Abstract
Massage is anecdotally associated with many health benefits, but physiological and clinically relevant mechanisms recently have begun to be investigated in a controlled manner. Herein, we describe research supporting our hypothesis that massage can be used as a mechanotherapy imparting biologically relevant adaptations in skeletal muscle and improving muscle properties.
Keywords: massage, mechanotherapy, skeletal muscle, atrophy, exercise
INTRODUCTION
There is a long-standing notion that massage can be applied as a therapeutic modality to attenuate etiology and symptoms of a variety of diseases and musculoskeletal impairments. Over 130 yr ago, Dr. Joseph Schreiber compiled a scientific and medical guide summarizing the anecdotal evidence of the beneficial applications of massage, entitled A Manual of Treatment by Massage and Methodical Muscle Exercise (1). Although written long ago, the introductory statement of the manual aptly captured the sentiment of not only the purpose of this review but also the past decade of our research on massage:
“The growing tendency of modern therapeutics to do away as far as possible with the use of drugs, and to seek to cure disease by application of the laws of hygiene, has made it seem desirable to present to the medical public a practical work on that oldest branch of the healing art, namely, mechanotherapy.”
The search for alternative and complimentary methods of medicine to heal ailments has certainly not dissipated over the past century. Massage often is used as a treatment or prevention of musculoskeletal conditions and pain relief (2). As of 2007, the annual medical costs related to complimentary medicine, such as massage, totaled $34 billion in the United States (3). Yet, despite the number of individuals seeking massage as a treatment, and its prominent use in rehabilitation strategies, there are limited rigorously performed scientific studies examining the impact of massage on the molecular, cellular, and physiological function of muscle. In fact, in a recent review on the physiological effects of manual therapies, Lima et al. (4) concluded that the high variability and lack of rigor in methodology, terminology, and study parameters limit the relevancy and clinical translatability of many studies of massage therapy.
This review proposes the novel hypothesis that massage is a mechanotherapy that provides biologically relevant adaptations in skeletal muscle across a variety of conditions. We will present work collected over the last decade from our laboratories that supports this novel hypothesis (Fig.). In this review, we first describe our controlled and reproducible methods to apply a massage-mimetic to skeletal muscle in animal models. We then discuss how massage as a mechanotherapy can improve skeletal muscle recovery from exercise and combat atrophy through its effects on protein turnover, ribosome turnover, extracellular matrix (ECM) turnover, satellite cell proliferation, and immunomodulation.
Figure.
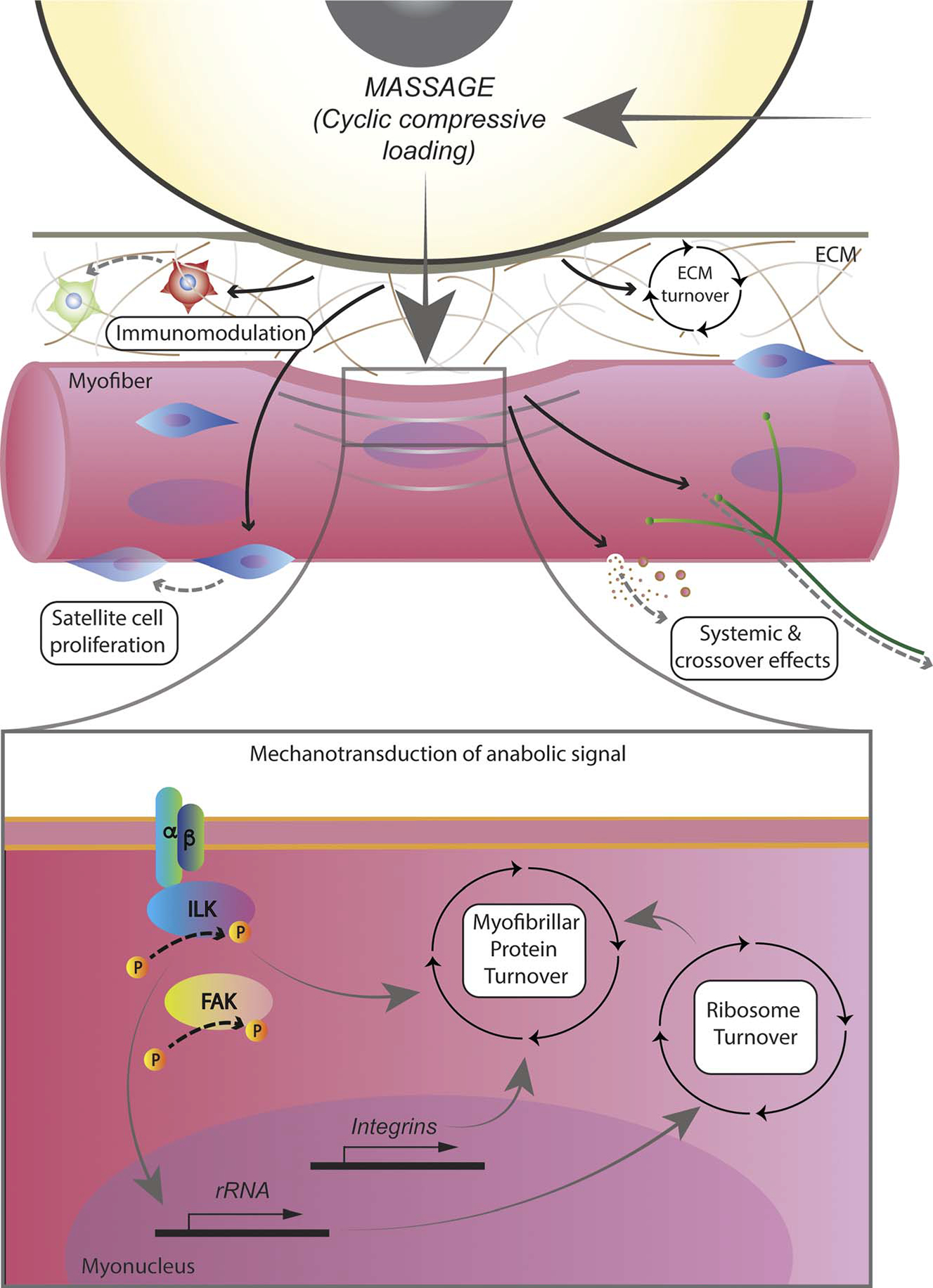
Massage as a mechanotherapy for muscle: physiological responses of muscle to massage in the form of cyclic compressive loading (CCL). Massage applied to muscle through CCL in a controlled manner affects physiological processes such as immunoregulation, satellite cell proliferation, and extracellular matrix (ECM) turnover, and leads to intracellular responses in the form of protein and ribosome turnover as well as integrin-related signaling. Crossover effects also occur through yet to be identified mechanisms.
CYCLIC COMPRESSIVE LOADING AS A MASSAGE MIMETIC
A major problem that undermined our understanding of the efficacy of massage was the inconsistent and uncontrolled manner in which massage was applied [i.e., massage type and magnitude, duration, and frequency of applied force, as reviewed in (5) and (4)]. Most previous research has focused on the application of massage for improving muscular performance and recovery from exercise and an in-depth discussion of the shortcomings of these research studies is beyond the scope of this review. However, we refer readers to a recent meta-analysis by Davis et al. (6) that adequately demonstrates the inconsistencies and variability in study design and results in human studies examining the impact of massage on muscular strength, fatigue, recovery from exercise, and more. The inconclusive nature of these studies affirms the need for well-controlled and repeatable methodologies in massage research. Furthermore, development of methodologies to perform massage studies with animals in a controlled fashion allows for uncovering the mechanisms by which massage may improve skeletal muscle function and recovery, thus allowing better tailoring of massage treatments in humans.
In 2008, Butterfield et al. (7) created a tool that facilitated the application of a controlled massage-like treatment to rabbits. The authors used allometric scaling to estimate the forces needed to mimic Swedish massage in humans when applied to rabbit muscle (7). We then used the same scaling to adapt the tool to use as a massage-mimetic for rats (8). We refer to this application of massage as cyclic compressive loading (CCL), which our group continues to use for the application of precise and well-controlled massage-like treatment to both rabbits and rats. For CCL, a cylindrical device is rolled over the muscle at a fixed frequency, force, and duration. We examined various duration and loading combinations for optimizing the application of CCL (8,9). Allometric scaling of the massage force that was optimal for muscle recovery from eccentric exercise in rabbits was used to calculate the massage force for rats. In addition, we leveraged data from the rabbits to estimate the optimal frequency and duration of massage application. In rats, we found that a force of 4.5 N at a frequency of 0.5 Hz applied over 30 min was the best application of CCL of the combinations we tried, and this is the schema used for most of our studies (8,10–12). Microarray analysis demonstrated a more robust immune response when 4.5 N was used when compared with 1.4-N massage force; however, use of a higher force of 11 N for massage caused tissue damage as evidenced by elevated edema (13). Thus, the 4.5-N regimen produced physiologically meaningful changes, while being comparable and translatable to a force, based on allometric scaling, and time commitment often allotted for massage treatments in humans. The complete details of the how the CCL device works can be found in our previous publications (7,8). Using CCL allowed us to overcome pitfalls of previous massage studies that demonstrated little rigor pertaining to the force, frequency, and duration of the mechanotherapy. Furthermore, CCL has allowed us to compare the impact of massage across multiple studies and species in a standardized manner.
MASSAGE ACCELERATES FUNCTIONAL RECOVERY FROM ECCENTRIC EXERCISE
High-intensity or strenuous exercise sometimes results in muscle damage (14), soreness (15), and inflammation (16), particularly when the exercise contains repetitive eccentric muscle contractions (17). Depending on the physical conditioning of the individual, these symptoms can last up to 1 wk (18). Several strategies, including massage, have been implemented to reduce these symptoms to reduce time lost from sports participation or to reduce the impact these symptoms may have on physical performance (19–22). To investigate the underlying mechanisms of the effect of massage on recovery from muscle damage, Butterfield et al. used CCL with a rabbit model of eccentric exercise (7). Four days after the exercise bout, nonmassaged tibialis anterior muscle exhibited classic signs of severe muscle damage resulting from a high-load eccentric exercise challenge such as widening of endomyseal space between myofibers, disruption of myofiber membranes, damage within myofibers, and a large infiltration of inflammatory cells (7). In stark contrast, the muscle that was massaged immediately postexercise, and every 24 h during the 4-d recovery, exhibited minimal disruption of myofibers and much less inflammatory cell infiltration, demonstrating a striking improvement in muscle remodeling and recovery with the mechanotherapy. The vastly improved recovery of the muscle structural integrity coincided with an approximately fourfold increase in peak torque recovery compared with no massage. Important for clinical application however, we found that the beneficial impact of massage on recovery from eccentric muscle damage is most effective when applied immediately after the exercise bout, with less efficacy when applied 48 h postexercise (23). In 2015, Cezar et al. (24) substantiated our findings by showing that cyclic mechanical compressions applied to muscle via a pressure cuff or biphasic ferrogel greatly improved muscle regeneration and function in mice during a 2-wk recovery period from very severe muscle injury. Thus, the use of massage as a mechanotherapy after muscle damage is highly effective at accelerating recovery of muscle structural integrity and function, which is of significance for athletes who need to return to performing at high levels after training or games as quickly as possible, but could also be used in the clinical population.
MASSAGE IMPROVES RECOVERY OF MUSCLE MASS AFTER DISUSE ATROPHY
Previous research suggested that massage can combat atrophy and improve regrowth after atrophy, although these studies lacked the rigor to conclude that massage was beneficial under these circumstances. For example, in 1946, it was stated that massage treatment applied to the gastrocnemius muscles of cats twice per day attenuated the amount of muscle mass and strength loss in response to denervation (25). However, there were no details provided on the massage regimen (how it was applied, length of massage bouts, etc) or any other details on making this treatment repeatable. Wood et al. (26) tried a massage intervention to combat denervation atrophy in dogs in 1948 and showed there was no impact of massage in preventing denervation related atrophy. Wood et al. described the massage treatment as a 10-min treatment consisting of approximately one-third stroking and two-thirds kneading of the entire denervated limb, but no other details were provided. In addition, Wood et al. killed the dogs between 8 and 34 wk and provided no analysis on whether the different lengths of time after denervation had any impact on the results. There have also been claims that massage lessened masticatory muscle atrophy in edentulous or partially edentulous patients, but none of the actual data were presented in this reporting (27). Mechanical vibration has been demonstrated as a mechanotherapy that slowed atrophy after a plexus lesion or unloading (28,29), respectively, indicating that mechanical manipulation can influence muscle size. The compilation of this limited evidence prompted us to investigate the potential use of massage to combat atrophy in a more controlled and rigorous manner.
We investigated whether massage could augment recovery of muscle mass after a period of disuse atrophy. Ten-month-old (young adult) Fisher 344/Brown Norway (F344/BN) rats were subjected to 2 wk of hindlimb suspension to induce severe disuse atrophy of the hindlimb musculature. The disuse was followed by either an 8-d reloading (return to normal ambulation) period (10) or CCL was applied to the gastrocnemius muscle for 30min every other day for a total of four bouts during the 8-d reloading phase. Massage was clearly beneficial for accelerating the recovery of muscle mass as evidenced by significantly higher muscle fiber cross-sectional area (CSA) in massaged muscle compared with no massage at the end of the reloading period. This study was our first to show that massage may be a clinically viable intervention for improving the time course of recovering muscle mass after periods of disuse (i.e., bed rest, injury).
In addition to improving recovery after reloading, it also is important to find interventions that can prevent the loss of muscle mass in a scenario in which a patient may not be able to ambulate or exercise. We tested whether massage could prevent loss of muscle mass by administering CCL for 30 min every other day during a 7-d hindlimb-suspension protocol in F344/BN rats (11). There was less atrophy with massage, although the difference was not statistically significant. However, the results suggested that massage is a mechanotherapy that can have an impact on disuse atrophy. Furthermore, we found that massage applied during disuse had other important effects on skeletal muscle protein and ribosome turnover that may be beneficial during the subsequent recovery period (discussed later).
MECHANISMS DICTATING THE BENEFICIAL EFFECTS OF MASSAGE
Massage Has an Immunomodulatory Effect in Skeletal Muscle
In the 2008 study by Butterfield et al., there was a striking reduction in the infiltration of immune cells in the massaged muscle, suggesting that massage provided an immunomodulatory effect when there is muscle damage (7). In fact, when CCL was applied to unperturbed muscle in rats, microarray analysis revealed that expression of genes related to immune response was the most affected (8). The abundance of CD68+ and CD163+ immune cells responded in a dose-dependent manner to massage, with the abundance of immune cells increasing as the applied massage force was increased. The effectiveness of massage to promote recovery from strenuous exercise via immunomodulatory mechanisms in humans was demonstrated in a study by Crane et al. (30). Eleven human subjects performed aerobic exercise on a cycle ergometer to exhaustion followed by massage for 10 min in the vastus lateralis muscle of one leg. A major finding from the study was that massage mitigated the muscle inflammatory response by attenuating the rise in tumor necrosis factor-α (TNF-α), interleukin-6, and nuclear factor κB (p65) nuclear accumulation. In addition, massage inhibited the elevation of heat shock protein 27 (HSP27) phosphorylation, which is a marker of cellular stress response. The immune cell response to massage-like mechanotherapies may be a critical aspect of massage's ability to combat atrophic processes (31). Although more work is needed in this area, it is evident that massage can alter the skeletal muscle inflammatory response in a manner that may promote recovery from exercise and atrophy.
Protein Synthesis and Degradation
Muscle size is ultimately determined by the balance of protein synthesis and degradation. Although increasing protein synthesis is important, increasing breakdown also is crucial because eliminating damaged and unnecessary proteins facilitates remodeling. To understand changes in protein turnover with massage, we used long-term deuterium oxide (D2O) labeling. We found that massage applied during reloading increased overall protein turnover compared with reloading alone (10). These changes in turnover coincided with a greater regain of muscle mass with massage compared with no massage. Massage applied during disuse partially attenuated the large drop in protein synthesis seen with hindlimb suspension, but protein degradation was not impacted (11).
Although massage applied during disuse and during reloading impacted protein turnover differently, there was indication of improved remodeling in both conditions. This insight would not have been apparent by looking at protein synthesis only and illustrates the importance of both processes for recovering from a disuse event. A limitation was that these studies largely focused on the contractile proteins, although we also examined cytosolic and mitochondrial proteins, as well as cell proliferation rates (10,11). Ongoing and future studies will examine the remodeling of the ECM, which is a principal contributor to mechanosensitivity, as well as the individual proteins whose increases or decreases are not apparent from an integrated bulk measurement. We hypothesize that massage will have a beneficial impact on ECM turnover.
Ribosome Biogenesis and Degradation
The rates of protein synthesis in muscle largely are determined by the translational efficiency and capacity of ribosomes (32). Translational capacity of the ribosome often is inferred from changes in ribosome content (33,34). Similar to proteins, ribosomal content is determined by the balance of ribosome biogenesis and degradation. Furthermore, like protein turnover, it is possible to determine ribosomal turnover using D2O. Disuse atrophy is associated with a loss of ribosome content, that is, translational capacity (10,11,35), and a novel finding from our work is that the majority of the loss in ribosomal content with disuse atrophy is because of an increase in ribosome degradation (11). Furthermore, massage partially attenuates the elevation in ribosome degradation during disuse in rats, which demonstrates that massage may be beneficial for preserving translational capacity of muscle in patients undergoing muscle disuse (11). The mechanisms that regulate these changes in ribosomal turnover, especially the process of ribosomal breakdown, with disuse and mechanotherapy are almost completely undescribed. However, we propose that preservation of translational capacity during disuse may be important for improving recovery of the muscle once reloading occurs. More work is needed to test this hypothesis.
Mechanosensitive and Anabolic Signaling
The alterations in protein and ribosome turnover that occur with massage may derive from mechanisms that sense mechanical cues at the interface of the ECM and cell membrane and translate them into intracellular anabolic signaling (36). Integrins are considered the primary receptors for transmission of mechanical signals to induce muscle adaptations such as hypertrophy (37). The expression of integrins is elevated in response to resistance exercise, and having a higher level of intramuscular integrins is associated with a greater hypertrophic response to overload (38–40). Interestingly, the transmission of mechanical forces via integrins may also directly convert the mechanical cues into biochemical signals that promote growth through scaffolding connected to the nucleus (41). We showed that massage causes sustained elevations in mechanosensitive signaling through integrin-linked kinase (ILK) and focal adhesion kinase, both of which are primary proteins by which integrins transmit mechanical cues into the cell (10,12). Massage increases the expression of integrin beta 2 subunit (ITGB2) and promotes ILK signaling in young adult rats, but not aged rats (12). β2-integrins are linked to muscle hypertrophy and have a prominent role in neutrophil and macrophage activity; thus, changes in the expression of ITGB2 may cause an altered anabolic response in muscle via mechanosensitive and inflammatory pathways (39). Aged rats have lower expression of integrins compared with younger rats, which may explain their lack of ILK signaling and lack of anabolic response to massage. Interestingly, massage does not seem to activate many of the canonical anabolic signaling pathways such Akt/mTOR signaling despite its impact on other mechanotransduction-related factors (10–12). However, it should be noted that in all of these referenced studies, the muscles were examined 24 h after the last bout of massage, and it is possible that the changes caused by the massage stimulus to anabolic signaling have subsided at that time point.
Massage Increases Satellite Cell Abundance
Satellite cells play an integral role in muscle regeneration and adaptation (42); therefore, interventions that augment the abundance or function of satellite cells may benefit muscle recovery after a damaging eccentric bout of exercise. Interestingly, we observed higher satellite cell abundance (Pax7+ cells) when massage was applied to healthy, unperturbed muscle as well as during regrowth after disuse atrophy (10,43). Massage elevated rates of DNA synthesis when applied during regrowth, which we attributed to the proliferative response of satellite cells to the mechanotherapy (10). As such, muscle recovery and regrowth may be improved by the influence of massage on satellite cell proliferation and function. The proliferation of satellite cells may provide a larger satellite cell pool and augment fusion of satellite cells into myofibers during muscle remodeling processes. In addition, satellite cells communicate with fibrogenic cells within the muscle via extracellular vesicles to promote ECM deposition and muscle remodeling (44). It is currently unknown what the signals are for the increase in satellite cell abundance. We found no signs of damage after massage in unperturbed muscle (43), so it is unlikely that injury-related signals are responsible. It is possible that the satellite cells themselves are responding to the mechanical stimulus, because stem cells are known to be mechanosensitive (45). More work is needed to address the question of what signals satellite cell proliferation in response to massage. Nonetheless, the proliferation of satellite cells in response to massage may optimize the muscle's regrowth capacity.
CROSSOVER EFFECT: MASSAGE IMPACTS CONTRALATERAL NONMASSAGED HOMOLOGOUS MUSCLE
A striking finding from our massage studies is the potential for the effects of massage to be transferred to the nonmassaged homologous muscle in the contralateral limb (10,11). Multiple studies established the potential for unilateral exercise interventions in a healthy limb to transfer beneficial adaptations, such as preservation of muscle strength and size, to an immobilized limb (46–49). This cross-transfer of massage adaptations is important because there are many clinical scenarios in which a critically ill or injured patient does not have the capacity to exercise the injured limb but can withstand the low-impact massage treatment on the other limb. In our studies, the gastrocnemius muscle in the contralateral limb showed the greatest beneficial effect compared with other hindlimb muscles when the ipsilateral gastrocnemius was massaged (10). Massage applied during regrowth elevated myofibrillar protein turnover and improved recovery of CSA in the nonmassaged gastrocnemius, which mimicked the beneficial impacts observed in the directly massaged muscle (10). Massage applied during disuse atrophy also exhibited crossover effects on protein and RNA turnover (11). Altogether, it has become clear that the beneficial effects of massage have the possibility to transfer to the nonmassaged limb. It is generally thought that the mechanism driving the crossover effect is neurally mediated (50,51). However, it also is possible that massage to skeletal muscle releases myokines or extracellular vesicles that impart physiologically meaningful outcomes (52,53). Future work should further investigate the mechanisms driving the crossover effect, especially with the high clinical value it imparts.
MASSAGE AND AGED SKELETAL MUSCLE
Aged skeletal muscle exhibits hampered regrowth after atrophy compared with younger muscle that is attributed to anabolic resistance due to dampened rates of protein synthesis (54), reduced activation of Akt/mTOR signaling (55), reduced sensitivity to amino acids (56), blunted ribosome biogenesis (33), reduced insulin sensitivity (57), and increased stiffness (58) compared with adult muscle. However, there also are studies demonstrating that aged skeletal muscle can adequately respond to a mechanical stimulus (59–61); thus, debate remains as to exactly why aged skeletal muscle does not recover muscle mass as well as younger muscle after periods of disuse.
We investigated whether massage is a viable therapeutic intervention to promote anabolism and regrowth in aged skeletal muscle and found that aged muscle demonstrated some responsiveness to mechanotherapy, albeit not as much as adult muscle (12,43,62). After a single bout of CCL, aged muscle elevated satellite cell abundance to a similar degree as adult muscle (43). This may be beneficial for improving the regeneration and remodeling capabilities of aged muscle, which is typically not as robust when compared with younger muscle (63). In addition, a single bout of massage via CCL in rats caused a unique stress response in aged muscle as evidenced by an elevation in the HSP25 and HSP70 and cold shock protein RNA-binding motif 3 (RBM3) (12). All three of these proteins have been found to be protective against atrophy when overexpressed in skeletal muscle (64–66). A single bout of massage certainly shows promise for instigating beneficial adaptations in aged skeletal muscle that may be protective against atrophy and help with muscle remodeling.
We followed up these findings by reproducing in aged rats our study designs that used massage during unloading and reloading in adult rats. Despite elevating protein synthesis and lowering protein degradation during hindlimb suspension in aged rats, application of massage failed to attenuate the loss of muscle mass (62). In addition, massage applied during recovery from atrophy did not improve recovery of muscle mass (62). Our results agree with others that demonstrate that aged muscle can effectively respond at a molecular level to regrowth or anabolic stimuli, but it does not translate into muscle growth (59–61). Interestingly, aged muscle exhibited higher ribosome degradation with massage when it was applied during weight bearing and disuse atrophy, which is different to the response of adult muscle in which ribosome degradation was lowered with massage (62).
The differential response of aged muscle to massage as a mechanotherapy may be due to a more fibrotic and stiffer ECM that hampers mechanosensing (58). As mentioned previously, aged muscle has a lower expression of integrins compared with adult muscle and lower activation of ILK in response to massage (12). In addition, adult muscle exhibits higher myofiber membrane permeability in response to a single bout of massage compared with aged, which we believe is the result of excessive ECM in the aged muscle that is shielding the transduction of mechanical force from effectively reaching the myofibers (43). A more abundant and stiffer ECM may prevent the proper transmission of mechanical signals in the muscle and lower mechanosensitivity. There is some evidence that higher loads or doses of mechanical stimulus can overcome the anabolic resistance in aged muscle (67), and it is possible that a higher applied load may improve the aged muscle's response to massage. Future studies should investigate the optimization of the load to overcome the limitations of aged muscle to mechanotherapy.
MASSAGE DOES NOT CAUSE HYPERTROPHY IN UNPERTURBED MUSCLE
We have consistently demonstrated that although massage may impart beneficial adaptations in skeletal muscle during disuse atrophy and during recovery from atrophy, it does not cause muscle growth when applied to unperturbed, homeostatic muscle (11,12,62). The lack of robust hypertrophic or anabolic effects suggests that massage is only a useful mechanotherapy for muscle growth under nonhomeostatic conditions (11,12,62). That is, massage can be best leveraged to augment or improve regrowth or other applied anabolic interventions in a synergistic fashion. Although there are no robust or consistent effects of massage on protein or ribosomal turnover in unperturbed muscle, there may still be some benefits. For example, the aforementioned immunomodulatory and satellite cell adaptations (8,10,43) may augment muscle's remodeling capacity. In addition, massage increases cold shock and heat shock proteins in aged muscle, which may provide protective adaptations against cellular stressors (12). These data combined have led us to conclude that massage can be a useful intervention during atrophy or when recovering from atrophy or strenuous exercise and should not be considered as an independent hypertrophic stimulus.
FUTURE DIRECTIONS FOR UNDERSTANDING CLINICAL APPLICATION OF MASSAGE
Although there is a plethora of research investigating the application of massage for sports performance and recovery after exercise in humans (6), there has been no progress in understanding how massage may protect muscle from atrophy or improve recovery from atrophy. As mentioned, the current evidence surrounding the utility of massage for sports performance is complicated by inconsistent research design and massage application, which may underlie the highly heterogeneous results regarding the success of massage for improving muscle recovery from exercise. Future research should incorporate strict methods to control the force, duration, and frequency of the massage application as well as ensuring that the massage intervention is applied at the correct intervals. For example, our laboratory has shown in animal models that the massage intervention is only effective for improving recovery from eccentric muscle damage when applied immediately after the exercise bout (23).
The evidence that massage may benefit muscle atrophy before our laboratory's studies was primarily anecdotal in nature, with little or no details provided to allow repeatability of the research or translation to a clinical setting. Allometric scaling can be used to estimate the appropriate force to apply with a roller device on human muscle tissue to elicit the same effects in muscle that we see in animal studies. The application of massage can be performed during bed rest, limb immobilization, or during recovery from these scenarios in human subjects to ascertain whether the positive effects of massage on muscle atrophy that we observe in animal studies can be adequately transferred to combating muscle atrophy in human subjects.
CONCLUSION
In conclusion, massage is a mechanotherapy that can modulate skeletal muscle satellite cell proliferation, immune response, ribosome turnover, and protein turnover in a manner that benefits recovery from eccentric exercise and disuse atrophy (Fig.). Massage also can impart crossover effects as evidenced by adaptations in contralateral muscles that were not subjected to massage. Although the work from our group and others have certainly shown great promise for massage as a useful intervention, there is still much work needed to optimize massage for the aging population. Furthermore, there is a need to further understand these mechanisms, such as potential ECM remodeling, that may impact the muscle response to disuse and reloading. The findings described in this review are clinically relevant and support the notion that massage as a mechanotherapy imparts biologically and physiologically relevant adaptations in skeletal muscle.
Key Points.
Massage has been described as a therapeutic modality to attenuate a number of ailments in skeletal muscle, and only recently has rigorously designed research determined its biological impacts and clinical potential.
Recovery of muscle function after muscle-damaging exercise bouts can be greatly accelerated by application of massage.
Massage can promote accelerated regrowth of muscle mass during recovery from disuse atrophy, which may highly benefit clinical populations.
The development of a well-controlled application of massage in animal models has helped show that the beneficial impact of massage on skeletal muscle is due to its impact on satellite cell proliferation, immune response, ribosome turnover, and protein turnover.
The summary of the current work on massage has illuminated that it is a mechanotherapy that offers a multitude of biologically relevant adaptations for both athletes and clinical populations.
Acknowledgments
This study was supported by National Institutes of Health grants AG042699 and AT009268.
References
- 1.Schrieber J. A Manual of Treatment by Massage and Methodical Muscle Exercise. Philadelphia: Lea Brothers & Co.; 1887. [Google Scholar]
- 2.Barnes PM, Powell-Griner E, McFann K, Nahin RL. Complementary and alternative medicine use among adults: United States, 2002. Semin. Integr. Med. 2004; 2(2):54–71. [PubMed] [Google Scholar]
- 3.Nahin RL, Barnes PM, Stussman BJ, Bloom B. Costs of complementary and alternative medicine (CAM) and frequency of visits to CAM practitioners: United States, 2007. Natl Health Stat Report. 2009; (18):1–14. [PubMed] [Google Scholar]
- 4.Lima CR, Martins DF, Reed WR. Physiological responses induced by manual therapy in animal models: a scoping review. Front Neurosci 2020; 14:430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Best TM, Hunter R, Wilcox A, Haq F. Effectiveness of sports massage for recovery of skeletal muscle from strenuous exercise. Clin. J. Sport Med. 2008; 18(5):446–60. [DOI] [PubMed] [Google Scholar]
- 6.Davis HL, Alabed S, Chico TJA. Effect of sports massage on performance and recovery: a systematic review and meta-analysis. BMJ Open Sport Exerc. Med. 2020; 6(1):e000614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Butterfield TA, Zhao Y, Agarwal S, Haq F, Best TM. Cyclic compressive loading facilitates recovery after eccentric exercise. Med. Sci. Sports Exerc. 2008; 40(7):1289–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Waters-Banker C, Butterfield TA, Dupont-Versteegden EE. Immunomodulatory effects of massage on nonperturbed skeletal muscle in rats. J. Appl. Physiol. 2014; 116(2):164–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Haas C, Butterfield TA, Zhao Y, Zhang X, Jarjoura D, Best TM. Dose-dependency of massage-like compressive loading on recovery of active muscle properties following eccentric exercise: rabbit study with clinical relevance. Br. J. Sports Med. 2013; 47(2):83–8. [DOI] [PubMed] [Google Scholar]
- 10.Miller BF, Hamilton KL, Majeed ZR, et al. Enhanced skeletal muscle regrowth and remodelling in massaged and contralateral non-massaged hindlimb. J. Physiol. 2018; 596(1):83–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lawrence MM, Van Pelt DW, Confides AL, et al. Massage as a mechanotherapy promotes skeletal muscle protein and ribosomal turnover but does not mitigate muscle atrophy during disuse in adult rats. Acta Physiol (Oxf) 2020; 229:e13460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Van Pelt DW, Confides AL, Abshire SM, Hunt ER, Dupont-Versteegden EE, Butterfield TA. Age-related responses to a bout of mechanotherapy in skeletal muscle of rats. J. Appl. Physiol. 2019; 127(6):1782–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Waters-Banker C, Dupont-Versteegden EE, Kitzman PH, Butterfield TA. Investigating the mechanisms of massage efficacy: the role of mechanical immunomodulation. J. Athl. Train. 2014; 49(2):266–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Malm C, Sjödin TL, Sjöberg B, et al. Leukocytes, cytokines, growth factors and hormones in human skeletal muscle and blood after uphill or downhill running. J. Physiol. 2004; 556(Pt 3):983–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hough T. Ergographic studies in muscular soreness. Am. J. Physiol. 1902; 7(1):16. [Google Scholar]
- 16.Paulsen G, Crameri R, Benestad HB, et al. Time course of leukocyte accumulation in human muscle after eccentric exercise. Med. Sci. Sports Exerc. 2010; 42(1):75–85. [DOI] [PubMed] [Google Scholar]
- 17.Butterfield TA. Eccentric exercise in vivo: strain-induced muscle damage and adaptation in a stable system. Exerc. Sport Sci. Rev. 2010; 38(2):51–60. [DOI] [PubMed] [Google Scholar]
- 18.Fridén J, Sjöström M, Ekblom B. Myofibrillar damage following intense eccentric exercise in man. Int. J. Sports Med. 1983; 4(3):170–6. [DOI] [PubMed] [Google Scholar]
- 19.Johansson PH, Lindstrom L, Sundelin G, Lindstrom B. The effects of preexercise stretching on muscular soreness, tenderness and force loss following heavy eccentric exercise. Scand. J. Med. Sci. Sports. 1999; 9(4):219–25. [DOI] [PubMed] [Google Scholar]
- 20.Ferreira-Junior JB, Bottaro M, Loenneke JP, Vieira A, Vieira CA, Bemben MG. Could whole-body cryotherapy (below −100°C) improve muscle recovery from muscle damage? Front. Physiol. 2014; 5:247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kong PW, Chua YH, Kawabata M, Burns SF, Cai C. Effect of post-exercise massage on passive muscle stiffness measured using myotonometry — a double-blind study. J. Sports Sci. Med. 2018; 17(4):599–606. [PMC free article] [PubMed] [Google Scholar]
- 22.Dupuy O, Douzi W, Theurot D, Bosquet L, Dugué B. An evidence-based approach for choosing post-exercise recovery techniques to reduce markers of muscle damage, soreness, fatigue, and inflammation: a systematic review with meta-analysis. Front. Physiol. 2018; 9:403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Haas C, Butterfield TA, Abshire S, et al. Massage timing affects postexercise muscle recovery and inflammation in a rabbit model. Med. Sci. Sports Exerc. 2013; 45(6):1105–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cezar CA, Roche ET, Vandenburgh HH, Duda GN, Walsh CJ, Mooney DJ. Biologic-free mechanically induced muscle regeneration. Proc. Natl. Acad. Sci. U. S. A. 2016; 113(6):1534–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Suskind MI, Hajek NM, Hines HM. Effects of massage on denervated skeletal muscle. Arch. Phys. Med. Rehabil. 1946; 27:133–5. [PubMed] [Google Scholar]
- 26.Wood EC, Kosman AJ, Osborne SL. Effects of massage in delaying atrophy in denervated skeletal muscle of the dog. Phys. Ther. Rev. 1948; 28(6):284. [DOI] [PubMed] [Google Scholar]
- 27.Balogh K. Corrective massage for atrophic masticatory and mimetic muscles. Dent. Dig. 1970; 76(8):347–8. [PubMed] [Google Scholar]
- 28.Kyparos A, Feeback DL, Layne CS, Martinez DA, Clarke MS. Mechanical stimulation of the plantar foot surface attenuates soleus muscle atrophy induced by hindlimb unloading in rats. J. Appl. Physiol. 2005; 99(2):739–46. [DOI] [PubMed] [Google Scholar]
- 29.Mei RJ, Xu YY, Li Q. Experimental study on mechanical vibration massage for treatment of brachial plexus injury in rats. J. Tradit. Chin. Med. 2010; 30(3):190–5. [DOI] [PubMed] [Google Scholar]
- 30.Crane JD, Ogborn DI, Cupido C, et al. Massage therapy attenuates inflammatory signaling after exercise-induced muscle damage. Sci Transl Med. 2012; 4(119):119ra13. [DOI] [PubMed] [Google Scholar]
- 31.Saitou K, Tokunaga M, Yoshino D, et al. Local cyclical compression modulates macrophage function in situ and alleviates immobilization-induced muscle atrophy. Clin. Sci. (Lond.). 2018; 132(19):2147–61. [DOI] [PubMed] [Google Scholar]
- 32.Figueiredo VC, McCarthy JJ. Regulation of ribosome biogenesis in skeletal muscle hypertrophy. Physiology (Bethesda) 2019; 34(1):30–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kirby TJ, Lee JD, England JH, Chaillou T, Esser KA, McCarthy JJ. Blunted hypertrophic response in aged skeletal muscle is associated with decreased ribosome biogenesis. J. Appl. Physiol. 2015; 119(4):321–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.West DWD, Marcotte GR, Chason CM, et al. Normal ribosomal biogenesis but shortened protein synthetic response to acute eccentric resistance exercise in old skeletal muscle. Front. Physiol. 2018; 9:1915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Haddad F, Adams GR, Bodell PW, Baldwin KM. Isometric resistance exercise fails to counteract skeletal muscle atrophy processes during the initial stages of unloading. J. Appl. Physiol. 2006; 100(2):433–41. [DOI] [PubMed] [Google Scholar]
- 36.Kirby TJ. Mechanosensitive pathways controlling translation regulatory processes in skeletal muscle and implications for adaptation. J. Appl. Physiol. 2019; 127(2):608–18. [DOI] [PubMed] [Google Scholar]
- 37.Boppart MD, Mahmassani ZS. Integrin signaling: linking mechanical stimulation to skeletal muscle hypertrophy. Am. J. Physiol. Cell Physiol.2019; 317(4):C629–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ogasawara R, Nakazato K, Sato K, Boppart MD, Fujita S. Resistance exercise increases active MMP and β1-integrin protein expression in skeletal muscle. Physiol. Rep. 2014; 2(11):e12212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Marino JS, Tausch BJ, Dearth CL, et al. Beta2-integrins contribute to skeletal muscle hypertrophy in mice. Am. J. Physiol. Cell Physiol. 2008; 295(4): C1026–36. [DOI] [PubMed] [Google Scholar]
- 40.Zou K, Meador BM, Johnson B, et al. The α7β1 -integrin increases muscle hypertrophy following multiple bouts of eccentric exercise. J. Appl. Physiol. 2011; 111(4):1134–41. [DOI] [PubMed] [Google Scholar]
- 41.Wang N, Tytell JD, Ingber DE. Mechanotransduction at a distance: mechanically coupling the extracellular matrix with the nucleus. Nat. Rev. Mol. Cell Biol. 2009; 10(1):75–82. [DOI] [PubMed] [Google Scholar]
- 42.Murach KA, Fry CS, Kirby TJ, et al. Starring or supporting role? Satellite cells and skeletal muscle fiber size regulation. Physiology (Bethesda) 2018; 33(1):26–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hunt ER, Confides AL, Abshire SM, Dupont-Versteegden EE, Butterfield TA. Massage increases satellite cell number independent of the age-associated alterations in sarcolemma permeability. Physiol. Rep. 2019; 7(17):e14200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fry CS, Kirby TJ, Kosmac K, McCarthy JJ, Peterson CA. Myogenic progenitor cells control extracellular matrix production by fibroblasts during skeletal muscle hypertrophy. Cell Stem Cell. 2017; 20(1):56–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Smith LR, Cho S, Discher DE. Stem cell differentiation is regulated by extracellular matrix mechanics. Physiology (Bethesda). 2018; 33(1):16–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Andrushko JW, Lanovaz JL, Björkman KM, Kontulainen SA, Farthing JP. Unilateral strength training leads to muscle-specific sparing effects during opposite homologous limb immobilization. J. Appl. Physiol. 2018; 124(4):866–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Farthing JP, Krentz JR, Magnus CR. Strength training the free limb attenuates strength loss during unilateral immobilization. J. Appl. Physiol. 2009; 106(3):830–6. [DOI] [PubMed] [Google Scholar]
- 48.Magnus CR, Barss TS, Lanovaz JL, Farthing JP. Effects of cross-education on the muscle after a period of unilateral limb immobilization using a shoulder sling and swathe. J. Appl. Physiol. 2010; 109(6):1887–94. [DOI] [PubMed] [Google Scholar]
- 49.Pearce AJ, Hendy A, Bowen WA, Kidgell DJ. Corticospinal adaptations and strength maintenance in the immobilized arm following 3 weeks unilateral strength training. Scand. J. Med. Sci. Sports. 2013; 23(6):740–8. [DOI] [PubMed] [Google Scholar]
- 50.Mason J, Frazer AK, Horvath DM, et al. Ipsilateral corticomotor responses are confined to the homologous muscle following cross-education of muscular strength. Appl. Physiol. Nutr. Metab. 2018; 43(1):11–22. [DOI] [PubMed] [Google Scholar]
- 51.Frazer AK, Pearce AJ, Howatson G, Thomas K, Goodall S, Kidgell DJ. Determining the potential sites of neural adaptation to cross-education: implications for the cross-education of muscle strength. Eur. J. Appl. Physiol. 2018; 118(9):1751–72. [DOI] [PubMed] [Google Scholar]
- 52.Vechetti IJ Jr. Emerging role of extracellular vesicles in the regulation of skeletal muscle adaptation. J. Appl. Physiol. 2019; 127(2):645–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Piccirillo R. Exercise-induced myokines with therapeutic potential for muscle wasting. Front. Physiol. 2019; 10:287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fry CS, Drummond MJ, Glynn EL, et al. Aging impairs contraction-induced human skeletal muscle mTORC1 signaling and protein synthesis. Skelet Muscle. 2011; 1(1):11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Funai K, Parkington JD, Carambula S, Fielding RA. Age-associated decrease in contraction-induced activation of downstream targets of Akt/mTor signaling in skeletal muscle. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2006; 290(4):R1080–6. [DOI] [PubMed] [Google Scholar]
- 56.Burd NA, Gorissen SH, van Loon LJ. Anabolic resistance of muscle protein synthesis with aging. Exerc. Sport Sci. Rev. 2013; 41(3):169–73. [DOI] [PubMed] [Google Scholar]
- 57.Rasmussen BB, Fujita S, Wolfe RR, et al. Insulin resistance of muscle protein metabolism in aging. FASEB J. 2006; 20(6):768–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lacraz G, Rouleau AJ, Couture V, et al. Increased stiffness in aged skeletal muscle impairs muscle progenitor cell proliferative activity. PLoS One. 2015; 10(8):e0136217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hornberger TA, Mateja RD, Chin ER, Andrews JL, Esser KA. Aging does not alter the mechanosensitivity of the p38, p70S6k, and JNK2 signaling pathways in skeletal muscle. J. Appl. Physiol. 2005; 98(4):1562–6. [DOI] [PubMed] [Google Scholar]
- 60.White JR, Confides AL, Moore-Reed S, Hoch JM, Dupont-Versteegden EE. Regrowth after skeletal muscle atrophy is impaired in aged rats, despite similar responses in signaling pathways. Exp. Gerontol. 2015; 64:17–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Miller BF, Baehr LM, Musci RV, et al. Muscle-specific changes in protein synthesis with aging and reloading after disuse atrophy. J. Cachexia. Sarcopenia Muscle. 2019; 10(6):1195–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lawrence MM, Van Pelt DW, Confides AL, et al. Muscle from aged rats is resistant to mechanotherapy during atrophy and reloading. Geroscience. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Conboy IM, Conboy MJ, Smythe GM, Rando TA. Notch-mediated restoration of regenerative potential to aged muscle. Science. 2003; 302(5650):1575–7. [DOI] [PubMed] [Google Scholar]
- 64.Van Pelt DW, Confides AL, Judge AR, Vanderklish PW, Dupont-Versteegden EE. Cold shock protein RBM3 attenuates atrophy and induces hypertrophy in skeletal muscle. J. Muscle Res. Cell Motil. 2018; 39(1–2):35–40. [DOI] [PubMed] [Google Scholar]
- 65.Dodd SL, Hain B, Senf SM, Judge AR. Hsp27 inhibits IKKbeta-induced NF-kappaB activity and skeletal muscle atrophy. FASEB J. 2009; 23(10):3415–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Senf SM, Dodd SL, McClung JM, Judge AR. Hsp70 overexpression inhibits NF-kappaB and Foxo3a transcriptional activities and prevents skeletal muscle atrophy. FASEB J. 2008; 22(11):3836–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bickel CS, Cross JM, Bamman MM. Exercise dosing to retain resistance training adaptations in young and older adults. Med. Sci. Sports Exerc. 2011; 43(7):1177–87. [DOI] [PubMed] [Google Scholar]


