Abstract
Background
Korean traditional food (KTF), originated from ancestral agriculture and the nomadic traditions of the Korean peninsula and southern Manchuria, is based on healthy food that balances disease prevention and treatment. Fermented foods that include grains, herbs, fruits, and mushrooms are also an important practice in KTF, providing high levels of Lactobacilli, which confer relevant health benefits, including antiviral properties. Some of these probiotics may also protect against the Influenza virus through the modulation of innate immunity.
Scope and approach
The emerging of the COVID-19 pandemic, in addition to other diseases of viral origin, and the problems associated with other respiratory disorders, highlight how essential is a healthy eating pattern to strengthen our immune system.
Key Findings and Conclusions: The present review covers the information available on edible plants, herbs, mushrooms, and preparations used in KTF to outline their multiple medicinal effects (e.g., antidiabetic, chemopreventive, antioxidative, anti-inflammatory, antibacterial), emphasizing their role and effects on the immune system with an emphasis on modulating properties of the gut microbiota that further support strong respiratory immunity. Potential functional foods commonly used in Korean cuisine such as Kimchi (a mixture of fermented vegetables), Meju, Doenjang, Jeotgal, and Mekgeolli and fermented sauces, among others, are highlighted for their great potential to improve gut-lung immunity. The traditional Korean diet and dietary mechanisms that may target viruses ACE-2 receptors or affect any step of a virus infection pathway that can determine a patient's prognosis are also highlighted. The regular oral intake of bioactive ingredients used in Korean foods can offer protection for some viral diseases, through protective and immunomodulatory effects, as evidenced in pre-clinical and clinical studies.
Keywords: Antiviral, Pathogens, Coronavirus, COVID-19, SARS-CoV-2, Functional foods, Plant extracts, Respiratory infections, Viruses, Korean traditional diet
1. Introduction
The positive health effects of several beverages and foodstuffs have been recorded and studied during the last decades. These effects are due to the presence of specific naturally-occurring compounds, whose particular levels and proportions influence the observed health benefits (Visioli et al., 2011). In this context, several traditional Korean foodstuffs are currently recognized for their beneficial properties (Im Kim, Sim, & Choi, 2010). This fact is rationalized since, for Koreans, the low risk of diseases and feeding starts from the same root, based on the philosophy called “Yaksikdongwon,” which defends that good health is part of an appropriate diet, in order to keep the human body in balance (Oktay & Ekinci, 2019). According to this philosophy, medicinal therapy would only be required if a positive evolution of the disease is not observed after ‘treatment’ with food, since health starts with diet (Oh, Park, Daily III, & Lee, 2014). Therefore, in the same concept, medicine and food converge (Leem & Park, 2007). In other words, Korean traditional food (KTF) is based on a notion of healthy foods, and these healthy properties cover food materials and their preparation (Park & Kim, 2014; Park, Jeong, Lee, & Daily, 2014). Such features have specialized over centuries owing to geographical, social, and political factors (Oh, Park, Daily, & Lee, 2014).
Originating in ancient agriculture and nomadic traditions of the Korean peninsula and southern Manchuria, KTF has evolved through complex interactions between the environment and different cultural trends, developing a distinct ethnicity and a matchless culture (e.g., language and food) (Hyun Kim, Song, & Potter, 2006). In order to resist long winters within a land isolated by rugged mountains and rocky ocean fronts, KTF developed out of the obligation to preserve good food by fermentation (e.g., fish, seaweed, vegetables, and salted beans, among others) in clay pots (Dharaneedharan & Heo, 2016; Patra, Das, Paramithiotis, & Shin, 2016).
Apart from the fact that Koreans have learned a reasonable balance between nutrition and disease prevention/treatment, an increasing interest in South Korea opened up a market that includes dietary supplements and health products of natural origin (namely, functional health foods (FHF)) (Kim, Kim, & Lee, 2008; Yoon et al., 2012). Thus, a regulatory venue for such products in South Korea (one of the largest Asian markets for FHF) was introduced and regulated by the Korea Food and Drug Administration (KFDA) (Zawistowski, 2014).
Some herbs and fruits (such as ginseng, cinnamon, wormwood, ginger, pomegranate, and adlay) are actually used as food, but their therapeutic effects are also exploited. Therefore, KTF usually includes some herbs/fruits for their medicinal properties and, correspondingly, health benefits in KTF are attributed to various common ingredients (Kim et al., 2006; Park & Kim, 2014). Based on this fact, KTFs are a subject of growing interest worldwide (Baeg & So, 2013; Kim, 2013). Particular KTF ingredients (broadly consumed in Korea) are currently being placed on global FHF markets, owing to the increasing interest beyond the characteristic examples (e.g., ginseng preparations, mushrooms, fermented sauces and mixed rice). Such attention comes from increasing research supporting their several health benefits (Park & Kim, 2014), including antiviral properties (Kang et al., 2011; Lee, Lee, Lee, & Choi, 2013).
Antivirals are the only drugs traditionally used to treat infectious diseases of viral origin (Gonçalves et al., 2020). However, like other drugs, they also have associated risks or harmful health effects, such as phlebitis, hematuria, hypocalcemia, creatininemia, and, in the worst cases, mutagenesis, and teratogenesis. Additionally, they develop resistance due to the change and decreased affinity of viral enzymes, especially polymerases and reverse transcriptases (Goldhill et al., 2018; Gonçalves et al., 2020). Therefore, the antiviral capacity of phytochemicals present in extracts of certain medicinal and edible plants and mushrooms (in common/dietary use) is a good choice, since they have been shown to be active in vitro and in vivo against many different types of viruses, including those that cause respiratory diseases (Nugraha, Ridwansyah, Ghozali, Khairani, & Atik, 2020; Verma et al., 2020).
In this regard, acute respiratory infections present morbidity and mortality of approximately 4 million children annually. These infections are considered a major cause of early childhood mortality, including diarrhea and malnutrition (Rodríguez, Cervantes, & Ortiz, 2011). In fact, a shocking surprise in the scientific and global community was caused by the epidemiological situation initiated by the influenza A subtype H1N1. It is a swine-origin influenzavirus strain producing critical respiratory infection in some places, which was identified for the first time in April 2009 (Phaswana-Mafuya et al., 2020). However, the current situation (i.e., 2021) is even worse due to the pandemic generated by COVID-19, an emerging disease triggered by SARS-COV-2 (severe acute respiratory syndrome coronavirus 2) (Rajaiah, Abhilasha, Shekar, Vogel, & Vishwanath, 2020). This disease has caused 3.03 million deaths worldwide as of this writing (i.e., 18/04/2021).
Therefore, without a cure or well-proven vaccine until now against SARS-COV-2 and the problems associated with other respiratory infections, a healthy eating pattern is essential to strengthen our body's defenses and improve response to these respiratory disease-causing agents (BourBour et al., 2020; Lin et al., 2016). Faced with the restrictions that we are currently experiencing, it is crucial to take care of our diet to maintain our health and ensure the strengthening of our immune system, that is, our body's natural defense against respiratory infections (Jawhara, 2020; Junaid et al., 2020; Roy et al., 2020). Therefore, it is easy comprehensible that a well-balanced diet is key to body homeostasis and in to preventing respiratory infections. Various nutrients in the diet on a regular basis can help strengthening the respiratory system. Moreover, more recently, studies have also highlighted the role of probiotics in promoting a healthy gut microbiota and how that can affect general health, reducing inflammation and strengthening the immune system. Adding to this, many people have to live with chronic conditions such as asthma, chronic obstructive pulmonary diseases (COPD) and pulmonary fibrosis that affect their quality of life. Lifestyle modification can help to protect the respiratory system and even reduce lung damage and disease's symptoms. Specific nutrients and foods have been identified to be particularly beneficial for lung function. Clinical studies have recommended that zinc, curcumin, and zinc-ionophores have significant antiviral potentials. And thus, intake of these food supplements can be helpful in the treatment of COVID-19 in the form of considerable immunity support, inhibition of replication of RNA of the SARS-COV-2 virus and by prevention of the virus entry into the cell (Celik, Gencay, & Ocsoy, 2021; Ghati et al., 2021; Roy et al., 2020). Moreover, KTF offers good edible alternatives to help us in this purpose through prophylactic and direct actions (Park & Kim, 2014). Accordingly, the present narrative review covers the information available on edible plants, herbs, and preparations used in KTF to outline the potential of KFTs and their ingredients based on their antiviral and anti-respiratory effects.
2. Korean traditional foods in brief and their beneficial effects
Korean traditional foods (KTF) are known worldwide for being spicy, tasty, and delicious, but KTF are also able to promote wellbeing and a balanced health. This KTF characteristic is often linked with the obesity percentage of South Koreans (i.e., 3.5%), which differs substantially from other countries such as the United States, the United Kingdom, Brazil, Mexico or New Zealand (25–34%) (Gupta et al., 2012; Groneberg et al., 2015). This is a good starting point since such a low obesity rate can be attributed to the genetic factors of Asians compared to Caucasians since the latter are greater than the former, but this is also not entirely true. Nutritional habits of Koreans make them healthier than other people since the fat content of Korean food is generally 13% lower compared to American and European diets (Lee, Sobal, & Frongillo, 1999). This condition can be justified as Korean food culture has evolved from very ancient times and adheres to a significant classification of various philosophical actions. The recognition that consuming healthy food can prevent and cure diseases is deep-rooted Korean thinking (Oh et al., 2014).
In this sense, edible plants and mushrooms in KTF are exploited for their medicinal properties that support their health benefits. However, each KTF exhibits particular benefits on health due to certain active principles and the use of special preparation/cooking procedures and practices (Patra et al., 2016). In addition, some KTF-used ingredients are also employed in traditional medicine to treat different health events. For instance, stomach problems are treated with chives or raw potato juice, whereas garlic is used for digestive purposes and blood cleansing. Bellflower roots and hazelnut are good for coughing/cold and skin/pregnant women, respectively, while patients are strengthened with pine nuts or rice porridge (Oktay & Ekinci, 2019).
The facts mentioned above supported several of the informed benefits of KTF and their ingredients (e.g., red ginseng, aloe, zedoary, turmeric, red sage, astragalus, among others), which comprise several effects, such as lower risks of cardiovascular diseases (Jovanovski et al., 2014), as well as neurological diseases (Leem & Park, 2007), reduced probabilities of developing some cancer types due to anticancer properties (Yoon, Jeong, Kim, & Aggarwal, 2013), more efficient and stronger internal organs (mainly liver and kidneys) (Choi, 2008), healthier digestion due to enhanced inclination for more digestible foods (Kim, Song, & Potter, 2006a), stronger bones by consuming isoflavones occurred in mushrooms/beans (Youn et al., 2008), and healthier skin (Pazyar, Omidian, & Jamshydian, 2012).
A previous review categorized the impact of the ingredients of Korean edible herbs involving properties such as antidiabetic, chemopreventive, and antioxidative and effects on the immune system (Park & Kim, 2014). In the case of mushrooms (e.g., Agaricus blazei, A. bisporus, Flammulina v elutipes, Ganoderma lucidum, Hericium erinaceus, Inonotus obliquus, Lentinus edodes, Phellinus linteus, Pleurotus eryngii, P. ostreatus, Sparassis crispa, among several others), they were traditionally used in Korea for their health benefits (i.e., digestive effects, risk of cancer, lowering cholesterol, losing weight, improving the immune system, preventing diabetes and anemia) (Thu et al., 2020), but also as food due to the good content of digestible carbohydrates and proteins and lower fat (Fu, Shieh, & Ho, 2002; Kim, Song, Kwon, Kim, & Heo, 2008). Indeed, the antiviral effects of I. obliquus have been recognized, even against SARS-CoV-2 (Shahzad, Anderson, & Najafzadeh, 2020).
Apart from using medicinal ingredients in KTFs within the healthy philosophy, the preparation is also important for them. In such preparations, preservation by fermentation is also an important practice in KTF, despite the country's different traditions (Han, Lucas, & Kunz, 1993). As one of the cheapest methods to increase the shelf-life of several highly-perishable foods (e.g., vegetables, fruits, fish, and meat), fermentation is a microorganism-mediated transformation process of organic compounds (Lee, Jang, Lee, Park, & Choi, 2015). The importance of this process in KTF is the action of particular microorganisms (such as bacteria and yeasts), not only for transform organic compounds (particularly carbohydrates) but also for the role as probiotics, conferring important health benefits to feeders, and regular consumption is recommended (Park, Jeong, et al., 2014).
The most recognized Korean fermented probiotic food is Kimchi (a Korean mixture of Fermented Vegetables), which has more than 3000 years of history (Chang, 2018). It is widely consumed in South Korea and, together with Indian lentils, Spanish olive oil, Greek yogurt, Japanese soybeans, it is considered one of the five healthiest foods in the world (Dharaneedharan & Heo, 2016). In fact, a strain of Lactiplantibacillus plantarum isolated from Kimchi can modulate innate immunity and protect against IV (influenza virus) (Park et al., 2013) and produces cyclic dipeptides that inhibit IV proliferation (Kwak et al., 2013). However, other traditional Korean fermented foods can be highlighted, such as Meju, Doenjang, Jeotgal, and Mekgeolli, which exhibit several medicinal effects (e.g., anticancer, antiobesity, antioxidant, anti-inflammatory, antidiabetes, among others) and involve different beneficial microorganisms, such as Leuconostoc mesenteroides, L. plantarum, Aspergillus sp., Bacillus sp., Bacillus siamensis, Halomonas sp., Kocuria sp., Saccharomyces cerevisiae, among others (Dharaneedharan & Heo, 2016).
3. Korean traditional foods with effects against the respiratory diseases
Fermented foods have been widely studied, and their benefits for human health have been elucidated. In this sense, it has been shown that fermented foods can affect the modulation of the immune system; therefore, fermented foods may improve the immunitary response to certain diseases or infections, such as those caused by viruses. Fermented food by lactic acid bacteria has been described to produce metabolites with antiviral effects (Table 1 ). Some of the antiviral mechanisms of lactic acid bacteria are the stimulation of the immune system and the production of antiviral metabolites. This type of fermented foods has shown an antagonist effect on respiratory viruses, such as influenza virus by stimulating the immune response, such as increased levels of interferon (INF-α and INF-β) and interleukin (IL-6), as well as the high secretion of cytokines IL-2, IL-12, and IFN-γ (Özel & Öztürk, 2020).
Table 1.
Potential beneficial effect of Korean foods against respiratory diseases.
| Korean food | Respiratory and antiviral models | Inhibitory effect | Reference |
|---|---|---|---|
| Chongkukjang extract | Influenza A virus | Neuroraminidase inhibitory effect of 4565.9 to 28,242.4 by IC50 (μg/mL). | Wei et al. (2015) |
| Ethanol extract from Cheonggukjang | Allergic asthma in a murine model | 70% ethanol extract (100 mg/kg/day) decreased degranulation and histamine release from mast cells | Bae et al. (2014a) |
| L. plantaruma (YML009) strain isolated from Kimchi | Influenza H1N1 virus | Antiviral activity at concentrations of 2a - 2c 10x cell-free supernatant. | Rather et al. (2015) |
| L. plantaruma DK119 isolated from Kimchi | Influenza H1N1 virus | A 109 CFU/mouse diary dose prevented weight loss of mice and maintained 100% survival. | (Park et al., 2013) |
| Lactobacillus strains isolated from Korean fermented foods | Mice model | Mice were fed a lyophilized powder (dose of 2.5 × 1010 CFU day−1) and induced the T-lymphocytes proliferation and IFN-γ production | Won et al. (2011) |
| L. citreumb HJ-P4 isolated from kimchi | Allergic mice model | A dose of 2 × 108 CFU/mL enhanced the secretion of IFN-γ and decreased IL-4 and IL-5 | (Kang et al., 2009) |
| L. citreumb EFEL2061 isolated from kimchi | Allergic mice model | A dose of 1 × 1010 UFC was given daily and reduced the bystander B cell activation | (Kang, Moon, Lee, & Han, 2016) |
| S. succinuc 14BME20 isolated from doenjang | Allergic asthma in a murine model | A dose of 5 × 107 UFC reduced cytokines and induced the accumulation of anti-inflammatory cells | (Song et al., 2019) |
Lactiplantibacillus plantarum.
Leuconostoc citreum.
Staphylococcus succinus.
Kimchi is a well-known fermented traditional Korean food rich in vitamins, minerals and dietary fiber, containing lactic acid bacteria and other compounds. Kimchi's effects on health, extend to anti-obesity, anti-aging, anti-mutagenic, anti-cancer, antioxidant, and anti-diabetic roles (Kim, Ha, Choi, & Ju, 2016; Kwon, Park, Chang, & Ju, 2016; Park, Jeong, et al., 2014). Likewise, Kimchi contains lactic acid bacteria, which are related to the content of beneficial compounds to health, as well as probiotic effects, decreased lipid peroxidation, and enhanced immune system response, reported in vitro and in vivo (Jang, Yu, Lee, & Paik, 2020; Lee, Shim, Park, Heo , Kim, & Ham, 2016; Yang et al., 2019). Similarly, Rather et al., 2015 demonstrated that a strain of Lactiplantibacillus plantarum (YML009) isolated from Kimchi has antiviral activity against the influenza virus H1N1 is hypothesized to be more effective than Tamiflu. Also, Park et al. (2013) found that Lactiplantibacillus plantarum DK119 (DK119) isolated from Kimchi improves protection for mice against the H1N1influenza virus by intranasal and oral administration. Jang et al. have reported the immune-stimulating potential of the Lactobacillus plantarum Ln1 strains isolated from the Kimchi (Jang, Yu, Lee, & Paik, 2020).
On the other hand, Kwon et al. (2016) reported that K imchi intake might be associated with rhinitis prevention because Korean adults who consume Kimchi are less prone to asthma (Kim et al., 2014). These effects may be related to vegetables and lactic acid composition of Kimchi, since the consumption of this food provides a significant amount of vitamins, such as ascorbic acid, which is associated with the improvement of the immune system and prevents allergenic diseases such as rhinitis and asthma (Kim, Ha, Choi, & Ju, 2016; Kim et al., 2014). In this sense, Kang et al. (2009) demonstrated that bacteria presented in Kimchi could be responsible for the reduction of allergenic diseases (such as rhinitis and asthma) in a murine model, as they have found that Leuconostoc citreum HJ-P4 (KACC 91035) isolated from Kimchi improves the immune system by decreasing serum levels and enhancing the secretion of antigen-specific IFN-γ. Likewise, Kang et al. (2016) have found that the bacteriium Leuconostoc citreum EFEL2061, isolated from Kimchi, induced cytokines and decreased the serum IgE in an allergic mice model. They reported that this could be due to the enhance in innate immune cells and reduced the bystander B cell activation. Also, Won et al. (2011) reported that strains of Lactobacillus isolated from Korean fermented foods (Kimchi and Deodoek Muchim) have a positive effect on improving the immune system in the murine model; they found that this effect could be due to the increased secretion of IL-2 which could induce T-lymphocytes proliferation and IFN-γ production; therefore these bacteria can affect diseases like asthma (Ya et al., 2008).
On the other hand, fermented Korean food, such as “doenjang” has been studied for the prevention of asthma; in this sense, Song et al. (2019) demonstrated that a bacterium isolated from the fermented Korean food “doenjang” has a protective effect against allergic asthma in a murine model. They found that the administration of Staphylococcus succinus strain 14BME20 significantly reduced cytokines and induced the accumulation of anti-inflammatory cells, enhancing the immune response to allergens. Likewise, Bae, Shin, See, Chai, and Shon (2014a) reported that an ethanol extract from the traditional Korean fermented soybean food, namely “Cheonggukjang” decreased the symptoms of allergic asthma in a murine model; they found that this extract decreased Ca2+ input to mast cells and decreased degranulation and histamine release from mast cells. Wei et al. (2015) also reported the antiviral effect of extracts of chongkukjang against the influenza A virus. They found that ethyl acetate extract from chongkukjang showed inhibitory activity of neuraminidase in vitro. Also, chongkukjang extracts maintained higher body weight and decreased the mortality of influenza in infected mice. Besides, Kim and Ju (2019) found that the consumption of seaweed and fish could be related to the prevention of asthma in Korean adults because these foods have a great content of polyunsaturated fatty acids and vitamins, related to anti-inflammatory precursors and pro-inflammatory mediators, such as protectins, resolvins, prostaglandins, and leukotrienes.
Besides, the Korean Red Ginseng which is obtained by the processing of the ginseng using repeated steaming and drying has shown nemorous potential activity against the human pathogenic viruses such as human immunodeficiency virus, human herpes virus, influenza virus, respiratory syncytial virus, hepatitis virus, norovirus, enterovirus, rhinovirus, coxsackievirus and rotavirus (Im, Kim, & Min, 2016). It is stated that the ginseng could possibly exert a direct antiviral potential by a number of mechanism of action such as inhibiting the attachment of the virus, penetration and replication, and also through the enhancement of the host immunity (Im et al., 2016). In another study it has been reported that the major compound in ginseng called the ginsenosides are highly effective against the H1N1, H3N2, H5N1 and H9N2 influenza viruses by the stimulation of the antiviral cytokines (Chan et al., 2011; Dae-Goon; Yoo et al., 2012; Park, Jeong, et al., 2014; Ratan et al., 2020). A clinical study has also reported that the Korean red ginseng have reduced the depletion of CD4 T-cells and have weakened the serum soluble CD8 antigen level in patients infected with the HIV type-1 viruses (Cho & Kim, 2017; Ratan et al., 2020). Another herb, Geranii Herba (Geranium thunbergii Siebold et Zuccarini), which is has been included in the Korean traditional foods, have been reported to possesses antiviral effects against the influenza viruses through the mechanism of neuraminidase enzyme inhibition (Choi, Kim, Kim, & Chung, 2019). In another study, the authors have reported that Platycodin D, a natural bioactive compound found in Platycodon grandiflorum, are helpful in preventing both the lysosome- and TMPRSS2-driven COVID-19 infection by obstructing the membrane fusion mechanism and or by briefly disrupting the distribution of membrane cholesterol (Kim et al., 2021). Similarly another herbal formulation, Qingfei Paidu decoction included in both Korean and Chinese Pharmacopea, are helpful in the treatment of COVID-19, as it could inhibit and alleviate the excessive immune responses and eliminate the inflammation by regulating the immune-related pathways and cytokine action–related pathways (Wu et al., 2020; Zhao, Tian, Yang, Liu, & Zhang, 2020). This herbal formulation increases the immunity and reduces inflammation by targeting the lung and spleen in COVID-19 patients (Wu et al., 2020; Zhao et al., 2020).
3.1. Korean traditional foods and beneficial effects on the COVID-19
Korean meals are rich in grains and vegetables, and many publications have arisen describing their pharmacological properties. A number of traditional foods have been reported to possess beneficial antiviral effects against the COVID-19 virus and these foods and herbs could be taken as dietary supplements and thus could be helpful in preventing the infection and strengthening the immunity against these viruses (Panyod, Ho, & Sheen, 2020). Besides, a number of research has been reported on the numerous medicinal plants and their potential active components which are highly effective against the COVID-19 viruses by possibly blocking the life-cycle related proteins, such as the cellular receptor ACE2, papain-like or chymotrypsin-like proteinases or by other possible mode of actions (Ang, Lee, Kim, Choi, & Lee, 2021; Benarba & Pandiella, 2020). Cabbage and fermented vegetables, for instance, fall under the scope of anti-viral properties (and severe anti-COVID-19 symptoms) (Bousquet et al., 2020). In the case of COVID-19 (SARS-CoV-2 infections), there is a correlation with lower mortality rates due to the high consumption of fermented vegetables, common in East Asia, Central Europe, and the Balkans.
These foods contain many Lactobacilli, which are also effective activators of nuclear factor (erythroid-derived 2)-like 2 (Nrf2). Additionally, Lactobacilli, present in large amounts in the fermented foods with strong probiotic properties are effective against the foodborne pathogenic microfloras (Behera, Ray, & Zdolec, 2018). Previously, heat-killed Lactobacilli have already been reported to effectively protect against influenza A and prevent secondary infections by stimulating immunity (Jung et al., 2017). A number of recent publications already exist, establishing a relationship between diet, gut microbiota, and viral and respiratory infections (Alkhatib, 2020; Chaari, Bendriss, Zakaria, & McVeigh, 2020). This literature attempts to draw correlations and make practical recommendations for the use of antiviral functional foods and lifestyle approaches to tackle the ongoing COVID-19 pandemic. They based their reports on the latest knowledge on the immunology of COVID-19 (Vabret et al., 2020) and the reported effects of the gut microbiome on health and disease.
However, before the pandemic of COVID-19 eruption, there were already comprehensive reports on the value of foods for their nutritional, antioxidant activity, and phenolic composition, including beans, which are widely used by Koreans (Carbas et al., 2020). Moreover, many publications have also highlighted the role of microbiota on viral infections (Dominguez-Diaz, Garcia-Orozco, Riera-Leal, Padilla-Arellano, & Fafutis-Morris, 2019; Planès & Goujon, 2020; Roth, Grau, & Karst, 2019; Wilks & Golovkina, 2012). Indeed, much has been postulated about how the gut microbiome can influence the severity of COVID-19. The greatest risk is often associated with pre-existing conditions as hypertension, diabetes, and obesity (Richardson et al., 2020), as already recognized by official health organizations such as the Centers for Disease Control and Prevention, CDC, U.S.A. (https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/people-with-medical-conditions.html accessed Dec 29th, 2020). A relationship between all of these conditions and alterations in the gut microbiome composition was reported (Aoun, Darwish, & Hamod, 2020; Gurung et al., 2020; Jose & Raj, 2015). The robustness of these associations raise the possibility that the mechanisms underlying hypertension, diabetes, and obesity may be responsible for developing severe respiratory infections associated with COVID-19; the gut microbiome at the time of infection may be determinant for patients outcome.
COVID-19 disease is caused by the new beta-coronavirus, now designated SARS-CoV-2 (Severe Acute Respiratory Syndrome coronavirus 2). The global COVID-19 pandemic has started an unprecedented race to find therapeutic targets and treatments for this disease and related serious pulmonary infections; thus, everything about SARS-CoV-2 has been under tight scrutiny. SARS-CoV-2 shares 79% sequence identity with SARS-CoV. This virus provoked a previous outbreak in early 2000, and with MERS-CoV, the Middle East Respiratory Syndrome Coronavirus (~50%) that appeared in 2012 (Lake, 2020; Lu et al., 2020). In common, all of these pathogens use the angiotensin-converting enzyme II (ACE-2) receptor to enter into the cell and have distinctive spike proteins. ACE-2 receptors are also expressed in cardiovascular tissues and kidneys, and gastrointestinal (GI) tracts, besides the airways (Harmer, Gilbert, Borman, & Clark, 2002; Leung et al., 2020). Thus, the expression of receptors for this peptidase is decisive for COVID-19 patient outcomes and has been pinpointed as a high-potential target for an effective vaccine. Moreover, it has been reported that SARS-CoV2 RNA can be detected in the stool of some patients with COVID-19 long after the live virus can be cultured from patients’ secretions and feces. Together with the fact that many patients develop diarrhea as a symptom of COVID-19, this suggests a distinct gut-lung link that may encompass gut microbiota (Dhar & Mohanty, 2020; Zuo et al., 2020).
The gut-lung or axis connection was reported long before the COVID-19 outbreak (Anand & Mande, 2018). A recent review points to the role of gut-lung axis in modulating the immune response and its implication in respiratory pathologies (Enaud et al., 2020). The gut microbiota may likely predict the patient's prognosis for SARS-CoV-2 infection, particularly when considering reports of gut symptoms (such as diarrhea and nausea) in patients with worse outcomes (Shang et al., 2020). Therefore, a healthy gut microbiome may support a better immune response to viral infections, thus inducing an effective protective response to COVID-19. Moreover, the state of this immune system-microbiota partnership may justify people's susceptibility to infections and inflammations, which in turn can be associated with a regular diet (Belkaid & Hand, 2014). A healthy gut or intestinal microbiome is known to be associated with a diet rich in fibers and a variety of fruits, vegetables, whole grains, legumes, nuts and seeds, and herbs and spices, as well as a few fermented foods (such as Kefir, kimchi and sauerkraut) (Heinen, Ahnen, & Slavin, 2020; Singh et al., 2017). Thus, the diet can also assist patients impaired with COVID-19 to recover and improve clinical outcomes, in addition to being behind lower infection rates or better disease prognosis (associated with lower mortality and morbidity rates) in certain regions of the planet or regions with characteristic cuisines or diets.
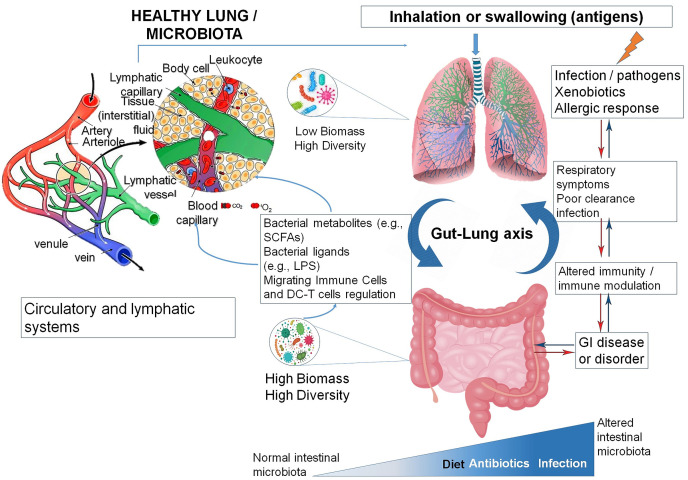
A scheme of the gut-lung connection is depicted in Fig. 1 . Succinctly, antigens or microbes in the intestine are ‘scrutinized’ by Dendritic cells (DC) directly from the lumen or after translocation through M cells to the GALT (Gut-associated lymphoid tissue). A combination of microbial cues leads to phenotypic changes in dendritic cells and migration to the draining lymph node. Dendritic cells then induce the activation of several T cells subsets within the MLN (mesenteric lymph nodes) and the production of several regulatory cytokines, such as IL-10, TGF-β, INFγ and IL-6. T cells then gain immune homing molecules (CCR9, CCR4). As a consequence of the immune challenge in the respiratory tract, cells activated in the GALT and MLN travel to the respiratory mucosa via CCR4/6 and induced protective and anti-inflammatory responses. Additionally, bacterial-derived products (e.g., LPS) can bind to TLR (Toll-like Receptors) in both intestinal epithelium and macrophages, which results in the production of several cytokines and chemokines. TLR activation can also encompass the expression of NF-kB in macrophages. The formation of several bacterial metabolites (as SCFAs) also affects the gut-lung connection. These products are conducted to the lung, where they modulate inflammation levels (Samuelson, Welsh, & Shellito, 2015).
Fig. 1.
- Potential connection between the gut-lung axis and nutrition related to the host-defense by the intestinal microbiota and lung immunity. Adapted from Samuelson et al. (Samuelson et al., 2015). The gastrointestinal tract (GI) or gut and lungs influence their homeostasis reciprocally. The unbalanced gut microbiota is correlated with lung disorders and infections. For example, antibiotic abuse can cause changes in the structure of the microbial intestinal community structure, which can result in altered immunity and changes in microbial growth conditions and, in turn, cause respiratory responses; or in the other way, a viral infection or inhalation of antigens/pathogens can alter immunity and microbial communities, resulting in changes in the gut. SCFAs, short-chain fatty acids; LPS, lipopolysaccharide; DC-T cells, Dendritic cell-T cell. Some of the intervenient immunity cells include: IL-6 (interleukin-6), IFN γ (interferon-gamma) and TNF-α (Tumour Necrosis Factor alpha), as well as migrating immune and DC-T cells, CCR 4/6 (Chemokine Receptor 4/6), CD4+ (Cluster of Differentiation antigen 4) and Th1 (T-Helper Cell type 1) that are carried in the circulatory vessels.
The diet-intestinal microbiota and the gut-lung connection have been extensively reviewed (e.g. (Anand & Mande, 2018; Nurmatov, Devereux, & Sheikh, 2011). It is now widely accepted that food intake affects the intestinal flora (or microbiota), resulting in bacterial metabolites that modulate immunity, namely by stimulating immune cells and the movement of sensitized immune cells or metabolites through the circulatory and lymphatic systems, can reach various distal organs, like the lungs or the brain. Therefore, unsurprisingly literature on nutrition, respiratory health, and gut flora increased substantially during the COVID-19 pandemic (Chaari et al., 2020; Dhar & Mohanty, 2020). Additional relationships have been established between dietary-related pre-conditions, such as obesity and severity of COVID-19 infection (Aoun et al., 2020). However, the opposite is why some regions or countries have not been as affected by the COVID-19 pandemic as Western countries like Spain or U.S.A. remain underexplored. Here, we will now focus on the traditional Korean diet and dietary mechanisms that may target viruses ACE-2 receptors or affect any step of a virus infection pathway, determining the patient's prognosis. Potential functional foods commonly used in Korean cuisine will be highlighted for their potential to improve gut-lung immunity (Table 2 ).
Table 2.
Some functional foods used in Korean traditional cuisine and their potential health benefits. Results from a literature survey reporting antiviral properties in the food are also indicated.
*Properties reported in pasteurized carrot juice; No-lack of information.
As mentioned above, traditional Korean food is rich in fermented fruits and vegetables as cabbage. This has already been reported to help mitigate the COVID-19 severity (Bousquet et al., 2020). It may also be associated with the richness of phenolic compounds and antioxidants in these foods, as well as with the high levels of Lactobacilli present and ingested upon consumption (Bousquet et al., 2020; Park et al., 2013). Therefore, the Korean diet has various fronts on which it can offer better outcomes for patients with COVID-19: high content of important bacteria for a healthy gut, high levels of antioxidants and phenolic compounds that can act as an anti-inflammatory and nutritionally rich in whole grains, fibers, and vitamins that further support a healthy balance for immunity and gut microbiota. In fact, antiviral properties have already been highlighted in these functional foods, particularly for their immune-promoting nutraceutical properties, through antioxidation and anti-inflammation features as summarized by Alkhatib (Alkhatib, 2020).
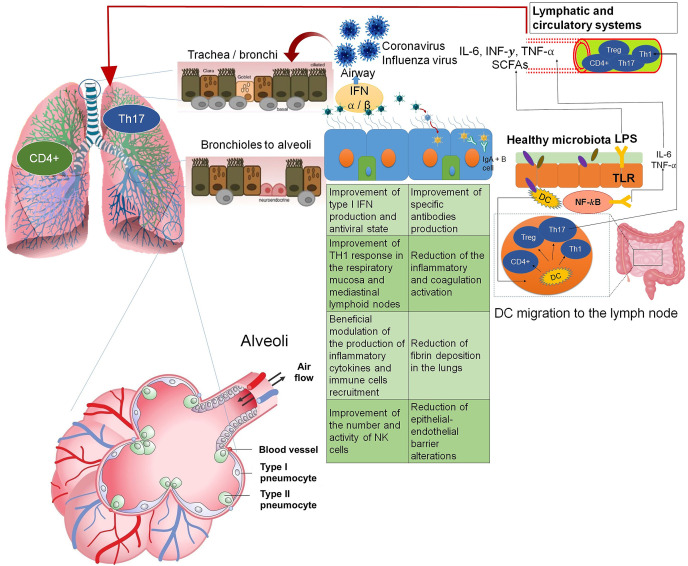
The lung epithelium in the adult is arranged in different types of cells. The tracheo-bronchial epithelium is a pseudo-stratified layer with ciliated cells and secretory epithelial cells (Clara), and goblet cells. Human basal cells (potential epithelial progenitor cells) appear in high numbers below this layer, decreasing as the lung proceeds into the alveolar space. Neuroendocrine cells may also occur and be innervated by ganglion cells, which are described to regulate cell proliferation and differentiation. Respiratory bronchioles’ structure is not very well described, and these lead to the alveoli, which are known to be linked mainly by type I and type II alveolar cells (Camelo, Dunmore, Sleeman, & Clarke, 2014). Therefore, lungs epithelia may differentiate into different cell types, including types I and II pneumocytes (Fig. 2 ). Type II pneumocytes make important proteins that reduce the surface tension of the alveoli, preventing them from collapsing. While type I pneumocytes are thin, flat cells that grant gas exchange between the alveolus and the surrounding blood capillaries.
Fig. 2.
– Focused effects of gut or gastrointestinal microbiome in lung immunity. Adapted from Camelo et al. (2014) and Zelaya et al. (2016a).
The SARS-CoV-2 virus can attach and infect alveolar pneumocytes. These primary human epithelial cells are their ACE-2 receptor, the gateway portal for SARS-CoV-2, and a different histological structure that may respond differently to virus infection. Therefore, a potential antiviral that can reduce the virus titter, an improvement in the survival rate, and a reduction in the rate of symptoms accumulated from the moment of contagion will be considered beneficial for future medical applications. This can occur via different pathways or mechanisms, as suggested in Fig. 2. An innate immune response against a virus in the respiratory tract is mediated by recognizing molecular patterns associated with viruses by specific receptors expressed in epithelial cells.
Intake of immunobiotics as yogurts or fermented/preserved foods can induce resistance and effective immune responses against viruses infection in the respiratory tract (Zelaya, Alvarez, Kitazawa, & Villena, 2016). Moreover, the respiratory tract also has significant remodeling capacity that can originate in an aberrant epithelial cell (genetic mutations) or exposure to external irritants and viruses or cigarette smoke, and this follows similar pathways to those that describe the benefits of immunobiotics (e.g., Lactobacillus probiotics) (Camelo et al., 2014; Zelaya et al., 2016).
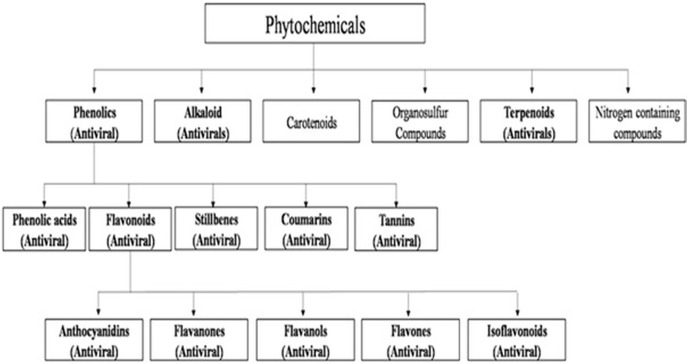
The effect of the gut microbiome on the immune response at distal sites or organs and its effects on the outcome of respiratory infections or disorders is now increasingly relevant and clear; gut microbiota assists in regulating the antiviral immunity (Abt et al., 2012; Ichinohe et al., 2011), and this has been especially exposed in infants nutrition (Berni Canani et al., 2017; Goehring et al., 2016) and adults with chronic illness as asthma (Williams et al., 2016). Potential plant-based drugs have been continuously screened for their antibacterial, antiviral, anti-cancerous, and antioxidant activities. More recently, antiviral properties have also been evaluated as plant-based antiviral natural compounds that may offer less toxicity and may be coupled with pre-existing therapies, along with improved delivery methods for greater effectiveness and bioavailability. Common plant compounds include flavonoids, phenolics, carotenoids, terpenoids, alkaloids, and many others, many of which have documented antiviral activities (Fig. 3 ).
Fig. 3.
- Classification of phytochemicals used as antivirals. Reproduced from Ghildiyal et al. (Ghildiyal, Prakash, Chaudhary, Gupta, & Gabrani, 2020), under the PMC Open Access Subset for unrestricted research re-use and secondary analysis in any form or by any means with acknowledgement of the original source (originally Fig. 1).
The medical and scientific communities are increasingly interested in ethnophytopharmaceuticals, as they offer complementary therapeutic options, with easy and inexpensive access along with the low potential for toxicity. The traditional Korean diet is rich in ingredients with a long history of ingredients and elements with strong antioxidant, anti-inflammatory, antiviral, antibacterial, fat metabolism, and modulating gut microbiota properties that further support strong respiratory immunity. However, research on nutraceuticals, plants, and food elements remains inconsistent and very confusing. Collaborations across several fields, from agriculture to chemistry and biomedical, will be important to fill the gap. Different comprehensive studies are needed to corroborate and validate associations between consuming a food and the specific health status or conditions.
Moreover, well-planned preclinical, in vitro and in vivo, and chemical investigations are still lacking. In controlled feeding trials, validation, as well as small populations and non-standard evaluation systems hold limitations. Thus, adequate, comprehensive study designs are required to validate the clinical functions of any particular food. Additionally, before the COVID-19 pandemic, universal systems were proposed to study coronavirus-host interaction in vitro in an attempt to respond to the need for identification of antivirals, evaluation of compound toxicity, and viral inhibition (e.g. (Jonsdottir & Dijkman, 2016)). However, these systems have not been explored enough when it comes to report the antiviral properties of plants and foods. To adds up to the current information, recently a clinical practice guideline has also been developed for the treatment of the SARS-CoV-2 using the Korean herbal medicine (Lee, Lee, Kim, Choi, & Jung, 2020). Further a number of panel discussion has also been published on the role of the Korean Medicine including the traditional herbal medicine that includes plant based components for the treatment in the post COVID-19 era (Kwon et al., 2020; Park et al., 2020).
4. Chemical constituents in the Korean tradition foods with antiviral and anti-respiratory medicinal effects
4.1. Chemical constituents in kimchi
Kimchi is the most widely distributed and popular Korean fermented food, which is prepared with napa cabbage or the Chinese cabbage or with radish as its main ingredients (Patra, Das, & Paramithiotis, 2017). Kimchi is prepared by mixing baechu, radish, powdered red pepper, salt, garlic, ginger, scallion, fermented fish, sugar and flavor enhancers. The addition of all these ingredients enhances the nutritional value of kimchi by incorporating carotenoids, vitamin C, chlorophyll, capsaicin, sulfur-containing compounds, phenolic compounds, dietary fiber, and fermentation metabolites such as lactic acid, glycoproteins, and bacteriocin (Patra et al., 2017). In addition, the literature research states that most of the chemical constituents present in fermented Korean foods are metabolites of fermentation (Table 3 ). For instance, when amino acids are evaluated, most of the measured free amino acids are produced due to the proteolytic action of microbial enzymes (Patra et al., 2016).
Table 3.
Chemical constituents of Kimchi.
| Vegetable source | Compounds | References |
|---|---|---|
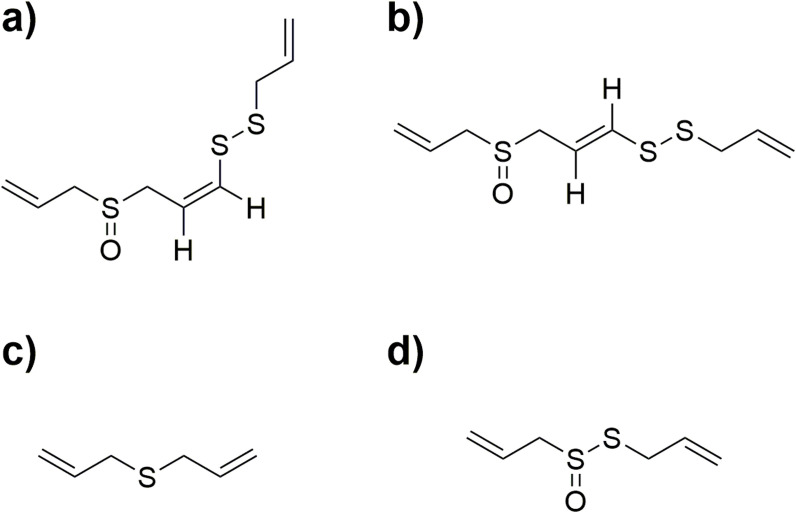
| Kimchi cabbages | Organic acids: acetic acid, citric acid, succinic acid, lactic acid, fumaric acid. Free sugars: fructose, glucose, sucrose, mannitol. Volatile compounds: allyl methyl disulfide, dimethyl trisulfide, diallyl tetrasulfide, 4-ethyl-5-methylthiazole, allyl methyl trisulfide, 3-vinyl-[4H]-1,2-dithin, and 2-phenylethyl isothiocyanate | (Choi, Yong, et al., 2019) |
| Kimchi fermented with L. mesenteroidesa | Lactate, ethanol, acetate, mannitol, diacetyl, acetoin, and 2,3-butanediol | (Chun, Lee, Jeon, Kim, & Jeon, 2017) |
| Kimchi fermented with red pepper | Glucose, fructose, lactate, acetate, mannitol | (Jeong, Lee, Jung, Lee, et al., 2013) |
| Metabolite composition of Kimchi during long-term storage | Decreased levels of free sugars fructose and glucose during storage. Increased levels of lactate, acetate, succinate, gamma-aminobutyric acid, and mannitol. | (Jeong, Lee, Jung, Choi, & Jeon, 2013) |
| Metabolic features of W. koreensisb during Kimchi fermentation | Fermentation metabolites: d-lactate, ethanol, acetate, D-sorbitol, thiamine, folate. Carbohydrates: glucose, mannose, lactose, malate, xylose, arabinose, ribose, N-acetyl-glucosamine, and gluconate | (Jeong et al., 2018) |
| Kimchi prepared with napa cabbage and different salts | Volatile metabolites: α-pinene, camphen, myrcene, 1-phellan, dimethyl trisulfide, diallyl disulfide, dipropyl disulfide, 1-butene-4-isothiocyanate, phenethyl isothiocyanate. Nonvolatile metabolites: alanine, valine, proline, serine, threonine, glutamate, phenylalanine, mannitol, tryptophan, stearidonic acid, pinolenic acid, capsaicin, dihydrocapsaicin. | (Kim et al., 2017) |
| Thirteen Korean Kimchi samples from Jeonju | Resveratrol (from the conversion of polydatin into resveratrol by the strain Lactobacillus kimchi JB301), isorhapontigenin, oxyresveratrol. | Ko et al. (2014) |
| Kimchi fermented with L. plantarumc | Increased lactic acid levels, glycerol, pyrotartaric acid, pentanedioic acid, 2-keto-1-gluconic acid, ribonic acid, isocitric acid, and palmitic acid. | (Park et al., 2016) |
| Mustard leaf kimchi extracts | Catechin, chlorogenic acid, epicatechin, epigallocatechin gallate, p-coumaric acid, gallocatechin gallate, ferulic acid, epicatechin gallate, rutin, catechin gallate, naringin. | (Park et al., 2017) |
| L. pentosusd isolated from Kimchi from Gyeonggi-do, Korea | Ginsenoside Rd, ginsenoside F2, compound K | Quan et al. (2010) |
| L. plantarumc HD1 isolated from Kimchi from home, restaurants, and temples located in South Korea | 5-oxododecanoic acid, 3-hydroxy decanoic acid, 3-hydroxy-5-dodecanoic acid | Ryu, Yang, Woo, & Chang (2014) |
| Kimchi prepared with different salt contents (0 and 5%) | Lactic acid, acetic acid, xylitol, and fumaric acid. | (Seo et al., 2018) |
Leuconostoc mesenteroides.
Weissella koreensis.
Lactiplantibacillus plantarum.
Lactobacillus pentosus.
4.2. Chemical constituents in chongkukjang, doenjang, and Gochujang
Chongkukjang is a traditional Korean food prepared in rice straw and boiled soybean fermented with Bacillus species rich in polyglutamic acid, isoflavones, phosphatide, and phenolic acids (Chang, 2018; Kim, Song, et al., 2008). Moreover, Kwon, Chung, and Jang (2019) stated that, unlike Doenjang and Gochujang, Chongkukjang fermentation takes only 2–4 days. Other reports mention that Chongkukjang has anti-inflammatory and antidiabetic properties, which are partially attributed to the chemical constituents of the soybean and its fermentative metabolites (Kwon et al., 2019; Wei et al., 2015). Also, Doenjang is a traditional Korean food made by mixing and fermenting soybeans for about six months using Bacillus subtillis, Rhizopus spp., and Aspergillus spp. (Lee, Kim, Park, Song, Nam, & Ahn, 2018).
Furthermore, Gochujang is a Korean traditional fermented food based on a paste of red pepper-soybean and is commonly used as a sauce in spicy Korean cooking. The main ingredients of Gochujang are red hot pepper powder, waxy rice flour, and fermented soybean (known as meju). Gochujang is usually fermented using Aspergillus sp. and Bacillus sp. strains (Cho et al., 2013). Due to the inclusion of red pepper and meju, some of the main constituents of Gochujang are capsaicin and isoflavones derivatives. The chemical constituents present in Chongkukjang, Doenjang, and Gochujang are shown in Table 4 .
Table 4.
Chemical constituents of Chongkukjang, Doenjang, and Gochujang.
| Vegetable source | Compounds | References |
|---|---|---|
| Chongkukjang | Poly-γ-glutamic acid | Ratha & Jhon, 2019 |
| Chongkukjang | Genistein, daidzein | (Kim, Song, et al., 2008) |
| Doenjang prepared with different content of garlic (2%, 6%, 10%), and thermal processes (heat-drying and freeze-drying) | Essential amino acids: isoleucine, leucine, lysine, methionine, phenylalanine, valine. Non-essential amino acids: arginine, proline, tyrosine, glycine, alanine, serine, glutamic acid, aspartic acid. Non-proteinogenic amino acids: ornithine, o-phosphoserine, taurine, sarcosine, l-citrulline, y-aminobutyric acid, ethanolamine, hydroxylysine. | Bahuguna et al. (2019) |
| Doenjang | Amino acids: glutamate, serine, valine, glycine, leucine, phenylalanine | (Chun, Kim, Jeong, & Jeon, 2020) |
| Soybean koji Doenjang | Acids: 2-methylpropanoic acid, acetic acid, 2-methylbutanoic acid, 3-methylbutanoic acid. Alcohols: 3-methylbutan-1-ol, pentan-1-ol. Carbonyls: benzaldehyde, butane-2,3-dione. Phenols: 2-methoxy phenol. | (Jeong et al., 2020) |
| Doenjang | Daidzein, glycitein, genistein | (Kim & Kim, 2014) |
| Doenjang | Genistein, daidzein, glycitein, genistein | (Kim et al., 2018) |
| Doenjang | Apigenin, soyasaponin A2, trihydroxyflavone, luteolin, daidzein, glycitein, genistein, soyasaponin, soyasaponin I, soyasaponin III, soyasaponin βg | (Kim, Kwak, & Kim, 2020) |
| Doenjang | Daidzein, glycitein, genistein, daidzin-β-glucoside, glycitin- β-glucoside, genistein- β-glucoside, daidzin malonylglucoside, glycitin malonylglucoside, genistein malonylglucoside, daidzin acetylglucoside, glycitin acetylglucoside, genistein acetylglucoside | Kwak, Son, Chung, and Kwon (2015) |
| Doenjang submitted to steaming, drying, meju fermentation, brining, and aging | Isoflavones: malonyldaidzin, malonyglucitin, malonygenistin, acetyldaidzin, acetylglycitin, acetylgenistin, daidzin, glycitin, genistein, daidzein, genistein. Soyasaponins: soyasaponin I–V, soyasaponin γg, soyasaponin γa, soyasaponin Bd, soyasaponin Be | (Lee, Lee, et al., 2014) |
| Doenjang and metabolites in rat plasma | Isoflavones: daidzein, genistein, glycitein, malonyl daidzin, malonyl genistein, malonyl glycitin, acetyl daidzin, acetyl genistein, acetyl glycitin. Isoflavone metabolites: 3-hydroxygenistein, hydroxydihydrogenistein, daidzein-4′-glucuronide, daidzein-7-glucuronide, daidzein-4′-sulfate, genistein-4′-glucuronide, genistein-7-glucuronide, genistein diglucuronide, genistein-4′-sulfate, genistein-7-sulfate-4′-glucuronide | (Lee et al., 2018) |
| Gochujang fermented with A. oryzae | Genistein, daidzin, apigenin 7-O-β-d-glucopyranoside, quercetin 3-O-α-l-rhamnopyranoside | (Cho et al., 2013) |
| Gochujang | Capsaicin, dihydrocapsaicin | Ha et al. (2010) |
| Three Gochujang types (white rice, brown rice, and wheat) | Isoflavones: daidzin, genistein, daidzein, glycitein, genistein. Soya saponins: soya saponin. | (Jang, Shin, et al., 2017) |
| Gochujang prepared with different cereals (wheat, brown rice, and white rice) and peppers (Capsicum annuum, C. annuum cv. Chung-yang, and C. frutescens) | Apigenin-C-hexoside-C-pentoside, dihydrocapsiate, linoleic ethanolamide, luteolin-C-hexoside, quercetin-O-rhamnoside, dihydrocapsaicin | (Lee, Suh, Jung, & Lee, 2016) |
| Gochujang products prepared with combinations of fungal rice koji with B. amyloliquefaciens CJ 3–27 and B. amyloliquefaciens CJ 14-6 | Genistein, acetylgenistin, daidzin, luteolin-diglucoside, genistein, apigenin-diglucoside, apigenin-glucoside, isovitexin-glucoside, daidzein, glycitein, luteolin, hydroxydaidzein, capsaicin, dihydrocapsaicin | Shin et al. (2016) |
4.3. Chemical bioactive ingredients used in Korean Foods
Some of the most common ingredients in fermented Korean cuisine are napa cabbage, garlic, and ginger. These ingredients are a rich source of bioactive compounds like phytochemicals, which are secondary metabolites produced as a defense mechanism in plants against biotic and abiotic stresses. Some of the most common phytochemicals found in food-stuff are alkaloids, terpenes, glucosinolates, and phenolic compounds (Croteau, Kutchan, & Lewis, 2015). Regular intake of these compounds in the diet has been related to a decreased incidence of non-communicable diseases, and some have also been used to treat infectious diseases. In this regard, some studies point to the antiviral potential of some Korean ingredients.
For instance, Dong, Farooqui, Leon, and Kelvin (2017) reported that ginsenosides commonly found in ginseng could interact with a viral hemagglutinin protein, preventing the attachment of the H1N1 virus to host cells. The chemical structure of ginsenoside was determined to be crucial as the sugar moieties in these molecules are pivotal for their bonds with viral hemagglutinin. Moreover, fresh ginger showed in vitro antiviral interest against the human respiratory syncytial virus, preventing viral attachment and internalization (Chang, Wang, Yeh, Shieh, & Chiang, 2013). Some studies also hypothesize that bioactive compounds from garlic have antiviral properties against influenza A and V, rhinovirus, viral pneumonia, and rotavirus (Bayan, Koulivand, & Gorji, 2014). Also, obesity, cardiovascular diseases, and diabetes have been described to increase the risk of some respiratory viral diseases. On this subject, Korean fermented foods have been reported with the potential to decrease the formation of macrophage foam cells by inhibiting lipid peroxidation induced by oxidized low-density lipoprotein (Yun, Kim, & Song, 2014). Furthermore, in vitro studies have shown that garlic extracts have antiviral properties against the influenza A and B virus (Mehrbod et al., 2009; Sharma, 2019). It is important to mention that the reported compounds commonly found in ingredients used in the preparation of traditional Korean fermented foods go through a lactic acid fermentative process, which metabolizes the compounds to produce different metabolites. In this sense, some reports state that Korean fermented foods have potential beneficial health effects, like antiviral potential against the influenza virus (Park et al., 2013), as stated in section 1.
4.3.1. Cabbage
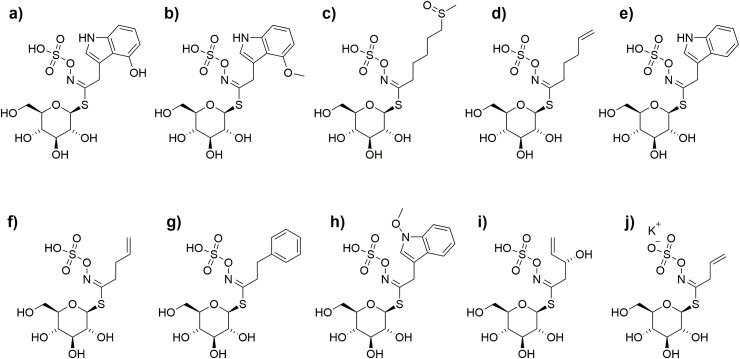
Chinese cabbage or napa cabbage (Brassica rapa L.) is used to prepare Kimchi, the most popular fermented Korean food. Chinese cabbage is a rich source of bioactive compounds, and these constituents may be related to the antioxidant and health-promoting properties of Kimchi. Some of the bioactive constituents most commonly identified in Chinese cabbage are glucosinolates, carotenoids, tocopherols, sterols, policosanols. Some of the most common glucosinolates found in Chinese cabbage are 4-hydroxyglucobrassicin, 4-methoxyglucobrassicin, glucoalyssin, glucobrassicanapin, glucobrassicin, gluconapin, gluconasturtiin, neoglucobrassicin, progotrin, and sinigrin (Fig. 4 ) (Baek, Jung, Lim, Park, & Kim, 2016). Some of the most commonly identified bioactive compounds in Chinese cabbage are shown in Table 5 .
Fig. 4.
Glucosinolates commonly found in Chinese cabbage. a) 4-hydroxyglucobrassicin, b) 4-methoxyglucobrassicin, c) glucoalyssin, d) glucobrassicanapin, e) glucobrassicin, f) gluconapin, g) gluconasturtiin, h) neoglucobrassicin, i) progotrin, j) sinigrin.
Table 5.
Metabolites with potential health-promoting effects in Chinese cabbage used in the preparation of traditional Korean foods.
| Sample | Identified compounds | Method of identification | References |
|---|---|---|---|
| Chinese cabbage | Glucosinolates: progoitrin, sinigrin, glucoalyssin, gluconapin, glucobrassicanapin, glucoeurucin, glucocochlearin, 4-hydroxyglucobrassicin, glucobrassicin, 4-methoxyglucobrassicin, neoglucobrassicin, gluconasturtiin. Carotenoids: violaxanthin, antheraxanthin, lutein, α-carotene, β-carotene, chlorophyll. | LC-qTOF-MS | Baek et al. (2016) |
| Hairy roots of Chinese cabbage induced by CuO nanoparticles | Glucosinolates: gluconasturtiin, glucobrassicin, 4-methoxyglucobrassicin, neoglucobrassicin, 4-hydroxyglucobrassicin, glucoallysin, glucobrassicanapin, sinigrin, progoitrin, and gluconapin. Hydroxycinnamic acids: p-hydroxybenzoic acid, protocatechuic acid, syringic acid, gentisic acid, vanillin. Hydroxycinnamic acids: p-coumaric acid, ferulic acid, chlorogenic acid, t-cinnamic acid. Flavonols: myricetin, quercetin, kaempferol, catechin, naringenin, rutin, hesperidin. | UHPLC and UHPLC-TQMS | Chung, Rekha, Rajakumar, and Thiruvengadam (2018) |
| Non-heading Chinese cabbage | Phenolic compounds: kaempferol-O-sophoroside-O-hexoside, kaempferol-dihexoside, kaempferol-sophoroside, kaempferol hexoside, myricetin-O-arabinoside, ferulic acid, quinic acid, protocatechuoyl hexose | UPLC-MS | Managa, Remize, Garcia, & Sivakumar (2019) |
| Chinese cabbage sprouts | Aliphatic glucosinolates: progoitrin, sinigrin, glucoalyssin, gluconapin, glucobrassicanapin. Indolic glucosinolates: 4-hydroxyglucobrassicin, glucobrassicin, 4-methoxyglucobrassicin, neoglucobrassicin | HPLC-DAD | Samec, Pavlovic, Redovnikovic, and Salopek-Sondi (2018) |
| Chinese cabbage (leaves and roots) | Glucosinolates: glucobrassicin, 4-methoxy glucobrassicin, neoglucobrassicin | HPLC | Zang et al. (2015) |
| Chinese cabbage | Glucosinolates: glucoallysin, sinigrin, progoitrin, gluconapin, glucobrassicanapin, gluconasturtin, glucobrassicin, 4-methoxyglucobrassicin, neoglucobrassicin, 4-hydroxyglucobrassicin | UPLC | Thiruvengadam, Kim, & Chung (2015) |
Abbreviations: HPLC - High Performance Liquid Chromatography; HPLC-DAD - high-performance liquid chromatography with a diode-array detector LC-qTOF-MS - liquid chromatography in combination with hybrid quadrupole time-of-flight mass spectrometry; UPLC - ultra performance liquid chromatography; UPLC-MS - Ultra performance liquid chromatography - tandem mass spectrometer; UHPLC-TQMS - ultra-high performance liquid chromatography coupled with a triple quadrupole mass spectrometry.
4.3.2. Garlic
Garlic contains many bioactive compounds, such as organosulfur and phenolic constituents. Some of them are phenolic compounds, like hydroxybenzoic acid derivatives, such as gallic and vanillic acid; and hydroxycinnamic acid derivatives, such as chlorogenic acid, caffeic acid, ferulic acid, p-hydroxybenzoic acid, m-coumaric acid, o-coumaric acid, p-coumaric acid (Beato, Orgaz, Mansilla, & Montano, 2011; Kim, Kang, & Gweon, 2013). Garlic is also the source of flavonoids like the flavanols catechin, epicatechin, and epigallocatechin gallate; flavonones like quercitrin and apigenin; flavonols like myricetin, resveratrol, morin, quercetin, and kaempferol (Fig. 5 ) (Kim et al., 2013). Furthermore, one the most important bioactive compounds identified in garlic is the non-volatile amino acids containing sulfur-like alliin, allicin, (E)-ajoene, diallyl sulfide, (Z)-ajoene, and 1,2-vinyldithiin (Lu et al., 2011; Martins, Petropoulos, & Ferreira, 2016).
Fig. 5.
Graphical representation of some bioactive compounds in garlic: a) (Z)-ajoene, b) ajoene, c) diallyl sulfide, d) allicin.
4.3.3. Ginger
Ginger is one of the main ingredients in many food preparations in Korea. Ginger is a rich source of phytochemicals with potential health-promoting effects. Some of the main bioactive constituents found in this root are phenolic compounds, such as gingerols, like 6-gingerol, which is the compound responsible for the pungency of fresh ginger; while shogaols are the main causes of pungency in dry gingers, and these compounds are derived from gingerols; some other constituents are zingerone, paradols like 6-deoxy gingerol and methyl paradols (Fig. 6 ) (Alsherbiny et al., 2019). Some other gingerols found in ginger are 8-gingerol, 10-gingerol, 6-shogoal (Brahmbhatt, Gundala, Asif, Shamsi, & Aneja, 2013). Also, different ginger plants of the Boesenbergia species have phenolic compounds like quercetin, kaempferol, rutin, naringin, hesperidin, caffeic acid, p-coumaric acid, sinapic acid, chlorogenic acid, gallic acid, luteolin, and diosmin (Jing, Mohamed, Rahmat, & Abu Bakar, 2010). Some terpenes found in ginger are β-bisabolene, α-curcumene, zingiberene, α-farnesene, and β-sesquiphellandrene.
Fig. 6.
Graphical representation of some bioactive compounds found in ginger: a) gingerol, b) zingerone, c) hesperidin, d) naringin.
5. Pre-clinical and clinical effectiveness in humans
Dietary measures are widely used as adjuvant treatments against influenza infections. Many fermented foods have antioxidant abilities, which promote the inhibition of essential enzymes and the disruption of cell membranes, thus avoiding viral binding and enhancing the capacity of the immune system. As stated in previous sections, fermented Korean foods are of great value by the multiplicity of microorganisms with a positive impact on the microbiota (protect the intestinal mucosa) and confer greater resistance to the immune system – immunobiotics and/or probiotics. Several microorganisms from fermented foods have been described to improve human immune response against viral infections (Arena et al., 2018). Antiviral effects of lactic acid bacteria (LAB) may be due to increased intestinal barrier function or stimulation of the immune system through the increase of macrophage activity and the production of antiviral inhibitory substances (H2O2, organic acids). The potential of heat-killed Lactobacillus probiotics against influenza was highlighted (Park et al., 2018).
Patra et al. (2016) reviewed many health benefits of Kimchi. Among different pharmacological properties of these foods, namely Kimchi, are their role in modulating the immune system. Activators for the potent transcription factor Nrf2 (nuclear factor erythroid-2-related factor 2) presented in some fermented Korean vegetables, as Kimchi, are suggested to improve health and metabolism. Fermented cabbage was recently proposed to increase the Nrf2-related antioxidant effects against COVID-19, modulating the severity of the symptoms and patient outcomes (Bousquet et al., 2020). The potential of dietary use of some fermented foods, probiotics, and prebiotics are being discussed as strategies to promote gut and upper respiratory tract immunity to better face a possible viral infection as by Sars-Cov-2, including in patients with COVID-related gastro-intestinal symptoms (Antunes, Vinderola, Xavier-Santos, & Sivieri, 2020).
The Lactiplantibacillus plantarum (previously designated Lactobaccilus plantarum) strain YU (LpYU), originally isolated from traditional Japanese fermented foods and one of the lactic acid bacteria (LAB) well known for its positive effects on immune function, was reported to activate Th1 immune responses on mice and prevent infection by the influenza A virus (A/NWS/33, H1N1) (Kawashima et al., 2011). In this work, mice were dosed with 0.011, 0.21, or 2.1 mg/day for 14 d, prior to intranasal inoculation of influenza A virus (IFV). Lungs and bronchoalveolar lavage fluids (BALFs) were studied to demonstrate that LpYU activated the innate and acquired immune systems.
The intake effects of two Korean traditional fermented soybean products, doenjang and cheonggukjang, or a mixture of both, have also been evaluated on the mice's immune response. However, no antiviral properties were explored in this study (Lee, Paek, Shin, Lee, Moon, & Park, 2017). The conclusion pointed to the increase in humoral and cellular immunity via Th1 responses in mice fed both products, suggesting an improved synergic effect. However, the antiviral properties of Lactiplantibacillus plantarum DK119, a microorganism isolated from fermented Korean cabbage, has been studied against the influenza virus, using a mouse model in a dose and route -dependent way ( Park et al., 2013). In this study, animals infected with influenza virus were intranasally or orally exposed to Lactiplantibacillus plantarum DK119, which effectively lowered lung viral loads. The bronchoalveolar lavage fluids from mice showed elevated concentrations of cytokines IL-12 and IFN-γ, and a low degree of inflammation, which indicates the bacteria's antiviral effects by modulating innate immunity.
Other pre-clinical studies demonstrated the antiviral effects of some microorganisms from fermented Korean food on the influenza virus. Table 6 displays some representative studies involving lactic acid bacteria (LAB) isolated from fermented foods and their immunobiological activity on animal models infected with the virus under different conditions. The preclinical studies presented in Table 6 demonstrate that the intake of Korean fermented foods, such as K imchi and Cheonggukjang or even Lactobacillus isolated from these foods, improves the immune system, not only of healthy animals but also in models infected with various types of virus or with asthma. The microorganisms present in this type of foods can offer protection for some viral diseases. They have been shown to have protective and immunomodulatory effects, namely upon regular oral intake of heat-killed forms isolated from K imchi.
Table 6.
Pre-clinical studies on the effects of microorganisms from Korean fermented foods on laboratory animal models. Abbreviations: BALFs - bronchoalveolar lavage fluids; CGJ - Cheonggukjang; EPS - exopolysaccharides; IAV - influenza A virus; IFV- influenza vírus; i.p. intraperitoneal; nF1 - heat-killed Lactiplantibacillus plantarum; OVA - ovalbumin; MLD50 - 50% mouse lethal dose; CFU - colony-forming unit; FFU – fan filter unit; IFN-α - interferon alpha; IgA – immunoglobulin A antibody; PFU – plaque-forming unit.
| Aims | Animal model | Exposure route | Evaluated Parameters | Dosing and period | Main Results | Reference |
|---|---|---|---|---|---|---|
| Evaluate L. brevisa KB290 impacts against influenza virus infection in mice | Mice | Oral | Lyophilized KB290 suspended in PBS for 14 days and then intranasally infected with 50% mouse lethal dose of IFV | L. brevisa alleviated clinical symptoms, by production of IFN-α and increase of IFV-specific IgA production | Waki et al. (2014) | |
| Evaluate if pretreatment of mice with L. plantarumb from the fermented Korean cabbage can increase protection against influenza virus infection | Mice | Intranasal or oral exposure | BALFs and lungs | Animals were treated once with (107 CFU/mouse) of L. plantarum DK119 strain (, 4 days prior infection with a lethal dose of influenza virus | L. plantarumb showed to be a beneficial probiotic against influenza virus infection byintranasal or oral exposure | (Park et al., 2013) |
| Evaluate the therapeutic effect of CGJ on a mouse model of ovalbumin (OVA)-induced asthma by the suppression of histamine release | Mice | I.p. | BALFs, lungs | After sensitized by i.p. of OVA and then turned with OVA inhalation, animals were administered i.p. ethanol-extracted CGJ (100 mg/kg/day) for 16 days. | Efficacy of CGJEs as a dietary therapy of histamine-mediated allergic diseases, probably by inhibition of mast cell activation. | Bae, Shin, See, Chai, and Shon (2014b) |
| Evaluate the health benefits of regular oral intake of nF1 against influenza virus infection | Mice | Oral | Daily oral intake (10 mg) of nF1 for 14 days followed by intranasally MLD50 of influenza A and B viruses, and the same feeding regimen for 14 days | Daily oral intake of nF1 delayed death of infected mice; increased survival rates | ( Park et al., 2018) | |
| Evaluate anti-rotavirus activity by the bacterial supernatant, lysate, and the EPS from L. plantarumb | Mice | Oral | Blood, heart, and small intestine | EPS (1 mg/mouse) for 2 days prior and 5 days after pups infection with the murine rotavirus epidemic diarrhea (10 μL of 2 × 10d FFU) | Decreased the duration of diarrhea, limited epithelial lesions, reduced rotavirus replication in the small intestine, and better animal recovery by EPS | ( Kim et al., 2018) |
| Evaluate the immune-stimulatory effects of L. bulgaricusc on the OSV-induced suppression of local and systemic humoral immunity in mice infected with IAV | Mice | Oral | BALFs, lungs | Daily single oral dose of 400 μL of L. bulgaricusc for 35 days. On d22, intranasal infection with 0.5 pfu IAV; followed by oral 50 μg of OSV in 100 μL of 5% methylcellulose (MC) as a vehicle or MC alone, twice daily | Regular intake of L. bulgaricusc can stimulate humoral immunity of anti-PR8-specific S-IgA and IgG in BALF and anti-PR8-specific IgG and IgA in serum against IAV infection | Takahashi, Sawabuchi, Kimoto, Sakai, & Kido, 2019 |
| Evaluate if S. succinusd from doenjang normalises immune response and benefits allergic diseases | Mice | Oral | BALFs, lung, mediastinal lymph nodes, mesenchymal lymph nodes and spleen | S. succinusd (5 × 107 CFU/mouse) every other day to day 20, then sensitized and replaced by ovalbumin as an allergen | Therapeutic potential for allergic asthma, due to suppression of airway inflammation by increase in Treg (regulatory T cells) responses | ( Kim, Song, Lim, Lee, & Lee, 2019) |
Levilactobacillus brevis.
Lactiplantibacillus plantarum.
Lactobacillus bulgaricus.
Staphylococcus succinus.
As the polyherbal formula Mahwangyounpae-tang (MT), other Korean dietary supplements have been used to treat respiratory diseases, including asthma ( Park, Choi, Kim, Lee, & Ku, 2010). This supplement, which contains 22 types of herbal extracts, was orally given to different groups of rats for 28 d at different doses (800, 400, and 200 mg/kg per day) and showed no significant toxic effects, confirming, therefore, the safety of these products. Previous studies investigated the pharmacological efficacy for the asthma treatment in mice only using 30 mg/kg of MT ( Kim, Kim, & Kam, 2003). Also, in humans, Korean fermented foods as kimchi were investigated for potential immune system-stimulating effects ( Lee, Kim, Lee, Jang, & Choue, 2014). In this study, healthy college students aged over 20 and having a body mass index of 18.5–23.0 kg/m2 took 100 g of Kimchi once a day for one month. However, no significant immunomodulatory effects were detected, possibly due to the short period of intake of Kimchi.
Clinical trials involving school-aged children were also conducted by Huang, Chie, & Wang (2018), focusing on the therapeutic impacts of Lacticaseibacillus paracasei (LP), Limosilactobacillus fermentum (LF), and the co-exposure (LP and LF) on asthma, biomarkers of immune function, and fecal microbiota or flora. These probiotics with well-known immunomodulatory effects were given to the children for three months. A co-exposure was reported most effective, resulting in risen peak expiratory flow rates and decreased IgE levels. As Korean fermented foods are rich in several of these pro- and pre-biotics, it offers a possible nutraceutical/supplement or functional food value that is yet to be characterized to treat respiratory disorders and infections, including in children, holding it at their fair safety. Clinical studies are still few to elucidate better the role of these foods in infections of the respiratory system.
6. Conclusion
Korean traditional foods are widely recognized for their delicious and spicy flavor, as well as health benefits. One of the most recognized Korean fermented foods is Kimchi (a mixture of fermented vegetables) among others such as Meju, Doenjang, Jeotgal, and Mekgeolli, which exhibit several medicinal effects (e.g., anticancer, antiobesity, antioxidant, anti-inflammatory, antidiabetes) and involve several beneficial microorganisms (e.g., Leuconostoc mesenteroides, Lactiplantibacillus plantarum, Aspergillus sp., Bacillus sp., Halomonas sp., Kocuria sp., Saccharomyces cerevisiae). It should be noted that other epidemiological situations caused by the new influenza A (H1N1) and more recently the pandemic COVID-19, induced by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has been devastating, causing more than 1.21 million deaths worldwide. In addition, edible plants and mushrooms are used in Korean traditional medicine to treat different health events. The consumption of traditional Korean foods, such as those fermented by lactic acid bacteria, is recognized to produce metabolites with antiviral effects against respiratory diseases (e.g., influenza virus H1N1) by stimulating the immune system. In fact, increased levels of interferons (INF-α and INF-β) and interleukins (IL-6), in addition to the high secretion of cytokines IL-2, IL-12 and IFN-γ are produced upon, for example, Lactobacilli intake. Adding to this, fermented foods such as kimchi and doenjang also provide a set of antioxidants and vitamins (e.g., ascorbic acid), strengthening the immune system and preventing or modulate allergic reactions such as rhinitis and asthma.
The role of gut microbiota on viral infections has been highlighted in the literature. The gut-lung or axis relationship already reported before the COVID-19 outbreak may predict the patient's prognosis for SARS-CoV-2 infection, particularly when considering gut symptoms (such as diarrhea and nausea) in patients with worse outcomes. Thus, the Korean diet, rich in fibers and a variety of vegetables, fruits, whole grains, nuts and seeds, herbs and spices, and fermented foods (such as Kefir, kimchi, and sauerkraut) may lead to a healthy gut microbiome, which may support a better immune response to viral infections, thus inducing an effective protective response to COVID-19.
In addition, plant-based compounds with antiviral properties (e.g., flavonoids, phenolics, carotenoids, terpenoids, alkaloids, and many others) may aid with an improved immune response besides the nutritional and medicinal values already well recognized. Moreover, this type of diet can also help COVID-19 patients to recover and improve clinical outcomes due to the high levels of antioxidants and phenolic compounds that further support a healthy balance for immunity and gut microbiota. In summary, the regular consumption of a healthy diet that includes, e.g., the Korean functional foods can support human respiratory health, namely via the antiviral properties of its ingredients, particularly due to their immune-promoting nutraceutical properties. It has been suggested by the clinical research that the food supplements such as curcumin and zinc have great potentials in terms of their antiviral activities and thus these types of food supplements can be useful in the treatment of COVID-19 to boost the immune system. Although these types of food supplements with their immense medicinal potentials proves helpful in the treatment of COVID-19, however the food supplement-drug interaction should be taken into consideration in terms of increasing toxicity and their drug efficiency. Although much is already known on the properties of functional foods, well-planned preclinical and clinical studies are required to elucidate their role in infections of the respiratory tract. In addition, chemical investigations on the nutraceutical/biomedical properties of both these ingredients and fully prepared foods may offer a template for improved therapeutics for respiratory infections.
Declaration of competing interest
The authors declare no conflict of interest with the manuscript.
Acknowledgments
The authors are grateful to their respective institutions for support. GD, HSS and JKP acknowledges Dongguk University, Republic of Korea for support. This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (No. 2020R1G1A1004667), the Republic of Korea. MLP acknowledges the project CICECO-Aveiro Institute of Materials, UIDB/50011/2020 and UIDP/50011/2020, national funds by FCT/MCTES.
References
- Aboubakr H.A., Nauertz A., Luong N.T., Agrawal S., El-Sohaimy S.A.A., Youssef M.M., et al. In vitro antiviral activity of clove and ginger aqueous extracts against feline calicivirus, a surrogate for human norovirus. Journal of Food Protection. 2016;79:1001–1012. doi: 10.4315/0362-028X.JFP-15-593. [DOI] [PubMed] [Google Scholar]
- Abt M.C., Osborne L.C., Monticelli L.A., Doering T.A., Alenghat T., Sonnenberg G.F., et al. Commensal bacteria calibrate the activation threshold of innate antiviral immunity. Immunity. 2012;37:158–170. doi: 10.1016/j.immuni.2012.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akhtar N.M.Y., Jantan I., Arshad L., Haque M.A. Standardized ethanol extract, essential oil and zerumbone of Zingiber zerumbet rhizome suppress phagocytic activity of human neutrophils. BMC Complementary and Alternative Medicine. 2019;19 doi: 10.1186/s12906-019-2748-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alkhatib A. Antiviral functional foods and exercise lifestyle prevention of coronavirus. Nutrients. 2020;12 doi: 10.3390/nu12092633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alshammari G.M., Balakrishnan A. Pumpkin (Cucurbita ficifolia Bouche) extract attenuate the adipogenesis in human mesenchymal stem cells by controlling adipogenic gene expression. Saudi Journal of Biological Sciences. 2019;26:744–751. doi: 10.1016/j.sjbs.2018.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alsherbiny M.A., Abd-Elsalam W.H., El Badawy S.A., Taher E., Fares M., Torres A., et al. Ameliorative and protective effects of ginger and its main constituents against natural, chemical and radiation-induced toxicities: A comprehensive review. Food and Chemical Toxicology. 2019;123:72–97. doi: 10.1016/j.fct.2018.10.048. [DOI] [PubMed] [Google Scholar]
- Anand S., Mande S.S. Diet, microbiota and gut-lung connection. Frontiers in Microbiology. 2018;9:2147. doi: 10.3389/fmicb.2018.02147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anantikulchai P., Emprom P., Pringproa K., Yamsakul P. In vitro cytotoxicity test and antiviral activity of curcuminoids from turmeric extract against PRRS virus. Veterinary Integrative Sciences. 2017;15:199–205. [Google Scholar]
- Anggakusuma, Colpitts C.C., Schang L.M., Rachmawati H., Frentzen A., Pfaender S., et al. Turmeric curcumin inhibits entry of all hepatitis C virus genotypes into human liver cells. Gut. 2014;63:1137–1149. doi: 10.1136/gutjnl-2012-304299. [DOI] [PubMed] [Google Scholar]
- Ang L., Lee H.W., Kim A., Choi J.-Y., Lee M.S. Network analysis of herbs recommended for the treatment of COVID-19. Infection and Drug Resistance. 2021;14:1833. doi: 10.2147/IDR.S305176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anh N.H., Kim S.J., Long N.P., Min J.E., Yoon Y.C., Lee E.G., et al. Ginger on human health: A comprehensive systematic review of 109 randomized controlled trials. Nutrients. 2020;12 doi: 10.3390/nu12010157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antunes A.E., Vinderola G., Xavier-Santos D., Sivieri K. Potential contribution of beneficial microbes to face the COVID-19 pandemic. Food Research International. 2020;136:109577. doi: 10.1016/j.foodres.2020.109577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoun A., Darwish F., Hamod N. The influence of the gut microbiome on obesity in adults and the role of probiotics, prebiotics, and synbiotics for weight loss. Prev Nutr Food Sci. 2020;25:113–123. doi: 10.3746/pnf.2020.25.2.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arena M.P., Capozzi V., Russo P., Drider D., Spano G., Fiocco D. Immunobiosis and probiosis: Antimicrobial activity of lactic acid bacteria with a focus on their antiviral and antifungal properties. Applied Microbiology and Biotechnology. 2018;102:9949–9958. doi: 10.1007/s00253-018-9403-9. [DOI] [PubMed] [Google Scholar]
- Asgary S., Afshani M.R., Sahebkar A., Keshvari M., Taheri M., Jahanian E., et al. Improvement of hypertension, endothelial function and systemic inflammation following short-term supplementation with red beet (Beta vulgaris L.) juice: A randomized crossover pilot study. Journal of Human Hypertension. 2016;30:627–632. doi: 10.1038/jhh.2016.34. [DOI] [PubMed] [Google Scholar]
- Baeg I.-H., So S.-H. The world ginseng market and the ginseng (Korea) Journal of ginseng research. 2013;37:1. doi: 10.5142/jgr.2013.37.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bae M.-J., Shin H.S., See H.-J., Chai O.H., Shon D.-H. Cheonggukjang ethanol extracts inhibit a murine allergic asthma via suppression of mast cell-dependent anaphylactic reactions. 2014;17:142–149. doi: 10.1089/jmf.2013.2997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bae M.-J., Shin H.S., See H.-J., Chai O.H., Shon D.-H. Cheonggukjang ethanol extracts inhibit a murine allergic asthma via suppression of mast cell-dependent anaphylactic reactions. Journal of Medicinal Food. 2014;17:142–149. doi: 10.1089/jmf.2013.2997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baek S.A., Jung Y.H., Lim S.H., Park S.U., Kim J.K. Metabolic profiling in Chinese cabbage (Brassica rapa L. Subsp pekinensis) cultivars reveals that glucosinolate content is correlated with carotenoid content. Journal of Agricultural and Food Chemistry. 2016;64:4426–4434. doi: 10.1021/acs.jafc.6b01323. [DOI] [PubMed] [Google Scholar]
- Bahuguna A., Shukla S., Lee J.S., Bajpai V.K., Kim S.Y., Huh Y.S., et al. Garlic augments the functional and nutritional behavior of Doenjang, a traditional Korean fermented soybean paste. Scientific Reports. 2019;9 doi: 10.1038/s41598-019-41691-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardaa S., Turki M., Ben Khedir S., Mzid M., Rebai T., Ayadi F., et al. The effect of prickly pear, pumpkin, and linseed oils on biological mediators of acute inflammation and oxidative stress markers. BioMed Research International. 2020;2020:5643465. doi: 10.1155/2020/5643465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayan L., Koulivand P.H., Gorji A. Garlic: A review of potential therapeutic effects. Avicenna Journal of Phytomedicine. 2014;4:1–14. [PMC free article] [PubMed] [Google Scholar]
- Beato V.M., Orgaz F., Mansilla F., Montano A. Changes in phenolic compounds in garlic (Allium sativum L.) owing to the cultivar and location of growth. Plant Foods for Human Nutrition. 2011;66:218–223. doi: 10.1007/s11130-011-0236-2. [DOI] [PubMed] [Google Scholar]
- Behera S.S., Ray R.C., Zdolec N. Lactobacillus plantarum with functional properties: An approach to increase safety and shelf-life of fermented foods. BioMed Research International. 2018;2018:9361614. doi: 10.1155/2018/9361614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belkaid Y., Hand T.W. Role of the microbiota in immunity and inflammation. Cell. 2014;157:121–141. doi: 10.1016/j.cell.2014.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benarba B., Pandiella A. Medicinal plants as sources of active molecules against COVID-19. Frontiers in Pharmacology. 2020;11:1189. doi: 10.3389/fphar.2020.01189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berni Canani R., De Filippis F., Nocerino R., Laiola M., Paparo L., Calignano A., et al. Specific signatures of the gut microbiota and increased levels of butyrate in children treated with fermented cow's milk containing heat-killed lactobacillus paracasei CBA L74. Applied and Environmental Microbiology. 2017;83 doi: 10.1128/AEM.01206-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisen S., P, Emerald M. Nutritional and therapeutic potential of garlic and onion (Allium sp.) Current Nutrition & Food Science. 2016;12:190–199. [Google Scholar]
- Bolling B.W., McKay D.L., Blumberg J.B. The phytochemical composition and antioxidant actions of tree nuts. Asia Pacific Journal of Clinical Nutrition. 2010;19:117–123. [PMC free article] [PubMed] [Google Scholar]
- Bondonno N.P., Bondonno C.P., Blekkenhorst L.C., Considine M.J., Maghzal G., Stocker R., et al. Flavonoid-rich apple improves endothelial function in individuals at risk for cardiovascular disease: A randomized controlled clinical trial. Molecular Nutrition & Food Research. 2018;62 doi: 10.1002/mnfr.201700674. [DOI] [PubMed] [Google Scholar]
- BourBour F., Mirzaei Dahka S., Gholamalizadeh M., Akbari M.E., Shadnoush M., Haghighi M., et al. Nutrients in prevention, treatment, and management of viral infections; special focus on Coronavirus. Archives of Physiology and Biochemistry. 2020:1–10. doi: 10.1080/13813455.2020.1791188. [DOI] [PubMed] [Google Scholar]
- Bousquet J., Anto J.M., Czarlewski W., Haahtela T., Fonseca S.C., Iaccarino G., et al. Cabbage and fermented vegetables: From death rate heterogeneity in countries to candidates for mitigation strategies of severe COVID-19. Allergy. 2021;76:735–750. doi: 10.1111/all.14549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brahmbhatt M., Gundala S.R., Asif G., Shamsi S.A., Aneja R. Ginger phytochemicals exhibit synergy to inhibit prostate cancer cell proliferation. Nutrition and Cancer-an International Journal. 2013;65:263–272. doi: 10.1080/01635581.2013.749925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buendia J.R., Li Y., Hu F.B., Cabral H.J., Bradlee M.L., Quatromoni P.A., et al. Long-term yogurt consumption and risk of incident hypertension in adults. Journal of Hypertension. 2018;36:1671–1679. doi: 10.1097/HJH.0000000000001737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camelo A., Dunmore R., Sleeman M.A., Clarke D.L. The epithelium in idiopathic pulmonary fibrosis: Breaking the barrier. Frontiers in Pharmacology. 2014;4:173. doi: 10.3389/fphar.2013.00173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbas B., Machado N., Oppolzer D., Ferreira L., Queiroz M., Brites C., et al. Vol. 9. 2020. (Nutrients, antinutrients, phenolic composition, and antioxidant activity of common bean cultivars and their potential for food applications). Antioxidants (Basel) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavarretta E., Peruzzi M., Del Vescovo R., Di Pilla F., Gobbi G., Serdoz A., et al. Dark chocolate intake positively modulates redox status and markers of muscular damage in elite football Athletes: A randomized controlled study. Oxid Med Cell Longev. 2018:4061901. doi: 10.1155/2018/4061901. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celik C., Gencay A., Ocsoy I. Can food and food supplements be deployed in the fight against the COVID 19 pandemic? Biochimica et Biophysica Acta (BBA) - General Subjects. 2021;1865:129801. doi: 10.1016/j.bbagen.2020.129801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaari A., Bendriss G., Zakaria D., McVeigh C. Importance of dietary changes during the coronavirus pandemic: How to upgrade your immune response. Front Public Health. 2020;8:476. doi: 10.3389/fpubh.2020.00476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang H.C. Healthy and safe Korean traditional fermented foods: Kimchi and chongkukjang. Journal of Ethnic Foods. 2018;5:161–166. [Google Scholar]
- Chan L.Y., Kwok H.H., Chan R.W.Y., Peiris M.J.S., Mak N.K., Wong R.N.S., et al. Dual functions of ginsenosides in protecting human endothelial cells against influenza H9N2-induced inflammation and apoptosis. Journal of Ethnopharmacology. 2011;137:1542–1546. doi: 10.1016/j.jep.2011.08.022. [DOI] [PubMed] [Google Scholar]
- Chang J.S., Wang K.C., Yeh C.F., Shieh D.E., Chiang L.C. Fresh ginger (Zingiber officinale) has anti-viral activity against human respiratory syncytial virus in human respiratory tract cell lines. Journal of Ethnopharmacology. 2013;145:146–151. doi: 10.1016/j.jep.2012.10.043. [DOI] [PubMed] [Google Scholar]
- Cheng H.-Y., Lin C.-C., Lin T.-C. Antiviral properties of prodelphinidin B-2 3′-O-gallate from green tea leaf. Antiviral Chemistry and Chemotherapy. 2002;13:223–229. doi: 10.1177/095632020201300403. [DOI] [PubMed] [Google Scholar]
- Choi H.-J., Song J.-H., Ahn Y.-J., Baek S.-H., Kwon D.-H. Antiviral activities of cell-free supernatants of yogurts metabolites against some RNA viruses. European Food Research and Technology. 2009;228:945–950. [Google Scholar]
- Choi H.-J., Song J.-H., Park K.-S., Baek S.-H., Lee E.-S., Kwon D.-H. Antiviral activity of yogurt against enterovirus 71 in vero cells. Food Science and Biotechnology. 2010;19:289–295. [Google Scholar]
- Choi J.-G., Kim Y.S., Kim J.H., Chung H.-S. Antiviral activity of ethanol extract of Geranii Herba and its components against influenza viruses via neuraminidase inhibition. Scientific Reports. 2019;9:1–12. doi: 10.1038/s41598-019-48430-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi K. Botanical characteristics, pharmacological effects and medicinal components of Korean Panax ginseng C A Meyer. Acta Pharmacologica Sinica. 2008:1109–1118. doi: 10.1111/j.1745-7254.2008.00869.x. [DOI] [PubMed] [Google Scholar]
- Choi Y.J., Yong S., Lee M.J., Park S.J., Yun Y.R., Park S.H., et al. Changes in volatile and non-volatile compounds of model kimchi through fermentation by lactic acid bacteria. Lwt-Food Science and Technology. 2019;105:118–126. [Google Scholar]
- Cho Y.-K., Kim J.-E. Effect of Korean Red Ginseng intake on the survival duration of human immunodeficiency virus type 1 patients. Journal of ginseng research. 2017;41:222–226. doi: 10.1016/j.jgr.2016.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho J.Y., Lee H.J., Shin H.C., Lee J.M., Park K.H., Moon J.H. Behavior of flavonoid glycosides contained in Korean red pepper paste (gochujang) during fermentation: Participation of a beta-glucosidase inhibitor. Food Science and Biotechnology. 2013;22:1245–1252. [Google Scholar]
- Chun B.H., Lee S.H., Jeon H.H., Kim D.-W., Jeon C.O. Complete genome sequence of Leuconostoc suionicum DSM 20241T provides insights into its functional and metabolic features. Standards in Genomic Sciences. 2017;12:38. doi: 10.1186/s40793-017-0256-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung I.M., Rekha K., Rajakumar G., Thiruvengadam M. Production of bioactive compounds and gene expression alterations in hairy root cultures of Chinese cabbage elicited by copper oxide nanoparticles. Plant Cell, Tissue and Organ Culture. 2018;134:95–106. [Google Scholar]
- Chun B.H., Kim K.H., Jeong S.E., Jeon C.O. The effect of salt concentrations on the fermentation of doenjang, a traditional Korean fermented soybean paste. Food Microbiology. 2020;86 doi: 10.1016/j.fm.2019.103329. [DOI] [PubMed] [Google Scholar]
- Cicero A.F.G., Caliceti C., Fogacci F., Giovannini M., Calabria D., Colletti A., et al. Effect of apple polyphenols on vascular oxidative stress and endothelium function: A translational study. Molecular Nutrition & Food Research. 2017;61 doi: 10.1002/mnfr.201700373. [DOI] [PubMed] [Google Scholar]
- Corzo-Martínez M., Corzo N., Villamiel M. Biological properties of onions and garlic. Trends in Food Science & Technology. 2007;18:609–625. [Google Scholar]
- Croteau R., Kutchan T.M., Lewis N.G. In: Biochemistry & molecular biology of plants. Buchanan B., Gruissem W., Jones R., editors. American Society of Plants; Rockville, Maryland: 2015. Natural products (secondary metabolites) pp. 1250–1318. [Google Scholar]
- Dhar D., Mohanty A. Gut microbiota and Covid-19- possible link and implications. Virus Research. 2020;285:198018. doi: 10.1016/j.virusres.2020.198018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dharaneedharan S., Heo M.-S. Korean Traditional fermented foods-a potential resource of beneficial microorganisms and their applications. Journal of Life Sciences. 2016;26:496–502. [Google Scholar]
- Dominguez-Diaz C., Garcia-Orozco A., Riera-Leal A., Padilla-Arellano J.R., Fafutis-Morris M. Microbiota and its role on viral evasion: Is it with us or against us? Front Cell Infect Microbiol. 2019;9:256. doi: 10.3389/fcimb.2019.00256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong W., Farooqui A., Leon A.J., Kelvin D.J. Inhibition of influenza A virus infection by ginsenosides. PloS One. 2017;12 doi: 10.1371/journal.pone.0171936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elkholy Y.M., Hamza A.S., Masoud M.S., Badr S.E.A., El Safty M.M. Chemical composition for rind, flesh and seeds of pumpkin (Cucurbita pepo L.) and their antiviral activities. Journal of Agricultural Chemistry and Biotechnology. 2009;34:39–56. [Google Scholar]
- Enaud R., Prevel R., Ciarlo E., Beaufils F., Wieërs G., Guery B., et al. The gut-lung Axis in health and respiratory diseases: A place for inter-organ and inter-kingdom crosstalks. Frontiers in Cellular and Infection Microbiology. 2020;10 doi: 10.3389/fcimb.2020.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferretti A., Judd J.T., Taylor P.R., Nair P.P., Flanagan V.P. Ingestion of marine oil reduces excretion of 11-dehydrothromboxane B2, an index of intravascular production of thromboxane A2. Prostaglandins Leukotrienes and Essential Fatty Acids. 1993;48:305–308. doi: 10.1016/0952-3278(93)90220-q. [DOI] [PubMed] [Google Scholar]
- Fu HY, Shieh DE, Ho CT. Antioxidant and free radical scavenging activities of edible mushrooms. Journal of Food Lipids. 2002;9:35–43. doi: 10.1111/j.1745-4522.2002.tb00206.x. [DOI] [Google Scholar]
- Ganesan K., Xu B. Polyphenol-rich dry common beans (Phaseolus vulgaris L.) and their health benefits. International Journal of Molecular Sciences. 2017;18 doi: 10.3390/ijms18112331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganesan K., Xu B. Polyphenol-rich lentils and their health promoting effects. International Journal of Molecular Sciences. 2017;18 doi: 10.3390/ijms18112390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gennaro G., Claudino M., Cestari T.M., Ceolin D., Germino P., Garlet G.P., et al. Green tea modulates cytokine expression in the periodontium and attenuates alveolar bone resorption in type 1 diabetic rats. PloS One. 2015;10 doi: 10.1371/journal.pone.0134784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghati A., Dam P., Tasdemir D., Kati A., Sellami H., Sezgin G.C., et al. Exogenous pulmonary surfactant: A review focused on adjunctive therapy for severe acute respiratory syndrome coronavirus 2 including SP-A and SP-D as added clinical marker. Current Opinion in Colloid & Interface Science. 2021;51:101413. doi: 10.1016/j.cocis.2020.101413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghildiyal R., Prakash V., Chaudhary V.K., Gupta V., Gabrani R. Plant-derived bioactives. 2020. Phytochemicals as antiviral agents: Recent updates. [Google Scholar]
- Goehring K.C., Marriage B.J., Oliver J.S., Wilder J.A., Barrett E.G., Buck R.H. Similar to those who are breastfed, infants fed a formula containing 2'-fucosyllactose have lower inflammatory cytokines in a randomized controlled trial. Journal of Nutrition. 2016;146:2559–2566. doi: 10.3945/jn.116.236919. [DOI] [PubMed] [Google Scholar]
- Goldhill D.H., Te Velthuis A.J., Fletcher R.A., Langat P., Zambon M., Lackenby A., et al. The mechanism of resistance to favipiravir in influenza. Proceedings of the National Academy of Sciences. 2018;115:11613–11618. doi: 10.1073/pnas.1811345115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes A.P.O., Ferreira M.A., Camargo J.M., Araújo M.O., Mortoza A.S., Mota J.F., et al. Organic beet leaves and stalk juice attenuates hdl-C reduction induced by high-fat meal in dyslipidemic patients: A pilot randomized controlled trial. Nutrition. 2019;65:68–73. doi: 10.1016/j.nut.2019.03.004. [DOI] [PubMed] [Google Scholar]
- González-Peña D., Checa A., de Ancos B., Wheelock C.E., Sánchez-Moreno C. New insights into the effects of onion consumption on lipid mediators using a diet-induced model of hypercholesterolemia. Redox Biology. 2017;11:205–212. doi: 10.1016/j.redox.2016.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Pena D., Dudzik D., Garcia A., Ancos B., Barbas C., Sanchez-Moreno C. Metabolomic fingerprinting in the comprehensive study of liver changes associated with onion supplementation in hypercholesterolemic wistar rats. International Journal of Molecular Sciences. 2017;18 doi: 10.3390/ijms18020267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez S., Fernandez-Navarro T., Arboleya S., de Los Reyes-Gavilan C.G., Salazar N., Gueimonde M. Fermented dairy foods: Impact on intestinal microbiota and health-linked biomarkers. Frontiers in Microbiology. 2019;10:1046. doi: 10.3389/fmicb.2019.01046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonçalves B.C., Lopes Barbosa M.G., Silva Olak A.P., Belebecha Terezo N., Nishi L., Watanabe M.A., et al. Fundamental & Clinical Pharmacology; 2020. Antiviral therapies: Advances and perspectives. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graf D., Monk J.M., Lepp D., Wu W., McGillis L., Roberton K., et al. Cooked red lentils dose-dependently modulate the colonic microenvironment in healthy C57Bl/6 male mice. Nutrients. 2019;11 doi: 10.3390/nu11081853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurung M., Li Z., You H., Rodrigues R., Jump D.B., Morgun A., et al. Role of gut microbiota in type 2 diabetes pathophysiology. EBioMedicine. 2020;51:102590. doi: 10.1016/j.ebiom.2019.11.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Y., Yu S., Lambert J.D. Dietary cocoa ameliorates obesity-related inflammation in high fat-fed mice. European Journal of Nutrition. 2014;53:149–158. doi: 10.1007/s00394-013-0510-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han M., Zhao G., Wang Y., Wang D., Sun F., Ning J., et al. Safety and anti-hyperglycemic efficacy of various tea types in mice. Scientific Reports. 2016;6:31703. doi: 10.1038/srep31703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han YS, Lucas J, Kunz B. Traditional Korean fermented foods. Chemie Mikrobiologie Technologie der Lebensmittel. 1993;15:150–160. [Google Scholar]
- Harmer D., Gilbert M., Borman R., Clark K.L. Quantitative mRNA expression profiling of ACE 2, a novel homologue of angiotensin converting enzyme. FEBS Letters. 2002;532:107–110. doi: 10.1016/s0014-5793(02)03640-2. [DOI] [PubMed] [Google Scholar]
- Ha J., Seo H.Y., Shim Y.S., Nam H.J., Seog H., Ito M., et al. Rapid method for the determination of capsaicin and dihydrocapsaicin in gochujang using ultra-high-performance liquid chromatography. Journal of AOAC International. 2010;93:1905–1911. [PubMed] [Google Scholar]
- Heinen E., Ahnen R.T., Slavin J. Fermented foods and the gut microbiome. Nutrition Today. 2020;55:163–167. [Google Scholar]
- Hermsdorff H.H., Zulet M., Abete I., Martínez J.A. A legume-based hypocaloric diet reduces proinflammatory status and improves metabolic features in overweight/obese subjects. European Journal of Nutrition. 2011;50:61–69. doi: 10.1007/s00394-010-0115-x. [DOI] [PubMed] [Google Scholar]
- Hobbs T., Caso R., McMahon D., Nymark M. A novel, multi-ingredient supplement to manage elevated blood lipids in patients with no evidence of cardiovascular disease: A pilot study. Alternative Therapies in Health & Medicine. 2014;20:18–23. [PubMed] [Google Scholar]
- Hossain M.A., Lee S.J., Park N.H., Birhanu B.T., Mechesso A.F., Park J.Y., et al. Enhancement of lipid metabolism and hepatic stability in fat-induced obese mice by fermented cucurbita moschata extract. Evid Based Complement Alternat Med. 2018:3908453. doi: 10.1155/2018/3908453. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu R., Zeng F., Wu L., Wan X., Chen Y., Zhang J., et al. Fermented carrot juice attenuates type 2 diabetes by mediating gut microbiota in rats. Food Funct. 2019;10:2935–2946. doi: 10.1039/c9fo00475k. [DOI] [PubMed] [Google Scholar]
- Huang C.-F., Chie W.-C., Wang I.-J. Efficacy of Lactobacillus administration in school-age children with asthma: A randomized, placebo-controlled trial. Nutrients. 2018;10:1678. doi: 10.3390/nu10111678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichinohe T., Pang I.K., Kumamoto Y., Peaper D.R., Ho J.H., Murray T.S., et al. Microbiota regulates immune defense against respiratory tract influenza A virus infection. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:5354–5359. doi: 10.1073/pnas.1019378108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Im Kim S., Sim K.H., Choi H.-Y. A comparative study of antioxidant activity in some Korean medicinal plant used as food materials. Molecular & Cellular Toxicology. 2010;6:279–285. [Google Scholar]
- Im K., Kim J., Min H. Ginseng, the natural effectual antiviral: Protective effects of Korean Red Ginseng against viral infection. Journal of ginseng research. 2016;40:309–314. doi: 10.1016/j.jgr.2015.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang H.J., Yu H.S., Lee N.K., Paik H.D. Immune-stimulating effect of Lactobacillus plantarum Ln1 isolated from the traditional Korean fermented food, Kimchi. Journal of Microbiology and Biotechnology. 2020;30:926–929. doi: 10.4014/jmb.2001.01038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang S., Lakshman S., Beshah E., Xie Y., Molokin A., Vinyard B.T., et al. Flavanol-rich cocoa powder interacts with Lactobacillus rhamnossus LGG to alter the antibody response to infection with the parasitic nematode Ascaris suum. Nutrients. 2017;9 doi: 10.3390/nu9101113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang Y.K., Shin G.R., Jung E.S., Lee S., Lee S., Singh D., et al. Process specific differential metabolomes for industrial gochujang types (pepper paste) manufactured using white rice, brown rice, and wheat. Food Chemistry. 2017;234:416–424. doi: 10.1016/j.foodchem.2017.04.154. [DOI] [PubMed] [Google Scholar]
- Jawhara S. How to boost the immune defence prior to respiratory virus infections with the special focus on coronavirus infections. Gut Pathogens. 2020;12:1–6. doi: 10.1186/s13099-020-00385-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong S.E., Chun B.H., Kim K.H., Park D., Roh S.W., Lee S.H., et al. Genomic and metatranscriptomic analyses of Weissella koreensis reveal its metabolic and fermentative features during kimchi fermentation. Food Microbiology. 2018;76:1–10. doi: 10.1016/j.fm.2018.04.003. [DOI] [PubMed] [Google Scholar]
- Jeong D.W., Jeong K., Lee H., Kim C.T., Heo S., Oh Y., et al. Effects of Enterococcus faecium and Staphylococcus succinus starters on the production of volatile compounds during doenjang fermentation. Lebensmittel-Wissenschaft & Technologie. 2020;122 [Google Scholar]
- Jeong S.H., Lee S.H., Jung J.Y., Choi E.J., Jeon C.O. Microbial succession and metabolite changes during long-term storage of kimchi. Journal of Food Science. 2013;78:M763–M769. doi: 10.1111/1750-3841.12095. [DOI] [PubMed] [Google Scholar]
- Jeong S.H., Lee H.J., Jung J.Y., Lee S.H., Seo H.Y., Park W.S., et al. Effects of red pepper powder on microbial communities and metabolites during kimchi fermentation. International Journal of Food Microbiology. 2013;160:252–259. doi: 10.1016/j.ijfoodmicro.2012.10.015. [DOI] [PubMed] [Google Scholar]
- Jing J.L., Mohamed M., Rahmat A., Abu Bakar M.F. Phytochemicals, antioxidant properties and anticancer investigations of the different parts of several ginger species (Boesenbergia rotunda, Boesenbergia pulchella var attenuata and Boesenbergia armeniaca) Journal of Medicinal Plants Research. 2010;4:27–32. [Google Scholar]
- Jo S.H., Cho C.Y., Lee J.Y., Ha K.S., Kwon Y.I., Apostolidis E. In vitro and in vivo reduction of post-prandial blood glucose levels by ethyl alcohol and water Zingiber mioga extracts through the inhibition of carbohydrate hydrolyzing enzymes. BMC Complementary and Alternative Medicine. 2016;16 doi: 10.1186/s12906-016-1090-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonsdottir H.R., Dijkman R. Coronaviruses and the human airway: A universal system for virus-host interaction studies. Virology Journal. 2016;13:24. doi: 10.1186/s12985-016-0479-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jose P.A., Raj D. Gut microbiota in hypertension. Current Opinion in Nephrology and Hypertension. 2015;24:403–409. doi: 10.1097/MNH.0000000000000149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junaid K., Ejaz H., Abdalla A.E., Abosalif K.O., Ullah M.I., Yasmeen H., et al. Effective immune functions of micronutrients against Sars-Cov-2. Nutrients. 2020;12:2992. doi: 10.3390/nu12102992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung Y.J., Lee Y.T., Ngo V.L., Cho Y.H., Ko E.J., Hong S.M., et al. Heat-killed Lactobacillus casei confers broad protection against influenza A virus primary infection and develops heterosubtypic immunity against future secondary infection. Scientific Reports. 2017;7:17360. doi: 10.1038/s41598-017-17487-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaczmarek J.L., Liu X., Charron C.S., Novotny J.A., Jeffery E.H., Seifried H.E., et al. Broccoli consumption affects the human gastrointestinal microbiota. The Journal of Nutritional Biochemistry. 2019;63:27–34. doi: 10.1016/j.jnutbio.2018.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang K.-D., Cho Y.S., Song J.H., Park Y.S., Lee J.Y., Hwang K.Y., et al. Identification of the genes involved in 1-deoxynojirimycin synthesis in Bacillus subtilis MORI 3K-85. Journal of Microbiology. 2011;49:431–440. doi: 10.1007/s12275-011-1238-3. [DOI] [PubMed] [Google Scholar]
- Kang H., Moon J.S., Lee M.-G., Han N.S. Immunomodulatory effects of Leuconostoc citreum EFEL2061 isolated from kimchi, a traditional Korean food, on the Th2 type-dominant immune response in vitro and in vivo. Journal of Functional Foods. 2016;20:79–87. [Google Scholar]
- Kang H., Oh Y.J., Ahn K.S., Eom H.J., Han N., Kim Y.B., et al. Leuconostoc citreum HJ-P4 (KACC 91035) regulates immunoglobulin E in an ovalbumin-induced allergy model and induces interleukin-12 through nuclear factor-kappa B and p38/c-Jun N-terminal kinases signaling in macrophages. Microbiology and Immunology. 2009;53:331–339. doi: 10.1111/j.1348-0421.2009.00131.x. [DOI] [PubMed] [Google Scholar]
- Kawashima T., Hayashi K., Kosaka A., Kawashima M., Igarashi T., Tsutsui H., et al. Lactobacillus plantarum strain YU from fermented foods activates Th1 and protective immune responses. International Immunopharmacology. 2011;11:2017–2024. doi: 10.1016/j.intimp.2011.08.013. [DOI] [PubMed] [Google Scholar]
- Kellingray L., Tapp H.S., Saha S., Doleman J.F., Narbad A., Mithen R.F. Consumption of a diet rich in Brassica vegetables is associated with a reduced abundance of sulphate-reducing bacteria: A randomised crossover study. Molecular Nutrition & Food Research. 2017;61 doi: 10.1002/mnfr.201600992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim E.Y. World Institute of Kimchi as a leading global institute of fermented foods. Biotechnology Journal. 2013;8:759–760. doi: 10.1002/biot.201300184. [DOI] [PubMed] [Google Scholar]
- Kim T.S., Song J., Lim H.X., Lee A., Lee J.-H. Staphylococcus succinus 14BME20 prevents allergic airway inflammation by induction of regulatory T cells via interleukin-10. Frontiers in Immunology. 2019;10:1269. doi: 10.3389/fimmu.2019.01269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim T.Y., Jeon S., Jang Y., Gotina L., Won J., Ju Y.H., et al. Platycodin D, a natural component of Platycodon grandiflorum, prevents both lysosome-and TMPRSS2-driven SARS-CoV-2 infection by hindering membrane fusion. Experimental & Molecular Medicine. 2021;53:956–972. doi: 10.1038/s12276-021-00624-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim E.-K., Ha A.-W., Choi E.-O., Ju S.-Y. Analysis of kimchi, vegetable and fruit consumption trends among Korean adults: Data from the Korea national health and nutrition examination survey (1998-2012) Nutrition Research Practice. 2016;10:188–197. doi: 10.4162/nrp.2016.10.2.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim E.K., Ju S.Y. Asthma and dietary intake of fish, seaweeds, and fatty acids in Korean adults. Nutrients. 2019;11:12. doi: 10.3390/nu11092187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J.S., Kang O.J., Gweon O.C. Comparison of phenolic acids and flavonoids in black garlic at different thermal processing steps. Journal of Functional Foods. 2013;5:80–86. [Google Scholar]
- Kim H, Song MJ, Potter D. Medicinal efficacy of plants utilized as temple food in traditional Korean Buddhism. Journal of Ethnopharmacology. 2006;104:32–46. doi: 10.1016/j.jep.2005.08.041. [DOI] [PubMed] [Google Scholar]
- Kim H.E., Kim Y.S. Biological activities of fermented soybean paste (Doenjang) prepared using germinated soybeans and germinated black soybeans during fermentation. Food Science and Biotechnology. 2014;23:1533–1540. [Google Scholar]
- Kim J.Y., Kim J.D., Kam C.W. Effects of mahwangyoonpye-tang on asthma induced by ovalbumin in mouse. Journal of Physiology & Pathology in Korean Medicine. 2003;17:1453–1462. [Google Scholar]
- Kim J.Y., Kim D.B., Lee H.J. Nutraceutical and functional food regulations in the United States and around the world. Elsevier; 2008. Regulations on health/functional foods in Korea. [Google Scholar]
- Kim D.W., Kim B.M., Lee H.J., Jang G.J., Song S.H., Lee J.I., et al. Effects of different salt treatments on the fermentation metabolites and bacterial profiles of kimchi. Journal of Food Science. 2017;82:1124–1131. doi: 10.1111/1750-3841.13713. [DOI] [PubMed] [Google Scholar]
- Kim M.J., Kwak H.S., Kim S.S. Effects of salinity on bacterial communities, Maillard reactions, isoflavone composition, antioxidation and antiproliferation in Korean fermented soybean paste (doenjang) Food Chemistry. 2018;245:402–409. doi: 10.1016/j.foodchem.2017.10.116. [DOI] [PubMed] [Google Scholar]
- Kim S.S., Kwak H.S., Kim M.J. The effect of various salinity levels on metabolomic profiles, antioxidant capacities and sensory attributes of doenjang, a fermented soybean paste. Food Chemistry. 2020;328 doi: 10.1016/j.foodchem.2020.127176. [DOI] [PubMed] [Google Scholar]
- Kim S., Lee M.S., Jung S., Son H.Y., Park S., Kang B., et al. Ginger extract ameliorates obesity and inflammation via regulating MicroRNA-21/132 expression and AMPK activation in white Adipose tissue. Nutrients. 2018;10 doi: 10.3390/nu10111567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K., Lee G., Thanh H.D., Kim J.-H., Konkit M., Yoon S., et al. Exopolysaccharide from Lactobacillus plantarum LRCC5310 offers protection against rotavirus-induced diarrhea and regulates inflammatory response. Journal of Dairy Science. 2018;101:5702–5712. doi: 10.3168/jds.2017-14151. [DOI] [PubMed] [Google Scholar]
- Kim H., Oh S.Y., Kang M.H., Kim K.N., Kim Y., Chang N. Association between kimchi intake and asthma in Korean adults: The fourth and fifth Korea national health and nutrition examination survey (2007-2011) Journal of Medicinal Food. 2014;17:172–178. doi: 10.1089/jmf.2013.3013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim N.Y., Song E.J., Kwon D.Y., Kim H.P., Heo M.Y. Antioxidant and antigenotoxic activities of Korean fermented soybean. Food and Chemical Toxicology. 2008;46:1184–1189. doi: 10.1016/j.fct.2007.12.003. [DOI] [PubMed] [Google Scholar]
- Kim H., Song M.-J., Potter D. Medicinal efficacy of plants utilized as temple food in traditional Korean Buddhism. Journal of Ethnopharmacology. 2006;104:32–46. doi: 10.1016/j.jep.2005.08.041. [DOI] [PubMed] [Google Scholar]
- Ko J.A., Park J.Y., Kwon H.J., Ryu Y.B., Jeong H.J., Park S.J., et al. Purification and functional characterization of the first stilbene glucoside-specific beta-glucosidase isolated from Lactobacillus kimchi. Enzyme and Microbial Technology. 2014;67:59–66. doi: 10.1016/j.enzmictec.2014.09.001. [DOI] [PubMed] [Google Scholar]
- Kuang A., Erlund I., Herder C., Westerhuis J.A., Tuomilehto J., Cornelis M.C. Lipidomic response to coffee consumption. Nutrients. 2018;10 doi: 10.3390/nu10121851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwak C.S., Son D., Chung Y.S., Kwon Y.H. Antioxidant activity and anti-inflammatory activity of ethanol extract and fractions of Doenjang in LPS-stimulated RAW 264.7 macrophages. Nutrition Research and Practice. 2015;9:569–578. doi: 10.4162/nrp.2015.9.6.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwak MK, Liu R, Kwon JO, Kim MK, Kim AH, Kang SO. Cyclic dipeptides from lactic acid bacteria inhibit proliferation of the influenza a virus. Journal of Microbiology. 2013;51 doi: 10.1007/s12275-013-3521-y. 863–843. [DOI] [PubMed] [Google Scholar]
- Kwon D.Y., Chung K.R., Jang D.-J. The history and science of Chongkukjang, a Korean fermented soybean product. Journal of Ethnic Foods. 2019;6:5. [Google Scholar]
- Kwon S., Chung H., Kang Y., Jang I., Choi J.-Y., Jung I.C., et al. The role of Korean medicine in the post-COVID-19 era: An online panel discussion part 1–clinical research. Integrative medicine research. 2020;9:100478. doi: 10.1016/j.imr.2020.100478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon Y.S., Park Y.K., Chang H.J., Ju S.Y. Relationship between plant food (fruits, vegetables, and kimchi) consumption and the prevalence of rhinitis among Korean adults: Based on the 2011 and 2012 Korea national health and nutrition examination survey data. Journal of Medicinal Food. 2016;19:1130–1140. doi: 10.1089/jmf.2016.3760. [DOI] [PubMed] [Google Scholar]
- Labban L. Medicinal and pharmacological properties of turmeric (Curcuma longa): A review. International Journal of Pharma Bio Sciences. 2014;5:17–23. [Google Scholar]
- Lake M.A. What we know so far: COVID-19 current clinical knowledge and research. Clinical Medicine. 2020;20:124–127. doi: 10.7861/clinmed.2019-coron. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasker S., Rahman M.M., Parvez F., Zamila M., Miah P., Nahar K., et al. High-fat diet-induced metabolic syndrome and oxidative stress in obese rats are ameliorated by yogurt supplementation. Scientific Reports. 2019;9:20026. doi: 10.1038/s41598-019-56538-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y., Cha Y.S., Park Y., Lee M. PPARγ2 C1431T polymorphism interacts with the Antiobesogenic effects of kochujang, a Korean fermented, soybean-based red pepper paste, in overweight/obese subjects: A 12-week, double-blind randomized clinical trial. Journal of Medicinal Food. 2017;20:610–617. doi: 10.1089/jmf.2016.3911. [DOI] [PubMed] [Google Scholar]
- Lee H.Y., Kim S.W., Lee G.H., Choi M.K., Chung H.W., Lee Y.C., et al. Curcumin and Curcuma longa L. extract ameliorate lipid accumulation through the regulation of the endoplasmic reticulum redox and ER stress. Scientific Reports. 2017;7:6513. doi: 10.1038/s41598-017-06872-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H.Y., Kim S.W., Lee G.H., Choi M.K., Jung H.W., Kim Y.J., et al. Turmeric extract and its active compound, curcumin, protect against chronic CCl4-induced liver damage by enhancing antioxidation. BMC Complementary and Alternative Medicine. 2016;16:316. doi: 10.1186/s12906-016-1307-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H., Kim D.Y., Lee M., Jang J.-Y., Choue R. Immunomodulatory effects of kimchi in Chinese healthy college students: A randomized controlled trial. Clinical Nutrition Research. 2014;3:98–105. doi: 10.7762/cnr.2014.3.2.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee B.-J., Lee J.A., Kim K.-I., Choi J.-Y., Jung H.-J. A consensus guideline of herbal medicine for coronavirus disease 2019. Integrative Medicine Research. 2020;9:100470. doi: 10.1016/j.imr.2020.100470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee D.H., Kim M.J., Park S.H., Song E.J., Nam Y.D., Ahn J., et al. Bioavailability of isoflavone metabolites after Korean fermented soybean paste (doenjang) ingestion in estrogen-deficient rats. Journal of Food Science. 2018;83:2212–2221. doi: 10.1111/1750-3841.14214. [DOI] [PubMed] [Google Scholar]
- Lee ME, Jang JY, Lee JH, Park HW, Choi HJ. Starter Cultures for Kimchi Fermentation. Journal of Microbiology and Biotechnology. 2015;25:559–568. doi: 10.4014/jmb.1501.01019. [DOI] [PubMed] [Google Scholar]
- Lee M.H., Lee B.H., Lee S., Choi C. Reduction of hepatitis A virus on FRhK‐4 cells treated with Korean red ginseng extract and ginsenosides. Journal of Food Science. 2013;78:M1412–M1415. doi: 10.1111/1750-3841.12205. [DOI] [PubMed] [Google Scholar]
- Lee SK, Sobal J, Frongillo EA. Acculturation and Dietary Practices Among Korean Americans. Journal of the American Dietetic Association. 1999;99:1084–1085. doi: 10.1016/S0002-8223(99)00258-8. [DOI] [PubMed] [Google Scholar]
- Lee S.Y., Lee S., Lee S., Oh J.Y., Jeon E.J., Ryu H.S., et al. Primary and secondary metabolite profiling of doenjang, a fermented soybean paste during industrial processing. Food Chemistry. 2014;165:157–166. doi: 10.1016/j.foodchem.2014.05.089. [DOI] [PubMed] [Google Scholar]
- Lee J.-B., Miyake S., Umetsu R., Hayashi K., Chijimatsu T., Hayashi T. Anti-influenza A virus effects of fructan from Welsh onion (Allium fistulosum L.) Food Chemistry. 2012;134:2164–2168. doi: 10.1016/j.foodchem.2012.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leem K.-H., Park H.-K. Traditional Korean medicine: Now and the future. Neurological Research. 2007;29:3–4. doi: 10.1179/016164107X172392. [DOI] [PubMed] [Google Scholar]
- Lee J.H., Paek S.H., Shin H.W., Lee S.Y., Moon B.S., Park J.E., et al. Effect of fermented soybean products intake on the overall immune safety and function in mice. Journal of Veterinary Science. 2017;18:25–32. doi: 10.4142/jvs.2017.18.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K.W., Shim J.M., Park S.-K., Heo H.-J., Kim H.-J., Ham K.-S., et al. Isolation of lactic acid bacteria with probiotic potentials from kimchi, traditional Korean fermented vegetable. Lebensmittel-Wissenschaft und -Technologie- Food Science and Technology. 2016;71:130–137. [Google Scholar]
- Lee G.M., Suh D.H., Jung E.S., Lee C.H. Metabolomics provides quality characterization of commercial gochujang (fermented pepper paste) Molecules. 2016;21:14. doi: 10.3390/molecules21070921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung J.M., Yang C.X., Tam A., Shaipanich T., Hackett T.L., Singhera G.K., et al. ACE-2 expression in the small airway epithelia of smokers and COPD patients: Implications for COVID-19. European Respiratory Journal. 2020;55 doi: 10.1183/13993003.00688-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y.-P., Kao Y.-C., Pan W.-H., Yang Y.-H., Chen Y.-C., Lee Y.L. Associations between respiratory diseases and dietary patterns derived by factor analysis and reduced rank regression. Annals of Nutrition and Metabolism. 2016;68:306–314. doi: 10.1159/000447367. [DOI] [PubMed] [Google Scholar]
- Lobo R., Prabhu K.S., Shirwaikar A., Shirwaikar A. Curcuma zedoaria rosc. (white turmeric): A review of its chemical, pharmacological and ethnomedicinal properties. Journal of Pharmacy and Pharmacology. 2009;61:13–21. doi: 10.1211/jpp/61.01.0003. [DOI] [PubMed] [Google Scholar]
- Loftfield E., Shiels M.S., Graubard B.I., Katki H.A., Chaturvedi A.K., Trabert B., et al. Associations of coffee drinking with systemic immune and inflammatory markers. Cancer Epidemiology Biomarkers & Prevention. 2015;24:1052–1060. doi: 10.1158/1055-9965.EPI-15-0038-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Chillón M.T., Carazo-Díaz C., Prieto-Merino D., Zafrilla P., Moreno D.A., Villaño D. Effects of long-term consumption of broccoli sprouts on inflammatory markers in overweight subjects. Clinical Nutrition. 2019;38:745–752. doi: 10.1016/j.clnu.2018.03.006. [DOI] [PubMed] [Google Scholar]
- Lorenzon Dos Santos J., Quadros A.S., Weschenfelder C., Garofallo S.B., Marcadenti A. Oxidative stress biomarkers, nut-related antioxidants, and cardiovascular disease. Nutrients. 2020;12 doi: 10.3390/nu12030682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu X.N., Rasco B.A., Jabal J.M.F., Aston D.E., Lin M.S., Konkel M.E. Investigating antibacterial effects of garlic (Allium sativum) concentrate and garlic-derived organosulfur compounds on Campylobacter jejuni by using fourier transform infrared spectroscopy, Raman spectroscopy, and electron microscopy. Applied and Environmental Microbiology. 2011;77:5257–5269. doi: 10.1128/AEM.02845-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu R., Zhao X., Li J., Niu P., Yang B., Wu H., et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: Implications for virus origins and receptor binding. The Lancet. 2020;395:565–574. doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Managa M.G., Remize F., Garcia C., Sivakumar D. Effect of moist cooking blanching on colour, phenolic metabolites and glucosinolate content in Chinese cabbage (Brassica rapa L. subsp. chinensis) Foods. 2019;8:18. doi: 10.3390/foods8090399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martins N., Petropoulos S., Ferreira I. Chemical composition and bioactive compounds of garlic (Allium sativum L.) as affected by pre- and post-harvest conditions: A review. Food Chemistry. 2016;211:41–50. doi: 10.1016/j.foodchem.2016.05.029. [DOI] [PubMed] [Google Scholar]
- Massot-Cladera M., Franch A., Castell M., Perez-Cano F.J. Cocoa polyphenols and fiber modify colonic gene expression in rats. European Journal of Nutrition. 2017;56:1871–1885. doi: 10.1007/s00394-016-1230-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massot-Cladera M., Perez-Berezo T., Franch A., Castell M., Perez-Cano F.J. Cocoa modulatory effect on rat faecal microbiota and colonic crosstalk. Archives of Biochemistry and Biophysics. 2012;527:105–112. doi: 10.1016/j.abb.2012.05.015. [DOI] [PubMed] [Google Scholar]
- Mathew D., Hsu W.-L. Antiviral potential of curcumin. Journal of Functional Foods. 2018;40:692–699. [Google Scholar]
- McCrea C.E., West S.G., Kris-Etherton P.M., Lambert J.D., Gaugler T.L., Teeter D.L., et al. Effects of culinary spices and psychological stress on postprandial lipemia and lipase activity: Results of a randomized crossover study and in vitro experiments. Journal of Translational Medicine. 2015;13:7. doi: 10.1186/s12967-014-0360-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehrbod P., Amini E., Tavassoti-Kheiri M. Antiviral activity of garlic extract on Influenza virus. Iranian Journal of Virology. 2009;3:19–23. [Google Scholar]
- Moghadamtousi S.Z., Kadir H.A., Hassandarvish P., Tajik H., Abubakar S., Zandi K. A review on antibacterial, antiviral, and antifungal activity of curcumin. BioMed Research International. 2014:186864. doi: 10.1155/2014/186864. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motawi T.K., Hamed M.A., Shabana M.H., Hashem R.M., Aboul Naser A.F. Zingiber officinale acts as a nutraceutical agent against liver fibrosis. Nutrition and Metabolism. 2011;8:40. doi: 10.1186/1743-7075-8-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller L., Meyer M., Bauer R.N., Zhou H., Zhang H., Jones S., et al. Effect of broccoli sprouts and live attenuated influenza virus on peripheral blood natural killer cells: A randomized, double-blind study. PloS One. 2016;11 doi: 10.1371/journal.pone.0147742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newsome B.J., Petriello M.C., Han S.G., Murphy M.O., Eske K.E., Sunkara M., et al. Green tea diet decreases PCB 126-induced oxidative stress in mice by up-regulating antioxidant enzymes. The Journal of Nutritional Biochemistry. 2014;25:126–135. doi: 10.1016/j.jnutbio.2013.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolle C., Cardinault N., Aprikian O., Busserolles J., Grolier P., Rock E., et al. Effect of carrot intake on cholesterol metabolism and on antioxidant status in cholesterol-fed rat. European Journal of Nutrition. 2003;42:254–261. doi: 10.1007/s00394-003-0419-1. [DOI] [PubMed] [Google Scholar]
- Nilsson A., Johansson E., Ekström L., Björck I. Effects of a brown beans evening meal on metabolic risk markers and appetite regulating hormones at a subsequent standardized breakfast: A randomized cross-over study. PloS One. 2013;8 doi: 10.1371/journal.pone.0059985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noah T.L., Zhang H., Zhou H., Glista-Baker E., Müller L., Bauer R.N., et al. Effect of broccoli sprouts on nasal response to live attenuated influenza virus in smokers: A randomized, double-blind study. PloS One. 2014;9 doi: 10.1371/journal.pone.0098671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nugraha R.V., Ridwansyah H., Ghozali M., Khairani A.F., Atik N. 2020. Traditional herbal medicine candidates as complementary treatments for COVID-19: A review of their mechanisms, pros and cons. Evidence-Based Complementary and Alternative Medicine, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nurmatov U., Devereux G., Sheikh A. Nutrients and foods for the primary prevention of asthma and allergy: Systematic review and meta-analysis. The Journal of Allergy and Clinical Immunology. 2011;127:724–733. doi: 10.1016/j.jaci.2010.11.001. e721-730. [DOI] [PubMed] [Google Scholar]
- Oh S.-H., Park K.W., Daily J.W., III, Lee Y.-E. Mary ann liebert, inc. 140 huguenot street. 3rd Floor New Rochelle; NY 10801 USA: 2014. Preserving the legacy of healthy Korean food. [Google Scholar]
- Oktay S., Ekinci E.K. Medicinal food understanding in Korean gastronomic culture. Journal of Ethnic Foods. 2019;6:4. [Google Scholar]
- Özel Z., Öztürk H. Antiviral mechanisms related to lactic acid bacteria and fermented food products. Biotech Studies. 2020;29:21–31. [Google Scholar]
- Panyod S., Ho C.-T., Sheen L.-Y. Dietary therapy and herbal medicine for COVID-19 prevention: A review and perspective. Journal of traditional and complementary medicine. 2020;10:420–427. doi: 10.1016/j.jtcme.2020.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park M.-Y., Choi H.-Y., Kim J.-D., Lee H.-S., Ku S.-K. 28 Days repeated oral dose toxicity test of aqueous extracts of mahwangyounpae-tang, a polyherbal formula. Food and Chemical Toxicology. 2010;48:2477–2482. doi: 10.1016/j.fct.2010.06.017. [DOI] [PubMed] [Google Scholar]
- Park S., Hahm D.-H., Joo M., Kim K., Kwon S., Choi H., et al. The role of Korean medicine in the post-COVID-19 era: An online panel discussion part 2–basic research and education. Integrative Medicine Research. 2020;9:100488. doi: 10.1016/j.imr.2020.100488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S.Y., Jang H.L., Lee J.H., Choi Y., Kim H., Hwang J., et al. Changes in the phenolic compounds and antioxidant activities of mustard leaf (Brassica juncea) kimchi extracts during different fermentation periods. Food Science and Biotechnology. 2017;26:105–112. doi: 10.1007/s10068-017-0014-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park K.Y., Jeong J.K., Lee Y.E., Daily J.W., 3rd Health benefits of kimchi (Korean fermented vegetables) as a probiotic food. Journal of Medicinal Food. 2014;17:6–20. doi: 10.1089/jmf.2013.3083. [DOI] [PubMed] [Google Scholar]
- Park H., Kim H.-S. Korean traditional natural herbs and plants as immune enhancing, antidiabetic, chemopreventive, and antioxidative agents: A narrative review and perspective. Journal of Medicinal Food. 2014;17:21–27. doi: 10.1089/jmf.2013.3059. [DOI] [PubMed] [Google Scholar]
- Park S., Kim J.I., Bae J.-Y., Yoo K., Kim H., Kim I.-H., et al. Effects of heat-killed Lactobacillus plantarum against influenza viruses in mice. Journal of Microbiology. 2018;56:145–149. doi: 10.1007/s12275-018-7411-1. [DOI] [PubMed] [Google Scholar]
- Park M.-K., Ngo V., Kwon Y.-M., Lee Y.-T., Yoo S., Cho Y.-H., et al. Lactobacillus plantarum DK119 as a probiotic confers protection against influenza virus by modulating innate immunity. PloS One. 2013;8 doi: 10.1371/journal.pone.0075368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park M.K., Ngo V., Kwon Y.M., Lee Y.T., Yoo S., Cho Y.H., et al. Lactobacillus plantarum DK119 as a probiotic confers protection against influenza virus by modulating innate immunity. PloS One. 2013;8 doi: 10.1371/journal.pone.0075368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S.E., Yoo S.A., Seo S.H., Lee K.I., Na C.S., Son H.S. GC-MS based metabolomics approach of Kimchi for the understanding of Lactobacillus plantarum fermentation characteristics. Lwt-Food Science and Technology. 2016;68:313–321. [Google Scholar]
- Park E.H., Yum J., Ku K.B., Kim H.M., Kang Y.M., Kim J.C., et al. Red Ginseng-containing diet helps to protect mice and ferrets from the lethal infection by highly pathogenic H5N1 influenza virus. Journal of Ginseng Research. 2014;38:40–46. doi: 10.1016/j.jgr.2013.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patra J.K., Das G., Paramithiotis S. Lactic acid fermentation of fruits and vegetables. CRC Press; 2017. Kimchi: A well-known Korean traditional fermented food. [Google Scholar]
- Patra J.K., Das G., Paramithiotis S., Shin H.-S. Kimchi and other widely consumed traditional fermented foods of Korea: A review. Frontiers in Microbiology. 2016;7:1–15. doi: 10.3389/fmicb.2016.01493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pazyar N, Omidian M, Jamshydian N. Ginseng as a potential novel addition to the antikeloid weaponry. Phytotherapy Research. 2012;26:1579–1580. doi: 10.1002/ptr.4598. [DOI] [PubMed] [Google Scholar]
- Peterson C.T., Vaughn A.R., Sharma V., Chopra D., Mills P.J., Peterson S.N., et al. Effects of turmeric and curcumin dietary supplementation on human gut microbiota: A double-blind, randomized, placebo-controlled pilot study. J Evid Based Integr Med. 2018;23 doi: 10.1177/2515690X18790725. 2515690X18790725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phaswana-Mafuya N., Shisana O., Gray G., Zungu N., Bekker L.-G., Kuonza L., et al. The utility of 2009 H1N1 pandemic data in understanding the transmission potential and estimating the burden of COVID-19 in RSA to guide mitigation strategies. South African Medical Journal: South African Medical Journal. 2020;110:1–2. doi: 10.7196/SAMJ.2020.v110i7.14935. [DOI] [PubMed] [Google Scholar]
- Pimentel G., Burton K.J., von Ah U., Butikofer U., Pralong F.P., Vionnet N., et al. Metabolic footprinting of fermented milk consumption in serum of healthy men. Journal of Nutrition. 2018;148:851–860. doi: 10.1093/jn/nxy053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Planès R., Goujon C. The surprising importance of gut microbiota in tackling flu infection. Biota. 2020;4:3. [Google Scholar]
- Poole R., Kennedy O.J., Roderick P., Fallowfield J.A., Hayes P.C., Parkes J. Coffee consumption and health: Umbrella review of meta-analyses of multiple health outcomes. BMJ. 2017;359:j5024. doi: 10.1136/bmj.j5024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poutahidis T., Kleinewietfeld M., Smillie C., Levkovich T., Perrotta A., Bhela S., et al. Microbial reprogramming inhibits Western diet-associated obesity. PloS One. 2013;8 doi: 10.1371/journal.pone.0068596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Praditya D., Kirchhoff L., Brüning J., Rachmawati H., Steinmann J., Steinmann E. Anti-infective properties of the golden spice curcumin. Frontiers in Microbiology. 2019;10 doi: 10.3389/fmicb.2019.00912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quan L.H., Cheng L.Q., Kim H.B., Kim J.H., Son N.R., Kim S.Y., et al. Bioconversion of ginsenoside rd into compound K by lactobacillus pentosus DC101 isolated from kimchi. Journal of Ginseng Research. 2010;34:288–295. [Google Scholar]
- Rajaiah R., Abhilasha K.V., Shekar M.A., Vogel S.N., Vishwanath B.S. Evaluation of mechanisms of action of re-purposed drugs for treatment of COVID-19. Cellular Immunology. 2020:104240. doi: 10.1016/j.cellimm.2020.104240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao N.Z., Fuller M. Acidity and antioxidant activity of cold brew coffee. Scientific Reports. 2018;8:16030. doi: 10.1038/s41598-018-34392-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratan Z.A., Haidere M.F., Hong Y.H., Park S.H., Lee J.-O., Lee J., et al. Pharmacological potential of ginseng and its major component ginsenosides. Journal of Ginseng Research. 2020;45:199–210. doi: 10.1016/j.jgr.2020.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratha P., Jhon D.-Y. Factors increasing poly-γ-glutamic acid content of cheongguk-jang fermented by Bacillus subtilis 168. Food Science and Biotechnology. 2019;28:103–110. doi: 10.1007/s10068-018-0424-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rather I., Choi K.-H., Bajpai V.K., Park Y.-H. Antiviral mode of action of Lactobacillus plantarum YML009 on Influenza virus H1N1. Bangladesh Journal of Pharmacology. 2015;10:475–482. [Google Scholar]
- Relja A., Miljkovic A., Gelemanovic A., Boskovic M., Hayward C., Polasek O., et al. Nut consumption and cardiovascular risk factors: A cross-sectional study in a mediterranean population. Nutrients. 2017;9 doi: 10.3390/nu9121296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rettedal E.A., Altermann E., Roy N.C., Dalziel J.E. The effects of unfermented and fermented cow and sheep milk on the gut microbiota. Frontiers in Microbiology. 2019;10:458. doi: 10.3389/fmicb.2019.00458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reverri E.J., Randolph J.M., Steinberg F.M., Kappagoda C.T., Edirisinghe I., Burton-Freeman B.M. Black beans, fiber, and antioxidant capacity pilot study: Examination of whole foods vs. Functional components on postprandial metabolic, oxidative stress, and inflammation in adults with metabolic syndrome. Nutrients. 2015;7:6139–6154. doi: 10.3390/nu7085273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson S., Hirsch J.S., Narasimhan M., Crawford J.M., McGinn T., Davidson K.W., et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York city Area. Journal of the American Medical Association. 2020;323:2052–2059. doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez L., Cervantes E., Ortiz R. Malnutrition and gastrointestinal and respiratory infections in children: A public health problem. International Journal of Environmental Research and Public Health. 2011;8:1174–1205. doi: 10.3390/ijerph8041174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth A.N., Grau K.R., Karst S.M. Diverse mechanisms underlie enhancement of enteric viruses by the mammalian intestinal microbiota. Viruses. 2019;11 doi: 10.3390/v11080760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy A., Sarkar B., Celik C., Ghosh A., Basu U., Jana M., et al. Can concomitant use of zinc and curcumin with other immunity-boosting nutraceuticals be the arsenal against COVID-19? Phytotherapy Research : PT. 2020;34:2425–2428. doi: 10.1002/ptr.6766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryu E.H., Yang E.J., Woo E.R., Chang H.C. Purification and characterization of antifungal compounds from Lactobacillus plantarum HD1 isolated from kimchi. Food Microbiology. 2014;41:19–26. doi: 10.1016/j.fm.2014.01.011. [DOI] [PubMed] [Google Scholar]
- Samec D., Pavlovic I., Redovnikovic I.R., Salopek-Sondi B. Comparative analysis of phytochemicals and activity of endogenous enzymes associated with their stability, bioavailability and food quality in five Brassicaceae sprouts. Food Chemistry. 2018;269:96–102. doi: 10.1016/j.foodchem.2018.06.133. [DOI] [PubMed] [Google Scholar]
- Samuelson D.R., Welsh D.A., Shellito J.E. Regulation of lung immunity and host defense by the intestinal microbiota. Frontiers in Microbiology. 2015;6:1085. doi: 10.3389/fmicb.2015.01085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarria B., Gomez-Juaristi M., Martinez Lopez S., Garcia Cordero J., Bravo L., Mateos Briz M.R. Cocoa colonic phenolic metabolites are related to HDL-cholesterol raising effects and methylxanthine metabolites and insoluble dietary fibre to anti-inflammatory and hypoglycemic effects in humans. PeerJ. 2020;8 doi: 10.7717/peerj.9953. e9953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwager J., Richard N., Mussler B., Raederstorff D. Tomato aqueous extract modulates the inflammatory profile of immune cells and endothelial cells. Molecules. 2016;21:168. doi: 10.3390/molecules21020168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sener B., Orhan I., Ozcelik B., Kartal M., Aslan S., Ozbilen G. Antimicrobial and antiviral activities of two seed oil samples of Cucurbita pepo L. And their fatty acid analysis. Natural Product Communications. 2007;2 1934578X0700200409. [Google Scholar]
- Seo M.Y., Kim K.R., Lee J.J., Ryu G., Lee S.H., Hong S.D., et al. Therapeutic effect of topical administration of red onion extract in a murine model of allergic rhinitis. Scientific Reports. 2019;9:2883. doi: 10.1038/s41598-019-39379-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo S.H., Park S.E., Kim E.J., Lee K.I., Na C.S., Son H.S. A GC-MS based metabolomics approach to determine the effect of salinity on Kimchi. Food Research International. 2018;105:492–498. doi: 10.1016/j.foodres.2017.11.069. [DOI] [PubMed] [Google Scholar]
- Shahzad F, Anderson D, Najafzadeh M. The Antiviral, Anti-Inflammatory Effects of Natural Medicinal Herbs and Mushrooms and SARS-CoV-2 Infection. Nutrients. 2020;12 doi: 10.3390/nu12092573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shang H., Bai T., Chen Y., Huang C., Zhang S., Yang P., et al. Outcomes and implications of diarrhea in patients with SARS-CoV-2 infection. Scandinavian Journal of Gastroenterology. 2020;55:1049–1056. doi: 10.1080/00365521.2020.1800078. [DOI] [PubMed] [Google Scholar]
- Sharma N. Efficacy of garlic and onion against virus. International Journal of Research in Pharmacy and Science. 2019;10:3578–3586. [Google Scholar]
- Shepherd A.I., Costello J.T., Bailey S.J., Bishop N., Wadley A.J., Young-Min S., et al. Beet" the cold: Beetroot juice supplementation improves peripheral blood flow, endothelial function, and anti-inflammatory status in individuals with raynaud's phenomenon. Journal of Applied Physiology. 2019;127:1478–1490. doi: 10.1152/japplphysiol.00292.2019. 1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin H.W., Jang E.S., Moon B.S., Lee J.J., Lee D.E., Lee C.H., et al. Anti-obesity effects of gochujang products prepared using rice koji and soybean meju in rats. Journal of Food Science and Technology-Mysore. 2016;53:1004–1013. doi: 10.1007/s13197-015-2162-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh R.K., Chang H.W., Yan D., Lee K.M., Ucmak D., Wong K., et al. Influence of diet on the gut microbiome and implications for human health. Journal of Translational Medicine. 2017;15:73. doi: 10.1186/s12967-017-1175-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song J.-M., Lee K.-H., Seong B.-L. Antiviral effect of catechins in green tea on influenza virus. Antiviral Research. 2005;68:66–74. doi: 10.1016/j.antiviral.2005.06.010. [DOI] [PubMed] [Google Scholar]
- Song J., Lim H.X., Lee A., Kim S., Lee J.H., Kim T.S. Staphylococcus succinus 14BME20 prevents allergic airway inflammation by induction of regulatory T cells via interleukin-10. Frontiers in Immunology. 2019;10:14. doi: 10.3389/fimmu.2019.01269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi E., Sawabuchi T., Kimoto T., Sakai S., Kido H. Lactobacillus delbrueckii ssp. bulgaricus OLL1073R-1 feeding enhances humoral immune responses, which are suppressed by the antiviral neuraminidase inhibitor oseltamivir in influenza A virus–infected mice. Journal of Dairy Science. 2019;102:9559–9569. doi: 10.3168/jds.2019-16268. [DOI] [PubMed] [Google Scholar]
- Tenore G.C., Caruso D., Buonomo G., D'Avino M., Ciampaglia R., Maisto M., et al. Lactofermented Annurca apple puree as a functional food indicated for the Control of plasma lipid and oxidative amine levels: Results from a randomised clinical trial. Nutrients. 2019;11 doi: 10.3390/nu11010122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiruvengadam M., Kim S.H., Chung I.M. Exogenous phytohormones increase the accumulation of health-promoting metabolites, and influence the expression patterns of biosynthesis related genes and biological activity in Chinese cabbage (Brassica rapa spp. pekinensis) Scientia Horticulturae. 2015;193:136–146. [Google Scholar]
- Thu ZM, Myo KK, Aung HT, Clericuzio M, Armijos C, Vidari G. Bioactive Phytochemical Constituents of Wild Edible Mushrooms from Southeast Asia. Molecules. 2020;25 doi: 10.3390/molecules25081972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian M., Wu X., Hong Y., Wang H., Deng G., Zhou Y. Comparison of chemical composition and bioactivities of essential oils from fresh and dry rhizomes of Zingiber zerumbet (L.) smith. BioMed Research International. 2020 doi: 10.1155/2020/9641284. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torky Z.A. Antiviral activity of polyphenols extracts from Daucus carota against herpes simplex virus type 1. Turkish Online Journal of Science and Technology. 2013;3:20–32. [Google Scholar]
- Trošt K., Ulaszewska M.M., Stanstrup J., Albanese D., De Filippo C., Tuohy K.M., et al. Host: Microbiome co-metabolic processing of dietary polyphenols - an acute, single blinded, cross-over study with different doses of apple polyphenols in healthy subjects. Food Research International. 2018;112:108–128. doi: 10.1016/j.foodres.2018.06.016. [DOI] [PubMed] [Google Scholar]
- Tsai Y., Cole L.L., Davis L.E., Lockwood S.J., Simmons V., Wild G.C. Antiviral properties of garlic: In vitro efects on influenza B,herpes simplex and coxsackie viruses. Planta Medica. 1985:460–461. doi: 10.1055/s-2007-969553. [DOI] [PubMed] [Google Scholar]
- Tsujimoto K., Sakuma C., Uozaki M., Yamasaki H., Utsunomiya H., Oka K., et al. Antiviral effect of pyridinium formate, a novel component of coffee extracts. International Journal of Molecular Medicine. 2010;25:459–463. doi: 10.3892/ijmm_00000365. [DOI] [PubMed] [Google Scholar]
- Tsutomu A., Hisashi Y., Keiko I., Daisuke E., Takeshi N., Koyama A.H. Antiviral and virucidal activities of natural products. Current Medicinal Chemistry. 2009;16:2485–2497. doi: 10.2174/092986709788682065. [DOI] [PubMed] [Google Scholar]
- Utsunomiya H., Ichinose M., Uozaki M., Tsujimoto K., Yamasaki H., Koyama A.H. Antiviral activities of coffee extracts in vitro. Food and Cosmetics Toxicology. 2008;46:1919–1924. doi: 10.1016/j.fct.2008.01.031. [DOI] [PubMed] [Google Scholar]
- Vabret N., Britton G.J., Gruber C., Hegde S., Kim J., Kuksin M., et al. & Sinai Immunology Review, P. Immunology of COVID-19: Current state of the science. Immunity. 2020;52 doi: 10.1016/j.immuni.2020.05.002. 910-941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valderas-Martinez P., Chiva-Blanch G., Casas R., Arranz S., Martinez-Huelamo M., Urpi-Sarda M., et al. Tomato sauce enriched with olive oil exerts greater effects on cardiovascular disease risk factors than raw tomato and tomato sauce: A randomized trial. Nutrients. 2016;8:170. doi: 10.3390/nu8030170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentina S. Coffee: A rich source of antimicrobial and antiviral compounds. Clinical Immunology, Endocrine & Metabolic Drugs (Discontinued) 2017;4:19–32. [Google Scholar]
- Verma S., Twilley D., Esmear T., Oosthuizen C.B., Reid A.-M., Nel M., et al. Anti-SARS-CoV natural products with the potential to inhibit SARS-CoV-2 (COVID-19) Frontiers in Pharmacology. 2020;11:1514. doi: 10.3389/fphar.2020.561334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visioli F., Lastra C.A.D.L., Andres-Lacueva C., Aviram M., Calhau C., Cassano A., et al. Polyphenols and human health: A prospectus. Critical Reviews in Food Science and Nutrition. 2011;51:524–546. doi: 10.1080/10408391003698677. [DOI] [PubMed] [Google Scholar]
- Vitaglione P., Mazzone G., Lembo V., D'Argenio G., Rossi A., Guido M., et al. Coffee prevents fatty liver disease induced by a high-fat diet by modulating pathways of the gut-liver axis. Journal of Nutrition Sciences. 2019;8:e15. doi: 10.1017/jns.2019.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waki N., Yajima N., Suganuma H., Buddle B., Luo D., Heiser A., et al. Oral administration of Lactobacillus brevis KB 290 to mice alleviates clinical symptoms following influenza virus infection. Letters in Applied Microbiology. 2014;58:87–93. doi: 10.1111/lam.12160. [DOI] [PubMed] [Google Scholar]
- Weber N.D., Andersen D.O., North J.A., Murray B.K., Lawson L.D., Hughes B.G. In vitro virucidal effects of Allium sativum (garlic) extract and compounds. Planta Medica. 1992;58:417–423. doi: 10.1055/s-2006-961504. [DOI] [PubMed] [Google Scholar]
- Weber J.M., Ruzindana-Umunyana A., Imbeault L., Sircar S. Inhibition of adenovirus infection and adenain by green tea catechins. Antiviral Research. 2003;58:167–173. doi: 10.1016/s0166-3542(02)00212-7. [DOI] [PubMed] [Google Scholar]
- Wei B., Cha S.-Y., Kang M., Kim Y.J., Cho C.-W., Rhee Y.K., et al. Antiviral activity of Chongkukjang extracts against influenza A virus in vitro and in vivo. Journal of Ethnic Foods. 2015;2:47–51. [Google Scholar]
- Wilks J., Golovkina T. Influence of microbiota on viral infections. PLoS Pathogens. 2012;8 doi: 10.1371/journal.ppat.1002681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams N.C., Johnson M.A., Shaw D.E., Spendlove I., Vulevic J., Sharpe G.R., et al. A prebiotic galactooligosaccharide mixture reduces severity of hyperpnoea-induced bronchoconstriction and markers of airway inflammation. British Journal of Nutrition. 2016;116:798–804. doi: 10.1017/S0007114516002762. [DOI] [PubMed] [Google Scholar]
- Won T.J., Kim B., Oh E.S., Bang J.S., Lee Y.J., Yoo J.S., et al. Immunomodulatory activity of Lactobacillus strains isolated from fermented vegetables and infant stool. Canadian Journal of Physiology and Pharmacology. 2011;89:429–434. doi: 10.1139/y11-047. [DOI] [PubMed] [Google Scholar]
- Wu R., Wang L., Kuo H.-C.D., Shannar A., Peter R., Chou P.J., et al. An update on current therapeutic drugs treating COVID-19. Current pharmacology reports. 2020;6:56–70. doi: 10.1007/s40495-020-00216-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wylie L.J., Kelly J., Bailey S.J., Blackwell J.R., Skiba P.F., Winyard P.G., et al. Beetroot juice and exercise: Pharmacodynamic and dose-response relationships. Journal of Applied Physiology. 2013;115:325–336. doi: 10.1152/japplphysiol.00372.2013. 1985. [DOI] [PubMed] [Google Scholar]
- Yamashita S., Hirashima A., Lin I.C., Bae J., Nakahara K., Murata M., et al. Saturated fatty acid attenuates anti-obesity effect of green tea. Scientific Reports. 2018;8:10023. doi: 10.1038/s41598-018-28338-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S.-J., Lee J.-E., Lim S.-M., Kim Y.-J., Lee N.-K., Paik H.-D. Antioxidant and immune-enhancing effects of probiotic Lactobacillus plantarum 200655 isolated from kimchi. Food Science and Biotechnology. 2019;28:491–499. doi: 10.1007/s10068-018-0473-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C., Li L., Yang L., Lu H., Wang S., Sun G. Anti-obesity and Hypolipidemic effects of garlic oil and onion oil in rats fed a high-fat diet. Nutrition and Metabolism. 2018;15:43. doi: 10.1186/s12986-018-0275-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ya T., Zhang Q., Chu F., Merritt J., Bilige M., Sun T., et al. Immunological evaluation of Lactobacillus casei Zhang: A newly isolated strain from koumiss in inner Mongolia, China. BMC Immunology. 2008;9:68. doi: 10.1186/1471-2172-9-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo D.-G., Kim M.-C., Park M.-K., Song J.-M., Quan F.-S., Park K.-M., et al. Protective effect of Korean red ginseng extract on the infections by H1N1 and H3N2 influenza viruses in mice. Journal of Medicinal Food. 2012;15:855–862. doi: 10.1089/jmf.2012.0017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon J.Y., Park H.A., Kang J.H., Kim K.W., Hur Y.I., Park J.J., et al. Prevalence of dietary supplement use in Korean children and adolescents: Insights from Korea national health and nutrition examination survey 2007-2009. Journal of Korean Medical Science. 2012;27:512–517. doi: 10.3346/jkms.2012.27.5.512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon SW, Jeong JS, Kim JH, Aggarwal BB. Cancer Prevention and Therapy: Integrating Traditional Korean Medicine Into Modern Cancer Care. Integrative Cancer Therapies. 2013;13:310–331. doi: 10.1177/1534735413510023. [DOI] [PubMed] [Google Scholar]
- Youn YN, Lim E, Lee N, Kim YS, Koo MS, Choi SY. Screening of Korean medicinal plants for possible osteoclastogenesis effects in vitro. Genes Nutrition. 2008;2:375–380. doi: 10.1007/s12263-007-0062-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yun Y.-R., Kim H.-J., Song Y.-O. Kimchi methanol extract and the kimchi active compound, 3′-(4′-hydroxyl-3′,5′-Dimethoxyphenyl)Propionic acid, downregulate CD36 in THP-1 macrophages stimulated by oxLDL. Journal of Medicinal Food. 2014;17:886–893. doi: 10.1089/jmf.2013.2943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zang Y.X., Ge J.L., Huang L.H., Gao F., Lv X.S., Zheng W.W., et al. Leaf and root glucosinolate profiles of Chinese cabbage (Brassica rapa ssp pekinensis) as a systemic response to methyl jasmonate and salicylic acid elicitation. Journal of Zhejiang University - Science B. 2015;16:696–708. doi: 10.1631/jzus.B1400370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zawistowski J. Nutraceutical and Functional Food Regulations in the United States and Around the World (Second Edition) Food Science and Technology. Elsevier; 2014. Chapter 24 - Regulation of functional foods in selected Asian countries in the pacific rim; pp. 419–463. [DOI] [Google Scholar]
- Zelaya H., Alvarez S., Kitazawa H., Villena J. Respiratory antiviral immunity and immunobiotics: Beneficial effects on inflammation-coagulation interaction during influenza virus infection. Frontiers in Immunology. 2016;7:633. doi: 10.3389/fimmu.2016.00633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J., Tian S.S., Yang J., Liu J.F., Zhang W.D. Investigating mechanism of Qing-Fei-Pai-Du-Tang for treatment of COVID-19 by network pharmacology (51) Chin. Trad. Herbal Drugs. 2020;4(51):829–835. 20200228. [Google Scholar]
- Zuo T., Liu Q., Zhang F., Lui G.C., Tso E.Y., Yeoh Y.K., et al. Depicting SARS-CoV-2 faecal viral activity in association with gut microbiota composition in patients with COVID-19. Gut. 2020;70:276–284. doi: 10.1136/gutjnl-2020-322294. [DOI] [PMC free article] [PubMed] [Google Scholar]