Abstract
Background
This secondary image analysis of a randomized trial of proton radiotherapy (PT) versus photon intensity-modulated radiotherapy (IMRT) compares tumor progression based on clinical radiological assessment versus Response Assessment in Neuro-Oncology (RANO).
Methods
Eligible patients were enrolled in the randomized trial and had MR imaging at baseline and follow-up beyond 12 weeks from completion of radiotherapy. “Clinical progression” was based on a clinical radiology report of progression and/or change in treatment for progression.
Results
Of 90 enrolled patients, 66 were evaluable. Median clinical progression-free survival (PFS) was 10.8 (range: 9.4–14.7) months; 10.8 months IMRT versus 11.2 months PT (P = .14). Median RANO-PFS was 8.2 (range: 6.9, 12): 8.9 months IMRT versus 6.6 months PT (P = .24). RANO-PFS was significantly shorter than clinical PFS overall (P = .001) and for both the IMRT (P = .01) and PT (P = .04) groups. There were 31 (46.3%) discrepant cases of which 17 had RANO progression more than a month prior to clinical progression, and 14 had progression by RANO but not clinical criteria.
Conclusions
Based on this secondary analysis of a trial of PT versus IMRT for glioblastoma, while no difference in PFS was noted relative to treatment technique, RANO criteria identified progression more often and earlier than clinical assessment. This highlights the disconnect between measures of tumor response in clinical trials versus clinical practice. With growing efforts to utilize real-world data and personalized treatment with timely adaptation, there is a growing need to improve the consistency of determining tumor progression within clinical trials and clinical practice.
Keywords: glioblastoma, imaging endpoints, progression-free survival, radiation therapy, RANO
Key Points.
RANO progression precedes clinical progression in GBM patients treated with RT.
Quantitative tools to accurately determine progression are needed to guide GBM trials.
Importance of the Study.
Timely and confident determination of tumor progression for high-grade gliomas is critical for enabling meaningful interpretation of novel treatments and guiding personalized care for patients. Findings from this study suggest that the assessment of tumor progression and treatment response using RANO criteria precedes the assessment done with clinical radiological determinants. Further development of standardized quantitative tools that improve the consistency and accuracy of determining disease progression is essential to guide therapeutic trials and timely adjustments to clinical treatments in patients with GBM.
A common problem in clinical trials for both therapeutics and treatment strategies in high-grade glioma patients is the selection of surrogate endpoints.1–3 The endpoint of overall survival (OS) remains the gold standard for these subjects. However, this requires a longer trial duration and may be confounded by the use of salvage therapies.3,4 In order to accelerate therapeutic discovery and reduce costs, alternative endpoints are selected such as neuroimaging ones. It has been anticipated that a strategy that combines neuroimaging and neurocognitive/clinical function for assessing progression-free survival (PFS) would better predict for OS.
As imaging modalities have advanced, imaging criteria of response have also evolved. In 1990, the McDonald criteria were developed and proposed as the standard for assessment of tumor response and progression in patients with high-grade glioma. These criteria classified response based on two-dimensional (2D) measurements on contrast-enhanced CT or MRI scans, using the tumor’s largest cross-sectional area, ie, the product of the maximal perpendicular diameters. This first standardized clinical response measurement tool in neuro-oncology also incorporated steroid use and clinical symptoms.5
Particularly with the increased use of concurrent chemoradiation, pseudo-progression, in which there is an initial increase in the size of the contrast-enhancing abnormality followed by regression, in the absence of true tumor progression, has been reported and is now commonly recognized.6–9 The use of anti-angiogenic salvage therapy, such as bevacizumab, can induce a pseudo-response, in which there is a rapid decrease in tumor enhancement on CT/MRI without true reduction in tumor burden attributed to the normalization of vascular permeability.7,8 In response to these new imaging phenomena associated with the evolution of therapy, the Response Assessment in Neuro-Oncology (RANO) criteria were established in 2010. Unlike McDonald criteria, RANO accounts for differences in fluid-attenuated inversion recovery (FLAIR)/T2 intensities in addition to T1 enhancement changes. By incorporating T2 FLAIR changes, timely follow-up imaging for response assessment and equivocal progression, as well as clinical considerations into its criteria, RANO improved the evaluation of pseudo-phenomena of disease response and progression.10,11
In view of the importance of neuroimaging endpoints in clinical trials and the pivotal role they can play in clinically meaningful evaluation of new drugs or treatment techniques in high-grade glioma, this secondary analysis of a randomized controlled trial was completed to evaluate response assessment strategies and tumor progression by comparing clinical radiological assessment and RANO criteria in glioblastoma (GBM) patients treated with proton radiotherapy (PT) versus photon intensity-modulated radiotherapy (IMRT).
Materials and Methods
This manuscript is a secondary analysis of the data from a randomized trial of PT versus IMRT.12 This trial was approved by The University of Texas MD Anderson Cancer Center (MDACC) institutional review board. All patients provided written informed consent before enrollment. On the primary phase II randomized trial (ClinicalTrials.gov identifier: NCT01854554), eligible patients were randomly assigned to 1 of 2 groups, PT versus IMRT, in a 1:1 ratio and stratified by age (< and ≥65 years), Radiation Therapy Oncology Group (RTOG) recursive partitioning analysis (RPA) of gliomas class (III or IV vs V),13 and Mini Mental Status Examination (MMSE) score (21–26 vs 27–30).14 Randomization was performed utilizing Clinical Oncology Research (CORe) system clinical trials management system.
Adult patients (18 years of age or older) with newly diagnosed GBM or gliosarcoma (WHO Grade IV), Karnofsky Performance Status score 70 or greater, RPA class III, IV, or V were eligible for this trial. Additional eligibility criteria for the primary trial included MMSE score of 21 or greater, ability to complete a MRI with contrast, aspartate amino transferase <3 times normal limit, alanine amino transferase <3 times normal limit, alkaline phosphatase <3 times normal limit, creatinine <1.7 mg/dL, blood urea nitrogen <35 mg/dL, absolute neutrophil count >1800 cells/mm3, hemoglobin >10 g/dL, and platelet count >100 000, and able to adequately read, write, and speak to participate in the cognitive and patient-reported outcome assessments, allowing for mild to moderate deficits in these functions due to tumor. Exclusion criteria included prior brain radiation, pregnancy, prior resection of other brain tumors, gliomatosis, or implantation of Carmustine (BCNU) wafers.
The primary phase II trial protocol included MR imaging at baseline then follow-up clinical visits and MR imaging at 2-month intervals (±30 days) for a total of 24 months after the completion of the assigned treatment. While the primary manuscript reports the intent-to-treat PFS endpoint along with the other primary endpoints, this secondary analysis investigates whether there are differences in PFS when progression is determined using 2 different methods for patients receiving 2 different treatment regimens. The first method consisted of 2 blinded observers—1 experienced neuroradiologist and 1 radiation oncologist trained for RANO assessment—applying RANO criteria to determine disease progression. Agreement between the 2 clinicians applying RANO criteria was assessed using descriptive statistics. The second method of assessment, termed “clinical progression,” was based on a clinical radiology report of progressive disease in combination with changes in treatment due to suspected disease progression.
RANO-based PFS (RANO-PFS) time was calculated from the date of study registration to the date of progressive disease based on RANO criteria, death (event), or last follow-up (censored). For this endpoint, RANO-PFS was determined using the data from the experienced neuroradiologist. “Clinical” PFS time was calculated from the date of study registration to date of clinical progression or death (event) or last follow-up (censored). Concordance and discordance between RANO and clinical determinations of progression are reported. The 2 determinations of progression were said to be discordant when progression was determined more than 1 month apart or when progression was noted by 1 definition and not by the other
Median RANO-based and clinical PFS times were estimated by Kaplan–Meier method and log-rank test was applied to compare their distributions between the proton therapy and IMRT arms. Gray’s test15 was also used to compare the 1- and 2-year cumulative incidence rates of progressive disease between the proton therapy and IMRT arms, based on each of RANO and clinical criteria, when death without progressive disease was considered a competing risk. A univariate proportional hazard Cox regression model was applied to compare PFS by RANO or clinical criteria overall and in each treatment group, taking intrapatient correlation into considerations. A landmark analysis was also employed to evaluate whether 6-month progressive disease (PD) status was predictive of OS time.
Results
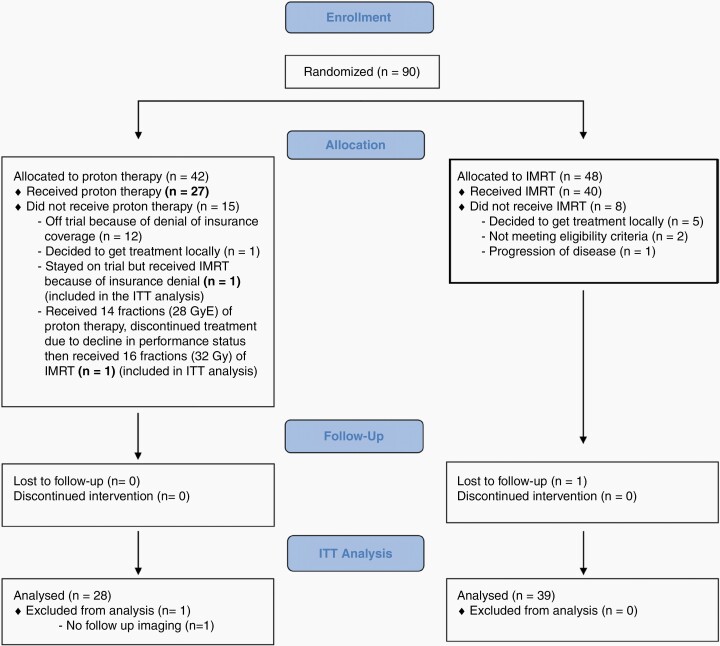
Of 90 enrolled patients, 66 were evaluable (Figure 1), with median follow-up of 48.7 months (95% CI, 39, 62.5) months. Patient and treatment characteristics as well as primary trial endpoints are described in the primary manuscript.12 One- and 2-year cumulative incidence rates of progressive disease as per RANO criteria were 46% (95% CI, 26%–64%) and 77% (95% CI, 54%–89%), respectively, for patients treated with proton therapy, and 58% (95% CI, 42%–72%) and 73% (95% CI, 56%–85%), respectively, for patients treated with IMRT (P = .84). One- and 2-year cumulative incidence rates of progressive disease based on clinical progression criteria were 54% (95% CI, 33%–71%) and 85% (95% CI, 62%–94%), respectively, for patients treated with proton therapy, and 39% (95% CI, 24%–54%) and 56% (95% CI, 39%–70%), respectively, for patients treated with IMRT (P = .01).
Figure 1.
CONSORT diagram.
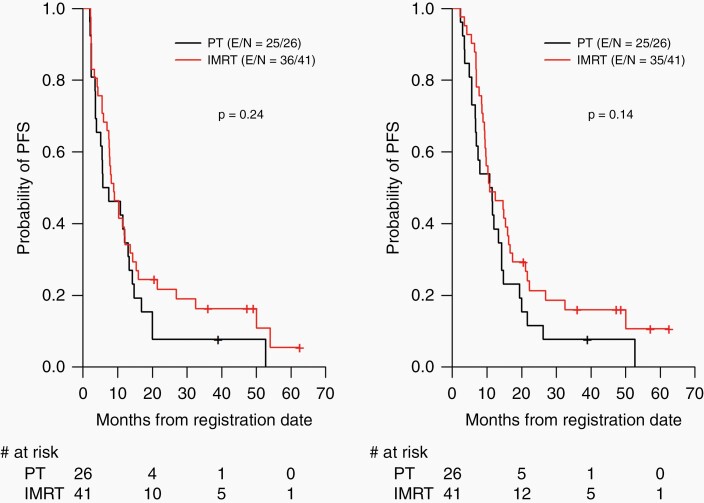
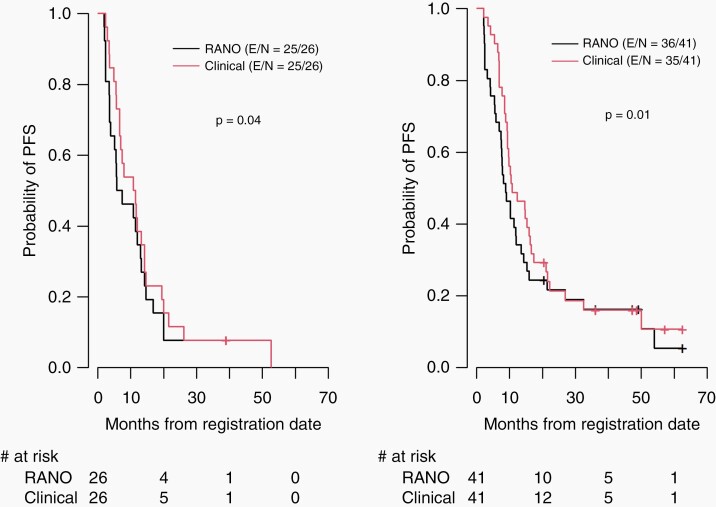
In the overall evaluable patient cohort, the median RANO-PFS was 8.2 (range: 6.9–12) months: 6.6 months for patients treated with proton therapy versus 8.9 months for patients treated with IMRT (P = .24). Median clinical PFS was 10.8 (range: 9.4–14.7) months; 11.2 months for patients treated with proton therapy versus 10.8 months for patients treated with IMRT(P = .14) (Figure 2). Notably, RANO-PFS time was significantly shorter than clinical PFS time overall (P = .001), and for both the IMRT and PT groups (P = .01 and P = .04, respectively) (Figure 3). There were 31 (46.3%) discrepant cases: of those, 17 had progression determined by RANO criteria more than a month prior to the determination of progression by clinical criteria, and 14 cases were called progression by RANO criteria but not by clinical criteria. For all the patients who had progression determined by both criteria (regardless of time difference), 21 had progression determined at around the same time (less than a month apart), and 24 had RANO progression preceding clinical progression by a median of 1.69 (interquartile range, 0.94–3.76) months.
Figure 2.
Progression-free survival based on Response Assessment in Neuro-Oncology (RANO) criteria (left) and on clinical criteria (right) in patients treated with PT versus IMRT. IMRT, intensity-modulated radiation therapy; PFS, progression-free survival; PT, proton therapy.
Figure 3.
Progression-free survival for patients treated with PT (left) and IMRT (right) based on Response Assessment in Neuro-Oncology (RANO) criteria versus clinical criteria. IMRT, intensity-modulated radiation therapy; PFS, progression-free survival; PT, proton therapy.
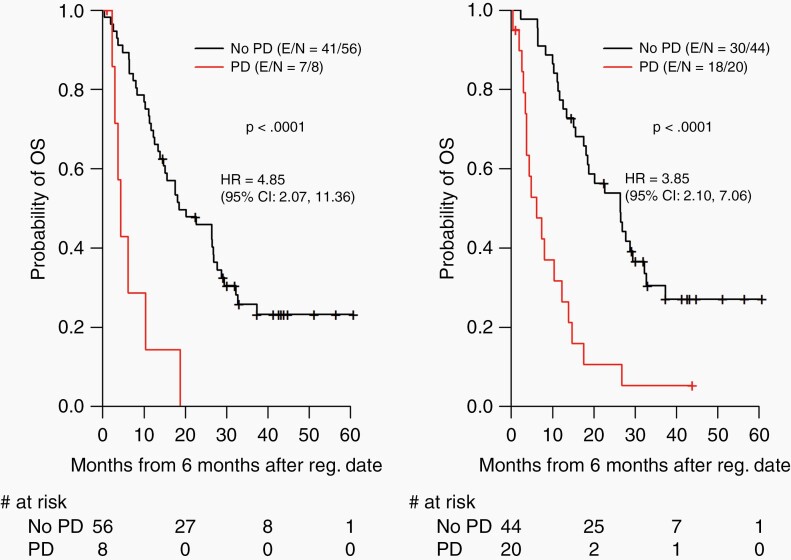
While the neuroradiologist RANO determination of progression was used for RANO-PFS in comparison to clinical PFS, it was noted that there was disagreement between the 2 observers applying RANO criteria on 4 cases. Upon further review and discussion of these 4 cases, it appeared that 2 had a new nodular enhancement that developed 3 months after they completed treatment, that one observer considered as progression, and another observer considered as treatment-related effect. The 2 other cases had increase in tumor enhancement that, according to one observer’s measurements did not meet RANO criteria of progression but that, based on the second observer’s measurements did meet RANO criteria of progression for the other observer. Employing landmark analysis based on our designated landmark time point of 6 months, patients with progressive disease at 6 months had significantly shorter OS time than patients without progressive disease at 6 months using both RANO and clinical criteria (P < .0001 and P < .0001, respectively) (Figure 4).
Figure 4.
Overall survival as a function of 6-month progressive disease (PD) status based on Response Assessment in Neuro-Oncology (RANO) criteria (left) and on clinical criteria (right)—landmark analysis. HR, hazard ratio; OS, overall survival; Reg., registration.
Discussion
The optimal endpoints in clinical trials for GBM have been a topic of debate and major challenge over the last few decades.1–3 Although the endpoint of OS is considered the most objective and straightforward endpoint, it does come with important limitations including increased clinical trial duration and confounders of subsequent salvage treatment. In pursuit of precision medicine approaches to treatment, reliable, timely, and quantitative response assessment and detection of tumor progression is critical for early treatment adaptation to gain maximal benefit in outcomes for patients. Therefore, endpoints such as PFS and objective response rates have been proposed as alternatives, relying on neuroimaging findings.3,4
As a result, a workshop in 2014 sponsored by the Jumpstarting Brain Tumor Drug Development Coalition was held on the use of endpoints in clinical trials for intracranial high-grade gliomas.16 During a neuroimaging discussion at this workshop, it was acknowledged that challenges extend beyond the response criteria and that variability in image acquisition itself could contribute to inaccurate response assessments. This resulted in the creation of the Brain Tumor Imaging Standardization Steering Committee, which has been leading an effort to standardize imaging acquisition parameters and protocols used in clinical trials for high-grade gliomas. The recommended consensus protocols were designed to allow for both current RANO measurements and for potential future utilization of volumetric analyses.17 This standardization is essential, especially in multi-institutional trials for interpretability and reproducibility of results.
In this secondary analysis of a prospective phase II trial, RANO determination of progression preceded clinical determination of progression in patients treated with proton therapy and IMRT. PFS and determination of progression were different between RANO and clinical determination in both IMRT and proton arms. There were 48% discordant cases, meaning that progression was determined >1 month apart based on the 2 definitions (RANO progression preceding clinical determination by a mean of around 2.6 months), and more rarely that there was progression based on 1 definition and no progression by another. There were no patients in this study who had clinical determination of progression but no progression by RANO. This may be due to the very small sample size, bias during RANO evaluation (readers knowing how many scans patients have), and “back-dating” when using RANO criteria by clinicians/investigators.
The fact that the clinician did not change the course of treatment at the first evidence of progression indicates that clinicians may have a tendency to question the possibility of pseudo-progression (treatment effect) and take a conservative approach with close follow-up imaging rather than acting upon the first evidence of imaging progression. This reflects the lack of confidence in image-based assessment to detect true tumor progression. Integrating neurocognitive function, patient-reported outcomes, and other patient-centered outcomes may enhance determination of patient status beyond imaging-based assessment alone for clinical trials and may help improve treatment decision making.18,19
Further, landmark analysis using 6 months as the landmark time point revealed that both RANO and clinical determination of progression at 6 months predicted for OS to a similar extent. This may reflect that there is similar uncertainty whether a rule-based approach using 2D measurements such as RANO or a rule-based algorithm by a clinician are used. A first step toward improving the certainty of determining progression that better predicts survival is to develop a more quantitative approach that allows us to measure and account for the degree of uncertainty. Efforts to improve the precision of tumor progression assessment may guide more timely treatment adjustments as well as better prediction of survival.
Imaging endpoints of response and progression are central in clinical trials for high-grade gliomas testing the therapeutic efficacy of systemic agents or radiation treatment modalities; reducing variability and ensuring interpretability of the imaging results is of utmost importance. In this analysis, the fact that there was disagreement in RANO determination of progression for few cases between the 2 observers is not entirely an unexpected finding, as RANO criteria are open to some to interpretation. Further development of tumor assessment tools that improve consistency and accuracy of determining tumor progression are needed to guide therapeutic trials in GBM. Current rule-based classification approaches for tumor response, largely using conventional imaging, can be biased by intra- or interobserver variability in linear or volumetric measurements of brain tumors.20 One approach to potentially improving the reproducibility is to use automated measurement tools, such as AutoRANO.21 Currently, deep learning algorithms for automated tumor segmentation are being developed to facilitate consistent and efficient cross-dimensional and 3-dimensional volumetric tumor measurements across time.22–24 Automated tools help eliminate intra- and interobserver variability. In order to evaluate the current status of automated brain tumor segmentation and compare between different methods, a Multimodal Brain Tumor Image Segmentation Benchmark (BRATS) challenge was organized in 2012–2013 in conjunction with the international conference on Medical Image Computing and Computer Assisted Interventions (MICCAI). A dataset of MR scans of low- and high-grade glioma patients was made available, and experts performed manual tumor delineations that were compared to realistically generated synthetic brain tumor datasets. The BRATS study revealed considerable disagreement between the human raters. It also found that combining all of the automated segmentation tools outperformed any individual segmentation algorithm. It concluded that continued algorithmic development was warranted.23
Moreover, appropriate incorporation of functional imaging such as diffusion weighted imaging and/or perfusion imaging with the anatomical imaging data has strong potential to improve the differentiation of treatment-related effects from true tumor progression.25 Ideally imaging response measurement should be based on integrated 3-dimensional volumetric tumor changes that incorporate spatially coregistered multiparametric MR data such as MR spectroscopy,26,27 diffusion weighted imaging,28,29 dynamic contrast-enhanced MRI,30 and dynamic susceptibility contrast MRI.31,32 Advanced multiparametric MRI data (anatomic, perfusion, diffusion) can offer a noninvasive assessment of the tumor itself and its surrounding environment in order to capture information about the biological and physiological changes after local or systemic therapy that may be useful early biomarkers of treatment response before any measurable changes in tumor size. However, validation of biomarkers is critically dependent on consistent, reliable outcome measures such as tumor response or progression.
In conclusion, while there was no significant difference in tumor progression relative to treatment technique based on either RANO or clinical criteria, this secondary analysis of the randomized trial of PT versus IMRT for GBM demonstrated difference in tumor progression between RANO criteria and clinical assessment with tumor progression noted more often and earlier using RANO compared with clinical assessment. Nonetheless, tumor progression at 6 months based on RANO or clinical criteria had similar prediction for OS. This study points to the disconnect between objective measures of tumor response used in clinical trials versus clinical practice. With growing efforts to utilize real-world data and to apply personalized treatment with timely adjustments of treatment to improve outcomes, there is a growing need to develop quantitative tools that improve the consistency and accuracy of determining tumor progression within therapeutic clinical trials and in clinical care of patients with GBM.
Funding
This work was supported by MD Anderson Cancer Center Support Grant P30CA01667 and by a Radiation Oncology Strategic Initiatives (ROSI) grant from the University of Texas MD Anderson Cancer Center, Division of Radiation Oncology.
Conflict of interest statement. None declared.
Authorship Statement. Study design: P.B., C.C., J.W., and N.G.; literature review: K.A. and C.C.; prepared data for analysis: K.A., J.R., P.B., C.C., and N.G.; statistical analysis: D.L.; interpreted results: K.A., S.B., H.E., N.C., and C.C.; wrote manuscript: K.A., D.L., P.B., and C.C.; edited manuscript: K.A., J.R., D.L., J.W., P.B., D.G., S.M., J.L., S.L.M., M.M., M.P., J.W., J.D., A.H., T.A., M.G., A.M., N.G., and C.C.; approved final version of manuscript: K.A., J.R., D.L., J.W., P.B., D.G., S.M., J.L., S.L.M., M.M., A.G., A.P., E.S., M.P., J.W., J.D., A.H., T.A., M.G., A.M., N.G., and C.C.
Oral presentations at the 2019 American Society of Radiation Oncology (ASTRO) annual meeting in Chicago, IL (September 2019) and at the 2019 Society on Neuro-Oncology (SNO) meeting in Phoenix, AZ (November 2019).
References
- 1. Wen PY, Chang SM, Van den Bent MJ, Vogelbaum MA, Macdonald DR, Lee EQ. Response assessment in neuro-oncology clinical trials. J Clin Oncol. 2017;35(21):2439–2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Eisele SC, Wen PY, Lee EQ. Assessment of brain tumor response: RANO and its offspring. Curr Treat Options Oncol. 2016;17(7):35. [DOI] [PubMed] [Google Scholar]
- 3. Taylor JW, Molinaro AM, Butowski N, Prados M. Clinical trial endpoints for patients with gliomas. Neurooncol Pract. 2017;4(4):201–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Polley M-YC, Lamborn KR, Chang SM, Butowski N, Clarke JL, Prados M. Six-month progression-free survival as an alternative primary efficacy endpoint to overall survival in newly diagnosed glioblastoma patients receiving temozolomide. Neuro Oncol. 2010;12(3):274–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Macdonald DR, Cascino TL, Schold SC Jr, Cairncross JG. Response criteria for phase II studies of supratentorial malignant glioma. J Clin Oncol. 1990;8(7):1277–1280. [DOI] [PubMed] [Google Scholar]
- 6. Brandes AA, Franceschi E, Tosoni A, et al. MGMT promoter methylation status can predict the incidence and outcome of pseudoprogression after concomitant radiochemotherapy in newly diagnosed glioblastoma patients. J Clin Oncol. 2008;26(13):2192–2197. [DOI] [PubMed] [Google Scholar]
- 7. Brandsma D, Stalpers L, Taal W, Sminia P, van den Bent MJ. Clinical features, mechanisms, and management of pseudoprogression in malignant gliomas. Lancet Oncol. 2008;9(5):453–461. [DOI] [PubMed] [Google Scholar]
- 8. Brandsma D, van den Bent MJ. Pseudoprogression and pseudoresponse in the treatment of gliomas. Curr Opin Neurol. 2009;22(6):633–638. [DOI] [PubMed] [Google Scholar]
- 9. Radbruch A, Fladt J, Kickingereder P, et al. Pseudoprogression in patients with glioblastoma: clinical relevance despite low incidence. Neuro Oncol. 2015;17(1):151–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wen PY, Macdonald DR, Reardon DA, et al. Updated response assessment criteria for high-grade gliomas: response assessment in neuro-oncology working group. J Clin Oncol. 2010;28(11):1963–1972. [DOI] [PubMed] [Google Scholar]
- 11. Chukwueke UN, Wen PY. Use of the Response Assessment in Neuro-Oncology (RANO) criteria in clinical trials and clinical practice. CNS Oncol. 2019;8(1):CNS28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Brown PD, Chung C, Liu DD, et al. A prospective phase II randomized trial of proton radiotherapy vs. intensity modulated radiotherapy for patients with newly diagnosed glioblastoma [published online ahead of print February 27, 2021]. Neuro Oncol. doi: 10.1093/neuonc/noab040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Curran WJ Jr, Scott CB, Horton J, et al. Recursive partitioning analysis of prognostic factors in three Radiation Therapy Oncology Group malignant glioma trials. J Natl Cancer Inst. 1993;85(9):704–710. [DOI] [PubMed] [Google Scholar]
- 14. Brown PD, Jensen AW, Felten SJ, et al. Detrimental effects of tumor progression on cognitive function of patients with high-grade glioma. J Clin Oncol. 2006;24(34):5427–5433. [DOI] [PubMed] [Google Scholar]
- 15. Gray RJ. A class of K-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat. 1988;16(3):1141–1154. [Google Scholar]
- 16. Helfer JL, Wen PY, Blakeley J, Gilbert MR, Armstrong TS. Report of the Jumpstarting Brain Tumor Drug Development Coalition and FDA clinical trials clinical outcome assessment endpoints workshop (October 15, 2014, Bethesda MD). Neuro Oncol. 2016;18(suppl 2):ii26–ii36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ellingson BM, Bendszus M, Boxerman J, et al. Consensus recommendations for a standardized Brain Tumor Imaging Protocol in clinical trials. Neuro Oncol. 2015;17(9):1188–1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Blakeley JO, Coons SJ, Corboy JR, Kline Leidy N, Mendoza TR, Wefel JS. Clinical outcome assessment in malignant glioma trials: measuring signs, symptoms, and functional limitations. Neuro Oncol. 2016;18(suppl 2):ii13–ii20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bergo E, Lombardi G, Guglieri I, Capovilla E, Pambuku A, Zagone V. Neurocognitive functions and health-related quality of life in glioblastoma patients: a concise review of the literature. Eur J Cancer Care (Engl). 2019;28(1):e12410. [DOI] [PubMed] [Google Scholar]
- 20. Provenzale JM, Ison C, Delong D. Bidimensional measurements in brain tumors: assessment of interobserver variability. AJR Am J Roentgenol. 2009;193(6):W515–W522. [DOI] [PubMed] [Google Scholar]
- 21. Chang K, Beers AL, Bai HX, et al. Automatic assessment of glioma burden: a deep learning algorithm for fully automated volumetric and bidimensional measurement. Neuro Oncol. 2019;21(11):1412–1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sun L, Zhang S, Chen H, Luo L. Brain tumor segmentation and survival prediction using multimodal MRI scans with deep learning. Front Neurosci. 2019;13:810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Menze BH, Jakab A, Bauer S, et al. The Multimodal Brain Tumor Image Segmentation Benchmark (BRATS). IEEE Trans Med Imaging. 2015;34(10):1993–2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zhuge Y, Ning H, Mathen P, et al. Automated glioma grading on conventional MRI images using deep convolutional neural networks. Med Phys. 2020;47(7):3044–3053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Pope WB, Brandal G. Conventional and advanced magnetic resonance imaging in patients with high-grade glioma. Q J Nucl Med Mol Imaging. 2018;62(3):239–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kazda T, Bulik M, Pospisil P, et al. Advanced MRI increases the diagnostic accuracy of recurrent glioblastoma: single institution thresholds and validation of MR spectroscopy and diffusion weighted MR imaging. Neuroimage Clin. 2016;11:316–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kamada K, Houkin K, Abe H, Sawamura Y, Kashiwaba T. Differentiation of cerebral radiation necrosis from tumor recurrence by proton magnetic resonance spectroscopy. Neurol Med Chir (Tokyo). 1997;37(3):250–256. [DOI] [PubMed] [Google Scholar]
- 28. Chu HH, Choi SH, Ryoo I, et al. Differentiation of true progression from pseudoprogression in glioblastoma treated with radiation therapy and concomitant temozolomide: comparison study of standard and high-b-value diffusion-weighted imaging. Radiology. 2013;269(3):831–840. [DOI] [PubMed] [Google Scholar]
- 29. Lee WJ, Choi SH, Park CK, et al. Diffusion-weighted MR imaging for the differentiation of true progression from pseudoprogression following concomitant radiotherapy with temozolomide in patients with newly diagnosed high-grade gliomas. Acad Radiol. 2012;19(11):1353–1361. [DOI] [PubMed] [Google Scholar]
- 30. Thomas AA, Arevalo-Perez J, Kaley T, et al. Dynamic contrast enhanced T1 MRI perfusion differentiates pseudoprogression from recurrent glioblastoma. J Neurooncol. 2015;125(1):183–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Shiroishi MS, Boxerman JL, Pope WB. Physiologic MRI for assessment of response to therapy and prognosis in glioblastoma. Neuro Oncol. 2016;18(4):467–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Schmainda KM, Prah M, Connelly J, et al. Dynamic-susceptibility contrast agent MRI measures of relative cerebral blood volume predict response to bevacizumab in recurrent high-grade glioma. Neuro Oncol. 2014;16(6):880–888. [DOI] [PMC free article] [PubMed] [Google Scholar]