKASSVPTELSAISTLYL, Genscript Biotech, Piscataway, NJ) to the coating which was previously shown to bind and activate the receptors of BMPs. 24–27 5% (w/v) of PLGA in DCM with or without containing peptide (5 mg) was used for electrospray deposition to the bone allografts. The polymer to peptide weight ratio was 50:1. The bone allografts were weighed initially (W0) and weighed after coating (W1), to maintain the uniform coating (W1-W0 ≈ 3 mg) for both in vitro and in vivo studies.
2.3. Characterization of bone allograft coatings and peptide release.
The coated bone allograft was characterized by scanning electron microscopy (SEM) for measuring the coating thickness. Profilm 3D technology, conducted by Filmetrics, KLA Co., was used to examine the polymer coating on the bone substrates via 3D optical profiler. The 3D optical profiler consisted of various objectives from 5X to 100X. Here, a 20X (Nikon CF IC Epi Plan) objective with a working distance of 4.7 mm was used to measure the coating thickness. The resulting graph was stitched using software provided by Filmetrics.
For quantification of peptide loading and release kinetics, 5 mg FITC labeled BMP-2 peptide was dispersed in 5% (w/v) of PLGA in DCM in glass vials and used for electrospray-mediated allograft coating as above described. The amount of BMP-2 peptide loading per graft was calculated from the difference in the weights of the bone samples before and after electrospray coating, assuming the polymer: peptide ratio of 50:1. The duration of the electrospray coating was adjusted to achieve an increase of 3 mg in the weights of the coated bone samples as compared to the corresponding uncoated ones. The FITC-labeled peptide release from the bone allograft was recorded by measuring the fluorescence intensities of the buffer aliquots at regular intervals using excitation and emission filters of 485 and 528 nm, respectively.
To measure the peptide loading and release kinetics from PCL-gelatin coated allografts, the PCL-gelatin HFIP solution containing 5 mg FITC labeled BMP-2 peptide was used for coating. PCL-gelatin (2.5 wt% each) and 5 mg of BMP-2 peptide were mixed by ultrasonication. The polymer to peptide weight ratio was 50:1. The above solution was used to coat bone allografts via electrospray deposition as above described. In the case of PCL-gelatin coatings, the samples were cross-linked with glutaraldehyde vapors from a 25 wt% ethanolic solution overnight for ~24 h. The peptide release from the PCL-gelatin bone allograft was recorded by measuring the fluorescence intensities of the buffer aliquots at regular intervals as previously described. 28
2.4. Experimental animals.
All in vivo experiments were performed using 2-month-old C57BL/6 mice housed in pathogen-free, temperature and humidity-controlled facilities with a 12-hour day-night cycle in the vivarium at the University of Rochester Medical Center. All cages contained wood shavings and bedding for environmental enrichment. All experimental procedures were reviewed and approved by the University Committee on Animal Resources. General anesthesia and analgesia procedures were performed based on the mouse formulary provided by the University Committee on Animal Resources. The animals’ health status was monitored throughout the experiments by experienced veterinarians according to the Guide for the Care and Use of Laboratory Animals outlined by the National Institute of Health.
2.5. Surgical procedures for segmental femoral bone allograft model.
A 4-mm segmental femoral bone graft transplantation model was used to evaluate the efficacy of coated allografts for reconstruction of long bone defect repair (Figure 1B). 6, 13, 29. Briefly, mice were anesthetized via intraperitoneal injection with a combination of Ketamine and Xylazine. A transverse unilateral osteotomy was performed to remove a 4 mm femoral diaphyseal shaft using a bone saw. A devitalized allograft or a devitalized allograft with coating was used to repair the defect. The grafts were secured by a 22-gauge metal pin placed through the intramedullary marrow cavity. Allografts were prepared from an outbred strain of FVB and washed extensively with phosphate buffered saline to remove periosteum, bone marrow cells and cell debris. The grafts were further washed with 70% ethanol, soaked in PBS containing a cocktail of penicillin and streptomycin and frozen at −80° for at least 1 week. Devitalized allografts were confirmed via histologic sections demonstrating empty lacunae with missing osteocytes in the allografted bone. During the postoperative period, pain was relieved by a subcutaneous administration of Buprenorphine SR (Pfizer Animal Health; 5 mg/kg twice daily for 2 days). Based on our prior experience with the murine allograft model, which demonstrates poor graft integration and biomechanics up to 9 weeks post-surgery, we chose week 5 post-surgery to conduct MicroCT and histology analyses and week 7 for biomechanical testing.10,18 A total of 18 mice in 3 groups (allograft, allograft with PLGA coating, and allograft with BMP-2 peptide loaded PLGA, n=6) were included in the MicroCT analyses. These same samples were used for analysis of allograft surface mineralization using Amira software as well as histologic and histomorphometric analyses to determine graft healing. Additional groups of samples were used for torsional biomechanical testing at week 7 to determine the functional integration of the allograft with host bone. At least five mice per group were used for evaluation.
2.6. Evaluation of femoral allograft healing by MicroCT.
Samples were scanned by ScancoVivaCT 40 system (Scanco Medical AG, Bassersdorf, Switzerland) at 12.5-micron isotropic resolution. Images were reconstructed to allow 3-dimensional structural rendering of the calluses at a standardized threshold corresponding to 750 mgHA/cm3 based on a phantom of known HA concentrations. To evaluate bone formation, contour lines were drawn in the 2-dimensional slice images to exclude the allograft and the old host cortical bone. New bone volume on the side of the host and donor bone, as well as in the total callus in grafted samples was calculated, respectively. 6, 13
Measurement of allograft surface deposited with newly formed bone was performed based on high resolution MicroCT scans using Amira 6.3 (Thermo Fisher Scientific) and custom algorithms. Briefly, a new bone shell formed within 100 μm distance from the allograft surface was isolated via density thresholding to include newly formed bone (lower density) but exclude allograft bone (higher density). The new bone shells covering the allograft in three experimental groups were generated. The inner surface area of the new bone shell and the outer surface area of the allograft were then calculated by pixel counting using Marching Cubes algorithm and triangle mesh calculation of the surface area. 30–31 The ratio of the two surface areas reflects the fraction of the surface of the bone allograft overlaid by new bone. To further determine the allograft surface that was directly in contact with the new bone, a Watershed Segmentation module built in Amira 6.3 was used to calculate the allograft surface that was directly mineralized with the newly formed host bone. The minimal area fraction of the allograft in direct contact with new bone was further calculated to determine the integration of the allograft with host bone. 32
2.7. Evaluation of femoral allograft healing via histology and histomorphometric analyses.
At the end point of the experiment, mice were perfused with 4% paraformaldehyde followed by an additional tissue fixation for 2 days. The specimens were decalcified in 10% EDTA and processed for paraffin embedding and sectioning. Mid-sagittal frozen sections (20 microns thick) or paraffin-embedded tissue sections (6 microns thick) were stained with Hematoxylin & Eosin plus Alcian blue hematoxylin/orange G as previously described. 6, 13, 29 Tissue sections were digitalized via Olympus VS110™ Virtual Slide Scanning System (Olympus, Tokyo, Japan). Histomorphometric analyses of bone, cartilage, and fibrotic tissue formation were performed in the VisioPharm Image Analysis Software via color-based semi-manual segmentation of different tissue component of the healing callus (Hørsholm, Denmark). 33–34 Percent area of bone, cartilage, bone marrow and fibrotic tissue within the area of callus were calculated to illustrate the difference among each group of samples (Figure 4A).
Figure 4. Histologic analyses of allograft healing.

Illustration of quantitative histomorphometric analyses of bone, cartilage and fibrotic tissue in the donor and host callus in the segmental bone allograft transplantation model (A). Areas of bone, cartilage and fibrotic tissue were isolated, color-coded and measured via VisioPharm. H&E Alcian Blue staining of the sections from allograft (B), PLGA coated allograft (C), and PLGA/BMP-2 peptide coated allograft (D). Quantitative histomorphometric analyses of the percent bone, cartilage and fibrotic tissue area at donor side (E), host side (F) and total callus (G). n=6, * indicate p<0.05.
2.8. Evaluation of femoral allograft healing via torsional biomechanical testing.
Following sacrifice, the tibia was isolated and cleaned of excess soft tissue. Tibias were stored at 4°C in phosphate saline buffer overnight, prior to torsional biomechanical testing. The ends of the tibias were cemented (Bosworth Company) in aluminum tube holders and tested using an EnduraTec TestBench™ system (Bose Corporation, Eden Prairie, MN). The tibias were tested at a rate of 1deg/sec, in torsion, until failure. The ultimate torque was determined based on the load-to-failure curve generated.
2.9. Immunofluorescent and immunohistochemical analysis.
For pSMAD3, pSMAD1/5 immunofluorescence staining, the slides were deparaffinized, and rehydrated. Next, the samples were treated with 3% bovine albumin in PBS and then stained with p-Smad3 (1:100 dilution, Roackland, PA) or p-Smad5 antibody (1:100 dilution, Cell Signaling Technology, Danvers, MA) overnight at 4 °C. The samples were incubated with secondary antibody (Alexa Fluor 546 dye, Thermofisher, Waltham, MA) for 2 h at room temperature after 3 times of wash. The samples were imaged via Olympus VS110™ Virtual Slide Scanning System (Olympus, Tokyo, Japan). Quantification of pSMAD3+ area and intensity were performed in ImageJ using digitalized 20X images obtained from Olympus VS110™ Virtual Slide Scanning System (Olympus, Tokyo, Japan). Phosphorylated-SMAD3+ fluorescence intensity and area in three experimental groups were evaluated in a double-blinded fashion within the defect region. Two regions of each sample from three treatment groups (n=6) were used for semi-quantitative analysis of the fluorescence signals. The average fluorescence signal intensity was expressed as the mean of all the pixel intensities, i.e., the integrated density, in the obtained images (320x246 pixels=160x123microns). Percent area of pSMAD3+ cells were also calculated in ImageJ and presented as the percent area ± SEM.
2.10. Statistical analysis.
All data are shown as the Mean ± Standard Deviation (SD). Statistical analysis was analyzed by one-way ANOVA in GraphPad Prism (GraphPad Prism, San Diego, CA). A p value < 0.05 was considered statistically significant.
3. RESULTS
3.1. Fabrication and characterization of the BMP-2 peptide-loaded periosteum mimetic coating on bone allografts.
Compared with autograft, which leads to extensive bone formation along the bone graft surface, the allograft surface is largely inert and extremely poorly mineralized. 5–6, 12–13 Studies have shown that periosteal signaling molecules, specifically BMP-2, are essential for the initiation and induction of bone formation on autograft surface. 5–6, 8, 35 To mimic the periosteal paracrine function, an osteogenic short-chain BMP-2 mimetic peptide, which was previously shown to bind and activate the receptors of BMPs, was used to create a periosteum mimetic coating via polymer-mediated electrospray deposition. 24–27
To ensure timely release of the BMP-2 peptide, a rapidly degradable PLGA polymer with a ratio of 50:50 lactide and glycolide monomers was selected as the carrier. PLGA, a biodegradable and biocompatible copolymer, has been used to fabricate a number of FDA-approved therapeutic devices. The degradation rate of PLGA can be easily adjusted by varying the ratio of lactide and glycolide monomers. 36 This strategy could be used to tailor the release profiles of the embedded peptides from the PLGA coatings. PLGA copolymer (50:50) was selected as the carrier for peptide coating in this study due to its faster degradation half-life that is compatible to the rate of allograft healing (~4-6 weeks). Compared to PLGA, PCL has a much longer degradation half-life, which could result in a much slower release of the loaded peptides. 37 To enhance the hydrophilicity and promote the peptide release, gelatin was blended with PCL as a coating material and used as a control in the peptide release experiment. The efficient and uniform coatings on the surface of bone allografts were achieved by optimizing the collector distance, solution viscosity and speed of the rotator. The thickness of the allograft coating can be precisely controlled in the range of 1-100 μm by varying the duration of the deposition time.
To characterize the coating, a portion of allografts was covered with a tape during the electrospray deposition. Subsequent removal of the tape revealed the morphology and thickness of coatings on the surface of bone allografts. It is observed that uniform PLGA coating was formed on the surface of bone allografts (Figure 2A). The SEM images show the cross-section of the coating, indicating that the thickness was approximately 100 μm (Figure 2B and 2C). To further characterize the coating, optical profilometry was performed by the Profilm3D Filmetrics system with state-of-the-art white light interferometry (WLI) and capability of measuring the surface profiles and roughness down to 0.05 μm. Figure 2D shows Profilm 3D images, suggesting that PLGA coated bone allografts had a uniform distribution of polymer coatings on bone allografts. The coating was calculated to be around 100 μm thick, which was consistent with the SEM results.
Figure 2. Characterization of the polymer coating on allografts.

(A) SEM images of half of a bone allograft uniformly coated with BMP-2 peptide-loaded PLGA. (B-C) High magnified cross-section images of the coated allograft illustrating the thickness of the PLGA coating. (D) The half of allograft coated with BMP-2 peptide-loaded PLGA imaged with Profile 3D filmetrics system. (E) BMP-2 loadings and in vitro release profiles from engineered bone allografts: comparison of PCL-gelatin (red triangles) and PLGA coatings (black circles and squares).
Due to the hydrophobic nature of the BMP-2 peptide, the release of the peptide could be largely dependent on the degradation rate of coated materials. To determine the release kinetics of peptide from the coated allografts, FITC-labeled BMP-2 peptide was incorporated to PLGA (50:50) or PCL-gelatin coatings. Here, we demonstrated the coatings made of these two distinct materials with vastly different degradation profiles. 37–38 The peptide loading and release were quantified following incubation of the coated allografts at 37 °C in Tris-buffered saline over a period of 30 days. As shown in Figure 2E, the loadings of FITC-labeled peptide (ca. 50 μg) on allograft were similar in both PLGA and PCL-gelatin coatings. A sustained release profile of BMP-2 peptide was recorded for PLGA coated bone allografts with >90% of the peptide released over 30 days. In comparison, only ~30% BMP-2 peptide release was observed from the PCL-gelatin coating within the same time period, indicating that the PCL-gelatin peptide coatings exhibited a retarded peptide release due to the prolonged degradation of PCL as well as glutaraldehyde cross-linking of the coatings. Taken together, these results suggest a strategy to control the release of therapeutic peptide using materials with varying degradation profiles.
3.2. Efficacy of BMP-2 peptide-coated bone allografts in repair of segment femoral bone defects in mice.
Our previous studies established a murine segmental bone graft model that recapitulates the most prominent features of bone graft healing in humans. 6, 39 This model allows us to study the molecular and cellular events that govern allograft healing and remodeling. 35, 40–42 This model also allows us to test tissue-engineered bone allografts for repair of large bone defects. 5, 10–13, 29, 43 In this study, bone allografts with or without BMP-2 peptide/PLGA coating were examined in 4-mm segmental defects created in the femurs of C57BL6 mice. The longitudinal X-ray examination showed significantly more callus formation close to or on the surface of bone allografts with BMP-2 peptide/PLGA coating (Figure 3A). MicroCT reconstruction of the defect at week 5 post-surgery showed that compared with allografts and allografts coated with PLGA, PLGA/BMP-2 peptide-coated allografts demonstrated significantly enhanced bone formation at the repair sites (Figure 3B). Newly formed bone was found immediately adjacent to the allograft surface in all samples examined (Figure 3B, arrows). Quantitative MicroCT analyses showed 3.1 and 2.3-fold increase of new bone at the donor and host side callus, respectively, at week 5 post-surgery (Figure 3C and 3D, n=6, p<0.05).
Figure 3. X-ray and MicroCT analyses of allograft healing.

(A) Examination of allograft healing by X-ray at weeks 0, 3, and 5 post-surgery. (B) MicroCT of allograft healing at week 5 post-surgery. Compared with allograft PLGA control, PLGA/BMP-2 peptide coated allograft induced new bone formation along the surface of allograft (arrows in B). MicroCT quantification of new bone volume at donor side (C) and host side callus (D). n=6. * indicates p<0.05.
Further histologic analysis was conducted at week 5 post-implantation. Significant fibrotic tissue and immature fibrocartilage on the surface of allografts and at the cortical bone junctions was observed in all three groups of samples (Figure 4). However, compared with the control groups, PLGA/BMP-2 peptide-coated grafts showed remarkable osteoinductive activity leading to enhanced bone callus formation directly on top of the bone allografts (Figure 4D, arrows). Quantitative histomorphometric analyses showed 4.2- and 2.3-fold induction of bone formation at the donor and host site, respectively (Figure 4E&F, n=6, p<0.05). The percentage of new bone formed in the area of total callus was also significantly enhanced by ~2 fold (Figure 4G, p<0.05). With increased bone formation, the percent area of fibrotic tissue was reduced in the PLGA/BMP-2 treated group, suggesting antagonism between osteogenesis and fibrosis.
To further determine osseointegration of bone allografts, new bone formation close to the allograft surface (red) as well as new bone formation directly on the surface of the allograft (yellow) were analyzed using high resolution MicroCT 2D image stacks obtained from the three experimental groups (Figure 5A). Based upon segmented 2D images, new bones close to allograft surface (red) as well as new bone directly integrated with the allograft surface (yellow) were reconstructed into 3D images around allograft outer surfaces (Figure 5B&C). As shown, allograft coated with PLGA/BMP-2 peptide had significantly increased new bone coverage over the allograft outer surface as compared to allograft alone or allograft coated with PLGA (Figure 5B, marked as red area on allografts). The minimal surface area that was in direct contact with the allograft bone was also increased in the allograft coated with PLGA/BMP-2 peptide (Figure 5C, marked as yellow area on surface of allografts). Quantitative analyses showed a near 5-fold increase of the allograft surface overlaid by new bone in PLGA/BMP-2 peptide-coated grafts as compared to the other two groups (Figure 5D, p<0.05, n=6). The fraction of the allograft surface in direct contact with new bone had more than 5-fold increase compared with the other two groups (Figure 5E, p<0.05, n=6), suggesting a significant improvement of osseointegration and incorporation of allograft to host bone.
Figure 5. Analysis of allograft integration via MicroCT and biomechanical testing.

(A) The representative MicroCT 2D cross-sectional slice views of bone allografts. New bone formed within 100μm distance from allograft surface was isolated in three experimental groups (red regions in A). The surface directly in contact with the allograft surface was further segmented (yellow lines in A). (B) The isolated new bone overlaid on allograft surface (red region) on the 3D reconstructed allografts. (C) The new bone surface directly in contact with allograft surface (yellow regions) on 3D reconstructed allografts. (D) Quantitative analysis of the percent allograft surface area covered by new bone. (E) Quantitative analysis of the percent allograft surface area in direct contact with new bone. (F) The ultimate torque in allografts, PLGA coated allografts, and PLGA/BMP-2 coated allografts. n=4, * indicates p<0.05.
Finally, torsional biomechanical testing was conducted at week 7 post surgery to determine the functional healing of the three groups of allografts. As shown, compared to allografts and allografts coated with PLGA, allografts coated with BMP-2 peptide/PLGA demonstrated significantly improved ultimate torque (Figure 5F). Compared with unfractured normal bone, BMP-2 coated allografts restored about 50% percent of the strength of bone at 7 weeks post-implantation.
3.3. Activation of TGF-β signaling pathway by PLGA coating and antagonism of BMP-2 peptide to TGF-β signaling pathway.
To further understand the potential mechanism of fibrotic tissue formation induced at the site of allograft repair, immunofluorescence staining of pSMAD 3 and 5 were conducted in allograft samples. High level of pSMAD3 was found in fibrotic tissue and immature cartilage along the surface of allografts and at the cortical bone junctions (Figure 6A and 6B), suggesting a role of TGF-β signaling in fibrotic tissue formation during allograft healing. In contrast, pSMAD5 was absent in fibrotic tissue but strongly positive in chondrocytes and bone cells (Figure 6C and 6D). Comparison of pSMAD3 expression was performed across the three groups of allograft samples. While all three groups showed increased pSMAD3 level in fibrotic tissue, PLGA coated allograft demonstrated the highest level of pSMAD3 in fibrotic tissue adjacent to bone. In comparison, PLGA/BMP-2 peptide coated allografts showed reduced level of pSMAD3, suggesting potential antagonism of BMP-2 peptide to TGF-β signaling pathways during allograft healing (Figure 6E–M). Further quantification of the pSMAD3+ fluorescence intensity in callus (Figure 6N) as well as the percent area of the pSMAD3+ cells (Figure 6O) using ImageJ show a significant induction of pSMAD3+ level in PLGA coated allografts while loading BMP peptide significantly suppressed the level of pSMAD3 (n=6, p<0.05).
Figure 6. pSmads immunofluorescence staining at the host-graft interface.

(A) H&E staining of a PLGA coated allograft. (B) Immunofluorescence image of pSMAD3 staining, and (C & D) Immunofluorescence images of pSMAD5 staining in respective boxed regions in (A) at the cortical bone junctions. (E-G) Represented microscopic images of H&E staining. (H-M) pSMAD3 staining in allograft. Allograft coated with PLGA (F, I, L) showed enhanced pSMAD3 staining as compared to allograft alone (E, H, and K) and allograft coated with PLGA/BMP-2 peptide (G, J, and M) at the indicated magnification. Boxed regions in E, F, and G are shown at 10x and 20x magnification in the fluorescent images as indicated. (N) Quantification of the mean pSMAD3+ fluorescence intensity as expressed as the integrated density of the fluorescence signal. (O) Quantification of the percent area of pSMAD3+ cells (n=6, p<0.05).
4. DISCUSSION
Structural bone allografts are commonly used in orthopedic reconstruction and revision surgeries for repair of large defects and for treatment of acute fractures and nonunions in which mechanical and structural stability is required. 44–48 Currently available allografts are not ideal due to the lack of osteogenic and angiogenic properties as well as the inert nature of the cortical allografts. To develop an off-the-shelf allograft capable of enhancing new bone formation and osseointegration, here we demonstrated a new and versatile approach for creating a periosteum mimetic coating on the surface of allografts via polymer-mediated electrospray deposition. The resultant BMP-2 peptide/polymer coated allografts showed a significant improvement in allograft healing in a murine segmental femoral bone defect model, as evidenced by more new bone formation, less fibrotic tissue formation, and better osseointegration of the grafts to host bone compared to the ones without coating or coating with polymer alone.
In lieu of the essential role of periosteum in bone graft healing and incorporation, a tissue engineering strategy has been proposed to create a periosteum mimetic to restore the missing molecular and cellular function of periosteum for bone graft healing and repair. 5, 11–13, 49–52 Most of the technologies used for this purpose involve complex fabrication procedures and often require osteogenic and angiogenic cells for transplantation. These cumbersome and costly fabrication processes significantly hamper the translation of modified allografts to clinical uses. Several methodologies namely dip-coating, physical adsorption and growth factor conjugation have been used to modify the surface of the allograft and to deliver growth factors to the healing site to enhance host-dependent repair. The dip-coating method is laborious, less efficient and may not achieve a uniform coating on the allograft. The physical adsorption method often leads to burst release which cannot sustain the desired level of growth factor at the defect site. Growth factor conjugation generally is limited by allograft surface area and could potentially compromise the bioactivity of growth factors due to the formation of new chemical bonds. 53 Compared to the other approaches, electrospray deposition offers a significant advantage in the fabrication of a periosteum mimetic coating to allografts as it is simple, practical, and efficient and allows well-controlled deposition of a variety of polymers and bioactive materials for surface modification and for delivery of periosteum-derived growth factors and chemokines for enhanced repair and reconstruction. This approach could be used alone or in conjunction with other fabrication techniques to deliver multiple components of periosteum to achieve better efficacy in repair and reconstruction of large bone defects.
To establish electrospray deposition as a platform technology for allograft surface coating and drug delivery, we utilized PLGA (50:50) as a carrier to deliver an osteogenic BMP-2 peptide to the healing site as it has been used in many FDA approved drug products. Our data showed that nearly all loaded BMP-2 peptide was released during the first 4 weeks, coincided with the early osteogenic and chondrogenic phase of allograft healing. As a result, implantation of the PLGA/BMP-2 peptide coated allografts led to improved allograft healing. In addition to overall enhanced bone formation, more bone was observed near or directly on the allograft surface, suggesting that the coating and modification of allograft surface were effective in stimulating allograft surface mineralization, enhancing the osseointegration and biomechanical function of the grafted bone. Other than delivery of various growth factors and therapeutic agents, selective modification of the allograft surface with biomimetic nano- and micro-materials could also be explored by incorporation of selective material/matrix into the periosteum mimetic coating for improved repair and regeneration. 54–57 In addition, simultaneous or sequential delivery of multiple osteogenic and angiogenic factors can also be used to control the integration of the tissue 16–18. These modifications could further enhance the interaction between allograft and host osteogenic and angiogenic cells, facilitating the revitalization of bone allografts.
While significantly enhanced healing was observed in BMP-2 peptide coated allografts, bone formation along the surface of allograft was found to be uneven. This could be the result of uneven degradation of the polymer coating or un-optimized dose or release kinetics of the BMP-2 mimetic peptide. In addition to the BMP-2 mimetic peptide, a number of novel osteogenic and angiogenic peptides published recently could be examined with our current technique to evaluate their efficacy of healing and graft incorporation. 58–62 The versatility of the electrospray-assisted approach, which permits layer-by-layer deposition of multiple polymer components on bone allograft surface, will enable delivery of multiple growth factors/peptides at different time scales, leading to the production of a multifunctional off-the-shelf allograft bone product for repair and reconstruction. Finally, while our current rodent model is efficient and cost-effective in testing novel treatment strategies, the rodent models cannot perfectly simulate the clinical allograft in human. Future studies using large animal models are warranted.
Periosteum mimetic has been proposed for coating of various implant surfaces. 63–68 The use of biomaterial coating enables prolonged release of bioactive agents from the implant surface, a desired modality that could lead to improved tissue integration. A body of literature has been shown that PLGA tubes and photopolymerisable polycapralactone scaffolds exhibiting osteoconductivity to bridge segmental defects. 69–70 By coating allograft with BMP peptide releasing PLGA, the periosteum mimetic coating could provide a conduit for bone growth and jump start the differentiation and recruitment of osteogenic cells to the healing site, leading to improved osseointegration. However, based upon the histology and mechanical testing, our data indicated that introducing a simple PLGA coating did not provide a conduit for bone growth, instead evoking a fibrotic encapsulation around bone allograft, suggesting that anti-fibrotic tissue formation is equally important when using biomaterials to coat graft surface. Our further immunofluorescence analyses pin point a role of TGFβ signaling in PLGA evoked fibrotic tissue response.
Bone allografts with or without coatings could trigger a host immune response that leads to chronic inflammation and fibrotic tissue formation. 71–72 Transforming growth factor-β (TGF-β), especially isoform 1, expressed by macrophages is known to promote fibrosis in many cells and organs, including the lungs, kidneys, liver, heart, and skin. TGF-β signaling is ubiquitously activated in fibrotic diseases and is sufficient and required for the induction of fibrosis. 73–74 In our current study, we found that TGF-β signaling, as indicated by p-SMAD3 expression, is enhanced in fibrotic tissue in allograft samples. There was stronger and more staining in allografts coated with PLGA, suggesting a role of TGF-β in polymer-induced fibrotic tissue formation at the healing site. Interestingly, the coating of BMP-2 peptide together with PLGA showed a tempered fibrotic response, accompanied by a significant reduction in the pSmad3 expression level. This data suggested BMP-2 peptide could antagonize TGF-β signaling in vivo.
The TGF-β/BMP superfamily of ligands is known to interact with their respective receptors and activate respective R-SMADs. The signaling pathway of BMPs activates R-SMADs 1, 5, and 8, whereas the signaling pathway of TGF-βs activates R-SMADs 2 and 3. Both pathways converge at the common transcription factor SMAD4, which may lead to synergistic or opposing effects depending on the stimulation strategies. 75 While both BMPs and TGF-βs have shown to play important roles in bone development and remodeling, a large amount of literature also demonstrates reciprocal and opposing effects of BMP and TGF-β signaling on osteoblast and chondrocyte differentiation. 76–78 Several in vivo studies have shown that when activin/TGF-β signaling is inhibited through genetic or pharmacologic approaches, bone formation rate and bone mass increase. 79–83 Inhibition of TGF-β signaling has also shown to enhance osteoblast differentiation of bone marrow stromal cells and pre-osteoblastic MC3T3-E1 cells, and further potentiate the effects of BMP-2.77,84 These studies, together with our current study, suggest a potential strategy to explore the antagonism between these two pathways for therapeutic purposes. 85
In addition to osseointegration, remodeling of the bone graft is an important mechanism for long-term repair of bone allografts. Comparing with bone autograft, cortical allograft is difficult to penetrate and resorb due to its dense nature and the lack of viable cells in bone tissue. 6, 13, 39 To enhance penetration of the repair tissue into allografts, perforated allografts are tested which show better remodeling and incorporation of the devitalized bone. 86 However, perforated allograft could compromise the mechanical strength of the cortical allografts. Our strategy has been centering on enhancing the overall bone forming activity of the mimetic periosteum, allowing the recruitment of progenitor and osteoclastic cells to the graft surface to gradually remodel bone allograft. Future work will explore the opportunities to enhance bone forming and resorptive activity of the mimetic periosteum in a spatiotemporally controlled manner to further improve the outcome of the allograft healing and remodeling.
5. CONCLUSION
We have demonstrated a simple and reproducible method of fabricating a periosteum mimetic coating on cortical allografts via polymer-mediated electrospray deposition. The BMP-2 peptide/PLGA coated allografts exhibited sustained release of BMP-2 peptide over 4 weeks and significantly improved bone allograft healing in a murine segmental bone defect model. While PLGA coated allografts showed enhanced fibrotic tissue formation associated with increased TGF-β signaling, the inclusion of BMP-2 peptide in the PLGA coating antagonized the TGF-β signaling and significantly reduced fibrotic tissue formation. Future studies could be devoted to the parallel or sequential delivery of multiple osteogenic and angiogenic factors as well as fibrotic inhibitors from the coatings of bone allografts to further enhance the new bone formation while reducing the fibrotic tissue formation and promoting osseointegration between graft surface and host bone. The strategy presented in this study can be readily extended to modify the surface of other types of implants or grafts as well.
ACKNOWLEDGEMENTS
This study is supported by grants from the National Institute of Health RO1AR067859, RO1DE019902, R21DE026256, RO1DE029790, P30AR069655 to XZ and R21AR076056 to XZ and JX. We thank Michael Thullen for performing MicroCT scan, Emma Gira and Christine Massie for conducting torsional biomechanical testing and Jeffrey Fox for tissue processing and preparation.
REFERENCES
- (1).Greenwald AS; Boden SD; Goldberg VM; Khan Y; Laurencin CT; Rosier RN Bone-Graft Substitutes: Facts, Fictions, and Applications. J Bone Joint Surg Am 2001, 83-A Suppl 2 Pt 2, 98–103. [DOI] [PubMed] [Google Scholar]
- (2).Grand View Research I Bone Grafts and Substitutes Market Size, Share & Trends Analysis Report by Material Type (Natural, Synthetic). Market Watch 2018. [Google Scholar]
- (3).Goldberg VM; Stevenson S Natural History of Autografts and Allografts. Clin Orthop 1987, (225), 7–16. [PubMed] [Google Scholar]
- (4).Wheeler DL; Enneking WF Allograft Bone Decreases in Strength in Vivo over Time. Clin Orthop Relat Res 2005, 435, 36–42. [DOI] [PubMed] [Google Scholar]
- (5).Zhang X; Awad HA; O’Keefe RJ; Guldberg RE; Schwarz EM A Perspective: Engineering Periosteum for Structural Bone Graft Healing. Clin Orthop Relat Res 2008, 466 (8), 1777–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Zhang X; Xie C; Lin AS; Ito H; Awad H; Lieberman JR; Rubery PT; Schwarz EM; O’Keefe RJ; Guldberg RE Periosteal Progenitor Cell Fate in Segmental Cortical Bone Graft Transplantations: Implications for Functional Tissue Engineering. J Bone Miner Res 2005, 20 (12), 2124–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Chang H; Knothe Tate ML Concise Review: The Periosteum: Tapping into a Reservoir of Clinically Useful Progenitor Cells. Stem cells translational medicine 2012, 1 (6), 480–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Colnot C; Zhang X; Knothe Tate ML Current Insights on the Regenerative Potential of the Periosteum: Molecular, Cellular, and Endogenous Engineering Approaches. J Orthop Res 2012, 30 (12), 1869–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Petrie Aronin CE; Shin SJ; Naden KB; Rios PD Jr.; Sefcik LS; Zawodny SR; Bagayoko ND; Cui Q; Khan Y; Botchwey EA The Enhancement of Bone Allograft Incorporation by the Local Delivery of the Sphingosine 1-Phosphate Receptor Targeted Drug Fty720. Biomaterials 2010, 31 (25), 6417–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Koefoed M; Ito H; Gromov K; Reynolds DG; Awad HA; Rubery PT; Ulrich-Vinther M; Soballe K; Guldberg RE; Lin AS; O’Keefe RJ; Zhang X; Schwarz EM Biological Effects of Raav-Caalk2 Coating on Structural Allograft Healing. Mol Ther 2005, 12 (2), 212–8. [DOI] [PubMed] [Google Scholar]
- (11).Hoffman MD; Xie C; Zhang X; Benoit DS The Effect of Mesenchymal Stem Cells Delivered Via Hydrogel-Based Tissue Engineered Periosteum on Bone Allograft Healing. Biomaterials 2013, 34 (35), 8887–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Wang T; Zhai Y; Nuzzo M; Yang X; Yang Y; Zhang X Layer-by-Layer Nanofiber-Enabled Engineering of Biomimetic Periosteum for Bone Repair and Reconstruction. Biomaterials 2018, 182, 279–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Xie C; Reynolds D; Awad H; Rubery PT; Pelled G; Gazit D; Guldberg RE; Schwarz EM; O’Keefe RJ; Zhang X Structural Bone Allograft Combined with Genetically Engineered Mesenchymal Stem Cells as a Novel Platform for Bone Tissue Engineering. Tissue Eng 2007, 13 (3), 435–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Goldberg VM; Stevenson S The Biology of Bone Grafts. Semin Arthroplasty 1993, 4 (2), 58–63. [PubMed] [Google Scholar]
- (15).Wheeler DL; Haynie JL; Berrey H; Scarborough M; Enneking W Biomechanical Evaluation of Retrieved Massive Allografts: Preliminary Results. Biomed Sci Instrum 2001, 37, 251–6. [PubMed] [Google Scholar]
- (16).Sharmin F; Adams D; Pensak M; Dukas A; Lieberman J; Khan Y Biofunctionalizing Devitalized Bone Allografts through Polymer-Mediated Short and Long Term Growth Factor Delivery. J Biomed Mater Res A 2015, 103 (9), 2847–54. [DOI] [PubMed] [Google Scholar]
- (17).Sharmin F; McDermott C; Lieberman J; Sanjay A; Khan Y Dual Growth Factor Delivery from Biofunctionalized Allografts: Sequential Vegf and Bmp-2 Release to Stimulate Allograft Remodeling. J Orthop Res 2017, 35 (5), 1086–1095. [DOI] [PubMed] [Google Scholar]
- (18).Sharmin F; O’Sullivan M; Malinowski S; Lieberman JR; Khan Y Large Scale Segmental Bone Defect Healing through the Combined Delivery of Vegf and Bmp-2 from Biofunctionalized Cortical Allografts. J Biomed Mater Res B Appl Biomater 2019, 107 (4), 1002–1010. [DOI] [PubMed] [Google Scholar]
- (19).Culpepper BK; Bonvallet PP; Reddy MS; Ponnazhagan S; Bellis SL Polyglutamate Directed Coupling of Bioactive Peptides for the Delivery of Osteoinductive Signals on Allograft Bone. Biomaterials 2013, 34 (5), 1506–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Culpepper BK; Morris DS; Prevelige PE; Bellis SL Engineering Nanocages with Polyglutamate Domains for Coupling to Hydroxyapatite Biomaterials and Allograft Bone. Biomaterials 2013, 34 (10), 2455–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Culpepper BK; Webb WM; Bonvallet PP; Bellis SL Tunable Delivery of Bioactive Peptides from Hydroxyapatite Biomaterials and Allograft Bone Using Variable-Length Polyglutamate Domains. J Biomed Mater Res A 2014, 102 (4), 1008–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Xie J; Jiang J; Davoodi P; Srinivasan MP; Wang CH Electrohydrodynamic Atomization: A Two-Decade Effort to Produce and Process Micro-/Nanoparticulate Materials. Chemical engineering science 2015, 125, 32–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Boda SK; Li XR; Xie JW Electrospraying an Enabling Technology for Pharmaceutical and Biomedical Applications: A Review. J Aerosol Sci 2018, 125, 164–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Kanie K; Kurimoto R; Tian J; Ebisawa K; Narita Y; Honda H; Kato R Screening of Osteogenic-Enhancing Short Peptides from Bmps for Biomimetic Material Applications. Materials (Basel) 2016, 9 (9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Saito A; Suzuki Y; Ogata S; Ohtsuki C; Tanihara M Activation of Osteo-Progenitor Cells by a Novel Synthetic Peptide Derived from the Bone Morphogenetic Protein-2 Knuckle Epitope. Biochim Biophys Acta 2003, 1651 (1-2), 60–7. [DOI] [PubMed] [Google Scholar]
- (26).Senta H; Bergeron E; Drevelle O; Park H; Faucheux N Combination of Synthetic Peptides Derived from Bone Morphogenetic Proteins and Biomaterials for Medical Applications. Can J Chem Eng 2011, 89 (2), 227–239. [Google Scholar]
- (27).Madl CM; Mehta M; Duda GN; Heilshorn SC; Mooney DJ Presentation of Bmp-2 Mimicking Peptides in 3d Hydrogels Directs Cell Fate Commitment in Osteoblasts and Mesenchymal Stem Cells. Biomacromolecules 2014, 15 (2), 445–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Boda SK; Almoshari Y; Wang H; Wang X; Reinhardt RA; Duan B; Wang D; Xie J Mineralized Nanofiber Segments Coupled with Calcium-Binding Bmp-2 Peptides for Alveolar Bone Regeneration. Acta Biomater 2019, 85, 282–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Huang C; Tang M; Yehling E; Zhang X Overexpressing Sonic Hedgehog Peptide Restores Periosteal Bone Formation in a Murine Bone Allograft Transplantation Model. Mol Ther 2014, 22 (2), 430–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).Lorensen W; Cline HE Marching Cubes: A High Resolution 3d Surface Construction Algorithm. . Computer Graphics (SIGGRAPH 87 Proceedings) 1987, 21 (4), 163–170. [Google Scholar]
- (31).Lewiner T; Lopes H; Vieira AW; Tavares G Efficient Implementation of Marching Cubes’ Cases with Topological Guarantees. Journal of Graphics Tools 2003, 8 (2), 1–15. [Google Scholar]
- (32).Reynolds DG; Shaikh S; Papuga MO; Lerner AL; O’Keefe RJ; Schwarz EM; Awad HA Micro-Ct-Based Measurement of Cortical Bone Graft-to-Host Union. J Bone Miner Res 2009, 24 (5), 899–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (33).Dhillon RS; Zhang L; Schwarz EM; Boyce BF; Xie C The Murine Femoral Bone Graft Model and a Semiautomated Histomorphometric Analysis Tool. Methods Mol Biol 2014, 1130, 45–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (34).Zhang L; Chang M; Beck CA; Schwarz EM; Boyce BF Analysis of New Bone, Cartilage, and Fibrosis Tissue in Healing Murine Allografts Using Whole Slide Imaging and a New Automated Histomorphometric Algorithm. Bone research 2016, 4, 15037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (35).Wang Q; Huang C; Xue M; Zhang X Expression of Endogenous Bmp-2 in Periosteal Progenitor Cells Is Essential for Bone Healing. Bone 2011, 48 (3), 524–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (36).Pamula E; Menaszek E In Vitro and in Vivo Degradation of Poly(L: -Lactide-Co-Glycolide) Films and Scaffolds. J Mater Sci Mater Med 2008, 19 (5), 2063–70. [DOI] [PubMed] [Google Scholar]
- (37).Woodruff MA; Hutmacher DW The Return of a Forgotten Polymer-Polycaprolactone in the 21st Century. Prog Polym Sci 2010, 35 (10), 1217–1256. [Google Scholar]
- (38).Makadia HK; Siegel SJ Poly Lactic-Co-Glycolic Acid (Plga) as Biodegradable Controlled Drug Delivery Carrier. Polymers 2011, 3 (3), 1377–1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (39).Tiyapatanaputi P; Rubery PT; Carmouche J; Schwarz EM; O’Keefe RJ; Zhang X A Novel Murine Segmental Femoral Graft Model. J Orthop Res 2004, 22 (6), 1254–60. [DOI] [PubMed] [Google Scholar]
- (40).Xie C; Ming X; Wang Q; Schwarz EM; Guldberg RE; O’Keefe RJ; Zhang X Cox-2 from the Injury Milieu Is Critical for the Initiation of Periosteal Progenitor Cell Mediated Bone Healing. Bone 2008, 43 (6), 1075–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (41).Xie C; Xue M; Wang Q; Schwarz EM; O’Keefe RJ; Zhang X Tamoxifen-Inducible Creer-Mediated Gene Targeting in Periosteum Via Bone-Graft Transplantation. J Bone Joint Surg Am 2008, 90 Suppl 1, 9–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (42).Wang Q; Huang C; Zeng F; Xue M; Zhang X Activation of the Hh Pathway in Periosteum-Derived Mesenchymal Stem Cells Induces Bone Formation in Vivo: Implication for Postnatal Bone Repair. Am J Pathol 2010, 177 (6), 3100–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (43).Ito H; Koefoed M; Tiyapatanaputi P; Gromov K; Goater JJ; Carmouche J; Zhang X; Rubery PT; Rabinowitz J; Samulski RJ; Nakamura T; Soballe K; O’Keefe RJ; Boyce BF; Schwarz EM Remodeling of Cortical Bone Allografts Mediated by Adherent Raav-Rankl and Vegf Gene Therapy. Nat Med 2005, 11 (3), 291–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (44).Berrey BH Jr.; Lord CF; Gebhardt MC; Mankin HJ Fractures of Allografts. Frequency, Treatment, and End-Results. J Bone Joint Surg Am 1990, 72 (6), 825–33. [PubMed] [Google Scholar]
- (45).Jaffe KA; Morris SG; Sorrell RG; Gebhardt MC; Mankin HJ Massive Bone Allografts for Traumatic Skeletal Defects. South Med J 1991, 84 (8), 975–82. [DOI] [PubMed] [Google Scholar]
- (46).Hornicek FJ; Zych GA; Hutson JJ; Malinin TI Salvage of Humeral Nonunions with Onlay Bone Plate Allograft Augmentation. Clin Orthop Relat Res 2001, (386), 203–9. [DOI] [PubMed] [Google Scholar]
- (47).Berkes MB; Little MT; Schottel PC; Pardee NC; Zuiderbaan A; Lazaro LE; Helfet DL; Lorich DG Outcomes of Schatzker Ii Tibial Plateau Fracture Open Reduction Internal Fixation Using Structural Bone Allograft. J Orthop Trauma 2014, 28 (2), 97–102. [DOI] [PubMed] [Google Scholar]
- (48).Van Houwelingen AP; McKee MD Treatment of Osteopenic Humeral Shaft Nonunion with Compression Plating, Humeral Cortical Allograft Struts, and Bone Grafting. J Orthop Trauma 2005, 19 (1), 36–42. [DOI] [PubMed] [Google Scholar]
- (49).Zou XH; Cai HX; Yin Z; Chen X; Jiang YZ; Hu H; Ouyang HW A Novel Strategy Incorporated the Power of Mesenchymal Stem Cells to Allografts for Segmental Bone Tissue Engineering. Cell Transplant 2009, 18 (4), 433–41. [DOI] [PubMed] [Google Scholar]
- (50).Zou XH; Cai HX; Yin Z; Chen X; Jiang YZ; Hu H; Ouyang HW A Novel Strategy Incorporated the Power of Mesenchymal Stem Cells to Allografts for Segmental Bone Tissue Engineering. Cell Transplant 2010, 19 (9), 1215. [DOI] [PubMed] [Google Scholar]
- (51).Zhao L; Zhao J; Wang S; Xia Y; Liu J; He J; Wang X Evaluation of Immunocompatibility of Tissue-Engineered Periosteum. Biomed Mater 2011, 6 (1), 015005. [DOI] [PubMed] [Google Scholar]
- (52).Gamie Z; Tran GT; Vyzas G; Korres N; Heliotis M; Mantalaris A; Tsiridis E Stem Cells Combined with Bone Graft Substitutes in Skeletal Tissue Engineering. Expert Opin Biol Ther 2012, 12 (6), 713–29. [DOI] [PubMed] [Google Scholar]
- (53).Mitchell AC; Briquez PS; Hubbell JA; Cochran JR Engineering Growth Factors for Regenerative Medicine Applications. Acta Biomater 2016, 30, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (54).Lee MH; You C; Kim KH Combined Effect of a Microporous Layer and Type I Collagen Coating on a Biphasic Calcium Phosphate Scaffold for Bone Tissue Engineering. Materials (Basel) 2015, 8 (3), 1150–1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (55).Mansour A; Abu-Nada L; Al-Waeli H; Mezour MA; Abdallah MN; Kinsella JM; Kort-Mascort J; Henderson JE; Ramirez-Garcialuna JL; Tran SD; Elkashty OA; Mousa A; El-Hadad AA; Taqi D; Al-Hamad F; Alageel O; Kaartinen MT; Tamimi F Bone Extracts Immunomodulate and Enhance the Regenerative Performance of Dicalcium Phosphates Bioceramics. Acta Biomater 2019, 89, 343–358. [DOI] [PubMed] [Google Scholar]
- (56).Park JW; Kang DG; Hanawa T New Bone Formation Induced by Surface Strontium-Modified Ceramic Bone Graft Substitute. Oral diseases 2016, 22 (1), 53–61. [DOI] [PubMed] [Google Scholar]
- (57).Wang T; Krieger J; Huang C; Das A; Dickinson M; Francis MP; Ogle R; Botchwey E Erratum To: Enhanced Osseous Integration of Human Trabecular Allografts Following Surface Modification with Bioactive Lipids. Drug delivery and translational research 2016, 6 (5), 630. [DOI] [PubMed] [Google Scholar]
- (58).Ciccarelli M; Campanile A; Santulli G; Galasso G; D’Andrea L; Del Gatto A; Altobelli GG; Cimini V; Piscione F; Trimarco B; Iaccarino G Proangiogenic Properties of Qk, a Peptide Mimicking Vegf/Kdr Binding Interface. Circulation 2006, 114 (18), 251–251. [Google Scholar]
- (59).Rahman HNA; Wu H; Dong Y; Pasula S; Wen A; Sun Y; Brophy ML; Tessneer KL; Cai X; McManus J; Chang B; Kwak S; Rahman NS; Xu W; Fernandes C; McDaniel JM; Xia L; Smith L; Srinivasan RS; Chen H Selective Targeting of a Novel Epsin-Vegfr2 Interaction Promotes Vegf-Mediated Angiogenesis. Circ Res 2016, 118 (6), 957–969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (60).Wang C; Liu Y; Fan Y; Li X The Use of Bioactive Peptides to Modify Materials for Bone Tissue Repair. Regenerative biomaterials 2017, 4 (3), 191–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (61).Bab I; Gazit D; Chorev M; Muhlrad A; Shteyer A; Greenberg Z; Namdar M; Kahn A Histone H4-Related Osteogenic Growth Peptide (Ogp): A Novel Circulating Stimulator of Osteoblastic Activity. EMBO J 1992, 11 (5), 1867–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (62).Gabet Y; Muller R; Regev E; Sela J; Shteyer A; Salisbury K; Chorev M; Bab I Osteogenic Growth Peptide Modulates Fracture Callus Structural and Mechanical Properties. Bone 2004, 35 (1), 65–73. [DOI] [PubMed] [Google Scholar]
- (63).Ardjomandi N; Klein C; Kohler K; Maurer A; Kalbacher H; Niederlander J; Reinert S; Alexander D Indirect Coating of Rgd Peptides Using a Poly-L-Lysine Spacer Enhances Jaw Periosteal Cell Adhesion, Proliferation, and Differentiation into Osteogenic Tissue. J Biomed Mater Res A 2012, 100 (8), 2034–44. [DOI] [PubMed] [Google Scholar]
- (64).Uezono M; Takakuda K; Kikuchi M; Suzuki S; Moriyama K Hydroxyapatite/Collagen Nanocomposite-Coated Titanium Rod for Achieving Rapid Osseointegration onto Bone Surface. J Biomed Mater Res B Appl Biomater 2013, 101 (6), 1031–8. [DOI] [PubMed] [Google Scholar]
- (65).Shi X; Fujie T; Saito A; Takeoka S; Hou Y; Shu Y; Chen M; Wu H; Khademhosseini A Periosteum-Mimetic Structures Made from Freestanding Microgrooved Nanosheets. Adv Mater 2014, 26 (20), 3290–6. [DOI] [PubMed] [Google Scholar]
- (66).Romero R; Chubb L; Travers JK; Gonzales TR; Ehrhart NP; Kipper MJ Coating Cortical Bone Allografts with Periosteum-Mimetic Scaffolds Made of Chitosan, Trimethyl Chitosan, and Heparin. Carbohydrate polymers 2015, 122, 144–51. [DOI] [PubMed] [Google Scholar]
- (67).Romero R; Travers JK; Asbury E; Pennybaker A; Chubb L; Rose R; Ehrhart NP; Kipper MJ Combined Delivery of Fgf-2, Tgf-Beta1, and Adipose-Derived Stem Cells from an Engineered Periosteum to a Critical-Sized Mouse Femur Defect. J Biomed Mater Res A 2017, 105 (3), 900–911. [DOI] [PubMed] [Google Scholar]
- (68).Vanderleyden E; Van Bael S; Chai YC; Kruth JP; Schrooten J; Dubruel P Gelatin Functionalised Porous Titanium Alloy Implants for Orthopaedic Applications. Mater Sci Eng C Mater Biol Appl 2014, 42, 396–404. [DOI] [PubMed] [Google Scholar]
- (69).Gogolewski S; Pineda L; Busing CM Bone Regeneration in Segmental Defects with Resorbable Polymeric Membranes: Iv. Does the Polymer Chemical Composition Affect the Healing Process? Biomaterials 2000, 21 (24), 2513–20. [DOI] [PubMed] [Google Scholar]
- (70).Henslee AM; Spicer PP; Yoon DM; Nair MB; Meretoja VV; Witherel KE; Jansen JA; Mikos AG; Kasper FK Biodegradable Composite Scaffolds Incorporating an Intramedullary Rod and Delivering Bone Morphogenetic Protein-2 for Stabilization and Bone Regeneration in Segmental Long Bone Defects. Acta Biomater 2011, 7 (10), 3627–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (71).Bannister SR; Powell CA Foreign Body Reaction to Anorganic Bovine Bone and Autogenous Bone with Platelet-Rich Plasma in Guided Bone Regeneration. Journal of periodontology 2008, 79 (6), 1116–20. [DOI] [PubMed] [Google Scholar]
- (72).Nuss KM; von Rechenberg B Biocompatibility Issues with Modern Implants in Bone - a Review for Clinical Orthopedics. The open orthopaedics journal 2008, 2, 66–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (73).Kajdaniuk D; Marek B; Borgiel-Marek H; Kos-Kudla B Transforming Growth Factor Beta1 (Tgfbeta1) in Physiology and Pathology. Endokrynol Pol 2013, 64 (5), 384–96. [DOI] [PubMed] [Google Scholar]
- (74).Meng XM; Nikolic-Paterson DJ; Lan HY Tgf-Beta: The Master Regulator of Fibrosis. Nature reviews . Nephrology 2016, 12 (6), 325–38. [DOI] [PubMed] [Google Scholar]
- (75).Wu M; Chen G; Li YP Tgf-Beta and Bmp Signaling in Osteoblast, Skeletal Development, and Bone Formation, Homeostasis and Disease. Bone research 2016, 4, 16009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (76).Mizuno Y; Tokuzawa Y; Ninomiya Y; Yagi K; Yatsuka-Kanesaki Y; Suda T; Fukuda T; Katagiri T; Kondoh Y; Amemiya T; Tashiro H; Okazaki Y Mir-210 Promotes Osteoblastic Differentiation through Inhibition of Acvr1b. FEBS Lett 2009, 583 (13), 2263–8. [DOI] [PubMed] [Google Scholar]
- (77).Maeda S; Hayashi M; Komiya S; Imamura T; Miyazono K Endogenous Tgf-Beta Signaling Suppresses Maturation of Osteoblastic Mesenchymal Cells. EMBO J 2004, 23 (3), 552–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (78).Li TF; Darowish M; Zuscik MJ; Chen D; Schwarz EM; Rosier RN; Drissi H; O’Keefe RJ Smad3-Deficient Chondrocytes Have Enhanced Bmp Signaling and Accelerated Differentiation. J Bone Miner Res 2006, 21 (1), 4–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (79).Edwards JR; Nyman JS; Lwin ST; Moore MM; Esparza J; O’Quinn EC; Hart AJ; Biswas S; Patil CA; Lonning S; Mahadevan-Jansen A; Mundy GR Inhibition of Tgf-Beta Signaling by 1d11 Antibody Treatment Increases Bone Mass and Quality in Vivo. J Bone Miner Res 2010, 25 (11), 2419–26. [DOI] [PubMed] [Google Scholar]
- (80).Qiu T; Wu X; Zhang F; Clemens TL; Wan M; Cao X Tgf-Beta Type Ii Receptor Phosphorylates Pth Receptor to Integrate Bone Remodelling Signalling. Nature cell biology 2010, 12 (3), 224–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (81).Koncarevic A; Cornwall-Brady M; Pullen A; Davies M; Sako D; Liu J; Kumar R; Tomkinson K; Baker T; Umiker B; Monnell T; Grinberg AV; Liharska K; Underwood KW; Ucran JA; Howard E; Barberio J; Spaits M; Pearsall S; Seehra J; Lachey J A Soluble Activin Receptor Type Iib Prevents the Effects of Androgen Deprivation on Body Composition and Bone Health. Endocrinology 2010, 151 (9), 4289–300. [DOI] [PubMed] [Google Scholar]
- (82).Ruckle J; Jacobs M; Kramer W; Pearsall AE; Kumar R; Underwood KW; Seehra J; Yang Y; Condon CH; Sherman ML Single-Dose, Randomized, Double-Blind, Placebo-Controlled Study of Ace-011 (Actriia-Igg1) in Postmenopausal Women. J Bone Miner Res 2009, 24 (4), 744–52. [DOI] [PubMed] [Google Scholar]
- (83).Pearsall RS; Canalis E; Cornwall-Brady M; Underwood KW; Haigis B; Ucran J; Kumar R; Pobre E; Grinberg A; Werner ED; Glatt V; Stadmeyer L; Smith D; Seehra J; Bouxsein ML A Soluble Activin Type Iia Receptor Induces Bone Formation and Improves Skeletal Integrity. Proc Natl Acad Sci U S A 2008, 105 (19), 7082–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (84).Takeuchi K; Abe M; Hiasa M; Oda A; Amou H; Kido S; Harada T; Tanaka O; Miki H; Nakamura S; Nakano A; Kagawa K; Yata K; Ozaki S; Matsumoto T Tgf-Beta Inhibition Restores Terminal Osteoblast Differentiation to Suppress Myeloma Growth. PLoS One 2010, 5 (3), e9870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (85).Hudnall AM; Arthur JW; Lowery JW Clinical Relevance and Mechanisms of Antagonism between the Bmp and Activin/Tgf-Beta Signaling Pathways. The Journal of the American Osteopathic Association 2016, 116 (7), 452–61. [DOI] [PubMed] [Google Scholar]
- (86).Delloye C; Simon P; Nyssen-Behets C; Banse X; Bresler F; Schmitt D Perforations of Cortical Bone Allografts Improve Their Incorporation. Clin Orthop Relat Res 2002, (396), 240–7. [DOI] [PubMed] [Google Scholar]


