Abstract
The epidemic of opioid use disorder (OUD) directly affects millions of women of child-bearing age. Unfortunately, parenting behaviors – among the most important processes for human survival – are vulnerable to the effects of OUD. The standard of care for pregnant women with OUD is opioid maintenance therapy (OMT), of which the primary objective is to mitigate addiction-related stress. The aim of this review is to synthesize current information specific to pregnancy and parenting that may be affected by OUD. We first summarize a model of the parental brain supported by animal research and human neuroimaging. We then review animal models of exogenous opioid effects on parental brain and behavior. We also present preliminary data for a unifying hypothesis that may link different effects of exogenous opioids on parenting across species and in the context of OMT. Finally, we discuss future directions that may inform research and clinical decision making for peripartum women with OUD.
Keywords: Opioid, Maternal brain, Neuroimaging, Hypothalamus, Periaqueductal gray, Animal models
1. Introduction to the parental brain
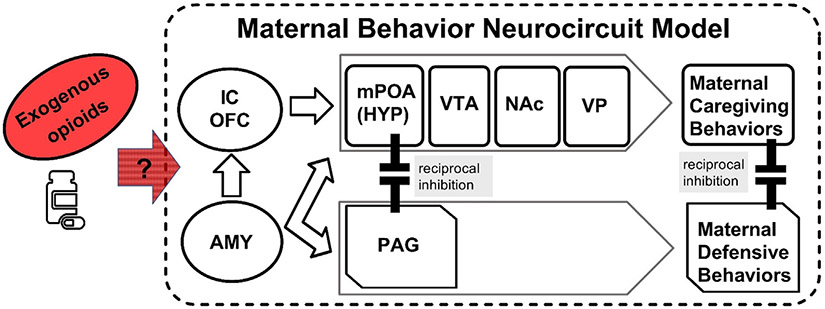
It has been established in animal (Numan and Young, 2016) and human (Swain and Ho, 2017) brain research that parenting involves an evolutionarily conserved Maternal Behavior Neurocircuit (MBN) under neuroendocrine control and opioid influence (Numan and Young, 2016; Brunton et al., 2008) (see Fig. 1).
Fig. 1.
The maternal behavior neurocircuit - a framework to consider the effects of exogenous opioids. Maternal behaviors may be activated by the amygdala (AMY). Insular cortex (IC), orbitofrontal cortex (OFC), medial preoptic area (mPOA) of the hypothalamus (HYP), ventral tegmental area (VTA), nucleus accumbens (NAc) and ventral pallidum (VP) activate caregiving. behaviors. Periaqueductal gray (PAG) activates defensive/aggressive behaviors. Sensitive maternal behavior requires that caregiving and defense processing reciprocally inhibit each other. Exogenous opioids, which affect multiple parts of the maternal brain, require further study in animals. We present preliminary data.on the effects of exogenous opioids on human mothers below in 4.3.
1.1. Parental brain in health
Healthy parenting is governed by the MBN with two reciprocally modulating subsystems that either activate maternal caregiving behaviors solicited by the offspring (care processing) or defensive and aggressive behaviors when the mother or offspring is threatened (defense processing). The former “care” subsystem consists of the medial preoptic area (mPOA) in hypothalamus (HYP), ventral tegmental area (VTA), nucleus accumbens (NAc) and ventral pallidum (VP), which activate sensitive maternal care behaviors across species (Lonstein et al., 2015). Examples of maternal care processing behaviors for rats include nursing, licking, grooming and characteristic vocalizations (Bayerl and Bosch, 2019). Among humans, sensitive parenting includes attuned interactions with appropriate tone of voice and resourcefulness in dealing with infant negative states in order to sustain infant life and optimize development (Swain and Ho, 2017; Bornstein et al., 2017). In the latter defense processing subsystem, which includes the periaqueductal gray (PAG), regulation of different opioid receptors in rodents can activate maternal “defense” or aggressive behaviors, such as hostility towards intruders or predatory behaviors in rats (Klein et al., 2014). The PAG is less-well studied in humans (preliminary data presented below in Section 4.3). These opposing care and defense behaviors must be reciprocally modulated to regulate sensitive maternal behavior according to contextual demands. The brain orchestrates these behaviors with the amygdala (AMY), insular cortex (IC), and orbitofrontal cortex (OFC) (Stolzenberg and Numan, 2011) and neuromodulators such as oxytocin and cortisol (Klampfl and Bosch, 2019).
Indeed, the healthy maternal brain undergoes tremendous neuroendocrinological adaptations in response to peripartum circumstances, including rising levels of progesterone, estrogens and modulation of oxytocin, endogenous opioids and stress-regulation hormones such as cortisol (Brunton et al., 2008; Feldman and Bakermans-Kranenburg, 2017; Swain et al., 2011; Brunton and Russell, 2008). For example, for virgin rodents, pups usually constitute an aversive stimulus that does not immediately induce care-giving behaviors. This aversion, particularly to the neonate’s odor, is mediated by olfactory bulb projections to the amygdala and from there to the anterior hypothalamus and PAG. In fact, maternal care-giving behaviors are not commonly present until late-pregnancy hormones induce changes in the MBN, including downregulation of pup-aversion projections to the amygdala and upregulating reward processing of pup cues. Rising levels and receptor densities of estrogens, progesterone, prolactin and oxytocin activate the hypothalamic mPOA and the adjacent ventral bed nucleus of stria terminalis (vBNST), overriding aversive reactions to pups and their odor that is present during early gestation. With this activation, glutamatergic projection from the mPOA and vBNST start to stimulate dopaminergic neurons in the VTA, which in turn increases release of dopamine in the NAc such that pup-specific stimuli (their odor, suckling behavior and touch) become rewarding and thus promote maternal care behaviors during the postpartum.
Among healthy human mothers, differential activation of the MBN has been shown to be a function of maternal sensitivity - the specific ability to perceive and respond to their infant’s behavioral signals (Elmadih et al., 2016). In this study, brain activation to infant cues was studied in 15 mothers with high sensitivity (HSMs) and 15 mothers with low sensitivity (LSMs). Mothers were selectively recruited from a pool based on mother–infant play interaction at 4–6 months postpartum. Brain responses to viewing silent videos of their “own” versus an “unknown” infant in 3 affective states (neutral, happy, and sad) were measured at 7–9 months postpartum. The participants' plasma oxytocin was measured immediately following free-play interactions with their infant. HSMs versus LSMs showed significantly greater brain activation in right superior temporal gyrus (STG) in response to own versus unknown neutral infant and to own-happy vs. own-neutral. Activation in the right STG in the latter contrast was negatively correlated with post-free-play oxytocin responses in HSMs mothers. By way of interpretation, the enhanced activation of STG for HSM mothers may indicate increased emotion regulation processing for HSM mothers – in accord with another report of STG activation to videos of own versus unknown infants among healthy mothers (Wan et al., 2014) and among synchronous vs. intrusive mothers (Atzil et al., 2011). Indeed, the STG has been widely implicated in the regulation of emotion, particularly facial emotion processing, and in empathizing with others “Theory of Mind” (Rizzolatti and Fabbri-Destro, 2008) – both facilities key for a mother to differentially express sensitivity to her infant’s needs over other demands on her resources (Strathearn et al., 2012). Other studies have also reported greater activation of STG in mothers who delivered vaginally compared with Caesarean section (Swain et al., 2008), for breastfeeding compared with bottle feeding mothers (Kim et al., 2011), and finally in accord with STG role in the intention to move and provide maternal care (Bornstein et al., 2017; Zebardast et al., 2013). The inversed correlation of post-free-play oxytocin and brain responses in HSMs mothers hints at the complex role for oxytocin – which may increase parent-child prosocial interaction under normal conditions, yet may also interfere through anxiety or erroneous recollection of maternal closeness and care during childhood with early life adversity (Szymanska et al., 2017; Bakermans-Kranenburg and Van, 2013). In sum, these data suggest that there are variations in MBN activation that correlate with hormone levels and different parenting phenotypes among healthy populations according to stress (Kim et al., 2016; Mayes et al., 2005; Leckman et al., 2004). We proceed with consideration of early life adversity and frank psychopathology in the parental brain.
1.2. Parental brain in psychopathology
Unsurprisingly, activation of the MBN in humans is highly sensitive to severe environmental stress, psychopathology, and early life adversity. For instance, the chronic stress of childhood poverty, well established to interfere with normal brain physiology for emotion response and executive function (Duval et al., 2017; Evans et al., 2016; Javanbakht et al., 2016; Javanbakht et al., 2015; Liberzon et al., 2015; Sripada et al., 2014; Sripada et al., 2014), has also been shown to impact parental MBN function – interestingly, in a sex-specific way (Kim et al., 2015). Among females' activations to salient own infant cry (Swain et al., 2004) with childhood poverty was associated with increased neural in the posterior insula, striatum, calcarine sulcus, hippocampus and fusiform gyrus, but with decreased neural responses to own infant cry in the same regions in males. Furthermore, neural activation in these regions was associated with higher levels of perceived annoyance elicited by infant cries and reduced motivation to approach crying infants regardless of the gender of the participants (Kim et al., 2015). These results are surprising given that the striatum is associated positively with motivation and reward (Lee and Reeve, 2017). It may be that chronic poverty has adverse effects yet also induces compensatory changes – essentially reprogramming some circuits – especially for these non-parent participants without the experience of becoming a parent. Indeed, in a related study on mothers (Kim et al., 2016), lower income was associated with reduced responses to infant cry only in cortical brain circuits, including those that evaluate emotional valence (medial prefrontal gyrus), regulate affect (middle prefrontal gyrus) and process sensory information (superior temporal gyrus). Furthermore, lower positive perceptions of parenting were associated with reductions in infant-cry response in the right middle frontal gyrus and superior temporal gyrus. Perhaps some poverty effects are themselves a function of the adaptation to becoming a parent.
Maternal depression is a serious mental disorder that affects approximately 12–20% of new mothers. From a recent review (Pawluski et al., 2017), brain activation patterns that are affected by depression and anxiety include cortical (dmPFC, dlPFC, IFG, SFG, OFC, STG), ACC, PCC) and subcortical regions (striatum, thalamus, hippocampus, SN, VTA, PAG) – highlighting the amygdala which is differentially active according to critically important experimental specifics. For example, it has been shown that depressed mother’s amygdala may be hypo-responsive to certain standard cognitive neuroimaging challenges (Moses-Kolko et al., 2014) and that mothers with chronic unresolved attachment trauma exhibit dampened amygdala responses to viewing their own (but not unknown) infant’s crying faces (Kim et al., 2014). Amygdala responses have also been positively correlated with maternal attribution of intentionality to their infant in response to own versus other infant cry (Hipwell et al., 2015). On the other hand, depressed compared to healthy mothers displayed greater reactivity of the right amygdala using a child face empathy task (Lenzi et al., 2016) – perhaps indicating emotional dysregulation in this task. Amygdala reactivity was also increased in a self-focused baby-cry task designed to provoke brain responses in participants with a history of adverse early life experiences sometimes described as a malevolent background “shark music” (Ho and Swain, 2017). This study demonstrated depression effects on amygdala connectivity, which also seem to change with anxiety (Guo et al., 2018). Variance in the properties of infant stimuli and context of presentation, along with research using hormone challenges will be helpful to clarify the role of the amygdala in depression – especially given that often-used depression measures may not perfectly capture real-life parental dysfunction. For example, intranasal oxytocin effects on amygdala response to infant crying were moderated by attachment security of the mothers, with oxytocin decreasing emotional and amygdala reactivity only in mothers with insecure attachment representations (Riem et al., 2016). Thus, parents with insecure attachment – perhaps different from other attachment classifications, may have different brain mechanisms that render them amenable to oxytocin interventions. Such future interventions may apply to many stress-related syndromes. However, more work using both animal and human models is needed to confirm findings over the complete range of hormone physiology and neuroanatomy that relate to sensitive parenting behaviors and mentalizing for applications to better understand and safely treat parental psychopathology.
2. Opioids use disorder and treatment during pregnancy
Opioid use disorder (OUD) has reached epidemic proportions in the United States recently and opioid dependence among pregnant women has more than doubled in the last 10 years. Approximately one-third of all women of reproductive age have filled a prescription for an opioid medication in the previous year, suggesting that affected women may be on opioid medication when they conceive. Indeed, the opioid epidemic in the U.S. affects millions of women – with about 20% of all pregnant women prescribed opioids and 2.5% chronically using (Krans and Patrick, 2016), and the number of pregnant women with OUD more than quadrupled from 1999 to 2014 (from 1.5 per 1000 delivery hospitalizations to 6.5) (Haight et al., 2018). In addition, many pregnant women with OUD are among those estimated to also use other drugs, licit drugs that are known to be harmful during pregnancy, alcohol and tobacco (Substance Abuse and Mental Health Services Administration, 2014; Hayes and Brown, 2012; Forray, 2016). Finally, neonatal abstinence syndrome (NAS), the signs and symptoms from the cessation of prenatal exposure (via placental transfer) to various substances – especially if untreated – can be associated with infant morbidity and unclear long-term consequences of opioid exposure – currently a subject of ongoing study (Coyle et al., 2018; Johnson and Jones, 2018; Kaltenbach et al., 2018).
Since the publication of the NIDA-funded Maternal Opioid Treatment: Human Experimental Research (MOTHER) study in 2010 (Jones et al., 2010) the use of Buprenorphine Treatment (BT) as a way of alleviating withdrawal symptoms has become the gold standard opioid maintenance therapy (OMT) (Jones et al., 2017; Nanda et al., 2015; Krans et al., 2016; Zedler et al., 2016) – partly due to reports of milder and more treatable withdrawal and NAS (Jones et al., 2010; Zedler et al., 2016; Cleary et al., 2011; Unger et al., 2011; Fischer et al., 2006; Kayemba-Kay's and Laclyde, 2003; Schindler et al., 2003; Jones et al., 2005; Laslo et al., 2017). For minimizing fetal stress, there are clear advantages to BT compared with detoxification (Whitten, 2012; Kakko et al., 2008), and for BT compared with methadone treatment (Johnson and Martin, 2017; Meyer et al., 2015; Gaalema et al., 2012; Hall et al., 2016; Mucke et al., 2017; Lemon et al., 2017; Brogly et al., 2014) although findings are controversial (Brogly et al., 2014; Lund et al., 2012; Jones et al., 2012) and BT is not without risks. For example, recent findings maintain that BT maternal buprenorphine dose is indeed associated with NAS symptoms (Velez et al., 2018) as well as lower birth weight and length over and above substance use severity (Jansson et al., 2017). Findings from our own research indicated that 89% of infants prenatally exposed to buprenorphine were diagnosed with NAS and 36% were admitted to the NICU.
Despite increased levels of stress due to parenthood-related psychosocial, physiological and economical demands (Barclay et al., 1997), parents typically find themselves highly motivated to take care of their offspring’s needs and find the interactions with their infants to be rewarding (Mercer, 1985; Goodman, 2002). Notably, the adaptation of brain stress systems is integral to such parenting behaviors. While the first few months postpartum are especially stressful for mothers affected by OUD, very little is known about the effects of BT on postpartum women with OUD. BT does help to break the repetitive cycle of quit attempts and hence, to some extent, desensitize core stress systems. Nevertheless, at least one in three mothers receiving BT still suffer relapse to illicit opioids and other harmful substances (Jones et al., 2012). Further, aspects of buprenorphine exposure, including dosing parameters, withdrawal stress pertaining to induction as well as pre- and post-natal effects (Jones et al., 2008) are also not as well understood compared with methadone treatment (Kaltenbach et al., 2012). In addition, there are theoretical concerns regarding the unique pharmacokinetic and dynamic properties of buprenorphine that may predispose higher occurrence of NAS (Kennedy-Hendricks et al., 2016) due to elevated dosing needed to offset higher clearance rates (Kacinko et al., 2009) and accelerated maternal metabolism during pregnancy (Welsh and Valadez-Meltzer, 2005). Although BT should reduce the risks of exposing fetus to repeated cycles of acute withdrawal (World Health Organization, 2014), a systematic risk/benefit assessment of BT is still required in order to accurately inform optimal parameters of use in treating pregnant women with OUD. To date, a central challenge pertaining to risk/benefit assessment has arisen from the fact that buprenorphine maintenance exposure has been difficult to quantify, because variables commonly affecting intra-uterine stability and postnatal environment have been difficult to account for. These include, but are not limited to, severity and pattern of maternal licit, illicit and prescription drug use, as well as maternal socio-economic and environmental factors. These problems have made it challenging to fully determine the extent to which neurodevelopmental risk from greater NAS severity is offset by the more stable intrauterine environment.
There is also a dearth of knowledge about the optimal timing for switching to a maintenance opioid during pregnancy and whether the switch or opioid withdrawal during certain sensitive periods may have a negative impact for the mother and/or her offspring. Finally, the prevalence of relapse with BT may be even higher during the first few months postpartum when additional parenting stresses of childcare are salient, such as having an infant go through NAS. As such, it is essential to better understand how BT may mediate stress system adaptations and motivation for opioid use during early postpartum periods for mothers with OUD.
In the current paper we propose that the MBN provides an effective mechanistic model which is applicable to early postpartum parental adaptation and accounts for the common neural circuitry underlying maternal behavior, stress system dysregulation and relapse to illicit opioids in BT mothers. Given that the motivation for opioid use and relapse have been linked to dysregulated stress system neurocircuits (Koob and Volkow, 2016), which are also integral to parenting behavior, the utility of this model may help to identify overlapping mechanisms relating to the impact of BT upon parenting. The importance of addressing this knowledge gap is magnified by risks to the infants of BT mothers who relapse to illicit drug use.
Addressing this gap is challenging because of the common co-morbidities of depression (Conway et al., 2006; Davis et al., 2017) and polysubstance use (Substance Abuse and Mental Health Services Administration, 2009) that may mask the effects of BT in OUD discussed above. Postpartum depression has a prevalence of 15–20% in general population (Gavin et al., 2005; Gaynes et al., 2005; O'Hara and McCabe, 2013), adversely affecting infants – even for subthreshold symptoms (Murray and Cooper, 1997; Righetti-Veltema et al., 2003; McLearn et al., 2006) – with long-term adverse effects on emotion and behavior regulation (Hay et al., 2008; Grace et al., 2003; Halligan et al., 2007; Goodman et al., 2011). Furthermore, maternal substance use places their infants at fourfold increased risk for abuse or neglect, contributing to as much as 80% of child maltreatment cases (Barth, 2009) and 60% of infant out-of-home placements (Child Welfare Information Gateway, 2014). Indeed, even during intermittent periods of sobriety, mothers with addiction are observed to be less keenly attentive and contingently responsive, while more intrusive and hostile toward their infants (Strathearn and Mayes, 2010) and may find infant cues to be less gratifying and more stressful, as compared to non-addicted mothers (Rutherford et al., 2011; Rutherford and Mayes, 2017; Kim et al., 2017). Additionally, maternal behaviors can be adversely influenced by infantile NAS that can still occur with BT (Jones and Fielder, 2015).
Relapse in BT mothers to illicit drug use is another significant concern. For women through pregnancy and the postpartum with OUD, co-occurring anxiety, mood and other substance use disorders, guilt, poor nutrition, impaired health, homelessness, violence, confusion regarding available medical options, and a lack of social support are more prevalent (Johnson and Jones, 2018; Young and Martin, 2012; McCarthy et al., 2017; Bishop et al., 2017; Jansson et al., 1996). From a neuroendocrine perspective, such chronic stressors alongside the cyclical use of short-term opioids and other drugs, reciprocally sensitize neurohormonal stress systems (Rutherford et al., 2011; Landi et al., 2011). This, in turn, may lead to further use of opioids and other drugs, worsening dysphoria, negative affect, anxiety and the relapse cycle (Koob, 2017a, 2017b; Hyman et al., 2007). Research on non-pregnant substance abusers has shown that stress system sensitivity is a key predictor of stress response dysregulation, elevated anxiety and depressive symptomatology as well as craving and relapse to various drugs of abuse during attempts at abstinence (Fox et al., 2008; Fox et al., 2007; Sinha et al., 2011; Sinha et al., 2006). Given a stress-sensitized and negatively reinforcing environment, the additional parenting stress (Suchman and Luthar, 2001) and the frequently rapid metabolism of opioids that characterize pregnancy (McCarthy et al., 2017), it is not surprising that OUD in peripartum women is characterized by numerous failed quit attempts (Guille et al., 2017). Indeed, In the MOTHER Study (Maternal Opioid Treatment: Human Experimental Research) (Jones et al., 2012), 33% of BT mothers relapsed to illicit opioids (Jones et al., 2012). This is likely driven by stress system pathophysiology and repeated cycles of opioid intoxication and acute withdrawal.
BT improves both maternal and fetal outcomes by limiting psychobiological changes associated with withdrawal (Klaman et al., 2017). The first few months postpartum for BT mothers are nevertheless fraught with depressive stress symptoms commonly comorbid with drug dependency (Oei et al., 2009). Indeed, pilot data from our study site suggest ~50% prevalence of significant depression in BT mothers (EPDS > 10). Finally, the children of affected mothers are vulnerable to long-term effects of early maternal depressive stress (Murray and Cooper, 1997; Righetti-Veltema et al., 2003; McLearn et al., 2006; Hay et al., 2008; Grace et al., 2003; Halligan et al., 2007; Goodman et al., 2011). Thus, early postpartum comorbid maternal opioid use and stress disorders can exert transgenerational consequences – likely through maladaptive maternal parenting behaviors (Stanley et al., 2004; Feldman et al., 2009; Field et al., 1988; Murray and Cooper, 1997; Weissman et al., 2004). For mothers with OUD, adaptative parenting behaviors depend on their perceived parenting stress (Suchman and Luthar, 2001), which depends on how well they can emotional attune to the child’s psychological needs (Borelli et al., 2012).
3. Animal research on the parental brain and opioids
3.1. Effects of opioids on the maternal brain and behavior
It is well known that gestational opioid exposure results in alterations in opioid receptor binding and functionality in dams during pregnancy and the early postpartum (Hou et al., 2004; Darmani et al., 1992), however only a few comparative studies have investigated the effects of opioids on a parental brain compared to a virgin or non-postpartum brain. Though changes in pharmacokinetics and thus plasma and tissue distribution of opioids are well established during pregnancy (Shah et al., 1976), the effects of these changes on the maternal brain are not well documented. In one study, pregnant mice had 96% lower brain-to plasma ratios of methadone after a single dose compared to non-pregnant mice, but levels of buprenorphine in brain or plasma were no different between pregnant and non-pregnant animals (Coles et al., 2009). To further complicate our understanding of the effects of opioids on the maternal brain, many of the early toxicology studies were performed in male animals only and there is accumulating evidence of substantial sex difference in opioid pharmacology (Rasakham and Liu-Chen, 2011; Dahan et al., 2008).
Additionally, opioids are well established to be involved in the healthy regulation of maternal behavior and to play an essential role in mediating some of the processes involved in the initiation of maternal care through the activation of the MBN (Brunton and Russell, 2008; Bridges and Grimm, 1982; Felicio et al., 1991; Grimm and Bridges, 1983; Moura et al., 2010). Endorphins exhibit an inhibitory effect on oxytocin and prolactin-releasing neurons in preparation for parturition, that allows an accumulation and then surge of these hormones during birth. This withdrawal of the inhibitory control of the opioid system is believed to be crucial for facilitating rapid initiation of maternal behaviors at birth (Byrnes et al., 2000). Further; this system is also involved in opioid-regulated switching from maternal to predatory behaviors for lactating rats (Klein et al., 2014). This regulation involves activation of different subtypes of opioid receptors in the PAG, as evidenced by the elegant demonstration that μ opioid receptor activation inhibits maternal behaviors, while blocking κ receptor increases predatory and hunting behaviors (Klein et al., 2014). Fast switching between caring and predation behaviors are essential for dams to increase the chances of survival for her pups and herself. The complex interaction of multiple opioid receptor systems in the regulation of these maternal behaviors suggests that endogenous opioids play crucial roles in this essential behavioral selection process.
Studies using the opioid antagonist naloxone have confirmed the importance of the opioid system in the activation of the maternal networks, as treatment with naloxone increases prolactin as well as oxytocin levels in the maternal brain (Brunton and Russell, 2008; Brunton, 2018; Brunton et al., 2005). Further, naloxone treatment after the birth of the first pup can increase oxytocin levels as well as adrenocorticotropic hormone and corticosterone, demonstrating that endogenous opioids usually inhibit the release of these hormones during parturition (Wigger et al., 1999). However, less is known as to whether exposure to opioid agonists (such as morphine, heroin or methadone), or partial agonists (such as buprenorphine) during gestation and throughout parturition can alter normal hormonal regulations during birth. Many animal models of gestational opioid exposure have focused on the outcome of the offspring, and not on the effects on the maternal brain or processes during labor (Byrnes and Vassoler, 2018). In line with this, many animal models cross-fostered pups after birth and have thus not monitored the initiation of maternal care behaviors. However, opioid-induced deficits in maternal behaviors have been documented in animal models (Bridges and Grimm, 1982; Slamberova et al., 2001) and there is evidence that the decrease in maternal care behaviors due to morphine and other μ-opioid receptor agonists is mediated through the activating of μ-opioid receptors in the mPOA, PAG and other brain areas (Mann et al., 1991; Rubin and Bridges, 1984; Stafisso-Sandoz et al., 1998). Additionally, more recent findings implicate the activation of select opioid receptor subtypes within the PAG in regulating select maternal behavior. Interestingly, μ-opioid receptor agonists infused into the PAG disrupts the balance of maternal care and aggression, while as mentioned above, κ-opioid receptor antagonism can shift maternal behaviors from caring to predatory behaviors in rodents (Klein et al., 2014). In accord with our central hypothesis, these findings are particularly relevant to the potential effects of buprenorphine given its antagonistic effects on κ receptors. Accordingly, preliminary data from the Brummelte lab (Wallin et al., 2018) has shown that low (0.3 mg/kg) but especially high (1 mg/kg) doses of buprenorphine given prior to and throughout gestation and continued in the postpartum in rat dams significantly impairs maternal care-giving behaviors. In fact, high dose buprenorphine resulted in complete neglect of the offspring in some dams. This supports the idea that exogenous opioids can interfere with the crucial role of endorphins in neural adaptation in the transition to motherhood. Opioid levels right at the time of parturition or during the very early postpartum period may be particularly important for determining the effect on maternal behaviors, given a report on buprenorphine exposure from 7 to 21 days of pregnancy (Hung et al., 2013). In this study, there were no significant neurobehavioral or physical (body/brain mass) alterations in dams that received buprenorphine (0.3 or 1 mg/kg) but there were depression-like neurobehaviors for pups at 1 mg/kg. It appears that these behaviors are regulated by protein phosphorylation and dephosphorylation mechanisms that are emerging in the regulation of animal neurotransmitter release that have consistently been shown to play roles in depression and addiction (Swain et al., 1991; Wu et al., 2017; Liu et al., 2016; Xu et al., 2006).
Endogenous opioids in offspring are released during social contact and low levels have been shown to induce behaviors to seek out contact from a caregiver (Nelson and Panksepp, 1998). Indeed, it has long been known, that proper attachment and maternal care have a tremendous impact on infant development and the adult phenotype (Denenberg, 1999; Denenberg and Bell, 1960; Denenberg et al., 1962; Rosenberg et al., 1970; Bayart et al., 1990; Hennessy et al., 1980; Joffe et al., 1972; Levine, 1967; Levine et al., 1988). However, we are far from fully understanding the underlying mechanisms of how responsive and sensitive parenting is initiated and how it can affect the offspring in the long-term (Kentner et al., 2018). Inhibitory effects of opioids on oxytocin secretion in postpartum females have been reported in numerous mammalian species (Tancin et al., 2000; Russell et al., 1991; Haldar and Sawyer, 1978; Haldar et al., 1982; Wright et al., 1983), including humans (Lindow et al., 1999). Furthermore, the role of endogenous opioids in attenuating the prosocial effects of oxytocin has been supported by data from both animals and humans (Dal Monte et al., 2017). While buprenorphine and other opioids clearly impinge upon the MBN, the precise nature of these relationships and effects on parenting remain unclear given the complex effects of BT and opioids on receptor occupancy, buffering postpartum stress and depression, and ultimately breaking the relapse cycle. It is clear though, that maternal behaviors are crucial in mediating offspring outcome, suggesting that infants from mothers with OUD may be suffering from a ‘double-hit’ of early adversity with gestational drug exposure as well as altered maternal care due to maternal opioid use.
3.2. Gestational opioid animal models: Need for better translational value
Although numerous animal studies have investigated the effects of gestational opioid exposure on the offspring, there is considerable variation in reported outcomes probably due to several factors, including species used, type and dose of drug and time of exposure (preconceptional, start during pregnancy, continued through postpartum or not). For instance, endogenous opioids and prescription opioids have been associated with normal maturational processes and can upregulate brain-derived neurotrophic factor as well as oligodendrocyte maturation and myelination through activation of opioid receptors (Zhang et al., 2006; Vestal-Laborde et al., 2014). Therefore, it is conceivable that prenatal exposure to exogenous opioids may interfere with proper brain development. In fact, recent data suggests that early opioid exposure may lead to accelerated brain development that could disrupt the complex sequence of synchronized neurochemical events leading to normal connectivity in the developing brain (Vestal-Laborde et al., 2014).
It has also been suggested that some of the variability in offspring outcome after gestational opioid exposure could be explained by the distinctive role that endogenous opioids play in regulating hypothalamic and pituitary outflow during pregnancy and the postpartum period (Byrnes and Vassoler, 2018). In other words, the physiological reaction of the mother to the drug is a crucial factor in determining the effects on the offspring. Unfortunately, many previous animal models, which typically began opioid exposure during pregnancy, failed to account for the fact that most women will already be taking opioids by the time of conception (either abusing, prescription or maintenance opioids) rather than starting to use them during pregnancy. As a woman’s body develops tolerance to a set opioid level and goes through withdrawal once that opioid level drops, the effect on the fetus could be very different in an ‘established’ user compared to a ‘new’ user. This crucial aspect of drug use patterns has previously been mostly ignored in animal studies that commonly start exposure after gestational day 7 when the opioid system started to develop in the fetus (Zhu et al., 1998; Khachaturian et al., 1983). Thus, there is a lack of research on the effects of pre-conceptional drug use and drug withdrawal, in addition to relapse or switching opioids during pregnancy. Importantly, there is also a lack of data concerning the short and long-term effects of opioids during these critical developmental periods on the dam’s brain, behavior and physiology – critical to determine the long-term consequences for the dam and her offspring.
Although perinatal exposure to buprenorphine has not been shown to produce severe maternal and fetal or neonatal mortality, it was associated with significant perinatal mortality and perturbations of pup development in the rat (Robinson and Wallace, 2001). These effects included increases in maternal and fetal mortality and morbidity, in addition to the perturbations in the acquisition of several pup developmental milestones, weight gain, precipitated withdrawal, and the antinociceptive effect of morphine. Specifically, it was shown that 1 and 3 mg/kg/day of buprenorphine suppressed food intake over the first few days after minipump implantation on gestational day 7, but then the rats seemed to develop a tolerance for that effect. The resulting reduction in maternal weight gain during those first days again highlights the importance of considering the timing of exposure since a nutrition deficit during pregnancy has long been known to have its own detrimental effects on maternal behavior and offspring (Wiener et al., 1976; Connor et al., 2012). Similar concerns come from rat research showing that the relative amount of morphine in placental and fetal tissue was reduced significantly following continuous administration compared to a single administration (DeVane et al., 1999). Taken together, future animal and human research must address critical issues of exposure timing and duration in addition to amount, since opioids are associated with many adaptive responses and many co-morbidities, such as stress, that could contribute to offspring outcome.
4. Research on the parental brain and opioids
4.1. Opioid-mediated regulation of stress during pregnancy
Endogenous opioids are well known to have a robust effect on extended amygdala morphology, which contributes to Hypothalamic-Pituitary-Adrenal/Sympathetic-Adrenal-Medullary (HPA/SAM) axis regulation. Interestingly, chronic opioid use seems to suppress HPA axis function in humans (Delitala et al., 1983), but rodents often show activation and increased cortisol levels after opioid treatment (Buckingham and Cooper, 1984). This paradox produces a challenge for translational studies of opioid effects, especially during stressful periods such as pregnancy and the postpartum. Despite this, experimental animal studies have been essential in determining how endogenous opioids suppress HPA axis function during gestation (Brunton and Russell, 2008). Briefly, increased inhibition of noradrenergic terminals in the hypothalamus by endogenous opioids prevents CRH neuron activation. In turn, decreased CRH neuron activity results in less CRH release which eventually translates into less corticosterone (rodents) or cortisol (humans) released from the adrenal glands and thus a reduced stress response to external stressors. This opioid-induced down-regulation of the HPA axis is believed to protect the fetus from maternal stress and negative effects of elevated glucocorticoid levels. Thus, in addition to regulating emotions and reward processes (Benarroch, 2012), opioids play a central role in regulating objective and subjective stress that women experience during pregnancy and lactation (Brunton, 2018). This further highlights the importance of assessing the behavioral effects of long-term opioid-related HPA-axis suppression, which may result in response dysregulation of the stress response system. This is particularly germane in terms of parenting behavior as appropriate response to stress may be key in the face of prenatal maternal stressors including fears regarding impending labor and delivery, health and welfare of the fetus, inactivity, concerns pertaining to child rearing and elevated levels of anxiety, obsessions and depressive symptomatology (Lobel et al., 2008; Lobel et al., 2016; Lobel and Ibrahim, 2018; Mahaffey et al., 2018; Alderdice et al., 2012; Feygin et al., 2006). Interestingly, several research studies have indicated that pregnancy-specific stress is a more robust indicator of fetal activity and adverse developmental outcome than general stress (Lobel et al., 2008; Dunkel Schetter, 2011; Huizink et al., 2004) potentially due to its lack of parenting specificity.
Finally, maternal stress and cortisol levels are associated with brain activation patterns in response to emotional stimuli in the mother. For example, dispositional personal distress was associated with greater cortisol reactivity to social evaluation stress in mothers, and mother's ventral ACC response to positive versus negative child feedback to their parenting decisions was inversely related to parenting-related cortisol reactivity (Ho et al., 2014). This suggests that opioids and BT may impact the mother-infant dyad and infant development beyond gestation, via their effects on external stressors, pregnancy specific stress and HPA-axis function.
4.2. Assessing maternal stress in the context of opioid maintenance therapy
The severity of stress as a behavioral teratogen (Dipietro, 2012) is compounded in women with addictive disorders, including OUD. For examples, OUD has co-occurring anxiety and mood related disorders, guilt, poor nutrition, impaired health, homelessness, violence, confusion regarding available medical options, and a lack of social support (McCarthy et al., 2017; Bishop et al., 2017; Jansson et al., 1996). From a neuroendocrine perspective, such chronic stressors alongside the repeated use of short-term opioids reciprocally sensitize HPA/SAM system activity. This in turn contributes to further use of opioids and other drugs, which further worsens dysphoria, negative affect, anxiety and the “relapse cycle” (Koob, 2017a, 2017b; Hyman et al., 2007). Indeed, research on non-pregnant substance abusers has indicated stress system sensitivity to be a key predictor of stress response dysregulation, elevated anxiety and depressive symptomatology as well as craving and relapse to various drugs of abuse during early abstinence (Fox et al., 2008; Fox et al., 2007; Sinha et al., 2011, 2006). Given this sensitized and negatively reinforcing environment, and added to the fact that pregnancy is frequently characterized by a rapid metabolism of opioids (McCarthy et al., 2017), it is not surprising that OUD during pregnancy is characterized by numerous failed quit attempts (Guille et al., 2017) potentially driven by aberrations in stress system pathophysiology, exacerbating the repeating cycle of opioid intoxication and acute withdrawal.
OMT such as BT may attenuate prenatal maternal stress in several ways. First, extensive clinical and preclinical data have indicated that the analgesic effects of buprenorphine dampen stress and reduce central levels of cortisol and ACTH in laboratory animals, clinical populations and healthy humans (Bershad et al., 2016). In addition, buprenorphine demonstrates both anti-depressive and anxiolytic properties in patients with opioid dependence (Kosten et al., 1990), treatment-resistant depression disorders (Bodkin et al., 1995; Kosten, 2016; Karp et al., 2014), and suicidal ideation (Yovell et al., 2016; Norelli et al., 2013). Healthy volunteers also show attenuation of cortisol and social stress with low buprenorphine which attenuates anxiety, rejection stress and response to fearful faces (Bershad et al., 2016; Bershad et al., 2015). Second, as BT provides a constant level of maternal μ receptor occupancy (McCarthy et al., 2017), it may effectively “break” cyclic withdrawal and relapse-related behaviors, decreasing bio-psychological aspects of the allostatic withdrawal state including anxiety, negative affect and sensitized HPA/SAM function – all shown to underpin relapse factors as well as negatively impact health outcomes in the mother-child dyad (Lobel et al., 2008, 2016; Lobel and Ibrahim, 2018; LeWinn et al., 2009; Entringer et al., 2009).
4.3. Toward a unifying hypothesis of opioids as treatment with side effects
In humans, MBN regions respond to ethologically pertinent own-baby cry in correlation with adaptive caregiving thoughts and behaviors during early postpartum (Swain et al., 2007; Barrett and Fleming, 2011; Swain, 2011). Many of these are same brain regions known to be regulated by endogenous opioids and dysregulated by stress and OUD. For example, in animal models, addiction affects reward processing in the OFC, VTA, and nucleus NAc (Rutherford et al., 2011). Furthermore, human neuroimaging has shown that amygdala, insula, ACC, and OFC are dysregulated in stress-related disorders like depression (Groenewold et al., 2012).
We previously presented preliminary data on the effects of BT on the response of human maternal brain systems to own baby-cry (Swain and Ho, 2017), which we update here: We studied 46 mothers in early postpartum who completed structural and functional magnetic resonance imaging (MRI) scans at two time-points: 1-month postpartum (T1) and 4-month postpartum (T2). The participants reported depressive symptoms using Beck Depression Inventory (BDI) (Beck et al., 1997) and parenting stress using Parenting Stress Index (PSI) (Abidin, 1995) T1 & T2. According to the BDI at T1 and prescription opioid usage, we divided the participants into three groups, including a group of 7 mothers who were on prescription buprenorphine treatment (BT) from the first trimester until the time of study (4–20 mg), a group of 7 non-OUD mothers who had elevated depressive symptoms similar to BT mothers (Depressed Controls, DC), and a group of 32 non-OUD mothers who were not depressed nor receiving BT (Healthy Controls, HC). At T1 and T2, the participants underwent functional magnetic resonance imaging (fMRI) scans during a baby-cry task, wherein they listened to own and other's baby cry and respective control sounds in a block design (30 s per block, 5 blocks per condition), and an 8-minute resting state task. The fMRI images were processed and analyzed in SPM 8 to examine the differential neural responses to Own vs. Other’s Baby Cry, functional connectivity during own vs. other baby cry and resting state functional connectivity (rsFC).
This preliminary data on mothers during the early postpartum shows that adaptation of maternal brain physiology and behaviors are influenced by opioid exposure. First, BT mothers with OUD showed greater PAG and hypothalamus responses related to Own vs. Other’s Baby-Cry as compared to healthy mothers. Second, Own vs. Other’s Baby-Cry responses in the hypothalamus were associated with greater parenting stress (PSI). Lastly, both differential functional connectivity in Own vs. Other’s Baby-Cry and rsFC between the PAG and hypothalamus were associated with PSI, suggesting a role of PAG in driving hypothalamus as a function of parenting stress. Although preliminary, these findings suggest that neuroimaging may reveal both mechanisms for therapeutic benefit from BT, as well as potential risks of parenting stress according to dysregulation in the MBN.
Based on our preliminary data and MBN model, we propose a unifying hypothesis to explain how BT may be helpful in the treatment of OUD, although also posing side-effect risks to maternal behavior, consistent with preclinical research. In OUD, BT increases activity in both care (hypothalamus) and defense (PAG) subsystems of MBN – both potentially supporting maternal care behaviors. However, we also reveal potential dysregulation of the normal reciprocal inhibition between these subsystems by exogenous opioids. The latter effect may result in excessive infant-oriented defensive/aggressive thoughts and behaviors that may manifest as anxiety or aggression during maternal behavior and responding to their baby-cry. This compelling yet simple idea could explain how exogenous opioids such as BT may be therapeutic role, yet also risk side-effect of diminished care with intrusive or insensitive parental behaviors in the case of OUD with exogenous opioid treatment, and as a function of stress (Bridges and Grimm, 1982; Slamberova et al., 2001). Evaluation of this hypothesis requires a currently lacking characterization of maternal phenomenology for BT mothers as to whether they are overly focused on defense of the infant, lacking in caregiving motivation, or more stress-sensitive during maternal behaviors.
5. Summary and future directions
Opioid use has reached epidemic proportions in the United States. Besides the health consequences for the using mother, there is also a significant concern following the use of opioids in pregnancy for adverse infant outcomes (Broussard et al., 2011; CSAT, 2006), as evidenced by a 5-fold increase in the prevalence of NAS in recent years. The use of OMT is the highly recommended “gold standard” for treatment despite its own risks. In addition to mitigating withdrawal, physicians are currently more likely to prescribe buprenorphine because emerging data suggest that neonatal withdrawal symptomology appears to be less frequent and less severe with buprenorphine than methadone (Kakko et al., 2008; Jones et al., 2005, 2010; Hall et al., 2018). OMT is designed to prevent relapse to illicit opioid drug use (i.e., heroin or morphine) and to avoid the cycling of intoxication and withdrawal resulting in inconsistent blood opioid levels and high fetal stress (Fischer et al., 2000). However, there is still a lack of data on the long-term consequences of OMT exposure for the fetus as well as a lack of consensus about appropriate dosing regimens especially around the time of parturition. Further, the impact of stress as a co-morbidity with opioid abuse is not well studied and deserves further attention. Animal models also raise concerns for adverse parenting behavior effects. Considering the role of endogenous opioids in regulating maternal behaviors and the stress response in pregnant women (Swain et al., 2005), exogenous opioids may have a unique effect on maternal physiology by impacting the HPA axis functioning and brain circuits involved in maternal caregiving behaviors. It is possible that opioids disrupt the normal regulation (reciprocal inhibition) of caring and defensive/aggressive brain circuits in mothers suffering from OUDs and concurrent high levels of stress. More research is needed to better understand how we can administer opioids to help mothers reduce their withdrawal symptoms without affecting important MBN brain networks leading to negative consequences in maternal–infant interactions and parenting. Parental brain models of reward-stress dysregulation by addiction present opportunities to understand how caregiving may be compromised in addicted parents and assisted by opioids. Future studies should further investigate the impact of opioids on parenting behavior in mothers and fathers (Swain et al., 2014) and test whether parenting interventions can alleviate some of the negative consequence for exposed children.
For example, the Mom Power (MP) parenting intervention, which aims to promote maternal empathy, reflective functioning, and stress reduction skills (Muzik et al., 2015, 2016) has been associated with decreased parenting stress and increased child-focused responses in social brain areas (Swain et al., 2017). This kind of parenting intervention may be optimized specifically to treat OUD or OUD-treatment related neurophysiological deficits for mothers affected.
Animal and human parental brain models may also lead to new and improved pharmacological and psychological interventions. For example, exogenous oxytocin may improve maternal caregiving motivation in OUD since opioids inhibit oxytocin – with due care for the timing and context of therapeutic administration of oxytocin to decrease maternal aggression (Szymanska et al., 2017; Bosch and Young, 2018). Although animal and human models can help disentangle the complex relationships between the direct and indirect effects of opioids on the maternal brain and behavior and the outcome of the offspring, we need better translational approaches that take these complexities into consideration and focus more on the dam and offspring. For example, it will be important to consider the fact that most women do not start abusing opioids during pregnancy but are rather already suffering from opioid abuse and likely related stress system dysregulation before conception. Given the opioid epidemic, there is also an urgent need to investigate the impact of switching during pregnancy – from drugs of abuse to therapeutic maintenance drugs such as buprenorphine. Indeed, the impact of experiencing recurring withdrawal during gestation and high levels of circulating opioids at the time of parturition is not well understood for maternal brain or behavior. Finally, brain-based models may help explore the underlying mechanisms of changes to maternal caregiving behaviors in women with OUD, as well as consequences of poly-drug use or frequent relapse for the mother and her offspring that may inform treatment.
Acknowledgments
This work has been supported by the Research Foundation of SUNY (JES, SSH) and the National Institutes for Health – National Center for Advanced Translational Sciences via the Michigan Institute for Clinical Health Research UL1TR000433 (JES, SSH).
Footnotes
Editorial disclosure
Given her role as Guest Editor, Dr. Susanne Brummelte was not involved in the peer-review of this article and has no access to information regarding its peer-review. Full responsibility for the editorial process for this article was delegated to Guest Editor, Dr. Benedetta Leuner.
Appendix A. Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.yfrne.2019.100766.
References
- Abidin R, 1995. Parenting Stress Index. Psychological Assessment Resources Inc, Lutz (FL). [Google Scholar]
- Alderdice F, Lynn F, Lobel M, 2012. A review and psychometric evaluation of pregnancy-specific stress measures. J. Psychosom. Obstet. Gynaecol 33, 62–77. [DOI] [PubMed] [Google Scholar]
- Atzil S, Hendler T, Feldman R, 2011. Specifying the neurobiological basis of human attachment: brain, hormones, and behavior in synchronous and intrusive mothers. Neuropsychopharmacology 36, 2603–2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakermans-Kranenburg MJ, Van IJMH, 2013. Sniffing around oxytocin: review and meta-analyses of trials in healthy and clinical groups with implications for pharmacotherapy. Transl. Psychiatry 3, e258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barclay L, Everitt L, Rogan F, Schmied V, Wyllie A, 1997. Becoming a mother–an analysis of women's experience of early motherhood. J. Adv. Nurs 25, 719–728. [DOI] [PubMed] [Google Scholar]
- Barrett J, Fleming AS, 2011. Annual Research Review: All mothers are not created equal: neural and psychobiological perspectives on mothering and the importance of individual differences. J. Child Psychol. Psychiatry 52, 368–397. [DOI] [PubMed] [Google Scholar]
- Barth RP, 2009. Preventing child abuse and neglect with parent training: evidence and opportunities. Future Child. 19, 95–118. [DOI] [PubMed] [Google Scholar]
- Bayart F, Hayashi KT, Faull KF, Barchas JD, Levine S, 1990. Influence of maternal proximity on behavioral and physiological responses to separation in infant rhesus monkeys (Macaca mulatta). Behav. Neurosci 104, 98–107. [PubMed] [Google Scholar]
- Bayerl DS, Bosch OJ, 2019. Brain vasopressin signaling modulates aspects of maternal behavior in lactating rats. Genes Brain Behav 18, e12517. [DOI] [PubMed] [Google Scholar]
- Beck AT, Guth D, Steer RA, Ball R, 1997. Screening for major depression disorders in medical inpatients with the beck depression inventory for primary care. Behav. Res. Ther 35, 785–791. [DOI] [PubMed] [Google Scholar]
- Benarroch EE, 2012. Endogenous opioid systems: current concepts and clinical correlations. Neurology 79, 807–814. [DOI] [PubMed] [Google Scholar]
- Bershad AK, Jaffe JH, Childs E, de Wit H, 2015. Opioid partial agonist buprenorphine dampens responses to psychosocial stress in humans. Psychoneuroendocrinology 52, 281–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bershad AK, Seiden JA, de Wit H, 2016. Effects of buprenorphine on responses to social stimuli in healthy adults. Psychoneuroendocrinology 63, 43–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop D, Borkowski L, Couillard M, Allina A, Baruch S, Wood S, 2017. Pregnant women and substance use: overview of research & policy in the United States. The George Washinton University. [Google Scholar]
- Bodkin JA, Zornberg GL, Lukas SE, Cole JO, 1995. Buprenorphine treatment of refractory depression. J. Clin. Psychopharmacol 15, 49–57. [DOI] [PubMed] [Google Scholar]
- Borelli JL, West JL, Decoste C, Suchman NE, 2012. Emotionally avoidant language in the parenting interviews of substance-dependent mothers: associations with reflective functioning, recent substance use, and parenting behavior. Infant Mental Health J 33, 506–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornstein MH, Putnick DL, Rigo P, Esposito G, Swain JE, Suwalsky JTD, Su X, Du X, Zhang K, Cote LR, De Pisapia N, Venuti P, 2017. Neurobiology of culturally common maternal responses to infant cry. Proc Natl Acad Sci U S A 114, E9465–E9473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosch OJ, Young LJ, 2018. Oxytocin and social relationships: from attachment to bond disruption. Curr. Top. Behav. Neurosci 35, 97–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridges RS, Grimm CT, 1982. Reversal of morphine disruption of maternal behavior by concurrent treatment with the opiate antagonist naloxone. Science 218, 166–168. [DOI] [PubMed] [Google Scholar]
- Brogly SB, Saia KA, Walley AY, Du HM, Sebastiani P, 2014. Prenatal buprenorphine versus methadone exposure and neonatal outcomes: systematic review and meta-analysis. Am. J. Epidemiol 180, 673–686. [DOI] [PubMed] [Google Scholar]
- Broussard CS, Rasmussen SA, Reefhuis J, Friedman JM, Jann MW, Riehle-Colarusso T, Honein MA, Study NBDP, 2011. Maternal treatment with opioid analgesics and risk for birth defects. Am. J. Obstet. Gynecol 314, 204 e1–314. e11. [DOI] [PubMed] [Google Scholar]
- Brunton PJ, 2018. Endogenous opioid signalling in the brain during pregnancy and lactation. Cell Tissue Res. [DOI] [PubMed] [Google Scholar]
- Brunton PJ, Russell JA, 2008. The expectant brain: adapting for motherhood. Nat. Rev. Neurosci 9, 11–25. [DOI] [PubMed] [Google Scholar]
- Brunton PJ, Meddle SL, Ma S, Ochedalski T, Douglas AJ, Russell JA, 2005. Endogenous opioids and attenuated hypothalamic-pituitary-adrenal axis responses to immune challenge in pregnant rats. J. Neurosci 25, 5117–5126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunton PJ, Russell JA, Douglas AJ, 2008. Adaptive responses of the maternal hypothalamic-pituitary-adrenal axis during pregnancy and lactation. J. Neuroendocrinol 20, 764–776. [DOI] [PubMed] [Google Scholar]
- Buckingham JC, Cooper TA, 1984. Differences in hypothalamo-pituitary-adrenocortical activity in the rat after acute and prolonged treatment with morphine. Neuroendocrinology 38, 411–417. [DOI] [PubMed] [Google Scholar]
- Byrnes EM, Rigero BA, Bridges RS, 2000. Opioid receptor antagonism during early lactation results in the increased duration of nursing bouts. Physiol. Behav 70, 211–216. [DOI] [PubMed] [Google Scholar]
- Byrnes EM, Vassoler FM, 2018. Modeling prenatal opioid exposure in animals: Current findings and future directions. Front. Neuroendocrinol 51, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Child Welfare Information Gateway, 2014. Parental Substance Use and the Child Welfare System. U.S. Department of Health and Human Services, Washington, DC. [Google Scholar]
- Cleary BJ, Donnelly JM, Strawbridge JD, Gallagher PJ, Fahey T, White MJ, Murphy DJ, 2011. Methadone and perinatal outcomes: a retrospective cohort study. Am. J. Obstet. Gynecol 204 (139), e1–e9. [DOI] [PubMed] [Google Scholar]
- Coles LD, Lee IJ, Hassan HE, Eddington ND, 2009. Distribution of saquinavir, methadone, and buprenorphine in maternal brain, placenta, and fetus during two different gestational stages of pregnancy in mice. J. Pharm. Sci 98, 2832–2846. [DOI] [PubMed] [Google Scholar]
- Connor KL, Vickers MH, Beltrand J, Meaney MJ, Sloboda DM, 2012. Nature, nurture or nutrition? Impact of maternal nutrition on maternal care, offspring development and reproductive function. J. Physiol 590, 2167–2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conway KP, Compton W, Stinson FS, Grant BF, 2006. Lifetime comorbidity of DSM-IV mood and anxiety disorders and specific drug use disorders: results from the National Epidemiologic Survey on Alcohol and Related Conditions. J. Clin. Psychiatry 67, 247–257. [DOI] [PubMed] [Google Scholar]
- Coyle MG, Brogly SB, Ahmed MS, Patrick SW, Jones HE, 2018. Neonatal abstinence syndrome. Nat. Rev. Dis. Primers 4, 47. [DOI] [PubMed] [Google Scholar]
- CSAT, 2006. Substance Abuse: Clinical Issues in Intensive Outpatient Treatment. Substance Abuse and Mental Health Services Administration, Rockville (MD, USA). [PubMed] [Google Scholar]
- Dahan A, Kest B, Waxman AR, Sarton E, 2008. Sex-specific responses to opiates: animal and human studies. Anesth. Analg 107, 83–95. [DOI] [PubMed] [Google Scholar]
- Dal Monte O, Piva M, Anderson KM, Tringides M, Holmes AJ, Chang SWC, 2017. Oxytocin under opioid antagonism leads to supralinear enhancement of social attention. Proc. Natl. Acad. Sci. USA 114, 5247–5252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darmani NA, Schnoll SH, Pandey U, Martin BR, 1992. Chronic prenatal methadone exposure alters central opioid mu-receptor affinity in both fetal and maternal brain. Neurotoxicol. Teratol 14, 265–271. [DOI] [PubMed] [Google Scholar]
- Davis MA, Lin LA, Liu H, Sites BD, 2017. Prescription opioid use among adults with mental health disorders in the United States. J. Am. Board Family Med 30, 407–417. [DOI] [PubMed] [Google Scholar]
- Delitala G, Devilla L, Musso NR, 1983. On the role of dopamine receptors in the naloxone-induced hormonal changes in man. J. Clin. Endocrinol. Metab 56, 181–184. [DOI] [PubMed] [Google Scholar]
- Denenberg VH, 1999. Commentary: is maternal stimulation the mediator of the handling effect in infancy? Dev. Psychobiol 34, 1–3. [DOI] [PubMed] [Google Scholar]
- Denenberg VH, Bell RW, 1960. Critical periods for the effects of infantile experience on adult learning. Science 131, 227–228. [DOI] [PubMed] [Google Scholar]
- Denenberg VH, Ottinger DR, Stephens MW, 1962. Effects of maternal factors upon growth and behavior of the rat. Child Dev. 33, 65–71. [DOI] [PubMed] [Google Scholar]
- DeVane CL, Simpkins JW, Boulton DW, Laizure SC, Miller RL, 1999. Disposition of morphine in tissues of the pregnant rat and foetus following single and continuous intraperitoneal administration to the mother. J. Pharm. Pharmacol 51, 1283–1287. [DOI] [PubMed] [Google Scholar]
- Dipietro JA, 2012. Maternal stress in pregnancy: considerations for fetal development. J. Adolesc. Health 51, S3–S8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunkel Schetter C, 2011. Psychological science on pregnancy: stress processes, biopsychosocial models, and emerging research issues. Annu. Rev. Psychol 62, 531–558. [DOI] [PubMed] [Google Scholar]
- Duval ER, Garfinkel SN, Swain JE, Evans GW, Blackburn EK, Angstadt M, Sripada CS, Liberzon I, 2017. Childhood poverty is associated with altered hippocampal function and visuospatial memory in adulthood. Dev. Cogn. Neurosci 23, 39–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmadih A, Wan MW, Downey D, Elliott R, Swain JE, Abel KM, 2016. Natural variation in maternal sensitivity is reflected in maternal brain responses to infant stimuli. Behav. Neurosci 130, 500–510. [DOI] [PubMed] [Google Scholar]
- Entringer S, Kumsta R, Hellhammer DH, Wadhwa PD, Wust S, 2009. Prenatal exposure to maternal psychosocial stress and HPA axis regulation in young adults. Horm. Behav 55, 292–298. [DOI] [PubMed] [Google Scholar]
- Evans GW, Swain JE, King AP, Wang X, Javanbakht A, Ho SS, Angstadt M, Phan KL, Xie H, Liberzon I, 2016. Childhood cumulative risk exposure and adult amygdala volume and function. J. Neurosci. Res 94, 535–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman R, Bakermans-Kranenburg MJ, 2017. Oxytocin: a parenting hormone. Curr. Opin. Psychol 15, 13–18. [DOI] [PubMed] [Google Scholar]
- Feldman R, Granat A, Pariente C, Kanety H, Kuint J, Gilboa-Schechtman E, 2009. Maternal depression and anxiety across the postpartum year and infant social engagement, fear regulation, and stress reactivity. J. Am. Acad. Child Adolesc. Psychiatry 48, 919–927. [DOI] [PubMed] [Google Scholar]
- Felicio LF, Mann PE, Bridges RS, 1991. Intracerebroventricular cholecystokinin infusions block beta-endorphin-induced disruption of maternal behavior. Pharmacol. Biochem. Behav 39, 201–204. [DOI] [PubMed] [Google Scholar]
- Feygin DL, Swain JE, Leckman JF, 2006. The normalcy of neurosis: evolutionary origins of obsessive-compulsive disorder and related behaviors. Prog. NeuroPsychopharmacol. Biol. Psychiatry 30, 854–864. [DOI] [PubMed] [Google Scholar]
- Field T, Healy B, Goldstein S, Perry S, Bendell D, Schanberg S, Zimmerman EA, Kuhn C, 1988. Infants of depressed mothers show “depressed” behavior even with nondepressed adults. Child Dev. 59, 1569–1579. [DOI] [PubMed] [Google Scholar]
- Fischer G, Johnson RE, Eder H, Jagsch R, Peternell A, Weninger M, Langer M, Aschauer HN, 2000. Treatment of opioid-dependent pregnant women with buprenorphine. Addiction 95, 239–244. [DOI] [PubMed] [Google Scholar]
- Fischer G, Ortner R, Rohrmeister K, Jagsch R, Baewert A, Langer M, Aschauer H, 2006. Methadone versus buprenorphine in pregnant addicts: a double-blind, double-dummy comparison study. Addiction 101, 275–281. [DOI] [PubMed] [Google Scholar]
- Forray A, 2016. Substance use during pregnancy. F1000 Res. 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox HC, Bergquist KL, Hong KI, Sinha R, 2007. Stress-induced and alcohol cue-induced craving in recently abstinent alcohol-dependent individuals. Alcohol. Clin. Exp. Res 31, 395–403. [DOI] [PubMed] [Google Scholar]
- Fox HC, Hong KI, Siedlarz K, Sinha R, 2008. Enhanced sensitivity to stress and drug/alcohol craving in abstinent cocaine-dependent individuals compared to social drinkers. Neuropsychopharmacology 33, 796–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaalema DE, Scott TL, Heil SH, Coyle MG, Kaltenbach K, Badger GJ, Arria AM, Stine SM, Martin PR, Jones HE, 2012. Differences in the profile of neonatal abstinence syndrome signs in methadone- versus buprenorphine-exposed neonates. Addiction 107 (Suppl 1), 53–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavin NI, Gaynes BN, Lohr KN, Meltzer-Brody S, Gartlehner G, Swinson T, 2005. Perinatal depression: a systematic review of prevalence and incidence. Obstet. Gynecol 106, 1071–1083. [DOI] [PubMed] [Google Scholar]
- Gaynes BN, Gavin N, Meltzer-Brody S, Lohr KN, Swinson T, Gartlehner G, Brody S, Miller WC, 2005. Perinatal depression: prevalence, screening accuracy, and screening outcomes. Evid. Rep. Technol. Assess. (Summ.) 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman JH, 2002. Becoming an involved father of an infant. Jognn-J. Obstetr. Gynecol. Neonatal Nurs 34, 190–200. [DOI] [PubMed] [Google Scholar]
- Goodman SH, Rouse MH, Connell AM, Broth MR, Hall CM, Heyward D, 2011. Maternal depression and child psychopathology: a meta-analytic review. Clin. Child. Fam. Psychol. Rev 14, 1–27. [DOI] [PubMed] [Google Scholar]
- Grace SL, Evindar A, Stewart DE, 2003. The effect of postpartum depression on child cognitive development and behavior: a review and critical analysis of the literature. Arch. Womens Ment. Health 6, 263–274. [DOI] [PubMed] [Google Scholar]
- Grimm CT, Bridges RS, 1983. Opiate regulation of maternal behavior in the rat. Pharmacol. Biochem. Behav 19, 609–616. [DOI] [PubMed] [Google Scholar]
- Groenewold NA, Opmeer EM, de Jonge P, Aleman A, Costafreda SG, 2012. Emotional valence modulates brain functional abnormalities in depression: evidence from a meta-analysis of fMRI studies. Neurosci. Biobehav. Rev 37, 152–163. [DOI] [PubMed] [Google Scholar]
- Guille C, Barth KS, Mateus J, McCauley JL, Brady KT, 2017. Treatment of prescription opioid use disorder in pregnant women. Am. J. Psychiatry 174, 208–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo C, Moses-Kolko E, Phillips M, Swain JE, Hipwell AE, 2018. Severity of anxiety moderates the association between neural circuits and maternal behaviors in the postpartum period. Cogn. Affect Behav. Neurosci 18, 426–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haight S, Ko J, Tong V, Bohm M, Callaghan W, 2018. Opioid use disorder documented at delivery hospitalization—United States, 1999–2014. MMWR Morbid. Mortal Week. Rep 845–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haldar J, Hoffman DL, Zimmerman EA, 1982. Morphine, beta-endorphin and [D-Ala2] Met-enkephalin inhibit oxytocin release by acetylcholine and suckling. Peptides 3, 663–668. [DOI] [PubMed] [Google Scholar]
- Haldar J, Sawyer WH, 1978. Inhibition of oxytocin release by morphine and its analogs. Exp. Biol. Med 157 (3), 476–480. [DOI] [PubMed] [Google Scholar]
- Hall ES, Isemann BT, Wexelblatt SL, Meinzen-Derr J, Wiles JR, Harvey S, Akinbi HT, 2016. A cohort comparison of buprenorphine versus methadone treatment for neonatal abstinence syndrome. J Pediatr 170 39–44 e1. [DOI] [PubMed] [Google Scholar]
- Hall ES, Rice WR, Folger AT, Wexelblatt SL, 2018. Comparison of neonatal abstinence syndrome treatment with sublingual buprenorphine versus conventional opioids. Am. J. Perinatol 35, 405–412. [DOI] [PubMed] [Google Scholar]
- Halligan SL, Murray L, Martins C, Cooper PJ, 2007. Maternal depression and psychiatric outcomes in adolescent offspring: a 13-year longitudinal study. J. Affect. Disord 97, 145–154. [DOI] [PubMed] [Google Scholar]
- Hay DF, Pawlby S, Waters CS, Sharp D, 2008. Antepartum and postpartum exposure to maternal depression: different effects on different adolescent outcomes. J. Child Psychol. Psychiatry 49, 1079–1088. [DOI] [PubMed] [Google Scholar]
- Hayes MJ, Brown MS, 2012. Epidemic of prescription opiate abuse and neonatal abstinenceprescription opiate abuse and neonatal abstinence. JAMA 307, 1974–1975. [DOI] [PubMed] [Google Scholar]
- Hennessy MB, Li J, Lowe EL, Levine S, 1980. Maternal behavior, pup vocalizations, and pup temperature changes following handling in mice of 2 inbred strains. Dev. Psychobiol 13, 573–584. [DOI] [PubMed] [Google Scholar]
- Hipwell AE, Guo C, Phillips ML, Swain JE, Moses-Kolko EL, 2015. Right frontoinsular cortex and subcortical activity to infant cry is associated with maternal mental state talk. J. Neurosci 35, 12725–12732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho SS, Konrath S, Brown S, Swain JE, 2014. Empathy and stress related neural responses in maternal decision making. Front. Neurosci 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho SS, Swain JE, 2017. Depression alters maternal extended amygdala response and functional connectivity during distress signals in attachment relationship. Behav. Brain Res 325, 290–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou Y, Tan Y, Belcheva MM, Clark AL, Zahm DS, Coscia CJ, 2004. Differential effects of gestational buprenorphine, naloxone, and methadone on mesolimbic mu opioid and ORL1 receptor G protein coupling. Brain Res. Dev. Brain Res 151, 149–157. [DOI] [PubMed] [Google Scholar]
- Huizink AC, Mulder EJ, Robles de Medina PG, Visser GH, Buitelaar JK, 2004. Is pregnancy anxiety a distinctive syndrome? Early Hum. Dev 79, 81–91. [DOI] [PubMed] [Google Scholar]
- Hung CJ, Wu CC, Chen WY, Chang CY, Kuan YH, Pan HC, Liao SL, Chen CJ, 2013. Depression-like effect of prenatal buprenorphine exposure in rats. PLoS ONE 8, e82262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman SM, Fox H, Hong KI, Doebrick C, Sinha R, 2007. Stress and drug-cue-induced craving in opioid-dependent individuals in naltrexone treatment. Exp. Clin. Psychopharmacol 15, 134–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansson LM, Svikis D, Lee J, Paluzzi P, Rutigliano P, Hackerman F, 1996. Pregnancy and addiction. A comprehensive care model. J. Subst. Abuse Treat 13, 321–329. [DOI] [PubMed] [Google Scholar]
- Jansson LM, Velez ML, McConnell K, Spencer N, Tuten M, Jones H, Rios R, King VL, Gandotra N, Millio L, DiPietro JA, 2017. Maternal buprenorphine treatment and infant outcome. Drug Alcohol Depend. 180, 56–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javanbakht A, King AP, Evans GW, Swain JE, Angstadt M, Phan KL, Liberzon I, 2015. Childhood poverty predicts adult amygdala and frontal activity and connectivity in response to emotional faces. Front. Behav. Neurosci 9, 154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javanbakht A, Kim P, Swain JE, Evans GW, Phan KL, Liberzon I, 2016. Sex-specific effects of childhood poverty on neurocircuitry of processing of emotional cues: a neuroimaging study. Behav. Sci. (Basel) 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joffe JM, Milkovic K, Levine S, 1972. Effects of changes in maternal pituitary-adrenal function on behavior of rat offspring. Physiol. Behav 8, 425–430. [DOI] [PubMed] [Google Scholar]
- Johnson AJ, Jones CW, 2018. Opioid use disorders and pregnancy. Obstet. Gynecol. Clin. North Am 45, 201–216. [DOI] [PubMed] [Google Scholar]
- Johnson S, Martin PR, 2017. Transitioning from methadone to buprenorphine maintenance in management of opioid use disorder during pregnancy. Am. J. Drug Alcohol Abuse 1–7. [DOI] [PubMed] [Google Scholar]
- Jones HE, Fielder A, 2015. Neonatal abstinence syndrome: Historical perspective, current focus, future directions. Prev. Med 80, 12–17. [DOI] [PubMed] [Google Scholar]
- Jones HE, Johnson RE, Jasinski DR, O'Grady KE, Chisholm CA, Choo RE, Crocetti M, Dudas R, Harrow C, Huestis MA, Jansson LM, Lantz M, Lester BM, Milio L, 2005. Buprenorphine versus methadone in the treatment of pregnant opioid-dependent patients: effects on the neonatal abstinence syndrome. Drug Alcohol Depend. 79, 1–10. [DOI] [PubMed] [Google Scholar]
- Jones HE, Fischer G, Heil SH, Kaltenbach K, Martin PR, Coyle MG, Selby P, Stine SM, O'Grady KE, Arria AM, 2012. Maternal Opioid Treatment: Human Experimental Research (MOTHER)–approach, issues and lessons learned. Addiction 107 (Suppl 1), 28–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones HE, Heil SH, Baewert A, Arria AM, Kaltenbach K, Martin PR, Coyle MG, Selby P, Stine SM, Fischer G, 2012. Buprenorphine treatment of opioid-dependent pregnant women: a comprehensive review. Addiction 107 (Suppl 1), 5–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones H, Johnson R, Jasinski DR, O’Grady KE, Chisholm CA, Choo RE, Crocetti M, Dudas R, Harrow C, Huestis MA, 2005. Buprenorphine versus methadone in the treatment of pregnant opioid-dependent patients: effects on the neonatal abstinence syndrome. Drug Alcohol Depend. 79, 1–10. [DOI] [PubMed] [Google Scholar]
- Jones H, Kaltenbach K, Heil SH, Stine SM, Coyle MG, Arria AM, O’grady KE, Selby P, Martin PR, Fischer G, 2010. Neonatal abstinence syndrome after methadone or buprenorphine exposure. N. Engl. J. Med 363, 2320–2331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones HE, Martin PR, Heil SH, Kaltenbach K, Selby P, Coyle MG, Stine SM, O'Grady KE, Arria AM, Fischer G, 2008. Treatment of opioid-dependent pregnant women: clinical and research issues. J. Subst. Abuse Treat 35, 245–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones HE, Kaltenbach K, Heil SH, Stine SM, Coyle MG, Arria AM, O'Grady KE, Selby P, Martin PR, Fischer G, 2010. Neonatal abstinence syndrome after methadone or buprenorphine exposure. N. Engl. J. Med 363, 2320–2331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones HE, Terplan M, Meyer M, 2017. Medically assisted withdrawal (detoxification): considering the mother-infant dyad. J. Addict. Med 11, 90–92. [DOI] [PubMed] [Google Scholar]
- Kacinko SL, Jones HE, Johnson RE, Choo RE, Concheiro-Guisan M, Huestis MA, 2009. Urinary excretion of buprenorphine, norbuprenorphine, buprenorphine-glucuronide, and norbuprenorphine-glucuronide in pregnant women receiving buprenorphine maintenance treatment. Clin. Chem 55, 1177–1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakko J, Heilig M, Sarman I, 2008. Buprenorphine and methadone treatment of opiate dependence during pregnancy: comparison of fetal growth and neonatal outcomes in two consecutive case series. Drug Alcohol Depend. 96, 69–78. [DOI] [PubMed] [Google Scholar]
- Kaltenbach K, Holbrook AM, Coyle MG, Heil SH, Salisbury AL, Stine SM, Martin PR, Jones HE, 2012. Predicting treatment for neonatal abstinence syndrome in infants born to women maintained on opioid agonist medication. Addiction 107 (Suppl 1), 45–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaltenbach K, O'Grady KE, Heil SH, Salisbury AL, Coyle MG, Fischer G, Martin PR, Stine S, Jones HE, 2018. Prenatal exposure to methadone or buprenorphine: early childhood developmental outcomes. Drug Alcohol Depend. 185, 40–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karp JF, Butters MA, Begley AE, Miller MD, Lenze EJ, Blumberger DM, Mulsant BH, Reynolds CF 3rd, 2014. Safety, tolerability, and clinical effect of low-dose buprenorphine for treatment-resistant depression in midlife and older adults. J. Clin. Psychiatry 75, e785–e793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayemba-Kay's S, Laclyde JP, 2003. Buprenorphine withdrawal syndrome in newborns: a report of 13 cases. Addiction 98, 1599–1604. [DOI] [PubMed] [Google Scholar]
- Kennedy-Hendricks A, McGinty EE, Barry CL, 2016. Effects of competing narratives on public perceptions of opioid pain reliever addiction during pregnancy. J. Health Polit. Policy Law 41, 873–916. [DOI] [PubMed] [Google Scholar]
- Kentner AC, Cryan JF, Brummelte S, 2018. Resilience priming: translational models for understanding resiliency and adaptation to early life adversity. Dev. Psychobiol [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khachaturian H, Alessi NE, Munfakh N, Watson SJ, 1983. Ontogeny of opioid and related peptides in the rat cns and pituitary: an immunocytochemical study. Life Sci. 33 (Suppl 1), 61–64. [DOI] [PubMed] [Google Scholar]
- Kim P, Feldman R, Mayes LC, Eicher V, Thompson N, Leckman JF, Swain JE, 2011. Breastfeeding, brain activation to own infant cry, and maternal sensitivity. J. Child Psychol. Psychiatry 52, 907–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S, Fonagy P, Allen J, Strathearn L, 2014. Mothers' unresolved trauma blunts amygdala response to infant distress. Soc. Neurosci 9, 352–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim P, Ho SS, Evans GW, Liberzon I, Swain JE, 2015. Childhood social inequalities influences neural processes in young adult caregiving. Dev. Psychobiol 57, 948–960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim P, Strathearn L, Swain JE, 2016. The maternal brain and its plasticity in humans. Horm. Behav 77, 113–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim P, Capistrano C, Congleton C, 2016. Socioeconomic disadvantages and neural sensitivity to infant cry: role of maternal distress. Soc. Cogn. Affect Neurosci [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S, Iyengar U, Mayes LC, Potenza MN, Rutherford HJV, Strathearn L, 2017. Mothers with substance addictions show reduced reward responses when viewing their own infant's face. Hum. Brain Mapp 38, 5421–5439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klaman SL, Isaacs K, Leopold A, Perpich J, Hayashi S, Vender J, Campopiano M, Jones HE, 2017. Treating women who are pregnant and parenting for opioid use disorder and the concurrent care of their infants and children: literature review to support national guidance. J. Addict. Med 11, 178–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klampfl SM, Bosch OJ, 2019. Mom doesn't care: when increased brain CRF system activity leads to maternal neglect in rodents. Front. Neuroendocrinol [DOI] [PubMed] [Google Scholar]
- Klein MO, Cruz Ade M, Machado FC, Picolo G, Canteras NS, Felicio LF, 2014. Periaqueductal gray mu and kappa opioid receptors determine behavioral selection from maternal to predatory behavior in lactating rats. Behav. Brain Res 274, 62–72. [DOI] [PubMed] [Google Scholar]
- Koob GF, 2017a. Antireward, compulsivity, and addiction: seminal contributions of Dr. Athina Markou to motivational dysregulation in addiction. Psychopharmacology 234, 1315–1332. [DOI] [PubMed] [Google Scholar]
- Koob GF, 2017b. The dark side of addiction: the Horsley Gantt to Joseph Brady connection. J. Nerv. Ment. Dis 205, 270–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Volkow ND, 2016. Neurobiology of addiction: a neurocircuitry analysis. Lancet Psychiatry 3, 760–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosten TR, 2016. An opioid for depression? Am. J. Psychiatry 173, 446–447. [DOI] [PubMed] [Google Scholar]
- Kosten TR, Krystal JH, Charney DS, Price LH, Morgan CH, Kleber HD, 1990. Opioid antagonist challenges in buprenorphine maintained patients. Drug Alcohol Depend. 25, 73–78. [DOI] [PubMed] [Google Scholar]
- Krans EE, Patrick SW, 2016. Opioid use disorder in pregnancy: health policy and practice in the midst of an epidemic. Obstet. Gynecol 128, 4–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krans EE, Bogen D, Richardson G, Park SY, Dunn SL, Day N, 2016. Factors associated with buprenorphine versus methadone use in pregnancy. Subst. Abus 37, 550–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landi N, Montoya J, Kober H, Rutherford HJ, Mencl WE, Worhunsky PD, Potenza MN, Mayes LC, 2011. Maternal neural responses to infant cries and faces: relationships with substance use. Front. Psychiatry 2, 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laslo J, Brunner JM, Burns D, Butler E, Cunningham A, Killpack R, Pyeritz C, Rinard K, Childers J, Horzempa J, 2017. An overview of available drugs for management of opioid abuse during pregnancy. Matern. Health Neonatol. Perinatol 3, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leckman JF, Feldman R, Swain JE, Eicher V, Thompson N, Mayes LC, 2004. Primary parental preoccupation: circuits, genes, and the crucial role of the environment. J. Neural Transm 111, 753–771. [DOI] [PubMed] [Google Scholar]
- Lee W, Reeve J, 2017. Identifying the neural substrates of intrinsic motivation during task performance. Cogn. Affect Behav. Neurosci 17, 939–953. [DOI] [PubMed] [Google Scholar]
- Lemon LS, Caritis SN, Venkataramanan R, Platt RW, Bodnar LM, 2017. Methadone versus buprenorphine for opioid use dependence and risk of neonatal abstinence syndrome. Epidemiology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenzi D, Trentini C, Macaluso E, Graziano S, Speranza AM, Pantano P, Ammaniti M, 2016. Mothers with depressive symptoms display differential brain activations when empathizing with infant faces. Psychiatry Res. 249, 1–11. [DOI] [PubMed] [Google Scholar]
- Levine S, 1967. Maternal and environmental influences on the adrenocortical response to stress in weanling rats. Science 156, 258–260. [DOI] [PubMed] [Google Scholar]
- Levine S, Stanton ME, Gutierrez YR, 1988. Maternal modulation of pituitary-adrenal activity during ontogeny. Adv. Exp. Med. Biol 245, 295–310. [DOI] [PubMed] [Google Scholar]
- LeWinn KZ, Stroud LR, Molnar BE, Ware JH, Koenen KC, Buka SL, 2009. Elevated maternal cortisol levels during pregnancy are associated with reduced childhood IQ. Int. J. Epidemiol 38, 1700–1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberzon I, Ma ST, Okada G, Ho SS, Swain JE, Evans GW, 2015. Childhood poverty and recruitment of adult emotion regulatory neurocircuitry. Soc. Cogn. Affect Neurosci 10, 1596–1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindow SW, Hendricks MS, Nugent FA, Dunne TT, van der Spuy ZM, 1999. Morphine suppresses the oxytocin response in breast-feeding women. Gynecol. Obstet. Invest 48, 33–37. [DOI] [PubMed] [Google Scholar]
- Liu X, Guo H, Sayed MD, Lu Y, Yang T, Zhou D, Chen Z, Wang H, Wang C, Xu J, 2016. cAMP/PKA/CREB/GLT1 signaling involved in the antidepressant-like effects of phosphodiesterase 4D inhibitor (GEBR-7b) in rats. Neuropsychiatr. Dis. Treat 12, 219–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobel M, Ibrahim SM, 2018. Emotions and mental health during pregnancy and postpartum. Women's Reprod. Health 5, 13–19. [Google Scholar]
- Lobel M, Dunkel-Schetter C, 2016. Pregnancy and prenatal stress. In: Friedman HS (Ed.), Encyclopedia of Mental Health. Academic Press, Waltham (MA), pp. 318–329. [Google Scholar]
- Lobel M, Cannella DL, Graham JE, DeVincent C, Schneider J, Meyer BA, 2008. Pregnancy-specific stress, prenatal health behaviors, and birth outcomes. Health Psychol. 27, 604–615. [DOI] [PubMed] [Google Scholar]
- Lonstein JS, Levy F, Fleming AS, 2015. Common and divergent psychobiological mechanisms underlying maternal behaviors in non-human and human mammals. Horm. Behav 73, 156–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund IO, Fitzsimons H, Tuten M, Chisolm MS, O'Grady KE, Jones HE, 2012. Comparing methadone and buprenorphine maintenance with methadone-assisted withdrawal for the treatment of opioid dependence during pregnancy: maternal and neonatal outcomes. Subst. Abuse Rehabil 3, 17–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahaffey BL, Lobel M, 2018. Mental health and emotional distress during pregnancy. In: Revenson T, Gurung RAR (Eds.), The New Handbook of Health Psychology. Taylor & Francis/Routledge. [Google Scholar]
- Mann PE, Kinsley CH, Bridges RS, 1991. Opioid receptor subtype involvement in maternal behavior in lactating rats. Neuroendocrinology 53, 487–492. [DOI] [PubMed] [Google Scholar]
- Mayes LC, Swain JE, Leckman JF, 2005. Parental attachment systems: neural circuits, genes, and experiential contributions to parental engagement. Clin. Neurosci. Res 4, 301–313. [Google Scholar]
- McCarthy JJ, Leamon MH, Finnegan LP, Fassbender C, 2017. Opioid dependence and pregnancy: minimizing stress on the fetal brain. Am. J. Obstet. Gynecol 216, 226–231. [DOI] [PubMed] [Google Scholar]
- McLearn KT, Minkovitz CS, Strobino DM, Marks E, Hou W, 2006. Maternal depressive symptoms at 2 to 4 months post partum and early parenting practices. Arch. Pediatr. Adolesc. Med 160, 279–284. [DOI] [PubMed] [Google Scholar]
- Mercer RT, 1985. The process of maternal role attainment over the first year. Nurs. Res 34, 198–204. [PubMed] [Google Scholar]
- Meyer MC, Johnston AM, Crocker AM, Heil SH, 2015. Methadone and buprenorphine for opioid dependence during pregnancy: a retrospective cohort study. J. Addict. Med 9, 81–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moses-Kolko EL, Horner MS, Phillips ML, Hipwell AE, Swain JE, 2014. In search of neural endophenotypes of postpartum psychopathology and disrupted maternal caregiving. J. Neuroendocrinol 26, 665–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moura LM, Canteras NS, Sukikara MH, Felicio LF, 2010. Morphine infusions into the rostrolateral periaqueductal gray affect maternal behaviors. Braz. J. Med. Biol. Res 43, 899–905. [DOI] [PubMed] [Google Scholar]
- Mucke S, Nagel M, Siedentopf J, Buhrer C, Huseman D, 2017. Neonatal abstinence syndrome: twelve years of experience at a regional referral center. Klin. Padiatr 229, 32–39. [DOI] [PubMed] [Google Scholar]
- Murray L, Cooper PJ, 1997. Postpartum Depression and Child Development. Guilford Press, New York. [DOI] [PubMed] [Google Scholar]
- Murray L, Cooper P, 1997. Effects of postnatal depression on infant development. Arch. Dis. Child 77, 99–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muzik M, Rosenblum KL, Alfafara EA, Schuster MM, Miller NM, Waddell RM, Stanton Kohler E, 2015. Mom Power: preliminary outcomes of a group intervention to improve mental health and parenting among high-risk mothers. Arch. Womens Ment. Health 18, 507–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muzik M, Rosenblum KL, Schuster MM, Kohler ES, Alfafara EA, Miller NM, 2016. A mental health and parenting intervention for adolescent and young adult mothers and their infants. J. Depress Anxiety 5, 233–239. [Google Scholar]
- Nanda S, Brant R, Regier M, Yossuck P, 2015. Buprenorphine: a new player in neonatal withdrawal syndrome. W. V. Med. J 111, 16–21. [PubMed] [Google Scholar]
- Nelson EE, Panksepp J, 1998. Brain substrates of infant-mother attachment: contributions of opioids, oxytocin, and norepinephrine. Neurosci. Biobehav. Rev 22, 437–452. [DOI] [PubMed] [Google Scholar]
- Norelli LJ, Smith HS, Sher L, Blackwood TA, 2013. Buprenorphine in the treatment of non-suicidal self-injury: a case series and discussion of the literature. Int. J. Adolesc. Med. Health 25, 323–330. [DOI] [PubMed] [Google Scholar]
- Numan M, Young LJ, 2016. Neural mechanisms of mother-infant bonding and pair bonding: similarities, differences, and broader implications. Horm. Behav 77, 98–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oei JL, Abdel-Latif ME, Craig F, Kee A, Austin MP, Lui K, NSW, A.N.E. Group, 2009. Short-term outcomes of mothers and newborn infants with comorbid psychiatric disorders and drug dependency. Aust. N. Z. J. Psychiatry 43, 323–331. [DOI] [PubMed] [Google Scholar]
- O'Hara MW, McCabe JE, 2013. Postpartum depression: current status and future directions. Annu. Rev. Clin. Psycho 9, 379–407. [DOI] [PubMed] [Google Scholar]
- Pawluski JL, Lonstein JS, Fleming AS, 2017. The neurobiology of postpartum anxiety and depression. Trends Neurosci. 40, 106–120. [DOI] [PubMed] [Google Scholar]
- Rasakham K, Liu-Chen LY, 2011. Sex differences in kappa opioid pharmacology. Life Sci. 88, 2–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riem MM, Bakermans-Kranenburg MJ, Van IMH, 2016. Intranasal administration of oxytocin modulates behavioral and amygdala responses to infant crying in females with insecure attachment representations. Attach. Hum. Dev 18, 213–234. [DOI] [PubMed] [Google Scholar]
- Righetti-Veltema M, Bousquet A, Manzano J, 2003. Impact of postpartum depressive symptoms on mother and her 18-month-old infant. Eur. Child Adolesc. Psychiatry 12, 75–83. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Fabbri-Destro M, 2008. The mirror system and its role in social cognition. Curr. Opin. Neurobiol 18, 179–184. [DOI] [PubMed] [Google Scholar]
- Robinson SE, Wallace MJ, 2001. Effect of perinatal buprenorphine exposure on development in the rat. J. Pharmacol. Exp. Ther 298, 797–804. [PubMed] [Google Scholar]
- Rosenberg KM, Denenberg VH, Zarrow MX, 1970. Mice (Mus musculus) reared with rat aunts: the role of rat-mouse contact in mediating behavioural and physiological changes in the mouse. Anim. Behav 18, 138–143. [DOI] [PubMed] [Google Scholar]
- Rubin BS, Bridges RS, 1984. Disruption of ongoing maternal responsiveness in rats by central administration of morphine sulfate. Brain Res. 307, 91–97. [DOI] [PubMed] [Google Scholar]
- Russell JA, Leng G, Coombes JE, Crockett SA, Douglas AJ, Murray I, Way S, 1991. Pethidine (meperidine) inhibition of oxytocin secretion and action in parturient rats. Am. J. Physiol 261, R358–R368. [DOI] [PubMed] [Google Scholar]
- Rutherford HJ, Mayes LC, 2017. Parenting and addiction: neurobiological insights. Curr. Opin. Psychol 15, 55–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutherford HJ, Williams SK, Moy S, Mayes LC, Johns JM, 2011. Disruption of maternal parenting circuitry by addictive process: rewiring of reward and stress systems. Front. Psychiatry 2, 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindler SD, Eder H, Ortner R, Rohrmeister K, Langer M, Fischer G, 2003. Neonatal outcome following buprenorphine maintenance during conception and throughout pregnancy. Addiction 98, 103–110. [DOI] [PubMed] [Google Scholar]
- Shah NS, Donald AG, Bertolatus JA, Hixson B, 1976. Tissue distribution of levomethadone in nonpregnant and pregnant female and male mice: effect of SKF 525-A 1,2. J. Pharmacol. Exp. Ther 199, 103–116. [PubMed] [Google Scholar]
- Sinha R, Garcia M, Paliwal P, Kreek MJ, Rounsaville BJ, 2006. Stress-induced cocaine craving and hypothalamic-pituitary-adrenal responses are predictive of cocaine relapse outcomes. Arch. Gen. Psychiatry 63, 324–331. [DOI] [PubMed] [Google Scholar]
- Sinha R, Fox HC, Hong KI, Hansen J, Tuit K, Kreek MJ, 2011. Effects of adrenal sensitivity, stress- and cue-induced craving, and anxiety on subsequent alcohol relapse and treatment outcomes. Arch. Gen. Psychiatry 68, 942–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slamberova R, Szilagyi B, Vathy I, 2001. Repeated morphine administration during pregnancy attenuates maternal behavior. Psychoneuroendocrinology 26, 565–576. [DOI] [PubMed] [Google Scholar]
- Sripada C, Angstadt M, Kessler D, Phan KL, Liberzon I, Evans GW, Welsh RC, Kim P, Swain JE, 2014. Volitional regulation of emotions produces distributed alterations in connectivity between visual, attention control, and default networks. Neuroimage 89, 110–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sripada RK, Swain JE, Evans GW, Welsh RC, Liberzon I, 2014. Childhood poverty and stress reactivity are associated with aberrant functional connectivity in default mode network. Neuropsychopharmacology 39, 2244–2251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stafisso-Sandoz G, Polley D, Holt E, Lambert KG, Kinsley CH, 1998. Opiate disruption of maternal behavior: morphine reduces, and naloxone restores, c-fos activity in the medial preoptic area of lactating rats. Brain Res. Bull 45, 307–313. [DOI] [PubMed] [Google Scholar]
- Stanley C, Murray L, Stein A, 2004. The effect of postnatal depression on mother-infant interaction, infant response to the still-face perturbation, and performance on an Instrumental Learning task. Dev. Psychopathol 16, 1–18. [DOI] [PubMed] [Google Scholar]
- Stolzenberg DS, Numan M, 2011. Hypothalamic interaction with the mesolimbic DA system in the control of the maternal and sexual behaviors in rats. Neurosci. Biobehav. Rev 35, 826–847. [DOI] [PubMed] [Google Scholar]
- Strathearn L, Mayes LC, 2010. Cocaine addiction in mothers: potential effects on maternal care and infant development. Ann. N. Y. Acad. Sci 1187, 1–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strathearn L, Iyengar U, Fonagy P, Kim S, 2012. Maternal oxytocin response during mother-infant interaction: associations with adult temperament. Horm. Behav 61, 429–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration, 2009. The NSDUH Report: Substance use Among Women During Pregnancy and Following Childbirth. NSDUH Services, Rockville (MD). [Google Scholar]
- Substance Abuse and Mental Health Services Administration, 2014. Results from the 2013 National Survey on Drug Use and Health: Summary of National Findings. NSDUH Services, Rockville (MD). [Google Scholar]
- Suchman NE, Luthar SS, 2001. The mediating role of parenting stress in methadone-maintained mothers’ parenting. Parent. Sci. Pract 1, 285–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swain JE, 2011. The human parental brain: in vivo neuroimaging. Prog. NeuroPsychopharmacol. Biol. Psychiatry 35, 1242–1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swain JE, Ho SS, 2017. Neuroendocrine mechanisms for parental sensitivity: overview, recent advances and future directions. Curr. Opin. Psychol 15, 105–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swain JE, Ho SS, 2017. Postpartum depression and opiate exposure affect maternal brain physiology according to parenting measures. Neuropsychopharmacology 42, S283. [Google Scholar]
- Swain JE, Robitaille R, Dass GR, Charlton MP, 1991. Phosphatases modulate transmission and serotonin facilitation at synapses: studies with the inhibitor okadaic acid. J. Neurobiol 22, 855–864. [DOI] [PubMed] [Google Scholar]
- Swain JE, Mayes LC, Leckman JF, 2004. The development of parent-infant attachment through dynamic and interactive signaling loops of care and cry. Behav. Brain Sci 27, 472–473. [Google Scholar]
- Swain JE, Mayes LC, Leckman JF, 2005. Endogenous and exogenous opiates modulate the development of parent–infant attachment. Behav. Brain Sci 28, 364–365. [Google Scholar]
- Swain JE, Lorberbaum JP, Kose S, Strathearn L, 2007. Brain basis of early parent-infant interactions: psychology, physiology, and in vivo functional neuroimaging studies. J. Child Psychol. Psychiatry 48, 262–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swain JE, Tasgin E, Mayes LC, Feldman R, Constable RT, Leckman JF, 2008. Maternal brain response to own baby-cry is affected by cesarean section delivery. J. Child Psychol. Psychiatry 49, 1042–1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swain JE, Kim P, Ho SS, 2011. Neuroendocrinology of parental response to baby-cry. J. Neuroendocrinol 23, 1036–1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swain JE, Dayton CJ, Kim P, Tolman RM, Volling BL, 2014. Progress on the paternal brain: theory, animal models, human brain research, and mental health implications. Infant Ment Health J 35, 394–408. [DOI] [PubMed] [Google Scholar]
- Swain JE, Ho SS, Rosenblum KL, Morelen D, Dayton CJ, Muzik M, 2017. Parent-child intervention decreases stress and increases maternal brain responses and connectivity in response to own baby-cry. Dev. Psychopathol 29, 535–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szymanska M, Schneider M, Chateau-Smith C, Nezelof S, Vulliez-Coady L, 2017. Psychophysiological effects of oxytocin on parent-child interactions: A literature review on oxytocin and parent-child interactions. Psychiatry Clin. Neurosci 71, 690–705. [DOI] [PubMed] [Google Scholar]
- Tancin V, Kraetzl WD, Schams D, 2000. Effects of morphine and naloxone on the release oxytocin and on milk ejection in dairy cows. J. Dairy Res 67, 13–20. [DOI] [PubMed] [Google Scholar]
- Unger A, Jagsch R, Bawert A, Winklbaur B, Rohrmeister K, Martin PR, Coyle M, Fischer G, 2011. Are male neonates more vulnerable to neonatal abstinence syndrome than female neonates? Gend. Med 8, 355–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velez ML, McConnell K, Spencer N, Montoya L, Tuten M, Jansson LM, 2018. Prenatal buprenorphine exposure and neonatal neurobehavioral functioning. Early Hum. Dev 117, 7–14. [DOI] [PubMed] [Google Scholar]
- Vestal-Laborde AA, Eschenroeder AC, Bigbee JW, Robinson SE, Sato-Bigbee C, 2014. The opioid system and brain development: effects of methadone on the oligodendrocyte lineage and the early stages of myelination. Dev. Neurosci 36, 409–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallin C, Richardson L, Bowen S, Brummelte S, 2018. Effects of Preconceptional and Gestational Opioid (Buprenorphine) Exposure in Rats on Neurodevelopment in Male and Female Offspring. International Society for Developmental Psychobiology, San Diego, CA, pp. 72–75. [Google Scholar]
- Wan MW, Downey D, Strachan H, Elliott R, Williams SR, Abel KM, 2014. The neural basis of maternal bonding. PLoS ONE 9, e88436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissman MM, Feder A, Pilowsky DJ, Olfson M, Fuentes M, Blanco C, Lantigua R, Gameroff MJ, Shea S, 2004. Depressed mothers coming to primary care: maternal reports of problems with their children. J. Affect. Disord 78, 93–100. [DOI] [PubMed] [Google Scholar]
- Welsh C, Valadez-Meltzer A, 2005. Buprenorphine: a (relatively) new treatment for opioid dependence. Psychiatry (Edgmont) 2, 29–39. [PMC free article] [PubMed] [Google Scholar]
- Whitten L, 2012. Buprenorphine during pregnancy reduces neonate distress. NIDA Notes. NIH-NIDA. http://www.drugabuse.gov/news-events/nida-notes/2012/07/buprenorphine-during-pregnancyreduces-neonate-distress. [Google Scholar]
- Wiener SG, Smotherman WP, Levine S, 1976. Influence of maternal malnutrition on pituitary-adrenal responsiveness to offspring. Physiol. Behav 17, 897–901. [DOI] [PubMed] [Google Scholar]
- Wigger A, Lorscher P, Oehler I, Keck ME, Naruo T, Neumann ID, 1999. Nonresponsiveness of the rat hypothalamo-pituitary-adrenocortical axis to parturition-related events: inhibitory action of endogenous opioids. Endocrinology 140, 2843–2849. [DOI] [PubMed] [Google Scholar]
- World Health Organization, 2014. Guidelines for the Identification and Management of Substance Use and Substance Use Disorders in Pregnancy. WHO Press, Geneva (Switzerland). [PubMed] [Google Scholar]
- Wright DM, Pill CE, Clarke G, 1983. Effect of ACTH on opiate inhibition of oxytocin release. Life Sci. 33 (Suppl 1), 495–498. [DOI] [PubMed] [Google Scholar]
- Wu CC, Hung CJ, Lin SY, Wang YY, Chang CY, Chen WY, Liao SL, Raung SL, Yang CP, Chen CJ, 2017. Treadmill exercise alleviated prenatal buprenorphine exposure-induced depression in rats. Neurochem. Int 110, 91–100. [DOI] [PubMed] [Google Scholar]
- Xu Y, Ku B, Tie L, Yao H, Jiang W, Ma X, Li X, 2006. Curcumin reverses the effects of chronic stress on behavior, the HPA axis, BDNF expression and phosphorylation of CREB. Brain Res. 1122, 56–64. [DOI] [PubMed] [Google Scholar]
- Young JL, Martin PR, 2012. Treatment of opioid dependence in the setting of pregnancy. Psychiatr. Clin. North Am 35, 441–460. [DOI] [PubMed] [Google Scholar]
- Yovell Y, Bar G, Mashiah M, Baruch Y, Briskman I, Asherov J, Lotan A, Rigbi A, Panksepp J, 2016. Ultra-low-dose buprenorphine as a time-limited treatment for severe suicidal ideation: a randomized controlled trial. Am. J. Psychiatry 173, 491–498. [DOI] [PubMed] [Google Scholar]
- Zebardast N, Crowley MJ, Bloch MH, Mayes LC, Wyk BV, Leckman JF, Pelphrey KA, Swain JE, 2013. Brain mechanisms for prepulse inhibition in adults with Tourette syndrome: initial findings. Psychiatry Res. 214, 33–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zedler BK, Mann AL, Kim MM, Amick HR, Joyce AR, Murrelle EL, Jones HE, 2016. Buprenorphine compared with methadone to treat pregnant women with opioid use disorder: a systematic review and meta-analysis of safety in the mother, fetus and child. Addiction 111, 2115–2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Torregrossa MM, Jutkiewicz EM, Shi YG, Rice KC, Woods JH, Watson SJ, Ko MC, 2006. Endogenous opioids upregulate brain-derived neurotrophic factor mRNA through delta- and micro-opioid receptors independent of antidepressant-like effects. Eur. J. Neurosci 23, 984–994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Hsu MS, Pintar JE, 1998. Developmental expression of the mu, kappa, and delta opioid receptor mRNAs in mouse. J. Neurosci 18, 2538–2549. [DOI] [PMC free article] [PubMed] [Google Scholar]