ABSTRACT
Researchers and counselors need diet-assessment tools that characterize diet at baseline and over time in diet counseling and coaching interventions. Among possible tools, the Healthy Eating Index (HEI) is of interest in cardiometabolic treatment as it has undergone significant validation and development. The objective of this study was to systematically review relevant intervention studies using the HEI and its adaptations to examine whether diet interventions improve diet quality as measured by the HEI and the magnitude of change in included diet-quality scores following dietary intervention. Two databases [Cumulative Index to Nursing and Allied Health Literature (CINAHL) and PubMed] were searched for articles published from January 1995 to December 2019. The review included intervention studies in adults presenting with overweight/obesity and obesity-related chronic disease (metabolic syndrome, diabetes, prediabetes, hypertension, dyslipidemia) who received education or counseling, and the HEI was evaluated from baseline to follow-up (US or Canadian version) or Alternate HEI. Study quality was assessed using Cochrane risk of bias for randomized controlled trials (RCTs) or Cochrane Risk of Bias for Nonrandomized interventions (ROBINS-I). A total of 25 studies were included: 15 RCTs, 3 quasi-experimental studies, and 7 pre-post studies. Eight different versions of the HEI were used. Results demonstrated that diet quality assessed by HEI and its adaptations improved to a clinically relevant degree, especially in studies where multiple food behaviors/food-behavior goals were the focus and where an intensive, long-term intervention was compared with a no-treatment control group. There was wide variation in magnitude of change in included diet-quality indicators. Use of the HEI and its adaptations and other diet-quality tools is promising for better characterization of diet-counseling interventions and results when multiple food behaviors are a focus. Additional development is encouraged.
Keywords: HEI, cardiometabolic, obesity, diet quality, intervention
This systematic review examines whether diet quality, assessed by Healthy Eating Index and its adaptations, improved during diet interventions targeted to adults presenting with overweight/obesity and cardiometabolic disease.
Introduction
Diet assessment is a core activity in the clinical nutrition care process and in various diet interventions offered in health education programs in the community. Accurately assessing dietary intake of individuals is a well-recognized and longstanding challenge in dietetics (1).
Tools developed to assess diet quality in epidemiologic studies have several desirable features that suggest they may also be helpful in intervention research. These indices aim to evaluate intake of several key nutrients and/or foods together and compile them into an overall summary score, defining a healthy diet, compared with some standard. They can be calculated from food-frequency questionnaires, recalls, or food records (2). Higher scores on several diet-quality tools are known to be associated with reduced risk for cardiovascular and overall mortality (3, 4). Various tools are preferred and used for different populations, health contexts, and outcomes, and no one tool is currently preferred. In North America, the Healthy Eating Index (HEI), the Dietary Approaches to Stop Hypertension (DASH) score, and the Alternate Healthy Eating Index (AHEI) are most often calculated.
Researchers conducting implementation studies of diet therapy in the health care system need feasible tools that can be completed during a client encounter or online, will characterize an individual's diet at baseline relative to their peers, and will be responsive to the food changes being promoted by the intervention program or counselor(s). Tools that can be summarized as a score would be helpful in comparing results over time and across programs or studies. Cardiometabolic risk conditions [i.e., various combinations of risk factors, as well as clinical cardiovascular diseases (CVD), prediabetes, type 2 diabetes (T2D), or metabolic syndrome] are a major focus of lifestyle intervention programs in the health system, as these conditions are very prevalent (5, 6) and efficacy and effectiveness of lifestyle (diet and exercise) in reducing the incidence of diabetes and CVD are proven (7). In terms of diet therapy, 2 main approaches can be discerned in the literature; weight-loss focus, as exemplified by the Diabetes Prevention Program (8) and subsequent adaptations, versus a focus on diet quality, as exemplified by the Prevención con Dieta Mediterránea (PREDIMED) study (9). Both approaches have shown merit; so many researchers and practitioners are becoming interested in collecting data on diet quality as well as traditional clinical measures and body weight.
Among possible candidate diet-quality assessment tools, the HEI is of particular interest, as it is scored against the benchmark of the US Dietary Guidelines (the basis of nutrition policy in the United States), has had extensive development, and population data are available for comparison (10). The first version, HEI-1995 was developed by the USDA Center for Nutrition Policy and Promotion, based on the work of Kennedy and colleagues (11). Diet adequacy and moderation components were scored based on intakes of servings of foods and required an estimate of overall energy intake for the calculation of percentage of energy from total and saturated fat. The 1994–1996 Continuing Survey of Food Intake by Individuals for the US population aged ≥2 y based on 1 d of 24-h recalls calculated a mean HEI score of 64, with 70% classified as “needs improvement” with scores in the range between 51 and 80 (12). From 2005, the USDA versions of the HEI were based on an energy density approach, which requires estimation of total energy intake with a mean reported HEI 2005 score of 58 based on NHANES 2001–2002 (13, 14). Average scores obtained from US populations have varied with each revision of the HEI, with scores for 2010 ∼6 points lower than 2005 (10), while scores for 2015 were ∼1 point higher than 2010 (15, 16).
There have been modifications and adjustments made to the original HEI in North America by other groups. The AHEI was developed by the Harvard nutrition epidemiology group, and is related to the risk of chronic disease in the Nurses’ Health Study and the Health Professionals Follow-Up Study, starting in 2002 (17) and updated in 2010 (18). In addition, a Canadian HEI version (HEI-C), broadly based on the HEI-2005 (but not energy density), with recommendations expressed as servings from the 2007 Canada's Food Guide, was published in 2009 (19). Population mean HEI-C from the 2004 Canadian Community Health Survey was 59 using the 24-h multi-pass method of the National Cancer Institute (20). A comparison of the various HEI versions and its adaptations is shown in Supplemental Table 1.
No previous systematic review has examined the use of the HEI in dietary intervention studies. As a possible diet-quality assessment tool in implementation studies for cardiometabolic conditions, we explored the use of the various versions of the HEI and its adaptations in studies of obesity and obesity-related cardiometabolic risk conditions, including diabetes and hypertension. We sought to systematically review whether diet quality, assessed by the HEI, HEI-C, AHEI, and their adaptations/modifications, improved during diet intervention studies and to describe the magnitude of change in score following the diet intervention.
Methods
The systematic review examined the following research question: In adults presenting with overweight/obesity or at cardiometabolic risk, what is the impact of dietary interventions delivered by a health care professional on diet quality as measured by the HEI? The review was registered with PROSPERO (International Prospective Register of Systematic Reviews; CRD 42,017,073,507). The search strategy was developed in consultation with a librarian. Keyword search terms related to intervention, diet measures, and conditions were combined to search PubMed and Cumulative Index to Nursing and Allied Health Literature (CINAHL) databases published from 1 January 1995 (after publication of HEI 1995) (11) to December 2019 (see Supplemental Table 2). Searches were limited to English-language publications.
Eligibility criteria
Intervention studies in adults (≥18 y) were eligible for inclusion, including randomized controlled trials (RCTs), nonrandomized studies, interventional studies without concurrent controls, and pre-post studies. The focus was lifestyle management of overweight/obesity and obesity-related chronic disease (metabolic syndrome, diabetes, prediabetes, CVD, hypertension, dyslipidemia etc.). Exclusions were studies of adolescents (<18 y), pregnant women, and individuals undergoing or previously treated for the following conditions: type 1 diabetes, gastrointestinal surgeries, cancer, lupus, or psychotic disorders.
Interventions were dietary interventions defined as any approaches that aimed to provide participants with nutrition education with the intention to change dietary behaviors. Interventions may have been delivered by a health care professional for individuals or groups in clinical or community settings, and could be face-to-face, over the phone, via e-mail, or through mailings. The study interventions did not need to have diet as a sole focus and may also have included other lifestyle components (physical activity, stress management, smoking cessation, etc.). There were no restrictions regarding length of intervention or sample size. Comparator/control groups could include participants who received standard care at the time of the study as well as control interventions such as general healthy lifestyle education or an active control group. The main outcome was change in diet-quality score from baseline to follow-up(s), measured using the HEI, HEI-C, AHEI, and their adaptations/modifications.
Selection process and quality assessment
Titles, abstracts, and full texts were reviewed independently by 2 researchers, and discrepancies were resolved through discussion. Two reviewers assessed risk of bias using the Cochrane risk-of-bias tool for RCTs (21) or the Cochrane Risk of Bias for Non-randomized Studies (ROBINS-I) (22). Discrepancies were resolved through discussion.
Data synthesis
One reviewer extracted relevant data from all included articles, which were verified by another reviewer. The following information was extracted from all studies: study design, country, sample size, population characteristics, eligibility criteria (including health condition), duration of intervention and follow-up, attrition, description of intervention and control condition (focus, frequency, and delivery), outcomes examined, HEI outcome results, and analysis. We organized the results according to type of HEI tool or its adaptation. Due to substantial differences in patient populations, study designs, interventions, and HEI outcomes, it was not possible to combine study findings in a meta-analysis. HEI results were summarized in a table for each study as reported by authors at various time periods, indicating P values and reported as mean (SD), mean (SD) change, mean (SEM), mean (95% CI) change, and/or between-group difference.
Results
Description of diet-quality tools and effect size
Most of the HEI indexes are scored from 0 to 100, except for the AHEI 2002, which is scored from 0 to 90, and the AHEI 2010, which is scored from 0 to 110. From large-population surveys, Kirkpatrick and colleagues (10) have noted that the SD of usual distribution of the HEI since 2005 has been ∼11–12 among adults; if the effect size of an intervention is ∼0.5, then a change of 5–6 points could be considered as clinically relevant.
Description of studies
A total of 1320 studies were found and screened for title and abstract; 72 articles were included for full-text review, and after analysis 25 studies were included in the systematic review (Figure 1). No studies were excluded due to missing or incomplete data. Of the included studies, 15 were RCTs (including 1 crossover RCT), 3 were quasi-experimental studies, and 7 were pre-post studies. Eight different versions of the HEI were used, including HEI 1995 [US (23–26) and Canadian version (27)], HEI 2005 [US (28–35) and Canadian version (7, 36)], AHEI [2002 (37, 38) and 2010 (39)], HEI 2010 (40–44), and HEI 2015 (45, 46). A comparison of the various HEI tools is shown in Supplemental Table 1.
FIGURE 1.
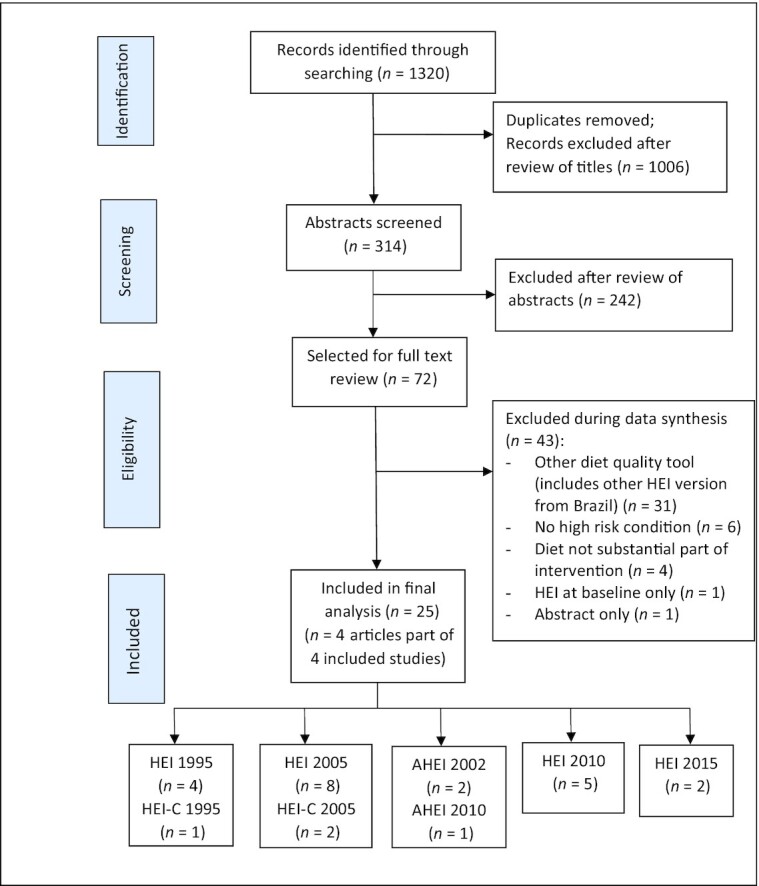
Flowchart for selection of studies. AHEI, Alternate Healthy Eating Index; HEI, Healthy Eating Index; HEI-C, Canadian Healthy Eating Index.
Study characteristics are summarized in Table 1. A majority of studies focused exclusively or predominantly (>70%) on women (45–60 y of age) presenting with overweight/obesity and other cardiometabolic risk factors (including hypertension, dyslipidemia, prediabetes, T2D, and metabolic syndrome). The remainder of studies included men and a lesser proportion (30–69%) of women (7, 24, 29, 30, 32, 35–37, 42, 44, 45). One study focused on men only (46). Most studies (n = 17) were conducted in the United States, 3 were from Canada (7, 27, 36), and 1 study each from Australia (23), Brazil (24), Spain (25), Greece (32), and Iran (31). Most of the diet interventions promoted healthy eating with a goal of weight loss. For studies in which there was a comparison condition, several utilized enhanced usual care (e.g., pamphlet and some additional contact) (26, 30–32, 35, 39, 44, 46). Some active-care comparisons utilized lifestyle interventions to promote healthy eating/weight loss through another intervention (23, 25, 28, 29, 37, 38, 42). The duration of active intervention ranged from 6 wk to 12 mo, with the majority of studies reporting HEI outcomes at 3 to 6 mo.
TABLE 1.
Characteristics of studies1
| Study (year) (ref) | Study design | Country | Sample size | Health condition | Intervention | Control | HEI score2 |
|---|---|---|---|---|---|---|---|
| HEI-1995 | |||||||
| Keogh et al. (2014) (23) | RCT | Australia | 75 women (n = 36 at 12 mo) | Overweight/obesity | Intermittent energy restriction (low energy for 1 wk then usual diet for 1 wk)—every 2 wk for 8 wk, then follow-up visit at 52 wk | Continuous energy restriction (low energy continuously)—every 2 wk for 8 wk, then follow-up visit at 52 wk | Intervention:baseline: 69 (12); 12 mo: 69 (12)Control:baseline: 62 (10); 12 mo: 71 (13)**(P < 0.01 compared to baseline); time by treatment effect** (P < 0.01) |
| Marques-Rocha et al.(2016) (24) | Pre-post | Brazil | 40; 50% women | MetS | Low-energy, frequent meals, higher protein (25% of energy): daily individual counselling by nutritionist × 15 d and 2 group sessions over 6 mo | NA | Baseline: 55.9 (11.8); 8 wk: 71.4 (10.2)** (P < 0.01) |
| Ortega et al. (2006) (25) | RCT | Spain | 67 women (n = 57 at 6 wk) | Overweight/obesity | Weight loss—increase vegetables (mode/frequency of delivery not reported in English) | Weight loss—increase cereals | Intervention:baseline: 55.7 (11.0); 6 wk: 82.5 (10.1)** (P < 0.01 compared to baseline)Control:baseline: 57.01 (13.6); 6 wk: 87.8 (7.1)** (P < 0.01 compared to baseline); difference between groups at 6 wk* (P < 0.05) |
| Stolley et al. (2009) (26) | RCT | USA | 213 African-American women (n = 198 at 6 mo) | Obesity | Weight loss (culturally proficient)—small group 2×/wk (included physical activity), plus monthly individual motivational interview sessions (in person or phone) | General health and safety newsletter—weekly plus monthly phone call | Intervention:baseline: 52.3 (12.6); 6 mo: 63.0 (12.3)3Control:baseline: 56.0 (10.6); 6 mo: 58.9 (11.6)3Mean (95% CI) difference: 5.6 (2.4 to 8.8)*** (P < 0.001) |
| HEI-C 1995 | |||||||
| Carbonneau et al.(2017) (27) | Quasi-experimental | Canada | 307 women (n = 248 at 4 mo) | Overweight/obesity | HAES intervention: 13 × 3 h weekly meetings + 6 h intensive day | Waiting list | Intervention:baseline: 73.3 (12.7); 4 mo: 76.8 (11.6)*,4 (P < 0.05 compared to baseline)Control:baseline: 73.7 (10.2); 4 mo: 72.1 (11.4)4 |
| HEI-C 2005 | |||||||
| Asaad et al. (2016) (36) | Pre-post | Canada | 73; 47% women (n = 42 at 6 mo) | T2D | Canadian Diabetes Association nutrition guidelines: weekly meetomgs (n = 5) with RD | NA | Baseline: 68.7 (8.9); 6 mo: 2.1 (0.1 to 4.1)* (P < 0.05) |
| Jeejeebhoy et al.(2017) (7) | Pre-post | Canada | 305; 52% women (n = 253 at 12 mo) | MetS | Healthy eating ± Mediterranean diet: weekly for 3 mo, then monthly for 9 mo with RD | NA | Baseline: 57.9 (14.2); 12 mo: 68.0 (14.1)Mean (95% CI) change: 9.6 (7.6 to 11.6)**** (P < 0.0001) |
| AHEI-2002 | |||||||
| Turner-McGrievy et al.(2008) (37) | RCT | USA | 99; 60% women (n = 88 at 22 wk) | T2D | Low-fat vegan; individualized meal plan with RD then weekly group sessions for 22 wk | ADA diet; including low-energy; individualized meal plan with RD then weekly group sessions for 22 wk | Intervention:baseline: 31.6 (11.8); 22 wk: 54.1 (17.9)Mean (SD) change: 22.5 (18.7)*** (P < 0.001)Control:baseline: 35.1 (10.1); 22 wk: 34.2 (17.9)Mean (SD) change: −0.9 (18.1)Between-group difference: 22 wk: mean (95% CI) = 23.5 (16.1 to 30.8)*** (P < 0.0001) |
| Ma et al. (2015) (38) | RCT | USA | 240; 71% women | Metabolic syndrome; obesity | Weight loss: AHA diet guidelines; RD individual (×2) then monthly group sessions (14 sessions) | High fiber: 14 sessions (same as AHA group) | Intervention:baseline: 37.2 (11.1); 12 mo: mean (95% CI) change: 5.4 (2.9 to 8.0)Control:baseline: 36.6 (10.8); 12 mo: mean (95% CI) change: 5.2 (2.6 to 7.8)No between-group difference; 12 mo: mean (95% CI) = −0.2 (−3.9 to 3.4) |
| AHEI-2010 | |||||||
| Lynch et al. (2019) (39) | RCT | USA | 211; 77% African-American women (n = 196 at 12 mo) | Uncontrolled T2D | Improve glycemic control: group self-management and telephone peer support; 28 group sessions by RD (weekly × 4 mo, biweekly × 2 mo, then monthly × 2 mo) and 28 peer-support phone calls | 2 group sessions by RD in 6 mo | Intervention:12 mo: mean change: 4** (P = 0.018 compared to control)3Control:12 mo: mean change: −0.53 |
| HEI-2005 | |||||||
| Landry et al. (2017)(28) | Quasi RCT (randomized organizaton between clusters) | USA | 319; >90% African American women (n = 241 at 6 mo) | HT (65%); DYSL (40%) | Weight loss—multiple messages (including healthy foods); 5 group sessions | Weight loss—single message (calories only); 5 group sessions | Intervention:baseline: 61.0 (SEM 1.1); 6 mo: 63.7 (1.1)** (P = 0.0034 time effect)Control:baseline: 58.6 (SEM 1.1); 6 mo: 61.0 (1.1)** (P = 0.0034 time effect)No treatment effect (P = 0.729) |
| Lewis et al. (2015) (29) | RCT | USA | 55; 64% women, 87% African American (n = 50 at 3 mo) | Obesity | Weight loss—grocery store (3× one-on-one visit with RD) | Weight loss—clinic (3× one-on-one visit with RD) | Intervention:baseline: 61.3 (11.9); 3 mo: mean 5* (improved, but P value not reported)Control:baseline: 61.9 (8.5); 3 mo: mean 5* (improved, but P value not reported)No treatment effect |
| Lin et al. (2013) (30) | RCT—2 × 2 | USA | 574; 62% women | HT | Lifestyle behavior change patient intervention—small group for 20 wk by RDs, with or without MD education (RD intervention: MD-I and MD-C) | Usual care—general advice and brochures: with or without MD education (RD control: MD-I and MD-C) | RD intervention:baseline MD-I: 61.3 (12.8); baseline MD-C: 59.6 (12.1); 6 mo change MDI-I: 8.9 (11.5)*; 6 mo change MDI-C: 5 (12.2)**RD control:baseline MD-I: 60.8 (12.2); baseline MD-C: 60.9 (11.7); 6 mo change MDI-I: 0.9 (10.5)**; 6 mo change MDI-C: −0.2 (9.4)*RD intervention vs. control3: *P < 0.05, **P < 0.001 |
| Mohammadshahiet al. (2014) (31) | RCT | Iran | 60 women | Obesity | Weight loss—nutrition education; weekly group sessions | No education | Intervention:baseline: 60.6 (6.3); 3 mo: 83.3 (5.1)*** (P = 0.000 compared to baseline)Control:baseline: 62.1 (5.7); 3 mo: 63.4 (7.5)Difference between groups by ANCOVA at 3 mo*** (P = 0.000) |
| Petrogianni et al.(2013) (32) | RCT | Greece | 115; 46% women (n = 108 at 3 mo) | DYSL | Lifestyle counseling: 9 biweekly group sessions ± milk with phytosterols | Healthy lifestyle | Intervention:baseline: 60.2 (13.3); 3 mo: 65.7 (11.6)0 Mean (95% CI) change: 5.5 (2.7 to 8.4)* (P < 0.05)Control:baseline: 58.5 (13.3); 3 mo: 58.9 (10.3)Mean (95% CI) change: 0.1 (−4.3 to 4.6)Treatment by time effect* (P = 0.045) |
| Thomson et al. (2015) (33) | Quasi RCT—by church; n = 5 intervention, n = 3 control | USA | 409; 70% African-American women (n = 321 at 6 mo) | HT (54%), DYSL (25%), T2D (21%) | Weight loss—lifestyle group education and physical activity (faith based) | No intervention—collect data only | Intervention:baseline: 55.8 (10.2)Mean (SEM) 6 mo change: 4.0 (0.9)* (P < 0.05 compared to baseline)Control:baseline: 56.1 (11.0)Mean (SEM) 6 mo change: 0.8 (1.3)No between-group difference (P = 0.096) |
| Webber and Lee (2011) (34) | Pre-post from RCT | USA | 66 women | Overweight/obesity | Weight loss—Diabetes Prevention Program curriculum | NA | Baseline: 53.9 (9.9); 4 mo: 57.4 (10.6)Mean (SD) change: 3.5 (8.8)** (P = 0.002) |
| Webel et al. (2018) (35) | RCT | USA | 107; 35% women, 86% African American (n = 101 at 6 mo) | HIV+ and CVD risk | Lifestyle behavior change: weekly group sessions × 6 wk | Enhanced usual care—AHA pamphlet and brief generic phone call | Intervention:baseline: 49.0 (11.6); 3 mo: no change (value not reported)5Control:baseline: 48.3 (12.6); 3 mo: no change (value not reported)5 |
| HEI-2010 | |||||||
| Arnold et al. (2018)(40) | Pre-post | USA | 14 postmenopausal women | Obesity | Improve diet quality and decrease inflammation: weekly individual in-person and phone with RD | NA | Baseline: 68 (12); 6 mo: 76 (11)***(P = 0.0003) |
| Hedrick et al. (2017)(41) | RCT | USA | 301; 80% female (n = 292 at 6 mo) | High SSB; overweight/obesity (79%) | Decrease SSBs—3 group classes, 1 live teach-back call, and 11 IVR calls | Increase physical activity—3 group classes, 1 live teach-back call, and 11 IVR calls | Intervention:baseline: 42.3 (12.4); 6 mo: 45.0 (13.4)Mean (95% CI) 6 mo change: 2.6 (0.9 to 4.3)** (P < 0.01 compared to baseline)Control:baseline: 45.4 (12.8); 6 mo: 44.3 (13.7) Mean (95% CI) 6 mo change: −1.1 (−2.6 to 0.3)No between-group difference (P = 0.096) |
| Njike et al. (2016) (42) | Randomized crossover | USA | 112; 72% women (n = 102 at 6 mo) | Pre-DM or MetS | Walnuts and low calorie—individual sessions with RD (number of sessions not specified) | Walnuts and no calorie restriction—individual with RD | Intervention:baseline: 57.1 (17.4) Mean (SD) 6 mo change: 9.1 (17.7)** (P < 0.001 compared to baseline)Control:baseline: 54.0 (15.7) Mean (SD) 6 mo change: 7.0 (15.9)* (P < 0.05 compared to baseline) No between-group difference (P = 0.60) Mean (95% CI) 6 mo change: −1.1 (−2.6 to 0.3) No between-group difference (P = 0.096) |
| Ptomey et al. (2018) (43) | Pre-post | USA | n = 197; 67% women | Overweight/obesity | Weight loss—phone or in-person; weekly for 6 mo | NA | Baseline: 46.4 (8.9); 6 mo: 66.6 (9.4)3Mean (SD) change: 20.3 (11.9)** (P < 0.001) |
| Swoboda et al. (2017) (44) | RCT | USA | 60; 69% women (n = 54 at 4 mo) | T2D + overweight/obesity + CVD risk factor | Decision support + goal setting (multiple goal or single goal)—in-person then biweekly by phone with RD | Attention-control group; received calls and info re: community resources | Intervention:baseline: 67.2 (8.2) Mean (SD) 4 mo change: 3.2 (7.6)* (P = 0.02 compared to baseline)Control:baseline: 66.7 (11.9) Mean (SD) 4 mo change: −0.2 (10.4)No between-group difference in change (P = 0.20) |
| HEI-2015 | |||||||
| Chang et al. (2019) (45) | Pre-post | USA | 16; 31% women (n = 14 at 2 mo) | CKD stage 1–3; HT (81%); DM (69%), DYSL (63%) | Decrease sodium; remote counselling with smartphone | NA | Baseline: 58 (10); 2 mo: 62 (9.6)Mean (95% CI) change: 3.97 (0.03 to 7.9)* (P < 0.05) |
| Ventura Marra et al.(2019) (46) | RCT | USA | 59 men (n = 56 at 3 mo) | Obesity; DYSL (90%), HT (83%), DM or pre-DM (53%) | Weight loss: weekly support via video (×3) and phone (×9) with RD | Usual care: educational material | Intervention:baseline: 51.0 (10.9); 3 mo: 71.3 (13.9)*** (P < 0.0001 compared to baseline)Control:baseline: 51.1 (14.0); 3 mo: 63.9 (14.8)*** (P < 0.0001 compared to baseline)No group or group by time difference (P = 0.110) |
ADA, American Diabetes Association; AHA, American Heart Association; CKD, chronic kidney disease; CVD, cardiovascular disease; DM, diabetes mellitus; DYSL, dyslipidemia; HAES, Health at Every Size; HEI, Healthy Eating Index; HT, hypertension; IVR, interactive voice response; MD-C, medical doctor control; MD-I, medical doctor intervention; MetS, metabolic syndrome; NA, not applicable; RCT, randomized controlled trial; RD, registered dietitian; SSB, sugar-sweetened beverage; T2D, type 2 diabetes. *P< 0.05, **P< 0.01, ***P< 0.001.
HEI scores as reported by authors at time periods shown are mean (SD) unless otherwise indicated as mean (SEM) or mean (95% CI).
Results also reported to 18 mo.
Results also reported to 16 mo.
Results also reported to 6 mo.
Effects of diet intervention on HEI
Of the 4 studies that reported using the 1995 version of the HEI, 3 studies were RCTs (23, 25, 26) and 1 study was a pre-post study (24). All 4 studies reported significant and clinically relevant improvements in HEI (Table 1). Ortega et al. (25) showed substantive increases in HEI in both groups, where the focus was on weight loss with increased vegetables versus cereals. However, the study by Keogh et al. (23) showed results opposite to those expected. There were no improvements in the intervention arm consisting of an intermittent energy-restricted diet [mean baseline HEI (SD) = 69 (12) to 12-month HEI = 69 (12)], in contrast to significant improvements (9 points) reported in the control group given a continuous energy-restricted diet. The difference could be explained by lower mean baseline HEI in the control group compared with the intervention group (62 vs. 69). Although this difference was not reported to be statistically significant, the study was not adequately powered to detect differences, given the high drop-out rate.
All 3 studies from Canada used adapted versions of HEI and reported significant improvements. Using HEI-C 1995, Carbonneau et al. (27) assessed diet quality in the Health at Every Size Study and reported modest but significant improvements over 4 mo compared with no change in a waitlist control group. Two pre-post studies using the 2005 HEI-C were found. Jeejeebhoy et al. (7) showed a 10-point increase in HEI from a baseline of 58 to 68, which was close to the baseline values reported by Asaad et al. (36); however, Asaad and colleagues reported a very modest 2-point increase. Exploratory analysis in the Jeejeebhoy et al. study revealed even greater changes in the subjects with initially poor diets (HEI <52) (47).
Three studies used the AHEI to assess diet quality. Turner-McGrievy et al. (37) and Ma et al. (38) used the AHEI-2002 version. Subjects of the Turner-McGrievy et al. (37) study had T2D. The intervention group received an intensive vegan diet intervention, while the control group received the American Diabetes Association diet. Intervention subjects increased scores by 22 points, while the control group had no change. Ma et al. (38) compared weight loss versus fiber focus in people with metabolic syndrome and both groups improved by ∼5 points. Lynch et al. (39) used the 2010 version; no baseline values were provided, and change was 4 points at 12 mo in the intensive intervention group of subjects with uncontrolled T2D, with no change in the control group receiving a more limited intervention.
Of the 8 studies using the HEI-2005, 5 had no or minimal intervention control groups, and of these, 3 showed changes in the intervention groups in the clinically relevant range while the control groups did not change (31–33). Changes were more modest for the pre-post study of Webber and Lee (34) and did not change for Webel et al. (35), which was a lifestyle behavior-change trial focused on individuals with HIV+ and CVD risk factors. Of the other 3 studies, Landry et al. (28) and Lewis et al. (29) both included active weight-loss control groups, so there was no statistical difference by treatment, but both reported improvements in HEI compared with baseline. Lin et al. (30) used a 2-by-2 study of dietitian counseling versus usual care with and without physician education. Dietitian counseling improved diet quality by 5 to 9 points at 6 mo compared with usual care, while physician education had minor effects (0–1 points).
Five studies used the HEI-2010 version. Two pre-post studies showed marked improvements of 8 to 20 points in studies that focused on improving diet quality (40) or promoted weight loss in weekly meetings for 6 mo (43). Improvements were modest (3-point increase in HEI scores) for a study that focused on reducing sugar-sweetened beverages (41) and in a decision-support telephone coaching intervention for T2D (44). However, no significant between-group differences were observed in HEI change compared with control groups in these studies. Njike et al. (42) saw 7- to 9-point increases in both intervention and control groups, but both groups received dietitian counseling, and calorie restriction versus no restriction was the main focus.
For the 2 studies that used the HEI-2015 version, the pre-post study by Chang et al. (45) focused on decreasing sodium and an increase in HEI of 4 points was reported at 2 mo. In the second study (the only one focused on men only), both the tele-nutrition and usual-care groups had clinically relevant improvements in diet-quality score (20- and 13-point increase, respectively), but treatment and usual-care effects were not statistically different between groups (46).
Quality assessment
For the RCTs, 2 were at low risk of bias in all domains (30, 38). The remainder had an unclear or a high risk of bias, particularly relating to allocation concealment, blinding of participants and personnel, and other sources of bias (including industry funding and study design) (Supplemental Table 3). For the quasi-experimental and pre-post studies, 5 had an overall rating of moderate risk of bias; 4 were serious and 1 was critical. Risk-of-bias issues related predominantly to confounding, selection bias, and measurement of outcomes (Supplemental Table 3).
Discussion
This narrative systematic review examined the change in diet quality (assessed by the HEI, HEI-C, AHEI, and their adaptations) during diet interventions in cardiovascular risk conditions, including overweight/obesity, metabolic syndrome, diabetes, prediabetes, hypertension, and dyslipidemia. Of the 25 included studies, 8 different versions of the included diet-quality indicator were used. Recognizing that scoring varied across the different versions, we considered all 25 studies together. A clinically relevant improvement in score (i.e., 5- to 6-point increase) for the included diet-quality indicator was observed in several studies, particularly those where multiple food behaviors or food-behavior goals were the focus and where an intensive long-term intervention was compared with a no-treatment control group.
Some key methodological challenges in nutrition research have been highlighted in recent commentaries in the literature (48, 49) and are relevant to interpretation of these findings. Dietary interventions cannot be controlled with true placebos, and context and cultural norms as well as changes in dietary patterns over time need to be considered in interpreting our results. Other limitations include lack of double blinding, variable and sometimes poor compliance and adherence, and high drop-out rates (50). Subjects of such studies are often not representative of the population of interest (51). Another fundamental problem is that diet-measurement challenges persist at every level (52).
With these considerations in mind, assessment of diet quality that attempts to provide a summary of several aspects of diet may be a promising “intermediate level” approach for characterizing diet when multiple food-behavior changes are likely to be promoted. This is particularly relevant to diet interventions for cardiometabolic risk, as current clinical practice guidelines offer multiple possible food-behavior changes (53, 54). There were 2 studies where only 1 nutrient or type of food was promoted. Hedrick et al. (41) focused on reduction in sugar-sweetened beverages in an RCT, while Chang et al. (45) focused on sodium reduction in chronic kidney disease in a pre-post study. HEI changes ranged from 2.6 to 4 in the intervention groups, lower than our estimated meaningful effect size (10). The use of the HEI and other diet-quality tools would be most suitable when multiple food behaviors are the focus.
Results most directly answering the systematic review question were in the 4 studies (27, 31, 33, 44) where the intervention involved an intensive long-term dietary intervention compared with a no-treatment control group. In each of these studies, there was no or minimal change in the HEI score in the control groups, irrespective of the HEI version. The degree of change in treatment groups within these 4 studies varied widely (3–23 points), however, which could be related to the known issues in diet intervention research already discussed.
Diet interventions for cardiometabolic risk vary widely in focus, such that a focus on weight loss alone may or may not include a change in diet quality. Similarly, it is possible to focus on diet quality without limiting energy intake. Generally, interventions were not described in enough detail to understand if diet-quality changes were an explicit goal. Four studies (7, 25, 37, 40), however, did indicate food goals that would be expected to change diet quality. Ortega et al. (25) reported a mean increase of 27 points in a study that focused on promotion of vegetables versus cereals in a weight-loss context; Jeejeebhoy et al. (7) observed a 10-point increase in a study focused on the Canada's Food Guide and Mediterranean diet using a care map approach to client-centered counseling (55); Turner-McGrievy et al. (37) tested a vegan diet in participants with T2D and reported a mean 23-point increase; and Arnold et al. (40) observed an 8-point increase in a study focused on diet quality in women presenting with obesity. These positive changes in HEI demonstrate a wide variation in the magnitude of change.
Thus, there is emerging evidence from this review that diet quality assessed by the HEI, HEI-C, AHEI, and their adaptations can improve to a clinically relevant degree. The only study with unexpected results was the study by Keogh et al. (23); however, substantive differences in mean baseline HEI suggest that the study may not have been truly randomized.
Unfortunately, few conclusions on expected magnitude of change using included diet-quality scores following various health-improvement interventions can be inferred from the studies to date. It has been observed by others that baseline diet is likely to be an issue in diet interventions, in that people with high baseline scores may not have many changes to make that can be detected with a diet-quality tool compared with those with initially low scores (51). For example, if the mean baseline HEI in an intervention group is 1 SD above the estimated comparable population mean (assuming the HEI is approximately normally distributed), then mean intake will be close to the 85th percentile of the standard normal curve (56). Similarly, studies of specific subgroups with poor diet quality may be enhanced by comparison to population data at baseline, if available for the relevant time period of data collection. Data on population HEI and AHEI are accumulating from the NHANES and other large surveys (10, 17), which will allow greater understanding of the degree to which intervention groups are representative of the population of interest in future studies.
Strengths of this review were the use of current systematic review methods, which revealed potential for the use of the HEI, HEI-C, AHEI, and their modifications as diet-quality indicators in intervention studies for multiple chronic conditions. The evidence base is currently quite limited. Although several RCTs were identified, most were at unclear or high risk of bias related to challenges in conducting blinded nutrition research. Bias due to confounding was common in nonrandomized studies. Other limitations include the changes in HEI over time, which decreased the comparability of studies. In addition, calculation of the HEI currently requires estimation of nutrient and total energy intake, which adds to data-collection costs and may be infeasible in some community-based studies; use of solely food-focused assessment criteria could make this tool appropriate for practice settings. It was not possible to draw conclusions regarding the magnitude of change in diet quality attributed to the interventions. Interpretation of results was also limited due to the variety of interventions offered (e.g., dietary components, intensity, duration). A better description of diet-counseling and coaching interventions with respect to focus and content is needed to drive improvement in the description of diet interventions. To avoid volunteer bias, greater emphasis on recruiting subjects from the population of interest is also important to better understand which diet interventions and counseling methods are most effective in which types of clients.
Conclusions
The review has revealed that assessment of diet quality using the HEI, HEI-C, AHEI, and their adaptations may be useful in intervention studies but that further development is needed, preferably in concert with other nutrition research in population contexts. Diet assessment tools need to be feasible and able to be completed in the course of counseling if they are to be widely useful in the clinical and community context. Currently, we cannot compare diverse studies in different groups across countries or over time. Current efforts are encouraged to improve and develop diet-quality assessment tools both for surveillance and for intervention studies to guide counseling practice.
Supplementary Material
ACKNOWLEDGEMENTS
The authors’ responsibilities were as follows—PB, DR, and AR: conducted the research and analyzed the data; PB and DR: wrote the manuscript and had primary responsibility for the final content of the manuscript; and all authors: read and approved the final manuscript.
Notes
The authors reported no funding received for this work.
Author disclosures: The authors report no conflicts of interest.
Supplemental Tables 1–3 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/advances.
Abbreviations used: AHEI, Alternate Healthy Eating Index; CVD, cardiovascular disease; HEI, Healthy Eating Index; HEI-C, Canadian Healthy Eating Index; RCT, randomized controlled trial; T2D, type 2 diabetes.
Contributor Information
Paula Brauer, Department of Family Relations and Applied Nutrition, University of Guelph, Guelph, Ontario, Canada.
Dawna Royall, Department of Family Relations and Applied Nutrition, University of Guelph, Guelph, Ontario, Canada.
Ariellia Rodrigues, Department of Family Relations and Applied Nutrition, University of Guelph, Guelph, Ontario, Canada.
References
- 1. Kirkpatrick SI, Vanderlee L, Raffoul A, Stapleton J, Csizmadi I, Boucher BA, Massarelli I, Rondeau I, Robson PJ. Self-report dietary assessment tools used in Canadian research: a scoping review. Adv Nutr. 2017;8(2):276–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Reedy J, Subar AF. 90th anniversary commentary: diet quality indexes in nutritional epidemiology inform dietary guidance and public health. J Nutr. 2018;148(10):1695–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Liese AD, Krebs-Smith SM, Subar AF, George SM, Harmon BE, Neuhouser ML, Boushey CJ, Schap TE, Reedy J. The Dietary Patterns Methods Project: synthesis of findings across cohorts and relevance to dietary guidance. J Nutr. 2015;145(3):393–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Schwingshackl L, Bogensberger B, Hoffmann G. Diet quality as assessed by the Healthy Eating Index, Alternate Healthy Eating Index, Dietary Approaches to Stop Hypertension Score, and health outcomes: an updated systematic review and meta-analysis of cohort studies. J Acad Nutr Diet. 2018;118(1):74–100, e11. [DOI] [PubMed] [Google Scholar]
- 5. Hosseini Z, Whiting SJ, Vatanparast H. Type 2 diabetes prevalence among Canadian adults—dietary habits and sociodemographic risk factors. App Physiol Nutr Metab. 2019;44(10):1099–104. [DOI] [PubMed] [Google Scholar]
- 6. Menke A, Casagrande S, Geiss L, Cowie CC. Prevalence of and trends in diabetes among adults in the United States, 1988–2012. JAMA. 2015;314(10):1021. [DOI] [PubMed] [Google Scholar]
- 7. Jeejeebhoy K, Dhaliwal R, Heyland DK, Leung R, Day AG, Brauer P, Royall D, Tremblay A, Mutch DM, Pliamm Let al. . Family physician-led, team-based, lifestyle intervention in patients with metabolic syndrome: results of a multicentre feasibility project. CMAJ Open. 2017;5(1):E229–e36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, Nathan DM. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346(6):393–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Estruch R, Ros E, Salas-Salvado J, Covas MI, Corella D, Aros F, Gomez-Gracia E, Ruiz-Gutierrez V, Fiol M, Lapetra Jet al. . Primary prevention of cardiovascular disease with a Mediterranean diet supplemented with extra-virgin olive oil or nuts. N Engl J Med. 2018;378(25):389. [DOI] [PubMed] [Google Scholar]
- 10. Kirkpatrick SI, Reedy J, Krebs-Smith SM, Pannucci TE, Subar AF, Wilson MM, Lerman JL, Tooze JA. Applications of the Healthy Eating Index for surveillance, epidemiology, and intervention research: considerations and caveats. J Acad Nutr Diet. 2018;118(9):1603–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kennedy ET, Ohls J, Carlson S, Fleming K. The Healthy Eating Index: design and applications. J Am Diet Assoc. 1995;95(10):1103–8. [DOI] [PubMed] [Google Scholar]
- 12. Bowman SA, Lino M, Gerrior SA, Basiotis PP. The Healthy Eating Index: 1994–96. 1998, U.S. Department of Agriculture, Center for Nutrition Policy and Promotion, Alexandria (VA). [Google Scholar]
- 13. Guenther PM, Reedy J, Krebs-Smith SM. Development of the Healthy Eating Index-2005. J Am Diet Assoc. 2008;108(11):1896–901. [DOI] [PubMed] [Google Scholar]
- 14. USDA, Centre for Nutrition Policy and Promotion . Diet quality of Americans in 1994–96 and 2001–02 as measured by the Healthy Eating Index-2005. Nutrition Insight 37; December 2007 [revised August 2008; cited 2020 Nov 6] [Internet]. Available from: https://nesr.usda.gov/sites/default/files/2019-05/Reviewing%20the%20Science%20on%20Nutrition%20and%20Health.pdf. [Google Scholar]
- 15. Reedy J, Lerman JL, Krebs-Smith SM, Kirkpatrick SI, Pannucci TE, Wilson MM, Subar AF, Kahle LL, Tooze JA. Evaluation of the Healthy Eating Index-2015. J Acad Nutr Diet. 2018;118(9):1622–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Freedman LS, Guenther PM, Krebs-Smith SM, Kott PS. A population's mean Healthy Eating Index-2005 scores are best estimated by the score of the population ratio when one 24-hour recall is available. J Nutr. 2008;138(9):1725–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. McCullough ML, Feskanich D, Stampfer MJ, Giovannucci EL, Rimm EB, Hu FB, Spiegelman D, Hunter DJ, Colditz GA, Willett WC. Diet quality and major chronic disease risk in men and women: moving toward improved dietary guidance. Am J Clin Nutr. 2002;76(6):1261–71. [DOI] [PubMed] [Google Scholar]
- 18. Chiuve SE, Fung TT, Rimm EB, Hu FB, McCullough ML, Wang M, Stampfer MJ, Willett WC. Alternative dietary indices both strongly predict risk of chronic disease. J Nutr. 2012;142(6):1009–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Garriguet D. Diet quality in Canada. Health Rep. 2009;20(3):41–52. [PubMed] [Google Scholar]
- 20. Jessri M, Ng AP, L'Abbe MR. Adapting the Healthy Eating Index 2010 for the Canadian population: evidence from the Canadian National Nutrition Survey. Nutrients. 2017;9(8):910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Higgins JPT, Altman DG, Sterne JAC. Assessing risk of bias in included studies. In: Higgins JPT, Churchill R, Chandler J, Cumpston MS, editors. Cochrane Handbook for Systematic Reviews of Interventions. Version 5.20 (updated June 2017). London (UK): Cochrane Collaboration; 2017, pg. 8.1–8.73. [Google Scholar]
- 22. Sterne JA, Hernan MA, Reeves BC, Savovic J, Berkman ND, Viswanathan M, Henry D, Altman DG, Ansari MT, Boutron Iet al. . ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355:i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Keogh JB, Pedersen E, Petersen KS, Clifton PM. Effects of intermittent compared to continuous energy restriction on short-term weight loss and long-term weight loss maintenance. Clin Obes. 2014;4(3):150–6. [DOI] [PubMed] [Google Scholar]
- 24. Marques-Rocha JL, Milagro FI, Mansego ML, Zulet MA, Bressan J, Martinez JA. Expression of inflammation-related miRNAs in white blood cells from subjects with metabolic syndrome after 8 wk of following a Mediterranean diet-based weight loss program. Nutrition. 2016;32(1):48–55. [DOI] [PubMed] [Google Scholar]
- 25. Ortega RM, Rodriguez-Rodriguez E, Aparicio A, Marin-Arias LI, Lopez-Sobaler AM. Responses to two weight-loss programs based on approximating the diet to the ideal: differences associated with increased cereal or vegetable consumption. Int J Vitam Nutr Res. 2006;76(6):367–76. [DOI] [PubMed] [Google Scholar]
- 26. Stolley MR, Fitzgibbon ML, Schiffer L, Sharp LK, Singh V, Van Horn L, Dyer A. Obesity reduction black intervention trial (ORBIT): six-month results. Obesity (Silver Spring). 2009;17(1):100–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Carbonneau E, Begin C, Lemieux S, Mongeau L, Paquette MC, Turcotte M, Labonte ME, Provencher V. A Health at Every Size intervention improves intuitive eating and diet quality in Canadian women. Clin Nutr. 2017;36(3):747–54. [DOI] [PubMed] [Google Scholar]
- 28. Landry AS, Thomson JL, Huye HF, Yadrick K, Connell CL. Mississippi Communities for Healthy Living. Health Educ Behav. 2017;44(2):316–25. [DOI] [PubMed] [Google Scholar]
- 29. Lewis KH, Roblin DW, Leo M, Block JP. The personal shopper—a pilot randomized trial of grocery store-based dietary advice. Clin Obes. 2015;5(3):154–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lin PH, Yancy WS Jr., Pollak KI, Dolor RJ, Marcello J, Samsa GP, Batch BC, Svetkey LP. The influence of a physician and patient intervention program on dietary intake. J Acad Nutr Diet. 2013;113(11):1465–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mohammadshahi M, Haidari F, Karandish M, Ebrahimi S, Haghighizadeh MH. A randomized clinical trial of nutrition education for improvement of diet quality and inflammation in Iranian obese women. J Nutr Metab. 2014;2014:605782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Petrogianni M, Kanellakis S, Kallianioti K, Argyropoulou D, Pitsavos C, Manios Y. A multicomponent lifestyle intervention produces favourable changes in diet quality and cardiometabolic risk indices in hypercholesterolaemic adults. J Hum Nutr Diet. 2013;26(6):596–605. [DOI] [PubMed] [Google Scholar]
- 33. Thomson JL, Goodman MH, Tussing-Humphreys L. Diet quality and physical activity outcome improvements resulting from a church-based diet and supervised physical activity intervention for rural, southern, African American adults: Delta Body and Soul III. Health Promot Pract. 2015;16(5):677–88. [DOI] [PubMed] [Google Scholar]
- 34. Webber KH, Lee E. The diet quality of adult women participating in a behavioural weight-loss programme. J Hum Nutr Diet. 2011;24(4):360–9. [DOI] [PubMed] [Google Scholar]
- 35. Webel AR, Moore SM, Longenecker CT, Currie J, Horvat Davey C, Perazzo J, Sattar A, Josephson RA. Randomized controlled trial of the SystemCHANGE intervention on behaviors related to cardiovascular risk in HIV+ adults. J Acquir Immune Defic Syndr. 2018;78(1):23–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Asaad G, Soria-Contreras DC, Bell RC, Chan CB. Effectiveness of a lifestyle intervention in patients with type 2 diabetes: the Physical Activity and Nutrition for Diabetes in Alberta (PANDA) Trial. Healthcare (Basel). 2016;4(4):73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Turner-McGrievy GM, Barnard ND, Cohen J, Jenkins DJ, Gloede L, Green AA. Changes in nutrient intake and dietary quality among participants with type 2 diabetes following a low-fat vegan diet or a conventional diabetes diet for 22 weeks. J Am Diet Assoc. 2008;108(10):1636–45. [DOI] [PubMed] [Google Scholar]
- 38. Ma Y, Olendzki BC, Wang J, Persuitte GM, Li W, Fang H, Merriam PA, Wedick NM, Ockene IS, Culver ALet al. . Single-component versus multicomponent dietary goals for the metabolic syndrome: a randomized trial. Ann Intern Med. 2015;162(4):248–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lynch EB, Mack L, Avery E, Wang Y, Dawar R, Richardson D, Keim K, Ventrelle J, Appelhans BM, Tahsin Bet al. . Randomized trial of a lifestyle intervention for urban low-income African Americans with type 2 diabetes. J Gen Intern Med. 2019;34(7):1174–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Arnold K, Weinhold KR, Andridge R, Johnson K, Orchard TS. Improving diet quality is associated with decreased inflammation: findings from a pilot intervention in postmenopausal women with obesity. J Acad Nutr Diet. 2018;118(11):2135–43. [DOI] [PubMed] [Google Scholar]
- 41. Hedrick VE, Davy BM, You W, Porter KJ, Estabrooks PA, Zoellner JM. Dietary quality changes in response to a sugar-sweetened beverage-reduction intervention: results from the Talking Health randomized controlled clinical trial. Am J Clin Nutr. 2017;105(4):824–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Njike VY, Yarandi N, Petraro P, Ayettey RG, Treu JA, Katz DL. Inclusion of walnut in the diets of adults at risk for type 2 diabetes and their dietary pattern changes: a randomized, controlled, cross-over trial. BMJ Open Diabetes Res Care. 2016;4(1):e000293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ptomey LT, Steger FL, Lee J, Sullivan DK, Goetz JR, Honas JJ, Washburn RA, Gibson CA, Donnelly JE. Changes in energy intake and diet quality during an 18-month weight-management randomized controlled trial in adults with intellectual and developmental disabilities. J Acad Nutr Diet. 2018;118(6):1087–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Swoboda CM, Miller CK, Wills CE. Impact of a goal setting and decision support telephone coaching intervention on diet, psychosocial, and decision outcomes among people with type 2 diabetes. Patient Educ Couns. 2017;100(7):1367–73. [DOI] [PubMed] [Google Scholar]
- 45. Chang AR, Bailey-Davis L, Hetherington V, Ziegler A, Yule C, Kwiecen S, Graboski E, Melough MM, Collins C, Anderson C. Remote dietary counseling using smartphone applications in patients with stages 1–3a chronic kidney disease: a mixed methods feasibility study. J Ren Nutr. Published online 13 May 2019. doi: 10.1053/j.jrn.2019.03.080. PMID: 31078403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ventura Marra M, Lilly CL, Nelson KR, Woofter DR, Malone J. A pilot randomized controlled trial of a telenutrition weight loss intervention in middle-aged and older men with multiple risk factors for cardiovascular disease. Nutrients. 2019;11(2):229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Brauer P, Royall D, Li A, Rodrigues A, Green J, Macklin S, Craig A, Pasanen J, Brunelle L, Maitland Set al. . Nutrient intake and dietary quality changes within a personalized lifestyle intervention program for metabolic syndrome in primary care. Appl Physiol Nutr Metab. 2019;44(12):1297–304. [DOI] [PubMed] [Google Scholar]
- 48. Ioannidis JPA. Neglecting major health problems and broadcasting minor, uncertain issues in lifestyle science. JAMA. 2019:1–2., Oct. 18. doi: 10.1001/jama.2019.17576. [DOI] [PubMed] [Google Scholar]
- 49. Ioannidis JPA. Dissenting opinions in nutrition research—reply. JAMA. 2020;323(10):1000–1. [DOI] [PubMed] [Google Scholar]
- 50. Schwingshackl L, Knuppel S, Schwedhelm C, Hoffmann G, Missbach B, Stelmach-Mardas M, Dietrich S, Eichelmann F, Kontopantelis E, Iqbal Ket al. . Perspective: NutriGrade: a scoring system to assess and judge the meta-evidence of randomized controlled trials and cohort studies in nutrition research. Adv Nutr. 2016;7(6):994–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Weaver CM, Miller JW. Challenges in conducting clinical nutrition research. Nutr Rev. 2017;75(7):491–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Trepanowski JF, Ioannidis JPA. Perspective: limiting dependence on nonrandomized studies and improving randomized trials in human nutrition research: why and how. Adv Nutr. 2018;9(4):367–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Arnett DK, Blumenthal RS, Albert MA, Buroker AB, Goldberger ZD, Hahn EJ, Himmelfarb CD, Khera A, Lloyd-Jones D, McEvoy JWet al. . 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease. Executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;140(11):e563–e95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Tobe SW, Stone JA, Anderson T, Bacon S, Cheng AYY, Daskalopoulou SS, Ezekowitz JA, Gregoire JC, Gubitz G, Jain Ret al. . Canadian Cardiovascular Harmonized National Guidelines Endeavour (C-CHANGE) guideline for the prevention and management of cardiovascular disease in primary care: 2018 update. CMAJ. 2018;190(40):E1192–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Royall D, Brauer P, Bjorklund L, O'Young O, Tremblay A, Jeejeebhoy K, Heyland D, Dhaliwal R, Klein D, Mutch DM. Development of a dietary management care map for metabolic syndrome. Can J Diet Pract Res. 2014;75(3):132–9. [DOI] [PubMed] [Google Scholar]
- 56. Rosner B. Fundamentals of biostatistics. 8th ed. Boston (MA): Cengage Learning; 2016. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


