Graphical abstract
Keywords: Liver cells, Detoxification, Pig, Model, Gene-expression, RT-PCR
Highlights
-
•
CYP mRNA induction were compared between human and porcine primary hepatocytes.
-
•
Both human and porcine primary hepatocytes responded to prototypical CYP inducers.
-
•
CYP mRNA induction displayed similar patterns in human and porcine primary hepatocytes.
Abstract
The hepatic cytochrome p450’s (CYP) are of major importance for the metabolism of xenobiotics and knowledge about their regulation is crucial. This knowledge often originates from cell models; primary human hepatocytes (PHH) being the gold standard. However, due to limited availability of high-quality human donor organs, basic knowledge on alternative models are needed. Primary porcine hepatocytes (PPH) have been suggested as an alternative to PHH. Unfortunately, data comparing the response in gene-transcription to standard CYP inducers between PHH and PPH are missing. In the present study we, cultured PHH and PPH under the same conditions, treated them with standard inducers of the CYP1-3 and determined the response in gene and protein expression. The results demonstrated that in both species TCDD and omeprazole caused an increase in CYP1A/B expression. In PPH, CITCO increased the content of CYP1A/B. For the CYP2B/C/D’s, phenobarbital and rifampicin caused increases in expression. For the CYP2D’s, TCDD and omeprazole caused increased gene expression in PPH, which were not the case for PHH. Both phenobarbital, rifampicin and omeprazole increased CYP3A expression in PHH and PPH. Moreover, TCDD increased the gene expression of CYP3A in PPH; this was not the case for PHH. Multivariate data analysis found no difference in gene expression between PHH and PPH for phenobarbital, rifampicin and CITCO. However, differential clustering was observed for TCDD and omeprazole. In conclusion, despite model specificity, there are a high number of similar responses, and experiments investigating mRNA regulation made in PPH permits for a reliable translation into human setting.
1. Introduction
The cytochrome P450s (CYP) are a major family of enzymes capable of conducting oxidative biotransformation of a broad range of drugs and xenobiotics. The CYPs are highly expressed in the liver and adapts rapidly to the challenges experienced by the individual. In humans, the CYP family consist of 57 genes (Lewis, 2004), of which subfamily 1, 2 and 3 are the most important ones for detoxification. Therefore, knowledge about the regulation of the CYP enzymes is of great importance to the scientific community and pharmacological industry. Most of this knowledge is generated using different model-systems like animal models, liver slices, primary hepatocytes, immortalized cell-line and re-constitutive enzymes.
For decades, human primary hepatocytes have been used to investigate the dynamic regulation of gene expression of the CYP families. Due to great resemblance with the in vivo condition, primary human hepatocytes (PHHs) are often considered a gold standard model compared to well establish cell lines like HepG2. Unfortunately, there use is limited by the cost and the scarce availability of donor liver tissue. Thus, several animal models have been used as donors to obtain primary cells. However, translation from animal models to humans are challenging due to species-specific differences in gene regulation. This has motivated several studies comparing different animal models to humans with respect to CYP activity and expression. Most focus has been on comparing constitutive CYP dependent metabolism in different species (Donato et al., 1999, Bogaards et al., 2000, Thörn et al., 2011), while comparison of gene induction is limited. In a previous study, Lu and Li (2001) compared the induction of the CYP1A and CYP3A dependent activity in hepatocytes isolated from both humans, rats and minipigs (Yukata-type). They observed a greater resemblance between human and porcine hepatocytes than between human and rat hepatocytes.
The transcriptional regulation of the CYPs is controlled by specific ligand activated transcription factors named xenoreceptors. Aryl hydrocarbon receptor (AhR) from the bHLH/PAS (basic helix-loop-helix-Per-ARNT-Sim) family regulates CYP family 1. Constitutive Androstane Receptor (NR1I3, CAR) and Pregnane X Receptor (NR1I2, PXR) belonging to the nuclear receptor family are the most important regulators of CYPs family 2 and 3, respectively.
The cross-talk between PXR and CAR create a complex network protecting against xenobiotics exposure. For instance, CYP2B6, a prototypical target of human CAR, can be efficiently induced by rifampicin, a selective agonist of human PXR. On the other hand, phenobarbital, a well-known inducer of CYP2B6, activates both CAR and PXR in humans. Rifampicin was therefore selected in our study as a specific ligand and activator of hPXR. Phenobarbital was selected for its ability to activate both hPXR and hCAR, while CITCO at low concentration specifically activates hCAR. Finally, TCDD and omeprazole were selected; TCDD for its ligand dependent ability to activate AhR, and omeprazole, that can activate AhR without binding to this receptor (Daujat et al., 1992). The used human xenoreceptors activators were selected to explore corresponding activities in pig counterpart.
The present study was then undertaken to evaluate the regulation of a comprehensive selection of CYPs, representing family 1, 2 and 3, in human and porcine primary hepatocytes isolated and cultured under similar conditions. Following exposure to prototypical ligands or activators for AhR, CAR and PXR, the response in gene and protein expression were analyzed using RT-PCR and western blotting, respectively.
2. Materials and methods
2.1. Donors
PHHs were isolated as described previously (Pichard et al., 2006) from liver resections performed in adult patients for medical reasons unrelated to our research program. Liver samples were obtained from the Biologic Resource Center of Montpellier University Hospital (CRB-CHUM; http://www.chumontpellier.fr; Biobank ID: BB-0033-00031), and this study benefitted from the expertise of Dr Benjamin Rivière (hepatogastroenterology sample collection) and Dr Edouard Tuaillon (CRB-CHUM manager). The patients’ clinical characteristics are presented in Table 1. The procedure was approved by the French Ethics Committee and written or oral consent was obtained from the patients.
Table 1.
Clinical characteristics of the liver donors.
| Liver | Age | Sex | Pathology |
|---|---|---|---|
| H-Liv1 | 81 | M | Metastasis of colic adenocarcinoma |
| H-Liv2 | 49 | F | Cholangiocarcinoma |
| H-Liv3 | 67 | M | Hepatocellular carcinoma |
| H-Liv4 | 74 | M | Metastasis of kidney cancer |
Hepatocytes from livers of four crossbreed (Landrace × Yorkshire sire and Duroc boar) female pigs (14.5 ± 0.6 kg) were isolated according to Rasmussen (2017). Immediately after killing with a bolt pistol the liver was removed and transferred to the lab for further processing. All pigs were treated according to Danish rules and regulation, and showed no sign of illness or abnormalities.
2.2. Culturing and treatment of hepatocytes
Following isolation, the hepatocytes were immediately seeded at a density of 250.000 cells pr. cm2 onto collagen coated culture dishes. Cells were seeded in Williams Media Essential (WME) supplemented with bovine serum albumin (3.75 mg/ml), insulin (5 µg/ml), transferrin (10 µg/ml), ascorbic acid (250 µg/ml), hydrocortisone (0.1 µM), glutamine (2 mM), penicillin and streptomycin (de Boussac et al., 2018) in the presence of 2% fetal bovine serum (FBS). Twenty-four hours after seeding media was renewed omitting FBS. Following additional 48 h, cells were treated with 10 nM TCDD (2,3,7,8-tetrachlorodibenzo-p-dioxine), 10 µM rifampicin, 500 µM phenobarbital, 1 µM CITCO (6-(4-chlorophenyl)imidazo[2,1-b][1,3]thiazole-5-carbaldehyde O-(3,4-dichlorobenzyl) oxime) or 100 µM omeprazole. mRNA and protein expression analysis were performed following 24 h and 48 h incubation with compounds, respectively.
2.3. Real-time PCR
Total RNA was extracted using the Trizol-reagent according to the protocol provided by the manufacture (Sigma-Aldrich). Equal amounts of RNA were reverse transcribed using either the MMLV Reverse Transcriptase kit (for human hepatocytes, Invitrogen) or iScript (for porcine hepatocytes, Bio-Rad.). For gene expression analysis of human hepatocytes, SyberGreen based RT-PCR were used according to Rasmussen et al. (2016), while for porcine hepatocytes TaqMan based RT-PCR were conducted according to Rasmussen et al. (2011). Human primers for SyberGreen and porcine primers and TaqMan probes are given in Supplementary data.
The mRNA expression was normalized to the mRNA expression of ribosomal protein large P0 (RPLP0). The Ct-value for RPLP0 was not different between treatments.
2.4. Western blotting
Before lysing of the hepatocytes in RIPA buffer (50 mM Tris, 150 mM NaCl, 2 mM EDTA, 1% Triton X 100, 0.5% sodium deoxycolate, 0.1% SDS; pH 7.4) containing 2 mM phenylmethylsulfonyl, the wells were washed with PBS. Equal amount of total protein was mixed 1:1 with Leammli-buffer and loaded onto Any KD gels (Bio-Rad). Following gel-electrophoresis, the proteins were electro-blotted onto PVDF membranes using the Turbo Transfer system (Bio-Rad). Membranes were blocked in TBST (50 mM Tris, 500 mM NaCl, 0.1% Tween 20; pH 7.4) supplemented with 2% dry-milk powder for 1 h at room temperature. Membranes were incubated over-night with primary antibodies, followed by carefully washing before incubating with horseradish peroxidase conjugated secondary antibodies at room temperature for 2 h. Following several washings, the specific protein was visualized using ECL substrate (Bio-Rad) and the ChemiDoc-XRS + workstation (Bio-Rad). Relative protein content was quantified using Image Lab (Bio-Rad) and normalized to the content of α-Tubulin (representative blots for α-Tubulin are given in Supplementary data).
The used antibodies were CYP1A (Santa Cruz 53241), CYP2B (Bio-Rad, WMA00171), CYP2C (Sigma-Aldrich, SAB2104858), CYP2D (Santa Cruz 130366), CYP3A (Millipore ab1254), and α-tubulin (Calbiochem CP06).
2.5. Statistics
For the comparison of mRNA and protein levels between treated and untreated cells, the untreated were arbitrary given the value of 1.0 within each donor. All data are given as mean ± standard error of the mean. For evaluating effects of treatments on mRNA levels Students paired t-test on delta Ct-values (Cttarget − CtRPLP0) were used.
Multivariate data analysis was performed using SIMCA 16.0. Principal component analysis was performed on UV-scaled reciproc-transformed data of fold changes compared to control. For analysis, the orthologs of the human and porcine CYP’s were as follows; hCYP1A1 = pCYP1A1; hCYP1A2 = pCYP1A2; hCYP1B1 = pCYP1B1; hCYP2B6 = pCYP2B22; hCYP2C8 = pCYP2C42; hCYP2D6 = pCYP2D25 and hCYP3A4 = pCYP3A22.
3. Results
To reduce the influence inherent to culture conditions, human and porcine hepatocytes were seeded at the same density and in the same culture medium. Forty-eight hours later they were incubated or not with 10 nM TCDD, 10 µM rifampicin, 500 mM phenobarbital, 1 µM CITCO or 100 µM omeprazole for 24 (gene expression analysis) or 48 h (protein expression analysis).
3.1. Regulation of CYP1 family
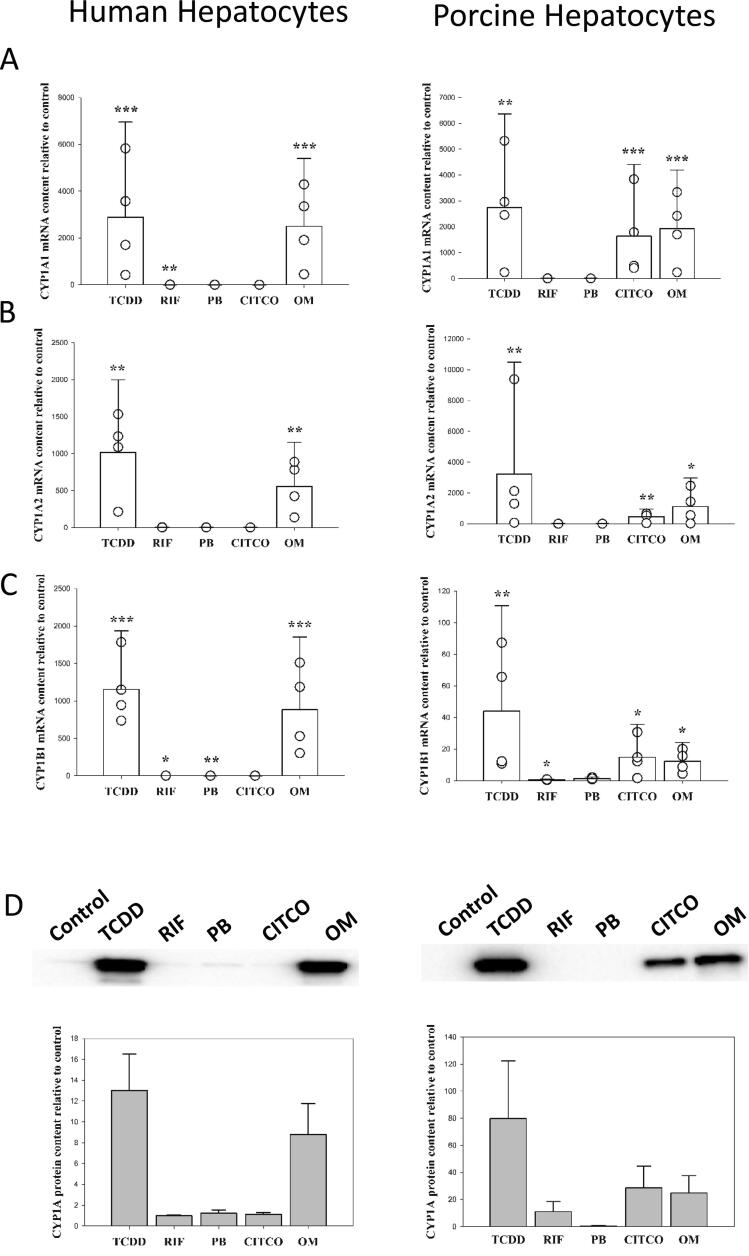
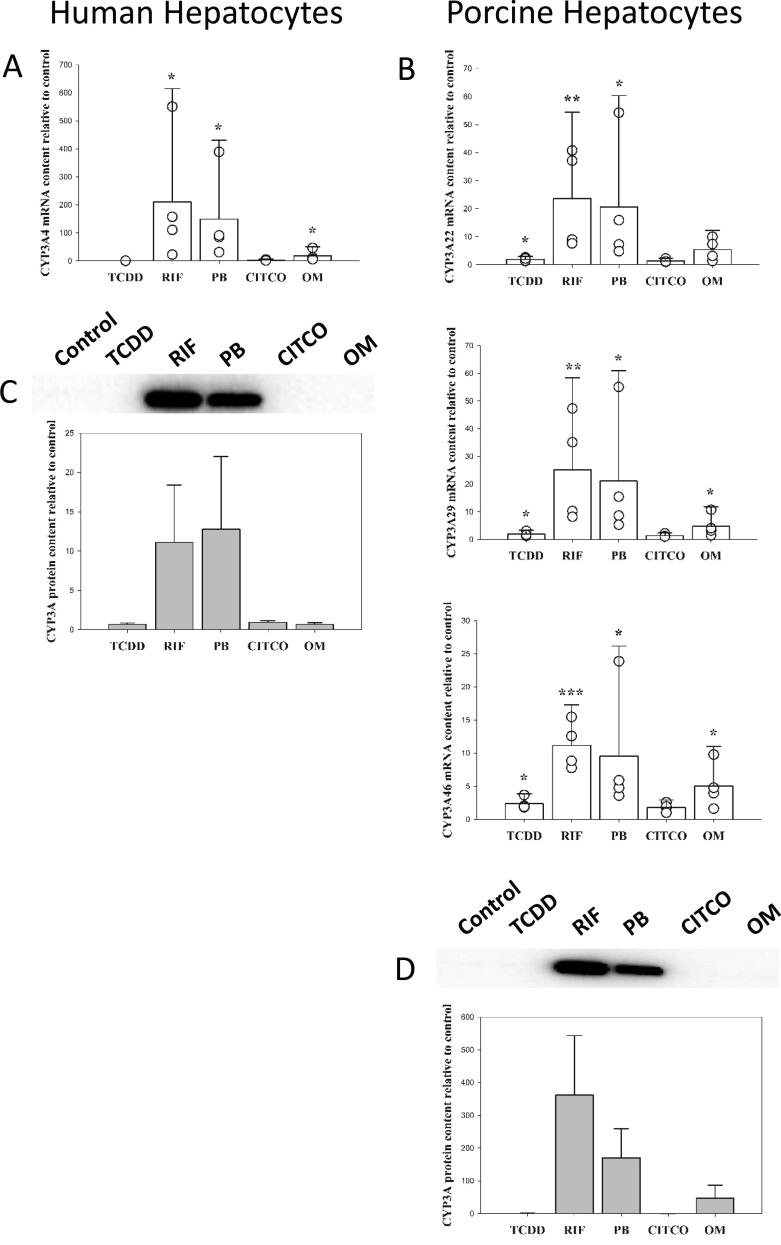
Both human and porcine hepatocytes responded to 24 h TCDD treatment by a substantial increase (1016.5 ± 981.0 and 3219.0 ± 7261.9-fold, respectively) of CYP1A2 mRNA expression (Fig. 1B). Even though, the average fold CYP1A2 induction observed were greater in the porcine hepatocytes compared to humans, this was not significantly different (p > 0.5), and large variation between donors within species was observed. In both species, treatment with omeprazole also caused a substantial increase (557.7 ± 595.6-fold for human and 1118.7 ± 1852.8-fold for porcine) of CYP1A2 mRNA expression (Fig. 1B). Surprisingly, CITCO caused a significant increase (460.8 ± 496.6 fold) of CYP1A2 mRNA content in porcine hepatocytes. The same effect of CITCO was not observed in human hepatocytes. Rifampicin and phenobarbital had no effect on CYP1A2 mRNA expression in either human or porcine hepatocytes (Fig. 1B). Similar responses in mRNA content following treatments were also observed for CYP1A1 (Fig. 1A) and CYP1B1 (Fig. 1C). Although it should be noticed that rifampicin decreased the mRNA content of porcine CYP1B1 (0.6 ± 0.3 fold), which was not the case for human CYP1B1.
Fig. 1.
mRNA and protein expression of the CYP1A and CYP1B family in primary hepatocytes from human and porcine donors following treatment with 10 nM TCDD (2,3,7,8-tetrachlorodibenzo-p-dioxine), 10 µM rifampicin (RIF), 500 µM phenobarbital (PB), 1 µM CITCO (6-(4-chlorophenyl)imidazo[2,1-b][1,3]thiazole-5-carbaldehyde O-(3,4-dichlorobenzyl) oxime) and 100 µM omeprazole (OM) for 24 (mRNA) or 48 (protein) hours. Data are given as mean + SEM relative to control. N = 4 for both species. *** (p < 0.001), ** (p < 0.01) and * (p < 0.05) significantly different from control treated hepatocytes.
The observed changes in mRNA expression corresponded with the observed induction in CYP1A protein level (Fig. 1D). In agreement, CYP1A expression were increased by TCDD and omeprazole in human hepatocytes and by TCDD, omeprazole and CITCO in porcine hepatocytes.
3.2. Regulation of CYP2 family
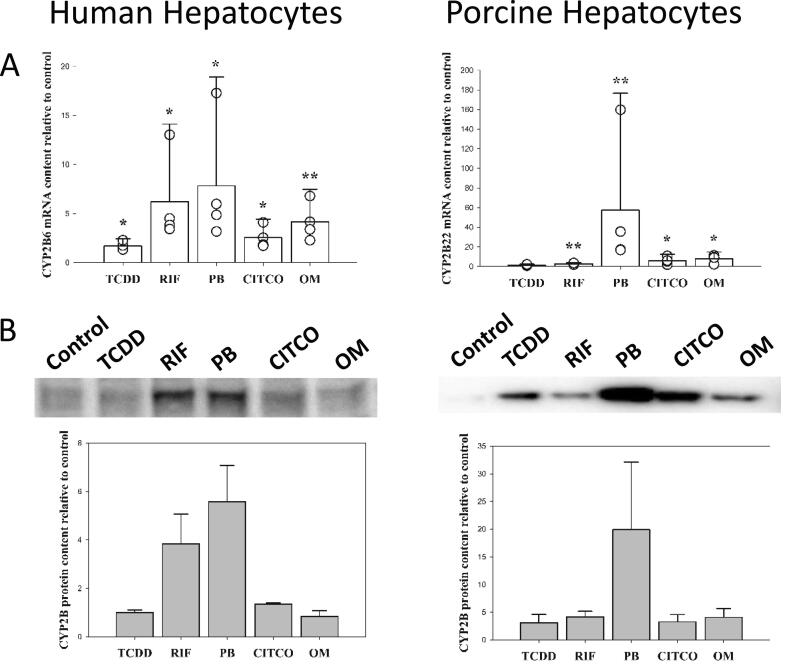
The porcine orthologue to human CYP2B6 is CYP2B22 (Puccinelli et al., 2011). In human and porcine hepatocytes all treatments, except TCDD for CYP2B22, caused a significant increase in CYP2B mRNA content (Fig. 2A). However, the observed increases in porcine hepatocytes were greater compared to what was observed in human hepatocytes, especially for phenobarbital (7.8 ± 11.1-fold vs. 57.3 ± 119.2-fold in human and porcine hepatocytes, respectively). At the protein level, induction of CYP2B6 and CYP2B22 by phenobarbital was observed (Fig. 2B). As expected, human CYP2B6 mRNA expression was induced following incubation with rifampicin (6.2 ± 7.9-fold). Induction by rifampicin of porcine CYP2B22 mRNA is of lower amplitude (2.7 ± 1.2-fold) and is not accompanied by an increase of CYP2B22 protein, in contrast to human CYP2B6 (Fig. 2B).
Fig. 2.
mRNA and protein expression of the CYP2B family in primary hepatocytes from human and porcine donors following treatment with 10 nM TCDD (2,3,7,8-tetrachlorodibenzo-p-dioxine), 10 µM rifampicin (RIF), 500 µM phenobarbital (PB), 1 µM CITCO (6-(4-chlorophenyl)imidazo[2,1-b][1,3]thiazole-5-carbaldehyde O-(3,4-dichlorobenzyl) oxime) and 100 µM omeprazole (OM) for 24 (mRNA) or 48 (protein) hours. Data are given as mean + SEM relative to control. N = 4 for both species. ** (p < 0.01) and * (p < 0.05) significantly different from control treated hepatocytes.
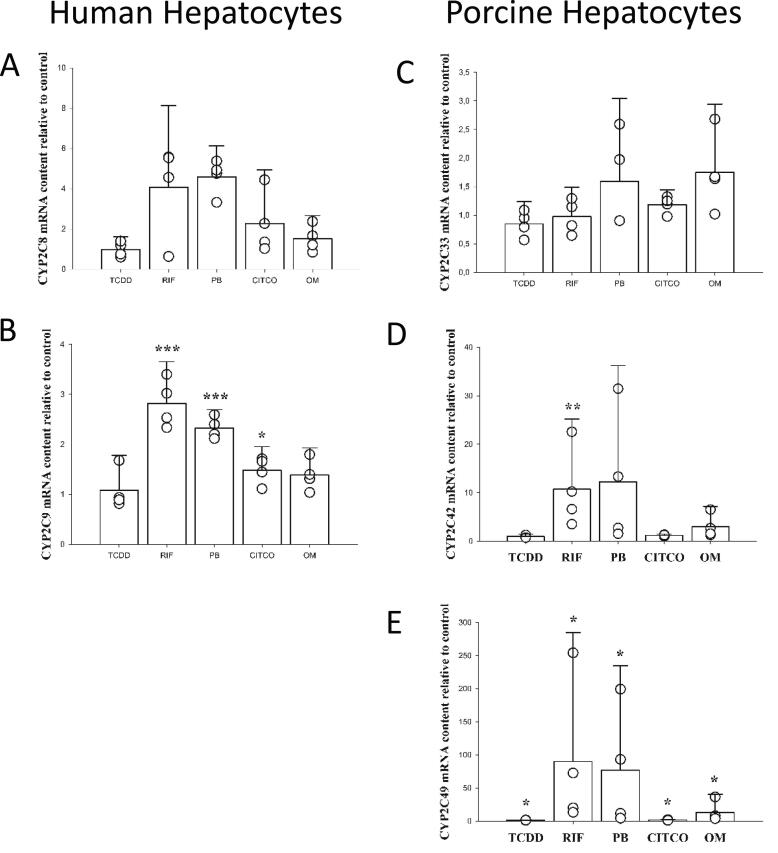
Human CYP2C8 and CYP2C9 mRNA expression was induced by phenobarbital (4.9 ± 1.5 and 2.3 ± 0.4-fold, respectively) and rifampicin (4.1 ± 4.1 and 2.8 ± 0.8-fold, respectively) (Fig. 3A-B). For the porcine CYP2C33, none of the used treatments caused significant induction (Fig. 3C). However, interestingly porcine CYP2C42 (Fig. 3D) and CYP2C49 (Fig. 3E) were induced by phenobarbital (12.3 ± 24.0 and 77.1 ± 157.3-fold, respectively) and rifampicin (10.7 ± 14.5 and 90.0 ± 195.0-fold, respectively). Although with a small magnitude, TCDD, CITCO and omeprazole also significantly increased the mRNA content of CYP2C49. The used antibody was not able to detect a band corresponding to human or porcine CYP2C.
Fig. 3.
mRNA expression of the CYP2C family in primary hepatocytes from human and porcine donors following treatment with 10 nM TCDD (2,3,7,8-tetrachlorodibenzo-p-dioxine), 10 µM rifampicin (RIF), 500 µM phenobarbital (PB), 1 µM CITCO (6-(4-chlorophenyl)imidazo[2,1-b][1,3]thiazole-5-carbaldehyde O-(3,4-dichlorobenzyl) oxime) and 100 µM omeprazole (OM) for 24 h. Data are given as mean + SEM relative to control. N = 4 for both species. *** (p < 0.001), ** (p < 0.01) and * (p < 0.05) significantly different from control treated hepatocytes.
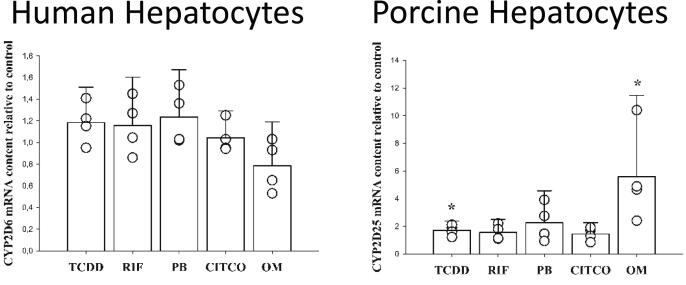
Treatment with TCDD and omeprazole caused a small but significant induction (1.7 ± 0.7 and 5.6 ± 5.9-fold, respectively) induction in the porcine CYP2D25 mRNA content, while all other treatments had no significant effect (Fig. 4). There was no effect of the used treatments on human CYP2D6 mRNA levels (Fig. 4). Protein expression of human CYP2D6 and porcine CYP2D25 were not detected by the used antibody.
Fig. 4.
mRNA expression of the CYP2D family in primary hepatocytes from human and porcine donors following treatment with 10 nM TCDD (2,3,7,8-tetrachlorodibenzo-p-dioxine), 10 µM rifampicin (RIF), 500 µM phenobarbital (PB), 1 µM CITCO (6-(4-chlorophenyl)imidazo[2,1-b][1,3]thiazole-5-carbaldehyde O-(3,4-dichlorobenzyl) oxime) and 100 µM omeprazole (OM) for 24 h. Data are given as mean + SEM relative to control. N = 4 for both species. * (p < 0.05) significantly different from control treated hepatocytes.
3.3. Regulation of CYP3 family
In human hepatocytes, rifampicin, phenobarbital, and omeprazole induced the mRNA content of CYP3A4 (210.2 ± 404.6; 149.0 ± 281.3 and 17.7 ± 33.6-fold, respectively), while TCDD reduces its expression (0.6 ± 0.3fold) (Fig. 5A). CITCO induces CYP3A4 mRNA expression in 2 out of 4 tested samples. Rifampicin and phenobarbital also caused significant induction in the mRNA content of the porcine CYP3A22 (23.6 ± 30.8 and 20.6 ± 39.8 fold, respectively), CYP3A29 (25.2 ± 33.1 and 21.1 ± 39.9- fold, respectively) and CYP3A46 (11.2 ± 6.1 and 9.5 ± 16.7-fold, respectively) (Fig. 5B). Moreover, TCDD also caused increased mRNA content of CYP3A22, CYP3A29 and CYP3A46, while only CYP3A29 and CYP3A46 was increased by omeprazole (Fig. 5B). At the protein level, human CYP3A4 (Fig. 5C) and porcine CYP3A (Fig. 5D) content was increased by rifampicin and phenobarbital in both species.
Fig. 5.
mRNA and protein expression of the CYP3A family in primary hepatocytes from human and porcine donors following treatment with 10 nM TCDD (2,3,7,8-tetrachlorodibenzo-p-dioxine), 10 µM rifampicin (RIF), 500 µM phenobarbital (PB), 1 µM CITCO (6-(4-chlorophenyl)imidazo[2,1-b][1,3]thiazole-5-carbaldehyde O-(3,4-dichlorobenzyl) oxime) and 100 µM omeprazole (OM) for 24 (mRNA) or 48 (protein) hours. Data are given as mean + SEM relative to control. N = 4 for both species. *** (p < 0.001), ** (p < 0.01) and * (p < 0.05) significantly different from control treated hepatocytes.
3.4. PCA of gene expression
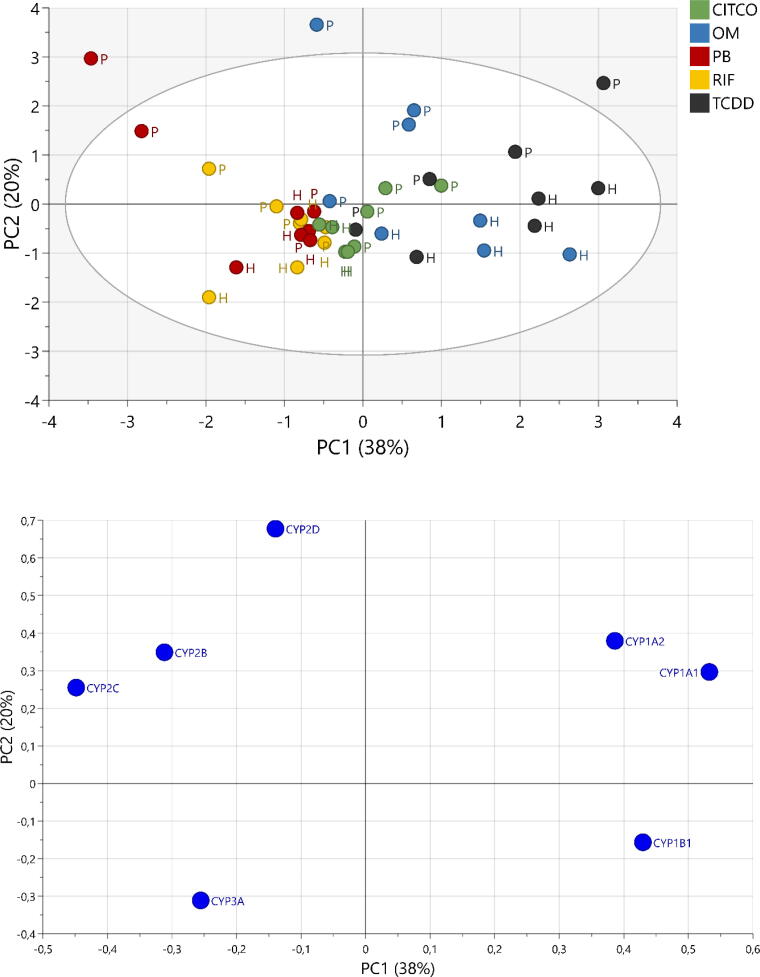
Principal component analysis (PCA) was performed to summarize and explore the responses to the used treatments (Fig. 6). The PCA on expression profile for the investigated genes were not able to distinguish between human and porcine hepatocytes treated with Rifampicin, Phenobarbital and CITCO. However, for TCDD and OM there were a clear distinguishing between human and porcine hepatocytes. Moreover, the human hepatocytes treated with TCDD clustered together with the ones treated with OM, while this was not the case for the porcine hepatocytes, as they clustered into two distinct groups.
Fig. 6.
Principal component analysis. The PCA analysis was based on the gene expression human and porcine primary hepatocytes after following treatment with 10 nM TCDD (2,3,7,8-tetrachlorodibenzo-p-dioxine), 10 µM rifampicin (RIF), 500 µM phenobarbital (PB), 1 µM CITCO (6-(4-chlorophenyl)imidazo[2,1-b][1,3]thiazole-5-carbaldehyde O-(3,4-dichlorobenzyl) oxime) and 100 µM omeprazole (OM) for 24 h. A) Score plot showing the clustering of human and porcine hepatocyte following treatment based on gene expression. The ellipse shows Hotellings T2. B) Loading plot showing the distribution of gene expression level.
4. Discussion
Robust and reliable cell-models are a prerequisite for predicting events like drug-drug and food-drug interactions. Moreover, the models should be of a nature allowing for an easy and accurate translation into in vivo conditions. Therefore, the choice of cell-models is very important when interpreting the outcome of basic and pre-clinical experiments. In order to choose the best cell-model, basic knowledge about the response in gene and protein regulation to prototypical treatments, needs to be known. Thus, the present study was conducted to compare the response in CYP gene regulation between primary human and porcine hepatocytes isolated and cultured using the same settings.
In agreement with the literature, TCDD and omeprazole strongly induce CYP1A1, CYP1A2 and CYP1B1 mRNA and CYP1A protein expression in primary human hepatocytes. The same pronounced increase of CYP1A1, CYP1A2 and CYP1B1 mRNA was observed in porcine hepatocytes. Species differences in hepatocytes induction of CYP1A1 and CYP1A2 by omeprazole has been described (Shih et al., 1999). Rat and mouse hepatocytes, the most frequently used in vitro models, are the least sensitive to omeprazole induction. Porcine hepatocytes mimic human situation in response to omeprazole.
CITCO strongly increased the expression of CYP1A1, CYP1A2 and CYP1B1 mRNA in porcine but not human hepatocytes. Treatment with CITCO, even though it is not considered a standard inducer of AhR regulated genes, has previously been shown to increase the expression of porcine CYP1A2 mRNA (Rasmussen et al., 2014). In Rasmussen et al (2014), 24 h incubation with 100 µM CITCO increases CYP1A2 mRNA expression 4-fold, a value close to that observed in response to the prototypical AhR activator β-naphtoflavone (induction 5.1-fold). In the present study incubation for 24 h with 1 µM CITCO induces CYP1A2 mRNA expression approx. 450 fold, while induction with TCDD reach above 3000 fold changes. Hence, responses to the same inducers are very different from one study to the other. We suggests that composition of culture medium and time of treatment following cell isolation may influence CYP basal expression (Rasmussen et al. 2014) as well as AhR activity, might due to variation between donors, and therefore the magnitude of the induction. CYP1A1 whose regulation in response to AhR activation is close to that of CYP1A2 presents a maximal observed mRNA induction of 1.71 in response to CITCO in cryopreserved human hepatocytes (Moscovitz et al., 2018) and was induced 1.78 times in freshly isolated cells (Kandel et al., 2016). Induction at the protein level was not established. This suggests that in contrast to human hepatocytes, CITCO is a strong inducer of CYP1A2 mRNA and protein expression in porcine hepatocytes. In a human AhR transactivation assay, CITCO was not an activator of AhR (Moscovitz et al., 2018), and CITCO-mediated CYP1A1/2 induction in HepaRG cells was increased following down-regulation of CAR using siRNA (Li et al., 2015) suggesting that CITCO dependent CYP1A2 induction is not mediated by CAR nor AhR in human. The mechanism of CITCO-mediated CYP1A genes induction in porcine needs to be explored.
CITCO, described as a specific ligand for human CAR (Maglich et al., 2003), increased the mRNA content of CYP2B6, a known CAR target gene (Sueyoshi et al., 1999), in human hepatocytes. However, the induction occurs at a low magnitude (fold changes < 2.0) in 2 out of 4 primary human hepatocytes tested. In parallel, CYP2B6 mRNA expression was strongly induced (fold change > 3) in response to rifampicin and phenobarbital in all donors. It was previously observed that CITCO-mediated induction of CYP2B6 mRNA expression was variable among donors and was lower than the effect mediated by phenobarbital (de Boussac et al., 2018). As phenobarbital can activate both CAR and PXR, it was proposed that a switch from CAR to PXR dependency is observed when CAR expression is low.
In primary porcine hepatocytes, CYP2B22 mRNA was strongly induced in response to phenobarbital and to a lesser extent to CITCO. In contrast to human hepatocytes, rifampicin-mediated induction was low (<3-fold compared to approx. 6-fold for human hepatocytes). This indicates that in contrast to human CYP2B6, porcine CYP2B22 depends preferentially on CAR-mediated regulation. The same pattern of induction was described by others (Giantin et al., 2012). We could not exclude that the culture conditions may also affect CAR and PXR expression and activity in favor to CAR in pig primary hepatocytes.
Finally, omeprazole induces both human CYP2B6 and porcine CY2B22 mRNA expression. As CYP2Bs mRNA expression was not affected by TCDD, this suggest that omeprazole-mediated induction is independent of AhR. CYP2B6 induction following 48 h incubation with 50 µM omeprazole was also observed in differentiated HepaRG (Anthérieu et al., 2012). In a human PXR transactivation reporter cell line omeprazole activates PXR at a concentration >10 µM (Moscovitz et al., 2018). Therefore, the observed CYP2B6 and CYP2B22 mRNA induction can occurred though PXR activation.
CYP2C8 and CYP2C9 expression parallels that of CYP2B6, with an induction in response to rifampicin, phenobarbital and to a lesser extent to CITCO and omeprazole. In contrast to the investigated human CYP2C’s, porcine CYP2C33 expression was not induced by the used treatments. However, porcine CYP2C42 and CYP2C49 were induced by both rifampicin and phenobarbital. CYP2C-isoform specific response to PXR activation has also been described in human hepatocytes (Gerbal-Chaloin et al., 2001). In human hepatocytes rifampicin and phenobarbital did not induced CYP2C18, suggesting that porcine CYP2C33 might corresponds to human CYP2C8 and cYP2C9 with respect to regulation mode. The CYP2C family is complex in human with 4 isoforms that are differentially regulated (Gerbal-Chaloin et al., 2001) and in pigs with 3 isoforms (Burkina et al., 2017). The level of expression differs between pig breed (Kojima and Degawa, 2016) and sex (Rasmussen et al., 2019). For example, CYP2C33 isoform is weakly expressed in Landrace breed (Kojima and Degawa, 2016) and female Gottingen minipigs (Rasmussen et al., 2019).
It was observed that in cryopreserved porcine hepatocytes CYP2C49 mRNA and protein were induced following 72 h incubation with phenobarbital (2 mM), rifampicin (10 µM) and dexamethasone (10 µM) (Giantin et al., 2012). Injection of phenobarbital to male castrated Large White × Landrace hybrid pigs resulted in an up-regulation of mRNA levels of liver CYP2C33, CYP2C42 and CYP2C49 (Puccinelli et al., 2010).
As expected, among the prototypical P450 inducers tested none affected human CYP2D6 mRNA expression (Zanger and Schwab, 2013). Apart from the role of main modulator mediated by hepatic nuclear factors 4α (HNF4α), very little is known regarding CYP2D6 regulation. Surprisingly, porcine CYP2D25 mRNA expression was significantly increased in response to omeprazole (approx. 6-fold) and to a lesser extent by TCDD (approx. 2-fold). As other compounds have no effect, this suggest that omeprazole acts in a PXR- and CAR- independent manner. Moreover, the low induction mediated by TCDD in comparison to the response mediated by omeprazole suggest that it is independent of AhR. Underlying mechanisms of porcine CYP2D regulation should be explored in more details.
In agreement with previous results, treatment with rifampicin increased the mRNA and protein expression of the human CYP3A4 and porcine CYP3A’s (Rasmussen, 2017, Rasmussen, 2020, Rasmussen et al., 2017). The same pattern was observed in response to phenobarbital but not to CITCO which is a ligand for CAR. This suggest that as in human for CYP3A4, PXR is the main regulator of porcine CYP3A induction. However, the decrease of CYP3A4 mRNA expression in response to AhR activation (TCDD) observed in human hepatocytes was not observed in porcine hepatocytes (Rasmussen et al., 2017).
In the present study, we did a direct comparison between the response in regulation of the CYPs to prototypical inducers in human between human and porcine CYPs. Similarities and differences emerge between the models.
AhR is activated in both models in response to prototypical activator TCDD, but also by omeprazole that activate human AhR without binding. As this is not the case in rodent model, porcine hepatocytes, represents an appropriate animal model for prediction of CYP1A induction in humans. However, it should be noticed that, based on the PCA, the overall response in CYP gene regulation between human and porcine hepatocytes differed for both TCDD and omeprazole. This is probably caused differences in the response in other genes than CYP1A1/2 and CYP1B1.
Porcine CYPs are induced by rifampicin, phenobarbital and high doses of dexamethasone but not pregnenolone 16α-carbonitrile (Giantin et al., 2012) a prototypical mouse PXR activator (Moore et al., 2003). Human and porcine PXR shares 87% sequence similarity in their ligand binding domain, while human and mice only shares 77% (Moore et al., 2002). This suggest that pig PXR ligands mimic what is observed in human as previously described (Moore et al., 2002). Correspondingly, CAR activation can be studied unambiguously through the effect of low concentration of CITCO on CAR target genes expression. CITCO induces human and pig CYP2Bs, the prototypical CAR targets demonstrating the role of CAR in porcine CYP genes regulation. CAR target genes are induced by human CAR agonist CITCO but not mouse CAR agonist TCPOBOP (1,4-bis-[2-(3,5-dichloropyridyloxy)]benzene) in a model of pig brain capillary endothelial cells (Lemmen et al., 2013). Moreover, ligand binding domain (LBD) of pig CAR shows 84% homology with the LBD of human CAR. Pig CAR and human CAR responded similarly to more ligands than did human CAR and mouse CAR making of primary pig hepatocyte a better model than mouse cells to study CAR activation (Gray et al., 2009). Based on the PCA the response in overall CYP regulation to CAR and PXR activation did not differ between human and porcine hepatocytes.
The results showed high similarities between human and porcine xenoreceptors activities. This is supported by the sequence (Gray et al., 2009) and activity homologies (Moore et al., 2002) between these receptors in human and pig. Moreover, it is well known that human and porcine CYPs shares many characteristics compared to other animal models (Burkina et al., 2017).
In conclusion, despite model specificity, there are a high number of corresponding responses, and experiments made in porcine hepatocytes allows for an easy and reliable translation into human settings. However, it should be noticed that this only implies to the regulation of mRNA contents as investigated in the present study. Further studies focusing on the regulation of activity of a comprehensive selection of the CYP’s in PPH and PHH is needed in order to fully evaluate the applicability of PPH’s as a model for humans.
CRediT authorship contribution statement
Sabine Gerbal-Chaloin: Conceptualization, Methodology, Formal analysis, Investigation. Philippe Briolotti: Formal analysis. Martine Daujat-Chavanieu: Writing‐review & editing. Martin Krøyer Rasmussen: Methodology, Formal analysis, Investigation, Funding acquisition.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
This study was funded by the Lundbeck Foundation (Grant ID: R-181-2014-1825).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.crtox.2021.03.002.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- Anthérieu S., Chesné C., Li R., Guguen-Guillouzo C., Guillouzo A. Optimization of the HepaRG cell model for drug metabolism and toxicity studies. Toxicol. In Vitro. 2012;26(8):1278–1285. doi: 10.1016/j.tiv.2012.05.008. [DOI] [PubMed] [Google Scholar]
- Bogaards J.J.P., Bertrand M., Jackson P., Oudshoorn M.J., Weaver R.J., Van Bladeren P.J., Walther B. Determining the best animal model for human cytochrome P450 activities: a comparison of mouse, rat, rabbit, dog, micropig, monkey and man. Xenobiotica. 2000;30:1131–1152. doi: 10.1080/00498250010021684. [DOI] [PubMed] [Google Scholar]
- Burkina V., Rasmussen M.K., Pilipenko N., Zamaratskaia G. Comparison of xenobiotic-metabolising human, porcine, rodent, and piscine cytochrome P450. Toxicology. 2017;375:10–27. doi: 10.1016/j.tox.2016.11.014. [DOI] [PubMed] [Google Scholar]
- Daujat M., Peryt B., Lesca P., Fourtanier G., Domergue J., Maurel P. Omeprazole, an inducer of human CYP1A1 and 1A2, is not a ligand for the Ah receptor. Biochem. Biophys. Res. Commun. 1992;188:820–825. doi: 10.1016/0006-291x(92)91130-i. [DOI] [PubMed] [Google Scholar]
- de Boussac H., Gondeau C., Briolotti P., Duret C., Treindl F., Römer M., Fabre J.-M., Herrero A., Ramos J., Maurel P., Templin M., Gerbal-Chaloin S., Daujat-Chavanieu M. Epidermal growth factor represses constitutive androstane receptor expression in primary human hepatocytes and favors regulation by pregnane X receptor. Drug Metab. Dispos. 2018;46:223–236. doi: 10.1124/dmd.117.078683. [DOI] [PubMed] [Google Scholar]
- Donato M.T., Castell J.V., Gómez-Lechón M.J. Characterization of drug metabolizing activities in pig hepatocytes for use in bioartificial liver devices: comparison with other hepatic cellular models. J. Hepatol. 1999;31:542–549. doi: 10.1016/s0168-8278(99)80049-x. [DOI] [PubMed] [Google Scholar]
- Gerbal-Chaloin S., Pascussi J.M., Pichard-Garcia L., Daujat M., Waechter F., Fabre J.M., Carrere N., Maurel P. Induction of CYP2C genes in human hepatocytes in primary culture. Drug Metab. Dispos. 2001;29:242–251. [PubMed] [Google Scholar]
- Giantin M., Zancanella V., Lopparelli R.M., Granato A., Carletti M., Vilei M.T., Muraca M., Baratto C., Dacasto M. Effects of time culture and prototypical cytochrome P450 3A (CYP3A) inducers on CYP2B22, CYP2C, CYP3A and nuclear receptor (NR) mRNAs in long-term cryopreserved pig hepatocytes (CPHs) Drug Metab. Pharmacokinet. 2012;27:495–505. doi: 10.2133/dmpk.dmpk-11-rg-146. [DOI] [PubMed] [Google Scholar]
- Gray M.A., Peacock J.N., Squires E.J. Characterization of the porcine constitutive androstane receptor (CAR) and its splice variants. Xenobiotica. 2009;39:915–930. doi: 10.3109/00498250903330348. [DOI] [PubMed] [Google Scholar]
- Kandel B.A., Thomas M., Winter S., Damm G., Seehofer D., Burk O., Schwab M., Zanger U.M. Genomewide comparison of the inducible transcriptomes of nuclear receptors CAR, PXR and PPARalpha in primary human hepatocytes. BBA. 2016;1859:1218–1227. doi: 10.1016/j.bbagrm.2016.03.007. [DOI] [PubMed] [Google Scholar]
- Kojima M., Degawa M. Sex differences in constitutive mRNA levels of CYP2B22, CYP2C33, CYP2C49, CYP3A22, CYP3A29 and CYP3A46 in the pig liver: comparison between Meishan and Landrace pigs. Drug Metab. Pharmacokinet. 2016;31:185–192. doi: 10.1016/j.dmpk.2016.02.001. [DOI] [PubMed] [Google Scholar]
- Lemmen J., Tozakidis I.E.P., Bele P., Galla H.-J. Constitutive androstane receptor upregulates Abcb1 and Abcg2 at the blood-brain barrier after CITCO activation. Brain Res. 2013;1501:68–80. doi: 10.1016/j.brainres.2013.01.025. [DOI] [PubMed] [Google Scholar]
- Lewis D.FV. 57 varieties: the human cytochromes P450. Pharmacogenomics. 2004;5:305–318. doi: 10.1517/phgs.5.3.305.29827. [DOI] [PubMed] [Google Scholar]
- Li D., Mackowiak B., Brayman T.G., Mitchell M., Zhang L., Huang S.-M., Wang H. Genome-wide analysis of human constitutive androstane receptor (CAR) transcriptome in wild-type and CAR-knockout HepaRG cells. Biochem. Pharmacol. 2015;98:190–202. doi: 10.1016/j.bcp.2015.08.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu C., Li A.P. Species comparison in P450 induction: effects of dexamethasone, omeprazole, and rifampin on P450 isoforms 1A and 3A in primary cultured hepatocytes from man, Sprague-Dawley rat, minipig, and beagle dog. Chem. Biol. Interact. 2001;134:271–281. doi: 10.1016/s0009-2797(01)00162-4. [DOI] [PubMed] [Google Scholar]
- Maglich J.M., Parks D.J., Moore L.B., Collins J.L., Goodwin B., Billin A.N., Stoltz C.A., Kliewer S.A., Lambert M.H., Willson T.M., Moore J.T. Identification of a Novel Human Constitutive Androstane Receptor (CAR) agonist and its use in the identification of CAR target genes. J. Biol. Chem. 2003;278:17277–17283. doi: 10.1074/jbc.M300138200. [DOI] [PubMed] [Google Scholar]
- Moore L.B., Maglich J.M., McKee D.D., Wisely B., Willson T.M., Kliewer S.A., Lambert M.H., Moore J.T. Pregnane X receptor (PXR), constitutive androstane receptor (CAR), and benzoate X receptor (BXR) define three pharmacologically distinct classes of nuclear receptors. Mol. Endocrinol. 2002;16:977–986. doi: 10.1210/mend.16.5.0828. [DOI] [PubMed] [Google Scholar]
- Moore J.T., Moore L.B., Maglich J.M., Kliewer S.A. Functional and structural comparison of PXR and CAR. BBA. 2003;1619:235–238. doi: 10.1016/s0304-4165(02)00481-6. [DOI] [PubMed] [Google Scholar]
- Moscovitz J.E., Kalgutkar A.S., Nulick K., Johnson N., Lin Z., Goosen T.C., Weng Y. Establishing transcriptional signatures to differentiate PXR-, CAR-, and AhR-mediated regulation of drug metabolism and transport genes in cryopreserved human hepatocytes. J. Pharmacol. Exp. Ther. 2018;365:262–271. doi: 10.1124/jpet.117.247296. [DOI] [PubMed] [Google Scholar]
- Pichard L., Raulet E., Fabre G., Ferrini J.B., Ourlin J.C., Maurel P. Human hepatocyte culture. Method Mol. Biol. 2006;320:283–293. doi: 10.1385/1-59259-998-2:283. [DOI] [PubMed] [Google Scholar]
- Puccinelli E., Gervasi P.G., Marca M.L., Beffy P., Longo V. Expression and inducibility by phenobarbital of CYP2C33, CYP2C42, CYP2C49, CYP2B22, and CYP3As in porcine liver, kidney, small intestine, and nasal tissues. Xenobiotica. 2010;40:525–535. doi: 10.3109/00498254.2010.489125. [DOI] [PubMed] [Google Scholar]
- Puccinelli E., Gervasi P.G., Longo V. Xenobiotic metabolizing cytochrome P450 in pig, a promising animal model. Curr. Drug Metab. 2011;12:507–525. doi: 10.2174/138920011795713698. [DOI] [PubMed] [Google Scholar]
- Rasmussen M.K. Induction of cytochrome P450 mRNA in porcine primary hepatocytes cultured under serum free conditions: comparison of freshly isolated cells and cryopreserved. Exp. Cell Res. 2017;360:218–225. doi: 10.1016/j.yexcr.2017.09.011. [DOI] [PubMed] [Google Scholar]
- Rasmussen M.K. Porcine cytochrome P450 3A: current status on expression and regulation. Arch. Toxicol. 2020;94:1899–1914. doi: 10.1007/s00204-020-02710-9. [DOI] [PubMed] [Google Scholar]
- Rasmussen M.K., Zamaratskaia G., Ekstrand B. Gender-related differences in cytochrome P450 in porcine liver - implication for activity, expression and inhibition by testicular steroids. Reprod. Domestic Anim. 2011:616–623. doi: 10.1111/j.1439-0531.2010.1714.x. [DOI] [PubMed] [Google Scholar]
- Rasmussen M.K., Klausen C.L., Ekstrand B.o. Regulation of cytochrome P450 mRNA expression in primary porcine hepatocytes by selected secondary plant metabolites from chicory (Cichorium intybus L.) Food Chem. 2014;146:255–263. doi: 10.1016/j.foodchem.2013.09.068. [DOI] [PubMed] [Google Scholar]
- Rasmussen M.K., Balaguer P., Ekstrand B.o., Daujat-Chavanieu M., Gerbal-Chaloin S., Ryffel B. Skatole (3-Methylindole) is a partial aryl hydrocarbon receptor agonist and induces CYP1A1/2 and CYP1B1 expression in primary human hepatocytes. PLoS ONE. 2016;11:e0154629. doi: 10.1371/journal.pone.0154629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen M.K., Daujat-Chavanieu M., Gerbal-Chaloin S. Activation of the aryl hydrocarbon receptor decreases rifampicin-induced CYP3A4 expression in primary human hepatocytes and HepaRG. Toxicol. Lett. 2017;277:1–8. doi: 10.1016/j.toxlet.2017.05.029. [DOI] [PubMed] [Google Scholar]
- Rasmussen M.K., Scavenius C., Gerbal-Chaloin S., Enghild J. Sex dictates the constitutive expression of hepatic cytochrome P450 isoforms in Gottingen minipigs. Toxicol. Lett. 2019;314:181–186. doi: 10.1016/j.toxlet.2019.08.008. [DOI] [PubMed] [Google Scholar]
- Shih H., Pickwell G.V., Guenette D.K., Bilir B., Quattrochi L.C. Species differences in hepatocyte induction of CYP1A1 and CYP1A2 by omeprazole. Hum. Exp. Toxicol. 1999;18:95–105. doi: 10.1177/096032719901800206. [DOI] [PubMed] [Google Scholar]
- Sueyoshi T., Kawamoto T., Zelko I., Honkakoski P., Negishi M. The repressed nuclear receptor CAR responds to phenobarbital in activating the human CYP2B6 gene. J. Biol. Chem. 1999;274:6043–6046. doi: 10.1074/jbc.274.10.6043. [DOI] [PubMed] [Google Scholar]
- Thörn H.A., Lundahl A., Schrickx J.A., Dickinson P.A., Lennernäs H. Drug metabolism of CYP3A4, CYP2C9 and CYP2D6 substrates in pigs and humans. Eur. J. Pharm. Sci. 2011;43:89–98. doi: 10.1016/j.ejps.2011.03.008. [DOI] [PubMed] [Google Scholar]
- Zanger U.M., Schwab M. Cytochrome P450 enzymes in drug metabolism: regulation of gene expression, enzyme activities, and impact of genetic variation. Pharmacol. Ther. 2013;138:103–141. doi: 10.1016/j.pharmthera.2012.12.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.