Abstract
Study Objectives:
Although obstructive sleep apnea (OSA) is a known risk factor for atrial fibrillation (AF), there is a paucity of data around its diagnosis and management in patients with AF. The objectives of this study were to compare the diagnostic accuracy of commonly used OSA screening tools in an AF population, including a level 3 portable sleep study device, and to examine the epidemiology of OSA in a hospital cohort with AF.
Methods:
One hundred seven patients with AF recruited from 2 tertiary centers underwent a panel of OSA screening tools and in-laboratory polysomnography in randomized order.
Results:
Oxygen desaturation index derived from a level 3 portable sleep study device performed best for moderate to severe and severe OSA, with excellent diagnostic accuracy (area under the curve, 0.899; 95% confidence interval, 0.838–0.960 and area under the curve, 0.925; 95% confidence interval, 0.859–0.991, respectively). Sixty-seven patients (62.6%) were newly diagnosed with OSA (31.8% mild, 18.7% moderate, 12.1% severe).
Conclusions:
Undiagnosed OSA is highly prevalent in a hospital AF cohort. However, it is characterized by a relative paucity of symptoms, markedly limiting the usefulness of history or screening questionnaires. This is the first study to find that a level 3 home sleep study device shows excellent diagnostic accuracy in patients with AF. This finding may inform AF management guidelines.
Clinical Trial Registration:
Registry: Australian New Zealand Clinical Trials Registry; Name: The validity and reliability of a portable device for the diagnosis of Obstructive Sleep Apnoea in patients with Atrial Fibrillation; URL:https://www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=371024; Identifier: ACTRN12616001016426.
Citation:
Mohammadieh AM, Sutherland K, Kanagaratnam LB, Whalley DW, Gillett MJ, Cistulli PA. Clinical screening tools for obstructive sleep apnea in a population with atrial fibrillation: a diagnostic accuracy trial. J Clin Sleep Med. 2021;17(5):1015–1024.
Keywords: polygraphy, level 3 sleep study, apnea-hypopnea index, oxygen desaturation index, ApneaLink
BRIEF SUMMARY
Current Knowledge/Study Rationale: Obstructive sleep apnea (OSA) is a known risk factor for atrial fibrillation (AF). However, international AF guidelines lack clarity around the approach to the diagnosis and management of OSA.
Study Impact: Our study shows that traditional OSA symptoms including snoring, self-reported sleepiness, and obesity are inadequate for the detection of moderate to severe OSA in an AF population. A level 3 portable sleep study device showed excellent diagnostic accuracy in a hospital-based AF population and may be useful as a screening tool in patients with AF.
INTRODUCTION
The association between obstructive sleep apnea (OSA) and atrial fibrillation (AF) is well described.1–3 Studies have consistently shown a higher prevalence of OSA among patients with AF patients as compared with control patients—for example, 62% vs 38%.4 Animal and human experimental studies have confirmed that OSA increases AF inducibility, both acutely, in response to individual apneic events, and via chronic atrial remodeling pathways.5–7 Several nonrandomized studies have reported that OSA increases the risk of AF recurrence after cardioversion and ablative procedures, a risk that appears to be mitigated by effective OSA treatment.8–12 OSA is an independent risk factor for ischemic stroke in patients with AF.13
Although clinical guidelines acknowledge the role of OSA as a risk factor for AF, they lack clarity in the approach to its diagnosis and management.14–17 Guidelines from the American Heart Association/American College of Cardiology/Heart Rhythm Society suggest that weight loss is recommended for patients with AF who are obese along with modification of risk factors, including OSA.16 However, the diagnostic strategy to identify OSA is not elaborated in the guidelines, and the treatment of OSA in patients who are not obese is not discussed. Recently published guidelines from the European Heart Rhythm Association/European Society of Cardiology (EHRA/ESC) recommend that optimal management of OSA may be considered to reduce AF incidence, progression, recurrence, and symptoms. However, they also report a gap in current knowledge about how and when to test for OSA in patients with AF.17 These inconsistencies highlight a paucity of evidence around the screening and management of OSA in patients with AF.
The gold standard investigation for the diagnosis of OSA, in-laboratory polysomnography (PSG), is a resource-intensive investigation requiring overnight admission in a specialist center and may be inaccessible to many patients. In practice, patients with AF may be referred for PSG at the discretion of their treating physician on the basis of clinical suspicion of OSA. However, it is unclear how to identify which patients with AF are most at risk of OSA. A recently published study highlighted the prevalence of undiagnosed OSA in patients awaiting AF ablation and concluded that more research is required to identify the optimal method to test for sleep apnea in patients with AF.18
The primary aim of this study was to evaluate the validity of a number of commonly used OSA screening tools in patients with AF, including snoring, obesity, daytime hypersomnolence, and airway crowding using symptom-based questionnaires and a level 3 portable sleep study performed at home. A secondary aim was to examine the epidemiology of OSA in a hospital cohort with AF.
METHODS
Approval was obtained from the Northern Sydney (Australia) Local Health District Human Research Ethics Committee (HREC/16/HAWKE/25) at Royal North Shore Hospital (Sydney) and the North Shore Private Hospital (Sydney) Ethics Committee (approval number 2016-012). All patients gave their informed written consent to participate in the study. The trial was registered with the Australian New Zealand Clinical Trials Registry (Identifier: 12616001016426).
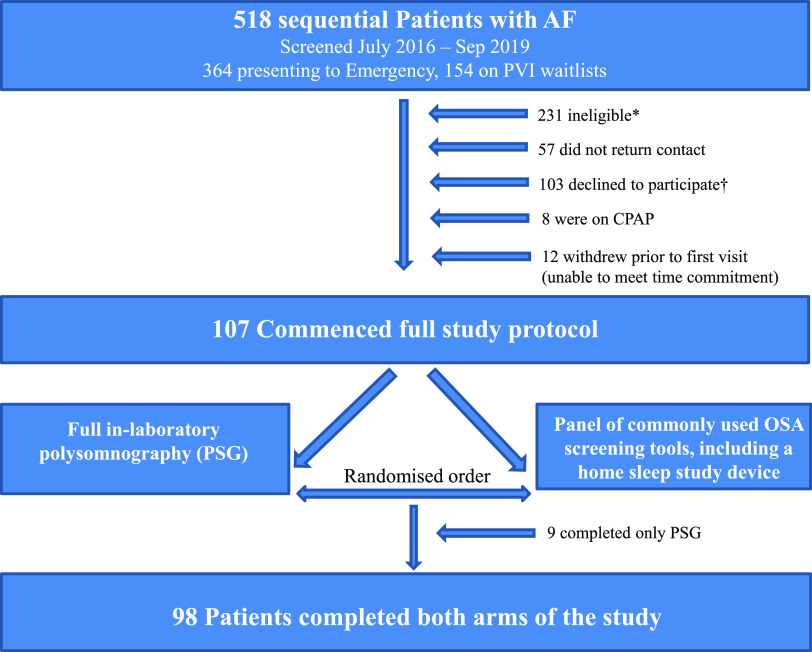
To compare the presence and severity of OSA across clinical AF phenotypes, patients were recruited via 2 hospital-based clinical pathways: (1) emergency department (ED) presentations with confirmed AF and (2) pulmonary vein isolation (PVI) waitlist in the departments of cardiology at 2 specialist centers. All patients had an electrocardiogram-documented history of ≥ 1 episode of AF in the last 12 months. Patients waiting for PVI were sequentially recruited over the duration of the study period (July 2016–September 2019). Because of large numbers of ED presentations during the study period, patients in the ED were sequentially recruited within 3 discrete time intervals during the study period (July 2016–November 2016, September 2017–February 2018, and July 2018–December 2018). Exclusion criteria are outlined in the study flowchart (Figure 1).
Figure 1. Study flowchart.
*Reasons for study ineligibility (n = 231): 67 (29.0%) patients had significant geriatric health issues (eg, falls, frequent urinary tract infections, impaired mobility); 43 (18.6%) had no definite history of documented AF; 29 (12.6%) had active malignancy; 25 (10.8%) had repeat presentation; 24 (10.4%) were geographically remote and unable to travel to the study; 12 (5.2%) had end-stage organ disease; 8 (3.5%) had significant cognitive impairment; 7 (3.0%) had significant mental illness; 6 (2.6%) had active substance abuse; 1 (0.4%) was deceased; 1 (0.4%) was intubated; and 8 (3.5%) were otherwise unable to provide written informed consent. †Patient-reported reasons for declining study participation (n = 103): 30 (29.1%) patients had no reason given; 22 (21.4%) were too busy/had no time; 16 (15.6%) were not interested; 9 (8.7%) said a diagnostic sleep study was already performed; 7 (6.8%) were too unwell to participate/had too many medical appointments; 6 (5.8%) had no OSA symptoms; 4 (3.9%) were already diagnosed with OSA (not on treatment); and 10 (1.0%) disclosed “other reason.” AF = atrial fibrillation, CPAP = continuous positive airway pressure, OSA = obstructive sleep apnea, PSG = polysomnography, PVI = pulmonary vein isolation.
All patients undertook a baseline interview for the collection of demographic and anthropometric data including age, sex, ethnicity, body mass index, and neck circumference. Comorbidities and AF risk factors were documented from patient interviews and corroborating medical record reviews, including the presence of diabetes, ischemic heart disease, hypertension, congestive cardiac failure, CHA2DS2-VASc (congestive heart failure, hypertension, age ≥ 75 years, diabetes mellitus, stroke or transient ischemic attack (TIA), vascular disease, age 65 to 74 years, sex category) score a predictor of stroke risk in atrial fibrillation, AF type (paroxysmal vs persistent/permanent), family history of AF, thyroid disease, and alcohol excess. Paroxysmal AF was defined as an episode of AF self-terminating within 7 days. Other forms of AF were considered as persistent/permanent. Echocardiographic data, when available, were reviewed for patient ejection fraction, atrial size, and valvulopathies.
All patients underwent a panel of commonly used OSA screening tools and full in-laboratory PSG as the diagnostic reference within a week of one another, in randomized order. Randomization was conducted using computer-generated random-number software. The presence and severity of OSA was assessed in all patients with AF. The diagnostic accuracy of each screening tool was compared to in-laboratory PSG for various levels of OSA severity. OSA screening tools were as follows: the presence of patient-reported snoring, obesity (body mass index ≥ 30 kg/m2), airway crowding (modified Mallampati score ≥ 3), self-reported daytime somnolence as measured by the Epworth Sleepiness Scale, the STOP-BANG questionnaire score, the Berlin questionnaire “high risk” category, and results from a level 3 portable home sleep apnea test (HSAT) (ApneaLink Air, ResMed Ltd, San Diego) performed in the patient’s home. Clinical screening tools are summarized in Table S1 (150.4KB, pdf) in the supplemental material. Test characteristics (sensitivity, specificity, positive and negative predictive values, and receiver operating characteristic areas under the curve [AUC]) were compared with the results from PSG.
PSG
PSG was performed and analyzed in a single tertiary referral center by experienced technicians who were blinded to the screening tool results. PSG was scored according to American Academy of Sleep Medicine 2012 criteria.19 An apnea was defined as a complete (≥ 90%) reduction in airflow, lasting ≥ 10 seconds. A hypopnea was defined as a partial (≥ 30%) reduction in airflow, lasting ≥ 10 seconds, associated with either an arousal from sleep or an oxygen desaturation of ≥ 3% from baseline.
Sleep apnea severity definitions
The apnea-hypopnea index (AHI) is the primary metric used to classify sleep apnea severity. It is defined as the number of apneas plus hypopneas occurring per hour of sleep time (for PSG) or recording time (for level 3 or 4 devices that do not have electroencephalogram capability and are therefore unable to detect the presence of sleep). An AHI of 5 to < 15 events/h is classified as mild OSA, an AHI of 15 to < 30 events/h is classified as moderate OSA, and an AHI ≥ 30 events/h is classified as severe OSA.
Level 3 portable sleep study device
The ApneaLink Air device (ResMed) is a portable, level 3 sleep study device comprising 4 channels: 1. heart rate, 2. oxygen saturation (both captured via pulse oximeter), 3. airflow via a nasal cannula pressure transducer, and 4. respiratory effort measured via a chest band with a pneumatic sensor. Application of the device was shown to patients in a clinic setting, after which patients were issued with a loan device to use overnight in their own home. Automated software analysis was used to derive sleep parameters including the AHI and a 3% oxygen desaturation index (ODI). The ODI, measured by pulse oximetry, is the average number of oxygen desaturations (3%) from baseline per hour of sleep time (for PSG) or recording time (for level 3 or 4 devices). ApneaLink automatic scoring software has previously shown good diagnostic accuracy compared with concurrent PSG in a sleep center population (AUC, 0.87; standard error, 0.06).20
Patient-centered assessments
A subset of patients (n = 29) completed paired visual analog questionnaires relating to patient-centered perceived qualities of in-laboratory PSG vs a level 3 portable sleep study device (questionnaire reproduced in Figure S1 (150.4KB, pdf) ). Outcomes included test comfort, test convenience, perceived similarity to the patient’s usual sleep pattern during the test, and patient confidence in the test results.
Statistical analysis
Data are presented as the mean ± standard deviation or as a percentage of the total group. Continuous variables were compared between dichotomous groups using independent t tests. The Levene test was used to assess the homogeneity of variances. Categorical variables were compared using χ2 tests or the Fisher exact test, as applicable. A P value of < .05 was considered statistically significant. Sensitivities, specificities, receiver operating characteristic curves, and positive and negative predictive values were calculated using the statistical software package IBM SPSS Statistics for Windows, Armonk, NY. Receiver operating characteristic AUC was used to assess diagnostic accuracy according to the following thresholds: excellent, 0.9–1.0; good, 0.8–0.9; fair, 0.7–0.8; poor, 0.6–0.7; very poor, 0.5–0.6.
Sample size calculation
Sample size estimates were obtained by evaluating confidence intervals (CIs) for likelihood ratios for diagnostic test studies.21 We hypothesized that ApneaLink would be clinically useful as a screening tool if the upper limit of the CI for the negative likelihood ratio was no greater than 0.2. We used an estimated AF prevalence of 65%, based on a previous study of OSA in an Australian AF population (although the study was limited to patients with AF with a normal ejection fraction).4 The sensitivity and specificity for the ApneaLink device for the detection of moderate or severe OSA (AHI > 15) have been documented at 0.947 and 1.0, respectively, in a non-AF population.22 Therefore, we required at least 40 patients with OSA and 40 patients without OSA to complete the final analysis. Considering the expected prevalence of 65%, a total of 88 patients was required. To offset an expected withdrawal rate of up to 20%, we aimed to recruit a total of 110 patients for the study.
RESULTS
The study flowchart is outlined in Figure 1. Baseline characteristics are summarized in Table 1 by the presence of moderate to severe OSA. Baseline characteristics are also reported in the supplemental material by recruitment stream (ED vs PVI, Table S2 (150.4KB, pdf) ) and according to OSA of differing severities (Table S3 (150.4KB, pdf) and Table S4 (150.4KB, pdf) ). A Bland-Altman plot comparing AHI from PSG and from ApneaLink is presented in Figure S2 (150.4KB, pdf) .
Table 1.
Baseline characteristics of patients with AF with and without moderate to severe OSA (AHI ≥ 15 events/h).
| Characteristic | Total (n = 107) | AHI < 15/h (n = 74) | AHI ≥ 15/h (n = 33) | P Value |
|---|---|---|---|---|
| General demographics | ||||
| Recruitment stream: ED | 58 (54.2) | 40 (54.1) | 18 (54.5) | .962 |
| Age (y) | 61.3 ± 11.7 | 60.2 ± 12.1 | 63.88 ± 10.3 | .130 |
| Male | 70 (65.4) | 45 (60.8) | 25 (75.8) | .133 |
| Ethnicity: Caucasian | 99 (92.5) | 68 (91.2) | 31 (93.9) | .870 |
| Phenotypic characteristics | ||||
| BMI (kg/m2) | 27.2 ± 4.2 | 25.8 ± 3.3 | 30.4 ± 4.2 | < .001 |
| Neck circumference, cm (n = 105) | 40.0 ± 4.7 | 39.1 ± 3.9 | 42.1 ± 5.8 | .003 |
| Modified Mallampati score (n = 106) | 2.7 ± 0.9 | 2.5 ± 0.9 | 3.1 ± 0.7 | .001 |
| OSA symptoms | ||||
| ESS score | 6.1 (3.4) | 5.7 ± 3.3 | 6.9 ± 3.4 | .079 |
| Self-reported snoring | 69 (64.5) | 43 (58.1) | 26 (78.8) | .039 |
| Comorbidities/AF risk factors | ||||
| Alcohol excess* (n = 105) | 26 (24.2) | 17 (23.3) | 9 (28.1) | .730 |
| Thyroid disease | 17 (15.9) | 15 (20.3) | 2 (6.1) | .135 |
| Family history of AF | 33 (30.8) | 27 (36.5) | 6 (18.9) | .015 |
| Moderate-severe MS/prosthetic heart valve | 3 (2.8) | 2 (2.7) | 1 (3.0) | .865 |
| Hypertension | 44 (41.1) | 22 (29.7) | 22 (66.7) | < .001 |
| Diabetes | 5 (4.7) | 2 (2.7) | 3 (9.1) | .169 |
| IHD | 5 (4.7) | 2 (2.7) | 3 (9.1) | .169 |
| CCF | 18 (16.8) | 8 (10.8) | 10 (30.3) | .013 |
| Cerebrovascular disease | 2 (1.8) | 2 (2.7) | 0 (0) | .476 |
| Peripheral vascular disease | 3 (2.8) | 3 (4.1) | 0 (0) | .327 |
| CHA2DS2-VASc score | 1.6 ± 1.3 | 1.5 ± 1.2 | 2.0 ± 1.4 | .038 |
| AF characteristics | ||||
| Paroxysmal | 102 (95.3) | 73 (98.6) | 29 (87.9) | .015 |
| Persistent/permanent | 5 (4.7) | 1 (1.4) | 4 (12.1) | .015 |
| High burden† | 34 (31.8) | 24 (32.4) | 10 (30.3) | .827 |
| Antiarrhythmic therapy | 88 (82.2) | 60 (81.0) | 28 (84.8) | .638 |
| Anticoagulant therapy | 87 (81.3) | 60 (81.0) | 27 (81.8) | .730 |
| Echocardiographic parameters | ||||
| Cardiac ejection fraction, % (n = 79) | 57.5 ± 8.6 | 58.8 ± 7.7 | 54.6 ± 9.8 | .075 |
| Left atrial diameter, cm (n = 57) | 4.1 ± 0.6 | 4.0 ± 0.6 | 4.3 ± 0.6 | .211 |
| Left atrial area, cm2 (n = 50) | 24.3 ± 5.2 | 23.4 ± 5.2 | 26.6 ± 4.7 | .048 |
| Questionnaires | ||||
| Berlin high-risk score (n = 106) | 44 (41.5) | 19 (25.7) | 25 (75.8) | < .001 |
| STOP-BANG score | 3.5 ± 1.7 | 3.0 ± 1.3 | 4.7 ± 1.7 | < .001 |
| Sleep parameters: from PSG | ||||
| AHI | 13.5 ± 15.5 | 5.2 ± 4.3 | 31.9 ± 15.5 | < .001 |
| ODI | 7.1 ± 10.6 | 2.1 ± 2.3 | 18.4 ± 13.1 | < .001 |
| CAI | 0.6 ± 1.5 | 0.2 ± 0.5 | 1.4 ± 2.5 | < .001 |
Values are presented as mean ± SD or n (%). *Ten or more standard drinks/week. †Ten or more episodes of AF in the last 12 months. AF = atrial fibrillation, AHI = apnea-hypopnea index, BMI = body mass index, CAI = central apnea index, CHA2DS2-VASc = (congestive heart failure, hypertension, age ≥ 75 years, diabetes mellitus, stroke or transient ischemic attack (TIA), vascular disease, age 65 to 74 years, sex category), CCF = congestive cardiac failure, ED = emergency department, ESS = Epworth Sleepiness Scale, IHD = ischemic heart disease, MS = mitral stenosis, ODI = oxygen desaturation index, OSA = obstructive sleep apnea, PSG = polysomnography, PVI = pulmonary vein isolation procedure waitlist.
OSA clinical screening tools
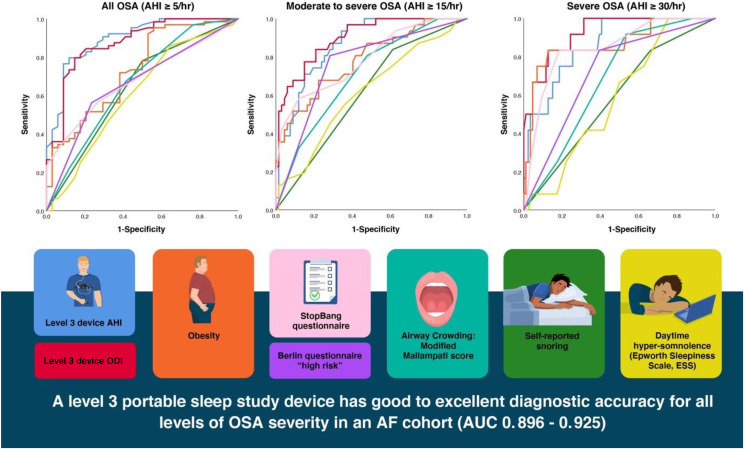
A level 3 HSAT was the only screening tool that performed with good to excellent diagnostic accuracy across all severity categories. AHI derived from a level 3 portable sleep study device performed best for the detection of OSA of any severity (AHI ≥ 5 events/h; AUC, 0.896; 95% CI, 0.830–0.961). ODI derived from a level 3 portable sleep study device performed best for the detection of moderate to severe OSA (AHI ≥ 15 events/h; AUC, 0.899; 95% CI, 0.838–0.960) and for severe OSA only (AHI ≥ 30 events/h; AUC, 0.925; 95% CI, 0.859–0.991).
For moderate to severe OSA, snoring and self-reported hypersomnolence measured via the Epworth Sleepiness Scale both performed with poor diagnostic accuracy. Obesity, a modified Mallampati score ≥ 3, and a STOP-BANG or Berlin questionnaire score in the high-risk category performed with fair diagnostic accuracy.
For severe OSA, 2 screening tools performed with good diagnostic accuracy: obesity (body mass index ≥ 30 kg/m2), with an AUC of 0.865 (95% CI, 0.741–0.990), and a STOP-BANG questionnaire score in the high-risk range (≥ 5; AUC, 0.847; 95% CI, 0.733–0.961). A Berlin questionnaire in the high-risk category performed with fair diagnostic accuracy, and snoring, self-reported hypersomnolence, and a modified Mallampati score ≥ 3 all performed with poor to very poor diagnostic accuracy.
Across all severity categories, the presence of snoring was a highly sensitive but not specific tool for the detection of OSA, although overall the presence of snoring showed poor to very poor diagnostic accuracy. The Epworth Sleepiness Scale also showed poor to very poor diagnostic accuracy across all severity categories. Screening tool diagnostic accuracy characteristics are reported in Table 2 and in Figure 2.
Table 2.
Sensitivity and specificity of clinical OSA screening tools in an AF cohort, at various levels of OSA severity (n = 98).
| Any OSA (n = 64) | Moderate to Severe OSA (n = 31) | Severe OSA (n = 12) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sns (%) | Spc (%) | PPV | NPV | AUC (95% CI) | Sns (%) | Spc (%) | PPV | NPV | AUC (95% CI) | Sns (%) | Spc (%) | PPV | NPV | AUC (95% CI) | |
| Self-reported scoring | 78.1 | 50.0 | 74.6 | 54.8 | 0.641 (0.22–0.522) | 83.9 | 38.8 | 38.8 | 83.9 | 0.613 (0.498–0.729) | 83.3 | 33.8 | 14.9 | 93.5 | 0.585 (0.424–0.746) |
| Obesity (BMI ≥ 30 kg/m2) | 32.8 | 94.1 | 91.3 | 42.7 | 0.732 (0.627–0.837) | 51.6 | 89.6 | 69.6 | 80.0 | 0.785 (0.686–0.883) | 83.3 | 84.9 | 43.5 | 97.3 | 0.865 (0.741–0.990) |
| Modified Mallampati score ≥ 3 | 67.2 | 58.8 | 75.4 | 48.8 | 0.665 (0.548–0.782) | 80.6 | 52.2 | 43.9 | 85.4 | 0.708 (0.602–0.813) | 91.7 | 46.5 | 19.3 | 97.6 | 0.683 (0.554–0.812) |
| Elevated ESS score (≥ 11) | 8.8 | 91.2 | 66.7 | 34.8 | 0.608 (0.487–0.730) | 16.1 | 94.0 | 55.6 | 70.8 | 0.615 (0.495–0.735) | 8.3 | 90.7 | 11.1 | 87.6 | 0.584 (0.441–0.726) |
| STOP-BANG score, intermediate- to high-risk (≥ 3) | 77.6 | 38.5 | 68.4 | 50.0 | 0.580 (0.465–0.695) | 90.9 | 37.0 | 39.5 | 90.0 | 0.639 (0.532–0.747) | 92.3 | 31.2 | 15.8 | 96.7 | 0.617 (0.474–0.761) |
| STOP-BANG score, high-risk (≥ 5) | 36.0 | 91.2 | 88.5 | 43.1 | 0.683 (0.579–0.787) | 58.1 | 88.1 | 69.2 | 82.0 | 0.787 (0.688–0.886) | 83.3 | 81.4 | 38.5 | 97.2 | 0.847 (0.733–0.961) |
| Berlin questionnaire, high-risk | 56.3 | 76.5 | 81.8 | 48.1 | 0.664 (0.552–0.775) | 80.7 | 71.6 | 56.8 | 88.9 | 0.761 (0.659–0.864) | 83.3 | 60.5 | 22.7 | 96.3 | 0.719 (0.577–0.861) |
| Level 3 sleep study: AHI | |||||||||||||||
| Cutoff AHI, 5.15 events/h | 79.7 | 88.2 | 92.7 | 69.8 | 0.896 (0.830–0.961) | ||||||||||
| Cutoff AHI, 8.75 events/h | 80.6 | 74.6 | 59.5 | 89.2 | 0.868 (0.799–0.937) | ||||||||||
| Cutoff AHI, 10.95 events/h | 83.3 | 74.4 | 31.2 | 97.0 | 0.866 (0.774– 0.958) | ||||||||||
| Level 3 sleep study: ODI | |||||||||||||||
| Cutoff ODI, 4.95 | 84.4 | 79.4 | 88.5 | 73.0 | 0.874 (0.799–0.948) | ||||||||||
| Cutoff ODI, 8.30 | 83.9 | 79.1 | 65.0 | 91.4 | 0.899 (0.838–0.960) | ||||||||||
| Cutoff ODI, 13.75 | 83.3 | 87.2 | 47.6 | 97.4 | 0.925 (0.859–0.991) | ||||||||||
Sensitivity and specificity values defined as excellent: 0.9–1.0, good: 0.8–0.9, fair: 0.7–0.8, poor: 0.6–0.7, and very poor: 0.5–0.6. OSA severity defined as: Any OSA (AHI ≥ 5/h), Moderate to severe OSA (AHI ≥ 15/h), Severe OSA (AHI ≥ 30/h). AF = atrial fibrillation, AHI = apnea-hypopnea index, AUC = area under the curve, BMI = body mass index, CI = confidence interval, ESS = Epworth Sleepiness Scale, NPV = negative predictive value, ODI oxygen desaturation index, OSA = obstructive sleep apnea, PPV = positive predictive value, Sns = sensitivity, Spc = specificity.
Figure 2. ROC curves depicting the diagnostic accuracy of OSA screening tools at various levels of severity.
A level 3 portable sleep study device had good to excellent diagnostic accuracy for all levels of OSA severity (AUC, 0.896–0.925). For moderate to severe OSA, snoring and self-reported hypersomnolence measured via the ESS both performed with poor diagnostic accuracy. All ROC AUC results are detailed in Table 2. AF = atrial fibrillation, AHI = apnea-hypopnea index, AUC = area under the curve, ESS = Epworth Sleepiness Scale, ODI = oxygen desaturation index, OSA = obstructive sleep apnea, ROC = receiver operating characteristic.
Patient-centered evaluation of sleep tests
A level 3 HSAT was perceived by patients as significantly more comfortable, more convenient, and more conducive to replicating their usual sleep pattern when compared to in-laboratory PSG (Table 3). There was no difference in patient confidence in the test results.
Table 3.
Self-reported patient assessment of in-laboratory PSG vs a level 3 portable sleep study device at home.
| PSG | Level 3 Device | Mean Difference (95% CI) | P Value | |
|---|---|---|---|---|
| How comfortable did you find the study? | 5.9 ± 2.4 | 7.2 ± 1.7 | −1.2 (−2.3 to −0.2) | .018 |
| How convenient did you find the study? | 7.0 ± 2.5 | 8.2 ± 1.1 | −1.3 (−2.2 to −0.4) | .008 |
| How closely did your sleep on the study night match your normal sleep pattern at home? | 5.1 ± 2.3 | 7.3 ± 2.1 | −2.2 (−3.3 to −1.1) | < .001 |
| How confident were you in the results of the study? | 7.5 ± 1.9 | 7.5 ± 1.7 | 0.0 (−0.8 to 0.8) | .99 |
Results from paired visual analog scales (1–10) in a subset of 29 patients. CI = confidence interval, PSG = polysomnography.
OSA diagnosis
We found that 67 patients (62.6%) were newly diagnosed with OSA (31.8% mild, 18.7% moderate, 12.1% severe). The average AHI in the OSA group fell within the moderate range (20.4 ± 15.8 events/h). There was no significant difference in the presence of moderate to severe OSA between the ED and PVI recruitment streams (31.0% vs 30.1%; P = .962).
DISCUSSION
This is the first study to validate a level 3 HSAT (also known as polygraphy) in an AF population, indicating its superiority to other OSA screening tools. Overall, a level 3 HSAT showed the highest diagnostic accuracy at all levels of OSA severity: mild, moderate, and severe. Previously, these devices have been validated only in patients in sleep clinics or in patients suspected to have OSA.20,22–24 We have shown that patients with AF represent a distinct clinical phenotype when compared with a sleep clinic population and are less likely to have daytime symptoms or obesity, thus supporting the need and clinical relevance of test validation in the target population.
Notably, many traditional OSA risk factors did not perform well as screening tools in this AF cohort, including some that are recommended in clinical guidelines. This finding gives caution to the use of self-reported snoring and daytime hypersomnolence, both of which performed with poor diagnostic accuracy for moderate to severe OSA and with very poor diagnostic accuracy for severe OSA. Similarly, the presence of obesity, which is linked to OSA treatment in the American Heart Association/American College of Cardiology/Heart Rhythm Society guidelines,16 performed with only fair diagnostic accuracy for moderate to severe OSA, whereas it performed with good diagnostic accuracy for severe OSA.
Self-reported hypersomnolence via the Epworth Sleepiness Scale has been shown in 3 prior studies of patients with AF to be poorly predictive of OSA,25–27 although ours is the first study to report the diagnostic accuracy of self-reported snoring and obesity as OSA screening tools in an AF population against the gold standard of PSG.
Diagnostic accuracy of questionnaires and patient-reported symptoms
This study adds to the very limited available data on the diagnostic accuracy of screening questionnaires in patients with AF. Both the STOP-BANG questionnaire scores ≥ 5 and the Berlin questionnaire overall performed with only fair diagnostic accuracy for moderate to severe OSA (AUC, 0.787; 95% CI; 0.688–0.886; AUC, 0.761; 95% CI, 0.659–0.864, respectively), results that are broadly consistent with a previously published study.27 The diminished performance of these questionnaires likely reflects the paucity of traditional OSA symptoms in an AF group.
Despite an AUC of only 0.639, a STOP-BANG score ≥ 3 (intermediate to high risk) had a very high negative predictive value for the exclusion of moderate to severe OSA (negative predictive value, 90.0 for AHI ≥ 15 events/h). This characteristic in particular would make a STOP-BANG score ≥ 3 a useful screening tool for regional or underresourced areas where a level 3 device may not be accessible. In addition, the high negative predictive value lends itself to a tiered screening strategy whereby STOP-BANG could be utilized initially, followed by a level 3 device for those patients with a STOP-BANG score ≥ 3. For every 100 patients screened with this approach, 29 would return a negative STOP-BANG result and therefore could forego the ApneaLink test. However, for every 100 patients screened with this tiered strategy, 3 patients with an AHI > 15 events/h would be missed.
Some screening tools, notably the presence of obesity, performed differently across different levels of OSA severity. This finding raises the question of what level of OSA severity is clinically significant in AF, an issue that is confounded by variable OSA definitions and diagnostic methods in the literature to date. The significance of OSA severity in patients with AF is an area for future research.
Level 3 HSAT (polygraphy) vs overnight oximetry
In this study, ODI and AHI were derived from a level 3 portable sleep study device. Two prior studies assessed ODI from overnight oximetry alone as a screening tool for OSA in patients with cardiac concerns, including 1 study in an AF population.28,29 In a group of 439 patients with AF, Linz et al28 showed an AUC of 0.951 (95% CI, 0.929–0.972) for the detection of moderate to severe OSA. In both of these studies, pulse oximetry data were collected as a component of PSG in a sleep laboratory setting: a highly controlled environment less susceptible to signal loss than the home setting. In addition, when studies are performed concurrently, there is no effect from night-to-night variability. These 2 factors are likely to at least partly explain the slightly lower AUC we found for ODI derived from a level 3 device, performed on a different night than PSG and in the home setting. Our study of ODI via polygraphy, performed in a home setting, represents an important next step for OSA diagnosis in AF patients. Unlike overnight oximetry alone, a level 3 device also has the capacity to distinguish obstructive from central apneas, which may be advantageous in a patient cohort with cardiovascular concerns.
Epidemiology of OSA in a hospital-based AF cohort
We confirm that the prevalence of OSA in patients with AF was very high, with a total of 62.6% of patients newly diagnosed with OSA (AHI ≥ 5 events/h) and 30.8% diagnosed with moderate to severe OSA (AHI ≥ 15 events/h). This is the first study to compare OSA prevalence between two different AF hospital populations, with no significant difference found for moderate to severe OSA. Notably, patients were recruited solely on the presence of AF and not on the basis of any sleep symptoms or OSA risk factors. The OSA prevalence in our cohort was somewhat lower than in a recently published study, which used home peripheral arterial tonometry rather than the gold standard investigation (in-laboratory PSG) to diagnose OSA.18
Despite the very high population prevalence of OSA, only a small proportion of patients (8 out of 230 contacted patients, 4.3%) was excluded on the basis of active OSA treatment. This finding highlights the significant underdiagnosis of OSA in this population and the need for a high index of suspicion among treating clinicians. Furthermore, this finding is consistent with a large cohort study of patients with AF, which found that only 17% had a diagnosis of OSA on the basis of physician report and medical record review, without active OSA screening.30 By contrast, studies that have sequentially screened patients with AF, regardless of OSA symptoms, have identified a much higher prevalence eg 82%18, 81%25,18,25 in keeping with our study.
Taken together, the high population prevalence of OSA among patients with AF, its underdiagnosis in this group, and the paucity of traditional daytime symptoms emphasize the need for a simple, cost-effective, and accurate OSA screening tool in this population.
Patient-centered assessments
Patients’ self-reported experience of a level 3 portable sleep study performed in their own home was that it resulted in sleep more similar to their usual sleep pattern. This assessment is biologically plausible because the level 3 device allows the study to be performed in the patient’s usual sleeping environment, less encumbered by leads. This finding aligns with previous studies showing that in-laboratory PSG artificially increases the severity of sleep-disordered breathing by increasing supine sleep.31 There was also a statistically significant increase in patient comfort and perceived convenience with the level 3 device when compared with in-laboratory PSG.
Limitations
Although our study provides novel findings, we acknowledge some important limitations. We performed PSG on 1 night only, which could not account for variations in sleep apnea severity from night to night, such as those caused by variations in sleep position. Note that the ApneaLink device used in this study does not record sleeping position, so it could not be compared to PSG use. It is possible that this study underestimated the diagnostic accuracy of a level 3 HSAT because it was performed on a different night and different setting than PSG. However, using an HSAT is necessary in a real-world scenario, so to allow for translation to clinical practice, we performed the portable sleep study in the patient’s own home rather than the sleep laboratory.
Although we documented the patient reasons given (Figure 1) for the 20% who declined to participate, it is possible that this percentage introduced a bias into the study that may have impacted the prevalence data in particular. We cannot exclude the possibility that patients with OSA symptoms or risk factors may have been more likely to participate. We recruited patients with AF from 2 streams: ED presentations and PVI waitlists. Because there were some significant differences between these groups (Table S2 (150.4KB, pdf) ), a bias may have been introduced. We employed this strategy to replicate a clinically relevant range of patients with AF and to allow comparisons between AF groups. Finally, our study does not address the question of whether treating OSA in patients with AF improves health outcomes, a critically important factor when considering screening strategies, and this inquiry warrants further research. Randomized control trials addressing this question are ongoing.
CONCLUSIONS
Our study shows that traditional OSA symptoms including snoring, self-reported sleepiness, and obesity are inadequate for the detection of moderate to severe OSA in an AF population. A level 3 HSAT showed excellent diagnostic accuracy in a hospital-based AF population and may be useful as a screening tool in patients with AF. Testing with a level 3 HSAT was perceived by patients as more comfortable and convenient and more closely matched to their usual sleep pattern than in-laboratory PSG.
DISCLOSURE STATEMENT
All authors have seen and approved the manuscript. Work for this study was performed at the Royal North Shore Hospital, Sydney, Australia, and the Charles Perkins Centre, University of Sydney, Sydney, Australia. AMM received research grants from the Ramsay Research and Teaching Fund, Sydney, Australia, and the ResMed Foundation/Sleep Health Foundation Research Entry Scholarship administered through the Royal Australasian College of Physicians, Sydney, Australia. AMM reports that in-kind support for this study (loan of ApneaLink Air diagnostic study devices) was received from Resmed Pty Ltd, Sydney, Australia. Resmed played no role in the design or implementation of the study. PAC has an appointment to an endowed academic chair at the University of Sydney that was created from ResMed funding; he receives no personal fees, and this relationship is managed by an oversight committee of the university. PAC has received research support from ResMed, SomnoMed, Zephyr Sleep Technologies, and Bayer; is a consultant/adviser to Zephyr Sleep Technologies, ResMed, SomnoMed, and Signifier Medical Technologies; and has a pecuniary interest in SomnoMed related to a previous role in research and development. KS reports in-kind support from SomnoMed in donation of oral appliances for a previous investigator-initiated research study. The remaining authors report no conflicts of interest.
SUPPLEMENTARY MATERIAL
ACKNOWLEDGMENTS
The authors gratefully acknowledge the following contributors: Mohammad Ahmadi, Gary Cohen, Aimee Lowth, Melanie Madronio, Bernie McGrath, Tessa Milne, Magas Ragupathy, Nina Sarkissian, and William Wood.
ABBREVIATIONS
- AF
atrial fibrillation
- AHI
apnea-hypopnea index
- AUC
area under the curve
- CI
confidence interval
- ED
emergency department
- HSAT
home sleep apnea test
- ODI
oxygen desaturation index
- OSA
obstructive sleep apnea
- PSG
polysomnography
- PVI
pulmonary vein isolation
REFERENCES
- 1.Gami AS, Pressman G, Caples SM, et al. Association of atrial fibrillation and obstructive sleep apnea. Circulation. 2004;110(4):364–367. 10.1161/01.CIR.0000136587.68725.8E [DOI] [PubMed] [Google Scholar]
- 2.Mehra R, Benjamin EJ, Shahar E, et al. Association of nocturnal arrhythmias with sleep-disordered breathing: the Sleep Heart Health Study. Am J Respir Crit Care Med. 2006;173(8):910–916. 10.1164/rccm.200509-1442OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gami AS, Hodge DO, Herges RM, et al. Obstructive sleep apnea, obesity, and the risk of incident atrial fibrillation. J Am Coll Cardiol. 2007;49(5):565–571. 10.1016/j.jacc.2006.08.060 [DOI] [PubMed] [Google Scholar]
- 4.Stevenson IH, Teichtahl H, Cunnington D, Ciavarella S, Gordon I, Kalman JM. Prevalence of sleep disordered breathing in paroxysmal and persistent atrial fibrillation patients with normal left ventricular function. Eur Heart J. 2008;29(13):1662–1669. 10.1093/eurheartj/ehn214 [DOI] [PubMed] [Google Scholar]
- 5.Iwasaki YK, Kato T, Xiong F, et al. Atrial fibrillation promotion with long-term repetitive obstructive sleep apnea in a rat model. J Am Coll Cardiol. 2014;64(19):2013–2023. 10.1016/j.jacc.2014.05.077 [DOI] [PubMed] [Google Scholar]
- 6.Linz D, Schotten U, Neuberger HR, Böhm M, Wirth K. Negative tracheal pressure during obstructive respiratory events promotes atrial fibrillation by vagal activation. Heart Rhythm. 2011;8(9):1436–1443. 10.1016/j.hrthm.2011.03.053 [DOI] [PubMed] [Google Scholar]
- 7.Dimitri H, Ng M, Brooks AG, et al. Atrial remodeling in obstructive sleep apnea: implications for atrial fibrillation. Heart Rhythm. 2012;9(3):321–327. 10.1016/j.hrthm.2011.10.017 [DOI] [PubMed] [Google Scholar]
- 8.Kanagala R, Murali NS, Friedman PA, et al. Obstructive sleep apnea and the recurrence of atrial fibrillation. Circulation. 2003;107(20):2589–2594. 10.1161/01.CIR.0000068337.25994.21 [DOI] [PubMed] [Google Scholar]
- 9.Fein AS, Shvilkin A, Shah D, et al. Treatment of obstructive sleep apnea reduces the risk of atrial fibrillation recurrence after catheter ablation. J Am Coll Cardiol. 2013;62(4):300–305. 10.1016/j.jacc.2013.03.052 [DOI] [PubMed] [Google Scholar]
- 10.Patel D, Mohanty P, Di Biase L, et al. Safety and efficacy of pulmonary vein antral isolation in patients with obstructive sleep apnea: the impact of continuous positive airway pressure. Circ Arrhythm Electrophysiol. 2010;3(5):445–451. 10.1161/CIRCEP.109.858381 [DOI] [PubMed] [Google Scholar]
- 11.Shukla A, Aizer A, Holmes D, et al. Effect of obstructive sleep apnea treatment on atrial fibrillation recurrence: a meta-analysis. JACC Clin Electrophysiol. 2015;1(1–2):41–51. 10.1016/j.jacep.2015.02.014 [DOI] [PubMed] [Google Scholar]
- 12.Qureshi WT, Nasir UB, Alqalyoobi S, et al. Meta-analysis of continuous positive airway pressure as a therapy of atrial fibrillation in obstructive sleep apnea. Am J Cardiol. 2015;116(11):1767–1773. 10.1016/j.amjcard.2015.08.046 [DOI] [PubMed] [Google Scholar]
- 13.Yaranov DM, Smyrlis A, Usatii N, et al. Effect of obstructive sleep apnea on frequency of stroke in patients with atrial fibrillation. Am J Cardiol. 2015;115(4):461–465. 10.1016/j.amjcard.2014.11.027 [DOI] [PubMed] [Google Scholar]
- 14.Gorenek B, Pelliccia A, Benjamin EJ, et al. European Heart Rhythm Association (EHRA)/European Association of Cardiovascular Prevention and Rehabilitation (EACPR) position paper on how to prevent atrial fibrillation endorsed by the Heart Rhythm Society (HRS) and Asia Pacific Heart Rhythm Society (APHRS). Eur J Prev Cardiol. 2017;24(1):4–40. 10.1177/2047487316676037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brieger D, Amerena J, Attia J, et al.; NHFA CSANZ Atrial Fibrillation Guideline Working Group . National Heart Foundation of Australia and the Cardiac Society of Australia and New Zealand: Australian Clinical Guidelines for the Diagnosis and Management of Atrial Fibrillation 2018. Heart Lung Circ. 2018;27(10):1209–1266. 10.1016/j.hlc.2018.06.1043 [DOI] [PubMed] [Google Scholar]
- 16.January CT, Wann LS, Calkins H, et al. 2019 AHA/ACC/HRS focused update of the 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Heart Rhythm. 2019;16(8):e66–e93. 10.1016/j.hrthm.2019.01.024 [DOI] [PubMed] [Google Scholar]
- 17.Hindricks G, Potpara T, Dagres N, et al.; ESC Scientific Document Group . 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association of Cardio-Thoracic Surgery (EACTS). Eur Heart J. 2021;42(5):373–498. [DOI] [PubMed] [Google Scholar]
- 18.Shapira-Daniels A, Mohanty S, Contreras-Valdes FM, et al. Prevalence of undiagnosed sleep apnea in patients with atrial fibrillation and its impact on therapy. JACC Clin Electrophysiol. 2020;6(12):1499–1506. 10.1016/j.jacep.2020.05.030 [DOI] [PubMed] [Google Scholar]
- 19.Berry RB, Budhiraja R, Gottlieb DJ, et al. Rules for scoring respiratory events in sleep: update of the 2007 AASM Manual for the Scoring of Sleep and Associated Events. Deliberations of the Sleep Apnea Definitions Task Force of the American Academy of Sleep Medicine. J Clin Sleep Med. 2012;8(5):597–619. 10.5664/jcsm.2172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nigro CA, Dibur E, Malnis S, Grandval S, Nogueira F. Validation of ApneaLink Ox for the diagnosis of obstructive sleep apnea. Sleep Breath. 2013;17(1):259–266. 10.1007/s11325-012-0684-4 [DOI] [PubMed] [Google Scholar]
- 21.Simel DL, Samsa GP, Matchar DB. Likelihood ratios with confidence: sample size estimation for diagnostic test studies. J Clin Epidemiol. 1991;44(8):763–770. 10.1016/0895-4356(91)90128-V [DOI] [PubMed] [Google Scholar]
- 22.Ng SS, Chan TO, To KW, et al. Validation of a portable recording device (ApneaLink) for identifying patients with suspected obstructive sleep apnoea syndrome. Intern Med J. 2009;39(11):757–762. 10.1111/j.1445-5994.2008.01827.x [DOI] [PubMed] [Google Scholar]
- 23.El Shayeb M, Topfer LA, Stafinski T, Pawluk L, Menon D. Diagnostic accuracy of level 3 portable sleep tests vs level 1 polysomnography for sleep-disordered breathing: a systematic review and meta-analysis. CMAJ. 2014;186(1):E25–E51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gottlieb DJ, Punjabi NM. Diagnosis and management of obstructive sleep apnea: a review. JAMA. 2020;323(14):1389–1400. 10.1001/jama.2020.3514 [DOI] [PubMed] [Google Scholar]
- 25.Kadhim K, Middeldorp ME, Elliott AD, et al. Self-reported daytime sleepiness and sleep-disordered breathing in patients with atrial fibrillation: SNOozE-AF. Can J Cardiol. 2019;35(11):1457–1464. 10.1016/j.cjca.2019.07.627 [DOI] [PubMed] [Google Scholar]
- 26.Albuquerque FN, Calvin AD, Sert Kuniyoshi FH, et al. Sleep-disordered breathing and excessive daytime sleepiness in patients with atrial fibrillation. Chest. 2012;141(4):967–973. 10.1378/chest.11-0975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.May AM, Wang L, Kwon DH, et al. Sleep apnea screening instrument evaluation and novel model development and validation in the paroxysmal atrial fibrillation population [published online ahead of print 2020 Sep 4]. Int J Cardiol Heart Vasc. 10.1016/j.ijcha.2020.100624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Linz D, Kadhim K, Brooks AG, et al. Diagnostic accuracy of overnight oximetry for the diagnosis of sleep-disordered breathing in atrial fibrillation patients. Int J Cardiol. 2018;272:155–161. 10.1016/j.ijcard.2018.07.124 [DOI] [PubMed] [Google Scholar]
- 29.Ward NR, Cowie MR, Rosen SD, et al. Utility of overnight pulse oximetry and heart rate variability analysis to screen for sleep-disordered breathing in chronic heart failure. Thorax. 2012;67(11):1000–1005. 10.1136/thoraxjnl-2012-201684 [DOI] [PubMed] [Google Scholar]
- 30.Holmqvist F, Guan N, Zhu Z, et al. Impact of obstructive sleep apnea and continuous positive airway pressure therapy on outcomes in patients with atrial fibrillation—results from the Outcomes Registry for Better Informed Treatment of Atrial Fibrillation (ORBIT-AF). Am Heart J. 2015;169(5):647–654.e2. 10.1016/j.ahj.2014.12.024 [DOI] [PubMed] [Google Scholar]
- 31.Vonk PE, de Vries N, Ravesloot MJL. Polysomnography and sleep position, a Heisenberg phenomenon? A large-scale series. HNO. 2019;67(9):679–684. 10.1007/s00106-019-0678-7 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.