Abstract
Background:
Septic shock is a serious condition leading to increased mortality. Despite previous report of no benefit, thiamine has emerged as potential therapy to reduce mortality in septic shock patients. This study aimed to investigate the effect of thiamine in mortality rate in patients with septic shock.
Methods:
Eight databases, including MEDLINE, EMBASE, Science Direct, Scopus, Cochrane, CINAHL, Open Grey, and Dart-Europe, were systematically searched from the inception of the database up to August 21, 2020. Studies evaluating the effectiveness of thiamine on mortality rate in septic shock patients compared between thiamine and placebo were included. We used random-effects model to analyze the mortality with risk ratio (RR) and 95% confidence interval (95% CI). The subgroup and sensitivity analysis were performed to examine the influence of variables. Publication bias was considered using funnel plot, Begg's test, and Egger's test.
Results:
A total of 3,658 studies were retrieved and reviewed. Five studies were included for meta-analysis. In random-effects meta-analysis of the randomized controlled trials, although not statistically significant, there was a trend which suggested that thiamine may reduce mortality rate in septic shock patients (RR, 0.96; 95% CI: 0.72–1.28, P = 0.774). The result of sensitivity and subgroup analyses also supported the suggestion that thiamine may decrease mortality in septic shock patients. The Begg's test (P = 0.624) and Egger's test (P = 0.777) revealed no publication bias.
Conclusions:
Although not statistically significant, thiamine may reduce mortality rate in septic shock patients. Further prospective studies with larger sample size are warranted.
Key Words: Mortality, septic shock, thiamine
INTRODUCTION
Septic shock is emergency conditions leading to causes of hospital morbidity and mortality.[1] To prevent multiple organ failure such as brain, renal, liver, respiratory system, and cardiovascular system, management for septic shock patients must be done urgently and appropriately. According to the World Health Organization, the global epidemiological burden of sepsis is estimated to affect more than 30 million people worldwide every year, potentially leading to 6 million deaths.[2] In addition, sepsis was the most common immediate cause of death in the United States. The rate of mortality from sepsis was as high as 88% in various hospitals across the United States.[3]
For the management of patients with sepsis and septic shock, the Surviving Sepsis Campaign recommended administration of broad-spectrum antibiotic, initial resuscitation using fluid therapy, and utilizing vasopressor when clinically appropriate. At present, there are limited data suggesting the mortality benefit of thiamine in patients with septic shock.[4] In patients with septic shock, thiamine deficiency is associated with an increased lactate level caused by anaerobic metabolism. Hyperlactatemia will accumulate within cells, leading to cell damage and organ failure.[5] In addition, thiamine is an important cofactor in aerobic metabolism. Thiamine deficiency will slow the change of pyruvate to Acetyl-CoA pathway, which leads to abnormal energy and carbohydrate metabolism, thus increasing anaerobic metabolism and lactate level accumulation, resulting in lactic acidosis.[6] Therefore, there is a hypothesis that receiving thiamine can reduce mortality in patients with septic shock.
Early studies found that there was no relationship between thiamine level and mortality outcome. This finding has been challenged as some recent trials showed no benefit of thiamine in mortality rate in septic shock patients, whereas some studies showed a significant benefit in reducing mortality rate in septic shock patients treated with thiamine.[4,7]
Currently, there are no conclusive evidence addressing the relationship between thiamine therapy and the mortality rate in patients with septic shock. To better assess the impact of thiamine therapy, our objective was to address the following research question: in patients with septic shock (Population), does administration of thiamine (Intervention) compared to either untreated or treated with a placebo (Comparator) affect mortality (Outcome)?
METHODS
Experimental procedures
All steps of systematic review and meta-analysis in this study were in line with the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA).[8] The study protocol was registered with PROSPERO (CRD42019123412).
Data sources and search strategy
We conducted systematic searches for article indexed in EMBASE, MEDLINE, Science Direct, Scopus, Cochrane, CINAHL, Open Grey, and Dart-Europe without language, publication status, and study design restriction. All databases were searched from their inception to August 21, 2020. The following keywords were used for search strategy: thiamine OR vitamin B1 AND sepsis OR (septic, shock) AND mortality OR death, in combination with trial or study. To assure appropriateness of the obtained records, the bibliographies of identified studies were also searched. Gray literature and the unpublished database (such as Open Grey and Dart-Europe) were reviewed. Once relevant studies were identified, we contacted the authors of gray literature and/or unpublished trials for further information. If the inquiry was not responded, the studies were excluded from our meta-analysis.
Study selection
From these articles, the studies eligible for the meta-analysis were prospective cohorts, retrospective cohort, and randomized studies evaluating thiamine in patients aged over 18 years with septic shock. Only studies with thiamine group, either untreated or treated with a placebo, were considered. Studies including thiamine concomitantly with other agents (such as ascorbic acid, steroid, or both) were excluded. Selected studies must include risk ratio (RR), odds ratio (OR), hazard ratio (HR), or number of mortality rate with P value or a 95% confidence interval (95% CI). Studies were excluded if they were animal studies, cross-over studies, review articles, editorials, expert opinion, comments, letters, surveys, case–control, systematic review, meta-analysis, case series, or case report. We also excluded studies that enrolled the same patients, studies that excluded patients with thiamine deficiency, and studies that did not have sufficient data to measure effect estimates.
EndNote software is used to assist with managing bibliographies and citations, eliminating duplicated record and selecting the eligible studies in accordance with the inclusion/exclusion criteria.
Outcome measures and definitions
The primary outcome of this study was mortality. The terms of mortality included 28-day mortality, in-hospital mortality, and intensive care unit (ICU) mortality. The 28-day mortality was described as death within 28 days of the start of the diagnosed to sepsis. ICU mortality was defined as death occurred only during ICU admission and not during early resuscitation in the emergency department upon hospital arrival. Septic shock was defined according to the Survival Sepsis Campaign 2012 and Sepsis-3 criteria.[9,10] STATA version 16.0 was used to calculate RR and 95% CI to evaluate mortality rate in our study.
Data extraction and quality assessment
Eligible titles, abstracts, and full texts were screened by two independent reviewers (S. K. and P. S.). Disagreement was resolved using a consultant of the third reviewer (W. S.). We extracted the following information: author, country, study design, sample size, effect size including RR, characteristic of participants (such as age and definition of shock), comorbidity of participants, details of treatment regimen, comparison group, alcohol use, and outcome measurement by three independent reviewers (S. K., P. S., and C. S.). We contacted corresponding authors when information was incomplete or not reported. If the corresponding author did not respond, the article was excluded.
Three reviewers (S. K., M. N., and V. C.) independently assessed the risk of bias utilizing the Cochrane Risk-of-bias tool 2.0 (RoB 2.0) for randomized control trials.[11] This tool includes 6 domains for methodological evaluation: (1) random sequence generation, (2) allocation concealment, (3) blinding of participants and personnel, (4) blinding of outcome assessment, (5) incomplete outcome data, and (6) selective reporting. Each study was categorized as high, low, or unclear risk of bias. The Risk Of Bias In Non-randomized Studies of Interventions (ROBINS-I) tool was utilized to evaluate the quality of the observational studies.[12] Seven domains used for assessment included (1) bias due to judgment confounding, (2) bias in selection of participants, (3) bias in measurement of interventions, (4) bias due to departures from intended interventions, (5) bias due to missing data, (6) bias in measurement of outcomes, and (7) bias in the selection of reported results. Each study was classified as low, moderate, and serious risk of bias.
Quality of evidence
The Grading of Recommendations, Assessment, Development and Evaluation (GRADE) approach was used to rate the quality of evidence of estimates. In this meta-analysis, GRADEpro GDT software online version was used to determine the quality of evidence for outcome according to 5 domains as follows: risk of bias, inconsistency, indirectness, imprecision, and publication bias. The levels of evidence can be categorized into four levels: high, moderate, low, and very low.[13,14]
Data synthesis and statistical analysis
To explain the efficacy of thiamine therapy for mortality in septic shock patients, overall RR and 95% CI were analyzed using the DerSimonian–Laird random-effects models.[15] Heterogeneity was investigated using Cochrane's Q statistic. An alpha value of 0.10 was selected to define heterogeneity among trials for each analysis.[16]
Statistical heterogeneity was assessed by the I2 values. I2 values lower than 25%, 25%–75%, and >75% indicated low, moderate, and high heterogeneity, respectively.[16,17] Nevertheless, it is recognized that Cochran's Chi-square suffers from poor power when the number of included studies is small. Moreover, outlying studies can have a great impact on conventional heterogeneity methods and on the conclusions of a meta-analysis. Consequently, the Tau-squared (τ2) was utilized as a second means to establish the between-study variance.[18] The funnel plot, Begg's test, and Egger's test were utilized to detect the publication bias in this study.[19,20,21] In addition, the trim-and-fill method was performed to adjust for publication bias.[22]
Sensitivity and subgroup analysis
Subgroup and sensitivity analysis were analyzed to examine the influence of each variable, based on baseline characteristics of each study, including (1) models, (2) patients age, (3) alcohol drinking, (4) diabetes mellitus (DM), (5) methodological quality tools, and (6) definition of septic shock. Results were calculated by the random-effects model. In addition, we analyzed sensitivity using influence plot to determine the cause of heterogeneity.
RESULTS
Study selection
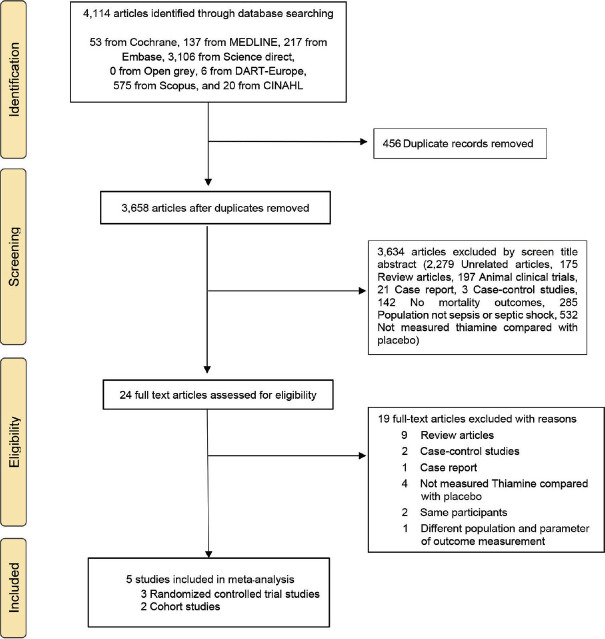
A total of 4,114 studies were found in eight databases. After eliminating 456 duplicate articles, we evaluated titles and abstracts. Of these, 3,634 articles were found to be irrelevant and excluded. After assessing the full texts, five articles were considered for synthesis of this systematic review and meta-analysis. A PRISMA flow diagram is shown in Figure 1. The full details of our search are presented in Supplement Appendix Table S1. Factors leading to article elimination are outlined in Supplement Appendix Table S2.
Figure 1.
The PRISMA flow chart of the study selection process
Study characteristics
Among five studies that included 645 patients, three articles were randomized controlled trial studies (RCTs),[23,24,25] whereas the remaining articles were cohort studies.[26,27] Of the five included articles, four studies were performed in the United States.[23,25,26,27] Only one study was conducted in Malaysia.[24] The mean age of the participants in the included studies was 67 years (range from 43.0 to 83.5). More than 50% of participants were male. All five studies compared mortality rate between thiamine groups to comparator groups. Of these, one study included patients given either oral or parenteral thiamine.[26] The other four studies included patients given only parenteral thiamine.[23,24,25,27] Of five studies, only one study reported duration of treatment.[23] The essential characteristics of included studies are exhibited in Table 1. The definition of septic shock in each study is outlined in Supplement Appendix Table S3. Description of included studies regarding location, duration of study, type of participants, number of participants, treatment onset, dose of thiamine, outcome, and details of treatment for each study are outlined in Supplement Appendix Table S4. Description of treatment interventions of included studies regarding dose, solution, and duration of treatment are outlined in Supplement Appendix Table S5.
Table 1.
Characteristics of studies included in the meta-analysis
| Characteristic | Author (years) |
||||
|---|---|---|---|---|---|
| Donnino M.W. et al (2016) | Moskowitz A, et al (2017) | Holmberg M.J. et al (2018) | Woolum J.A. et al (2018) | Harun N.F. et al (2019) | |
| Region | USA | USA | USA | USA | Malaysia |
| Study design | Randomized controlled trial | Randomized controlled trial | Cohort study | Cohort study | Randomized controlled trial |
| Sample size | 88 | 70 | 53 | 369 | 65 |
| Age (years) | 67.5±15.5a | 67.0±16.5a | Thiamine group=57.0 (49.0-64.0)b Placebo group=62.0 (44.0-68.0)b |
Thiamine group=52.0 (43.0-61.0)b Placebo group=54.0 (45.0-61.0)b |
Thiamine group=63.5 (50.5-70.5)b Placebo group=67.0 (55.0-77.0)b |
| Male (%) | 59.09 | 57.14 | 64.15 | 56.10 | 58.46 |
| Characteristic of participants | Patients aged ≥18 years with sepsis, lactate >3 mmol/L and hypotension | Adult patients presenting with sepsis (defined as the presence of two or more SIRS criteria with documented or suspected infection), lactate >3 mmol/L, and hypotension (systolic pressure <90 mmHg) after a minimum of a 2 L fluid bolus followed by vasopressor-dependence | Patients aged≥18 years with septic shock and an ICD-9 code for alcohol use disorders and admitted to the ICUs | Patients aged ≥18 years with septic shock were initially identified using the ICD-9 or ICD-10 diagnosis code criteria and admitted to either the medical or surgical ICU | Patients aged ≥18 years with septic shock, defined ≥2 SIRS or suspected infection, hypotension, and serum lactate ≥2 mmol/L |
| Comorbidity of participants | Coronary artery disease Congestive heart failure Hypertension Diabetes Chronic obstructive pulmonary disease |
Hypertension Coronary artery disease Congestive heart failure Chronic pulmonary disease Diabetes Renal disease |
Hypertension Congestive heart failure Diabetes Renal disease Liver disease Chronic obstructive pulmonary disease |
Liver disease | Coronary artery disease Congestive heart failure Hypertension Diabetes Chronic kidney disease Chronic pulmonary disease |
| Alcohol use, n (%) | N/A | N/A | Thiamine group=34 (64.2) Placebo group=19 (35.8) |
N/A | N/A |
| Treatment regimen | Parenteral thiamine 200 mg | Parenteral thiamine 200 mg | Oral or parenteral thiamine any dose, most of patients received 100 mg (97% of total dose) | Parenteral thiamine any dose including 100 mg, 100-400 mg and 500 mg | Parenteral thiamine 200 mg |
| Comparison group | Placebo | Placebo | No-thiamine | No-thiamine | Placebo |
| Effect size (95% CI) | 0.92 (0.61-1.38) | 0.79 (0.42-1.48) | 0.56 (0.36-0.87) | 0.91 (0.74-1.12) | 1.20 (0.69-2.09) |
| Outcome measurement | Lactate levels at 24 h Relative lactate change from baseline to 24 hTime to shock Mortality |
In-hospital mortality Creatinine levels |
Mortality Hospital-free day ICU-free day |
Lactate clearance 28 days mortality |
Lactate levels at 24 h Duration for weaning off the vasopressor SOFA score changes over 72 h ICU length of stay ICU mortality |
aMean±SD, bMedian (IQR). SOFA: Sequential organ failure assessment, SIRS: Systemic inflammatory response syndrome, N/A: Not available, CI: Confidence interval, ICD: International classification of disease, ICUs: Intensive care units, IQR: Interquartile range
Quality assessment
For quality assessment, all five articles were evaluated for risk of bias. RoB 2.0 was used to analyze the risk of bias for the three included RCT studies. Risk of bias from the RCT studies was considered low as presented in Supplement Appendix Figure S1. The two cohort studies were evaluated by ROBINS-I tool; all studies had a moderate risk of bias as shown in Supplement Appendix Table S6.
Mortality
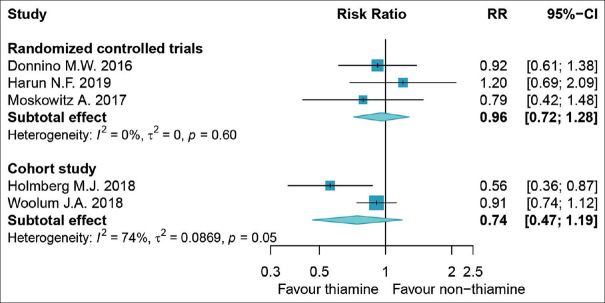
The forest plot showing RR of mortality rate in 645 patients with septic shock using thiamine compared to comparators is shown in Figure 2. Using random-effects model, data analyzed from the RCT demonstrated that thiamine was insignificantly associated with lower mortality rate as compared to comparators (RR, 0.96; 95% CI: 0.72–1.28), respectively, with the I2 0%, P = 0.60, τ2 = 0. The measurement of heterogeneity test represented low heterogeneity among RCTs. Thiamine did not significantly lower mortality rate according to data analyzed from cohort studies (RR, 0.74; 95% CI: 0.47–1.19), respectively, with the I2 74%, P = 0.05, τ2 = 0.0869. Graphical overview for evidence reviews is shown in Figure 3.
Figure 2.
Result of forest plot using random-effects model (risk ratio) comparing thiamine with non-thiamine use in patients with septic shock
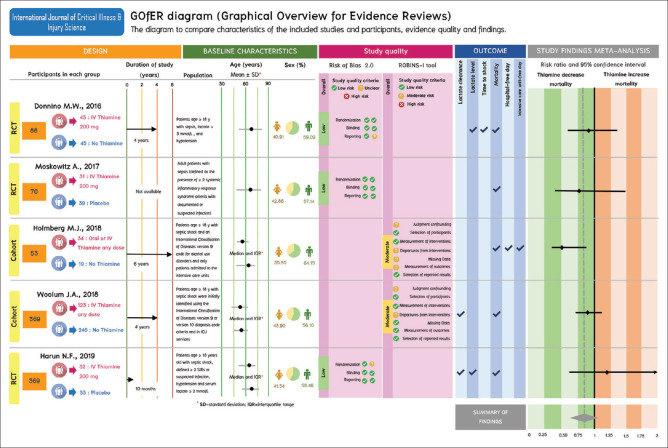
Figure 3.
Graphical overview for evidence reviews
Sensitivity and subgroup analysis
Results of sensitivity and subgroup analysis is shown in Table 2. For sensitivity and subgroup analysis, the data were stratified by study design. The RRs of RCT were 0.96 (95% CI, 0.72–1.28) for random-effects model and fixed-effects model, whereas the RRs of cohort studies were 0.74 (95% CI, 0.47–1.19) for random-effects model and 0.83 (95% CI, 0.69–1.01) for fixed-effects model. The data were stratified not only by study design but also by model, age, alcohol drinking, DM, methodological quality tools, and definition of septic shock. Compared between patients aged ≥65 years or <65 years, thiamine did not significantly lower risk of death (RR 0.96 [95% CI, 0.72–1.28] for age ≥65 years and 0.74 [95% CI, 0.47–1.19] for age <65 years). Thiamine was more effective than comparators in lowering mortality rate if patients had a history of alcohol intake (RR 0.56 [95% CI, 0.36–0.87] in patients with a history of alcohol intake and 0.93 [95% CI, 0.78–1.10] in patients with no alcohol intake). We did not find a significant reduction in mortality rate from thiamine use in DM patients (RR 0.82 [95% CI, 0.60–1.13] and 0.91 [95% CI, 0.74–1.12] in group of DM and no DM, respectively). Methodological quality tools did not result in differences in mortality rate (RR: 0.96 [95% CI, 0.72–1.28] and 0.74 [95% CI, 0.47–1.19] in group of RoB 2.0 and ROBINS-I tool, respectively). Definition of septic shock used among trials did not lead to differences in mortality rate (RR: 0.74 [95% CI, 0.47–1.19] and 0.96 [95% CI, 0.72–1.28] in group of Sepsis-3 criteria and SIRS criteria, respectively). Sensitivity analysis by influence plot is shown in Supplement Appendix Figure S2.
Table 2.
Subgroup and sensitivity analysis
| Subgroup | All studies |
Randomized controlled trials studies |
Cohort studies |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Risk ratio (95% CI) | Heterogeneity |
Risk ratio (95% CI) | Heterogeneity |
Risk ratio (95% CI) | Heterogeneity |
||||
| I2 (%) | P | I2 (%) | P | I2 (%) | P | ||||
| Models | |||||||||
| Random-effects model | 0.86 (0.69-1.05) | 27 | 0.24 | 0.96 (0.72-1.28) | 0 | 0.60 | 0.74 (0.47-1.19) | 74 | 0.05 |
| Fixed-effects model | 0.87 (0.74-1.12) | 27 | 0.24 | 0.96 (0.72-1.28) | 0 | 0.60 | 0.83 (0.69-1.01) | 74 | 0.05 |
| Age (years) | |||||||||
| ≥65 | 0.96 (0.72-1.28) | 0 | 0.60 | 0.96 (0.72-1.28) | 0 | 0.60 | N/A | N/A | N/A |
| <65 | 0.74 (0.47-1.19) | 74 | 0.05 | N/A | N/A | N/A | 0.74 (0.47-1.19) | 74 | 0.05 |
| Alcohol drinking | |||||||||
| Yes | 0.56 (0.36-0.87) | N/A | N/A | N/A | N/A | N/A | 0.56 (0.36-0.87) | N/A | N/A |
| No | 0.93 (0.78-1.10) | 0 | 0.77 | 0.96 (0.72-1.28) | 0 | 0.60 | 0.91 (0.74-1.12) | N/A | N/A |
| Diabetes mellitus | |||||||||
| Yes | 0.82 (0.60-1.13) | 40 | 0.17 | 0.96 (0.72-1.28) | 0 | 0.60 | 0.56 (0.36-0.87) | N/A | N/A |
| No | 0.91 (0.74-1.12) | N/A | N/A | N/A | N/A | N/A | 0.91 (0.74-1.12) | N/A | N/A |
| Methodological quality tools | |||||||||
| RoB 2.0 | 0.96 (0.72-1.28) | 0 | 0.60 | 0.96 (0.72-1.28) | 0 | 0.60 | N/A | N/A | N/A |
| ROBINS-I | 0.74 (0.47-1.19) | 74 | 0.05 | N/A | N/A | N/A | 0.74 (0.47-1.19) | 74 | 0.05 |
| Definition of septic shock | |||||||||
| Sepsis-3 criteria | 0.74 (0.47-1.19) | 74 | 0.05 | N/A | N/A | N/A | 0.74 (0.47-1.19) | 74 | 0.05 |
| SIRS criteria | 0.96 (0.72-1.28) | 0 | 0.60 | 0.96 (0.72-1.28) | 0 | 0.60 | N/A | N/A | N/A |
CI: Confidence interval, N/A: Not available, RoB: Risk of bias, ROBINS-I: Risk Of Bias In Nonrandomized Studies of Interventions, SIRS: Systemic inflammatory response syndrome
Publication bias of include studies
There was no obvious publication bias as shown by a symmetric funnel plot [Supplement Appendix Figure S3]. Moreover, Begg's and Egger's test exhibited no significant difference (P = 0.624 and P = 0.777, respectively). Egger's test is shown in Supplement Appendix Figure S4.
Quality of evidence
The quality of evidence of the RCT and cohort studies included for meta-analysis is shown in Table 3. Randomized studies without significant limitations are considered high on the GRADE scale. All included RCT studies were rated as low risk of bias. In addition to low heterogeneity, it was estimated that the intervention, population, and outcome measurement were similar among the included RCT studies. Level of certainty for risk of bias, inconsistency, and indirectness were considered not serious. Since the CI displayed a probable benefit from intervention and comparator approaches, we consequently decided to lower the rate for imprecision.
Table 3.
The quality of evidence of the randomized controlled trial and cohort studies included for meta-analysis
| Outcome | Quality assessment |
Number of patients |
Relative effect (95% CI) | Quality | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Number of studies | Study design | Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerations | Thiamine (%) | Non-thiamine (%) | |||
| Mortality | 3 | RCT | Not seriousa | Not seriousb | Not seriousc | Seriousd | Nonee | 45/106 (42.5) | 52/117 (44.4) | RR 0.96 (0.72-1.28) | ⊕⊕⊕◯Moderate |
| 2 | Cohort | Seriousf | Seriousg | Serioush | Seriousd | Nonee | 78/157 (49.7) | 153/265 (57.7) | RR 0.74 (0.47-1.19) | ⊕◯◯◯Very low | |
aTrials were low risk of bias, bInconsistency explained by I2 value as 0%; low heterogeneity; not serious, cThe intervention, population, and outcome measured were similar in included studies, dThe confidence interval includes possible benefit from intervention and comparator approaches, eNone: Publication bias is not likely; no large effect and dose response gradient; no plausible confounding, fThe include trials were moderate risk of bias, gInconsistency explained by I2 value as 74%; moderate heterogeneity; serious, hThe interventions were delivered in different dose and the dosage form of thiamine varied among these studies. RCT: Randomized control trial, CI: Confidence interval, RR: Risk ratio
Cohort studies without major limitations are rated low on the GRADE scale. All of the included cohort studies were found to have moderate risks of bias. Since the included cohort studies were noted to have moderate heterogeneity, we decided to rate down for the risk of bias and inconsistency. The interventions given in the included cohort studies were inconsistent in dosage regimen and dosage form. Hence, we downgraded the rating of indirectness. According to the CI which represented the possible benefit from intervention and comparator approaches, imprecision was considered serious.
DISCUSSION
To the best of our knowledge, this is the first systematic review and meta-analysis to appraise the effectiveness of thiamine therapy in mortality rate in patients with septic shock. Although not statistically significant, our study found that thiamine therapy has a trend to reduce mortality rate in septic shock patients. It has been proposed that thiamine therapy could likely benefit patients with septic shock since thiamine is an essential cofactor in cellular metabolism. In animal models, thiamine improved hemodynamic stability regardless of the status of thiamine deficiency.[28] In a cardiac arrest mouse model, thiamine improved mitochondrial function as measured by cellular oxygen consumption, restored cerebral pyruvate dehydrogenase activity, decreased histologic signs of brain injury, and improved neurologic outcomes.[29]
The prevalence of thiamine deficiency among critically ill patients varies substantially. Among patients with sepsis, thiamine deficiency was reported in a range from 10% to 70%. The risk of thiamine deficiency was considered higher in patients with a history of alcoholism, DM, and gastric/intestinal resection.[30] Our subgroup analysis found that thiamine was more effective than comparators in lowering mortality rate if patients had a history of alcohol intake. It has been long established that inadequate nutritional intake associated with alcohol consumption can lead to reduced absorption of thiamine from the gastrointestinal tract, decreased uptake into cells, and reduced utilization of thiamine within the cell.[30] Thiamine is known to play an essential role in the pentose phosphate pathway and other key metabolic processes. Thiamine deficiency, one of the known causes of lactic acidosis, will slow the change of pyruvate to Acetyl-CoA pathway which leads to lactate level accumulation. In more recent years, thiamine has also been estimated as a potential contributor to refractory lactic acidosis and organ injury in septic shock and other shock states. To improve survival rate, it is important to recognize sign and symptoms of thiamine deficiency early. If treatment was not initiated promptly and appropriately, it could lead to multiple organ failure and death.[1]
Strengths and limitations
The strengths of this study should be highlighted. Our study is the first meta-analysis addressing the relationship between thiamine therapy and mortality rate in patients with septic shock. An exhaustive literature search using eight reputable peer-reviewed databases was performed. Our search method included all published articles internationally. During screening process, particular attention was paid to include all articles that met inclusion criteria. Missing information was directly requested from the investigators. Articles were excluded if data were deemed incomplete. Meeting articles were then carefully extracted, analyzed, and reported in accordance with the PRISMA statement.
This study still has some limitations. As thiamine administration in septic shock patients is considered a relatively new concept, the published studies for our consideration were limited. Despite extensive literature research, our systematic review and meta-analysis was only able to include 5 studies that met criteria, thus leading to small sample size. We also decided to include observational studies in our analysis but only after risk of bias was assessed and deemed low. Heterogeneity of observational studies was tested and deemed moderate. The funnel plot has shown asymmetric graph likely secondary to a small number of studies included in our study. It is important to note that, after using Begg's and Egger's test, we found no evidence of publication bias. It is important to note that heterogeneity could occur if septic shock patients from the included studies were defined differently. While it could be a limitation of this meta-analysis, we performed subgroup analysis and discovered that the effects of thiamine on mortality were similar and consistent despite the difference in the definition.
During the screening process, we found studies that discussed relationship between thiamine treatment in septic shock patients and mortality rate, but we had to exclude those studies from our final evaluation. Of these, one study excluded patients with thiamine deficiency including refeeding syndrome, beriberi, and Wernicke encephalopathy. The investigators of this study also used risk difference as outcome measurement. RR was not reported.[31] Therefore, this study was excluded from our consideration. Another study reported that thiamine deficiency in sepsis may be associated with ICU mortality.[32] The investigators reported OR of 0.98 (95% CI, 0.96–1.00) for thiamine group. However, after careful review, we found that this study did not meet our inclusion criteria because thiamine was not given to the patients. In addition, there was no comparators. This study aimed to assess thiamine level using blood sample; therefore, it was excluded from our analysis.
While this meta-analysis did not include a librarian during the literature search, the authors involved in this process were trained and skilled in the principle of search methods for systematic reviews. We have carefully selected database to search, choose the appropriate keywords and MeSH term, build search strategies, and organize citations to ensure the appropriateness of the obtained record. The eligible title, abstracts, and full text were screened by two independent reviewers to ensure that the inclusion criteria were met. In addition, we contacted the corresponding authors when information of the outcome was missing. If the corresponding author did not respond to our inquiry, the article was excluded from our analysis.
Suggestion for practice and future research
Upon literature review, we are in agreement with the previous suggestion to consider thiamine use in patients with septic shock. The administration of thiamine is beneficial in septic shock patients with severe thiamine deficiency (thiamine level ≤7 nmol/L). The dose of thiamine varies and is often left to the discretion of the physicians. The usual dose for adults is 100 mg, given once daily for patients with mild thiamine deficiency. Thiamine dose could be titrated to 100 mg, given two or three times daily in severe thiamine deficiency. The most commonly used regimen in clinical setting for septic shock is 200 mg intravenous given every 12 h for 72 h.[23,24,25,33] Despite the suggestion of thiamine use as noted above, the exact mechanism and benefit of thiamine in clinical setting is unknown at this time. Our study found that there was a trend that suggested that thiamine may reduce mortality rate in patients with septic shock. However, our findings were not statistically significant. It would be interesting to examine further if the impact of thiamine observed in patients with septic shock is similar or different if patients were also diagnosed with thiamine deficiency. It would be beneficial to conduct further studies to evaluate lactate level, time to hospitalization, thiamine dosage regimen, as well as ICU free days in this population. Further prospective studies with larger sample size are warranted.
CONCLUSIONS
Although not statistically significant, our study found that there was a trend that suggested that thiamine may reduce mortality rate in patients with septic shock. Further prospective studies with larger sample size are warranted.
Research quality and ethics statement
The systematic review or meta-analysis is exempt from ethics approval because it collecting and synthesizing data from the previous studies. In addition, patient data are anonymized and data are available in the public domain so that ethical permission is not needed. The authors followed applicable EQUATOR Network (https://www. equator-network. org) guidelines during the conduct of research project.
Financial support and sponsorship
This work was supported by a grant from the Unit of Excellence on Clinical Outcomes Research and IntegratioN (UNICORN) (Grant number: FF64-UoE003), School of Pharmaceutical Sciences, University of Phayao. The funding source had no role in the study design, collection, analysis, and interpretation of data.
Conflicts of interest
There are no conflicts of interest.
Acknowledgement
We thank Miss Kanyanut Chongsub and Miss Kannika Chumpiw for the technical support in the construction of the figures.
REFERENCES
- 1.Hotchkiss RS, Moldawer LL, Opal SM, Reinhart K, Turnbull IR, Vincent JL. Sepsis and septic shock. Nat Rev Dis Primers. 2016;2:16045. doi: 10.1038/nrdp.2016.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization. Report on the Burden of Endemic Health Care-Associated Infection Worldwide. WHO Libr Cat Data. 2011. [Last accessed on 2020 Sep 01]. Available from: https://apps.who.int/iris/handle/10665/80135 .
- 3.Rhee C, Jones TM, Hamad Y, Pande A, Varon J, O'Brien C, et al. Prevalence, underlying causes, and preventability of sepsis – Associated mortality in US acute care hospitals. JAMA Netw Open. 2019;2:e187571. doi: 10.1001/jamanetworkopen.2018.7571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Donnino MW, Carney E, Cocchi MN, Barbash I, Chase M, Joyce N, et al. Thiamine deficiency in critically ill patients with sepsis. J Crit Care. 2010;25:576–81. doi: 10.1016/j.jcrc.2010.03.003. [DOI] [PubMed] [Google Scholar]
- 5.Garcia-Alvarez M, Marik P, Bellomo R. Sepsis-associated hyperlactatemia. Crit Care. 2014;18:503. doi: 10.1186/s13054-014-0503-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Manzanares W, Hardy G. Thiamine supplementation in the critically ill. Curr Opin Clin Nutr Metab Care. 2011;14:610–7. doi: 10.1097/MCO.0b013e32834b8911. [DOI] [PubMed] [Google Scholar]
- 7.Costa NA, Gut AL, de Souza Dorna M, Pimentel JA, Cozzolino SM, Azevedo PS, et al. Serum thiamine concentration and oxidative stress as predictors of mortality in patients with septic shock. J Crit Care. 2014;29:249–52. doi: 10.1016/j.jcrc.2013.12.004. [DOI] [PubMed] [Google Scholar]
- 8.Stewart LA, Clarke M, Rovers M, Riley RD, Simmonds M, Stewart G, et al. Preferred reporting items for systematic review and meta-analyses of individual participant data: The PRISMA-IPD statement. 2015;313:1657–65. doi: 10.1001/jama.2015.3656. [DOI] [PubMed] [Google Scholar]
- 9.Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, Opal SM, et al. Surviving sepsis campaign: International guidelines for management of severe sepsis and septic shock: 2012. Crit Care Med. 2013;41:580–637. doi: 10.1097/CCM.0b013e31827e83af. [DOI] [PubMed] [Google Scholar]
- 10.Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3) JAMA. 2016;315:801–10. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The cochrane collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sterne JA, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M, et al. ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355:i4919. doi: 10.1136/bmj.i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Atkins D, Best D, Briss PA, Eccles M, Falck-Ytter Y, Flottorp S, et al. Grading quality of evidence and strength of recommendations. BMJ. 2004;328:1490. doi: 10.1136/bmj.328.7454.1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al. GRADE: An emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336:924–6. doi: 10.1136/bmj.39489.470347.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–88. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 16.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–60. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Melsen WG, Bootsma MC, Rovers MM, Bonten MJ. The effects of clinical and statistical heterogeneity on the predictive values of results from meta-analyses. Clin Microbiol Infect. 2014;20:123–9. doi: 10.1111/1469-0691.12494. [DOI] [PubMed] [Google Scholar]
- 18.Lin L, Chu H, Hodges JS. Alternative measures of between-study heterogeneity in meta-analysis: Reducing the impact of outlying studies. Biometrics. 2017;73:156–66. doi: 10.1111/biom.12543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sterne JA, Egger M. Funnel plots for detecting bias in meta-analysis: Guidelines on choice of axis. J Clin Epidemiol. 2001;54:1046–55. doi: 10.1016/s0895-4356(01)00377-8. [DOI] [PubMed] [Google Scholar]
- 20.Begg CB, Berlin JA. Publication bias and dissemination of clinical research. J Natl Cancer Inst. 1989;81:107–15. doi: 10.1093/jnci/81.2.107. [DOI] [PubMed] [Google Scholar]
- 21.Egger M, Smith GD. Bias in location and selection of studies. BMJ. 1998;316:61–6. doi: 10.1136/bmj.316.7124.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jin ZC, Wu C, Zhou XH, He J. A modified regression method to test publication bias in meta-analyses with binary outcomes. BMC Med Res Methodol. 2014;14:132. doi: 10.1186/1471-2288-14-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Donnino MW, Andersen LW, Chase M, Berg KM, Tidswell M, Giberson T, et al. Randomized, double-blind, placebo-controlled trial of thiamine as a metabolic resuscitator in septic shock: A pilot study. Crit Care Med. 2016;44:360–7. doi: 10.1097/CCM.0000000000001572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Harun NF, Cheah SK, Yusof AM, Lau CL, Masdar A, Mahdi SN, et al. Intravenous thiamine as an adjuvant therapy for hyperlactatemia in septic shock patients. Crit Care Shock. 2019;22:288–98. [Google Scholar]
- 25.Moskowitz A, Andersen LW, Cocchi MN, Karlsson M, Patel PV, Donnino MW. Thiamine as a renal protective agent in septic shock. A secondary analysis of a randomized, double-blind, placebo-controlled trial. Ann Am Thorac Soc. 2017;14:737–41. doi: 10.1513/AnnalsATS.201608-656BC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Holmberg MJ, Moskowitz A, Patel PV, Grossestreuer AV, Uber A, Stankovic N, et al. Thiamine in septic shock patients with alcohol use disorders: An observational pilot study. J Crit Care. 2018;43:61–4. doi: 10.1016/j.jcrc.2017.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Woolum JA, Abner EL, Kelly A, Thompson Bastin ML, Morris PE, Flannery AH. Effect of thiamine administration on lactate clearance and mortality in patients with septic shock. Crit Care Med. 2018;46:1747–52. doi: 10.1097/CCM.0000000000003311. [DOI] [PubMed] [Google Scholar]
- 28.Lindenbaum GA, Larrieu AJ, Carroll SF, Kapusnick RA. Effect of cocarboxylase in dogs subjected to experimental septic shock. Crit Care Med. 1989;17:1036–40. doi: 10.1097/00003246-198910000-00014. [DOI] [PubMed] [Google Scholar]
- 29.Moskowitz A, Donnino MW. Thiamine (vitamin B1) in septic shock: A targeted therapy. J Thorac Dis. 2020;12:S78–83. doi: 10.21037/jtd.2019.12.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Martin PR, Singleton CK, Hiller-Sturmhöfel S. The role of thiamine deficiency in alcoholic brain disease. Alcohol Res Health. 2003;27:134–42. [PMC free article] [PubMed] [Google Scholar]
- 31.Miyamoto Y, Aso S, Iwagami M, Yasunaga H, Matsui H, Fushimi K, et al. Association between IV thiamine and mortality in patients with septic shock: A nationwide observational study. Crit Care Med. 2020;48:1135–9. doi: 10.1097/CCM.0000000000004394. [DOI] [PubMed] [Google Scholar]
- 32.Heming N, Salah A, Meng P, Sivanandamoorthy S, Bounab R, Chevret S, et al. Thiamine status and lactate concentration in sepsis: A prospective observational study. Medicine (Baltimore) 2020;99:e18894. doi: 10.1097/MD.0000000000018894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Marik PE, Khangoora V, Rivera R, Hooper MH, Catravas J. Hydrocortisone, vitamin c, and thiamine for the treatment of severe sepsis and septic shock: A retrospective before-after study. Chest. 2017;151:1229–38. doi: 10.1016/j.chest.2016.11.036. [DOI] [PubMed] [Google Scholar]