ABSTRACT
Immoderate calorie intake coupled with a sedentary lifestyle are major determinants of health issues and inflammatory diseases in modern society. The balance between energy consumption and energy expenditure is critical for longevity. Excessive energy intake and adiposity cause systemic inflammation, whereas calorie restriction (CR) without malnutrition, exerts a potent anti-inflammatory effect. The objective of this review was to provide an overview of different strategies used to reduce calorie intake, discuss physiological mechanisms by which CR might lead to improved health outcomes, and summarize the present knowledge about inflammatory diseases. We discuss emerging data of observational studies and randomized clinical trials on CR that have been shown to reduce inflammation and improve human health.
Keywords: inflammatory disease, fasting, caloric restriction, gut microbiota, endoplasmic reticulum stress, autophagy, metabolic switch
Introduction
Modern society has brought profound changes in lifestyle. Diets have become less healthy with the overconsumption of calories (e.g. Western diet). This along with sedentary behavior has led to weight gain and metabolic alterations, increasing the vulnerability to inflammation-driven chronic diseases (1, 2). A growing body of evidence has demonstrated that the balance between energy consumption and energy expenditure is critical not only for longevity but also for improved quality of life across the lifespan (3, 4). It would be naïve to posit that starvation is a key to reverse the onset and development of chronic diseases because a balanced diet is critical for the proper maintenance of healthy physiological and metabolic functions. Nevertheless, the “hormesis hypothesis” suggests that the adaptive responses of cells and organs to a moderate stress may prevent worse damage caused by a stronger similar stress. Within this context, calorie restriction (CR) (called “caloric restriction” or “calorie restriction”) is considered to have many beneficial effects on health (5, 6). Indeed, McCay et al. first reported in 1935 that rats fed a CR diet lived longer (7). Accumulating data from observational cohort and randomized clinical trials show that CR results in some metabolic and molecular adaptations that have been shown to improve health and delay the accumulation of molecular damage in inflammatory disorders. Studies published during the last decade have conclusively demonstrated that CR slows the progression of multiple age-related conditions, including diabetes, cardiovascular diseases, neurological disorders, chronic inflammatory diseases, and cancer (8–10).
Because many chronic diseases ultimately arise from diet-induced inflammation, a logical approach to minimize the impact of these inflammation-related conditions is to follow anti-inflammatory diets. Excessive energy intake and consistent adiposity cause systemic inflammation, whereas moderate CR without malnutrition exerts a potent anti-inflammatory effect (11). But what does CR actually mean? The most widely accepted view is that the health benefits of CR are attributed to eating fewer calories, whatever the source of those fewer calories might be, whether protein, carbohydrate, or fat (12, 13). Several CR strategies were developed to reduce calorie intake (14). Sustained periods of CR or fasting are commonly used to maintain human health, to manage overweight and pathological states, and consequently improve aging circumstances. Improvement of overall health and well-being as well as the physiological effects of CR have been documented for rodents, monkeys, and humans (8–10). These effects involve shaping of the gut microbiota (15) and adaptive cellular responses that optimize energy metabolism, favor cellular protection, improve insulin sensitivity and glucose homeostasis, induce functional changes in the neuroendocrine systems, and reduce oxidative damage and inflammation (8, 11, 14, 16).
In this review, we discuss the different dietary strategies to achieve CR, the cellular and physiological response to these diets as well as their impact on the gut microbiota, with a particular interest in anti-inflammatory effects. Finally, we discuss the potential use of CR strategies in the management of human inflammatory diseases.
Current Status of Knowledge
Different strategies of CR
Extreme restriction in macronutrients, such as a nutritional ketogenic diet (17) are beyond the scope of this review. CR consists of a balanced and moderated decrease in the intake of all nutrients. For the first time, the data presented by McCay et al. described that the restriction of calories without malnutrition prolongs the lifespan in rats compared with ad libitum feeding (7). Subsequently, the reports published during 1946 to 1955 evaluating the effect of CR on development and lifespan focused primarily on defining the experimental diet ingredients and testing different restriction protocols (18, 19). One of the first publications to discuss the appropriateness of CR for humans appeared in 1946 (18). Carlson and Hoelzel (18) speculated that the abundance of food presented to humans in modern society is concomitant for drive us to eat which would make daily CR difficult. The authors suggested that a more realistic method of CR in humans would be to fast on a periodic schedule. Although questions surrounding the effectiveness of CR in humans have yet to be answered, Carson and Hoelzel did establish a new method for CR, i.e. intermittent fasting (IF), one that is currently being tested for use in humans (20, 21). Currently, different strategies that do not result in malnutrition are used to reduce calorie intake (Table 1). Continuous energy restriction (CER) consists of limiting daily caloric intake below energy needs (22). Fasting manipulates meal timing or eating frequency and involves a severe or complete restriction of calorie intake for a consistent window of 8 to 12 h. Fasting-related strategies can be categorized into 4 approaches: IF, alternate-day fasting (ADF), alternate-day modified fasting (ADMF), and time-restricted feeding (TRF) (14, 23, 24). Modern lifestyle reduces the duration of time spent fasting and maintains individuals in a persistent postprandial state (25). The concept of TRF arose within the context of circadian rhythms and is defined as the provision of food for ≤12 h during the active phase (26–28). The majority of TRF studies have also initiated the eating window early in the active phase, presumably to maximize the metabolic benefits (14, 27). A fasting mimicking diet (FMD), which is a combination of CR and IF, consists of the consumption of a hypocaloric diet for 5 consecutive days. Considering the role of ketone bodies (see below) and the 3 d of delay for their endogenous production (26), this strategy seems to be the most efficient.
TABLE 1.
Different calorie restriction protocols
| Dietary regimens | Description |
|---|---|
| Normal balanced diet | 55% carbohydrate, 30% lipids, 15% protein.Caloric intake according to daily energy needs |
| Continuous energy restriction (CER) | ↓ daily caloric intake for ≤10–30% of energy needs |
| Intermittent fasting (IF) | Severe energy restriction (≤25% of energy needs) on 2 or 3 d per wk (5:2-IF or 4:3-IF).Consecutive or nonconsecutive fasting days.Ad libitum eating for the remaining days |
| Alternate day fasting (ADF) | Alternates days of ad libitum eating with fasting days (≃0 calories) |
| Alternate day modified fasting (ADMF) | Alternates days of ad libitum eating with fasting days (≤25% of energy needs) |
| Time-restricted fasting (TRF) or periodic fasting (PF) | Restricts food intake to a feeding time window(≤12 h per d) during the waking phase |
| Fasting mimicking diet (FMD) | ↓ daily caloric intake for 5 consecutive days (≃30% of energy needs) with low carbohydrate/low protein intake + micronutrient supplementation Ad libitum eating for the remaining days |
| Nutritional ketogenic diet | Extreme restriction in carbohydrates 4% carbohydrates, 6% proteins, 90% fat |
These different strategies of intermittent energy restriction (IER) work just like CER with it focusing more on weekly calorie averages than daily calorie averages. However, regardless of the strategy, long-term adherence and compliance for IER are better than CER. Overeating on the “feed day” due to elevated hunger followed on from the “fast day” is obviously a concern with these approaches. However, studies on IER have concluded that even after fasting every other day, participants report high levels of satiety throughout the duration of the study and no compensatory eating. This observation probably reflects an adaptation to the IER achieved within a few weeks (29). Overall, IER is novel and a potentially more efficacious intervention for weight loss, preservation of lean mass, and improved metabolic health. Indeed, moderate and short-term CER or IER does not compromise quality of life and are tolerable, but their influence on appetite as well as difficulties in adherence question their long-term feasibility and efficiency. Fasting strategies are considered to have a better adherence than CER. However, there is no “standard” protocol for fasting at this time. A lot of research was reviewed for this article and almost all used a different fasting definition. Indeed, most studies use ad libitum diets as control groups, making it harder to determine whether one fasting protocol is more advantageous than another. Taking into account that all CR protocols investigated have shown comparable metabolic benefits, it is suggested that choosing a protocol that can best fit an individual's lifestyle will likely increase compliance and long-term success.
Mechanisms contributing to the anti-inflammatory effect of CR
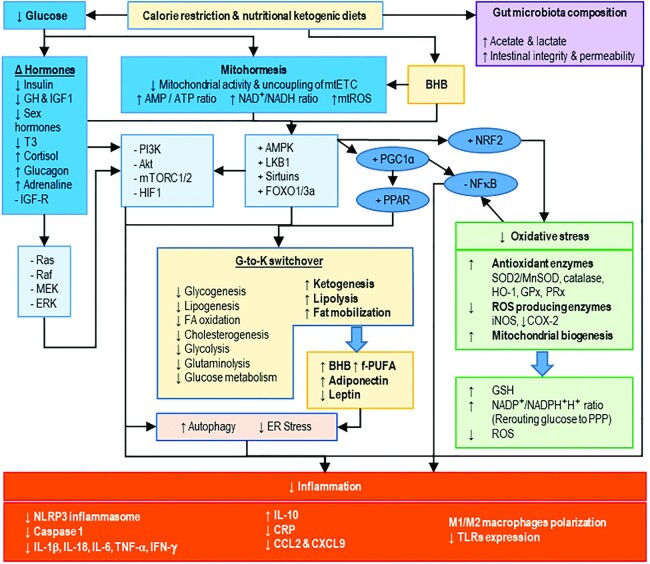
Several studies that were conducted on animal models support the observation that CR has the capacity to reduce inflammation. Accordingly, evidence supporting the antioxidant and anti-inflammatory properties, using mainly animal models, has shown a rapid growth during the last decade and has been previously reviewed (30). CR strategies decrease serum glucose concentrations within the organism and trigger both molecular and cellular adaptations, which induce a robust metabolic switching in major organs and highly affect inflammatory responses (Figure 1) (31–33).
FIGURE 1.
Calorie restriction and anti-inflammatory effects. Calorie restriction (CR) promotes a switch in gut microbiota composition and favors protecting bacteria which produce anti-inflammatory SCFAs, improve intestinal integrity and permeability, and limit bacterial toxin internalization. CR is detected by the decrease in serum glucose concentration and subsequent decrease of mitochondrial activity. On the one hand, hypoglycemia decreases anabolic hormones (e.g. insulin, GH, and IGF1), as well as sex and thyroid hormones, increases the expression of the catabolic cortisol, and subsequently inhibits the MAPK pathway (i.e. RAS/RAF/MEK/ERK) and the PI3K/Akt/mTOR pathway. On the other hand, the inhibition of ERK avoids mTOR activation and subsequently induces autophagy activity, which contributes to the suppression of inflammation by downregulation of both IFN and proinflammatory cytokine responses. Inhibition of mTOR also inhibits HIF1, a transcription factor involved in the upregulation of the inflammation related genes (e.g. cytokines, chemokines, iNOS, and COX-2) as well as in the mediation of the proinflammatory effect of ROS and the activation of NF-κB (34, 35). Moreover, the decrease of mitochondrial activity activates AMPK and downstream regulators such as sirtuins and transcription factors (e.g. FoxO3A and FoxO1) and subsequently activates PGC-1α. PGC-1α is a major inhibitor of NF-kB and activates the anti-inflammatory nuclear receptor PPAR. The activation of AMPK activates the nuclear factor-E2 related-factor 2 (NRF2)-dependent response to oxidative stress, which extends the inhibition of NF-kB and promotes autophagy-dependent repression of inflammation. Moreover, activation of AMPK decreases reticulum stress and triggers the switch from glucose to ketones which is a global metabolism modification consisting of 1) the decrease of the anabolic pathways and glucose utilization, 2) the increase of adipose tissue lipolysis and the production of ketone bodies (e.g. BHB), and also 3) modulation of adipokine and hormone secretion by adipose tissue. In summary, BHB and adiponectin inhibit inflammation through activation of the AMPK regulation network. In contrast, circulating amounts of leptin, a proinflammatory hormone produced by the white adipose tissue decreased. Therefore, CR-dependent inhibition of NF-kB and of PI3K signaling pathways contribute to the maintenance of the oxidative status and have an anti-inflammatory effect through the inhibition of NLRP3, the decrease of proinflammatory markers, the increase of anti-inflammatory IL-10, and the improvement of anti-inflammatory Treg and M2 cells polarization. Akt, AKT serine/threonine kinase; AMPK, AMP-activated protein kinase; BHB, β-hydroxybutyrate; CCL2, C-C motif chemokine ligand 2; COX-2, cyclooxygenase-2; CR, calorie restriction; CRP, C-reactive protein; CXCL9, C-X-C motif chemokine ligand 9; ER stress, endoplasmic reticulum stress; ERK, extracellular signal-regulated kinase; FOXO, forkhead box O; f-PUFA, free-PUFAs; GH, growth hormone; GPx, glutathione peroxidase; GSH, glutathione; G-to-K switchover, glucose-ketone switchover; HIF1, hypoxia-inducible factor 1; HO-1, heme oxygenase-1; IGF-1, insulin-like growth factor-1; IGF-R, insulin-like growth factor-receptor; iNOS, inductible nitric oxide synthase; LKB1, liver kinase B1; MEK, Raf, Ras, serine/threonine kinase; MnSOD, manganese superoxide dismutase; mtETC, mitochondrial electron transport chain; mTORC1/2, mammalian target of rapamycin-1/2; mtROS, mitochondrial reactive oxygen species; NLRP3, pyrin-containing receptor 3; NRF2, nuclear factor erythroid 2-related factor 2; PGC1-α, peroxisome proliferator-activated receptor-γ coactivator 1-α; PI3K, phosphatidylinositol 3-kinase; PPAR, peroxisome proliferator-activated receptor; PPP, pentose phosphate pathway; PRx, peroxiredoxin; ROS, reactive oxygen species; SOD2, superoxide dismutase 2; T3, triiodothyronine; TLR, toll-like receptor.
Sensing of CR and downstream signaling pathways
CR-induced hypoglycemia decreases anabolic hormones, inhibits insulin-dependent anabolic metabolism through the inhibition of the phosphatidylinositol 3-kinase (PI3K) and mitogen-activated protein kinase (MAPK) signaling pathways, which finally avoids the activation of mammalian target of rapamycin (mTOR). The inactivity of mTOR induces autophagy, which contributes to the suppression of inflammation by downregulation of both IFN and proinflammatory cytokines secretion and also by inflammasome inhibition (6). Inactive mTOR also prevents hypoxia inducible factor 1 (HIF-1)-dependent activation of genes related to inflammation, proinflammatory effects of reactive oxygen species (ROS), and NF-κB activation (34, 35).
CR-induced hypoglycemia reduces mitochondrial activity and leads to a decrease in ATP synthesis, an accumulation of oxidized NAD+, and a low production of ROS in order to maintain a low-grade oxidative stress which is considered to be protective according to the mitohormesis hypothesis (36). Therefore, CR-dependent maintenance of low levels of ROS limits the production of proinflammatory molecules.
The accumulation of AMP and NAD+, as well as inhibition of PI3K signaling pathways, activate sirtuin 1 (SIRT1) and AMP-activated protein kinase (AMPK)-dependent regulatory proteins and subsequently activate peroxisome proliferator-activated receptor-γ coactivator 1-α (PGC-1α), a coregulator of numerous transcription factors. PGC-1α inhibits NF-κB, a major activator of the expression of several proinflammatory genes (37). Moreover, PGC-1α activates peroxisome proliferator-activated receptor (PPAR)-α and PPAR-γ, which mediate anti-inflammatory effects (38).
CR-dependent regulation of the PI3K pathway increases apoptosis and autophagy (allowing recycling of biochemical compounds) and decreases reticulum endoplasmic stress (24, 39–41). PGC-1α does not seem to be required for the fasting regulation of unfolded protein response (UPR) and the autophagy process but may be involved in regulating basal hepatic autophagy (42).
Steroid hormones also participate in CR-dependent regulation of inflammation. CR activates the hypothalamic-pituitary-adrenal (HPA) axis, increases the production of glucocorticoids, and thus counteracts inflammation. The anti- or pro-inflammatory effects of glucocorticoids are context dependent, with variable responses depending upon concentration, time of exposure, the compound type, and also the nature of the stimulus (43). According to the hormesis theory, glucocorticoids mediate the anti-inflammatory effect under physiological stress, such as CR, due to the inhibition of key inflammatory transcriptional regulators [e.g. activator protein-1 (AP-1) and NF-κB] (44). Cortisol reduces the degradation and phosphorylation of nuclear factor of kappa light polypeptide gene enhancer in B-cells inhibitor-alpha (IκBα) in a dose-dependent manner, demonstrating a significant inhibitory effect on NF-κB and MAPK pathway activities (45).
In summary, CR modulates hormonal activities, induces mild to moderate oxidative stress according to the hormesis hypothesis, and subsequently triggers several intracellular signaling pathways resulting in the regulation of UPR, autophagy activity, and thus the inhibition of inflammation.
Role of CR in the maintenance of both oxidative and inflammatory homeostasis
A delicate balance between the protective and damaging redox effects of glucose exists (46). Beside their role in oxidative defense at low concentration under nonpathological conditions, high concentrations of ROS and other reactive species (RS) have deleterious effects via inducing an uncontrolled oxidative stress. Mitochondrial metabolism results in the production of numerous ROS (36, 47). This uncontrolled oxidative stress is tightly associated with the establishment of inflammation. Evidence suggests that the mechanisms by which intensive oxidative stress induces chronic inflammation relies on the ROS ability to activate cell signaling cascades that include IκB kinase and MAPKs, which further turn on NF-κB.
CR-induced hypoglycemia and subsequent activation of SIRT1/AMPK regulating network activates Nuclear Factor E2 related Factor (NRF2) to promote a response to oxidative stress through increasing the expression of antioxidant enzymes [e.g. superoxide dismutase 2 (SOD2), catalase, glutathione peroxidase (GPx), and peroxiredoxin (PRx)], decreasing the expression of RS productive enzymes [e.g. inductible nitric oxide synthase (iNOS)], and increasing mitochondrial biogenesis. Moreover, redirecting of glucose into the pentose phosphate pathway (PPP) reduces NADP+ concentration and maintains redox homeostasis under CR conditions (46).
Thus, CR favors a protective redox state and limits systemic inflammation by the activation of antioxidant enzymes.
CR-induced metabolic switch and regulation of inflammation by lipid compounds
The activation of both “SIRT1/AMPK regulatory network” and PPAR receptors, and conversely inhibition of the PI3K signaling pathway have critical metabolic consequences such as the increase of lipolysis and ketogenesis and the shift of substrate utilization for energy production from glucose to fatty acids and ketone bodies (24). This metabolic switch [named Glucose-Ketone (G-to-K) switchover] improves cellular metabolic flexibility and bioenergetic efficiency. Thereby, CR increases circulating concentrations of ketone bodies and free fatty acids (FFAs) (48).
β-hydroxybutyrate (BHB) is a major endogenous ketone body produced under CR conditions (49). Besides being an important substitute to glucose as an energy substrate, BHB is also a signaling molecule that plays a key role in the regulation of numerous proteins and physiological processes by its ability to bind to histones, transcription factors and transcription coregulators, or enzymes (e.g. SIRT) to regulate their activities. In particular, high concentrations of BHB resulting from the G-to-K switchover activates PGC-1α and maintains the suppression of NF-κB activity (24, 50, 51). Inhibition of the nucleotide-binding domain leucin-rich repeat (LRR) and pyrin-containing receptor 3 (NLRP3) inflammasome with BHB is independent of the classical starvation regulated mechanisms such as AMPK, ROS, autophagy, or the inhibition of glycolysis (51). Regarding their structure, FFAs have a differential effect on NLRP3 inflammasome activation. SFAs promote inflammasome activation and IL-1β secretion. High concentrations of ω-3 PUFAs compete with ω-6 PUFAs for the same enzymes, thus reducing the production of arachidonic acid-derived proinflammatory eicosanoids (e.g. prostaglandin E2, leukotriene B4, and the thromboxane 2 series) that have chemotactic and procoagulant actions, and increasing the synthesis of anti-inflammatory eicosanoids (e.g. prostaglandin E3, leukotriene B5, and the thromboxane 3 series) that have immunomodulatory effects (52, 53).
Briefly, metabolic adaptation to CR leads to the production of lipids with anti-inflammatory proprieties.
CR-induced adipose tissue remodeling and inhibition of inflammation
CR-dependent lipolysis leads to an important remodeling of the adipose tissue. Beside its major function in energy storage, white adipose tissue (WAT) is also a major endocrine tissue by secreting adipokines. Their secretory profile differs according to the size of adipocytes. Indeed, small adipocytes secrete more adiponectin, less monocyte chemotactic protein 1 (MCP-1), and less TNFα than large adipocytes (which characterize obesity) (54). CR-triggered lipolysis promotes a decrease of fat mass, WAT remodeling, and increases circulating concentrations of adiponectin, which prevents inflammation through the activation of AMPK signaling pathways and the subsequent inhibition of NF-κB (24, 55–59). In addition, several studies report that CR decreases the production of both leptin and proinflammatory cytokines, which contribute significantly to the low-grade inflammatory state in obese patients (56, 58, 60–63).
In brief, CR induces adipose tissue remodeling and changes WAT endocrine functions that correct the chronic metainflammation.
Role of CR in the regulation of the immune response and inflammatory markers
CR-dependent downregulation of the PI3K and NF-κB pathways promotes the inhibition of the NLRP3 inflammasome and restricts the production of proinflammatory cytokines (Figure 1) (64–66). Numerous studies have reported that CR strategies correlate with a decrease of proinflammatory markers [e.g. C-reactive protein (CRP), IL-6, and TNF-α] at the circulating level, as well as at the tissue level [e.g. liver (33, 67), brain (65, 68, 69), or intestine (69)] in the context of different types of diseases (56, 70, 71).
A high concentration of adiponectin inhibits macrophage differentiation and shifts macrophage polarization from proinflammatory macrophages 1 (M1) to a macrophages 2 (M2) state (59, 72, 73). M2 macrophages mediate anti-inflammatory effects by restraining M1 proinflammatory activities, protecting adipocyte functions, and maintaining adipose tissue metabolic homeostasis by their involvement in adipose tissue remodeling following body weight loss. M2 macrophages are also involved in the browning of WAT which has several beneficial metabolic effects such as increasing energy expenditure and reducing adiposity. However, the mode of CR differentially alters macrophage infiltration in adipose tissue and might explain the contradictory results such as infiltration of M1 macrophages in obese women (74) or inflammatory inflexibility in obese mice (75). Finally, adiponectin has anti-inflammatory effects on endothelial cells, cardiomyocytes, and fibroblasts (55, 76).
Additionally, CR modulates the immune response to antigenic stimuli. Nutritional glucose and lipids activate both leukocytes toll-like receptor (TLR)-2 and TLR-4 and thus trigger acute postprandial inflammatory responses, which attenuates anti-inflammatory molecules such as IL-10. Noteworthy, the compensatory response of immune cells to macronutrients is less effective in obese patients. Short-term CR prevents an exacerbated inflammatory process (77, 78). Indeed, CR decreases the expression of TLR-4 in the liver and similarly, adiponectin decreases TLR-4 expression on the macrophage surface which inhibits the production of proinflammatory chemokines and upregulates the production of anti-inflammatory cytokines (33, 67, 79, 80).
On the whole, CR induces the production of anti-inflammatory rather than proinflammatory macrophages, resulting in the decrease of proinflammatory markers as well as the decrease of the TLR response to antigenic stimulation.
Gut microbiota changes induced by CR
Many studies have shown the role of gut microbiota as drivers of chronic inflammatory diseases (15, 50, 81). The intestinal microbiota is a key actor of the maintenance of a healthy status and its composition depends on many environmental conditions and particularly on nutritional intake (82–85). Indeed, diet plays a fundamental role in shaping gut microbiome composition and function. It is well known that the Westernized diet, characterized by a high dietary intake of saturated fats and refined sugars together with a low intake of fiber, promotes deleterious gut microbiota, impacts intestinal permeability, and represents a growing risk factor associated with chronic inflammation (86, 87).
Over the last decade, knowledge of gut microbiota and metabolic changes that result from CR has substantially increased. Diet composition and age of models are the major factors that may influence the CR impact on gut microbiota (88, 89). Studies of the CR effect on gut microbiota have been performed in animal, as well as human models. The fasting regimens utilized were 10% to 40% calorie restricted based on either a normal or high-fat diet for animal studies (89, 90), or 700 to 1500 kcal/d for human studies (91, 92). Firmicutes, Bacteroidetes, Proteobacteria, and Actinobacteria are the main phyla in the gut microbiota; however, several studies have shown that CR-induced alterations in the relative abundances of these bacteria varied (93, 94). Some studies reported that IF (24 h feeding/24 h fasted) reduced the Bacteroidetes population at the expense of Firmicutes (95), whereas CR (25% less than the daily ration) enriched Bacteroidetes and greatly reduced the Firmicutes/Bacteroidetes ratio, which in turn enhances metabolic and oxidative parameters (94). The inconsistent results might be due to the variable diversity of the microbes present under a specific phylum, and dietary intervention may have led to changes in low-level taxa without affecting the relative abundance of a major phylum. Indeed, CR restructures the intestinal microbiota composition of diabetic mice with enrichment of species of the genus Lactobacillus, Oscillospira, and Ruminococcus and reduction of species of the genus Akkermansia,Bacteroides, and Bifidobacterium (95). These changes favor a healthy microbiota and the production of both SCFAs and lactate (81), which improve the regeneration of the intestinal crypts (96, 97) and permeability and thus prevents gut leakage (98). Other studies have shown an increased relative abundance of probiotic microbes, such as Bifidobacterium and Lactobacillus in CR-treated mammals which may explain some of the benefits of CR given the acknowledged role of these genera in promoting intestinal homeostasis (90, 94, 99). Moreover, the increased abundances of these probiotics correlated with decreases in body weight, total cholesterol, and triglycerides, and thus Lactobacillus growth might be correlated to a diet-dependent effect on lipid metabolism in subjects under CR conditions (90, 99, 100). The circulating LPS-binding protein (LBP), an inflammatory biomarker was also reduced after CR intervention (45-d 25% restricted diet for mice and 28-d 800 kcal/d diet for humans). The antigen translocation from the intestine to the blood might be considerably reduced with CR intervention, due to the decreased abundance of Gram-positive bacteria (91, 101).
In summary, shaping gut microbiota by CR suggests that subjects can establish a balanced intestinal microbiota composition which is efficient in promoting intestinal homeostasis and attenuating local and systemic inflammation, and thus providing health advantages to the host.
CR in humans: feasibility and effects on inflammatory markers
Evidence for the potential anti-inflammatory mechanisms of CR in humans is more limited (Table 2), and most of the studies addressing this aspect have been developed in obese patients. Circulating concentrations of serum amyloid A protein, IL-6, CRP, TNF-α, and IFN-γ were reduced in obese patients after CR, improving their general inflammatory profile (102–104). Nevertheless, whether the reduction in the systemic concentrations of proinflammatory molecules is due to the reduction in adipose tissue mass and adipocyte-secreted cytokines (i.e. adipokines), or involves a direct effect on immune cells (i.e. macrophages, lymphocytes) after CR (102–104) is still controversial. We focused here on landmark studies addressing this topic and studies with data on inflammatory markers.
TABLE 2.
Human studies on effects of caloric restriction on inflammatory markers
| Subjects | Caloric restriction strategy | Inflammatory markers | Reference |
|---|---|---|---|
| 68 healthy individuals: 40 (20 men & 20 women) in CR group vs. 28 (14 men & 14 women) in ad libitum (AL) group.Age: 20–40 y BMI <25 | Food and beverage restriction during 12 h/d for 1 mo | ↓ CRP↓ IL-6↓ total cholesterol/HDL ratio (HDL risk factor)↓ homocysteine | Aksungar et al. (2007) (106) |
| 29 individuals with type 2 diabetes (15 men & 14 women).Age: 45–70 yBMI >30 | Time-restricted fasting (TRF) (Ramadan) for 15 d | ↓ Hemoglobin A1c (HbA1c) (↑ glycemic control)↓ body fat mass↓ visceral adiposity | Yeoh et al. (2015) (108) |
| 10 individuals with asthma.Age: N/A BMI >30 | Alternate day calorie restriction (ADCR) with <20% of their normal calorie intake on the intervening days for 8 wk | ↓ Serum cholesterol↓ TG↓ oxidative stress markers (8-isoprostane, nitrotyrosine, protein carbonyls, and 4-hydroxynonenal adducts)↓ TNF-α↓ BDNF | Johnson et al. (2007) (21) |
| 36 healthy individuals with risk factors for atherosclerosis: 18 (15 men and 3 women) in CR group vs. 18 consuming Western diet.Age: 35–82 y BMI <25 | Caloric restriction (CR) with ∼30% less energy as compared to a Western diet group for 3–15 y | ↓ CRP↓ systolic & diastolic blood pressure (cardiometabolic risk factor)↓ TNF-α↓ IL-6 | Fontana et al. (2004) (109) |
| 56 healthy individuals with risk factors for age-associated diseases: 28 (24 men & 4 women) in CR group vs. 28 (24 men & 4 women) consuming Western diet.Age: 42–64 y BMI <25 | Caloric restriction (CR) with ∼30% less energy as compared to a Western diet group for an average of 7 y | ↓ HDL-C↓ TG/HDL-C↓ total cholesterol↓ adiponectin↓ fasting glucose↓ fasting insulin | Fontana et al. (2010) (110) |
| 48 healthy nonobese and sedentary individuals.Age: 26–48 y 25 < BMI <30 | Caloric restriction (CR) with: 12 assigned to control group, 12 assigned to CR (25%) group, 12 assigned to CR (12.5%) / exercise (12.5%) group, and 12 assigned to low-calorie liquid diet group for 6 mo | ↓ DNA damage↓ fasting insulin↓ oxidative stress markers | Heilbronn et al. (2006) (111)Larson-Meyer et al. (2006) (112)Redman et al. (2007) (113)Civitarese et al. (2007) (114) |
| 48 healthy nonobese individuals (18 men & 30 women).Age: 50–60 y 23 < BMI <30 | Caloric restriction (CR) with: 10 assigned to control group, 19 assigned to CR (20%) group, and 19 assigned to exercise (20%) group for 1 y | ↓ CRP↓ oxidative damage↓ LDL-cholesterol↓ total cholesterol/HDL ratio (HDL risk factor)↓ leptin↓ insulin↑ insulin sensitivity↑ adiponectin | Racette et al. (2006) (115)Villareal et al. (2006) (116)Fontana et al. (2007) (117)Hofer et al. (2008) (118) |
| 46 healthy nonobese individuals. Age: 24–42 y 25 < BMI <30 | Caloric restriction (30% CR) for 1 y | ↓ CRP↓ PGE2↑ T-cell functions | Pittas et al. (2006) (119)Das et al. (2007) (120)Ahmed et al. (2009) (121) |
| 218 healthy nonobese individuals.Age: 21–51 y (men aged 21–50 y whereas women aged 21–47 y to avoid menopause) 22 < BMI <28 | Caloric restriction (25% CR) for 2 y | ↓ CRP↓ TNF-α↓ LDL-cholesterol↓ TG↓ total cholesterol↓ systolic & diastolic blood pressure (cardiometabolic risk factor) | Rickman et al. (2011) (122)Rochon et al. (2011) (123)Ravussin et al. (2015) (124) |
BDNF, brain-derived neurotrophic factor; CRP, C-reactive protein; N/A, not available; PGE2, prostaglandin E2; TG, triglyceride.
Observational studies
A 2014 meta-analysis of 30 cohort studies that included healthy young men and women examined whether Ramadan fasting altered biomarkers in addition to body weight (105). Some of these studies have reported that Ramadan fasts are associated with significantly lower concentrations of inflammatory markers, such as CRP, IL-6, and TNF-α (106, 107). Previous studies have shown that Ramadan fasting practiced by patients with type 2 diabetes (T2D) for 15 to 21 d leads to a statistically and clinically significant reduction in hemoglobin A1c (HbA1c) concentrations, suggesting that glycemic control is improved substantially during Ramadan fasting in this population (108). Ramadan is the most common form of TRF, and it results in transitory body weight loss, with mixed evidence for improvements in inflammatory marker concentrations.
On the other hand, human asthma studies involving 10 subjects with a BMI over 30 kg/m2 which were maintained for 8 wk on an alternate day calorie restriction (ADCR) dietary regimen in which they are at ad libitum, whereas consuming <20% of their normal calorie intake on the intervening days resulted in the improvement of asthma-related symptoms. Nine of the subjects adhered to the diet and lost an average of 8% of their initial weight during the study. Regarding their asthma-related symptoms, control improved significantly within 2 wk of diet initiation; these changes persisted for the duration of the study. The improved clinical findings were associated with decreased concentrations of serum cholesterol and triglycerides, as well as striking reductions in markers of oxidative stress. Indicators of inflammation, including TNF-α and brain-derived neurotrophic factor (BDNF), were also significantly decreased by ADCR. Compliance with the ADCR diet was high, symptoms and pulmonary function improved, and oxidative stress and inflammation declined in response to the dietary intervention (21).
An ancillary study which was carried out on data collected in members of the CR Society whose participants follow severe self-imposed CR with Optimal Nutrition, called the CRON study, showed that individuals following severe self-imposed CR are lean (BMI 19.7 ± 1.8), voluntary restricting their caloric intake (∼1800 kcal/d) for an average of 15 y, and consuming ∼30% less energy compared with a group of individuals (matched for age, sex, and socioeconomic status) consuming a regular Western diet (109). All cardiometabolic risk factors in the members of the CR Society were lower than in the general population. Interestingly, several serum proinflammatory markers such as CRP (109), TNF-α, and IL-6 were low (110). At the molecular level, the positive impact on several pathways such as PI3K/AKT and AMPK/SIRT further supports the anti-inflammatory potential of CR (125) (Table 2).
Randomized controlled trials
The CALERIE (Comprehensive Assessment of Long-term Effects of Reducing Intake of Energy) trials initiated by the US National Institute of Aging were the first controlled clinical trials of CR (111, 113, 115, 124, 122, 123).
The CALERIE-1 project was composed of 3 pilot studies looking at the short- and mid-term effects of CR at 6 (111, 113) and 12 mo (115). CR was achieved through different modalities: 1) reduced calorie intake (CR), 2) increased exercise energy expenditure, or 3) a combination of both CR and exercise (113, 115, 114, 116–118, 120, 119).
In the CALERIE-1 trial conducted at Pennington Biomedical in Louisiana, a reduction of energy intake alone (25% CR) was compared to a combined reduction in energy intake (12.5%) and a 12.5% increase in energy expenditure through exercise (−12.5% energy intake +12.5% energy expenditure = 25% CR), a positive weight loss control group that through a very low-calorie diet achieved a 15 kg weight loss, and a weight-maintenance control group (111, 113). Although the metabolic profile of study participants was significantly improved with CR, various factors that are associated with cardiovascular disease (e.g. blood pressure, LDL, HDL, fibrinogen, homocysteine), and proinflammatory markers (e.g. CRP and TNF-α) were not influenced by this diet (126, 127). This is likely explained by the young age of individuals enrolled in this trial as well as their relatively good health status at inclusion. However, a decrease in markers of oxidative stress was reported in subjects following a CR especially DNA damage and SOD activity (111, 114).
In the CALERIE-1 trial conducted at Washington University in St. Louis, 48 overweight (BMI: 23.5–29.9) individuals, aged 50–60 y, were randomized for 1 y to 20% CR or 20% increase in energy expenditure by means of endurance exercise or allocated to a control group of healthy lifestyle (115). CR reduced the serum concentration of CRP (116, 117). Furthermore, both CR and exercise-induced weight loss resulted in a significant reduction in oxidative damage to DNA and RNA measured ex vivo in white blood cells (118).
In the CALERIE-1 trial conducted at Tufts University in Boston, 46 young (aged 24–42 y) overweight (BMI: 25–29.9) individuals were randomized to low- versus high-glycemic load during 30% CR (120). Serum concentrations of CRP were reduced in the 30% low-glycemic CR group, but not in the 30% high-glycemic CR group (119). Moreover, 30% CR significantly improved T-cell functions (i.e. delayed-type hypersensitivity response and proliferative response of T cells to T-cell mitogens) and reduced prostaglandin E2 (PGE2) production (121).
Thereafter, a phase 2 multicenter trial (i.e. CALERIE-2) was conducted to investigate the effects at 2 y of a 25% CR in leaner and younger individuals. The CALERIE-2 study enrolled 218 healthy, young, and middle-aged (21–51 y), nonobese men and women (124, 122, 123). This large trial demonstrated that mild CR improves cardiometabolic risk factors, even when implemented in healthy lean or slightly overweight young and middle-aged individuals. Many metabolic and inflammatory markers such as total cholesterol, LDL-cholesterol, triglycerides, CRP, TNF-α, and blood pressure decreased significantly and inversely HDL-cholesterol increased in the CR group (124, 122, 123).
In summary, beyond the beneficial effects of CR on the metabolic and cardiovascular profile, multiple lines of evidence indicate that CR also has anti-inflammatory effects in humans. Trials of CR in patients with immune-mediated inflammatory diseases are eagerly awaited (Table 2).
Conclusion
CR which reduces calorie intake without malnutrition has been shown to exert an anti-inflammatory effect and to extend lifespan in rodent and primate models, and it has been an area of active research for >80 y.
CR appears to promote weight loss and may improve metabolic health. There are a variety of fasting diets which manipulate meal timing or eating frequency and involve a severe or complete restriction of energy intake for a consistent window of 8 to 12 h. Data from observational, experimental, and clinical studies strongly indicate that maintaining a healthy body weight and preventing the accumulation of abdominal fat are essential to prevent multiple chronic diseases and to promote healthy aging. However, there is insufficient data to determine the optimal CR, including the length of the fasting interval, the number of fasting days per week, the degree of energy restriction needed on fasting days, and recommendations for dietary behavior on nonfasting days. Moreover, one may assume that there may be great interindividual and intraindividual variation in the human response to a CR.
Measuring tissue-specific effects of CR using genomic, proteomic, and metabolomic techniques in both animals and humans will foster understanding of the complex biological processes involved in the anti-inflammatory and antiaging effects of this dietary regimen. A growing body of literature suggests that CR can trigger several biological pathways (i.e. increased autophagy and mitochondrial respiratory efficiency), which can result in a host of beneficial biological effects including modifications in energy metabolism, oxidative stress, insulin sensitivity, inflammation, autophagy, neuroendocrine function, and induction of hormesis response, in addition, these CR periods have also been shown to have antimutagenic, anticarcinogenic, and antibacterial effects (128). Indeed, CR favors anti-inflammatory intestinal microbiota, reduces gut permeability, and results in blunted postprandial endotoxemia (129, 130) and systemic inflammation (131), which are typically elevated in obesity.
To conclude, CR has opened new approaches to assess the effects of fasting on metabolism, physiology, and behavior. Although animal experiments have produced great results in preventing or reversing chronic metabolic diseases, the underlying mechanisms remain to be explored. More rigorous human studies are also needed to assess the mechanisms and efficacy of CR in a wide range of diseases. In the coming years, research will continue to explore many unresolved questions. What are the long-term benefits and risks of the various eating patterns? Which fasting-related strategies are feasible as a long-term practice? What specific biological effects on inflammatory diseases are triggered by a particular CR strategy? If a specific way of CR is recommended, at what age is it best to start, for which diseases, and is it safe to continue as you get older?
Whether long-term CR is feasible, safe, and effective for reducing inflammation in humans is not known, and publications of these comprehensive data from both the observational studies and randomized controlled trials will go a long way toward providing suitable information for evaluation. If proven to be efficient, these dietary regimens may offer promising nongenetic, nonpharmacological experimental intervention to improve healthspan at the population level with multiple public health benefits.
ACKNOWLEDGEMENTS
We thank Maryann Odusanya-Borraccino for revising the manuscript.
The authors’ responsibilities were as follows—TK: designed the analyses and wrote the manuscript; LP-B: had primary responsibility for the final content; FH, NCN, ACH, DQ, ND and DA: read and approved the final manuscript.
Notes
TK was funded by grants from the Association François Aupetit (AFA) and by the French Investments for the Future Programme (PIA) project « Lorraine Université d'Excellence » (reference ANR-15-IDEX-04-LUE).
DA was funded by a grant from the “Fondation pour la Recherche Médicale” (reference ECO20170637494).
Author disclosures: The authors report no conflicts of interest.
Abbreviations used: ADCR, alternate day calorie restriction; ADF, alternate-day fasting; ADMF, alternate-day modified fasting; AMPK, AMP-activated protein kinase; BHB, beta-hydroxybutyrate; CALERIE, comprehensive assessment of long-term effects of reducing intake of energy; CER, continous energy restriction; CR, calorie restriction; CRP, C-reactive protein; FFA, free fatty acid; FMD, fasting mimicking diet; G-to-K, glucose-ketone switchover; HIF-1, hypoxia inductible factor 1; IER, intermittent energy restriction; IF, intermittent fasting; MAPK, mitogen-activated protein kinase; mTOR, mammalian target of rapamycin; NLRP3, pyrin-containing receptor 3; PGC-1α, peroxisome proliferator-activated receptor-gamma coactivator 1-alpha; PIK3, phosphatidylinositol 3-kinase; PPAR, peroxisome proliferator-activated receptor; ROS, reactive oxygen species; RS, reactive species; SIRT1, sirtuin 1; SOD, superoxide dismutase; TLR, toll-like receptor; TRF, time-restricted feeding; UPR, unfolded protein response; WAT, white adipose tissue.
Contributor Information
Tunay Kökten, Université de Lorraine, Inserm U1256 NGERE (Nutrition—Genetics and Exposure to Environmental Risks), Nancy, France.
Franck Hansmannel, Université de Lorraine, Inserm U1256 NGERE (Nutrition—Genetics and Exposure to Environmental Risks), Nancy, France.
Ndeye Coumba Ndiaye, Université de Lorraine, Inserm U1256 NGERE (Nutrition—Genetics and Exposure to Environmental Risks), Nancy, France.
Anne-Charlotte Heba, Université de Lorraine, Inserm U1256 NGERE (Nutrition—Genetics and Exposure to Environmental Risks), Nancy, France.
Didier Quilliot, Université de Lorraine, Inserm U1256 NGERE (Nutrition—Genetics and Exposure to Environmental Risks), Nancy, France; Université de Lorraine, Centre Hospitalier Régional Universitaire (CHRU)-Nancy, Department of Diabetology-Endocrinology-Nutrition, Nancy, France.
Natacha Dreumont, Université de Lorraine, Inserm U1256 NGERE (Nutrition—Genetics and Exposure to Environmental Risks), Nancy, France.
Djésia Arnone, Université de Lorraine, Inserm U1256 NGERE (Nutrition—Genetics and Exposure to Environmental Risks), Nancy, France.
Laurent Peyrin-Biroulet, Université de Lorraine, Inserm U1256 NGERE (Nutrition—Genetics and Exposure to Environmental Risks), Nancy, France; Université de Lorraine, Centre Hospitalier Régional Universitaire (CHRU)-Nancy, Department of Gastroenterology, Nancy, France.
References
- 1. Christ A, Lauterbach M, Latz E. Western diet and the immune system: an inflammatory connection. Immunity. 2019;51:794–811. [DOI] [PubMed] [Google Scholar]
- 2. Christ A, Latz E. The Western lifestyle has lasting effects on metaflammation. Nat Rev Immunol. 2019;19:267–8. [DOI] [PubMed] [Google Scholar]
- 3. Fontana L, Partridge L. Promoting health and longevity through diet: from model organisms to humans. Cell. 2015;161:106–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Minor RK, Allard JS, Younts CM, Ward TM, de Cabo R. Dietary interventions to extend life span and health span based on calorie restriction. J Gerontol A Biol Sci Med Sci. 2010;65A:695–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Picca A, Pesce V, Lezza AMS. Does eating less make you live longer and better? An update on calorie restriction. Clin Interv Aging. 2017;12:1887–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Yong-Quan Ng G, Yang-Wei Fann D, Jo D-G, Sobey CG, Arumugam TV. Dietary restriction and epigenetics: part I. Cond Med. 2019;2:284–99. [PMC free article] [PubMed] [Google Scholar]
- 7. McCay CM, Crowell MF, Maynard LA. The effect of retarded growth upon the length of life span and upon the ultimate body size. 1935. Nutrition. 1989;5(3):155–71.;discussion 172. [PubMed] [Google Scholar]
- 8. Muñoz-Hernández L, Márquez-López Z, Mehta R, Aguilar-Salinas CA. Intermittent fasting as part of the management for T2DM: from animal models to human clinical studies. Curr Diab Rep. [Internet]. 2020; [cited 2020 May 21];20. Available from: http://link.springer.com/10.1007/s11892-020-1295-2. [DOI] [PubMed] [Google Scholar]
- 9. Skaznik-Wikiel ME, Polotsky AJ. The health pros and cons of continuous versus intermittent calorie restriction: more questions than answers. Maturitas. 2014;79:275–8. [DOI] [PubMed] [Google Scholar]
- 10. Varady KA, Hellerstein MK. Alternate-day fasting and chronic disease prevention: a review of human and animal trials. Am J Clin Nutr. 2007;86:7–13. [DOI] [PubMed] [Google Scholar]
- 11. Opalach K, Rangaraju S, Madorsky I, Leeuwenburgh C, Notterpek L. Lifelong calorie restriction alleviates age-related oxidative damage in peripheral nerves. Rejuvenation Res. 2010;13:65–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Masoro EJ. Overview of caloric restriction and ageing. Mech Ageing Dev. 2005;126:913–22. [DOI] [PubMed] [Google Scholar]
- 13. Weindruch R, Naylor PH, Goldstein AL, Walford RL. Influences of aging and dietary restriction on serum thymosin alpha 1 levels in mice. J Gerontol. 1988;43:B40–2. [DOI] [PubMed] [Google Scholar]
- 14. Dorling JL, Martin CK, Redman LM. Calorie restriction for enhanced longevity: the role of novel dietary strategies in the present obesogenic environment. Ageing Res Rev. 2020;64:101038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hills RD, Pontefract BA, Mishcon HR, Black CA, Sutton SC, Theberge CR. Gut microbiome: profound implications for diet and disease. Nutrients. 2019;11:1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Martín-Montalvo A, Villalba JM, Navas P, de Cabo R. NRF2, cancer and calorie restriction. Oncogene. 2011;30:505–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Boison D. New insights into the mechanisms of the ketogenic diet. Curr Opin Neurol. 2017;30:187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Carlson AJ, Hoelzel F. Apparent prolongation of the life span of rats by intermittent fasting. J Nutr. 1946;31:363–75. [DOI] [PubMed] [Google Scholar]
- 19. Riesen WH, Herbst EJ. The effect of restricted caloric intake on the longevity of rats. Am J Physiol. 1947;148:614–7. [DOI] [PubMed] [Google Scholar]
- 20. Johnson JB, Laub DR, John S. The effect on health of alternate day calorie restriction: eating less and more than needed on alternate days prolongs life. Med Hypotheses. 2006;67:209–11. [DOI] [PubMed] [Google Scholar]
- 21. Johnson JB, Summer W, Cutler RG, Martin B, Hyun D-H, Dixit VD, Pearson M, Nassar M, Tellejohan R, Maudsley Set al. Alternate day calorie restriction improves clinical findings and reduces markers of oxidative stress and inflammation in overweight adults with moderate asthma. Free Radic Biol Med. 2007;42:665–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Michalczyk MM, Maszczyk A, Stastny P. The effects of low-energy moderate-carbohydrate (MCD) and mixed (MixD) diets on serum lipid profiles and body composition in middle-aged men: a randomized controlled parallel-group clinical trial. IJERPH. 2020;17:1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Anton SD, Moehl K, Donahoo WT, Marosi K, Lee SA, Mainous AG, Leeuwenburgh C, Mattson MP. Flipping the metabolic switch: understanding and applying the health benefits of fasting. Obesity. 2018;26:254–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Malinowski B, Zalewska K, Węsierska A, Sokołowska MM, Socha M, Liczner G, Pawlak-Osińska K, Wiciński M. Intermittent fasting in cardiovascular disorders—an overview. Nutrients. 2019;11:673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Monnier L. Is postprandial glucose a neglected cardiovascular risk factor in type 2 diabetes?. Eur J Clin Invest. 2000;30:3. [PubMed] [Google Scholar]
- 26. Paoli A, Tinsley G, Bianco A, Moro T. The influence of meal frequency and timing on health in humans: the role of fasting. Nutrients. 2019;11:719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Parr EB, Devlin BL, Radford BE, Hawley JA. A delayed morning and earlier evening time-restricted feeding protocol for improving glycemic control and dietary adherence in men with overweight/obesity: a randomized controlled trial. Nutrients. 2020;12:505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Froy O. Circadian rhythms, nutrition and implications for longevity in urban environments. Proc Nutr Soc. 2018;77:216–22. [DOI] [PubMed] [Google Scholar]
- 29. Ravussin E, Gilmore LA, Redman LM. Chapter 48—Calorie restriction in humans: impact on human health. In: Malavolta M, Mocchegiani E, editors. Molecular Basis of Nutrition and Aging. [Internet]. San Diego: Academic Press; 2016. [cited 2020 Jun 29].pp. 677–92. Available from: http://www.sciencedirect.com/science/article/pii/B9780128018163000480. [Google Scholar]
- 30. Fontana L, Partridge L, Longo VD. Extending healthy life span—from yeast to humans. Science. 2010;328:321–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Madkour MI, El-Serafi TA, Jahrami HA, Sherif NM, Hassan RE, Awadallah S, Faris MAE. Ramadan diurnal intermittent fasting modulates SOD2, TFAM, Nrf2, and sirtuins (SIRT1, SIRT3) gene expressions in subjects with overweight and obesity. Diabetes Res Clin Pract. 2019;155:107801. [DOI] [PubMed] [Google Scholar]
- 32. Rahbar AR, Safavi E, Rooholamini M, Jaafari F, Darvishi S, Rahbar A. Effects of intermittent fasting during Ramadan on insulin-like growth factor-1, interleukin 2, and lipid profile in healthy Muslims. Int J Prev Med. 2019; 10:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Miyauchi T, Uchida Y, Kadono K, Hirao H, Kawasoe J, Watanabe T, Ueda S, Okajima H, Terajima H, Uemoto S. Up-regulation of FOXO1 and reduced inflammation by β-hydroxybutyric acid are essential diet restriction benefits against liver injury. Proc Natl Acad Sci U S A. 2019;116:13533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. D'Ignazio L, Bandarra D, Rocha S. NF-κB and HIF crosstalk in immune responses. FEBS J. 2016;283:413–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Eltzschig HK, Carmeliet P. Hypoxia and inflammation. N Engl J Med. 2011;364:656–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Miller VJ, Villamena FA, Volek JS. Nutritional ketosis and mitohormesis: potential implications for mitochondrial function and human health. J Nutr Metab. 2018;2018:1–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Mulero MC, Huxford T, Ghosh G. NF-κB, IκB, and IKK: integral components of immune system signaling. Adv Exp Med Biol. 2019;1172:207–26. [DOI] [PubMed] [Google Scholar]
- 38. Mandard S, Patsouris D. Nuclear control of the inflammatory response in mammals by peroxisome proliferator-activated receptors. PPAR Res. 2013;2013:613864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Chaube R. Can UPR integrate fasting and stem cell regeneration?. Front Chem. [Internet]. 2015; [cited 2020 May 21];3. Available from: http://journal.frontiersin.org/Article/10.3389/fchem.2015.00005/abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ding S, Jiang J, Zhang G, Bu Y, Zhang G, Zhao X. Resveratrol and caloric restriction prevent hepatic steatosis by regulating SIRT1-autophagy pathway and alleviating endoplasmic reticulum stress in high-fat diet-fed rats. PLoS One. 2017;12:e0183541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. González-Rodríguez Á, Mayoral R, Agra N, Valdecantos MP, Pardo V, Miquilena-Colina ME, Vargas-Castrillón J, Lo Iacono O, Corazzari M, Fimia GMet al. Impaired autophagic flux is associated with increased endoplasmic reticulum stress during the development of NAFLD. Cell Death Dis. 2014;5:e1179–e1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kristensen CM, Olsen MA, Jessen H, Brandt N, Meldgaard JN, Pilegaard H. PGC-1α in exercise and fasting-induced regulation of hepatic UPR in mice. Pflüg Arch - Eur J Physiol. 2018;470:1431–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Sorrells SF, Caso JR, Munhoz CD, Sapolsky RM. The stressed CNS: when glucocorticoids aggravate inflammation. Neuron. 2009;64:33–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Rodrigues Vasconcelos A, Cabral-Costa JV, Mazucanti CH, Scavone C, Mitiko Kawamoto E. The role of steroid hormones in the modulation of neuroinflammation by dietary interventions. Front Endocrinol (Lausanne). 2016;7:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Dong J, Qu Y, Li J, Cui L, Wang Y, Lin J, Wang H. Cortisol inhibits NF-κB and MAPK pathways in LPS activated bovine endometrial epithelial cells. Int Immunopharmacol. 2018;56:71–7. [DOI] [PubMed] [Google Scholar]
- 46. Cherkas A, Holota S, Mdzinarashvili T, Gabbianelli R, Zarkovic N. Glucose as a major antioxidant: when, what for and why it fails?. Antioxidants. [Internet]. 2020; [cited 2020 Mar 25];9. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7070274/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Massudi H, Grant R, Guillemin GJ, Braidy N. NAD+ metabolism and oxidative stress: the golden nucleotide on a crown of thorns. Redox Report. 2012;17:28–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Abdellatif M, Sedej S. Cardiovascular benefits of intermittent fasting. Cardiovasc Res. 2020;116:e36–8. [DOI] [PubMed] [Google Scholar]
- 49. Dąbek A, Wojtala M, Pirola L, Balcerczyk A. Modulation of cellular biochemistry, epigenetics and metabolomics by ketone bodies. Implications of the ketogenic diet in the physiology of the organism and pathological states. Nutrients. 2020;12:788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Nandivada P, Fell GL, Pan AH, Nose V, Ling P-R, Bistrian BR, Puder M. Eucaloric ketogenic diet reduces hypoglycemia and inflammation in mice with endotoxemia. Lipids. 2016;51:703–14. [DOI] [PubMed] [Google Scholar]
- 51. Youm Y-H, Nguyen KY, Grant RW, Goldberg EL, Bodogai M, Kim D, D'Agostino D, Planavsky N, Lupfer C, Kanneganti TDet al. The ketone metabolite β-hydroxybutyrate blocks NLRP3 inflammasome-mediated inflammatory disease. Nat Med. 2015;21:263–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Martínez-Micaelo N, González-Abuín N, Pinent M, Ardévol A, Blay M. Dietary fatty acid composition is sensed by the NLRP3 inflammasome: omega-3 fatty acid (DHA) prevents NLRP3 activation in human macrophages. Food Funct. 2016;7:3480–7. [DOI] [PubMed] [Google Scholar]
- 53. Ringseis R, Ringseis E, Mooren F, Mooren K. Metabolic signals and innate immune activation in obesity and exercise [Internet]. Exerc Immunol Rev. 2015; [cited 2020 Apr 21]. Available from: https://pubmed.ncbi.nlm.nih.gov/25825956/?from_term = nefa+inflammasome&from_pos = 2. [PubMed] [Google Scholar]
- 54. Engin AB. Adipocyte-Macrophage Cross-Talk in Obesity. In: Engin AB, Engin A, editors. Obesity and Lipotoxicity [Internet]. Cham: Springer International Publishing; 2017; [cited 2020 May 6].pp. 327–43. Available from: http://link.springer.com/10.1007/978-3-319-48382-5_14. [DOI] [PubMed] [Google Scholar]
- 55. Fang H, Judd RL. Adiponectin Regulation and Function. In: Pollock DM, editor. Comprehensive Physiology [Internet]. Hoboken (NJ): John Wiley & Sons, Inc; 2018; [cited 2020 May 21]. pp. 1031–63. Available from: http://doi.wiley.com/10.1002/cphy.c170046. [DOI] [PubMed] [Google Scholar]
- 56. Lettieri-Barbato D, Giovannetti E, Aquilano K. Effects of dietary restriction on adipose mass and biomarkers of healthy aging in human. Aging. 2016;8:3341–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. McAllister MJ, Pigg BL, Renteria LI, Waldman HS. Time-restricted feeding improves markers of cardiometabolic health in physically active college-age men: a 4-week randomized pre-post pilot study. Nutr Res. 2020;75:32–43. [DOI] [PubMed] [Google Scholar]
- 58. Moro T, Tinsley G, Bianco A, Marcolin G, Pacelli QF, Battaglia G, Palma A, Gentil P, Neri M, Paoli A. Effects of eight weeks of time-restricted feeding (16/8) on basal metabolism, maximal strength, body composition, inflammation, and cardiovascular risk factors in resistance-trained males. J Transl Med. [Internet]. 2016; [cited 2020 May 21];14. Available from: http://translational-medicine.biomedcentral.com/articles/10.1186/s12967-016-1044-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Choi HM, Doss HM, Kim KS. Multifaceted physiological roles of adiponectin in inflammation and diseases. IJMS. 2020;21:1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Cipryan L, Maffetone PB, Plews DJ, Laursen PB. Effects of a four-week very low-carbohydrate high-fat diet on biomarkers of inflammation: non-randomised parallel-group study. Nutr Health. 2020;26:35–42. [DOI] [PubMed] [Google Scholar]
- 61. La Cava A. Leptin in inflammation and autoimmunity. Cytokine. 2017;98:51–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Cho Y, Hong N, Kim K, Cho S, Lee M, Lee Y, Lee Y, Kang E, Cha B-S, Lee B-W. The effectiveness of intermittent fasting to reduce body mass index and glucose metabolism: a systematic review and meta-analysis. JCM. 2019;8:1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Chung H, Chou W, Sears DD, Patterson RE, Webster NJG, Ellies LG. Time-restricted feeding improves insulin resistance and hepatic steatosis in a mouse model of postmenopausal obesity. Metabolism. 2016;65:1743–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Liu B, Page AJ, Hatzinikolas G, Chen M, Wittert GA, Heilbronn LK. Intermittent fasting improves glucose tolerance and promotes adipose tissue remodeling in male mice fed a high-fat diet. Endocrinology. 2019;160(1):169–80. [DOI] [PubMed] [Google Scholar]
- 65. Singh H, Kaur T, Manchanda S, Kaur G. Intermittent fasting combined with supplementation with Ayurvedic herbs reduces anxiety in middle aged female rats by anti-inflammatory pathways. Biogerontology. 2017;18:601. [DOI] [PubMed] [Google Scholar]
- 66. Vasconcelos AR, Yshii LM, Viel TA, Buck HS, Mattson MP, Scavone C, Kawamoto EM. Intermittent fasting attenuates lipopolysaccharide-induced neuroinflammation and memory impairment. J Neuroinflammation. 2014;11:85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Marinho T de S, Ornellas F, Barbosa-da-Silva S, Mandarim-de-Lacerda CA, Aguila MB. Beneficial effects of intermittent fasting on steatosis and inflammation of the liver in mice fed a high-fat or a high-fructose diet. Nutrition. 2019;65:103–12. [DOI] [PubMed] [Google Scholar]
- 68. Zhou Z-L, Jia X-B, Sun M-F, Zhu Y-L, Qiao C-M, Zhang B-P, Zhao L-P, Yang Q, Cui C, Chen Xet al. Neuroprotection of fasting mimicking diet on MPTP-induced Parkinson's disease mice via gut microbiota and metabolites. Neurotherapeutics. 2019;16(3):741–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Zhang X, Zou Q, Zhao B, Zhang J, Zhao W, Li Y, Liu R, Liu X, Liu Z. Effects of alternate-day fasting, time-restricted fasting and intermittent energy restriction DSS-induced on colitis and behavioral disorders. Redox Biol. 2020;32:101535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Elliott RM, de Roos B, Duthie SJ, Bouwman FG, Rubio-Aliaga I, Crosley LK, Mayer C, Polley AC, Heim C, Coort SLet al. Transcriptome analysis of peripheral blood mononuclear cells in human subjects following a 36 h fast provides evidence of effects on genes regulating inflammation, apoptosis and energy metabolism. Genes Nutr. [Internet]. 2014; [cited 2020 May 21];9. Available from: http://link.springer.com/10.1007/s12263-014-0432-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Razavi R, Parvaresh A, Abbasi B, Yaghoobloo K, Hassanzadeh A, Mohammadifard N, Clark CCT, Morteza Safavi S. The alternate-day fasting diet is a more effective approach than a calorie restriction diet on weight loss and hs-CRP levels. Int J Vitam Nutr Res. 2020;1–9. [DOI] [PubMed] [Google Scholar]
- 72. Fabbiano S, Suárez-Zamorano N, Rigo D, Veyrat-Durebex C, Stevanovic Dokic A, Colin DJ, Trajkovski M. Caloric restriction leads to browning of white adipose tissue through type 2 immune signaling. Cell Metab. 2016;24:434–46. [DOI] [PubMed] [Google Scholar]
- 73. Kumari M, Heeren J, Scheja L. Regulation of immunometabolism in adipose tissue. Semin Immunopathol. 2018;40:189–202. [DOI] [PubMed] [Google Scholar]
- 74. Liu B, Hutchison AT, Thompson CH, Lange K, Heilbronn LK. Markers of adipose tissue inflammation are transiently elevated during intermittent fasting in women who are overweight or obese. Obes Res Clin Pract. 2019;13:408–15. [DOI] [PubMed] [Google Scholar]
- 75. Lacerda DR, Costa KA, Silveira ALM, Rodrigues DF, Silva AN, Sabino JL, Pinho V, Menezes GB, Soares DD, Teixeira MMet al. Role of adipose tissue inflammation in fat pad loss induced by fasting in lean and mildly obese mice. J Nutr Biochem. 2019;72:108208. [DOI] [PubMed] [Google Scholar]
- 76. Fujisaka S, Usui I, Nawaz A, Takikawa A, Kado T, Igarashi Y, Tobe K. M2 macrophages in metabolism. Diabetol Int. 2016;7:342–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Martínez-García MÁ, Moncayo S, Insenser M, Montes-Nieto R, Fernández-Durán E, Álvarez-Blasco F, Luque-Ramírez M, Escobar-Morreale HF. Postprandial inflammatory responses after oral glucose, lipid and protein challenges: influence of obesity, sex and polycystic ovary syndrome. Clin Nutr. 2020;39:876–85. [DOI] [PubMed] [Google Scholar]
- 78. Martínez-García MÁ, Ojeda-Ojeda M, Rodríguez-Martín E, Insenser M, Moncayo S, Álvarez-Blasco F, Luque-Ramírez M, Escobar-Morreale HF. TLR2 and TLR4 surface and gene expression in white blood cells after fasting and oral glucose, lipid and protein challenges: influence of obesity and sex hormones. Biomolecules. 2020;10:111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Yang W, Cao M, Mao X, Wei X, Li X, Chen G, Zhang J, Wang Z, Shi J, Huang Het al. Alternate-day fasting protects the livers of mice against high-fat diet-induced inflammation associated with the suppression of toll-like receptor 4/nuclear factor κB signaling. Nutr Res. 2016;36:586–93. [DOI] [PubMed] [Google Scholar]
- 80. Zhang Y, Higgins CB, Fortune HM, Chen P, Stothard AI, Mayer AL, Swarts BM, DeBosch BJ. Hepatic arginase 2 (Arg2) is sufficient to convey the therapeutic metabolic effects of fasting. Nat Commun. 2019;10:1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Li G, Xie C, Lu S, Nichols RG, Tian Y, Li L, Patel D, Ma Y, Brocker CN, Yan Tet al. Intermittent fasting promotes white adipose browning and decreases obesity by shaping the gut microbiota. Cell Metab. 2017;26:672–85.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Barko PC, McMichael MA, Swanson KS, Williams DA. The gastrointestinal microbiome: a review. J Vet Intern Med. 2018;32:9–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Thaiss CA, Zeevi D, Levy M, Zilberman-Schapira G, Suez J, Tengeler AC, Abramson L, Katz MN, Korem T, Zmora Net al. Transkingdom control of microbiota diurnal oscillations promotes metabolic homeostasis. Cell. 2014;159:514–29. [DOI] [PubMed] [Google Scholar]
- 84. Thursby E, Juge N. Introduction to the human gut microbiota. Biochem J. 2017;474:1823–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Zarrinpar A, Chaix A, Yooseph S, Panda S. Diet and feeding pattern affect the diurnal dynamics of the gut microbiome. Cell Metab. 2014;20:1006–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Francescangeli F, De Angelis ML, Zeuner A. Dietary factors in the control of gut homeostasis, intestinal stem cells, and colorectal cancer. Nutrients. 2019;11:2936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Leech B, McIntyre E, Steel A, Sibbritt D. Risk factors associated with intestinal permeability in an adult population: a systematic review. Int J Clin Pract. [Internet]. 2019; [cited 2020 May 1];73. Available from: 10.1111/ijcp.13385. [DOI] [PubMed] [Google Scholar]
- 88. Ruiz A, Cerdó T, Jáuregui R, Pieper DH, Marcos A, Clemente A, García F, Margolles A, Ferrer M, Campoy Cet al. One-year calorie restriction impacts gut microbial composition but not its metabolic performance in obese adolescents. Environ Microbiol. 2017;19:1536–51. [DOI] [PubMed] [Google Scholar]
- 89. Zhang C, Li S, Yang L, Huang P, Li W, Wang S, Zhao G, Zhang M, Pang X, Yan Zet al. Structural modulation of gut microbiota in life-long calorie-restricted mice. Nat Commun. 2013;4:2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Fraumene C, Manghina V, Cadoni E, Marongiu F, Abbondio M, Serra M, Palomba A, Tanca A, Laconi E, Uzzau S. Caloric restriction promotes rapid expansion and long-lasting increase of Lactobacillus in the rat fecal microbiota. Gut Microbes. 2018;9:104–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Ott B, Skurk T, Hastreiter L, Lagkouvardos I, Fischer S, Büttner J, Kellerer T, Clavel T, Rychlik M, Haller Det al. Effect of caloric restriction on gut permeability, inflammation markers, and fecal microbiota in obese women. Sci Rep. 2017;7:11955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Pataky Z, Genton L, Spahr L, Lazarevic V, Terraz S, Gaïa N, Rubbia-Brandt L, Golay A, Schrenzel J, Pichard C. Impact of hypocaloric hyperproteic diet on gut microbiota in overweight or obese patients with nonalcoholic fatty liver disease: a pilot study. Dig Dis Sci. 2016;61:2721–31. [DOI] [PubMed] [Google Scholar]
- 93. Damms-Machado A, Mitra S, Schollenberger AE, Kramer KM, Meile T, Königsrainer A, Huson DH, Bischoff SC. Effects of surgical and dietary weight loss therapy for obesity on gut microbiota composition and nutrient absorption. Biomed Res Int. 2015;2015:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Russo M, Fabersani E, Abeijón-Mukdsi MC, Ross R, Fontana C, Benítez-Páez A, Gauffin-Cano P, Medina RB. Lactobacillus fermentum CRL1446 ameliorates oxidative and metabolic parameters by increasing intestinal feruloyl esterase activity and modulating microbiota in caloric-restricted mice. Nutrients. 2016;8:415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Beli E, Yan Y, Moldovan L, Vieira CP, Gao R, Duan Y, Prasad R, Bhatwadekar A, White FA, Townsend SDet al. Restructuring of the gut microbiome by intermittent fasting prevents retinopathy and prolongs survival in db / db mice. Diabetes. 2018;67:1867–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Okada T, Otsubo T, Hagiwara T, Inazuka F, Kobayashi E, Fukuda S, Inoue T, Higuchi K, Kawamura YI, Dohi T. Intermittent fasting prompted recovery from dextran sulfate sodium-induced colitis in mice. J Clin Biochem Nutr. 2017;61:100–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Okada T, Fukuda S, Hase K, Nishiumi S, Izumi Y, Yoshida M, Hagiwara T, Kawashima R, Yamazaki M, Oshio Tet al. Microbiota-derived lactate accelerates colon epithelial cell turnover in starvation-refed mice. Nat Commun. 2013;4:1654. [DOI] [PubMed] [Google Scholar]
- 98. Liu Z, Dai X, Zhang H, Shi R, Hui Y, Jin X, Zhang W, Wang L, Wang Q, Wang Det al. Gut microbiota mediates intermittent-fasting alleviation of diabetes-induced cognitive impairment. Nat Commun. 2020;11(1):855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Bernardeau M, Guguen M, Vernoux JP. Beneficial lactobacilli in food and feed: long-term use, biodiversity and proposals for specific and realistic safety assessments. FEMS Microbiol Rev. 2006;30:487–513. [DOI] [PubMed] [Google Scholar]
- 100. Sun J, Buys N. Effects of probiotics consumption on lowering lipids and CVD risk factors: a systematic review and meta-analysis of randomized controlled trials. Ann Med. 2015;47:430–40. [DOI] [PubMed] [Google Scholar]
- 101. Zhang C, Zhang M, Wang S, Han R, Cao Y, Hua W, Mao Y, Zhang X, Pang X, Wei Cet al. Interactions between gut microbiota, host genetics and diet relevant to development of metabolic syndromes in mice. ISME J. 2010;4:232–41. [DOI] [PubMed] [Google Scholar]
- 102. Lee IS, Shin G, Choue R. A 12-week regimen of caloric restriction improves levels of adipokines and pro-inflammatory cytokines in Korean women with BMIs greater than 23 kg/m2. Inflamm Res. 2010;59(5):399–405. [DOI] [PubMed] [Google Scholar]
- 103. Salas-Salvadó J, Bulló M, García-Lorda P, Figueredo R, Del Castillo D, Bonada A, Balanzà R. Subcutaneous adipose tissue cytokine production is not responsible for the restoration of systemic inflammation markers during weight loss. Int J Obes. 2006;30:1714–20. [DOI] [PubMed] [Google Scholar]
- 104. Viguerie N, Poitou C, Cancello R, Stich V, Clément K, Langin D. Transcriptomics applied to obesity and caloric restriction. Biochimie. 2005;87:117–23. [DOI] [PubMed] [Google Scholar]
- 105. Kul S, Savaş E, Öztürk ZA, Karadağ G. Does Ramadan fasting alter body weight and blood lipids and fasting blood glucose in a healthy population? A meta-analysis. J Relig Health. 2014;53:929–42. [DOI] [PubMed] [Google Scholar]
- 106. Aksungar FB, Topkaya AE, Akyildiz M. Interleukin-6, C-reactive protein and biochemical parameters during prolonged intermittent fasting. Ann Nutr Metab. 2007;51:88–95. [DOI] [PubMed] [Google Scholar]
- 107. Faris MA-IE, Kacimi S, Al-Kurd RA, Fararjeh MA, Bustanji YK, Mohammad MK, Salem ML. Intermittent fasting during Ramadan attenuates proinflammatory cytokines and immune cells in healthy subjects. Nutr Res. 2012;32:947–55. [DOI] [PubMed] [Google Scholar]
- 108. Yeoh ECK, Zainudin SB, Loh WN, Chua CL, Fun S, Subramaniam T, Sum CF, Lim SC. Fasting during Ramadan and associated changes in glycaemia, caloric intake and body composition with gender differences in Singapore. Ann Acad Med Singap. 2015;44(6):202–6. [PubMed] [Google Scholar]
- 109. Fontana L, Meyer TE, Klein S, Holloszy JO. Long-term calorie restriction is highly effective in reducing the risk for atherosclerosis in humans. Proc Natl Acad Sci. 2004;101:6659–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Fontana L, Klein S, Holloszy JO. Effects of long-term calorie restriction and endurance exercise on glucose tolerance, insulin action, and adipokine production. AGE. 2010;32:97–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Heilbronn LK, de Jonge L, Frisard MI, DeLany JP, Larson-Meyer DE, Rood J, Nguyen T, Martin CK, Volaufova J, Most MMet al. Effect of 6-month calorie restriction on biomarkers of longevity, metabolic adaptation, and oxidative stress in overweight individuals: a randomized controlled trial. JAMA. 2006;295:1539–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Larson-Meyer DE, Heilbronn LK, Redman LM, Newcomer BR, Frisard MI, Anton S, Smith SR, Alfonso A, Ravussin E. Effect of calorie restriction with or without exercise on insulin sensitivity, beta-cell function, fat cell size, and ectopic lipid in overweight subjects. Diabetes Care. 2006;29:1337–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Redman LM, Heilbronn LK, Martin CK, Alfonso A, Smith SR, Ravussin E, Pennington CALERIE Team. Effect of calorie restriction with or without exercise on body composition and fat distribution. J Clin Endocrinol Metab. 2007;92:865–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Civitarese AE, Carling S, Heilbronn LK, Hulver MH, Ukropcova B, Deutsch WA, Smith SR, Ravussin E, CALERIE Pennington Team. Calorie restriction increases muscle mitochondrial biogenesis in healthy humans. PLoS Med. 2007;4:e76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Racette SB, Weiss EP, Villareal DT, Arif H, Steger-May K, Schechtman KB, Fontana L, Klein S, Holloszy JO, Washington University School of Medicine CALERIE Group. One year of caloric restriction in humans: feasibility and effects on body composition and abdominal adipose tissue. J Gerontol A Biol Sci Med Sci. 2006;61:943–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Villareal DT, Fontana L, Weiss EP, Racette SB, Steger-May K, Schechtman KB, Klein S, Holloszy JO. Bone mineral density response to caloric restriction-induced weight loss or exercise-induced weight loss: a randomized controlled trial. Arch Intern Med. 2006;166:2502–10. [DOI] [PubMed] [Google Scholar]
- 117. Fontana L, Villareal DT, Weiss EP, Racette SB, Steger-May K, Klein S, Holloszy JO, Washington University School of Medicine CALERIE Group. Calorie restriction or exercise: effects on coronary heart disease risk factors. A randomized, controlled trial. Am J Physiol Endocrinol Metab. 2007;293:E197–202. [DOI] [PubMed] [Google Scholar]
- 118. Hofer T, Fontana L, Anton SD, Weiss EP, Villareal D, Malayappan B, Leeuwenburgh C. Long-term effects of caloric restriction or exercise on DNA and RNA oxidation levels in white blood cells and urine in humans. Rejuvenation Res. 2008;11:793–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Pittas AG, Roberts SB, Das SK, Gilhooly CH, Saltzman E, Golden J, Stark PC, Greenberg AS. The effects of the dietary glycemic load on type 2 diabetes risk factors during weight loss. Obesity. 2006;14:2200–9. [DOI] [PubMed] [Google Scholar]
- 120. Das SK, Gilhooly CH, Golden JK, Pittas AG, Fuss PJ, Cheatham RA, Tyler S, Tsay M, McCrory MA, Lichtenstein AHet al. Long-term effects of 2 energy-restricted diets differing in glycemic load on dietary adherence, body composition, and metabolism in CALERIE: a 1-y randomized controlled trial. Am J Clin Nutr. 2007;85:1023–30. [DOI] [PubMed] [Google Scholar]
- 121. Ahmed T, Das SK, Golden JK, Saltzman E, Roberts SB, Meydani SN. Calorie restriction enhances T-cell-mediated immune response in adult overweight men and women. J Gerontol A Biol Sci Med Sci. 2009;64:1107–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Rickman AD, Williamson DA, Martin CK, Gilhooly CH, Stein RI, Bales CW, Roberts S, Das SK. The CALERIE Study: design and methods of an innovative 25% caloric restriction intervention. Contemp Clin Trials. 2011;32:874–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Rochon J, Bales CW, Ravussin E, Redman LM, Holloszy JO, Racette SB, Roberts SB, Das SK, Romashkan S, Galan KMet al. Design and conduct of the CALERIE study: comprehensive assessment of the long-term effects of reducing intake of energy. J Gerontol A Biol Sci Med Sci. 2011;66:97–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Ravussin E, Redman LM, Rochon J, Das SK, Fontana L, Kraus WE, Romashkan S, Williamson DA, Meydani SN, Villareal DTet al. A 2-year randomized controlled trial of human caloric restriction: feasibility and effects on predictors of health span and longevity. J Gerontol A Biol Sci Med Sci. 2015;70:1097–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Mercken EM, Crosby SD, Lamming DW, JeBailey L, Krzysik-Walker S, Villareal DT, Capri M, Franceschi C, Zhang Y, Becker Ket al. Calorie restriction in humans inhibits the PI3K/AKT pathway and induces a younger transcription profile. Aging Cell. 2013;12:645–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Lefevre M, Redman LM, Heilbronn LK, Smith JV, Martin CK, Rood JC, Greenway FL, Williamson DA, Smith SR, Ravussin Eet al. Caloric restriction alone and with exercise improves CVD risk in healthy non-obese individuals. Atherosclerosis. 2009;203:206–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Tam CS, Covington JD, Ravussin E, Redman LM, Pennington CALERIE Team. Little evidence of systemic and adipose tissue inflammation in overweight individuals(†). Front Genet. 2012;3:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Lee C, Longo VD. Fasting vs dietary restriction in cellular protection and cancer treatment: from model organisms to patients. Oncogene. 2011;30:3305–16. [DOI] [PubMed] [Google Scholar]
- 129. Laugerette F, Alligier M, Bastard J-P, Drai J, Chanséaume E, Lambert-Porcheron S, Laville M, Morio B, Vidal H, Michalski M-C. Overfeeding increases postprandial endotoxemia in men: inflammatory outcome may depend on LPS transporters LBP and sCD14. Mol Nut Food Res. 2014;58:1513–8. [DOI] [PubMed] [Google Scholar]
- 130. Moreira APB, Texeira TFS, Ferreira AB, Peluzio M do CG, Alfenas R de CG. Influence of a high-fat diet on gut microbiota, intestinal permeability and metabolic endotoxaemia. Br J Nutr. 2012;108:801–9. [DOI] [PubMed] [Google Scholar]
- 131. Tilg H, Kaser A. Gut microbiome, obesity, and metabolic dysfunction. J Clin Invest. 2011;121:2126–32. [DOI] [PMC free article] [PubMed] [Google Scholar]