Abstract
Objective
To investigate the relationship between LH and testosterone (T), which characteristics associate with the strength of this relationship, and their interrelationships with GH, TSH, cortisol, and ACTH.
Design
Hormones were measured in serum samples collected every 10 minutes during 24 hours from 20 healthy men, comprising 10 offspring of long-lived families and 10 control subjects, with a mean (SD) age of 65.6 (5.3) years. We performed cross-correlation analyses to assess the relative strength between 2 timeseries for all possible time shifts.
Results
Mean (95% CI) maximal correlation was 0.21 (0.10-0.31) at lag time of 60 minutes between LH and total T concentrations. Results were comparable for calculated free, bioavailable, or secretion rates of T. Men with strong LH-T cross-correlations had, compared with men with no cross-correlation, lower fat mass (18.5 [14.9-19.7] vs. 22.3 [18.4-29.4] kg), waist circumference (93.6 [5.7] vs. 103.1 [12.0] cm), high-sensitivity C-reactive protein (0.7 [0.4-1.3] vs. 1.8 [0.8-12.3] mg/L), IL-6 (0.8 [0.6-1.0] vs. 1.2 [0.9-3.0] pg/mL), and 24-hour mean LH (4.3 [2.0] vs. 6.1 [1.5] U/L), and stronger LH-T feedforward synchrony (1.5 [0.3] vs. 1.9 [0.2]). Furthermore, T was positively cross-correlated with TSH (0.32 [0.21-0.43]), cortisol (0.26 [0.19-0.33]), and ACTH (0.26 [0.19-0.32]).
Conclusions
LH is followed by T with a delay of 60 minutes in healthy older men. Men with a strong LH-T relationship had more favorable body composition, inflammatory markers, LH levels, and LH-T feedforward synchrony. We observed positive correlations between T and TSH, cortisol, and ACTH.
Keywords: luteinizing hormone, testosterone, pituitary hormones, cross-correlation, ageing, men
Serum levels of several parameters from the hypothalamic–pituitary–gonadal (HPG) axis change with age in men, which has been reviewed by others [1, 2]. Total testosterone (T) levels decline moderately, but progressively with age, starting around the age of 30 to 40 years, whereas levels of SHBG gradually increase with age, resulting in a steeper decline in serum levels of free T [2–5]. The decline in T is a multifactorial process. Intervention studies have shown that aging in healthy men is accompanied by diminished GnRH output, resulting in less LH drive on the Leydig cell and diminished testicular responsiveness to LH, whereas T feedback on the hypothalamus-pituitary unit is also decreased [6, 7]. The decline in T (free T) levels in males together with several sexual symptoms, when not caused by hypothalamus-pituitary disorders, including tumors, infections, and trauma, has been named late-onset hypogonadism [8, 9]. Androgen deficiency is worsened by comorbidities, including diabetes mellitus, cardiac failure, renal disease, chronic obstructive lung disease, obesity, medication, unhealthy lifestyle, and increased aromatase activity [1, 9, 10]. Aside from age-related T decline, the increase of SHBG levels may mask a low serum free T concentration. Factors influencing SHBG positively are thyroxine, estrogens, and antiepileptic drugs, whereas insulin, IGF-1, prolactin, pro-inflammatory cytokines, and adiponectin decrease SHBG levels [11, 12]. With advancing age, mean population LH levels increase, which can be attributed to diminished feedback on the gonadotrophic cells and hypothalamus centers involved in the secretion of GnRH. The diminished feedback is also revealed by amplified LH frequency and low amplitude pulses [13, 14]. Studies have shown that obesity, comorbidities, and lifestyle factors might be even better predictors for low T levels than age [3, 5, 15, 16]. In line, T levels were not lower in older men compared with younger men in a study with exceptionally healthy men nor in a study with men reporting themselves as having good or excellent health [17, 18].
Although the underlying cellular mechanisms of the decline in T levels with age are not entirely clear, it is commonly thought that the age-related decline in T is a large contributor to many problems in older men [13]. Therefore, T administration became a popular intervention in both hypogonadal and eugonadal men, and in both middle-aged and older men, especially in the United States [19, 20]. T administration might influence the secretion of hormones from other hypothalamic–pituitary–“target gland” axes. For example, 1 study in healthy older men showed that long-term low-dose T administration resulted in increased nocturnal GH secretion [21], but not in spontaneous nocturnal cortisol secretion [22]. Besides, with age, not only levels of LH and T change, but levels of other pituitary hormones also change concomitantly with age. For example, elevated TSH levels and a decline in GH secretion have been observed with aging [23–25]. It could be hypothesized that these hormonal changes are synchronized with each other, potentially because these endocrine systems share the ability to respond to changes in the environment to maintain homeostasis. In support of this hypothesis, anterior pituitary cells share the same embryonic origin, and there is evidence for crosstalk between pituitary cells [26–29]. Recently, we observed interrelations between hormones from different hypothalamic–pituitary–target gland axes, specifically between cortisol and TSH, GH and TSH, and between GH and cortisol concentrations, in healthy older men and women [30].
In a previous publication, we compared 24-hour LH and T secretion parameters and the LH-T relationship between 10 healthy older male offspring of long-lived families with 10 healthy older male controls from the Leiden Longevity Study [31]. We did not find an association between LH-T parameters and familial longevity. In the present study, we investigated the relationship between 24-hour serum LH and T concentrations in the total population of 20 healthy older men from the Leiden Longevity Study and which health characteristics associate with the strength of this relationship. Furthermore, we aimed to determine the interrelationships between LH and T with GH, TSH, cortisol, and ACTH over 24 hours. To this end, we performed cross-correlation analyses to assess the relative strength between 2 24-hour hormone concentration series at intervals of 10 minutes for all possible time shifts. FSH was not included in this study because its pulsatility is less pronounced due to its long half-life and low amplitude [32].
Materials and Methods
Study population
Participants were recruited from the Leiden Longevity Study, which is a family-based study consisting of 421 families with at least 2 long-lived Caucasian siblings (men ≥ 89 years and women ≥ 91 years) without any selection on health or demographics together with their offspring and the offsprings’ partners [33]. In the Switchbox Leiden Study, 24-hour blood samples were collected from 38 healthy older individuals between June 2012 and July 2013 [34]. This study comprised 20 offspring of long-lived families, including 10 men and 10 women, and 18 partners of the offspring as a control group, including 10 men and 8 women. Exclusion criteria included a fasting plasma glucose above 7 mmol/L, the presence of any significant chronic, renal, hepatic, or endocrine disease, or the use of medication known to influence any hormonal axis [34]. For the present analysis, we only included the male participants from the Switchbox Leiden Study. Consequently, 20 participants were included in the analyses, comprising 10 male offspring of long-lived families and 10 male controls. None of the participants indicated that they were using any biotin or vitamin B8 supplements, which otherwise could have interfered with the ACTH assay [35]. The Switchbox Leiden Study protocol P11.116 was approved by the Medical Ethical Committee of the Leiden University Medical Centre and performed according to the Helsinki declaration. All participants gave written informed consent for participation.
Clinical protocol
Full details on the 24-hour blood sampling procedure have been described previously [36]. In short, a catheter was placed in a vein of the forearm of the nondominant hand and blood was collected every 10 minutes starting around 09:00 am. The participants received standardized feeding consisting of 600 kcal Nutridrink (Nutricia Advanced Medical Nutrition, Zoetermeer, The Netherlands) at 3 fixed times during the day (between 9:00 and 10:00 am, 12:00 pm and 1:00 pm, and 6:00 pm and 7:00 pm). Lights were switched off for approximately 9 hours (circa between 11:00 pm and 8:00 am) to allow the participants to sleep. Height and weight were measured in the research center. Body mass index (BMI) was calculated as weight (in kilograms) divided by the square of height (in meters). Body composition was determined by Bioelectrical Impedance Analysis at a fixed frequency of 50 kHz (Bodystat 1500 Ltd, Isle of Man, UK). Waist circumference was measured with a measuring tape midway between the uppermost border of the iliac crest and the lower border of the costal margin. The Pittsburgh Sleep Quality Index questionnaire was used to collect data on habitual bedtime and getting up time during the past month [37].
Hormone assays
All laboratory measurements were performed with fully automated equipment and diagnostics from Roche Diagnostics (Almere, The Netherlands) and Siemens Healthcare diagnostics (The Hague, The Netherlands) at the Department of Clinical Chemistry and Laboratory Medicine of the Leiden University Medical Center in The Netherlands. Full details on the procedures of the hormone assays have been described previously [31, 34, 38, 39].
Levels of LH, T, GH, TSH, cortisol, and ACTH were all measured in blood samples collected every 10 minutes from all 20 participants. For each participant, all samples from 1 time series were measured with the same lot number in the same batch. LH (Roche, catalog #11732234-122, RRID:AB_2800498) and T (Roche, catalog #05200067-190, RRID:AB_2783736) were measured in EDTA plasma samples collected every 10 minutes using electrochemiluminescence (ECLIA) on a Roche Modular E170 immunoanalyzer. Human growth hormone with a molecular mass of 22000 Da (Siemens, catalog #L2KGRH2, RRID: AB_2811291) was measured in serum samples using an IMMULITE 2000 Xpi Immunoassay system (Siemens Healthcare Diagnostics). TSH (Roche, catalog #11731459, RRID:AB_2756377) and cortisol (Roche, catalog #11875116, RRID:AB_2811288) were measured in serum samples by ECLIA using Cobas reagents and a Roche Modular E170 Immunoanalyzer. ACTH (Siemens, catalog #L2KAC2, RRID:AB_2783635) was measured in EDTA samples using an IMMULITE 2000 Xpi Immunoassay system (Siemens Healthcare Diagnostics). The coefficients of variation (CV) in our study ranged between 4.5% and 2.8% for LH, between 4.1% and 3.8% for T, between 5.4% and 7.2% for GH, between 1.4% and 4.2% for TSH, between 2.4% and 5.1% for cortisol, and between 3.8% and 7.7% for ACTH. The data were checked for obvious outliers by 4 reviewers with expert knowledge in endocrinology by visual inspection of a graphical display of individual hormone profiles from all 20 participants [40].
fT4 (catalog #6437281190) was measured in serum samples with 1-hour intervals by ECLIA using a Modular E170 Immunoanalyzer in 1 batch. For fT4, the CV range in our study was 2.4% to 3.5%. IGF-1 (catalog #IS-3900) was measured in 6 plasma EDTA samples with 4-hour intervals for each participant using an iSYS Immunoassay system of ImmunoDiagnostic Systems (IDS GMBH, Frankfurt am Main, Germany) with a CV range of 1.4% to 1.8%.
Glucose and insulin were measured in a fasting serum sample withdrawn around 08:30 am at the second day of the 24-hpir blood sampling. Glucose (catalog #11876899216) was measured using Roche Hitachi Modular P800 and insulin (catalog #L2KIN2) was measured using IMMULITE 2000 Xpi Immunoassay. Testosterone is mostly bound to SHBG and albumin. SHBG (catalog #03052001190) and albumin (catalog #11970909216) were measured on Roche Modular analyzers in 6 EDTA plasma samples with 4-hour intervals for each participant around 10:00 am, 2:00 pm, 6:00 pm, 10:00 pm, 2:00 am, and 6:00 am. Bioavailable and free T concentrations were calculated as described in the appendix of Takahashi et al. [41]. Calculations were based on total T concentrations, which were measured every 10 minutes, and SHBG and albumin concentrations, which were measured every 4 hours. Therefore, for the calculation of bioavailable and free T concentrations for the period from 9:00 am to 1:00 pm, the SHBG and albumin levels measured at 10:00 am were used. Subsequently, for the time period from 1:00 pm to 5:00 pm, the SHBG and albumin levels measured at 2:00 pm were used, and so on. For each participant, 3 μL of all (144) samples taken during the 24-hour blood sampling were pooled. In this mixture, levels of estradiol (catalog #06656021190) with a CV range between 6.2% and 10.4% and prolactin (catalog #03203093190) with a CV range between 1.6% and 2.0% were determined using ECLIA and the E170 module of Modular Analytics from Roche Diagnostics.
Additional blood measurements
On the study day, total cholesterol, triglycerides, and high-density lipoprotein cholesterol were measured on the Roche Cobas 8000 Modular. Approximately 2 weeks before the study day, fasting serum was withdrawn to screen for baseline factors. Dehydroepiandrosterone sulfate (catalog #L2KDS2) was measured using a solid-phase competitive chemiluminescent enzyme immunoassay with an Immulite 2000 XPi system from Siemens Healthcare diagnostics (The Hague, The Netherlands). High-sensitivity C-reactive protein (hsCRP) (catalog #04628918190) was determined by a particle-enhanced turbidimetric assay using Cobas Integra 800 from Roche Diagnostics. Interleukin 6 (catalog #SS600B) and TNF-α (catalog #SSTA00D) were measured by ELISA from R&D Systems. All measurements were performed at the Department of Clinical Chemistry and Laboratory Medicine of the Leiden University Medical Center in The Netherlands.
Secretion rate
To determine underlying components of LH and T secretion, 24-hour serum LH and T concentration profiles were analyzed by validated deconvolution analysis [42–44]. By deconvolution analysis, a hormone concentration profile is decomposed into underlying secretory bursts, basal secretion, elimination of previously secreted hormone, and random experimental variability. The algorithm in the software program MATLAB (the Mathworks, Inc., Natick, MA) first detrends the data and normalizes concentrations by converting them to values between 0 and 1. Second, successive potential pulse-time sets, each containing 1 fewer burst, were created by a smoothing process. Finally, a maximum-likelihood expectation deconvolution method estimated all secretion and elimination rates simultaneously for each candidate pulse-time set. For LH, fast half-life was fixed to 6.93 minutes and slow half-life was estimated as unknown variable between 40 and 120 minutes [45]. For testosterone, fast and slow half-lives were fixed to 1.4 and 27 minutes, respectively [46]. Main outcome parameters are the secretion rate per minute and the pulse frequency. LH concentrations are cross-correlated with the secretion rates of T, which is a measure for the feedforward drive of LH on T [47].
Cross-correlation
Cross-correlation assesses the relative strength between 2 24-hour time series for all possible time shifts, by calculating linear Pearson’s correlation coefficients, as explained in more detail elsewhere [48, 49]. For example, hormone concentrations in time series A are compared pairwise with those of series B measured simultaneously (0 lag) or measured earlier or later (with a time lag). The unit of 1 lag time is the interval between 2 sampling points, so a lag time of 1 means that there is a delay of 10 minutes between 2 time series. Cross-correlation analyses were performed using the CCF function in the software program R, version 3.6.2 (The R Foundation for Statistical Computing, Vienna, Austria). The range of tested lag times depends on the number of data points in 1 time series; the range is lag –18 to 18 (360 minutes in total) for 144 data points. A correlation is considered significant when the absolute value is greater than 2/(√n – │k│), where n is the number of data points in 1 time series and k is the maximal possible lag [50]. Thus, for a time series of 144 data points and a maximal lag of 18, the significance level is 0.18.
Cross-correlation analyses were also performed after stratifying the 24-hour data for lights-on period, which is the data from time point 9:00 am up to and including 10:50 pm, and lights-off period (11:00 pm to 8:00 am). Data from 8:00 to 9:00 am were excluded from these stratified analyses because the lights were switched on at 8:00 am and the remaining hour of the data is too short to perform separate analyses on. For these subanalyses, the lag range and the significance level changed accordingly to a lag range of –16 to 16 (320 minutes) and significance level of 0.24 for the lights-on period, and a lag range of –14 to 14 (280 minutes) and significance level of 0.31 for the lights-off period.
Cross-approximate entropy
Bivariate cross-approximate entropy (cross-ApEn) is a scale- and model-independent regularity statistic, which quantifies the joint pattern synchrony between 2 simultaneously measured time series, with lower cross-ApEn values signifying greater synchrony [51]. Cross-ApEn of hormones A-B is different from a cross-ApEn of hormones B-A because A is leading in the first case and following in the second. Changes in the cross-ApEn reflect feedback and/or feedforward alterations within an interlinked axis, with the cross-ApEn of LH-T representing feedforward (a)synchrony and cross-ApEn of T-LH indicating feedback (a)synchrony [47]. Cross-ApEn was calculated with a window length of m = 1 and a margin of r = 0.2 (20% of the SD of the individual subject’s hormone time series) with standardized data using a Matlab-based algorithm (Mathworks, Inc., Natick, MA). Because cross-ApEn analyses cannot deal with missing data, missing data points were linearly interpolated.
Statistical analysis
Characteristics of the study participants were calculated using descriptive statistics. Normally distributed variables were presented as mean with standard deviation and nonnormally distributed variables were presented as median with interquartile ranges. Differences in participant characteristics between subgroups were assessed by independent-samples t tests for variables that were normally distributed and by Mann-Whitney U tests for not normally distributed variables. Categorical variables were compared between subgroups using a chi-squared test. Repeated measures ANOVA, which is a model-independent method, was used to test whether levels of albumin and SHBG were different between 6 time points. Repeated measures ANOVA was performed with time (10:00 am vs 2:00 pm vs 6:00 pm vs 10:00 pm vs 2:00 am vs 6:00 am) as within-subjects factor and albumin and SHBG levels as dependent variables. All statistical analyses were performed using SPSS for Windows, version 23 (SPSS, Chicago, IL). Fig. 1 was made using GraphPad Prism version 8 (GraphPad, San Diego, CA) and Figs 2, 3, and 4 were made using R, version 3.6.2.
Figure 1.
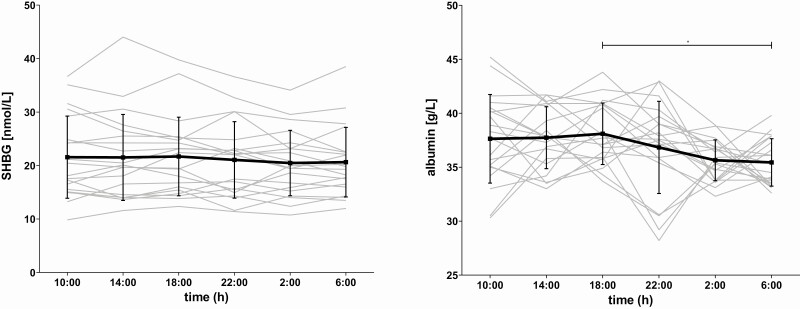
Concentration profiles of SHBG and albumin over 24 hours. Serum concentrations of SHBG in nmol/L and albumin in g/L were measured in blood that was sampled during 24 hour with 4-hour intervals for each participant. The individual concentrations profiles are plotted as gray lines. The black line represents the mean per timepoint together with the standard error bars. The asterisk indicates that mean albumin levels at 6:00 pm are significantly higher than levels at 6:00 am (P = 0.02).
Figure 2.
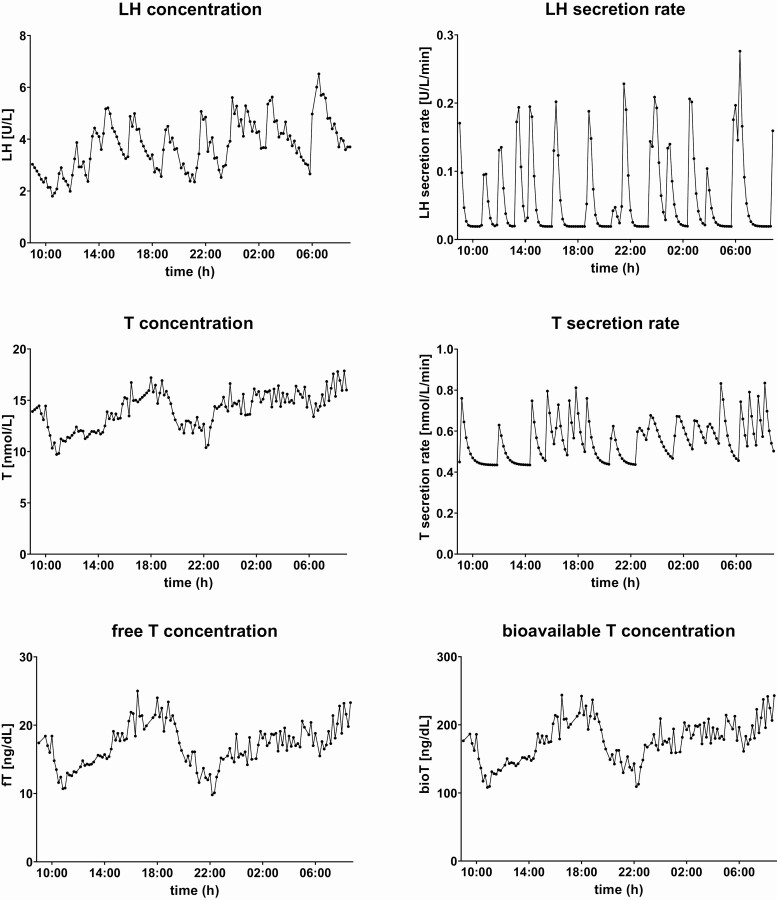
Twenty-four-hour concentration profiles and secretion rates of LH and (free and bioavailable) T from 1 individual. The concentration profiles of LH and testosterone (T), together with the calculated secretion rates, and the calculated concentration profiles of free T and bioavailable T from 1 representative participant are plotted over 24 hour. LH and T concentrations were measured in serum which was sampled every 10 minutes during 24 hour. Secretion rates were calculated using deconvolution analysis and free T and bioavailable T concentrations were calculated using total T, SHBG, and albumin levels.
Figure 3.
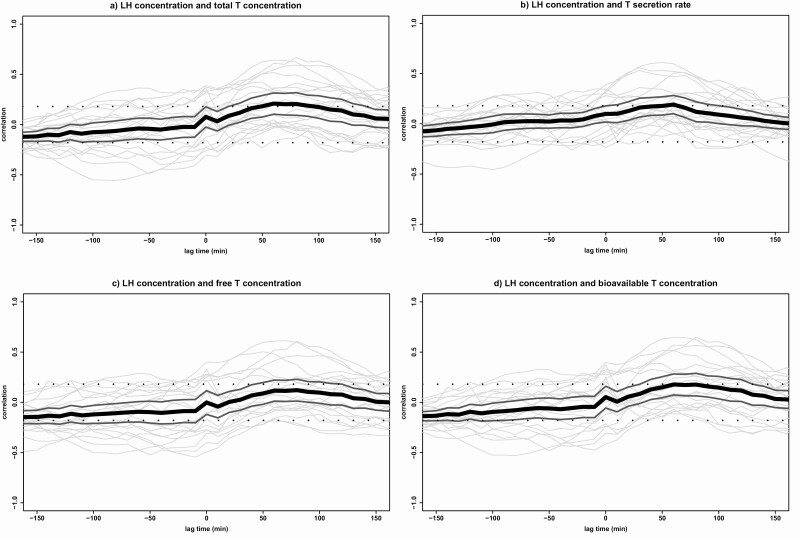
Cross-correlations between LH and T. Cross-correlations between LH concentrations and (A) total T concentrations, (B) T secretion rates, (C) free T concentrations, and (D) bioavailable T concentrations in all 20 participants. Cross-correlation assesses the relative strength between 2 hormone time series for all possible time shifts. The graph displays the correlation (y-axis) at a lag time in minutes (x-axis) with each gray line corresponding with 1 participant. The black line indicates the mean correlation for all participants and the 2 dark gray lines indicate the 95% CI. The significance level is indicated by 2 straight dotted lines at correlations –0.18 and +0.18. Negative lag times represent a correlation in which hormone 2 is followed by hormone 1 and positive lag times represent a correlation in which hormone 1 is followed by hormone 2. T, testosterone.
Figure 4.
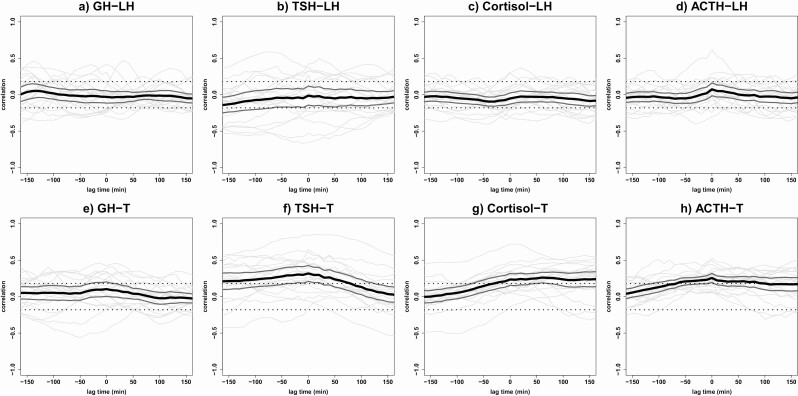
Cross-correlations between LH and T concentrations with GH, TSH, cortisol, and ACTH concentrations. Results of the cross-correlation analyses between LH concentrations and (A) GH, (B) TSH, (C) cortisol, and (D) ACTH concentrations are plotted, together with the cross-correlations between total T concentrations and (E) GH, (F) TSH, (G) cortisol, and (H) ACTH concentrations. Cross-correlation assesses the relative strength between 2 hormone time series for all possible time shifts. The graph displays the correlation (y-axis) at a lag time in minutes (x-axis) with each gray line corresponding with 1 participant. The black line indicates the mean correlation for all participants and the 2 dark gray lines indicate the 95% CI. The significance level is indicated by 2 straight dotted lines at correlations –0.18 and +0.18. Negative lag times represent a correlation in which hormone 2 is followed by hormone 1 and positive lag times represent a correlation in which hormone 1 is followed by hormone 2. T, testosterone.
Results
Characteristics of study participants
Characteristics of study participants are presented in Table 1 for all 20 participants. Participants had a mean (SD) age of 65.6 (5.3) years with a range of 52 to 76 years. The observed mean (SD) BMI of 25.8 (3.2) kg/m2 and other anthropometric measurements are normal for this age category. Participants were regular nocturnal sleepers in the month prior to the study day with a median habitual bedtime of 10:30 pm (11:00 pm-11:45 pm) and getting up time of 7:45 am (7:00-8:15 am), which is similar to the time schedule of the study protocol during the 24-hour blood sampling. Fasting glucose and insulin levels were for all participants within the reference range of our laboratory. Two men had hsCRP levels above 10.0 mg/L approximately 2 weeks before the study day, which may indicate the presence of a low-grade infection. SHBG and albumin levels were within our laboratory reference range for all participants. None of the participants were diagnosed with hypogonadism, but 1 man had slightly low T levels, with a fasting concentration of 7.50 nmol/L at 9:00 am, and another participant had somewhat high LH levels with a 24-hour mean of 9.53 U/L. None of the participants were currently smoking and 2 participants were drinking more than 3 units of alcohol per day or more than 20 units per week. In addition, we reported these variables separately for offspring of long-lived families and control subjects and compared these in Table 1, which was partly previously performed [31]. We did not find any significant difference between groups in age, anthropometrics, usual bedtime, usual getting up time, metabolic markers, inflammatory markers, hormones, or LH-T related markers. Evidently, only the median (interquartile range) age of the parents differed significantly (offspring: 89.0 [83.4-95.0] vs. controls: 79.5 [74.6-84.3], P = 0.02).
Table 1.
Characteristics of study participants, for all subjects and stratified for offspring of long-lived families and controls
| Category | Characteristics | All (N = 20) | Offspring of long-lived families (N = 10) | Controls (N = 10) | P value |
|---|---|---|---|---|---|
| Age | Age, y | 65.6 (5.3) | 66.6 (6.4) | 64.6 (4.0) | 0.41 |
| Family history | Mean age of parents, ya | 84.5 (75.6-89.8) | 89.0 (83.4-95.0) | 79.5 (74.6-84.3) | 0.02 |
| Anthropometrics | BMI, kg/m2b | 25.8 (3.2) | 26.0 (3.4) | 25.7 (3.2) | 0.84 |
| Height, cma,b | 178 (175-182) | 177 (175-182) | 181 (175-184) | 0.60 | |
| Fat mass, kga,b | 19.1 (18.0-24.1) | 19.8 (16.4-25.2) | 18.5 (18.1-23.6) | 0.84 | |
| Lean body mass, kgb | 61.7 (4.9) | 61.5 (5.4) | 61.9 (4.7) | 0.89 | |
| Waist circumference, cmb | 99 (11) | 99 (12) | 98 (9) | 0.96 | |
| Sleep | Usual bedtimea | 11:30 (11:00-11:45) | 11:30 (11:11-12:00) | 11:30 (10:53-11:30) | 0.28 |
| Usual getting up timea | 7:45 (7:00-8:15) | 8:00 (7:30-8:30) | 7:15 (6:53-8:04) | 0.06 | |
| Metabolic markers | Fasting glucose, mmol/L | 4.9 (0.7) | 5.0 (0.8) | 4.8 (0.4) | 0.48 |
| Fasting insulin, mU/La | 6.2 (3.4-10.1) | 8.0 (7.5-8.5) | 7.3 (6.9-8.1) | 0.80 | |
| Total cholesterol, mmol/L | 5.8 (0.9) | 5.7 (0.9) | 5.8 (1.0) | 0.74 | |
| Triglycerides, mmol/La | 1.2 (0.9-1.3) | 1.0 (0.8-1.3) | 1.2 (0.8-1.4) | 0.74 | |
| HDL cholesterol, mmol/L | 1.5 (0.3) | 1.6 (0.4) | 1.4 (0.3) | 0.36 | |
| Cholesterol/HDL ratio | 4 (1.2) | 3.8 (1.2) | 4.2 (1.2) | 0.45 | |
| Inflammatory markers | hsCRP, mg/La | 0.9 (0.7-2.5) | 0.7 (0.6-3.0) | 1.0 (0.7-7.6) | 0.58 |
| IL-6, pg/mLa | 1.0 (0.8-1.3) | 0.8 (0.6-1.3) | 1.1 (0.9-1.6) | 0.22 | |
| TNF-a, pg/mLa | 1.5 (1.3-2.5) | 1.7 (1.4-4.0) | 1.4 (1.3-2.2) | 0.22 | |
| Hormones | DHEAS, μmol/La | 3.3 (1.7-5.9) | 4.0 (1.5-5.9) | 2.6 (2.0-6.4) | 0.74 |
| Estradiol (24-h pool), pmol/L | 81.0 (20.3) | 80.8 (23.2) | 81.1 (18.1) | 0.98 | |
| Prolactin (24-h pool), µg/La | 8.9 (7.5-9.4) | 8.9 (7.8-9.5) | 8.4 (7.4-9.7) | 0.63 | |
| IGF-1 (24-h mean), nmol/L | 16.0 (2.9) | 15.5 (3.0) | 16.4 (2.9) | 0.47 | |
| fT4 (24-h mean), pmol/L | 13.8 (1.9) | 13.4 (1.6) | 14.3 (2.1) | 0.33 | |
| LH-T related markers | SHBG (24-h mean), nmol/L | 21.2 (6.9) | 21.3 (7.9) | 21.1 (6.3) | 0.95 |
| Albumin (24-h mean), g/L | 36.9 (1.8) | 36.7 (1.8) | 37.1 (1.8) | 0.58 | |
| LH (24-h mean), U/L | 5.2 (2.0) | 5.3 (2.0) | 5.1 (2.1) | 0.82 | |
| T (24-h mean), nmol/L | 14.4 (3.6) | 14.8 (3.5) | 14.0 (3.8) | 0.63 | |
| Calculated free T (24-h mean), ng/dLa | 12.0 (11.2-9.4) | 13.7 (11.3-17.9) | 11.6 (11.0-14.6) | 0.32 | |
| Calculated bioavailable T (24-h mean), ng/dLa | 122 (116-175) | 144 (115-195) | 119 (117-159) | 0.85 | |
| LH pulse frequency | 12.8 (2.1) | 13.2 (1.9) | 12.3 (2.3) | 0.35 | |
| T pulse frequencya | 21.0 (16.0-25.0) | 22.0 (14.8-25.3) | 20.5 (16.0-25.3) | 0.99 | |
| LH-T cross-ApEn (feedforward asynchrony) | 1.7 (0.3) | 1.8 (0.2) | 1.6 (0.3) | 0.27 | |
| T-LH cross-ApEn (feedback asynchrony) | 1.9 (0.3) | 2.0 (0.2) | 1.8 (0.4) | 0.17 |
Unless indicated otherwise, data are presented as mean with SD and groups were compared using independent-samples t tests. Boldface type indicates P < 0.05.
Abbreviations: BMI, body mass index; cross-ApEn, cross-approximate entropy; HDL, high-density lipoprotein; hsCRP, high-sensitivity C-reactive protein; T, testosterone.
a Data are presented as median with interquartile range and groups were compared using Mann-Whitney U tests.
b Data were not available for 1 control subject.
Concentration profiles of SHBG and albumin over 24 hours
The concentration profiles of SHBG and albumin are plotted over 24 hours in Fig. 1 for each of the 20 individual participants, together with the mean and standard error per timepoint. To investigate whether levels of SHBG and albumin differed over time, repeated-measures ANOVA was performed with time as within-subjects factor and protein levels as dependent variable. No significant difference in mean SHBG levels over time was observed (F(3.9,73.5) = 1.4, P = 0.25), but mean albumin levels differed significantly between time points (F(3.8,71.4) = 3.1, P = 0.02). Post hoc t tests indicated that specifically mean albumin levels at 6:00 am were significantly lower than mean albumin levels at 6:00 pm (P = 0.02).
Concentration profiles and secretion rates of LH and total, free, and bioavailable T from 1 individual
In Fig. 2, the 24-hour serum concentration profiles of LH and T as measured in blood samples withdrawn every 10 minutes from 1 random participant are plotted. The secretion rates of LH and T, which were calculated by deconvolution analysis, of the same participant are also visualized over 24 hours. Furthermore, 24-hour concentration profiles of free T and bioavailable T, calculated using total T, SHBG, and albumin levels, from the same participant are plotted.
Relationship between LH and T
For the cross-correlation of LH and total T concentrations, a mean (95% CI) maximal correlation of 0.21 (0.10-0.31) was found at lag time 60 minutes, with all positive cross-correlations between lag times 50 and 90 minutes (see Fig. 3A). These results indicate that LH concentrations are followed by T concentrations with a delay of 50 to 90 minutes. As published previously [31], comparable results were obtained in offspring of long-lived families and controls.
Stratified for lights-on and lights-off periods
When stratifying for lights-on and lights-off periods, it was found that the mean maximal cross-correlation between LH and T is stronger during the lights-on period (0.30 [0.21-0.39]) at lag time 60 minutes than during the complete 24-hour period (data not shown). The significant cross-correlation disappeared during the lights-off period.
LH concentrations with T secretion rates
If the secretion rates of T are used in the cross-correlation analysis instead of T concentrations, similar results are found. The mean maximal correlation coefficient for LH concentrations with T secretion rates was 0.19 (0.10-0.28) at lag time 60 minutes (Fig. 3B).
Calculated free T and bioavailable T
When using 24-hour free T data (Fig. 3C), the maximal positive correlation was found at lag time 80 minutes, but this mean correlation coefficient (95% CI) between LH and free T concentrations (0.12 [0.01-0.23]) was not significant. The cross-correlations between LH and bioavailable T (Fig. 3D) were similar to the results using total T concentrations, with a mean (95% CI) maximal correlation coefficient of 0.18 (0.07-0.28) at lag time 60 minutes, which is borderline significant.
Strong vs. no LH and T cross-correlation
One-half of the participants had a strong maximal cross-correlation between LH and total T concentrations, with correlation coefficients above 0.20 (range, 0.20-0.64) at lag time 60 minutes. The other half of the participants had no significant maximal cross-correlation between LH and T concentrations, with correlation coefficients between –0.16 and 0.05. Therefore, in Table 2, participant characteristics are stratified for men with a strong cross-correlation and men without a significant cross-correlation between LH and T concentrations, to compare these 2 groups. Groups did not differ in chronological age or in their family history, indicated by the distribution of offspring of long-lived families and controls and the mean age of the parents. Although BMI and lean body mass did not significantly differ between groups, fat mass (18.5 [14.9-19.7] vs. 22.3 [18.4-29.4], P = 0.02) and waist circumference (93.6 [5.7] vs. 103.1 [12.0], P = 0.04) were significantly lower in men with a strong LH-T correlation compared with men with no LH-T cross-correlation. Groups did not differ significantly in their metabolic markers, nor in other hormones. However, both mean hsCRP (0.7 [0.4-1.3] vs. 1.8 [0.8-12.3], P = 0.02) and IL-6 (0.8 [0.6-1.0] vs. 1.2 [0.9-3.0], P = 0.02) levels were lower in men with a strong LH-T correlation than in men with no correlation between LH and T concentrations. Although 24-hour mean total, free, and bioavailable T levels were comparable between groups, 24-hour mean LH levels were significantly (P = 0.04) lower in men with a strong LH-T correlation with a mean (SD) level of 4.3 (2.0) U/L compared with men without a LH-T cross-correlation (6.1 [1.5] U/L). LH-T cross-ApEn differed significantly (P = 0.009) between groups, with lower cross-ApEn values signifying greater synchrony, which indicates that men with a strong LH-T correlation also had a stronger feedforward synchrony (1.5 [0.3] vs. 1.9 [0.2]) than men with no correlation between LH and T concentrations. T-LH cross-ApEn, which is a measure for feedback asynchrony, was not significantly different between the groups.
Table 2.
Characteristics of men with a strong cross-correlation and of men with no cross-correlation between LH and T concentrations
| Category | Characteristics | Strong LH-T correlation (N = 10) | No LH-T correlation (N = 10) | P value |
|---|---|---|---|---|
| Age | Age, y | 64.6 (6.9) | 66.6 (3.0) | 0.39 |
| Family history | Offspring of long-lived family, N (%) | 4 (40) | 6 (60) | 0.37c |
| Mean age of parents, ya | 82.8 (75.5-86.8) | 85.3 (76.3-92.4) | 0.53 | |
| Anthropometrics | BMI, kg/m2b | 24.6 (1.7) | 27.0 (3.9) | 0.11 |
| Height, cma,b | 176 (175-184) | 180 (176-182) | 0.66 | |
| Fat mass, kga,b | 18.5 (14.9-19.7) | 22.3 (18.4-29.4) | 0.02 | |
| Lean body mass, kgb | 61.5 (3.8) | 61.8 (6.0) | 0.91 | |
| Waist circumference, cmb | 93.6 (5.7) | 103.1 (12.0) | 0.04 | |
| Sleep | Usual bedtimea | 11:30 (11:00-11:45) | 11:15 (11:00-11:30) | 0.58 |
| Usual getting up timea | 7:15 (6:45-8:15) | 8:00 (7:30-8:15) | 0.32 | |
| Metabolic markers | Fasting glucose, mmol/L | 5.1 (0.6) | 4.7 (0.7) | 0.28 |
| Fasting insulin, mU/La | 4.2 (2.8-7.5) | 8.1 (3.7-11.1) | 0.12 | |
| Total cholesterol, mmol/L | 5.4 (0.9) | 6.1 (0.9) | 0.12 | |
| Triglycerides, mmol/La | 1.1 (0.8-1.4) | 1.2 (0.8-1.3) | 0.74 | |
| HDL cholesterol, mmol/L | 1.5 (0.3) | 1.5 (0.4) | 0.98 | |
| Cholesterol/HDL ratio | 3.8 (1.2) | 4.2 (1.2) | 0.45 | |
| Inflammatory markers | hsCRP, mg/La | 0.7 (0.4-1.3) | 1.8 (0.8-12.3) | 0.02 |
| IL-6, pg/mLa | 0.8 (0.6-1.0) | 1.2 (0.9-3.0) | 0.02 | |
| TNF-a, pg/mLa | 1.5 (1.3-2.2) | 1.8 (1.3-3.3) | 0.48 | |
| Hormones | DHEAS, μmol/La | 2.6 (1.9-5.9) | 3.6 (1.5-6.0) | 0.99 |
| Estradiol (24-h pool), pmol/L | 75.4 (19.1) | 86.5 (20.8) | 0.23 | |
| Prolactin (24-h pool), µg/La | 9.2 (7.5-10.2) | 8.6 (7.5-9.2) | 0.58 | |
| IGF-1 (24-h mean), nmol/L | 16.7 (3.6) | 15.3 (2.0) | 0.29 | |
| fT4 (24-h mean), pmol/L | 13.6 (1.9) | 14.1 (1.9) | 0.61 | |
| LH-T related markers | SHBG (24-h mean), nmol/L | 22.4 (7.8) | 19.9 (6.1) | 0.45 |
| Albumin (24-h mean), g/L | 37.3 (1.5) | 36.5 (2.0) | 0.30 | |
| LH (24-h mean), U/L | 4.3 (2.0) | 6.1 (1.5) | 0.04 | |
| T (24-h mean), nmol/L | 14.6 (2.7) | 14.2 (4.4) | 0.80 | |
| Calculated free T (24-h mean), ng/dLa | 12.3 (11.0-16.0) | 12.0 (11.3-18.4) | 0.58 | |
| Calculated bioavailable T (24-h mean), ng/dLa | 129 (117-171) | 120 (115-198) | 0.68 | |
| LH pulse frequency | 12.1 (2.3) | 13.4 (1.6) | 0.17 | |
| T pulse frequencya | 24.5 (18.3-26.0) | 18.5 (14.8-23.5) | 0.09 | |
| LH-T cross-correlation | 0.36 (0.30-0.56) | 0.01 (-0.02 to 0.06) | <0.001 | |
| LH-T cross-ApEn (feedforward asynchrony) | 1.5 (0.3) | 1.9 (0.2) | 0.009 | |
| T-LH cross-ApEn (feedback asynchrony) | 1.8 (0.3) | 2.0 (0.3) | 0.18 |
Unless indicated otherwise, data are presented as mean with standard deviation and groups were compared using independent-samples t tests. Boldface type indicates P < 0.05.
Abbreviations: BMI, body mass index; cross-ApEn, cross-approximate entropy; HDL, high-density lipoprotein; hsCRP, high-sensitivity C-reactive protein; T, testosterone.
a Data are presented as median with interquartile range and groups were compared using Mann-Whitney U tests.
b Data were not available for 1 participant.
c Groups were compared using a chi-squared test.
Association of LH-T parameters with age
To investigate whether LH-T parameters and their relationship are associated with age, we dichotomized the group in men with an age below the median age and above the median age to create equally sized groups and compare LH-T parameters between these groups. Results of these comparisons can be found in Table 3. No differences were found in 24-hour mean total T levels, but older men had significantly higher SHBG levels and consequently lower calculated 24-hour mean levels of free and bioavailable T. Furthermore, albeit not significant, older men tended to have higher 24-hour mean levels of LH. No significant differences were found in the strength of the LH-T relationship, described by cross-correlation analyses between LH and total T concentrations.
Table 3.
LH-T characteristics of men aged below and above median age
| Characteristics | Age below median (N = 10) | Age above median (N = 10) | P value |
|---|---|---|---|
| Age, y | 61.9 (4.5) | 69.3 (3.0) | <0.001 |
| SHBG (24-h mean), nmol/L | 17.9 (5.3) | 24.4 (7.1) | 0.03 |
| Albumin (24-h mean), g/L | 37.5 (1.6) | 36.3 (1.8) | 0.15 |
| LH (24-h mean), U/L | 4.4 (1.6) | 6.0 (2.1) | 0.08 |
| T (24-h mean), nmol/L | 14.4 (3.9) | 14.5 (3.5) | 0.93 |
| Calculated free T (24-h mean), ng/dLa | 15.5 (12.5-18.2) | 11.3 (11.0-11.9) | 0.02 |
| Calculated bioavailable T (24-h mean), ng/dLa | 167 (132-199) | 117 (115-121) | 0.02 |
| LH pulse frequency | 12.8 (2.0) | 12.7 (2.3) | 0.92 |
| T pulse frequencya | 22.5 (17.3-25.0) | 19.5 (15.5-26.3) | 0.91 |
| LH-T cross-correlationa | 0.15 (0.01-0.54) | 0.16 (-0.02 to 0.35) | 0.58 |
| LH-T cross-ApEn (feedforward asynchrony) | 1.7 (0.3) | 1.7 (0.2) | 0.65 |
| T-LH cross-ApEn (feedback asynchrony) | 1.9 (0.3) | 2.0 (0.3) | 0.64 |
Unless indicated otherwise, data are presented as mean with standard deviation and groups were compared using independent-samples t tests. Boldface type indicates P < 0.05.
Abbreviations: cross-ApEn, cross-approximate entropy; T, testosterone
a Data are presented as median with interquartile range and groups were compared using Mann-Whitney U tests.
Cross-correlations of LH and T with GH, TSH, cortisol, and ACTH
Results of the cross-correlations of LH and T concentrations with GH, TSH, cortisol, and ACTH concentrations are presented in Fig. 4 with a graphical summary in Fig. 5. No significant cross-correlations were found between LH and GH, LH and TSH, LH and cortisol, LH and ACTH, and between T and GH concentrations (Fig. 4A-E). This was also not the case when stratified for lights-on and lights-off periods or for offspring of long-lived families vs. controls.
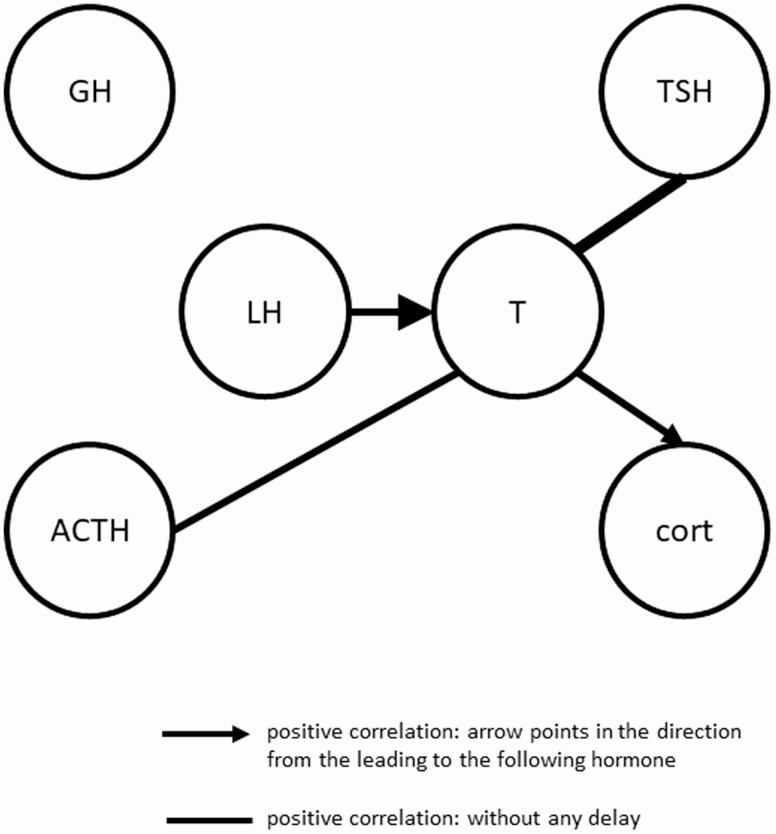
Figure 5.
Summary of cross-correlations between LH and T concentrations with GH, TSH, cortisol, and ACTH concentrations. A graphical summary of cross-correlation analyses in all 20 participants. Solid lines represent positive correlations between hormones, which is strongest at lag time 0, so without a delay. Solid arrows represent positive correlations between hormones, which is strongest at a certain lag time, with the arrow directed towards the hormone which is following the leading hormone. The weight of the line/arrow represents the strength of the correlation.
A strong cross-correlation was observed between T and TSH (Fig. 4F), with a mean (95% CI) maximal correlation coefficient in all men of 0.32 (0.21-0.43) at lag time 0. All cross-correlations between lag times –180 and 60 minutes were significantly positive. When stratifying for offspring of long-lived families and controls, strong cross-correlations were observed in both offspring (0.30 [0.14-0.46]) and controls (0.34 [0.18-0.49]) at lag time 0. Similar results for the maximal cross-correlation between T and TSH concentrations were obtained in men with a strong LH-T cross-correlation (0.33 [0.15-0.50]) compared with men with a weak LH-T cross-correlation (0.31 [0.17-0.45]). No significant correlations between T and TSH was found when stratifying for lights-on and lights-off periods.
For T and cortisol (Fig. 4G), the maximal cross-correlation coefficient was 0.26 (0.19-0.33) at lag time 60, indicating that T concentrations are followed by cortisol concentrations after 60 minutes. Between lag times –30 and 180 minutes, all correlations were positive. Results for offspring (0.28 [0.16-0.40]) and controls (0.24 [0.16-0.33]) were comparable. When stratifying for men with strong vs. weak LH-T cross-correlation, similar results were found (0.24 [0.11-0.36] vs. 0.29 [0.21-0.37]). No significant correlations between T and cortisol were found when stratifying for lights-on and lights-off periods.
The mean (95% CI) maximal cross-correlation coefficient between T and ACTH (Fig. 4H) was 0.26 (0.19-0.32) at lag time 0. All correlations between lag times –60 and 100 minutes were weak but significantly positive. Comparable results were obtained for offspring (0.28 [0.17-0.38]) and controls (0.23 [0.17-0.30]) at lag time 0. Men with a strong LH-T cross-correlation had a similar mean maximal T-ACTH cross-correlation (0.27 [0.17-0.36]) compared with men with a weak LH-T cross-correlation (0.24 [0.16-0.33]). No significant correlations between T and ACTH were found when stratifying for lights-on and lights-off periods.
Discussion
In this study, we aimed to determine the relationship between LH and T concentrations over 24 hours in 20 healthy older men. Besides, we aimed to determine which health characteristics are associated with the strength of this LH-T relationship. Furthermore, we explored the interrelationships between serum concentrations of LH and T with GH, TSH, cortisol, and ACTH concentrations using 24-hour time series data with intervals of 10 minutes.
We observed a significant positive correlation between LH concentrations and total T concentrations with a lag time of 60 minutes, which was strongest during daytime. Comparable results were obtained when using calculated free, bioavailable, or secretion rates of T. This indicates that LH concentrations are followed by (free/bioavailable) T concentrations/secretion with a delay of 60 minutes, which agrees with other studies investigating this relationship in healthy young and older men [52–54]. Although the magnitude of the correlation between LH-T that we observed here is in line with that found in other studies [52–54], a correlation coefficient of circa 0.20 is relatively weak compared with that found for other hormones within an interlinked hormonal axis, notably ACTH and cortisol. In a bigger, but partly overlapping, population as the present study, a maximal mean (95% CI) correlation coefficient of 0.78 (0.74-0.81) was found between 24-hour serum ACTH and cortisol concentrations [30]. We hypothesized that calculated free or bioavailable T concentrations might give stronger cross-correlation results than using total T concentrations, but results were similar.
With aging, LH levels rise whereas T levels decline, although this decline in T levels could also be caused by health status, including body composition, inflammation, and comorbidities [1, 3]. Therefore, although this is a healthy population, we wanted to investigate which health factors, including body composition, metabolic and inflammatory markers, and LH-T related markers are associated with the strength of a LH-T relationship in older men. We found that men with a strong LH-T relationship had more favorable body composition, inflammatory markers, LH levels, and LH-T feedforward synchrony, which is a novel finding. It is known that higher BMI and waist circumference are associated with higher levels of chronic inflammation and low T levels [55, 56], but an unfavorable body composition, including higher fat mass and waist circumference, has not yet been found to be associated with a weaker LH-T cross-correlation. In line, inflammatory markers also differed between men with a strong vs. no significant LH-T cross-correlation, with lower hsCRP and IL-6 levels in men with a strong relationship between LH and T concentrations. Inflammation is strongly related to the HPG axis, with an inverse relationship between T levels and hsCRP and IL-6 levels [57, 58]. The importance of the anti-inflammatory action of T is also recently seen in male COVID-19 patients, among which men expressing a genetic polymorphism in the androgen receptor resulting in them being unable to increase their serum T levels had a more severe clinical outcome and the need for intensive care [59]. Furthermore, healthy male subjects who received an injection with a dose of 3 or 6 million U of IL-2 had a reduction in feedforward drive of LH on T secretion, which led to a decrease in T secretory-burst frequency [60]. This finding is in line with our observation that men with no LH-T correlation had reduced feedforward synchrony compared with men with a strong LH-T relationship, which was expected because the cross-correlation of LH concentrations with T secretion rates is another measure for the feedforward drive of LH on T [47]. Men with no LH-T relationship did not have significantly lower 24-hour mean total T levels, but 24-hour mean LH levels were significantly higher, although still within the reference range. This observation suggests that LH concentrations play a bigger role in determining the strength of LH-T cross-correlations than T concentrations. Thereby, it could indicate a possible more significant LH-receptor resistance and/or diminished testicular responsiveness in men with no LH-T relationship. Chronological age did not significantly differ between groups of strong vs. no LH-T relationship, which could be due to the limited age range of 52 to 76 years. However, all the significant differences found between men with a strong vs. no LH-T correlation are pointing toward the hypothesis that men with no LH-T correlation are being biologically older than men with a strong LH-T relationship. Furthermore, when comparing LH-T parameters between men with an age below and above the median age, we found that older men had significantly higher SHBG levels, lower calculated 24-hour mean levels of free and bioavailable T, and tended to have higher 24-hour mean levels of LH. Although participants in this study had T levels within the normal range, these results are according to literature because higher age is a risk factor for developing primary hypogonadism, which is characterized by low free T and high LH levels.
In our exploratory analysis of cross-correlations with other pituitary hormones, we observed positive correlations between T and TSH concentrations with no delay. Because the cross-correlation plots of T and TSH did not show a sharp peak at lag time 0, but positive correlations at a broad window of lag times, this could indicate that both T and TSH are driven by a common regulator instead of 1 hormone driving the other hormone. Animals that breed seasonally have elevated levels of both T and TSH during winter seasons. TSH and/or thyroid hormones might stimulate the secretion of GnRH and gonadotropin leading to gonadal growth, which is important for reproduction [61, 62]. Humans are however not seasonal breeders, so these mechanisms could be of greater importance in animals than in humans. The positive correlation between T and TSH concentrations is in contrast to other human studies, which found higher total T levels in subjects with lower TSH levels and lower T levels in men with subclinical or primary hypothyroidism [63, 64]. Furthermore, we observed positive correlations between T and ACTH concentrations with no delay, whereas the positive cross-correlation between T and cortisol concentrations was maximal at lag time 60 minutes, indicating that T is followed by cortisol after 60 minutes. It was found that hypercortisolism such as Cushing syndrome or chronic long-term glucocorticoid therapy, could result in (secondary hypogonadotropic) hypogonadism [65]. This is, however, in the case of severe cortisol excess, whereas our study is performed in healthy older men with normal hormonal levels. In the cross-correlation plots, not 1 sharp peak is shown, but a low peak at a broad window of lag times, which could indicate that these hormones are driven by a common regulator instead of 1 hormone driving the other hormone. Both the HPG axis and the hypothalamic–pituitary–adrenal axis are highly influenced by inflammation, which could be such a common regulator. For example, healthy male subjects who received a low-dose IL-2 administration had an increase in cortisol secretion [66]. However, in contrast to the hypothalamic–pituitary–adrenal axis, the HPG axis responds to inflammation by decreasing its levels [60].
Last, we established the 24-hour concentration profiles of SHBG and albumin and observed a low-amplitude circadian rhythm in albumin levels with higher levels during the day compared to nighttime. Albumin has a half-life of circa 3 weeks and is not dependent on nutritional status, so this cannot explain the moderate circadian rhythm in albumin levels [67, 68].
Limitations of this study were that, although measurements are extensive, the sample size is relatively small with a limited age range, so we do not have data on the oldest old. Furthermore, liquid chromatography tandem mass spectometry is generally recommended for measuring sex steroids. However, this is only required when levels are below the detection limit, which was not the case for the participants in the current study.
Summarizing, LH concentrations were followed by T concentrations/secretion with a delay of 60 minutes in healthy older men. The strength of a LH-T relationship was mainly associated with health factors, including body composition and inflammation markers, LH levels and its feedforward drive, whereas chronological age and T levels were not associated with the strength of the LH-T relationship. Future research should aim to determine the role of the hypothalamus in this LH-T relationship and determine the importance of a strong LH-T relationship in ageing men. Furthermore, we found that T concentrations were positively correlated with TSH, ACTH, and cortisol concentrations. These exploratory analyses could indicate that T and other hormones are driven by a common regulator or that there is crosstalk between these hormones. More research is needed to determine the biological meaning and clinical consequences of these interrelationships between T and other hormones.
Acknowledgments
The authors thank all participants of the Switchbox Leiden Study, laboratory personnel, and secretarial staff; Dr. A.A. Akintola and Dr. S.W. Jansen for setting up the Switchbox Leiden Study; and Prof. Dr. P.E. Slagboom and Prof. Dr. R.G.J. Westendorp for setting up the Leiden Longevity Study.
Financial Support: This research is funded by 2 European Commission projects, Switchbox (FP7, Health-F2-2010–259772) and THYRAGE (Horizon 2020 research and innovation programme, 666869). E.v.d.S. was supported by a personal PhD grant from the Leiden University Medical Center.
Glossary
Abbreviations
- BMI
body mass index
- cross-ApEn
cross-approximate entropy
- CV
coefficient of variation
- ECLIA
electrochemiluminescence
- HPG
hypothalamic–pituitary–gonadal
- hsCRP
high-sensitivity C-reactive protein
- T
testosterone.
Additional Information
Disclosures: The authors have nothing to disclose.
Data Availability
The datasets analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.
References
- 1. Kaufman JM, Lapauw B, Mahmoud A, T’Sjoen G, Huhtaniemi IT. Aging and the male reproductive system. Endocr Rev. 2019;40(4):906-972. [DOI] [PubMed] [Google Scholar]
- 2. Veldhuis JD. Aging and hormones of the hypothalamo-pituitary axis: gonadotropic axis in men and somatotropic axes in men and women. Ageing Res Rev. 2008;7(3):189-208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wu FC, Tajar A, Pye SR, et al. ; European Male Aging Study Group . Hypothalamic-pituitary-testicular axis disruptions in older men are differentially linked to age and modifiable risk factors: the European Male Aging Study. J Clin Endocrinol Metab. 2008;93(7):2737-2745. [DOI] [PubMed] [Google Scholar]
- 4. Camacho EM, Huhtaniemi IT, O’Neill TW, et al. ; EMAS Group . Age-associated changes in hypothalamic-pituitary-testicular function in middle-aged and older men are modified by weight change and lifestyle factors: longitudinal results from the European Male Ageing Study. Eur J Endocrinol. 2013;168(3):445-455. [DOI] [PubMed] [Google Scholar]
- 5. Travison TG, Araujo AB, Kupelian V, O’Donnell AB, McKinlay JB. The relative contributions of aging, health, and lifestyle factors to serum testosterone decline in men. J Clin Endocrinol Metab. 2007;92(2):549-555. [DOI] [PubMed] [Google Scholar]
- 6. Roelfsema F, Liu PY, Takahashi PY, Yang RJ, Veldhuis JD. Dynamic interactions between LH and testosterone in healthy community-dwelling men: impact of age and body composition. J Clin Endocrinol Metab. 2020;105(3):e628-e641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Veldhuis JD, Liu PY, Keenan DM, Takahashi PY. Older men exhibit reduced efficacy of and heightened potency downregulation by intravenous pulses of recombinant human LH: a study in 92 healthy men. Am J Physiol Endocrinol Metab. 2012;302(1):E117-E122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wu FC, Tajar A, Beynon JM, et al. ; EMAS Group . Identification of late-onset hypogonadism in middle-aged and elderly men. N Engl J Med. 2010;363(2):123-135. [DOI] [PubMed] [Google Scholar]
- 9. Dudek P, Kozakowski J, Zgliczyński W. Late-onset hypogonadism. Prz Menopauzalny. 2017;16(2):66-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Allan CA, McLachlan RI. Androgens and obesity. Curr Opin Endocrinol Diabetes Obes. 2010;17(3):224-232. [DOI] [PubMed] [Google Scholar]
- 11. Hammond GL. Plasma steroid-binding proteins: primary gatekeepers of steroid hormone action. J Endocrinol. 2016;230(1):R13-R25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Thaler MA, Seifert-Klauss V, Luppa PB. The biomarker sex hormone-binding globulin - from established applications to emerging trends in clinical medicine. Best Pract Res Clin Endocrinol Metab. 2015;29(5):749-760. [DOI] [PubMed] [Google Scholar]
- 13. Liu PY, Iranmanesh A, Nehra AX, Keenan DM, Veldhuis JD. Mechanisms of hypoandrogenemia in healthy aging men. Endocrinol Metab Clin North Am. 2005;34(4):935-55, ix. [DOI] [PubMed] [Google Scholar]
- 14. Liu PY, Pincus SM, Takahashi PY, et al. Aging attenuates both the regularity and joint synchrony of LH and testosterone secretion in normal men: analyses via a model of graded GnRH receptor blockade. Am J Physiol Endocrinol Metab. 2006;290(1):E34-E41. [DOI] [PubMed] [Google Scholar]
- 15. Tajar A, Forti G, O’Neill TW, et al. ; EMAS Group . Characteristics of secondary, primary, and compensated hypogonadism in aging men: evidence from the European Male Ageing Study. J Clin Endocrinol Metab. 2010;95(4):1810-1818. [DOI] [PubMed] [Google Scholar]
- 16. Harman SM, Metter EJ, Tobin JD, Pearson J, Blackman MR; Baltimore Longitudinal Study of Aging . Longitudinal effects of aging on serum total and free testosterone levels in healthy men. Baltimore Longitudinal Study of Aging. J Clin Endocrinol Metab. 2001;86(2):724-731. [DOI] [PubMed] [Google Scholar]
- 17. Sartorius G, Spasevska S, Idan A, et al. Serum testosterone, dihydrotestosterone and estradiol concentrations in older men self-reporting very good health: the healthy man study. Clin Endocrinol (Oxf). 2012;77(5):755-763. [DOI] [PubMed] [Google Scholar]
- 18. Harman SM, Tsitouras PD. Reproductive hormones in aging men. I. Measurement of sex steroids, basal luteinizing hormone, and Leydig cell response to human chorionic gonadotropin. J Clin Endocrinol Metab. 1980;51(1):35-40. [DOI] [PubMed] [Google Scholar]
- 19. Rao PK, Boulet SL, Mehta A, et al. Trends in testosterone replacement therapy use from 2003 to 2013 among reproductive-age men in the United States. J Urol. 2017;197(4):1121-1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Baillargeon J, Kuo YF, Westra JR, Urban RJ, Goodwin JS. Testosterone prescribing in the United States, 2002-2016. Jama. 2018;320(2):200-202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Muniyappa R, Sorkin JD, Veldhuis JD, et al. Long-term testosterone supplementation augments overnight growth hormone secretion in healthy older men. Am J Physiol Endocrinol Metab. 2007;293(3):E769-E775. [DOI] [PubMed] [Google Scholar]
- 22. Muniyappa R, Veldhuis JD, Harman SM, Sorkin JD, Blackman MR. Effects of testosterone administration on nocturnal cortisol secretion in healthy older men. J Gerontol A Biol Sci Med Sci. 2010;65(11):1185-1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bremner AP, Feddema P, Leedman PJ, et al. Age-related changes in thyroid function: a longitudinal study of a community-based cohort. J Clin Endocrinol Metab. 2012;97(5):1554-1562. [DOI] [PubMed] [Google Scholar]
- 24. Ehrenkranz J, Bach PR, Snow GL, et al. Circadian and circannual rhythms in thyroid hormones: determining the TSH and free T4 reference intervals based upon time of day, age, and sex. Thyroid. 2015;25(8):954-961. [DOI] [PubMed] [Google Scholar]
- 25. Bartke A, Sun LY, Longo V. Somatotropic signaling: trade-offs between growth, reproductive development, and longevity. Physiol Rev. 2013;93(2):571-598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Melmed S, Polonsky K, Larsen PR, Kronenberg HM.. Williams Textbook of Endocrinology. 13th ed. Philadelphia, PA: Elsevier; 2016:1335. [Google Scholar]
- 27. Denef C. Paracrinicity: the story of 30 years of cellular pituitary crosstalk. J Neuroendocrinol. 2008;20(1):1-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Villalobos C, Núñez L, Frawley LS, García-Sancho J, Sánchez A. Multi-responsiveness of single anterior pituitary cells to hypothalamic-releasing hormones: a cellular basis for paradoxical secretion. Proc Natl Acad Sci U S A. 1997;94(25):14132-14137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Villalobos C, Núñez L, Garcia-Sancho J. Functional glutamate receptors in a subpopulation of anterior pituitary cells. Faseb J. 1996;10(5):654-660. [DOI] [PubMed] [Google Scholar]
- 30. van der Spoel E, Roelfsema F, Akintola AA, et al. Interrelationships between pituitary hormones as assessed from 24-hour serum concentrations in healthy older subjects. J Clin Endocrinol Metab. 2020;105(4):e1201-e1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. van der Spoel E, Roelfsema F, Jansen SW, et al. Familial longevity is not associated with major differences in the hypothalamic-pituitary-gonadal axis in healthy middle-aged men. Front Endocrinol (Lausanne). 2016;7:143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Boron WFB, Boulpaep EL.. Medical Physiology: a Cellular and Molecular Approach. 2nd ed. Philadelphia, PA: Saunders Elsevier; 2009. [Google Scholar]
- 33. Westendorp RG, van Heemst D, Rozing MP, et al. ; Leiden Longevity Study Group . Nonagenarian siblings and their offspring display lower risk of mortality and morbidity than sporadic nonagenarians: the Leiden Longevity Study. J Am Geriatr Soc. 2009;57(9):1634-1637. [DOI] [PubMed] [Google Scholar]
- 34. Jansen SW, Akintola AA, Roelfsema F, et al. Human longevity is characterised by high thyroid stimulating hormone secretion without altered energy metabolism. Sci Rep. 2015;5:11525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Donegan DM, Algeciras-Schimnich A, Hamidi O, et al. Corticotropin hormone assay interference: a case series. Clin Biochem. 2019;63:143-147. [DOI] [PubMed] [Google Scholar]
- 36. Akintola AA, Jansen SW, Wilde RB, Hultzer G, Rodenburg R, van Heemst D. A simple and versatile method for frequent 24 h blood sample collection in healthy older adults. Methodsx. 2015;2:33-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Buysse DJ, Reynolds CF 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28(2):193-213. [DOI] [PubMed] [Google Scholar]
- 38. Jansen SW, Roelfsema F, Akintola AA, et al. Characterization of the hypothalamic-pituitary-adrenal-axis in familial longevity under resting conditions. Plos One. 2015;10(7):e0133119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. van der Spoel E, Jansen SW, Akintola AA, et al. Growth hormone secretion is diminished and tightly controlled in humans enriched for familial longevity. Aging Cell. 2016;15(6):1126-1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. van der Spoel E, Choi J, Roelfsema F, Cessie SL, van Heemst D, Dekkers OM. Comparing methods for measurement error detection in serial 24-h hormonal data. J Biol Rhythms. 2019;34(4):347-363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Takahashi PY, Votruba P, Abu-Rub M, Mielke K, Veldhuis JD. Age attenuates testosterone secretion driven by amplitude-varying pulses of recombinant human luteinizing hormone during acute gonadotrope inhibition in healthy men. J Clin Endocrinol Metab. 2007;92(9):3626-3632. [DOI] [PubMed] [Google Scholar]
- 42. Liu PY, Keenan DM, Kok P, Padmanabhan V, O’Byrne KT, Veldhuis JD. Sensitivity and specificity of pulse detection using a new deconvolution method. Am J Physiol Endocrinol Metab. 2009;297(2):E538-E544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Keenan DM, Licinio J, Veldhuis JD. A feedback-controlled ensemble model of the stress-responsive hypothalamo-pituitary-adrenal axis. Proc Natl Acad Sci U S A. 2001;98(7):4028-4033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Keenan DM, Roelfsema F, Biermasz N, Veldhuis JD. Physiological control of pituitary hormone secretory-burst mass, frequency, and waveform: a statistical formulation and analysis. Am J Physiol Regul Integr Comp Physiol. 2003;285(3):R664-R673. [DOI] [PubMed] [Google Scholar]
- 45. Veldhuis JD, Liu PY, Takahashi PY, Weist SM, Wigham JR. Analysis of the impact of intravenous LH pulses versus continuous LH infusion on testosterone secretion during GnRH-receptor blockade. Am J Physiol Regul Integr Comp Physiol. 2012;303(10):R994-R1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Veldhuis JD, Keenan DM, Liu PY, Takahashi PY. Kinetics of removal of intravenous testosterone pulses in normal men. Eur J Endocrinol. 2010;162(4):787-794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Liu PY, Pincus SM, Keenan DM, Roelfsema F, Veldhuis JD. Analysis of bidirectional pattern synchrony of concentration-secretion pairs: implementation in the human testicular and adrenal axes. Am J Physiol Regul Integr Comp Physiol. 2005;288(2):R440-R446. [DOI] [PubMed] [Google Scholar]
- 48. Veldhuis JD, Keenan DM, Pincus SM. Motivations and methods for analyzing pulsatile hormone secretion. Endocr Rev. 2008;29(7):823-864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Veldhuis JD, Pincus SM, Garcia-Rudaz MC, Ropelato MG, Escobar ME, Barontini M. Disruption of the joint synchrony of luteinizing hormone, testosterone, and androstenedione secretion in adolescents with polycystic ovarian syndrome. J Clin Endocrinol Metab. 2001;86(1):72-79. [DOI] [PubMed] [Google Scholar]
- 50. Minitab. 2017. https://support.minitab.com/en-us/minitab/18/help-and-how-to/modeling-statistics/time-series/how-to/cross-correlation/interpret-the-results/all-statistics-and-graphs/. Accessed November 2020.
- 51. Pincus S, Singer BH. Randomness and degrees of irregularity. Proc Natl Acad Sci U S A. 1996;93(5):2083-2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Mulligan T, Iranmanesh A, Johnson ML, Straume M, Veldhuis JD. Aging alters feed-forward and feedback linkages between LH and testosterone in healthy men. Am J Physiol. 1997;273(4):R1407-R1413. [DOI] [PubMed] [Google Scholar]
- 53. Veldhuis JD, Iranmanesh A, Keenan DM. Erosion of endogenous testosterone-driven negative feedback on pulsatile luteinizing hormone secretion in healthy aging men. J Clin Endocrinol Metab. 2004;89(11):5753-5761. [DOI] [PubMed] [Google Scholar]
- 54. Bridges NA, Hindmarsh PC, Pringle PJ, Matthews DR, Brook CG. The relationship between endogenous testosterone and gonadotrophin secretion. Clin Endocrinol (Oxf). 1993;38(4):373-378. [DOI] [PubMed] [Google Scholar]
- 55. Tajar A, Huhtaniemi IT, O’Neill TW, et al. ; EMAS Group . Characteristics of androgen deficiency in late-onset hypogonadism: results from the European Male Aging Study (EMAS). J Clin Endocrinol Metab. 2012;97(5):1508-1516. [DOI] [PubMed] [Google Scholar]
- 56. Lainez NM, Coss D. Obesity, neuroinflammation, and reproductive function. Endocrinology. 2019;160(11):2719-2736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Pastuszak AW, Kohn TP, Estis J, Lipshultz LI. Low plasma testosterone is associated with elevated cardiovascular disease biomarkers. J Sex Med. 2017;14(9):1095-1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Mohamad NV, Wong SK, Wan Hasan WN, et al. The relationship between circulating testosterone and inflammatory cytokines in men. Aging Male. 2019;22(2):129-140. [DOI] [PubMed] [Google Scholar]
- 59. Baldassarri M, Picchiotti N, Fava F, et al. ; Spanish Covid HGE, GEN-COVID Multicenter Study . Shorter androgen receptor polyQ alleles protect against life-threatening COVID-19 disease in European males. Ebiomedicine. 2021;65:103246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Veldhuis J, Yang R, Roelfsema F, Takahashi P. Proinflammatory cytokine infusion attenuates LH’s feedforward on testosterone secretion: modulation by age. J Clin Endocrinol Metab. 2016;101(2):539-549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Nakayama T, Yoshimura T. Seasonal rhythms: the role of thyrotropin and thyroid hormones. Thyroid. 2018;28(1):4-10. [DOI] [PubMed] [Google Scholar]
- 62. Nakao N, Ono H, Yamamura T, et al. Thyrotrophin in the pars tuberalis triggers photoperiodic response. Nature. 2008;452(7185):317-322. [DOI] [PubMed] [Google Scholar]
- 63. Corona G, Wu FC, Forti G, et al. ; EMAS Study Group . Thyroid hormones and male sexual function. Int J Androl. 2012;35(5):668-679. [DOI] [PubMed] [Google Scholar]
- 64. Sharma LK, Sharma N, Gadpayle AK, Dutta D. Prevalence and predictors of hyperprolactinemia in subclinical hypothyroidism. Eur J Intern Med. 2016;35:106-110. [DOI] [PubMed] [Google Scholar]
- 65. Shibli-Rahhal A, Van Beek M, Schlechte JA. Cushing’s syndrome. Clin Dermatol. 2006;24(4):260-265. [DOI] [PubMed] [Google Scholar]
- 66. Roelfsema F, Liu PY, Yang R, Takahashi P, Veldhuis JD. Interleukin-2 drives cortisol secretion in an age-, dose-, and body composition-dependent way. Endocr Connect. 2020;9(7):637-648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Nilsen J, Trabjerg E, Grevys A, et al. An intact C-terminal end of albumin is required for its long half-life in humans. Commun Biol. 2020;3(1):181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Lee JL, Oh ES, Lee RW, Finucane TE. Serum albumin and prealbumin in calorically restricted, nondiseased individuals: a systematic review. Am J Med. 2015;128(9):1023.e1-1023.22. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.