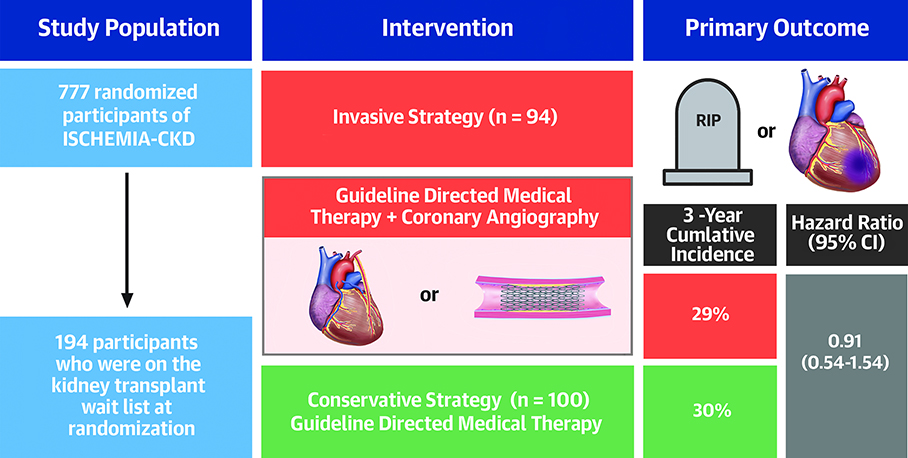
Abstract
Background:
Patients with chronic kidney disease (CKD) and coronary artery disease frequently undergo preemptive revascularization before kidney transplant listing.
Objectives:
In this post-hoc analysis from ISCHEMIA-CKD, we compared outcomes of patients not listed versus those listed according to management strategy.
Methods:
In ISCHEMIA-CKD (n=777), 194 patients (25%) with chronic coronary syndromes and at least moderate ischemia were listed for transplant. The primary (all-cause mortality or nonfatal myocardial infarction [MI]) and secondary (death, nonfatal MI, hospitalization for unstable angina, heart failure, resuscitated cardiac arrest, or stroke) outcomes were analyzed using Cox multivariable modeling. Heterogeneity of randomized treatment effect between listed versus not listed groups was assessed.
Results:
Compared with those not listed, listed patients were younger (60 versus 65 years), less likely of Asian race (15% versus 29%), more likely on dialysis (83% versus 44%), had fewer anginal symptoms, and more likely to have coronary angiography and coronary revascularization irrespective of treatment assignment. Among patients assigned to an invasive strategy versus conservative strategy, the adjusted hazard ratios (aHR) (95% confidence interval [CI]) for the primary outcome were 0.91 (0.54–1.54) and 1.03 (0.78–1.37) for those listed and not listed, respectively (pinteraction=0.68). Adjusted HR for secondary outcomes were 0.89 (0.55–1.46) in listed and 1.17 (0.89–1.53) in those not listed (pinteraction=0.35).
Conclusions:
In ISCHEMIA-CKD, an invasive strategy in kidney transplant candidates did not improve outcomes compared with conservative management. These data do not support routine coronary angiography or revascularization in patients with advanced CKD and chronic coronary syndromes listed for transplant.
Keywords: chronic kidney disease, kidney transplantation, ischemic heart disease, coronary angiography, coronary revascularization, medical therapy
Condensed Abstract
In this post-hoc analysis from ISCHEMIA-CKD (n=777), we compared patients listed for kidney transplant (n=194 [25%]) versus those not listed. The primary outcome was all-cause mortality or nonfatal myocardial infarction. Heterogeneity of randomized treatment effect between listed versus not listed groups was assessed. We found that an invasive strategy did not improve outcomes compared with conservative management regardless of kidney transplant candidacy. These data do not support routine coronary angiography or revascularization in patients with chronic kidney disease and myocardial ischemia on stress testing listed for transplant.
Candidates for kidney transplant are often at high risk for adverse cardiovascular events, including perioperative myocardial infarction (MI). Screening for occult coronary artery disease (CAD) is a primary component of pre-kidney transplant evaluation. The support for this strategy consists of one small randomized controlled trial conducted 3 decades ago, observational studies, and consensus statements primarily focused on correlating risk of cardiovascular events post-kidney transplant with pre-transplant cardiac risk stratification (including coronary angiography).(1–6) In the one trial, Manske et al. examined preemptive coronary revascularization compared with medical therapy in 26 candidates for kidney transplant with insulin-dependent diabetes mellitus and >75% stenosis in at least 1 epicardial coronary artery. The study was stopped early (median follow-up 8.4 months) for benefit in the revascularization arm for the primary composite endpoint of unstable angina, myocardial infarction, or cardiac death. Despite the rapidly evolving and improving medical armamentarium for the management of chronic coronary disease, there have been no further randomized controlled trials conducted to confirm Manske et al.’s findings supporting preemptive coronary revascularization in kidney transplant candidates.
ISCHEMIA-CKD (International Study of Comparative Health Effectiveness of Medical and Invasive Approaches–Chronic Kidney Disease) is the only randomized trial to prospectively compare an initial invasive strategy of coronary angiography and revascularization plus optimal medical therapy versus an initial conservative strategy of optimal medical therapy alone in patients with advanced CKD and stable coronary disease.(7) There was no evidence of benefit for the invasive strategy on the primary outcome of all-cause death or nonfatal MI at 3 years. The ISCHEMIA-CKD study did not specifically seek patients being evaluated for kidney transplant; however eligibility criteria were such that patients undergoing pre-transplant evaluation were included in the trial. This report describes the subset of patients in ISCHEMIA-CKD who underwent cardiac stress testing prior to randomization as part of their evaluation for kidney transplant listing.
METHODS
Patient Population
The rationale and design of ISCHEMIA-CKD (NCT01985360) have been published.(8) A total of 802 participants were recruited between April 29, 2014 and January 31, 2018. Of these, 777 were randomized at 118 participating sites in 30 countries.(7,8) Principal inclusion criteria included end-stage renal disease on dialysis or estimated glomerular filtration rate (eGFR) <30 mL/min/1.73m2, chronic coronary syndromes with well-controlled angina or silent ischemia, and at least moderate or severe myocardial ischemia as determined by the site investigators. Major exclusion criteria included known left main disease or non-obstructive CAD, LVEF ≤35% or NYHA class III or IV heart failure, acute coronary syndromes within 2 months or unacceptable level of angina despite maximal medical therapy. The study protocol was approved by institutional review boards at participating sites and all patients provided written informed consent.
For the current post-hoc analysis, we categorized patients by their transplant list status at baseline. Patients listed for kidney transplant (n=179) were identified from the study database, which included patients both previously listed and those having a stress test to qualify for listing. In addition, patients identified during follow-up as having received a kidney transplant (n=15) post-randomization without indication of transplant list status at baseline were considered to be on the transplant list at enrollment. The final transplant list analysis cohort thus consisted of 194 participants (25% of the total ISCHEMIA-CKD cohort); median duration of follow-up for listed participants was 2.4 years (25th, 75th percentiles [1.6, 3.1]).
Clinical Outcomes
The primary outcome was a composite of all-cause death or nonfatal MI. The secondary outcome was a composite of death, nonfatal MI, stroke, or hospitalization for unstable angina, heart failure, or resuscitated cardiac arrest (For the present analysis, stroke was included in the secondary outcome).(8) Among other renal endpoints, receipt of a kidney transplant was queried at each study visit.
Statistical Methods
Descriptive statistics of patient characteristics at baseline were analyzed by kidney transplant list status (not listed versus listed). We assessed associations of patient characteristics with list status using the chi-squared test for categorical variables and the Wilcoxon test for continuous variables. For categorical variables involving 2-by-2 comparisons, we substituted Fisher’s exact test if the expected cell size was less than 5. We estimated cumulative event rates of the primary and secondary outcomes using the Kaplan-Meier method.
Multivariable Cox proportional hazards models were used to examine the association of each outcome with list status, adjusting for the following patient characteristics at baseline: age at randomization, sex, dialysis status, eGFR, ejection fraction, diabetes status, and treatment strategy. eGFR was controlled for only among non-dialysis patients by including an indicator for no dialysis at baseline (equal to 1 if a participant was not on dialysis, and 0 otherwise) and an interaction term between no dialysis at baseline and eGFR at baseline. Hazard ratios (HRs) and associated 95% confidence intervals (CIs) were estimated. To examine whether the treatment effect differed by list status, we estimated the models adding an interaction between treatment and list status. Continuously measured variables were modeled as restricted cubic splines with 3 knots at the 10th, 50th, and 90th percentiles. Proportional hazards assumptions were checked by inspecting the Schoenfeld residuals and testing the null hypothesis of no interaction of time with transplant list status and treatment strategy. For outcomes subject to competing risks, we used cause-specific hazard models.
All analyses were conducted using SAS 9.4 software (SAS Institute, Inc., Cary, NC) and R 3.5.3 (R Foundation for Statistical Computing, Vienna, Austria). For all analyses, a 2-tailed p<0.05 denoted statistical significance.
RESULTS
Baseline Characteristics
Baseline characteristics of ISCHEMIA-CKD participants not listed (n=583) and listed (n=194) for transplant are presented in Table 1. Fifty-one patients (26%) in the listed group received a kidney transplant during the course of the trial. Compared with those not listed, patients listed for transplant were younger, less likely of Asian race, less likely to have cerebrovascular or peripheral artery disease, more likely receiving dialysis, had a lower proportion with severe ischemia on stress testing, had less angina and better functional status based on the Seattle Angina Questionnaire and Canadian Cardiovascular Society Angina Class, had lower New York Heart Association class, and were more likely to undergo an imaging stress test.
Table 1.
Participant Baseline Characteristics by Transplant Listing Status
| Not listed | Listed | P-value* | |
|---|---|---|---|
| (n=583) | (n=194) | ||
| Age at randomization, years | 0.000 | ||
| N | 583 | 194 | |
| Median (Q1, Q3) | 65 (57, 72) | 60 (54, 64) | |
| Female | 0.051 | ||
| 193/583 (33%) | 49/194 (25%) | ||
| Race | 0.002 | ||
| American Indian or Alaskan Native | 2/567 (0%) | 3/180 (2%) | |
| Asian | 164/567 (29%) | 27/180 (15%) | |
| Native Hawaiian or Other Pacific Islander | 3/567 (1%) | 3/180 (2%) | |
| Black or African American | 45/567 (8%) | 18/180 (10%) | |
| White | 352/567 (62%) | 129/180 (72%) | |
| Multiple Races Reported | 1/567 (0%) | 0/180 (0%) | |
| Degree of ischemia on stress test | 0.008 | ||
| Moderate | 340/575 (59%) | 136/194 (70%) | |
| Severe | 235/575 (41%) | 58/194 (30%) | |
| Hypertension | 0.520 | ||
| 537/581 (92%) | 174/192 (91%) | ||
| Diabetes | 0.658 | ||
| 330/583 (57%) | 114/194 (59%) | ||
| Smoking status | 0.030 | ||
| Never smoked | 280/583 (48%) | 91/194 (47%) | |
| Former smoker | 231/583 (40%) | 91/194 (47%) | |
| Current Smoker | 72/583 (12%) | 12/194 (6%) | |
| Previous myocardial infarction | 0.095 | ||
| 108/583 (19%) | 25/193 (13%) | ||
| Previous heart failure | 0.255 | ||
| 107/583 (18%) | 28/194 (14%) | ||
| Previous stroke | 0.467 | ||
| 54/583 (9%) | 14/194 (7%) | ||
| History of cerebrovascular disease or PAD† | 0.016 | ||
| 112/583 (19%) | 22/194 (11%) | ||
| Previous PCI‡ | 0.992 | ||
| 109/583 (19%) | 37/194 (19%) | ||
| Previous CABG§ | 0.119 | ||
| 17/583 (3%) | 11/194 (6%) | ||
| Ejection fraction (%) | 0.398 | ||
| N | 463 | 156 | |
| Median (Q1, Q3) | 58 (50, 64) | 59 (51, 64) | |
| On dialysis | 0.000 | ||
| 254/583 (44%) | 161/194 (83%) | ||
| Duration of dialysis (years) | 0.004 | ||
| N | 224 | 145 | |
| Median (Q1, Q3) | 3 (1, 6) | 2 (1, 4) | |
| Dialysis type | 0.039 | ||
| Peritoneal dialysis | 29/247 (12%) | 31/157 (20%) | |
| eGFR among those not receiving dialysis (ml/min/1.73m2) | 0.000 | ||
| N | 329 | 33 | |
| Median (Q1, Q3) | 23 (18, 27) | 16 (14, 20) | |
| SAQ# Summary score | 0.000 | ||
| N | 536 | 188 | |
| Median (Q1, Q3) | 74 (58, 88) | 92 (77, 100) | |
| SAQ# Angina Frequency score | 0.000 | ||
| N | 536 | 188 | |
| Median (Q1, Q3) | 90 (80, 100) | 100 (90, 100) | |
| SAQ# Physical Limitation score | 0.000 | ||
| N | 416 | 159 | |
| Median (Q1, Q3) | 75 (50, 100) | 92 (71, 100) | |
| SAQ# Quality of Life score | 0.000 | ||
| N | 535 | 188 | |
| Median (Q1, Q3) | 63 (38, 88) | 88 (63, 100) | |
| Canadian Cardiovascular Society angina class | 0.000 | ||
| None | 171/583 (29%) | 121/193 (63%) | |
| I | 118/583 (20%) | 37/193 (19%) | |
| II | 264/583 (45%) | 34/193 (18%) | |
| III | 30/583 (5%) | 1/193 (1%) | |
| New York Heart Association class | 0.009 | ||
| I | 99/337 (29%) | 37/78 (47%) | |
| II | 237/337 (70%) | 41/78 (53%) | |
| III | 1/337 (0%) | 0/78 (0%) | |
| Type of stress testing | 0.002 | ||
| Nuclear | 358/581 (62%) | 121/194 (62%) | |
| ECHO | 100/581 (17%) | 52/194 (27%) | |
| CMR†† | 1/581 (0%) | 0/194 (0%) | |
| ETT‡‡ | 122/581 (21%) | 21/194 (11%) | |
| Treatment strategy | 0.694 | ||
| Initial Invasive | 294/583 (50%) | 94/194 (48%) |
When the denominator is smaller than the column title population size, it represents the non-missing statistics
P-values were obtained using the Wilcoxon Rank Sum test for continuous variables. For categorical variables, Pearson’s chi-squared test was used
PAD: peripheral arterial disease
PCI: percutaneous coronary intervention
CABG: coronary artery bypass graft
eGFR: estimated glomerular filtration rate
SAQ: Seattle Angina Questionnaire
ECHO: echocardiography
CMR: Cardiac Magnetic Resonance Imaging
ETT: exercise treadmill test
Baseline characteristics of participants by kidney transplant list status and treatment strategy are presented in Table 2. Balance of measured baseline covariates between treatment strategies in each listing group was maintained, with the exception of the slightly younger age of those in the invasive strategy not listed for transplant. Supplemental Tables 1a and 1b present baseline and follow-up physiologic measurements, risk factors, and medications by list status and treatment strategy. Both not listed and listed patients had improved attainment of risk factor goals (systolic blood pressure <140 mm Hg; 55% increasing to 69% in those not listed and 56% to 68% in those listed) and greater medication adherence (62% to 70% in not listed and 70% to 84% in listed) from baseline to last study visit.
Table 2.
Participant Baseline Characteristics by Listing Status and Treatment Strategy.
| Not Listed | Listed | |||||
|---|---|---|---|---|---|---|
| CON | INV | P-value* | CON | INV | P-value* | |
| (n=289) | (n=294) | (n=100) | (n=94) | |||
| Age at randomization, years | 0.040 | 0.907 | ||||
| N | 289 | 294 | 100 | 94 | ||
| Median (Q1, Q3) | 66 (58, 73) | 64 (56, 71) | 61 (53, 65) | 59 (54, 64) | ||
| Female | 0.748 | 0.802 | ||||
| Female | 98/289 (34%) | 95/294 (32%) | 24/100 (24%) | 25/94 (27%) | ||
| Race | 0.433 | 0.922 | ||||
| American Indian or Alaskan Native | 2/281 (1%) | 0/286 (0%) | 1/93 (1%) | 2/87 (2%) | ||
| Asian | 87/281 (31%) | 77/286 (27%) | 13/93 (14%) | 14/87 (16%) | ||
| Native Hawaiian or Other Pacific Islander | 2/281 (1%) | 1/286 (0%) | 2/93 (2%) | 1/87 (1%) | ||
| Black or African American | 20/281 (7%) | 25/286 (9%) | 10/93 (11%) | 8/87 (9%) | ||
| White | 170/281 (60%) | 182/286 (64%) | 67/93 (72%) | 62/87 (71%) | ||
| Multiple Races Reported | 0/281 (0%) | 1/286 (0%) | 0/93 (0%) | 0/87 (0%) | ||
| Degree of ischemia on stress test | 0.540 | 0.850 | ||||
| Moderate | 165/286 (58%) | 175/289 (61%) | 69/100 (69%) | 67/94 (71%) | ||
| Severe | 121/286 (42%) | 114/289 (39%) | 31/100 (31%) | 27/94 (29%) | ||
| Hypertension | 0.184 | 0.665 | ||||
| 270/287 (94%) | 267/294 (91%) | 92/100 (92%) | 82/92 (89%) | |||
| Diabetes | 0.989 | 0.341 | ||||
| 163/289 (56%) | 167/294 (57%) | 55/100 (55%) | 59/94 (63%) | |||
| Smoke status | 0.616 | 0.951 | ||||
| Never smoked | 139/289 (48%) | 141/294 (48%) | 46/100 (46%) | 45/94 (48%) | ||
| Former smoker | 118/289 (41%) | 113/294 (38%) | 48/100 (48%) | 43/94 (46%) | ||
| Current Smoker | 32/289 (11%) | 40/294 (14%) | 6/100 (6%) | 6/94 (6%) | ||
| Previous myocardial infarction | 0.528 | 0.815 | ||||
| 57/289 (20%) | 51/294 (17%) | 14/100 (14%) | 11/93 (12%) | |||
| Previous heart failure | 0.599 | 1.000 | ||||
| 56/289 (19%) | 51/294 (17%) | 14/100 (14%) | 14/94 (15%) | |||
| Previous stroke | 0.350 | 0.476 | ||||
| 23/289 (8%) | 31/294 (11%) | 9/100 (9%) | 5/94 (5%) | |||
| History of cerebrovascular disease or PAD† | 0.291 | 0.599 | ||||
| 50/289 (17%) | 62/294 (21%) | 13/100 (13%) | 9/94 (10%) | |||
| Previous PCI‡ | 0.910 | 1.000 | ||||
| 53/289 (18%) | 56/294 (19%) | 19/100 (19%) | 18/94 (19%) | |||
| Previous CABG§ | 1.000 | 1.000 | ||||
| 8/289 (3%) | 9/294 (3%) | 6/100 (6%) | 5/94 (5%) | |||
| Ejection fraction (%) | 0.484 | 0.184 | ||||
| N | 221 | 242 | 79 | 77 | ||
| Median (Q1, Q3) | 58 (50, 64) | 57 (50, 63) | 60 (52, 65) | 58 (50, 61) | ||
| On dialysis | 0.549 | 0.180 | ||||
| 130/289 (45%) | 124/294 (42%) | 87/100 (87%) | 74/94 (79%) | |||
| Duration of dialysis (years) | 0.333 | 0.414 | ||||
| N | 114 | 110 | 78 | 67 | ||
| Median (Q1, Q3) | 2 (1, 5) | 3 (1, 6) | 2 (1, 3) | 2 (1, 5) | ||
| Dialysis type | 0.676 | 0.551 | ||||
| Peritoneal dialysis | 13/124 | 16/123 | 15/86 | 16/71 | ||
| (10%) | (13%) | (17%) | (23%) | |||
| eGFRǁ among those not receiving dialysis (ml/min/1.73m2) | 0.848 | 0.567 | ||||
| N | 159 | 170 | 13 | 20 | ||
| Median (Q1, Q3) | 23 (18, 27) | 23 (17, 27) | 16 (13, 19) | 16 (15, 20) | ||
| #SAQ Summary score | 0.611 | 0.961 | ||||
| N | 269 | 267 | 97 | 91 | ||
| Median (Q1, Q3) | 75 (60, 88) | 72 (54, 92) | 90 (75, 100) | 92 (78, 98) | ||
| #SAQ Angina Frequency score | 0.424 | 0.202 | ||||
| N | 269 | 267 | 97 | 91 | ||
| Median (Q1, Q3) | 90 (80, 100) | 90 (70, 100) | 100 (90, 100) | 100 (100, 100) | ||
| #SAQ Physical Limitation score | 0.739 | 0.360 | ||||
| N | 200 | 216 | 83 | 76 | ||
| Median (Q1, Q3) | 75 (56, 92) | 75 (50, 100) | 92 (67, 100) | 100 (75, 100) | ||
| #SAQ Quality of Life score | 0.685 | 0.738 | ||||
| N | 268 | 267 | 97 | 91 | ||
| Median (Q1, Q3) | 63 (38, 88) | 63 (38, 88) | 88 (50, 100) | 88 (63, 100) | ||
| Canadian Cardiovascular Society angina class | 0.964 | 0.240 | ||||
| None | 82/289 (28%) | 89/294 (30%) | 56/99 (57%) | 65/94 (69%) | ||
| I | 60/289 (21%) | 58/294 (20%) | 23/99 (23%) | 14/94 (15%) | ||
| II | 132/289 (46%) | 132/294 (45%) | 19/99 (19%) | 15/94 (16%) | ||
| III | 15/289 (5%) | 15/294 (5%) | 1/99 (1%) | 0/94 (0%) | ||
| New York Heart Association class | 0.579 | 0.338 | ||||
| I | 48/168 (29%) | 51/169 (30%) | 23/43 (53%) | 14/35 (40%) | ||
| II | 119/168 (71%) | 118/169 (70%) | 20/43 (47%) | 21/35 (60%) | ||
| III | 1/168 (1%) | 0/169 (0%) | 0/43 (0%) | 0/35 (0%) | ||
| Type of stress testing | 0.377 | 0.755 | ||||
| Nuclear | 185/288 (64%) | 173/293 (59%) | 60/100 (60%) | 61/94 (65%) | ||
| ECHO** | 44/288 (15%) | 56/293 (19%) | 29/100 (29%) | 23/94 (24%) | ||
| CMR†† | 1/288 (0%) | 0/293 (0%) | 0/100 (0%) | 0/94 (0%) | ||
| ETT‡‡ | 58/288 (20%) | 64/293 (22%) | 11/100 (11%) | 10/94 (11%) | ||
P-values were obtained using the Wilcoxon Rank Sum test for continuous variables. For categorical variables, Pearson’s chi-squared test was used. Initial Invasive (INV) and Initial Conservative (CON) strategy arms.
PAD: peripheral arterial disease
PCI: percutaneous coronary intervention
CABG: coronary artery bypass graft
eGFR: estimated glomerular filtration rate
SAQ: Seattle Angina Questionnaire
ECHO: echocardiography
CMR: Cardiac Magnetic Resonance Imaging
ETT: exercise treadmill test;
Coronary Angiography and Revascularization by List Status
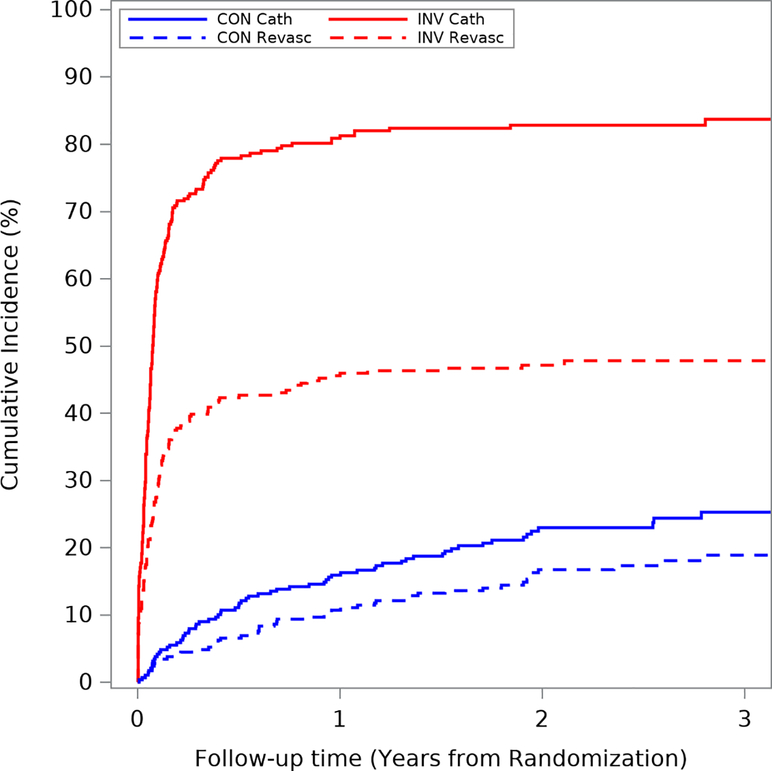
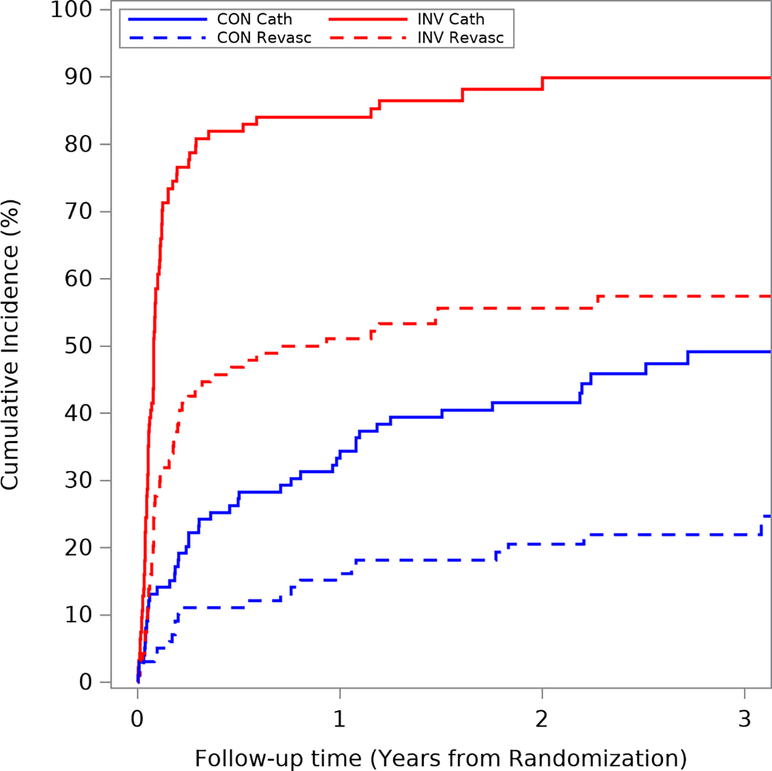
In ISCHEMIA-CKD, the estimated 3-year cumulative incidence (accounting for competing risk of death) of coronary angiography and coronary revascularization was 31.6% and 19.6% in the conservative strategy group and 85.2% and 50.2% in the invasive strategy group. The cumulative incidence of coronary angiography and revascularization are presented in Figures 1a–b and Supplemental Figures 1a–b. There was greater use of coronary angiography and revascularization in patients listed for transplant, regardless of treatment strategy, compared with those not listed. Among patients assigned to the conservative strategy, the estimated 1-year cumulative incidence of coronary angiography and coronary revascularization was 15.9% and 10.7% for those not listed and 33.3% and 16.2% for those listed. For patients assigned to the invasive strategy, the 1-year cumulative incidence of coronary angiography and coronary revascularization was 80.9% and 45.6% for those not listed and 84.0% and 51.1 % for those listed.
Figure 1a. Coronary angiography and revascularization in patients not listed for transplant.
Cumulative incidence of coronary angiography and revascularization by treatment arm in patients not listed for kidney transplant. The blue lines represent patients in the conservative arm and the red lines represent those in the invasive arm. The solid lines represent coronary angiography and the dashed lines represent revascularization. Abbreviations: CON cath=angiography/catheterization in conservative arm; CON revasc=revascularization in conservative arm; INV cath=angiography/catheterization in invasive arm; INV revasc=revascularization in invasive arm.
Figure 1b. Coronary angiography and revascularization in patients listed for transplant.
Unadjusted cumulative incidence of coronary angiography and revascularization in patients who are listed for kidney transplant. The blue lines represent patients in the conservative arm and the red lines represent those in the invasive arm. The solid lines represent coronary angiography and the dashed lines represent revascularization. Abbreviations: CON cath=angiography/catheterization in conservative arm; CON revasc=revascularization in conservative arm; INV cath=angiography/catheterization in invasive arm; INV revasc=revascularization in invasive arm.
The indications for coronary angiography and revascularization in the conservative strategy group at 3 years are presented in Supplemental Figure 2; 59% of angiograms and 46% of revascularizations were not protocol-specified. In the conservative strategy, 4.2% of those not listed for transplant had angiography for non–protocol-specified indications compared with 29.2% of listed patients—an approximately 7-fold difference.
Clinical Outcomes by Kidney Transplant List Status and Treatment Strategy
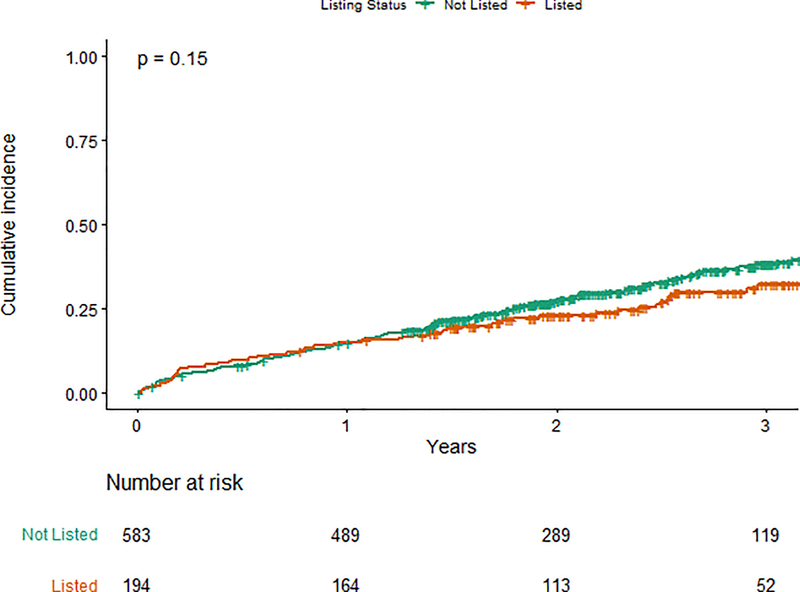
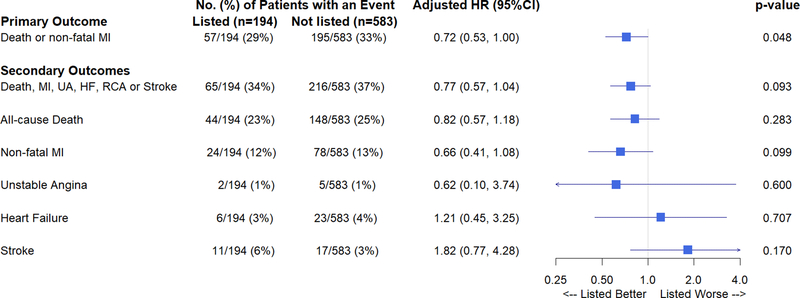
In an unadjusted analysis, the primary outcome of all-cause mortality or nonfatal MI at 3 years was not significantly different between those not listed and listed for kidney transplant, 38.1% versus 32.1%, respectively (log rank p=0.15) (Figure 2a). In a Cox proportional hazards adjusted model, listed participants demonstrated a lower hazard for the primary outcome compared with those not listed (adjusted HR [aHR] 0.72, 95% CI 0.53–1.00; p=0.048) (Figure 2b).
Figure 2a. Primary outcome by list status.
Cumulative incidence function for the primary outcome (all-cause mortality/non-fatal myocardial infarction) in patients not listed (green) and listed (orange) for kidney transplant at study enrollment; number at risk at each time point is listed beneath the graph.
Figure 2b. Forest plot of outcomes by transplant list status.
Forest plot presenting adjusted hazard ratios for the primary outcome and individual secondary outcomes in patients listed and not listed for kidney transplant; p<0.05 was considered statistically significant. Abbreviations: CI=confidence interval; HF=heart failure; HR=hazard ratio; MI=myocardial infarction; RCA=resuscitated cardiac arrest; UA=unstable angina.
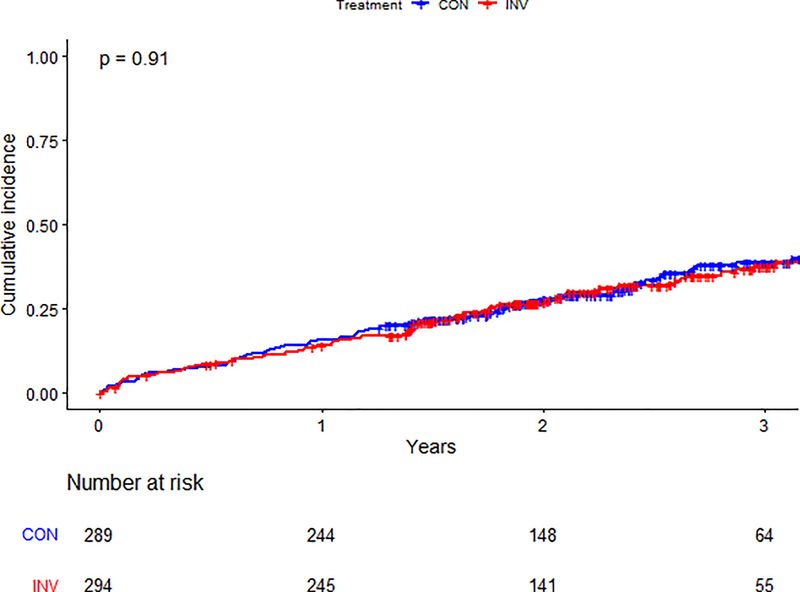
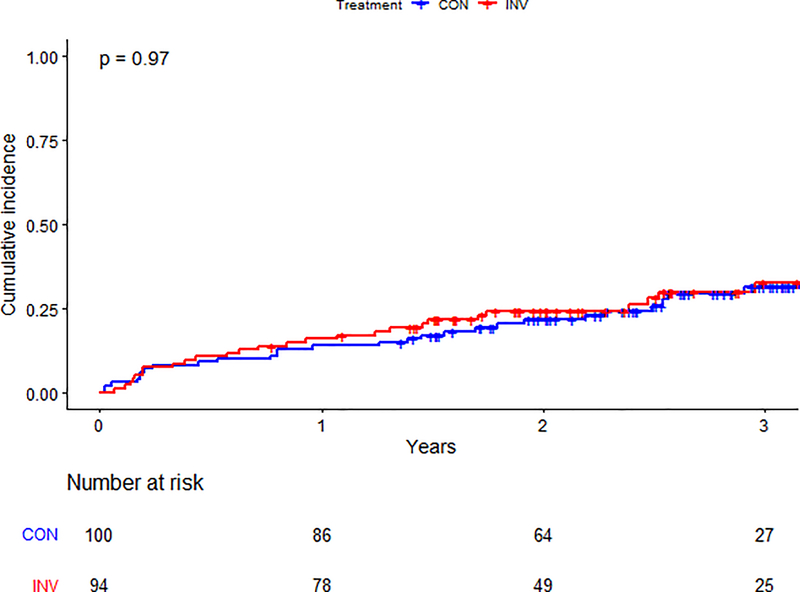
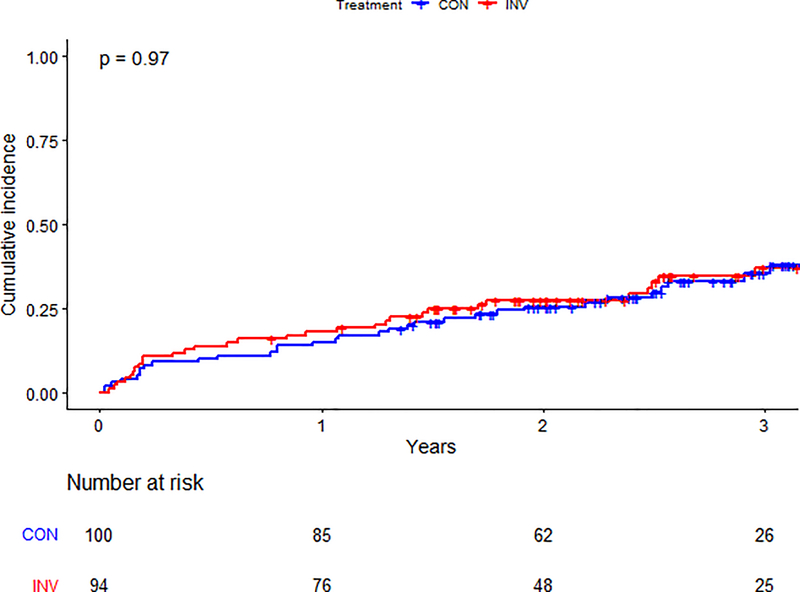
There was no statistical evidence of an interaction between treatment strategy and list status for the primary composite (p=0.68) or secondary outcomes (p=0.35) (Supplemental Figure 3). Among participants not listed, the primary outcome was observed in 96/294 (33%) patients in the invasive-strategy group and 99/289 (34%) in the conservative-strategy group (aHR 1.03, 95% CI 0.78–1.37) (Table 3). Among those listed for transplant, the primary outcome occurred in 27/94 (28%) participants in the invasive strategy group and 30/100 (30%) in the conservative-strategy group (aHR 0.91, 95% CI 0.54–1.54) (Central Illustration). The estimated cumulative incidence for the primary outcome by list status and treatment strategy is presented in Figures 3a–b.
Table 3.
Adjusted hazard ratios for invasive versus conservative strategy by renal transplant candidacy status
| No. (%) of Patients with an Event | Adj. HRa (95% CI) | P-value for Interaction | ||
|---|---|---|---|---|
| CON (n=389) | INV(n=388) | |||
| Primary Outcome | ||||
| Death or non-fatal MI | 0.68 | |||
| Not Listed | 99/289 (34%) | 96/294 (33%) | 1.03 (0.78, 1.37) | |
| Listed | 30/100 (30%) | 27/94 (29%) | 0.91 (0.54, 1.54) | |
| Secondary Outcomes | ||||
| Death, non-fatal MI, Unstable angina, Heart failure, RCA or Stroke | 0.35 | |||
| Not Listed | 106/289 (37%) | 110/294 (37%) | 1.17 (0.89, 1.53) | |
| Listed | 34/100 (34%) | 31/94 (33%) | 0.89 (0.55, 1.46) | |
Estimated from Cox proportional hazards models of the primary and secondary composite outcomes. All models are adjusted for age, sex, kidney function, left ventricular ejection fraction, and diabetes. The hazard ratio is for the invasive-strategy group as compared with the conservative-strategy group
Central Illustration: Kidney Transplant List Status and Outcomes in ISCHEMIA-CKD.
Estimated cumulative incidence for primary and secondary outcomes by treatment strategy for participants listed for kidney transplant. Abbreviations: MI = Myocardial Infarction; UA = Unstable Angina; HF = Heart Failure; RCA = Resuscitated Cardiac Arrest; HR = Hazard Ratio; CI = Confidence Interval
Figure 3a. Primary outcome by treatment arm in patients not listed.
Cumulative incidence of the primary outcome between randomized treatment arms in patients not listed for kidney transplant. The blue line represents those in the conservative arm; the red line represents those in the invasive arm. Number at risk at each time point is listed below the graph. Abbreviations: CON=conservative arm; INV=invasive arm.
Figure 3b. Primary outcome by treatment arm in patients listed for transplant.
Cumulative incidence of the primary outcome between randomized treatment arms in patients listed for kidney transplant. The blue line represents those in the conservative arm; the red line represents those in the invasive arm. Number at risk at each time point is listed below the graph. Abbreviations: CON=conservative arm; INV=invasive arm.
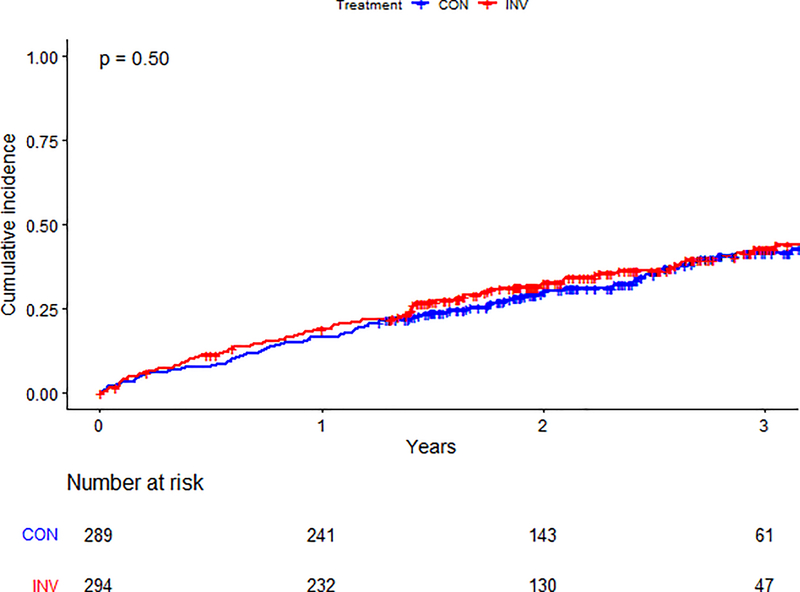
The proportion of patients with the composite secondary outcome (all-cause death, nonfatal MI, hospitalization for unstable angina, heart failure, resuscitated cardiac arrest, or stroke) by treatment strategy is presented in Table 3. As with the primary outcome, no significant difference in event rates was seen between the invasive and conservative strategies, regardless of list status. Figures 3c–d illustrate the estimated cumulative incidence for the secondary outcome by list status and treatment strategy. Table 3 and Supplemental Table 2 provide additional data on primary and secondary outcomes by randomized group and kidney transplant list status.
Figure 3c. Secondary outcome by treatment arm in patients not listed.
Cumulative incidence of secondary outcome between randomized treatment arms in patients not listed for kidney transplant. The blue line represents those in the conservative arm; the red line represents those in the invasive arm. Number at risk at each time point is listed below the graph. Abbreviations: CON=conservative arm; INV=invasive arm.
Figure 3d. Secondary outcome by treatment arm in patients listed for transplant.
Cumulative incidence of secondary outcome between randomized treatment arms in patients not listed for kidney transplant. The blue line represents those in the conservative arm; the red line represents those in the invasive arm. Number at risk at each time point is listed below the graph. Abbreviations: CON=conservative arm; INV=invasive arm.
Two sensitivity analyses were performed: 1. exclusion of the 15 transplanted participants assumed to have been listed (but not indicated in the database at baseline), and 2. assignment of the same 15 transplanted participants to the not listed group. There was no change in the primary outcome based on these sensitivity analyses (data not shown).
DISCUSSION
In this post-hoc analysis of 194 patients listed for kidney transplant in the ISCHEMIA-CKD trial, we found no evidence of a difference in either the primary or secondary outcome at 3 years by treatment strategy (invasive versus conservative), similar to the results from the overall trial.(7) Nor was a significant interaction between assigned treatment strategy and transplant list status observed. After adjustment for baseline covariates, patients listed for transplant had a better outcome than those not listed—a finding supporting the impact of potential selection bias for healthier patients in the process of identifying kidney transplant candidates.
We believe the findings of the present study and those of the overall ISCHEMIA-CKD trial should prompt a reexamination of the current clinical practice of preemptive noninvasive assessment and coronary revascularization of potential kidney transplant recipients with chronic coronary disease, particularly those with asymptomatic myocardial ischemia and those without left main CAD.
The results of this analysis differ from the prior, smaller study of Manske et al. that set the foundation for pre-emptive revascularization among potential kidney transplant recipients with stable CAD.(1) In the present study and the overall ISCHEMIA-CKD trial, the invasive and conservative strategies were equally efficacious; however, a crucial feature of the trial was the implementation of optimal medical therapy in both groups, including intensive treatment of dyslipidemia, with high treatment goal achievement.(7) In Manske et al., reflecting decades-old medical practice, medical therapy included only short-acting nifedipine and aspirin without statin therapy, angiotensin-receptor blockers/angiotensin converting enzyme inhibitors, or beta blockers, a regimen that would now be considered sub-standard.(9,10) Of note, patients with advanced CKD and stable coronary disease are often undertreated, with less frequent use of guideline-based medical therapy.(11,12)
Other studies that supported preemptive revascularization among potential kidney transplant recipients or patients with advanced CKD were observational and often single-center experiences with no comparator group.(12–15) Lentine et al. examined Medicare claims of patients undergoing kidney transplant.(16) Among those at high risk for cardiovascular events, cardiac evaluation (stress testing or angiography) was associated with a higher likelihood of post-transplant acute MI; however, only 10% of all high-risk patients undergoing cardiac evaluation actually received surgical or percutaneous coronary revascularization (a finding consistent with Konig et al (17)). The authors concluded that clinical risk status was the strongest predictor of post-transplant MI, regardless of evaluation or intervention. A more recent single-center study had similar findings, placing greater weight on clinical risk stratification than abnormal cardiac stress testing or extent and severity of stenoses on coronary angiography.(4)
A meta-analysis of the COURAGE (Clinical Outcomes Utilizing Revascularization and Aggressive Drug Evaluation), BARI 2D (Bypass Angioplasty Revascularization Investigation 2 Diabetes), and FREEDOM (Future Revascularization Evaluation in Patients with Diabetes Mellitus: Optimal Management of Multivessel Disease) trials found no convincing advantage of coronary revascularization compared with optimal medical therapy in patients with CKD.(18) Furthermore, in CARP (Coronary Artery Revascularization Prophylaxis) trial and the DECREASE-V (Dutch Echocardiographic Cardiac Risk Evaluation Applying Stress Echo-V) pilot study, a strategy of pre-emptive coronary artery revascularization with percutaneous coronary intervention or coronary artery bypass grafting before elective high-risk vascular surgery was not beneficial.(3,19,20) In both studies, pre-emptive revascularization compared with optimal medical therapy alone was not associated with improved 30-day post-operative survival.
These findings highlight that screening to detect potentially critical coronary artery stenoses identifies kidney transplant candidates at especially high risk for adverse events, specifically during the perioperative period. However, whether an abnormal screening result warrants angiography and revascularization to reduce risk appears unlikely from the present study, at least in patients with well-controlled symptoms and without heart failure. Given the similar risk profiles of vascular surgery and kidney transplantation coupled with recent improvements in medical therapy for CAD, the strategy of pre-transplant screening for occult CAD (and preemptive revascularization) needs to be re-evaluated.
Although there is broader consensus among the kidney transplant community about the need for screening for asymptomatic CAD among those at high risk, there is significant heterogeneity in revascularization practices, which is likely a reflection of the lack of compelling evidence.(16,21,22) We believe our data support the Kidney Disease: Improving Global Outcomes (KDIGO) clinical practice guidelines and the American Heart Association/American College of Cardiology statement on evaluating and managing cardiac disease in candidates for kidney and liver transplantation. Both recommend that asymptomatic candidates with known CAD should not undergo routine coronary revascularization exclusively to reduce perioperative cardiac events, and that such therapy be reserved for high-risk anatomic subsets in whom revascularization confers survival benefits.(21,22) In a single-center observational study of 3698 patients evaluated for kidney transplantation, coronary revascularization was associated with enhanced survival only in patients with 3-vessel disease (23).
Our study does not directly address the issue of whether kidney transplant candidates should be routinely screened for obstructive CAD before planned live donor transplant, wait-listing for deceased donor transplant, or as part of routine surveillance of patients previously listed for transplantation (the latter recommended in KDOQI [Kidney Disease Outcomes Quality Initiative] and societal guidelines(24,25)). The strategy of routine surveillance is the subject of the ongoing CARSK (Canadian-Australian Randomized trial of Screening Kidney transplant candidates for coronary artery disease) trial (26).
In the invasive strategy group in ISCHEMIA-CKD, the frequency of participants with coronary anatomy undergoing revascularization was lower than in the ISCHEMIA trial. This is attributable to suboptimal target vessels and “false positive” stress tests without pre-test computed tomographic angiography to exclude non-obstructive CAD. In addition, the majority of cardiac stress testing in ISCHEMIA-CKD employed nuclear scintigraphy (61.8%), which has been shown to have lower diagnostic accuracy in patients with end-stage kidney disease (27,28).
It is noteworthy that twice as many patients in the conservative strategy listed for transplant underwent angiography compared with those not listed (33% versus 16% at 1 year). We hypothesize that this may reflect a differing approach in the clinical management of listed patients randomized to the conservative strategy (i.e., rational, but unproven, lingering clinician concerns regarding non-intervention in waitlisted patients with evidence for inducible moderate or severe myocardial ischemia). This hypothesis is supported by the nearly 7-fold difference in non–protocol-specified indications for angiography in the conservative strategy in patients listed versus those not listed (and a greater proportion receiving revascularization).
The strategy of preemptive coronary revascularization in kidney transplant candidates is based on the unproven assumption of improved perioperative (and possibly long-term) post-transplant outcomes, and potentially improved outcomes of waitlisted patients (particularly those with prolonged waitlist times, which can easily extend to 5 years or more). The reduction in all-cause mortality associated with kidney transplant, as compared with waitlist status,(9) may be denied to those patients with extended waitlist times (i.e., they do not survive long enough to receive a kidney, and cardiovascular mortality is the most common cause of death). In the present study, we find no evidence to support the concept of improved waitlist survival from preemptive coronary revascularization. Only 51 patients underwent transplantation, preventing definitive comparison of the invasive versus conservative strategies for outcomes.
There are several important limitations to the present study. It is a post-hoc secondary analysis of a subset of 194 of 777 participants enrolled in ISCHEMIA-CKD. The modest sample size reduced the ability to detect a difference in outcomes related to treatment strategy in the kidney transplant-listed patients. Moreover, a substantial proportion of those listed for transplant randomized to the conservative strategy (cumulative incidence of 22% at 3 years) underwent coronary revascularization. The potential difference in outcome between the invasive and conservative strategies in this post-hoc analysis of listed patients could have been diluted by the fact that revascularization was only performed in 50% of invasive strategy participants, and further diluted by revascularization of 20% of conservative strategy participants. The follow-up time was relatively short (median 2.4 years), particularly when compared with waitlist times of many kidney transplant candidates, which often extends beyond 5 years.(29) In ISCHEMIA-CKD, noninvasive coronary computed tomography angiography to exclude left main disease was not performed. However, individual sites could exclude patients from study entry if the stress test results were concerning for left main disease, and only 2.5% of invasively managed patients in ISCHEMIA-CKD had left main disease. Conceivably, patients with large ischemic burdens not due to left main disease were not entered in the trial. Patients with a left ventricular ejection fraction <35%, recent acute coronary syndrome, percutaneous coronary intervention or coronary artery bypass grafting within the past year, severe angina, and heart failure were excluded, so any conclusions do not apply to these patient subsets. Finally, due to the limitations of data collection, accrued waitlist time of the 194 listed patients in our study could not be determined, nor accrued time post-transplantation, and the effect of perioperative major adverse cardiac events on those patients who underwent transplantation could not be ascertained.
In conclusion, in patients with stable coronary disease and advanced chronic kidney disease listed for kidney transplant, our study found no overall difference in all-cause death or nonfatal MI between an initial invasive strategy and an initial conservative strategy. Our findings do not support routine coronary angiography or coronary revascularization in patients waitlisted for kidney transplant exclusively for the purposes of reducing cardiac events or mortality.
Supplementary Material
Perspectives.
Competency in Medical Knowledge:
In patients listed for kidney transplantation who have stable ischemic heart disease and inducible myocardial ischemia on stress testing, management with optimal medical therapy is associated with outcomes comparable to those with an invasive strategy of coronary angiography and revascularization along with optimal medical therapy.
Translational Outlook:
Further studies are needed to compare the outcomes after renal transplantation in patients managed with these two strategies for ischemic heart disease.
Acknowledgments
Funding:
This work was funded by the National Institutes of Health (grants U01HL117904 and U01HL117905). This project was supported by grants from Arbor Pharmaceuticals, LLC. and AstraZeneca Pharmaceuticals, LP. Devices or medications were provided by Abbott Vascular (previously St. Jude Medical, Inc.); Medtronic, Inc.; Phillips (previously Volcano Corporation); and Omron Healthcare, Inc. Medications provided by Arbor Pharmaceuticals, LLC; AstraZeneca Pharmaceuticals, LP; Espero Pharmaceuticals; Merck Sharp & Dohme Corp.; and Sunivion Pharmaceuticals.
The contents are solely the responsibility of the authors and do not necessarily represent official views of the National Heart, Lung, and Blood Institute, the National Institutes of Health, or the Department of Health and Human Services.
Disclosures:
Herzog: Personal fees from NHLBI/NIH, during the conduct of the study; research grants from Amgen, Relypsa, Bristol-Myers Squibb, NIDDK/NIH, University of British Columbia; other from Abbvie, Amgen, AstraZeneca, Corvidia, Diamedica, FibroGen, Janssen, NxStage, Pfizer, Relypsa, Sanifit, University of Oxford, Bristol-Myers Squibb, UpToDate, Boston Scientific, General Electric, Johnson & Johnson, Merck, Hennepin Healthcare. Simegn: Research grants from National Heart, Lung, and Blood Institute, during the conduct of the study. Xu: Research grants from National Heart, Lung, and Blood Institute, during the conduct of the study. Costa: Research grants from National Heart, Lung, and Blood Institute, during the conduct of the study. Mathew: Research grants from National Heart, Lung, and Blood Institute, during the conduct of the study. El-Hajjar: Research grants from National Heart, Lung, and Blood Institute, during the conduct of the study. Gulati: Research grants from National Heart, Lung, and Blood Institute, during the conduct of the study. Maldonado: Research grants from National Heart, Lung, and Blood Institute, during the conduct of the study. Daugas: Personal fees Amgen, GSK, AstraZeneca; grants from Agence Nationale pour la Recherche/ Direction Générale de l’Offre de Soins; and non-financial support from Amgen. Madero: Research grants from National Heart, Lung, and Blood Institute, during the conduct of the study. Fleg: Employment by the NHLBI, during the conduct of the study. Anthopolos: Research grants from NHLBI, during the conduct of the study. Stone: has received speaker or other honoraria from Cook and Terumo; has served as a consultant to Valfix, TherOx, Vascular Dynamics, Robocath, HeartFlow, Gore, Ablative Solutions, Miracor, Neovasc, V-Wave, Abiomed, Ancora, MAIA Pharmaceuticals, Vectorious, Reva, Matrizyme, Cardiomech, Elucid Bio, Occlutech; and has Equity/options from Ancora, Cagent, Applied Therapeutics, Biostar family of funds, SpectraWave, Orchestra Biomed, Aria, Cardiac Success, MedFocus family of funds, Valfix. Sidhu: Research grants from NHLBI, during the conduct of the study; personal fees from AstraZeneca, Sanofi-Regeneron. Maron: Research grants from NHLBI, during the conduct of the study. Hochman: PI for ISCHEMIA trial for which, in addition to support by NHLBI, devices and medications were provided by Abbott Vascular, Medtronic, Inc., St. Jude Medical, Inc., Volcano Corporation, Arbor Pharmaceuticals, LLC, AstraZeneca Pharmaceuticals, LP, Merck Sharp & Dohme Corp., Omron Healthcare, Inc.; financial donations from Arbor Pharmaceuticals LLC and AstraZeneca Pharmaceuticals LP. Bangalore: Research grants from NHLBI, during the conduct of the study; research grants from Abbott Vascular; personal fees from Abbott Vascular, Biotronik, Pfizer, Amgen, and Reata.
Abbreviations
- BARI-2D
Bypass Angioplasty Revascularization Investigation 2 Diabetes
- CAD
coronary artery disease
- CARP
Coronary Artery Revascularization Prophylaxis Trial
- CARSK
Canadian-Australian Randomized trial of Screening Kidney Transplant candidates for coronary disease
- COURAGE
Clinical Outcomes Utilizing Revascularization and Aggressive Drug Evaluation Trial
- DECREASE-V
Dutch Echocardiographic Cardiac Risk Evaluation Applying Stress Echo-V
- FREEDOM
Future Revascularization Evaluation in Patients with Diabetes Mellitus: Optimal Management of Multivessel Disease
- ISCHEMIA-CKD
International Study of Comparative Health Effectiveness of Medical and Invasive Approaches-Chronic Kidney Disease Trial
- KDIGO
Kidney Disease Improving Global Outcomes
- KDOQI
Kidney Disease Outcomes Quality Initiative
Footnotes
Clinical Trial Registration: ClinicalTrials.gov (NCT01985360)
Twitter: @sripalbangalore @nyulangone @nyugrossman
Tweet: Patients listed for kidney transplant in ISCHEMIA-CKD trial had similar outcomes with a conservative or invasive strategy
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Manske CL, Wang Y, Rector T, Wilson RF, White CW. Coronary revascularisation in insulin-dependent diabetic patients with chronic renal failure. Lancet 1992;340:998–1002. [DOI] [PubMed] [Google Scholar]
- 2.Abbud-Filho M, Adams PL, Alberú J et al. A Report of the Lisbon Conference on the Care of the Kidney Transplant Recipient. Transplantation 2007;83:S1–S22. [DOI] [PubMed] [Google Scholar]
- 3.Fleisher LA, Fleischmann KE, Auerbach AD et al. 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014;130:2215–45. [DOI] [PubMed] [Google Scholar]
- 4.Arantes RL, Gowdak LH, Paula FJ et al. Myocardial scintigraphy and clinical stratification as predictors of events in renal transplant candidates. J Nephrol 2010;23:314–20. [PubMed] [Google Scholar]
- 5.Kahn MR, Fallahi A, Kim MC, Esquitin R, Robbins MJ. Coronary artery disease in a large renal transplant population: implications for management. Am J Transplant 2011;11:2665–74. [DOI] [PubMed] [Google Scholar]
- 6.Herzog CA, Marwick TH, Pheley AM, White CW, Rao VK, Dick CD. Dobutamine stress echocardiography for the detection of significant coronary artery disease in renal transplant candidates. Am J Kidney Dis 1999;33:1080–90. [DOI] [PubMed] [Google Scholar]
- 7.Bangalore S, Maron DJ, O’Brien SM et al. Management of Coronary Disease in Patients with Advanced Kidney Disease. N Engl J Med 2020;382:1608–1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bangalore S, Maron DJ, Fleg JL et al. International Study of Comparative Health Effectiveness with Medical and Invasive Approaches-Chronic Kidney Disease (ISCHEMIA-CKD): Rationale and design. Am Heart J 2018;205:42–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fihn SD, Blankenship JC, Alexander KP et al. 2014. ACC/AHA/AATS/PCNA/SCAI/STS Focused Update of the Guideline for the Diagnosis and Management of Patients With Stable Ischemic Heart Disease. J Am Coll Cardiol. 2014 November 4;64(18):1929–49. [DOI] [PubMed] [Google Scholar]
- 10.Knuuti J, Wijns W, Saraste A et al. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes: The Task Force for the diagnosis and management of chronic coronary syndromes of the European Society of Cardiology (ESC). Eur Heart J 2019;41:407–477. [DOI] [PubMed] [Google Scholar]
- 11.Keeley EC, Kadakia R, Soman S, Borzak S, McCullough PA. Analysis of long-term survival after revascularization in patients with chronic kidney disease presenting with acute coronary syndromes. Am J Cardiol 2003;92:509–14. [DOI] [PubMed] [Google Scholar]
- 12.Reddan DN, Szczech LA, Tuttle RH et al. Chronic Kidney Disease, Mortality, and Treatment Strategies among Patients with Clinically Significant Coronary Artery Disease. J Am Soc Nephrol 2003;14:2373–2380. [DOI] [PubMed] [Google Scholar]
- 13.Kumar N, Baker CS, Chan K et al. Cardiac survival after pre-emptive coronary angiography in transplant patients and those awaiting transplantation. Clin J Am Soc Nephrol 2011;6:1912–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Patel RK, Mark PB, Johnston N et al. Prognostic value of cardiovascular screening in potential renal transplant recipients: a single-center prospective observational study. Am J Transplant 2008;8:1673–83. [DOI] [PubMed] [Google Scholar]
- 15.Hemmelgarn BR, Southern D, Culleton BF, Mitchell LB, Knudtson ML, Ghali WA. Survival after coronary revascularization among patients with kidney disease. Circulation 2004;110:1890–5. [DOI] [PubMed] [Google Scholar]
- 16.Lentine KL, Schnitzler MA, Brennan DC et al. Cardiac evaluation before kidney transplantation: a practice patterns analysis in Medicare-insured dialysis patients. Clinical journal of the American Society of Nephrology : CJASN 2008;3:1115–1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Konig J, Mockel M, Mueller E et al. Risk-stratified cardiovascular screening including angiographic and procedural outcomes of percutaneous coronary interventions in renal transplant candidates. J Transplant 2014;2014:854397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Farkouh ME, Sidhu MS, Brooks MM et al. Impact of Chronic Kidney Disease on Outcomes of Myocardial Revascularization in Patients With Diabetes. J Am Coll Cardiol 2019;73:400–411. [DOI] [PubMed] [Google Scholar]
- 19.McFalls EO, Ward HB, Moritz TE et al. Coronary-Artery Revascularization before Elective Major Vascular Surgery. N Engl J Med 2004;351:2795–2804. [DOI] [PubMed] [Google Scholar]
- 20.Schouten O, van Kuijk JP, Flu WJ et al. Long-term outcome of prophylactic coronary revascularization in cardiac high-risk patients undergoing major vascular surgery (from the randomized DECREASE-V Pilot Study). Am J Cardiol 2009;103:897–901. [DOI] [PubMed] [Google Scholar]
- 21.Lentine KL, Costa SP, Weir MR et al. Cardiac disease evaluation and management among kidney and liver transplantation candidates: a scientific statement from the American Heart Association and the American College of Cardiology Foundation. J Am Coll Cardiol 2012;60:434–80. [DOI] [PubMed] [Google Scholar]
- 22.Chadban SJ, Ahn C, Axelrod DA et al. KDIGO Clinical Practice Guideline on the Evaluation and Management of Candidates for Kidney Transplantation. Transplantation 2020;104:S11–s103. [DOI] [PubMed] [Google Scholar]
- 23.Hage FG, Smalheiser S, Zoghbi GJ et al. Predictors of survival in patients with end-stage renal disease evaluated for kidney transplantation. Am J Cardiol 2007;100:1020–5. [DOI] [PubMed] [Google Scholar]
- 24.K/DOQI clinical practice guidelines for cardiovascular disease in dialysis patients. Am J Kidney Dis 2005;45:S1–153. [PubMed] [Google Scholar]
- 25.Gaston RS, Danovitch GM, Adams PL et al. The Report of a National Conference on the Wait List for Kidney Transplantation. Am J Transplant 2003;3:775–785. [DOI] [PubMed] [Google Scholar]
- 26.Ying T, Gill J, Webster A et al. Canadian-Australasian Randomised trial of screening kidney transplant candidates for coronary artery disease-A trial protocol for the CARSK study. Am Heart J 2019;214:175–183. [DOI] [PubMed] [Google Scholar]
- 27.Sarnak MJ, Amann K, Bangalore S et al. Chronic Kidney Disease and Coronary Artery Disease: JACC State-of-the-Art Review. J Am Coll Cardiol 2019;74:1823–1838. [DOI] [PubMed] [Google Scholar]
- 28.Dilsizian V, Gewirtz H, Marwick TH et al. Cardiac Imaging for Coronary Heart Disease Risk Stratification in Chronic Kidney Disease. JACC Cardiovasc Imaging 2021;14:669–682. [DOI] [PubMed] [Google Scholar]
- 29.Hart A, Smith JM, Skeans MA et al. OPTN/SRTR 2016 Annual Data Report: Kidney. Am J Transplant 2018;18 Suppl 1:18–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.