Abstract
Tests of grammar, repetition and semantics were administered to 62 prospectively enrolled right-handed participants with primary progressive aphasia (PPA). Structural brain images were obtained at the time of testing. Regression analyses uncovered 3 clearly delineated non-overlapping left hemisphere clusters where cortical thinning (atrophy) was significantly correlated with impaired performance. A morphosyntactic cluster associated with the grammaticality of sentence construction was located predominantly within the middle and inferior frontal gyri; a phonolexical cluster associated with language repetition was located in the temporoparietal junction; a lexicosemantic cluster associated with object naming and single word comprehension was located within the middle and anterior parts of the temporal lobe and extended into insular, orbitofrontal, and mediotemporal cortices. Commonality analyses were undertaken to explore whether these three clusters were as modular as indicated by the regression analyses or whether some underlying functional granularity could be uncovered. Modularity was defined as the exclusive association of an anatomical cluster with a single type of language task whereas granularity was defined as the association of a single anatomical cluster with more than one type of language task. The commonality analyses revealed a predominantly modular organization with quantitatively minor instances of inter-cluster granularity. The results also reconfirmed previous work on PPA which had shown that Wernicke’s area is not essential for word comprehension, that naming impairments can be based either on deficits of lexical retrieval or word comprehension, and that the essential substrates of word comprehension encompass much wider areas of the temporal lobe than the temporal pole. The anatomy of the language network has traditionally been explored through patients with focal cerebrovascular accidents and experiments based on functional activation. Investigations on PPA are showing that focal neurodegenerations can add new perspectives to existing models of the language network.
Keywords: dementia, grammar, semantics, repetition, word comprehension
The Russian psychologist Lev Vygotsky compared thought to “a cloud shedding a shower of words” (Vygotsky, 1962). Within the context of this analogy, the language network can be said to occupy the interface between Vygotsky’s cloud and the raindrops. It enables arbitrary symbols known as words to communicate and elaborate the verbally expressible components of thought and experience (Fedorenko & Varley, 2016; Mesulam, 1998). Despite vast differences in phonology, semantics and syntax, the cerebral anatomy of the underlying network remains essentially identical for all languages.
A blueprint for the organization of this universal network emerged by the end of the 19th century. It was characterized by a left hemisphere dominance for language and the polarization of function into a frontal component essential for fluency and a temporoparietal component essential for comprehension (Broca, 1865; Dax, 1836; Lichtheim, 1885; Wernicke, 1874). This model was reformulated in modern neuroscientific idiom by Geschwind and became the canonical language network described in textbooks of cognitive neuroscience (Geschwind, 1972). During the 50 years since then, advances in cognitive neuroscience and brain mapping have further refined the computational and anatomical architecture of this network (Binder, 2015; Dronkers, Wilkins, van Valin Jr., Redfern, & Jaeger, 2004; Fedorenko & Varley, 2016; Friederici, 2011; Hagoort, 2013; Hickok & Poeppel, 2007; Hillis, Rorden, & Fridriksson, 2017).
The approaches most commonly used for mapping the anatomy of language can be divided into those based on focal cerebrovascular lesions and those based on electrophysiological and hemodynamic activation sites. The lesion approach assumes that a cortical area makes a critical contribution to the function it disrupts upon being damaged. However, vascular territories encompass not only cortical areas but also deep white matter tracts that interconnect intact regions outside the zone of destruction. Conclusions based on cerebrovascular accidents may therefore overestimate the essential functions of the lesion site (Mesulam, Rader, et al., 2019b; Mesulam, Thompson, Weintraub, & Rogalski, 2015).
Alternative approaches based on functional activation have allowed the mapping of specific language functions with spatial resolution in millimeters (for fMRI) and temporal resolution in milliseconds (for electrocorticography). However, these approaches cannot distinguish critical from ancillary correlates of the functional domain. For example, language tasks regularly activate right hemisphere areas even though right hemisphere lesions do not impair basic language domains such as grammar, language repetition, or word comprehension. The lesion and activation approaches are therefore both subject to systemic limitations. The former approach tends to inflate the essential functions of the lesion site while the latter approach tends to inflate the number of areas that might be deemed essential for a given function.
A third and relatively more recent approach has been based on focal cortical neurodegenerations such as those associated with the syndrome of primary progressive aphasia (PPA) (Forkel et al., 2020; Mesulam, Rader, et al., 2019b; Mesulam et al., 2015; Rogalski et al., 2011; Wilson, Dronkers, et al., 2010). According to this line of investigation, a region where cortical atrophy correlates with impaired performance can be said to have a critical role in maintaining the integrity of the corresponding language function. Atrophy in areas with ancillary roles are not expected to display such correlations. If there is white matter loss, the assumption can be made that it is mostly confined to Wallerian degeneration of axons located within the atrophied cortical area. It would appear, therefore, that focal atrophies can avoid the fiber-of-passage problem inherent in stroke and the conflation of essential with ancillary functionality inherent in activation mapping.
However, clinico-anatomical correlations based on focal cortical neurodegenerations are not without limitations (Mesulam et al., 2020). For one, the slow evolution of the lesion is likely to trigger reactive plasticity. Second, even areas of peak atrophy contain residual neurons that could sustain some functionality. Thirdly, atrophy tends to expand concentrically and along projection pathways so that some sites located on the path of this expansion may spuriously appear critical for the relevant function. Despite these caveats, approaches based on neurodegenerative diseases have offered new perspectives that can complement and clarify those based on cerebrovascular lesions and functional mapping (Catani et al., 2013; Forkel et al., 2020; Mesulam, Rader, et al., 2019b).
In this investigation, we mapped the distribution of cortical atrophy associated with performance on 5 language tests in 62 longitudinally enrolled and uniformly tested right-handed PPA patients with different patterns of aphasia. Regression and commonality analyses were used to determine the preponderance of modularity versus granularity in the resultant functional landscape. Modularity was defined as a map where a given language function is associated with a dedicated critical area. The term granularity was used in a descriptive sense to designate an arrangement where individual areas play critical roles for more than one function, albeit with differential weights for each. The results of the regression and commonality analyses were interpreted in light of existing information on the neuroanatomy of language.
METHODS
The research participants were enrolled in the Northwestern Primary Progressive Aphasia Research Program. Only patients who had mild aphasia (defined as a Western Aphasia Battery (WAB) aphasia quotient (AQ) of ≥80), who had completed all 5 tests that were used to evaluate language functions, and who had structural MRI scans of sufficient quality to generate whole-hemisphere cortical thickness maps were included. There were 62 such participants. The initial clinical diagnosis of PPA was made by the same clinician (M-M.M.) in all cases. The diagnosis was further supported by a review of previous records, evaluation of the history obtained from a reliable informant, administration of standardized neuropsychological measures, and neuroimaging. The Clinical Dementia Rating Scale (CDR) (Morris, 1993) was used to assess global aspects of functionality in daily life. All participants were Caucasian, native English speakers, and right-handed. The study was approved by the Institutional Review Board at Northwestern University and informed consent was obtained from all participants.
Language Tests
The Aphasia Quotient (AQ) of the revised WAB (WAB-R) was used to measure aphasia severity (Kertesz, 2006). The WAB-R offers a comprehensive tool for testing speech fluency, communicative content, comprehension, repetition, naming, reading, and writing. A subset of these tests (fluency, content, comprehension, repetition, and naming) is used to derive the AQ, which has a maximum score of 100. The quantitative clinico-anatomical analyses were based on 5 tests of language function. Scores for each measure were transformed into percentages of maximum performance.
NAT-NAVS- The ability to construct non-canonical sentences was assessed with a composite score derived from two tests. One component was based on the Sentence Priming Production Test of the Northwestern Assessment of Verbs and Sentences (NAVS) (Thompson, 2011). In this test, the patient is shown reversible action pictures and asked to produce sentences of varying complexity according to primes provided by the examiner. A subset of 15 non-canonical sentences (passive voice, object-extracted Wh-questions, object relatives) were used to derive the NAVS half of the NAT-NAVS score. Performance on the NAVS can potentially be influenced by impaired fluency. We therefore used the Northwestern Anagram Test (NAT) to derive the other half of the composite score. In the NAT, the patient is asked to order single words, each printed on a separate movable tile, to be syntactically consistent with an action depicted in a target picture (Weintraub et al., 2010). No verbal output is required. A subset of 15 items from the NAT, representing the same non-canonical sentence types as those we chose for the NAVS were used to derive the NAT component of the score. Performance on these two sets of 15 noncanonical sentences were averaged to derive a composite score of syntax during sentence construction. The NAT-NAVS score was validated for use in the classification of PPA patients according to the 2011 consensus guidelines (Gorno-Tempini et al., 2011; Mesulam, Wieneke, Thompson, Rogalski, & Weintraub, 2012).
%G- The grammaticality of free narrative was assessed by asking participants to view a wordless picture book of the story of Cinderella and tell the story to the examiner. The narrative was recorded using the Praat software (version 5.0, http://www.praat.org) and transcribed by experienced personnel in the Aphasia and Neurolinguistics Research Laboratory at Northwestern University (Thompson, Ballard, Tait, Weintraub, & Mesulam, 1997; Thompson et al., 1995). Transcripts were coded using the Northwestern Narrative Language Analysis system and entered into the Systematic Analysis of Language Transcripts (SALT) for quantification of codes (Hsu & Thompson, 2018; Miller & Chapman, 2000; Thompson et al., 2012). Assessment of morphosyntactic errors (of noun morphology, verb morphology, argument structure, word order) in the recorded Cinderella narrative helped to determine the percentage of phrases and sentences that were grammatically accurate.
REP- The WAB-R Repetition subtest contains 15 items of ascending difficulty ranging from the repetition of single words, to word strings, phrases, and sentences. The first 9 items were too easy and were not failed by any of the subjects. We therefore selected the 6 most difficult items to generate the REP score.
PPVT- Word comprehension was tested with a subset of 36 moderately difficult items (157–192) of the Peabody Picture Vocabulary test, PPVT-IV (Dunn & Dunn, 2006). Each item requires the patient to match a word representing an object, action or attribute to one of 4 picture choices. Performance in the PPVT-IV correlates with word-word association tasks but not with tests of fluency (Mesulam et al., 2009).
BNT- The Boston Naming Test (BNT) was used to assess the naming of objects (Kaplan, Goodglass, & Weintraub, 1983). It is a 60-item standardized and challenging test in which items are administered in order of increasing frequency of occurrence in the English language.
Structural Imaging of Atrophy
For structural imaging, T1-weighted 3D MP-RAGE sequences (TR=2300 ms, TE=2.91ms, TI=900 ms, flip angle=9°, FoV=256 mm) were used to acquire 176 slices at a slice thickness of 1.0 mm on a 3T Siemens TIM Trio using a 12-channel birdcage head coil. Reconstruction was done with the FreeSurfer image analysis suite, version 5.1 (Fischl & Dale, 2000; Fischl, Sereno, Tootell, & Dale, 1999). Geometric inaccuracies and topological defects were corrected using manual and automatic methods based on validated guidelines (Segonne, Pacheco, & Fischl, 2007). Cortical thickness maps of the PPA patients were statistically contrasted against 35 previously described right-handed cognitively healthy volunteers with a similar range of age- and education to identify peak patterns of atrophy (Rogalski et al., 2014). Differences in cortical thickness between groups were calculated by conducting a general linear model on every vertex along the cortical surface. False Discovery Rate (FDR) for individual patient maps was applied at 0.05 to adjust for multiple comparisons and to detect areas of peak cortical thinning (i.e., atrophy) (Genovese, Lazar, & Nichols, 2002).
Regression and Commonality Analyses
Surface-based group analyses were conducted using a general linear model at every vertex along the whole cortex to identify significant correlations between cortical thickness measures and performance on each of the 5 language measures described above. Cortical vertices with Pearson’s r ≥ 0.3 were used to create the corresponding regions of interest (ROI) associated with each of the language tests. Commonality analyses was undertaken to explore additional relationships of each ROI to performance on the 5 tests. Commonality analysis is a statistical technique that decomposes the explained variance of a dependent variable by all independent variables in a multiple linear regression model (Ozdemir, 2015). The resultant commonality coefficients from each ROI’s model include unique effects that are explained by each independent variable and common effects that are shared in each possible combination of the independent variables. The sum of adjusted unique and common effects represented 100% of the explained variance in each ROI. Average thickness values from each ROI were used as the dependent variable in a multiple linear regression where the scores of the 5 tests were used as the independent variables. Parameters with positive β weights from the 5-parameter regression were retained, and the resultant model was used for the commonality analysis. The process was repeated for each ROI, independently.
RESULTS
Age at testing varied from 48 to 80 with a mean and standard deviation of 65.1±6.8 years (Table 1). Symptom duration at testing was 3.4±1.8 years. In keeping with the root criteria for the diagnosis of PPA, domains outside of language were preserved as documented by global CDR scores that ranged from 0 to 1. The WAB-AQ ranged from 80.1 to 97.1 with a mean of 89.6±4.7 indicating that the patients had been tested at early stages of aphasia when other areas of functionality were relatively preserved. The group of 62 participants contained 27 agrammatic, 19 logopenic, 13 semantic and 3 unclassifiable PPA variants according to the 2011 consensus guidelines (Gorno-Tempini et al., 2011). All language tests revealed scores that varied from nearly intact to severely impaired (Table 2). Intercorrelations among tests are shown in Table 3. The structural MRI maps showed that significant thinning of the cerebral cortex encompassed all left hemisphere regions that have been considered part of the language network (Fig. 1).
Table 1.
Characteristics of the Group of 62 Participants.
| Age at Examination: Mean±SD (Range) | 65.1±6.8 (48–80) |
| Symptom Duration at Examination: Mean±SD (Range) | 3.4±1.8 (1–10) |
| Gender Distribution | 33 Male, 29 Female |
| 3 Other | |
| Global CDR: Median (Range) | 0 (0–1) |
| WAB AQ: Mean±SD (Range) | 89.6±4.7 (80.1–97.1) |
Abbreviations: CDR- Clinical Dementia Rating Score; PPA-G- agrammatic/dysfluent variant primary progressive aphasia; PPA-L- logopenic variant primary progressive aphasia; PPA-S- semantic variant primary progressive aphasia; WAB AQ- Western Aphasia Battery Aphasia Quotient.
Table 2.
Test Results for the Group of 62 Participants.
| Test | Mean±SD (%) | Range (%) |
|---|---|---|
| REP | 81.9±12.4 | 43.9–100 |
| BNT | 71.2±29.7 | 10–98.3 |
| PPVT | 86.8±18.1 | 19.4–100 |
| NAT-NAVS | 76.8±21.2 | 6.7–100 |
| %G | 76.2±18.1 | 9.1–100 |
All subjects had all 5 tests. Abbreviations: BNT-Boston Naming Test; %G-Percentage of grammatically correct sentences; NAT-NAVS- a composite test of sentence construction combining non-canonical sentences on the Northwestern Anagram Test and the Northwestern Assessment of Verbs and Sentences; REP- test of repetition; PPVT- the Peabody Picture Vocabulary test of word comprehension.
Table 3.
Intercorrelations of the 5 tests in the group of 62 participants.
| WAB-Rep66 | BNT | PPVTb | NATNAVSnc | %Gram Correct Sentences | |
|---|---|---|---|---|---|
| REP | 1 | −.239 | −.126 | .465** | .375** |
| BNT | −.239 | 1 | .750** | −.352** | −.146 |
| PPVT | −.126 | .750** | 1 | −.166 | .079 |
| NAT-NAVS | .465** | −.352** | −.166 | 1 | .426** |
| %G | .375** | −.146 | .079 | .426** | 1 |
Pearson’s r, ** p<0.01. Abbreviations: BNT-Boston Naming Test; %G-Percentage of grammatically correct sentences; NAT-NAVS- a composite test of sentence construction combining non-canonical sentences on the Northwestern Anagram Test and the Northwestern Assessment of Verbs and Sentences; REP- test of repetition; PPVT- the Peabody Picture Vocabulary test of word comprehension.
Figure 1-.
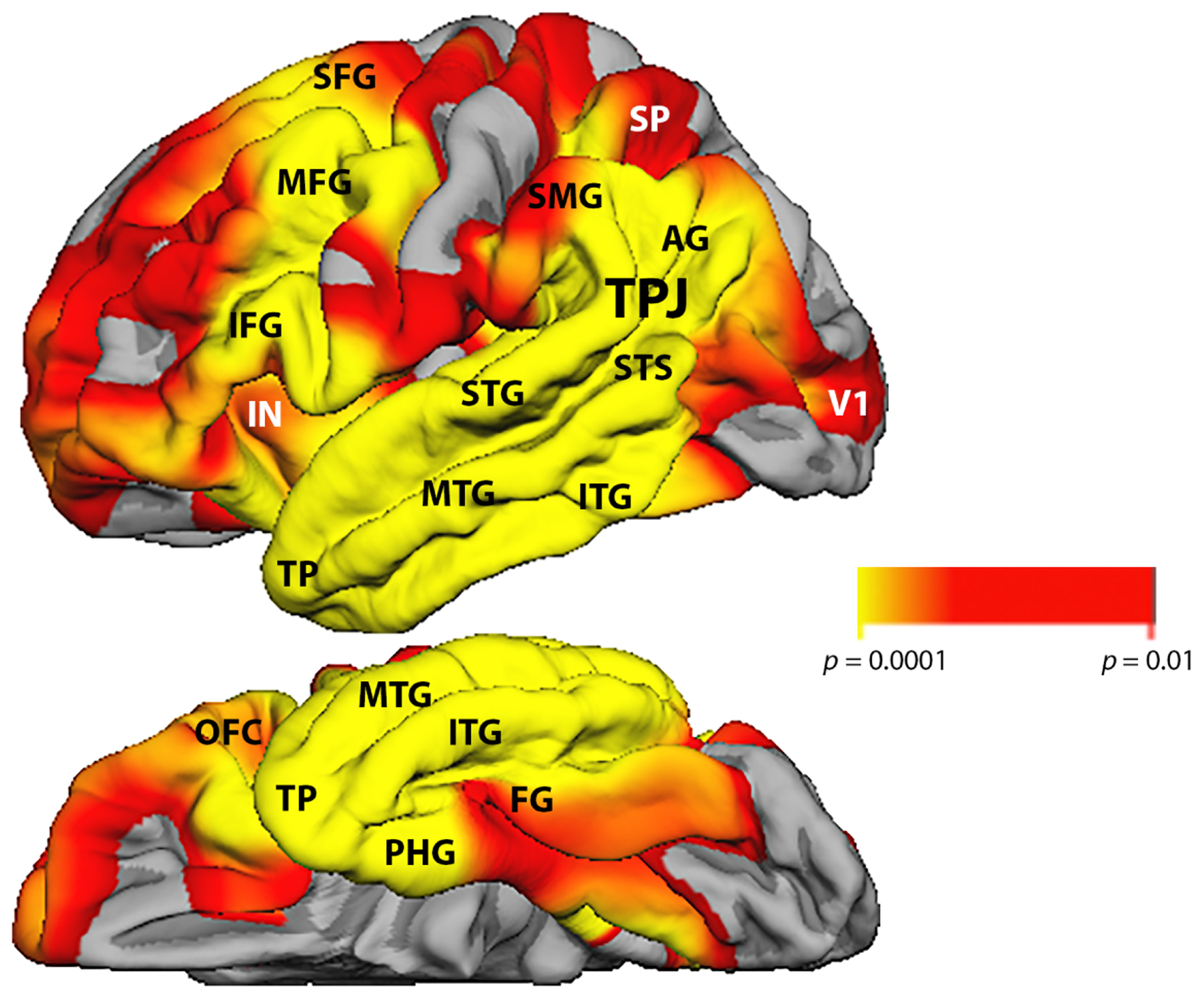
Composite atrophy map for the entire full cohort of 62 PPA participants. Cortical thickness maps of the PPA participants were statistically contrasted against 35 previously described right-handed cognitively healthy volunteers with a similar range of age- and education to identify peak patterns of atrophy. The color scale reflects significance levels. Lateral view of the left hemisphere is on top, and ventral view on the bottom. Abbreviations: AG- angular gyrus; FG- fusiform gyrus; IFG- inferior frontal gyrus; IN- insula; ITG- inferior temporal gyrus; MFG- middle frontal gyrus; MTG- middle temporal gyrus; OFC- orbitofrontal cortex; PHG- parahippocampal gyrus; SFG- superior frontal gyrus; SMG- supramarginal gyrus; SP- superior parietal lobule; STG: superior temporal gyrus; STS- superior temporal sulcus; TP- temporopolar cortex; TPJ- temporoparietal junction; V1- primary visual cortex.
The regression analysis identified regions where the magnitude of cortical thinning (atrophy) was significantly correlated with the magnitude of impairment. Four distinct sets of regions of interest (ROI) were identified (Figs. 2A, C; Fig. 3A, C). Worse performance on the NAT-NAVS task was correlated with atrophy predominantly within the posterior part of the middle frontal gyrus (MFG). Smaller areas of correlation were located in premotor cortex just posterior to the inferior frontal gyrus (IFG) and in the superior frontal gyrus (SFG). The percentage of grammatically correct statements in speech (%G) was inversely correlated with atrophy in the IFG, including the pars opercularis and the junction of the pars triangularis with the pars orbitalis. Lower scores on the BNT and PPVT were correlated with greater atrophy in nearly identical areas of the anterior temporal lobe, extending into the temporal pole, the midsections of the middle and inferior temporal gyri (MTG, ITG), parahippocampal cortex, anterior fusiform gyrus, insula, and adjacent orbitofrontal areas. Lower scores on the REP was correlated with atrophy in the posterior half of the STG and STS as well as the adjacent parts of the supramarginal and angular gyri. These areas of significant correlation were designated NAT-NAVS-ROI, %G-ROI, PPVT/BNT-ROI, and REP-ROI. The ROI for %G, NAT-NAVS and REP were almost exclusively left-sided and the PPVT/BNT ROI was overwhelmingly left-sided but had a small mirror region on the right anterior temporal lobe.
Figure 2 -.
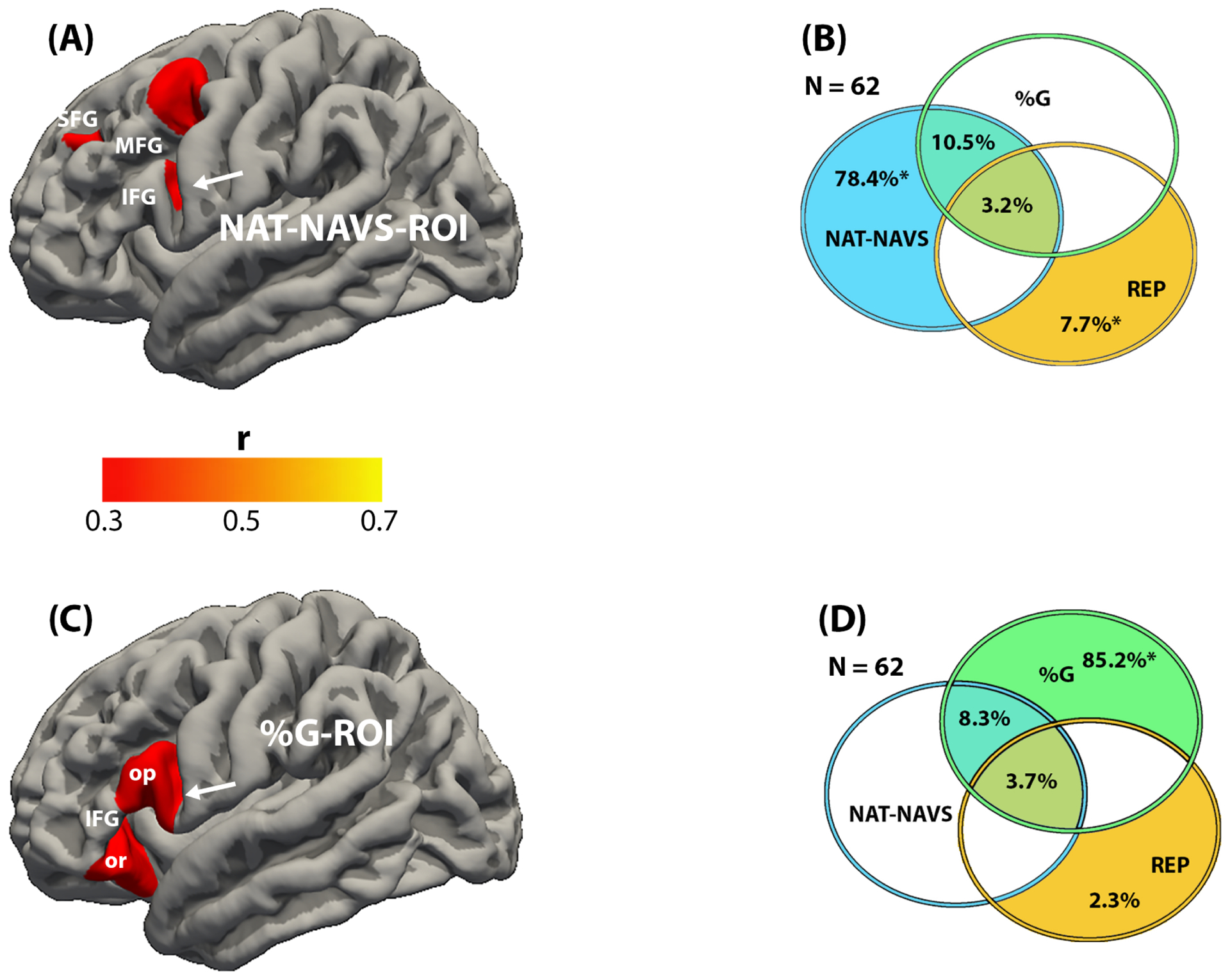
Results of the regression (A, C), and commonality (B, D) analyses in the cohort of 62 PPA participants. A. The areas in red indicate regions where cortical thinning (atrophy) was correlated with impaired performance on the NAT-NAVS test of syntactically correct sentence construction (r range 0.3–0.412). These areas are collectively designated NAT-NAVS-ROI. The heat map indicates the scale used to illustrate significance levels. B. Commonality analysis indicates the task-specific unique and shared contributions to the explained variance of thickness in the NAT-NAVS-ROI. Each circle reflects the contribution of a specific task, blue for NAT-NAVS, green for %G, gold for REP. The numbers represent the contribution of a specific task or of a combination of tasks to the explained variance of thickness within the ROI. Overlapping areas represent shared contributions. Those with an asterisk were statistically significant. Absence of detectable contribution to variance of ROI thickness is indicated by the uncolored spaces. The figure offers a conceptual illustration of the results but is not drawn to scale so there is no correspondence between the magnitude of contributions and the size of the areas encompassed by overlapping or non-overlapping parts of the circles. C. The red areas indicate regions where cortical thinning (atrophy) was correlated with impaired performance on the %G score of grammatically correct sentences in speech (r range 0.3–0.397). These areas are collectively designated %G-ROI. The heat map indicates the scale used to illustrate significance levels. The arrows in A and C indicate the small region of overlap between the two ROI. D. Commonality analysis indicates the task-related unique and shared contributions to the explained variance of thickness in the %G-ROI. Each circle reflects the contribution of a specific task, blue for NAT-NAVS, green for %G, gold for REP. The numbers represent the percentage of contribution to the explained variance of the ROI. Overlapping areas represent shared contributions. Those with an asterisk were statistically significant. Absence of detectable contribution to variance of ROI thickness is indicated by the uncolored spaces. The figure offers a conceptual illustration of the results but is not drawn to scale so there is no correspondence between the magnitude of contribution and the areas encompassed by overlapping or non-overlapping parts of the circles. Abbreviations: %G- percentage of grammatically correct sentences in running speech; IFG- inferior frontal gyrus; MFG- middle frontal gyrus; NAT-NAVS- test of syntactically correct sentence construction; op- opercularis component of IFG; or- orbitalis component of IFG; REP- test of language repetition; SFG- superior frontal gyrus.
Figure 3-.
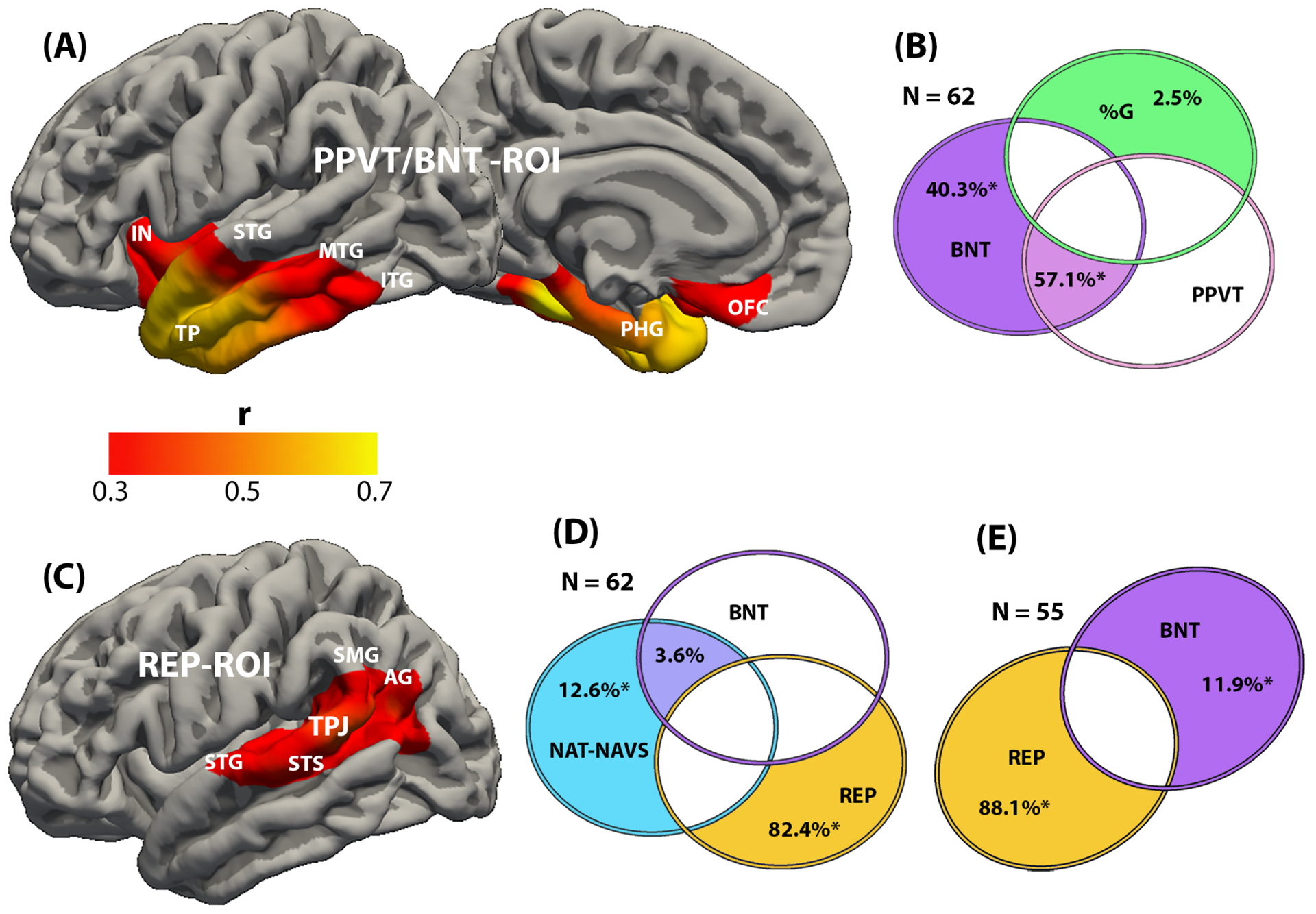
Results of the regression (A, C), and commonality (B, D) analyses in the cohort of 62 PPA participants. For the analysis in E, participants with the lowest scores of word comprehension (tested by the PPVT) were excluded to generate a subgroup of 55. A. The red and yellow areas indicate regions where cortical thinning (atrophy) was correlated with worse performance on the BNT and PPVT tests of object naming and single word comprehension (r range 0.3–0.743). The two tests led to regional correlations that were almost entirely overlapping. These areas are collectively designated PPVT/BNT-ROI. The heat map indicates the scale used to illustrate significance levels. B. Commonality analysis indicates the task-specific unique and shared contributions to the explained variance of thickness in the PPVT/BNT-ROI. Each circle reflects the contribution of a specific task, green for %G, orange purple BNT, magenta for PPVT. The numbers represent the percentage of contribution to the explained variance of the ROI. Overlapping areas represent shared contributions. Those with an asterisk were statistically significant. Absence of detectable contribution to variance of ROI thickness is indicated by the uncolored spaces. The figure offers a conceptual illustration of the results but is not drawn to scale so there is no correspondence between the magnitude of contribution and the areas encompassed by overlapping or non-overlapping parts of the circles. C. The red and yellow areas indicate regions where cortical thinning (atrophy) was correlated with impaired performance on the REP test of language repetition (r range 0.3–0.584). These areas are collectively designated REP-ROI. The heat map indicates the scale used to illustrate significance levels. D. Commonality analysis indicates the task-specific unique and shared contributions to the explained variance of thickness in the REP-ROI. Each circle reflects the contribution of a specific task, blue for NAT-NAVS, purple for BNT, gold for REP. The numbers represent the percentage of contribution to the explained variance of the ROI. Overlapping areas represent shared contributions. Those with an asterisk were statistically significant. Absence of detectable contribution to variance of ROI thickness is indicated by the uncolored spaces. The figure offers a conceptual illustration of the results but is not drawn to scale so there is no correspondence between the magnitude of contribution and the areas encompassed by overlapping or non-overlapping parts of the circles. E. Same analysis as in D but on a cohort of 55, including only those with a PPVT score above 60%. Abbreviations: AG- angular gyrus; BNT- test of object naming; %G- percentage of grammatically correct sentences in running speech; IN- insula; ITG- inferior temporal gyrus; MTG- middle temporal gyrus; NAT-NAVS- test of syntactically correct sentence construction; OFC- orbitofrontal cortex; PPVT- test of word comprehension; REP- test of language repetition; SMG- supramarginal gyrus; STG- superior temporal gyrus; STS- superior temporal sulcus; TPJ- temporoparietal junction.
Commonality Analyses
The regression analysis based on 5 language tasks revealed a modular map with no regional overlap of functionality except for the narrow sliver of premotor cortex where atrophy was correlated with performance both on the NAT-NAVS and the %G (arrows in Figs. 2 A and C). If the remaining ROI components represent modular ‘centers’, the variance in their thickness should be explained fully by variations in the performance of the corresponding tasks. This hypothesis was tested with commonality analyses. The goal was to use multiple linear regressions to decompose the explained variance in the thickness of each functional ROI in Figures 2 and 3 into those that are uniquely explained by each language task (unique effects) and those that are shared in each possible combination of all tasks (common effects). Varimax-rotated principal component analyses had been used to address similar questions in other groups of aphasic patients (Ingram et al., 2020).
When a full model is used for each ROI (thickness ~ NAT-NAVS + %G + REP + BNT + PPVT), the proportion of explained variance (R2) within each ROI was 0.32 for the NAT-NAVS-ROI, 0.19 for the %G-ROI, 0.81 for the PPVT/BNT-ROI, and 0.53 for the REP-ROI. Independently, for each ROI’s model, once only positive β weight parameters were retained, the commonality analysis decomposed the variance explained.
The results of the commonality analyses are illustrated in the form of overlapping circles in Figures 2 and 3, each circle representing the contribution of a single function to the explained variance of the relevant ROI. The magnitudes of the common and unique contributions to the explained variance of cortical thickness in each ROI are indicated numerically within corresponding sectors of the circles. Contributions to the explained variance that are significant (p≤0.05) are indicated with an asterisk.
According to the analysis within the NAT-NAVS-ROI (Fig. 2A), for the model thickness ~ NAT-NAVS + %G + REP (R2 = 0.27; p < 0.001), NAT-NAVS uniquely explains 78.4% and REP uniquely explains 7.7% of the explained variance. The shared contributions indicated within the regions of overlap in Fig. 2B did not reach significance. For the %G-ROI (Fig. 2B), 85.2% of the variance in the model thickness ~ NAT-NAVS + %G + REP (R2 = 0.17; p = 0.011) was explained uniquely by the %G score. No other unique or shared contribution reached significance. It appears that the narrow overlap of the NAT-NAVS and %G-ROI shown by arrows in Figures 2A and 2C is not robust enough to be demonstrated by the commonality analysis. In the PPVT/BNT-ROI, for the model thickness ~ %G + PPVT + BNT (R2 = 0.78; p < 0.001), 40.3% of the variance in thickness was uniquely explained by performance on the BNT task and 57.1% shared by the performance on the BNT and PPVT tasks. In the REP-ROI, with the model thickness ~ NAT-NAVS + REP + BNT (R2 = 0.47; p < 0.001), 82.4% of the thickness variance was uniquely explained by performance on the REP task and 12.6% uniquely by performance on the NAT-NAVS task. A further analysis was performed on a subgroup obtained by eliminating those with PPVT scores at or below 60%. Commonality analysis in this subgroup of 55 participants with relatively spared word comprehension revealed a small but significant unique variance explained by BNT scores within the REP-ROI (Fig. 3E).
DISCUSSION
The current study involved 62 PPA participants prospectively enrolled in a longitudinal research program. Testing was done at early stages of impairment as shown by the mean AQ of 89.6±4.7. The same 5 language tests were administered with uniform methodology to all 62 participants and revealed a full range of performance levels, from severely impaired to intact. All major PPA variants were represented so that the pattern of impaired language functions and cortical atrophy varied widely from one participant to the other. Each participant was right-handed. Consequently, language dominance in all cases was almost certainly located within the left hemisphere where significant atrophy encompassed all language network components.
As noted in the introduction, one goal was to use regression and commonality analyses to explore whether the functional anatomy of language reflected modularity or granularity. The analyses were guided by the premise that an area where atrophy is linearly correlated with impairment contains an essential substrate for performance on the corresponding language test. The regression analyses identified four such areas of interest (ROI), each linked to specific language tests. They were designated the NAT-NAVS-ROI (related to sentence construction), %G-ROI (related to grammar of connected speech), PPVT/BNT-ROI (related to naming and word comprehension) and REP-ROI (related to sentence repetition). The four ROI were sharply delineated from each other. The subsequent commonality analyses reinforced the presence of a predominantly modular landscape with only minor granularity of functional anatomy.
Two caveats are relevant to the interpretation of these results. First, the analyses do not allow us to determine if the critical substrate of a given function encompasses the corresponding ROI in its entirety or only in part. Secondly, the commonality analyses showed that performance in the 5 language tasks explained only a fraction of the variance in the thickness of the relevant cortical areas, ranging from a high of 81% in the PPVT/BNT-ROI to a low of 19% in the %G-ROI. In the case of the %G-ROI, the unexplained variance of 81% suggests that this region is likely to have extensive affiliations either with language functions that were not tested or with domains unrelated to language, as has been shown for the IFG within which the %G-ROI was located (Fedorenko & Blank, 2020; Griffiths, Marslen-Wilson, Stamatakis, & Tyler, 2013; Hagoort, 2014; Koechlin & Jubault, 2006; Lau, Phillips, & Poeppel, 2008; Long et al., 2016; Müller, 2004; Poldrack et al., 1999). Similar considerations apply to the NAT-NAVS-ROI, REP-ROI, and the PPVT/BNT-ROI where unexplained variances were 68%, 47%, and 19%, respectively. An alternative possibility underlying the high unexplained variance in the IFG may be related to the type of neuropathology (usually a tauopathy) that causes agrammatic forms of PPA in which the dysfunction may be more reflective of impaired functional connectivity than of cortical atrophy (Bonakdarpour et al., 2017). Within the constraints of these caveats, the results of the regression and commonality analyses lent themselves to a general review based on three functional clusters that were designated morphosyntactic, phonolexical, and lexicosemantic.
The Morphosyntactic Cluster- the Relationship of Word to Word
Cortical areas where atrophy was linked to impaired production of grammatical sentences, as determined by the NAT-NAVS and %G, constituted a morphosyntactic cluster that was located predominantly in the IFG and MFG. The agrammatism of PPA patients can be quite striking. The following is a transcript of a voicemail left by a PPA patient thanking one of us (C.C.) for information on PPA. - “Thank you …um…PPA article help..so much…um…never know wrong with me doctors never know…um…this PPA explain thing every everything relief tears thank you. Research going still participate me yes. Call me feel free but even not if thank you so much for explain this thank you.” To extend Vygotsky’s analogy, the cloud has shed droplets that carry the appropriate meaning but in unruly sprinkles.
The relationship of the IFG to grammar is in keeping with multiple lines of evidence linking Broca’s area to production of syntax, grammatical morphology, sequencing of verbal output, word category awareness, and verb tense agreement (Friederici, 2011; Hagoort, 2014; Heim, Opitz, & Friederici, 2003; Kielar, Milman, Bonakdarpour, & Thompson, 2011; Leminen, Smolka, Duñabetia, & Pliatsikas, 2019; Nelson et al., 2017; Sahin, Pinker, Cash, Schomer, & Halgren, 2009; Wilson, Henry, et al., 2010). Although the MFG site is outside the traditional boundaries of Broca’s area, it has been linked to grammaticality and syntactic complexity of connected speech (Matchin & Hickok, 2020; Wilson, Henry, et al., 2010). The anatomical segregation of atrophy sites associated with the two measures of grammar was somewhat unexpected. The %G score is based on free narrative and therefore influenced by fluency. As agrammatic PPA patients also tend to be dysfluent, the related ‘economy of expression’ is likely to have promoted the production of statements devoid of functors and morphological markers. The NAT-NAVS score, on the other hand, is a more specific marker of syntax, namely the ability to sequence lexical elements to fit the intended meaning (e.g., the boy kissed the girl versus the girl kissed the boy). Furthermore, the NAT component of the composite score is independent of fluency because it is performed by manually positioning words written on tiles. The %G score can therefore be said to be more reflective of morphology while the NAT-NAVS score is more sensitive to syntax. While this distinction could account for the separation of the anatomical sites associated with the two measures, it is also important to note that investigations using the English and Italian versions of NAT had previously linked performance of this task to atrophy of the IFG rather than the MFG (Canu et al., 2019; Rogalski et al., 2011). Although more information is needed to settle these relationships, it could be suggested that the specializations of the IFG encompass morphology as well as syntax whereas the MFG component may be more narrowly specialized for the sequencing of lexical elements into syntactically correct sentences. Intraoperative recordings within components of the morphosyntactic cluster have started to generate important insights into the electrophysiological correlates of syntax and morphology (Nelson et al., 2017; Sahin et al., 2009).
The Phonolexical Cluster – Repetition and Sentence Comprehension
Repetition helps to gauge processes that transform phonological input into verbal output. We coined the descriptive term phonolexical to designate a cluster of areas where atrophy was associated with impaired repetition as tested by the REP. Intact repetition requires the phonological encoding and on-line holding of the input, its transformation into a pre-articulatory code (response buffer), and the subsequent monitoring of the resultant verbal output (efference copy). Perturbations in any of these components would impair repetition and promote phonemic paraphasias (Herman, Houde, Vinogradov, & Nagarajan, 2013). The on-line linkage of mental content (i.e., thought, inner speech) to pre-articulatory phonological representations may also suffer, giving rise to the characteristic word retrieval deficits and circumlocutions that accompany repetition impairments in aphasic patients. In the current cohort, atrophy sites correlated with performance on the REP were clustered within the posterior STG-STS and the inferior parietal lobule, a territory that includes Wernicke’s area and that can be designated the ‘temporoparietal junction’. These areas are commonly damaged in logopenic PPA and conduction aphasia, two syndromes characterized by poor repetition and the associated features noted above (Fridriksson et al., 2010; Gorno-Tempini et al., 2004; Selnes, Knopman, Niccum, & Rubens, 1985). In keeping with these relationships, numerous investigations have demonstrated the importance of the temporoparietal junction for verbal working memory (i.e., phonological loop function), sensorimotor integration of speech, representation of phonetic sequences, attentional capture of verbal information, and phonological retrieval (Binder, 2015; Herman et al., 2013; Ravizza, Hazeltine, Ruiz, & Zhu, 2011; Wise et al., 2001).
The commonality analysis revealed one instance of inter-cluster granularity by detecting a small but significant effect that linked the area critical for REP to performance on the NAT-NAVS task, and reciprocally the area critical for NAT-NAVS to performance on the REP task (Fig. 2B and 3D). These commonality effects may be reflecting the shared dependence of both syntax and repetition on working memory and sequencing. However, the impact of this granularity on naturalistic language function is likely to be quite subtle since logopenic PPA patients, characterized by the presence of impaired repetition abilities and atrophy of the REP-ROI, tend to have relatively intact sentence construction abilities (Gorno-Tempini et al., 2011; Mesulam et al., 2012).
Sentence comprehension was not included in the current analyses but is obviously a key aspect of language. It represents the collective outcome of at least three components, the on-line holding of incoming auditory verbal information, the recognition of the constituent lexical elements, and the decoding of their grammatical interrelationships. Investigations on PPA have shown that the semantic component can be dissociated from the other two so that patients with severely impaired single word comprehension can have spared comprehension of non-canonical sentences as long as the constituent nouns and verbs (e.g., ‘point to the boy that the girl kissed’) are familiar enough to be recognized (Mesulam et al., 2015). It is therefore possible to identify a sentence comprehension (syntax knowledge) faculty independent of semantics. This functionality would appear to straddle the morphosyntactic and phonolexical clusters of the language network. In fact, regression analyses in PPA had shown sentence comprehension impairments to be associated with widespread atrophy that extends both into the temporoparietal areas correlated with REP scores and the lateral frontal areas correlated with NAT-NAVS and %G scores (Mesulam et al., 2015). This organization is in keeping with multiple investigations that link comprehension at the sentence (but not word) level to the integrity of both temporoparietal and frontal components of the language network (Dapretto & Bookheimer, S. Y., 1999; Dronkers et al., 2004; Hartwigsen, Golombek, & Obleser, 2015; Keller, Carpenter, & Just, 2001; Nelson et al., 2017; Siegelman, Blank, Mineroff, & Fedorenko, 2019; Towle et al., 2008; Xu, Wu, & D’uann, 2020).
The Lexicosemantic Cluster- from Word to Meaning
Impairments of object naming (assessed by the BNT) and of word comprehension (assessed by the PPVT) were associated with nearly identical regions of atrophy, reflecting the dependence of naming on word comprehension. The resultant lexicosemantic cluster was the largest of the 3 and extended from middle parts of the MTG and ITG to the temporal pole, entorhinal cortex, the anterior fusiform gyrus, insula, and orbitofrontal cortex. Surprisingly, the insula displayed correlations that placed it in the lexicosemantic rather than morphosyntactic cluster, a relationship that has been reported previously and that may reflect the connections of this area with components of the temporal lobe (Cloutman, Binney, Drakesmith, Parker, & Lambon Ralph, 2012; Kümmerer et al., 2013; Mesulam & Mufson, 1985). The extension of the lexicosemantic cluster into medial temporal cortex is consistent with previous functional imaging and electrical stimulation studies that have implicated the fusiform gyrus and surrounding areas in naming and word comprehension (Lüders et al., 1991; Moss, Rodd, Stamatakis, Bright, & Tyler, 2005; Nobre, Allison, & McCarthy, 1994). Furthermore, quantitative analyses of autopsied PPA cases had revealed a correlation between word comprehension and orbitofrontal neurodegeneration while MEG investigations in neurologically intact participants had shown a relationship between semantics and ventromedial prefrontal cortex activity (Giannini et al., 2019; Pylkkänen, 2021).
The regression and commonality analyses linked word comprehension to anterior and middle temporal cortices rather than temporoparietal regions that contain Wernicke’s area. This pattern, while inconsistent with classic aphasiology, is no longer surprising based on numerous investigations in PPA, stroke aphasia, and neurologically intact participants (Binder, 2017; Binder, 2015; Mesulam, Rader, et al., 2019b; Pylkkänen, 2021). The lack of association between object naming impairments and atrophy within the temporoparietal junction was surprising because this area encompasses the core atrophy site for logopenic PPA, a syndrome where word retrieval impairments constitute key features (Gorno-Tempini et al., 2004). This consideration led to a secondary analysis on a subgroup of 55 participants with relatively preserved word comprehension. The commonality analysis in this subgroup revealed a small but significant linkage between temporopolar junction atrophy and BNT performance (Fig. 3E). This relationship appears to have been obscured in the larger group of participants where the most severe BNT impairments were associated with PPVT impairments and acted as markers of anterior temporal atrophy. There are thus at least two distinct routes to anomia. One is based on impaired word comprehension, arises in association with anterior and middle temporal atrophy, and becomes the leading cause of anomia in semantic PPA. The second route, linked to the temporoparietal junction, is more heavily influenced by impaired retrieval and becomes a principal cause of anomia in patients with relatively intact word comprehension as in the case of logopenic PPA (Hurley, Paller, Rogalski, & Mesulam, 2012).
The size of the lexicosemantic cluster reflects the complexity of the neural interactions that underlie word comprehension. According to neuroanatomical investigations in monkeys and functional connectivity in humans, the temporal lobe offers a synaptic matrix for the sequential transfer of visual and auditory information from primary sensory cortices into unimodal, heteromodal, paralimbic, and limbic areas (Mesulam, 1998; Sepulcre, Sabuncu, Yeo, Hesheng, & Johnson, 2013). The latter three, also known as transmodal areas, offer templates for transforming percepts such as sensory word-forms, faces, and objects into multimodal representations that reflect their experiential and semantic attributes (Mesulam, 2000). Each stage in the hierarchy offers a gateway for the preferential shunting of specific classes of information into the next level so that general surface properties (e.g., round, red, etc.) can be transformed into generic (e.g., wearable), specific (e.g., a hat), and unique (e.g., my hat) levels of recognition. The point of origin for the activation is determined by the modality of the percept while the subsequent spread is guided by task-specific top-down projections (Bitan et al., 2005; Friston, 2005; Seeck et al., 1995; Summerfield et al., 2006). The process need not be conceptualized as an assembly line where each stage passes a finished product onto the next for further elaboration but, instead, as the collective outcome of activation ripples reverberating throughout the lexicosemantic cluster (Mesulam, 2008; Tovèe, 1994).
Within this framework, the process of word comprehension starts in primary auditory cortex where neurons respond to pure tones and pitch. This information provides the input for the mid-to-posterior STG, which is relatively unresponsive to non-linguistic sounds but which responds to specific phonetic parameters of spoken language (Démonet et al., 1992; Petersen, Fox, Posner, Mintun, & Raichle, 1988; Zatorre, Evans, & Meyer, 1994). These neurons respond to spoken real words as readily as to distorted backward speech and represent a pre-semantic auditory word form area selectively tuned to word-like percepts (Creutzfeldt, Ojemann, & Lettich, 1989; DeWitt & Rauschecker, 2013; DeWitt & Rauschecker, 2016; Mesulam, Nelson, et al., 2019). A subsequent step in linking percept to concept takes place in MTG where neurons are selectively activated by understandable speech but not distorted speech (Creutzfeldt et al., 1989). These MTG neurons are positioned to shunt inputs with features of understandable speech into downstream transmodal cortices of the inferior and anterior temporal lobe. Through this process of selective tuning, gating, and shunting, an auditory input that is both word-like and intelligible (i.e., familiar) gains access to transmodal areas of the left temporal lobe and evokes the associative linkages that collectively encode its meaning (Mesulam, Nelson, et al., 2019). The question may be asked why atrophy in mid-to-posterior STG (i.e., Wernicke’s area), where the putative auditory word form area is located, does not play a more critical role in undermining word comprehension abilities in PPA. The answer may lie in the fact that the pre-semantic auditory word-form area is represented bilaterally so that the asymmetric left-sided atrophy of Wernicke’s area in PPA is not sufficient to undermine the subsequent steps of word recognition. In support of this possibility, listening to non-words leads to bilateral STG activation (Wise et al., 1991). In contrast, the transformation of word to meaning is strongly lateralized to the left hemisphere, making left anterior and middle temporal lobe atrophy sufficient for causing severe word comprehension impairments. An analogous but bilateral multisynaptic hierarchy links non-verbal percepts, including faces and objects, into the distributed transmodal associations that collectively encode their attributes. Transmodal nodes of the two systems overlap and establish the linkages of verbal to non-verbal representations that underlie object naming and aspects of word comprehension.
According to this synaptic organization, the flow of information during performance on the PPVT is initiated through the auditory word-form area of the STG and propagates anteriorly to transmodal nodes. In the BNT, the process originates in bilateral fusiform visual association areas and propagates through multisynaptic transmodal relays into the posterior left STG for lexical and phonologic retrieval. The resultant ability to verbally name the object can therefore be disrupted either at the level of transmodal associations (as in semantic PPA) or at the level of retrieval (as in the case of logopenic PPA). The process of word comprehension also involves top-down signals from downstream parts of the hierarchy so that the interpretation of the multi-synaptic feed-forward pathways described above becomes constrained and facilitated by contextually relevant top-down expectations (Damasio, 1989; Friston, 2005; Grabowski et al., 2001; Mesulam, 2008; Summerfield et al., 2006). The anterograde progression of feed-forward pathways and the top-down guidance emanating at least in part from downstream components of the synaptic chain explain why the anterior temporal lobe plays such a critical role in word comprehension and object naming (Hurley et al., 2012; Mesulam et al., 2013; Schwartz et al., 2009).
According to the commonality analyses, as much as 81% of the variance in the thickness of the PPVT/BNT area in our cohort was explained by performance on the language parameters that were assessed, a much higher percentage than what was seen in the phonolexical and morphosyntactic clusters. It appears therefore that the dependence of object naming and word comprehension on the integrity of temporal cortex is much higher than the dependence of %G, NAT-NAVS and REP on the integrity of frontal and temporoparietal cortices, helping to explain why anomia is such a sensitive marker of even minor left hemisphere damage.
Essential (critical) and Ancillary (participating) Functionalities of Large-Scale Networks
A map of language based on only three functional clusters appears spartan compared to more elaborate accounts that incorporate more numerous clusters and extensive granularity (Dapretto & Bookheimer, S. Y., 1999; Fedorenko & Blank, 2020; Hickok & Poeppel, 2007; Lau et al., 2008; Matchin & Hickok, 2020; Nelson et al., 2017). The difference may lie in the distinction between essential and ancillary functionalities. Lesion-deficit analyses are designed to reveal the essential functional role of the lesioned area. In contrast, maps based on functional imaging, event related potentials and electrocorticography reveal areas of both essential and ancillary functionality without being able to distinguish one from the other. The relationship of Wernicke’s area (temporoparietal junction) to word comprehension illustrates this distinction. As noted above, severe atrophy in Wernicke’s area can leave word comprehension intact. Furthermore, anterior temporal lobe atrophy impairs word comprehension even when Wernicke’s area is intact (Mesulam, Rader, et al., 2019a). These results indicate that Wernicke’s area is neither essential nor sufficient for word comprehension. Nonetheless, functional imaging during a word comprehension task showed activation not only in the anterior temporal lobe but also within Wernicke’s area (Gitelman, Nobre, Sonty, Parrish, & Mesulam, 2005). Wernicke’s area can therefore be said to have an ancillary (participatory) role in verbal semantics. Its destruction leaves basic word comprehension intact but nonetheless leads to subtle distortions of word recognition when the computational demands are high (Hurley et al., 2012; Hurley et al., 2009; Rogalski, Rademaker, Mesulam, & Weintraub, 2008; C. Thompson et al., 2012). Additional examples of ancillary functionality, detected by activation methods, include word comprehension and phoneme identification in Broca’s area (IFG) (Booth et al., 2002; Dapretto & Bookheimer, S. Y., 1999; Ojemann, 1983; Poldrack et al., 1999), and syntax comprehension in the anterior temporal lobe (Brennan et al., 2012; Friederici, 2011). Lesions confined to either one of these areas do not cause behaviorally consequential impairments of ancillary functionalities as tested by off-line tasks which have relatively low computational demands. In the course of naturalistic language functions, however, the heightened computational demands are likely to induce the additional recruitment of ancillary functionalities inherent in network components. At such activated states, the network becomes more granular and more tightly interdigitated through multiple parallel and serial connections that are known to exist within the language network (Bitan et al., 2005; Bonakdarpour et al., 2019; Catani, Jones, & ffytche, 2005; Catani & Mesulam, 2008; Catani & Thiebaut de Schotten, 2012; Forkel et al., 2020; Hagoort, 2013; Hurley, Bonakdarpour, Wang, & Mesulam, 2015; Moritz-Gasser, Herbet, & Duffau, 2013).
Conclusions
The process of verbalizing a thought activates a host of pre-lexical representations that vie for selection and positioning so that the utterance resonates with prevailing context and intent. Although the physiological foundations of these dynamic processes are poorly understood, great progress has been made in identifying the neuroanatomy of the underlying anchor points. In keeping with this line of development, the results of the current study are in agreement with the proposed subdivision of the language network into dorsal (frontoparietal) and ventral (parietotemporal) routes of processing (Hickok & Poeppel, 2007; Pylkkänen, 2021; Saur et al., 2008; Ueno, Saito, Rogers, & Lambon Ralph, 2011). The morphosyntactic and phonolexical clusters would fit predominantly into the dorsal route and the lexicosemantic cluster into the ventral route. At the risk of excessive generalization, the dorsal route could be said to mediate ‘concatenation’ (e.g., the rule-based sequencing of phonemes into words, words into sentences) whereas the ventral route could be said to mediate ‘amalgamation’ (e.g., the combinatorial linking of word-forms to their experiential correlates). The three functional clusters act in concert during the transformation of thoughts into words and words into thoughts, a faculty that is uniquely human (Fig. 4). No single method or model is likely to settle all the questions related to the computational architecture of the underlying neural processes (Patterson & Lambon Ralph, 1999; Pylkkänen, 2021; Seidenberg & McClelland, 1989; Ueno et al., 2011). For over a century, focal cerebrovascular lesions offered the major insights, many of which are still valid. Spectacular advances in functional imaging and event-related electrophysiological recordings led to the subsequent stage of progress. It now appears that focal neurodegenerative disorders, such as the one causing PPA, are poised to make additional contributions that complement those based on stroke and functional imaging. In specific, cortical neurodegenerations can obviate the fiber-of-passage problem of cerebrovascular lesions and are less vulnerable to the conflation of essential with ancillary functionality seen in activation methods. Research on PPA has already modified the classic models of language by revealing the critical importance of the anterior temporal lobe for word comprehension and by redefining the functional properties of Wernicke’s area. The next phase of progress associated with focal neurodegenerative diseases may entail greater integration of structural with functional, metabolic and molecular imaging as well as analytical methods that allow reliable single subject investigations so that individual variations of brain anatomy can be taken into account (Braga & Buckner, 2017; Fedorenko, Hsieh, Nieto-Castañón, Whitfield-Gabrieli, & Kanwisher, 2010; Ricci, Cimini, Chiaravalloti, Filippi, & Schillaci, 2020).
Figure 4-.
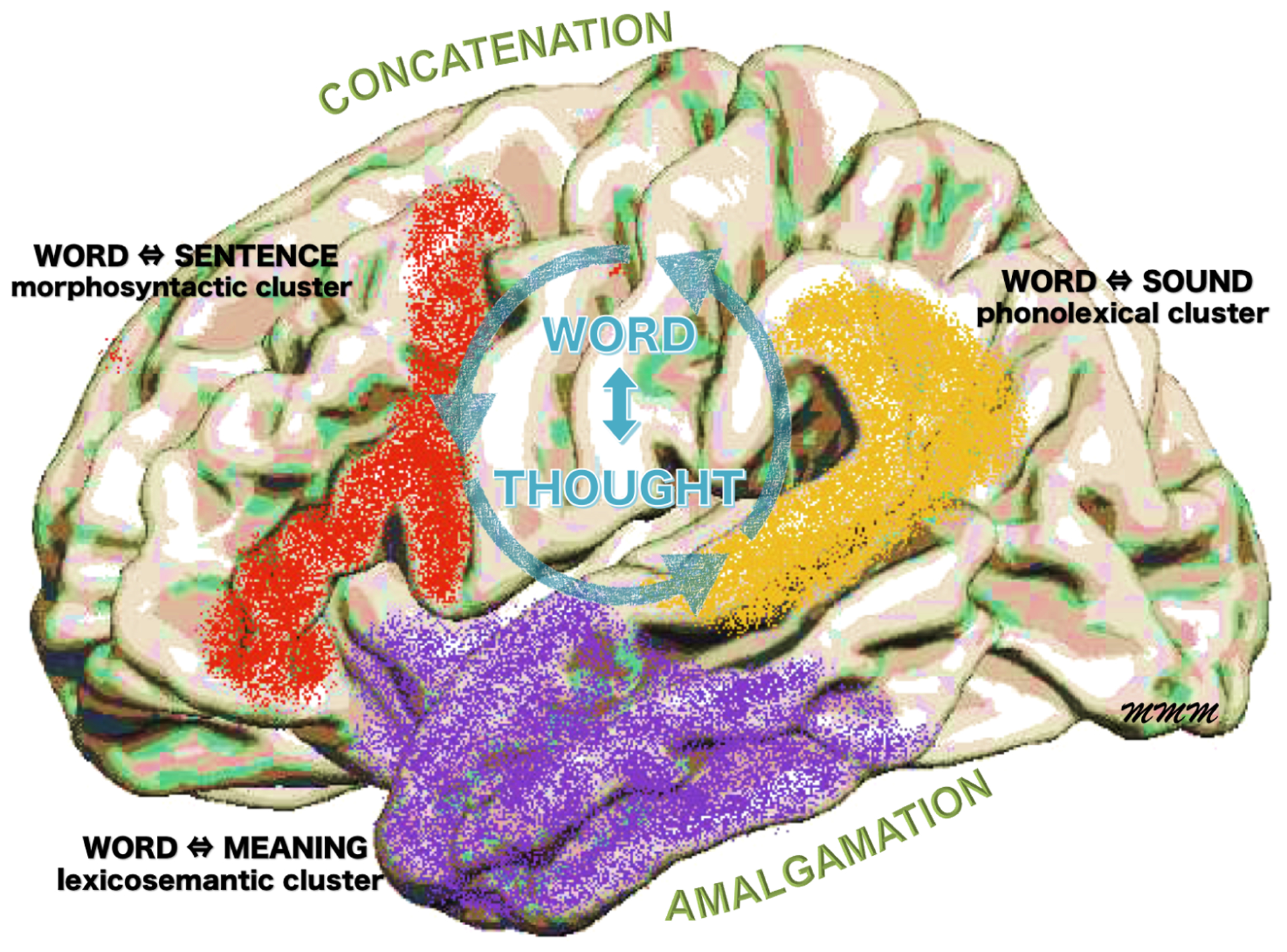
The network for spoken language from the perspective of PPA. The partitioning into clusters and the further organization into dorsal (concatenation) and ventral (amalgamation) routes reflect critical (sine qua non) functionalities. Naturalistic language, which entails exceedingly rapid interactions of word with thought, engages the collaborative participation of all clusters.
ACKNOWLEDGMENTS
Funding was provided by R01DC008552 from the National Institute on Deafness and Communication Disorders, R01NS075075 by the National Institute on Neurological Disorders and Stroke, R01AG056258 and P30AG013854 from the National Institute on Aging, the Davee Foundation, and the Jeanine Jones Fund.
This research was supported in part through the computational resources and staff contributions provided for the Quest high performance computing facility at Northwestern University which is jointly supported by the Office of the Provost, the Office for Research, and Northwestern University Information Technology.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DATA SHARING
All data used in this report will be available to the research community pending a data request and collaboration agreement that abide by the Northwestern University and Mesulam Center data use policies. The conditions of our ethics approval do not permit public archiving of anonymised study data. Legal copyright restrictions prevent public archiving of test materials, which can be obtained from the copyright holders in the cited references. No part of the study procedures or analyses was pre-registered prior to the research being conducted. We report how we determined our sample size, all data exclusions (if any), all inclusion/exclusion criteria, whether inclusion/exclusion criteria were established prior to data analysis, all manipulations, and all measures in the study.
Conflict of Interest: None by any of the authors.
REFERENCES
- Binder J (2017). Current controversies on Wernicke’s area and its role in language. Current Neurology and Neuroscience Reports, 17, 1–10. [DOI] [PubMed] [Google Scholar]
- Binder JR (2015). The Wernicke area. Neurology, 85, 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitan T, Booth JR, Choy J, Burman DD, Gitelman DR, & Mesulam M-M (2005). Shifts of effective connectivity within a language network during rhyming and spelling. Journal of Neuroscience, 25, 5397–5403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonakdarpour B, Hurley RS, Wang A, Fereira HR, Basu A, A., C., … Mesulam M-M (2019). Perturbations of language network connectivity in primary progressive aphasia. Cortex, 121, 468–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonakdarpour B, Rogalski E, Wang A, Sridhar J, Mesulam M-M, & Hurley RS (2017). Functional connectivity is reduced in early-stage primary progressive aphasia when atrophy is not prominent. Alzheimer’s Disease and Associated Disorders, 31, 101–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth JR, Burman DD, Meyer JR, GItelman D, Parrish T, & Mesulam M-M (2002). Modality independence of word comprehension. Human Brain Mapping, 16, 251–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braga RM, & Buckner RL (2017). Parallel interdigitated distributed networks within the individual estimated by intrinsic functional connectivity. Neuron, 95, 457–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan J, Nir Y, Hasson U, Malach R, Heeger DJ, & Pylkkänen L (2012). Syntactic structure building in the anterior temporal lobe during natural story listening. Brain & Language, 120, 163–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broca P (1865). Sur le siège de la faculté du language articulé. Bulletins de la Société d’anthropologie de Paris, 6, 377–379. [Google Scholar]
- Canu E, Agosta F, Imperiale F, Ferraro PM, Fontana A, Magnani G, … Filippi M (2019). Northwestern anagram test-Italian (NAT-I) for primary progressive aphasia. Cortex, 119, 497–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catani M, Jones DK, & ffytche DH (2005). Perisylvian language networks of the human brain. Annals of Neurology, 57, 8–16. [DOI] [PubMed] [Google Scholar]
- Catani M, & Mesulam M (2008). The arcuate fasciculus and the disconnection theme in language and aphasia: history and current state. Cortex, 44, 953–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catani M, Mesulam M-M, Jacobsen E, Malik F, Martersteck A, Wieneke C, … Rogalski E (2013). A novel frontal pathway underlies verbal fluency in primary progressive aphasia. Brain, 136, 2619–2628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catani M, & Thiebaut de Schotten M (2012). Atlas of Human Brain Connections. Oxford, UK: Oxford University Press. [Google Scholar]
- Cloutman LL, Binney RJ, Drakesmith M, Parker GJM, & Lambon Ralph MA (2012). The variations of function across the human insula mirrors its patterns of structural connectivity: Evidence from in vivo probabilistic tractography. NeuroImage, 59, 3514–3521. [DOI] [PubMed] [Google Scholar]
- Creutzfeldt O, Ojemann G, & Lettich E (1989). Neuronal activity in the human lateral temporal lobe. I. Responses to speech. Brain Research, 77, 451–475. [DOI] [PubMed] [Google Scholar]
- Damasio AR (1989). The brain binds entities and events by multiregional activation from convergence zones. Neural Computation, 1, 123–132. [Google Scholar]
- Dapretto M, & Bookheimer SY (1999). Form and content: dissociating syntax and semantics in sentence comprehension. Neuron, 24, 427–432. [DOI] [PubMed] [Google Scholar]
- Dax M (1836). Lésions de la moitié gauche de l’encéphale coïncident avec l’oublie des signes de la pensée. Paper presented at the Congres Meridional, Montpellier. [Google Scholar]
- Démonet J-F, Chollet F, Ramsay S, Cardebat D, Nespoulous J-L, Wise R, … Frackowiak R (1992). The anatomy of phonological and semantic processing in normal subjects. Brain, 115. [DOI] [PubMed] [Google Scholar]
- DeWitt I, & Rauschecker JP (2013). Wernicke’s area revisited: Parallel streams of word processing. Brain & Language, 127, 181–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeWitt I, & Rauschecker JP (2016). Convergent evidence for the causal involvement of anterior superior temporal gyrus in auditory single-word comprehension. Cortex, 77, 164–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dronkers NF, Wilkins DP, van Valin RD Jr., Redfern BB, & Jaeger JJ (2004). Lesion analysis of the brain areas involved in language comprehension. Cognition, 92, 145–177. [DOI] [PubMed] [Google Scholar]
- Dunn LA, & Dunn LM (2006). Peabody Picture Vocabulary Test-4: Pearson.
- Fedorenko E, & Blank IA (2020). Broca’s area is not a natural kind. Trends in Cognitive Sciences, 24, 270–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedorenko E, Hsieh P-J, Nieto-Castañón A, Whitfield-Gabrieli S, & Kanwisher N (2010). New method for fMRI investigations of language: Defining ROIs functionally in individual subjects. Journal of Neurophysiology, 104, 1177–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedorenko E, & Varley R (2016). Language and thought are not the same thing: evidence from neuroimaging and neurological patients. Annals of the New York Academy of Science, 1369, 132–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, & Dale A (2000). Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proceedings of the National Academy of Science (USA), 97, 11050–11055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Sereno MI, Tootell RB, & Dale AM (1999). High-resolution intersubject averaging and a coordinate system for the cortical surface. Human Brain Mapping, 8, 272–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forkel SJ, Rogalski E, Sancho ND, D’Anna L, L. LP, Sridhar J, … Catani M (2020). Anatomical evidence of an indirect pathway for word repetition. Neurology, 94, e594–e606. doi: doi: 10.1212/WNL.0000000000008746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fridriksson J, Kjartansson O, Morgan PS, Hjaltason H, Magnusdottir S, Bonilha L, & Rorden C (2010). Impaired speech repetition and left parietal lobe damage. The Journal of Neuroscience, 30, 11057–11061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friederici AD (2011). The brain basis of language processing: from structure to function. Physiological Reviews, 91, 1357–1392. [DOI] [PubMed] [Google Scholar]
- Friston K (2005). A theory of cortical responses. Philosophical Transactions of the Royal Society B, 360, 815–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genovese CR, Lazar NA, & Nichols TE (2002). Thresholding of statistical maps in functional imaging using the false discovery rate. NeuroImage, 15, 870–878. [DOI] [PubMed] [Google Scholar]
- Geschwind N (1972). Language and the Brain. Scientific American, 226, 76–83. [DOI] [PubMed] [Google Scholar]
- Giannini LAA, Xie SX, McMillan CT, Liang M, Williams A, Jester C, … Irwin DJ (2019). Divergent patterns of TDP-43 and tau pathologies in primary progressive aphasia. Annals of Neurology, 85, 630–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gitelman DR, Nobre AC, Sonty S, Parrish TB, & Mesulam M-M (2005). Language network specializations: An analysis with parallel task design and functional magnetic resonance imaging. NeuroImage, 26, 975–985. [DOI] [PubMed] [Google Scholar]
- Gorno-Tempini ML, Dronkers NF, Rankin KP, Ogar JM, Phengrasamy L, Rosen HJ, … Miller BL (2004). Cognition and anatomy in three variants of primary progressive aphasia. Annals of Neurology, 55, 335–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorno-Tempini ML, Hillis A, Weintraub S, Kertesz A, Mendez MF, Cappa SF, … Grossman M (2011). Classification of primary progressive aphasia and its variants. Neurology, 76, 1006–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabowski TJ, Damasio H, Tranel D, Boles Ponto LL, Hichwa RD, & Damasio AR (2001). A role for left temporal pole in the retrieval of words for unique entities. Human Brain Mapping, 13, 199–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths J, Marslen-Wilson WD, Stamatakis EA, & Tyler LK (2013). Functional organization of the neural language system: Dorsal and ventral pathways are critical for syntax. Cerebral Cortex, 23, 139–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagoort P (2013). MUC (Memory, Unification, Control) and beyond. Frontiers in Psychology, 4, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagoort P (2014). Nodes and networks in the neural architecture for language: Broca’s region and beyond. Current Opinion in Neurobiology, 28, 136–141. [DOI] [PubMed] [Google Scholar]
- Hartwigsen G, Golombek T, & Obleser J (2015). Repetitive transcranial magnetic stimulation over left angular gyrus modulates the predictability gain in degraded speech comprehension. Cortex, 68, 100–110. [DOI] [PubMed] [Google Scholar]
- Heim S, Opitz B, & Friederici AD (2003). Distributed cortical networks for syntax processing: Broca’s area as the common denominator. Brain and Language, 85, 402–408. [DOI] [PubMed] [Google Scholar]
- Herman AB, Houde JF, Vinogradov S, & Nagarajan SS (2013). Parsing the phonological loop: Activation timing in the dorsal speech stream determines accuracy in speech reproduction. The Journal of Neuroscience, 33, 5439–5453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickok G, & Poeppel D (2007). The cortical organization of speech processing. Nature Reviews Neuroscience, 8, 293–402. [DOI] [PubMed] [Google Scholar]
- Hillis AE, Rorden C, & Fridriksson J (2017). Brain regions essential for word comprehension: drawing inferences from patients. Annals of Neurology, 81, 759–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu C-J, & Thompson CK (2018). Manual versus automated narrative analysis of agrammatic production patterns: The Northwestern Narrative Language Analysis and Computerized Language Analysis. Journal of Speech, Language and Hearing Research, 61, 373–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurley RS, Bonakdarpour B, Wang X, & Mesulam M-M (2015). Asymmetric connectivity between the anterior temporal lobe and the language network. Journal of Cognitive Neuroscience, 27, 464–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurley RS, Paller K, Rogalski E, & Mesulam M-M (2012). Neural mechanisms of object naming and word comprehension in primary progressive aphasia. Journal of Neuroscience, 32, 4848–4855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurley RS, Paller K, Wieneke C, Weintraub S, Thompson C, Federmeier K, & M.-M. M (2009). Electrophysiology of object naming in primary progressive aphasia. Journal of Neuroscience, 16, 15762–15769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingram RU, Halai AD, Pobric G, Sajjadi SA, Patterson DK, & Lambon Ralph MA (2020). Graded multidimensional intra- and intergroup variations in primary progressive aphasia and post-stroke aphasia. Brain, 143, 3121–3135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan E, Goodglass H, & Weintraub S (1983). The Boston Naming Test. Philadelphia: Lea & Febiger. [Google Scholar]
- Keller TA, Carpenter PA, & Just MA (2001). The neural bases of sentence comprehension: a fMRI examination of syntactic and lexical processing. Cerebral Cortex, 11, 223–237. [DOI] [PubMed] [Google Scholar]
- Kertesz A (2006). Western Aphasia Battery- Revised (WAB-R). Austin, Texas: Pearson. [Google Scholar]
- Kielar A, Milman L, Bonakdarpour B, & Thompson CK (2011). Neural correlates of covert and overt production of tense and agreement morphology: Evidence from fMRI. Journal of Neurolinguistics, 24, 183–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koechlin E, & Jubault T (2006). Broca’s area and the hierarchical organization of human behavior. Neuron, 50, 963–974. [DOI] [PubMed] [Google Scholar]
- Kümmerer D, Hartwigsen G, Kellmeyer P, Glauche V, Mader I, Klöppel S, … Saur D (2013). Damage to ventral and dorsal language pathways in acute aphasia. Brain, 136, 619–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau EF, Phillips C, & Poeppel D (2008). A cortical network for semantics: (de)constructing the N400. Nature Reviews Neuroscience, 9, 920–933. [DOI] [PubMed] [Google Scholar]
- Leminen A, Smolka E, Duñabetia JA, & Pliatsikas C (2019). Morphological processing in the brain: The good (inflection), the bad (derivation) and the ugly (compounding). Cortex, 116, 4–44. [DOI] [PubMed] [Google Scholar]
- Lichtheim L (1885). Über Aphasie. Deutsches Archiv für Klinische Medicin, 36, 204–268. [Google Scholar]
- Long MA, Katlowitz KA, Svirsky MA, Clary RC, Byun TM, Majaj N, … Greenlee JDW (2016). Functional segregation of cortical regions underlying speech timing and articulation. Neuron, 89, 1187–1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lüders H, Lesser RP, Hahn J, Dinner DS, Morris HH, Wyllie E, & Godoy J (1991). Basal temporal language area. Brain, 114, 743–754. [DOI] [PubMed] [Google Scholar]
- Matchin W, & Hickok G (2020). The cortical organization of syntax. Cerebral Cortex, 30, 1481–1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesulam M-M (1998). From sensation to cognition. Brain, 121, 1013–1052. [DOI] [PubMed] [Google Scholar]
- Mesulam M-M (2000). Behavioral neuroanatomy: large-scale networks, association cortex, frontal syndromes, the limbic system and hemispheric specialization. In Mesulam M-M (Ed.), Principles of Behavioral and Cognitive Neurology (pp. 1–120). New York: Oxford University Press. [Google Scholar]
- Mesulam M-M, Coventry C, Bigio E, Geula C, Thompson C, Bonakdarpour B, … Weintraub S (2020). Nosology of primary progressive aphasia and the neuropathology of language. In Ghetti B, Boeve B, Buratti E & Rademakers R (Eds.), Frontotemporal Dementias-Emerging Milestones of the 21st century: Springer Nature. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesulam M-M, & Mufson EJ (1985). The insula of Reil in man and monkey. In Peters A & Jones EG (Eds.), Cerebral Cortex (Vol. 4, pp. 179–226). New York: Plenum Press. [Google Scholar]
- Mesulam M-M, Nelson MJ, Hyun J, Rader B, Hurley RS, Rademakers R, … Weintraub S (2019). Preferential disruption of auditory word representations in primary progressive aphasia with the neuropathology of FTLD-TDP type A. Cognitive and Behavioral Neurology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesulam M-M, Rader B, Sridhar J, Nelson MJ, Hyun J, Rademaker A, … Rogalski E (2019a). Word comprehension in temporal cortex and Wernicke area: A PPA perspective. Neurology, 92. doi: doi: 10.1212/WNL.0000000000006788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesulam M-M, Rader B, Sridhar J, Nelson MJ, Hyun J, Rademaker A, … Rogalski EJ (2019b). Word comprehension in temporal cortex and Wernicke area: A PPA perspective. Neurology, 92. doi: 10.1212/WNL.0000000000006788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesulam M-M, Rogalski E, Wieneke C, Cobia D, Rademaker A, Thompson C, & Weintraub S (2009). Neurology of anomia in the semantic subtype of primary progressive aphasia. Brain, 2553–2565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesulam M-M, Thompson CK, Weintraub S, & Rogalski EJ (2015). The Wernicke conundrum and the anatomy of language comprehension in primary progressive aphasia. Brain, 138, 2423–2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesulam M-M, Wieneke C, Hurley RS, Rademaker A, Thompson CK, Weintraub S, & Rogalski E (2013). Words and objects at the tip of the left temporal lobe in primary progressive aphasia. Brain, 136, 601–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesulam M-M, Wieneke C, Thompson C, Rogalski E, & Weintraub S (2012). Quantitative classification of primary progressive aphasia at early and mild impairment stages. Brain, 135, 1537–1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesulam MM (2008). Representation, inference and transcendent encoding in neurocognitive networks of the human brain. Annals of Neurology, 64, 367–378. [DOI] [PubMed] [Google Scholar]
- Miller JW, & Chapman R (2000). Systematic analysis of language transcripts (SALT) (Version Research Version 6.1]) Madison, WI: University of Wisconsin, Language Analysis Lab. [Google Scholar]
- Moritz-Gasser S, Herbet G, & Duffau H (2013). Mapping the connectivity underlying multimodal (verbal and non-verbal) semantic processing: A brain electrostimulation study. Neuropsychologia, 51, 1814–1822. [DOI] [PubMed] [Google Scholar]
- Morris JC (1993). The clinical dementia rating (CDR): Current version and scoring rules. Neurology, 43, 2412–2414. [DOI] [PubMed] [Google Scholar]
- Moss HE, Rodd JM, Stamatakis EA, Bright P, & Tyler LK (2005). Anteromedial temporal cortex supports fine-grained differentiation among objects. Cerebral Cortex, 15, 616–627. [DOI] [PubMed] [Google Scholar]
- Müller R-A (2004). Are nonlinguistic functions in “Broca’s area” prerequisites for language acquisition? FMRI findings from an ontogenetic viewpoint. Brain and Language, 89, 329–336. [DOI] [PubMed] [Google Scholar]
- Nelson MJ, I. EK, Giber K, Yang X, Cohen L, Koopman H, … Dehaene S (2017). Neuropsysiological dynamics of phrase-structure building during sentence processing Proceedings of the National Academy of Science (USA). doi: doi/ 10.1073/pnas.1701590114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nobre AC, Allison T, & McCarthy G (1994). Word recognition in the human inferior temporal lobe. Nature, 372, 260–263. [DOI] [PubMed] [Google Scholar]
- Ojemann GA (1983). Brain organization for language from the perspective of electrical stimulation. Behavioral and Brain Science, 6, 189–230. [Google Scholar]
- Ozdemir B (2015). Partitioning variance into constituents in multiple regression models: Commonality analysis. In Millsap RE, Bolt DM, van der Ark LA & Wang W-C (Eds.), Quantitative Psychology Reserach (Vol. 89, pp. 395–405). New York: Springer. [Google Scholar]
- Patterson DK, & Lambon Ralph MA (1999). Selective disorders of reading? Current Opinion in Neurobiology, 9, 235–239. [DOI] [PubMed] [Google Scholar]
- Petersen SE, Fox PT, Posner MI, Mintun M, & Raichle ME (1988). Positron emission tomographic studies of the cortical anatomy of single word processing. Nature, 331, 585–589. [DOI] [PubMed] [Google Scholar]
- Poldrack RA, Wagner AD, Prull MW, Desmond JE, Glover GH, & Gabrieli JDE (1999). Functional specialization for semantic and phonological processing in the left inferior prefrontal cortex. NeuroImage, 10, 15–35. [DOI] [PubMed] [Google Scholar]
- Pylkkänen L (2021). The neural basis of combinatory syntax and semantics. Science, 366, 62–66. [DOI] [PubMed] [Google Scholar]
- Ravizza SM, Hazeltine E, Ruiz S, & Zhu DC (2011). Left TPJ activity in verbal working memory: Implications for storage- and sensory-specific models of short term memory. NeuroImage, 55, 1836–1846. [DOI] [PubMed] [Google Scholar]
- Ricci M, Cimini A, Chiaravalloti A, Filippi L, & Schillaci O (2020). Positron emission tomography (PET) and neuroimaging in the personalized appeoach to neurodegenerative causes of dementia. International Journal of Molecular Sciences, 21. doi: doi: 10.3390/ijms21207481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogalski E, Cobia D, Harrison TM, Wieneke C, Thompson C, Weintraub S, & Mesulam M-M (2011). Anatomy of language impairments in primary progressive aphasia. Journal of Neuroscience, 31, 3344–3350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogalski E, Cobia D, Martersteck AC, Rademaker A, Wieneke CA, Weintraub S, & Mesulam M-M (2014). Asymmetry of cortical decline in subtypes of Peimary Progressive Aphasia. Neurology, 83, 1184–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogalski E, Rademaker A, Mesulam M, & Weintraub S (2008). Covert processing of words and pictures in nonsemantic variants of primary progressive aphasia. Alzheimer’s Disease and Associated Disorders, 21, 343–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahin NT, Pinker S, Cash SS, Schomer DL, & Halgren E (2009). Sequential processing of lexical, grammatical, and phonological information within Broca’s area. Science, 326, 445–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saur D, Kreher BW, Schnell S, Kümmerer D, Kellmeyer P, Vry M-S, … Weiller C (2008). Ventral and dorsal pathways for language. Proceedings of the National Academy of Science (USA), 105, 18035–18040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz MF, Kimberg DY, Walker GM, Faseyitan O, Brecher A, Dell GS, & Coslett HB (2009). Anterior temporal involvement in semantic word retrieval: voxel-based lesion-symptom mapping evidence from aphasia. Brain, 132, 3411–3427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeck M, Schomer D, Mainwaring N, Ives J, Dubuissson D, Blume H, … Mesulam M-M (1995). Selectively distributed processing of visual object recognition in the temporal and frontal lobes of the human brain. Annals of Neurology, 37, 538–545. [DOI] [PubMed] [Google Scholar]
- Segonne F, Pacheco J, & Fischl B (2007). Geometrically accurate topology-correction of cortical surfaces using nonseparating loops. IEEE Transactions of Medical Imaging, 26, 518–529. [DOI] [PubMed] [Google Scholar]
- Seidenberg MS, & McClelland JL (1989). A distributed, developmental model of word recognition and naming. Psychological Review, 96, 523–568. [DOI] [PubMed] [Google Scholar]
- Selnes OA, Knopman D, Niccum N, & Rubens AB (1985). The critical role of Wernicke’s area in sentence repetition. Annals of Neurology, 17, 549–557. [DOI] [PubMed] [Google Scholar]
- Sepulcre J, Sabuncu MR, Yeo TB, Hesheng L, & Johnson KA (2013). Spetwise connectivity of the modal cortex reveals the multimodal organization of the human brain. Journal of Neuroscience, 32, 10649–10661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegelman M, Blank IA, Mineroff Z, & Fedorenko E (2019). An attempt to conceptually replicate the dissociation between syntax and semantics dueing sentence comprehension. Neuroscience, 413, 219–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summerfield C, Egner T, Greene M, Koechlin E, Mangels J, & Hirsch J (2006). Predictive codes for forthcoming perception in the frontal cortex. Science, 314, 1311–1314. [DOI] [PubMed] [Google Scholar]
- Thompson C, Cho S, Rogalski E, Wieneke C, Weintraub S, & Mesulam M-M (2012). Semantic interference during object naming in agrammatic and logopenic primary progressive aphasia (PPA). Brain and Language, 120, 237–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson CK (2011). Northwestern Assessment of Verbs and Sentences (NAVS). Aphasia and Neurolinguistics Laboratory, Northwestern University School of Communication. Evanston, IL. [Google Scholar]
- Thompson CK, Ballard KJ, Tait ME, Weintraub S, & Mesulam M (1997). Patterns of language decline in non-fluent primary progressive aphasia. Aphasiology, 11, 297–321. [Google Scholar]
- Thompson CK, Cho S, Hsu C-J, Wieneke C, Rademaker A, Weitner BB, … Weintraub S (2012). Dissociations between fluency and agrammatism in primary progressive aphasia. Aphasiology, 26, 20–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson CK, Shapiro LP, Tait ME, Jacobs B, Schneider SL, & Ballard K (1995). A system for the linguistic analysis of agrammatic language production. Brain and Language, 51, 124–129. [Google Scholar]
- Tovèe MJ (1994). How fast is the speed of thought? Current Biology, 4, 1125–1127. [DOI] [PubMed] [Google Scholar]
- Towle VL, Yoon H-L, Castelle M, Edgar JC, Biassou NM, Frim DM, … Kohrman MH (2008). ECoG gamma activity during a language task: differentiating expressive and receptice speech areas. Brain, 131, 2013–2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueno T, Saito S, Rogers TT, & Lambon Ralph MA (2011). Lichtheim 2: Synthesizing aphasia and the neural basis of language in a neurocomputational model of the dual doesal ventral language pathways. Neuron, 72, 385–396. [DOI] [PubMed] [Google Scholar]
- Vygotsky LS (1962). Thought and Language (Hanfmann E & Vakar G, Trans.). Cambridge, Massachusetts: MIT Press. [Google Scholar]
- Weintraub S, Mesulam M-M, Wieneke C, Rademaker A, Rogalski EJ, & Thompson CK (2010). The Northwestern Anagram Test: measuring sentence production in primary progressive aphasia. American Journal of Alzheimer’s Disease and Other Dementias, 24, 408–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wernicke C (1874). Der Aphasische Symptomencomplex (Eggert GH, Trans.). Breslau: Cohn and Weigert. [Google Scholar]
- Wilson SM, Dronkers NF, Ogar J, Jung J, Growdon ME, Agosta F, … Gorno-Tempini ML (2010). Neural correlates of syntactic processing in the nonfluent variant of primary progressive aphasia. Journal of Neuroscience, 30, 16845–16854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson SM, Henry ML, Besbris M, Ogar JM, Dronkers NF, Jarrold W, … Gorno Tempini ML (2010). Connected speech production in three variants of primary progressive aphasia. Brain, 133, 2069–2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise R, Chollet F, Hadar U, Friston K, Hoffner E, & Frackowiak RSJ (1991). Distribution of cortical neural networks involved in word comprehension and word retrieval. Brain, 114, 1803–1817. [DOI] [PubMed] [Google Scholar]
- Wise RJ, Scott SK, Blank C, Mummery CJ, Murphy K, & Warbuton EA (2001). Separate neural subsystems within “Wernicke’s area”. Brain, 124, 83–95. [DOI] [PubMed] [Google Scholar]
- Xu K, Wu DH, & D’uann JR A. S (2020). Enhanced left inferior frontal to left superior temporal effective connectivity for complex sentence comprehension: fMRI evisdence from Chinese relative clause processing. Brain & Language, 200, 1–10. doi: 10.1016/j.bandl.2019.104712 [DOI] [PubMed] [Google Scholar]
- Zatorre RJ, Evans AC, & Meyer E (1994). Neural mechanisms underlying melodic perception and memory for pitch. Journal of Neuroscience, 14, 1908–1919. [DOI] [PMC free article] [PubMed] [Google Scholar]


