Keywords: brain, diabetes, hypoglycemia, hypothalamus, insulin
Abstract
The brain has been traditionally thought to be insensitive to insulin, primarily because insulin does not stimulate glucose uptake/metabolism in the brain (as it does in classic insulin-sensitive tissues such as muscle, liver, and fat). However, over the past 20 years, research in this field has identified unique actions of insulin in the brain. There is accumulating evidence that insulin crosses into the brain and regulates central nervous system functions such as feeding, depression, and cognitive behavior. In addition, insulin acts in the brain to regulate systemic functions such as hepatic glucose production, lipolysis, lipogenesis, reproductive competence, and the sympathoadrenal response to hypoglycemia. Decrements in brain insulin action (or brain insulin resistance) can be observed in obesity, type 2 diabetes (T2DM), aging, and Alzheimer’s disease (AD), indicating a possible link between metabolic and cognitive health. Here, we describe recent findings on the pleiotropic actions of insulin in the brain and highlight the precise sites, specific neuronal population, and roles for supportive astrocytic cells through which insulin acts in the brain. In addition, we also discuss how boosting brain insulin action could be a therapeutic option for people at an increased risk of developing metabolic and cognitive diseases such as AD and T2DM. Overall, this perspective article serves to highlight some of these key scientific findings, identify unresolved issues, and indicate future directions of research in this field that would serve to improve the lives of people with metabolic and cognitive dysfunctions.
HOW INSULIN ENTERS THE BRAIN
After being secreted from pancreatic β-cells, insulin crosses into the brain by either, 1) circumventing the blood-brain barrier (BBB) crossing the median eminence, or 2) crossing the vascular endothelium via transport proteins (1–3). Transport of insulin into the brain is highly regulated and may be altered by states such as obesity, diabetes mellitus, fasting, and Alzheimer’s disease (AD) (1). The mechanism(s) of altered insulin transport by these disease states is incompletely understood, but in diabetes, BBB disruption may be mediated by altered insulin transport proteins, pericytes loss, and altered cerebral microvessel expression of tight junction proteins (2, 4, 5). Insulin actions on the cerebral blood flow is region specific and may also be altered in insulin-resistant states (6–10). Regional-specific actions of insulin in the brain may be based on sites of increased insulin receptor expression within the olfactory bulb, hypothalamus, hippocampus, cerebral cortex, and cerebellum (11, 12). Further specificity of brain insulin actions are likely dependent on the regional distribution of insulin receptor isoforms and cell-type differences in receptor density (i.e., neurons vs. glia) (13).
Early studies in rodents (and subsequent human studies) identified insulin mRNA in the central nervous system (CNS), thus hinting at the possibility that a small amount of insulin may be synthesized, de novo, within the brain (14–20). Physiological roles for localized insulin synthesized in the brain have been identified (21–23). Recently, Lee et al. (21) detected insulin mRNA in the paraventricular nucleus and identified its role in the regulation of pituitary growth hormone production.
To specifically study insulin action in the brain, animal studies often make use of (albeit nonphysiological) insulin infusion into the brain or targeted genetic manipulations of the insulin receptor, whereas human studies make use of intranasal insulin dosing to selectively increase brain insulin levels. The pros and cons of these techniques have been reported (and include issues with possible supratherapeutic dosing, regional-specific effects, and issues of systemic spillage); however, the consistency of results using different techniques and across species strengthen the body of work demonstrating insulin action in the brain (24–30).
BRAIN INSULIN ACTION REGULATES CNS FUNCTIONS
Neuronal Development and Neuronal Survival
Insulin action is critically important in the developing nervous system, directing differentiation, proliferation, and neurite growth (31–33). Insulin acts via its receptor to stimulate brain growth, as noted by increased expression and activity in developing brain tissues, both within neurons and surrounding glial cells (34, 35). Insulin also acts as a neuroprotector, preventing damage induced by ischemia, β-amyloid toxicity, oxidative stress, and apoptosis (36–39). Thus, brain insulin action may play a role in preventing the development of dementia. Conversely, these protective actions of insulin may be diminished in the setting of impaired brain insulin signaling (40), thereby increasing the risk for both neuropsychiatric and neurogenerative disorders (40–42), and thereby linking brain insulin resistance (i.e., lack of insulin action in brain) with cognitive dysfunction and depression.
Insulin Effects on Anxiety and Depression
Studies in animals support the concept that insulin action in the brain can regulate anxiety and depression. The knockdown of insulin receptors in the hypothalamus in animals has shown to trigger depressive and anxiety-like behaviors (43). Similarly, neuronal-specific insulin receptor knockout (NIRKO) mice demonstrated depression-like behaviors (42). Indeed, the knockdown of insulin receptors, specifically in astrocytes, also results in increased anxiety and depressive-like behavior in mice, via decreasing dopamine release (44). Consistent with these rodent findings, postmortem brain tissue of patients with mood and psychotic disorders demonstrated a lower expression of insulin receptor-related signaling genes, again associating decreased insulin action with a downregulation of dopaminergic signaling (45). Clinically, intranasal administration of insulin (which specifically increases brain insulin levels) has been shown to be beneficial in blunting stress in healthy subjects (46, 47). Collectively, these studies support the notion that insulin action in the brain may be an important component of mood regulation and neuropsychiatric disorders.
Insulin Action in the Brain Regulates Cognition/Alzheimer’s Disease
Evidence from human and animal studies indicate that decreased insulin action in the brain (i.e., brain insulin resistance) and/or deficiency of brain insulin is a pathological feature of metabolic and cognitive dysfunctions, including obesity, type 2 diabetes (T2DM), and Alzheimer’s disease (AD) (48, 49). Specifically, the impairment in brain insulin signaling has shown to increase hyperphosphorylation of tau protein (50) and β-amyloid (Aβ) accumulation (51), which are the neuropathological hallmarks of AD. People with T2DM are at a higher risk of developing AD and cognitive decline (52, 53). Brain insulin resistance has been documented in patients with AD (54) and the term “type 3 diabetes” has been used to identify this brain type of T2DM (55). Given the beneficial action of insulin in the brain, intranasal insulin administration has been shown to provide cognitive benefits in healthy humans as well as in patients with T2DM and AD (56–62).
Insulin Action on Brain Cholesterol Synthesis
Cholesterol is an important constituent of cell membrane, which plays an important role in cellular physiology and signaling (63, 64). Changes in brain cholesterol metabolism have been implicated in diabetes (65) and AD (66), suggesting that altered brain cholesterol metabolism is another possible link between diabetes and AD. The impaired brain cholesterol biosynthesis that occurs in diabetes can be restored with increased insulin action in the brain (65). The action of insulin on brain cholesterol metabolism is not only restricted to neurons but also occur in glial cells, especially astrocytes, and plays an important role in brain development and behavioral functions (65, 67).
Body Weight and Food Intake
Of all insulin’s actions in the brain, insulin’s action in regulating body weight and feeding behavior has been most convincingly established (68). Insulin action in the brain to reduce food intake and body weight have been linked to insulin’s ability to alter the expression of critical body weight regulating neuropeptides [e.g., neuropeptide Y, agouti-related peptide, pro-opiomelanocortin (POMC), and amphetamine-regulated transcript] in the arcuate nucleus of the hypothalamus (69–72).
Following intranasal administration, insulin has been shown to decrease appetite, food palatability, and reduce food intake and body weight (73, 74). Imaging studies have shown that intranasal insulin administration in the presence of food-related images leads to a reduction of activity in areas of the brain involved in memory and object processing, suggesting that insulin reduces the wanting of food (70).
Although insulin action in the brain has been shown to reduce body weight, treatment with insulin often leads to weight gain (75), indicating that when delivered peripherally, insulin’s anabolic actions on peripheral tissues (e.g., adipocytes) are dominant.
BRAIN INSULIN ACTION REGULATES PERIPHERAL FUNCTIONS
Hepatic Glucose Production
Insulin acts to lower blood glucose levels by its suppressive effects on hepatic glucose production (HGP). In addition to direct effects on the liver, there is increasing evidence that insulin acts in the brain to indirectly suppress HGP, as recently reviewed (76, 77).
Studies in rodents consistently demonstrated that insulin acts in the brain to suppress HGP (27, 78) via several mechanisms including action on pro-opiomelanocortin neurons (79), agouti-related protein neurons (80), and hepatic vagal efferents (81). In contrast to rodent studies, an elegant series of studies in dogs demonstrate that the insulin actions to acutely suppress HGP are attributable to insulin’s direct effect on the liver and that indirect effects of insulin are redundant (28, 29, 82, 83). Thus, there is ongoing debate regarding the physiological relevance of brain insulin action in regulating HGP (84, 85). In humans, some (74, 86) but not all (87, 88) studies demonstrated an effect of intranasal insulin to augment suppression of HGP, although early studies were possibly confounded by spillover of insulin into the systemic circulation. More recent studies that controlled for insulin spillover did demonstrate an effect of intranasal insulin to suppress HGP (24, 25). Particularly interesting was the evidence for brain insulin resistance in obese and type 2 diabetic subjects, as these subjects failed to demonstrate an effect of increased brain insulin in suppressing HGP, which was noted in healthy controls (25, 87). These findings support the concept that the brain can sense insulin and alter hepatic glucose production, but whether this represents a physiological role of insulin remains to be established.
Lipolysis and Lipogenesis
In rodents, intracerebroventricular infusion of insulin induces lipogenesis (89), and insulin infusion into the mediobasal hypothalamus suppresses systemic lipolysis by dampening of sympathetic nervous system outflow to white adipose tissue (WAT) (90). This suppressive effect on lipolysis may be mediated by insulin action on POMC neurons (91). Consistent with this effect of brain insulin action, NIRKO mice lacking the neuronal insulin receptor showed increased WAT lipolysis and disrupted lipogenesis (90). Corroborating these animal studies, intranasal insulin administration in humans was shown to suppress systemic lipolysis and reduce levels of circulating free fatty acids (92).
Reproductive Competence
In addition to regulating glucose homeostasis and adiposity, insulin action in the brain regulates reproductive competence. Studies in NIRKO mice have demonstrated that the lack of insulin action in the brain leads to hypothalamic luteinizing hormone (LH) dysregulation that results in reduced fertility due to decreased spermatogenesis in males and impaired ovarian follicle maturation in females (93). Consistent with these observations, intracerebroventricular insulin infusion in rats with diabetes has been shown to restore and normalize LH surges that are necessary for ovulation (94). Another recent study in mice demonstrated that impaired insulin signaling in astrocytes dramatically reduces adult reproductive competence due to dysfunction of the hypothalamic-pituitary-gonadotropin axis (95). Thus, independent of neuronal insulin signaling, insulin signaling in astrocytes appears to play an important role in regulating reproductive competence.
Role of Insulin in Mediating the Counterregulatory Response to Hypoglycemia and CNS Glucose Sensing
Many animal and human studies (96–99), but not all (100–102), have shown that insulin acts acutely in the brain to augment the counterregulatory response to hypoglycemia. Consistent with the positive effect of central insulin action in augmenting counterregulation, NIRKO mice have impaired CNS glucose sensing and a blunted sympathoadrenal response to insulin-induced hypoglycemia (99). This blunted CNS glucose sensing may be mediated by insulin action on neuronal glucose transporter 4 (99, 103, 104) or astrocyte insulin signaling (105, 106). Thus, in addition to acute actions of insulin in the brain to augment the counterregulatory response to hypoglycemia, insulin also acts chronically in the brain to regulate CNS glucose sensing, and thus, the counterregulatory response to hypoglycemia. To support this notion, we recently demonstrated that in rats with diabetes, insulin deficiency impairs the sympathoadrenal response to hypoglycemia and that chronic insulin infusion into the ventromedial hypothalamus alone is sufficient to normalize the sympathoadrenal response to hypoglycemia (107). These studies suggest that insulin action, particularly in hypothalamic region, is required for brain glucose sensing. Thus, insulin acts in a paradoxical fashion by both causing hypoglycemia via dominant glucose lowering actions on peripheral tissues (e.g., liver, muscle, and fat), yet also acts to protect against hypoglycemia by augmenting the brain’s ability to detect and mount a counterregulatory response.
PERSPECTIVE AND FUTURE DIRECTIONS
Scientists no longer question whether insulin acts in the brain but now seek to delineate specific actions of insulin in the brain (Fig. 1, Table 1). Identifying mechanisms of insulin transport into the brain and specific areas of insulin action will help to enhance our understanding in the field. Future novel transgenic models will continue to advance the field by precisely identifying sites (i.e., neuronal and/or astrocytic) and mechanisms by which insulin exerts its actions in the brain. Though studies in humans have been limited, targeting brain insulin action via intranasal insulin delivery has validated earlier preclinical findings regarding brain insulin action. Unfortunately, the role of insulin action in the brain has been less well characterized under pathophysiological settings in humans (i.e., insulin-resistant T2DM). Since clinical studies are often confounded by comorbid conditions, a clearer link between brain insulin resistance and cognitive dysfunction/AD is needed. Another unanswered question is whether interventions known to increase peripheral insulin sensitivity (exercise, weight loss, and insulin-sensitizing drugs) act similarly in the brain to reap the benefits of improved CNS insulin signaling. In addition to further studies with intranasal insulin delivery, future efforts to create insulin analogs that preferentially target brain action are needed. Future strategies to exploit the multiple unique and beneficial actions of insulin in the brain seem warranted.
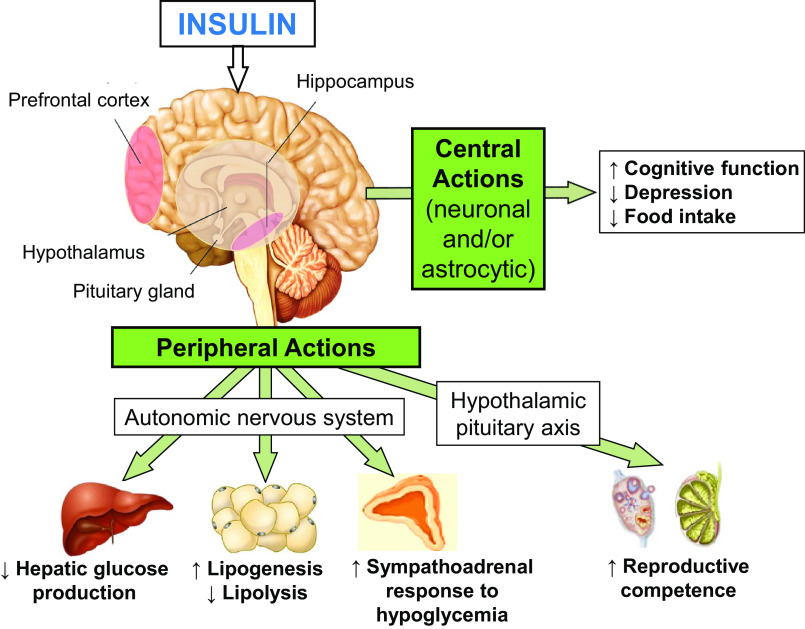
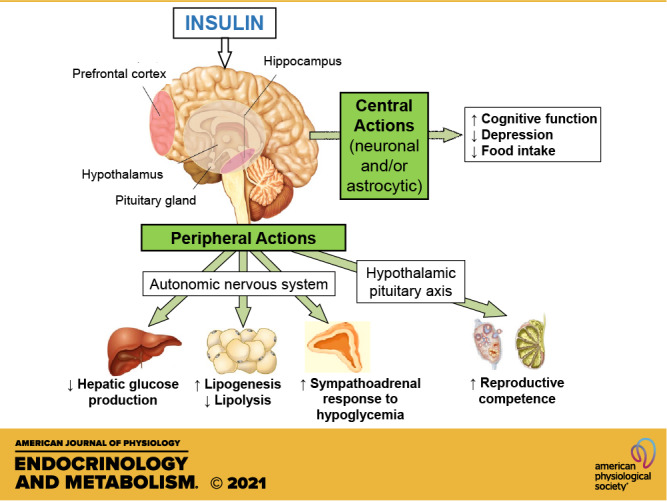
Figure 1.
In addition to regulating CNS (via neurons and/or astrocytes) functions (such as cognition, depression, and food intake), insulin acts in the brain to regulate peripheral functions via the autonomic nervous system (ANS) and the hypothalamic pituitary axis (HPA). Specifically, insulin acts in the prefrontal cortex and hippocampus to improve cognitive function and reduce depressive symptoms. Insulin acts in the hypothalamic nuclei to decrease food intake and reduce body weight. Via the efferent autonomic nervous system to target organs, insulin acts in the brain to decrease hepatic glucose production, increase lipogenesis, decrease lipolysis, and increase the sympathoadrenal response to hypoglycemia. Insulin acts via the hypothalamic-pituitary-gonadal axis to improve reproductive competence. CNS, central nervous system.
Table 1.
Contrasting effects of insulin action on peripheral organs versus action in the central nervous system
| Function | Peripheral Action | Central Action |
|---|---|---|
| Metabolism | Anabolic (75, 108) | Catabolic (109) |
| Muscle glucose uptake | ↑ (110,111) | – |
| Lipolysis | ↓ (112) | ↓ (90–92) |
| Lipogenesis | ↑ (113) | ↑ (89, 90) |
| Hypoglycemia | ↑ (114) | ↓*(97–99, 107) |
| Hepatic glucose production | ↓(82) | ↓ (24, 25, 27, 74, 76–79, 84–86, 115, 116) |
| Reproductive competence | ↑ (117) | ↑ (93–95, 118) |
| Neuroprotective | – | ↑ (36–39, 119, 120) |
| Cognition | – | ↑ (56–59, 61, 73, 121) |
| Depression | – | ↓ (42–46, 122) |
| Food intake | ↑ (123, 124) | ↓ (70, 71, 73, 74, 125) |
In addition to insulin’s direct effects on classic insulin sensitive tissues (muscle, fat, and liver), insulin acts in the brain to regulate peripheral tissues indirectly (via the autonomic nervous system and the hypothalamic pituitary axis). Some of insulin’s actions in the brain parallel its peripheral actions, but, perhaps acting in a homeostatic fashion, some of insulin’s action in the brain oppose its peripheral action.
*Insulin acts centrally to augment the counterregulatory responses, thus acting to reduce hypoglycemia.
GRANTS
This work was supported by funding from the National Institutes of Health Grants NS070235 and DK118082 (to S.J.F), by Juvenile Diabetes Research Foundation Grant 1-FAC-2020-984-A-N (to C.M.R.), and by the University of Utah’s Diabetes and Metabolism Research Center.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
R.A. conceived and designed research; R.A. and S.J.F. prepared figures; R.A., C.M.R., S.S., Y.H., and S.J.F. drafted manuscript; R.A., C.M.R., S.S., C.C., Y.H., and S.J.F. edited and revised manuscript; R.A., C.M.R., S.S., C.C., Y.H., and S.J.F. approved final version of manuscript.
ACKNOWLEDGMENTS
We sincerely thank collaborators, mentors, and all members of our laboratory, past and present.
REFERENCES
- 1.Banks WA, Kastin AJ. Differential permeability of the blood-brain barrier to two pancreatic peptides: insulin and amylin. Peptides 19: 883–889, 1998. doi: 10.1016/s0196-9781(98)00018-7. [DOI] [PubMed] [Google Scholar]
- 2.Gray SM, Barrett EJ. Insulin transport into the brain. Am J Physiol Cell Physiol 315: C125–C136, 2018. doi: 10.1152/ajpcell.00240.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Haddad-Tovolli R, Dragano NRV, Ramalho AFS, Velloso LA. Development and function of the blood-brain barrier in the context of metabolic control. Front Neurosci 11: 224, 2017. doi: 10.3389/fnins.2017.00224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Banks W. The blood-brain barrier interface in diabetes mellitus: dysfunctions, mechanisms and approaches to treatment. Curr Pharm Design 26: 1438–1447, 2020. doi: 10.2174/1381612826666200325110014. [DOI] [PubMed] [Google Scholar]
- 5.Sun YN, Liu LB, Xue YX, Wang P. Effects of insulin combined with idebenone on blood-brain barrier permeability in diabetic rats. J Neurosci Res 93: 666–677, 2015. doi: 10.1002/jnr.23511. [DOI] [PubMed] [Google Scholar]
- 6.Akintola AA, van Opstal AM, Westendorp RG, Postmus I, van der Grond J, van Heemst D. Effect of intranasally administered insulin on cerebral blood flow and perfusion; a randomized experiment in young and older adults. Aging (Albany NY) 9: 790–802, 2017. doi: 10.18632/aging.101192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ryan JP, Sheu LK, Verstynen TD, Onyewuenyi IC, Gianaros PJ. Cerebral blood flow links insulin resistance and baroreflex sensitivity. PLoS One 8: e83288, 2013. doi: 10.1371/journal.pone.0083288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schilling TM, Ferreira de Sa DS, Westerhausen R, Strelzyk F, Larra MF, Hallschmid M, Savaskan E, Oitzl MS, Busch HP, Naumann E, Schachinger H. Intranasal insulin increases regional cerebral blood flow in the insular cortex in men independently of cortisol manipulation. Hum Brain Mapp 35: 1944–1956, 2014. doi: 10.1002/hbm.22304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Williams VJ, Trombetta BA, Jafri RZ, Koenig AM, Wennick CD, Carlyle BC, Ekhlaspour L, Ahima RS, Russell SJ, Salat DH, Arnold SE. Task-related fMRI BOLD response to hyperinsulinemia in healthy older adults. JCI Insight 4: e129700, 2019. doi: 10.1172/jci.insight.129700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wingrove JO, O'Daly O, Forbes B, Swedrowska M, Amiel SA, Zelaya FO. Intranasal insulin administration decreases cerebral blood flow in cortico-limbic regions: a neuropharmacological imaging study in normal and overweight males. Diabetes Obes Metab 23: 175–185, 2021. doi: 10.1111/dom.14213. [DOI] [PubMed] [Google Scholar]
- 11.Havrankova J, Roth J. Insulin receptors are widely distributed in central nervous-system of rat. Nature 272: 827–829, 1978. doi: 10.1038/272827a0. [DOI] [PubMed] [Google Scholar]
- 12.Unger J, Mcneill TH, Moxley RT, White M, Moss A, Livingston JN. Distribution of insulin receptor-like immunoreactivity in the rat forebrain. Neuroscience 31: 143–157, 1989. doi: 10.1016/0306-4522(89)90036-5. [DOI] [PubMed] [Google Scholar]
- 13.Pomytkin I, Costa-Nunes JP, Kasatkin V, Veniaminova E, Demchenko A, Lyundup A, Lesch KP, Ponomarev ED, Strekalova T. Insulin receptor in the brain: mechanisms of activation and the role in the CNS pathology and treatment. CNS Neurosci Ther 24: 763–774, 2018. doi: 10.1111/cns.12866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Deltour L, Leduque P, Blume N, Madsen O, Dubois P, Jami J, Bucchini D. Differential expression of the two nonallelic proinsulin genes in the developing mouse embryo. Proc Natl Acad Sci USA 90: 527–531, 1993. doi: 10.1073/pnas.90.2.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Devaskar SU, Giddings SJ, Rajakumar PA, Carnaghi LR, Menon RK, Zahm DS. Insulin gene expression and insulin synthesis in mammalian neuronal cells. J Biol Chem 269: 8445–8454, 1994. doi: 10.1016/S0021-9258(17)37214-9. [DOI] [PubMed] [Google Scholar]
- 16.Devaskar SU, Singh BS, Carnaghi LR, Rajakumar PA, Giddings SJ. Insulin II gene expression in rat central nervous system. Regul Pept 48: 55–63, 1993. doi: 10.1016/0167-0115(93)90335-6. [DOI] [PubMed] [Google Scholar]
- 17.Dorn A, Rinne A, Hahn HJ, Bernstein HG, Ziegler M. C-peptide immunoreactive neurons in human brain. Acta Histochem 70: 326–330, 1982. doi: 10.1016/S0065-1281(82)80080-9. [DOI] [PubMed] [Google Scholar]
- 18.Frolich L, Blum-Degen D, Bernstein HG, Engelsberger S, Humrich J, Laufer S, Muschner D, Thalheimer A, Turk A, Hoyer S, Zochling R, Boissl KW, Jellinger K, Riederer P. Brain insulin and insulin receptors in aging and sporadic Alzheimer's disease. J Neural Transm (Vienna) 105: 423–438, 1998. doi: 10.1007/s007020050068. [DOI] [PubMed] [Google Scholar]
- 19.Havrankova J, Schmechel D, Roth J, Brownstein M. Identification of insulin in rat brain. Proc Natl Acad Sci USA 75: 5737–5741, 1978. doi: 10.1073/pnas.75.11.5737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Young WS, 3rd. Periventricular hypothalamic cells in the rat brain contain insulin mRNA. Neuropeptides 8: 93–97, 1986. doi: 10.1016/0143-4179(86)90035-1. [DOI] [PubMed] [Google Scholar]
- 21.Lee J, Kim K, Cho JH, Bae JY, O’Leary TP, Johnson JD, Bae YC, Kim E-K. Insulin synthesized in the paraventricular nucleus of the hypothalamus regulates pituitary growth hormone production. JCI Insight 5: e135412, 2020. doi: 10.1172/jci.insight.135412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mazucanti CH, Liu Q-R, Lang D, Huang N, O’Connell JF, Camandola S, Egan JM. Release of insulin produced by the choroid plexis is regulated by serotonergic signaling. JCI Insight 4: e131682, 2019. doi: 10.1172/jci.insight.131682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Molnar G, Farago N, Kocsis AK, Rozsa M, Lovas S, Boldog E, Baldi R, Csajbok E, Gardi J, Puskas LG, Tamas G. GABAergic neurogliaform cells represent local sources of insulin in the cerebral cortex. J Neurosci 34: 1133–1137, 2014. doi: 10.1523/JNEUROSCI.4082-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dash S, Xiao C, Morgantini C, Koulajian K, Lewis GF. Intranasal insulin suppresses endogenous glucose production in humans compared with placebo in the presence of similar venous insulin concentrations. Diabetes 64: 766–774, 2015. doi: 10.2337/db14-0685. [DOI] [PubMed] [Google Scholar]
- 25.Heni M, Wagner R, Kullmann S, Gancheva S, Roden M, Peter A, Stefan N, Preissl H, Haring HU, Fritsche A. Hypothalamic and striatal insulin action suppresses endogenous glucose production and may stimulate glucose uptake during hyperinsulinemia in lean but not in overweight men. Diabetes 66: 1797–1806, 2017. doi: 10.2337/db16-1380. [DOI] [PubMed] [Google Scholar]
- 26.Lochhead JJ, Thorne RG. Intranasal delivery of biologics to the central nervous system. Adv Drug Deliver Rev 64: 614–628, 2012. doi: 10.1016/j.addr.2011.11.002. [DOI] [PubMed] [Google Scholar]
- 27.Obici S, Zhang BB, Karkanias G, Rossetti L. Hypothalamic insulin signaling is required for inhibition of glucose production. Nat Med 8: 1376–1382, 2002. doi: 10.1038/nm1202-798. [DOI] [PubMed] [Google Scholar]
- 28.Ramnanan CJ, Kraft G, Smith MS, Farmer B, Neal D, Williams PE, Lautz M, Farmer T, Donahue EP, Cherrington AD, Edgerton DS. Interaction between the central and peripheral effects of insulin in controlling hepatic glucose metabolism in the conscious dog. Diabetes 62: 74–84, 2013. doi: 10.2337/db12-0148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ramnanan CJ, Saraswathi V, Smith MS, Donahue EP, Farmer B, Farmer TD, Neal D, Williams PE, Lautz M, Mari A, Cherrington AD, Edgerton DS. Brain insulin action augments hepatic glycogen synthesis without suppressing glucose production or gluconeogenesis in dogs. J Clin Invest 121: 3713–3723, 2011. doi: 10.1172/JCI45472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Woods SC, Lotter EC, Mckay LD, Porte D. Chronic intracerebroventricular infusion of insulin reduces food-intake and body-weight of baboons. Nature 282: 503–505, 1979. doi: 10.1038/282503a0. [DOI] [PubMed] [Google Scholar]
- 31.Apostolatos A, Song S, Acosta S, Peart M, Watson JE, Bickford P, Cooper DR, Patel NA. Insulin promotes neuronal survival via the alternatively spliced protein kinase CdeltaII isoform. J Biol Chem 287: 9299–9310, 2012. doi: 10.1074/jbc.M111.313080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chiu SL, Cline HT. Insulin receptor signaling in the development of neuronal structure and function. Neural Dev 5: 7, 2010. doi: 10.1186/1749-8104-5-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mamik MK, Asahchop EL, Chan WF, Zhu Y, Branton WG, McKenzie BA, Cohen EA, Power C. Insulin treatment prevents neuroinflammation and neuronal injury with restored neurobehavioral function in models of HIV/AIDS neurodegeneration. J Neurosci 36: 10683–10695, 2016. doi: 10.1523/JNEUROSCI.1287-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Clarke DW, Boyd FT, Jr., Kappy MS, Raizada MK. Insulin stimulates macromolecular synthesis in cultured glial cells from rat brain. Am J Physiol Cell Physiol 249: C484–C489, 1985. doi: 10.1152/ajpcell.1985.249.5.C484. [DOI] [PubMed] [Google Scholar]
- 35.Wozniak M, Rydzewski B, Baker SP, Raizada MK. The cellular and physiological actions of insulin in the central nervous system. Neurochem Int 22: 1–10, 1993. doi: 10.1016/0197-0186(93)90062-a. [DOI] [PubMed] [Google Scholar]
- 36.Duarte AI, Santos MS, Seica R, de Oliveira CR. Insulin affects synaptosomal GABA and glutamate transport under oxidative stress conditions. Brain Res 977: 23–30, 2003. doi: 10.1016/s0006-8993(03)02679-9. [DOI] [PubMed] [Google Scholar]
- 37.Reno CM, Tanoli T, Bree A, Daphna-Iken D, Cui C, Maloney SE, Wozniak DF, Fisher SJ. Antecedent glycemic control reduces severe hypoglycemia-induced neuronal damage in diabetic rats. Am J Physiol Endocrinol Metab 304: E1331–E1337, 2013. doi: 10.1152/ajpendo.00084.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rensink AA, Otte-Holler I, de Boer R, Bosch RR, ten Donkelaar HJ, de Waal RM, Verbeek MM, Kremer B. Insulin inhibits amyloid beta-induced cell death in cultured human brain pericytes. Neurobiol Aging 25: 93–103, 2004. doi: 10.1016/s0197-4580(03)00039-3. [DOI] [PubMed] [Google Scholar]
- 39.Ryu BR, Ko HW, Jou I, Noh JS, Gwag BJ. Phosphatidylinositol 3-kinase-mediated regulation of neuronal apoptosis and necrosis by insulin and IGF-I. J Neurobiol 39: 536–546, 1999. [PubMed] [Google Scholar]
- 40.Soto M, Cai W, Konishi M, Kahn CR. Insulin signaling in the hippocampus and amygdala regulates metabolism and neurobehavior. Proc Natl Acad Sci USA 116: 6379–6384, 2019. doi: 10.1073/pnas.1817391116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Holscher C. Insulin signaling impairment in the brain as a risk factor in Alzheimer's disease. Front Aging Neurosci 11: 88, 2019. doi: 10.3389/fnagi.2019.00088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kleinridders A, Cai W, Cappellucci L, Ghazarian A, Collins WR, Vienberg SG, Pothos EN, Kahn CR. Insulin resistance in brain alters dopamine turnover and causes behavioral disorders. Proc Natl Acad Sci USA 112: 3463–3468, 2015. doi: 10.1073/pnas.1500877112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Grillo CA, Piroli GG, Kaigler KF, Wilson SP, Wilson MA, Reagan LP. Downregulation of hypothalamic insulin receptor expression elicits depressive-like behaviors in rats. Behav Brain Res 222: 230–235, 2011. doi: 10.1016/j.bbr.2011.03.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cai W, Xue C, Sakaguchi M, Konishi M, Shirazian A, Ferris HA, Li ME, Yu R, Kleinridders A, Pothos EN, Kahn CR. Insulin regulates astrocyte gliotransmission and modulates behavior. J Clin Invest 128: 2914–2926, 2018. doi: 10.1172/JCI99366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mansur RB, Fries GR, Subramaniapillai M, Frangou S, De Felice FG, Rasgon N, McEwen B, Brietzke E, McIntyre RS. Expression of dopamine signaling genes in the post-mortem brain of individuals with mental illnesses is moderated by body mass index and mediated by insulin signaling genes. J Psychiatr Res 107: 128–135, 2018. doi: 10.1016/j.jpsychires.2018.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bohringer A, Schwabe L, Richter S, Schachinger H. Intranasal insulin attenuates the hypothalamic-pituitary-adrenal axis response to psychosocial stress. Psychoneuroendocrinology 33: 1394–1400, 2008. doi: 10.1016/j.psyneuen.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 47.Kern W, Born J, Schreiber H, Fehm HL. Central nervous system effects of intranasally administered insulin during euglycemia in men. Diabetes 48: 557–563, 1999. doi: 10.2337/diabetes.48.3.557. [DOI] [PubMed] [Google Scholar]
- 48.Kullmann S, Heni M, Hallschmid M, Fritsche A, Preissl H, Haring HU. Brain insulin resistance at the crossroads of metabolic and cognitive disorders in humans. Physiol Rev 96: 1169–1209, 2016. doi: 10.1152/physrev.00032.2015. [DOI] [PubMed] [Google Scholar]
- 49.Laws SM, Gaskin S, Woodfield A, Srikanth V, Bruce D, Fraser PE, Porter T, Newsholme P, Wijesekara N, Burnham S, Dore V, Li QX, Maruff P, Masters CL, Rainey-Smith S, Rowe CC, Salvado O, Villemagne VL, Martins RN, Verdile G. Insulin resistance is associated with reductions in specific cognitive domains and increases in CSF tau in cognitively normal adults. Sci Rep 7: 9766, 2017. doi: 10.1038/s41598-017-09577-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schubert M, Gautam D, Surjo D, Ueki K, Baudler S, Schubert D, Kondo T, Alber J, Galldiks N, Kustermann E, Arndt S, Jacobs AH, Krone W, Kahn CR, Bruning JC. Role for neuronal insulin resistance in neurodegenerative diseases. Proc Natl Acad Sci USA 101: 3100–3105, 2004. doi: 10.1073/pnas.0308724101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chua LM, Lim ML, Chong PR, Hu ZP, Cheung NS, Wong BS. Impaired neuronal insulin signaling precedes Abeta42 accumulation in female AbetaPPsw/PS1DeltaE9 mice. J Alzheimers Dis 29: 783–791, 2012. doi: 10.3233/JAD-2012-111880. [DOI] [PubMed] [Google Scholar]
- 52.Chatterjee S, Mudher A. Alzheimer's disease and type 2 diabetes: a critical assessment of the shared pathological traits. Front Neurosci 12: 383, 2018. doi: 10.3389/fnins.2018.00383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Han W, Li C. Linking type 2 diabetes and Alzheimer's disease. Proc Natl Acad Sci USA 107: 6557–6558, 2010. doi: 10.1073/pnas.1002555107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Talbot K, Wang HY, Kazi H, Han LY, Bakshi KP, Stucky A, Fuino RL, Kawaguchi KR, Samoyedny AJ, Wilson RS, Arvanitakis Z, Schneider JA, Wolf BA, Bennett DA, Trojanowski JQ, Arnold SE. Demonstrated brain insulin resistance in Alzheimer's disease patients is associated with IGF-1 resistance, IRS-1 dysregulation, and cognitive decline. J Clin Invest 122: 1316–1338, 2012. doi: 10.1172/JCI59903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.de la Monte SM, Wands JR. Alzheimer's disease is type 3 diabetes-evidence reviewed. J Diabetes Sci Technol 2: 1101–1113, 2008. doi: 10.1177/193229680800200619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Benedict C, Hallschmid M, Schmitz K, Schultes B, Ratter F, Fehm HL, Born J, Kern W. Intranasal insulin improves memory in humans: superiority of insulin aspart. Neuropsychopharmacology 32: 239–243, 2007. doi: 10.1038/sj.npp.1301193. [DOI] [PubMed] [Google Scholar]
- 57.Brunner YF, Kofoet A, Benedict C, Freiherr J. Central insulin administration improves odor-cued reactivation of spatial memory in young men. J Clin Endocrinol Metab 100: 212–219, 2015. doi: 10.1210/jc.2014-3018. [DOI] [PubMed] [Google Scholar]
- 58.Claxton A, Baker LD, Hanson A, Trittschuh EH, Cholerton B, Morgan A, Callaghan M, Arbuckle M, Behl C, Craft S. Long-acting intranasal insulin detemir improves cognition for adults with mild cognitive impairment or early-stage Alzheimer's disease dementia. J Alzheimers Dis 44: 897–906, 2015. doi: 10.3233/JAD-141791. [DOI] [PubMed] [Google Scholar]
- 59.Craft S, Baker LD, Montine TJ, Minoshima S, Watson GS, Claxton A, Arbuckle M, Callaghan M, Tsai E, Plymate SR, Green PS, Leverenz J, Cross D, Gerton B. Intranasal insulin therapy for Alzheimer disease and amnestic mild cognitive impairment: a pilot clinical trial. Arch Neurol 69: 29–38, 2012. doi: 10.1001/archneurol.2011.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Novak V, Milberg W, Hao Y, Munshi M, Novak P, Galica A, Manor B, Roberson P, Craft S, Abduljalil A. Enhancement of vasoreactivity and cognition by intranasal insulin in type 2 diabetes. Diabetes Care 37: 751–759, 2014. doi: 10.2337/dc13-1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Reger MA, Watson GS, Green PS, Wilkinson CW, Baker LD, Cholerton B, Fishel MA, Plymate SR, Breitner JC, DeGroodt W, Mehta P, Craft S. Intranasal insulin improves cognition and modulates beta-amyloid in early AD. Neurology 70: 440–448, 2008. doi: 10.1212/01.WNL.0000265401.62434.36. [DOI] [PubMed] [Google Scholar]
- 62.Zhang H, Hao Y, Manor B, Novak P, Milberg W, Zhang J, Fang J, Novak V. Intranasal insulin enhanced resting-state functional connectivity of hippocampal regions in type 2 diabetes. Diabetes 64: 1025–1034, 2015. doi: 10.2337/db14-1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Korinek M, Gonzalez-Gonzalez IM, Smejkalova T, Hajdukovic D, Skrenkova K, Krusek J, Horak M, Vyklicky L. Cholesterol modulates presynaptic and postsynaptic properties of excitatory synaptic transmission. Sci Rep 10: 12651, 2020. doi: 10.1038/s41598-020-69454-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Smith AJ, Sugita S, Charlton MP. Cholesterol-dependent kinase activity regulates transmitter release from cerebellar synapses. J Neurosci 30: 6116–6121, 2010. doi: 10.1523/JNEUROSCI.0170-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Suzuki R, Lee K, Jing E, Biddinger SB, McDonald JG, Montine TJ, Craft S, Kahn CR. Diabetes and insulin in regulation of brain cholesterol metabolism. Cell Metab 12: 567–579, 2010. doi: 10.1016/j.cmet.2010.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Vance JE. Dysregulation of cholesterol balance in the brain: contribution to neurodegenerative diseases. Dis Model Mech 5: 746–755, 2012. doi: 10.1242/dmm.010124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ferris HA, Perry RJ, Moreira GV, Shulman GI, Horton JD, Kahn CR. Loss of astrocyte cholesterol synthesis disrupts neuronal function and alters whole-body metabolism. Proc Natl Acad Sci USA 114: 1189–1194, 2017. doi: 10.1073/pnas.1620506114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Roh E, Song DK, Kim MS. Emerging role of the brain in the homeostatic regulation of energy and glucose metabolism. Exp Mol Med 48: e216, 2016. doi: 10.1038/emm.2016.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Dodd GT, Tiganis T. Insulin action in the brain: roles in energy and glucose homeostasis. J Neuroendocrinol 29: e12513, 2017. doi: 10.1111/jne.12513. [DOI] [PubMed] [Google Scholar]
- 70.Guthoff M, Grichisch Y, Canova C, Tschritter O, Veit R, Hallschmid M, Haring HU, Preissl H, Hennige AM, Fritsche A. Insulin modulates food-related activity in the central nervous system. J Clin Endocrinol Metab 95: 748–755, 2010. doi: 10.1210/jc.2009-1677. [DOI] [PubMed] [Google Scholar]
- 71.Loh K, Zhang L, Brandon A, Wang Q, Begg D, Qi Y, Fu M, Kulkarni R, Teo J, Baldock P, Bruning JC, Cooney G, Neely GG, Herzog H. Insulin controls food intake and energy balance via NPY neurons. Mol Metab 6: 574–584, 2017. doi: 10.1016/j.molmet.2017.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Varela L, Horvath TL. Leptin and insulin pathways in POMC and AgRP neurons that modulate energy balance and glucose homeostasis. EMBO Rep 13: 1079–1086, 2012. doi: 10.1038/embor.2012.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Benedict C, Kern W, Schultes B, Born J, Hallschmid M. Differential sensitivity of men and women to anorexigenic and memory-improving effects of intranasal insulin. J Clin Endocrinol Metab 93: 1339–1344, 2008. doi: 10.1210/jc.2007-2606. [DOI] [PubMed] [Google Scholar]
- 74.Hallschmid M, Higgs S, Thienel M, Ott V, Lehnert H. Postprandial administration of intranasal insulin intensifies satiety and reduces intake of palatable snacks in women. Diabetes 61: 782–789, 2012. doi: 10.2337/db11-1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Carver C. Insulin treatment and the problem of weight gain in type 2 diabetes. Diabetes Educ 32: 910–917, 2006. doi: 10.1177/0145721706294259. [DOI] [PubMed] [Google Scholar]
- 76.Lewis GF, Carpentier AC, Pereira S, Hahn M, Giacca A. Direct and indirect control of hepatic glucose production by insulin. Cell Metab 33: 709–720, 2021. doi: 10.1016/j.cmet.2021.03.007. [DOI] [PubMed] [Google Scholar]
- 77.Ribeiro IMR, Antunes VR. The role of insulin at brain-liver axis in the control of glucose production. Am J Physiol Gastrointest Liver Physiol 315: G538–G543, 2018. doi: 10.1152/ajpgi.00290.2017. [DOI] [PubMed] [Google Scholar]
- 78.Pocai A, Lam TKT, Gutierrez-Juarez R, Obici S, Schwartz GJ, Bryan J, Aguilar-Bryan L, Rossetti L. Hypothalamic K-ATP channels control hepatic glucose production. Nature 434: 1026–1031, 2005. doi: 10.1038/nature03439. [DOI] [PubMed] [Google Scholar]
- 79.Dodd GT, Michael NJ, Lee-Young RS, Mangiafico SP, Pryor JT, Munder AC, Simonds SE, Bruning JC, Zhang ZY, Cowley MA, Andrikopoulos S, Horvath TL, Spanswick D, Tiganis T. Insulin regulates POMC neuronal plasticity to control glucose metabolism. eLife 7: e38704, 2018. doi: 10.7554/eLife.38704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lin HV, Plum L, Ono H, Gutierrez-Juarez R, Shanabrough M, Borok E, Horvath TL, Rossetti L, Accili D. Divergent regulation of energy expenditure and hepatic glucose production by insulin receptor in agouti-related protein and POMC neurons. Diabetes 59: 337–346, 2010. doi: 10.2337/db09-1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kimura K, Tanida M, Nagata N, Inaba Y, Watanabe H, Nagashimada M, Ota T, Asahara S, Kido Y, Matsumoto M, Toshinai K, Nakazato M, Shibamoto T, Kaneko S, Kasuga M, Inoue H. Central insulin action activates Kupffer cells by suppressing hepatic vagal activation via the nicotinic alpha 7 acetylcholine receptor. Cell Rep 14: 2362–2374, 2016. doi: 10.1016/j.celrep.2016.02.032. [DOI] [PubMed] [Google Scholar]
- 82.Edgerton DS, Kraft G, Smith M, Farmer B, Williams PE, Coate KC, Printz RL, O'Brien RM, Cherrington AD. Insulin's direct hepatic effect explains the inhibition of glucose production caused by insulin secretion. JCI Insight 2: e91863, 2017. doi: 10.1172/jci.insight.91863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Edgerton DS, Lautz M, Scott M, Everett CA, Stettler KM, Neal DW, Chu CA, Cherrington AD. Insulin's direct effects on the liver dominate the control of hepatic glucose production. J Clin Invest 116: 521–527, 2006. doi: 10.1172/JCI27073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Dash S, Xiao CT, Morgantini C, Koulajian K, Lewis GF. Is insulin action in the brain relevant in regulating blood glucose in humans? J Clin Endocr Metab 100: 2525–2531, 2015. doi: 10.1210/jc.2015-1371. [DOI] [PubMed] [Google Scholar]
- 85.Levin BE, Sherwin RS. Peripheral glucose homeostasis: does brain insulin matter? J Clin Invest 121: 3392–3395, 2011. doi: 10.1172/JCI59653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Heni M, Kullmann S, Ketterer C, Guthoff M, Linder K, Wagner R, Stingl KT, Veit R, Staiger H, Haring HU, Preissl H, Fritsche A. Nasal insulin changes peripheral insulin sensitivity simultaneously with altered activity in homeostatic and reward-related human brain regions. Diabetologia 55: 1773–1782, 2012. doi: 10.1007/s00125-012-2528-y. [DOI] [PubMed] [Google Scholar]
- 87.Gancheva S, Koliaki C, Bierwagen A, Nowotny P, Heni M, Fritsche A, Haring HU, Szendroedi J, Roden M. Effects of intranasal insulin on hepatic fat accumulation and energy metabolism in humans. Diabetes 64: 1966–1975, 2015. doi: 10.2337/db14-0892. [DOI] [PubMed] [Google Scholar]
- 88.Ott V, Lehnert H, Staub J, Wonne K, Born J, Hallschmid M. Central nervous insulin administration does not potentiate the acute glucoregulatory impact of concurrent mild hyperinsulinemia. Diabetes 64: 760–765, 2015. doi: 10.2337/db14-0931. [DOI] [PubMed] [Google Scholar]
- 89.Koch L, Wunderlich FT, Seibler J, Konner AC, Hampel B, Irlenbusch S, Brabant G, Kahn CR, Schwenk F, Bruning JC. Central insulin action regulates peripheral glucose and fat metabolism in mice. J Clin Invest 118: 2132–2147, 2008. doi: 10.1172/JCI31073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Scherer T, O'Hare J, Diggs-Andrews K, Schweiger M, Cheng B, Lindtner C, Zielinski E, Vempati P, Su K, Dighe S, Milsom T, Puchowicz M, Scheja L, Zechner R, Fisher SJ, Previs SF, Buettner C. Brain insulin controls adipose tissue lipolysis and lipogenesis. Cell Metab 13: 183–194, 2011. doi: 10.1016/j.cmet.2011.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Shin AC, Filatova N, Lindtner C, Chi T, Degann S, Oberlin D, Buettner C. Insulin receptor signaling in POMC, but not AgRP, neurons controls adipose tissue insulin action. Diabetes 66: 1560–1571, 2017. doi: 10.2337/db16-1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Iwen KA, Scherer T, Heni M, Sayk F, Wellnitz T, Machleidt F, Preissl H, Haring HU, Fritsche A, Lehnert H, Buettner C, Hallschmid M. Intranasal insulin suppresses systemic but not subcutaneous lipolysis in healthy humans. J Clin Endocrinol Metab 99: E246–E251, 2014. doi: 10.1210/jc.2013-3169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bruning JC, Gautam D, Burks DJ, Gillette J, Schubert M, Orban PC, Klein R, Krone W, Muller-Wieland D, Kahn CR. Role of brain insulin receptor in control of body weight and reproduction. Science 289: 2122–2125, 2000. doi: 10.1126/science.289.5487.2122. [DOI] [PubMed] [Google Scholar]
- 94.Kovacs P, Parlow AF, Karkanias GB. Effect of centrally administered insulin on gonadotropin-releasing hormone neuron activity and luteinizing hormone surge in the diabetic female rat. Neuroendocrinology 76: 357–365, 2002. doi: 10.1159/000067585. [DOI] [PubMed] [Google Scholar]
- 95.Manaserh IH, Chikkamenahalli L, Ravi S, Dube PR, Park JJ, Hill JW. Ablating astrocyte insulin receptors leads to delayed puberty and hypogonadism in mice. PLoS Biol 17: e3000189, 2019. doi: 10.1371/journal.pbio.3000189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Davis MR, Mellman M, Shamoon H. Physiologic hyperinsulinemia enhances counterregulatory hormone responses to hypoglycemia in IDDM. J Clin Endocrinol Metab 76: 1383–1385, 1993. doi: 10.1210/jcem.76.5.8496333. [DOI] [PubMed] [Google Scholar]
- 97.Davis SN, Dunham B, Walmsley K, Shavers C, Neal D, Williams P, Cherrington AD. Brain of the conscious dog is sensitive to physiological changes in circulating insulin. Am J Physiol Endocrinol Physiol 272: E567–E575, 1997. doi: 10.1152/ajpendo.1997.272.4.E567. [DOI] [PubMed] [Google Scholar]
- 98.Diggs-Andrews KA, Zhang X, Song Z, Daphna-Iken D, Routh VH, Fisher SJ. Brain insulin action regulates hypothalamic glucose sensing and the counterregulatory response to hypoglycemia. Diabetes 59: 2271–2280, 2010. doi: 10.2337/db10-0401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Fisher SJ, Bruning JC, Lannon S, Kahn CR. Insulin signaling in the central nervous system is critical for the normal sympathoadrenal response to hypoglycemia. Diabetes 54: 1447–1451, 2005. doi: 10.2337/diabetes.54.5.1447. [DOI] [PubMed] [Google Scholar]
- 100.Davis SN, Shavers C, Collins L, Cherrington AD, Price L, Hedstrom C. Effects of physiological hyperinsulinemia on counterregulatory response to prolonged hypoglycemia in normal humans. Am J Physiol Endocrinol Physiol 267: E402–E410, 1994. doi: 10.1152/ajpendo.1994.267.3.E402. [DOI] [PubMed] [Google Scholar]
- 101.Diamond MP, Hallarman L, Starick-Zych K, Jones TW, Connolly-Howard M, Tamborlane WV, Sherwin RS. Suppression of counterregulatory hormone response to hypoglycemia by insulin per se. J Clin Endocrinol Metab 72: 1388–1390, 1991. doi: 10.1210/jcem-72-6-1388. [DOI] [PubMed] [Google Scholar]
- 102.Paranjape SA, Chan O, Zhu W, Horblitt AM, McNay EC, Cresswell JA, Bogan JS, McCrimmon RJ, Sherwin RS. Influence of insulin in the ventromedial hypothalamus on pancreatic glucagon secretion in vivo. Diabetes 59: 1521–1527, 2010. doi: 10.2337/db10-0014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ren H, Vieira-de-Abreu A, Yan S, Reilly AM, Chan O, Accili D. Altered central nutrient sensing in male mice lacking insulin receptors in Glut4-expressing neurons. Endocrinol 160: 2038–2048, 2019. doi: 10.1210/en.2019-00341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Reno CM, Puente EC, Sheng Z, Daphna-Iken D, Bree AJ, Routh VH, Kahn BB, Fisher SJ. Brain GLUT4 knockout mice have impaired glucose tolerance, decreased insulin sensitivity, and impaired hypoglycemic counterregulation. Diabetes 66: 587–597, 2017. doi: 10.2337/db16-0917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Fernandez AM, Hernandez-Garzon E, Perez-Domper P, Perez-Alvarez A, Mederos S, Matsui T, Santi A, Trueba-Saiz A, Garcia-Guerra L, Pose-Utrilla J, Fielitz J, Olson EN, Fernandez de la Rosa R, Garcia Garcia L, Pozo MA, Iglesias T, Araque A, Soya H, Perea G, Martin ED, Torres Aleman I. Insulin regulates astrocytic glucose handling through cooperation with IGF-I. Diabetes 66: 64–74, 2017. doi: 10.2337/db16-0861. [DOI] [PubMed] [Google Scholar]
- 106.Garcia-Caceres C, Quarta C, Varela L, Gao Y, Gruber T, Legutko B, Jastroch M, Johansson P, Ninkovic J, Yi CX, Le Thuc O, Szigeti-Buck K, Cai W, Meyer CW, Pfluger PT, Fernandez AM, Luquet S, Woods SC, Torres-Aleman I, Kahn CR, Gotz M, Horvath TL, Tschop MH. Astrocytic insulin signaling couples brain glucose uptake with nutrient availability. Cell 166: 867–880, 2016. doi: 10.1016/j.cell.2016.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Agrawal R, Vieira-de-Abreu A, Durupt G, Taylor C, Chan O, Fisher SJ. Insulin regulates GLUT4 in the ventromedial hypothalamus to restore the sympathoadrenal response to hypoglycemia in diabetic rats. Am J Physiol Endocrinol Metab 315: E1286–E1295, 2018. doi: 10.1152/ajpendo.00324.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Schwartsburd PM. Catabolic and anabolic faces of insulin resistance and their disorders: a new insight into circadian control of metabolic disorders leading to diabetes. Future Sci OA 3: FSO201, 2017. doi: 10.4155/fsoa-2017-0015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Benoit SC, Air EL, Coolen LM, Strauss R, Jackman A, Clegg DJ, Seeley RJ, Woods SC. The catabolic action of insulin in the brain is mediated by melanocortins. J Neurosci 22: 9048–9052, 2002. doi: 10.1523/JNEUROSCI.22-20-09048.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Cartee GD. Mechanisms for greater insulin-stimulated glucose uptake in normal and insulin-resistant skeletal muscle after acute exercise. Am J Physiol Endocrinol Metab 309: E949–E959, 2015. doi: 10.1152/ajpendo.00416.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Deshmukh AS. Insulin-stimulated glucose uptake in healthy and insulin-resistant skeletal muscle. Horm Mol Biol Clin Investig 26: 13–24, 2016. doi: 10.1515/hmbci-2015-0041. [DOI] [PubMed] [Google Scholar]
- 112.Chakrabarti P, Kim JY, Singh M, Shin YK, Kim J, Kumbrink J, Wu Y, Lee MJ, Kirsch KH, Fried SK, Kandror KV. Insulin inhibits lipolysis in adipocytes via the evolutionarily conserved mTORC1-Egr1-ATGL-mediated pathway. Mol Cel Biol 33: 3659–3666, 2013. doi: 10.1128/MCB.01584-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Wong RH, Sul HS. Insulin signaling in fatty acid and fat synthesis: a transcriptional perspective. Curr Opin Pharmacol 10: 684–691, 2010. doi: 10.1016/j.coph.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Herzog RI, Sherwin RS, Rothman DL. Insulin-induced hypoglycemia and its effect on the brain unraveling metabolism by in vivo nuclear magnetic resonance. Diabetes 60: 1856–1858, 2011. doi: 10.2337/db11-0498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Inoue H, Ogawa W, Asakawa A, Okamoto Y, Nishizawa A, Matsumoto M, Teshigawara K, Matsuki Y, Watanabe E, Hiramatsu R, Notohara K, Katayose K, Okamura H, Kahn CR, Noda T, Takeda K, Akira S, Inui A, Kasuga M. Role of hepatic STAT3 in brain-insulin action on hepatic glucose production. Cell Metab 3: 267–275, 2006. doi: 10.1016/j.cmet.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 116.Rojas JM, Schwartz MW. Control of hepatic glucose metabolism by islet and brain. Diabetes, Obes Metab 16: 33–40, 2014. doi: 10.1111/dom.12332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Das D, Arur S. Conserved insulin signaling in the regulation of oocyte growth, development, and maturation. Mol Reprod Dev 84: 444–459, 2017. doi: 10.1002/mrd.22806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Sliwowska JH, Fergani C, Gawalek M, Skowronska B, Fichna P, Lehman MN. Insulin: its role in the central control of reproduction. Physiol Behav 133: 197–206, 2014. doi: 10.1016/j.physbeh.2014.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Duarte AI, Moreira PI, Oliveira CR. Insulin in central nervous system: more than just a peripheral hormone. J Aging Res 2012: 384017, 2012. doi: 10.1155/2012/384017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Duarte AI, Proenca T, Oliveira CR, Santos MS, Rego AC. Insulin restores metabolic function in cultured cortical neurons subjected to oxidative stress. Diabetes 55: 2863–2870, 2006. doi: 10.2337/db06-0030. [DOI] [PubMed] [Google Scholar]
- 121.McNay EC, Recknagel AK. Brain insulin signaling: a key component of cognitive processes and a potential basis for cognitive impairment in type 2 diabetes. Neurobiol Learn Mem 96: 432–442, 2011. doi: 10.1016/j.nlm.2011.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Zou XH, Sun LH, Yang W, Li BJ, Cui RJ. Potential role of insulin on the pathogenesis of depression. Cell Prolif 53: e12806, 2020. doi: 10.1111/cpr.12806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Rodin J. Insulin levels, hunger, and food intake: an example of feedback loops in body weight regulation. Health Psychol 4: 1–24, 1985. doi: 10.1037//0278-6133.4.1.1. [DOI] [PubMed] [Google Scholar]
- 124.Woods SC, Lutz TA, Geary N, Langhans W. Pancreatic signals controlling food intake; insulin, glucagon and amylin. Philos Trans R Soc Lond B Biol Sci 361: 1219–1235, 2006. doi: 10.1098/rstb.2006.1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Kullmann S, Kleinridders A, Small DM, Fritsche A, Haring HU, Preissl H, Heni M. Central nervous pathways of insulin action in the control of metabolism and food intake. Lancet Diabetes Endocrinol 8: 524–534, 2020. doi: 10.1016/S2213-8587(20)30113-3. [DOI] [PubMed] [Google Scholar]