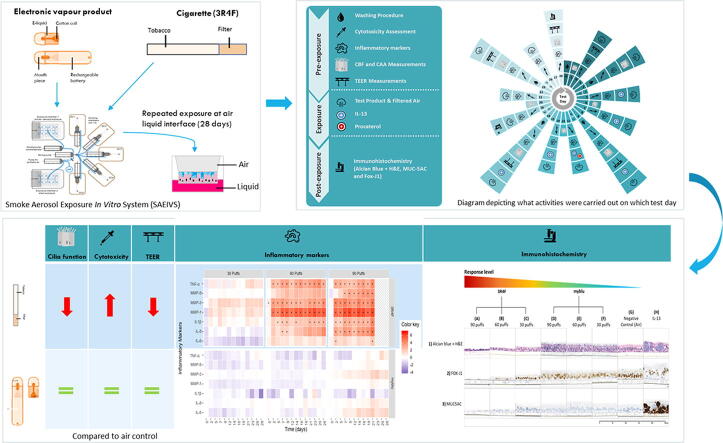
Graphical abstract
Abbreviations: 2D, Two Dimensional; 3D, Three Dimensional; 3R4F, Scientific Reference Tobacco Cigarette (University of Kentucky); ALI, Air-Liquid Interface; ANOVA, Analysis of Variance; AOP, Adverse Outcome Pathway; CBF, Cilia Beat Frequency; CAA, Cilia Active Area; COPD, Chronic Obstructive Pulmonary Disease; CYP450, Cytochrome P450; DPBS, Dulbecco's phosphate-buffered saline containing Ca2+ and Mg2+; EVP, Electronic Vapor Product; EGFR, Epidermal Growth Factor Receptor; FOX-J1, Forkhead Box J1 protein; H&E, Hematoxylin and Eosin; IIVS, Institute for In Vitro Sciences; IL-1β, Interleukin 1 Beta; IL-6, Interleukin-6; IL-8, Interleukin-8; IL-13, Interleukin 13; ISO, International Organization for Standardization; KEs, Key Events; KERs, Key Event Relationships; LDH, Lactate Dehydrogenase; MIE, Molecular Initiating Event; MMP-1, Matrix Metalloproteinase-1; MMP-3, Matrix Metalloproteinase-3; MMP-9, Matrix Metalloproteinase-9; MUC5AC, Mucin 5AC Protein; MWP, Multi-Well Plate; NKT, Natural Killer T Cells; PBS, Phosphate Buffered Saline; PMN, polymorphonuclear; SAEIVS, Smoke Aerosol Exposure In Vitro System; TEER, Transepithelial Electrical Resistance; THR, Tobacco Harm Reduction; TNF-α, Tumor Necrosis Factor Alpha; TPM, Total Particulate Matter
Keywords: Cigarette, Electronic vapor product, Organotypic tissue model, Cilia, Immunohistochemistry, Pro-inflammatory markers
Abstract
Smoking is a cause of serious diseases in smokers including chronic respiratory diseases. This study aimed to evaluate the tobacco harm reduction (THR) potential of an electronic vapor product (EVP, myblu™) compared to a Kentucky Reference Cigarette (3R4F), and assessed endpoints related to chronic respiratory diseases. Endpoints included: cytotoxicity, barrier integrity (TEER), cilia function, immunohistochemistry, and pro-inflammatory markers. In order to more closely represent the user exposure scenario, we have employed the in vitro 3D organotypic model of human airway epithelium (MucilAir™, Epithelix) for respiratory assessment. The model was repeatedly exposed to either whole aerosol of the EVP, or whole 3R4F smoke, at the air liquid interface (ALI), for 4 weeks to either 30, 60 or 90 puffs on 3-exposure-per-week basis. 3R4F smoke generation used the ISO 20778:2018 regime and EVP aerosol used the ISO 20768:2018 vaping regime.
Exposure to undiluted whole EVP aerosol did not trigger any significant changes in the level of pro-inflammatory mediators, cilia beating function, barrier integrity and cytotoxicity when compared with air controls. In contrast, exposure to diluted (1:17) whole cigarette smoke caused significant changes to all the endpoints mentioned above. To our knowledge, this is the first study evaluating the effects of repeated whole cigarette smoke and whole EVP aerosol exposure to a 3D lung model at the ALI. Our results add to the growing body of scientific literature supporting the THR potential of EVPs relative to combustible cigarettes and the applicability of the 3D lung models in human-relevant product risk assessments.
1. Introduction
Chronic respiratory diseases are diseases of the airways and other lung structures (World Health Organization, 2020). Public health bodies report that smoking is a cause of serious diseases in smokers (National Center for Chronic Disease, 2014, World Health Organization, 2020). There is scientific consensus that these detrimental effects of smoking cigarettes are due to the inhalation of toxicants from tobacco combustion and not by nicotine (Institute of Medicine, 2001, Royal College of Physicians, 2016). For this reason, a growing number of public health bodies and governments worldwide have indicated that electronic vapor products (EVPs) are likely to have a major role to play in tobacco harm reduction (THR) for adult smokers who would otherwise continue to smoke. Public Health England estimate that EVPs are at least 95% less harmful than combustible cigarettes (McNeill et al., 2018, McNeill et al., 2015). The best action adult smokers can take to improve their health is to stop tobacco and nicotine use entirely. Numerous public health bodies have encouraged adult cigarette smokers who are uninterested or unwilling to stop cigarette consumption to transition to nicotine-containing products that potentially pose fewer health risks (McNeill et al., 2018, McNeill et al., 2015). EVPs are battery powered devices that deliver an aerosol to the user by heating an e-liquid of known chemical composition, which typically contains propylene glycol and/or glycerol and may contain nicotine and flavorings (Eaton et al., 2018). EVPs do not contain or burn tobacco, therefore EVP aerosol contains substantially fewer and significantly lower levels of harmful chemicals when compared to cigarettes (Rudd et al., 2020, Margham et al., 2016, Kucharska et al., 2016). Recent clinical studies have shown that adult smokers who have transitioned to EVPs, are exposed to substantially fewer and significantly lower levels of carcinogens and toxicants found in cigarette smoke, with levels of biomarkers of exposure largely indistinguishable from complete smoking cessation or use of approved pharmaceutical nicotine replacement products (O’Connell et al., 2016, Goniewicz et al., 2017, Shahab et al., 2017).
The lungs are the primary route of exposure to inhaled compounds, such as tobacco smoke, occupational chemicals, and air pollution. The pseudostratified lung epithelium consists of four primary cell types. These are ciliated cells (50–70%), mucus (MUC5AC and MUC5B) producing goblet cells (25%), basal stem cells and Club cells (~11%), that express club cell-specific 10 kDa secretory proteins with both anti-inflammatory and immune-modulating properties (Rayner et al., 2019a). In situ, the ciliated cells beat in synchronicity and transport mucus up the respiratory tract, where it is swallowed. This ‘mucociliary escalator’ enables clearance in the human lungs and is the first line of defense, to remove inhaled harmful/irritating compounds, pathogens, toxins, and moves non-respirable particles away from the lung surface (Rayner et al., 2019a). With this basic knowledge of the key physiological processes, the functional measurements made in this study focused on alterations to cilia beating, mucus production, histology and cell–cell communication as recommended by the Institute for In Vitro Sciences (IIVS) working group (Behrsing et al., 2016), to pick up changes due to repeated EVP or smoke exposure for 28 days.
Physiologically relevant models of the human airway have been used in many studies to assess the effects of airborne toxicants and the relative safety and biological impact of inhalable products, such as EVP aerosols and cigarette smoke (Iskandar et al., 2016, Iskandar et al., 2019, Murphy et al., 2017, Cervena et al., 2018). These models have also been applied in human disease relevant research, including that into chronic obstructive pulmonary disease (COPD), asthma and cystic fibrosis (Leclercq et al., 2016). The model consists of primary human cells originated either from the nasal, tracheal or bronchial region, and cultured at the ALI. The reconstructed tissues contain basal cells, ciliated cells and goblet cells that mimic the morphological characteristics of human lung epithelium. Additionally, the model is physiologically relevant, with functional beating cilia, the ability to produce mucus, tight junctions, metabolic competence (CYP450s) and the ability to produce inflammatory mediators such as cytokines, chemokines and metalloproteinases (Epithelix, 2020). These characteristics offer many advantages for the in vitro evaluation of functional and mechanistic responses in comparison to conventional 2D submerged cell cultures (Shamir and Ewald, 2014). Organotypic respiratory model combined with ALI exposure resembles a more clinically relevant exposure setup. The distribution and deposition of the smoke/ aerosol can also be mimicked more closely compared to the use of monolayer cell cultures, where a bubbled smoke/ aerosol extract (in an aqueous medium) is added into the culture medium or applied directly on to the cellular surface (Pezzulo et al., 2011, Dvorak et al., 2011). Biologically relevant features such as beating cilia and cell barrier functionality allow a more physiologically relevant assessment of the effects of exposures on overall tissue functioning, therefore contributing to the reduced need for animal testing for such information (Kuehn et al., 2015).
Our previous work has shown that acute exposures of EVP aerosols to a 3D organotypic bronchial tissue at the air liquid interface (ALI) was not cytotoxic and did not result in tissue damage when compared to control conditions (Czekala, et al., 2019). The present study aimed to evaluate the THR potential of EVPs compared to reference cigarettes under the condition of repeated exposure to EVP aerosol or cigarette smoke. Building upon our previous study, the range of endpoints was extended to evaluate those particularly observed in COPD, along with other chronic respiratory diseases. This study intended to deliver sub-cytotoxic concentrations of aerosol or smoke to the tissues on a puff-by-puff basis to determine if any functional impairments could be detected prior to the loss of the model function due to cytotoxicity. The viability of the tissue model and levels of cytotoxicity were assessed based on a range of functional cellular endpoints used by other authors (Haswell et al., 2017, Iskandar et al., 2018, Schamberger et al., 2015, Gagliardo et al., 2016) and recommended following a series of workshops held by the IIVS (Behrsing et al., 2016).
2. Materials and methods
2.1. 3D lung model
MucilAir™ (Epithelix Sàrl, Geneva, Switzerland) tissues were produced using primary cells from a pathology-free, non-smoker, 41-year-old male Caucasian donor (Batch No. MD072001). It is a fully differentiated 3D in vitro reconstituted upper airway human epithelium. Prior to the start of the experiment, the tissues were acclimatized in an incubator at standard culture conditions (37 °C; 5% CO2) for 7 days according to manufacturer instructions. Additionally, to prevent microbial contamination, standard manufacturer’s culture medium (Epithelix Sàrl) was supplemented with 1% Amphotericin B (Sigma-Aldrich; A2942) (final concentration in medium 2.5 µg/ml).
2.2. Test products
The test products were a commercially available EVP (manufactured by Fontem Ventures, the Netherlands) and the 3R4F Kentucky Reference Cigarette (University of Kentucky, Centre for Tobacco Reference Products, Lexington, KY). Commercial EVPs from the brand myblu™ contained 1.6% [w/w] (equivalent to 18 mg/ml [w/v]) nicotine; tobacco flavor and were obtained from the UK market at the time of the study (flavor bulk liquid code V-FL0170). The myblu device is a rechargeable closed pod system: The rechargeable battery has a capacity of 350 mAh and the pod has a 1.5 mL liquid volume and 1.3 Ω coil resistance. The myblu device and e-liquid combination delivers approximately 85 µg nicotine per puff, and a device mass loss of approximately 7.7 mg per puff (Rudd et al., 2020). The comparator cigarette was the Kentucky 3R4F Reference Cigarette (USB tobacco blend with cellulose acetate filter, particulate matter 11 mg/cig, tar 9.4 mg/cig, nicotine 0.73 mg/cig) (lot number V351X61B5).
3R4F cigarettes were stored in an air-tight container at 4 °C until use. The cigarette container was acclimatized to room temperature for 15 min before opening. The cigarettes were then conditioned for at least 48 h in a humidified chamber following the International Organization for Standardization (ISO) 3402 guideline (ISO, 1999). The EVPs were stored at room temperature and the batteries were fully charged before use.
2.3. Smoke and aerosol generation
Fresh, whole smoke/aerosol was generated as detailed in Table 1, using a bespoke 5-port smoking machine, ‘Smoke Aerosol Exposure In Vitro System’ (SAEIVS) (Burghart Tabaktechnik, Wedel, Germany), which consists of the exposure device, smoking chambers and smoke ‘distributers’ (Rudd et al., 2020, Wieczorek et al., 2020). The smoking atmosphere was generated using 3 cigarettes at a time with 10 puffs from each cigarette. The smoke from all parallel puffs, on a per puff basis, were mixed in a mixing pump and diluted with filtered air to achieve the final homogenous concentration. Each 10-puff run was repeated 3 times to achieve the 30-puff dose (lowest reference cigarette dose in the study) with a total of 9 reference cigarettes. The 60-puff dose was achieved with mixing of 18 reference cigarettes and 90-puff dose with 27 cigarettes respectively. For the EVP it was conducted in a similar manner to minimize the possible variation between the devices. Three devices were used to deliver 30/60/90 puffs each, which were then mixed in a mixing pump, without the air dilution, and delivered to the 24 multi-well plate (MWP) as a dose of 30/60/90 homogenous puffs. The maximum achievable number of puffs from the EVP used in the study is up to 240 puffs, therefore using 90 puffs, a dry puffing scenario is very unlikely. For further details on the SAEIVS smoking robot operations please see Wieczorek et al. (2020). Cigarette smoke was diluted with filtered humidified air 1:17 times, whilst EVP aerosol was exposed to the cells undiluted.
Table 1.
Summary of the smoking and vaping regimes used for the reference cigarettes and myblu product.
| Test Product | Smoking and Vaping Standards | Puff Volume (ml) | Puff Duration (s) | Puff Interval (s) | Ventilation Blocking | Puff Profile |
|---|---|---|---|---|---|---|
| 3R4F Reference Cigarette | ISO 20778:2018 (ISO, 2018a) | 55 | 2 | 30 | Yes | Bell |
| myblu EVP | ISO 20768:2018 (ISO, 2018b) | 55 | 3 | 30 | N/A | Square |
2.4. Smoke and aerosol exposure
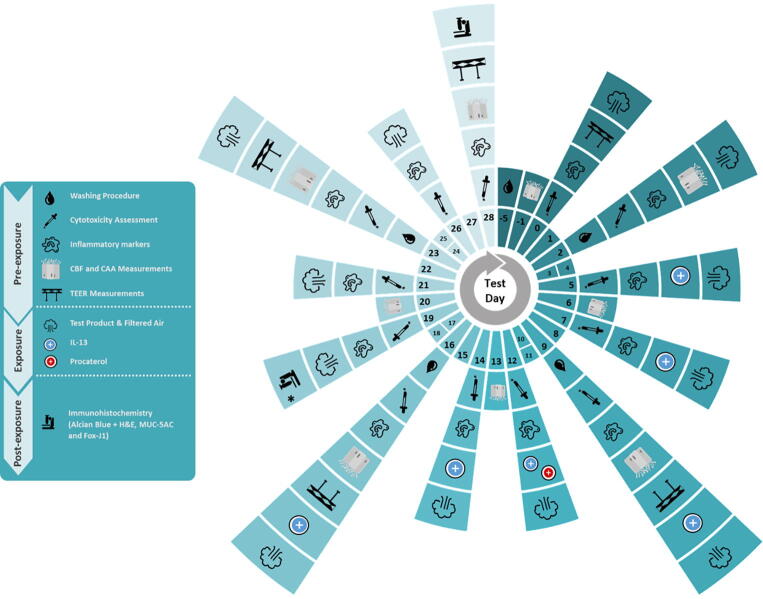
The tissues were repeatedly exposed (3 times per week) at the ALI for 28 days to 30, 60 or 90 puffs of EVP aerosol (undiluted), cigarette smoke (1:17 smoke: air dilution) or filtered humidified air (air control, 3 repeats) (see Fig. 1). Inserts were tested in triplicate. The puff doses were chosen based on a preliminary study with cells exposed on multiple occasions. Based on cytotoxicity in a previous study (not shown) it was believed that 90-puffs was the highest dose of 3R4F, diluted 1:17 in filtered air that could be sustained for 28 days. The 30 and 60 puffs were lower fractions of the highest dose. Following test article exposure, tissues were incubated for 2 days (weekday) or over the weekend under standard culture conditions before the next exposure.
Fig. 1.
Schematic diagram of the experimental design, depicting which experimental steps was carried on each specific test day (T) from day −5 (before the first time of exposure) to 28. * T19, Immunohistochemistry was carried out only for tissues exposed to IL- 13.
2.5. Nicotine dosimetry
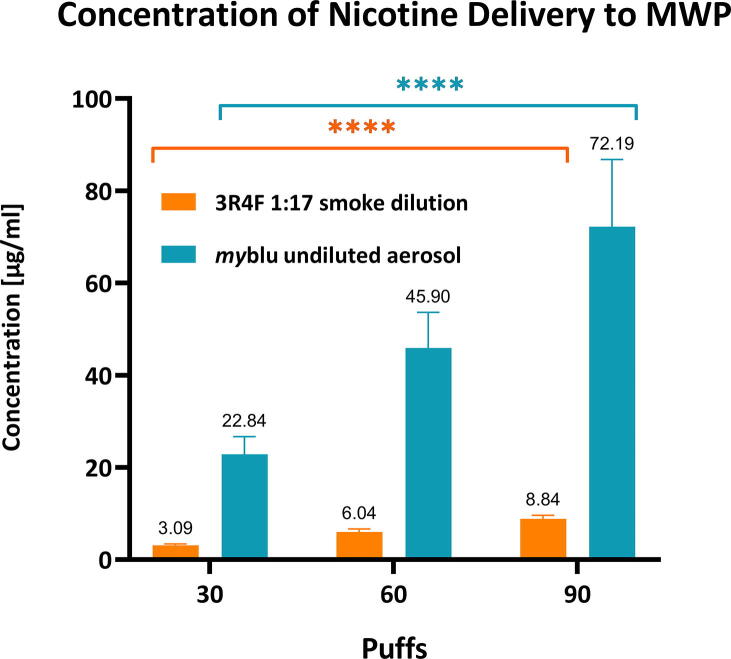
To confirm efficient delivery of cigarette smoke or EVP aerosol to the exposure chambers, nicotine deposition was quantified using a blank well in the MWP containing phosphate buffered saline (PBS). The nicotine deposition was conducted on two separate 24 MWPs with 4 repeats (wells) per plate and the individual values are a mean of 8 repeats per puff dose (Fig. 2). Nicotine was quantified using an AB Sciex API 6500 (Q-Trap) with an Agilent (1290 Infinity) HPLC system (Agilent LC Systems). The Electrospray Ionization and multiple reaction monitoring in positive ion mode was used. The quantification was performed with an external calibration of nicotine (concentration range 2.5–100 ng/ml).
Fig. 2.
Mean concentration of whole smoke and myblu aerosol nicotine delivery to the exposure chambers by quantification of nicotine in blank well MWP containing PBS. The individual values are a mean of 8 repeats per puff dose. Mean values for concentration are plotted above the corresponding bar. Key to significance. **** p ≤ 0.0001. Error bars are the standard deviation.
2.6. Cytotoxicity assay
To assess the cytotoxicity of cigarette smoke or EVP aerosol on the 3D lung model, basal culture medium was collected before every exposure and lactate dehydrogenase (LDH) level was quantified (see Fig. 1). In brief, LDH levels in the medium were quantified using the CytoTox 96® assay (Promega, # G1780) according to the manufacturer’s recommendations. Visible wavelength absorbance data at 490 nm were collected using a standard 96-well plate reader (Sunrise™ Reader, TECAN) and raw absorbance data were recorded automatically.
Relative cytotoxicity was determined using the following equation:
Eq. (1) Cytotoxicity calculation from the LDH quantification
where: LDH - lactate dehydrogenase; HuOlm –human organotypic lung model
2.6.1. Pro-Inflammatory markers quantification
The quantitative analysis of the pro-inflammatory markers (Tumor necrosis factor alpha (TNF-α), matrix metalloproteinases (MMPs) 1; 3; 9 and interleukin (IL)-1β; 6; 8) secreted into the basal medium was performed in sub-samples collected from each well prior to each exposure and were stored at −80 °C until thawed (once) for further analysis. Assessment of pro-inflammatory markers was performed with MSD® Multi-Spot Assay System MESO Scale QuickPlex™ (MSD Maryland, Rockville USA) on custom made plates (MSD) according to the manufacturer’s recommendations (Meso Scale Discovery®).
2.7. Immunohistochemistry analysis and quantification
2.7.1. Tissue preparations
A dedicated set of three tissues were exposed to interleukin 13 (IL-13) (10 ng/ml) on six alternative days to induce goblet cell hyperplasia (Fig. 1) (Evans et al., 2015). A single tissue from each smoke or aerosol treatment group was harvested for immunohistochemical staining at test day T28. Tissues were fixed in 10% neutral buffered formalin for 30 min. The membrane which the tissue sits on in the insert were removed with a scalpel. The tissue disc was laid flat on a cutting plate, with cells facing upwards, then cut from the center into two half discs with a scalpel. Dehydration (with ethanol, 70%) and paraffin-embedding of the tissue discs were processed in the PELORIS Rapid Tissue Processor (Leica Biosystem) following a 1 h protocol. Slides were prepared from formalin-fixed and paraffin-embedded tissues; sectioned at a thickness of 3 μm.
2.7.2. Immunohistochemistry staining
The tissue sections were first rehydrated. After they were incubated in alcian blue at room temperature for 40 min. After 3 washes in PBS, the sections were counter-stained with hematoxylin & eosin (H&E) for 15 min at room temperature. Mucin-5AC-Protein (MUC5AC) (Thermofisher; MA5-12178; 1/800; 1 h) and forkhead box transcription factor (FOX-J1) (Novus BIO; NBP1-87928; 2 µg/mL; 1 h) staining were performed using a Benchmark robot (Ventana-Roche) with the kit Ultraview DAB for revelation. The tissue sections were treated with CC1 buffer (Tris/EDTA, pH 8.4) at 95 °C (30 mins) prior to primary antibody incubation (1 hr). Stained slides were imaged using an Olympus VS120 Virtual Slide microscope (Olympus, Tokyo, Japan).
2.7.3. Quantification
All staining quantifications were performed using the program Image Pro Plus from Media Cybernetics (version 6.2). The results were shown as percentage of specific staining.
2.8. Cilia beat frequency and active area
Cilia activity was measured prior to exposure to smoke or aerosol (Fig. 1). The tissues were acclimated 20 min before and during the measurement at 37 °C in ibidi Heating System for MWP (ibidi GmbH, Martinsried, Germany). Cilia beat frequency (CBF) and cilia active area (CAA) were recorded under the microscope (4x; Olympus IX53P1F inverted; Olympus, Tokyo, Japan) and analyzed using Sisson-Ammons Video Analysis software. The captured image was divided into 2 blocks, each block was analyzed using the same routine analysis. Each video was recorded at 200 frames per second with a total of 1024 frames per video. The results of the analysis were displayed in two intensity graphs, one for frequency and one for beat amplitude. This was done in two core steps: the first was to extract the pixel intensities of a region of interest over time and the second to use this data for fast Fourier transformation. Additionally, noise reduction was performed by using a Gaussian distribution.
A positive control, procaterol hydrochloride (Sigma-Aldrich, 10 μM) was added to the basolateral culture media of a dedicated set of three tissues to show an increase in CBF. The tissues were rested on the microscope stage for 15 min to achieve equilibrium of CBF before measurements were taken. Procaterol was removed after an hour.
2.9. Transepithelial electrical resistance measurements
The 3D lung model tissue’s barrier integrity and tight junctions were assessed by measuring the transepithelial electrical resistance (TEER) using an EVOM2 Epithelial Volt/Ohm Meter in combination with the ENDOHM-6 electrode (World Precision Instruments, Sarasota, FL, USA). Prior to TEER measurement, each tissue surface was rinsed with PBS to remove mucus layer (according to the tissue manufacturer protocol). Background readings of Dulbecco's phosphate-buffered saline (DPBS) containing Ca2+ and Mg2+ (Sigma-Aldrich, Germany) were taken and subtracted from each subsequent resistance value. TEER measurements were conducted according to the manufacturer’s protocol, using DPBS. These corrected values were then multiplied by the surface area of the tissue insert (0.33 cm2); mean ohms*cm for each treatment group was then calculated. The tissues were classified as having intact barrier function if their TEER values were equal to or greater than 2000 ohms/cm; as per the 3D tissue manufacturer’s guidance. In addition, the mean percentage TEER change value over time was calculated for both controls and aerosol/smoke treatment groups.
2.10. Data and statistical analysis
All data and statistical analyses were conducted using Microsoft Excel and GraphPad Prism (Version 8.1.1). Statistically significant differences were calculated using one-way analysis of variance (ANOVA) with the appropriate post-hoc tests (Bonferroni's or Dunnett’s post-hoc test). A difference was considered statistically significant with a p-value ≤ 0.05.
Pro-inflammatory marker assessment and comparative analysis was performed using the R version 3.5.1 with “dplyr” package for data ranking. The “stat” package was used for statistical testing and p-value adjustment, and the “ggplot2” package for graphical representation of the data. For a proper selection of differentially expressed inflammatory mediators, a statistical model based on t-test using a false discovery rate correction (Benjamini and Hochberg, 1995, R Core Team, 2018) was applied. The purpose of the correction is to control the risk of false positive results when multiple T tests are tested simultaneously. The level of statistical significance is expressed as the adjusted p-value between 0 and 1. The smaller the p-value, the stronger the evidence that you should reject the null hypothesis that there is no difference in protein expression between the exposed cultures and the control for given treatment conditions. The adjusted p-value was compared to a threshold of 5%, and if the adjusted p-value was lower than 5% the difference of expression was considered as significant.
3. Results
3.1. Nicotine dosimetry
The total mean concentration of nicotine delivered per treatment dose is shown in Fig. 2. The concentration is an average of 8 repeats. The 30 puffs of 1:17 diluted 3R4F smoke delivered on average 3.09 µg of nicotine per ml of trapping media (PBS), which is approximately 7 times lower than corresponding 30 puffs of undiluted myblu aerosol (22.84 µg/ml). The mean concentration of nicotine delivered for both 3R4F and myblu increased consistently with increasing puff dose.
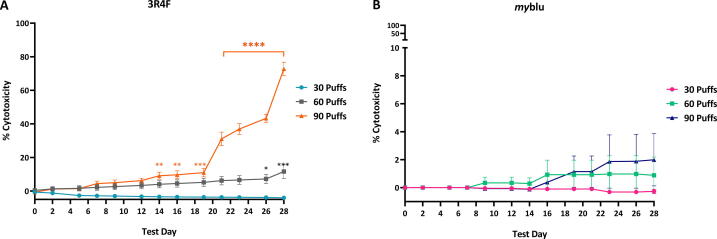
3.2. Cytotoxicity assessment of smoke or aerosol exposed to 3D lung models
LDH levels in the basal culture medium were quantified and the percent cytotoxicity relative to the air control was calculated using Equation 1. The maximum percentage cytotoxicity of myblu aerosol did not exceed 2% (Fig. 3B). Compared to air control, the percentage cytotoxicity was not significantly different. Exposure to the sub-cytotoxic dose of 30 puffs of 3R4F smoke did not induce a significant difference in percentage cytotoxicity versus air controls. For the 60 puff 3R4F dose, a statistically significant difference in percentage cytotoxicity was observed on T26 (7%; p ≤ 0.05) and T28 (12%; p ≤ 0.005). For the highest 90 puff 3R4F dose a statistically significant difference in the cytotoxicity levels was noted earlier in the assay timeframe, from T14 to 16 (p ≤ 0.01), and from T19 (p ≤ 0.0001) onwards a steep increase in the relative cytotoxicity levels was observed (Fig. 3A).
Fig. 3.
The 3R4F smoke and the myblu aerosol cytotoxicity assessment quantifying LDH levels at different time points in the culture medium over the 28 days. Key to significance. * p ≤ 0.05, ** p ≤ 0.01, *** p ≤ 0.005, **** p ≤ 0.0001. N = 3 per time point. Error bars are the standard error of the mean.
3.3. Analysis of pro-inflammatory markers secreted by the 3D lung model
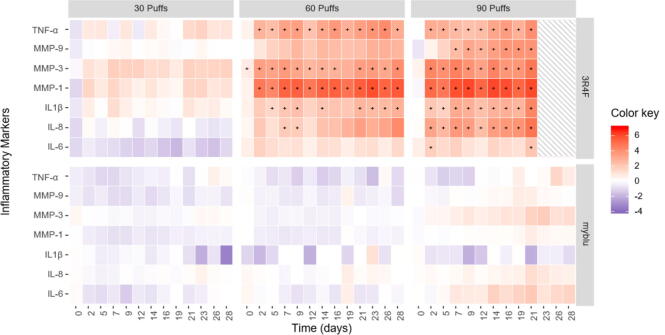
The cytokine concentration in the basal medium of each treatment group was expressed as normalized mean fold change over the air control treatment. For the comparison of the changes in pro-inflammatory mediators compared to the air control values, an inflammatory markers heat-map was generated to visualize the key differences between tested groups (Fig. 4). To determine differentially expressed inflammatory mediators, a statistical model based on t-test using a false discovery rate correction was applied. Analysis of pro-inflammatory markers from T23-28 were excluded for 90 puffs of 3R4F only. This is due to significantly high levels of cytotoxicity compared to air control after T21 (p ≤ 0.0001, See Fig. 3), and TEER measurements at T23 and after (Fig. 9) suggests the tissue model was no longer viable.
Fig. 4.
Heat-maps of the differences in expression of inflammatory markers, calculated using the difference between log intensity of the test product and the control (log fold change). A total of six heat-maps are plotted, corresponding to three treatment conditions (30, 60 & 90 puffs) with diluted 3R4F reference cigarette smoke or undiluted myblu aerosol. Analysis of pro-inflammatory markers from T23-28 were excluded for 90 puffs of 3R4F smoke, due to significantly high levels of cytotoxicity. Each map represents the log-ratio of the seven inflammatory mediators (y-axis) measured over a period of 28 days (x-axis). The color represents the change in expression of the inflammatory mediator compared to control. Expression level is plotted according to a continuous color scale, indicated in the ‘Color key’, provided at the right of the figure. Red-scale color represents an inflammatory mediator over-expression, whilst blue-scale color represents an inflammatory mediator under-expression relative to the air control. White color corresponds to a log fold change equal to zero, i.e., no difference in expression observed between the control and the product assessed. The significance threshold in the statistical test was adjusted to 5% (p-value < 0.05), and significant responses are indicated by ‘+’. Note that the MMP-3 at T0 is deemed significantly different from air control, this is due to the very low in-treatment group variability, where even a small change was deemed significant. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Fig. 9.
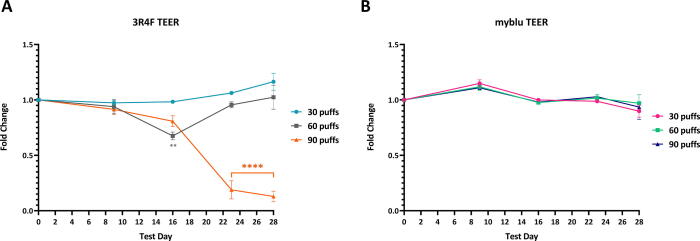
Fold change of 3D lung TEER value over time (T0 - T28) following repeated exposures to 30, 60 or 90 puffs of 3R4F smoke (A) and myblu aerosol (B) compared to matching air controls. Key to significance. ** p ≤ 0.01; **** p ≤ 0.0001. Error bars are the standard error of the mean. To note, significantly high levels of cytotoxicity for the 90 puffs of 3R4F smoke was observed from T23-28 (see Fig. 3).
3.3.1. TNF-α
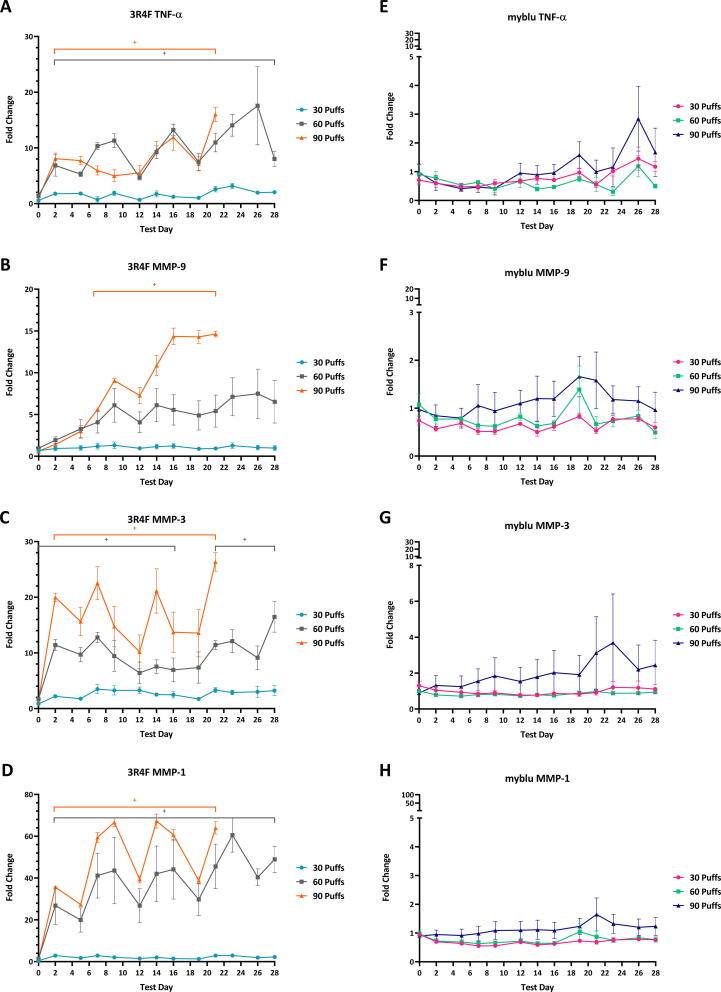
TNF-α is a potent pro-inflammatory cytokine (Horiuchi et al., 2010). Repeated exposure to different puff doses of myblu aerosols did not induce elevated TNF-α secretion from the 3D lung models under the conditions of the test. The levels of TNF-α in the culture medium were of similar levels to air control exposure, with no statistically significant difference throughout the course of the study (Fig. 5E). The highest fold change observed was on T26 for 90 puffs dose (3-fold change compared to air); however, this level of change was not significant when compared to air control. Exposure to a sub-cytotoxic level of 3R4F smoke (30 puffs/exposure) did not cause the 3D lung tissues to significantly increase secretion of TNF-α throughout the entire 28-day exposure. By contrast, 60 and 90 puff doses of 3R4F smoke caused a significant increase in TNF-α at T2 and levels continued to be significantly higher than control throughout the study (adjusted p < 5%) (Fig. 5A).
Fig. 5.
TNF-α, MMP-9, MMP-3, and MMP-1 levels in culture media over 28 days during which repeated exposures of whole aerosol/smoke (1:17 dilution) /humidified air were applied. Analysis of pro-inflammatory markers from T23-28 were excluded for 90 puffs of 3R4F smoke, due to significantly high levels of cytotoxicity. Levels are expressed as fold change compared to air control response. Note the y-axis scale differences between 3R4F and myblu graphs. Key to significance. +, indicates a significant difference where the adjusted p < 0.05. Error bars are the standard error of the mean.
3.3.2. MMPs
Repeated exposure to different puff doses of myblu aerosols did not induce the 3D lung model to elevate MMPs (MMP-1, 3 & 9) secretion under the experimental conditions. The levels of MMPs in the culture medium were of similar levels to air control, with no statistically significant differences throughout the course of the study (Fig. 5F–H). The fold change never exceeded 4-fold over the 28 days and the level of change was not significant when compared to air control. Exposure to 30 puffs of 3R4F smoke did not significantly elevate the secretion of MMPs throughout the 28 days of exposure. On the other hand, 60 puffs doses of 3R4F smoke caused significant increases in MMP-3 and MMP-1 secretion (adjusted p < 5%) (Fig. 5C & D). The 90 puffs dose caused all three of the MMPs quantified in this experiment to significantly increase, although the 90-puff dose was not sustainable for the duration of the study. MMP-1 levels showed the largest fold change, peaking at approximately 63-fold. The largest fold change observed for MMP-3 and MMP-9 were 26 and 14 respectively.
3.3.3. Interleukins
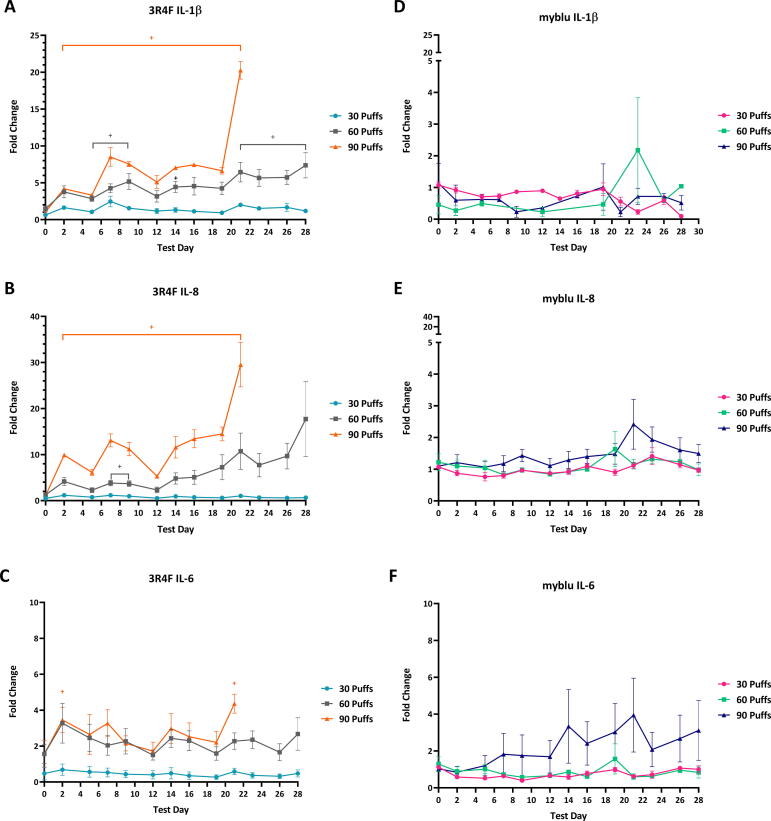
Different puff doses of myblu aerosols did not induce the 3D lung model to elevate secretion of selected interleukins (IL-1β, IL-6, IL-8) under the test conditions. The levels of interleukins in the culture medium were of similar levels to air control, with no statistically significant differences throughout the course of the study (Fig. 6D–F). Exposure to 30 puffs of 3R4F smoke did not significantly elevate the secretion of interleukins. The 60 puff doses of 3R4F smoke caused a significant increase in IL-1β (T5-T9, T21-T28) and IL-8 (T7 & T9 only) (adjusted p < 5%) (Fig. 6A & C). The 90 puffs dose caused all the interleukins quantified in this experiment to significantly increase. IL-8 showed the largest fold change followed by IL-1β, then IL-6 (T2 and T21 only).
Fig. 6.
IL-1β, IL-6 and IL-8 levels in culture media over 28 days during which repeated exposures of whole aerosol/smoke (1:17 dilution)/humidified air were applied. Analysis of pro-inflammatory markers from T23-28 were excluded for 90 puffs of 3R4F smoke, due to significantly high levels of cytotoxicity. Levels are expressed as fold change compared to air control response. Note the y-axis scale differences between 3R4F and myblu graphs. Key to significance. +, indicates a significant difference where the adjusted p < 0.05. Error bars are the standard error of the mean.
3.4. Immunohistochemistry
3.4.1. Tissue morphology
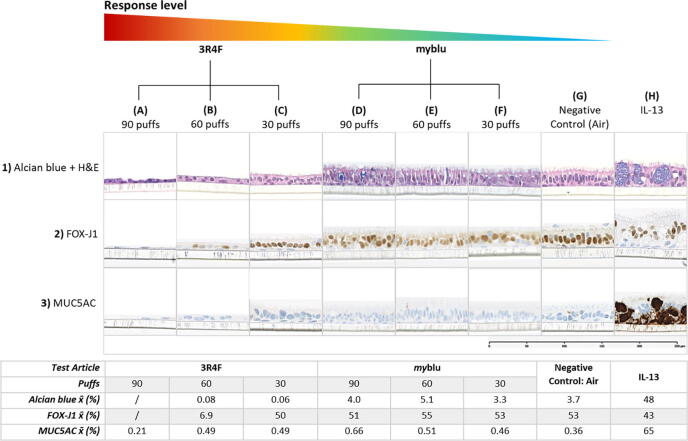
The tissue morphology was assessed by alcian blue and H&E staining at the end of the 28-day exposure. Normal 3D lung model tissue is pseudostratified, with the presence of cilia on the apical surface (Fig. 7.1G). Exposure to myblu aerosol did not cause any visible reduction in tissue thickness or cilia appearance when compared to the air control (Fig. 7.1D-F). By contrast, a reduction in epithelial thickness and the number of visible cilia was prominent in tissues exposed to 3R4F smoke. As the 3R4F puff dose increased, the columnar shaped cells lost its structure and appeared more squamous-like, therefore reducing the thickness of the tissue (Fig. 7.1C), indicating a clear dose response to the treatment.
Fig. 7.
Representative immunohistochemistry images (alcian blue + H&E, MUC-5AC and FOX-J1) of 3D lung models after 28 days of repeated exposure to 30, 60 or 90 puffs of myblu aerosol (undiluted), 3R4F cigarette smoke (dilution 1:17 smoke: air) or negative control (90 puffs of humidified filtered air). Tissues exposed to IL-13 was evaluated at T19. x̄ in table (Quantified staining average). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
3.4.2. Ciliated cells quantification
An abundant number of ciliated cells were present after exposure to different puff quantities of myblu aerosols. FOX-J1 stains the nucleus of ciliated cells, 53% of the cells in air-controlled tissues were ciliated; tissues exposed to myblu aerosols contained very similar percentages of ciliated cells (30 puffs: 53%; 60 puffs: 55%; 90 puffs: 51%). A dose dependent decrease in the percentage of ciliated cells was observed for the 3R4F smoke exposed group. As shown in Fig. 7.1, where the tissues were stained with alcian blue and H&E, no visible cilia were observed on the tissue surfaces exposed to 60 and 90 puffs of 3R4F smoke. FOX-J1 staining reflected this observation (Fig. 7.2A-C). After 28 days of repeated exposure to 30 puffs of 3R4F smoke, 50% of cells were ciliated. Exposure to 60 puffs showed a dramatic decrease in FOX-J1 staining (6.9%), and quantification was unable to be performed in the tissues exposed to 90 puffs, as they were severely damaged, with only a few basal cells remaining.
3.4.3. Mucin secretion
IL-13 induces hyperplasia of the epithelium and mucous hypersecretion. Repeated addition of IL-13 to the 3D lung model caused the tissue to thicken due to the accumulation of mucin and cell proliferation. Non-specific mucin (stained with alcian blue) and MUC5AC secretion were both elevated as reflected by intensive staining in Fig. 7.1H & 3H.
Based upon the alcian blue staining, exposure to humidified air (3.7%) and different doses of myblu aerosols showed relatively similar staining intensities (30 puffs: 3.3%; 60 puffs: 5.1%; 90 puffs: 4.0%). However, tissues exposed to 3R4F smoke presented very low alcian blue staining, this may be due to severe tissue disruption, leading to loss of secretory cells and in general there is less tissue material to quantify.
MUC5AC is secreted from goblet cells and often used as a maker for goblet cell hyperplasia (Song et al., 2007). Compared with air (0.36%), exposure to myblu aerosol caused a small increase in MUC5AC secretion as the puff dose increased (30 puffs: 0.46%; 60 puffs: 0.51%; 90 puffs: 0.66%). Exposure to the 30 puffs dose of 3R4F smoke also induced a small increase in MUC5AC secretion compared to air, however at the 60 and 90 puffs doses, the secretion did not increase which was likely caused by the loss of goblet cells and tissue matter, leaving only basal cells as seen in the histology sections for 60 and 90 puffs.
3.5. Functional assessment of cilia
3.5.1. Cilia beat frequency
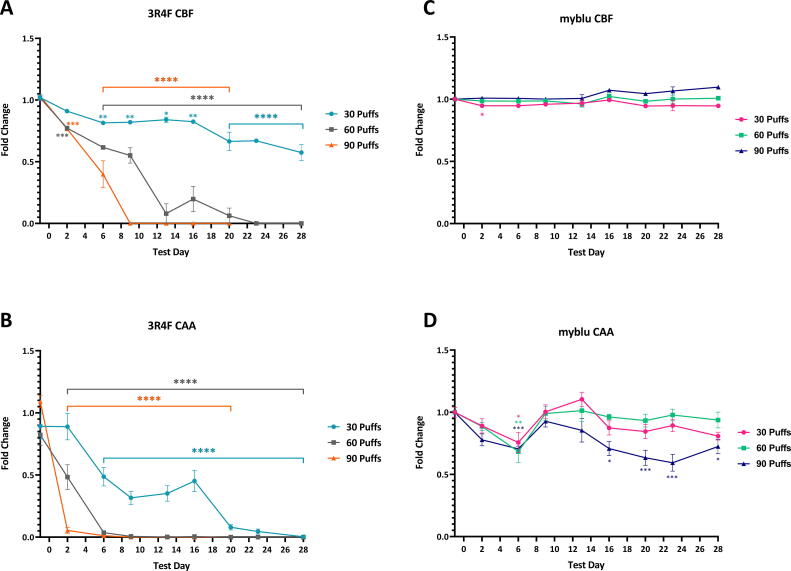
The 3D lung models were exposed to myblu aerosol or cigarette smoke on T0. The CBF fold change for 30 puffs of myblu aerosol at T2 (0.95) was deemed statistically significant different (p ≤ 0.05) versus air control. However, this fold change was the same as T6, yet it was not significant, it should be noted that there was variability within the air control treated models that contribute to this observation. Overall, CBF was not significantly altered after repeated exposure to myblu aerosol over the course of the study (Fig. 8C).
Fig. 8.
Fold change of CBF and CAA over time (T0 - T28) following repeated exposures to 30, 60 or 90 puffs of 3R4F smoke (A &B) and myblu aerosol (C & D) compared to matching air controls. Key to significance. Key to significance. * p ≤ 0.05, ** p ≤ 0.01, *** p ≤ 0.005, **** p ≤ 0.0001. Error bars are the standard error of the mean. Analysis of CBF and CAA were excluded for 90 puffs of 3R4F smoke from T23-28 due to significantly high levels of cytotoxicity (see Fig. 3).
By contrast CBF was significantly affected by exposure to different puff doses of 3R4F smoke (Fig. 8A). After the second exposure, a decline in CBF was observed for all 3R4F smoke treated groups. At sub-cytotoxic exposures (30 puffs) significant inhibition of CBF was observed from T6 (p ≤ 0.01), followed by a larger decrease in CBF after T20 (p ≤ 0.0001) resulting in a decrease of 0.57-fold relative to the air control at the end of the experiment at T28. The 60 puffs dose caused a significant reduction in CBF at T3 (p ≤ 0.005), a steep decline at T13 (p ≤ 0.0001), followed by the complete loss of beating cilia after T22. Repeated exposure to 90 puffs of 3R4F smoke caused a drastic decline in the CBF at T6 (p ≤ 0.0001), with a complete loss of CBF signal by T9.
3.5.2. Cilia active area
Exposure to myblu aerosol caused CAA measurements to initially decrease. At T6, the decline was statistically significantly different verses air control (30 puffs: p ≤ 0.05; 60 puffs: p ≤ 0.01; 90 puffs: p ≤ 0.005). After T6, CAA measurements reverted to similar values as air control baseline for 30 and 60 puffs dose until the end of the study. The 90 puffs dose followed a similar trend until T16 where the CAA fold change begins to drop, at the last evaluation point T28 a fold change of 0.72 (p ≤ 0.05).
The repeated exposure to 3R4F cigarette smoke resulted in a steep, statistically significant decrease in CAA (Fig. 8B), indicating severe damage to the cilia. Measurements taken at T2 showed a single exposure to 30 puffs of cigarette smoke, followed by 2 days recovery did not reduce CAA. Both measurements at T0 and T2 showed a fold change of 0.89 vs air control. A further two exposures caused the fold change to drop to 0.48 at T6 (45% reduction, p ≤ 0.0001). By T20, the fold change was close to 0. A single exposure to 60 and 90 puffs of cigarette smoke caused CAA to decline by 50% and 100% at T2 respectively (p ≤ 0.0001).
3.6. Assessment of barrier integrity
Barrier integrity of the 3D lung model was assessed by measuring TEER. The values from each exposure condition are expressed as fold change compared to the air controls (Fig. 9). Based upon this, exposure to myblu aerosols at all dose levels did not disrupt the barrier integrity of the 3D lung model. As shown in Fig. 7.1D-F, the tissues were intact, and no visible damage was observed. Throughout the study, the TEER values were similar to the air control, and no statistically significant difference was found (Fig. 9B). Tissues exposed to 30 puffs of 3R4F smoke, which is a sub-cytotoxic concentration, did not induce any significant fold change in TEER over the 28-day study. A significant drop in TEER values was recorded in tissues exposed to 60 puffs of 3R4F smoke on T16 (p ≤ 0.01) (Fig. 9A). However, measurements taken at T23 and T28 (see Fig. 9) showed that the TEER values had recovered back to air control baseline values. Fig. 9A shows that by T23, 90 puffs per exposure dose of 3R4F smoke caused a drastic decline in TEER compared to air control (p ≤ 0.0001), reaching a 0.87-fold decrease in TEER at this timepoint due to excessive cytotoxicity (Fig. 3). Further cross comparison with histological images showed that the tissue structure was severely disrupted, with only a few basal cells remaining on T28 (Fig. 7.1A).
4. Discussion
This study built upon on our previous work, which acutely exposed a respiratory epithelial model to aerosols of EVPs and combustible cigarette smoke and demonstrated that acute exposure to EVP aerosol resulted in no significant in vitro toxicity in any of the measured endpoints compared to air controls (Czekala, et al., 2019). In contrast to the previous study, which focused on the acute exposure period, the present study repeatedly exposed the 3D lung model over 28 days (3 days a week) to whole myblu aerosol or diluted cigarette (3R4F) smoke and employed a multi-endpoint approach to assess the biological impact following exposure at various time points. The focus was on selected endpoints related to the outcomes of the IIVS working group on functional and biological endpoints, including ciliary beating frequency, mucus production, and goblet cell hyperplasia identified by multi-laboratory studies (Behrsing et al., 2016). The aim was to assess the relative THR potential of EVPs when compared to cigarette smoke over an extended period (28 days).
4.1. Dosimetry
To determine effective delivery of aerosol or smoke to the cellular surface of the 3D lung model, the nicotine delivery in smoke/aerosol in the SAEIVS system was quantified from blank wells of MWP filled with PBS. Nicotine is often used as a marker of smoke/aerosol delivery, and this method of quantification assumes that the other components of the smoke/aerosol are delivered in the same ratios as nicotine (Bishop et al., 2019). The individual puffs were distributed evenly over 24 MWPs each containing an insert with 3D organotypic lung models, with an insert surface area of 0.33 cm2. The exposure, in terms of dosage per unit surface area is likely to be considerably higher than that seen in humans with a predicted tracheal and bronchial surface area of approximately 2,471 ± 320 cm2 (Mercer et al., 1994). Based on preliminary study (data not shown) and previous work (Czekala, et al., 2019), the 3R4F cigarette smoke was diluted with filtered humidified air at 1:17 ratio to obtain sub-cytotoxic concentrations, which allows the observation of biologically relevant changes to the tissue model without the influence of severe cytotoxic responses. The lowest, 30-puff dose of diluted 3R4F smoke (which delivered on average 3.09 µg/ml of nicotine) was the sub-cytotoxic concentration, exposure to this dose did not increase LDH secretion or pro-inflammatory markers, barrier integrity was not disrupted, however, ciliary functions (CBF & CAA) were significantly reduced compared to the air control, and a visible reduction of thickness of the 3D lung model was observed at the end of the study period. Repeated myblu aerosol exposure, despite a lack of the dilution step, and even at the highest dose evaluated, did not induce any major statistically significant perturbations to the cellular function measured in this work, even with the notable higher delivery of nicotine (see Fig. 2). Consistent with the scientific literature, these results suggest that the toxicity of cigarette smoke is not solely linked to the presence or concentration of nicotine but due to the by-products of tobacco combustion (Institute of Medicine, 2001, Levitz et al., 2004, Royal College of Physicians, 2012).
4.2. Cytotoxicity assessment
Various methodologies for in vitro cytotoxicity determination in 3D lung models have been reported in the literature (Elson et al., 2015, Iskandar et al., 2017, Neilson et al., 2015, Czekala et al., 2019). In general, cytotoxicity assays are colorimetric and fluorometric-based and can either be destructive (e.g., MTT assay) or non-destructive (e.g., LDH assay). The LDH assay was selected for our study as it enables the continued use of the tissue for repeat exposure (Nachlas et al., 1960).
Whole cigarette smoke at 1L/minute dilution rate, as previously shown in a single, acute exposure scenario, can lead to complete tissue disruption (Czekala, et al., 2019). In the current study, the 30 puffs of 3R4F (1:17 dilution) dose group cytotoxicity levels did not differ from the air controls throughout the course of the experiment. The lowest doses of 3R4F were sub-cytotoxic to allow for functional impairments to be detected prior to the loss of the model structure. However, the 90 puffs dose triggered severe cytotoxicity from T19 onwards, leading to a complete tissue disruption towards the end of the study. Similar results were also reported by Ito et al. (2020), where the 3D lung model was exposed to cigarette smoke total particulate matter (TPM) for up to 40 days. A significant increase in cytotoxicity was observed from day 22 until study termination (day 40) (Ito et al., 2020). In the present study, marked cytotoxicity was observed 3 days earlier than in the study by Ito et al.; however, here the tissue models were exposed to whole cigarette smoke rather than the TPM fraction only, which may have played a role in triggering an earlier response. In contrast, even the highest 90 puff dose of EVP aerosol did not impact the tissue viability in this (sub-acute) repeated exposure study under the conditions of test. To our knowledge, this is the first study which has evaluated the effects of repeated whole cigarette smoke and whole EVP aerosol exposure on the 3D lung model, and therefore a direct comparison to available literature is not possible. In an acute exposure scenario, EVP exposures have been shown to be significantly less cytotoxic than the reference or commercial cigarette in the 3D lung model (Bishop et al., 2019).
4.3. Molecular responses
4.3.1. Pro-Inflammatory markers
Long term exposure to noxious agents or inhaling cigarette smoke exacerbates chronic inflammation in the lungs (Aghapour et al., 2018, Brusselle et al., 2011, Devine, 2008). Oxidative stress induced by cigarette smoke amplifies airway inflammation, disrupts junctions between epithelial cells, induces mucus hypersecretion and causes ciliary malfunction (Aghapour et al., 2018, Brusselle et al., 2011). Imbalance of proteinases and anti-proteinases drives airway tissue remodeling and alveolar tissue destruction (Brusselle et al., 2011). The innate immune system has been shown to play a key role in the pathogenesis of COPD (Brandsma et al., 2020). To quantify pro-inflammatory mediators secreted by the 3D lung model in response to repeated exposure of 3R4F smoke or myblu aerosol, an analysis of the collected basolateral media was performed. Data for pro-inflammatory markers from 90 puff 3R4F, from T23 onwards, were excluded due to overtly high cytotoxicity and lack of model viability.
4.3.2. TNF-α
TNF-α is a pleiotropic cytokine and a master regulator of the immune system, and most cells in the body show at least some TNF-α responsiveness. Studies have revealed two opposite functions of TNF-α in host defense. At low levels, TNF-α has been shown to have beneficial homeostatic functions, such as for host defense mechanisms. However, at high concentrations, TNF-α can be deleterious to tissues and promotes inflammation and organ injury (Steeland et al., 2018). During disease states, TNF-α is predominantly secreted by monocytes and macrophages, but many other cells including epithelial cells and airway smooth muscle cells can also produce TNF-α under certain circumstances (Steeland et al., 2018). TNF-α was significantly increased at all time points for 60 and 90 puffs of cigarette smoke. The significantly elevated TNF-α seen with 60 and 90 puffs of 3R4F smoke exposure appears to indicate cellular stress. Exposure to myblu EVP aerosol did not increase TNF-α secretion under the conditions of test.
4.3.3. MMPs
MMPs contribute to inflammatory processes by regulating physical barriers, modulating the inflammatory mediators such as cytokines and chemokines, and establishing a chemokine gradient in tissues that are inflamed (Manicone and McGuire, 2008). Pathogens, environmental toxins, growth factors and cytokines can stimulate airway epithelium, fibroblasts and smooth muscle to express MMPs (Fujita, 2014). In human studies, MMP-1 and −3 (and −7, −10 and −12) are elevated in COPD patients (Kraen et al., 2019, Navratilova et al., 2012). Cigarette smoking has also been shown to influence MMP expression with MMP-1, −3 and −9, amongst others (Kraen et al., 2019). The changes of the MMP-1 expression levels in lung epithelial cells after the cigarette smoke exposure has been linked with the emphysema (Mercer et al., 2009). We observed no significant increase in MMPs for 30 puffs of 3R4F exposure over the duration of the study. However, exposure to 60 and 90 puff doses of 3R4F resulted in a significant elevation in all MMP-1, −3 and −9 measured. These findings are consistent with clinical data showing cigarette smoking increases MMP expression (Huang, 2016, Kraen et al., 2019, Navratilova et al., 2012). The most significant change was observed for MMP-1, with the highest 3R4F dose tested resulting in a 63-fold increase over air control. Repeated exposure to myblu aerosol, at all doses tested, did not significantly induce the 3D lung model to elevate MMP (-1, 3 & 9) secretion, although at 90-puffs, MMP-3 secretion appeared to have an increasing trend, however, this was not statically significant.
4.3.4. IL-1β
IL-1β levels increased significantly throughout the study after exposure to 90 puffs 3R4F, and to a lesser extent after 60-puffs. By contrast, no significant changes were seen following any dose of myblu aerosol, although sporadic and non-significant decreases in IL-1β levels were recorded throughout the study duration. IL-1β is an inducible cytokine and is not generally expressed in healthy cells or tissue (Borthwick, 2016). In the case of adult smokers, the development of the chronic inflammatory airway pathology is thought to be a response triggered by the inhalation of noxious particles within cigarette smoke. Healthy adult smokers have been reported to have elevated levels of IL-1β in their bronchoalveolar lavage fluid, when compared to healthy non-smokers, with measured IL-1β levels correlating with cigarette usage in a cigarette-dose-dependent manner (Kuschner, 1996). IL-1β release markedly activates macrophages isolated from patients with COPD to secrete inflammatory cytokines, chemokines, and MMP-9 (Barnes, 2009).
4.3.5. Il-6
IL-6 is a pro-inflammatory cytokine featuring pleiotropic activity and together with other cytokines provides a link between innate and acquired immunity. In addition to its role in the acute phase response to the environmental stress factors such as lung injuries and infection, IL-6 has diverse roles in driving chronic inflammation, endothelial cell dysfunction, fibrogenesis and autoimmunity (Barnes et al., 2011). IL-6 is also involved in acquired immunity response via the stimulation of antibody production and of effector T-cell development. The continual, dysregulated synthesis of IL-6 plays a key pathological role in autoimmunity and chronic inflammation leading to the onset or development of multiple disease states (Tanaka et al., 2014).
In the present study, IL-6 levels were decreased after 30 puffs of myblu, although this was not deemed as a significant change. Neither exposure to 60 nor 90 puffs of myblu resulted in a significant change in IL-6 secretion when compared to the air controls. A significant increase in IL-6 was seen with the 90 puffs of 3R4F at T2 and T21 only. IL-6 was marginally reduced at 30 puffs and marginally increased at 60 puffs 3R4F; however, the levels were not statistically different from the air controls. Although a general increasing trend in IL-6 was observed for the 90-puff dose of 3R4F and myblu, the 3R4F smoke was substantially diluted (1:17) compared to the undiluted myblu aerosol, whilst delivering less nicotine to the model. A steady increase in the IL-6 secretion level may be associated with a tissue damage and a response to the cigarette smoke is recognized as a stress factor. Results reported here are in keeping with an increase of the IL-6 levels secreted by 3D tissues after exposure to the cigarette smoke from other studies (Banerjee et al., 2017, Haswell et al., 2018, Iskandar et al., 2017, Czekala et al., 2019).
4.3.6. Il-8
IL-8 is known to be secreted from bronchial epithelial cells under stress, and has been demonstrated to have an important role in neutrophilic inflammation (Barnes, 2007). IL-8, a CXC chemokine, is a known neutrophil chemoattractant and activator; IL-8 release induces a transient shape change in neutrophils, a rise in intracellular calcium concentration, exocytosis with release of enzymes and proteins from intracellular storage organelles, and respiratory burst (Chung, 2001). Several studies in animal models focusing on lung inflammation and injury have shown that IL-8 is the main polymorphonuclear (PMN) cell family chemoattractant compound. Animal models pre-treated with monoclonal antibodies to IL-8 were found to significantly reduce lung injury and PMN migration in endotoxemia models (Goodman et al., 2003). The high levels of IL-8 release particularly at 90 puffs of 3R4F indicate the potential for inflammation and potential remodeling of the tissue; however, this did not occur likely due to the lack of interaction with other immune cells.
In summary, all three doses of myblu undiluted whole aerosol and the sub-cytotoxic dose (30 puffs) of diluted 3R4F cigarette smoke did not elevate any of the pro-inflammatory mediators as mentioned above. Although a few isolated cytokines and MMPs were increased at 30 puffs 3R4F and 90 Puffs myblu, these were not deemed statically significant. In contrast, the 60 and 90 puffs dose of the reference cigarette which significantly increased all pro-inflammatory mediators in this study. This suggests that myblu aerosol did not irritate or damage the 3D lung model, thus no significant inflammatory response was induced. By contrast, 3R4F cigarette smoke displayed toxic effects to the model which triggered an inflammatory response at 60 and 90 puffs.
4.4. Tissue responses
4.4.1. Assessment of goblet cell hyperplasia and mucus secretion
Mucous cell hyperplasia is a pathophysiological characteristic of chronic bronchitis, and increased numbers of goblet cells have been observed in the lungs of adult smokers (Haswell et al., 2010). In COPD patients, chronic mucus hypersecretion is linked with a more rapid decline in lung function (Kim and Criner, 2013). Mucin is a family of high molecular weight glycoproteins that is predominantly found in mucus. MUC5AC, mainly secreted by goblet cells and MUC5B, secreted by mucus cells of the submucosal glands are the two main mucins in airway mucus (Haswell et al., 2010). In the airway of smokers, MUC5AC was reported to be upregulated both at gene and protein levels (Haswell et al., 2010).
We assessed the level of MUC5AC secreted by the 3D lung model after repeated exposure to myblu aerosol or 3R4F cigarette smoke by immunohistochemistry. Exposure to the 30 puffs dose of 3R4F smoke resulted in a small increase in secretion. By comparison, the 60 and 90 puff dose treatment did not increase MUC5AC secretion. In reference to the histology images (Fig. 7) for 60 and 90 puffs of 3R4F smoke, the reduction in MUC5AC production could be due to the loss of tissue infrastructure and functional cells, resulted from high levels of cytotoxicity. Exposure to myblu aerosol did result in a small increase in MUC5AC secretion as the puff dose increased. As may be expected, MUC5AC concentration in induced sputum samples from EVP users (58 pmol/ml ± 21 SD) has been shown to be higher compared to non-smokers; however, cross referencing the MUC5AC concentration to cigarette smokers samples (132 pmol/ml ± 58 SD) showed a 56% decrease (Reidel et al., 2018).
The repeated exposure of IL-13, directly to the 3D lung model, induced a clear increase in both mucus production (alcian blue) and goblet cell proliferation (MUC5AC) in histopathological slides (see Fig. 7). MUC5AC gene expression was induced by IL-13 through direct stimulation of airway epithelial cells (Evans et al., 2015). In comparison to the IL-13 control group, neither 3R4F smoke nor myblu aerosol triggered goblet cell hyperplasia or hypersecretion of mucus in this study; similar results were also reported by Iskikawa et al. (Ishikawa and Ito, 2017). In the human body, foreign stimuli such as allergens and pollutants irritate the epithelial cells, causing the release of cytokines. These cytokines mediate the activation of a variety of immune cells (ILC2, Th2 cells, mast cells, macrophages & B cells) which subsequently produce several cytokines including IL-13. IL-13 stimulates various immune cells (ILC2, Th2 cells, mast cells, macrophages, basophils, eosinophils, B cells) as well as inducing goblet cell hyperplasia and airway smooth muscle cell hyperplasia (Marone et al., 2019). The lack of mucin production and goblet cell hyperplasia after repeated exposure to smoke in this study may be related to the absence of immune cells in the 3D lung model.
4.4.2. Cilia function in the 3D lung model
In this study, the viability and functionality of ciliated cells in the 3D lung model were assessed by measuring CBF, CAA and staining for FOX-J1. Cilia function is impaired in chronic bronchitis due to hypersecretion of mucus and inflammation, resulting in squamous metaplasia of the bronchial epithelium, causing the loss of ciliated cells (Sapey and Stockley, 2011). The FOX-J1 protein is a master regulator of motile ciliogenesis, and cigarette smoke has been demonstrated to inhibit FOX-J1 expression (Brekman et al., 2014, You et al., 2004). Normal airway epithelium consists of 50–70% ciliated cells (Schamberger et al., 2015). Approximately 53% of the cells in the air-controlled tissues in this study were stained positive for FOX-J1.
It is known that cigarette smoke induces inflammation in the airways, thus damaging the ciliated cell. These findings are consistent with other acute exposure studies assessing the impact of cigarette smoke on ciliary function in vitro (Gindele et al., 2020, Iskandar et al., 2019). 3R4F exposure significantly reduced both cilia number and their functionality. Both CAA and CBF were significantly impaired after repeated exposure to all three puff doses of 3R4F smoke (compared to air controls). Due to significantly high levels of cytotoxicity, the CBF and CAA measured on T23-T28 for 90 puffs of 3R4F smoke was excluded from analysis (see Fig. 8).The 60 puffs dose of 3R4F cigarette smoke resulted in a dramatic decrease in FOX-J1 staining in the tissues (see Fig. 7), indicating a reduction in ciliated cells. For the 90 puffs dose of 3R4F cigarette smoke, severe tissue loss was observed towards the end of the experiment due to high cytotoxicity, and it was not possible to obtain any FOX-J1 staining. The loss of cilia from tissues due to smoke exposure has been well described in literature, more recently Valencia-Gattas et al. (2016) reported that treatment of normal human primary bronchial epithelial (NHBE) cells with the epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor, gefitinib, during smoke exposure prevents ciliated cell loss and promotes ciliated cell differentiation from basal cells. The authors propose that the loss of ciliated cells and lack of differentiation of basal cells is due to the activation of EGFR (Valencia-Gattas et al., 2016).
By contrast, exposure to myblu aerosol had substantially less impact on cilia number and function. The percentage of cells expressing FOX-J1 was not reduced in any of the three treatment groups compared to air controls. Repeated exposure to 30 or 60 puffs of myblu aerosol did not affect the CAA and CBF. A small but significant decrease in CAA was only observed at T6. However, CBF was not affected and CAA recovered to similar levels as the air controls after T6. The 90-puff dose resulted in a significant reduction in CAA at T6 then T16 onwards until the end of the study (see Fig. 8), a partial recovery was observed on T28 and the fold change was consistently less, when compared to the drastic effects of 3R4F smoke on CAA and CBF. It should also be noted that CBF was normal throughout the exposure period, cilia number and morphology was also not significantly affected by the exposure. In addition, no dose–response effect on CAA was observed between the 30- and 60 puff dose of EVP aerosol.
Further research is required to address precisely what mechanism caused the response observed for the 90-puff dose of myblu aerosol, such as the reduced CAA from day 16 onwards. Sensitive methods, including ‘omics technologies, could be incorporated into future studies to further define mechanisms driving the observed effect for both myblu aerosol and cigarette smoke. It is possible that the reduction in CAA could be linked to the amount of nicotine or the mass of aerosol delivered to the organotypic tissues, since the EVP aerosol was tested undiluted, whereas cigarette smoke was diluted (1:17). Research to date suggests nicotine can impact cilia activity. Hahn et al. (1992) observed a transient increase in cilia beat frequency upon exposure of ferret tracheal epithelial strips to nicotine, which was not attributed to an increase in mucus secretion (Hahn et al., 1992). Similar results were observed by Perniss et al. (2020) in explanted mouse trachea acutely exposed to 100 µM nicotine, with nicotine transiently increasing cilia beat frequency and particle transport speed. The authors propose the α3β4 nicotinic acetylcholine receptor stimulation via nicotine is crucial for response observed. However, it should be noted that marked desensitization of particle transport speed was observed within 30 min of initial nicotine dosing (Perniss et al., 2020). In contrast, Chung et al. (2019) have shown that both nicotine and EVP aerosol impaired parameters of mucociliary function in ciliated airway epithelial cultures and concluded that the main nicotine effect on mucociliary function is mediated by transient receptor potential ankyrin 1 (TRPA1) and not nicotinic acetylcholine receptors. Within our study, we did not see an impact of myblu aerosol on cilia beating frequency, which is a key measurement of cilia function (Chung et al., 2019). It is possible that chronic, or repeated exposure to nicotine may convert the transient cilio-activating effects to cilio-depressive effects as shown for normal human bronchial epithelial cells cultured at an air–liquid interface (Rayner et al., 2019b), however further research is required to understand the mechanism of this possible response. In addition, a number of Harmful and Potentially Harmful Constituents have been shown to adversely affect cilia morphology and function. This includes acetaldehyde, formaldehyde, acrolein, and ammonia (Sisson et al., 1991, Tilley et al., 2015). These volatile compounds are found within tobacco smoke, with considerable reductions or undetectable levels in EVP aerosol (Rudd et al., 2020).
4.4.3. Barrier integrity
Cigarette smoke can induce barrier dysfunction via oxidative stress and inhibiting the expression of apical junction genes (Aghapour et al., 2018). The 60 and 90 puffs dose of 3R4F reduced the TEER measurement over time, mainly due to cytotoxicity (see Fig. 3A); histological images (see Fig. 7) showed severe tissue damage for both treatments. The TEER values were significantly reduced from T23 onwards for 90-puff 3R4F smoke group due to severe cytotoxicity (T23, 37% cytotoxicity). Repeated exposure to all doses of myblu aerosol did not impact barrier integrity under the experimental conditions. The results of the present study are consistent with findings in published literature, where TEER decreased in a time and dose dependent manner upon exposure to cigarette smoke whereas acute exposure to EVP aerosol did not impact barrier integrity (Balharry et al., 2008, Fields et al., 2017, Neilson et al., 2015). Therefore, myblu aerosol does not initiate barrier dysfunction, whereas several mechanisms have been associated with cigarette smoke (Aghapour et al., 2018). The expression of various cytokines including TNF-α and IL-8 have also been show to affect barrier integrity in diseases and may be another reason for the reduced TEER responses seen at both 60 and 90 puffs of 3R4F (Shen et al., 2017, Ye et al., 2006).
4.5. Overall summary of the results in terms of an AOP approach
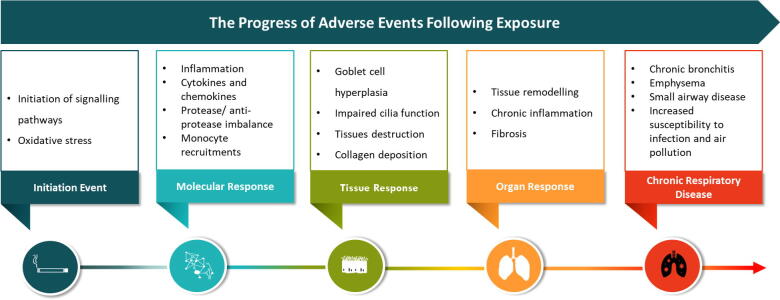
Another way to look at the overall data of this study is via an Adverse Outcome Pathway (AOP) approach. An AOP is considered to be a practical analytical tool for collecting, synthesizing, reviewing and disseminating knowledge about a particular toxicological process (Vinken et al., 2017). An AOP in its entirety represents those key elements and the basic mechanisms of the pathway, a chain of sequential causally related key events (KEs) linked by key event relationships (KERs). The molecular initiating event (MIE) triggers the start of the process that, under the appropriate conditions (e.g., based on the duration and size of the insult), leads to, a series of linked KEs to the final adverse outcome of regulatory concern, for example specific organ toxicity in a human or at a population level (Krewski et al., 2020). Essentially, a KE reflects the state of the system at a particular stage or time, while a KER describes the reasons or conditions for the system transiting from one KE (upstream) to another KE further (downstream) (Krewski et al., 2020). The typical progression of adverse events in terms of following exposure at the ALI is shown in Fig. 10.
Fig. 10.
The progression of adverse events following exposure at the ALI based on Peitsch et al., 2018.
Luettich et al. (2017) proposed an AOP for reduced lung function through the activation of EGFR via reactive oxygen species, leading to excessive mucous production and ultimately leading to decreased lung function. Whilst we had not conducted transcriptomics with this study or focused on the AOP as one of the initial goals of the study, we did see evidence of oxidative stress with the elevation of MMPs and cytokines. EGFR activation from oxidative stress has been demonstrated previously by several authors (cited in (Luettich et al., 2017). We did not measure decreased apoptosis in goblet cells, and only saw minor increases in mucus production for 3R4F and EVP, this however may have been related to increasing cytotoxicity at the 90 puffs of 3R4F and possible irritation at lower doses. With the positive treatment using IL-13, there was a large increase in mucus production demonstrating that given the correct stimulus, the 3D model cells are able to undergo goblet cell hyperplasia.
Exposure to 60 and 90 puffs of 3R4F lead to loss of cilia measured in terms of FOX-J1, CAA and CBF. The loss of cilia and conversion of cells from ciliated epithelial cells to goblet cells was seen with the positive control (IL-13), leading to a decreased FOX-J1 and increased MUC5AC. The source of IL-13 in humans has an immune component particularly macrophages being activated by natural killer T cells (NKT). This innate immune axis is also activated in the lungs of humans with chronic airway disease, such as asthma or COPD (Kim et al., 2008). The lack of an immune element in the 3D model could explain the lack of goblet cell hyperplasia seen in our studies. Our repeated exposure in vitro study demonstrated significant elements of the AOP described in Fig. 10, minus the involvement of the immune system, proposed by Luettich et al. Other non-IL-13 based pathways could also exist. The evidence to support this key event KE2 to KE3 (decreased epithelial cell apoptosis leading directly to trans differentiation into goblet cells) was stated to be weak (Luettich et al., 2017). Further studies to target this exact mechanism could be performed in the future. The derivation of goblet cells from ciliated progenitor cells has been demonstrated by Turner et al. (2011), with human bronchial epithelial cells expressing FOX-J1 following IL-13 treatment (Turner et al., 2011).
4.6. Long term effects of using EVPs are uncertain
This study looked the sub-acute effects of repeated exposure of 3D lung tissues to EVP. We believe that 28 days with repeated in vitro exposure is getting toward the limits of this study, however obviously longer term human clinical studies do exist but the results of these are not entirely clear for the THR potential of EVP. Various expert bodies have reviewed the long-term potential risks of switching from combusted cigarettes to EVPs. The National Academies of Science Engineering and Medicine on their review of the Health Effects of Electronic Nicotine Delivery Systems concluded, “Much of the research on e-cigarettes suffers from methodological flaws, and many important areas have not yet been researched. Nonetheless, the committee found sufficient literature to suggest that, while there are risks associated with e-cigarettes, compared with combustible tobacco cigarettes, e-cigarettes contain fewer toxicants; can deliver nicotine in a manner similar to combustible tobacco cigarettes; show significantly less biological activity in a number of in vitro, animal, and human systems; and might be useful as a cessation aid to smokers who use e-cigarettes exclusively.” (Eaton et al., 2018). We conclude with this and believe that EVPs can play an important role in THR for adult cigarette smokers. They are not without their own risks, however, are potentially reduced risk when compared cigarettes. People with asthma and COPD have generally shown improvements in respiratory health following the switch to EVPs from smoking (Polosa et al., 2016, Polosa et al., 2018).
A recent clinical trial found that adult smokers who switched to EVPs for 1 month showed significant improvements in endothelial function, arterial stiffness and systolic blood pressure compared with continuing smoking, and that these effects may be greater for females (George et al., 2019). Hartmann-Boyce et al., 2020 reviewed 50 completed studies, representing 12,430 participants, of which 26 were randomized clinical trials and found that for adverse events and serious adverse events and other safety markers, the confidence intervals were wide, however, the overall the incidence of serisous adverse events was low across all study arms (Hartmann-Boyce et al., 2020). The authors did not detect any clear evidence of harm from nicotine EVPs but noted that the longest study follow‐up was two years and the overall number of studies was small. In a smaller study with 209 particiapnts, the safety profile of Puritane™, a closed system EVP, was evaluated when used by smokers of conventional cigarettes for 2 years in a real-life setting. Mesured outcomes included adverse events, vital signs, electrocardiogram, lung function tests, exposure to nicotine and selected smoke constituents, nicotine withdrawal effects and smoking desire. The authors reported no serious adverse events related to EVP use were observed. The most frequently reported adverse events were headache, nasopharyngitis, sore throat and cough, reported by 28.7%,to 16.7% of subjects, respectively, which decreased with incresing study duration. No clinically relevant findings were observed in the other safety parameters measured (Walele et al., 2018).
4.7. Study limitations
The modelling of a complex disease state such as COPD and related disease pathologies may not be possible with a single in vitro model, despite it being more physiologically relevant and an improvement in comparison to monolayer cultures. Although we assessed endpoints related to chronic respiratory diseases, the model tested is mainly composed of bronchial epithelial cells, therefore the bronchial 3D model cannot fully reflect the pathophysiology of lower respiratory tract conditions, such as emphysema. To do so, we may need to incorporate an alveolar model into the future testing strategies aiming at this specific disease state. As discussed above, the lack of cigarette smoke induced goblet cell hyperplasia may be related to the absence of immune cells in the 3D lung model. Marescotti et al. (2019) reported that increasing the number of different cell types in a lung epithelial mode will increase the human physiological relevance of a model. This could be addressed in future studies by the utilization of co-culture systems with immune cells, or a combined multi-organ model. Within this study, we have assessed the closed pod system EVP device with one, commercially available e-liquids with tobacco flavoring. Subsequent studies could include e-liquids with and without flavors and nicotine to identify which, if any, components were driving the activity observed in the assessed endpoints.
In response to the high levels of cytotoxicity observed for the 90-puff dose of 3R4F from T23 onwards, the authors excluded the pro-inflammatory marker analysis, CBF and CAA from T23 onwards. The high cytotoxicity is very likely to have driven the reduced tissue functionality as measured by cilia activity and supported by TEER results. Despite 1:17 dilution, the 90 puff 3R4F dose could not be sustained for the full duration of the study. Future studies should consider a lower sub-cytotoxic dose of repeated cigarette smoke to enable multiple exposures for the full duration of the study.
Although we have not looked at transcriptomics in this study, in a related study by Phillips et al. (2021) assessed the acute response to non-cytotoxic doses of myblu and 3R4F. No pathological changes were observed at either recovery time point from any exposure (48 h). Air and EVP aerosol exposure had no effect on CBF, CAA nor TEER at 48 h. Exposure to cigarette smoke resulted in a decrease in TEER and an increase in CBF and the release of proinflammatory cytokines at both recovery time points. Although the number of significantly expressed genes was minimal following exposure to EVP aerosol, exposure to 3R4F smoke resulted in a significant upregulation of several disease relevant pathways (oxidative stress and inflammation) (Phillips et al., 2021). These functional findings as expected are mirrored in the current longer-term study we report here (28 days) with EVP and 3R4F. Ito et al. (2020) conducted transcriptomics analysis during 40 days exposure to cigarette TPM. The canonical pathway analysis with ingenuity pathway analysis software revealed that perturbed biological pathways were similar throughout the cigarette TPM exposure, including inflammatory response-related and oxidative stress (e.g., IL-6, IL-8 and NF-κB-signalling pathway) similar effects were seen in Philips et al. (2021) and this paper. Future studies will consider the uses of transcriptomics.
A single tissue donor was utilized in this study. As with all in vitro studies this will not fully reflect the response variability within a population. A recent study by Bovard et al. (2020) compared 3D lung cultures derived from various donors, in terms of tissue morphology, functionality and metabolic capacity. The authors found that models from different donors presented with diverse morphology but similar functionality and metabolic activity, with certain variability in their response to stimulation. In addition, Bovard et al. (2020) reported that studies assessing donor variation have shown there is a preserved response to certain stimuli across donors, despite variations in intensity, including proinflammatory mediators and gene expression changes following cigarette smoke exposure (Banerjee et al., 2017, Bovard et al., 2020, Haswell et al., 2017, Iskandar et al., 2017). Future studies could consider assessing the long-term repeated exposure on a diseased tissue, e.g. derived from a COPD patient, and compare its response to that of a healthy donor.
5. Conclusions
Repeated exposure of 3D lung model at the ALI for 4 weeks better recapitulates the product use than a single point acute exposure. Chronic respiratory diseases develop over many years, and as expected cannot be completely replicated in the current model. Overall, these results add to the growing weight-of-evidence in the scientific literature showing EVPs are likely to be potentially less harmful than combustible cigarettes due to reduced potential exposure to toxicants when used as part of a larger set of studies (Czekala, et al., 2019). The data highlights a clear difference in the in vitro toxicological responses between cigarette smoke and EVP aerosol under the study conditions. Exposure to EVP aerosol did not trigger any significant changes in the level of pro-inflammatory mediators, when compared with the air controls. Although there appears to be an upward trend for the higher EVP concentration at the later timepoints, the changes were small, temporary, and consistently less when compared to diluted 3R4F smoke. CAA was significantly decreased after repeated exposure to 90 puffs of EVP aerosol from T16, however, the mechanism of action is unclear. Other endpoints such as CBF, TEER, mucus production and cytotoxicity were not significantly different compared with air controls. By contrast, exposure to diluted cigarette smoke led to dramatic changes in tissue morphology (T26 for 60 puffs and T14 for 90 puffs 3R4F), significantly decreased ciliary functions and cilia numbers at all doses tested. Whilst exposure to 60 and 90 puffs of 3R4F did not induce goblet cell hyperplasia histologically, increased levels of pro-inflammatory mediators indicates an inflammatory response in response to increasing cytotoxicity. Exposure to the sub-cytotoxic dose of 30 puffs 3R4F smoke, did not induce goblet cell hyperplasia.
Due to non-dilution, EVP aerosol delivered significantly more nicotine to the 3D lung model than 3R4F cigarette smoke. However, as mentioned above most endpoints were not significantly different compared to air controls. Thereby demonstrating it is potentially not nicotine, but other harmful and potentially harmful constituents found in cigarette smoke that cause, or potentially cause, smoking-related diseases. This supports the inclusion of EVPs in THR strategies for adult smokers who would otherwise continue to smoke. The in vitro data presented here indicates a reduced risk potential for EVP aerosol when compared to cigarette smoke, however, it is important to note that no tobacco or nicotine-containing product is absolutely risk free.
6. Funding sources
This work was funded and supported by Imperial Brands PLC.
CRediT authorship contribution statement
Lukasz Czekala: Conceptualization, Project administration, Methodology, Writing - original draft, Writing - review & editing. Roman Wieczorek: Project administration, Methodology, Visualization, Supervision. Liam Simms: Methodology, Writing - original draft, Writing - review & editing. Fan Yu: Writing - original draft, Writing - review & editing, Visualization. Jessica Budde: Investigation, Formal analysis, Visualization. Edgar Trelles Sticken: Investigation, Methodology, Visualization, Data curation. Kathryn Rudd: Writing - original draft, Writing - review & editing. Thomas Verron: Formal analysis, Visualization, Software. Oleg Brinster: Investigation. Matthew Stevenson: Conceptualization, Writing - original draft, Writing - review & editing. Tanvir Walele: Supervision, Funding acquisition.
Declaration of Competing Interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: All authors were employees of Imperial Brands PLC or subsidiaries at the time of this study. Imperial Brands PLC is the sole source of funding and sponsor of this project. Fontem Ventures B.V., the manufacturer of the EVP used in this study, is a wholly owned subsidiary of Imperial Brands PLC.
Acknowledgments
Acknowledgements
The authors would like to acknowledge the staff at Imperial Brands PLC Laboratory Network and Scientific Research & Harm Reduction teams for their contribution in conducting these experiments and data analysis. The authors would also like to thank Imperial Brands PLC internal Reading Committee for their critical review of the manuscript.
Lukasz Czekala conducted part of this work whilst also working towards a Ph.D. in Translational Biomedicine at the Catania University.
References
- Aghapour M. Airway epithelial barrier dysfunction in chronic obstructive pulmonary disease: role of cigarette smoke exposure. Am. J. Respir. Cell. Mol. Biol. 2018;58(2):157–169. doi: 10.1165/rcmb.2017-0200TR. [DOI] [PubMed] [Google Scholar]
- Balharry D., Sexton K., BéruBé K.A. An in vitro approach to assess the toxicity of inhaled tobacco smoke components: Nicotine, cadmium, formaldehyde and urethane. Toxicology. 2008;244(1):66–76. doi: 10.1016/j.tox.2007.11.001. [DOI] [PubMed] [Google Scholar]
- Banerjee A. Differential gene expression using RNA sequencing profiling in a reconstituted airway epithelium exposed to conventional cigarette smoke or electronic cigarette aerosols. Appl. In Vitro Toxicol. 2017;3(1):84–98. [Google Scholar]
- Barnes P.J. Chronic Obstructive Pulmonary Disease: A Growing but Neglected Global Epidemic. PLOS Medicine. 2007;4(5) doi: 10.1371/journal.pmed.0040112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes P.J. The cytokine network in chronic obstructive pulmonary disease. Am. J. Respirat. Cell Mol. Biol. 2009;41(6):631–638. doi: 10.1165/rcmb.2009-0220TR. [DOI] [PubMed] [Google Scholar]
- Barnes T.C., Anderson M.E., Moots R.J. The many faces of interleukin-6: the role of il-6 in inflammation, vasculopathy, and fibrosis in systemic sclerosis. Int. J. Rheumatol. 2011;2011 doi: 10.1155/2011/721608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrsing Holger, Raabe Hans, Manuppello Joseph, Bombick Betsy, Curren Rodger, Sullivan Kristie, Sethi Sanjay, Phipps Richard, Tesfaigzi Yohannes, Yan Sherwin, D'Ruiz Carl, Tarran Robert, Constant Samuel, Phillips Gary, Gaça Marianna, Hayden Patrick, Cao Xuefei, Mathis Carole, Hoeng Julia, Braun Armin, Hill Erin. Assessment of in vitro COPD models for tobacco regulatory science: workshop proceedings, conclusions and paths forward for in vitro model use. Alternat. Labor. Anim. 2016;44(2):129–166. doi: 10.1177/026119291604400206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamini Y., Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R. Stati. Soc. Ser. B (Methodol.) 1995;57(1):289–300. [Google Scholar]
- Bishop E. An approach to testing undiluted e-cigarette aerosol in vitro using 3D reconstituted human airway epithelium. Toxicol. In Vitro. 2019;54:391–401. doi: 10.1016/j.tiv.2018.01.010. [DOI] [PubMed] [Google Scholar]
- Borthwick L.A. The IL-1 cytokine family and its role in inflammation and fibrosis in the lung. Semin. Immunopathol. 2016;38(4):517–534. doi: 10.1007/s00281-016-0559-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bovard D. Comparison of the basic morphology and function of 3D lung epithelial cultures derived from several donors. Curr. Res. Toxicol. 2020;1:56–69. doi: 10.1016/j.crtox.2020.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandsma C.-A. Recent advances in chronic obstructive pulmonary disease pathogenesis: from disease mechanisms to precision medicine. J. Pathol. 2020;250(5):624–635. doi: 10.1002/path.5364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brekman A. FOXJ1 prevents cilia growth inhibition by cigarette smoke in human airway epithelium in vitro. Am. J. Respirat. Cell Mol. Biol. 2014;51(5):688–700. doi: 10.1165/rcmb.2013-0363OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brusselle G.G., Joos G.F., Bracke K.R. New insights into the immunology of chronic obstructive pulmonary disease. Lancet. 2011;378(9795):1015–1026. doi: 10.1016/S0140-6736(11)60988-4. [DOI] [PubMed] [Google Scholar]
- Cervena T., Vrbova K., Topinka J., Rössner P., Jr. In vitro cytotoxicity and gene expression analysis of air-liquid interface model (Mucilair™) after exposure to PAHs. Toxicol. Lett. 2018;295:S131. doi: 10.1016/j.toxlet.2018.06.695. [DOI] [Google Scholar]
- Chung K.F. Cytokines in chronic obstructive pulmonary disease. European Respiratory Journal. 2001;18(34 suppl):50s–59s. [PubMed] [Google Scholar]
- Chung S. Electronic cigarette vapor with nicotine causes airway mucociliary dysfunction preferentially via TRPA1 receptors. Am. J. Respirat. Crit. Care Med. 2019;200(9):1134–1145. doi: 10.1164/rccm.201811-2087OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czekala, L., et al., Toxicological comparison of cigarette smoke and e-cigarette aerosol using a 3D in vitro human respiratory model. Regul Toxicol Pharmacol, 2019. 103: p. 314-324 [DOI] [PubMed]
- Devine J.F. Chronic obstructive pulmonary disease: an overview. Am. Health Drug Benefits. 2008;1(7):34–42. [PMC free article] [PubMed] [Google Scholar]
- Dvorak A. Do airway epithelium air-liquid cultures represent the in vivo airway epithelium transcriptome? Am. J. Respir. Cell Mol. Biol. 2011;44(4):465–473. doi: 10.1165/rcmb.2009-0453OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Academies of Sciences, E., et al., in Public Health Consequences of E-Cigarettes, D.L. Eaton, L.Y. Kwan, and K. Stratton, Editors. 2018, National Academies Press (US) Copyright 2018 by the National Academy of Sciences. All rights reserved: Washington (DC). [PubMed]
- National Academies of Sciences Engineering and Medicine, et al., in Public Health Consequences of E-Cigarettes, D.L. Eaton, L.Y. Kwan, and K. Stratton, Editors. 2018, National Academies Press (US): Washington (DC). [PubMed]
- Elson K.M. Non-destructive monitoring of viability in an ex vivo organ culture model of osteochondral tissue. Eur. Cell Mater. 2015;29:356–369. doi: 10.22203/ecm.v029a27. discussion 369. [DOI] [PubMed] [Google Scholar]
- Epithelix. MucilAir™: A unique 3D Human Airway Epithelia reconstituted in vitro. 2020 [cited 2020 26/05/2020]; Available from: http://www.epithelix.com/products/mucilair.
- Evans C.M. The polymeric mucin Muc5ac is required for allergic airway hyperreactivity. Nat. Commun. 2015;6(1):6281. doi: 10.1038/ncomms7281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields W. Characterization and application of the VITROCELL VC1 smoke exposure system and 3D EpiAirway models for toxicological and e-cigarette evaluations. Appl. In Vitro Toxicol. 2017;3(1):68–83. [Google Scholar]
- Fujita, M. The Role of MMPs in the Progression of Chronic Lung Inflammatory Diseases, Lung Inflammation. 2014 [cited 2020 27/05/2020]; Available from: https://www.intechopen.com/books/lung-inflammation/the-role-of-mmps-in-the-progression-of-chronic-lung-inflammatory-diseases.
- Gagliardo R. Cigarette smoke alters primary human bronchial epithelial cell (PBEC) differentiation at air-liquid interface (ALI) and induces expression of CD105 and CD146. Eur. Respirat. J. 2016;48(suppl 60):PA949. [Google Scholar]
- George J. Cardiovascular effects of switching from tobacco cigarettes to electronic cigarettes. J. Am. Coll. Cardiol. 2019;74(25):3112–3120. doi: 10.1016/j.jacc.2019.09.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gindele J.A. Intermittent exposure to whole cigarette smoke alters the differentiation of primary small airway epithelial cells in the air-liquid interface culture. Sci. Rep. 2020;10(1):6257. doi: 10.1038/s41598-020-63345-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goniewicz Maciej L., Gawron Michal, Smith Danielle M., Peng Margaret, Jacob Peyton, III, Benowitz Neal L. Exposure to nicotine and selected toxicants in cigarette smokers who switched to electronic cigarettes: a longitudinal within-subjects observational study. Nicotine Tob. Res. 2017;19(2):160–167. doi: 10.1093/ntr/ntw160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman R.B. Cytokine-mediated inflammation in acute lung injury. Cytokine Growth Factor Rev. 2003;14(6):523–535. doi: 10.1016/s1359-6101(03)00059-5. [DOI] [PubMed] [Google Scholar]
- Hahn H.L., Kleinschrot D., Hansen D. Nicotine increases ciliary beat frequency by a direct effect on respiratory cilia. Clin. Investig. 1992;70(3–4):244–251. doi: 10.1007/BF00184658. [DOI] [PubMed] [Google Scholar]
- Hartmann-Boyce, J., et al., 2020. Electronic cigarettes for smoking cessation. Cochrane Database Syst Rev. 10: p. Cd010216 [DOI] [PMC free article] [PubMed]
- Haswell L.E. Cigarette smoke total particulate matter increases mucous secreting cell numbers in vitro: a potential model of goblet cell hyperplasia. Toxicol. In Vitro. 2010;24(3):981–987. doi: 10.1016/j.tiv.2009.12.019. [DOI] [PubMed] [Google Scholar]
- Haswell L.E. In vitro RNA-seq-based toxicogenomics assessment shows reduced biological effect of tobacco heating products when compared to cigarette smoke. Sci. Rep. 2018;8(1):1145. doi: 10.1038/s41598-018-19627-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haswell Linsey E., Baxter Andrew, Banerjee Anisha, Verrastro Ivan, Mushonganono Jessica, Adamson Jason, Thorne David, Gaça Marianna, Minet Emmanuel. Reduced biological effect of e-cigarette aerosol compared to cigarette smoke evaluated in vitro using normalized nicotine dose and RNA-seq-based toxicogenomics. Sci. Rep. 2017;7(1) doi: 10.1038/s41598-017-00852-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horiuchi T. Transmembrane TNF-α: structure, function and interaction with anti-TNF agents. Rheumatology. 2010;49(7):1215–1228. doi: 10.1093/rheumatology/keq031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang B. Effects of cigarette smoking on cardiovascular-related protein profiles in two community-based cohort studies. Atherosclerosis. 2016;254:52–58. doi: 10.1016/j.atherosclerosis.2016.09.014. [DOI] [PubMed] [Google Scholar]
- Institute of Medicine, Clearing the Smoke: Assessing the Science Base for Tobacco Harm Reduction, ed. K. Stratton, et al. 2001, Washington, DC: The National Academies Press. 656. [PubMed]
- Ishikawa S., Ito S. Repeated whole cigarette smoke exposure alters cell differentiation and augments secretion of inflammatory mediators in air-liquid interface three-dimensional co-culture model of human bronchial tissue. Toxicol. In Vitro. 2017;38:170–178. doi: 10.1016/j.tiv.2016.09.004. [DOI] [PubMed] [Google Scholar]
- Iskandar A.R. A systems toxicology approach for comparative assessment: Biological impact of an aerosol from a candidate modified-risk tobacco product and cigarette smoke on human organotypic bronchial epithelial cultures. Toxicol. In Vitro. 2017;39:29–51. doi: 10.1016/j.tiv.2016.11.009. [DOI] [PubMed] [Google Scholar]
- Iskandar Anita R., Gonzalez-Suarez Ignacio, Majeed Shoaib, Marescotti Diego, Sewer Alain, Xiang Yang, Leroy Patrice, Guedj Emmanuel, Mathis Carole, Schaller Jean-Pierre, Vanscheeuwijck Patrick, Frentzel Stefan, Martin Florian, Ivanov Nikolai V., Peitsch Manuel C., Hoeng Julia. A framework for in vitro systems toxicology assessment of e-liquids. Toxicol. Mech. Methods. 2016;26(6):392–416. doi: 10.3109/15376516.2016.1170251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iskandar Anita R., Martin Florian, Leroy Patrice, Schlage Walter K., Mathis Carole, Titz Bjorn, Kondylis Athanasios, Schneider Thomas, Vuillaume Grégory, Sewer Alain, Guedj Emmanuel, Trivedi Keyur, Elamin Ashraf, Frentzel Stefan, Ivanov Nikolai V., Peitsch Manuel C., Hoeng Julia. Comparative biological impacts of an aerosol from carbon-heated tobacco and smoke from cigarettes on human respiratory epithelial cultures: a systems toxicology assessment. Food Chem. Toxicol. 2018;115:109–126. doi: 10.1016/j.fct.2018.02.063. [DOI] [PubMed] [Google Scholar]
- Iskandar Anita R., Zanetti Filippo, Kondylis Athanasios, Martin Florian, Leroy Patrice, Majeed Shoaib, Steiner Sandro, Xiang Yang, Ortega Torres Laura, Trivedi Keyur, Guedj Emmanuel, Merg Celine, Frentzel Stefan, Ivanov Nikolai V., Doshi Utkarsh, Lee Kyeonghee Monica, McKinney Willie J., Jr, Peitsch Manuel C., Hoeng Julia. A lower impact of an acute exposure to electronic cigarette aerosols than to cigarette smoke in human organotypic buccal and small airway cultures was demonstrated using systems toxicology assessment. Int. Emerg. Med. 2019;14(6):863–883. doi: 10.1007/s11739-019-02055-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito S. In vitro long-term repeated exposure and exposure switching of a novel tobacco vapor product in a human organotypic culture of bronchial epithelial cells. J. Appl. Toxicol. 2020 doi: 10.1002/jat.3982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim E.Y. Persistent activation of an innate immune response translates respiratory viral infection into chronic lung disease. Nat. Med. 2008;14(6):633–640. doi: 10.1038/nm1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim V., Criner G.J. Chronic bronchitis and chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2013;187(3):228–237. doi: 10.1164/rccm.201210-1843CI. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraen M. Matrix Metalloproteinases in COPD and atherosclerosis with emphasis on the effects of smoking. PLOS ONE. 2019;14(2) doi: 10.1371/journal.pone.0211987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krewski D. Toxicity testing in the 21st century: progress in the past decade and future perspectives. Arch Toxicol. 2020;94(1):1–58. doi: 10.1007/s00204-019-02613-4. [DOI] [PubMed] [Google Scholar]
- Kucharska Małgorzata, Wesołowski Wiktor, Czerczak Sławomir, Soćko Renata. Testing of the composition of e-cigarette liquids – Manufacturer-declared vs. true contentsin a selected series of productsBadanie składu płynów do e-papierosów – deklaracje producentaa stan rzeczywisty w wybranej serii wyrobów. Med. Pr. 2016;67(2):239–253. doi: 10.13075/mp.5893.00365. [DOI] [PubMed] [Google Scholar]
- International Organization for Standardization, ISO 3402:1999, in Tobacco and tobacco products — Atmosphere for conditioning and testing. 1999: https://www.iso.org/standard/28324.html. p. 5.
- Kuehn, D., et al., Impact assessment of repeated exposure of organotypic 3D bronchial and nasal tissue culture models to whole cigarette smoke. J Vis Exp, 2015(96). [DOI] [PMC free article] [PubMed]
- Kuschner W.G. Dose-dependent cigarette smoking-related inflammatory responses in healthy adults. Eur. Respir. J. 1996;9(10):1989–1994. doi: 10.1183/09031936.96.09101989. [DOI] [PubMed] [Google Scholar]
- Leclercq B., Happillon M., Antherieu S., Hardy E.M., Alleman L.Y., Grova N., Perdrix E., Appenzeller B.M., Lo Guidice J.-M., Coddeville P., Garçon G. Differential responses of healthy and chronic obstructive pulmonary diseased human bronchial epithelial cells repeatedly exposed to air pollution-derived PM4. Environ. Pollut. 2016;218:1074–1088. doi: 10.1016/j.envpol.2016.08.059. [DOI] [PubMed] [Google Scholar]
- Levitz, J.S., T.P. Bradley, and A.L. Golden, Overview of smoking and all cancers. Med Clin North Am, 2004. 88(6): p. 1655-75, xiii [DOI] [PubMed]
- Luettich Karsta, Talikka Marja, Lowe Frazer, J, Haswell Linsey, E, Park Jennifer, Gaca Marianna, D, Hoeng Julia. The Adverse Outcome Pathway for Oxidative Stress-Mediated EGFR Activation Leading to Decreased Lung Function. Applied In Vitro Toxicology. 2017;3(1):99–109. doi: 10.1089/aivt.2016.0032. [DOI] [Google Scholar]
- Manicone A.M., McGuire J.K. Matrix metalloproteinases as modulators of inflammation. Semin. Cell Dev. Biol. 2008;19(1):34–41. doi: 10.1016/j.semcdb.2007.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marescotti D. How complex should an in vitro model be? Evaluation of complex 3D alveolar model with transcriptomic data and computational biological network models. Altex. 2019;36(3):388–402. doi: 10.14573/altex.1811221. [DOI] [PubMed] [Google Scholar]
- Margham Jennifer, McAdam Kevin, Forster Mark, Liu Chuan, Wright Christopher, Mariner Derek, Proctor Christopher. Chemical composition of aerosol from an e-cigarette: a quantitative comparison with cigarette smoke. Chem. Res. Toxicol. 2016;29(10):1662–1678. doi: 10.1021/acs.chemrestox.6b00188.s002. [DOI] [PubMed] [Google Scholar]
- Marone, G., et al., The Intriguing Role of Interleukin 13 in the Pathophysiology of Asthma. Frontiers in pharmacology, 2019. 10: p. 1387-1387. [DOI] [PMC free article] [PubMed]
- McNeill, A., et al., E-cigarettes: an evidence update, in A report commissioned by Public Health England. 2015, Public Health England: London.
- McNeill, A., et al., Evidence review of e-cigarettes and heated tobacco products 2018, in A report commissioned by Public Health England. London: Public Health England. 2018.
- Mercer R.R. Cell number and distribution in human and rat airways. Am. J. Respir. Cell Mol. Biol. 1994;10(6):613–624. doi: 10.1165/ajrcmb.10.6.8003339. [DOI] [PubMed] [Google Scholar]
- Mercer B.A. Identification of a cigarette smoke-responsive region in the distal MMP-1 promoter. Am. J. Respir. Cell Mol. Biol. 2009;40(1):4–12. doi: 10.1165/rcmb.2007-0310OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy, J., et al., Assessing modified risk tobacco and nicotine products: Description of the scientific framework and assessment of a closed modular electronic cigarette. Regul Toxicol Pharmacol, 2017. 90: p. 342-357 [DOI] [PubMed]
- Nachlas M.M. The determination of lactic dehydrogenase with a tetrazolium salt. Anal. Biochem. 1960;1(4):317–326. doi: 10.1016/0003-2697(60)90029-4. [DOI] [PubMed] [Google Scholar]
- National Center for Chronic Disease, P., S. Health Promotion Office on, and Health, Reports of the Surgeon General, in The Health Consequences of Smoking—50 Years of Progress: A Report of the Surgeon General. 2014, Centers for Disease Control and Prevention (US): Atlanta (GA).
- Navratilova Z. Simultaneous up-regulation of matrix metalloproteinases 1, 2, 3, 7, 8, 9 and tissue inhibitors of metalloproteinases 1, 4 in serum of patients with chronic obstructive pulmonary disease. Respirology. 2012;17(6):1006–1012. doi: 10.1111/j.1440-1843.2012.02197.x. [DOI] [PubMed] [Google Scholar]
- Neilson L. Development of an in vitro cytotoxicity model for aerosol exposure using 3D reconstructed human airway tissue; application for assessment of e-cigarette aerosol. Toxicol. In Vitro. 2015;29(7):1952–1962. doi: 10.1016/j.tiv.2015.05.018. [DOI] [PubMed] [Google Scholar]
- O’Connell Grant, Graff Donald W., D’Ruiz Carl D. Reductions in biomarkers of exposure (BoE) to harmful or potentially harmful constituents (HPHCs) following partial or complete substitution of cigarettes with electronic cigarettes in adult smokers. Toxicol. Mech. Methods. 2016;26(6):453–464. doi: 10.1080/15376516.2016.1196282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peitsch, M.C., et al., Next-generation tobacco and nicotine products: Substantiating harm reduction and supporting tobacco regulatory science. Toxicology Research and Application, 2018. 2: p. 2397847318773701
- Perniss A. Acute nicotine administration stimulates ciliary activity via α3β4 nAChR in the mouse trachea. Int. Immunopharmacol. 2020;84 doi: 10.1016/j.intimp.2020.106496. [DOI] [PubMed] [Google Scholar]
- Pezzulo Alejandro A., Starner Timothy D., Scheetz Todd E., Traver Geri L., Tilley Ann E., Harvey Ben-Gary, Crystal Ronald G., McCray Paul B., Jr., Zabner Joseph. The air-liquid interface and use of primary cell cultures are important to recapitulate the transcriptional profile of in vivo airway epithelia. Am. J. Physiol. Lung Cell. Mol. Physiol. 2011;300(1):L25–L31. doi: 10.1152/ajplung.00256.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips G. Acute electronic vapour product whole aerosol exposure of 3D human bronchial tissue results in minimal cellular and transcriptomic responses when compared to cigarette smoke. Toxicol. Res. Appl. 2021;5:1–19. [Google Scholar]
- Polosa R. Persisting long term benefits of smoking abstinence and reduction in asthmatic smokers who have switched to electronic cigarettes. Discov. Med. 2016;21(114):99–108. [PubMed] [Google Scholar]
- Polosa R. Health effects in COPD smokers who switch to electronic cigarettes: a retrospective-prospective 3-year follow-up. Int. J. Chron Obstruct Pulmon Dis. 2018;13:2533–2542. doi: 10.2147/COPD.S161138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing 2018; Available from: https://www.R-project.org/.
- Rayner R.E. Cigarette and ENDS preparations differentially regulate ion channels and mucociliary clearance in primary normal human bronchial 3D cultures. Am. J. Physiol. Lung Cell. Mol. Physiol. 2019;317(2):L295–L302. doi: 10.1152/ajplung.00096.2019. [DOI] [PubMed] [Google Scholar]
- Rayner Rachael E., Makena Patrudu, Prasad Gaddamanugu L., Cormet-Boyaka Estelle. Optimization of normal human bronchial epithelial (NHBE) cell 3D cultures for in vitro lung model studies. Sci. Rep. 2019;9(1) doi: 10.1038/s41598-018-36735-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reidel B. E-cigarette use causes a unique innate immune response in the lung, involving increased neutrophilic activation and altered mucin secretion. Am. J. Respir. Crit. Care Med. 2018;197(4):492–501. doi: 10.1164/rccm.201708-1590OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royal College of Physicians, Fifty years since Smoking and health. Progress, lessons and priorities for a smoke-free UK, in Report of conference proceedings. 2012, Royal College of Physicians,: London.
- Royal College of Physicians, Nicotine without smoke: Tobacco harm reduction. 2016, Royal College of Physicians,: London.
- Rudd Kathryn, Stevenson Matthew, Wieczorek Roman, Pani Jutta, Trelles-Sticken Edgar, Dethloff Ole, Czekala Lukasz, Simms Liam, Buchanan Francesca, O'Connell Grant, Walele Tanvir. Chemical composition and in vitro toxicity profile of a pod-based e-cigarette aerosol compared to cigarette smoke. Appl. In Vitro Toxicol. 2020;6(1):11–41. doi: 10.1089/aivt.2019.0015. [DOI] [Google Scholar]
- Sapey, E. and R.A. Stockley, The Importance of Chronic Bronchitis in Chronic Obstructive Pulmonary Disease, in Bronchitis, I. Martin-Loeches, Editor. 2011, IntechOpen.
- Schamberger Andrea C., Staab-Weijnitz Claudia A., Mise-Racek Nikica, Eickelberg Oliver. Cigarette smoke alters primary human bronchial epithelial cell differentiation at the air-liquid interface. Sci. Rep. 2015;5(1) doi: 10.1038/srep08163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahab Lion, Goniewicz Maciej L., Blount Benjamin C., Brown Jamie, McNeill Ann, Alwis K. Udeni, Feng June, Wang Lanqing, West Robert. Nicotine, carcinogen, and toxin exposure in long-term E-cigarette and nicotine replacement therapy users: a cross-sectional study. Ann. Int. Med. 2017;166(6):390. doi: 10.7326/M16-1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shamir Eliah R., Ewald Andrew J. Three-dimensional organotypic culture: experimental models of mammalian biology and disease. Nat. Rev. Mol. Cell. Biol. 2014;15(10):647–664. doi: 10.1038/nrm3873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Y. miR-200b inhibits TNF-α-induced IL-8 secretion and tight junction disruption of intestinal epithelial cells in vitro. Am. J. Physiol. Gastrointest Liver Physiol. 2017;312(2):G123–G132. doi: 10.1152/ajpgi.00316.2016. [DOI] [PubMed] [Google Scholar]
- Sisson J.H., Tuma D.J., Rennard S.I. Acetaldehyde-mediated cilia dysfunction in bovine bronchial epithelial cells. Am. J. Physiol. 1991;260(2 Pt 1):L29–L36. doi: 10.1152/ajplung.1991.260.2.L29. [DOI] [PubMed] [Google Scholar]
- Song J.S. Nitric oxide induces MUC5AC mucin in respiratory epithelial cells through PKC and ERK dependent pathways. Respirat. Res. 2007;8(1):28. doi: 10.1186/1465-9921-8-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steeland S., Libert C., Vandenbroucke R.E. A new venue of TNF targeting. Int. J. Mol. Sci. 2018;19(5):1442. doi: 10.3390/ijms19051442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka T., Narazaki M., Kishimoto T. IL-6 in inflammation, immunity, and disease. Cold Spring Harb. Perspect. Biol. 2014;6(10) doi: 10.1101/cshperspect.a016295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilley A.E. Cilia dysfunction in lung disease. Annu. Rev. Physiol. 2015;77:379–406. doi: 10.1146/annurev-physiol-021014-071931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner J. Goblet cells are derived from a FOXJ1-expressing progenitor in a human airway epithelium. Am. J. Respir. Cell Mol. Biol. 2011;44(3):276–284. doi: 10.1165/rcmb.2009-0304OC. [DOI] [PubMed] [Google Scholar]
- Valencia-Gattas M., Conner G.E., Fregien N.L. Gefitinib, an EGFR tyrosine kinase inhibitor, prevents smoke-mediated ciliated airway epithelial cell loss and promotes their recovery. PLoS One. 2016;11(8) doi: 10.1371/journal.pone.0160216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinken M. Adverse outcome pathways: a concise introduction for toxicologists. Arch. Toxicol. 2017;91(11):3697–3707. doi: 10.1007/s00204-017-2020-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walele Tanvir, Bush Jim, Koch Annelize, Savioz Rebecca, Martin Claire, O’Connell Grant. Evaluation of the safety profile of an electronic vapour product used for two years by smokers in a real-life setting. Regulatory Toxicology and Pharmacology. 2018;92:226–238. doi: 10.1016/j.yrtph.2017.12.010. [DOI] [PubMed] [Google Scholar]
- Wieczorek R. A comparative in vitro toxicity assessment of electronic vaping product e-liquids and aerosols with tobacco cigarette smoke. Toxicol. In Vitro. 2020;66 doi: 10.1016/j.tiv.2020.104866. [DOI] [PubMed] [Google Scholar]
- ISO 20778:2018, Cigarettes — Routine analytical cigarette smoking machine — Definitions and standard conditions with an intense smoking regime. 2018 [cited 2020 15th September]; Available from: https://www.iso.org/standard/69065.html.
- ISO 20768:2018, Vapour products — Routine analytical vaping machine — Definitions and standard conditions. 2018 [cited 2020 15th September]; Available from: https://www.iso.org/standard/69019.html.
- World Health Organization. Chronic respiratory diseases. 2020 12/11/2020]; Available from: https://www.who.int/health-topics/chronic-respiratory-diseases#tab=tab_1.
- Ye D., Ma I., Ma T.Y. Molecular mechanism of tumor necrosis factor-alpha modulation of intestinal epithelial tight junction barrier. Am. J. Physiol. Gastrointest. Liver Physiol. 2006;290(3):G496–G504. doi: 10.1152/ajpgi.00318.2005. [DOI] [PubMed] [Google Scholar]
- You Y. Role of f-box factor foxj1 in differentiation of ciliated airway epithelial cells. Am. J. Physiol. Lung Cell Mol. Physiol. 2004;286(4):L650–L657. doi: 10.1152/ajplung.00170.2003. [DOI] [PubMed] [Google Scholar]