Abstract
Background
Despite effective combination antiretroviral therapy (cART), people living with HIV (PLWH) remain at risk for developing neurocognitive impairment primarily due to systemic inflammation that persists despite virologic suppression, albeit the mechanisms underlying such inflammation are poorly understood.
Methods
Herein, we evaluate the predictive capacity of the mitochondrial redox environment on circulating neuro- and T-lymphocyte-related inflammation and concomitant cognitive function in 40 virally-suppressed PLWH and 40 demographically-matched controls using structural equation modeling. We used state-of-the-art systems biology approaches including Seahorse Analyzer of mitochondrial function, electron paramagnetic resonance (EPR) spectroscopy to measure superoxide levels, antioxidant activity assays, and Meso Scale multiplex technology to quantify inflammatory proteins in the periphery.
Findings
We observed disturbances in mitochondrial function and the redox environment in PLWH compared to controls, which included reduced mitochondrial capacity (t(76) = −1.85, p = 0.034, 95% CI: −∞,−0.13), elevated levels of superoxide (t(75) = 1.70, p = 0.047, 95% CI: 8.01 E 3, ∞) and alterations in antioxidant defense mechanisms (t(74) = 1.76, p = 0.041, 95% CI: −710.92, ∞). Interestingly, alterations in both superoxide- and hydrogen peroxide-sensitive redox environments were differentially predictive of neuro-, but not T-lymphocyte-related inflammatory profiles in PLWH and controls, respectively (ps < 0.026). Finally, when accounting for superoxide-sensitive redox pathways, neuroinflammatory profiles significantly predicted domain-specific cognitive function across our sample (β = −0.24, p = 0.034, 95% CI: −0.09, −0.004 for attention; β = −0.26, p = 0.018, 95% CI: −0.10, −0.01 for premorbid function).
Interpretation
Our results suggest that precursors to neuroinflammation apparent in PLWH (i.e., mitochondrial function and redox environments) predict overall functionality and cognitive dysfunction and importantly, may serve as a proxy for characterizing inflammation-related functional decline in the future.
Keywords: Seahorse Analyzer, EPR Spectroscopy, Inflammation, Mitochondrial Respiration, Superoxide, Hydrogen Peroxide
Research in Context.
Evidence before this study
While the advent of combination antiretroviral therapy (cART) has dramatically decreased the prevalence of HIV-associated dementia, 35-70% of all people living with HIV (PLWH) are still at risk for developing some forms of cognitive impairment. One well theorized contributor to this cognitive dysfunction is inflammation that persists in the modern cART era, primarily due to the imperfect delivery of anti-HIV drugs to the central nervous system. However, the mechanisms underlying persistent and systemic inflammation in HIV-infected adults remains unknown.
The evaluation of the molecular precursors, including mitochondrial function and associated redox interactions, may aid our understanding of these pathways in the context of chronic HIV-infection and further, may serve as effective targets for inflammation-related cognitive decline in PLWH.
Added value of this study
This study uses state-of-the-art systems biology approaches to comprehensively quantify features of mitochondrial function, superoxide and hydrogen peroxide-sensitive redox systems, and inflammatory profiles in PLWH and uninfected controls. Herein, we identify the precise features of the mitochondrial redox environment that differentially predict elevated neuro- and t-lymphocyte-related inflammatory profiles. Further, this study is the first to demonstrate the contribution of redox-regulated neuroinflammatory profiles in domain-specific cognitive function in humans with and without HIV-infection.
Implications of all the available evidence
This study provides key evidence for a redox-regulated neuroinflammatory profile sensitive to superoxide redox systems that predict overall functionality and cognitive dysfunction in a large sample of virally-suppressed PLWH. Importantly, this study provides further evidence that precursors to persistent inflammation (i.e., the mitochondrial redox environment) may serve as effective proxies for other instances of chronic inflammation and cognitive dysfunction in the future.
Alt-text: Unlabelled box
1. Introduction
Effective combination antiretroviral therapy (cART) has made HIV-infection a chronic manageable condition, with life expectancies now approaching that seen in the seronegative population. However, this longevity often coincides with a host of age-related comorbidities, including cognitive dysfunction. In fact, while cART has dramatically decreased the prevalence of HIV-associated dementia (HAD), 35–70% of all people living with HIV (PLWH) still develop some form of cognitive impairment [1], [2], [3], [4], [5], [6], [7]. With the population of HIV-infected adults living longer than ever before, and often exhibiting premature or accelerated aging phenotypes [8], the mechanisms underlying HIV-related cognitive decline are of particular importance.
Persistent inflammation is the most widely theorized contributor to the cognitive decline observed in PLWH, despite the wide-spread use of cART [9]. Essentially, the imperfect delivery of anti-HIV drugs to the central nervous system likely enables ongoing viral replication and downstream release of inflammatory cytokines that effectively disrupt neuronal processing [10]. Importantly, this elevated inflammatory profile may also be mirrored in the periphery, suggesting that persistent inflammation may be systemic in PLWH [10]. However, the mechanisms underlying this tenacious inflammatory response in PLWH is poorly understood. One proposed mechanism is a mitochondrial-induced redox imbalance in the system. Under normal physiologic conditions, effective responses to invading pathogens and acute infections requires mitochondrial-produced energy as well as reactive oxygen species (ROS) to initiate an appropriate immune response and to promote inflammation [11], [12], [13], [14]. This physiological shift often results in dynamic changes to mitochondrial respiratory profiles, including increases in basal and maximal oxygen consumption rates (OCR), as well as increased spare respiratory capacity (SRC), reflecting the increased energy reserve available to meet changing bioenergetic demands and subsequent ROS production [11,12,14]. In regard to HIV-infection, only a few studies have evaluated changes in the mitochondrial redox environment in response to these chronic immunocompromised states, especially in the context of human physiology. In contrast to acute immune system activation, mitochondrial respiration seems to decrease in the context of HIV-infection, such that seropositive adults exhibit decreases in basal and maximal respiration, leading to reduced SRC and increased ROS production in the periphery [15]. However, these results should be considered with caution, as these studies included adults with and without cART-induced viral suppression, and cART has been shown to directly impact cellular respiratory and redox profiles, regardless of HIV exposure [16,17]. Importantly, these data, combined with the well-established literature linking mitochondrial functionality and redox interactions (e.g., ROS and antioxidant defense mechanisms) to a host of age- and disease-related comorbidities [18], suggest that analysis of mitochondrial function and redox environment may provide novel mechanistic insight into systemic inflammation and further, the cognitive dysfunction observed in PLWH.
Thus, the goal of the current study was to comprehensively quantify features of mitochondrial function and the redox environment, and to assess the predictive capacity of these parameters on inflammatory profiles and cognitive dysfunction in PLWH using structural equation modeling. To this end, we recruited 40 PLWH with virologic suppression who were on a stable regimen of cART and 40 demographically-matched uninfected controls. Importantly, we chose to study a relatively healthy sample of PLWH in hopes to parse apart the unique effects of HIV-infection on these mitochondrial redox-inflammatory pathways above and beyond the potential confounding effects of comorbidities (e.g., substance abuse, neurological or psychiatric disorders), which is relatively understudied in the context of HIV. In addition, unlike previous studies using qualitative or indirect measures of the redox environment (e.g., fluorescence), we used state-of-the-art systems biology approaches to directly quantify mitochondrial respiration in real time using Seahorse Analyzer, total intracellular superoxide levels using Electron Paramagnetic Resonance (EPR) Spectroscopy, enzymatic and non-enzymatic antioxidant activity assays, and 54 circulating cytokines using Meso Scale multiplex technology. We hypothesized [1] that oxygen consumption rates of mitochondria would be reduced in PLWH, suggesting a less efficient bioenergetic capacity which [2] leads to an increased generation of superoxide concomitant with decreases in antioxidant defense mechanisms (e.g., superoxide dismutase, catalase, glutathione), indicative of a redox imbalance in the system. In addition, we hypothesized that elevated levels of circulating inflammatory proteins would be differentially modulated by these features of the redox environment in PLWH compared to uninfected controls and further, would index cognitive and behavioral dysfunction across the sample. Importantly, our results provide novel evidence that superoxide-sensitive redox environments are pertinent to the neuroinflammatory-related, but not T-lymphocyte-related inflammatory profiles observed in PLWH and further, are associated with deficits in attention and premorbid functioning in a healthy sample of virally-suppressed PLWH.
2. Methods
2.1. Participant demographics
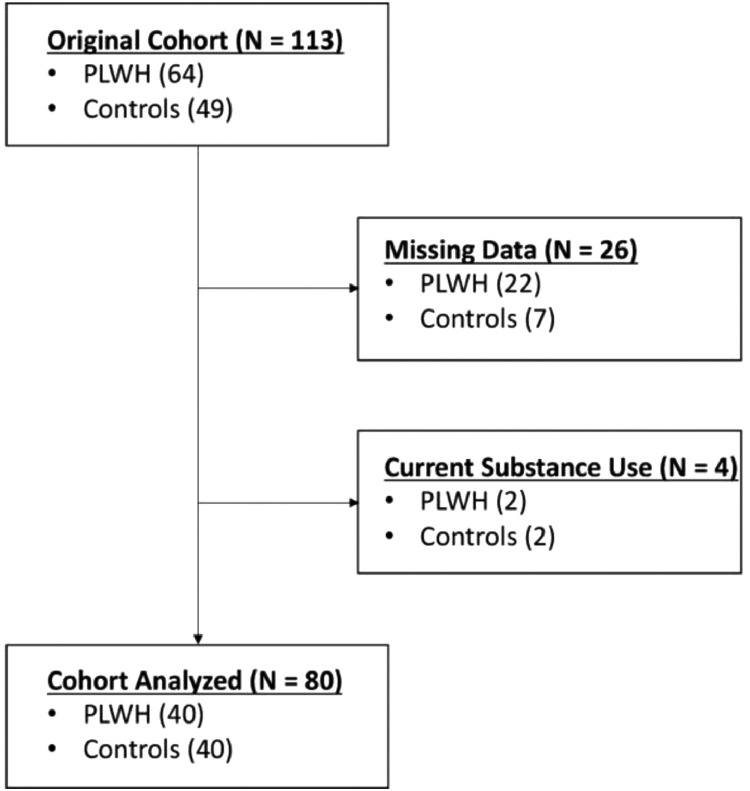
We enrolled 113 adults (range: 20–66 years old; 64 PLWH and 49 controls) in this study. Recruitment began for this study in February 2019. All PLWH were receiving effective cART and had viral suppression defined as <50 copies/mL. Exclusion criteria included any medical illness affecting CNS function (other than HIV), any psychiatric or neurological disorder, history of head trauma, current pregnancy and current substance use. Following all exclusions (Fig. 1), 80 participants remained, including 40 PLWH and 40 healthy controls. Uninfected controls were enrolled to demographically match PLWH based on their age (t(78) = 0.18, p = 0.857, 95% CI: −6.71, 5.59; [independent-samples t-test]), sex (X2 = 3.65, p = 0.095, 95% CI: [Chi-square test]), handedness (X2 = 0.16, p = 0.999, 95% CI: [Chi-square test]), and body mass index (t(78) = −1.12, p = 0.267, 95% CI: −1.14, 4.04; [independent-samples t-test]) (see Table 1). Based on previous relevant studies from our laboratory evaluating virally-supressed PLWH and healthy, demographically-matched uninfected controls[19], [20], [21], [22], concomitant with post hoc power analyses based on Monte Carlo simulations (see Supplementary Materials), we had a reasonable sample size per group for all redox, inflammatory and behavioural comparisons.
Fig. 1.
Flow diagram. A total of 113 participants were enrolled, including 64 PLWH and 49 controls following initial screening for eligibility. Further exclusions were made for current substance use and missing data. The final sample included 40 PLWH and 40 controls.
Table 1.
Participant demographics and neuropsychological assessments of cohort analyzed. Independent samples t-tests were used to evaluate cognitive function (demographically-adjusted composite z-scores) in PLWH compared to uninfected controls. *p = 0.014, ***p = 0.002. PLWH exhibited significant reductions in attention and premorbid function normed z-scores compared to demographically-matched uninfected controls, indicative of worse performance on these neuropsychological domains.
| Controls | PLWH | |
| Demographics: Median (Interquartile Range) | ||
| N | 40 | 40 |
| Age (yrs) | 45.5 (22.1) | 47.7 (24.9) |
| Sex (% males) | 57.5 | 77.5 |
| Handedness (% right handed) | 92.5 | 90.0 |
| Education (yrs) | 16.0 (4.0) | 14.0 (4.0) |
| BMI | 26.7 (6.6) | 28.2 (7.6) |
| CD4 Nadir (cells/μL) | – | 241 (274) |
| Current CD4 (cells/μL) | – | 710 (362) |
| Time since diagnosis (yrs) | – | 11[16] |
| Time on ART (yrs) | – | 8[12] |
| Neuropsychological Assessments (Normed Z-scores) | ||
| Learning | 0.58 (0.79) | 0.32 (1.1) |
| Memory | 0.50 (0.69) | 0.21 (1.25) |
| Processing Speed | 0.35 (0.78) | 0.19 (0.92) |
| Attention*** | 0.33 (1.21) | −0.17 (0.87) |
| Executive Function | 0.58 (0.78) | 0.23 (1.06) |
| Language | -0.10 (0.91) | −0.23 (1.13) |
| Motor Function | 0.00 (1.24) | −0.10 (1.23) |
| Premorbid Function* | 0.33 (0.93) | −0.07 (1.02) |
2.2. Ethics
The University of Nebraska Medical Center Institutional Review Board approved the study (403-18-EP) and all participants provided written informed consent. Data from this study will be made available to qualified investigators upon reasonable request to the corresponding author.
2.3. Isolation of peripheral blood mononuclear cells and respiration analysis
Whole blood (~45 mL) was collected into EDTA tubes by venous puncture for all participants. Buffy coats were submitted to a Ficoll-Paque Plus (GE Healthcare) gradient centrifugation for isolation of the mononuclear fraction. PBMCs were cryopreserved in Fetal Bovine Serum with 10% DMSO. Cells were thawed within 6 weeks of isolation and underwent assessment using the Seahorse XF96 Analyzer (Seahorse Bioscience) to quantify oxygen consumption rate (OCR) using the mitochondrial stress test assay. Specifically, PBMCs were plated at 500,000 cells/well and 3 OCR measurements were taken sequentially on 5-6 technical replicate wells prior to and upon serial injection of 3.5 μM oligomycin (Sigma; complex V inhibitor), 1 μM fluoro-carbonyl cyanide phenylhydrazone (FCCP; Sigma; mitochondrial oxidative phosphorylation uncoupler) and 14 μM rotenone + 14 μM antimycin A (Sigma; complex I and III inhibitors, respectively) to evaluate features of mitochondrial respiration including basal respiration, proton leak, maximal respiration, and non-mitochondrial respiration. Importantly, each feature was average of the 3 OCR measurements following each serial injection. Additionally, the spare respiratory capacity of the mitochondria was quantified by subtracting average basal respiration from the average maximal respiration and reflects the cell's adaptability to changing energy demands induced by specific electron transport chain inhibitors and uncouplers. Finally, mitochondrial respiration associated with ATP production (i.e., ATP-linked respiration) was quantified by subtracting the average proton leak from the average basal respiration observed in the PBMCs. All bioenergetic data were normalized to protein in the well and treated as independent, continuous measures of mitochondrial function for subsequent analyses. For data calculation, the Seahorse Wave software (v2.2.0) was used.
2.4. Quantification of the redox environment
Cellular levels of superoxide were assessed using Electron Paramagnetic Resonance (EPR) Spectroscopy of whole blood incubated with a superoxide-sensitive spin probe (1-hydroxy-3-methoxycarbonyl1-2,2,5,5-tetramethylpyrrolidine: CMH) for 1 h under physiologic conditions (37 ˚C), as previously described [23]. Specifically, immediately after sample collection, 200 μM of CMH was reconstituted into EPR buffer (Krebs Hepes Buffer) supplemented with metal chelators (5 μM sodium diethyldithiocarbamate trihydrate and 25 μM deferoxamine) and incubated with 200 μL of whole blood. EPR measurements were performed with a Bruker eScan EPR spectrometer (Bruker BioSpin GmbH, Rheinstetten/Karlsruhe, Germany), with the following parameters: field sweep width, 100.0 G; center field, 3482 G; microwave frequency, 9.75 kHz; microwave power, 1.10 mW; modulation amplitude, 5.94 G; conversion time, 10.24 ms; time constant, 40.96 ms. The resulting EPR spectra amplitude is expressed as arbitrary units (a.u.) that are directly proportional to the amount of total cellular superoxide in the sample.
Antioxidant activity levels were quantified in erythrocytes for key enzymatic and non-enzymatic contributors to the mitochondrial redox environment including superoxide dismutase (SOD), catalase, and glutathione. Specifically, we used the SOD Assay Kit-WST (DOJINDO, Inc.) to measure total SOD activity, the OxiSelect Catalase Activity Assay Kit (Cell Biolabs, Inc.) for catalase, and the GSSG/GSH Quantification kit (DOJINDO, Inc.) for total (tGSH), oxidized (GSSG) and reduced glutathione (GSH) according to the manufacturers’ guidelines.
2.5. Evaluation of inflammatory profiles
Comprehensive quantification of cytokine levels were conducted based on electrochemiluminescence-based multiplex immunoassays in plasma. Specifically, samples were prepared on multispot 96-well plates from the V-PLEX Human Cytokine 54-Plex Kit (Meso Scale Discovery) according to manufacturer instructions. Plates were analyzed using a Meso Scale QuickPlex SQ 120 and sample concentrations were calculated using the Discovery Workbench 4.0 software using a 4-PL curve model. Any concentrations below the lower level of detection based on 2.5 SD above the assay background blank were reported as 0 pg/mL.
2.6. Neuropsychological assessment
Cognitive function was based on a neuropsychological battery that assessed functionality across six domains (i.e., learning, memory, executive function, attention, processing speed, motor function, premorbid function). The battery included the following tests for each domain: learning (Wechsler Memory Scale (WMS-III) Logical Memory Initial Recall [24] California Verbal Learning Test (CVLT-II) Learning Trials 1-5 [25]), memory (CVLT-II Delayed Recall and Recognition Discriminability Index [25], WMS-III Logical Memory II Delayed Recall[24]), executive function (Comalli Stroop Test Interference Trial [26] and Trail Making Test Part B [27]), processing speed (Comalli Stroop Test Color and Word Trials [26], Wechsler Adult Intelligence Scale (WAIS-III) Digit Symbol Coding [28], and Trail Making Part A [27]), attention (WAIS-III Letter Number Sequencing [28], WAIS-III Digit Span Forward and Backward Trials [28], CVLT-II Trial 1 [25]), motor (Grooved Pegboard, Dominant and Non-Dominant Hands [29]), and premorbid function (Wide Range Achievement Test 4 (WRAT-4) Word Reading [30]). Demographically-adjusted Z-scores were computed using published normative data and composite domain-specific scores were calculated by averaging the Z-scores of assessments comprising each domain respectively.
2.7. Statistical analysis
To evaluate HIV-related changes in mitochondrial redox environment, relevant parameters of mitochondrial function (e.g., basal respiration) and the redox environment (e.g., superoxide levels) underwent independent samples t-tests following standard data evaluation procedures. Specifically, bioenergetic and redox data underwent standard data evaluation protocols such that measures exceeding 2.5 standard deviations above or below the group's mean were excluded from subsequent analyses. Next, we evaluated assumptions of equal variance using the Levene's test (p < 0.05) and conducted independent-samples t-tests (one-tailed) on these mitochondrial redox parameters as a function of HIV-infection assuming non-equal variance where appropriate. In order to characterize comprehensive changes in the inflammatory markers as a function of HIV, a profile analysis of HIV-infection was conducted using multivariate analysis of variance (MANOVA) with Bonferroni correction for multiple comparisons. Specifically, 48 inflammatory cytokines measured in plasma were assigned as dependent variables, with group designation (i.e., PLWH vs. controls) identified as an independent variable with 2 levels.
Next, we aimed to evaluate the predictive path by which the mitochondrial redox environment leads to changes in inflammatory cytokines elevated as a function of HIV-infection (see MANOVA model described above). To begin, we conducted principal components analyses to define a single component of inflammatory profiles using the compilation of cytokines exhibiting significant alterations as a function of HIV-infection that are known to contribute to disparate physiological processes (e.g., neuro-, T-lymphocyte-related, vascular and chemokine inflammatory functions; Table S1). Given our study population, we focused our latent variable extraction on those cytokines known to contribute to neuro and T-lymphocyte-related inflammatory processes in the system (for achieved loadings, see Table S2). As such, inflammatory profile scores were subsequently extracted per participant for neuro and T-lymphocyte markers, separately and entered as dependent variables in our structural equation model (all two-tailed p-values reported). We tested the hypothesis that alterations in mitochondrial respiration (i.e., SRC, ATP-linked respiration, non-mitochondrial respiration) would predict an imbalance in the redox environment (for superoxide and H2O2-sensitive markers assessed separately) and subsequent pro-inflammatory profiles differentially in PLWH versus uninfected controls using structural equation modeling. Specifically, we conducted a group-based path analysis with measures of mitochondrial respiration as predictors of the redox environment, which yields parameter estimates for PLWH versus uninfected controls (all two-tailed p-values reported). Of note, we did not include measures of basal OCR in this analysis as other parameters of mitochondrial function (i.e., SRC, ATP-linked OCR) are directly dependent on levels of basal respiration and thus could lead to multicollinearity and inaccurate model estimations. Finally, we also tested for any mediating effects of the redox environment on the mitochondrial-inflammatory pathways through changes in the redox environment (i.e., superoxide and SOD; catalase activity and GSSG/tGSH ratio) by assessing the standard errors and p-values based on 1000 bootstrapped samples. All principal components and path analyses were conducted using the lavaan package in R with full information maximum likelihood estimation employed for missing data.
Finally, we aimed to examine whether mitochondrial redox-regulated inflammatory profiles were predictive of cognitive function assessed using demographically-adjusted neuropsychological test scores. Essentially, we computed a predicted neuroinflammatory profile score per participant accounting for levels of mitochondrial function and the redox environment using the regression equation described above, with higher values indicative of elevated neuroinflammatory cytokines. Next, we conducted a series of linear regressions to determine the predictive capacity of redox-regulated neuroinflammatory profiles on cognitive domains and premorbid function. Importantly, our study ensured that multiplicity (i.e., potential inflation of Type 1 error rate) was not allowed through stringent correction for multiple comparisons where appropriate.
2.8. Role of funding source
The funding sources for this study had no role in study design, data collection, data analyses or interpretation nor writing of the report as it is presented herein.
3. Results
3.1. Mitochondrial respiration in PLWH
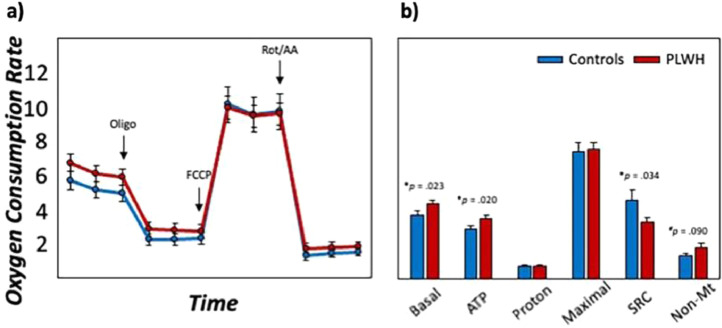
Bioenergetic data of PBMCs revealed distinct alterations in mitochondrial respiration as a function of HIV-infection. Interestingly, we observed statistically significant increases in basal oxygen consumption rates (OCR; t(74) = 2.03, p = 0.023, 95% CI: 0.12, ∞; [independent-samples t-test]), ATP-linked OCR (i.e., basal respiration—proton leak; t(76) = 2.09, p = 0.020, 95% CI: 0.13, ∞; [independent-samples t-test]) and marginally increased levels of non-mitochondrial respiration in PLWH compared to uninfected controls (t(74) = 1.35, p = 0.090, 95% CI: −0.11, ∞; [independent-samples t-test]; Fig. 2, Table S3). In contrast, PLWH exhibited statistically significant reductions in the spare respiratory capacity (SRC; maximal respiration – basal respiration; t(76) = -1.85, p = 0.034, 95% CI: -∞,−0.13; [independent-samples t-test]) of the mitochondria compared to controls, suggesting that mitochondria in PLWH have an impaired adaptability when faced with increased energy demands.
Fig. 2.
Mitochondrial energetics disrupted in PLWH. (a): Oxygen consumption rates (OCR) were recorded using Seahorse XF96 Analyzer during serial injections of electron transport chain inhibitors (i.e., oligo, rot/AA) and a mitochondrial oxidative phosphorylation uncoupler (i.e., FCCP) to quantify mitochondrial respiration. (b): Measures of mitochondrial function in response to mitochondrial stress tests included basal respiration (basal), ATP-linked respiration (ATP; i.e., basal respiration—proton leak), proton leak (proton), maximal respiration (maximal), spare respiratory capacity (SRC; i.e., maximal respiration—basal respiration), and non-mitochondrial respiration (non-Mt) for uninfected controls (shown in blue) and PLWH (shown in red). Y-axes are fixed for both graphs. N = 80, #p < .01, *p < .05.
3.2. Superoxide and H2O2 redox profiles
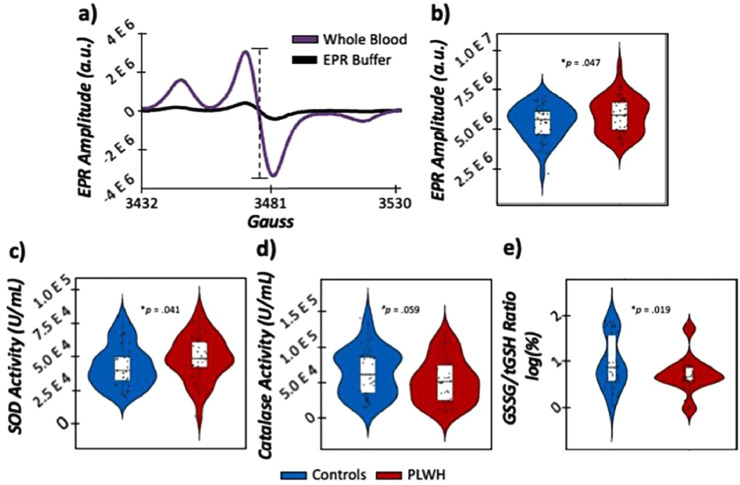
In order to comprehensively quantify the redox environment in our participants, we conducted EPR spectroscopy on whole blood incubated with a superoxide-sensitive spin probe (i.e., CMH) to evaluate total levels of cellular superoxide in the periphery. In addition, commercially-available assays were collected on erythrocytes for key antioxidants known to scavenge ROS. In regard to superoxide-sensitive redox environments, we observed statistically significant elevations in superoxide concentrations (i.e., EPR amplitude; t(75) = 1.70, p = 0.047, 95% CI: 8.01 E 3, ∞; [independent-samples t-test]), concomitant with elevations in total SOD activity (i.e., the antioxidant important for scavenging superoxide; t(74) = 1.76, p = 0.041, 95% CI: −710.92, ∞; [independent-samples t-test]) in PLWH compared to controls (Fig. 3, Table S3). In contrast, antioxidants important for scavenging H2O2 were differentially modulated as a function of HIV-infection. Specifically, we observed marginal reductions in catalase activity in PLWH (t(71) = −1.59, p = 0.059, 95% CI: -∞, 627.68; [independent-samples t-test]). Additionally, we assessed levels of total (tGSH), oxidized (GSSG), and reduced (GSH) forms of glutathione, and observed statistically significant reductions in GSSG in PLWH (t(75) = −3.03, p = 0.002, 95% CI: -∞, −94.20; [independent-samples t-test]), while tGSH and GSH were unaffected by HIV-infection (ps > .183; [independent-samples t-test]). Ultimately, this reduction in GSSG likely resulted in the significantly reduced GSSG/tGSH ratios also observed in PLWH compared to controls (t(72) = −2.123, p = 0.019, 95% CI: −∞, −0.05; [independent-samples t-test]), with lower values indicative of larger reducing capacity of glutathione in PLWH. Taken together, these results suggest that both superoxide- and H2O2-sensitive redox profiles are disrupted in PLWH.
Fig. 3.
Evaluation of the redox environment in PLWH. (a): EPR spectra of whole blood (shown in purple) incubated with a superoxide-sensitive spin probe compared to EPR buffer incubated with the same spin probe (shown in black) for a representative subject. The x-axis denotes magnetic field strength (gauss) and the y-axis denotes EPR amplitude (in arbitrary units (a.u.)), with the absolute peak-to-trough distance (denoted in the dashed line) directly proportional to the amount of superoxide in the sample. (b): EPR amplitude was significantly elevated in PLWH (shown in red) compared to controls (shown in blue), indicative of greater superoxide concentrations in PLWH. (Bottom row): Commercially-available antioxidant activity assays revealed elevated levels of SOD (c), reduced levels of catalase (d) and reduced GSSG/tGSH ratios (e) in PLWH compared to controls. N = 80, #p < 0.01, *p < 0.05.
3.3. Profile analysis of inflammation in HIV
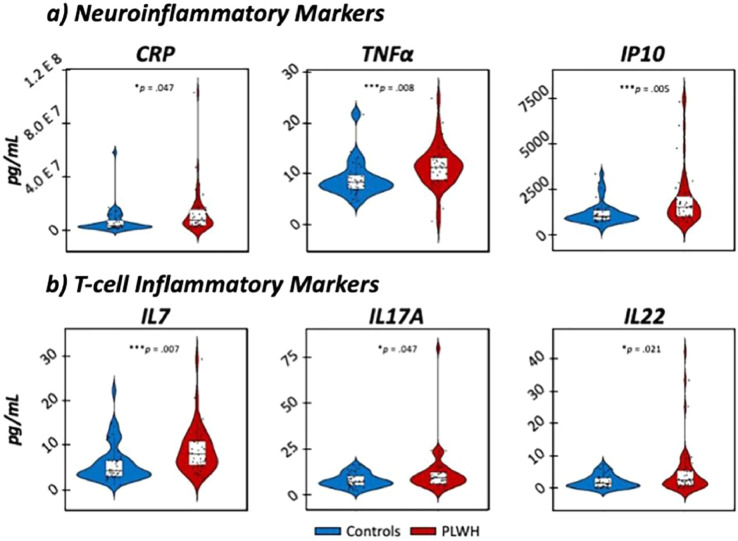
Statistical analysis of electrochemiluminescence-based immunoassays in plasma revealed substantial elevations in inflammatory cytokines in virally-suppressed PLWH compared to uninfected controls (for a statistical summary, see Table S1). Specifically, a MANOVA of the comprehensive 54-biomarker assay revealed elevations in markers important for neuroinflammatory processes (i.e., CRP, TNFα, IP10), T-lymphocyte development and inflammation (i.e., IL-17A, IL-22, IL-7), vascular functionality (i.e., PIGF, SAA, sICAM1) and chemotaxis (i.e., M IP3α, MCP1), all of which survived stringent Bonferroni correction for multiple comparisons (all ps corrected < .047; [MANOVA]; Figure 4). As described in the Supplementary Materials, we focused our subsequent analyses on markers co ntributing to neuro- and T-lymphocyte-related inflammatory responses and conducted principal component analyses to yield a single component comprising neuro and T-cell cytokines, separately (for achieved loadings, see the Supplementary Materials). Importantly, our factor defined by neuroinflammatory markers accounted for 54.90% of the total variance in neuroinflammatory profiles (eigenvalue = 1.65), while our component defined by T-cell cytokines accounted for 46.45% of the total variance in T-cell inflammatory profiles (eigenvalue = 1.39), with higher values for both extracted scores indicative of greater levels of inflammatory cytokines.
Fig. 4.
Elevated neuroinflammatory and t-lymphocyte inflammatory markers in PLWH. MANOVA models of 54 inflammatory cytokines revealed significant elevations in key contributors to neuroinflammatory (a: i.e., CRP, TNFα, IP10) and T-cell regulatory (b: i.e., IL-7, IL-17A, IL-22) immune responses in PLWH (shown in red) compared to controls (shown in blue). N = 80, *p corrected < 0.05, ***p corrected < 0.005.
3.4. Superoxide-sensitive pathways predict neuroinflammation in PLWH
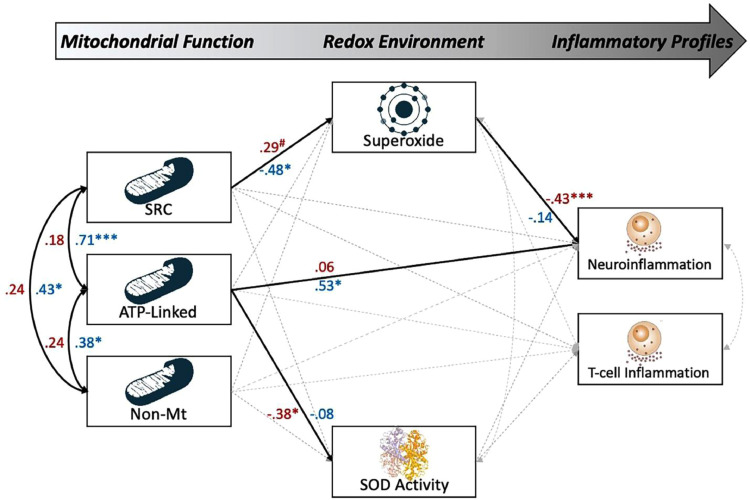
To interrogate the predictive capacity of the redox environment on neuro and T-lymphocyte inflammatory profiles in our sample, we conducted a path analysis with parameters disrupted by the superoxide and mitochondrial function (i.e., SRC, ATP-linked respiration, non-mitochondrial respiration, superoxide concentration, SOD activity) as continuous predictors of neuro- and T-lymphocyte inflammatory scores in PLWH and controls (for a full model visualization, see Fig. 5). Interestingly, we observed statistically significant direct effects of mitochondrial function on neuroinflammatory scores in controls, such that increases in ATP-linked respiration were associated with elevated neuroinflammatory cytokines (β = 0.53, b = 0.28, p = 0.013, 95% CI: 0.06, 0.51; [SEM]; Fig. 5). In addition, we observed a robust direct effect of superoxide on neuroinflammation in PLWH (β = −0.43, b = −0.40, p = 0.002, 95% CI: -0.68, −0.12; [structural equation model: SEM]), but not controls (p = .459; [SEM]), such that greater levels of superoxide were predictive of decreased levels of inflammation. In addition, levels of superoxide were differentially predicted by the SRC of the mitochondria for PLWH (β = .29, p = 0.062, 95% CI: 0.03, 0.54; [SEM]) and controls (β = −0.48, b = −0.17, p = 0.021, 95% CI: −0.32, −0.03; [SEM]), suggesting that in controls, decreases in SRC led to elevated levels of superoxide, while increases in SRC led to this elevation in PLWH. Finally, we observed no statistically significant mediating effects in either group. Importantly, superoxide and mitochondrial function accounted for 22% of the variance in neuroinflammatory profiles of PLWH, but only 18% in controls.
Fig. 5.
Superoxide-sensitive mitochondrial redox environment predicts neuroinflammatory profiles in PLWH. Results of the group-based structural equation model exploring the predictive capacity of superoxide-sensitive mitochondrial redox environments on neuro and T-lymphocyte inflammatory scores. Single-headed arrows denote predictive paths and double-headed curved arrows denote correlations. Statistically significant estimates (p < 0.05, two-tailed) are denoted with solid lines, while non-significant paths are denoted with dashed gray lines. All listed parameters are standardized coefficients for controls (shown in blue) and PLWH (shown in red).Together, superoxide-sensitive pathways accounted for 22% of the variance in neuroinflammation in PLWH, but not in controls. N = 80, #p < 0.10, *p < 0.05, ***p < 0.005.
3.5. H2O2-sensitive pathways predict neuroinflammation in controls
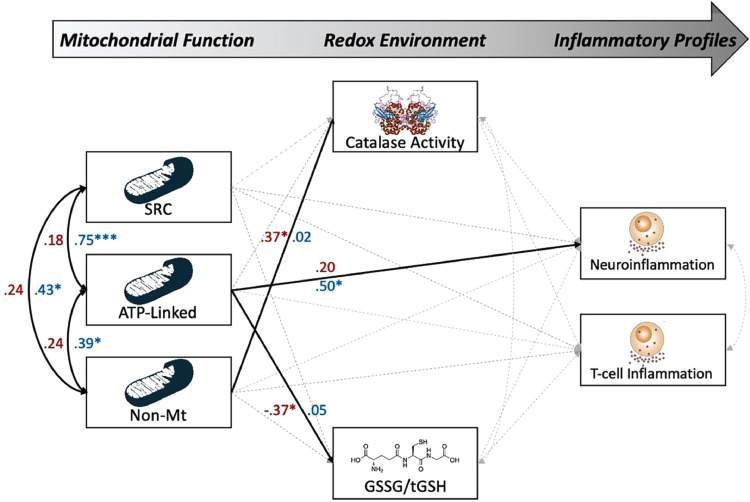
Next, we evaluated the predictive capacity of H2O2-sensitive redox environments and mitochondrial function on inflammatory profiles using the same regression model described above. However, our measures of the redox environment were now comprised of catalase activity and GSSG/tGSH ratios as continuous predictors of neuro- and T-lymphocyte-related inflammatory profiles. For a full model visualization, see Fig. 6. Similar to our model of superoxide-sensitive pathways, we observed a statistically significant direct effect of ATP-linked respiration on neuroinflammatory scores in controls (β = 0.50, b = 0.27, p = 0.021, 95% CI: 0.04, 0.50; [SEM], Fig. 6), but not in PLWH (p = 0.253; [SEM]), such that increases in ATP-linked OCR were predictive of more inflammation. In addition, we observed statistically significant direct effects of mitochondrial respiration on H2O2-sensitive parameters of the redox environment in PLWH, such that increases in ATP-linked OCR were predictive of reduced GSSG/tGSH ratios (i.e., more reducing capacity of GSH (β = −0.37, b = -0.12, p = 0.024, 95% CI: −0.22, −0.02; [SEM]), while increases in non-mitochondrial respiration were predictive of increased catalase activity (β = 0.37, b = 0.01, p = 0.026, 95%CI: 0.00, 0.01; [SEM]). Finally, we observed no statistically significant mediating effects of the redox environment in either group. Taken together, H2O2-sensitive redox environments and mitochondrial function accounted for 23% of the variance in neuroinflammatory profiles in controls, but only 7.9% in PLWH.
Fig. 6.
H2O2-Sensitive mitochondrial redox environment predicts neuroinflammatory profiles in PLWH. Results of the group-based structural equation model exploring the predictive capacity of H2O2-sensitive mitochondrial redox environments on neuro and T-lymphocyte inflammatory scores. Single-headed arrows denote predictive paths and double-headed curved arrows denote correlations. Statistically significant estimates (p < .05, two-tailed) are denoted with solid lines, while non-significant paths are denoted with dashed gray lines. All listed parameters are standardized coefficients for controls (shown in blue) and PLWH (shown in red). Together, measures of the H2O2-sensitive mitochondrial redox environment accounted for 23% of the variance in neuroinflammatory profiles in controls, but not in PLWH. N = 80, *p < 0.05, ***p < 0.005.
3.6. Cognitive function is modulated by mitochondrial redox-regulated neuroinflammation
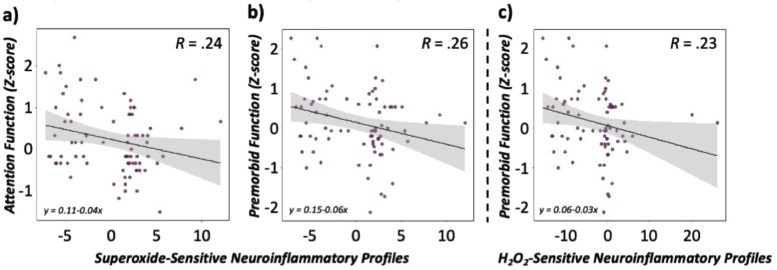
Finally, we assessed whether neuroinflammatory profiles regulated by the redox environment were associated with cognitive function assessed using an extensive neuropsychological battery. As described in the methods, we computed a predicted neuroinflammatory score for PLWH and controls using the regression equation described above for superoxide- and H2O2-sensitive pathways, separately. This yielded a predicted value of neuroinflammation, with higher values indicative of greater redox-regulated inflammatory profiles that were specific to both groups. Interestingly, neuroinflammatory profiles predicted by changes in superoxide-sensitive redox environments were significantly associated with demographically-adjusted attention composite scores and premorbid functioning (Z-scores), such that increases in neuroinflammatory profiles were associated with worse performance on these domains regardless of group (β = −0.24, b = −0.05, p = 0.034, 95% CI: -0.09, −0.004; [regression] for attention; Fig. 6), (β = −0.26, b = −0.06, p = 0.018, 95% CI: -0.10, −0.01; [regression] for premorbid function; Fig. 7). In contrast, increased neuroinflammatory profiles accounting for changes in the H2O2-sensitive redox environment were only associated with deficits in premorbid functioning regardless of group (β = −0.23, b = −0.03, p = 0.037, 95% CI: −0.06, −0.002; [regression]; Fig. 7).
Fig. 7.
Redox-sensitive neuroinflammatory profiles predict cognitive function. (a) Predicted values of neuroinflammation accounting for levels of the superoxide-sensitive redox environment (a,b: left, middle) and H2O2-sensitive environment (c: right) are denoted on the x-axis, with higher values indicative of elevated inflammatory markers. The y-axis denotes demographically-adjusted domain composite z-scores for attention (left) and premorbid function (middle, right). Data are presented across all participants (scatter points shown in purple) with 95% confidence intervals (shown in grey) surrounding the regression line denoted in black. N = 80.
4. Discussion
Using advanced, quantitative measurements of mitochondrial function, the redox environment, inflammatory profiles, and extensive neuropsychological testing, we interrogated the predictive capacity of the redox environment and mitochondrial function on elevated inflammatory profiles and cognitive dysfunction in a group of PLWH with viral-suppression and uninfected controls. Specifically, we observed disruptions in mitochondrial respiration, such that basal, ATP-linked and non-mitochondrial respiration were increased in concert with a marked reduction in mitochondrial energetic reserves (i.e., spare respiratory capacity) in PLWH compared to controls. In regard to the redox environment, we observed significant increases in superoxide-sensitive features, including elevated levels of superoxide and the antioxidant important for scavenging it (i.e., superoxide dismutase). In contrast, PLWH exhibited reductions in catalase and oxidized forms of glutathione, suggesting that H2O2-involved pathways may also be disrupted in PLWH. Finally, our study was the first to effectively link key features of mitochondrial function and redox status to elevated levels of the inflammatory cytokines important for neuro-related, but not T-lymphocyte-related immune responses, and cognitive function in our sample. We discuss the implications of these novel findings below.
Previous investigations regarding the role of mitochondria in immune system integrity suggest a preferential shift of functionality in response to foreign pathogens/infections. Amongst these changes in mitochondrial metabolism are increases in the consumption of oxygen concomitant with increases in ROS production for downstream signaling mechanisms upon T-cell activation, although the opposite directionality (i.e., decreased oxygen consumption with increased ROS) has been shown during instances of chronic infection as seen in HIV [15,31,32]. In line with our hypotheses, we observed robust reductions in the SRC of mitochondria in PLWH, indicative of reduced bioenergetic capacities when faced with changing energy demands. Interestingly, this result was likely attributable to the increases in basal OCR observed in PLWH, which contradicts previous reports in humans and animals. For example, only one prior study has evaluated the dynamic changes in the OCR of immune cells in PLWH and found significant decreases in basal and maximal OCR, leading to impaired SRC [15], while other reports have corroborated this trajectory in animal models treated with the HIV protein gp-120 [31,32]. One factor that could contribute to this discrepancy is likely the cellular architecture comprising our PBMC samples. For instance, previous work suggests that immune cells (e.g., T-lymphocytes, B-lymphocytes) have disparate respiratory profiles relating to subsequent changes in cellular activation [15]. Thus, the discrepant trajectories observed in the current study could be attributable, at least in part, to the cellular composition of our PBMC samples that were not further purified into their respective cell types for subsequent respiratory analyses. Future work could use methods such as flow cytometry to directly quantify the contribution of these cellular profiles. Nevertheless, our data and previous reports suggest that immune cell mitochondrial adaptability is impaired in PLWH (i.e., reduced SRC), albeit the mechanism leading to this inefficiency may be dependent on sample characteristics.
In regard to the redox environment, we observed increases in total cellular superoxide concomitant with increased activity of the antioxidant important for scavenging it (i.e., total SOD) in PLWH compared to uninfected controls, suggesting that elevated levels of SOD activity may be a compensatory strategy employed to combat the increased levels of superoxide in PLWH [15,33,34]. In addition, we observed differential modulations of H2O2-involved scavengers, such that activity levels of catalase and oxidized glutathione were reduced in HIV-infected adults. These results were somewhat surprising, as previous studies have shown either no change or opposing trajectories of catalase and glutathione levels, indicative of an overall decrease in the reducing capacity of H2O2 in PLWH [33], [34], [35]. Nevertheless, the diverse alterations in antioxidant defense systems observed in the current sample potentially point to a H2O2-pertinent mechanism also contributing to the oxidative environment in PLWH. As the direct product of the superoxide-SOD scavenging reaction is H2O2, increases in both superoxide and SOD may result in elevated levels of H2O2, which aligns well with the reductions observed for the main scavenger of it (i.e., catalase)[36]. However, the stronger reducing capacity of glutathione (i.e., GSSG/tGSH ratio) observed in the current study may be working to assist the catalase-deficient scavenging of H2O2. Although we did not directly quantify levels of H2O2 in our sample, our study is the first to provide comprehensive evidence for alterations in both superoxide- and H2O2-sensitive mitochondrial redox environments as a function of HIV-infection, and future work directly evaluating levels of H2O2 will be invaluable to fully unravel this relationship.
Our most important finding was likely the differential modulation of elevated inflammatory profiles by superoxide- and H2O2-sensitive redox environments and mitochondrial function in PLWH compared to controls. Briefly, using structural equation modeling we observed separable paths by which superoxide-sensitive mechanisms significantly predicted neuroinflammatory profiles in HIV-infected adults (22% of the variance in neuroinflammation), while H2O2-sensitive paths were predictive of this change in controls (23% of the variance in neuroinflammation). Importantly, this effect seems to be specific to neuroinflammatory responses (i.e., component score derived from levels of CRP, TNFα, IP10), as neither path significantly predicted T-lymphocyte-related inflammatory profiles (i.e., component score derived from levels of IL17, IL17A, IL22) in either group. It is not surprising that superoxide-sensitive measures of the redox environment directly predicted inflammatory profiles in PLWH. Although not the direct defender of invading pathogens, increases in mitochondrial energetic capacities and subsequent superoxide production has been shown to be critical for normative immune responses (e.g., T-lymphocyte maturation and activation, secretion of circulating cytokines) to both acute and chronic infections [[11], [12], [13], [14],37]. However, ours is the first study to directly link measures of superoxide-sensitive redox profiles to instances of neuro-related, but not T-lymphocyte-related inflammatory responses, which has been reported extensively elsewhere [[11], [12], [13], [14], [15],37]. In addition, H2O2-sensitive mechanisms were predictive of this change in controls, although this result requires careful interpretation, as neither measure of catalase nor glutathione reducing capacity (i.e., H2O2-sensitive redox metrics) were direct modulators of neuroinflammation above and beyond levels of ATP-linked mitochondrial respiration alone.
Finally, our study was the first to effectively link redox-regulated inflammatory profiles to performance on neuropsychological tests in a large sample of seropositive and seronegative adults. Specifically, using predicted values of neuroinflammation accounting for levels of superoxide- and H2O2-sensitive paths separately, we observed significant associations with attention and premorbid function, such that increased levels of superoxide-sensitive neuroinflammatory profiles were associated with worse performance on these domains, while increased levels of H2O2-sensitive predicted neuroinflammation was associated with deficits in premorbid function across the entire sample. These results are not surprising, considering the markers comprising our extracted score of neuroinflammation. For example, increases in circulating levels of CRP, TNFα, and IP10 have long been revered as important correlates of age- and disease-related cognitive dysfunction [38], [39], [40], [41], [42], [43], [44], [45], [46]. While previous reports have linked elevations in these inflammatory cytokines to HIV-related disease metrics (e.g., CD4 count, time to AIDS progression, etc.), mortality rates and opportunistic diseases [47], [48], [49], [50], our study is the first to demonstrate a relationship between these markers and cognitive performance in a relatively healthy sample of virally-suppressed PLWH and uninfected controls. In addition, the role of superoxide and related mitochondrial oxidative environments appear to be valuable, but separable contributors to inflammation-related cognitive decline, which had yet to be reported in the context of human physiology.
In conclusion, our study was the first to directly link quantitative measures of mitochondrial function and redox environment to the inflammatory profiles modulating cognitive function in virally-suppressed PLWH and uninfected adults. Importantly, our results provide evidence for a redox-regulated neuroinflammatory profile in PLWH, which is directly related to alterations in superoxide-sensitive redox environments as opposed to other ROS-dependent mechanisms in the system. While the existing literature provided valuable insight regarding elevated inflammatory and redox profiles in PLWH, the connection between redox-regulated systemic inflammation and HIV-related cognitive impairment was poorly understood. Essentially, elevated levels of superoxide and associated efficiencies in its primary producer (i.e., mitochondria) accounted for a substantial portion of the variance in neuroinflammatory profiles in PLWH, but not controls, and further were associated with deficits in attention and premorbid functioning regardless of group. Finally, it is important to consider several methodological differences and limitations that could be attributable to some of the discrepant findings reported in this study. First, our study was the first to evaluate a large sample of HIV-infected adults with virologic suppression. This is an important consideration, as prior work has evaluated these mitochondrial-redox constructs in smaller samples of HIV-infected adults with and without virologic suppression [15], which is unfortunate, as various regimens of cART have been directly tied to alterations in mitochondrial toxicity and subsequent redox imbalances even in the absence of HIV exposure [16,17,51,52]. Alternatively, while the inclusion of virally-suppressed PLWH is often considered a strength (i.e., fewer confounding factors), it may introduce some selection bias which could limit the generalizability of our findings to the population of PLWH as a whole, who often present with numerous comorbidities (e.g., depression, psychiatric symptoms, substance abuse [53,54]). Thus, future studies should consider including those with comorbid conditions. Second, our sample was considerably larger (~4x larger) than prior studies in humans, which could have also contributed to some of the directionality differences when comparing to the current literature. In addition, future work would greatly benefit from the direct intracellular quantification of H2O2, as our results suggest a differential involvement of H2O2-sensitive scavengers in HIV-infection. Similarly, it will be valuable for future work to quantify levels of ROS and antioxidants directly within the mitochondria (e.g., MnSOD: mitochondrial origin of SOD) to further corroborate our evidence for aberrant mitochondrial-specific redox environments. Finally, our study aimed to focus on the contribution of neuro- and t-lymphocyte-related inflammatory profiles given the extensive literature implicating these markers and their cellular targets (e.g., CD4 t-cell activation/maturation) in the development of neurocognitive impairment in PLWH, albeit markers important for other physiological processes (e.g., chemotaxis: MCP1, vascular-related inflammation: PIGF, SAA, sICAM1) also exhibited significant elevations as a function of HIV. In contrast, certain markers hypothesized to be involved in the development of neurocognitive ability (e.g., IL-6) exhibited no change as a function of serostatus. This specificity in inflammation suggests that the neuroinflammatory profiles as identified in the current study may be especially pertinent to the development of domain-specific cognitive ability (i.e., attention, premorbid function), as opposed to global advancements in neurocognitive dysfunction that have been previously described. Additionally, other cellular profiles may also contribute to this process (e.g., monocytes). For example, as one of the primary infected cells in the HIV brain due to their permeability across the blood brain barrier [55,56], the study of monocytes has become an attractive avenue of investigation as of late, as recent work suggests that increased activation markers in monocytes (e.g., HIV DNA levels, soluble CD163/14) are associated with worse cognitive impairment in PLWH[55,57,58]. Thus, future work will undoubtably benefit from the characterization of monocyte-related inflammatory profiles to expand upon the current study. Nevertheless, our study provides unique, mechanistic insight into the role of inflammation in cognitive dysfunction in PLWH and healthy controls. Importantly, precursors to pro-inflammatory profiles (i.e., parameters of the mitochondrial redox environment) as evaluated in the current study may provide effective proxies for other instances of chronic inflammation (e.g., aging, neurodegenerative diseases) and further, may serve as effective targets to modulate downstream functional decline in the future.
Contributors
All authors have read and approved the final version of the manuscript.
*Authors that have verified the underlying data presented in this study.
*Rachel K. Spooner: Design and conceptualization of the study, acquisition of data, analysis and interpretation of the data, drafting and revising the manuscript for intellectual content.
Brittany K. Taylor: Analysis and interpretation of the data, revising the manuscript for intellectual content.
Cassandra M. Moshfegh: Analysis and interpretation of the data.
Iman M. Ahmad: Analysis and interpretation of the data.
Kelsey Dyball: Analysis and interpretation of the data.
Kathleen Emanuel: Analysis and interpretation of the data.
Sarah L. Schlichte: Analysis and interpretation of the data.
Mikki Schantell: Acquisition of data, analysis and interpretation of the data.
Pamela E. May: Analysis and interpretation of the data.
Jennifer O'Neill: Acquisition of data.
Maureen Kubat: Acquisition of data.
Sara H. Bares: Acquisition of data.
Susan Swindells: Acquisition of data, revising the manuscript for intellectual content.
Howard S. Fox: Acquisition of data.
Kelly L. Stauch: Analysis and interpretation of the data.
*Tony W. Wilson: Design and conceptualization of the study, acquisition of data, interpretation of the data, revising the manuscript for intellectual content.
*Adam J. Case: Design and conceptualization of the study, analysis and interpretation of the data, revising the manuscript for intellectual content.
*Matthew C. Zimmerman: Design and conceptualization of the study, acquisition of data, interpretation of the data, revising the manuscript for intellectual content.
Data Sharing Statement
Data from this study will be made available to qualified investigators upon reasonable request to the corresponding author.
Declaration of Competing Interest
Dr. Swindells reports grants from ViiV Healthcare, outside the submitted work. Dr. Bares reports grants from ViiV, Janssen, and Gilead, outside the submitted work. All other authors have declared that no conflict of interest exists.
Acknowledgments
Acknowledgments
This research was supported by grants R01-MH116782 (TWW), R01-MH118013 (TWW), R01-DA047828 (TWW), RF1-MH117032 (TWW), P30-MH062261 (HSF), and T32-NS105594 (RKS) from the National Institutes of Health (NIH), and grant #1539067 from the National Science Foundation (TWW). EPR Spectroscopy data was collected in the University of Nebraska's EPR Spectroscopy Core, which was initially established with support from a Center of Biomedical Research Excellence grant from the National Institute of General Medical Sciences of the National Institutes of Health (P30-GM103335) awarded to the University of Nebraska's Redox Biology Center. We would like to thank all of the volunteers for participating in the study, as well as our staff and collaborators for their contributions.
Funding
National Institute of Mental Health, National Institute for Neurological Disorders and Stroke, National Institute on Drug Abuse, National Science Foundation.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.ebiom.2021.103487.
Contributor Information
Adam J. Case, Email: adam.case@unmc.edu.
Matthew C. Zimmerman, Email: mczimmerman@unmc.edu.
Appendix. Supplementary materials
References
- 1.Antinori A, Arendt G, Becker JT, Brew BJ, Byrd DA, Cherner M. Updated research nosology for HIV-associated neurocognitive disorders. Neurology. 2007 Oct;69(18):1789–1799. doi: 10.1212/01.WNL.0000287431.88658.8b. 2007/10/03 ed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cysique LA, Brew BJ. Neuropsychological functioning and antiretroviral treatment in HIV/AIDS: a review. Neuropsychol Rev. 2009;19(2):169–185. doi: 10.1007/s11065-009-9092-3. 2009/05/09 ed. [DOI] [PubMed] [Google Scholar]
- 3.Heaton RK, Franklin DR, Ellis RJ, McCutchan JA, Letendre SL, Leblanc S. HIV-associated neurocognitive disorders before and during the era of combination antiretroviral therapy: differences in rates, nature, and predictors. J Neurovirol. 2011;17(1):3–16. doi: 10.1007/s13365-010-0006-1. 2010/12/21 ed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Heaton RK, Clifford DB, Franklin DR, Woods SP, Ake C, Vaida F. HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER Study. Neurology. 2010;75(23):2087–2096. doi: 10.1212/WNL.0b013e318200d727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Robertson KR, Smurzynski M, Parsons TD, Wu K, Bosch RJ, Wu J. The prevalence and incidence of neurocognitive impairment in the HAART era. AIDS. 2007;21(14):1915–1921. doi: 10.1097/QAD.0b013e32828e4e27. [DOI] [PubMed] [Google Scholar]
- 6.Saylor D, Dickens A, Sacktor N, Haughey N, Slusher B, Pletnikov M. HIV-associated neurocognitive disorder — pathogenesis and prospects for treatment. Nat Rev Neurol. 2016;12:15. doi: 10.1038/nrneurol.2016.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Simioni S, Cavassini M, Annoni JM, Rimbault Abraham A, Bourquin I, Schiffer V. Cognitive dysfunction in HIV patients despite long-standing suppression of viremia. AIDS. 2010;24(9):1243–1250. doi: 10.1097/QAD.0b013e3283354a7b. [DOI] [PubMed] [Google Scholar]
- 8.Gross AM, Jaeger PA, Kreisberg JF, Licon K, Jepsen KL, Khosroheidari M. Methylome-wide analysis of chronic HIV infection reveals five-year increase in biological age and epigenetic targeting of HLA. Mol Cell. 2020;62(2):157–168. doi: 10.1016/j.molcel.2016.03.019. 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hong S, Banks WA. Role of the immune system in HIV-associated neuroinflammation and neurocognitive implications. Brain Behav Immun. 2015;45:1–12. doi: 10.1016/j.bbi.2014.10.008. 2014/10/22 ed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Varatharajan L, Thomas SA. The transport of anti-HIV drugs across blood-CNS interfaces: summary of current knowledge and recommendations for further research. Antiviral Res. 2009;82(2):A99–109. doi: 10.1016/j.antiviral.2008.12.013. 2009/01/25 ed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Akkaya B, Roesler AS, Miozzo P, Theall BP, Al Souz J, Smelkinson MG. Increased mitochondrial biogenesis and reactive oxygen species production accompany prolonged CD4. J Immunol. 2018;201(11):3294–3306. doi: 10.4049/jimmunol.1800753. 10/29 ed. 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Franchina DG, Dostert C, Brenner D. Reactive oxygen species: involvement in T cell signaling and metabolism. Trends Immunol. 2018;39(6):489–502. doi: 10.1016/j.it.2018.01.005. /02/13 ed. 6. [DOI] [PubMed] [Google Scholar]
- 13.Sena LA, Li S, Jairaman A, Prakriya M, Ezponda T, Hildeman DA. Mitochondria are required for antigen-specific T cell activation through reactive oxygen species signaling. Immunity. 2013;38(2):225–236. doi: 10.1016/j.immuni.2012.10.020. 2013/02/15 ed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van der Windt GJ, Everts B, Chang CH, Curtis JD, Freitas TC, Amiel E. Mitochondrial respiratory capacity is a critical regulator of CD8+ T cell memory development. Immunity. 2012;36(1):68–78. doi: 10.1016/j.immuni.2011.12.007. 2011/12/28 ed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Korencak M, Byrne M, Richter E, Schultz BT, Juszczak P, Ake JA. Effect of HIV infection and antiretroviral therapy on immune cellular functions. JCI Insight. 2019;4(12) doi: 10.1172/jci.insight.126675. https://www.ncbi.nlm.nih.gov/pubmed/31217351 2019/06/20 ed. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Akay C, Cooper M, Odeleye A, Jensen BK, White MG, Vassoler F. Antiretroviral drugs induce oxidative stress and neuronal damage in the central nervous system. J Neurovirol. 2014;20(1):39–53. doi: 10.1007/s13365-013-0227-1. 2014/01/14 ed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stauch KL, Emanuel K, Lamberty BG, Morsey B, Fox HS. Central nervous system-penetrating antiretrovirals impair energetic reserve in striatal nerve terminals. J Neurovirol. 2017;23(6):795–807. doi: 10.1007/s13365-017-0573-5. 2017/09/11 ed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harman D. Free radical theory of aging: an update: increasing the functional life span. Ann N Y Acad Sci. 2006;1067:10–21. doi: 10.1196/annals.1354.003. [DOI] [PubMed] [Google Scholar]
- 19.Spooner RK, Wiesman AI, Mills MS, O'Neill J, Robertson KR, Fox HS. Aberrant oscillatory dynamics during somatosensory processing in HIV-infected adults. Neuroimage Clin. 2018;20:85–91. doi: 10.1016/j.nicl.2018.07.009. 2018/07/10 ed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wiesman AI, O'Neill J, Mills MS, Robertson KR, Fox HS, Swindells S. Aberrant occipital dynamics differentiate HIV-infected patients with and without cognitive impairment. Brain. 2018;141(6):1678–1690. doi: 10.1093/brain/awy097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lew BJ, McDermott TJ, Wiesman AI, O'Neill J, Mills MS, Robertson KR. Neural dynamics of selective attention deficits in HIV-associated neurocognitive disorder. Neurology. 2018;91(20):e1860–e1869. doi: 10.1212/WNL.0000000000006504. 2018/10/17 ed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Casagrande CC, Lew BJ, Taylor BK, Schantell M, O'Neill J, May PE. Impact of HIV-infection on human somatosensory processing, spontaneous cortical activity, and cortical thickness: a multimodal neuroimaging approach. Hum Brain Mapp. 2021;42(9):2851–2861. doi: 10.1002/hbm.25408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ahmad IM, Temme JB, Abdalla MY, Zimmerman MC. Redox status in workers occupationally exposed to long-term low levels of ionizing radiation: a pilot study. Redox Rep. 2016;21(3):139–145. doi: 10.1080/13510002.2015.1101891. 2016/02/05 ed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wechsler D. The Psychological Corporation; San Antonio, TX: 1997. Wechsler Memory Scale - Third Edition. [Google Scholar]
- 25.Woods PS, Delis DC, Cobb Scott J, Kramer JH, Holdnack JA. The California Verbal Learning Test – second edition: test-retest reliability, practice effects, and reliable change indices for the standard and alternate forms. Arch Clin Neuropscyhol. 2006;21(5):8. doi: 10.1016/j.acn.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 26.Comalli PE, Wapner S, Werner H. Interference effects of Stroop color-word test in childhood, adulthood, and aging. J Genet Psychol. 1962;100:47–53. doi: 10.1080/00221325.1962.10533572. [DOI] [PubMed] [Google Scholar]
- 27.Heaton R, Miller S, Taylor M, Grant I. Revised comprehensive norms for an expanded Halstead-Reitan battery. Demographically Adjusted Neuropsychological Norms for African American and Caucasian adults. 2004 [Google Scholar]
- 28.Wechsler D. Psychological Corporation; San Antonio, TX: 1997. Wechsler Adult Intelligence Scale - Third Edition. [Google Scholar]
- 29.Klove H. Pegboard; 1963. Grooved. [Google Scholar]
- 30.Wilkinson G.S., Roberson G.J. Psychological Assessment Resources., Inc; Lutz, FL: 2006. Wide Range Achievement Test 4 professional manual. [Google Scholar]
- 31.Avdoshina V, Fields JA, Castellano P, Dedoni S, Palchik G, Trejo M. The HIV Protein gp120 alters mitochondrial dynamics in neurons. Neurotox Res. 2016;29(4):583–593. doi: 10.1007/s12640-016-9608-6. 2016/03/02 ed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shah A, Kumar A. HIV-1 gp120-mediated mitochondrial dysfunction and HIV-associated neurological disorders. Neurotox Res. 2016;30(2):135–137. doi: 10.1007/s12640-016-9619-3. [DOI] [PubMed] [Google Scholar]
- 33.Gil L, Martínez G, González I, Tarinas A, Alvarez A, Giuliani A. Contribution to characterization of oxidative stress in HIV/AIDS patients. Pharmacol Res. 2003 Mar;47(3):217–224. doi: 10.1016/s1043-6618(02)00320-1. [DOI] [PubMed] [Google Scholar]
- 34.Repetto M, Reides C, Gomez Carretero ML, Costa M, Griemberg G, Llesuy S. Oxidative stress in blood of HIV infected patients. Clin Chim Acta. 1996;255(2):107–117. doi: 10.1016/0009-8981(96)06394-2. [DOI] [PubMed] [Google Scholar]
- 35.Pace GW, Leaf CD. The role of oxidative stress in HIV disease. Free Radic Biol Med. 1995;19(4):523–528. doi: 10.1016/0891-5849(95)00047-2. [DOI] [PubMed] [Google Scholar]
- 36.Liochev S, Fridovich I. The effects of superoxide dismutase on H2O2 formation. Free Radic Biol Med. 2007;42(10):5. doi: 10.1016/j.freeradbiomed.2007.02.015. [DOI] [PubMed] [Google Scholar]
- 37.Moshfegh CM, Collins CW, Gunda V, Vasanthakumar A, Cao JZ, Singh PK. Mitochondrial superoxide disrupts the metabolic and epigenetic landscape of CD4. Redox Biol. 2019;27 doi: 10.1016/j.redox.2019.101141. /02/21 ed. 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Deeks SG. HIV infection, inflammation, immunosenescence, and aging. Ann Rev Med. 2011;62:141–155. doi: 10.1146/annurev-med-042909-093756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Roberts RO, Geda YE, Knopman DS, Boeve BF, Christianson TJ, Pankratz VS. Association of C-reactive protein with mild cognitive impairment. Alzheimers Dement. 2009;5(5):398–405. doi: 10.1016/j.jalz.2009.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Noble JM, Manly JJ, Schupf N, Tang MX, Mayeux R, Luchsinger JA. Association of C-reactive protein to cognitive impairment. Arch Neurol. 2010;67(1):87–92. doi: 10.1001/archneurol.2009.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Watanabe Y, Kitamura K, Nakamura K, Sanpei K, Wakasugi M, Yokoseki A. Elevated C-reactive protein is associated with cognitive decline in outpatients of a general hospital: the Project in Sado for Total Health (PROST) Dement Geriatr Cogn Dis Extra. 2016;6(1):10–19. doi: 10.1159/000442585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rubin LH, Benning L, Keating SM, Norris PJ, Burke-Miller J, Savarese A. Variability in C-reactive protein is associated with cognitive impairment in women living with and without HIV: a longitudinal study. J Neurovirol. 2018;24(1):41–51. doi: 10.1007/s13365-017-0590-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gendelman HE. Predictive biomarkers for cognitive decline during progressive HIV infection. EBioMedicine [Internet] 2020 doi: 10.1016/j.ebiom.2019.10.064. https://www.thelancet.com/journals/ebiom/article/PIIS2352-3964(19)30741-8/abstract [cited 2021 May 3];51. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Holmes C, Cunningham C, Zotova E, Woolford J, Dean C, Kerr S. Systemic inflammation and disease progression in Alzheimer disease. Neurology. 2009;73(10):768–774. doi: 10.1212/WNL.0b013e3181b6bb95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tarkowski E. Intrathecal inflammation precedes development of Alzheimer's disease. J Neurol Neurosurg Psychiatry. 2003;74(9):1200–1205. doi: 10.1136/jnnp.74.9.1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tegeler C, O'Sullivan JL, Bucholtz N, Goldeck D, Pawelec G, Steinhagen-Thiessen E. The inflammatory markers CRP, IL-6, and IL-10 are associated with cognitive function—data from the Berlin Aging Study II. Neurobiol Aging. 2016;38:112–117. doi: 10.1016/j.neurobiolaging.2015.10.039. [DOI] [PubMed] [Google Scholar]
- 47.Kumar P, Liu C, Suliburk JW, Minard CG, Muthupillai R, Chacko S. Supplementing glycine and N-acetylcysteine (GlyNAC) in Aging HIV patients improves oxidative stress, mitochondrial dysfunction, inflammation, endothelial dysfunction, insulin resistance, genotoxicity, strength, and cognition: results of an open-label clinical trial. Biomedicines. 2020;8(10) doi: 10.3390/biomedicines8100390. https://www.ncbi.nlm.nih.gov/pubmed/33007928 2020/09/30 ed. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lau B, Sharrett AR, Kingsley LA, Post W, Palella FJ, Visscher B. C-reactive protein is a marker for human immunodeficiency virus disease progression. Arch Intern Med. 2006;166(1):64–70. doi: 10.1001/archinte.166.1.64. [DOI] [PubMed] [Google Scholar]
- 49.Nixon D, Landay A. Biomarkers of immune dysfunction in HIV. Curr Opin HIV AIDS. 2011;5(6):5. doi: 10.1097/COH.0b013e32833ed6f4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Noel N, Boufassa F, Lécuroux C, Saez-Cirion A, Bourgeois C, Dunyach-Remy C. Elevated IP10 levels are associated with immune activation and low CD4+ T-cell counts in HIV controller patients. AIDS. 2014;28(4):467–476. doi: 10.1097/QAD.0000000000000174. [DOI] [PubMed] [Google Scholar]
- 51.Funes HA, Apostolova N, Alegre F, Blas-Garcia A, Alvarez A, Marti-Cabrera M. Neuronal bioenergetics and acute mitochondrial dysfunction: a clue to understanding the central nervous system side effects of efavirenz. J Infect Dis. 2014;210(9):1385–1395. doi: 10.1093/infdis/jiu273. 2014/05/09 ed. [DOI] [PubMed] [Google Scholar]
- 52.Polo R, Martinez S, Madrigal P, Gonzalez-Muñoz M. Factors associated with mitochondrial dysfunction in circulating peripheral blood lymphocytes from HIV-infected people. J Acquir Immune Defic Syndr. 2003;34(1):32–36. doi: 10.1097/00126334-200309010-00004. [DOI] [PubMed] [Google Scholar]
- 53.Mimiaga MJ, Reisner SL, Grasso C, Crane HM, Safren SA, Kitahata MM. Substance use among HIV-infected patients engaged in primary care in the United States: findings from the centers for AIDS research network of integrated clinical systems cohort. Am J Public Health. 2013;103(8):1457–1467. doi: 10.2105/AJPH.2012.301162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rabkin JG. HIV and depression: 2008 review and update. Curr HIV/AIDS Rep. 2008;5(4):163–171. doi: 10.1007/s11904-008-0025-1. [DOI] [PubMed] [Google Scholar]
- 55.Anzinger JJ, Butterfield TR, Angelovich TA, Crowe SM, Palmer CS. Monocytes as regulators of inflammation and HIV-related comorbidities during cART. Journal of Immunology Research. 2014;2014 doi: 10.1155/2014/569819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kaul M, Garden GA, Lipton SA. Pathways to neuronal injury and apoptosis in HIV-associated dementia. Nature. 2001;410(6831):988–994. doi: 10.1038/35073667. [DOI] [PubMed] [Google Scholar]
- 57.Valcour VG, Shiramizu BT, Sithinamsuwan P, Nidhinandana S, Ratto-Kim S, Ananworanich J. HIV DNA and cognition in a Thai longitudinal HAART initiation cohort. Neurology. 2009;72(11):992–998. doi: 10.1212/01.wnl.0000344404.12759.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Imp BM, Rubin LH, Tien PC, Plankey MW, Golub ET, French AL. Monocyte activation is associated with worse cognitive performance in HIV-infected women with virologic suppression. J Infect Dis. 2017;215(1):114–121. doi: 10.1093/infdis/jiw506. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.