Abstract
Background
Evidence for the impact of body size and composition on cancer risk is limited. This mendelian randomisation (MR) study investigates evidence supporting causal relationships of body mass index (BMI), fat mass index (FMI), fat-free mass index (FFMI), and height with cancer risk.
Methods and findings
Single nucleotide polymorphisms (SNPs) were used as instrumental variables for BMI (312 SNPs), FMI (577 SNPs), FFMI (577 SNPs), and height (293 SNPs). Associations of the genetic variants with 22 site-specific cancers and overall cancer were estimated in 367,561 individuals from the UK Biobank (UKBB) and with lung, breast, ovarian, uterine, and prostate cancer in large international consortia. In the UKBB, genetically predicted BMI was positively associated with overall cancer (odds ratio [OR] per 1 kg/m2 increase 1.01, 95% confidence interval [CI] 1.00–1.02; p = 0.043); several digestive system cancers: stomach (OR 1.13, 95% CI 1.06–1.21; p < 0.001), esophagus (OR 1.10, 95% CI 1.03, 1.17; p = 0.003), liver (OR 1.13, 95% CI 1.03–1.25; p = 0.012), and pancreas (OR 1.06, 95% CI 1.01–1.12; p = 0.016); and lung cancer (OR 1.08, 95% CI 1.04–1.12; p < 0.001). For sex-specific cancers, genetically predicted elevated BMI was associated with an increased risk of uterine cancer (OR 1.10, 95% CI 1.05–1.15; p < 0.001) and with a lower risk of prostate cancer (OR 0.97, 95% CI 0.94–0.99; p = 0.009). When dividing cancers into digestive system versus non-digestive system, genetically predicted BMI was positively associated with digestive system cancers (OR 1.04, 95% CI 1.02–1.06; p < 0.001) but not with non-digestive system cancers (OR 1.01, 95% CI 0.99–1.02; p = 0.369). Genetically predicted FMI was positively associated with liver, pancreatic, and lung cancer and inversely associated with melanoma and prostate cancer. Genetically predicted FFMI was positively associated with non-Hodgkin lymphoma and melanoma. Genetically predicted height was associated with increased risk of overall cancer (OR per 1 standard deviation increase 1.09; 95% CI 1.05–1.12; p < 0.001) and multiple site-specific cancers. Similar results were observed in analyses using the weighted median and MR–Egger methods. Results based on consortium data confirmed the positive associations between BMI and lung and uterine cancer risk as well as the inverse association between BMI and prostate cancer, and, additionally, showed an inverse association between genetically predicted BMI and breast cancer. The main limitations are the assumption that genetic associations with cancer outcomes are mediated via the proposed risk factors and that estimates for some lower frequency cancer types are subject to low precision.
Conclusions
Our results show that the evidence for BMI as a causal risk factor for cancer is mixed. We find that BMI has a consistent causal role in increasing risk of digestive system cancers and a role for sex-specific cancers with inconsistent directions of effect. In contrast, increased height appears to have a consistent risk-increasing effect on overall and site-specific cancers.
Mathew Vithayathil and colleagues study associations of body mass index and other measures with incidence of specific cancers.
Author summary
Why was this study done?
The causal relevance of body size and composition as risk factors for specific cancers is unclear based on traditional observational studies.
By considering the relationships between genetically predicted values of body size and composition with cancer risk, our estimates are less influenced by confounding variables, and, hence, more reliably reflect the underlying causal relationships between these measures and cancer risk.
What did the researchers do and find?
We assessed the associations between genetically predicted body mass index (BMI), fat mass index (FMI), fat-free mass index (FFMI), and height with 22 specific cancers in the UK Biobank (UKBB), a population-based sample of the United Kingdom residents.
Although genetically predicted height was consistently associated with increased risk of site-specific cancers, genetically predicted BMI was associated with an increased risk of certain digestive system cancers (esophageal, stomach, liver, and pancreas), plus lung and uterine cancer, but a decreased risk of breast and prostate cancer.
When dividing cancers into digestive system cancers versus non-digestive system cancers, genetically predicted BMI was associated with increased risk of digestive system cancers, but not associated with non-digestive system cancers.
What do these findings mean?
Our findings suggest that BMI is a causal risk factor for some cancers, but is not a generic risk factor for all cancers.
Body fat may play a role in development of specific cancers and should be studied further to identify future targets to prevent cancer.
Public health strategies should focus on reducing obesity as a risk factor for cancer, but should be clear that benefit may be limited to certain cancers.
Background
Obesity is a global epidemic [1] that is predicted to affect 20% of the world’s population by 2025 [2]. The relationship between obesity and cancer risk has been subject to extensive investigation. Body mass index (BMI) is the most commonly measured marker for obesity and correlates most strongly with fat mass [3]. Observational studies have shown that raised BMI is associated with increased risk [4–7], no risk [4,7], and even reduced risk [4,8] of cancers. In particular, consistent positive associations have been observed between BMI and risk of colorectal, stomach, esophagus, liver, gallbladder, breast, endometrial, ovarian, kidney, and pancreatic cancers [9–11]. However, traditional epidemiological studies are influenced by confounding factors, such as smoking [12,13], and reverse causation due to subclinical disease [14,15]. Thus, the true relationship between obesity and cancer remains unclear.
Mendelian randomisation (MR) uses genetic variants as instrumental variables for an exposure to investigate evidence for a causal effect of the exposure on a disease outcome [16]. MR estimates represent associations of genetically predicted levels of risk factors with outcomes, as opposed to standard epidemiological estimates, which represent associations of the risk factor levels with outcomes. As a result of Mendel’s laws of segregation and independent assortment, estimates from MR are less susceptible to bias due to confounding factors than those from conventional observational epidemiological studies [17]. As the genetic code cannot be influenced by environmental factors or preclinical disease, MR estimates are also less susceptible to bias due to reverse causation.
MR investigations have previously been performed to investigate the effect of obesity on various cancer types. Studies have suggested risk-increasing effects of BMI for cancers of the colorectum, pancreas, kidney, lung, uterus, and ovary, for adenocarcinoma in the esophagus, and for overall cancer [18–20]. A separate study evidenced a risk-increasing effect of BMI on renal cell carcinoma [21]. Risk-decreasing effects of BMI have been evidenced for both pre- and postmenopausal breast cancer [22] and for prostate cancer in one study [23], but not others [19,24]. However, a comprehensive MR investigation into the effect of BMI on a wide range of cancer types has not been performed. Additionally, previous investigations have not considered the specific contribution of fat mass and fat-free mass to cancer risk.
Our aim in this paper is to perform a wide-angled MR investigation to provide independent evidence that replicates and extends analyses for the impact of obesity on cancer outcomes where MR investigations have already been performed and provides new evidence for cancer outcomes that have not previously been investigated using this approach. In particular, we want to assess the consistency of findings across different site-specific cancers. We define fat mass index (FMI) and fat-free mass index (FFMI) analogously to BMI, as fat mass or fat-free mass divided by height squared. As a comparative analysis, we consider height as a risk factor, as this has also been suggested to have a causal effect on multiple cancer types [25–27]. We used MR to investigate the causal roles of genetically predicted BMI, FMI, FFMI, and height on the risk of developing 22 cancers in 367,561 individuals from the UK Biobank (UKBB) study. We supplement our investigation with publicly available genetic association data from large international consortia for certain site-specific cancers. We aimed to elucidate the causal role of body composition for site-specific cancers and so extend and focus the evidence base for targeted public health strategies.
Methods
Study population
Data for genetic associations with site-specific cancer risk were obtained from the UKBB. The UKBB comprises demographic, clinical, biochemical, and genetic data from around 500,000 adults (aged 37 to 73 years old at baseline) recruited between 2006 and 2010 and followed up until June 30, 2020 [28]. Only unrelated individuals of European ancestries (defined by self-report and genetics) were included in our analysis in order to reduce population stratification bias. For each group of related individuals (third-degree relatives or closer), only 1 individual was included in analyses. After performing quality control filters as described previously [29], 367,561 individuals were included in analyses (S1 Fig). We defined cancer outcomes in the UKBB for the 22 most common site-specific cancers in the UK using the International Classification of Diseases (ICD)-9 and ICD-10 coding (S1 Table). Overall cancer analyses included individuals with any of the 22 site-specific cancers. Cancer outcomes were obtained from electronic health records, hospital episodes statistics (HES) data, the National Cancer Registry, death certification data, and self-reporting validated by nurse interview. Both prevalent and incident events were included in analyses. Genetic association estimates were obtained for each cancer outcome by logistic regression adjusting for age, sex, and 10 genetic principal components. Associations for sex-specific cancers (breast, ovarian, cervical, and uterine for women and testicular and prostate for men) were estimated in participants of the relevant sex only (198,825 women and 168,736 men).
In addition, publicly available data were extracted from the MR-Base platform [30] for lung, breast, ovarian, uterine, and prostate cancer from the International Lung Cancer Consortium (11,348 cases and 15,861 controls) [31], Breast Cancer Association Consortium (BCAC) (122,977 cases and 105,974 controls) [32], the Ovarian Cancer Association Consortium (25,509 cases and 40,941 controls) [33], a meta-analysis of genome-wide association studies (GWASs) for endometrial cancer (12,906 cases and 108,979 controls from 17 studies identified from the Endometrial Cancer Association Consortium, the Epidemiology of Endometrial Cancer Consortium, and the UKBB) [34], and the Prostate Cancer Association Group to Investigate Cancer Associated Alterations in the Genome consortium (79,148 cases and 61,106 controls) [35].
Genetic instruments
The genetic instrument for BMI compromised 312 uncorrelated single nucleotide polymorphisms (SNPs) (linkage disequilibrium R2 < 0.001) associated with BMI at genome-wide significance (p < 5 × 10−8) in a GWAS of up to 806,834 individuals of European ancestries [36] (S2 Table). For fat mass and fat-free mass (measured using bioelectrical impedance), we used 577 uncorrelated SNPs associated with body composition among 331,291 UKBB participants [37] (S3 Table). FMI and FFMI were computed by dividing fat mass or fat-free mass by the square of height. A GWAS of 253,288 European ancestry individuals identified 697 genome-wide significant SNPs (p < 5 × 10−8) for adult height [38], of which 293 were uncorrelated (linkage disequilibrium R2 < 0.001) and were used as instrumental variables (S4 Table). Genetic associations with these body composition measures were obtained from the relevant discovery GWAS and were adjusted for age, sex, and genetic principal components.
Statistical analysis
Associations of genetically predicted BMI and height with the 22 site-specific cancers and overall cancer for the UKBB cohort were obtained using the random-effects inverse-variance weighted method [39]. We performed additional analyses using the weighted median [39] and MR–Egger [40] methods. The analyses of FMI and FFMI were based on the multivariable random-effects inverse-variance weighted method with both exposures included in the same model. Although overall cancer is a composite outcome comprising malignancies with different etiologies, analyses for overall cancer are important from a public health perspective to estimate the overall impact of the risk factors on cancer risk.
Our analysis did not have an explicit prespecified analysis plan. The analysis was conducted similarly to previous published efforts for investigating the causal relationships of BMI, FMI, and FFMI with cardiovascular diseases [37,41]. In response to comments from peer reviewers, we made a number of changes to the analysis: We changed the overall cancer outcome from including all cancers to including any of the 22 named site-specific cancers, we updated the genetic variants used as instrumental variables to those from the latest GWAS for the relevant risk factor, and we added analyses based on large-scale consortia for lung, breast, ovarian, uterine, and prostate cancer.
The odds ratios (ORs) are expressed per 1 kg/m2 increase in genetically predicted BMI, FMI, and FFMI and per 1 standard deviation (approximately 6.5 cm) increase in height. As the number of cases and thus statistical power differed between analyses, we did not set a fixed threshold for statistical significance. Statistical analyses were performed in Stata/SE 14.2 using the mrrobust package [42] and R 3.6.0 software using the MendelianRandomization package [39]. Power calculations were performed using the web-based tool at https://sb452.shinyapps.io/power/ [43].
This study is reported as per the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guideline (S1 Checklist).
Results
Baseline characteristics of the 367,561 participants in the UKBB are provided in Table 1. The mean age was 57.2 years, with 45.9% males. Moreover, 10.3% of participants were current smokers. In the UKBB, the 312 SNPs explained 4.1% of the variance in BMI, whereas the 577 SNPs for body composition explained 3.1% of the variance in FMI and 2.3% of the variance in FFMI. The 293 SNPs for height explained around 5.5% of the variance in height. The phenotypic correlation of BMI with FMI was 0.84 and with FFMI was 0.66. The correlation between FMI and FFMI was 0.14. The relatively low correlation between FMI and FFMI means multivariable analyses can likely differentiate between these 2 risk factors. A total of 59,647 participants had one of the 22 defined site-specific cancers. When excluding outcomes for which only self-reported data were available, 55 674 events remained.
Table 1. Baseline characteristics of the UKBB participants included in this study.
| Mean (SD) or n (%)† | |
|---|---|
| Sample size | 367,561 (100) |
| Male | 168,736 (45.9) |
| Age at baseline (years) | 57.2 (8.1) |
| Body composition | |
| BMI (kg/m2) | 27.4 (4.8) |
| FMI (kg/m2) | 8.8 (3.6) |
| FFMI (kg/m2) | 18.6 (2.6) |
| Height (m) | 1.69 (0.07) |
| Blood pressure (mm Hg) | |
| Systolic blood pressure | 137.6 (18.6) |
| Diastolic blood pressure | 82.0 (10.1) |
| Smoking status | |
| Current | 37,866 (10.3) |
| Ex | 185,704 (50.5) |
| Never | 143,777 (39.1) |
| Alcohol status | |
| Current | 342,797 (93.2) |
| Ex | 12,732 (3.5) |
| Never | 11,646 (3.2) |
| Type 2 diabetes | 15,834 (4.3) |
† Mean (standard deviation) for continuous variables; n (%) for categorical variables.
A total of 214 participants had missing data on smoking status, and 386 participants had missing data on alcohol status.
BMI, body mass index; FFMI, fat-free mass index; FMI, fat mass index; SD, standard deviation; UKBB, UK Biobank.
Power calculations are provided in S2 Fig. For BMI, there was 80% power to detect an OR of 1.2 per 1 kg/m2 increase in genetically predicted BMI even for outcomes with only 300 events and an OR of 1.05 for outcomes with 3,800 events. Power was lower for FMI and FFMI, although still adequate to detect a moderate effect size for more common cancers.
BMI and cancer risk
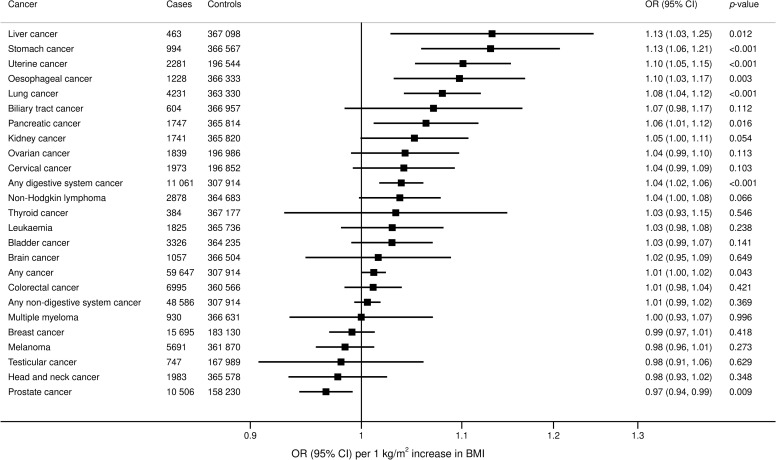
Associations between genetically predicted BMI and site-specific and overall cancer in the UKBB are shown in Fig 1. Genetically predicted BMI was associated with an increased risk of overall cancer (OR 1.01, 95% confidence interval [CI] 1.00 to 1.02; p = 0.043); certain digestive system cancers, including stomach (OR 1.13, 95% CI 1.06 to 1.21; p < 0.001), esophagus (OR 1.10, 95% CI 1.10 (1.03, 1.17; p = 0.003), liver (OR 1.13, 95% CI 1.03 to 1.25; p = 0.012), and pancreas (OR 1.06, 95% CI 1.01 to 1.12; p = 0.016); and lung cancer (OR 1.08, 95% CI 1.04 to 1.12; p < 0.001). For sex-specific cancers, elevated BMI was associated with an increased risk of uterine cancer (OR 1.10, 95% CI 1.05 to 1.15; p < 0.001) and with a lower risk of prostate cancer (OR 0.97, 95% CI 0.94 to 0.99; p = 0.009). After omission of self-reported cancer events, the strength of association with risk of overall cancer was diminished (OR 1.01, 95% CI 1.00 to 1.02; p = 0.055). Results for site-specific cancers excluding self-reported outcomes were generally similar but marginally less precise (S3 Fig). Complementary analyses based on the weighted median and MR–Egger methods revealed similar but less precise estimates (S5 Table).
Fig 1. Associations of genetically predicted BMI with overall and site-specific cancers in the UKBB.
ORs are expressed per 1 kg/m2 increase in BMI. Results are obtained from the random-effects inverse-variance weighted method. BMI, body mass index; CI, confidence interval; OR, odds ratio; UKBB, UK Biobank.
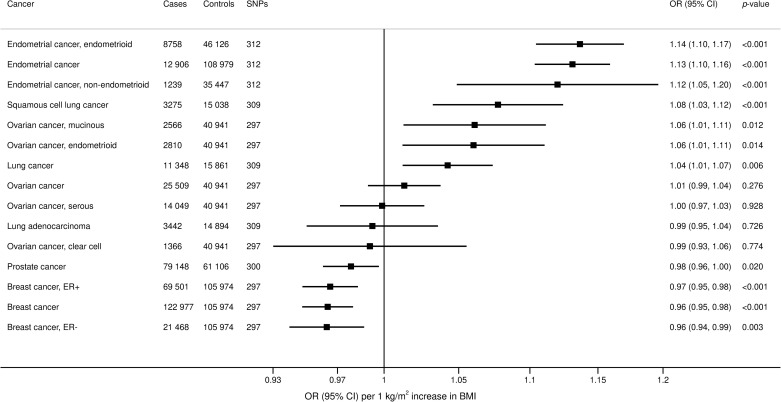
In analyses based on consortium data, genetically predicted BMI was positively associated with increased risk of overall lung cancer (OR 1.04, 95% CI 1.01 to 1.07; p = 0.006) and the squamous cell cancer subtype (OR 1.08, 95% CI 1.03 to 1.12; p < 0.001) (Fig 2). For sex-specific cancers, elevated BMI was associated with increased risk of uterine cancer (OR 1.13, 95% CI 1.10 to 1.16; p < 0.001), but with lower risk of breast (OR 0.96, 95% CI 0.95 to 0.98; p < 0.001) and prostate cancer (OR 0.98, 95% CI 0.96 to 1.00; p = 0.020). Inverse associations with similar magnitude were observed for both estrogen receptor positive (ER+) and estrogen receptor negative (ER−) breast cancer. Positive associations were observed for mucinous and endometrioid ovarian cancer, but not overall ovarian cancer.
Fig 2. Associations of genetically predicted BMI with site-specific cancers in large international consortia.
ORs are expressed per 1 kg/m2 increase in BMI. Results are obtained from the random-effects inverse-variance weighted method. BMI, body mass index; CI, confidence interval; ER−, estrogen receptor negative; ER+, estrogen receptor positive; OR, odds ratio; SNP, single nucleotide polymorphism.
FMI and FFMI and cancer risk
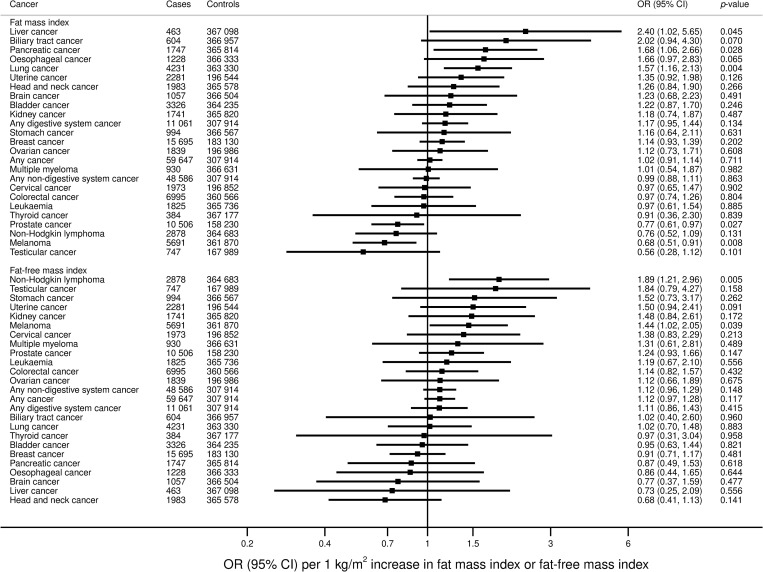
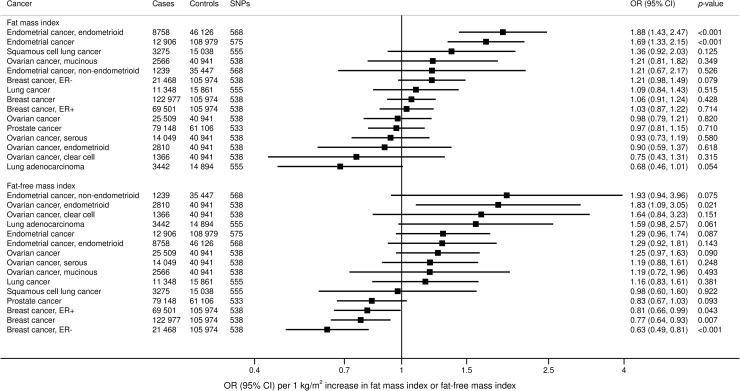
Similar to BMI, genetically predicted FMI was associated with an increased risk of liver (OR 2.40, 95% CI 1.02 to 5.65; p = 0.045), pancreatic (OR 1.68, 95% CI 1.06 to 2.66; p = 0.028), and lung cancer (OR 1.57, 95% CI 1.16 to 2.13; p = 0.004) in the UKBB (Fig 3). Elevated FMI was associated with lower risk of prostate cancer (OR 0.77, 95% CI 0.61 to 0.97; p = 0.027) and melanoma (OR 0.68, 95% CI 0.51 to 0.91; p = 0.008). From the consortia, elevated FMI was associated with increased uterine cancer risk (OR 1.69, 95% CI 1.33 to 2.15; p < 0.001) (Fig 4).
Fig 3. Associations of genetically predicted FMI and FFMI with overall and site-specific cancers in the UKBB.
ORs are expressed per one 1 kg/m2 increase in FMI. Results are obtained from the multivariable random-effects inverse-variance weighted method. CI, confidence interval; FFMI, fat-free mass index; FMI, fat mass index; OR, odds ratio; UKBB, UK Biobank.
Fig 4. Associations of genetically predicted FMI and FFMI with site-specific cancers in large international consortia.
ORs are expressed per one 1 kg/m2 increase in FMI. Results are obtained from the multivariable random-effects inverse-variance weighted method. CI, confidence interval; ER−, estrogen receptor negative; ER+, estrogen receptor positive; FFMI, fat-free mass index; FMI, fat mass index; OR, odds ratio; SNP, single nucleotide polymorphism.
Genetically predicted FFMI was associated with increased risk of non-Hodgkin lymphoma (OR 1.89, 95% CI 1.21 to 2.96; p = 0.005) and melanoma (OR 1.44, 95% CI 1.02 to 2.05; p = 0.039) in the UKBB (Fig 3). From the consortia, elevated FFMI was associated with a decreased risk of breast cancer (0.77, 95% CI 0.64 to 0.93; p = 0.007) (Fig 4).
Height and cancer risk
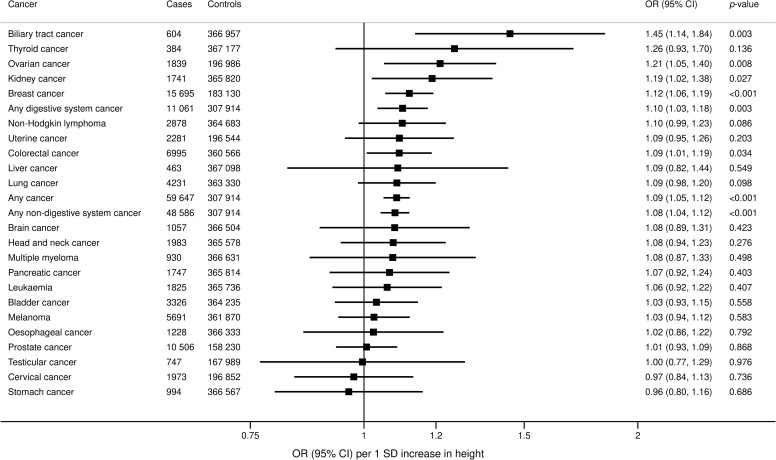
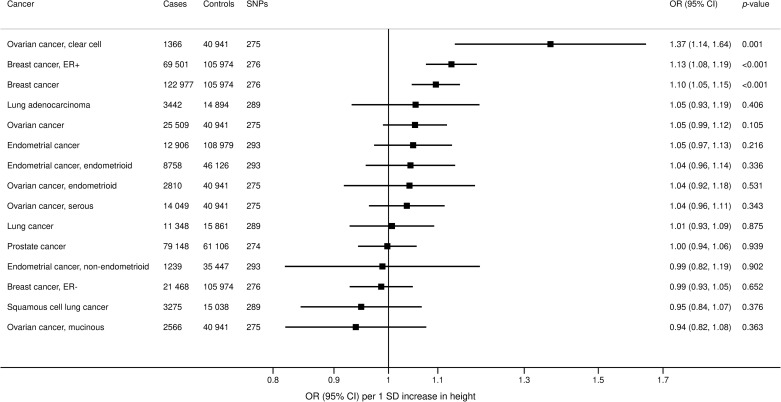
In the UKBB, genetically predicted height was positively associated with overall cancer (OR 1.09; 95% 1.05 to 1.12; p < 0.001) and multiple site-specific cancers, including kidney (OR 1.19, 95% CI 1.02 to 1.38; p = 0.027), colorectal (OR 1.09, 95% CI 1.01 to 1.19; p = 0.034), biliary tract (OR 1.45, 95% CI 1.14 to 1.84; p = 0.003), breast (OR 1.12, 95% CI 1.06 to 1.19; p < 0.001), and ovarian cancer (OR 1.21, 95% CI 1.05 to 1.40; p = 0.008) (Fig 5). Estimates were generally similar in additional analyses using the MR–Egger and weighted median methods (S6 Table). From the consortia, genetically predicted height was positively associated with ovarian (OR 1.37, 95% CI 1.14 to 1.64; p = 0.001) and breast cancer (OR 1.10, 95% CI 1.05 to 1.15; p < 0.001), similar to the UKBB (Fig 6).
Fig 5. Associations of genetically predicted height with overall and site-specific cancers in the UKBB.
ORs are expressed per 1 standard deviation (6.5 cm) increase in height. Results are obtained from the random-effects inverse-variance weighted method. CI, confidence interval; OR, odds ratio; UKBB, UK Biobank.
Fig 6. Associations of genetically predicted height with site-specific cancers in large international consortia.
ORs are expressed per 1 standard deviation (6.5 cm) increase in height. Results are obtained from the random-effects inverse-variance weighted method. CI, confidence interval; ER−, estrogen receptor negative; ER+, estrogen receptor positive; OR, odds ratio; SNP, single nucleotide polymorphism.
Digestive system versus non-digestive system cancer risk
When dividing cancers into digestive system (esophagus, stomach, colorectum, liver, biliary tract, and pancreas; 11,061 cases) versus non-digestive system (48,586 cases), estimates for BMI were OR 1.04 (95% CI 1.02 to 1.06; p < 0.001) for digestive system and OR 1.01 (95% CI 0.99 to 1.02; p = 0.37) for non-digestive system cancers. For FMI, estimates were OR 1.17 (95% CI 0.95 to 1.44; p = 0.13) for digestive system and OR 0.99 (95% CI 0.88 to 1.11; p = 0.86) for non-digestive system cancers. For FFMI, estimates were OR 1.11 (95% CI 0.86 to 1.43; p = 0.42) for digestive system and OR 1.12 (95% CI 0.96 to 1.29; p = 0.15) for non-digestive system cancers. Genetically predicted height was positively associated with both digestive system and non-digestive system cancers (Fig 5).
Discussion
This MR study investigated the causal role of clinically relevant measures of body composition for a wide range of site-specific cancers. Genetically predicted BMI was associated with risk of overall cancer. Elevated BMI was positively associated with several digestive system cancers, including at the esophagus, stomach, liver, and pancreas. Additionally, BMI was positively associated with lung and uterine cancer, but inversely associated with breast cancer (only in the BCAC) and prostate cancer. Genetically predicted FMI was positively associated with lung, liver, and pancreatic cancer, with inverse associations seen for melanoma and prostate cancer. FFMI was positively associated with non-Hodgkin lymphoma, melanoma, and uterine cancer, with inverse associations seen for breast cancer. Genetically predicted height was positively associated with overall and multiple site-specific cancers.
The relationship between adiposity and cancer has been assessed in many traditional observational studies. An umbrella review of 204 meta-analyses found adiposity to be consistently associated with 10 site-specific cancers: esophageal, gastric, colorectal, biliary tract, pancreas, breast, uterine, ovarian, and kidney cancer as well as multiple myeloma [11]. Current MR studies have replicated the associations with esophageal, colorectal, pancreas, pancreas, uterine, ovarian, and kidney cancer [18–21,44,45]. However, further positive associations were observed in MR analyses for lung cancer [19,20], and inverse associations were seen with breast and prostate cancer [22,23]. These discrepancies between observational and MR studies may be due to the effect of environmental confounders (such as smoking) and reverse causation bias in traditional observational studies.
In our study, we observed a positive association between genetically predicted BMI and digestive system cancers. This link between BMI and adiposity with risk of certain digestive system cancers replicates and extends previous findings. High BMI has been associated with esophageal and stomach cancers in a meta-analysis of observational studies [46] and MR studies [20]. Increased adipose tissue is associated with insulin resistance and hyperinsulinemia [47], with raised circulating insulin enhancing colorectal epithelial cell proliferation in rat models [48]. Additionally, ghrelin is a gut hormone produced in the stomach, with reduced levels seen in obesity [49]. Ghrelin reduces pro-inflammatory cytokines and inflammatory stress [50], and reduced levels are associated with increased risk of esophageal [51] and stomach [52] cancers. Adiposity is also well established in causing nonalcoholic fatty liver disease and has been implicated in its progression to hepatocellular carcinoma [53]. In line with this, we observed an increased risk of liver cancer with raised BMI and FMI. Similarly, we observed a positive association between elevated BMI and FMI and pancreas cancer, in line with adipose tissue driving low grade inflammation and carcinogenesis in pancreatic tissue through pro-inflammatory cytokines [54]. We also observe low-precision positive associations between elevated BMI and colorectal and biliary tract cancers, suggesting a causal role between adiposity and digestive system cancers. Further research should assess the impact of weight loss interventions and dietary interventions in reducing cancers of the digestive system.
In this MR study, elevated genetically predicted BMI was associated with sex-specific cancers: increased risk of uterine cancer and decreased risk of breast and prostate cancer. BMI and breast cancer has been extensively studied in previous MR studies [19,22,55,56]. A previous MR study based on data from the BCAC and DRIVE consortia of 46,325 cases of breast cancer found that genetically predicted BMI based on 84 SNPs was inversely associated with breast cancer risk in both pre- and postmenopausal women [22], consistent with the findings of our study based on data from the BCAC. A further MR study of 98,842 cases of breast cancer confirmed this finding [57]. These MR results contrast with observational study findings that have demonstrated adult obesity to be associated with increased risk of postmenopausal breast cancer [10,46]. Furthermore, our study corroborates the findings of a previous MR study of 6,609 cases of uterine cancer, showing genetically predicted BMI was positively associated with incidence [58]. In males, our findings are in line with a previous MR of 22 European cohorts, which showed weak evidence for an inverse association between genetically predicted BMI and prostate cancer [24], as well as a larger analysis showing stronger evidence for an association [23]. The association of BMI and these sex-specific cancers is likely to be at least in part hormonally mediated. In males, elevated BMI reduces serum testosterone [59], with androgens recognised as promoting prostate cancer. In premenopausal women, increased BMI is associated with anovulation, reducing lifetime exposure of circulating estrogen and progesterone [60], and thus a consequent reduction in breast cancer risk [61] and an increase in uterine cancer risk [62]. While observational associations of BMI with pre- and postmenopausal breast cancers are directionally discordant, MR estimates have consistent direction. This may be because the association of genetically predicted BMI is mediated via lifelong exposure to elevated estrogen levels, the majority of which is premenopausal. We report an inverse association between FFMI and breast cancer risk, suggesting that increased non-adipose tissue density may have a protective role against malignancy. Observational studies have demonstrated that sarcopenia is associated with increased breast cancer mortality [63,64], although the association may be subject to reverse causation. However, reduced muscle mass and atrophy is associated with systemic inflammation [65,66] and increased TNF-alpha levels [66], which may drive carcinogenesis, although the mechanistic links need to be assessed further.
We report a positive association between genetically predicted BMI and squamous cell lung cancer risk, consistent with previous MR findings [19]. Our findings of no association between BMI and melanoma risk are consistent with findings of a recent MR investigation [67]. However, we observed an inverse association between FMI and melanoma risk, but a positive association for FFMI. These findings suggest that body composition influences melanoma risk, with increased adiposity being protective against melanoma. This may be because those with higher FFMI and lower FMI spend more time outdoors and so have greater exposure to the sun. Previous observational studies have shown that obesity is associated with improved survival of melanoma patients [68], with a down-regulation of key lipid genes shown in melanoma cells [69], suggesting that lipids play a role in carcinogenesis.
We observed a positive association between genetically predicted height and overall cancer risk, which was consistent across a wide range of site-specific cancers, including kidney, colorectal, biliary tract, ovarian, and breast cancers. Our findings are consistent with previous MR studies showing a positive association of height with colorectal [70–72] and breast cancer [72–74]. Increased height is associated with elevated insulin-like growth factor 1 (IGF1) [75], which is a growth factor that drives cellular proliferation and survival and has thus been implicated in carcinogenesis of IGF-responsive tissues. Increased expression of IGF-1 and its cellular receptors are present in cancer tumours [76,77]. Our recent MR investigation demonstrated that genetically predicted IGF-1 was associated with increased risk of colorectal cancer, and, possibly, breast cancer, but not associated with overall or other site-specific cancers [78]. This suggests that the effect of height on cancer risk operates via pathways independent of IGF-1.
We observed a positive association between elevated genetically predicted BMI and overall cancer risk. This result replicates a recent MR investigation using 520 genetic variants for BMI in the UKBB that showed that overall cancer risk (excluding nonmelanoma skin cancer) and mortality was associated with elevated genetically predicted BMI. However, when dividing cancers into digestive system versus non-digestive system, genetically predicted BMI was only associated with digestive system cancers. This result has important clinical implications. Previous public health recommendations have advocated obesity as a generic risk factor for cancer prevention [79,80]. While our research supports a causal role of obesity in driving and protecting against certain cancers, it suggests differential effects of BMI and obesity in different malignancies, which should be explored further. A more nuanced message public health message with regard to obesity as a risk factor for digestive system cancers may be more appropriate.
Our study has several strengths. The MR design minimises the influence of environmental confounding factors and reverse causality, allowing for causal relationships to be better characterised. The UKBB is a large prospective cohort, allowing multiple cancer types to be studied in a single dataset and comparisons of estimates across cancers to be made. However, there are some limitations. The main limitation is the assumption that the genetic associations with cancer risk are mediated via the proposed risk factors. Additionally, estimates for some lower frequency cancer types are subject to low precision, and, therefore, results should be interpreted based on the magnitude of the associations rather than on p-values alone. Another shortcoming is that our findings may not be applicable to other ethnic groups as we confined the study population to individuals of European ancestries to minimise bias from population stratification. As we analysed middle- to early late-aged individuals, some cancers, particularly those with early onset and poor survival, may not be well captured in our analysis. Associations in the UKBB may be subject to selection bias [81], as participants in the UKBB are overall more healthy and better educated compared to the overall UK population [82]. Another potential source of bias is detection bias. The probability of diagnosis for less severe cancers (such as prostate cancer) may be more likely if the individual has comorbidities and so has more extensive contact with health services. While we wanted to perform analyses for specific cancer subtypes, we were unable to define these in a reliable way for the majority of site-specific cancers in the data available. Results for overall cancer are dependent on the characteristics of the analytic sample and the relative prevalence of different cancer types. In particular, cancers with greater survival chances will be overrepresented in the case sample. Selection of genetic variants was based on datasets that include the UKBB participants, and for FMI and FFMI, on a dataset comprised solely of the UKBB participants. This may lead to bias due to sample overlap and winner’s curse. However, results were similar when genetic associations with cancers were taken from independent consortia. Our analysis assumes a linear relationship between the risk factors and the outcome. Quantitative estimates may be misleading if the true relationship is nonlinear, although estimates are still reflective of the presence and direction of the population-averaged causal effect [83]. As with all methodologies that aim to assess causal relationships, MR makes untestable assumptions. The approach relies on the genetic associations with cancer risk being mediated via the body composition measures. While we were able to perform robust methods to assess sensitivity to this assumption, it remains a possibility that some genetic variants may influence cancer risk through other pathways than obesity. A further limitation is that the relationship between obesity and cancer risk may change over the life course. Typically, MR estimates reflect the impact of a lifelong difference in the trajectory of a risk factor, as they represent associations between genetically predicted levels of risk factors and outcomes. Finally, our study does not provide understanding of the physiological pathways by which obesity and height may affect carcinogenesis.
Conclusions
In conclusion, this comprehensive MR study provides evidence that elevated BMI increases the risk of digestive system cancers, and BMI increases the risk of uterine cancer but is protective for other sex-specific cancers, including breast and prostate. We showed that elevated genetically predicted FMI is associated with liver, lung, and pancreatic cancer, with FFMI inversely associated with breast cancer. In contrast, genetically predicted height was consistently positively associated with overall cancer and several site-specific cancers. These findings suggest that obesity and body composition have particular causal relevance to specific cancer types.
Supporting information
STROBE, Strengthening the Reporting of Observational Studies in Epidemiology.
(DOCX)
PCA, principal component analysis; SD, standard deviation; UKBB, UK Biobank.
(PDF)
As power calculators are not available for multivariable MR, all calculations are performed for univariable MR analyses based on each risk factor in turn. BMI, body mass index; FFMI, fat-free mass index; FMI, fat mass index; MR, mendelian randomisation; OR, odds ratio.
(PDF)
ORs are expressed per 1 kg/m2 increase in BMI. Results are obtained from the random-effects inverse-variance weighted method. BMI, body mass index; CI, confidence interval; OR, odds ratio; UKBB, UK Biobank.
(PDF)
(PDF)
(PDF)
MR, mendelian randomisation; SNP, single nucleotide polymorphism.
(PDF)
(PDF)
BMI, body mass index.
(PDF)
(PDF)
Acknowledgments
The authors thank all investigators from the UK Biobank (UKBB), where data were conducted under application 29202.
Disclaimers: The views expressed are those of the authors and not necessarily those of the National Health Service, the National Institute for Health Research, or the Department of Health and Social Care.
Abbreviations
- BCAC
Breast Cancer Association Consortium
- BMI
body mass index
- CI
confidence interval
- ER−
estrogen receptor negative
- ER+
estrogen receptor positive
- FFMI
fat-free mass index
- FMI
fat mass index
- GWAS
genome-wide association study
- HES
hospital episodes statistics
- ICD
International Classification of Diseases
- IGF1
insulin-like growth factor 1
- MR
mendelian randomisation
- OR
odds ratio
- SNP
single nucleotide polymorphism
- STROBE
Strengthening the Reporting of Observational Studies in Epidemiology
- UKBB
UK Biobank
Data Availability
All primary data are available from the UK Biobank on application to any bona fide researcher (url: https://www.ukbiobank.ac.uk/). Genetic associations with cancer outcomes have been deposited at https://figshare.com/articles/dataset/Genetic_associations_with_cancer_outcomes/14806638.
Funding Statement
Stephen Burgess is supported by Sir Henry Dale Fellowship jointly funded by the Wellcome Trust and the Royal Society (Grant Number 204623/Z/16/Z). This work was supported by the UK National Institute for Health Research Cambridge Biomedical Research Centre (BRC-1215-20014). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Obesity: preventing and managing the global epidemic. Report of a WHO consultation. World Heal Organ—Tech Rep Ser. 2000. [PubMed]
- 2.Di Cesare M, Bentham J, Stevens GA, Zhou B, Danaei G, Lu Y, et al. Trends in adult body-mass index in 200 countries from 1975 to 2014: A pooled analysis of 1698 population-based measurement studies with 19.2 million participants. Lancet. 2016 (16):30054–X. doi: 10.1016/S0140-6736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Flegal KM, Shepherd JA, Looker AC, Graubard BI, Borrud LG, Ogden CL, et al. Comparisons of percentage body fat, body mass index, waist circumference, and waist-stature ratio in adults. Am J Clin Nutr. 2009. doi: 10.3945/ajcn.2008.26847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Song X, Pukkala E, Dyba T, Tuomilehto J, Moltchanov V, Männistö S, et al. Body mass index and cancer incidence: The FINRISK study. Eur J Epidemiol. 2014. doi: 10.1007/s10654-014-9934-z [DOI] [PubMed] [Google Scholar]
- 5.Johnson CM, Wei C, Ensor JE, Smolenski DJ, Amos CI, Levin B, et al. Meta-Analyses of colorectal cancer risk factors. Cancer Causes Control. 2013. doi: 10.1007/s10552-013-0201-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kawachi A, Shimazu T, Budhathoki S, Sawada N, Yamaji T, Iwasaki M, et al. Association of BMI and height with the risk of endometrial cancer, overall and by histological subtype: A population-based prospective cohort study in Japan. Eur J Cancer Prev. 2019. doi: 10.1097/CEJ.0000000000000449 [DOI] [PubMed] [Google Scholar]
- 7.Renehan AG, Tyson M, Egger M, Heller RF, Zwahlen M. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet. 2008. doi: 10.1016/S0140-6736(08)60269-X [DOI] [PubMed] [Google Scholar]
- 8.Schoemaker MJ, Nichols HB, Wright LB, Brook MN, Jones ME, O’Brien KM, et al. Association of Body Mass Index and Age with Subsequent Breast Cancer Risk in Premenopausal Women. JAMA Oncol. 2018. doi: 10.1001/jamaoncol.2018.1771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bernard WS, Christopher PW. World cancer report 2020. World Health Organization. 2020. [Google Scholar]
- 10.Fund WCR. Diet, Nutrition, Physical Activity and Cancer: a Global Perspective. Continuous Update Project Expert Report. 2018;2018. doi: 10.1016/j.scienta.2014.02.005 [DOI] [Google Scholar]
- 11.Kyrgiou M, Kalliala I, Markozannes G, Gunter MJ, Paraskevaidis E, Gabra H, et al. Adiposity and cancer at major anatomical sites: Umbrella review of the literature. BMJ (Online). 2017. doi: 10.1136/bmj.j477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Samet JM. Lung Cancer, Smoking, and Obesity: It’s Complicated. Journal of the National Cancer Institute. 2018. doi: 10.1093/jnci/djy019 [DOI] [PubMed] [Google Scholar]
- 13.Song M, Giovannucci E. Estimating the influence of obesity on cancer risk: Stratification by smoking is critical. J Clin Oncol. 2016. doi: 10.1200/JCO.2016.67.6916 [DOI] [PubMed] [Google Scholar]
- 14.Flegal KM, Graubard BI, Yi SW. Comparative effects of the restriction method in two large observational studies of body mass index and mortality among adults. Eur J Clin Investig. 2017. doi: 10.1111/eci.12756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee DH, Giovannucci EL. The Obesity Paradox in Cancer: Epidemiologic Insights and Perspectives. Curr Nutr Rep. 2019. doi: 10.1007/s13668-019-00280-6 [DOI] [PubMed] [Google Scholar]
- 16.Davey Smith G, Hemani G. Mendelian randomization: genetic anchors for causal inference in epidemiological studies. Hum Mol Genet. 2014;23:R89–98. doi: 10.1093/hmg/ddu328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Davies NM, Holmes MV, Davey Smith G. Reading Mendelian randomisation studies: A guide, glossary, and checklist for clinicians. BMJ. 2018. doi: 10.1136/bmj.k601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mariosa D, Carreras-Torres R, Martin RM, Johansson M, Brennan P. Commentary: What can Mendelian randomization tell us about causes of cancer? Int J Epidemiol. 2019;48:816–21. doi: 10.1093/ije/dyz151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gao C, Patel CJ, Michailidou K, Peters U, Gong J, Schildkraut J, et al. Mendelian randomization study of adiposity-related traits and risk of breast, ovarian, prostate, lung and colorectal cancer. Int J Epidemiol. 2016. doi: 10.1093/ije/dyw129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gharahkhani P, Ong J-S, An J, Law MH, Whiteman DC, Neale RE, et al. Effect of increased body mass index on risk of diagnosis or death from cancer. Br J Cancer. 2019;120:565–70. doi: 10.1038/s41416-019-0386-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Johansson M, Carreras-Torres R, Scelo G, Purdue MP, Mariosa D, Muller DC, et al. The influence of obesity-related factors in the etiology of renal cell carcinoma-A mendelian randomization study. PLoS Med. 2019;e1002724:16. doi: 10.1371/journal.pmed.1002724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guo Y, Warren Andersen S, Shu XO, Michailidou K, Bolla MK, Wang Q, et al. Genetically Predicted Body Mass Index and Breast Cancer Risk: Mendelian Randomization Analyses of Data from 145,000 Women of European Descent. PLoS Med. 2016. doi: 10.1371/journal.pmed.1002105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kazmi N, Haycock P, Tsilidis K, Lynch BM, Truong T, Martin RM, et al. Appraising causal relationships of dietary, nutritional and physical-activity exposures with overall and aggressive prostate cancer: two-sample Mendelian-randomization study based on 79 148 prostate-cancer cases and 61 106 controls. Int J Epidemiol. 2019. doi: 10.1093/ije/dyz235 [DOI] [PubMed] [Google Scholar]
- 24.Davies NM, Gaunt TR, Lewis SJ, Holly J, Donovan JL, Hamdy FC, et al. The effects of height and BMI on prostate cancer incidence and mortality: a Mendelian randomization study in 20,848 cases and 20,214 controls from the PRACTICAL consortium. Cancer Causes Control. 2015. doi: 10.1007/s10552-015-0654-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Green J, Cairns BJ, Casabonne D, Wright FL, Reeves G, Beral V. Height and cancer incidence in the Million Women Study: prospective cohort, and meta-analysis of prospective studies of height and total cancer risk. Lancet Oncol. 2011. doi: 10.1016/S1470-2045(11)70154-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lin ZW. Analysis of the height dependence of site-specific cancer risk in relation to organ mass. Ann Transl Med. 2016. doi: 10.21037/atm.2016.03.04 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Choi YJ, Lee DH, Do HK, Yoon H, Shin CM, Park YS, et al. Adult height in relation to risk of cancer in a cohort of 22,809,722 Korean adults. Br J Cancer. 2019. doi: 10.1038/s41416-018-0371-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sudlow C, Gallacher J, Allen N, Beral V, Burton P, Danesh J, et al. UK Biobank: An Open Access Resource for Identifying the Causes of a Wide Range of Complex Diseases of Middle and Old Age. PLoS Med. 2015. doi: 10.1371/journal.pmed.1001779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Allara E, Morani G, Carter P, Gkatzionis A, Zuber V, Foley CN, et al. Genetic Determinants of Lipids and Cardiovascular Disease Outcomes: A Wide-Angled Mendelian Randomization Investigation. Circ Genomic Precis Med. 2019;12:e002711. doi: 10.1161/CIRCGEN.119.002711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hemani G, Zheng J, Elsworth B, Wade KH, Haberland V, Baird D, et al. The MR-base platform supports systematic causal inference across the human phenome. elife. 2018. doi: 10.7554/eLife.34408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang Y, McKay JD, Rafnar T, Wang Z, Timofeeva MN, Broderick P, et al. Rare variants of large effect in BRCA2 and CHEK2 affect risk of lung cancer. Nat Genet. 2014. doi: 10.1038/ng.3002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Michailidou K, Lindström S, Dennis J, Beesley J, Hui S, Kar S, et al. Association analysis identifies 65 new breast cancer risk loci. Nature. 2017. doi: 10.1038/nature24284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Phelan CM, Kuchenbaecker KB, Tyrer JP, Kar SP, Lawrenson K, Winham SJ, et al. Identification of 12 new susceptibility loci for different histotypes of epithelial ovarian cancer. Nat Genet. 2017. doi: 10.1038/ng.3826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.O’Mara TA, Glubb DM, Amant F, Annibali D, Ashton K, Attia J, et al. Identification of nine new susceptibility loci for endometrial cancer. Nat Commun. 2018. doi: 10.1038/s41467-018-05427-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schumacher FR, Al Olama AA, Berndt SI, Benlloch S, Ahmed M, Saunders EJ, et al. Association analyses of more than 140,000 men identify 63 new prostate cancer susceptibility loci. Nat Genet. 2018. doi: 10.1038/s41588-018-0142-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pulit SL, Stoneman C, Morris AP, Wood AR, Glastonbury CA, Tyrrell J, et al. Meta-Analysis of genome-wide association studies for body fat distribution in 694 649 individuals of European ancestry. Hum Mol Genet. 2019. doi: 10.1093/hmg/ddy327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Larsson SC, Burgess S. Fat mass and fat-free mass in relation to cardiometabolic diseases: a two-sample Mendelian randomization study. J Intern Med. 2020. doi: 10.1111/joim.13078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wood AR, Esko T, Yang J, Vedantam S, Pers TH, Gustafsson S, et al. Defining the role of common variation in the genomic and biological architecture of adult human height. Nat Genet. 2014. doi: 10.1038/ng.3097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yavorska OO, Burgess S. MendelianRandomization: An R package for performing Mendelian randomization analyses using summarized data. Int J Epidemiol. 2017. doi: 10.1093/ije/dyx034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Burgess S, Thompson SG. Interpreting findings from Mendelian randomization using the MR-Egger method. Eur J Epidemiol. 2017. doi: 10.1007/s10654-017-0255-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Larsson SC, Bäck M, Rees JMB, Mason AM, Burgess S. Body mass index and body composition in relation to 14 cardiovascular conditions in UK Biobank: A Mendelian randomization study. Eur Heart J. 2020. doi: 10.1093/eurheartj/ehz388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Spiller W, Davies NM, Palmer TM. Software application profile: Mrrobust—A tool for performing two-sample summary Mendelian randomization analyses. Int J Epidemiol. 2019. doi: 10.1093/ije/dyy195 [DOI] [Google Scholar]
- 43.Burgess S. Sample size and power calculations in Mendelian randomization with a single instrumental variable and a binary outcome. Int J Epidemiol. 2014. doi: 10.1093/ije/dyu005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Langdon RJ, Richmond RC, Hemani G, Zheng J, Wade KH, Carreras-Torres R, et al. A phenome-wide Mendelian randomization study of pancreatic cancer using summary genetic data. Cancer Epidemiol Biomark Prev. 2019. doi: 10.1158/1055-9965.EPI-19-0036 [DOI] [PubMed] [Google Scholar]
- 45.Cornish AJ, Law PJ, Timofeeva M, Palin K, Farrington SM, Palles C, et al. Modifiable pathways for colorectal cancer: a mendelian randomisation analysis. Lancet Gastroenterol Hepatol. 2020. doi: 10.1016/S2468-1253(19)30294-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kalliala I, Markozannes G, Gunter MJ, Paraskevaidis E, Gabra H, Mitra A, et al. Obesity and gynaecological and obstetric conditions: umbrella review of the literature. BMJ. 2017. doi: 10.1136/bmj.j4511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bezemer ID, Rinaldi S, Dossus L, Van Gils CH, Peeters PHM, Van Noord PAH, et al. C-peptide, IGF-I, sex-steroid hormones and adiposity: A cross-sectional study in healthy women within the European Prospective Investigation into Cancer and Nutrition (EPIC). Cancer Causes Control. 2005. doi: 10.1007/s10552-004-7472-9 [DOI] [PubMed] [Google Scholar]
- 48.Tran TT, Naigamwalla D, Oprescu AI, Lam L, McKeown-Eyssen G, Bruce WR, et al. Hyperinsulinemia, but not other factors associated with insulin resistance, acutely enhances colorectal epithelial proliferation in vivo. Endocrinology. 2006. doi: 10.1210/en.2005-1012 [DOI] [PubMed] [Google Scholar]
- 49.Shiiya T, Nakazato M, Mizuta M, Date Y, Mondal MS, Tanaka M, et al. Plasma ghrelin levels in lean and obese humans and the effect of glucose on ghrelin secretion. J Clin Endocrinol Metab. 2002. doi: 10.1210/jcem.87.1.8129 [DOI] [PubMed] [Google Scholar]
- 50.Baatar D, Patel K, Taub DD. The effects of ghrelin on inflammation and the immune system. Molecular and Cellular Endocrinology. 2011. doi: 10.1016/j.mce.2011.04.019 [DOI] [PubMed] [Google Scholar]
- 51.De Martel C, Haggerty TD, Corley DA, Vogelman JH, Orentreich N, Parsonnet J. Serum ghrelin levels and risk of subsequent adenocarcinoma of the esophagus. Am J Gastroenterol. 2007. doi: 10.1111/j.1572-0241.2007.01116.x [DOI] [PubMed] [Google Scholar]
- 52.Murphy G, Kamangar F, Dawsey SM, Stanczyk FZ, Weinstein SJ, Taylor PR, et al. The relationship between serum ghrelin and the risk of gastric and esophagogastric junctional adenocarcinomas. J Natl Cancer Inst. 2011. doi: 10.1093/jnci/djr194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yao K-F, Ma M, Ding G-Y, Li Z-M, Chen H-L, Han B, et al. Meta-analysis reveals gender difference in the association of liver cancer incidence and excess BMI. Oncotarget. 2017. doi: 10.18632/oncotarget.20127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Brocco D, Florio R, De Lellis L, Veschi S, Grassadonia A, Tinari N, et al. The role of dysfunctional adipose tissue in pancreatic cancer: A molecular perspective. Cancers. 2020. doi: 10.3390/cancers12071849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Benn M, Tybjærg-Hansen A, Smith GD, Nordestgaard BG. High body mass index and cancer risk—a Mendelian randomisation study. Eur J Epidemiol. 2016. doi: 10.1007/s10654-016-0147-5 [DOI] [PubMed] [Google Scholar]
- 56.Ooi BNS, Loh H, Ho PJ, Milne RL, Giles G, Gao C, et al. The genetic interplay between body mass index, breast size and breast cancer risk: A Mendelian randomization analysis. Int J Epidemiol. 2019. doi: 10.1093/ije/dyz124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shu X, Wu L, Khankari NK, Shu XO, Wang TJ, Michailidou K, et al. Associations of obesity and circulating insulin and glucose with breast cancer risk: A Mendelian randomization analysis. Int J Epidemiol. 2019. doi: 10.1093/ije/dyy201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Painter JN, O’Mara TA, Marquart L, Webb PM, Attia J, Medland SE, et al. Genetic risk score mendelian randomization shows that obesity measured as body mass index, but not waist:hip ratio, is causal for endometrial cancer. Cancer Epidemiol Biomark Prev. 2016. doi: 10.1158/1055-9965.EPI-16-0147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Eriksson J, Haring R, Grarup N, Vandenput L, Wallaschofski H, Lorentzen E, et al. Causal relationship between obesity and serum testosterone status in men: A bidirectional mendelian randomization analysis. PLoS ONE. 2017. doi: 10.1371/journal.pone.0176277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hambridge HL, Mumford SL, Mattison DR, Ye A, Pollack AZ, Bloom MS, et al. The influence of sporadic anovulation on hormone levels in ovulatory cycles. Hum Reprod. 2013. doi: 10.1093/humrep/det090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Key TJ, Appleby PN, Reeves GK, Roddam A, Dorgan JF, Longcope C, et al. Body mass index, serum sex hormones, and breast cancer risk in postmenopausal women. J Natl Cancer Inst. 2003. doi: 10.1093/jnci/djg022 [DOI] [PubMed] [Google Scholar]
- 62.Kim JJ, Chapman-Davis E. Role of progesterone in endometrial cancer. Seminars in Reproductive Medicine. 2010. doi: 10.1055/s-0029-1242998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhang XM, Dou QL, Zeng Y, Yang Y, Cheng ASK, Zhang WW. Sarcopenia as a predictor of mortality in women with breast cancer: A meta-analysis and systematic review. BMC Cancer. 2020. doi: 10.1186/s12885-020-6645-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Aleixo GFP, Williams GR, Nyrop KA, Muss HB, Shachar SS. Muscle composition and outcomes in patients with breast cancer: meta-analysis and systematic review. Breast Cancer Research and Treatment. 2019. doi: 10.1007/s10549-019-05352-3 [DOI] [PubMed] [Google Scholar]
- 65.Feliciano EMC, Kroenke CH, Meyerhardt JA, Prado CM, Bradshaw PT, Kwan ML, et al. Association of Systemic Inflammation and Sarcopenia With Survival in Nonmetastatic Colorectal Cancer: Results From the C SCANS Study. JAMA Oncol. 2017. doi: 10.1001/jamaoncol.2017.2319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bian AL, Hu HY, Rong YD, Wang J, Wang JX, Zhou XZ. A study on relationship between elderly sarcopenia and inflammatory factors IL-6 and TNF-α. Eur J Med Res. 2017. doi: 10.1186/s40001-017-0266-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dusingize JC, Olsen CM, An J, Pandeya N, Law MH, Thompson BS, et al. Body mass index and height and risk of cutaneous melanoma: Mendelian randomization analyses. Int J Epidemiol. 2020. doi: 10.1093/ije/dyaa009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.McQuade JL, Daniel CR, Hess KR, Mak C, Wang DY, Rai RR, et al. Association of body-mass index and outcomes in patients with metastatic melanoma treated with targeted therapy, immunotherapy, or chemotherapy: a retrospective, multicohort analysis. Lancet Oncol. 2018. doi: 10.1016/S1470-2045(18)30078-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Giampietri C, Tomaipitinca L, Scatozza F, Facchiano A. Expression of Genes Related to Lipid Handling and the Obesity Paradox in Melanoma: Database Analysis. JMIR Cancer. 2020. doi: 10.2196/16974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Khankari NK, Shu XO, Wen W, Kraft P, Lindström S, Peters U, et al. Association between Adult Height and Risk of Colorectal, Lung, and Prostate Cancer: Results from Meta-analyses of Prospective Studies and Mendelian Randomization Analyses. PLoS Med. 2016. doi: 10.1371/journal.pmed.1002118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Thrift AP, Gong J, Peters U, Chang-Claude J, Rudolph A, Slattery ML, et al. Mendelian randomization study of height and risk of colorectal cancer. Int J Epidemiol. 2015. doi: 10.1093/ije/dyv082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ong JS, An J, Law MH, Whiteman DC, Neale RE, Gharahkhani P, et al. Height and overall cancer risk and mortality: Evidence from a Mendelian randomisation study on 310,000 UK Biobank participants /692/308/174 /692/499 article. Br J Cancer. 2018. doi: 10.1038/s41416-018-0063-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Qian F, Wang S, Mitchell J, McGuffog L, Barrowdale D, Leslie G, et al. Height and body mass index as Modifiers of breast cancer risk in BRCA1/2 mutation carriers: A Mendelian randomization study. J Natl Cancer Inst. 2019. doi: 10.1093/jnci/djy132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhang B, Shu XO, Delahanty RJ, Zeng C, Michailidou K, Bolla MK, et al. Height and breast cancer risk: Evidence from prospective studies and mendelian randomization. J Natl Cancer Inst. 2015. doi: 10.1093/jnci/djv219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rosenfeld RG. Insulin-like Growth Factors and the Basis of Growth. N Engl J Med. 2003. doi: 10.1056/NEJMp038156 [DOI] [PubMed] [Google Scholar]
- 76.Sell C, Dumenil G, Deveaud C, Miura M, Coppola D, DeAngelis T, et al. Effect of a null mutation of the insulin-like growth factor I receptor gene on growth and transformation of mouse embryo fibroblasts. Mol Cell Biol. 1994. doi: 10.1128/mcb.14.6.3604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Simpson A, Petnga W, Macaulay VM, Weyer-Czernilofsky U, Bogenrieder T. Insulin-Like Growth Factor (IGF) Pathway Targeting in Cancer: Role of the IGF Axis and Opportunities for Future Combination Studies. Target Oncol. 2017. doi: 10.1007/s11523-017-0514-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Larsson SC, Carter P, Vithayathil M, Kar S, Mason AM, Burgess S. Insulin-like growth factor-1 and site-specific cancers: A Mendelian randomization study. Cancer Med. 2020. doi: 10.1002/cam4.3345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lauby-Secretan B, Scoccianti C, Loomis D, Grosse Y, Bianchini F, Straif K. Body fatness and cancer—Viewpoint of the IARC working group. N Engl J Med. 2016. doi: 10.1056/NEJMsr1606602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Cancer Research UK: Obesity, weight and cancer. [cited 9 Jan 2020]. Available: https://www.cancerresearchuk.org/about-cancer/causes-of-cancer/obesity-weight-and-cancer.
- 81.Hughes RA, Davies NM, Davey Smith G, Tilling K. Selection Bias When Estimating Average Treatment Effects Using One-sample Instrumental Variable Analysis. Epidemiology. 2019. doi: 10.1097/EDE.0000000000000972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Haworth S, Mitchell R, Corbin L, Wade KH, Dudding T, Budu-Aggrey A, et al. Apparent latent structure within the UK Biobank sample has implications for epidemiological analysis. Nat Commun. 2019. doi: 10.1038/s41467-018-08219-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Burgess S, Davies NM, Thompson SG. Instrumental variable analysis with a nonlinear exposure-outcome relationship. Epidemiology. 2014. doi: 10.1097/EDE.0000000000000161 [DOI] [PMC free article] [PubMed] [Google Scholar]