Abstract
BACKGROUND:
The North Carolina Breast and Cervical Cancer Control Program (NC BCCCP) provides breast cancer screening services to underserved women to mitigate disparities in access to care. The authors sought to characterize this understudied population.
METHODS:
Women 21 years old or older who underwent their first breast cancer screen through NC BCCCP from 2008 to 2018 were included. Demographic factors associated with the timeline of care and odds of a breast cancer diagnosis were identified with negative binomial and logistic regression, respectively.
RESULTS:
Of the 88,893 women identified, 45.5% were non-Hispanic (NH) White, 30.9% were NH Black, 19.6% were Hispanic, 1.7% were American Indian, and 1.1% were Asian. Breast cancer was diagnosed in 2.5% of the women (n = 2255). Hispanic women were the least likely to be diagnosed with breast cancer (odds ratio vs NH White women, 0.40; 95% confidence interval [CI], 0.34–0.47). Among patients with breast pathology, the median time to diagnosis was 19 days (interquartile range [IQR], 10–33 days), and the time to treatment was 33 days (IQR, 19–54 days). After adjustments, a longer time to diagnosis was significantly associated with age (incidence rate ratio [IRR], 1.01; 95% CI, 1.01–1.02) and being NH Black (vs NH White; IRR, 1.17; 95% CI, 1.06–1.29). A longer time to treatment was significantly associated with age (IRR, 1.01; 95% CI, 1.01–1.01), being NH Black (vs NH White; IRR, 1.20; 95% CI, 1.10–1.31), and being Hispanic (vs NH White; IRR, 1.22; 95% CI, 1.05–1.41).
CONCLUSIONS:
NC BCCCP participants with breast cancer received treatment within approximately 1 month of presentation, and this finding aligns with quality care benchmarks. Nevertheless, racial/ethnic disparities in timeliness of care persist, and this suggests opportunities for improvement.
Keywords: breast neoplasms, cancer screening, health care disparities, insurance
INTRODUCTION
Breast cancer is the cancer most commonly diagnosed among women in the United States, with an estimated 279,100 new cases of invasive breast cancer and 42,690 breast cancer–related deaths anticipated in 2020 alone.1 Breast cancer mortality has decreased over the past several decades, in large part because of advances in screening and systemic therapy.2–4 However, racial and ethnic disparities in breast cancer mortality have widened.5,6 Black women, in particular, have been shown to have worse disease-specific survival and a higher incidence of late-stage disease than their White counterparts.7–9 Access to insurance is a significant contributor to these disparities.10
The North Carolina Breast and Cervical Cancer Control Program (NC BCCCP) is the North Carolina state division of the National Breast and Cervical Cancer Early Detection Program. NC BCCCP provides free and low-cost breast cancer screening services to underserved women for whom no other source of health care reimbursement is available. Specifically, NC BCCCP offers breast cancer screening services to women aged 40 to 75 years (or younger than 40 years if they are symptomatic or at high risk) who are uninsured or underinsured, do not have Medicare Part B or Medicaid, and have a household income level ≤ 250% of the federal poverty line.11 Services offered include clinical breast examinations, screening and diagnostic imaging, biopsies, and treatment referrals. Importantly, NC BCCCP provides a pathway toward eligibility for Breast and Cervical Cancer Medicaid should participants be diagnosed with cancer as part of their screening.11
The patients served by NC BCCCP represent an understudied patient population. Given the vital role that NC BCCCP plays in filling gaps in care for underserved women, we sought both to characterize the patients who underwent breast cancer screening through NC BCCCP and to evaluate concordance with timeliness benchmarks set forth by the Centers for Disease Control and Prevention (CDC).12 Finally, we aimed to identify factors associated with the diagnosis and timely workup of breast cancer to inform future efforts aimed at addressing persistent barriers to care.
MATERIALS AND METHODS
Study Population
Female patients 21 years old or older who underwent their first breast cancer screening through NC BCCCP from 2008 to 2018 were identified. Two distinct analytic cohorts were created: 1) an all-comers cohort that included all patients who underwent screening and 2) a subset of the all-comers that included only patients diagnosed with breast pathology (ie, atypia or malignancy) during their first screening cycle. Atypia included atypical ductal hyperplasia, atypical lobular hyperplasia, and lobular carcinoma in situ, whereas malignancy included both ductal carcinoma in situ (DCIS) and invasive carcinoma. For both cohorts, records were excluded if they were missing the diagnosis date or the imaging funding source or if the time from enrollment to diagnosis (TTD) was >3 years. For the breast pathology cohort, records missing the treatment start date were also excluded. A data use agreement was executed between our institution and the North Carolina Department of Health and Human Services. This study received approval from our institutional review board.
Statistical Analysis
Patient demographic and clinical data, including age, race/ethnicity, personal and family history of breast cancer, imaging modality, year of screening, funding source, Breast Imaging Reporting and Data System imaging classifications, final pathologic diagnosis, and timeline of workup, were collected. Race and ethnicity were combined into 1 variable as follows: Asian/Pacific Islander (PI), American Indian (AI), non-Hispanic (NH) Black, NH White, and Hispanic. In terms of funding, all eligible patients who were enrolled in BCCCP by a BCCCP-affiliated provider received funding support as part of their participation in the program; however, the source of funding for each individual patient differed, with some patients fully funded by BCCCP (state and/or federal funds) and some funded by alternative sources, including local county, nonprofit, and charity funds. Determination of the funding source was a complex process based on both patient-level factors (eg, age and total cost of services) and provider-level factors (eg, annual allocated screening targets and provider-dependent availability of alternative funds). Patients whose services were covered in full by BCCCP funds were coded as fully funded. Patients whose services were covered in full by alternative funding sources were coded as not funded. Patients whose services were covered by both BCCCP and additional outside funds were coded as partially funded. The timeline of workup represented the time from enrollment (the date on which the patient was enrolled in NC BCCCP by an affiliated provider for the specific screening cycle of interest) to several key dates of interest, including first imaging (the date of mammography and/or ultrasound), biopsy (the date of biopsy), diagnosis (the date-tissue diagnosis was reported), and treatment (the date on which treatment was initiated). The primary outcome measures were 1) the rate of breast cancer diagnoses (DCIS or invasive carcinoma), 2) the TTD, 3) the time from enrollment to treatment (TTT), 4) the time from first imaging to treatment (ITT), and 5) the time from diagnosis to treatment (DTT).
Descriptive statistics were used to summarize the study cohort. Continuous and categorical variables were described as medians and interquartile ranges (IQR) and as numbers and percentages, respectively. Trends in program participation over time among racial/ethnic groups were tested with the Cochran-Armitage trend test. For the all-comers cohort, differences in the timeline of care and the rate of breast cancer diagnosis were compared by level of BCCCP funding support (non-funded vs partially funded vs fully funded), age (<50 vs ≥50 years), and race/ethnicity (NH White vs NH Black vs Hispanic vs AI vs Asian/PI) with the Wilcoxon rank sum test and the χ2 test, respectively. In the breast pathology subgroup, differences in the timeline of care were compared by funding source, age, race/ethnicity, and pathologic diagnosis (atypia vs carcinoma) with the Wilcoxon rank sum test. Logistic regression was used to identify factors independently associated with a breast cancer diagnosis among all-comers; we report odds ratios (ORs) and 95% confidence intervals (CIs). Negative binomial regression was used to identify factors associated with TTD, TTT, ITT, and DTT in the breast pathology cohort; we report incidence rate ratios (IRRs) and 95% CIs. For all analyses, 2-tailed tests were used, and the threshold for significance was set at level α = .05. No adjustments were made for multiple comparisons. All statistical analyses were performed with SAS software (version 9.4; SAS Institute, Cary, North Carolina).
RESULTS
Demographic Characteristics
A total of 88,893 patients were included in the all-comers cohort (Supporting Fig. 1A), and 1653 patients were included in the breast pathology cohort (Supporting Fig. 1B). Clinical and demographic characteristics of both cohorts are presented in Table 1. Within the all-comers cohort, 1.1% of patients (n = 961) were Asian/PI, 19.6% (n = 17,382) were Hispanic, 1.7% (n = 1492) were AI, 30.9% (n = 27,476) were NH Black, 45.5% (n = 40,411) were NH White, and 1.3% (n = 1171) were of unknown race. The median age was 50 years (IQR, 44–56 years). In the breast pathology cohort, 1.2% of the patients (n = 20) were Asian/PI, 9.3% (n = 153) were Hispanic, 1.2% (n = 20) were AI, 35.3% (n = 584) were NH Black, 52.3% (n = 864) were NH White, and 0.7% (n = 12) were of unknown race. The median age was 52 years (IQR, 45–57 years). Of the 1653 patients in the breast pathology cohort, 1% (n = 16) were diagnosed with atypical ductal hyperplasia or atypical lobular hyperplasia, 4.4% (n = 72) were diagnosed with lobular carcinoma in situ, 23.4% (n = 386) were diagnosed with DCIS, and 71.3% (n = 1179) were diagnosed with invasive carcinoma.
TABLE 1.
North Carolina Breast and Cervical Cancer Control Program, 2008–2018
| All Patients (n = 88,893) | Breast Pathology Subgroup (n = 1653) | |
|---|---|---|
| Age, No. (%) | ||
| <50 y | 39,509 (44.4) | 701 (42.4) |
| ≥50 y | 49,384 (55.5) | 952 (57.6) |
| Age, median (IQR), y | 50 (44–56) | 52 (45–57) |
| Race/ethnicity, No. (%) | ||
| Asian/Pacific Islander | 961 (1.1) | 20 (1.2) |
| Hispanic | 17,382 (19.6) | 153 (9.3) |
| American Indian | 1492 (1.7) | 20 (1.2) |
| Non-Hispanic Black | 27,476 (30.9) | 584 (35.3) |
| Non-Hispanic White | 40,411 (45.5) | 864 (52.3) |
| Unknown | 1171 (1.3) | 12 (0.7) |
| Breast cancer history, No. (%) | ||
| No personal/family history | 72,798 (81.9) | 1264 (76.5) |
| Family history | 9695 (10.9) | 276 (16.7) |
| Personal history | 1793 (2) | 62 (3.8) |
| Personal and family history | 267 (0.3) | 11 (0.7) |
| Refused/unable to answer | 4316 (4.9) | 40 (2.4) |
| Funding source, No. (%) | ||
| Full BCCCP funds | 71,795 (80.8) | 972 (58.8) |
| Partial BCCCP funds | 8852 (10.0) | 470 (28.4) |
| No BCCCP funds | 8246 (9.3) | 211 (12.8) |
| First imaging modality, No. (%) | ||
| Mammogram | 76,749 (86.3) | 424 (25.7) |
| Ultrasound | 76 (0.1) | 3 (0.2) |
| Both | 12,068 (13.6) | 1226 (74.2) |
| BI-RADS score, No. (%) | ||
| 0: assessment incomplete/technically unsatisfactory | 16,886 (19) | 610 (36.9) |
| 1: negative | 41,226 (46.4) | 8 (0.5) |
| 2: benign | 25,179 (28.3) | 10 (0.6) |
| 3: probably benign | 2480 (2.8) | 10 (0.6) |
| 4: suspicious abnormality | 2372 (2.7) | 444 (26.9) |
| 5: highly suggestive of malignancy | 750 (0.8) | 571 (34.5) |
| Pathologic diagnosis, No. (%) | ||
| Benign | 82,893 (92.4) | — |
| Unknown | 4773 (5.4) | — |
| Nonbreast malignancy | 11 (0) | — |
| Atypia | ||
| Atypical ductal hyperplasia/atypical lobular hyperplasia | 33 (0) | 16 (1) |
| Lobular carcinoma in situ | 85 (0.1) | 72 (4.4) |
| Malignancy | ||
| Ductal carcinoma in situ | 549 (0.6) | 386 (23.4) |
| Invasive carcinoma | 1349 (1.5) | 1179 (71.3) |
Abbreviations: BCCCP, Breast and Cervical Cancer Control Program; BI-R ADS, Breast Imaging Reporting and Database System; IQR, interquartile range.
Program Participation
Annual enrollment in NC BCCCP decreased from 14,315 participants in 2008 to 5808 participants in 2018. Concomitantly, participation decreased among Asian/PI women (–4 6.6%; P = .01), AI women (–76.2%; P < .001), NH Black women (–69.0%; P < .001), and NH White women (–70.2%; P < .001; Fig. 1). The proportion of Hispanic women enrolled increased over time but in a nonlinear fashion with a resultant net change from 2008 to 2018 of +1.3% (trend test P < .001).
Figure 1.
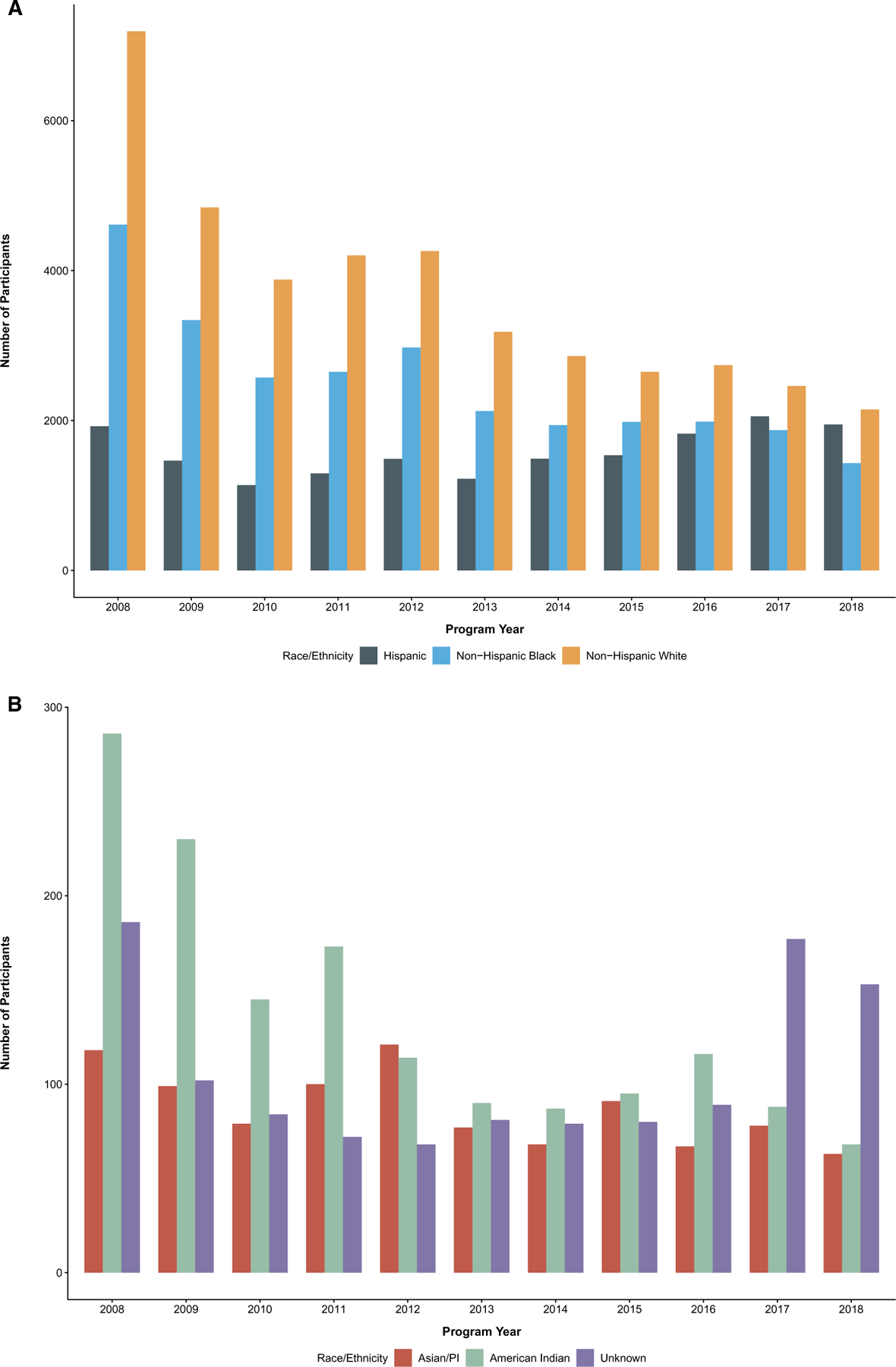
Program participation over time by race/ethnicity, North Carolina Breast and Cervical Cancer Control Program, 2008–2018. PI indicates Pacific Islander.
Timeline of Care
For all-comers, the median time from enrollment to first imaging was 8 days (IQR, 1–21 days), and among those who underwent biopsy, the median time to biopsy was 27 days (IQR, 14–48 days; Fig. 2). In a univariate analysis (Supporting Table 1), there were significant differences in the time to imaging by race/ethnicity (P < .001), with AI patients having the longest time (15 days; IQR, 6–32 days) and Asian/PI patients having the shortest time (7 days; IQR, 0–20 days). Similarly, there were significant differences in the time to biopsy by race/ethnicity (P < .001), with Hispanic patients having the longest time (29 days; IQR, 15–50 days) and AI patients having the shortest time (23 days; IQR, 14–36 days). Patients younger than 50 years had a slightly shorter timeline of care than patients 50 years old or older: the time to imaging was 8 days (IQR, 1–21 days) versus 9 days (IQR, 1–22 days), and the time to biopsy was 26 days (IQR, 14–44 days) versus 28 days (IQR, 14–50 days; both P values < .001). Finally, there were significant differences in the times to imaging and biopsy by funding source (both P values < .001), with fully funded patients having the longest time to imaging (9 days; IQR, 2–21 days) and partially funded patients having the longest time to biopsy (34 days; IQR, 20–56 days).
Figure 2.
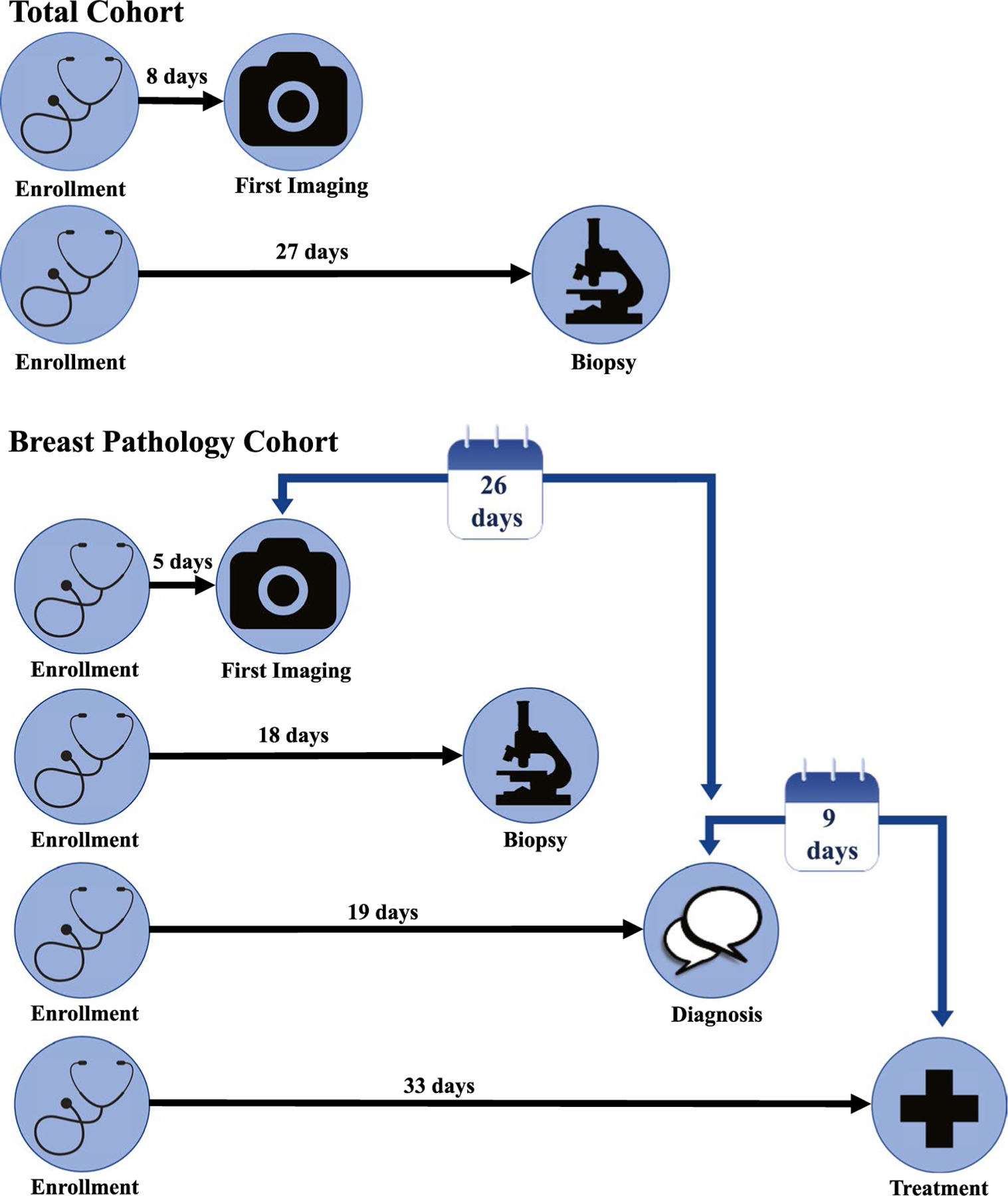
Median timeline of care from enrollment, North Carolina Breast and Cervical Cancer Control Program, 2008–2018.
For patients with breast pathology, the median time to imaging was 5 days (IQR, 0–11 days), the median time to biopsy was 18 days (IQR, 9–31 days), the median TTD was 19 days (IQR, 10–33 days), the median TTT was 33 days (IQR, 19–54 days), the median ITT was 26 days (IQR, 14–44 days), and the median DTT was 9 days (IQR, 1–20 days; Fig. 2). In a univariate analysis (Table 2), there were no significant differences in TTD, TTT, ITT, or DTT by race/ethnicity (P = .54, P = .23, P = .09, and P = .13, respectively). The median TTD and TTT values for all groups fell within the CDC’s 60-day standard.12 Similarly, there were no significant differences in TTD, TTT, ITT, or DTT between patients with atypia and malignancy (TTD, 18 days [IQR, 12–37 days] vs 20 days [IQR, 10–33 days], P = .68; TTT, 34 days [IQR, 16–57 days] vs 33 days [IQR, 19–54 days], P = .98; ITT, 28 days [IQR, 14–47 days] vs 26 days [IQR, 14–44 days], P = .66; DTT, 9 days [IQR, 0–19 days] vs 8 days [IQR, 1–21 days], P = .45). An age < 50 years was associated with a shorter timeline of care in comparison with an age ≥ 50 years (TTD, 18 days [IQR, 8–30 days] vs 21 days [IQR, 11–36 days]; TTT, 30 days [IQR, 17–48 days] vs 35 days [IQR, 20–58 days]; ITT, 24 days [IQR, 12–40 days] vs 28 days [IQR, 15–48 days]; all P values < .001), although there was no significant difference in DTT (P = .14). There were also significant differences in the timeline of care by funding source (all P values < .01), with nonfunded patients having the shortest TTD (12 days; IQR, 5–22 days), TTT (22 days; IQR, 12–38 days), ITT (18 days; IQR, 10–34 days), and DTT (7 days; IQR, 2–15 days), whereas fully funded patients had the longest TTD (21 days; IQR, 12–36 days) and TTT (35 days; IQR, 20–57 days) and partially funded patients had the longest ITT (29 days; IQR, 19–48 days) and DTT (12 days; IQR, 3–21 days).
TABLE 2.
Timeline of Care by Race/Ethnicity, Pathologic Diagnosis, Age, and Funding Among Patients With Breast Pathology, North Carolina Breast and Cervical Cancer Control Program, 2008–2018
| Time to Diagnosis, Median (IQR), d | P | Time to Treatment, Median (IQR), d | P | Time From Imaging to Treatment, Median (IQR), d | P | Time From Diagnosis to Treatment, Median (IQR), d | P | |
|---|---|---|---|---|---|---|---|---|
| Race/ethnicity | .54 | .23 | .09 | .13 | ||||
| Asian/Pacific Islander | 13 (6–40) | 30 (11–57) | 31 (14–45) | 7 (0–18) | ||||
| Hispanic | 20 (10–35) | 34 (19–60) | 25 (12–44) | 8 (2–21) | ||||
| American Indian | 15 (12–27) | 36 (24–49) | 29 (17–41) | 21 (4–31) | ||||
| Non-Hispanic Black | 20 (10–36) | 35 (19–57) | 28 (14–48) | 10 (2–21) | ||||
| Non-Hispanic White | 19 (10–31) | 31 (19–49) | 25 (14–41) | 8 (0–19) | ||||
| Unknown | 22 (17–33) | 28 (23–60) | 27 (14–49) | 11 (2–21) | ||||
| Pathologic diagnosis | .68 | .98 | .66 | .45 | ||||
| Atypia | 18 (12–37) | 34 (16–57) | 28 (14–47) | 9 (0–19) | ||||
| Malignancy | 20 (10–33) | 33 (19–54) | 26 (14–44) | 8 (1–21) | ||||
| Age | <.001 | <.001 | <.001 | .14 | ||||
| <50 y | 18 (8–30) | 30 (17–48) | 24 (12–40) | 8 (0–19) | ||||
| ≥50 y | 21 (11–36) | 35 (20–58) | 28 (15–48) | 9 (2–21) | ||||
| Funding | <.001 | <.001 | <.001 | .001 | ||||
| Fully funded | 21 (12–36) | 35 (20–57) | 26 (13–43) | 8 (0–21) | ||||
| Partially funded | 19 (10–32) | 34 (20–52) | 29 (19–48) | 12 (3–21) | ||||
| Not funded | 12 (5–22) | 22 (12–38) | 18 (10–34) | 7 (2–15) |
Abbreviation: IQR, interquartile range.
In multivariate models (Table 3), NH Black race (vs NH White; IRR, 1.17; 95% CI, 1.06–1.29), age (IRR, 1.01; 95% CI, 1.01–1.02), and funding status (vs not funded; IRR for fully funded status, 1.75; 95% CI, 1.52–2.02; IRR for partially funded status, 1.55; 95% CI, 1.33–1.80) were associated with longer TTD (all P values < .01). Similarly, NH Black race (IRR, 1.2; 95% CI, 1.1–1.31), Hispanic ethnicity (IRR, 1.22; 95% CI, 1.05–1.41), age (IRR, 1.01; 95% CI, 1.01–1.01), and funding status (vs not funded; IRR for fully funded status, 1.52; 95% CI, 1.34–1.73; IRR for partially funded status, 1.44; 95% CI, 1.26–1.65) were associated with longer TTT (all P values < .01). Additionally, NH Black race (vs NH White; IRR, 1.2; 95% CI, 1.09–1.31), age (IRR, 1.01; 95% CI, 1–1.01), and funding status (vs not funded; IRR for fully funded status, 1.31; 95% CI, 1.15–1.49; IRR for partially funded status, 1.41; 95% CI, 1.22–1.62) were associated with longer ITT (all P values < .01). Finally, NH Black race (vs NH White; IRR, 1.27; 95% CI, 1.09–1.48) was associated with longer DTT (P < .01).
TABLE 3.
Factors Associated With the Timeline of Care Among Patients With Breast Pathology, North Carolina Breast and Cervical Cancer Control Program, 2008–2018
| Time to Diagnosis, IRR (95% CI) | P | Time to Treatment, IRR (95% CI) | P | Time From Imaging to Treatment, IRR (95% CI) | P | Time From Diagnosis to Treatment, IRR (95% CI) | P | |
|---|---|---|---|---|---|---|---|---|
| Age (y) | 1.01 (1.01–1.02) | <.001 | 1.01 (1.01–1.01) | <.001 | 1.01 (1–1.01) | <.001 | 1.01 (1–1.02) | .08 |
| Race/ethnicity | ||||||||
| Non-Hispanic White | Reference | .02 | Reference | .001 | Reference | .006 | Reference | .03 |
| Asian/Pacific Islander | 1.09 (0.72–1.64) | .69 | 1.05 (0.73–1.50) | .80 | 1.28 (0.87–1.88) | .20 | 0.91 (0.48–1.74) | .78 |
| Hispanic | 1.16 (0.98–1.37) | .08 | 1.22 (1.05–1.41) | .01 | 1.14 (0.98–1.33) | .10 | 1.27 (0.97–1.66) | .08 |
| American Indian | 0.73 (0.48–1.10) | .13 | 1.02 (0.71–1.46) | .92 | 1.12 (0.76–1.64) | .56 | 1.61 (0.85–3.06) | .14 |
| Non-Hispanic Black | 1.17 (1.06–1.29) | .002 | 1.20 (1.10–1.31) | <.001 | 1.2 (1.09–1.31) | <.001 | 1.27 (1.09–1.48) | .002 |
| Unknown | 0.99 (0.58–1.69) | .98 | 1.04 (0.65–1.66) | .86 | 1.09 (0.66–1.78) | .74 | 1.11 (0.48–2.54) | .81 |
| Program year | 0.96 (0.95–0.98) | <.001 | 0.97 (0.95–0.98) | <.001 | 0.97 (0.95–0.98) | <.001 | 0.97 (0.95–0.99) | .01 |
| Funding | <.001 | <.001 | <.001 | .13 | ||||
| Not funded | Reference | Reference | Reference | Reference | ||||
| Partially funded | 1.55 (1.33–1.8) | <.001 | 1.44 (1.26–1.65) | <.001 | 1.41 (1.22–1.62) | <.001 | 1.29 (1.01–1.64) | .04 |
| Fully funded | 1.75 (1.52–2.02) | <.001 | 1.52 (1.34–1.73) | <.001 | 1.31 (1.15–1.49) | <.001 | 1.21 (0.97–1.52) | .09 |
| Pathologic diagnosis | .50 | .93 | .77 | .78 | ||||
| Atypia | Reference | Reference | Reference | Reference | ||||
| Malignancy | 1.07 (0.88–1.31) | .50 | 1.01 (0.84–1.21) | .93 | 0.97 (0.8–1.18) | .77 | 0.95 (0.69–1.32) | .78 |
Abbreviations: CI, confidence interval; IRR, incidence rate ratio.
Breast Pathology
Among all-comers, breast cancer was diagnosed in 2255 women (2.5%). Rates of breast cancer diagnosis varied significantly among racial/ethnic groups (P < .001; Supporting Table 1), ranging from 3.0% in NH White patients to 1.1% in Hispanic patients. Rates of breast cancer diagnosis also differed significantly by funding source (P < .001), with the highest rates of cancer observed among nonfunded patients (5.5%) and the lowest rates observed among partially funded patients (1.6%). There were higher rates of breast cancer diagnosis in patients 50 years old or older in comparison with patients younger than 50 years (2.7% vs 2.4%; P = .006). After adjustments, age (OR, 1.01; 95% CI, 1.01–1.02), a personal history of breast cancer (OR, 3.25; 95% CI, 2.7–3.89), a family history of breast cancer (OR, 1.55; 95% CI, 1.39–1.74), and a nonfunded status (OR, 2.75; 95% CI, 2.46–3.07; all P values < .001) remained significantly associated with breast cancer diagnosis (Supporting Table 2). Hispanic patients had the lowest odds of breast cancer diagnosis (vs NH White patients; OR, 0.40; 95% CI, 0.34–0.47; P < .001).
DISCUSSION
In our review of nearly 90,000 women, we found that NC BCCCP provides care to a diverse cohort of North Carolinians. The diversity of the program has increased over time, with increasing participation among Hispanic women reflecting the growing North Carolina Hispanic population,13 although total annual enrollment has declined since the passage of the Patient Protection and Affordable Care Act (ACA) in 2010.14,15 Of the 88,893 patients who underwent breast cancer screening from 2008 to 2018, breast cancer was diagnosed in 2255 women, or 2.5% of the program participants. Concordant with national data,1 the incidence of breast cancer was highest among older and NH White women.
When considered both as a whole and by racial/ethnic groups, the vast majority of NC BCCCP participants received timely care, with patients receiving their diagnosis and treatment within the benchmarks set forth by both the CDC (a 60-day standard for both diagnosis and treatment) and other national entities, such as the National Quality Measures for Breast Centers (a 14-day standard from diagnosis to treatment and a 28-day standard from abnormal mammogram to treatment).12,16 However, after adjustments, we did observe relative racial/ethnic disparities in the timeliness of workup, which highlight opportunities to improve equity in breast cancer detection and treatment. NH Black and Hispanic women experienced delays in workup in comparison with NH White women, with NH Black women having longer TTD, TTT, ITT, and DTT and Hispanic women having longer TTT. Thus, even within a program designed to target populations in which the barriers of cost and insurance are disproportionately experienced, racial/ethnic disparities in quality of care persist, and this indicates that nonfinancial barriers must also be mitigated to achieve equitable outcomes. We believe that ours is both the first large-scale study to analyze the NC BCCCP patient population and one of the first to characterize the vital care provided by a division of the National Breast and Cervical Cancer Early Detection Program in the post-ACA era.
Despite advances in breast cancer care, socioeconomic factors such as insurance status continue to play a key role in driving disparities in outcomes.17 The ACA expanded eligibility for Medicaid14; however, 12 states, including North Carolina, have yet to adopt Medicaid expansion, and this has left a substantial number of people in the coverage gap with an individual income above that required for Medicaid eligibility but below the federal poverty line.18 Safety net programs such as NC BCCCP provide essential breast cancer screening services to patients who fall within this coverage gap and are at risk for poor outcomes.19 These programs are understudied; therefore, the characteristics of the patients that they serve, the quality of the services that they provide, and the disparities that may exist therein are unknown.
Racial/ethnic differences in the timeliness of workup have been demonstrated in several previous studies.20–22 In a retrospective analysis of the National Cancer Database, Fedewa et al23 demonstrated that NH Black and Hispanic women had a higher risk of treatment delays following biopsy, with such delays serving to perpetuate disparities in outcomes.24,25 Although the authors enumerated a myriad of factors that contributed to delays in care, insurance status was highlighted as a key variable that was independently associated with treatment delays.
Programs such as NC BCCCP play an important role in providing care to uninsured and underinsured women.10,19 However, our findings demonstrate that expanding access to care is not a panacea, and mitigating insurance as a barrier is not enough to achieve equity. Previous work has identified numerous other potentially modifiable barriers to timely treatment, including a lack of support with childcare, household responsibilities, job-related demands, and care coordination.26–28 Thus, the racial/ethnic minority women served by NC BCCCP may still disproportionately face other challenges that keep them from attaining equitable outcomes. Importantly, the existing relationships between NC BCCCP, its providers, and the communities that they serve will be vital in mediating efforts toward health equity and providing the infrastructure needed to address the specific needs demonstrated by these patients.
Interestingly, NC BCCCP funding was associated with longer TTD, TTT, and ITT in adjusted analyses. Because the allocation of patient-level NC BCCCP funding is a multistep process based on both patient- and provider-level characteristics, the factors contributing to these discrepancies in timeliness are likely multiple and interrelated. Prior work has demonstrated that provider-level aspects such as geographic distance from the screening facility and scheduling availability can affect breast cancer workup.29–31 Additionally, patient-level factors, including attitudes toward breast cancer screening, comorbidities, and compliance, can contribute to workup delays.31,32 Further work is needed to investigate the discrepancies in timeliness associated with patient-level funding support within NC BCCCP.
Notably, although timeliness of workup is a validated quality metric,16 it is not the only measure of care quality. In fact, implementation of certain quality care metrics recommended by clinical practice guidelines, such as multidisciplinary care coordination,33 can lengthen the time to treatment. Thus, there may be benefits associated with NC BCCCP participation that are not reflected in the data presented here. Future work is needed to fully capture NC BCCCP participant experiences, including a qualitative assessment of patient perspectives.
Our study included several limitations. First, there was a high rate of missingness in the provider-reported NC BCCCP data, reflecting a lack of consistent reporting and limiting our ability to describe differences in key clinical characteristics. Similarly, there was a high rate of data entry error (eg, diagnosis before enrollment and a tissue diagnosis discordant with the diagnostic disposition) that limited our analyzable sample size. Furthermore, important patient-level characteristics such as the location of residence and structural characteristics such as the provider or screening site were not available within the database and thus could not be included in our regression models. Finally, data were entered directly by individual providers and were subject to provider-level interpretation and variation.
In summary, our study is the first to analyze the large, diverse cohort served by NC BCCCP, a program that aims to provide breast cancer screening services to underinsured and uninsured women. We have found that NC BCCCP provides high-quality care, meeting national benchmarks for time to diagnosis and treatment. However, we have also found that disparities in the timeliness of workup persist along racial/ethnic lines even within a program that eliminates the barriers of cost and lack of insurance that prevent many women from accessing screening services. Future efforts must be directed toward identifying unmet needs within this patient population and deploying interventions to target those needs via established relationships between NC BCCCP, its providers, and the patients that they serve with the ultimate goal of achieving racial/ethnic parity and improving equity for breast cancer screening and treatment.
Supplementary Material
LAY SUMMARY:
This review of approximately 90,000 participants in a breast cancer screening program for uninsured and underinsured women highlights the importance of safety net programs in providing timely care to underserved patients.
The authors found that the North Carolina Breast and Cervical Cancer Control Program met timeliness benchmarks from the Centers for Disease Control and Prevention across all racial/ethnic groups. However, non-Hispanic Black women experienced relative delays in the time to diagnosis, and both non-Hispanic Black women and Hispanic women experienced relative delays in the time to treatment.
These findings demonstrate how racial/ethnic disparities in the timeliness of care can persist even within a program intended to reduce barriers to access.
FUNDING SUPPORT
This work was supported by the National Institutes of Health (award 1K08CA241390; principal investigator Oluwadamilola M. Fayanju) and the Duke Cancer Institute (National Institutes of Health grant P30CA014236; principal investigator Michael B. Kastan). The content is solely the authors’ responsibility and does not represent the official views of the NIH.
Footnotes
The findings and conclusions presented in this article are those of the researchers and do not represent the views of the Division of Public Health of the North Carolina Department of Health and Human Services.
Additional supporting information may be found in the online version of this article.
CONFLICT OF INTEREST DISCLOSURES
Cushanta C. Horton, Sherry Wright, and Debi Nelson are employed by the North Carolina Department of Health and Human Services. The findings and conclusions presented are those of the researchers and do not represent the views of the North Carolina Department of Health and Human Services, Division of Public Health. Terry Hyslop and Samantha M. Thomas report personal fees from AbbVie outside the submitted work. The other authors made no disclosures.
REFERENCES
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin 2020;70:7–30. [DOI] [PubMed] [Google Scholar]
- 2.Berry DA, Cronin KA, Plevritis SK, et al. Effect of screening and adjuvant therapy on mortality from breast cancer. N Engl J Med 2005;353:1784–1792. [DOI] [PubMed] [Google Scholar]
- 3.Plevritis SK, Munoz D, Kurian AW, et al. Association of screening and treatment with breast cancer mortality by molecular subtype in US women, 2000–2012. JAMA 2018;319:154–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Malmgren JA, Parikh J, Atwood MK, Kaplan HG. Impact of mammography detection on the course of breast cancer in women aged 40–49 years. Radiology 2012;262:797–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hunt BR, Whitman S, Hurlbert MS. Increasing Black:White disparities in breast cancer mortality in the 50 largest cities in the United States. Cancer Epidemiol 2014;38:118–123. [DOI] [PubMed] [Google Scholar]
- 6.van Ravesteyn NT, Schechter CB, Near AM, et al. Race-specific impact of natural history, mammography screening, and adjuvant treatment on breast cancer mortality rates in the United States. Cancer Epidemiol Biomarkers Prev 2011;20:112–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.DeSantis CE, Ma J, Goding Sauer A, Newman LA, Jemal A. Breast cancer statistics, 2017, racial disparity in mortality by state. CA Cancer J Clin 2017;67:439–448. [DOI] [PubMed] [Google Scholar]
- 8.Shoemaker ML, White MC, Wu M, Weir HK, Romieu I. Differences in breast cancer incidence among young women aged 20–49 years by stage and tumor characteristics, age, race, and ethnicity, 2004–2013. Breast Cancer Res Treat 2018;169:595–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Iqbal J, Ginsburg O, Rochon PA, Sun P, Narod SA. Differences in breast cancer stage at diagnosis and cancer-specific survival by race and ethnicity in the United States. JAMA 2015;313:165–173. [DOI] [PubMed] [Google Scholar]
- 10.Jemal A, Robbins AS, Lin CC, et al. Factors that contributed to Black-White disparities in survival among nonelderly women with breast cancer between 2004 and 2013. J Clin Oncol 2018;36:14–24. [DOI] [PubMed] [Google Scholar]
- 11.NC Department of Health and Human Services.Breast and Cervical Cancer Control Program Accessed April 1, 2020. https://bcccp.nc-dhhs.gov
- 12.Richardson LC, Royalty J, Howe W, Helsel W, Kammerer W, Benard VB. Timeliness of breast cancer diagnosis and initiation of treatment in the National Breast and Cervical Cancer Early Detection Program, 1996–2005. Am J Public Health 2010;100:1769–1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.US Census Bureau. North Carolina Hispanic or Latino population Accessed July 3, 2020. https://www.census.gov/quickfacts/fact/table/NC/RHI725219
- 14.Kominski GF, Nonzee NJ, Sorensen A. The Affordable Care Act’s impacts on access to insurance and health care for low-income populations. Annu Rev Public Health 2017;38:489–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tangka F, Kenny K, Miller J, Howard DH. The eligibility and reach of the National Breast and Cervical Cancer Early Detection Program after implementation of the Affordable Care Act. Cancer Causes Control 2020;31:473–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kaufman CS, Shockney L, Rabinowitz B, et al. National Quality Measures for Breast Centers (NQMBC): a robust quality tool. Ann Surg Oncol 2010;17:377–385. [DOI] [PubMed] [Google Scholar]
- 17.Ward E, Jemal A, Cokkinides V, et al. Cancer disparities by race/ethnicity and socioeconomic status. CA Cancer J Clin 2004;54:78–93. [DOI] [PubMed] [Google Scholar]
- 18.Garfield R, Damico A, Stephens J, Rouhani S. The Coverage Gap: Uninsured Poor Adults in States That Do Not Expand Medicaid—An Update. Kaiser Family Foundation; 2016.
- 19.Ward E, Halpern M, Schrag N, et al. Association of insurance with cancer care utilization and outcomes. CA Cancer J Clin 2008;58:9–31. [DOI] [PubMed] [Google Scholar]
- 20.Smith EC, Ziogas A, Anton-Culver H. Delay in surgical treatment and survival after breast cancer diagnosis in young women by race/ethnicity. JAMA Surg 2013;148:516–523. [DOI] [PubMed] [Google Scholar]
- 21.Gwyn K, Bondy ML, Cohen DS, et al. Racial differences in diagnosis, treatment, and clinical delays in a population-based study of patients with newly diagnosed breast carcinoma. Cancer 2004;100:1595–1604. [DOI] [PubMed] [Google Scholar]
- 22.Reeder-Hayes KE, Mayer SE, Olshan AF, et al. Race and delays in breast cancer treatment across the care continuum in the Carolina Breast Cancer Study. Cancer 2019;125:3985–3992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fedewa SA, Edge SB, Stewart AK, Halpern MT, Marlow NM, Ward EM. Race and ethnicity are associated with delays in breast cancer treatment (2003–2006). J Health Care Poor Underserved 2011;22:128–141. [DOI] [PubMed] [Google Scholar]
- 24.McLaughlin JM, Anderson RT, Ferketich AK, Seiber EE, Balkrishnan R, Paskett ED. Effect on survival of longer intervals between confirmed diagnosis and treatment initiation among low-income women with breast cancer. J Clin Oncol 2012;30:4493–4500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Richards MA, Westcombe AM, Love SB, Littlejohns P, Ramirez AJ. Influence of delay on survival in patients with breast cancer: a systematic review. Lancet 1999;353:1119–1126. [DOI] [PubMed] [Google Scholar]
- 26.Carroll JK, Humiston SG, Meldrum SC, et al. Patients’ experiences with navigation for cancer care. Patient Educ Couns 2010;80:241–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.George P, Chandwani S, Gabel M, et al. Diagnosis and surgical delays in African American and White women with early-stage breast cancer. J Womens Health (Larchmt) 2015;24:209–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fayanju OM, Ren Y, Stashko I, et al. Patient-reported causes of distress predict disparities in time to evaluation and time to treatment after breast cancer diagnosis. Cancer Published online November 11, 2020. doi: 10.1002/cncr.33310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mishra SI, DeForge B, Barnet B, Ntiri S, Grant L. Social determinants of breast cancer screening in urban primary care practices: a community-engaged formative study. Womens Health Issues 2012;22:e429–e438. [DOI] [PubMed] [Google Scholar]
- 30.Allen JD, Shelton RC, Harden E, Goldman RE. Follow-up of abnormal screening mammograms among low-income ethnically diverse women: findings from a qualitative study. Patient Educ Couns 2008;72:283–292. [DOI] [PubMed] [Google Scholar]
- 31.Caplan LS, Helzlsouer KJ, Shapiro S, Wesley MN, Edwards BK. Reasons for delay in breast cancer diagnosis. Prev Med 1996;25:218–224. [DOI] [PubMed] [Google Scholar]
- 32.Rojas M, Mandelblatt J, Cagney K, Icerner J, Freeman H; Cancer Control Center of Harlem. Barriers to follow-up of abnormal screening mammograms among low-income minority women. Ethn Health 1996;1:221–228. [DOI] [PubMed] [Google Scholar]
- 33.Carlson RW, Allred DC, Anderson BO, et al. Breast cancer. Clinical practice guidelines in oncology. J Natl Compr Canc Netw 2009;7:122–192. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


