Abstract
Microbial metabolites produced by the gut microbiome, e.g. short-chain fatty acids (SCFA), have been found to influence lung physiology and injury responses. However, how lung immune activity is regulated by SCFA is unknown. We examined fresh human lung tissue and observed the presence of SCFA with interindividual variability. In vitro, SCFA were capable of modifying the metabolic programming in LPS-exposed alveolar macrophages (AM). We hypothesized that lung immune tone could be defined by baseline detection of lung intracellular IL-1β. Therefore, we interrogated naïve mouse lungs with intact gut microbiota for IL-1β mRNA expression and localized its presence within alveolar spaces, specifically within AM subsets. We established that metabolically active gut microbiota, which produce SCFA, can transmit LPS and SCFA to the lung and thereby could create primed lung immunometabolic tone. To understand how murine lung cells sensed and upregulated IL-1β in response to gut microbiome-derived factors, we determined that, in vitro, AM and alveolar type II (AT2) cells expressed SCFA receptors, free fatty acid receptor 2 (FFAR2), free fatty acid receptor 3 (FFAR3), and IL-1β but with distinct expression patterns and different responses to LPS. Finally, we observed that IL-1β, FFAR2, and FFAR3 were expressed in isolated human AM and AT2 cells ex vivo, but in fresh human lung sections in situ, only AM expressed IL-1β at rest and after LPS challenge. Together, this translational study using mouse and human lung tissue and cells point to an important role for the gut microbiome and their SCFA in establishing and regulating lung immune tone.
Keywords: gut-lung axis, gut microbiome, lung immune tone, lung injury, SCFA
INTRODUCTION
The human gut (and lung) microbiomes have been linked to host health and immune development (1–5). Key microbial metabolites such as short-chain fatty acids (SCFA), which originate via gut microbiome fermentation of dietary fiber or directly from the diet, have been shown to regulate local tissue inflammation, and act as communication signals to extraintestinal organs (6–8). SCFA can influence allergic and inflammatory lung diseases including asthma (9–12). However, the lung microbiome’s low biomass (103–105 bacteria/g) (13) and lack of significant metabolic activity suggest that these lung bacteria are unlikely sources of SCFA production. Consequently, we and other investigators have proposed that SCFA modulate lung immune function via direct or indirect mechanisms (11, 14, 15), but thus far no clear consensus has been established on how gut-originating SCFA regulate lung inflammatory responses.
The lung likely exists in a state of immune responsiveness that is influenced by numerous interrelated factors, including environmental exposures, genetics, diet, medications, prior disease/injury exposure, and microbiome composition. These factors in combination likely contribute to dynamic “lung immune tone” that forms the basis for the host lung response to injury or infection. Microbiome-sourced bacterial signatures, such as LPS, and metabolites, such as SCFA, might mediate the gut microbiome’s role in creating this lung immune tone via the gut-lung immune axis. The primary metabolite receptors for SCFA are two free fatty acid receptors (FFAR): FFAR2 (GPR43) and FFAR3 (GPR 41) (16–19). FFAR2 is engaged primarily by acetate and propionate, and FFAR3 by propionate and butyrate (20, 21). Expression of these FFAR has been described in intestinal stromal and immune cells, mainly in the colon, but FFAR2 and FFAR3 have also been detected extraintestinally (6, 18, 22).
We previously demonstrated that sensing of LPS and SCFA produced by the gut microbiome could strongly influence the course of lung injury and infections, thereby supporting the existence of the gut-lung axis of immune communication (11, 23). Moreover, we reported that coexposure of bacterial products, such as LPS or lipoteichoic acid (LTA), with SCFA enhanced or repressed inflammatory responses depending on their concentrations or levels within the lung (11) (reflecting the presence of specific types of gram-negative and gram-positive fermenting bacteria in the gut microbiome). We further reported detectable resting IL-1β expression in lung tissue and interpreted this as gut microbiome-mediated lung immune priming (11). The inflammasome complex, which regulates IL-1β release, was also required for appropriate host response to lung injury and infection (24).
In the present study, we characterized both terminal points of the gut-lung immune axis and elucidated how gut SCFA and LPS presence as well as lung SCFA sensing may contribute to steady-state lung immune tone, which can in turn vary dramatically in human lungs. To do this, we assessed human lungs for presence of gut-derived SCFA; we also studied how LPS and propionate specifically could transit along the gut-lung axis to the lung, and in combination alter lung AM metabolic programming. Further, we examined the expression and regulation of FFAR2 and FFAR3 using mouse cell lines and human lung cells—both freshly isolated and cultured in vitro. We also examined intact mouse or human lung tissue to identify cellular source(s) of IL-1β, and expression of FFAR2 and FFAR3 as a means of assessing individual lung immune tone. By identifying lung cell types that receive the gut-derived signals (LPS, SCFA, etc.) and directly or indirectly establish lung baseline IL-1β expression and thereby lung immune tone, we hope to advance understanding of the interconnected gastrointestinal and pulmonary systems in health and disease.
MATERIALS AND METHODS
Animal Care
All studies were approved by the institutional animal care and use committee at University of California, San Francisco (IACUC protocol no. AN181709). All mice were purchased from the Jackson Laboratory (Bar Harbor, ME) or bred in the animal facility at University of California, San Francisco. Wild-type (WT) C57BL/6, C3H/HeOuJ mice, and germ-free (GF aka gnotobiotic) mice were used in this study (8–10-wk-old GF or 10–15-wk-old WT C57BL/6J and C3H/HeOuJ adult male mice). Commercially purchased mice were allowed to acclimatize to their new housing for at least 1 wk before any experiments were conducted on them.
GF mice (C57BL/6 background) were obtained from the UCSF Gnotobiotic Facility. In accordance with Biosafety Level 2 guidelines from the Centers for Disease Control and Prevention, GF mice were maintained in sterile gnotobiotic isolators under a strict 12-h light cycle and were fed an autoclaved standard diet.
Human Lung Tissue Samples
Lung tissue samples were collected through an associated lung cancer screening and treatment program, where noncancerous (“healthy”) portions of the resected lung tissue were excised for this purpose from patients undergoing surgery as part of their definitive lung cancer treatment. The UCSF Institutional Review Board (IRB) approved the protocols and performance of this study (PI: Mehrdad Arjomandi). Written informed consent and Health Insurance Portability and Accountability Act (HIPAA) was obtained from all patients. For metabolomic analyses, lung tissue was immediately frozen on dry ice and stored for later submission for metabolomic analyses (see below). Lung tissue for RNAScope was either fixed right away in formalin or equivalent sections were incubated in PBS or LPS for 2 h before fixation (see RNAScope section below for more details for subsequent steps).
Human rejected donor lungs were obtained with explicit approval for the use of donor lungs for research from each donor’s family by Donor Network West and were kindly provided for our analyses by Michael Matthay (UCSF). All donor lung tissue originated from the right upper lobe of the rejected lungs.
Reagents and Cell Lines
Propionate SCFA and lipopolysaccharide (LPS) were purchased from Sigma-Aldrich (St. Louis, MO). Seahorse XF calibrant solution (part number 100840-000), Seahorse XF RPMI medium, pH 7.4 (part number 103576-100), and Seahorse XF cell mito stress test kit (part number 103015-100, contains 1 each of oligomycin, FCCP, and rotenone/antimycin A) were all purchased from Agilent Technologies, Inc. (Santa Clara, CA).
The following cell lines were used in this study: MLE 12 (WT FVB/N mouse lung epithelial AT2 cell), MH-S (wild-type BALB/c alveolar macrophage), EOMA (AWT 129 background endothelial cell)—all purchased from ATCC (Manassas, VA); HUVEC (primary human umbilical vein endothelial cells, used at passage 6 or less), HPMEC-M or HPMEC-F (primary human pulmonary microvascular endothelial cells-male or female, used at passage 2–6) were purchased from Promocell (Heidelberg, Germany). Human lung alveolar macrophages (AM) were collected by bronchoalveolar lavage (BAL) from human donor lungs. Human alveolar type II (AT2) cells were isolated from elastase-digested human donor lung tissue using negative selection (anti-CD14 and IgG antibodies) at a purity of 90%–95% (25) and were a kind gift from Xiaohui Fang and Michael Matthay (UCSF). Unless used freshly after isolation, all cells were incubated at 37°C under humidified 5% CO2 and then treated with 10 ng/mL or 100 ng/mL LPS for 24 h as indicated.
Bacteroides thetaiotaomicron Strains Inoculation Experiment
Two Bacteroides thetaiotaomicron (B. theta) strains: B.theta Delta TDK (WT) and B.theta Delta 1686-89 (del propionate) strains were a kind gift from Eric Martens (University of Michigan) (26, 27). Both B. theta strains were grown in brain-heart infusion (BHI) broth in an anerobic chamber. Both B. theta strains were used to gavage the GF adult C57BL/6 mice and each individual mouse was colonized with 100-µL bacterial suspension and each mouse effectively received ∼109 CFUs. Two weeks after inoculation, samples of lung tissue, fecal pellets and cecal contents were collected, homogenized for endotoxin and SCFA measurement (see below).
Endotoxin Measurement
Limulus Amebocyte Lysate (LAL) test cartridges (Endosafe nexgen-PTS Charles River Laboratories)—a rapid, point-of-use handheld spectrophotometer—was used for real-time endotoxin testing in this study for mice lung tissue and stool/intestinal tissue sample endotoxin measurement. Lung tissue and stool were homogenized and diluted in pyrogen-free PBS until the solution was clear and passed Endosafe nexgen-PTS QC.
SCFA Measurement
Short-chain fatty acids of human lung samples were quantified at two independent Metabolomic Core facilities at Baylor University and University of Michigan.
The Baylor University Metabolomic Core uses a Water Acquity uPLC System with a Photodiode Array Detector and an autosampler (192 sample capacity). Samples were analyzed on HSS T3 1.8 mm 2.1 150 mm column. Flow rate: 0.25 mL/min, injection volume: 5 mL, run-time: 25 min per sample. Eluent A was 100 mM sodium phosphate monobasic, pH 2.5, eluent B was methanol, the weak needle wash was 0.1% formic acid in water, the strong needle wash was 0.1% formic acid in acetonitrile, and the seal wash was 10% acetonitrile in water. The gradient was 100% eluent A for 5 min, gradient to 70% eluent B from 5 to 22 min, and then 100% eluent A for 3 min. The photodiode array was set to read absorbance at 215 nm with 4.8 nm resolution. Samples were quantified against standard curves of at least five points run in triplicate. Standard curves were run at the beginning and end of each metabolomics run. Quality control checks (blanks and standards) were run every eight samples. Results were rejected if the standards deviated by greater than 5%. Concentrations in the samples were calculated as the measured concentration minus the concentration of the solvent; the range of detection was at least 1–100 mmol/g stool. Stool samples were similarly analyzed for SCFA levels. Further details are available at: https://media.bcm.edu/documents/2016/82/scfa-protocol-vs.docx.
The University of Michigan Metabolomic core performed sample extraction using aqueous extraction solvent containing 3% 1 M HCl (v/v) and isotope-labeled internal standards (d7-buytric acid and d11-hexanoic acid). Samples were then homogenized and centrifuged. Supernatants were transferred to new Eppendorf tubes for extraction by diethyl ether. After layer separation, the upper layer was transferred to an autosampler vial for GC-MS analysis. GC (Agilent 6890, Wilmington, DE) separation was performed using a ZB-Wax plus column, 0.25 μm × 0.25 mm × 30 m (Phenomenex, Torrance, CA). A single quadrupole mass spectrometer (Agilent, 5973 inert MSD) was used to identify and quantify SCFA using Agilent Masshunter software, version B.06 (1). Absolute quantities of SCFA were normalized to sample mass.
Seahorse Extracellular Flux Metabolism Analysis
To examine metabolic response in the MH-S alveolar macrophage (AM) cell lines exposed to LPS and SCFA, Agilent Seahorse XFe24 analyzer platform (Agilent Technologies, Inc.) was used per manufacturer’s instructions. Briefly, MH-S cells were seeded into XFe24 cell culture plate, and 40 K/well were grown in RPMI medium supplemented with 10% FBS and 1% P/S. All treatments were given 4 h after seeding cells. As noted, cells were treated with combinations of LPS (10 ng/mL) and propionate (0.1 mM or 5 mM).
RNA Scope In Situ Hybridization for RNA Transcript Detection
Lungs from naïve wild-type C3H mice or mice that underwent LPS challenge (10 mg/kg IV for 1 h) (23, 24) were formalin-fixed and paraffin embedded (FFPE). Human lung tissue was obtained following a lobectomy for tumor resection; the section studied was the furthest section away from the isolated nodule and thus reflective of nontumor/”normal” lung. Lung sections were either fixed in formalin immediately (fresh) or incubated in PBS or 10 μg/mL LPS solution for 2 h before fixing. Paraffin embedded sections were stained using RNAScope (from Advanced Cell Diagnostics—ACDBio, Newark, CA) using specific human or mouse RNA probes for IL-1β, IL-18, and for cell markers, such as surfactant C (AT2 cells), CD11c (AM and dendritic cells), CD45 (hematopoietic cells), and CD206/MRC1 (macrophages). Probes were visualized as two colorimetric dyes (dots or clusters) via light microscopy (mouse) or three fluorescent dyes via fluorescent microscopy (human).
Briefly, 5 μm formalin fixed, paraffin-embedded tissue sections were pretreated with heat and protease before hybridization with the target oligo probes. Preamplifier, amplifier, and AP-labeled oligos were then hybridized sequentially, followed by chromogenic precipitate development. Each sample was quality controlled for RNA integrity with a positive control probe specific to the housekeeping gene peptidylprolyl isomerase B and for background with a negative control probe specific to bacterial Bacillus subtilis gene dihydrodipicolinate reductase (dapB). Optimization of pretreatment conditions was performed to establish the maximum signal to noise ratio. All probes used were inventoried and prevalidated for specific detection of corresponding gene transcripts. Specific RNA staining signal was identified as yellow, red, or blue-green punctate dots. Samples were counterstained with Gill’s Hematoxylin with nuclei appearing light purple (colormetric) or DAPI (fluorescence).
Reverse Transcription Quantitative Real-Time Polymerase Chain Reaction
TaqMan-specific inventoried gene primers for glyceraldehyde 3-phosphate dehydrogenase (GAPDH), beta actin, β-glucuronidase (GUSB), interleukin (IL)-6, IL-1β, chemokine (C-X-C motif) ligand (CXCL) 1, CXCL2, free fatty acid receptor (FFAR) 2, FFAR3, and toll like receptor (TLR) 4 (Life Technologies, Carlsbad, CA) were used to measure the mRNA levels of these human or mouse genes in cells or lung tissue.
Lung tissue was homogenized (Tissue-Tearor—Biospec Products, Bartlesville, OK) and total RNA isolated using Trizol (Invitrogen—Thermo Fisher Scientific, Waltham MA). We used the High Capacity RNA-to-cDNA reverse transcription Kit (Life Technologies) using 1 μg messenger RNA per reaction. Quantitative real-time polymerase chain reaction was performed using the ABI Prism 7000 Sequence Detection System (Life Technologies). Run method: Polymerase chain reaction activation at 95°C for 20 s was followed by 40 cycles of 1 s at 95°C and 20 s at 60°C.
The average threshold counts (Ct) value of two to three technical replicates were used in all calculations. The average Ct values of the internal controls (GAPDH, beta actin) was used to calculate ΔCt values for the array samples. Data analysis was performed using the 2−ΔΔCt method, data presented as relative quantification (RQ), and the data were corrected for statistical analysis using log transformation, mean centering, and autoscaling (28, 29).
Sandwich Enzyme-Linked Immunosorbent Assay
Concentrations of IL-6 in cell culture supernatant were determined using the mouse DuoSet kit (R&D Systems, Minneapolis, MN). All assays were performed according the manufacturer’s supplied protocol. Standard curves were generated and used to determine the concentrations of IL-6 in the sample. Results are presented as means ± SD.
Statistical analysis
Data in the figures are expressed as means ± SD. Data from in vivo studies comparing two conditions were analyzed using two-tailed nonparametric Mann–Whitney analyses. Data from in vitro studies comparing two conditions were analyzed using standard Student’s t test with equal SD to generate P values. GraphPad Prism was used for statistical analyses (GraphPad Software, La Jolla, CA). P values < 0.05 were considered significant. Experiments were repeated two or more times, as indicated in the figure legends, and representative data are shown. For Seahorse, ELISA, and qPCR data, each experiment includes three biological replicates per condition and three technical replicates per biological replicate. No samples were excluded from analysis.
RESULTS
Human Lung Tissue Contains Variable Micromolar Levels of Acetate and Propionate
Human lung tissue from five patients undergoing lung lobectomies were assayed for SCFA (including medium and branch-chain fatty acids: C2-C8) levels. We noted significant variability in the relative levels of all SCFA between patients (Fig. 1A) as well as when comparing the absolute levels of the SCFA (Fig. 1B, left) or as a percentage of total SCFA (Fig. 1C, left). Previously, we had reported that ratios of C2:C3 can regulate lung inflammatory responses (11) and noted here that C2:C3 lung tissue ratios varied from 3:1 to 10:1 in this small human patient cohort (Fig. 1B, right, and Fig. 1C, right).
Figure 1.
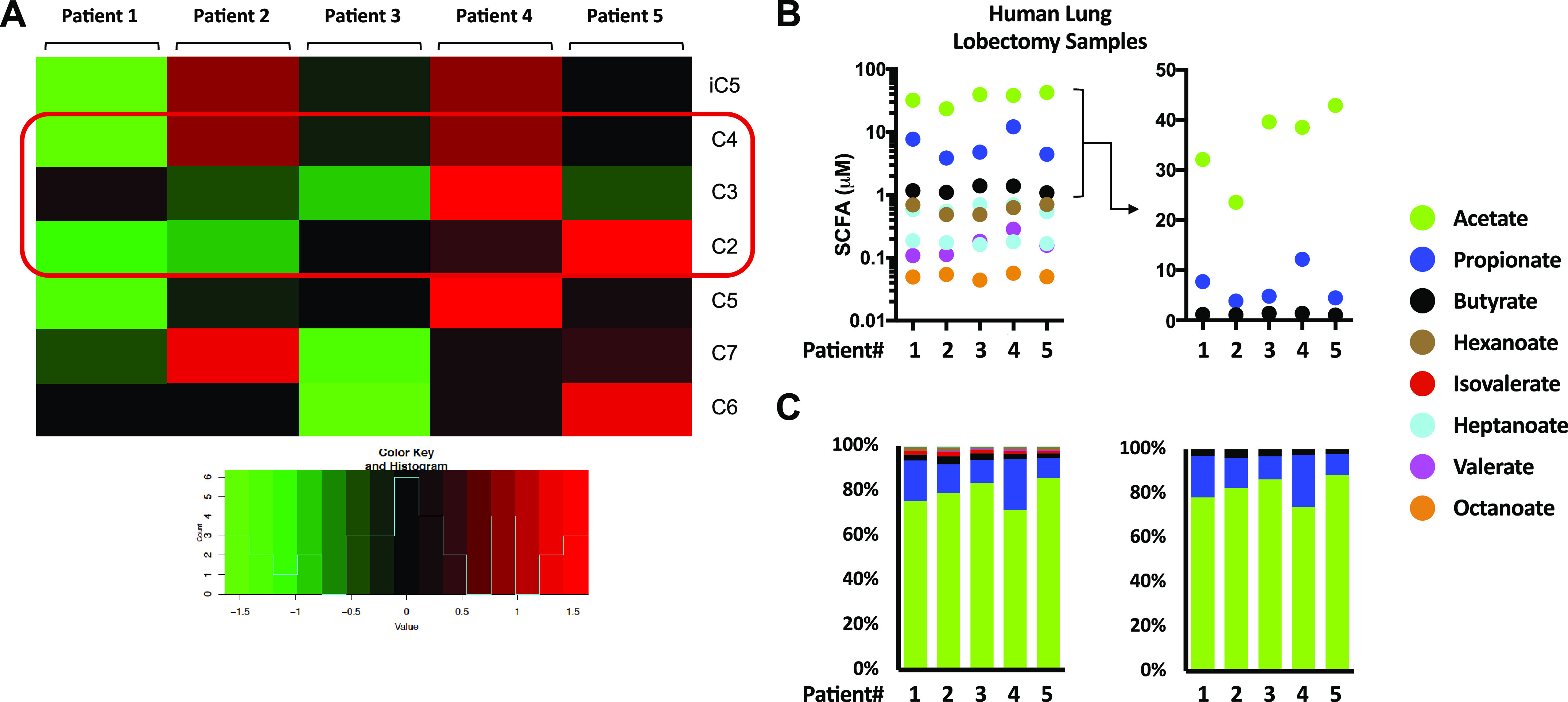
SCFA levels in human lung tissue vary from patient to patient. A: relative levels of measured SCFA from human lung tissue (n = 5 patients) obtained from surgical resections (lung lobectomies) for isolated tumor nodules. Tissue sections used for metabolomic analysis were the furthest sections away from the tumor nodules so as to be representative of patients’ steady state lung metabolome. B: absolute concentrations of all measured SCFA in each of the lung sections (left) and focusing only on C2–4 SCFA (right). C: data presented as a percentage of total SCFA (all SCFA, left; only C2–4, right). SCFA (C2–4) are highlighted in red. C2, acetate; C3, propionate; C4, butyrate; C5, valerate; C6, hexanoate; C7, heptanoate; C8, octanoate; iC5, isovalerate; SCFA, short-chain fatty acid.
LPS and SCFA Presence in Naïve Murine Lungs Depends on the Presence of an Intact Gut Microbiome
We next wanted to directly verify that the gut-lung axis communicates LPS and SCFA from the gut to the lung and thereby confirm that the gut microbiome was the source of lung SCFA. To do this, we compared the metabolomic profile of colons/stool and lung tissue of mice that originated from UCSF’s germ-free (GF)/gnotobiotic and SPF (specific pathogen free, conventionally housed) facilities, the former having no microbiome niche occupied and the latter with fully occupied microbiome niches. We observed that GF mice had low to absent levels of SCFA and other FA metabolites (C2-8) confirming the current understanding that SCFA are 1) not generated in mammalian cells, 2) derived from dietary fiber-fermenting metabolically active gut bacterial species (C3 and C4), or 3) originate from the diet (Fig. 2A). We also measured the levels of LPS present in GF versus SPF mouse stool and lung tissue and noted the presence of LPS in the lungs of SPF mice (Fig. 2B). Although it was most plausible that the lung LPS and SCFA we detected originated from the gut microbiome, we could not formally exclude contribution from the low biomass and less metabolically active lung/airway microbiome. Therefore, to attempt to demonstrate the gut to lung transit of LPS and SCFA metabolites, we monocolonized GF mice with a gram-negative bacterial species (B. theta) and found that gut colonization led 2 wk later to an increase in LPS within the intestine compared to GF mice as well as a large relative increase in lung LPS levels (albeit extremely low absolute LPS concentrations; Fig. 2B), consistent with the amounts of LPS expected to transit from the gut microbiome to the lung. Next, we used a B. theta mutant (del propionate) that was unable to produce C3/propionate to demonstrate that propionate from the intestine could travel to lung tissue: gut colonization by gavage of GF mice with C3 mutant B. theta resulted in significantly less propionate present in the lungs of mice colonized compared to colonization with wildtype B. theta (Fig. 2C). Interestingly, a few other measured SCFA and metabolites were reduced as well but without any effect on growth characteristics of the B. theta mutant (data not shown). Baseline propionate (and other SCFA) levels detected in B. theta (del propionate) colonized mice (and GF mice in general) reflect the residual SCFA levels present within the diet. We also do not believe that direct lung colonization by either B. theta strain can account for our findings given the gut to lung gradient of LPS levels detected two weeks after colonization, absence of any evidence of bacterial infection in the lung, and the lack of fermentable energy sources present in the naïve mouse lung.
Figure 2.
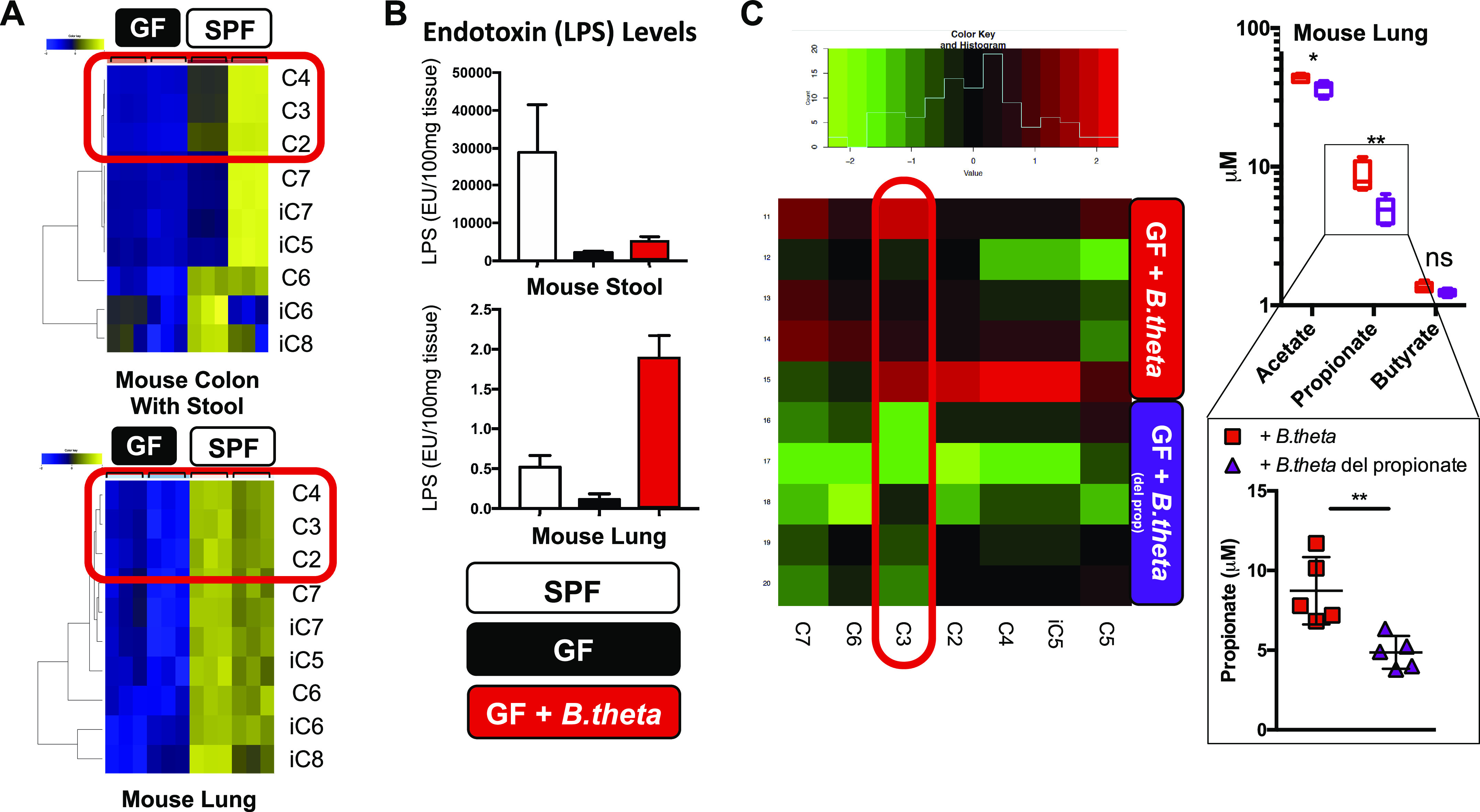
SCFA and LPS levels in mouse lung tissue depend on the presence of metabolically active gut microbiota. A: profiling of SCFA from germ-free (GF) and SPF mice (n = 2 littermates, and three independent colon/stool and lung tissue samples/mouse). C2–4 are highlighted in red. B: endotoxin (LPS) levels measured in stool and lungs from three of each of the following mice: SPF, GF, and GF mice colonized with WT gram-negative B. theta (∼109 CFU). C: relative levels of SCFA were profiled from lung tissue from GF mice (5 mice each) 2 wk after mono-colonization with either WT B. theta (GF + B. theta) or a mutant B. theta deficient in propionate-production (GF + B. theta del propionate; ∼109 CFU) (left). C3 is highlighted in red. Measured absolute levels of only C2–4 (acetate, propionate, and butyrate) are shown (right top) with a focus on C3 levels (right bottom). *P < 0.5, **P < 0.01. CFU, colony-forming unit; C2, acetate; C3, propionate; C4, butyrate; C5, valerate; C6, hexanoate; C7, heptanoate; iC5, isovalerate; iC6, isocaproic; iC7, isoheptanoate; iC8, isooctanoate; SCFA, short-chain fatty acid; SPF, specific pathogen free.
SCFA and LPS Can Alter Mouse Alveolar Macrophage Metabolism and Affect Cellular Responses to Metabolic Stress
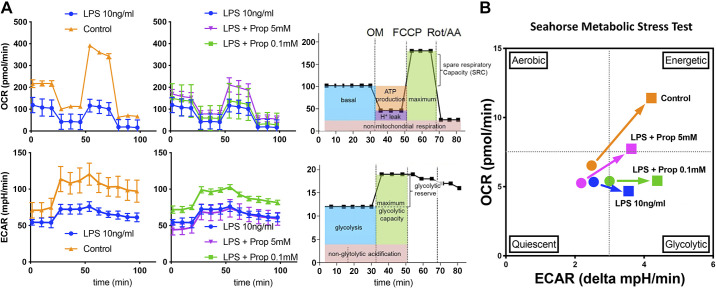
We had previously reported that SCFA could significantly modulate AM response to gram-negative LPS (or gram-positive lipoteichoic acid) in vitro (11). Additionally, we reported that antibiotic treatment in vivo resulted in dysbiosis and potentially millimolar levels of propionate accumulating in the lung (11). We also found that a significant enrichment of gram-positive propionate-producing gut microbiome Lachnospiraceae species (11) was associated with diminished lung inflammatory responses. Therefore, here we sought to understand the effects of LPS on lung AM metabolism (oxidative phosphorylation and glycolysis) and the modulation of this metabolic reprogramming by low and high dose C3/propionate (0.1 mM and 5 mM) using a Seahorse extracellular flux analyzer (Fig. 3A). Focusing on mitochondrial respiration, we observed that low dose LPS exposure (10 ng/mL) significantly reduced basal, maximal respiration and spare respiratory capacity of AM (Fig. 3A, top left, blue tracing). Interestingly, high dose propionate (Fig. 3A, top middle, 5 mM, purple tracing) but not low dose propionate (Fig. 3A, top middle, 0.1 mM, green tracing) could partially reverse these LPS effects. When examining glycolytic metabolism in these AM, we observed that LPS reduced basal glycolysis as well as glycolytic reserve (Fig. 3A, bottom left, blue tracing). However, in contrast to their effects on LPS reduction of oxidative phosphorylation, low dose and not high dose propionate was able to restore some glycolytic capacity in AM (Fig. 3A, bottom middle, purple versus green tracings).
Figure 3.
Propionate can metabolically reprogram LPS-exposed AM in a dose dependent manner. A: control MH-S cells (AM cell line) or after challenged with LPS (10 ng/mL) with or without 0.1 mM propionate (C3) or 5 mM propionate were assessed for oxygen consumption rate (OCR, top) and extracellular acidification rage (ECAR, bottom) using the Seahorse XFe24 analyzer platform. Top and bottom left panels compare control conditions (orange) against LPS (10 ng/mL) treatment (blue). Top and bottom middle panels compare LPS treatment (blue) against LPS + 0.1 mM propionate (green) and LPS + 5 mM propionate (purple). Top and bottom right sided panels represent reference schematic for metabolic measurements. B: metabolic stress tests were run to measure maximal levels of mitochondrial respiration and glycolysis and basal and stressed states plotted as shown within the four denoted quadrants: quiescent, glycolytic, aerobic, and energetic. Circular symbols represent the basal metabolic state, and square symbols represent the metabolic stressed state. Experiment was repeated twice and representative data shown. AM, alveolar macrophages; MH-S, wild-type BALB/c alveolar macrophage; Prop, propionate; OM, oligomycin; FCCP, carbonyl cyanide-4 (trifluoromethoxy) phenylhydrazone; Rot, rotenone; AA, antimycin.
By plotting basal and stressed mitochondrial respiration and glycolysis, we estimated the energetic state transitions of AM under these LPS and propionate exposure conditions. In Fig. 3B, we observed that control AM transitioned into an energetic state (simultaneous aerobic and glycolytic metabolic states) compared to LPS-exposed AM which exhibited primarily a nonaerobic glycolytic program after metabolic stress (orange arrow transition versus blue arrow transition). Within the context of this metabolic stress test, we observed that low-dose propionate further pushed LPS-exposed AM into glycolysis, whereas high-dose propionate could partially reprogram AM to respond more akin to control AM with increased mitochondrial aerobic respiration and increased glycolysis (Fig. 3B, green arrow transition versus purple arrow transition). Lung infections may create a similar state of metabolic stress and therefore the baseline lung immunemetabolic tone could define the type of host defense response triggered by infection.
Specific Subsets of Naïve and LPS-Stimulated Mouse AM Subsets Express IL-1β
To further understand the molecular basis of basal lung immune tone, we examined the baseline expression of IL-1β within the mouse lung. Per our model of gut-lung axis immune communication, baseline IL-1β expression would be a marker of the lung immune tone established by the gut microbiome. Using validated highly specific spatial transcriptomic RNA probe pools (RNAScope) to detect IL-1β and surfactant C mRNA, we had previously showed that resting, LPS-induced, and lung ischemia-reperfusion injury-induced IL-1β mRNA was expressed along the alveolar surface and in close proximity to AT2 cells but not in AT1 cells (24). Here, we followed up on our previous findings and using more RNAScope probe pools, we observed that some but not all CD11c+ AM residing within the alveolar space expressed IL-1β mRNA transcripts within naïve lungs suggesting that a subset of AM are responsible for IL-1β-dependent lung immune tone (Fig. 4A, left). These IL-1β+ cells were confirmed to be CD45+ (hematopoietically derived; Fig. 4B, left). Further imaging revealed punctate IL-1β mRNA expression in the alveolar space appearing in close conjunction to AT2 cells (Surfactant C expressing) cells (Fig. 4C, left) possibly suggesting some form of coordination between AT2 and AM with respect to IL-1β expression and secretion/release. We also probed mouse naïve lung for IL-18 mRNA presence. IL-18 is a member of the IL-1β family of cytokines (also regulated by the inflammasome) and has been shown to be important in certain mouse lung disease models and in human disease (30–33). We propose that IL-18 too could serve as another potentially independent contributor to lung immune tone. Interestingly, we noted that IL-18 and IL-1β were rarely coexpressed or colocalized (Fig. 4D), raising the intriguing possibility that IL-1β and IL-18 specialized AM subsets exist within the naïve lung.
Figure 4.
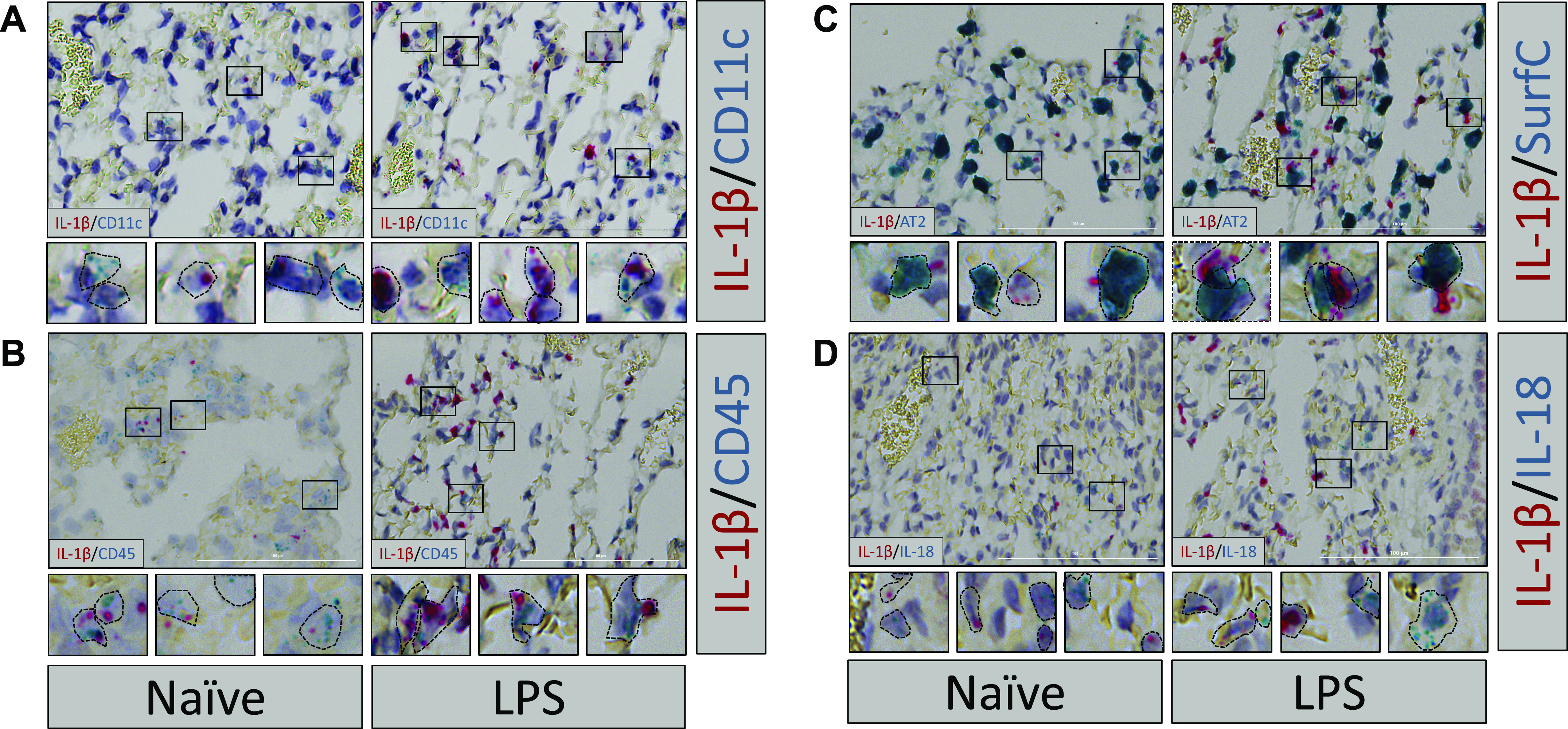
Basal IL-1β levels detected in naïve mouse AM subsets and in same AM subsets after LPS challenge. Lungs from naïve WT C3H mice (left) or WT C3H mice after LPS in vivo challenge (10 mg/kg IV for 1 h, right) were harvested, fixed, and stained using specific mouse RNAscope probes for IL-1β in combination with (A) CD11c (AM and dendritic cells), (B) CD45 (hematopoietic cells), (C) surfactant C (SurfC; AT2 cells), and (D) IL-18. Dashed lines represent approximate estimations of cellular outlines of positively staining cells. Three naïve and three LPS challenged lungs were probed and representative images are shown. AM, alveolar macrophages; AT2, alveolar type II; CD11c, AM and dendritic cells; CD45, hematopoietic cells; WT, wild-type.
In vivo exposure of mice to LPS resulted in increased expression of IL-1β within a similar AM population as seen in the naïve mouse lung—namely, within the alveolar space, CD11c+, CD45+, in close proximity to AT2 (Surfactant C+) cells, and distinct from IL-18+ cells (Fig. 4, A–D, right).
FFAR2 and FFAR3 Are Expressed in AT2 and AM Mouse Cell Lines and Are Inducible by LPS
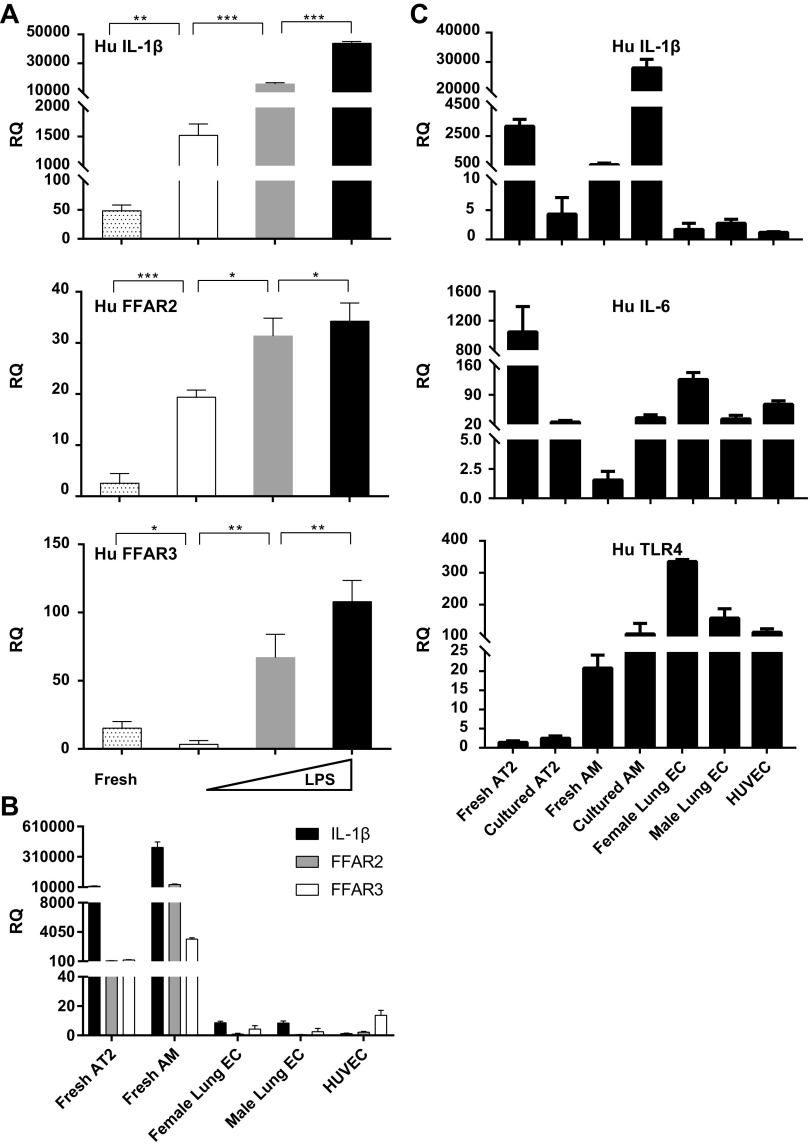
How lungs sense SCFA signals transmitted from the gut is not well understood. We examined different mouse lung cell lines, including MLE-12 (AT2 epithelial cell line), MH-S (AM cell line), and EOMA (endothelial cell (EC) line) for IL-1β, FFAR2, and FFAR3 expression. We first studied the LPS responsiveness of these cell lines in vitro and found that EC and AM were major sources of IL-6 production (Fig. 5A), confirming literature reports on EC IL-6 production (34, 35). Baseline expression of IL-1β and FFAR2 mRNA were highest in AM; FFAR3 was equivalently expressed in both AT2 and AM (Fig. 5B). Low-dose LPS (10 ng/mL) resulted in a large increase in AM IL-1β, and AT2 FFAR2 and FFAR3 expression (Fig. 5C). Higher dose LPS (100 ng/mL) further increased the AM IL-1β and FFAR2 expression, and AT2’s FFAR3 expression (Fig. 5C). As reported by others (36), CXCL-1 was primarily made by AT2 and EC and CXCL-2 by AM (Fig. 5C). Overall, our data suggest that low lung LPS levels (e.g., baseline levels via gut-lung axis communication) and high lung LPS levels (e.g., in infection or dysbiosis) resulted in very different profiles of FFAR2 and FFAR3 expression in alveolar resident cell types in vitro. These variable FFAR expression profiles may result in altered responsiveness to gut-lung axis messengers, such as SCFA as well as altered downstream lung inflammatory and host defense responses.
Figure 5.
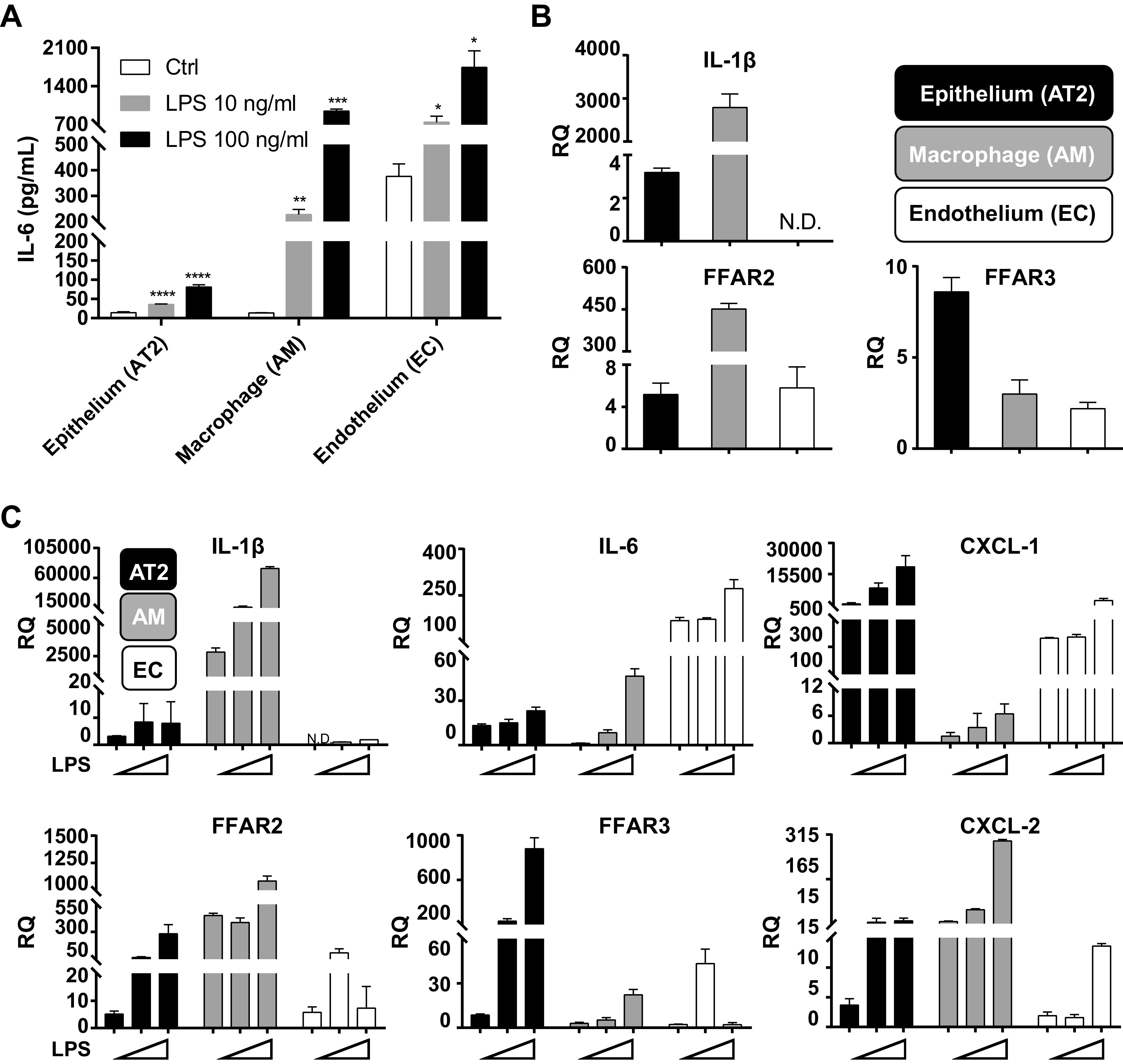
In vitro mouse AM and AT2 cells express IL-1β, FFAR2, and FFAR3 but with differential expression patterns at baseline and after LPS challenge. A: MLE 12 (epithelial AT2), MH-S (WT AM), and EOMA (EC) cell lines were treated with 10 ng/mL or 100 ng/mL LPS for 24 h and concentration of IL-6 in cell culture supernatant were measured by ELISA. B: in separate experiments, total RNA was extracted from these three cell types and basal expression of IL-1β, FFAR2, and FFAR3 was measured by RT-qPCR. C: similar to B, the three cell types were challenged with 10 ng/mL or 100 ng/mL LPS for 24 h and IL-1β, FFAR2, FFAR3, IL-6, CXCL-1, and CXCL-2 mRNA levels were measured by RT-qPCR. Experiments were repeated at least thrice and representative data shown. *P < 0.5, **P < 0.01, ***P < 0.001. AM, alveolar macrophages; AT2, alveolar type II; CXCL, chemokine (C-X-C motif) ligand; EC, endothelial cell; FFAR, free fatty acid receptors; RQ, relative quantification.
Expression of IL-1β, FFAR2, and FFAR3 in Human Lung Alveolar Primary Cells Ex Vivo and after In Vitro Culture
Next, we attempted to translate our mouse findings to human lung cells. We measured IL-1β and FFAR2 and FFAR3 mRNA levels in human BAL cells (AM) obtained from rejected human donor lungs ex vivo (fresh) and after in vitro culture. We found that cells after overnight culture expressed more IL-1β and FFAR2 and less FFAR3 mRNA, and LPS exposure in vitro led to large and significant increases in all three genes’ expression levels (Fig. 6A). Comparing freshly isolated primary AT2 cells, BAL AM, commercially purchased primary ECs from male and female cadaveric lungs, and HUVEC, we noted that AT2 and AM (and not EC) were the primary source of IL-1β, FFAR2, and FFAR3 mRNA (Fig. 6B). Interestingly, overnight cultured AT2 (versus the same cells freshly isolated) expressed less IL-1β and IL-6 and consistently low TLR4 mRNA levels; in contrast cultured AM expressed more IL-1β, IL-6, and TLR4 versus freshly isolated BAL AM, and TLR4 expression was highest in ECs (Fig. 6C).
Figure 6.
A: human AM express IL-1β, FFAR2, and FFAR3. Freshly isolated AM (from BAL performed on human donor lungs) were assayed without further manipulation (fresh, gray dots) or cultured for 24 h under control conditions (white), 10 ng/mL LPS (grey), or 100 ng/mL LPS (black). Total RNA was isolated and assayed for levels of IL-1β (top), FFAR2 (middle), and FFAR3 (bottom) by RT-qPCR. B: freshly isolated (from different human donor lungs) AT2 cells (fresh AT2), BAL AM (fresh AM), and commercially obtained primary human pulmonary microvascular endothelial cells—male or female (female or male lung EC) or primary human umbilical vein endothelial cells (HUVEC) were assayed for expression of IL-1β, FFAR2, and FFAR3 by RT-qPCR. C: human BAL AM (either freshly isolated or after 24 h in culture, from the same donor lung), and AT2 (either freshly isolated or after 24 h in culture, from the same donor lung), female and male lung EC, and HUVEC were assayed for IL-1β (Hu IL-1β), IL-6 (Hu IL-6), and TLR-4 (Hu TLR4) expression by RT-qPCR. Experiments were repeated at least thrice and representative data shown. *P < 0.5, **P < 0.01, ***P < 0.001. AM, alveolar macrophages; AT2, alveolar type II; BAL, bronchoalveolar lavage; EC, endothelial cell; FFAR, free fatty acid receptors; Hu, human; RQ, relative quantification; TLR4, toll like receptor 4.
Human Cellular Expression of IL-1β and FFAR2 and FFAR3 In Situ in Fresh Human Lung Tissue and BAL AM
Our previous results suggested taking alveolar cells out of their natural lung microenvironment may result in significant alteration of their expression of IL-1β, FFAR2, and FFAR3 and perhaps other genes. Therefore, we decided to switch our focus from in vitro cell culture experiments to examining the expression of these lung immune priming genes along with cell-specific markers in situ in freshly obtained human lung tissue. We noted that IL-1β signal in naïve human lungs was present in a subset of AM that were CD11c/ITGAX positive (Fig. 7A, inset a) and CD206/MRC1 positive (Fig. 7B, insets a and b). Once again, we noted the close proximity of naïve lung IL-1β mRNA signal to AT2 cells (SFTPC/surfactant C+) (Fig. 7A, inset b). FFAR2 and FFAR3 were coexpressed in MRC1/CD206 positive AM and possibly in other as yet unidentified cell types (Fig. 7C) but not AT2 cells (Fig. 7D). We exposed sections of the human lung tissue to LPS for 2 h to stimulate the expression of IL-1β. We found that the previously observed subset of CD11c+ CD206+ AM that expressed baseline IL-1β increased their IL-1β levels after LPS challenge (Fig. 7, E and F). To begin to assess the level of variability in human lung immune tone, we assayed two different donor lung BAL cells for IL-1β, FFAR2 and FFAR3 expression and found two to three orders of magnitude differences in all three genes’ expression levels (Fig. 7G).
Figure 7.
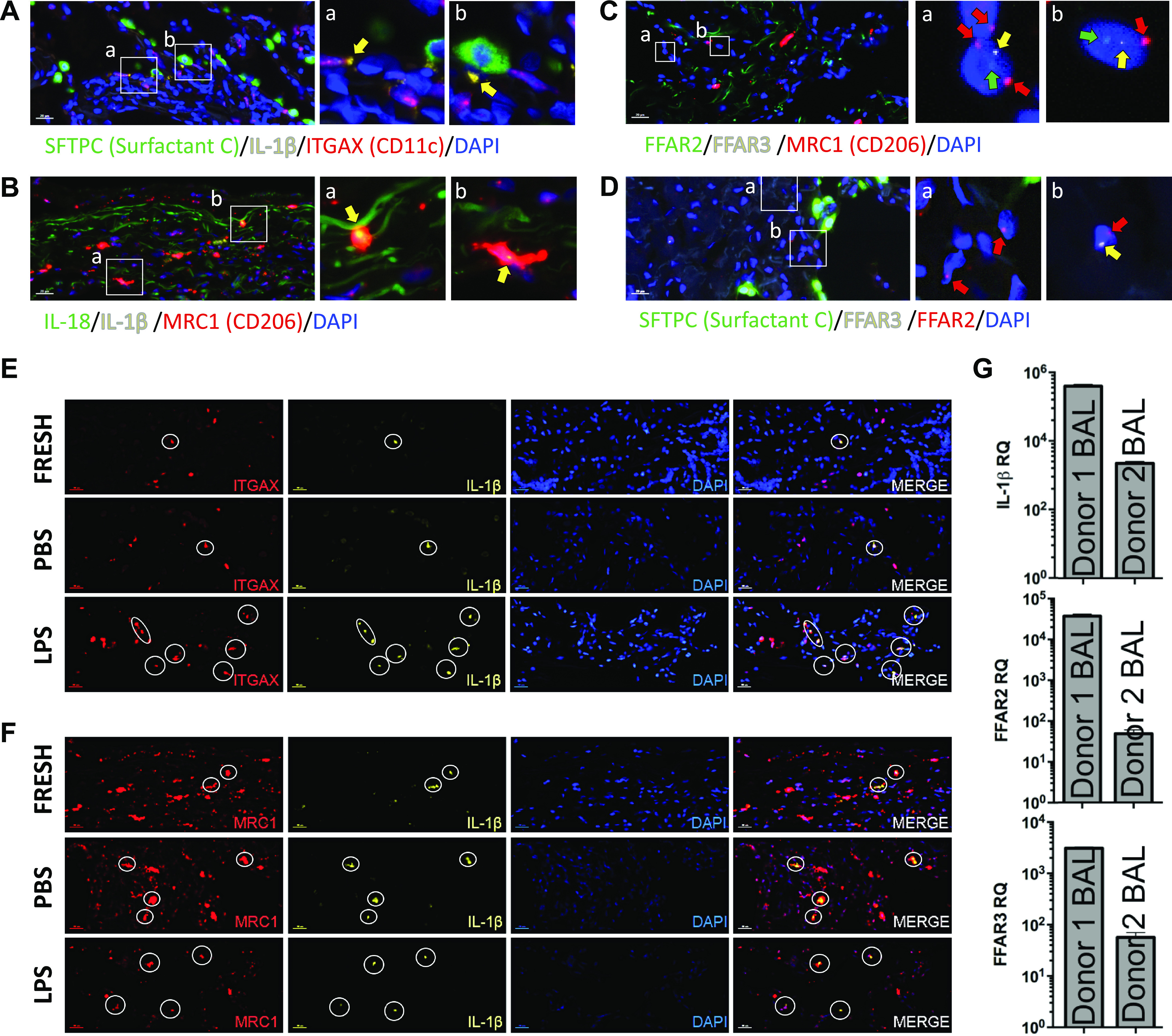
Human IL-1β, FFAR2, and FFAR3 are expressed in AM in vivo and lung immune tone can vary greatly between individuals. A–D: freshly obtained human lung tissue was fixed in formalin immediately after surgical excision and then later probed with fluorescent probes for DAPI (blue) and combinations of (A) surfactant C (AT2 cells, green), IL-1β (yellow), and ITGAX/CD11c (AM and dendritic cells, red); or (B) IL-18 (green), IL-1β (yellow), and CD206/MRC1 (macrophages, red); or (C) FFAR2 (green), FFAR3 (yellow), MRC1/CD206 (macrophages, red); or (D) SFTPC (Surfactant C, green), FFAR2 (yellow), and FFAR3 (red). E and F: human tissue from the same patient was simultaneously either formalin fixed immediately (FRESH, as in A–D) or first incubated with PBS or LPS for 2 h prior to fixation and then stained/probed with fluorescent probes for DAPI (blue) and (E) ITGAX/CD11c (red channel) and IL-1β (yellow channel) or (F) MRC1/CD206 (red channel) and IL-1β (yellow channel). G: human IL-1β, FFAR2, and FFAR3 mRNA relative expression were determined by RT-qPCR in freshly isolated BAL alveolar macrophages (AM) from two different human donor lungs. AM, alveolar macrophages; AT2, alveolar type II; BAL, bronchoalveolar lavage; FFAR, free fatty acid receptors.
The relative expression levels of these three genes appear to be similar between the two individuals perhaps suggesting coordinated gene expression. Interestingly, FFAR2 and FFAR3 are located very close to each other in genomic space (within 30MB of each other on chromosome 2), and close proximity in linear or 3 D genomic space has been associated with coordinated gene expression (37).
DISCUSSION
The major conclusions of this study are that human lungs contain gut-derived metabolites and other bacterial signals and how these signals are sensed within the lung likely contribute to the immune tone of the lung, namely, the resting steady-state that the lungs exist in before injury or infection. These conclusions are based on the following experimental evidence. First, we demonstrated that gut-derived SCFA metabolites exist in variable amounts within human lung tissue and using germ-free mice, we confirmed that lung SCFA levels depended on functionally intact gut microbiome. Next, we demonstrated LPS and the SCFA propionate profoundly metabolically reprogrammed lung alveolar macrophages especially in the context of metabolic stress. We then examined naïve mouse lungs and found clear presence of lung immune tone via detection of IL-1β expression within alveolar macrophage subsets. SCFA receptors, namely FFAR2 and FFAR3 were expressed in vitro in alveolar macrophages (AM) and alveolar type 2 epithelial (AT2) cells and exposure to LPS regulated this expression. Confirming these findings in human lung cells, we detected IL-1β, FFAR2, and FFAR3 expression in alveolar cells in vitro and in vivo, however with a high degree of inter-individual variability. Collectively, our experimental evidence strongly supports the concept of dynamic lung immune tone as defined by key factors, including: the basal expression of IL-1β mRNA, levels of LPS and metabolites in the lung, and expression of SCFA receptors like FFAR2 and FFAR3. These factors are strongly influenced by the composition of the gut microbiome and transport of the bacterial products and SCFA metabolites they produce along the gut-lung immune axis.
We previously demonstrated through the use of antibiotics in mice that the gut microbiome regulates sterile lung inflammation (23). Furthermore, we reported that IL-1β and its downstream regulation by the NLRP3 inflammasome was required for both sterile inflammatory and infectious immune responses (24). We also uncovered a connection between propionate-producing gut bacteria and reduced sterile lung inflammation and observed that direct propionate introduction into the lung reduced lung anti-bacterial defense (11). Other groups have also described the gut-lung axis (reviewed in Refs. 14 and 15). Seminal studies from the Marsland and Lynch groups reported strong indirect and direct effects of the gut microbiome on allergic asthma in mice and humans (12, 38–41). FFAR2 and FFAR3 expression has recently been reported in multiple cells in lung tissue including airway smooth muscle (42), airway epithelium (43, 44), mesenchymal cells (45), neutrophils, and alveolar macrophages (46). Finally, evidence for SCFA contributing to immune tone exists in the literature: SCFA can have proinflammatory effects (47); LPS can upregulate FFAR2 and FFAR3 in monocytes (48); and acetate (via FFAR2) can affects type I interferon responses after RSV infection (49).
Although we cannot formally disprove a role for the lung microbiome in creating or contributing to lung immune tone, the high metabolic activity and fiber fermentation requirements for SCFA production made the gut microbiome and the gut-lung axis the primary focus in this study. In fact, the distal ileum and colon contain a 106–109 fold more bacteria per gram of organ weight as compared to the healthy lung (5). That said, many groups have strongly supported the role of the lung microbiome in influencing ARDS, sepsis, and other lung disease outcomes (50–53). This may be explained by the fact that diseased lungs can support greater lung microbial biomass (via favorable growth conditions) or by increased gut to lung bacterial translocation in critical illness (reviewed in Refs. 4 and 13). Separately, the effects of the gut microbiome and SCFA on Treg development and dendritic cell function locally in the colon, and remotely in the lung, may also contribute to the gut-lung axis (12, 54–56). Our experiments examined baseline steady-state immune tone in healthy lungs and immediate injury responses when gut-originating SCFA likely have the strongest influence on early lung inflammatory responses. Also, although we focused in this study on SCFA signaling via FFAR, it may be important to consider propionate’s and butyrate’s immunomodulatory effects via histone deacetylase (HDAC) inhibition (57), especially when their levels in the lung are in the high millimolar levels. Additionally, FFAR2-independent effects of SCFA have been reported in lung eosinophils and mast cells in asthma and allergic airway disease (9, 58).
Lung immune tone for some may represent an important and novel concept and yet for others may be an obvious fact of lung biology; nonetheless, it has not been defined or explored in any real depth. It is clear that as individuals age, their lungs are exposed to numerous potentially injurious factors/agents and after each exposure/injury, the lungs will transition to a “new normal” state/immune tone which would in turn set the stage for the response to subsequent challenge. We propose that the gut microbiome produces factors that signal to the lung, to influence its health and host defense capability and can be provide a lens through which to understand varied patient responses to similar forms of lung injury. In severe systemic disease, such as sepsis, the phenomenon of the intestinal barrier disruption or “leaky gut” is well known but the influence of intestinal contents in extraintestinal organ physiology is less familiar. In fact, the immune tone of other organs, such as the placenta, has been proposed to be influenced by gut microbiome-derived SCFA and LPS (59). Studying the gut microbiome’s rheostat-like dynamic control of lung inflammatory and immune responses could lead to better understanding of lung immune tone, how it is controlled, and potentially how it could be therapeutically manipulated.
Our study has limitations: we focused on the two major SCFA receptors, namely, FFAR2 and FFAR3, and not on others, such as GPR109a and OLFR78 (60). Our reasoning was that GPR109a primarily resides in the gut where its primary ligand (butyrate) is present (20), and the olfactory chemo sensor OLFR78, which binds propionate, acetate, and lactate, has been found only in upper airways (60–63). Other metabolites, such as 12,13-dihydroxy-9Z-octadecenoic acid (12, 13-diHOME), primary and secondary bile acids, may also participate in the gut-lung immune axis and establishment of lung immune tone (40). This study does not definitively demonstrate that interrupting or short-circuiting the gut-lung immune axis and lung sensing of SCFA affects lung inflammatory and immune responses. Some of these findings have been reported by others, specifically through use of global FFAR knockout mice and cells and studying bacterial pneumonia (46), RSV infection (49), or influenza infection (44) and by us through the direct introduction of propionate into mouse lungs (11). Further studies will require the generation of lung-specific FFAR knockout mice and use of specific FFAR pharamcologic inhibitors, such as pertussis toxin.
The major conclusions of this study are that lungs possess the capacity to sense the metabolic output and composition of the gut microbiome. These microbial products, such as LPS and propionate, can influence and regulate lung immune tone and cellular metabolism. We believe that each individual’s unique lung immune tone can drive how they respond to lung injury and infection. The implication of these findings are that diet, medication, and lifestyle influences on the gut microbiome may indirectly have large effects on lung inflammatory and immune responses. Furthermore, SCFA supplementation, diets including fermentable fiber, or fecal microbiota transplantation (FMT) may be an avenue toward reprogramming lung immune tone for therapeutic benefit. Overall, the gut-lung immune axis may play a critical role in regulating lung immune tone both in health and disease.
GRANTS
A.P. is funded by an R01 award from the NIH/NHLBI (1R01HL146753) as well as departmental funding from UCSF and SFGH. Part of this work was conducted while A.P. was funded by a K08 award from the NIH/NIGMS (K08GM110497). M.A. reports grants from the Departments of Defense (W81XWH-20-1-0158) and Veterans Affairs (CXV-00125), the Flight Attendant Medical Research Institute (012500WG and CIA190001), and the California Tobacco-related Disease Research Program (T29IR0715) during the conducting of this study. Q.L. was supported by the China Scholarship Council (CSC NO. 201806210414) for 1 year study at UCSF.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
A.P. conceived and designed research; Q.L., X.T., and A.P. performed experiments; Q.L., X.T., and A.P. analyzed data; Q.L. and A.P. interpreted results of experiments; Q.L., X.T., and A.P. prepared figures; A.P. drafted manuscript; Q.L., X.T., D.M., M.A., and A.P. edited and revised manuscript; Q.L., X.T., D.M., M.A., and A.P. approved final version of manuscript.
ACKNOWLEDGMENTS
We acknowledge the following individuals for assistance with providing lung tissue, reagents, mice, advice, helpful discussions, and critical reading and editing of the manuscript: Arthur Hill (UCSF), Judith Hellman (UCSF), Michael Matthay (UCSF), Xiaohui (Jenny) Fang (UCSF), Mervyn Maze (UCSF), Kevin Wilhelmsen (BioAge Labs), Eric Martens (University of Michigan), UCSF Gnotobiotics Core and UCSF Mouse Metabolism Core. The authors also acknowledge the continuing support of THFC (COYS) on these studies.
Meetings at which portions of this work have been presented: American Thoracic Society (ATS) Annual meeting, May 2019, Dallas, TX; Association of University Anesthesiologists (AUA) Annual Meeting, May 2019, Montreal, CA; Lung Development, Injury and Repair Gordon Conference, August 2019, Lewiston, ME.
Former address of Q. Liu: Department of Anesthesiology, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China.
REFERENCES
- 1.Dickson RP, Huffnagle GB. The lung microbiome: new principles for respiratory bacteriology in health and disease. PLoS Pathog 11: e1004923, 2015. doi: 10.1371/journal.ppat.1004923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gollwitzer ES, Marsland BJ. Microbiota abnormalities in inflammatory airway diseases - potential for therapy. Pharmacol Ther 141: 32–39, 2014. doi: 10.1016/j.pharmthera.2013.08.002. [DOI] [PubMed] [Google Scholar]
- 3.Korpela K, Salonen A, Virta LJ, Kekkonen RA, Forslund K, Bork P, De Vos WM. Intestinal microbiome is related to lifetime antibiotic use in Finnish pre-school children. Nat Commun 7: 1–8, 2016. doi: 10.1038/ncomms10410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Man WH, De Steenhuijsen Piters WAA, Bogaert D. The microbiota of the respiratory tract: gatekeeper to respiratory health. Nat Rev Microbiol 15: 259–270, 2017. doi: 10.1038/nrmicro.2017.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sender R, Fuchs S, Milo R. Revised estimates for the number of human and bacteria cells in the body. PLoS Biol 14: e1002533, 2016. doi: 10.1371/journal.pbio.1002533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kimura I, Ichimura A, Ohue-Kitano R, Igarashi M. Free fatty acid receptors in health and disease. Physiol Rev 100: 171–210, 2020. doi: 10.1152/physrev.00041.2018. [DOI] [PubMed] [Google Scholar]
- 7.Rooks MG, Garrett WS. Gut microbiota, metabolites and host immunity. Nat Rev Immunol 16: 341–352, 2016. doi: 10.1038/nri.2016.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shapiro H, Thaiss CA, Levy M, Elinav E. The cross talk between microbiota and the immune system: metabolites take center stage. Curr Opin Immunol 30: 54–62, 2014. doi: 10.1016/j.coi.2014.07.003. [DOI] [PubMed] [Google Scholar]
- 9.Folkerts J, Redegeld F, Folkerts G, Blokhuis B, van den Berg MPM, de Bruijn MJW, van IJcken WFJ, Junt T, Tam SY, Galli SJ, Hendriks RW, Stadhouders R, Maurer M. Butyrate inhibits human mast cell activation via epigenetic regulation of FcεRI-mediated signaling. Allergy 75: 1966–1978, 2020. doi: 10.1111/all.14254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ghorbani P, Santhakumar P, Hu Q, Djiadeu P, Wolever TMS, Palaniyar N, Grasemann H. Short-chain fatty acids affect cystic fibrosis airway inflammation and bacterial growth. Eur Respir J 46: 1033–1045, 2015. doi: 10.1183/09031936.00143614. [DOI] [PubMed] [Google Scholar]
- 11.Tian X, Hellman J, Prakash A. Elevated gut microbiome-derived propionate levels are associated with reduced sterile lung inflammation and bacterial immunity in mice. Front Microbiol 10: 159, 2019. doi: 10.3389/fmicb.2019.00159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Trompette A, Gollwitzer ES, Yadava K, Sichelstiel AK, Sprenger N, Ngom-Bru C, Blanchard C, Junt T, Nicod LP, Harris NL, Marsland BJ. Gut microbiota metabolism of dietary fiber influences allergic airway disease and hematopoiesis. Nat Med 20: 159–166, 2014. doi: 10.1038/nm.3444. [DOI] [PubMed] [Google Scholar]
- 13.Wypych TP, Wickramasinghe LC, Marsland BJ. The influence of the microbiome on respiratory health. Nat Immunol 20: 1279–1290, 2019. doi: 10.1038/s41590-019-0451-9. [DOI] [PubMed] [Google Scholar]
- 14.Budden KF, Gellatly SL, Wood DLA, Cooper MA, Morrison M, Hugenholtz P, Hansbro PM. Emerging pathogenic links between microbiota and the gut–lung axis. Nat Rev Microbiol 15: 55–63, 2017. doi: 10.1038/nrmicro.2016.142. [DOI] [PubMed] [Google Scholar]
- 15.Dang AT, Marsland BJ. Microbes, metabolites, and the gut–lung axis. Mucosal Immunol 12: 843–850, 2019. doi: 10.1038/s41385-019-0160-6. [DOI] [PubMed] [Google Scholar]
- 16.Brown AJ, Goldsworthy SM, Barnes AA, Eilert MM, Tcheang L, Daniels D, Muir AI, Wigglesworth MJ, Kinghorn I, Fraser NJ, Pike NB, Strum JC, Steplewski KM, Murdock PR, Holder JC, Marshall FH, Szekeres PG, Wilson S, Ignar DM, Foord SM, Wise A, Dowell SJ. The orphan G protein-coupled receptors GPR41 and GPR43 are activated by propionate and other short chain carboxylic acids. J Biol Chem 278: 11312–11319, 2003. doi: 10.1074/jbc.M211609200. [DOI] [PubMed] [Google Scholar]
- 17.Le Poul E, Loison C, Struyf S, Springael JY, Lannoy V, Decobecq ME, Brezillon S, Dupriez V, Vassart G, Van Damme J, Parmentier M, Detheux M. Functional characterization of human receptors for short chain fatty acids and their role in polymorphonuclear cell activation. J Biol Chem 278: 25481–25489, 2003. doi: 10.1074/jbc.M301403200. [DOI] [PubMed] [Google Scholar]
- 18.Melhem H, Kaya B, Ayata CK, Hruz P, Niess JH. Metabolite-sensing G protein-coupled receptors connect the diet-microbiota-metabolites axis to inflammatory bowel disease. Cells 8: 450, 2019. doi: 10.3390/cells8050450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nilsson NE, Kotarsky K, Owman C, Olde B. Identification of a free fatty acid receptor, FFA2R, expressed on leukocytes and activated by short-chain fatty acids. Biochem Biophys Res Commun 303: 1047–1052, 2003. doi: 10.1016/s0006-291x(03)00488-1. [DOI] [PubMed] [Google Scholar]
- 20.Jobin C. GPR109a: the missing link between microbiome and good health. Immunity 40: 8–10, 2014. doi: 10.1016/j.immuni.2013.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Singh N, Gurav A, Sivaprakasam S, Brady E, Padia R, Shi H, Thangaraju M, Prasad PD, Manicassamy S, Munn DH, Lee JR, Offermanns S, Ganapathy V. Activation of Gpr109a, receptor for niacin and the commensal metabolite butyrate, suppresses colonic inflammation and carcinogenesis. Immunity 40: 128–139, 2014. doi: 10.1016/j.immuni.2013.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bonini JA, Anderson SM, Steiner DF. Molecular cloning and tissue expression of a novel orphan G protein-coupled receptor from rat lung. Biochem Biophys Res Commun 234: 190–193, 1997. doi: 10.1006/bbrc.1997.6591. [DOI] [PubMed] [Google Scholar]
- 23.Prakash A, Sundar S, Zhu Y-G, Tran A, Lee J-W, Lowell C, Hellman J. Lung ischemia reperfusion (IR) is a sterile inflammatory process influenced by commensal microbiota in mice. Shock 44: 272–279, 2015. doi: 10.1097/SHK.0000000000000415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tian X, Sun H, Casbon A-J, Lim E, Francis KP, Hellman J, Prakash A. NLRP3 inflammasome mediates dormant neutrophil recruitment following sterile lung injury and protects against subsequent bacterial pneumonia in mice. Front Immunol 8: 1337, 2017. doi: 10.3389/fimmu.2017.01337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fang X, Neyrinck AP, Matthay MA, Lee JW. Allogeneic human mesenchymal stem cells restore epithelial protein permeability in cultured human alveolar type II cells by secretion of angiopoietin-1. J Biol Chem 285: 26211–26222, 2010. doi: 10.1074/jbc.M110.119917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jacobson A, Lam L, Rajendram M, Tamburini F, Honeycutt J, Pham T, Van Treuren W, Pruss K, Stabler SR, Lugo K, Bouley DM, Vilches-Moure JG, Smith M, Sonnenburg JL, Bhatt AS, Huang KC, Monack D. A gut commensal-produced metabolite mediates colonization resistance to Salmonella infection. Cell Host Microbe 24: 296–307.e7, 2018. doi: 10.1016/j.chom.2018.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kovatcheva-Datchary P, Nilsson A, Akrami R, Lee YS, De Vadder F, Arora T, Hallen A, Martens E, Björck I, Bäckhed F. Dietary fiber-induced improvement in glucose metabolism is associated with increased abundance of Prevotella. Cell Metab 22: 971–982, 2015. doi: 10.1016/j.cmet.2015.10.001. [DOI] [PubMed] [Google Scholar]
- 28.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 25: 402–408, 2001. doi: 10.1006/meth.2001.1262S1046-2023(01)91262-9. [DOI] [PubMed] [Google Scholar]
- 29.Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method [Online]. Nat Protoc 3: 1101–1108, 2008. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- 30.Abdel Fattah E, Bhattacharya A, Herron A, Safdar Z, Eissa NT. Critical role for IL-18 in spontaneous lung inflammation caused by autophagy deficiency. J Immunol 194: 5407–5416, 2015. doi: 10.4049/jimmunol.1402277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jordan JA, Guo RF, Yun EC, Sarma V, Warner RL, Crouch LD, Senaldi G, Ulich TR, Ward PA. Role of IL-18 in acute lung inflammation. J Immunol 167: 7060–7068, 2001. doi: 10.4049/jimmunol.167.12.7060. [DOI] [PubMed] [Google Scholar]
- 32.Nakajima T, Owen CA. Interleukin-18: the master regulator driving destructive and remodeling processes in the lungs of patients with chronic obstructive pulmonary disease? Am J Respir Crit Care Med 185: 1137–1139, 2012. doi: 10.1164/rccm.201204-0590ED. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yasuda K, Nakanishi K, Tsutsui H. Interleukin-18 in health and disease. Int J Mol Sci 20: 649, 2019. doi: 10.3390/ijms20030649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shin H-SS, Xu F, Bagchi A, Herrup E, Prakash A, Valentine C, Kulkarni H, Wilhelmsen K, Warren S, Hellman J. Bacterial lipoprotein TLR2 agonists broadly modulate endothelial function and coagulation pathways in vitro and in vivo. J Immunol 186: 1119–1130, 2011. doi: 10.4049/jimmunol.1001647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wilhelmsen K, Mesa KRR, Prakash A, Xu F, Hellman J. Activation of endothelial TLR2 by bacterial lipoprotein upregulates proteins specific for the neutrophil response. Innate Immun 18: 602–616, 2012. doi: 10.1177/1753425911429336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kolaczkowska E, Kubes P. Neutrophil recruitment and function in health and inflammation. Nat Rev Immunol 13: 159–175, 2013. doi: 10.1038/nri3399. [DOI] [PubMed] [Google Scholar]
- 37.Thévenin A, Ein-Dor L, Ozery-Flato M, Shamir R. Functional gene groups are concentrated within chromosomes, among chromosomes and in the nuclear space of the human genome. Nucleic Acids Res 42: 9854–9861, 2014. doi: 10.1093/nar/gku667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fujimura KE, Demoor T, Rauch M, Faruqi AA, Jang S, Johnson CC, Boushey HA, Zoratti E, Ownby D, Lukacs NW, Lynch SV. House dust exposure mediates gut microbiome Lactobacillus enrichment and airway immune defense against allergens and virus infection. Proc Natl Acad Sci USA 111: 805–810, 2014. doi: 10.1073/pnas.1310750111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fujimura KE, Sitarik AR, Havstad S, Lin DL, Levan S, Fadrosh D, Panzer AR, LaMere B, Rackaityte E, Lukacs NW, Wegienka G, Boushey HA, Ownby DR, Zoratti EM, Levin AM, Johnson CC, Lynch SV. Neonatal gut microbiota associates with childhood multisensitized atopy and T cell differentiation. Nat Med 22: 1187–1191, 2016. doi: 10.1038/nm.4176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Levan SR, Stamnes KA, Lin DL, Panzer AR, Fukui E, McCauley K, Fujimura KE, McKean M, Ownby DR, Zoratti EM, Boushey HA, Cabana MD, Johnson CC, Lynch SV. Elevated faecal 12,13-diHOME concentration in neonates at high risk for asthma is produced by gut bacteria and impedes immune tolerance. Nat Microbiol 4: 1851–1861, 2019. doi: 10.1038/s41564-019-0498-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Marsland BJ, Trompette A, Gollwitzer ES. The gut-lung axis in respiratory disease. Ann Am Thorac Soc 12 Suppl 2: S150–S156, 2015. doi: 10.1513/AnnalsATS.201503-133AW. [DOI] [PubMed] [Google Scholar]
- 42.Mizuta K, Sasaki H, Zhang Y, Matoba A, Emala CW. The short-chain free fatty acid receptor FFAR3 is expressed and potentiates contraction in human airway smooth muscle. Am J Physiol Lung Cell Mol Physiol 318: L1248–L1260, 2020. doi: 10.1152/ajplung.00357.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Imoto Y, Kato A, Takabayashi T, Sakashita M, Norton JE, Suh LA, Carter RG, Weibman AR, Hulse KE, Stevens W, Harris KE, Peters AT, Grammer LC, Tan BK, Welch K, Conley DB, Kern RC, Fujieda S, Schleimer RP. Short-chain fatty acids induce tissue plasminogen activator in airway epithelial cells via GPR41&43. Clin Exp Allergy 48: 544–554, 2018. doi: 10.1111/cea.13119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang G, Jiang L, Wang J, Zhang J, Kong F, Li Q, Yan Y, Huang S, Zhao Y, Liang L, Li J, Sun N, Hu Y, Shi W, Deng G, Chen P, Liu L, Zeng X, Tian G, Bu Z, Chen H, Li C. The G protein-coupled receptor FFAR2 promotes internalization during influenza A virus entry. J Virol 94: e01707-19, 2019. doi: 10.1128/JVI.01707-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rutting S, Xenaki D, Malouf M, Horvat JC, Wood LG, Hansbro PM, Oliver BG. Short-chain fatty acids increase tnfα-induced inflammation in primary human lung mesenchymal cells through the activation of p38 mapk. Am J Physiol Lung Cell Mol Physiol 316: L157–L174, 2019. doi: 10.1152/ajplung.00306.2018. [DOI] [PubMed] [Google Scholar]
- 46.Galvão I, Tavares LP, Corrêa RO, Fachi JL, Rocha VM, Rungue M, Garcia CC, Cassali G, Ferreira CM, Martins FS, Oliveira SC, Mackay CR, Teixeira MM, Vinolo MAR, Vieira AT. The metabolic sensor GPR43 receptor plays a role in the control of Klebsiella pneumoniae infection in the lung. Front Immunol 9: 142, 2018. doi: 10.3389/fimmu.2018.00142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kim MH, Kang SG, Park JH, Yanagisawa M, Kim CH. Short-chain fatty acids activate GPR41 and GPR43 on intestinal epithelial cells to promote inflammatory responses in mice. Gastroenterology 145: 396–406.e1–10, 2013. doi: 10.1053/j.gastro.2013.04.056. [DOI] [PubMed] [Google Scholar]
- 48.Ang Z, Er JZ, Tan NS, Lu J, Liou YC, Grosse J, Ding JL. Human and mouse monocytes display distinct signalling and cytokine profiles upon stimulation with FFAR2/FFAR3 short-chain fatty acid receptor agonists. Sci Rep 6: 34145, 2016. doi: 10.1038/srep34145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Antunes KH, Fachi JL, de Paula R, da Silva EF, Pral LP, dos Santos AÁ, Malaquias Dias GB, Vargas JE, Puga R, Mayer FQ, Maito F, Zarate-Blades CR, Ajami NJ, Sant’Ana MR, Candreva T, Rodrigues HG, Schmiele M, Pedrosa Silva Clerici MT, Proenca-Modena JL, Vieira AT, Mackay CR, Mansur D, Caballero MT, Marzec J, Li J, Wang X, Bell D, Polack FP, Kleeberger SR, Stein RT, Ramirez Vinolo MA, Duarte de Souza AP. Microbiota-derived acetate protects against respiratory syncytial virus infection through a GPR43-type 1 interferon response. Nat Commun 10: 3273, 2019. doi: 10.1038/s41467-019-11152-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Barcik W, Boutin RCT, Sokolowska M, Finlay BB. The role of lung and gut microbiota in the pathology of asthma. Immunity 52: 241–255, 2020. doi: 10.1016/j.immuni.2020.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dickson RP. The lung microbiome and ARDS it is time to broaden the model. Am J Respir Crit Care Med 197: 549–551, 2018. doi: 10.1164/rccm.201710-2096ED. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dickson RP, Erb-Downward JR, Martinez FJ, Huffnagle GB. The microbiome and the respiratory tract. Annu Rev Physiol 78: 481–504, 2016. doi: 10.1146/annurev-physiol-021115-105238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.O’Dwyer DN, Dickson RP, Moore BB. The lung microbiome, immunity, and the pathogenesis of chronic lung disease. J Immunol 196: 4839–4847, 2016. doi: 10.4049/jimmunol.1600279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Smith PM, Howitt MR, Panikov N, Michaud M, Gallini CA, Bohlooly-Y M, Glickman JN, Garrett WS. The microbial metabolites, short-chain fatty acids, regulate colonic T reg cell homeostasis. Science 341: 569–573, 2013. doi: 10.1126/science.1241165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yang W, Xiao Y, Huang X, Chen F, Sun M, Bilotta AJ, Xu L, Lu Y, Yao S, Zhao Q, Liu Z, Cong Y. Microbiota metabolite short-chain fatty acids facilitate mucosal adjuvant activity of cholera toxin through GPR43. J Immunol 203: 282–292, 2019. doi: 10.4049/jimmunol.1801068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zaiss MM, Rapin A, Lebon L, Dubey LK, Mosconi I, Sarter K, Piersigilli A, Menin L, Walker AW, Rougemont J, Paerewijck O, Geldhof P, McCoy KD, Macpherson AJ, Croese J, Giacomin PR, Loukas A, Junt T, Marsland BJ, Harris NL. The intestinal microbiota contributes to the ability of helminths to modulate allergic inflammation. Immunity 43: 998–1010, 2015. doi: 10.1016/j.immuni.2015.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chang PV, Hao L, Offermanns S, Medzhitov R. The microbial metabolite butyrate regulates intestinal macrophage function via histone deacetylase inhibition. Proc Natl Acad Sci USA 111: 2247–2252, 2014. doi: 10.1073/pnas.1322269111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Theiler A, Bärnthaler T, Platzer W, Richtig G, Peinhaupt M, Rittchen S, Kargl J, Ulven T, Marsh LM, Marsche G, Schuligoi R, Sturm EM, Heinemann A. Butyrate ameliorates allergic airway inflammation by limiting eosinophil trafficking and survival. J Allergy Clin Immunol 144: 764–776, 2019. doi: 10.1016/j.jaci.2019.05.002. [DOI] [PubMed] [Google Scholar]
- 59.Voltolini C, Battersby S, Etherington SL, Petraglia F, Norman JE, Jabbour HN. A novel antiinflammatory role for the short-chain fatty acids in human labor. Endocrinology 153: 395–403, 2012. doi: 10.1210/en.2011-1457. [DOI] [PubMed] [Google Scholar]
- 60.Corrêa-Oliveira R, Fachi JL, Vieira A, Sato FT, Vinolo MAR. Regulation of immune cell function by short-chain fatty acids. Clin Transl Immunol 5: e73, 2016. doi: 10.1038/cti.2016.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chang AJ, Ortega FE, Riegler J, Madison DV, Krasnow MA. Oxygen regulation of breathing through an olfactory receptor activated by lactate. Nature 527: 240–244, 2015. doi: 10.1038/nature15721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pluznick JL, Protzko RJ, Gevorgyan H, Peterlin Z, Sipos A, Han J, Brunet I, Wan LX, Rey F, Wang T, Firestein SJ, Yanagisawa M, Gordon JI, Eichmann A, Peti-Peterdi J, Caplan MJ. Olfactory receptor responding to gut microbiotaderived signals plays a role in renin secretion and blood pressure regulation. Proc Natl Acad Sci USA 110: 4410–4415, 2013. doi: 10.1073/pnas.1215927110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Torres-Torrelo H, Ortega-Sáenz P, Macías D, Omura M, Zhou T, Matsunami H, Johnson RS, Mombaerts P, López-Barneo J. The role of Olfr78 in the breathing circuit of mice. Nature 561: E33–E40, 2018. doi: 10.1038/s41586-018-0545-9. [DOI] [PubMed] [Google Scholar]