Abstract
In 2018, the AHA/ACC Multisociety Guideline on the Management of Blood Cholesterol was released. Less than one year later, the 2019 ESC/EAS Dyslipidemia Guideline was published. While both provide important recommendations for managing atherosclerotic cardiovascular disease (ASCVD) risk through lipid management, differences exist. Prior to the publication of both guidelines, important randomized clinical trial data emerged on non-statin lipid lowering therapy and ASCVD risk reduction. To illustrate important differences in guideline recommendations, we use this data to help answer three key questions: 1) Are ASCVD event rates similar in high-risk primary and stable secondary prevention? 2) Does imaging evidence of subclinical atherosclerosis justify aggressive use of statin and non-statin therapy (if needed) to reduce LDL-C levels below 55 mg/dL as recommended in the European Guideline? 3) Do LDL-C levels below 70 mg/dL achieve a large absolute risk reduction in secondary ASCVD prevention? The US guideline prioritizes both the added efficacy and cost implications of non-statin therapy, which limits intensive therapy to individuals with the highest risk of ASCVD. The European approach broadens the eligibility criteria by incorporating goals of therapy in both primary and secondary prevention. The current cost and access constraints of healthcare worldwide, especially amidst a COVID-19 pandemic, makes the European recommendations more challenging to implement. By restricting non-statin therapy to a subgroup of high- and, in particular, very high-risk individuals, the US guideline provides primary and secondary ASCVD prevention recommendations that are more affordable and attainable. Ultimately, finding a common ground for both guidelines rests on our ability to design trials that assess cost-effectiveness in addition to efficacy and safety.
Keywords: ASCVD Risk assessment, ASCVD Prevention, Lipid-lowering therapy
1. Introduction
In 2018, the American Heart Association (AHA)/American College of Cardiology (ACC) Multisociety Guideline on the Management of Blood Cholesterol was released [1]. Less than one year later, the 2019 European Society of Cardiology (ESC)/European Atherosclerosis Society (EAS) Dyslipidemia Guideline was published [2]. Both guidelines highlight approaches for atherosclerotic cardiovascular disease (ASCVD) risk reduction through lipid management. While agreement exists that low density lipoprotein-cholesterol (LDL-C) is the primary target for risk reduction and statin therapy is the first-line therapy to reduce LDL-C levels and ASCVD risk, they diverge at some important clinical decision points in the management of ASCVD risk for both primary and secondary prevention.
This narrative review examines both guidelines and focuses on three key questions:
-
1.
Are ASCVD event rates similar in high-risk primary and stable secondary prevention?
-
2.
Does imaging evidence of subclinical atherosclerosis justify aggressive use of statin and non-statin therapy (if needed) to reduce LDL-C levels below 55 mg/dL as recommended in the ESC/EAS Guideline?
-
3.
Do LDL-C levels below 70 mg/dL achieve a large absolute risk reduction (ARR) in secondary ASCVD prevention?
1.1. Examining the AHA/ACC Multisociety and ESC/EAS guidelines
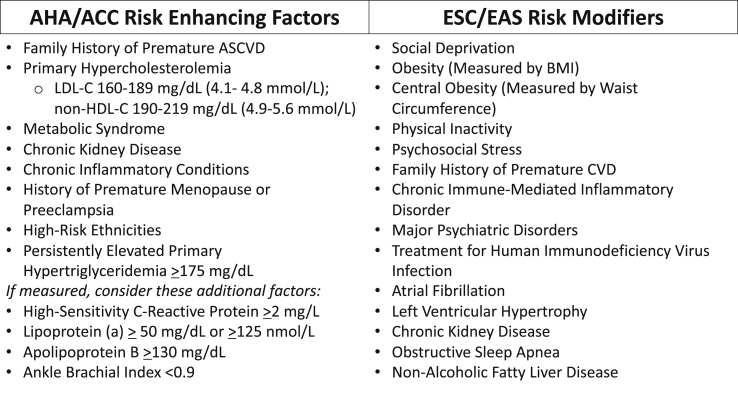
The AHA/ACC Multisociety Guideline utilizes established criteria in primary prevention to help determine when statin therapy should be initiated or intensified (Table 1). If treatment decisions remain uncertain after a clinician-patient risk discussion, risk-enhancing factors (Fig. 1) and a coronary artery calcium (CAC) score may be used to further guide decision-making. Incorporation of these other factors into the clinician-patient risk discussion promotes personalized treatment decisions for reducing risk in the primary prevention of ASCVD [3].
Table 1.
AHA/ACC Multisociety & ESC/EAS approach to LDL-C level and ASCVD risk Reduction.
Fig. 1.
List of AHA/ACC risk enhancing factors and ESC/EAS risk Modifiers
Abbreviations: AHA – American heart association; ACC – American college of cardiology; ESC – european society of cardiology; EAS – european atherosclerosis society; ASCVD – atherosclerotic cardiovascular disease; LDL-C – low-density lipoprotein cholesterol; CVD – cardiovascular disease; BMI – body mass index.
For secondary prevention and high-risk primary prevention patients, maximally tolerated statin therapy is recommended to reduce LDL-C by at least 50%. For the highest risk secondary prevention patients, whose LDL-C remains at or above the treatment threshold of 70 mg/dL despite maximally tolerated statin therapy, non-statin therapy is recommended with preference given to ezetimibe as the first line therapy, based on its ease of accessibility and low cost. If the LDL-C level remains ≥70 mg/dL (or non-high density lipoprotein cholesterol (non-HDL-C) remains ≥100 mg/dL) on maximally tolerated statin therapy and ezetimibe, then the addition of a proprotein convertase subtilisin/kexin type 9 inhibitor (PCSK9i) is reasonable, though this is more likely to be cost-effective if the LDL-C is ≥ 100 mg/dL.
The ESC/EAS Dyslipidemia Guideline has prioritized treatment goals, including percent LDL-C reduction and fixed targets, for guiding lipid-lowering therapy (Table 1). If a maximally tolerated statin is unable to achieve an LDL-C goal based on an individual’s estimated risk or co-morbidities, non-statin therapy should be considered for both primary and secondary prevention to achieve that desired target. While the ESC/EAS Guideline recommends consideration of risk modifiers (Fig. 1) and a CAC score for additional risk stratification, it also includes carotid and femoral plaque imaging, which does not carry the same negative predictive value as a CAC score of zero [4].
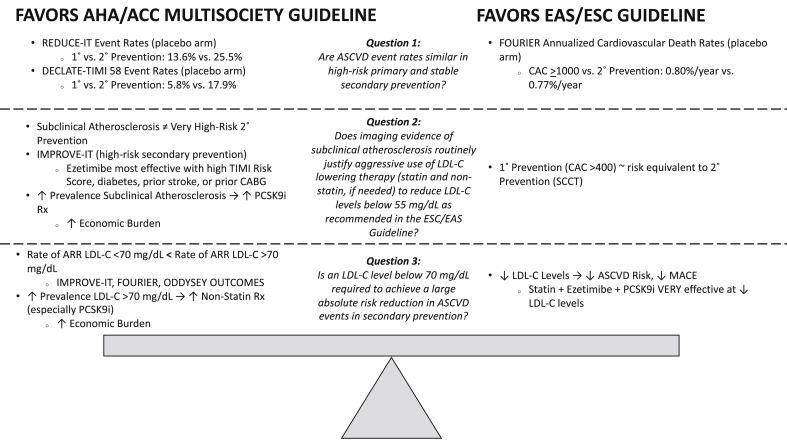
While the ESC/EAS Guideline includes the option to add on non-statin therapy for all subgroups to achieve specific LDL-C goals based on data that ‘lower is better’, the AHA/ACC Multisociety Guideline defines a smaller, more specific subgroup where use of non-statin therapy is most beneficial. Ultimately, constructing a framework to optimize the use of lipid-lowering therapy requires careful consideration of an individual’s absolute risk over 10 years, LDL-C levels on optimized treatment regimens, and the cost of different forms of non-statin therapy. A ‘highest risk-highest benefit’ matrix has been proposed as one approach [5]. It should be noted that both guidelines are largely in agreement on the general principle of tailoring the intensity of interventions to the level of risk. However, there are some differences regarding which aspects should be prioritized when determining their recommendations. In an attempt to further inform the comparative reviews of both guidelines [6,7], important differences between the two guidelines can be clarified by answering three specific questions (Fig. 2).
-
Question 1
Are ASCVD event rates similar in high-risk primary and stable secondary prevention?
Fig. 2.
A Comparison of the Evidence Favoring the AHA/ACC and ESC/EAS Approach to the Three Key Questions
Abbreviations: AHA – American Heart Association; ACC – American College of Cardiology; ESC – European Society of Cardiology; EAS – European Atherosclerosis Society; ASCVD – Atherosclerotic Cardiovascular Disease; LDL-C – Low-Density Lipoprotein Cholesterol; REDUCE-IT – Reduction of Cardiovascular Events with Icosapent Ethyl-Intervention Trial; DECLARE-TIMI 58 – Dapaglifozin and Cardiovascular Outcomes in Type 2 Diabetes Trial; 1˚- Primary; 2° - Secondary; IMPROVE-IT – Improved Reduction of Outcomes: Vytorin Efficacy International Trial; TIMI – Thrombolysis In Myocardial Infarction; CABG – Coronary Artery Bypass Graft; PCSK9i – proprotein convertase subtilisin/kexin type 9 inhibitor; Rx – Prescriptions; ARR – Absolute Risk Reduction; FOURIER – Further Cardiovascular Outcomes Research With PCSK9 Inhibition in Subjects with Elevated Risk; ODDYSEY OUTCOMES – Evaluation of Cardiovascular Outcomes after an Acute Coronary Syndrome During Treatment with Alirocumab; CAC – Coronary Artery Calcium; SCCT – Society of Cardiovascular Computed Tomography; MACE – Major Adverse Cardiovascular Events.
Both guidelines leverage scoring systems derived from population-based studies to estimate an individual’s risk for total and fatal ASCVD events – the Pooled Cohort Equations in the United States (US) and the Systematic Coronary Risk Estimation in Europe, respectively. While there are imprecisions with risk estimation at the individual level [8], both scoring systems are based on the principle that the intensity of prevention efforts should match the absolute ASCVD risk of the individual [9].
Currently, there is no randomized clinical trial (RCT) data to support the use of non-statin therapy as an add-on for primary prevention other than in individuals with a baseline LDL-C ≥190 mg/dL or heterozygous familial hypercholesterolemia. The AHA/ACC Multisociety Guideline adheres closely to the trial evidence related to this approach, whereas the ESC/EAS Guideline extends use of non-statin therapy in primary prevention based on the belief that event rates are similar in high-risk primary and stable secondary prevention.
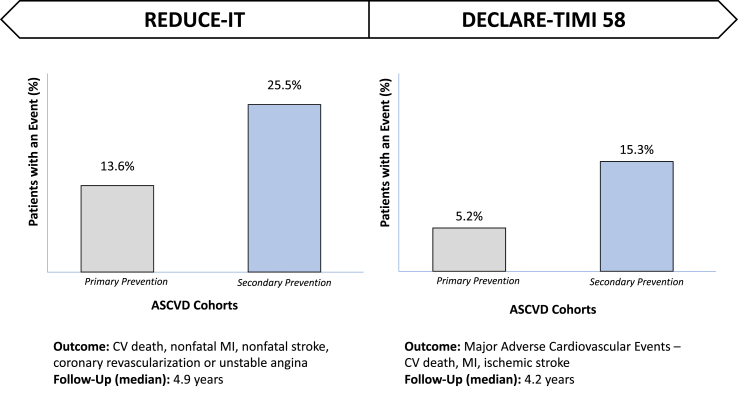
Data from two key trials can be helpful in clarifying this (Fig. 3). In the Reduction of Cardiovascular Events with Icosapent Ethyl-Intervention Trial (REDUCE-IT) [10], which enrolled patients on statin therapy with established ASCVD or diabetes and additional cardiovascular risk factors and followed them over an ~5-year period, the percentage of patients with an adverse cardiovascular event was almost double in the placebo arm (25.5%) of the secondary prevention cohort (patients with established ASCVD) versus the placebo arm (13.6%) of the high-risk primary prevention cohort, which included patients with diabetes and other cardiovascular risk factors, but without ASCVD.
Fig. 3.
The percentage of patients with an event in primary and secondary ASCVD prevention – a comparison using data from reduce-it and declare-timi 58
Abbreviations: REDUCE-IT – reduction of cardiovascular events with icosapent ethyl-intervention trial; declare-timi 58 – dapaglifozin and cardiovascular outcomes in type 2 diabetes trial; ASCVD – atherosclerotic cardiovascular disease; CV – cardiovascular; MI – myocardial infarction.
In the Dapaglifozin and Cardiovascular Outcomes in Type 2 Diabetes Trial (DECLARE-TIMI 58), which enrolled patients with type 2 diabetes on statin therapy or ezetimibe and followed them over an ~4 year period, the percentage of patients with a major adverse cardiovascular events (MACE) was almost three times greater in the placebo arm of the secondary (15.3%) versus primary (5.2%) prevention cohort [11]. While both groups included patients with diabetes, this trial still allows for comparison of event rates among high-risk primary and stable secondary prevention patients.
First, both RCTs include a unique population with significant lipid and inflammatory risk, which may limit the generalizability to all primary and secondary cohorts. Collectively though, without adjusting for baseline differences between both subgroups, these studies indicate that event rates in secondary prevention are considerably higher than in high-risk primary prevention. In addition, despite similar risk factor burden in these patients, development of an ASCVD event in one identifies inherent host susceptibility to those risk factors or yet unknown factors that may make a host more susceptible to another event.
As a result, the AHA/ACC Multisociety Guideline largely limits drug treatment to statin therapy for primary prevention, with the exception of ezetimibe and/or a PCSK9i in patients with severe hypercholesterolemia and an LDL-C ≥100 mg/dL despite adherence to high-intensity statin therapy. Non-statin therapy is more likely to be used in secondary prevention but is informed by the level of ASCVD risk. The more conservative use of non-statin therapy is based on a desire to add value by considering both net clinical benefit and cost, with a number needed to treat (NNT) ≤50 being reasonably indicative of this [12].
This concept when applied to ezetimibe and PCSK9i was previously validated and clarifies the thresholds to initiate non-statin therapy utilized in the AHA/ACC Multisociety Guideline [13]. Use of ezetimibe (with an approximate 15–24% reduction in LDL-C) has been suggested to be reasonable at a NNT ≤50 for very high-risk patients with a LDL-C ≥130 mg/dL or high-risk patients with a LDL-C ≥190 mg/dL. The addition of a PCSK9i (with a 50–65% reduction in LDL-C) is felt to be reasonable at a NNT ≤50 for very high-risk patients with a LDL-C ≥70 mg/dL or high-risk patients with a LDL-C ≥100 mg/dL.
In contrast, the ESC/EAS Guideline maintains that adding generic ezetimibe is reasonable to achieve lower LDL-C levels in primary and secondary prevention, which is often separated by a fine line with just an abrupt and adverse event being the only transition. They cite the heterogeneity of these patients, whom upon further stratification reveals a group with a wide range of risk based on varying levels of subclinical atherosclerosis. For example, based on retrospective cohort data, the ACC/AHA previously described individuals with a CAC >400 as highest risk for ASCVD events among a primary prevention cohort [14,15]. More recently, patients with a CAC ≥1000 were felt to represent a unique very-high risk primary prevention population where annualized cardiovascular death rates (0.80%/year) were very similar to those of a stable secondary prevention cohort (0.77%/year) [16,17]. These comparisons do not account, however, for variation in the follow-up duration or the use and intensity of statin therapy, which was much higher in the stable secondary prevention cohort. These factors could lower the observed event rates in secondary prevention patients compared to those with significant subclinical atherosclerosis.
With improvement in early initiation, adherence and intensification of risk factor modification, including diet, exercise and lipid lowering, hypoglycemic and anti-hypertensive medications, high-risk primary prevention individuals with significant subclinical atherosclerosis can further reduce their ASCVD risk [[18], [19], [20]]. Therefore, if more current practice patterns, specifically appropriate statin therapy allocation and optimal blood pressure control, were implemented equitably among the high-risk primary and stable secondary prevention patients, the event rates may more accurately reflect modern RCT data.
Based on the REDUCE-IT and DECLARE-TIMI 58 trial findings, as well as cohort data from high-risk primary prevention patients with significant subclinical atherosclerosis, a spectrum of risk in primary and secondary prevention exists. Varying interpretation of the event rates in high-risk primary and stable secondary prevention patients has led to differences in recommendations for the treatment of ASCVD risk in the US and Europe.
According to the AHA/ACC Multisociety Guideline, ezetimibe, based on its ease of accessibility, cost, and ability to reduce LDL-C levels by 15–24% on top of statin therapy, is currently considered first-line non-statin add-on therapy only in secondary prevention or select primary prevention groups. The EVAPORATE trial showed that icosapent ethyl can reduce low attenuation plaque volume providing mechanistic insight into the benefits of icosapent ethyl in REDUCE-IT [21]. However, further confirmatory RCT evidence of the benefit of icosapent ethyl in other subgroups such as high-risk primary prevention patients without diabetes is needed. As access to icosapent ethyl improves and additional RCT data emerges, it will be important to consider this therapy given its significant effect on hard ASCVD events in patients with a triglycerides level ≥150 mg/dL. Based on initial data, the effect of icosapent ethyl is greater for secondary than primary prevention (NNT 17 vs. 61) as well [10]. Additional analyses have also demonstrated icosapent ethyl’s benefit in not only reducing initial but also subsequent events in a population at high risk for ischemic events with an annualized placebo primary end point rate of 5.7% [22]. While these effects are more pronounced than that noted with ezetimibe in the Improved Reduction of Outcomes: Vytorin Efficacy International Trial (IMPROVE-IT), the current cost of icosapent ethyl is likely to limit broader use.
-
Question 2
Does imaging evidence of subclinical atherosclerosis justify aggressive use of statin and non-statin therapy (if needed) to reduce LDL-C levels below 55 mg/dL as recommended in the ESC/EAS Guideline?
In the AHA/ACC Multisociety Guideline, imaging to detect subclinical atherosclerosis is limited to a CAC score when the decision to initiate statin therapy in primary prevention is uncertain. If CAC is present, statin therapy is recommended, especially if CAC is ≥ 100. However, once statin therapy is initiated, CAC is no longer indicated for guiding treatment decisions, including adding non-statin therapy.
The ESC/EAS Guideline recommends that a CAC score be used to help guide decision-making in individuals with low to moderate risk for ASCVD. However, by having a combined treatment goal of both a ≥50% reduction in LDL-C and a LDL-C level <55 mg/dL, non-statin therapy is likely to be needed frequently.
While extremely high levels of calcified coronary plaque (a CAC score ≥1000), which is strongly correlated with total plaque, can pose substantial risk in primary prevention [16], the US guideline maintains that patients with advanced subclinical atherosclerosis on appropriate statin therapy are unlikely to derive significant enough clinical benefit to justify routine non-statin therapy as part of aggressive LDL-C lowering.
For example, in IMPROVE-IT [23], which enrolled a very high-risk secondary prevention population with an acute coronary syndrome (ACS) within the preceding 10 days, the addition of ezetimibe to statin therapy (32.7%) resulted in a ~2% ARR compared to statin therapy alone (34.7%) for the primary outcome (CV mortality, major CV event, or nonfatal stroke) over a 7-year period. Even among this very high-risk population, the ARR of adding ezetimibe to statin therapy was most pronounced in patients with recent ACS and additional high-risk features, including those with a high Thrombolysis In Myocardial Infarction (TIMI) Risk Score, diabetes, and age ≥ 75 years, as well as prior stroke, or prior coronary artery bypass graft surgery [24].
Therefore, the approach in the ESC/EAS Guideline to target LDL-C levels below 55 mg/dL based on the presence of subclinical atherosclerosis is unlikely to be cost-effective if ezetimibe and/or PCSK9i are needed to get the LDL-C this low. In most cases, the presence of moderate or advanced subclinical atherosclerosis in those on optimal medical therapy does not usually raise ASCVD risk to the level of a patient with clinical ASCVD especially after moderate-to high-intensity statin therapy is used. Therefore, the use of non-statin therapy (especially with a PCSK9i) in this population is likely to have a NNT considerably >50.
-
Question 3
Do LDL-C levels below 70 mg/dL achieve a large ARR in secondary ASCVD prevention?
The Cholesterol Treatment Trialists’ Collaboration demonstrated an ~22% relative risk reduction in major ASCVD events per mmol/L reduction in LDL-C (~39 mg/dL) [25]. This analysis was pivotal in validating the benefits of LDL-C lowering (with statin therapy) to reduce ASCVD risk. As can be seen, multiplying relative risk reduction (~22%/mmol) from LDL-C lowering therapies by the patient’s absolute risk determines the absolute risk reduction achieved. Additionally, when LDL-C is higher, the greater the LDL-C reduction from such therapies leads to greater absolute risk reduction and therefore increased benefit.
Based on this, the AHA/ACC Multisociety Guideline defines a secondary prevention population that is most likely to benefit from addition of non-statin therapy and further reduction in LDL-C levels. This includes individuals with a history of multiple major ASCVD events or 1 major ASCVD event plus multiple high-risk conditions. According to the AHA/ACC Multisociety Guideline, for these very high-risk individuals, it is reasonable to consider adding ezetimibe to maximally tolerated statin therapy when the LDL-C level remains ≥70 mg/dL; it is also reasonable to consider adding a PCSK9i to maximally tolerated statin therapy and ezetimibe when the LDL-C level remains ≥70 mg/dL or non-HDL-C level remains ≥100 mg/dL.
The ESC/EAS Guideline broadly extends the addition of non-statin therapy to a much larger group – primary prevention patients with moderate atherosclerosis on imaging and patients with stable ASCVD. The most relevant data documenting the benefit of intensifying LDL-C lowering therapy beyond statin therapy comes from the IMPROVE-IT, Further Cardiovascular Outcomes Research With PCSK9 Inhibition in Subjects with Elevated Risk (FOURIER), and Evaluation of Cardiovascular Outcomes after an Acute Coronary Syndrome During Treatment with Alirocumab (ODYSSEY OUTCOMES) trials – studies which specifically enrolled patients with clinical ASCVD and prior events. Acknowledging that these studies provide support that ‘lower is better’, the degree to which LDL-C should be lowered is of key importance.
In IMPROVE-IT, which included a high-risk secondary prevention population with a recent ACS, addition of ezetimibe (compared to placebo) was associated with a LDL-C level difference of 24% (LDL-C 53.2 mg/dL vs. 69.9 mg/dL) at 1 year and an ARR of 2.0% (32.7% vs. 34.7%) at 7 years. The between group difference in LDL-C levels (12.8 mg/dL) and proportional reduction in rate of major vascular events (7.2%) was also consistent with the reduction that occurs with statin therapy.
If this population was further risk stratified, those with three or more high-risk features had a much higher recurrent event rate (40% vs. 14% for 0–1 high-risk features) and greater risk reduction with the addition of ezetimibe (6.3% vs. 2.2% ARR in patients with two high-risk features) [21]. This data suggest a clear benefit of ezetimibe in a very high-risk secondary prevention population, where the reduction in LDL-C is directly proportional to the relative risk reduction in ASCVD events [26]. However, once LDL-C levels are below 70 mg/dL, the ARR from LDL-C lowering is less significant. In fact, the addition of ezetimibe did not result in a statistically significant graded decrease in the hazard ratio for the primary outcome with lower LDL-C strata (<30 mg/dL or 30–49 mg/dL) once below 70 mg/dL (50–69 mg/dL) [27].
In the FOURIER trial, patients with established ASCVD on statin therapy with a LDL-C ≥70 mg/dL were randomized to evolocumab or placebo [17]. The primary outcome of incident cardiovascular death, MI, stroke, hospitalization for unstable angina or coronary revascularization occurred in 12.6% in the evolocumab versus 14.6% in the placebo group over a mean of 2.2 years. While LDL-C levels were reduced on average by 56 mg/dL in those on evolocumab, with an on-treatment mean LDL-C level of 30 mg/dL (down from a baseline median LDL-C level of 92 mg/dL), the magnitude of ARR was more pronounced among individuals with an MI within 2 years (3.4%), ≥2 MIs (3.7%), or residual multivessel coronary artery disease (3.6%) [28].
While a prespecified secondary analysis of the FOURIER trial also demonstrated a linear association with the rate of primary and secondary endpoints and LDL-C levels to very low values, once LDL-C levels reached below 70 mg/dL, the ARR significantly diminished [29]. In IMPROVE-IT and FOURIER, while the ARR of non-statin add-on therapy extends to all secondary prevention patients, there is a significant increase in the ARR when LDL-C levels are >70 mg/dL as the baseline risk of the individual increases. This highlights that the benefit of LDL-C lowering diminishes with lower LDL-C levels–a 50% relative reduction in LDL-C at lower baseline LDL-C levels of 60 mg/dL and 80 mg/dL leads to 30 and 40 mg/dL absolute reductions from baseline [30]. Therefore, adding non-statin therapy at lower LDL-C levels results in less LDL-C reduction and lower relative risk reduction for ASCVD events [31].
In the ODYSSEY OUTCOMES trial, patients with an ACS within the preceding 1–12 months on statin therapy and an LDL-C ≥ 70 mg/dL were randomized to alirocumab or placebo [32]. The primary endpoint, which was a composite of death from coronary heart disease, nonfatal MI, fatal or nonfatal ischemic stroke, or unstable angina requiring hospitalization, occurred in 9.5% in the alirocumab versus 11.1% in the placebo group over a median of 2.8 years.
In a secondary analysis, the population was classified as very high-risk and non-very high-risk according to the AHA/ACC Multisociety Guideline. Very high-risk participants, despite an incidence rate of MACE per 100 patient-years more than double that of non-very high-risk individuals, benefited most from alirocumab with an ARR of 2.1% [33].
Consistent with the AHA/ACC Multisociety Guideline, these very high-risk individuals represent those most likely to benefit from addition of non-statin therapy with a PCSK9i to achieve an LDL-C level <70 mg/dL. Acknowledging that adherence to an appropriate intensity of statin therapy is the first step in lipid lowering, statin therapy alone is often insufficient to lower LDL-C levels and ASCVD risk enough in those at very high-risk.
In a simulated analysis of the SWEDEHEART registry, it was estimated that half of MI patients would require PCSK9i therapy after maximizing statin intensity and adding ezetimibe to achieve a LDL-C level <55 mg/dL [34]. In The Pravastatin or Atorvastatin Evaluation and Infection Therapy-Thrombolysis in Myocardial Infarction (PROVE IT-TIMI 22) study, ~55% and ~75% of all individuals LDL-C levels would be above the AHA/ACC (≥70 mg/dL) and ESC/EAS (≥55 mg/dL) very high-risk LDL-C thresholds, respectively, with high-intensity statin therapy alone [35].
In a more modern data set from the Veterans Affairs health care system, 1,038,903 individuals with ASCVD were identified, of which ~43% met criteria for very high-risk ASCVD [36]. Despite use of statin therapy in 82% of these very high-risk individuals, 67% of them had an LDL-C level ≥70 mg/dL. Even after titrating to a high-intensity statin, ~37% of the very high-risk individuals were found to have an LDL-C level ≥70 mg/dL. After the addition of ezetimibe to high-intensity statin, 24% of these individuals still had a LDL-C level ≥70 mg/dL, and therefore were candidates for a PCSK9i.
Data from IMPROVE-IT, FOURIER and ODYSSEY OUTCOMES support an additional ARR when individuals LDL-C levels fall below 70 mg/dL. Enrichment of this population with those at highest risk, though, results in a more pronounced ARR and likely, more cost-effective approach. The extent to which LDL-C level thresholds should be lowered in the very high-risk secondary prevention population depends not only on efficacy but also “cost efficacy”?
Despite a significant reduction in PCSK9i cost [37], which still varies significantly by region, the affordability of therapy remains a major issue for many healthcare systems and patients alike. It is financially challenging to recommend an LDL-C level <55 mg/dL for all patients with ASCVD, as a sizeable proportion of individuals are likely to require ezetimibe and a PCSK9i to achieve this goal. To minimize the cost associated with PCSK9i therapy, all high-risk individuals should first be titrated to a high-intensity statin with strong emphasis on adherence [38,39], which in doing so will also reduce clinical inertia [40]. Then individuals with the highest risk of recurrent ASCVD events among those with clinical ASCVD should be considered for additional non-statin therapies if LDL-C levels are >70 mg/dL, as recommended in the AHA/ACC Multisociety Guideline. This approach identifies a subgroup likely to derive the greatest benefit and reduces the NNT and economic challenges of achieving the ESC/EAS guideline recommendations [41].
2. Conclusions
Both guidelines incorporate advances from recent RCTs. The 2018 AHA/ACC Multisociety Guideline adheres closely to trial evidence and strongly considers both the added efficacy and cost implications of broadly reducing LDL-C levels in all high-risk individuals. This provides the basis for its more conservative recommendations pertaining to the highest risk subgroup where addition of non-statin therapy should be considered. In contrast, the 2019 ESC/EAS Guideline focuses primarily on trial data demonstrating that lower LDL-C levels resulted in lower recurrent ASCVD event rates, without strongly weighing the additive benefit and financial cost. Given current challenges, where issues with cost and access to some non-statin therapies exist, the ESC/EAS guideline provides recommendations that may be difficult to attain both in the US (if implemented) and in Europe, particularly in a context of limited healthcare resources. This provides support for the US guideline that selectively recommends non-statin therapy use in high- and, in particular, very high-risk individuals optimized on statin therapy. Future studies that incorporate cost-effectiveness in addition to efficacy and safety may help answer questions that could bring the guidelines closer together.
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: DIF: None; EDM: None; NJS: None; TJG: None; MCA: None; SSV: Grant support: Department of Veterans Affairs, World Heart Federation, Tahir and Jooma Family, Honorarium: American College of Cardiology (Associate Editor for Innovations, acc.org), Steering Committee Member: Provider and Patient Assessment of Lipid Management (PALM) registry at the Duke Clinical Research Institute (no financial remuneration); RSB: None.
Acknowledgements
Michael J. Blaha, MD, MPH & Christie M. Ballantyne, MD for their review and comments regarding the paper.
References
- 1.Grundy S.M., Stone N.J., Bailey A.L. AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American College of Cardiology/American heart association task force on clinical practice guidelines. Circulation. 2018;139(25):e1082–e1143. doi: 10.1161/CIR.0000000000000625. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mach F., Baigent C., Catapano A.L. ESC Scientific Document Group. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J. 2020;41(1):111–188. doi: 10.1093/eurheartj/ehz455. [DOI] [PubMed] [Google Scholar]
- 3.Michos E.D., McEvoy J.W., Blumenthal R.S. Lipid management for the prevention of atherosclerotic cardiovascular disease. N Engl J Med. 2019;381:1557–1567. doi: 10.1056/NEJMra1806939. [DOI] [PubMed] [Google Scholar]
- 4.Mortensen M.B., Fuster V., Muntendam P. A simple disease-guided approach to personalize ACC/AHA-recommended statin allocation in elderly people: the BioImage study. J Am Coll Cardiol. 2016;68:881–891. doi: 10.1016/j.jacc.2016.05.084. [DOI] [PubMed] [Google Scholar]
- 5.Annemans L., Packard C.J., Briggs A. ‘Highest risk-highest benefit’ strategy: a pragmatic, cost-effective approach to targeting use of PCSK9 inhibitor therapies. Eur Heart J. 2018;39(27):2546–2550. doi: 10.1093/eurheartj/ehx710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stone N.J., Blumenthal R.S., Lloyd-Jones D. Comparing primary prevention recommendations: a focused look at United States and European guidelines on dyslipidemia. Circulation. 2020;141(14):1117-1120. doi: 10.1161/CIRCULATIONAHA.119.044562. [DOI] [PubMed] [Google Scholar]
- 7.Virani S.S., Smith S.C., Jr., Stone N.J., Grundy S.M. Secondary prevention for atherosclerotic cardiovascular disease: comparing recent US and European guidelines on dyslipidemia. Circulation. 2020;141(14):1121-1123. doi: 10.1161/CIRCULATIONAHA.119.044282. [DOI] [PubMed] [Google Scholar]
- 8.Amin N.P., Martin S.S., Blaha M.J. Headed in the right direction but at risk for miscalculation: a critical appraisal of the 2013 ACC/AHA risk assessment guidelines. J Am Coll Cardiol. 2014;63:2789–2794. doi: 10.1016/j.jacc.2014.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fuster V., Pearson T.A. 27th Bethesda Conference: matching the intensity of risk factor management with hazard for coronary disease events. J Am Coll Cardiol. 1996;27:957–1047. [PubMed] [Google Scholar]
- 10.Bhatt D.L., Steg P.G., Miller M. Cardiovascular risk reduction with icosapent ethyl for hypertriglyceridemia. N Engl J Med. 2019;380:11–22. doi: 10.1056/NEJMoa1812792. [DOI] [PubMed] [Google Scholar]
- 11.Wiviott S.D., Raz I., Bonaca M.P. Dapaglifozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2019;380:347–357. doi: 10.1056/NEJMoa1812389. [DOI] [PubMed] [Google Scholar]
- 12.Steel N. Thresholds for taking antihypertensive drugs in different professional and lay groups: questionnaire survey. BMJ. 2000;320:1446–1447. doi: 10.1136/bmj.320.7247.1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Robinson J.G., Huijgen R., Ray K. Determining when to add nonstatin therapy: a quantitative approach. J Am Coll Cardiol. 2016;68(22):2412–2421. doi: 10.1016/j.jacc.2016.09.928. [DOI] [PubMed] [Google Scholar]
- 14.Hecht H., Blaha M.J., Berman D.S. Clinical indications for coronary artery calcium scoring in asymptomatic patients: expert consensus statement from the Society of Cardiovascular Computed Tomography. J Cardiovasc Comput Tomogr. 2017;11(2):157-168. doi: 10.1016/j.jcct.2017.02.010. [DOI] [PubMed] [Google Scholar]
- 15.Arnson Y., Rozanski A., Gransar H. Comparison of the coronary artery calcium score and number of calcified coronary plaques for predicting patient mortality risk. Am J Cardiol. 2017;120(12):2154–2159. doi: 10.1016/j.amjcard.2017.09.001. [DOI] [PubMed] [Google Scholar]
- 16.Peng A.W., Mirbolouk M., Orimoloye O.A. Long-term all cause and cause-specific mortality in asymptomatic patients with CAC >1000; Results from the CAC Consortium. JACC Cardiovasc Imaging. 2020;13(1):83–93. doi: 10.1016/j.jcmg.2019.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sabatine M.S., Giugliano R.P., Keech A.C. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med. 2017;376:1713–1722. doi: 10.1056/NEJMoa1615664. [DOI] [PubMed] [Google Scholar]
- 18.Ritchey M.D., Maresh S., McNeely J. Tracking cardiac rehabilitation participation and completion among medicare beneficiaries to inform the efforts of a national initiative. Circ Cardiovasc Qual Outcomes. 2020 Jan;13(1) doi: 10.1161/CIRCOUTCOMES.119.005902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bhatti S.K., DiNicolantonio J.J., Captain B.K. Neutralizing the adverse prognosis of coronary artery calcium. Mayo Clin Proc. 2013;88(8):806–812. doi: 10.1016/j.mayocp.2013.05.019. [DOI] [PubMed] [Google Scholar]
- 20.Muntner P., Hardy S.T., Fine L.J. Trends in blood pressure control among US adults with hypertension, 1999-2000 to 2017-2018. J Am Med Assoc. 2020 Sep 9;324(12):1–12. doi: 10.1001/jama.2020.14545. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Budoff M.J., Bhatt D.L., Kinninger A. Effect of icosapent ethyl on progression of coronary atherosclerosis in patients with elevated triglycerides on statin therapy: final results of the EVAPORATE trial. Eur Heart J. 2020 Aug 29 doi: 10.1093/eurheartj/ehaa652. ehaa652. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bhatt D.L., Steg P.G., Miller M. Effects of icosapent ethyl on total ischemic events; from REDUCE-IT. J Am Coll Cardiol. 2019;73(22):2791–2802. doi: 10.1016/j.jacc.2019.02.032. [DOI] [PubMed] [Google Scholar]
- 23.Cannon C.P., Blazing M.A., Giugliano R.P. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med. 2015;372:2387–2397. doi: 10.1056/NEJMoa1410489. [DOI] [PubMed] [Google Scholar]
- 24.Bohula E.A., Morrow D.A., Giugliano R.P. Atherothrombotic risk stratification and ezetimibe for secondary prevention. J Am Coll Cardiol. 2017;69(8):911–921. doi: 10.1016/j.jacc.2016.11.070. [DOI] [PubMed] [Google Scholar]
- 25.Cholesterol Treatment Trialists’ (CTT) Collaboration Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170 000 participants in 26 randomised trials. Lancet. 2010;376(9753):1670–1681. doi: 10.1016/S0140-6736(10)61350-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Silverman M.G., Ference B.A., Im K. Association between lowering LDL-C and cardiovascular risk reduction among different therapeutic interventions: a systematic review and meta-analysis. J Am Med Assoc. 2016;316:1289–1297. doi: 10.1001/jama.2016.13985. [DOI] [PubMed] [Google Scholar]
- 27.Giugliano R.P., Wiviott S.D., Blazing M.A. Long-term safety and efficacy of achieving very low levels of low-density lipoprotein cholesterol: a prespecified analysis of the IMPROVE-IT trial. JAMA Cardiol. 2017;2:547–555. doi: 10.1001/jamacardio.2017.0083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sabatine M.S., De Ferrari G.M., Giugliano R.P. Clinical benefit of evolocumab by severity and extent of coronary artery disease: analysis from FOURIER. Circulation. 2018;138(8):756–766. doi: 10.1161/CIRCULATIONAHA.118.034309. [DOI] [PubMed] [Google Scholar]
- 29.Giugliano R.P., Pedersen T.R., Park J.G. Clinical efficacy and safety of achieving very low LDL-cholesterol concentrations with the PCSK9 inhibitor evolocumab: a prespecified secondary analysis of the FOURIER trial. Lancet. 2017;390(10106):1962–1971. doi: 10.1016/S0140-6736(17)32290-0. [DOI] [PubMed] [Google Scholar]
- 30.Ballantyne C.M., Virani S.S. Real-world data, theoretical application of guidelines, cost, and access: how do we optimize non-statin therapy for LDL-C/non-HDL-C/Apo-B? Eur Heart J. 2020:1–3. doi: 10.1093/eurheartj/ehaa139. 0. [DOI] [PubMed] [Google Scholar]
- 31.Navarese E.P., Robinson J.G., Kowalewski M. Association between baseline ldl-C level and total and cardiovascular mortality after ldl-C lowering: a systematic review and meta-analysis [published correction appears in JAMA. 2018 Oct 2;320(13):1387] J Am Med Assoc. 2018;319(15):1566–1579. doi: 10.1001/jama.2018.2525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schwartz G.G., Steg P.G., Szarek M. Alirocumab and cardiovascular outcomes after acute coronary syndrome. N Engl J Med. 2018;379:2097–2107. doi: 10.1056/NEJMoa1801174. [DOI] [PubMed] [Google Scholar]
- 33.Roe M.T., Li Q.H., Bhatt D.L. Risk categorization using new American College of Cardiology/American Heart Association Guidelines for Cholesterol Management and its relation to alirocumab treatment following acute coronary syndromes. Circulation. 2019;140(19):1578–1589. doi: 10.1161/CIRCULATIONAHA.119.042551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Allahyari A., Jernberg T., Hagstrom E. Application of the 2019 ESC/EAS dyslipidaemia guidelines to nationwide data of patients with a recent myocardial infarction: a simulation study. Eur Heart J. 2020:1–10. doi: 10.1093/eurheartj/ehaa034. 0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wiviott S.D., Cannon C.P., Morrow D.A. Can low-density lipoprotein be too low? The safety and efficacy of achieving very low low-density lipoprotein with intensive statin therapy: a PROVE IT-TIMI 22 Substudy. J Am Coll Cardiol. 2005;46(8):1411–1416. doi: 10.1016/j.jacc.2005.04.064. [DOI] [PubMed] [Google Scholar]
- 36.Virani S.S., Akeroyd J.M., Smith S.S. Very high-risk ASCVD and eligibility for nonstatin therapies based on 2018 AHA/ACC Cholesterol Guidelines. J Am Coll Cardiol. 2019;74(5):712–714. doi: 10.1016/j.jacc.2019.05.051. [DOI] [PubMed] [Google Scholar]
- 37.Kazi D.S., Moran A.E., Coxson P.G. Cost-effectiveness of PCSK9 inhibitor therapy in patients with heterozygous familial hypercholesterolemia or atherosclerotic cardiovascular disease. J Am Med Assoc. 2016;316(7):743–753. doi: 10.1001/jama.2016.11004. [DOI] [PubMed] [Google Scholar]
- 38.Virani S.S., Akeroyd J.M., Nambi V. Applicability and cost implications for proprotein convertase subtilisin/kexin type 9 inhibitors based on the ODYSSEY outcomes trial. Circulation. 2019;139:410–412. doi: 10.1161/CIRCULATIONAHA.118.034993. [DOI] [PubMed] [Google Scholar]
- 39.Virani S.S., Akeroyd J.M., Nambi V. Estimation of eligibility for proprotein convertase subtilisin/kexin type 9 inhibitors and associated costs based on the FOURIER trial (further cardiovascular outcomes Research with PCSK9 inhibition in Subjects with elevated risk): insights from the department of Veterans Affairs. Circulation. 2017;135(25):2572–2574. doi: 10.1161/CIRCULATIONAHA.117.028503. [DOI] [PubMed] [Google Scholar]
- 40.Dixon D.L., Sharma G., Sandesara P.B. Therapeutic inertia in cardiovascular disease prevention: time to move the bar. J Am Coll Cardiol. 2019 Oct 1;74(13):1728–1731. doi: 10.1016/j.jacc.2019.08.014. [DOI] [PubMed] [Google Scholar]
- 41.Robinson J.G., Jayanna M.B., Brown A.S. Enhancing the value of PCSK9 monoclonal antibodies by identifying patients most likely to benefit. A consensus statement from the National Lipid Association. J Clin Lipidol. 2019;13(4):525–537. doi: 10.1016/j.jacl.2019.05.005. [DOI] [PubMed] [Google Scholar]