ABSTRACT
The tumor microenvironment is a complex mix of cancerous and noncancerous cells (especially immune cells and fibroblasts) with distinct metabolisms. These cells interact with each other and are influenced by the metabolic disorders of the host. In this review, we discuss how metabolic pathways that sustain biosynthesis in cancer cells could be targeted to increase the effectiveness of cancer therapies by limiting the nutrient uptake of the cell, inactivating metabolic enzymes (key regulatory ones or those linked to cell cycle progression), and inhibiting ATP production to induce cell death. Furthermore, we describe how the microenvironment could be targeted to activate the immune response by redirecting nutrients toward cytotoxic immune cells or inhibiting the release of waste products by cancer cells that stimulate immunosuppressive cells. We also examine metabolic disorders in the host that could be targeted to inhibit cancer development. To create future personalized therapies for targeting each cancer tumor, novel techniques must be developed, such as new tracers for positron emission tomography/computed tomography scan and immunohistochemical markers to characterize the metabolic phenotype of cancer cells and their microenvironment. Pending personalized strategies that specifically target all metabolic components of cancer development in a patient, simple metabolic interventions could be tested in clinical trials in combination with standard cancer therapies, such as short cycles of fasting or the administration of sodium citrate or weakly toxic compounds (such as curcumin, metformin, lipoic acid) that target autophagy and biosynthetic or signaling pathways.
Keywords: metabolism, glycolysis, tumor microenvironment, immunity, body composition, drug resistance
Introduction
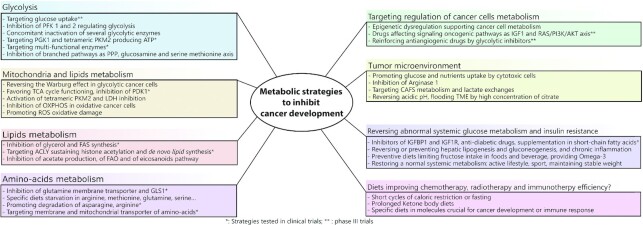
The incidence of various cancers [colorectal cancer (CRC), hepatocellular carcinoma (HCC), etc.] is strongly correlated with metabolic disorders such as diabetes, fatty liver disease, and obesity; hence, counteracting these conditions is mandatory for the prevention of cancer and the optimization of treatment (1). The metabolism of cancer cells differs from that of normal cells due to epigenetic defects, gene mutations, and metabolic reprogramming, resulting in the upregulation of oncogenic proteins and signaling pathways, and the inactivation of key suppressors such as tumor protein 53 (TP53) (2, 3). Current anti-cancer treatments (chemotherapy, immunotherapy, and targeted therapies) that target a specific aspect of cancer cell development, such as uncontrolled replication or deregulated molecular pathways, frequently show poor or transient efficacy, thus emphasizing the need for new strategies. Current research is focused on the interaction between the intrinsic metabolism of cancer cells, the tumor microenvironment (TME), and the host to develop strategies needed to improve standard therapies. This review aims to clarify current issues (summarized in Figure 1), presenting their rationales, proof of concepts, limits, and drawbacks.
FIGURE 1.
Key figure summarizing the different metabolic strategies which enable inhibition of cancer development.
Current Status of Knowledge
Cancer cell metabolism
The Warburg effect
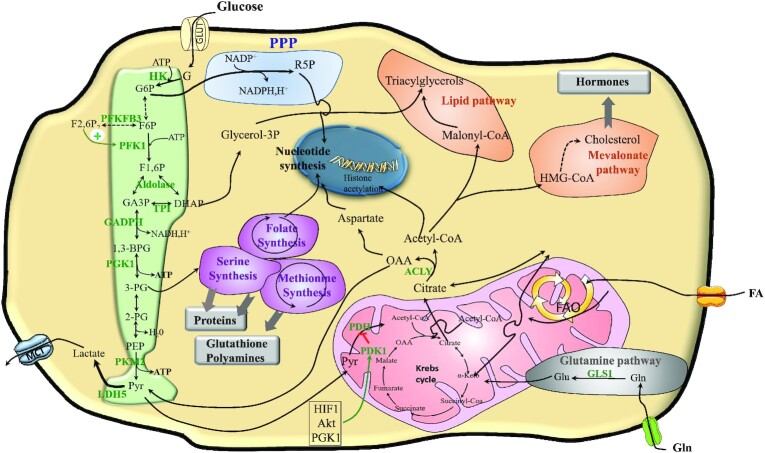
Cancer cells display enhanced glucose uptake and convert a significant amount of glucose into lactic acid, even in the presence of oxygen. In this so-called “Warburg effect” (4), a substantial part of glycolysis intermediates sustains biosynthesis processes. Inappropriate expression of pyruvate kinase (PK) in its PK muscle embryonic isozyme 2 (PKM2) embryonic dimeric form (less active than the adult tetrameric form) creates a bottleneck at the end of glycolysis, thus promoting the accumulation of glycolytic intermediates upstream. Therefore, the main branched pathways of glycolysis are promoted, in particular, the hexosamine biosynthetic pathway (HBP), the pentose phosphate pathway (PPP), the glycerol pathway, and the serine-glycine-folate-methionine pathway (SGFMP) (5) (Figure 2). Of note, the oxidative PPP generates reduced NAD(P)H H+, which is required for redox balance, nucleotides, and fatty acid (FA) synthesis (FAS) (6).
FIGURE 2.
The metabolism of cancer cells relying on the Warburg effect. Cancer cell metabolism is supported by glycolysis, glutaminolysis, and/or FAO. Glycolysis is enhanced: PFK1 is promoted by F2,6P produced by PFKFB3. Dimeric embryonic PKM2 creates a bottleneck at the end of glycolysis promoting branched pathway activities: PPP provides R5P for nucleotide synthesis, the DHAP pathway provides G3P for triglyceride synthesis, and SGFMP sustains protein and glutathione synthesis, as well as one-carbon metabolism required for methylation processes (in particular of epigenome and genome), and for polyamine formation. The Warburg effect is related to PDH inhibition by PDK1, a process stimulated by HIF1 and AKT. Lactate produced by LDH5 is expulsed by MCT4. Due to PDH inactivation, acetyl-CoA is produced by FAO or derives from oxidation of AKGα, which enters the Krebs cycle (also named the TCA cycle). Cytosolic citrate derives from mitochondrial export or from carboxylation of AKG deriving from glutaminolysis. ACLY transforms citrate into OAA and acetyl-CoA. Acetyl-CoA sustains lipid synthesis and histone acetylation while OAA sustains aspartate synthesis or pyruvate and lactate formation. Glycolysis is green, PPP is blue, amino acid synthesis is purple, lipid and hormone pathways are orange, and the glutamine pathway is gray. ACLY, ATP citrate lyase; AKG, α-ketoglutarate; Akt, protein kinase B; DHAP, dihydroxyacetone phosphate; FA, fatty acids; FAO, fatty acid β-oxidation; F6P, fructose 6-phosphate; F1,6P, fructose-1,6-bisphosphate; F2,6BP, fructose-2,6-biphosphate; G, glucose; G6P, glucose 6-phosphate; GA3P, glyceraldehyde 3-phosphate; GLS1, glutaminase 1; GLUT1, membrane glucose transporter 1; Glycerol-3P, glycerol-3-phosphate; HIF1α, hypoxia inducible factor 1 alpha; HK, hexokinase; HMG-CoA, 3-hydroxy-3-methylglutaryl-CoA; LDH5, lactate dehydrogenase 5; MCT, monocarboxylate transporter; NADPH,H+, nicotinamide adenine dinucleotide phosphate; OAA, oxaloacetate; PDH, pyruvate dehydrogenase; PDK1, pyruvate dehydrogenase kinase 1; PEP, phosphoenolpyruvate; PFK1, phosphofructokinase 1; PFKFB3, 6-phosphofructo-2-kinase/fructose-2,6-biphosphatase 3; PGK1, phosphoglycerate kinase 1; PK, pyruvate kinase; PKM2, embryonic pyruvate kinase; PPP, pentose phosphate pathway; R5P, ribose 5-phosphate; SGFMP, serine-glycine-folate-methionine pathway; TCA, tricarboxylic acid; TPI, triosephosphate isomerase; 2-PG, 2 phosphoglycerate; 3-PG, 3 phosphoglycerate; 1,3-BPG, 1,3-bisphosphogylcerate.
Abnormal glucose consumption by cancer cells also sustains fast ATP production. Glycolysis is regulated by phosphofructokinase-1 (PFK1), the activity of which is stimulated by low cytosolic concentrations of ATP, citrate, and basic intracellular pH induced by decreased mitochondrial functioning (7). The extracellular acid pH favors cancer invasiveness and resistance to chemotherapy (CT) (7).
From a molecular point of view, the Warburg effect is related to pyruvate dehydrogenase (PDH) inhibition by pyruvate dehydrogenase kinase 1 (PDK1), a process promoted by hypoxia-inducible factor 1 alpha (HIF-1α) and protein kinase B (PKB or AKT) (2). Several oncogenes, in particular the myelocytomatosis viral oncogene (MYC) and the Kristen rat sarcoma viral oncogene homolog (RAS), promote HIF-1α and the Warburg effect, a reductive metabolism also sustained by signaling pathways such as phosphoinositide-3-kinase–PKB/Akt (PI3K-PKB/Akt), and mammalian target of rapamycin (mTOR) (8–11). Concomitantly, key suppressors, such as TP53, inhibiting glycolysis, and phosphatase and tensin homolog (PTEN), inhibiting PI3K/AKT/mTOR, are inactivated (8–11). Key oncogenic drivers involved in cancer cell metabolism reprogramming are listed in Table 1.
TABLE 1.
Key oncogenic drivers and signaling pathways involved in cancer cell metabolism1
| Pathway (reference) | Anomaly | Target | Effect |
|---|---|---|---|
| PI3K-AKT-mTOR (48–50) | Activation | ↑PDK1↑GLUT1 (51)↑HK2 (51)↑SREBPInduction of HIF-1 | Stimulation of glucose transport and glycolysis Increased FAS Suppression of autophagy with increased net protein synthesis |
| RAS-RAF-MEK-MAPK (52) | Activation | Induction of HIF-1 | Increased glucose transportInhibition of OXPHOSIncreased FAS |
| PTEN (51) | Loss | Activation of AKT | Stimulation of glucose transport and glycolysisIncreased FAS |
| HIF-1 (49, 53, 54) | Increase | ↑GLUT1 (51)↑HK2 (51)↑PDK1↑SREBP↑SHMT2 | Increased glucose transportInhibition of OXPHOSIncreased FASSerine-glycine conversion and increased one-carbon metabolism |
| c-MYC (55, 56) | Amplification | ↑GLUT1 (51)↑LDH-A↑SHMT2↑HK2 (51)↑PFK1↑MCT4↑OXPHOS | Stimulation of glucose transport and glycolysisIncreased lactate synthesis and extrusionIncreased GLUT1 (51) glutaminolysisIncreased FAOSerine-glycine conversion and increased one-carbon metabolism |
| p53 (57–59) | Loss/inactivation | ↓TIGAR (51)↑PGM↓OXPHOS↑G6PD↑SREBP | Stimulation of glucose transport and glycolysisIncreased PPPIncreased FAS |
| LKB1/AMPK (60, 61) | Loss/inactivation | Activation of mTOR and HIF1Inhibition of p53↓OXPHOS↑SREBP | Stimulation of glycolysisIncreased oxidative stress during glucose deprivalIncreased FAS |
| STAT3 (62) | Activating mutation | Induction of HIF-1↑PDK1↓OXPHOS | Increased glucose transportInhibition of OXPHOSIncreased FAS |
AKT, protein kinase B; AMPK, AMP-activated protein kinase; c-MYC, c-myelomatosis viral oncogene; FAO, fatty acid oxidation; FAS, fatty acid synthesis; GLUT1, glucose transporter 1; G6PD, glucose-6-phosphate 1-dehydrogenase; HIF-1, hypoxia-inducible factor; HK2, hexokinase 2; LDH-A, lactate dehydrogenase A; LKB1, liver kinase B1; MAPK, mytogen-activated protein kinase; MCT4, monocarboxylate transporter 4; MEK, mitogen-activated protein kinase; mTOR, mammalian target of rapamycin; OXPHOS, oxidative phosphorylation; PDK1, pyruvate dehydrogenase kinase 1; PFK1, phosphofructokinase 1; PGM, phosphoglycerate mutase; PI3K, phosphatidylinositol-3-kinase; PPP, pentose phosphate pathway; PTEN, phosphatase and tensin homolog; RAF, rapidly accelerated fibrosarcoma; RAS, Kristen rat sarcoma viral oncogene homolog; SHMT2, serine hydroxymethyltransferase; SREBP, sterol regulatory element-binding protein; STAT3, signal transducer and activator of transcription 3; TIGAR, tumor protein 53–induced glycolysis and apoptosis regulator.
Importantly, lactate excreted in the TME by monocarboxylate transporter (MCT) 4 (MCT4) promotes invasiveness and angiogenesis and suppresses the immune response (12, 13). Aerobic glycolysis is associated with concomitant mitochondria downregulation, a process that limits the production of reactive oxygen species (ROS) and allows active cell proliferation (14).
In summary, the Warburg effect is associated with resistance to apoptosis, CT, and radiotherapy (RT). A high uptake of 18F-fluoro-2-deoxyglucose (18F-FDG) in positron emission tomography/computed tomography scanning reflects an increase in glucose consumption in the tumor, and correlates with higher resistance to treatment and poor survival (15). Therefore, the high reliance on glycolysis can constitute a vulnerability in many aggressive cancer cells, which is especially important to target.
Glutaminolysis
Glutamine metabolism provides the molecules and amine groups for nucleotide synthesis, in particular during the S phase progression (16), and reloads the tricarboxylic acid (TCA) cycle into α-ketoglutarate (AKG), a molecule derived from glutamate. Glutamine can also sustain lipid synthesis, by supporting citrate formation either by the TCA cycle or by a pathway involving the carboxylation of AKG and a reverse isocitrate dehydrogenase (IDH) reaction (9). Therefore, glutaminase 1, regulating the conversion of glutamine to glutamate, appears to be a key target for specific inhibition.
ATP citrate lyase links glycolysis with de novo lipid synthesis
ATP citrate lyase (ACLY) has a pivotal role in cancer cell metabolism because its activity links glycolysis and/or glutaminolysis with lipid and sterol synthesis, protein acetylation, isoprenylation, and glycosylation of proteins (17). ACLY converts citrate into acetyl-CoA and oxaloacetate (OAA). Acetyl-CoA sustains histone acetylation allowing transcription (18) and/or the de novo FAS required for membrane replication and the modification of proteins. Acetyl-CoA carboxylase (ACC) catalyzes and regulates the first step of FAS, a pathway sustained by fatty acid synthase (FASN) and leading to palmitate (19). Although palmitate, the most common FA in the human body (20–30% of total FAs), can be provided by the diet, most cancer cells synthesize de novo palmitate and FA independently of nutrient availability (19).
OAA can undergo a transamination reaction to form aspartate, a molecule required for nucleotide and polyamine synthesis. OAA metabolism also sustains the regeneration of pyruvate by the malic enzyme (ME) reaction. This reaction regenerates NADPH H+, a cofactor required for FAS and nucleotide synthesis, and the regeneration of reduced glutathione, an antioxidant molecule. Pyruvate can sustain lactate production, the TCA cycle, or gluconeogenesis. Opened by pyruvate carboxylase (PC), this later pathway is truncated in cancer cells because fructose-1,6-bisphosphatase (FBPase; regulating the exit) is inactivated, in particular, because of a low citrate concentration, a physiological activator of FBPase (20). Thus, in cells lacking glucose, PC redirects the carbon flux from the glutaminolysis and/or β-oxidation of FAs (FAO) toward gluconeogenesis sustaining nucleotide synthesis.
Cancer cell metabolism is not inherently glycolytic
Several studies have demonstrated that numerous cancer cells may rely on predominant oxidative or intermediate metabolism (21–23). In particular, the lactate that is released by cancer cells expressing MCT4 can be assimilated by cells expressing MCT1. In these cells, lactate is recycled as fuel for mitochondrial oxidation after conversion into pyruvate by reversed lactate dehydrogenase-5 (LDH-5) functioning (22, 24). This mode of ATP production can efficiently support the metabolism of oxidative cancer cells and immune-suppressive cells, a glucose-sparing process for cancer cells relying on dominantly glycolytic metabolism (25).
Furthermore, cells growing in a lipid-rich environment (such as ovarian cancer cells, triple-negative breast cancer cells) can be supported by active FAO (26, 27). Cancer stem cells frequently show both glycolytic and mitochondrial functioning (28), increasing their survival and maintaining their stemness properties by the upregulation of FAO (29).
Targeting cancer cell metabolism
The inhibition of metabolic pathways is a promising strategy to inhibit growth of cancer cells and enhance the efficacy of current therapy.
Inhibition of aerobic glycolysis
Glucose uptake can be targeted by inhibitors of glucose transporter 1 (GLUT1) (30) or hexokinase 2 (HK2) (31, 32). Theoretically, the inactivation of 1 of the 10 glycolytic enzymes may disrupt glycolysis [for lists of inhibitors, see Akins et al. (33), Abdel-Wahab et al. (34), and Table 2]. Targeting PFK1, the key regulatory enzyme of glycolysis, as well as GAPDH, a limiting enzyme at the end of glycolysis (because its functioning requires NAD+), provides a rational strategy to limit glycolytic flow. Similar considerations apply to the inhibition of the 2 enzymes sustaining ATP production [phosphoglycerate kinase 1 (PGK1) and tetrameric PKM2], since their blockade may cause an energy crisis leading to cell death in clones that are strongly reliant on glycolysis (35). The inhibition of branched pathways can be attempted to counterbalance the nucleotide and polyamine biosynthesis required for cell growth: 1) HBP sustaining protein glycosylation (required for transcription, epigenetics, signaling, and bioenergetics) can be inhibited by quercetin, a natural flavonoid (36); 2) oxidative PPP can be targeted by glucose 6-phosphate dehydrogenase (G6P) inhibition, and nonoxidative PPP by transketolase 1 (TKL1) inactivation (37); 3) SGFMP can be targeted by the concomitant inactivation of PGK1 and phosphoglycerate dehydrogenase (PHGDH) regulating this pathway (38). Targeting the entrance and exit of aerobic glycolysis is particularly effective as shown by the concomitant inhibition of glucose-6-phosphate isomerase (GPI), LDH-5, and lactate export regulated by MCT1/4 (39). Pan glycolytic inhibitors could be particularly effective as the alkaline agent 3-bromopyruvate (3-BP), targeting several enzymes such as HK2 and PKM2 (40). However, to date, its toxicity has not been determined in phase 1 studies.
TABLE 2.
List of metabolic inhibitors tested in preclinical models1
| Main targeted pathways | Metabolic inhibitors tested in vitro (reference) | Metabolic inhibitors tested in vivo | Experimental tumor type |
|---|---|---|---|
| Glycolysis | |||
| GLUTs | Fasentin (76)STF-31 (77) | Ritonavir (78)Silybin (79)Phloretin (80)WZB-117 (81) | Breast cancer Bladder cancer Breast cancer NSCLC |
| HK2 | Astragalin (82)Resveratrol (83) | Lonidamine (84)2-Deoxyglucose (85)Genistein (86)Benserazide (87) | Prostate and ovarian cancersBreast and prostate cancersHCCCRC |
| PFK1 PFK2/PFKFB3 GAPDH PKM2 LDH-A | Oxamate (88) | Sulforaphane (89)Citrate (90)PFK158, 3PO (91)3-Bromopyruvate (92)Apigenin (93)FX11 (94) | Triple-negative breast cancer Pancreatic cancer Ovarian and cervical cancers Gastric cancer HCC Esophageal cancer |
| IGF signaling | |||
| IGF-1R/IR | NVP-AEW541 (95)BMS-536924 (96)AG-1024 (97) | BMS-754807 (98)GSK1904529A (99) GSK1838705A (100)Picropodophyllin (101)PQ 401 (102) | Pancreatic cancer Osteosarcoma Glioma Rhabdomyosarcoma Glioma |
| Mitochondrial functioning | |||
| PDK1 | — | Dichloroacetate (103) | NSCLC |
| PDH | — | Lipoic acid (PDH activator) (104) | Breast cancer |
| OXPHOS inhibition | Niclosamide (105) | Gamatrinib (106) | Prostate cancer |
| Complex I | Menadione (107) | Metformin (108)Phenformin (109) | CRCOvarian cancer |
| Complex II (or SDH) | 3-Bromopyruvate (110) | — | — |
| Complex III | — | Antimycin A (111) | Lung cancer |
| Complex V | Oligomycin (112) | Bedaquiline (113) | Lung cancer |
| Mitochondrial biogenesis | DoxycyclineTetracycline (114, 115) | Azithromycin (116) | CRC |
| Lactate exchanges | |||
| MCT1 | — | AZD-3965 (117) | Burkitt's lymphoma |
| Amino-acid metabolism | |||
| ASCT2 (SLC1A5) | Benzylserine (118) | V-9302 (119)GPNA (120) | CRCNSCLC |
| GLS1 | Acivicin (121)Zaprinast (122) | CB-839 (123) | NSCLC |
| IDH | — | Ivosidenib (IDH1) (124)Enasidenib (IDH2) (125) | AMLAML |
| Lipid metabolism | |||
| CPT1 | Etoximir (126) | Avocatin B (127) | AML |
| ACLY | Cucurbitacin B (128) | Hydroxycitrate (129) | NSCLCBladder cancer and melanoma |
| FAS inhibition | Orlistat (130) | Epigallocatechin-3-gallate (131)Cerulenin (132) | Breast cancerCRC |
| Mevalonate and cholesterol | — | Statins (133) | Ovarian cancer |
| Redox homeostasis | |||
| Antioxidants inhibition | Auranofin (134) | Disulfiram (135)Arsenic trioxide (136)Gossypol (137) | Testicular cancerHCCHNC |
| Glutathione biosynthesis | Imexon (138) | Buthionine sulfoximine (139) | Lung cancer |
ACLY, ATP citrate lyase; AML, acute myeloid leukemia; CRC, colorectal cancer; FAS, fatty acid synthesis; GLS1, glutaminase 1; GLUT, glucose transporter; GPNA, L-γ-glutamyl-p-nitroanilide; HCC, hepatocellular carcinoma; HK2, hexokinase 2; HNC, head and neck carcinoma; IDH, isocitrate dehydrogenase; IGF, insulin-like growth factor; LDH-A, lactate dehydrogenase A; MCT1, monocarboxylate transporter 1; NSCLC, non–small cell lung carcinoma; OXPHOS, oxidative phosphorylation; PDH, pyruvate dehydrogenase; PDK1, pyruvate dehydrogenase kinase 1; PFK, phosphofructokinase; PFKFB3, 6-phosphofructo-2-kinase/fructose-2,6-biphosphatase 3; PFK158, 1-(4-pyridinyl)-3-(2-quinolinyl)-2-propen-1-one; SDH, succinate dehydrogenase; V-9302, 2-amino-4-bis(aryloxybenzyl)aminobutanoic acid; 3PO, 3-(3-pyridinyl)-1-(4-pyridinyl)-2-propen-1-one.
Most notably, several enzymes such as PKM2, GAPDH [and also 6-phosphofructo-2-kinase/fructose-2,6-biphosphatase 3 (PFKFB3) promoting PFK1 activity] display nonglycolytic functions, promoting cell cycle progression by periodical translocation (directly or through their products) into the nucleus [for review, see Icard et al. (41)]. Hence, pharmacological inhibition of these multifunctional enzymes can be particularly effective in reinforcing cyclin-dependent kinase (CDK) inhibitors. For example, GAPDH sustains entrance in mitosis (42), and therefore its inactivation could reinforce inhibitors of CDK1, aurora kinase, and polo-like kinase 1, regulating cell cycle progression toward mitosis. The efficiency of anti-angiogenic drugs could also be increased by glycolysis inhibitors. Indeed, the benefit of anti-angiogenic therapy is generally short-term in both preclinical models and clinical trials, because these agents induce chronic hypoxia in tissues, which favors the emergence of high glucose-dependent phenotypes through HIF-1 activation and/or possibly the selection of pre-existing resistant cancer clones (43).
Modulation of mitochondrial functioning and autophagy
Promoting mitochondrial activity can reverse the Warburg effect, counteracting tumor progression (44). This can be obtained by redirecting the glycolytic carbon flux toward mitochondria through the promotion of PKM2 tetrameric functioning (45) and/or PDH activity either by lipoic acid (46) or dichloroacetate [a molecule reversing the inhibition of PDK1 (47)]. The inhibition of lactate production, export, or recycling can also favor the reactivation of mitochondrial functioning (39).
However, promoting oxidative functioning can be deleterious, particularly if it stimulates: 1) the activity of mutated enzymes in the TCA cycle, with overproduction of molecules acting as oncometabolites (such as succinate and fumarate), which alter the methylation of the genome and promote resistance to CT (63), and 2) the mobility of some cellular subclones as shown in breast cancer models (64, 65), probably by increasing ROS production because high concentrations of ROS play a major role in the metastatic process (66). Importantly, metformin could limit ROS production by inhibiting the complex I of oxidative phosphorylation (OXPHOS) (67), a major source of ROS, with complex III.
However, the benefit of metformin is controversial [as shown in renal cancer (68)], probably because this molecule can activate the key sensor of energy, AMP-activated protein kinase (AMPK), which might favor cell survival in some contexts (69). Indeed, AMPK promotes ATP generation by several processes favoring survival, such as 1) FAO activation (70), 2) mitochondrial biogenesis through activation of the peroxisome proliferator-activated receptor γ coactivator (PGC)-1ɑ (71), 3) and autophagy, providing molecules for catabolic pathways and thus an energy supply to endure metabolic and cytotoxic stress (72). Autophagy is a cellular process that recycles damaged organelles, superfluous proteins, and lipids. Initially regarded as a cancer-suppressive mechanism, autophagy is now most often considered to be a survival process, which allows cancer cells, especially “autophagy-dependent” cells such as RAS-induced lung cancer cells (73), to overcome stressful conditions such as exposure to CT (74, 75).
The complex mechanisms supporting autophagy include preserving the quality and abundance of mitochondria. Autophagy is regulated by the mammalian ortholog of the yeast autophagy-related gene 6 (ATG6/BECN1) and TP53, which enhances mitochondrial function and ATP production (75). Combining autophagy inhibitors (such as chloroquine) with glycolysis and/or OXPHOS inhibitors (such as metformin or curcumin) can result in anti-cancer activity as shown in studies in vitro and in vivo, probably by depriving cancer cells of energy (140). Curcumin has demonstrated multiple anti-cancer mechanisms, and in association with metformin can result in a synergistic effect by suppressing signaling pathways that promote glycolysis such as PI3K/AKT/mTOR and epidermal growth factor receptor/signal transducer and activator of transcription 3 (EGFR/STAT3) (141, 142). Metformin is currently being tested in trials with chloroquine in solid cancers with IDH mutated genes (high-grade chondrosarcoma, glioma, and intrahepatic cholangiocarcinoma) (143). Metformin is also currently being tested with EGFR tyrosine kinase inhibitors in advanced or metastatic non–small cell lung cancer (NSCLC) with EGFR mutations (NCT01864681).
Inhibition of amino acid and FA metabolism (uptake and transformation)
l-Asparaginase dramatically improved the treatment of acute lymphoblastic leukemia by transforming l-asparagine (an essential molecule for these cells) into aspartate and ammonia (NH4+) (144, 145). Thus, dietary deprivation of specific amino acids, and inhibition of membrane transporters, can be efficient in counteracting cancer cell growth.
For example, glutamine starvation can inhibit the proliferation of cancer cells, especially those that are highly consuming of this amino acid. Platinum-resistant ovarian and lung cancer cells (146–149) appear particularly sensitive to glutamine deprivation, a strategy restoring the cisplatin response (146). In ovarian cancer cells, MYC promotes glutamine addiction by increasing glutamine uptake and GLS1 expression and also promotes cisplatin resistance (147, 148). Thus, targeting the main plasma membrane transporter of glutamine [alanine serine cysteine preferring transporter 2 (ASCT2) also known as SLC1A5] and GLS1 could be an important strategy to inhibit platinum-resistant cancer cells (147–149). Importantly, in several models of tumor-bearing mice (colon, lymphoma, and melanoma xenograft cancers), GLS1 inhibition by 6-diazo-5-oxo-L-norleucine (DON) reduced tumor growth and activated cytotoxic effector T cells (150). DON inactivated oxidative and glycolytic metabolism of cancer cells while it promoted oxidative metabolism and the activation of effector T cells (150).
Arginine deprivation arrests the growth of various cancer cells (such as sarcomas and HCC, malignant melanoma and pleural mesothelioma, prostate and renal cancer cells, and cisplatin-resistant ovarian cancer), because these cancer cells do not synthesize sufficient arginine (108, 151). This results from the epigenetic silencing of the gene promoter of argininosuccinate synthetase (ASS1), the rate-limiting enzyme of arginine synthesis; therefore, these cancer cells become auxotrophic to arginine (151). Consequently, an arginine deprivation diet [as realized by arginine deiminase (ADI-PEG20) administration] can inhibit the growth of cancer cells with ASS1 deficiency, as shown by preclinical studies—in particular, in sarcomas (152).
Methionine starvation can promote the therapeutic response of CT-resistant RAS CRC cancer xenografts and RT-resistant mutated KRAS (Kristen rat sarcoma viral oncogene homolog) soft tissue sarcoma with TP53 deficiency (153). However, methionine starvation diets may have a dual effect, promoting liver cancer in some experimental studies (154). Therefore, diet strategies targeting amino acids must be conducted with a clear understanding of the specific metabolism of the various cancer tumors.
Inhibiting membrane transporters might also be an efficient strategy in altering the proliferation of cancer cells and/or stimulating the immune response. For example, l-cysteine is imported into cancer cells and myeloid-derived suppressor cells (MDSCs) by the l-cystine (L-CSSC) transporter, a carrier not expressed in CD8+ T cells (155). Therefore, inhibition of this transporter can suppress cancer cell growth and promote a cytotoxic immune response (156).
Counteracting anaplerosis, the process of reloading the TCA cycle with intermediates (OAA, AKG, and fumarate) that have been extracted for biosynthesis (in what are called anaplerotic reactions) can inhibit cancer cell growth. For example, the inhibition of PC (the enzyme that produces OAA) decreases the growth of breast and lung cancer cells (157, 158) while the inhibition of the mitochondrial pyruvate carrier (MPC) arrests the proliferation of various cancer cells lines (159). However, in apparent contrast, promoting the activity of MPC can decrease the growth of cancer cell lines that predominantly rely on glutamine (160, 161). Further studies must clarify the specific metabolism supporting cancer tumor development in vitro and in vivo. Of note, translocases of the outer and inner mitochondrial membrane (TOMM and TIMM, respectively) carry hundreds of proteins into the mitochondria (162). Targeting these translocases, in particular TOMM20, could be useful in reducing the growth of cancer cells, as shown by knockdown of TOMM20 in a xenograft mouse model of colon cancer (163).
Inhibition of lipid metabolism can also be an important strategy as many cancer cells activate de novo FAS (164). Thus, ACLY and FASN are key targets for specific inhibition (17, 90, 164), as well as all enzymes sustaining cholesterol and mevalonate pathways (for a list of inhibitors, see Table 2 and Table 3). Targeting FAO can also be an option, especially for counteracting cancer cells sustained by lipid catabolism (26, 27).
TABLE 3.
Clinical trials with metabolic inhibitors1
| Metabolic target | Clinical trial | Main drug | Associated treatment | Cancer type | Results |
|---|---|---|---|---|---|
| GLUTs | NCT01009437 (phase I/II)NCT00487721 (phase I/II) | RitonavirSilybin-phytosome | SurgerySurgery | Breast cancerProstatic cancer | Unpublished16.7% AEs |
| HK2 | (165) (phase III) | Lonidamine | CT (mitomycin-C/vindesine) | NSCLC | Higher 1-y survival with CT + lonidamide vs. CT alone (32% vs. 20%) |
| PFKs | (166) (phase II) NCT00735332 (phase II) | SulforaphaneTLN-232 | Single agentSingle agent | Prostatic cancerMelanoma | PSA level decreased in the sulforaphane group vs. placeboWell tolerated |
| GAPDH | NCT02044861 (phase I) | ACT-PFK-158 | Single agent | Solid malignancies | Unpublished |
| IGF-1R/IR | (167) (phase III) (168) (phase II) | LinsitinibAXL1717 | Single agentSingle agent | Adrenocortical carcinomaNSCLC | No increased OS vs. placeboNo difference in PFS and OS vs. docetaxel |
| PDK1 | NCT01386632 (phase II) | Dichloroacetate | Cisplatin+RT | HNC | 32.0% vs. 24.0% severe AE in arms DCA vs. placebo |
| PDH | (169) (phase I/III) | Devimistat | Folfirinox | Pancreatic cancer | 61.0% RR in phase I; phase III ongoing |
| Complex I | NCT01101438 (phase III)NCT01864681 (phase II) | Metformin | Single agentTyrosine-kinase inhibitorsChloroquine | Breast cancerNSCLCIDH mutated solid tumors | Unpublished; ongoing |
| Complex V | (170) (phase II) | Curcumin | Folfox | CRC | Curcumin use was safe and tolerableNo difference between the 2 arms in QoL |
| MCT1 | NCT01791595 (phase I) | AZD3965 | Single agent | Solid malignancies and lymphoma | Ongoing |
| GLS1 | NCT03965845 (phase I/II) | Telaglenastat | Palbociclib | NSCLC and CRC | Ongoing |
| IDH | NCT02977689 (phase II)(171) (phase III) | IDH305Ivosidenib | Single agentSingle agent | GliomaCholangiocarcinoma | UnpublishedImproved PFS vs. placebo |
| l-Asparagine | (172) (phase II) | Erythrocyte encapsulated asparaginase | CT | Pancreatic cancer | Improved OS and PFS vs. CT aloneWell tolerated |
| Arginine | NCT01910012 (phase II) | Arginine deiminase | Single agent | AML | The duration of arginine depletion was correlated with disease controlWell tolerated |
| FAS | NCT03808558 (phase II) | TVB-2640 | Single agent | K-RAS mutated NSCLC | Ongoing |
| HMG-CoA reductase | NCT03971019 (phase III) | Statins (simvastatin and atorvastatin) | Single agent | Operated breast cancer | Ongoing |
| Antioxidants | NCT03323346 (phase II) | Antabuse (disulfiram) | Copper | Breast cancer | Unpublished |
| Glutathione | (173) (phase I)NCT00165867 (phase II) | Buthionine sulfoximineIndisulam | MelphalanIdarubicin and cytarabine | Solid malignanciesAML | No major toxicity35.0% RR |
AE, adverse event; AML, acute myeloid leukemia; CRC, colorectal cancer; CT, chemotherapy; FAS, fatty acid synthesis; GLS1, glutamine synthase 1; GLUT, glucose transporter; HK2, hexokinase 2; HMG-CoA, 3-hydroxy-3-methylglutaryl-CoA; HNC, head and neck carcinoma; IDH, isocitrate dehydrogenase; IGF, insulin-like growth factor; MCT1, monocarboxylate transporter 1; QoL, quality of life; NSCLC, non–small cell lung carcinoma; OS, overall survival; PDH, pyruvate dehydrogenase; PDK1, pyruvate dehydrogenase kinase 1; PFK, phosphofructokinase; PFS, progression-free survival; PSA, prostate-specific antigen; RR, response rate; RT, radiation therapy.
The “citrate strategy” recapitulates many advantages of metabolic interventions
The rationale of the “citrate strategy” is based on the decrease in citrate cytosolic concentration induced by the Warburg effect (90) and/or upregulation of ACLY observed in numerous cancer cells (17). Low citrate concentrations promote PFK1 activity and FBPase inactivity since citrate physiologically regulates these key enzymes. Through fructose-1,6-bisphosphate (PFK1 product), the proliferative RAS-PI3K-AKT pathway is promoted, and therefore the Warburg effect and ACLY are concomitantly activated in a feedback loop (174–176). In various cancer models, the administration of high concentrations of citrate (∼50 times the physiological concentration) inhibits cancer cell proliferation by several mechanisms: the promotion of apoptosis by caspase 3 and 9 activation with the extinction of the anti-apoptotic factor Mcl-1 (177); increasing sensitivity to cisplatin (177, 178); inactivation of PFK1 with decreased ATP production (179); inhibition of the insulin-like growth factor (IGF) I (IGF-I) and its type I receptor (IGF-IR); inactivation of PI3/AKT pathway and activation of the PTEN suppressor; reversion of dedifferentiation and increased T-lymphocyte response (180–182).
Citrate is a product of very low toxicity as it is an endogenous metabolite characterized by a very short half-life and a rapid and complete metabolism (183, 184). Its only toxicity in humans and mammals is due to its chelating properties on calcium and other divalent cations. Administered in excess, citrate induces hypocalcemia, which causes muscle spasms and convulsions, and also a risk of hemorrhage (183, 184). The potentially severe and eventually lethal effects of acute hypocalcemia are usually reversed and cured by the intravenous administration of calcium chloride (184, 185). Citrate is commonly used in continuous veno-venous hemofiltration, in which clinical signs of hypocalcemia (such as tingling) are detected regularly; blood calcium concentrations are monitored and hypocalcemia is avoided by administering a calcium-containing liquid as a preventive measure (185, 186). Extrapolating the results of preclinical models using citrate as anti-cancer treatment (179–182), the active dose in humans is probably much lower than that used in continuous venous hemofiltration. However, the citrate strategy should only be tested in phase 1/2 clinical trials to determine its adverse effects and toxicity, mode and duration of administration, and its efficacy. Disturbance of the acid-base balance will also be avoided by the appropriate addition of bicarbonate (187).
Bridging cell metabolism to the TME
The emergence of immunotherapy as an efficient treatment in some cancers demonstrated the impact of targeting the TME. Tumor stroma contains a wide array of noncancerous cells, including cancer-associated fibroblasts (CAFs), adipocytes, endothelial cells, macrophages, myeloid-derived cells, natural killer (NK) cells, and other immune cells, including T and B lymphocytes. Schematically, cytotoxic immune cells comprise the following: CD8+ T, T-helper (Th) 1 and Th17 subpopulations of CD4+ T lymphocytes (CD4+Th1/Th17), dendritic cells (DCs), NK cells, and proinflammatory tumor-associated macrophage (TAM) 1 (TAM1). Immunosuppressive cells comprise regulatory T cells (Tregs; a subgroup of CD4+ T cells), MDSCs, and anti-inflammatory TAM2 cells [for review, see Caruana et al. (188)]. Of note, tumor-infiltrating lymphocytes (TILs) are often organized to form tertiary lymphoid structures together with DCs (189). The high consumption of nutrients by cancer cells contributes to exhaustion (nonresponsiveness) of cytotoxic immune cells, while waste products secreted by cancer cells (lactate, NO, polyamines, adenosine, and NH4+) further stimulate local immunosuppression and/or the proliferation of cancer cells (190). For example, adenosine generated from ATP degradation exerts local immunosuppression (191). The manipulation of the chemical milieu can promote a hostile TME for cancer development and may be an “ecological” strategy, which could improve cancer therapy.
How to improve the cytotoxic immune response?
The development of cancer cells is influenced by the number, nature, and activity of immune cells within tumors. Immunotherapy aims to restore the activity of cytotoxic T lymphocytes, and this strategy has dramatically improved the prognosis in certain types of cancers. The immune checkpoints programmed death (PD)-1 (PD-1) and its ligand (PD-L1), as well as T-lymphocyte–associated protein 4 (CTLA-4) are located on the membrane of T cells; they favor the inhibition of the cytotoxic function of TILs and stimulate immunosuppressive Tregs (188).
The dense infiltration of tumors with active TILs and proinflammatory TAM1 correlates with a better outcome (192, 193). Shifting T cells from a quiescent state to a highly active effector phenotype (activation, proliferation, migration, and differentiation with cytokine secretion) is a process that requires the large availability of nutrients (glucose, glutamine, and FA) and the rapid production of energy provided by glycolysis and OXPHOS (194, 195). The inhibition of PD-1 and PD-L1 results in an intense activation of glycolysis-dependent CD8+ T cells, leading to the secretion of IFN-γ (196, 197). TAM1 activation also results from the activation of glycolysis, PPP, and FAS (198). However, cancer cells outsmart the proliferation and activation of cytotoxic TILs and TAM1 by diverting nutrients for their own profit—in particular, glucose and amino acids (196–198). As a result, the loss of nutrients in the TME alters the cytotoxic function of effector T cells while it favors the anti-inflammatory effect of immunosuppressive cells. Of note, immunosuppressive cells are less demanding in glucose than cytotoxic T cells, and are mainly supported by FAO (26). Deprived in nutrients, T-cytotoxic cells progressively enter a dysfunctional and exhausted state, associated with the downregulation of glycolysis and OXPHOS, and loss of mitochondria induced by endoplasmic reticulum stress (199, 200). In this context, immune tolerance is promoted, while glucose and other nutrients are left for use in cancer cell proliferation.
Two main strategies can counteract the imbalance that is beneficial for cancer cells, and these strategies can be combined if they are well tolerated: 1) counteracting the absorption of nutrients by cancer cells and immunosuppressive cells, thus redirecting these nutrients to cytotoxic cells, and 2) suppressing secretion and recycling of waste products in the TME where they promote immunosuppressive cell activation.
The first strategy—that is, inhibition of nutrient uptake (glucose, glutamine, arginine, methionine, and FAs) and/or their conversion in cancer cells—has been partly described above. In this setting, the inhibition of arginase 1 (ARG1) promotes the activation of cytotoxic and proinflammatory cells, while anti-inflammatory and immunosuppressive cells are inhibited (199). Another example is targeting tryptophan metabolism, which can both counteract cancer cell growth and promote the activation of cytotoxic cells. Indeed, tryptophan is transformed by indoleamine 2–3 dioxygenase (IDO) into kynurenine, an immunosuppressive molecule. IDO can be inhibited by molecules such as indoximod (201, 202), but also by inhibitors of cyclooxygenase (COX)-2 (COX-2) as it produces prostaglandin E2 (PGE2), which upregulates IDO. COX-2 inhibition can be of particular importance because it concomitantly blocks ARG1 in MDSCs (203). PGE2 also binds to E type prostanoid 4 (EP4) promoting activation of immunosuppressive cells such as MDSCs; thus, several EP4 antagonists are currently being tested in clinical trials (203). In addition to amino acid metabolism, it could be important to target lipid metabolism, since lipid accumulation in the TME promotes cancer development, suppressing the immune response, particularly in prostate cancer (204). FAO inhibition could act against immunosuppressive cells (such as Tregs), relying preferentially on FA catabolism for their activation and/or maturation (205).
The second main strategy targets waste products secreted and recycled in the TME where they favor immunosuppression by various mechanisms described in detail elsewhere (206, 207). As an example, the adenosine pathway can be blocked by inhibitors of adenosine-generating enzymes (such as CD73) and/or adenosine receptors [for a list of ongoing trials, see Allard et al. (208)]. The inhibition of lactic acid metabolism can be attempted by using diclofenac (209), LDH inhibitors such as oxamate (210), MCT1/4 inhibitors (159), or by promoting PDH functioning by molecules such as lipoic acid (46) or dichloroacetate (211). Lactate transporter inhibition, in particular, targeting MCT1, was found to be a promising strategy for stopping cancer cell growth (159, 211), reducing the risk of metastasis in melanoma mouse models (212). Targeting an extracellular acid pH could be attempted as this pH decreases drug penetration in cancer cells and stimulates immunosuppression (213). Reversing the pH on both sides of the cellular membrane by the inhibition of Na+/H+ exchanger (NHE) 1 (NHE1) can slow down the proliferation process (214, 215). However, the redundancy of membrane pH-regulating proteins including carbonic anhydrases (CAs), NHEs, Na+/HCO3− co-transporters (NBCs), and MCTs prevents effective pH reversing if a sole individual protein is targeted (215). Interestingly, the oral administration of bicarbonate prevents the occurrence of metastases in breast cancer mouse models (216). In preclinical studies, the administration of sodium bicarbonate favors the penetration of cytotoxic drugs (doxorubicin, in particular) (217, 218), increases the response to mTOR inhibitors (218), and stimulates TILs in tumors (219). Sodium citrate is also a basic salt, increasing cytotoxic response to drugs such as cisplatin and T-cell infiltration in tumors (179–182). Co-administration or pretreatment of clinical doses of proton pump inhibitors in patients receiving cisplatin and fluorouracil results in lower extracellular acidification, enhancing the sensitivity of the tumor cells to anti-cancer agents (220).
Targeting nonimmune cancer-associated cells, such as CAFs
CAFs produce fibronectin and rich proline molecules (such as type I and III collagen), which, along with type IV collagen (entering the basement membrane produced by cancer cells) (221, 222), form a fibrotic tumor stroma stimulated by hypoxia and HIF-1 (223, 224). A thick tumor stoma leads to a “desmoplastic reaction” of poor prognosis, which increases interstitial fluid pressure and constitutes a physical barrier against drug delivery and antitumor immune response (221). Thus, targeting these cells can be important in establishing a TME hostile to cancer cell development. As CAFs use glycolysis to produce lactate for cancer cells, a process known as the “reverse Warburg effect” (224), targeting lactate secretion and exchanges by the aforementioned strategies can participate in the inhibition of CAF activity, thus counteracting cancer cell development.
Targeting the perverted metabolism of the host supporting the growth of the “cancer parasite”
Beyond the vicious exchanges occurring between cancer cells and their environmental niche, the tumor takes advantage of aberrant metabolic exchanges with its host, consuming muscles and fat reserves, and leading to cachexia (225). Cancer tumor can be viewed as a metabolic “parasite” sustained by daily food intake and liver gluconeogenesis, the latter pathway being supplied by glycerol (derived from lipolysis), alanine (derived from proteolysis), and lactate secreted by hypoxic tumor cells and muscles. Alanine consumption promotes proteolysis, loss of muscle, and finally the occurrence of sarcopenia (225), whereas lactate secreted by tumors can constitute a primary source of carbons for the TCA cycle of many cells in tissues and organs, except for the brain (226). This general recycling participates in glucose sparing for cancer cells—in particular, for highly glycolytic cells, which are often hypoxic and resistant to therapies (227). However, the metabolic requirements of the tumor-parasites should be less important than the distant metabolic effects exercised by the tumor on the “host,” and the tumor burden is <1% in many cancers, even at an advanced stage (228). The relations between cancer and the host are complex and involve numerous interconnected factors, including the following 1) chronic inflammation with deregulated mixes of cytokines (in particular IL-6) secreted by various cells (cancer cells, immune cells, CAFs, and organs cells, in particular, of the liver) (229); 2) nutritional status and adipose tissue composition and conversion of white into brown fat, promoted in particular by PGC1ɑ, a central modulator of cell metabolism (230); and 3) endogenous hormone (in particular insulin and IGFs) synthesized by almost any tissue and supporting insulin resistance, diabetes, and obesity (231).
Targeting the deregulated lipid metabolism sustaining cancer development
Excess consumption of caloric food promotes obesity, insulin resistance with increased circulating concentrations of insulin and IGFs, triglycerides, and nonesterified FAs (NEFAs). NEFAs trigger nonalcoholic fatty liver disease by endoplasmic reticulum stress, inflammation, necrosis, and fibrosis; all of these factors promote carcinogenesis and the development of liver cancer in particular (232, 233). Many factors contribute to this lipotoxic pathogenic sequence, such as free lipotoxic FA, several arachidonic and sphingolipid molecules, high expression of altered triglyceride lipase and acylcarnitines, as well as insulin resistance, cytokine production, micro-RNA (miRNA) dysregulation, NSCLC, and altered intestinal microbiota (234–236). The arachidonic pathway that promotes tumor growth could be targeted by natural products such as curcumin, resveratrol, and berberine (236). Upregulating the concentrations of proapoptotic sphingolipids (as ceramide and sphingosine) could also be beneficial (237).
Targeting the altered insulin–IGF-I pathway that promotes cancer development
The insulin–IGF-I axis and its downstream effectors are involved in cancer metabolism as observed in several cancer models and cohorts of patients (238–240). Insulin resistance is frequent in cancer patients, a process characterized by increased hepatic gluconeogenesis. Unlike in type 2 diabetes, cancer patients have normal fasting glucose with high, normal, or low concentrations of insulin (241). The complex role of the IGF family (IGF-I and IGF-II) ligands, IGF-IR1–3, insulin receptor (IR), and IGF-binding proteins (IGFBP1–6) in the mechanisms of insulin resistance in cancer patients still requires exploration [for review, see Denduluri et al. (238) and Bowers et al. (239)]. The increased expression of IGF-I, IGF-II, and IGF-IR has been observed in a variety of malignancies (242, 243). Of note, IGF-IR gene mutations have been rarely documented in cancer and no evidence links IGF-IR mutation with cancer prognosis (242, 244). IGF-I signaling activates PI3K/AKT, thus promoting glucose metabolism with the inhibition of glucagon expression and secretion (239, 244). IGFBPs regulate IGF-I availability and have an essential role in subverting glucose metabolism and promoting cancer growth and insulin resistance (239, 240). In a mouse model of acute myeloid leukemia, leukemia cells induced a high secretion of IGFBP1 in adipose tissue, which promoted a diabetic state and leukemia progression; thus, in this situation, anti-IGFBP1 and antidiabetic drugs could be beneficial (244). Of note, IGF-IR–specific inhibitors were disappointing in trials—in particular, because IGF-IR is expressed ubiquitously and shares high homology with IR, while compensatory growth factor signaling demonstrates some redundancy with IGF-IR signaling (245). Interestingly, in vitro studies showed that several miRNAs and lipoic acid inhibits IGF-IR (246, 247), while small molecules can displace IGF-I from the IGF-I–IGFBP complex, thus suppressing IGF-I–induced proliferation (248).
Dietary interventions could protect healthy cells from the cytotoxic effects induced by chemotherapeutic drugs on cancer cells
The maintenance of a sufficient high BMI and/or a stable weight is a pledge of quality of life and response to anti-cancer therapies. In this context, proposing a diet may appear illogical and conterproductive. However, preclinical experiments have shown that fasting (FS) and caloric restriction (CR) increase the effectiveness of CT and RT by promoting cytotoxic stress, acute inflammation, and immune responses (249–251). In mice, 48–72 h of FS protects from the toxic side effects of CT (such as platinum-based drug combinations or doxorubicin and etoposide), reverses CT-induced DNA damage on healthy cells, improves regeneration of hematopoietic stem cells, and favors an effective immune response (251–254). Interestingly, FS and CR downregulate IGF-IR and PI3K/mTOR signaling pathways, thus arresting the proliferation of healthy cells (such as cells of the bone marrow, gastrointestinal tract, hair follicles, and heart), which switches their metabolism in an oxidative mode for repair and survival (252, 253, 255). In contrast, cancer cells continue to proliferate as they are strongly programmed by oncogenic factors to replicate. This distinct reaction supports the concept of “differential stress resistance” (DSR) (250, 251, 255–257).
The switch from glycolysis to oxidative metabolism—supporting DSR—is promoted by AMPK, which enhances FAO and consequently supplies cells with ATP and NAD+ molecules (258). In healthy cells, proliferation is arrested and repair is regulated by various genes such as TP53, CDKN1, sirtuin 3 (SIRT3), and the protein kinase forkhead box protein O3 (FOXO3) (259, 260). Concomitantly, ROS neutralization is promoted by the mitochondrial NAD+-dependent protein Sirt3, a deacetylase that stimulates superoxide dismutase 2 (MnSOD2) and inhibits the Warburg effect (260). In contrast, these censoring controls are frequently altered in cancer cells, which survive and continue to replicate, supported by altered mechanisms of autophagy, AMPK, and poly (ADP ribose) polymerases (PARPs) (261–263). However, repeated cycles of CR or FS associated with RT and CT could be lethal for cancer cells, in contrast to healthy cells that could better recover from metabolic stress (257, 264, 265). Interestingly, such metabolic strategies could improve the immune cytotoxic response in tumors, as suggested experimentally (265). DSR suggests therapeutic windows for metabolic interventions that could be associated or interspersed between CT, RT, or immunotherapy sessions to improve the cytotoxic effect on the tumor while protecting healthy cells.
In this setting, the significance of ketone body (KB) diets (KBDs) may appear more problematic, although proposed as a “metabolic therapy” in particular for brain tumors (266). The rationale of these diets—low in carbohydrates and rich in fat—is to starve tumor cells in glucose because KBs (produced by the liver) cannot be metabolized by cancer cells, which lack relative mitochondrial enzymes (267). In contrast to tumor cells, many tissues and organs, in particular the brain, heart, and muscles, can use 3β-hydroxybutyrate (3β-OHB)—the main KB—as an efficient source of energy. Furthermore, 3β-OHB inhibits histone deacetylation in cancer cells, thus arresting their division (23). Accordingly, some studies have shown that KBDs reduce tumor growth [for review, see Branco et al. (267)], deplete TME in immunosuppressive cells, ad decrease the expression of inhibitory checkpoints (268). However, in other preclinical studies, KBDs had no effect and might even accelerate breast cancer growth (21, 23). These contradictory results could be related to the fact that many cancer cells display an oxidative metabolism and thus likely consume FA and KBs (269, 270). Furthermore, it is noteworthy that few studies (mainly carried out in cultured murine cells) demonstrate the assumption that cancer cells have lost enzymes metabolizing KBs (271). Moreover, some subclones can adapt to glucose starvation by promoting their glucose uptake, in particular through the overexpression of GLUT1 (272, 273) or by increasing their glutamine dependency (274). Therefore, the efficiency and relevance of KBDs warrant further demonstration by rigorous clinical trials, taking also into account that these diets are not easy to follow, often inducing significant weight loss. The results (not currently available) of randomized trials testing KBDs with CT and RT will clarify the benefits and relevance of KBDs [for recent lists of clinical trials associating KBDs and RT, see Icard et al. (275)].
Concluding Remarks and Future Perspectives
There is a novel and increasing interest in metabolic interventions to improve the results of conventional therapies. However, as we have described, preclinical studies have shown that there is no universal cancer cell metabolism, and thus no universal metabolic strategy capable to arrest the growth of all cancer cells, every time. However, the addiction of numerous cancer cells to glucose could be their Achilles heel, a vulnerability that should be targeted, especially in cells relying on strong aerobic glycolysis. This is of primary interest because these cells are often the most hypoxic and treatment-refractory cells, and thus sustain recurrence and metastasis (276). Otherwise, several experimental studies have shown that drug-resistant cancer cells can be re-sensitized by targeting metabolic pathways supporting their development (277). As an example, 5-fluorouracil (5-FU)–resistant CRC cancer cells are destroyed if 5-FU is combined with an inhibitor of OXPHOS (278). Similar considerations apply to the glutamine addiction in platinum-resistant cancer cells such as ovarian cancer cells (74). However, the metabolism of cancer cells can adapt to various changes related to nutritional conditions or enzymatic inhibitions by developing alternative pathways. For example, glucose deprivation induces a decrease in ATP production, which can be counterbalanced by an increasing production sustained by OXPHOS and FAO (26), and this condition can sometimes induce the selection of mutations that increase glucose uptake and glutaminolysis (272, 274). Acetyl-CoA—a key molecule in cell metabolism—can be obtained from several sources such as citrate and/or acetate (279), but also from glycolysis through transketolase-like 1 activity (37). Thus, reducing the concentration of acetyl-CoA in cancer cells may be difficult, almost impossible. The same is true for ribose 5-phosphate (R5P) because its synthesis can be supported by the oxidative part and/or the nonoxidative part of PPP, sustained by glycolysis or truncated gluconeogenesis. This latter pathway can be sustained by glutaminolysis and/or FAO. However, despite this adaptability, the metabolism of cancer cells can be inhibited by several strategies that can be simplified as follows: limiting the absorption of nutrients essential for cell growth; inactivating key regulatory and/or limiting enzymatic reactions; targeting metabolic enzymes linked with other processes that sustain cancer proliferation, such as cell cycle progression; stopping the production of ATP to cause an energy crisis; redirecting nutrients to cytotoxic immune cells; and suppressing waste secretion and recycling in TME.
However, without a comprehensive picture of whether metabolism supports the development of a particular tumor in vivo at a specific time, it is quite impossible to define the pathways or enzymes, which should be targeted in priority, considering also that the supplementation or starvation (in particular for KBs and several amino acids) may have dual effects. Furthermore, promoting oxidative metabolism instead of reductive metabolism (and vice versa) may paradoxically select resistant and/or metastatic cell clones (64, 65). Thus, while awaiting the development of new methods capable of specifying the metabolism of tumors in vivo, it may be advisable to concomitantly or sequentially target the glycolytic and oxidative behavior of cancer cells, and also to favor short cycles of “metabolic interventions” rather than prolonged interventions, which can select resistant clones and require stricter compliance. Further studies and clinical trials will define the modalities of metabolic interventions, their place among current treatments, their toxicity, and their repercussion on body physiology and immune response. It is time to develop new classifications including markers of cancer cell metabolism, immune response, and metabolic disorders to develop personalized treatments based on deeper knowledge of the specific metabolic vulnerabilities of both tumor and host, and actively test simple and inexpensive metabolic strategies that could improve the results of anti-cancer treatments.
ACKNOWLEDGEMENTS
The authors’ responsibilities were as follows—PI: manuscript design and writing, table, and final content approval; ML and AG: table design and writing; ZW and HL: figure design and writing; ER: table design and references; AC: writing and manuscript reviewing; DB: language and reviewing; LF: table design, writing, and editing; MA: manuscript review and final content approval; and all authors: read and approved the final manuscript.
Notes
The authors reported no funding received for this work.
Author disclosures: The authors report no conflicts of interest.
Abbreviations used: ACC, acetyl-CoA carboxylase; ACLY, ATP citrate lyase; AKG, α-ketoglutarate (also known as 2-oxoglutarate); AKT, protein kinase B; AMPK, AMP-activated protein kinase; ARG1, arginase 1; ASS1, arginosuccinate synthase 1; CAF, cancer-associated fibroblast; CA, carbonic anhydrase; CDK, Cyclin-dependent kinase; COX, cyclooxygenase; CR, caloric restriction; CRC, colorectal cancer; CT, chemotherapy; DC, dendritic cell; DON, 6-diazo-5-oxo-L-norleucine; DSR, differential stress resistance; EGFR, epidermal growth factor receptor; EP4, E type prostanoid 4; FA, fatty acid; FAO, fatty acid β-oxidation; FAS, fatty acid synthesis; FASN, fatty acid synthase; FBPase, fructose-1,6-bisphosphatase; FS, fasting; GLS1, glutaminase 1; GLUT1, glucose transporter 1; HBP, hexosamine biosynthetic pathway; HCC, hepatocellular carcinoma; HIF-1α, hypoxia-inducible factor alpha; HK2, hexokinase 2; IDH, isocitrate dehydrogenase; IDO, indoleamine 2–3 dioxygenase; IGF, insulin-like growth factor; IGF-IR, insulin-like growth factor receptor; IGFBP, insulin-like growth factor binding protein; IR, insulin receptor; KB, ketone body; KBD, ketone body diet; LDH-5, lactate dehydrogenase-5; MCT, monocarboxylate transporter; MDSC, myeloid-derived suppressor cell; ME, malic enzyme; miRNA, microRNA; MPC, mitochondrial pyruvate carrier; mTOR, mammalian target of rapamycin; MYC, myelocytomatosis viral oncogene; NADPH,H+, nicotinamide adenine dinucleotide phosphate; NEFA, nonesterified fatty acid; NHE, Na+/H+ exchanger; NK, natural killer; NSCLC, non–small cell lung cancer; OAA, oxaloacetate; OXPHOS, oxidative phosphorylation; PC, pyruvate carboxylase; PD, programmed death; PDH, pyruvate dehydrogenase; PDK1, pyruvate dehydrogenase kinase 1; PD-L1, programmed death ligand 1; PFK1, phosphofructokinase 1; PGC, proliferator-activated receptor γ coactivator; PGE2, prostaglandin E2; PGK1, phosphoglycerate kinase 1; PI3K/AKT, phosphatidylinositol-3-kinase/Akt; PK, pyruvate kinase; PKM2, pyruvate kinase muscle embryonic isozyme 2; PPP, pentose phosphate pathway; PTEN, phosphatase and tensin homolog; ROS, reactive oxygen species; RT, radiotherapy; SGFMP, serine-glycine-folate-methionine pathway; SIRT3, sirtuin 3; TAM, tumor-associated macrophage; TCA, tricarboxylic acid; Th, T-helper; TIL, tumor-infiltrating lymphocyte; TME, tumor microenvironment; TOMM, translocases of the outer mitochondrial membrane; TP53, tumor protein 53; Treg, regulatory T cell; 3β-OHB, 3β-hydroxybutyrate.
Contributor Information
Philippe Icard, Université Caen Normandie, Medical School, CHU de Caen, Caen, France; Normandie Université, UNICAEN, INSERM U1086, Interdisciplinary Research Unit for Cancer Prevention and Treatment, Centre de Lutte Contre le Cancer Centre François Baclesse, Caen, France; Service de Chirurgie Thoracique, Hôpital Cochin, Hôpitaux Universitaires Paris Centre, AP-HP, Paris-Descartes University, Paris, France.
Mauro Loi, Radiotherapy Department, Humanitas Cancer Center, Rozzano, Milan, Italy.
Zherui Wu, School of Medicine, Shenzhen University, Shenzhen, Guangdong, China; INSERM UMR-S 1124, Cellular Homeostasis and Cancer, Paris-Descartes University, Paris, France.
Antonin Ginguay, Service de Biochimie, Hôpital Cochin, Hôpitaux Universitaires Paris-Centre, AP-HP, Paris, France; EA4466 Laboratoire de Biologie de la Nutrition, Faculté de Pharmacie de Paris, Université Paris-Descartes, Sorbonne Paris Cité, Paris, France.
Hubert Lincet, INSERM U1052, CNRS UMR5286, Cancer Research Center of Lyon (CRCL), France; ISPB, Faculté de Pharmacie, Université Lyon 1, Lyon, France.
Edouard Robin, Service de Chirurgie Thoracique, Hôpital Cochin, Hôpitaux Universitaires Paris Centre, AP-HP, Paris-Descartes University, Paris, France.
Antoine Coquerel, INSERM U1075, Comete “Mobilités: Attention, Orientation, Chronobiologie”, Université Caen, Caen, France.
Diana Berzan, Service de Chirurgie Thoracique, Hôpital Cochin, Hôpitaux Universitaires Paris Centre, AP-HP, Paris-Descartes University, Paris, France.
Ludovic Fournel, Service de Chirurgie Thoracique, Hôpital Cochin, Hôpitaux Universitaires Paris Centre, AP-HP, Paris-Descartes University, Paris, France; INSERM UMR-S 1124, Cellular Homeostasis and Cancer, Paris-Descartes University, Paris, France.
Marco Alifano, Service de Chirurgie Thoracique, Hôpital Cochin, Hôpitaux Universitaires Paris Centre, AP-HP, Paris-Descartes University, Paris, France; INSERM U1138, Integrative Cancer Immunology, Paris, France.
References
- 1. Kang C, LeRoith D, Gallagher EJ. Diabetes, obesity, and breast cancer. Endocrinology. 2018;159(11):3801–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324(5930):1029–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Vander Linden C, Corbet C. Reconciling environment-mediated metabolic heterogeneity with the oncogene-driven cancer paradigm in precision oncology. Semin Cell Dev Biol. 2020;98:202–10. [DOI] [PubMed] [Google Scholar]
- 4. Koppenol WH, Bounds PL, Dang CV. Otto Warburg's contributions to current concepts of cancer metabolism. Nat Rev Cancer. 2011;11(5):325–37. [DOI] [PubMed] [Google Scholar]
- 5. Yang M, Vousden KH. Serine and one-carbon metabolism in cancer. Nat Rev Cancer. 2016;16(10):650–62. [DOI] [PubMed] [Google Scholar]
- 6. Vander Heiden MG, DeBerardinis RJ. Understanding the intersections between metabolism and cancer biology. Cell. 2017;168(4):657–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Icard P, Shulman S, Farhat D, Steyaert JM, Alifano M, Lincet H. How the Warburg effect supports aggressiveness and drug resistance of cancer cells?. Drug Resist Updat. 2018;38:1–11. [DOI] [PubMed] [Google Scholar]
- 8. Cairns RA. Drivers of the Warburg phenotype. Cancer J. 2015;21(2):56–61. [DOI] [PubMed] [Google Scholar]
- 9. DeBerardinis RJ, Chandel NS. Fundamentals of cancer metabolism. Sci Adv. 2016;2(5):e1600200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chen JQ, Russo J. Dysregulation of glucose transport, glycolysis, TCA cycle and glutaminolysis by oncogenes and tumor suppressors in cancer cells. Biochim Biophys Acta. 2012;1826(2):370–84. [DOI] [PubMed] [Google Scholar]
- 11. Martinez-Outschoorn UE, Peiris-Pagés M, Pestell RG, Sotgia F, Lisanti MP. Cancer metabolism: a therapeutic perspective. Nat Rev Clin Oncol. 2017;14(1):11–31. [DOI] [PubMed] [Google Scholar]
- 12. Dhup S, Dadhich RK, Porporato PE, Sonveaux P. Multiple biological activities of lactic acid in cancer: influences on tumor growth, angiogenesis and metastasis. Curr Pharm Des. 2012;18(10):1319–30. [DOI] [PubMed] [Google Scholar]
- 13. Husain Z, Huang Y, Seth P, Sukhatme VP. Tumor-derived lactate modifies antitumor immune response: effect on myeloid-derived suppressor cells and NK cells. J Immunol. 2013;191(3):1486–95. [DOI] [PubMed] [Google Scholar]
- 14. Rodic S, Vincent MD. Reactive oxygen species (ROS) are a key determinant of cancer's metabolic phenotype. Int J Cancer. 2018;142(3):440–8. [DOI] [PubMed] [Google Scholar]
- 15. Na F, Wang J, Li C, Deng L, Xue J, Lu Y. Primary tumor standardized uptake value measured on F18-fluorodeoxyglucose positron emission tomography is of prediction value for survival and local control in non-small-cell lung cancer receiving radiotherapy: meta-analysis. J Thorac Oncol. 2014;9(6):834–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tudzarova S, Colombo SL, Stoeber K, Carcamo S, Williams GH, Moncada S. Two ubiquitin ligases, APC/C-Cdh1 and SKP1-CUL1-F (SCF)-beta-TrCP, sequentially regulate glycolysis during the cell cycle. Proc Natl Acad Sci USA. 2011;108(13):5278–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Icard P, Wu Z, Fournel L, Coquerel A, Lincet H, Alifano M. ATP citrate lyase: a central metabolic enzyme in cancer. Cancer Lett. 2020;471:125–34. [DOI] [PubMed] [Google Scholar]
- 18. Wellen KE, Hatzivassiliou G, Sachdeva UM, Bui TV, Cross JR, Thompson CB. ATP-citrate lyase links cellular metabolism to histone acetylation. Science. 2009;324(5930):1076–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Menendez JA, Lupu R. Fatty acid synthase and the lipogenic phenotype in cancer pathogenesis. Nat Rev Cancer. 2007;7(10):763–77. [DOI] [PubMed] [Google Scholar]
- 20. Icard P, Wu Z, Alifano M, Fournel L. Gluconeogenesis of cancer cells is disrupted by citrate. Trends Cancer. 2019;5(5):265–6. [DOI] [PubMed] [Google Scholar]
- 21. Bonuccelli G, Tsirigos A, Whitaker-Menezes D, Pavlides S, Pestell RG, Chiavarina B, Frank PG, Flomenberg N, Howell A, Martinez-Outschoorn UEet al. . Ketones and lactate “fuel” tumor growth and metastasis: evidence that epithelial cancer cells use oxidative mitochondrial metabolism. Cell Cycle. 2010;9(17):3506–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Curry JM, Tuluc M, Whitaker-Menezes D, Ames JA, Anantharaman A, Butera A, Leiby B, Cognetti DM, Sotgia F, Lisanti MPet al. . Cancer metabolism, stemness and tumor recurrence: MCT1 and MCT4 are functional biomarkers of metabolic symbiosis in head and neck cancer. Cell Cycle. 2013;12(9):1371–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rodrigues LM, Uribe-Lewis S, Madhu B, Honess DJ, Stubbs M, Griffiths JR. The action of beta-hydroxybutyrate on the growth, metabolism and global histone H3 acetylation of spontaneous mouse mammary tumours: evidence of a beta-hydroxybutyrate paradox. Cancer Metab. 2017;5:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wilde L, Roche M, Domingo-Vidal M, Tanson K, Philp N, Curry J, Martinez-Outschoorn U. Metabolic coupling and the reverse Warburg effect in cancer: implications for novel biomarker and anticancer agent development. Semin Oncol. 2017;44(3):198–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ho PC, Liu PS. Metabolic communication in tumors: a new layer of immunoregulation for immune evasion. J Immunother Cancer. 2016;4:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Nieman KM, Kenny HA, Penicka CV, Ladanyi A, Buell-Gutbrod R, Zillhardt MR, Romero IL, Carey MS, Mills GB, Hotamisligil GSet al. . Adipocytes promote ovarian cancer metastasis and provide energy for rapid tumor growth. Nat Med. 2011;17(11):1498–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Camarda R, Zhou AY, Kohnz RA, Balakrishnan S, Mahieu C, Anderton B, Eyob H, Kajimura S, Tward A, Krings Get al. . Inhibition of fatty acid oxidation as a therapy for MYC-overexpressing triple-negative breast cancer. Nat Med. 2016;22(4):427–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Vannini N, Girotra M, Naveiras O, Nikitin G, Campos V, Giger S, Roch A, Auwerx J, Lutolf MP. Specification of haematopoietic stem cell fate via modulation of mitochondrial activity. Nat Commun. 2016;7:13125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Visweswaran M, Arfuso F, Warrier S, Dharmarajan A. Aberrant lipid metabolism as an emerging therapeutic strategy to target cancer stem cells. Stem Cells. 2020;38(1):6–14. [DOI] [PubMed] [Google Scholar]
- 30. Granchi C, Tuccinardi T, Minutolo F. Design, synthesis, and evaluation of GLUT inhibitors. Methods Mol Biol. 2018;1713:93–108. [DOI] [PubMed] [Google Scholar]
- 31. Lis P, Dylag M, Niedzwiecka K, Ko YH, Pedersen PL, Goffeau A, Ulaszewski S. The HK2 dependent “Warburg effect” and mitochondrial oxidative phosphorylation in cancer: targets for effective therapy with 3-bromopyruvate. Molecules. 2016;21(12):1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Rempel A, Mathupala SP, Perdersen PL. Glucose catabolism in cancer cells: regulation of the type II hexokinase promoter by glucose and cyclic AMP. FEBS Lett. 1996;385(3):233–7. [DOI] [PubMed] [Google Scholar]
- 33. Akins NS, Nielson TC, Le HV. Inhibition of glycolysis and glutaminolysis: an emerging drug discovery approach to combat cancer. Curr Top Med Chem. 2018;18(6):494–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Abdel-Wahab AF, Mahmoud W, Al-Harizy RM. Targeting glucose metabolism to suppress cancer progression: prospective of anti-glycolytic cancer therapy. Pharmacol Res. 2019;150:104511. [DOI] [PubMed] [Google Scholar]
- 35. Lepleux C, Abeilard-Lemoisson E, Duval M, Icard P, Lincet H. siPGK1 sensitizes chemoresistant human ovarian cancer cell lines to cisplatin. Anticancer Res. 2012;32(10):4277–86. [PubMed] [Google Scholar]
- 36. Ali A, Kim MJ, Kim MY, Lee HJ, Roh GS, Kim HJ, Cho GJ, Choi WS. Quercetin induces cell death in cervical cancer by reducing O-GlcNAcylation of adenosine monophosphate-activated protein kinase. Anat Cell Biol. 2018;51(4):274–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Diaz-Moralli S, Aguilar E, Marin S, Coy JF, Dewerchin M, Antoniewicz MR, Meca-Cortes O, Notebaert L, Ghesquiere B, Eelen Get al. . A key role for transketolase-like 1 in tumor metabolic reprogramming. Oncotarget. 2016;7(32):51875–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Mullarky E, Lucki NC, Beheshti Zavareh R, Anglin JL, Gomes AP, Nicolay BN, Wong JC, Christen S, Takahashi H, Singh PKet al. . Identification of a small molecule inhibitor of 3-phosphoglycerate dehydrogenase to target serine biosynthesis in cancers. Proc Natl Acad Sci USA. 2016;113(7):1778–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Zdralevic M, Marchiq I, de Padua MMC, Parks SK, Pouyssegur J. Metabolic plasticity in cancers—distinct role of glycolytic enzymes GPI, LDHs or membrane transporters MCTs. Front Oncol. 2017;7:313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ko YH, Smith BL, Wang Y, Pomper MG, Rini DA, Torbenson MS, Hullihen J, Pedersen PL. Advanced cancers: eradication in all cases using 3-bromopyruvate therapy to deplete ATP. Biochem Biophys Res Commun. 2004;324(1):269–75. [DOI] [PubMed] [Google Scholar]
- 41. Icard P, Fournel L, Wu Z, Alifano M, Lincet H. Interconnection between metabolism and cell cycle in cancer. Trends Biochem Sci. 2019;44(6):490–501. [DOI] [PubMed] [Google Scholar]
- 42. Carujo S, Estanyol JM, Ejarque A, Agell N, Bachs O, Pujol MJ. Glyceraldehyde 3-phosphate dehydrogenase is a SET-binding protein and regulates cyclin B-cdk1 activity. Oncogene. 2006;25(29):4033–42. [DOI] [PubMed] [Google Scholar]
- 43. Curtarello M, Zulato E, Nardo G, Valtorta S, Guzzo G, Rossi E, Esposito G, Msaki A, Pasto A, Rasola Aet al. . VEGF-targeted therapy stably modulates the glycolytic phenotype of tumor cells. Cancer Res. 2015;75(1):120–33. [DOI] [PubMed] [Google Scholar]
- 44. Lu J, Tan M, Cai Q. The Warburg effect in tumor progression: mitochondrial oxidative metabolism as an anti-metastasis mechanism. Cancer Lett. 2015;356(2 Pt A):156–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kung C, Hixon J, Choe S, Marks K, Gross S, Murphy E, DeLaBarre B, Cianchetta G, Sethumadhavan S, Wang Xet al. . Small molecule activation of PKM2 in cancer cells induces serine auxotrophy. Chem Biol. 2012;19(9):1187–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Kafara P, Icard P, Guillamin M, Schwartz L, Lincet H. Lipoic acid decreases Mcl-1, Bcl-xL and up regulates Bim on ovarian carcinoma cells leading to cell death. J Ovarian Res. 2015;8(1):36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Papandreou I, Goliasova T, Denko NC. Anticancer drugs that target metabolism: is dichloroacetate the new paradigm?. Int J Cancer. 2011;128(5):1001–8. [DOI] [PubMed] [Google Scholar]
- 48. Elstrom RL, Bauer DE, Buzzai M, Karnauskas R, Harris MH, Plas DR, Zhuang H, Cinalli RM, Alavi A, Rudin CMet al. . Akt stimulates aerobic glycolysis in cancer cells. Cancer Res. 2004;64(11):3892–9. [DOI] [PubMed] [Google Scholar]
- 49. Furuta E, Pai SK, Zhan R, Bandyopadhyay S, Watabe M, Mo YY, Hirota S, Hosobe S, Tsukada T, Miura Ket al. . Fatty acid synthase gene is up-regulated by hypoxia via activation of Akt and sterol regulatory element binding protein-1. Cancer Res. 2008;68(4):1003–11. [DOI] [PubMed] [Google Scholar]
- 50. Porstmann T, Santos CR, Griffiths B, Cully M, Wu M, Leevers S, Griffiths JR, Chung YL, Schulze A. SREBP activity is regulated by mTORC1 and contributes to Akt-dependent cell growth. Cell Metab. 2008;8(3):224–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Sansal I, Sellers WR. The biology and clinical relevance of the PTEN tumor suppressor pathway. J Clin Oncol. 2004;22(14):2954–63. [DOI] [PubMed] [Google Scholar]
- 52. Sang N, Stiehl DP, Bohensky J, Leshchinsky I, Srinivas V, Caro J. MAPK signaling up-regulates the activity of hypoxia-inducible factors by its effects on p300. J Biol Chem. 2003;278(16):14013–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Semenza GL, Roth PH, Fang HM, Wang GL. Transcriptional regulation of genes encoding glycolytic enzymes by hypoxia-inducible factor 1. J Biol Chem. 1994;269(38):23757–63. [PubMed] [Google Scholar]
- 54. Denko NC. Hypoxia, HIF1 and glucose metabolism in the solid tumour. Nat Rev Cancer. 2008;8(9):705–13. [DOI] [PubMed] [Google Scholar]
- 55. Miller DM, Thomas SD, Islam A, Muench D, Sedoris K. c-Myc and cancer metabolism. Clin Cancer Res. 2012;18(20):5546–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Goetzman ES, Prochownik EV. The role for Myc in coordinating glycolysis, oxidative phosphorylation, glutaminolysis, and fatty acid metabolism in normal and neoplastic tissues. Front Endocrinol. 2018;9:129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Madan E, Gogna R, Bhatt M, Pati U, Kuppusamy P, Mahdi AA. Regulation of glucose metabolism by p53: emerging new roles for the tumor suppressor. Oncotarget. 2011;2(12):948–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Jiang P, Du W, Wang X, Mancuso A, Gao X, Wu M, Yang X. p53 regulates biosynthesis through direct inactivation of glucose-6-phosphate dehydrogenase. Nat Cell Biol. 2011;13(3):310–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Parrales A, Iwakuma T. p53 as a regulator of lipid metabolism in cancer. Int J Mol Sci. 2016;17(12):2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Shackelford DB, Shaw RJ. The LKB1-AMPK pathway: metabolism and growth control in tumour suppression. Nat Rev Cancer. 2009;9(8):563–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Faubert B, Boily G, Izreig S, Griss T, Samborska B, Dong Z, Dupuy F, Chambers C, Fuerth BJ, Viollet Bet al. . AMPK is a negative regulator of the Warburg effect and suppresses tumor growth in vivo. Cell Metab. 2013;17(1):113–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Darnell JE Jr. STAT3, HIF-1, glucose addiction and Warburg effect. Aging (Albany NY). 2010;2(12):890–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Collins RRJ, Patel K, Putnam WC, Kapur P, Rakheja D. Oncometabolites: a new paradigm for oncology, metabolism, and the clinical laboratory. Clin Chem. 2017;63(12):1812–20. [DOI] [PubMed] [Google Scholar]
- 64. LeBleu VS, O'Connell JT, Gonzalez Herrera KN, Wikman H, Pantel K, Haigis MC, de Carvalho FM, Damascena A, Domingos Chinen LT, Rocha RMet al. . PGC-1alpha mediates mitochondrial biogenesis and oxidative phosphorylation in cancer cells to promote metastasis. Nat Cell Biol. 2014;16(10):992–1003., 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Andrzejewski S, Klimcakova E, Johnson RM, Tabaries S, Annis MG, McGuirk S, Northey JJ, Chenard V, Sriram U, Papadopoli DJet al. . PGC-1alpha promotes breast cancer metastasis and confers bioenergetic flexibility against metabolic drugs. Cell Metab. 2017;26(5):778–87 e5. [DOI] [PubMed] [Google Scholar]
- 66. Porporato PE, Payen VL, Baselet B, Sonveaux P. Metabolic changes associated with tumor metastasis, part 2: mitochondria, lipid and amino acid metabolism. Cell Mol Life Sci. 2016;73(7):1349–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Martín-Rodríguez S, de Pablos-Velasco P, Calbet JAL. Mitochondrial complex I inhibition by metformin: drug-exercise interactions. Trends Endocrinol Metab. 2020;31(4):269–71. [DOI] [PubMed] [Google Scholar]
- 68. Liu M, Zhang Z, Wang H, Chen X, Jin C. Activation of AMPK by metformin promotes renal cancer cell proliferation under glucose deprivation through its interaction with PKM2. Int J Biol Sci. 2019;15(3):617–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Agius L, Ford BE, Chachra SS. The metformin mechanism on gluconeogenesis and AMPK activation: the metabolite perspective. Int J Mol Sci. 2020;21(9):240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Hampsch RA, Wells JD, Traphagen NA, McCleery CF, Fields JL, Shee K, Dillon LM, Pooler DB, Lewis LD, Demidenko Eet al. . AMPK activation by metformin promotes survival of dormant ER(+) breast cancer cells. Clin Cancer Res. 2020;26(14):3707–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Bost F, Kaminski L. The metabolic modulator PGC-1alpha in cancer. Am J Cancer Res. 2019;9(2):198–211. [PMC free article] [PubMed] [Google Scholar]
- 72. Jiang S, Wang Y, Luo L, Shi F, Zou J, Lin H, Ying Y, Luo Y, Zhan Z, Liu Pet al. . AMP-activated protein kinase regulates cancer cell growth and metabolism via nuclear and mitochondria events. J Cell Mol Med. 2019;23(6):3951–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Guo JY, White E. Autophagy is required for mitochondrial function, lipid metabolism, growth, and fate of KRAS(G12D)-driven lung tumors. Autophagy. 2013;9(10):1636–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Zhang XY, Zhang M, Cong Q, Zhang MX, Zhang MY, Lu YY, Xu CJ. Hexokinase 2 confers resistance to cisplatin in ovarian cancer cells by enhancing cisplatin-induced autophagy. Int J Biochem Cell Biol. 2018;95:9–16. [DOI] [PubMed] [Google Scholar]
- 75. White E. Autophagy and p53. Cold Spring Harb Perspect Med. 2016;6(4):a026120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Wood TE, Dalili S, Simpson CD, Hurren R, Mao X, Saiz FS, Gronda M, Eberhard Y, Minden MD, Bilan PJet al. . A novel inhibitor of glucose uptake sensitizes cells to FAS-induced cell death. Mol Cancer Ther. 2008;7(11):3546–55. [DOI] [PubMed] [Google Scholar]
- 77. Chan DA, Sutphin PD, Nguyen P, Turcotte S, Lai EW, Banh A, Reynolds GE, Chi JT, Wu J, Solow-Cordero DEet al. . Targeting GLUT1 and the Warburg effect in renal cell carcinoma by chemical synthetic lethality. Sci Transl Med. 2011;3(94):94ra70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Yan Q, Hruz PW. Direct comparison of the acute in vivo effects of HIV protease inhibitors on peripheral glucose disposal. J Acquir Immune Defic Syndr. 2005;40(4):398–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Prack Mc Cormick B, Langle Y, Belgorosky D, Vanzulli S, Balarino N, Sandes E, Eijan AM. Flavonoid silybin improves the response to radiotherapy in invasive bladder cancer. J Cell Biochem. 2018;119(7):5402–12. [DOI] [PubMed] [Google Scholar]
- 80. Wu KH, Ho CT, Chen ZF, Chen LC, Whang-Peng J, Lin TN, Ho YS. The apple polyphenol phloretin inhibits breast cancer cell migration and proliferation via inhibition of signals by type 2 glucose transporter. J Food Drug Anal. 2018;26(1):221–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Liu Y, Cao Y, Zhang W, Bergmeier S, Qian Y, Akbar H, Colvin R, Ding J, Tong L, Wu Set al. . A small-molecule inhibitor of glucose transporter 1 downregulates glycolysis, induces cell-cycle arrest, and inhibits cancer cell growth in vitro and in vivo. Mol Cancer Ther. 2012;11(8):1672–82. [DOI] [PubMed] [Google Scholar]
- 82. Chen M, Cai F, Zha D, Wang X, Zhang W, He Y, Huang Q, Zhuang H, Hua ZC. Astragalin-induced cell death is caspase-dependent and enhances the susceptibility of lung cancer cells to tumor necrosis factor by inhibiting the NF-кB pathway. Oncotarget. 2017;8(16):26941–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Zhang B, Yin X, Sui S. Resveratrol inhibited the progression of human hepatocellular carcinoma by inducing autophagy via regulating p53 and the phosphoinositide 3–kinase/protein kinase B pathway. Oncol Rep. 2018;40(5):2758–65. [DOI] [PubMed] [Google Scholar]
- 84. Nath K, Nelson DS, Heitjan DF, Leeper DB, Zhou R, Glickson JD. Lonidamine induces intracellular tumor acidification and ATP depletion in breast, prostate and ovarian cancer xenografts and potentiates response to doxorubicin. NMR Biomed. 2015;28(3):281–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Tagg SL, Foster PA, Leese MP, Potter BV, Reed MJ, Purohit A, Newman SP. 2-Methoxyoestradiol-3,17-O,O-bis-sulphamate and 2-deoxy-D-glucose in combination: a potential treatment for breast and prostate cancer. Br J Cancer. 2008;99(11):1842–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Li S, Li J, Dai W, Zhang Q, Feng J, Wu L, Liu T, Yu Q, Xu S, Wang Wet al. . Genistein suppresses aerobic glycolysis and induces hepatocellular carcinoma cell death. Br J Cancer. 2017;117(10):1518–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Li W, Zheng M, Wu S, Gao S, Yang M, Li Z, Min Q, Sun W, Chen L, Xiang Get al. . Benserazide, a dopadecarboxylase inhibitor, suppresses tumor growth by targeting hexokinase 2. J Exp Clin Cancer Res. 2017;36(1):58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Elwood JC. Effect of oxamate on glycolysis and respiration in sarcoma 37 ascites cells. Cancer Res. 1968;28(10):2056–60. [PubMed] [Google Scholar]
- 89. Castro NP, Rangel MC, Merchant AS, MacKinnon G, Cuttitta F, Salomon DS, Kim YS. Sulforaphane suppresses the growth of triple-negative breast cancer stem-like cells in vitro and in vivo. Cancer Prev Res (Phila). 2019;12(3):147–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Icard P, Poulain L, Lincet H. Understanding the central role of citrate in the metabolism of cancer cells. Biochim Biophys Acta. 2012;1825(1):111–6. [DOI] [PubMed] [Google Scholar]
- 91. Mondal S, Roy D, Sarkar Bhattacharya S, Jin L, Jung D, Zhang S, Kalogera E, Staub J, Wang Y, Xuyang Wet al. . Therapeutic targeting of PFKFB3 with a novel glycolytic inhibitor PFK158 promotes lipophagy and chemosensitivity in gynecologic cancers. Int J Cancer. 2019;144(1):178–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Xian SL, Cao W, Zhang XD, Lu YF. 3-Bromopyruvate inhibits human gastric cancer tumor growth in nude mice via the inhibition of glycolysis. Oncol Lett. 2016;12(6):5377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Bhattacharya S, Mondal L, Mukherjee B, Dutta L, Ehsan I, Debnath MC, Gaonkar RH, Pal MM, Majumdar S. Apigenin loaded nanoparticle delayed development of hepatocellular carcinoma in rats. Nanomedicine. 2018;14(6):1905–17. [DOI] [PubMed] [Google Scholar]
- 94. Im DK, Cheong H, Lee JS, Oh MK, Yang KM. Protein kinase CK2-dependent aerobic glycolysis-induced lactate dehydrogenase A enhances the migration and invasion of cancer cells. Sci Rep. 2019;9(1):5337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Wolf S, Lorenz J, Mössner J, Wiedmann M. Treatment of biliary tract cancer with NVP-AEW541: mechanisms of action and resistance. World J Gastroenterol. 2010;16(2):156–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Litzenburger BC, Kim HJ, Kuiatse I, Carboni JM, Attar RM, Gottardis MM, Fairchild CR, Lee AV. BMS-536924 reverses IGF-IR-induced transformation of mammary epithelial cells and causes growth inhibition and polarization of MCF7 cells. Clin Cancer Res. 2009;15(1):226–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Wu H, Sun T, Bi R. Inhibition of insulin-like growth factor 1 signaling synergistically enhances the tumor suppressive role of triptolide in triple-negative breast cancer cells. Oncol Lett. 2019;18(1):822–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Awasthi N, Zhang C, Ruan W, Schwarz MA, Schwarz RE. BMS-754807, a small-molecule inhibitor of insulin-like growth factor-1 receptor/insulin receptor, enhances gemcitabine response in pancreatic cancer. Mol Cancer Ther. 2012;11(12):2644–53. [DOI] [PubMed] [Google Scholar]
- 99. Fei HD, Yuan Q, Mao L, Chen FL, Cui ZH, Tao S, Ji F. Assessment of GSK1904529A as a promising anti-osteosarcoma agent. Oncotarget. 2017;8(30):49646–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Zhou X, Shen F, Ma P, Hui H, Pei S, Chen M, Wang Z, Zhou W, Jin B. GSK1838705A, an IGF-1R inhibitor, inhibits glioma cell proliferation and suppresses tumor growth in vivo. Mol Med Rep. 2015;12(4):5641–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Tarnowski M, Tkacz M, Zgutka K, Bujak J, Kopytko P, Pawlik A. Picropodophyllin (PPP) is a potent rhabdomyosarcoma growth inhibitor both in vitro and in vivo. BMC Cancer. 2017;17(1):532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Zhou X, Zhao X, Li X, Ping G, Pei S, Chen M, Wang Z, Zhou W, Jin B. PQ401, an IGF-1R inhibitor, induces apoptosis and inhibits growth, proliferation and migration of glioma cells. J Chemother. 2016;28(1):44–9. [DOI] [PubMed] [Google Scholar]
- 103. Lu X, Zhou D, Hou B, Liu QX, Chen Q, Deng XF, Yu ZB, Dai JG, Zheng H. Dichloroacetate enhances the antitumor efficacy of chemotherapeutic agents via inhibiting autophagy in non-small-cell lung cancer. Cancer Manag Res. 2018;10:1231–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Feuerecker B, Pirsig S, Seidl C, Aichler M, Feuchtinger A, Bruchelt G, Senekowitsch-Schmidtke R. Lipoic acid inhibits cell proliferation of tumor cells in vitro and in vivo. Cancer Biol Ther. 2012;13(14):1425–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Lu W, Lin C, Roberts MJ, Waud WR, Piazza GA, Li Y. Niclosamide suppresses cancer cell growth by inducing Wnt co-receptor LRP6 degradation and inhibiting the Wnt/beta-catenin pathway. PLoS One. 2011;6(12):e29290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Kang BH, Siegelin MD, Plescia J, Raskett CM, Garlick DS, Dohi T, Lian JB, Stein GS, Languino LR, Altieri DC. Preclinical characterization of mitochondria-targeted small molecule hsp90 inhibitors, gamitrinibs, in advanced prostate cancer. Clin Cancer Res. 2010;16(19):4779–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Marchionatti AM, Picotto G, Narvaez CJ, Welsh J, Tolosa de Talamoni NG. Antiproliferative action of menadione and 1,25(OH)2D3 on breast cancer cells. J Steroid Biochem Mol Biol. 2009;113(3-5):227–32. [DOI] [PubMed] [Google Scholar]
- 108. McAlpine JA, Lu HT, Wu KC, Knowles SK, Thomson JA. Down-regulation of argininosuccinate synthetase is associated with cisplatin resistance in hepatocellular carcinoma cell lines: implications for PEGylated arginine deiminase combination therapy. BMC Cancer. 2014;14:621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Jackson AL, Sun W, Kilgore J, Guo H, Fang Z, Yin Y, Jones HM, Gilliam TP, Zhou C, Bae-Jump VL. Phenformin has anti-tumorigenic effects in human ovarian cancer cells and in an orthotopic mouse model of serous ovarian cancer. Oncotarget. 2017;8(59):100113–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Queiros O, Preto A, Pacheco A, Pinheiro C, Azevedo-Silva J, Moreira R, Pedro M, Ko YH, Pedersen PL, Baltazar Fet al. . Butyrate activates the monocarboxylate transporter MCT4 expression in breast cancer cells and enhances the antitumor activity of 3-bromopyruvate. J Bioenerg Biomembr. 2012;44(1):141–53. [DOI] [PubMed] [Google Scholar]
- 111. Yeh CT, Su CL, Huang CY, Lin JK, Lee WH, Chang PM, Kuo YL, Liu YW, Wang LS, Wu CHet al. . A preclinical evaluation of antimycin a as a potential antilung cancer stem cell agent. Evid Based Complement Alternat Med. 2013;2013:910451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Lonardo E, Cioffi M, Sancho P, Sanchez-Ripoll Y, Trabulo SM, Dorado J, Balic A, Hidalgo M, Heeschen C. Metformin targets the metabolic Achilles heel of human pancreatic cancer stem cells. PLoS One. 2013;8(10):e76518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Wu X, Li F, Wang X, Li C, Meng Q, Wang C, Huang J, Chen S, Zhu Z. Antibiotic bedaquiline effectively targets growth, survival and tumor angiogenesis of lung cancer through suppressing energy metabolism. Biochem Biophys Res Commun. 2018;495(1):267–72. [DOI] [PubMed] [Google Scholar]
- 114. Sharda DR, Yu S, Ray M, Squadrito ML, De Palma M, Wynn TA, Morris SM Jr, Hankey PA. Regulation of macrophage arginase expression and tumor growth by the Ron receptor tyrosine kinase. J Immunol. 2011;187(5):2181–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Zhong W, Chen S, Zhang Q, Xiao T, Qin Y, Gu J, Sun B, Liu Y, Jing X, Hu Xet al. . Doxycycline directly targets PAR1 to suppress tumor progression. Oncotarget. 2017;8(10):16829–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Qiao X, Wang X, Shang Y, Li Y, Chen SZ. Azithromycin enhances anticancer activity of TRAIL by inhibiting autophagy and up-regulating the protein levels of DR4/5 in colon cancer cells in vitro and in vivo. Cancer Commun (Lond). 2018;38(1):43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Curtis NJ, Mooney L, Hopcroft L, Michopoulos F, Whalley N, Zhong H, Murray C, Logie A, Revill M, Byth KFet al. . Pre-clinical pharmacology of AZD3965, a selective inhibitor of MCT1: DLBCL, NHL and Burkitt's lymphoma anti-tumor activity. Oncotarget. 2017;8(41):69219–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. van Geldermalsen M, Quek LE, Turner N, Freidman N, Pang A, Guan YF, Krycer JR, Ryan R, Wang Q, Holst J. Benzylserine inhibits breast cancer cell growth by disrupting intracellular amino acid homeostasis and triggering amino acid response pathways. BMC Cancer. 2018;18(1):689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Schulte ML, Fu A, Zhao P, Li J, Geng L, Smith ST, Kondo J, Coffey RJ, Johnson MO, Rathmell JCet al. . Pharmacological blockade of ASCT2-dependent glutamine transport leads to antitumor efficacy in preclinical models. Nat Med. 2018;24(2):194–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Hassanein M, Qian J, Hoeksema MD, Wang J, Jacobovitz M, Ji X, Harris FT, Harris BK, Boyd KL, Chen Het al. . Targeting SLC1a5-mediated glutamine dependence in non-small cell lung cancer. Int J Cancer. 2015;137(7):1587–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Kreuzer J, Bach NC, Forler D, Sieber SA. Target discovery of acivicin in cancer cells elucidates its mechanism of growth inhibition: synthesis, cloning, protein expression, purification and biochemical assays. Chem Sci. 2014;6(1):237–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Drees M, Zimmermann R, Eisenbrand G. 3',5'-Cyclic nucleotide phosphodiesterase in tumor cells as potential target for tumor growth inhibition. Cancer Res. 1993;53(13):3058–61. [PubMed] [Google Scholar]
- 123. Boysen G, Jamshidi-Parsian A, Davis MA, Siegel ER, Simecka CM, Kore RA, Dings RPM, Griffin RJ. Glutaminase inhibitor CB-839 increases radiation sensitivity of lung tumor cells and human lung tumor xenografts in mice. Int J Radiat Biol. 2019;95(4):436–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Popovici-Muller J, Lemieux RM, Artin E, Saunders JO, Salituro FG, Travins J, Cianchetta G, Cai Z, Zhou D, Cui Det al. . Discovery of AG-120 (ivosidenib): a first-in-class mutant IDH1 inhibitor for the treatment of IDH1 mutant cancers. ACS Med Chem Lett. 2018;9(4):300–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Yen K, Travins J, Wang F, David MD, Artin E, Straley K, Padyana A, Gross S, DeLaBarre B, Tobin Eet al. . AG-221, a first-in-class therapy targeting acute myeloid leukemia harboring oncogenic IDH2 mutations. Cancer Discov. 2017;7(5):478–93. [DOI] [PubMed] [Google Scholar]
- 126. Pike LS, Smift AL, Croteau NJ, Ferrick DA, Wu M. Inhibition of fatty acid oxidation by etomoxir impairs NADPH production and increases reactive oxygen species resulting in ATP depletion and cell death in human glioblastoma cells. Biochim Biophys Acta. 2011;1807(6):726–34. [DOI] [PubMed] [Google Scholar]
- 127. Lee EA, Angka L, Rota SG, Hanlon T, Mitchell A, Hurren R, Wang XM, Gronda M, Boyaci E, Bojko Bet al. . Targeting mitochondria with avocatin B induces selective leukemia cell death. Cancer Res. 2015;75(12):2478–88. [DOI] [PubMed] [Google Scholar]
- 128. Dandawate P, Subramaniam D, Panovich P, Standing D, Krishnamachary B, Kaushik G, Thomas SM, Dhar A, Weir SJ, Jensen RAet al. . Cucurbitacin B and I inhibits colon cancer growth by targeting the Notch signaling pathway. Sci Rep. 2020;10(1):1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Schwartz L, Guais A, Israel M, Junod B, Steyaert JM, Crespi E, Baronzio G, Abolhassani M. Tumor regression with a combination of drugs interfering with the tumor metabolism: efficacy of hydroxycitrate, lipoic acid and capsaicin. Invest New Drugs. 2013;31(2):256–64. [DOI] [PubMed] [Google Scholar]
- 130. Shiragami R, Murata S, Kosugi C, Tezuka T, Yamazaki M, Hirano A, Yoshimura Y, Suzuki M, Shuto K, Koda K. Enhanced antitumor activity of cerulenin combined with oxaliplatin in human colon cancer cells. Int J Oncol. 2013;43(2):431–8. [DOI] [PubMed] [Google Scholar]
- 131. Abd El-Rahman SS, Shehab G, Nashaat H. Epigallocatechin-3-gallate: the prospective targeting of cancer stem cells and preventing metastasis of chemically-induced mammary cancer in rats. Am J Med Sci. 2017;354(1):54–63. [DOI] [PubMed] [Google Scholar]
- 132. Huang P, Zhu S, Lu S, Li L, Dai Z, Jin Y. [Cerulenin inhibits growth of human colonic carcinoma in nude mice] Zhonghua Bing Li Xue Za Zhi. 2000;29(6):435–8. [PubMed] [Google Scholar]
- 133. Stine JE, Guo H, Sheng X, Han X, Schointuch MN, Gilliam TP, Gehrig PA, Zhou C, Bae-Jump VL. The HMG-CoA reductase inhibitor, simvastatin, exhibits anti-metastatic and anti-tumorigenic effects in ovarian cancer. Oncotarget. 2016;7(1):946–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Li H, Hu J, Wu S, Wang L, Cao X, Zhang X, Dai B, Cao M, Shao R, Zhang Ret al. . Auranofin-mediated inhibition of PI3K/AKT/mTOR axis and anticancer activity in non-small cell lung cancer cells. Oncotarget. 2016;7(3):3548–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Schmidtova S, Kalavska K, Gercakova K, Cierna Z, Miklikova S, Smolkova B, Buocikova V, Miskovska V, Durinikova E, Burikova Met al. . Disulfiram overcomes cisplatin resistance in human embryonal carcinoma cells. Cancers (Basel). 2019;11(9):1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Beauchamp EM, Ringer L, Bulut G, Sajwan KP, Hall MD, Lee YC, Peaceman D, Ozdemirli M, Rodriguez O, Macdonald TJet al. . Arsenic trioxide inhibits human cancer cell growth and tumor development in mice by blocking Hedgehog/GLI pathway. J Clin Invest. 2011;121(1):148–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Wolter KG, Wang SJ, Henson BS, Wang S, Griffith KA, Kumar B, Chen J, Carey TE, Bradford CR, D'Silva NJ. (-)-Gossypol inhibits growth and promotes apoptosis of human head and neck squamous cell carcinoma in vivo. Neoplasia. 2006;8(3):163–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Dorr RT, Raymond MA, Landowski TH, Roman NO, Fukushima S. Induction of apoptosis and cell cycle arrest by imexon in human pancreatic cancer cell lines. Int J Gastrointest Cancer. 2005;36(1):15–28. [DOI] [PubMed] [Google Scholar]
- 139. Cilurzo F, Cristiano MC, Da Pian M, Cianflone E, Quintieri L, Paolino D, Pasut G. Overcoming cancer cell drug resistance by a folic acid targeted polymeric conjugate of buthionine sulfoximine. Anticancer Agents Med Chem. 2019;19(12):1513–22. [DOI] [PubMed] [Google Scholar]
- 140. Yang B, Ding L, Chen Y, Shi J. Augmenting tumor-starvation therapy by cancer cell autophagy inhibition. Adv Sci (Weinh). 2020;7(6):1902847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Zhang HH, Zhang Y, Cheng YN, Gong FL, Cao ZQ, Yu LG, Guo XL. Metformin in combination with curcumin inhibits the growth, metastasis, and angiogenesis of hepatocellular carcinoma in vitro and in vivo. Mol Carcinog. 2018;57(1):44–56. [DOI] [PubMed] [Google Scholar]
- 142. Zhang P, Lai ZL, Chen HF, Zhang M, Wang A, Jia T, Sun WQ, Zhu XM, Chen XF, Zhao Zet al. . Curcumin synergizes with 5-fluorouracil by impairing AMPK/ULK1-dependent autophagy, AKT activity and enhancing apoptosis in colon cancer cells with tumor growth inhibition in xenograft mice. J Exp Clin Cancer Res. 2017;36(1):190. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 143. Molenaar RJ, Coelen RJS, Khurshed M, Roos E, Caan MWA, van Linde ME, Kouwenhoven M, Bramer JAM, Bovee J, Mathot RAet al. . Study protocol of a phase IB/II clinical trial of metformin and chloroquine in patients with IDH1-mutated or IDH2-mutated solid tumours. BMJ Open. 2017;7(6):e014961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Silverman LB, Gelber RD, Dalton VK, Asselin BL, Barr RD, Clavell LA, Hurwitz CA, Moghrabi A, Samson Y, Schorin MAet al. . Improved outcome for children with acute lymphoblastic leukemia: results of Dana-Farber Consortium Protocol 91-01. Blood. 2001;97(5):1211–8. [DOI] [PubMed] [Google Scholar]
- 145. Batool T, Makky EA, Jalal M, Yusoff MM. A comprehensive review on L-asparaginase and its applications. Appl Biochem Biotechnol. 2016;178(5):900–23. [DOI] [PubMed] [Google Scholar]
- 146. Obrist F, Michels J, Durand S, Chery A, Pol J, Levesque S, Joseph A, Astesana V, Pietrocola F, Wu GSet al. . Metabolic vulnerability of cisplatin-resistant cancers. EMBO J. 2018;37(14):597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Reyes-Gonzalez JM, Armaiz-Pena GN, Mangala LS, Valiyeva F, Ivan C, Pradeep S, Echevarria-Vargas IM, Rivera-Reyes A, Sood AK, Vivas-Mejia PE. Targeting c-Myc in platinum-resistant ovarian cancer. Mol Cancer Ther. 2015;14(10):2260–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Wise DR, Thompson CB. Glutamine addiction: a new therapeutic target in cancer. Trends Biochem Sci. 2010;35(8):427–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Hudson CD, Savadelis A, Nagaraj AB, Joseph P, Avril S, DiFeo A, Avril N. Altered glutamine metabolism in platinum resistant ovarian cancer. Oncotarget. 2016;7(27):41637–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Leone RD, Zhao L, Englert JM, Sun IM, Oh MH, Sun IH, Arwood ML, Bettencourt IA, Patel CH, Wen Jet al. . Glutamine blockade induces divergent metabolic programs to overcome tumor immune evasion. Science. 2019;366(6468):1013–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Delage B, Fennell DA, Nicholson L, McNeish I, Lemoine NR, Crook T, Szlosarek PW. Arginine deprivation and argininosuccinate synthetase expression in the treatment of cancer. Int J Cancer. 2010;126(12):2762–72. [DOI] [PubMed] [Google Scholar]
- 152. Bean GR, Kremer JC, Prudner BC, Schenone AD, Yao JC, Schultze MB, Chen DY, Tanas MR, Adkins DR, Bomalaski Jet al. . A metabolic synthetic lethal strategy with arginine deprivation and chloroquine leads to cell death in ASS1-deficient sarcomas. Cell Death Dis. 2016;7(10):e2406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Gao X, Sanderson SM, Dai Z, Reid MA, Cooper DE, Lu M, Richie JP Jr., Ciccarella A, Calcagnotto A, Mikhael PGet al. . Dietary methionine influences therapy in mouse cancer models and alters human metabolism. Nature. 2019;572(7769):397–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Ghoshal AK, Farber E. The induction of resistant hepatocytes during initiation of liver carcinogenesis with chemicals in rats fed a choline deficient methionine low diet. Carcinogenesis. 1983;4(7):801–4. [DOI] [PubMed] [Google Scholar]
- 155. Srivastava MK, Sinha P, Clements VK, Rodriguez P, Ostrand-Rosenberg S. Myeloid-derived suppressor cells inhibit T-cell activation by depleting cystine and cysteine. Cancer Res. 2010;70(1):68–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Cramer SL, Saha A, Liu J, Tadi S, Tiziani S, Yan W, Triplett K, Lamb C, Alters SE, Rowlinson Set al. . Systemic depletion of L-cyst(e)ine with cyst(e)inase increases reactive oxygen species and suppresses tumor growth. Nat Med. 2017;23(1):120–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Phannasil P, Thuwajit C, Warnnissorn M, Wallace JC, MacDonald MJ, Jitrapakdee S. Pyruvate carboxylase is up-regulated in breast cancer and essential to support growth and invasion of MDA-MB-231 cells. PLoS One. 2015;10(6):e0129848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Sellers K, Fox MP, Bousamra M 2nd, Slone SP, Higashi RM, Miller DM, Wang Y, Yan J, Yuneva MO, Deshpande Ret al. . Pyruvate carboxylase is critical for non-small-cell lung cancer proliferation. J Clin Invest. 2015;125(2):687–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Corbet C, Bastien E, Draoui N, Doix B, Mignion L, Jordan BF, Marchand A, Vanherck JC, Chaltin P, Schakman Oet al. . Interruption of lactate uptake by inhibiting mitochondrial pyruvate transport unravels direct antitumor and radiosensitizing effects. Nat Commun. 2018;9(1):1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Yang C, Ko B, Hensley CT, Jiang L, Wasti AT, Kim J, Sudderth J, Calvaruso MA, Lumata L, Mitsche Met al. . Glutamine oxidation maintains the TCA cycle and cell survival during impaired mitochondrial pyruvate transport. Mol Cell. 2014;56(3):414–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Rauckhorst AJ, Taylor EB. Mitochondrial pyruvate carrier function and cancer metabolism. Curr Opin Genet Dev. 2016;38:102–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Heinemeyer T, Stemmet M, Bardien S, Neethling A. Underappreciated roles of the translocase of the outer and inner mitochondrial membrane protein complexes in human disease. DNA Cell Biol. 2019;38(1):23–40. [DOI] [PubMed] [Google Scholar]
- 163. Park SH, Lee AR, Choi K, Joung S, Yoon JB, Kim S. TOMM20 as a potential therapeutic target of colorectal cancer. BMB Rep. 2019;52(12):712–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Jones SF, Infante JR. Molecular pathways: fatty acid synthase. Clin Cancer Res. 2015;21(24):5434–8. [DOI] [PubMed] [Google Scholar]
- 165. Gatzemeier U, Cavalli F, Häussinger K, Kaukel E, Koschel G, Martinelli G, Neuhauss R, von Pawel J. Phase III trial with and without lonidamine in non-small cell lung cancer. Semin Oncol. 1991;18(2 Suppl 4):42–8. [PubMed] [Google Scholar]
- 166. Alumkal JJ, Slottke R, Schwartzman J, Cherala G, Munar M, Graff JN, Beer TM, Ryan CW, Koop DR, Gibbs Aet al. . A phase II study of sulforaphane-rich broccoli sprout extracts in men with recurrent prostate cancer. Invest New Drugs. 2015;33(2):480–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Fassnacht M, Berruti A, Baudin E, Demeure MJ, Gilbert J, Haak H, Kroiss M, Quinn DI, Hesseltine E, Ronchi CLet al. . Linsitinib (OSI-906) versus placebo for patients with locally advanced or metastatic adrenocortical carcinoma: a double-blind, randomised, phase 3 study. Lancet Oncol. 2015;16(4):426–35. [DOI] [PubMed] [Google Scholar]
- 168. Bergqvist M, Holgersson G, Bondarenko I, Grechanaya E, Maximovich A, Andor G, Klockare M, Thureson M, Jerling M, Harmenberg J. Phase II randomized study of the IGF-1R pathway modulator AXL1717 compared to docetaxel in patients with previously treated, locally advanced or metastatic non-small cell lung cancer. Acta Oncol. 2017;56(3):441–7. [DOI] [PubMed] [Google Scholar]
- 169. Philip PA, Buyse ME, Alistar AT, Rocha Lima CM, Luther S, Pardee TS, Van Cutsem E. A phase III open-label trial to evaluate efficacy and safety of CPI-613 plus modified Folfirinox (mFFX) versus Folfirinox (FFX) in patients with metastatic adenocarcinoma of the pancreas. Future Oncol. 2019;15(28):3189–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Howells LM, Iwuji COO, Irving GRB, Barber S, Walter H, Sidat Z, Griffin-Teall N, Singh R, Foreman N, Patel SRet al. . Curcumin combined with FOLFOX chemotherapy is safe and tolerable in patients with metastatic colorectal cancer in a randomized phase IIa trial. J Nutr. 2019;149(7):1133–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Abou-Alfa GK, Macarulla T, Javle MM, Kelley RK, Lubner SJ, Adeva J, Cleary JM, Catenacci DV, Borad MJ, Bridgewater Jet al. . Ivosidenib in IDH1-mutant, chemotherapy-refractory cholangiocarcinoma (ClarIDHy): a multicentre, randomised, double-blind, placebo-controlled, phase 3 study. Lancet Oncol. 2020;21(6):796–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Hammel P, Fabienne P, Mineur L, Metges JP, Andre T, De La Fouchardiere C, Louvet C, El Hajbi F, Faroux R, Guimbaud Ret al. . Erythrocyte-encapsulated asparaginase (eryaspase) combined with chemotherapy in second-line treatment of advanced pancreatic cancer: an open-label, randomized phase IIb trial. Eur J Cancer. 2020;124:91–101. [DOI] [PubMed] [Google Scholar]
- 173. Villablanca JG, Volchenboum SL, Cho H, Kang MH, Cohn SL, Anderson CP, Marachelian A, Groshen S, Tsao-Wei D, Matthay KKet al. . A phase I new approaches to neuroblastoma therapy study of buthionine sulfoximine and melphalan with autologous stem cells for recurrent/refractory high-risk neuroblastoma. Pediatr Blood Cancer. 2016;63(8):1349–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Chae YC, Vaira V, Caino MC, Tang HY, Seo JH, Kossenkov AV, Ottobrini L, Martelli C, Lucignani G, Bertolini Iet al. . Mitochondrial Akt regulation of hypoxic tumor reprogramming. Cancer Cell. 2016;30(2):257–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Peeters K, Van Leemputte F, Fischer B, Bonini BM, Quezada H, Tsytlonok M, Haesen D, Vanthienen W, Bernardes N, Gonzalez-Blas CBet al. . Fructose-1,6-bisphosphate couples glycolytic flux to activation of Ras. Nat Commun. 2017;8(1):922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Bauer DE, Hatzivassiliou G, Zhao F, Andreadis C, Thompson CB. ATP citrate lyase is an important component of cell growth and transformation. Oncogene. 2005;24(41):6314–22. [DOI] [PubMed] [Google Scholar]
- 177. Lincet H, Kafara P, Giffard F, Abeilard-Lemoisson E, Duval M, Louis MH, Poulain L, Icard P. Inhibition of Mcl-1 expression by citrate enhances the effect of Bcl-xL inhibitors on human ovarian carcinoma cells. J Ovarian Res. 2013;6(1):72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Zhang X, Varin E, Allouche S, Lu Y, Poulain L, Icard P. Effect of citrate on malignant pleural mesothelioma cells: a synergistic effect with cisplatin. Anticancer Res. 2009;29(4):1249–54. [PubMed] [Google Scholar]
- 179. Guo X, Zhang X, Wang T, Xian S, Lu Y. 3-Bromopyruvate and sodium citrate induce apoptosis in human gastric cancer cell line MGC-803 by inhibiting glycolysis and promoting mitochondria-regulated apoptosis pathway. Biochem Biophys Res Commun. 2016;475(1):37–43. [DOI] [PubMed] [Google Scholar]
- 180. Hanai J, Doro N, Sasaki AT, Kobayashi S, Cantley LC, Seth P, Sukhatme VP. Inhibition of lung cancer growth: ATP citrate lyase knockdown and statin treatment leads to dual blockade of mitogen-activated protein kinase (MAPK) and phosphatidylinositol-3-kinase (PI3K)/AKT pathways. J Cell Physiol. 2012;227(4):1709–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Wang TA, Zhang XD, Guo XY, Xian SL, Lu YF. 3-Bromopyruvate and sodium citrate target glycolysis, suppress survivin, and induce mitochondrial-mediated apoptosis in gastric cancer cells and inhibit gastric orthotopic transplantation tumor growth. Oncol Rep. 2016;35(3):1287–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Ren JG, Seth P, Ye H, Guo K, Hanai JI, Husain Z, Sukhatme VP. Citrate suppresses tumor growth in multiple models through inhibition of glycolysis, the tricarboxylic acid cycle and the IGF-1R pathway. Sci Rep. 2017;7(1):4537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. National Library of Medicine, National Center for Biotechnology Information . Citrate. [Internet]. [Accessed 2020 Mar 26]. Available from: https://pubchem.ncbi.nlm.nih.gov/compound/Citric-acid#section=Toxicity. [Google Scholar]
- 184. Dzik WH, Kirkley SA. Citrate toxicity during massive blood transfusion. Transfus Med Rev. 1988;2(2):76–94. [DOI] [PubMed] [Google Scholar]
- 185. Zhang L, Liao Y, Xiang J, Qin W, Wu X, Tang Y, Yang Y, Chen Z, Fu P. Simplified regional citrate anticoagulation using a calcium-containing replacement solution for continuous venovenous hemofiltration. J Artif Organs. 2013;16(2):185–92. [DOI] [PubMed] [Google Scholar]
- 186. Bihorac A, Ross EA. Continuous venovenous hemofiltration with citrate-based replacement fluid: efficacy, safety, and impact on nutrition. Am J Kidney Dis. 2005;46(5):908–18. [DOI] [PubMed] [Google Scholar]
- 187. Morgera S, Schneider M, Slowinski T, Vargas-Hein O, Zuckermann-Becker H, Peters H, Kindgen-Milles D, Neumayer HH. A safe citrate anticoagulation protocol with variable treatment efficacy and excellent control of the acid-base status. Crit Care Med. 2009;37(6):2018–24. [DOI] [PubMed] [Google Scholar]
- 188. Caruana I, Simula L, Locatelli F, Campello S. T lymphocytes against solid malignancies: winning ways to defeat tumours. Cell Stress. 2018;2(8):200–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Goc J, Germain C, Vo-Bourgais TK, Lupo A, Klein C, Knockaert S, de Chaisemartin L, Ouakrim H, Becht E, Alifano Met al. . Dendritic cells in tumor-associated tertiary lymphoid structures signal a Th1 cytotoxic immune contexture and license the positive prognostic value of infiltrating CD8+ T cells. Cancer Res. 2014;74(3):705–15. [DOI] [PubMed] [Google Scholar]
- 190. Puleston DJ, Villa M, Pearce EL. Ancillary activity: beyond core metabolism in immune cells. Cell Metab. 2017;26(1):131–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Vijayan D, Young A, Teng MWL, Smyth MJ. Targeting immunosuppressive adenosine in cancer. Nat Rev Cancer. 2017;17(12):709–24.. Erratum in: Nat Rev Cancer 2017;17(12):765. [DOI] [PubMed] [Google Scholar]
- 192. Pages F, Galon J, Dieu-Nosjean MC, Tartour E, Sautes-Fridman C, Fridman WH. Immune infiltration in human tumors: a prognostic factor that should not be ignored. Oncogene. 2010;29(8):1093–102. [DOI] [PubMed] [Google Scholar]
- 193. Remark R, Lupo A, Alifano M, Biton J, Ouakrim H, Stefani A, Cremer I, Goc J, Regnard JF, Dieu-Nosjean MCet al. . Immune contexture and histological response after neoadjuvant chemotherapy predict clinical outcome of lung cancer patients. Oncoimmunology. 2016;5(12):e1255394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194. Buck MD, Sowell RT, Kaech SM, Pearce EL. Metabolic instruction of immunity. Cell. 2017;169(4):570–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195. Marelli-Berg FM, Jangani M. Metabolic regulation of leukocyte motility and migration. J Leukoc Biol. 2018;104(2):285–93. [DOI] [PubMed] [Google Scholar]
- 196. Sukumar M, Roychoudhuri R, Restifo NP. Nutrient competition: a new axis of tumor immunosuppression. Cell. 2015;162(6):1206–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197. Chang CH, Qiu J, O'Sullivan D, Buck MD, Noguchi T, Curtis JD, Chen Q, Gindin M, Gubin MM, van der Windt GJet al. . Metabolic competition in the tumor microenvironment is a driver of cancer progression. Cell. 2015;162(6):1229–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198. O'Neill LA, Pearce EJ. Immunometabolism governs dendritic cell and macrophage function. J Exp Med. 2016;213(1):15–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199. Steggerda SM, Bennett MK, Chen J, Emberley E, Huang T, Janes JR, Li W, MacKinnon AL, Makkouk A, Marguier Get al. . Inhibition of arginase by CB-1158 blocks myeloid cell-mediated immune suppression in the tumor microenvironment. J Immunother Cancer. 2017;5(1):101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200. Hurst KE, Lawrence KA, Essman MT, Walton ZJ, Leddy LR, Thaxton JE. Endoplasmic reticulum stress contributes to mitochondrial exhaustion of CD8(+) T cells. Cancer Immunol Res. 2019;7(3):476–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201. Zamanakou M, Germenis AE, Karanikas V. Tumor immune escape mediated by indoleamine 2,3-dioxygenase. Immunol Lett. 2007;111(2):69–75. [DOI] [PubMed] [Google Scholar]
- 202. Rodriguez PC, Hernandez CP, Quiceno D, Dubinett SM, Zabaleta J, Ochoa JB, Gilbert J, Ochoa AC. Arginase I in myeloid suppressor cells is induced by COX-2 in lung carcinoma. J Exp Med. 2005;202(7):931–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203. Ching MM, Reader J, Fulton AM. Eicosanoids in cancer: prostaglandin E(2) receptor 4 in cancer therapeutics and immunotherapy. Frontiers in pharmacology. 2020;11:819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204. Dang Q, Chen YA, Hsieh JT. The dysfunctional lipids in prostate cancer. Am J Clin Exp Urol. 2019;7(4):273–80. [PMC free article] [PubMed] [Google Scholar]
- 205. Rueda CM, Rodríguez-Perea AL, Moreno-Fernandez M, Jackson CM, Melchior JT, Davidson WS, Chougnet CA. High density lipoproteins selectively promote the survival of human regulatory T cells. J Lipid Res. 2017;58(8):1514–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206. Vijayan D, Young A, Teng MWL, Smyth MJ. Targeting immunosuppressive adenosine in cancer. Nat Rev Cancer. 2017;17(12):709–24. [DOI] [PubMed] [Google Scholar]
- 207. Silva LS, Poschet G, Nonnenmacher Y, Becker HM, Sapcariu S, Gaupel AC, Schlotter M, Wu Y, Kneisel N, Seiffert Met al. . Branched-chain ketoacids secreted by glioblastoma cells via MCT1 modulate macrophage phenotype. EMBO Rep. 2017;18(12):2172–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208. Allard B, Allard D, Buisseret L, Stagg J. The adenosine pathway in immuno-oncology. Nat Rev Clin Oncol. 2020;17:611–29. [DOI] [PubMed] [Google Scholar]
- 209. Chirasani SR, Leukel P, Gottfried E, Hochrein J, Stadler K, Neumann B, Oefner PJ, Gronwald W, Bogdahn U, Hau Pet al. . Diclofenac inhibits lactate formation and efficiently counteracts local immune suppression in a murine glioma model. Int J Cancer. 2013;132(4):843–53. [DOI] [PubMed] [Google Scholar]
- 210. Moreno-Sánchez R, Marín-Hernández Á, Del Mazo-Monsalvo I, Saavedra E, Rodríguez-Enríquez S. Assessment of the low inhibitory specificity of oxamate, aminooxyacetate and dichloroacetate on cancer energy metabolism. Biochim Biophys Acta Gen Subj. 2017;1861(1 Pt A):3221–36. [DOI] [PubMed] [Google Scholar]
- 211. Payen VL, Mina E, Van Hée VF, Porporato PE, Sonveaux P. Monocarboxylate transporters in cancer. Mol Metab. 2020;33:48–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212. Tasdogan A, Faubert B, Ramesh V, Ubellacker JM, Shen B, Solmonson A, Murphy MM, Gu Z, Gu W, Martin Met al. . Metabolic heterogeneity confers differences in melanoma metastatic potential. Nature. 2020;577(7788):115–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213. Corbet C, Feron O. Tumour acidosis: from the passenger to the driver's seat. Nat Rev Cancer. 2017;17(10):577–93. [DOI] [PubMed] [Google Scholar]
- 214. Dykes SS, Gao C, Songock WK, Bigelow RL, Woude GV, Bodily JM, Cardelli JA. Zinc finger E-box binding homeobox-1 (Zeb1) drives anterograde lysosome trafficking and tumor cell invasion via upregulation of Na+/H+ exchanger-1 (NHE1). Mol Carcinog. 2017;56(2):722–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215. Parks SK, Pouyssegur J. Targeting pH regulating proteins for cancer therapy—progress and limitations. Semin Cancer Biol. 2017;43:66–73. [DOI] [PubMed] [Google Scholar]
- 216. Robey IF, Baggett BK, Kirkpatrick ND, Roe DJ, Dosescu J, Sloane BF, Hashim AI, Morse DL, Raghunand N, Gatenby RAet al. . Bicarbonate increases tumor pH and inhibits spontaneous metastases. Cancer Res. 2009;69(6):2260–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217. Raghunand N, He X, van Sluis R, Mahoney B, Baggett B, Taylor CW, Paine-Murrieta G, Roe D, Bhujwalla ZM, Gillies RJ. Enhancement of chemotherapy by manipulation of tumour pH. Br J Cancer. 1999;80(7):1005–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218. Faes S, Duval AP, Planche A, Uldry E, Santoro T, Pythoud C, Stehle JC, Horlbeck J, Letovanec I, Riggi Net al. . Acidic tumor microenvironment abrogates the efficacy of mTORC1 inhibitors. Mol Cancer. 2016;15(1):78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219. Pilon-Thomas S, Kodumudi KN, El-Kenawi AE, Russell S, Weber AM, Luddy K, Damaghi M, Wojtkowiak JW, Mule JJ, Ibrahim-Hashim Aet al. . Neutralization of tumor acidity improves antitumor responses to immunotherapy. Cancer Res. 2016;76(6):1381–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220. Ikemura K, Hiramatsu S, Okuda M. Drug repositioning of proton pump inhibitors for enhanced efficacy and safety of cancer chemotherapy. Front Pharmacol. 2017;8:911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221. Egeblad M, Rasch MG, Weaver VM. Dynamic interplay between the collagen scaffold and tumor evolution. Curr Opin Cell Biol. 2010;22(5):697–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222. Ohlund D, Franklin O, Lundberg E, Lundin C, Sund M. Type IV collagen stimulates pancreatic cancer cell proliferation, migration, and inhibits apoptosis through an autocrine loop. BMC Cancer. 2013;13:154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223. Cho Y, Cho EJ, Lee JH, Yu SJ, Kim YJ, Kim CY, Yoon JH. Hypoxia enhances tumor-stroma crosstalk that drives the progression of hepatocellular carcinoma. Dig Dis Sci. 2016;61(9):2568–77. [DOI] [PubMed] [Google Scholar]
- 224. Martinez-Outschoorn UE, Balliet RM, Rivadeneira DB, Chiavarina B, Pavlides S, Wang C, Whitaker-Menezes D, Daumer KM, Lin Z, Witkiewicz AKet al. . Oxidative stress in cancer associated fibroblasts drives tumor-stroma co-evolution: a new paradigm for understanding tumor metabolism, the field effect and genomic instability in cancer cells. Cell Cycle. 2010;9(16):3256–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225. Porporato PE. Understanding cachexia as a cancer metabolism syndrome. Oncogenesis. 2016;5:e200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226. Hui S, Ghergurovich JM, Morscher RJ, Jang C, Teng X, Lu W, Esparza LA, Reya T, Le Z, Yanxiang Guo Jet al. . Glucose feeds the TCA cycle via circulating lactate. Nature. 2017;551(7678):115–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227. Icard P, Kafara P, Steyaert JM, Schwartz L, Lincet H. The metabolic cooperation between cells in solid cancer tumors. Biochim Biophys Acta. 2014;1846(1):216–25. [DOI] [PubMed] [Google Scholar]
- 228. Fearon KC, Glass DJ, Guttridge DC. Cancer cachexia: mediators, signaling, and metabolic pathways. Cell Metab. 2012;16(2):153–66. [DOI] [PubMed] [Google Scholar]
- 229. Remark R, Lupo A, Alifano M, Biton J, Ouakrim H, Stefani A, Cremer I, Goc J, Régnard JF, Dieu-Nosjean MCet al. . Immune contexture and histological response after neoadjuvant chemotherapy predict clinical outcome of lung cancer patients. Oncoimmunology. 2016;5(12):e1255394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230. Avgerinos KI, Spyrou N, Mantzoros CS, Dalamaga M. Obesity and cancer risk: Emerging biological mechanisms and perspectives. Metabolism. 2019;92:121–35. [DOI] [PubMed] [Google Scholar]
- 231. Hu B, Lin JZ, Yang XB, Sang XT. Aberrant lipid metabolism in hepatocellular carcinoma cells as well as immune microenvironment: a review. Cell Prolif. 2020;53(3):e12772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232. Hayes CN, Zhang P, Chayama K. The role of lipids in hepatocellular carcinoma. In: Tirnitz-Parker JEEeditor. Hepatocellular carcinoma. Brisbane (Australia: ): Codon Publications; 2019. [PubMed] [Google Scholar]
- 233. Liu X, Liang Y, Song R, Yang G, Han J, Lan Y, Pan S, Zhu M, Liu Y, Wang Yet al. . Long non-coding RNA NEAT1-modulated abnormal lipolysis via ATGL drives hepatocellular carcinoma proliferation. Mol Cancer. 2018;17(1):90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234. Schooneman MG, Vaz FM, Houten SM, Soeters MR. Acylcarnitines: reflecting or inflicting insulin resistance?. Diabetes. 2013;62(1):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235. Noureldein MH, Eid AA. Gut microbiota and mTOR signaling: insight on a new pathophysiological interaction. Microb Pathog. 2018;118:98–104. [DOI] [PubMed] [Google Scholar]
- 236. Yarla NS, Bishayee A, Sethi G, Reddanna P, Kalle AM, Dhananjaya BL, Dowluru KS, Chintala R, Duddukuri GR. Targeting arachidonic acid pathway by natural products for cancer prevention and therapy. Semin Cancer Biol. 2016;40–41:48–81. [DOI] [PubMed] [Google Scholar]
- 237. Adan-Gokbulut A, Kartal-Yandim M, Iskender G, Baran Y. Novel agents targeting bioactive sphingolipids for the treatment of cancer. Curr Med Chem. 2013;20(1):108–22. [PubMed] [Google Scholar]
- 238. Denduluri SK, Idowu O, Wang Z, Liao Z, Yan Z, Mohammed MK, Ye J, Wei Q, Wang J, Zhao Let al. . Insulin-like growth factor (IGF) signaling in tumorigenesis and the development of cancer drug resistance. Genes Dis. 2015;2(1):13–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239. Bowers LW, Rossi EL, O'Flanagan CH, deGraffenried LA, Hursting SD. The role of the insulin/IGF system in cancer: lessons learned from clinical trials and the energy balance-cancer link. Front Endocrinol. 2015;6:77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240. Werner H, Sarfstein R, LeRoith D, Bruchim I. Insulin-like growth factor 1 signaling axis meets p53 genome protection pathways. Front Oncol. 2016;6:159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241. Dev R, Bruera E, Dalal S. Insulin resistance and body composition in cancer patients. Ann Oncol. 2018;29(Suppl 2):ii18–26. [DOI] [PubMed] [Google Scholar]
- 242. Vigneri R, Goldfine ID, Frittitta L. Insulin, insulin receptors, and cancer. J Endocrinol Invest. 2016;39(12):1365–76. [DOI] [PubMed] [Google Scholar]
- 243. Mancuso E, Mannino GC, Fatta CD, Fuoco A, Spiga R, Andreozzi F, Sesti G. Insulin-like growth factor-1 is a negative modulator of glucagon secretion. Oncotarget. 2017;8(31):51719–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244. Ye H, Adane B, Khan N, Alexeev E, Nusbacher N, Minhajuddin M, Stevens BM, Winters AC, Lin X, Ashton JMet al. . Subversion of systemic glucose metabolism as a mechanism to support the growth of leukemia cells. Cancer Cell. 2018;34(4):659–73.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245. Yee D. A tale of two receptors: insulin and insulin-like growth factor signaling in cancer. Clin Cancer Res. 2015;21(4):667–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246. Liu W, Kang L, Han J, Wang Y, Shen C, Yan Z, Tai Y, Zhao C. miR-342-3p suppresses hepatocellular carcinoma proliferation through inhibition of IGF-1R-mediated Warburg effect. Onco Targets Ther. 2018;11:1643–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247. Farhat D, Leon S, Ghayad SE, Gadot N, Icard P, Le Romancer M, Hussein N, Lincet H. Lipoic acid decreases breast cancer cell proliferation by inhibiting IGF-1R via furin downregulation. Br J Cancer. 2020;122(6):885–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248. Chen C, Zhu YF, Liu XJ, Lu ZX, Xie Q, Ling N. Discovery of a series of nonpeptide small molecules that inhibit the binding of insulin-like growth factor (IGF) to IGF-binding proteins. J Med Chem. 2001;44(23):4001–10. [DOI] [PubMed] [Google Scholar]
- 249. Saleh AD, Simone BA, Palazzo J, Savage JE, Sano Y, Dan T, Jin L, Champ CE, Zhao S, Lim Met al. . Caloric restriction augments radiation efficacy in breast cancer. Cell Cycle. 2013;12(12):1955–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250. Simone BA, Palagani A, Strickland K, Ko K, Jin L, Lim MK, Dan TD, Sarich M, Monti DA, Cristofanilli Met al. . Caloric restriction counteracts chemotherapy-induced inflammation and increases response to therapy in a triple negative breast cancer model. Cell Cycle. 2018;17(13):1536–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251. Lee C, Safdie FM, Raffaghello L, Wei M, Madia F, Parrella E, Hwang D, Cohen P, Bianchi G, Longo VD. Reduced levels of IGF-I mediate differential protection of normal and cancer cells in response to fasting and improve chemotherapeutic index. Cancer Res. 2010;70(4):1564–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252. de Groot S, Pijl H, van der Hoeven JJM, Kroep JR. Effects of short-term fasting on cancer treatment. J Exp Clin Cancer Res. 2019;38(1):209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253. Raffaghello L, Lee C, Safdie FM, Wei M, Madia F, Bianchi G, Longo VD. Starvation-dependent differential stress resistance protects normal but not cancer cells against high-dose chemotherapy. Proc Natl Acad Sci USA. 2008;105(24):8215–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254. Cheng CW, Adams GB, Perin L, Wei M, Zhou X, Lam BS, Da Sacco S, Mirisola M, Quinn DI, Dorff TBet al. . Prolonged fasting reduces IGF-1/PKA to promote hematopoietic-stem-cell-based regeneration and reverse immunosuppression. Cell Stem Cell. 2014;14(6):810–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255. Jelluma N, Yang X, Stokoe D, Evan GI, Dansen TB, Haas-Kogan DA. Glucose withdrawal induces oxidative stress followed by apoptosis in glioblastoma cells but not in normal human astrocytes. Mol Cancer Res. 2006;4(5):319–30. [DOI] [PubMed] [Google Scholar]
- 256. Buono R, Longo VD. Starvation, stress resistance, and cancer. Trends Endocrinol Metab. 2018;29(4):271–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257. Shaw RJ. LKB1 and AMP-activated protein kinase control of mTOR signalling and growth. Acta Physiol (Oxf). 2009;196(1):65–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258. Matoba S, Kang JG, Patino WD, Wragg A, Boehm M, Gavrilova O, Hurley PJ, Bunz F, Hwang PM. p53 Regulates mitochondrial respiration. Science. 2006;312(5780):1650–3. [DOI] [PubMed] [Google Scholar]
- 259. Haigis MC, Deng CX, Finley LW, Kim HS, Gius D. SIRT3 is a mitochondrial tumor suppressor: a scientific tale that connects aberrant cellular ROS, the Warburg effect, and carcinogenesis. Cancer Res. 2012;72(10):2468–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260. Qiu X, Brown K, Hirschey MD, Verdin E, Chen D. Calorie restriction reduces oxidative stress by SIRT3-mediated SOD2 activation. Cell Metab. 2010;12(6):662–7. [DOI] [PubMed] [Google Scholar]
- 261. Jeon SM, Hay N. The double-edged sword of AMPK signaling in cancer and its therapeutic implications. Arch Pharm Res. 2015;38(3):346–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262. Jeon SM, Chandel NS, Hay N. AMPK regulates NADPH homeostasis to promote tumour cell survival during energy stress. Nature. 2012;485(7400):661–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263. Simons AL, Mattson DM, Dornfeld K, Spitz DR. Glucose deprivation-induced metabolic oxidative stress and cancer therapy. J Cancer Res Ther. 2009;5(Suppl 1):S2–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264. Lo Re O, Panebianco C, Porto S, Cervi C, Rappa F, Di Biase S, Caraglia M, Pazienza V, Vinciguerra M. Fasting inhibits hepatic stellate cells activation and potentiates anti-cancer activity of Sorafenib in hepatocellular cancer cells. J Cell Physiol. 2018;233(2):1202–12. [DOI] [PubMed] [Google Scholar]
- 265. Kharazi AI, James SJ, Taylor JM, Lubinski JM, Nakamura LT, Makinodan T. Combined chronic low dose radiation-caloric restriction: a model for regression of spontaneous mammary tumor. Int J Radiat Oncol Biol Phys. 1994;28(3):641–7. [DOI] [PubMed] [Google Scholar]
- 266. Seyfried TN, Flores R, Poff AM, D'Agostino DP, Mukherjee P. Metabolic therapy: a new paradigm for managing malignant brain cancer. Cancer Lett. 2015;356(2 Pt A):289–300. [DOI] [PubMed] [Google Scholar]
- 267. Branco AF, Ferreira A, Simoes RF, Magalhaes-Novais S, Zehowski C, Cope E, Silva AM, Pereira D, Sardao VA, Cunha-Oliveira T. Ketogenic diets: from cancer to mitochondrial diseases and beyond. Eur J Clin Invest. 2016;46(3):285–98. [DOI] [PubMed] [Google Scholar]
- 268. Lussier DM, Woolf EC, Johnson JL, Brooks KS, Blattman JN, Scheck AC. Enhanced immunity in a mouse model of malignant glioma is mediated by a therapeutic ketogenic diet. BMC Cancer. 2016;16:310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269. Peiris-Pages M, Martinez-Outschoorn UE, Pestell RG, Sotgia F, Lisanti MP. Cancer stem cell metabolism. Breast Cancer Res. 2016;18(1):55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270. Zu XL, Guppy M. Cancer metabolism: facts, fantasy, and fiction. Biochem Biophys Res Commun. 2004;313(3):459–65. [DOI] [PubMed] [Google Scholar]
- 271. Tisdale MJ, Brennan RA. Loss of acetoacetate coenzyme A transferase activity in tumours of peripheral tissues. Br J Cancer. 1983;47(2):293–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272. Yun J, Rago C, Cheong I, Pagliarini R, Angenendt P, Rajagopalan H, Schmidt K, Willson JK, Markowitz S, Zhou Set al. . Glucose deprivation contributes to the development of KRAS pathway mutations in tumor cells. Science. 2009;325(5947):1555–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273. Brown RS, Wahl RL. Overexpression of Glut-1 glucose transporter in human breast cancer: an immunohistochemical study. Cancer. 1993;72(10):2979–85. [DOI] [PubMed] [Google Scholar]
- 274. Romero R, Sayin VI, Davidson SM, Bauer MR, Singh SX, LeBoeuf SE, Karakousi TR, Ellis DC, Bhutkar A, Sanchez-Rivera FJet al. . Keap1 loss promotes Kras-driven lung cancer and results in dependence on glutaminolysis. Nat Med. 2017;23(11):1362–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275. Icard P, Ollivier L, Forgez P, Otz J, Alifano M, Fournel L, Loi M, Thariat J. Perspective: do fasting, caloric restriction, and diets increase sensitivity to radiotherapy? A literature review. Adv Nutr. 2020;11:1089–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276. Riester M, Xu Q, Moreira A, Zheng J, Michor F, Downey RJ. The Warburg effect: persistence of stem-cell metabolism in cancers as a failure of differentiation. Ann Oncol. 2018;29(1):264–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277. Butler EB, Zhao Y, Munoz-Pinedo C, Lu J, Tan M. Stalling the engine of resistance: targeting cancer metabolism to overcome therapeutic resistance. Cancer Res. 2013;73(9):2709–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278. Denise C, Paoli P, Calvani M, Taddei ML, Giannoni E, Kopetz S, Kazmi SM, Pia MM, Pettazzoni P, Sacco Eet al. . 5-Fluorouracil resistant colon cancer cells are addicted to OXPHOS to survive and enhance stem-like traits. Oncotarget. 2015;6(39):41706–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279. Schug ZT, Vande Voorde J, Gottlieb E. The metabolic fate of acetate in cancer. Nat Rev Cancer. 2016;16(11):708–17. [DOI] [PMC free article] [PubMed] [Google Scholar]