Abstract
Although recognized as an important endocrine organ, little is known about the mechanisms through which adipose tissue can regulate inflammatory responses in distant tissues, such as lung that are affected by obesity. To explore potential mechanisms, male C57BL/6J mice were provided either high-fat diet, low-fat diet, or were provided a high-fat diet then switched to the low-fat diet to promote weight loss. Visceral adipocytes were then cultured in vitro to generate conditioned media (CM) that was used to treat both primary (mouse tracheal epithelial cells; MTECs) and immortalized (mouse-transformed club cells; MTCCs) airway epithelial cells. Adiponectin levels were greatly depressed in the CM from both obese and diet-switched adipocytes relative to mice continually fed the low-fat diet. MTECs from mice with obesity secreted higher baseline levels of inflammatory cytokines than MTECs from lean or diet-switched mice. MTECs treated with obese adipocyte CM increased their secretion of these cytokines compared with MTECs treated with lean CM. Diet-switched CM modestly decreased the production of cytokines compared with obese CM, and these effects were recapitulated when the CM was used to treat MTCCs. Adipose stromal vascular cells from mice with obesity expressed genes consistent with an M1 macrophage phenotype and decreased eosinophil abundance compared with lean stromal vascular fraction, a profile that persisted in the lean diet-switched mice despite substantial weight loss. Soluble factors secreted from obese adipocytes exert a proinflammatory effect on airway epithelial cells, and these alterations are attenuated by diet-induced weight loss, which could have implications for the airway dysfunction related to obese asthma and its mitigation by weight loss.
Keywords: cytokines, epithelium, mice, obese, weight loss
INTRODUCTION
Obesity has become a worldwide epidemic and is linked to an increased risk for multiple diseases, including atherosclerosis (1), type 2 diabetes (2), and nonalcoholic fatty liver disease (3). Obesity also profoundly influences the lung and can not only worsen existing pulmonary diseases such as chronic obstructive pulmonary disease (4) and allergic asthma (5) but also drive the development of a nonatopic, intrinsic airway hyperresponsiveness (6, 7). Recent genome-wide association studies (GWAS) indicate that high-body mass index (BMI) single nucleotide polymorphisms (SNPs) are highly correlated with SNPs associated with increased asthma risk. The endotype of adult-onset, inherent asthma is often noneosinophilic, nonallergic, and steroid resistant, and as such presents unique therapeutic challenges, leading to a higher morbidity and mortality rate for these patients (8). Although weight loss has proven beneficial in alleviating this condition, it is difficult for patients to maintain in the long term, and often does not fully correct symptoms of the disease (9, 10).
Adipocytes function to store fat in the form of triglycerides, regulate body temperature, and act as endocrine cells that regulate energy and glucose homeostasis (11). It has become appreciated that white adipocytes and brown adipocytes arise from separate progenitor lineages, with white adipocytes functioning primarily as fat storage and protection from mechanical injury, whereas mitochondria-rich brown adipocytes regulate thermogenesis and energy dissipation (12, 13). The alterations in adipose tissue during obesity include an excessive accumulation of fat in visceral adipose tissue, leading to both adipocyte hypertrophy and hyperplasia. In addition, adipose tissue-resident immune leukocytes undergo phenotypic alterations under conditions of obesity (14, 15). Obese adipocytes lose healthy production of adiponectin, increase their release of free fatty acids (FFAs), and produce elevated levels of leptin, interleukin-6 (IL-6), monocyte chemoattractant protein-1 (MCP-1), and other proinflammatory cytokines (16). This increase in tissue inflammatory tone drives remodeling of the extracellular matrix to allow for angiogenesis supporting adipose expansion (11). Lean adipose tissue leukocytes are characterized by eosinophils and innate lymphoid type 2 (ILC2) cells that control thermogenesis and a small, anti-inflammatory population of resident macrophages that express resistin-like α (Retnla, also known as Fizz1), cluster of differentiation 163 (Cd163), cluster of differentiation 209f (Cd209f), peroxisome proliferator-activated receptor γ (Pparg1), and peroxisome proliferator-activated receptor γ coactivator-1 α (Pgc1a) (17). In the obese state, eosinophils and ILCs are diminished and resident macrophages form “crown-like structures” around adipocytes and become proinflammatory “M1” cells. RNA-seq approaches have identified a class of adipose tissue-resident macrophages present only under diet-induced obese conditions that are characterized by enhanced expression of Cd9, Trem2, and Lgals3, among other genes (18). Weight loss can lead to so-called “browning” of adipocytes, a process in which white adipose tissue adipocytes express higher levels of genes associated with brown adipose tissue such as lipocalin-2 (Lcn2), sirtuin-1 (Sirt1), and uncoupling protein 1 (Ucp1). These cells are often considered beige or “brite” (brown in white) adipocytes (19, 20).
Although the correlation between obesity and asthma is well established (6, 21), the contribution of adipose tissue and the mechanism, whereby it modulates distant organs and cellular responses is less well understood. In this study, we analyzed visceral adipose tissue from diet-induced lean mice and mice with obesity, and cultured primary adipocytes from these animals for generating adipocyte-conditioned media (Ad-CM). This Ad-CM was then used to determine how adipocyte-derived products could influence the baseline inflammatory profiles of airway epithelial cells, as well as their ability to respond to stimulation. Finally, we examined whether several secreted mediators from the adipocytes, including adiponectin, IL-10, and IL-6, were necessary and/or sufficient to influence airway epithelial cells.
MATERIALS AND METHODS
Mice
C57BL/6J male mice were purchased from The Jackson Laboratory (Bar Harbor, ME) at 12 wk of age. These mice had already been maintained on either low-fat diet (LFD; 10% kCal from fat) or high-fat diet (HFD; 60% kCal from fat) (Research Diets, Inc., New Brunswick, NJ) at The Jackson Laboratory. After a week of acclimation, some of the HFD-fed mice were switched to the LFD for 6 wk. All experiments were reviewed and approved by the University of Vermont Institutional Animal Care and Use Committee (IACUC) and conducted in accordance with the University of Vermont IACUC and the Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC). Mice were maintained on their special diets ad libitum and given water ad libitum, and maintained on a 12-h light/dark cycle.
Diet-Induced Obesity Model
At 13 wk of age, half of the mice on high-fat diet (HFD) were switched to the low-fat diet (LFD) for further 6 wk, whereas other mice were maintained on their original diets for the remainder of the study. Mice were weighed weekly. After the 6-wk diet change period, all mice were euthanized. Visceral omental adipose tissue and tracheas (for generation of mouse tracheal epithelial cells) were collected.
Primary Adipocyte Generation
Adipose tissue was minced with razor blades in a digestion buffer (100 mM HEPES, 120 mM NaCl, 50 mM glucose, 1 mM CaCl2, 1.5% BSA Fraction V) containing 2 mg/mL collagenase IV (StemCell Technologies, Vancouver, BC Canada) and incubated at 37°C for 1 h before centrifugal separation (100 g and 500 g) of floating adipocytes from the pelleted stromal vascular fraction (SVF). Adipocytes were washed twice in Dulbecco’s phosphate-buffered saline (DPBS) and plated at 10 × 106 cells/mL for 48 h in complete media, containing DMEM (Life Technologies, Cat. No. 11995), 20% FBS, penicillin-streptomycin, and 1 mM sodium pyruvate (all components from Life Technologies, Carlsbad, CA). At 48 h, floating cells were removed from the conditioned media via centrifugation, and conditioned media was frozen for later use. All cell cultures tested negative for the presence of mycoplasma when analyzed by the MycoDect Mycoplasma Detection Kit (Alstem, Richmond, CA).
Stromal Vascular Fraction Enrichment
The stromal vascular fraction (SVF) was collected during isolation of the primary adipocytes, and red blood cells were lysed from the pellet using ACK Lysis Buffer (Life Technologies). The clean pellet was then resuspended in RNA lysis buffer and RNA was isolated using the GE Illustra Spin kit (GE Healthcare Systems, Chicago, IL).
Preparation of Mouse Tracheal Epithelial Cell Cultures
Mouse tracheal epithelial cells (MTECs) were prepared as previously described (22). Briefly, tracheas were digested with a protease solution (1 mg/mL) in MEM at 37°C for 16 h before washing and centrifugation. Pelleted cells were resuspended in culture media (DMEM F12 supplemented with 50 U/mL penicillin, 50 μg/mL streptomycin, 2 mM l-glutamine, 20 ng/mL cholera toxin, 15 μg/mL bovine pituitary extract, 10 ng/mL external growth factor, 5 μg/mL insulin, 5 μg/mL transferrin, and 0.1 μM dexamethasone) and grown on flasks coated with rat tail collagen (BD Biosciences, San Jose, CA). Cells were fed every 3–4 days until they reached confluency, and then fed every other day thereafter. Cells were collected from the flasks using trypsin and collagenase to digest the collagen matrix. For air-liquid interface experiments, cells were differentiated over the course of 28 days, using PneumaCult-ALI Medium (StemCell Technologies, Inc. Cat. No. 05001) and following the manufacturer’s protocol. Briefly, cells were plated at 1 × 106 cells/mL on 0.4-μm polycarbonate transwells (Corning, Kennebunk, ME). Cells reached transepithelial electrical resistance (TEER) around 7 days of culture, at which point media was supplied only in the basal chamber, and changed every other day. Beginning 2 wk after ALI induction, mucus was removed from the apical chamber by washing with DPBS once a week. Transepithelial electrical resistance (TEER) was measured by the brief addition of DPBS to the apical chamber during washes, using an EVOM 2 Epithelial Voltohmmeter (World Precision Instruments, Sarasota, FL).
Mouse-Transformed Club Cells
Murine bronchiolar epithelial cells [mouse-transformed club cells (MTCCs), SV40 (23)] obtained from Dr. Francisco DeMayo were cultured at 37°C in 95% humidified air containing 5% CO2 using DMEM (Life Technologies) containing 10% FBS (Life Technologies), 2 mM l-glutamine, 50 U/mL penicillin, and 50 μg/mL streptomycin. For conditioned media experiments, MTCC were plated at 1 × 106 cells/mL and grown to confluency, at which point they were washed and treated with Ad-CM for 24 h. In separate experiments, MTCCs treated with Ad-CM for 24 h were subsequently washed and stimulated with 64 ng/mL Ultra Pure lipopolysaccharide from Escherichia coli 0111:B4 (Invivogen, San Diego, CA) for further 24 h.
3T3-L1 Cell Culture
3T3-L1-MBX mouse embryonic fibroblasts were purchased from American Type Culture Collection (RRID:CVCL_0A20, Manassas, VA) and cultured and differentiated according to manufacturer recommendations. Briefly, cells were cultured in high-glucose DMEM (Cat. No. 11995) with 10% FBS, 1 mM sodium pyruvate, and 50 U/mL penicillin-5 μg/mL streptomycin. All media and base components were purchased from Life Technologies. Cells were differentiated in base media with the addition of 1 μM dexamethasone, 1 μg/mL insulin, and 0.5 μM 3-isobutyl-1-methylxanthine (IBMX) (Sigma-Aldrich, St. Louis, MO) on day 0. Cells were then fed every other day in base media with 1 μg/mL insulin and collected on day 8. In some experiments, capsaicin (Sigma-Aldrich) was added in the differentiation media on day 0 only, in the presence or absence of capsazepine (Sigma-Aldrich).
Real-Time Quantitative PCR
Total RNA was isolated using the GE Illustra RNAspin Mini RNA Isolation kit (GE Healthcare Bio-Sciences) according to manufacturer’s recommendations. One microgram of RNA was reversed transcribed to cDNA using the iScript cDNA synthesis kit (Bio-Rad Technologies, Hercules, CA). Quantitative PCR was performed using SYBR Green iTaq Universal master mix (Bio-Rad) and primer pairs designed using PrimerBLAST and custom made by Integrated DNA Technologies (Coralville, IA). Primer sequences are presented in Table 1. Gene expression analysis was performed on the CFX 1000 Thermocycler (Bio-Rad) and expression was determined relative to either the housekeeping gene Actb, Gapdh, or Rpl13a using the ΔΔCT method.
Table 1.
Primer sequences
| Gene | Name | Forward Primer | Reverse Primer |
|---|---|---|---|
| Adipoq | Adiponectin | TGTTCCTCTTAATCCTGCCCA | CCAACCTGCACAAGTTCCCTT |
| Bactin | β-Actin | TAGGCACCAGGGTGTGATG | GGTGTGGTGCCAGATCTTCT |
| Cd9 | Cluster of differentiation 9 | CAGCGGGAAACACTCAAAGC | GGCAGGGCTTAACCTGGAAA |
| Cd209f | Cluster of differentiation 209f | GTCACATCTGGAGAGCAGGG | TGTCCCTGTGAGTATGCACG |
| Clca1 | Calcium-activated chloride channel regulator-1 (Gob5) | AAGCAAACCACTCCCATGAC | TGCGAAAGCATCAACAAGAC |
| Il5ra | Interleukin 5 receptor α | TGGCTCTGTATCTAATGTGTTCA | CATGGTAGCCGAATGCTGGA |
| Il6 | Interleukin-6 | CCGGAGAGGAGACTTCACAG | GAGCATTGGAAATTGGGGTA |
| Lcn2 | Lipocalin-2 | TGAGTGTCATGTGTCTGGGC | AACTGATCGCTCCGGAAGTC |
| Lgals3 | Galectin-3 | TAATCAGGTGAGCGGCACAG | GATAAGCAGCCCCTGGGTAG |
| Nos2 | Nitric oxide synthase 2 | AGTTCGTCCCCTTCTCCTGT | CCTTGTTCAGCTACGCCTTC |
| Pgc1α | Peroxisome proliferator-activated receptor γ coactivator-1 α | ATGTGTCGCCTTCTTGCTCT | ATCTACTGCCTGGGGACCTT |
| Pparg1 | Peroxisome proliferator-activated receptor γ1 | CCACCAACTTCGGAATCAGCT | TTTGTGGATCCGGCAGTTAAGA |
| Pref1 | Preadipocyte factor 1 | GACCCACCCTGTGACCCC | CAGGCAGCTCGTGCACCCC |
| Retnla | Resistin-like α (Fizz1) | CCTGCTGGGATGACTGCTA | TGGGTTCTCCACCTCTTCAT |
| Rpl13a | Ribosomal protein L13a | CCCTCCACCCTATGACAAGA | CTGCCTGTTTCCGTAACCTC |
| Saa3 | Serum amyloid A3 | CAGGATGAAGCCTTCCATTG | CATGACTGGGAACAACAGGA |
| SiglecF | Sialic acid binding Ig-like lectin F | GATGACCATCAGGGTGTCCT | GACTCACCCTCTTGGATCTGC |
| Sirt1 | Sirtuin 1 | CTCTAGTGACTGGACTCCGC | CCGCAAGGCGAGCATAGATA |
| Trem2 | Triggering receptor expressed on myeloid cells 2 | CGGAATGGGAGCACAGTCAT | CTTGATTCCTGGAGGTGCTGT |
Antibodies and Recombinant Proteins
Anti-IL-10 (RRID:AB_2125085) and isotype control antibodies were purchased from R&D Systems (Minneapolis, MN). Anti-IL-6 (RRID:AB_1107709) and isotype control antibodies were purchased from Bio X Cell (West Lebanon, NH). All antibodies were used at a concentration of 10 μg/mL, and conditioned media was treated 30 min before addition to cells. Recombinant adiponectin was purchased from R&D Technologies, whereas both recombinant IL-6 and IL-10 were purchased from StemCell Technologies. In single-stimulation experiments, rAdiponectin was used at 10 μg/mL, rIL-6 was used at 20 ng/mL, and rIL-10 was used at 20 ng/mL. In separate experiments, cytokine cocktails were made to represent levels observed in the LFD and HFD-fed Ad-CM. The “LFD” cocktail contained 10 ng/mL rAdiponectin and 100 pg/mL rIL-10, whereas the “HFD” cocktail contained 2 ng/mL rAdiponectin, 30 ng/mL rIL-6, and 10 pg/mL rIL-10.
Cytokine Analysis
All cytokines and adipokines were analyzed by ELISA, using DuoSet kits purchased from R&D Systems. Kits were utilized according to manufacturer’s instructions and read on a plate reader (BioTek, Winooski, VT).
Free Fatty Acid ELISA
Free fatty acids were measured from adipocyte-conditioned media using the Free Fatty Acid Flurometric Kit (Cayman Chemical, Ann Arbor, MI) according to manufacturer’s instructions. The kit was capable of measuring between 25 and 250 μM concentrations and measured via plate-based fluorometric analysis (ex 530–540 nm, em 585–595 nm) on a BioTek plate reader.
Statistics
Data were analyzed by two-tailed unpaired t test or one-way or two-way ANOVA and Bonferroni post hoc test using GraphPad Prism 8.2.1 for Windows (GraphPad Software, Inc, La Jolla, CA). A P value smaller than 0.05 was considered statistically significant. Asterisks (*) were used to signify significant differences between the LFD and all other groups. Hash marks (#) were used to signify significant differences between the HFD and HFD to LFD groups.
RESULTS
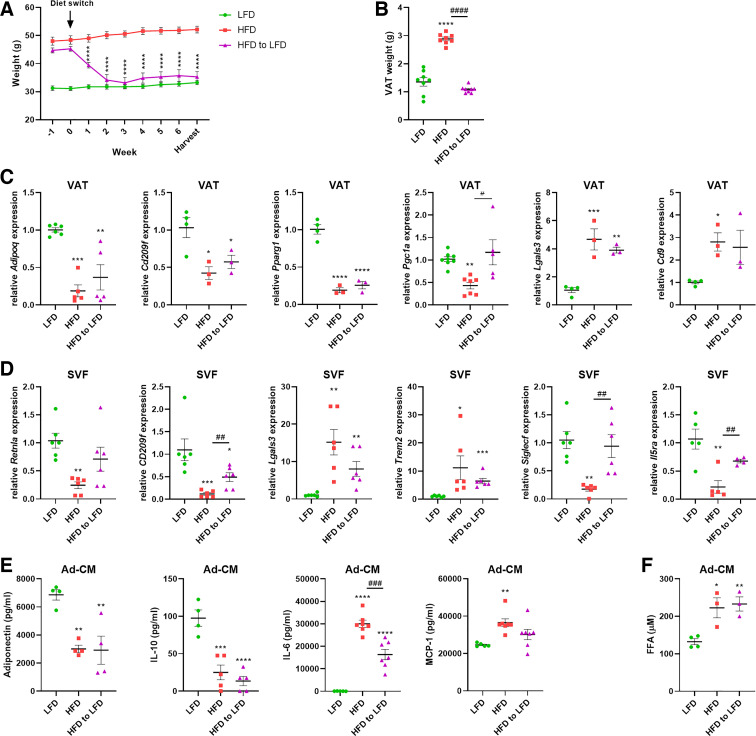
Mice were maintained on lean or obese diets (Table 2) until 13 wk of age, at which point half of the animals with obesity were switched to the low-fat diet (LFD). Diet-switched mice rapidly lost weight, becoming comparable to lean animals within 2 wk. Weight loss persisted as mice were maintained on the low-fat diet for a total of 6 wk, at which point animals were analyzed (Fig. 1A). The diet-induced loss in total weight was also reflected in the weight of the collected visceral adipose tissue (VAT) (Fig. 1B). Analysis of VAT revealed significant alterations in gene expression from the lean-to-obese state. Mice maintained on the HFD had diminished expression of Adipoq, consistent with previous reports (24, 25), as well as Cd209f, Pparg1, and Pgc1a, as well as increases in Lgals3 and Cd9 (Fig. 1C). VAT was further enriched separately into the stromal vascular fraction and primary adipocytes. Gene expression analysis of the SVF revealed a decrease in the anti-inflammatory macrophage cell markers Retnla and CD209f and concomitant increases in the proinflammatory M1 markers Lgals3 and Trem2 (Fig. 1D). Consistent with the hypothesis that diet-induced obesity is associated with a loss of homeostatic tissue-resident eosinophils (26, 27), we observed significant decreases in the gene expression of Siglecf and Il5ra in the SVF (Fig. 1D). The adipocytes were separated from the SVF and cultured for 48 h. Analysis of the cell-free conditioned media (Ad-CM) demonstrated impaired production of adiponectin and interleukin-10 (IL-10) by the obese adipocytes, an effect that persisted in the diet-switched (HFD to LFD) mice despite 6 wk of diet-induced weight loss (Fig. 1E). Obese adipocytes, as well as those from previously obese, diet-switched mice, demonstrated increased production of the cytokines IL-6 and MCP-1 compared with lean adipocytes (Fig. 1E), as well as increased free fatty acids (FFAs) (Fig. 1F). There were no differences in secreted lactate dehydrogenase, a surrogate marker for cell damage/death, between any groups (data not shown).
Table 2.
Special diet information
| Diet | Source | Cat. No. | % kCal from Fat |
|---|---|---|---|
| Low-fat diet (LFD) | Research diets | D12450B | 10 (Soybean oil > lard) |
| High-fat diet (HFD) | Research diets | D12492 | 60 (Lard > soybean oil) |
Figure 1.
Diet-induced obesity and weight loss model in mice. Low-fat (LFD) and high-fat (HFD) diets were obtained preformulated from Research Diets and contain 10% and 60% kCal from fat, respectively. Male mice, maintained on these diets, were purchased from Jackson Labs, and one group of HFD-fed mice was switched to LFD for 6 wk. Diet-switched (HFD to LFD) mice rapidly lost weight and maintained body weights comparable to the LFD group for 6 wk before analysis (A). Diet-switched mice also exhibited a loss of total visceral adipose tissue (VAT) weight (B). mRNA expression was analyzed from VAT (C). Gene expression of myeloid cell markers was analyzed from the stromal vascular fraction (SVF) of VAT (D). Enriched primary adipocytes were cultured for 48 h and cell-free conditioned media (Ad-CM) was analyzed for cytokine secretion (E) and free fatty acid (FFA) content (F). n = 3–8 mice/group. Data were analyzed by 2-way ANOVA and Tukey’s multiple comparison test (A) or 1-way ANOVA and Dunnett’s multiple comparison test (B–F). *P < 0.05, **P < 0.01, ***P < 0.005, ****P < 0.001: significant differences compared with LFD. #P < 0.05, ##P < 0.01, ###P < 0.005, ####P < 0.001: significant differences between HFD and HFD to LFD.
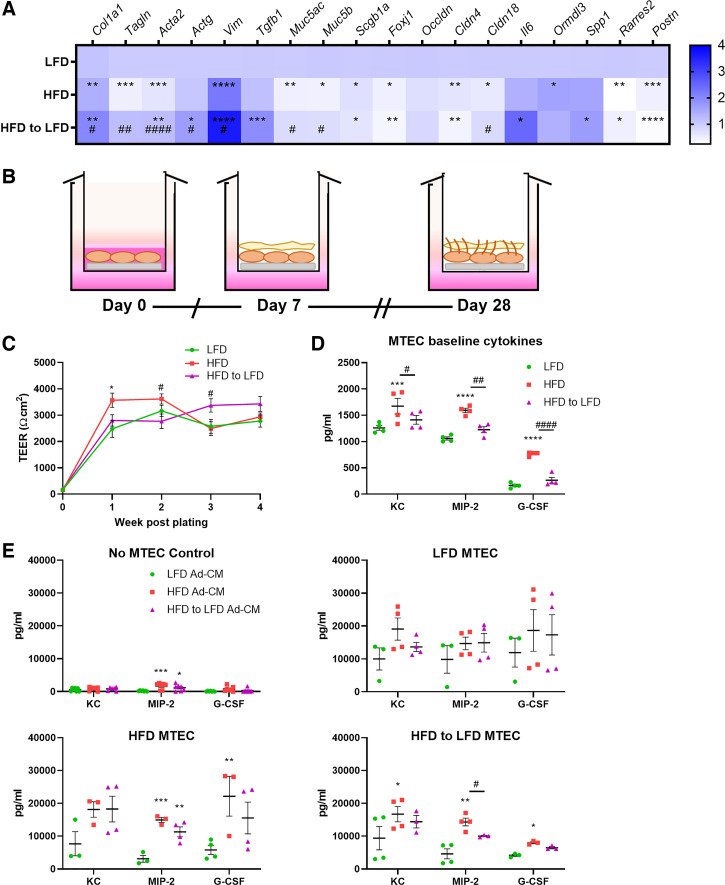
To examine the effects of adipocyte-derived products on airway epithelial cells, we first assessed the phenotypes of mouse tracheal epithelial cells (MTECs) from lean, obese, and diet-switched mice. Freshly isolated MTECs from the three groups were analyzed for gene expression by RT-qPCR, revealing differences between the lean, obese, and diet-switched MTECs (Fig. 2A). MTECs from mice with obesity exhibited a downregulation in genes involved in smooth muscle development (Tagln, Acta2) and goblet cell differentiation (Muc4ac, Muc5b), whereas fibrotic remodeling genes (Col1a1 and Vim) were significantly increased; and these increases were observed even more strongly in the diet-switched mice. Also observed from the obese and diet-switched mice were increases in gene expression of the asthma susceptibility gene Ormdl3, the proinflammatory cytokine Il6, and Spp1 (osteopontin), a secreted matricellular protein that is increased in obesity and is thought to play roles in remodeling and leukocyte recruitment. When cultured at air-liquid interface (ALI) (Fig. 2B), MTECs from all three groups were able to establish and maintain transepithelial electrical resistance (TEER) (Fig. 2C). After 28 days of differentiation, cells were analyzed for the production of the proinflammatory cytokines keratinocyte-derived chemoattractant (KC), macrophage inflammatory protein-2 (MIP-2), and granulocyte colony-stimulating factor (G-CSF). All three cytokines were significantly elevated in the MTEC supernatants from the diet-induced obese animals compared with the lean and diet-switched MTECs (Fig. 2D). In separate experiments, MTECs from all three groups were plated and then treated with Ad-CM from lean, obese, and diet-switched animals for 24 h. The Ad-CM from the obese adipocytes increased cytokine secretion from MTECs, regardless of whether the MTECs were sourced from lean, obese, or diet-switched animals, with the effects of HFD Ad-CM being the most substantial on HFD MTECs. Interestingly, Ad-CM from the lean animals had a slight dampening effect on cytokine secretion from the obese and diet-switched MTECs, with the HFD-to-LFD Ad-CM attenuating KC production from LFD MTECs and MIP-2 and G-CSF production from HFD and HFD-to-LFD MTECs compared with the effect of HFD-CM. (Fig. 2E).
Figure 2.
Diet-induced obesity increases proinflammatory cytokine secretion from primary mouse tracheal epithelial cells (MTECs). MTECs from diet-induced lean mice and mice with obesity were analyzed for gene expression by RT-qPCR immediately after isolation (A). In separate experiments, isolated MTECs were cultured at air-liquid interface for 28 days of differentiation (B), during which transepithelial electrical resistance (TEER) was measured each week (C) and baseline cytokine secretion was analyzed at day 28 (D). MTECs were plated separately for 24-h challenge with adipocyte-conditioned media (Ad-CM) from lean (LFD), obese (HFD), or diet-switched (HFD to LFD) mice, and cytokine secretion was measured (E). n = 3–11 mice/group. Data were analyzed by 2-way ANOVA and Tukey’s multiple comparison test (C) or 2-way ANOVA and Dunnett’s multiple comparison test (D and E). *P < 0.05, **P < 0.01, ***P < 0.005, ****P < 0.001: significant differences compared with LFD. #P < 0.05, ##P < 0.01, ####P < 0.001: significant differences between HFD and HFD to LFD. HFD, high-fat diet; LFD, low-fat diet.
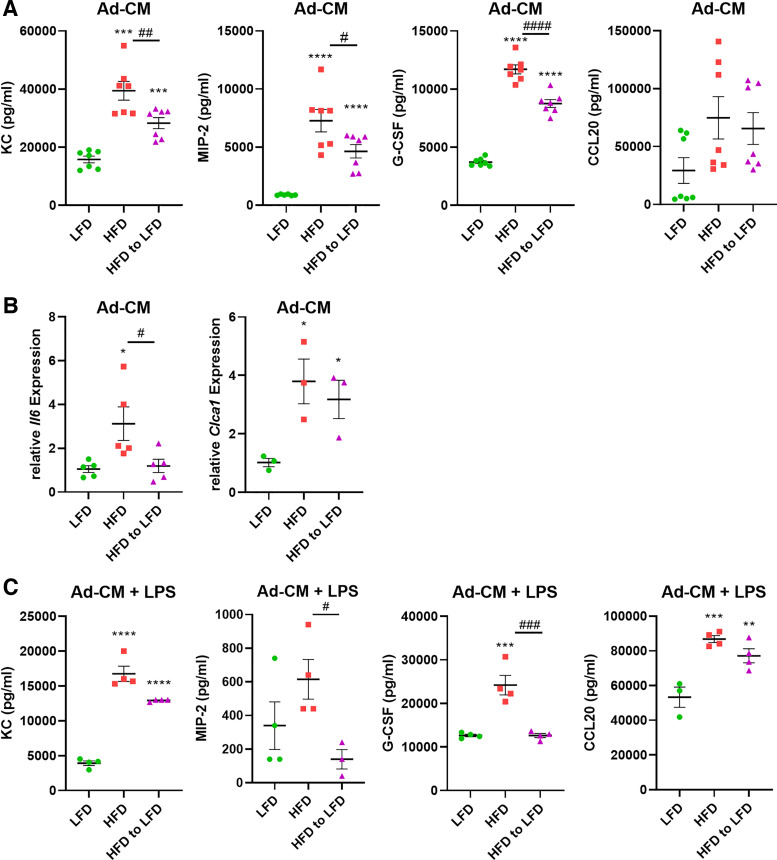
To conduct additional mechanistic studies of the effects of adipocyte-conditioned media on airway epithelial cells, we utilized the mouse-transformed club cell (MTCC) line. Treating MTCC with the Ad-CM from lean, obese, and diet-switched primary adipocytes recapitulated our results in the primary airway epithelial cells. The obese Ad-CM significantly increased the baseline secretion of KC, MIP-2, and G-CSF from the MTCCs (Fig. 3A). Ad-CM from the diet-switched mice significantly reduced cytokine secretion from MTCCs, although not to the lower levels produced by MTCCs exposed to lean Ad-CM. Furthermore, treatment with HFD Ad-CM increased the gene expression of interleukin-6 (Il6) and the mucin gene Clca1 (Fig. 3B), with no significant decrease from the MTCCs exposed to lean Ad-CM. In another study, following a 24-h treatment with the Ad-CM, MTCCs were washed and stimulated with LPS for an additional 24 h. Diet-induced obese adipocyte CM predisposed the MTCCs to respond more vigorously to LPS, inducing increased levels of KC, MIP-2, G-CSF, and chemokine (C-c motif) ligand 20 (CCL20) compared with lean Ad-CM. (Fig. 3C). mRNA analysis of Tlr4 expression, the cell receptor for LPS, revealed no differences between the LFD, HFD, and HFD-to-LFD Ad-CM treated cells (data not shown).
Figure 3.
Primary adipocyte-conditioned media (Ad-CM) modulates airway epithelial cell cytokine secretion and responses to lipopolysaccharide. Mouse-transformed club cells (MTCCs) were treated with Ad-CM from diet-induced lean and obese mice for 24 h and assessed for proinflammatory cytokine secretion (A) and gene expression (B). Twenty-four hours after Ad-CM treatment, MTCCs were challenged with 64 ng/mL lipopolysaccharide (LPS) for further 24 h and supernatants were analyzed by ELISA (C). n = 3–7 mice/group. Data were analyzed by 1-way ANOVA and Dunnett’s multiple comparison test. *P < 0.05, **P < 0.01, ***P < 0.005, ****P < 0.001: significant differences compared with LFD. #P < 0.05, ##P < 0.01, ###P < 0.005, ####P < 0.001: significant differences between HFD and HFD to LFD. HFD, high-fat diet; LFD, low-fat diet.
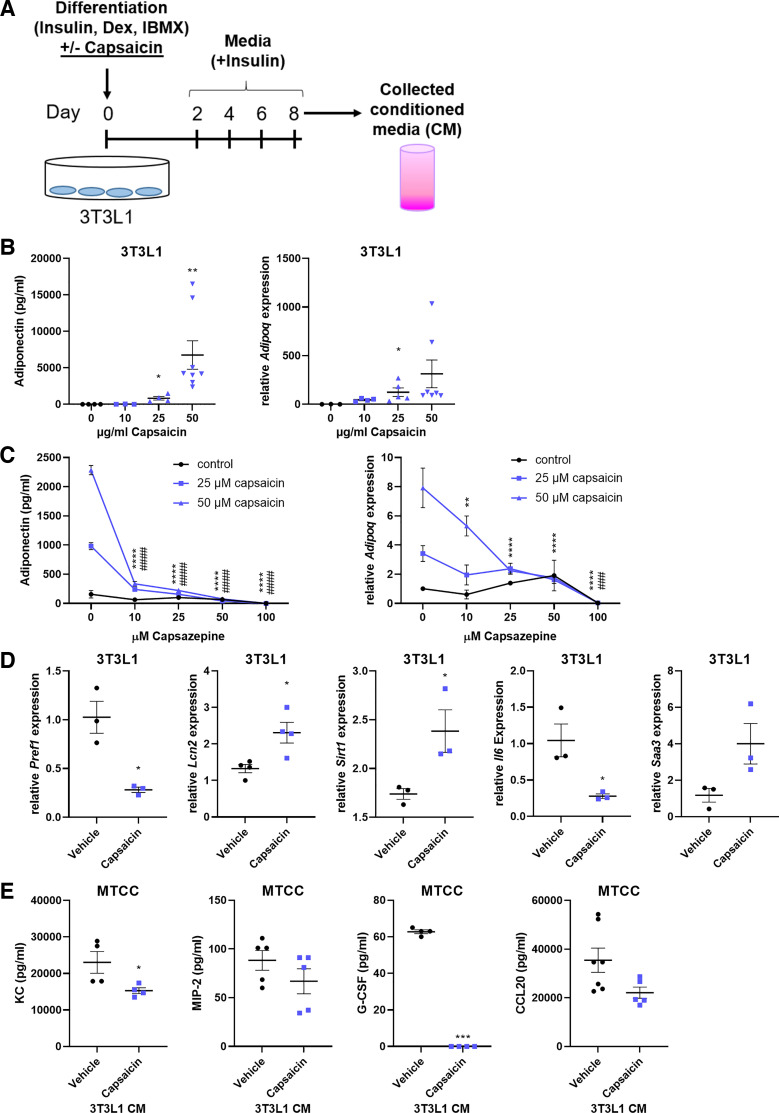
The 3T3-L1 cell line is a valuable tool to study adipocyte differentiation and function. Treatment with the TRPV1 agonist, capsaicin, during adipocyte differentiation drives a brown adipocyte phenotype (28, 29) and could perhaps be used as a model for healthy, lean fat cells. We therefore treated 3T3-L1 cells with increasing doses of capsaicin during differentiation (Fig. 4A), and this revealed a robust, dose-dependent increase in both adiponectin secretion and mRNA expression (Fig. 4B). These increases were blocked by the TRPV1 antagonist, capsazepine, indicating that the capsaicin is acting through its receptor (Fig. 4C). Capsaicin-differentiated 3T3-L1 cells decreased their expression of preadipocyte factor-1 (Pref1), indicating that they are in fact mature adipocytes, and they increased expression of the brown adipose genes lipocalin-2 (Lcn2) and sirtuin-1 (Sirt1) (Fig. 4D). These cells also decreased gene expression of Il6 and increased serum amyloid A3 (Saa3), which we have previously demonstrated is required for healthy adipose homeostasis (30). As with the lean Ad-CM, treatment of MTCCs with the conditioned media from capsaicin-differentiated 3T3-L1 adipocytes diminished the secretion of KC, MIP-2, and G-CSF (Fig. 4E).
Figure 4.
3T3L1 adipocytes differentiated in the presence of capsaicin acquire a lean adipocyte phenotype with immunosuppressive capabilities. 3T3L1-MBX cells were differentiated into adipocytes in the presence of capsaicin or equivalent volume vehicle control (ethanol) (A) and were analyzed for adiponectin secretion and gene expression (B). The effects of capsaicin differentiation on adiponectin were performed in conjunction with the capsaicin inhibitor capsazepine (C). 3T3L1 cells were treated with 50-µM capsaicin or vehicle control and analyzed for mRNA expression of adipocyte differentiation and browning genes was performed (D). MTCCs were challenged for 24 h with cell-free conditioned media from 3T3L1 cells differentiated with capsaicin, and cytokine secretion was analyzed by ELISA (E). n = 3–8 samples/group. Data were analyzed by 1-way ANOVA and Dunnett’s multiple comparison test (B), 2-way ANOVA and Tukey’s multiple comparison test (C), or unpaired t test (D and E). *P < 0.05, **P < 0.01, ***P < 0.005, ****P < 0.001: significant differences between capsazepine doses of the 50-μM capsaicin treatment. ###P < 0.005, ####P < 0.001: significant differences between capsazepine doses of the 25-μM capsaicin treatment. MTCC, mouse-transformed club cells.
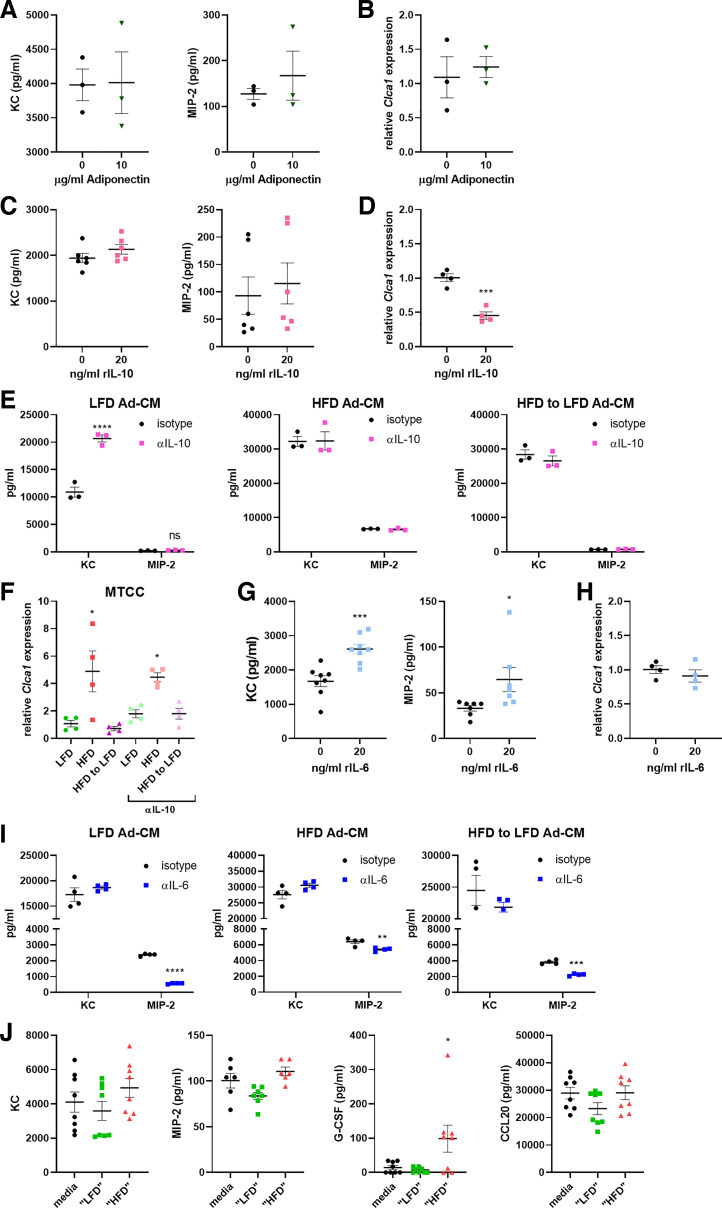
Since we observed that both lean primary adipocytes (Fig. 1E) and capsaicin-primed 3T3-L1 cells (Fig. 4B) secreted significantly more adiponectin, we sought to determine whether adiponectin was sufficient to inhibit proinflammatory cytokine production from the airway epithelial cells. From MTCCs cultured with increasing doses of recombinant adiponectin, we observed no decrease in proinflammatory cytokine secretion (Fig. 5A) or Clca1 gene expression (Fig. 5B), even at a high (10 μg/mL) dose. As interleukin-10 (IL-10) has been proposed to be an anti-inflammatory cytokine made by resident macrophages of healthy, lean adipose tissue, and is found to be produced at lower levels from HFD adipocytes (Fig. 1E), we next examined whether IL-10 may be serving in an immunomodulatory capacity in our system. Although there were no decreases in proinflammatory cytokine secretion from MTCCs treated with IL-10 for 24 h (Fig. 5C), IL-10 treatment induced decreases in Clca1 expression (Fig. 5D). However, inhibition of IL-10 in the Ad-CM did not affect cytokine secretion or gene expression in the MTCCs (Fig. 5, E and F). Finally, to reflect the immense increases in IL-6 observed in the Ad-CM from obese adipocytes (Fig. 1E), we treated MTCCs with recombinant IL-6 and observed modest but significant increases in proinflammatory cytokine secretion (Fig. 5G) and no changes in Clca1 expression (Fig. 5H). When a neutralizing IL-6 antibody was added to the Ad-CM, MIP-2 secretion was reduced from each of the LFD, HFD, and HFD-to-LFD Ad-CM groups (Fig. 5I). Finally, MTCCs were treated with a cocktail of cytokines that represented average cytokine values observed in the Ad-CM from either the LFD- or HFD-fed mice. Addition of the “HFD” cytokine cocktail significantly increased the levels of G-CSF secreted from MTCCs (Fig. 5J).
Figure 5.
Adiponectin and IL-10 are not responsible for the immunomodulatory effects of adipocytes upon airway epithelial cells, whereas IL-6 controls secretion of MIP-2. Mouse-transformed club cells (MTCCs) were treated with recombinant adiponectin and analyzed for proinflammatory cytokine secretion (A) and gene expression of Clca1 (B). MTCCs were treated with recombinant IL-10 and analyzed for cytokine secretion (C) and gene expression of Clca1 (D). In separate experiments, MTCCs were treated with lean and obese adipocyte-conditioned media (Ad-CM) in the presence or absence of anti-IL-10 neutralizing antibody, and analyzed for proinflammatory cytokine secretion (E) and Clca1 expression (F). MTCCs were treated with recombinant IL-6 and analyzed for cytokine secretion (G) and gene expression of Clca1 (H). In separate experiments, MTCCs were treated with lean and obese Ad-CM in the presence or absence of anti-IL-6 neutralizing antibody, and cytokine secretion was analyzed by ELISA (I). In separate experiments, MTCCs were treated with cytokine cocktails representative of their content as measured in LFD or HFD CM and analyzed for proinflammatory cytokine secretions (J). n = 3–8 samples/group. Data were analyzed by unpaired t test (A–D and G–I), 2-way ANOVA and Sidak’s multiple comparison test (E and F), or 1-way ANOVA and Dunnett’s multiple comparison test J). *P < 0.05, **P < 0.01, ***P < 0.005, and ****P < 0.001.
DISCUSSION
Though weight loss and lifestyle changes can aid in easing the symptoms of intrinsic obese asthma, a complete cure is not always achieved. Recent publications have lamented the findings that weight loss alone cannot reverse the damaging effects of morbid obesity (31). To better understand the effects of obese adipose tissue on intrinsic airway epithelial dysfunction, we generated conditioned media (CM) from the primary adipocytes of diet-induced lean mice, mice with obesity, and diet-induced mice with obesity that had undergone 6 wk of weight loss by switching to a low-fat diet. This CM was then used to challenge both primary epithelial cells (MTECs) and a bronchiolar epithelial cell line (MTCC). Dietary interventions reverse obesity-associated intrinsic and allergic methacholine hyperresponsiveness in the mice, but it is not known if any residual effects of obesity on airway function persist even after weight loss (32).
In earlier studies, we analyzed mice after only 3 wk of diet switching, as this was sufficient time for the mice with obesity (HFD) to lose weight and become comparable to lean, low-fat diet (LFD)-fed mice. However, analysis of the adipocyte CM at the 3-wk time point revealed no differences between the HFD mice and the HFD-to-LFD mice (data not shown). Adiponectin levels were still extremely low, and VAT and SVF characterization revealed no return to lean phenotypes. An extended study, in which mice were diet-switched for 12 wk, demonstrated a more thorough recovery from the obese phenotype. We therefore chose 6 wk as a feasible amount of time to examine the effects of diet-switch-induced weight loss.
Our results, presented herein, indicate a shift in adipose tissue composition under conditions of obesity that is consistent with previous reports (27, 33). Obese adipocytes from mice maintained on a high-fat diet lose the ability to produce adiponectin and IL-10 and increase secretion of the proinflammatory cytokines IL-6 and MCP-1. Analysis of the visceral adipose tissue stromal vascular fraction revealed a loss of eosinophils, as evidenced by the marked decrease in the presence of Siglecf and IL5ra mRNA, an increase in obesity-associated proinflammatory macrophages that express Lgals3 and Trem2, and a decrease in their expression of Retnla and Cd209f. After 6 wk of weight loss, the HFD-to-LFD mice demonstrated only a partial recovery to the lean state in these parameters.
Analysis of the primary tracheal epithelial cells from lean, obese, and diet-switched mice indicated fundamental differences in gene expression, suggesting an alteration in phenotype that persisted after 4 wk of differentiation at air-liquid interface. Fully differentiated MTECs revealed elevated proinflammatory cytokine production from the mice with obesity and an increased susceptibility to proinflammatory effects of the conditioned adipocyte media from the obese adipocytes. Mice that had undergone weight loss were able to partially mitigate some of these effects, but were not equivalent to the lean mice. These results were recapitulated in the MTCC cell line and also indicated that pre-exposure to HFD Ad-CM led to heightened inflammatory response to LPS (that was not due to altered expression of Tlr4).
We used the cell line 3T3L1-MBX to differentiate cells into adipocytes. When differentiation was performed in the presence of capsaicin, cells took on a mature brown adipocyte phenotype, as has been previously reported (29), with augmented production of adiponectin. Conditioned media from these adipocytes was able to reduce levels of KC and G-CSF secretion from MTCCs. This corroborates our findings that secreted factors from adipocytes can alter the responses of airway epithelial cells. The most likely candidate for immune alteration was adiponectin, with the literature indicating that adiponectin exerts important anti-inflammatory effects in addition to its critical function as a metabolic modulator (34). However, treatment of MTCCs with high doses of recombinant adiponectin failed to reduce proinflammatory cytokine production or gene expression. Another possible candidate for immunomodulation, produced by both healthy lean adipocytes and lean adipose tissue-resident macrophages, was interleukin-10. Although IL-10 was sufficient to decrease Clca1 expression, it did not dampen cytokine production from MTCCs, and addition of an IL-10-neutralizing antibody revealed that it could not alleviate the proinflammatory capabilities of the HFD Ad-CM.
Though MCP-1 production was increased in our HFD Ad-CM, we did not conduct mechanistic studies with MCP-1 augmentation or neutralization due to the lack of Ccr5 (the receptor for MCP-1) expression on airway epithelial cells (35, 36). However, it has been reported that IL-6 plays a key role as an exacerbating factor in the inflammation observed in obese adipose tissue (37) and that IL-6 is a modulator of airway epithelial cells and airway hyperresponsiveness (38–40). Addition of recombinant IL-6 to our MTCCs induced increases in KC and MIP-2 secretion, and neutralization of IL-6 in the Ad-CM was able to inhibit MIP-2 release. Combination of the cytokines in doses that represent the levels of those observed in the Ad-CM of LFD- and HFD-fed mice did not result in more significant modulation of KC and MIP-2 secretion; however, the “HFD” cocktail (low doses of rAdiponectin and IL-10 and a higher dose of rIL-6) did lead to augmented secretion of G-CSF. Consequently, IL-6 represents a rational biomarker and soluble mediator of the proinflammatory effects of visceral adipose tissue on airway epithelial cells.
Perspectives and Significance
The data presented herein indicate a critical role for adipocyte-secreted factors in the modulation of airway epithelial inflammation, and that although IL-6 emerges as important, the key players may not be predominantly soluble protein based. We have previously reported major differences in the microbiome of diet-induced lean and obese animals, and a body of literature suggests that microbiome-produced short-chain fatty acids (SCFAs) can circulate in the bloodstream and modulate processes at distal organ sites (41). Even more intriguing is the growing number of studies that have implicated roles for extracellular vesicles (EVs) such as exosomes that are differentially produced between the lean and obese state (42, 43). These non-protein secreted factors provide opportunity for further study. Our results also recapitulate that weight loss alone is not sufficient to completely restore a lean, healthy phenotype to the adipose tissue, and that immune-modulating therapies may be of benefit, or even required, to reverse obesity-induced immunometabolic dysfunction. A novel finding from our studies is that weight loss alone may not be sufficient to restore a lean, healthy phenotype to airway epithelium, indicating that effects of obesity on airway dysfunction may persist even after weight loss.
GRANTS
This work was funded by grants R01 HL133920 (to M.E.P. and A.E.D.) and R01 HL142081 (to M.E.P.)
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
J.L.A. and M.E.P. conceived and designed research; J.L.A., K.E.V.D.V, M.M.M., and L.F.R. performed experiments; J.L.A., M.M.M., and M.E.P. analyzed data; J.L.A. and M.E.P. interpreted results of experiments; J.L.A. prepared figures; J.L.A. drafted manuscript; J.L.A., A.E.D., and M.E.P. edited and revised manuscript; J.L.A., K.E.V.D.V., M.M.M., A.E.D., and M.E.P. approved final version of manuscript.
REFERENCES
- 1.Lovren F, Teoh H, Verma S. Obesity and atherosclerosis: mechanistic insights. Can J Cardiol 31: 177–183, 2015. doi: 10.1016/j.cjca.2014.11.031. [DOI] [PubMed] [Google Scholar]
- 2.Kahn SE, Hull RL, Utzschneider KM. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature 444: 840–846, 2006. doi: 10.1038/nature05482. [DOI] [PubMed] [Google Scholar]
- 3.Polyzos SA, Kountouras J, Mantzoros CS. Obesity and nonalcoholic fatty liver disease: From pathophysiology to therapeutics. Metabolism 92: 82–97, 2019. doi: 10.1016/j.metabol.2018.11.014. [DOI] [PubMed] [Google Scholar]
- 4.Wouters EFM. Obesity and metabolic abnormalities in chronic obstructive pulmonary disease. Ann Am Thorac Soc 14: S389–S94, 2017. doi: 10.1513/AnnalsATS.201705-371AW. [DOI] [PubMed] [Google Scholar]
- 5.Holguin F, Bleecker ER, Busse WW, Calhoun WJ, Castro M, Erzurum SC, Fitzpatrick AM, Gaston B, Israel E, Jarjour NN, Moore WC, Peters SP, Yonas M, Teague WG, Wenzel SE. Obesity and asthma: an association modified by age of asthma onset. J Allergy Clin Immunol 127: 1486–1493.e2, 2011. doi: 10.1016/j.jaci.2011.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Peters U, Dixon AE, Forno E. Obesity and asthma. J Allergy Clin Immunol 141: 1169–1179, 2018. doi: 10.1016/j.jaci.2018.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Granell R, Rodriguez S. Confirmed causal effect of obesity on asthma and new insights on potential underlying shared genetic mechanisms. J Allergy Clin Immunol 145: 484–486, 2020. doi: 10.1016/j.jaci.2019.11.033. [DOI] [PubMed] [Google Scholar]
- 8.Mohanan S, Tapp H, McWilliams A, Dulin M. Obesity and asthma: pathophysiology and implications for diagnosis and management in primary care. Exp Biol Med (Maywood) 239: 1531–1540, 2014. doi: 10.1177/1535370214525302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.MacLean PS, Higgins JA, Giles ED, Sherk VD, Jackman MR. The role for adipose tissue in weight regain after weight loss. Obes Rev 16, Suppl 1: 45–54, 2015. doi: 10.1111/obr.12255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van Baak MA, Mariman ECM. Mechanisms of weight regain after weight loss—the role of adipose tissue. Nat Rev Endocrinol 15: 274–287, 2019. doi: 10.1038/s41574-018-0148-4. [DOI] [PubMed] [Google Scholar]
- 11.Schoettl T, Fischer IP, Ussar S. Heterogeneity of adipose tissue in development and metabolic function. J Exp Biol 221: jeb162958, 2018. doi: 10.1242/jeb.162958. [DOI] [PubMed] [Google Scholar]
- 12.Carpentier AC, Blondin DP, Virtanen KA, Richard D, Haman F, Turcotte EE. Brown adipose tissue energy metabolism in humans. Front Endocrinol (Lausanne) 9: 447, 2018. doi: 10.3389/fendo.2018.00447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rosell M, Kaforou M, Frontini A, Okolo A, Chan YW, Nikolopoulou E, Millership S, Fenech ME, Maclntyre D, Turner JO, Moore JD, Blackburn E, Gullick WJ, Cinti S, Montana G, Parker MG, Christian M. Brown and white adipose tissues: intrinsic differences in gene expression and response to cold exposure in mice. Am J Physiol Endocrinol Metab 306: E945–E964, 2014. doi: 10.1152/ajpendo.00473.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schipper HS, Prakken B, Kalkhoven E, Boes M. Adipose tissue-resident immune cells: key players in immunometabolism. Trends Endocrinol Metab 23: 407–415, 2012. doi: 10.1016/j.tem.2012.05.011. [DOI] [PubMed] [Google Scholar]
- 15.Priceman SJ, Kujawski M, Shen S, Cherryholmes GA, Lee H, Zhang C, Kruper L, Mortimer J, Jove R, Riggs AD, Yu H. Regulation of adipose tissue T cell subsets by Stat3 is crucial for diet-induced obesity and insulin resistance. Proc Natl Acad Sci USA 110: 13079–13084, 2013. doi: 10.1073/pnas.1311557110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sideleva O, Black K, Dixon AE. Effects of obesity and weight loss on airway physiology and inflammation in asthma. Pulm Pharmacol Ther 26: 455–458, 2013. doi: 10.1016/j.pupt.2012.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nussbaum JC, Van Dyken SJ, von Moltke J, Cheng LE, Mohapatra A, Molofsky AB, Thornton EE, Krummel MF, Chawla A, Liang H-E, Locksley RM. Type 2 innate lymphoid cells control eosinophil homeostasis. Nature 502: 245–248, 2013. doi: 10.1038/nature12526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jaitin DA, Adlung L, Thaiss CA, Weiner A, Li B, Descamps H, Lundgren P, Bleriot C, Liu Z, Deczkowska A, Keren-Shaul H, David E, Zmora N, Eldar SM, Lubezky N, Shibolet O, Hill DA, Lazar MA, Colonna M, Ginhoux F, Shapiro H, Elinav E, Amit I. Lipid-associated macrophages control metabolic homeostasis in a Trem2-dependent manner. Cell 178: 686–698.e14, 2019. doi: 10.1016/j.cell.2019.05.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Giralt M, Villarroya F. White, brown, beige/brite: different adipose cells for different functions? Endocrinology 154: 2992–3000, 2013. doi: 10.1210/en.2013-1403. [DOI] [PubMed] [Google Scholar]
- 20.Shabalina IG, Petrovic N, de Jong JM, Kalinovich AV, Cannon B, Nedergaard J. UCP1 in brite/beige adipose tissue mitochondria is functionally thermogenic. Cell Rep 5: 1196–1203, 2013. doi: 10.1016/j.celrep.2013.10.044. [DOI] [PubMed] [Google Scholar]
- 21.Shore SA, Cho Y. Obesity and asthma: microbiome-metabolome interactions. Am J Respir Cell Mol Biol 54: 609–617, 2016. doi: 10.1165/rcmb.2016-0052PS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Alcorn JF, Guala AS, van der Velden J, McElhinney B, Irvin CG, Davis RJ, Janssen-Heininger YMW. Jun N-terminal kinase 1 regulates epithelial-to-mesenchymal transition induced by TGF-β1. J Cell Sci 121: 1036–1045, 2008. doi: 10.1242/jcs.019455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Magdaleno SM, Wang G, Jackson KJ, Ray MK, Welty S, Costa RH, DeMayo FJ. Interferon-gamma regulation of Clara cell gene expression: in vivo and in vitro. Am J Physiol Lung Cell Mol Physiol 272: L1142–L1151, 1997. doi: 10.1152/ajplung.1997.272.6.L1142. [DOI] [PubMed] [Google Scholar]
- 24.Landrier JF, Kasiri E, Karkeni E, Mihaly J, Beke G, Weiss K, Lucas R, Aydemir G, Salles J, Walrand S, de Lera AR, Ruhl R. Reduced adiponectin expression after high-fat diet is associated with selective up-regulation of ALDH1A1 and further retinoic acid receptor signaling in adipose tissue. FASEB J 31: 203–211, 2017. doi: 10.1096/fj.201600263RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bullen JW Jr, Bluher S, Kelesidis T, Mantzoros CS. Regulation of adiponectin and its receptors in response to development of diet-induced obesity in mice. Am J Physiol Endocrinol Metab 292: E1079–E1086, 2007. doi: 10.1152/ajpendo.00245.2006. [DOI] [PubMed] [Google Scholar]
- 26.Bolus WR, Kennedy AJ, Hasty AH. Obesity-induced reduction of adipose eosinophils is reversed with low-calorie dietary intervention. Physiol Rep 6: e13919, 2018. doi: 10.14814/phy2.13919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wu D, Molofsky AB, Liang HE, Ricardo-Gonzalez RR, Jouihan HA, Bando JK, Chawla A, Locksley RM. Eosinophils sustain adipose alternatively activated macrophages associated with glucose homeostasis. Science 332: 243–247, 2011. doi: 10.1126/science.1201475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Baskaran P, Krishnan V, Ren J, Thyagarajan B. Capsaicin induces browning of white adipose tissue and counters obesity by activating TRPV1 channel-dependent mechanisms. Br J Pharmacol 173: 2369–2389, 2016. doi: 10.1111/bph.13514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fan L, Xu H, Yang R, Zang Y, Chen J, Qin H. Combination of capsaicin and capsiate induces browning in 3T3-L1 white adipocytes via activation of the peroxisome proliferator-activated receptor γ/β3-adrenergic receptor signaling pathways. J Agric Food Chem 67: 6232–6240, 2019. doi: 10.1021/acs.jafc.9b02191. [DOI] [PubMed] [Google Scholar]
- 30.Ather JL, Poynter ME. Serum amyloid A3 is required for normal weight and immunometabolic function in mice. PLoS One 13: e0192352, 2018. doi: 10.1371/journal.pone.0192352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Abdullah A, Wolfe R, Mannan H, Stoelwinder JU, Stevenson C, Peeters A. Epidemiologic merit of obese-years, the combination of degree and duration of obesity. Am J Epidemiol 176: 99–107, 2012. doi: 10.1093/aje/kwr522. [DOI] [PubMed] [Google Scholar]
- 32.Ather JL, Chung M, Hoyt LR, Randall MJ, Georgsdottir A, Daphtary NA, Aliyeva MI, Suratt BT, Bates JHT, Irvin CG, Russell SR, Forgione PM, Dixon AE, Poynter ME. Weight loss decreases inherent and allergic methacholine hyperresponsiveness in mouse models of diet-induced obese asthma. Am J Respir Cell Mol Biol 55: 176–187, 2016. doi: 10.1165/rcmb.2016-0070OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lumeng CN, Bodzin JL, Saltiel AR. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest 117: 175–184, 2007. doi: 10.1172/JCI29881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Okamoto Y, Kihara S, Funahashi T, Matsuzawa Y, Libby P. Adiponectin: a key adipocytokine in metabolic syndrome. Clin Sci (Lond) 110: 267–278, 2006. doi: 10.1042/CS20050182. [DOI] [PubMed] [Google Scholar]
- 35.Du Y, Kitzmiller JA, Sridharan A, Perl AK, Bridges JP, Misra RS, Pryhuber GS, Mariani TJ, Bhattacharya S, Guo M, Potter SS, Dexheimer P, Aronow B, Jobe AH, Whitsett JA, Xu Y. Lung Gene Expression Analysis (LGEA): an integrative web portal for comprehensive gene expression data analysis in lung development. Thorax 72: 481–484, 2017. doi: 10.1136/thoraxjnl-2016-209598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Du Y, Guo M, Whitsett JA, Xu Y. ‘LungGENS’: a web-based tool for mapping single-cell gene expression in the developing lung. Thorax 70: 1092–1094, 2015. doi: 10.1136/thoraxjnl-2015-207035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ouchi N, Parker JL, Lugus JJ, Walsh K. Adipokines in inflammation and metabolic disease. Nat Rev Immunol 11: 85–97, 2011. doi: 10.1038/nri2921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jevnikar Z, Ostling J, Ax E, Calven J, Thorn K, Israelsson E; Unbiased Biomarkers in Prediction of Respiratory Disease Outcomes study group,, et al. Epithelial IL-6 trans-signaling defines a new asthma phenotype with increased airway inflammation. J Allergy Clin Immunol 143: 577–590, 2019. doi: 10.1016/j.jaci.2018.05.026. [DOI] [PubMed] [Google Scholar]
- 39.Tadokoro T, Wang Y, Barak LS, Bai Y, Randell SH, Hogan BL. IL-6/STAT3 promotes regeneration of airway ciliated cells from basal stem cells. Proc Natl Acad Sci USA 111: E3641–E3649, 2014. doi: 10.1073/pnas.1409781111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gubernatorova EO, Gorshkova EA, Namakanova OA, Zvartsev RV, Hidalgo J, Drutskaya MS, Tumanov AV, Nedospasov SA. Non-redundant functions of IL-6 produced by macrophages and dendritic cells in allergic airway inflammation. Front Immunol 9: 2718, 2018. doi: 10.3389/fimmu.2018.02718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chambers ES, Preston T, Frost G, Morrison DJ. Role of gut microbiota-generated short-chain fatty acids in metabolic and cardiovascular health. Curr Nutr Rep 7: 198–206, 2018. doi: 10.1007/s13668-018-0248-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kim A, Shah AS, Nakamura T. Extracellular vesicles: a potential novel regulator of obesity and its associated complications. Children (Basel) 5: 152, 2018. )doi: 10.3390/children5110152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Castano C, Kalko S, Novials A, Parrizas M. Obesity-associated exosomal miRNAs modulate glucose and lipid metabolism in mice. Proc Natl Acad Sci USA 115: 12158–12163, 2018. doi: 10.1073/pnas.1808855115. [DOI] [PMC free article] [PubMed] [Google Scholar]