Abstract
BACKGROUND:
Dysfunctional reward processing is implicated in multiple mental disorders. Novelty seeking (NS) assesses preference for seeking novel experiences, which is linked to sensitivity to reward environmental cues.
METHODS:
A subset of 14-year-old adolescents (IMAGEN) with the top 20% ranked high-NS scores was used to identify high-NS–associated multimodal components by supervised fusion. These features were then used to longitudinally predict five different risk scales for the same and unseen subjects (an independent dataset of subjects at 19 years of age that was not used in predictive modeling training at 14 years of age) (within IMAGEN, n ≈ 1100) and even for the corresponding symptom scores of five types of patient cohorts (non-IMAGEN), including drinking (n = 313), smoking (n = 104), attention-deficit/hyperactivity disorder (n = 320), major depressive disorder (n = 81), and schizophrenia (n = 147), as well as to classify different patient groups with diagnostic labels.
RESULTS:
Multimodal biomarkers, including the prefrontal cortex, striatum, amygdala, and hippocampus, associated with high NS in 14-year-old adolescents were identified. The prediction models built on these features are able to longitudinally predict five different risk scales, including alcohol drinking, smoking, hyperactivity, depression, and psychosis for the same and unseen 19-year-old adolescents and even predict the corresponding symptom scores of five types of patient cohorts. Furthermore, the identified reward-related multimodal features can classify among attention-deficit/hyperactivity disorder, major depressive disorder, and schizophrenia with an accuracy of 87.2%.
CONCLUSIONS:
Adolescents with higher NS scores can be used to reveal brain alterations in the reward-related system, implicating potential higher risk for subsequent development of multiple disorders. The identified high-NS–associated multimodal reward-related signatures may serve as a transdiagnostic neuroimaging biomarker to predict disease risks or severity.
Adolescence is a critical development period in which personalities and behavioral tendencies that extend into personal adulthood are established. Risk taking and novelty seeking (NS) are among the most prominent behavioral changes observed in adolescence and are highly correlated (1). Novelty seeking assesses preference for seeking novel experiences and higher levels of rewarding stimulation, which is a hallmark of typical adolescent behavior (2), as well as a key personality trait in adolescents that is of critical importance in transitioning from a dependent child to an independent adult (3). Therefore, there might be an association between NS outlier scores in adolescents and risk of having developmental disorders. Coincidently, recent studies show that both adolescent smokers (4) and problematic drug users (5) exhibit significantly higher NS scores than normal control subjects. In addition, adolescents show more inclination to risk taking (6) and NS (7), possibly with a hypersensitivity to reward circuit.
In contrast, reward processing is impaired in multiple disorders, including substance use disorders (8,9), depression (10–13), and psychosis (including negative symptoms) (14). The changes of mechanisms underlying reward processing are important for NS behavior and are also likely to influence depression and psychosis, albeit in a different way. Nonetheless, there could still be core properties of reward processing that remain invariant to modulate distinct psychopathologies. Although there are links between NS and reward circuitry dysfunction through dopamine modulation (15) and between reward circuitry dysfunction and multiple disorders, the specific associations and longitudinal impact among NS, reward circuitry dysfunction, and risks for multiple disorders (including drinking, smoking, attention-deficit/hyperactivity disorder [ADHD], depression, and psychosis) remain unexplored in one study.
Recently, a meta-analysis showed that the personality trait is significantly correlated with almost every psychiatric disorder, including ADHD, bipolar disorder, major depressive disorder (MDD), and schizophrenia (SZ), based on heritability within the general population (16). Coincidently, symptomatic and epidemiological comorbidity (17–20) has also been reported for these mental disorders including bipolar disorder (21), MDD (22), autism spectrum disorder, and ADHD. Considering the well-validated linkage between NS and reward processing, we hypothesize that high-NS–associated multimodal brain networks in adolescents would represent a common dysfunctional circuit among smoking, drinking, hyperactivity, depression, and psychosis, and that these features are able to predict disease risk longitudinally in the follow-up adolescents and external patient cohorts.
It is known that NS depends on reinforcement through external or internal (drug experience, but perhaps also prayer/meditation) novel stimuli. In this study, we are trying to better understand the structural and functional brain mechanisms that underlie the individual cognitive components of reinforcement in relation to NS, namely, reward processing, impulsiveness, and emotional processing, which play an essential role in forming personality differences during adolescent development and are implicated in many neuropsychiatric disorders, including addiction, ADHD, SZ, and depression (23). Here, the IMAGEN (23) dataset, which is a large cross-sectional European multicenter study of reinforcement sensitivity in adolescents, was used as a discovery cohort. Many IMAGEN studies have identified risk biomarkers in healthy adolescents for individual disorders such as smoking, alcohol use (24), drug abuse (5), ADHD (25), depression (26), and psychosis-like experience (27), but mostly within one modality or without exploring imaging predictors of symptoms severity in multiple external patient cohorts. This work aims to address the following questions to test our hypothesis:
Which brain regions are associated with high NS in multimodal magnetic resonance imaging (MRI) (Figure 1A)?
Can high-NS–associated multimodal features identified in 14-year-old adolescents longitudinally predict the risks of drinking, smoking, hyperactivity, depression, and psychosis in 19-year-old adolescents (IMAGEN, Figure 1B)?
Can high-NS–associated multimodal features be generalized to predict the symptom scores spanning alcohol drinking, smoking, ADHD, MDD, and SZ in patient groups (non-IMAGEN, Figure 1C)?
How accurately can the high-NS–associated multimodal features classify multiple clinical disorders (non-IMAGEN, Figure 1D)?
Can the high-NS–associated multimodal reward system implicate a generalized dysfunctional brain circuit spanning these five patient groups (Figure 1E)?
Figure 1.
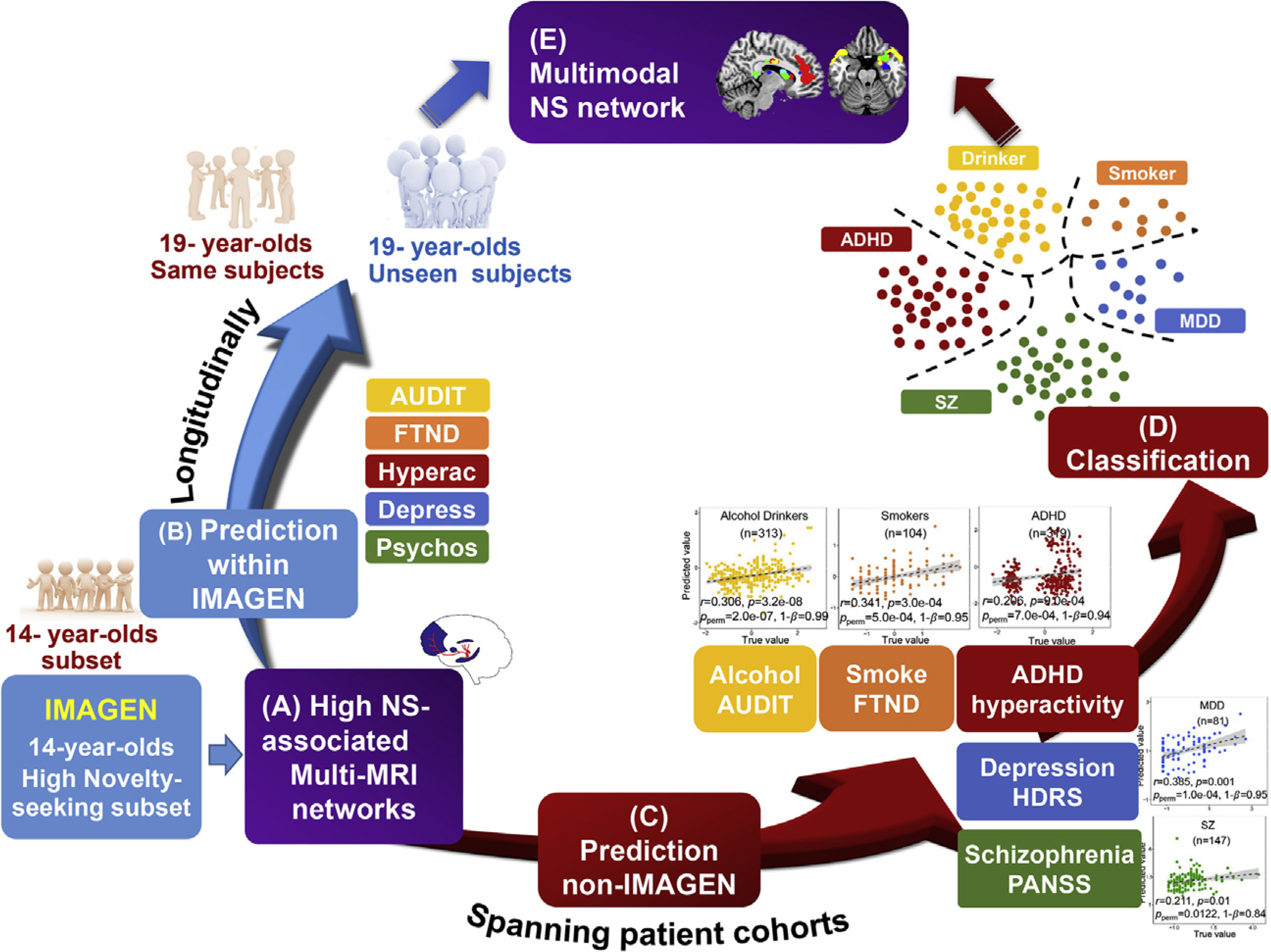
Study design. (A) Identify high-novelty seeking (NS)–associated multimodal brain networks on the top 20% NS scored adolescents at age 14 (239 out of 1378, IMAGEN). (B) Follow-up study within IMAGEN: to evaluate whether the identified high-NS–associated multimodal features can longitudinally predict five different risk scores for the same subjects (n = 239) and the unseen youth (n = 1100) at 19 years of age. (C) External patient cohort verification, n = 965: to determine whether high-NS–associated features can predict symptom scores for alcohol drinking (Alcohol Use Disorders Identification Test [AUDIT]), smoking (Fagerström Test for Nicotine Dependence [FTND]), attention-deficit/hyperactivity disorder (ADHD) (hyperactivity), major depressive disorder (MDD) (depression), and schizophrenia (SZ) (psychosis). (D) Classification between patients (n = 965) and control subjects (n = 1094) as well as among different patient groups. (E) A generalized dysfunctional multimodal brain circuit spanning alcohol, smoking, hyperactivity, depression, and psychosis. Depress, depression; HDRS, Hamilton Depression Rating Scale; Hyperac, hyperactivity; PANSS, Positive and Negative Syndrome Scale; Psychos, psychosis.
Addressing these questions goes beyond predicting the development of addiction and other mental health disorders, a worthwhile goal in itself for clinicians. It further allows the examination and refining of the neural basis common or specific to various psychiatric disorders.
Specifically, NS scores were used as reference to guide a four-way MRI fusion, including gray matter volume and three task-related functional MRI (fMRI) contrasts, to identify the high-NS–associated (top 20% scored) brain regions in 14-year-old adolescents. This supervised fusion model, multisite canonical correlation analysis with reference + joint independent component analysis (28), can identify multimodal imaging components associated with a specific measure of interest (NS). The three representative fMRI tasks that were used are 1) modified monetary incentive delay (anticipation of large gain vs. no gain), which relates to reward anticipation; 2) a faces task (angry vs. control) that relates to emotional processing; and 3) a stop signal task (stop failure vs. baseline) that relates to reinforcement in adolescents. The identified NS-associated multimodal features in 14-year-old adolescents were then used to build prediction models for both follow-up (19-year-old adolescents) disease risk prediction and transdiagnostic symptom severity evaluation, and even classification among multiple diseases.
METHODS AND MATERIALS
IMAGEN Participants
In the IMAGEN project (23), adolescents were recruited through local public schools at eight sites across Europe. The Temperament and Character Inventory test (29) was used to evaluate NS. Alcohol and nicotine consumption was assessed by the Alcohol Use Disorders Identification Test (AUDIT) (30) and Fagerström Test for Nicotine Dependence (FTND) (31). The subjects were screened for psychiatric disorders with the Development and Well-Being Assessment questionnaire (32), including risk rating scale scores of depression and psychosis. Gender differences on these clinical scores are shown in Table S1. The study was approved by the local ethics committees and adhered to the Declaration of Helsinki, and written informed consents were obtained. The IMAGEN adolescents completed a range of functional tasks related to reward (modified monetary incentive delay), emotion (faces task), and reinforcement (the stop signal task) and structural neuroimaging at 14 and 19 years. Details of each task design, imaging parameters, and preprocessing strategies are presented under Multimodal Imaging Parameters and Preprocessing in the Supplement.
Non-IMAGEN Cohorts
Patients with SZ (n = 147) were aggregated from Function Biomedical Informatics Research Network (33) subjects who had no current or past history of other psychiatric or neurological illness. Patients with MDD (n = 81) were recruited from the West China Hospital of Sichuan (34,35). ADHD (n = 320) data (http://fcon_1000.projects.nitrc.org/indi/adhd200/index.html) were obtained from the ADHD-200 project (18). Smoking and drinking subjects came from Erlangen, Germany. Diagnosis of SZ, MDD, and ADHD was based on the Structured Clinical Interview for DSM-IV. Demographic information of each diagnosed group and IMAGEN adolescents can be found in Table 1.
Table 1.
Demographic Information of Subjects Studied
| Groups | Age, Years, Mean ± SD | Gender | NS, Mean ± SD |
|---|---|---|---|
| 14-Year-Olds, n = 239, Selected From 1378 | 14.4 ± 0.4 | 100 M/139 F | 125.7 ± 4.9 |
| 19-Year-Olds, n = 239 | 19.0 ± 0.8 | 100 M/139 F | 114.7 ± 10.3 |
| Unseen 19-Year-Olds, n = 1134 | 19.1 ± 0.8 | 568 M/566 F | 106.6 ± 10.1 |
| Alcohol Drinkers, n = 313 | 32.0 ± 9.8 | 219 M/94 F | NA |
| Smokers, n = 104 | 26.4 ± 4.6 | 79 M/25 F | NA |
| Patients With ADHD, n = 320 | 11.1 ± 2.5 | 238 M/82 F | NA |
| Patients With MDD, n = 81 | 29.1 ± 9.8 | 28 M/53 F | NA |
| Patients With SZ, n = 147 | 42.8 ± 11.8 | 108 M/39 F | NA |
| HC, n = 1094 | 19.9 ± 13.0 | 591 M/503 F | NA |
ADHD, attention-deficit/hyperactivity disorder; F, female; HC, healthy control subjects; M, male; MDD, major depressive disorder; NA, not available; NS, novelty seeking; SZ, schizophrenia.
Multimodal Fusion With High NS Scores
As displayed in Figure 2, we answered the questions listed in the introduction by using a dedicated high-NS–guided multimodal fusion (28), multiple cross-prediction, and multigroup classification analyses. First, a subset of 14-year-old adolescents with top 20% ranked high-NS scores (correlation between NS and other clinical measures can be found in Table S2) was selected (125.7 ± 4.9, n = 239, 100 male) as a discovery cohort to study a homogeneity subset with the highest NS scores. These subset adolescents are assumed to have a higher risk of developing addiction or other psychotic disorders (5). Thereafter, NS scores were used as a reference to guide a four-way MRI fusion on these top 20% ranked high-NS adolescents, including gray matter volume and three task-related fMRI contrasts, to identify the high-NS–associated brain regions at 14 years. Multisite canonical correlation analysis with reference + joint independent component analysis imposes an additional constraint to maximize the column-wise correlations between loading parameters (Ak) and the reference NS scores (equation 1). Therefore, fusion with NS scores enables identification of a joint multimodal component(s) that has robust correlations with NS, which, however, cannot be detected by a blinded N-way multimodal fusion approach.
| (1) |
where corr(Ak,Aj) is the columnwise correlation between Ak and Aj, while corr(Ak,ref) is the columnwise correlation between Ak and the reference signal, k,j={1,2,3,4}.
Figure 2.

The analysis flowchart. Novelty-seeking (NS) scores were used as a reference to guide a four-way multimodal fusion to identify a set of multimodal imaging biomarkers, each of which was separated as positive and negative brain regions based on the z-scored brain maps, plus the corresponding biomarker loadings, resulting in 12 features for the following prediction analysis. Multiple linear regression models were constructed for each of the five risk scores including alcohol use, smoking, hyperactivity, depression, and psychosis in the subset of 14-year-old adolescents. Then, the same prediction models were applied to longitudinally predict each of the five risk scores of the same subjects and the large set of unseen healthy adolescents at 19 years of age. The same models were also used to predict corresponding symptom scores of five types of patients, including alcohol drinkers, smokers, and patients with attention-deficit/hyperactivity disorder (ADHD), major depressive disorder (MDD), or schizophrenia (SZ). Finally, binary class and multiclass classification analysis were performed to verify the classification ability of the identified high-NS–associated multimodal imaging features. FTND, Fagerström Test for Nicotine Dependence; GM, gray matter; HC, healthy control subjects.
After identifying the high-NS–associated multimodal components in the high-risk subset, each component was separated into positive and negative brain regions based on the z-scored brain maps. Thus, we can obtain separate positive and negative brain masks for each of the four modalities (eight brain imaging networks). The mean of the voxels within the masked region was calculated for each subject for each of the eight networks, generating a Nsubj × 8 feature vector (Reward_P, Reward_N, Emotion_P, Emotion_N, Inhibition_P, Inhibition_N, GM_P and GM_N). Apart from the aforementioned brain imaging features, we also included the corresponding biomarker loadings (contribution weight across subjects) of each target component (generating four loading features, i.e., Reward_L, Emotion_L, Inhibition_L; and GM_L). Thus, we formed a feature matrix of NS-associated multimodal brain features in dimension of Nsubj × 12 in total for the prediction analysis.
As for the patient cohorts, there are no task-related fMRI data available, but only resting-state fMRI. Thus, the brain networks identified from IMAGEN tasks were used as regions of interest (ROIs) to extract features from patients’ resting-state fMRI. The mean of the voxels within the ROI was calculated for each subject, generating a Nsubj × 8 feature vector for patient cohorts. Linear back reconstruction was performed from the IMAGEN to non-IMAGEN groups to get loading features (Nsubj × 4) for each patient group. Details can be found under Linear back reconstruction in the Supplement.
Thereafter, the identified multimodal features (Nsubj × 12) in 14-year-old adolescents were used to longitudinally predict five disease risks/symptoms of the same (n = 239) and the unseen (n ≈ 1100, within IMAGEN) subjects (an independent dataset of subjects at 19 years of age that was not used in predictive modeling training at 14 years of age), spanning drinking, smoking, hyperactivity, depression, and psychosis, and clinical symptoms in 5 independent patient cohorts, including alcohol drinkers (n = 313), smokers (n = 104), patients with ADHD (n = 320), patients with MDD (n = 81), and patients with SZ (n = 147). Multiple linear regression models (equation 2) were constructed for each of the 5 symptom/risk rating scale scores in the subset of 14-year-old adolescents, separately. The predictions of symptom scores in the subset of 19-year-old adolescents and the patient group were made using the same regression weights obtained from the subset of 14-year-old adolescents. Thus, no training/additional feature selection was performed on the sample of 19-year-old adolescents and patients. Details can be found under Prediction in the Supplement
| (2) |
The classification ability of the identified high-NS–associated multimodal imaging features was also tested by both binary class and multiclass classification on 5 patient cohorts. Linear support vector machine was used as the classification model for all the classification analyses, in which an unbiased 10-fold cross-validation framework was applied. Details can be found under Classification in the Supplement.
RESULTS
High-NS–Associated Multimodal Brain Networks at Age 14
As shown in Figure 3, one linked reward-emotion-inhibition-GM component was identified by supervised fusion, showing significant correlations between its loadings and the high-NS scores in all modalities (r = .280 [false discovery rate (FDR) corrected], pperm = 1.0 × 10−4; r = .254 [FDR corrected], pperm = 1.0 × 10−4; r = .366 [FDR corrected], pperm = 1.0 × 10−8; r = .349 [FDR corrected], pperm = 1.0 × 10−7 for reward, emotion, inhibition tasks, and GM, respectively). pperm represents the p values from the permutation test (Figure S1), with details provided under Permutation test in the Supplement. This joint component is also correlated with other personality domains (Figure S2). The identified brain regions are summarized in Table S3 for task components and GM (Talairach labels), respectively. To confirm that the extracted high-NS–associated multimodal patterns are specific to the NS measures and not a random pattern, we permuted the NS scores in the supervised fusion analysis. Note that the random pattern (Figure S3) is very different from the identified high-NS–associated network, supporting the specificity of the relationship to NS (Figure 3A).
Figure 3.

The identified high-novelty seeking–associated multimodal joint components in the subset of 14-year-old adolescents. (A) Spatial brain maps visualized at |Z| > 2. (B) Correlation scatter plot between novelty-seeking scores and loadings of component for each modality. GM, gray matter; IC, independent component.
Predictive Models Trained on 14-Year-Old Adolescents
Multiple linear regression models were constructed for each of the five symptom/risk rating scale scores in the subset of 14-year-old adolescents. Pearson correlation coefficients (r) of .394, .230, .389, .263, and .183 were achieved between the estimated symptom/risk scores and the true values for drinking, smoking, hyperactivity, depression, and psychosis, respectively, in the subset of 14-year-old adolescents (Figure 4B), with the corresponding permuted p values of pperm = 1.0 × 10–8, .0016, 3.0 × 10–4, 1.0 × 10–4, and .0089, respectively. Importantly, the prediction results remain significant even regressing out gender (Figure S4). Note that some of the predicted measures are with discrete values (e.g., Figure 4B, smoker); hence, Spearman correlation was also calculated in addition to the Pearson correlation, which remains significant for the five risk score predictions as well (Figure S5). We provide six different predictive accuracy estimation strategies, including 1) Pearson correlation, 2) Spearman correlation, 3) partial correlation by regressing out gender, 4) normalized root-mean-square prediction error, 5) permutation test, and 6) statistical power to evaluate the predictive performance, as summarized in Table S4.
Figure 4.

Prediction results based on the identified high-novelty seeking (NS)–associated multimodal brain imaging networks. (A) Positive (red) and negative (blue) brain maps of the z-scored components and the corresponding loading parameters (columns). Loadings represent the contribution weight of the corresponding component across subjects. (B) Regression models trained on the subset of 14-year-old high-risk adolescents on five different risk scores. (C) Within-IMAGEN longitudinal predictions on five risk scores for the same 19-year-old adolescents (n = 239) using the same prediction models as in panel (B). (D) Within-IMAGEN longitudinal predictions for the other unseen 19-year-old adolescents (n ≈ 1100). (E) Generalized prediction for independent patients diagnosed as drinking, smoking, or having attention-deficit/hyperactivity disorder (ADHD), major depressive disorder (MDD), or schizophrenia (SZ) (n = 964). The n in each subplot represents the number of subjects with that kind of risk scores. Here r represents correlation between true values and the predicted values; pperm represents the p values of permutation test, and 1−β represents the statistical power. *False discovery rate correction for multiple comparisons; ^Bonferroni correction. A summarized table on the prediction accuracy estimation by including 1) Pearson correlation, 2) Spearman correlation, 3) partial correlation by regressing out gender, 4) normalized root-mean-square prediction error, 5) permutation test, and 6) statistical power can be found in Table S4. AUDIT, Alcohol Use Disorders Identification Test; FTND, Fagerström Test for Nicotine Dependence; GM, gray matter.
Within IMAGEN: Longitudinal Risk Predictions in 19-Year-Old Adolescents
The same predictive models and the brain ROIs identified on the subset in 14-year-old adolescents were generalized to predict AUDIT (r = .263, pperm = 9.0 × 10−5), FTND (r = .178, pperm = .0081), hyperactivity (r = .285, pperm = .0084), depression (r = .268, pperm = 3.0 × 10−4), and psychosis (r = .325, pperm = .0011) rating scores on the same subjects at age 19 (Figure 4C), when most psychopathological symptoms become manifest at this age. More importantly, the same models can also be generalized to predict risks for a large number of new, previously unseen subjects at age 19 years on AUDIT (n = 1021, r = .153, pperm = 1.0 × 10−5), FTND (n = 1105, r = .102, pperm = 6.0 × 10−4), hyperactivity (n = 1037, r = .144, pperm = 1.0 × 10−3), depression (n = 1011, r = .132, pperm = 2.0 × 10−5), and psychosis (n = 444, r = .186, pperm = 5.0 × 10−4) ratings, as shown in Figure 4D. Similarly, the results calculated with Spearman correlations or gender controlled still remain significant (Figures S4 and S5). For psychosis, the variability of psychosis scores is not homogeneous, i.e., most of the adolescents do not have psychotic symptoms; a few of them have a risk for developing psychosis, but they are not diagnosed as having SZ. Thus, we also performed a two-sample t test of these outlier subjects between the other subjects. For the outliers with significantly higher true psychosis rating scales, the predicted values were also significantly higher (p < 1.0 × 10–20) than those of the others. Longitudinal prediction on the same subjects also holds sufficient statistical power.
Non-IMAGEN: External Disease Symptom Predictions
Both the prediction models and the brain ROIs identified in 14-year-old adolescents can be successfully generalized to predict symptoms for five types of diagnosed patients, spanning AUDIT for drinkers (r = .306, pperm = 2.0 × 10−7), FTND for smokers (r = .341, pperm = 5.0 × 10−4), inattentive for ADHD (r = .206, pperm = 7.0 × 10−4), Hamilton Depression Rating Scale for MDD (r = .385, pperm = 1.0 × 10−4) and Positive and Negative Syndrome Scale Negative for SZ (r = .211, pperm = .0122), as displayed in Figure 4E. The predictions were adequately powered (1−β = .99, .95, .94, .95, .84) and also remain significant with either Spearman correlation or gender controlled (Figures S4 and S5). Although the identified high-NS–associated brain ROIs can be used to predict five different types of symptom scores, the contribution weights (β1, β2,.., β12 as in equation 2) of imaging features for each disease model are different (Figure S6).
Potential to N-Way Classification
Besides predicting the continuous values such as disease severity, the potential of the identified high-NS–associated multimodal features to discriminate multiple types of patients (multiclass classification) were also tested. The same imaging features as used in symptom prediction were adopted, with the site, age, and gender regressed out before classification. As displayed in Figure 5A, the binary classification accuracy between control subjects (n = 1094) and all kinds of patients (n = 965) is 84.4%. For multigroup classification, accuracy of 74.9% (Figure 5B), 79.4% (Figure 5C), and 87.2% (Figure 5D) was achieved, respectively, for six-group, five-group, and three-group classifications. Particularly, the area under the curve is 0.98 for classifying ADHD, MDD, and SZ; in all cases, the area under the curve is higher than 0.91, showing promise for clinical utility of the identified NS-associated biomarkers. Furthermore, the classification results were not consistent with the null distribution (36) (Figure S7).
Figure 5.

Receiver operating characteristic curves and confusion matrices obtained from the classification based on the identified features. (A) Binary classification between all the patients and healthy control subjects (HC). (B) Six-group classification among HC, alcohol drinking, smoking, attention-deficit/hyperactivity disorder (ADHD), major depressive disorder (MDD), and schizophrenia (SZ). (C) Five-group classification among HC, alcohol drinking, ADHD, MDD, and SZ. (D) Three-group classification among ADHD, MDD, and SZ. The rows in each confusion matrix show the true group label, and the columns show the predicted label. The diagonal colorful cells (true positive rate) show where the true labels and predicted labels match. The off-diagonal cells (gray, false-negative rate) represent the misclassified percentage. ACC, accuracy; AUC, area under the curve; ROC, receiver operating characteristic.
A Shared Multimodal Network
To find a generalized dysfunctional brain network spanning drinking, smoking, hyperactivity, depression, and psychosis, we further overlay all the identified high-NS–associated brain regions across four modalities (Figure S8), with different task fMRI capturing different brain-activated subregions. Consequently, the prefrontal cortex (PFC), striatum, amygdala, and hippocampus are shown as the most consistent brain regions over the four modalities, which are also the key nodes of the human reward-related system.
DISCUSSION
Based on rigorous longitudinal and transdiagnostic cross-validation leveraging six independent datasets, our data-driven analysis revealed that there is a degree of dependence among drinking, smoking, ADHD, MDD, and SZ and suggested that an apparent partially shared deficit in addiction and psychiatric disorders may arise from a common NS-associated dysfunction (37). Results suggest that 1) high NS in adolescents is associated with alterations in the reward-related system that may implicate higher risk for subsequent development of the abovementioned disorders, and 2) the identified high-NS–associated multimodal network may serve as a transdiagnostic neuroimaging biomarker to predict disease severity as well as classify among ADHD, MDD, and SZ.
Specifically, the most spatially consistent brain regions identified associated with high NS in four modalities were the PFC, striatum, amygdala, and hippocampus. Both the ventral striatum and PFC are key components of the reward system (38–40), while the amygdala and hippocampus are core regions involved in the regulation of reward (41). Moreover, it is striking that a common set of imaging signatures involving the reward-related system may predict individuals on their subsequent development of the five dissimilar clinical syndromes here studied. One recent genetic study demonstrated that common variant risk for psychiatric disorders was shown to correlate significantly among ADHD, MDD, bipolar disorder, and SZ (16). Another large genome-wide association study also shows that alcohol dependence reveals a common genetic underpinning with psychiatric disorders, including SZ, ADHD, and MDD (42). Both suggest that there is shared genetic substrate among addiction and psychiatric disorders. Coincidently, we discovered imaging evidence of high-NS–guided brain alternations sharing among five disorders. Overall, these findings are consistent with the fact that addiction and psychiatric disorders share genetic and environmental risk factors in their critical neurodevelopmental period, which manifests as subtle brain abnormalities involving the reward system and subclinical behaviors during childhood/adolescence before the full clinical syndrome manifests at an older age.
There are studies that support the link between high NS and dopaminergic activity via the dopamine D4 receptor gene (DRD4) (43,44). Moreover, individuals who had longer alleles of DRD4 had higher NS scores than individuals with the shorter allele (45). The overall effect of dopamine when exposed to a novel stimulus is a mass release of the neurotransmitter in the reward systems of the brain (46). Because of this effect, NS has been linked to personality disorders as well as substance abuse and other addictive behaviors (5). At the behavioral level, both human and rodent models demonstrate that high NS can predict the initiation of addiction (15,47). At the molecular level, both NS and addiction are modulated by the reward system in the brain through dopamine (3). There is also robust evidence showing that SZ involves increased striatal presynaptic release, and all antipsychotic drugs used to treat SZ are dopamine D2 receptor blockers (48). Conversely, ADHD and MDD are associated with decreased dopamine activity (49,50). More specifically, stimulants are the standard treatment for ADHD, and they work by increasing the availability of synaptic dopamine (51). These findings are generally consistent with our results that the identified high-NS–associated multimodal features can predict risk/symptom scores for not only addiction but also psychiatric disorders, including ADHD, MDD, and SZ. Therefore, our results converge with this broad literature and support the premise that dopaminergic dysregulation of NS-associated brain networks underlies high NS behavior in addiction and psychiatric disorders.
This work also helps refocus the clinical community on the risk biomarker identification. That the high-NS–associated multimodal reward-related imaging features differentiate between patients and healthy control subjects with 84.4% accuracy is informative but not particularly useful. However, the 87.2% accuracy in classification among SZ, MDD, and ADHD groups is potentially clinically significant. Because approximately 50% of patients with psychiatric illness have at least one additional lifetime diagnosis of a comorbidity (18,52), reliable diagnostic classification remains challenging (53). For example, prevalence of depression in patients with SZ is 25% (54), and adolescent depression may progress to SZ (55). Furthermore, the comorbidity of SZ and depression makes the diagnosis of schizoaffective disorder particularly difficult (56), especially in adolescence (57). Although diagnostic classification may be straightforward in many patients, there remains a significant subgroup (58), often of hard-to-treat patients, for whom an additional biomarker analysis may increase diagnostic reliability (59) and support treatment decisions. Hence, this study’s ability to discriminate between depression and SZ may be developed into a useful clinical application. For these patients, performing a 10-minute structural MRI and resting-state fMRI acquisition is potentially feasible. Although we emphasized that the identified high-NS–associated multimodal reward circuit may serve as a common dysfunction underlying drinking, smoking, ADHD, MDD, and SZ, its specificity in discriminating among ADHD, MDD, and SZ indicates that this common reward network may work differently or alter to a different extent among these three disorders.
Note that for the external patient cohorts, we had only resting-state fMRI data instead of the same task-related fMRI data as in IMAGEN. Nonetheless, we extracted the same ROIs from resting-state fMRI as those identified from task-related fMRI. The structural scans of non-IMAGEN cohorts also show certain difference from the IMAGEN/ADNI protocol. However, our study shows excellent reproducible performance from task to rest brain networks, without specifically matching the imaging parameters, either in prediction or in classification. This suggests that it is the combination of the subregions of the reward network from each task modality playing the most important role in prediction, which is also verified when removing one predictor related to either emotion processing (Figure S9) or inhibition control (Figure S10); consequently, the prediction accuracy and generalizability in both longitudinal adolescents and external patient cohorts decreased. Nonetheless, replication in a new clinical cohort (non-IMAGEN) will be necessary with greater emphasis on specific recommendations for clinical implementation (60).
One possible limitation is that we used only NS as the reference to identify multimodal neuroimaging features. There may be other useful personality or social functional features to serve as a reference, such as harm avoidance, which is more associated with MDD than is NS (61). It is possible that the harm avoidance–associated multimodal features may achieve better prediction for a single disorder, such as MDD. However, our study focused on identifying a common and generalized imaging pattern emerging in adolescence that could predict the development of psychiatric disorders. We did not tailor the study to predict the development of one specific disorder. Note that though some of the predicted measures (Figure 4) involve discrete values, we also provide several different predictive accuracy estimation strategies (Table S2), and each of these criteria provides a different approach to measure the predictive accuracy. Although site, age, and gender were regressed out from the multimodal feature matrix before classification analysis, site and age (the ADHD group is younger than the other groups) should be considered as potential confounding factors when interpreting the classification results. Another limitation involves the impact of comorbidities. We confirm that the ADHD subjects had no current or past history of other psychiatric or neurological illness. However, for the other non-IMAGEN patient groups (SZ and MDD), comorbidity assessments for smoking and drinking were not available. Future analysis should examine the classification ability of the identified high-NS–associated multimodal features on comorbid psychiatric conditions.
Conclusions
In conclusion, a specific brain network involving reward-related structures (i.e., the PFC, striatum, amygdala, and hippocampus) appears to underlie the personality trait of NS in mid-adolescence. Variation in this network could predict the development of various dysfunctional behaviors in late adolescence. It also predicts symptom severity in the corresponding clinical populations (i.e., smokers, alcoholics, ADHD, SZ, and MDD). Finally, this network variation can accurately classify among the ADHD, SZ, and MDD groups, highlighting the potential of a multimodal neuroimaging approach for future biomarker development. Collectively, this study goes beyond a specific psychiatric condition to identify shared neuroimaging patterns in multiple brain disorders (62) by multimodal fusion and tests the role of transdiagnostic risk factors by both longitudinal risk prediction and cross-patient classification validation.
Supplementary Material
ACKNOWLEDGMENTS AND DISCLOSURES
This work was supported by the China Natural Science Foundation (Grant Nos. 82022035 and 61773380 [to JS]), the National Institutes of Health (NIH Grant Nos. R01EB005846, R01MH117107, P20GM103472, and P30GM122734 [to VDC]), the National Science Foundation (Grant No. 1539067 [to VDC]), Beijing Municipal Science and Technology Commission (Grant No. Z181100001518005 [to JS]), the European Union-funded FP6 Integrated Project IMAGEN (Reinforcement-related behavior in normal brain function and psychopathology) (Grant No. LSHM-CT-2007-037286 [to GS]), the Horizon 2020-funded ERC Advanced Grant ‘STRATIFY’ (Brain network based stratification of reinforcement-related disorders) (Grant No. 695313 [to GS]), ERANID (Understanding the Interplay between Cultural, Biological and Subjective Factors in Drug Use Pathways) (Grant No. PR-ST-0416-10004), BRIDGET (JPND: BRain Imaging, cognition Dementia and next generation GEnomics) (Grant No. MR/N027558/1), Human Brain Project (HBP SGA 2, Grant No. 785907), the FP7 project MATRICS (Grant No. 603016), the Medical Research Council Grant ‘c-VEDA’ (Consortium on Vulnerability to Externalizing Disorders and Addictions) (Grant No. MR/N000390/1), the National Institute for Health Research Biomedical Research Centre at South London and Maudsley NHS Foundation Trust and King’s College London, the Bundesministerium für Bildung und Forschung (Grant Nos. 01GS08152 and 01EV0711; Forschungsnetz AERIAL Grant Nos. 01EE1406A and 01EE1406B), the Deutsche Forschungsgemeinschaft (DFG Grant Nos. SM 80/7-2, SFB 940/2, and NE 1383/14-1), the Medical Research Foundation and Medical Research Council (Grant Nos. MR/R00465X/1 and MR/S020306/1), and the NIH-funded Enhancing Neuro Imaging Genetics through Meta Analysis (Grant Nos. 5U54EB020403-05 and 1R56AG058854-01). Further support was provided by grants from ANR (project AF12-NEUR0008-01 - WM2NA and ANR-12-SAMA-0004), the Fondation de France, the Fondation pour la Recherche Médicale, the Mission Interministérielle de Lutte-contre-les-Drogues-et-les-Conduites-Addictives (MILDECA), the Assistance-Publique-Hôpitaux-de-Paris and INSERM (interface grant), Paris Sud University IDEX 2012; the NIH, Science Foundation Ireland (Grant No. 16/ERCD/3797), U.S.A. (Axon, Testosterone and Mental Health during Adolescence; Grant No. RO1 MH085772-01A1), and by NIH Consortium Grant No. U54 EB020403, supported by a cross-NIH alliance that funds Big Data to Knowledge Centres of Excellence.
Footnotes
The authors report no biomedical financial interests or potential conflicts of interest.
See the Supplement for the full list of IMAGEN Consortium collaborators.
The supervised fusion code has been released and integrated in the Fusion ICA Toolbox (FIT, https://trendscenter.org/software/fit), which can be downloaded and used directly by users worldwide. The IMAGEN and ADHD multimodal data used in this study can be accessed upon application from IMAGEN and ADHD-200 Consortium. The SZ, MDD, drinking, and smoking data can be accessed upon request to the corresponding authors.
IMAGEN Consortium Authors: Gunter Schumann, M.D.; Tobias Banaschewski, M.D., Ph.D.; Gareth J. Barker, Ph.D.; Arun L.W. Bokde, Ph.D.; Erin Burke Quinlan, Ph.D.; Sylvane Desrivières, Ph.D.; Herta Flor, Ph.D.; Antoine Grigis, Ph.D.; Hugh Garavan, Ph.D.; Penny Gowland, Ph.D.; Andreas Heinz, M.D., Ph.D.; Jean-Luc Martinot, M.D.; Marie-Laure Paillère Martinot, M.D.; Eric Artiges, M.D.; Frauke Nees, Ph.D.; Dimitri Papadopoulos Orfanos, Ph.D.; Tomáš Paus, M.D., Ph.D.; Luise Poustka, M.D.; Sarah Hohmann, M.D.; Juliane H. Fröhner, M.S.; Michael N. Smolka, M.D.; Henrik Walter, M.D., Ph.D.; Robert Whelan, Ph.D.
Supplementary material cited in this article is available online at https://doi.org/10.1016/j.biopsych.2021.01.011.
Contributor Information
Shile Qi, Tri-institutional Center for Translational Research in Neuroimaging and Data Science, Georgia State University, Georgia Institute Technology, and Emory University, Atlanta, Georgia; Department of Computer Science and Engineering, Nanjing University of Aeronautics and Astronautics, Nanjing.
Gunter Schumann, Centre for Population Neuroscience and Stratified Medicine, Institute for Science and Technology of Brain-Inspired Intelligence, Fudan University, Shanghai.
Juan Bustillo, Department of Psychiatry, University of New Mexico, Albuquerque, New Mexico.
Jessica A. Turner, Department of Psychology, Georgia State University, Atlanta, Georgia
Rongtao Jiang, University of Chinese Academy of Sciences, Beijing; Brainnetome Center and National Laboratory of Pattern Recognition, Institute of Automation, Chinese Academy of Sciences, Beijing.
Dongmei Zhi, University of Chinese Academy of Sciences, Beijing; Brainnetome Center and National Laboratory of Pattern Recognition, Institute of Automation, Chinese Academy of Sciences, Beijing.
Zening Fu, Tri-institutional Center for Translational Research in Neuroimaging and Data Science, Georgia State University, Georgia Institute Technology, and Emory University, Atlanta, Georgia.
Andrew R. Mayer, Department of Psychiatry, University of New Mexico, Albuquerque, New Mexico
Victor M. Vergara, Tri-institutional Center for Translational Research in Neuroimaging and Data Science, Georgia State University, Georgia Institute Technology, and Emory University, Atlanta, Georgia
Rogers F. Silva, Tri-institutional Center for Translational Research in Neuroimaging and Data Science, Georgia State University, Georgia Institute Technology, and Emory University, Atlanta, Georgia
Armin Iraji, Tri-institutional Center for Translational Research in Neuroimaging and Data Science, Georgia State University, Georgia Institute Technology, and Emory University, Atlanta, Georgia.
Jiayu Chen, Tri-institutional Center for Translational Research in Neuroimaging and Data Science, Georgia State University, Georgia Institute Technology, and Emory University, Atlanta, Georgia.
Eswar Damaraju, Tri-institutional Center for Translational Research in Neuroimaging and Data Science, Georgia State University, Georgia Institute Technology, and Emory University, Atlanta, Georgia.
Xiaohong Ma, Psychiatric Laboratory and Mental Health Center, the State Key Laboratory of Biotherapy, West China Hospital of Sichuan University, Chengdu.
Xiao Yang, Psychiatric Laboratory and Mental Health Center, the State Key Laboratory of Biotherapy, West China Hospital of Sichuan University, Chengdu.
Michael Stevens, Olin Neuropsychiatry Research Center, Hartford, Connecticut.
Daniel H. Mathalon, Department of Psychiatry, University of California San Francisco, San Francisco, California
Judith M. Ford, Department of Psychiatry, University of California San Francisco, San Francisco, California
James Voyvodic, Department of Radiology, Duke University, Durham.
Bryon A. Mueller, Department of Psychiatry, University of Minnesota, Minneapolis, Minnesota
Aysenil Belger, Department of Psychiatry, University of North Carolina at Chapel Hill, Chapel Hill, North Carolina.
Steven G. Potkin, Department of Psychiatry, University of California Irvine, Irvine, California
Adrian Preda, Department of Psychiatry, University of California Irvine, Irvine, California.
Chuanjun Zhuo, Department of Psychiatric-Neuroimaging-Genetics and Morbidity Laboratory, Nankai University Affiliated Anding Hospital, Tianjin.
Yong Xu, Department of Humanities and Social Science, Shanxi Medical University, Taiyuan, China.
Congying Chu, Centre for Population Neuroscience and Stratified Medicine, Institute for Science and Technology of Brain-Inspired Intelligence, Fudan University, Shanghai.
Tobias Banaschewski, Department of Child and Adolescent Psychiatry and Psychotherapy, Central Institute of Mental Health, Medical Faculty Mannheim, Heidelberg University, Mannheim.
Gareth J. Barker, Department of Neuroimaging, Institute of Psychiatry, Psychology & Neuroscience, King’s College London, London
Arun L.W. Bokde, Discipline of Psychiatry, School of Medicine and Trinity College Institute of Neuroscience, Trinity College Dublin, Dublin, Ireland
Erin Burke Quinlan, Centre for Population Neuroscience and Stratified Medicine, Institute for Science and Technology of Brain-Inspired Intelligence, Fudan University, Shanghai.
Sylvane Desrivières, Centre for Population Neuroscience and Stratified Medicine, Institute for Science and Technology of Brain-Inspired Intelligence, Fudan University, Shanghai.
Herta Flor, Department of Psychology, School of Social Sciences, University of Mannheim, Mannheim, Germany.
Antoine Grigis, NeuroSpin, CEA, Université Paris-Saclay, Gif-sur-Yvette.
Hugh Garavan, Departments of Psychiatry and Psychology, University of Vermont, Burlington, Vermont.
Penny Gowland, Sir Peter Mansfield Imaging Centre School of Physics and Astronomy, University of Nottingham, University Park, Nottingham, United Kingdom.
Andreas Heinz, Department of Psychiatry and Psychotherapy, Charité – Universitätsmedizin Berlin, Campus Charité Mitte, corporate member of Freie Universität Berlin, Humboldt-Universität zu Berlin, and Berlin Institute of Health, Department of Psychiatry and Psychotherapy, Campus Charité Mitte, Berlin.
Jean-Luc Martinot, Institut National de la Santé et de la Recherche Médicale, INSERM Unit 1000 “Neuroimaging & Psychiatry,” University Paris-Saclay, Paris, France.
Marie-Laure Paillère Martinot, Institut National de la Santé et de la Recherche Médicale, INSERM Unit 1000 “Neuroimaging & Psychiatry,” University Paris-Saclay, Paris, France.
Eric Artiges, Institut National de la Santé et de la Recherche Médicale, INSERM Unit 1000 “Neuroimaging & Psychiatry,” University Paris-Saclay, Paris, France.
Frauke Nees, Department of Child and Adolescent Psychiatry and Psychotherapy, Central Institute of Mental Health, Medical Faculty Mannheim, Heidelberg University, Mannheim.
Dimitri Papadopoulos Orfanos, NeuroSpin, CEA, Université Paris-Saclay, Gif-sur-Yvette.
Tomáš Paus, Bloorview Research Institute, Holland Bloorview Kids Rehabilitation Hospital and Departments of Psychology and Psychiatry, University of Toronto, Toronto, Ontario, Canada..
Luise Poustka, Department of Child and Adolescent Psychiatry and Psychotherapy, University Medical Centre Göttingen, Göttingen.
Sarah Hohmann, Department of Child and Adolescent Psychiatry and Psychotherapy, Central Institute of Mental Health, Medical Faculty Mannheim, Heidelberg University, Mannheim.
Juliane H. Fröhner, Department of Psychiatry and Neuroimaging Center, Technische Universität Dresden, Dresden
Michael N. Smolka, Department of Psychiatry and Neuroimaging Center, Technische Universität Dresden, Dresden
Henrik Walter, Department of Psychiatry and Psychotherapy, Charité – Universitätsmedizin Berlin, Campus Charité Mitte, corporate member of Freie Universität Berlin, Humboldt-Universität zu Berlin, and Berlin Institute of Health, Department of Psychiatry and Psychotherapy, Campus Charité Mitte, Berlin.
Robert Whelan, PONS Research Group, Department of Psychiatry and Psychotherapy, Campus Charité Mitte, Humboldt University, Berlin.
Vince D. Calhoun, Tri-institutional Center for Translational Research in Neuroimaging and Data Science, Georgia State University, Georgia Institute Technology, and Emory University, Atlanta, Georgia Department of Psychology, Georgia State University, Atlanta, Georgia.
Jing Sui, Tri-institutional Center for Translational Research in Neuroimaging and Data Science, Georgia State University, Georgia Institute Technology, and Emory University, Atlanta, Georgia; University of Chinese Academy of Sciences, Beijing; State Key Laboratory of Cognitive Neuroscience and Learning, Beijing Normal University, Beijing.
REFERENCES
- 1.Wang Y, Liu Y, Yang L, Gu F, Li X, Zha R, et al. (2015): Novelty seeking is related to individual risk preference and brain activation associated with risk prediction during decision making. Sci Rep 5:10534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nees F, Tzschoppe J, Patrick CJ, Vollstädt-Klein S, Steiner S, Poustka L, et al. (2012): Determinants of early alcohol use in healthy adolescents: The differential contribution of neuroimaging and psychological factors. Neuropsychopharmacology 37:986–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wingo T, Nesil T, Choi JS, Li MD (2016): Novelty seeking and drug addiction in humans and animals: From behavior to molecules. J Neuroimmune Pharmacol 11:456–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Peters J, Bromberg U, Schneider S, Brassen S, Menz M, Banaschewski T, et al. (2011): Lower ventral striatal activation during reward anticipation in adolescent smokers. Am J Psychiatry 168:540–549. [DOI] [PubMed] [Google Scholar]
- 5.Büchel C, Peters J, Banaschewski T, Bokde ALW, Bromberg U, Conrod PJ, et al. (2017): Blunted ventral striatal responses to anticipated rewards foreshadow problematic drug use in novelty-seeking adolescents. Nat Commun 8:14140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wan Z, Rolls ET, Cheng W, Feng J (2020): Sensation-seeking is related to functional connectivities of the medial orbitofrontal cortex with the anterior cingulate cortex. Neuroimage 215:116845. [DOI] [PubMed] [Google Scholar]
- 7.Ernst M, Pine DS, Hardin M (2006): Triadic model of the neurobiology of motivated behavior in adolescence. Psychol Med 36:299–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cheng W, Rolls ET, Robbins TW, Gong W, Liu Z, Lv W, et al. (2019): Decreased brain connectivity in smoking contrasts with increased connectivity in drinking. eLife 8:e40765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Navarri X, Afzali MH, Lavoie J, Sinha R, Stein DJ, Momenan R, et al. (2020): How do substance use disorders compare to other psychiatric conditions on structural brain abnormalities? A cross-disorder meta-analytic comparison using the ENIGMA consortium findings [published online ahead of print Jul 9]. Hum Brain Mapp. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kerestes R, Davey CG, Stephanou K, Whittle S, Harrison BJ (2013): Functional brain imaging studies of youth depression: A systematic review. NeuroImage Clin 4:209–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Olino TM, McMakin DL, Morgan JK, Silk JS, Birmaher B, Axelson DA, et al. (2014): Reduced reward anticipation in youth at high-risk for unipolar depression: A preliminary study. Dev Cogn Neurosci 8:55–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cheng W, Rolls ET, Qiu J, Liu W, Tang Y, Huang CC, et al. (2016): Medial reward and lateral non-reward orbitofrontal cortex circuits change in opposite directions in depression. Brain 139:3296–3309. [DOI] [PubMed] [Google Scholar]
- 13.Cheng W, Rolls ET, Qiu J, Yang D, Ruan H, Wei D, et al. (2018): Functional connectivity of the precuneus in unmedicated patients with depression. Biol Psychiatry Cogn Neurosci Neuroimaging 3:1040–1049. [DOI] [PubMed] [Google Scholar]
- 14.Santucci K (2012): Psychiatric disease and drug abuse. Curr Opin Pediatr 24:233–237. [DOI] [PubMed] [Google Scholar]
- 15.Zald DH, Cowan RL, Riccardi P, Baldwin RM, Ansari MS, Li R, et al. (2008): Midbrain dopamine receptor availability is inversely associated with novelty-seeking traits in humans. J Neurosci 28:14372–14378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brainstorm Consortium, Anttila V, Bulik-Sullivan B, Finucane HK, Walters RK, Bras J, et al. (2018): Analysis of shared heritability in common disorders of the brain. Science 360:eaap8757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barch DM, Sheffield JM (2014): Cognitive impairments in psychotic disorders: Common mechanisms and measurement. World Psychiatry 13:224–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Qi S, Bustillo J, Turner JA, Jiang R, Zhi D, Fu Z, et al. (2020): The relevance of transdiagnostic shared networks to the severity of symptoms and cognitive deficits in schizophrenia: A multimodal brain imaging fusion study. Transl Psychiatry 10:149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sha Z, Wager TD, Mechelli A, He Y (2019): Common dysfunction of large-scale neurocognitive networks across psychiatric disorders. Biol Psychiatry 85:379–388. [DOI] [PubMed] [Google Scholar]
- 20.Plana-Ripoll O, Pedersen CB, Holtz Y, Benros ME, Dalsgaard S, de Jonge P, et al. (2019): Exploring comorbidity within mental disorders among a Danish national population. JAMA Psychiatry 76:259–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hirschfeld RM, Calabrese JR, Weissman MM, Reed M, Davies MA, Frye MA, et al. (2003): Screening for bipolar disorder in the community. J Clin Psychiatry 64:53–59. [DOI] [PubMed] [Google Scholar]
- 22.Pan A, Sun Q, Okereke OI, Rexrode KM, Hu FB (2011): Depression and risk of stroke morbidity and mortality: A meta-analysis and systematic review. JAMA 306:1241–1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schumann G, Loth E, Banaschewski T, Barbot A, Barker G, Büchel C, et al. (2010): The IMAGEN study: Reinforcement-related behaviour in normal brain function and psychopathology. Mol Psychiatry 15:1128–1139. [DOI] [PubMed] [Google Scholar]
- 24.Heinrich A, Müller KU, Banaschewski T, Barker GJ, Bokde ALW, Bromberg U, et al. (2016): Prediction of alcohol drinking in adolescents: Personality-traits, behavior, brain responses, and genetic variations in the context of reward sensitivity. Biol Psychol 118:79–87. [DOI] [PubMed] [Google Scholar]
- 25.Albaugh MD, Orr C, Chaarani B, Althoff RR, Allgaier N, D’Alberto N, et al. (2017): Inattention and reaction time variability are linked to ventromedial prefrontal volume in adolescents. Biol Psychiatry 82:660–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Quinlan EB, Cattrell A, Jia T, Artiges E, Banaschewski T, Barker G, et al. (2017): Psychosocial stress and brain function in adolescent psychopathology. Am J Psychiatry 174:785–794. [DOI] [PubMed] [Google Scholar]
- 27.Bourque J, Spechler PA, Potvin S, Whelan R, Banaschewski T, Bokde ALW, et al. (2017): Functional neuroimaging predictors of self-reported psychotic symptoms in adolescents. Am J Psychiatry 174:566–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Qi S, Calhoun VD, van Erp TGM, Bustillo J, Damaraju E, Turner JA, et al. (2018): Multimodal fusion with reference: Searching for joint neuromarkers of working memory deficits in schizophrenia. IEEE Trans Med Imaging 37:93–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cloninger CR, Svrakic DM, Przybeck TR (1993): A psychobiological model of temperament and character. Arch Gen Psychiatry 50:975–990. [DOI] [PubMed] [Google Scholar]
- 30.Saunders JB, Aasland OG, Babor TF, de la Fuente JR, Grant M (1993): Development of the Alcohol Use Disorders Identification Test (AUDIT): WHO Collaborative project on early detection of persons with harmful alcohol consumption–II. Addiction 88:791–804. [DOI] [PubMed] [Google Scholar]
- 31.Heatherton TF, Kozlowski LT, Frecker RC, Fagerström KO (1991): The Fagerström Test for Nicotine Dependence: A revision of the Fagerström Tolerance Questionnaire. Br J Addict 86:1119–1127. [DOI] [PubMed] [Google Scholar]
- 32.Goodman R, Ford T, Richards H, Gatward R, Meltzer H (2000): The Development and Well-Being Assessment: Description and initial validation of an integrated assessment of child and adolescent psychopathology. J Child Psychol Psychiatry 41:645–655. [PubMed] [Google Scholar]
- 33.Sui J, Qi S, van Erp TGM, Bustillo J, Jiang R, Lin D, et al. (2018): Multimodal neuromarkers in schizophrenia via cognition-guided MRI fusion. Nat Commun 9:3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhi D, Calhoun VD, Lv L, Ma X, Ke Q, Fu Z, et al. (2018): Aberrant dynamic functional network connectivity and graph properties in major depressive disorder. Front Psychiatry 9:339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Qi S, Yang X, Zhao L, Calhoun VD, Perrone-Bizzozero N, Liu S, et al. (2018): MicroRNA132 associated multimodal neuroimaging patterns in unmedicated major depressive disorder. Brain 141:916–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stelzer J, Chen Y, Turner R (2013): Statistical inference and multiple testing correction in classification-based multi-voxel pattern analysis (MVPA): Random permutations and cluster size control. Neuroimage 65:69–82. [DOI] [PubMed] [Google Scholar]
- 37.Keshavan MS (2019): Impaired insight in psychotic disorder: An unmet need in treatment. Schizophr Res 206:2–3. [DOI] [PubMed] [Google Scholar]
- 38.Reuter J, Raedler T, Rose M, Hand I, Gläscher J, Büchel C (2005): Pathological gambling is linked to reduced activation of the mesolimbic reward system. Nat Neurosci 8:147–148. [DOI] [PubMed] [Google Scholar]
- 39.Schneider S, Peters J, Bromberg U, Brassen S, Miedl SF, Banaschewski T, et al. (2012): Risk taking and the adolescent reward system: A potential common link to substance abuse. Am J Psychiatry 169:39–46. [DOI] [PubMed] [Google Scholar]
- 40.Xu Q, Liu F, Qin W, Jiang T, Yu C (2020): Multiscale neurobiological correlates of human neuroticism. Hum Brain Mapp 41:4730–4743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Haber SN, Knutson B (2010): The reward circuit: Linking primate anatomy and human imaging. Neuropsychopharmacology 35:4–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Walters RK, Polimanti R, Johnson EC, McClintick JN, Adams MJ, Adkins AE, et al. (2018): Transancestral GWAS of alcohol dependence reveals common genetic underpinnings with psychiatric disorders. Nat Neurosci 21:1656–1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ebstein RP, Novick O, Umansky R, Priel B, Osher Y, Blaine D, et al. (1996): Dopamine D4 receptor (D4DR) exon III polymorphism associated with the human personality trait of novelty seeking. Nat Genet 12:78–80. [DOI] [PubMed] [Google Scholar]
- 44.Buskila D, Cohen H, Neumann L, Ebstein RP (2004): An association between fibromyalgia and the dopamine D4 receptor exon III repeat polymorphism and relationship to novelty seeking personality traits. Mol Psychiatry 9:730–731. [DOI] [PubMed] [Google Scholar]
- 45.Lusher JM, Chandler C, Ball D (2001): Dopamine D4 receptor gene (DRD4) is associated with Novelty Seeking (NS) and substance abuse: The saga continues. Mol Psychiatry 6:497–499. [DOI] [PubMed] [Google Scholar]
- 46.Volkow ND, Wang GJ, Fowler JS, Tomasi D, Telang F (2011): Addiction: beyond dopamine reward circuitry. Proc Natl Acad Sci U S A 108:15037–15042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Laucht M, Becker K, Blomeyer D, Schmidt MH (2007): Novelty seeking involved in mediating the association between the dopamine D4 receptor gene exon III polymorphism and heavy drinking in male adolescents: Results from a high-risk community sample. Biol Psychiatry 61:87–92. [DOI] [PubMed] [Google Scholar]
- 48.Howes OD, Kapur S (2009): The dopamine hypothesis of schizophrenia: Version III–The final common pathway. Schizophr Bull 35:549–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Volkow ND, Wang GJ, Kollins SH, Wigal TL, Newcorn JH, Telang F, et al. (2009): Evaluating dopamine reward pathway in ADHD: Clinical implications. JAMA 302:1084–1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dunlop BW, Nemeroff CB (2007): The role of dopamine in the pathophysiology of depression. Arch Gen Psychiatry 64:327–337. [DOI] [PubMed] [Google Scholar]
- 51.Wang GJ, Volkow ND, Wigal T, Kollins SH, Newcorn JH, Telang F, et al. (2013): Long-term stimulant treatment affects brain dopamine transporter level in patients with attention deficit hyperactive disorder. PLoS One 8:e63023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Buckley PF, Miller BJ, Lehrer DS, Castle DJ (2009): Psychiatric comorbidities and schizophrenia. Schizophr Bull 35:383–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Peters J, Bromberg U, Schneider S, Brassen S, Menz M, Banaschewski T, et al. (2011): Lower ventral striatal activation during reward anticipation in adolescent smokers. Am J Psychiatry 168:540–549. [DOI] [PubMed] [Google Scholar]
- 54.Siris SG (2000): Management of schizophrenia with depression. Program and abstracts from the 153rd Annual American Psychiatric Association Meeting, May 13–18, 2000; Chicago, Illinois. Abstract 56A. [Google Scholar]
- 55.Musliner KL, Munk-Olsen T, Mors O, Østergaard SD (2017): Progression from unipolar depression to schizophrenia. Acta Psychiatr Scand 135:42–50. [DOI] [PubMed] [Google Scholar]
- 56.Keller J, Schatzberg AF, Maj M (2007): Current issues in the classification of psychotic major depression. Schizophr Bull 33:877–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Salamon S, Santelmann H, Franklin J, Baethge C (2018): Test-retest reliability of the diagnosis of schizoaffective disorder in childhood and adolescence - A systematic review and meta-analysis. J Affect Disord 230:28–33. [DOI] [PubMed] [Google Scholar]
- 58.Feczko E, Miranda-Dominguez O, Marr M, Graham AM, Nigg JT, Fair DA (2019): The heterogeneity problem: Approaches to identify psychiatric subtypes. Trends Cogn Sci 23:584–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mcgorry PD, Mihalopoulos C, Henry L, Dakis J, Jackson HJ, Flaum M, et al. (1995): Spurious precision: Procedural validity of diagnostic assessment in psychotic disorders. Am J Psychiatry 152:220–223. [DOI] [PubMed] [Google Scholar]
- 60.Botvinik-Nezer R, Holzmeister F, Camerer CF, Dreber A, Huber J, Johannesson M, et al. (2020): Variability in the analysis of a single neuroimaging dataset by many teams. Nature 582:84–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chen CY, Lin SH, Li P, Huang WL, Lin YH (2015): The role of the harm avoidance personality in depression and anxiety during the medical internship. Medicine (Baltimore) 94:e389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Thompson PM, Jahanshad N, Ching CRK, Salminen LE, Thomopoulos SI, Bright J, et al. (2020): ENIGMA and global neuroscience: A decade of large-scale studies of the brain in health and disease across more than 40 countries. Transl Psychiatry 10:100. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


